Abstract
The principal objective of this study was to screen and verify reference genes appropriate for gene expression evaluation during plant growth and development under distinct growth conditions. Nine candidate reference genes were screened based on garlic transcriptome sequence data. RT-qPCR was used to detect the expression levels of the aforementioned reference genes in specific tissues under drought and cold stress. Then, geNorm, NormFinder, BestKeeper, and ReFinder were used to consider the consistency of the expression levels of candidate reference genes. Finally, the stress-responsive gene expression of ascorbate peroxidase (APX) was quantitatively evaluated to confirm the chosen reference genes. Our results indicated that there were variations in the abundance and stability of nine reference gene transcripts underneath cold and drought stress, among which ACT and UBC-E2 had the highest transcript abundance, and 18S rRNA and HIS3 had the lowest transcript abundance. UBC and UBC-E2 were the most stably expressed genes throughout all samples; UBC and UBC-E2 were the most stably expressed genes during cold stress, and ACT and UBC were the most stably expressed genes under drought stress. The most stably expressed genes in roots, pseudostems, leaves, and cloves were EF1, ACT, HIS3, UBC, and UBC-E2, respectively, while GAPDH was the most unstable gene during drought and cold stress conditions and in exclusive tissues. Taking the steady reference genes UBC-E2, UBC, and ACT as references during drought and cold stress, the reliability of the expression levels was further demonstrated by detecting the expression of AsAPX. Our work thereby offers a theoretical reference for the evaluation of gene expression in garlic in various tissues and under stress conditions.
Keywords: garlic, reference gene, normalization, RT-qPCR, gene expression
1. Introduction
Garlic (Allium sativum L.), a medicinal and edible vegetable, is widely cultivated throughout the world. The shallow root system in garlic seedlings causes its weak abilities which are relevant to drought and cold tolerance [1]. Especially in Qinghai plateau, the climate in spring is characterized by dry weather, low rainfall, and continuous low temperature, which results in decreased yield and low quality of garlic. Therefore, drought and cold stress have become the key problems that are hindering plateau garlic production [2] as plants are often restricted from realizing their full genetic potential [3]. Under cold stress, plants often show dwarfed seedlings and yellow leaves [4]; the damage to the plasma membrane is caused by low solute concentration [5,6]. Drought stress may cause the decomposition of photosynthetic pigments and reactive oxygen species accumulation [7,8], which can damage macromolecules and encourage membrane lipid peroxidation [9]. Plants could resist drought and cold stress by enhancing antioxidant systems [10,11,12], including the trigger of differential expression of antioxidant-related gene ascorbate peroxidase (APX) [13,14], transcription of stress-related proteins [15,16,17], and regulation of hormone levels in response to stress [18].
Real time quantitative PCR (RT-qPCR) couples a fluorescent chemical reporter to DNA amplification, thereby monitoring the amount of total product after each PCR cycle and quantitatively analyzing the abundance of transcripts of interest [19]. According to its advantages of outstanding repeatability, excellent sensitivity, and quantitative accuracy [20], it has become an effective method for detecting and quantifying gene expression levels in molecular biology research [21]. Since the quantitative process is easily restricted by the quality of RNA and cDNA, the specificity of primers, and the efficiency of PCR amplification [22], the quantitative analysis of the target gene needs to introduce a stable reference gene for calibration [23,24]. Reference genes used to explore the relative levels of gene expression generally consist of cytoskeletal proteins and essential components involved in the basic biochemical metabolic pathways of cells [25], such as actin (ACT) [26], 18S somatic ribose RNA (18S rRNA), and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) [27]. Although the above genes might be stably expressed under any conditions in theory [28], their expression level is not always consistent with the changes in experimental conditions, plant tissues, and physiological states [29]. Therefore, the selection of appropriate reference genes for the calibration and standardization of expression levels according to specific species and different processing methods is necessary to improve the accuracy of RT-qPCR [30]. At present, geNorm, NormFinder, and BestKeeper are common methods for the screening of stable reference genes and authoritative methods in this part [31,32], which were widely used in the stable reference genes of various plants, such as larch [33] and jujube [34]. Despite the screening methods of reference genes being relatively mature and developed, few studies detail the screening of reference genes in plateau garlic and its specific tissues under different treatments, which greatly limits the efficacy of reference genes in garlic.
Toward the evaluation of garlic reference genes, garlic varieties (A. sativm L. cv. Ershuizao et al.) were used as materials to screen for stable reference genes at different developmental stages and under salt stress [35,36]. However, knowledge of garlic reference genes during drought and cold stresses remains deficient. In this study, nine widely used reference genes were initially selected from the garlic transcriptomic data of our previous laboratory. The expression levels of reference genes in roots, pseudostems, leaves, and cloves under cold and drought stresses were detected by RT-qPCR. The stability and reliability of the selected reference genes were analyzed and validated by evaluation software. Based on the above, our study will provide a foundation for expanding the selection of reference genes in garlic and analyzing the mechanisms of garlic responses to cold and drought stress.
2. Results
2.1. RNA Quality Detection and Primer Specificity Analysis of Reference Genes
All the RNA samples were detected by electrophoresis, and the results (Figure 1A) showed that the 5S, 18S, and 28S rRNA bands were intact without obvious tailing, indicating that the extracted RNA had suitable integrity. RNA concentration and quality were determined, and the A260/A280 and A260/A230 of RNA were between 1.9~2.1 and 2.1~2.3, respectively, which could be used for cDNA synthesis.
Figure 1.
Garlic RNA samples (A) and amplification products of candidate reference genes (B) detected by agarose electrophoresis.
PCR amplification products detected by agarose electrophoresis were performed on the nine candidate reference genes using the cDNA of different tissues at 0 h treatment as the template. The results (Figure 1B) showed that the fragments of each reference gene were between 100 and 200 bp, which was consistent with the expected fragment sizes. Each product was well amplified with no primer dimers and secondary bands evident, indicating that the selected reference gene primers could be used in subsequent experiments.
2.2. Assessment of Primer Amplification Efficiency and Specificity
Based on the garlic transcriptome data, primer sequences for the expression analysis of nine candidate reference genes (ACT, 18S rRNA, HIS3, GAPDH, RPS5, UBC-E2, UBC, UBQ, and EF1) were designed (Table 1). The primers’ generated amplicons of the reference gene were between 97–147 bp. The amplification efficiency (E) was generated via the standard curve, accompanied by a linear relationship (R2 > 0.93); the amplification efficiency was between 83.24% and 157.45% (Table 1).
Table 1.
Information on the candidate reference genes of garlic.
| Genename | Primer Sequence (Forward/Reverse) |
Amplicon Length (bp) |
Tm (℃) | E (%) |
Slope | R2 |
|---|---|---|---|---|---|---|
| ACT | ATTAGTGTCGCCATTCTT | 117 | 54 | 91.25413 | −3.551 | 0.999 |
| Actin | TTGACGCACATTACCATC | |||||
| 18S rRNA(18 S) | CGCTGGTGGCGTAGTTGT | 101 | 58 | 109.1765 | −3.12 | 0.996 |
| 18S ribosomal RNA | TGGGAAGGGTGGTTTGTG | |||||
| HIS3 | CCCGTCACAGAGGAAAGA | 121 | 63 | 157.4409 | −2.435 | 0.936 |
| HistoneH3 | GAGCAGCAGGGATAAGCA | |||||
|
GAPDH
Glyceraldehyde-3-phosphatedehydrogenase |
CCCTGGCAAAGGTGAT | 106 | 53 | 96.06391 | −3.42 | 0.999 |
| AAGGCAGTTGGTGGTG | ||||||
| RPS5 | TACCGACCAGAACCCTA | 103 | 53 | 90.62968 | −3.569 | 0.998 |
| RibosomalproteinS5 | CTGCCTGACGCCTAAC | |||||
| UBC-E2 | CGGTTTGTATGAATGTGC | 97 | 54 | 97.83189 | −3.375 | 0.992 |
| Ubiquitin-conjugating enzyme E2 | TTAGGGTAAGAAAGGAGTTG | |||||
| UBC | TTCGGGTTCGGTTTGTAT | 105 | 56 | 83.23965 | −3.802 | 0.996 |
| Ubiquitin-conjugating enzyme | TTAGGGTAAGAAAGGAGTTGAG | |||||
| UBQ | GGAAGATGGCAGAACG | 139 | 50 | 94.88171 | −3.451 | 0.999 |
| Polyubiquitin | GCACAAGATGAAGGGTA | |||||
| EF1 | GCATAAAGAAGGAGGGT | 147 | 56 | 85.79506 | −3.717 | 0.992 |
| elongationfactor1 | CTGGTTCGGCAGTAAG |
2.3. RT-qPCR Analysis of Candidate Reference Genes in Garlic
2.3.1. Melting Curve Analysis
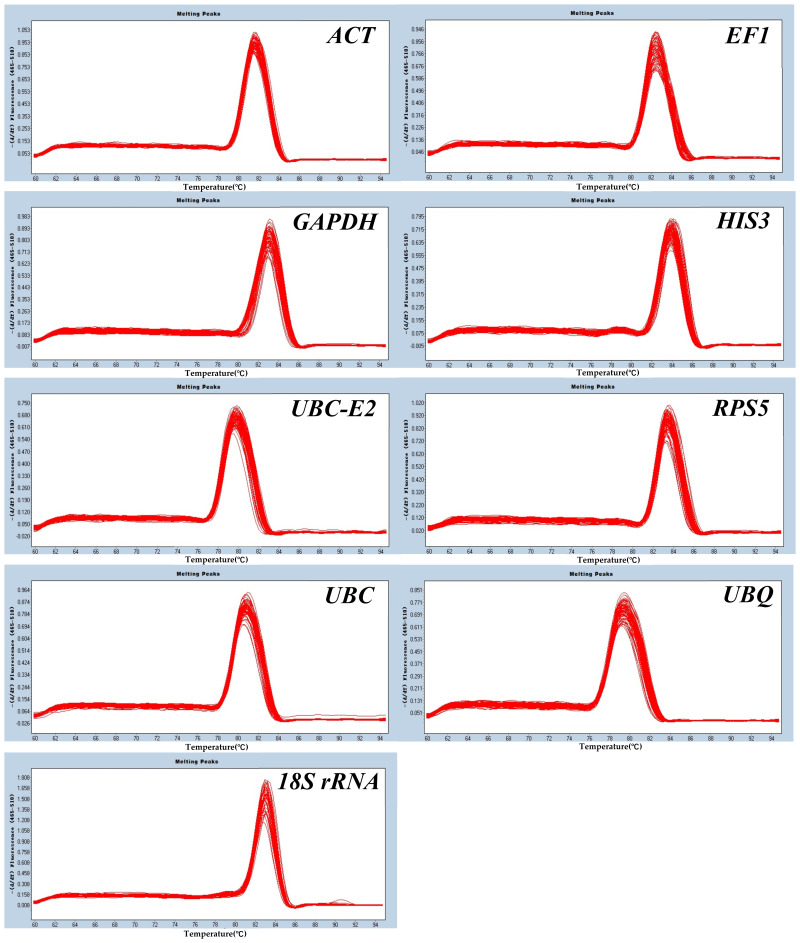
cDNA was used as the template for RT-qPCR amplification and the melting curve of the candidate reference gene was further analyzed. The melting curves of the nine candidate reference genes were all single peaks, indicating that the primers were specific and that the amplification curves were reproducible between samples (Figure 2).
Figure 2.
RT−qPCR melting curves of the nine candidate reference genes in garlic.
2.3.2. Expression Profile Analysis of Candidate Reference Genes
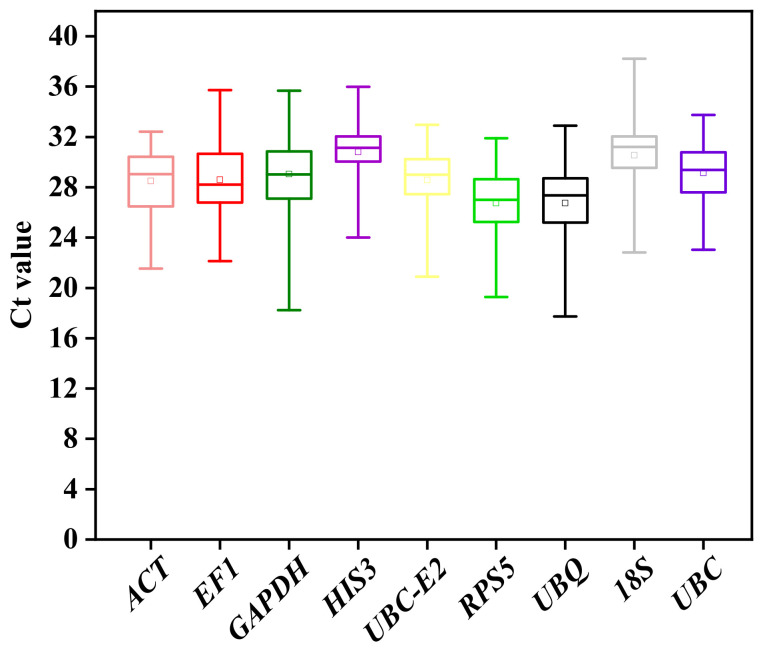
The expression levels of candidate reference genes can be reflected by measuring the cycle threshold (Ct) values based on RT- qPCR. The Ct value is inversely proportional to the gene expression abundance, which indicated that lower Ct value corresponds to higher gene expression abundance. Among all the tested samples under different tissues and stress conditions, the Ct values of nine candidate reference genes were between 24.26 and 34.69. The Ct values of ACT and UBC-E2 were relatively low, while the Ct values of HIS3 and 18S rRNA were relatively high (Figure 3).
Figure 3.
Distribution of Ct values of the nine candidate reference genes in garlic among all the tested samples. The boxes indicate the 25th and 75th percentiles. The line across the box and the inner square in each box indicate the median and mean Ct values, respectively.
2.4. Expression Stability Analysis of the Reference Genes
2.4.1. geNorm Analysis
The geNorm software determines the expression stability of reference genes by calculating the M value (Expression Stability) of nine candidate reference genes under different experimental conditions and in specific tissues. The program uses M = 1.5 as a threshold; the smaller M value indicated higher stability of the candidate reference gene. In Figure 4, the changes in M value in different conditions and tissues were variant. The expression stability values of all reference samples were evaluated and seven candidate reference genes had M values less than 1.5 (UBC-E2 < UBC < RPS5 < EF1 < UBQ < 18S rRNA < HIS3). There were six reference genes with M values lower than 1.5 (UBC-E2 < UBC < 18S rRNA < HIS3 < EF1 < RPS5) and nine genes (UBC-E2 < UBC < ACT < UBQ < 18S rRNA < RPS5 < HI3 < EF1) that met this criterion under cold and drought stress, respectively. The results of stable reference genes in different tissues showed that EF1 and UBC were the most stable in roots; ACT and UBC were the most stable in pseudostems; EF1 and UBC were the most stable in leaves; UBC-E2 and UBC were the most stable in cloves. The stability of GAPDH was generally the worst in different experimental conditions and tissues.
Figure 4.
Mean expression stability values (M) of candidate reference genes. Note: The most stable genes are listed on the right and the most unstable genes are listed on the left.
geNorm determined the optimal number of candidate reference genes by calculating the Vn/n+1 = 0.15 value to obtain more accurate and reliable results. When Vn/n+1 is lower than 0.15, there are no candidate reference genes that meet the requirements for correcting the expression level of the target gene. The Vn/n+1 values of the total samples, samples under drought and cold treatments, roots, leaves, and cloves were all higher than 0.15, and the optimal number of reference genes could not be determined. Therefore, the minimum value of Vn/n+1 should be selected as the optimal inner parameter under the above conditions. It can be seen from Figure 5 that V2/3 = 0.043 < 0.15 in the pseudostem, which indicates that selecting two reference genes in the pseudostem could provide an accurate reference for the detection of the target gene.
Figure 5.
Determination of optimal parameters for candidate reference genes.
2.4.2. NormFinder Analysis
NormFinder determines the stability of the reference gene by the value of gene expression stability (S); the lower S value represented a more suitable reference gene. From the above ranking results, the most stable reference genes in all samples were UBC-E2 and EF1 and the most stable reference genes under cold and drought were HIS3, UBC-E2, EF1, and ACT. Among different tissues, the most stable reference genes in roots, pseudostems, and cloves were ACT, 18S rRNA, UBQ, HIS3, UBC, EF1, and UBC-E2 (Table 2).
Table 2.
Stable values of nine candidate reference genes in different treatments and tissues by NormFinder.
| Rank | Total | Cold Stress | Drought Stress | Root | Pseudostem | Leaf | Clove | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | Gene | Stability | |
| 1 | UBC-E2 | 0.035 | HIS3 | 0.48 | EF1 | 0.566 | ACT | 0.582 | UBQ | 0.063 | UBC | 0.482 | UBC-E2 | 0.7 |
| 2 | EF1 | 0.041 | UBC-E2 | 0.633 | ACT | 0.579 | 18S rRNA | 0.645 | HIS3 | 0.072 | EF1 | 0.527 | UBC | 0.722 |
| 3 | HIS3 | 0.042 | UBC | 0.692 | UBC | 0.607 | EF1 | 0.72 | ACT | 0.072 | UBC-E2 | 0.564 | RPS5 | 0.73 |
| 4 | UBQ | 0.046 | EF1 | 0.832 | UBC-E2 | 0.65 | HIS3 | 0.744 | UBC-E2 | 0.073 | UBQ | 0.637 | EF1 | 0.772 |
| 5 | 18S rRNA | 0.047 | UBQ | 0.901 | RPS5 | 0.657 | UBC-E2 | 0.802 | UBC | 0.083 | RPS5 | 0.657 | UBQ | 0.837 |
| 6 | ACT | 0.05 | ACT | 0.923 | UBQ | 0.658 | UBC | 0.81 | GAPDH | 0.085 | 18S rRNA | 0.784 | 18S rRNA | 0.857 |
| 7 | RPS5 | 0.051 | 18S rRNA | 1.03 | HIS3 | 0.689 | UBQ | 0.923 | EF1 | 0.096 | HIS3 | 0.882 | HIS3 | 0.985 |
| 8 | UBC | 0.062 | RPS5 | 1.132 | 18S rRNA | 0.772 | RPS5 | 1.052 | 18S rRNA | 0.108 | GAPDH | 0.943 | GAPDH | 1.006 |
| 9 | GAPDH | 0.069 | GAPDH | 1.341 | GAPDH | 1.525 | GAPDH | 1.589 | RPS5 | 0.113 | ACT | 1.09 | ACT | 1.009 |
2.4.3. BestKeeper Analysis
BestKeeper evaluates the stability of candidate reference genes by the standard deviation (SD) and coefficient of variation (CV). Lower SD and CV indicate better stability of reference genes. As shown in Table 3, the stabilities of HIS3 and 18S rRNA were the best among all samples; the stabilities of UBC-E2 and HIS3 were the highest under cold stress; the expression of both HIS3 and RPS5 were relatively stable under drought stress. The stability in different tissues of garlic was evaluated and the transcript stability in the root was ranked as ACT and HIS3; the stability of pseudostem was best in 18S rRNA and HIS3; the stability of leaf was best in 18S rRNA and GAPDH, and the stability in cloves was best in UBC-E2 and 18S rRNA. The stability of gene transcripts varied greatly under different experimental conditions, so the reference gene needs to be carefully considered depending on different experimental conditions.
Table 3.
Stable values of nine candidate reference genes in different treatments and tissues in BestKeeper.
| Rank | Total | Cold Stress | Drought Stress | Root | Pseudostem | Leaf | Clove | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Gene | SD | CV | Gene | SD | CV | Gene | SD | CV | Gene | SD | CV | Gene | SD | CV | Gene | SD | CV | Gene | SD | CV | |
| 1 | HIS3 | 0.773 | 2.451 | UBC-E2 | 0.787 | 2.751 | HIS3 | 0.711 | 2.271 | ACT | 0.309 | 1.07 | 18S rRNA | 0.367 | 1.142 | 18S rRNA | 0.344 | 1.073 | UBC-E2 | 0.742 | 2.45 |
| 2 | 18S rRNA | 0.845 | 2.676 | HIS3 | 0.927 | 2.921 | RPS5 | 0.752 | 2.824 | HIS3 | 0.744 | 2.375 | HIS3 | 0.554 | 1.762 | GAPDH | 0.617 | 2.273 | 18S rRNA | 0.784 | 2.478 |
| 3 | UBC-E2 | 1.112 | 3.775 | UBC | 0.933 | 3.23 | 18S rRNA | 0.801 | 2.564 | 18S rRNA | 0.83 | 2.721 | UBC-E2 | 0.621 | 2.137 | HIS3 | 0.625 | 1.982 | UBC | 0.846 | 2.761 |
| 4 | UBC | 1.26 | 4.218 | UBQ | 0.945 | 3.524 | EF1 | 0.858 | 3.047 | UBQ | 1.373 | 5.065 | GAPDH | 0.659 | 2.39 | RPS5 | 0.956 | 3.389 | UBQ | 1.17 | 4.149 |
| 5 | RPS5 | 1.284 | 4.648 | 18S rRNA | 0.97 | 3.081 | ACT | 0.885 | 2.944 | GAPDH | 1.525 | 5.046 | UBC | 0.961 | 3.218 | UBC-E2 | 1.079 | 3.623 | HIS3 | 1.267 | 3.98 |
| 6 | UBQ | 1.321 | 4.747 | RPS5 | 1.329 | 4.887 | UBC-E2 | 0.994 | 3.419 | RPS5 | 1.531 | 5.717 | RPS5 | 1.274 | 4.647 | UBC | 1.12 | 3.726 | RPS5 | 1.311 | 4.672 |
| 7 | ACT | 1.484 | 5.066 | EF1 | 1.513 | 5.357 | UBC | 1.023 | 3.494 | EF1 | 1.588 | 5.592 | UBQ | 1.295 | 4.564 | EF1 | 1.287 | 4.385 | GAPDH | 1.391 | 4.584 |
| 8 | GAPDH | 1.67 | 5.794 | ACT | 1.677 | 6.149 | UBQ | 1.145 | 4.227 | UBC-E2 | 1.609 | 5.6 | ACT | 2.037 | 6.956 | UBQ | 1.413 | 5.113 | ACT | 1.397 | 4.719 |
| 9 | EF1 | 1.712 | 5.84 | GAPDH | 1.883 | 6.604 | GAPDH | 2.046 | 7.101 | UBC | 1.723 | 5.968 | EF1 | 2.042 | 7.092 | ACT | 1.951 | 6.66 | EF1 | 1.596 | 5.195 |
2.4.4. ReFinder Analysis
Finally, ReFinder was used to comprehensively rank reference genes in different treatment conditions and tissues. UBC and UBC-E2 were the most stably expressed genes in all samples, while UBC and UBC-E2 were the most stably expressed genes under cold stress, and ACT and UBC were the most stably-expressed genes under drought stress. In different tissues, the most stably expressed genes in roots, pseudostems, leaves, and cloves were EF1 and ACT, HIS3 and UBC, UBC and EF1, and UBC-E2 and UBC, respectively. GAPDH was the most unstable gene expressed in drought, cold stress, and different tissues (Table 4).
Table 4.
Stability values of nine candidate reference genes in different treatments and tissues in BestKeeper.
| Rank | Total | Cold Stress | Drought Stress | Root | Pseudostem | Leaf | Clove | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Genes | Rank | Genes | Rank | Genes | Rank | Genes | Rank | Genes | Rank | Genes | Rank | Genes | Rank | |
| 1 | UBC | 1.41 | UBC | 1.68 | ACT | 1.57 | EF1 | 1.97 | HIS3 | 1.41 | UBC | 1.50 | UBC-E2 | 1.00 |
| 2 | UBC-E2 | 1.86 | UBC-E2 | 1.73 | UBC | 2.30 | ACT | 2.34 | UBC | 1.86 | EF1 | 2.30 | UBC | 1.68 |
| 3 | RPS5 | 3.94 | HIS3 | 1.86 | UBC-E2 | 2.71 | 18S rRNA | 3.13 | UBC-E2 | 2.06 | UBQ | 3.83 | RPS5 | 3.71 |
| 4 | HIS3 | 3.96 | 18S rRNA | 4.36 | HIS3 | 4.30 | UBC | 3.20 | GAPDH | 4.23 | RPS5 | 4.00 | 18S rRNA | 4.16 |
| 5 | UBQ | 4.05 | EF1 | 4.95 | 18S rRNA | 4.61 | UBC-E2 | 3.46 | 18S rRNA | 4.82 | HIS3 | 4.30. | EF1 | 5.03 |
| 6 | 18S rRNA | 4.36 | UBQ | 5.38 | UBQ | 4.76 | HIS3 | 5.12 | UBQ | 5.48 | GAPDH | 4.56 | UBQ | 5.96 |
| 7 | EF1 | 6.05 | RPS5 | 6.96 | RPS5 | 5.18 | UBQ | 5.18 | ACT | 7.24 | UBC-E2 | 5.23 | HIS3 | 6.05 |
| 8 | ACT | 7.74 | ACT | 7.74 | EF1 | 7.11 | RPS5 | 8.00 | EF1 | 8.24 | 18S rRNA | 6.26 | ACT | 7.20 |
| 9 | GAPDH | 9.00 | GAPDH | 9.00 | GAPDH | 9.00 | GAPDH | 9.00 | RPS5 | 8.45 | ACT | 9.00 | GAPDH | 9.00 |
2.5. Stability Verification of CANDIDATE Reference Genes
To verify the reliability of candidate reference genes, the gene expression of APX was calculated using the identified reference genes. APX, one of the important antioxidant enzymes in plant reactive oxygen species’ metabolism, especially the key enzyme for scavenging H2O2 in the chloroplast, has been shown to be related to stress tolerance. The relatively stable reference genes (UBC-E2 and UBC, and ACT and UBC, respectively) and unstable reference genes (GAPDH) were used to detect the expression of AsAPX in garlic at 12 h under cold and drought stress, thereby verifying the stability and accuracy of the selected reference genes (Figure 6). The expression levels of AsAPX under drought and cold stress were the same when the stable reference genes were used, but the expression levels determined by the unstable reference gene GAPDH were significantly different. Thus, these data further prove the reliability of the experimental results of this study.
Figure 6.
AsAPX expression in different parts of garlic under drought(A) and cold(B)stress.
3. Discussion
With the advancement of molecular biology, research on the expression of functional genes in plants has become a hot topic of research [37]. Although gene expression can be detected by genome and transcriptome sequencing, RT-qPCR is still a vital method due to its advantages of high sensitivity, flexible detection, and excellent efficiency [38,39]. Nevertheless, the accuracy of RT-qPCR detection results is often affected by the specificity of primers, length of amplified products, quality of RNA, and consistent reference genes [40,41]. Meanwhile, the use of common reference genes such as 18S rRNA and ACTIN without screening also reduce the accuracy of quantitative results and even lead to incorrect conclusions [42].
To explore the changes in gene expression levels in organisms, the screening of stable reference genes is essential. geNorm, NormFinder, and BestKeeper were widely used in the evaluation of the transcript stability of reference genes but determining the stability of most reference genes could be dependent on different software parameters [43]. In this study, the most stable reference genes screened by geNorm under cold stress were UBC-E2 and UBC, while HIS3, UBC-E2, and HIS3 were the reliable reference genes as determined by NormFinder and BestKeeper, respectively. geNorm, NormFinder, and BestKeeper found that the most stable reference genes were UBC-E2, UBC, EF1, ACT, HIS3, and RPS5 under drought stress. Similar results were obtained in Cynomorium [44] and Chrysanthemum [45]. To improve the detection accuracy of the target gene and resolve the diversities between different software, Vandesompele [46] recommends using the V value (Pairwise variation value) generated from geNorm as the threshold to further reduce errors by using a combination of multiple reference genes. This program usually takes Vn/n+1 equal to 0.15 as a threshold. When the threshold was less than 0.15, there were no candidate reference genes to satisfy the requirements of correcting the expression of the target gene. Due to 0.15 being an ideal value, which mainly depends on the number of genes and the type of test samples, there were still large differences in the accuracy of screening results [47]. When V = 0.15 was used for calibration in garlic, only the value of V2/3 in cloves was lower than 0.15 and two genes were needed to achieve the purpose of accurate calibration. However, more than two reference genes were required to be used together in different experimental treatments and tissues (Figure 2), which not only amplifies the experimental error but also increases the experimental budget [36]. Therefore, in order to avoid errors caused by differences in software analysis and human selection, integrated analysis by comprehensively evaluating the results obtained from different software was used as an important means to reflect the stability of each reference gene and reduce the differences caused by software evaluation. ReFinder software was used to comprehensively evaluate the results of the three conventional softwares based on previous studies, and the most stably expressed genes under cold and drought stress were UBC and UBC-E2, and ACT and UBC, respectively (Table 4). Some researchers have also used ReFinder technology to evaluate the stability of reference genes in different varieties of pears [48] and rice [49], which means that the reference genes comprehensively screened by ReFinder software were stable and reliable.
In garlic, Liu [35] screened CYP as the most suitable reference gene for abiotic stress conditions but lacked specific reference genes for cold stress. Wang [36] identified ACTIN and SAND as stable reference genes in garlic under salt stress. However, there are still relatively few stable reference genes in garlic under plateau climate stress, which greatly limits genetic resources in garlic for the detection of gene expression levels. In our study, the stable reference genes in garlic under cold and drought stress were UBC-E2, UBC and ACT, and UBC, respectively. Similarly, the UBC and ACTIN genes in Psathyrostachys huashanica under abiotic and biotic stresses were confirmed to be consistently expressed in all samples [50]. Schmidt combined geNorm, NormFinder, BestKeeper, and other software to screen out eight candidate reference genes of tobacco and found that UBC-E2 was a highly stable reference gene in different tissues and throughout the entrained circadian rhythm(s) [51]. Wang screened for the most stable reference gene in Iris germanica L., which was UBC [52]. Actin is a reference gene that has been widely used in many plant species at present [53], which could be proved in Kosteletzkya Virginica’s research [54]. However, actin was demonstrated to be the best-performing reference gene in all test samples under drought stress in this study. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH), as a key component in plant response to abiotic stress, has unique advantages as a probe due to its low homology across phyla [55]. GAPDH was used as a stable reference gene in cotton [56] and Arabidopsis [57], providing an important reference for the expression of key genes. However, in this study, GAPDH had the lowest expression stability in different treatments and tissues, which was consistent with the screening results of reference genes in garlic under salt stress by Wang [36], indicating that GAPDH was not suitable as a reference gene for garlic.
APX is one of the key antioxidant-related genes, which plays the curial role during reactive oxygen species (ROS) signal transduction in plants [58,59,60]. Both Verma [61] and Lee [62] have demonstrated that APX was directly involved in ROS detoxification, protecting cells from oxidative bursts. ROS signal transduction is not steady under abiotic stress, but the reference gene can be stably expressed, which guarantees accurate detection of ROS inducing APX differential expression level [63]. For garlic, Wani [64] found that the transcript expression of cold responsive genes (APX, GR) was detected to be upregulated under cold stress. Fones [65] evaluated the suitability of the selected reference gene by analyzing the expression levels in APX. Compared with the method of Wang [36] to verify the selected reference gene with the garlic AO gene, the stable reference genes in this study were verified by AsAPX and were found to be excellent reference genes in garlic, which is consistent with the identification of reference genes in kiwifruit through APX [66].
4. Conclusions
The identification of reliable reference genes is the prerequisite for qualifying gene expression under specific experimental conditions. In the present study, a systematic analysis validates a set of reference genes by RT-qPCR in garlic various tissues subject to stressful environments. Based on the above results, ACT, UBC, and UBC-E2 were recommended as the most stable reference genes in garlic under drought and cold stress. Current research may help to accurately quantify the target genes in garlic and reveal the molecular mechanisms of garlic stress tolerance.
5. Materials and Methods
5.1. Plant Material
Allium sativum L. variety ‘Ledu Purple Skin Garlic’, as a special Qinghai-plateau vegetable, preserved at the Horticulture Institute of Academy of Agriculture and Forestry Sciences in Qinghai University, Xining, China, was used as experimental material in the present study. Garlic cloves released from dormancy with similar size were selected and planted into plastic pots (top diameter 10cm, bottom diameter 8 cm,) filled with commercial substrate (peat: perlite = 1: 1). Then, the plants were cultured in a growth chamber with a 14-h artificial light (25 °C): 10-h dark (10 °C) cycle and 65–75% relative humidity. Cultivated until the garlic seedling stage (the seedling height reached approximately 10 cm), the treatments were performed as follows: for drought stress, seedlings were cultivated by controlling substrate moisture content (45–55%); for cold stress, seedlings were cultivated at the growth chamber with temperature of 4 °C. Different parts of garlic (root, pseudostem, leaf, clove) were then collected at 0 h, 1 h, 4 h, 12 h, and 1 d after the respective treatments. Each experiment was completed with three replicates, each comprised of five seedlings per treatment. All collected samples were immediately frozen in liquid nitrogen and stored at −80 °C until analyzed.
5.2. Total RNA Isolation and cDNA Synthesis
Total RNA was extracted from frozen samples using the Trizol Reagent (DP405, Tiangen Biotech, Beijing, China). The integrity and quality of extracted RNA were evaluated by 1% agarose gel electrophoresis and a TGem micro-spectrophotometer (OSE-260, Tiangen Biotech, Beijing, China). Only the A260/A280 value of the total RNA of each sample ranging between 1.8–2.1 meet the requirements of subsequent experiments. The above RNA samples as a template referring to the cDNA synthesized were carried out using the Fast Quant cDNA First Strand Synthesis Kit (KR106, Tiangen Biotech Beijing, Beijing, China). The products were stored at −20 °C until analyzed.
5.3. Primer Design
The nine candidate genes that were selected for screening based on their role as reference genes in other plants were aligned with the garlic transcriptome established by our group, including ACT(Actin), EF1(elongationfactor1), UBC-E2(Ubiquitin-conjugating enzyme-E2), GAPDH(Glyceraldehyde-3-phosphatedehydrogenase), HIS3 (Histone H3), RPS5(ribosomalprotein S5), UBC(Ubiquitin-conjugating enzyme), UBQ(Polyubiquitin), 18S rRNA(18S ribosomal RNA). All RT-qPCR primers were designed with the Primer 5.0.
5.4. PCR and RT-qPCR Analysis of Reference Genes
According to the instructions provided by Tiangen Biotech, Beijing, China, for the Super Real Fluorescence Quantitative Premix Reagent-Enhanced Kit (SYBR Green, FP205), we set the mixture into the Bio-RadiQ5 Real-Time Fluorescence Quantitative Instrument for PCR amplification. The reaction mixture was comprised of 10 μL 2×SYBR Green RT-qPCR Master Mix, 8.2 μL ddH2O, 1 μL cDNA, and 0.4 µL each of forward and reverse amplification primers for a final volume of 20 μL. The amplification conditions were as follows: Pre-denaturation at 95 °C for 5 min, denaturation at 95 °C for 10 s, TM (53–63 °C) annealing, extension for 30 s, 40 cycles. A blank control lacking a template was also included at the same time to ensure amplification quality and gene expression levels for each sample were determined based on three replicates. The amplification specificity of each pair of primers was evaluated by 1% agarose gel electrophoresis and melting curve analysis, and the PCR amplification efficiency (E) and correlation coefficient (R2) were calculated using a 5-fold cDNA dilution series.
5.5. Analysis of Reference Gene Expression Stability
The cycle threshold (Ct) value was recorded for each RT-qPCR experiment. Through geNorm [46], NormFinder [67], and BestKeeper [68] analyses, we analyzed the transcript stability of garlic candidate reference genes under drought and cold stress. Among them, GeNorm and NormFinder used the 2-∆∆CT method [37] to convert the CT value into the relative expression value for their respective analysis, while BestKeeper used the original CT value to evaluate consistency. Finally, ReFinder [69] was used for comprehensive evaluation [38] and the most stable reference gene(s) were determined. According to the selected reference genes, the expression patterns of APX during garlic development under drought and cold stress were analyzed to further verify the selected stable reference genes.
Author Contributions
Conceptualization, J.T.; methodology, S.Y. and Q.Z.; formal analysis, Q.W. and C.G.; writing—original draft preparation, Q.W. and C.G.; writing-review and editing, Q.W. and J.T. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
Not applicable.
Conflicts of Interest
All authors have read and approved this version of the article. No conflicts of interest exist in the submission of this manuscript.
Funding Statement
This research was funded by [National Natural Science Foundation of Natural Science Foundation of China] grant number [31960590], [the Chinese Academy of Sciences (CAS) Western Light Program] grant number [2019 1-2] and [the Key Laboratory Project of Qinghai Science & Technology Department] grant number [2022-ZJ-Y01].
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ried K., Frank O., Stocks N. Aged garlic extract reduces blood pressure in hypertensives: A dose–response trial. Eur. J. Clin. Nutr. 2013;67:64–70. doi: 10.1038/ejcn.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhu J.-K. Salt and drought stress signal transduction in plants. Annu. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farahani H.A., Valadabadi S.A., Daneshian J., Shiranirad A.H., Khalvati M.A. Medicinal and aromatic plants farm-ing under drought conditions. J. Hortic. For. 2009;1:086–092. [Google Scholar]
- 4.Ji L., Zhou P., Zhu Y., Liu F., Li R., Qiu Y. Proteomic Analysis of Rice Seedlings Under Cold Stress. Protein J. 2017;36:299–307. doi: 10.1007/s10930-017-9721-2. [DOI] [PubMed] [Google Scholar]
- 5.Feng D.-R., Liu B., Li W.-Y., He Y.-M., Qi K.-B., Wang H.-B., Wang J.-F. Over-expression of a cold-induced plasma membrane protein gene (MpRCI) from plantain enhances low temperature-resistance in transgenic tobacco. Environ. Exp. Bot. 2009;65:395–402. doi: 10.1016/j.envexpbot.2008.12.009. [DOI] [Google Scholar]
- 6.Khan A.A., Vincent J.F. Mechanical damage induced by controlled freezing in apple and potato. J. Texture Stud. 1996;27:143–157. doi: 10.1111/j.1745-4603.1996.tb00065.x. [DOI] [Google Scholar]
- 7.Ma D., Sun D., Wang C., Ding H., Qin H., Hou J., Huang X., Xie Y., Guo T. Physiological Responses and Yield of Wheat Plants in Zinc-Mediated Alleviation of Drought Stress. Front. Plant Sci. 2017;8:860. doi: 10.3389/fpls.2017.00860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razi K., Muneer S. Drought stress-induced physiological mechanisms, signaling pathways and molecular response of chloroplasts in common vegetable crops. Crit. Rev. Biotechnol. 2021;41:669–691. doi: 10.1080/07388551.2021.1874280. [DOI] [PubMed] [Google Scholar]
- 9.Wu D., Yang C., Zhang X., Hou X., Zhang S., Dai X., Zhang X., Igarashi Y., Luo F. Algicidal effect of tryptoline against Microcystis aeruginosa: Excess reactive oxygen species production mediated by photosynthesis. Sci. Total. Environ. 2022;806:150719. doi: 10.1016/j.scitotenv.2021.150719. [DOI] [PubMed] [Google Scholar]
- 10.Taji T., Ohsumi C., Iuchi S., Seki M., Kasuga M., Kobayashi M., Yamaguchi-Shinozaki K., Shinozaki K. Important roles of drought- and cold-inducible genes for galactinol synthase in stress tolerance in Arabidopsis thaliana. Plant J. 2002;29:417–426. doi: 10.1046/j.0960-7412.2001.01227.x. [DOI] [PubMed] [Google Scholar]
- 11.Bartels D., Sunkar R. Drought and Salt Tolerance in Plants. Crit. Rev. Plant Sci. 2005;24:23–58. doi: 10.1080/07352680590910410. [DOI] [Google Scholar]
- 12.Seki M., Umezawa T., Urano K., Shinozaki K. Regulatory metabolic networks in drought stress responses. Curr. Opin. Plant Biol. 2007;10:296–302. doi: 10.1016/j.pbi.2007.04.014. [DOI] [PubMed] [Google Scholar]
- 13.Jiang Y., Watkins E., Liu S., Yu X., Luo N. Antioxidative Responses and Candidate Gene Expression in Prairie Junegrass under Drought Stress. J. Am. Soc. Hortic. Sci. 2010;135:303–309. doi: 10.21273/JASHS.135.4.303. [DOI] [Google Scholar]
- 14.Lü X.-P., Gao H.-J., Zhang L., Wang Y.-P., Shao K.-Z., Zhao Q., Zhang J.-L. Dynamic responses of Haloxylon ammodendron to various degrees of simulated drought stress. Plant Physiol. Biochem. 2019;139:121–131. doi: 10.1016/j.plaphy.2019.03.019. [DOI] [PubMed] [Google Scholar]
- 15.Huang L., Hong Y., Zhang H., Li D., Song F. Rice NAC transcription factor ONAC095 plays opposite roles in drought and cold stress tolerance. BMC Plant Biol. 2016;16:203. doi: 10.1186/s12870-016-0897-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.El-Esawi M.A., Al-Ghamdi A.A., Ali H.M., Ahmad M. Overexpression of AtWRKY30 Transcription Factor Enhances Heat and Drought Stress Tolerance in Wheat (Triticum aestivum L.) Genes. 2019;10:163. doi: 10.3390/genes10020163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamaguchi-Shinozaki K., Shinozaki K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006;57:781–803. doi: 10.1146/annurev.arplant.57.032905.105444. [DOI] [PubMed] [Google Scholar]
- 18.Jogawat A., Yadav B., Chhaya, Lakra N., Singh A.K., Narayan O.P. Crosstalk between phytohormones and secondary metabolites in the drought stress tolerance of crop plants: A review. Physiol. Plant. 2021;172:1106–1132. doi: 10.1111/ppl.13328. [DOI] [PubMed] [Google Scholar]
- 19.Kulcheski F.R., Marcelino-Guimaraes F.C., Nepomuceno A.L., Abdelnoor R.V., Margis R. The use of microRNAs as reference genes for quantitative polymerase chain reaction in soybean. Anal. Biochem. 2010;406:185–192. doi: 10.1016/j.ab.2010.07.020. [DOI] [PubMed] [Google Scholar]
- 20.Wen L., Tan B., Guo W.-W. Estimating transgene copy number in precocious trifoliate orange by TaqMan real-time PCR. Plant Cell, Tissue Organ Cult. (PCTOC) 2012;109:363–371. doi: 10.1007/s11240-011-0101-x. [DOI] [Google Scholar]
- 21.Zhang C., Lei Z., Li Y., Yi L., Shang Y. Identification of Tps2 Used as an Endogenous Reference Gene in Qualitative and Real-time Quantitative PCR Detection of Flammulina velutipes. Food Anal. Methods. 2021;14:2152–2160. doi: 10.1007/s12161-021-02043-y. [DOI] [Google Scholar]
- 22.Bustin S.A., Benes V., Garson J.A., Hellemans J., Huggett J., Kubista M., Mueller R., Nolan T., Pfaffl M.W., Shipley G.L., et al. The MIQE Guidelines: Minimum Information for Publication of Quantitative Real-Time PCR Experiments. Clin. Chem. 2009;55:611–622. doi: 10.1373/clinchem.2008.112797. [DOI] [PubMed] [Google Scholar]
- 23.Martins M.Q., Fortunato A.S., Rodrigues W.P., Partelli F.L., Campostrini E., Lidon F.C., DaMatta F.M., Ramalho J.C., Ribeiro-Barros A.I. Selection and Validation of Reference Genes for Accurate RT-qPCR Data Normalization in Coffea spp. under a Climate Changes Context of Interacting Elevated [CO2] and Temperature. Front. Plant Sci. 2017;8:307. doi: 10.3389/fpls.2017.00307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Nolan T., Hands R.E., Bustin S.A. Quantification of mRNA using real-time RT-PCR. Nat. Protoc. 2006;1:1559–1582. doi: 10.1038/nprot.2006.236. [DOI] [PubMed] [Google Scholar]
- 25.Libault M., Thibivilliers S., Bilgin D.D., Radwan O., Benitez M., Clough S.J., Stacey G. Identification of Four Soybean Reference Genes for Gene Expression Normalization. Plant Genome. 2008;1 doi: 10.3835/plantgenome2008.02.0091. [DOI] [Google Scholar]
- 26.Thomas C., Meyer D., Wolff M., Himber C., Alioua M., Steinmetz A. Molecular characterization and spatial expression of the sunflower ABP1 gene. Plant Mol. Biol. 2003;52:1025–1036. doi: 10.1023/A:1025482432486. [DOI] [PubMed] [Google Scholar]
- 27.Sikand K., Singh J., Ebron J.S., Shukla G.C. Housekeeping Gene Selection Advisory: Glyceraldehyde-3-Phosphate Dehydrogenase (GAPDH) and β-Actin Are Targets of miR-644a. PLoS ONE. 2012;7:e47510. doi: 10.1371/journal.pone.0047510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Radonić A., Thulke S., Mackay I.M., Landt O., Siegert W., Nitsche A. Guideline to reference gene selection for quantitative real-time PCR. Biochem. Biophys. Res. Commun. 2004;313:856–862. doi: 10.1016/j.bbrc.2003.11.177. [DOI] [PubMed] [Google Scholar]
- 29.Ding Y., Sun H., Zhang R., Yang Q., Liu Y., Zang X., Zhang X. Selection of reference gene from Gracilaria lemaneiformis under temperature stress. J. Appl. Phycol. 2015;27:1365–1372. doi: 10.1007/s10811-014-0423-2. [DOI] [Google Scholar]
- 30.Yi S., Lu H., Tian C., Xu T., Song C., Wang W., Wei P., Gu F., Liu D., Cai Y., et al. Selection of Suitable Reference Genes for Gene Expression Normalization Studies in Dendrobium huoshanense. Genes. 2022;13:1486. doi: 10.3390/genes13081486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Yang Q., Yin J., Li G., Qi L., Yang F., Wang R., Li G. Reference gene selection for qRT-PCR in Caragana korshinskii Kom. under different stress conditions. Mol. Biol. Rep. 2014;41:2325–2334. doi: 10.1007/s11033-014-3086-9. [DOI] [PubMed] [Google Scholar]
- 32.Wang Q., Ishikawa T., Michiue T., Zhu B.L., Guan D.W., Maeda H. Stability of endogenous reference genes in postmortem human brains for normalization of quantitative real-time PCR data: Comprehensive evaluation using geNorm, NormFinder, and BestKeeper. Int. J. Leg. Med. 2012;126:943–952. doi: 10.1007/s00414-012-0774-7. [DOI] [PubMed] [Google Scholar]
- 33.Zhang D., Li J., Li B., Li C., Chen X., Ouyang K. Internal Reference Gene Selection under Different Hormone Stresses in Multipurpose Timber Yielding Tree Neolamarckia cadamba. Forests. 2020;11:1014. doi: 10.3390/f11091014. [DOI] [Google Scholar]
- 34.Bu J., Zhao J., Liu M. Expression Stabilities of Candidate Reference Genes for RT-qPCR in Chinese Jujube (Ziziphus jujuba Mill.) under a Variety of Conditions. PloS ONE. 2016;11:e0154212. doi: 10.1371/journal.pone.0154212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liu M., Wu Z., Jiang F. Selection and validation of garlic reference genes for quantitative real-time PCR normalization. Plant Cell Tissue Organ Cult. (PCTOC) 2015;122:435–444. doi: 10.1007/s11240-015-0780-9. [DOI] [Google Scholar]
- 36.Wang G., Tian C., Wang Y., Wan F., Hu L., Xiong A., Tian J. Selection of reliable reference genes for quantitative RT-PCR in garlic under salt stress. Peerj. 2019;7:e7319. doi: 10.7717/peerj.7319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 38.Xie F., Sun G., Stiller J., Zhang B. Genome-Wide Functional Analysis of the Cotton Transcriptome by Creating an Integrated EST Database. PLoS ONE. 2011;6:e26980. doi: 10.1371/journal.pone.0026980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jiang B., Xie D., Liu W., Peng Q., He X. De Novo Assembly and Characterization of the Transcriptome, and Development of SSR Markers in Wax Gourd (Benicasa hispida) PLoS ONE. 2013;8:e71054. doi: 10.1371/journal.pone.0071054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Udvardi M.K., Czechowski T., Scheible W.-R. Eleven Golden Rules of Quantitative RT-PCR. Plant Cell. 2008;20:1736–1737. doi: 10.1105/tpc.108.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gutierrez L., Mauriat M., Guénin S., Pelloux J., Lefebvre J.-F., Louvet R., Rusterucci C., Moritz T., Guerineau F., Bellini C., et al. The lack of a systematic validation of reference genes: A serious pitfall undervalued in reverse transcription-polymerase chain reaction (RT-PCR) analysis in plants. Plant Biotechnol. J. 2008;6:609–618. doi: 10.1111/j.1467-7652.2008.00346.x. [DOI] [PubMed] [Google Scholar]
- 42.Yu M., Liu D., Li Y.-C., Sui C., Chen G.-D., Tang Z.-K., Yang C.-M., Hou D.-B., Wei J.-H. Validation of reference genes for expression analysis in three Bupleurum species. Biotechnol. Biotechnol. Equip. 2019;33:154–161. doi: 10.1080/13102818.2018.1557556. [DOI] [Google Scholar]
- 43.Huggett J.F., Dheda K., Bustin S., Zumla A. Real-time RT-PCR normalisation; strategies and considerations. Genes Immun. 2005;6:279–284. doi: 10.1038/sj.gene.6364190. [DOI] [PubMed] [Google Scholar]
- 44.Marum L., Miguel A., Ricardo C.P., Miguel C. Reference Gene Selection for Quantitative Real-time PCR Normalization in Quercus suber. PLoS ONE. 2012;7:e35113. doi: 10.1371/annotation/13c5a136-9db4-43a9-aad3-f73acb064d0a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Qi S., Yang L., Wen X., Hong Y., Song X., Zhang M., Dai S. Reference Gene Selection for RT-qPCR Analysis of Flower Development in Chrysanthemum morifolium and Chrysanthemum lavandulifolium. Front. Plant Sci. 2016;7:287. doi: 10.3389/fpls.2016.00287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.1. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Hong S.-Y., Seo P.J., Yang M.-S., Xiang F., Park C.-M. Exploring valid reference genes for gene expression studies in Brachypodium distachyonby real-time PCR. BMC Plant Biol. 2008;8:112. doi: 10.1186/1471-2229-8-112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wang G., Guo Z., Wang X., Guan S.L., Gao H., Qi K., Gu C., Zhang S. Identification and testing of reference genes for qRT-PCR analysis during pear fruit development. Biologia. 2022;77:2763–2777. doi: 10.1007/s11756-022-01087-7. [DOI] [Google Scholar]
- 49.Wu J., Fan H., Hu Y., Guo H., Lin H., Jiao Y., Lu Z., Du S., Liu X., Shahid M.Q. Identification of stable pollen development related reference genes for accurate qRT-PCR analysis and morphological variations in autotetraploid and diploid rice. PLoS ONE. 2021;16:e0253244. doi: 10.1371/journal.pone.0253244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shen C., Li J., Zhang X., Wei C., Wu Y. Transcriptome-based identification and validation of optimal reference genes for quantitative real-time PCR normalisation in Psathyrostachys huashanica. Plant Gene. 2021;27:100306. doi: 10.1016/j.plgene.2021.100306. [DOI] [Google Scholar]
- 51.Schmidt G.W., Delaney S.K. Stable internal reference genes for normalization of real-time RT-PCR in tobacco (Nicotiana tabacum) during development and abiotic stress. Mol. Genet. Genom. 2010;283:233–241. doi: 10.1007/s00438-010-0511-1. [DOI] [PubMed] [Google Scholar]
- 52.Wang Y., Zhang Y., Liu Q., Tong H., Zhang T., Gu C., Liu L., Huang S., Yuan H. Selection and validation of appropriate reference genes for RT-qPCR analysis of flowering stages and different genotypes of Iris germanica L. Sci. Rep. 2021;11:1–10. doi: 10.1038/s41598-021-89100-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wang G.-L., Tian C., Jiang Q., Xu Z.-S., Wang F., Xiong A.-S. Comparison of nine reference genes for real-time quantitative PCR in roots and leaves during five developmental stages in carrot (Daucus carota L.) J. Hortic. Sci. Biotechnol. 2016;91:264–270. doi: 10.1080/14620316.2016.1148372. [DOI] [Google Scholar]
- 54.Tang X., Wang H., Shao C., Shao H. Reference Gene Selection for qPCR Normalization of Kosteletzkya virginica under Salt Stress. BioMed Res. Int. 2015;2015:1–8. doi: 10.1155/2015/823806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Stürzenbaum S.R., Kille P. Control genes in quantitative molecular biological techniques: The variability of invariance. Comp. Biochem. Physiol. Part B: Biochem. Mol. Biol. 2001;130:281–289. doi: 10.1016/S1096-4959(01)00440-7. [DOI] [PubMed] [Google Scholar]
- 56.Wang M., Wang Q., Zhang B. Evaluation and selection of reliable reference genes for gene expression under abiotic stress in cotton (Gossypium hirsutum L.) Gene. 2013;530:44–50. doi: 10.1016/j.gene.2013.07.084. [DOI] [PubMed] [Google Scholar]
- 57.Jin Y., Liu F., Huang W., Sun Q., Huang X. Identification of reliable reference genes for qRT-PCR in the ephemeral plant Arabidopsis pumila based on full-length transcriptome data. Sci. Rep. 2019;9:8408. doi: 10.1038/s41598-019-44849-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dudziak K., Zapalska M., Börner A., Szczerba H., Kowalczyk K., Nowak M. Analysis of wheat gene expression related to the oxidative stress response and signal transduction under short-term osmotic stress. Sci. Rep. 2019;9:1–14. doi: 10.1038/s41598-019-39154-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Liu J., Wang J., Lee S., Wen R. Copper-caused oxidative stress triggers the activation of antioxidant enzymes via ZmMPK3 in maize leaves. PLoS ONE. 2018;13:e0203612. doi: 10.1371/journal.pone.0203612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gupta S., Dong Y., Dijkwel P.P., Mueller-Roeber B., Gechev T.S. Genome-Wide Analysis of ROS Antioxidant Genes in Resurrection Species Suggest an Involvement of Distinct ROS Detoxification Systems during Desiccation. Int. J. Mol. Sci. 2019;20:3101. doi: 10.3390/ijms20123101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Verma D., Upadhyay S.K., Singh K. Characterization of APX and APX-R gene family in Brassica juncea and B. rapa for tolerance against abiotic stresses. Plant Cell Rep. 2021;41:571–592. doi: 10.1007/s00299-021-02726-0. [DOI] [PubMed] [Google Scholar]
- 62.Lee H.-J., Yang H.Y., Choi J.-I. Study of functional verification to abiotic stress through antioxidant gene transformation of Pyropia yezoensis (Bangiales, Rhodophyta) APX and MnSOD in Chlamydomonas. J. Microbiol. Biotechnol. 2018;28:1217–1224. doi: 10.4014/jmb.1802.02024. [DOI] [PubMed] [Google Scholar]
- 63.Shi J., Liu M., Shi J., Zheng G., Wang Y., Wang J., Chen Y., Lu C., Yin W. Reference gene selection for qPCR in Ammopiptanthus mongolicus under abiotic stresses and expression analysis of seven ROS-scavenging enzyme genes. Plant Cell Rep. 2012;31:1245–1254. doi: 10.1007/s00299-012-1245-9. [DOI] [PubMed] [Google Scholar]
- 64.Wani M.A., Jan N., Qazi H.A., Andrabi K.I., John R. Cold stress induces biochemical changes, fatty acid profile, antioxidant system and gene expression in Capsella bursa pastoris L. Acta Physiol. Plant. 2018;40:167. doi: 10.1007/s11738-018-2747-z. [DOI] [Google Scholar]
- 65.Fones H., Preston G.M. Reactive oxygen and oxidative stress tolerance in plant pathogenic Pseudomonas. FEMS Microbiol. Lett. 2012;327:1–8. doi: 10.1111/j.1574-6968.2011.02449.x. [DOI] [PubMed] [Google Scholar]
- 66.Petriccione M., Mastrobuoni F., Zampella L., Scortichini M. Reference gene selection for normalization of RT-qPCR gene expression data from Actinidia deliciosa leaves infected with Pseudomonas syringae pv. actinidiae. Sci. Rep. 2015;5:16961. doi: 10.1038/srep16961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Andersen C.L., Jensen J.L., Ørntoft T.F. Normalization of real-time quantitative reverse transcription-PCR data: A model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64:5245–5250. doi: 10.1158/0008-5472.CAN-04-0496. [DOI] [PubMed] [Google Scholar]
- 68.Pfaffl M.W., Tichopad A., Prgomet C., Neuvians T.P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel–based tool using pair-wise correlations. Biotechnol. Lett. 2004;26:509–515. doi: 10.1023/B:BILE.0000019559.84305.47. [DOI] [PubMed] [Google Scholar]
- 69.Xie F., Xiao P., Chen D., Xu L., Zhang B. miRDeep Finder: A miRNA analysis tool for deep sequencing of plant small RNAs. Plant Mol. Biol. 2012;80:75–84. doi: 10.1007/s11103-012-9885-2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.