Abstract
The severe acute respiratory syndrome coronavirus 2 main protease (SARS-CoV-2-Mpro) plays an essential role in viral replication, transcription, maturation, and entry into host cells. Furthermore, its cleavage specificity for viruses, but not humans, makes it a promising drug target for the treatment of coronavirus disease 2019 (COVID-19). In this study, a fragment-based strategy including potential antiviral quinazolinone moiety and glutamine- or glutamate-derived peptidomimetic backbone and positioned nitro functional groups was used to synthesize putative Mpro inhibitors. Two compounds, G1 and G4, exhibited anti-Mpro enzymatic activity in a dose-dependent manner, with the calculated IC50 values of 22.47 ± 8.93 μM and 24.04 ± 0.67 μM, respectively. The bio-layer interferometer measured real-time binding. The dissociation kinetics of G1/Mpro and G4/Mpro also showed similar equilibrium dissociation constants (KD) of 2.60 × 10−5 M and 2.55 × 10−5 M, respectively, but exhibited distinct association/dissociation curves. Molecular docking of the two compounds revealed a similar binding cavity to the well-known Mpro inhibitor GC376, supporting a structure−function relationship. These findings may open a new avenue for developing new scaffolds for Mpro inhibition and advance anti-coronavirus drug research.
Keywords: peptidomimetic, quinazolinone, main protease Mpro, SARS-CoV-2, targeted covalent inhibitor
1. Introduction
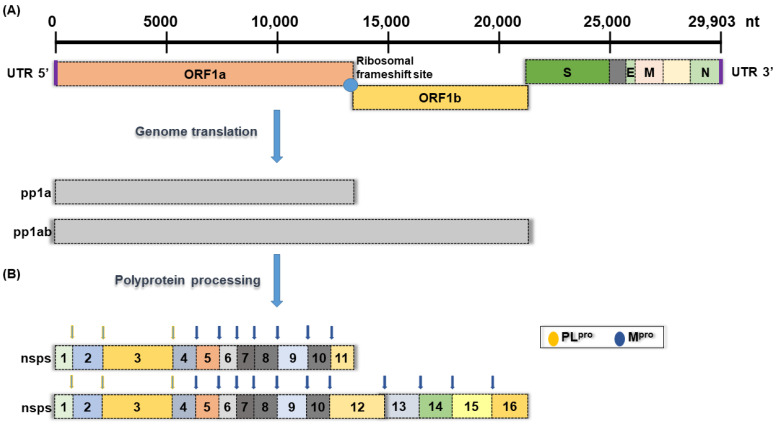
The 2019−present global pandemic of coronavirus disease (COVID-19) has brought permanent changes to human daily life and interpersonal interactions around the world, as well as serious impacts and loss of economic activity. The disease is caused by a novel coronavirus called severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), which is more contagious than the closely related 2002 severe acute respiratory syndrome coronavirus (SARS-CoV) and 2012 Middle East respiratory syndrome coronavirus (MERS-CoV) infections [1]. Among various coronaviruses, SARS-CoV-2 and SARS-CoV enter host cells by binding to angiotensin-converting enzyme 2 (ACE 2), while MERS-CoV binds to dipeptidyl peptidase 4 (DPP4) receptors [2,3,4]. Coronaviruses are a group of enveloped single-stranded positive-sense RNA viruses with genomes ranging from 26 kb to 32 kb, encoding at least six open reading frames (ORFs) (Figure 1) [5]. Once inside the cell, genomic RNA is translated into nonstructural proteins (nsps) from two open reading frames (ORFs), namely ORF1a and ORF1b, and structural proteins from the rest of the RNA genome. ORF1a produces polyprotein 1a (pp1a) and is cleaved into 11 nsps. At the same time, a −1 ribosomal frameshift occurs upstream of the ORF1a stop codon and allows continued translation to ORF1b to generate a large polyprotein 1ab (pp1ab), which is cleaved into 15 nsps. Proteolytic cleavage is mediated by the viral proteases nsp3 and nsp5, which have a papain-like protease domain (PLpro) and a 3C-like protease domain (Mpro or 3CLpro), respectively (Figure 1) [5,6].
Figure 1.
Schematic presentation of the SARS-CoV-2 genome organization. (A) Full-length genomic RNA (29,903 nt) that serves as an mRNA, containing ORF1a, ORF1b, and nine subgenomic RNAs. (B) Schematic representation of non-structural polyprotein cleavage sites. There are two viral proteases: a papain-like protease (PLpro) cleaves virus nonstructural polyprotein at three sites, and the other main protease (Mpro) recognizes and cleaves the virus non-structural polyprotein at 11 sites.
Current treatments for COVID-19 mainly focus on conserved proteins and enzymes, such as the spike protein (S), main proteases (Mpro or 3CLpro, nsp5), papain-like protease (PLpro), RNA-dependent RNA polymerase (RdRP, nsp12), and nucleoside-triphosphate hydrolase and helicase (NTPase/helicase, nsp13). For example, effective vaccines from Pfizer and Moderna, as well as antiviral drugs such as remdesivir and molnupiravir (MK-4482 and EIDD-2801), have been developed and have been widely available since the end of 2020 [7,8]. However, the failure of the public to comply with regulations and containment protocols, as well as the high mutation rate of the virus, has spawned multiple variants, namely Alpha, Beta, Gamma, Delta, and Omicron, which have higher transmission rates and/or antigen escape capabilities, resulting in breakthrough infections. Therefore, new treatments are still needed to reduce the risk of the progression of COVID-19 [7,8].
Main protease (Mpro), also called 3C-like protease, is involved in its own replication, transcription, and maturation and has a role in viral entry into host cells [9,10]. It has unique substrate specificity and recognizes and cleaves 11 conserved glutamine residues (Leu-Gln↓(Ser, Ala, Gly)) in polyproteins. No known human protease has this substrate specificity, so the inhibition of Mpro activity blocks the replication of many coronaviruses with no adverse effect on human host cells. The essential functions of Mpro in the viral life cycle, conserved sequence and structural suitability, unique substrate recognition profile, and role in host cell entry make Mpro a promising drug target for the treatment of COVID-19. Up-to-date, many potential Mpro inhibitors have been screened based on structures, fragments, in silico, and databases [11] and references therein. On 21 December 2021, the FDA issued Emergency Use Authorization for Paxlovid, the first oral antiviral drug for the treatment of mild to moderate COVID-19 in adults and pediatric patients. Paxlovid consists of nirmatrelvir (PF-07321332), which targets the Mpro to stop viral replication, and ritonavir, which slows the former’s breakdown and helps it stay longer in the body at higher concentrations [12]. However, the use of Paxlovid with certain other drugs may cause a decrease in its own activity or an increase in blood levels of the other drugs, which may be harmful. In addition, the drug is also contraindicated in patients with severely reduced renal or hepatic function or in pregnant women. This suggests an urgent need to discover more Mpro inhibitors as drugs for COVID-19.
A compound design that integrates fragments with various functional structures has become a powerful tool for drug discovery. Quinazolinones are heterocyclic compounds bearing a carbonyl group on the C4N2 ring and are constituents of approximately 150 natural alkaloids that have shown attractive pharmacological activities, including antibacterial activity [13], antifungal activity [14], antimalarial activity [15], antiviral activity [13], anticancer activity [16], and sedative effects [17]. For example, febrifugine and its derivatives have been used to treat malaria, cancer, fibrosis, and inflammatory disease [18]. In parallel, the previous elucidation of putative Mpro inhibitors has revealed structural features such as peptidomimetic or amide structures and/or structural features containing aza, nitrile, amino, or nitro moieties as binding moieties to active site cysteine residues [19]. For example, the dipeptide-based Mpro inhibitor GC376 is a broad-spectrum anti-coronavirus drug [20,21]. Furthermore, an example of the formation of a covalent nitrosothiol adduct by the active site Cys191 of Mycobacterium tuberculosis isocitrate lyase (ICL) with 3-nitropropionic acid shows that appropriately positioned weakly electrophilic groups forming covalent adducts with active site nucleophilic residues of proteins is an effective and rapid strategy for the development of designed inhibitors [22,23,24,25]. Combining a quinazolinone-derived antiviral activity, a non-human glutamine-specific recognition mode, and a nitro-directed covalent binding to the cysteine active site may yield highly active and specific Mpro inhibitors. In this study, we created a series of quinazolinone derivative compounds containing glutamine- or glutamate-derived peptidomimetic backbone and nitro/amino-functional derivatives to mimic Mpro substrate specificity and evaluated their effects on the inhibition of Mpro enzymatic activity. The results show that the synthesized compounds have obvious inhibitory activity against Mpro and their molecular docking results have better binding energies than GC376 and are similar to the binding sites of the active region of the GC376−Mpro complex.
2. Materials and Methods
2.1. Synthesis of G Compounds
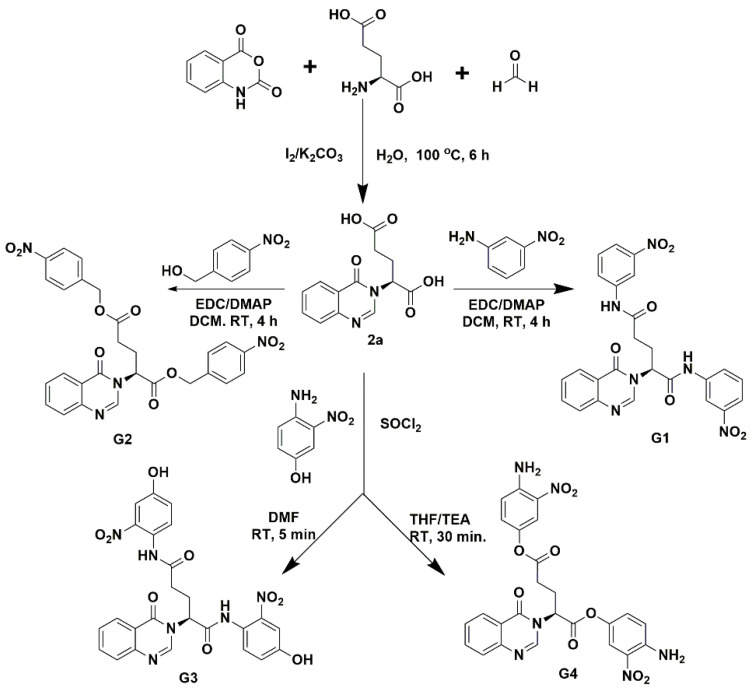
The synthesis of G compounds, G1–G4, is through intermediate 2a, that is, isatoic anhydride (1 mmol, 0.16 g), L-glutamic acid (1.2 mmol, 0.177 g), potassium carbonate (4 mmol, 0.55 g), and 10 mL H2O were mixed in a round bottom flask at room temperature for 30 min, then iodine (1 mmol, 0.25 g) and formaldehyde (10 mmol, 0.37 mL) were added, and the mixtures were stirred at reflux for 6 h. After the reaction, the excess I2 was removed with 5 mL of saturated Na2S2O3, the reaction was partially purified, and the solution was neutralized to pH = 3 with a 10% HCl solution. The reaction mixture was extracted with 20 mL of ethyl acetate for 5 times, the organic layer was washed with a saturated NaCl solution, and the water was removed with anhydrous MgSO4. After the filtration and extraction of the organic layer, the crude yellow-orange product was obtained (234 mg, 85% yield).
To synthesize G1, 2a (0.5 mmol, 138.12 mg), 4-dimethylaminopyridine (DMAP) (0.5 mmol, 61.09 mg), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (1.5 mmol, 232.87 mg) were added to 10 mL of dichloromethane (DCM), cooled to 4 °C and stirred for 60 min before adding 3-nitroanilline (1 mmol, 138.14 mg), and the solution was stirred at room temperature for 4 h. The reaction solution was evaporated under vacuum, and the product was re-dissolved in 20 mL of ethyl acetate (EA) and then washed 3 times with 6 mL of ddH2O. The solvent in the organic layer was evaporated under vacuum, and the crude product was purified by column chromatography with an EA:Hex volume ratio of 2:1. The yield of G1 was 70%.
1H NMR (400 MHz, DMSO-d6): δ 10.83 (s, 1H), 10.36 (s, 1H), 8.60 (t, J = 2.2 Hz, 1H), 8.51–8.45 (m, 2H), 8.13–8.08 (m, 1H), 7.99–7.92 (m, 2H), 7.86–7.79 (m, 2H), 7.74 (dd, J = 8.5, 2.0 Hz, 1H), 7.68 (d, J = 8.1 Hz, 1H), 7.62 (t, J = 8.2 Hz, 1H), 7.52 (td, J = 8.1, 2.1 Hz, 2H), 5.62 (dd, J = 10.0, 5.2 Hz, 1H), 2.66 (p, J = 6.9 Hz, 2H), 2.48–2.39 (m, 2H). 13C NMR (151 MHz, DMSO-d6) δ 170.80, 168.64, 160.78, 148.29 (d, J = 9.4 Hz), 147.86, 147.01, 140.50, 140.03, 135.03, 130.69, 130.43, 127.59, 126.73, 126.20, 125.32, 121.66, 118.81, 118.00, 114.44, 113.48, 57.60, 33.25, 26.04. The calculated electrospray ionization-high resolution mass (ESI-HRMS) of G1 (C25H20N6O7) was 516.1393 g/mol. The experimental peak of m/z = 517.1472 was considered as [M + H]+.
To synthesize G2, 2a (0.5 mmol, 138.12 mg), 4-dimethylaminopyridine (DMAP) (0.5 mmol, 61.09 mg), and 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDC) (1.5 mmol, 232.87 mg) were added to 10 mL of dichloromethane (DCM), cooled to 4 °C and stirred for 60 min before adding 4-nitrobenzyl alcohol (1 mmol, 153.40 mg), and the solution was stirred at room temperature for 4 h. The reaction solution was evaporated under vacuum, and the product was re-dissolved in 20 mL of ethyl acetate (EA) and then washed 3 times with 6 mL of ddH2O. The solvent in the organic layer was evaporated under vacuum, and the crude product was purified by column chromatography with an EA:Hex volume ratio of 2:1. The yield of G2 was 75%.
G2: 1H NMR (400 MHz, DMSO-d6) δ 8.42 (s, 1H), 8.25–8.04 (m, 5H), 7.87 (t, J = 7.4 Hz, 1H), 7.71 (d, J = 8.1 Hz, 1H), 7.57 (dd, J = 8.5, 2.4 Hz, 5H), 5.37 (s, 1H), 5.33 (s, 2H), 5.12 (s, 2H), 2.57 (dd, J = 16.1, 1.4 Hz, 4H). 13C NMR (151 MHz, DMSO-d6) δ 172.19, 169.09, 160.59, 147.97, 147.64, 147.58, 147.49, 144.17, 143.74, 135.31, 128.94, 128.91, 127.90, 127.74, 126.63, 123.92, 121.72, 65.96, 64.90, 58.91, 30.47, 24.56. The expected ESI-HRMS of G2 (C27H22N4O9) was about 546.1387 g/mol. The experimental peak in (m/z = 547.1458) was considered as [M + H]+.
Synthesis of G3: In a 50 mL round bottom flask containing 5 mL of DMF, 2a (0.5 mmol, 137.5 mg) and 4-nitro-3-aminophenol (1 mmol, 154.1 mg) were added followed by 100 μL of thionyl chloride via a syringe. The solution was stirred at room temperature for 5 min and then quenched with 10 mL of water. The solution was stirred for 30 min, and the precipitate was washed with 6 mL of water. The crude product was purified by silica gel column chromatography with an elution solution (EA:Hex volume ratio = 3:1). The yield of G3 was 38%.
1H NMR (400 MHz, DMSO-d6) δ 10.40 (s, 1H), 10.32 (s, 1H), 10.21 (s, 1H), 9.90 (s, 1H), 8.36 (s, 1H), 8.18 (dd, J = 8.2, 1.5 Hz, 1H), 7.89–7.84 (m, 1H), 7.71 (dt, J = 8.2, 1.0 Hz, 1H), 7.58 (ddd, J = 8.1, 7.1, 1.2 Hz, 1H), 7.37 (d, J = 8.9 Hz, 1H), 7.29 (d, J = 2.8 Hz, 1H), 7.25–7.21 (m, 2H), 7.12–7.08 (m, 1H), 7.03 (dd, J = 8.8, 2.8 Hz, 1H), 5.56 (d, J = 9.5 Hz, 1H), 2.34 (q, J = 8.4, 5.8 Hz, 4H). 13C NMR (151 MHz, DMSO-d6) δ 170.21, 168.10, 160.68, 155.65, 155.11, 147.84, 146.71, 144.24, 144.05, 135.08, 128.64, 128.13, 127.65, 126.87, 122.76, 122.19, 121.76, 121.47, 121.31, 111.08, 56.81, 32.31, 29.46. The expected ESI-HRMS of G3 (C25H20N6O9) was about 548.1292 g/mol. The experimental peak in (m/z = 549.1363) was considered as [M + H]+.
To obtain compound G4, 2a (0.5 mmol, 137.5 mg) was added in a 50 mL two-necked round bottom flask containing 5 mL of DCM under argon. Then, thionyl chloride (100 µL) was added into the solution by a syringe, and the solution was kept at room temperature for 4 h. After removed the solvent, a dark brown oil product was obtained. The product was dissolved in 5 mL of a solution with a THF:TEA ratio of 1:1 and then cooled to 4 °C before adding 4-nitro-3-aminephenol (0.5 mmol, 77 mg). The solution was kept at room temperature for 30 min before quenched with 6 mL of ddH2O. The product was extracted with EA (10 mL) and washed with ddH2O (6 mL). Following the removal of the solvent, the crude product was purified with column chromatography with an elution solution (EA:Hex volume ratio = 2:1). The yield of G4 was 42%.
1H NMR (400 MHz, Methylene Chloride-d2) δ 8.32 (d, J = 8.0 Hz, 1H), 8.15 (s, 1H), 7.86–7.78 (m, 3H), 7.76 (d, J = 8.0 Hz, 1H), 7.56 (s, 1H), 7.19 (dd, J = 9.1, 2.8 Hz, 1H), 7.13 (dd, J = 9.1, 2.7 Hz, 1H), 6.85 (dd, J = 9.1, 6.0 Hz, 2H), 6.11 (s, 4H), 5.39 (dd, J = 9.2, 5.5 Hz, 1H), 2.91–2.84 (m, 1H), 2.79–2.68 (m, 2H), 2.62–2.55 (m, 1H). The expected mass of G4 (C25H20N6O9) was about 548.1292 g/mol. 13C NMR (151 MHz, DMSO-d6) δ 171.68, 168.52, 160.71, 148.06, 147.72, 145.08, 144.82, 139.23, 139.04, 135.39, 131.10, 130.76, 129.36, 128.02, 127.82, 126.67, 121.75, 120.62, 120.27, 58.98, 30.46, 24.51. The ESI-HRMS experimental peak in (m/z = 549.1366) was considered as [M + H]+.
2.2. Molecular Cloning and Protein Expression of Mpro
The SARS-CoV-2 Mpro gene from Addgene was constructed on the vector pGEX-5X, which was linked to a glutathione S-transferase (GST) tag at the N-terminus of the recombinant Mpro protein. The recombinant plasmid pGEX-5X−SARS-CoV-2−Mpro was transformed into E. coli BL21 (DE3) for protein expression. Cell cultures were spread on LB agar plates containing 100 μg/mL ampicillin and incubated overnight at 37 °C. The single colony was picked and transferred to a 3 mL LB medium tube supplemented with 100 μg/mL ampicillin and incubated at 37 °C at 220 rpm for 16 h. The overnight culture was diluted into 1 L LB broth (volume ratio: 1:100) and incubated at 37 °C and at 220 rpm until OD600 reached 0.6–0.7. Isopropyl thio-β-Dthiogalactopyranoside (IPTG) (0.5 mM) was added to induce Mpro protein expression for 10 h at 16 °C and 180 rpm. Cells were pelleted at 6500 × g for 30 min at 4 °C and resuspended in 20 mL lysis buffer for protein purification.
The suspension was sonicated for 1 h at a 30% energy using a Vibra-Cell™ Ultrasonic Liquid Processors VCX 500, pulsing 1 s “on” and 3 s “off” on the ice. Cell lysate was centrifuged at 10,000× g for 40 min at 4 °C to separate the supernatant and the pellet. The supernatant was filtered through a 0.45 µm pore size filter to obtain a crude solution. To purify Mpro, the crude solution was mixed with 1 mL of Glutathione Sepharose 4 Fast Flow resin and incubated for 12 h at 4 °C by shaking. Afterwards, the resin was washed 10 times with 10 mL of lysis buffer and re-suspended in 500 µL of lysis buffer and 10 µL of Factor Xa. The mixture was incubated at 4 °C for 36 h. The Mpro was collected by flow-through fractions and analyzed by SDS-PAGE.
2.3. In Vitro Mpro Inhibition Assay
Mpro activity assays were performed in a 384-well black flat-bottomed microtiter plate (Thermo Scientific™ Nunc™) in a final volume of 25 μL. Non-GST-tagged Mpro protein was added at a final concentration of 50 nM at 37 °C with various concentrations (3.125, 6.25, 12.5, 25, 50, and 100 μM) of compounds and assay buffer (final concentration: 20 mM Tris, 100 mM NaCl, 1% DTT, 1% EDTA, and 1% DMSO) for 30 min. The FRET substrate DABCYL-KTSAVLQSGFRKME-EDANS (50 µM) was added, and the solution was incubated at 37 °C for 1 h. Blank wells had the same compound concentrations as the substrate but did not contain Mpro protein. Inhibition was calculated by comparison to control wells to which no inhibitor was added. Fluorescence signals (excitation/emission: 355 nm/460 nm) of the released EDANS were measured using a fluorometer (Fluoroskan Ascent FL). IC50 values were determined by nonlinear regression (GraphPad Prism 8.0.1). For calculations, 100% active enzyme was assumed.
2.4. Bio-Layer Interferometry Binding Kinetics Assay
Label-free bio-layer interferometry (BLI) assays were performed by the Octet K2 two-channel system (FortéBio) at the Center for Emergent Functional Matter Science, National Yang Ming Chiao Tung University. BLI measurements were performed at a shaking speed of 1000 rpm and a temperature of 30 °C. A phosphate buffer with 0.1% Tween-20 (PBS-t) was a kinetic buffer. To prepare Mpro binding test probes, Ni-NTA optical fiber probes were run at baseline for 60 s, loaded into 200 µL of 50 µg/mL Mpro-His diluted in PBS-T for 600 s and then run again at baseline in PBS-T for 60 s. The Mpro-loaded probes were stored in PBS-T at 4 °C until kinetic analysis. For kinetic binding assays, serial dilutions of five concentrations of G compounds in PBS-T were added to one row of wells in a black polypropylene 96-well microplate (Greiner Bio-one), and one row of wells filled with PBS-T was used as a reference control in parallel. One assay cycle consisting of 60 s of baseline normalization in PBS-T and 150 s of association in compound solution, followed by 150 s of dissociation in PBS-T and 30 s of regeneration in 10 mM glycine buffer (pH 1.7) was performed for each compound concentration with Mpro-loaded probes and blank probes. BLI results were analyzed using FortéBio Data Analysis High Throughput 12.0. The curves were aligned with the dissociation step, the y-axis was aligned with the last 5 s of the baseline step, and the last 5 s of the association step was considered steady-state. Savitzky−Golay filtering was performed on the corrected curve to remove high-frequency noise from the data. Specific binding to Mpro was subtracted from the observed blank probes and reference control curves by selecting the “Double References” mode. A 1:1 binding model was assumed in the binding kinetics analysis.
2.5. Molecular Docking
Molecular docking of G compounds was performed using iGemdock software and following the instructions provided by the authors. The structures of G1−G4 were drawn with ChemDraw 12.0 software and converted into Mol files with ChemBio3D Ultra software. The Mpro X-ray structure was retrieved from the Protein Data Bank (https://www.rcsb.org/structure/6W63 accessed on 7 July 2021) in the PDB format. For the docking experiments, amino acid residues including His41, Cys44, Leu141, Asn142, Gly143, Ser144, Cys145, His163, His164, Met165, Glu166, Arg188, and Gln189 were used as active sites. Following ligand and binding site preparation, virtual screening, post-screening analysis, and pharmacological interactions analysis, the docking conformation of the ligand was determined by selecting the pose with the lowest binding free energy. Structural analysis of system and graphics were performed using PyMOL2 2.3.3 (Accelrys Software Inc., San Diego, CA, USA) and Discovery Studio.
3. Results and Discussion
3.1. Synthesis of Fragment-Based G-Compounds
Two types of fragment-based compounds were synthesized, with one glutamine-derived and one glutamate-derived peptidomimetic backbone (Figure 2). Additionally, either the side chain amide group of glutamine or the side chain carboxyl group of glutamic acid of the peptidomimetic backbones was attached to a nitro-functionalized phenyl derivative, because nitro groups of synthetic compounds often show binding interactions in predicted docking poses. The synthesized compounds (G1–G4) were separated by column chromatography and structurally characterized by high-resolution electrospray ionization mass spectrometry (HPLC/HR-ESI-MS) and 1H/13C nuclear magnetic resonance (NMR). These compounds were tested for their inhibitory effects on Mpro enzymatic activity.
Figure 2.
Synthesis of fragment-based compounds containing glutamine- or glutamic acid-derived peptidomimetic backbone, quinazolinone moiety, and nitro-functionalized phenyl derivatives.
3.2. Mpro Inhibition Assay
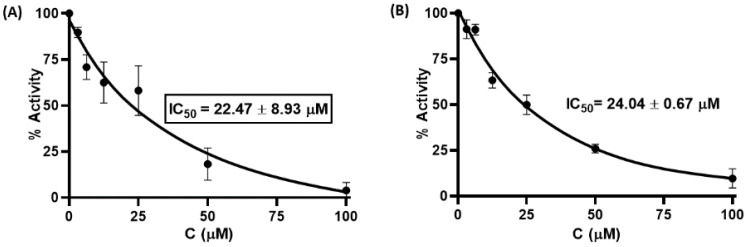
The effect of the synthesized compounds on Mpro inhibitory activity was examined in an in vitro Förster resonance energy transfer (FRET) assay using Dabcyl-KNSTLQSGLRKE-Edans as the fluorogenic substrate peptide. This 12-amino acid fluorescent quenching paired peptide carries Dabcyl at the N-terminus and Edans at the C-terminus, which were paired, and when uncleaved, most of the energy emitted by Edans was quenched by Dabcyl. Recognition and cleavage of the peptide fragment by Mpro resulted in the separation of the two compounds and the release of fluorescence by Edans, which can be excited at 355 nm and detected at 460 nm. In parallel, GC376, a preclinical cysteine protease inhibitor that binds to the Mpro of the feline coronavirus (FCoV) was used as a positive control to validate the assay [21]. The G1–G4 compounds were initially screened at 50 μM in an assay buffer (20 mM Tris, 100 mM NaCl, 1% DTT, 1% EDTA, and 1% DMSO) at a Mpro concentration of 50 nM. The results showed that two compounds (G1 and G4) exhibited more than 50% inhibitory activity against Mpro. We next quantified the inhibitory activities of different inhibitor concentrations (100, 50, 25, 12.5, 6.25, and 3.125 μM). As shown in Figure 3, G1 and G4 inhibited Mpro enzymatic activity in a dose-dependent manner with calculated IC50 values of 22.47 ± 8.93 μM and 24.04 ± 0.67 μM. These results suggest that glutamine- or glutamate-derived peptidomimetic backboned quinazolinone derivatives, combined with nitro-functionalized phenyl derivatives on the side chain or main chain, may play an important role in inhibiting Mpro enzymatic activity.
Figure 3.
Inhibitory activity of serial dilutions of G1 (A) and G4 (B) against SARS-CoV-2 Mpro.
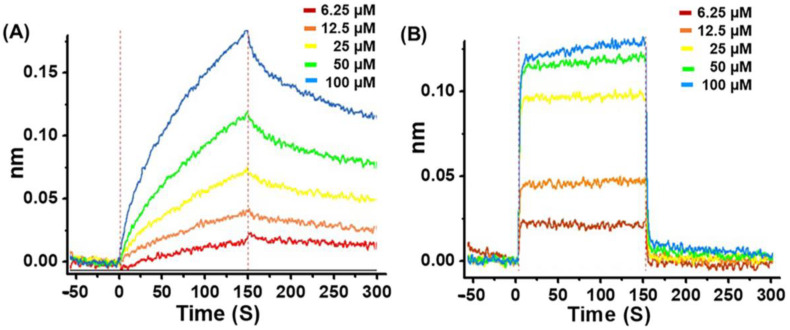
To determine the inhibitory interaction of G1 or G4 with Mpro, the kinetics of G1/Mpro and G4/Mpro association and dissociation were measured using the bio-layer interferometry (BLI) technique, an optical biosensing technique for real-time analysis biomolecular interactions without fluorescent labels [26,27]. Figure 4 shows the real-time binding and dissociation BLI kinetics of G1/Mpro and G4/Mpro. Both displayed a similar equilibrium dissociation constant (KD = koff/kon), at 2.60 × 10−5 M and 2.55 × 10−5 M, respectively, but exhibited distinct association/dissociation curves (Figure 4 and Table 1). G1 bound logarithmically to the Mpro-loaded probe in the binding segment of the kinetic cycle and slowly dissociated from the probe during the dissociation segment (Figure 4A). In contrast, G4 bound strongly to the Mpro-loaded probe in the first 5 s of the association segment, rapidly reached steady-state and dissociated from the probe in the first 5 s of the dissociation segment (Figure 4B). The kon and koff values for G1 and G4, respectively, also demonstrate the same trend. These results suggest that G1 may be a long-term potent inhibitor to Mpro, while G4 is a fast-acting inhibitor to the protease.
Figure 4.
Bio-layer interferometry analysis of G1 and G4 with Mpro. (A) Association and dissociation data of G1 with Mpro. (B) Association and dissociation data of G4 with Mpro.
Table 1.
Bio-layer interferometry analysis data of G1/Mpro and G4/Mpro. KD = koff/kon; kon—association constant; koff—dissociation constant; R2—coefficient of determination.
| KD (M) | kon (1/Ms) | koff (1/s) | R2 | |
|---|---|---|---|---|
| G1 | 2.60 × 10−5 ± 1.49 × 10−7 | 1.25 × 102 ± 4.99 × 10−1 | 3.25 × 10−3 ± 1.33 × 10−5 | 0.9913 |
| G4 | 2.55 × 10−5 ± 7.59 × 10−7 | 1.62 × 104 ± 3.50 × 102 | 4.13 × 10−1 ± 8.44 × 10−3 | 0.971 |
3.3. Pharmacological and Bioavailability of the Synthesized Compounds
The G1–G4 were evaluated for physicochemical properties and bioavailability for potential oral active drugs using the Adsorption, Distribution, Metabolism and Excretion (ADME) assay from SwissADME (Table 2) [28]. Although G1–G4 do not satisfy Lipinski’s rule of five due to their molecular weights exceeding 500 Da, the computational physicochemical properties can be compensated by intramolecular H-bonding and formulation that allow the significant expansion of the molecular weight (MW), the polar surface area (PSA), and the hydrogen bond acceptor atom (HBA) [20,29,30,31]. For example, there are many macrolides and peptide structures such as erythronolides, leucomycins, and vancomycins of antibacterial, antiviral, antifungal, immunosuppressive, and anticancer agents with an MW of >500 Da and high PSAs and HBAs that do not satisfy Lipinski’s rule of five but still observe excellent human bioavailability [29,30,31]. In addition, the hepatitis C virus NS3/4A protease inhibitor designed with the weak heptapeptide leader also has a linear or macrocyclic peptidomimetic structure with a molecular weight of >700 Da [32]. One explanation is that passive permeability across cell membranes is inversely proportional to the hydrodynamic radius of the compound; that is, it depends on the size and the shape of the compound. Thus, although the pharmacological data for the above compounds are beyond the rule of five, with poor solubility, cell permeability, metabolism, and toxicity, this can be compensated by intramolecular H-bonding and formulation that allow the significant expansion of the MW, the PSA, and the HBA [20,29,31 and references therein].
Table 2.
Physico-chemical properties of the synthesized compounds in terms of the drug likeness and the bioavailability score.
| Name | Lipinski’s Rules | |||||
|---|---|---|---|---|---|---|
| MW <500 |
HBA <10 |
HBD ≤5 |
Mlog P ≤4.15 |
Lipinski’s Volations | Bioavailability Score | |
| G1 | 516.46 | 8 | 2 | 1.63 | 2 | 0.17 |
| G2 | 546.48 | 10 | 0 | 2.31 | 2 | 0.17 |
| G3 | 548.46 | 10 | 4 | −0.13 | 2 | 0.17 |
| G4 | 548.46 | 10 | 2 | 0.41 | 2 | 0.17 |
3.4. Molecular Docking
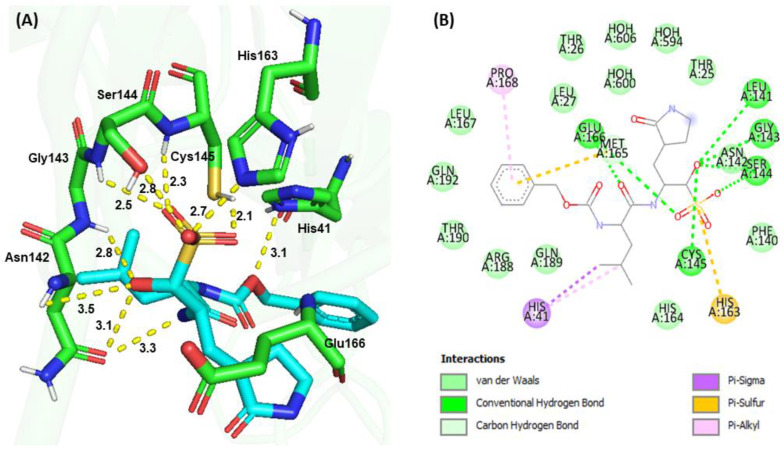
Molecular docking is a popular drug discovery method often used to predict protein-ligand affinity and the stability of interactions before conducting experiments. To elucidate the structure−function relationship of Mpro inhibition with synthesized compounds, docking simulations of GC376, G1, and G4 were performed and compared with the crystal structure of the GC376−Mpro complex [20,21]. Similar binding pocket and active site cavity packing, electrostatic, hydrogen bonding, and hydrophobic interactions were observed between the molecular docking and the X-ray structure of the GC376−Mpro complex, supporting the accuracy of the docking model (Figure 5). A binding affinity analysis indicated that GC376 bound to the active site cavity of Mpro with a binding energy of −158.75 kJ/mol. The ligand GC376 bound to the active site pocket Gly143-Ser144-Cys145 oxyanion hole and forms conserved H-bond interactions with the backbone amide donors of His163 and Glu166. Additionally, Cys145 interacted with His41 to form a catalytic dyad and formed hydrogen bonds and hydrophobic interactions with amino acid residues Thr25, His41, Phe140, Leu141, Asn142, Gly143, Ser144, Cys145, His163, His164, Met165, Glu166, Leu167, Pro168, Arg188, Gln189, Thr190, and Gln192 in the active site cavity. The glutamine surrogate ring at the P1 position of GC376 was embedded into the S1 pocket and formed an H-bond with the Nε of the imidazole ring of His163 and the side chain carboxyl group of Glu166. The Leu at the P2 position was inserted into the S2 hydrophobic pocket consisting of Arg40, His41, Met49, Tyr54, Met165, Arg188, and Gln189, as reported by Fu et al. [20]. The carbonyl group on the benzyl ring at the P3 position of GC376 was also inserted into the S1 binding site to form an H-bond with the backbone amide group of Glu166.
Figure 5.
Molecular docking of GC376 and SARS-CoV-2 Mpro. (A) 3D interactions between GC376 (Cyan) and Mpro (Green). (B) 2D interactions, including H-bonding, hydrophobic, π−sulfur, π−π, and π−alkyl interactions between GC376 and Mpro, identified by Discovery Studio.
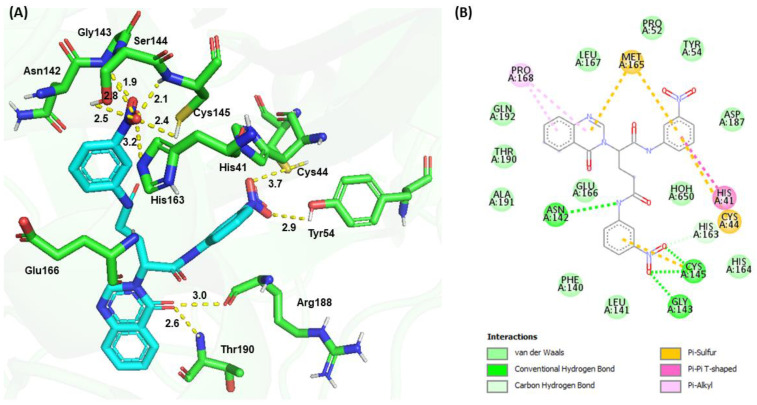
The molecular docking of G1 showed a binding energy of −187.80 kJ/mol. The −NO2 (1) group at the P1 position of G1 formed H-bonds with the side chain sulfhydryl group (3.7 Å) of Cys44 and the phenolic group (2.9 Å) of Tyr54, while the −NO2 (2) group at the P2 position formed several H-bond interactions, including the main chain amide groups of Gly143 (1.9 Å), Ser144 (2.8 Å), and Cys145 (2.1 Å), the side chain sulfhydryl group (2.4 Å) of Cys145, and the hydroxyl group of Ser144 (2.5 Å), as well as the Nε of the imidazole ring (3.2 Å) of His163. The Nγ of the quinazolinone ring formed an H-bond with the backbone carbonyl group of Glu166 (Figure 6). The carbonyl group of the quinazolinone ring interacted with the backbone carbonyl group (3.0 Å) of Arg188 and formed an H-bond with the backbone amide group (2.6 Å) of Thr190. Additional π−sulfur interactions were observed between the quinazolinone ring and Met165, the aromatic ring at the P1 position and Cys44, and the aromatic ring at the P2 position and Cys145. The phenyl group at P1 interacted with the imidazole ring of His41 through a π−π interaction. Pro168 formed π−alkyl interactions with the quinazolinone ring.
Figure 6.
Molecular docking of G1 and SARS-CoV-2 Mpro. (A) 3D interaction between G1 (cyan) and Mpro (green). (B) 2D interactions between G1 and Mpro, including H-bonding, hydrophobic, π−sulfur, π−π, and π−alkyl interactions, as determined by Discovery Studio.
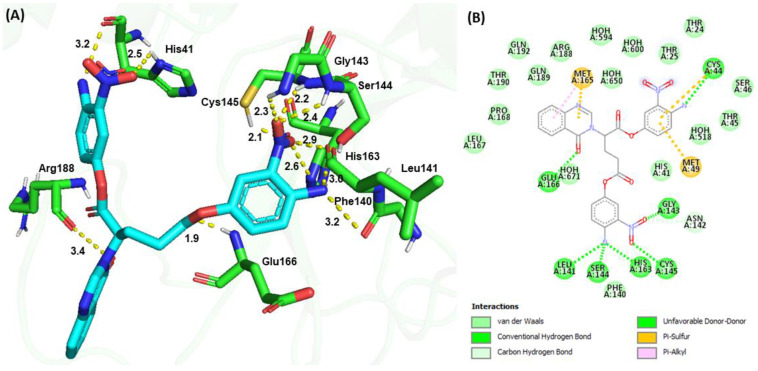
Molecular docking revealed a binding energy of −188.67 kJ/mol for G4 and a similar binding configuration to G1. The −NO2 (1) group and −NH2 (1) at the P1 position formed H-bonds with the Nδ of the imidazole ring (2.5 Å) and the carbonyl group (3.2 Å) of His41, respectively. The −NO2 (2) group at the P2 position formed H-bonds with the backbone amide groups of Gly143 (2.3 Å), backbone amide (2.4 Å), and carbonyl (2.9 Å) groups of Ser144; the backbone amide group (2.2 Å) and the side chain sulfhydryl group (2.1 Å) of Cys145; and the Nε (2.6 Å) of the imidazole ring of His163. The −NH2 (2) group at the P2 position formed an H-bond with the backbone carbonyl group (3.0 Å) of Leu141 and the backbone carbonyl group (3.2 Å) of Phe140. The carboxylate group of the peptidomimetic backbone formed an H-bond with the backbone amide group (1.9 Å) of Glu166 (Figure 7). The carbonyl group of the quinazolinone ring interacted with the backbone carbonyl group (3.4 Å) of Arg188. Additional π−sulfur interactions were observed between the quinazolinone ring and Met165 and between the aromatic ring at the P1 position and Cys44 and Met49. The phenyl moiety of the quinazolinone ring exhibited π−alkyl interactions with Met165. G4 also exhibited numerous van der Waals interactions with Thr24, Thr25, His41, Thr45, Ser46, Met49, Phe140, Leu167, Pro168, Arg188, Gln189, Thr190, and Gln192.
Figure 7.
Molecular docking of G4 and SARS-CoV-2 Mpro. (A) 3D H-bond interactions between G4 (cyan) and Mpro (green). (B) 2D interactions between G4 and Mpro, including H-bond, hydrophobic, π−sulfur, and π−π interactions, as determined by Discovery Studio.
Numerous in silico Mpro inhibitors have been identified; however, only a few in vitro experiments have been performed to validate virtual screening results [33,34,35]. Meanwhile, two clinical approved drugs, GSK-256066 and bicalutamide, showed more than 30% Mpro inhibitory activity at a concentration of 50 μM [36]. Coelho et al. reported a biochemical high-throughput screening of a 2,400-drug library and identified 13 inhibitors with IC50 values between 0.2 and 23 μM [37]. GC376 is a pre-clinical inhibitor against feline infectious peritonitis coronavirus (FIPV) that inhibits Mpro in vitro with an IC50 of 0.15 ± 0.03 μM [20,21]. However, the side effects of GC376, such as delayed adult teeth development in animals, prevent it from being approved by the FDA as a Mpro inhibitor [38]. In this study, a series of glutamine- or glutamate-derived peptidomimetic backbone with quinazolinone moieties and nitro-functionalized derivatives were synthesized, and their inhibitory effects on the Mpro protease of SARS-CoV-2 were investigated. Among the synthesized G-compounds, G1 and G4 were determined to have good IC50 inhibitory activity in an approximately 20 μM range, indicating a comparable performance to the published results, although their oral bioavailability predictions exceeded that of Lipinski’s rule of five.
Previous studies have shown that the putative Mpro inhibitor GC376 binds to the active site pocket Gly143-Ser144-Cys145 oxyanion hole and forms conserved H-bond interactions with the backbone amide donors of His163 and Glu166 [20,21]. Molecular docking of G1 and G4 with Mpro showed that both compounds showed similar binding configurations, namely the P1 moiety pointed to His41 and Cys44, the P2 moiety pointed to the Gly143-Ser144-Cys145 oxyanion hole, and formed electrostatic and hydrogen bonding interactions with amino acid residues around the cavity. Although −NO2 groups are used relatively conservatively in drug development due to their metabolic instability, nitro-containing ligands have been increasingly used as biological probes with appropriately positioned cysteine and acidic side chains to form covalent inhibitors with acceptable potencies and no apparent toxicity [23,25]. Interestingly, the −NO2 group at the P1 position pointed to Cys44, while the −NO2 group at the P2 position pointed to Cys145, which is reminiscent of the hypothesis of the formation of a targeted covalent inhibitor from an appropriately positioned weak electrophilic group and the active site nucleophilic residues of proteins. Perhaps the simultaneous introduction of −NO2 functional groups at the P1 and P2 positions could form stable bidentate chelating interactions with active site residues, thereby facilitating ligand binding and synergistically increasing inhibitory activity. Consistent with the observations was a dramatic drop of inhibitory activity when the nitrophenyl group at the P2 position was deleted. The quinazolinone moiety at the P3 position formed a π−sulfur or π−alkyl interaction with Met165, Glu166, Arg188, or Thr190 and enhanced its binding affinity.
4. Conclusions
Using a fragment-based strategy including a potential antiviral quinazolinone moiety, a glutamine- or glutamate-derived peptidomimetic backbone, and positioned nitro-functionalized phenyl groups to form targeted covalent inhibitors, we successfully synthesized and identified G1 and G4 to have in vitro Mpro inhibitory activity with IC50 values of 22.47 ± 8.93 μM and 24.04 ± 0.67 μM, respectively. G1/Mpro and G4/Mpro displayed similar KDs values of 2.60 × 10−5 M and 2.55 × 10−5 M, respectively, but exhibited different association/dissociation curves. The molecular docking results of G1 and G4 suggest that they bind to the same active region as GC376 and may form targeted covalent ligand-protein complexes. These results support the new scaffold as a candidate for Mpro inhibition and advance anti-coronavirus drug research. Experimental validation to further confirm the inhibitory potential of these compounds against COVID-19 is ongoing.
Acknowledgments
This work was financially supported by the Ministry of Science and Technology, Taiwan (grant No. MOST 111-2634-F-A49-007), the Center for Emergent Functional Matter Science and the Center for Intelligent Drug Systems and Smart Bio-devices (IDS2B) of National Yang Ming Chiao Tung University from The Featured Areas Research Center Program within the framework of the Higher Education Sprout Project by the Ministry of Education (MOE) in Taiwan.
Author Contributions
H.-N.-H.G. and F.-P.C. contributed equally. Conceptualization, H.-N.-H.G. and T.-K.W.; investigation, H.-N.-H.G., F.-P.C., C.-Y.C. and S.-C.C.; data analysis, S.-C.H. and T.W.; supervision, B.-T.-B.H. and H.-C.L.; writing, T.-K.W. All authors have read and agreed to the published version of the manuscript.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Conflicts of Interest
The authors declare no competing financial interest.
Funding Statement
This research was funded by Ministry of Science and Technology, Taiwan grant No. MOST 111-2634-F-A49-007.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Gordon D.E., Hiatt J., Bouhaddou M., Rezelj V.V., Ulferts S., Braberg H., Jureka A.S., Obernier K., Guo J.Z., Batra J., et al. Comparative host-coronavirus protein interaction networks reveal pan-viral disease mechanisms. Science. 2020;370:eabe9403. doi: 10.1126/science.abe9403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Fehr A.R., Perlman S. Coronaviruses: An overview of their replication and pathogenesis. In: Maier H.J., Bickerton E., Britton P., editors. Coronaviruses. Springer; New York, NY, USA: 2015. pp. 1–23. Methods in Molecular Biology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ge X.Y., Li J.L., Yang X.L., Chmura A.A., Zhu G., Epstein J.H., Mazet J.K., Hu B., Zhang W., Peng C. Isolation and characterization of a bat SARS-like coronavirus that uses the ACE2 receptor. Nature. 2013;503:535–538. doi: 10.1038/nature12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lu R., Zhao X., Li J., Niu P., Yang B., Wu H., Wang W., Song H., Huang B., Zhu N., et al. Genomic characterisation and epidemiology of 2019 novel coronavirus: Implications for virus origins and receptor binding. Lancet. 2020;395:565–574. doi: 10.1016/S0140-6736(20)30251-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ullrich S., Nitsche C. The SARS-CoV-2 main protease as drug target. Bioorg. Med. Chem. Lett. 2020;30:127377. doi: 10.1016/j.bmcl.2020.127377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yang H., Rao Z. Structural biology of SARS- CoV-2 and implications for therapeutic development. Nat. Rev. Microbiol. 2021;19:685–700. doi: 10.1038/s41579-021-00630-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Saha A., Sharma A.R., Bhattacharya M., Sharma G., Lee S.-S., Chakraborty C. Probable molecular mechanism of remdesivir for the treatment of COVID-19: Need to Know more. Arch. Med. Res. 2020;51:585–586. doi: 10.1016/j.arcmed.2020.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Painter W.P., Holman W., Bush J.A., Almazedi F., Malik M., Eraut N.C.J.E., Morin M.J., Szewczyk L.J., Painter G.R. Human Safety, Tolerability, and Pharmacokinetics of Molnupiravir, a Novel Broad-Spectrum Oral Antiviral Agent with Activity against SARS-CoV-2. Antimicrob. Agents Chem. 2021;65:e02428-20. doi: 10.1128/AAC.02428-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Du Q.S., Wang S.Q., Zhu Y., Wei D.Q., Guo H., Sirois S., Chou K.C. Polyprotein cleavage mechanism of SARS-CoV Mpro and chemical modification of the octapeptide. Peptides. 2004;25:1857–1864. doi: 10.1016/j.peptides.2004.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jain R., Mujwar S. Repurposing metocurine as main protease inhibitor to develop novel antiviral therapy for COVID-19. Struct. Chem. 2020;36:2487–2499. doi: 10.1007/s11224-020-01605-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mengist H.M., Dilnessa T., Jin T. Structural Basis of Potential Inhibitors Targeting SARS-CoV-2 Main Protease. Front. Chem. 2021;9:622898. doi: 10.3389/fchem.2021.622898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Li J., Lin C., Zhou X., Zhong F., Zeng P., Yang Y., Zhang Y., Yu B., Fan X., McCormick P.J., et al. Structural Basis of the Main Proteases of Coronavirus Bound to Drug Candidate PF-07321332. J. Virol. 2022;96:e0201321. doi: 10.1128/jvi.02013-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khodarahmi G.A., Rahmani Khajouei M., Hakimelahi G.H., Abedi D., Jafari E., Hassanzadeh F. Antibacterial, antifungal and cytotoxic evaluation of some new 2,3-disubstituted 4(3H)-quinazolinone derivatives. Res. Pharm. Sci. 2012;7:151–158. [PMC free article] [PubMed] [Google Scholar]
- 14.Khan I., Zaib S., Batool S., Abbas N., Ashraf Z., Iqbal J., Saeed A. Quinazolines and quinazolinones as ubiquitous structural fragments in medicinal chemistry: An update on the development of synthetic methods and pharmacological diversification. Bioorg. Med. Chem. 2016;24:2361–2381. doi: 10.1016/j.bmc.2016.03.031. [DOI] [PubMed] [Google Scholar]
- 15.Birhan Y.S., Bekhit A.A., Hymete A. In vivo antimalarial evaluation of some 2,3-disubstituted-4(3H)-quinazolinone derivatives. BMC Res. Notes. 2015;8:589. doi: 10.1186/s13104-015-1578-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toledo-Sherman L., Deretey E., Slon-Usakiewicz J.J., Ng W., Dai J.R., Foster J.E., Redden P.R., Uger M.D., Liao L.C., Pasternak A., et al. Frontal affinity chromatography with MS detection of EphB2 tyrosine kinase receptor. 2. Identification of small-molecule inhibitors via coupling with virtual screening. J. Med. Chem. 2005;48:3221–3230. doi: 10.1021/jm0492204. [DOI] [PubMed] [Google Scholar]
- 17.Gupta A., Kashaw S.K., Jain N., Rajak H., Soni A., Stables J. Design and synthesis of some novel 3-[5-(4-substituted) phenyl-1, 3, 4-oxadiazole-2yl]-2-phenylquinazoline-4 (3 H)-ones as possible anticonvulsant agent. Med. Chem. Res. 2011;20:1638–1642. doi: 10.1007/s00044-010-9475-2. [DOI] [Google Scholar]
- 18.Keller T.L., Zocco D., Sundrud M.S., Hendrick M., Edenius M., Yum J., Kim Y.J., Lee H.K., Cortese J.F., Wirth D., et al. Halofuginone and other febrifugine derivatives inhibit prolyl-tRNA synthetase. Nat. Chem. Biol. 2012;8:311–317. doi: 10.1038/nchembio.790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yamamoto K.Z., Yasuo N., Sekijima M. Screening for Inhibitors of Main Protease in SARS-CoV-2: In Silico and In Vitro Approach Avoiding Peptidyl Secondary Amides. J. Chem. Inf. Model. 2022;62:350–358. doi: 10.1021/acs.jcim.1c01087. [DOI] [PubMed] [Google Scholar]
- 20.Fu L., Ye F., Feng Y., Yu F., Wang Q., Wu Y., Zhao C., Sun H., Huang B., Niu P., et al. Both Boceprevir and GC376 efficaciously inhibit SARS-CoV-2 by targeting its main protease. Nat. Commun. 2020;11:4417. doi: 10.1038/s41467-020-18233-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vuong W., Khan M.B., Fischer C., Arutyunova E., Lamer T. Feline coronavirus drug inhibits the main protease of SARS-CoV-2 and blocks virus replication. Nat. Commun. 2020;11:4282. doi: 10.1038/s41467-020-18096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Singh J., Petter R.C., Baillie T.A., Whitty A. The resurgence of covalent drugs. Nat. Rev. Drug Discov. 2011;10:307–317. doi: 10.1038/nrd3410. [DOI] [PubMed] [Google Scholar]
- 23.Moynihan M.M., Murkin A.S. Cysteine Is the General Base That Serves in Catalysis by Isocitrate Lyase and in Mechanism-Based Inhibition by 3-Nitropropionate. Biochemistry. 2014;53:178–187. doi: 10.1021/bi401432t. [DOI] [PubMed] [Google Scholar]
- 24.Baillie T.A. Targeted Covalent Inhibitors for Drug Design. Angew. Chem. Int. Ed. 2016;55:13408–13421. doi: 10.1002/anie.201601091. [DOI] [PubMed] [Google Scholar]
- 25.Ray S., Kreitler D.F., Gulick A.M., Murkin A.S. The Nitro Group as a Masked Electrophile in Covalent Enzyme Inhibition. ACS Chem. Biol. 2018;13:1470–1473. doi: 10.1021/acschembio.8b00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Abdiche Y., Malashock D., Pinkerton A., Pons J. Determining kinetics and affinities of protein interactions using a parallel realtime label-free biosensor, the Octet. Anal. Biochem. 2008;377:209–217. doi: 10.1016/j.ab.2008.03.035. [DOI] [PubMed] [Google Scholar]
- 27.Shah N.B., Duncan T.M. Bio-layer Interferometry for Measuring Kinetics of Protein-protein Interactions and Allosteric Ligand Effects. J. Vis. Exp. 2014;84:e51383. doi: 10.3791/51383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lipinski C.A., Lombardo F., Dominy B.W., Feeney P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 2001;46:3–26. doi: 10.1016/S0169-409X(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 29.Doak B.C., Over B., Giordanetto F., Kihlberg J. Oral druggable space beyond the rule of 5: Insights from drugs and clinical candidates. Chem. Biol. 2014;21:1115–1142. doi: 10.1016/j.chembiol.2014.08.013. [DOI] [PubMed] [Google Scholar]
- 30.Doak B.C., Kihlberg J. Drug discovery beyond the rule of 5—Opportunities and challenges. Expert Opin. Drug Discov. 2017;12:115–119. doi: 10.1080/17460441.2017.1264385. [DOI] [PubMed] [Google Scholar]
- 31.de Oliveira E.C., Santana K., Josino L., Lima E., Lima A.H., de Souza de Sales J.C. Predicting cell-penetrating peptides using machine learning algorithms and navigating in their chemical space. Sci. Rep. 2021;11:7628. doi: 10.1038/s41598-021-87134-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kügler J., Schmelz S., Gentzsch J., Haid S., Pollmann E., van den Heuvel J., Franke R., Pietschmann T., Heinz D.W., Collins J. High affinity peptide inhibitors of the hepatitis C virus NS3-4A protease refractory to common resistant mutants. J. Biol. Chem. 2012;287:39224–39232. doi: 10.1074/jbc.M112.393843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Choudhary M.I., Shaikh M., Tul-Wahab A., Ur-Rahman A. In silico identification of potential inhibitors of key SARS-CoV-2 3CL hydrolase (Mpro) via molecular docking, MMGBSA predictive binding energy calculations, and molecular dynamics simulation. PLoS ONE. 2020;15:e0235030. doi: 10.1371/journal.pone.0235030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elmezayen A.D., Al-Obaidi A., Sahin A.T., Yelekci K. Drug repurposing for coronavirus (COVID-19): In silico screening of known drugs against coronavirus 3CL hydrolase and protease enzymes. J. Biomol. Struct. Dyn. 2020;38:2980–2992. doi: 10.1080/07391102.2020.1758791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Mittal L., Kumari A., Srivastava M., Singh M., Asthana S. Identification of potential molecules against COVID-19 main protease through structure-guided virtual screening approach. J. Biomol. Struct. Dyn. 2020;38:3662–3680. doi: 10.1080/07391102.2020.1768151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shitrit A., Zaidman D., Kalid O., Bloch I., Doron D., Yarnizky T., Buch I., Segev I., Ben-Zeev E., Segev E., et al. Conserved interactions required for inhibition of the main protease of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) Sci. Rep. 2020;10:20808. doi: 10.1038/s41598-020-77794-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coelho C., Gallo G., Campos C.B., Hardy L., Würtele M. Biochemical screening for SARS-CoV-2 main protease inhibitors. PLoS ONE. 2020;15:e0240079. doi: 10.1371/journal.pone.0240079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pedersen N.C., Kim Y., Liu H., Kankanamalage A.C.G., Eckstrand C., Groutas W.C., Bannasch M., Meadows J.M., Chang K.O. Efficacy of a 3C-like protease inhibitor in treating various forms of acquired feline infectious peritonitis. J. Feline Med. Surg. 2017;20:378–392. doi: 10.1177/1098612X17729626. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.