Abstract
Mesenchymal stem/stromal cells (MSCs) are found in almost all postnatal organs. Under appropriate environmental cues, multipotency enables MSCs to serve as progenitors for several lineage-specific, differentiated cell types. In vitro expansion and differentiation of MSCs give the opportunity to obtain hardly available somatic cells, such as neurons. The neurogenic potential of MSCs makes them a promising, autologous source to restore damaged tissue and as such, they have received much attention in the field of regenerative medicine. Several stem cell pool candidates have been studied thus far, but only a few of them showed neurogenic differentiation potential. Due to their embryonic ontology, stem cells residing in the stroma of the dental pulp chamber are an exciting source for in vitro neural cell differentiation. In this study, we review the key properties of dental pulp stem cells (DPSCs), with a particular focus on their neurogenic potential. Moreover, we summarize the various presently available methods used for neural differentiation of human DPSCs also emphasizing the difficulties in reproducibly high production of such cells. We postulate that because DPSCs are stem cells with very close ontology to neurogenic lineages, they may serve as excellent targets for neuronal differentiation in vitro and even for direct reprogramming.
Keywords: dental pulp stem cells, neural differentiation, mesenchymal stem cells, reprogramming, neural crest
Introduction
Cell fate determination is a general process in differentiated living organisms (Furlong, 2010). Since cellular differentiation leads to specialized, functionally active cells, understanding and influencing these processes can potentially help restore tissue integrity and functionality (Ratcliffe et al, 2013).
During embryonic development, the derivatives of the fertilized oocyte are differentiated into specified cells while losing their potential for producing other cell types (Bertrand and Hobert, 2010). Stem cells, the immature precursors of specialized cells, are able to self-renew and differentiate into several lineages (Kolios and Moodley, 2013). Embryonic stem cells (ESCs) were the first that were differentiated into specific lineages, although their availability is limited due to biological and ethical issues (Charitos et al, 2021; Martello and Smith, 2014). Later on, the discovery and widespread usage of patient-derived induced pluripotent stem cells (iPSCs) retrieved from adult human tissues completely revolutionized the reprogramming field by allowing the use of different cell types such as blood cells and fibroblasts as cell sources and thus avoiding ethical issues (Andrews, 2021).
As a result, several novel cellular reprogramming techniques have emerged such as direct reprogramming or so-called transdifferentiation of fibroblasts into other cell types, including neurons (Bocchi et al, 2022; Grath and Dai, 2019; Vierbuchen et al, 2010; Wapinski et al, 2013). Direct conversion provides access to donor-derived, in vitro reprogrammed cells, which, in contrast to iPSCs, are nonclonal, and do not undergo a PSC or progenitor stage at any point during the conversion, therefore preserving the epigenetic aging signatures of the donor cells (Drouin-Ouellet et al, 2017b; Frobel et al, 2014; Kane and Sinclair, 2019; Mertens et al, 2016; Pircs et al, 2022; Yang et al, 2015).
Similar to iPSCs, the most commonly used cell pool for direct conversion up to date are fibroblasts, almost terminally differentiated somatic cells. The embryonic ontology of cells is the key aspect of tissue repair and engineering. Consequently, the use of fibroblasts as a source for differentiation and reprogramming is challenging (Kolios and Moodley, 2013; Perczel-Kovach et al, 2021; Shi et al, 2001).
Mesenchymal stem/stromal cells (MSCs) are multipotent cells found in almost all postnatal organs and represent an attractive cell pool for reprogramming methods. Their multipotency makes it possible after extraction and under appropriate environmental cues, to differentiate them into germ line-specific lineages (Pittenger et al, 2019). Various types of MSCs have been described according to their adherence to plastic, specific surface antigen expression, and multipotency (Dominici et al, 2006). MSCs derived from bone marrow (BM) and Wharton jelly are widely used for research and therapeutic purposes (Hong, 2022; Liau et al, 2020). MSCs originating from other tissues, including the dental pulp isolated from third molars (wisdom teeth), have also been studied and used.
In this review, we describe the origin and cellular specification of the ectomesenchymal human dental pulp stem cells (hDPSCs). We summarize how these cells were previously differentiated in vitro to a neuronal fate. All protocols used allogenic cell sources and presented neurogenic differentiation of hDPSCs. The reviewed differentiation protocols demonstrate indirect ways to get insight into the epigenetic landscape of hDPSCs. Lastly, we propose an exciting alternative method, direct reprogramming of hDPSCs for neuronal differentiation in the future.
Dental Pulp Stem Cells
The dental pulp is a soft tissue, located in the pulp chamber of the teeth. Most importantly, it comprises dentin producing odontoblasts, fibroblast-like cells, neural fibers, and blood vessels. Similar to other stromal organs, the stroma of the dental pulp and their neighboring tissues contains stem cells with MSC properties. These dental-derived cell pools include stem cells from human exfoliated deciduous teeth, stem cells from apical papilla, periodontal ligament stem cells, and also DPSCs, which are able to differentiate into several lineages showing similar embryonic ontology, differentiation potency, and cellular morphology.
In this review, we focus on hDPSCs, which are the most widely used and understood dental-derived stem cell type (Bansal and Jain, 2015; Campanella, 2018; Marrelli et al, 2015; Mayo et al, 2014; Sharpe, 2016). Human DPSCs are easily harvested neural crest-derived mesenchymal-like stem cells residing in the dental pulp tissue in vivo. They have a spindle-shaped morphology and are easily available from impacted third molars (Fig. 1) (Huang et al, 2009). Their biological role is well described. Dentin is formed by odontoblasts, which are terminally differentiated hard-tissue forming cells in the dentin-pulp complex. Postnatal cells in the dental papilla constitute the stem cell pool called DPSCs, which differentiate into odontoblasts. Therefore, DPSCs play a pivotal role in dentinogenesis (Gronthos et al, 2000). DPSCs are an ideal source of MSCs in vitro, due to their high proliferation rate, self-renewal capability, and easy accessibility.
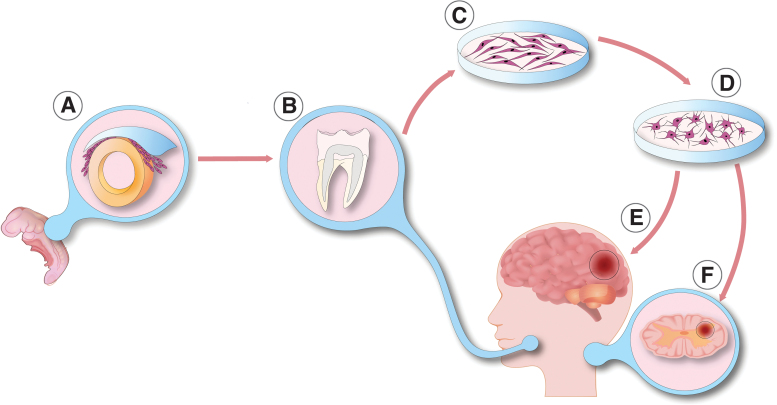
FIG. 1.
Neuronal differentiation of dental pulp cells. (A) During the fourth week of embryonic life—within the ectoderm—dorsally from the neural tube, neural crest cells start to migrate into distinct parts of the developing organs. Later, derivatives of neural crest cells take part in the formation of different craniofacial organs, including the dental pulp complex. (B) Stromal cells from the dental pulp are an easily obtainable source of cells extracted from deciduous or permanent tooth dental. (C) Dental pulp stem cells are spindle-shaped, multipotent stem cells, residing in the stroma of the pulp chamber. After isolation, dental pulp stem cells can start to proliferate in optimal culture conditions. (D) These cells are multipotent and in accordance with their embryonic ontology, under appropriate environmental circumstances, dental pulp stem cells can differentiate into functionally active neuronal cells. (E, F) These in vitro differentiated neuronal cells provide a promising alternative cell source for central nervous system regeneration after ischemic brain damage (E) or spinal cord injury (F).
According to the cross-lineage boundaries, hDPSCs may provide a potential cell pool for regenerative medicine and clinical tissue engineering (Fang et al, 2013; Naz et al, 2022). In line with this, many of the hDPSC-based researches demonstrate their beneficial effect in endodontic therapies (Nakashima et al, 2022). Moreover, hDPSCs promote in vivo tissue regeneration after transplantation into animal models with pathologies associated with cardiological, neurological, and ophthalmological disorders (Anitua et al, 2018; Fang et al, 2013; Shi et al, 2020). Focusing on neurological injuries, Yang et al (2017) transplanted different types of dental stem cells (DSCs), including hDPSCs, into transected rat spinal cord and observed pure functional recovery after 8 weeks. They hypothesized that DSCs, due to inhibition of interleukin-1beta, reduce inflammation in the spinal cord of the rats. DSCs also inhibited injury-induced neuronal, astrocytic, and oligodendrocytic apoptosis.
Multiple inhibitions of axon growth inhibitors through paracrine mechanisms were also described. Interestingly after spinal cord injury, DSCs were able to differentiate into mature neurons and oligodendrocytes in the damaged tissue. This phenomenon has also been reported by others (Sakai et al, 2012; Yamamoto et al, 2014; Yang et al, 2017).
Although several methods were developed to isolate hDPSCs from the dental pulp, isolated hDPSCs—similarly to other MSCs—invariably comprise many various subpopulations according to their biological and regenerative characteristics (Gronthos et al, 2002). These traits present a major obstacle to the regenerative utilization of hDPSC-based therapies. By including divergent proliferation potentials, differentiation properties to lineage-specific fate determination, and cell surface markers, hDPSCs represent a heterogeneous source of MSCs (Kok et al, 2022). Consequently, one of the main challenges using hDPSCs as a cellular source for routine regenerative therapy is the identification of particular cell surface markers and discrimination of different hDPSC subpopulations in terms of their proliferation and differentiation potential, immunomodulatory effect, and other regenerative properties.
Several studies reported candidate markers of hDPSCs, however, no specific hDPSC marker has been identified thus far (Tatullo et al, 2015). The main MSC markers are CD29, CD44, CD73, CD90, CD105, CD146, CD166, CD271, and STRO-1, which are all widely expressed across hDPSC subpopulations (Gronthos et al, 2002; Perczel-Kovach et al, 2021). These markers exemplify the heterogeneous nature of DPSCs (Kawashima, 2012). According to the neighboring persistence of the perivascular niche, subpopulations of hDPSCs express STRO-1, STRO-3, platelet-derived growth factor-beta, vascular endothelial growth factor 1, and CD146 (Shi and Gronthos, 2003). Several studies reported ESC marker expression in hDPSCs: octamer binding transcription factor 4 (OCT-4), homeobox transcription factor nanog, SRY-box transcription factor 2 (SOX-2), stage-specific embryo antigen 4 (SSEA4), and snail family transcriptional repressor 2 (Slug), which influence self-renewal capacity and multipotency (Huang et al, 2009; Kunimatsu et al, 2018).
In addition to the differentiation and proliferation characteristics, subpopulations of hDPSCs can also exhibit anti-inflammatory and immunomodulatory properties (Demircan et al, 2011; Foldes et al, 2016; Racz et al, 2014). Accordingly, hDPSCs induce lymphocyte activation through the secretion of pleiotropic regulators, modulation of antigen-presenting cells, and by building cell-to-cell interactions involved in immune cell adhesion and migration (Ma and Chan, 2016). Several studies reported an increased expression of CD90 on distinct subpopulations of hDPSCs, a commonly used bone marrow-derived MSC marker (Agha-Hosseini et al, 2010; Alongi et al, 2010).
DSCs, including DPSCs, are derived from the neural crest. During embryological development, the central nervous system (CNS) is formed by the ectoderm, the outermost germ layer of the gastrulated embryo (Nikolopoulou et al, 2017). In chordate, CNS precursors are related to the neural tube, which is formed by a certain process called neurulation (Schoenwolf and Smith, 1990). Dorsally from the neural tube during neurulation, certain types of neurectoderm cells disconnect from the epidermis and start to migrate into distinct parts of the developing organism. These migrating cells are the earliest precursors of neural crest cells (Fig. 1). Derivatives of the neural crest are melanocytes, peripheral and enteric neurons, glial cells, different types of connective tissue, and stromal cells mostly in the cardiac and the craniofacial region (Rothstein and Simoes-Costa, 2022; Song et al, 2022). The dental pulp is also formed from neural crest cells.
Progenitors of DPSCs migrate from the cranial neural crest into the pharyngeal arches, and after an ectomesenchymal transition, DPSCs reside in the dental pulp (Ibarretxe et al, 2012; Miletich and Sharpe, 2004). Indeed, derivatives of these cells express both ectodermal and mesodermal markers (Lee et al, 2011). The expression of various neural lineage markers were reported: CD117 (c-Kit), CD271, nestin (NES), glial fibrillary acidic protein (GFAP), beta-III tubulin (TUBB3), S100, Notch 2, Musashi RNA binding protein 1 (MSI1), synaptophysin (SYP), and microtubule associated protein 2 (MAP2) (Karaoz et al, 2011; Kawashima, 2012; Kiraly et al, 2011; Kiraly et al, 2009). In accordance with their embryonic ontology, DPSCs also express cell surface marker CD271, also known as low-affinity nerve growth factor (NGF) receptor, NGF receptor, or p75NTR (neurotrophin receptor) (Alvarez et al, 2015; Martens et al, 2012).
The presence of CD271 correlates with CD105 and neurogenic locus notch homolog protein 2 (Notch 2) expression, low proliferation, and high neurogenic differentiation potential (Alaidaroos et al, 2021; Alraies et al, 2017; Waddington et al, 2009). The lack of this central cell surface antigen marker on DPSCs is associated with high proliferation and colony-forming efficiency (Alaidaroos et al, 2021; Alraies et al, 2017). Altogether, these data suggest the importance of the appropriate identification and purification of DPSCs (Fig. 1).
Numerous studies have reported that hDPSCs are multipotent and able to differentiate into osteogenic (Liu et al, 2009), neurogenic (Arthur et al, 2008), adipogenic (Iohara et al, 2006), and chondrogenic lineages (Iohara et al, 2006). Multipotency closely relates to the biological roles of DPSCs as well as providing the stem cell pool for odontoblasts and for soft tissue regeneration in the dental pulp (Fawzy El-Sayed et al, 2013). Moreover, DPSCs can differentiate into neurogenic lineages that may correlate with their embryonic ontology (Arthur et al, 2008; Kiraly et al, 2011; Kiraly et al, 2009).
In comparison with other mesenchymal stromal stem cells, DPSCs have a higher neurogenic differentiation potential although the tissue heterogeneity leads to differences between the neurogenic DPSC-derived neurons. Using appropriate inductors, DPSCs can differentiate into functional neurons (Fig. 1) (Arthur et al, 2008; Kiraly et al, 2011; Kiraly et al, 2009).
Neural Differentiation
Neurodegenerative diseases cause a progressive loss of functionally active, mature neurons. The replacement of damaged neurons as a therapeutic strategy has been extensively studied, and for regenerative medicine, it is crucial to find autologous, transplantable sources of neurons and glial cells (Barker et al, 2018; Bjorklund and Parmar, 2020; Volkman and Offen, 2017). Neural stem cells (NSCs) are stem cells found in the adult CNS and are able to differentiate into neurons, astrocytes, and oligodendrocytes. They reside in specific brain regions called neurogenic niches (Ming and Song, 2011). An adult human brain contains two neurogenic niches; (i) the subventricular zone of the lateral ventricles and (ii) the subgranular layer of the dentate gyrus. Although NSCs might be a source of transplantable neurons, their availability from the adult human brain is very limited.
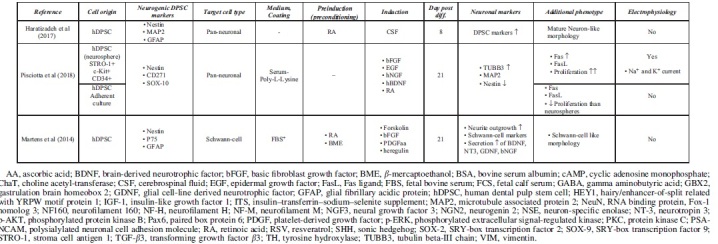
Accessible stem cell sources can be found outside of the CNS (Ming and Song, 2011). Multipotent MSCs, including DPSCs, are able to differentiate into lineage-specific cell types (Dominici et al, 2006). This high differentiation potential correlates with great self-renewal capacity, easy accessibility, and high proliferation ability. Under standard culture conditions, growth factors and chemical inductors were used to differentiate hDPSCs into the neural lineage. During the last two decades, various differentiation strategies were developed to classify functionally active and subtype-specific neurons derived from hDPSCs. Table 1 summarizes the methodologies for neuronal differentiation of human DPSCs (hDPSCs). In general, neuronal induction and maturation of hDPSCs take between 1 and 3 weeks and were all isolated from non-inflamed human third molars.
Table 1.
Summary of Neural Differentiation Methodologies Using Human Dental Pulp Stem Cells

|
Growth factor-mediated approaches activate signal transduction pathways that may promote with neural lineage-specific gene expression (Karaoz et al, 2011). Chemical inductors such as forskolin or valproic acid significantly change the gene expression regulation of hDPSCs due to epigenetic modifications and signaling activation, therefore enhancing the conversion efficiency during neuronal differentiation (Heng et al, 2019). The separate usage of chemical inductors (butylated hydroxyanisole and β-mercaptoethanol) and growth factors, basic fibroblast growth factor (bFGF), epidermal growth factor (EGF), and retinoic acid (RA) was also studied, only resulting in a neuronal-like morphology on the differentiated DPSCs in case of growth factors (Table 1). The usage of chemical inductors, without growth factors, showed false-positive neural differentiation. Interestingly, gamma aminobutyric acid receptor expression was present after the growth factor-mediated differentiation. Functional activation of Notch signaling pathway was also observed in growth factor differentiated DPSCs (Osathanon et al, 2014).
Using chemical inductors and growth factors simultaneously, pan-neuronal and subtype-specific neuronal cells were differentiated, presented in detail in Table 1. Priming stimulation of the FGF/FGFR signaling pathway initiates cell cycle progression and proliferation through activating mitogen activated protein kinase/extracellular signal-regulated kinase (ERK) signaling in hDPSCs (Chang et al, 2017). Several studies reported that FGF/FGFR signaling also promotes neural differentiation in hDPSCs. In the presence of bFGF, increased hDPSC neurosphere size, neural morphology, and neurogenic marker upregulation were observed (Gonmanee et al, 2021; Li et al, 2019a; Nagashima et al, 2017; Osathanon et al, 2011). FGF stimulation supplemented with RA and EGF could not differentiate hDPSCs into subtype-specific neurons, but they were able to induce crucial morphological changes, which led to the functional activation of differentiated neurons by demonstrating their ability to respond directly to their surrounding environment (Arthur et al, 2008).
The presence and functional role of neurotrophic factors such as NGF are well reported from the dental pulp in vivo. NGF is a key regulator of the development of neuronal and non-neuronal cells. Low- and high-affinity NGF receptors, p75, and Tropomyosin receptor kinase (TrkA) respectively, were described at the cell surface of hDPSCs. In injured teeth, NGF and TrkA expression is upregulated, which shows that NGF signaling is strongly linked to the pathological and regenerative process of hDPSCs (Mitsiadis et al, 2017; Pisciotta et al, 2020). Following pretreatment with the demethylating agent 5-azacytidine and bFGF, activation of NGF and neurotrophin 3 (NT-3) signaling transduction pathway differentiated hDPSCs to functionally active, neuron-like cells in the presence of cyclic adenosine monophosphate (cAMP) and protein kinase C activators, such as IMBX (3-isobutyl-1-metilxanthin), forskolin, and tissue plasminogen activator (TPA) (Kiraly et al, 2009).
In the presence of RSV, NGF and bFGF promote the expression of neuronal markers (Nestin, TUBB3, MAP2) and induce neural differentiation through the ERK and protein kinase B signaling pathways (Zhang et al, 2017). Another neurotrophic factor, brain derived neurotrophic factor (BDNF), also plays a pivotal role in the neural commitment of DPSCs (Zhang et al, 2018). Luzuriaga et al (2019b) reported that BDNF and NT-3 are responsible for the differentiation of hDPSCs into NSCs in vitro.
Several studies investigated DPSC differentiation into subtype-specific neurons and glial cells including Schwann cells (Martens et al, 2014). Pre-exposure to bFGF and EGF and activation of MAPK/ERK signaling pathways, at the induction phase, appropriate neurotrophins from the embryonic development of spinal motor neurons or dopaminergic neurons are able to differentiate premature hDPSC-derived neurons to subtype-specific derivatives (Gonmanee et al, 2018; Vescovi et al, 1993). It has been reported that growth factors such as BDNF, glial cell-line derived neurotrophic factor (GDNF), NT-3, and NGF contribute to differentiate dopaminergic neurons in vitro (Fujii et al, 2015; Trzaska et al, 2009). In comparison with bone-marrow derived MSCs (BM-MSCs), neural-differentiated hDPSCs yield better cells in terms of neural-specific gene expression including the dopaminergic marker tyrosine hydroxylase (TH) (Singh et al, 2017).
Spinal motor neurons are localized at the ventral part of the spinal cord and during their embryonic development, several ventralization agents are contributing to their morphological fate determination, including sonic hedgehog (SHH) (Ravanelli and Appel, 2015; Yang et al, 2019). Using SHH in hDPSCs for the preinduction phase with their synergistic chemical inductor RA has promoted spinal motor neuron-like morphology and gene expression after being cultured with BDNF, GDNF, insulin-like growth factor 1 (IGF-1), and cAMP activators (Chang et al, 2014; Hua et al, 2021). Darabi et al (2019) also studied and presented a cholinergic differentiation of hDPSCs.
To maximize neuronal maturity and avoid undesired immune reactivity of transplanted DPSCs in vivo, the usage of xenogeneic serum elements is discouraged, although the growth rates of DPSCs are usually lower in serum-free environments than in the FBS-containing media (Gregory et al, 2006; Luo et al, 2018b; Luzuriaga et al, 2019a; Solis-Castro et al, 2020). Several protocols have been published to optimize the culture conditions of serum-free differentiated hDPSCs (Madanagopal et al, 2020; Sonoda et al, 2022; Zhang et al, 2017). Serum-free approaches are also applied to culture hDPSCs in spheroids, which may act as microniches providing a better culture environment to differentiate DPSCs (Bonnamain et al, 2013; Gonmanee et al, 2021). In comparison with adherent culture conditions, hDPSC spheroids show significant differences in neural gene expression, maturity, and proliferation potential (Gervois et al, 2015; Luzuriaga et al, 2019a; Xiao and Tsutsui, 2013).
In spheroid culture conditions, the expression of neural crest-derived genes (Nestin, CD2171, SOX-10) is notably higher than in adherent cultures, suggesting that spheres are favorable for the stemness and neuro-ectomesenchymal properties. In this process, insulin-like growth factor binding protein 5 (IGFBP5) plays a pivotal role by inducing the overexpression of neural cell adhesion molecule (NCAM), a key regulator of axon growth that promotes cell adhesion and migration. Under IGFBP5 stimulation, the number of Nestin- and TUBB3-positive neurospheres also increased (Chernyshova et al, 2011; Li et al, 2019b). It is noteworthy that differences between progenitor and mature neural gene expression were also described in spheres in comparison with adherent culture-derived hDPSCs. The relative expression of TUBB3 and MAP2, which are both markers of neural lineage commitment, is higher at the same passage numbers than in adherent cultured hDPSCs.
In addition, differences in proliferation ability are also observed between adherent and spheroid conditions (Pisciotta et al, 2018). Notwithstanding, the expansion of hDPSCs cultivated on microspheres is also a promising direction for future studies (Foldes et al, 2021).
Interestingly, the cerebrospinal fluid (CSF) also provided a favorable microenvironment for differentiating hDPSCs into neurogenic lineages (Goudarzi et al, 2020). As the CSF is in direct contact with NSCs in the CNS, it contains neurotrophic and growth factors with other nutrients and cytokines, which are able to induce neural differentiation of hDPSCs with the simultaneous usage of RA. In addition, these RA/CSF-treated neurons passed the preneuronal stage and differentiated into mature neurons in induction culture due to the simultaneous activation of multiple molecular signaling transduction cascades. This condition is similar to the in vivo proceedings during embryonic development (Haratizadeh et al, 2017).
In summary, spindle-shaped hDPSCs can efficiently be differentiated into neuronal-like cells with neurite outgrowth and bulbous soma that express several neuronal markers (e.g., NSE, NCAM, TUBB3, MAP2, NeuN). Electrophysiological analyses confirmed the functional presence of voltage-gated sodium and potassium channels involved in action potentials in hDPSC-derived neurons (Arimura et al, 2021; Arthur et al, 2008; Kiraly et al, 2009; Li et al, 2019a). Arimure et al (2021) found the presence of transient receptor potential (TRP) channels in the differentiated hDPSCs, by also examining the role of TRP channels during intracellular Ca2+ response. Ullah et al (2016) examined the functional maturity of differentiated hDPSCs and found a subpopulation-dependent electrophysiological maturation of hDPSC-derived neuronal cells. These data outline the importance of dental pulp tissue heterogeneity and how this affects the neurogenic differentiation of DPSCs.
Discussion
Functionally active, mature neurons are anticipated to restore neural cells after injury or progressive loss of neurons in the CNS. To obtain hDPSC-derived neurons as an alternative cell source in regenerative medicine, various differentiation methodologies have been described. Cytoskeleton-associated proteins such as TUBB3, Nestin, GFAP, and MAP2 have been widely used in DPSC-based studies as neuronal markers (Govindasamy et al, 2010). It is important to emphasize that even these commonly used markers show a temporal expression profile during in vivo maturation of neurons. Undifferentiated hDPSCs, due to their neural crest origin, express both immature and mature neural markers (Rafiee et al, 2020). After neural differentiation, the change of relative marker expression levels is apparent, although there is no commonly accepted gold standard in the field so far as to which protocol leads to the optimal decrease of immature and increase of mature neural markers.
Another key aspect in cell therapy is the effective transplantation of differentiated hDPSCs to the target area in vivo. Scaffolds are biomaterials intended to cause desirable cellular interactions and support the three-dimensional formation of in vitro seeded cells (Henriques et al, 2019). hDPSCs conditioned with growth factors were able to grow and differentiate into neurons in highly porous chitosan scaffolds. In vivo transplantation of hDPSCs/chitosan scaffolds may provide a more physiologic microenvironment during attachment for transplanted hDPSCs (Zhang et al, 2016). In vivo injection of hDPSCs improved behavioral dysfunctions and survived several weeks in a neonatal hypoxic–ischemic brain damaged rat model (Fang et al, 2013). Combining hDPSCs with scaffold materials increased the expression of several neurotrophins such as BDNF, NT-3, or NGF, all of which are reported to be important for an enhanced tissue regeneration.
Synthetic and natural polymers such as heparin-poloxamer, poly-lactide-co-glycolide, and even chitosan polysaccharide promoted peripheral nerve regeneration in murine models (Luo et al, 2018a; Sasaki et al, 2011). Enhancing the neural maturity, functional activity, neurotrophin secretion, and transplantation efficiency of hDPSC-derived neurons into the host organism requires more research efforts to maximize the regenerative potential of hDPSCs. In line with this, scaffolds may serve as a promising solution for transplantation and integrating cells into the host organism.
Similar to other tissues, the dental pulp represents a heterogenous source of expanded cells, including a variable proliferation rate and cellular senescence. Alraies et al (2017) reported that there is a significant variability in the proliferative potential and cellular senescence between different patient-derived hDPSCs within a similar age range, and even identified inherent differences between hDPSC subpopulations derived from the same patient. Proliferative and regenerative heterogeneity is closely related to the replicative senescence (telomere length) and, interestingly, to the expression of the low-affinity NGF receptor, CD271. CD271+ cells showed a lower proliferation rate and a higher neurogenic potential suggesting that the presence of this specific neural crest marker was related to neuronal cell fate lineage restriction of hDPSCs (Alraies et al, 2017; Kok et al, 2022).
Selective screening for neurogenic niches of DPSCs within these subpopulations or the transplantation of nondifferentiated CD271+ cells for regenerative issues may lead to better results in the long term in DPSC-derived therapies. Overall, these data suggest the importance of population selection and selective screening from dental pulp tissue for in vitro expansion, aiding for more effective hDPSC-based therapies for clinical application.
During the last two decades, transcription-factor-mediated neuronal reprogramming approaches were developed. hDPSCs might be a promising cell source for pluripotent phase-mediated and direct reprogramming strategies (Tamaoki et al, 2010; Yan et al, 2010). Using fibroblasts as a cell source, the combinatorial expression of neural lineage-specific transcription factors led to directly converted neurons in vitro (Vierbuchen et al, 2010). These cells preserve donor age-dependent epigenetic and transcriptomic signatures and are able to model human aging in several late-stage neurodegenerative diseases such as Alzheimer's disease, Huntington's disease, and Parkinson's disease (Drouin-Ouellet et al, 2017a, Drouin-Ouellet et al, 2017b; Legault et al, 2022; Mertens et al, 2015; Pircs et al, 2022). In the case of DPSCs, transcription factor-mediated conversions were used to induce overexpression of POU domain class 5, transcription factor 1 (OCT-4), and SOX-2.
However, studies focused on the forced overexpression of specific neural lineage commitment transcription factors such as achaete-scute family BHLH transcription factor 1 (ASCL1), POU domain class 3 transcription factor 2 (BRN2), and myelin transcription factor 1 like (MYT1L) are not described yet (Liu et al, 2015a, Liu et al, 2015b).
Cellular heterogeneity is an important property of the dental pulp cell niche. Although individual hDPSC subpopulations share certain similarities, heterogeneity is a crucial factor considering the therapeutic benefits of DPSC usage (Kok et al, 2022). The previously mentioned cell marker CD271 certainly set an example for this phenomenon. High cellular proliferating ability is crucial in regenerative applications but does not simultaneously mean high neurogenic potential. In comparison with other transcription factor-mediated approaches or growth factor-mediated differentiation strategies, one of the main advantages of direct reprogramming is that transdifferentiated cells represent the whole cellular diversity of the donor tissue (Mertens et al, 2016). We propose that direct neuronal reprogramming using hDPSCs would be beneficial when cellular diversity is preferred while preserving the genetic and epigenetic identity of the donor. In addition, directly reprogrammed cells preserve the age-related signatures of donor cells (Drouin-Ouellet et al, 2017b; Mertens et al, 2016; Pircs et al, 2022).
Since hDPSCs can be harvested from extracted third molars, these cells can be easily accessed without ethical issues to obtain neuronal cells from young patients used for further studies (Yamada et al, 2019). Therefore, directly converted hDPSCs could provide an excellent cellular source for future studies focusing on neuronal aging and neuronal rejuvenation by using cells from donors of all ages. We postulate that transcription factor-mediated approaches with simultaneous usage of growth factor-mediated differentiation protocols may allow an increased neural maturity and differentiation efficiency. In summary, hDPSC-derived neurons can potentially overcome various limitations of stem cell differentiation by providing an efficient, easily accessible cellular source for neural cell restoration. This can greatly induce the development of novel regenerative therapeutic strategies in the future.
Acknowledgments
We are grateful to Janelle Drouin-Ouellet for excellent comments on the article and to Anna Anoir Abbas for valuable help with illustrations.
Authors' Contributions
B.S.: Visualization, writing—original draft, and writing—review and editing. A.F.: Writing—review and editing. K.K.: Writing—review and editing. G.V.: Writing—review and editing. Á.Z.: Writing—review and editing. K.P.: Supervision, writing—original draft, and writing—review and editing.
Author Disclosure Statement
The authors declare they have no conflicting financial interests.
Funding Information
This research was supported, in whole or in part, by the STIA-KFI-2020, STIA-PoC-2020, TKP2021-EGA-23, TKP-NVA-20, ICGEB CRP/HUN21-05_EC. TKP-NVA-20 has been implemented with the support provided by the Ministry of Innovation and Technology of Hungary from the National Research, Development and Innovation Fund, financed under the TKP-NVA funding scheme. The project has received funding from the EU Horizon 2020 research and innovation program under grant agreement number 739593. This study was also supported by a Hungarian National Research, Development and Innovation Office grant (NKFIH K-125161). This research work was also conducted with the support of the National Academy of Scientist Education Program of the National Biomedical Foundation under the sponsorship of the Hungarian Ministry of Culture and Innovation (FEIF/646-4/2021-ITM_SZERZ).
References
- Agha-Hosseini F, Jahani MA, Jahani M, et al. In vitro isolation of stem cells derived from human dental pulp. Clin Transplant 2010;24(2):E23–E28; doi: 10.1111/j.1399-0012.2009.01137.x [DOI] [PubMed] [Google Scholar]
- Alaidaroos NYA, Alraies A, Waddington RJ, et al. Differential SOD2 and GSTZ1 profiles contribute to contrasting dental pulp stem cell susceptibilities to oxidative damage and premature senescence. Stem Cell Res Ther 2021;12(1):142; doi: 10.1186/s13287-021-02209-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alongi DJ, Yamaza T, Song Y, et al. Stem/progenitor cells from inflamed human dental pulp retain tissue regeneration potential. Regen Med 2010;5(4):617–631; doi: 10.2217/rme.10.30 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alraies A, Alaidaroos NY, Waddington RJ, et al. Variation in human dental pulp stem cell ageing profiles reflect contrasting proliferative and regenerative capabilities. BMC Cell Biol 2017;18(1):12; doi: 10.1186/s12860-017-0128-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarez R, Lee HL, Hong C, et al. Single CD271 marker isolates mesenchymal stem cells from human dental pulp. Int J Oral Sci 2015;7(4):205–212; doi: 10.1038/ijos.2015.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews PW. Human pluripotent stem cells: Tools for regenerative medicine. Biomater Transl 2021;2(4):294–300; doi: 10.12336/biomatertransl.2021.04.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anitua E, Troya M, Zalduendo M. Progress in the use of dental pulp stem cells in regenerative medicine. Cytotherapy 2018;20(4):479–498; doi: 10.1016/j.jcyt.2017.12.011 [DOI] [PubMed] [Google Scholar]
- Arimura Y, Shindo Y, Yamanaka R, et al. Peripheral-neuron-like properties of differentiated human dental pulp stem cells (hDPSCs). PLoS One 2021;16(5):e0251356; doi: 10.1371/journal.pone.0251356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arthur A, Rychkov G, Shi S, et al. Adult human dental pulp stem cells differentiate toward functionally active neurons under appropriate environmental cues. Stem Cells 2008;26(7):1787–1795; doi: 10.1634/stemcells.2007-0979 [DOI] [PubMed] [Google Scholar]
- Bansal R, Jain A. Current overview on dental stem cells applications in regenerative dentistry. J Nat Sci Biol Med 2015;6(1):29–34; doi: 10.4103/0976-9668.149074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker RA, Gotz M, Parmar M. New approaches for brain repair-from rescue to reprogramming. Nature 2018;557(7705):329–334; doi: 10.1038/s41586-018-0087-1 [DOI] [PubMed] [Google Scholar]
- Bertrand V, Hobert O. Lineage programming: Navigating through transient regulatory states via binary decisions. Curr Opin Genet Dev 2010;20(4):362–368; doi: 10.1016/j.gde.2010.04.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjorklund A, Parmar M. Neuronal Replacement as a Tool for Basal Ganglia Circuitry Repair: 40 Years in Perspective. Front Cell Neurosci 2020;14:146; doi: 10.3389/fncel.2020.00146 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bocchi R, Masserdotti G, Gotz M. Direct neuronal reprogramming: Fast forward from new concepts toward therapeutic approaches. Neuron 2022;110(3):366–393; doi: 10.1016/j.neuron.2021.11.023 [DOI] [PubMed] [Google Scholar]
- Bonnamain V, Thinard R, Sergent-Tanguy S, et al. Human dental pulp stem cells cultured in serum-free supplemented medium. Front Physiol 2013;4:357; doi: 10.3389/fphys.2013.00357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campanella V. Dental Stem Cells: Current research and future applications. Eur J Paediatr Dent 2018;19(4):257; doi: 10.23804/ejpd.2018.19.04.1 [DOI] [PubMed] [Google Scholar]
- Chang CC, Chang KC, Tsai SJ, et al. Neurogenic differentiation of dental pulp stem cells to neuron-like cells in dopaminergic and motor neuronal inductive media. J Formos Med Assoc 2014;113(12):956–965; doi: 10.1016/j.jfma.2014.09.003 [DOI] [PubMed] [Google Scholar]
- Chang YC, Chang MC, Chen YJ, et al. Basic Fibroblast Growth Factor Regulates Gene and Protein Expression Related to Proliferation, Differentiation, and Matrix Production of Human Dental Pulp Cells. J Endod 2017;43(6):936–942; doi: 10.1016/j.joen.2017.01.024 [DOI] [PubMed] [Google Scholar]
- Charitos IA, Ballini A, Cantore S, et al. Stem Cells: A Historical Review about Biological, Religious, and Ethical Issues. Stem Cells Int 2021;2021:9978837; doi: 10.1155/2021/9978837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernyshova Y, Leshchyns'ka I, Hsu SC, et al. The neural cell adhesion molecule promotes FGFR-dependent phosphorylation and membrane targeting of the exocyst complex to induce exocytosis in growth cones. J Neurosci 2011;31(10):3522–3535; doi: 10.1523/JNEUROSCI.3109-10.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darabi S, Tiraihi T, Nazm Bojnordi M, et al. Trans-Differentiation of Human Dental Pulp Stem Cells Into Cholinergic-Like Neurons Via Nerve Growth Factor. Basic Clin Neurosci 2019;10(6):609–617; doi: 10.32598/bcn.10.6.609 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demircan PC, Sariboyaci AE, Unal ZS, et al. Immunoregulatory effects of human dental pulp-derived stem cells on T cells: Comparison of transwell co-culture and mixed lymphocyte reaction systems. Cytotherapy 2011;13(10):1205–1220; doi: 10.3109/14653249.2011.605351 [DOI] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006;8(4):315–317; doi: 10.1080/14653240600855905 [DOI] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Lau S, Brattås PL, et al. REST suppression mediates neural conversion of adult human fibroblasts via microRNA-dependent and -independent pathways. EMBO Mol Med 2017a;9(8):1117–1131; doi: 10.15252/emmm.201607471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Pircs K, Barker RA, et al. Direct Neuronal Reprogramming for Disease Modeling Studies Using Patient-Derived Neurons: What Have We Learned? Front Neurosci 2017b;11:530; doi: 10.3389/fnins.2017.00530 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CZ, Yang YJ, Wang QH, et al. Intraventricular injection of human dental pulp stem cells improves hypoxic-ischemic brain damage in neonatal rats. PLoS One 2013;8(6):e66748; doi: 10.1371/journal.pone.0066748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fawzy El-Sayed KM, Dorfer C, Fandrich F, et al. Adult mesenchymal stem cells explored in the dental field. Adv Biochem Eng Biotechnol 2013;130:89–103; doi: 10.1007/10_2012_151 [DOI] [PubMed] [Google Scholar]
- Foldes A, Kadar K, Keremi B, et al. Mesenchymal Stem Cells of Dental Origin-Their Potential for Antiinflammatory and Regenerative Actions in Brain and Gut Damage. Curr Neuropharmacol 2016;14(8):914–934; doi: 10.2174/1570159x14666160121115210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foldes A, Reider H, Varga A, et al. Culturing and Scaling up Stem Cells of Dental Pulp Origin Using Microcarriers. Polymers (Basel) 2021;13(22); doi: 10.3390/polym13223951 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frobel J, Hemeda H, Lenz M, et al. Epigenetic rejuvenation of mesenchymal stromal cells derived from induced pluripotent stem cells. Stem Cell Reports 2014;3(3):414–422; doi: 10.1016/j.stemcr.2014.07.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii H, Matsubara K, Sakai K, et al. Dopaminergic differentiation of stem cells from human deciduous teeth and their therapeutic benefits for Parkinsonian rats. Brain Res 2015;1613:59–72; doi: 10.1016/j.brainres.2015.04.001 [DOI] [PubMed] [Google Scholar]
- Furlong EE. The importance of being specified: Cell fate decisions and their role in cell biology. Mol Biol Cell 2010;21(22):3797–3798; doi: 10.1091/mbc.E10-05-0436 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gervois P, Struys T, Hilkens P, et al. Neurogenic maturation of human dental pulp stem cells following neurosphere generation induces morphological and electrophysiological characteristics of functional neurons. Stem Cells Dev 2015;24(3):296–311; doi: 10.1089/scd.2014.0117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonmanee T, Arayapisit T, Vongsavan K, et al. Optimal culture conditions for neurosphere formation and neuronal differentiation from human dental pulp stem cells. J Appl Oral Sci 2021;29:e20210296; doi: 10.1590/1678-7757-2021-0296 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonmanee T, Thonabulsombat C, Vongsavan K, et al. Differentiation of stem cells from human deciduous and permanent teeth into spiral ganglion neuron-like cells. Arch Oral Biol 2018;88:34–41; doi: 10.1016/j.archoralbio.2018.01.011 [DOI] [PubMed] [Google Scholar]
- Goudarzi G, Hamidabadi HG, Bojnordi MN, et al. Role of cerebrospinal fluid in differentiation of human dental pulp stem cells into neuron-like cells. Anat Cell Biol 2020;53(3):292–300; doi: 10.5115/acb.19.241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindasamy V, Abdullah AN, Ronald VS, et al. Inherent differential propensity of dental pulp stem cells derived from human deciduous and permanent teeth. J Endod 2010;36(9):1504–1515; doi: 10.1016/j.joen.2010.05.006 [DOI] [PubMed] [Google Scholar]
- Grath A, Dai G. Direct cell reprogramming for tissue engineering and regenerative medicine. J Biol Eng 2019;13:14; doi: 10.1186/s13036-019-0144-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory CA, Reyes E, Whitney MJ, et al. Enhanced engraftment of mesenchymal stem cells in a cutaneous wound model by culture in allogenic species-specific serum and administration in fibrin constructs. Stem Cells 2006;24(10):2232–2243; doi: 10.1634/stemcells.2005-0612 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Brahim J, Li W, et al. Stem cell properties of human dental pulp stem cells. J Dent Res 2002;81(8):531–535; doi: 10.1177/154405910208100806 [DOI] [PubMed] [Google Scholar]
- Gronthos S, Mankani M, Brahim J, et al. Postnatal human dental pulp stem cells (DPSCs) in vitro and in vivo. Proc Natl Acad Sci U S A 2000;97(25):13625–13630; doi: 10.1073/pnas.240309797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haratizadeh S, Nazm Bojnordi M, Darabi S, et al. Condition medium of cerebrospinal fluid and retinoic acid induces the transdifferentiation of human dental pulp stem cells into neuroglia and neural like cells. Anat Cell Biol 2017;50(2):107–114; doi: 10.5115/acb.2017.50.2.107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heng BC, Jiang S, Yi B, et al. Small molecules enhance neurogenic differentiation of dental-derived adult stem cells. Arch Oral Biol 2019;102:26–38; doi: 10.1016/j.archoralbio.2019.03.024 [DOI] [PubMed] [Google Scholar]
- Henriques D, Moreira R, Schwamborn J, et al. Successes and Hurdles in Stem Cells Application and Production for Brain Transplantation. Front Neurosci 2019;13:1194; doi: 10.3389/fnins.2019.01194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong IS. Enhancing Stem Cell-Based Therapeutic Potential by Combining Various Bioengineering Technologies. Front Cell Dev Biol 2022;10:901661; doi: 10.3389/fcell.2022.901661 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hua TT, Bejoy J, Song L, et al. Cerebellar Differentiation from Human Stem Cells Through Retinoid, Wnt, and Sonic Hedgehog Pathways. Tissue Eng Part A 2021;27(13–14):881–893; doi: 10.1089/ten.TEA.2020.0135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang GT, Gronthos S, Shi S. Mesenchymal stem cells derived from dental tissues vs. those from other sources: Their biology and role in regenerative medicine. J Dent Res 2009;88(9):792–806; doi: 10.1177/0022034509340867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibarretxe G, Crende O, Aurrekoetxea M, et al. Neural crest stem cells from dental tissues: A new hope for dental and neural regeneration. Stem Cells Int 2012;2012:103503; doi: 10.1155/2012/103503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iohara K, Zheng L, Ito M, et al. Side population cells isolated from porcine dental pulp tissue with self-renewal and multipotency for dentinogenesis, chondrogenesis, adipogenesis, and neurogenesis. Stem Cells 2006;24(11):2493–2503; doi: 10.1634/stemcells.2006-0161 [DOI] [PubMed] [Google Scholar]
- Kane AE, Sinclair DA. Epigenetic changes during aging and their reprogramming potential. Crit Rev Biochem Mol Biol 2019;54(1):61–83; doi: 10.1080/10409238.2019.1570075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karaoz E, Demircan PC, Saglam O, et al. Human dental pulp stem cells demonstrate better neural and epithelial stem cell properties than bone marrow-derived mesenchymal stem cells. Histochem Cell Biol 2011;136(4):455–473; doi: 10.1007/s00418-011-0858-3 [DOI] [PubMed] [Google Scholar]
- Kawashima N. Characterisation of dental pulp stem cells: A new horizon for tissue regeneration? Arch Oral Biol 2012;57(11):1439–1458; doi: 10.1016/j.archoralbio.2012.08.010 [DOI] [PubMed] [Google Scholar]
- Kiraly M, Kadar K, Horvathy DB, et al. Integration of neuronally predifferentiated human dental pulp stem cells into rat brain in vivo. Neurochem Int 2011;59(3):371–381; doi: 10.1016/j.neuint.2011.01.006 [DOI] [PubMed] [Google Scholar]
- Kiraly M, Porcsalmy B, Pataki A, et al. Simultaneous PKC and cAMP activation induces differentiation of human dental pulp stem cells into functionally active neurons. Neurochem Int 2009;55(5):323–332; doi: 10.1016/j.neuint.2009.03.017 [DOI] [PubMed] [Google Scholar]
- Kok ZY, Alaidaroos NYA, Alraies A, et al. Dental Pulp Stem Cell Heterogeneity: Finding Superior Quality “Needles” in a Dental Pulpal “Haystack” for Regenerative Medicine-Based Applications. Stem Cells Int 2022;2022:9127074; doi: 10.1155/2022/9127074 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolios G, Moodley Y. Introduction to stem cells and regenerative medicine. Respiration 2013;85(1):3–10; doi: 10.1159/000345615 [DOI] [PubMed] [Google Scholar]
- Kunimatsu R, Nakajima K, Awada T, et al. Comparative characterization of stem cells from human exfoliated deciduous teeth, dental pulp, and bone marrow-derived mesenchymal stem cells. Biochem Biophys Res Commun 2018;501(1):193–198; doi: 10.1016/j.bbrc.2018.04.213 [DOI] [PubMed] [Google Scholar]
- Lee S, An S, Kang TH, et al. Comparison of mesenchymal-like stem/progenitor cells derived from supernumerary teeth with stem cells from human exfoliated deciduous teeth. Regen Med 2011;6(6):689–699; doi: 10.2217/rme.11.95 [DOI] [PubMed] [Google Scholar]
- Legault EM, Bouquety J, Drouin-Ouellet J. Disease Modeling of Neurodegenerative Disorders Using Direct Neural Reprogramming. Cell Reprogram 2022;24(5); doi: 10.1089/cell.2021.0172 [DOI] [PubMed] [Google Scholar]
- Li D, Zou XY, El-Ayachi I, et al. Human Dental Pulp Stem Cells and Gingival Mesenchymal Stem Cells Display Action Potential Capacity In Vitro after Neuronogenic Differentiation. Stem Cell Rev Rep 2019a;15(1):67–81; doi: 10.1007/s12015-018-9854-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Diao S, Yang H, et al. IGFBP5 promotes angiogenic and neurogenic differentiation potential of dental pulp stem cells. Dev Growth Differ 2019b;61(9):457–465; doi: 10.1111/dgd.12632 [DOI] [PubMed] [Google Scholar]
- Liau LL, Ruszymah BHI, Ng MH, et al. Characteristics and clinical applications of Wharton's jelly-derived mesenchymal stromal cells. Curr Res Transl Med 2020;68(1):5–16; doi: 10.1016/j.retram.2019.09.001 [DOI] [PubMed] [Google Scholar]
- Liu L, Ling J, Wei X, et al. Stem cell regulatory gene expression in human adult dental pulp and periodontal ligament cells undergoing odontogenic/osteogenic differentiation. J Endod 2009;35(10):1368–1376; doi: 10.1016/j.joen.2009.07.005 [DOI] [PubMed] [Google Scholar]
- Liu L, Wu L, Wei X, et al. Induced overexpression of Oct4A in human dental pulp cells enhances pluripotency and multilineage differentiation capability. Stem Cells Dev 2015a;24(8):962–972; doi: 10.1089/scd.2014.0388 [DOI] [PubMed] [Google Scholar]
- Liu P, Cai J, Dong D, et al. Effects of SOX2 on Proliferation, Migration and Adhesion of Human Dental Pulp Stem Cells. PLoS One 2015b;10(10):e0141346; doi: 10.1371/journal.pone.0141346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, Albashari AA, Wang X, et al. Effects of Transplanted Heparin-Poloxamer Hydrogel Combining Dental Pulp Stem Cells and bFGF on Spinal Cord Injury Repair. Stem Cells Int 2018a;2018:2398521; doi: 10.1155/2018/2398521 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luo L, He Y, Wang X, et al. Potential Roles of Dental Pulp Stem Cells in Neural Regeneration and Repair. Stem Cells Int 2018b;2018:1731289; doi: 10.1155/2018/1731289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga J, Pastor-Alonso O, Encinas JM, et al. Human Dental Pulp Stem Cells Grown in Neurogenic Media Differentiate Into Endothelial Cells and Promote Neovasculogenesis in the Mouse Brain. Front Physiol 2019a;10:347; doi: 10.3389/fphys.2019.00347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzuriaga J, Pineda JR, Irastorza I, et al. BDNF and NT3 Reprogram Human Ectomesenchymal Dental Pulp Stem Cells to Neurogenic and Gliogenic Neural Crest Progenitors Cultured in Serum-Free Medium. Cell Physiol Biochem 2019b;52(6):1361–1380; doi: 10.33594/000000096 [DOI] [PubMed] [Google Scholar]
- Ma OK, Chan KH. Immunomodulation by mesenchymal stem cells: Interplay between mesenchymal stem cells and regulatory lymphocytes. World J Stem Cells 2016;8(9):268–278; doi: 10.4252/wjsc.v8.i9.268 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madanagopal TT, Franco-Obregon A, Rosa V. Comparative study of xeno-free induction protocols for neural differentiation of human dental pulp stem cells in vitro. Arch Oral Biol 2020;109:104572; doi: 10.1016/j.archoralbio.2019.104572 [DOI] [PubMed] [Google Scholar]
- Marrelli M, Paduano F, Tatullo M. Human periapical cyst-mesenchymal stem cells differentiate into neuronal cells. J Dent Res 2015;94(6):843–852; doi: 10.1177/0022034515570316 [DOI] [PubMed] [Google Scholar]
- Martello G, Smith A. The nature of embryonic stem cells. Annu Rev Cell Dev Biol 2014;30:647–675; doi: 10.1146/annurev-cellbio-100913-013116 [DOI] [PubMed] [Google Scholar]
- Martens W, Sanen K, Georgiou M, et al. Human dental pulp stem cells can differentiate into Schwann cells and promote and guide neurite outgrowth in an aligned tissue-engineered collagen construct in vitro. FASEB J 2014;28(4):1634–1643; doi: 10.1096/fj.13-243980 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martens W, Wolfs E, Struys T, et al. Expression pattern of basal markers in human dental pulp stem cells and tissue. Cells Tissues Organs 2012;196(6):490–500; doi: 10.1159/000338654 [DOI] [PubMed] [Google Scholar]
- Mayo V, Sawatari Y, Huang CY, et al. Neural crest-derived dental stem cells—where we are and where we are going. J Dent 2014;42(9):1043–1051; doi: 10.1016/j.jdent.2014.04.007 [DOI] [PubMed] [Google Scholar]
- Mertens J, Marchetto MC, Bardy C, et al. Evaluating cell reprogramming, differentiation and conversion technologies in neuroscience. Nat Rev Neurosci 2016;17(7):424–437; doi: 10.1038/nrn.2016.46 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mertens J, Paquola ACM, Ku M, et al. Directly Reprogrammed Human Neurons Retain Aging-Associated Transcriptomic Signatures and Reveal Age-Related Nucleocytoplasmic Defects. Cell Stem Cell 2015;17(6):705–718; doi: 10.1016/j.stem.2015.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miletich I, Sharpe PT. Neural crest contribution to mammalian tooth formation. Birth Defects Res C Embryo Today 2004;72(2):200–212; doi: 10.1002/bdrc.20012 [DOI] [PubMed] [Google Scholar]
- Ming GL, Song H. Adult neurogenesis in the mammalian brain: Significant answers and significant questions. Neuron 2011;70(4):687–702; doi: 10.1016/j.neuron.2011.05.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsiadis TA, Magloire H, Pagella P. Nerve growth factor signalling in pathology and regeneration of human teeth. Sci Rep 2017;7(1):1327; doi: 10.1038/s41598-017-01455-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagashima K, Miwa T, Soumiya H, et al. Priming with FGF2 stimulates human dental pulp cells to promote axonal regeneration and locomotor function recovery after spinal cord injury. Sci Rep 2017;7(1):13500; doi: 10.1038/s41598-017-13373-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakashima M, Fukuyama F, Iohara K. Pulp Regenerative Cell Therapy for Mature Molars: A Report of 2 Cases. J Endod 2022;48(10):1334–1340.e1; doi: 10.1016/j.joen.2022.07.010 [DOI] [PubMed] [Google Scholar]
- Naz S, Khan FR, Khan I, et al. Comparative analysis of dental pulp stem cells and stem cells from human exfoliated teeth in terms of growth kinetics, immunophenotype, self-renewal and multi lineage differentiation potential for future perspective of calcified tissue regeneration. Pak J Med Sci 2022;38(5):1228–1237; doi: 10.12669/pjms.38.5.5187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikolopoulou E, Galea GL, Rolo A, et al. Neural tube closure: Cellular, molecular and biomechanical mechanisms. Development 2017;144(4):552–566; doi: 10.1242/dev.145904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osathanon T, Nowwarote N, Pavasant P. Basic fibroblast growth factor inhibits mineralization but induces neuronal differentiation by human dental pulp stem cells through a FGFR and PLCgamma signaling pathway. J Cell Biochem 2011;112(7):1807–1816; doi: 10.1002/jcb.23097 [DOI] [PubMed] [Google Scholar]
- Osathanon T, Sawangmake C, Nowwarote N, et al. Neurogenic differentiation of human dental pulp stem cells using different induction protocols. Oral Dis 2014;20(4):352–358; doi: 10.1111/odi.12119 [DOI] [PubMed] [Google Scholar]
- Perczel-Kovach K, Hegedus O, Foldes A, et al. STRO-1 positive cell expansion during osteogenic differentiation: A comparative study of three mesenchymal stem cell types of dental origin. Arch Oral Biol 2021;122:104995; doi: 10.1016/j.archoralbio.2020.104995 [DOI] [PubMed] [Google Scholar]
- Pircs K, Drouin-Ouellet J, Horvath V, et al. Distinct subcellular autophagy impairments in induced neurons from Huntington's disease patients. Brain 2022;145(9):3035–3057; doi: 10.1093/brain/awab473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisciotta A, Bertoni L, Riccio M, et al. Use of a 3D Floating Sphere Culture System to Maintain the Neural Crest-Related Properties of Human Dental Pulp Stem Cells. Front Physiol 2018;9:547; doi: 10.3389/fphys.2018.00547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pisciotta A, Bertoni L, Vallarola A, et al. Neural crest derived stem cells from dental pulp and tooth-associated stem cells for peripheral nerve regeneration. Neural Regen Res 2020;15(3):373–381; doi: 10.4103/1673-5374.266043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittenger MF, Discher DE, Peault BM, et al. Mesenchymal stem cell perspective: Cell biology to clinical progress. NPJ Regen Med 2019;4:22; doi: 10.1038/s41536-019-0083-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racz GZ, Kadar K, Foldes A, et al. Immunomodulatory and potential therapeutic role of mesenchymal stem cells in periodontitis. J Physiol Pharmacol 2014;65(3):327–339. [PubMed] [Google Scholar]
- Rafiee F, Pourteymourfard-Tabrizi Z, Mahmoudian-Sani MR, et al. Differentiation of dental pulp stem cells into neuron-like cells. Int J Neurosci 2020;130(2):107–116; doi: 10.1080/00207454.2019.1664518 [DOI] [PubMed] [Google Scholar]
- Ratcliffe E, Glen KE, Naing MW, et al. Current status and perspectives on stem cell-based therapies undergoing clinical trials for regenerative medicine: Case studies. Br Med Bull 2013;108:73–94; doi: 10.1093/bmb/ldt034 [DOI] [PubMed] [Google Scholar]
- Ravanelli AM, Appel B. Motor neurons and oligodendrocytes arise from distinct cell lineages by progenitor recruitment. Genes Dev 2015;29(23):2504–2515; doi: 10.1101/gad.271312.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothstein M, Simoes-Costa M. On the evolutionary origins and regionalization of the neural crest. Semin Cell Dev Biol 2022; doi: 10.1016/j.semcdb.2022.06.008 [DOI] [PubMed] [Google Scholar]
- Sakai K, Yamamoto A, Matsubara K, et al. Human dental pulp-derived stem cells promote locomotor recovery after complete transection of the rat spinal cord by multiple neuro-regenerative mechanisms. J Clin Invest 2012;122(1):80–90; doi: 10.1172/JCI59251 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki R, Aoki S, Yamato M, et al. PLGA artificial nerve conduits with dental pulp cells promote facial nerve regeneration. J Tissue Eng Regen Med 2011;5(10):823–830; doi: 10.1002/term.387 [DOI] [PubMed] [Google Scholar]
- Schoenwolf GC, Smith JL. Mechanisms of neurulation: Traditional viewpoint and recent advances. Development 1990;109(2):243–270; doi: 10.1242/dev.109.2.243 [DOI] [PubMed] [Google Scholar]
- Sharpe PT. Dental mesenchymal stem cells. Development 2016;143(13):2273–2280; doi: 10.1242/dev.134189 [DOI] [PubMed] [Google Scholar]
- Shi S, Gronthos S. Perivascular niche of postnatal mesenchymal stem cells in human bone marrow and dental pulp. J Bone Miner Res 2003;18(4):696–704; doi: 10.1359/jbmr.2003.18.4.696 [DOI] [PubMed] [Google Scholar]
- Shi S, Robey PG, Gronthos S. Comparison of human dental pulp and bone marrow stromal stem cells by cDNA microarray analysis. Bone 2001;29(6):532–539; doi: 10.1016/s8756-3282(01)00612-3 [DOI] [PubMed] [Google Scholar]
- Shi X, Mao J, Liu Y. Pulp stem cells derived from human permanent and deciduous teeth: Biological characteristics and therapeutic applications. Stem Cells Transl Med 2020;9(4):445–464; doi: 10.1002/sctm.19-0398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Singh M, Kakkar A, Sharma R, et al. Synergistic Effect of BDNF and FGF2 in Efficient Generation of Functional Dopaminergic Neurons from human Mesenchymal Stem Cells. Sci Rep 2017;7(1):10378; doi: 10.1038/s41598-017-11028-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solis-Castro OO, Boissonade FM, Rivolta MN. Establishment and neural differentiation of neural crest-derived stem cells from human dental pulp in serum-free conditions. Stem Cells Transl Med 2020;9(11):1462–1476; doi: 10.1002/sctm.20-0037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W, Bo X, Ma X, et al. Craniomaxillofacial derived bone marrow mesenchymal stem/stromal cells (BMSCs) for craniomaxillofacial bone tissue engineering: A literature review. J Stomatol Oral Maxillofac Surg 2022;123(6):e650–e659; doi: 10.1016/j.jormas.2022.06.002 [DOI] [PubMed] [Google Scholar]
- Sonoda S, Yamaza H, Yoshimaru K, et al. Protocol to generate xenogeneic-free/serum-free human dental pulp stem cells. STAR Protoc 2022;3(2):101386; doi: 10.1016/j.xpro.2022.101386 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki N, Takahashi K, Tanaka T, et al. Dental pulp cells for induced pluripotent stem cell banking. J Dent Res 2010;89(8):773–778; doi: 10.1177/0022034510366846 [DOI] [PubMed] [Google Scholar]
- Tatullo M, Marrelli M, Shakesheff KM, et al. Dental pulp stem cells: Function, isolation and applications in regenerative medicine. J Tissue Eng Regen Med 2015;9(11):1205–1216; doi: 10.1002/term.1899 [DOI] [PubMed] [Google Scholar]
- Trzaska KA, King CC, Li KY, et al. Brain-derived neurotrophic factor facilitates maturation of mesenchymal stem cell-derived dopamine progenitors to functional neurons. J Neurochem 2009;110(3):1058–1069; doi: 10.1111/j.1471-4159.2009.06201.x [DOI] [PubMed] [Google Scholar]
- Ullah I, Subbarao RB, Kim EJ, et al. In vitro comparative analysis of human dental stem cells from a single donor and its neuronal differentiation potential evaluated by electrophysiology. Life Sci 2016;154:39–51; doi: 10.1016/j.lfs.2016.04.026 [DOI] [PubMed] [Google Scholar]
- Vescovi AL, Reynolds BA, Fraser DD, et al. bFGF regulates the proliferative fate of unipotent (neuronal) and bipotent (neuronal/astroglial) EGF-generated CNS progenitor cells. Neuron 1993;11(5):951–966; doi: 10.1016/0896-6273(93)90124-a [DOI] [PubMed] [Google Scholar]
- Vierbuchen T, Ostermeier A, Pang ZP, et al. Direct conversion of fibroblasts to functional neurons by defined factors. Nature 2010;463(7284):1035–1041; doi: 10.1038/nature08797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman R, Offen D. Concise Review: Mesenchymal Stem Cells in Neurodegenerative Diseases. Stem Cells 2017;35(8):1867–1880; doi: 10.1002/stem.2651 [DOI] [PubMed] [Google Scholar]
- Waddington RJ, Youde SJ, Lee CP, et al. Isolation of distinct progenitor stem cell populations from dental pulp. Cells Tissues Organs 2009;189(1–4):268–274; doi: 10.1159/000151447 [DOI] [PubMed] [Google Scholar]
- Wapinski OL, Vierbuchen T, Qu K, et al. Hierarchical mechanisms for direct reprogramming of fibroblasts to neurons. Cell 2013;155(3):621–635; doi: 10.1016/j.cell.2013.09.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao L, Tsutsui T. Characterization of human dental pulp cells-derived spheroids in serum-free medium: Stem cells in the core. J Cell Biochem 2013;114(11):2624–2636; doi: 10.1002/jcb.24610 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Nakamura-Yamada S, Kusano K, et al. Clinical Potential and Current Progress of Dental Pulp Stem Cells for Various Systemic Diseases in Regenerative Medicine: A Concise Review. Int J Mol Sci 2019;20(5); doi: 10.3390/ijms20051132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto A, Sakai K, Matsubara K, et al. Multifaceted neuro-regenerative activities of human dental pulp stem cells for functional recovery after spinal cord injury. Neurosci Res 2014;78:16–20; doi: 10.1016/j.neures.2013.10.010 [DOI] [PubMed] [Google Scholar]
- Yan X, Qin H, Qu C, et al. iPS cells reprogrammed from human mesenchymal-like stem/progenitor cells of dental tissue origin. Stem Cells Dev 2010;19(4):469–480; doi: 10.1089/scd.2009.0314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Li S, Li X, et al. Effect of sonic hedgehog on motor neuron positioning in the spinal cord during chicken embryonic development. J Cell Mol Med 2019;23(5):3549–3562; doi: 10.1111/jcmm.14254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C, Li X, Sun L, et al. Potential of human dental stem cells in repairing the complete transection of rat spinal cord. J Neural Eng 2017;14(2):026005; doi: 10.1088/1741-2552/aa596b [DOI] [PubMed] [Google Scholar]
- Yang Y, Jiao J, Gao R, et al. Enhanced Rejuvenation in Induced Pluripotent Stem Cell-Derived Neurons Compared with Directly Converted Neurons from an Aged Mouse. Stem Cells Dev 2015;24(23):2767–2777; doi: 10.1089/scd.2015.0137 [DOI] [PubMed] [Google Scholar]
- Zhang J, Lian M, Cao P, et al. Effects of Nerve Growth Factor and Basic Fibroblast Growth Factor Promote Human Dental Pulp Stem Cells to Neural Differentiation. Neurochem Res 2017;42(4):1015–1025; doi: 10.1007/s11064-016-2134-3 [DOI] [PubMed] [Google Scholar]
- Zhang J, Lu X, Feng G, et al. Chitosan scaffolds induce human dental pulp stem cells to neural differentiation: Potential roles for spinal cord injury therapy. Cell Tissue Res 2016;366(1):129–142; doi: 10.1007/s00441-016-2402-1 [DOI] [PubMed] [Google Scholar]
- Zhang X, Zhou Y, Li H, et al. Intravenous administration of DPSCs and BDNF improves neurological performance in rats with focal cerebral ischemia. Int J Mol Med 2018;41(6):3185–3194; doi: 10.3892/ijmm.2018.3517 [DOI] [PMC free article] [PubMed] [Google Scholar]