Significance
Humans express 10 ATP-independent protein chaperones called small heat-shock proteins (sHSPs). The highly abundant sHSP chaperone present in eye lens, α-crystallin, delays lens protein aggregation and, therefore, development of cataract, over an individual’s lifetime. α-Crystallin components, HSPB4 and HSPB5, are highly similar to each other yet differ in their chaperone activity toward a lens protein proxy that mimics a UV-damaged, aggregate-prone species. We identified the source of the differential activity as a short sequence within the large intrinsically-disordered regions of the two sHSPs. This stretch determines local secondary structure and dynamics throughout the disordered region and modulates chaperone activity. Our findings support the notion that short segments within highly variable disordered regions of sHSPs can control chaperone function.
Keywords: chaperones, intrinsic disorder, small heat-shock proteins, protein aggregation
Abstract
Small heat-shock proteins (sHSPs) are a widely expressed family of ATP-independent molecular chaperones that are among the first responders to cellular stress. Mechanisms by which sHSPs delay aggregation of client proteins remain undefined. sHSPs have high intrinsic disorder content of up to ~60% and assemble into large, polydisperse homo- and hetero-oligomers, making them challenging structural and biochemical targets. Two sHSPs, HSPB4 and HSPB5, are present at millimolar concentrations in eye lens, where they are responsible for maintaining lens transparency over the lifetime of an organism. Together, HSPB4 and HSPB5 compose the hetero-oligomeric chaperone known as α-crystallin. To identify the determinants of sHSP function, we compared the effectiveness of HSPB4 and HSPB5 homo-oligomers and HSPB4/HSPB5 hetero-oligomers in delaying the aggregation of the lens protein γD-crystallin. In chimeric versions of HSPB4 and HSPB5, chaperone activity tracked with the identity of the 60-residue disordered N-terminal regions (NTR). A short 10-residue stretch in the middle of the NTR (“Critical sequence”) contains three residues that are responsible for high HSPB5 chaperone activity toward γD-crystallin. These residues affect structure and dynamics throughout the NTR. Abundant interactions involving the NTR Critical sequence reveal it to be a hub for a network of interactions within oligomers. We propose a model whereby the NTR critical sequence influences local structure and NTR dynamics that modulate accessibility of the NTR, which in turn modulates chaperone activity.
The ATP (adenosine triphosphate)-independent small heat-shock protein (sHSP) chaperone family plays important roles in protein homeostasis in all tissues in humans (1). Interactions with client proteins are generally believed to be transient, and detailed understanding of sHSPs is limited by their tendency to form large, heterogeneous, and structurally polydisperse oligomers (2). Two highly related sHSPs with such properties, HSPB4 and HSPB5, are present at millimolar levels in eye lens and form mixed oligomers in a 3:1 molar ratio (α-crystallin; 3). Together, HSPB4 and HSPB5 promote proper vision by preventing the formation of insoluble protein aggregates, or cataract, a leading cause of blindness worldwide (4). It is of great interest to understand how these sHSPs interact with lens clients and how such interactions are modulated.
All sHSPs consist of a central α-crystallin domain (ACD) that is structurally conserved and N- and C-terminal regions (NTR and CTR) that differ in both sequence and length and are presumed to be disordered (5–7). The ACD forms an antiparallel dimer that is considered the building block of sHSP oligomers. Interactions involving the NTR and CTR drive oligomer formation, and the NTR has been reported to be required for productive interactions with some client proteins (8–10). The involvement of the NTR in chaperone activity is supported by its sequence characteristics: hydrophobic- and aromatic-rich segments interspersed by proline residues. We showed that the NTR of HSPB5 is required for productive interactions with lens client protein γD-crystallin (11). Among human sHSPs, the NTRs of HSPB4 and HSPB5 are most similar (67.6% similarity vs. 49.3% for next most similar sHSP pair; SI Appendix, Fig. S1). As HSPB4 and HSPB5 are both expressed in lens, we hypothesized that comparing their ability to chaperone lens clients could offer insight into their mechanism of action.
High stability of lens proteins prevents their use in sHSP activity assays at physiological temperature, but the development of a γD-crystallin mutant that mimics a photodamaged, aggregation-prone state enables such assessments (γD-W130E; 12). Although γD-W130E remains folded at 37 °C, it can undergo rare conformational excursions that lead to aggregation in a time and concentration-dependent manner. We used γD-W130E to compare the chaperone activity of HSPB4 and HSPB5 under mildly acidic conditions as found in lens (13, 14). We find that HSPB4 and HSPB5 have markedly different abilities to prevent the aggregation of γD-W130E and that mixed HSPB4/HSPB5 oligomers have activity most closely resembling that of HSPB4. Through use of chimeric constructs, we have identified three NTR residues that account for most of the observed difference in chaperone activity. The identity of these residues influences structure and dynamics of the entire NTR in a manner that trends with chaperone activity. Our results reveal that NTR structure, dynamics, and interactions can be modulated by a remarkably small number of amino acid residues within a long, disordered region and these in turn affect the accessibility of binding sites for client.
Results
HSPB4 and HSPB5 Differ in Chaperone Activity Toward Lens Client γD-Crystallin.
The major eye lens chaperone is α-crystallin, a hetero-oligomer composed of two sHSPs, HSPB4 and HSPB5, in a 3:1 ratio (3). We compared the ability of HSPB4 and HSPB5, both individually and as mixed oligomers, to delay and/or inhibit aggregation of a γD-crystallin mutant (γD-W130E) that is aggregation-prone at 37 °C (12). In all experiments, we used HSPB4 with its two native cysteine residues mutated to serine (hereto referred to as HSPB4) to prevent possible disulfide exchange with client, as was observed for clients p53 and MDH during in vitro chaperone activity assessments (15).
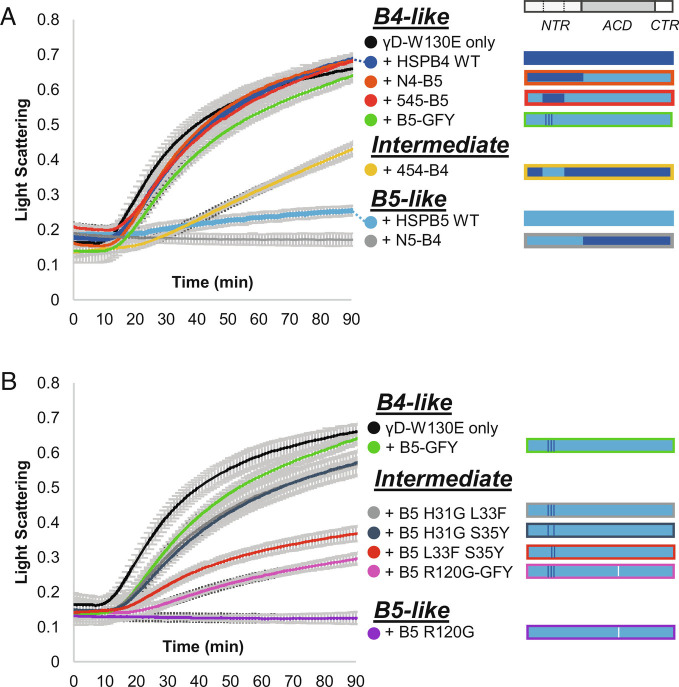
At pH 6.5 and 37 °C, γD-W130E aggregates spontaneously as evidenced by time-dependent increase of light scattering, monitored as A360 (Fig. 1A; black). The presence of HSPB4 or HSPB5 (15 μM) had markedly different effects on the aggregation time course (Fig. 1A). Whereas HSPB5 delayed aggregation for over 2 h, HSPB4 had little effect and led to increased absorbance at 360 nm (A360) at later stages of the assay, indicating that only HSPB5 has high chaperone activity against γD-W130E under the experimental conditions. As HSPB4 is present at higher levels than HSPB5 in lens, we assessed HSPB4 activity at a fourfold higher concentration (60 μM) and confirmed that it displays activity, albeit lower than HSPB5 under assay conditions (SI Appendix, Fig. S2). To test the chaperone activity of HSPB4/HSPB5 hetero-oligomers, HSPB5 (15 μM) and HSPB4 (45 μM) were equilibrated for 1 h at 37 °C prior to addition to γD-W130E to allow hetero-oligomers to form. Surprisingly, the HSPB4/5 mixture exhibited chaperone activity more like HSPB4, despite containing a concentration of HSPB5 that is highly effective on its own (Table 1). The results demonstrate that HSPB4 and HSPB5 have substantially different intrinsic chaperone activity toward γD-W130E and that HSPB4-like (“B4-like”) chaperone activity dominates in a physiologically relevant mixture of HSPB4 and HSPB5.
Fig. 1.
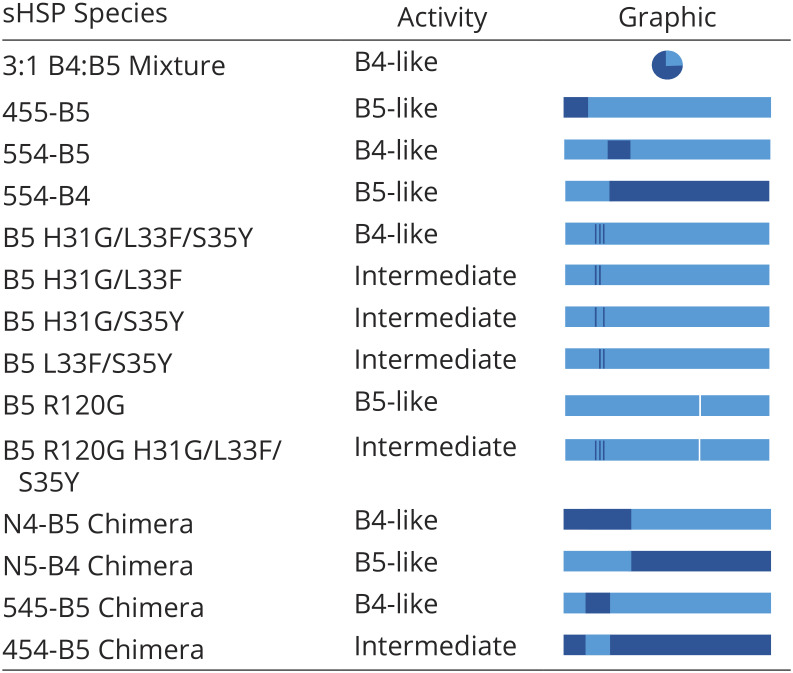
The Middle NTR subregion has a dominant effect on chaperone activity. (A) Left: Aggregation of γD-W130E in the absence and presence of HSPB5 and HSPB4 WT (wild-type) and NTR swap chimeras. Time courses of aggregation are shown as the increase in light scattering (monitored as absorbance at 360 nm over time. Species are classified by activity level: high—“B5-like”, “Intermediate”, or low—“B4-like”. Right: key depicts the identities of the NTR subregions, ACD, and CTR for each sample, with light blue bars representing HSPB5 regions and dark blue bars representing HSPB4 regions. (B) Analogous assays to those described in A) showing the influence of Middle NTR mutations on HSPB5 chaperone activity. Right: A white line shows the approximate location of the R120G mutation in the ACD of B5 R120G and R120G-GFY. N = 3 for all samples, average light scattering value ±1 SD is shown.
Table 1.
Summary of chaperone activity of all constructs tested
|
The above results raise the question of what region(s) and/or properties of HSPB5 contribute to the observed enhancement in chaperone activity. HSPB4 and HSPB5 are the two most highly related of the 10 human sHSPs, with highest sequence similarity within the structurally conserved ACD and highest sequence divergence within the disordered NTR and CTR. Based on previous studies that revealed that the NTR of HSPB5 is required for its chaperone activity against γD-W130E (11), chimeric constructs were created in which the NTRs of HSPB4 and HSPB5 were swapped(“N4-B5” has the NTR of HSPB4 swapped onto HSPB5, “N5-B4” is the alternate chimera; SI Appendix, Fig. S3). Care was taken to design the boundary between the NTR and ACD to retain the entire structured ACD. A previous study created chimeras using a restriction endonuclease site within the HSPB4 and HSPB5 genes to facilitate cloning, resulting in the first ~15 ACD residues being swapped (16). Under identical experimental conditions as above, N4-B5 has B4-like activity and N5-B4 has B5-like activity (Fig. 1A). Thus, the observed chaperone activity follows the NTR identity. Furthermore, the results suggest that the activity afforded by the HSPB5 NTR is modulated in the context of mixed HSPB4/HSPB5 oligomers.
Sequence Identity of the Middle NTR Subregion Has a Dominant Effect on Chaperone Activity.
To further parse the NTRs of HSPB4 and HSPB5, the ~60-residue region was sub-divided into three essentially equal parts, named based on their position relative to the structured ACD: the Distal subregion (residues 1 to 19), the Middle subregion (residues 20 to 42), and the Proximal subregion (residues 43 to 62) (SI Appendix, Fig. S1). New chimeric proteins were generated in which a single subregion from one sHSP was swapped into the other sHSP, creating, for example, “455-B5” which contains the Distal residues of HSPB4 and the Middle and Proximal residues of HSPB5 in the context of HSPB5 ACD/CTR and so forth. Relative to HSPB5, 455-B5 has 10 amino acid substitutions (all within the first 19 residues), 545-B5 has six (all within residues 20 to 42), and 554-B5 has 14 (all within residues 43 to 62).
The chaperone activity of each chimera toward γD-W130E was compared to those of HSPB4 and HSPB5. Swapping the Distal subregion has no discernable effect, as 455-B5 has B5-like activity (Table 1). In contrast, swapping either the Middle or Proximal subregions of HSPB4 into HSPB5 (545-B5 and 554-B5, respectively) resulted in B4-like activity (Fig. 1 A and B), suggesting that these NTR subregions could be responsible for the observed difference in activity. Importantly, the C-terminal portion of the HSPB5 Proximal NTR is a transiently-ordered continuation of the ACD β-sandwich structure, raising the possibility that installing the Proximal subregion of one sHSP onto the ACD of a different sHSP could result in a “mismatch” at the edge of the β-sheet (17). To test this possibility explicitly, a 554-B4 chimera was generated. The 554-B4 chimera exhibited high chaperone activity (i.e., was B5-like; Table 1), providing strong support for the notion that the low activity observed for 554-B5 likely results from a mismatch between the ACD and Proximal NTR rather than the Proximal subregion being directly responsible for chaperone activity. Taken together, we conclude that the Middle subregion is predominantly responsible for the lower activity that results from installing the NTR of HSPB4 into HSPB5.
To test whether the converse is true, i.e., whether swapping the Middle subregion of HSPB5 into HSPB4 enhances chaperone activity, we installed the HSPB5 Middle subregion into HSPB4 (454-B4). Although this alteration does not completely restore chaperone activity, the construct is substantially more active than HSPB4 and is therefore more B5-like (Fig. 1A). The first half of the Middle subregion contains the only conserved sequence among sHSP NTRs (“Conserved” sequence), whereas the second half contains sequence that is only partly conserved in HSPB4 and HSPB5 (SI Appendix, Fig. S1). Thus, the enhancement of activity afforded by installation of HSPB5’s NTR Middle subregion is brought about by a small number of amino acid differences in the second half of this subregion.
Comparing the two sHSPs, there are six differences within the 10-residue sequence of the C-terminal half of the Middle subregion, at positions H31G/L33F/S35Y/F38L/T40L and an inserted phenylalanine between residue 39 and 40. A triple mutant that substitutes three residues of HSPB4 into HSPB5 (H31G/L33F/S35Y; “B5-GFY”) had a similar effect to swapping the entire Middle subregion (i.e., 545-B5; Fig. 1A). We tested all combinations of double mutants at these positions; all exhibited lower activity than HSPB5 WT, but all were more active than B5-GFY and HSPB4 WT (Fig. 1B). Thus, the B5-GFY triple mutant contains a minimal set of sequence substitutions necessary for the observed difference in chaperone activity between HSPB4 and HSPB5 WT. The results clearly reveal a dominant role for the C-terminal half of the Middle NTR, which we will refer to from here on as the Critical sequence. Putting this new insight into the context of mixed HSPB4/HSPB5 (wild-type) oligomers, we hypothesize that the observed modulation of HSPB5 chaperone activity is due to the action of the HSPB4 Critical sequence on HSPB5 subunits.
To test the generality of the effect of the Critical sequence on chaperone activity, we installed the Critical sequence swaps H31G/L33F/S35Y into the HSPB5 cataract- and myopathy-associated mutant R120G (“R120G-GFY”; 18). This mutant is an enhanced chaperone of γD-W130E and has strongly decreased protection from hydrogen/deuterium exchange (HDX) in the NTR and ACD (11). The Critical sequence substitutions substantially decrease R120G chaperone activity (Fig. 1B). Therefore, the identity of these three Critical sequence residues can affect the activity of an activated sHSP mutant that has a more dynamic and/or less structured NTR.
NTR Critical Sequence Affects Structure and Dynamics of the Entire NTR.
Potential changes in local secondary structure resulting from substitutions in the NTR were assessed by circular dichroism (CD) (SI Appendix, Figs. S4–S7). Spectra of all species are consistent with primarily β-structure, with negative ellipticity that spans from roughly 200 to 250 nm and a minimum near 215 nm. Concentration-matched spectra of HSPB4, HSPB5, N4-B5, and N5-B4 differ from each other both in the position of their minima and in the absolute negative ellipticities (SI Appendix, Fig. S4). The two species that contain the ACD of HSPB4 have the strongest negative ellipticity, with minima at ~216 nm. Comparison of spectra of middle subregion swaps and critical sequence substitutions similarly show small differences (SI Appendix, Fig. S5). To assess whether these small differences could be accounted for by small errors in protein concentration, the mutant spectra were scaled to the amplitude of the WT (SI Appendix, Fig. S6 A and B). This procedure revealed that the spectra of 454-B4 and B4 are nearly identical, while the HSPB5 spectrum still differs from those of 545-B5 or B5-GFY between 212 and 200 nm. Altogether, the spectra suggest that substitution of as few as three residues within an intrinsically disordered NTR alters secondary structure content within an sHSP oligomer.
Secondary structural content changes in hetero-oligomers relative to mixtures of the two homo-oligomers were assessed by comparison of the CD spectrum of a 3:1 ratio of HSPB4:HSPB5 with a theoretical spectrum calculated from the experimental HSPB4- or HSPB5-only spectra (3× HSPB4 + 1× HSPB5 = B4/B5 mixture). The spectrum of the mixed oligomers has ~20% more CD signal at the minimum relative to the theoretical spectrum, consistent with increased secondary structure content in the hetero-oligomer (SI Appendix, Fig. S7). These results indicate that the presence of HSPB4 NTRs has an effect on local secondary structure content of HSPB5, and/or vice versa.
The large differences in chaperone activity associated with sequence changes in the Middle NTR suggest that the subregion may modulate availability of client-binding sites. HDX coupled with mass spectrometry (MS) was used to probe how the sequence changes affect structure and/or dynamics of the entire molecule. HDX measures exchange of backbone amide hydrogens with deuterium when incubated in D2O-based buffer. The extent of exchange in a region is reflective of its local structure and dynamics. Less structured and/or more dynamic regions exchange rapidly, whereas regions with stable structure and/or low dynamics exchange slowly and are described as protected. Differences in the extent of deuteration for a given region indicate changes in structure and dynamics (19). We confirmed that all samples experienced highly similar forward- and back-exchange conditions using internal standards (SI Appendix, Figs. S8–S11).
A range of deuterium uptake was observed for homo-oligomeric assemblies of HSPB4 and HSPB5, with similar patterns of exchange to other reports (SI Appendix, Figs. S12 and S13; HSPB1, 20; HSPB4, 15; and HSPB5 at pH 7.5, 11). CTR-derived peptides exchange very rapidly, ACD-derived peptides exchange slowly reflecting the stable β-sandwich structure, and peptides from the NTR have intermediate exchange rates. Many more peptides could be followed in the data for HSPB4 and no peptides had identical sequence between HSPB4 and HSPB5, but the exchange patterns of the two sHSPs can be compared qualitatively. A particularly notable difference is observed for peptides from the Middle NTR, which had deuterium uptake values of ~32 to 42% in HSPB5 and ~26% in HSPB4 at the 4-s timepoint. Thus, in its native context, the HSPB4 Middle subregion appears more sequestered or structured than the analogous subregion in HSPB5. Differences in Middle NTR peptide lengths (and sequence) preclude a more detailed interpretation. The proximal NTR and peptides at the beginning of the ACD are also more protected in HSPB4.
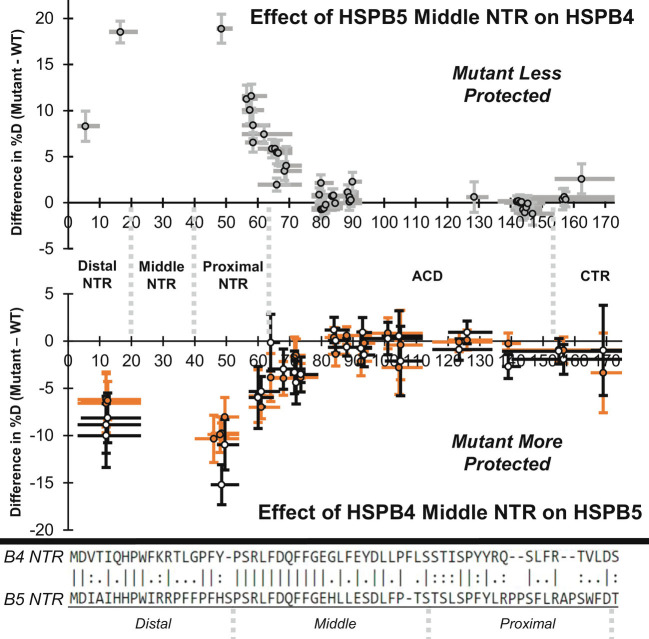
To assess the effect of substituting the entire HSPB4 Middle subregion or three HSPB4 Middle residues into HSPB5, HDX profiles of 545-B5 and B5-GFY were compared to HSPB5. The overall exchange profiles are similar (SI Appendix, Fig. S13). For each peptide of identical sequence observed in the HSPB5 and 545-B5 or B5-GFY datasets, the difference in percent deuteration of each mutant relative to wild-type was calculated (ΔHDX = Mutant %D—WT %D; Fig. 2, Lower panel; Fig. 2, Lower panel and SI Appendix, Figs. S14–S16, Lower panels). In all cases where the difference plot shows a significant difference (we consider |ΔHDX| values of ≥5% to be significant), ΔHDX values are below zero, meaning that the peptide is more protected in the mutant than in the wild-type context. The entire NTR is affected in both constructs, with the 545-B5 Middle subregion swap mutant having larger effects than the B5-GFY triple mutant. Higher protection of the Distal and Proximal NTR subregions was observed at 4-s, 1-min, and 30-min exchange time points (Fig. 2 Lower; SI Appendix, Figs. S14 and S15, Lower). In addition, most of the ACD-derived peptides had lower deuterium uptake after 20-h exchange, though none of the differences was greater than 5% (SI Appendix, Fig. S16, Lower). Increased protection at long exchange timepoints is suggestive of increased stability of existing structure. Overall, the HDX results reveal that the identity of as few as three residues in the NTR Critical sequence dictates the degree to which the entire NTR is sequestered, structured, and/or dynamic.
Fig. 2.
The NTR Middle subregion affects HDX throughout the NTR. Data shown are for a 4-s exchange time point. Top panel: The effect of swapping HSPB5’s Middle NTR subregion into HSPB4 (454-B4) is shown in gray as the difference in percent deuteration (mean 454-B4 %D—mean HSPB4 %D; N = 3 to 7). Values above 0 indicate 454-B4 is less protected from exchange than HSPB4. Gray circles outlined in black represent the peptide center, horizontal bars represent peptide length (minus the first two residues), and vertical error bars show error (±1 SD). Lower panel: The effect of swapping HSPB4’s Middle NTR subregion (545-B5) or the residue swaps H31G/L33F/S35Y (B5-GFY) in HSPB5 are shown in black and orange as the difference in percent deuteration (mutant mean %D—HSPB5 mean %D). Values below 0 indicate the mutants are more protected from HDX. N = 3 to 7, change in mean %D value ±1 SD is shown. Boundaries of the Distal NTR, Middle NTR, Proximal NTR, ACD, and CTR are shown between the panels. Bottom: Alignment of HSPB4 and HSPB5 NTR with the Distal, Middle, and Proximal NTR subregions shown.
The observation that the HSPB4 Middle subregion can alter the properties of the entire NTR when installed into HSPB5 raises the question of whether the Middle subregion of HSPB5 can similarly affect the properties of HSPB4. An analogous analysis was performed on HDX data collected for HSPB4 and the Middle subregion swap chimera, 454-B4. The ΔHDX plot for the 4-s exchange timepoint is shown in the Top panel of Fig. 2. Substitution of the Middle subregion of HSPB5 into HSPB4 results in lower protection of all peptides derived from the NTR at 4-s, 1-min, and 30-min time points (i.e., ΔHDX > 0; Fig. 2, Top panel; SI Appendix, Figs. S14 and S15, Top). Differences are largest near the mutation sites and lowest at the extreme ends of the NTR. Peptic digestion of HSPB4 yielded higher resolution and redundancy than for HSPB5 and revealed extensive changes in the ACD at 30-min and 20-h exchange times. Two clusters of peptides with ΔHDX ≥5% were evident around residues ~60 to 90 and ~128 to 155, corresponding to the first 30 ACD residues and the last two ACD β-strands (SI Appendix, Figs. S15 and S16, Top). We note that these residues are near each other in the ACD structure and constitute the entire “top” sheet of the ACD β-sandwich and the ACD edge groove (strands 2, 3, 4, 8, and 9; SI Appendix, Fig. S16). Low coverage at the ACD dimer interface in fully-deuterated control samples prevented quantitation of changes in percent deuteration there, but direct comparison of deuteration values indicates that swapping the HSPB5 Middle subregion into HSPB4 causes increased HDX at the ACD dimer interface at long exchange times (20 h; SI Appendix, Fig. S18). Altogether, the results demonstrate that sequence changes in the Middle NTR subregion affect structure and dynamics throughout the entire NTR and in regions of the ACD, with the extent of exchange trending with chaperone activity against γD-W130E.
HSPB5 NTR Critical Sequence Interacts Directly with Other NTR Subregions.
The observation that mutations in the Middle NTR subregion affect HDX throughout the NTR implies that the subregion is involved in interactions with other subregions within an oligomer. Polydispersity and high heterogeneity of HSPB5 oligomers are refractory to standard structural investigation, so we adopted a targeted cross-linking MS approach to identify interactions involving the Middle NTR. We installed p-benzoyl-l-phenylalanine (BPA), a non-natural amino acid, at single sites in HSPB5 NTR using amber-codon suppression (21). When excited by UV light, BPA forms covalent bonds to CH- groups in direct contact (within 3 Å), overcoming the relatively long spacer arms of commonly used chemical cross-linkers. The BPA excited state quickly decays to the ground state if no cross-link is generated, allowing for repeated excitation and multiple opportunities for cross-links to form (22).
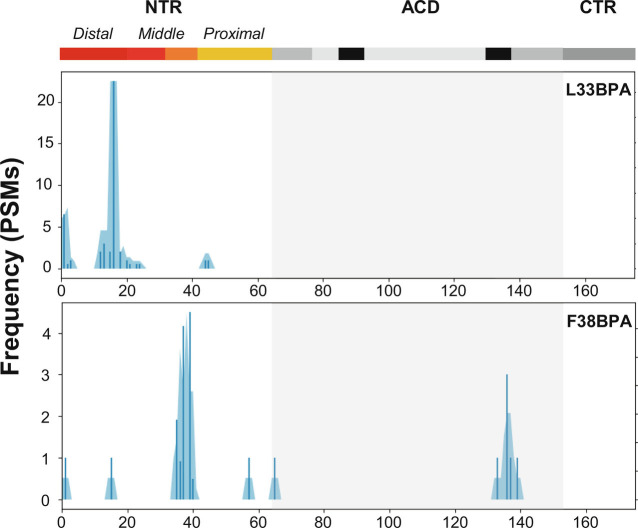
To probe inter-subunit interactions within HSPB5 oligomers that involve the Critical sequence, BPA was installed at amino acid positions 33 or 38 (L33BPA and F38BPA, respectively). Oligomers composed of each variant were equilibrated for 1 h at 37 °C prior to UV irradiation at 4 °C for 30 min. The resulting samples were resolved by SDS-PAGE (sodium dodecyl sulfate polyacrylamide gel electrophoresis), revealing a band containing two HSPB5 subunits with one intermolecular cross-link as the major product, with similar amounts of higher-order species representing three or more covalently linked HSPB5 chains (SI Appendix, Figs. S19 and S20). Qualitatively, L33BPA produced lower amounts of cross-linked material than F38BPA. In-gel digest was performed on the singly cross-linked band and the resulting peptides were analyzed by MS. Bioinformatic analysis of the fragmentation data identified positions of cross-links formed by BPA (SI Appendix, Fig. S21).
Cross-linked positions identified in L33BPA and F38BPA samples are shown as histograms across the HSPB5 sequence in Fig. 3. Although similar numbers of cross-linked peptides could be identified in the two species, the cross-linking patterns are remarkably distinct. Cross-links from BPA at position 33 cluster mainly in the Distal NTR while links from position 38 are mainly to the Critical sequence and to a region on the structured ACD known as the edge groove. To further expand the view provided by the above results, BPA cross-linking was performed under identical conditions on HSPB5 variants with BPA individually placed at positions in the Distal, Middle, and Proximal NTR (specifically, residues 9, 17, 24, or 61). Each BPA position results in a distinct and discrete pattern of cross-links, consistent with the notion that NTRs are not completely disordered within oligomers (SI Appendix, Figs. S22–S24). Notably, every BPA variant generated cross-links to the Critical sequence, suggesting that the Critical sequence serves as a hub for a network of NTR-to-NTR interactions in HSPB5 (Fig. 4B). The results provide a rationale for how the critical sequence, located in the middle of the ~60-residue NTR, can influence the structure and dynamics of the entire NTR.
Fig. 3.
HSPB5 NTR Critical sequence contacts other NTR subregions. BPA was individually installed at position 33 or 38 within the HSPB5 NTR Critical sequence. Positions cross-linked to BPA (X-axis) at site 33 or 38 are shown as blue bars that represent the number of PSMs (peptide spectral counts) identified using tandem MS data; blue shading shows the number of cross-links in a 3-residue sliding window. Domain boundaries are shown above the plots for the NTR (Distal subregion: maroon, Middle NTR Conserved sequence: red, Middle NTR Critical sequence: orange, Proximal subregion: gold), ACD (gray, with locations of the ACD edge groove in black and ACD central groove in light gray), and CTR (dark gray). Boundaries of the ACD are shown by the light gray Inset. All plots are the result of MS identification in one cross-linking reaction.
Fig. 4.
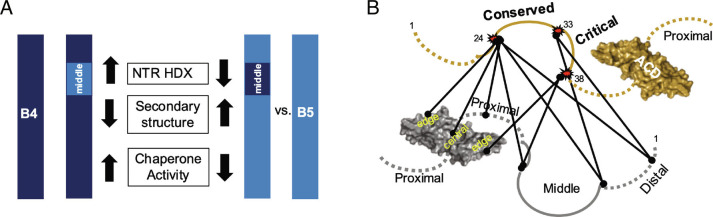
Summary of the effects of Middle NTR swaps in HSPB4 or HSPB5. (A) Installation of HSPB5 Middle NTR in HSPB4 increases HDX throughout the NTR at short exchange time points (4 s, 1 min, 30 min) and is associated with decreased secondary structure and increased chaperone activity (454-B4 vs. HSPB4). Installation of HSPB4 Middle NTR in HSPB5 decreases HDX throughout the NTR at short exchange time points (4 s, 1 min, 30 min) and is associated with increased secondary structure and decreased chaperone activity (B5-GFY or 545-B5 vs. HSPB5). (B) Summary of BPA cross-links identified from Middle subregion sites 24, 33, and 38 indicate the Critical sequence is a hub of NTR interactions.
Discussion
sHSPs remain among the most enigmatic of protein chaperones, largely due to intrinsic properties that confound detailed structural and functional studies under physiologically relevant experimental conditions (23). A mimic of a UV-damaged version of the major eye lens client protein γD-crystallin (i.e., γD-W130E) that spontaneously aggregates at 37 °C has enabled direct comparison of the chaperone activity of the two most abundant sHSPs in lens, HSPB4 and HSPB5 (12). We found that HSPB4 and HSPB5 have markedly different chaperone activity toward γD-crystallin and that the high intrinsic activity of HSPB5 is dampened when present with HSPB4 in the ratio that exists in lens. Using a series of chimeric constructs, we identified the source of activity difference to be a short sequence in the middle of the NTR. We dub this the Critical sequence (residues ~31 to 42), as substitution of the HSPB5 sequence into HSPB4 changes the chaperone activity from B4-like (i.e., very low) to B5-like (i.e., very high), and vice versa.
Targeted inter-subunit UV cross-linking of HSPB5 oligomers revealed that the Critical sequence is close to all NTR subregions, at least transiently. The identity of the Critical sequence alters CD spectra, consistent with altered secondary structure content, and it alters HDX protection patterns throughout the NTR. Taking all observations together, a B4-like Critical sequence is associated with increased β-structure content, higher protection of the NTR from HDX, and lower intrinsic chaperone activity toward γD-W130E. A B5-like Critical sequence is associated with lower β-structure content, lower protection of the NTR, and higher intrinsic chaperone activity. Finally, observation of interactions between the Critical sequence and the ACD edge groove in addition to small but significant effects of Critical sequence identity on the HDX protection in regions of the ACD near the edge groove indicate that the Critical sequence serves as the interaction network hub of the NTR. Thus, even small changes in the structure or dynamics of the Critical sequence can affect properties and activity of the entire NTR. The associations observed in this study lead us to conclude that a small section of the disordered NTR (~10 residues out of 60) is largely responsible for the dynamics, local structure(s), and activity of the sHSPs HSPB4 and HSPB5 (Fig. 4 A and B).
Although HSPB5 is highly expressed in eye lens, muscle, and neurons, HSPB4 is only expressed in high abundance in lens (24, 25). Despite its high level of similarity to HSPB5, evolutionary analysis suggests that HSPB4 may be specialized for a role in lens (26). HSPB4 forms mixed oligomers with HSPB5 in lens, and studies have found that the ratio of HSPB4 to HSPB5 that exists in lens (3:1) is optimal for stabilization of structure against temperature-induced changes (3, 27). Our CD data on HSPB4/HSPB5 hetero-oligomers indicate that they have increased β-structure content, which may contribute to the reported stabilization. The B4-like chaperone activity of hetero-oligomers suggests that such stabilization may limit or alter interactions between HSPB5 and γD-W130E, thereby dampening productive chaperone activity as observed under laboratory conditions. We cannot rule out the alternative possibility that HSPB4 outcompetes HSPB5 interactions with γD-W130E in the context of hetero-oligomers. The observation of low HSPB4 intrinsic chaperone activity against γD-W130E may seem counterintuitive. However, client interactions involving highly activated HSPB5 species including known disease/cataract-associated mutants such as R120G (Fig. 1A) result in long-lived co-complexes that eventually co-aggregate (11). Such observations indicate that there are negative consequences associated with constitutive high HSPB5 activity, suggesting there may be pressure to modulate client binding in lens.
Relatively few structural details are known for the NTR of sHSPs. Sparse structural data exist for HSPB1, HSPB2/HSPB3, HSPB4, HSPB5, and HSPB6 (11, 15, 20, 28, 29). In all cases, portions of NTRs are observed or inferred to contact the structurally conserved ACD. In particular, Distal regions are inserted into ACD edge grooves in structures of HSPB2/3 and HSPB6 and the Conserved sequence of the Middle region is inserted into the central ACD groove in HSPB6 (28, PDB (Protein Data Bank) 6F2R; 29, PDB 5LTW). A similar interaction appears in the tetramer of HSPB2/HSPB3, though the resolution was insufficient to unambiguously identify the exact NTR residues involved (28, PDB 6F2R). The HSPB4 NTR was not well resolved in cryo-EM studies, suggesting it is either highly heterogenous or highly dynamic, but NTR to ACD interactions were implied by BS3 cross-linking experiments that link the Distal, Middle, and Proximal NTR to the ACD (15). Our studies reveal that all three HSPB5 NTR subregions interact with the ACD edge groove, and we also detect an interaction between the Middle NTR Conserved sequence and the central groove (Fig. 4B; 11). Finally, multiple NTR-to-ACD edge groove and Conserved sequence-to-ACD central groove interactions have been reported for HPSB1 (20). Thus, evidence from a growing number of human sHSPs appears to converge on a model in which there are multiple possible interactors within NTRs onto ACDs, and the number of interactors outnumbers the number of available grooves. Each such interaction is likely weak and transient, giving rise to the known high heterogeneity of sHSPs.
Compared to other sHSPs, more is known about local structure that exists in the HSPB5 NTR (17). Solid-state NMR (ssNMR) provided evidence for two short β-strands, “β1” and “β2” in the Proximal subregion that associate with each other and with the first stably structured portion of the ACD, strand β3. Additionally, ssNMR revealed evidence of α-helical structure for HSPB5 residues 23 to 32, spanning the Conserved sequence and ending at the start of the Critical sequence (17). It is noteworthy that a helical conformation will not fit into an ACD central groove, implying that the Conserved sequence must be non-helical when bound to the groove. Importantly, the ssNMR data revealed high heterogeneity within the NTR, indicating that the local structures implied by the data represent examples of what is likely a multitude of coexisting local structures.
As pointed out previously, there are far more NTR subregions capable of interacting with an ACD than there are available ACD sites in an oligomer (11, 20). This situation suggests that NTRs also take part in interactions with other NTRs. Previous evidence for such interactions includes HDX and NMR experiments performed on HSPB1 (20) and single cysteine disulfide formation for Cys residues installed in the Distal and Proximal subregions of HSPB5 (residues 19, 45, 57, 59, and 63; 8, 17). Our targeted BPA cross-linking results build on the prior data and reveal numerous novel NTR cross-links. Altogether, current available data are consistent with a highly interactive and interconnected NTR in the context of oligomers.
We find that a short 10-residue stretch in the middle of the NTR (Critical sequence) dictates chaperone activity, HDX protection, and local secondary structure of HSPB4 and HSPB5. Remarkably, as few as three amino acid swaps are required to change the behavior of HSPB5 into that of HSPB4. What is special about the Critical sequence and the three amino acids in question? We offer two speculations. First, the HSPB4 Critical sequence has alternating hydrophobic residues (F32, Y34, L36) suggestive of a short β-strand with a hydrophobic face; the corresponding residues in HSPB5 are L33, S35, L37. The triple residue-swap mutant yields F33, Y35, L37 in HSPB5, creating a more hydrophobic strand should it exist. Second, the first residue of the Critical sequence is a glycine in HSPB4 and a histidine in HSPB5. Gly30 at the boundary between the Conserved and Critical sequences may serve to disfavor helical structure, whereas the His in HSPB5 would not. The swap of this residue could therefore disfavor helical structure (observed in ssNMR) and favor adoption of extended β-structure, consistent with the observed change in CD. Drawing from the observation that Conserved sequences (with virtually identical sequences) of sHSPs have been captured in at least three different conformations (extended, loop, and helical; 15, 17, 29), we propose that which conformers are favored depends on both the surrounding sequence (i.e., the Critical sequence) and on available interactions. In this way, substitution of the Critical sequence alters the local structure and consequently the interactions involved. Thus, changes in one region are expected to be transmitted to all other regions directly (via interaction) or indirectly (via changes in relative structure/dynamics). The functional consequences of such changes may depend on the identity of the client. In the case of lens client γD-W130E, changes that yield increased NTR accessibility are associated with enhanced ability to delay aggregation, and vice versa. The most parsimonious explanation is that increased NTR accessibility exposes client-binding sites that reside within the NTR. Alternatively, increased NTR exposure could provide better access to the structured ACD and its binding grooves. Future studies will be needed to determine which model is in play; both may be relevant, depending on the client in question.
Finally, we note that the ability of the HSPB4 Critical sequence to transform HSPB5 into a lower activity γD-W130E chaperone does not require that the sequence be installed directly into HSPB5 in the form of a chimera or mutant. Hetero-oligomers of HSPB4/HSPB5, as they exist in lens, display the same ability, leading to the conclusion that the modulation can work in trans. This new insight could have broad implications, as many cell types express more than one of the 10 human sHSPs and numerous examples of heteromeric sHSP species have been reported (30–32). It is reasonable to expect that different combinations and cellular levels of sHSPs will have differing levels of activity and sensitivity to changes in cellular conditions and client needs, as has been shown for various mixed oligomers in vitro (33, 34). A fuller understanding of how sHSP activity can be modulated endogenously may ultimately lead to ways to modulate activity exogenously.
Materials and Methods
Growth and Purification of HSPB4 or HSPB5 WT and Mutants.
HSPB4 or HSPB5 WT and mutants were cloned into pET23 vector and expressed in Escherichia coli BL21 DE3 cells (New England BioLabs (NEB)). HSPB5 BPA mutants were created by installing the amber codon TAG at target sites and were co-expressed with pEVOL-pBpF. pEVOL-pBpF was a gift from Peter Schultz (Addgene plasmid # 31190; http://n2t.net/addgene:31190; RRID:Addgene_31190). Cells were grown at 37 °C until reaching an OD600 nm of 0.4 to 0.8, then temperature was adjusted to 22 °C and expression was induced with 1 mM IPTG or 1 mM IPTG and 1 mM arabinose in the case of BPA mutants. Cells were grown overnight, harvested by centrifugation, and resuspended in lysis buffer (20 mM Tris, 100 mM NaCl, 10 mM EDTA pH 8). Cells were lysed using a french press after adding DNase, RNase, and lysozyme. PEI (0.1% v/v) was added to the lysate and then the lysate was clarified by centrifugation. Ammonium sulfate (30% w/v) was added to the clarified lysate, then the ammonium sulfate pellet was isolated by centrifugation and the supernatant was discarded. The ammonium sulfate pellet was resuspended in 20 mM Tris pH 8 (buffer A) and applied to a G25 column equilibrated with buffer A. Desalted protein was pooled and applied to a DEAE column equilibrated with buffer A and eluted by a sodium chloride gradient. Protein eluted from the DEAE column was diluted in buffer A and then applied to a HiTrap Q column (Cytiva) equilibrated in buffer A. Protein was eluted from the Q column using a sodium chloride gradient and then was pooled, concentrated, and applied to a SDX200 column equilibrated with 25 mM NaPO4, 150 mM NaCl pH 6.5. Pure protein was flash-frozen in liquid nitrogen and stored at −80 °C. The concentrations of BPA-containing proteins were quantified using a BCA assay, while those without BPA were quantified via absorbance at 280 nm.
Growth and Purification of γD-W130E.
γD-W130E was cloned into pET28a vector and expressed in E. coli BL21 DE3 cells (NEB) as a fusion on the C terminus of 6xHis-SUMO. Cells were grown at 37 °C until reaching an OD600 nm of 0.4 to 0.8, then temperature was adjusted to 20 °C and expression was induced with 0.5 mM IPTG. Cells were grown overnight, harvested by centrifugation, and resuspended in 20 mM Tris, 200 mM NaCl, 10 mM imidazole pH 7.6 (lysis buffer). Cells were lysed using a french press after adding DNAse, RNAse, and lysozyme. PEI (0.1% v/v) was added to the lysate and then the lysate was clarified by centrifugation. Clarified lysate was filtered (0.45 μm) and then applied to a HisTrap column (Cytiva) equilibrated with lysis buffer. Bound protein was eluted with imidazole. SUMO protease (GST-SENP3) was added to the eluted protein and then the mixture was dialyzed overnight in lysis buffer +2 mM β-Mercaptoethanol. Dialyzed protein was re-applied to a HisTrap column equilibrated with lysis buffer. The flow through was concentrated and then injected on an SDX75 column equilibrated with 20 mM Tris, 50 mM NaCl, 10 mM EDTA, 5 mM β-mercaptoethanol pH 8. Fractions containing purified protein were pooled and stored in this buffer until the day before it was used for chaperone activity assays, at which point it was buffer exchanged to 25 mM NaPO4, 150 mM NaCl, 2 mM EDTA pH 6.5. Concentrations were determined by measuring absorbance at 280 nm.
Chaperone Activity Assay.
Chaperone activity of HSPB4 or HSPB5 WT and mutants was assessed using γD-W130E as client. The aggregation of γD-W130E was measured at 37 °C in a 96-well plate reader (BioTek) by monitoring absorbance at 360 nm. All sHSP samples were incubated for 1 h at 37 °C before adding to assay wells. Wells containing sHSPs (or buffer only) were assembled and then incubated at 37 °C for 5 min, then ice-cold γD-W130E was added (γD-W130E final concentration 500 μM; sHSP final concentration 15 μM for homo-oligomers, 60 μM for mixed HSPB4/HSPB5 oligomers; final buffer conditions 25 mM NaPO4, 150 mM NaCl, 1 mM EDTA pH 6.5).
CD Spectroscopy.
CD spectra were collected using a Jasco J-1500 instrument. Samples (10 μM in species of interest as determined by A280 nm) were equilibrated at 37 °C for 1 h in 5 mM NaPO4, 30 mM NaCl, 0.2 mM EDTA pH 6.5. 10 wavelength scans from 300 nm to 200 nm were accumulated at 37 °C and averaged automatically. The 3:1 HSPB4:HSPB5 mixture contained 40 μM protein in total and the plot is truncated at low wavelengths because the high tension limit was reached at 206 nm.
Hydrogen/Deuterium Exchange Mass Spectrometry.
Samples (20 μM) were equilibrated in water-based buffer (25 mM NaPO4, 150 mM NaCl, 1 mM EDTA pH 6.5) for 1 h at 37 °C and then cooled to room temperature. Internal exchange standards Tyr-Pro-Ile “YPI” (35) and Tyr-Gly-Gly-Phe-Leu “Leucine-enkephalin” were added to samples. Samples were diluted 10-fold into 25 mM NaPO4, 150 mM NaCl, 1 mM EDTA pH 6.5 buffer made in 95% D2O (85% D2O final, 2 μM protein) and incubated for 4 s, 1 min, 30 min, or 20 h at room temperature. Fully deuterated samples were created by incubating in 85% D2O at 75 °C for 30 min. The exchange was quenched by the addition of ice-cold quench buffer (1.4% v/v formic acid, final concentration 0.7%) and flash frozen in liquid nitrogen. Samples were automatically thawed, digested by immobilized pepsin, and injected on a Waters Synapt G2-Si mass spectrometer coupled to a custom HDX-LC (liquid chromatography) system with automated sample thawing (36). Peptides from pepsin digests were identified by MS/MS on a Thermo Orbitrap Fusion Tribrid mass spectrometer using data-dependent acquisition followed by analysis using ProteinProspector (Univ. of California, San Francisco). Deuterium uptake was analyzed in HDExaminer 3.0 (Sierra Analytics) and HX-Express (37). Percent deuteration at each time point was calculated relative to the fully deuterated control and compared for each peptide with identical sequence.
BPA Cross-linking Mass Spectrometry.
BPA was incorporated in HSPB5 using amber codon suppression technology (21). The amber codon was inserted at HSPB5 residue 9, 17, 24, 33, 38, or 61 using QuikChange mutagenesis (Agilent). Single-BPA HSPB5 mutants were purified using the standard protocol while taking steps to limit exposure to ambient light. Sample concentrations were determined using BCA. 50 μM HSPB5 BPA mutants were incubated for 1 h at 37 °C, cooled to 4 °C, placed in a 96-well flat-bottomed half-area plate, and then exposed to UV for 30 min at 4 °C. Samples were subjected to SDS-PAGE on a 4 to 20% acrylamide gradient gel (Bio-Rad). The no-UV monomer and plus-UV dimer bands were excised and were each digested in-gel with MS grade Trypsin (Thermo Scientific) and GluC (New England Biolabs) in ammonium bicarbonate buffer (Thermo Manual–In-Gel Tryptic Digestion Kit). Digests were cleaned up with C18 spin columns (Thermo Scientific) and dried using a speed-vac. Samples were resuspended in 95% water 5% ACN with 0.1% FA with volumes adjusted based on sample weight. Data were collected with an Easy Nano LC coupled to a Thermo Orbitrap Fusion Lumos Tribrid. 0.5 µg of protein was loaded onto an 8-cm trap column. The sample was then then separated on a 25-cm analytical column with a 75 µm inner diameter using an 85-min gradient from 6% B to 45% B, where A was water and B was 80% acetonitrile, at 300 nL min−1. The column was then flushed and regenerated for 35 min. Spectra were acquired across the entire LC method using data-dependent acquisition with dynamic exclusion after one time for a duration of 30 s and an intensity threshold of 2.0 × 104. Orbitrap detection and Higher-energy collisional dissociation fragmentation (30% normalized collision energy) were used with a target value of 1.00 × 105, maximum injection time of 22 ms, top N of 20, and isolation width of 1.6. MS1 were acquired at a resolution of 120,000 over the range of 400 to 2000 m/z, and MS2 were acquired with a resolution of 15,000. The mass spectrometry proteomics data have been deposited to the ProteomeXchange Consortium (38) via the PRIDE partner repository (39) with the dataset identifier PXD035668.
Identification of Cross-links Using the Trans Proteomic Pipeline.
First, Comet (40) was used to search for non-cross-linked peptides in the non-UV treated control to construct the protein database. The Comet search database contained the BL21 E. coli database from UniProt (UP000431028), the common Repository of Adventitious Proteins (cRAP) database from the Global Proteome Machine with all five levels of proteins (cRAP Protein Sequences), the pertinent HSPB5 BPA mutant, and reverse-sequence decoys. In addition, the wild-type HSPB5 was included in the database for samples that were analyzed on the same date as other samples containing that protein. The Comet searches were enzyme nonspecific using a peptide mass tolerance of 20.0 ppm. The isotope error offset was 3, and BPA was defined as an additional amino acid, B, that has a mass of 251.09462859 Da. Methionine oxidation and cysteine iodoacetamide alkylation were variable modifications. Results were validated using Peptide Prophet (41). After filtering using a 1% False Discovery Rate (FDR, based on PeptideProphet probabilities) and a minimum of two Peptide Spectral Matches (PSM), this yields a protein database for the sample.
Second, cross-links in the UV-treated samples were identified using Kojak (42) and the protein database for the sample. The Kojak search settings matched those described for the Comet searches expect the precursor tolerance was 15 ppm and enzyme selection rules were used. For the trypsin digested samples, the preexisting trypsin setting was used. For the trypsin-GluC digested samples, the cleavage sites of D and E were added to the trypsin settings. Cross-links were defined as from BPA to any residue with no mass change. Cross-link results were validated using PeptideProphet. For ions of each charge state, probabilities were determined using PeptideProphet and were used to define the thresholds used to filter results to a 1% FDR. For histograms, each PSM was associated with the residue that was assigned the highest probability of participating in a cross-link with BPA. When more than one residue was assigned the same probability, an equal fraction of that PSM was assigned to each of those residues.
Supplementary Material
Appendix 01 (PDF)
Acknowledgments
We thank M. Janowska, D. Wilburn, P. Brzovic for helpful discussions and N. Stone for technical support. Portions of this work appear in the Ph.D. thesis “Modulation of Human Small Heat Shock Proteins via Changes in Quasi-ordered Networks of Interactions” by CN Woods (University of Washington, 2022). The study was supported by NIH grant R01 EY017370 to R.E.K. and T32 GM008268 to C.N.W. R.E.K. is the Edmond H Fischer/Washington Research Foundation Endowed Chair in Biochemistry.
Author contributions
C.N.W. and R.E.K. designed research; C.N.W. and L.D.U. performed research; L.D.U., M.G., and M.F.B. contributed new reagents/analytic tools; C.N.W., L.D.U., and R.E.K. analyzed data; and C.N.W., L.D.U., M.G., M.F.B., and R.E.K. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
Reviewers: U.H., Max-Planck-Institut fur Biochemie; and S.M., University of California.
Data, Materials, and Software Availability
Mass spec/Proteomic data have been deposited in PRIDE (PDX035668) (43).
Supporting Information
References
- 1.Carra S., et al. , The growing world of small heat shock proteins: From structure to functions. Cell Stress Chaperones 22, 601–611 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Janowska M. K., Baughman H. E. R., Woods C. N., Klevit R. E., Mechanisms of small heat shock proteins. Cold Spring Harb. Perspect. Biol. 11, a034025 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Horwitz J., Bova M. P., Ding L.-L., Haley D. A., Stewart P. L., Lens a-crystallin: Function and structure. Eye 6, 403–409 (1999). [DOI] [PubMed] [Google Scholar]
- 4.Hashemi H., et al. , Global and regional prevalence of age-related cataract: A comprehensive systematic review and meta-analysis. Eye 34, 1357–1370 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Delbecq S. P., Klevit R. E., One size does not fit all: The oligomeric states of αB crystallin. FEBS Lett. 587, 1073–1080 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Clark A. R., Naylor C. E., Bagnéris C., Keep N. H., Slingsby C., Crystal structure of R120G disease mutant of human αB-crystallin domain dimer shows closure of a groove. J. Mol. Biol. 408, 118–134 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bagnéris C., et al. , Crystal structures of α-crystallin domain dimers of αB-crystallin and Hsp20. J. Mol. Biol. 392, 1242–1252 (2009). [DOI] [PubMed] [Google Scholar]
- 8.Peschek J., et al. , Regulated structural transitions unleash the chaperone activity of αB-crystallin. Proc. Natl. Acad. Sci. U.S.A. 110, E3780–E3789 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baughman H. E. R., Pham T.-H.T., Adams C. S., Nath A., Klevit R. E., Release of a disordered domain enhances HspB1 chaperone activity toward tau. Proc. Natl. Acad. Sci. U.S.A. 117, 2923–2929 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mainz A., et al. , The chaperone αB-crystallin uses different interfaces to capture an amorphous and an amyloid client. Nat. Struct. Mol. Biol. 22, 898–905 (2015). [DOI] [PubMed] [Google Scholar]
- 11.Woods C. N., et al. , HSPB5 disease-associated mutations have long-range effects on structure and dynamics through networks of quasi-ordered interactions. bioRxiv [Preprint] (2022). https://www.biorxiv.org/content/10.1101/2022.05.30.493970v1 (Accessed 30 May 2022).
- 12.Serebryany E., et al. , Aggregation of Trp > Glu point mutants of human gamma-D crystallin provides a model for hereditary or UV-induced cataract. Protein Sci. 25, 1115–1128 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bassnett S., Duncan G., Direct measurement of pH in the rat lens using ion-sensitive microelectrodes. Exp. Eye. Res. 40, 585–590 (1985). [DOI] [PubMed] [Google Scholar]
- 14.Mathias R. T., Riquelme G., Rae J. L., Cell to cell communication and pH in the frog lens. J. Gen. Physiol. 98, 1085–1103 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaiser C. J. O., et al. , The structure and oxidation of the eye lens chaperone αA-crystallin. Nat. Struct. Mol. Biol. 26, 1141–1150 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kumar L. V. S., Rao Ch. M., Domain swapping in human αA and αB crystallins affects oligomerization and enhances chaperone-like activity. J. Biol. Chem. 275, 22009–22013 (2000). [DOI] [PubMed] [Google Scholar]
- 17.Jehle S., et al. , N-terminal domain of αB-crystallin provides a conformational switch for multimerization and structural heterogeneity. Proc. Natl. Acad. Sci. U.S.A. 108, 6409–6414 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vicart P., et al. , A missense mutation in the αB-crystallin chaperone gene causes a desmin-related myopathy. Nat. Genet. 20, 92–95 (1998). [DOI] [PubMed] [Google Scholar]
- 19.Masson G. R., et al. , Recommendations for performing, interpreting and reporting hydrogen deuterium exchange mass spectrometry (HDX-MS) experiments. Nat. Methods 16, 595–602 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Clouser A. F., et al. , Interplay of disordered and ordered regions of a human small heat shock protein yields and ensemble of ‘quasi-ordered’ states. Elife 8, e50259 (2019), 10.7554/eLife.50259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chin J. W., Martin A. B., King D. S., Wang L., Schultz P. G., Addition of a photocrosslinking amino acid to the genetic code of Escherichia coli. Proc. Natl. Acad. Sci. U.S.A. 99, 11020–11024 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dorman G., Prestwich G. D., Benzophenone photophores in biochemistry. Biochemistry 33, 5661–5673 (1994). [DOI] [PubMed] [Google Scholar]
- 23.Haslbeck M., Weinkauf S., Buchner J., Small heat shock proteins: Simplicity meets complexity. J. Biol. Chem. 294, 2121–2132 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bartelt-Kirbach B., Slowik A., Beyer C., Golenhofen N., Upregulation and phosphorylation of HspB1/Hsp25 and HspB5/αB-crystallin after transient middle cerebral artery occlusion in rats. Cell Stress Chaperones 22, 653–663 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimauro I., Antonioni A., Mercatelli N., Caporossi D., The role of αB-crystallin in skeletal and cardiac muscle tissues. Cell Stress Chaperones 23, 491–505 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wistow G., The human crystallin gene families. Hum. Genomics 6, 26 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Selivanova O. M., Galzitskaya O. V., Structural and functional peculiarities of α-crystallin. Biology 9, 85 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clark A. R., et al. , Terminal regions confer plasticity to the tetrameric assembly of human HspB2 and HspB3. J. Mol. Biol. 430, 3297–3310 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sluchanko N. N., et al. , Structural basis for the interaction of a human small heat shock protein with the 14-3-3 universal signaling regulator. Structure 25, 305–316 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zantema A., Verlaan-De Vries M., Maasdam D., Bol S., van der Eb A., Heat shock protein 27 and alpha B-crystallin can form a complex, which dissociates by heat shock. J. Biol. Chem. 267, 12936–12941 (1992). [PubMed] [Google Scholar]
- 31.Kato K., et al. , Copurification of small heat shock protein with αB crystallin from human skeletal muscle. J. Biol. Chem. 267, 7718–7725 (1992). [PubMed] [Google Scholar]
- 32.Mymrikov E. V., Seit-Nebi A. S., Gusev N. B., Heterooligomeric complexes of human small heat shock proteins. Cell Stress Chaperones 17, 157–169 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Mymrikov E. V., et al. , Regulation of small heat-shock proteins by hetero-oligomer formation. J. Biol. Chem. 295, 158–169 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Preis W., Bestehorn A., Buchner J., Haslbeck M., An alternative splice variant of human αA-crystallin modulates the oligomer ensemble and the chaperone activity of α-crystallins. Cell Stress Chaperones 22, 541–552 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Toth R. T. IV, et al. , Empirical correction for differences in chemical exchange rates in hydrogen exchange-mass spectrometry measurements. Anal. Chem. 89, 8931–8941 (2017). [DOI] [PubMed] [Google Scholar]
- 36.Watson M. J., et al. , Simple platform for automating decoupled LC–MS analysis of hydrogen/deuterium exchange samples. J. Am. Soc. Mass Spectrom. 32, 597–600 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Guttman M., Weis D. D., Engen J. R., Lee K. K., Analysis of overlapped and noisy hydrogen/deuterium exchange mass spectra. J. Am. Soc. Mass Spectrom. 24, 1906–1912 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Deutsch E. W., et al. , The ProteomeXchange consortium in 2020: Enabling ‘big data’ approaches in proteomics. Nucleic Acids Res. 48, D1145–D1152 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Perez-Riverol Y., et al. , The PRIDE database and related tools and resources in 2019: Improving support for quantification data. Nucleic Acids Res. 47, D442–D450 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Eng J. K., Jahan T. A., Hoopmann M. R., Comet: An open-source MS/MS sequence database search tool. Proteomics 13, 22–24 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Keller A., Nesvizhskii A. I., Kolker E., Aebersold R., Empirical statistical model to estimate the accuracy of peptide identifications made by MS/MS and database search. Anal. Chem. 74, 5383–5392 (2002). [DOI] [PubMed] [Google Scholar]
- 42.Hoopmann M. R., et al. , Kojak: Efficient analysis of chemically cross-linked protein complexes. J. Proteome Res. 14, 2190–2198 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ulmer L. D., Bush M. F., Disordered region encodes α-crystallin chaperone activity toward lens client γD-crystallin. Proteomics Identifications Database (PRIDE) https://www.ebi.ac.uk/pride/archive/projects/PXD035668 Deposited 1 August 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Data Availability Statement
Mass spec/Proteomic data have been deposited in PRIDE (PDX035668) (43).