Abstract
Herniaria hemistemon J.Gay is widely used in folk medicine to treat hernia. The present study aimed to annotate the phytoconstituents of H. hemistemon aerial-part extract and investigate its in vivo anticryptosporidial activity. The chemical characterization was achieved via the LC–ESI–MS/MS technique resulting in the annotation of 37 phytocompounds comprising flavonoids and phenolic acids. Regarding the anticryptosporidial activity, fifty dexamethasone-immunosuppressed mice were separated into five groups: GI, un-infected (normal control); GII, infected but not treated (model); GIII, infected and received NTZ, the reference drug; GIV, infected and received H. hemistemon extract (100 mg/kg); and GV, infected and received H. hemistemon extract (200 mg/kg). When GIII, GIV, and GV were compared to GII, parasitological analyses displayed highly significant differences in the mean numbers of Cryptosporidium parvum oocysts in the stool between the different groups. GV demonstrated the highest efficacy of 79%. Histopathological analyses displayed improvement in the small intestine and liver pathology in the treated groups (GIII, IV, and V) related to the model (GII), with GV showing the highest efficacy. Moreover, the docking-based study tentatively highlighted the potential of benzoic acid derivatives as lactate dehydrogenase inhibitors. The docked compounds showed the same binding interactions as oxamic acid, where they established H-bond interactions with ARG-109, ASN-140, ASP-168, ARG-171, and HIS-195. To sum up, H. hemistemon is a promising natural therapeutic agent for cryptosporidiosis.
Keywords: Herniaria hemistemon J.Gay, nitazoxanide, anticryptosporidiosis, polyphenols, LC–ESI–MS/MS, lactate dehydrogenase inhibitors
1. Introduction
Cryptosporidium spp. are intracellular protozoan of the phylum Apicomplexa that can invade the microvillous area of epithelial cells in the digestive tract of humans and other mammals [1]. In immunocompromised patients, cryptosporidiosis can produce major complications such as severe diarrhea, dehydration, electrolyte imbalance, and hepatic and respiratory disorders [2]. Although the nature of cryptosporidiosis is widely understood, the therapeutic options are still restricted. Supportive therapy and specific treatments by Nitazoxanide (NTZ), the only Food and Drug Administration (FDA)-approved medicine in the United States, are still the only options for these serious infections. Nitazoxanide has an impact on the parasite by preventing its metabolism-necessary anaerobic energy, which makes its clearance easier [3]. Unfortunately, NTZ’s impact on children suffering from malnutrition is limited, and it has no effect on immunocompromised hosts [4].
Medicinal herbs have been widely employed in the treatment of parasitic illnesses, among them cryptosporidiosis [5,6]. These include Egyptian propolis [7], Verbena Officinalis [8], Ficus carica [9], Olea europaea [9], and Asafoetida [1]. The genus Herniaria, family Caryophyllaceae, comprises nearly 89 genera and 2070 species with five widespread species of this genus in Egypt, namely H. Cyrenaica, H. fontanesii, H. hemistemon, H. hirsute, and H. arabica [10,11]. Traditionally, Herniaria spp. have been utilized as antispasmodic, hypotensive, litholytic, and antidiuretic agents [12,13]. Herniaria hemistemon J.Gay is an herb native to North Africa and Asia, spanning from Morocco to Iran and the Arabian Peninsula but currently introduced to other parts of the world. The plant has been traditionally used in the management of hernia [12,14]. It has also shown remarkable biological activities including antioxidant [15,16], antimicrobial [16], and in vitro antischistosomal [17]. Phytochemically, different categories of compounds have been reported in the herb such as flavonoids [15], phenolic acids, and coumarins [16].
Despite its prevalent utility and numerous traditional uses, there are no adequate reports regarding the effects of H. hemistemon against cryptosporidiosis in a murine model. Therefore, this study aimed to annotate the chemical constituents of H. hemistemon aerial-part extract and to explore its activity against cryptosporidiosis in a murine model and possible modes of action via a molecular docking study.
2. Materials and Methods
2.1. Plant Material, Extraction, and Fractionation
Aerial parts (1.2 Kg) of Herniaria hemistemon J.Gay were collected after permission from and in compliance with relevant international guidelines and legislation of Matrouh governorate, Egypt in April 2020. The identification and authentication of the plant were performed by specialists at El-Orman Botanical Garden, Giza, Egypt. A voucher specimen was preserved at the Medicinal Chemistry Department, TBRI under the accession code (H.h.ap.2020). The dry, powdered plant materials were extracted with 85% methanol (4 × 2 L) at room temperature. The obtained extract was concentrated under reduced pressure using Rotavapor (Buchi R-300) at 40 °C. The total extract was then defatted using petroleum ether to afford 103 g (the extraction yield was 8.5%).
2.2. Phytochemical Analysis, Total Phenolic (TPC) and Total Flavonoid (TFC) Contents and Antioxidant Properties
The chemical components of H. hemistemon aerial-part extract were tentatively identified using high-performance liquid chromatography–mass spectrometry (HPLC–PDA-MS/MS). The LC system was SHIMADZU LC MS 8050 (Shimadzu, Japan, USA) coupled with a triple quadruple spectrometer with an ESI source. The separation was performed via a C18 reversed-phase column (Zorbax Eclipse XDB-C18, rapid resolution, 4.6 × 150 mm, 3.5 µm, Agilent, USA). A gradient of water and acetonitrile (ACN) (0.1% formic acid each) was applied from 5% to 30% ACN over 45 and increased to 60 over the last 15 min with a flow rate of 1 mL/min. The samples were automatically injected using autosamplerSIL-40C xs. The instrument was controlled by LC solution software (Shimadzu, Japan). The MS operated in the negative mode. TPC, TFC, DPPH (2,2-diphenyl-1-picrylhydrazyl) and total antioxidant capacity (TAC) were assayed as previously described [18].
2.3. In Vivo Anticryptosporidial Activity
2.3.1. Animals
The experimental work was permitted by the ethical committee of Theodor Bilharz Research Institute (TBRI-REC) in accordance with internationally valid guidelines (protocol serial number: PT: 612). TBRI-REC operates in a manner consistent with the National Institute of Health (NIH) guide for the care and use of laboratory animals (Eighth edition) and adhered to the ARRIVE guidelines. Theodor Bilharz Biological Center provided laboratory male-bred, CDI-strain white Albino mice, which were about 4–6 weeks old and weighed 20–25 g. The experiments were conducted in the TBRI’s Animal House, in a well-ventilated plastic cage in conditioned rooms (24 ± 2 °C) with clean wood-chip bedding that were distant from direct sunlight, keeping a clean environment.
2.3.2. Immunosuppression and Induction of Infection
A total of 50 laboratory-bred white albino male mice were orally given 0.25 µg/g/day dexamethasone sodium phosphate (Dexazone) by gavage through an esophageal tube to suppress their immune systems. Dexazone was applied daily for two weeks before oral inoculation of Cryptosporidium oocysts, and it was given once per week for the entire study duration for each group [19].
The mice were separated into five groups. GI: immunosuppressed and uninfected (negative control). Mice (GII–GV) were gavaged and orally infected using an esophageal tube (day 0) with C. parvum oocysts (concentrated from the feces of naturally infected neonatal calves and genetically identified as C. parvum [8]). The infection dose was approximately 1000 oocysts of C. parvum dissolved in 200 μL PBS for each mouse. To ensure infection establishment, fecal pellets were collected and studied after one week of mice infection (7th day post-infection (PI)).
At the 7th day PI, drugs were administered via oral gavage:
-
-
GII: Immunosuppressed and infected (model);
-
-
GIII: Immunosuppressed, infected, and received NTZ (Nanazoxid, 100 mg/5 mL suspension, Medizen Pharmaceutical industries for Utopia Pharmaceuticals) at 100 mg/kg every day for 5 successive days [20]. The doses were derived by extrapolating therapeutic human doses to animal doses [21];
-
-
GIV: Immunosuppressed, infected, and received H. hemistemon extract at a dose of 100 mg/kg every day for 5 days;
-
-
GV: Immunosuppressed, infected, and received H. hemistemon extract at a dose of 200 mg/kg every day for 5 days.
2.3.3. Parasitological Examination
Fecal pellet collection was performed on the 12th day PI (the time of the end of therapeutic doses), stained using modified Zheil Nelsen stain (MZN), and examined with the x100 oil-immersion lens according to the reported procedures [22]. The parasite number was detected per gram of feces [23]. The efficacy percentages of NTZ and H. hemistemon were determined according to the following equation: efficacy (%) = mean value of infected non-treated group-mean value of infected treated group × 100/mean value of infected non-treated group [24].
2.4. Histopathological Examination
All mice were sacrificed 12 days after infection under light anesthesia by using isoflurane inhalation (Forane®, Baxter, UK). The jejunum, ileum, and sections from all segments of the liver tissue were collected and placed in a 10% buffered formalin solution for fixation, embedded in paraffin wax blocks, sectioned, and stained in the TBRI pathology lab with hematoxylin and eosin (H&E) to detect the pathological abnormalities [25].
2.5. Docking-Based Virtual Screening
The annotated compounds of the H. hemistemon aerial-part extract were virtually screened for their ability to inhibit lactate dehydrogenase (PDB codes: 4ND1) [26] using AutoDock Vina software [27]. Detailed methods are provided in the Supplementary File.
2.6. Statistical Analysis
Data analysis was performed using Microsoft Excel 2016 and the statistical package for social science ‘IBM SPSS Statistics for Windows, version 26 (IBM Corp., Armonk, NY, USA)’. Continuous, normally distributed variables were represented as mean ± SE with a confidence interval of 95%. The ANOVA test was used to compare the means of normally distributed variables between groups. p-values ≤ 0.05 were considered statistically significant.
3. Results
3.1. Chemical Profiling
The LC–ESI–MS/MS analysis of H. hemistemon aerial-part extract furnished 37 secondary metabolites belonging to organic and phenolic acids, coumarins, flavonoids, and their glycosides and sulfate derivatives (Figure 1 and Table 1). Noteworthy, three coumarin derivatives were annotated in the tested extract. They furnished an [M-H]- m/z at 339, 257, and 177, and daughter ions at 177, 121, and 133; they were assigned as esculetin glucoside, esculetin sulfate and their aglycone, esculetin, Figure 2. Several other compounds containing sulfate derivatives were also annotated in the extract. For instance, a signal furnished an [M-H]- m/z at 247 and daughter ions at 167 ([M-H-80], typical loss of sulfate moiety) along with other fragment ions (108, 123, 155, typical daughter ions of vanillic acid); it was assigned to vanillic acid sulfate (Table 1). Another signal demonstrated an [M-H]- m/z at 395 and daughter ions at 151, 255, 300 and 315; it was tentatively identified as isorhamnetin sulfate (Table 1).
Figure 1.
LC chromatogram of H. hemistemon aerial-part extract.
Table 1.
Chemical constituents of H. hemistemon aerial-part extract.
| No. | Rt | M-H | MS/MS | Proposed Compounds |
|---|---|---|---|---|
| 1 | 1.80 | 191 | 111 | Quinic acid |
| 2 | 3.30 | 169 | 125 | Gallic acid |
| 3 | 4.86 | 315 | 153 | Protocatechuic acid glucoside |
| 4 | 7.20 | 353 | 191 | Chlorogenic acid |
| 5 | 7.59 | 341 | 135, 179 | Caffeoyl glucose |
| 6 | 8.05 | 285 | 153 | Protocatechuic acid pentoside |
| 7 | 8.67 | 339 | 177 | Esculetin glucoside |
| 8 | 9.01 | 137 | 108 | Hydroxybenzoic acid |
| 9 | 10.33 | 447 | 153, 315 | Protocatechuic acid caffeoyl pentoside |
| 10 | 10.31 | 183 | 125 | Methyl gallate |
| 11 | 10.51 | 257 | 121 | Esculetin sulfate |
| 12 | 11.05 | 353 | 191 | Neochlorogenic acid |
| 13 | 12.37 | 177 | 121, 133 | Esculetin |
| 14 | 12.67 | 329 | 167 | Vanillic acid glucoside |
| 15 | 13.75 | 329 | 167 | Caffeoyl vanillic acid |
| 16 | 14.59 | 431 | 153 | Protocatechuic acid coumaroyl pentoside |
| 17 | 15.34 | 337 | 163, 191 | Coumaroylquinic acid |
| 18 | 15.44 | 247 | 167 | Vanillic acid sulfate |
| 19 | 16.46 | 305 | 151, 287 | Gallocatechin |
| 20 | 18.50 | 337 | 191 | Coumaroylquinic acid |
| 21 | 18.62 | 303 | 137 | Hydroxybenzoic acid methyl gallate |
| 22 | 18.92 | 563 | 353, 383 | Schaftoside |
| 23 | 19.66 | 755 | 255, 301 | Quercetin rhamnosyl-rutinoside |
| 24 | 19.85 | 563 | 353, 383 | Vicenin 1 |
| 25 | 19.88 | 625 | 179, 317 | Myricetin rutinoside |
| 26 | 21.93 | 739 | 179, 285, 575 | Kaempferol dirhamnosyl-glucoside |
| 27 | 22.61 | 769 | 299, 315 | Isorhamnetin rhamnosyl-rutinoside |
| 28 | 23.15 | 771 | 299, 315, 477 | Isorhamnetin digalactosyl-pentoside |
| 29 | 23.67 | 609 | 271, 301 | Rutin |
| 30 | 24.18 | 463 | 271, 301 | Quercetin glucoside |
| 31 | 25.65 | 593 | 285 | Kaempferol rutinoside |
| 32 | 27.12 | 515 | 161, 179, 191 | Dicaffeoylquinic acid |
| 33 | 27.45 | 623 | 300, 315 | Isorhamnetin rutinoside |
| 34 | 28.66 | 395 | 300, 315 | Isorhamnetin sulfate |
| 35 | 29.98 | 593 | 179, 271, 315 | Isorhamnetin rhamnosyl-pentoside |
| 36 | 30.64 | 515 | 161, 179, 353 | Dicaffeoylquinic acid |
| 37 | 32.74 | 409 | 271, 299, 314 | Isorhamnetin derivative |
Figure 2.
MS/MS spectra of esculetin glucoside ((a), compound 7), esculetin sulfate ((b), compound 11), and esculetin ((c), compound 13) from Table 1.
3.2. Total Polyphenols, Total Flavonoids and Antioxidant Properties
The H. hemistemon aerial-part extract displayed considerable total phenolic (TPC) and total flavonoid (TFC) contents when assayed using the Folin–Ciocalteu and aluminum chloride methods (Table 2). As expected, it also furnished promising antioxidant potential in two assays: DPPH and TAC (Table 2). These results might be attributed to the presence of a series of polyphenolics with antioxidant properties, among them kaempferol, quercetin, isorhamnetin and myricetin, along with their glycosides and sulfate derivatives, as well as several phenolic acids (Table 1).
Table 2.
Results of TPC, TFC, DPPH and TAC of H. hemistemon aerial-part extract.
| Sample | TPC | TFC | DPPH | TAC |
|---|---|---|---|---|
| mg GAE/g Plant Extract | mg RE/g Plant Extract | IC50 (µg/mL) | mg AAE/g Extract | |
| H. hemistemon extract | 163.84 ± 3.91 | 61.54 ± 3.07 | 9.53 ± 0.67 | 438.67 ± 3.05 |
| Ascorbic acid | - | 3.39 ± 1.52 | - |
Data are presented as mean ± S.D., n = 3. GAE: gallic acid equivalent; RE: rutin equivalent; AAE: ascorbic acid equivalent.
3.3. Parasitological Examination
C. parvum was not found in the uninfected mice (GI, the negative control group). On the other hand, all infected mice began excreting C. parvum oocysts, which were confirmed after seven days post-infection (PI). At the end of the experiment (12 days PI), the model (GII, infected, untreated) developed the highest oocyst intensity with a mean score of 90.4. In contrast, all treated groups showed lower oocyst intensities compared to the model (GII), with GV showing the best efficacy with an inhibition of 79% (Table 3).
Table 3.
Results from Cryptosporidium spp. oocyst in stool.
| Animal Groups | Cryptosporidium Oocysts (Mean Count/g Stool) | Inhibition % |
|---|---|---|
| GI (normal control) | - | - |
| GII (model) | 90.4 ± 1.33 | - |
| GIII (mice received the reference drug NTZ) | 29.6 ± 1.35 a | 67% |
| GIV (mice received H. hemistemon extract (100 mg/kg) | 35.7 ± 1.24 a,b | 61% |
| GV (mice received H. hemistemon extract (200 mg/kg) | 19.1 ± 1.2 a,b,c | 79% |
Data are expressed as mean ± SE × 103, n = 10. a,b,c Significantly different from the model, the reference drug, and GIV at p ≤ 0.5, respectively.
3.4. Effects of H. hemistemon Aerial-Part Extract on the Small Intestine
Following C. parvum oocyst induction, several deleterious pathological changes in the small intestine were observed. These included a marked broadening of the villi with a decreased ratio of villous height to crypt length, a dense infiltration of mononuclear inflammatory cells inside the villous core, villous tip-region degeneration, and high mucin production. Cryptosporidium oocysts were also present along the villi brush border and in the intestinal lumen as oval-to-rounded bodies. NTZ (the reference drug) and the extract, at the two dose levels, attenuated the aforementioned effects of the extract, and at 200 mg/kg, retained a nearly normal villous pattern (Figure 3).
Figure 3.
Histological photomicrographs of small intestinal sections (stained with H&E, unless mentioned otherwise, 200×). (a) Sections from normal mice (GI) displayed the normal structure of the villi with a preserved brush border and normal pattern of mucin secretion. (b) Sections from infected mice (GII) revealed a significant broadening of villi (red line), dense infiltration by mononuclear inflammatory cells (yellow arrows), and villous tip-region degeneration (green arrows). (c) Sections from infected mice (GII) revealed numerous adherent (green arrow) and separate cryptosporidium oocysts (red arrow, H&E stain, stained purple, 4–6 µm in diameter, 1000×). (d) Sections from infected mice (GIII) treated with the reference drug (NTZ) demonstrated mild villi broadening with focal tip-region degeneration and mild mononuclear inflammatory cell infiltration. (e) Sections from infected mice (GIV) treated with H. hemistemon extract (100 mg/kg) demonstrated moderate villous broadening (red line), infiltration by mononuclear inflammatory cells (yellow arrow), and focal degeneration of the villous tip regions (green arrow). (f) Sections from infected mice (GV) treated with H. hemistemon extract (200 mg/kg) showed a retained villous architecture, such as the recovery of an almost normal villous pattern with the occasional appearance of inflammatory cells.
3.5. Effects of H. hemistemon Aerial-Part Extract on the Liver
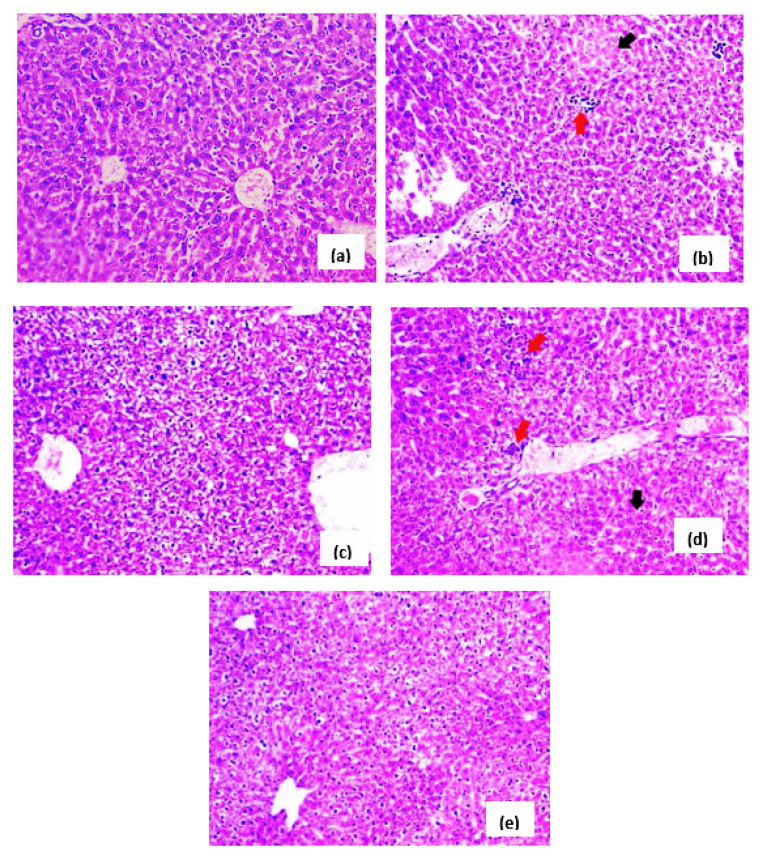
Similar to the small intestine, the induction of C. parvum oocysts induced caused pathological changes to the liver tissue. These included hepatocyte degeneration and focal infiltration with mononuclear cells. The reference drug (NTZ) and the extract, at 100 mg/kg, moderately improved the above-mentioned effects. Interestingly, the extract, at 200 mg/kg, retained the hepatic architecture (Figure 4).
Figure 4.
Histological photomicrographs of liver tissue (stained with H&E, unless mentioned otherwise, 200×). (a) Sections from normal mice (GI) showing a normal architecture of hepatic lobules. (b) Sections from infected mice (GII) displaying moderate degeneration of hepatocytes (black arrow) and focal infiltration with mononuclear cells (red arrow). (c) Sections from infected mice (GIII) treated with the reference drug, NTZ, displayed mild degeneration of hepatocytes. (d) Sections from infected mice (GIV) treated with H. hemistemon extract (100 mg/kg) showed moderate hepatocellular degeneration (black arrow) and focal infiltration by mononuclear cells (red arrow). (e) Sections from infected mice (GIV) treated with H. hemistemon extract (200 mg/kg) showed a retained hepatic lobular architecture.
3.6. Docking-Based Virtual Screening
Molecular modeling has become integral in biomedical research, minimizing lab work and facilitating the discovery of the most probable molecular targets and/or signaling pathways [28]. To putatively determine the mode of action of the H. hemistemon aerial-part extract, we subjected the structures of all annotated compounds to molecular inverse docking experiments using the idTarget platform (http://idtarget.rcas.sinica.edu.tw accessed on 1 October 2022) [29]. We found lactate dehydrogenase (PDB ID: 4ND1) as a probable target for gallic acid, hydroxybenzoic acid, and methyl gallate with affinity scores of −9.35, −8.89, and −8.73, respectively. As a validation step, the whole annotated structures were then re-docked into the enzyme’s active site using AutoDock Vina [30]. From the docked structures, quinic acid, hydroxybenzoic acid, gallic acid, and methyl gallate relatively better scores compared co-crystalized inhibitor (oxamic acid, Table 4). They also showed several interactions such as the co-crystalized inhibitor oxamic acid, including H-bond interactions with ARG-109, ASN-140, ASP-168, ARG-171, and HIS-195 (Figure 5). This docking-based study tentatively highlighted the potential of benzoic acid derivatives as C. parvum-derived lactate dehydrogenase inhibitors.
Table 4.
Docking scores of the annotated compounds in H. hemistemon aerial-part extract inside the active site of C. parvum-derived lactate dehydrogenase (PDB ID: 4ND1).
| Compound | Docking Score |
|---|---|
| Hydroxybenzoic acid | −7.4 |
| Quinic acid | −7.4 |
| Gallic acid | −7.3 |
| Methyl gallate | −7.1 |
| Esculetin | −4.6 |
| Dicaffeoylquinic acid | −4.4 |
| Isorhamnetin rhamnosyl-rutinoside | −4.1 |
| Hydroxybenzoic acid methyl gallate | −3.9 |
| Myricetin rutinoside | −3.8 |
| Kaempferol rutinoside | −3.6 |
| Kaempferol dirhamnosyl-glucoside | −3.4 |
| Isorhamnetin rutinoside | −3.3 |
| Rutin | −3.2 |
| Isorhamnetin sulfate | −3.2 |
| Caffeoyl glucose | −3.1 |
| Protocatechuic acid pentoside | −3.1 |
| Neochlorogenic acid | −3.1 |
| Vanillic acid glucoside | −3.1 |
| Quercetin glucoside | −3.1 |
| Caffeoyl vanillic acid | −3.0 |
| Chlorogenic acid | −2.8 |
| Vanillic acid sulfate | −2.8 |
| Gallocatechin | −2.7 |
| Coumaroylquinic acid | −2.6 |
| Vicenin 1 | −2.6 |
| Protocatechuic acid glucoside | −2.5 |
| Esculetin sulfate | −2.5 |
| Esculetin glucoside | −2.2 |
| Quercetin rhamnosyl-rutinoside | −2.1 |
| Schaftoside | −1.9 |
| Oxamic acid * | −6.1 |
* The reported co-crystalized inhibitor.
Figure 5.
(A–D): Binding modes of quinic acid, gallic acid, hydroxybenzoic acid, and methyl gallate inside the active site of C. parvum-derived lactate dehydrogenase (PDB ID: 4ND1). The four structures (in brick-red color) are aligned with the structure of the co-crystalized inhibitor oxamic acid (in orange color). The structure of the co-factor nicotinamide adenine dinucleotide (NAD) is shown in pink color.
4. Discussion
Cryptosporidiosis represents a serious health issue that can cause life-threatening diarrhea in immunocompromised people [2]. The lack of effective cryptosporidiosis therapies and vaccinations had led to the quest for an effective and safe anticryptosporidiosis therapy, particularly for immunocompromised hosts [4]. H. hemistemon has been proven to have antifungal effects against Aspergillus niger and Candida albicans, and antibacterial properties against both Gram-positive bacteria such as Enterococcus faecalis, Staphylococcus aureus, and Bacillus subtilis and Gram-negative bacteria such as Escherichia coli and Pseudomonas aeruginosa [16].
In this study, Herniaria hemistemon (H. hemistemon), which belongs to the family Caryophyllaceae, was tested against cryptosporidiosis in immunocompromised mice. The administration of H. hemistemon (GIV (100 mg/kg) and GV (200 mg/kg)) following challenged infection resulted in marked reductions in Cryptosporidium oocyst shedding (reduction of 61% and 79%, respectively) related to the infected control group. Interestingly, the H. hemistemon extract, at a dose of 200 mg/kg, displayed better activities than the reference drug, Nitazoxanide (reduction of 79% versus 67% in favor of the extract).
Additionally, a histopathological examination of the small intestine of GII (immunosuppressed and infected) revealed a deleterious effect on the structure of the intestinal mucosa compared to GI (immunosuppressed and not infected). Villous shortening and atrophy were observed, as well as a reduction in the ratio of villous height to mucosal ulceration, goblet cell depletion, crypt length, and infiltration of the lamina propria with inflammatory cells, primarily eosinophils and lymphocytes, as well as a diffuse loss of the brush border microvillous surface area. This was in line with several earlier studies [8,9]. Interestingly, these deleterious changes were inversed in GV (immunosuppressed, infected, and treated with H. hemistemon extract at a dose of 200 mg/kg). Noteworthily, H. hemistemon attenuated the adverse effects of cryptosporidiosis on the liver, where it retained a normal structure at the high dose of the extract.
The H. hemistemon aerial-part extract exhibited promising antioxidant activities, as well. The demonstrated activities (anticryptosporidiosis and antioxidant) could be explained by the presence of the 37 phytocompounds, detected in the extract, with reported antiparasitic and antioxidant properties. This was also supported by the docking study, which suggested that benzoic acid derivatives in the H. hemistemon extract (i.e., gallic acid, hydroxybenzoic acid, and methyl gallate) could act as C. parvum lactate dehydrogenase inhibitors and may serve as promising starting skeletons for the further development of more potent anti-C. parvum agents. Additionally, previous reports revealed that some identified compounds in the H. hemistemon extract showed potent antiparasitic effects against various types of parasites. For instance, rutin was evaluated in vitro for its ability to inhibit the replication of C. parvum [31]. Moreover, caffeoylquinic acids exhibited anthelmintic effects against Entamoeba histolytica [32]. Peña-Espinoza and his co-workers reported the antiparasitic effects of hydroxycinnamic acids, quercetin, and kaempferol derivatives against gastrointestinal parasites [33]. Furthermore, it was reported that polyphenolic compounds could be responsible for the anticryptosporidial activity of plant extracts through numerous modes of action, including interfering with essential parasite enzymes [34].
In general, the positive therapeutic effects of Herniaria in this study are consistent with previous reports that documented the medicinal use of the genus Herniaria as antispasmodic, antihypertensive, lithophytic, and diuretic agents. They demonstrated antioxidant and antimicrobial activities, as well [14]. Additionally, similar results were observed from several polyphenolic-rich extracts. These included pomegranate peels (red and white), Egyptian propolis, Verbena Officinalis, Ficus carica, and Olea europaea extracts; they significantly diminished the C. parvum oocyst count in infected mice with comparable activities to the reference drug, NTZ, and displayed a potential improvement in the shape and structure of the villi of ileal sections, as well [8,9,35].
5. Conclusions
The current work annotated 37 secondary metabolites from the H. hemistemon aerial-part extract via LC–ESI–MS/MS. It also suggested H. hemistemon as a promising anticryptosporidiosis agent, since it markedly decreased the C. parvum oocyst count in infected mice with better activities at 200 mg/kg than the reference drug, NTZ, and noticeably improved and retained the normal structure of the small intestine and liver tissue. To sum up, H. hemistemon could be considered as a potential candidate for further evaluation as an antiparasitic agent, food supplement, and livestock feed. Further experiments are needed to determine its toxicity and explore its individual components as well the involved mechanisms.
Acknowledgments
The authors would like to thank Ahmed M. Sayed for performing docking studies.
Abbreviations
HIV: human immunodeficiency virus; FDA: Food and Drug Administration; NTZ: Nitazoxanide; LC–ESI–MS/MS: liquid chromatography electrospray ionization mass spectrometry; m/z: mass-to-charge; Rt: retention time; TPC: total phenolic content; TFC: total flavonoid content; DPPH: 2,2-diphenyl-1-picrylhydrazyl; TAC: total antioxidant capacity; GAE: gallic acid equivalent; RE: rutin equivalent; AAE: ascorbic acid equivalent; S.D.: standard deviation; PI: post-infection; SE: standard error: TBRI: Theodor Bilharz Research Institute; H & E: hematoxylin and eosin; ANOVA: analysis of variance.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15020415/s1, Docking-based virtual screening [26,36,37,38,39,40].
Author Contributions
M.A.G.: Conceptualization, visualization, plant collection, extraction, antioxidant activity evaluation, phytochemical investigation, writing original draft-review & editing. M.S.: Investigation, visualization, writing original draft-review & editing. T.A.: Perform histopathological study, investigation, writing-original draft, review & editing. M.E.: Parasitological investigation, writing-original draft, review & editing. H.S.M.: Visualization, investigation & editing. E.S.E.-W.: Conceptualization, perform parasitological study, investigation, methodology, writing-original draft, review & editing. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All animal experiments were carried out under Institutional Ethical Committee rules for care and use of experimental animals, authorized by Theodor Bilharz Research Institute’s Animal Ethics Committee in Giza, Egypt (PT: (612)/TBRI-REC).
Informed Consent Statement
Not applicable.
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Abdelmaksoud H.F., El-Ashkar A.M., Elgohary S.A., El-Wakil E.S. Potential Therapeutic and Prophylactic Effects of Asafoetida in Murine Cryptosporidiosis. J. Parasit. Dis. 2020;44:646–653. doi: 10.1007/s12639-020-01241-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Guk S.-M., Seo M., Park Y.-K., Oh M.-D., Choe K.-W., Kim J.-L., Choi M.-H., Hong S.-T., Chai J.-Y. Parasitic Infections in HIV-Infected Patients Who Visited Seoul National University Hospital during the Period 1995–2003. Korean J. Parasitol. 2005;43:1. doi: 10.3347/kjp.2005.43.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.El-Wakil E.S., Salem A.E., Al-Ghandour A.M. Evaluation of Possible Prophylactic and Therapeutic Effect of Mefloquine on Experimental Cryptosporidiosis in Immunocompromised Mice. J. Parasit. Dis. 2021;45:380–393. doi: 10.1007/s12639-020-01315-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Diptyanusa A., Sari I.P. Treatment of Human Intestinal Cryptosporidiosis: A Review of Published Clinical Trials. Int. J. Parasitol. Drugs Drug Resist. 2021;17:128–138. doi: 10.1016/j.ijpddr.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Krstin S., Sobeh M., Braun M.S., Wink M. Anti-Parasitic Activities of Allium sativum and Allium cepa against Trypanosoma brucei and Leishmania tarentolae. Medicines. 2018;5:37. doi: 10.3390/medicines5020037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Krstin S., Sobeh M., Braun M.S., Wink M. Tulbaghia violacea and Allium ursinum Extracts Exhibit Anti-Parasitic and Antimicrobial Activities. Molecules. 2018;23:313. doi: 10.3390/molecules23020313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Soufy H., Nadia M., Nasr S.M., Abd El-Aziz T.H., Khalil F.A., Ahmed Y.F., Abou Zeina H.A. Effect of Egyptian Propolis on Cryptosporidiosis in Immunosuppressed Rats with Special Emphasis on Oocysts Shedding, Leukogram, Protein Profile and Ileum Histopathology. Asian Pac. J. Trop. Med. 2017;10:253–262. doi: 10.1016/j.apjtm.2017.03.004. [DOI] [PubMed] [Google Scholar]
- 8.El-Wakil E.S., El-Shazly M.A., El-Ashkar A.M., Aboushousha T., Ghareeb M.A. Chemical Profiling of Verbena officinalis and Assessment of Its Anti-Cryptosporidial Activity in Experimentally Infected Immunocompromised Mice. Arab. J. Chem. 2022;15:103945. doi: 10.1016/j.arabjc.2022.103945. [DOI] [Google Scholar]
- 9.Abd El-Hamed W.F., Yousef N.S., Mazrou Y.S., Elkholy W.A., El-Refaiy A.I., Elfeky F.A., Albadrani M., El-Tokhy A.I., Abdelaal K. Anticryptosporidium Efficacy of Olea europaea and Ficus carica Leaves Extract in Immunocompromised Mice Associated with Biochemical Characters and Antioxidative System. Cells. 2021;10:2419. doi: 10.3390/cells10092419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghazanfar S.A. Handbook of Arabian Medicinal Plants. CRC Press; Boca Raton, FL, USA: 1994. [Google Scholar]
- 11.Tackholm V., Boulos L. Students’ Flora of Egypt. Cairo University; Giza, Egypt: 1974. [Google Scholar]
- 12.Sabitha S., Maher K., Mohamed M. Medicinal Plants Diversity and Their Conservation Status in the United Arab Emirates (UAE) J. Med. Plants Res. 2012;6:1304–1322. doi: 10.5897/JMPR11.1412. [DOI] [Google Scholar]
- 13.Chandra S., Rawat D.S. Medicinal Plants of the Family Caryophyllaceae: A Review of Ethno-Medicinal Uses and Pharmacological Properties. Integr. Med. Res. 2015;4:123–131. doi: 10.1016/j.imr.2015.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kozachok S., Kolodziejczyk-Czepas J., Marchyshyn S., Wojtanowski K.K., Zgórka G., Oleszek W. Comparison of Phenolic Metabolites in Purified Extracts of Three Wild-Growing Herniaria L. Species and Their Antioxidant and Anti-Inflammatory Activities In Vitro. Molecules. 2022;27:530. doi: 10.3390/molecules27020530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Radwan N.M., Nazif L.M., Setta A. The Lipid and Flavonoidal Constituents of Herniaria nemistemon J. Gay and Their Biological Activity. Egypt. J. Pharm. Sci. 2006;47:29–41. [Google Scholar]
- 16.Elhagali G.A., Abozeed A.E., Youssif Y.M. Investigation of Bioactive Constituents and Biological Activities of Different Fractions from Herniaria hemistemon J. Gay. Al-Azhar Bull. Sci. 2019;30:67–80. doi: 10.21608/absb.2019.67894. [DOI] [Google Scholar]
- 17.Yousif F., Hifnawy M.S., Soliman G., Boulos L., Labib T., Mahmoud S., Ramzy F., Yousif M., Hassan I., Mahmoud K., et al. Large-Scale in Vitro. Screening of Egyptian Native and Cultivated Plants for Schistosomicidal Activity. Pharm. Biol. 2007;45:501–510. doi: 10.1080/13880200701389425. [DOI] [Google Scholar]
- 18.Ghareeb M.A., Mohamed T., Saad A.M., Refahy L.A.-G., Sobeh M., Wink M. HPLC-DAD-ESI-MS/MS Analysis of Fruits from Firmiana simplex (L.) and Evaluation of Their Antioxidant and Antigenotoxic Properties. J. Pharm. Pharmacol. 2018;70:133–142. doi: 10.1111/jphp.12843. [DOI] [PubMed] [Google Scholar]
- 19.Tarazona R., Blewett D.A., Carmona M.D. Cryptosporidium parvum Infestion in Experimentally Infected Mice: Infection Dynamics and Effect of Immunosuppression. Folia Parasitol. 1998;45:101–107. [PubMed] [Google Scholar]
- 20.Li X., Brasseur P., Agnamey P., Leméteil D., Favennec L., Ballet J.-J., Rossignol J.-F. Long-Lasting Anticryptosporidial Activity of Nitazoxanide in an Immunosuppressed Rat Model. Folia Parasitol. 2003;50:19–22. doi: 10.14411/fp.2003.003. [DOI] [PubMed] [Google Scholar]
- 21.Barnes J.M., Paget G.E. 2 Mechanisms of Toxic Action. Prog. Med. Chem. 1965;4:18–38. doi: 10.1016/s0079-6468(08)70166-8. [DOI] [PubMed] [Google Scholar]
- 22.Henriksen S.A., Pohlenz J.F.L. Staining of Cryptosporidia by a Modified Ziehl-Neelsen Technique. Acta Vet. Scand. 1981;22:594. doi: 10.1186/BF03548684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Benamrouz S., Guyot K., Gazzola S., Mouray A., Chassat T., Delaire B., Chabé M., Gosset P., Viscogliosi E., Dei-Cas E., et al. Cryptosporidium parvum Infection in SCID Mice Infected with Only One Oocyst: QPCR Assessment of Parasite Replication in Tissues and Development of Digestive Cancer. PLoS ONE. 2012;7:e51232. doi: 10.1371/journal.pone.0051232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hosking B.C., Watson T.G., Leathwick D.M. Multigeneric Resistance to Oxfendazole by Nematodes in Cattle. Vet. Rec. 1996;138:67. doi: 10.1136/vr.138.3.67. [DOI] [PubMed] [Google Scholar]
- 25.Feldman A.T., Wolfe D. Histopathology. Humana Press; New York, NY, USA: 2014. Tissue Processing and Hematoxylin and Eosin Staining; pp. 31–43. [DOI] [PubMed] [Google Scholar]
- 26.Cook W.J., Senkovich O., Hernandez A., Speed H., Chattopadhyay D. Biochemical and Structural Characterization of Cryptosporidium Parvum Lactate Dehydrogenase. Int. J. Biol. Macromol. 2015;74:608–619. doi: 10.1016/j.ijbiomac.2014.12.019. [DOI] [PubMed] [Google Scholar]
- 27.Trott O., Olson A.J. AutoDock Vina: Improving the Speed and Accuracy of Docking with a New Scoring Function, Efficient Optimization, and Multithreading. J. Comput. Chem. 2010;31:455–461. doi: 10.1002/jcc.21334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fan J., Fu A., Zhang L. Progress in Molecular Docking. Quant. Biol. 2019;7:83–89. doi: 10.1007/s40484-019-0172-y. [DOI] [Google Scholar]
- 29.Wang J.-C., Chu P.-Y., Chen C.-M., Lin J.-H. IdTarget: A Web Server for Identifying Protein Targets of Small Chemical Molecules with Robust Scoring Functions and a Divide-and-Conquer Docking Approach. Nucleic Acids Res. 2012;40:W393–W399. doi: 10.1093/nar/gks496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Huey R., Morris G.M., Forli S. Using AutoDock 4 and AutoDock Vina with AutoDockTools: A Tutorial. Vol. 10550. The Scripps Research Institute Molecular Graphics Laboratory; La Jolla, CA, USA: 2012. p. 92037. [Google Scholar]
- 31.Mead J.R., McNair N. Antiparasitic Activity of Flavonoids and Isoflavones against Cryptosporidium parvum and Encephalitozoon Intestinalis. FEMS Microbiol. Lett. 2006;259:153–157. doi: 10.1111/j.1574-6968.2006.00263.x. [DOI] [PubMed] [Google Scholar]
- 32.Scholz E., Heinrich M., Hunkler D. Caffeoylquinic Acids and Some Biological Activities of Pluchea symphytifolia. Planta Med. 1994;60:360–364. doi: 10.1055/s-2006-959501. [DOI] [PubMed] [Google Scholar]
- 33.Peña-Espinoza M., Valente A.H., Thamsborg S.M., Simonsen H.T., Boas U., Enemark H.L., López-Muñoz R., Williams A.R. Antiparasitic Activity of Chicory (Cichorium intybus) and Its Natural Bioactive Compounds in Livestock: A Review. Parasites Vectors. 2018;11:475. doi: 10.1186/s13071-018-3012-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Teichmann K., Kuliberda M., Schatzmayr G., Pacher T., Zitterl-Eglseer K., Joachim A., Hadacek F. In Vitro Inhibitory Effects of Plant-Derived by-Products against Cryptosporidium parvum. Parasite. 2016;23:41. doi: 10.1051/parasite/2016050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Aboelsoued D., Abo-Aziza F.A.M., Mahmoud M.H., Abdel Megeed K.N., Abu El Ezz N.M.T., Abu-Salem F.M. Anticryptosporidial Effect of Pomegranate Peels Water Extract in Experimentally Infected Mice with Special Reference to Some Biochemical Parameters and Antioxidant Activity. J. Parasit. Dis. 2019;43:215–228. doi: 10.1007/s12639-018-01078-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.O’Boyle N.M., Banck M., James C.A., Morley C., Vandermeersch T., Hutchison G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011;3:33. doi: 10.1186/1758-2946-3-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Morris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;30:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Eastman P., Friedrichs M.S., Chodera J.D., Radmer R.J., Bruns C.M., Ku J.P., Beauchamp K.A., Lane T.J., Wang L.-P., Shukla D., et al. OpenMM 4: A Reusable, Extensible, Hardware Independent Library for High Performance Molecular Simulation. J. Chem. Theory Comput. 2013;9:461–469. doi: 10.1021/ct300857j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dallakyan S., Olson A.J. In: Chemical Biology. Hempel J.E., Williams C.H., Hong C.C., editors. Springer; New York, NY, USA: 2015. pp. 243–250. [DOI] [Google Scholar]
- 40.Seeliger D., de Groot B.L. Ligand docking and binding site analysis with PyMOL and Autodock/Vina. J. Comput. Aided Mol. Des. 2010;24:417–422. doi: 10.1007/s10822-010-9352-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All data generated or analyzed during this study are included in this published article and its supplementary information files.