Abstract
Fruit rot caused by phytopathogenic fungi is one of the major diseases affecting watermelons (Citrullus lanatus) around the world, which can result in unmarketable fruits and significant economic losses. Fruit rot was observed on watermelons throughout the postharvest storage periods in Phayao Province, northern Thailand in 2022. For the present study, a total of ten fungal isolates were isolated from the rot lesions of watermelons. All obtained fungal isolates were then characterized in terms of their pathogenicity. The results indicated that only four fungal isolates caused rot disease with similar symptoms during the postharvest storage period. Based on their morphological characteristics, these four fungal isolates were identified as belonging to the genus Fusarium. Using multi-gene phylogenetic analyses with a combination of the translation elongation factor 1-alpha (tef-1), calmodulin (cam), and RNA polymerase second largest subunit (rpb2) genes, the fungal isolates were subsequently identified as Fusarium compactum and F. paranaense. Taken together, the results of this study indicate that F. compactum and F. paranaense cause fruit rot disease in watermelons. To the best of our knowledge, this is the first study to report F. compactum and F. paranaense as novel pathogens of watermelon fruit rot both in Thailand and elsewhere in the world.
Keywords: fruit rot, fungal disease, Fusarium, pathogen identification, watermelon disease
1. Introduction
Watermelon (Citrullus lanatus) is one of the most significant economic fruits within the family Cucurbitaceae. It has successfully been planted and farmed in subtropical and tropical regions throughout the world [1,2,3]. In 2022, the Food and Agriculture Organization Statistical Database (FAOSTAT) [4] demonstrated that China was the world’s largest producer of watermelons producing 60.25 million tonnes (with global production recorded at 101.62 million tonnes), followed by Turkey, India, Iran, and Algeria. This increasing trend in watermelon production is expected to continue into the future. In Southeast Asia, watermelon production in the area is led by Vietnam followed by Indonesia, the Lao People’s Democratic Republic, Thailand, and the Philippines [4]. Many scientific studies have reported that watermelon fruits are a good source of nutrition for humans. They contain a variety of important nutrients, including amino acids, carbohydrates, fiber, minerals, organic acids, proteins, sugars, and vitamins [5,6,7]. Furthermore, watermelon fruits contain several beneficial chemical compounds, including alkaloids, flavonoids, glycosides, phenols, tannins, terpenoids, saponins, and steroids, which possess advantageous pharmacological properties [8,9]. These compounds can be utilized in therapeutic approaches due to their antimicrobial, anticancer, antiulcer, antioxidant, anti-inflammatory, antihypertensive, analgesic, and antigiardial properties, which allow them to function against prosthetic hyperplasia and serve as atherosclerosis, gastroprotective, hepatoprotective, and laxative agents [8,9,10,11].
In Thailand, watermelon is currently an economical crop, and the area of cultivation for watermelons is continually increasing [12]. The majority of watermelon production in the northern region is located in the Provinces of Chiang Mai, Phayao, Kamphaeng Phet, Phichit, Sukhothai, and Phitsanulok [13]. Watermelon is cultivated and harvested twice a year in Thailand (from January to May and from mid-October to December). The damage to watermelons caused by fruit rot diseases can result in significant losses for farms in terms of productivity and quality [14]. Watermelons can be affected by a variety of diseases caused by bacteria, fungi, and viruses throughout the growing season, harvest procedure, and postharvest storage period [13,15,16]. Fruit rot disease is known to be the most common and widespread disease in watermelon fruits during the pre and postharvest periods (e.g., storage and transportation) [17,18,19]. This disease can be caused by a number of fungal pathogens within the genera Aspergillus, Alternaria, Fusarium, Curvularia, Macrophomina, Phytophthora, Lasiodiplodia, Sclerotium, and Pythium [13,20,21,22,23,24,25]. The symptoms are characterized by the presence of spots, water-soaked lesions, and often depressions. The lesions enlarge gradually, eventually covering most of the entire fruit. Then, the insides of the infected fruit are completely decayed [19,26]. Due to the formation of water-soaked lesions on the fruit surface, rot disease reduces the quality of the fruit and causes them to appear unattractive to consumers, which significantly reduces their market value [27].
Several synthetic fungicides are commonly used to prevent disease infections in watermelons in order to safeguard crop yield and quality, as they are typically affordable, easy to apply, and effective [28,29]; for example, copper hydroxide, cyazofamid, dimethomorph, ethaboxam, fluopicolide, mandipropamid, mefenoaxam, oxathiapiprolin, phthalimide, and potassium phosphite have been used to control fungi causing fruit rot disease in watermelons by spraying [19,27,30]. However, it has been widely recognized that such synthetic fungicides are hazardous to the environment, the health of farmers and consumers, and may contribute to the development of fungicide-resistant strains [31,32].
The global demand for watermelon fruit continues to increase in accordance with the rapid growth of the world’s population [6] resulting in a significant increase in the cultivation area of watermelons. However, the practice of growing crops in unsuitable environments has also increased the prevalence and severity of certain fungal diseases [33,34]. In the context of this study, fruit rot disease was observed in watermelons during the two postharvest storage period phases in Phayao province, northern Thailand in the year 2022 (February to May and mid-October to December) with a percentage of affected fruits that ranges between 15% and 20%. Consequently, a significant amount of that fruit crop became unmarketable. Consequently, the aim of this investigation was to isolate the causative fungi responsible for this disease. The obtained fungi were characterized and identified using a combination of morphological features and molecular data. Pathogenicity tests were performed, and Koch’s postulates were used to evaluate the asymptomatic watermelon fruits with isolated fungi.
2. Results
2.1. Disease Symptoms
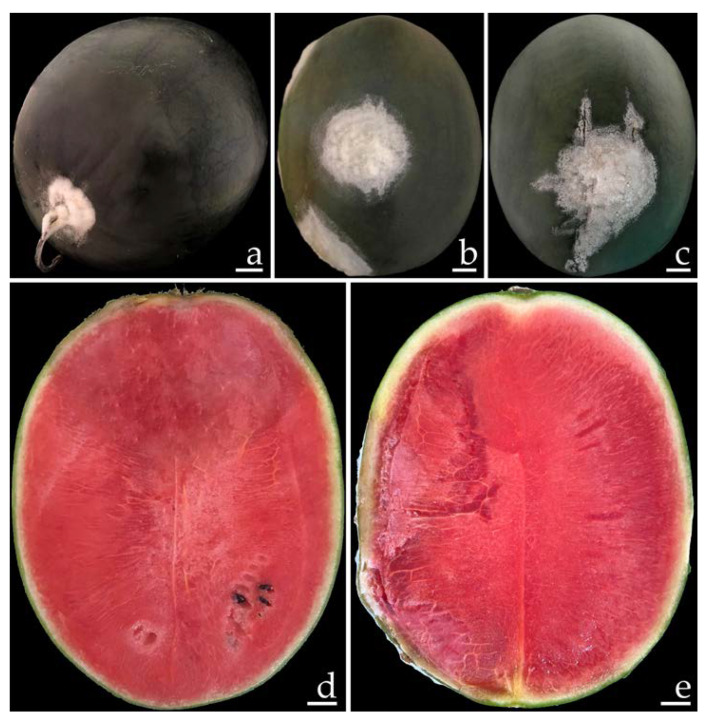
Ten samples of watermelon with fruit rot were collected from the markets in Phayao Province, northern Thailand for each time period. The primary symptoms of disease during the postharvest storage period appeared as green bruised spots on the watermelon fruits (Figure 1a). These spots then grew into dark green bruised spots surrounded by white mycelia (Figure 1b,c). One week after collection, the infected fruits exhibited mild to moderate (22–40% of disease infection on fruit areas) infection by rot symptoms. The lesions on the watermelon fruit gradually expand and combine to encompass the whole fruit, providing the infected fruit with a bruised, decayed, and broken appearance. The internal area of decomposition seemed obviously degraded and the surrounding tissues were soaked with water (Figure 1d,e).
Figure 1.
Naturally occurring symptoms of fruit rot in watermelon (a–c). A cross section of the infected watermelon fruits shows the internal decaying areas (d,e). Scale bars: (a) = 30 mm; (b,c) = 20 mm; (d,e) = 15 mm.
2.2. Fungal Isolation
Ten fungal isolates (FPY1 to FPY10) were isolated from the watermelons that were collected and which exhibited the typical rot symptoms. Subsequently, all fungal isolates were inoculated into asymptomatic commercial watermelons. The conidia collected from two-week-old cultures on potato dextrose agar (PDA) of each fungal isolate were used as the inoculum. A conidial suspension of each fungal isolate was individually dropped onto the wounded fruits at the equator of each fruit. After three days of conidial inoculation, only four fungal isolates—namely, FPY1, FPY4, FPY7, and FPY9—led to the development of rot lesions. The disease symptoms of these four fungal isolates are provided below. All four fungal causal agents—namely, FPY1, FPY7, FPY4, and FPY9—were stored in 20% glycerol and submitted to the culture collection of the Sustainable Development of Biological Resources (SDBR-CMU), Faculty of Science, Chiang Mai University, Thailand, with the numbers SDBR-CMU461, SDBR-CMU462, SDBR-CMU463, and SDBR-CMU464, respectively. These four fungal isolates were selected for further species identification.
2.3. Morphological Observations
Four fungal isolates (SDBR-CMU461, SDBR-CMU462, SDBR-CMU463, and SDBR-CMU464) were selected and used in this experiment. Fungal colonies of each isolate were observed on oatmeal agar (OA), PDA, and synthetic nutrient-poor agar (SNA) at 25 °C for one week. According to the fungal colony characteristics, the isolate SDBR-CMU461 was related to the isolate SDBR-CMU462, whereas the isolate SDBR-CMU463 was related to the isolate SDBR-CMU464. All fungal isolates produced both macro- and micro-conidia, as well as chlamydospores. Based on these morphological features, all the isolated fungi were initially determined to be members of the genus Fusarium [35,36,37,38]. The identification of the fungi was subsequently confirmed by multi-gene phylogenetic analyses.
2.4. Phylogenetic Analysis
The sequences derived from the four fungal isolates obtained in this investigation were submitted to the GenBank database (Table 1 and Table 2). Based on the BLAST results, two fungal isolates—namely, SDBR-CMU461 and SDBR-CMU462—belonged to the F. incarnatum-equiseti species complex, whereas the fungal isolates SDBR-CMU463 and SDBR-CMU464 belonged to the F. solani species complex. Fungal identification was further confirmed through subsequent multi-gene phylogenetic analyses. Two phylogenetic trees (for F. incarnatum-equiseti and F. solani species complexes) were constructed in this study. The results of both phylogenetic analyses revealed that the topological results of both the maximum likelihood (ML) and Bayesian inference (BI) analyses employed in each analysis were similar (data not shown). Consequently, the phylogenetic trees generated by the ML analysis are presented.
Table 1.
Details of the Fusarium incarnatum-equiseti species complex sequences used in the molecular phylogenetic analysis.
| Fungal Taxa | Strain/Isolate | GenBank Accession Number | Reference | ||
|---|---|---|---|---|---|
| tef-1 | cam | rpb2 | |||
| Fusarium aberrans | CBS 131385 T | MN170445 | MN170311 | MN170378 | [37] |
| Fusarium arcuatisporum | LC12147 T | MK289584 | MK289697 | MK289739 | [35] |
| Fusarium arcuatisporum | LC11639 | MK289586 | MK289658 | MK289736 | [35] |
| Fusarium brevicaudatum | NRRL 43638 T | GQ505665 | GQ505576 | GQ505843 | [39] |
| Fusarium bubalinum | CBS 161.25 T | MN170448 | MN170314 | MN170381 | [37] |
| Fusarium caatingaense | URM 6779 T | LS398466 | − | LS398495 | [40] |
| Fusarium cateniforme | CBS 150.25 T | MN170451 | MN170317 | MN170384 | [37] |
| Fusarium citrullicola | SDBR-CMU422 T | OP020920 | OP020924 | OP020928 | [13] |
| Fusarium clavum | CBS 126202 T | MN170456 | MN170322 | MN170389 | [37] |
| Fusarium coffeatum | CBS 635.76 T | MN120755 | MN120696 | MN120736 | [41] |
| Fusarium coffeatum | CBS 430.81 | MN120756 | MN120697 | MN120737 | [41] |
| Fusarium compactum | CBS 186.31 ET | GQ505648 | GQ505560 | GQ505826 | [39] |
| Fusarium compactum | CBS 185.31 | GQ505646 | GQ505558 | GQ505824 | [39] |
| Fusarium compactum | SDBR-CMU461 | OQ108468 | OQ108472 | OQ108474 | This study |
| Fusarium compactum | SDBR-CMU462 | OQ108469 | OQ108473 | OQ108475 | This study |
| Fusarium duofalcatisporum | CBS 384.94 T | GQ505652 | GQ505564 | GQ505830 | [39] |
| Fusarium duofalcatisporum | CBS 264.50 | GQ505651 | GQ505563 | GQ505829 | [39] |
| Fusarium equiseti | CBS 307.94 NT | GQ505599 | GQ505511 | GQ505777 | [39] |
| Fusarium flagelliforme | CBS 162.57 T | GQ505645 | GQ505557 | GQ505823 | [39] |
| Fusarium flagelliforme | CBS 259.54 | GQ505650 | GQ505562 | GQ505828 | [39] |
| Fusarium guilinense | LC12160 T | MK289594 | MK289652 | MK289747 | [35] |
| Fusarium hainanense | LC11638 T | MK289581 | MK289657 | MK289735 | [35] |
| Fusarium incarnatum | CBS 132.73 NT | MN170476 | MN170342 | MN170409 | [37] |
| Fusarium incarnatum | NRRL 32866 | GQ505615 | GQ505527 | GQ505793 | [39] |
| Fusarium ipomoeae | LC12165 T | MK289599 | MK289704 | MK289752 | [35] |
| Fusarium ipomoeae | LC12166 | MK289600 | MK289706 | MK289753 | [35] |
| Fusarium irregulare | LC7188 T | MK289629 | MK289680 | MK289783 | [35] |
| Fusarium lacertarum | NRRL 20423 T | GQ505593 | GQ505505 | GQ505771 | [39] |
| Fusarium lacertarum | LC7942 | MK289643 | MK289696 | MK289797 | [35] |
| Fusarium longicaudatum | CBS 123.73 T | MN170481 | MN170347 | MN170414 | [37] |
| Fusarium luffae | LC12167 T | MK289601 | MK289698 | MK289754 | [35] |
| Fusarium melonis | SDBR-CMU424 T | OP020922 | OP020926 | OP020930 | [13] |
| Fusarium multiceps | CBS 130386 T | GQ505666 | GQ505577 | GQ505844 | [39] |
| Fusarium nanum | LC12168 T | MK289602 | MK289651 | MK289755 | [35] |
| Fusarium neoscirpi | CBS 610.95 T | GQ505601 | GQ505513 | GQ505779 | [39] |
| Fusarium pernambucanum | URM 7559 T | LS398489 | − | LS398519 | [40] |
| Fusarium persicinum | CBS 479.83 T | MN170495 | MN170361 | MN170428 | [37] |
| Fusarium scirpi | CBS 447.84 NT | GQ505654 | GQ505566 | GQ505832 | [39] |
| Fusarium scirpi | CBS 448.84 | GQ505592 | GQ505504 | GQ505770 | [39] |
| Fusarium serpentinum | CBS 119880 T | MN170499 | MN170365 | MN170432 | [37] |
| Fusarium sulawesiense | InaCC F940 T | LS479443 | LS479422 | LS479855 | [42] |
| Fusarium tanahbumbuense | InaCC F965 T | LS479448 | LS479432 | LS479863 | [42] |
| Fusarium toxicum | CBS 406.86 T | MN170508 | MN170374 | MN170441 | [37] |
| Fusarium camptoceras | CBS 193.65 ET | MN170450 | MN170316 | MN170383 | [37] |
| Fusarium neosemitectum | CBS 189.60 T | MN170489 | MN170355 | MN170422 | [37] |
Ex-type, epi-type, and neotype species are indicated by the superscript letters as “T”, “ET,” and “NT,” respectively. The symbol “−“ indicates the absence of sequencing information in GenBank.
Table 2.
Details of the Fusarium solani species complex sequences used in the molecular phylogenetic analysis.
| Fungal Taxa | Strain/Isolate | GenBank Accession Number | Reference | |
|---|---|---|---|---|
| tef-1 | rpb2 | |||
| Fusarium azukicola | NRRL 54364 T | JQ670137 | KJ511287 | [43] |
| Fusarium bataticola | NRRL 22402 T | AF178344 | FJ240381 | [44] |
| Fusarium bataticola | NRRL 22400 | AF178343 | EU329509 | [44] |
| Fusarium bostrycoides | CBS 144.25 NT | LR583597 | LR583818 | [45] |
| Fusarium brasiliense | NRRL 31757 T | EF408409 | EU329565 | [44] |
| Fusarium breve | CBS 144387 T | LR583601 | LR583822 | [45] |
| Fusarium cuneirostrum | NRRL 31157 T | EF408414 | FJ240389 | [44] |
| Fusarium diminutum | CBS 144390 T | LR583607 | LR583828 | [45] |
| Fusarium diminutum | LC13825 | MW620164 | MW474689 | [36] |
| Fusarium falciforme | CBS 475.67 T | LT906669 | LT960558 | [45] |
| Fusarium falciforme | NRRL 32778 | DQ247088 | EU329616 | [44,46] |
| Fusarium helgardnirenbergiae | NRRL 22387 T | AF178339 | EU329505 | [44] |
| Fusarium hypothenemi | NRRL 52782 T | JF740850 | JF741176 | [47] |
| Fusarium keratoplasticum | NRRL 22661 T | JN235712 | JN235897 | [48] |
| Fusarium keratoplasticum | NRRL 46437 | GU170623 | GU170588 | [49] |
| Fusarium liriodendri | NRRL 22389 T | AF178340 | EU329506 | [44] |
| Fusarium metavorans | CBS 135789 T | LR583627 | LR583849 | [45] |
| Fusarium metavorans | CBS 143219 | LR583629 | LR583851 | [45] |
| Fusarium mori | NRRL 22230 T | AF178358 | EU329499 | [44] |
| Fusarium mori | NRRL 22157 | AF178359 | EU329493 | [44] |
| Fusarium paraeumartii | NRRL 13997 T | DQ247549 | LR583855 | [45,46] |
| Fusarium paraeumartii | LC13835 | MW620180 | MW474705 | [36] |
| Fusarium paranaense | CML 1830 T | KF597797 | KF680011 | [50] |
| Fusarium paranaense | CML 1993 | KF597800 | KF680004 | [50] |
| Fusarium paranaense | SDBR-CMU463 | OQ108470 | OQ108476 | This study |
| Fusarium paranaense | SDBR-CMU464 | OQ108471 | OQ108477 | This study |
| Fusarium parceramosum | CBS 115695 T | JX435149 | JX435249 | [51] |
| Fusarium perseae | CBS 144142 T | LT991902 | LT991909 | [45] |
| Fusarium pseudoradicicola | NRRL 25137 T | JF740757 | JF741084 | [47] |
| Fusarium pseudoradicicola | NRRL 25138 | JF740758 | JF741085 | [47] |
| Fusarium quercinum | NRRL 22652 T | DQ247634 | LR583869 | [38,46] |
| Fusarium regulare | CBS 230.34 T | LR583643 | MW834029 | [38,45] |
| Fusarium samuelsii | CBS 114067 T | LR583644 | LR583874 | [45] |
| Fusarium silvicola | CBS 123846 T | LR583646 | LR583876 | [45] |
| Fusarium solani | NRRL 66304 ET | KT313611 | KT313623 | [52] |
| Fusarium solani | NRRL 43474 | EF452945 | EF469984 | [53] |
| Fusarium vanettenii | NRRL 45880 ET | FJ240352 | EU329640 | [44] |
| Fusarium waltergamsii | NRRL 32323 T | DQ246951 | EU329576 | [44,46] |
| Fusarium yamamotoi | NRRL 22277 ET | AF178336 | FJ240380 | [44] |
| Fusarium decemcellulare | LC13606 | MW580428 | MW474374 | [36] |
| Fusarium setosum | CBS 635.92 ET | MW834294 | JX171651 | [38,54] |
Ex-type, epi-type, and neotype species are indicated by the superscript letters as “T”, “ET”, and “NT”, respectively. The symbol “−” indicates the absence of sequencing information in GenBank.
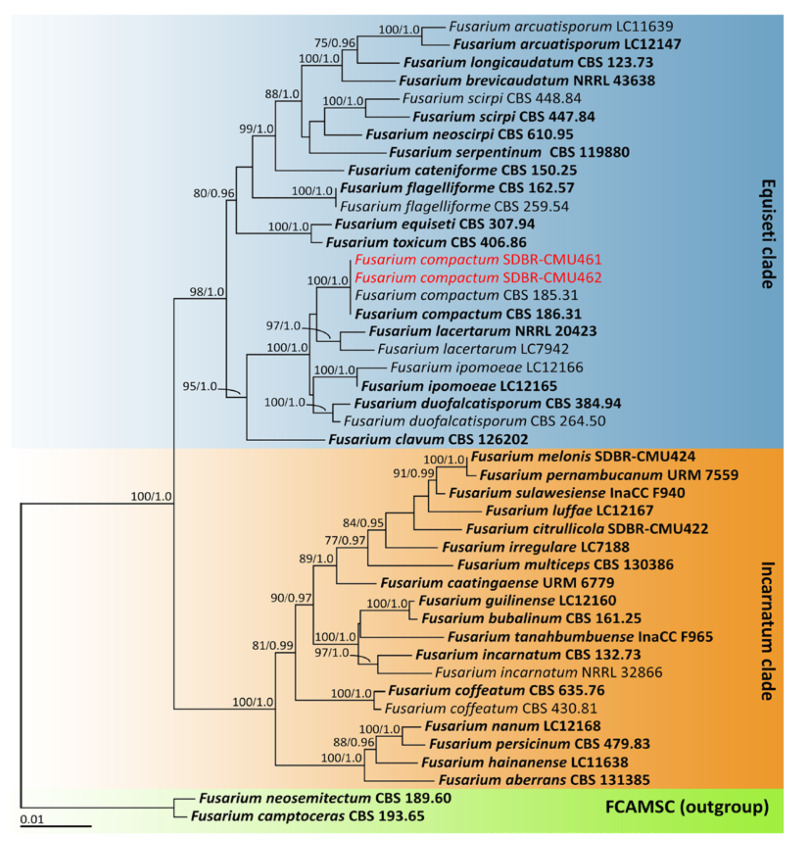
For phylogenetic analysis of the F. incarnatum-equiseti species complex, the combined tef-1, cam, and rpb2 sequence data set was used, according to the identification techniques used in previous studies [35,36,37,38]. The aligned data set contained 2181 bp including gaps (tef-1: 1–704, cam: 705–1288, and 127 rpb2: 1289–2181) with 45 taxa. The outgroup consisted of F. camptoceras and F. neosemitectum from the F. camptoceras species complex (FCAMSC). A phylogenetic tree is represented in Figure 2. Our phylogenetic tree was constructed with the aim of having similar outcomes to previous phylogenetic studies [13,35,36,37,38]. The phylogenetic tree assigned the two fungal isolates (SDBR-CMU461 and SDBR-CMU462) assessed in this investigation within the same clade of F. compactum, which consisted of the type species CBS 186.31 in the F. equiseti clade. This clade established a monophyletic clade with high statistical support (100% BS and 1.0 PP). Fusarium compactum formed a species that was phylogenetically related to F. lacertarum. Therefore, these two fungal isolates (SDBR-CMU461 and SDBR-CMU462) were identified as F. compactum.
Figure 2.
Phylogenetic tree derived from maximum likelihood analysis of Fusarium incarnatum-equiseti species complex of the combined tef-1, cam and rpb2 sequences of 45 taxa. The outgroup included F. camptoceras CBS 193.65 and F. neosemitectum CBS 189.60. Numbers above branches are the bootstrap percentages (left) and Bayesian posterior probabilities (right). Branches with bootstrap and Bayesian posterior probabilities values greater than or equal to 75% and 0.95, respectively, are shown at each branch. The scale bar displays the expected number of nucleotide substitutions per site. The sequences of the fungal species derived in this study are shown in red. Type species are shown in bold.
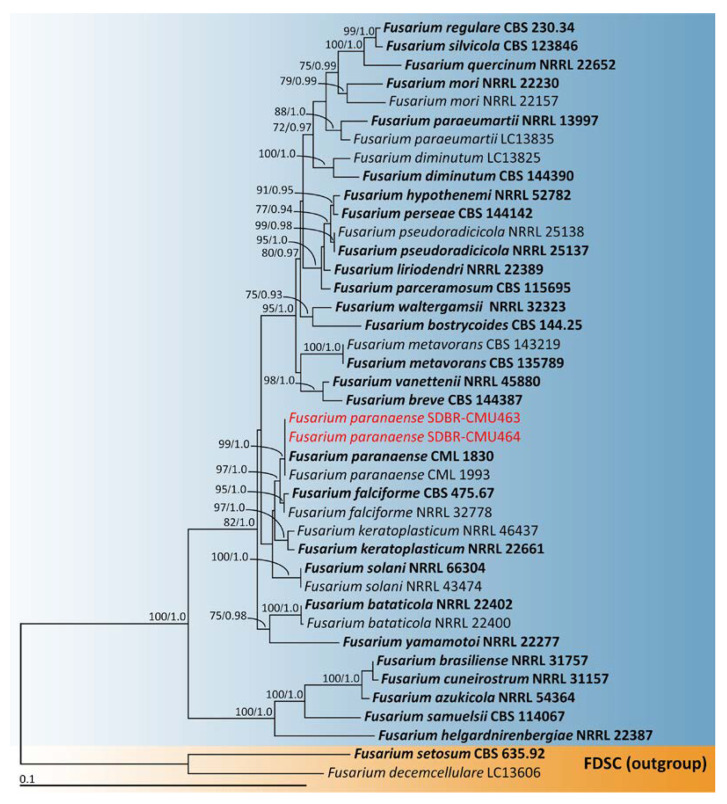
The combined tef-1 and rpb2 sequence data set was used for phylogenetic analysis of the F. solani species complex, following the identification techniques employed in earlier studies [50,55]. This phylogenetic analysis included 41 taxa and the aligned data set contained 1415 bp including gaps (tef-1: 1–603 and rpb2: 604–1415). The outgroup consisted of F. decemcellulare and F. setosum from the F. decemcellulare species complex (FDSC). A phylogenetic tree of the F. solani species complex is shown in Figure 3. Our phylogenetic tree was constructed with the aim of being similar to those in previous phylogenetic studies [50,55,56]. The phylogenetic tree successfully assigned the two fungal isolates (SDBR-CMU463 and SDBR-CMU464) assessed in this investigation within the same clade of F. paranaense, which consisted of the type species CML 1830. This clade established a monophyletic clade with high statistical support (99% BS and 1.0 PP). Fusarium paranaense formed a sister taxon to F. falciforme with high statistical support (97% BS and 1.0 PP). Thus, both fungal isolates (SDBR-CMU463 and SDBR-CMU464) were recognized as F. paranaense.
Figure 3.
Phylogenetic tree derived from maximum likelihood analysis of Fusarium solani species complex of the combined tef-1 and rpb2 sequences of 41 taxa. The outgroup included F. decemcellulare LC13606 and F. setosum CBS 635.92. Numbers above branches are the bootstrap percentages (left) and Bayesian posterior probabilities (right). Branches with bootstrap and Bayesian posterior probabilities values greater than or equal to 75% and 0.95, respectively, are shown at each branch. The scale bar displays the expected number of nucleotide substitutions per site. The sequences of the fungal species derived in this study are shown in red. Type species are shown in bold.
2.5. Morphological Descriptions
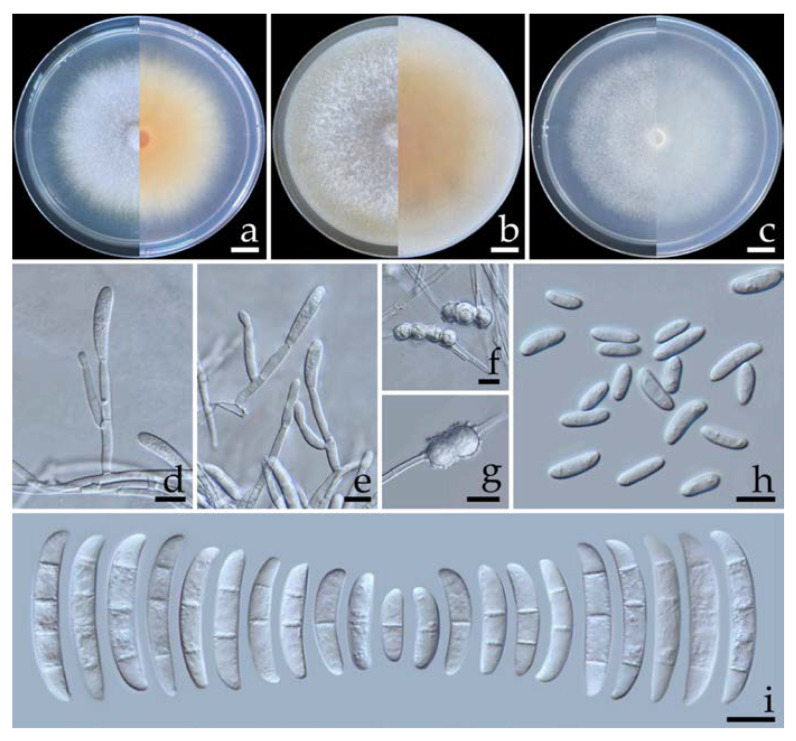
2.5.1. Fusarium compactum (Wollenw.) Raillo, Fungi of the Genus Fusarium: 180 (1950) (Figure 4)
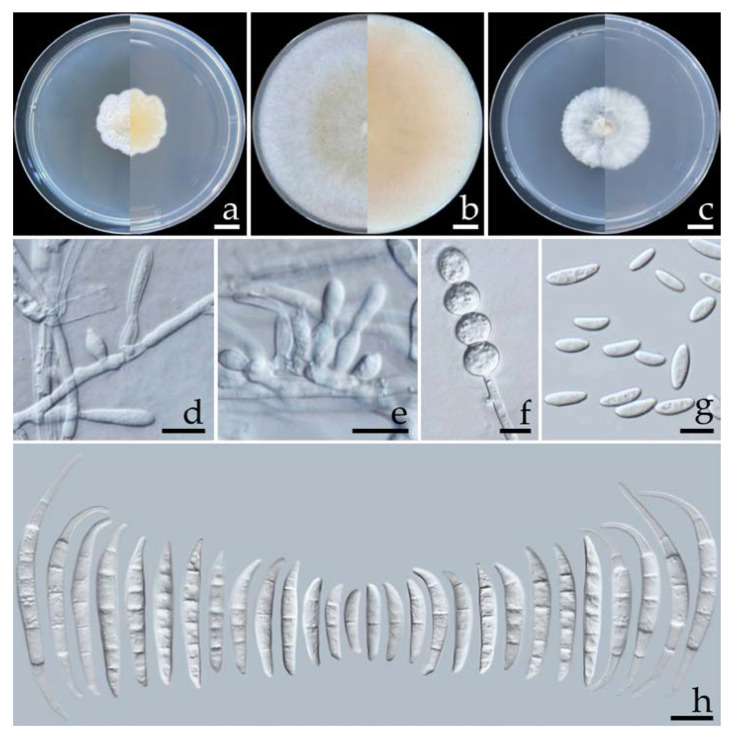
Colonies on OA, PDA, and SNA grew to >85.0, 25.0–3.25, and 32.0–36.0 mm in diameter, respectively, at 25 °C in the dark for one week. Colonies on PDA were yellowish white in the center, white at the margins, and flat with undulated edges that were pale yellow. Colonies on OA were greyish yellow in the center, white at the margins, had dense aerial mycelia, and were flat with entire edges that were greyish orange. Colonies on SNA were white and umbilicated with entire edges that were white. Pigment and odor were not present. Sporodochia were not found in any agar media. Conidiophores were formed on aerial mycelium, of a size of 12.5–100 × 2.8–4.2 µm, which appeared as branched, and bore terminal or lateral phialides. Phialides were monophialidic, subulate to sub-cylindrical, hyaline, smooth and thin-walled, and of a size of 13.1–31.6 × 2.6–4.3 µm. Chlamydospores were abundant, globose, ellipsoid, intercalarily or terminal, hyaline to pale yellow with age, smooth-walled, solitary, in chains or clusters, and of a size of 6.6–17.4 × 6.1–16.7 µm (av. ± SD: 11.1 ± 2.5 × 11.0 ± 2.4 µm). Microconidia were abundant, hyaline, oval to ellipsoidal, straight to slightly curved, aseptate, and of a size of 5.3–13.5 × 2.1–3.7 µm (av. ± SD: 9.6 ± 1.9 × 2.8 ± 0.3 µm). Macroconidia hyaline were thick-walled, strongly curved, had 1–7-septa, and were of a size of 13.3–72.5 × 3.3–6.4 µm (av. ± SD: 33.0 ± 13.1 × 4.6 ± 0.6 µm).
Figure 4.
Fusarium compactum (SDBR-CMU461). Colony on potato dextrose agar (a), oatmeal agar (b) and synthetic nutrient-poor agar (c) (left, surface view and right, reverse view) after incubation at 25 °C for seven days. Phialides on aerial mycelium (d,e). Chlamydospores (f). Aerial microconidia (g). Aerial macroconidia (h). Scale bars: (a–c) = 10 mm; (d–h) = 10 µm.
Note: Morphologically, the two isolates of F. compactum obtained in this study could produce microconidia, which has not been recorded in previous studies [57,58]. However, their other morphological characteristics agreed well with the previous descriptions of F. compactum [57,58]. Phylogenetically, F. compactum forms a species that is phylogenetically related to F. lacertarum. However, F. lacertarum may be distinguished from F. compactum by its shorter conidiophores (up to 7.0 µm long) and phialides (2.5–4.0 × 1.0–1.5 µm) [59].
2.5.2. Fusarium paranaense Costa, Matos & Pfenning, Fungal Biology 120: 55 (2015) (Figure 5)
Colonies on OA, PDA, and SNA grew to 80–83, 75.0–78.0, and 77.0–80.5 mm in diameter, respectively, at 25 °C in the dark for one week. Colonies on PDA were orange–white in the center, white at the margins, and flat with entire edges that were light yellow. Colonies on OA were brownish orange in the center and white at the margins with aerial mycelia that were dense and flat with entire edges that were brownish orange. Colonies on SNA were white and raised with entire edges that were white. Pigment and odor were not present. Sporodochia were not found in any agar media. Conidiophores were formed on aerial mycelium, of a size of 12–105 × 2.5–4.1 µm, were verticillately branched, and bore terminal or lateral phialides. Phialides were monophialidic, subulate to sub-cylindrical, hyaline, smooth and thin-walled, and of a size of 10.8–38.9 × 2.3–5.4 µm. Chlamydospores were abundant, hyaline, globose, intercalarily or terminal, ellipsoid, smooth to rough-walled, solitary, or were present in pairs or formed chains, and of a size of 6.2–11.3 × 6.2–11.6 µm (av. ± SD: 9.2 ± 1.4 × 8.9 ± 1.3 µm). Microconidia were abundant, hyaline, thin-walled, elongated to ellipsoidal, straight to slightly curved, aseptate, and of a size of 5.3–20.1 × 2.3–5.2 µm (av. ± SD: 11.4 ± 3.4 × 4.0 ± 0.7 µm). Macroconidia were hyaline, cylindrical to fusiform, 1–4-septate, and of a size of 16.0–40.6 × 3.5–5.4 µm (av. ± SD: 29.1 ± 6.5 × 4.7 ± 0.4 µm).
Figure 5.
Fusarium paranaense (SDBR-CMU463). Colony on potato dextrose agar (a), oatmeal agar (b) and synthetic nutrient-poor agar (c) (left, surface view and right, reverse view) after incubation at 25 °C for seven days. Conidiophores on aerial mycelium (d). Phialides on aerial mycelium (e). Smooth and rough-walled chlamydospores (f,g). Aerial microconidia (h). Aerial macroconidia (i). Scale bars: (a–c) = 10 mm; (d–i) = 10 µm.
Note: The morphological characteristics of isolates SDBR-CMU463 and SDBR-CMU464 corresponded to descriptions of F. paranaense [50]. Phylogenetically, F. paranaense forms a sister taxon to F. falciforme; however, the growth of F. paranaense appeared to be slower than that of F. falciforme (85.0 mm) on PDA for one week at 25 °C [18]. In addition, F. paranaense produces elongated to ellipsoidal microconidia, whereas F. falciforme produces oval microconidia [18].
2.6. Pathogenicity Test
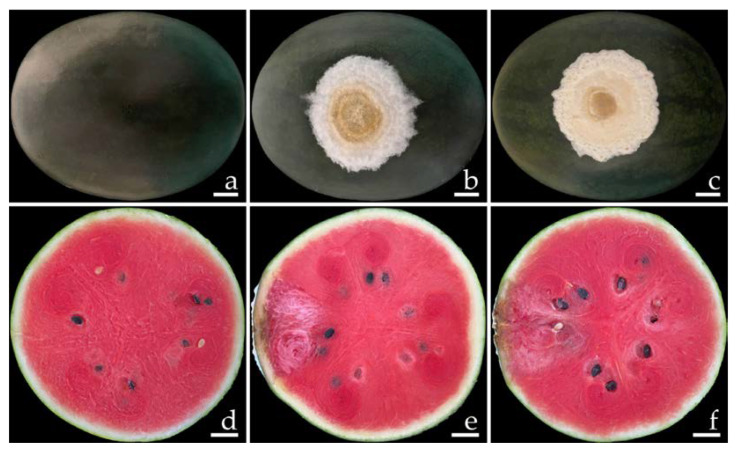
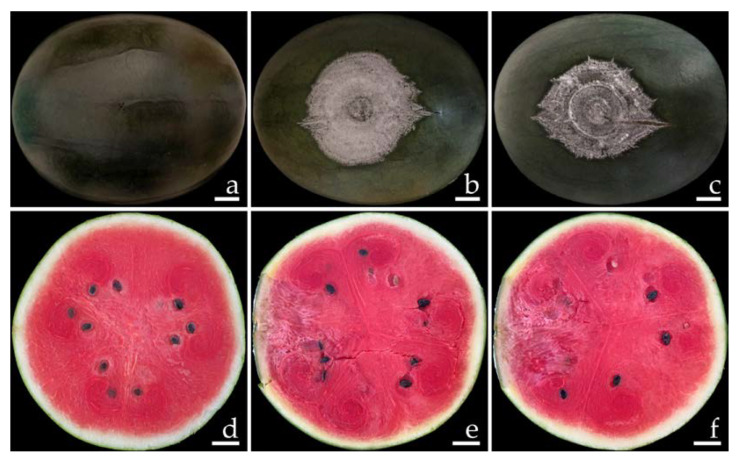
The disease symptoms of F. compactum (SDBR-CMU461 and SDBR-CMU462) and F. paranaense (SDBR-CMU463 and SDBR-CMU464) are shown in Figure 6 and Figure 7, respectively. Primary symptoms appeared on the wounded fruits as small light-brown to brown spots and developed into green bruises. After that, these spots developed into dark green bruised spots that were covered with a dense white mycelia for F. compactum (Figure 6b,c) and a thin white mycelia for F. paranaense (Figure 7b,c) surrounding each lesion. The inoculated fruits displayed moderate infections, as characterized by rot symptoms after one week of incubation. A cross section of a mature lesion indicated that the interior lesion area seemed to be decomposing and was encircled by water-soaked tissue (Figure 6e,f and Figure 7e,f). Following a 14-day inoculation period, the lesions covered the entire fruit. The fruits eventually became extremely rotten and squashy. The symptoms of the disease were consistent with those observed during the postharvest storage period. Nevertheless, no disease symptoms were observed on wounded fruits treated with sterile distilled water (Figure 6a,d and Figure 7a,d). Each fungal isolate was consistently re-isolated from all inoculated tissues and re-identified using both morphological methods of characterization in order to fulfill Koch’s postulates.
Figure 6.
Pathogenicity test with F. compactum SDBR-CMU461 and SDBR-CMU462 on watermelon fruits after seven days of inoculation. Control fruit inoculated with sterile water (a,d); and disease symptoms after inoculation with isolate SDBR-CMU461 (b,e) and isolate SDBR-CMU462 (c,f). Scale bars = 20 mm.
Figure 7.
Pathogenicity test with F. paranaense SDBR-CMU463 and SDBR-CMU464 on watermelon fruits after seven days of inoculation. Control fruit inoculated with sterile water (a,d); and disease symptoms after inoculation with isolate SDBR-CMU463 (b,e) and isolate SDBR-CMU464 (c,f). Scale bars = 20 mm.
3. Discussion
Fusarium is considered to be one of the most important genera of plant pathogens, as it is known to cause serious diseases in several economic plants—including watermelons—grown around the world [16,60,61]. Traditional approaches to the characterization and identification of the Fusarium species are mainly based on morphological characteristics [38,58,62]. Due to the wide variety of morphological differences, it is impossible to distinguish between the closely related Fusarium species based on morphological characteristics alone [38,58]. Therefore, molecular methods are essential to concretely identify Fusarium at the species level. An effective method for identifying Fusarium species has been designed using protein-coding (β-tubulin, cam, tef-1, and RNA polymerase largest sub-unit) and ribosomal DNA (the internal transcribed spacer and the large sub-unit regions) genes [35,38,42,63,64,65,66]. However, several previous studies have reported that species-level identification of Fusarium cannot be achieved using only the ribosomal DNA gene [67,68]. Therefore, the accurate identification of Fusarium species is currently carried out using a combination of morphological characteristic and multi-gene molecular phylogenetic analyses [35,36,37,38,40,63,64,66]. In this study, two isolates of F. compactum (SDBR-CMU461 and SDBR-CMU462) and two isolates of F. paranaense (SDBR-CMU463 and SDBR-CMU464) were isolated from fruit rot lesions on watermelons from northern Thailand. These four fungal isolates were identified using a combination of their morphological features and phylogenetic analysis of multiple genes, according to the identification techniques used in previous studies [35,36,37,38,50,55,56]. Prior to this study, F. compactum and F. paranaense had previously been identified as plant pathogens; for example, F. compactum was found to be the cause of leaf spot on sweet cherry (Prunus avium L.) [69] and leaf blight on maize (Zea mays L.) [70] in China, root rot of banana (Musa sp.) in Greece [71], and canker of Italian cypress (Cupressus sempervirens) trees in Israel [72]. In Brazil, F. paranaense caused root rot in soybeans [Glycine max (L.) Merr.] [50].
The pathogenicity of all F. compactum and F. paranaense isolates in this study was examined in order to confirm Koch’s postulates. According to the results, both fungal species can be regarded as causal agents of fruit rot disease in watermelons. Our results are supported by previous studies that reported that Fusarium species are the cause of various disease symptoms in watermelons in tropical and subtropical regions around the world [73,74,75,76]. Prior to this study, F. solani, F. oxysporum, F. verticillioides, and F. chlamydosporum were considered to be the causal agents of fruit rot in watermelons in Nigeria [16,24,77]. In particular, F. equiseti was found to cause fruit rot in watermelons cultivated in China [78], Malaysia [26], and the United States [79]. Postharvest fruit rot found on watermelons that was caused by F. falciforme and F. oxysporum has also been reported in Malaysia [18]. Furthermore, other Fusarium species have also been associated with the severity of several watermelon diseases. For example, F. equiseti and F. oxysporum f. sp. niveum have been observed to cause Fusarium wilt disease in fruits grown in Korea [76] and Malaysia [74], respectively. On the other hand, Fusarium brachygibbosum and F. oxysporum have been shown to lead to vine decline symptoms in the United States [73] and root rot in China [75], respectively. Furthermore, other fungal species from the genera Alternaria, Aspergillus, Curvularia, Fusarium, Macrophomina, Phytophthora, Lasiodiplodia, Sclerotium, and Pythium have also been associated with fruit rot in watermelons. For example, Pythium aphanidermatum and P. debaryanum caused fruit rot disease in watermelons collected in China [20]; Phytophthora capsici was found to cause fruit rot in watermelons in China [20] and the United States [22]; Kwon and Park [21] found that Sclerotium rolfsii caused postharvest fruit rot in watermelons in South Korea and, in Nigeria, Alternaria cucumeria, Aspergillus flavus, Curvularia lunata, Lasiodiolodia theobromae, and Macrophomina phaseolina have been identified as causal agents of postharvest fruit rot in watermelon [23,24].
In Thailand, the Fusarium species has been associated with symptoms of fruit rot in a number of fruits. For example, fruit rot in cantaloupes and muskmelons has been associated with F. equiseti [34], F. incarnatum [80], and F. melonis [13]. Fusarium fabicercianum caused fruit rot disease in mangoes (Mangifera indica Linn.) [81]. Cases of fruit rot in lychee (Litchi chinensis Sonn) [82] and durian (Durio zibethinus Murray) fruits [83] have been found to be caused by F. solani. Prior to this study, only incidences of watermelon fruit rot caused by F. citrullicola have been reported in Thailand [13]. The symptoms of fruit rot disease caused by F. compactum and F. paranaense in watermelons are similar to those determined to have been caused by known fungal pathogens [13,21,22,25]; however, to date, there have been no reports of watermelon fruit rot caused by F. compactum and F. paranaense. Therefore, we propose that F. compactum and F. paranaense should be identified as new pathogens of watermelon fruit rot in Thailand and throughout the world. Follow-up study is required to clarify the source of the disease inoculum and how weather conditions influence infection and disease development with respect to these pathogens. Furthermore, determination of the incidence of this disease in other areas of Thailand and throughout the world is a necessary task.
4. Materials and Methods
4.1. Sample Collection
Ten watermelon fruits (Citrullus lanatus) with typical rot symptoms were collected during the postharvest storage periods in Phayao Province, northern Thailand (19°08′20″ N, 99°54′42″ E) in 2022 (two periods: February to May and mid-October to December). All symptomatic fruits were randomly selected and placed in sterile plastic boxes. After being transported to the laboratory, the symptomatic fruits were described and assessed under a stereomicroscope (Nikon H55OS, Tokyo, Japan).
4.2. Fungal Isolation
All symptomatic fruits were processed to isolate the fungal causal agents by storing them in a plastic container with moistened filter paper to stimulate fungal conidia production. The single conidial isolation technique was used to isolate the causal fungi from rot lesions on 1.0% water agar supplemented with streptomycin (0.5 mg/L) under a stereomicroscope, following the methods established by Choi et al. [84]. After 24–48 h of incubation at 25 °C in the dark, individual germ conidia were selected and transferred directly onto PDA (CONDA, Madrid, Spain) including streptomycin (0.5 mg/L). Pure fungal isolates were kept in 20% glycerol and submitted to the culture collection of the SDBR-CMU, Chiang Mai Province, Thailand.
4.3. Pathogenicity Tests
Conidia collected from two-week-old cultures on PDA of each fungal isolate were used in this experiment. Asymptomatic commercial watermelons were thoroughly washed and their surfaces were disinfected by immersion in sterile 1.5% (v/v) NaOCl solution for 5 min. Subsequently, sterile distilled water was used to rinse them three times. After being surface-disinfected, the fruits were air-dried for 10 min at room temperature (25 ± 2 °C) [85]. The equator of each fruit received a uniform wound (5 pores, 1 mm width and 1 cm depth) with an aseptic needle after being air-dried [13]. A conidial suspension (500 µL, 1 × 106 conidia/mL) of each fungal isolate was separately dropped onto the wounded fruits. Subsequently, the wounded fruits were inoculated with a drop of sterile distilled water as a control. The inoculated fruit was then kept under conditions of 80% relative humidity in a separate sterile plastic container (26 × 35.5 × 20 cm). The plastic containers were kept in a growth chamber at 25 °C during a 12 h light phase for a week. All treatments were repeated twice with ten replicates of each treatment. The samples were assessed according to the degree of disease infection on the damaged fruit areas, with scores ranging from 1–25% (mild), 26–50% (moderate), 51–75% (severe), to 76–100% (extremely severe) [86]. To confirm Koch’s postulates, the fungi were again isolated from any lesions that appeared on the inoculated fruits using the single spore isolation technique described above. The single spore isolation technique previously mentioned was employed to re-isolate the fungi from any lesions that appeared on the inoculated fruits in order to confirm Koch’s postulates.
4.4. Fungal Identification
4.4.1. Morphological Studies
Colony characteristics of the fungal isolates on OA (Difco, Le Pont de Claix, France), PDA, and SNA were observed following incubation in darkness at 25 °C for a week, according to the methods described in previous studies [35,36,38]. Micromorphological features were assessed and photographed using a light microscope (Nikon Eclipse Ni-U, Tokyo, Japan). The size information related to the anatomical properties (e.g., chlamydospores, conidiogenous cells, conidiophores, phialides and conidia) were measured with at least 50 numbers of each structure using the Tarosoft (R) Image Frame Work program.
4.4.2. DNA Extraction, Amplification, and Sequencing
The genomic DNA of each week-old fungal isolate cultivated on PDA at 25 °C was extracted using a DNA extraction kit (FAVORGEN, Ping-Tung, Taiwan). Polymerase chain reaction (PCR) was employed to amplify the tef-1, cam, and rpb2 genes using the primer pairs EF1/EF2 [87], CAL-228F/CAL-2Rd [88], and RPB2-5F2/RPB2-7cR [65], respectively. The three genes’ amplification programs were carried out in independent PCR reactions, consisting of an initial denaturation for 3 min at 95 °C, followed by 35 cycles of denaturation for 30 s at 95 °C, annealing steps for 50 s at 60 °C (tef-1), 30 s at 59 °C (cam) or 1 min at 52 °C (rpb2), and a final extension step for 1 min at 72 °C on a peqSTAR thermal cycler (PEQLAB Ltd., Fareham, U.K.). PCR products were checked and purified using a PCR clean-up Gel Extraction NucleoSpin® Gel and a PCR Clean-up Kit (Macherey-Nagel, Düren, Germany), according to the manufacturer’s instructions. Following final purification, the PCR products were directly sequenced. Sequencing reactions were carried out and the above-mentioned PCR primers were employed to automatically determine the sequences in the Genetic Analyzer at the 1st Base Company (Kembangan, Malaysia).
4.4.3. Sequence Alignment and Phylogenetic Analyses
The resulting tef-1, cam, and rpb2 sequences were assessed for similarity analysis via the BLAST program available from the NCBI (http://blast.ncbi.nlm.nih.gov, accessed on 10 December 2022). Multiple sequence alignment was performed using MUSCLE [89], and any necessary modifications were made using BioEdit version 6.0.7. [90]. The combined data set of tef-1, cam, and rpb2 data was employed to conduct a multi-gene phylogenetic analysis. Phylogenetic trees were constructed using the maximum likelihood (ML) and Bayesian inference (BI) methods. The ML analysis was performed using 25 categories and 1000 bootstrap (BS) replicates with the GTRCAT model of nucleotide substitution [91] on RAxML-HPC2 version 8.2.12 [92] at the CIPRES web portal [93]. The optimal model for substitution of nucleotides was derived using the jModeltest v.2.3 [94] according to the Akaike Information Criterion (AIC) method. BI analysis was performed using the MrBayes v. 3.2.6 software [95]. For BI analysis, six simultaneous Markov chains with random starting trees were run for a million generations, with 1000 generations of each chain being sampled. The first 2000 trees were removed using a burn-in phase, and then the remaining trees were utilized to construct a phylogenetic tree using the 50% majority rule consensus. The Bayesian posterior probabilities (PPs) were subsequently calculated. The phylogenetic trees were visualized using FigTree v1.4.0 [96].
5. Conclusions
Watermelon fruit rot caused by Fusarium species is typically spread either in the field or during storage and is occurring in many countries around the world. In the present study, we reported F. compactum and F. paranaense to be pathogens of watermelon fruit rot for the first time, in Thailand and worldwide. These fungi were obtained from rot lesions taken from watermelons and identified on the basis of morphological features and multi-gene phylogenetic analyses. In pathogenicity tests under artificial inoculation conditions, the same symptoms as those seen during the postharvest storage period were observed. Therefore, F. compactum and F. paranaense were concluded to be novel pathogens of fruit rot diseases in watermelons. Further investigation of the epidemiology of these diseases in other areas of Thailand, as well as for the purposes of establishing effective management practices, is required. Moreover, in the future, the development of efficient monitoring and preventative strategies will be necessary in order to prevent the significant financial losses introduced by fruit rot disease.
Acknowledgments
The authors are grateful to Russell Kirk Hollis for proofreading the English and Tanapol Thitla for phylogenetic tree preparation.
Author Contributions
Conceptualization, W.N., J.K. and N.S.; methodology, W.N., J.K., S.K. and N.S.; software, S.K. and J.K.; validation, S.K., J.K. and N.S.; formal analysis, W.N., J.K. and N.S.; investigation, W.N., S.K., J.K. and N.S.; resources, W.N., S.K. and N.S.; data curation, S.K., J.K. and N.S.; writing—original draft, W.N., S.K., J.K. and N.S.; writing—review and editing, W.N., J.K., S.K., S.L. and N.S.; supervision, N.S. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The DNA sequence data obtained from this study have been deposited in GenBank under accession numbers; tef-1 (OQ108468, OQ108469, OQ108470, OQ108471), cam (OQ108472, OQ108473), and rpb2 (OQ108474, OQ108475, OQ108476, OQ108477).
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by grants from University of Phayao, Phayao and Chiang Mai University, Chiang Mai, Thailand.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Olayinka B.U., Etejere E.O. Proximate and chemical compositions of watermelon (Citrullus lanatus (Thunb.) Matsum and Nakai cv red and cucumber (Cucumis sativus L. cv Pipino) Int. Food Res. J. 2018;25:1060–1066. [Google Scholar]
- 2.Saediman H., Alwi L.O., Rianse I.S., Taridala S.A.A., Salahuddin S., Indarsyih Y., Astuti R.W. Comparative profitability of melon and watermelon production in South Konawe District of Southeast Sulawesi. WSEAS Trans. Bus. Econ. 2020;17:933–939. doi: 10.37394/23207.2020.17.91. [DOI] [Google Scholar]
- 3.Masika F.B., Alicai T., Shimelis H., Ddamulira G., Athman S.Y., Ipulet P., Andama A., Tugume A.K. Pumpkin and watermelon production constraints and management practices in Uganda. CABI Agric. Biosci. 2022;3:39. doi: 10.1186/s43170-022-00101-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Food and Agriculture Organization of the United Nations. [(accessed on 23 August 2022)]. Available online: http://faostat.fao.org.
- 5.Perkins-Veazie P., Collins J.K., Davis A.R., Roberts W. Carotenoid content of 50 watermelon cultivars. J. Agric. Food Chem. 2006;54:2593–2597. doi: 10.1021/jf052066p. [DOI] [PubMed] [Google Scholar]
- 6.Assefa A.D., Hur O.S., Ro N.Y., Lee J.E., Hwang A.J., Kim B.S., Rhee J.H., Yi J.Y., Kim J.H., Lee H.S., et al. Fruit morphology, citrulline, and arginine levels in diverse watermelon (Citrullus lanatus) germplasm collections. Plants. 2020;9:1054. doi: 10.3390/plants9091054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Aslam A., Zhao S., Lu X., He N., Zhu H., Malik A.U., Azam M., Liu W. High-throughput LC-ESI-MS/MS metabolomics approach reveals regulation of metabolites related to diverse functions in mature fruit of grafted watermelon. Biomolecules. 2021;11:628. doi: 10.3390/biom11050628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deshmukh C.D., Jain A., Tambe M.S. Phytochemical and pharmacological profile of Citrullus lanatus (THUNB) Biolife. 2015;3:483–488. [Google Scholar]
- 9.Manivannan A., Lee E.S., Han K., Lee H.E., Kim D.S. Versatile nutraceutical potentials of watermelon—A modest fruit loaded with pharmaceutically valuable phytochemicals. Molecules. 2020;25:5258. doi: 10.3390/molecules25225258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Maoto M.M., Beswa D., Jideani A.I.O. Watermelon as a potential fruit snack. Int. J. Food Prop. 2019;22:355–370. doi: 10.1080/10942912.2019.1584212. [DOI] [Google Scholar]
- 11.Nadeem M., Navida M., Ameer K., Iqbal A., Malik F., Nadeem M.A., Fatima H., Ahmed A., Din A. A comprehensive review on the watermelon phytochemical profile and their bioactive and therapeutic effects. Korean J. Food Preserv. 2022;29:546–576. doi: 10.11002/kjfp.2022.29.4.546. [DOI] [Google Scholar]
- 12.Johnson G.I., Weinberger K., Wu M.H. The Vegetable Industry in Tropical Asia: Thailand, an Overview of Production and Trade. AVRDC—The World Vegetable Center; Tainan, Taiwan: 2008. pp. 1–59. [Google Scholar]
- 13.Khuna S., Kumla J., Thitla T., Nuangmek W., Lumyong S., Suwannarach N. Morphology, molecular identification, and pathogenicity of two novel Fusarium species associated with postharvest fruit rot of cucurbits in Northern Thailand. J. Fungi. 2022;8:1135. doi: 10.3390/jof8111135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suwannarach N., Kumla J., Lumyong S. Leaf spot on cattleya orchid caused by Neoscytalidium orchidacearum in Thailand. Can. J. Plant Pathol. 2018;40:109–114. doi: 10.1080/07060661.2017.1414882. [DOI] [Google Scholar]
- 15.Reingold V., Lachman O., Sela N., Luria N., Dombrovsky A. Watermelon fruit rot disease in Israel is caused by a distinct Squash vein yellowing virus (SqVYV) strain. Plant Dis. 2016;100:1176–1183. doi: 10.1094/PDIS-09-15-1040-RE. [DOI] [PubMed] [Google Scholar]
- 16.Oyedeji E.O., Arogundade O., Tairu F.M., Elum C.G. Identification and characterization of fungi pathogen causing fruit rot disease of watermelon (Citrullus lanatus) Arch. Phytopathol. Plant Prot. 2022;55:344–354. doi: 10.1080/03235408.2021.2019990. [DOI] [Google Scholar]
- 17.Kousik C.S., Ikerd J.L., Turechek W.W. Development of phytophthora fruit rot caused by Phytophthora capsici on resistant and susceptible watermelon fruit of different ages. Plant Dis. 2017;102:370–374. doi: 10.1094/PDIS-06-17-0898-RE. [DOI] [PubMed] [Google Scholar]
- 18.Balasubramaniam J., Goh K.S., Sani S.F., Alam M.W., Ismail N.A., Gleason M.L., Rosli H. Fusarium falciforme and F. oxysporum causing postharvest fruit rot of watermelon (Citrullus lanatus) in Malaysia: A first report. Crop Prot. 2023;163:106115. doi: 10.1016/j.cropro.2022.106115. [DOI] [Google Scholar]
- 19.Kousik C.S., Ikerd J.L., Harrison H.F. Development of pre- and postharvest Phytophthora fruit rot on watermelons treated with fungicides in the field. Plant Health Prog. 2014;15:145–151. doi: 10.1094/PHP-RS-14-0025. [DOI] [Google Scholar]
- 20.Wei S.Q., Zhong Y., Ma Z.T., Jiang H. A survey on watermelon diseases in the northern China. China Fruits. 1991;1:36–37. [Google Scholar]
- 21.Kwon J.H., Park C.S. Occurrence of fruit rot of watermelon (Citrullus lanatus) caused by Sclerotium rolfsii. Res. Plant Dis. 2009;15:51–53. doi: 10.5423/RPD.2009.15.1.051. [DOI] [Google Scholar]
- 22.Kousik C.S., Ikerd J.L., Wechter P., Harrison H., Levi A. Resistance to phytophthora fruit rot of watermelon caused by Phytophthora capsici in U.S. Plant Introductions. HortScience. 2012;47:1682–1689. doi: 10.21273/HORTSCI.47.12.1682. [DOI] [Google Scholar]
- 23.Ojo O.A., Oladipo S.O., Odelade K.A. In vitro assessment of fungicidal activity of Ageratum conyzoides and Cybopogon flexuosus weed extracts against some phytopathogenic fungi associated with fruit rot of watermelon (Citrullus lunatus (Thunb.)) Arch. Phytopathol. Plant Prot. 2014;47:2421–2428. doi: 10.1080/03235408.2014.880559. [DOI] [Google Scholar]
- 24.Abiala M.A., Ayandeko F.M., Odebode A.C. Antifungal effects of selected botanicals on fungal pathogens of watermelon fruit. Arch. Phytopathol. Plant Prot. 2015;48:569–577. doi: 10.1080/03235408.2015.1075297. [DOI] [Google Scholar]
- 25.Jidda M.B., Adamu M.I. Rot inducing fungi of watermelon (Citrullus lanatus) fruits in storage and fruit stalls in Maiduguri, Nigeria. Int. J. Adv. Acad. Res. 2017;3:11–18. [Google Scholar]
- 26.Rahman M.Z., Ahmad K., Siddiqui Y., Saad N., Hun T.G., Hata E.M., Rashed O., Hossain M.I. First report of Fusarium equiseti, causing fruit rot disease of watermelon in Malaysia. Plant Dis. 2022;106:326. doi: 10.1094/PDIS-05-21-1027-PDN. [DOI] [PubMed] [Google Scholar]
- 27.Kousik C.S., Ji P., Egel D.S., Quesada-Ocampo L.M. Fungicide rotation programs for managing Phytophthora fruit rot of watermelon in Southeastern United States. Plant Health Prog. 2017;18:28–34. doi: 10.1094/PHP-RS-16-0059. [DOI] [Google Scholar]
- 28.Keinath A.P. Minimizing yield and quality losses in watermelon with multi-site and strobilurin fungicides effective against foliar and fruit anthracnose. Crop Prot. 2018;106:72–78. doi: 10.1016/j.cropro.2017.12.012. [DOI] [Google Scholar]
- 29.Thambugala K.M., Daranagama D.A., Phillips A.J.L., Kannangara S.D., Promputtha I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020;10:604923. doi: 10.3389/fcimb.2020.604923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kousik C.S., Adams M.L., Jester W.R., Hassell R., Harrison H.F., Holmes D.J. Effect of cultural practices and fungicides on Phytophthora fruit rot of watermelon in the Carolinas. Crop Prot. 2011;30:888–894. doi: 10.1016/j.cropro.2011.03.012. [DOI] [Google Scholar]
- 31.Yang C. Fungicide: Modes of action and possible impact on nontarget microorganisms. ISRN Ecol. 2011;8:130289. doi: 10.5402/2011/130289. [DOI] [Google Scholar]
- 32.Hahn M. The rising threat of fungicide resistance in plant pathogenic fungi: Botrytis as a case study. J. Chem. Biol. 2014;7:133–141. doi: 10.1007/s12154-014-0113-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wilkinson K., Grant W.P., Green L.E., Hunter S., Jeger M.J., Lowe P., Medley G.F., Mills P., Phillipson J., Poppy G.M., et al. Infectious diseases of animals and plants: An interdisciplinary approach. Phil. Trans. R. Soc. B. 2011;366:1933–1942. doi: 10.1098/rstb.2010.0415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Nuangmek W., Aiduang W., Suwannarach N., Kumla J., Kiatsiriroat T., Lumyong S. First report of fruit rot on cantaloupe caused by Fusarium equiseti in Thailand. J. Gen. Plant Pathol. 2019;85:295–300. doi: 10.1007/s10327-019-00841-1. [DOI] [Google Scholar]
- 35.Wang M.M., Chen Q., Diao Y.Z., Duan W.J., Cai L. Fusarium incarnatum-equiseti complex from China. Persoonia. 2019;43:70–89. doi: 10.3767/persoonia.2019.43.03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang M.M., Crous P.W., Sandoval-Denis M., Han S.L., Liu F., Liang J.M., Duan W.J., Cai L. Fusarium and allied genera from China: Species diversity and distribution. Persoonia. 2022;48:1–53. doi: 10.3767/persoonia.2022.48.01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Xia J.W., Sandoval-Denis M., Crous P.W., Zhang X.G., Lombard L. Numbers to names–restyling the Fusarium incarnatum-equiseti species complex. Persoonia. 2019;43:186–221. doi: 10.3767/persoonia.2019.43.05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Crous P.W., Lombard L., Sandoval-Denis M., Seifert K.A., Schroers H.-J., Chaverri P., Gené J., Guarro J., Hirooka Y., Bensch K., et al. Fusarium: More than a node or a foot-shaped basal cell. Stud. Mycol. 2021;98:100116. doi: 10.1016/j.simyco.2021.100116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O’Donnell K., Sutton D.A., Rinaldi M.G., Gueidan C., Crous P.W., Geiser D.M. Novel multilocus sequence typing scheme reveals high genetic diversity of human pathogenic members of the Fusarium incarnatum—F. equiseti and F. chlamydosporum species complexes within the United States. J. Clin. Microbiol. 2009;47:3851–3861. doi: 10.1128/JCM.01616-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Santos A.C.S., Trindade J.V.C., Lima C.S., Barbosa R.N., Costa A.F., Tiago P.V., Oliveira N.T. Morphology, phylogeny, and sexual stage of Fusarium caatingaense and Fusarium pernambucanum, new species of the Fusarium incarnatum-equiseti species complex associated with insects in Brazil. Mycologia. 2019;111:244–259. doi: 10.1080/00275514.2019.1573047. [DOI] [PubMed] [Google Scholar]
- 41.Lombard L., van Doorn R., Crous P.W. Neotypification of Fusarium chlamydosporum—A reappraisal of a clinically important species complex. Fungal Syst. Evol. 2019;4:183–200. doi: 10.3114/fuse.2019.04.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Maryani N., Sandoval-Denis M., Lombard L., Crous P.W., Kema G.H.J. New endemic Fusarium species hitch-hiking with pathogenic Fusarium strains causing Panama disease in small-holder banana plots in Indonesia. Persoonia. 2019;43:48–69. doi: 10.3767/persoonia.2019.43.02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aoki T., Scandiani M.M., O’Donnell K. Phenotypic, molecular phylogenetic, and pathogenetic characterization of Fusarium crassistipitatum sp. nov., a novel soybean sudden death syndrome pathogen from Argentina and Brazil. Mycoscience. 2012;53:167–186. doi: 10.1007/S10267-011-0150-3. [DOI] [Google Scholar]
- 44.O’Donnell K., Sutton D.A., Fothergill A., McCarthy D., Rinaldi M.G., Brandt M.E., Zhang N., Geiser D.M. Molecular phylogenetic diversity, multilocus haplotype nomenclature, and in vitro antifungal resistance within the Fusarium solani species complex. J. Clin. Microbiol. 2008;46:2477–2490. doi: 10.1128/JCM.02371-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sandoval-Denis M., Lombard L., Crous P.W. Back to the roots: A reappraisal of Neocosmospora. Persoonia. 2019;43:90–185. doi: 10.3767/persoonia.2019.43.04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang N., O’Donnell K., Sutton D.A., Nalim F.A., Summerbell R.C., Padhye A.A., Geiser D.M. Members of the Fusarium solani species complex that cause infections in both humans and plants are common in the environment. J. Clin. Microbiol. 2006;44:2186–2190. doi: 10.1128/JCM.00120-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.O’Donnell K., Humber R.A., Geiser D.M., Kang S., Park B., Robert V.A., Crous P.W., Johnston P.R., Aoki T., Rooney A.P., et al. Phylogenetic diversity of insecticolous fusaria inferred from multilocus DNA sequence data and their molecular identification via FUSARIUM-ID and Fusarium MLST. Mycologia. 2012;104:427–445. doi: 10.3852/11-179. [DOI] [PubMed] [Google Scholar]
- 48.Short D.P.G., O’Donnell K., Zhang N., Juba J.H., Geiser D.M. Widespread occurrence of diverse human pathogenic types of the fungus Fusarium detected in plumbing drains. J. Clin. Microbiol. 2011;49:4264–4272. doi: 10.1128/JCM.05468-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Migheli Q., Balmas V., Harak H., Sanna S., Scherm B., Aoki T., O’Donnell K. Molecular phylogenetic diversity of dermatologic and other human pathogenic fusarial isolates from hospitals in northern and central Italy. J. Clin. Microbiol. 2010;48:1076–1084. doi: 10.1128/JCM.01765-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Costa S.S., Matos K.S., Tessmann D.J., Seixas C.D., Pfenning L.H. Fusarium paranaense sp. nov., a member of the Fusarium solani species complex causes root rot on soybean in Brazil. Fungal Biol. 2016;120:51–60. doi: 10.1016/j.funbio.2015.09.005. [DOI] [PubMed] [Google Scholar]
- 51.Debourgogne A., Gueidan C., de Hoog S., Lozniewski A., Machouart M. Comparison of two DNA sequence-based typing schemes for the Fusarium solani species complex and proposal of a new consensus method. J. Microbiol. Methods. 2012;91:65–72. doi: 10.1016/j.mimet.2012.07.012. [DOI] [PubMed] [Google Scholar]
- 52.Schroers H.J., Samuels G.J., Zhang N., Short D.P., Juba J.H., Geiser D.M. Epitypification of Fusisporium (Fusarium) solani and its assignment to a common phylogenetic species in the Fusarium solani species complex. Mycologia. 2016;108:806–819. doi: 10.3852/15-255. [DOI] [PubMed] [Google Scholar]
- 53.O’Donnell K., Sarver B.A., Brandt M., Chang D.C., Noble-Wang J., Park B.J., Sutton D.A., Benjamin L., Lindsley M., Padhye A., et al. Phylogenetic diversity and microsphere array-based genotyping of human pathogenic Fusaria, including isolates from the multistate contact lens-associated U.S. keratitis outbreaks of 2005 and 2006. J. Clin. Microbiol. 2007;45:2235–2248. doi: 10.1128/JCM.00533-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.O’Donnell K., Rooney A.P., Proctor R.H., Brown D.W., McCormick S.P., Ward T.J., Frandsen R.J., Lysoe E., Rehner S.A., Aoki T., et al. Phylogenetic analyses of RPB1 and RPB2 support a middle Cretaceous origin for a clade comprising all agriculturally and medically important fusaria. Fungal Genet. Biol. 2013;52:20–31. doi: 10.1016/j.fgb.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 55.Carreras-Villaseñor N., Rodríguez-Haas J.B., Martínez-Rodríguez L.A., Pérez-Lira A.J., Ibarra-Laclette E., Villafán E., Castillo-Díaz A.P., Ibarra-Juárez L.A., Carrillo-Hernández E.D., Sánchez-Rangel D. Characterization of two Fusarium solani species complex isolates from the ambrosia beetle Xylosandrus morigerus. J. Fungi. 2022;8:231. doi: 10.3390/jof8030231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.James J.E., Santhanam J., Zakaria L., Mamat Rusli N., Abu Bakar M., Suetrong S., Sakayaroj J., Abdul Razak M.F., Lamping E., Cannon R.D. Morphology, phenotype, and molecular identification of clinical and environmental Fusarium solani species complex isolates from Malaysia. J. Fungi. 2022;8:845. doi: 10.3390/jof8080845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Raillo A.I. Griby Roda Fusarium. Gosudarstv. Izd. Sel’skochoz. Lit.; Moscow, Russia: 1950. [Google Scholar]
- 58.Leslie J.F., Summerell B.A. The Fusarium Laboratory Manual. 1st ed. Blackwell Publishing Professional; Ames, IA, USA: 2006. pp. 8–240. [Google Scholar]
- 59.Subrahmanyam A. Fusarium laceratum. Mykosen. 1983;26:478–480. doi: 10.1111/j.1439-0507.1983.tb03242.x. [DOI] [PubMed] [Google Scholar]
- 60.Tuttle McGrath M. Diseases of Cucurbits and their Management. In: Naqvi S.A.M.H., editor. Diseases of Fruits and Vegetables Volume I. Springer; Dordrecht, The Netherlands: 2004. pp. 455–510. [Google Scholar]
- 61.Ajmal M., Hussain A., Ali A., Chen H., Lin H. Strategies for controlling the sporulation in Fusarium spp. J. Fungi. 2023;9:10. doi: 10.3390/jof9010010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Rahjoo V., Zad J., Javan-Nikkhah M., Mirzadi Gohari A., Okhovvat S.M., Bihamta M.R., Razzaghian J., Klemsdal S.S. Morphological and molecular identification of Fusarium isolated from maize ears in Iran. J. Plant Pathol. 2008;90:463–468. [Google Scholar]
- 63.Geiser D.M., Jiménez-Gasco M.M., Kang S., Makalowska I., Veeraraghavan N., Ward T.J., Zhang N., Kuldau G.A., O’Donnell K. FUSARIUM-ID v. 1.0: A DNA sequence database for identifying Fusarium. Eur. J. Plant Pathol. 2004;110:473–479. doi: 10.1023/B:EJPP.0000032386.75915.a0. [DOI] [Google Scholar]
- 64.Nitschke E., Nihlgard M., Varrelmann M. Differentiation of eleven Fusarium spp. isolated from sugar beet, using restriction fragment analysis of a polymerase chain reaction-amplified translation elongation factor 1 gene fragment. Phytopathology. 2009;99:921–929. doi: 10.1094/PHYTO-99-8-0921. [DOI] [PubMed] [Google Scholar]
- 65.O’Donnell K., Sutton D.A., Rinaldi M.G., Sarver B.A.J., Balajee S.A., Schroers H.-J., Summerbell R.C., Robert V.A.R.G., Crous P.W., Zhang N., et al. Internet-accessible DNA sequence database for identifying fusaria from human and animal infections. J. Clin. Microbiol. 2010;48:3708–3718. doi: 10.1128/JCM.00989-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Jedidi I., Jurado M., Cruz A., Trabelsi M.M., Said S., González-Jaén M.T. Phylogenetic analysis and growth profiles of Fusarium incarnatum-equiseti species complex strains isolated from Tunisian cereals. Int. J. Food Microbiol. 2021;353:109297. doi: 10.1016/j.ijfoodmicro.2021.109297. [DOI] [PubMed] [Google Scholar]
- 67.Balajee S.A., Borman A.M., Brandt M.E., Cano J., Cuenca-Estrella M., Dannaoui E., Guarro J., Haase G., Kibbler C.C., Meyer W., et al. Sequence-based identification of Aspergillus, Fusarium, and Mucorales species in the clinical mycology laboratory: Where are we and where should we go from here? J. Clin. Microbiol. 2009;47:877–884. doi: 10.1128/JCM.01685-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.O’Donnell K., Ward T.J., Robert V.A.R.G., Crous P.W., Geiser D.M., Kang S. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica. 2015;43:583–595. doi: 10.1007/s12600-015-0484-z. [DOI] [Google Scholar]
- 69.Zhou Y., Zhang W., Li X., Ji S., Chethana K.W.T., Hyde K.D., Yan J. Fusarium species associated with cherry leaf spot in China. Plants. 2022;11:2760. doi: 10.3390/plants11202760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Xu X., Zhang L., Yang X., Shen G., Wang S., Teng H., Yang C., Liu X., Wang X., Zhao J., et al. Fusarium species associated with maize leaf blight in Heilongjiang Province, China. J. Fungi. 2022;8:1170. doi: 10.3390/jof8111170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Frisullo S., Logrieco A., Moretti A., Grammatikaki G., Bottalico A. Banana corm and root rot by Fusarium compactum, in Crete. Phytopathol. Mediterr. 1994;33:78–82. [Google Scholar]
- 72.Madar Z., Kimchi M., Solel Z. Fusarium canker of Italian cypress. Eur. J. For. Path. 1996;26:107–112. doi: 10.1111/j.1439-0329.1996.tb00715.x. [DOI] [Google Scholar]
- 73.Pisani C., Adkins S.T., Turechek W., Patel P.C., Rosskopf E.N. First report of Macrophomina phaseolina, Fusarium brachygibbosum, and Lasiodiplodia theobromae causing fungal watermelon vine decline in southwest and west-central Florida. Plant Health Prog. 2021;22:544–551. doi: 10.1094/PHP-09-20-0077-RS. [DOI] [Google Scholar]
- 74.Rahman M.Z., Ahmad K., Siddiqui Y., Saad N., Hun T.G., Hata E.M., Rashed O., Hossain M.I., Kutawa A.B. First report of fusarium wilt disease on watermelon caused by Fusarium oxysporum f. sp. niveum in Malaysia. Plant Dis. 2021;105:4169. doi: 10.1094/PDIS-04-21-0780-PDN. [DOI] [PubMed] [Google Scholar]
- 75.Zhang M., Yang X., Xu J., Liu G., Yao X., Ren R. Characterization of Fusarium root rot disease in grafted watermelon. Eur. J. Plant Pathol. 2021;159:1–11. doi: 10.1007/s10658-020-02013-w. [DOI] [Google Scholar]
- 76.Han Y.K., Dumin W., Park M.J., Bae Y.S., Park J.H., Back C.G. First report of fusarium wilt disease caused by Fusarium equiseti on grafted watermelon in Korea. Plant Dis. 2022;106:2989. doi: 10.1094/PDIS-08-21-1745-PDN. [DOI] [PubMed] [Google Scholar]
- 77.Ikediugwu F.E.O., Ogieva W.O. Fruit rot of Citrullus lanatus in Nigeria caused by Fusarium solani. Trans. Br. Mycol. Soc. 1978;71:209–213. doi: 10.1016/S0007-1536(78)80100-4. [DOI] [Google Scholar]
- 78.Li Y.G., Song X.L., Wang X.Q., Zhang H., Tian S., Ji P. First report of fruit rot of watermelon caused by Fusarium equiseti in China. Plant Dis. 2018;102:1852. doi: 10.1094/PDIS-01-18-0199-PDN. [DOI] [Google Scholar]
- 79.Li Y., Ji P. First report of fruit rot of watermelon caused by Fusarium equiseti in Georgia in the United States. Plant Dis. 2015;99:1272. doi: 10.1094/PDIS-10-14-1074-PDN. [DOI] [Google Scholar]
- 80.Wonglom P., Sunpapao A. Fusarium incarnatum is associated with postharvest fruit rot of muskmelon (Cucumis melo) J. Phytopathol. 2020;168:204–210. doi: 10.1111/jph.12882. [DOI] [Google Scholar]
- 81.Saepaisan S., Chawengphaophan S., Falab S. Identification of the fungal fruit rot causal agents of Khiew Sawoey mango of production area in Loei province for export. Khon Kaen Agr. J. 2020;48:1360–1373. (In Thai) [Google Scholar]
- 82.Thaenthanee S., Sukprasert J., Daosukho S., Rodprasert S. The study on efficiency of Acorus calamus L. extract against fruit rot fungi isolated from lychee. Bulletin Appl. Sci. 2014;3:88–101. (In Thai) [Google Scholar]
- 83.Piasai R., Chalmers P., Piasai O., Khewkhom N. Postharvest fungicide dips to control fruit rot of ‘Monthong’ durian (Durio zibethinus) Eur. J. Plant Pathol. 2021;160:325–336. doi: 10.1007/s10658-021-02246-3. [DOI] [Google Scholar]
- 84.Choi Y.W., Hyde K.D., Ho W.H. Single spore isolation of fungi. Fungal Divers. 1999;3:29–38. [Google Scholar]
- 85.De Oliveira M.J., Laranjeira D., Câmara M.P.S., Laranjeira F.F., Armengol J., Michereff S.J. Effects of wounding, humidity, temperature, and inoculum concentrations on the severity of corky dry rot caused by Fusarium semitectum in melon fruits. Acta Sci. Agron. 2014;36:281–289. doi: 10.4025/actasciagron.v36i3.17656. [DOI] [Google Scholar]
- 86.Safari Z.S., Ding P., Nakasha J.J., Yuso S.F. Combining chitosan and vanillin to retain postharvest quality of tomato fruit during ambient temperature storage. Coatings. 2020;10:1222. doi: 10.3390/coatings10121222. [DOI] [Google Scholar]
- 87.O’Donnell K., Kistler H.C., Cigelnik E., Ploetz R.C. Multiple evolutionary origins of the fungus causing Panama disease of banana: Concordant evidence from nuclear and mitochondrial gene genealogies. Proc. Natl. Acad. Sci. USA. 1998;95:2044–2049. doi: 10.1073/pnas.95.5.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Carbone I., Kohn L.M. A method for designing primer sets for speciation studies in filamentous ascomycetes. Mycologia. 1999;91:553–556. doi: 10.1080/00275514.1999.12061051. [DOI] [Google Scholar]
- 89.Edgar R.C. MUSCLE: A multiple sequence alignment method with reduced time and space complexity. BMC Bioinform. 2004;5:113. doi: 10.1186/1471-2105-5-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Hall T. Bioedit Version 6.0.7. 2004. [(accessed on 20 August 2022)]. Available online: http://www.mbio.ncsu.edu/bioedit/bioedit.html.
- 91.Felsenstein J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution. 1985;39:783–791. doi: 10.2307/2408678. [DOI] [PubMed] [Google Scholar]
- 92.Stamatakis A. RAxML-VI-HPC: Maximum likelihood-based phylogenetic analyses with thousands of taxa and mixed models. Bioinformatics. 2006;22:2688–2690. doi: 10.1093/bioinformatics/btl446. [DOI] [PubMed] [Google Scholar]
- 93.Miller M.A., Pfeiffer W., Schwartz T. Creating the cipres science gateway for inference of large phylogenetic trees; Proceedings of the 2010 Gateway Computing Environments Workshop (GCE); New Orleans, LA, USA. 14 November 2010; Manhattan, NY, USA: IEEE; pp. 1–8. [Google Scholar]
- 94.Darriba D., Taboada G.L., Doallo R., Posada D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods. 2012;9:772. doi: 10.1038/nmeth.2109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Ronquist F., Teslenko M., van der Mark P., Ayres D.L., Darling A., Höhna S., Larget B., Liu L., Suchard M.A., Huelsenbeck J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012;61:539–542. doi: 10.1093/sysbio/sys029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rambaut A. FigTree Tree Figure Drawing Tool Version 13. Institute of Evolutionary 623 Biology, University of Edinburgh; Edinburgh, Scotland: 2019. [(accessed on 10 August 2022)]. Available online: http://treebioedacuk/software/figtree/ [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The DNA sequence data obtained from this study have been deposited in GenBank under accession numbers; tef-1 (OQ108468, OQ108469, OQ108470, OQ108471), cam (OQ108472, OQ108473), and rpb2 (OQ108474, OQ108475, OQ108476, OQ108477).