Abstract
Selenium (Se) is a microelement that plays an important nutrient role by influencing various physiological and biochemical traits in plants. It has been shown to stimulate plant metabolism, enhancing secondary metabolites and lowering abiotic and biotic stress in plants. Globally, the enormous applications of nanotechnology in the food and agricultural sectors have vastly expanded. Nanoselenium is more active than bulk materials, and various routes of synthesis of Se nanoparticles (Se-NPs) have been reported in which green synthesis using plants is more attractive due to a reduction in ecological issues and an increase in biological activities. The Se-NP-based biofortification is more significant because it increases plant stress tolerance and positively impacts their metabolism. Se-NPs can enhance plant resistance to various oxidative stresses, promote growth, enhance soil nutrient status, enhance plant antioxidant levels, and participate in the transpiration process. Additionally, they use a readily available, biodegradable reducing agent and are ecologically friendly. This review concentrates on notable information on the different modes of Se-NPs’ synthesis and characterization, their applications in plant growth, yield, and stress tolerance, and their influence on the metabolic process.
Keywords: selenium nanoparticles, SeNPssynthesis, plant growth, stress tolerance, physiology, yield
1. Introduction
Materials with a dimension smaller than 100 nm are referred to as nanomaterials (NMs). NMs have improved surface-to-volume proportions owing to their small size, which gives the man edge over their traditional equivalents. Since they are tiny in size and have an enhanced surface area, chemical composition, stability, and form, an aggregation of nanoparticles (NPs) has distinctive physicochemical properties: limited surface area, unusual surface structure, and heightened reactivity. Applying diverse NPs and nanomaterials (NMs) effectively improved crop plant nutrition compared to standard fertilizers. Additionally, compared to their bulkier counterparts, NPs less than 100 nm exhibit unique physicochemical, electrical, optical, and biological characteristics [1]. Due to their unique characteristics, such as their substantial surface-to-volume quotient, solubility, and multifunctionality, selenium nanoparticles (Se-NPs) are distinguishable from other materials.
The use of nanotechnology in creating nutraceuticals has many benefits, including enhancing the bioavailability of encapsulated bioactive natural compounds and promoting their controlled release and targeted delivery, both of which increase their biological efficacy, as reviewed in [2]. Additionally, NPs modify gene expression, which changes the molecular pathways of plants. Silver (Ag), gold (Au), ferric oxide (Fe2O3), zinc oxide (ZnO), selenium (Se), titanium dioxide (TiO2), aluminum oxide (Al2O3), silicon dioxide (SiO2), carbon NPs, nanotubes, nanowires, and quantum dots are examples of metal and non-metal oxides of NPs that play vital roles in plant germination, repair, and stress reduction. Applying nanotechnology in agricultural distribution to plant technologies holds the promise of targeted release of different types of macromolecules for increased immunity to plant diseases, efficient nutrient usage, and enriched plant development [3]. The synthesis of NPs in a unique, cost-efficient, eco-friendly way has been made possible using phyto-nanotechnology. The advantages of phyto-nanotechnology include scalability, biocompatibility, and the synthesis of NPs using a global solvent (water) as a reducing agent. Phyto-nanotechnology uses plants and their distinct plant parts, including roots, fruits, stems, seeds, and leaves, to create nanoparticles. Owing to their microscopic size and distinctive surface properties, nanoparticles are an alluring approach to regulating agricultural production systems [4,5], resulting in the recent focus on the impacts of NPs and NMs such as macro, micro, and nanocarrier-mediated fertilizers and plant-growth increasing NPs with unknown mechanisms, application concentration/rate, particle size, mode of action, and hazardous effects [6].
A forthcoming technology called nano-assisted agriculture has the potential to advance plant breeding and agricultural practices as well as increase tolerance to biotic and abiotic challenges [7]. Slow nutrient release from NPs may encourage plant development by maximizing nutrient supply and boosting the antioxidant defense system [8]. A new field, “plant nanoscience,” outlines the interplay between plants and nanotechnology. Nanosensors, nanopesticides, nanofertilizers, and nano-plant genetic engineering can all help to increase agricultural yields significantly.
Selenium (Se) is a non-metal or metalloid element that is present in a diverse range of forms, particularly as selenide [Se (-II)], elemental selenium [Se (0)], selenite [Se (IV)], or selenate [Se (VI)], all of which exist naturally, occurring in the environment, and accumulate in a variety of organisms [9]. The content of Se in soil has been in the range between 0.01 and 2 mg kg−1 [10]. Plant growth, biochemistry, and productivity changes caused by selenium are determined by many parameters, notably Se source, experimental techniques, growth rate, and variety of plant species. In a limited extent of doses (below 10 mg L−1), application of Se may increase growth rates, enhance nutritional content, not alter nuclear transcription profiles, increase photosynthesis efficacy, induce antioxidant profile, alter hormonal balances, enhance primary and secondary metabolism, and enhance plant accustomed to unfavorable environmental conditions [11]. Typically, selenium-rich agricultural products are regarded as a practical and efficient strategy to raise selenium levels in human bodies [12]. Se-NPs have various advantages, including low toxicity, high degradability, and excellent anticancer, antimicrobial, and antiviral properties [13,14].
The Se accumulated within plants is greatly impacted by varied circumstances, including the levels of selenium in the soil ecosystem and the concentrations plant species uptake themselves [14,15]. Plant-mediated generation of Se-NPs is superior to conventional biosynthesis strategies. Natural stabilizing and reducing agents found in plants can produce Se-NPs in an economically feasible and eco-friendly safe manner. Among other nanomaterials, plant-derived Se-NPs have shown promise as potent antibacterial and antioxidant agents to lessen the detrimental impacts of plant diseases in many crops [16]. Se NPs have shown promising clinical benefits in a range of oxidative stress and inflammation-mediated diseases, such as diabetes, cancer, nephropathy, and arthritis [17].
The biocompatibility, bioavailability, low toxicity, biodegradability, and environmental friendliness of Se-NPs derived from plants have all been demonstrated to be quite high. This is due to the presence in the plant extract of secondary metabolites such as phenols, tannin sesquiterpenes, cinnamic acid, and monoterpenes that serve as reducing agents and stabilize capping during the production of Se-NPs and sustain them as environmentally friendly [18]. Depending on the synthesis conditions, their wide use in biomedical applications is determined by their size, structure, and biochemical characteristics [18]. In this review, we highlighted the different routes of the synthesis of Se-NPs and their imminent applications in plant growth and development. Further, their role in the regulation of plant metabolites was discussed in detail.
2. Modes of SeNP Synthesis
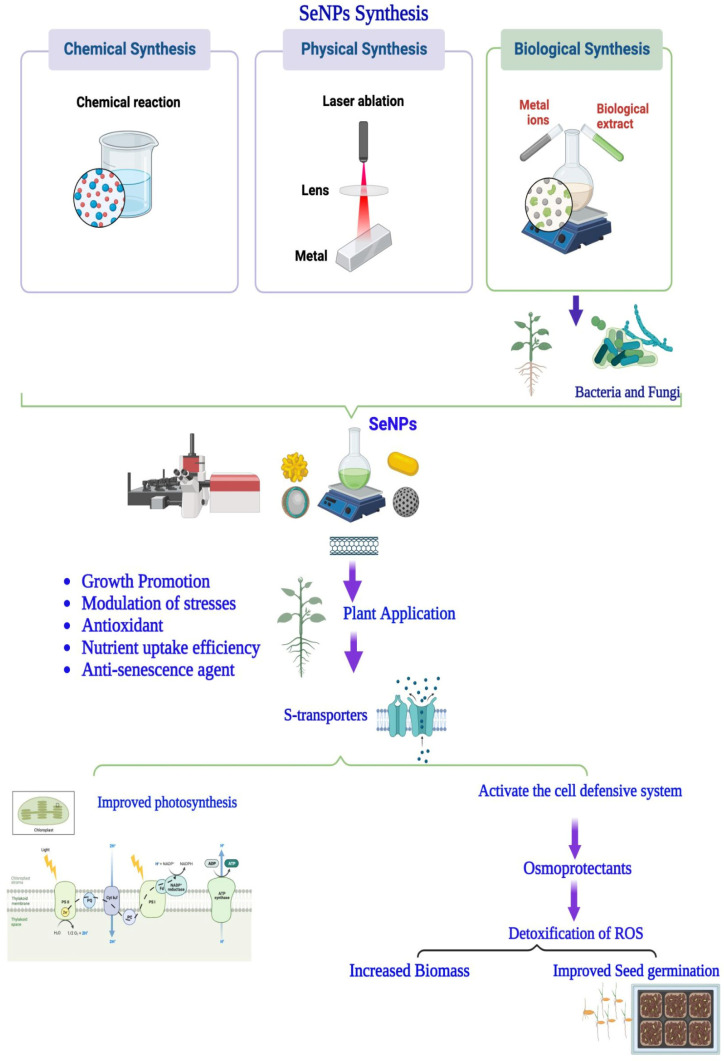
NPs have emerged recently due to their distinctive qualities, including morphological propensity, chemical reactivity, competitive binding ability, and optical activity. SeNPs are synthesized by three basic methodologies, namely physical, chemical, and biological approaches (Figure 1).
Figure 1.
An overview of various modes of synthesis and application of SeNPs in plants.
Several investigations have suggested physical means of synthesizing Se-NPs by employing pulsed laser ablation and ultrasound-based synthesis, vapor deposition, and hydrothermal and solvothermal techniques for synthesizing Se-NPs [19]. Gudkov et al. [20] proposed a method for producing Se-NPs in the zero-valent state. Se-NPs were formed by laser ablation of Se in water utilizing a fiberytterbium laser at a wavelength of (b/w 1060 and 1070 nm), where the repetition of the pulse rate is 20 kHz (Kilohertz). The fundamental particle mass component altered from a size of 800 nm to less than 100 nm due to increased laser fragmentation durations. The resulting SeNPs were mono-dispersed in both mass and size.
Se-NPs are synthesized chemically from precursors that are inorganic forms of selenium. To prevent nanoparticle aggregation, reducing agents such as glucose, ascorbic acid, fructose, cysteine, glutathione, sodium metasulfate, and the ionic liquid 1-ethyl-3-methylimidazolium thiocyanate have been employed in Se-NP synthesis. Panahi-Kalamuei et al. [21] used chemical substances, which include SDS (Sodium dodecyl sulfate), CTAB (Cetyl Trimethyl Ammonium Bromide), and polyethylene glycol (PEG 600) to synthesize Se-NPs. The initial material was SeCl4 (Selenium tetrachloride), which, when dissolved in water, creates selenious acid and is reduced with hydrazine hydrate. Se4+/Se has a reduction potential of 0.74 eV, while N2H4H2O/N2 (Hydrazine Hydrate/Nitrogen Gas) has a reduction potential of 1.16 eV. Ragavan et al. [22] exploited sodium selenite and ascorbic acid to produce Se-NPs by a precipitation process, and the physical properties of Se-NPs were characterized by scanning electronic microscopy (SEM). The size of the SeNPs ranged between 9.09 mm to 9.07 mm. The chemical properties of the Se-NPs analyzed with the Energy Dispersive X-Ray Analysis (EDAX) spectrum were recorded as two peaks between 1.6 Kev and 10.8 Kev. The diffraction peaks of the XRD (X-ray diffraction) include 22.0158°, 6.9573°, 2.7332°, and the Fourier-transform infrared spectroscopy (FTIR) spectrum in the region of 4000 to 400 cm−1, and O-H stretch, free hydroxyl-C-H stretch, H-C-H stretch = C-O bend, and C-O stretch bands were recorded at 3441 cm−1, 2920 cm−1, 2858 cm−1, 1625 cm−1, 1537 cm−1, 1324 cm−1, 1025 cm−1, and 1032 cm−1, which are coupled to each another.
El-ghazaly et al. [23] produced spherical Se-NPs around 13 nm in size in an ice-cold solution using SeO2 as the precursor and polyvinylpyrrolidone (PVP) as the stabilizing agent and KBH4 as the reducing compound. The presence of orange color suggested the creation of Se-NPs, and according to a transmission electron microscopy (TEM) study, the particle shape and size were 10 nm in diameter. Vahdati et al. [24] observed a distinctive Ultraviloiet (UV) absorption peak at 265 nm, comparable with prior reports but with considerably smaller sizes in the region of 35–45 nm as compared to polyvinyl acetate or chitosan.
Se-NPs are created from selenate using a powerful reducing cum stabilizing agent, ascorbic acid (AA). The typical surface plasmon absorption peak at 296 nm in the UV-vis spectrum verified the synthesis of nanosized sodium selenate (Na2SeO4) at 10 mm using 1.5% AA. The TEM results of the produced Se-NPs revealed that Se-NPs are generally spherical with a diameter of 33.4 nm. The XRD pattern validated that Se-NPs are crystalline and have a particle size of 42.92 nm [25].
El Lateef Gharib et al.’s [25] study compared the treated supplementation of Na2SeO4 and Se-NPs up to 25 M and untreated plants and recorded that entire plants treated with Se-NPs observed a significant increase in foliar growth, including different plant parts along with total leaf area cm2/plant. With Na2SeO4 and Se-NP application, particularly at 6.25 M concentration, the biochemical observations of the total content of photosynthetic pigments (TPP), carbohydrates (TC), soluble proteins (TSP), and other minerals in leaves were improved. In these studies, the cowpea leaves were treated with Se-NPs at 6.25 M, which elevated levels of the growth gibberellic acid (GA3), indole acetic acid (IAA), and cytokines (CKs), then with Na2SeO4 at 6.25 M. This explains how plants treated with Se-NPs and Na2SeO4 had higher growth parameters and heavier seeds than untreated plants.
The microbially synthesized Se-NPs were examined using dynamic light scattering (DLS), which showed that the size varied. Particle size ranged between 120 and 260 nm, with an average diameter range of 10–55 nm. Se-NPs zeta potential analysis revealed a negative potential (32.42 mV) in ddH2O (double distilled water), and TEM analysis revealed that all Se-NPs were spherical NPs [26].
Allium sativum L. clove extract-based Se-NPs were validated using the UV-visible spectrum between 200 and 800 nm, demonstrating and analyzing the bio-fabricated Se-NPs’ peak between 200 and 400 nm. The maximal characterization peaks of selenium measured using energy dispersive X-rays ranged from 2.5 to 3.5 Kev. SEM studies on green-synthesized Se-NP particles revealed that they are spherical, cylindrical, or rectangular, with most being 40–100 nm in size [27]. The production of Se-NPs via green nanotechnology, notably employing microbes and plants or their associated by-products (microbially synthesized proteins/enzymes, secondary metabolites, and lipids), as well as diverse biotechnological processes, is indeed gaining popularity [13]. These strategies are eco-friendly, cost-effective, eliminate hazardous and harsh chemicals, and use subtle energy. Plants serve as both stabilizing and reducing agents, allowing for fine control of nanoparticle size and distribution. Due to the massive importance of the biological generation of Se-NPs, currently, several researchers have examined the use of plant parts (Table 1). Numerous studies have investigated the photosynthesis of Se-NPs utilizing raisin extract as a capping, stabilizing, and reducing agent. The adequate concentration of plant extract employed for it and the physicochemical properties characterizing the diverse surface plasmon resonance bands affect Se-NPs’ biocompatibility, stability, and adaptability, as reviewed in [16]. Se-NPs biosynthesis encompasses the use of living microorganisms and plants to convert sodium selenite salt to Se-NPs, including the fungus Mariannaea for Se-NP biogenesis [28]. Biosynthetic Se-NPs have many advantages over both organic and inorganic selenium compounds. As a result, the biological synthesis of Se-NPs is preferred for increased biocompatibility and stability. In Se-NPs synthesized with an extract from the Vitis vinifera (raisin), Sharma et al. [29] found the least zeta potential values equal to 36 V, followed by Java tea extract (34.9 V) [30], and the cocoa bean shell was 28.6 V [31]. Joshi et al. [32] synthesized the antifungal activity of mycogenic Se-NPs and, through SEM-EDS (Scanning Electron Microscopy—Energy Dispersive X-ray Spectroscopy analysis) of bioactive Se-NPs revealed that they are spherical in size (60.48 nm to 123.16 nm).
The biosynthesis of Se-NPs has also been found to involve a wide variety of bacterial strains, including Klebsiella pneumoniae and Pseudomonas aeruginosa, as reviewed in [16]. According to Seliem et al. [33], Se-NPs of 50–100 nm size were produced using Lactobacillus casei, and the biosynthesized Se-NPs improved the physiological and biochemical profiles of two sensitive chrysanthemum cultivars—namely, Sensuous and Francofone under heat stress by increasing antioxidant enzyme activity.
The Se-NPs synthesized using glutathione as a reducing agent exhibit a size of 12.7 nm. The resultant biosynthesized Se-NPs showed inhibitory activity against the Sclerospora graminicola and Alternaria solani [34]. According to Khalifa and Sameer [35], Se-NPs inhibited the mycelia growth of fungi Penicillium digitatum to 5.56% at 100 ppm, and 85.22% at 500 ppm concentration, which is the cause of green mold disease of orange fruit. The Sclerospora graminicola biosynthesized Se-NPs inhibit vegetative growth, sporulation, spore viability, and proliferation [36]. El Badri et al. [37] synthesized biogenic Se-NPs (bio Se-NPs) with sizes ranging from 120 to 260 nm, with a mean size of 167 nm. Six species of Trichoderma were examined for their ability to produce Se-NPs, including T. asperellum, T. harzianum, T. atroviride, T. virens, T. longibrachiatum, and T. brevicompactum. These Se-NPs were then used to control the severity of the Sclerospora graminicola-induced downy mildew disease in pearl millet. In the UV-visible spectral range from 200 and 400 nm, Se-NP solutions exhibited the highest absorption [36]. Employing leaf extract from the Pelargonium zonale against P. digitatum, Se-NPs demonstrated remarkable potential. P. digitatum, a plant pathogen, is a significant cause of the post-harvest fungi known as green mold in citrus [38].
Table 1.
Green synthesis of Se-NPs using different plant extracts and their characterization methods.
| Method of Synthesis | Name of Plant Source | Size | Shape | Operational Parameters | References |
|---|---|---|---|---|---|
| Green-synthesis | Vitis vinifera Extract | 3–18 nm | Spherical | 10 mL of Extract added with 4 × 10−5 M selenous acid for refluxed for 15 min and centrifuged at 15,000× g rpm | [29] |
| A. sativum | 48–87 nm | Spherical | Garlic extract (5 mL) mixed with 20 mM Na2SeO3 solution (50 mL) and stirred at 150 rpm at 60 °C. | [39] | |
| Clausena dentate leaf extracts | 46.32–78.88 nm | Spherical | 10 g of C. dentata leaf powder with 100 mL of DH2O boiled at 60 °C for 5 min. This broth (12 mL) was added to 1 mM aqueous selenium powder (88 mL) for the synthesis of Se-NPs |
[40] | |
| Crataegus monogyna | 113 nm | Spherical | 0.01M Na2SeO3 was mixed with 2 mg mL−1 hawthorn fruits lyophilized powder for 12 hrs and dialyzed (MWCO 8000–14,000) for 48 hrs. |
[41] | |
| Emblica officinalis | 20 to 60 nm | Spherical | 2 mL of aqueous fruit extract of E. officinalis was added with 10 mL of 10 mM Na2SeO3 under stirring. The mixture was allowed in dark conditions at 27 ± 2 °C and 120 rpm for 24 hrs. | [42] | |
|
Catharanthus roseus and Peltophorumpterocarpum flowers |
23.2 nm and 30.44 nm | Spherical | 10 gm of the flowers was added with 90 mL of 10 mM aqueous solution of Na2SeO4 and incubated at 250 rpm at 36 °C for 7 days | [43] | |
| Withania somnifera leaves extract | 15 nm | Crystalline | One hundred milli liters of plant extract was mixed with 50 mM selenious acid and stirred and washed with distilled water and acetone at overnight | [44] | |
|
Aloe vera leaf extract (ALE) Fabricated with Se-NPs |
50 nm | Spherical fabricated Se-NPs |
20 mL of culture filtrates, cell lysate, and crude cell wall from six Trichoderma spp was added with 70 mL of sterile distilled water containing 25 mM Na2SeO3 and stored 28 ± 1 °C on a shaker at 150 rpm for 6 days | [45] | |
| Zingiber officinale | 100 to 150 nm | Spherical | 1% ginger extract was added to 10 mM Na2SeO4 solution (9:1 ratio) with pH 9 at 37 °C for 75 hrs at 130 rpm | [46] | |
| Wheat (Triticum aestivum L.) | 140 ± 10 nm | Spherical |
Biosynthesized SeNPs: Rahnella aquatilis HX2 cell broth filtered in Na2SeO3 at 5 mM for 48 hrs at 28 °C then centrifuged at 8000× g for 30 min and washed with 1 M NaOH for 20 min in a boiling water bath. |
[47] | |
| Vigna unguiculata L. | 33.4 nm | Spherical | 10 mM of aq. solution of 10 mM Na2SeO4 added to the ascorbic acid powder 1.5% (w/v) under stirring at room temperature for 15 min. | [25] | |
| Cyamopsis tetragonoloba | 50–150 nm | Oval | 700 mg of Na2SeO3 in 50 mL of distilled water for 20 min, added with 50 mL of ascorbic acid solution | [22] | |
| Raphanus sativus var. sativus, Eruca sativa, Solanum melongena, Cucumis sativus and Capsicum annuum. | 100 nm | X-ray diffractograms | Laser ablation processes of solid Se then fiber ytterbium laser (1060 nm and 1070 nm), pulse rate 20 KHz in 80 nanoseconds in 20 W | [48] | |
| Brassica napus | 10–55 nm | Spherical | 1%ComamonastestosteroniS44 culture added with 10 mM Na2SeO3 for 72 hrs. Then, 2 M NaOH solution was added to this under stirring | [26] | |
| Allium sativum | 40 to 100 nm | Spherical | Cassia auriculata in fine powder added with 100 mL of 10 mM Na2SeO3 at a concentration (10 × 10−3 M) under magnetic stirring, then incubated at 37 °C | [49] |
3. Mode of Action
The diversification of selenium, which occurs in several environmental compartments in various forms, impacts its accessibility and distribution based on numerous characteristics, including pH, natural organic matter (NOM), microbial activity, redox potential, ionic elements, soil quality, temperature, and moisture [50]. Plant species, age, and selenium availability all affect how toxic selenium is to plants. Young plants are substantially more susceptible to Se toxicity than adult ones, whereas SeO32− is a more phytotoxic form of SeO42− [51]. Variations exist across plant species in Se absorption and accumulation and the production of volatile Se-compounds to prevent Se toxicity. Plants are divided into three types based on their ability to absorb, use, and accumulate Se. The primary Se accumulators, which contain 1000 µg Se g−1 DW, are followed by secondary Se accumulators, which contain 100 to 1000 µg Se g−1 DW, and non-accumulators, which contain less than 100 µg Se g−1 DW, as reviewed in [51]. Many studies have suggested that Se participates in upregulating antioxidant defense in hyperaccumulator species, where enzymatic and non-enzymatic antioxidants and phytohormones, such as jasmonic acid (JA), salicylic acid (SA), and ethylene (ET), play crucial roles in Se tolerance [52,53].
Plants take up selenium from the soil, and the Se-containing plants can be split into two categories: first, those with selenium concentrations equivalent to those present in the soil, and then those with selenium in significantly greater amounts than the concentrations present in the soil [54].
Despite the fact that there is still much to learn about this phenomenon, several directions have been suggested to investigate Se-NP absorption and uptake into plant systems [47]. The plant’s cell wall prevents external factors, such as Se-NPs, from entering the plant’s cell walls. Wang et al. [55] disclosed that Se-NPs could enter the plasma membrane and cross a cell wall. Se-NPs may adhere to plant roots and impact a plant’s chemical and physical absorption. The most universally acknowledged theory is that NPs are absorbed intra- and extracellularly via the tissues till they attain the xylem [11].
Selenite most probably enters the plant root via a phosphate transport channel, a metabolically energetic process. Selenite is converted to selenide and subsequently integrated into selenocysteine during the Se assimilation process (SeCys). Zhu et al. [56] investigated selenium and iodine intake by spinach (Spinacia oleracea L.) dispersed in a liquid and proposed that the cell wall performs as a physical blockade; moreover, it possesses pores with diameters of 5 to 20 nm where the NPs smaller than this can enter.
White and Broadley [57] stated that selenocysteine (SeCys) most likely occurs in the cytosol of the cell, chloroplasts, and mitochondria, and therein SeCys is transformed into selenomethionine (SeMet). It [58] was hypothesized that in the transfer of nanoparticulate materials to plants was also conceivable that nanoparticles larger than 20 nm widen the pores, causing holes through which endocytosis or transmembrane proteins or ionic channels could enter. Earlier research by Domokos-Szabolcsy et al. [59] showed that tobacco could absorb Se-NPs to root and callus cultures, and significant effects of Se-NPs in plant tissue culture varied from selenate. The uptake of Se-NPs is not simply based on cell wall pore diameter; additional mechanisms might well be implicated. According to Schiavon and Pilon-Smits [60], selenate is taken into the cell via a sulphate transporter in the root plasma membrane and subsequently transformed into Se-amino acids in plant metabolism.
Se-NPs may also be absorbed by plants, which then convert them into selenite and selenate in the roots and shoots, proving that Se-NPs are bioavailable to plants. The twenty-fold increased uptake rate of SeCys and SeMet by wheat and canola is greater than selenate or selenite, indicating that plants likely absorb organic forms of Se through amino acid permease, as reviewed in [47]. Hu et al. [47] demonstrated that after being absorbed by wheat roots, Se-NPs were promptly oxidized to Se (IV) and transformed into organic forms of either selenocysteine (SeCys2) or se-methyl-selenocysteine (MeSeCys), or selenomethionine (SeMet). Absorption and bio-transformation experiments on chemically and biologically synthesized 5 M nSe treated wheat seedlings discovered that nSe was absorbed by root aquaporins, and thereby SeMet and SeIV were accumulated in roots [47]. Aquaporins inhibitor prevented wheat roots from absorbing chemically synthesized Se-NPs (Che Se-NPs) and biologically produced Se-NPs (Bio Se-NPs) by 92.5 and 93.4%, respectively. Wheat roots absorbed Se-NPs at 1.8 and 2.2 times greater rates than 140 and 240 nm [47].
4. Impact of Se-NPs on Growth and Physiology
The impact of NPs varies, subject to the plant type, physiochemical characteristics, and application concentration. Different NPs affect the plant to improve its physiology, germination rate, and biomass productivity. Numerous NPs have distinct physical and chemical characteristics that make it simple for them to penetrate plant cells and can change their metabolism through a number of interactions to affect plant growth and development, which activates the capacity to withstand stressful circumstances [61].
In accordance with a number of research studies, a foliar supply of metal NPs significantly increases the amount of chlorophyll in plants, allowing them to produce more complexes for light harvesting in order to increase the absorption of light energy [62]. The impacts of NPs have also been thoroughly studied by looking at how they affect chlorophyll and photosynthetic efficiency and growth inhibition. Se-NPs operate as catalysts and strengthen plants’ antioxidant defense mechanisms, improving their ability to withstand biotic stress as reviewed in [16]. Chlorophyll a and b contents are minimized by tannic acid-capped Se-NPs at 20 and 80 mg L−1, respectively. This occurs as a result of the reactive oxygen species (ROS) produced by biogenic Se-NPs, which raise hydrogen peroxide (H2O2) and malondialdehyde (MDA) while lowering Peroxidase (POD) and other antioxidant defense enzymes [63]. Mozafariyan et al. [64] reported that when compared to control plants, 5 g of Se enhanced root growth and relative water content in hot pepper plants by 13%.
The vital roles of Se include stimulating plant growth by enhancing glucose metabolism, recovering chloroplast ultrastructure, accelerating chlorophyll biosynthesis, and preventing chlorophyll degradation [65]. According to Djanaguiraman et al. [66], Se-NPs decrease high-temperature mediated stress by boosting seed yield, pollen germination, percentage of seed setting, photosynthetic rate, and lowering oxidative stress. Cowpea seeds were treated with foliar applications of Na2SeO4 or Se-NPs at 6.25 and 12.5 M, which resulted in higher levels of Total Carbohydrate (TC) and Crude Protein (CP) than controls at 105 days after planting. The Se-NP supplementation at 6.25 M concentration, followed by Na2SeO4 at the identical concentration, produced remarkable mineral content values for N, P, K, Ca, S, and Mg in dry cowpea leaves [25]. According to Al-Deriny et al. [67], Se-NPs at low concentrations cause changes in jasmonic acid, salicylic acid, and ethylene, and their signaling causes changes in metabolism along with the expression of defensive genes. Trichoderma asperelleum + Se-NP application enhanced plant height (24.9 cm), tiller count (3.40 tillers/seedling), and content of chlorophyll (3.76 mg g−1) in pearl millet seedlings, according to Nandini et al. [36]. Se-NP concentrations between 50 and 100 mg kg−1 greatly boosted the root system (>40%) and organogenesis [68]. Additionally, Se-NPs support organogenesis and root formation. A trace level of Se has been demonstrated to promote growth in Brassica oleracea, potato, lettuce, and ryegrass plants [69]. Dai et al. [70] examined the beneficial effects of Se in Brassica campestris sp. Pekinensis grew under two different Se and Zinc accumulation treatments, revealing that Superoxide dismutases (SOD), POD, Catalase (CAT), Glutathione peroxidase (GR), Ascorbate peroxidase (APX), and proline concentration were enhanced when Se was present.
El-Kinany et al. [71] revealed that applying foliar nSe (25 and 50 mg L−1) with the surfactant tween 80 (0.005%) twice every 15 days enhanced yield and ascorbic content in NaCl salt-stressed coriander (Coriandrum sativum L.) plants. Additionally, Alves et al. [72] observed that when the treatment of 1.0 M of selenite or selenate was added to cadmium (Cd) toxicity (0.5 mM CdCl2)-stressed tomato plants, they exhibited an increase in photosynthesis and biomass. While Se and Zn (10, 20, and 40 mg L−1) were applied together, wheat’s Cd-induced loss in photosynthesis, stem growth, and pigmentation was significantly reduced [73]. Maize growth and chlorophyll content were raised by selenium treatment (20 mg L−1) under increased saline stress, which causes high MDA and H2O2 [74]. APX, SOD, and CAT were upregulated by 44%, 56%, and 57%, respectively. Rady et al. [75] found that tomatoes treated with 40 M of Se had enhanced drought tolerance.
Xu et al. [76] demonstrated a rise in CAT levels following Se supplementation in rice under Cd toxicity stress, indicating that Se has a beneficial effect in improving plant resistance to stress. Behbahani et al. [11] investigated nSe (0, 1, 4, 10, 30, and 50 mg L−1) or bulk (selenate) treatments in bitter melon seedlings and concluded that they dramatically increased to an average of 52% in leaf nitrate reductase activity compared to the control. Micro-measurement analyses at 1 mg L−1 induced the growth of primary and secondary tissues. However, the nSe-applied seedlings had considerably enhanced Pheylalanine ammonia lyase (PAL) activity in the roots and leaf-soluble phenols compared to the control. The impact of selenium on Vicia faba L. minor roots exposed to lead (Pb) stress was analyzed by examining root growth, root viability, and antioxidant enzyme activity, according to Mroczek-Zdyrska and Wójcik [77]. Molnár et al. [78] reported that selenite sensitivity in Arabidopsis resulted in plant alterations such as the opening of stomatal openings, aggregation of callose, severe oxidative stress, and moderate nitrosative changes. These changes were also associated with reduced stomatal density. Cowpea (Vigna unguiculata L) plants that adapted to foliar sprays of either Na2SeO4 up to 25 M or Se-NPs up to 50 M concentration were investigated by El Lateef Gharib et al. [25]. They found that both nanocomposite treatments significantly increased the Chlorophyll a and b and carotenoid levels more than the control till 75 days after sowing. The increment in chlorophyll and carotenoid content in the cowpea leaf was found with Se-NPs at 6.25 M concentrations, followed by Na2SeO4 at 6.25 M concentrations. According to Skalickova et al. [79], applying Se-NPs in agricultural practice protects against salt and high temperatures. Se-NPs and SeO32 ameliorate Brassica napus L. growth under Cd toxicity stress due to Se-NPs. However, Yu et al. [80] found that in Brassica chinensis, 10 M of either SeO32− or SeO42− improved antioxidant defense and prevented the formation of H2O2 and MDA. By limiting the development of NADPH (Nicotinamide adenine dinucleotide phosphate) oxidases (BnaRBOHD1, BnaRBOHC, and BnaRBOHF1) and glycolate oxidase (BnaGLO), the form of Se-NPs reduced Cd-induced reactive oxygen species generation, hence reducing oxidative protein and membrane lipid degradation.
Plant growth and development, K+ content, and photosynthetic rate all increased with one mM Se, while the ratio of Na+ decreased. Additionally, by lowering oxidative damage caused by high MDA and H2O2 under enhanced salinity stress, selenium treatment (20 mg L−1) enhanced maize growth and chlorophyll level [74]. Se-NPs also increased resistance to Cd toxicity stress conditions by lowering Cd accumulation, facilitating the formation of disulfide bonds, sustaining intracellular calcium homeostasis, and regenerating the waxy outer layer of the leaf surface. These results showed that applying SeO32⁻ at an enhanced level (20 mg L−1) had negative impacts on the growth of B. napus, while applying 20 mg L−1 Se-NPs caused a dose-dependent increase in chlorophyll content [81]. Se treatment increased SOD and glutathione reductase (GR) activity in the rice plant under Cd toxicity stress conditions because lipid peroxidation was reduced [82]. Se treatment elevated CAT and POD activity ROS, antioxidant enzymes, and isozymes of rice plants [83].
Se (10 mg L−1), CS (0.1%), and a combination of two concentrations of CS-Se NPs (5 and 10 mg L−1) were administered to Moldavian balm (Dracocephalum moldavica L.) plants at 0, 2.5, and 5 mg kg−1 Cd-stress conditions. The outcomes revealed that the administration of Se and CS-Se NPs might reduce the adverse impacts of Cdstress by improving agronomic qualities, antioxidant enzyme activities, photosynthetic pigments, proline, and phenols, and reducing MDA and H2O2 [84]. They concluded that a 5 mg L−1 concentration might be the most effective strategy. Under 2.5 mg kg−1 cadmium toxicity stress, the administration of Se and CS-Se NPs increased the amount of chlorophyll b and carotenoids. Ikram et al. [18] studied the Allium sativum L. clove extract-based Se-NPs synthesized at different concentrations (25, 50, 75, and 100 mg L−1), which were externally supplied to huanglongbing (HLB)-affected Kinnow mandarin trees. Comparing treated and untreated diseased plants, Se-NPs at 75 mg L−1 demonstrated a remarkable enhancement in the levels of chlorophyll a (72.61%), chlorophyll b (65.66%), total chlorophyll (50.62%), carotenoid (64.58%), membrane stability index (66.81%), relative water (70.53%), and increased sugar (65.16%). Significantly, proline content (70.96%), H2O2 (64.89%), and MDA (66.81%) concentrations decreased when compared to untreated mandarin trees.
The biogenic Se-NPs (200 mg L−1) increased heat tolerance in Chrysanthemum by increasing the activity of peroxidase and catalase and decreasing polyphenol oxidase [33]. El Badri et al. [37] observed that 150 mol L−1 bio Se-NPs enhanced the length of shoots and roots by 8.47 and 24.74%. The enhanced level of Se (bio Se-NPs 150 mol L−1) also enhanced the total chlorophyll and chlorophyll a and b. Additionally, proline content significantly increased at conditions of 50, 100, and 150 mol L−1 by 21.60, 29.23, and 33.14% (bio Se-NPs), and 86.40, 125.69, and 243.38% (Na2SeO3), accordingly. In conclusion, it was observed that seedlings treated with bio Se-NPs surpassed Se (IV) in maintaining total leaf K+ concentration during salt stress [37]. Cui et al. [85] studied the medicinal plant Glycyrrhiza uralensis Fisch. in pot culture experiments under salt stress such as NaCl, SiO2 kg−1 and found that it enhanced sucrose synthetase (SS) and sucrose phosphate synthetase (SPS) activity.
According to Neysanian et al. [86], tomato plants exposed to foliar sprays of nSe at 0, 3, and 10 mg L−1 or equivalent dosages of sodium selenate showed elevated shoot and root biomass at 3 mg L−1 but considerably decreased biomass accumulation at 10 mg L−1. The BSe/nSe treatments transcriptionally increased the levels of miR172 (3.5-fold) and the bZIP transcription factor (9.7-fold) in leaves. In comparison, the leaves and fruits of the nSe-treated seedlings had an average transcriptional increase of 5.5 times in the carotene isomerase (CRTISO) gene.
The effectof nano-priming on the transcription levels of genes of GA biosynthesis, such as BnGA3ox2, BnGA20ox1, BnGA20ox2, BnGA3ox1, BnGA20ox3, and BnCPS, during rape seed germination under salt treatment, were examined. Those genes showed distinct expression levels, notably after being nano-primed by Se-NPs and ZnONPs after the salt stress subjection.
Furthermore, only BnGA20ox1 showed the least expression among the BnGA20-oxidase genes, and nano-priming with Se-NPs and ZnONPs raised their levels of countenance by 61.97% and 110.72% (salt-tolerant variety—Yangyou 9), 36.37% and 65.35% (salt-sensitive variety—Zhongshuang 11), respectively, in comparison to unprimed seeds and hydroprimed seeds at 24 h after sowing [26]. Recent research has focused on the transcriptional responses to nSe in several different plant species, including wheat Heat shock transcription factor A4a, Melissa officinalis (Hydroxyphenylpyruvate reductase and rosmarinicacid synthase, pepper (bZIP transcription factor), chicory (Hydroxycinnamoyl-CoA shikimate transferase 1, PAL, Dehydration responsive element binding factor-1, and hydroxycinnamoyl quinate hydroxycinnamoyltransferase genes), and bitter melon (WRKY1, PAL, and 4-Coumarate:CoA ligase, as reviewed in [86]. Soliman et al. [87] investigated physiological alterations in leaves and their impact on wheat crop yield in salt-affected soils. Se-NPs significantly stimulate water transporter genes in these studies, specifically P1P1, NIP, and N1P1. These genes were stimulated in saline stress and were considerably enhanced when straw biochar (SB) was administered. Wheat exhibited improved carbon absorption and biomass formation in SB and treatments, which might be associated with the activation of water transporter genes [87].
Influence on Primary and Secondary Metabolites
Plant secondary metabolism in response to NPs is necessary for plant performance, communication, and adaptation. Modifications in plant secondary metabolism brought on by NPs may have an impact on how well plants interact with their environment, which may have an impact on growth and productivity [88]. NPs can modulate plant secondary metabolism by interfering with numerous different signaling pathways. Plants’ initial responses to NPs often include increased levels of ROS, cytoplasmic Ca2+, and activation of mitogen-activated protein kinase (MAPK) cascades, substantially like other abiotic stresses. The induction of ROS in response to NP interactions has been evidenced throughout species of plants. The established link among ROS and secondary signaling messengers leads to secondary metabolism transcriptional regulation. The existing connection between secondary signaling molecules and ROS results in transcriptional regulation of secondary metabolisms.
Se may alter the secondary metabolism and biomass in plants, resulting in a higher concentration of certain health-promoting phytocompounds [15]. Battin et al. [89] stated aconcern regardingprimary metabolites; there is a rise in the activity of glutathione peroxidase (GPX), an enzyme whose catalytic cycle includes selenic acid (PSeOH). This combines with glutathione, which acts as a coenzyme in this process, to generate a selenyl-sulfide adduct [89]. Ascorbate peroxidase, catalase, superoxide dismutase, dehydroascorbate reductase, glutathione reductase, and monodehydroascorbate reductase are only a few of the enzymes with an antioxidant capacity that are made more active by selenium treatment [90].
Schiavon et al. [90] examined the impact of two selenium fertilization strategies on levels of anticarcinogenic seleno compounds in radish roots and discovered that the thiols cysteine and glutathione were existent at two-to-three-fold higher levels in Se-treated plants’ roots. Increases in glucoraphanin led to 35% higher levels of total glucosinolate. Se-methyl-SeCys is the only seleno-aminoacid present in Se-treated plants (100 mg kg−1 FW in leaves and 33 mg kg−1 FW in roots) that was noticed. They found that foliar Se administration in radish (Raphanus sativus) altered the synthesizing phenolic chemicals individually in leaves and roots in distinct ways. There are 11 major polyphenols found in leaves; five were flavonols and kaempferol derivatives, while the remaining six of the compounds constituted hydroxycinnamic acids. Despite the fact that sinapic acid, caffeic acid, kaempferol-3-O-arabinoside-7-O-rhamnoside, kaempferol-3-ramnosil glucoside, and kaempferol-3,7-diramnoside, which were explored to increase at particular Se dosages, the other identified phenolics (cumaric acid, kaempferol-7-O-rhamnoside) did not exhibit any increase (kaempherol-3-glucoside, ferulic acid, feruilmalatesinapoil-malate). In the past few years, countless researchers have examined the role of nanoparticles as an elicitor for stimulating the expression of genes that participate in secondary metabolite biosynthesis [91]. Elicitors may induce gene expression to create enzymes that boost the route of secondary metabolites [92]. Existing studies by Hussein et al. [93] and Zahedi et al. [94] have also revealed that Se-NP application promotes plants to generate higher secondary metabolites. Corresponding to this, Ciccolini et al. [95] showed that Se-induced accumulation of phenols and flavonoids in lettuce increased antioxidant effectiveness and stress adaptation. According to Li et al. [96], application of foliar treatment of 5 mg L−1 Se-NPs boosted celery’s overall antioxidant capacity by 46.7%, total flavonoids by 50%, particularly apigenin, p-coumaric acid, ferulic acid, luteolin, kaempferol, total phenols (21.4%), and vitamin C (26.7%). The effects of Se-NPs on the biochemical characteristics of cluster beans (Cyamopsis tetragonoloba) were studied by Ragavan et al. [22]. In these studies, cluster bean pot culture tests were conducted with varying concentrations of selenium nanoparticles (0, 100, 200, 300, 400, and 500 mg for treatment T0 (Control), T1, T2, T3, T4, and T5), and growth biochemical and yield estimates were performed after 60 days. They observed that T4 plants had higher chlorophyll a, b total chlorophyll, carotenoids, anthocyanin, protein, L-proline, free amino acids, and leaf nitrate. Over all, T4 has the highest yield of cluster beans among the treatments, while T0 has the lowest. Tian et al. [97] analyzed selenium-enriched broccoli and used 25 M Na2SeO4 to treat two broccoli cultivars. Se supplementation was observed to decrease the formation of total glucosinolates, particularly glucoraphanin, the direct precursor of a strong anti-cancer agent, in broccoli florets and leaves in this investigation. This work demonstrates that after Se supplementation, concentrations of the glucosinolate precursors methionine and phenylalanine, along with the expression of genes associated with glucosinolate production, were significantly lowered. The addition of Se-NPs at a concentration of 100 mg L−1 resulted in a significant accumulation of Se in the leaves of barley (Hordeum vulgare) cultivated under saline stress, an increase in the amount of aggregate phenolic composites, and a reduction in the content of ROS-mediated cellular membrane injury markers such as MDA, which could affect the metabolism and be the cause of nutrient deficiencies [98]. Soliman et al. [87] found that SB and Se-NP significantly increased phenols, osmolytes, and flavonoid levels in salt-stressed wheat plants, including glycine betaine, proline, and carbohydrates. The enhanced accumulation of phenols and flavonoids improved the antioxidant system, reducing ROS damage to cellular organelles.
5. Influence of Se-NPs on Crop Yield
Garca Márquez et al. [99] examined the impact of foliar supplementation of selenium (Na2SeO3 and Na2SeO4) in cereal crops at various concentrations between 30 and 300 g ha−1. They reported that the selenium application results in the deposition of Se in grains, and the metabolism might be stimulated. In a hydroponic method, the magnification of Se and antioxidants in edible parts of crops such as lettuce, tomato, and strawberry is increased in the form of Na2SeO3 (0.86 to 5 mg L−1) and Na2SeO4 (0.5 to 18 mg L−1), as reviewed in [99]. Se biofortification has beneficial actions on plant metabolism, resulting in the development of molecules with reduced power, which promotes stress tolerance [100], plant growth, and fruits with better nutraceutical qualities. Different crops have improved from various biofortification techniques, which include the exogenous inclusion of ionic forms [15]. Se biofortification of food crops has shown positive outcomes, including a rise in metabolites that improve tolerance from the nutraceutical standpoint.
In wheat roots and shoots, Se-NPs were transported from roots to shoots and promptly absorbed into Se (IV) and organic forms, whereas the smaller diameter bio Se-NPs made by microorganisms may indeed enhance the wheat field to the uptake of Se-NPs. As a result, Se-NPs could therefore be used as a cutting-edge fertilizer in the green revolution [47]. The manufacture and application of nSe as a nutrient and biofortifier has been indicated to be an innovative method since it has been discovered to have superior biocompatibility, chemical stability, quick absorption, and lower toxicity than ionic forms of this element [79,96]. Foliar application of sodium selenate to leaves (up to 5 mg L−1) under normal and salt stress circumstances in canola plants dramatically enhanced the plant pods, seed numbers, and seed weight [101].
In pot culture experiments, with and without Si NP foliar supplement, Golubkina et al. [102] evaluated the individual and combined foliar application of chervil plants with potassium iodide (KI, 150 mg L−1) and Na2SeO4 (10 mg L−1). In all treatments, nano-Si (14 mg L−1) enhanced the shoot biomass compared to control plants: 4.8 times with Si, 2.8 times with KI + Si, 5.6 times with Se + Si, and 4.0 times with KI + Se + Si. Se, KI, and KI + Se treatments had a growth-stimulating impact on chervil shoots and roots, which increased by 2.7, 3.5, and 3.6 times, correspondingly. The foliar application of Na2SeO4(1 mg L−1) in tomatoes considerably delayed the fruit ripening and preserved the fruit quality. Lower ethylene synthesis and respiration rate were recorded with inhibiting ethylene biosynthesis genes such as 1-aminocyclopropane-1-carboxylic acid (ACC) synthase and ACC oxidase [103].
Foliar Se treatments (50 g ha−1) carried out on Cowpea (Vigna unguiculata L. Walp.) plants that grow in acidic and weathered soil conditions such as in tropical soils increased chlorophyll production and photosynthesis in addition to the antioxidant activities and productivity of the plant [104]. Cd-contaminated soil was used to grow the wheat plants, whereas they accumulated more biomass and had increased antioxidant enzyme function as a result of Se (Na2SeO4) foliar spraying at various growth stages. While leaf SOD, CAT, POD, and APX are dramatically increased with the supply of Se, lipid peroxidation is reduced [105]. By promoting the activity of glutamate synthase and nitrate reductase in lettuce, the foliar application of Se improved nitrogen metabolism [106]. Se-NP concentrations of 265–530 µM noticeably prompted organogenesis and root system development (40%) in tobacco callus cultures, whereas the form of selenate completely inhibited organogenesis and root system development. During an in vitro tobacco tissue culture (Nicotiana tabacum), it was reported that the administration of Se-NPs (265–530 M) enhanced the organogenesis and expansion of the root system [59].
The uniformly sized mature seeds of Brassica napus types such asthesalt-tolerant variety (Yangyou 9) and the salt-sensitive variety (Zhongshuang 11) were hydro-primed (HP) in ddH2O with 150 mol L−1 Se-NPs and 100 mg L−1 ZnONPs for 8 h. Se-NPs (150 mol L−1) enhanced CAT by 11.51% and 17.31% compared to Hydro priming in Yangyou 9 and Zhongshuang 11, respectively. Se-NPs also increased SOD activity by 3.386% and 3.981%, POD activity by 12.33% and 12.50%, APX activity by 15.78% and 12.20%, and SOD activity by 3.386% and 3.981% [26].
Apart from the Se-NPs, other nanomaterials are excessively incorporated into the environmental system. It is crucial to treat or recycle excess Se-NPs once they have been used in suitable farmlands to prevent environmental problems. In this regard, Padervand [107] worked on the 3-Glycidoxypropyltrimethoxysilane (GPTMS) decorated magnetic core—aluminosilicate shell Na (SiA1)06.XH2O/NiFe04) prepared hydrothermally. This method may successfully remove the dangerous lead and cadmium ions from waste fluids. The different types of applications of biosynthesized Se-NPs in plant growth, yield and stress tolerance are shown in Table 2.
Table 2.
The dose, mode of application, and the impact of biosynthesized Se-NPs onplant growth and development.
| Plant Species | Se-NPsSource (Selenium and Their Combination) | Dosage/Size | Mode of Application | Impact of Se-NPs | Biochemical/ Molecular Function |
References |
|---|---|---|---|---|---|---|
| Lactuca sativa | Na2SeO3 and SeO3 | 50 mg L−1 | Foliar spray | Plant growth | 1. Chlorophyll content was elevated at the early stages of plant germination. 2. Senescence prevention |
[108] |
| Brassica napus | Na2SeO4 | 2.5 and 5.0 mg L−1 | Foliar spray | Plant growth and yield | Increased in yield, shoot length, and number of leaves plant−1 under salt stress conditions | [101] |
| Solanum lycopersicum | N-Se, Na2SeO4 | 1 µM and 2.5 µM | Foliar spray | Plant growth at low and high-temperature conditions | Improved 27.5% green pigments content in hydroponic culture | [109] |
| Triticum aestivum | Se | 7.06 μM | Foliar spray | Plant growth | Maintaining higher growth, fresh and dry matter content | [110] |
| Brassica juncea | Na2SeO4 | 10 µM | Foliar spray | Plant growth improvement | Enhancing growth and photosynthesis efficacy | [111] |
| Nicotiana tabacum | Na2SeO3 | 6 mg kg−1 | Foliar spray | Plant growth improvement | Improved plant growth by uplifting plant photosynthesis | [112] |
| S. lycopersicum | Na2SeO4 | 10 to 20 mg kg−1 | Directly to peat | Plant growth improvement | Increased vitamin A content in tomato fruit | [113] |
| S. lycopersicum | Na2SeO4 | 1 mg L−1 | Foliar | Improved plant growth and disease management | 1. Increased antioxidant activity. 2. Control of gray mold rot infection |
[114] |
| Raphanus sativus cv. Saxa | Se | 5 mg | Foliar spray | Improved plant growth | Increasing the polyphenol content (flavanols, kaempferol derivative, and hydroxycinnamic acids) up to 10% higher than the control. | [90] |
| Se | 10 or 20µM | Foliar spray | Improved plant growth | 1. Enhanced in Glucosinolates, 2. Dimeric-4 mercaptobutyl (DMB-GLS) concentration | ||
| Se | 40 µM | Foliar spray | Improved plant growth | In plant root systems, the stimulation of high-affinity sulfate transporter genes (Sultr1;1 and Sultr1;2) | ||
| S. lycopersicum | Na2SeO4 | 1 mg L−1 | Foliar pre-treatment | Improved plant growth | 1. Delay in tomato fruit 2. Inhibition of ethylene biosynthetic genes—ACC synthase |
[103] |
| Fragaria × ananassa | Na2SeO4 | 1.9 and 19 mg L−1 | Nutrient solution | Plant growth and fruit yield | Growth regulator’s upregulation, biomass, and nutraceutical quality | [115] |
| Ocimum basilicum | Na2SeO4 | 4, 8, and 12 mg L−1 | Nutrient solution | Plant growth | Biofortification (Se enrichment in the leaves) | [116] |
| Oryza sativa | Na2SeO3 and Na2SeO4 | 120–300 g ha−1 | Foliar | Improved yield | Enhanced Se content in the rice grains | [117] |
| Cyamopsis tetragonoloba | Se-NPs | 400 mg | Foliar spray | Enhancing the growth yield | Enhancing the biochemical activity | [22] |
| Fragaria × ananassa | Se | 100 µM | Nutrient solution | Enhancing the growth | Enhanced the accumulation of anthocyanins | [115] |
| L. sativa | SeO2 | 5 mg L−1 | Nutrient solution | Enhancing the growth and biomass | Accumulation of 24 mg Kg−1 of Se in leaves (Dry Weight) | [118] |
| Citrus aurantifolia | Se-NPs | 50 mg L−1 | Imbibition of seeds | Plant growth improvement | Plant growth was improved | [119] |
| T. aestivum | Se-NPs | 5 µM | Imbibition | Plant growth improvement | Enhance the root aquaporins | [47] |
| Daucus carota | Na2SeO4 | 1 mg L−1 | Foliar apply | Enhancing the yield | Increased yield, decreased fruit ripening | [120] |
| S. lycopersicum | Na2SeO4 | 1 and 1.5 L−1 | Hydroponics | Yield improvement | Delayed postharvest ripening | [121] |
| T. aestivum | Na2SeO4 | 10 g ha−1 | Soil application | Yield improvement | 50% accumulation of Se in grains | [122] |
| T. aestivum | Na2SeO4 + surfactant | 120 g ha−1 | Foliar apply | Enhancing the growth and biomass | 1. Increased production by 48% and biomass by 30% 2. Increasing the grain weight (DW) |
[123] |
| Arachis hypogaea | nSe | 40 mg L−1 | Foliar | Enhanced plant growth | Improved antioxidant potential | [93] |
| S. lycopersicum | nSe | 10 mg L−1 | Foliar | Plant growth improvement | 1. Induce the salinity tolerance of growth 2. Enhanced antioxidant enzyme activity |
[124] |
| Lubia | nSe | 1.18 mg L−1 | Imbibition of seeds | Plant growth improvement | The total proteins, sugars, and increased seedling enzyme activity (α, β amylase, and protease) | [125] |
| Coriandrum sativum | nSe | 25 and 50 mg L−1 | Foliar + surfactant tween 80–0.005 | Plant growth improvement | Ascorbic acid content was improved. | [71] |
| Vicia faba | nSe | 10 and 20 mg L−1 | Imbibition of seeds | Yield improvement | The cytotoxicity activity | [126] |
| (Fragaria × ananassa) | Se-NPs | 10 and 20 mg L−1 | Foliar spray | Yield improvement | Enhanced organic acids and sugars content | [94] |
| Brassica chinensis | SeO32− | 10 μM | Hydroponic | Plant growth improvement | Enhanced antioxidant activity | [80] |
| Vigna unguiculata | Se-NPs | 6.25 µM | Foliar applications | Plant growth improvement | Increased the level of Indole Acetic Acid (IAA), Gibberellic Acid, and Cytokinins | [25] |
| Apium graveolens | SeNPs | 5 mg L−1, 50–78 nm | Foliar spray (Once in 10 days, 3 times application | Plant growth improvement | Increased primary and secondary metabolites | [96] |
| T. aestivum | Bio Se-NPs | 100 µg mL−1 | Mixture with the soil at the rate of 5% (v/w).) | Plant germination and yield improvement | Improvement of plant growth and 5–40% enhancement of the grain quantity and quality | [127] |
| Dracocephalum moldavicum | Cs–Se NPs | 5 mg L−1 | Exogenously applied | Plant growth and yield improvement | Enhance the improvement of the agronomic traits | [84] |
| Momordica charantia | Cs–Se NPs | 10, and 20 mg L−1 | Foliar spray | Plant growth enhancement | Increasing antioxidant enzyme activity, proline, and relative water content. | [128] |
| Hordeum vulgare | Se-NPs | 100 mg L−1 | Foliar mode | Enhancing plant growth | 1. Increase in phenolic composites under saline conditions. 2. Decrease in ROS-mediated cellular membrane harm markers |
[98] |
| H. vulgare | Se-NPs | 4.65 g mL−1 | Dosage | Improvement of seedling growth | Greatest seed germination percentage | [68] |
| Citrus nobilis × Citrus deliciosa | Se-NPs | 25, 50, 75, and 100 mg L−1 | Bio-Fabrication | Improvement of yield | Improving the content of carotenoids, chlorophyll, flavonoid, and soluble sugar) | [27] |
| Brassica napus | Bio Se-NPs | 150 µmol L−1 | Exogenously applied | Improvement in growth | Increased the shoot and root length under salt stress conditions. | [37] |
| T. aestivum | Se-NPs | 30 ppm (once a week) | Soybean straw biochar mixed with soil media | Improved growth | Significantly increased PSII efficiency under salt treatments | [87] |
6. Impact of Se-NPs on Abiotic and Biotic Stress Tolerance
NPs can be employed to minimize the aggregation and toxicity of heavy metals as well as protect plants against environmental effects such as salt or drought stress. NPs can act as a supply of micronutrients, boosting fitness and assisting plants in surviving stressful situations. Exogenous applications of plant-mediated Se-NPs boosted the function of APX, SOD, and CAT enzymes and subsequently stimulated the expression of antioxidant defense-related genes in maize and strawberry plants, enhancing their ability to withstand a/biotic stresses [129]. Meanwhile, according to a different study, exogenous foliar spray of Se-NPs reduced H2O2 and lipid peroxidation levels while increasing the activity of anti-oxidant enzymes, including peroxidase and SOD, in strawberry plants under salt stress [94].
Several antioxidant enzymes, namely SOD, APX, and CAT, increased drought tolerance in tomatoes treated with 40 M of selenium, according to Rady et al. [75]. APX accelerated drought tolerance by 44%, SOD increased drought tolerance by 56%, and CAT enhanced drought tolerance by 57%. The foliar mode administration of groundnut plants with nSe (40 mg L−1) for 45 days results in enhanced unsaturated fatty acids and antioxidant capability, which greatly increases plant growth [93]. Pomegranate plants under drought stress had better growth and yield owing to the Se-NP (10 nm) concentrations, and apart from that Se-NPs (10 nm) also improve the activity of photosynthetic pigments, nutritional status, antioxidant activity, and total phenolic content levels during drought conditions [94]. The Se-NPs are biologically synthesized by reduction with leaf extract obtained from the barley (Hordeum vulgare L.) plant used to lessen the harmful effects of saline stress. One of the main abiotic factors that impact plant crops is salinity, which affects 30% of the world’s irrigated agriculture and 7% of rainfed agriculture, ultimately resulting in a 65% loss in agricultural productivity. P3+ and Mg2+ concentrations in canola plants under control and saline treatments respond positively to Se treatment at 5 mg L−1 [101]. The growth and yield metrics of strawberry plants grown under normal and drought stress conditions (30, 60, and 100% foot-candles) were increased by spraying solutions of nanoparticles of SiO2, Se, and Se/SiO2 (50 and 100 mg L−1). The benefits of nanoparticles of SiO2, Se, and Se/SiO2 (SiO2-NPs, Se-NPs, and Se/SiO2-NPs) were shown in moderating the adverse effects of drought on the growth and yield of strawberry plants [130].
During high and low-temperature stress, a lower dosage of Se (2.5 µM) and nSe (1 µM) can increase plant growth performance more significantly than a high proportion of Se/N-Se (2.5 µM) [109]. The administration of 20 g Se to the Brassica napus L. plants resulted in a considerable increase in the dry weight of vegetative portions and pods and seeds [131]. At the same time, 20 ppm Se-supplied B. napus L. plants exhibited superior photosynthetic rate and protein content than control plants [131]. Dong et al. [132] reported that Lycium chinense L. leaf contents such as chlorophyll, chlorogenic acid, and carotenoids were increased substantially by 200–400% when selenium (10–50 ppm Se) was applied.
The supply of nano-SiO2 (10 mg L−1) to cotton soil did not affect plant Si concentration, whereas it substantially enhanced the IAA production [133]. Se-NPs might sustain Se beneficial actions at lesser concentrations to protect plants from metal toxicity via a reduction in oxidative stress, decreased metal uptake, and translocation [134]. According to Wu et al. [105], Se boosted the enzymatic activities of SOD and CAT levels in the roots and leaves of Chinese cabbage under Cd-stress conditions. The desirable features and distinctive bioactivities of Se-NPs, which have drawn interest in agricultural applications [135], have principally shown considerable economic benefits in helping plants to withstand heavy metal or other abiotic stress situations [136].
Gao et al. [137] reported that Se prevents hazardous metals from being absorbed and transported from the roots to the top plant parts, including shoots, leaves, and grain. Se-NPs(10–40 nm) were used to study the toxicity, physiological, and biological impacts of heat stress on Sorghum bicolor (L.) Moench [66]. Enhanced pollen germination rates and larger amounts of unsaturated phospholipids were accomplished, which also increased seed yield under heat stress. Administration of Se-NPs raised the percent seed yield (14%), seed set (19%), and pollen germination (26%). Selenium boosted the CAT, POD, and SOD activities in the drought-stressed cucumber roots [74]. This implies that the Se-NPs increased proline production by increasing nitrate reductase activity, which is necessary for proline synthesis. Se supplementation increases pectin and hemicellulose content as well as cell wall thickness, which improves harmful metal binding by the cell wall [65]. Babajani et al. [138] and Soleymanzadeh et al. [139] reported that an important mechanism that enhances plant defense against abiotic stress conditions is the Se-mediated stimulation of the antioxidant system. A study by Morales-Espinoza [124] investigated the impact of Se-NPs on tomato antioxidant responses, plant growth, and fruit quality under NaCl stress. The effects of four dosages of Se-NPs (1, 5, 10, and 20 mg L−1) were studied under sodium chloride stress-treated and control tomato plants. The aerial biomass and fruit mass responded favorably to the treatment (10 mg L−1 of Se-NPs + NaCl). Treatment with 20 mg L−1 of Se-NPs with NaCl resulted in the greatest levels of total chlorophyll and chlorophylls a and b. Compared to the other treatments, 10 mg L−1 of Se-NPs + NaCl and 20 mg L−1 of Se-NPs + NaCl were preferable [124]. Ikram et al. [18] investigated the effects of Se-NPs produced from Allium sativum L. gloves extract against drought stress in wheat plants. Remarkably, it was found that wheat plants under drought stress exhibited enhanced agronomic attributes (number of leaves per plant, shoot length, root length, and plant height) when exposed to plant-mediated Se-NPs at a concentration of 30 mg L−1.
The lesser level of Se-NPs promotes the development of A. niger, which may be related to trace elements’ role in microbial growth promotion. Low levels of Se also enhance the development and yield of ryegrass, Brassica, soybean, and potato plants. According to earlier findings, applying Se-NPs at a lesser level coupled with Trichoderma as a seed treatment improves plant growth characteristics, as reviewed in [36]. Plant pathogen Sclerospora graminicola affects maize and pearl millet crops. According to a previous study, S. graminicola’s growth, spore viability, and sporulation are inhibited by biosynthesized Se-NPs [36]. The development and photosynthetic ability of Nicotiana tabacum were shown to be negatively impacted by the supplementation of selenate at 10 mg L−1. However, no adverse effects were seen when selenate 1 mg L−1 was applied at higher concentrations (100 mg L−1), according to Zsiros et al. [140]. Inductively coupled plasma mass spectrometry (ICP-MS) determines strawberry plants’nSe absorption and accumulation rates under salt conditions [141]. Effects of nSe (10 and 100 µM) on strawberry plants subjected to salt stress were assessed in terms of photosynthetic performance, ion homeostasis, antioxidant system, and phenylpropanoids level and water-splitting complex (Fv/Fo), and the results revealed that the above parameters were improved as a result of the nSe treatment at 10 µM. In strawberry plants under drought stress, Se and SiO2 NPs had enhanced photosynthetic pigments compared to many other treatments. Additionally, they showed that 100 mg L−1 Se/SiO2 NPs enhanced the relative water content, water usage efficiency, and membrane stability [130].
Matraszek-Gawron [142] assessed the effects of Ni (5 and 10 mM)-subjected lettuce plants receiving 2 or 6 mM of various types of Se (selenite IV and selenate VI administered as Na2SeO3 or Na2SeO4). Se administration can reduce the absorption of metal by roots and transformation to shoots, a critical metal/metalloid stress tolerance mechanism [51]. Lower Se concentrations can activate the production of auxin, which imposes root architectural modification and enhanced root development, resulting in lower metal uptake [143]. Se nanoparticle treatment of tomato plants increased enzymatic antioxidants in tomatoes and indicated that Se-NPs activate the antioxidant system more effectively than selenate. Compared to control plants, Se and plant growth-promoting rhizobacteria (PGPR) are highly active at enhancing the antioxidant system, which helps to explain the elevated activity of antioxidant enzymes (APX, GPX, and CAT) in pretreated plants [86].
Mohammadhassan et al. [144] demonstrated that the NanoFe (10 mg L−1) and NanoSe (1 mg L−1) as the most appropriate treatment for the biosynthesis of maximum antioxidant activity (11,974 µg mL−1) in in vitro studies on Asparagus officinalis. Se-NPs are particularly effective against many diseases, including the downy mildew in pearl millet caused by Sclerospora graminicola, as reviewed in [99], tomato leaf blight induced by Alternaria alternata, and late blight of tomatoes caused by Phytopthora infestans. According to El-Saadony et al. [127], green-produced Se-NPs are highly efficient towards Fusarium species-caused root rot and crown diseases in wheat crops.
Biofortification is a process that tries to improve the Se level in edible plant parts to prevent Se deficiency in humans and cattle, and Se-NPs could be employed for Se biofortification. Investigations on Nicotiana tabacum and Allium sativum have demonstrated that Se-NPs are less damaging to plants than ionic Se salts (SeO42− and SeO32−) [96]. Application of 50 M selenite (Se IV) to B. juncea managed to improve germination by increasing seedling vigor and germination rate as well as growth by decreasing the As-based (As III) harmful actions in shoot and root length, fresh and dry mass of the plant, and root/shoot ratio by about 9, 12, 5, and 9%, respectively [145]. The Se (8 mM) administration boosted Ca and K levels and salt stress resistance in bread wheat exposed to salt stress [146]. Se-NPs have been linked to significantly improved Cd-mediated oxidative damage in Brassica napus [81]. When selenium was applied to quinoa plants in limited concentrations (2.5 and 5 mg L−1), the plant’s growth parameters and antioxidative enzymes all significantly increased under diverse abiotic stressors [147]. In particular, Se-NP treatment boosted the proline level in control and Cd-stressed plants [148].
In a previous study [149], Se-NPs (1 mg L−1), various bacteria (Bacillus cereus, and Pseudomonas fluorescens), and a combination of both were used to prime foxmillet seeds before they were planted in pots and subjected to salinity stress (0, 100, and 200 mM) after two weeks. The results revealed that the ability of a plant to handle salinity stress could be improved by soaking millet seeds in Se-NP solution, improving photosynthetic pigments, lowering oxidative stress, preserving the cell membrane and suitable solutes, and lowering sodium uptake. When PGPRs bacteria develop, these effects are accelerated. Secondary stresses, including oxidative stress, which is detrimental to plant cells due to steep ROS, osmotic disparity, and water deficits, frequently occur along with salinity-induced osmotic stress, leading to ion toxicity in stressed plants, as reviewed in [37]. Se-biofortified tomato fruits were produced when selenate was applied as a foliar spray (0, 2, or 20 mg Se); as a result, the selenate level is sufficiently low and is not adverse to human health. Schiavon et al. [150] illustrated that Se-biofortified fruits had higher amounts of antioxidants.
Commercially available Se-NPs (80 to 100 nm) enhanced the growth recovery of wheat plants subjected to salt stress more effectively than soybean straw biochar (SB), according to Soliman et al. [87]. Their results depicted that the salt-stressed plants applied with SB and biochar + led to 25%, 33%, and 44% more root dry weight, respectively. Designing a feasible and ecologically sustainable method for producing selenium nanoparticles has gradually gained more attention [151]. Se-NPs’ ability to adsorb substances makes them useful for the remediation of soil and water contaminated with metals and heavy metals. Regarding their use as medicinal agents and food additives, SeNPs have drawn particular attention [152].
7. Conclusions
Selenium is a crucial microelement vital for the growth and development of various life forms, including plants. Nanomaterials are potentially active compared to their bulk form due to their large surface area and tiny size. This review presented the various types of Se-NP synthesis approaches, optimal conditions for synthesis, and their characterization methods. Se-NPs’ influence on the plants’ growth and physiology was discussed in detail. Their impact on the production of primary and secondary metabolites and their functions concerning stress tolerance were provided. This review will help researchers to understand the mechanistic functions of Se-NPs in the growth and yield, nutraceutical quality, and metal, biotic, and abiotic stress tolerance in plants, and will provide insight for using Se-NPs in agriculture. Detailed molecular research on Se-NPs’ uptake, translocation, distribution, and how they influence growth and stress tolerance in plants is warranted to increase their application in sustainable modern agriculture. Additionally, their utilization in nano-agrochemicals is also essential in future modern agricultural applications.
Acknowledgments
This article was supported by the KU Research Professor Program of Konkuk University.
Author Contributions
Conceptualization, R.S. and B.V.; data curation, R.S. and B.V.; writing—original draft preparation, P.S.K. and S.T.; Data curation and formal analysis; K.R., P.M. and H.S.; writing—review and editing, and I.S.; funding acquisition; review and editing, I.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in the present study are available in the article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Ingle A.P., Duran N., Rai M. Bioactivity, mechanism of action, and cytotoxicity of copper-based nanoparticles: A review. Appl. Microbiol. Biotechnol. 2014;98:1001–1009. doi: 10.1007/s00253-013-5422-8. [DOI] [PubMed] [Google Scholar]
- 2.Arshad R., Gulshad L., Haq I.U., Farooq M.A., Al-Farga A., Siddique R., Manzoor M.F., Karrar E. Nanotechnology: A novel tool to enhance the bioavailability of micronutrients. Food Sci. Nutr. 2021;9:3354–3361. doi: 10.1002/fsn3.2311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sun J., Jia Q., Li Y., Zhang T., Chen J., Ren Y., Dong K., Xu S., Shi N.N., Fu S. Effects of Arbuscular Mycorrhizal Fungi and Biochar on Growth, Nutrient Absorption, and Physiological Properties of Maize (Zea mays L.) J. Fungi. 2022;8:1275. doi: 10.3390/jof8121275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li J., Charles L.S., Yang Z., Du G., Fu S. Differential mechanisms drive species loss under artificial shade and fertilization in the alpine meadow of the Tibetan Plateau. Front. Plant Sci. 2022;13:63. doi: 10.3389/fpls.2022.832473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pramanik P.K.D., Solanki A., Debnath A., Nayyar A., El-Sappagh S., Kwak K.S. Advancing modern healthcare with nanotechnology, nanobiosensors, and internet of nano things: Taxonomies, applications, architecture, and challenges. IEEE Access. 2020;8:65230–65266. doi: 10.1109/ACCESS.2020.2984269. [DOI] [Google Scholar]
- 6.Wu Z., Li T. Nanoparticle-mediated cytoplasmic delivery of messenger RNA vaccines: Challenges and future perspectives. Pharm. Res. 2021;38:473–478. doi: 10.1007/s11095-021-03015-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pulizzi F. Nano in the future of crops. Nat. Nanotechnol. 2019;14:507. doi: 10.1038/s41565-019-0475-1. [DOI] [PubMed] [Google Scholar]
- 8.Hamouda R.A., Hussein M.H., Abo-Elmagd R.A., Bawazir S.S. Synthesis and biological characterization of silver nanoparticles derived from the cyanobacterium Oscillatoria limnetica. Sci. Rep. 2019;9:113071. doi: 10.1038/s41598-019-49444-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dissanayake C.B., Chandrajith R. Introduction to Medical Geology. Springer; Berlin/Heidelberg, Germany: 2009. Selenium-A New Entrant to Medical Geology; pp. 205–222. [Google Scholar]
- 10.Winkel L.H.E., Vriens B., Jones G.D., Schneider L.S., Pilon-Smits E., Bañuelos G.S. Selenium Cycling Across Soil-Plant-Atmosphere Interfaces: A Critical Review. Nutrients. 2015;7:4199–4239. doi: 10.3390/nu7064199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.RajaeeBehbahani S., Iranbakhsh A., Ebadi M., Majd A., Ardebili Z.O. Red elemental selenium nanoparticles mediated substantial variations in growth, tissue differentiation, metabolism, gene transcription, epigenetic cytosine DNA methylation, and callogenesis in bittermelon (Momordica charantia); an in vitro experiment. PLoS ONE. 2020;15:e0235556. doi: 10.1371/journal.pone.0235556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bhatia P., Aureli F., D’Amato M., Prakash R., Cameotra S.S., Nagaraja T.P., Cubadda F. Selenium bioaccessibility and speciation in biofortified Pleurotus mushrooms grown on selenium-rich agricultural residues. Food Chem. 2013;140:225–230. doi: 10.1016/j.foodchem.2013.02.054. [DOI] [PubMed] [Google Scholar]
- 13.Wadhwani S.A., Shedbalkar U.U., Singh R., Chopade B.A. Biogenic selenium nanoparticles: Current status and future prospects. Appl. Microbiol. Biotechnol. 2016;100:2555–2566. doi: 10.1007/s00253-016-7300-7. [DOI] [PubMed] [Google Scholar]
- 14.Hosnedlova B., Kepinska M., Skalickova S., Fernandez C., Ruttkay-Nedecky B., Peng Q., Baron M., Melcova M., Opatrilova R., Zidkova J., et al. Nano-selenium and its nanomedicine applications: A critical review. Int. J. Nanomed. 2018;13:2107–2128. doi: 10.2147/IJN.S157541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Malagoli M., Schiavon M., Dall’Acqua S., Pilon-Smits E.A.H. Effects of selenium biofortification on crop nutritional quality. Front. Plant Sci. 2015;6:280. doi: 10.3389/fpls.2015.00280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zohra E., Ikram M., Omar A.A., Hussain M., Satti S.H., Raja N.I., Ehsan M. Potential applications of biogenic selenium nanoparticles in alleviating biotic and abiotic stresses in plants: A comprehensive insight on the mechanistic approach and future perspectives. Green Process. Synth. 2021;10:456–475. doi: 10.1515/gps-2021-0047. [DOI] [Google Scholar]
- 17.Khurana A., Tekula S., Saifi M.A., Venkatesh P., Godugu C. Therapeutic applications of selenium nanoparticles. Biomed. Pharmacother. 2019;111:802–812. doi: 10.1016/j.biopha.2018.12.146. [DOI] [PubMed] [Google Scholar]
- 18.Ikram M., Javed B., Raja N.I., Mashwani Z.-u.-R. Biomedical potential of plant-based selenium nanoparticles: A comprehensive review on therapeutic and mechanistic aspects. Int. J. Nanomed. 2021;16:249. doi: 10.2147/IJN.S295053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Khan I., Zaneb H., Masood S., Yousaf M.S., Rehman H.F., Rehman H. Effect of Moringa oleifera leaf powder supplementation on growth performance and intestinal morphology in broiler chickens. J. Anim. Physiol. Anim. Nutr. 2017;101:114–121. doi: 10.1111/jpn.12634. [DOI] [PubMed] [Google Scholar]
- 20.Pyrzynska K., Sentkowska A. Biosynthesis of selenium nanoparticles using plant extracts. J. Nanostruct. Chem. 2021;12:467–480. doi: 10.1007/s40097-021-00435-4. [DOI] [Google Scholar]
- 21.Panahi-Kalamuei M., Salavati-Niasari M., Hosseinpour-Mashkani S.M. Facile microwave synthesis, characterization, and solar cell application of selenium nanoparticles. J. Alloys Compd. 2014;617:627–632. doi: 10.1016/j.jallcom.2014.07.174. [DOI] [Google Scholar]
- 22.Ragavan P., Ananth A., Rajan M.R. Impact of selenium nanoparticles on growth, biochemical characteristics and yield of cluster bean Cyamopsis tetragonoloba. Int. J. Environ. Agric. Biotechnol. 2017;2:238983. doi: 10.22161/ijeab/2.6.19. [DOI] [Google Scholar]
- 23.El-Ghazaly M.A., Fadel N., Rashed E., El-Batal A., Kenawy S.A. Anti-inflammatory effect of selenium nanoparticles on the inflammation induced in irradiated rats. Can. J. Physiol. Pharmacol. 2017;95:101–110. doi: 10.1139/cjpp-2016-0183. [DOI] [PubMed] [Google Scholar]
- 24.Vahdati M., Tohidi Moghadam T. Synthesis and characterization of selenium nanoparticles-lysozyme nanohybrid system with synergistic antibacterial properties. Sci. Rep. 2020;10:510. doi: 10.1038/s41598-019-57333-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.El Lateef Gharib F.A., Zeid I.M., Ghazi S.M., Ahmed E.Z. The response of cowpea (Vigna unguiculata L) plants to foliar application of sodium selenate and selenium nanoparticles (SeNPs) J. Nanomater. Mol. Nanotechnol. 2019;8:4. [Google Scholar]
- 26.El-Badri A.M., Batool M., Wang C., Hashem A.M., Tabl K.M., Nishawy E., Kuai J., Zhou G., Wang B. Selenium and zinc oxide nanoparticles modulate the molecular and morpho-physiological processes during seed germination of Brassica napus under salt stress. Ecotoxicol. Environ. Saf. 2021;225:112695. doi: 10.1016/j.ecoenv.2021.112695. [DOI] [PubMed] [Google Scholar]
- 27.Ikram M., Raja N.I., Mashwani Z.U.R., Omar A.A., Mohamed A.H., Satti S.H., Zohra E. Phytogenic Selenium Nanoparticles Elicited the Physiological, Biochemical, and Antioxidant Defense System Amelioration of Huanglongbing-Infected ‘Kinnow’ Mandarin Plants. Nanomaterials. 2022;12:356. doi: 10.3390/nano12030356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang H., Zhou H., Bai J., Li Y., Yang J., Ma Q., Qu Y. Biosynthesis of selenium nanoparticles mediated by fungus Mariannaea sp. HJ and their characterization. Colloids Surf. A Physicochem. Eng. Asp. 2019;571:9–16. doi: 10.1016/j.colsurfa.2019.02.070. [DOI] [Google Scholar]
- 29.Sharma G., Sharma A.R., Bhavesh R., Park J., Ganbold B., Nam J.S., Lee S.S. Biomolecule-mediated synthesis of selenium nanoparticles using dried Vitis vinifera (raisin) extract. Molecules. 2014;19:2761–2770. doi: 10.3390/molecules19032761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Anu K., Devanesan S., Prasanth R., AlSalhi M.S., Ajithkumar S., Singaravelu G. Biogenesis of selenium nanoparticles and their anti-leukemia activity. J. King Saud. Univ. Sci. 2020;32:2520–2526. doi: 10.1016/j.jksus.2020.04.018. [DOI] [Google Scholar]
- 31.Alam H., Khatoon N., Raza M., Ghosh P.C., Sardar M. Synthesis and Characterization of Nano Selenium Using Plant Biomolecules and Their Potential Applications. Bio Nano Sci. 2019;9:96–104. doi: 10.1007/s12668-018-0569-5. [DOI] [Google Scholar]
- 32.Joshi S.M., De Britto S., Jogaiah S., Ito S.I. Mycogenic selenium nanoparticles as potential new generation broad spectrum antifungal molecules. Biomolecules. 2019;9:419. doi: 10.3390/biom9090419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Seliem M.K., Hafez Y., El-Ramady H. Using Nano-selenium in reducing the negative effects of high temperature stress on Chrysanthemum morifolium Ramat. J. Sustain. Agric. Sci. 2020;46:47–60. doi: 10.21608/jsas.2020.23905.1203. [DOI] [Google Scholar]
- 34.Ismail A.W.A., Sidkey N.M., Arafa R.A., Fathy R.M., El-Batal A.I. Evaluation of in vitro antifungal activity of silver and selenium nanoparticles against Alternaria solani caused early blight disease on potato. Br. Biotechnol. J. 2016;12:1–11. doi: 10.9734/BBJ/2016/24155. [DOI] [Google Scholar]
- 35.Khalifa H.M.S., Sameer W.M. Control of the green mold of orange using fungicides alone and in combination with antioxidants. Middle East J. Appl Sci. 2014;4:200–206. [Google Scholar]
- 36.Nandini B., Hariprasad P., Prakash H.S., Shetty H.S., Geetha N. Trichogenic-selenium nanoparticles enhance disease suppressive ability of Trichoderma against downy mildew disease caused by Sclerosporagraminicola in pearl millet. Sci. Rep. 2017;7:2612. doi: 10.1038/s41598-017-02737-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.El-Badri A.M., Hashem A.M., Batool M., Sherif A., Nishawy E., Ayaad M., Hassan H.M., Elrewainy I.M., Wang J., Kuai J., et al. Comparative efficacy of bio-selenium nanoparticles and sodium selenite on morpho-physiochemical attributes under normal and salt stress conditions, besides selenium detoxification pathways in Brassica napus L. J. Nanobiotechnol. 2022;20:163. doi: 10.1186/s12951-022-01370-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Korde P., Ghotekar S., Pagar T., Pansambal S., Oza R., Mane D. Plant extract assisted eco-benevolent synthesis of selenium nanoparticles-a review on plant parts involved, characterization and their recent applications. J. Chem. Rev. 2020;2:157–168. [Google Scholar]
- 39.Satgurunathan T., Bhavan P.S., Komathi S. Green synthesis of selenium nanoparticles from sodium selenite using garlic extract and its enrichment on Artemia nauplii to feed the freshwater prawn Macrobrachiumrosenbergiipost-larvae. Res. J. Chem. Environ. 2017;21:1–12. [Google Scholar]
- 40.Sowndarya P., Ramkumar G., Shivakumar M.S. Green synthesis of selenium nanoparticles conjugated Clausena dentata plant leaf extract and their insecticidal potential against mosquito vectors. Artif. Cells Nanomed. Biotechnol. 2017;45:1490–1495. doi: 10.1080/21691401.2016.1252383. [DOI] [PubMed] [Google Scholar]
- 41.Cui D., Liang T., Sun L., Meng L., Yang C., Wang L., Liang T., Li Q. Green synthesis of selenium nanoparticles with extract of hawthorn fruit induced HepG2 cells apoptosis. Pharm. Biol. 2018;56:528–534. doi: 10.1080/13880209.2018.1510974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gunti L., Dass R.S., Kalagatur N.K. Phytofabrication of selenium nanoparticles from Emblica officinalis fruit extract and exploring its biopotential applications: Antioxidant, antimicrobial, and biocompatibility. Front. Microbiol. 2019;10:931. doi: 10.3389/fmicb.2019.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Deepa B., Ganesan V. Bioinspiredsynthesis of selenium nanoparticles using flowers of Catharanthus roseus (L.) G. Don.; Peltophorumpterocarpum (DC.) Backer ex Heyne–a comparison. Int. J. Chem. Technol. Res. 2015;7:725–733. [Google Scholar]
- 44.Alagesan V., Venugopal S. Green synthesis of selenium nanoparticle using leaves extract of Withaniasomnifera and its biological applications and photocatalytic activities. Bionanoscience. 2019;9:105–116. doi: 10.1007/s12668-018-0566-8. [DOI] [Google Scholar]
- 45.Fardsadegh B., Jafarizadeh-Malmiri H. Aloe vera leaf extract mediated green synthesis of selenium nanoparticles and assessment of their in vitro antimicrobial activity against spoilage fungi and pathogenic bacteria strains. Green Process. Synth. 2019;8:399–407. doi: 10.1515/gps-2019-0007. [DOI] [Google Scholar]
- 46.Menon S., KS S.D., Agarwal H., Shanmugam V.K. Efficacy of biogenic selenium nanoparticles from an extract of ginger towards evaluation on anti-microbial and anti-oxidant activities. Colloid Interface Sci. Commun. 2019;29:1–8. doi: 10.1016/j.colcom.2018.12.004. [DOI] [Google Scholar]
- 47.Hu T., Li H., Li J., Zhao G., Wu W., Liu L., Wang Q., Guo Y. Absorption and bio-transformation of selenium nanoparticles by wheat seedlings (Triticum aestivum L.) Front. Plant Sci. 2018;9:597. doi: 10.3389/fpls.2018.00597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Gudkov S.V., Shafeev G.A., Glinushkin A.P., Shkirin A.V., Barmina E.V., Rakov I.I., Simakin A.V., Kislov A.V., Astashev M.E., Vodeneev V.A., et al. Production and use of selenium nanoparticles as fertilizers. ACS Omega. 2020;5:17767–17774. doi: 10.1021/acsomega.0c02448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Anu K., Singaravelu G., Murugan K., Benelli G. Green-synthesis of selenium nanoparticles using garlic cloves (Allium sativum): Biophysical characterization and cytotoxicity on vero cells. J. Clust. Sci. 2017;28:551–563. doi: 10.1007/s10876-016-1123-7. [DOI] [Google Scholar]
- 50.El-Ramady H., Abdalla N., Taha H.S., Alshaal T., El-Henawy A., Faizy S.E.D.A., Shams M.S., Youssef S.M., Shalaby T., Bayoumi Y., et al. Selenium and nano-selenium in plant nutrition. Environ. Chem. Lett. 2016;14:123–147. doi: 10.1007/s10311-015-0535-1. [DOI] [Google Scholar]
- 51.Hasanuzzaman M., Bhuyan M.B., Raza A., Hawrylak-Nowak B., Matraszek-Gawron R., Nahar K., Fujita M. Selenium toxicity in plants and environment: Biogeochemistry and remediation possibilities. Plants. 2020;9:1711. doi: 10.3390/plants9121711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasanuzzaman M., Hossain M.A., da Silva J.A.T., Fujita M. Crop Stress and Its Management: Perspectives and Strategies. Springer; Dordrecht, The Netherlands: 2012. Plant response and tolerance to abiotic oxidative stress: Antioxidant defense is a key factor; pp. 261–315. [Google Scholar]
- 53.Malik J.A., Goel S., Kaur N., Sharma S., Singh I., Nayyar H. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exp. Bot. 2012;77:242–248. doi: 10.1016/j.envexpbot.2011.12.001. [DOI] [Google Scholar]
- 54.Shahbaz M., Fatima N., Mashwani Z.U.R., Akram A., Mehak A., Abasi F., Ajmal M., Yousaf T., Raja N.I., UlHassan H., et al. Effect of Phytosynthesized Selenium and Cerium Oxide Nanoparticles on Wheat (Triticum aestivum L.) against Stripe Rust Disease. Molecules. 2022;27:8149. doi: 10.3390/molecules27238149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Wang K., Wang Y., Li K., Wan Y., Wang Q., Zhuang Z., Guo Y., Li H. Uptake, translocation and biotransformation of selenium nanoparticles in rice seedlings (Oryza sativa L.) J. Nanobiotechnol. 2020;18:103. doi: 10.1186/s12951-020-00659-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zhu Y.G., Huang Y., Hu Y., Liu Y., Christie P. Interactions between selenium and iodine uptake by spinach (Spinacia oleracea L.) in solution culture. Plant Soil. 2004;261:99–105. doi: 10.1023/B:PLSO.0000035539.58054.e1. [DOI] [Google Scholar]
- 57.White P.J., Broadley M.R. Biofortification of crops with seven mineral elements often lacking in human diets–iron, zinc, copper, calcium, magnesium, selenium and iodine. New Phytol. 2009;182:49–84. doi: 10.1111/j.1469-8137.2008.02738.x. [DOI] [PubMed] [Google Scholar]
- 58.Nair R., Varghese S.H., Nair B.G., Maekawa T., Yoshida Y., Kumar D.S. Nanoparticulate material delivery to plants. Plant Sci. 2010;179:154–163. doi: 10.1016/j.plantsci.2010.04.012. [DOI] [Google Scholar]
- 59.Domokos-Szabolcsy E., Alshaal T., Elhawat N., Abdalla N., Reis A., El-Ramady H. The interactions between selenium, nutrients and heavy metals in higher plants under abiotic stresses. Environ. Biodivers. Soil Secur. 2017;1:5–31. [Google Scholar]
- 60.Schiavon M., Pilon-Smits E.A. The fascinating facets of plant selenium accumulation–biochemistry, physiology, evolution and ecology. New Phytol. 2017;213:1582–1596. doi: 10.1111/nph.14378. [DOI] [PubMed] [Google Scholar]
- 61.Rastogi A., Tripathi D.K., Yadav S., Chauhan D.K., Živčák M., Ghorbanpour M., El-Sheery N.I., Brestic M. Application of silicon nanoparticles in agriculture. 3 Biotech. 2019;9:90. doi: 10.1007/s13205-019-1626-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Ali A., Shah T., Ullah R., Zhou P., Guo M., Ovais M., Tan Z., Rui Y. Review on recent progress in magnetic nanoparticles: Synthesis, characterization, and diverse applications. Front. Chem. 2021;9:629054. doi: 10.3389/fchem.2021.629054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Tarrahi R., Mahjouri S., Khataee A. A review on in vivo and in vitro nanotoxicological studies in plants: A headlight for future targets. Ecotoxicol. Environ. Saf. 2021;208:111697. doi: 10.1016/j.ecoenv.2020.111697. [DOI] [PubMed] [Google Scholar]
- 64.Mozafariyan M., Shekari L., Hawrylak-Nowak B., Kamelmanesh M.M. Protective role of selenium on pepper exposed to cadmium stress during reproductive stage. Biol. Trace Elem. Res. 2014;160:97–107. doi: 10.1007/s12011-014-0028-2. [DOI] [PubMed] [Google Scholar]
- 65.Zhao Y., Hu C., Wang X., Qing X., Wang P., Zhang Y., Zhang X., Zhao X. Selenium alleviated chromium stress in Chinese cabbage (Brassica campestris L. ssp. Pekinensis) by regulating root morphology and metal element uptake. Ecotoxicol. Environ. Saf. 2019;173:314–321. doi: 10.1016/j.ecoenv.2019.01.090. [DOI] [PubMed] [Google Scholar]
- 66.Djanaguiraman M., Belliraj N., Bossmann S.H., Prasad P.V. High-temperature stress alleviation by selenium nanoparticle treatment in grain sorghum. ACS Omega. 2018;3:2479–2491. doi: 10.1021/acsomega.7b01934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Al-Deriny S.H., Dawood M.A., Elbialy Z.I., El-Tras W.F., Mohamed R.A. Selenium nanoparticles and spirulina alleviate growth performance, hemato-biochemical, immune-related genes, and heat shock protein in Nile tilapia (Oreochromis niloticus) Biol. Trace Elem. Res. 2020;19:661–668. doi: 10.1007/s12011-020-02096-w. [DOI] [PubMed] [Google Scholar]
- 68.Siddiqui S.A., Blinov A.V., Serov A.V., Gvozdenko A.A., Kravtsov A.A., Nagdalian A.A., Raffa V.V., Maglakelidze D.G., Blinova A.A., Kobina A.V., et al. Effect of selenium nanoparticles on germination of Hordéumvulgáre barley seeds. Coatings. 2021;11:862. doi: 10.3390/coatings11070862. [DOI] [Google Scholar]
- 69.Bideshki A., Arvin M.J., Aien A., Hasandokht M.R., Khalighi A. Interactive effects of Foliar 24-Epibrassinolide and selenium applications on yield, reduce nitrate accumulation and selenium enrichment in potato tuber in field. Cogent Food Agric. 2019;5:1690315. doi: 10.1080/23311932.2019.1690315. [DOI] [Google Scholar]
- 70.Dai Z., Imtiaz M., Rizwan M., Yuan Y., Huang H., Tu S. Dynamics of selenium uptake, speciation, and antioxidant response in rice at different panicle initiation stages. Sci. Total Environ. 2019;691:827–834. doi: 10.1016/j.scitotenv.2019.07.186. [DOI] [PubMed] [Google Scholar]
- 71.El-Kinany R.G., Brengi S.H., Nassar A.K., El-Batal A. Enhancement of Plant Growth, Chemical Composition and Secondary Metabolites of Essential Oil of Salt-Stressed Coriander (Coriandrum sativum L.) Plants Using Selenium, Nano-Selenium, and Glycine Betaine. Sci. J. Flowers Ornam. Plants. 2019;6:151–173. doi: 10.21608/sjfop.2019.84973. [DOI] [Google Scholar]
- 72.Alves L.R., Rossatto D.R., Rossi M.L., Martinelli A.P., Gratão P.L. Selenium improves photosynthesis and induces ultrastructural changes but does not alleviate cadmium-stress damages in tomato plants. Protoplasma. 2020;257:597–605. doi: 10.1007/s00709-019-01469-w. [DOI] [PubMed] [Google Scholar]
- 73.Lyu L., Wang H., Liu R., Xing W., Li J., Man Y.B., Wu F. Size-dependent transformation, uptake, and transportation of SeNPs in a wheat–soil system. J. Hazard. Mater. 2022;424:127323. doi: 10.1016/j.jhazmat.2021.127323. [DOI] [PubMed] [Google Scholar]
- 74.Xu H., Yan J., Qin Y., Xu J., Shohag M.J.I., Wei Y., Gu M. Effect of different forms of selenium on the physiological response and the cadmium uptake by rice under cadmium stress. Int. J. Environ. Res. Public Health. 2020;17:6991. doi: 10.3390/ijerph17196991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rady M.M., Belal H.E., Gadallah F.M., Semida W.M. Selenium application in two methods promotes drought tolerance in Solanum lycopersicum plant by inducing the antioxidant defense system. Sci. Hortic. 2020;266:109290. doi: 10.1016/j.scienta.2020.109290. [DOI] [Google Scholar]
- 76.Xu J., Gong Y., Sun Y., Cai J., Liu Q., Bao J., Yang J., Zhang Z. Impact of selenium deficiency on inflammation, oxidative stress, and phagocytosis in mouse macrophages. Biol. Trace Elem. Res. 2020;194:237–243. doi: 10.1007/s12011-019-01775-7. [DOI] [PubMed] [Google Scholar]
- 77.Mroczek-Zdyrska M., Wójcik M. The influence of selenium on root growth and oxidative stress induced by lead in Vicia faba L. minor plants. Biol. Trace Elem. Res. 2012;147:320–328. doi: 10.1007/s12011-011-9292-6. [DOI] [PubMed] [Google Scholar]
- 78.Molnár Á., Kolbert Z., Kéri K., Feigl G., Ördög A., Szőllősi R., Erdei L. Selenite-induced nitro-oxidative stress processes in Arabidopsis thaliana and Brassica juncea. Ecotoxicol. Environ. Saf. 2018;148:664–674. doi: 10.1016/j.ecoenv.2017.11.035. [DOI] [PubMed] [Google Scholar]
- 79.Skalickova S., Milosavljevic V., Cihalova K., Horky P., Richtera L., Adam V. Selenium nanoparticles as a nutritional supplement. Nutrition. 2017;33:83–90. doi: 10.1016/j.nut.2016.05.001. [DOI] [PubMed] [Google Scholar]
- 80.Yu Y., Fu P., Huang Q., Zhang J., Li H. Accumulation, subcellular distribution, and oxidative stress of cadmium in Brassica chinensis supplied with selenite and selenate at different growth stages. Chemosphere. 2019;216:331–340. doi: 10.1016/j.chemosphere.2018.10.138. [DOI] [PubMed] [Google Scholar]
- 81.Qi W.Y., Li Q., Chen H., Liu J., Xing S.F., Xu M., Yan Z., Song C., Wang S.G. Selenium nanoparticles ameliorate Brassica napus L. cadmium toxicity by inhibiting the respiratory burst and scavenging reactive oxygen species. J. Hazard. Mater. 2021;417:125900. doi: 10.1016/j.jhazmat.2021.125900. [DOI] [PubMed] [Google Scholar]
- 82.Pereira A.G., Gerolis L.G.L., Gonçalves L.S., Moreira L.M.C., Gastelois P.L., Neves M.J. Radiolytic synthesis and characterization of selenium nanoparticles: Comparative biosafety evaluation with selenite and ionizing radiation. World J. Microbiol. Biotechnol. 2022;38:33. doi: 10.1007/s11274-021-03218-9. [DOI] [PubMed] [Google Scholar]
- 83.Wang K., Wang Y., Wan Y., Mi Z., Wang Q., Wang Q., Li H. The fate of arsenic in rice plants (Oryza sativa L.): Influence of different forms of selenium. Chemosphere. 2021;264:128417. doi: 10.1016/j.chemosphere.2020.128417. [DOI] [PubMed] [Google Scholar]
- 84.Azimi F., Oraei M., Gohari G., Panahirad S., Farmarzi A. Chitosan-selenium nanoparticles (Cs–Se NPs) modulate the photosynthesis parameters, antioxidant enzymes activities and essential oils in Dracocephalummoldavica L. under cadmium toxicity stress. Plant Physiol. Biochem. 2021;167:257–268. doi: 10.1016/j.plaphy.2021.08.013. [DOI] [PubMed] [Google Scholar]
- 85.Cui J., Zhang E., Zhang X., Wang Q. Silicon alleviates salinity stress in licorice (Glycyrrhiza uralensis) by regulating carbon and nitrogen metabolism. Sci. Rep. 2021;11:1115. doi: 10.1038/s41598-020-80739-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Neysanian M., Iranbakhsh A., Ahmadvand R., OraghiArdebili Z., Ebadi M. Comparative efficacy of selenate and selenium nanoparticles for improving growth, productivity, fruit quality, and postharvest longevity through modifying nutrition, metabolism, and gene expression in tomato; potential benefits and risk assessment. PLoS ONE. 2020;15:e0244207. doi: 10.1371/journal.pone.0244207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Soliman M.H., Alnusairi G.S., Khan A.A., Alnusaire T.S., Fakhr M.A., Abdulmajeed A.M., Aldesuquy H.S., Yahya M., Najeeb U. Biochar and Selenium Nanoparticles Induce Water Transporter Genes for Sustaining Carbon Assimilation and Grain Production in Salt-Stressed Wheat. J. Plant Growth Regul. 2022:1–22. doi: 10.1007/s00344-022-10636-y. [DOI] [Google Scholar]
- 88.Marslin G., Sheeba C.J., Franklin G. Nanoparticles alter secondary metabolism in plants via ROS burst. Front. Plant Sci. 2017;8:832. doi: 10.3389/fpls.2017.00832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Battin E.E., Brumaghim J.L. Antioxidant activity of sulfur and selenium: A review of reactive oxygen species scavenging, glutathione peroxidase, and metal-binding antioxidant mechanisms. Cell Biochem. Biophys. 2009;55:1–23. doi: 10.1007/s12013-009-9054-7. [DOI] [PubMed] [Google Scholar]
- 90.Schiavon M., Lima L.W., Jiang Y., Hawkesford M.J. Selenium in Plants. Springer; Cham, Switzerland: 2017. Effects of selenium on plant metabolism and implications for crops and consumers; pp. 257–275. [Google Scholar]
- 91.Yarizade K., Hosseini R. Expression analysis of ADS, DBR2, ALDH1 and SQS genes in Artemisia vulgaris hairy root culture under nano cobalt and nano zinc elicitation. Ext. J. App. Sci. 2015;3:69–76. [Google Scholar]
- 92.Zhai X., Jia M., Chen L., Zheng C.J., Rahman K., Han T., Qin L.P. The regulatory mechanism of fungal elicitor-induced secondary metabolite biosynthesis in medical plants. Crit. Rev. Microbiol. 2017;43:238–261. doi: 10.1080/1040841X.2016.1201041. [DOI] [PubMed] [Google Scholar]
- 93.Hussain S.M., Khalid A., Shahzad M.M., Rasul A., Akram A.M., Ahmad N., Khalid F. Effect of dietary supplementation of selenium nanoparticles on growth performance and nutrient digestibility of common carp (Cyprinus carpio Linnaeus, 1758) fingerlings fed sunflower meal-based diet. Indian J. Fish. 2019;66:55–61. doi: 10.21077/ijf.2019.66.3.87585-07. [DOI] [Google Scholar]
- 94.Zahedi S.M., Abdelrahman M., Hosseini M.S., Hoveizeh N.F., Tran L.S.P. Alleviation of the effect of salinity on growth and yield of strawberry by foliar spray of selenium-nanoparticles. Environ. Pollut. 2019;253:246–258. doi: 10.1016/j.envpol.2019.04.078. [DOI] [PubMed] [Google Scholar]
- 95.Ciccolini V., Pellegrino E., Coccina A., Fiaschi A.I., Cerretani D., Sgherri C. Biofortification with Iron and Zinc Improves Nutritional and Nutraceutical Properties of Common Wheat Flour and Bread. J. Agric. Food Chem. 2017;65:5443–5452. doi: 10.1021/acs.jafc.7b01176. [DOI] [PubMed] [Google Scholar]
- 96.Li D., An Q., Wu Y., Li J.Q., Pan C. Foliar application of selenium nanoparticles on celery stimulates several nutrient component levels by regulating the α-linolenic acid pathway. ACS Sustain. Chem. Eng. 2020;8:10502–10510. doi: 10.1021/acssuschemeng.0c02819. [DOI] [Google Scholar]
- 97.Tian M., Xu X., Liu F., Fan X., Pan S. Untargeted metabolomics reveals predominant alterations in primary metabolites of broccoli sprouts in response to pre-harvest selenium treatment. Food Res. Int. 2018;111:205–211. doi: 10.1016/j.foodres.2018.04.020. [DOI] [PubMed] [Google Scholar]
- 98.Garza-García J.J., Hernández-Díaz J.A., Zamudio-Ojeda A., León-Morales J.M., Guerrero-Guzmán A., Sánchez-Chiprés D.R., López-Velázquez J.C., García-Morales S. The role of selenium nanoparticles in agriculture and food technology. Biol. Trace Elem. Res. 2021;200:2528–2548. doi: 10.1007/s12011-021-02847-3. [DOI] [PubMed] [Google Scholar]
- 99.García Márquez V., Morelos Moreno Á., Benavides Mendoza A., Medrano Macías J. Ionic selenium and nanoselenium as biofortifiers and stimulators of plant metabolism. Agronomy. 2020;10:1399. doi: 10.3390/agronomy10091399. [DOI] [Google Scholar]
- 100.Paciolla C., de Leonardis S., Dipierro S. Effects of selenite and selenate on the antioxidant systems in Senecio scandens L. Plant Biosyst. 2011;145:253–259. doi: 10.1080/11263504.2010.509942. [DOI] [Google Scholar]
- 101.Hashem H.A., Hassanein R.A., Bekheta M.A., El-Kady F.A. Protective role of selenium in canola (Brassica napus L.) plant subjected to salt stress. Egypt. J. Exp. Biol. 2013;9:199–211. [Google Scholar]
- 102.Golubkina N., Moldovan A., Fedotov M., Kekina H., Kharchenko V., Folmanis G., Alpatov A., Caruso G. Iodine and Selenium Biofortification of Chervil Plants Treated with Silicon Nanoparticles. Plants. 2021;10:2528. doi: 10.3390/plants10112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhu L., Peng X., Li H., Zhang Y., Yao S. On–off–on fluorescent silicon nanoparticles for recognition of chromium (VI) and hydrogen sulfide based on the inner filter effect. Sens. Actuators B Chem. 2017;238:196–203. doi: 10.1016/j.snb.2016.07.029. [DOI] [Google Scholar]
- 104.Silva V.M., Boleta E.H.M., Lanza M.G.D.B., Lavres J., Martins J.T., Santos E.F., dos Santos F.L.M., Putti F.F., Junior E.F., White P.J., et al. Physiological, biochemical, and ultrastructural characterization of selenium toxicity in cowpea plants. Environ. Exp. Bot. 2018;150:172–182. doi: 10.1016/j.envexpbot.2018.03.020. [DOI] [Google Scholar]
- 105.Wu Z., Liu S., Zhao J., Wang F., Du Y., Zou S., Li H., Wen D., Huang Y. Comparative responses to silicon and selenium in relation to antioxidant enzyme system and the glutathione-ascorbate cycle in flowering Chinese cabbage (Brassica campestris L. ssp. chinensis var. utilis) under cadmium stress. Environ. Exp. Bot. 2017;133:1–11. doi: 10.1016/j.envexpbot.2016.09.005. [DOI] [Google Scholar]
- 106.Bian Z.-H., Lei B., Cheng R.-F., Wang Y., Li T., Yang Q.-C. Selenium distribution and nitrate metabolism in hydroponic lettuce (Lactuca sativa L.): Effects of selenium forms and light spectra. J. Integr. Agric. 2020;19:133–144. doi: 10.1016/S2095-3119(19)62775-9. [DOI] [Google Scholar]
- 107.Padervand M. Reusable Porous Na (SiAl) O6. xH2O/NiFe2O4 Structure for Selective Removal of Heavy Metals from Waste Waters. 11,014,082. U.S. Patent. 2021 May 25;
- 108.Duma M., Alsina I., Dubova L., Stroksa L., Smiltina Z. The effect of sodium selenite and selenate on the quality of lettuce; Proceedings of the 6th Baltic Conference on Food Science and Technology Foodbalt; Jelgava, Latvia. 5–6 May 2011. [Google Scholar]
- 109.Haghighi M., Abolghasemi R., da Silva J.A.T. Low and high temperature stress affect the growth characteristics of tomato in hydroponic culture with Se and nano-Se amendment. Sci. Hortic. 2014;178:231–240. doi: 10.1016/j.scienta.2014.09.006. [DOI] [Google Scholar]
- 110.Nawaz F., Ashraf M.Y., Ahmad R., Waraich E.A., Shabbir R.N., Hussain R.A. Selenium supply methods and time of application influence spring wheat (Triticum aestivum L.) yield under water deficit conditions. J. Agric. Sci. 2017;155:643–656. doi: 10.1017/S0021859616000836. [DOI] [Google Scholar]
- 111.Naz F.S., Yusuf M., Khan T.A., Fariduddin Q., Ahmad A. Low level of selenium increases the efficacy of 24-epibrassinolide through altered physiological and biochemical traits of Brassica juncea plants. Food Chem. 2015;185:441–448. doi: 10.1016/j.foodchem.2015.04.016. [DOI] [PubMed] [Google Scholar]
- 112.Jiang C., Zu C., Shen J., Shao F., Li T. Effects of selenium on the growth and photosynthetic characteristics of flue-cured tobacco (Nicotiana tabacum L.) Acta Soc. Bot. Pol. 2015;84:71–77. doi: 10.5586/asbp.2015.006. [DOI] [Google Scholar]
- 113.Pezzarossa B., Rosellini I., Borghesi E., Tonutti P., Malorgio F. Effects of Se-enrichment on yield, fruit composition and ripening of tomato (Solanum lycopersicum) plants grown in hydroponics. Sci. Hortic. 2014;165:106–110. doi: 10.1016/j.scienta.2013.10.029. [DOI] [Google Scholar]
- 114.Zhu Z., Chen Y., Zhang X., Li M. Effect of Foliar Treatment of Sodium Selenate on Postharvest Decay and Quality of Tomato Fruits. Sci. Hortic. 2016;198:304–310. doi: 10.1016/j.scienta.2015.12.002. [DOI] [Google Scholar]
- 115.Mimmo T., Tiziani R., Valentinuzzi F., Lucini L., Nicoletto C., Sambo P., Scampicchio M., Pii Y., Cesco S. Selenium biofortification in Fragaria × ananassa: Implications on strawberry fruits quality, content of bioactive health beneficial compounds and metabolomic profile. Front. Plant Sci. 2017;8:1887. doi: 10.3389/fpls.2017.01887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Puccinelli M., Pezzarossa B., Pintimalli L., Malorgio F. Selenium biofortification of three wild species, Rumex acetosa L.; Plantago coronopus L.; and Portulaca oleracea L.; grown as microgreens. Agronomy. 2021;11:1155. doi: 10.3390/agronomy11061155. [DOI] [Google Scholar]
- 117.Lidon F., Oliveira K., Galhano C., Guerra M., Ribeiro M., Pelica J., Pataco I., Ramalho J., Leitão A., Almeida A. Selenium biofortification of rice through foliar application with selenite and selenate. Exp. Agric. 2018;55:528–542. doi: 10.1017/S0014479718000157. [DOI] [Google Scholar]
- 118.Leija-Martínez P., Benavides-Mendoza A., Cabrera-De La Fuente M., Robledo-Olivo A., Ortega-Ortíz H., Sandoval-Rangel A., González-Morales S. Lettuce biofortification with selenium in chitosan-polyacrylic acid complexes. Agronomy. 2018;8:275. doi: 10.3390/agronomy8120275. [DOI] [Google Scholar]
- 119.Ahmed H.S., Ahmed M.F., Shoala T., Salah M. Impact of Single or Fractionated Radiation and Selenium Nano-particles on Acid Lime (Citrus aurantifolia L.) Seed Germination Ability and Seedlings Growth. Adv. Agric. Environ. Sci. Open Access. 2018;1:91–100. doi: 10.30881/aaeoa.00016. [DOI] [Google Scholar]
- 120.De Oliveira V.C., Faquin V., Guimarães K.C., Andrade F.R., Pereira J., Guilherme L.R.G. Agronomic biofortification of carrot with selenium. Cienc. Agrotecnol. 2018;42:138–147. doi: 10.1590/1413-70542018422031217. [DOI] [Google Scholar]
- 121.Puccinelli M., Malorgio F., Terry L.A., Tosetti R., Rosellini I., Pezzarossa B. Effect of selenium enrichment on metabolism of tomato (Solanum lycopersicum) fruit during postharvest ripening. J. Sci. Food Agric. 2019;99:2463–2472. doi: 10.1002/jsfa.9455. [DOI] [PubMed] [Google Scholar]
- 122.Ramkissoon C., Degryse F., da Silva R.C., Baird R., Young S.D., Bailey E.H., McLaughlin M.J. Improving the efficacy of selenium fertilizers for wheat biofortification. Sci. Rep. 2019;9:19520. doi: 10.1038/s41598-019-55914-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Lara T.S., de Lima Lessa J.H., de Souza K.R.D., Corguinha A.P.B., Martins F.A.D., Lopes G., Guilherme L.R.G. Selenium biofortification of wheat grain via foliar application and its effect on plant metabolism. J. Food Compos. Anal. 2019;81:10–18. doi: 10.1016/j.jfca.2019.05.002. [DOI] [Google Scholar]
- 124.Morales-Espinoza M.C., Cadenas-Pliego G., Pérez-Alvarez M., Hernández-Fuentes A.D., De La Fuente M.C., Benavides-Mendoza A., Valdés-Reyna J., Juárez-Maldonado A. Se nanoparticles induce changes in the growth, antioxidant responses, and fruit quality of tomato developed under NaCl stress. Molecules. 2019;24:3030. doi: 10.3390/molecules24173030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zeid I.M., Gharib Z.F.A.E., Ghazi S.M., Ahmed E.Z. Promotive effect of ascorbic acid, gallic acid, selenium and nano-selenium on seed germination, seedling growth and some hydrolytic enzymes activity of cowpea (Vigna unguiculata) seedling. J. Plant Physiol. Pathol. 2019;7:1000193. [Google Scholar]
- 126.Amina Z., Samar O. Nano Selenium: Reduction of severe hazards of Atrazine and promotion of changes in growth and gene expression patterns on Vicia faba seedlings. Afr. J. Biotechnol. 2019;18:502–510. doi: 10.5897/AJB2019.16773. [DOI] [Google Scholar]
- 127.El-Saadony M.T., Saad A.M., Najjar A.A., Alzahrani S.O., Alkhatib F.M., Shafi M.E., Selem E., Desoky E.S.M., Fouda S.E., El-Tahan A.M., et al. The use of biological selenium nanoparticles to suppress Triticum aestivum L. crown and root rot diseases induced by Fusarium species and improve yield under drought and heat stress. Saudi J. Biol. Sci. 2021;28:4461–4471. doi: 10.1016/j.sjbs.2021.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sheikhalipour M., Esmaielpour B., Behnamian M., Gohari G., Giglou M.T., Vachova P., Rastogi A., Brestic M., Skalicky M. Chitosan–selenium nanoparticle (Cs–Se NP) foliar spray alleviates salt stress in bitter melon. Nanomaterials. 2021;11:684. doi: 10.3390/nano11030684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Huang Y., Tan J., Cui L., Zhou Z., Zhou S., Zhang Z., Zheng R., Xue Y., Zhang M., Li S., et al. Graphene and Au NPs co-mediated enzymatic silver deposition for the ultrasensitive electrochemical detection of cholesterol. Biosens. Bioelectron. 2018;102:560–567. doi: 10.1016/j.bios.2017.11.037. [DOI] [PubMed] [Google Scholar]
- 130.Zahedi S.M., Moharrami F., Sarikhani S., Padervand M. Selenium and silica nanostructure-based recovery of strawberry plants subjected to drought stress. Sci. Rep. 2020;10:17672. doi: 10.1038/s41598-020-74273-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Hajiboland R. Selenium supplementation stimulates vegetative and reproductive growth in canola (Brassica napus L.) plants. Acta Agric. Slov. 2012;99:13. doi: 10.2478/v10014-012-0002-7. [DOI] [Google Scholar]
- 132.Dong J.Z., Wang Y., Wang S.H., Yin L.P., Xu G.J., Zheng C., Lei C., Zhang M.Z. Selenium increases chlorogenic acid, chlorophyll and carotenoids of Lyciumchinense leaves. J. Sci. Food Agric. 2013;93:310–315. doi: 10.1002/jsfa.5758. [DOI] [PubMed] [Google Scholar]
- 133.Le V.N., Rui Y., Gui X., Li X., Liu S., Han Y. Uptake, Transport, distribution and bio-effects of SiO2 nanoparticles in Bt-transgenic cotton. J. Nanobiotechnol. 2014;12:50. doi: 10.1186/s12951-014-0050-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Sarraf M., Vishwakarma K., Kumar V., Arif N., Das S., Johnson R., Janeeshma E., Puthur J.T., Aliniaeifard S., Chauhan D.K., et al. Metal/metalloid-based nanomaterials for plant abiotic stress tolerance: An overview of the mechanisms. Plants. 2022;11:316. doi: 10.3390/plants11030316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.El-Ramady H., Abdalla N., Alshaal T., El-Henawy A., Elmahrouk M., Bayoumi Y., Shalaby T., Amer M., Shehata S., Fári M., et al. Plant nano-nutrition: Perspectives and challenges. In: Gothandam K., Ranjan S., Dasgupta N., Ramalingam C., Lichtfouse E., editors. Nanotechnology, Food Security and Water Treatment. Springer; Cham, Switzerland: 2018. pp. 129–161. [Google Scholar]
- 136.Hawrylak-Nowak B., Hasanuzzaman M., Matraszek-Gawron R. Mechanisms of selenium-induced enhancement of abiotic stress tolerance in plants. In: Hasanuzzaman M., Fujita M., Oku H., Nahar K., Hawrylak-Nowak B., editors. Plant Nutrients and Abiotic Stress Tolerance. Springer; Singapore: 2018. pp. 269–295. [Google Scholar]
- 137.Gao M., Zhou J., Liu H., Zhang W., Hu Y., Liang J., Zhou J. Foliar spraying with silicon and selenium reduces cadmium uptake and mitigates cadmium toxicity in rice. Sci. Total Environ. 2018;631:1100–1108. doi: 10.1016/j.scitotenv.2018.03.047. [DOI] [PubMed] [Google Scholar]
- 138.Babajani A., Iranbakhsh A., OraghiArdebili Z., Eslami B. Differential growth, nutrition, physiology, and gene expression in Melissa officinalis mediated by zinc oxide and elemental selenium nanoparticles. Environ. Sci. Pollut. Res. 2019;26:24430–24444. doi: 10.1007/s11356-019-05676-z. [DOI] [PubMed] [Google Scholar]
- 139.Soleymanzadeh R., Iranbakhsh A., Habibi G., Ardebili Z.O. Selenium nanoparticle protected strawberry against salt stress through modifications in salicylic acid, ion homeostasis, antioxidant machinery, and photosynthesis performance. Acta Biol. Crac. S. Bot. 2020;62:33–42. [Google Scholar]
- 140.Zsiros O., Nagy V., Párducz Á., Nagy G., Ünnep R., El-Ramady H., Prokisch J., Lisztes-Szabó Z., Fári M., Csajbók J., et al. Effects of selenate and red Se-nanoparticles on the photosynthetic apparatus of Nicotiana tabacum. Photosynth. Res. 2019;139:449–460. doi: 10.1007/s11120-018-0599-4. [DOI] [PubMed] [Google Scholar]
- 141.IranBakhsh A., Soleymanzadeh R., Habibi G., OraghiArdebili Z. Soil supplementation with silicon nanoparticles to alleviate toxicity signs of salinity in strawberry. Iran. J. Plant Physiol. 2022;12:4099–4109. [Google Scholar]
- 142.Matraszek-Gawron R., Hawrylak-Nowak B. Micronutrient status and selected physiological parameters of roots in nickel-exposed Sinapis alba L. affected by different sulphur levels. Plants. 2019;8:440. doi: 10.3390/plants8110440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Feng R., Wang L., Yang J., Zhao P., Zhu Y., Li Y., Yu Y., Liu H., Rensing C., Wu Z. Underlying mechanisms responsible for restriction of uptake and translocation of heavy metals (metalloids) by selenium via root application in plants. J. Hazard. Mater. 2021;402:123570. doi: 10.1016/j.jhazmat.2020.123570. [DOI] [PubMed] [Google Scholar]
- 144.Mohammadhassan R., Ferdosi A., Seifalian A.M., Seifalian M., Malmir S. Nanoelicitors Application Promote Antioxidant Capacity of Asparagus officinalis (In Vitro) J. Trop. Life Sci. 2021;11:259–265. doi: 10.11594/jtls.11.03.01. [DOI] [Google Scholar]
- 145.Sahay S., Khan E., Praveen A., Panthri M., Mirza Z., Gupta M. Sulphur potentiates selenium to alleviate arsenic-induced stress by modulating oxidative stress, accumulation and thiol-ascorbate metabolism in Brassica juncea L. Environ. Sci. Pollut. Res. 2020;27:11697–11713. doi: 10.1007/s11356-019-07520-w. [DOI] [PubMed] [Google Scholar]
- 146.Desoky E.S.M., Merwad A.R.M., Abo El-Maati M.F., Mansour E., Arnaout S.M., Awad M.F., Ramadan M.F., Ibrahim S.A. Physiological and biochemical mechanisms of exogenously applied selenium for alleviating destructive impacts induced by salinity stress in bread wheat. Agronomy. 2021;11:926. doi: 10.3390/agronomy11050926. [DOI] [Google Scholar]
- 147.Khalofah A., Migdadi H., El-Harty E. Antioxidant enzymatic activities and growth response of quinoa (Chenopodium quinoa willd.) to exogenous selenium application. Plants. 2021;10:719. doi: 10.3390/plants10040719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Sardar R., Ahmed S., Shah A.A., Yasin N.A. Selenium nanoparticles reduced cadmium uptake, regulated nutritional homeostasis and antioxidative system in Coriandrum sativum grown in cadmium toxic conditions. Chemosphere. 2022;287:132332. doi: 10.1016/j.chemosphere.2021.132332. [DOI] [PubMed] [Google Scholar]
- 149.Nasibi F., Aminian F., Mohammadinejad G., Hassanshahian M. Seed priming with selenium nanoparticle and plant growth promoting rhizobacteria improve seedling development of foxtail millet (Setaria italica) under salinity stress. Res. Sq. 2022 doi: 10.21203/rs.3.rs-1809244/v1. [DOI] [Google Scholar]
- 150.Schiavon M., dall’Acqua S., Mietto A., Pilon-Smits E.A., Sambo P., Masi A., Malagoli M. Selenium fertilization alters the chemical composition and antioxidant constituents of tomato (Solanum lycopersicon L.) J. Agric. Food Chem. 2013;61:10542–10554. doi: 10.1021/jf4031822. [DOI] [PubMed] [Google Scholar]
- 151.Samadi S., Lajayer B.A., Moghiseh E., Rodríguez-Couto S. Effect of carbon nanomaterials on cell toxicity, biomass production, nutritional and active compound accumulation in plants. Environ. Technol. Innov. 2021;21:101323. doi: 10.1016/j.eti.2020.101323. [DOI] [Google Scholar]
- 152.Qasim U., Osman A.I., Al-Muhtaseb A.A.H., Farrell C., Al-Abri M., Ali M., Vo D.V.N., Jamil F., Rooney D.W. Renewable cellulosic nanocomposites for food packaging to avoid fossil fuel plastic pollution: A review. Environ. Chem. Lett. 2021;19:613–641. doi: 10.1007/s10311-020-01090-x. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in the present study are available in the article.