Abstract
Paleontology has provided invaluable basic knowledge on the history of life on Earth. The discipline can also provide substantial knowledge to societal challenges such as climate change. The long-term perspective of climate change impacts on natural systems is both a unique selling point and a major obstacle to becoming more pertinent for policy-relevant bodies like the Intergovernmental Panel on Climate Change (IPCC). Repeated experiments on the impacts of climate change without anthropogenic disturbance facilitate the extraction of climate triggers in biodiversity changes. At the same time, the long timescales over which paleontological changes are usually assessed are beyond the scope of policymakers. Based on first-hand experience with the IPCC and a quantitative analysis of its cited literature, we argue that the differences in temporal scope are less of an issue than inappropriate framing and reporting of most paleontological publications. Accepting that some obstacles will remain, paleontology can quickly improve its relevance by targeting climate change impacts more directly and focusing on effect sizes and relevance for projections, particularly on higher-end climate change scenarios.
Keywords: paleobiology, climate change, IPCC, biodiversity
The effects of climate change are increasing across systems (1–3). A rigorous synthesis of all scientific disciplines is crucial for understanding past and future climates, their impacts on human and natural systems, and possibilities of adaptation and mitigation. Due to its unique perspective on past biological changes, paleontology has much to offer in this regard to organizations like the Intergovernmental Panel on Climate Change (IPCC).
Specifically, paleontology allows a systematic assessment of past impacts of climate change, permitting us to find generalities of past responses to climate change, which can be incorporated in projections. For example, studies of the youngest fossil record from the last several decades, centuries, and millennia in the emerging field of conservation paleobiology can be used to evaluate biotic responses to the most recent episode of climate warming (4–6). Similarly, the relatively high temporal resolution of the Quaternary fossil record from the last tens and hundreds of thousands of years has yielded perspectives on range shifts including local extinctions and biome shifts (7–9) and community turnover (10–12) driven by climate variability during glacial–interglacial cycles. Examination of the more ancient fossil record provides a still more unique perspective during past intervals of climatic upheaval that are unparalleled in the near-time record (Pleistocene to prehistorical Holocene, the last 2.58 million y). Particularly, ancient hyperthermal events, brief episodes of massive climate warming, were similarly driven by (volcanically induced) greenhouse gas emissions as modern climate change (13, 14) and are thus potentially more representative of scenarios we may face in the near future than near-time paleo patterns, when climate change was largely driven by orbital cycles (15). The deep-time pattern is also better suited to evaluate the potential for climate change to drive mass extinction events, which is an urgent matter in the context of catastrophic climate change scenarios (16).
Even though paleoclimatic data on the physical evidence of climate change have long been incorporated in the IPCC assessment reports (ARs) of Working Group (WG) I, paleontological findings on biotic responses to climate change are rarely considered by the IPCC or by other policy-relevant organizations such as the Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (IPBES). We argue that, despite showing promise (17–19), paleontology does not currently fulfill its potential to provide policy-relevant information on climate change impacts. In our opinion, this unrealized potential is only partly due to the most commonly cited factor: the vastly longer timescales considered in paleontology than are considered relevant for conservationists and policymakers. As has been discussed broadly in the conservation science literature (e.g., refs. 20–22), we suggest that the manner in which paleontological research is prioritized and communicated also contributes substantially to the lack of policy relevance.
We provide guidelines for increasing the policy relevance of paleontological contributions on biotic responses to climate change. Two authors of this paper (W.K. and N.B.R.) were involved in the latest IPCC AR of WG II, which was published in February 2022 (3). Hence, this paper gives a WG II perspective. These guidelines are based on the personal experience of the first author and a systematic survey of paleontological papers comparing those which have been cited in the Fifth Assessment Report of the IPCC (AR5) (1) with those that were not considered despite having climate change in the keywords.
The IPCC
The IPCC assesses the world’s scientific knowledge of all facets of climate change. The IPCC is a scientific panel acting on behalf of the United Nations—and thus has a political mandate. Although perhaps the most interdisciplinary scientific panel on the planet, the IPCC’s task is to assess research, not to conduct original research. Authors of the IPCC thus depend on the most pertinent scientific literature for their assessment. The IPCC ARs are produced by three working groups. WG II, focusing on observed and projected impacts of climate change, vulnerabilities, and adaptation options, is the natural home for paleontological contributions to the IPCC. Paleoclimate data considering paleontological data are assessed in WG I (15) but biodiversity information, which is the output of many paleontological studies, is only included in WG II. The recent AR of WG II (3) focused more strongly than previous reports on the interactions of climate change, ecosystems, and human society. The reaffirmation that climate change and biodiversity loss are tightly intertwined (23) offers new impetus for paleontology to contribute to this discussion. With the opportunity to study general patterns of ecological and evolutionary dynamics during past climate changes, paleontology has the potential to contribute to projections of climate impacts under different scenarios such as the shared socioeconomic pathways (24). Similarly, climate changes in the near-time geologic record can help to fine-tune the role of rates in biological response (25).
An IPCC assessment is fundamentally different from a review in that the pertinent literature is not simply surveyed and discussed but evaluated in a semiquantitative fashion: Every substantial statement in the IPCC reports has to be associated with a likelihood or confidence statement. The confidence language of the IPCC can be difficult to grasp (26) but is nevertheless necessary to maintain scientific standards and increase policy relevance. Key statements emerging from individual chapters in IPCC reports are included in the summary for policymakers (SPM), but only after government review and intense negotiations. To put it pointedly, the whole purpose of the very long assessment reports is to provide the scientific basis for the SPM.
Thus, it is disconcerting that the sole paleo-related SPM statement in the previous assessment cycle, AR5, was “While only a few recent species extinctions have been attributed as yet to climate change (high confidence), natural global climate change at rates slower than current anthropogenic climate change caused significant ecosystem shifts and species extinctions during the past millions of years (high confidence)” (1). This statement is not even fully accurate. There are challenges to comparing rates of climate change across timescales, owing to a temporal scaling effect that leads to artificially reduced rates at longer time spans of observation (27). It might be that warming rates were indeed slower during past hyperthermals, but confidence in estimates of slower rates is low given the dramatic consequences of some ancient hyperthermals such as the end-Permian crisis (28). The lack of paleontological contributions is especially noteworthy because WG II filled two volumes in AR5 (rather than only one, as in the current assessment cycle, AR6) and involved two paleontologists who contributed substantially to both the terrestrial (29) and ocean (30) chapters. The SPM of the current assessment cycle (31) is not any better: Reference to past observations is made twice but there is not a single explicit reference to paleo.
Policy-Relevant Contributions from the Study of Past Hyperthermals
Hyperthermal events are here defined as geologically brief episodes of substantial warming, namely “a geologically constrained interval of time when global mean temperature rose significantly above background.” In this context, “geologically constrained” refers to a time interval smaller than a geological stage (one unit of time commonly spanning several millions of years) and “background” translates to a time interval of the length of a geological stage or longer. Foster et al. (13) provided more specific commonalities of hyperthermals such as onset durations of less than 100 thousand years, a total duration of less than 2 million years, a negative carbon-isotope excursion, a reduction of oxygen content, ocean acidification, and an increased hydrological cycle and continental erosion. As not all these circumstances are evident from all known past warming events, we focus on the same six hyperthermal events in the last 300 million years that were defined by Foster et al. (13) (Fig. 1). Taking past hyperthermal events as natural laboratories, paleontology can contribute policy-relevant knowledge along four major routes.
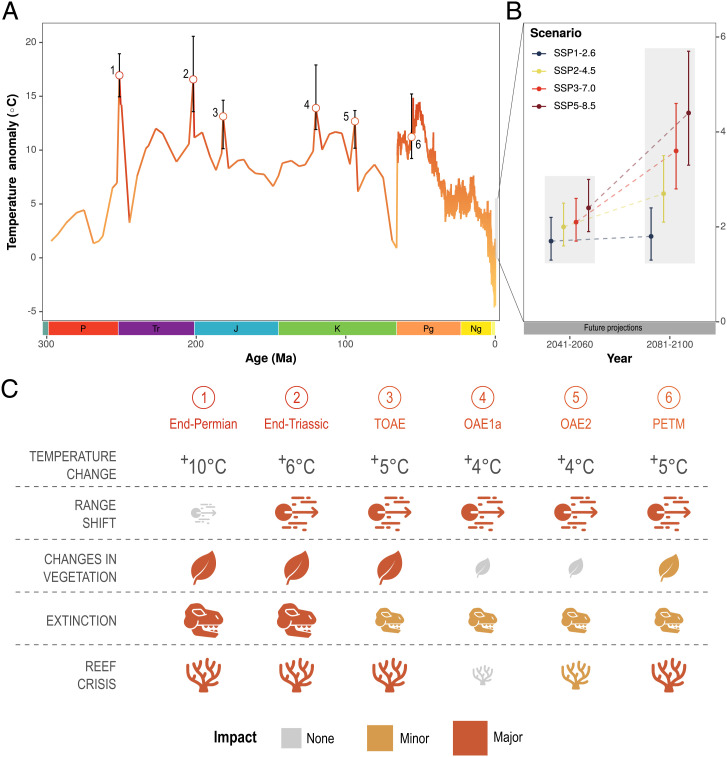
Fig. 1.
(A) Temperature anomalies (temperature difference from preindustrial [1850 to 1900]; solid orange curve) derived from climate modeling (300 to 66 million years ago; Ma) (89, 90) and deep-sea proxy data (66 to 0.1 million years ago) (91). Temperature peaks below the gray bars indicate well-known hyperthermals with temperature anomalies derived from temperature-sensitive proxy data (Dataset S1). Error bars indicate uncertainties in peak warming events (ranges in the literature). Geological period abbreviations: P: Permian, Tr: Triassic, J: Jurassic, K: Cretaceous, Pg: Paleogene, Ng: Neogene. (B) Temperature anomalies as per the shared socioeconomic pathway (SSP) scenarios (15). (C) Biological responses to rapid warming events (hyperthermals) over the last 300 million years (92). Hyperthermal abbreviations: TOAE: Toarcian Oceanic Anoxic Event, OAE: Oceanic Anoxic Event, PETM: Paleocene-Eocene Thermal Maximum.
Confronting Climate Skepticism.
Climate skeptics often rely on the argument that climate is always changing and the current warming trend is therefore not alarming. Paleontology can provide insights into the catastrophic effects of ancient climate change and critical temperatures at which tipping points might occur across ecosystems. For example, a recent analysis revealed that a change in mean global temperature of 5.2 °C or more between geological stages resulted in mass extinction events (32). This represents the type of pertinent quantitative information the IPCC seeks. Indeed, the IPCC incorporated this information to make a confidence statement: “Paleorecords indicate that at extreme global warming levels (>5.2 °C), mass extinction of marine species may occur (medium confidence)” (33). Increasing the confidence of this statement and quantifying the expected extinction toll at various degrees of warming should be a high research priority. Other tipping points could also be defined with fossil data.
Defining Limits of Natural Adaptation.
Limits to natural adaptation are key to predicting the consequences of climate change not only in terms of rates but also in terms of magnitude. Most paleontological contributions already support niche conservatism with respect to climatic tolerances (34, 35) and habitat affinity (36, 37). They also suggest that natural adaptation is unlikely to be able to keep pace with rapid climate change, although neither rates of adaptation nor rates of climate change can be compared directly across timescales (27, 38). There is a negative power-law relationship between time spans of observation and observed rates, probably because rates of climate change and evolution are not monotonic but the likelihood of transient stagnations or even reversals increases over time (27). Therefore, attempts to compare deep-time adaptation rates with projected rates of warming (39) are flawed. That said, the relative rate of climate forcing and ecological response may still be informative when analyzed at comparable temporal resolution (25).
Despite mismatches in rates, paleontology can still provide valuable insights into the effectiveness of adaptation under rapid climate change. Every extinction in the fossil record signposts a failed adaptation. During hyperthermal events, the parsimonious explanation is that a warming-related stressor was responsible for the extinction (40). Global extinction of a species is often preceded by regional population extinctions (extirpations) and such extirpations are an integral part of range shifts (41). Together with phenological changes, latitudinal and altitudinal range shifts are the most widely documented response of life to current (42) and near-time past climate change (7, 43). It is not clearly defined whether range shifts are part of climate adaptation or indicate failure to adapt; however, extirpations obviously signify a failure to adapt to local environmental changes. In that sense, range shifts could be used to track patterns of adaptation. Unfortunately, range shifts are very hard to trace in deep time (the record older than the Pleistocene; more than 2.58 million years ago) due to sampling issues (44, 45). At the level of individual species, only global extinctions signpost the exceedance of adaptation limits.
Any aspect of performance loss, such as changes in abundance, can also be viewed as an adaptation limit in the IPCC sense: “the change in climate where adaptation is unable to prevent damaging impacts and further risk” (46). Measuring abundance changes within populations is challenging in the near- and deep-time fossil record alike but, as an extreme case of abundance loss, the failure of entire ecosystems can be easily detected. For example, the collapse of ancient coral reefs and some plant biomes has been associated with hyperthermal events (45, 47).
Reductions of body size can also be viewed as an adaptive strategy against warming and elevated CO2 levels (48). Assessments of body size reductions due to climate change are common in the paleontological literature (e.g., refs. 49–52), although the explicit link to adaptation is rare (53). Quantifying the interplay of range shifts, body size, and extinction would be a worthwhile agenda for future research.
Learning from the Past to Predict Winners and Losers of the Current Climate Change.
Identifying vulnerabilities to climate change across systems is one of the key goals of IPCC WG II. Vulnerability is defined as the propensity to be adversely affected by climate change and depends on exposure to climate hazards, the intensity of the hazards, and the adaptive capacity of organisms and systems. Most paleontological studies of climate change focus on some aspect of vulnerability (e.g., refs. 54–58). Paleontology can elucidate which species, traits, and ecosystems were most vulnerable to past climate changes (Fig. 1) and identify commonalities that allow projection of such patterns into the future.
Geographic patterns of climate-induced extinction in the fossil record can also contribute to projections of climate risks. For example, Penn et al. (55) combined the observation of increased extinction tolls in high latitudes and reduced extinctions in the tropics with modeling techniques to reveal that the combination of warming and hypoxia explains the biogeography of the end-Permian mass extinction. The predictive capacity of this finding is, however, compromised by the observation that latitudinal extinction selectivity varied across hyperthermals (59). For example, the end-Triassic hyperthermal exhibits an opposite latitudinal extinction selectivity: Extinction risk was substantially greater in the tropics than extratropics (59, 60). More comprehensive approaches to assessing geographic patterns of vulnerability are required to explain these differences.
Trait-based approaches are very promising for the identification of vulnerabilities (6). For example, a trait-based assessment of extinction risk of reef corals, using machine-learning techniques and calibrated against observed coral extinctions in the Caribbean Plio-Pleistocene (61), produced a more realistic map than a previous assessment of coral extinction risk, which largely relied on loss of reef areas as a surrogate for population trends (62). As previously established in paleontological studies, vulnerability of reefs to thermal stress is not necessarily indicative of an elevated extinction risk of corals (47). One study has also suggested that among marine life, photosymbiotic, actively burrowing or swimming taxa, and taxa with larger body sizes were especially prone to extinction in hyperthermal events (63). Evaluating which traits are particularly vulnerable to hyperthermal events as opposed to background and nonhyperthermal mass extinctions is key to identifying specific vulnerabilities (57).
Improving Attribution.
Attribution of current ecological change to rising greenhouse gases is confounded by the signal of direct human impacts (64). An oft-cited strength of paleontology is that it can provide knowledge on the response of ecosystems to abiotic stressors without the “noise” of direct human impacts such as habitat destruction, overexploitation, and pollution (4–6, 57, 65). Recording nonanthropogenically biased patterns is indeed a major strength of paleo. Ancient hyperthermals are free of anthropogenic impacts but the interaction of abiotic drivers is hard to constrain. For example, the Siberian Trap volcanism that is thought to have caused the end-Permian mass extinction not only released greenhouse gases but also halogens, which may have amplified the extinction toll (28, 66). Attribution to temperature change is facilitated in the near-time fossil record, suggesting mild effects of climate changes (5, 11), but again near-time climate change was largely forced by astronomical cycles rather than greenhouse gases (15). Quantitative tests ranging from simple correlation to more complex tests of causality (67) will improve the rigor of attribution across temporal scales.
Obstacles
Several paleontological contributions cited above were included in the IPCC ARs. There are many more paleontological publications, which have not been considered by the IPCC, even though they focus on climate change. The usual scapegoat for the nonconsideration of paleontological contributions in conservation-relevant literature is the vast difference in temporal scale at which patterns are explored (17, 18). Temporal scale is indeed a major problem, as we have highlighted above when referring to rates of change. However, scale is less problematic when magnitudes are the focus. For example, the rank order of sensitivity to climate-related stressors among animal groups in experimental studies is positively correlated with the rank order of average extinction rates in the fossil record (68). In addition, timescales are less of an issue in the near-time record, where rates of change are addressable (25). We hypothesize that, in addition to the obstacle of scale, there are two less often considered but equally important obstacles: 1) the lack of targeted research and 2) incongruent reporting of results.
-
1)
The paleontological literature is rich in documenting patterns and processes of extinction. However, publications often target general extinction risks rather than risks that are specific to climate change. Thus, they cannot be used to inform the future of biodiversity under climate change. For example, Finnegan et al. (65) defined baselines for extinction risk in the modern ocean based on Neogene extinction patterns. Models were calibrated based on extinctions occurring during a long-term cooling trend, which may compromise their application for projections under climate warming (69). Similarly, many publications assess the selectivity of extinctions in background and mass extinction events without linking to specific causes (e.g., refs. 70–73). In order to be specific to climate change, dichotomizing hyperthermals and nonhyperthermals may be more useful than dichotomizing, for example, mass extinction and background extinctions (e.g., refs. 74 and 75).
Most paleontologists are not producing data with the end goal of seeing their work used for political decisions. However, many discuss their results in the context of modern climate change, maybe in an attempt to remain topical to funders and journals. This creates a misalignment in the relevance of information generated by paleontologists and the information needed for policy-relevant documents, potentially undermining attempts to maintain or increase the funding and perceived relevance of paleo. Starting research projects with policy relevance and organizations like the IPCC in mind would certainly help to align research outputs with policy needs and justify funding for paleontology (20, 22).
-
2)
The most important shortcoming in most paleontological contributions is probably the way results are reported. Reporting issues are perhaps best illustrated with the end-Permian mass extinction as an example. Whereas there is no shortage of paleontological publications emphasizing the relationship between global warming and extinction, with several discussing potential analogs to 21st-century warming (e.g., refs. 76 and 77), it is very difficult to extract from this literature even the most basic numbers that could be used by the IPCC such as extinction tolls, ecological changes, and their links to global warming.
Extinction rates of the end-Permian mass extinction have been quantified at different taxonomic levels using different data sources and techniques. Marine extinction tolls at the species level vary between 81 and 96%. A survey of the literature suggests that numbers of 90 to 95% are still more widely stated than lower numbers, although the higher estimates originated in the 1970s (78) and Stanley (79) made a convincing case that the actual extinction rate was closer to 81%. Continued reporting of the higher numbers may either reflect an uncritical repetition of older numbers or the human preference for drama. Regardless, the current climate crisis, as acute as it may be, should be assessed based on the best rather than most dramatic estimates from the fossil record.
The extinction toll in plants is even more highly disputed. A recent publication hinted toward severe biases in the plant fossil record at the Permian–Triassic boundary and concluded that there was no mass extinction of plants at the genus level (80). However, for the data to be useful for the IPCC, a revised number for extinction toll at the species level is required, as the IPCC does not refer to genera.
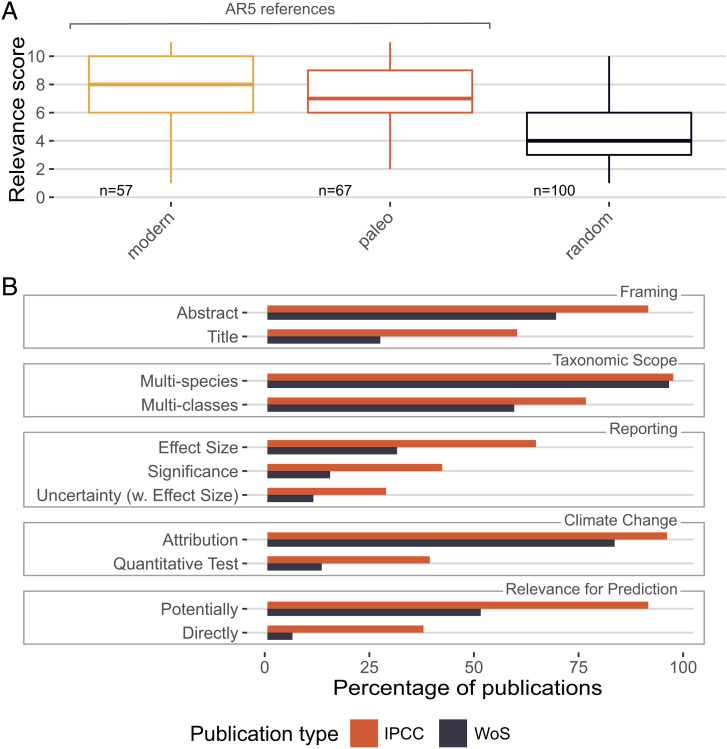
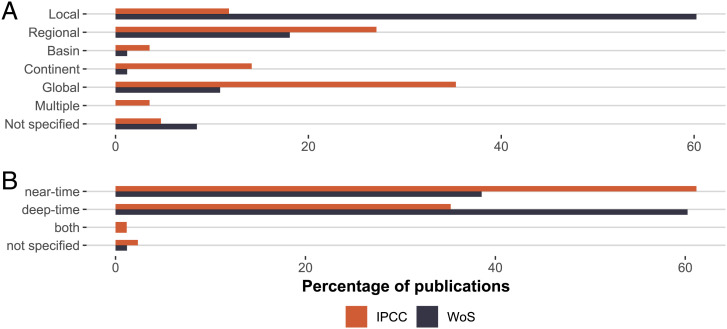
Based on these observations and inspired by a similar approach to strengthening the confidence in climate change impact science (81), we developed a scoring system for publications and calculated a relevance score (RS) for a suite of references cited or not cited by the IPCC (Materials and Methods). The results are clear (Fig. 2). Modern ecological literature cited in the terrestrial and ocean chapters of IPCC AR5 (29, 30) performed highest (median RS = 8) but not significantly higher than cited paleo literature (median RS = 7; Wilcoxon test, W = 2133.5; P = 0.259). However, the cited paleontological literature scored massively higher than a random subset of the noncited paleontological literature, which also had “climate change” as a keyword (median RS = 4, W = 5354.5, P = 4.254e-11). The cited literature had a greater spatial scope and tended to focus more on historical and near-time than deep-time patterns (Fig. 3). Nevertheless, 37% of IPCC citations with reference to the past refer to deep-time paleontological papers, suggesting that a substantial part of the paleontological literature could become relevant for the IPCC. The RS of near-time literature cited by the IPCC (median 8) is only marginally greater than the RS of deep-time literature (median RS = 7, W = 353, P = 0.35). And the pattern is even inverse for the noncited random subset, with deep-time RS scoring higher than near-time RS (median 8 and 7, respectively, W = 1,255, P = 0.057).
Fig. 2.
(A) Relevance scores of cited references in two IPCC AR5 publications with a focus on ecological impacts of climate change (29, 30) and a random subset of the paleontological literature with a focus on climate change (from Web of Science; WoS). Scores of modern and paleo data are statistically not distinguishable, whereas the random subset is substantially lower (see text for statistics). (B) Percentages of IPCC AR5 and WoS paleontological publications, which score in the respective categories. For example, 42% of all paleontological publications cited by the IPCC provide direct relevance for the prediction of climate change impacts, whereas this relevance is only shown in 6% of the uncited random WoS subset.
Fig. 3.
Scope of publications varies profoundly between paleontological publications cited by the IPCC and those that are not cited. (A) Spatial scope. (B) Temporal scope. Percentages are provided for IPCC and WoS publications. For example, 63% of publications refer to near-time paleo and historical data, whereas 37% refer to the pre-Pleistocene deep-time record.
The IPCC and policymakers rely on the quantitative reporting of effect sizes rather than significance tests obtained through statistical tests (e.g., P values). Statements that are useful for the IPCC include the observed effects on an organism or system given a certain level of warming or provide a quantifiable comparison among organisms or systems: “Global mass bleaching and destruction of coral reefs occurred at X °C (X = measured temperature anomaly) of global warming” or “species in the Y group (a clade or functional group) were on average more vulnerable to warming than species in the Z group (another or all other clades or groups) by ES (a measure of effect size).” The communication of results using such statements is usually lacking in the paleontological literature. Nolan et al. (10) provide a good-practice example to reporting paleontological data. They provide empirical quantitative evidence for compositional and structural vegetation changes at different levels of regional warming. Nevertheless, the abstract only makes qualitative statements about the sensitivity of terrestrial ecosystems to climate warming and the excellent study was cited only once in a regional chapter of WG II (82). The framing of publications with regard to their titles and abstracts contributes to their omission in IPCC reports. Many publications focus on a specific location or fossil group and only mention climate change in passing, rather than featuring it prominently in the title. Framing publications to highlight the impacts of climate change improves the likelihood they are found and incorporated in the IPCC (Fig. 2).
Two publications may showcase the ideal structure and reporting for use in policy matters. As is typical for the IPCC, in the process of writing, lead authors not only identify research gaps but also aim to fill such gaps with new research. For example, Warren et al. (83) filled a knowledge gap concerning the difference in effects between 1.5 °C and larger magnitudes of global warming on terrestrial biodiversity by modeling and quantifying the effects of projected warming on regional biodiversity. The results of this paper were included in the SPM of the Special Report on the Impacts of Global Warming of 1.5 °C (84). Similarly, authors of the cross-chapter paper of biodiversity hotspots in AR6 (85) realized that the climate change impacts on these regions had not yet been documented in the scientific literature and published a targeted paper focusing on projected extinction risks in such areas (86). The key finding of this paper, a roughly 10-fold increase in extinction risk of endemic species with an increase from <1.5 to 3 °C warming, was also included in the SPM (31). Because these publications were tailored for the IPCC, they serve to showcase best practices for reporting. Both papers are framed to specifically provide data on ecological responses to warming under different scenarios. They thoroughly quantify these responses and the key numbers are reported in the abstract. In addition, the spatial distribution of risks is shown in global maps, and uncertainties are clearly reported.
Concluding Remarks
There are limits to the spatiotemporal resolution of paleontological data. For example, time averaging in most sedimentary rocks limits the temporal resolution that paleontology can achieve (87). However, this limitation does not necessarily compromise the potential of making policy-relevant contributions along the four routes indicated above. The contribution of paleontology to policy-relevant climate impact research can be increased by more focused research, explicitly considered scaling issues, and especially improved framing and reporting. The potential contributions of paleontology to conservation and climate policy are many; however, much of that potential remains unrealized and all branches of paleo can become more relevant through self-assessment to better align with the needs of practitioners and policymakers (6, 88).
Materials and Methods
Assessment of Reporting Practices of Publications.
We developed a framework to evaluate publications in the IPCC AR5 (chapters 4 and 6 from WG II) (29, 30) with a historical or paleontological focus. We focus on AR5, because it was more comprehensive than the recent AR6 (3) and because none of the present authors were involved in that report; thereby we avoid conflict of interest. Our framework is aimed to not only assess publications but also to provide guidelines for paleontologists when reporting results in a climate change–related study. We developed a scoring system that specifically evaluates and assigns a publication a score, named the relevance score, based on the presence of relevant information such as the framing of the article, taxonomic scope of the study (one or more classes), reporting of quantitative effect sizes, clear attribution to climate change, and relevance of the study to predicting future climate impacts on biodiversity (SI Appendix, Table S1). This assessment is not evaluating the quality of the publication but rather the extent to which it may be relevant for policy-related bodies such as the IPCC. The maximum RS of 11 describes a publication that provides evidence-based conclusions (through statistical and other quantitative assessments) on how a range of organisms respond to climate change and either provides crucial information (in the form of effect sizes, rates, or proportions) on the extent of the response given a certain amount of warming or relates to near-future scenarios.
We assessed publications that used long-term data (more than 30 y and up to millions of years) and categorized them according to their temporal scope (Dataset S2). Paleontological papers were categorized as near-time (Pleistocene-Holocene; less than 2.58 million years ago) or deep-time (older than the Pleistocene; greater than 2.58 million years ago). We only included papers that had a biodiversity context. We also compared the paleontological papers included in the AR5 with a subset of those that were not cited in the AR. We pulled a random list of 100 publications published between 2003 and 2013 from the Web of Science. The list was drawn from a download of references using the search terms “(paleontology OR paleobiology OR palaeontology OR palaeobiology OR paleoecology OR palaeoecology) AND climate change” (Dataset S2).
Supplementary Material
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Acknowledgments
We thank the German Aerospace Center for supporting travels to the lead author’s meetings. This work was supported by the Volkswagen-Stiftung and the Deutsche Forschungsgemeinschaft (KI 806/15-2, KI 806/17-1) and is embedded in the Research Unit TERSANE (FOR 2332: Temperature-Related Stressors as a Unifying Principle in Ancient Extinctions).
Author contributions
W.K. designed research; J.A.S. and N.B.R. performed research; W.K. and N.B.R. analyzed data; and W.K., J.A.S., and N.B.R. wrote the paper.
Competing interests
The authors declare no competing interest.
Footnotes
This article is a PNAS Direct Submission.
Data, Materials, and Software Availability
Data and code reported in this article have been deposited in Zenodo (https://doi.org/10.5281/zenodo.7236301) (93). All study data are included in the article and/or supporting information.
Supporting Information
References
- 1.IPCC, Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change (Cambridge University Press, Cambridge, UK, 2014). [Google Scholar]
- 2.IPCC, IPCC Special Report on the Ocean and Cryosphere in a Changing Climate (Cambridge University Press, Cambridge, UK, 2019). [Google Scholar]
- 3.IPCC, Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel of Climate Change (IPCC), Pörtner H.-O., et al., Eds. (Cambridge University Press, Cambridge, UK, 2022). [Google Scholar]
- 4.Dietl G. P., et al. , Conservation paleobiology: Leveraging knowledge of the past to inform conservation and restoration. Annu. Rev. Earth Planet. Sci. 43, 79–103 (2015). [Google Scholar]
- 5.Kidwell S. M., Biology in the Anthropocene: Challenges and insights from young fossil records. Proc. Natl. Acad. Sci. U.S.A. 112, 4922–4929 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barnosky A. D., et al. , Merging paleobiology with conservation biology to guide the future of terrestrial ecosystems. Science 355, eaah4787 (2017). [DOI] [PubMed] [Google Scholar]
- 7.Davis M. B., Shaw R. G., Range shifts and adaptive responses to Quaternary climate change. Science 292, 673–679 (2001). [DOI] [PubMed] [Google Scholar]
- 8.Williams J. W., Shuman B. N., Webb T. III, Bartlein P. J., Leduc P. L., Late-Quaternary vegetation dynamics in North America: Scaling from taxa to biomes. Ecol. Monogr. 74, 309–334 (2004). [Google Scholar]
- 9.Kiessling W., Simpson C., Beck B., Mewis H., Pandolfi J. M., Equatorial decline of reef corals during the last Pleistocene interglacial. Proc. Natl. Acad. Sci. U.S.A. 109, 21378–21383 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nolan C., et al. , Past and future global transformation of terrestrial ecosystems under climate change. Science 361, 920–923 (2018). [DOI] [PubMed] [Google Scholar]
- 11.Scarponi D., et al. , Resilient biotic response to long-term climate change in the Adriatic Sea. Glob. Chang. Biol. 28, 4041–4053 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Staples T. L., Kiessling W., Pandolfi J. M., Emergence patterns of locally novel plant communities driven by past climate change and modern anthropogenic impacts. Ecol. Lett. 25, 1497–1509 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Foster G. L., Hull P., Lunt D. J., Zachos J. C., Placing our current ‘hyperthermal’ in the context of rapid climate change in our geological past. Philos. Trans. A Math. Phys. Eng. Sci. 376, 20170086 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gernon T. M., et al. , Transient mobilization of subcrustal carbon coincident with Palaeocene–Eocene thermal maximum. Nat. Geosci. 15, 573–579 (2022). [Google Scholar]
- 15.IPCC, Climate Change 2021: The Physical Science Basis. Contribution of Working Group I to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Masson-Delmotte V., et al. (Cambridge University Press, Cambridge, UK, 2021). [Google Scholar]
- 16.Kemp L., et al. , Climate endgame: Exploring catastrophic climate change scenarios. Proc. Natl. Acad. Sci. U.S.A. 119, e2108146119 (2022). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith J. A., Durham S. R., Dietl G. P., “Conceptions of long-term data among marine conservation biologists and what conservation paleobiologists need to know” in Marine Conservation Paleobiology, Tyler C. L., Schneider C. L., Eds. (Springer, 2018), pp. 23–54. [Google Scholar]
- 18.Kiessling W., Raja N. B., Roden V. J., Turvey S. T., Saupe E. E., Addressing priority questions of conservation science with palaeontological data. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20190222 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fordham D. A., et al. , Using paleo-archives to safeguard biodiversity under climate change. Science 369, eabc5654 (2020). [DOI] [PubMed] [Google Scholar]
- 20.Bremer S., Meisch S., Co‐production in climate change research: Reviewing different perspectives. Wiley Interdiscip. Rev. Clim. Change 8, e482 (2017). [Google Scholar]
- 21.Jarvis R. M., et al. , Navigating spaces between conservation research and practice: Are we making progress? Ecol. Solut. Evid. 1, e12028 (2020). [Google Scholar]
- 22.Buxton R. T., et al. , Avoiding wasted research resources in conservation science. Conserv. Sci. Pract. 3, e329 (2021). [Google Scholar]
- 23.Pörtner H.-O., et al. , Scientific Outcome of the IPBES-IPCC Co-Sponsored Workshop on Biodiversity and Climate Change (IPBES Secretariat, Bonn, 2021). [Google Scholar]
- 24.Riahi K., et al. , The shared socioeconomic pathways and their energy, land use, and greenhouse gas emissions implications: An overview. Glob. Environ. Change 42, 153–168 (2017). [Google Scholar]
- 25.Williams J. W., Ordonez A., Svenning J.-C., A unifying framework for studying and managing climate-driven rates of ecological change. Nat. Ecol. Evol. 5, 17–26 (2021). [DOI] [PubMed] [Google Scholar]
- 26.Barkemeyer R., Dessai S., Monge-Sanz B., Renzi B. G., Napolitano G., Linguistic analysis of IPCC summaries for policymakers and associated coverage. Nat. Clim. Chang. 6, 311–316 (2016). [Google Scholar]
- 27.Kemp D. B., Eichenseer K., Kiessling W., Maximum rates of climate change are systematically underestimated in the geological record. Nat. Commun. 6, 8890 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dal Corso J., et al. , Environmental crises at the Permian–Triassic mass extinction. Nat. Rev. Earth Environ. 3, 197–214 (2022). [Google Scholar]
- 29.Settele J., et al. , “Terrestrial and inland water systems” in Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change, Field C. B., et al., Eds. (Cambridge University Press, Cambridge, UK, 2014), pp. 271–359. [Google Scholar]
- 30.Pörtner H. O., et al. , “Ocean systems” in Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part A: Global and Sectoral Aspects, Field C. B., et al., Eds. (Cambridge University Press, Cambridge, UK, 2014), pp. 411–484. [Google Scholar]
- 31.IPCC, “Summary for policymakers” in Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel of Climate Change (IPCC), Pörtner H.-O., et al., Eds. (Cambridge University Press, Cambridge, UK, 2022), pp. 3–33. [Google Scholar]
- 32.Song H., et al. , Thresholds of temperature change for mass extinctions. Nat. Commun. 12, 4694 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cooley S., et al. , “Ocean and coastal ecosystems and their services” in Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Pörtner H.-O., et al., Eds. (Cambridge University Press, Cambridge, UK, 2022), pp. 379–550. [Google Scholar]
- 34.Stigall A. L., Using ecological niche modelling to evaluate niche stability in deep time. J. Biogeogr. 39, 772–781 (2012). [Google Scholar]
- 35.Tomašových A., Jablonski D., Decoupling of latitudinal gradients in species and genus geographic range size: A signature of clade range expansion. Glob. Ecol. Biogeogr. 26, 288–303 (2017). [Google Scholar]
- 36.Holland S. M., Zaffos A., Niche conservatism along an onshore-offshore gradient. Paleobiology 37, 270–286 (2011). [Google Scholar]
- 37.Hopkins M. J., Simpson C., Kiessling W., Differential niche dynamics among major marine invertebrate clades. Ecol. Lett. 17, 314–323 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gingerich P. D., Rates of evolution. Annu. Rev. Ecol. Evol. Syst. 40, 657–675 (2009). [Google Scholar]
- 39.Quintero I., Wiens J. J., Rates of projected climate change dramatically exceed past rates of climatic niche evolution among vertebrate species. Ecol. Lett. 16, 1095–1103 (2013). [DOI] [PubMed] [Google Scholar]
- 40.Bijma J., Pörtner H.-O., Yesson C., Rogers A. D., Climate change and the oceans—What does the future hold? Mar. Pollut. Bull. 74, 495–505 (2013). [DOI] [PubMed] [Google Scholar]
- 41.Román-Palacios C., Wiens J. J., Recent responses to climate change reveal the drivers of species extinction and survival. Proc. Natl. Acad. Sci. U.S.A. 117, 4211–4217 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lenoir J., et al. , Species better track climate warming in the oceans than on land. Nat. Ecol. Evol. 4, 1044–1059 (2020). [DOI] [PubMed] [Google Scholar]
- 43.Greenstein B. J., Pandolfi J. M., Escaping the heat: Range shifts of reef coral taxa in coastal Western Australia. Glob. Chang. Biol. 14, 513–528 (2008). [Google Scholar]
- 44.Reddin C. J., Kocsis Á. T., Kiessling W., Marine invertebrate migrations trace climate change over 450 million years. Glob. Ecol. Biogeogr. 27, 704–713 (2018). [Google Scholar]
- 45.McElwain J. C., Paleobotany and global change: Important lessons for species to biomes from vegetation responses to past global change. Annu. Rev. Plant Biol. 69, 761–787 (2018). [DOI] [PubMed] [Google Scholar]
- 46.IPCC, “Annex II: Glossary” in Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel of Climate Change (IPCC), Pörtner H.-O., et al., Eds. (Cambridge University Press, Cambridge, UK, 2022), pp. 2897–2930. [Google Scholar]
- 47.Kiessling W., Simpson C., On the potential for ocean acidification to be a general cause of ancient reef crises. Glob. Chang. Biol. 17, 56–67 (2011). [Google Scholar]
- 48.Garilli V., et al. , Physiological advantages of dwarfing in surviving extinctions in high-CO2 oceans. Nat. Clim. Chang. 5, 678–682 (2015). [Google Scholar]
- 49.Smith F. A., Betancourt J. L., Brown J. H., Evolution of body size in the woodrat over the past 25,000 years of climate change. Science 270, 2012–2014 (1995). [Google Scholar]
- 50.D’Ambrosia A. R., Clyde W. C., Fricke H. C., Gingerich P. D., Abels H. A., Repetitive mammalian dwarfing during ancient greenhouse warming events. Sci. Adv. 3, e1601430 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Nätscher P. S., Dera G., Reddin C. J., Rita P., De Baets K., Morphological response accompanying size reduction of belemnites during an Early Jurassic hyperthermal event modulated by life history. Sci. Rep. 11, 14480 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Piazza V., Ullmann C. V., Aberhan M., Temperature-related body size change of marine benthic macroinvertebrates across the Early Toarcian anoxic event. Sci. Rep. 10, 4675 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Martin J. M., Mead J. I., Barboza P. S., Bison body size and climate change. Ecol. Evol. 8, 4564–4574 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Dunhill A. M., Foster W. J., Azaele S., Sciberras J., Twitchett R. J., Modelling determinants of extinction across two Mesozoic hyperthermal events. Proc. Biol. Sci. 285, 20180404 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Penn J. L., Deutsch C., Payne J. L., Sperling E. A., Temperature-dependent hypoxia explains biogeography and severity of end-Permian marine mass extinction. Science 362, eaat1327 (2018). [DOI] [PubMed] [Google Scholar]
- 56.Foster W. J., et al. , Resilience of marine invertebrate communities during the Early Cenozoic hyperthermals. Sci. Rep. 10, 2176 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Clapham M. E., Conservation evidence from climate-related stressors in the deep-time marine fossil record. Philos. Trans. R. Soc. Lond. B Biol. Sci. 374, 20190223 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Calosi P., Putnam H. M., Twitchett R. J., Vermandele F., Marine metazoan modern mass extinction: Improving predictions by integrating fossil, modern, and physiological data. Annu. Rev. Mar. Sci. 11, 369–390 (2019). [DOI] [PubMed] [Google Scholar]
- 59.Reddin C. J., Kocsis Á. T., Kiessling W., Climate change and the latitudinal selectivity of ancient marine extinctions. Paleobiology 45, 70–84 (2019). [Google Scholar]
- 60.Kiessling W., Aberhan M., Environmental determinants of marine benthic biodiversity dynamics through Triassic-Jurassic times. Paleobiology 33, 414–434 (2007). [Google Scholar]
- 61.Raja N. B., et al. , Morphological traits of reef corals predict extinction risk but not conservation status. Glob. Ecol. Biogeogr. 30, 1597–1608 (2021). [Google Scholar]
- 62.Carpenter K. E., et al. , One-third of reef-building corals face elevated extinction risk from climate change and local impacts. Science 321, 560–563 (2008). [DOI] [PubMed] [Google Scholar]
- 63.Reddin C. J., Kocsis Á. T., Aberhan M., Kiessling W., Victims of ancient hyperthermal events herald the fates of marine clades and traits under global warming. Glob. Chang. Biol. 27, 868–878 (2021). [DOI] [PubMed] [Google Scholar]
- 64.Parmesan C., Duarte C., Poloczanska E., Richardson A. J., Singer M. C., Overstretching attribution. Nat. Clim. Chang. 1, 2–4 (2011). [Google Scholar]
- 65.Finnegan S., et al. , Extinctions. Paleontological baselines for evaluating extinction risk in the modern oceans. Science 348, 567–570 (2015). [DOI] [PubMed] [Google Scholar]
- 66.Broadley M. W., Barry P. H., Ballentine C. J., Taylor L. A., Burgess R., End-Permian extinction amplified by plume-induced release of recycled lithospheric volatiles. Nat. Geosci. 11, 682–687 (2018). [Google Scholar]
- 67.Hannisdal B., Liow L. H., Causality from palaeontological time series. Palaeontology 61, 495–509 (2018). [Google Scholar]
- 68.Reddin C. J., Nätscher P. S., Kocsis Á. T., Pörtner H.-O., Kiessling W., Marine clade sensitivities to climate change conform across timescales. Nat. Clim. Chang. 10, 249–253 (2020). [Google Scholar]
- 69.Mathes G. H., van Dijk J., Kiessling W., Steinbauer M. J., Extinction risk controlled by interaction of long-term and short-term climate change. Nat. Ecol. Evol. 5, 304–310 (2021). [DOI] [PubMed] [Google Scholar]
- 70.Bush A. M., Wang S. C., Payne J. L., Heim N. A., A framework for the integrated analysis of the magnitude, selectivity, and biotic effects of extinction and origination. Paleobiology 46, 1–22 (2020). [Google Scholar]
- 71.Cole S. R., Hopkins M. J., Selectivity and the effect of mass extinctions on disparity and functional ecology. Sci. Adv. 7, eabf4072 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Payne J. L., Bush A. M., Heim N. A., Knope M. L., McCauley D. J., Ecological selectivity of the emerging mass extinction in the oceans. Science 353, 1284–1286 (2016). [DOI] [PubMed] [Google Scholar]
- 73.Vázquez P., Clapham M. E., Extinction selectivity among marine fishes during multistressor global change in the end-Permian and end-Triassic crises. Geology 45, 395–398 (2017). [Google Scholar]
- 74.Jablonski D., Flessa K. W., The taxonomic structure of shallow-water marine faunas: Implications for Phanerozoic extinctions. Malacologia 27, 43–66 (1986). [Google Scholar]
- 75.Rego B. L., Wang S. C., Altiner D., Payne J. L., Within- and among-genus components of size evolution during mass extinction, recovery, and background intervals: A case study of Late Permian through Late Triassic foraminifera. Paleobiology 38, 627–643 (2012). [Google Scholar]
- 76.Payne J. L., Clapham M. E., End-Permian mass extinction in the oceans: An ancient analog for the twenty-first century? Annu. Rev. Earth Planet. Sci. 40, 89–111 (2012). [Google Scholar]
- 77.Benton M. J., Hyperthermal-driven mass extinctions: Killing models during the Permian-Triassic mass extinction. Philos. Trans. A Math. Phys. Eng. Sci. 376, 20170076 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Raup D. M., Size of the Permo-Triassic bottleneck and its evolutionary implications. Science 206, 217–218 (1979). [DOI] [PubMed] [Google Scholar]
- 79.Stanley S. M., Estimates of the magnitudes of major marine mass extinctions in Earth history. Proc. Natl. Acad. Sci. U.S.A. 113, E6325–E6334 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Nowak H., Schneebeli-Hermann E., Kustatscher E., No mass extinction for land plants at the Permian-Triassic transition. Nat. Commun. 10, 384 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.O’Connor M. I., et al. , Strengthening confidence in climate change impact science. Glob. Ecol. Biogeogr. 24, 64–76 (2015). [Google Scholar]
- 82.Hicke J. A., et al. , “North America” in Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel of Climate Change (IPCC), Pörtner H.-O., et al., Eds. (Cambridge University Press, Cambridge, UK, 2022), pp. 1929–2042. [Google Scholar]
- 83.Warren R., Price J., Graham E., Forstenhaeusler N., VanDerWal J., The projected effect on insects, vertebrates, and plants of limiting global warming to 1.5 °C rather than 2 °C. Science 360, 791–795 (2018). [DOI] [PubMed] [Google Scholar]
- 84.IPCC, “Summary for policymakers” in Global Warming of 1.5 °C. An IPCC Special Report on the Impacts of Global Warming of 1.5 °C above Pre-Industrial Levels and Related Global Greenhouse Gas Emission Pathways, in the Context of Strengthening the Global Response to the Threat of Climate Change, Sustainable Development, and Efforts to Eradicate Poverty, Masson-Delmotte V., et al., Eds. (Cambridge University Press, Cambridge, UK, 2018), pp. 3–24. [Google Scholar]
- 85.Costello M. J., et al. , “Cross-chapter paper 1: Biodiversity hotspots” in Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Pörtner H.-O., et al., Eds. (Cambridge University Press, Cambridge, UK, 2022), pp. 2123–2161. [Google Scholar]
- 86.Manes S., et al. , Endemism increases species’ climate change risk in areas of global biodiversity importance. Biol. Conserv. 257, 109070 (2021). [Google Scholar]
- 87.Kidwell S. M., Time-averaging and fidelity of modern death assemblages: Building a taphonomic foundation for conservation palaeobiology. Palaeontology 56, 487–522 (2013). [Google Scholar]
- 88.Smith J. A., Dietl G. P., Durham S. R., Increasing the salience of marine live–dead data in the Anthropocene. Paleobiology 46, 279–287 (2020). [Google Scholar]
- 89.Haywood A. M., et al. , What can palaeoclimate modelling do for you? Earth Syst. Environ. 3, 1–18 (2019). [Google Scholar]
- 90.Valdes P. J., Scotese C. R., Lunt D. J., Deep ocean temperatures through time. Clim. Past 17, 1483–1506 (2021). [Google Scholar]
- 91.Hansen J., Sato M., Russell G., Kharecha P., Climate sensitivity, sea level and atmospheric carbon dioxide. Philos. Trans. A Math. Phys. Eng. Sci. 371, 20120294 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Kiessling W., et al. , “Cross-chapter box PALEO: Vulnerability and adaptation to past climate changes” in Climate Change 2022: Impacts, Adaptation and Vulnerability. Contribution of Working Group II to the Sixth Assessment Report of the Intergovernmental Panel on Climate Change, Pörtner H.-O., et al., Eds. (Cambridge University Press, Cambridge, UK, 2022). [Google Scholar]
- 93.W. Kiessling, J. A. Smith, N. B. Raja, Data and code for: “Improving the relevance of paleontology to climate change policy.” 10.5281/zenodo.7236302. Deposited 21 October 2022. [DOI] [PMC free article] [PubMed]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix 01 (PDF)
Dataset S01 (XLSX)
Dataset S02 (XLSX)
Data Availability Statement
Data and code reported in this article have been deposited in Zenodo (https://doi.org/10.5281/zenodo.7236301) (93). All study data are included in the article and/or supporting information.