Abstract
Adenylate kinase (AK; ATP:AMP phosphotransferase, EC 2.7.4.3) is a ubiquitous enzyme that contributes to the homeostasis of adenine nucleotides in eukaryotic and prokaryotic cells. AK catalyzes the reversible reaction Mg · ATP + AMP ↔ Mg · ADP + ADP. In this study we show that AK secreted by the pathogenic strains of Pseudomonas aeruginosa appears to play an important role in macrophage cell death. We purified and characterized AK from the growth medium of a cystic fibrosis isolate strain of P. aeruginosa 8821 and hyperproduced it as a fusion protein with glutathione S-transferase. We demonstrated enhanced macrophage cell death in the presence of both the secreted and recombinant purified AK and its substrates AMP plus ATP or ADP. These data suggested that AK converts its substrates to a mixture of AMP, ADP, and ATP, which are potentially more cytotoxic than ATP alone. In addition, we observed increased macrophage killing in the presence of AK and ATP alone. Since the presence of ATPase activity on the macrophages was confirmed in the present work, external macrophage-effluxed ATP is converted to ADP, which in turn can be transformed by AK into a cytotoxic mixture of three adenine nucleotides. Evidence is presented in this study that secreted AK was detected in macrophages during infection with P. aeruginosa. Thus, the possible role of secreted AK as a virulence factor is in producing and keeping an intact pool of toxic mixtures of AMP, ADP, and ATP, which allows P. aeruginosa to exert its full virulence.
Pseudomonas aeruginosa is a dominant pathogen in the respiratory tract of cystic fibrosis patients (35). Unlike other bacterial infections, P. aeruginosa is more difficult to control through antibiotic therapy (26). To survive in the hostile environment of the human body, this pathogen utilizes an impressive arsenal of weapons (30). Macrophages constitute the first line of defense against infections, and the ability of P. aeruginosa to kill macrophages and other phagocytic cells such as mast cells may explain this bacterium's capability to persist and disseminate in the host. It is well known that P. aeruginosa can avoid phagocytosis by encapsulating itself with an exopolysaccharide coating, called alginate, which confers on the nonmucoid cells a mucoid phenotype (11, 27, 31). Earlier, it was demonstrated that a mucoid, alginate-producing strain of P. aeruginosa isolated from the lungs of a cystic fibrosis patient secretes nucleoside diphosphate kinase (Ndk), ATPase, adenylate kinase (AK), 5′-nucleotidase, and ATP-modifying enzymatic activities that can modulate the external ATP level of macrophages and enhance their cell death through P2Z receptor activation (40). The P2Z receptor is responsible for ATP-dependent cell death of macrophages through the formation of membrane pores permeable to molecules of up to 900 daltons in size (4, 37).
The role of individual enzymes secreted by the mucoid strain of P. aeruginosa (40) in the killing of macrophages is, however, unknown. In this article, we report the role of a single secreted enzyme, AK, as a virulence factor of P. aeruginosa in triggering macrophage cell death. Our demonstration of its role as a cytotoxic factor thus delineates for the first time a novel role of this enzyme in bacterial virulence.
MATERIALS AND METHODS
Bacterial strains and plasmids.
P. aeruginosa mucoid strain 8821 (3), a strain 8821 ndk deletion mutant (40), and nonmucoid strain 808 (41) were grown as described before (40, 41). The Escherichia coli strains DH5α (Gibco BRL) and BL21 (Amersham Pharmacia) were used for routine subcloning and for the glutathione S-transferase (GST) fusion protein expression, respectively. Plasmid pGEX-5X-3 (Amersham Pharmacia) was used for GST-AK fusion construct preparation.
Purification of P. aeruginosa AK.
Secreted AK was purified from the ndk knockout mutant strain of P. aeruginosa 8821 (40). Cells were grown in 4 liters of L broth containing 300 μg of chloramphenicol per ml to an optical density at 600 nm (OD600) of 1 to 1.2 at 37°C and then centrifuged (8,000 rpm for 20 min at 4°C), and the supernatant was concentrated using the QuixStand Benchtop System (A/T Technology Corp.). The concentrated supernatant (50 ml) was mixed with 15 ml of Dyematrex Gel Blue A (Millipore) equilibrated in TM buffer (50 mM Tris-HCl, 10 mM MgCl2 [pH 7.5]) and gently shaken at room temperature for 2 h. The supernatant was removed after centrifugation (500 × g for 5 min), and the packed resin was washed with TM buffer until no protein was observed in the flow-through fraction. The bound proteins were eluted with TM buffer containing 10 mM ATP and 10 mM AMP, and the eluted fractions were analyzed on sodium dodecyl sulfate–12% polyacrylamide gel electrophoresis (SDS–12% PAGE). For AK enzymatic assay, the samples were desalted on Micro Bio-Spin 6 columns (Bio-Rad). Protein concentration was determined according to the Bradford method by using a Pierce kit. SDS-PAGE was performed as described by Laemmli (16).
Protein sequencing and mass spectrometric analysis of AK.
The purified AK was subjected to SDS-PAGE and electroblotted to a polyvinylidene difluoride membrane from Bio-Rad as described earlier (21). The stained band (30 kDa) was cut out and subjected to N-terminal sequencing on an Applied Biosystems 475A protein sequencer (UIC Protein Research facility). Mass spectrometric analysis was done on mass spectrometer-MALDI (matrix-assisted laser desorption ionization) in the Macromolecular Resources Facility at Colorado State University.
Antibody production and immunoblot analysis.
The P. aeruginosa AK amino acid sequence deduced from genomic DNA was analyzed for its antigenicity profile (38). Two immunogenic peptides corresponding to AK amino acid sequence VYHTEHNPPKVAA (132 to 144) and EQITAKVLSALS (205 to 216) were synthesized that contained an additional Cys amino acid residue for coupling to keyhole limpet hemocyanin with maleimido-benzoyl-N-hydroxysuccinimide. Immunizations of the rabbits were done according to standard protocol, and the titer for antibodies (Ab2 and Ab4) in the antiserum was determined by indirect enzyme-linked immunosorbent assay. The 96-well plates (Sigma) were coated either with purified recombinant GST-AK or with secreted AK (5 μg/ml), and the bound antibodies were detected with anti-rabbit immunoglobulin G labeled with horseradish peroxidase. Immunoblot analysis was performed by electrotransfer of proteins after SDS-PAGE to a polyvinylidene diflouride membrane (Millipore) followed by incubation with primary antibodies. Detection was performed by using anti-rabbit immunoglobulin G labeled with horseradish peroxidase and using an ECL system (Amersham).
Expression and purification of GST fusion protein with AK.
A plasmid was constructed to express AK as a fusion protein with GST. Two primers, which included 5′ BamHI and 3′ EcoRI restriction sites 5′-GGGGATCCCCATGCGTGTGATTCTG-3′ and 5′-GGGAATTCTCAGCTCAGGGCCGA-3′ were designed from the AK DNA sequence retrieved from the P. aeruginosa database. They were used to amplify the AK gene (adk) from the genomic DNA by PCR using Pfu DNA polymerase (Stratagene). The amplification product was purified after agarose gel electrophoresis by using a GeneClean II kit (Bio 101, Inc.), digested with EcoRI and BamHI, and ligated into BamHI-EcoRI-linearized pGEX-5X-3 plasmid to create the plasmid pGEX-5X-3/ak. This plasmid was transformed into E. coli BL21 cells to create the strain BL21/ak. The BL21/ak cells were grown in 0.5 liters of L broth with ampicillin at 37°C to late log phase (A600 = 0.8) and induced with 1 mM isopropyl-β-d-thiogalactopyranoside for 5 h. The cells were centrifuged and resuspended in 20 ml of phosphate-buffered saline (PBS) containing a protease inhibitor cocktail from Boehringer. After mild sonication and addition of Triton X-100 (1%), the suspension was gently shaken at room temperature for 30 min. The debris was removed from the lysed cells by centrifugation (20,000 rpm, 20 min, 4°C), and the resulting supernatant was used for purification of the GST-AK fusion protein by using a GST purification module kit (Amersham Pharmacia) as described in the manufacturer's instructions.
Enzyme assays.
AK activity was measured by spectrophotometry at 340 nm, where ATP formation from ADP was coupled to NADP reduction with glucose (10 mM), hexokinase (2.5 U/ml), and glucose-6-phosphate dehydrogenase (1.25 U/ml) in 1 ml of reaction mixture at 37°C as described earlier (6). A molar absorbance value of 6.22 × 103 was used for NADPH. Lactate dehydrogenase (LDH) activity was measured at 490 nm by using a Cytotox 96 nonradioactive cytotoxicity assay kit (Promega). Enzyme activities were expressed as absorbance per minute per milliliter of solution. ATPase activity of macrophages was assayed by measurement of the production of inorganic phosphate from ATP. Macrophages adhered to 96-well plates were prepared as described below. The reaction was performed in 100 μl of buffer (50 mM Tris-HCl, pH 7.5) containing 1 mM ATP at 37°C, and inorganic phosphate was determined by measurement of the absorption at 820 nm as described earlier (43).
Macrophage cytotoxicity assay.
The macrophage cytotoxicity assays were performed at 37°C in the presence of 5% CO2 and RPMI medium containing 10 mM HEPES buffer, pH 7.0. Macrophages derived from J774 cells were cultured and plated on 96-well plates (Beckton Dickinson Labware) at a final concentration of 105 cells/well in 200 μl of medium and were allowed to adhere to the wells for 2 h at 37°C. After being rinsed to remove nonadherent cells, the macrophages were activated with 50 ng of cell wall lipopolysaccharide (LPS) (Sigma) per ml for 12 h. LPS-primed cells were washed and incubated for 2 h in the presence of different concentrations of AMP, ADP, and ATP, singly or in combination, with or without purified GST-AK or secreted AK. At the end of the incubation, 50 μl of the supernatant was transferred into the 96-well plate and LDH activity was determined. Triplicate samples were tested for each data point. Prior to challenge with macrophages, the reactions of enzymes with nucleotides were allowed to proceed for 2 h at room temperature. AMP, ADP, and ATP used in these studies were of the highest purity and were purchased from Sigma.
Macrophage infection with P. aeruginosa cells.
J774 cell line macrophages were cultured on a mini petri dish at a concentration of 105 cells per ml in RPMI 1640 medium. The cells, adhered to dishes, were infected with L broth-grown P. aeruginosa 808 cells at a multiplicity of 50:1 (bacteria and macrophages). As a control, heat-inactivated P. aeruginosa cells were also used at the same multiplicity. Infections were also carried out with cells in the presence of 10 mM CaCl2, since CaCl2 (5 mM) was previously shown to inhibit secretion of AK and other enzymes (40). At various times, the macrophages were washed thrice with PBS to remove external bacteria and treated with 0.5 ml of 1× SDS sample buffer (without β-mercaptoethanol), and the lysates were mechanically scraped and 40-μl aliquots were examined by immunoblotting using anti-AK antibodies.
RESULTS
Secretion of AK during growth of P. aeruginosa.
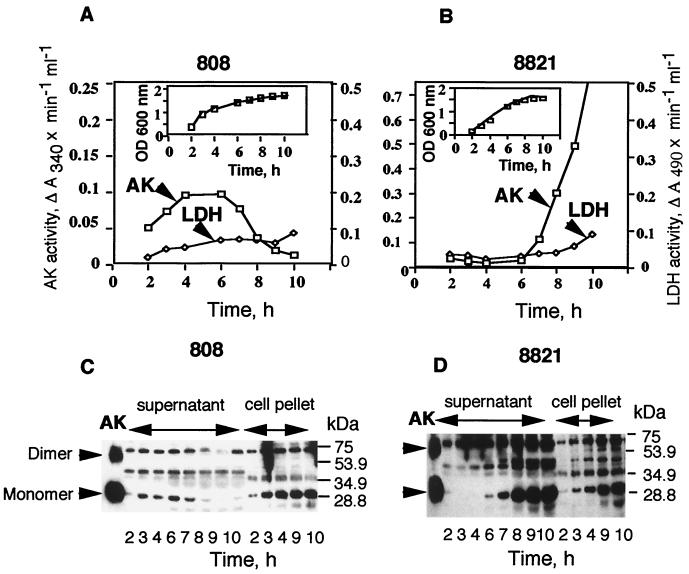
We recently reported the secretion of ATP-utilizing enzymes, such as Ndk, AK, 5′-nucleotidase, etc., both by mucoid cystic fibrosis isolate strain 8821 (40) and by nonmucoid burn patient isolate strain 808 (41) of P. aeruginosa. We further demonstrated that the secreted enzymes modulated the ATP levels effluxed from the macrophages to activate various macrophage surface-associated purinergic receptors, thereby enhancing macrophage cell death (40, 41). It was not clear, however, if all the ATP-utilizing enzymes, or if any single enzyme, contributed to macrophage cytotoxicity. In order to delineate the role of individual enzymes in this process we decided to study the role of AK, since it is a widely studied enzyme (23) without any reported role in phagocytic cell death. We first wanted to determine if AK is, in fact, secreted from both the nonmucoid and mucoid cells of P. aeruginosa. We therefore compared the level of AK in the growth medium with that of a known cytoplasmic enzyme, LDH, which is not known to be secreted and is thought to be present in the growth medium due to cell lysis. The amounts of AK were discerned both by enzymatic assays as well as by immunoblotting. AK was detected in the growth medium of nonmucoid strain 808 at an early log phase (2 h) (Fig. 1A) and increased continuously up to entry into stationary phase (6 h), after which it steadily declined and became almost nondetectable at the late stationary phase (10 h) (Fig. 1A). In contrast, LDH activity in the growth medium was low for 2 to 9 h, after which it started to increase, presumably due to cell lysis. Western blotting data (Fig. 1C) showed low levels of monomeric AK at 2 h but a steadily increasing amount up to 6 h, as was observed in enzymatic assays (Fig. 1A). The amount of monomeric AK protein was found to diminsh after 7 h (Fig. 1C) commensurate with enzymatic assay data (Fig. 1A), presumably because of proteolysis. The amount of the dimeric form of AK also was high from 2 to 7 h, after which it decreased slightly (Fig. 1C). The amounts of both the monomeric and dimeric forms of AK increased steadily in the cell pellet extract (Fig. 1C). The decreasing enzymatic activity for AK from 6 h onwards is reflected in the decreasing amounts of AK monomer more than that of the dimer, suggesting that the monomeric form is predominantly enzymatically active. When purified 30-kDa monomeric AK was electroeluted from the gel and rerun on SDS-PAGE, dimeric forms were again observed, suggesting that dimerization is a spontaneous event. When the level of LDH or AK in the supernatant of strain 808 was determined as percent of total activities (supernatant plus cell extract), the LDH activity during the log phase of growth was 1 to 2%, while that of AK was 15 to 20%. During stationary phase, LDH activity in the supernatant was about 20% of the total, while that of AK was 2 to 3%. In contrast to nonmucoid strain 808, mucoid strain 8821 secreted AK only after 6 h of growth, when the cell density reached an OD600 of 1.0. This finding confirms our previous observation that mucoid strain 8821 secretes ATP-utilizing enzymes only at high cell density (OD600 > 0.9), while nonmucoid strain 808 secretes ATP-utilizing enzymes much more efficiently at lower cell densities (40, 41). The release of LDH in the growth medium of mucoid strain 8821 occurs predominantly at the stationary phase (9 to 10 h). Unlike nonmucoid strain 808, however, the secretion of AK by mucoid strain 8821 did not decrease after 7 h of growth (Fig. 1B and D) but kept on increasing up to 10 h (Fig. 1D), when the cells reached stationary phase (Fig. 1B, inset). Since the antibodies used in these studies were obtained against a 13-mer peptide derived from residues 132 to 144 of the AK sequence, we believe that antibodies are specific and the bands between the monomeric and dimeric forms are the result of proteolytic digestion. The differences in AK enzyme and protein profiles between nonmucoid strain 808 and mucoid strain 8821 most likely reflect the levels of proteases in the growth medium, since the mucoid strains are known to release much less protease to the outside medium than the nonmucoid strains (25, 39).
FIG. 1.
Time course of AK appearance in the extracellular medium of P. aeruginosa strains 808 and 8821. Aliquots of the growing culture of strain 808 (A) and strain 8821 (B) were centrifuged, and AK and LDH activities were assayed in the supernatant. For immunoblot analysis, 1 ml of the growing culture of P. aeruginosa 808 (C) and 8821 (D) was centrifuged, and the supernatant proteins were precipitated with trichloroacetic acid. The residue was boiled with 50 μl of 1 × SDS sample buffer, and 20 μl was loaded on the 12% SDS polyacrylamide gel. The cell pellet was treated with 100 μl of 1 × SDS sample buffer, and 2 μl was loaded on the same gel. The 30-kDa AK protein band was detected by immunoblotting with anti-AK antibodies (AK2) as described in Materials and Methods. Panel A inset, growth curve for strain 808; panel B inset, growth curve for strain 8821.
Purification of secreted AK and GST-AK.
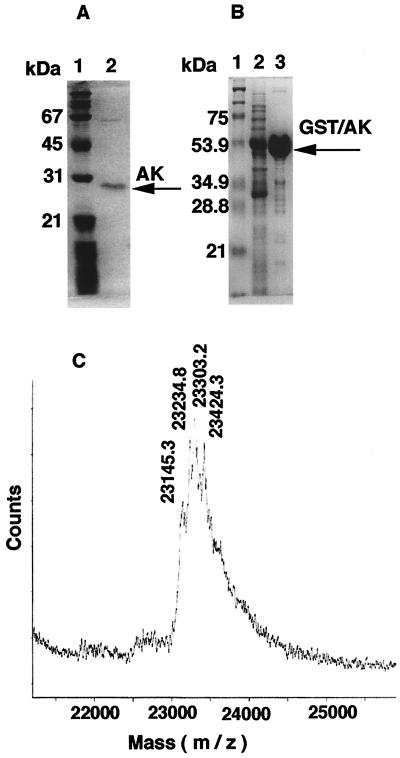
In view of the central role AK plays in homeostasis of adenine nucleotides in prokaryotic and eukaryotic cells (23) and to evaluate more fully its potential role in macrophage cell death, we have purified secreted AK from the P. aeruginosa mucoid strain 8821. Since P. aeruginosa 8821 also secretes Ndk, which copurifies with AK, causing complications during purification, we used the ndk knockout mutant strain of P. aeruginosa 8821 (40) for AK purification. Extracellular medium of this strain was concentrated and fractionated on a Blue A column as described in Materials and Methods. The eluted fractions, analyzed for AK activity and by SDS-PAGE, showed a good correlation between the enzymatic activity and the intensity of a 30-kDa band on the gel (data not shown). To determine if this 30-kDa band (Fig. 2A) corresponds to AK, the sequence of the first 10 N-terminal amino acids of this band was determined (MRVILLGAPX), which showed 100% identity with the N-terminal amino acid sequence of the adk gene product deduced from the P. aeruginosa DNA database (Integrated Genomics, Inc., Chicago, Illinois). The purification procedure yielded 100 μg of AK, with a specific activity of 122 μmol of NADPH/min/mg of protein. To further characterize AK and to obtain an amount sufficient for further studies, we overexpressed P. aeruginosa AK in E. coli as a fusion protein with GST. The expressed protein of about 55 kDa (Fig. 2B) was purified to approximately 99% purity, with a specific activity of 280 μmol of NADPH/min/mg of protein. Its identity was established by measuring its enzymatic activity and by immunoblot analysis with anti-AK and anti-GST antibodies.
FIG. 2.
Purification of P. aeruginosa 8821 AK and GST-AK overexpressed in E. coli. Purified AK from P. aeruginosa 8821 (A) and GST-AK from E. coli (B) after SDS–12% PAGE and staining with Coomassie Blue are shown. Panel A lane 1, molecular size markers; lane 2, purified AK. Panel B lane 1, molecular size markers; lane 2, cell lysate; lane 3, purified GST-AK. (C) Mass spectrometric analysis of purified AK from P. aeruginosa strain 8821.
Molecular and catalytic properties of P. aeruginosa AK.
From the primary structure of the P. aeruginosa AK deduced from the nucleotide sequence of the gene, the molecular weight (Mr) of the protein would be 23,107.30. Measurement of the molecular size by SDS-PAGE, as shown in Fig. 2A, gave a value of about 30 kDa, which could be due to an aberrant migration of the enzyme on the SDS gel. Indeed, a mass spectrometric analysis of the purified AK showed four peaks at m/z 23,145.3, 23,234.8, 23,303.2, and 23,424.3, with a maximum at m/z 23,303.2 (Fig. 2C). The heterogeneity of the AK peaks on the MALDI spectrum and discrepancy between their molecular masses and the theoretical mass of AK may reflect a potential posttranslational modification of the secreted enzyme. Glycosylation was not found in AK when a glycoprotein detection kit from Sigma, with a detection limit of 25 ng of carbohydrates, was used. Lipidation might be a possibility, as is the case for a number of bacterial protein toxins, including adenylate cyclase from Bordetella pertussis (13).
Since we intended to use purified recombinant and secreted AK for functional studies, we compared some of their catalytic properties. Both enzymes were active over a wide range of pH values, with a broad pH optimum between pH 7.0 and 10.0. However, specific activity of the purified GST-AK assayed in the direction of ATP formation with 2 mM ADP was two times higher than that of the purified secreted AK. Secreted AK from P. aeruginosa showed its maximal activity at a higher temperature (60°C) than GST-AK (50°C). In thermostability studies, both enzymes were incubated for 10 min at various temperatures between 37 and 60°C, after which their residual activity was determined at 37°C. Heating at 60°C completely inactivated GST-AK, while secreted AK retained 30% of its activity. It was recently reported that AK-based fusion proteins show up to a 20°C increase or decrease in stability where 88% of the AK sequence was maintained (14). Inhibition studies of GST-AK and P. aeruginosa AK showed that 250 μM AP5A [P1,P5-di(adenosine-5′) pentaphosphate], which is a mixed noncompetitive inhibitor for AK (32), inactivated more than 90% of both enzymes' activities.
The effects of different adenine nucleotides and AK on macrophage cell death.
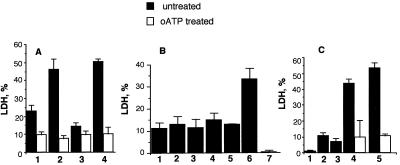
In previous studies it was shown that secreted ATP-utilizing enzymes enhanced ATP-inducible macrophage cell death, although the role of individual enzymes in this enhancement was unknown (40, 41). To examine whether secretion of AK by P. aeruginosa strains may have any effect on adenine nucleotide-induced macrophage cell death, we determined the extent of macrophage death in the presence of different adenine nucleotides with or without purified AK. We used the GST-AK fusion protein isolated from E. coli to decipher the role of AK in macrophage cell death. Results of these investigations are presented in Fig. 3. Similar cytotoxicity effects on macrophages were observed when we used purified secreted AK instead of GST-AK (data not shown). Among the adenine nucleotides tested (ATP, ADP, AMP), only ATP at a concentration of 1 mM caused significant macrophage cell death (about 22%), which is consistent with data reported in the literature (7, 17, 40). The same concentration of ATP treated with GST-AK (5 μg/ml) accelerated macrophage cell death to 45% (Fig. 3A). GST-AK by itself had no cytotoxicity (Fig. 3B, lane 7). Similar enhancement of macrophage cell death by GST-AK also was noted in the case of ATP plus AMP (Fig. 3A, columns 3 and 4). Macrophages pretreated with oxidized ATP (oATP) did not respond appreciably to the enhanced cytotoxicity of the nucleotides by GST-AK (Fig. 3A; columns 2 and 4, empty columns). In the case of ADP at concentrations of 1 and 0.5 mM, we could not detect differences in cell death with or without GST-AK. However, at a lower concentration of ADP (0.33 mM), treatment with GST-AK enhanced cell death from 12 to 33% (Fig. 3B, columns 5 and 6), although lower concentrations, such as 0.1 mM, had no effect. The exact mechanism of this effect is unknown. However, it is interesting to note that the MgSO4 concentration in RPMI medium during the cytotoxicity assay was around 0.4 mM. Enhanced macrophage death was observed when the concentration of magnesium exceeded the ADP concentration. There are two nucleotide-binding sites of AK, one for the magnesium-ADP complexes and the other for uncomplexed nucleotides. Free ADP is known to inhibit AK activity (32). Thus, at the 0.33 mM ADP concentration, which is lower than the magnesium concentration in the reaction mixture, there is no free ADP to inhibit AK activity, thereby promoting AK-induced macrophage cell death. The results of the above studies indicated that accelerated macrophage death was achieved in the presence of AK and its substrates. Therefore, we concluded that the combination of adenine nucleotides might lead to higher macrophage cell death than individual adenine derivatives alone. When low concentrations (0.33 mM) of nucleotides (AMP, ADP, or ATP) were used singly, very little macrophage killing was observed; however, a combination of the nucleotides had significant cell killing activity (Fig. 3C, column 4) which could be further enhanced in the presence of GST-AK (Fig. 3C, column 5). Pretreatment of the macrophages with oATP significantly reduced macrophage death, suggesting that macrophage surface-associated purinergic receptors involved in such cell death are rendered nonamenable to the nucleotide action when bound with oATP. Whether receptors other than P2Z are involved in macrophage cell death in the presence of the combination of nucleotides is unknown.
FIG. 3.
Adenine nucleotide-mediated killing of macrophages. (A) AMP- and ATP-mediated cytotoxicity in the presence of 1 μg of GST-AK. Column 1, 1 mM ATP; column 2, 1 mM ATP treated with GST-AK; column 3, 0.5 mM AMP plus 0.5 mM ATP; column 4, 0.5 mM AMP plus 0.5 mM ATP treated with GST-AK. AMP alone, GST-AK alone, or AMP treated with GST-AK had no cytotoxicity towards macrophages (not shown). oATP-pretreated macrophages were not amenable to cytotoxicity to ATP or ATP plus AMP with or without GST-AK treatment. (B) ADP-mediated killing of the macrophages in the presence of 1 μg of GST-AK. Columns 1, 3, and 5, 1, 0.5, and 0.33 mM ADP, respectively; columns 2, 4, and 6, the same concentrations, respectively, of ADP but treated with 1 μg of GST-AK; column 7, GST-AK only. (C) Cooperative effect of AMP, ADP, and ATP mixtures on macrophage killing. Column 1, 0.33 mM AMP; column 2, 0.33 mM ADP; column 3, 0.33 mM ATP; column 4, 0.33 mM AMP plus 0.33 mM ADP plus 0.33 mM ATP; column 5, 0.33 mM AMP plus 0.33 mM ADP plus 0.33 mM ATP treated with 1 μg of GST-AK. Greatly reduced cell killing by the nucleotide mixture in the absence or presence of GST-AK in oATP-pretreated macrophages is also shown. Details of the cytotoxicity assays measured by LDH release and pretreatment of macrophages with oATP are given in Materials and Methods as well as in references 40 and 41. Results represent the means of three experiments; error bars indicate the standard errors of the means.
Association of P. aeruginosa AK with macrophages during infection.
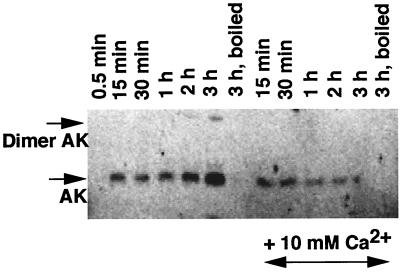
The secretion of AK, as well as its cytotoxicity toward macrophages in the presence of its substrates, ATP plus AMP or ADP, raises interesting questions regarding its secretion during infection of host tissues. Is AK secreted by P. aeruginosa during its exposure to macrophages? It was previously reported that secretion of ATP-utilizing enzymes, including AK, is inhibited in the presence of 5.0 mM CaCl2 (40). Since we showed that P. aeruginosa strain 808 secretes AK more efficiently at lower cell densities, and because of the fact that this nonmucoid strain lacks a sticky alginate layer to prevent nonspecific binding to macrophages, we used it for infection studies. Macrophages were infected with P. aeruginosa strain 808 cells at a multiplicity of infection of 1:50 (macrophage:bacteria). After incubation for various periods of time, the external bacteria were washed out with RPMI media and the presence of macrophage-associated AK was estimated by Western blotting. During the early period of infection (0.5 min), very little AK was found to be associated with macrophages (Fig. 4). After 3 h of incubation, increasing amounts of macrophage-associated AK became detectable, suggesting that AK was continually secreted during this period of incubation. Infection with heat-killed (boiled) cells did not demonstrate the presence of macrophage-associated AK even up to an incubation period of 3 h, suggesting that AK secretion is a metabolically active process (Fig. 4; lane 3 h, boiled). Inclusion of 10 mM Ca2+ in the infection mixture severely inhibited AK secretion after 15 min (Fig. 4), confirming the role of Ca2+ as an inhibitor of the secretion machinery (40). Most of the AK was present as enzymatically active monomers, although dimeric forms were also detected after 2 and 3 h of infection (Fig. 4, lanes 2 h and 3 h).
FIG. 4.
Time course of macrophage infection with P. aeruginosa 808 cells. Macrophages were infected with live or heat-killed bacteria for various periods as indicated. At the end of each period, the macrophages were washed thrice to remove external bacteria and lysed with an SDS-containing buffer, and an aliquot was loaded on SDS-PAGE and developed by Western blotting using anti-AK antibodies (AK2). A similar infection experiment was conducted in the presence of CaCl2 (10 mM) to examine its effect on AK secretion during infection.
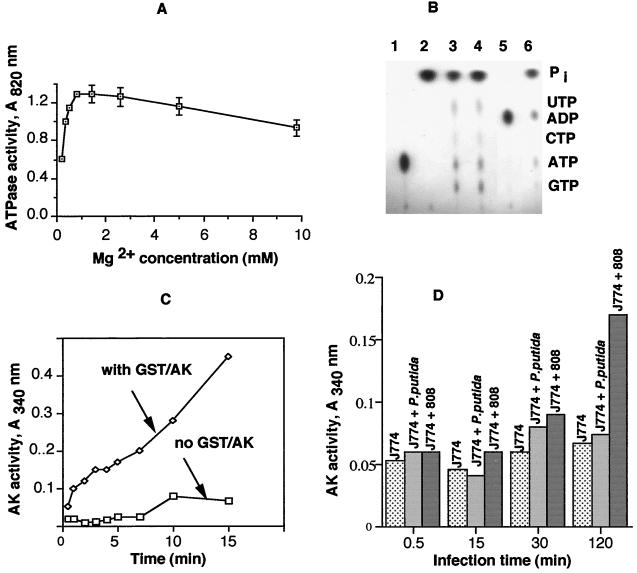
The apparent secretion of AK during infection of macrophages with live cells of P. aeruginosa begged the question of whether purified AK in the absence of live cells could also bind macrophage cells. This would be similar to the presence of AK as an ectoenzyme on the macrophage surface. We therefore examined the presence of ectoenzymes such as ATPase and AK on the macrophage surface. J774 cell line-derived macrophages demonstrated the presence of strong Mg2+-activated ATPase activity (Fig. 5A). When the macrophages were incubated with [γ-32P]ATP alone, release of 32Pi (inorganic orthophosphate) as a function of ecto-ATPase activity was apparent even within 1 min (Fig. 5B, lane 3). Weak bands of UTP, CTP, and GTP were also observed, even though no exogenous UDP, CDP, and GDP were added, suggesting that an ecto-Ndk activity, along with small amounts of nucleoside diphosphates effluxed from the macrophages, led to the formation of small amounts of nucleoside triphosphates (Fig. 5B, lanes 3 and 4). However, when excess (100 μM) AMP was present, small amounts of ADP could be detected (Fig. 5B, lane 6), indicating the presence of weak ecto-AK activity. When, however, macrophages were incubated with purified GST-AK, a considerable amount of bound GST-AK was detected (Fig. 5C), confirming the binding of purified enzyme on macrophage surfaces. To confirm that AK secreted from live cells of P. aeruginosa was responsible for its binding with macrophages, we looked at Pseudomonas putida strain 700412. This strain had significant intracellular AK activity, but very little AK was found to be secreted (data not shown). When macrophages were infected separately with P. putida 700412 and P. aeruginosa strain 808 for varying periods of time and examined for the presence of bound AK, very little was found in P. putida-infected macrophages up to a period of 2 h but significant surface-associated AK activity was detected, particularly at 2 h in P. aeruginosa-infected macrophages (Fig. 5D), clearly demonstrating the secretion of AK from P. aeruginosa and its subsequent association with macrophages. When AK was fluorescently tagged with Alexa Fluor 488 (Molecular Probes, Inc.) and examined by confocal microscopy, a considerable amount of surface-associated fluorescent AK was detected, while fluorescently tagged cytochrome c did not demonstrate much binding (data not shown), suggesting that AK is preferentially bound on macrophage surfaces, thereby modulating external adenine nucleotide levels for enhanced killing of such macrophages.
FIG. 5.
Presence of ATPase and AK as ectoenzymes on macrophage surfaces. (A) Mg2+-dependent ATPase activity. Ninety-six-well plates coated with 2 × 105 macrophages per well were incubated with 1 mM ATP for 30 min at 37°C in the presence of different concentrations of MgCl2, and ATPase activity was measured as described in Materials and Methods. (B) ATPase assay on thin-layer chromatography plates. Reactions were performed in 96-well plates without or coated with 2 × 105 macrophages per well in RPMI-HEPES medium at 37°C. Thin-layer chromatography analyses of the nucleotide products were done as described previously (40, 41). All lanes contained 0.07 μM [γ-32P]ATP. Lane 1, no macrophages added; lane 2, no macrophages but with 1 μg of commercial ATPase; lane 3, macrophages incubated with [γ-32P]ATP for 1 min; lane 4, macrophages incubated with [γ-32P]ATP for 15 min; lane 5, no macrophages but with 1 μg of GST-AK plus 100 μM unlabeled AMP (standard AK reaction control); lane 6, macrophages incubated with 100 μM unlabeled AMP for 15 min. (C) Binding of purified GST-AK with macrophages. Macrophages adhered to the wells were incubated with or without GST-AK (400 μg/ml) in RPMI-HEPES buffer for 30 min at 37°C. After being extensively washed with PBS, AK activity in the wells was measured as described in Materials and Methods. (D) Secreted AK activity during contact with macrophages. Macrophages adhered to the wells were infected with P. putida or P. aeruginosa 808. At different infection times, as indicated, wells were thoroughly washed with PBS to remove nonadherent bacteria and AK activity was measured as described above.
DISCUSSION
AK is a well-studied enzyme which interconverts ADP to AMP and ATP, thus maintaining adenine nucleotide balance within cells (23). The present study reveals that AK secreted by pathogenic strains of P. aeruginosa in the presence of its substrates or ATP alone promotes macrophage killing. This conclusion is supported by the evidence that during macrophage infection with P. aeruginosa, AK was found to be associated with macrophages.
P. aeruginosa AK, which has not been previously studied, migrated on SDS-PAGE as a protein with an apparent molecular mass of 30 kDa, even though the predicted molecular mass from DNA sequence analysis and mass spectrometric analysis showed its true mass at around 23 kDa. This aberrant electrophoretic migration has been observed for AK from E. coli (29) and B. pertussis (9). We compared the deduced primary structure of the P. aeruginosa adk gene product with the known sequences of bacterial AKs by using the protein database search program BLAST. Analysis revealed that P. aeruginosa AK has 81% identity with the enzyme from P. putida and about 65% identity with the AKs from such pathogens as Neisseria meningitidis, Vibrio cholerae, B. pertussis, and Salmonella enterica serovar Typhimurium. It is likely that under certain conditions these pathogens may also release AK activity as one way of exerting their virulence on macrophages as P. aeruginosa secreted AK. It is interesting to note that AK is efficiently secreted not only by P. aeruginosa but also by other pathogens, such as Burkholderia cepacia (22) and V. cholerae (28).
Extracellular ATP is an important signal nucleotide that triggers a variety of biological activities, especially those in the immune system (4, 5). The biological activities of extracellular ATP are diverse and include induction of cell death. The macrophages are known to efflux ATP when exposed to bacterial LPS or intact bacteria (8, 33). Preferential expression and activation of purinergic receptors such as P2Z occur in the presence of external ATP (15), and activated P2Z receptors allow macrophage cell death via pore formation on macrophage membranes (4, 37). Under normal conditions, host cells negate the deleterious effect of ATP by expression of surface-located ectoenzymes. A number of ectoenzymes involved in extracellular purine metabolism have been identified (42). However, the relative functional importance of these different enzymatic activities has not been defined for a given cell type. It remains to be understood how these ectoenzymes work in concert and maintain a balanced level of nucleotides. Recently, metabolism of endogenous ATP in the extracellular medium of four epithelial cell lines was studied (18). The results indicated that, in addition to the ecto-ATPase activity, two other enzymatic activities, ecto-ATP pyrophosphatase and nucleoside diphosphokinase, might play a role in defining a balanced level of extracellular nucleotides. It is interesting to note that AK was not detected as an ectoenzyme in these cells (18).
In the present work we showed that the enhancement of macrophage cell killing was caused by a mixture of AMP, ADP, and ATP formed by the AK-catalyzed forward and reverse reactions. The combination of these three nucleotides has a more profound effect on macrophage cytotoxicity than individual adenine nucleotides alone (Fig. 3C). Since macrophages have ecto-ATPase activity on their surface (10, 36) (Fig. 5A) and have only traces of ecto-AK activity (Fig. 5B, lane 6), the macrophage-effluxed ATP can be converted to ADP. We assume that this ectoenzyme degrades extracellular ATP and counteracts, to some extent, the deleterious effect of high concentrations of external ATP. Since AK has not been found as an ectoenzyme in epithelial cells (18) and since we showed negligible AK activity on the macrophages (Fig. 5B), the pathogens may derive an advantage by secreting AK in the external milieu of host cells, thereby altering the adenine nucleotide levels and facilitating host cell death.
Finally, the role of AK as well as other ATP-utilizing enzymes in the colonization by mucoid P. aeruginosa of lungs with cystic fibrosis is unknown. Since AK and other ATP-utilizing enzymes are secreted by a number of pathogens such as B. cepacia (22), V. cholerae (28), and both mucoid (40) and nonmucoid (41) P. aeruginosa, leading in all cases to enhanced killing of macrophages and mast cells, it is clear that secretion of such enzymes is a common weapon in the arsenal of many pathogens for dealing with phagocytic cells. It is likely that soon after infection in the upper respiratory tract, P. aeruginosa secretes these enzymes to contend with alveolar macrophages and mast cells to be able to form a biofilm. There is clear evidence that nonmucoid P. aeruginosa forms biofilms (20), and biofilms have been shown to be present in lungs with cystic fibrosis (34). Once the biofilms are formed, neutrophils are recruited at the site to eliminate the pathogens. Neutrophils are abundant in lungs with cystic fibrosis. AK and other ATP-utilizing enzymes that enhance phagocytic cell death through purinergic receptor activation are ineffective against neutrophils, since neutrophils and platelets are known to possess only extremely weak P2Z receptor activity (12). However, polymorphonuclear leukocytes and the accompanying oxygen radicals, including hydrogen peroxide, produced by the neutrophils are known to trigger mucoidy to the nonmucoid cells in the biofilms through mutational activation of mucA (20). The mucoid cells having the capsular polysaccharide alginate coating not only resist polymorphonuclear leukocyte actions (1) and antibiotic treatment (1) and detoxify the oxygen radicals via formation of a number of superoxide dismutases, catalases, and other enzymes that are involved in hydroperoxide resistance (19, 24), but they are also believed to contribute to further colonization and biofilm formation (2, 20, 34). Thus, P. aeruginosa uses different virulence factors to contend with different phagocytic cells for successful colonization of lungs with cystic fibrosis.
ACKNOWLEDGMENTS
We thank Integrated Genomics, Inc., for generously providing the P. aeruginosa genomic database.
This work was supported by PHS grant AI 16790-21 from the National Institutes of Health.
REFERENCES
- 1.Bayer A S, Speert D P, Park S, Tu J, Witt M, Nast C C, Norman D C. Functional role of mucoid exopolysaccharide (alginate) in antibiotic-induced and polymorphonuclear leukocyte-mediated killing of P. aeruginosa. Infect Immun. 1991;59:302–308. doi: 10.1128/iai.59.1.302-308.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Boyd A, Chakrabarty A M. Pseudomonas aeruginosa biofilms: role of the alginate exopolysaccharide. J Ind Microbiol. 1995;15:162–168. doi: 10.1007/BF01569821. [DOI] [PubMed] [Google Scholar]
- 3.Darzins A, Chakrabarty A M. Cloning of genes controlling alginate biosynthesis from a mucoid cystic fibrosis isolate of Pseudomonas aeruginosa. J Bacteriol. 1984;159:9–18. doi: 10.1128/jb.159.1.9-18.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Di Virgilio F. The P2Z purinoceptor: an intriguing role in immunity, inflammation and cell death. Immunol Today. 1995;16:524–528. doi: 10.1016/0167-5699(95)80045-X. [DOI] [PubMed] [Google Scholar]
- 5.Dubyak G R, El-Moatassum C. Signal transduction via P2-purinergic receptors for extracellular ATP and other nucleotides. Am J Physiol. 1993;265:C577–C606. doi: 10.1152/ajpcell.1993.265.3.C577. [DOI] [PubMed] [Google Scholar]
- 6.Elvir-Mairena J R, Jovanovic A, Gomez L A, Alekseev A E, Terzic A. Reversal of the ATP-liganded state of ATP-sensitive K+ channels by adenylate kinase activity. J Biol Chem. 1996;271:31903–31908. doi: 10.1074/jbc.271.50.31903. [DOI] [PubMed] [Google Scholar]
- 7.Ferrari D, Chiozzi P, Falzoni S, Dal Susino M, Melchiorri L, Baricordi O R, Di Virgillio F. Extracellular ATP triggers IL-1 beta release by activating the purinergic P2Z receptor or human macrophages. J Immunol. 1997;159:1451–1458. [PubMed] [Google Scholar]
- 8.Ferrari D, Chiozzi P, Falzoni S, Hanau S, Di Virgilio F. Purinergic modulation of interleukin-1 beta release from microglial cells stimulated with bacterial endotoxin. J Exp Med. 1997;185:579–582. doi: 10.1084/jem.185.3.579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilles A M, Sismeiro O, Munier H, Fabian H, Mantsch H H, Surewicz W K, Craescu C C, Barzu O, Danchin A. Structural and physico-chemical characteristics of Bordetella pertussis adenylate kinase, a tryptophan-containing enzyme. Eur J Biochem. 1993;218:921–927. doi: 10.1111/j.1432-1033.1993.tb18448.x. [DOI] [PubMed] [Google Scholar]
- 10.Gordon S, Cohn Z. Macrophage-melanocyte heterokaryons. I. Purification and properties. J Exp Med. 1970;131:981–1003. doi: 10.1084/jem.131.5.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Govan J R, Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol Rev. 1996;60:539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gu B J, Zhang W Y, Bendall L J, Chessel I P, Buell G N, Willey J S. Expression of P2X (7) purino receptors on human lymphocytes and monocytes: evidence for nonfunctional P2X (7) receptors. Am J Cell Physiol. 2000;279:C1189–C1197. doi: 10.1152/ajpcell.2000.279.4.C1189. [DOI] [PubMed] [Google Scholar]
- 13.Hackett M, Guo L, Shabanowitz J, Hunt D F, Hewlett E L. Internal lysine palmitoylation in adenylate cyclase toxin from Bordetella pertussis. Science. 1994;266:433–435. doi: 10.1126/science.7939682. [DOI] [PubMed] [Google Scholar]
- 14.Haney P J, Steers M, Konisky J. Analysis of thermal stabilizing interactions in mesophilic and thermophilic adenylate kinases from the genus Methanococcus. J Biol Chem. 1999;274:28453–28458. doi: 10.1074/jbc.274.40.28453. [DOI] [PubMed] [Google Scholar]
- 15.Humphreys B D, Dubyak G R. Induction of the P2Z/P2X7 nucleotide receptor and associated phospholipase D activity by lipopolysaccharide and IFN-gamma in the human THP-1 monocytic cell line. J Immunol. 1996;157:5627–5637. [PubMed] [Google Scholar]
- 16.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 17.Lammas D A, Stober C, Harvey C J, Kendrick N, Panchalingam S, Kumararatne D S. ATP-induced killing of mycobacteria by human macrophages is mediated by purinergic P2Z (P2X7) receptors. Immunity. 1997;7:433–444. doi: 10.1016/s1074-7613(00)80364-7. [DOI] [PubMed] [Google Scholar]
- 18.Lazarowski E, Boucher R C, Harden T K. Constitutive release of ATP and evidence for major contribution of ecto-nucleotide pyrophosphatase and nucleoside diphosphokinase to extracellular nucleotide concentrations. J Biol Chem. 2000;275:31061–31068. doi: 10.1074/jbc.M003255200. [DOI] [PubMed] [Google Scholar]
- 19.Ma J F, Ochsner U A, Klotz M G, Nanayakkara V K, Howell M L, Johnson Z, Posey J E, Vasil M L, Monaco J J, Hassett D J. Bacterioferritin A modulates catalase A (KatA) activity and resistance to hydrogen peroxide in Pseudomonas aeruginosa. J Bacteriol. 1999;181:3730–3742. doi: 10.1128/jb.181.12.3730-3742.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Mathee K, Ciofu O, Sternberg C, Lindum P W, Campbell J I, Jensen P, Johnsen A H, Givskov M, Ohman D E, Molin S, Hoiby N, Kharazmi A. Mucoid conversion of Pseudomonas aeruginosa by hydrogen peroxide: a mechanism for virulence activation in the cystic fibrosis lung. Microbiology. 1999;145:1349–1357. doi: 10.1099/13500872-145-6-1349. [DOI] [PubMed] [Google Scholar]
- 21.Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987;262:10035–10038. [PubMed] [Google Scholar]
- 22.Melnikov A, Zaborina O, Dhiman N, Prabhakar B S, Chakrabarty A M, Hendrickson W. Clinical and environmental isolates of Burkholderia cepacia exhibit differential cytotoxicity towards macrophages and mast cells. Mol Microbiol. 2000;36:1481–1493. doi: 10.1046/j.1365-2958.2000.01976.x. [DOI] [PubMed] [Google Scholar]
- 23.Noda L. Adenylate kinase. In: Boyer P, editor. The enzymes. 3rd ed. New York, N.Y: Academic Press; 1973. pp. 279–305. [Google Scholar]
- 24.Ochsner U A, Hassett D J, Vasil M I. Genetic and physiological characterization of ohr, encoding a protein involved in organic hydroperoxide resistance in Pseudomonas aeruginosa. J Bacteriol. 2001;183:773–778. doi: 10.1128/JB.183.2.773-778.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ohman D E, Chakrabarty A M. Utilization of human respiratory secretions by mucoid Pseudomonas aeruginosa of cystic fibrosis origin. Infect Immun. 1982;37:662–669. doi: 10.1128/iai.37.2.662-669.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Oliver A, Canton R, Campo P, Baquero F, Blazquez J. High frequency of hypermutable Pseudomonas aeruginosa in cystic fibrosis lung infection. Science. 2000;288:1251–1254. doi: 10.1126/science.288.5469.1251. [DOI] [PubMed] [Google Scholar]
- 27.Pier G B. Pseudomonas aeruginosa: a key problem in cystic fibrosis. ASM News. 1998;64:339–347. [Google Scholar]
- 28.Punj V, Zaborina O, Dhiman N, Falzari K, Bagdasarian M, Chakrabarty A M. Phagocytic cell killing mediated by secreted cytotoxic factors of Vibrio cholerae. Infect Immun. 2000;68:4930–4937. doi: 10.1128/iai.68.9.4930-4937.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saint Girons I, Gilles A M, Margarita D, Michelson S, Monnot M, Fermandjian S, Danchin A, Barzu O. Structural and catalytic characteristics of Escherichia coli adenylate kinase. J Biol Chem. 1987;262:622–629. [PubMed] [Google Scholar]
- 30.Salyers A A, Whitt D D. Bacterial pathogenesis: a molecular approach. Washington, D.C.: ASM Press; 1994. [Google Scholar]
- 31.Shankar S, Ye R W, Schlictman D, Chakrabarty A M. Exopolysaccharide alginate synthesis in Pseudomonas aeruginosa: enzymology and regulation of gene expression. Adv Enzymol. 1995;70:221–255. doi: 10.1002/9780470123164.ch4. [DOI] [PubMed] [Google Scholar]
- 32.Sheng X R, Li X, Pan X M. An iso-random Bi Bi mechanism for adenylate kinase. J Biol Chem. 1999;274:22238–22242. doi: 10.1074/jbc.274.32.22238. [DOI] [PubMed] [Google Scholar]
- 33.Sikora A, Liu J, Brosnan C, Buell G, Chessel I, Bloom B R. Cutting edge: purinergic signaling regulates radical-mediated bacterial killing mechanisms in macrophages through a P2X7-independent mechanism. J Immunol. 1999;163:558–561. [PubMed] [Google Scholar]
- 34.Singh P K, Schaefer A L, Parsek M R, Moninger T O, Welsh M J, Greenberg E P. Quorum-sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature. 2000;407:762–764. doi: 10.1038/35037627. [DOI] [PubMed] [Google Scholar]
- 35.Stover C K, Pham X Q, Erwin A L, Mizoguchi S D, Warrener P, et al. Complete genome sequence of Pseudomonas aeruginosa PAO1, an opportunistic pathogen. Nature. 2000;406:959–964. doi: 10.1038/35023079. [DOI] [PubMed] [Google Scholar]
- 36.Sung S S, Young J D, Origlio A M, Heiple J M, Kaback H R, Silverstein S C. Extracellular ATP perturbs transmembrane ion fluxes, elevates cytosolic [Ca2+], and inhibits phagocytosis in mouse macrophages. J Biol Chem. 1985;260:13442–13449. [PubMed] [Google Scholar]
- 37.Surprenant A, Rassendren F, Kawashima E, North R A, Buell G. The cytolytic P2Z receptor for extracellular ATP identified as a P2X receptor (P2X7) Science. 1996;272:735–738. doi: 10.1126/science.272.5262.735. [DOI] [PubMed] [Google Scholar]
- 38.Welling G W, Weijer W J, van der Zee R, Welling-Wester S. Prediction of sequential antigenic regions in proteins. FEBS Lett. 1985;188:215–218. doi: 10.1016/0014-5793(85)80374-4. [DOI] [PubMed] [Google Scholar]
- 39.Woods D E, Sokol P A, Bryan L E, Storey D G, Mattingly S J, Vogel H J, Ceri H. In vivo regulation of virulence in Pseudomonas aeruginosa associated with genetic rearrangement. J Infect Dis. 1991;163:143–149. doi: 10.1093/infdis/163.1.143. [DOI] [PubMed] [Google Scholar]
- 40.Zaborina O, Misra N, Kostal J, Kamath S, Kapatral V, El-Azami El-Idrissi M, Prabhakar B S, Chakrabarty A M. P2Z-independent and P2Z receptor-mediated macrophage killing by Pseudomonas aeruginosa isolated from cystic fibrosis patients. Infect Immun. 1999;67:5231–5242. doi: 10.1128/iai.67.10.5231-5242.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zaborina O, Dhiman N, Chen M L, Kostal J, Holder I A, Chakrabarty A M. Secreted products of a nonmucoid Pseudomonas aeruginosa strain induce two modes of macrophage killing: external-ATP-dependent, P2Z-receptor-mediated necrosis and ATP-independent, caspase-mediated apoptosis. Microbiology. 2000;146:2521–2530. doi: 10.1099/00221287-146-10-2521. [DOI] [PubMed] [Google Scholar]
- 42.Zimmermann H. Extracellular purine metabolism. Drug Dev Res. 1996;39:337–352. [Google Scholar]
- 43.Zwikl P, Ng D, Woo K M, Klenk H P, Goldberg A L. An archaebacterial ATPase, homologous to ATPases in the eukaryotic 26 S proteasome, activates protein breakdown by 20 S proteasomes. J Biol Chem. 1999;274:26008–26014. doi: 10.1074/jbc.274.37.26008. [DOI] [PubMed] [Google Scholar]