Abstract
Moderate osmolality can stimulate bacterial growth at temperatures near the upper limit for growth. We investigated the mechanism by which high osmolality enhances the thermotolerance of Salmonella enterica serovar Typhimurium, by isolating bacteriophage MudI1734-induced insertion mutations that blocked the growth-stimulatory effect of 0.2 M NaCl at 45°C. One of these mutations proved to be in the seqA gene (a regulator of initiation of DNA synthesis). Because this gene is cotranscribed with pgm (which encodes phosphoglucomutase), it is likely to be polar on the expression of the pgm gene. Pgm catalyzes the conversion of glucose-6-phosphate to glucose-1-phosphate during growth on glucose, and therefore loss of Pgm results in a deficiency in a variety of cellular constituents derived from glucose-1-phosphate, including trehalose. To test the possibility that the growth defect of the seqA::MudI1734 mutant at high temperature in medium of high osmolality is due to the block in trehalose synthesis, we determined the effect of an otsA mutation, which inactivates the first step of the trehalose biosynthetic pathway. The otsA mutation caused a growth defect at 45°C in minimal medium containing 0.2 M NaCl that was similar to that caused by the pgm mutation, but otsA did not affect growth rate in this medium at 37°C. These results suggest that the growth defect of the seqA-pgm mutant at high temperature could be a consequence of the block in trehalose synthesis. We found that, in addition to the well-known osmotic control, there is a temperature-dependent control of trehalose synthesis such that, in medium containing 0.2 M NaCl, cells grown at 45°C had a fivefold higher trehalose pool size than cells grown at 30°C. Our observations that trehalose accumulation is thermoregulated and that mutations that block trehalose synthesis cause a growth defect at high temperature in media of high osmolality suggested that this disaccharide is crucial for growth at high temperature either for turgor maintenance or for protein stabilization.
Although high osmolality is generally regarded as a source of “stress” (3), this is not necessarily always the case, because raising the osmotic strength of the medium can increase the thermotolerance of bacteria (5, 16, 19, 32).
In bacteria, thermotolerance can be quantified by assays of at least two responses: viability at lethal high temperatures and growth rate at nonlethal but inhibitory high temperatures. Moderate or high osmolality enhances both of these aspects of thermotolerance in Escherichia coli and Salmonella enterica serovar Typhimurium. It is not clear whether these two osmoregulated responses, namely enhancement of viability at lethal high temperatures and stimulation of growth near the upper limit of nonlethal temperatures, are different manifestations of a single osmotically controlled thermotolerance mechanism or they represent two independent responses. Hengge-Aronis et al. (19) observed that the high-osmolality-dependent acquisition of increased viability at high temperatures in exponentially growing cells was abolished by an rpoS::Tn10 mutation, indicating that the stationary-phase factor ςS is required for this response. However, there have been no studies of the mechanism that regulates the second response, the stimulation of growth by high osmolality at high but nonlethal temperatures. We investigated this issue by isolating MudI1734-induced mutations in serovar Typhimurium, which blocked this response. Our analysis of mutants revealed that trehalose (O-α-d-glucosyl-[1→1]-α-d-glucoside) is crucial for the cells to grow in media of moderate osmolality at limiting high temperature.
MATERIALS AND METHODS
Media.
The rich medium was Luria-Bertani medium (LB) (11), and the minimal medium was M63 (8), containing the indicated concentrations of d-glucose or d-galactose as carbon sources. Because the biosynthesis of methionine is impaired at temperatures above 42.5°C (29), this amino acid was added at 0.5 mM to M63. When used, glycine betaine · HCl was added at 1 mM. The osmotic strength of M63 was increased with 0 to 0.2 M NaCl, and the pH of the medium was adjusted with NaOH to 7.2. The osmolality of M63 and M63 plus 0.2 M NaCl is 0.2 and 0.6 osm, respectively (12). When used, sodium ampicillin was at 25 μg/ml and kanamycin sulfate was at 75 μg/ml. Solid media contained 20 g of agar per liter (Difco). For the determination of trehalose levels and growth rates at different temperatures, cultures were grown with aeration in a Julabo shaking water bath, model SW-21C (guaranteed temperature stability of ±0.1°C), with a shaking frequency of 150 oscillations/min. Unless stated otherwise, all chemicals were purchased from Sigma (St. Louis, Mo.).
Bacterial strains.
The experiments described in this paper were carried out with derivatives of Salmonella enterica serovar Typhimurium strain TL1, a line of wild-type strain LT2 propagated in this laboratory (10). Phage P22 HT105/1 int201 (designated P22 hereafter) was used as the generalized transducing phage (11). Strains SF1005 (rpoS::amp) (15) and XF373 (otsA::MudI1734) (14) were obtained from F. Fang. Strains TL3131 (rpoS::amp) and TL3354 (otsA::MudI1734) were derived by transduction of TL1 to Ampr and Kanr by P22 grown on SF1005 and XF373, respectively. Strain TL3356 (rpoS::amp otsA::MudI1734) is a Kanr transductant of TL3131 by P22 grown on TL3354. The presence of the rpoS mutation in strains TL3131 and TL3356 was verified by reaction with H2O2. Treatment of colonies of the wild-type strain on LB plates with a drop of 30% H2O2 results in vigorous bubbling, due to the generation of O2 by catalases encoded by the katE and katG genes. Because the transcription of these genes is dependent on RpoS, mutants lacking this transcriptional activator have reduced catalase activity (20). As expected, the rpoS mutants TL3131 and TL3356 showed a greatly reduced efficiency of bubble formation upon treatment with H2O2 compared to that of control rpoS+ strains.
The seqA101::MudI1734 insertion was isolated as a mutation that impaired growth stimulation by high osmolality at high temperature. Approximately 105 derivatives of TL1 carrying random insertions of MudI1734 (24), obtained on LB plus kanamycin plates, were replica plated to two M63 plates containing 0.2 M NaCl and methionine, one of which was incubated at 48°C and the other at 30°C. Derivatives which could not grow at 48°C but could grow at 30°C were collected. (48°C was used for the screening of the mutants, because we found that the wild type was able to form colonies in replica plates at this high temperature.) The MudI1734 insertion from potential mutants was transduced with P22 into strain TL1, and the transductants were tested for their thermotolerance at 45°C in liquid M63–0.2 M NaCl–glucose–methionine medium. We identified 12 strains that showed various gradations of impaired growth at 45°C but grew normally at 37°C in the same medium. Strain TL3283 (seqA101::MudI1734) was obtained as one of these transductants.
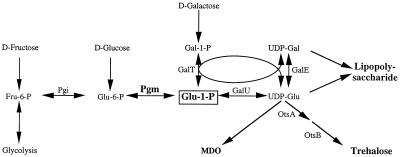
The location of this MudI1734 insertion was determined by cloning and DNA sequencing. Cloning steps were carried out according to procedures described by Sambrook et al. (30). In phage MudI1734, the Kanr gene is located between HindIII and BamHI sites that are 1 and 2.8 kbp from the “left” (or c) end of the phage (6). To clone the DNA flanking the MudI1734 insertions, total DNA was isolated from strain TL3283 and was subjected to a partial digestion with Sau3AI (New England Biolabs, Beverly, Mass.). The fragments were ligated into the BamHI site of pBluescript IIKS (Stratagene, La Jolla, Calif.) and introduced into strain DH5α (Gibco-BRL, Grand Island, N.Y.) by electroporation, and then Kanr derivatives were selected. In phage MudI1734, there is an NsiI site 0.5 kbp from the above HindIII site. As an initial screening for constructs that were likely to contain the left end of MudI1734 and hence, the flanking host DNA, the plasmids were digested with NsiI and HindIII, and plasmids that contained both of these sites were used for DNA sequence analysis. The sequences of the inserts were determined at the Iowa State University DNA sequencing facility, Ames, Iowa. The sequencing reactions employed the primer 5′-CCAATGTCCTCCCGGTTTTT-3′, which directs the sequence determination outward from phage MudI1734, starting at nucleotide 25 from the left end of the phage. Sequence analysis revealed that the left end of the phage in TL3283 is immediately upstream of nucleotide 358 of the seqA gene of serovar Typhimurium (see http://genome.wustl.edu/gsc/Blast/client.pl for the S. enterica serovar Typhimurium genomic sequence) and is thus likely to be polar on the expression of the pgm gene (23). As expected (2), the seqA101::MudI1734 insertion was cotransducible with the kdp locus in P22 transduction (∼50% linkage). The seqA101::MudI1734 mutation resulted in a defect in galactose utilization in M63 and in MacConkey galactose medium, similar to that described in E. coli by Lu and Kleckner (23). This insertion also conferred resistance to phage P22, which could be reversed by galactose, similar to mutations in other genes encoding enzymes of the pathway between UDP-galactose and glucose-6-phosphate (Fig. 1) (13). These observations, which we confirmed further by trehalose measurements (see below), indicated that the seqA101::MudI1734 insertion diminishes the expression of the pgm gene by transcriptional polarity.
FIG. 1.
Metabolic role of phosphoglucomutase.
Determination of growth rates at 45°C.
Cells from single colonies grown on LB plates at 37°C were inoculated into LB and grown to saturation at 30°C. The cells were then subcultured at a dilution of 1:100 into M63–10 mM glucose–methionine and the indicated concentrations of NaCl, with or without glycine betaine or 5 mM galactose, and were grown to saturation overnight at 30°C. The cells were then subcultured again at a 1:8 dilution into 8 ml of medium of an identical composition to that used for the overnight growth, except the glucose concentration was increased to 20 mM and galactose to 10 mM, and then these cultures were grown for 120 min at 30°C (to an optical density at 600 nm [OD600] of 0.4 to 0.6). At this point, the cells were diluted to an OD600 of 0.10 into 20 ml of medium which had the same composition as was used for the previous step and which had been prewarmed to 45°C. The cells were grown for the growth curve measurements at this temperature in the Julabo water bath. At different time intervals, samples were withdrawn, and the cell density was monitored by determining the OD600. Because the light scattering was not proportional to cell density above an OD600 of >1, at high cell densities, the OD600 was determined for a 1:10 dilution of the cultures. Specific growth rates were calculated from the best exponential fit of the corrected OD600 versus time for the exponential portion of the growth curves, using the Cricket Graph III, version 1.01 Macintosh application. We used 30°C as the growth temperature prior to the final growth phase at 45°C as a precaution to minimize possible protective effects that might arise from the induction of the heat shock system (35). However, pilot experiments indicated that cultures that were grown first at 42°C and shifted to 45°C had growth rates at the latter temperature that were within experimental error equal to those of cultures that were grown at 30°C and shifted to 45°C (data not shown). For determination of the growth rates of control cultures at 37°C and 42°C, the same pregrowth steps were used, except that the final cultures were incubated at these latter temperatures.
Determination of trehalose levels.
Cells were grown in liquid LB, and then two successive subcultures were grown in M63 plus glucose plus methionine plus the indicated concentrations of NaCl, glycine betaine, or galactose, as described above for the determination of the growth rates at 45°C, except that these cultures were grown at 30, 37, or 42°C, depending on the desired temperature at which the cellular trehalose level was to be determined. After growth for 120 min and verification that the cultures were in exponential growth, the cells were diluted to an OD600 of 0.10 in 20 ml of medium of the same composition used for the previous step (M63–20 mM glucose–methionine plus the indicated concentrations of NaCl, galactose, or glycine betaine). These last cultures were incubated at the same temperature used for the pregrowth, with the exception of the experiment conducted at 45°C, for which the inocula were taken from cultures grown at 42°C. This temperature shift in the 45°C experiment was performed because the latter temperature would not have supported the growth of control cultures with ≤0.10 M NaCl, whereas 42°C was permissive for growth, regardless of the NaCl concentration.
When the cell density in the final cultures reached an OD600 of 0.4 to 0.6 (2 to 2.6 doublings), 10-ml samples were removed, and the cells were sedimented by centrifugation at 10,000 × g for 15 min at 4°C. In order to reduce the carryover of glucose from culture media that would interfere with the trehalose determination (see below), the cells were resuspended in 10 ml of glucose-free M63 plus methionine plus the same concentration of NaCl used for the growth and were sedimented by centrifugation, as above. The cells were suspended in 1 ml of 80% (vol/vol) ethanol and heated to 65°C for 15 min. The cell debris was removed by centrifugation in a microfuge, and the ethanol extract was transferred to new tubes and dried under vacuum at 37°C in a SpeedVac (Savant). The residue was dissolved in 0.75 ml of H2O.
The trehalose contents of the above aqueous extracts were determined by a modification of the procedure recommended by the manufacturer (Sigma, St. Louis, Mo.) for the assay of trehalase. In this procedure, trehalose is hydrolyzed to two molecules of glucose, which are converted to glucose-6-phosphate and oxidized by NADP, producing two molecules of NADPH per molecule of trehalose input. Samples of 0.05, 0.10, and 0.15 ml of the aqueous cell extracts were brought to 0.20 ml with H2O, and 0.0054 U of trehalase was added in 0.02 ml of 25 mM potassium phosphate, pH 6.5. The mixtures were incubated for 4 h at 37°C, during which the trehalose was hydrolyzed to glucose. Then 1 ml of a mixture of 0.25 M triethanolamine · HCl (pH 7.6), 10 mM MgCl2 · 6H2O, 0.95 mM Na-ATP, 1.4 mM NADP, 0.32 U of hexokinase, and 0.18 U of glucose-6-phosphate dehydrogenase was added to each sample and incubated at 37°C for 60 min. The NADPH produced was measured by its A340.
The extracts could contain a number of compounds that would interfere with the measurement of trehalose, such as glucose, glucose-6-phosphate, NADH, or NADPH. In order to nullify the effects of any contribution from these sources, we carried out control assays, from which trehalase was omitted, and the A340 of these controls was subtracted from the A340 of the assays with trehalase. The factor for conversion of A340 to trehalose concentration was determined from standard curves generated with various concentrations of trehalose, subjected to identical assays as described above. In the standard curves, A340 was linear with the input concentration of trehalose in the range of 0 to 160 mM (r2 > 0.98). The trehalose levels were expressed as nanomoles per milligram of protein, with the latter calculated from the OD600 of the cultures at the time of harvest and standard curves relating OD600 to milligrams of cellular protein per milliliter, determined with the Bradford assay for cells grown in media of appropriate NaCl concentrations. It has been reported in E. coli that if the periplasmic TreA trehalase is not inactivated, measurements of trehalose levels might be artifactually low because of the hydrolysis of the solute in the cell extracts (22). To test whether this is a problem in our procedure in which the cell extracts were prepared by ethanol lysis, we compared the trehalose levels in a treA152::MudQ insertion mutant with those in the wild type. Within experimental error, the treA mutation did not have an effect on the trehalose levels measured in cells grown in M63 or in M63–0.2 M NaCl at 37 or 42°C (data not shown); therefore, we concluded that the trehalase activity did not cause a significant interference with our measurements.
β-Galactosidase assays.
For β-galactosidase measurements, strains carrying rpoS-lacZ fusions were grown at the indicated temperatures in M63–20 mM glucose–methionine, with or without 0.2 M NaCl and glycine betaine, as described above for growth rate measurements. The β-galactosidase activities were determined at two points in the growth cycle: in exponential phase (OD600, 0.2 to 0.25) and in stationary phase, after 24 h of incubation (corrected OD600, 3.9 to 4.2). The β-galactosidase activities were assayed as described by Miller (25), with the specific activity expressed as nanomoles of product formed per minute per milligram of protein, with the protein concentration determined as described above for the trehalose level measurements.
RESULTS
Trehalose synthesis was required for high-osmolality-dependent growth stimulation at 45°C.
As described above, our search for mutations that abolish the osmotic stimulation of growth at high temperature yielded the seqA101::MudI1734 insertion. The seqA gene, which encodes a negative regulator of initiation of chromosomal DNA replication, is upstream of the pgm gene in a single operon (23). The product of the latter gene, phosphoglucomutase, catalyzes the formation of glucose-1-phosphate from glucose-6-phosphate during growth on glucose (Fig. 1). Glucose-1-phosphate is the precursor of a variety of saccharide moieties in a number of cellular constituents (Fig. 1). In principle, the growth defect of the seqA::MudI1734 mutant at 45°C in the presence of high salt might be due to the loss of function of the SeqA protein or to reduced expression of Pgm because of transcriptional polarity. One of the metabolic consequences of the lack of Pgm is the inability to make trehalose. Because this disaccharide has been suggested to have thermoprotective functions (9, 33) and because its cellular levels increase in response to high osmolality (17), we investigated the possibility that it is essential for growth at very high temperatures in media of moderate osmolality.
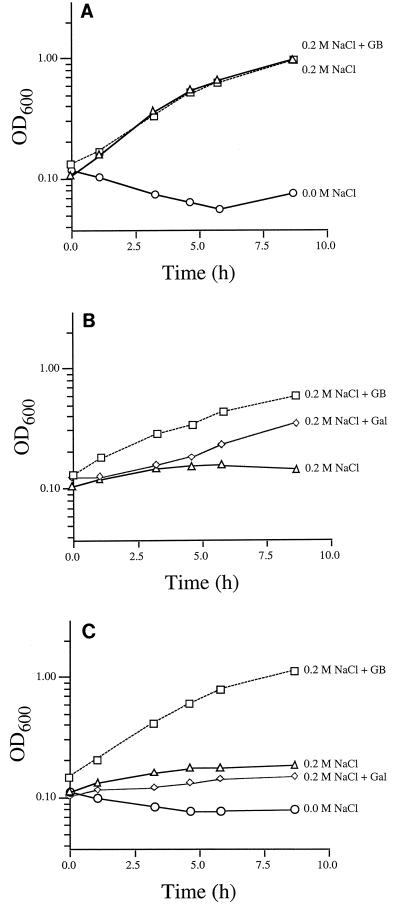
Trehalose is derived from UDP-glucose and glucose-6-phosphate by two reactions catalyzed by the otsA and otsB gene products (Fig. 1). In order to test the possibility that the growth defect of the seqA mutant at high temperature might be connected to trehalose deficiency, we compared the growth rates of this mutant with that of an otsA::MudI1734 mutant at 45°C (Fig. 2). Typical results, obtained with wild-type serovar Typhimurium, are shown in Fig. 2A. This strain was unable to grow in M63 plus methionine at 45°C, but supplementation with 0.2 M NaCl stimulated it to grow at a specific growth rate of 0.38 generation/h. As reported previously (16), glycine betaine did not have an effect on the growth rate of the wild type in the presence of 0.2 M NaCl. The seqA::MudI1734 mutant had a severe growth defect in M63–methionine–0.2 M NaCl (Fig. 2B). The otsA mutant showed a similar growth defect to that of the seqA mutant at 45°C (Fig. 2C). These results demonstrated that trehalose is necessary for the cells to respond to growth stimulation by increased osmolality at high temperature.
FIG. 2.
seqA::MudI1734 and otsA mutations block the high-osmolality-dependent growth stimulation at 45°C. The growth rates of the wild-type strain TL1 (A), the seqA::MudI1734 mutant TL3283 (B), and the otsA::MudI1734 mutant TL3354 were determined at 45°C, as described in Materials and Methods. Symbols: ○, M63–20 mM glucose–0.5 mM methionine; ▵, medium supplemented with 0.2 M NaCl; ▫, medium supplemented with 0.2 M NaCl and 1 mM glycine betaine (GB); ⋄, medium supplemented with 10 mM galactose.
The effects of the seqA::MudI1734 mutation might be complicated, not only because it could impair the expression of the pgm gene but conceivably because the lack of the seqA gene product could have pleiotropic consequences. Supplementation of the growth medium with galactose or glycine betaine restored growth to this mutant at 45°C (Fig. 2B). Because galactose is metabolized to glucose-1-phosphate (Fig. 1), it is possible to supply this intermediate in strains lacking Pgm by providing them with galactose during growth on glucose (13). The result, that galactose improved the growth of the seqA::MudI1734 mutant in M63–0.2 M NaCl at 45°C, indicated that the high-temperature growth defect of this mutant could be ascribed to the loss of metabolic product(s) derived from glucose-1-phosphate, rather than to the loss of the SeqA protein. The fact that glycine betaine could also alleviate the growth defect at high temperature in this mutant suggested that the problem arose from the lack of trehalose, whose function as a compatible solute could be supplanted by glycine betaine. These conclusions were supported by the observations (Fig. 2C) that in the otsA mutant, the growth defect at high temperature was not repaired by galactose, which could not be converted in this mutant to trehalose, but it was corrected by glycine betaine, which could replace trehalose as a compatible solute.
Mutations blocking the synthesis of trehalose (otsA, otsB, and galU) (see Fig. 1) have been isolated previously by their phenotype of sensitivity to high osmolality (≥0.45 M NaCl) (17, 28). Although these previous experiments indicated that the loss of the ability to accumulate trehalose does not cause a significant growth defect at 37°C at NaCl concentrations below 0.45 M, conceivably there might be an increased need for this disaccharide at higher temperatures in media of moderate osmolality. Therefore, we determined the growth rates of the relevant strains in media containing 0.2 M NaCl at various temperatures (Table 1). At 37°C, the growth rate of the otsA mutant in the presence of 0.2 M NaCl was only 2% lower than that of the wild-type strain, in accord with the earlier conclusions in E. coli (17, 28) that at this temperature, trehalose is not required for cytoplasmic osmoregulation at moderate osmolalities. However, the otsA mutation caused a more substantial growth defect in the presence of 0.2 M NaCl as the temperature was increased. At 42°C, this mutation caused an ∼25% decrease in the growth rate, and at 45°C, it resulted in a >90% reduction in growth rate in M63–0.2 M NaCl, compared to the wild type. The growth rate defect caused by the loss of trehalose synthesis at these higher temperatures could be corrected by glycine betaine. These results suggested that trehalose becomes more crucial for growth at moderate osmolality with increasing temperature.
TABLE 1.
Growth rates of trehalose-deficient strains at different temperatures
| Temperature (°C) | Strain (genotype) | Growth ratea (generation/h ± SE) in medium plus:
|
||
|---|---|---|---|---|
| 0.0 M NaCl | 0.2 M NaCl | 0.2 M NaCl + GB | ||
| 37 | TL1 (wild type) | 1.31 ± 0.01 | 0.92 ± 0.01 | 1.07 ± 0.02 |
| TL3354 (otsA::MudI1734) | 1.18 ± 0.09 | 0.90 ± 0.01 | 1.12 ± 0.02 | |
| TL3283 (seqA::MudI1734) | 0.87 ± 0.07 | 0.77 ± 0.01 | 0.99 ± 0.01 | |
| 42 | TL1 (wild type) | 1.04 ± 0.08 | 0.88 ± 0.11 | 1.08 ± 0.03 |
| TL3354 (otsA::MudI1734) | 0.97 ± 0.02 | 0.67 ± 0.17 | 1.11 ± 0.02 | |
| TL3283 (seqA::MudI1734) | 0.68 ± 0.06 | 0.45 ± 0.03 | 0.82 ± 0.03 | |
| 45 | TL1 (wild type) | <0.03 | 0.40 ± 0.07 | 0.35 ± 0.05 |
| TL3354 (otsA::MudI1734) | <0.01 | <0.03 | 0.45 ± 0.04 | |
| TL3283 (seqA::MudI1734) | <0.02 | 0.02 | 0.21 ± 0.02 | |
The specific exponential growth rates of the strains were determined, as described in Materials and Methods, at the indicated temperatures in M63 medium–20 mm glucose–Met plus the indicated concentrations of NaCl or 1 mM glycine betaine (GB). The data are averages ± standard errors of results obtained in three independent experiments.
Like the otsA mutant, the seqA mutant also exhibited a growth defect in M63–0.2 M NaCl at 42°C and especially at 45°C. However, the seqA mutant had a lower growth rate than the wild type even in M63 without NaCl at 37°C and 42°C, and the growth rate of this strain was not completely restored by glycine betaine in M63–0.2 M NaCl to wild-type values at any temperature. The latter result suggested that, while the growth defect in the seqA mutant could at least in part be due to the block in trehalose synthesis, the Pgm or SeqA deficiency could also cause a more pleiotropic growth reduction. Loss of the ability to grow at 45°C in M63–methionine–0.2 M NaCl in the SeqA/Pgm mutant was not due to the defect in the normal composition of the outer membrane, because a galE mutation, which also interferes with the synthesis of the sugar moieties in the lipopolysaccharide (Fig. 1), did not cause a detectable defect in the osmotically induced growth stimulation at high temperature (data not shown).
The seqA::MudI1734 mutation impairs the synthesis of trehalose.
In order to verify that the seqA::MudI1734 mutation blocks the synthesis of trehalose, we measured the levels of this disaccharide in various strains at low and high osmolality (Table 2). Our results confirmed observations in E. coli (31) that high osmolality stimulated the accumulation of trehalose in wild-type serovar Typhimurium and that this response was blocked by glycine betaine. As expected, the otsA mutation prevented the high-osmolality-dependent accumulation of trehalose. The seqA mutation had a similar consequence, demonstrating that indeed this insertion is polar on the expression of pgm. The results shown in Table 2 were obtained at 37°C, but we found that the seqA insertion mutation eliminated trehalose synthesis at 42°C also (data not shown). Supplementation with galactose restored the synthesis of trehalose in the seqA mutant in M63–glucose–0.2 M NaCl (Table 2), in accord with the notion that the metabolic defect in this Pgm-deficient mutant can be circumvented by galactose.
TABLE 2.
Trehalose levels in the wild-type strain and in the seqA::MudI1734 and otsA mutantsa
| Strain (genotype) | Medium (M63 +) | Trehalose (nmol · mg protein−1) in medium with
|
|
|---|---|---|---|
| 0.0 M NaCl | 0.2 M NaCl | ||
| TL1 (wild type) | 0.8 ± 0.3 | 20 ± 4 | |
| Glycine betaine | NDb | <1.7 | |
| Galactose | NDb | 15 ± 1 | |
| TL3283 (seqA::MudI1734) | 0.7 ± 0.2 | 3 ± 2 | |
| Galactose | NDb | 29 ± 1 | |
| TL3354 (otsA::MudI1734) | NDb | <0.7 | |
The trehalose levels were determined in mid-exponential-phase cells grown in M63 medium + 20 mM glucose and the indicated concentrations of NaCl at 37°C as described in Materials and Methods. When used, glycine betaine was 1 mM and galactose was 10 mM.
ND, not determined.
High-osmolality-dependent accumulation of trehalose was enhanced at high temperature.
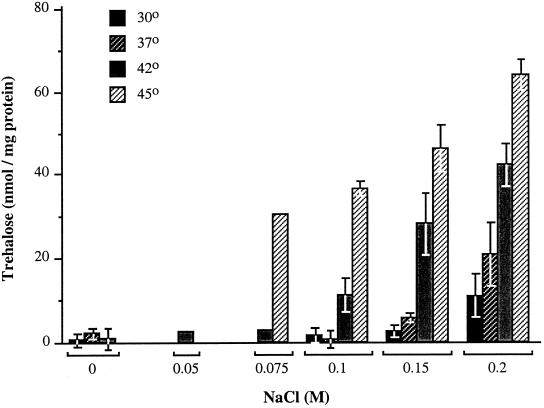
Because of our finding that the synthesis of trehalose was critical for growth at high temperature in the presence of 0.2 M NaCl, we examined whether the levels of trehalose were regulated by temperature. This experiment (Fig. 3) revealed that the accumulation of trehalose was subject to a temperature-dependent control in media of moderate osmolality; in M63–0.2 M NaCl, cells that were grown at 45°C had ∼fivefold higher trehalose levels than cells that were grown at 30°C. Because there was a requirement for at least 0.075 M NaCl for growth at 45°C, we could not determine whether this high temperature would be sufficient signal by itself to elevate the trehalose pool size, but at lower temperatures, high osmolality was required to observe trehalose accumulation. The effect of increasing temperature was to lower the threshold osmolality that was required to elevate the trehalose pool size; at 45°C, 0.075 M NaCl was sufficient to induce trehalose accumulation, whereas at 30°C, 0.2 M NaCl was required.
FIG. 3.
Regulation of trehalose levels by osmolality and temperature in the wild-type serovar Typhimurium. The trehalose levels were determined in serovar Typhimurium strain TL1 in M63 plus glucose plus methionine, containing the indicated concentrations of NaCl at the indicated temperatures, as described in Materials and Methods. The data represent the averages and standard errors of results obtained in at least two independent measurements for all conditions, except for the results obtained with 0.05 and 0.075 M NaCl at 42 and 45°C, which were obtained in single measurements.
Thermoregulation of expression of otsA.
In E. coli K-12 derivatives, the otsAB operon is induced by high osmolality or by stationary phase under the control of ςS (18, 21). In addition, it has been shown that the otsAB operon exhibits a three- to fourfold “heat shock” induction upon shift of the temperature from 30 to 42.5°C (26). Because we found that the trehalose levels increased as a function of increasing temperature in cells that were grown at constant temperature and salinity for more than nine generations (Fig. 3), we determined whether there is a thermoregulation of expression of the otsA-lacZ fusion in serovar Typhimurium grown continuously at 30 or 42°C. As can be seen in Table 3, the expression of the otsA-lacZ fusion was five- to sixfold higher at 42 than at 30°C, both in the absence and in the presence of 0.2 M NaCl. Thus, the otsA operon remained induced continuously at high temperature, unlike the typical genes in the heat shock regulon, which are transcribed maximally only for a short time after a temperature upshift (35). As has been seen in E. coli (18, 21), the expression of the otsA-lacZ fusion in serovar Typhimurium was also induced in exponential phase about threefold by 0.2 M NaCl. Glycine betaine reversed the high-osmolality-dependent induction at both temperatures in exponentially growing cells. We observed stationary-phase-dependent induction of the fusion at 30 but not at 42°C. Induction of the otsA gene by high temperature in midexponential phase was eliminated by the rpoS::amp insertion, as was the induction by high osmolality in stationary phase. This result, which was observed earlier in E. coli, indicated that the ςS factor is required for the thermoregulation of expression of otsA (18, 26).
TABLE 3.
Thermoregulation of otsA expression
| Strain (genotype) | Growth phase | Medium (M63 +) | β-Galactosidase specific activitya (nmol min−1 mg protein−1) ± SE at temperature in medium with
|
|||
|---|---|---|---|---|---|---|
| 30°C
|
42°C
|
|||||
| 0.0 M NaCl | 0.2 M NaCl | 0.0 M NaCl | 0.2 M NaCl | |||
| TL3354 (otsA-lacZ) | Exponential | 6 ± 2 | 20 ± 1 | 39 ± 8 | 97 ± 16 | |
| Stationary | 22 ± 2 | 32 ± 2 | 35 ± 2 | 38 ± 8 | ||
| Exponential | Glycine betaine | NDb | 6 ± 1 | NDb | 26 ± 1 | |
| TL3356 (rpoS::amp otsA-lacZ) | Exponential | 3 ± 2 | 4 ± 2 | 2 ± 1 | 2 ± 1 | |
| Stationary | 3 ± 1 | 4 ± 1 | 2 ± 1 | 2 ± 1 | ||
The regulation of expression of the otsA gene was monitored in strains carrying an otsA-lacZ by β-galactosidase assays, as described in Materials and Methods. The β-galactosidase-specific activities were determined in exponential phase (OD600 = 0.2 to 0.25) and in stationary phase (OD600 = 3.9 to 4.2).
ND, not determined.
DISCUSSION
Moderate or high osmolality generated by ≥0.2 M NaCl elevates the upper limit of growth for serovar Typhimurium to 45°C (16). In this work, we found that the synthesis of trehalose was necessary for this high-osmolality-dependent growth stimulation. It has been documented that trehalose is one of the compatible solutes that is synthesized by bacteria in response to osmotic stress, but we discovered that high temperature greatly enhanced the high-osmolality-dependent accumulation of this disaccharide.
As discussed in the introduction, moderate osmolality imparts increased thermotolerance to serovar Typhimurium and E. coli by two different mechanisms: enhancement of resistance to lethal high temperatures (19) and elevation of the upper limit of temperature for growth (32). Hengge-Aronis et al. (19) reported that otsAB mutations do not impair the high-osmolality-dependent induction of increased thermotolerance in exponentially growing E. coli cells, which we confirmed in serovar Typhimurium with the trehalose-deficient seqA and otsA mutants (S. A. Fletcher and L. N. Csonka, unpublished data). In contrast, the ability to respond to growth stimulation by high osmolality at elevated temperature was abolished by mutations that blocked the synthesis of trehalose (Fig. 2 and Table 1).
With our enzymatic assay, we found that the trehalose level in wild-type serovar Typhimurium grown in M63–0.2 M NaCl at 37°C was 20 nmol/mg of protein (Fig. 3 and Table 2). Laboratory lines of E. coli K-12 showed large variation in their trehalose levels at low osmolalities. Wild-type K-12 carries an in-frame amber codon in the rpoS gene. In this strain and in related amber suppressor-free derivatives, trehalose was undetectable at 37°C in M63–0.2 M NaCl (21). In strains that contain an amber suppressor tRNA, the trehalose pool size was ∼200 nmol/mg of protein at 37°C in medium containing 0.2 M NaCl. Welsh et al. (34) reported that the trehalose content of E. coli NCIB9484 was ∼5 nmol/mg of protein at 0.2 M NaCl. Thus, the trehalose level we measured with the enzymatic assay in serovar Typhimurium at 37°C in M63–0.2 M NaCl was within the range reported for wild-type E. coli strains grown at the low osmolalities (≤0.2 M NaCl).
Previously, we found that a minimum 0.15 M NaCl is required to induce growth stimulation at 45°C (16). This response can be elicited by NaCl concentrations up to 0.6 M, but above 0.3 M, the stimulatory effects of high osmolality at high temperature are counteracted by its adverse effect due to osmotic inhibition that are seen at all temperatures. In the present study, we used 0.2 M NaCl because it is nearly optimal for stimulating the growth of serovar Typhimurium at 45°C (16). At this temperature, the trehalose content of cells grown with this concentration of NaCl was 64 nmol/mg of protein (Fig. 3). The water-accessible cytoplasmic volume of E. coli grown at an osmolality comparable to that of M63–0.2 M NaCl was reported to be ∼1.7 μl/mg dry weight or ∼3.4 μl/mg of protein (7). Using this value as the cytoplasmic volume of serovar Typhimurium, we calculated that the trehalose concentration at 45°C and 0.2 M NaCl was approximately 0.02 M.
Trehalose has been suggested to have a number of functions, which are not mutually exclusive. By accumulating this disaccharide to high levels, cells can generate the necessary turgor pressure in media of high osmolality. Solutes that are accumulated at high concentrations in intracellular compartments for turgor maintenance are “compatible” because they do not interfere with cellular processes (4). In general, compatible solutes are excluded from the water of hydration surrounding the proteins, and therefore such compounds promote macromolecular stability under conditions of low water activity (1, 27). Trehalose also has been found at very high levels (up to 20% of dry weight) in a number of desiccation-tolerant plants under extreme dehydration (9). It has been suggested that under extremely anhydrobiotic conditions, trehalose at very high concentrations may promote the integrity of membranes and other macromolecules by functioning as a water substitute (9). Although our results demonstrate that the synthesis of trehalose is required in serovar Typhimurium for growth at high temperature in media of moderate osmolality and that high temperature stimulates the accumulation of trehalose, the basis for its thermoprotective function is not clear in serovar Typhimurium. At 0.02 M internal concentration, trehalose could balance only about 3% of the osmotic pressure generated by M63–0.2 M NaCl. At such modest concentrations, it is not plausible that trehalose could have the stabilizing effects on membranes that have been proposed at trehalose contents approaching 20%. To our knowledge, there has been no investigation of the temperature dependence of the turgor maintenance by compatible solutes or their exclusion from the water of hydration around proteins, but our observation that glycine betaine could compensate for the growth defect at high temperature arising from the block in trehalose production suggests that there is a greater need for compatible solutes at high temperature even at moderate osmolality. While our results demonstrated that trehalose was required for growth near the limiting high temperatures in media of elevated osmolality, further experiments are necessary to determine the role of this disaccharide.
This work was supported by the U.S. Department of Agriculture grant 98–35201-6219. David Cánovas was supported by a fellowship from the Ministerio de Educación y Cultura of Spain.
ACKNOWLEDGMENTS
We thank K. O'Connor for critical comments on the manuscript, D. S. Cayley, D. Fraenkel, and M. T. Record, Jr., for thought-provoking discussions, N. C. Carpita for suggesting to us the method for measurement of the trehalose levels, and E. Groisman for providing us with the sequence of the Mu sequencing primer.
ADDENDUM IN PROOF
After this paper was accepted, we became aware of a large body of literature on the accumulation and function of trehalose in response to heat stress in yeast. Examples are the work of DeVirgilio et al. (C. DeVirgilio, T. Hottinger, J. Dominguez, T. Boller, and A. Wiemken, Eur. J. Biochem. 219:179–186, 1994) and Hottinger et al. (T. Hottinger, C. DeVirgilio, M. N. Hall, T. Boller, and A. Wiemken, Eur. J. Biochem. 219:187–193, 1994). In addition, prior use of trehalase to assay trehalose has been reported (I. Kienle, M. Burgert, and H. Holzer, Yeast 9:607–611, 1993).
REFERENCES
- 1.Arakawa T, Timasheff S N. The stabilization of proteins by osmolytes. Biophys J. 1985;47:411–414. doi: 10.1016/S0006-3495(85)83932-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berlyn M, Low K B, Rudd K E. Linkage map of Escherichia coli K-12, edition 9. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella typhimurium: cellular and molecular biology. Washington D.C.: ASM Press; 1996. pp. 1715–1902. [Google Scholar]
- 3.Bremer E, Krämer R. Coping with osmotic challenges: osmoregulation through accumulation and release of compatible solutes in bacteria. In: Storz G, Hengge-Aronis R, editors. Bacterial stress. Washington D.C.: ASM Press; 2000. pp. 79–97. [Google Scholar]
- 4.Brown A D. Microbial water stress physiology: principles and perspectives. Chichester, United Kingdom: John Wiley & Sons, Ltd.; 1990. [Google Scholar]
- 5.Calhoun C L, Frazier W C. Effect of available water on thermal resistance of three nonsporeforming species of bacteria. Appl Microbiol. 1966;14:416–420. doi: 10.1128/am.14.3.416-420.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Castilho B, Olfson P, Casadaban M J. Plasmid insertion mutagenesis and lac gene fusion with mini-Mu bacteriophage transposon. J Bacteriol. 1984;158:488–495. doi: 10.1128/jb.158.2.488-495.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cayley S, Lewis B A, Guttman H J, Record M T., Jr Characterization of the cytoplasm of Escherichia coli K-12 as a function of external osmolarity. J Mol Biol. 1991;222:281–300. doi: 10.1016/0022-2836(91)90212-o. [DOI] [PubMed] [Google Scholar]
- 8.Cohen G N, Rickenberg R H. Concentration specifique reversible des amino acides chez E. coli. Ann Inst Pasteur (Paris) 1956;91:693–720. [PubMed] [Google Scholar]
- 9.Crowe J H, Hoekstra F A, Crowe L M. Anhydrobiosis. Annu Rev Physiol. 1992;54:579–599. doi: 10.1146/annurev.ph.54.030192.003051. [DOI] [PubMed] [Google Scholar]
- 10.Csonka L N. Proline over-production results in enhanced osmotolerance in Salmonella typhimurium. Mol Gen Genet. 1981;182:82–86. doi: 10.1007/BF00422771. [DOI] [PubMed] [Google Scholar]
- 11.Davis R W, Botstein D, Roth J R. Advanced bacterial genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1980. [Google Scholar]
- 12.Dunlap V J, Csonka L N. Osmotic regulation of l-proline transport in Salmonella typhimurium. J Bacteriol. 1985;163:296–304. doi: 10.1128/jb.163.1.296-304.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Enomoto M, Stocker B A D. Transduction by phage P1kc in Salmonella typhimurium. Virology. 1974;60:503–514. doi: 10.1016/0042-6822(74)90344-4. [DOI] [PubMed] [Google Scholar]
- 14.Fang F C, Chen C Y, Guiney D G, Xu Y. Identification of ςs-regulated genes in Salmonella typhimurium: complementary regulatory interactions between sigma ςs and cyclic AMP-receptor protein. J Bacteriol. 1995;178:5112–5120. doi: 10.1128/jb.178.17.5112-5120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fang F C, Libby S J, Buchmeier N A, Loewen P C, Switala J, Harwood J, Guiney D G. The alternative ς-factor KatF (RpoS) regulates Salmonella virulence. Proc Natl Acad Sci USA. 1992;89:11978–11982. doi: 10.1073/pnas.89.24.11978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fletcher S A, Csonka L N. Characterization of the induction of increased thermotolerance by high osmolality in Salmonella typhimurium. Food Microbiol. 1998;15:307–317. [Google Scholar]
- 17.Giæver H M, Styrvold O B, Kaasen I, Strøm A R. Biochemical and genetic characterization of osmoregulatory trehalose synthesis in Escherichia coli. J Bacteriol. 1988;170:2841–2849. doi: 10.1128/jb.170.6.2841-2849.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hengge-Aronis R, Klein W, Lange R, Rimmele M, Boos W. Trehalose synthesis genes are controlled by the putative sigma factor encoded by rpoS and are involved in stationary-phase thermotolerance in Escherichia coli. J Bacteriol. 1991;173:7918–7924. doi: 10.1128/jb.173.24.7918-7924.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hengge-Aronis R, Lange R, Henneberg N, Fischer D. Osmotic regulation of rpoS-dependent genes in Escherichia coli. J Bacteriol. 1993;175:259–265. doi: 10.1128/jb.175.1.259-265.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hengge-Aronis R. The general stress response of Escherichia coli. In: Storz G, Hengge-Aronis R, editors. Bacterial stress. Washington, D.C.: ASM Press; 2000. pp. 161–178. [Google Scholar]
- 21.Kaasen I, Falkenberg P, Styrvold O B, Strøm A R. Molecular cloning and physical mapping of the otsBA genes, which encode the osmoregulatory trehalose pathway of Escherichia coli: evidence that transcription is activated by KatF (AppR) J Bacteriol. 1992;174:889–898. doi: 10.1128/jb.174.3.889-898.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Larsen P I, Sydnes L K, Landfald B, Strøm A R. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987;147:1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- 23.Lu M, Kleckner N. Molecular cloning and characterization of the pgm gene encoding phosphoglucomutase of Escherichia coli. J Bacteriol. 1994;176:5847–5851. doi: 10.1128/jb.176.18.5847-5851.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Maloy S R. Experimental techniques in bacterial genetics. Boston, Mass: Jones and Bartlett; 1990. [Google Scholar]
- 25.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1991. [Google Scholar]
- 26.Muffler A, Barth M, Marschall C, Hengge-Aronis R. Heat shock regulation of ςs turnover: role for DnaK and relationship between stress responses mediated by ςs and ς32 in Escherichia coli. J Bacteriol. 1997;179:445–452. doi: 10.1128/jb.179.2.445-452.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Record T M, Jr, Courtenay E S, Cayley D S, Guttman H J. Biophysical compensation mechanisms buffering E. coli protein-nucleic acid interactions against changing environments. Trends Biochem Sci. 1998;23:190–194. doi: 10.1016/s0968-0004(98)01207-9. [DOI] [PubMed] [Google Scholar]
- 28.Rod L M, Alam K Y, Cunningham P R, Clark D P. Accumulation of trehalose by Escherichia coli K-12 at high osmotic pressure depends on the presence of amber suppressors. J Bacteriol. 1988;170:3601–3610. doi: 10.1128/jb.170.8.3601-3610.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ron E Z, Shani M. Growth rate of Escherichia coli at elevated temperatures: reversible inhibition of homoserine trans-succinylase. J Bacteriol. 1971;107:397–400. doi: 10.1128/jb.107.2.397-400.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 31.Styrvold O B, Strøm A R. Synthesis, accumulation, and excretion of trehalose in osmotically stressed Escherichia coli K-12 strains: influence of amber suppressors and function of periplasmic trehalase. J Bacteriol. 1991;173:1187–1192. doi: 10.1128/jb.173.3.1187-1192.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tesone S, Hughes A, Hurst A. Salt extends the upper temperature limit for growth of food-poisoning bacteria. Can J Microbiol. 1981;27:970–972. doi: 10.1139/m81-154. [DOI] [PubMed] [Google Scholar]
- 33.Van Laere A. Trehalose, reserve and/or stress metabolite? FEMS Microbiol Rev. 1989;63:201–210. [Google Scholar]
- 34.Welsh D T, Reed R H, Herbert R A. The role of trehalose in the osmoadaptation of Escherichia coli NCIB 9484: interaction of trehalose, K+ and glutamate during osmoadaptation in continuous culture. J Gen Microbiol. 1991;137:745–750. doi: 10.1099/00221287-137-4-745. [DOI] [PubMed] [Google Scholar]
- 35.Yura T, Kanemori M, Morita M T. The heat shock response: regulation and function. In: Storz G, Hengge-Aronis R, editors. Bacterial stress. Washington, D.C.: ASM Press; 2000. pp. 3–18. [Google Scholar]