Abstract
Specialized pro-resolving mediators (SPMs) are endogenous small molecules produced mainly from dietary omega-3 polyunsaturated fatty acids by both structural cells and cells of the active and innate immune systems. Specialized pro-resolving mediators have been shown to both limit acute inflammation and promote resolution and return to homeostasis following infection or injury. There is growing evidence that chronic immune disorders are characterized by deficiencies in resolution and SPMs have significant potential as novel therapeutics to prevent and treat chronic inflammation and immune system disorders. This review focuses on important breakthroughs in understanding how SPMs are produced by, and act on, cells of the adaptive immune system, specifically macrophages, B cells and T cells. We also highlight recent evidence demonstrating the potential of SPMs as novel therapeutic agents in topics including immunization, autoimmune disease and transplantation.
Introduction
Inflammation is a normal host response to harmful stimuli, including injury and infection. Inflammation includes steps to mitigate the damage, contain the injury to a localized area if possible, and to clear pathogens, excess immune cells and debris in preparation for a return to homeostasis. Resolution of inflammation is a critical step in the injury response. Failure to resolve inflammation leads to chronic inflammation, which contributes to the pathology of serious diseases including chronic obstructive pulmonary disease (COPD) (1), asthma (2, 3), rheumatoid arthritis (RA) (4), and multiple sclerosis (MS) (5). Of the top ten leading causes of death world-wide in 2019 (pre-pandemic), at least 5 include chronic inflammation as a contributory factor (heart disease, COPD, Alzheimer’s disease, diabetes and kidney disease) (1, 6–9).
Previously, return to homeostasis was attributed to passive clearing of immune cells and cytokines by way of dilution. It is now known that resolution of inflammation is an active process mediated largely by specialized pro-resolving lipid mediators (SPMs). SPMs are derived from the dietary ω−3 polyunsaturated fatty acids (PUFAs) docosahexaenoic acid (DHA) and eicosapentaenoic acid (EPA), and from arachidonic acid through key enzymes such as cyclooxygenase 2 (COX-2), arachidonate 5-lipooxygenase (ALOX-5), ALOX-12 and ALOX-15 (10, 11). DHA gives rise to the D-series resolvins (RvD1 through RvD6 are currently known), protectins (PD1) and maresins (MaR1), while EPA gives rise to the E-series resolvins (RvE1, RvE2, RvE3 and RvE4). Lipoxins (LXA4 and LXB4), on the other hand, are derived from arachidonic acid. SPMs can also be modified through the addition of glutathione derivatives to their structures, giving rise to new classes including maresin conjugates of tissue repair (MCTR), protectin conjugates of tissue repair (PCTR) and resolvin conjugates of tissue repair (RCTRs). Production of SPMs occurs in a variety of cell types, including but not restricted to innate (12–15) and adaptive leukocytes (16), platelets (17), epithelial cells (18), and endothelial cells (19). Production of SPMs is temporally regulated, and cells can undergo class-switching, from producing pro-inflammatory lipids such as leukotrienes and prostaglandins to pro-resolving eicosanoids by changing expression of synthetic enzymes (20–22). SPMs can also be produced via trans-cellular biosynthesis, in which one cell type produces an intermediate which is converted to the final effector molecule by a second cell (17, 19, 23, 24).
SPM effects are mediated by G-protein coupled receptors (GPCRs). Receptors identified to date include LTB4R/BLT1 (binds RvE1 and RvE2), CMKLR1/ChemR23/ERV1 (bind RvE1) ALX/FPR2 (binds RvD1, RvD3 and LXA4), 23,24, GPR32/DRV1 (RvD1, RvD3, and RvD5), GPR18/DRV2 (binds RVD2), GPR37 (binds PD1), LGR6 (binds MaR1), and GPR101 (binds n-3 D-resolvins) (10, 25). The downstream mechanism of receptor-ligand binding remains unclear, and will not be discussed in this review. The effector functions of SPMs include promoting nonphlogistic phagocytosis of pathogens (phagocytosis that does not provoke release of proinflammatory cytokines), increased phagocytosis and clearance of apoptotic inflammatory cells (efferocytosis), and decreased production of pro-inflammatory cytokines and chemokines (26). Specific effects of SPMs on structural cells and innate immune cells have been reviewed elsewhere (10, 27, 28). Here, we review the production and action of SPMs on key cells of the adaptive immune system: B cells, T cells and macrophages.
SPMs are especially interesting as potential therapies for diseases involving chronic inflammation, as they promote resolution without being immunosuppressive (reviewed in (27) and (29)). This is in stark contrast to NSAIDs and corticosteroids—which are typically the first line of defense against inflammation—but are known to suppress immune responses to pathogens. Furthermore, it is now known that corticosteroids and NSAIDs dampen SPM production, potentially delaying resolution. SPMs may represent novel therapies that promote resolution without suppressing the beneficial effects of inflammatory and immune responses. This concept will be further explored at the end of the review.
Macrophages
It has been more than 30 years since the significant role of macrophages in the resolution of inflammation was first suggested (30). These pluripotent cells act as the “Pac-Men” of the immune system, killing pathogens by phagocytosis, engulfing cellular remnants (a process known as efferocytosis), shaping the adaptive and innate immune response, and releasing a vast array of mediators, including cytokines, lipid mediators, and reactive oxygen species (23, 31). Their role is being increasingly recognized in a number of acute and chronic processes such as COPD, asthma, cystic fibrosis, and pulmonary fibrosis (32). Depending on outside signals/cytokines and the local conditions, macrophages can develop different phenotypes; these different phenotypes were initially described as M1 and M2 based on in vitro data, although this does not necessarily perfectly reflect the in vivo experience. M1 polarized macrophages are thought to promote inflammation, whereas M2 cells promote tissue healing and inflammation resolution (33, 34).
SPM production by macrophages
The first evidence for the interaction between SPMs and macrophages was obtained in the late 1990s, when it was demonstrated that lipoxins promote the clearance of necrotic cellular debris (35, 36), and it is now well-known that macrophages are major producers of SPMs. Dalli et al. demonstrated that exposure of macrophages to apoptotic neutrophils and microparticles produced by neutrophils resulted in increased production of resolvins and lipoxins. The precursors for SPM production are provided from apoptotic neutrophils, and the macrophages perform the final stages of the mediator biosynthesis, a process termed transcellular biosynthesis (37). Peripheral blood mononuclear cells (PBMCs) differentiated to M1 phenotype via IFN-y and LPS produced mostly inflammatory prostanoids and leukotrienes, whereas PBMCs differentiated to M2 phenotype produced mostly SPMs including D-series resolvins, maresins and lipoxins (37).
In addition to exposure to apoptotic cells or cellular debris, exposure to bacterial pathogens themselves can also promote SPM production (13). Human monocytes were differentiated to the M1 or M2 phenotype in vitro and challenged with E. coli or S. aureus. M1 macrophages predominantly expressed 5-LOX and Cox-2 products LTB4 and PGE2, while M2 macrophages predominantly expressed SPMs including RvD2, RvD5 and maresin-1. Furthermore, treatment with exogenous RvD5 but not LTB4 enhanced macrophage phagocytosis of target bacteria (13). The mechanism responsible for this response has yet to be elucidated. However it seems to be linked to the relative abundance and localization of 5-LOX/FLAP and 15-LOX-1 in M1 and M2 polarized cells respectively (13, 38).
SPM effects on macrophage function
Beyond contributing as a major area for SPM production, macrophages themselves are greatly affected by SPMs. Activation of SPM receptors results in changes in a number of pathways, including MAPK and NF-kB (39). Interestingly, ChemR23 (ERV1), the receptor for RvE1, is expressed on M1 macrophages but not M2 macrophages; signaling by RvE1 drives M1 macrophages to a less-inflammatory and more pro-resolving M2-like phenotype (40).
Many studies have demonstrated pro-resolving and anti-inflammatory effects of SPMs on macrophage function. Exposure of both alveolar- and monocyte-derived macrophages to RvD1 and RvD2 resulted in a profound reduction in the production of proinflammatory cytokines, along with polarization towards an M2 phenotype (39). Moreover, it restored the phagocytic activity of those cells, restoring the defects caused by exposure to cigarette smoke. Both RvD1 and RvD2 reduced the expression of IL-1β and IL-18 in both bone marrow-derived and peritoneal macrophages, indicating that resolvins block the priming of the NLRP3 inflammasome (41). RvD1 and MaR1 have also been shown to reduce the expression of TNF-α by macrophages exposed to M. tuberculosis and to promote mycobacterial-killing ability (42).
In vivo data
RvE1 and PD1 promoted resolution in a mouse model of zymosan-induced peritonitis by enhancing macrophage phagocytosis of zymosan and efferocytosis of neutrophils, while blocking lipoxygenase activity resulted in increased inflammation that could be rescued by exogenous SPMs (43). DHA and RvD1 inhibited inflammatory cytokine production and increased nonphlogistic phagocytosis in adipose tissue in a mouse model of diet-induced obesity (44). RvD1 also increased phagocytosis of P. aeruginosa and efferocytosis of leukocytes in macrophages obtained from patients with cystic fibrosis (CF) and CF mice without increasing the expression of proinflammatory cytokines (45). Profound changes to the transcriptome of these cells were noted, with suppression of inflammatory genes and increased expression of genes related to phagocytosis and inflammation resolution. RvD1, RvD5 and PD1 also enhanced bacterial phagocytosis and efferocytosis in a mouse model of E. coli infection (46).
Maresin-1 is produced by macrophages from DHA via 12-LOX. It increases the uptake of apoptotic neutrophils in mouse models of peritonitis, as well as macrophage phagocytosis (23, 47). MaR1 also promotes macrophage phenotype switching towards M2 both in vitro and in vivo and reduces TNF-α production in macrophages exposed to Mycobacterium tuberculosis, while at the same time enhancing bactericidal activity (23, 42, 48, 49). In LPS-stimulated macrophages, MaR1 suppressed the inflammatory response by reducing the expression of IL-1β and IL-6 (50). Maresin-2 is a recently identified maresin that has also been demonstrated to increase macrophage phagocytosis, perhaps to a higher degree than MaR1 (47).
Mechanism of SPM function
The biological mechanisms underlying these effects are beginning to be elucidated. RvD1 reduced lung inflammation in mice with P. aeruginosa infection in part by increasing levels of miR-155 and miR-21, which have MyD88 and toll-like receptor 2 as downstream targets, suggesting that one mechanism by which SPMs work is attenuating the sensitivity of macrophages to pro-inflammatory signals (51). RvD2 reduced the activity of caspase-1 in peritoneal macrophages and the release of IL-1β from LPS/ATP stimulated macrophages (41). It also decreased the expression of TLR4 and other accessory proteins in macrophages, mediated partially through upregulation of miR-146a. Moreover, RvD2 reduced the expression of IL-6 and TNF-α and upregulated IL-10 expression in murine models of peritonitis (41). Among the different SPMs, RvD2 is the most potent inhibitor of IL-1β production, and it may also protect macrophages from pyroptosis, a form of programmed cell death mediated by caspase-1 (41). Lipoxin A4 inhibits the secretion of TNF-α and IL-6 in human and mouse macrophages, promotes autophagy and inhibits apoptosis acting through MAPK, JNK and NF-κB (52–56). Efferocytosis of apoptotic cells may stimulate production of SPMs via upregulation of ALOX5 mediated by the binding of apoptotic cells to the MerTK receptor (57, 58).
Novel SPM conjugates in tissue repair
Three new classes of SPMs have recently been discovered, termed maresin conjugates of tissue repair (MCTR), protectin conjugates of tissue repair (PCTR) and resolvin conjugates of tissue repair (RCTR). These compounds represent MaR1, PD-1 or RvD1 conjugated to mono-, di- or tripeptides derived from glutathione (reviewed in (59)). Macrophages are an important site of synthesis of CTRs, and macrophages can also respond to signaling by exogenous CTRs (60, 61). MCTRs in particular have been shown to promote macrophage phagocytosis and efferocytosis, increase expression of M2 markers, and decrease production of inflammatory cytokines and eicosanoids in mouse models of E coli. peritonitis and LPS-induced ARDS (62, 63). Production of MCTRs by alveolar macrophages may be able to reduce inflammatory lung injury by protecting epithelial and endothelial cells (64, 65).
Macrophages are perhaps the key cell type in which the synthesis and functions of SPMs is most well-understood. Even so, there are still knowledge gaps regarding endogenous production of SPMs by macrophages or by other cell types that act on macrophages to modify their function, and further studies are ongoing.
B cells
Specialized pro-resolving mediators (SPMs) have many effects on the adaptive immune system, that include modulating B-cell development and function. While this section will focus specifically on direct actions of SPMs on B cells, it is important to remember that B-cell function and development is greatly influenced by other cells of both the innate and adaptive immune system (66, 67). Therefore, the actions of SPMs on cells involved in the innate immune response, such as antigen presenting cells (APCs) or other cells involved in adaptive immunity, including all classes of T cells, will ultimately affect the antigen-induced activation and differentiation of B cells.
B cells are derived from common lymphoid progenitor cells in the fetal liver and bone marrow (67). B-cell differentiation creates numerous, unique, functional B-cell receptors. The primary step in B-cell differentiation occurs by rearrangement of loci on the H and L chain of the immunoglobulin receptor (67, 68). This step begins with pro-B cells, which do not express pre-B-cell receptors or immunoglobulin and become pre-B cells when they utilize surrogate L chains to form pre-B-cell receptors. Following this step, immature B cells undergo auto-reactivity testing, pass into circulation, and travel to the spleen where they form various transitional B cells before ultimately forming follicular or marginal zone B cells (66, 67). Marginal zone B cells remain in the spleen, and follicular B cells move to lymph nodes, where co-stimulatory action from T cells in the presence of antigen will produce germinal centers where antigen specificity is improved via various mechanisms. In addition, B cells can differentiate into plasma cells through many transcription factors, which include PAX-5, BLIMP1 and BLC-6. They also can develop into memory B cells in various sites throughout the body (67). Aside from development into plasma and memory B cells, B cells can also act as APCs and produce cytokines throughout their life cycle in response to various stimuli (69). SPMs have been found in all sites of B cell development, including the bone marrow, peripheral blood, spleen, and lymph nodes; furthermore, B cells can express SPM receptors including ALX/FPR2, ERV1, DRV2 and BLT1 (70–72). These facts spurred research into the effects of SPMs and polyunsaturated fatty acids (PUFAs) on B cells. In this section we will discuss the effects of PUFAs and SPMs on the sequential steps of B-cell life, including development, migration, maturation, and function.
SPMs in B-cell development:
While much of the role of SPMs on hematopoiesis and early B-cell development is yet to be investigated, SPMs are present in bone marrow, and enzymes involved in SPM biosynthesis are present in hematopoietic stem cells (73, 74). In one murine study, Kinder et al. inhibited the 5-LOX and 12/15-LOX enzymes in bone marrow. This resulted in lower peripheral white blood cell count largely driven by decreased lymphoid development in bone marrow and thymus. More specifically, bone marrow contained reduced pro-B, pre-B, and immature B cells (75). This work suggests that early development of B cells from common lymphoid progenitor cells may be partially regulated by SPMs. Studies have also shown that dietary supplementation with fish-derived omega-3 fatty acids, the precursor for EPA and DPA, may result in increased numbers of T1 transitional B cells (created during migration from the bone marrow to the spleen) compared to standard diets or to diets with more specific EPA or DPA supplementation (68, 76).
SPMs in B cell migration:
Once pro-B cells exit the bone marrow, they travel to peripheral lymphatic structures to mature. This migration is directed by chemokines, and their retention in the spleen is directed by integrins. There is evidence that SPMs may influence the migration of some classes of B cells under certain conditions. A study of murine sepsis showed that administration of LXA4 promoted migration of innate response activator (IRA) CD19+ B cells, which contain the LXA4 receptor ALX/FPR2, from the peritoneum to the spleen via STAT-5 upregulation of integrins (77). This migration was independent of macrophage signaling. The implications of this finding hinge on the ability of activated B cells to produce GM-CSF, which has been tied to sepsis survival. This study suggests that the SPM LXA4 promotes IRA B-cell migration to the spleen, where they increase amounts of GM-CSF locally in response to LPS. This likely orchestrates the immune response during sepsis. More research is needed to see if this finding would translate to better sepsis outcomes with exogenous SPM administration. Another study showed that RvD1 impairs B cell migration in a rat model of lipopolysaccharide (LPS) induced uveitis (78). It was not clear exactly how this was mediated, but there were changes in miRNA levels associated with reduced integrin expression, which would decrease lymphocyte adhesion and ocular infiltration (78, 79).
Effect of SPMs on B-cell function and differentiation
While it seems likely that SPMs affect early B-cell development in the bone marrow and influence migration, there is more conclusive evidence that SPMs can affect mature B-cell function. This includes antibody class switching, quantitative changes in antibody and cytokine production, and directing mature B-cell differentiation (70, 76, 80–86). One of the first studies to demonstrate an effect of SPMs on B-cell function showed that exogenous supplementation of the SPMs 17-HDHA (an intermediate SPM involved in production of RvD1) and RvD1 itself increased human B-cell IgM and IgG production (81). This increase in antibody production appears to be mediated by increased differentiation of activated B cells to antibody secreting CD38+ B cells and facilitated by B-cell differentiation factors BLIMP1 and Xpb1. This study also confirmed that 17-HDHA and RvD1 were present in the spleen, providing evidence that these SPMs are spatiotemporally available to affect B-cell function in vivo. A similar increase in IgG and IgM production via BLIMP1- and Xpb1-mediated B-cell differentiation was also demonstrated in a study of 17-HDHA as an adjuvant in a pre-clinical influenza vaccine mouse model (83). Perhaps most importantly, this increase in IgG and IgM also translated to increased protection for influenza infection in mice treated with 17-HDHA during immunization. 17-HDHA and RvD1 also increased antibody production in a model of non-typeable Haemophilus influenza infection in mice and rescued immunosuppressive effects of chronic cigarette smoke exposure (87, 88). LXB4 increased the production of IgG specifically from human memory B cells through a COX2 mediated increase in Xpb1 and BLIMP1 expression (80). These studies highlight a potential therapeutic role for the SPMs LXB4, RvD1, and 17-HDHA as possible adjuvants for vaccines or to induce higher antibody levels and greater protection against influenza, and perhaps other viral infections.
While the SPMs RvD1 and 17-HDHA can increase secretion of IgG and IgM in primary immune responses, this is in contrast to the “classical” view of SPMs as being anti-inflammatory and pro-resolving. There is evidence that SPMs can reduce antibody secretion, but this is dependent on context (70, 84, 85). LXA4 decreased proliferation and antibody production of human and mouse memory B cells in vitro, with a profound decrease in IgM and IgG production; this effect was mediated by ALX/FPR2 receptors, but LXA4 did not have a similar effect on naïve B cells (70). The researchers also showed that LXA4 reduced IgG and IgM response to OVA immunization by two and fourfold, respectively, in a mouse model. These studies seem to suggest opposing actions of RvD1 and 17-HDHA compared to LXA4 on memory B cells, which is somewhat confusing given that both RvD1 and LXA4 bind the ALX/FPR2 receptor. This may be due to differing levels of the ALX/FPR2 receptors or the presence of additional SPM receptors on certain classes of B cells. More work is necessary to understand how SPMs interact with memory and naïve B cells in the micro-environment under certain stressors including viral infection, immunization, and other inflammatory stimuli.
In addition to IgM and IgG, IgE production seems to be modulated by SPM concentrations. This is of particular interest given the important role of IgE in allergic and atopic disorders such as asthma, in which there are elevated levels of B cell derived IgE in circulation (85). In studies using in vitro B cells from donors (which were stimulated to produce IgE) and from B cells in asthmatic patients, there was a profound inhibitory effect of RvD1 and 17-HDHA on the number of B cells undergoing class switching to produce IgE (84, 85). This effect was not due to changes in B-cell viability or reduced IgE secretion, but is rather caused by changes in BLC-6 function, which decreased activity of the STAT6 pathway and reduced expression of genes necessary for IgE class switching. Of potential clinical interest, this reduction in IgE from RvD1 and 17-HDHA was not seen in subjects treated with oral steroids or in cells treated with steroids, likely due to reduced levels of BLC-6. This may indicate a counterintuitive and unwanted effect of steroid treatment on refractory asthma, one that leads to the inability of SPMs to modulate IgE production (84).
There is also evidence for alterations in cytokine production by B cells via SPM signaling. In the aforementioned, landmark study showing increased IgG and IgM with RvD1 and 17-HDHA stimulation in human and murine B cells, the authors also noted reduced IL-6 and IL-10 production without a change in tumor necrosis factor alpha (TNF-α) levels (81). This differs from a study evaluating 17-HDHA in a murine influenza immunization model which showed increase in IL-10 levels and no change in IL-6 and TNF-α (83).
Effects of diet on B cell SPMs and function
The majority of endogenous PUFAs are derived from the diet, suggesting that dietary intake of PUFAs, via a diet naturally rich in fish and certain plants, or via supplementation, may beneficially modify B cell function. Studies in mice have shown that diets rich in DHA and EPA increased spleen levels of resolvin precursors 14-HDHA and 17-HDHA, protectin DX and RvD2, and also increased IgM-expressing splenic B cells following antigen stimulation, but not increased circulating IgG (68, 76, 89, 90). In contrast, mice fed a diet rich in omega-6 fatty acids exhibited diminished antibody titers and increased mortality to influenza challenge (91). Increased circulating levels of SPMs have been found in people taking PUFA supplements (92, 93). However, there is no current evidence that dietary omega-3 fatty acids improve antibody production or B cell function in humans.
In summary, the effect of SPMs on B cell maturation and function is complex and context-specific. SPMs and SPM synthetic enzymes are required for efficient B cell development. RvD1 and 17-HDHA enhance maturation of IgG responses upon primary immunization but inhibit class switching to IgE. LXB4 promotes reactivation of memory B cells while LXA4 inhibits memory responses. Further SPM-specific and context-specific effects may be uncovered with future study.
T cells
T cells make up the other arm of adaptive immunity and play a crucial role in the orchestration of pathogen elimination and regulation of inflammation and autoimmunity. CD8+ T cells, also known as cytotoxic T cells, release perforin and granzymes to directly kill pathogen-infected cells. CD4+ T cells perform a wide variety of immune regulatory effects including production of inflammatory cytokines, regulation of B cell differentiation and antibody production, and recruitment of neutrophils and macrophages to kill pathogens and remove damaged or infected cells. CD4+ T cells have been divided into 4 phenotypes based on expression of surface receptors and distinct effector functions upon stimulation. Th1 cells strengthen the immune response by recruiting and activating macrophages and promoting increased pathogen detection (via toll-like receptors) and antigen presentation (major histocompatibility complexes I and II) and are characterized by the release of interferon gamma (IFN-γ) and TNF-α (94). Th2 cells are characterized by expression of IL-4, IL-5, and IL-13, drive B cell to class-switch to IgE, can recruit mast cells and eosinophils, and are implicated in allergies and asthma (95). Th17 cells are associated with bacterial and fungal infections, are important for recruiting neutrophils, and are characterized by production of IL-17, IL-22, IL-25, and IL-26 (94, 96). Finally, Treg cells act to regulate and suppress immune responses. Tregs are involved in suppressing activated T cells and reducing inflammation via the production of IL-10 and tumor-growth factor beta (TGF-β), which affects almost all circulating immune cell types (94). They are crucial in ensuring that the inflammatory response is timely and appropriate.
With their myriad of important effector functions in both promoting and resolving inflammatory and immune responses, it is not surprising that SPMs regulate T cell differentiation and function in profound ways.
T cell production of SPMs
Surprisingly, there has been little reported to date concerning the production of SPMs by T cells. In one study, peripheral blood mononuclear cells driven to a Th2 phenotype in vitro produced protectin D1 (PD1), while Th1 cells did not. Since PD1 can block T cell migration and synthesis of TNF-α, this might represent a means by which T cells exert autologous or paralogous effects on other T cells (97). A more recent study showed that pharmacologic inhibition or genetic deletion of ALOX15 (involved in production of most SPMs) in Tregs resulted in impaired function. Exogenous RvD3 was able to rescue the effect of ALOX15 deletion, suggesting that autologous production of SPMs is required for proper Treg function (16).
SPM receptor expression on T cell subsets
T cells have long been known to express BLT1, the receptor for leukotriene B4 (LTB4), which is generally understood to have pro-inflammatory effects. LTB4 signaling promotes T cell migration and CD8 T cell effector functions in activated but not naïve or memory T cells (98, 99), and BLT1 expression is higher in chronic inflammatory conditions like asthma (100). BLT1 is also a receptor for RvE1 (101), and there is recent evidence that signaling through BLT1 promotes recruitment of Tregs to the alveoli and resolution of an experimental model of acute lung injury (86). Therefore, it appears that BLT1 can participate in both pro-inflammatory and pro-resolution pathways depending on its ligand. T cells also express CMKLR1 (ChemR23, ERV1), a receptor for both chemerin (a pro-inflammatory adipokine) and RvE1 (102, 103). RvE1 can interact with the receptor to block binding of chemerin and LTB4 (101). Similarly, activated and memory CD4 T cells express high levels of ALX/FPR2, which is a receptor for both the pro-inflammatory cytokine serum amyloid A (104) and RvD1 (71). (71). These seemingly oppositional actions of SPM receptors demonstrate an important concept in resolution biology, that the effects of receptors can be ligand and situation-specific. In this context, strategies to block receptor expression or ligation to reduce pro-inflammatory signaling may ultimately be counter-productive as such inhibitors may also reduce pro-resolving signals. Finally, T cells express GPR32 (a second receptor for RvD1) and GPR18 (DRV2), a receptor for RvD2, and loss of receptor expression is associated with failure to resolve inflammation in chronic heart failure (105, 106).
SPMs as modifiers of T cell function
Early studies reported that dietary supplementation with PUFAs or DHA increased T cell proliferation and decreased Th2 cytokine production in mice (107). Multiple SPMs, including RvD1, RvD2, Mar1, RvD3, LXA4 and LXB4, can block production of IFN-γ and TNF-α by Th1 and Th17 cells (97, 105, 108). RvD1, RvD2 and MaR1 also suppressed differentiation of human peripheral blood mononuclear cells into Th1 and Th17 cells in vitro and enhanced differentiation to Tregs (105), while MaR1 enhanced the ability of Tregs to suppress inflammatory cytokine production by ILC2s (109). RvD1 also enhanced antigen responses and decreased lung inflammation in a mouse model of respiratory infection following exposure to secondhand smoke, in part by suppressing generation of antigen-specific Th17 cells and enhancing generation of antigen-specific Th2 cells (87). RvE1 also inhibited ex vivo differentiation of mouse splenic T cells to Th17 cells (103). There is less evidence for direct suppressing effects of SPMs on Th2 cells, although RvE1 can reduce inflammation and cytokine production by Th2 cells in a mouse model of allergic airways hyperresponsiveness (110, 111). RvE1, LXA4 and PD1 were also shown to enhance clearance of apoptotic T cells via upregulation of the chemokine receptor CCR5 (97, 112).
SPMs in disease models
Inflammatory and autoimmune diseases are often characterized by an imbalance between Th1 and Th17 responses (excessive and pathologic) and Tregs (regulatory and pro-resolving). As a result, studies aimed at understanding this imbalance and shifting it toward Tregs have high translational relevance, and SPMs have been identified as important factors in disease and potential therapies.
Patients with systemic lupus erythematosus (SLE) exhibited significantly lower levels of RvD1 in plasma compared to healthy controls, and treatment with exogenous RvD1 improved disease activity scores in a mouse model, with decreased Th17 and increased Tregs (113). RvD1 also improved saliva secretion and reduced Th17 infiltration in a mouse model of Sjögren’s syndrome (114). MarR1 promoted resolution of lung inflammation in a mouse sepsis model, which was associated with increased Tregs/FoxP3 expression and decreased Th17/RORC expression (115). In separate studies, patients with inactive rheumatoid arthritis had higher circulating levels of MaR1 and protectin DX and higher expression of the Treg transcription factor FoxP3 in CD4+ T cells than patients with active disease, as well as lower levels of the Th17 transcription factor RORC (116, 117). A separate study also found that rheumatoid arthritis patients with active disease had lower circulating levels of LXA4, RvD1 and RvE1 compared to patients in remission or healthy controls (118). MaR1 and PDX were investigated as therapies in mice with collagen induced arthritis, and reduced disease severity scores, decreased joint inflammation and pro-inflammatory cytokines, and increased FoxO1/RORC and Treg/Th17 ratios (116, 117). RvE1 and PD1 both decreased T cell production of IL-17A and numbers of Th17 cells in a mouse psoriasis model (119, 120). In contrast, RvE1 inhibition of T cell mediated responses in a mouse periodontal model was associated with decreases in both Th17 and Treg cell numbers (121). RvD2 also inhibited bone loss in the periodontal disease model, associated with reduced secretion of IFN-γ from Th1 cells and reduced numbers of Th1 and Tregs (122). This reduction of Tregs as well as Th1 and Th17 cells in this model is in contrast to other SPMs and disease models and deserves more study.
LXA4 has also been shown to suppress Th1 and Th17 responses in autoimmune uveitis and experimental autoimmune encephalomyelitis. In the uveitis model, ALOX15-deficient mice (which can’t produce LXA4) developed worse inflammation than controls, with increased Th1 and Th17 cells in ocular infiltrates. Treatment with exogenous LXA4 rescued these phenotypes (123). Similarly, in the murine experimental autoimmune encephalomyelitis model, administration of LXA4 ameliorated clinical signs with decreased spinal cord Th1 and Th17 cells, leading to decreased production of IFN-γ and IL-17 (124).
An area of particular interest to the authors is the field of lung transplantation. Lung transplantation is currently limited by poor long-term outcomes, with long-term graft survival falling well behind that of other solid organ transplants due in large part to chronic rejection mediated by Th17 cells (125). While investigation of SPMs in lung transplant models have not been reported to date, SPMs have been shown to inhibit T cell mediated inflammatory processes other transplant models. RvD1 protected mice from ischemia/reperfusion-induced acute kidney injury, with less renal tubule injury, lower circulating levels of IFN-γ and TNF-α, and increased Tregs. The effect was mediated by the RvD1 receptor ALX/FPR2 but not GPR32 (126). Similarly, RvE1 improved allograft survival in a mouse model of corneal transplant, with reduced infiltration of Th1 and Th17 cells and decreased production of inflammatory cytokines (127). These results support the concept of further exploring the role for SPMs to prevent chronic rejection after transplant.
Discussion
One caveat with any animal model study is that, unlike in vitro experiments on isolated cell types, the effects of SPMs can’t be assigned to a specific cell type with certainty. SPMs can have effects on immune cells and structural cells, and it is possible that their effects on B or T cells in a given disease model could be due, in part or wholly, to indirect effects mediated by endothelial cells, epithelial cells, DCs, neutrophils or others, as well as to direct effects. For example, we reported that RvD1 prevents cigarette smoke-induced airspace enlargement in mice, associated with decreases in CD45+ cells, macrophages, neutrophils, and oxidative stress (128), and that RvD1 altered both T and B cell function in a model of smoke exposure plus chronic bacterial infection (87), but the exact location and nature of the effector cells responsible for this effect was undetermined.
Although dietary supplementation with fish oil, highly purified DHA or EPA or other sources of omega-3 PUFAs has been shown to increase circulating levels of their metabolites in animals and humans, several completed clinical trials of omega-3 PUFAs for diseases including heart disease, metabolic syndrome and obesity have shown mixed results, and no consensus has been reached regarding definite benefits (129–132). Additional trials are underway. To date, two clinical trials have been completed involving pharmaceutical derivatives of RvE1 (ClinicalTrials.gov Identifier: NCT00799552, NCT00941018) but the results have not been reported. A lipoxin analogue BLXA4-Me is in a clinical trial to treat gingivitis (ClinicalTrials.gov Identifier: NCT02342691). Further trials are exploring whether SPMs can be used as biomarkers of disease progression or treatment efficacy following other interventions.
Regardless, SPMs remain a promising area for pharmaceutical development for a number of reasons. First, since they are endogenously produced, administration of SPMs is expected to be well-tolerated. Secondly, they can be derivatized to increase stability or better target specific receptors. Finally, they are active in the nanomolar to picomolar range, which is readily achievable with typical routes of administration. The principal difficulty is likely to be targeting SPM therapies to the right location at the right time, since SPMs act over short distances, and their effects are temporally restricted and dependent on specific context. A better understanding of how the G-protein coupled receptors translate ligand binding into specific changes in gene expression and cellular activity is also necessary.
Finally, the authors think it is appropriate to briefly consider the role of SPMs in management of the current Sars-Cov-2/COVID-19 pandemic. Severe cases of COVID-19 commonly manifest with respiratory failure due to acute respiratory distress syndrome (ARDS) and cytokine storm. A recent study reported that patients with mild COVID-19 had elevated levels of SPMs in their serum, while patients with severe COVID-19 pneumonia had depressed serum SPMs. (133) Corticosteroids have been adopted as a front-line defense and have been proven to have mortality benefit in clinical trials (134). As noted previously, corticosteroids can also suppress adaptive immune responses and production of some SPMs in cell culture and some animal models (27, 29), suggesting that targeted SPM-based therapies might be more beneficial in the ongoing pandemic than a non-targeted immunosuppressive approach (135). On the other hand, dexamethasone was noted to increased serum SPM levels in COVID-19 patients (133), so more information is clearly needed. SPMs have successfully reduced lung inflammation in animal models of sepsis, ARDS, cigarette smoking, and bacterial infection (51, 62, 63, 87, 128, 136–138). To date, two small pilot studies have reported a modest survival benefit of dietary omega-3 PUFAs in COVID-19 (139, 140), and several authors have speculated on the benefits of dietary omega-3 PUFAs and SPMs in treating COVID-19 (135, 141, 142). SPMs might also be used to treat long-COVID syndrome, as they have potent abilities to resolve chronic inflammatory conditions. Ultimately, SPMs represent a new window into understanding and treating acute and chronic inflammatory and immune disorders characterized by failure to appropriately resolve inflammation and acute immune responses.
Figure 1. SPMs act to promote M2 macrophage differentiation and function.
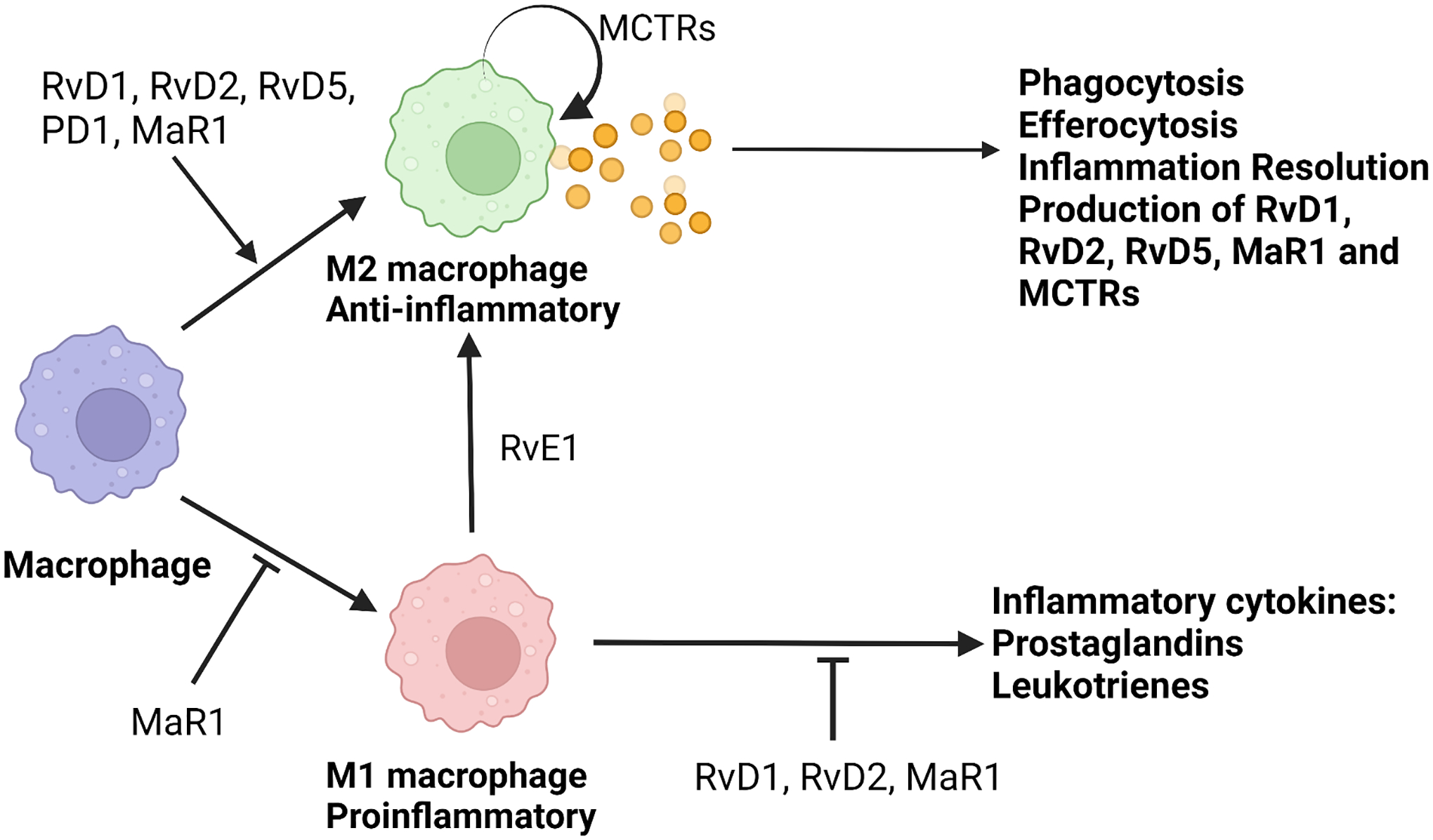
This figure summarizes current knowledge on the effects of SPMs on macrophages. SPMs have been shown to promote differentiation of M2 macrophages and inhibit differentiation of M1 macrophages. SPMs also inhibit production of pro-inflammatory mediators by M1s, while they are produced by M2s. MCTRs are both produced by and act on M2 macrophages.
Figure 2. B cell differentiation and antibody production are modulated by SPMs.
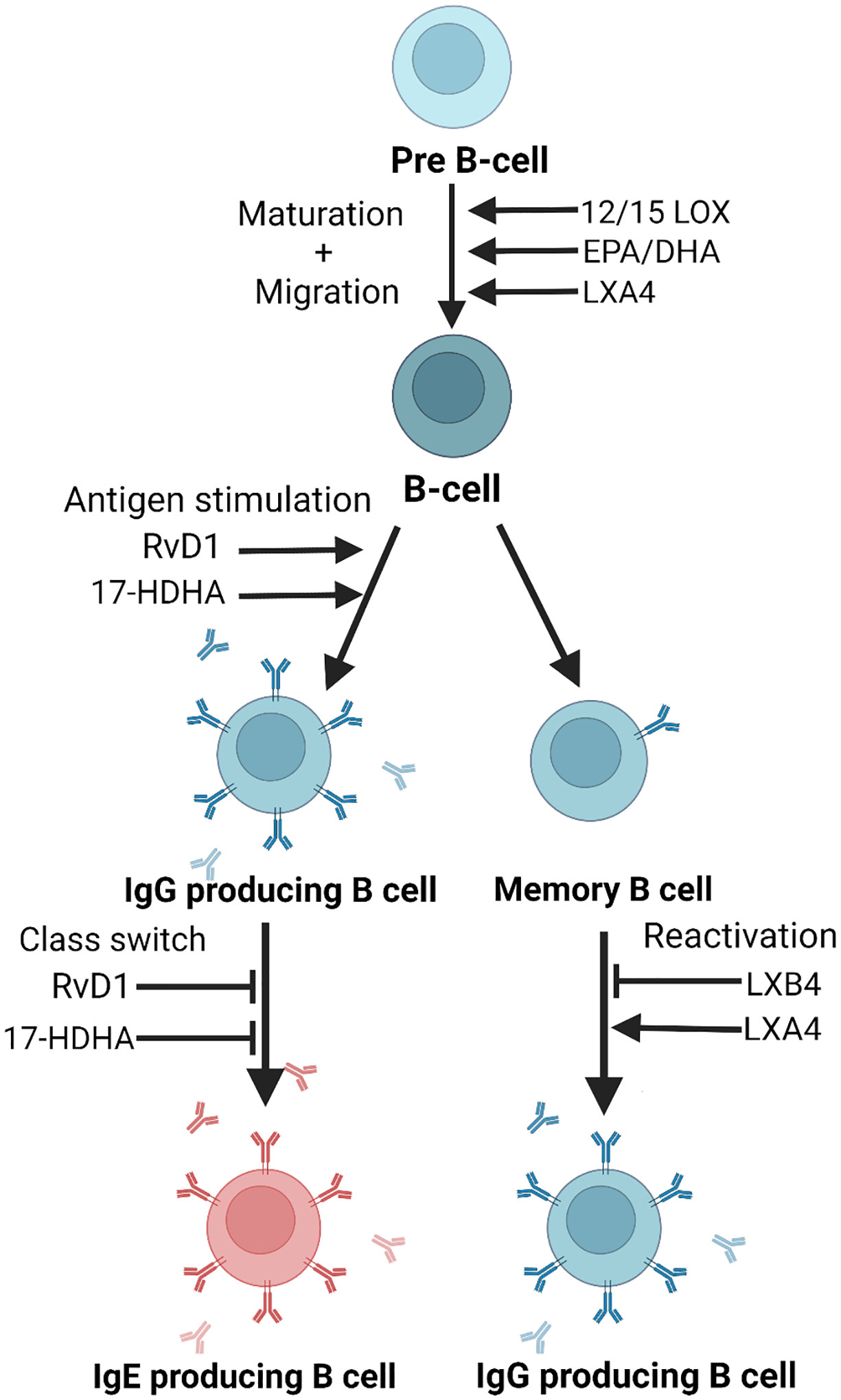
SPMs can either up- or down-regulate antibody production depending on the B cell subset and specific SPM involved.
Figure 3. SPMs regulate T cell differentiation and function.
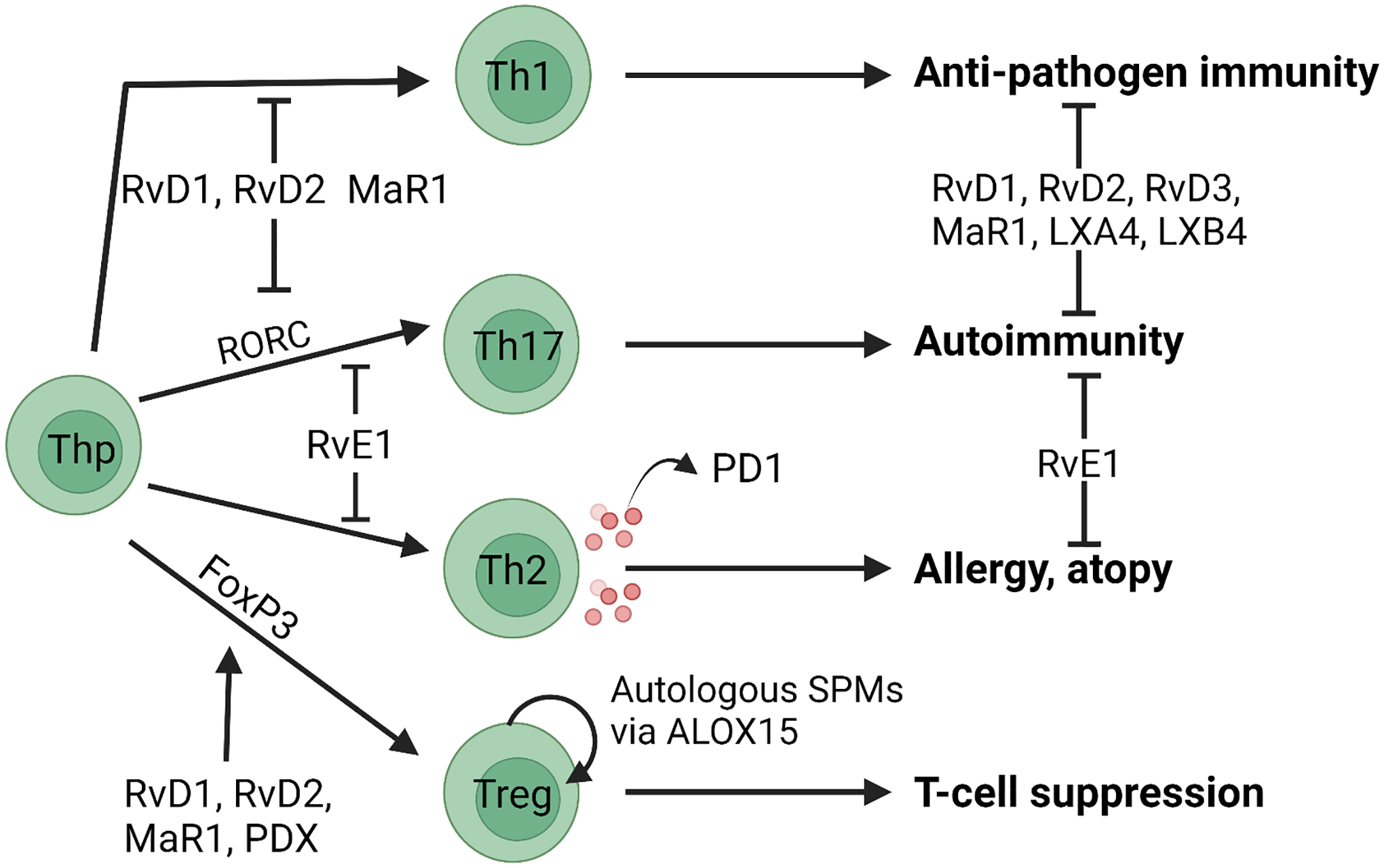
The indicated SPMs have been reported to inhibit differentiation of Th1 and Th17 cells and promote differentiation of Tregs, as shown. SPMs also inhibit the effector functions of Th1, Th17 and Th2 cells as shown. Production of SPMs by T cells has only been reported to date for PD1.
Table 1.
SPM effects in vitro
| Cell Type | Experimental condition | SPM (Dose) | Effect | Reference |
|---|---|---|---|---|
| Monocyte/Macrophages | N/A | LXA4 and analogs (1–100 nM) | Increased chemotaxis but not cytotoxicity | [35] |
| Monocyte/Macrophages | Cigarette smoke extract | RvD1 and RvD2 (1–100 nM) | Decreased IL-6, IL-8, TNF-α, and PGE2 | [39] |
| Monocyte/Macrophages | M. tuberculosis infection | LXA4, RvD1, RvD2, PD1, Mar1 (100 nM) | Decreased TNF-α, and mycobacteria infection | [42] |
| Monocyte/Macrophages | E. coli infection | RvD1, RvD5 and PD1 (0.01–100 nM) | Increased phagocytosis | [46] |
| Monocyte/Macrophages | LPS | MaR1 (0.1–100 nM) | Decreased IL-6, IL-1β, TNF-α and increased PPAR-γ activation | [50] |
| Monocyte/Macrophages | Oxidized LDL | LXA4 (100 nM) | Decreased foam cells | [52] |
| Monocyte/Macrophages | LPS | LXA4 (1–300 nM) | Decreased NFκB activation and TNF-α. | [53] |
| Monocyte/Macrophages | IFNγ plus LPS | RvD1 (1–100 nM), DHA (10–100 μM) | Inhibited pro-inflammatory cytokines and increased phagocytosis | [44] |
| Monocyte/Macrophages | E. coli | MCTR1, MCTR2, MCTR3 (0.001–10 nM) | Increased macrophage phagocytosis | [62] |
| B-cells (human peripheral blood) | N/A | LXA4 (10–100 nM) | Decreased antibody production by memory B cells. | [70] |
| B-cells (human peripheral blood) | N/A | LXB4 (10–100 nM) | Increased memory B cell activation | [80] |
| B-cells (human peripheral blood) | N/A | 17-HDHA (10–100 nM) | Increased antibody production | [81] |
| B-cells (mouse spleen) | Influenza vaccination | 17-HDHA (10–100 nM) | Increased antibody production and B cell differentiation. | [83] |
| B-cells (human peripheral blood) | N/A | 17-HDHA and RvD1 (10–100 nM) | Decreased IgE production through decreased class-switching. | [85] |
| T-cells (human peripheral blood) | N/A | PD1 (0.01–10 nM) | Decreased TNF-α and INF-γ production, increased T-cell apoptosis | [97] |
| T-cells (human peripheral blood) | N/A | RvD1, RvD2 and Mar1 (10 nM) | Decreased TNF-α, INF-γ, IL-17, and IL-2 production, increased Treg polarization | [105] |
Table 2.
Effects and doses of SPMs in vivo.
| Animal Model | SPM (Dose) | Effect | Reference |
|---|---|---|---|
| Tissue regeneration (Planaria) | MCTR1, MCTR2, MCTR3 (1 nM–100 nM) | Faster tissue regeneration | [62] |
| Ear inflammation (mouse) | LXA4 analogs (120–240 nM, topical) | Decreased neutrophil infiltration | [36] |
| Peritonitis (mouse) | RvD1 and RvD2 (300 ng, one time) | Inhibition of NLRP3 and increased M2 polarization | [41] |
| Peritonitis (mouse) | RvE1, PD1 and AT-LXA4 (300 ng, one time) | Increased macrophage phagocytosis | [43] |
| Peritonitis (mouse) | MCTR1, MCTR2, MCTR3 (50 ng, one time) | Decreased neutrophil infiltration, increased macrophage efferocytosis. | [62] |
| Peritonitis (mouse) | RvD1 and RvD5 (50–100 ng, one time) | Decreased E. coli infection | [46] |
| Sepsis (mouse) | LXA4 (10 μg/kg/day) | Increased survival and B cell generation | [77] |
| Sepsis-induced lung injury (mouse) | MaR1 (0.5–1 ng, one time) | Increased survival rate, Tregs, decreased Th17 | [115] |
| Sepsis-induced cardiac injury (mouse) | MaR1 (100 ng, one time) | Decreased cardiac damage, increased M2 polarization | [49] |
| Obesity (mouse) | DHA (4 mg/kg/day for 10 days) | Increased M2 polarization | [44] |
| Cystic Fibrosis (mouse) | RvD1 (100 ng/mouse/day) | Decreased neutrophil infiltration, increased macrophage phagocytosis | [45] |
| Acute Respiratory Distress Syndrome (mouse) | MCTR1 (100 ng/mouse, one time) | Decreased lung injury, neutrophils and increased M2 polarization | [63] |
| Uveitis (rat) | RvD1 (10–1000 ng/kg, one time) | Decreased B-cell and T-cell recruitment, increased M2 polarization. | [78] |
| Experimental autoimmune uveitis (mouse) | LXA4 (1 μg/mouse/day) | Decreased uveitis pathology, decreased T cell recruitment | [123] |
| Influenza vaccination (mouse) | 17-HDHA (1 μg/mouse, one time) | Increased antibody titers | [83] |
| Ova immunization (mouse) | LXA4 (1 μg/mouse, one time) | Decreased ova-specific IgM and IgG production. | [70] |
| Infection after second-hand smoke exposure (mouse) | AT-RvD1 (1 μg/mouse/twice per week) | Decreased neutrophil, macrophage and T cell recruitment | [87] |
| Allergic lung inflammation (mouse) | MaR1 (1 ng/mouse/day) | Increased Treg polarization, shortened allergic inflammation period. | [109] |
| Allergic lung inflammation (mouse) | RvE1 (1 μg/mouse/day) | Decreased eosinophil and lymphocyte recruitment and airway hyperresponsiveness | [110] |
| Periodontitis (mouse) | RvE1 (1 μM applied daily) | Decreased bone loss and T cell recruitment | [121] |
| Periodontitis (mouse) | RvD2 (0.5 μg then 0.1 μg/mouse/every 3 days) | Decreased bone loss, decreased neutrophil recruitment to gingiva | [122] |
| Ischemia/reperfusion-induced acute kidney injury (mouse) | RvD1 (5 μg/kg/day) | Decreased renal damage, increased Tregs. | [126] |
| Lupus (mouse) | RvD1 (5 μg/kg/every 3 days) | Increased Treg/Th17 ratio, decreased autoantibodies and kidney damage | [113] |
| Sjogren’s syndrome (mouse) | AT-RvD1 (100 ng/kg/twice per week) | Decreased Th17 cells in submandibular gland, increased saliva production | [114] |
| Collagen-induced arthritis (mouse) | MaR1 (100 ng/mouse/every 3 days) | Decreased joint pathology, decreased Th17 and increased Treg | [116] |
| Collagen-induced arthritis (mouse) | PDX (160 ng/mouse/every 3 days) | Decreased joint pathology, decreased Th17 and increased Treg | [117] |
| Psoriasis-like model (mouse) | MaR1 (100 ng topically, daily) | Decreased swelling, IL-17 production | [119] |
| Corneal transplant (mouse) | RvE1 (1 μg, subconjunctivally, day 0 and 7) | Decreased transplant rejection, neutrophils, Th1 and Th17. | [127] |
Acknowledgements
This work was funded in part by NIH grant HL120908. Figures created with BioRender.com.
References
- [1].Barbu C, Iordache M, Man MG: Inflammation in COPD: pathogenesis, local and systemic effects. Rom J Morphol Embryol 2011, 52:21–7. [PubMed] [Google Scholar]
- [2].Fahy JV: Type 2 inflammation in asthma--present in most, absent in many. Nat Rev Immunol 2015, 15:57–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Mims JW: Asthma: definitions and pathophysiology. Int Forum Allergy Rhinol 2015, 5 Suppl 1:S2–6. [DOI] [PubMed] [Google Scholar]
- [4].McInnes IB, Schett G: The pathogenesis of rheumatoid arthritis. N Engl J Med 2011, 365:2205–19. [DOI] [PubMed] [Google Scholar]
- [5].Lassmann H, van Horssen J, Mahad D: Progressive multiple sclerosis: pathology and pathogenesis. Nat Rev Neurol 2012, 8:647–56. [DOI] [PubMed] [Google Scholar]
- [6].Sarnak MJ, Amann K, Bangalore S, Cavalcante JL, Charytan DM, Craig JC, Gill JS, Hlatky MA, Jardine AG, Landmesser U, Newby LK, Herzog CA, Cheung M, Wheeler DC, Winkelmayer WC, Marwick TH, Conference P: Chronic Kidney Disease and Coronary Artery Disease: JACC State-of-the-Art Review. J Am Coll Cardiol 2019, 74:1823–38. [DOI] [PubMed] [Google Scholar]
- [7].Golia E, Limongelli G, Natale F, Fimiani F, Maddaloni V, Pariggiano I, Bianchi R, Crisci M, D’Acierno L, Giordano R, Di Palma G, Conte M, Golino P, Russo MG, Calabro R, Calabro P: Inflammation and cardiovascular disease: from pathogenesis to therapeutic target. Curr Atheroscler Rep 2014, 16:435. [DOI] [PubMed] [Google Scholar]
- [8].Calsolaro V, Edison P: Neuroinflammation in Alzheimer’s disease: Current evidence and future directions. Alzheimers Dement 2016, 12:719–32. [DOI] [PubMed] [Google Scholar]
- [9].Furman D, Campisi J, Verdin E, Carrera-Bastos P, Targ S, Franceschi C, Ferrucci L, Gilroy DW, Fasano A, Miller GW, Miller AH, Mantovani A, Weyand CM, Barzilai N, Goronzy JJ, Rando TA, Effros RB, Lucia A, Kleinstreuer N, Slavich GM: Chronic inflammation in the etiology of disease across the life span. Nat Med 2019, 25:1822–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Chiang N, Serhan CN: Specialized pro-resolving mediator network: an update on production and actions. Essays Biochem 2020, 64:443–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Panigrahy D, Gilligan MM, Serhan CN, Kashfi K: Resolution of inflammation: An organizing principle in biology and medicine. Pharmacol Ther 2021, 227:107879. [DOI] [PubMed] [Google Scholar]
- [12].Kretzer C, Jordan PM, Meyer KPL, Hoff D, Werner M, Hofstetter RK, Koeberle A, Cala Peralta A, Viault G, Seraphin D, Richomme P, Helesbeux JJ, Stuppner H, Temml V, Schuster D, Werz O: Natural chalcones elicit formation of specialized pro-resolving mediators and related 15-lipoxygenase products in human macrophages. Biochem Pharmacol 2022, 195:114825. [DOI] [PubMed] [Google Scholar]
- [13].Werz O, Gerstmeier J, Libreros S, De la Rosa X, Werner M, Norris PC, Chiang N, Serhan CN: Human macrophages differentially produce specific resolvin or leukotriene signals that depend on bacterial pathogenicity. Nat Commun 2018, 9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Snodgrass RG, Benatzy Y, Schmid T, Namgaladze D, Mainka M, Schebb NH, Lutjohann D, Brune B: Efferocytosis potentiates the expression of arachidonate 15-lipoxygenase (ALOX15) in alternatively activated human macrophages through LXR activation. Cell death and differentiation 2021, 28:1301–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Mainka M, George S, Angioni C, Ebert R, Goebel T, Kampschulte N, Krommes A, Weigert A, Thomas D, Schebb NH, Steinhilber D, Kahnt AS: On the biosynthesis of specialized pro-resolving mediators in human neutrophils and the influence of cell integrity. Biochim Biophys Acta Mol Cell Biol Lipids 2022, 1867:159093. [DOI] [PubMed] [Google Scholar]
- [16].Marques RM, Gonzalez-Nunez M, Walker ME, Gomez EA, Colas RA, Montero-Melendez T, Perretti M, Dalli J: Loss of 15-lipoxygenase disrupts Treg differentiation altering their pro-resolving functions. Cell death and differentiation 2021, 28:3140–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Serhan CN, Sheppard KA: Lipoxin formation during human neutrophil-platelet interactions. Evidence for the transformation of leukotriene A4 by platelet 12-lipoxygenase in vitro. J Clin Invest 1990, 85:772–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Planaguma A, Pfeffer MA, Rubin G, Croze R, Uddin M, Serhan CN, Levy BD: Lovastatin decreases acute mucosal inflammation via 15-epi-lipoxin A4. Mucosal immunology 2010, 3:270–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Claria J, Serhan CN: Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A 1995, 92:9475–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Liu T, Xiang A, Peng T, Doran AC, Tracey KJ, Barnes BJ, Tabas I, Son M, Diamond B: HMGB1-C1q complexes regulate macrophage function by switching between leukotriene and specialized proresolving mediator biosynthesis. Proc Natl Acad Sci U S A 2019, 116:23254–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Pace S, Zhang K, Jordan PM, Bilancia R, Wang W, Borner F, Hofstetter RK, Potenza M, Kretzer C, Gerstmeier J, Fischer D, Lorkowski S, Gilbert NC, Newcomer ME, Rossi A, Chen X, Werz O: Anti-inflammatory celastrol promotes a switch from leukotriene biosynthesis to formation of specialized pro-resolving lipid mediators. Pharmacol Res 2021, 167:105556. [DOI] [PubMed] [Google Scholar]
- [22].Levy BD, Clish CB, Schmidt B, Gronert K, Serhan CN: Lipid mediator class switching during acute inflammation: signals in resolution. Nat Immunol 2001, 2:612–9. [DOI] [PubMed] [Google Scholar]
- [23].Dalli J, Serhan CN: Pro-Resolving Mediators in Regulating and Conferring Macrophage Function. Frontiers in immunology 2017, 8:1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Carlo T, Levy BD: Molecular circuits of resolution in airway inflammation. ScientificWorldJournal 2010, 10:1386–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Chiang N, Serhan CN: Structural elucidation and physiologic functions of specialized pro-resolving mediators and their receptors. Mol Aspects Med 2017, 58:114–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Godson C, Mitchell S, Harvey K, Petasis NA, Hogg N, Brady HR: Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J Immunol 2000, 164:1663–7. [DOI] [PubMed] [Google Scholar]
- [27].Basil MC, Levy BD: Specialized pro-resolving mediators: endogenous regulators of infection and inflammation. Nat Rev Immunol 2016, 16:51–67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Thornton JM, Yin K: Role of Specialized Pro-Resolving Mediators in Modifying Host Defense and Decreasing Bacterial Virulence. Molecules 2021, 26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Serhan CN, Chiang N, Dalli J: The resolution code of acute inflammation: Novel pro-resolving lipid mediators in resolution. Semin Immunol 2015, 27:200–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Savill JS, Wyllie AH, Henson JE, Walport MJ, Henson PM, Haslett C: Macrophage phagocytosis of aging neutrophils in inflammation. Programmed cell death in the neutrophil leads to its recognition by macrophages. J Clin Invest 1989, 83:865–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Ortega-Gomez A, Perretti M, Soehnlein O: Resolution of inflammation: an integrated view. EMBO Mol Med 2013, 5:661–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lee JW, Chun W, Lee HJ, Min JH, Kim SM, Seo JY, Ahn KS, Oh SR: The Role of Macrophages in the Development of Acute and Chronic Inflammatory Lung Diseases. Cells 2021, 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Mantovani A, Biswas SK, Galdiero MR, Sica A, Locati M: Macrophage plasticity and polarization in tissue repair and remodelling. The Journal of pathology 2013, 229:176–85. [DOI] [PubMed] [Google Scholar]
- [34].Martinez FO, Sica A, Mantovani A, Locati M: Macrophage activation and polarization. Front Biosci 2008, 13:453–61. [DOI] [PubMed] [Google Scholar]
- [35].Maddox JF, Hachicha M, Takano T, Petasis NA, Fokin VV, Serhan CN: Lipoxin A4 stable analogs are potent mimetics that stimulate human monocytes and THP-1 cells via a G-protein-linked lipoxin A4 receptor. J Biol Chem 1997, 272:6972–8. [DOI] [PubMed] [Google Scholar]
- [36].Takano T, Fiore S, Maddox JF, Brady HR, Petasis NA, Serhan CN: Aspirin-triggered 15-epi-lipoxin A4 (LXA4) and LXA4 stable analogues are potent inhibitors of acute inflammation: evidence for anti-inflammatory receptors. J Exp Med 1997, 185:1693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Dalli J, Serhan CN: Specific lipid mediator signatures of human phagocytes: microparticles stimulate macrophage efferocytosis and pro-resolving mediators. Blood 2012, 120:e60–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Ebert R, Cumbana R, Lehmann C, Kutzner L, Toewe A, Ferreiros N, Parnham MJ, Schebb NH, Steinhilber D, Kahnt AS: Long-term stimulation of toll-like receptor-2 and −4 upregulates 5-LO and 15-LO-2 expression thereby inducing a lipid mediator shift in human monocyte-derived macrophages. Biochim Biophys Acta Mol Cell Biol Lipids 2020, 1865:158702. [DOI] [PubMed] [Google Scholar]
- [39].Croasdell A, Thatcher TH, Kottmann RM, Colas RA, Dalli J, Serhan CN, Sime PJ, Phipps RP: Resolvins attenuate inflammation and promote resolution in cigarette smoke-exposed human macrophages. Am J Physiol Lung Cell Mol Physiol 2015, 309:L888–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Herova M, Schmid M, Gemperle C, Hersberger M: ChemR23, the receptor for chemerin and resolvin E1, is expressed and functional on M1 but not on M2 macrophages. J Immunol 2015, 194:2330–7. [DOI] [PubMed] [Google Scholar]
- [41].Lopategi A, Flores-Costa R, Rius B, Lopez-Vicario C, Alcaraz-Quiles J, Titos E, Claria J: Frontline Science: Specialized proresolving lipid mediators inhibit the priming and activation of the macrophage NLRP3 inflammasome. J Leukoc Biol 2019, 105:25–36. [DOI] [PubMed] [Google Scholar]
- [42].Ruiz A, Sarabia C, Torres M, Juarez E: Resolvin D1 (RvD1) and maresin 1 (Mar1) contribute to human macrophage control of M. tuberculosis infection while resolving inflammation. International immunopharmacology 2019, 74:105694. [DOI] [PubMed] [Google Scholar]
- [43].Schwab JM, Chiang N, Arita M, Serhan CN: Resolvin E1 and protectin D1 activate inflammation-resolution programmes. Nature 2007, 447:869–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Titos E, Rius B, Gonzalez-Periz A, Lopez-Vicario C, Moran-Salvador E, Martinez-Clemente M, Arroyo V, Claria J: Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol 2011, 187:5408–18. [DOI] [PubMed] [Google Scholar]
- [45].Isopi E, Mattoscio D, Codagnone M, Mari VC, Lamolinara A, Patruno S, D’Aurora M, Cianci E, Nespoli A, Franchi S, Gatta V, Dubourdeau M, Moretti P, Di Sabatino M, Iezzi M, Romano M, Recchiuti A: Resolvin D1 Reduces Lung Infection and Inflammation Activating Resolution in Cystic Fibrosis. Frontiers in immunology 2020, 11:581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Chiang N, Fredman G, Backhed F, Oh SF, Vickery T, Schmidt BA, Serhan CN: Infection regulates pro-resolving mediators that lower antibiotic requirements. Nature 2012, 484:524–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Deng B, Wang CW, Arnardottir HH, Li Y, Cheng CY, Dalli J, Serhan CN: Maresin biosynthesis and identification of maresin 2, a new anti-inflammatory and pro-resolving mediator from human macrophages. PLoS One 2014, 9:e102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Tang S, Wan M, Huang W, Stanton RC, Xu Y: Maresins: Specialized Proresolving Lipid Mediators and Their Potential Role in Inflammatory-Related Diseases. Mediators of inflammation 2018, 2018:2380319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [49].Li D, Wang M, Ye J, Zhang J, Xu Y, Wang Z, Zhao M, Ye D, Wan J: Maresin 1 alleviates the inflammatory response, reduces oxidative stress and protects against cardiac injury in LPS-induced mice. Life Sci 2021, 277:119467. [DOI] [PubMed] [Google Scholar]
- [50].Wang W, Xu RL, He P, Chen R: MAR1 suppresses inflammatory response in LPS-induced RAW 264.7 macrophages and human primary peripheral blood mononuclear cells via the SIRT1/PGC-1alpha/PPAR-gamma pathway. J Inflamm (Lond) 2021, 18:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Codagnone M, Cianci E, Lamolinara A, Mari VC, Nespoli A, Isopi E, Mattoscio D, Arita M, Bragonzi A, Iezzi M, Romano M, Recchiuti A: Resolvin D1 enhances the resolution of lung inflammation caused by long-term Pseudomonas aeruginosa infection. Mucosal immunology 2018, 11:35–49. [DOI] [PubMed] [Google Scholar]
- [52].Mai J, Liu W, Fang Y, Zhang S, Qiu Q, Yang Y, Wang X, Huang T, Zhang H, Xie Y, Lin M, Chen Y, Wang J: The atheroprotective role of lipoxin A4 prevents oxLDL-induced apoptotic signaling in macrophages via JNK pathway. Atherosclerosis 2018, 278:259–68. [DOI] [PubMed] [Google Scholar]
- [53].Kure I, Nishiumi S, Nishitani Y, Tanoue T, Ishida T, Mizuno M, Fujita T, Kutsumi H, Arita M, Azuma T, Yoshida M: Lipoxin A(4) reduces lipopolysaccharide-induced inflammation in macrophages and intestinal epithelial cells through inhibition of nuclear factor-kappaB activation. The Journal of pharmacology and experimental therapeutics 2010, 332:541–8. [DOI] [PubMed] [Google Scholar]
- [54].Liu H, Zhou K, Liao L, Zhang T, Yang M, Sun C: Lipoxin A4 receptor agonist BML-111 induces autophagy in alveolar macrophages and protects from acute lung injury by activating MAPK signaling. Respiratory research 2018, 19:243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [55].Huang YH, Wang HM, Cai ZY, Xu FY, Zhou XY: Lipoxin A4 inhibits NF-kappaB activation and cell cycle progression in RAW264.7 cells. Inflammation 2014, 37:1084–90. [DOI] [PubMed] [Google Scholar]
- [56].Zhao J, Geng W, Wan K, Guo K, Xi F, Xu X, Xiong X, Huang X, Liu J, Kuang X: Lipoxin A4 promotes autophagy and inhibits overactivation of macrophage inflammasome activity induced by Pg LPS. J Int Med Res 2021, 49:300060520981259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Cai B, Thorp EB, Doran AC, Subramanian M, Sansbury BE, Lin CS, Spite M, Fredman G, Tabas I: MerTK cleavage limits proresolving mediator biosynthesis and exacerbates tissue inflammation. Proc Natl Acad Sci U S A 2016, 113:6526–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].Cai B, Kasikara C, Doran AC, Ramakrishnan R, Birge RB, Tabas I: MerTK signaling in macrophages promotes the synthesis of inflammation resolution mediators by suppressing CaMKII activity. Sci Signal 2018, 11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Serhan CN, Chiang N, Dalli J: New pro-resolving n-3 mediators bridge resolution of infectious inflammation to tissue regeneration. Mol Aspects Med 2018, 64:1–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Dalli J, Vlasakov I, Riley IR, Rodriguez AR, Spur BW, Petasis NA, Chiang N, Serhan CN: Maresin conjugates in tissue regeneration biosynthesis enzymes in human macrophages. Proc Natl Acad Sci U S A 2016, 113:12232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [61].Jouvene CC, Shay AE, Soens MA, Norris PC, Haeggstrom JZ, Serhan CN: Biosynthetic metabolomes of cysteinyl-containing immunoresolvents. FASEB J 2019, 33:13794–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Dalli J, Sanger JM, Rodriguez AR, Chiang N, Spur BW, Serhan CN: Identification and Actions of a Novel Third Maresin Conjugate in Tissue Regeneration: MCTR3. PLoS One 2016, 11:e0149319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Wang Q, Zhang HW, Mei HX, Ye Y, Xu HR, Xiang SY, Yang Q, Zheng SX, Smith FG, Jin SW: MCTR1 enhances the resolution of lipopolysaccharide-induced lung injury through STAT6-mediated resident M2 alveolar macrophage polarization in mice. J Cell Mol Med 2020, 24:9646–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [64].Li H, Hao Y, Yang LL, Wang XY, Li XY, Bhandari S, Han J, Liu YJ, Gong YQ, Scott A, Smith FG, Jin SW: MCTR1 alleviates lipopolysaccharide-induced acute lung injury by protecting lung endothelial glycocalyx. J Cell Physiol 2020, 235:7283–94. [DOI] [PubMed] [Google Scholar]
- [65].Han J, Li H, Bhandari S, Cao F, Wang XY, Tian C, Li XY, Zhang PH, Liu YJ, Wu CH, Smith FG, Jin SW, Hao Y: Maresin Conjugates in Tissue Regeneration 1 improves alveolar fluid clearance by up-regulating alveolar ENaC, Na, K-ATPase in lipopolysaccharide-induced acute lung injury. J Cell Mol Med 2020, 24:4736–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [66].Hardy RR, Hayakawa K: B cell development pathways. Annual review of immunology 2001, 19:595–621. [DOI] [PubMed] [Google Scholar]
- [67].LeBien TW, Tedder TF: B lymphocytes: how they develop and function. Blood 2008, 112:1570–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [68].Gutierrez S, Svahn SL, Johansson ME: Effects of Omega-3 Fatty Acids on Immune Cells. Int J Mol Sci 2019, 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [69].Duffney PF, Falsetta ML, Rackow AR, Thatcher TH, Phipps RP, Sime PJ: Key roles for lipid mediators in the adaptive immune response. J Clin Invest 2018, 128:2724–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Ramon S, Bancos S, Serhan CN, Phipps RP: Lipoxin A(4) modulates adaptive immunity by decreasing memory B-cell responses via an ALX/FPR2-dependent mechanism. Eur J Immunol 2014, 44:357–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [71].Spurr L, Nadkarni S, Pederzoli-Ribeil M, Goulding NJ, Perretti M, D’Acquisto F: Comparative analysis of Annexin A1-formyl peptide receptor 2/ALX expression in human leukocyte subsets. International immunopharmacology 2011, 11:55–66. [DOI] [PubMed] [Google Scholar]
- [72].Crouch MJ, Kosaraju R, Guesdon W, Armstrong M, Reisdorph N, Jain R, Fenton J, Shaikh SR: Frontline Science: A reduction in DHA-derived mediators in male obesity contributes toward defects in select B cell subsets and circulating antibody. J Leukoc Biol 2019, 106:241–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Shear MK, Fyer AJ, Ball G, Josephson S, Fitzpatrick M, Gitlin B, Frances A, Gorman J, Liebowitz M, Klein DF: Vulnerability to sodium lactate in panic disorder patients given cognitive-behavioral therapy. Am J Psychiatry 1991, 148:795–7. [DOI] [PubMed] [Google Scholar]
- [74].Romano M, Patruno S, Pomilio A, Recchiuti A: Proresolving Lipid Mediators and Receptors in Stem Cell Biology: Concise Review. Stem Cells Transl Med 2019, 8:992–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Kinder M, Wei C, Shelat SG, Kundu M, Zhao L, Blair IA, Pure E: Hematopoietic stem cell function requires 12/15-lipoxygenase-dependent fatty acid metabolism. Blood 2010, 115:5012–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].Teague H, Fhaner CJ, Harris M, Duriancik DM, Reid GE, Shaikh SR: n-3 PUFAs enhance the frequency of murine B-cell subsets and restore the impairment of antibody production to a T-independent antigen in obesity. Journal of lipid research 2013, 54:3130–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [77].Cheng Q, Wang Z, Ma R, Chen Y, Yan Y, Miao S, Jiao J, Cheng X, Kong L, Ye D: Lipoxin A4 protects against lipopolysaccharide-induced sepsis by promoting innate response activator B cells generation. International immunopharmacology 2016, 39:229–35. [DOI] [PubMed] [Google Scholar]
- [78].Rossi S, Di Filippo C, Gesualdo C, Potenza N, Russo A, Trotta MC, Zippo MV, Maisto R, Ferraraccio F, Simonelli F, D’Amico M: Protection from endotoxic uveitis by intravitreal Resolvin D1: involvement of lymphocytes, miRNAs, ubiquitin-proteasome, and M1/M2 macrophages. Mediators of inflammation 2015, 2015:149381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Settimio R, Clara DF, Franca F, Francesca S, Michele D: Resolvin D1 reduces the immunoinflammatory response of the rat eye following uveitis. Mediators of inflammation 2012, 2012:318621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [80].Kim N, Lannan KL, Thatcher TH, Pollock SJ, Woeller CF, Phipps RP: Lipoxin B4 Enhances Human Memory B Cell Antibody Production via Upregulating Cyclooxygenase-2 Expression. J Immunol 2018, 201:3343–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Ramon S, Gao F, Serhan CN, Phipps RP: Specialized proresolving mediators enhance human B cell differentiation to antibody-secreting cells. J Immunol 2012, 189:1036–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [82].Pal A, Gowdy KM, Oestreich KJ, Beck M, Shaikh SR: Obesity-Driven Deficiencies of Specialized Pro-resolving Mediators May Drive Adverse Outcomes During SARS-CoV-2 Infection. Frontiers in immunology 2020, 11:1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [83].Ramon S, Baker SF, Sahler JM, Kim N, Feldsott EA, Serhan CN, Martinez-Sobrido L, Topham DJ, Phipps RP: The specialized proresolving mediator 17-HDHA enhances the antibody-mediated immune response against influenza virus: a new class of adjuvant? J Immunol 2014, 193:6031–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [84].Kim N, Thatcher TH, Sime PJ, Phipps RP: Corticosteroids inhibit anti-IgE activities of specialized proresolving mediators on B cells from asthma patients. JCI insight 2017, 2:e88588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [85].Kim N, Ramon S, Thatcher TH, Woeller CF, Sime PJ, Phipps RP: Specialized proresolving mediators (SPMs) inhibit human B-cell IgE production. Eur J Immunol 2016, 46:81–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [86].Wang L, Zhao L, Lv J, Yin Q, Liang X, Chu Y, He R: BLT1-dependent alveolar recruitment of CD4(+)CD25(+) Foxp3(+) regulatory T cells is important for resolution of acute lung injury. Am J Respir Crit Care Med 2012, 186:989–98. [DOI] [PubMed] [Google Scholar]
- [87].Bhat TA, Kalathil SG, Bogner PN, Lehmann PV, Thatcher TH, Sime PJ, Thanavala Y: AT-RvD1 Mitigates Secondhand Smoke-Exacerbated Pulmonary Inflammation and Restores Secondhand Smoke-Suppressed Antibacterial Immunity. J Immunol 2021, 206:1348–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [88].Bhat TA, Kalathil SG, Miller A, Thatcher TH, Sime PJ, Thanavala Y: Specialized Proresolving Mediators Overcome Immune Suppression Induced by Exposure to Secondhand Smoke. J Immunol 2020, 205:3205–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [89].Teague H, Harris M, Fenton J, Lallemand P, Shewchuk BM, Shaikh SR: Eicosapentaenoic and docosahexaenoic acid ethyl esters differentially enhance B-cell activity in murine obesity. Journal of lipid research 2014, 55:1420–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Gurzell EA, Teague H, Harris M, Clinthorne J, Shaikh SR, Fenton JI: DHA-enriched fish oil targets B cell lipid microdomains and enhances ex vivo and in vivo B cell function. J Leukoc Biol 2013, 93:463–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [91].Kosaraju R, Guesdon W, Crouch MJ, Teague HL, Sullivan EM, Karlsson EA, Schultz-Cherry S, Gowdy K, Bridges LC, Reese LR, Neufer PD, Armstrong M, Reisdorph N, Milner JJ, Beck M, Shaikh SR: B Cell Activity Is Impaired in Human and Mouse Obesity and Is Responsive to an Essential Fatty Acid upon Murine Influenza Infection. J Immunol 2017, 198:4738–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].Colas RA, Shinohara M, Dalli J, Chiang N, Serhan CN: Identification and signature profiles for pro-resolving and inflammatory lipid mediators in human tissue. Am J Physiol Cell Physiol 2014, 307:C39–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Mas E, Croft KD, Zahra P, Barden A, Mori TA: Resolvins D1, D2, and other mediators of self-limited resolution of inflammation in human blood following n-3 fatty acid supplementation. Clin Chem 2012, 58:1476–84. [DOI] [PubMed] [Google Scholar]
- [94].Raphael I, Nalawade S, Eagar TN, Forsthuber TG: T cell subsets and their signature cytokines in autoimmune and inflammatory diseases. Cytokine 2015, 74:5–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Walker JA, McKenzie ANJ: TH2 cell development and function. Nat Rev Immunol 2018, 18:121–33. [DOI] [PubMed] [Google Scholar]
- [96].Korn T, Bettelli E, Oukka M, Kuchroo VK: IL-17 and Th17 Cells. Annual review of immunology 2009, 27:485–517. [DOI] [PubMed] [Google Scholar]
- [97].Ariel A, Li PL, Wang W, Tang WX, Fredman G, Hong S, Gotlinger KH, Serhan CN: The docosatriene protectin D1 is produced by TH2 skewing and promotes human T cell apoptosis via lipid raft clustering. J Biol Chem 2005, 280:43079–86. [DOI] [PubMed] [Google Scholar]
- [98].Tager AM, Bromley SK, Medoff BD, Islam SA, Bercury SD, Friedrich EB, Carafone AD, Gerszten RE, Luster AD: Leukotriene B4 receptor BLT1 mediates early effector T cell recruitment. Nat Immunol 2003, 4:982–90. [DOI] [PubMed] [Google Scholar]
- [99].Goodarzi K, Goodarzi M, Tager AM, Luster AD, von Andrian UH: Leukotriene B4 and BLT1 control cytotoxic effector T cell recruitment to inflamed tissues. Nat Immunol 2003, 4:965–73. [DOI] [PubMed] [Google Scholar]
- [100].Dakhama A, Collins ML, Ohnishi H, Goleva E, Leung DY, Alam R, Sutherland ER, Martin RJ, Gelfand EW: IL-13-producing BLT1-positive CD8 cells are increased in asthma and are associated with airway obstruction. Allergy 2013, 68:666–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [101].Arita M, Ohira T, Sun YP, Elangovan S, Chiang N, Serhan CN: Resolvin E1 selectively interacts with leukotriene B4 receptor BLT1 and ChemR23 to regulate inflammation. J Immunol 2007, 178:3912–7. [DOI] [PubMed] [Google Scholar]
- [102].Arita M, Bianchini F, Aliberti J, Sher A, Chiang N, Hong S, Yang R, Petasis NA, Serhan CN: Stereochemical assignment, antiinflammatory properties, and receptor for the omega-3 lipid mediator resolvin E1. J Exp Med 2005, 201:713–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [103].Oner F, Alvarez C, Yaghmoor W, Stephens D, Hasturk H, Firatli E, Kantarci A: Resolvin E1 Regulates Th17 Function and T Cell Activation. Frontiers in immunology 2021, 12:637983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [104].Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, Flower RJ, Perretti M: Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A 2013, 110:18232–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [105].Chiurchiu V, Leuti A, Dalli J, Jacobsson A, Battistini L, Maccarrone M, Serhan CN: Proresolving lipid mediators resolvin D1, resolvin D2, and maresin 1 are critical in modulating T cell responses. Science translational medicine 2016, 8:353ra111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [106].Chiurchiu V, Leuti A, Saracini S, Fontana D, Finamore P, Giua R, Padovini L, Incalzi RA, Maccarrone M: Resolution of inflammation is altered in chronic heart failure and entails a dysfunctional responsiveness of T lymphocytes. FASEB J 2019, 33:909–16. [DOI] [PubMed] [Google Scholar]
- [107].Arrington JL, Chapkin RS, Switzer KC, Morris JS, McMurray DN: Dietary n-3 polyunsaturated fatty acids modulate purified murine T-cell subset activation. Clin Exp Immunol 2001, 125:499–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [108].Ariel A, Chiang N, Arita M, Petasis NA, Serhan CN: Aspirin-triggered lipoxin A4 and B4 analogs block extracellular signal-regulated kinase-dependent TNF-alpha secretion from human T cells. J Immunol 2003, 170:6266–72. [DOI] [PubMed] [Google Scholar]
- [109].Krishnamoorthy N, Burkett PR, Dalli J, Abdulnour RE, Colas R, Ramon S, Phipps RP, Petasis NA, Kuchroo VK, Serhan CN, Levy BD: Cutting edge: maresin-1 engages regulatory T cells to limit type 2 innate lymphoid cell activation and promote resolution of lung inflammation. J Immunol 2015, 194:863–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [110].Aoki H, Hisada T, Ishizuka T, Utsugi M, Ono A, Koga Y, Sunaga N, Nakakura T, Okajima F, Dobashi K, Mori M: Protective effect of resolvin E1 on the development of asthmatic airway inflammation. Biochem Biophys Res Commun 2010, 400:128–33. [DOI] [PubMed] [Google Scholar]
- [111].Aoki H, Hisada T, Ishizuka T, Utsugi M, Kawata T, Shimizu Y, Okajima F, Dobashi K, Mori M: Resolvin E1 dampens airway inflammation and hyperresponsiveness in a murine model of asthma. Biochem Biophys Res Commun 2008, 367:509–15. [DOI] [PubMed] [Google Scholar]
- [112].Ariel A, Fredman G, Sun YP, Kantarci A, Van Dyke TE, Luster AD, Serhan CN: Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol 2006, 7:1209–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Cheng T, Ding S, Liu S, Li X, Tang X, Sun L: Resolvin D1 Improves the Treg/Th17 Imbalance in Systemic Lupus Erythematosus Through miR-30e-5p. Frontiers in immunology 2021, 12:668760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [114].Dean S, Wang CS, Nam K, Maruyama CL, Trump BG, Baker OJ: Aspirin Triggered Resolvin D1 reduces inflammation and restores saliva secretion in a Sjogren’s syndrome mouse model. Rheumatology (Oxford) 2019, 58:1285–92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [115].Xia H, Wang F, Wang M, Wang J, Sun S, Chen M, Huang S, Chen X, Yao S: Maresin1 ameliorates acute lung injury induced by sepsis through regulating Th17/Treg balance. Life Sci 2020, 254:117773. [DOI] [PubMed] [Google Scholar]
- [116].Jin S, Chen H, Li Y, Zhong H, Sun W, Wang J, Zhang T, Ma J, Yan S, Zhang J, Tian Q, Yang X, Wang J: Maresin 1 improves the Treg/Th17 imbalance in rheumatoid arthritis through miR-21. Ann Rheum Dis 2018, 77:1644–52. [DOI] [PubMed] [Google Scholar]
- [117].Jin S, Sun S, Ling H, Ma J, Zhang X, Xie Z, Zhan N, Zheng W, Li M, Qin Y, Zhao H, Chen Y, Yang X, Wang J: Protectin DX restores Treg/Th17 cell balance in rheumatoid arthritis by inhibiting NLRP3 inflammasome via miR-20a. Cell Death Dis 2021, 12:280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [118].Ozgul Ozdemir RB, Soysal Gunduz O, Ozdemir AT, Akgul O: Low levels of pro-resolving lipid mediators lipoxin-A4, resolvin-D1 and resolvin-E1 in patients with rheumatoid arthritis. Immunol Lett 2020, 227:34–40. [DOI] [PubMed] [Google Scholar]
- [119].Saito-Sasaki N, Sawada Y, Mashima E, Yamaguchi T, Ohmori S, Yoshioka H, Haruyama S, Okada E, Nakamura M: Maresin-1 suppresses imiquimod-induced skin inflammation by regulating IL-23 receptor expression. Sci Rep 2018, 8:5522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [120].Park KD, Kim N, Kang J, Dhakal H, Kim JY, Jang YH, Lee WJ, Lee SJ, Kim SH: Protectin D1 reduces imiquimod-induced psoriasiform skin inflammation. International immunopharmacology 2021, 98:107883. [DOI] [PubMed] [Google Scholar]
- [121].Alvarez C, Abdalla H, Sulliman S, Rojas P, Wu YC, Almarhoumi R, Huang RY, Galindo M, Vernal R, Kantarci A: RvE1 Impacts the Gingival Inflammatory Infiltrate by Inhibiting the T Cell Response in Experimental Periodontitis. Frontiers in immunology 2021, 12:664756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Mizraji G, Heyman O, Van Dyke TE, Wilensky A: Resolvin D2 Restrains Th1 Immunity and Prevents Alveolar Bone Loss in Murine Periodontitis. Frontiers in immunology 2018, 9:785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Wei J, Mattapallil MJ, Horai R, Jittayasothorn Y, Modi AP, Sen HN, Gronert K, Caspi RR: A novel role for lipoxin A4 in driving a lymph node-eye axis that controls autoimmunity to the neuroretina. Elife 2020, 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Derada Troletti C, Enzmann G, Chiurchiu V, Kamermans A, Tietz SM, Norris PC, Jahromi NH, Leuti A, van der Pol SMA, Schouten M, Serhan CN, de Vries HE, Engelhardt B, Kooij G: Pro-resolving lipid mediator lipoxin A4 attenuates neuro-inflammation by modulating T cell responses and modifies the spinal cord lipidome. Cell Rep 2021, 35:109201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [125].Chen QR, Wang LF, Xia SS, Zhang YM, Xu JN, Li H, Ding YZ: Role of interleukin-17A in early graft rejection after orthotopic lung transplantation in mice. Journal of thoracic disease 2016, 8:1069–79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [126].Luan H, Wang C, Sun J, Zhao L, Li L, Zhou B, Shao S, Shen X, Xu Y: Resolvin D1 Protects Against Ischemia/Reperfusion-Induced Acute Kidney Injury by Increasing Treg Percentages via the ALX/FPR2 Pathway. Front Physiol 2020, 11:285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [127].Wang H, Zhao Q, Luo D, Yin Y, Li T, Zhao M: Resolvin E1 Inhibits Corneal Allograft Rejection in High-Risk Corneal Transplantation. Invest Ophthalmol Vis Sci 2018, 59:3911–9. [DOI] [PubMed] [Google Scholar]
- [128].Hsiao HM, Thatcher TH, Colas RA, Serhan CN, Phipps RP, Sime PJ: Resolvin D1 Reduces Emphysema and Chronic Inflammation. Am J Pathol 2015, 185:3189–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [129].Yu H, Su X, Lei T, Zhang C, Zhang M, Wang Y, Zhu L, Liu J: Effect of Omega-3 Fatty Acids on Chronic Obstructive Pulmonary Disease: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. International journal of chronic obstructive pulmonary disease 2021, 16:2677–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [130].Chatterjee D, Chatterjee A, Kalra D, Kapoor A, Vijay S, Jain S: Role of adjunct use of omega 3 fatty acids in periodontal therapy of periodontitis. A systematic review and meta-analysis. J Oral Biol Craniofac Res 2022, 12:55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [131].Schonenberger KA, Schupfer AC, Gloy VL, Hasler P, Stanga Z, Kaegi-Braun N, Reber E: Effect of Anti-Inflammatory Diets on Pain in Rheumatoid Arthritis: A Systematic Review and Meta-Analysis. Nutrients 2021, 13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [132].Zhang HJ, Gao X, Guo XF, Li KL, Li S, Sinclair AJ, Li D: Effects of dietary eicosapentaenoic acid and docosahexaenoic acid supplementation on metabolic syndrome: A systematic review and meta-analysis of data from 33 randomized controlled trials. Clin Nutr 2021, 40:4538–50. [DOI] [PubMed] [Google Scholar]
- [133].Koenis DS, Beegun I, Jouvene CC, Aguirre GA, Souza PR, Gonzalez-Nunez M, Ly L, Pistorius K, Kocher HM, Ricketts W, Thomas G, Perretti M, Alusi G, Pfeffer P, Dalli J: Disrupted Resolution Mechanisms Favor Altered Phagocyte Responses in COVID-19. Circ Res 2021, 129:e54–e71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [134].Group RC, Horby P, Lim WS, Emberson JR, Mafham M, Bell JL, Linsell L, Staplin N, Brightling C, Ustianowski A, Elmahi E, Prudon B, Green C, Felton T, Chadwick D, Rege K, Fegan C, Chappell LC, Faust SN, Jaki T, Jeffery K, Montgomery A, Rowan K, Juszczak E, Baillie JK, Haynes R, Landray MJ: Dexamethasone in Hospitalized Patients with Covid-19. N Engl J Med 2021, 384:693–704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [135].Panigrahy D, Gilligan MM, Huang S, Gartung A, Cortes-Puch I, Sime PJ, Phipps RP, Serhan CN, Hammock BD: Inflammation resolution: a dual-pronged approach to averting cytokine storms in COVID-19? Cancer Metastasis Rev 2020, 39:337–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].Hsiao HM, Sapinoro RE, Thatcher TH, Croasdell A, Levy EP, Fulton RA, Olsen KC, Pollock SJ, Serhan CN, Phipps RP, Sime PJ: A novel anti-inflammatory and pro-resolving role for resolvin D1 in acute cigarette smoke-induced lung inflammation. PLoS One 2013, 8:e58258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [137].Kim KH, Park TS, Kim YS, Lee JS, Oh YM, Lee SD, Lee SW: Resolvin D1 prevents smoking-induced emphysema and promotes lung tissue regeneration. International journal of chronic obstructive pulmonary disease 2016, 11:1119–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [138].Posso SV, Quesnot N, Moraes JA, Brito-Gitirana L, Kennedy-Feitosa E, Barroso MV, Porto LC, Lanzetti M, Valenca SS: AT-RVD1 repairs mouse lung after cigarette smoke-induced emphysema via downregulation of oxidative stress by NRF2/KEAP1 pathway. International immunopharmacology 2018, 56:330–8. [DOI] [PubMed] [Google Scholar]
- [139].Doaei S, Gholami S, Rastgoo S, Gholamalizadeh M, Bourbour F, Bagheri SE, Samipoor F, Akbari ME, Shadnoush M, Ghorat F, Mosavi Jarrahi SA, Ashouri Mirsadeghi N, Hajipour A, Joola P, Moslem A, Goodarzi MO: The effect of omega-3 fatty acid supplementation on clinical and biochemical parameters of critically ill patients with COVID-19: a randomized clinical trial. Journal of translational medicine 2021, 19:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Asher A, Tintle NL, Myers M, Lockshon L, Bacareza H, Harris WS: Blood omega-3 fatty acids and death from COVID-19: A pilot study. Prostaglandins Leukot Essent Fatty Acids 2021, 166:102250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Simopoulos AP, Serhan CN, Bazinet RP: The need for precision nutrition, genetic variation and resolution in Covid-19 patients. Mol Aspects Med 2021, 77:100943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [142].Cagnina RE, Duvall MG, Nijmeh J, Levy BD: Specialized pro-resolving mediators in respiratory diseases. Curr Opin Clin Nutr Metab Care 2021. [DOI] [PMC free article] [PubMed] [Google Scholar]


