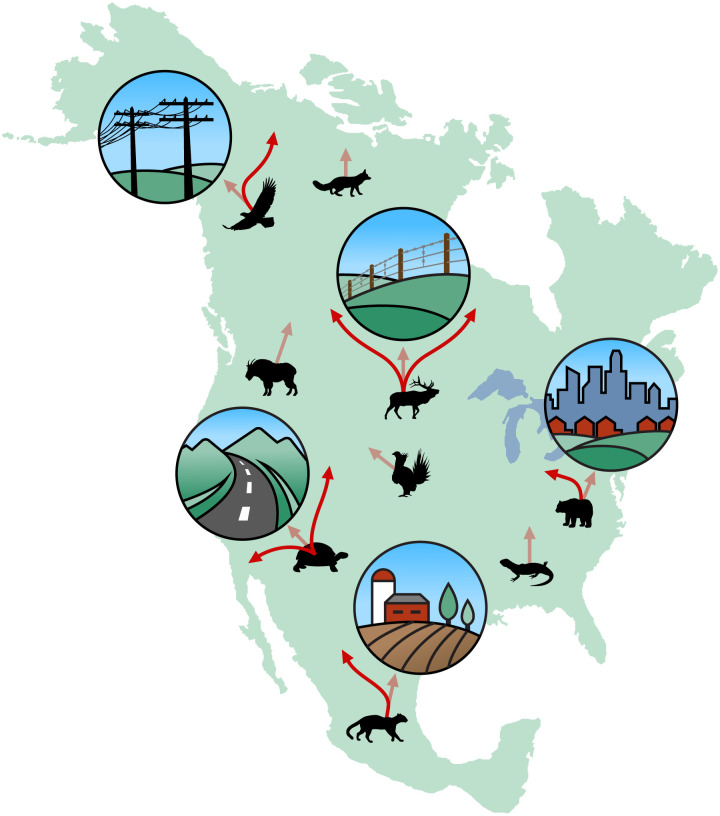
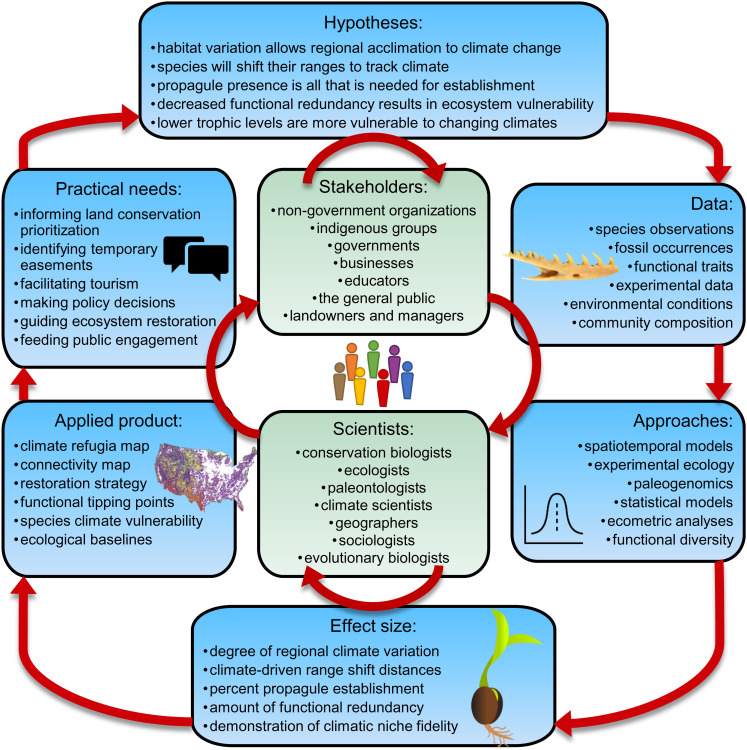
We are in the midst of a major biodiversity crisis, with deep impacts on the functioning of ecosystems and derived benefits to people (1, 2). But we still have time to pull back. To do so, it is imperative that we learn from plants’ and animals’ past actions (3, 4). Conservation biology, ecology, and paleontology all emphasize that natural systems must exhibit resilience and dynamic responses to rapid environmental changes (3, 5, 6). Both climate and land-use change have accelerated over the past decades, underscoring the urgency for increased understanding and action (7–9). The cumulative effects of these disruptions are not additive or systematic; rather, they pose complex, dynamic environmental challenges to ecological systems (see “dynamic systems” Table 1). With the dramatic ecological effects from climate fluctuations and increasing instability of the fabric of life (10–12), we anticipate that biota will dramatically shift their ranges, reconfiguring ecological communities across Earth’s natural landscapes (13) (Fig. 1). Today’s most prevalent conservation approaches focus on the maintenance of static reserves. These approaches need to be supplemented by approaches that facilitate dynamic ecological shifts using flexible strategies that involve local stakeholders (14–17). In addition, given the magnitude, rates, and complex interactions of anthropogenic and climatic change occurring today, these conservation approaches must be informed by research that spans time scales to infer likely responses (18). This special feature integrates research from across spatial and temporal scales to explore how ecosystems and communities function dynamically to respond to large-scale environmental change, highlighting proposed solutions for conserving biodiversity on a rapidly changing planet (Fig. 2).
Table 1.
Key concepts and terms as used in special feature articles
| Key concept | Definition | Special Feature Article |
|---|---|---|
| Dynamic systems | “A theoretical framework that is used to understand and predict self-organizing phenomena in complex systems that are constantly changing, reorganizing, and progressing over time” (19) | This paper |
| Resilient landscape | A landscape that sustains “biodiversity and ecological functions over time in the face of climate change and other anthropogenic and natural stressors” (20) | (21) |
| Ecosystem connectivity | The capacity of habitat configuration to allow the “unimpeded movement of species and the flow of natural processes that sustain life on Earth” (22) | (21) |
| Ecological restoration | The process of reinstating communities to support ecosystem functioning and services, ecosystem connectivity, and biodiversity (23, 24) | (25) |
| Functional diversity | The “number, type and distribution of functions performed by organisms within an ecosystem” (26) | (27) |
| Functional trait | A “measurable property of organisms … that strongly influences organismal performance” (28, 29) | (27, 30, 31, 32) |
| Climate fidelity | The ability of a taxon to shift its range to track their realized climatic niche as climate changes (33) | (33) |
| Functional redundancy | The relative number of organisms that “occupy the same functional space” within an ecosystem (34) | (27, 30) |
| Ecometrics | “The study of the distribution of functional traits within and between communities in relation to environment” | (31, 32) |
| Effect size | A statistical estimate of “the magnitude of an effect” or the “biological importance of the effect” (35) | (36) |
| Socioecological gap | A geospatial representation that “includes both threat and resource components to determine if existing capacities are sufficient to promote stable geographic ranges” (37) | (37) |
Fig. 1.
Species will need to shift their geographic ranges in response to rapidly changing climates (transparent red arrows). However, anthropogenic landscape features—such as cities, farms, roads, fences, and power lines—can act as filters or barriers necessitating alternate paths (solid red arrows). These features and other impacts can lead to geographic range contraction (e.g., imposed on taxa with only transparent arrows). Figure by Duncan MacGruer.
Fig. 2.
Knowledge coproduction occurs through dialog between stakeholders and scientists. Together, they select the appropriate data and approaches to determine applicable effects, which are translated into applied products that can inform the practical needs of stakeholders. Images, from top-right: a fossil snake dentary, a statistical model, a seedling indicating propagule establishment rate, and a map of possible corridors to facilitate climate tracking (modified from ref. 38).
To accommodate migration, adaptation, and acclimation in response to rapid change, we must formulate effective, long-term dynamic conservation approaches. These involve the protection and restoration of resilient landscapes (Table 1) that can serve as regional climate refugia, allowing plants and animals to shift locally to acclimate to changing climates (18, 39, 40). Once climate change is extreme enough that the buffering capacity of these regional climate refugia is exceeded, plants and animals will need to traverse broader landscapes, requiring larger-scale ecosystem connectivity (17, 39, 40) (Table 1). Simultaneously, effective ecological restoration (Table 1) strategies must maintain ecosystem function, allowing primary productivity to continue and diverse taxa to persist despite ongoing geographic shifts and taxonomic turnover. The restoration of landscapes and conservation of individual species requires integrating the rapidly shifting environmental, social, and political landscapes into our risk considerations and engaging with local stakeholders (Fig. 2) to ensure success (16, 41, 42).
Given that 75% of terrestrial landscapes are strongly affected by human impacts while simultaneously undergoing rapidly changing climates (1, 2), there are many outstanding ecological questions that need to be addressed to identify the most effective dynamic conservation strategies. For example, although we anticipate that species will need to shift their ranges to track changing climates (43, 44) (Fig. 1), we do not know the extent to which individual species shift their ranges as ecosystems rapidly turnover (45) versus exhibiting climate flexibility, acclimating or adapting in place (46). Neither do we know the extent to which those species are influenced by different types of anthropogenic landscape changes (47–49) (Fig. 1). Biodiversity of all sorts, including functional diversity (Table 1), is decreasing as populations are extirpated and community compositions change (50). Functional traits (Table 1) have been used as indicators of past ecological function and environments due to their fidelity of signal. But as traits are lost within a community, ecosystem functions will likely shift due to offsets in those trait–environment relationships. However, we do not know the degree of diversity or combination of species traits that are necessary to maintain ecosystem function (51).
To address questions relevant to conserving biodiversity in the face of these large, dynamic, changes, we need to understand complex, large-scale interactions. This brings several challenges, notably that we must consider ecological responses over varying spatial and temporal scales in a rapidly changing system. Studies that expand over longer timescales can test hypotheses and models based on ecological principles using empirical data from past events (Fig. 2). Best practices mean knowledge coproduction of both questions and models for addressing conservation needs (52–54) and reporting of findings that can be easily translated for conservation applications (55) (Fig. 2). In this way, studies that span multiple spatiotemporal scales can serve to refine and refocus on-the-ground efforts for conserving biodiversity in a dynamically changing landscape.
The aim of this special feature is to capture approaches that transcend fields. Conservation practitioners highlight the latest dynamic conservation strategies, which underscore the ecological theory and hypotheses that should be examined to effectively translate historical findings into actionable conservation practices. Ecologists and paleoecologists, working across temporal scales, demonstrate approaches that have already begun to effectively inform the conservation of biodiversity on a dynamically changing planet. Studies herein identify resilient and connected landscapes, explore ecological movement dynamics, and evaluate how extinctions and range-shifting species lead to the erosion of functional groups that affect trait–environment dynamics through space and time (Fig. 1). It is clear that we need to continue identifying a path forward for the meaningful integration of approaches across fields for the conservation of biodiversity and ecosystem function. Ecosystem preservation requires a clear understanding of trait–environment relationships across trophic levels. We highlight work from across a series of trophic levels from plants to herbivores to carnivores to demonstrate the strategies being used to assess how functional groups within ecosystems are structured, and the major drivers of change.
Connected and Resilient Landscapes
Today’s changing planet presents challenges for conservation strategies. Previously, when climate or land availability changed dramatically, for example, during previous glacial–interglacial cycles, plants and animals traversed the landscape without experiencing the resistance that results from our highly fragmented and human-impacted landscapes (Fig. 1). Although both species' geographic ranges and ecosystem compositions have changed substantially in the past, overall biodiversity persisted in ecosystems often without a current analog or within climate refugia (13, 56, 57). Projections of migration requirements in the future compared to those of previous glacial-interglacial cycles show that much-accelerated migration velocities will be required to reach the nearest climate refugia (58). Additionally, given the extent of terrestrial human impacts (2), both climate refugia and migration tracts are being extensively impinged (38, 59) (Fig. 1). Identifying strategies to allow plants and animals to navigate this dynamic landscape requires conservation strategies that recognize and integrate the complexity of these issues in a spatially and socially explicit way.
As species ranges are encroached upon by new climate regimes and expanding human impacts, plants and animals will need to shift their ranges to track suitable habitats as they have done in the past (61, 62). To facilitate this, Anderson et al. (21) demonstrate a new framework for characterizing modern landscapes that promotes both landscape resilience and broader connectivity. The resultant maps will serve as a crucial tool for conservation prioritization in a changing world. The authors pinpoint places in the modern landscape where resilience to changing climates is highest. Diverse topography, bedrock, and soil are used to characterize high landscape resilience that will sustain biodiversity and ecological function into the future. They consider how habitat configurations promote the regional movement of plants, animals, and whole ecosystems. Conserving these areas will ensure that our dynamically changing planet harbors the climate refugia of the future.
Global human footprints are expanding because human needs are expanding. Thus, we must develop more nuanced strategies for considering both the threat to plants’ and animals’ ranges and potential directions forward for maintaining those species. This means working with a variety of landowners, land users, governments, agencies, NGOs, and conservation organizations (60). Public perceptions play a strong role in the ability of these groups to achieve their goals. For example, the public’s fear of and negative associations with carnivores makes full trophic restoration challenging in some areas. Harris et al. (37) introduce the next generation of species-level gap analysis that considers these global dynamics. Their work integrates not only climate and environmental changes into species protection consideration, but it also considers socioecological threats and resources that could affect how species are protected to identify any socioecological gap (Table 1). When this is applied to 91 African carnivores, they find that the threats of range contractions are on average 15% more extreme than previously thought, yielding many suggestions for shifts in the threatened or endangered statuses of carnivores, particularly for smaller carnivores. But more importantly, this work identifies potential social and political partners to help conserve threatened carnivore species.
Identifying the spatially explicit vulnerabilities of individual species and protecting resilient and connected landscapes to allow those species to adapt to global change dynamics are important steps for conserving biodiversity. However, to effectively support these strategies, we must continue to identify intrinsic species traits and extrinsically driven dispersal and migration dynamics that will affect how, when, and if they will need to respond to dynamic environmental changes.
Ecological Movement Dynamics
Climate is a critical component for predicting where species are geographically distributed (63, 64). Because the climate is changing so rapidly, we assume that plants and animals will need to shift their distributions across climate gradients to track those changes (65, 66). However, although we know that both plants and animals have previously shifted their distributions to track climate, we also know that climate tracking often lags behind climatic changes (67, 68). Some organisms, in fact, possess distributions that are not directly affected by climate (69). Lags could result from dispersal limitations, particularly in long-lived plants, and today may be further limited by increasing habitat loss and fragmentation (49, 70). An essential challenge for evaluating species’ responses to environmental changes is that it may take tens to thousands of years for geographic range shifts or other adjustments to occur (71, 72). To effectively predict the future movement needs and capabilities of plants and animals, we must integrate a temporal component into our assessments.
Identifying the geographic dispersal challenges faced by plants has been difficult for ecologists because most studies are conducted across relatively short time scales (days, weeks, months, or years). Ecological dynamics resulting from multiyear and decadal fluctuations in climate and management practices require a longer temporal perspective. Orrock et al. (25) report findings from a near-decade-long regional experiment to identify taxa, ecosystems, and their characteristics that benefit most from connected landscapes. They conducted a long-term and large-scale replicated experiment across the southeastern United States, manipulating seed arrival and neighboring competitors to evaluate joint impacts of timber densities, prescribed fire strategies, soil conditions, and climate on establishment and persistence. Species establishment and persistence dynamics depend on arrival, dispersal, and migration capability, potentially confounded by neighboring competitors. Orrock et al. (25) identify specific plant traits and environmental conditions that promote resilience under changing climates, maximizing conservation and restoration potential. Notably, they find that the strongest factor to influence plant establishment is seeding, or the presence of seeds in an area, while warming temperatures inhibit establishment. These findings point to the need for relatively long-distance climate tracking with warming, but a high potential for success given sufficient dispersal.
Orrock et al. (25) identify how to best promote climate tracking in plants, whereas Wang et al. (33) evaluate the extent to which different taxa have geographic ranges that will be strictly dictated by climate. To construct a comprehensive plan for responses to global change, we need to evaluate which taxa exhibit climatic niche fidelity (Table 1) versus climatic niche flexibility (33, 46). These questions will help focus our efforts on those taxa that will be most affected by changing temperature or precipitation patterns versus those that may be more affected by other components of its niche, including biotic interactions or other abiotic components. Wang et al. (33) introduce a climate fidelity metric, which captures the degree to which species maintain their climate niches through time. They evaluate climate fidelity in North American plants over the past 18,000 years using the palynological record. They find that 75% of plant taxa exhibit high climate fidelity. These species have tracked climate for 18,000 years through relatively rapid changes in climate; they will require resilient and connected landscapes to keep doing so into the future.
Erosion of Functional Groups
As plant and animal communities are geographically shifting and reconfiguring in response to global change, so too do the traits and the functional diversity conferred by component species (28, 73). These changes happen locally as a result of redistributions or extirpations and globally due to extinctions. Given that roughly 25% of vascular plant and vertebrate species are currently threatened with extinction (2), functional diversity will continue to be eroded. Understanding the mechanisms underlying the loss of global biodiversity is critical for its conservation and the preservation of functioning ecosystems.
Over the past million years, glacial–interglacial cycles led to rapid expansions and contractions of ecosystems as they experienced considerable changes in both temperature and precipitation across the Northern Hemisphere (74). Germain and colleagues (27) evaluate the relative abundances and functional diversity of global bird clades over the past million years in response to these large-scale environmental changes. They find that functional diversity of bird species alive today, overall, has changed very little. However, there has been a gradual loss of functional groups in some of the more divergent aspects of trait space, specifically the loss of birds that exhibit more unusual trait sets, especially large-bodied birds that lay many large eggs (27). The losses of these traits that have been previously associated with hunting or predation by invasive species, implicates potential anthropogenic drivers, and pinpoints historically vulnerable clades (27). Given that this trait set is energetically costly, they may be the harbinger of losses to come as birds continue to experience increased stress from increasing global change.
This slow erosion of functional trait spaces can be contrasted with work by Kemp (30), which evaluates how the functional diversity of Caribbean reptiles changed during the late Quaternary. When evaluating functional diversity at these smaller spatial and temporal scales, Kemp finds that the number of functional entities (functionally distinct species groups) has decreased by 70% on small Caribbean islands, a much more rapid and dramatic loss than observed in the global bird dataset. Reptile functional trait losses dramatically decrease the resilience of these small islands’ ecological function and community-level adaptability to global change. However, on those small islands with few human occupants, functional entities are maintained. Large islands exhibited sufficient functional redundancy (Table 1) to maintain a larger pool of functional diversity. These findings highlight the need for maintaining resilient systems through the preservation of functional entities and restoring those functional entities when possible.
Biotas tend to become more homogenous in areas of high human impact through the extinction of native species or the introduction of nonnative ones (75). Often species within functional groups at the highest trophic levels (e.g., predators) are more sensitive to land conversion, habitat destruction, and fragmentation (49, 76). Similarly, species with larger body sizes, compared to small-bodied species, are more likely to be lost (77), which has led to missing portions of ecological space (78). Areas of high human impact have distinctive influences on different types of functional groups (79). Both of these articles find evidence of anthropogenic impacts in restructuring functionality within biotic systems, either through land-use conversion or direct harvesting. In both reptiles and birds, shifts in functional diversity, whether slow or fast, thus far appear to be driven by human land use and exploitation rather than changes in climate. Understanding how anthropogenic impacts restructure functionality within biotic systems will increase our understanding of the ecological consequences of biodiversity loss.
Trait–Environment Dynamics through Space and Time
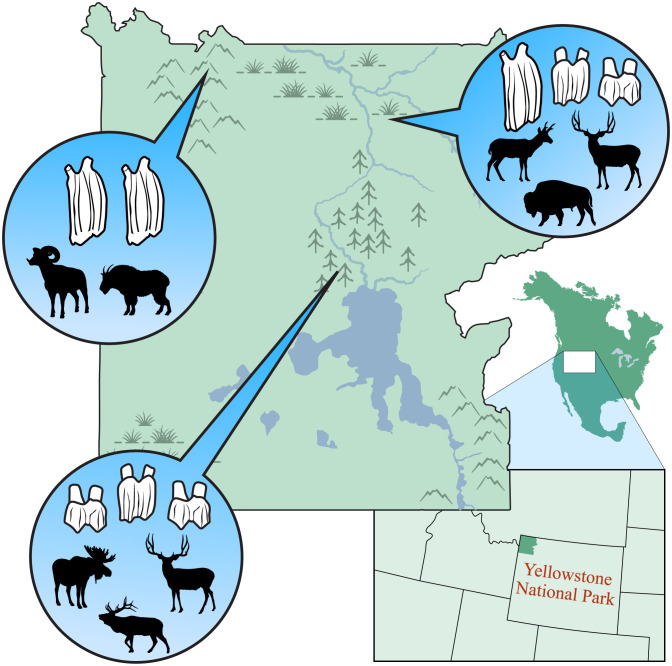
Functional trait losses have important implications for overall ecosystem function, which can be assessed by evaluating trait–environment relationships for communities (Fig. 3). For example, the loss of grazing species with high-crowned teeth, which are adapted for consuming coarse plants in harsh environments, can theoretically affect the relative abundances of the plants growing in certain ecosystems, altering overall ecological function (80, 81). However, the complex interactions between functional groups, global environmental change, and ecosystem function require much more extensive investigation. Two papers in this special feature use a compelling approach, ecometrics (Table 1), for contextualizing consistent and disrupted trait–environment relationships (82–84). Parker et al. (31) and Short et al. (32) examine how well community-level functional traits align with the environments where they are found as change occurs across space and through time.
Fig. 3.
Equilibrium communities possess traits that are well suited to the habitats where they are found. For example, bovids in Yellowstone National Park, Wyoming, United States, possess teeth that effectively process plants in their habitats. Mountain goats and bighorn sheep have high-crowned teeth for eating coarse, gritty grasses found on mountain tops. Moose, elk, and deer have lower tooth crowns, needing less durable teeth to process foods in forests. In addition, the mixed habitats around river systems are occupied by a variety of taxa, including pronghorn, bison, and elk. Figure by Duncan MacGruer.
Physiological models of the mechanistic relationship between body size and temperature have been useful tools for estimating paleotemperature and evaluating functional trait–environment relationships through time. Parker et al. (31) use a well-established physiological relationship between turtle body size and temperature to examine trait–environment patterns globally. To do this, they calculate the body sizes of turtle communities from every continent and compare them to anticipated body sizes based on physiological models. They find that globally, the predicted relationship holds for only the largest turtles. However, continentally and through time, patterns do broadly align. Like the papers above, this paper theorizes that anthropogenic land use changes and other biotic impacts may be the cause of observed discrepancies. They emphasize the utility of this method for assessing anthropogenic effects on trait–environment relationships.
Empirically-estimated models of the functional relationship between locomotor strategy and vegetation cover have been useful tools for assessing change in vertebrate functional traits and estimating past vegetation cover. Short et al. (32) evaluate the geographic patterns of ecometric relationships where mismatches occur between locomotor strategies of artiodactyls and carnivorans with vegetation cover. They demonstrate that multitrophic models, including both primary and secondary consumers, better capture trait–environment relationships than calibrated models of either group independently. Applying their multitrophic model to several fossil localities, they demonstrate the potential of the approach to estimate past vegetation cover and track functional trait change through time. They find that some functional relationships may have been compromised either due to nonrandom extirpation of species or change in vegetation cover.
Identifying a Path Forward
Effective conservation strategies in the midst of rapid change require that we understand past responses to large-scale changes. This special feature demonstrates how one can build models and construct hypotheses relevant to conservation questions that can then be tested using empirical data from long-term experiments and historical and fossil records (Fig. 2). Many of the results described here improve our ecological understanding in a way that can directly inform conservation frameworks. These studies are most effective when they specifically target real, on-the-ground conservation questions.
Knowledge coproduction happens when researchers work with stakeholders to develop research questions and directions most relevant to the needs of the stakeholders (85, 86). This paradigm moves past researchers evaluating what they consider the most relevant problems in their discipline and puts the focus on problems identified by other relevant stakeholders (Fig. 2). In a fully coproduced scientific endeavor, both researchers and stakeholders design research methodology and evaluate and interpret the results (86). Working with stakeholders in knowledge coproduction will help scientists working at longer temporal scales and larger spatial extents produce the kinds of results that conservation organizations need to make prioritization decisions. Even though this approach is time consuming, it may imbue more rapid conservation action from research findings. For example, Wang et al. (33) consulted with The Nature Conservancy to develop a strategy to assess plant range dynamics and climate fidelity, in an effort to align planned research to relevant mission objectives for their resilience and connectivity framework (21).
To make paleontological findings more broadly applicable beyond a single study system, practitioners must be able to access and use the results in pragmatic ways. However, much of the paleontological literature focuses on establishing the significance of historical baselines or purported drivers. These foci fall short of potential contributions of paleontology to conservation, where the most promising contributions result from addressing questions about biotic responses to climate change (55). Kiessling et al. (36) discuss several gaps in the current paleontological literature that could be filled to improve translational science from paleontological findings to decision-making. They point to insufficient reporting of effect size (Table 1; often only P-values are reported) and insufficient consideration of scaling effects (Fig. 2). They suggest a roadmap for future research to refine the paleontological contribution to conservation science under a changing climate.
Going forward, integration and translatability will be critical for improving our framework for conserving terrestrial biodiversity. To identify land-purchasing, land-sharing, and other strategies that will allow biodiversity to persist and shift in the face of changing climates, we must know which taxa are most affected by rapidly changing climates and habitat alteration (Fig. 2). To promote and restore functional ecosystems in the midst of these dynamic shifts, we must understand trait–environment relationships and the emergent properties of those relationships that result in a healthy ecosystem (Fig. 3). We must further identify the appropriate partners for establishing these conservation practices and coproduce the knowledge necessary to succeed. Finally, for practitioners to translate these findings into practice, it will be imperative to communicate not only the presence of change but also the magnitude of the effects that result. This will ensure that plant and animal communities maintain sufficient functional trait variety to form new assemblages that will be both connected and resilient under novel conditions.
Acknowledgments
This special feature is the result of presentations shared at a virtual symposium and round table titled, “The past as a lens for biodiversity conservation on a dynamically changing planet” held on December 2 to 3, 2021. We thank the symposium presenters and participants for thoughtfully sharing ideas and insights and for sparking important discussions around the topics in this special feature. We especially thank the authors for their important contributions. We are grateful to DA Lauer, J Schap, BR Shipley, K Slenker, RP Dorn, BA Richey, LM Siciliano-Martina, and RA Short for written suggestions; to J Head, J Müller, the Spatial Ecology and Paleontology Lab at Georgia Tech, and the Paleoecology, Evolution, and Climate Lab at Texas A&M University for discussions; and to D MacGruer for his creative work on two images. We thank M Bastian and A Schroeder for guiding us through the editorial process. IUBS funded figures and provided support to iCCB and CPiA, which was crucial for consolidating ideas. Work was supported by the following grants: NSF 1655898, 1945013, 2124770, 2124836, NERC NE/W007576/1, and CONICET PIP 11220130100103.
Author contributions
J.L.M, A.M.L., and N.C.S. designed research; J.L.M and A.M.L. performed research; and J.L.M., A.M.L., S.D., and N.C.S. wrote the paper.
Competing interests
The authors declare no competing interest.
Contributor Information
Jenny L. McGuire, Email: jmcguire@gatech.edu.
A. Michelle Lawing, Email: alawing@tamu.edu.
References
- 1.Díaz S., et al. , Pervasive human-driven decline of life on Earth points to the need for transformative change. Science 366, eaax3100 (2019). [DOI] [PubMed] [Google Scholar]
- 2.IPBES, Global assessment report on biodiversity and ecosystem services of the intergovernmental science-policy platform on biodiversity and ecosystem services, Brondizio J. S. E. S., Díaz S., Ngo H. T., Eds. (IPBES, Bonn, Germany, 2019), p. 1148. [Google Scholar]
- 3.Barnosky A. D., et al. , Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science 355, eaah4787 (2017). [DOI] [PubMed] [Google Scholar]
- 4.Barnosky A. D., et al. , Has the Earth’s sixth mass extinction already arrived? Nature 471, 51–57 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Hannah L., et al. , Conservation of biodiversity in a changing climate. Conserv. Biol. 16, 264–268 (2002). [DOI] [PubMed] [Google Scholar]
- 6.Reynolds M. D., et al. , Dynamic conservation for migratory species. Sci. Adv. 3, e1700707 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.IPCC, “An IPCC special report on climate change, desertification, land degradation, sustainable land management, food security, and greenhouse gas fluxes in terrestrial ecosystems” in Climate Change and Land, Shukla P. R., et al., Eds. (2019), in press.
- 8.Smith S. J., Edmonds J., Hartin C. A., Mundra A., Calvin K., Near-term acceleration in the rate of temperature change. Nat. Clim. Chang. 5, 333–336 (2015). [Google Scholar]
- 9.Herrmann S. M., Brandt M., Rasmussen K., Fensholt R., Accelerating land cover change in West Africa over four decades as population pressure increased. Commun. Earth Environ. 1, 1–10 (2020). [Google Scholar]
- 10.Stenseth N. C., et al. , Ecological effects of climate fluctuations. Science 297, 1292–1296 (2002). [DOI] [PubMed] [Google Scholar]
- 11.Dong Y., et al. , Paleorecords reveal the increased temporal instability of species diversity under biodiversity loss. Quat. Sci. Rev. 269, 107147 (2021). [Google Scholar]
- 12.Díaz S., A fabric of life view of the world. Science 375, 1204–1204 (2022). [DOI] [PubMed] [Google Scholar]
- 13.Williams J. W., Jackson S. T., Novel climates, no-analog communities, and ecological surprises. Front Ecol. Environ. 5, 475–482 (2007). [Google Scholar]
- 14.Gillson L., Dawson T. P., Jack S., McGeoch M. A., Accommodating climate change contingencies in conservation strategy. Trends Ecol. Evol. 28, 135–142 (2013). [DOI] [PubMed] [Google Scholar]
- 15.Keough H. L., Blahna D. J., Achieving integrative, collaborative ecosystem management. Conserv. Biol. 20, 1373–1382 (2006). [DOI] [PubMed] [Google Scholar]
- 16.Magness D. R., et al. , Management foundations for navigating ecological transformation by resisting, accepting, or directing social–ecological change. BioScience 72, 30–44 (2022). [Google Scholar]
- 17.McGuire J. L., Shipley B. R., Dynamic priorities for conserving species. Science 376, 1048–1049 (2022). [DOI] [PubMed] [Google Scholar]
- 18.Gill J. L., et al. , A 2.5-million-year perspective on coarse-filter strategies for conserving nature’s stage. Conserv. Biol. 29, 640–648 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Connell J., DiMercurio A., Corbetta D., Vonk J., Shackelford T., "Dynamic systems theory" in Encyclopedia of Animal Cognition and Behavior, Vonk J., Shackelford T., Eds. (Springer, Cham, 2017), pp. 1–8. [Google Scholar]
- 20.Beller E. E., et al. , Building ecological resilience in highly modified landscapes. BioScience 69, 80–92 (2019). [Google Scholar]
- 21.Anderson M. G., et al. , A resilient and connected network of sites to sustain biodiversity under a changing climate. Proc. Natl. Acad. Sci. U.S.A. (2022), this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.CMS, Improving ways of addressing connectivity in the conservation of migratory species, resolution 12.26 (REV. COP-13), Gandhinagar, India (17-22 February 2020) (UNEP/CMS/COP-13/CRP 26.4. 4, 2020).
- 23.Guiden P. W., et al. , Effects of management outweigh effects of plant diversity on restored animal communities in tallgrass prairies. Proc. Natl. Acad. Sci. U.S.A. 118, e2015421118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Temperton V. M., et al. , Step back from the forest and step up to the Bonn Challenge: How a broad ecological perspective can promote successful landscape restoration. Restor. Ecol. 27, 705–719 (2019). [Google Scholar]
- 25.Orrock J. L., et al. , Long-term, large-scale experiment reveals the effects of seed limitation, climate, and anthropogenic disturbance on restoration of plant communities in a biodiversity hotspot. Proc. Natl. Acad. Sci. U.S.A. (2022), this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.S. Díaz, Cabido M., Vive la différence: Plant functional diversity matters to ecosystem processes. Trends Ecol. Evol. 16, 646–655 (2001). [Google Scholar]
- 27.Germain R. R., et al. , Changes in global avian functional diversity over the last million years. Proc. Natl. Acad. Sci. U.S.A. (2022), this issue. [Google Scholar]
- 28.McGill B. J., Enquist B. J., Weiher E., Westoby M., Rebuilding community ecology from functional traits. Trends Ecol. Evol. 21, 178–185 (2006). [DOI] [PubMed] [Google Scholar]
- 29.Violle C., et al. , Let the concept of trait be functional! Oikos 116, 882–892 (2007). [Google Scholar]
- 30.Kemp M. E., Long-term functional trait diversity in insular reptiles, the last bastions of key ecosystem services. Proc. Natl. Acad. Sci. U.S.A. (2022), this issue. [Google Scholar]
- 31.Parker A. K., Müller J., Boisserie J.-R., Head J. J., The utility of body size as a functional trait to link the past and present in a diverse reptile clade. Proc. Natl. Acad. Sci. U.S.A. (2022), this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Short R. A., McGuire J. L., Polly P. D., Lawing A. M., Anthropogenic impact disrupts the functional relationship between traits and environment in large mammals. Proc. Natl. Acad. Sci. U.S.A. (2022), this issue. [Google Scholar]
- 33.Wang Y., Pineda-Munoz S., McGuire J. L., Plants maintain climate fidelity in the face of dynamic climate change. Proc. Natl. Acad. Sci. U.S.A. (2022), this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Carmona C. P., De Bello F., Mason N. W., Lepš J., Traits without borders: Integrating functional diversity across scales. Trends Ecol. Evol. 31, 382–394 (2016). [DOI] [PubMed] [Google Scholar]
- 35.Nakagawa S., Cuthill I. C., Effect size, confidence interval and statistical significance: A practical guide for biologists. Biol. Rev. 82, 591–605 (2007). [DOI] [PubMed] [Google Scholar]
- 36.Kiessling W., Smith J. A., Raja N. B., Improving the relevance of paleontology to climate change policy. Proc. Natl. Acad. Sci. U.S.A. (2022), this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harris N. C., et al. , Socio-ecological gap analysis to forecast species range contractions for conservation. Proc. Natl. Acad. Sci. U.S.A. 27, e202201942R (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.McGuire J. L., Lawler J. J., McRae B. H., Nuñez T. A., Theobald D. M., Achieving climate connectivity in a fragmented landscape. Proc. Natl. Acad. Sci. U.S.A. 113, 7195–7200 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bull J. W., Suttle K. B., Singh N. J., Milner-Gulland E., Conservation when nothing stands still: Moving targets and biodiversity offsets. Front. Ecol. Environ. 11, 203–210 (2013). [Google Scholar]
- 40.D’Aloia C. C., et al. , Coupled networks of permanent protected areas and dynamic conservation areas for biodiversity conservation under climate change. Front. Ecol. Evol. 7, 27 (2019). [Google Scholar]
- 41.Harris N. C., Gámez S., Gadsden G. I., Malhotra R., Textured species range maps enhance interdisciplinary science capacity across scales. Front. Ecol. Environ. 20, 319–326 (2022). [Google Scholar]
- 42.Santos A. M., et al. , Current climate, but also long-term climate changes and human impacts, determine the geographic distribution of European mammal diversity. Glob. Ecol. Biogeogr. 29, 1758–1769 (2020). [Google Scholar]
- 43.Lawing A. M., Polly P. D., Hews D. K., Martins E. P., Including fossils in phylogenetic climate reconstructions: A deep time perspective on the climatic niche evolution and diversification of spiny lizards (Sceloporus). Am. Nat. 188, 133–148 (2016). [DOI] [PubMed] [Google Scholar]
- 44.Lawler J., Ruesch A., Olden J., McRae B., Projected climate-driven faunal movement routes. Ecol. Lett. 16, 1014–1022 (2013). [DOI] [PubMed] [Google Scholar]
- 45.Wang Y., Shipley B. R., Lauer D. A., Pineau R. M., McGuire J. L., Plant biomes demonstrate that landscape resilience today is the lowest it has been since end-Pleistocene megafaunal extinctions. Glob. Chang. Biol. 26, 5914–5927 (2020). [DOI] [PubMed] [Google Scholar]
- 46.Antell G. S., Fenton I. S., Valdes P. J., Saupe E. E., Thermal niches of planktonic foraminifera are static throughout glacial–interglacial climate change. Proc. Natl. Acad. Sci. U.S.A. 118, e2017105118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Chyn K., et al. , Fine-scale roadkill risk models: Understanding the intersection of wildlife and roads. Biodivers. Conserv. 30, 139–164 (2021). [Google Scholar]
- 48.Heim N., Fisher J. T., Volpe J., Clevenger A. P., Paczkowski J., Carnivore community response to anthropogenic landscape change: Species-specificity foils generalizations. Landsc. Ecol. 34, 2493–2507 (2019). [Google Scholar]
- 49.Pineda-Munoz S., Wang Y., Lyons S. K., Toth A. B., McGuire J. L., Mammal species occupy different climates following the expansion of human impacts. Proc. Natl. Acad. Sci. U.S.A. 118, e1922859118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.McGill B. J., Dornelas M., Gotelli N. J., Magurran A. E., Fifteen forms of biodiversity trend in the Anthropocene. Trends Ecol. Evol. 30, 104–113 (2015). [DOI] [PubMed] [Google Scholar]
- 51.Heilpern S. A., Weeks B. C., Naeem S., Predicting ecosystem vulnerability to biodiversity loss from community composition. Ecology 99, 1099–1107 (2018). [DOI] [PubMed] [Google Scholar]
- 52.Gillson L., Paleoecology reveals lost ecological connections and strengthens ecosystem restoration. Proc. Natl. Acad. Sci. U.S.A. 119, e2206436119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Reeder-Myers L., et al. , Indigenous oyster fisheries persisted for millennia and should inform future management. Nat. Commun. 13, 1–13 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Smith J. A., Dietl G. P., Durham S. R., Increasing the salience of marine live–dead data in the Anthropocene. Paleobiology 46, 279–287 (2020). [Google Scholar]
- 55.Kiessling W., Raja N. B., Roden V. J., Turvey S. T., Saupe E. E., Addressing priority questions of conservation science with palaeontological data. Philos. Trans. R. Soc. B Biol. Sci. 374, 20190222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Williams J. W., et al. , Model systems for a no-analog future: Species associations and climates during the last deglaciation. Ann. N. Y. Acad. Sci. 1297, 29–43 (2013). [DOI] [PubMed] [Google Scholar]
- 57.Brown S. C., Wigley T. M. L., Otto-Bliesner B. L., Rahbek C., Fordham D. A., Persistent quaternary climate refugia are hospices for biodiversity in the anthropocene. Nat. Clim. Chang. 10, 244–248 (2020). [Google Scholar]
- 58.Roberts D. R., Hamann A., Climate refugia and migration requirements in complex landscapes. Ecography 39, 1238–1246 (2016). [Google Scholar]
- 59.Schloss C. A., Cameron D. R., McRae B. H., Theobald D. M., Jones A., “No-regrets” pathways for navigating climate change: Planning for connectivity with land use, topography, and climate. Ecol. Appl. 32, e02468 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Fitzgerald L. A., Stronza A. L., Applied biodiversity science: Bridging ecology, culture, and governance for effective conservation. Interciencia 34, 563–570 (2009). [Google Scholar]
- 61.Davis M. B., Shaw R. G., Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679 (2001). [DOI] [PubMed] [Google Scholar]
- 62.Williams J. E., Blois J. L., Range shifts in response to past and future climate change: Can climate velocities and species’ dispersal capabilities explain variation in mammalian range shifts? J. Biogeogr. 45, 2175–2189 (2018). [Google Scholar]
- 63.Ackerly D., et al. , The geography of climate change: Implications for conservation biogeography. Divers. Distrib. 16, 476–487 (2010). [Google Scholar]
- 64.Burrows M. T., et al. , Geographical limits to species-range shifts are suggested by climate velocity. Nature 507, 492–495 (2014). [DOI] [PubMed] [Google Scholar]
- 65.Schloss C. A., Nuñez T. A., Lawler J. J., Dispersal will limit ability of mammals to track climate change in the Western Hemisphere. Proc. Natl. Acad. Sci. U.S.A. 109, 8606–8611 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Lawing A. M., Polly P. D., Pleistocene climate, phylogeny, and climate envelope models: An integrative approach to better understand species’ response to climate change. PLoS One 6, e28554 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Menéndez R., et al. , Species richness changes lag behind climate change. Proc. Biol. Sci. 273, 1465–1470 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nathan R., et al. , Spread of North American wind-dispersed trees in future environments. Ecol. Lett. 14, 211–219 (2011). [DOI] [PubMed] [Google Scholar]
- 69.Wisz M. S., et al. , The role of biotic interactions in shaping distributions and realised assemblages of species: Implications for species distribution modelling. Biol. Rev. 88, 15–30 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Seliger B. J., McGill B. J., Svenning J.-C., Gill J. L., Widespread underfilling of the potential ranges of North American trees. J. Biogeogr. 48, 359–371 (2021). [Google Scholar]
- 71.Knight C. A., et al. , Community assembly and climate mismatch in late quaternary eastern North American pollen assemblages. Am. Nat. 195, 166–180 (2020). [DOI] [PubMed] [Google Scholar]
- 72.Ordonez A., Realized climatic niche of North American plant taxa lagged behind climate during the end of the Pleistocene. Am. J. Botany 100, 1255–1265 (2013). [DOI] [PubMed] [Google Scholar]
- 73.Cadotte M. W., Carscadden K., Mirotchnick N., Beyond species: Functional diversity and the maintenance of ecological processes and services. J. Appl. Ecol. 48, 1079–1087 (2011). [Google Scholar]
- 74.Batchelor C. L., et al. , The configuration of Northern Hemisphere ice sheets through the Quaternary. Nat. Commun. 10, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Su G., et al. , Human impacts on global freshwater fish biodiversity. Science 371, 835–838 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Ingeman K. E., et al. , Glimmers of hope in large carnivore recoveries. Sci. Rep. 12, 10005 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Newbold T., et al. , Ecological traits affect the response of tropical forest bird species to land-use intensity. Proc. R Soc. Lond B Biol. Sci. 280, 20122131 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smith F. A., et al. , Late Pleistocene megafauna extinction leads to missing pieces of ecological space in a North American mammal community. Proc. Natl. Acad. Sci. U.S.A. 119, e2115015119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Newbold T., et al. , Global effects of land use on biodiversity differ among functional groups. Funct. Ecol. 34, 684–693 (2020). [Google Scholar]
- 80.Mendoza M., Palmqvist P., Hypsodonty in ungulates: An adaptation for grass consumption or for foraging in open habitat? J. Zool. 274, 134–142 (2008). [Google Scholar]
- 81.Damuth J., Janis C. M., On the relationship between hypsodonty and feeding ecology in ungulate mammals, and its utility in palaeoecology. Biol. Rev. 86, 733–758 (2011). [DOI] [PubMed] [Google Scholar]
- 82.Eronen J. T., et al. , Ecometrics: The traits that bind the past and present together. Integr. Zool. 5, 88–101 (2010). [DOI] [PubMed] [Google Scholar]
- 83.Polly P. D., et al. , History matters: Ecometrics and integrative climate change biology. Proc. R. Soc. B: Biol. Sci. 278, 1131–1140 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Vermillion W. A., Polly P. D., Head J. J., Eronen J. T., Lawing A. M., "Ecometrics: A trait-based approach to paleoclimate and paleoenvironmental reconstruction" in Methods in Paleoecology, (Springer, 2018), pp. 373–394. [Google Scholar]
- 85.United States Geological Survey, Advisory Committee on Climate Change and Natural Resource Science (ACCCNRS). https://www.sciencebase.gov/catalog/item/5c1d05d3e4b0708288c9bc2a. Accessed 3 June 2022.
- 86.Beier P., Hansen L. J., Helbrecht L., Behar D., A how-to guide for coproduction of actionable science. Conserv. Lett. 10, 288–296 (2017). [Google Scholar]