Abstract
Juglans regia Linn. is a valuable medicinal plant that possesses the therapeutic potential to treat a wide range of diseases in humans. It has been known to have significant nutritional and curative properties since ancient times, and almost all parts of this plant have been utilized to cure numerous fungal and bacterial disorders. The separation and identification of the active ingredients in J. regia as well as the testing of those active compounds for pharmacological properties are currently of great interest. Recently, the naphthoquinones extracted from walnut have been observed to inhibit the enzymes essential for viral protein synthesis in the SARS-CoV-2. Anticancer characteristics have been observed in the synthetic triazole analogue derivatives of juglone, and the unique modifications in the parent derivative of juglone have paved the way for further synthetic research in this area. Though there are some research articles available on the pharmacological importance of J. regia, a comprehensive review article to summarize these findings is still required. The current review, therefore, abridges the most recent scientific findings about antimicrobial, antioxidant, anti-fungal, and anticancer properties of various discovered and separated chemical compounds from different solvents and different parts of J. regia.
Keywords: antibacterial, antifungal, antioxidant, anticancer, juglone, Juglans regia
1. Introduction
Juglans regia Linn. belongs to the family Juglandaceae and is an aromatic transient tree that grows in abundance in the North-Western Himalayas of Kashmir that produces most of the world’s walnuts, accounting for around 88% of total walnut production [1]. The bark of the tree is gray, and the tree has longitudinally fissured leaves that are alternating, imparipinnate, sessile, elliptic, or oblong-lanceolate. Flowers are unisexual and there are around two genera and fifteen species in the family. The leaves are evenly organized, measuring 25–40 cm in length, unevenly pinnate with five to nine leaflets and placed in a regular pattern. The medicinal plant Juglans regia Linn. has been used extensively in traditional medicine for a variety of illnesses, including helminthiasis, diarrhea, sinusitis, stomach aches, arthritis, asthma, eczema, scrofula, skin disorders, and various endocrine diseases such as diabetes mellitus, anorexia, thyroid dysfunctions, cancer, and infectious diseases [2,3]. The green fruit ripens in the autumn, when the entire fruit, including the husk, drops from the tree. The huge seed contains a rich flavor and a thin edible shell.
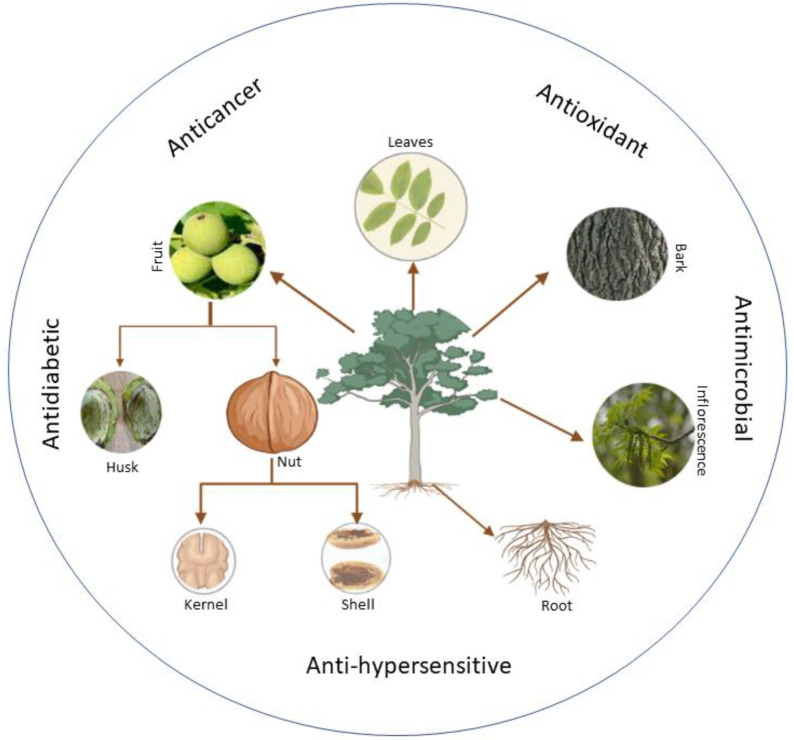
The phytochemistry of the tree has extensively been studied and many important phytochemical activities have been exploited [3]. However, the number of elements may vary from one species to another in a different place based on several parameters, such as geographical location, time, temperature, genetic makeup, and other factors. Several studies on the phytochemical examination of the tree’s various components with numerous health benefits have been performed (Figure 1 and Table 1). According to these studies, the chemical components found in walnuts fluctuate depending on the climate. The oil is rich in polyunsaturated fatty acids, tocopherols and phytosterols. The walnut fruits are valuable, and tasty walnut leaves contain many chemicals, the most prominent of which are Aesculin, Taxifolin-pantocid, Quercetin-glucronide, Kaempferol-rhamnoside, Syringetin-O-Hexoside, Myricetin-3-O-glucoside, Myricetin-3-O-pantocid, and Epicatechin [3,4].
Figure 1.
Different parts of Juglans regia with medicinal properties.
Table 1.
List of compounds in Juglans regia and their pharmacological or biological activities.
| Compound | Class | Structure | Biological Activity | References |
|---|---|---|---|---|
| Gallic Acid | Phenolic |
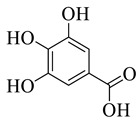
|
Anti-inflammatory | [5,6] |
| Protocatechuic acid | Phenolic |
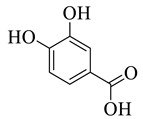
|
Anti-inflammatory and antiapoptotic activities. | [7] |
| Ferulic acid | Phenolic |
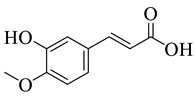
|
Anticancerous | [8] |
| Sinapate | Phenolic |
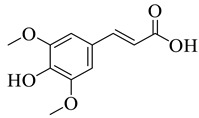
|
Antioxidant, Antimicrobial | [9] |
| Protocatechuic acid derivative | Phenolic |
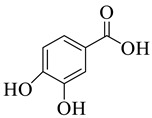
|
Anti-inflammatory | [10] |
| Ellagic acid | Phenolic |
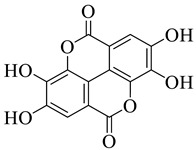
|
Cytotoxic, Anti-proliferative |
[11] |
| p-hydroxybenzoic acid | Phenolic |
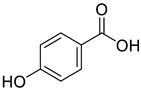
|
Antimicrobial | [12] |
| p-coumaric acid | Phenolic |
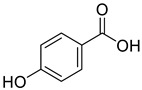
|
Antioxidant | [13] |
| Quercetin 3-galactosid | Phenolic |
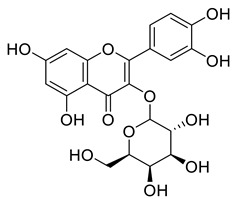
|
Antimicrobial, Antioxidant | [14] |
| Galactose | Phenolic |
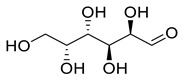
|
Anticancer, Antidiabetic | [15] |
| Pentose | Phenolic |
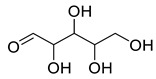
|
Amino acid synthesis | [16,17] |
| Arabinose | Phenolic |
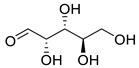
|
Antimicrobial | [18] |
| Xylose | Phenolic |
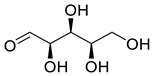
|
Antimicrobial | [19,20] |
| Rhamnose | Phenolic |
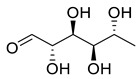
|
Antioxidant, Antimicrobial | [21] |
| Juglone | Phenolic |
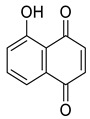
|
Antibacterial, Anticancer, Antioxidant | [22] |
| Caffeic acid | Phenolic |
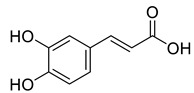
|
Antioxidant, Anti-inflammatory and Anticarcinogenic activity |
[23] |
| Vannilic acid | Phenolic |
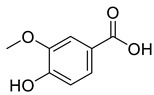
|
Antibacterial, Antioxidant | [24] |
| Quercetin | Flavonoid |
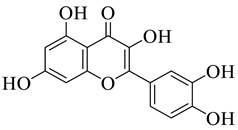
|
Analgesic, Antibacterial, Antiviral | [25] |
| Myricetin | Flavonoid |
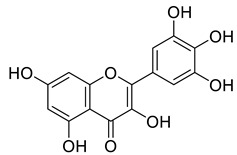
|
Anticancer, Antidiabetic, Antibacterial, Antiviral | [26,27] |
| Kaempferol | Flavonoid |
|
Acute and chronic inflammation | [28] |
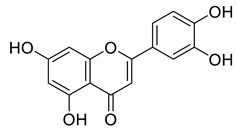
| Apigenin | Flavone |
|
Anti-inflammatory, Antioxidant, Neuroprotective |
[29] |
| Luteolin | Flavone |
|
Anticancer, Anti-inflammatory | [30] |
| Daidzein | Isoflavonoid |
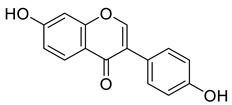
|
Neurobiological activities | [31] |
| Naringenin | Flavanone |
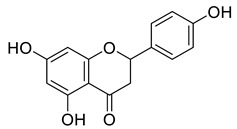
|
Antiviral, Antibacterial, Antioxidant | [32] |
| Hesperetin | Flavonone |
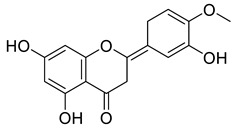
|
Scavenging activity and Cardioprotective activity | [33,34] |
| Gallocatechin | Flavanol |
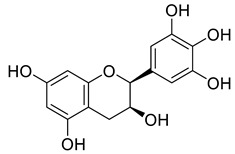
|
Antioxidant, Antitumor | [35] |
| Sitosterol | Steroid |
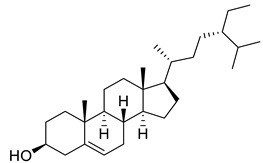
|
Anticancer, Antidiabetic, Antimicrobial | [36] |
| Stigmast-5-en-3β,7α- diol | Steroid |
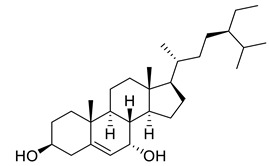
|
Antimicrobial | [37] |
| Campesterol | Steroid |
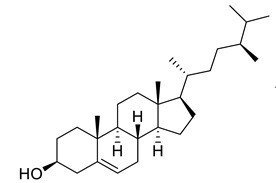
|
Anti-inflammatory, Anticancer, Antidiabetic | [38] |
| Stigmasterol | Steroid |
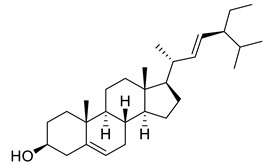
|
Anticancerous Dyslipidemia, diabetes and metabolic syndrome |
[39] |
| Oleanic acid | Tereponoid |
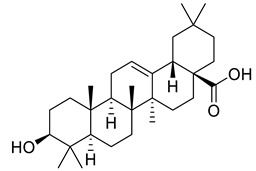
|
Dyslipidemia, diabetes, and metabolic syndrome | [40] |
| 3-alpha-Corosolic acid | Tereponoid |
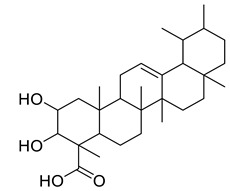
|
Antidiabetic, Anti-obesity, Anti-inflammatory | [41] |
| Urosolic acid | Tereponoid |
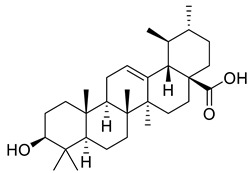
|
Anti-inflammatory, Anticancer, Antidiabetic, Antioxidant | [42] |
| 3-Epikatonic acid | Tereponoid |
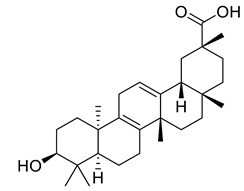
|
Cytotoxic | [43] |
2. Traditional and Ethnobotanical Uses
For ages, the plant has been utilized in tropical medicine to treat cutaneous irritation and excessive perspiration in the hands and feet [44]. The leaves are traditionally used to treat sinusitis and stomach aches and are also used throughout the world as an antibacterial, anthelmintic, antidiarrheal, hypoglycemic, tonic, and depurative medicine [45,46]. In Turkish traditional medicine, fresh leaves are applied to the naked body or the forehead to reduce fever or to swollen joints to treat rheumatic agony [47]. The wood is durable and perfect for furniture and contains essential oils for aromatic essence. As a supplementary ointment, the leaves of this plant are applied directly to cure sunburn, superficial burns, scalp itching, dandruff, and other skin disorders [48]. The plant has been used to treat inflammatory diseases, diabetes, and cardiac illness in Palestine [49] as well as to help older men’s vascular and prostate health [50]. In addition to using bark and unripe fruit for piscicidal action, the Lotha, Angami, and Sumi tribes of Kohima (Nagaland) also employed leaves of J. regia as astringents, anthelmintics, and treatments for dermatitis [51].
3. Pharmacological Applications
The use of medicinal herbs has increased substantially in recent years, resulting in the utilization of both traditional remedies and medicinal plants for the novel drug discoveries, and the development of more effective pharmaceuticals and nutraceuticals with fewer side effects. The main focus of this review is to explore the pharmacological applications, phytochemistry, and the therapeutic benefits of the plant extracts on antimicrobial, antioxidant, anticancer, and anti-inflammatory activities and many others (Table 2).
Table 2.
Phytochemical analysis of different extracts, their effect, dosage, and model organism or experimental reference screened.
| Pharmacological Effect | Extract | Model/Experimental-Reference | Dosage-Range | References |
|---|---|---|---|---|
| Antibacterial | Hull extract of ethanol, ethyl-acetate and water | E. coli, B. subtilis, K. aerogenes, S. Aureus | 5 mg/mL | [52,53] |
| Antibacterial | Aqueous leaf extract | Gram-positive and Gram-negative bacteria | 0.1 mg/mL (MIC) | [54,55] |
| Antibacterial | Essential oil and components | Gram-positive and Gram-negative bacteria | Wide range | [56] |
| Antioxidant | Ethyl acetate, butanol, ether, and aq. Extracts of (kernels, husk, and leaves) | NR | 125 µL | [57] |
| Antioxidant | Leaf extract (ethanol and water) |
NR | 34.5 and 56.4 µg/mL | [58,59] |
| Analgesic and anti-inflammatory | Aqueous and ethanolic extract of leaf | Wistar rats, human arthritis | 250, 500, 1000, 1500 mg/kg | [60] |
| Antiviral | Methanolic extract | HSC, polio virus and SINV | 2 mg/mL | [61] |
| Antidiabetic | Leaf extract | Human type-ii diabetic patients | Wide range | [62] |
| Anticancer | Juglone | Intestinal carcinogenesis | Wide range | [63] |
| Anticancer | Methanol and aqueous extract of leaf | Mice melanoma (B16F10) and human melanoma (A375) cell lines | Wide range | [64] |
| Anticancer | Juglone and modified juglone | (NCl-H322 and A549) Lung cancers | Wide range | [65] |
| Anticancer | Chloroform leaf extract | Human oral and breast cancer cell lines | Wide range | [66] |
3.1. Antibacterial Activity
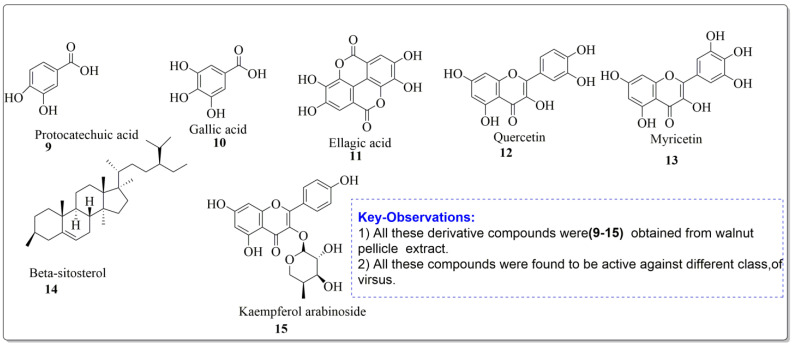
The disc diffusion method (zone of inhibition) was used to identify and examine the antibacterial activity of Juglans regia hull extracts and it was revealed that these extracts possessed an appreciable antibacterial activity against a variety of bacterial species, including E. coli, B. subtilis, K. aerogene, and S. aureus [67]. The findings also implied that J. regia green hull extract could be useful in the treatment of acne due to the virtue of having anti-inflammatory effects. Some leaf extracts of J. regia showed the ability to prevent the growth of K. pneumonia at minimum inhibitory concentrations (MIC) of 100 mg/mL [68]; other extracts from the hull prepared in different solvents were able to stop the development of P. aeruginosa and E. Coli at MIC of 50 and 100 mg/mL, respectively [69]. Several antibacterial chemicals identified from Juglans regia pellicle are represented (Figure 2).
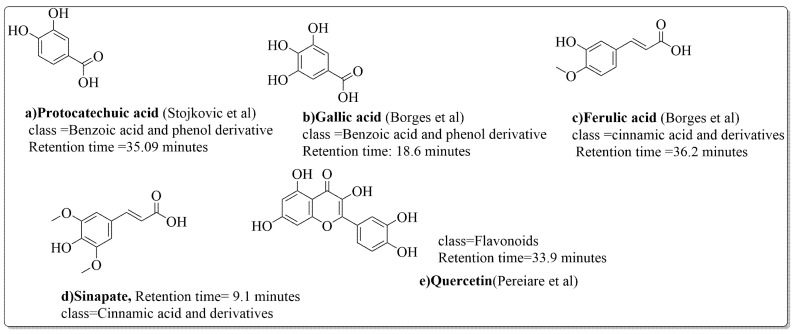
Figure 2.
Phenolic compounds having antibacterial activities from pellicle extract of J. regia, Nowicki et al. [69].
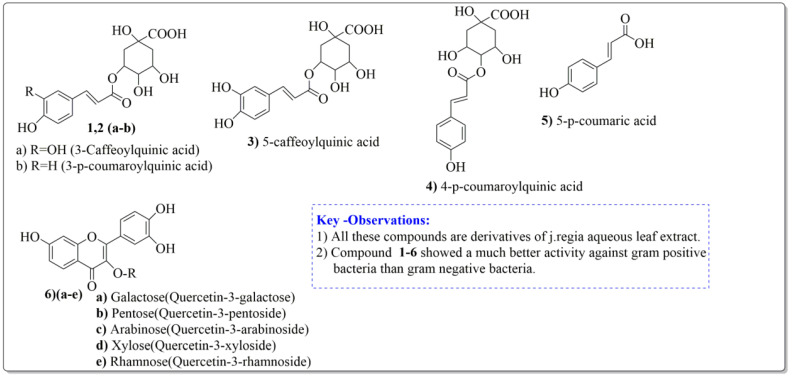
Pereira et al. [70] investigated the antibacterial activity of aqueous extracts from Juglans regia leaves against Gram-positive and Gram-negative microorganisms. The aqueous extracts mostly contained phenolic compounds which were obtained by shade drying the leaves, followed by the extraction of the compounds with various solvents, such as ether, alcohol, and water. By using HPLC-DAD, phenolic substances were identified and quantified. The chromatograms obtained at 320 and 350 nm revealed the presence of the different compounds (Figure 3, compounds 1-6). The obtained extracts were screened against Gram-positive (B. subtilis, Staphylococcus aureus, Bacillus cereus) and Gram-negative (Escherichia coli, Pseudomonas aeruginosa, Klebsiella pneumoniae) bacteria which revealed that Gram-positive bacteria development was inhibited to a greater extent than the inhibition in the case of Gram-negative bacteria, clearly indicating the fact that the ether, alcohol, and water extracts turned out to be favorable for the inhibition activity against Gram-positive bacteria. It was also observed that B. cereus was the most suspectable microorganism at MIC of 0.1 mg/mL. The order of inhibition of the above-made extracts from different solvents was found to be B. cereus > S. aureus > B. subtilis.
Figure 3.
Extracted phenolic compounds obtained from J. regia, Rahman et al. [70].
The antibacterial characteristics of the essential oil from the leaves and its constituents have been examined by Rather et al. [71]. In this study, the essential oil was extracted from the leaves of J. regia and analyzed using GC-MS, yielding a total of 38 compounds, accounting for 92.7% of the oil substance, including major components such as β-pinene (30.5%), α-pinene (15.1%), germacrene D (14.4%), β-caryophyllene (15.5%), and Limonene (3.6%). Disc diffusion and microdilution methods were used to test all essential oils and individual constituents for antibacterial activity against a group of Gram-positive (B. subtilis MTCC-441, S. epidermidis MTCC-43, S. aureus) and Gram-negative (B. subtilis MTCC-441, S. epidermidis MTCC-435, P. vulgaris MTCC-321, Salmonella typhi, Pseudomonas aeruginosa MTCC-1688, Klebsiella pneumonia, Shigella dysenteriae, and Escherichia coli) bacteria. It should be noted that the essential oil and its components were effective against all bacterial strains screened for antibacterial activity with a wide range of activity. However, it was discovered that Gram-positive bacteria were found to be more inhibited compared to Gram-negative bacteria in the development and cell division processes. The potent compounds at the maximum inhibition category include α-pinene, β pinene, β-caryophyllene, and Germacrene D. For the most inhibited Gram-positive bacteria (S. epidermidis, B. subtilis and S. aureus), the MIC recorded were 15.62, 15.62, and 15.62 g/mL for J. regia essential oil, 48.31, 47.21, and 45.62 g/mL for α-pinene, 46.55, 46.55, and 41.33 g/mL for β-pinene, respectively, clearly indicating the fact that the essential oil present in the leaves of the plant played a key role in the inhibition of different bacteria. Gentamicin had the lowest potency in this category, with MIC values of 5.95, 5.90, and 3.90 g/mL, respectively, for the above said Gram-positive bacteria. The findings indicate that these three bacterial species are the most susceptible to essential oils and components. The changes in the activity against different bacteria might be ascribed to structural differences between the microbes and the different developmental stages in different bacteria [71,72].
3.2. Antioxidant Activity
Juglans regia leaves are high in flavonoid content, which has been linked to regulating immunological function and boosting anticancer activity, which is a global threat nowadays [73]. The antioxidant potential of walnut kernels, husks, and leaf extracts formed in different solvents, such as ethyl acetate, butanol, methanol, ether, and aqueous methanol, have been evaluated using a variety of methods. Some common and highly placed methods include the reducing power method, lipid oxidation inhibition method, and DPPH radical scavenging activity. Moreover, various studies have validated the antioxidant activity of the flavonoids present in leaves and fruit of J. regia and it was established that almost all the extracts prepared in different solvents possessed this property [74,75]. Antioxidant phenolic components from J. regia walnut kernels, which were extracted and fractionalized based on the principle of polarity and interactions with the solvent system used (Petroleum ether, Ethyl-acetate, n-butanol, and aqueous solvent) and on the basis of which they were designated as petroleum ether fraction (PEF), ethyl-acetate fraction, n-butanol fraction, and others [76]. Column chromatography over silica gel eluted with increasing polarity was used to separate the more active fractions (EEF and BUF) (Figure 4).
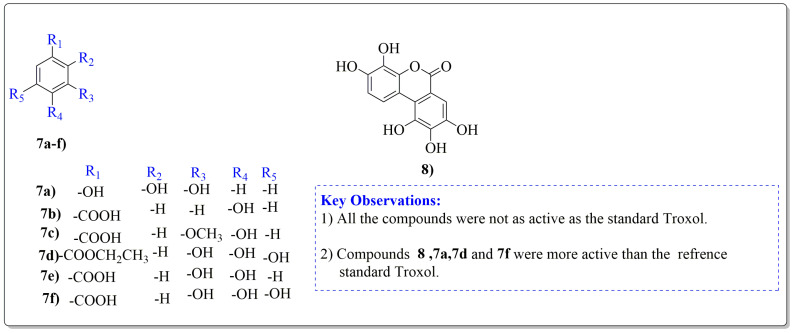
Figure 4.
Phenolic antioxidant compounds from walnut kernels, Zhai et al. [76].
All the derivative compounds (7a–f and 8) were screened for α, α-diphenyl-β-picrylhydrazyl (DPPH) free radical scavenging activity using Trolox IC50 = 0.026 mM as a reference standard. The IC50 values revealed that the EEF and BUF fractions with (IC50 = 0.83 and 0.88 µM) were the most active in terms of DPPH free radical scavenging among all the used fractions (PEF, EEF, BUF, and AF), as described earlier. Compounds 8, 7f, 7d, and 7a, however, demonstrated considerable DPPH scavenging capabilities as depicted by IC50 values of (0.007, 0.011, 0.013, and 0.015 µM), respectively, indicating that they were more active than the reference standard Trolox (IC50 = 0.026 µM). For the first time, spectroscopic methods were used to isolate and identify seven phenolic compounds with significant antioxidant activities in J. regia: pyrogallol (7a), p-hydroxybenzoic acid (7b), vanillic acid (7c), ethyl gallate (7d), protocatechuic acid (7e), gallic acid (7f), and 3,4,8,9,10-pentahydroxydibenzo [b, d] pyran-6-one (8). The DPPH scavenging capacity of these compounds was ranked in the following order: 7 > 6 ≥ 4 ≥1 > Trolox ≥ 5 > 3 > 2. The findings of this study suggested that the number of hydroxyls in these phenolic compounds’ aromatic rings may influence their antioxidant activities. Some important structure-activity relationships of the derivative compound (7f) indicate the essence of the COOH group as R1 with other groups as R3 = R4 = R5 = OH. This activity can be enhanced in the near future research by introducing some new acid electron withdrawing groups (EWG) as the replacement of R1 in the said derivative (7f), e.g., introduction of carbonyl, thiol, and aldehyde. The antioxidant activity, which also showed a sharp increase in case of the derivative (7d), can be reasonably attributed to the presence of R1 = COOCH2CH3 group with the aid of R3 = R4 = R5 = OH in the moiety.
J. regia leaf extracts are effective scavengers of pro-oxidant reactive species and can be used as a readily available source of natural antioxidants. The growing interest in replacing synthetic food antioxidants with natural ones has fueled research on vegetable sources and raw material screening in the search for new antioxidants. An ethanol:water (4:6) extract of Juglans regia leaves was evaluated in vitro for its putative scavenging effects on reactive oxygen species (ROS) [hydroxyl radical (HO.), superoxide radical (O2−, peroxyl radical (ROO.), hydrogen peroxide (H2O2.)] and reactive nitrogen species (RNS) [nitric oxide (NO.), and peroxynitrite anion (ONOO−)].The extract demonstrated strong scavenging activity against all reactive species tested, with all IC50s found at the lg/mL level. The IC50s (mean ± SE) for ROS O2− and H2O2 radical were found to be 47.6, 4.6 and 383, 17 lg/mL, respectively. In addition, the antioxidant activity discovered in this study can be used to validate the use of J. regia leaf extracts in the treatment of inflammatory diseases. The current study found that the J. regia leaf can be used as a readily available source of natural antioxidants. The extract under investigation could aid in the prevention of lipid peroxidation as well as the protection of food, excipient bases, and medicines from oxidative damage. Furthermore, the observed antioxidant activity can at least partially justify the use of J. regia leaves as a therapeutic agent in inflammatory diseases. Nonetheless, its potential toxicity should be addressed before any practical application. [77].
By using DPPH and hydroxyl radical scavenging assays, Zurek et al. [78] investigated the antioxidant activity of the leaf’s essential oil and some of its constituents. The antioxidant activity was measured using the 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging activity assay, the copper ion reduction assay (CUPRAC), metal chelating ability (ChA), and the ability to scavenge superoxide (O2−) and hydroxyl (OH−) radicals. These methods are regarded as important indicators of plant samples’ antioxidant potential. The free radical scavenging DPPH had the lowest IC50 value in this study, corresponding to the highest antioxidant activity. The calculated half maximum inhibitory concentration (IC50) value was 22.34, 2.70 g/mL, which was higher than the positive control ascorbic acid (5.00, 0.01 g/mL). The chelating capacity test (71.69, 0.02 g/mL), superoxide scavenging (147.06, 0.27 g/mL), and hydroxyl radicals test (41.85, 0.09 g/mL) all had low IC50 values, indicating high antioxidant activity. The findings revealed that J. regia flowers were found to have a high ability to scavenge free radicals, which could be attributed to their high content of bioactive compounds. Furthermore, the results revealed a strong relationship between the phenolic compounds studied and their antioxidant activities.
3.3. Analgesic and Anti-Inflammatory Properties
J. regia extracts from aqueous (2.87 and 1.64 g/kg) and ethanolic (2.044 and 1.17 g/kg) solutions have demonstrated antinociceptive effectiveness in a hot plate test, according to Hosseinzadeh et al. [79]. The hot-plate test was performed on eight male and female mice groups. The metal surface temperature was kept constant at 55 °C. Before and after drug administration, the latency to a discomfort reaction (licking paws or jumping) was measured. 30 min after the extracts were given to groups of eight male and female mice, they were given an intraperitoneal injection of 0.7% v/v acetic acid solution (volume of injection 0.1 mL/10 g body wt.). The number of writhing produced in these animals was counted for 30 min after acid injection. The anti-inflammatory activity against acute inflammation was evaluated in mice using the xylene-induced ear edema method. The mice were divided into eight groups. 30 min after the different doses of extract were injected intravenously, diclofenac and 0.03 mL of xylene were applied to the anterior and posterior surfaces of the right ear. The left ear was used as a control. The mice were sacrificed two hours after xylene application, and both ears were removed. Circular sections were excised and weighed using a cork borer with a 9 mm diameter. The weight gain caused by the irritant was calculated by subtracting the weight of the untreated left ear section from the weight of the treated right ear section. In mice, the anti-inflammatory activity against chronic inflammation was assessed using the cotton pellet granuloma method. The 30 mg dental cotton pellets were sterilized in an air oven at 121 °C for 20 min before being impregnated with 0.4 mL of an ampicillin aqueous solution. Under ketamine (65 mg/kg body weight) and xylazine (6.5 mg/kg body weight) anesthesia, two cotton pellets, one on each side, were implanted subcutaneously in the shoulder region of mice. The extract and diclofenac were administered once daily for seven days. The rats were killed on day 8, and the pellets and surrounding granulation tissue were dried at 60 °C for 24 h. In mice, the intraperitoneal LD50 values of J. regia aqueous and ethanolic leaves extract were 5.5 g/kg (4.1–6.5) and 3.3 g/kg (3.1–3.5), respectively, with maximum nonfatal doses of 4.1 g/kg and 2.93 g/kg. However, it should be noted that both extracts displayed an anti-inflammatory effect in xylene at lower dosages. The extracts demonstrated antinociceptive action in the Writhing test that was not inhibited by naloxone (lifesaving medication). The extracts were found to have anti-inflammatory properties in the case of chronic inflammation. J. regia leaves have been shown to have an antinociceptive action via non-opioid receptors as well as an anti-inflammatory impact against acute and chronic inflammation, making them an effective drug with analgesic and anti-inflammatory properties against rheumatoid arthritis [80].
3.4. Antidepressant Activity
Depression has been classified as a mood disorder and has been defined as a feeling of sadness, loss, or anger [81]. Treatments derived from the Juglans regia L. flower and its leaf extracts have been found to be highly successful in treating depression. The effects of the forced swimming test (FST) and tail suspension test (TST) in mice were quite evident [82].
3.4.1. Forced Swimming Test
The immobility time was calculated by observing the muscle movements of rats that were placed in a pool of water. A glass cylinder with a diameter of 25 cm and a height of 23 cm was filled with water to a height of 12 cm. The water temperature was 23 °C. Each rat was given a single injection of the extract. The animals were tested thirty minutes later. For the first two minutes, each animal was given time to get used to the new circumstances before having their immobility time recorded. For the following six minutes, conditions of increased muscle control interrupted with periods of immobility. For the next four minutes, immobility was timed using a stopwatch.
3.4.2. Tail Suspension Test
The tail-suspension test was the second method used to evaluate the extract’s antidepressant effect. Rats were tested thirty minutes after a single drug or vehicle injection. A cord which was about 50 cm long was stretched between two metal tripods at a height of 70 cm, to which the rats were taped by the tail. The rats became immobile after a period of vigorous motor activity, and the time was recorded with a stopwatch for a total of 4 min. When rats hung passively and completely motionless, they were considered immobile.
The mean duration of immobility in the control group was 188.33, 2.16 s, whereas it was 151.16, 2.56 s, in the fluoxetine group. The reduction in immobility was found to be statistically significant (p < 0.05). The decrease in immobility in the extract groups was also significant (p < 0.05) for both doses. For 100 mg/kg and 150 mg/kg body weight, the total duration of immobility was found to be 160.66, 3.76 s and 154.83, 4.32 s, respectively. Regarding the effect on immobility in the tail suspension test, in the control group, the mean duration of immobility was 193.33, 1.96 s, whereas in the fluoxetine group, it was 147.16, 2.48 s. The reduction in immobility was found to be statistically significant (p < 0.05). The total duration of immobility for 100 mg/kg body weight was found to be 168.39 s and 148.66 1.75 s for 150 mg/kg body weight. The decrease in immobility in the extract groups was also significant (p < 0.05) for both doses [83]. This remarkable antidepressant activity was attributed to the phenol and flavonoid contents, especially quercetin. In addition, the presence of omega-3 fatty acids in Juglans regia fruit extracts may have depressive properties [84]. More research is needed to fully understand the mechanism of antidepressant activity.
3.5. Antiviral Activities
Mouhajir et al. [85] studied the activity of Juglans regia methanol extracts. At noncytotoxic concentrations, the 2 mg/mL concentration was tested against Sindbis virus (SINV), herpes simplex virus (HSV) and poliovirus. Vardhini [86] also used a computational method to investigate juglone’s antiviral activities and the results were well supported by molecular docking studies. In this study, the ligand which had the highest binding affinity had a dock score of 114.967 against ASP 29, ASP 30, and ASP 30, which are the hydrogen bonds that existed between the ligand and the protein molecule. Using phytochemical and chromatographic methods to separate components from J. regia, other researchers [85] investigated the impact of anti-HIV activity in vitro. The impact of anti-HIV activity in vitro was assessed using MT4 cells and HIV-III B virus. The target research was conducted using BIACORE 3000 molecules linked equipment. Angeli et al. [87] studied the walnut pellicle extracts and derived some handful number of antiviral compounds which possessed the inhibitory activity against HSV-1 and HSV-2 replication. The ID50 (con. which inhibited 50% virus formation) were found to be 10 and 8 µg/mL for HSV-1 and HSV-2, respectively. However, the walnut pellicle extract was ineffective against Echovirus9 (ECHO-9), Poliovirus1 (polio1), Coxsackievirus B1 (coxsackie B1) and Adenovirus (Adenoid). The compounds extracted from walnut pellicle extract were found to be active against viral diseases (Figure 5).
Figure 5.
Compound derivatives from walnut pellicle extract, Alkhawajah et al. [87].
The global outbreak of COVID-19 was caused by SARS-CoV-2, a positive-sense single-stranded RNA coronavirus. The virus’s main protease (Mpro), the major enzyme processing viral polyproteins, contributed to SARS-CoV-2 replication and transcription in host cells and has been identified as an appealing target in drug discovery. A series of 1,4-naphthoquinones with juglone (compound isolated from leaves of walnut) skeletons were synthesized and tested for inhibitory efficacy against SARS-CoV-2 Mpro. At a concentration of 10 µM, more than half of the tested naphthoquinones effectively inhibited the target enzyme, with an inhibition rate of more than 90%. The characteristics of substituents and their position on the juglone core scaffold were identified as key ingredients for enzyme inhibitory activity in the structure-activity relationships (SARs) analysis. The most active compound, 2-acetyl-8-methoxy-1,4-naphthoquinone, had an IC50 value of 72.07–4.84 nM against Mpro-mediated hydrolysis of the fluorescently labelled peptide, which was much higher than shikonin as the positive control. In molecular docking studies, it fit well into the active site cavity of the enzyme by forming hydrogen bonds with adjacent amino acid residues. The results of in vitro antiviral activity testing revealed that the most potent Mpro inhibitor could significantly suppress SARS-CoV-2 replication in Vero E6 cells at low micromolar concentrations, with an EC50 value of about 4.55 µM. Under the conditions tested, it was non-toxic to the host Vero E6 cells. The current study suggested that the juglone skeleton could serve as a primary template for the development of potent Mpro inhibitors [88].
Enzyme Inhibition Mechanism
The inhibitory activity of the prepared quinones against Mpro of SARS-CoV-2 was evaluated using a fluorescently labelled short peptide containing a Q-S scissile bond. In the first library of compounds, we tested the enzymatic inhibition rate of several naturally occurring naphthoquinones (juglone 2, 7-methyl juglone, lawsone, plumbagin and shikonin), 9,10-anthraquinones (emodin, rhein and aloe emodin), and synthetic vitamin K3 against SARS-CoV-2 Mpro at 10 µM. The primary screening results showed that the majority of the natural quinones were ineffective, with inhibition rates of less than 10% at 10 µM. Vitamin K3 was also inactive, with a 12.7% inhibition rate. The positive control was the natural naphthoquinone shikonin, which had previously been identified as a strong Mpro inhibitor (IC50 = 15.75 8.22 µM). At a concentration of 10 M, it had a moderate inhibitory effect on the target enzyme. In the first library of naphthoquinones, juglone and 7-methyl juglone inhibited Mpro completely, resulting in the loss of its hydrolytic efficacy. The lead compounds for further structural modifications were the two natural naphthoquinones. In the second library, juglone and 7-methyl juglone derivatives were synthesized by adding a few groups to their naphthoquinone scaffold and modifying the phenolic hydroxyl group. The results indicated that almost all of the juglone derivatives in the second library retained their high inhibitory potency at concentrations of 10 M and 1 M. A few analogues exhibited significantly higher potency than the parent compounds juglone and 7-methyl juglone at 0.1 µM. The compounds with an enzymatic inhibition rate of more than 25% at 0.1 µM concentration were then subjected to IC50 value screening.
3.6. Antidiabetic Activity
J. regia leaf extract has been thought to be advantageous for the treatment of diabetes mellitus since ancient times, and this notion has been scientifically proven to be effective. Blood glucose, glycosylated hemoglobin, LDL, lipid, and cholesterol levels were found to be reduced by alcohol extracts from the plant’s leaves. Streptozotocin-treated rats were administered by treating with 200 and 400 mg/kg leaf extracts of J. regia leaf extract for 28 days, and it alleviated hyperglycemia through reduced glycosylated hemoglobin and enhanced insulin sensitivity [89]. They looked at how J. regia leaf extract affected hyperglycemia in type 2 diabetic patients. The leaves of J. regia were initially freshly harvested, then washed and dried at 25 °C temperature in the shade before being powdered, and the powder was extracted at room temperature using the percolation technique and 70% aqueous ethanol. The solvent was eliminated using Whatman paper filters and the crude extract was standardized by counting the total phenol concentration after evaporating at a temperature of no more than 40 °C under decreased pressure. Later, capsules of Juglans regia and a placebo with the same appearance were created. The J. regia capsules included 100 mg of leaf extract powder. In the manufacture of the leaf extract powder, toast powder was chosen as the placebo and as the excipient. The research was conducted on a group of individuals who were divided into two groups: those who had received J. regia and those who had received a placebo. The levels of the constituents fasting blood glucose (FBG), HbA1c, total cholesterol, and triglyceride were measured in J. regia-treated patients and they were found to be on a lower side than those in the placebo group, with no adverse effects. Finally, for type 2 diabetes, three months of treatment with 100 mg of Juglans regia leaf extract twice daily improved glucose control with no noticeable side effects. The results showed that FBG, HbA1c, total cholesterol, and triglyceride levels in Juglans regia-treated patients were significantly lower than in the baseline and placebo groups. When compared to the placebo group, patients in the Juglans regia group were significantly more satisfied with their treatment. Except for more GI events (especially mild diarrhea) associated with extract treatment at the start of the study, no liver, kidney, or other side effects were observed in the groups.
Testicular dysfunction is a complication of diabetes, and Juglans regia L. leaf extract contains phenolic compounds with hypoglycemic and antioxidative properties. Nasiry et al. investigated whether J. regia leaf extract could protect against the negative effects of diabetes on oxidative stress, testis histology and testosterone hormone production [90]. In their research, four groups of male rats were used: a control (nondiabetic) group given saline, a diabetic group, a diabetic + J. regia group that received J. regia leaf extract and a J. regia group (nondiabetic) that received J. regia leaf extract only. They looked at histopathological and histomorphometric changes, serum testosterone, malondialdehyde (MDA), glutathione (GSH), superoxide dismutase (SOD), and catalase (CAT) levels to see how J. regia L. leaf extract affected testicular functions in diabetic animals. In the testis of diabetic rats, there was a reduction in MDA as well as an improvement in antioxidant status; J. regia leaf extract attenuated these abnormalities. In diabetic rats, it was found that there was a significantly decrease in the levels of testosterone, GSH, SOD, and other antioxidant biomarkers; these levels were restored after the J. regia leaf extract was administrated. After administering the J. regia leaf extract, the MDA level and improved antioxidant status in the testis of diabetic rats were found. Because of its antioxidant, anti-inflammatory and anti-apoptotic properties, J. regia leaf extract may have protective effects against diabetic dysfunction in the testis, according to the findings.
3.7. Anticancer Activity
Cancer treatment has long been a difficulty for medical science, and research into a variety of medicinal plants has resulted in a potential treatment for the disease’s early stages [91,92]. Even while cancer has been identified as a burden on human civilization, no comprehensive cure has yet been established [93]. Juglone has been demonstrated to inhibit intestinal carcinogenesis in mice, suggesting that it might be a potential chemo preventive medication for neoplasia in human intestines [94]. The human carcinoma cells lines HCT-15 cells, HL-60 cells, and doxorubicin-resistant HL-60R cells have all demonstrated the potency of juglone as a strong cytotoxin [95]. Human cancer renal cell lines A-498 and 769-P, as well as the colon tumor cell lines Caco-2, were inhibited in a concentration-dependent way by walnut methanolic extract out from the seeds, greenish husks, and leaves of Juglans regia. All extracts inhibited the growth in 769-P renal and Caco-2 colon cancer cells (IC50 values of 0.352 and 0.229 mg/mL, respectively; range, 0.226 to 0.29 mg/mL), but the walnut extract of leaves was more effective at suppressing cell proliferation than green husks and extracts of seeds (IC50 values of 0.352 and 0.229 mg/mL, respectively).
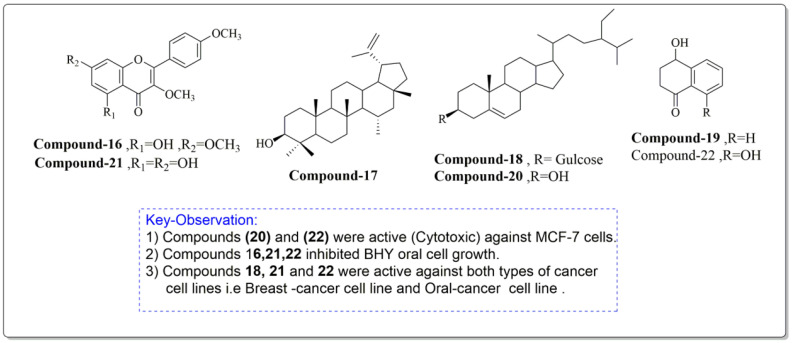
Constituents of J. regia chloroform leaf extract was tested for anti-proliferative and apoptotic effects on human breast (MCF-7) and oral tumor (BHY) cell lines. The components were extracted from shrub leaves which were air dried, pulverized, and then percolated in n-hexane for 24 h. After three days of extraction, all extracts were filtered and dried with a rotatory evaporator. At a lower pressure, the solvent, n-hexane, was extracted. The remaining powder was then suspended and extracted to obtain the chloroform fraction, which was further purified using chromatography (Figure 6). The proliferative and apoptotic activities of the compounds mentioned above (16–22) were investigated. Derivatives (20 and 22) were found to be significantly cytotoxic to MCF-7 cell lines, whereas compounds (16, 21, 22) significantly inhibited BHY cell proliferation. According to the IC50 values, MCF-7 cell lines were also the most sensitive to virtually all chemicals. Compounds (21) (IC50 = 50.98 µM) and (22) (IC50 = 21.30 µM) were selectively active against both cancer cell lines, MCF-7 and BHY, but were significantly less effective against normal cells. The compounds (16–22) suppressed cell population development in human tumor cell lines MCF-7 and BHY, as well as mouse fibroblast cell lines, using the MIT test at 24, 48, and 72 h (NIH-3T3). However, the best proliferation activities were obtained after 72 h. It is worth mentioning that compounds 21 and 22, which are plant flavonoids, and a plant naphthoquinone can be further studied well to explore their new biological activities such as antidiabetic and antibacterial activities. Moreover, these compounds induced apoptosis in MCF-7 cell lines by the well-known mechanism caspase-3 independent pathway [95].
Figure 6.
Compounds having antiproliferative and apoptotic activity against MCF-7 and BHY cancer cell lines, Noumi et al. [95].
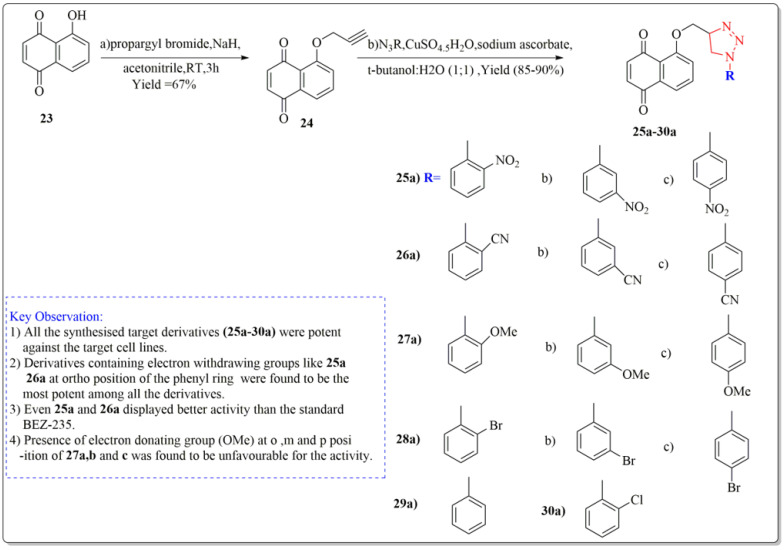
One of the important synthetic breakthroughs was given by Zhang et al. [96] as they developed modified Juglone from J. regia as a strong cytotoxic drug against carcinoma cell lines of lungs by isolating and modifying the compound. The synthesis started with a solution of Juglone (23) in acetonitrile (isolated from J. regia roots). At 20 °C, the solution was portioned with NaH and agitated continuously for 10 min. Propargyl bromide was added drop by drop, and the suspension was again agitated for two hours. Ethyl acetate was used to extract the reaction mixture, which was then purified on a silica gel column to get the derivative (24). The derivative (24) was then treated with organic azide, sodium ascorbate, water:butanol (1:1), and copper ) (ii) pentahydrate to yield the final target derivatives (25a–30a). Juglone derivates having cytotoxic effects are depicted in Figure 7. All the synthesized target derivatives (25a–30a) were evaluated through cytotoxic assays against lung cancer cell lines (NCl-H322 and A549) using BEZ-235 (IC50 = 9.80 µM) as a positive control. Among all the synthesized derivatives of Juglone, 25a and 26a bearing o-nitro and o-cyano phenyl moieties, respectively, displayed the most potency (IC50 = 4.72, 8.90 and 4.67, 7.94 µM) against both the lung cancer cell lines (NCl-H322 and A549), respectively. Both these derivatives even displayed better activity results than the standard positive control BEZ-235 (IC50 = 9.80 µM). Derivatives 27a, 27b, and 27c with ortho, meta, and para-methoxy R moieties, respectively, were very less potent towards the cancer cell lines. Similarly, derivatives (28 a, b, c) bearing o, m, and p-bromophenol moieties were lesser active than juglone (IC50 = 19.32 µM) and standard BEZ-235 as displayed by the IC50 values (24.34, 21.82, 24.55 µM), respectively. Moreover, derivatives 29a and 30a bearing a phenyl and o-chlorophenyl displayed slight better activity than juglone; however, they were less potent than the standard BEZ-235 against the lung cancer cell lines (NCl-H322 and A549). From the above results, it can be clearly stated that the introduction of EWG at ortho position of the phenyl ring in the said derivative (25a, 26a) turned out to be favorable for the net potency of the derivatives, which can be enhanced in the new research by introducing some other EWG’s at ortho position of the phenyl ring, such as the EWG group carbonyl, carboxyl, CF3, or fluorine at ortho position. Moreover, from structure activity relationship (SAR) studies, it is clear that changing the position of groups from ortho to meta in (25b and 26b) or from ortho to para in (25c and 26c) in the above structures showed a dramatic decrease in the activity as depicted by the IC50 values of 25b, 26b, 25c, 26c (IC50 = 15.6, 17.22, 10.96, 13.30 µM), respectively, providing clear proof that the respective position of the groups to influence the activity is necessary to impart activity to the new derivatives.
Figure 7.
Synthesis of modified juglone derivatives (triazole analogs) as cytotoxic agents, Haque et al. [96].
Shah et al. [97] also studied the anti-proliferative and cytotoxic effects of J. regia leaf extracts (methanol and aqueous extracts) at different concentrations on growth inhibitions of cell lines of mice melanoma (B16F10) and human melanoma (A375). The extract concentrations prepared in this experiment included (0.1, 0.15, 0.2, 0.25, 0.3, 0.35, 0.40, 0.45, and 0.5 mg/mL of extract/mL). The normal lymphocyte cell lines were observed to show negligible sensitivity towards the extracts. The cytotoxic activity was screened for 72 h of treatment to the (B16F10) and (A375) cell lines, and it was revealed that methanolic extracts at different concentrations showed potent activity (cell anti-proliferation) against mice melanoma with IC50 = 0.234 mg/mL compared to IC50 = 0.304 mg/mL on human melanoma cell lines. Likewise, aqueous extracts of the above-mentioned concentrations also showed good activity with (IC50 = 0.298 and 0.350 mg/mL) against mice and human cell melanoma, respectively. Even though both methanolic and aqueous extracts had significant anti-proliferative action against mice and human cancer cell lines, significant activity was detected for methanol extracts generated at the same concentrations in this investigation.
3.8. Antifungal Activity
The antifungal activity of aqueous and solvent extracts from leaf and bark helps in medicinal use because these extracts showed a wide range of activity against fungus using various methods such as agar dilution, disc diffusion, agar streak dilution, and the Radish method [98]. The antifungal activity was demonstrated by various research, which includes the major breakthrough contributed by D. Wianowska et al. by comparison of the antifungal activity of different extracts of Juglans regia cultivars and the major compound juglone. This study compares the antifungal effects of juglone and extracts from walnut green husks of the cultivars Lake, Koszycki, UO1, UO2, and non-grafted against plant pathogenic fungi such as Alternaria alternata, Rhizoctonia solani, Botrytis cinerea, Fusarium culmorum, Phytophthora infestans, as well as Ascosphaera apis. The data obtained demonstrate that the antifungal activities of the extracts can be modulated by their other constituents and are not always dependent on the antifungal activity of juglone. This enables us to draw the conclusion that juglone is not the only element in walnut green husk extracts that inhibits mycelial growth. It was discovered that phenolic compounds were in charge of the extracts’ activity and that they could change juglone’s antifungal properties [99]. Similarly, the activity was demonstrated by Hubert Sytykiewicz et al. [100] by conducting a study to assess the antifungal efficacy of four extract fractions (methanolic, ethyl acetate, alkaloid, and hydrolyzed methanolic) derived from Juglans regia (L.) leaves against pathogenic Candida albicans strains. One reference strain (C. albicans ATCC 900et 29) and 140 isolates from various biological samples, including skin lesions, sputum, urine, and feces, were used to test the yeasts. The highest anticandidal activity was found in the methanolic extract from walnut leaves, followed by the alkaloid fraction, which had a slightly lower antifungal efficacy. Etyl acetate and hydrolyzed methanolic preparate had the lowest levels of growth rate inhibition for the examined fungal pathogens [101].
Similar antifungal activity was assessed using the disc diffusion method with extract concentrations of 100, 200, and 300 g/mL/disc, using the standard ketoconazole (40 g/mL/disc). Selective fungistatic activity was demonstrated by the extracts against some species. Different levels of inhibitory activity were present in all extracts against all types of fungi. The study revealed that acetone and chloroform extracts significantly inhibited the growth of Alternaria alternata and Trichoderma virens, respectively. Moreover, methanolic extract demonstrated significant activity against Aspergillus niger [102]. The other studies which have been carried out by analyzing the different extract of Juglans regia and the antifungal activity have demonstrated that, no matter the type of extract evaluated for the antifungal activity, juglone is the primary component of walnut green husk extracts which demonstrated the maximum antifungal activity. However, the activity varied depending on the composition of other vital extract constituents and the kind of fungal infection that was treated [100,103].
3.9. Cardiovascular Activity
Walnuts have been found to have high quantities of omega-3 and omega-6 polyunsaturated fatty acids (PUFA). While some studies have connected omega-6 PUFA to an enhanced proinflammatory vascular response, the bulk of investigations have indicated that these components have no deleterious consequences on human cardiovascular health. It has been also discovered that in non-hyperlipidemic persons, eating walnuts regularly (30–100 g/day) lessened the cardiovascular risk factors [104]. Consuming nuts on a regular basis has been linked to a lower risk of both fatal and non-fatal myocardial infarction. According to epidemiological studies, compared to people who never consumed nuts, those who consumed nuts five or more times per week had a 50% lower risk of coronary heart disease [105,106]. Green walnut hull extract suppressed protein secretion and thrombin-induced platelet aggregation by 50% in an in vitro investigation, with no deleterious effects on platelets at a dosage of 50 mg/mL. Walnut green hull extract’s antiplatelet activity is most likely due to its polyphenolic components and antioxidant capabilities. As a result, it can be regarded a thrombotic disease candidate as well [107].
3.10. Brain Enhancing Activity
A healthy functioning brain requires sufficient amounts of water, vitamins (such as folate, thiamine, vitamins B6, and B12), lipoic acid, lutein, and n-3 fatty acids for proper and improved functioning. Walnuts are high in n-3-linolenic acid (ALA), as well as a variety of other potentially neuro-regenerative substances such as phenolic acid (ellagic acid), gamma tocopherol (vitamin E), folate, melatonin, flavonoids, and a plant-based omega-3 fatty acid. It is worth noting that walnuts placed in second place among 1113 foods that were tested for antioxidant levels [108]. Memory is the retaining process of a learning experience across time. By delivering the right stimuli, a single memory can be retrieved. Polyphenols have been demonstrated to exhibit properties which influence the key neuronal signaling pathways in memory and learning. Based on obesity, hypercholesterolemia, and oxidative stress, walnut polyphenolic extracts improved learning by 42% and memory in hypercholesterolemic rats [109]. A walnut diet containing 6% walnut oil protected male rats from neurotoxicity caused by the chemotherapeutic drug cisplatin, according to another study. The results demonstrated that walnut administration enhanced cognitive and motor skills, implying that including walnut in one’s diet may be beneficial in combating chemotherapy-induced motor and cognitive dysfunction [108]. It was also revealed that walnuts enhanced learning skills, locomotor activity, memory, anxiety, and motor coordination in the transgenic mice model against Alzheimer’s disease by 6–9 percent [110]. The above studies which were carried in vivo have clearly opened a new path of the research to demonstrate the same effect on humans while making sure the earlier conclusion and the ample number of dosages are delivered hand in hand alongside with the safety of the subject.
4. Toxicity Activity on Plants and Animals
After conducting toxicology research on humans and animals, it was discovered that in the acute oral toxicity trial, Wistar female rats were given several doses of methanolic extracts from 10 to 5000 mg/kg of J. regia septum for 14 days. In a sub chronic study, the Wistar rats were fed the extract orally at a rate of 1000 mg kg−1 for 28 days. No harmful effects or deaths were observed for the extract even at a concentration of 5000 mg kg−1. Further in the study, there were no notable morphological or histological alterations in the studied tissues [111]. According to the Yang et al. [112], walnut polyphenols increased immune function by lowering oxidative stress. Tropical walnut use has been also linked to skin irritation and discoloration in many cases reported in the past, clearly indicating the fact that taking an excess diet of walnut fruit is also harmful to human health [113]. This fact was also well supported by developing enormous blisters and skin discoloration in a 65-year-old woman after she consumed 15 kg of walnuts in three days [114]. It was also revealed that walnut aqueous extract decreased the cyclophosphamide toxicity and protected metabolizing and antioxidant enzymes at the time of treatment on the woman. The goal of the current study was to determine whether walnut extract can reduce the toxicity of the anticancer drug cyclophosphamide (CP) while also protecting against the disruption of enzymes that help the body break down drugs and fight free radicals [113]. Human erythrocyte forward scatter was significantly reduced after 24 h of exposure to juglone (5 µM). Juglone (1–5 µM) markedly raised the proportion of annexin V-binding cells. Juglone (5 µM) markedly increased the amount of ceramide at the erythrocyte surface and decreased the amount of erythrocyte adenosine triphosphate (ATP). Juglone stimulates suicidal erythrocyte death or eryptosis, at least in part, by increasing the abundance of ceramides (lipid molecule), depleting energy, and activating protein kinase (PKC) [114]. Walnut’s detrimental effects on animals, notably horses, have also been reported. Moreover, walnut heartwood can induce laminitis, an inflammatory disease in horses. As a result, the black walnut extract model has been created to study the many parameters connected to horse laminitis [115].
To investigate and validate the much promising study in relation to toxicity caused by the walnut excess diet, Iwamoto et al. investigated the effects of walnut consumption on serum lipids and blood pressure in Japanese subjects to determine whether it would also be beneficial as a component of the Japanese diet. In a crossover design, they randomly assigned 20 men and 20 women to one of two mixed natural diets for four weeks each. Both diets adhered to the typical Japanese diet (reference diet) and included the same foods and macronutrients, with the exception that the walnut diet provided 12.5% of its energy from walnuts (43–57 g/d) (offset by lesser amounts of fatty foods, meat, and visible fat). When comparing the walnut diet to the reference diet, total cholesterol levels were 0.21 mmol/L lower for women (p < 0.01) and 0.16 mmol/L lower for men (p = 0.05). When they followed the walnut diet, the LDL cholesterol levels were 0.18 mmol/L lower for the men (p = 0.13) and 0.22 mmol/L lower for the women (p < 0.01). The walnut diet also decreased the concentration of apolipoprotein B and the ratio of LDL to HDL cholesterol (p < 0.05) [116].
5. Conclusions
The current review addresses the most prominent published research on J. regia L. in medical sciences, the plant’s traditional and modern scientific applications, as well as scientific validation of the stated biological activity in vivo and in vitro. Synthetic triazole derivatives suggested in the manuscript can be modified to create new potent molecules that can be tested for a variety of biological activities such as anticancer, antidiabetic, antibacterial, and many others with promising results that will stand in the research gap. Furthermore, various extracts (aqueous or methanolic) from walnut leaves, flowers, or fruits might be utilized in concentration-dependent ways in a new study to make improvements in the relevant field. To the best of our knowledge, we have not reported any deceptive activity after working on our own green fruit and leaf extract of the plant, as well as manipulation of other work in the same field. However, more human trials are needed to determine all J. regia Linn extracts’ much anticipated and promising capabilities and activities.
Acknowledgments
All authors are grateful to King Abdulaziz University (KAU), University of Ha’il (UOH), Taif University (TU), Lovely Professional University (LPU), and Aligarh Muslim University (AMU) for providing the basic infrastructure to write this review article and especially KAU, UOH, and TU for giving access to the Saudi Digital Library (SDL).
Author Contributions
All authors equally contributed through initiating a draft of the review, performing the artwork, and editing the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Acarsoy Bilgin N. Morphological Characterization of Pollen in Some Varieties of Walnut (Juglans regia) Int. J. Fruit Sci. 2022;22:471–480. doi: 10.1080/15538362.2022.2060895. [DOI] [Google Scholar]
- 2.Hayes D., Angove M.J., Tucci J., Dennis C. Walnuts (Juglans regia) chemical composition and research in human health. Crit. Rev. Food Sci. Nutr. 2016;56:1231–1241. doi: 10.1080/10408398.2012.760516. [DOI] [PubMed] [Google Scholar]
- 3.Gupta A., Behl T., Panichayupakaranan P. A review of phytochemistry and pharmacology profile of Juglans regia. Obes. Med. 2019;16:100142. doi: 10.1016/j.obmed.2019.100142. [DOI] [Google Scholar]
- 4.Dawid-Pać R. Medicinal plants used in treatment of inflammatory skin diseases. Postep. Dermatol. Alergol. 2013;30:170–177. doi: 10.5114/pdia.2013.35620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bourais I., Elmarrkechy S., Taha D., Mourabit Y., Bouyahya A., El Yadini M., Machich O., El Hajjaji S., El Boury H., Dakka N., et al. A Review on Medicinal Uses, Nutritional Value, and Antimicrobial, Antioxidant, Anti-Inflammatory, Antidiabetic, and Anticancer Potential Related to Bioactive Compounds of J. regia. Food Rev. Int. 2022:1–51. doi: 10.1080/87559129.2022.2094401. [DOI] [Google Scholar]
- 6.Chand S.P., Arif H. StatPearls. StatPearls Publishing; Treasure Island, FL, USA: 2022. Depression. [Google Scholar]
- 7.Nabavi S., Ebrahimzadeh M., Nabavi S., Mahmoudi M., Rad S. Biological activities of Juglans regia flowers. Rev. Bras. Farmacogn. 2011;21:465–470. doi: 10.1590/S0102-695X2011005000092. [DOI] [Google Scholar]
- 8.Mahmoudi M., Ebrahimzadeh M., Ansaroudi F., Nabavi S.F., Nabavi S.M. Antidepressant and antioxidant activities of Artemisia absinthium L. at flowering stage. Afr. J. Biotechnol. 2009;8:7170–7175. doi: 10.4314/ajb.v8i24.68818. [DOI] [Google Scholar]
- 9.Rath B.P., Pradhan D. Antidepressant Activity of Juglans regia L. Fruit Extract. Int. J. Toxicol. Pharmacol. Res. 2009;1:24–29. [Google Scholar]
- 10.Mouhajir F., Hudson J.B., Rejdali M., Towers G.H.N. Multiple Antiviral Activities of Endemic Medicinal Plants Used by Berber Peoples of Morocco. Pharm. Biol. 2001;39:364–374. doi: 10.1076/phbi.39.5.364.5892. [DOI] [Google Scholar]
- 11.Vardhini S.R. Exploring the antiviral activity of juglone by computational method. J. Recept. Signal Transduct. 2014;34:456–457. doi: 10.3109/10799893.2014.917325. [DOI] [PubMed] [Google Scholar]
- 12.Liu Z.M., Wen R.X., Ma H.T., Yang Y.S., Wang X.L., Lv X.H., Li Z.L. Investigation on inhibition of HIV III B virus with extractions of Juglans regia. Zhongguo Zhong Yao Za Zhi. 2008;33:2535–2538. [PubMed] [Google Scholar]
- 13.D’Angeli F., Malfa G.A., Garozzo A., Li Volti G., Genovese C., Stivala A., Nicolosi D., Attanasio F., Bellia F., Ronsisvalle S., et al. Antimicrobial, Antioxidant, and Cytotoxic Activities of Juglans regia L. Pellicle Extract. Antibiotics. 2021;10:159. doi: 10.3390/antibiotics10020159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cui J., Jia J. Discovery of juglone and its derivatives as potent SARS-CoV-2 main proteinase inhibitors. Eur. J. Med. Chem. 2021;225:113789. doi: 10.1016/j.ejmech.2021.113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pan A., Sun Q., Manson J.E., Willett W.C., Hu F.B. Walnut Consumption Is Associated with Lower Risk of Type 2 Diabetes in Women. J. Nutr. 2013;143:512–518. doi: 10.3945/jn.112.172171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nasiry D., Khalatbary A.R., Ahmadvand H., Talebpour Amiri F.B. Effects of Juglans regia L. leaf extract supplementation on testicular functions in diabetic rats. Biotech. Histochem. 2021;96:41–47. doi: 10.1080/10520295.2020.1755893. [DOI] [PubMed] [Google Scholar]
- 17.Qanash H., Yahya R., Bakri M.M., Bazaid A.S., Qanash S., Shater A.F., TM A. Anticancer, antioxidant, antiviral and antimicrobial activities of Kei Apple (Dovyalis caffra) fruit. Sci. Rep. 2022;12:5914. doi: 10.1038/s41598-022-09993-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wani A.K., Akhtar N., Sharma A., El-Zahaby S.A. Fighting Carcinogenesis with Plant Metabolites by Weakening Proliferative Signaling and Disabling Replicative Immortality Networks of Rapidly Dividing and Invading Cancerous Cells. Curr. Drug Deliv. 2023;202:2. doi: 10.2174/1567201819666220414085606. [DOI] [PubMed] [Google Scholar]
- 19.Al-Rajhi A.M.H., Qanash H., Almuhayawi M.S., Al Jaouni S.K., Bakri M.M., Ganash M., Salama H.M., Selim S., Abdelghany T.M. Molecular Interaction Studies and Phytochemical Characterization of Mentha pulegium L. Constituents with Multiple Biological Utilities as Antioxidant, Antimicrobial, Anticancer and Anti-Hemolytic Agents. Molecules. 2022;27:4824. doi: 10.3390/molecules27154824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sugie S., Okamoto K., Rahman K.M., Tanaka T., Kawai K., Yamahara J., Mori H. Inhibitory effects of plumbagin and juglone on azoxymethane-induced intestinal carcinogenesis in rats. Cancer Lett. 1998;127:177–183. doi: 10.1016/S0304-3835(98)00035-4. [DOI] [PubMed] [Google Scholar]
- 21.Segura-Aguilar J., Jönsson K., Tidefelt U., Paul C. The cytotoxic effects of 5-OH-1,4-naphthoquinone and 5,8-diOH-1,4-naphthoquinone on doxorubicin-resistant human leukemia cells (HL-60) Leuk. Res. 1992;16:631–637. doi: 10.1016/0145-2126(92)90013-W. [DOI] [PubMed] [Google Scholar]
- 22.Salimi M., Ardestaniyan M.H., Mostafapour Kandelous H., Saeidnia S., Gohari A.R., Amanzadeh A., Sanati H., Sepahdar Z., Ghorbani S., Salimi M. Anti-proliferative and apoptotic activities of constituents of chloroform extract of Juglans regia leaves. Cell Prolif. 2014;47:172–179. doi: 10.1111/cpr.12090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang X.B., Zou C.L., Duan Y.X., Wu F., Li G. Activity guided isolation and modification of juglone from Juglans regia as potent cytotoxic agent against lung cancer cell lines. BMC Complement. Altern. Med. 2015;15:396. doi: 10.1186/s12906-015-0920-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shah T.I., Sharma E., Shah G.A. Anti-proliferative, Cytotoxicity and Anti-oxidant Activity of uglans regia Extract. Am. J. Cancer Prev. 2015;3:45–50. [Google Scholar]
- 25.Bazaid A.S., Aldarhami A., Patel M., Adnan M., Hamdi A., Snoussi M., Qanash H., Imam M., Monjed M.K., Khateb A.M. The Antimicrobial Effects of Saudi Sumra Honey against Drug Resistant Pathogens: Phytochemical Analysis, Antibiofilm, Anti-Quorum Sensing, and Antioxidant Activities. Pharmaceuticals. 2022;15:1212. doi: 10.3390/ph15101212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wianowska S., Garbaczewska A., Cieniecka-Roslonkiewicz A.L., Dawidowicz A., Jankowska A. Comparison of antifungal activity of extracts from different Juglans regia cultivars and juglone. Microb. Pathog. 2016;100:263–267. doi: 10.1016/j.micpath.2016.10.009. [DOI] [PubMed] [Google Scholar]
- 27.Choub V., Ajuna H.B., Won S.-J., Moon J.-H., Choi S.-I., Maung C.E.H., Kim C.-W., Ahn Y.S. Antifungal Activity of Bacillus velezensis CE 100 against Anthracnose Disease (Colletotrichum gloeosporioides) and Growth Promotion of Walnut (Juglans regia L.) Trees. Int. J. Mol. Sci. 2021;22:10438. doi: 10.3390/ijms221910438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Upadhyay V., Kambhoja S., Harshaleena K. Antifungal activity and preliminary phytochemical analysis of stem bark extracts of Juglans regia linn. IJPBA. 2010;1:442. [Google Scholar]
- 29.Sytykiewicz H., Chrzanowski G., Czerniewicz P., Leszczyński B., Sprawka I., Krzyżanowski R., Matok H. Antifungal Activity of Juglans regia (L.) Leaf Extracts Against Candida albicans Isolates. Pol. J. Environ. Stud. 2015;24:1339–1348. doi: 10.15244/pjoes/34671. [DOI] [Google Scholar]
- 30.Oliveira I., Sousa A., Ferreira I.C., Bento A., Estevinho L., Pereira J.A. Total phenols, antioxidant potential and antimicrobial activity of walnut (Juglans regia L.) green husks. Food Chem. Toxicol. 2008;46:2326–2331. doi: 10.1016/j.fct.2008.03.017. [DOI] [PubMed] [Google Scholar]
- 31.Hamazaki T., Okuyama H. The Japan Society for Lipid Nutrition recommends to reduce the intake of linoleic acid. A review and critique of the scientific evidence. World Rev. Nutr. Diet. 2003;92:109–132. doi: 10.1159/000073796. [DOI] [PubMed] [Google Scholar]
- 32.Czernichow S., Thomas D., Bruckert E. n-6 Fatty acids and cardiovascular health: A review of the evidence for dietary intake recommendations. Br. J. Nutr. 2010;104:788–796. doi: 10.1017/S0007114510002096. [DOI] [PubMed] [Google Scholar]
- 33.Simopoulos A.P. The importance of the ratio of omega-6/omega-3 essential fatty acids. Biomed. Pharmacother. 2002;56:365–379. doi: 10.1016/S0753-3322(02)00253-6. [DOI] [PubMed] [Google Scholar]
- 34.El Haouari M., Rosado J.A. Medicinal Plants with Antiplatelet Activity. Phytother. Res. 2016;30:1059–1071. doi: 10.1002/ptr.5619. [DOI] [PubMed] [Google Scholar]
- 35.Haider S., Batool Z., Tabassum S., Perveen T., Saleem S., Naqvi F., Javed H., Haleem D.J. Effects of walnuts (Juglans regia) on learning and memory functions. Plant Foods Hum. Nutr. 2011;66:335–340. doi: 10.1007/s11130-011-0260-2. [DOI] [PubMed] [Google Scholar]
- 36.Shi D., Chen C., Zhao S., Ge F., Liu D., Song H. Effects of Walnut Polyphenol on Learning and Memory Functions in Hypercholesterolemia Mice. J. Food Nutr. Res. 2014;2:450–456. doi: 10.12691/jfnr-2-8-4. [DOI] [Google Scholar]
- 37.Shabani M., Nazeri M., Parsania S., Razavinasab M., Zangiabadi N., Esmaeilpour K., Abareghi F. Walnut consumption protects rats against cisplatin-induced neurotoxicity. Neurotoxicology. 2012;33:1314–1321. doi: 10.1016/j.neuro.2012.08.004. [DOI] [PubMed] [Google Scholar]
- 38.Muthaiyah B., Essa M.M., Lee M., Chauhan V., Kaur K., Chauhan A. Dietary supplementation of walnuts improves memory deficits and learning skills in transgenic mouse model of Alzheimer’s disease. J. Alzheimers Dis. 2014;42:1397–1405. doi: 10.3233/JAD-140675. [DOI] [PubMed] [Google Scholar]
- 39.Ravanbakhsh A., Mahdavi M., Jalilzade-Amin G., Javadi S., Maham M., Mohammadnejad D., Rashidi M.R. Acute and Subchronic Toxicity Study of the Median Septum of Juglans regia in Wistar Rats. Adv. Pharm. Bull. 2016;6:541–549. doi: 10.15171/apb.2016.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yang L., Ma S., Han Y., Wang Y., Guo Y., Weng Q., Xu M. Walnut Polyphenol Extract Attenuates Immunotoxicity Induced by 4-Pentylphenol and 3-methyl-4-nitrophenol in Murine Splenic Lymphocyte. Nutrients. 2016;8:287. doi: 10.3390/nu8050287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hausen B.M. Woods Injurious to Human Health. De Gruyter; Berlin, Germany: Boston, MA, USA: 2016. [Google Scholar]
- 42.Bonamonte D., Foti C., Angelini G. Hyperpigmentation and contact dermatitis due to Juglans regia. Contact Dermat. 2001;44:101–102. doi: 10.1034/j.1600-0536.2001.440209-5.x. [DOI] [PubMed] [Google Scholar]
- 43.Utiérrez Ortiz A.L., Berti F., Navarini L., Crisafulli P., Colomban S., Forzato C. Aqueous extracts of walnut (Juglans regia L.) leaves: Quantitative analyses of hydroxycinnamic and chlorogenic acids. J. Chromatogr. Sci. 2018;56:753–760. doi: 10.1093/chromsci/bmy041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Calabrò S., Alzoubi K., Bissinger R., Jilani K., Faggio C., Lang F. Enhanced eryptosis following juglone exposure. Basic Clin. Pharmacol. Toxicol. 2015;116:460–467. doi: 10.1111/bcpt.12340. [DOI] [PubMed] [Google Scholar]
- 45.Belknap J.K. Black walnut extract: An inflammatory model. Vet. Clin. N. A. Equine Pract. 2010;26:95–101. doi: 10.1016/j.cveq.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 46.Carvalho M., Ferreira P.J., Mendes V.S., Silva R., Pereira J.A., Jerónimo C., Silva B.M. Human cancer cell antiproliferative and antioxidant activities of Juglans regia L. Food Chem. Toxicol. 2010;48:441–447. doi: 10.1016/j.fct.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 47.Arslan H., Ondul Koc E., Ozay Y., Canli O., Ozdemir S., Tollu G., Dizge N. Antimicrobial and antioxidant activity of phenolic extracts from walnut (Juglans regia L.) green husk by using pressure-driven membrane process. J. Food Sci. Technol. 2022;60:73–83. doi: 10.1007/s13197-022-05588-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Almeida I., Fernandes E., Lima J., Costa P., Bahia M. Walnut (Juglans regia) leaf extracts are strong scavengers of pro-oxidant reactive species. Food Chem. 2008;106:1014–1020. doi: 10.1016/j.foodchem.2007.07.017. [DOI] [Google Scholar]
- 49.Żurek N., Pawłowska A., Pycia K., Grabek-Lejko D., Kapusta I.T. Phenolic Profile and Antioxidant, Antibacterial, and Antiproliferative Activity of Juglans regia L. Male Flowers. Molecules. 2022;27:2762. doi: 10.3390/molecules27092762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hosseinzadeh H., Behravan E., Soleimani M.M. Antinociceptive and Anti-inflammatory Effects of Pistacia vera LeafExtract in Mice. Iran. J. Pharm. Res. 2011;10:821–828. [PMC free article] [PubMed] [Google Scholar]
- 51.Bhat A.A., Tandon N., Tandon R. Pyrrolidine derivatives as antibacterial agents, current status and future prospects: A patent review. Pharm. Patent Analyst. 2022 doi: 10.4155/ppa-2022-0015. [DOI] [PubMed] [Google Scholar]
- 52.Chiavaccini L., Hassel D.M., Shoemaker M.L., Charles J.B., Belknap J.K., Ehrhart E.J. Detection of calprotectin and apoptotic activity within the equine colon from horses with black walnut extract-induced laminitis. Vet. Immunol. Immunopathol. 2011;144:366–373. doi: 10.1016/j.vetimm.2011.08.006. [DOI] [PubMed] [Google Scholar]
- 53.Stacey Lockyer, Anne E de la Hunty, Simon Steenson, Ayela Spiro, Sara A Stanner, Walnut consumption and health outcomes with public health relevance—A systematic review of cohort studies and randomized controlled trials published from 2017 to present. Nutr. Rev. 2023;81:26–54. doi: 10.1093/nutrit/nuac040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Amaral J.S., Casal S., Pereira J.A., Seabra R.M., Oliveira B.P. Determination of sterol and fatty acid compositions, oxidative stability, and nutritional value of six walnut (Juglans regia L.) cultivars grown in Portugal. J. Agric. Food Chem. 2003;51:7698–7702. doi: 10.1021/jf030451d. [DOI] [PubMed] [Google Scholar]
- 55.Colaric M., Veberic R., Solar A., Hudina M., Stampar F. Phenolic acids, syringaldehyde, and juglone in fruits of different cultivars of Juglans regia L. J. Agric. Food Chem. 2005;53:6390–6396. doi: 10.1021/jf050721n. [DOI] [PubMed] [Google Scholar]
- 56.Trandafir I., Cosmulescu S., Botu M., Nour V. Antioxidant activity, and phenolic and mineral contents of the walnut kernel (Juglans regia L.) as a function of the pellicle color. Fruits. 2016;71:173–184. doi: 10.1051/fruits/2016006. [DOI] [Google Scholar]
- 57.Akbari V., Jamei R., Heidari R., Jahanban Esfahlan A. Antiradical activity of different parts of Walnut (Juglans regia L.) fruit as a function of genotype. Food Chem. 2012;135:2404–2410. doi: 10.1016/j.foodchem.2012.07.030. [DOI] [PubMed] [Google Scholar]
- 58.Owens N., Lee D. The use of micro bubble flotation technology in secondary and tertiary produced water treatment—A technical comparison with other separation technologies; Proceedings of the the TUV NEL, 5th Produced Water Workshop; Aberdeen, Scotland. 30–31 May 2007. [Google Scholar]
- 59.Altun T., Pehlivan E. Removal of Cr (VI) from aqueous solutions by modified walnut shells. Food Chem. 2012;132:693–700. doi: 10.1016/j.foodchem.2011.10.099. [DOI] [Google Scholar]
- 60.Ding D., Zhao Y., Yang S., Shi W., Zhang Z., Lei Z., Yang Y. Adsorption of cesium from aqueous solution using agricultural residue–Walnut shell: Equilibrium, kinetic and thermodynamic modeling studies. Water Res. 2013;47:2563–2571. doi: 10.1016/j.watres.2013.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Kazemipour M., Ansari M., Tajrobehkar S., Majdzadeh M., Kermani H.R. Removal of lead, cadmium, zinc, and copper from industrial wastewater by carbon developed from walnut, hazelnut, almond, pistachio shell, and apricot stone. J. Hazard. Mater. 2008;150:322–327. doi: 10.1016/j.jhazmat.2007.04.118. [DOI] [PubMed] [Google Scholar]
- 62.Köhler S.J., Cubillas P., Rodríguez-Blanco J.D., Bauer C., Prieto M. Removal of cadmium from wastewaters by aragonite shells and the influence of other divalent cations. Environ. Sci. Technol. 2007;41:112–118. doi: 10.1021/es060756j. [DOI] [PubMed] [Google Scholar]
- 63.Pehlivan E., Altun T. Biosorption of chromium (VI) ion from aqueous solutions using walnut, hazelnut and almond shell. J. Hazard. Mater. 2008;155:378–384. doi: 10.1016/j.jhazmat.2007.11.071. [DOI] [PubMed] [Google Scholar]
- 64.Saadat S., Karimi-Jashni A. Optimization of Pb (II) adsorption onto modified walnut shells using factorial design and simplex methodologies. Chem. Eng. J. 2011;173:743–749. doi: 10.1016/j.cej.2011.08.042. [DOI] [Google Scholar]
- 65.Saifuddin M., Kumaran P. Removal of heavy metal from industrial wastewater using chitosan coated oil palm shell charcoal. Electron. J. Biotechnol. 2005;8:43–53. [Google Scholar]
- 66.Saqib A.N.S., Waseem A., Khan A.F., Mahmood Q., Khan A., Habib A., Khan A.R. Arsenic bioremediation by low cost materials derived from Blue Pine (Pinus wallichiana) and Walnut (Juglans regia L.) Ecol. Eng. 2013;51:88–94. doi: 10.1016/j.ecoleng.2012.12.063. [DOI] [Google Scholar]
- 67.Shah J., Jan M.R., Haq A., Khan Y. Removal of Rhodamine B from aqueous solutions and wastewater by walnut shells: Kinetics, equilibrium and thermodynamics studies. Front. Chem. Sci. Eng. 2013;7:428–436. doi: 10.1007/s11705-013-1358-x. [DOI] [Google Scholar]
- 68.Soleimani M., Kaghazchi T. Low-Cost Adsorbents from Agricultural By-Products Impregnated with Phosphoric Acid. Adv. Chem. Eng. Res. 2014;3:34–41. [Google Scholar]
- 69.Nowicki P., Pietrzak R., Wachowska H. Sorption properties of active carbons obtained from walnut shells by chemical and physical activation. Catal. Today. 2010;150:107–114. doi: 10.1016/j.cattod.2009.11.009. [DOI] [Google Scholar]
- 70.Rahman S. Evaluation of filtering efficiency of walnut granules as deep-bed filter media. J. Petrol. Sci. Eng. 1992;7:239–246. doi: 10.1016/0920-4105(92)90021-R. [DOI] [Google Scholar]
- 71.Zare H., Najafpour G., Rahimnejad M., Tardast A., Gilani S. Biofiltration of ethyl acetate by Pseudomonas putida immobilized on walnut shell. Bioresour. Technol. 2012;123:419–423. doi: 10.1016/j.biortech.2012.07.036. [DOI] [PubMed] [Google Scholar]
- 72.Blumenschein C.D., Severing K., Boyle E. Walnut shell filtration for oil and solids removal from steel mill recycle systems. AISE Steel Technol. 2001;78:33–37. [Google Scholar]
- 73.Srinivasan A., Viraraghavan T. Removal of oil by walnut shell media. Bioresour. Technol. 2008;99:8217–8220. doi: 10.1016/j.biortech.2008.03.072. [DOI] [PubMed] [Google Scholar]
- 74.Srinivasan A., Viraraghavan T. Oil removal from water using biomaterials. Bioresour. Technol. 2010;101:6594–6600. doi: 10.1016/j.biortech.2010.03.079. [DOI] [PubMed] [Google Scholar]
- 75.Wei Q., Ma X., Zhao Z., Zhang S., Liu S. Antioxidant activities and chemical profiles of pyroligneous acids from walnut shell. J. Anal. Appl. Pyrolysis. 2010;88:149–154. doi: 10.1016/j.jaap.2010.03.008. [DOI] [Google Scholar]
- 76.Zhai M., Shi G., Wang Y., Mao G., Wang D., Wang Z. Chemical compositions and biological activites of pyroligneous acids from walnut shell. Bioresources. 2015;10:1715–1729. doi: 10.15376/biores.10.1.1715-1729. [DOI] [Google Scholar]
- 77.Meshkini A., Tahmasbi M. Anti-platelet aggregation activity of walnut hull extract via suppression of ROS generation and caspase activation. J. Acupunct. Meridian Stud. 2017;10:193–203. doi: 10.1016/j.jams.2017.02.007. [DOI] [PubMed] [Google Scholar]
- 78.Fernández-Agulló A., Pereira E., Freire M.S., Valentão P., Andrade P.B., González-Álvarez J., Pereira J.A. Influence of solvent on the antioxidant and antimicrobial properties of walnut (Juglans regia L.) green husk extracts. Ind. Crops Prod. 2013;42:126–132. doi: 10.1016/j.indcrop.2012.05.021. [DOI] [Google Scholar]
- 79.Van Hellemont J. Compendium de Phytotherapie. Association Pharmaceutique; Bruxelles, Belgium: 1986. [Google Scholar]
- 80.Bruneton J. Pharmacognosy, Phytochemistry, Medicinal Plants. Intercept; Hampshire, UK: 1999. [Google Scholar]
- 81.Girzu M., Carnat A., Privat A.-M., Fialip J., Carnat A.-P., Lamaison J.-L. Sedative effect of walnut leaf extract and juglone, an isolated constituent. Pharm. Biol. 1998;36:280–286. doi: 10.1076/phbi.36.4.280.4580. [DOI] [Google Scholar]
- 82.Hadis E., Mostafa H.-Z.S., Saeedeh N., Mehdi M. Study of the effects of walnut leaf on the levels of a number of Blood Biochemical Factors in normal male rats fed with high cholesterol diet. Clin. Biochem. 2011;44:S331. doi: 10.1016/j.clinbiochem.2011.08.818. [DOI] [Google Scholar]
- 83.Hosseini S., Fallah Huseini H., Larijani B., Mohammad K., Najmizadeh A., Nourijelyani K., Jamshidi L. The hypoglycemic effect of Juglans regia leaves aqueous extract in diabetic patients: A first human trial. DARU J. Pharm. Sci. 2014;22:19. doi: 10.1186/2008-2231-22-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Hosseini S., Mehrzadi S., Najmizadeh A.R., Kazem M., Alimoradi H., Fallah Huseini H. Effects of Juglans regia L. leaf extract on hyperglycemia and lipid profiles in type two diabetic patients: A randomized double-blind, placebo-controlled clinical trial. J. Ethnopharmacol. 2014;152:451–456. doi: 10.1016/j.jep.2014.01.012. [DOI] [PubMed] [Google Scholar]
- 85.Mahmoodi M., Eghbali H., Hosseini Zijoud S.M., Pourrashidi A., Mohamadi A., Borhani M., Hassanshahi G., Rezaeian M. Study of the effects of walnut leaf on some blood biochemical parameters in hypercholesterolemic rats. Biochem. AnalBiochem. 2011;1:1–2. doi: 10.4172/2161-1009.1000103. [DOI] [Google Scholar]
- 86.Pitschmann A., Zehl M., Atanasov A.G., Dirsch V.M., Heiss E., Glasl S. Walnut leaf extract inhibits PTP1B and enhances glucose-uptake in vitro. J. Ethnopharmacol. 2014;152:599–602. doi: 10.1016/j.jep.2014.02.017. [DOI] [PubMed] [Google Scholar]
- 87.Alkhawajah A.M. Studies on the antimicrobial activity of Juglans regia. Am. J. Chin. Med. 1997;25:175–180. doi: 10.1142/S0192415X97000202. [DOI] [PubMed] [Google Scholar]
- 88.Qa’dan F., Thewaini A.-J., Ali D.A., Afifi R., Elkhawad A., Matalka K.Z. The antimicrobial activities of Psidium guajava and Juglans regia leaf extracts to acne-developing organisms. Am. J. Chin. Med. 2005;33:197–204. doi: 10.1142/S0192415X05002783. [DOI] [PubMed] [Google Scholar]
- 89.Valdebenito D., Farías D., Oyanedel E., Castro M., Lampinen B., Tixier A., Saa S. The morphology of a Walnut (Juglans regia L.) shoot is affected by its position in the canopy and correlated to the number and size of its fruits. Sci. Hortic. 2017;220:303–309. doi: 10.1016/j.scienta.2017.03.046. [DOI] [Google Scholar]
- 90.Claudot A.-C., Ernst D., Sandermann H., Drouet A. Chalcone synthase activity and polyphenolic compounds of shoot tissues from adult and rejuvenated walnut trees. Planta. 1997;203:275–282. doi: 10.1007/s004250050192. [DOI] [Google Scholar]
- 91.Radix P., Seigle-Murandi F., Charlot G. Walnut blight: Development of fruit infection in two orchards. Crop. Prot. 1994;13:629–631. doi: 10.1016/0261-2194(94)90010-8. [DOI] [Google Scholar]
- 92.Mahoney N., Molyneux R.J., Campbell B.C. Regulation of aflatoxin production by naphthoquinones of walnut (Juglans regia) J. Agric. Food Chem. 2000;48:4418–4421. doi: 10.1021/jf0003449. [DOI] [PubMed] [Google Scholar]
- 93.Taha N.A., Al-wadaan M.A. Utility and importance of walnut, Juglans regia Linn: A review. Afr. J. Microbiol. Res. 2011;5:5796–5805. [Google Scholar]
- 94.Kale A., Shah S., Gaikwad S., Mundhe K., Deshpande N., Salvekar J. Elements from Stem Bark of Orchard Tree-Juglans regia. Int. J. Chemtech. Res. 2010;2:548–550. [Google Scholar]
- 95.Noumi E., Snoussi M., Hajlaoui H., Valentin E., Bakhrouf A. Antifungal properties of Salvadora persica and Juglans regia L. extracts against oral Candida strains. Eur. J. Clin. Microbiol. Infect. Dis. 2010;29:81–88. doi: 10.1007/s10096-009-0824-3. [DOI] [PubMed] [Google Scholar]
- 96.Haque R., Bin-Hafeez B., Parvez S., Pandey S., Sayeed I., Ali M., Raisuddin S. Aqueous extract of walnut (Juglans regia L.) protects mice against cyclophosphamide-induced biochemical toxicity. Hum. Exp. Toxicol. 2003;22:473–480. doi: 10.1191/0960327103ht388oa. [DOI] [PubMed] [Google Scholar]
- 97.Bhatia K., Rahman S., Ali M., Raisuddin S. In vitro antioxidant activity of Juglans regia L. bark extract and its protective effect on cyclophosphamide-induced urotoxicity in mice. Redox Rep. 2006;11:273–279. doi: 10.1179/135100006X155030. [DOI] [PubMed] [Google Scholar]
- 98.Nirmla Devi T., Apraj V., Bhagwat A., Mallya R., Sawant L., Pandita N. Pharmacognostic and Phytochemical Investigation of Juglans regia Linn. bark. Pharm. J. 2011;3:39–43. [Google Scholar]
- 99.Bhat A.A., Iqubal S., Nitin T., Runjhun T. Structure activity relationship (SAR) and anticancer activity of pyrrolidine derivatives: Recent developments and future prospects (A review) Eur. J. Med. Chem. 2022;246:114954. doi: 10.1016/j.ejmech.2022.114954. [DOI] [PubMed] [Google Scholar]
- 100.Abdelghany T.M., Yahya R., Bakri M.M., Ganash M., Amin B.H., Qanash H. Effect of Thevetia peruviana seeds extract for microbial pathogens and cancer control. Int. J. Pharmacol. 2021;17:643–655. doi: 10.3923/ijp.2021.643.655. [DOI] [Google Scholar]
- 101.Freeman P. Tyler’s Herbs of Choice-The Therapeutic Use of Phytomedicinals. J. E. Robbers and V. E. Tyler. New York, NY: Haworth Herbal Press. 2000. ISBN 0-7890- 0160-8. Br. J. Nutr. 2000;84:583. doi: 10.1017/S0007114500001902. [DOI] [Google Scholar]
- 102.Fujita T., Sezik E., Tabata M., Yesilada E., Honda G., Takeda Y., Tanaka T., Takaishi Y. Traditional medicine in Turkey VII. Folk medicine in middle and west Black Sea regions. Econ. Bot. 1995;49:406–422. doi: 10.1007/BF02863092. [DOI] [Google Scholar]
- 103.Kaileh M., Vanden Berghe W., Boone E., Essawi T., Haegeman G. Screening of indigenous Palestinian medicinal plants for potential anti-inflammatory and cytotoxic activity. J. Ethnopharmacol. 2007;113:510–516. doi: 10.1016/j.jep.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 104.Spaccarotella K.J., Kris-Etherton P.M., Stone W.L., Bagshaw D.M., Fishell V.K., West S.G., Lawrence F.R., Hartman T.J. The effect of walnut intake on factors related to prostate and vascular health in older men. Nutr. J. 2008;7:13. doi: 10.1186/1475-2891-7-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dominic R., Ramanujam S.N. Traditional knowledge and ethnobotanical uses of piscicidal plants of Nagaland, north east India. Indian J. Nat. Prod. Resour. 2012;3:582–588. [Google Scholar]
- 106.Bazaid A.S., Alamri A., Almashjary M.N., Qanash H., Almishaal A.A., Amin J., Binsaleh N.K., Kraiem J., Aldarhami A., Alafnan A. Antioxidant, Anticancer, Antibacterial, Antibiofilm Properties and Gas Chromatography and Mass Spectrometry Analysis of Manuka Honey: A Nature’s Bioactive Honey. Appl. Sci. 2022;12:9928. doi: 10.3390/app12199928. [DOI] [Google Scholar]
- 107.Sharma P., Verma P.K., Sood S., Pankaj N.K., Agarwal S., Raina R. Neuroprotective potential of hydroethanolic hull extract of Juglans regia L. on isoprenaline induced oxidative damage in brain of Wistar rats. Toxicol. Rep. 2021;8:223–229. doi: 10.1016/j.toxrep.2021.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Pereira J., Oliveira I., Sousa A., Valentão P., Andrade P., Ferreira I., Ferreres F., Bento A., Seabra R., Estevinho L. Walnut (Juglans regia L.) leaves: Phenolic compounds, antibacterial activity and antioxidant potential of different cultivars. Food Chem. Toxicol. 2007;45:2287–2295. doi: 10.1016/j.fct.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 109.Bennacer A., Sahir-Halouane F., Aitslimane-Aitkaki S., Oukali Z., Oliveira I.V., Rahmouni N., Aissaoui M. Structural characterization of phytochemical content, antibacterial, and antifungal activities of Juglans regia L. leaves cultivated in Algeria. Biocatal. Agric. Biotechnol. 2022;40:102304. doi: 10.1016/j.bcab.2022.102304. [DOI] [Google Scholar]
- 110.Pereira J.A., Oliveira I., Sousa A., Ferreira I.C., Bento A., Estevinho L. Bioactive properties and chemical composition of six walnut (Juglans regia L.) cultivars. Food Chem. Toxicol. 2008;46:2103–2111. doi: 10.1016/j.fct.2008.02.002. [DOI] [PubMed] [Google Scholar]
- 111.Rather M.A., Dar B.A., Dar M.Y., Wani B.A., Shah W.A., Bhat B.A., Ganai B.A., Bhat K.A., Anand R., Qurishi M.A. Chemical composition, antioxidant and antibacterial activities of the leaf essential oil of Juglans regia L. and its constituents. Phytomedicine. 2012;19:1185–1190. doi: 10.1016/j.phymed.2012.07.018. [DOI] [PubMed] [Google Scholar]
- 112.Fang Y.Z., Yang S., Wu G. Free radicals, antioxidants, and nutrition. Nutrition. 2002;18:872–879. doi: 10.1016/S0899-9007(02)00916-4. [DOI] [PubMed] [Google Scholar]
- 113.Gaunt M.W., Turner S.L., Rigottier-Gois L., Lloyd-Macgilp S.A., Young J.P. Phylogenies of atpD and recA support the small subunit rRNA-based classification of rhizobia. Int. J. Syst. Evol. Microbiol. 2001;51:2037–2048. doi: 10.1099/00207713-51-6-2037. [DOI] [PubMed] [Google Scholar]
- 114.Okatan V., Bulduk I., Kaki B., Gundesli M.A., Usanmaz S., Alas T., Hajizadeh H.S. Identification and quantification of biochemical composition and antioxidant activity of walnut pollens. Pak. J. Bot. 2021;53:1–10. doi: 10.30848/PJB2021-6(44). [DOI] [Google Scholar]
- 115.Wani A.K., Hashem N.M., Akhtar N., Singh R., Madkour M., Prakash A. Understanding microbial networks of farm animals through genomics, metagenomics and other meta-omic approaches for livestock wellness and sustainability—A Review. Ann. Anim. Sci. 2022;22:839–853. doi: 10.2478/aoas-2022-0002. [DOI] [Google Scholar]
- 116.Iwamoto M., Sato M., Kono M., Hirooka Y., Sakai K., Takeshita A., Imaizumi K. Walnuts lower serum cholesterol in Japanese men and women. J. Nutr. 2000;130:171–176. doi: 10.1093/jn/130.2.171. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.