Abstract
Thyroid neoplasms (tumors) are the most common pathology of the endocrine system that requires surgery, and in most cases changes are benign. The surgical treatment of thyroid neoplasms consists in total, subtotal, or one lobe excision. Our study aimed to assess the concentration of vitamin D and its metabolites in patients before thyroidectomy. The study included 167 patients with thyroid pathology. Before the thyroidectomy procedure calcidiol (25-OHD), calcitriol (1,25-(OH)2D), and vitamin D binding protein (VDBP), as well as basic biochemical parameters, were measured using an enzyme-linked immunosorbent assay kit. Data analysis showed that the cohort of patients has a significant 25-OHD deficiency and proper concentration of 1,25-(OH)2D. Before the surgery, more than 80% of patients have extreme vitamin D deficiency (<10 ng/mL), and only 4% of the study group has proper 25-OHD concentration. Patients undergoing thyroidectomy are exposed to many complications, including calcium reduction. Our research has shown that patients prior to surgery have a marked vitamin D deficiency, an indicator that may affect their subsequent convalescence and prognosis. The results suggest that determination of vitamin D levels prior to thyroidectomy may be useful for potential consideration of supplementation when vitamin D deficiency is marked and needs to be incorporated into the good clinical management of these patients.
Keywords: vitamin D; thyroidectomy; 25-hydroxycholecalciferol; 1,25-dihydroxycholecalciferol
1. Introduction
Thyroid diseases are a common health problem worldwide. Recent evidence has demonstrated an association between low vitamin D status and Hashimoto’s thyroiditis, Graves’ disease, and thyroid neoplasms [1]. Thyroid neoplasms (tumors) are the most common pathology of the endocrine system that requires surgery, and in most cases these changes are benign. Thyroid nodules are diagnosed in 1% of men and 5% of women worldwide [2]. Nodule incidence increases with age, iodine deficiency, and radiation exposure, and they are more frequent in women. Numerous studies suggest that the prevalence of thyroid tumors is diagnosed in 8 to 65% of autopsy data, 19 to 35% in ultrasound, and 2 to 6% in palpation examination [3]. Surgical treatment of benign nodules is considered mainly in the case of non-toxic goiter, especially nodular goiter [4,5]. The prevalence of thyroid cancer is estimated at 1–2% of thyroid neoplasms The surgical treatment of thyroid tumors consists of total, subtotal, or one lobe excision. In some cases, it is necessary to extend the scope of surgery to adjacent lymph nodes. Based on preoperative aspiration biopsy, it is possible to remove one lobe of the thyroid gland in benign or low-risk neoplasms [6,7]. The presence of multifocal lesions required the entire thyroid gland removal. The basics of thyroid cancer treatment are associated with the removal of the thyroid gland and excision of the middle neck lymph nodes. In the case of a microcarcinoma, the removal of one affected lobe of the thyroid gland is recommended [8,9]. Thyroidectomy has significant consequences and possible complications. The thyroid gland is located near the retrograde laryngeal nerves and the parathyroid glands. The retrograde laryngeal nerves are responsible for the mobility of the vocal cords. The parathyroid glands are responsible for the metabolism of calcium in the body. Patients after total thyroidectomy and most of them after lobectomy require the use of thyroid hormones for the rest of their lives. In some cases, surgical treatment is complicated by hypoparathyroidism and retrograde laryngeal paralysis [10,11].
Postoperative hypoparathyroidism is associated with a significantly reduced quality of life [12]. It is estimated that 7 to 30% of patients undergoing total thyroidectomy will have at least temporary hypocalcemia; therefore, it is important to maintain a consistent protocol for calcium management after total or subtotal thyroidectomy to minimize calcium reduction [13]. Permanent decreased calcium levels following a parathyroidectomy may contribute to a number of conditions related to renal failure and an increased risk of death [14,15]. One of the most important factors maintaining the adequate status of calcium in the blood is vitamin D. In the general population, the prevalence of vitamin D deficiency ranges from 20 to 80% [16]. Vitamin D level had a significant effect on hypocalcemia after thyroidectomy. Although post-thyroidectomy hypocalcemia is multifactorial, severe vitamin D deficiency is significantly associated with the development of biochemical and clinical hypocalcemia and its complication [1]. Choi et al. revealed that preoperative vitamin D deficiency is associated with a higher risk of hypocalcemia following total thyroidectomy, and the average concentration of vitamin D before surgery was 14.6 mg/mL [17]. Vitamin D supplementation can prevent further complications and reduce symptoms of transient hypocalcemia [1]. Therefore, our study aimed to assess the concentration of vitamin D in patients before thyroidectomy procedure.
2. Results
2.1. Vitamin D Status among All Patients before Thyroidectomy Procedure
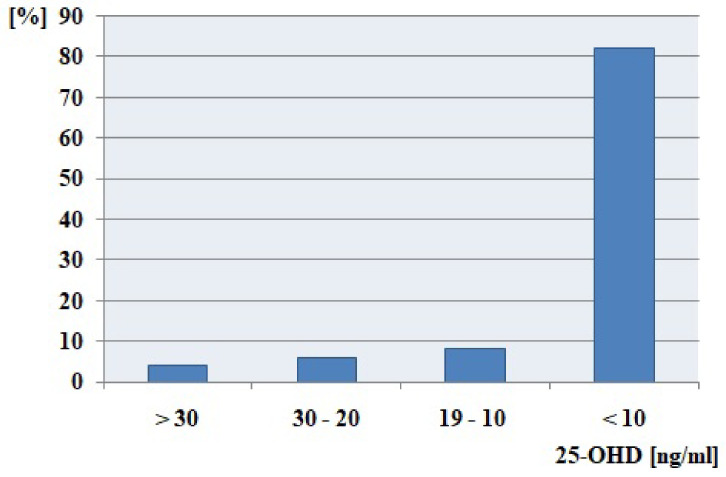
Before the surgery, more than 80% of patients have extreme vitamin D deficiency (<10 ng/mL), and only 4% of the study cohort has proper 25-OHD concentration (Figure 1).
Figure 1.
25-OHD deficiency level among all patients before thyroidectomy procedure.
2.2. Vitamin D Status between Patients with Normal and Increased BMI
The analysis revealed that patients with higher BMI have higher concentrations of 1.25-(OH)2D and PTH. There were no significant changes in 25-OHD and VDBR concentration (Table 1).
Table 1.
Vitamin D status between patients with normal and increased BMI.
| Vitamin D Status | Normal BMI | Incresed BMI | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| 25-OHD [ng/mL] | 8.55 | 40.04 | 4.25 | 44.21 | 0.17 |
| 1.25-(OH)2D [pg/mL] | 44.77 | 5.73 | 53.4 | 8.03 | 0.01 |
| VDBP [ng/mL] | 29.77 | 22.11 | 30.37 | 23.20 | 0.57 |
| PTH [pg/mL] | 15.45 | 17.72 | 24.4 | 24.6 | 0.02 |
2.3. Vitamin D Concentration and Type of Thyroid Tumor
There were no significant differences between the type of tumor/surgery and concentration of 25-OHD, 1,25-(OH)2D, and VDBP (Table 2).
Table 2.
Vitamin D status between patients with benign nodules and thyroid cancer.
| Vitamin D Status | Benign Nodules | Thyroid Cancers | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| 25-OHD [ng/mL] | 4.96 | 2.51 | 4.25 | 2.49 | 0.31 |
| 1.25-OHD [pg/mL] | 48.51 | 14.95 | 49.51 | 16.71 | 0.45 |
| VDBR [ng/mL] | 30.28 | 14.40 | 29.63 | 8.75 | 0.62 |
2.4. Calcium Status after Surgery
Calcium concentration after surgery was significantly reduced (Table 3) and decreased below the normal range. The analysis revealed also a very weak, positive correlation between 25-OHD before surgery and calcium concentration after thyroidectomy (p < 0.04, RHO = 0.2).
Table 3.
Calcium status before and after thyroidectomy.
| Calcium Status | Before Surgery | After Surgery | p | ||
|---|---|---|---|---|---|
| Median | IQR | Median | IQR | ||
| Ca [mmol/L] | 1.19 | 0.16 | 1.09 | 0.17 | 0.002 |
3. Materials and Methods
3.1. Patients
The study included 167 patients with thyroid pathology who were patients of the Department of Plastic, Endocrine and General Surgery in Szczecin, between 2020 and 2021. The patients underwent total or partial thyroidectomy. The main reasons for the surgery were single thyroid nodules, nodular goiter, hyperthyroidism due to Graves Basedow’s disease, autonomic nodules, suspected cancer, and the diagnosis of thyroid cancer. The concentration of basic biochemical parameters was measured before a surgery to assess the hormones changes and calcium status. In the order to protect the retrograde laryngeal nerves, all procedures were performed with neuromonitoring. Postoperatively, each patient was administered L-thyroxine hormones appropriately matched to body weight, parathyroid hormone (PTH), and calcium level. Exclusion criteria were renal diseases, liver diseases, and intestinal diseases. The study protocol was approved by the ethics committee of the Pomeranian Medical University and conformed to the ethical guidelines of the 1975 Declaration of Helsinki (KB-0012/195/19). The volunteers provided written informed consent before the study. Patient characteristics are presented in Table 4.
Table 4.
Patient characteristics before thyroidectomy procedure.
| Patients Characteristics n = 167 |
Median | IQR |
|---|---|---|
| Age [years] | 48 | 24 |
| Men [%] | 29 | - |
| Benign nodules [%] | 79 | - |
| Thyroid cancers [%] | 21 | - |
| Body mass index (BMI) [kg/m2], * (18.5–25) | 27.76 | 7.79 |
| TSH [uIU/mL], * (0.27–2.4) | 1.29 | 0.78 |
| Creatinine [mg/dL], * (0.5–0.9) | 0.72 | 0.18 |
| FT3 [pg/mL], * (2–4.4) | 3.16 | 0.46 |
| FT4 [ng/dL], * (0.93–1.7) | 1.28 | 0.29 |
| PTH [pg/mL], * (15–65) | 19.7 | 22.18 |
| Ca [mmol/L], * (1.0–1.3) | 1.19 | 0.16 |
| 25-OHD [ng/mL], * (0–30) | 6.13 | 2.51 |
| 1,25-(OH)2D [pg/mL] * (20–60) | 48.69 | 15.17 |
| VDBP [ng/mL] | 30.12 | 14.13 |
* ()—normal range.
3.2. Biochemical Analysis
All biochemical parameters were performed in the Laboratory of Independent Public Regional Hospital in Szczecin during routine analysis before thyroidectomy. For the determination of free calcium ion (ionized calcium), ion-selective electrodes was used. Calcidiol (25-OHD), calcitriol (1,25-(OH)2D), and vitamin D binding protein (VDBP) were measured using an enzyme-linked immunosorbent assay kit (EIAab science inc, Wuhan 430075, China).
3.3. Statistical Analysis
The statistical analysis was performed using the “R 4.0.3” software. The normality of continuous variables distribution by using Shapiro–Wilk test was evaluated, and non-parametric tests were used. The Mann–Whitney U test was used to analyze the differences between the groups. Data are presented as medians and interquartile ranges (IQR). The values of p < 0.05 were considered as statistically significant.
3.4. Vitamin D Suplemmentation
Patients were asked about cholecacyferol supplementation. A total of 15% of patients were supplementing vitamin D, with a total dose not exceeding 4000 IU/day, which is the highest acceptable dose for the general adult population.
4. Discussion
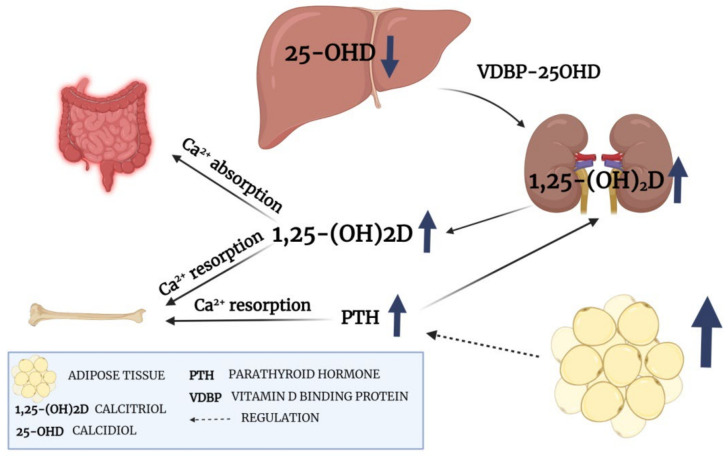
Vitamin D plays a crucial role in calcium homeostasis and the maintenance of bone health. First, Vitamin D binds to VDBP and transports to the liver. Then, hydroxylases convert inactive vitamin D to 25-OHD. In the next step 25-OHD is attached to VDBR and goes to the kidney, where an active metabolite (1,25-(OH)2D) is synthesized [18]. In contrast to 25-OHD, 1,25-(OH)2D has a short half-life period and is tightly regulated over a narrow range by PTH, calcium, and phosphate. The concentration of 1,25-(OH)2D is not a good predictor of vitamin D status unless the deficiency is severe. Therefore vitamin D status is evaluated according to 25-OHD concentration [19,20]. Our study revealed that almost all patients (96%) had insufficient 25-OHD concentration before the surgery procedure, and only 15% supplement vitamin D. It is estimated that patients with a lower concentration of 25-OH have a higher probability of hypocalcemia after thyroidectomy [21]. Tolone et al., revealed that compared to the control group, preoperative supplementation of vitamin D and calcium decreases the incidence of transient hypocalcemia after total thyroidectomy from 25.9% to 6.8% [22]. Calcium homeostasis depends on PTH and 1,25-(OH)2D concentration. PTH is responsible for the proper conversion of vitamin D and synthesis of active 1,25-(OH)2D. Lower PTH concentrations can reduce vitamin D conversion and decrease calcium absorption from the intestine [23]. Therefore, increased vitamin D before surgery can be important factor to maintain proper calcium concentration after surgery. Moreover, our study showed that patients with increased BMI has higher concentrations of 1,25-(OH)2D. This observation can be a consequence of the significant increase in PTH concentration. A high level of PTH is observed in obese and overweight patients. The correlation of PTH with a percentage of body fat seems to be independent of the plasma 25-OHD concentration, and the association between high fat level and PTH is still unclear [24]. However, one study suggests that increased PTH concentration in overweight and obese patients is associated with a lower level of 25-OHD and the need to intensify the conversion of 25-OHD to a biologically active form [25]. The PTH-vitamin D metabolism axis in our patients is presented in Figure 2
Figure 2.
PTH-vitamin D metabolism axis (created with BioRender).
In recent decades, many experimental studies have shown a wide range of anticancer effects of vitamin D compounds [26]. Cell culture studies revealed decreased thyroid cancer growth after the administration of 1,25-(OH)2D or its analogs in several thyroid cancer cell lines. The antitumor effect of 1,25-(OH)2D on thyroid cancers can be associated with proliferation inhibition [27]. It has been shown that 1,25-(OH)2D inhibits the expression of c-MYC, the proto-oncogene, causing an increased percentage of cells in the G0-G1 phase [28]. Roskies et al., showed that patients undergoing thyroidectomy had a higher malignancy rates in the vitamin D deficient group, suggesting that a lower level of 25-OHD is a potentially modifiable risk factor for thyroid cancer [29]. Sahin et al., reported that patients with papillary thyroid carcinoma had significantly lower vitamin D levels than controls, and decreases in 25-OHD concentration were more prevalent in the study group [30]. Kim et al., investigated 548 female individuals who underwent a total thyroidectomy due to thyroid cancer; the preoperative 25-OHD concentration was significantly lower in patients with a tumor size of less than 1 cm [31]. Stepien et al., revealed a significantly lower concentration of 1,25-OH2D in thyroid cancer compared to healthy controls [32]. Penna-Martinez et al., revealed that patients with thyroid carcinoma had lower serum 1,25-(OH)2D levels compared to healthy controls; there were no significant differences in 25-OHD levels in these group of patients [33,34]. Our study group revealed no significant differences in any of vitamin D metabolites or VDBP between benign nodules and thyroid cancers. However, the concentration of 25-OH in both groups was extremely low, and all patients suffered from deep vitamin D deficiency. The concentration of 25-OHD in more than 80% of patients was less than 10 ng/mL and only 4% had the proper level of vitamin D. Low vitamin D levels before surgery can have negative effects on calcium concentration after the procedure. Our study revealed weak positive correlation (RHO = 0.2, p = 0.04) between 25-OHD before thyroidectomy and calcium concentration after surgery. Moreover, after the procedure calcium concentration was significantly lower and decreased below the normal range. Decreased concentrations of vitamin D are associated with several health outcomes, such as increased bone loss, risk of fracture, muscle weakness, or insulin resistance [35]. Mayank et al. showed that a pre-operative concentration of 25-OHD has a positive correlation with calcium concentration in the early period after thyroidectomy. Patients with a 25-OHD concentration less of 20 ng/mL are highly likely to develop hypocalcemia [36]. As long as we investigate how to exploit vitamin D in cancer prevention and treatment, the basic recommendation to aim at a sufficient vitamin D level is still valid [37].
5. Conclusions
Patients undergoing thyroidectomy are exposed to many complications, including abnormal calcium concentration. Adequate vitamin D levels before the surgery appears to be an important factor in maintaining and preventing proper calcium concentration. Our research has shown that patients prior to surgery have a marked vitamin D deficiency, an indicator that may affect their subsequent convalescence and prognosis. Only 15% of them supplement vitamin D before surgery. The results suggest that the determination of vitamin D levels prior to thyroidectomy may be useful for potential consideration of supplementation when vitamin D deficiency is marked and needs to be incorporated into the good clinical management of these patients.
6. Limitations
Our study has several limitations. First of all, we did not have access to long-term measurements of calcium or 25-OHD concentration. Second of all, the size of the study group was determined by the number of patients admitted to the hospital. We did not carry out predetermined sample size estimates considering underlying hypotheses and sufficient underlying statistical power to answer them. Moreover, we do not have data about long-term complications after thyroidectomy procedure.
Author Contributions
Conceptualization, D.M.-M. and P.B.; Methodology K.J., J.K., M.M., A.S., S.H. and P.P. (Piotr Puchalski); Investigation, N.C., J.P. and Z.S.; Data Curation A.B.; Writing—Original Draft Preparation D.M.-M. and P.B.; Writing—Review and Editing, E.S.; Supervision, P.P. (Piotr Prowans). All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance ethics committee of the Pomeranian Medical University and conformed to the ethical guidelines of the 1975 Declaration of Helsinki (KB-0012/195/19).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Dağlar G., Kiliç M.Ö., Çelik C., Yüksel C., Terzioğlu S.G., Özden S., İçen D. Is There A Relationship Between Vitamin D Status And Hypocalcemia After Total Thyroidectomy? Acta Endocrinol. 2016;12:291–296. doi: 10.4183/aeb.2016.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Biello A., Kinberg E.C., Wirtz E.D. Thyroidectomy. StatPearls Publishing; Tampla, FL, USA: 2022. [PubMed] [Google Scholar]
- 3.Dean D.S., Gharib H. Epidemiology of Thyroid Nodules. Best Pract. Res. Clin. Endocrinol. Metab. 2008;22:901–911. doi: 10.1016/j.beem.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 4.Mackenzie E.J., Mortimer R.H. 6: Thyroid Nodules and Thyroid Cancer. Med. J. Aust. 2004;180:242–247. doi: 10.5694/j.1326-5377.2004.tb05894.x. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence W., Kaplan B.J. Diagnosis and Management of Patients with Thyroid Nodules. J. Surg. Oncol. 2002;80:157–170. doi: 10.1002/jso.10115. [DOI] [PubMed] [Google Scholar]
- 6.Seib C.D., Sosa J.A. Evolving Understanding of the Epidemiology of Thyroid Cancer. Endocrinol. Metab. Clin. N. Am. 2019;48:23–35. doi: 10.1016/j.ecl.2018.10.002. [DOI] [PubMed] [Google Scholar]
- 7.Molinaro E., Romei C., Biagini A., Sabini E., Agate L., Mazzeo S., Materazzi G., Sellari-Franceschini S., Ribechini A., Torregrossa L., et al. Anaplastic Thyroid Carcinoma: From Clinicopathology to Genetics and Advanced Therapies. Nat. Rev. Endocrinol. 2017;13:644–660. doi: 10.1038/nrendo.2017.76. [DOI] [PubMed] [Google Scholar]
- 8.Araque K.A., Gubbi S., Klubo-Gwiezdzinska J. Updates on the Management of Thyroid Cancer. Horm. Metab. Res. 2020;52:562–577. doi: 10.1055/a-1089-7870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. [(accessed on 2 November 2022)]; Available online: https://pubmed.ncbi.nlm.nih.gov/26462967.
- 10.Kant R., Davis A., Verma V. Thyroid Nodules: Advances in Evaluation and Management. Am. Fam. Physician. 2020;102:298–304. [PubMed] [Google Scholar]
- 11.Axelsson T.A., Hrafnkelsson J., Olafsdottir E.J., Jonasson J.G. Tall Cell Variant of Papillary Thyroid Carcinoma: A Population-Based Study in Iceland. Thyroid. 2015;25:216–220. doi: 10.1089/thy.2014.0075. [DOI] [PubMed] [Google Scholar]
- 12.Büttner M., Hinz A., Singer S., Musholt T.J. Quality of Life of Patients More than 1 Year after Surgery for Thyroid Cancer. Hormones. 2020;19:233–243. doi: 10.1007/s42000-020-00186-x. [DOI] [PubMed] [Google Scholar]
- 13.Balentine C.J., Sippel R.S. Outpatient Thyroidectomy: Is It Safe? Surg. Oncol. Clin. N. Am. 2016;25:61–75. doi: 10.1016/j.soc.2015.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Smulever A., Pitoia F. High Rate Incidence of Post-Surgical Adverse Events in Patients with Low-Risk Papillary Thyroid Cancer Who Did Not Accept Active Surveillance. Endocrine. 2020;69:587–595. doi: 10.1007/s12020-020-02310-8. [DOI] [PubMed] [Google Scholar]
- 15.Almquist M., Ivarsson K., Nordenström E., Bergenfelz A. Mortality in Patients with Permanent Hypoparathyroidism after Total Thyroidectomy. Br. J. Surg. 2018;105:1313–1318. doi: 10.1002/bjs.10843. [DOI] [PubMed] [Google Scholar]
- 16.Chen K.-W., Chen C.-W., Yuan K.-C., Wang I.-T., Hung F.-M., Wang A.-Y., Wang Y.-C., Kuo Y.-T., Lin Y.-C., Shih M.-C., et al. Prevalence of Vitamin D Deficiency and Associated Factors in Critically Ill Patients: A Multicenter Observational Study. Front. Nutr. 2021;8:768804. doi: 10.3389/fnut.2021.768804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Choi E.H.E., Qeadan F., Alkhalili E., Lovato C., Burge M.R. Preoperative Vitamin D Deficiency Is Associated with Increased Risk of Postoperative Hypocalcemia after Total Thyroidectomy. J. Investig. Med. 2021;69:1175–1181. doi: 10.1136/jim-2020-001644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lips P. Vitamin D Physiology. Prog. Biophys. Mol. Biol. 2006;92:4–8. doi: 10.1016/j.pbiomolbio.2006.02.016. [DOI] [PubMed] [Google Scholar]
- 19.Mackawy A.M.H., Al-ayed B.M., Al-rashidi B.M. Vitamin D Deficiency and Its Association with Thyroid Disease. Int. J. Health Sci. 2013;7:267–275. doi: 10.12816/0006054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawson-Hughes B., Heaney R.P., Holick M.F., Lips P., Meunier P.J., Vieth R. Estimates of Optimal Vitamin D Status. Osteoporos. Int. 2005;16:713–716. doi: 10.1007/s00198-005-1867-7. [DOI] [PubMed] [Google Scholar]
- 21.Malik M.Z., Mirza A.A., Farooqi S.A., Chaudhary N.A., Waqar M., Bhatti H.W. Role of Preoperative Administration of Vitamin D and Calcium in Postoperative Transient Hypocalcemia after Total Thyroidectomy. Cureus. 2019;11:e4579. doi: 10.7759/cureus.4579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tolone S., Roberto R., del Genio G., Brusciano L., Parmeggiani D., Amoroso V., Casalino G., Verde I., Bosco A., D’Alessandro A., et al. The Impact of Age and Oral Calcium and Vitamin D Supplements on Postoperative Hypocalcemia after Total Thyroidectomy. A Prospective Study. BMC Surg. 2013;13((Suppl. 2)):S11. doi: 10.1186/1471-2482-13-S2-S11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khundmiri S.J., Murray R.D., Lederer E. PTH and Vitamin D. Compr. Physiol. 2016;6:561–601. doi: 10.1002/cphy.c140071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pitroda A.P., Harris S.S., Dawson-Hughes B. The Association of Adiposity with Parathyroid Hormone in Healthy Older Adults. Endocrine. 2009;36:218–223. doi: 10.1007/s12020-009-9231-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valiña-Tóth A.L.B., Lai Z., Yoo W., Abou-Samra A., Gadegbeku C.A., Flack J.M. Relationship of Vitamin D and Parathyroid Hormone to Obesity and Body Composition in African Americans. Clin. Endocrinol. 2010;72:595–603. doi: 10.1111/j.1365-2265.2009.03676.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Muñoz A., Grant W.B. Vitamin D and Cancer: An Historical Overview of the Epidemiology and Mechanisms. Nutrients. 2022;14:1448. doi: 10.3390/nu14071448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim D. The Role of Vitamin D in Thyroid Diseases. Int. J. Mol. Sci. 2017;18:1949. doi: 10.3390/ijms18091949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Clinckspoor I., Verlinden L., Mathieu C., Bouillon R., Verstuyf A., Decallonne B. Vitamin D in Thyroid Tumorigenesis and Development. Prog. Histochem. Cytochem. 2013;48:65–98. doi: 10.1016/j.proghi.2013.07.001. [DOI] [PubMed] [Google Scholar]
- 29.Roskies M., Dolev Y., Caglar D., Hier M.P., Mlynarek A., Majdan A., Payne R.J. Vitamin D Deficiency as a Potentially Modifiable Risk Factor for Thyroid Cancer. J. Otolaryngol. Head Neck Surg. 2012;41:160–163. [PubMed] [Google Scholar]
- 30.Sahin M., Uçan B., Giniş Z., Topaloğlu O., Güngüneş A., Bozkurt N.Ç., Arslan M.S., Ünsal İ.Ö., Akkaymak E.T., Demirci T., et al. Vitamin D3 Levels and Insulin Resistance in Papillary Thyroid Cancer Patients. Med. Oncol. 2013;30:589. doi: 10.1007/s12032-013-0589-5. [DOI] [PubMed] [Google Scholar]
- 31.Kim J.R., Kim B.H., Kim S.M., Oh M.Y., Kim W.J., Jeon Y.K., Kim S.S., Lee B.J., Kim Y.K., Kim I.J. Low Serum 25 Hydroxyvitamin D Is Associated with Poor Clinicopathologic Characteristics in Female Patients with Papillary Thyroid Cancer. Thyroid. 2014;24:1618–1624. doi: 10.1089/thy.2014.0090. [DOI] [PubMed] [Google Scholar]
- 32.Stepien T., Krupinski R., Sopinski J., Kuzdak K., Komorowski J., Lawnicka H., Stepien H. Decreased 1-25 Dihydroxyvitamin D3 Concentration in Peripheral Blood Serum of Patients with Thyroid Cancer. Arch. Med. Res. 2010;41:190–194. doi: 10.1016/j.arcmed.2010.04.004. [DOI] [PubMed] [Google Scholar]
- 33.Penna-Martinez M., Ramos-Lopez E., Stern J., Kahles H., Hinsch N., Hansmann M.-L., Selkinski I., Grünwald F., Vorländer C., Bechstein W.O., et al. Impaired Vitamin D Activation and Association with CYP24A1 Haplotypes in Differentiated Thyroid Carcinoma. Thyroid. 2012;22:709–716. doi: 10.1089/thy.2011.0330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Penna-Martinez M., Ramos-Lopez E., Stern J., Hinsch N., Hansmann M.-L., Selkinski I., Grünwald F., Vorländer C., Wahl R.A., Bechstein W.O., et al. Vitamin D Receptor Polymorphisms in Differentiated Thyroid Carcinoma. Thyroid. 2009;19:623–628. doi: 10.1089/thy.2008.0388. [DOI] [PubMed] [Google Scholar]
- 35.Shapses S.A., Lee E.J., Sukumar D., Durazo-Arvizu R., Schneider S.H. The Effect of Obesity on the Relationship Between Serum Parathyroid Hormone and 25-Hydroxyvitamin D in Women. J. Clin. Endocrinol. Metab. 2013;98:E886–E890. doi: 10.1210/jc.2012-3369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Tripathi M., Karwasra R.K., Parshad S. Effect of Preoperative Vitamin D Deficiency on Postoperative Hypocalcemia after Thyroid Surgery. Thyroid Res. 2014;7:8. doi: 10.1186/1756-6614-7-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Henn M., Martin-Gorgojo V., Martin-Moreno J.M. Vitamin D in Cancer Prevention: Gaps in Current Knowledge and Room for Hope. Nutrients. 2022;14:4512. doi: 10.3390/nu14214512. [DOI] [PMC free article] [PubMed] [Google Scholar]