Abstract
Availability of artificial light and light-emitting devices have altered human temporal life, allowing 24-hour healthcare, commerce and production, and expanding social life around the clock. However, physiology and behavior that evolved in the context of 24 h solar days are frequently perturbed by exposure to artificial light at night. This is particularly salient in the context of circadian rhythms, the result of endogenous biological clocks with a rhythm of ~24 h. Circadian rhythms govern the temporal features of physiology and behavior, and are set to precisely 24 h primarily by exposure to light during the solar day, though other factors, such as the timing of meals, can also affect circadian rhythms. Circadian rhythms are significantly affected by night shift work because of exposure to nocturnal light, electronic devices, and shifts in the timing of meals. Night shift workers are at increased risk for metabolic disorder, as well as several types of cancer. Others who are exposed to artificial light at night or late mealtimes also show disrupted circadian rhythms and increased metabolic and cardiac disorders. It is imperative to understand how disrupted circadian rhythms alter metabolic function to develop strategies to mitigate their negative effects. In this review, we provide an introduction to circadian rhythms, physiological regulation of homeostasis by the suprachiasmatic nucleus (SCN), and SCN-mediated hormones that display circadian rhythms, including melatonin and glucocorticoids. Next, we discuss circadian-gated physiological processes including sleep and food intake, followed by types of disrupted circadian rhythms and how modern lighting disrupts molecular clock rhythms. Lastly, we identify how disruptions to hormones and metabolism can increase susceptibility to metabolic syndrome and risk for cardiovascular diseases, and discuss various strategies to mitigate the harmful consequences associated with disrupted circadian rhythms on human health.
Keywords: circadian rhythms, hormonal rhythms, SCN, sleep, metabolism, light, melatonin, jet lag, cardiovascular
1. Introduction
For the past three to four billion years, life on Earth has evolved under the predictable pattern of solar days, i.e., exposure to relatively bright light (10,000–100,000 lux) during the day and relatively dark (0.0001–0.5 lux) during the night. During the evolution of life, organisms internalized the temporal rhythm of Earth’s rotation and eventually developed self-sustaining biological clocks. These internal daily rhythms with periods of approximately 24 h are called circadian (from the Latin, circa = about and dies = day) rhythms. The processes and structures that generate circadian rhythms are called circadian clocks. The primary circadian clock in humans is a paired cluster of about 20,000 nerve cells in the hypothalamus at the base of the brain, named the suprachiasmatic nucleus (SCN). As suggested by their name, the period of circadian clocks is approximately 24 h; daily light exposure during the day sets these periods precisely to 24 h. Having circadian clocks set to the environmental light–dark rhythms optimizes physiology and behavior and allows preparation for predictable events, such as food or mate availability, sleep times, or predator activities throughout each day.
Circadian clocks are a nearly universal feature of life on this planet [1,2], yet since the invention and wide-spread adoption of electric lights during the past 140 years, humans have managed to manipulate light duration and intensity, in both outdoor and indoor environments, so much that disrupted circadian rhythms are rampant [3]. Light exposure outside of daytime disrupts the genetic machinery underlying these endogenous circadian clocks [4,5]. As detailed below, either too much light exposure at night or too little light exposure during the day can directly disrupt central and peripheral timing mechanisms, interfere with how internal rhythms are entrained to the external environment, and dysregulate the typical and optimal 24-hour physiological and behavioral functioning of individuals. Although no direct studies in humans examine the direct effects of artificial light at night (ALAN) on the human molecular lock, converging data from human correlative studies and a plethora of data from nocturnal and diurnal animal studies indicate that circadian rhythms are disrupted, leading to similarly poor outcomes in both nocturnal and diurnal mammals, indicating that the disruptive effects of ALAN can be uncoupled from altered rest activity cycles.
A second consequence of electric lighting has been the development of around the clock human activities. These activities can directly and indirectly affect circadian clock function and include night shift work, extended hours of recreation, voluntary shifts in bed and awakening times during weekends versus the work/school week (social jet lag; see below). For example, typical night shift workers are awake, active, and eating during the daily rest phase (i.e., the night). Night shift workers especially, but anyone extending light exposure outside of the daytime hours, typically shift the timing of food intake, and often change the nutritional value of their diet [5], which also affects circadian clocks and deranges metabolism. Night shift workers are prone to cardiometabolic disorders compared to their day shift counterparts [6]. Other forms of disruption to circadian rhythms, including transmeridian jet travel across several time zones, can provoke jet lag [7,8] and also disrupt circadian function [9]. However, relatively few individuals are exposed to chronic jet lag compared to the numbers of people exposed to night-shift work, mis-timed food intake, and light at night.
Obviously, individuals cannot perform all physiological and behavioral functions all the time. Respiration [10], blood pressure [11], and heart pulse rate [12] vary throughout the day in response to activity and clock-programmed signals. Notably, energetic requirements are relatively continuous, whereas energy production or consumption is somewhat sporadic. All organisms partition temporal energetic activities across the day. Indeed, temporal partitioning of photosynthesis, metabolism, gene expression, reproduction, defense, growth, activity, and inactivity is universal among plants and animals. Disrupted circadian rhythms impair function and can increase risk for, or may provoke, diseases [13,14,15]. Inadequate exposure to light during daytime hours, and exposure to bright electric lights during the night is associated with many health disorders [16,17,18]. Indeed, most of our time is currently spent indoors [19] under light levels during the day that are typically 100 times dimmer than natural daylight, and ~1000 times brighter than natural light intensities at night. We focus this review on the role of disrupted circadian rhythms on metabolic function.
2. Circadian Rhythms and the Molecular Clock
Nearly every organism on Earth is the result of evolution under fairly consistent, ~24 h cycles of alternating bright, sun-lit days and dark nights as a result of the Earth’s rotation on its axis [20]. These light–dark cycles, accompanied by temperature and climate fluctuations, provide predictable environmental patterns to which organisms have adapted. This internalization has permitted them to optimize energy intake and expenditure and be prepared for environmental changes. Optimal physiological, behavioral, and reproductive timing of these events improves fitness. Given this putative biological advantage [21], circadian rhythms are seen in virtually every species to provide a predictable temporal pattern in physiology and behavior. The molecular result of this internalization, the circadian molecular clock located in the SCN, is responsible for orchestrating optimally-timed biological events, by synchronizing endogenous physiology to exogenous cues, or zeitgebers, ensuring internal homeostasis. The most potent cue is light [22,23,24,25], which conveys temporal information to brain centers, which then relay this temporal information via neurotransmitter and hormone signals, to all cells and organ systems.
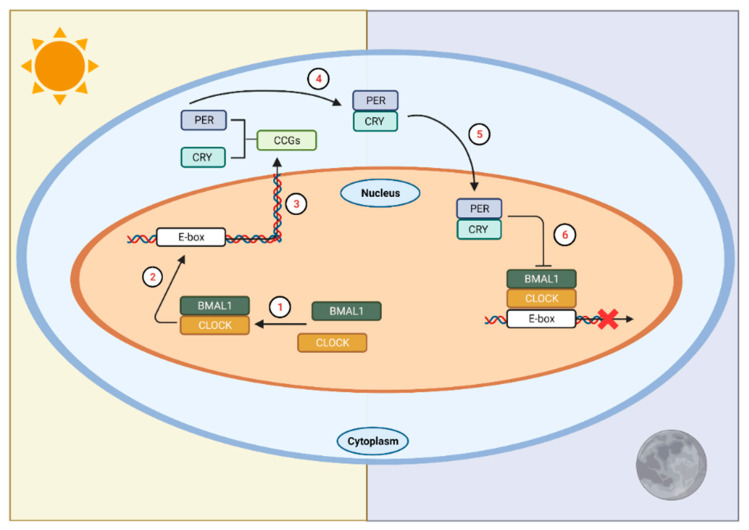
At the molecular level, a signaling cascade elicited by photic stimulation promotes the transcription of core clock proteins, which then initiates a series of interlocking, autoregulatory, transcriptional-translational feedback loops (TTFL) [26,27]. At the beginning of this cycle (during the biological morning), the core clock proteins circadian locomotor outputs kaput (CLOCK) and brain and muscle aryl hydrocarbon receptor nuclear translocator-like protein 1 (BMAL1) heterodimerize and translocate to the nucleus (Figure 1). The heterodimer binds to E-box sequences of their targets, cryptochrome (Cry) and period (Per), and transcription ensues. Upon accumulation of these gene products, they dimerize in the cytoplasm and eventually translocate back into the nucleus, where the cycle began. In the nucleus, they inhibit their own transcription by inhibiting transactivation of E-boxes by CLOCK and BMAL1. This process ultimately leads to the degradation and depletion of PER and CRY proteins, which disinhibits Per and Cry transcription, and the ~24 h cycle starts again.
Figure 1.
The circadian clock in mammals. In SCN neurons at the start of the circadian day, BMAL1 and CLOCK form a heterodimer (1) that binds to E-box sequences in the promoters of the Cry and Per genes (2) to activate their transcription (3). This marks the beginning of the circadian day. The gene products of Per and Cry accumulate in the cytoplasm, dimerize (4), and then form a complex that translocates into the nucleus (5) to interact with CLOCK and BMAL1, ultimately repressing their own transcription (6). This process takes approximately 24 h. LAN affects the timing of this transcription/translation cycle leading to temporal misalignments affecting physiology and behavior. CCGs = circadian clock genes. Figure was created using Biorender.com (accessed on 13 January 2022).
In order to maintain the strength of this biological rhythm, this TTFL is tightly regulated at multiple levels. Specifically, regulation has been documented at the transcription factor binding and histone modifications [28], at the chromosome organization [29,30], and at the post-transcriptional [31] and post-translational [32] modification levels (see [26] for a detailed review). Auxiliary interlocked loops also exist and aid in the regulation of Bmal1 and Clock transcription. These auxiliary loops function to strengthen the rhythmicity of the primary loops in two ways. First, they help entrain the rhythms to a narrow and specific temporal range [33]. Second, they provide redundancy to the system, presumably in case of genetic mutations or other loss of function changes [34,35]. Interestingly, this ‘failsafe’ only functions as such if the co-functional gene pairs (Per1/Cry1 or Per2/Cry2) are both lacking or deficient [35]; otherwise, the system develops arrhythmicity and is unable to entrain under constant conditions. Together, these data provide evidence of the highly precise nature and critically important role of the clock genes in the regulation of physiology.
2.1. Phototransduction to the SCN
In mammals, rhythms are generated and maintained by the bilateral nuclei of the SCN in the anterior hypothalamus. Here, a highly specialized network of cells work in concert to maintain these rhythms in the absence of external zeitgebers. However, to maintain precise 24 h rhythms, these cells must continually receive input from the environment to accurately time signaling and downstream effects [36]. Although other cues, such as food and locomotor activity [37,38,39] exist, the most potent cue is exposure to sunlight early in the biological day. Photic information enters the retina [40] and travels through the monosynaptic glutamatergic retinohypothalamic tract [41,42,43] via intrinsically photosensitive retinal ganglion cells (ipRGCs) [44,45]. The IpRGCs express melanopsin, a photopigment preferentially activated by short wavelength light (~460–480 nm; appears blue), and is critical in the process of circadian entrainment. Exposure to light of this wavelength resets the molecular clock and inhibits melatonin release [46,47,48,49,50,51], an endocrine marker of time for many organisms. Notably, deletion of melanopsin in mice impedes circadian phase shifting when light-pulsed under constant dark conditions [52,53,54], whereas complete ablation of the ipRGCs themselves produces deficits in both pupillary light reflex and circadian entrainment [55].
An additional pathway that transmits both photic and nonphotic (e.g., time of food intake), information to the SCN is the geniculohypothalamic tract (GHT) [56]. The GHT primarily signals via neuropeptide Y (NPY), which serves to phase shift circadian rhythms in vitro [57] and in vivo [58], and modulates photoperiod responsiveness [59]. The phase-shifting-inhibiting action of NPY is only observed in vivo during the late subjective night [60]. Moreover, NPY serves a sleep-promoting neuropeptide by modulating noradrenergic signaling [61], and inhibiting orexin signaling [62], which modulates alertness.
2.2. SCN Signaling
The primary clock in the SCN communicates within the brain and periphery via neural and humoral signaling to synchronize the multiple cellular oscillators throughout the organism. Although individual clocks have the ability to oscillate independently of the SCN, it is unique because it is the only mammalian biological clock that directly receives environmental photic information. Thus, it is positioned to synchronize internal timing with that of the environment. Broadly, by providing specific timing signals (neural or humoral), the SCN couples individual oscillators within an organ or system to each other, ensuring synchronicity within the system, and setting their phase relative to the environmental time. Both neural and humoral signaling are necessary for sustaining endocrine rhythms.
Despite its widespread effects on physiological and organismal function, the SCN interfaces directly with few regions, of which its primary connections remain within the hypothalamus. Upon photic activation, the mammalian SCN communicates with the subparaventricular zone (SPZ), and the dorsomedial hypothalamic nuclei (DMH), which then relay and amplify the cellular message to downstream areas. The dorsal SPZ projects to the medial preoptic area that regulates rhythms in body temperature [63,64,65], while the ventral SPZ projects to the DMH [66]. The SCN also sends direct GABAergic projections to the DMH [66], which promotes the release of hormonal and neurotransmitter factors to regions controlling corticosteroid release, arousal [67,68] and sleep [69,70,71,72]. Thus, the SCN sends both direct and indirect input to the DMH, underlining the relevance of the strict regulation of signaling to this region and the subsequent molecular relay to regions governing homeostatic control within the organism.
3. Hypothalamic Control of Circadian Homeostasis and Hormone Regulation
Biological function is organized in a rhythmic, often circadian, manner, and many key life-sustaining functions, such as regulation of body temperature, metabolism, food intake and sleep are controlled by the hypothalamus. The hypothalamus is one of the central brain regions involved in coordinating organ-to-organ system communication to maintain internal homeostasis. This coordination between the nervous and endocrine systems occurs through hormonal secretion. Physiological homeostasis is regulated from autonomic, somatic, and endocrine control through complexly and tightly regulated hormone production and communication across brain regions, including energy intake, and expenditure, thermoregulation, sleep, and hormone regulation [73]. Hypothalamic hormone release functions through a cascade system induced by input received from higher brain centers responding to environmental information. These hormones travel through the hypothalamic-hypophyseal portal system to the pituitary to produce or inhibit hormones that are then transported throughout the body to interact with target organs [73]. As discussed in subsequent sections, dysregulation of the circadian system, via any of various environmental circadian rhythm disruptors (i.e., exposure to artificial light at night, night shift work, jet lag/social jet lag, or mistimed eating; see Section 4), can severely impact basic homeostatic functions. In the next sections we will briefly discuss how the hypothalamus regulates food intake and sleep, as key examples of basic homeostatic functions that are regulated in a circadian manner. We will then discuss how the SCN mediates the release of hormones that contribute to the regulation of daily rest-activity states.
3.1. Food Intake
Food intake is another biological process important in maintaining a healthy metabolism, from nutrient intake to optimal energy expenditure. The hypothalamus regulates appetitive behavior, food intake, and energy expenditure through hormonal release and afferent autonomic nerves [74]. The ventromedial hypothalamus (VMH) is primarily involved in satiety by receiving inputs from hormones in the bloodstream and arcuate nucleus (ARC), along with neurotransmitters, neuropeptide Y (NPY), Agouti-related peptide (AgRP), and pro-opiomelanocotin (POMC) [75]. Leptin is a hormone involved in satiety, produced in adipose tissue, and its dysregulation or dysfunction may lead to overeating. These hormones function as signals to the brain as the proportional value of fat storage relative to blood secretion levels [76]. Other hormones directly involved in food intake, include ghrelin, which directly induces food consumption through stimulating GHS-R1a in the VMH, and through an indirect orexigenic pathway from AgRP and NPY from the ARC inhibiting anorexigenic signals [77], and hypocretins/orexins, that mediate energy storage after food intake [78].
3.2. Sleep
Sleep is an integral biological process that affects several biological functions, including cognition, development, energy conservation, immune modulation, and others [79]. Sleep is characterized by altered brain wave activity, and reduced body movement and responsiveness to the environment [80]. The hypothalamus is one of the main brain regions responsible for playing a central role in sleep-wake regulation. The preoptic area of the hypothalamus functions by promoting sleep onset and maintenance by inhibiting arousal systems baseline, and contains inhibitory neuromodulator/transmitters galanin and gamma-aminobutyric acid (GABA) [81]. Sleep control systems are also implicated in bi-directional pathways with the SCN of the anterior hypothalamus, involved in entrainment and regulation of circadian rhythms. Notably, hypothalamic SCN oscillators, circadian rhythms, and sleep regulation have been recently implicated in playing a role in food intake, metabolism, hormone release, and temperature, suggesting its broad importance in homeostatic physiological regulation [82].
3.3. SCN-Mediated Hormonal Release and Function
The SCN mediates the timing of most circadian rhythms, including the daily release of many hormones associated with metabolism. In common with other circadian regulated processes, precise timing is critical. Thus, changes in environmental signals that influence circadian clock function can ultimately influence endocrine function in maladaptive ways. Interactions with peripheral clocks in the liver and pancreas, for example, that are entrained to meal timing are also critical for optimal metabolic function. As already discussed, intrahypothalamic signaling mediates the temporal output of the SCN. In the next sections we provide examples of key hormones under circadian clock control and provide the current understanding on how they communicate with the SCN.
3.3.1. Melatonin
Perhaps the best known SCN-mediated endocrine response is pineal-melatonin production and secretion. Melatonin, sometimes referred to as a “sleep hormone”, at least in humans [83,84] and various diurnal species [85,86,87,88], promotes sleep. More accurately, it is a “dark” hormone, which signals night length (scotoperiod). Upon the onset of darkness (night), the SCN release of GABAergic inputs to the PVN are halted, and the paraventricular nucleus (PVN) sends projections to the intermediolateral cell column of the spinal cord [89]. From here, preganglionic noradrenergic (NEergic) neurons project to the superior cervical ganglion, which subsequently innervate the pineal gland. NE released from these fibers then promotes the biosynthesis of melatonin [90,91]. Melatonin release peaks near the middle of the night and decreases by morning [92]. Although the SCN regulates the timing of pineal melatonin release, melatonin also feeds back to the SCN, through melatonin receptor 1 (MT1) [93] and through melatonin receptor 2 (MT2) [94] signaling, to decrease neuronal firing or induce a circadian phase shift [95,96], respectively. In this context, melatonin signaling is sustained throughout much of the night and inhibited by light exposure [50,97,98].
Melatonin is not only a primary endocrine clock output, but also serves as a neuroendocrine synchronizer of molecular rhythms, both centrally and peripherally [99,100,101,102,103,104]. In rodents, melatonin induces rhythmic expression of Per1, Bmal1, Clock, and Cry in the pituitary [105,106]; pinealectomy of Syrian hamsters abolishes the rhythmicity of Per1 mRNA expression in the pituitary without affecting its expression in the SCN or VMH [107]. Rat pinealectomy, however, leads to long-term (after three months) desynchrony of Per1 and Per2 expression, and decreased Rev-erbα amplitude in the SCN [108], suggesting that long-term loss of endogenous melatonin weakens circadian rhythmicity. Clinical studies support this hypothesis [109,110,111].
As a result of its feedback to the SCN, melatonin also plays a role in circadian entrainment [112]. Studies in humans and other mammals have demonstrated that exogenous melatonin can phase shift [113] and entrain the circadian clock of blind subjects [109,110,111]. The role of melatonin in promoting sleep itself, however, is a source of debate in the field, due to some of the data acquired from studies using nocturnal rodents. A core argument against the role of melatonin in sleep promotion results from the observation that, in these animals, melatonin is associated with wakefulness. Notwithstanding, some studies suggest that melatonin functions as a sleep-promoting signal [114,115], and it induces sedation and lowers core body temperature [116,117]. Indeed, a review of the literature suggests that administration of melatonin agonists may help in reducing sleep latency and promote sleep in patients with insomnia; however, additional clinical trials are necessary before approval for broad clinical use [118,119]. Additionally, melatonin receptors are broadly distributed (not unique to the CNS), so administration of exogenous melatonin may cause off-target effects and/or interact with other drugs, such as observed with nifedipine, an anti-hypertensive [120], although more recent data suggest protective effects [121,122].
In the periphery, melatonin has been reported to play a role in blood pressure regulation in experimental and clinical settings [103,120]. Its actions are receptor-specific; MT1 activation mediates vasoconstriction in both cerebral and peripheral arteries [123,124,125,126,127], and vasodilation via MT2 [128,129]. Other work in this field presents promising data regarding the role of melatonin in regulating blood pressure, in vascular protection after ischemic injury, and other vascular injuries [121,122,130,131,132]. Finally, in adipose tissue, melatonin synchronizes metabolic and hormonal function [133] by regulating Per2, Clock and REV-ERBα, of which REV-ERBα is a documented requirement for daily balanced metabolism of carbohydrates and lipids [134].
Various additional possible roles for melatonin have emerged. For instance, melatonin plays a role in T cell activation [135], and overall immune function [136,137,138,139,140,141,142], reviewed in [136,137]. It has been widely proposed as an antioxidant [143,144,145,146], particularly in the context of recovery from ischemia/reperfusion injury [121,143].
A more nuanced role for melatonin has been proposed for the aged. Specifically, given the association between decreased melatonin production in the early stages of Alzheimer’s disease (AD), the role of melatonin in sleep promotion (in humans), and the function of sleep in clearing the brain from metabolites and toxins, melatonin has been proposed as a promising therapeutic for those at risk [147]. Moreover, a preclinical study analyzing the relationship between sleep and the accumulation of Aβ, determined that sleep deprivation or orexin administration increases Aβ in interstitial fluid, suggesting a role for sleep and wakefulness-regulating molecular players in AD pathology [148]. Further, in the context of AD, and considering its antioxidant capacity, melatonin has also been proposed as an ‘anti-aging’ agent, as it is capable of stimulating antioxidant enzymes and re-establishing the mitochondrial membrane integrity [149]. Further pre-clinical and clinical research is required to fully understand the interconnectedness of these pathways and the molecular mechanisms that mediate their effects.
Despite the mounting literature on the systemic effects of melatonin, more work is required to fully understand (1) endogenous versus exogenous melatonin effects, (2) its central versus peripheral effects, (3) differential effects on diurnal versus nocturnal species, and (4) sex differences in response to its administration.
3.3.2. Glucocorticoids
Glucocorticoids (GC) are steroid hormones produced by the adrenal cortex, and are involved in several physiological processes, such as metabolism [150], immune response [151], cardiovascular function [152,153], and reproduction [154]. As many homeostatic drivers of physiology, the production of GC is driven by circadian rhythms and is tightly regulated. Neuronal activity in the SCN stimulates the PVN to initiate the release of corticotropin-releasing hormone, which then signals to the anterior pituitary to release adrenocorticotrophin, which, in turn, communicates to the adrenal cortex to release GC. Over time, GC feed back to the hypothalamus and pituitary to block secretion of their precursors, and consequently, their production. There is no GC feedback directly to the SCN [155]; however, the arcuate nucleus serves as the “stress sensor”, providing feedback to the PVN about corticosterone levels, and thus, modulating its release [156]. As a result, GC are released at specific times of the day, and in response to specific events (e.g., corticosterone release as a result of restraint), and display cyclic decreases and increases in their circulating concentrations. Cortisol secretion, for instance, begins prior to awakening, rises in the morning, peaking around the time of awakening [157,158]; elevated GC increase glucose availability in anticipation of the increase in energy demands associated with wakefulness [159]. In rodents and other nocturnal animals, the cycle of corticosterone secretion is reversed, with the peak observed around dusk, but coinciding with the beginning of their active phase [160].
The GC play an important role in overall animal physiology, particularly in the stress response. During times of stress, the release of GC and epinephrine suppresses energy storage and shifts towards usage of adipose and liver stores [161,162]. Thus, GC released as part of the stress response may also shut down cellular processes that require ample energy resources, such as humoral immune responses, digestion, growth, and reproduction [161,163].
4. Changing Environment and Consequences on Hormonal Rhythms
Environmental changes such as widespread adoption of artificial light at night and the subsequent shift in human activities that affect the internal timing system cause a myriad of health disturbances, both immediately (such as lack of sleep or altered energy intake) and long-term (such as the accumulation of risk factors to cardiometabolic disorders and elevated risk of developing endocrine, gastrointestinal, and neurological disorders). Over the past few decades, the field of chronobiology has focused on some of these unintended consequences and has focused research efforts on understanding the interplay between these technological developments and their long-term effects on humans and other organisms. Because light has neuromodulatory effects on the SCN, and the SCN regulates downstream secondary oscillators, light and circadian rhythms have been central to this work.
The circadian clock has direct influence on hormonal rhythms in the endocrine system. As such, exposure to changing environmental conditions that affect circadian rhythms can have adverse physiological consequences on health, particularly affecting the hypothalamus and its function in communicating and modulating signaling between the nervous and endocrine systems. Imbalances or alterations to oscillatory hormonal rhythms can unfavorably affect cellular and molecular processes in physiology and increase risk for diabetes, metabolic disruption, and altered hormonal signaling [164]. Increasing evidence highlights the role of light pollution negatively affecting human and non-human animal health, including disruptions to molecular circadian clocks, metabolism, and hormonally-driven physiological functioning, in addition to, or in parallel with, disrupted sleep-wake cycles.
4.1. Artificial Light at Night
Circadian clocks rely on light as the primary zeitgeber responsible for synchronizing endogenous biological rhythms to the 24 h light–dark cycles. Therefore, mammals are particularly vulnerable to circadian disruption by exposure to low, or even dim, levels of artificial light at night (ALAN), below the threshold necessary to directly disrupt rest activity cycles. Exposure to ALAN is now ubiquitous. Humans undergoing hospital stays, transmeridian travel, or night shift work are exposed to constant lighting, which can disrupt circadian rhythms and clock genes resulting in misalignment of circadian-controlled physiology and overall physiological homeostasis [165,166,167]. Animal models of nocturnal light exposure have provided substantial evidence supporting the detrimental effects on cellular to behavioral physiology [166,168,169,170].
Metabolism and circadian rhythm disruption is well characterized. Time of day alters energy expenditure and disrupted circadian rhythms can increase metabolic abnormalities and elevate the risk for obesity. Chronic exposure to ALAN increases body mass and impairs glucose processing while maintaining equivalent caloric intake and locomotor activity patterns [169]. Insulin is another circulating hormone that is affected by ALAN and disrupted circadian rhythms, independent of food intake; although the precise mechanisms through which desynchronization of central and peripheral clocks contributes to insulin resistance has yet to be determined, a recent review of the literature [171] proposes two possible mediators of this interaction: the phosphatases belonging to the pleckstrin homology leucine-rich repeat protein phosphatase family and the deacetylase Sirtuin1 [172]. ALAN decreases glucose tolerance and insulin sensitivity in rodent models [168,173] and in human studies [174]. Just one night of 100 lux light exposure during sleep affects various cardiometabolic measures; specifically, ALAN increases nighttime heart rate, decreases heart rate variability, and increases next-morning insulin resistance in humans [174]. Further, other aspects of metabolism including energy expenditure regulated by brown adipose tissue (BAT), are altered with prolonged lighting duration in rodent models, by decreasing β3-adrenergic intracellular signaling/sympathetic input to BAT, and impairing fatty acid uptake from triglycerides [175]. Thermogenesis and body temperature are also affected by ALAN; nighttime light exposure increases brain and core body temperature in a rodent model [176].
ALAN has been directly implicated in altering central neuroendocrine rhythms such as the hypothalamo-pituitary-adrenal (HPA) axis, which comprises hormonal feedback mechanisms that regulate axis activity [177]. Corticotropin-releasing hormone (CRH) and arginine vasopressin (AVP) are involved in the daily release of corticosterone and are regulated by clock genes [178]. In mouse models of circadian rhythm disruption, exposure to bright or constant ALAN increases glucocorticoid concentrations [179], whereas dim ALAN (i.e., 5 lux) does not alter glucocorticoids [169]. It is possible that these null results in response to 5 lux reflect phase advanced plasma glucocorticoid concentrations after exposure to ALAN, so that low values reflect differences in biological time of day [180]. These studies suggest that the intensity of lighting plays an important role in hormonal and metabolic disruption, but species differences may also play a role in the observed effects.
Melatonin as one of the hormones predominantly involved in circadian cycles, relies on external lighting cues to regulate its production and secretion, and is affected by ALAN. In addition to melatonin’s role in nocturnal physiology, a core circadian rhythm, there are several receptor-mediated physiological involvements, including immune-modulation and free-radical scavenging [181]. In both human and rodent studies, the extent of suppression is dependent on duration, intensity, and wavelength [182]; notably, dim or constant lighting suppresses melatonin release onset, and shortens melatonin duration [183]. Circulating melatonin additionally plays a role in regulating glucose and plasma leptin rhythms [184], and suppressed melatonin due to exposure to ALAN can affect metabolic regulation. Further, disrupted melatonin concentrations have been associated with changes in thyroid, adrenal, and reproductive hormone rhythms [185,186], suggesting that the effects of ALAN and disruptions to melatonin rhythms may further exacerbate neuroendocrine and metabolic disruptions.
4.2. Shift Work and Jet Lag
Disruption of circadian rhythms can occur through other means such as shift work, erratic social schedules (i.e., social jet lag), and traveling across time zones (i.e., jet lag). These can impair sleep-wake cycles, and, in turn, can affect other physiological processes, resulting in a myriad of adverse health consequences [187], such as increased risk for weight gain and metabolic disruption, along with risk for diabetes, cardiovascular disease, and cancer.
Night shift work refers to jobs that encompass overnight work (e.g., 18:00 h–7:00 h), or more generally, beyond the common “9 to 5”. Presumably, because humans evolved to be active (i.e., work) during the day and inactive (i.e., sleep) during the night, their physiology also evolved to maximize metabolic efficiency during the day, and optimize recovery and removal of waste and toxins at night [188,189,190]. Indeed, data from both basic and clinical studies support the role for metabolic and hormonal dysregulation associated with night shift work [191,192]. Circadian misalignment from exposure to 8 h phase advance and/or delays, shifted glucose tolerance and altered insulin sensitivity during the light (inactive) phase in rodents [173,193]. Other manipulations that disrupt circadian rhythms, such as social jet lag, have also been implicated in dysregulating hormonal rhythms that contribute to metabolic syndrome. In rodents, shifting of light/dark cycles and/or persistence of dim ALAN can increase glucose intolerance, body mass [169], and white adipose tissue with changes to metabolic profiles in the liver [194], and social jet lag increases body mass gain by overconsumption [195].
In the context of clinical data, a study using ten 28 h “day” shift-work paradigm in humans reported dysregulated plasma leptin, insulin, glucose, and cortisol concentrations [196] and observational studies support the increased risk of obesity associated with night shift workers [197]. Other studies examining food intake in relation to social jet lag, noted that social jet lag is associated with later meal times and poor nutritional continent in meal choices [198]. Nonetheless, it is worth noting that the shift work schedule maintained, including the number of consecutive hours worked, the number of consecutive days worked in a nocturnal shift, and the pace with which the worker alternates between work and rest days, as well as how often they shift between day and night shifts, may affect health outcomes, and may differentially affect systems. Thus, the effects of night shift work may strongly depend on the type of rotating schedules [199,200,201].
4.3. Mistimed Food Intake
The molecular circadian clock is responsible for coupling the regulation of metabolic and cellular processes to time of day. Meal timing and food composition are additional zeitgebers that can entrain circadian rhythms [37] and are typically limited to the active phase of organisms. Thus, shifting meal timing as a result of social or work responsibilities (night-shift work or other social schedules), can also phase shift internal clocks to functionally alter endogenous circadian rhythms.
Unfortunately, for humans, mistimed eating usually indicates eating during the nighttime. However, because human physiology developed with sleep typically occurring at night, eating during the nighttime is inherently physiologically “incorrect”. Further, humans are adapted to dark nights, with physiology programmed to rest and recover during that time; thus, night shift workers’ exposure to artificial light also inherently affects the molecular framework of circadian rhythms, which ultimately impairs downstream physiology. Thus, disentangling the contributions of these individual cycle disruptors (nocturnal light exposure, lack of nocturnal sleep, etc.) from that of mistimed daily eating, becomes very complex. Nonetheless, regardless of the mechanism of how misalignment happens, data demonstrate that eating during the nighttime impairs circadian function [202,203], and overall decreases the nutritional value of the diet [198,204], increasing fat, saturated fats, and cholesterol consumption and total caloric value.
Studies using nocturnal rodents have provided some insights on the effects of light on feeding behavior independent of sleep. Studies using low-level ALAN in mice have determined that exposure to ALAN shifts the time of food consumption [169] to that of the biological inactive phase, without affecting sleep quality [205]. However, a more recent study using nocturnal rats reported that exposure to ALAN alters the amplitude of the rhythms of REM and non-REM sleep [206]. Nonetheless, many dysfunctional metabolic parameters arise, such as impaired glucose utilization [169,173,207,208] and insulin sensitivity [173]; however, when the timing of food intake is restricted to the active phase, some of the metabolic disorders are reversed [173,209]. Moreover, studies with diurnal rodents have also demonstrated misaligned rhythms and disturbed metabolic function when exposed to ALAN and/or shift work models [173,210]. Thus, it is possible that the metabolic effects of misaligned rhythms via exposure to ALAN are independent of sleep itself, especially when we consider that SCN neurons are most active during the daytime, irrespective of whether the organism is diurnal or nocturnal [211]. Indeed, the study [206], in which it was reported that ALAN impairs sleep in rats, also reported changes in SCN clock gene expression, without the typically reported changes in body mass and glucose tolerance observed in mice. Thus, the mechanisms mediating the effects of ALAN on sleep and metabolism may not be coupled or may differ by species. A recent review of the “light-at-night” literature in the context of mammals, provides evidence to suggest more nuanced effects on sleep in diurnal versus nocturnal creatures [212]. In sum, this body of work supports the notion that circadian disruptors can differentially affect individual circadian processes, and that the interaction of individually affected pathways and processes may result in compounding and complex effects that need to be further examined.
5. Changing Environments, Misaligned Circadian Rhythms, and Resulting Disorders
Circadian rhythms allow organisms to synchronize their physiology and behavior with cues from the external environment to maximize resources and reduce energy expenditure. Again, these rhythms have evolved to be aligned with the 24 h solar day. This provides a relatively simple, but powerful, cue that entrains physiology to efficiently use energetic resources when the body is optimized to do so (organism’s active phase), and effectively metabolize energy stores to maintain stable glucose supply during fasting (organism’s inactive phase). Thus, any stimulus that impairs the receipt of the temporal cue provided by light and the circadian timing system, threatens physiological homeostasis.
Non-natural environmental changes, as the ones described in the prior section, have a direct effect on various aspects of behavior, metabolism and overall physiological homeostasis. Next, we provide a brief overview of the relationship between circadian rhythms and endocrine, metabolic and cardiovascular disorders.
Endocrine, Metabolic, and Cardiovascular Diseases
The human metabolic and cardiovascular systems are especially vulnerable to nocturnal rhythm disruption, as during these hours, the body is not prepared for the energetic demands of activity and the processing of nutrients. As described, the rhythm of energy intake and expenditure is carefully regulated to coincide with the release and regulation of hormones that allow the body to signal the need for energy, (i.e., hunger, ghrelin), and enzymes that contribute to the extraction of nutrients (e.g., amylase, lipase, etc.), with that of the hormones that signal satiety (i.e., leptin) and promote digestion. Night shift workers, however, threaten the internal metabolic balance as they displace their sleep-wake and food consumption schedules to biologically incompatible times. For instance, consumption of a large meal at night uncouples the relationship between plasma glucose and insulin concentrations, which can lead to metabolic dysfunction [213]. Indeed, severing circadian and behavioral rhythms under a chronic night shift work paradigm, uncouples synchrony between hormones that follow an ~24 h cycle (endogenous rhythm, i.e., glucose, epinephrine, and cortisol), and those that shift as a result of behavior (i.e., leptin, insulin, and norepinephrine). Leptin production decreases, with an increase in glucose concentration, despite concurrent increased insulin production [196]. Decreased leptin increases hunger, which increases food intake, with a consequent decrease in energy expenditure. Together, these contribute to the increased plasma glucose concentrations and an obese phenotype. Moreover, under this chronic shift work paradigm, the cortisol peak occurs during the end of the wake period/beginning of sleep, which contributes to insulin resistance [214,215] and hyperglycemia [216,217].
In the context of night shift work, or otherwise shifted (human) behavioral schedules (i.e., being active/awake at night), biological routines such as food consumption are also altered. As evidenced from both preclinical and clinical data, glucose metabolism is also influenced by mistimed eating. For instance, night eating syndrome is associated with obesity [218], and in overweight female night shift workers, compared to overweight day shift workers (with no difference in BMI), the post-meal suppression of ghrelin production was blunted, resulting in elevated waist circumference, greater energy intake, impaired sleep, insulin insensitivity, increased triglycerides and increased C-reactive protein (CRP) [219]. These risk factors have all been associated with the development of type 2 diabetes [220,221,222,223] and pathological cardiovascular events (cardiovascular diseases; CVD) [224,225,226,227]. The CRP, an inflammatory biomarker that is present in most cardiac pathologies, and whose production may be triggered by metabolic hallmarks of diabetes—high glucose, adipokines, lipoproteins and free fatty acids—plays a role in the development and progression of most CVD, with immunomodulatory roles in the humoral innate immune and complement responses, as well as in vascular function [201]. Although it remains unspecified whether CRP acts as a regulator or amplifier of the immune response. Regardless, detection of CRP during risk assessment of CVD, namely, myocardial infarction, greatly improves its prognostic power [228], and can help prevent another event within the subsequent 10 years [229].
In the context of vascular function, CRP inhibits endothelial nitric oxide synthase (eNOS) production [230], which is necessary for the synthesis of nitric oxide (NO), the primary endogenous vasodilator. Thus, decreased production can lead to dysregulated vasoconstriction, leukocyte adherence, platelet activation, oxidation, impaired or uncontrolled coagulation, and vascular inflammation [231]. However, under exacerbated inflammatory states, also mediated by CRP, NO production increases, and along with other reactive oxygen species, can lead to oxidative stress, which in turn, promotes vascular pathologies such as atherosclerosis [232]. In the context of shifted sleep schedules or sleep deprivation, which also have detrimental effects on metabolic parameters, (e.g., exacerbated insulin resistance, [214], these data suggest possible overlapping mechanisms for the development and persistence of cardiometabolic disorders such as obesity [197,233,234,235], diabetes [6,236,237], hypertension [238,239], and other CVD. Further, a meta-analysis directly assessing the relationship between shift work and the development of CVD, determined that shift work increases the risk of myocardial infarction, cerebrovascular accidents and coronary disease [240], and increases the risk of reperfusion injury following myocardial infarction [241], lending evidence to support a relationship between disrupted circadian rhythms and the development of cardiovascular pathologies.
Lastly, lifestyle choices may also interact with disrupted rhythms to exacerbate metabolic pathology. Studies have documented shift workers’ elevated risks for overall smoking [242], and are also more prone to smoke during their shifts, in order to stay awake, curb hunger and/or relieve stress [242,243,244,245]. Similarly, alcohol overconsumption has been documented in night or rotating shift nurses, potentially as a sleep aid [246], with decreased activity (exercise) [247,248]. These behaviors, together with pre-existing risk factors, such as elevated triglycerides [249,250], uncontrolled hypertension [236,239], and/or dyslipidemia [251], compounded by the physiological dysfunctions resulting from circadian disruption through shift work, may interact to promote a prediabetic state [233].
Taken together, these converging lines of research provide ample support for the relationship of disrupted circadian rhythms and cardiometabolic dysfunction. However, the relationship, although seemingly direct (i.e., exacerbated metabolic function co-occurs with disrupted circadian rhythms) may not be linear. Multiple, otherwise innocuous, daily routines, such as food intake and sleep, may occur when other physiological processes are endogenously-timed to occur, thus impairing their biological function and homeostasis. Moreover, coupling those physiological aspects to behavioral nuances (exercise or lack thereof, dietary habits, disordered substance use, etc.), may further complicate the interrelations of all the above.
6. Strategies to Remediate Effects on Disrupted Rhythms in Humans
6.1. Dark Nights
Various strategies to mitigate the effects of our changing environment and their noxious effects on circadian rhythmicity can be taken, and may involve only slight modifications of behavior, at a low cost. During the night, exposure to light in the short wavelength (“blue light”) can phase-shift the body’s internal rhythms and delay melatonin release. Thus, limiting exposure to blue light or filtering it out can help retain synchronized rhythms. For instance, if the need arises to work late into the night and/or be exposed to blue-light-emitting devices, then one may employ the use of glasses that filter this wavelength light [252], and limit their disruptive effects [253]. These glasses function to reduce the component of light that stimulates the intrinsically photosensitive retinal ganglion cells, and thus, improving the dim-light melatonin onset. Their use has been demonstrated to improve sleep and mood [254]. In the bedroom, using blackout curtains may also help.
6.2. Decrease Blue Light Exposure in the Evening
A related strategy consists of implementing a “naturalistic light environment” approach. This strategy requires modifying environmental lighting (with the use of a specialized light system) to become “circadian lighting”, where daily illumination dynamically changes throughout the day to reflect the external day conditions. Specifically, under this paradigm, light is depleted of blue wavelength-rich light during the evening and night hours [255]. Using a cross-over design decreased suppression of melatonin levels, a dim-light melatonin onset phase-advance, and an increase in total sleep were observed in the blue-depleted light conditions, suggesting that modulating nocturnal artificial lighting to emulate that of the external environment, could be a promising strategy. However, this experimental protocol used only five days in each experimental arm, so longer-term experiments should be conducted to elucidate the sustained effects of this manipulation.
6.3. Early Morning Bright Light
Blue light, however, is not all “bad”. Exposure to bright light (bright light therapy; BLT) [256,257,258] early in the biological morning [259] can also help synchronize individuals’ rhythms in a variety of contexts [256,260,261,262]. Indeed, BLT has been used to treat the symptoms of seasonal affective disorder (SAD) when administered early in the biological day [256,257]. BLT has also been proven effective as a second line of treatment for non-seasonal depression [261], and has been used to treat symptoms such as lethargy, decreased alertness, and blunted mood in patients with dementia [260]. BLT has also been effective in resynchronizing rhythms in patients diagnosed with ADHD, an evening chronotype and sleep problems [263,264].
6.4. Food Restriction to the Active Phase
Another strategy to synchronize endogenous rhythms is limiting food intake to the active phase. As discussed, metabolism is tightly regulated by the circadian clock [134,168,202,265], and energetic cues that alter the internal balance threaten physiological homeostasis, leading to increased risk for obesity, glucose and insulin imbalance, and cardiovascular disorders [204,266,267,268,269,270]. Food restriction has been demonstrated to re-entrain rhythms and limit weight gain in rodents [169,271] and humans [37,272]. Moreover, a recent study demonstrated that caloric restriction in addition to circadian alignment of feeding further optimized the beneficial effects of timed-feeding in mice, viz., weight management and, importantly, extended the animal’s lifespan and delayed age-related gene expression changes in immune function and metabolism [273].
6.5. Melatonin Supplementation
Oral melatonin is commercially available in the US, marketed as a dietary supplement, specifically, as a sleep aid, which has been reported to reduce sleep latency onset and increase sleep duration [84]. However, because of its status as a dietary supplement, it is not regulated by the US Food and Drug Administration; thus, true melatonin content is not certified. A 2017 study analyzing the melatonin content of 31 commercially available supplements determined that melatonin greatly varied across supplements from 83–478% of the advertised melatonin content [274,275]. Further, 26% of the supplements contained serotonin, which can be a health risk. This, perhaps, is not surprising because the use of melatonin in the US has become commonplace as a relatively inexpensive and easily-accessible supplement. Indeed, the use of melatonin supplements has quintupled between 1999 and 2018 [276,277]. Overall, the documented increased use of melatonin in the US raises concerns of its safety as a result of the lack of regulation, especially in the context of vulnerable populations, such as children or the elderly. It is worth highlighting that the US is one of few countries that makes melatonin supplements accessible over the counter. In light of this information, the use of melatonin should probably only be used under the direct supervision of a physician and sourced from a vetted supplier.
7. Recommendations for Night Shift Workers and Future Work
It is unlikely that night shift work will disappear, yet it is very likely that many of the long-term effects of disrupted circadian rhythms on human function remain to be uncovered. The strategies that we provided to potentially remediate the effects of circadian rhythms on humans have been, thus far, demonstrated to be helpful in mitigating some of the effects of altered nocturnal rhythms, at least in the short-term and for non-chronic rhythm disruption. Their success in mitigating long-term pathological effects on night shift workers remains to be determined.
Additional strategies are currently recommended for night shift workers; these are primarily aimed at adjusting individuals’ daily environmental conditions in a manner that facilitates an appropriate circadian phase shift, and aligns their internal rhythms to their shifted 24 h light–dark schedule. For instance, recommendations include keeping a consistent schedule for at least 3+ days (not continually alternating between day and night shifts), and keeping shifts under 11 h. Other recommendations include not going to bed immediately after the night shift ends, but rather going to bed later in the day and waking up a few hours before the next night shift, in common with what most diurnal workers do. When it comes to preparation for sleep, it is recommended that one avoids caffeine 3–4 h before going to bed; consuming melatonin supplements 1–2 h before bedtime, and keeping the sleeping environment cool, with minimal exposure to light sources among others [278,279]. The use of sleep masks and black-out curtains in the sleeping rooms is also recommended. Together, the goal is to mimic the conditions one may encounter during the natural active/inactive phases (for instance, the nocturnal decrease in body temperature). Nonetheless, further longitudinal data need to be collected and analyzed to determine best practices and to develop standard operating procedures for widespread use in the night shift work industry. These studies should focus on strategies to best adapt to and properly function within the chosen 24 h rest-activity cycle, as well as investigate the long-term effects of such lifestyle, on metabolism and overall health.
8. Conclusions
Metabolism is a key homeostatic function that is integral to organismal functioning and regulated in a circadian-dependent manner. Any alteration that threatens metabolic temporal equilibrium could have potentially negative, long-term, and persistent repercussions. Here, we highlight the relationship between hormonal metabolism and misaligned rhythms as a result of exposure to light at night, night shift work, jet lag, and mistimed eating. Substantial data have been reviewed from preclinical and clinical settings that reveal interconnected relationships among factors that correlate with impaired rhythmic physiological function (e.g., night shift work), pre-existing conditions (e.g., obesity), and lifestyle choices (e.g., smoking) to exacerbate pathological states. Nonetheless, more research is required to further understand these phenomena, and how they differentially affect individuals by age and sex to develop strategies to mitigate the negative effects and develop appropriate policies to protect those in positions where environmental conditions are misaligned with endogenous physiology (e.g., night shift workers).
Various strategies to counter some of the sequelae of disrupted internal rhythms are available and promising, but further research into their effectiveness, long-term applicability, and the differential effects by age, sex, and possibly, chronotype (individuals’ natural inclination in reference to time of day when sleep is preferred or most alert) remain unspecified. Moreover, regulatory measures should be considered to ensure that both pharmacological and non-pharmacological treatments are as advertised (e.g., precise drug content and functional blue light filters). Given the increasing need to extend daily activity well into the nighttime for work, study, and social activities, awareness of preventative measures should be widely-disseminated for human health and safety.
Author Contributions
Conceptualization, O.H.M.-F. and R.J.N.; data curation, O.H.M.-F., J.A.L. and R.J.N.; writing—original draft preparation, O.H.M.-F., J.A.L. and R.J.N.; writing—review and editing, O.H.M.-F. and R.J.N.; creation of figures, O.H.M.-F.; funding acquisition, O.H.M.-F. and R.J.N. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Institutes of Health (NIH) grant #5RO1NS092388 to R.J.N. O.H.M.-F. was supported by the NIH National Institutes of General Medical Sciences predoctoral fellowship (T32 GM132494). The APC was funded by the Hazel Ruby McQuain Chair for Neurological Research fund to R.J.N.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Bell-Pedersen D., Cassone V.M., Earnest D.J., Golden S.S., Hardin P.E., Thomas T.L., Zoran M.J. Circadian rhythms from multiple oscillators: Lessons from diverse organisms. Nat. Rev. Genet. 2005;6:544–556. doi: 10.1038/nrg1633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kumar V., Sharma A. Common features of circadian timekeeping in diverse organisms. Curr. Opin. Physiol. 2018;5:58–67. doi: 10.1016/j.cophys.2018.07.004. [DOI] [Google Scholar]
- 3.Wright K.P., Jr., McHill A.W., Birks B.R., Griffin B.R., Rusterholz T., Chinoy E.D. Entrainment of the human circadian clock to the natural light-dark cycle. Curr. Biol. 2013;23:1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Roenneberg T., Merrow M. The Circadian Clock and Human Health. Curr. Biol. 2016;26:R432–R443. doi: 10.1016/j.cub.2016.04.011. [DOI] [PubMed] [Google Scholar]
- 5.Bonnell E.K., Huggins C.E., Huggins C.T., McCaffrey T.A., Palermo C., Bonham M.P. Influences on Dietary Choices during Day versus Night Shift in Shift Workers: A Mixed Methods Study. Nutrients. 2017;9:193. doi: 10.3390/nu9030193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gan Y., Yang C., Tong X., Sun H., Cong Y., Yin X., Li L., Cao S., Dong X., Gong Y., et al. Shift work and diabetes mellitus: A meta-analysis of observational studies. Occup. Environ. Med. 2014;72:72–78. doi: 10.1136/oemed-2014-102150. [DOI] [PubMed] [Google Scholar]
- 7.Rose D.M., Jung D., Parera D., Konietzko J. Time zone shifts and jet lag after long-distance flights. Z Arztl Fortbild Qualitatssich. 1999;93:485–490. [PubMed] [Google Scholar]
- 8.Sack R.L. The pathophysiology of jet lag. Travel Med. Infect. Dis. 2009;7:102–110. doi: 10.1016/j.tmaid.2009.01.006. [DOI] [PubMed] [Google Scholar]
- 9.Nagano M., Adachi A., Nakahama K.-I., Nakamura T., Tamada M., Meyer-Bernstein E., Sehgal A., Shigeyoshi Y. An Abrupt Shift in the Day/Night Cycle Causes Desynchrony in the Mammalian Circadian Center. J. Neurosci. 2003;23:6141–6151. doi: 10.1523/jneurosci.23-14-06141.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mortola J.P. Breathing around the clock: An overview of the circadian pattern of respiration. Eur. J. Appl. Physiol. 2004;91:119–129. doi: 10.1007/s00421-003-0978-0. [DOI] [PubMed] [Google Scholar]
- 11.Kawano Y. Diurnal blood pressure variation and related behavioral factors. Hypertens. Res. 2010;34:281–285. doi: 10.1038/hr.2010.241. [DOI] [PubMed] [Google Scholar]
- 12.Vandewalle G., Middleton B., Rajaratnam S.M.W., Stone B.M., Thorleifsdottir B., Arendt J., Dijk D. Robust CircadianRhythm in Heart Rate and Its Variability: Influence of Exogenous Melatonin and Photoperiod. J. Sleep Res. 2007;16:148–155. doi: 10.1111/j.1365-2869.2007.00581.x. [DOI] [PubMed] [Google Scholar]
- 13.Meléndez-Fernández O., Walton J., DeVries A., Nelson R. Clocks, Rhythms, Sex, and Hearts: How Disrupted Circadian Rhythms, Time-of-Day, and Sex Influence Cardiovascular Health. Biomolecules. 2021;11:883. doi: 10.3390/biom11060883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chellappa S., Vujovic N., Williams J.S., Scheer F.A. Impact of Circadian Disruption on Cardiovascular Function and Disease. Trends Endocrinol. Metab. 2019;30:767–779. doi: 10.1016/j.tem.2019.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bedrosian T.A., Fonken L.K., Nelson R.J. Endocrine Effects of Circadian Disruption. Annu. Rev. Physiol. 2016;78:109–131. doi: 10.1146/annurev-physiol-021115-105102. [DOI] [PubMed] [Google Scholar]
- 16.Walker W.H., Borniger J.C., Gaudier-Diaz M.M., Meléndez-Fernández O.H., Pascoe J.L., Devries A.C., Nelson R.J. Acute exposure to low-level light at night is sufficient to induce neurological changes and depressive-like behavior. Mol. Psychiatry. 2020;25:1080–1093. doi: 10.1038/s41380-019-0430-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fonken L.K., Nelson R.J. The effects of light at night on circadian clocks and metabolism. Endocr. Rev. 2014;35:648–670. doi: 10.1210/er.2013-1051. [DOI] [PubMed] [Google Scholar]
- 18.Walker W.H., Bumgarner J.R., Walton J.C., Liu J.A., Meléndez-Fernández O.H., Nelson R.J., Devries A.C. Light Pollution and Cancer. Int. J. Mol. Sci. 2020;21:9360. doi: 10.3390/ijms21249360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Klepeis N.E., Nelson W.C., Ott W.R., Robinson J.P., Tsang A.M., Switzer P., Behar J.V., Hern S.C., Engelmann W.H. The National Human Activity Pattern Survey (NHAPS): A resource for assessing exposure to environmental pollutants. J. Expo. Sci. Environ. Epidemiol. 2001;11:231–252. doi: 10.1038/sj.jea.7500165. [DOI] [PubMed] [Google Scholar]
- 20.Bhadra U., Thakkar N., Das P., Bhadra M.P. Evolution of circadian rhythms: From bacteria to human. Sleep Med. 2017;35:49–61. doi: 10.1016/j.sleep.2017.04.008. [DOI] [PubMed] [Google Scholar]
- 21.Vaze K.M., Sharma V.K. On the Adaptive Significance of Circadian Clocks for Their Owners. Chrono Int. 2013;30:413–433. doi: 10.3109/07420528.2012.754457. [DOI] [PubMed] [Google Scholar]
- 22.Refinetti R. Comparison of light, food, and temperature as environmental synchronizers of the circadian rhythm of activity in mice. J. Physiol. Sci. 2015;65:359–366. doi: 10.1007/s12576-015-0374-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wever R.A. Light Effects on Human Circadian Rhythms: A Review of Recent Andechs Experiments. J. Biol. Rhythm. 1989;4:49–73. doi: 10.1177/074873048900400206. [DOI] [PubMed] [Google Scholar]
- 24.Roenneberg T., Kumar C.J., Merrow M. The human circadian clock entrains to sun time. Curr. Biol. 2007;17:R44–R45. doi: 10.1016/j.cub.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 25.Roenneberg T., Foster R.G. Twilight Times: Light and the Circadian System. Photochem. Photobiol. 1997;66:549–561. doi: 10.1111/j.1751-1097.1997.tb03188.x. [DOI] [PubMed] [Google Scholar]
- 26.Rijo-Ferreira F., Takahashi J.S. Genomics of circadian rhythms in health and disease. Genome Med. 2019;11:82. doi: 10.1186/s13073-019-0704-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Takahashi J.S. Transcriptional architecture of the mammalian circadian clock. Nat. Rev. Genet. 2016;18:164–179. doi: 10.1038/nrg.2016.150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fang B., Everett L.J., Jager J., Briggs E., Armour S.M., Feng D., Roy A., Gerhart-Hines Z., Sun Z., Lazar M.A. Circadian Enhancers Coordinate Multiple Phases of Rhythmic Gene Transcription In Vivo. Cell. 2014;159:1140–1152. doi: 10.1016/j.cell.2014.10.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xu Y., Guo W., Li P., Zhang Y., Zhao M., Fan Z., Zhao Z., Yan J. Long-Range Chromosome Interactions Mediated by Cohesin Shape Circadian Gene Expression. PLOS Genet. 2016;12:e1005992. doi: 10.1371/journal.pgen.1005992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Arnal L.A., Hakim O., Patel V.R., Baldi P., Hager G.L., Sassone-Corsi P. Cycles in spatial and temporal chromosomal organization driven by the circadian clock. Nat. Struct. Mol. Biol. 2013;20:1206–1213. doi: 10.1038/nsmb.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kojima S., Shingle D.L., Green C.B. Post-transcriptional control of circadian rhythms. J. Cell Sci. 2011;124:311–320. doi: 10.1242/jcs.065771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mehra A., Baker C.L., Loros J.J., Dunlap J.C. Post-translational modifications in circadian rhythms. Trends Biochem. Sci. 2009;34:483–490. doi: 10.1016/j.tibs.2009.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Erzberger A., Hampp G., Granada A., Albrecht U., Herzel H. Genetic redundancy strengthens the circadian clock leading to a narrow entrainment range. J. R. Soc. Interface. 2013;10:20130221. doi: 10.1098/rsif.2013.0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Oster H., Baeriswyl S., van der Horst G.T., Albrecht U. Loss of circadian rhythmicity in aging mPer1-/-mCry2-/- mutant mice. Genes Dev. 2003;17:1366–1379. doi: 10.1101/gad.256103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Oster H., Yasui A., van der Horst G.T., Albrecht U. Disruption of mCry2 restores circadian rhythmicity in mPer2 mutant mice. Genes Dev. 2002;16:2633–2638. doi: 10.1101/gad.233702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Welsh D.K., Takahashi J.S., Kay S.A. Suprachiasmatic Nucleus: Cell Autonomy and Network Properties. Annu. Rev. Physiol. 2010;72:551–577. doi: 10.1146/annurev-physiol-021909-135919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewis P., Oster H., Korf H.W., Foster R.G., Erren T.C. Food as a circadian time Cue-Evidence from human studies. Nat. Rev. Endocrinol. 2020;16:213–223. doi: 10.1038/s41574-020-0318-z. [DOI] [PubMed] [Google Scholar]
- 38.Lewis P., Korf H., Kuffer L., Groß J.V., Erren T.C. Exercise time cues (zeitgebers) for human circadian systems can foster health and improve performance: A systematic review. BMJ Open Sport Exerc. Med. 2018;4:e000443. doi: 10.1136/bmjsem-2018-000443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stephan F.K. The "Other" Circadian System: Food as a Zeitgeber. J. Biol. Rhythm. 2002;17:284–292. doi: 10.1177/074873040201700402. [DOI] [PubMed] [Google Scholar]
- 40.Yamazaki S., Goto M., Menaker M. No Evidence for Extraocular Photoreceptors in the Circadian System of the Syrian Hamster. J. Biol. Rhythm. 1999;14:197–201. doi: 10.1177/074873099129000605. [DOI] [PubMed] [Google Scholar]
- 41.Ibata Y., Takahashi Y., Okamura H., Kawakami F., Terubayashi H., Kubo T., Yanaihara N. Vasoactive intestinal peptide (VIP)-like immunoreactive neurons located in the rat suprachiasmatic nucleus receive a direct retinal projection. Neurosci. Lett. 1989;97:1–5. doi: 10.1016/0304-3940(89)90129-8. [DOI] [PubMed] [Google Scholar]
- 42.Moore R.Y., Speh J.C., Card J.P. The retinohypothalamic tract originates from a distinct subset of retinal ganglion cells. J. Comp. Neurol. 1995;352:351–366. doi: 10.1002/cne.903520304. [DOI] [PubMed] [Google Scholar]
- 43.Moore R.Y., Lenn N.J. A retinohypothalamic projection in the rat. J. Comp. Neurol. 1972;146:1–14. doi: 10.1002/cne.901460102. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt T.M., Chen S.-K., Hattar S. Intrinsically photosensitive retinal ganglion cells: Many subtypes, diverse functions. Trends Neurosci. 2011;34:572–580. doi: 10.1016/j.tins.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schmidt T.M., Do M.T.H., Dacey D., Lucas R., Hattar S., Matynia A., Matynia A. Melanopsin-Positive Intrinsically Photosensitive Retinal Ganglion Cells: From Form to Function. J. Neurosci. 2011;31:16094–16101. doi: 10.1523/JNEUROSCI.4132-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Smith M.R., Revell V.L., Eastman C.I. Phase advancing the human circadian clock with blue-enriched polychromatic light. Sleep Med. 2009;10:287–294. doi: 10.1016/j.sleep.2008.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith M.R., Eastman C.I. Phase Delaying the Human Circadian Clock with Blue-Enriched Polychromatic Light. Chrono Int. 2009;26:709–725. doi: 10.1080/07420520902927742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wahl S., Engelhardt M., Schaupp P., Lappe C., Ivanov I.V. The inner clock—Blue light sets the human rhythm. J. Biophotonics. 2019;12:e201900102. doi: 10.1002/jbio.201900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tähkämö L., Partonen T., Pesonen A.-K. Systematic review of light exposure impact on human circadian rhythm. Chronobiol. Int. 2019;36:151–170. doi: 10.1080/07420528.2018.1527773. [DOI] [PubMed] [Google Scholar]
- 50.Lewy A.J., Wehr T.A., Goodwin F.K., Newsome D.A., Markey S.P. Light Suppresses Melatonin Secretion in Humans. Science. 1980;210:1267–1269. doi: 10.1126/science.7434030. [DOI] [PubMed] [Google Scholar]
- 51.Cajochen C., Münch M., Kobialka S., Kräuchi K., Steiner R., Oelhafen P., Orgül S., Wirz-Justice A. High Sensitivity of Human Melatonin, Alertness, Thermoregulation, and Heart Rate to Short Wavelength Light. J. Clin. Endocrinol. Metab. 2005;90:1311–1316. doi: 10.1210/jc.2004-0957. [DOI] [PubMed] [Google Scholar]
- 52.Panda S., Sato T.K., Castrucci A.M., Rollag M.D., DeGrip W.J., Hogenesch J.B., Provencio I., Kay S.A. Melanopsin ( Opn4 ) Requirement for Normal Light-Induced Circadian Phase Shifting. Science. 2002;298:2213–2216. doi: 10.1126/science.1076848. [DOI] [PubMed] [Google Scholar]
- 53.Ruby N.F., Brennan T.J., Xie X., Cao V., Franken P., Heller H.C., O’Hara B.F. Role of Melanopsin in Circadian Responses to Light. Science. 2002;298:2211–2213. doi: 10.1126/science.1076701. [DOI] [PubMed] [Google Scholar]
- 54.Panda S., Provencio I., Tu D.C., Pires S.S., Rollag M.D., Castrucci A.M., Pletcher M.T., Sato T.K., Wiltshire T., Andahazy M., et al. Melanopsin Is Required for Non-Image-Forming Photic Responses in Blind Mice. Science. 2003;301:525–527. doi: 10.1126/science.1086179. [DOI] [PubMed] [Google Scholar]
- 55.Güler A.D., Ecker J.L., Lall G.S., Haq S., Altimus C.M., Liao H.-W., Barnard A.R., Cahill H., Badea T.C., Zhao H., et al. Melanopsin cells are the principal conduits for rod–cone input to non-image-forming vision. Nature. 2008;453:102–105. doi: 10.1038/nature06829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Stopa E.G., Johnson J.K., I Friedman D., I Ryer H., Reidy J., Kuo-Leblanc V., E Albers H. Neuropeptide Y receptor distribution and regulation in the suprachiasmatic nucleus of the Syrian hamster (Mesocricetus auratus) Pept. Res. 1995;8:95–100. [PubMed] [Google Scholar]
- 57.Golombek D.A., Biello S.M., Rendon R.A., Harrington M.E. Neuropeptide Y phase shifts the circadian clock in vitro via a Y2 receptor. Neuroreport. 1996;7:1315–1319. doi: 10.1097/00001756-199605170-00020. [DOI] [PubMed] [Google Scholar]
- 58.Huhman K., Gillespie C., Marvel C., Albers H.E. Neuropeptide Y phase shifts circadian rhythms in vivo via a Y2 receptor. Neuroreport. 1996;7:1249–1252. doi: 10.1097/00001756-199605170-00005. [DOI] [PubMed] [Google Scholar]
- 59.Freeman D.A., Dhandapani K.M., Goldman B.D. The thalamic intergeniculate leaflet modulates photoperiod responsiveness in Siberian hamsters. Brain Res. 2004;1028:31–38. doi: 10.1016/j.brainres.2004.08.049. [DOI] [PubMed] [Google Scholar]
- 60.Weber E., A Rea M. Neuropeptide Y blocks light-induced phase advances but not delays of the circadian activity rhythm in hamsters. Neurosci. Lett. 1997;231:159–162. doi: 10.1016/S0304-3940(97)00559-4. [DOI] [PubMed] [Google Scholar]
- 61.Singh C., Rihel J., Prober D.A. Neuropeptide Y Regulates Sleep by Modulating Noradrenergic Signaling. Curr. Biol. 2017;27:3796–3811. doi: 10.1016/j.cub.2017.11.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Fu L.-Y., Acuna-Goycolea C., Pol A.N.V.D. Neuropeptide Y Inhibits Hypocretin/Orexin Neurons by Multiple Presynaptic and Postsynaptic Mechanisms: Tonic Depression of the Hypothalamic Arousal System. J. Neurosci. 2004;24:8741–8751. doi: 10.1523/JNEUROSCI.2268-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abrahamson E.E., Moore R.Y. Suprachiasmatic nucleus in the mouse: Retinal innervation, intrinsic organization and efferent projections. Brain Res. 2001;916:172–191. doi: 10.1016/s0006-8993(01)02890-6. [DOI] [PubMed] [Google Scholar]
- 64.Swanson L.W., Cowan W.M. The efferent connections of the suprachiasmatic nucleus of the hypothalamus. J. Comp. Neurol. 1975;160:1–12. doi: 10.1002/cne.901600102. [DOI] [PubMed] [Google Scholar]
- 65.Kriegsfeld L.J., Leak R.K., Yackulic C.B., LeSauter J., Silver R. Organization of suprachiasmatic nucleus projections in Syrian hamsters (Mesocricetus auratus): An anterograde and retrograde analysis. J. Comp. Neurol. 2003;468:361–379. doi: 10.1002/cne.10995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Vujovic N., Gooley J.J., Jhou T.C., Saper C.B. Projections from the subparaventricular zone define four channels of output from the circadian timing system. J. Comp. Neurol. 2015;523:2714–2737. doi: 10.1002/cne.23812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Leibowitz S.F. Reciprocal Hunger-Regulating Circuits Involving Alpha-and Beta-Adrenergic Receptors Located, Respectively, in the Ventromedial and Lateral Hypothalamus. Proc. Natl. Acad. Sci. USA. 1970;67:1063–1070. doi: 10.1073/pnas.67.2.1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Berthoud H.-R., Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: From electrical self-stimulation to opto-genetics. Physiol. Behav. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Arrigoni E., Chee M.J., Fuller P.M. To eat or to sleep: That is a lateral hypothalamic question. Neuropharmacology. 2018;154:34–49. doi: 10.1016/j.neuropharm.2018.11.017. [DOI] [PubMed] [Google Scholar]
- 70.Lu J., Zhang Y.-H., Chou T.C., Gaus S.E., Elmquist J.K., Shiromani P., Saper C.B. Contrasting Effects of Ibotenate Lesions of the Paraventricular Nucleus and Subparaventricular Zone on Sleep–Wake Cycle and Temperature Regulation. J. Neurosci. 2001;21:4864–4874. doi: 10.1523/jneurosci.21-13-04864.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Asala S.A., Okano Y., Honda K., Inoué S. Effects of medial preoptic area lesions on sleep and wakefulness in unrestrained rats. Neurosci. Lett. 1990;114:300–304. doi: 10.1016/0304-3940(90)90580-3. [DOI] [PubMed] [Google Scholar]
- 72.Mondino A., Hambrecht-Wiedbusch V.S., Li D., York A.K., Pal D., González J., Torterolo P., Mashour G.A., Vanini G. Glutamatergic Neurons in the Preoptic Hypothalamus Promote Wakefulness, Destabilize NREM Sleep, Suppress REM Sleep, and Regulate Cortical Dynamics. J Neurosci. 2021;41:3462–3478. doi: 10.1523/JNEUROSCI.2718-20.2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Hiller-Sturmhöfel S., Bartke A. The Endocrine System: An Overview. Alcohol Health Res. Alcohol Health Res World. 1998;22:153–164. [PMC free article] [PubMed] [Google Scholar]
- 74.Yousefvand S., Hamidi F. The Role of Ventromedial Hypothalamus Receptors in the Central Regulation of Food Intake. Int. J. Pept. Res. Ther. 2020;27:689–702. doi: 10.1007/s10989-020-10120-9. [DOI] [Google Scholar]
- 75.Sternson S., Shepherd G.M.G., Friedman J.M. Topographic mapping of VMH → arcuate nucleus microcircuits and their reorganization by fasting. Nat. Neurosci. 2005;8:1356–1363. doi: 10.1038/nn1550. [DOI] [PubMed] [Google Scholar]
- 76.Schwartz M.W., Woods S.C., Porte D., Jr., Seeley R.J., Baskin D.G. Central nervous system control of food intake. Nature. 2000;404:661–671. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 77.Hashiguchi H., Sheng Z., Routh V., Gerzanich V., Simard J.M., Bryan J. Direct versus indirect actions of ghrelin on hypothalamic NPY neurons. PLoS ONE. 2017;12:e0184261. doi: 10.1371/journal.pone.0184261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Barson J.R., Leibowitz S.F. Orexin/Hypocretin System: Role in Food and Drug Overconsumption. Int. Rev. Neurobiol. 2017;136:199–237. doi: 10.1016/bs.irn.2017.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zielinski M.R., Krueger J.M. Sleep and innate immunity. Front. Biosci. 2011;S3:632–642. doi: 10.2741/s176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zielinski M.R., McKenna J.T., McCarley R.W. Functions and Mechanisms of Sleep. AIMS Neurosci. 2016;3:67–104. doi: 10.3934/Neuroscience.2016.1.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Szymusiak R., Gvilia I., McGinty D. Hypothalamic control of sleep. Sleep Med. 2007;8:291–301. doi: 10.1016/j.sleep.2007.03.013. [DOI] [PubMed] [Google Scholar]
- 82.Cedernaes J., Waldeck N., Bass J. Neurogenetic basis for circadian regulation of metabolism by the hypothalamus. Genes Dev. 2019;33:1136–1158. doi: 10.1101/gad.328633.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Brzezinski A., Vangel M.G., Wurtman R.J., Norrie G., Zhdanova I., Ben-Shushan A., Ford I. Effects of exogenous melatonin on sleep: A meta-analysis. Sleep Med. Rev. 2005;9:41–50. doi: 10.1016/j.smrv.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 84.Zhdanova I.V. Melatonin as a hypnotic: Pro. Sleep Med. Rev. 2005;9:51–65. doi: 10.1016/j.smrv.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 85.Gandhi A.V., Mosser E.A., Oikonomou G., Prober D.A. Melatonin Is Required for the Circadian Regulation of Sleep. Neuron. 2015;85:1193–1199. doi: 10.1016/j.neuron.2015.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Zhdanova I.V., Geiger D.A., Schwagerl A.L., Leclair O.U., Killiany R., Taylor J.A., Rosene D.L., Moss M.B., Madras B.K. Melatonin promotes sleep in three species of diurnal nonhuman primates. Physiol. Behav. 2002;75:523–529. doi: 10.1016/s0031-9384(02)00654-6. [DOI] [PubMed] [Google Scholar]
- 87.Goldstein R., Pavel S. REM sleep suppression in cats by melatonin. Brain Res. Bull. 1981;7:723–724. doi: 10.1016/0361-9230(81)90126-X. [DOI] [PubMed] [Google Scholar]
- 88.Mintz E.M., Phillips N.H., Berger R.J. Daytime melatonin infusions induce sleep in pigeons without altering subsequent amounts of nocturnal sleep. Neurosci. Lett. 1998;258:61–64. doi: 10.1016/S0304-3940(98)00849-0. [DOI] [PubMed] [Google Scholar]
- 89.Kalsbeek A., Garidou M.-L., Palm I.F., Van Der Vliet J., Simonneaux V., Pévet P., Buijs R.M. Melatonin sees the light: Blocking GABA-ergic transmission in the paraventricular nucleus induces daytime secretion of melatonin. Eur. J. Neurosci. 2000;12:3146–3154. doi: 10.1046/j.1460-9568.2000.00202.x. [DOI] [PubMed] [Google Scholar]
- 90.Klein D.C. Arylalkylamine N-Acetyltransferase: “The Timezyme”. J. Biol. Chem. 2007;282:4233–4237. doi: 10.1074/jbc.R600036200. [DOI] [PubMed] [Google Scholar]
- 91.Chattoraj A., Liu T., Zhang L.S., Huang Z., Borjigin J. Melatonin formation in mammals: In vivo perspectives. Rev. Endocr. Metab. Disord. 2009;10:237–243. doi: 10.1007/s11154-009-9125-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Macchi M.M., Bruce J.N. Human pineal physiology and functional significance of melatonin. Front. Neuroendocr. 2004;25:177–195. doi: 10.1016/j.yfrne.2004.08.001. [DOI] [PubMed] [Google Scholar]
- 93.Reppert S.M., Weaver D.R., Ebisawa T. Cloning and characterization of a mammalian melatonin receptor that mediates reproductive and circadian responses. Neuron. 1994;13:1177–1185. doi: 10.1016/0896-6273(94)90055-8. [DOI] [PubMed] [Google Scholar]
- 94.Reppert S.M., Godson C., Mahle C.D., Weaver D.R., A Slaugenhaupt S., Gusella J.F. Molecular characterization of a second melatonin receptor expressed in human retina and brain: The Mel1b melatonin receptor. Proc. Natl. Acad. Sci. USA. 1995;92:8734–8738. doi: 10.1073/pnas.92.19.8734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dubocovich M.L., Rivera-Bermudez M.A., Gerdin M.J., Masana M.I. Molecular pharmacology regulation and function of mammalian melatonin receptors. Front. Biosci. 2003;8:1093–1108. doi: 10.2741/1089. [DOI] [PubMed] [Google Scholar]
- 96.Liu C., Weaver D.R., Jin X., Shearman L.P., Pieschl R.L., Gribkoff V.K., Reppert S.M. Molecular Dissection of Two Distinct Actions of Melatonin on the Suprachiasmatic Circadian Clock. Neuron. 1997;19:91–102. doi: 10.1016/s0896-6273(00)80350-5. [DOI] [PubMed] [Google Scholar]
- 97.Czeisler C.A., Allan J.S., Strogatz S.H., Ronda J.M., Sánchez R., Ríos C.D., Freitag W.O., Richardson G.S., Kronauer R.E. Bright Light Resets the Human Circadian Pacemaker Independent of the Timing of the Sleep-Wake Cycle. Science. 1986;233:667–671. doi: 10.1126/science.3726555. [DOI] [PubMed] [Google Scholar]
- 98.Brainard G.C., Lewy A.J., Menaker M., Fredrickson R.H., Miller L.S., Weleber R.G., Cassone V., Hudson D. Dose-response relationship between light irradiance and the suppression of plasma melatonin in human volunteers. Brain Res. 1988;454:212–218. doi: 10.1016/0006-8993(88)90820-7. [DOI] [PubMed] [Google Scholar]
- 99.Slotten H.A., Krekling S., Sicard B., Pévet P. Daily infusion of melatonin entrains circadian activity rhythms in the diurnal rodent Arvicanthis ansorgei. Behav. Brain Res. 2001;133:11–19. doi: 10.1016/s0166-4328(01)00411-9. [DOI] [PubMed] [Google Scholar]
- 100.Pévet P., Bothorel B., Slotten H., Saboureau M. The chronobiotic properties of melatonin. Cell Tissue Res. 2002;309:183–191. doi: 10.1007/s00441-002-0584-1. [DOI] [PubMed] [Google Scholar]
- 101.Slotten H.A., Pitrosky B., Krekling S., Pévet P. Entrainment of circadian activity rhythms in rats to melatonin administered at T cycles different from 24 hours. Neurosignals. 2002;11:73–80. doi: 10.1159/000058543. [DOI] [PubMed] [Google Scholar]
- 102.Xiang S., Mao L., Duplessis T., Yuan L., Dauchy R., Dauchy E., Blask D., Frasch T., Hill S. Oscillation of Clock and Clock Controlled Genes Induced by Serum Shock in Human Breast Epithelial and Breast Cancer Cells: Regulation by Melatonin. Breast Cancer Basic Clin. Res. 2012;6:137–150. doi: 10.4137/BCBCR.S9673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Pechanova O., Paulis L., Simko F. Peripheral and Central Effects of Melatonin on Blood Pressure Regulation. Int. J. Mol. Sci. 2014;15:17920–17937. doi: 10.3390/ijms151017920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Slominski R.M., Reiter R.J., Schlabritz-Loutsevitch N., Ostrom R.S., Slominski A.T. Melatonin membrane receptors in peripheral tissues: Distribution and functions. Mol. Cell. Endocrinol. 2012;351:152–166. doi: 10.1016/j.mce.2012.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Dardente H., Menet J.S., Poirel V.-J., Streicher D., Gauer F., Vivien-Roels B., Klosen P., Pévet P., Masson-Pévet M. Melatonin induces Cry1 expression in the pars tuberalis of the rat. Mol. Brain Res. 2003;114:101–106. doi: 10.1016/s0169-328x(03)00134-7. [DOI] [PubMed] [Google Scholar]
- 106.von Gall C., Weaver D.R., Moek J., Jilg A., Stehle J.H., Korf H.W. Melatonin plays a crucial role in the regulation of rhythmic clock gene expression in the mouse pars tuberalis. Ann. New York Acad. Sci. 2005;1040:508–511. doi: 10.1196/annals.1327.105. [DOI] [PubMed] [Google Scholar]
- 107.Messager S., Garabette M.L., Hastings M.H., Hazlerigg D.G. Tissue-specific abolition of Per1 expression in the pars tuberalis by pinealectomy in the Syrian hamster. Neuroreport. 2001;12:579–582. doi: 10.1097/00001756-200103050-00029. [DOI] [PubMed] [Google Scholar]
- 108.Agez L., Laurent V., Guerrero H.Y., Pévet P., Masson-Pévet M., Gauer F. Endogenous melatonin provides an effective circadian message to both the suprachiasmatic nuclei and the pars tuberalis of the rat. J. Pineal Res. 2009;46:95–105. doi: 10.1111/j.1600-079X.2008.00636.x. [DOI] [PubMed] [Google Scholar]
- 109.Lockley S.W., Skene D.J., James K., Thapan K., Wright J., Arendt J. Melatonin administration can entrain the free-running circadian system of blind subjects. J. Endocrinol. 2000;164:R1–R6. doi: 10.1677/joe.0.164r001. [DOI] [PubMed] [Google Scholar]
- 110.Sack R.L., Brandes R.W., Kendall A.R., Lewy A.J. Entrainment of Free-Running Circadian Rhythms by Melatonin in Blind People. N. Engl. J. Med. 2000;343:1070–1077. doi: 10.1056/NEJM200010123431503. [DOI] [PubMed] [Google Scholar]
- 111.Lewy A.J., Bauer V.K., Hasler B.P., Kendall A.R., Pires M.N., Sack R.L. Capturing the circadian rhythms of free-running blind people with 0.5 mg melatonin. Brain Res. 2001;918:96–100. doi: 10.1016/s0006-8993(01)02964-x. [DOI] [PubMed] [Google Scholar]
- 112.Kazimi N., Cahill G.M. Development of a circadian melatonin rhythm in embryonic zebrafish. Dev. Brain Res. 1999;117:47–52. doi: 10.1016/s0165-3806(99)00096-6. [DOI] [PubMed] [Google Scholar]
- 113.Lewy A.J., Ahmed S., Jackson J.M.L., Sack R.L. Melatonin Shifts Human Orcadian Rhythms According to a Phase-Response Curve. Chrono Int. 1992;9:380–392. doi: 10.3109/07420529209064550. [DOI] [PubMed] [Google Scholar]
- 114.Turek F., Gillette M. Melatonin, sleep, and circadian rhythms: Rationale for development of specific melatonin agonists. Sleep Med. 2004;5:523–532. doi: 10.1016/j.sleep.2004.07.009. [DOI] [PubMed] [Google Scholar]
- 115.Cipolla-Neto J., Amaral F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018;39:990–1028. doi: 10.1210/er.2018-00084. [DOI] [PubMed] [Google Scholar]
- 116.Dijk D.-J., Cajochen C. Melatonin and the Circadian Regulation of Sleep Initiation, Consolidation, Structure, and the Sleep EEG. J. Biol. Rhythm. 1997;12:627–635. doi: 10.1177/074873049701200618. [DOI] [PubMed] [Google Scholar]
- 117.Lavie P. Melatonin: Role in Gating Nocturnal Rise in Sleep Propensity. J. Biol. Rhythm. 1997;12:657–665. doi: 10.1177/074873049701200622. [DOI] [PubMed] [Google Scholar]
- 118.Srinivasan V., Pandi-Perumal S.R., Trahkt I., Spence D.W., Poeggeler B., Hardeland R., Cardinali D.P. Melatonin and Melatonergic Drugs on Sleep: Possible Mechanisms of Action. Int. J. Neurosci. 2009;119:821–846. doi: 10.1080/00207450802328607. [DOI] [PubMed] [Google Scholar]
- 119.Williams W.P., McLin D.E., Dressman M.A., Neubauer D.N. Comparative Review of Approved Melatonin Agonists for the Treatment of Circadian Rhythm Sleep-Wake Disorders. Pharmacother. J. Hum. Pharmacol. Drug Ther. 2016;36:1028–1041. doi: 10.1002/phar.1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Lusardi P., Piazza E., Fogari R. Cardiovascular effects of melatonin in hypertensive patients well controlled by nifedipine: A 24-hour study. Br. J. Clin. Pharmacol. 2000;49:423–427. doi: 10.1046/j.1365-2125.2000.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zhou H., Ma Q., Zhu P., Ren J., Reiter R.J., Chen Y. Protective role of melatonin in cardiac ischemia-reperfusion injury: From pathogenesis to targeted therapy. J. Pineal Res. 2018;64:e12471. doi: 10.1111/jpi.12471. [DOI] [PubMed] [Google Scholar]
- 122.Baker J., Kimpinski K. Role of melatonin in blood pressure regulation: An adjunct anti-hypertensive agent. Clin. Exp. Pharmacol. Physiol. 2018;45:755–766. doi: 10.1111/1440-1681.12942. [DOI] [PubMed] [Google Scholar]
- 123.Ting K.N., A Blaylock N., Sugden D., Delagrange P., Scalbert E., Wilson V.G. Molecular and pharmacological evidence for MT1 melatonin receptor subtype in the tail artery of juvenile Wistar rats. Br. J. Pharmacol. 1999;127:987–995. doi: 10.1038/sj.bjp.0702612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Krause D.N., Barrios V.E., Duckles S.P. Melatonin receptors mediate potentiation of contractile responses to adrenergic nerve stimulation in rat caudal artery. Eur. J. Pharmacol. 1995;276:207–213. doi: 10.1016/0014-2999(95)00028-J. [DOI] [PubMed] [Google Scholar]
- 125.Geary G.G., Duckles S.P., Krause D.N. Effect of melatonin in the rat tail artery: Role of K+ channels and endothelial factors. Br. J. Pharmacol. 1998;123:1533–1540. doi: 10.1038/sj.bjp.0701761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Viswanathan M., Scalbert E., Delagrange P., Guardiola-Lemaître B., Saavedra J.M. Melatonin receptors mediate contraction of a rat cerebral artery. Neuroreport. 1997;8:3847–3849. doi: 10.1097/00001756-199712220-00002. [DOI] [PubMed] [Google Scholar]
- 127.Geary G.G., Krause D.N., Duckles S.P. Melatonin directly constricts rat cerebral arteries through modulation of potassium channels. Am. J. Physiol. Circ. Physiol. 1997;273:H1530–H1536. doi: 10.1152/ajpheart.1997.273.3.H1530. [DOI] [PubMed] [Google Scholar]
- 128.Doolen S., Krause D.N., Dubocovich M.L., Duckles S.P. Melatonin mediates two distinct responses in vascular smooth muscle. Eur. J. Pharmacol. 1998;345:67–69. doi: 10.1016/s0014-2999(98)00064-8. [DOI] [PubMed] [Google Scholar]
- 129.Duckles S.P., Dubocovich M.L., Krause D.N. MT2 Melatonin Receptors Are Present and Functional in Rat Caudal Artery. [(accessed on 13 January 2023)];J. Pharmacol. Exp. Ther. 2002 doi: 10.1124/jpet.302.3.1295. Available online: https://jpet.aspetjournals.org/content/302/3/1295.short?casa_token=zrUt7JhvzmMAAAAA:5a6J1SvtfYHjtWNg-i9tVHY8fNYCYg1XlulSvfwajsXjhUVI3dub9b2IKwOrqtN3JhdnG8OgWHdn. [DOI] [PubMed] [Google Scholar]
- 130.Pandi-Perumal S.R., Bahammam A.S., Ojike N.I., Akinseye O.A., Kendzerska T., Buttoo K., Dhandapany P.S., Brown G.M., Cardinali D.P. Melatonin and Human Cardiovascular Disease. J. Cardiovasc. Pharmacol. Ther. 2016;22:122–132. doi: 10.1177/1074248416660622. [DOI] [PubMed] [Google Scholar]
- 131.Lochner A., Marais E., Huisamen B. Melatonin and cardioprotection against ischaemia/reperfusion injury: What’s new? A review. J. Pineal Res. 2018;65:e12490. doi: 10.1111/jpi.12490. [DOI] [PubMed] [Google Scholar]
- 132.Tobeiha M., Jafari A., Fadaei S., Mirazimi S.M.A., Dashti F., Amiri A., Khan H., Asemi Z., Reiter R.J., Hamblin M.R., et al. Evidence for the Benefits of Melatonin in Cardiovascular Disease. Front. Cardiovasc. Med. 2022;9:888319. doi: 10.3389/fcvm.2022.888319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Alonso-Vale M.I.C., Andreotti S., Mukai P.Y., Borges-Silva C.D.N., Peres S.B., Cipolla-Neto J., Lima F.B. Melatonin and the circadian entrainment of metabolic and hormonal activities in primary isolated adipocytes. J. Pineal Res. 2008;45:422–429. doi: 10.1111/j.1600-079X.2008.00610.x. [DOI] [PubMed] [Google Scholar]
- 134.Kennaway D.J., Owens J.A., Voultsios A., Wight N. Adipokines and Adipocyte Function inClockMutant Mice That Retain Melatonin Rhythmicity. Obesity. 2012;20:295–305. doi: 10.1038/oby.2011.276. [DOI] [PubMed] [Google Scholar]
- 135.Ren W., Liu G., Chen S., Yin J., Wang J., Tan B., Wu G., Bazer F.W., Peng Y., Li T., et al. Melatonin signaling in T cells: Functions and applications. J. Pineal Res. 2017;62:e12394. doi: 10.1111/jpi.12394. [DOI] [PubMed] [Google Scholar]
- 136.Bondy S.C. Melatonin and Regulation of Immune Function: Impact on Numerous Diseases. Curr. Aging Sci. 2020;13:92–101. doi: 10.2174/1874609813666200711153223. [DOI] [PubMed] [Google Scholar]
- 137.Carrillo-Vico A., Lardone P.J., Álvarez-Sánchez N., Rodríguez-Rodríguez A., Guerrero J.M. Melatonin: Buffering the Immune System. Int. J. Mol. Sci. 2013;14:8638–8683. doi: 10.3390/ijms14048638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Srinivasan V., Maestroni G.J.M., Cardinali D.P., Esquifino A.I., Pandi-Perumal S.R., Miller S.C. Melatonin, immune function and aging. Immun. Ageing. 2005;2:17. doi: 10.1186/1742-4933-2-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Fenn A.M., Fonken L.K., Nelson R.J. Sustained melatonin treatment blocks body mass, pelage, reproductive, and fever responses to short day lengths in female Siberian hamsters. J. Pineal Res. 2011;51:180–186. doi: 10.1111/j.1600-079X.2011.00874.x. [DOI] [PubMed] [Google Scholar]
- 140.Nelson R.J., Demas G.E., Klein S.L., Kriegsfeld L.J. Minireview The influence of season, photoperiod, and pineal melatonin on immune function. J. Pineal Res. 1995;19:149–165. doi: 10.1111/j.1600-079X.1995.tb00184.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Demas G.E., Nelson R.J. Exogenous Melatonin Enhances Cell-Mediated, but Not Humoral, Immune Function in Adult Male Deer Mice (Peromyscus maniculatus) J. Biol. Rhythm. 1998;13:245–252. doi: 10.1177/074873098129000084. [DOI] [PubMed] [Google Scholar]
- 142.Kriegsfeld L.J., Drazen D.L., Nelson R.J. In vitro melatonin treatment enhances cell-mediated immune function in male prairie voles (Microtus ochrogaster ) J. Pineal Res. 2001;30:193–198. doi: 10.1034/j.1600-079X.2001.300401.x. [DOI] [PubMed] [Google Scholar]
- 143.Reiter R.J., Mayo J.C., Tan D.-X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 144.Parisotto E.B., Vidal V., García-Cerro S., Lantigua S., Filho D.W., Sanchez-Barceló E.J., Martínez-Cué C., Rueda N. Chronic Melatonin Administration Reduced Oxidative Damage and Cellular Senescence in the Hippocampus of a Mouse Model of Down Syndrome. Neurochem. Res. 2016;41:2904–2913. doi: 10.1007/s11064-016-2008-8. [DOI] [PubMed] [Google Scholar]
- 145.Manchester L.C., Coto-Montes A., Boga J.A., Andersen L.P.H., Zhou Z., Galano A., Vriend J., Tan D.-X., Reiter R.J. Melatonin: An ancient molecule that makes oxygen metabolically tolerable. J. Pineal Res. 2015;59:403–419. doi: 10.1111/jpi.12267. [DOI] [PubMed] [Google Scholar]
- 146.Poeggeler B., Reiter R.J., Tan D.-X., Chen L.-D., Manchester L.C. Melatonin, hydroxyl radical-mediated oxidative damage, and aging: A hypothesis. J. Pineal Res. 1993;14:151–168. doi: 10.1111/j.1600-079x.1993.tb00498.x. [DOI] [PubMed] [Google Scholar]
- 147.Zisapel N. New perspectives on the role of melatonin in human sleep, circadian rhythms and their regulation. Br. J. Pharmacol. 2018;175:3190–3199. doi: 10.1111/bph.14116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kang J.-E., Lim M.M., Bateman R.J., Lee J.J., Smyth L.P., Cirrito J.R., Fujiki N., Nishino S., Holtzman D.M. Amyloid-β Dynamics Are Regulated by Orexin and the Sleep-Wake Cycle. Science. 2009;326:1005–1007. doi: 10.1126/science.1180962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Beaupre L.M.M., Brown G.M., Gonçalves V.F., Kennedy J.L. Melatonin’s neuroprotective role in mitochondria and its potential as a biomarker in aging, cognition and psychiatric disorders. Transl. Psychiatry. 2021;11:339. doi: 10.1038/s41398-021-01464-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Vegiopoulos A., Herzig S. Glucocorticoids, metabolism and metabolic diseases. Mol. Cell. Endocrinol. 2007;275:43–61. doi: 10.1016/j.mce.2007.05.015. [DOI] [PubMed] [Google Scholar]
- 151.Cruz-Topete D., Cidlowski J.A. One Hormone, Two Actions: Anti- and Pro-Inflammatory Effects of Glucocorticoids. Neuroimmunomodulation. 2014;22:20–32. doi: 10.1159/000362724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Cruz-Topete D., Myers P.H., Foley J.F., Willis M., Cidlowski J. Corticosteroids Are Essential for Maintaining Cardiovascular Function in Male Mice. Endocrinology. 2016;157:2759–2771. doi: 10.1210/en.2015-1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Burford N.G., Webster N.A., Cruz-Topete D. Hypothalamic-Pituitary-Adrenal Axis Modulation of Glucocorticoids in the Cardiovascular System. Int. J. Mol. Sci. 2017;18:2150. doi: 10.3390/ijms18102150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Whirledge S., Cidlowski J.A. Glucocorticoids and Reproduction: Traffic Control on the Road to Reproduction. Trends Endocrinol. Metab. 2017;28:399–415. doi: 10.1016/j.tem.2017.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Oster H., Damerow S., Kiessling S., Jakubcakova V., Abraham D., Tian J., Hoffmann M.W., Eichele G. The circadian rhythm of glucocorticoids is regulated by a gating mechanism residing in the adrenal cortical clock. Cell Metab. 2006;4:163–173. doi: 10.1016/j.cmet.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 156.Leon-Mercado L., Chao D.H.M., Basualdo M.D.C., Kawata M., Escobar C., Buijs R.M. The Arcuate Nucleus: A Site of Fast Negative Feedback for Corticosterone Secretion in Male Rats. Eneuro. 2017;4:e0350. doi: 10.1523/ENEURO.0350-16.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Weitzman E.D., Fukushima D., Nogeire C., Roffwarg H., Gallagher T.F., Hellman L. Twenty-four Hour Pattern of the Episodic Secretion of Cortisol in Normal Subjects. J. Clin. Endocrinol. Metab. 1971;33:14–22. doi: 10.1210/jcem-33-1-14. [DOI] [PubMed] [Google Scholar]
- 158.Selmaoui B., Touitou Y. Reproducibility of the circadian rhythms of serum cortisol and melatonin in healthy subjects: A study of three different 24-h cycles over six weeks. Life Sci. 2003;73:3339–3349. doi: 10.1016/j.lfs.2003.05.007. [DOI] [PubMed] [Google Scholar]
- 159.Kuo T., McQueen A., Chen T.C., Wang J.C. Regulation of Glucose Homeostasis by Glucocorticoids. Adv. Exp. Med. Biol. 2015;872:99–126. doi: 10.1007/978-1-4939-2895-8_5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Albers H.E., Yogev L., Todd R.B., Goldman B.D. Adrenal corticoids in hamsters: Role in circadian timing. Am. J. Physiol. Integr. Comp. Physiol. 1985;248:R434–R438. doi: 10.1152/ajpregu.1985.248.4.R434. [DOI] [PubMed] [Google Scholar]
- 161.Sapolsky R.M., Romero L.M., Munck A.U. How Do Glucocorticoids Influence Stress Responses? Integrating Permissive, Suppressive, Stimulatory, and Preparative Actions. Endocr. Rev. 2000;21:55–89. doi: 10.1210/edrv.21.1.0389. [DOI] [PubMed] [Google Scholar]
- 162.Dallman M.F., Strack A.M., Akana S.F., Bradbury M.J., Hanson E.S., Scribner K.A., Smith M. Feast and Famine: Critical Role of Glucocorticoids with Insulin in Daily Energy Flow. Front. Neuroendocr. 1993;14:303–347. doi: 10.1006/frne.1993.1010. [DOI] [PubMed] [Google Scholar]
- 163.McEwen B.S., Brinton R.E., Sapolsky R.M. Glucocorticoid Receptors and Behavior: Implications for the Stress Response. Adv. Exp. Med. Biol. 1988;245:35–45. doi: 10.1007/978-1-4899-2064-5_4. [DOI] [PubMed] [Google Scholar]
- 164.Urbanski H.F. Role of Circadian Neuroendocrine Rhythms in the Control of Behavior and Physiology. Neuroendocrinology. 2011;93:211–222. doi: 10.1159/000327399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takahashi J.S. Molecular Architecture of the Circadian Clock in Mammals. In: Sassone-Corsi P., Christen Y., editors. A Time for Metabolism and Hormones. Springer; Berlin/Heidelberg, Germany: 2016. [DOI] [PubMed] [Google Scholar]
- 166.Ohta H., Yamazaki S., McMahon D.G. Constant light desynchronizes mammalian clock neurons. Nat. Neurosci. 2005;8:267–269. doi: 10.1038/nn1395. [DOI] [PubMed] [Google Scholar]
- 167.Mazzoccoli G., Pazienza V., Vinciguerra M. Clock Genes and Clock-Controlled Genes in the Regulation of Metabolic Rhythms. Chrono Int. 2012;29:227–251. doi: 10.3109/07420528.2012.658127. [DOI] [PubMed] [Google Scholar]
- 168.Coomans C.P., Berg S.A.A., Houben T., Klinken J., Berg R., Pronk A.C.M., Havekes L.M., Romijn J.A., Dijk K.W., Biermasz N.R., et al. Detrimental effects of constant light exposure and high-fat diet on circadian energy metabolism and insulin sensitivity. FASEB J. 2012;27:1721–1732. doi: 10.1096/fj.12-210898. [DOI] [PubMed] [Google Scholar]
- 169.Fonken L.K., Workman J.L., Walton J.C., Weil Z.M., Morris J.S., Haim A., Nelson R.J. Light at night increases body mass by shifting the time of food intake. Proc. Natl. Acad. Sci. USA. 2010;107:18664–18669. doi: 10.1073/pnas.1008734107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Fonken L.K., Finy M.S., Walton J., Weil Z., Workman J.L., Ross J., Nelson R.J. Influence of light at night on murine anxiety- and depressive-like responses. Behav. Brain Res. 2009;205:349–354. doi: 10.1016/j.bbr.2009.07.001. [DOI] [PubMed] [Google Scholar]
- 171.Catalano F., De Vito F., Cassano V., Fiorentino T.V., Sciacqua A., Hribal M.L. Circadian Clock Desynchronization and Insulin Resistance. Int. J. Environ. Res. Public Health. 2022;20:29. doi: 10.3390/ijerph20010029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Zhou B., Zhang Y., Zhang F., Xia Y., Liu J., Huang R., Wang Y., Hu Y., Wu J., Dai C., et al. CLOCK/BMAL1 regulates circadian change of mouse hepatic insulin sensitivity by SIRT1. Hepatology. 2014;59:2196–2206. doi: 10.1002/hep.26992. [DOI] [PubMed] [Google Scholar]
- 173.Zhong L.-X., Li X.-N., Yang G.-Y., Zhang X., Li W.-X., Zhang Q.-Q., Pan H.-X., Zhang H.-H., Zhou M.-Y., Wang Y.-D., et al. Circadian misalignment alters insulin sensitivity during the light phase and shifts glucose tolerance rhythms in female mice. PLoS ONE. 2019;14:e0225813. doi: 10.1371/journal.pone.0225813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Mason I.C., Grimaldi D., Reid K.J., Warlick C.D., Malkani R.G., Abbott S.M., Zee P.C. Light exposure during sleep impairs cardiometabolic function. Proc. Natl. Acad. Sci. USA. 2022;119:e2113290119. doi: 10.1073/pnas.2113290119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kooijman S., Berg R.V.D., Ramkisoensing A., Boon M.R., Kuipers E.N., Loef M., Zonneveld T.C.M., Lucassen E.A., Sips H.C.M., Chatzispyrou I.A., et al. Prolonged daily light exposure increases body fat mass through attenuation of brown adipose tissue activity. Proc. Natl. Acad. Sci. USA. 2015;112:6748–6753. doi: 10.1073/pnas.1504239112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Song X., Rusak B. Acute effects of light on body temperature and activity in Syrian hamsters: Influence of circadian phase. Am. J. Physiol. Integr. Comp. Physiol. 2000;278:R1369–R1380. doi: 10.1152/ajpregu.2000.278.5.R1369. [DOI] [PubMed] [Google Scholar]
- 177.Jung C.M., Khalsa S.B., Scheer F., Cajochen C., Lockley S.W., Czeisler C.A., Wright K.P. Acute Effects of Bright Light Exposure on Cortisol Levels. J. Biol. Rhythm. 2010;25:208–216. doi: 10.1177/0748730410368413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Jones J.R., Chaturvedi S., Granados-Fuentes D., Herzog E.D. Circadian neurons in the paraventricular nucleus entrain and sustain daily rhythms in glucocorticoids. Nat. Commun. 2021;12:5763. doi: 10.1038/s41467-021-25959-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wilson A.-L., Downs C.T. Light interference and melatonin affects digestion and glucocorticoid metabolites in striped mouse. Biol. Rhythm. Res. 2015;46:929–939. doi: 10.1080/09291016.2015.1066546. [DOI] [Google Scholar]
- 180.Okuliarova M., Dzirbikova Z., Rumanova V.S., Foppen E., Kalsbeek A., Zeman M. Disrupted Circadian Control of Hormonal Rhythms and Anticipatory Thirst by Dim Light at Night. Neuroendocrinology. 2022;112:1116–1128. doi: 10.1159/000524235. [DOI] [PubMed] [Google Scholar]
- 181.Kennaway D.J., Wright H. Melatonin and circadian rhythms. Curr. Top. Med. Chem. 2002;2:199–209. doi: 10.2174/1568026023394380. [DOI] [PubMed] [Google Scholar]
- 182.Emmer K.M., Russart K.L.G., Walker W.H., Nelson R.J., DeVries A.C. Effects of light at night on laboratory animals and research outcomes. Behav. Neurosci. 2018;132:302–314. doi: 10.1037/bne0000252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Dauchy R.T., E Blask D., A Sauer L., Brainard G.C., A Krause J. Dim light during darkness stimulates tumor progression by enhancing tumor fatty acid uptake and metabolism. Cancer Lett. 1999;144:131–136. doi: 10.1016/S0304-3835(99)00207-4. [DOI] [PubMed] [Google Scholar]
- 184.Echakir I., Dumont S., Pã©Vet P., Eouarour A., Echallet E., Evuillez P. Pineal melatonin is a circadian time-giver for leptin rhythm in Syrian hamsters. Front. Neurosci. 2015;9:190. doi: 10.3389/fnins.2015.00190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Scheving E.L., Pauly E.J. Effect of light on corticosterone levels in plasma of rats. Am. J. Physiol. Content. 1966;210:1112–1117. doi: 10.1152/ajplegacy.1966.210.5.1112. [DOI] [PubMed] [Google Scholar]
- 186.Snyder S.H., Zweig M., Axelrod J. Control of the circadian rhythm in serotonin content of the rat pineal gland. Life Sci. 1964;3:1175–1179. doi: 10.1016/0024-3205(64)90137-7. [DOI] [PubMed] [Google Scholar]
- 187.Khan S., Duan P., Yao L., Hou H. Shiftwork-Mediated Disruptions of Circadian Rhythms and Sleep Homeostasis Cause Serious Health Problems. Int. J. Genom. 2018;2018:8576890. doi: 10.1155/2018/8576890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Iliff J.J., Wang M., Liao Y., Plogg B.A., Peng W., Gundersen G.A., Benveniste H., Vates G.E., Deane R., Goldman S.A., et al. A Paravascular Pathway Facilitates CSF Flow Through the Brain Parenchyma and the Clearance of Interstitial Solutes, Including Amyloid β. Sci. Transl. Med. 2012;4:147ra111. doi: 10.1126/scitranslmed.3003748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Xie L., Kang H., Xu Q., Chen M.J., Liao Y., Thiyagarajan M., O’Donnell J., Christensen D.J., Nicholson C., Iliff J.J., et al. Sleep Drives Metabolite Clearance from the Adult Brain. Science. 2013;342:373–377. doi: 10.1126/science.1241224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Fultz N.E., Bonmassar G., Setsompop K., Stickgold R.A., Rosen B.R., Polimeni J.R., Lewis L.D. Coupled electrophysiological, hemodynamic, and cerebrospinal fluid oscillations in human sleep. Science. 2019;366:628–631. doi: 10.1126/science.aax5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Gamble K.L., Resuehr D., Johnson C.H.S. Work and Circadian Dysregulation of Reproduction. Front. Endocrinol. 2013;4:92. doi: 10.3389/fendo.2013.00092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Yaw A.M., McLane-Svoboda A.K., Hoffmann H.M. Shiftwork and Light at Night Negatively Impact Molecular and Endocrine Timekeeping in the Female Reproductive Axis in Humans and Rodents. Int. J. Mol. Sci. 2020;22:324. doi: 10.3390/ijms22010324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Figueiro M.G., Steverson B., Heerwagen J., Kampschroer K., Hunter C.M., Gonzales K., Plitnick B., Rea M.S. The impact of daytime light exposures on sleep and mood in office workers. Sleep Health. 2017;3:204–215. doi: 10.1016/j.sleh.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 194.Oike H., Sakurai M., Ippoushi K., Kobori M. Time-fixed feeding prevents obesity induced by chronic advances of light/dark cycles in mouse models of jet-lag/shift work. Biochem. Biophys. Res. Commun. 2015;465:556–561. doi: 10.1016/j.bbrc.2015.08.059. [DOI] [PubMed] [Google Scholar]
- 195.Espitia-Bautista E., Velasco-Ramos M., Osnaya-Ramírez I., Ángeles-Castellanos M., Buijs R.M., Escobar C. Social jet-lag potentiates obesity and metabolic syndrome when combined with cafeteria diet in rats. Metabolism. 2017;72:83–93. doi: 10.1016/j.metabol.2017.04.006. [DOI] [PubMed] [Google Scholar]
- 196.Scheer F.A.J.L., Hilton M.F., Mantzoros C.S., Shea S.A. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc. Natl. Acad. Sci. USA. 2009;106:4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Liu Q., Shi J., Duan P., Liu B., Li T., Wang C., Li H., Yang T., Gan Y., Wang X., et al. Is shift work associated with a higher risk of overweight or obesity? A systematic review of observational studies with meta-analysis. Int. J. Epidemiol. 2018;47:1956–1971. doi: 10.1093/ije/dyy079. [DOI] [PubMed] [Google Scholar]
- 198.Mota M.C., Silva C.M., Balieiro L.C.T., Gonçalves B.F., Fahmy W.M., Crispim C.A. Association between social jetlag food consumption and meal times in patients with obesity-related chronic diseases. PLoS ONE. 2019;14:e0212126. doi: 10.1371/journal.pone.0212126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Yong M., Nasterlack M., Pluto R.-P., Elmerich K., Karl D., Knauth P. Is health, measured by work ability index, affected by 12-hour rotating shift schedules? Chrono Int. 2010;27:1135–1148. doi: 10.3109/07420528.2010.490111. [DOI] [PubMed] [Google Scholar]
- 200.Yong M., Nasterlack M., Messerer P., Oberlinner C., Lang S. A retrospective cohort study of shift work and risk of cancer-specific mortality in German male chemical workers. Int. Arch. Occup. Environ. Health. 2013;87:175–183. doi: 10.1007/s00420-013-0843-3. [DOI] [PubMed] [Google Scholar]
- 201.Bittner N., Korf H.-W., Stumme J., Jockwitz C., Moebus S., Schmidt B., Dragano N., Caspers S. Multimodal investigation of the association between shift work and the brain in a population-based sample of older adults. Sci. Rep. 2022;12:2969. doi: 10.1038/s41598-022-05418-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wehrens S.M., Christou S., Isherwood C., Middleton B., Gibbs M.A., Archer S.N., Skene D.J., Johnston J.D. Meal Timing Regulates the Human Circadian System. Curr. Biol. 2017;27:1768–1775.e3. doi: 10.1016/j.cub.2017.04.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Goel N., Stunkard A.J., Rogers N.L., Van Dongen H.P., Allison K.C., O’Reardon J.P., Ahima R.S., Cummings D.E., Heo M., Dinges D.F. Circadian Rhythm Profiles in Women with Night Eating Syndrome. J. Biol. Rhythm. 2009;24:85–94. doi: 10.1177/0748730408328914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Fleury G., Masís-Vargas A., Kalsbeek A. Metabolic Implications of Exposure to Light at Night: Lessons from Animal and Human Studies. Obesity. 2020;28:S18–S28. doi: 10.1002/oby.22807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Borniger J.C., Weil Z.M., Zhang N., Nelson R.J. Dim Light at Night Does Not Disrupt Timing or Quality of Sleep in Mice. Chrono Int. 2013;30:1016–1023. doi: 10.3109/07420528.2013.803196. [DOI] [PubMed] [Google Scholar]
- 206.Stenvers D.J., van Dorp R., Foppen E., Mendoza J., Opperhuizen A.-L., Fliers E., Bisschop P.H., Meijer J.H., Kalsbeek A., Deboer T. Dim light at night disturbs the daily sleep-wake cycle in the rat. Sci. Rep. 2016;6:35662. doi: 10.1038/srep35662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Figueiro M.G., Radetsky L., Plitnick B., Rea M.S. Glucose tolerance in mice exposed to light–dark stimulus patterns mirroring dayshift and rotating shift schedules. Sci. Rep. 2017;7:40661. doi: 10.1038/srep40661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Rumanova V.S., Okuliarova M., Foppen E., Kalsbeek A., Zeman M. Exposure to dim light at night alters daily rhythms of glucose and lipid metabolism in rats. Front. Physiol. 2022;13:973461. doi: 10.3389/fphys.2022.973461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Fonken L., Weil Z., Nelson R. Dark nights reverse metabolic disruption caused by dim light at night. Obesity. 2013;21:1159–1164. doi: 10.1002/oby.20108. [DOI] [PubMed] [Google Scholar]
- 210.Masís-Vargas A., Hicks D., Kalsbeek A., Mendoza J. Blue light at night acutely impairs glucose tolerance and increases sugar intake in the diurnal rodent Arvicanthis ansorgei in a sex-dependent manner. Physiol. Rep. 2019;7:e14257. doi: 10.14814/phy2.14257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Smale L., Lee T., Nunez A.A. Mammalian Diurnality: Some Facts and Gaps. J. Biol. Rhythm. 2003;18:356–366. doi: 10.1177/0748730403256651. [DOI] [PubMed] [Google Scholar]
- 212.Mendoza J. Nighttime Light Hurts Mammalian Physiology: What Diurnal Rodent Models Are Telling Us. Clocks Sleep. 2021;3:236–250. doi: 10.3390/clockssleep3020014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Qin L.-Q., Li J., Wang Y., Wang J., Xu J.-Y., Kaneko T. The effects of nocturnal life on endocrine circadian patterns in healthy adults. Life Sci. 2003;73:2467–2475. doi: 10.1016/s0024-3205(03)00628-3. [DOI] [PubMed] [Google Scholar]
- 214.Leproult R., Holmbäck U., Van Cauter E. Circadian Misalignment Augments Markers of Insulin Resistance and Inflammation, Independently of Sleep Loss. Diabetes. 2014;63:1860–1869. doi: 10.2337/db13-1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Wefers J., van Moorsel D., Hansen J., Connell N.J., Havekes B., Hoeks J., Lichtenbelt W.D.V.M., Duez H., Phielix E., Kalsbeek A., et al. Circadian misalignment induces fatty acid metabolism gene profiles and compromises insulin sensitivity in human skeletal muscle. Proc. Natl. Acad. Sci. USA. 2018;115:7789–7794. doi: 10.1073/pnas.1722295115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Dinneen S., Alzaid A., Miles J., Rizza R. Metabolic effects of the nocturnal rise in cortisol on carbohydrate metabolism in normal humans. J. Clin. Investig. 1993;92:2283–2290. doi: 10.1172/JCI116832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Rizza R.A., Mandarino L.J., Gerich J.E. Cortisol-Induced Insulin Resistance in Man: Impaired Suppression of Glucose Production and Stimulation of Glucose Utilization due to a Postreceptor Defect of Insulin Action. J. Clin. Endocrinol. Metab. 1982;54:131–138. doi: 10.1210/jcem-54-1-131. [DOI] [PubMed] [Google Scholar]
- 218.Stunkard A.J., Allison K.C. Two forms of disordered eating in obesity: Binge eating and night eating. Int. J. Obes. 2003;27:1–12. doi: 10.1038/sj.ijo.0802186. [DOI] [PubMed] [Google Scholar]
- 219.Schiavo-Cardozo D., Lima M.M.O., Pareja J.C., Geloneze B. Appetite-regulating hormones from the upper gut: Disrupted control of xenin and ghrelin in night workers. Clin. Endocrinol. 2013;79:807–811. doi: 10.1111/cen.12114. [DOI] [PubMed] [Google Scholar]
- 220.Taylor R. Insulin Resistance and Type 2 Diabetes. Diabetes. 2012;61:778–779. doi: 10.2337/db12-0073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Donin A.S., Nightingale C.M., Owen C.G., Rudnicka A.R., Jebb S.A., Ambrosini G.L., Stephen A.M., Cook D.G., Whincup P.H. Dietary Energy Intake Is Associated With Type 2 Diabetes Risk Markers in Children. Diabetes Care. 2013;37:116–123. doi: 10.2337/dc13-1263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Zhao J., Zhang Y., Wei F., Song J., Cao Z., Chen C., Zhang K., Feng S., Wang Y., Li W.-D. Triglyceride is an independent predictor of type 2 diabetes among middle-aged and older adults: A prospective study with 8-year follow-ups in two cohorts. J. Transl. Med. 2019;17:403. doi: 10.1186/s12967-019-02156-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Gottlieb D.J., Punjabi N.M., Newman A.B., Resnick H.E., Redline S., Baldwin C., Nieto F.J. Association of Sleep Time With Diabetes Mellitus and Impaired Glucose Tolerance. Arch. Intern. Med. 2005;165:863–867. doi: 10.1001/archinte.165.8.863. [DOI] [PubMed] [Google Scholar]
- 224.Li L., Renier G. The Connection Between C-Reactive Protein (CRP) and Diabetic Vasculopathy. Focus on Preclinical Findings. Curr. Diabetes Rev. 2010;6:27–34. doi: 10.2174/157339910790442628. [DOI] [PubMed] [Google Scholar]
- 225.Ormazabal V., Nair S., Elfeky O., Aguayo C., Salomon C., Zuñiga F.A. Association between insulin resistance and the development of cardiovascular disease. Cardiovasc. Diabetol. 2018;17:122. doi: 10.1186/s12933-018-0762-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Tune J.D., Goodwill A.G., Sassoon D.J., Mather K.J. Cardiovascular consequences of metabolic syndrome. Transl. Res. 2017;183:57–70. doi: 10.1016/j.trsl.2017.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Guembe M.J., Fernandez-Lazaro C.I., Sayon-Orea C., Toledo E., Moreno-Iribas C., Cosials J.B., Reyero J.B., Martínez J.D., Diego P.G., Uche A.M.G., et al. Risk for cardiovascular disease associated with metabolic syndrome and its components: A 13-year prospective study in the RIVANA cohort. Cardiovasc. Diabetol. 2020;19:195. doi: 10.1186/s12933-020-01166-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Ridker P.M., Glynn R.J., Hennekens C.H. C-Reactive Protein Adds to the Predictive Value of Total and HDL Cholesterol in Determining Risk of First Myocardial Infarction. Circulation. 1998;97:2007–2011. doi: 10.1161/01.CIR.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 229.Emerging Risk Factors Collaboration. Kaptoge S., Di Angelantonio E., Pennells L., Wood A.M., White I.R., Gao P., Walker M., Thompson A., Sarwar N., et al. C-Reactive Protein, Fibrinogen, and Cardiovascular Disease Prediction. N. Engl. J. Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Verma S., Wang C.-H., Li S.-H., Dumont A.S., Fedak P., Badiwala M.V., Dhillon B., Weisel R.D., Li R.-K., Mickle D.A., et al. A Self-Fulfilling Prophecy. Circulation. 2002;106:913–919. doi: 10.1161/01.cir.0000029802.88087.5e. [DOI] [PubMed] [Google Scholar]
- 231.Cyr A.R., Huckaby L.V., Shiva S.S., Zuckerbraun B.S. Nitric Oxide and Endothelial Dysfunction. Crit. Care Clin. 2020;36:307–321. doi: 10.1016/j.ccc.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Förstermann U., Xia N., Li H. Roles of Vascular Oxidative Stress and Nitric Oxide in the Pathogenesis of Atherosclerosis. Circ. Res. 2017;120:713–735. doi: 10.1161/CIRCRESAHA.116.309326. [DOI] [PubMed] [Google Scholar]
- 233.Bayon V., Berger M., Solelhac G., Haba-Rubio J., Marques-Vidal P., Strippoli M.-P., Preisig M., Leger D., Heinzer R. Impact of night and shift work on metabolic syndrome and its components: A cross-sectional study in an active middle-to-older-aged population-based sample. BMJ Open. 2022;12:e053591. doi: 10.1136/bmjopen-2021-053591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Brum M.C.B., Filho F.F.D., Schnorr C.C., Bertoletti O.A., Bottega G.B., Rodrigues T.D.C. Night shift work, short sleep and obesity. Diabetol. Metab. Syndr. 2020;12:13. doi: 10.1186/s13098-020-0524-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Sun M., Feng W., Wang F., Li P., Li Z., Li M., Tse G., Vlaanderen J., Vermeulen R., Tse L.A. Meta-analysis on shift work and risks of specific obesity types. Obes. Rev. 2017;19:28–40. doi: 10.1111/obr.12621. [DOI] [PubMed] [Google Scholar]
- 236.Park J., Shin S.-Y., Kang Y., Rhie J. Effect of night shift work on the control of hypertension and diabetes in workers taking medication. Ann. Occup. Environ. Med. 2019;31:e27. doi: 10.35371/aoem.2019.31.e27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Vetter C., Devore E.E., Ramin C.A., Speizer F.E., Willett W.C., Schernhammer E.S. Mismatch of Sleep and Work Timing and Risk of Type 2 Diabetes. Diabetes Care. 2015;38:1707–1713. doi: 10.2337/dc15-0302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Yeom J.H., Sim C.S., Lee J., Yun S.H., Park S.J., Yoo C.-I., Sung J.H. Effect of shift work on hypertension: Cross sectional study. Ann. Occup. Environ. Med. 2017;29:11. doi: 10.1186/s40557-017-0166-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Manohar S., Thongprayoon C., Cheungpasitporn W., Mao M.A., Herrmann S. Associations of rotational shift work and night shift status with hypertension. J. Hypertens. 2017;35:1929–1937. doi: 10.1097/HJH.0000000000001442. [DOI] [PubMed] [Google Scholar]
- 240.Vyas M.V., Garg A.X., Iansavichus A.V., Costella J., Donner A., Laugsand L.E., Janszky I., Mrkobrada M., Parraga G., Hackam D.G. Shift work and vascular events: Systematic review and meta-analysis. BMJ. 2012;345:e4800. doi: 10.1136/bmj.e4800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Zhao Y., Lu X., Wan F., Gao L., Lin N., He J., Wei L., Dong J., Qin Z., Zhong F., et al. Disruption of Circadian Rhythms by Shift Work Exacerbates Reperfusion Injury in Myocardial Infarction. J. Am. Coll. Cardiol. 2022;79:2097–2115. doi: 10.1016/j.jacc.2022.03.370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Bae M.-J., Song Y.-M., Shin J., Choi B.-Y., Keum J.-H., Lee E.-A. The Association Between Shift Work and Health Behavior: Findings from the Korean National Health and Nutrition Examination Survey. Korean J. Fam. Med. 2017;38:86–92. doi: 10.4082/kjfm.2017.38.2.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Knutsson A., Nilsson T. Tobacco use and exposure to environmental tobacco smoke in relation to certain work characteristics. Scand. J. Soc. Med. 1998;26:183–189. doi: 10.1177/14034948980260030801. [DOI] [PubMed] [Google Scholar]
- 244.Trinkoff A.M., Storr C.L. Work schedule characteristics and substance use in nurses. Am. J. Ind. Med. 1998;34:266–271. doi: 10.1002/(SICI)1097-0274(199809)34:3<266::AID-AJIM9>3.0.CO;2-T. [DOI] [PubMed] [Google Scholar]
- 245.Kivimäki M., Kuisma P., Virtanen M., Elovainio M. Does shift work lead to poorer health habits? A comparison between women who had always done shift work with those who had never done shift work. Work. Stress. 2001;15:3–13. doi: 10.1080/02678370118685. [DOI] [Google Scholar]
- 246.Richter K., Peter L., Rodenbeck A., Weess H.G., Riedel-Heller S.G., Hillemacher T. Shiftwork and Alcohol Consumption: A Systematic Review of the Literature. Eur. Addict. Res. 2020;27:9–15. doi: 10.1159/000507573. [DOI] [PubMed] [Google Scholar]
- 247.Brum M.C.B., Filho F.F.D., Schnorr C.C., Bottega G.B., Rodrigues T.C. Shift work and its association with metabolic disorders. Diabetol. Metab. Syndr. 2015;7:45. doi: 10.1186/s13098-015-0041-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Monnaatsie M., Biddle S.J., Khan S., Kolbe-Alexander T. Physical activity and sedentary behaviour in shift and non-shift workers: A systematic review and meta-analysis. Prev. Med. Rep. 2021;24:101597. doi: 10.1016/j.pmedr.2021.101597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Knutsson A., Akerstedt T., Jonsson B.G. Prevalence of risk factors for coronary artery disease among day and shift workers. Scand. J. Work. Environ. Heal. 1988;14:317–321. doi: 10.5271/sjweh.1913. [DOI] [PubMed] [Google Scholar]
- 250.Romon M., Nuttens M.-C., Fievet C., Pot P., Bard J.M., Furon D., Fruchart J.C. Increased triglyceride levels in shift workers. Am. J. Med. 1992;93:259–262. doi: 10.1016/0002-9343(92)90230-9. [DOI] [PubMed] [Google Scholar]
- 251.Dutheil F., Baker J.S., Mermillod M., De Cesare M., Vidal A., Moustafa F., Pereira B., Navel V. Shift work, and particularly permanent night shifts, promote dyslipidaemia: A systematic review and meta-analysis. Atherosclerosis. 2020;313:156–169. doi: 10.1016/j.atherosclerosis.2020.08.015. [DOI] [PubMed] [Google Scholar]
- 252.Shechter A., Kim E.W., St-Onge M.-P., Westwood A.J. Blocking nocturnal blue light for insomnia: A randomized controlled trial. J. Psychiatr. Res. 2017;96:196–202. doi: 10.1016/j.jpsychires.2017.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Chang A.-M., Aeschbach D., Duffy J.F., Czeisler C.A. Evening use of light-emitting eReaders negatively affects sleep, circadian timing, and next-morning alertness. Proc. Natl. Acad. Sci. USA. 2014;112:1232–1237. doi: 10.1073/pnas.1418490112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hester L., Dang D., Barker C.J., Heath M., Mesiya S., Tienabeso T., Watson K. Evening wear of blue-blocking glasses for sleep and mood disorders: A systematic review. Chrono Int. 2021;38:1375–1383. doi: 10.1080/07420528.2021.1930029. [DOI] [PubMed] [Google Scholar]
- 255.Vethe D., Scott J., Engstrøm M., Salvesen Ø., Sand T., Olsen A., Morken G., Heglum H.S., Kjørstad K., Faaland P.M., et al. The evening light environment in hospitals can be designed to produce less disruptive effects on the circadian system and improve sleep. Sleep. 2020;44:zsaa194. doi: 10.1093/sleep/zsaa194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Avery D.H., Eder D.N., Bolte M.A., Hellekson C.J., Dunner D.L., Vitiello M.V., Prinz P.N. Dawn simulation and bright light in the treatment of SAD: A controlled study. Biol. Psychiatry. 2001;50:205–216. doi: 10.1016/S0006-3223(01)01200-8. [DOI] [PubMed] [Google Scholar]
- 257.Campbell P.D., Miller A.M., Woesner M.E. Bright Light Therapy: Seasonal Affective Disorder and Beyond. Einstein J. Biol. Med. 2017;32:E13–E25. [PMC free article] [PubMed] [Google Scholar]
- 258.Rosenthal N.E., Sack D.A., Gillin J.C., Lewy A.J., Goodwin F.K., Davenport Y., Mueller P.S., Newsome D.A., Wehr T.A. Seasonal Affective Disorder. Arch. Gen. Psychiatry. 1984;41:72–80. doi: 10.1001/archpsyc.1984.01790120076010. [DOI] [PubMed] [Google Scholar]
- 259.Terman J.S., Terman M., Lo E.-S., Cooper T.B. Circadian Time of Morning Light Administration and Therapeutic Response in Winter Depression. Arch. Gen. Psychiatry. 2001;58:69–75. doi: 10.1001/archpsyc.58.1.69. [DOI] [PubMed] [Google Scholar]
- 260.Cibeira N., Maseda A., Lorenzo-López L., González-Abraldes I., López-López R., Rodríguez-Villamil J.L., Millán-Calenti J.C. Bright Light Therapy in Older Adults with Moderate to Very Severe Dementia: Immediate Effects on Behavior, Mood, and Physiological Parameters. Healthcare. 2021;9:1065. doi: 10.3390/healthcare9081065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Al-Karawi D., Jubair L. Bright light therapy for nonseasonal depression: Meta-analysis of clinical trials. J. Affect. Disord. 2016;198:64–71. doi: 10.1016/j.jad.2016.03.016. [DOI] [PubMed] [Google Scholar]
- 262.Elliott J.E., McBride A.A., Balba N.M., Thomas S.V., Pattinson C.L., Morasco B.J., Wilkerson A., Gill J.M., Lim M.M. Feasibility and preliminary efficacy for morning bright light therapy to improve sleep and plasma biomarkers in US Veterans with TBI. A prospective, open-label, single-arm trial. PLoS ONE. 2022;17:e0262955. doi: 10.1371/journal.pone.0262955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Korman M., Palm D., Uzoni A., Faltraco F., Tucha O., Thome J., Coogan A.N. ADHD 24/7: Circadian clock genes, chronotherapy and sleep/wake cycle insufficiencies in ADHD. World J. Biol. Psychiatry. 2018;21:156–171. doi: 10.1080/15622975.2018.1523565. [DOI] [PubMed] [Google Scholar]
- 264.van Andel E., Bijlenga D., Vogel S.W.N., Beekman A.T.F., Kooij J.J.S. Effects of chronotherapy on circadian rhythm and ADHD symptoms in adults with attention-deficit/hyperactivity disorder and delayed sleep phase syndrome: A randomized clinical trial. Chrono Int. 2020;38:260–269. doi: 10.1080/07420528.2020.1835943. [DOI] [PubMed] [Google Scholar]
- 265.Laposky A.D., Bass J., Kohsaka A., Turek F.W. Sleep and circadian rhythms: Key components in the regulation of energy metabolism. FEBS Lett. 2007;582:142–151. doi: 10.1016/j.febslet.2007.06.079. [DOI] [PubMed] [Google Scholar]
- 266.Rumanova V.S., Okuliarova M., Zeman M. Differential Effects of Constant Light and Dim Light at Night on the Circadian Control of Metabolism and Behavior. Int. J. Mol. Sci. 2020;21:5478. doi: 10.3390/ijms21155478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Froy O. Metabolism and Circadian Rhythms—Implications for Obesity. Endocr. Rev. 2009;31:1–24. doi: 10.1210/er.2009-0014. [DOI] [PubMed] [Google Scholar]
- 268.Asher G., Sassone-Corsi P. Time for Food: The Intimate Interplay between Nutrition, Metabolism, and the Circadian Clock. Cell. 2015;161:84–92. doi: 10.1016/j.cell.2015.03.015. [DOI] [PubMed] [Google Scholar]
- 269.Rana S., Prabhu S.D., Young M.E. Chronobiological Influence Over Cardiovascular Function. Circ. Res. 2020;126:258–279. doi: 10.1161/CIRCRESAHA.119.313349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Bray M.S., Shaw C.A., Moore M.W.S., Garcia R.A.P., Zanquetta M.M., Durgan D.J., Jeong W.J., Tsai J.-Y., Bugger H., Zhang D., et al. Disruption of the circadian clock within the cardiomyocyte influences myocardial contractile function, metabolism, and gene expression. Am. J. Physiol. Circ. Physiol. 2008;294:H1036–H1047. doi: 10.1152/ajpheart.01291.2007. [DOI] [PubMed] [Google Scholar]
- 271.Arble D., Bass J., Laposky A.D., Vitaterna M.H., Turek F.W. Circadian Timing of Food Intake Contributes to Weight Gain. Obesity. 2009;17:2100–2102. doi: 10.1038/oby.2009.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Challet E. The circadian regulation of food intake. Nat. Rev. Endocrinol. 2019;15:393–405. doi: 10.1038/s41574-019-0210-x. [DOI] [PubMed] [Google Scholar]
- 273.Acosta-Rodríguez V., Rijo-Ferreira F., Izumo M., Xu P., Wight-Carter M., Green C.B., Takahashi J.S. Circadian alignment of early onset caloric restriction promotes longevity in male C57BL/6J mice. Science. 2022;376:1192–1202. doi: 10.1126/science.abk0297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Grigg-Damberger M.M., Ianakieva D. Poor Quality Control of Over-the-Counter Melatonin: What They Say Is Often Not What You Get. J. Clin. Sleep Med. 2017;13:163–165. doi: 10.5664/jcsm.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Erland L., Saxena P.K. Melatonin Natural Health Products and Supplements: Presence of Serotonin and Significant Variability of Melatonin Content. J. Clin. Sleep Med. 2017;13:275–281. doi: 10.5664/jcsm.6462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Li J., Somers V.K., Xu H., Lopez-Jimenez F., Covassin N. Trends in Use of Melatonin Supplements Among US Adults, 1999–2018. JAMA. 2022;327:483–485. doi: 10.1001/jama.2021.23652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.National Institutes of Health (NIH) Use of Melatonin Supplements Rising Among Adults. [(accessed on 13 January 2023)];2022 Available online: https://www.nih.gov/news-events/nih-research-matters/use-melatonin-supplements-rising-among-adults.
- 278.Garde A.H., Begtrup L., Bjorvatn B., Bonde J.P., Hansen J., Hansen Å.M., Härmä M., Jensen M.A., Kecklund G., A Kolstad H., et al. How to schedule night shift work in order to reduce health and safety risks. Scand. J. Work. Environ. Health. 2020;46:557–569. doi: 10.5271/sjweh.3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 279.der Holst H.L.-V., Murphy A., Wise J., Duffy J. Sleep tips for shift workers in the time of pandemic. Southwest J Pulm Crit Care. Southwest J. Pulm. Crit. Care. 2020;20:128–130. doi: 10.13175/swjpcc024-20. [DOI] [PMC free article] [PubMed] [Google Scholar]