Abstract
Deletion of the (p)ppGpp synthetase gene, relA, of Streptomyces coelicolor A3(2) results in loss of production of the antibiotics actinorhodin (Act) and undecylprodigiosin (Red) and delayed morphological differentiation when the mutant is grown under conditions of nitrogen limitation. To analyze the role of (p)ppGpp as an intracellular signaling molecule for the initiation of antibiotic production, several C-terminally deleted derivatives of S. coelicolor relA that could potentially function in the absence of ribosome activation were placed under the control of the thiostrepton-inducible tipA promoter. While 0.82- and 1.28-kb N-terminal segments failed to restore (p)ppGpp and antibiotic production upon induction in a relA null mutant, 1.46- and 2.07-kb segments did. Under conditions of phosphate limitation, deletion of relA had little or no effect on Act or Red synthesis, potentially reflecting an alternative mechanism for ppGpp synthesis. A second S. coelicolor RelA homologue (RshA, with 42% identity to S. coelicolor RelA) was identified in the genome sequence. However, deletion of rshA had no effect on the ability of the relA mutant to make Act and Red when grown under conditions of phosphate limitation. While high-level induction of tipAp::rshA in the relA mutant resulted in growth inhibition, low-level induction restored antibiotic production and sporulation. In neither case, nor in the relA mutant that was grown under phosphate limitation and producing Act and Red, could (p)ppGpp synthesis be detected. Thus, a ppGpp-independent mechanism exists to activate antibiotic production under conditions of phosphate limitation that can be mimicked by overexpression of rshA.
Streptomycetes are gram-positive mycelial soil bacteria that produce a large number and wide variety of different secondary metabolites, many of which have important applications in human medicine and in agriculture as antibiotics or as compounds with other useful biological properties (29). Streptomyces coelicolor A3(2) is the most genetically characterized streptomycete and produces at least four chemically distinct antibiotics (16, 19), including the blue-pigmented polyketide actinorhodin (Act) and the red-pigmented tripyrolle undecylprodigiosin (Red). Production of the two pigmented compounds is generally confined to stationary phase in liquid culture and usually coincides with the onset of morphological differentiation in surface-grown cultures (5, 6). Expression of the act and red biosynthetic gene clusters is controlled by the pathway-specific regulatory genes actII-ORF4 and redD, respectively. Transcription of both of these genes increases markedly on entry into stationary phase and coincides with the intracellular accumulation of the highly phosphorylated guanosine nucleotides (p)ppGpp (43, 46). In Escherichia coli, (p)ppGpp has been implicated in the growth-rate control of gene expression and in the regulation of stationary-phase gene expression (reviewed in reference 2), roles that are consistent with a function for (p)ppGpp as an intracellular signal for antibiotic production in streptomycetes. E. coli possesses two enzymes, RelA and SpoT (with 32% amino acid sequence identity), that can synthesize (p)ppGpp from GTP and ATP. On nitrogen and amino acid starvation, uncharged tRNAs bind to the ribosomal A site and activate the ribosome-bound RelA, while carbon or phosphate starvation promotes SpoT-dependent (p)ppGpp synthesis in a ribosome-independent manner (2, 42). In both cases, the resulting (p)ppGpp is degraded to GDP by the (p)ppGpp hydrolase activity of SpoT, i.e., SpoT is a bifunctional enzyme capable of synthesizing and degrading (p)ppGpp (8, 28). Mutational analysis subsequently localized regions of SpoT responsible for the two competing activities. The catalytic sites are distinct but closely linked and located towards the N terminus of SpoT (8). In Streptococcus equisimilis (26, 27) and Corynebacterium glutamicum (49), there seems to be only one relA/spoT homologue, which is presumed to encode both synthetic and degradative functions. Moreover, inspection of the complete genome sequences of 10 bacterial species (Aquifex aeolicus, Bacillus subtilis, Borrelia burgdorferi, Campylobacter jejuni, Helicobacter pylori, Mycobacterium tuberculosis H37Rv, Mycoplasma genitalium G37, Synechocystis sp. strain PCC6803, Thermotoga maritima, and Ureaplasma urealyticum) on the website of the National Center for Biotechnology Information (http://www.ncbi.nlm.nih.gov/PMGifs /Genomes/bact.html) revealed only a single relA/spoT homologue in each genome.
The (p)ppGpp synthetase gene, relA, of S. coelicolor encodes a protein of 847 amino acids with a predicted molecular mass of 94.2 kDa that shows a high degree of similarity to all known RelA/SpoT homologues (3). S. coelicolor RelA, which clearly possesses synthetic activity (3, 4), shows a greater level of amino acid sequence identity to the SpoT homologue of E. coli (42.6% identity) than to its RelA counterpart (38.5% identity). Perhaps more significantly, S. coelicolor RelA shows more similarity to the region of E. coli SpoT deduced to encode degradative functions than to the corresponding region of RelA. Consistent with these sequence comparisons, S. coelicolor RelA has since been shown to possess both (p)ppGpp synthetase and hydrolase activities when expressed in E. coli (25). Replacement of most of the S. coelicolor relA coding region with a hygromycin resistance gene resulted in a relA null mutant (M570) that grew at the same rate as the parental strain (M600). It entered stationary phase at the same final optical density, but failed to produce (p)ppGpp on nitrogen limitation and was deficient in the production of both Act and Red. The latter was attributed to a marked decline in actII-ORF4 and redD transcription, respectively, on entry into stationary phase (3) and suggested a direct role for (p)ppGpp in activating transcription of the pathway-specific regulatory genes. Consistent with this, induction of (p)ppGpp synthesis by subjecting an exponentially growing culture to amino acid starvation resulted in activation of actII-ORF4 (43) and, if induced in late exponential growth, redD transcription (45). However, such a severe nutritional downshift subjects the culture to considerable physiological stress; the growth rate after amino acid starvation was markedly below that observed if the culture was grown ab initio in a modified form of the medium that lacked amino acids. Consequently, it was difficult to ascribe activation of actII-ORF4 and redD transcription to (p)ppGpp synthesis rather than to the severe physiological stress experienced by the culture. To circumvent this problem, we set out to establish a system in which (p)ppGpp synthesis could be induced under conditions of nutritional sufficiency. In earlier work, we had constructed a relA disruption mutant in which the 494 N-terminal amino acids of RelA had been retained. The mutant grew more slowly than the wild-type strain, reflecting elevated basal levels of (p)ppGpp synthesis that did not increase on amino acid starvation. This implied that the N-terminal region of S. coelicolor RelA possessed ribosome-independent (p)ppGpp synthetase activity. This prediction was confirmed by Martinez-Costa et al. (25), who examined expression of S. coelicolor relA, and deletion derivatives thereof, in E. coli. Both of these observations are consistent with work on E. coli relA which demonstrated that C-terminally deleted derivatives did indeed possess ribosome-independent (p)ppGpp synthetase activity (39). Here we describe a deletion analysis of S. coelicolor relA and the construction of an inducible system for (p)ppGpp synthesis under conditions of nutritional sufficiency that results in restoration of antibiotic production in a relA null mutant.
While the S. coelicolor relA null mutant fails to produce Act or Red under conditions of nitrogen limitation, under phosphate limitation both antibiotics are produced at or near wild-type levels. In principle, this might reflect an alternative mechanism for (p)ppGpp synthesis under conditions of phosphate limitation which is potentially analogous to SpoT-mediated (p)ppGpp production in E. coli under conditions of carbon and energy starvation. Intriguingly, a second member of the relA/spoT gene family, rshA (relA spoT homologue), was recently revealed through the S. coelicolor genome sequencing project (http://www.sanger.ac.uk/Projects/S.coelicolor). RshA shares 42% amino acid sequence identity with S. coelicolor RelA. To assess whether rshA might be responsible for (p)ppGpp synthesis, and hence antibiotic production, under conditions of phosphate limitation, the gene was subjected to mutational and overexpression analysis in S. coelicolor M600 (relA+) and M570 (ΔrelA).
MATERIALS AND METHODS
Bacterial strains and plasmids, culture conditions, and microbiological procedures.
E. coli DH5α (10) and ET12567/pUZ8002 (36) were used for routine subcloning and were grown and transformed according to methods described by Sambrook et al. (38). The S. coelicolor A3(2) strains used were M600 (SCP1− SCP2−) (21) and its derivative, M570 (ΔrelA) (3). pIJ8600 and pIJ2925 were as described previously (references 18 and 44, respectively). MS agar medium (also known as SFM [7]) was used to make spore suspensions and for plating out conjugations between E. coli and S. coelicolor. To assess antibiotic production on agar plates, nitrogen-limited SMMS, phosphate-limited R2, and complex R5 media were used (21). Modified Evan's medium containing 2 mM phosphate consisted of 5% (wt/vol) polyethylene glycol 6000, 0.01% (wt/vol) antifoam 289 (Sigma), 25 mM N-tris(hydroxymethyl)methyl-2-aminoethane sulfonic acid, 10 mM KCl, 2 mM Na2SO4, 2 mM citric acid, 1.25 mM MgCl2 · 6H2O, 0.25 mM CaCl2 · 2H2O, 1 μM Na2MO4 · 2H2O, 0.5% (vol/vol) trace metals, 139 mM glucose, 102 mM NaNO3, and 2 mM NaH2PO4 · 2H2O. For carbon-limited medium 28 mM glucose was used, and for nitrogen-limited medium 10.2 mM NaNO3 was used. Trace metal solution was prepared by dissolving 0.31 g of H3BO4, 27.0 g of FeCl3 · 6H2O, 10.0 g of MnCl2 · 4H2O, 0.85 g of CuCl2 · 2H2O, and 2.38 g of CoCl2 · 6H2O in 5 liters of water. Conjugations between E. coli and S. coelicolor were carried out as described previously (36).
Southern hybridization, PCR, and (p)ppGpp assays.
Southern blotting was carried out as described elsewhere (17). Probes were labeled with digoxigenin (DIG), using the DIG DNA-labeling kit of Boehringer Mannheim. PCR conditions were as described by Chakraburtty et al. (4). (p)ppGpp assays were carried out as described previously (43).
Construction of promoterless derivative of S. coelicolor relA.
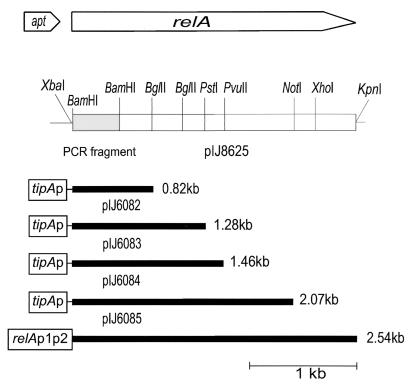
pIJ6054 (4), derived from pBluescript SK(+) and containing the S. coelicolor relA and upstream apt (encoding adenine phosphoribosyltransferase) genes, was used in the PCR with the synthetic oligonucleotides JS1 (5′-CCCGGATCCCGCACGAGGAGTCCTCTTG) and JS2 (5′-CGTACTCGGTGTCCTCGACGGTGTCGTG), whose 3′ ends were located at the end of the relA TTG start codon and 552 nucleotides (nt) inside the relA coding sequence, respectively. JS2 primes downstream of a BamHI site located within the relA coding region. A BamHI restriction site (shown above in bold italics) was incorporated in front of the relA ribosome binding site (GAGGA) by changing one base pair (a G to a C, corresponding to nt 8 of JS1). A 510-bp PCR product was isolated, digested with BamHI, and cloned in BamHI-cleaved pIJ2925, yielding pIJ8620. After sequence confirmation, the PCR product was excised as a BamHI fragment and an attempt was made to use it to replace the 5′ promoter region of relA from pIJ6054 after cleavage with BamHI. Insertion only occurred in the inappropriate orientation, i.e., the one that failed to reconstitute the relA coding region, presumably because transcriptional readthrough from the uninduced lacZ promoter (lacZp) caused deleterious expression of the S. coelicolor relA in E. coli. Consequently, to obtain a promoterless relA in the opposite orientation with respect to lacZp, relA was excised from pIJ6054 as a XbaI-KpnI fragment and cloned in similarly cleaved pBluescript KS(+), yielding pIJ8623. An 820-bp BamHI fragment containing the native relA promoter region and apt of pIJ8623 was then replaced with the 510-bp PCR product from pIJ8620 to yield pIJ8625 (Fig. 1).
FIG. 1.
Restriction map of relA in pIJ8625 and fragments thereof cloned in pIJ8600. apt, adenine phosphoribosyltransferase gene; relA, S. coelicolor relA; tipAp, thiostrepton-inducible tipA promoter; relAp1p2, the native relA promoters.
Construction of C-terminally deleted derivatives of S. coelicolor relA.
Convenient restriction sites in pIJ8625 enabled relA to be partitioned into four N-terminally nested segments of 0.82, 1.28, 1.46, and 2.07 kb (Fig. 1). The 0.82-kb segment was isolated as an XbaI-BglII fragment from pIJ8625 and inserted in the thiostrepton-inducible expression vector pIJ8600 that had also been cleaved with XbaI and BglII, yielding pIJ6082. The 1.28-, 1.46-, and 2.07-kb segments were isolated from pIJ8625 as NotI-PstI, NotI-PvuII, and NotI fragments, respectively, blunt-ended using the Klenow fragment of DNA polymerase I, and digested with XbaI, and the XbaI-blunt-end fragments were ligated with pIJ8600 that had been digested with BglII, blunt-ended, and cut with XbaI. The structures of each of the resulting constructs, pIJ6082, pIJ6083, pIJ6084, and pIJ6085 (Fig. 1), were confirmed by restriction analysis. The plasmids were introduced by transformation into the methylation-deficient E. coli strain ET12567 (dam dcm hsdS) (24) containing the helper plasmid pUZ8002 (36) and transferred to S. coelicolor strains M600 (relA+) and M570 (ΔrelA) by conjugation, selecting for apramycin resistance. Insertion of each of the plasmids at the S. coelicolor ΦC31 attP site was confirmed by Southern analysis.
Expression of full-length S. coelicolor relA under tipAp control.
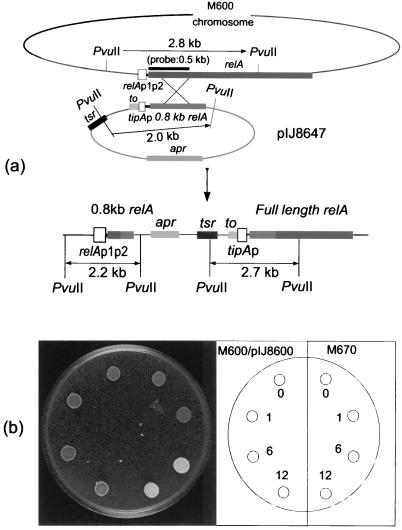
pOJ260 (1) is an E. coli vector that can be transferred to S. coelicolor by conjugation. Since it cannot replicate autonomously in streptomycetes and lacks any mechanism for site-specific recombination, stable inheritance requires recombination between a fragment cloned in the vector and a homologous host DNA sequence. Thus, the 2.7-kb BglII-EcoRI fragment of pIJ6085 that contained the λ t0 transcriptional terminator, tipAp fused to the 0.8-kb N-terminal region of relA, and tsr was ligated with pOJ260 that had been similarly cleaved to yield pIJ8647 (Fig. 2a). The plasmid was introduced by transformation into the methylation-deficient E. coli strain ET12567/pUZ8002 and then by conjugation into S. coelicolor strain M600 (relA+). Total DNA was isolated from two of the putative exconjugants, digested with PvuII, and subjected to Southern analysis using the 0.5-kb BamHI fragment of pIJ8625 as a DIG-labeled probe. Hybridizing PvuII fragments of 2.7 kb (from tipAp fused to full-length relA) and 2.2 kb (from the natural relA promoter fused to the 0.8-kb N-terminal region of relA) confirmed integration of pIJ8647 at the desired location (Fig. 2a). Interestingly, a 2.0-kb hybridizing band was also detected, presumably reflecting integration of two or more tandemly arrayed copies of pIJ8647 (and reflected in the stronger [twice or more] hybridization signal of the 2.0-kb fragment compared with that of the 2.7-kb and 2.2-kb bands). As expected, a band corresponding to the intact chromosomal copy of relA (2.8 kb) was not observed. One of the exconjugants was designated M670.
FIG. 2.
(a) Strategy for placing full-length S. coelicolor relA under tipAp control, and subsequent Southern analysis. Total DNA preparations from each strain were digested with PvuII and probed with the purified 0.5-kb BamHI fragment from pIJ8625 that contains the N-terminal region of relA and that had been labeled with DIG. relAp1p2, natural relA promoters; tipAp, thiostrepton-inducible promoter; tsr, thiostrepton resistance gene; t0, transcriptional terminator from phage λ; apr, apramycin resistance gene. (b) Effect of inducing expression of full-length relA on morphological differentiation in M670. For each strain, 106 spores in 10 μl of water were spotted four times on SMMS (nitrogen-limited) agar. The left four spots are M600 containing the vector pIJ8600, and the right four spots are M670. For induction, 0, 1, 6, or 12 μl of 1-mg ml−1 thiostrepton (i.e., 0, 1, 6, or 12 μg) were added to each spot immediately after inoculation and the plates were incubated at 30°C for 36 h.
RNA isolation and S1 nuclease protection studies.
RNA was extracted from cultures essentially as described previously (21) using the Kirby method. For each S1 nuclease reaction, 40 μg of RNA was hybridized in sodium trichloroacetate buffer (30) with about 0.2 pmol (approximately 105 Cerenkov min−1) of labeled probe. A uniquely end-labeled probe with a 12-bp nonhomologous tail was generated by PCR as follows. The primer internal to rshA 5′-GGCCGAGGCGGCGCAGGTCGAT was labeled with [γ-32P]ATP using T4 polynucleotide kinase (38) and employed in the PCR with the unlabeled primer 5′-ATGCGATCCTAGATCGCTCGAATGG GTGATCCGT and pIJ8613 as template. PCR conditions were 30 cycles of 45 s at 95°C, 45 s at 58°C, and 25 s at 72°C in the presence of 7% dimethyl sulfoxide. The resultant probe was 287 bp. Hybridizations were carried out at 45°C for 4 h following denaturation at 65°C for 15 min. S1 nuclease digestions and analyses of RNA-protected fragments were performed as previously described (19).
Construction of rshA null mutant.
The 4.5-kb PvuI-XhoI fragment containing rshA was isolated from cosmid 4H2 (EMBL accession number AL022268) and made blunt-ended using mung bean nuclease. The fragment was ligated with pIJ2925 that had been digested with SmaI to yield pIJ8613. A 1.75-kb AgeI fragment located entirely within the rshA coding region was removed from pIJ8613 by AgeI digestion and religation to yield pIJ8614; this resulted in the in-frame deletion of most of the rshA coding sequence. The 2.75-kb XbaI-EcoRI fragment of pIJ8614 was purified and ligated with pOJ260 that had been cleaved with XbaI and EcoRI to yield pIJ8616. pIJ8616 was introduced into the methylation-deficient E. coli strain ET12567/pUZ8002 by transformation and transferred to S. coelicolor strain M570 (ΔrelA) by conjugation. One exconjugant was grown on MS agar without apramycin for four rounds of sporulation. Serial dilutions of the resulting spores yielded colonies that were replica plated onto MS with and without apramycin for the isolation of apramycin-sensitive colonies. Total DNA was isolated from one of these derivatives, digested with XhoI, and probed with the DIG-labeled 1.5-kb AgeI-BglII fragment of pIJ8613, which contains the upstream region of rshA. Replacement of a 5.7-kb hybridizing XhoI fragment in M570 with a 3.9-kb fragment in the putative double mutant confirmed deletion of rshA. PCR analysis of the same DNA using the primers 5′-GATGGGCTCGATAGCATCAAG and 5′-GGATCAGACGGGTGAGGTAC, whose 3′ ends are located 93 nt downstream of the rshA ATG translation start codon and 149 nt upstream of the TGA stop codon, respectively, yielded the 663-bp fragment expected from deletion of rshA; the 2.4-kb band expected for an intact rshA was not observed. The resulting ΔrshA ΔrelA mutant was designated M680.
Construction of pIJ8618 for inducible expression of rshA in S. coelicolor.
The 1.7-kb DdeI fragment containing the N-terminal region of rshA, including its ribosome binding site, was isolated from pIJ8613 and made blunt-ended using mung bean nuclease. The fragment was ligated with pIJ2925 that had been digested with SmaI to yield pIJ8617. pIJ8671 is a derivative of pIJ8600 that can be used to create transcriptional fusions between tipAp and a gene of interest at its native chromosomal locus by recombination between a cloned N-terminal fragment of that gene and the homologous chromosomal sequence (44) (see Fig. 2a for an example of the principle). The 1.7-kb XbaI-BglII fragment of pIJ8617 was isolated and ligated with pIJ8671 that had been digested with XbaI and BamHI to yield pIJ8618. pIJ8618 was introduced into ET12567/pUZ8002 by transformation and into S. coelicolor M600 and M570 by conjugation. Approximately 5% of the putative M600 transconjugants failed to produce Act and Red on SMMS, the phenotype of a relA null mutant. This might reflect the integration of pIJ8618 into relA, rather than rshA (as the 1.7-kb of homologous sequence shows 64% nucleotide sequence identity), resulting in the production of a nonfunctional RshA::RelA chimera. Derivatives of M570 and M600 containing pIJ8618 integrated at the rshA locus were confirmed by Southern analysis. The resulting strains were designated M685 (tipAp::rshA ΔrelA) and M688 (tipAp::rshA relA+), respectively.
RESULTS
Construction and activity of thiostrepton-inducible C-terminally deleted derivatives of S. coelicolor relA.
To enable the substitution of the native relA promoter region by the thiostrepton-inducible tipAp, PCR was used to eliminate the relA p1 and p2 promoters while simultaneously providing a convenient restriction fragment that contained the entire relA coding region. The resulting construct (see Materials and Methods) was called pIJ8625 (Fig. 1). The (p)ppGpp synthetase domain of E. coli RelA is confined to the N-terminal region of the protein, with sequences responsible for ribosome-association located towards the C terminus (39). The aim of this study was to clone an N-terminal segment of S. coelicolor relA that functioned as a (p)ppGpp synthetase in a ribosome-independent manner. Convenient restriction sites in pIJ8625 enabled relA to be partitioned into four N-terminally nested segments of 0.82, 1.28, 1.46, and 2.07 kb (Fig. 1) that were inserted individually in the integrative thiostrepton-inducible expression vector pIJ8600. Integration of each of the plasmids at the S. coelicolor ΦC31 attP site in both M600 (relA+) and M570 (ΔrelA) was confirmed by Southern analysis.
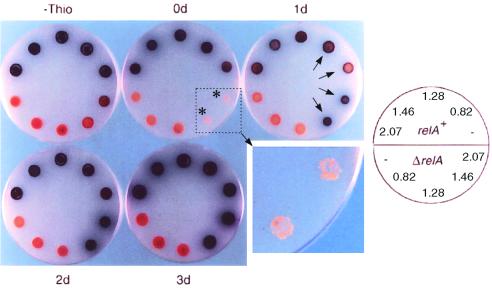
To assess the ability of the C-terminally deleted S. coelicolor relA derivatives to restore antibiotic production in M570 (ΔrelA), plate assays were carried out on nitrogen-limited SMMS agar. While pIJ6082 and pIJ6083 failed to restore antibiotic production and sporulation in the relA null mutant on thiostrepton induction, pIJ6084 and pIJ6085 did (Fig. 3). Even in the absence of induction, a low level of antibiotic production was detected in M570/pIJ6085 after 4 days of incubation; this was manifested as enhanced pigment production compared to that in M570 (ΔrelA) containing the vector alone. This presumably reflects a basal level of transcription initiation from tipAp or a low level of transcriptional readthrough from a vector promoter. Induction of M570/pIJ6084 and M570/pIJ6085 after 1 day resulted in a reduction in growth compared with M600/pIJ6084 and M600/pIJ6085, as evident from the smaller diameter of the patches of mycelium (Fig. 3). Furthermore, induction of M570/pIJ6084 and M570/pIJ6085 immediately after inoculation resulted initially in complete growth inhibition, presumably reflecting the accumulation of (p)ppGpp and sufficient inhibition of rRNA transcription to prevent colony formation. It thus seems likely that the 478-amino-acid N-terminal segment of S. coelicolor RelA encoded by pIJ6084 possesses ribosome-independent (p)ppGpp synthetase activity similar to that of the 455-amino-acid N-terminal segment of E. coli RelA (39). However, after prolonged incubation (4 to 5 days), small colonies appeared in the areas of the agar plate inoculated with M570/pIJ6084 and M570/pIJ6085 and induced immediately after inoculation (Fig. 3). About 90% of these “(p)ppGpp-resistant” colonies were subsequently shown to be deficient in (p)ppGpp synthesis, and several were shown to harbor deletions in the truncated relA genes. However, approximately 10% still produced (p)ppGpp on thiostrepton induction. Of these apparently truly (p)ppGpp-resistant mutants, some failed to make Act and Red regardless of thiostrepton induction, some made the antibiotics only in the presence of thiostrepton, while a third class produced Act and Red without thiostrepton induction.
FIG. 3.
Induction of the C-terminally deleted S. coelicolor relA derivatives in M600 (relA+) and M570 (ΔrelA). For each strain, 106 spores in 10 μl of water were spotted on SMMS (nitrogen-limited) agar. The five spots in the top half of each plate are M600 containing the vector pIJ8600, or derivatives of the latter with the 0.82-kb (pIJ6082), 1.28-kb (pIJ6083), 1.46-kb (pIJ6084), or 2.07-kb (pIJ6085) truncated relA fragments; equivalent M570 derivatives are shown in the bottom half of each plate. Next, 6 μl of 1-mg ml−1 thiostrepton (i.e., 6 μg of thiostrepton) was added to each spot, 0, 1, 2, or 3 days after inoculation; the plates were incubated at 30°C and photographed after 4 days. Arrows indicate the inhibitory effect of inducing (p)ppGpp synthesis in the ΔrelA but not relA+ strain. Asterisks highlight the appearance of (p)ppGpp-resistant mutants after early induction of (p)ppGpp synthesis in the ΔrelA mutant.
Expression of full-length S. coelicolor relA under tipAp control.
Attempts to construct a pIJ8600 derivative in E. coli that contained full-length S. coelicolor relA under tipAp control failed. All of the transformants analyzed contained a deletion in relA. The full-length relA was therefore placed under the control of tipAp directly in S. coelicolor using homologous recombination (15) (see Materials and Methods). The resulting strain was designated M670. In E. coli, overproduction of full-length RelA resulted in a reduction in growth rate, reflecting elevated levels of (p)ppGpp and a reduction in rRNA and tRNA synthesis (39). To assess whether this also applied to S. coelicolor RelA, plate assays were carried out on SMMS. Induction of full length relA did not provoke obvious growth inhibition, but it did elicit the precocious formation of aerial hyphae (Fig. 2b) and, on longer incubation, precocious Act production (see reference 48 for an example).
Assessment of (p)ppGpp synthesis after tipAp induction in M570/pIJ6082, M570/pIJ6083, M570/pIJ6084, M570/pIJ6085, and M670.
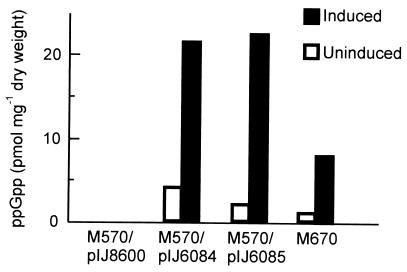
Thiostrepton was added to a final concentration of 25 μg ml−1 to mid-exponential-growth-phase cultures of M570/pIJ6082, M570/pIJ6083, M570/pIJ6084, M570/pIJ6085, and M670 grown in SMM, and the level of (p)ppGpp present in the cultures was monitored over a period of 8 h. The maximal levels of (p)ppGpp observed were 21 pmol mg−1 (dry weight) for M570/pIJ6084, 22 pmol mg−1 (dry weight) for M570/pIJ6085, and 8 pmol mg−1 (dry weight) for M670 (Fig. 4); none was detected in either M570/pIJ6082 or M570/pIJ6083, i.e., strains in which antibiotic production was not restored on thiostrepton induction. Thus, both the 1.46- and 2.07-kb segments of relA, but not the 0.82- and 1.28-kb fragments, possessed (p)ppGpp synthetase activity that was able to restore antibiotic production in the ΔrelA mutant.
FIG. 4.
Stimulation of ppGpp synthesis by inducing expression of the 1.46-kb (M570/pIJ6084) or 2.07-kb (M570/pIJ6085) N-terminal segments of relA, or the full-length gene (M670). Cultures were grown to an OD450 of 0.4 to 0.6 in SMM before addition of thiostrepton to 25 μg ml−1, and intracellular levels of ppGpp were measured by high-performance liquid chromatography analysis of nucleotides extracted 120 min after induction. A control set of cultures that were not induced by thiostrepton addition was similarly analyzed. No ppGpp synthesis was detected when the ΔrelA mutant harboring the vector alone (M570/pIJ8600) was induced.
GMP synthetase inhibitor decoyinine restores morphological differentiation, but not Act production, in ΔrelA mutant.
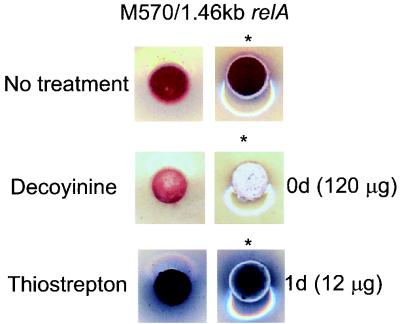
(p)ppGpp synthesis is accompanied by a marked decrease in the intracellular concentration of GTP (31, 32, 43), and in principle this, rather than the accumulation of (p)ppGpp, could be responsible for activation of antibiotic production. However, earlier work had shown that addition of the GMP-synthetase inhibitor decoyinine induced sporulation in several Streptomyces species, as well as in B. subtilis (23), but had no effect on antibiotic production. This led Ochi (33) to propose that while (p)ppGpp synthesis was required to activate antibiotic production, it was a fall in GTP levels that prompted morphological differentiation. To assess whether a similar effect could be observed in S. coelicolor, M570/pIJ6084 was treated with either thiostrepton or decoyinine. While thiostrepton induction after 1 day resulted in the formation of Act and aerial mycelium, addition of decoyinine at the time of inoculation restored morphological differentiation but not Act production (Fig. 5) (later addition of decoyinine had no effect). Addition of decoyinine to M570 gave the same results. These observations are entirely consistent with Ochi's hypothesis.
FIG. 5.
Decoyinine induces morphological differentiation but not antibiotic production in a ΔrelA mutant of S. coelicolor. A total of 106 spores of M570/pIJ6084 in 10 μl of water were spotted three times on SMMS agar. Then, 12 μl of 10-mg ml−1 decoyinine was added to one spot immediately after inoculation, and 12 μl of 1-mg ml−1 thiostrepton was added to another spot 1 day after inoculation; the plates were incubated at 30°C and photographed after 3 days from below and from on top (asterisks).
A bifunctional role for relA?
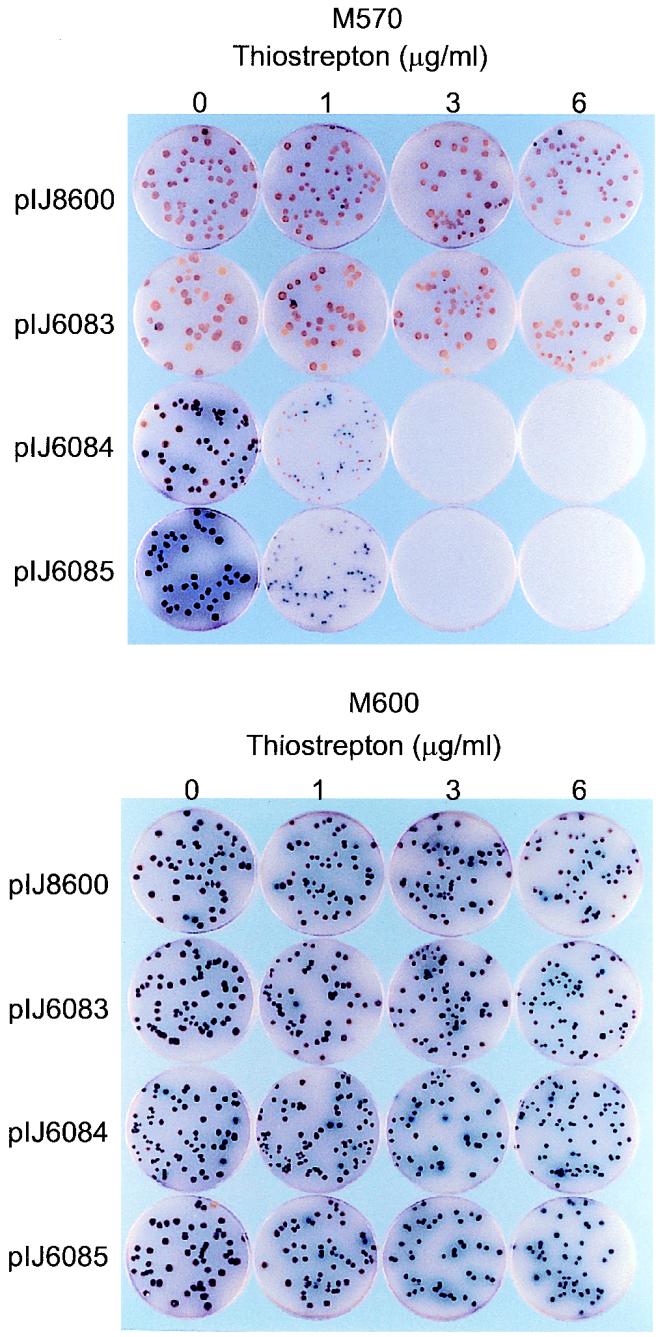
Early induction of the two complementing fragments (1.46 and 2.07 kb) in the relA null mutant M570 resulted in complete growth inhibition (Fig. 6), presumably reflecting the accumulation of (p)ppGpp and cessation of rRNA synthesis. However, there was little or no effect on growth in the congenic relA+ strain M600 (Fig. 6). These results are consistent with a bifunctional role for S. coelicolor RelA as both a (p)ppGpp synthetase and hydrolase. In M600, induction of (p)ppGpp synthesis by the truncated RelA derivatives would be catered for by the hydrolase activity of the wild-type RelA, while in M570 (ΔrelA) this would not be possible. This interpretation requires that the hydrolase function of the truncated RelA derivatives has been markedly impaired or destroyed while the synthetase function has been retained. Although this latter prediction has not been tested, analysis of (p)ppGpp decay in E. coli derivatives expressing S. coelicolor relA suggests that it does indeed encode a (p)ppGpp hydrolase activity (25). Thus, it seemed possible that, unlike E. coli, S. coelicolor might possess a single RelA homologue with both synthetic and degradative functions.
FIG. 6.
Effect of induction of the C-terminally deleted relA derivatives on growth of M570 (ΔrelA) and M600 (relA+). Spores were plated on SMMS agar containing different concentrations of thiostrepton. The plates were photographed after incubation for 4 days at 30°C.
Genome sequencing reveals rshA, a second relA/spoT homologue of S. coelicolor.
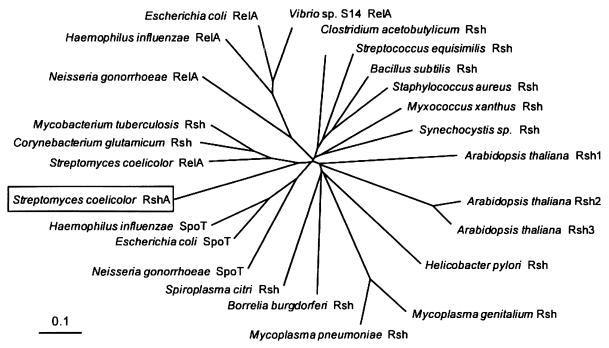
A BLAST search of the emerging S. coelicolor genome sequence revealed a second relA/spoT homologue, rshA, on cosmid 4H2 (EMBL accession number AL022268). The predicted 2,163-nt open reading frame begins with an ATG translational start codon preceded by a potential ribosome binding site (GGAGG) located 10 nt upstream. RshA (721 amino acids and predicted molecular mass of 78.4 kDa) shows 42% amino acid sequence identity to S. coelicolor RelA but lacks the N-terminal extension found in the latter (847 amino acids, 94.2 kDa). When compared with S. coelicolor RelA, it also has a C-terminally located deletion of 61 amino acids, potentially reflecting different regulatory properties (modulation of the activity of E. coli SpoT has been proposed to occur via interaction of low molecular weight effectors with the C-terminal domain of the protein [8]). RshA possesses the amino acid sequences required for the ppGpp synthetase and hydrolase activities of the RelA/SpoT family of proteins (8, 25). Phylogenetic analysis grouped RshA with other actinomycete RelA/SpoT homologues, but it appears to have diverged from its S. coelicolor counterpart early in the evolution of actinomycetes (Fig. 7).
FIG. 7.
Phylogenetic analysis of the RelA/SpoT family of proteins. Unless previously designated otherwise, proteins potentially possessing both ppGpp synthetase and hydrolase activities were termed Rsh. Nonconserved N and C termini, which also varied in length, were removed from the alignment before deriving the tree. ClustalW (47) was used to align the sequences and construct the tree, which was displayed using TreeView (35). The analysis is based on an alignment spanning 809 amino acids.
Transcription of rshA is growth-phase dependent and is induced upon ppGpp synthesis.
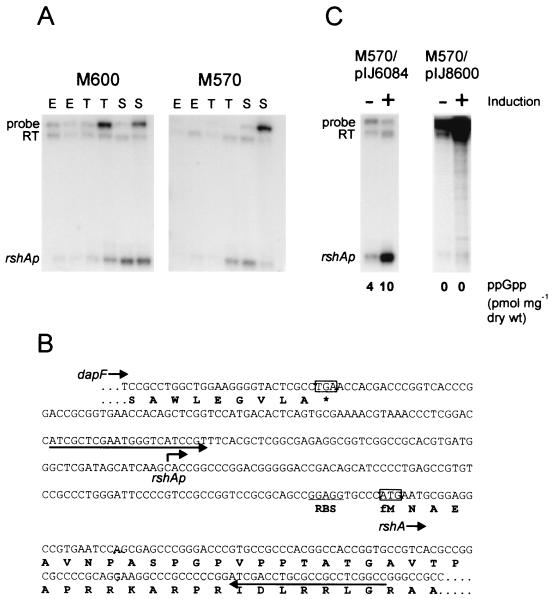
To assess the transcriptional profile of rshA in S. coelicolor, S1 nuclease protection experiments were carried out using RNA isolated from M600 and M570 at different stages of growth in SMM (Fig. 8A). A protected fragment of 211 nt indicated a transcriptional start site 90 nt upstream of the ATG translation start codon (Fig. 8B). This was verified by high-resolution S1 nuclease mapping (data not shown). A protected fragment corresponding in size to the full-length probe minus the 12-nt nonhomologous tail indicated additional transcription of rshA from a promoter 5′ of the upstream primer used to generate the labeled probe. Similar results were obtained when an S1 probe covering the entire intergenic region between the upstream dapF (37) homologue and rshA was used (data not shown). Thus, rshA is transcribed both from its own promoter and by transcriptional readthrough from dapF. In M600, while the level of readthrough transcription remained approximately constant throughout growth, transcription from rshAp was barely detectable during exponential growth but increased markedly during transition and stationary phase. M570 (ΔrelA) showed a similar pattern of transcription, but the level of transcripts detected in transition and stationary phase was noticeably reduced compared with that in M600 (relA+).
FIG. 8.
Transcription analysis of rshA. (A) Transcription of rshA during growth of M600 (relA+) and M570 (ΔrelA) in SMM. RNA was isolated during the exponential (E), transition (T), and stationary (S) phases of growth and subjected to S1 nuclease protection analysis using a uniquely end-labeled PCR-generated probe. Readthrough transcription from the upstream gene (dapF) is indicated (RT) and could be distinguished from probe reannealing by the presence of a 12-bp nonhomologous tail on the probe. (B) Sequence upstream of rshA indicating the transcription start point (bent arrow), the C terminus of dapF, and the primers used to generate the S1 probe (the arrows that underline the nucleotide sequence). (C) Effect of induction of ppGpp synthesis in M570/pIJ6084 on transcription from rshAp. The ΔrelA mutant harboring the vector alone (M570/pIJ8600) is shown as a control. Cultures were grown in SMM and induced (+) by the addition of thiostrepton to 25 mg ml−1 at an OD450 of 0.6. A parallel series of cultures were not induced (−). Mycelia were harvested for RNA isolation and ppGpp analysis 120 min after induction.
The elevation in transcription from rshAp in M600 coincides with the transition- and stationary-phase accumulation of ppGpp (3; A. Hesketh, unpublished data). Furthermore, this increase in transcription appears to be reduced in the ppGpp-deficient strain M570. Consequently, the effect of induction of ppGpp synthesis on transcription from rshAp was assessed using strain M570/pIJ6084 (Fig. 8C). Thiostrepton induction of an SMM-grown culture at an optical density at 450 nm (OD450) of 0.6 resulted in an intracellular ppGpp level of 10 pmol mg−1 (dry weight) 120 min following induction and correlated with a significant (approximately 10-fold) increase in rshAp transcription compared to that in the uninduced control, where only 4 pmol of ppGpp mg−1 (dry weight) was detected. In M570 harboring the vector alone (M570/pIJ8600), transcription from rshAp remained at a low level in both the induced and uninduced cultures, and ppGpp was not detected in either case.
Construction of rshA null mutant.
The S. coelicolor relA null mutant fails to produce Act or Red under conditions of nitrogen limitation, but under phosphate limitation both antibiotics are produced at or near wild-type levels. This might reflect an alternative mechanism for (p)ppGpp synthesis under conditions of phosphate limitation. To assess whether rshA might fulfill such a role, a rshA null mutant was made by introducing an in-frame deletion into the chromosomal copy of the gene in the ΔrelA strain M570. The deletion removed from nt 65 to nt 1817 of the 2,163-nt rshA coding sequence. To assess the effect of the rshA mutation on growth, antibiotic production, and morphological differentiation, the resulting ΔrshA ΔrelA mutant M680 and its parental strain M570 (ΔrelA) were grown on SMMS, R2, and R5 agar media. No difference was observed.
Construction of pIJ8618 for inducible expression of rshA in S. coelicolor.
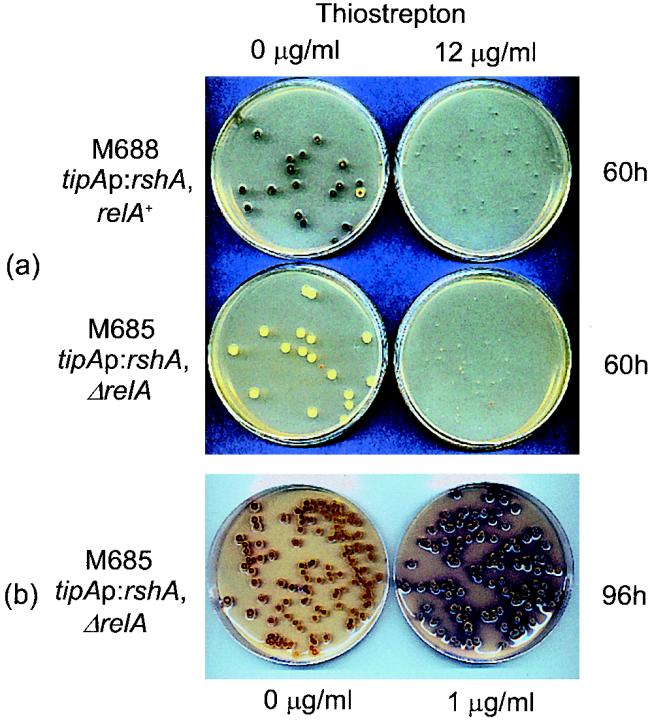
Since deletion of rshA in M570 had no obvious phenotypic effect, the gene was overexpressed in M570 and M600 in an attempt to deduce its function. Derivatives of M570 (ΔrelA) and M600 (relA+) containing rshA under tipAp transcriptional control were designated M685 (tipAp::rshA ΔrelA) and M688 (tipAp::rshA relA+), respectively. Spores of M688 and M685 were plated out for single colonies on SMMS plates containing no or 12 μg of thiostrepton ml−1. After 60 h of incubation at 30°C, the growth of both of the induced strains was markedly inhibited and antibiotic production was not restored to the ΔrelA mutant (Fig. 9a). While inhibition of M685 might have reflected (p)ppGpp synthesis in the predicted absence of (p)ppGpp hydrolysis in the ΔrelA mutant, this seemed unlikely in M688 unless the tipAp::rshA construction was capable of much higher levels of (p)ppGpp production than the functional tipAp::relA constructs, which did not inhibit growth of the relA+ host upon induction. However, since early induction of tipAp::rshA in M685 did not lead to complete growth inhibition, whereas similar induction of the tipAp::relA constructs did, this explanation seems unlikely. Interestingly, induction of tipAp::rshA in M685 (ΔrelA) on SMMS containing only 1 μg of thiostrepton ml−1 restored antibiotic production as well as sporulation (Fig. 9b). The effect of induction on sporulation was most noticeable around the edges of the colonies; it became more evident across the entire surface of the colonies upon further incubation. When a mid-exponential-growth-phase culture of M685 was induced with a final concentration of 25 μg of thiostrepton ml−1 (which has been shown to yield maximal tipAp induction) (14) and the cultures were left for a further 40 and 80 min, no (p)ppGpp synthesis (<1 pmol mg−1 [dry weight]) was observed.
FIG. 9.
Effect of induction of rshA on growth and antibiotic production in M688 (relA+) and M685 (ΔrelA). Spores were plated on SMMS agar with and without thiostrepton. The plates were photographed after 60 h (induction with 12 μg of thiostrepton ml−1) (a) or 96 h (induction with 1 μg of thiostrepton ml−1) (b) of incubation at 30°C
Antibiotic production occurs in ΔrelA mutant M570 grown under conditions of phosphate limitation in the absence of detectable (p)ppGpp synthesis.
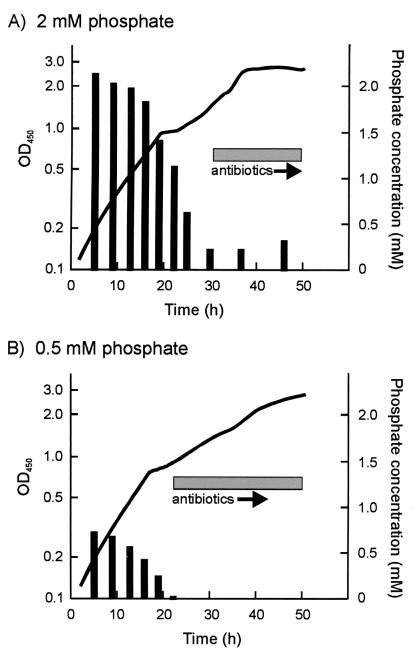
To assess whether there was any detectable (p)ppGpp synthesis in M570 under conditions of phosphate limitation (i.e., under conditions permissive for Act and Red production in the ΔrelA mutant), M600 and M570 were grown in a modified version of Evan's medium containing 2 or 0.5 mM phosphate. Carbon- and nitrogen-limited versions of the medium were also assessed. Antibiotic production and (p)ppGpp synthesis occurred in all four M600 cultures. In M570, (p)ppGpp synthesis was not detected under any of the four growth conditions (detection limit of approximately 1 pmol mg−1 [dry weight]), and Act and Red production was observed only in the 2 and 0.5 mM phosphate media. The appearance of antibiotics in these cultures coincided with a marked reduction in inorganic phosphate in the culture medium and occurred 8 h later in the medium with the higher initial phosphate concentration (Fig. 10); in both cases, antibiotic production commenced once the phosphate concentration had fallen below 0.25 mM. In M600 grown under the same conditions, there was no marked decrease in inorganic phosphate concentration and the onset of antibiotic production coincided with a peak in (p)ppGpp synthesis in each case (data not shown).
FIG. 10.
Antibiotic production and phosphate concentration during growth of the ΔrelA mutant M570 in Evan's medium containing 2 mM (A) or 0.5 mM (B) Na2HPO4. Curves indicate growth, measured as OD450. Solid vertical bars reflect phosphate concentrations in the culture supernatants, which were determined using the Sigma diagnostic inorganic phosphorus reagent. The occurrence of antibiotic production is denoted by the hatched box.
DISCUSSION
While no attempts were made to measure the abundance of the proteins produced, induction of the 1.46- and 2.07-kb C-terminally truncated derivatives of S. coelicolor relA in the relA null mutant M570 resulted in (p)ppGpp synthesis and restoration of antibiotic production. Although not assessed experimentally, it seems unlikely that either of these proteins binds to the ribosome. In E. coli, the ribosome-binding domain of RelA is localized to the carboxy-terminal domain (28), and single amino acid substitutions close to the C terminus are sufficient to prevent ribosome association (M. Cashel, personal communication).
Induction of the full-length relA resulted in approximately 8 pmol mg−1 (dry weight) of (p)ppGpp, which was markedly less than the levels obtained with the truncated derivatives (over 20 pmol mg−1 [dry weight]). This presumably reflects a requirement for ribosome activation of the full-length protein prior to (p)ppGpp synthesis and the possible lack of hydrolase activity in the truncated proteins. Alternatively, it is conceivable that the full-length protein possesses ribosome-independent activity of lower specific activity than that of the truncated RelA derivatives.
The ability to manipulate intracellular (p)ppGpp levels under conditions of nutritional sufficiency by thiostrepton induction of the truncated relA derivatives will provide a useful means of assessing quantitatively the role that (p)ppGpp plays in the initiation of antibiotic production. Moreover, the isolation of (p)ppGpp-resistant mutants in which antibiotic production no longer appears to require (p)ppGpp synthesis should greatly assist in future studies aimed at defining the targets of (p)ppGpp action.
Induction of full-length relA in M670 caused precocious formation of aerial hyphae, suggesting that (p)ppGpp might play a role in initiating morphological development. Expression of E. coli relA in Myxococcus xanthus led to the accumulation of (p)ppGpp and activation of early developmental gene expression even in the presence of nutrient levels sufficient to support growth; it also promotes the synthesis of an extracellular population density signal, Factor A (11, 41). In E. coli, (p)ppGpp enhances the activity of the stationary-phase-specific sigma factor, ςs (9), and in S. coelicolor alternative sigma factors play an important role in morphological differentiation (see reference 20 for a review). Thus, it is conceivable that elevated levels of (p)ppGpp could influence morphological differentiation through sigma factor activation. However, it is also possible that the effect on aerial hyphae formation reflects instead the drop in GTP levels that accompanies (p)ppGpp synthesis. Indeed, Ochi (33) has proposed a role for decreased GTP levels in the initiation of morphological differentiation in both streptomycetes and B. subtilis. Consistent with Ochi's proposal, while induction of (p)ppGpp synthesis in M570/pIJ6084 resulted in induction of antibiotic synthesis and morphological differentiation, addition of the GMP synthetase inhibitor decoyinine resulted only in morphological differentiation.
RshA contains sequences believed to be required for both the ppGpp synthetase and hydrolase activities of the RelA/SpoT family of proteins (8, 25). Transcriptional analysis revealed that rshA is transcribed in a growth phase-dependent manner in both M600 (relA+) and M570 (ΔrelA), but with the levels of transcripts significantly reduced in the latter. Consistent with the notion that RshA might possess (p)ppGpp synthetase activity, high-level induction of tipAp::rshA in M600 (relA+) resulted in growth inhibition, while a lower level of induction in M570 (ΔrelA) led to a restoration of antibiotic production and sporulation. However, deletion of rshA in either strain had no apparent phenotypic effect, and RshA-dependent (p)ppGpp synthesis could not be observed in the relA null mutant (M570) or on induction of strain M685 (tipAp::rshA, ΔrelA) (detection limit of <1 pmol mg−1 [dry weight]).
Recently, a new protein domain, the HD domain (named after the conserved doublet of predicted catalytic residues, histidine and aspartic acid), was identified that defines a new superfamily of metal-dependent phosphohydrolases (22). This superfamily is characterized by three motifs comprising the HD signature flanked by regions containing conserved histidine and aspartate residues, producing a predicted metal-chelating region believed to be involved in coordination of divalent metal cations essential for enzyme activity. Since removal of the 3′-pyrophosphate residue of (p)ppGpp by E. coli SpoT occurs in a manganese-dependent manner (12, 13), we examined the sequences of RelA and SpoT homologues for the presence of the HD domain. All of the putative bifunctional enzymes [the SpoT orthologues and the RelA/SpoT homologues that appear to catalyze both (p)ppGpp synthesis and hydrolysis] possess the HD domain, as does RshA. In contrast, none of the bona fide RelA proteins do. Thus, RshA might function as a (p)ppGpp hydrolase, and indeed rshA appears to be transcribed partly in response to an increase in the intracellular level of ppGpp; rshA transcript levels are noticeably lower in the ppGpp-deficient strain M570, and transcription is activated on induction of ppGpp synthesis in strain M570/pIJ6084. However, induction of tipAp::rshA in the ppGpp-producing strain M600 (relA+) had no detectable effect on the level of ppGpp synthesized, and induction of ppGpp synthesis using pIJ6084 (tipAp::relA [1.46 kb]) was similar in both M680 (ΔrelA ΔrshA) and M570 (ΔrelA rshA+) (data not shown). It therefore seems unlikely that RshA simply functions as a (p)ppGpp hydrolase, and its role in vivo remains to be elucidated.
Shima et al. (40) have isolated streptomycin-resistant mutants of M570 (ΔrelA) in which antibiotic production, but not (p)ppGpp synthesis, was restored. The mutations were located in rpsL, encoding ribosomal protein S12 (34). It is conceivable that the restoration of antibiotic production that occurs on thiostrepton induction of rshA results in a change in ribosome function that can be mimicked by alterations in S12. In any event, the isolation of ribosomal protein mutants that can suppress the requirement for an intracellular signaling molecule that stimulates transcription of regulatory genes for antibiotic biosynthesis suggests that the ribosome plays an important role in determining the onset of antibiotic production that is not currently understood.
ACKNOWLEDGMENTS
We thank Keith Chater and David Hopwood for their comments on the manuscript.
This work was supported by a Royal Society Fellowship Award to A. Hesketh, by a grant-in-aid to the John Innes Centre from the Biotechnology and Biological Sciences Research Council (BBSRC), by BBSRC grant 208/P10950 to M.J.B., and by the John Innes Foundation.
REFERENCES
- 1.Bierman M, Logan R, O'Brien K, Seno E T, Nagaraj R, Schoner B E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 2.Cashel M, Gentry D R, Hernandez V J, Vinella D. The stringent reponse. In: Neidhardt F C, editor. Escherichia coli and Salmonella typhimurium. 2nd ed. Vol. 1. Washington, D.C.: American Society for Microbiology; 1995. pp. 1458–1496. [Google Scholar]
- 3.Chakraburtty R, Bibb M J. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J Bacteriol. 1997;179:5854–5861. doi: 10.1128/jb.179.18.5854-5861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chakraburtty R, White J, Takano E, Bibb M J. Cloning, characterization and disruption of a (p)ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) Mol Microbiol. 1996;19:357–368. doi: 10.1046/j.1365-2958.1996.390919.x. [DOI] [PubMed] [Google Scholar]
- 5.Chater K F. Taking a genetic scalpel to the Streptomyces colony. Microbiology. 1998;144:1465–1478. doi: 10.1099/00221287-144-6-1465. [DOI] [PubMed] [Google Scholar]
- 6.Chater K F, Bibb M J. Regulation of bacterial antibiotic production. In: Kieinkauf H, von Dohren H, editors. Biotechnology. 7, Products of secondary metabolism. Weinheim, Germany: VCH; 1997. pp. 57–105. [Google Scholar]
- 7.Floriano B, Bibb M. afsR is a pleiotropic but conditionally required regulatory gene for antibiotic production in Streptomyces coelicolor A3(2) Mol Microbiol. 1996;21:385–396. doi: 10.1046/j.1365-2958.1996.6491364.x. [DOI] [PubMed] [Google Scholar]
- 8.Gentry D R, Cashel M. Mutational analysis of the Escherichia coli spoT gene identifies distinct but overlapping regions involved in ppGpp synthesis and degradation. Mol Microbiol. 1996;19:1373–1384. doi: 10.1111/j.1365-2958.1996.tb02480.x. [DOI] [PubMed] [Google Scholar]
- 9.Gentry D R, Hernandez V J, Nguyen L H, Jensen D B, Cashel M. Synthesis of the stationary-phase sigma factor ςS is positively regulated by ppGpp. J Bacteriol. 1993;175:7982–7989. doi: 10.1128/jb.175.24.7982-7989.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hanahan D. Studies on transformation of Escherichia coli with plasmids. J Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 11.Harris B Z, Kaiser D, Singer M. The guanosine nucleotide (p)ppGpp initiates development and A-factor production in Myxococcus xanthus. Genes Dev. 1998;12:1022–1035. doi: 10.1101/gad.12.7.1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heinemeyer E A, Richter D. Mechanism of the in vitro breakdown of guanosine 5′-diphosphate 3′-diphosphate in Escherichia coli. Proc Natl Acad Sci USA. 1978;75:4180–4183. doi: 10.1073/pnas.75.9.4180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Heinemeyer E A, Geis M, Richter D. Degradation of guanosine 3′-diphosphate 5′-diphosphate in vitro by the spoT gene product of Escherichia coli. Eur J Biochem. 1978;89:125–131. doi: 10.1111/j.1432-1033.1978.tb20904.x. [DOI] [PubMed] [Google Scholar]
- 14.Hesketh A, Sun J, Bibb M J. Induction of ppGpp synthesis in Streptomyces coelicolor A3(2) grown under conditions of nutritional sufficiency elicits actII-ORF4 transcription and actinorhodin biosynthesis. Mol Microbiol. 2001;39:136–144. doi: 10.1046/j.1365-2958.2001.02221.x. [DOI] [PubMed] [Google Scholar]
- 15.Hillemann D, Puhler A, Wohlleben W. Gene disruption and gene replacement in Streptomyces via single stranded DNA transformation of integration vectors. Nucleic Acids Res. 1991;19:727–731. doi: 10.1093/nar/19.4.727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hopwood D A, Chater K F, Bibb M J. Antibiotic production in Streptomyces coelicolor A3(2) In: Vining L C, Stuttard C, editors. Regulation and biochemistry of antibiotic production. Newton, Mass: Butterworth-Heinemann; 1994. pp. 71–108. [Google Scholar]
- 17.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M, Lydiate D J, Smith C P, Ward J M, Schrempf H. Genetic manipulation of Streptomyces: a laboratory manual. Norwich, United Kingdom: The John Innes Foundation; 1985. [Google Scholar]
- 18.Janssen G R, Bibb M J. Derivatives of pUC18 that have BglII sites flanking a modified multiple cloning site and that retain the ability to identify recombinant clones by visual screening of Escherichia coli colonies. Gene. 1993;124:133–134. doi: 10.1016/0378-1119(93)90774-w. [DOI] [PubMed] [Google Scholar]
- 19.Janssen G R, Ward J M, Bibb M J. Unusual transcriptional and translational features of the aminoglycoside phosphotransferase gene (aph) from Streptomyces fradiae. Genes Dev. 1989;3:415–429. doi: 10.1101/gad.3.3.415. [DOI] [PubMed] [Google Scholar]
- 20.Kelemen G H, Brown G L, Kormanec J, Potuckova L, Chater K F, Buttner M J. The positions of the sigma factor genes, whiG and sigF, in the hierarchy controlling the development of spore chains in the aerial hyphae of Streptomyces coelicolor A3(2) Mol Microbiol. 1996;21:593–603. doi: 10.1111/j.1365-2958.1996.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 21.Kieser T, Bibb M J, Chater K F, Hopwood D A. Practical Streptomyces genetics. Norwich, United Kingdom: John Innes Foundation; 2000. [Google Scholar]
- 22.Koonin E V. The HD domain defines a new superfamily of metal-dependent phosphohydrolases. Trends Biochem Sci. 1998;23:469–472. doi: 10.1016/s0968-0004(98)01293-6. [DOI] [PubMed] [Google Scholar]
- 23.Lopez J M, Dromerick A, Freese E. Response of guanosine 5′-triphosphate concentration to nutritional changes and its significance for Bacillus subtilis sporulation. J Bacteriol. 1981;146:605–613. doi: 10.1128/jb.146.2.605-613.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacNeil D J, Gewain K M, Ruby C L, Dezeny G, Gibbons P H, MacNeil T. Analysis of Streptomyces avermitilis genes required for avermectin biosynthesis utilizing a novel integration vector. Gene. 1992;111:61–68. doi: 10.1016/0378-1119(92)90603-m. [DOI] [PubMed] [Google Scholar]
- 25.Martinez-Costa O H, Fernandez-Moreno M A, Malpartida F. The relA/spoT-homologous gene in Streptomyces coelicolor encodes both ribosome-dependent (p)ppGpp-synthesizing and -degrading activities. J Bacteriol. 1998;180:4123–4132. doi: 10.1128/jb.180.16.4123-4132.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mechold U, Cashel M, Steiner K, Gentry D, Malke H. Functional analysis of a relA/sopT gene homologue from Streptococcus equisimilis. J Bacteriol. 1996;178:1401–1411. doi: 10.1128/jb.178.5.1401-1411.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mechold U, Malke H. Characterization of the stringent and relaxed responses of Streptococcus equisimilis. J Bacteriol. 1997;179:2658–2667. doi: 10.1128/jb.179.8.2658-2667.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Metzger S, Sarubbi E, Glaser G, Cashel M. Protein sequences encoded by the relA and the spoT genes of Escherichia coli are interrelated. J Biol Chem. 1989;264:9122–9125. [PubMed] [Google Scholar]
- 29.Miyadoh S. Research on antibiotic screening in Japan over the last decade: a producing microorganisms approach. Actinomycetologica. 1993;7:100–106. [Google Scholar]
- 30.Murray M G. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precision during transcript mapping. Anal Biochem. 1986;158:165–170. doi: 10.1016/0003-2697(86)90605-6. [DOI] [PubMed] [Google Scholar]
- 31.Ochi K. Metabolic initiation of differentiation and secondary metabolism by Streptomyces griseus: significance of the stringent response (ppGpp) and GTP content in relation to A factor. J Gen Microbiol. 1986;132:299–305. doi: 10.1128/jb.169.8.3608-3616.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ochi K. A decrease in GTP content is associated with aerial mycelium formation in Streptomyces MA406-A-1. J Gen Microbiol. 1986;132:299–305. doi: 10.1099/00221287-132-2-299. [DOI] [PubMed] [Google Scholar]
- 33.Ochi K. Metabolic initiation of differentiation and secondary metabolism by Streptomyces griseus: significance of the stringent response (ppGpp) and GTP content in relation to A factor. J Bacteriol. 1987;169:3608–3616. doi: 10.1128/jb.169.8.3608-3616.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ochi K, Zhang D, Kawamoto S, Hesketh A. Molecular and functional analysis of the ribosomal L11 and S12 protein genes (rplK and rpsL) of Streptomyces coelicolor A3(2) Mol Gen Genet. 1997;256:488–498. doi: 10.1007/pl00008614. [DOI] [PubMed] [Google Scholar]
- 35.Page R D M. TreeView: an application to display phylogenetic trees on personal computers. Comput Appl Biosci. 1996;12:357–358. doi: 10.1093/bioinformatics/12.4.357. [DOI] [PubMed] [Google Scholar]
- 36.Paget M S B, Chamberlin L, Atrih A, Foster S J, Buttner M J. Evidence that the extracytoplasmic function sigma factor ςE is required for normal cell wall structure in Streptomyces coelicolor A3(2) J Bacteriol. 1999;181:204–211. doi: 10.1128/jb.181.1.204-211.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Richaud C, Higgins W, Mengin-Lecreulx D, Stragier P. Molecular cloning, characterization, and chromosomal localization of dapF, the Escherichia coli gene for diaminopimelate epimerase. J Bacteriol. 1989;169:1454–1459. doi: 10.1128/jb.169.4.1454-1459.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 39.Schreiber G, Metzger S, Aizenman E, Roza S, Cashel M, Glaser G. Overexpression of the relA gene in Escherichia coli. J Biol Chem. 1991;266:3760–3767. [PubMed] [Google Scholar]
- 40.Shima J, Hesketh A, Okamoto S, Kawamoto S, Ochi K. Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2) J Bacteriol. 1996;178:7276–7284. doi: 10.1128/jb.178.24.7276-7284.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singer M, Kaiser D. Ectopic production of guanosine penta- and tetraphosphate can initiate early developmental gene expression in Myxococcus xanthus. Genes Dev. 1995;8:1633–1644. doi: 10.1101/gad.9.13.1633. [DOI] [PubMed] [Google Scholar]
- 42.Spira B, Yagil E. The relation between ppGpp and the PHO regulon in Escherichia coli. Mol Gen Genet. 1998;257:469–477. doi: 10.1007/s004380050671. [DOI] [PubMed] [Google Scholar]
- 43.Strauch E, Takano E, Baylis H A, Bibb M J. The stringent response in Streptomyces coelicolor A3(2) Mol Microbiol. 1991;5:289–298. doi: 10.1111/j.1365-2958.1991.tb02109.x. [DOI] [PubMed] [Google Scholar]
- 44.Sun J, Kelemen G H, Fernandez-Abalos J M, Bibb M J. Green fluorescent protein as a reporter for spatial and temporal gene expression in Streptomyces coelicolor A3(2) Microbiology. 1999;145:2221–2227. doi: 10.1099/00221287-145-9-2221. [DOI] [PubMed] [Google Scholar]
- 45.Takano E. ppGpp and antibiotic production in Streptomyces coelicolor A3(2). Ph.D. thesis. Norwich, United Kingdom: University of East Anglia; 1993. [Google Scholar]
- 46.Takano E, Gramajo H C, Strauch E, Andres N, White J, Bibb M J. Transcriptional regulation of the redD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2) Mol Microbiol. 1992;6:2797–2804. doi: 10.1111/j.1365-2958.1992.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 47.Thompson J D, Higgins D G, Gibson T J. CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994;22:4673–4680. doi: 10.1093/nar/22.22.4673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.van der Biezen E A, Sun J, Coleman M J, Bibb M J, Jones J D. Arabidopsis RelA/SpoT homologs implicate (p)ppGpp in plant signaling. Proc Natl Acad Sci USA. 2000;97:3747–3752. doi: 10.1073/pnas.060392397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wehmeier L, Schafer A, Burkovski A, Kramer R, Mechold U, Malke H, Puhler A, Kalinowski J. The role of the Corynebacterium glutamicum rel gene in (p)ppGpp metabolism. Microbiology. 1998;144:1853–1862. doi: 10.1099/00221287-144-7-1853. [DOI] [PubMed] [Google Scholar]