Abstract
Despite substantial efforts dedicated to the development of novel, non-addictive analgesics, success has been limited. Clinically available agents generally lack efficacy, creating pharmacoresistance, and are accompanied by undesirable side-effects. The traditional target-based drug discovery effort, while generating compounds with selectivity for single targets, has a high rate of attrition due to poor clinical efficacy. Here, we examine the problems associated with the current analgesic drug discovery model, and review evidence for a new approach for the identification of analgesic targets and compounds for the diverse forms of acute and chronic pain. Using human iPSC-derived nociceptors, genes selectively expressed in nociceptors, but not in other excitable cell types, can be identified, disease states can be modeled, phenotypic screens can identify compounds that selectively reduce nociceptor excitability and individual patient susceptibility to developing pain or responding to particular treatments can be identified. This human cell/genetics-based approach can identify nociceptor-specific and pain-disease selective compounds that modulate the function of multiple targets. This polypharmacology approach will enhance analgesic efficacy by broadening drug activity on complex pain mechanisms, while selective action on nociceptors will curb side-effects.
What is pain?
Although we use a single word, pain, to describe a broad range of unpleasant sensations that comprise feelings of soreness coupled with discomfort and unpleasantness, pain itself is complex and can be initiated by a range of quite distinct conditions. It is important to recognize this, as each pain condition may well require different therapeutic interventions(1, 2). There are aspects of pain that are truly physiological, our capacity to detect potentially damaging external stimuli: intense heat or cold, excessive mechanical force, and chemical irritants. This constitutes nociceptive pain, which is driven by the activation of high threshold nociceptor sensory neurons adapted to transduce such noxious stimuli into an inflow of sensory input into the central nervous system (CNS), where the acute pain the nociceptors evoke through activation of nociceptive circuits, helps us learn to avoid environmental danger(3–6). This pain is highly adaptive and essential for preventing damage in our daily interactions with the external world. Individuals who lack this damage warning system, such as those with congenital insensitivity to pain due to loss of function mutations in the Nav1.7 voltage-gated sodium channel or the TrkA receptor, typically damage their tongues and lips when they eat, their toes on walking, and fingers when exploring objects, and have no warning when they break a limb or have appendicitis, which reduces life expectancy(7, 8). Preserving nociceptive pain is essential, therefore, except during or post-surgery or immediately after major trauma.
Another adaptive pain is the inflammatory pain that occurs when tissue injury cannot be avoided or when inflammation occurs due to a pathogen invasion or pathological inflammation. Activation of the immune system in these cases leads to production of inflammatory mediators that act on nociceptors to both directly activate them(9–11) and sensitize them(12, 13), such that their threshold of activation drops so that low-intensity stimuli, such as light touch or movement of a joint, can now activate sensitized nociceptors, and these innocuous stimuli are now painful. This pain, at least in an acute setting, is adaptive in that the heightened pain and threshold drop promotes avoiding use of or contact with the inflamed area, which promotes healing and repair, however this pain typically needs to be reduced to a manageable level. In addition to the pain produced by the activation and sensitization of nociceptors, the augmented sensory inflow triggers use-dependent plasticity in the CNS, the phenomenon of central sensitization, that amplifies and spreads pain sensitivity beyond the primary area of damage/inflammation to neighboring secondary areas of non-inflamed areas, causing, for example, tenderness around a surgical wound or an inflamed joint, and prolonging the pain(14, 15).
For chronic inflammatory conditions, like rheumatoid arthritis, the ongoing inflammatory pain is not adaptive since the inflammation does not resolve and healing does not occur. In these situations, the combined chronic inflammation and pain constitute the clinical problem to be controlled, and the pain can be considered maladaptive or pathological(16). Finally, there are two categories of truly maladaptive pain, where the pain is not a reaction to some pathology but a disease state itself. The first of these is neuropathic pain, persistent pain due to lesions, most typically of the peripheral nervous system (PNS) (nerve injuries or neuropathies)(17, 18), but also of the CNS (after spinal cord injury or stroke)(19) that lead to pathological excitability in nociceptive circuits due to ectopic firing of injured neurons, a loss of the normal inhibitory circuits in the CNS - the phenomenon of disinhibition, neuroimmune interactions in the nerve and CNS, and structural and functional connectivity changes in the spinal cord and brain that alter circuit function and output(20–23).
The last major form of pain is dysfunctional or nociplastic pain, defined as chronic pain in the absence of noxious stimuli, active inflammation, or detectable damage to the nervous system(24–26). In individuals with this pain, there is abnormal functioning of the nervous system such that it amplifies and sustains essentially normal signals to a point at which they are perceived as being painful. The pain may localize to a particular organ, as in tension-type headaches, temporomandibular joint disease or irritable bowel syndrome, or may be diffuse as in fibromyalgia, a form of chronic widespread pain(27).
In addition to these quite distinct types of pain, we need to recognize that pain arising from the somatosensory system (skin/joints/muscle) may differ from that from visceral organs(28, 29), and that acute pain typically differs considerably from chronic pain in terms of mechanisms and response to analgesics(30). Furthermore, pain has several quite different manifestations, which reflect different mechanisms. Pain may arise spontaneously in the absence of a stimulus or detectable pathology or may need a stimulus to evoke it. Spontaneous pain is a major element of many clinical conditions, particularly neuropathic pain(31). Stimulus evoked pain includes exaggerated and prolonged pain in response to noxious stimuli, the phenomenon of hyperalgesia, as well as pain in response to normally innocuous mechanical or thermal stimuli, tactile or thermal allodynia(32). There is of course, a major difference between symptom suppression and disease modification in the setting of pain, the former requires ongoing treatment to reduce the experience of pain, while the latter has the potential to have long-lasting consequences, for example, interventions that can prevent the transition of acute to chronic pain or the development of axonal neuropathy, and hence neuropathic pain. To date, there are only symptom suppressor analgesic interventions.
We need to recognize the diverse nature and the multiple distinct specific mechanisms responsible for different pain conditions to be able to select those interventions with the greatest chance of efficacy. There is no point, for example, in blocking an inflammatory mediator or targeting a protein only induced by neuronal damage if a patient’s pain occurs in the absence of any inflammation or with a fully intact nervous system. We need to build an algorithm that enables identification of the most likely responders to particular therapies that act on distinct mechanisms, rather than relying on an empirical trial and error approach, as currently used. Such a precision pain medicine approach requires parallel efforts to 1. Understand and fully reveal all the mechanisms underlying different pains, 2. Define how to identify which mechanisms are operational in individual patients by careful phenotyping and utilization of biomarkers, and 3. The provision of a range of different therapies that interact specifically with each of the distinct mechanisms. To achieve the goal of developing analgesics with defined selective actions in particular patients will require an approach quite different from that conventionally used for the development of analgesic therapies. The nature of such a drug development approach and how to realize it using human stem cell-derived neuronal assays and screens, specifically for selectively targeting nociceptors, is the focus of this review.
Current pharmacological treatment
The current repertoire of analgesics (Figure 1) is limited to only a few of the defined types of acute or chronic pains and their underlying mechanisms, and overall have several major problems; generally low efficacy and multiple adverse effects, and for opioids, tolerance requiring increasing doses, dependency with withdrawal on discontinuation, and abuse liability(33–35).
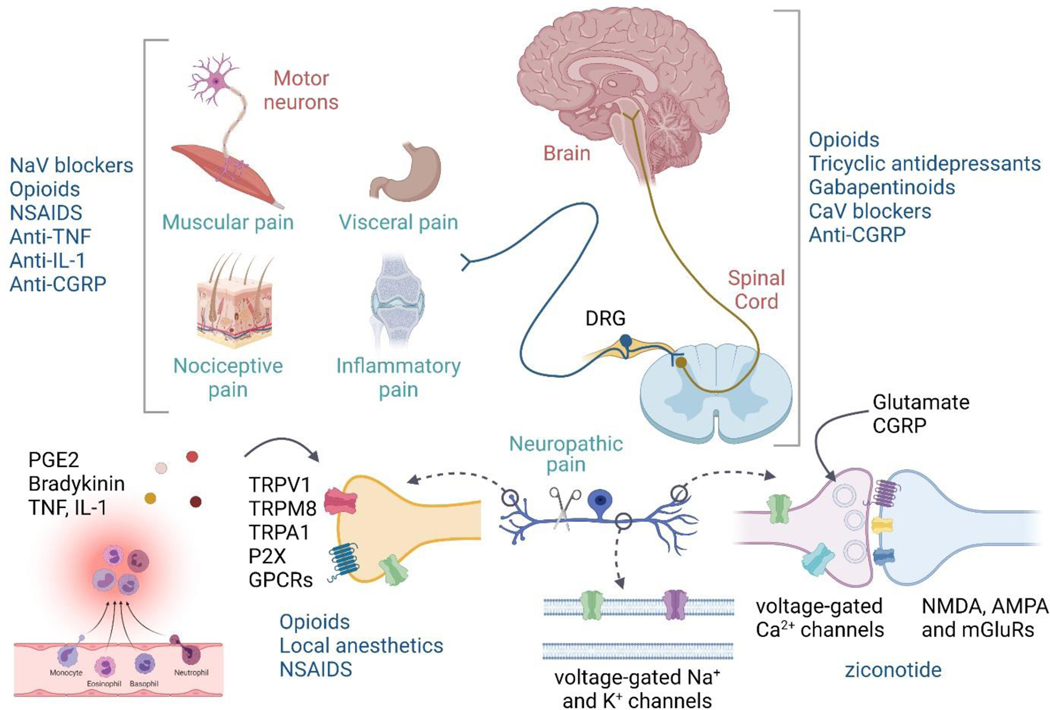
Fig. 1: Site of action of current analgesics.
Clinically used analgesics act on diverse targets in the central and peripheral nervous system contributing to their lack of efficacy and numerous side-effects, highlighting the need for nociceptor-selective targets.
Available analgesics operate in the following ways: 1. Block electrical activity in neurons with non-selective sodium channel blockers – either as local anesthetics such as lidocaine or bupivacaine, or systemic sodium channel blockers, like carbamazepine, mexiletine, lacosamide, and amitriptyline(36). The lack of selectivity means that all excitable cells exposed to the blockers will be impacted such that the therapeutic index is limited, and adverse effects due to activity on non-nociceptor neurons are inevitable. 2. Block the action of inflammatory mediators on nociceptors – most notably cyclooxygenase inhibiting NSAIDs, which inhibit PGE2 synthesis(37, 38), as well as anti-TNF or anti-IL1 antibodies in particular inflammatory conditions(12, 39). Cox2 is induced in macrophages during inflammation and therefore Cox inhibitors are only effective in reducing the sensitization of nociceptors in those conditions where Cox activity is a major driver of this(40–42). In the context of postoperative pain relief, the NSAIDS diclofenac, ibuprofen and naproxen only provide at best 50% maximum pain relief for 4–6 hrs(43) but reduce opioid use with moderate success(44–46). Also, inhibiting PGE2 production during inflammation, although a widely used analgesic strategy, has several disadvantages on prolonged use, including bleeding, GI and CVS issues(47). Combination therapy using lower doses of NSAIDS together with acetaminophen has proven beneficial(48). TNF-α levels are elevated in inflammatory conditions and anti-TNF therapies can be used for treatment of rheumatoid arthritis, Crohn’s disease and psoriasis(49). Anti-TNF antibodies reduce cytokine production in joints, particularly, IL-1, IL-6, and TNF itself(50). Injectable therapies include soluble TNF receptors such as etanercept and PEG-sTNFR1, or TNF-α antibodies such as infliximab, adalimumab, and CDP-870. Small molecule inhibitors of TNF-α synthesis, such as p38 MAP kinase and PDE4 inhibitors are also used(51, 52). However, all these options are associated with side effects(51), with increased risk of congestive heart failure and resurgence of latent tuberculosis. Anti-IL-1 therapies are also used for the treatment of specific inflammatory conditions. IL-1 blockers such as anakinra, rilonacept, or canakinumab, provide relief from broad-spectrum inflammatory diseases including rheumatoid arthritis, osteoarthritis, osteomyelitis, traumatic joint injury, systemic juvenile idiopathic arthritis and gout. However, suppressing innate immune activity increases the risk of bacterial infections(53). 3. Reduce synaptic transmission from nociceptors to dorsal horn neurons, but the action of the drugs used for this purpose are not restricted to this, and therefore they block transmission quite broadly in the CNS, causing adverse effects. Such blockers include mu opiate receptor opioid agonists(54), alpha2delta calcium channel subunit agonists - the gabapentinoids(55, 56), the Cv2.2 inhibitor ziconotide(57, 58), and for migraine, CGRP receptor antagonists(59, 60). While most options for CGRP are monoclonal antibodies, a small molecule antagonist for its receptor is also proving to be equally effective(61). The expression of the alpha2delta calcium channel subunit increases substantially in DRG neurons after nerve injury(62, 63) explaining why GABApentin and pregabalin, which bind to it, have efficacy in neuropathic pain. Pregabalin is also used to treat nociplastic pain(64). 4. Promote inhibition in the CNS by mimicking the activity of inhibitory transmitters, again at present with no selectivity of action for nociceptive circuits, this includes dual amine uptake inhibitors like duloxetine(64–66), opioids(67), and GABA mimetics, like the benzodiazepines. Specific GABA-A receptor subtypes expressed in lamina II inhibitory interneurons of the spinal dorsal horn have been linked to analgesia. However, the sedative effects of the available non-selective allosteric modulators, such as benzodiazepines, dimmish therapeutic potential as analgesics(68–72). The highly conserved nature of the transmembrane domains of ion channel families, and GPCR receptor subtypes, pose a significant problem in identifying subtype-selective allosteric pockets for generation of subtype selective molecules. Efficacy-selective molecules rather than affinity-selective ones might offer a partial solution to this problem(73). 5. Finally, there are drugs that can reduce use-dependent plasticity in CNS: especially NMDAR antagonists like ketamine, which reduce synaptic plasticity(74), but once again the actions are not limited to changes in nociceptive circuits and there are in consequence adverse effects(75). Timing of treatment needs to be considered, since in some situations, pre-emptive treatment can be more effective than treating established pain(76, 77).
The two major problems with existing therapies are then, first, their non-selectivity (e.g. sodium channel blockers act on all excitable cell types, while NMDA and MOR receptors are widely distributed in the CNS). This reduces therapeutic index and means that analgesia is accompanied by undesired actions. These non-selective drugs have multiple non-pain-related actions (e.g. opioids reduce activity in nociceptive circuits but also generate euphoria, respiratory depression and constipation; gabapentinoids, reduce cognition and produce sedation, as well as a reduction in neuropathic hyperactivation, NMDA receptor antagonists have psychotropic actions, as well as reducing central sensitization. The second problem is the drugs available often only block one of many parallel pain triggering signals e.g. Cox inhibitors only block PGE2 production but there are other inflammatory mediators (IL1 or TNF, plus others) that will continue to act on nociceptors in the presence of Cox2 inhibition, substantially reducing efficacy. There is, therefore, an enormous need to both improve selectivity and broaden action to improve efficacy and reduce adverse effects.
There are targets that have some promise to fill some of these gaps that have moved toward clinical development. These include TRP channel antagonists (TRPV1 and TRPA1) to block nociceptor transduction(78, 79), inhibitors of acid-sensitive ion channels(80) or purinergic receptors(81, 82); anti-NGF antibodies to neutralize the pain-promoting actions of NGF produced by immune cells(83, 84); JAK/STAT pathway inhibitors as nociceptive cytokine signaling inhibitors(85), K channel openers to suppress neuronal excitability(86, 87); Nav selective blockers that act only on sodium channels expressed by nociceptors (Nav1,7 and Nav1.8 blockers)(88, 89); GABA receptor subunit-specific compounds(90, 91), inhibitors of tetrahydrobiopterin synthesis(92), angiotensin type 2 receptors(93), and attempts to modify opioid action on opiate receptors to retain analgesia but reduce euphoria/tolerance/dependence/respiratory depression(94–97). Some have been abandoned because of failure in the clinic and some are still progressing(34), but none have yet resulted in a new drug.
Current obstacles in pain treatment
The obstacles for the development of effective pain treatments that have not yet been overcome include then, the very limited range of targets/mechanisms that available analgesics act on, resulting in pharmacoresistance – where administered analgesic drugs are commonly ineffective in patients – current FDA approved drugs typically only are clinically effective (>50% reduction in pain) in a minority of chronic pain patients. A useful metric of this is the number needed to treat (NNT) which defines how many patients need to be given a particular treatment to see a clinically meaningful reduction in pain. Ideally, this should be one, but usually is from 5 to 20 for chronic pain conditions(98, 99). Apart from low efficacy across the pain spectrum most available analgesics also have a low persistence of activity when used chronically including both opioids and NSAIDS(100, 101).
Targeting nociceptors for cell selectivity
Like all neurons, nociceptors express dozens of different kinds of sodium, potassium and calcium ion channels that together regulate their excitability. However, the expression levels of particular ion channels in primary mouse nociceptors are substantially different from those in other kinds of neurons, even when compared with other primary sensory neurons(102). The unique pattern of expression of ion channels in nociceptors results in distinct intrinsic electrical properties compared to other neurons. It should be possible to exploit these differences to differentially inhibit/modulate the excitability of nociceptors, with minimal inhibition of other kinds of neurons. So far, such efforts have largely focused on a “magic bullet” approach of developing highly selective blockers for either Nav1.7 and Nav1.8 channels, which are predominantly expressed in nociceptors, but these efforts have been disappointing, so far. It may well be that selectively targeting any single ion channel will only have limited efficacy given that excitability is driven by activity in multiple channels and the capacity for compensation in response to inhibition of a single channel. It is noteworthy that almost all ion channel-targeted drugs currently effective in clinical practice are “dirty” in the sense that they inhibit many different ion channels; for example, carbamazepine and phenytoin used to control hyperexcitability in epilepsy not only target almost all subtypes of voltage-gated sodium channels with similar efficacy but also have effects on many kinds of calcium and potassium channels(103). The efficacy of these drugs was established without knowledge of their full target profile, but we now realize that the overall electrical activity of neurons has a complex non-linear dependence on the dozens of different ion channel types they express, present in different combinations and proportions in different kinds of neurons. With this knowledge, we can set out to analyze and model the overall excitability of different neurons with the goal of more rationally identifying that particular combination of targets required for optimal differential cell-selective effects. Then, it should be possible to deliberately screen for compounds with actions only on the identified multiple specific targets - a “magic shotgun” approach, similar to that proposed for improved GPCR-targeted drugs for mood disorders(104).
An additional consideration is that many ion channels have distinctive patterns of expression in different regions of nociceptors – peripheral axon terminals, axons in the nerve or dorsal root, and central axon terminals – than in the cell bodies in the DRG where electrical properties are commonly studied. Our knowledge of the expression of individual ion channels in nerve endings, axons, and central terminals, is unfortunately very limited, because even substantial channel densities are difficult to detect using immunocytochemistry. The most detailed knowledge of regional expression, in the case of sodium channels, is currently based on the differential sensitivity of transduction, action potential propagation and transmission to local TTX administration, revealing that specific populations of nociceptors differ widely in the extent to which TTX, or more specific sodium channel blockers, either inhibit transduction at terminals or the propagation of action potentials. This has led to the conclusion that because Nav1.7 and Nav1.8 channels are differentially important for the generation and the propagation of action potentials respectively, in various nociceptors “a dual-targeting strategy of peripheral Nav1.7 and Nav1.8 inhibition would be more effective in providing analgesia” than either alone(105). While this could be done by combining selective Nav1.7 and Nav1.8 inhibitors, an alternative approach would be to find single compounds with such dual-target action, and with no or minimal effects on other neuronal, cardiac, or skeletal muscle sodium channels, or even compounds with a broader profile on more ion channels.
Identification of the cell-region specific expression of all ion channels in nociceptors will be really valuable, particularly for the many types of potassium channels important for regulating the excitability of nociceptors. There are compounds that can both inhibit sodium channels and enhance several types of potassium channels, like riluzole, used for treating ALS(106, 107), so it is not far-fetched to search for and generate single agents that are targeted specifically at multiple sodium, potassium or calcium channels important for different aspects of nociceptor function, and the changes in these that occur during inflammation or after nerve lesions. Potassium channels with substantial expression in nociceptors include Kv3.4, BK, Kv7 and TREK channels(102, 108), and Kv7 enhancing compounds are effective in multiple pain models(108, 109). In general, the pharmacology of potassium channel enhancement is an underdeveloped area, but one of future promise for targeting neurons in a cell-selective way, especially because of the widely different patterns of potassium channel expression in different kinds of neurons. Overall, a polypharmacology approach towards acting on that set of ion channels that determine nociceptor activity in health or disease, should both increase efficacy over single target-selective drugs, and because of cell selectivity, reduce the adverse effects inherent in non-selective drugs. There are two ways potentially to do this, either identify compounds with the desired activity on the identified set of targets by parallel screening of each channel separately, or screen directly for compounds (single-agent or in a combinatorial fashion) that have the desired excitability modulating action on nociceptors but not on other excitable cells, using iPSC-derived neuronal phenotypic screens.
New approaches to analgesic development can increasingly be based on the optimal utilization of human stem cell-derived neurons for disease modeling, cell type and disease state-specific screening, and identification of patient susceptibility to particular disease states, like neuropathy, or responsiveness to particular treatments(110–117). Three therapeutic approaches may be useful to explore. First, ways to block inputs to nociceptors need to be identified, to enable reducing either the activation or the sensitization of nociceptors in health, inflammatory conditions or after nerve lesions. Second, there is a need to block action potential transmission selectively only in nociceptors, using drugs that silence these neurons with no or minimal activity on other cells, to block pain-triggering signals entering into the CNS. The strategy of using permanently charged sodium channel blockers that only enter neurons through activated large pore ion channels, which are primarily only expressed by nociceptors, generates long-duration selective silencing of nociceptors, but can only be administered topically(118, 119). Third, tools are needed to selectively block activity only in nociceptive circuits in the CNS, by either reducing excitation or increasing inhibition and preventing/reversing maladaptive plasticity only in these circuits in the spinal cord or brain(15, 120–122). However, given the similarity of nociceptive circuits to many others in the CNS, it is likely the third approach will be the most challenging, and we will therefore focus here only on the first two approaches, reducing the activation/sensitization of nociceptors and selectively silencing them.
Developing nociceptor specific therapies - Identifying targets
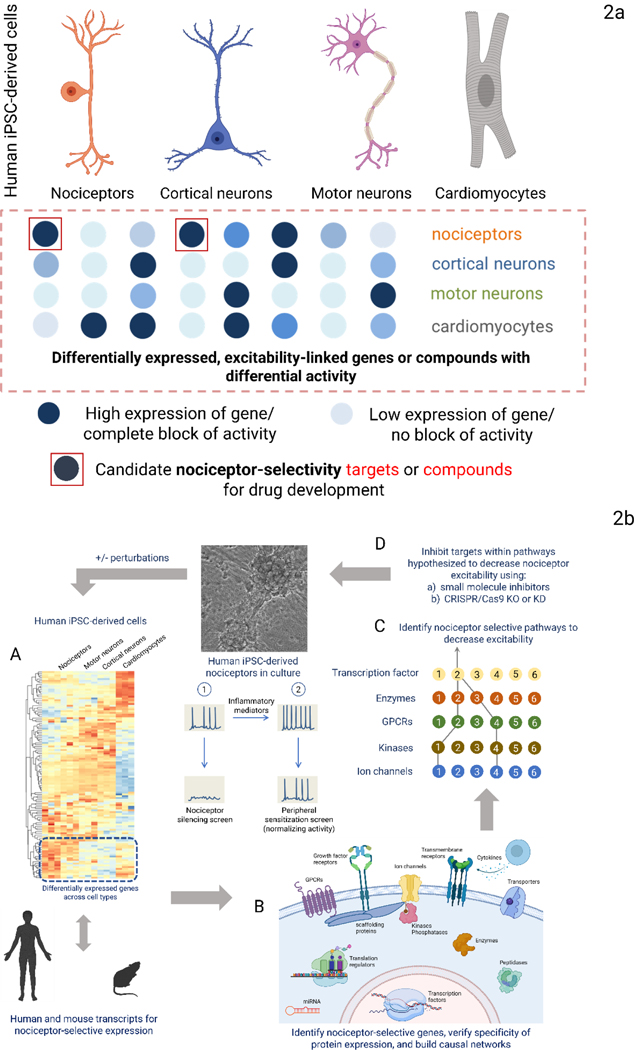
We propose two complementary approaches to identify novel pain targets. 1) Use differential gene and protein expression analyses to identify targets selectively expressed in nociceptors or those cells (e.g. immune cells or glia) linked to increased excitability in sensory neurons, and 2) use phenotypic screens of human iPSC-derived excitable cells against annotated libraries of compounds whose mechanism of action and targets are known, to identify those that selectively inhibit nociceptor excitability while sparing other excitable cells – such hits will reveal targets driving nociceptor-specific function (Figure 2a).
Figure 2:
Fig. 2a: Two complementary approaches to identify novel analgesic targets. Human iPSC-derived nociceptors can be used to identify novel analgesic targets. In one approach, differential gene expression in nociceptors is identified. In the second, an annotated library of compounds, whose primary targets of action are known, are screened for decreases in activity on multiple cell types. The annotated targets of compounds that selectively only inhibit nociceptor activity are potential pain targets. Fig. 2b: Identifying and validating candidate nociceptor-selective targets and pathways. Genes selectively expressed in iPSC-derived nociceptors can be identified (A). A similar comparison of primary mouse and human tissues will yield sets of genes selectively expressed in nociceptors for each species. These can be assembled into signaling pathways (B) predicted to affect nociceptor excitability selectively (C) and represent potential translatable target sets. Nociceptor-selective targets can be validated using small molecules that selectively modulate the function of targets or through CRISPR/Cas9 editing (D). The process can be repeated in the presence of inflammatory mediators, chemotherapeutic agents, or after axon injury to discover selective targets for each condition. Reporters of intracellular signaling can be used to garner information on pathways activated by disease perturbations.
The first approach will yield a list of gene transcripts and proteins selectively expressed in nociceptors and not in other neurons or excitable cells – using both human tissue samples and iPSC-derived cells (Figure 2b). Nociceptor selective proteins can then be assembled into causal networks that reveal signaling pathways that modulate excitability(123). From this approach, the best combination of network nodes predicted to diminish or block nociceptor activity can be determined and tested to establish if there is selective modulation of nociceptor excitability. Targeting selective pathways instead of a single protein could provide a polypharmacology approach to modulating nociceptor excitability(124–127).
Single-cell/nuclei RNA-seq enables the determination of the repertoire of genes expressed in nociceptors under physiological conditions, in response to disease-based manipulations, or as a result of the action of a drug. While some attempts have been made to identify targets enriched in primary human DRGs(128) relative to those expressed in other human cell types(129), single-cell profiling analyses are required to define exactly the human nociceptor transcript profile. Identifying nociceptor targets contributing to inflammatory and neuropathic pain will require access to DRG from patients with these conditions, which is a major challenge, especially for getting a temporal profile of induced changes. Data generated from human iPSC-derived neurons may provide an alternative means to identify inflammatory and neuropathic pain targets in a human “disease-in-a-dish” model system by detecting changes in gene expression under various pain-related conditions. A comparison of phenotypes and transcription profiles in nociceptors from individual patients in response to disease-related challenges may also assist in the identification of the drivers of disease susceptibility. Why do some patients with diabetes or on exposure to chemotherapy agents develop painful neuropathy and others do not? Phosphoproteomics can add an annotation to pathways of interest and help identify which nociceptor selective pathways actively impact excitability(130). While mouse DRG single-cell gene expression datasets may have some utility, there may be species differences both in cell identity and the response to inflammation/injury(102, 131–133). Comparing primary human and mouse nociceptor selective genes with iPSC-derived nociceptor selective genes will help determine how far iPSC-derived cells recapitulate pain-triggering pathways.
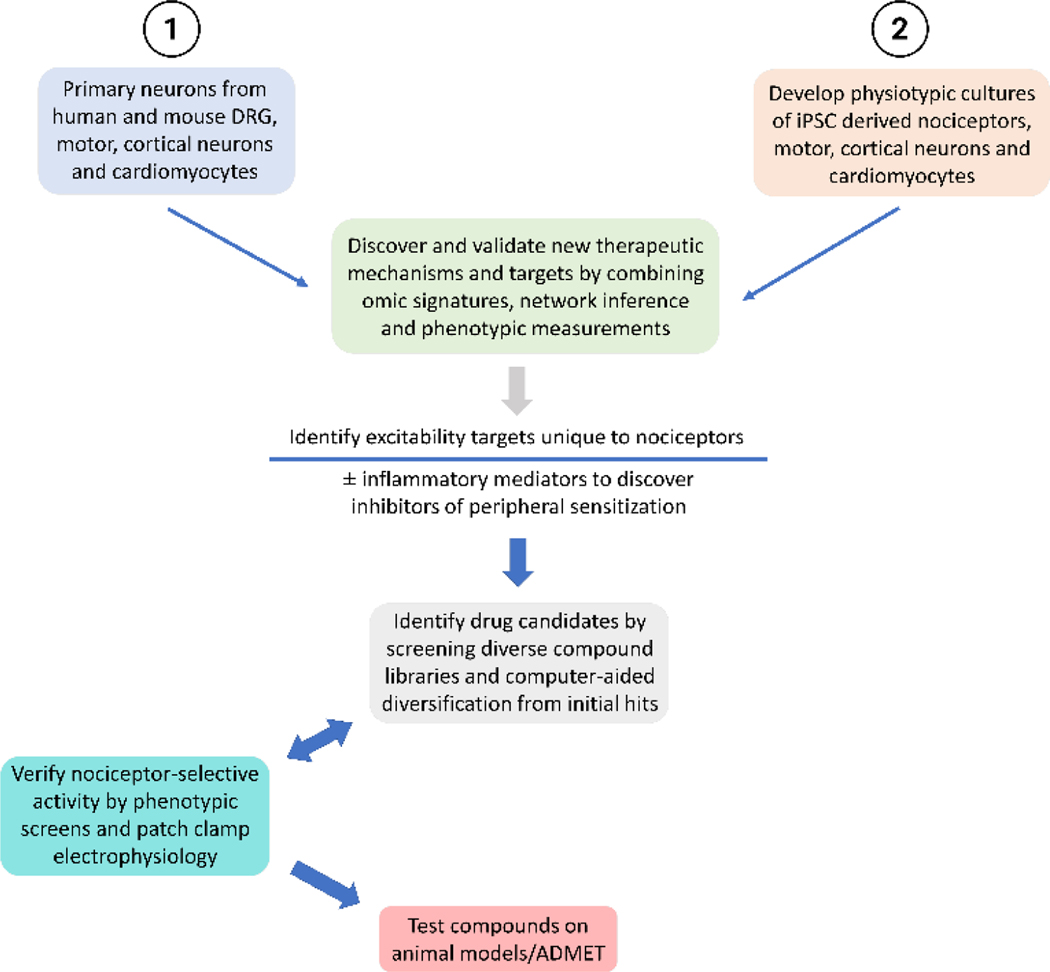
A phenotypic screen of bioactive compounds that yield hits that selectively modulate nociceptor excitability will enable deconvolution of a set of targets can then be and then tested in a primary mouse or human nociceptors (Figure 3).
Figure 3: Phenotypic screen-based discovery platform.
Schematic of discovery platform for novel pain targets using omics-based nociceptor selective targets and phenotypic screen-based compounds that decrease nociceptor excitability. The screen can be modified to discover targets that prevent peripheral sensitization by conducting phenotypic screens after nociceptors have been sensitized with inflammatory mediators. Validated nociceptor selective targets can be screened against diversity chemical libraries to identify scaffolds that modulate the activity of desired sets of targets. Structural and drug-like properties of these compounds can be enhanced and diversified using machine language assisted algorithms. New molecules designed from lead scaffolds can be tested for selective activity on nociceptor excitability by phenotypic screens and patch-clamp electrophysiology. Promising compounds with desired target modulation profile can be tested on preclinical animal models for efficacy, ADMET properties and safety.
Compounds that completely and selectively block action potential generation only in nociceptors will reveal targets for managing nociceptive pain. Compounds that decrease action potential firing only when nociceptors are treated with inflammatory mediators will reveal targets useful in managing inflammatory pain. Targets for neuropathic conditions can be identified by identifying compounds that diminish nociceptor excitability on laser cutting of axons, treatment with chemotherapeutic agents or other forms of axon injury. Genome-wide genetic editing screens in iPSC-derived neurons, could provide a more direct way to identify targets, providing the false positive and negative rate is small enough, and the genetic mutations do not affect the iPSC function or the differentiation into nociceptors. CRISPR/Cas9 is the method of choice for site-specific, inducible and reversible genetic manipulation(113, 134–139).
Once suitable targets are identified, their activity profile needs to be verified, either by using available small molecules selective for these targets or CRISPR/Cas9 gene KO or siRNA knockdown approaches, looking for nociceptor selectivity and disease normalization, with no activity in counter screens on other neurons/cells. Following such validation, heterologous expression systems can then be used to identify chemical entities that selectively modulate the function of the validated target in HEK or CHO cell lines. Large chemical diversity libraries can be screened against selected ion channel, GPCR or kinase targets to identify chemical scaffolds as starting material for medicinal chemistry campaigns for structure-activity relationship studies and ensuring drug-like properties. Target-based screening has the technical advantage that the screening assay is relatively straightforward and the signal typically robust (high Z-prime), a limitation though is that the activity of a target in a non-neuronal heterologous expression system may not match that in nociceptors because of a lack of post-translational processing or absence of interacting proteins that create a particular molecular architecture in native cells.
In vivo preclinical validation of putative targets is critical, if possible – if there is no species difference in target expression. Rodent pain models that are used for this need though to be accurate surrogates of human pain conditions and their response to specific interventions and measurements need to unbiased, specific and sensitive – something now possible using machine learning.
Using human genetic studies as the primary identifier of potential analgesic targets – where a loss of function mutation causes a clear pain phenotype(105), target-based screening has to date largely focused on developing single target-specific compounds for treating pain, e.g. Nav1.7 blockers(140). However, there is a high failure rate of target-based drug discovery in clinical trials, mainly due to lack of efficacy(141). Despite major efforts by multiple companies, Nav1.7 blockers have repeatedly failed in the clinic(142). It may be time to consider alternative strategies.
Cell and disease-based phenotypic screening using iPSC derived nociceptors
Unbiased phenotypic screens can be designed to identify compounds that act selectively only on a particular cell (e.g. silence only nociceptors) or change a specific disease-related phenotype (reduce inflammatory sensitization or neuropathic ectopic firing in nociceptors), something much more complex than interacting with a single target Figures 2 & 3). Nevertheless, recent advances in iPSC technology (Figure 4) now enable the design of disease-related phenotypic assays in physiologically relevant systems using human/patient cells(143). In addition, a hit in such assays may act on multiple targets and signal pathways, and such polypharmacology might well generate better efficacy in vivo than a compound that acts exclusively on one target.
Figure 4: Human iPSC technology in target discovery and translational applications.
Patient-derived somatic cells (e.g. skin or blood cells) can be reprogrammed into iPSCs using four defined transcription factors (OCT4, SOX2, KLF4, C-MYC). Once pluripotent stem cell lines are established, they can be expanded indefinitely and differentiated into nociceptors and other cell types of the human body relevant to pain (e.g. astrocytes, microglia and macrophages) enabling a broad range of applications for translational pain research including disease modeling, identification of novel targets, drug screening, cell therapy, gene therapy.
Currently there are several different types of image-based assays that can be used to identify compounds that act on nociceptors in high-content screens. The activity of the nociceptors can be measured using genetically encoded calcium indicators (GECIs) or voltage indicators (GEVIs). The primary advantage of measuring calcium signals as a surrogate for electrical activity, for example with GCaMP, is the strong magnitude of the signal, while a limitation is the slow kinetics of calcium entry(144). Furthermore, nociceptors are adapted to respond only to specific activation of their peripheral terminals by noxious stimuli, and therefore have low levels of spontaneous activity, which means that it is difficult to directly screen for nociceptor silencing compounds when there is little basal activity to inhibit. One way around this is to increase activity in the nociceptors, by for example enhancing sodium currents with veratridine(145), or by reducing potassium flux. Another more physiological way is incubating the iPSC-derived nociceptors with inflammatory mediators which sensitize TRPV1 channels, increase the current density of Nav1.8 and the trafficking of Nav1.7 channels to the membrane, mimicking inflammation-induced peripheral sensitization(146). Screening for a reduction of such inflammatory mediator triggered activity will identify compounds that either silence the nociceptors (e.g. by blocking action potential firing) or reduce the peripheral sensitization (e.g. by inhibiting kinases activated by the inflammatory mediators), and secondary screens are required to identify which specific action the compounds have. Alternatives to genetically encoded reporters are automated patch clamp and multi-well multielectrode array (MEA) recordings, which have though, a lower throughput screening capability and higher cost, but the direct measurement of membrane current or voltage makes them a useful option for secondary validation assays. Genetically encoded pH indicators (GEPIs) can identify compounds that block transmitter vesicle release from nociceptors. As GEPIs do not reveal the dynamics of specific neurotransmitters, genetically encoded transmitter indicators (GETIs) at the presynaptic or postsynaptic site can be used if specific release mechanisms (e.g. neuropeptide release) are being targeted(144). Automated neurite imaging systems in multi-well assay format can pick up compounds that rescue chemotherapy-induced axon degeneration or promote the regeneration of axons after axon injury(147). Finally, spatially multiplexed imaging of signaling reporter islands (SiRIs) as indicators of second messengers (Calcium and cCAMP) and kinases (PKA, PKC, pERK) in neuronal culture can unveil which signaling pathways drive changes in excitability. Such assays can reveal the signaling pathway of the hit compound and the dynamic changes caused by stimuli that cause sensitization/ectopic activity in nociceptors(148, 149).
A crucial element of phenotypic screening strategies is to run parallel counter screens. If the aim is to find compounds that act selectively on nociceptors, for example, then a clear demonstration of a lack of activity on other excitable cells is required, if the aim is to reduce spontaneous firing that drives neuropathic pain there must be no effect on non-injured nociceptors, if the goal is to develop a drug that reduces inflammatory pain, then disruption of immune cell interaction with nociceptors is required.
A direct comparison of target-based versus phenotypic strategies reveals that phenotypic screens are more successful in developing first-in-class drugs(150). Screening chemical libraries in miniaturized 384- and 1536-well plates and using different compound concentrations can establish valuable dose-response curves for thousands of small molecules and the use of multiple replicates can help minimize the number of false-positive and false-negative hits(151, 152). Similarly, taking advantage of innovative screening strategies, such as fast and precise low-volume liquid dispensing using acoustic sound energy, allows for the flexible testing of multiple small molecules in combinatorial drug screens(151, 153).
As an example of the promise of phenotypic screens Studer and colleagues(154) screened iPSC-derived neural crest precursors from patients with familial dysautonomia against 6,912 small molecule compounds and identified compounds that induce expression of IKBKAP, the gene affected in this rare disease, that among other symptoms leads to insensitivity to pain. While using undifferentiated cells might provide insights into particular diseases, the ultimate goal for drug screening is to use terminally differentiated neural cell types that are functional and express the relevant targets or phenotypes of interest. However, iPSC technology enables screening at different stages of cell differentiation or disease state, and it might be possible to elucidate key temporal features of pathological changes and perform chemical screens accordingly. For instance, precisely characterizing the transition from acute to chronic pain could lead to treatment options that prevent or reverse the development or maintenance of cellular pathology and aberrant cellular plasticity. While the standardized use of human iPSC-derived neural cell types generated by stepwise differentiation for high-throughput screening is still in its infancy, more efficient and scalable cell differentiation protocols should help overcome the current technical challenges.
Disease modeling using iPSC-derived neurons
Disease modeling and identification of underlying cellular and molecular disease mechanisms is an important and novel opportunity enabled by iPSC technology, constituting “disease-in-a-dish” or “patient-in-a-dish” for probing relevant phenotypes in cell types under precisely controlled laboratory conditions (Figure 4).
Establishing genotype-phenotype relationships and charting disease signatures that accurately reflect disease phenotypes represents a key strategy for translating basic research findings into clinical applications, especially in terms of phenotypic screening and target identification(150, 155). Genome engineering using CRISPR/Cas9 allows site-specific and precise manipulation of normal and disease alleles and vastly expands the translational toolbox when combined with iPSCs(134, 156). Of special interest for modeling monogenic diseases, and for target identification and validation, is the utilization of isogenic iPSC lines which are genetically matched with the parental cell line after gene editing. Use of isogenic iPSC lines increases confidence in that accurate comparisons between normal and diseased cellular phenotypes can be achieved, and can reduce technical and biological variability as potential experimental confounders(134, 157, 158).
Genome-wide association studies (GWAS) and study of rare familial disorders has provided new insight into the genetics and epigenetics of acute and chronic pain including susceptibility, interindividual differences, and has identified potential new targets(159–163). For instance, gain-of-function mutations of NaV1.7 lead to erythromelalgia, paroxysmal extreme pain disorder, and small fiber neuropathy(164, 165). By investigating iPSC-derived nociceptors of a family with the same NaV1.7 mutation (S241T) but different pain severity (mother with moderate pain and son with severe pain), Waxman and colleagues could correlate in vitro excitability of nociceptors with the clinical presentation. Interestingly, further analysis using whole-exome sequencing identified a variant in the potassium channel KCNQ2 that conferred the resilience to pain in the mother(166). This study shows that interindividual differences in pain phenotypes and the underlying molecular mechanism(s) can be identified using patient-derived nociceptors. Also taking advantage of iPSC-derived nociceptors from a patient with small fiber neuropathy, in vitro drug testing was able to predict that the anticonvulsant lacosamide would alleviate pain in the patient(114). Ex vivo testing of patient-derived cells can serve therefore, as a precision medicine approach to develop personalized treatments. However, while iPSC derived nociceptors preserve features on Nav1.7 gain of function mutations – such as enhanced activity and temperature dependence, such mutations in mice seem not to, presenting with no pain phenotype(167), a suggestion that mice may not be useful for modeling at least some pain conditions.
iPSC-derived cell types can be exploited for disease modeling in response to neurotoxicants, chemotherapeutic drugs, metabolites, inflammatory signals and pathogens. Chemotherapy-induced polyneuropathy (CIPN) is a common side effect of cancer treatment, including numbness and loss of proprioception as well as hyperalgesia or allodynia(168). To model CIPN, patient-derived iPSC neurons can be treated with paclitaxel, cisplatin, and vincristine(169, 170). Such studies provide invaluable information on mechanisms of neuronal damage and interindividual susceptibility differences and offer an opportunity for finding new therapeutic options.
iPSC derived neurons
Human pluripotent stem cells (hPSCs) are defined by their unlimited self-renewal capacity in the absence of senescence (i.e. high telomerase activity maintains telomere length and stability) and the potential to differentiate into all somatic cell types(171). Given their human origin and the ability to model disease using patient-derived disease-specific iPSC lines, these lines offer the advantage of providing the cell numbers required for phenotypic screens that are otherwise unattainable, together with a promise of personalized therapy. The two major types of hPSCs comprise embryonic stem cells (ESCs) and iPSCs. The first human ESC lines were derived from the inner cell mass of blastocyst-stage embryos over 20 years ago(172). However, because ESCs are directly derived from preimplantation human embryos, establishing new cell lines poses ethical challenges and limits the streamlined study of human genetics and diseases. In contrast, Yamanaka and colleagues(173, 174) established the iPSC lines by reprogramming skin fibroblasts to a pluripotent state by transient expression of the transcription factors OCT4, SOX2, KLF4, C-MYC.
Once cells are successfully reprogrammed to iPSCs, endogenous developmental genes (including the four transcription factors mentioned above) are activated and maintain the gene regulatory network of pluripotency. These cell lines can be created not only from skin fibroblasts but also from other somatic cells (e.g. blood cells, keratinocytes) of an healthy individual or patient, irrespective of age, genetic background, and disease type (e.g. common or rare, monogenic or polygenic). Importantly, iPSCs are highly similar to ESCs with regard to morphology, gene expression, self-renewal capacity, and functional differentiation into multiple cell types representing various developmental lineages including ectoderm (e.g. CNS, neural crest, skin), mesoderm (e.g. blood, bone, muscle), and endoderm (e.g. lung, gut, liver, pancreas).
While the first iPSC lines were established using retroviral and lentiviral methods, more advanced reprogramming techniques allow the generation of cell lines using non-integrating viruses or even virus-free methods (episomal DNAs, synthetic mRNAs, Sendai virus) thereby increasing safety and avoiding the risk of random genomic insertions and mutagenesis(175, 176). Neurons derived from human iPSCs are now widely used to model neurodegenerative, neuropsychiatric and neurodevelopmental diseases(177, 178).
Differentiating iPSCs into relevant cell types
Converting iPSCs into specialized functional cell types entails stepwise differentiation following the principles of developmental biology. By modulating cell signaling pathways with small molecules and recombinant proteins, the pluripotent cells are coaxed into lineage-committed precursor cells and then further patterned and differentiated into more specialized cell types that demonstrate gene expression signatures and functional properties similar to their in vivo counterpart (e.g. expression of marker proteins, cell-type-specific transcriptomes, intracellular signaling, response to natural ligands, electrophysiological properties). However, because most of our developmental biology knowledge is derived from animal models, not all available information on pathways controlling cell differentiation can be directly applied to human cells due to significant species-specific differences(128, 179–181). Specific protocols need, therefore, to be carefully developed and optimized for human iPSCs followed by comprehensive functional and molecular characterization of differentiated cell types to assess validity, maturation state, and usefulness for translational applications. Currently, a major challenge for utilizing iPSC technology for translational research and therapeutic development is the lack of highly efficient, reproducible, and scalable cell differentiation protocols. Other hurdles for iPSC application are cell-line-to-cell-line variability and technical variation of cell culture conditions that strongly influence the differentiation process(157, 182, 183). Nevertheless, progress has been made over the last decade in improving cell differentiation protocols. For instance, iPSCs can be converted into neural crest stem cells expressing the transcription factor SOX10, which then can be further differentiated into nociceptors by simultaneously manipulating specific cell signaling pathways. These iPSC-derived nociceptors express typical transcription factors (e.g. BRN3A, ISL1, PRDM12), neuropeptides (e.g. Substance P, CGRP), transmembrane receptors (e.g. TRPV1, TRKA, TRKB, P2RX3), sodium channels (e.g. NaV1.7, 1.8, 1.9) and display cell-type-specific features of peripheral sensory neurons including response to specific ligands such as capsaicin, menthol, mustard oil, and ATP(113, 166, 179, 184–187). Besides generation of nociceptors, various other cell types relevant for pain research have been derived from iPSCs, including spinal cord neurons(188), Schwann cells(189, 190), microglia(191), astrocytes(192–194), GABAergic neurons(195), and cortical neurons(196).
In summary, iPSCs represent an inexhaustible source of human cells that allow studying of the cellular diversity and disease state of the PNS and CNS for translational pain research, and the utilization of pain-specific phenotypic assays for drug screens. As an alternative to iPSCs, it is also possible to directly convert fibroblasts into nociceptors by forced expression of specific transcription factors(117). However, such transdifferentiation protocols are inefficient and not scalable for high-throughput screening.
Organoids and complex tissue models
Under two-dimensional (2D) cell culture conditions, cells grow attached to flat cell culture plates and therefore do not fully recapitulate in vivo physiology. In contrast, 3D models such as organoids or mini-organs more closely model dynamic in vivo cell function and behavior, including cell-cell interactions and biomechanical forces within the appropriate extracellular matrix(197). The use of 3D models can also help enhance cell maturation, a common challenge for iPSC-derived cell types. Organoid models with relevance for pain research have been developed for skin(198), DRG(199) and thalamus(200). The study of complex tissues may provide future opportunities to interrogate nociceptive circuits and test neuroactive drugs. However, the reproducibility and consistency of randomly self-organizing organoid models need to be improved before they can be used as robust assays compatible with drug discovery. Other innovative technologies being developed are based on advances in bioengineering and biomaterials and could lead to standardized microphysiological assays, organs-on-chips, and bioprinted tissues(201, 202). These complex systems are also being developed as platforms for ADME (absorption, distribution, metabolism and excretion) of small molecules, which may provide human pharmacokinetic (PK) predictions and potentially eventually replace animal experimentation. Another model is the “nerve-on-a-chip”, which takes advantage of culturing Schwann cells with human motor or sensory neurons to establish long axonal projections for measuring electrophysiological properties, drug effects, and response to injury(203).
Scalable biomanufacturing of human iPSC-derived cells
Standardized and reproducible production of large quantities of human cells is required for genetic and chemical screening projects. Manual culture and differentiation of iPSCs are variable and labor-intensive, which limits their utilization in industrial-scale screening projects and high-throughput applications. Recent progress has been made in generating and culturing iPSCs using bioreactors, automated liquid handlers, and robotic cell culture systems(204–206). For instance, it is possible to automate all essential steps of iPSC culture and directed differentiation into neurons, cardiomyocytes, and hepatocytes(206). This approach allows culture of up to 90 iPSC lines in parallel, and that may help overcome the problem that investigation of only a few cell lines per study may not be sufficient for drawing general population-wide conclusions. Robotic cell culture supports the standardized manufacturing of billions of human cells, which can be cryopreserved and then used as needed. Scalable cell culture is compatible with batch production as well as continuous biomanufacturing. Overall, automation not only increases scientific rigor by reducing the investigator bias inherent in the manual iPSC culture, but also provides access to cellular material for functional genomics and high-throughput/high-content screening of chemical libraries, natural products, and biologics.
Cell therapy
One of the great promises of iPSC technology is the potential to develop personalized cell/regenerative medicine therapies (Figure 4). Autologous non-immunogenic cell therapies are of particular interest because, unlike allogeneic approaches, they do not require long-term immunosuppression or donors with a matching human leucocyte antigen (HLA) signature. Although various cell products generated from human ESCs and iPSCs are currently being tested in clinical trials(156), none of the current clinical trials target chronic pain. However, cell therapy may become a therapeutic option for certain pains, since human iPSC-derived GABAergic neurons injected into the spinal cord of mice with chronic neuropathic pain reduces the pain phenotype for up to two months post-transplantation(207). Earlier reports had documented that grafting of GABAergic precursors isolated from fetal rodent brains or human ESC-derived GABAergic neurons mitigates pain after spinal cord or peripheral injury by overcoming disinhibition(208, 209). These studies are consistent with the observation that intrathecal application of the GABA agonist baclofen provides analgesia in chronic pain patients(210). Central neuropathic pain may also benefit from grafting neuronal cells with other inhibitory neurotransmitter phenotypes (e.g. glycine), oligodendrocytes(211) and astrocytes(193, 194) but more research is needed to define the mechanisms of action and the optimal time window for cell therapeutic intervention. iPSC-based cell therapies may prove useful for the treatment of peripheral neuropathy and neuropathic pain, since the complex interplay of multiple cell types including sensory neurons, Schwann cells, satellite glia, and immune cells contribute to axon loss, demyelination, ectopic firing and pathological pain(212). The grafting of undifferentiated neural crest stem cells supports axonal regrowth and myelination after sciatic nerve injury(213, 214). Although progress has been made in differentiating iPSC-derived neural crest stem cells into Schwann cells(189, 190, 215), more efficient and clinically relevant protocols are needed to generate large quantities of cells with myelination capacity. Satellite glia are specialized support cells that closely envelop the cell bodies of sensory neurons in the DRG and are activated during inflammation and nerve injury(216). Despite their unique anatomical location and potential importance for sensory neuron function, human satellite glia are largely understudied, and derivation of satellite glia from iPSCs and their detailed characterization should change this. There is also increasing interest in immune cells such as microglia and DRG-resident macrophages, which contribute to the induction and maintenance of chronic pain(217, 218). Derivation of microglia-like cells from human iPSCs(191) should enable a better understanding of their basic biology, immunomodulatory function, and therapeutic potential. Altogether, production of all disease-relevant cell types from iPSCs (neuronal and non-neuronal) would allow systematic neurotransplantation experiments and testing of the utility of replacement of these cells in animal models of central and peripheral neuropathic pain. However, all stem cell-derived products will require extensive safety measures to ensure that grafted cells are karyotypically normal and properly differentiated otherwise in vivo tumor formation may occur. Indeed, the use of undifferentiated or proliferative cells is particularly risky as they can continue to grow upon transplantation and form teratomas or other tumors(219).
Gene Therapy
Newly emerging insights into genetic mechanisms and cellular pathologies provide opportunities to devise efficacious and safe therapies for pain by directly targeting DNA or RNA. The rationale for gene therapy includes replacing or correcting a disease-causing gene/mutation in a specific cell type, overexpression of neurotransmitters or ligands to modulate specific receptors, expression of transcription factors to drive change in function, neuroprotection, change of cellular identity or recruitment of endogenous cells for repair or replacement. Relevant technologies for gene delivery use replication-incompetent viruses such as herpes simplex viral vector (HSV) and adeno-associated viral vector (AAV), which have been used in the context of pain(220). Other available gene therapy strategies are RNA interference (RNAi) and antisense oligonucleotide (ASO) technology that can degrade or inhibit the processing of specific mRNA molecules, thereby modulating or silencing the expression of a protein of interest(221–223). Application of CRISPR/Cas9 methods can correct a genetic defect, overexpress genes, or fine-tune gene expression via inducible systems that increase the level of control and avoid/reverse unwanted effects. More research is warranted though, to better understand off-target effects, neurotoxicity, and long-term consequences(224). Specifically, the use of iPSC-derived cell types in the context of gene therapy could become a reality in two scenarios: first, gene therapy therapeutic strategies could be directly tested in relevant human cell types expressing the target gene, as part of a predictive in vitro platform (Figure 4). For instance, modulating the expression of specific ion channels in human nociceptors and characterizing the resulting functional consequences would provide valuable information before moving into clinical application. Second, the combined use of gene and cell therapies would expand the therapeutic armamentarium of personalized medicine. In theory, any iPSC-derived somatic cell type and any gene could be manipulated in vitro and then given back to a patient donor. Using chemogenetic or optogenetic cell engineering approaches, only the genetically modified cells could be selectively activated or inhibited in the patient. Neuromodulation could also be achieved using prodrugs that become pharmacologically active after being metabolized in vivo or on photostimulation(225–228).
Perspectives and translational opportunities
To develop effective analgesic drugs with no abuse liability it is important to acknowledge pain as a major and diverse systems-level challenge and identify optimal strategies across the preclinical and clinical spectrum. The wealth of new bioengineering technologies, multi-omics platforms, bioinformatics and machine learning and artificial intelligence will certainly aid in generating and integrating information derived from genomics, iPSC-derived human cells, and animal models, with a better representation of human disease, and therefore a better predictor of outcome in clinical studies. As a major new frontier in therapeutics development, iPSC technology can bridge the gap between genomic data and personalized medicine by enabling disease modeling and phenotype characterization, new target identification, drug screening and experimental validation (Figure 4). The development of such therapeutic strategies requires running “clinical trials in a dish” and using iPSC lines from large patient cohorts (including different genders, ethnic backgrounds, disease histories) to identify novel therapeutics and patient responders and non-responders. Mechanism-based scientific findings using human cellular models can, we argue, be efficiently translated into clinical applications that improve the management of chronic pain and avoid adverse effects, including dependence and addiction.
Crucial to translating preclinical findings to clinical use is to understand exactly which conclusions drawn from preclinical studies apply to clinical settings. Until recently preclinical studies have largely used rodent models, even though important species differences exist, and pain cannot be directly measured in animals. The introduction of human iPSC-derived models provides an opportunity to begin to overcome these difficulties, although challenges remain in establishing which preclinical parameters positively correlate with clinical efficacy. Phenotypic screens allow for the determination of the specific action of compounds on particular cells and defined disease phenotypes, as well exploitation of polypharmacology opportunities. Counter screens provide a means of removing compounds with non-specific/undesired effects early in the discovery process. Overall, prospects utilizing iPSC-derived neurons are now higher for the development of effective nociceptor selective silencers suitable for managing surgical or post-traumatic pain, and eliminators of either the immune-mediated sensitization that drives inflammatory pain hypersensitivity or of the development of ectopic activity in injured nociceptors that triggers neuropathic pain.
Acknowledgments
We thank our funding sources for their generous support, all members of the DARPA Panacea Safe Therapeutic Options for Pain and Inflammation consortium for many discussions, and the NIH Medical Illustration Team.
Funding
DARPA (HR0011-19-2-0022 BPB & CJW); NIH (R35NS105076-01 and R01AT011447 CJW, NS036855 BPB); Regenerative Medicine Program (RMP) of the NIH Common Fund (I.S.); NIH HEAL Initiative (I.S.); Intramural Research Program, NCATS, NIH (I.S.)
National Research Foundation Fellowship (NRF-2018R1A6A3A03012431 JS)
Footnotes
Competing interests
I.S. JS, SJ have no competing financial interests. CJW and BPB are cofounders of Nocion Therapeutics, CJW is a founder of QurAlis.
Data and materials availability
Not applicable
References and Notes
- 1.Colloca L, Ludman T, Bouhassira D, Baron R, Dickenson AH, Yarnitsky D, Freeman R, Truini A, Attal N, Finnerup NB, Eccleston C, Kalso E, Bennett DL, Dworkin RH, Raja SN, Neuropathic pain. Nat Rev Dis Primers 3, 17002 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cohen SP, Vase L, Hooten WM, Chronic pain: an update on burden, best practices, and new advances. Lancet 397, 2082–2097 (2021). [DOI] [PubMed] [Google Scholar]
- 3.Woolf CJ, Ma Q, Nociceptors--noxious stimulus detectors. Neuron 55, 353–364 (2007). [DOI] [PubMed] [Google Scholar]
- 4.Rubin MR, Nguyen-Huu MC, Alternatively spliced Hox-1.7 transcripts encode different protein products. DNA Seq 1, 115–124 (1990). [DOI] [PubMed] [Google Scholar]
- 5.Wang M, Thyagarajan B, Pain pathways and potential new targets for pain relief. Biotechnol Appl Biochem, (2020). [DOI] [PubMed]
- 6.Khan A, Khan S, Kim YS, Insight into Pain Modulation: Nociceptors Sensitization and Therapeutic Targets. Curr Drug Targets 20, 775–788 (2019). [DOI] [PubMed] [Google Scholar]
- 7.Sun J, Li L, Yang L, Duan G, Ma T, Li N, Liu Y, Yao J, Liu JY, Zhang X, Novel SCN9A missense mutations contribute to congenital insensitivity to pain: Unexpected correlation between electrophysiological characterization and clinical phenotype. Mol Pain 16, 1744806920923881 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drissi I, Woods WA, Woods CG, Understanding the genetic basis of congenital insensitivity to pain. Br Med Bull 133, 65–78 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pan TT, Pan F, Gao W, Hu SS, Wang D, Involvement of Macrophages and Spinal Microglia in Osteoarthritis Pain. Curr Rheumatol Rep 23, 29 (2021). [DOI] [PubMed] [Google Scholar]
- 10.Shu CC, Zaki S, Ravi V, Schiavinato A, Smith MM, Little CB, The relationship between synovial inflammation, structural pathology, and pain in post-traumatic osteoarthritis: differential effect of stem cell and hyaluronan treatment. Arthritis Res Ther 22, 29 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kawabata A, Prostaglandin E2 and pain--an update. Biol Pharm Bull 34, 1170–1173 (2011). [DOI] [PubMed] [Google Scholar]
- 12.de Magalhaes SF, Manzo LP, de Faria FM, de Oliveira-Fusaro MC, Nishijima CM, Vieira WF, Bonet IJM, Dos Santos GG, Tambeli CH, Parada CA, Inflammatory pain in peripheral tissue depends on the activation of the TNF-alpha type 1 receptor in the primary afferent neuron. Eur J Neurosci 53, 376–389 (2021). [DOI] [PubMed] [Google Scholar]
- 13.Matsuda M, Huh Y, Ji RR, Roles of inflammation, neurogenic inflammation, and neuroinflammation in pain. J Anesth 33, 131–139 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Woolf CJ, Central sensitization: implications for the diagnosis and treatment of pain. Pain 152, S2–S15 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Woolf CJ, Salter MW, Neuronal plasticity: increasing the gain in pain. Science 288, 1765–1769 (2000). [DOI] [PubMed] [Google Scholar]
- 16.Minhas D, Clauw DJ, Pain Mechanisms in Patients with Rheumatic Diseases. Rheum Dis Clin North Am 47, 133–148 (2021). [DOI] [PubMed] [Google Scholar]
- 17.Costigan M, Scholz J, Woolf CJ, Neuropathic pain: a maladaptive response of the nervous system to damage. Annu Rev Neurosci 32, 1–32 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Finnerup NB, Kuner R, Jensen TS, Neuropathic Pain: From Mechanisms to Treatment. Physiol Rev 101, 259–301 (2021). [DOI] [PubMed] [Google Scholar]
- 19.Hunt C, Moman R, Peterson A, Wilson R, Covington S, Mustafa R, Murad MH, Hooten WM, Prevalence of chronic pain after spinal cord injury: a systematic review and meta-analysis. Reg Anesth Pain Med 46, 328–336 (2021). [DOI] [PubMed] [Google Scholar]
- 20.von Hehn CA, Baron R, Woolf CJ, Deconstructing the neuropathic pain phenotype to reveal neural mechanisms. Neuron 73, 638–652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Malcangio M, Role of the immune system in neuropathic pain. Scand J Pain 20, 33–37 (2019). [DOI] [PubMed] [Google Scholar]
- 22.Ashina S, Mitsikostas DD, Lee MJ, Yamani N, Wang SJ, Messina R, Ashina H, Buse DC, Pozo-Rosich P, Jensen RH, Diener HC, Lipton RB, Tension-type headache. Nat Rev Dis Primers 7, 24 (2021). [DOI] [PubMed] [Google Scholar]
- 23.Vardeh D, Mannion RJ, Woolf CJ, Toward a Mechanism-Based Approach to Pain Diagnosis. J Pain 17, T50–69 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fitzcharles MA, Cohen SP, Clauw DJ, Littlejohn G, Usui C, Hauser W, Nociplastic pain: towards an understanding of prevalent pain conditions. Lancet 397, 2098–2110 (2021). [DOI] [PubMed] [Google Scholar]
- 25.Siracusa R, Paola RD, Cuzzocrea S, Impellizzeri D, Fibromyalgia: Pathogenesis, Mechanisms, Diagnosis and Treatment Options Update. Int J Mol Sci 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kosek E, Clauw D, Nijs J, Baron R, Gilron I, Harris RE, Mico JA, Rice AS, Sterling M, Chronic nociplastic pain affecting the musculoskeletal system: clinical criteria and grading system. Pain, (2021). [DOI] [PubMed]
- 27.Creed F, A review of the incidence and risk factors for fibromyalgia and chronic widespread pain in population-based studies. Pain 161, 1169–1176 (2020). [DOI] [PubMed] [Google Scholar]
- 28.Drewes AM, Olesen AE, Farmer AD, Szigethy E, Rebours V, Olesen SS, Gastrointestinal pain. Nat Rev Dis Primers 6, 1 (2020). [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Mu J, Zhu M, Mukherjee A, Zhang H, Transient Receptor Potential Channels and Inflammatory Bowel Disease. Front Immunol 11, 180 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.van der Gaag WH, Roelofs PD, Enthoven WT, van Tulder MW, Koes BW, Non-steroidal anti-inflammatory drugs for acute low back pain. Cochrane Database Syst Rev 4, CD013581 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bouali-Benazzouz R, Landry M, Benazzouz A, Fossat P, Neuropathic pain modeling: Focus on synaptic and ion channel mechanisms. Prog Neurobiol 201, 102030 (2021). [DOI] [PubMed] [Google Scholar]
- 32.Jensen TS, Finnerup NB, Allodynia and hyperalgesia in neuropathic pain: clinical manifestations and mechanisms. Lancet Neurol 13, 924–935 (2014). [DOI] [PubMed] [Google Scholar]
- 33.Carter JA, Black LK, Sharma D, Bhagnani T, Jahr JS, Efficacy of non-opioid analgesics to control postoperative pain: a network meta-analysis. BMC Anesthesiol 20, 272 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Woolf CJ, Capturing Novel Non-opioid Pain Targets. Biol Psychiatry 87, 74–81 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yekkirala AS, Roberson DP, Bean BP, Woolf CJ, Breaking barriers to novel analgesic drug development. Nat Rev Drug Discov 16, 810 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alsaloum M, Higerd GP, Effraim PR, Waxman SG, Status of peripheral sodium channel blockers for non-addictive pain treatment. Nat Rev Neurol 16, 689–705 (2020). [DOI] [PubMed] [Google Scholar]
- 37.Schaible HG, Ebersberger A, Von Banchet GS, Mechanisms of pain in arthritis. Ann N Y Acad Sci 966, 343–354 (2002). [DOI] [PubMed] [Google Scholar]
- 38.Villarreal CF, Funez MI, Cunha Fde Q, Parada CA, Ferreira SH, The long-lasting sensitization of primary afferent nociceptors induced by inflammation involves prostanoid and dopaminergic systems in mice. Pharmacol Biochem Behav 103, 678–683 (2013). [DOI] [PubMed] [Google Scholar]
- 39.Richter F, Natura G, Loser S, Schmidt K, Viisanen H, Schaible HG, Tumor necrosis factor causes persistent sensitization of joint nociceptors to mechanical stimuli in rats. Arthritis Rheum 62, 3806–3814 (2010). [DOI] [PubMed] [Google Scholar]
- 40.Pogatzki-Zahn E, Chandrasena C, Schug SA, Nonopioid analgesics for postoperative pain management. Curr Opin Anaesthesiol 27, 513–519 (2014). [DOI] [PubMed] [Google Scholar]
- 41.Mazaleuskaya LL, Muzykantov VR, FitzGerald GA, Nanotherapeutic-directed approaches to analgesia. Trends Pharmacol Sci 42, 527–550 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jones P, Dalziel SR, Lamdin R, Miles-Chan JL, Frampton C, Oral non-steroidal anti-inflammatory drugs versus other oral analgesic agents for acute soft tissue injury. Cochrane Database Syst Rev, CD007789 (2015). [DOI] [PubMed]
- 43.Moore RA, Derry S, Aldington D, Wiffen PJ, Single dose oral analgesics for acute postoperative pain in adults - an overview of Cochrane reviews. Cochrane Database Syst Rev, CD008659 (2015). [DOI] [PMC free article] [PubMed]
- 44.McNicol ED, Ferguson MC, Schumann R, Single-dose intravenous ketorolac for acute postoperative pain in adults. Cochrane Database Syst Rev 5, CD013263 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Derry S, Karlin SM, Moore RA, Single dose oral ibuprofen plus codeine for acute postoperative pain in adults. Cochrane Database Syst Rev, CD010107 (2015). [DOI] [PubMed]
- 46.Derry S, Derry CJ, Moore RA, Single dose oral ibuprofen plus oxycodone for acute postoperative pain in adults. Cochrane Database Syst Rev, CD010289 (2013). [DOI] [PMC free article] [PubMed]
- 47.Laine L, Approaches to nonsteroidal anti-inflammatory drug use in the high-risk patient. Gastroenterology 120, 594–606 (2001). [DOI] [PubMed] [Google Scholar]
- 48.Derry CJ, Derry S, Moore RA, Single dose oral ibuprofen plus paracetamol (acetaminophen) for acute postoperative pain. Cochrane Database Syst Rev, CD010210 (2013). [DOI] [PMC free article] [PubMed]
- 49.Sbidian E, Chaimani A, Garcia-Doval I, Doney L, Dressler C, Hua C, Hughes C, Naldi L, Afach S, Le Cleach L, Systemic pharmacological treatments for chronic plaque psoriasis: a network meta-analysis. Cochrane Database Syst Rev 4, CD011535 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Feldmann M, Maini RN, Anti-TNF alpha therapy of rheumatoid arthritis: what have we learned? Annu Rev Immunol 19, 163–196 (2001). [DOI] [PubMed] [Google Scholar]
- 51.Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL, Anti-TNF-alpha therapies: the next generation. Nat Rev Drug Discov 2, 736–746 (2003). [DOI] [PubMed] [Google Scholar]
- 52.Jang DI, Lee AH, Shin HY, Song HR, Park JH, Kang TB, Lee SR, Yang SH, The Role of Tumor Necrosis Factor Alpha (TNF-alpha) in Autoimmune Disease and Current TNF-alpha Inhibitors in Therapeutics. Int J Mol Sci 22, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Dinarello CA, Simon A, van der Meer JW, Treating inflammation by blocking interleukin-1 in a broad spectrum of diseases. Nat Rev Drug Discov 11, 633–652 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Sun J, Chen SR, Chen H, Pan HL, mu-Opioid receptors in primary sensory neurons are essential for opioid analgesic effect on acute and inflammatory pain and opioid-induced hyperalgesia. J Physiol 597, 1661–1675 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.McAnally H, Bonnet U, Kaye AD, Gabapentinoid Benefit and Risk Stratification: Mechanisms Over Myth. Pain Ther 9, 441–452 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dolphin AC, Voltage-gated calcium channels: their discovery, function and importance as drug targets. Brain Neurosci Adv 2, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Deer TR, Pope JE, Hanes MC, McDowell GC, Intrathecal Therapy for Chronic Pain: A Review of Morphine and Ziconotide as Firstline Options. Pain Med 20, 784–798 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wang F, Yan Z, Liu Z, Wang S, Wu Q, Yu S, Ding J, Dai Q, Molecular basis of toxicity of N-type calcium channel inhibitor MVIIA. Neuropharmacology 101, 137–145 (2016). [DOI] [PubMed] [Google Scholar]
- 59.Deligianni CI, Mitsikostas DD, Ashina M, Safety and tolerability evaluation of erenumab for the preventive treatment of migraine. Expert Opin Drug Saf, (2021). [DOI] [PubMed]
- 60.Masoud AT, Hasan MT, Sayed A, Edward HN, Amer AM, Naga AE, Elfil M, Alghamdi BS, Perveen A, Ashraf GM, Bahbah EI, Efficacy of calcitonin gene-related peptide (CGRP) receptor blockers in reducing the number of monthly migraine headache days (MHDs): A network meta-analysis of randomized controlled trials. J Neurol Sci 427, 117505 (2021). [DOI] [PubMed] [Google Scholar]
- 61.Popoff E, Johnston K, Croop R, Thiry A, Harris L, Powell L, Coric V, L’Italien G, Moren J, Matching-adjusted indirect comparisons of oral rimegepant versus placebo, erenumab, and galcanezumab examining monthly migraine days and health-related quality of life in the treatment of migraine. Headache, (2021). [DOI] [PMC free article] [PubMed]
- 62.Kukkar A, Bali A, Singh N, Jaggi AS, Implications and mechanism of action of gabapentin in neuropathic pain. Arch Pharm Res 36, 237–251 (2013). [DOI] [PubMed] [Google Scholar]
- 63.Boroujerdi A, Zeng J, Sharp K, Kim D, Steward O, Luo DZ, Calcium channel alpha-2-delta-1 protein upregulation in dorsal spinal cord mediates spinal cord injury-induced neuropathic pain states. Pain 152, 649–655 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Serra E, Duloxetine and pregabalin: safe and effective for the long-term treatment of fibromyalgia? Nat Clin Pract Neurol 4, 594–595 (2008). [DOI] [PubMed] [Google Scholar]
- 65.Waitekus AB, Kirkpatrick P, Duloxetine hydrochloride. Nat Rev Drug Discov 3, 907–908 (2004). [DOI] [PubMed] [Google Scholar]
- 66.Branton MW, Hopkins TJ, Nemec EC, Duloxetine for the reduction of opioid use in elective orthopedic surgery: a systematic review and meta-analysis. Int J Clin Pharm 43, 394–403 (2021). [DOI] [PubMed] [Google Scholar]
- 67.Roques BP, Fournie-Zaluski MC, Wurm M, Inhibiting the breakdown of endogenous opioids and cannabinoids to alleviate pain. Nat Rev Drug Discov 11, 292–310 (2012). [DOI] [PubMed] [Google Scholar]
- 68.Todd AJ, Neuronal circuitry for pain processing in the dorsal horn. Nat Rev Neurosci 11, 823–836 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Knabl J, Witschi R, Hosl K, Reinold H, Zeilhofer UB, Ahmadi S, Brockhaus J, Sergejeva M, Hess A, Brune K, Fritschy JM, Rudolph U, Mohler H, Zeilhofer HU, Reversal of pathological pain through specific spinal GABAA receptor subtypes. Nature 451, 330–334 (2008). [DOI] [PubMed] [Google Scholar]
- 70.Ralvenius WT, Benke D, Acuna MA, Rudolph U, Zeilhofer HU, Analgesia and unwanted benzodiazepine effects in point-mutated mice expressing only one benzodiazepine-sensitive GABAA receptor subtype. Nat Commun 6, 6803 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Munro G, Ahring PK, Mirza NR, Developing analgesics by enhancing spinal inhibition after injury: GABAA receptor subtypes as novel targets. Trends Pharmacol Sci 30, 453–459 (2009). [DOI] [PubMed] [Google Scholar]
- 72.Zeilhofer HU, Ralvenius WT, Acuna MA, Restoring the spinal pain gate: GABA(A) receptors as targets for novel analgesics. Adv Pharmacol 73, 71–96 (2015). [DOI] [PubMed] [Google Scholar]
- 73.Rudolph U, Knoflach F, Beyond classical benzodiazepines: novel therapeutic potential of GABAA receptor subtypes. Nat Rev Drug Discov 10, 685–697 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.de Geus TJ, Patijn J, Joosten EAJ, Qualitative review on N-methyl-D-aspartate receptor expression in rat spinal cord during the postnatal development: Implications for central sensitization and pain. Dev Neurobiol 80, 443–455 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Caruso K, Tyler D, Lyden A, Ketamine for Pain Management: A Review of Literature and Clinical Application. Orthop Nurs 40, 189–193 (2021). [DOI] [PubMed] [Google Scholar]
- 76.Wang C, Fu H, Wang J, Huang F, Cao X, Preemptive analgesia using selective cyclooxygenase-2 inhibitors alleviates postoperative pain in patients undergoing total knee arthroplasty: A protocol for PRISMA guided meta-analysis of randomized controlled trials. Medicine (Baltimore) 100, e24512 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Raja SD, Shetty AP, Subramanian B, Kanna RM, Rajasekaran S, A prospective randomized study to analyze the efficacy of balanced pre-emptive analgesia in spine surgery. Spine J 19, 569–577 (2019). [DOI] [PubMed] [Google Scholar]
- 78.Souza D. Monteiro de Araujo, Nassini R, Geppetti P, De Logu F, TRPA1 as a therapeutic target for nociceptive pain. Expert Opin Ther Targets 24, 997–1008 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gao M, Wang Y, Liu L, Qiao Z, Yan L, A patent review of transient receptor potential vanilloid type 1 modulators (2014-present). Expert Opin Ther Pat 31, 169–187 (2021). [DOI] [PubMed] [Google Scholar]
- 80.Dulai JS, Smith ESJ, Rahman T, Acid-sensing ion channel 3: An analgesic target. Channels (Austin) 15, 94–127 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Inoue K, Tsuda M, Nociceptive signaling mediated by P2X3, P2X4 and P2X7 receptors. Biochem Pharmacol 187, 114309 (2021). [DOI] [PubMed] [Google Scholar]
- 82.Tam TH, Salter MW, Purinergic signalling in spinal pain processing. Purinergic Signal 17, 49–54 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wise BL, Seidel MF, Lane NE, The evolution of nerve growth factor inhibition in clinical medicine. Nat Rev Rheumatol 17, 34–46 (2021). [DOI] [PubMed] [Google Scholar]
- 84.Malfait AM, Miller RE, Miller RJ, Basic Mechanisms of Pain in Osteoarthritis: Experimental Observations and New Perspectives. Rheum Dis Clin North Am 47, 165–180 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Crispino N, Ciccia F, JAK/STAT pathway and nociceptive cytokine signalling in rheumatoid arthritis and psoriatic arthritis. Clin Exp Rheumatol 39, 668–675 (2021). [PubMed] [Google Scholar]
- 86.Smith PA, K(+) Channels in Primary Afferents and Their Role in Nerve Injury-Induced Pain. Front Cell Neurosci 14, 566418 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Huang L, Xu G, Jiang R, Luo Y, Zuo Y, Liu J, Development of Non-opioid Analgesics Targeting Two-pore Domain Potassium Channels. Curr Neuropharmacol, (2021). [DOI] [PMC free article] [PubMed]
- 88.Cheng X, Choi JS, Waxman SG, Dib-Hajj SD, Mini-review - Sodium channels and beyond in peripheral nerve disease: Modulation by cytokines and their effector protein kinases. Neurosci Lett 741, 135446 (2021). [DOI] [PubMed] [Google Scholar]
- 89.Chen R, Coppes OJM, Urman RD, Receptor and Molecular Targets for the Development of Novel Opioid and Non-Opioid Analgesic Therapies. Pain Physician 24, 153–163 (2021). [PubMed] [Google Scholar]
- 90.Di Lio A, Benke D, Besson M, Desmeules J, Daali Y, Wang ZJ, Edwankar R, Cook JM, Zeilhofer HU, HZ166, a novel GABAA receptor subtype-selective benzodiazepine site ligand, is antihyperalgesic in mouse models of inflammatory and neuropathic pain. Neuropharmacology 60, 626–632 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Lorenzo LE, Godin AG, Ferrini F, Bachand K, Plasencia-Fernandez I, Labrecque S, Girard AA, Boudreau D, Kianicka I, Gagnon M, Doyon N, Ribeiro-da-Silva A, De Koninck Y, Enhancing neuronal chloride extrusion rescues alpha2/alpha3 GABAA-mediated analgesia in neuropathic pain. Nat Commun 11, 869 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Latremoliere A, Latini A, Andrews N, Cronin SJ, Fujita M, Gorska K, Hovius R, Romero C, Chuaiphichai S, Painter M, Miracca G, Babaniyi O, Remor AP, Duong K, Riva P, Barrett LB, Ferreiros N, Naylor A, Penninger JM, Tegeder I, Zhong J, Blagg J, Channon KM, Johnsson K, Costigan M, Woolf CJ, Reduction of Neuropathic and Inflammatory Pain through Inhibition of the Tetrahydrobiopterin Pathway. Neuron 86, 1393–1406 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Pulakat L, Sumners C, Angiotensin Type 2 Receptors: Painful, or Not? Front Pharmacol 11, 571994 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Nagase H, Saitoh A, Research and development of kappa opioid receptor agonists and delta opioid receptor agonists. Pharmacol Ther 205, 107427 (2020). [DOI] [PubMed] [Google Scholar]
- 95.Gottesman-Katz L, Latorre R, Vanner S, Schmidt BL, Bunnett NW, Targeting G protein-coupled receptors for the treatment of chronic pain in the digestive system. Gut 70, 970–981 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kaski SW, White AN, Gross JD, Siderovski DP, Potential for Kappa-Opioid Receptor Agonists to Engineer Nonaddictive Analgesics: A Narrative Review. Anesth Analg 132, 406–419 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Che T, Dwivedi-Agnihotri H, Shukla AK, Roth BL, Biased ligands at opioid receptors: Current status and future directions. Sci Signal 14, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Kolber MR, Ton J, Thomas B, Kirkwood J, Moe S, Dugre N, Chan K, Lindblad AJ, McCormack J, Garrison S, Allan GM, Korownyk CS, Craig R, Sept L, Rouble AN, Perry D, PEER systematic review of randomized controlled trials: Management of chronic low back pain in primary care. Can Fam Physician 67, e20–e30 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Falk J, Thomas B, Kirkwood J, Korownyk CS, Lindblad AJ, Ton J, Moe S, Allan GM, McCormack J, Garrison S, Dugre N, Chan K, Kolber MR, Train A, Froentjes L, Sept L, Wollin M, Craig R, Perry D, PEER systematic review of randomized controlled trials: Management of chronic neuropathic pain in primary care. Can Fam Physician 67, e130–e140 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Gregori D, Giacovelli G, Minto C, Barbetta B, Gualtieri F, Azzolina D, Vaghi P, Rovati LC, Association of Pharmacological Treatments With Long-term Pain Control in Patients With Knee Osteoarthritis: A Systematic Review and Meta-analysis. JAMA 320, 2564–2579 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Busse JW, Wang L, Kamaleldin M, Craigie S, Riva JJ, Montoya L, Mulla SM, Lopes LC, Vogel N, Chen E, Kirmayr K, De Oliveira K, Olivieri L, Kaushal A, Chaparro LE, Oyberman I, Agarwal A, Couban R, Tsoi L, Lam T, Vandvik PO, Hsu S, Bala MM, Schandelmaier S, Scheidecker A, Ebrahim S, Ashoorion V, Rehman Y, Hong PJ, Ross S, Johnston BC, Kunz R, Sun X, Buckley N, Sessler DI, Guyatt GH, Opioids for Chronic Noncancer Pain: A Systematic Review and Meta-analysis. JAMA 320, 2448–2460 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zheng Y, Liu P, Bai L, Trimmer JS, Bean BP, Ginty DD, Deep Sequencing of Somatosensory Neurons Reveals Molecular Determinants of Intrinsic Physiological Properties. Neuron 103, 598–616 e597 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sills GJ, Rogawski MA, Mechanisms of action of currently used antiseizure drugs. Neuropharmacology 168, 107966 (2020). [DOI] [PubMed] [Google Scholar]
- 104.Roth BL, Sheffler DJ, Kroeze WK, Magic shotguns versus magic bullets: selectively non-selective drugs for mood disorders and schizophrenia. Nat Rev Drug Discov 3, 353–359 (2004). [DOI] [PubMed] [Google Scholar]
- 105.Goodwin G, McMahon SB, The physiological function of different voltage-gated sodium channels in pain. Nat Rev Neurosci 22, 263–274 (2021). [DOI] [PubMed] [Google Scholar]
- 106.Dimitriadi M, Kye MJ, Kalloo G, Yersak JM, Sahin M, Hart AC, The neuroprotective drug riluzole acts via small conductance Ca2+-activated K+ channels to ameliorate defects in spinal muscular atrophy models. J Neurosci 33, 6557–6562 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Duprat F, Lesage F, Patel AJ, Fink M, Romey G, Lazdunski M, The neuroprotective agent riluzole activates the two P domain K(+) channels TREK-1 and TRAAK. Mol Pharmacol 57, 906–912 (2000). [PubMed] [Google Scholar]
- 108.Tsantoulas C, McMahon SB, Opening paths to novel analgesics: the role of potassium channels in chronic pain. Trends Neurosci 37, 146–158 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Du X, Gao H, Jaffe D, Zhang H, Gamper N, M-type K(+) channels in peripheral nociceptive pathways. Br J Pharmacol 175, 2158–2172 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.La Montanara P, Hervera A, Baltussen LL, Hutson TH, Palmisano I, De Virgiliis F, Kong G, Chadwick J, Gao Y, Bartus K, Majid QA, Gorgoraptis N, Wong K, Downs J, Pizzorusso T, Ultanir SK, Leonard H, Yu H, Millar DS, Istvan N, Mazarakis ND, Di Giovanni S, Cyclin-dependent-like kinase 5 is required for pain signaling in human sensory neurons and mouse models. Sci Transl Med 12, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Schoepf CL, Zeidler M, Spiecker L, Kern G, Lechner J, Kummer KK, Kress M, Selected Ionotropic Receptors and Voltage-Gated Ion Channels: More Functional Competence for Human Induced Pluripotent Stem Cell (iPSC)-Derived Nociceptors. Brain Sci 10, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Pettingill P, Weir GA, Wei T, Wu Y, Flower G, Lalic T, Handel A, Duggal G, Chintawar S, Cheung J, Arunasalam K, Couper E, Haupt LM, Griffiths LR, Bassett A, Cowley SA, Cader MZ, A causal role for TRESK loss of function in migraine mechanisms. Brain 142, 3852–3867 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.McDermott LA, Weir GA, Themistocleous AC, Segerdahl AR, Blesneac I, Baskozos G, Clark AJ, Millar V, Peck LJ, Ebner D, Tracey I, Serra J, Bennett DL, Defining the Functional Role of NaV1.7 in Human Nociception. Neuron 101, 905–919 e908 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Namer B, Schmidt D, Eberhardt E, Maroni M, Dorfmeister E, Kleggetveit IP, Kaluza L, Meents J, Gerlach A, Lin Z, Winterpacht A, Dragicevic E, Kohl Z, Schuttler J, Kurth I, Warncke T, Jorum E, Winner B, Lampert A, Pain relief in a neuropathy patient by lacosamide: Proof of principle of clinical translation from patient-specific iPS cell-derived nociceptors. EBioMedicine 39, 401–408 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Schrenk-Siemens K, Rosseler C, Lampert A, Translational Model Systems for Complex Sodium Channel Pathophysiology in Pain. Handb Exp Pharmacol 246, 355–369 (2018). [DOI] [PubMed] [Google Scholar]
- 116.Sommer C, Exploring pain pathophysiology in patients. Science 354, 588–592 (2016). [DOI] [PubMed] [Google Scholar]
- 117.Wainger BJ, Buttermore ED, Oliveira JT, Mellin C, Lee S, Saber WA, Wang AJ, Ichida JK, Chiu IM, Barrett L, Huebner EA, Bilgin C, Tsujimoto N, Brenneis C, Kapur K, Rubin LL, Eggan K, Woolf CJ, Modeling pain in vitro using nociceptor neurons reprogrammed from fibroblasts. Nat Neurosci 18, 17–24 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tochitsky I, Jo S, Andrews N, Kotoda M, Doyle B, Shim J, Talbot S, Roberson D, Lee J, Haste L, Jordan SM, Levy BD, Bean BP, Woolf CJ, Inhibition of inflammatory pain and cough by a novel charged sodium channel blocker. Br J Pharmacol, (2021). [DOI] [PMC free article] [PubMed]
- 119.Roberson DP, Binshtok AM, Blasl F, Bean BP, Woolf CJ, Targeting of sodium channel blockers into nociceptors to produce long-duration analgesia: a systematic study and review. Br J Pharmacol 164, 48–58 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kuner R, Kuner T, Cellular Circuits in the Brain and Their Modulation in Acute and Chronic Pain. Physiol Rev 101, 213–258 (2021). [DOI] [PubMed] [Google Scholar]
- 121.Bannister K, Dickenson AH, Central Nervous System Targets: Supraspinal Mechanisms of Analgesia. Neurotherapeutics 17, 839–845 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hughes DI, Todd AJ, Central Nervous System Targets: Inhibitory Interneurons in the Spinal Cord. Neurotherapeutics 17, 874–885 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.O’Leary T, Williams AH, Franci A, Marder E, Cell types, network homeostasis, and pathological compensation from a biologically plausible ion channel expression model. Neuron 82, 809–821 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Sidders B, Karlsson A, Kitching L, Torella R, Karila P, Phelan A, Network-Based Drug Discovery: Coupling Network Pharmacology with Phenotypic Screening for Neuronal Excitability. J Mol Biol 430, 3005–3015 (2018). [DOI] [PubMed] [Google Scholar]
- 125.Jamieson DG, Moss A, Kennedy M, Jones S, Nenadic G, Robertson DL, Sidders B, The pain interactome: connecting pain-specific protein interactions. Pain 155, 2243–2252 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Csermely P, Agoston V, Pongor S, The efficiency of multi-target drugs: the network approach might help drug design. Trends Pharmacol Sci 26, 178–182 (2005). [DOI] [PubMed] [Google Scholar]
- 127.Brodie JS, Di Marzo V, Guy GW, Polypharmacology Shakes Hands with Complex Aetiopathology. Trends Pharmacol Sci 36, 802–821 (2015). [DOI] [PubMed] [Google Scholar]
- 128.Ray P, Torck A, Quigley L, Wangzhou A, Neiman M, Rao C, Lam T, Kim JY, Kim TH, Zhang MQ, Dussor G, Price TJ, Comparative transcriptome profiling of the human and mouse dorsal root ganglia: an RNA-seq-based resource for pain and sensory neuroscience research. Pain 159, 1325–1345 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Mele M, Ferreira PG, Reverter F, DeLuca DS, Monlong J, Sammeth M, Young TR, Goldmann JM, Pervouchine DD, Sullivan TJ, Johnson R, Segre AV, Djebali S, Niarchou A, Consortium GT, Wright FA, Lappalainen T, Calvo M, Getz G, Dermitzakis ET, Ardlie KG, Guigo R, Human genomics. The human transcriptome across tissues and individuals. Science 348, 660–665 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Goswami T, Li X, Smith AM, Luderowski EM, Vincent JJ, Rush J, Ballif BA, Comparative phosphoproteomic analysis of neonatal and adult murine brain. Proteomics 12, 2185–2189 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Usoskin D, Furlan A, Islam S, Abdo H, Lonnerberg P, Lou D, Hjerling-Leffler J, Haeggstrom J, Kharchenko O, Kharchenko PV, Linnarsson S, Ernfors P, Unbiased classification of sensory neuron types by large-scale single-cell RNA sequencing. Nat Neurosci 18, 145–153 (2015). [DOI] [PubMed] [Google Scholar]
- 132.Zeisel A, Hochgerner H, Lonnerberg P, Johnsson A, Memic F, van der Zwan J, Haring M, Braun E, Borm LE, La Manno G, Codeluppi S, Furlan A, Lee K, Skene N, Harris KD, Hjerling-Leffler J, Arenas E, Ernfors P, Marklund U, Linnarsson S, Molecular Architecture of the Mouse Nervous System. Cell 174, 999–1014 e1022 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Renthal W, Tochitsky I, Yang L, Cheng YC, Li E, Kawaguchi R, Geschwind DH, Woolf CJ, Transcriptional Reprogramming of Distinct Peripheral Sensory Neuron Subtypes after Axonal Injury. Neuron 108, 128–144 e129 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Hendriks D, Clevers H, Artegiani B, CRISPR-Cas Tools and Their Application in Genetic Engineering of Human Stem Cells and Organoids. Cell Stem Cell 27, 705–731 (2020). [DOI] [PubMed] [Google Scholar]
- 135.Koike-Yusa H, Li Y, Tan EP, Velasco-Herrera Mdel C, Yusa K, Genome-wide recessive genetic screening in mammalian cells with a lentiviral CRISPR-guide RNA library. Nat Biotechnol 32, 267–273 (2014). [DOI] [PubMed] [Google Scholar]
- 136.Mandegar MA, Huebsch N, Frolov EB, Shin E, Truong A, Olvera MP, Chan AH, Miyaoka Y, Holmes K, Spencer CI, Judge LM, Gordon DE, Eskildsen TV, Villalta JE, Horlbeck MA, Gilbert LA, Krogan NJ, Sheikh SP, Weissman JS, Qi LS, So PL, Conklin BR, CRISPR Interference Efficiently Induces Specific and Reversible Gene Silencing in Human iPSCs. Cell Stem Cell 18, 541–553 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Anzalone AV, Randolph PB, Davis JR, Sousa AA, Koblan LW, Levy JM, Chen PJ, Wilson C, Newby GA, Raguram A, Liu DR, Search-and-replace genome editing without double-strand breaks or donor DNA. Nature 576, 149–157 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Tian R, Gachechiladze MA, Ludwig CH, Laurie MT, Hong JY, Nathaniel D, Prabhu AV, Fernandopulle MS, Patel R, Abshari M, Ward ME, Kampmann M, CRISPR Interference-Based Platform for Multimodal Genetic Screens in Human iPSC-Derived Neurons. Neuron 104, 239–255 e212 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Black JB, McCutcheon SR, Dube S, Barrera A, Klann TS, Rice GA, Adkar SS, Soderling SH, Reddy TE, Gersbach CA, Master Regulators and Cofactors of Human Neuronal Cell Fate Specification Identified by CRISPR Gene Activation Screens. Cell Rep 33, 108460 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Eagles DA, Chow CY, King GF, Fifteen years of NaV 1.7 channels as an analgesic target: Why has excellent in vitro pharmacology not translated into in vivo analgesic efficacy? Br J Pharmacol, (2020). [DOI] [PubMed]
- 141.Arrowsmith J, Miller P, Trial watch: phase II and phase III attrition rates 2011–2012. Nat Rev Drug Discov 12, 569 (2013). [DOI] [PubMed] [Google Scholar]
- 142.Kushnarev M, Pirvulescu IP, Candido KD, Knezevic NN, Neuropathic pain: preclinical and early clinical progress with voltage-gated sodium channel blockers. Expert Opin Investig Drugs 29, 259–271 (2020). [DOI] [PubMed] [Google Scholar]
- 143.Yamanaka S, Induced pluripotent stem cells: past, present, and future. Cell Stem Cell 10, 678–684 (2012). [DOI] [PubMed] [Google Scholar]
- 144.Lin MZ, Schnitzer MJ, Genetically encoded indicators of neuronal activity. Nat Neurosci 19, 1142–1153 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Stacey P, Wassermann AM, Kammonen L, Impey E, Wilbrey A, Cawkill D, Plate-Based Phenotypic Screening for Pain Using Human iPSC-Derived Sensory Neurons. SLAS Discov 23, 585–596 (2018). [DOI] [PubMed] [Google Scholar]
- 146.Akin EJ, Higerd GP, Mis MA, Tanaka BS, Adi T, Liu S, Dib-Hajj FB, Waxman SG, Dib-Hajj SD, Building sensory axons: Delivery and distribution of NaV1.7 channels and effects of inflammatory mediators. Sci Adv 5, eaax4755 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Hughes RO, Bosanac T, Mao X, Engber TM, DiAntonio A, Milbrandt J, Devraj R, Krauss R, Small Molecule SARM1 Inhibitors Recapitulate the SARM1(−/−) Phenotype and Allow Recovery of a Metastable Pool of Axons Fated to Degenerate. Cell Rep 34, 108588 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Linghu C, Johnson SL, Valdes PA, Shemesh OA, Park WM, Park D, Piatkevich KD, Wassie AT, Liu Y, An B, Barnes SA, Celiker OT, Yao CC, Yu CJ, Wang R, Adamala KP, Bear MF, Keating AE, Boyden ES, Spatial Multiplexing of Fluorescent Reporters for Imaging Signaling Network Dynamics. Cell 183, 1682–1698 e1624 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Shemesh OA, Linghu C, Piatkevich KD, Goodwin D, Celiker OT, Gritton HJ, Romano MF, Gao R, Yu CJ, Tseng HA, Bensussen S, Narayan S, Yang CT, Freifeld L, Siciliano CA, Gupta I, Wang J, Pak N, Yoon YG, Ullmann JFP, Guner-Ataman B, Noamany H, Sheinkopf ZR, Park WM, Asano S, Keating AE, Trimmer JS, Reimer J, Tolias AS, Bear MF, Tye KM, Han X, Ahrens MB, Boyden ES, Precision Calcium Imaging of Dense Neural Populations via a Cell-Body-Targeted Calcium Indicator. Neuron 107, 470–486 e411 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Swinney DC, Anthony J, How were new medicines discovered? Nat Rev Drug Discov 10, 507–519 (2011). [DOI] [PubMed] [Google Scholar]
- 151.Inglese J, Auld DS, Jadhav A, Johnson RL, Simeonov A, Yasgar A, Zheng W, Austin CP, Quantitative high-throughput screening: a titration-based approach that efficiently identifies biological activities in large chemical libraries. Proc Natl Acad Sci U S A 103, 11473–11478 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Chen Y, Tristan CA, Chen L, Jovanovic VM, Malley C, Chu PH, Ryu S, Deng T, Ormanoglu P, Tao D, Fang Y, Slamecka J, Hong H, LeClair CA, Michael S, Austin CP, Simeonov A, Singec I, A versatile polypharmacology platform promotes cytoprotection and viability of human pluripotent and differentiated cells. Nat Methods 18, 528–541 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Mathews Griner LA, Guha R, Shinn P, Young RM, Keller JM, Liu D, Goldlust IS, Yasgar A, McKnight C, Boxer MB, Duveau DY, Jiang JK, Michael S, Mierzwa T, Huang W, Walsh MJ, Mott BT, Patel P, Leister W, Maloney DJ, Leclair C, Rai G, Jadhav A, Peyser BD, Austin CP, Martin SE, Simeonov A, Ferrer M, Staudt LM, Thomas CJ, High-throughput combinatorial screening identifies drugs that cooperate with ibrutinib to kill activated B-cell-like diffuse large B-cell lymphoma cells. Proc Natl Acad Sci U S A 111, 2349–2354 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Lee G, Ramirez CN, Kim H, Zeltner N, Liu B, Radu C, Bhinder B, Kim YJ, Choi IY, Mukherjee-Clavin B, Djaballah H, Studer L, Large-scale screening using familial dysautonomia induced pluripotent stem cells identifies compounds that rescue IKBKAP expression. Nat Biotechnol 30, 1244–1248 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gribkoff VK, Kaczmarek LK, The need for new approaches in CNS drug discovery: Why drugs have failed, and what can be done to improve outcomes. Neuropharmacology 120, 11–19 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Kimbrel EA, Lanza R, Next-generation stem cells - ushering in a new era of cell-based therapies. Nat Rev Drug Discov 19, 463–479 (2020). [DOI] [PubMed] [Google Scholar]
- 157.Choi J, Lee S, Mallard W, Clement K, Tagliazucchi GM, Lim H, Choi IY, Ferrari F, Tsankov AM, Pop R, Lee G, Rinn JL, Meissner A, Park PJ, Hochedlinger K, A comparison of genetically matched cell lines reveals the equivalence of human iPSCs and ESCs. Nat Biotechnol 33, 1173–1181 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Ortmann D, Vallier L, Variability of human pluripotent stem cell lines. Curr Opin Genet Dev 46, 179–185 (2017). [DOI] [PubMed] [Google Scholar]
- 159.Madian AG, Wheeler HE, Jones RB, Dolan ME, Relating human genetic variation to variation in drug responses. Trends Genet 28, 487–495 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Peters MJ, Broer L, Willemen HL, Eiriksdottir G, Hocking LJ, Holliday KL, Horan M, Meulenbelt I, Neogi T, Popham M, Schmidt CO, Soni A, Valdes AM, Amin N, Dennison EM, Eijkelkamp N, Harris TB, Hart DJ, Hofman A, Huygen FJ, Jameson KA, Jones GT, Launer LJ, Kerkhof HJ, de Kruijf M, McBeth J, Kloppenburg M, Ollier WE, Oostra B, Payton A, Rivadeneira F, Smith BH, Smith AV, Stolk L, Teumer A, Thomson W, Uitterlinden AG, Wang K, van Wingerden SH, Arden NK, Cooper C, Felson D, Gudnason V, Macfarlane GJ, Pendleton N, Slagboom PE, Spector TD, Volzke H, Kavelaars A, van Duijn CM, Williams FM, van Meurs JB, Genome-wide association study meta-analysis of chronic widespread pain: evidence for involvement of the 5p15.2 region. Ann Rheum Dis 72, 427–436 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Zorina-Lichtenwalter K, Parisien M, Diatchenko L, Genetic studies of human neuropathic pain conditions: a review. Pain 159, 583–594 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Meng W, Adams MJ, Palmer CNA, andMe Research T, Shi J, Auton A, Ryan KA, Jordan JM, Mitchell BD, Jackson RD, Yau MS, McIntosh AM, Smith BH, Genome-wide association study of knee pain identifies associations with GDF5 and COL27A1 in UK Biobank. Commun Biol 2, 321 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Naureen Z, Lorusso L, Manganotti P, Caruso P, Mazzon G, Cecchin S, Marceddu G, Bertelli M, Genetics of pain: From rare Mendelian disorders to genetic predisposition to pain. Acta Biomed 91, e2020010 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Bennett DL, Clark AJ, Huang J, Waxman SG, Dib-Hajj SD, The Role of Voltage-Gated Sodium Channels in Pain Signaling. Physiol Rev 99, 1079–1151 (2019). [DOI] [PubMed] [Google Scholar]
- 165.Yang Y, Wang Y, Li S, Xu Z, Li H, Ma L, Fan J, Bu D, Liu B, Fan Z, Wu G, Jin J, Ding B, Zhu X, Shen Y, Mutations in SCN9A, encoding a sodium channel alpha subunit, in patients with primary erythermalgia. J Med Genet 41, 171–174 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Mis MA, Yang Y, Tanaka BS, Gomis-Perez C, Liu S, Dib-Hajj F, Adi T, Garcia-Milian R, Schulman BR, Dib-Hajj SD, Waxman SG, Resilience to Pain: A Peripheral Component Identified Using Induced Pluripotent Stem Cells and Dynamic Clamp. J Neurosci 39, 382–392 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Chen L, Wimalasena NK, Shim J, Han C, Lee SI, Gonzalez-Cano R, Estacion M, Faber CG, Lauria G, Dib-Hajj SD, Woolf CJ, Waxman SG, Two independent mouse lines carrying the Nav1.7 I228M gain-of-function variant display dorsal root ganglion neuron hyperexcitability but a minimal pain phenotype. Pain 162, 1758–1770 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Brewer JR, Morrison G, Dolan ME, Fleming GF, Chemotherapy-induced peripheral neuropathy: Current status and progress. Gynecol Oncol 140, 176–183 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Komatsu M, Wheeler HE, Chung S, Low SK, Wing C, Delaney SM, Gorsic LK, Takahashi A, Kubo M, Kroetz DL, Zhang W, Nakamura Y, Dolan ME, Pharmacoethnicity in Paclitaxel-Induced Sensory Peripheral Neuropathy. Clin Cancer Res 21, 4337–4346 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Wheeler HE, Wing C, Delaney SM, Komatsu M, Dolan ME, Modeling chemotherapeutic neurotoxicity with human induced pluripotent stem cell-derived neuronal cells. PLoS One 10, e0118020 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Shi Y, Inoue H, Wu JC, Yamanaka S, Induced pluripotent stem cell technology: a decade of progress. Nat Rev Drug Discov 16, 115–130 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Thomson JA, Itskovitz-Eldor J, Shapiro SS, Waknitz MA, Swiergiel JJ, Marshall VS, Jones JM, Embryonic stem cell lines derived from human blastocysts. Science 282, 1145–1147 (1998). [DOI] [PubMed] [Google Scholar]
- 173.Takahashi K, Yamanaka S, Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126, 663–676 (2006). [DOI] [PubMed] [Google Scholar]
- 174.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, Yamanaka S, Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131, 861–872 (2007). [DOI] [PubMed] [Google Scholar]
- 175.Warren L, Manos PD, Ahfeldt T, Loh YH, Li H, Lau F, Ebina W, Mandal PK, Smith ZD, Meissner A, Daley GQ, Brack AS, Collins JJ, Cowan C, Schlaeger TM, Rossi DJ, Highly efficient reprogramming to pluripotency and directed differentiation of human cells with synthetic modified mRNA. Cell Stem Cell 7, 618–630 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Schlaeger TM, Daheron L, Brickler TR, Entwisle S, Chan K, Cianci A, DeVine A, Ettenger A, Fitzgerald K, Godfrey M, Gupta D, McPherson J, Malwadkar P, Gupta M, Bell B, Doi A, Jung N, Li X, Lynes MS, Brookes E, Cherry AB, Demirbas D, Tsankov AM, Zon LI, Rubin LL, Feinberg AP, Meissner A, Cowan CA, Daley GQ, A comparison of non-integrating reprogramming methods. Nat Biotechnol 33, 58–63 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Kampmann M, CRISPR-based functional genomics for neurological disease. Nat Rev Neurol 16, 465–480 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Rivetti di Val Cervo P, Besusso D, Conforti P, Cattaneo E, hiPSCs for predictive modelling of neurodegenerative diseases: dreaming the possible. Nat Rev Neurol 17, 381–392 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Davidson S, Copits BA, Zhang J, Page G, Ghetti A, Gereau R. W. t., Human sensory neurons: Membrane properties and sensitization by inflammatory mediators. Pain 155, 1861–1870 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Rossant J, Mouse and human blastocyst-derived stem cells: vive les differences. Development 142, 9–12 (2015). [DOI] [PubMed] [Google Scholar]
- 181.Rostock C, Schrenk-Siemens K, Pohle J, Siemens J, Human vs. Mouse Nociceptors - Similarities and Differences. Neuroscience 387, 13–27 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Osafune K, Caron L, Borowiak M, Martinez RJ, Fitz-Gerald CS, Sato Y, Cowan CA, Chien KR, Melton DA, Marked differences in differentiation propensity among human embryonic stem cell lines. Nat Biotechnol 26, 313–315 (2008). [DOI] [PubMed] [Google Scholar]
- 183.Schwartzentruber J, Foskolou S, Kilpinen H, Rodrigues J, Alasoo K, Knights AJ, Patel M, Goncalves A, Ferreira R, Benn CL, Wilbrey A, Bictash M, Impey E, Cao L, Lainez AJ Loucif, Whiting PJ, Consortium H, Gutteridge A, Gaffney DJ, Molecular and functional variation in iPSC-derived sensory neurons. Nat Genet 50, 54–61 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Chambers SM, Qi Y, Mica Y, Lee G, Zhang XJ, Niu L, Bilsland J, Cao L, Stevens E, Whiting P, Shi SH, Studer L, Combined small-molecule inhibition accelerates developmental timing and converts human pluripotent stem cells into nociceptors. Nat Biotechnol 30, 715–720 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Eberhardt E, Havlicek S, Schmidt D, Link AS, Neacsu C, Kohl Z, Hampl M, Kist AM, Klinger A, Nau C, Schuttler J, Alzheimer C, Winkler J, Namer B, Winner B, Lampert A, Pattern of Functional TTX-Resistant Sodium Channels Reveals a Developmental Stage of Human iPSC- and ESC-Derived Nociceptors. Stem Cell Reports 5, 305–313 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Cao L, McDonnell A, Nitzsche A, Alexandrou A, Saintot PP, Loucif AJ, Brown AR, Young G, Mis M, Randall A, Waxman SG, Stanley P, Kirby S, Tarabar S, Gutteridge A, Butt R, McKernan RM, Whiting P, Ali Z, Bilsland J, Stevens EB, Pharmacological reversal of a pain phenotype in iPSC-derived sensory neurons and patients with inherited erythromelalgia. Sci Transl Med 8, 335ra356 (2016). [DOI] [PubMed] [Google Scholar]
- 187.Meents JE, Bressan E, Sontag S, Foerster A, Hautvast P, Rosseler C, Hampl M, Schuler H, Goetzke R, Le TKC, Kleggetveit IP, Le Cann K, Kerth C, Rush AM, Rogers M, Kohl Z, Schmelz M, Wagner W, Jorum E, Namer B, Winner B, Zenke M, Lampert A, The role of Nav1.7 in human nociceptors: insights from human induced pluripotent stem cell-derived sensory neurons of erythromelalgia patients. Pain 160, 1327–1341 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Lippmann ES, Williams CE, Ruhl DA, Estevez-Silva MC, Chapman ER, Coon JJ, Ashton RS, Deterministic HOX patterning in human pluripotent stem cell-derived neuroectoderm. Stem Cell Reports 4, 632–644 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Kim HS, Lee J, Lee DY, Kim YD, Kim JY, Lim HJ, Lim S, Cho YS, Schwann Cell Precursors from Human Pluripotent Stem Cells as a Potential Therapeutic Target for Myelin Repair. Stem Cell Reports 8, 1714–1726 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Mukherjee-Clavin B, Mi R, Kern B, Choi IY, Lim H, Oh Y, Lannon B, Kim KJ, Bell S, Hur JK, Hwang W, Che YH, Habib O, Baloh RH, Eggan K, Brandacher G, Hoke A, Studer L, Kim YJ, Lee G, Comparison of three congruent patient-specific cell types for the modelling of a human genetic Schwann-cell disorder. Nat Biomed Eng 3, 571–582 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Hasselmann J, Blurton-Jones M, Human iPSC-derived microglia: A growing toolset to study the brain’s innate immune cells. Glia 68, 721–739 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Krencik R, Weick JP, Liu Y, Zhang ZJ, Zhang SC, Specification of transplantable astroglial subtypes from human pluripotent stem cells. Nat Biotechnol 29, 528–534 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Tcw J, Wang M, Pimenova AA, Bowles KR, Hartley BJ, Lacin E, Machlovi SI, Abdelaal R, Karch CM, Phatnani H, Slesinger PA, Zhang B, Goate AM, Brennand KJ, An Efficient Platform for Astrocyte Differentiation from Human Induced Pluripotent Stem Cells. Stem Cell Reports 9, 600–614 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Tchieu J, Calder EL, Guttikonda SR, Gutzwiller EM, Aromolaran KA, Steinbeck JA, Goldstein PA, Studer L, NFIA is a gliogenic switch enabling rapid derivation of functional human astrocytes from pluripotent stem cells. Nat Biotechnol 37, 267–275 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Maroof AM, Keros S, Tyson JA, Ying SW, Ganat YM, Merkle FT, Liu B, Goulburn A, Stanley EG, Elefanty AG, Widmer HR, Eggan K, Goldstein PA, Anderson SA, Studer L, Directed differentiation and functional maturation of cortical interneurons from human embryonic stem cells. Cell Stem Cell 12, 559–572 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Zhang Y, Pak C, Han Y, Ahlenius H, Zhang Z, Chanda S, Marro S, Patzke C, Acuna C, Covy J, Xu W, Yang N, Danko T, Chen L, Wernig M, Sudhof TC, Rapid single-step induction of functional neurons from human pluripotent stem cells. Neuron 78, 785–798 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Rowe RG, Daley GQ, Induced pluripotent stem cells in disease modelling and drug discovery. Nat Rev Genet 20, 377–388 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Lee J, Rabbani CC, Gao H, Steinhart MR, Woodruff BM, Pflum ZE, Kim A, Heller S, Liu Y, Shipchandler TZ, Koehler KR, Hair-bearing human skin generated entirely from pluripotent stem cells. Nature 582, 399–404 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Mazzara PG, Muggeo S, Luoni M, Massimino L, Zaghi M, Valverde PT, Brusco S, Marzi MJ, Palma C, Colasante G, Iannielli A, Paulis M, Cordiglieri C, Giannelli SG, Podini P, Gellera C, Taroni F, Nicassio F, Rasponi M, Broccoli V, Frataxin gene editing rescues Friedreich’s ataxia pathology in dorsal root ganglia organoid-derived sensory neurons. Nat Commun 11, 4178 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Xiang Y, Tanaka Y, Cakir B, Patterson B, Kim KY, Sun P, Kang YJ, Zhong M, Liu X, Patra P, Lee SH, Weissman SM, Park IH, hESC-Derived Thalamic Organoids Form Reciprocal Projections When Fused with Cortical Organoids. Cell Stem Cell 24, 487–497 e487 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Liu X, Michael S, Bharti K, Ferrer M, Song MJ, A biofabricated vascularized skin model of atopic dermatitis for preclinical studies. Biofabrication 12, 035002 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Low LA, Mummery C, Berridge BR, Austin CP, Tagle DA, Organs-on-chips: into the next decade. Nat Rev Drug Discov 20, 345–361 (2021). [DOI] [PubMed] [Google Scholar]
- 203.Sharma AD, McCoy L, Jacobs E, Willey H, Behn JQ, Nguyen H, Bolon B, Curley JL, Moore MJ, Engineering a 3D functional human peripheral nerve in vitro using the Nerve-on-a-Chip platform. Sci Rep 9, 8921 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Paull D, Sevilla A, Zhou H, Hahn AK, Kim H, Napolitano C, Tsankov A, Shang L, Krumholz K, Jagadeesan P, Woodard CM, Sun B, Vilboux T, Zimmer M, Forero E, Moroziewicz DN, Martinez H, Malicdan MC, Weiss KA, Vensand LB, Dusenberry CR, Polus H, Sy KT, Kahler DJ, Gahl WA, Solomon SL, Chang S, Meissner A, Eggan K, Noggle SA, Automated, high-throughput derivation, characterization and differentiation of induced pluripotent stem cells. Nat Methods 12, 885–892 (2015). [DOI] [PubMed] [Google Scholar]
- 205.Daniszewski M, Crombie DE, Henderson R, Liang HH, Wong RCB, Hewitt AW, Pebay A, Automated Cell Culture Systems and Their Applications to Human Pluripotent Stem Cell Studies. SLAS Technol 23, 315–325 (2018). [DOI] [PubMed] [Google Scholar]
- 206.Tristan CA, Ormanoglu P, Slamecka J, Malley C, Chu PH, Jovanovic VM, Gedik Y, Bonney C, Barnaeva E, Braisted J, Mallanna SK, Dorjsuren D, Iannotti MJ, Voss C, Michael S, Simeonov A, Singec I, Robotic High-Throughput Biomanufacturing and Functional Differentiation of Human Pluripotent Stem Cells. bioRxiv, (2020). [DOI] [PMC free article] [PubMed]
- 207.Manion J, Khuong T, Harney D, Littleboy JB, Ruan T, Loo L, Costigan M, Larance M, Caron L, Neely GG, Human induced pluripotent stem cell-derived GABAergic interneuron transplants attenuate neuropathic pain. Pain 161, 379–387 (2020). [DOI] [PubMed] [Google Scholar]
- 208.Fandel TM, Trivedi A, Nicholas CR, Zhang H, Chen J, Martinez AF, Noble-Haeusslein LJ, Kriegstein AR, Transplanted Human Stem Cell-Derived Interneuron Precursors Mitigate Mouse Bladder Dysfunction and Central Neuropathic Pain after Spinal Cord Injury. Cell Stem Cell 19, 544–557 (2016). [DOI] [PubMed] [Google Scholar]
- 209.Braz JM, Etlin A, Juarez-Salinas D, Llewellyn-Smith IJ, Basbaum AI, Rebuilding CNS inhibitory circuits to control chronic neuropathic pain and itch. Prog Brain Res 231, 87–105 (2017). [DOI] [PubMed] [Google Scholar]
- 210.Zuniga RE, Schlicht CR, Abram SE, Intrathecal baclofen is analgesic in patients with chronic pain. Anesthesiology 92, 876–880 (2000). [DOI] [PubMed] [Google Scholar]
- 211.Goldman SA, Kuypers NJ, How to make an oligodendrocyte. Development 142, 3983–3995 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Scholz J, Woolf CJ, The neuropathic pain triad: neurons, immune cells and glia. Nat Neurosci 10, 1361–1368 (2007). [DOI] [PubMed] [Google Scholar]
- 213.Huang CW, Huang WC, Qiu X, Fernandes F. Ferreira da Silva A. Wang, Patel S, Nesti LJ, Poo MM, Li S, The Differentiation Stage of Transplanted Stem Cells Modulates Nerve Regeneration. Sci Rep 7, 17401 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Kimura H, Ouchi T, Shibata S, Amemiya T, Nagoshi N, Nakagawa T, Matsumoto M, Okano H, Nakamura M, Sato K, Stem cells purified from human induced pluripotent stem cell-derived neural crest-like cells promote peripheral nerve regeneration. Sci Rep 8, 10071 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Lee G, Chambers SM, Tomishima MJ, Studer L, Derivation of neural crest cells from human pluripotent stem cells. Nat Protoc 5, 688–701 (2010). [DOI] [PubMed] [Google Scholar]
- 216.Hanani M, Spray DC, Emerging importance of satellite glia in nervous system function and dysfunction. Nat Rev Neurosci 21, 485–498 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Inoue K, Tsuda M, Microglia in neuropathic pain: cellular and molecular mechanisms and therapeutic potential. Nat Rev Neurosci 19, 138–152 (2018). [DOI] [PubMed] [Google Scholar]
- 218.Yu X, Liu H, Hamel KA, Morvan MG, Yu S, Leff J, Guan Z, Braz JM, Basbaum AI, Dorsal root ganglion macrophages contribute to both the initiation and persistence of neuropathic pain. Nat Commun 11, 264 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Andrews PW, Ben-David U, Benvenisty N, Coffey P, Eggan K, Knowles BB, Nagy A, Pera M, Reubinoff B, Rugg-Gunn PJ, Stacey GN, Assessing the Safety of Human Pluripotent Stem Cells and Their Derivatives for Clinical Applications. Stem Cell Reports 9, 1–4 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Guedon JM, Wu S, Zheng X, Churchill CC, Glorioso JC, Liu CH, Liu S, Vulchanova L, Bekker A, Tao YX, Kinchington PR, Goins WF, Fairbanks CA, Hao S, Current gene therapy using viral vectors for chronic pain. Mol Pain 11, 27 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Cai W, Cao J, Ren X, Qiao L, Chen X, Li M, Zang W, shRNA mediated knockdown of Nav1.7 in rat dorsal root ganglion attenuates pain following burn injury. BMC Anesthesiol 16, 59 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Rinaldi C, Wood MJA, Antisense oligonucleotides: the next frontier for treatment of neurological disorders. Nat Rev Neurol 14, 9–21 (2018). [DOI] [PubMed] [Google Scholar]
- 223.Vega-Loza A, Van C, M. M, Aleman F, Gene therapies to reduce chronic pain: are we there yet? Pain Manag 10, 209–212 (2020). [DOI] [PubMed] [Google Scholar]
- 224.Wang D, Zhang F, Gao G, CRISPR-Based Therapeutic Genome Editing: Strategies and In Vivo Delivery by AAV Vectors. Cell 181, 136–150 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Montgomery KL, Iyer SM, Christensen AJ, Deisseroth K, Delp SL, Beyond the brain: Optogenetic control in the spinal cord and peripheral nervous system. Sci Transl Med 8, 337rv335 (2016). [DOI] [PubMed] [Google Scholar]
- 226.Roth BL, DREADDs for Neuroscientists. Neuron 89, 683–694 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Weir GA, Middleton SJ, Clark AJ, Daniel T, Khovanov N, McMahon SB, Bennett DL, Using an engineered glutamate-gated chloride channel to silence sensory neurons and treat neuropathic pain at the source. Brain 140, 2570–2585 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Michoud F, Seehus C, Schonle P, Brun N, Taub D, Zhang Z, Jain A, Furfaro I, Akouissi O, Moon R, Meier P, Galan K, Doyle B, Tetreault M, Talbot S, Browne LE, Huang Q, Woolf CJ, Lacour SP, Epineural optogenetic activation of nociceptors initiates and amplifies inflammation. Nat Biotechnol 39, 179–185 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]