The plight of the western bumble bee (Bombus occidentalis) is a compelling, cautionary tale for the future of wild bee species under anthropogenic global change. Once a common species across much of western North America, dramatic declines of the western bumble bee were documented starting in the 1990s (1). These initial declines have been attributed to a variety of factors, including possible increased exposure to pathogens associated with commercial bumble bee rearing (1). Despite cessation of commercial rearing in the early 2000s, continued range-wide declines over the last two decades call for the careful examination of additional anthropogenic drivers and the need to identify regions of special concern (2).
In PNAS, Janousek et al. (3) use Bayesian hierarchical occupancy models to document continuing, recent declines of the western bumble bee while also quantifying the relative contributions of multiple anthropogenic stressors associated with declines (Fig. 1). High temperatures, followed by drought, and neonicotinoid pesticides were the strongest predictors of recent declines. The authors then project future occupancy of the western bumble bee across its range in the continental United States by combining their best models with 30-y projected values of key drivers under optimistic, moderate, and worst-case climate and land use scenarios. The future of the western bumble bee under realistic scenarios is concerning.
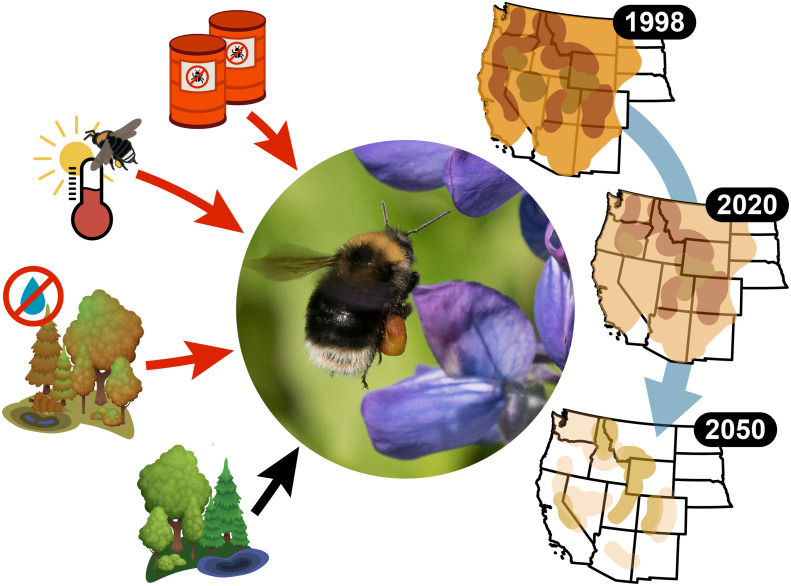
Fig. 1.
Historical and projected changes in occupancy of the western bumble bee (B. occidentalis) are impacted by a suite of climatological, land use, and management stressors. Left, Neonicotinoid use, drought severity and duration, and especially warmest summer temperatures had strong negative impacts on occupancy. Forest area, shrub area, and the amount of forest edge had positive effects on occupancy. Right, Over the past 20 y and under most realistic future scenarios, the western bumble bee (Center) shows substantial decline in occupancy across its range. Western bumble bee photo from Liz Osborn (iNaturalist observation).
As a group, bumble bees face multiple environmental stressors: climate and land use change, intensification of agriculture, extensive pesticide use, parasites, and disease—all of which likely drive species declines (4). Prior studies have examined individual stressors both in North America and elsewhere (5–7), but stressors often act simultaneously, and their additive and/or interactive effects determine outcomes for species (8). Recent experimental studies have revealed striking impacts of simultaneous stressors on bee survival and reproduction (9–11). However, studies exploring the impacts of multiple drivers on bumble bees or other bee species at larger scales are rare (12, 13). The modeling approach used by Janousek et al. provides a thorough assessment of multiple drivers at the scale of a species’ range and over multiple decades that is critical to understand ongoing trends and inform strategies to mitigate range-wide losses. As such, it adds important insights to a recent set of assessments of North American bumble bees (5, 14).
Occupancy modeling is a powerful tool for assessing the probability of species occurrence among sites and over time (15). The approach can be used to estimate a species’ distribution while also quantifying associations with various habitat and environmental factors. Hierarchical occupancy models are able to account for biases in detection probability, making them well suited for data compiled from multiple collections over time that use various methods and collectors (16).
Janousek et al. build on the framework laid out in a recent summary of knowledge gaps (2) and modeling approaches detailed for other regions (16). They model annual occupancy over two decades (1998 to 2020 for land use and climate; 2008 to 2014 for land use, climate, and pesticides) throughout the species’ range as a function of multiple climatic, land use, and pesticide variables. The authors identify trends in occupancy through time for 16 predefined ecoregions across the species range.
Janousek et al. use Bayesian hierarchical occupancy models to document continuing, recent declines of the western bumble bee while also identifying and quantifying the relative contributions of multiple anthropogenic stressors associated with declines.
The study reveals a striking 57% decline in average occupancy over the past two decades with considerable variation among the 16 ecoregions. The largest declines were in the arid Southwest and Pacific Northwest. Increasing temperature during the warmest part of the season and persistent, severe drought most strongly reduced occupancy. Landcover effects were more complex. Occupancy increased with forest cover but was greatest at intermediate levels of shrub cover and forest edge. Despite the considerable effect of agricultural land use on bees, this had no consistent effect on western bumble bee occupancy. It is worth noting that agriculture within the range of the western bumble bee and as categorized by Janousek et al. includes extensive rangelands, which differs from the Eastern United States and Europe, where most existing studies have linked agricultural land use with bumble bee declines (12, 13). Separating out different forms of agricultural land use (e.g., row crop, pasture, and rangeland) may provide additional insights. Despite no effect of agricultural land use generally, pesticide use, in particular, estimated neonicotinoid intensity, had a strong negative effect—similar in magnitude to effects of drought.
Future occupancy projections for the western bumble bee vary by ecoregion and scenario. The best-case scenario removed all nonclimate or land use-related factors, thus assuming full mitigation of pesticide, disease, and other unmeasured stressors. In this most optimistic projection, occupancy increased in five of 16 ecoregions. Critically, however, including these stressors negated all increases in occupancy. Under middle- and worst-case scenarios all regions are expected to experience substantial declines (except for two that already have modeled occupancy below 5%).
What do the findings mean for the western bumble bee, and how can they help support bees and other pollinators generally? Taken together, the results provide a clear picture of ongoing and future declines for the western bumble bee. Under the middle- and worst-case scenarios, which are likely the most realistic, the future of the western bumble bee appears bleak. Do we need to confront a difficult conclusion that it might be too late to rescue the species? For us, the answer is no. Janousek et al.'s results importantly help prioritize factors that can best mitigate these trends as well as give insight into conservation and management strategies that would bolster the western bumble bee and likely many other bumble bee species. For example, positive effects of forest and shrub area and forest edge on occupancy reinforce the value of habitat complexity—both habitat composition and configuration—in supporting bumble bees, as has been also found for other species (17). They also highlight the value of specific habitats that might buffer against effects of changing climate (18).
The assessment also provides compelling evidence to support more integrated pest and pollinator (IPPM) decisions regarding pesticide use (19, 20). The negative impacts of systemic insecticides, in particular neonicotinoids, on bees are undeniable (7, 10, 21, 22) and must be weighed against their utility (pest management) in agricultural production. Partial bans on neonicotinoids exist in multiple countries and some regions in the United States. More holistic approaches to integrated pest management that incorporate the known impacts of insecticides and seek to reduce their prophylactic use are critical. Growing evidence shows IPPM can reduce the need for extensive neonicotinoid use in field crops (23).
Janousek et al.’s analysis provides key information for ongoing status assessments of the western bumble bee, highlighting the importance of such research for informating federal and state policy. It and other recent analyses of bumble bees nationally (5) also emphasize the value of standardized, coordinated monitoring. Such coordination allows for streamlined data integration by minimizing biases associated with different sampling techniques, identification of critical spatial information gaps, and purposeful sampling designs that facilitate their use for multiple goals, including the assessment of species responses to the changing environment and human activities (24).
A critical next step for assessment will be accounting for the interacting effects of drivers (e.g., the strength of the neonicotinoid effect may depend on the magnitude of rising summer temperatures). Many experimental and observational studies describe strong interactions among bumble bee stressors (8). Quantifying such interactions in an occupancy framework could help further reveal regions of conservation concern and/or promise for the western bumble bee and bumble bees, generally.
The notable variation in current and predicted occupancy inspires some hope for the species. Moreover, the results provide important, pragmatic guidance as to where conservation actions are critical and may be most effective in addition to identifying areas where supporting populations of the western bumble bee may be untenable.
Finally, expanding these methods to additional bumble bee species [i.e., creating dynamic, multispecies occupancy models (5)] would provide critical insight into the population and range trends across the genus and allow for conservation efforts to include multiple species. Multispecies models may also help to quantify the degree to which bumble bees exhibit varied responses to multiple stressors, as has been shown for climate change (5). Fortunately, records of bumble bee occurrence are numerous relative to other insect taxa, and the database used by Janousek et al. (3) provides an excellent starting point.
Acknowledgments
Author contributions
N.M.W. and J.H. wrote the paper.
Competing interests
Williams was J. Mola's doctoral advisor.
Footnotes
See companion article, “Recent and future declines of a historically widespread pollinator linked to climate, land cover, and pesticides,” 10.1073/pnas.2211223120.
References
- 1.Cameron S. A., et al. , Patterns of widespread decline in North American bumble bees. Proc. Natl. Acad. Sci. U.S.A. 108, 662–667 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Graves T. A., et al. , Western bumble bee: Declines in the continental United States and range-wide information gaps. Ecosphere 11, e03141 (2020). [Google Scholar]
- 3.Janousek W. M., Douglas M. R., Cannings S., Clement M., Recent and future declines of a historically widespread pollinator linked to climate, land cover, and pesticides. Proc. Natl. Acad. Sci. U.S.A. 120, e2211223120 (2023). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron S. A., Sadd B. M., Global trends in bumble bee health. Annu. Rev. Entomol. 65, 209–232 (2020). [DOI] [PubMed] [Google Scholar]
- 5.Jackson H. M., et al. , Climate change winners and losers among North American bumblebees. Biol. Lett. 18, 20210551 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scheper J., et al. , Museum specimens reveal loss of pollen host plants as key factor driving wild bee decline in The Netherlands. Proc. Natl. Acad. Sci. U.S.A. 111, 17552–17557 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Woodcock B. A., et al. , Impacts of neonicotinoid use on long-term population changes in wild bees in England. Nat. Commun. 7, 1–8 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Siviter H., et al. , Agrochemicals interact synergistically to increase bee mortality. Nature 596, 389–392 (2021). [DOI] [PubMed] [Google Scholar]
- 9.Knauer A. C., et al. , Nutritional stress exacerbates impact of a novel insecticide on solitary bees’ behaviour, reproduction and survival. Proc. R. Soc. B 289, 20221013 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stuligross C., Williams N. M., Pesticide and resource stressors additively impair wild bee reproduction. Proc. R. Soc. B 287, 20201390 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vanderplanck M., et al. , Ensuring access to high-quality resources reduces the impacts of heat stress on bees. Sci. Rep. 9, 1–10 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Duchenne F., et al. , Long-term effects of global change on occupancy and flight period of wild bees in Belgium. Global Change Biol. 26, 6753–6766 (2020). [DOI] [PubMed] [Google Scholar]
- 13.Hemberger J., Crossley M. S., Gratton C., Historical decrease in agricultural landscape diversity is associated with shifts in bumble bee species occurrence. Ecol. Lett. 24, 1800–1813 (2021). [DOI] [PubMed] [Google Scholar]
- 14.Soroye P., Newbold T., Kerr J., Climate change contributes to widespread declines among bumble bees across continents. Science 367, 685–688 (2020). [DOI] [PubMed] [Google Scholar]
- 15.Royle J. A., Dorazio R. M., Hierarchical Modeling and Inference in Ecology: The Analysis of Data from Populations, Metapopulations and Communities (Elsevier, 2008). [Google Scholar]
- 16.Guzman L. M., Johnson S. A., Mooers A. O., M’Gonigle L. K., Using historical data to estimate bumble bee occurrence: Variable trends across species provide little support for community-level declines. Biol. Conserv. 257, 109141 (2021). [Google Scholar]
- 17.Martin E. A., et al. , The interplay of landscape composition and configuration: New pathways to manage functional biodiversity and agroecosystem services across Europe. Ecol. Lett. 22, 1083–1094 (2019). [DOI] [PubMed] [Google Scholar]
- 18.Mola J. M., Hemberger J., Kochanski J., Richardson L. L., Pearse I. S., The importance of forests in bumble bee biology and conservation. BioScience 71, 1234–1248 (2021). [Google Scholar]
- 19.Egan P. A., Dicks L. V., Hokkanen H. M., Stenberg J. A., Delivering integrated pest and pollinator management (IPPM). Trends Plant Sci. 25, 577–589 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Lundin O., Rundlöf M., Jonsson M., Bommarco R., Williams N. M., Integrated pest and pollinator management–Expanding the concept. Front Ecol. Environ. 19, 283–291 (2021). [Google Scholar]
- 21.Rundlöf M., et al. , Seed coating with a neonicotinoid insecticide negatively affects wild bees. Nature 521, 77–80 (2015). [DOI] [PubMed] [Google Scholar]
- 22.Willis Chan D. S., Raine N. E., Population decline in a ground-nesting solitary squash bee (Eucera pruinosa) following exposure to a neonicotinoid insecticide treated crop (Cucurbita pepo). Sci. Rep. 11, 1–11 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pecenka J. R., Ingwell L. L., Foster R. E., Krupke C. H., Kaplan I., IPM reduces insecticide applications by 95% while maintaining or enhancing crop yields through wild pollinator conservation. Proc. Natl. Acad. Sci. U.S.A. 118, e2108429118 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woodard S. H., et al. , Towards a US national program for monitoring native bees. Biol. Conserv. 252, 108821 (2020). [Google Scholar]