Abstract
Based on the complementarity of the initial coding region (downstream box [db]) of several bacterial and phage mRNAs to bases 1469 to 1483 in helix 44 of 16S rRNA (anti-downstream box [adb]), it has been proposed that db-adb base pairing enhances translation in a way that is similar to that of the Shine-Dalgarno (SD)/anti-Shine-Dalgarno (aSD) interaction. Computer modeling of helix 44 on the 30S subunit shows that the topography of the 30S ribosome does not allow a simultaneous db-adb interaction and placement of the initiation codon in the ribosomal P site. Thus, the db-adb interaction cannot substitute for the SD-aSD interaction in translation initiation. We have always argued that any contribution of the db-adb interaction should be most apparent on mRNAs devoid of an SD sequence. Here, we show that 30S ribosomes do not bind to leaderless mRNA in the absence of initiator tRNA, even when the initial coding region shows a 15-nucleotide complementarity (optimal fit) with the putative adb. In addition, an optimized db did not affect the translational efficiency of a leaderless λ cI-lacZ reporter construct. Thus, the db-adb interaction can hardly serve as an initial recruitment signal for ribosomes. Moreover, we show that different leaderless mRNAs are translated in heterologous systems although the sequence of the putative adb's within helix 44 of the 30S subunits of the corresponding bacteria differ largely. Taken our data together with those of others (M. O'Connor, T. Asai, C. L. Squires, and A. E. Dahlberg, Proc. Natl. Acad. Sci. USA 96:8973–8978, 1999; A. La Teana, A. Brandi, M. O'Connor, S. Freddi, and C. L. Pon, RNA 6:1393–1402, 2000), we conclude that the db does not base pair with the adb.
The downstream box (db) located 8 to 13 nucleotides (nt) downstream of the start codon has been originally proposed to base pair with the anti-downstream box (adb) spanning nt 1469 to 1483 within helix 44 of the 16S rRNA (Fig. 1A; 37). The db element was identified first in the highly expressed genes 0.3 and 10 of Escherichia coli phage T7 and has been reported to stimulate translation per se or in combination with the Shine-Dalgarno (SD) sequence (37, 38). Since the original proposal, there has been much publicity in favor of the proposed db-adb interaction (3, 6, 7, 8, 9, 16, 21, 23, 26, 33, 36, 43, 44). In some cases, the importance of the db-adb interaction in translation initiation was proposed solely based on computer analysis in the complete absence of any experimental data. The supporting experimental evidence for the db-adb base pairing rests entirely on manipulations of mRNAs containing a putative db and the observations that, in general, increases in complementarity of the db with the adb resulted in an increased expression and decreases in the db-adb complementarity had the corresponding downward effects on expression (9, 36, 38).
FIG. 1.
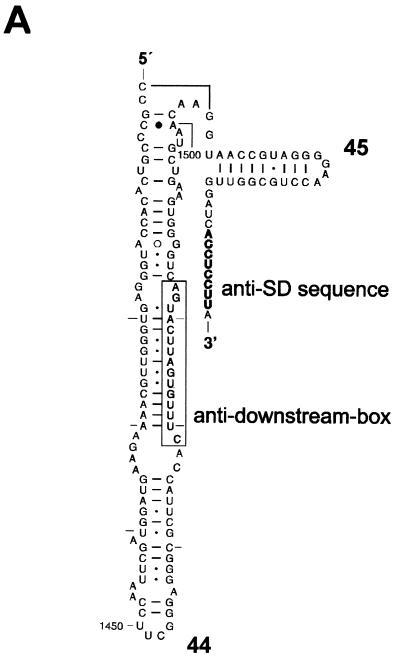
Localization of the putative adb in the penultimate stem of E. coli 16S rRNA and spatial localization of the adb in the 30S subunit of T. thermophilus. (A) Helices 44 and 45 of the E. coli 16S rRNA are enlarged. The adb sequence shown boxed (nt 1469 to 1483) in helix 44 has been suggested to base pair with the db in mRNA, i.e., with mRNA sequences downstream of the start codon. (B) Placement of the mRNA (nt −6 to +12; green tube) in the T. thermophilus 16S rRNA based on cross-linking studies (35). The positions of the aSD sequence and the adb in the 16S rRNA are shown. The aSD sequence and the putative adb region are depicted in magenta. The mRNA is shown as a green tube from nt −6 to +12 with regard to the A (+1) of the start codon. The P-site tRNA is shown in orange. (C) Stereoview of the structure shown in panel B added to the contour of the 30S subunit (see text).
Biochemical evidence for the db-adb interaction is lacking. Chemical protection studies on λ cI mRNA-70S initiation complexes (31) and on phage T4 gene 32 mRNA-30S and -70S complexes (15) have failed to show protection of the putative db. Mutational studies revealed that alterations on either side of the adb-containing helix 44 that disrupt the helical continuity had deleterious effects on ribosome function. These effects could be reversed upon introduction of compensatory mutations that restored base pairing within the helix (10). These studies indicated that a stable helix rather than a particular primary sequence is important for ribosome function. Recently, O'Connor et al. (28) performed a landmark experiment by reversing all 12 bp of the stem containing the putative adb, thereby creating a mutant 16S rRNA with a radically altered base pairing potential. This 16S rRNA allele with the adb-flip has been expressed in an E. coli strain in which all of the seven rrn operons had been deleted (2). The expression rates of several previously described db-containing reporter constructs were found to be indistinguishable in both the adb-flip mutant and the isogenic wild-type (wt) strain. These genetic studies showed that any db-associated enhancer activity does not involve db-adb base pairing (28). The db-adb interaction has been suggested to be instrumental for the translation of E. coli cspA mRNA during cold shock, and it seemed conceivable that the adb is particularly exposed in cold-shocked ribosomes (8, 21). Using the same approach as O'Connor et al. (28), La Teana et al. (18) have recently shown that 30S db-flip mutants translated the cspA mRNA with the same efficiency as wt ribosomes under cold shock as well as under non-cold shock conditions, suggesting again that the db-adb base pairing is irrelevant.
It has been suggested that the db serves as an independent translation initiation signal (38). In another report, it has been suggested that the db-adb and the SD/anti-SD (aSD) interactions act synergistically to enhance translation initiation (7). However, no direct in vitro experiments have been performed to address the question of whether an mRNA with an optimal fit to the adb has an increased affinity for the 30S subunit as would be expected regardless of whether the db provides per se a ribosomal recruitment signal, whether it acts in concert with the SD sequence, or whether it acts transiently to increase the concentration of the start codon close to the decoding center (39). Here, we present genetic and biochemical studies as well as topographical information on the ribosome which, taken together with previously performed genetic studies (18, 28), add strong evidence against the db-adb interaction.
MATERIALS AND METHODS
Bacterial strains and plasmids.
The E. coli strains MS59λ (31) and MC4100F′ (40) as well as the Bacillus subtilis strain 168 (1) have been described. They were grown in Luria-Bertani medium (20) supplemented with ampicillin (100 μg/ml) where appropriate to maintain selection of plasmids. Growth of the liquid cultures was monitored photometrically by measuring the optical density at a wavelength of 600 nm (OD600).
Plasmid pAXL7 harbors the full-length cI gene under transcriptional control of a T7 promoter (41).
Construction of plasmids used in this study.
Plasmids pRB381-1 and pRB381-3 are derivatives of plasmid pRB381 (4). They were constructed as follows. Plasmid pMCcI(63) (31) was digested with XbaI and SmaI, and the fragment was inserted into the corresponding sites of plasmid pUC18 (19). The resulting pUC18 derivative was then cleaved with XbaI and BamHI, and the fragment containing the λ pRM promoter and the first 63 codons of the cI gene was inserted into the corresponding sites of pRB381, resulting in pRB381-1.
The putative db in λ cI was optimized in plasmid pRB381-3 by using the PCR-based site-directed mutagenesis procedure described by Hall and Emery (14). Briefly, the immediate 5′-coding region of the cI gene was modified as shown in Fig. 3A, using complementary oligonucleotides comprising this region in conjunction with biotinylated oligonucleotides for the upstream and downstream region of the part of the cI gene present in plasmid pMCcI(63) (31). After hybridization of the two strands and the fill-in reaction with T4 DNA polymerase, a PCR with nonbiotinylated oligonucleotides was performed. The resulting fragment was cleaved with XbaI and BamHI and cloned into the corresponding sites of the vector pRB381, resulting in plasmid pRB381-3. The plasmids pRB381-1 and pRB381-3 are isogenic except for the initial coding region of the cI gene (optimized db*; see Fig. 3A).
FIG. 3.
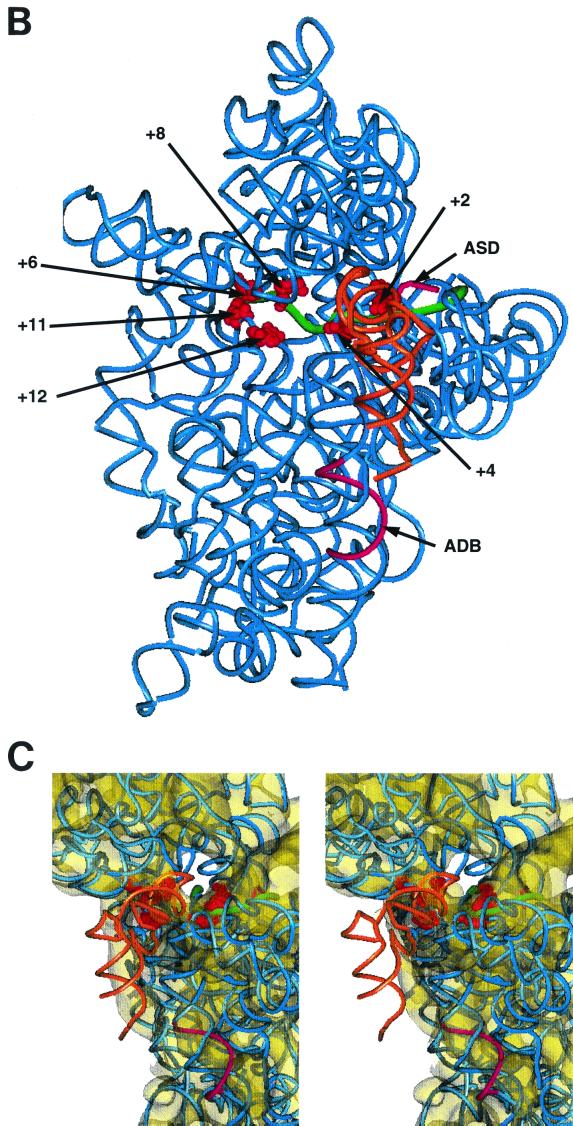
Expression rates of the cI-lacZ and the cIOptdb*-lacZ constructs. (A) Possible db-adb complementarity in the cI-lacZ and the cIOptdb*-lacZ mRNA, respectively. (B) E. coli strain MS59 (λwt) cells harboring plasmids pRB381-1 and pRB381-3, respectively, were grown in Luria-Bertani medium to an OD600 of 0.8. Then, triplicate samples were taken from each culture and the β-galactosidase (given in Miller units) values were determined. The two Lys codons (subsequence, 5′… AAGAAA… 3′) in λ cI wt mRNA following the putative db were omitted in the initial coding region of the cIOptdb*-lacZ construct.
Plasmid pRB381cI is a derivative of the E. coli-B. subtilis shuttle vector pRB381 and was constructed as follows. First, a PCR fragment containing the lac promoter (from nt −60 to +1) from plasmid pUHE21-2 (17) and the first 189 nt of the λ cI gene were inserted into the XbaI-SmaI sites of plasmid pKT35 (30). In the resulting plasmid, pKTplaccI, the lac promoter abutted the start codon of the cI gene via an NcoI site, which ensured that transcription of cI mRNA starts at the adenosine of the start codon. Plasmid pKTplaccI was then used as a template with an XbaI forward primer and a BamHI reverse primer. The PCR product was cleaved with XbaI and BamHI, and the fragment was inserted into the same sites of pRB381. In the resulting plasmid pRB381cI, the cI-lacZ mRNA, which contains the first 63 codons of the cI gene, is transcribed from the modified lacpo. The transcript starts with the A of the initiating codon of the cI gene. All DNA manipulations were verified by sequencing.
Filter-binding assay.
Filter-binding assays were performed using a Schleicher and Schuell SRC 072/0 Minifold II Slot Blot apparatus. The cI34 mRNA, cIOptdb mRNA, and cI34SD mRNA (Fig. 2) were obtained by hybridization of cDNA nucleotides containing the T7 φ10 promoter and either the first 34 bp (cI34 and cI34SD mRNAs, including the 5′ extension) of the cI gene or the first 33 bp of cI with the optimized db (cIOptdb mRNA) and subsequent T7 RNA polymerase-directed mRNA synthesis. All mRNAs were labeled with [α-32P]CTP. In a reaction volume of 50 μl, 0.5 pmol of 32P-labeled mRNA was incubated with 30S ribosomes at a molar ratio of 1:10 (Fig. 2). fMet-tRNAfMet was added in a twofold excess over 30S ribosomes. The reaction mixtures were incubated for 10 min at 37°C. Samples were added to the filter under vacuum and washed with 25 volumes of VD+ reaction buffer as previously described (41). The filters were exposed to a Molecular Dynamics PhosphorImager screen for quantitation of the retained mRNA. The total counts per minute values were corrected for the number of C's in each mRNA.
FIG. 2.
The cI34, cIOptdb, and cI34SD mRNAs are depicted. The putative db sequences as well as the putative adb in E. coli 16S rRNA are shown in bold. The start codons and the SD sequence in cI34SD mRNA are underlined. The total counts per minute values corrected for the number of C's in each mRNA were set at 100% (mRNA input). Ternary complexes were allowed to form in the presence of a twofold molar excess of fMet-tRNAfMet over ribosomes.
β-galactosidase assays.
The β-galactosidase activities were determined as described by Miller (20). Triplicate aliquots were taken of each culture at an OD600 of 0.8.
In vitro translation.
Full-length Lactococcus lactis phage r1t rro mRNA was obtained as described previously (22) except that the reverse “PCR-oligonucleotide” was complementary to a region downstream of the rro stop codon. For full-length synthesis of cI mRNA, plasmid pAXL7 was cleaved with PvuII. The PCR fragment and the linearized plasmid served as templates for in vitro transcription reactions with T7 RNA polymerase. The in vitro translation reactions with Sulfolobus solfataricus extracts were performed as described by Condo et al. (5). The samples contained the following in a final volume of 50 μl: 10 mM KCl, 20 mM Tris-HCl (pH 7.0), 18 mM MgOAc, 7 mM β-mercaptoethanol, 3 mM ATP, 1 mM GTP, 5 μg of bulk S. solfataricus tRNA, 20 μM amino acids (except for methionine), 2 μl of [35S]methionine (1,200 Ci/mmol), 20 μl of S. solfataricus S30 extract (preincubated for 10 min at 73°C), and 10 pmol of mRNA. The samples were incubated at 73°C for 40 min. The labeled proteins were separated on a sodium dodecyl sulfate (SDS)–12% polyacrylamide gel. The gel was dried under vacuum and exposed to an X-ray film.
Computer graphics and modeling of helix 44 on the 30S subunit.
All of the relevant procedures for modeling of helix 44 on the 30S subunit have been described in detail by Mueller and Brimacombe (24, 25).
RESULTS AND DISCUSSION
Spatial organization of mRNA-db and adb on the 30S subunit.
The recently published crystal structure of Thermus thermophilus 16S rRNA (42) is shown in Fig. 1B. Nucleotides in the E. coli 16S rRNA which have been cross-linked to downstream positions (+2, +4, etc.) in the mRNA (35) are highlighted in red as CPK models. It should be noted that attempts to cross-link the putative adb to mRNA have failed (R. Brimacombe, unpublished results).
In Fig. 1C, the structure shown in Fig. 1B was added to the contour of the 30S subunit obtained by electron cryomicroscopy (24). The stereo views show that helix 44 is far from the decoding site and runs down to the bottom of the interface site of the E. coli 30S subunit with the adb situated approximately in the middle of the body. In the translation initiation complex, the mRNA cross-links are grouped around the “hole,” and part of the putative db on mRNA would pass through the hole. Thus, the putative db is exposed to the solvent site and the whole shoulder of the body of the 30S subunit is situated between the putative db on the mRNA and the adb in helix 44 (Fig. 1C). Taken together with other topographical information on the 30S ribosome (11, 34, 42), models where the start codon can be placed in the ribosomal P site while the adjacent db interacts simultaneously with the adb (38) or where the db acts synergistically with the SD/aSD interaction (7) are not tenable. These topographical constraints are supported by previous chemical probing experiments which showed that the putative db on λ cI mRNA is not protected from modifying reagents in 70S initiation complexes (31).
An optimal db does not result in 30S subunit binding to leaderless λ cI mRNA.
Although the topography of the ribosome apparently excludes that the db can mechanistically substitute for the SD sequence, it seemed worth testing the hypothesis that the db-adb interaction could contribute to initial interactions between the 30S subunit and mRNA prior to formation of the translation initiation complex (39). We have argued that a contribution of the putative db-adb interaction in ribosome mRNA binding may be best assessed in the absence of an SD/aSD interaction (31). Therefore, the relative affinities of 30S subunits were determined for leaderless λ cI wt mRNA and for a derivative thereof containing an “optimized db” as well as for a cI mRNA derivative with a 5′-terminal extension containing an SD sequence (Fig. 2). λ cI34 RNA contains the first 34 nt, and 8 consecutive nucleotides are complementary to the putative adb (Fig. 2). The λ cIOptdb comprises 33 nt, of which nt 1 to 15 are fully complementary to the putative adb (Fig. 2). Both were added to a 10-fold molar excess of 30S subunits (conditions which reflect the relative affinity constants of the corresponding complexes). As shown in Fig. 2, only 0.37% of cI34 mRNA and 0.30% of cIOptdb mRNA formed a binary complex with 30S subunits. When the cI34SD mRNA (Fig. 2) was used as a binding substrate, approximately 46% of this mRNA formed a binary complex with ribosomes. Thus, in contrast to the SD/aSD interaction, an optimal base pairing potential did not increase the affinity of ribosomes for the cIOptdb mRNA. In other words, an optimized db cannot provide sufficient interactions with the 30S ribosome to stimulate initial binding of the mRNA to the ribosome. The addition of a 2-fold molar excess of fMet-tRNAfMet over ribosomes increased the amount of bound cI34 mRNA approximately 4-fold, that of cIOptdb mRNA 3-fold, and that of cI34SD mRNA 1.6-fold. Since the topography of the 30S subunits (Fig. 1) should restrict a simultaneous interaction of both the start codon and anti-codon in the P site and the db-adb, it was not surprising that the presence of the optimal db did not enhance ternary complex formation (Fig. 2).
The failure of 30S ribosomes to form a binary complex with λ cIOptdb mRNA is consistent with kinetic toeprint experiments (31) and with studies performed by La Teana et al. (18), who have recently shown that the adb is not accessible to a cDNA oligonucleotide while the anti-SD sequence, which served as a control, was fully accessible. Ever since the db-adb base pairing was first proposed, it has been perplexing that it would require extensive unwinding of the 16S rRNA stem comprising the adb. Again, La Teana et al. (18) have shown, using chemical probing, that the adb is in a double-stranded conformation and therefore is not available for base pairing.
An optimized db does not enhance translation of a leaderless λ cI-lacZ construct.
We have previously shown that elimination of 5 nt of the putative λ cI db did not affect the translational efficiency of the corresponding cI-lacZ construct (13, 31). However, this work has been criticized in that alternative db stretches would have been recreated (6, 39). Therefore, we next addressed the question whether an optimized db rather than a partial deletion of the db in cI mRNA would affect the translation of a reporter construct in vivo. Two different plasmids were used. Plasmid pRB381-1 contains the first 63 codons of the wt cI gene fused to the lacZ gene, whereas in pRB381-3, the immediate coding region of the cI-lacZ fusion gene was altered such that a consecutive stretch of 10 nt, including three G:C pairs, showed complementarity to the putative adb (Fig. 3A). In both plasmids, the λ pRM promoter abutted the start codon of the cI gene, which ensured that cI transcription started at the adenosine of the AUG initiating codon (see Materials and Methods). As shown in Fig. 3B, the optimized db did not increase translation of the cIOptdb*-lacZ construct in vivo when compared to the corresponding cI-lacZ fusion, which corroborates the in vitro results presented in Fig. 2.
Expression of leaderless mRNAs in heterologous translation systems.
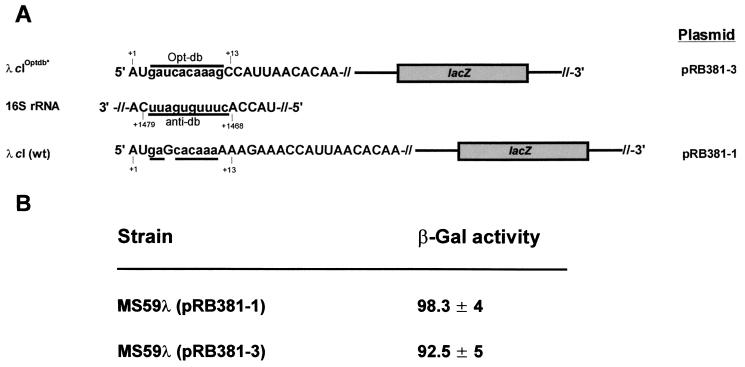
Helix 44 is a phylogenetically conserved element in ribosomes. However, the rRNA sequence of the region corresponding to the putative E. coli adb is dissimilar in different bacteria and archaea. If the db has any significance in translation initiation, the diminished db-adb base pairing potential in a heterologous system would be expected to affect translation in a negative manner. Depending on the alignment, the initial coding region of λ cI mRNA shows, at best, a 4-nt complementarity with the adb of B. subtilis (Fig. 4A). Therefore, we asked whether a cI-lacZ reporter gene carried by plasmid pRB381cI (see Materials and Methods) is expressed in B. subtilis. The cI63-lacZ fusion was translated in B. subtilis despite the insignificant complementarity between the putative db of cI and the putative adb of B. subtilis. The absolute expression levels of the reporter construct may vary in E. coli and B. subtilis due to differential transcriptional, posttranscriptional, or translational regulatory events. It is therefore not possible to compare the translational efficiencies in both organisms directly. However, the cI63-lacZ fusion is translated in B. subtilis at a rather high level (1,050 ± 45 Miller units), which would not be expected if the db-adb base pairing was essential. It seems worth noting that the coding sequences from +4 to +33 of all B. subtilis genes did not reveal a statistically significant motif, i.e., a db (32). Moreover, these authors randomized the sequences of the putative db region and observed that 34% of the sequences showed a db with eight matches while 9% had one with nine matches, which led them to conclude that the db “patterns” are statistically irrelevant.
FIG. 4.
Expression of leaderless mRNAs in heterologous translation systems. (A) Possible complementarity between the putative db of λ cI mRNA and the putative adb in B. subtilis. Multiple alignments are shown. (B) Alignment of the putative adb sequences of E. coli, L. lactis, and S. solfataricus (27, 29, 37). (C) Translation of equimolar amounts of λ cI and r1t rro mRNA in the in vitro translation system derived from S. solfataricus. Samples from the reaction mixtures were analyzed on an SDS–12% polyacrylamide gel. Lane 1, no mRNA added; lanes 2 and 3, translation of λ cI mRNA and rro mRNA, respectively.
We have recently shown that the λ cI mRNA is faithfully translated in an in vitro translation system derived from the archaeon S. solfataricus (12) although the region of helix 44 comprising the putative adb is highly dissimilar in E. coli and S. solfataricus (Fig. 4B). To extend the heterologous expression studies, the translation rates of both λ cI mRNA and L. lactis phage r1t rro mRNA encoding the phage repressor (27) were compared in the S. solfataricus in vitro translation system. As for E. coli, the putative adb of L. lactis is highly dissimilar compared to that of S. solfataricus. The in vitro translation system was programmed with equimolar amounts of λ cI mRNA and rro mRNA. Despite the differences in the respective adb sequences of E. coli, L. lactis, and S. solfataricus (Fig. 4B), both mRNAs were expressed with approximately the same efficiency (Fig. 4C). At first glance, this may be fortuitous. However, in light of the current view on translation initiation of leaderless mRNAs, it may not be surprising. Grill et al. (12) have recently shown that the start codon is the only constant and necessary element of the leaderless λ cI mRNA that is recognized by the ribosome. Moreover, since translation initiation factor 2 (IF2) selectively stimulated translation of a leaderless mRNA in vitro as well as in vivo, it has been concluded that the 5′-terminal start codon of leaderless mRNAs is recognized by a 30S-initiator-tRNA-IF2 complex, an intermediate equivalent to that obligatorily formed during translation initiation in eukaryotes (12). A homologue of both the eukaryal IF2 and the bacterial type IF2 is present in S. solfataricus (P. Londei, unpublished results). Therefore, the translation initiation pathway proposed for leaderless mRNAs in E. coli (12) could operate in this organism as well, which would explain the similar expression rates of both the λ cI and r1t rro mRNA observed with the S. solfataricus in vitro translation system. In agreement with these studies, Condo et al. (5) have shown that two different open reading frames of S. solfataricus were translated in the homologous in vitro translation system upon removal of the upstream leader sequence, including an SD sequence, despite the absence of any sequence motif in the 5′ initial coding region with complementarity to the corresponding adb. In addition, we have previously demonstrated the heterologous expression of cI mRNA) in a Bacillus stearothermophilus in vitro translation system (41) and of cI mRNA in a mammalian reticulocyte lysate (12), as well as in vitro ternary complex formation on rro mRNA with E. coli ribosomes (22). Again, the putative adb sequences are not conserved in these organisms. Our expression studies in different organisms are in agreement with the genetic studies of Firpo and Dahlberg (10) and of O'Connor et al. (28), who demonstrated that the continuity and stability of helix 44 is important for ribosome function rather than its primary sequence.
Conclusions.
In summary, there is neither biochemical nor genetic evidence for the proposed db-adb interaction in terms of ribosome recruitment via rRNA-mRNA contacts during translation initiation. The topography of the 30S subunit can explain why the db-adb interaction cannot act simultaneously with the SD/aSD interaction. In addition, the sequestration of the putative adb within helix 44 readily explains why ribosomes do not form a binary complex with an mRNA comprising an optimal-fit db or with a db-oligonucleotide. While these and other data (18, 28, 31) argue against the molecular mechanism originally proposed for the db (37–39), they do not invalidate studies wherein the db has been suggested to enhance translation initiation in particular mRNAs. Therefore, other models to explain the stimulating effects of this downstream enhancer should be tested.
ACKNOWLEDGMENTS
We thank A. Nauta and R. Brückner for providing plasmids.
This work was supported by grant P12065 from the Austrian Science Fund.
REFERENCES
- 1.Anagnostopoulos C, Spizizen J. Requirements for transformation in Bacillus subtilis. J Bacteriol. 1961;81:741–746. doi: 10.1128/jb.81.5.741-746.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asai T, Zaporojets D, Squires C, Squires C L. An Escherichia coli strain with all chromosomal rRNA operons inactivated: complete exchange of rRNA genes between bacteria. Proc Natl Acad Sci USA. 1999;96:1971–1976. doi: 10.1073/pnas.96.5.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bibb M J, White J, Ward J M, Janssen G R. The mRNA for the 23S rRNA methylase encoded by the ermE gene of Saccharopolyspora erythraea is translated in the absence of a conventional ribosome-binding site. Mol Microbiol. 1994;14:533–545. doi: 10.1111/j.1365-2958.1994.tb02187.x. [DOI] [PubMed] [Google Scholar]
- 4.Brückner R. A series of shuttle vectors for Bacillus subtilis and Escherichia coli. Gene. 1992;122:187–192. doi: 10.1016/0378-1119(92)90048-t. [DOI] [PubMed] [Google Scholar]
- 5.Condo I, Ciammaruconi A, Benelli D, Ruggero D, Londei P. Cis-acting signals controlling translational initiation in the thermophilic archaeon Sulfolobus solfataricus. Mol Microbiol. 1999;34:377–384. doi: 10.1046/j.1365-2958.1999.01615.x. [DOI] [PubMed] [Google Scholar]
- 6.Etchegaray J P, Inouye M. DB or not DB in translation? Mol Microbiol. 1999;33:438–439. doi: 10.1046/j.1365-2958.1999.01487.x. [DOI] [PubMed] [Google Scholar]
- 7.Etchegaray J P, Inouye M. Translational enhancement by an element downstream of the initiation codon in Escherichia coli. J Biol Chem. 1999;274:10079–10085. doi: 10.1074/jbc.274.15.10079. [DOI] [PubMed] [Google Scholar]
- 8.Etchegaray J P, Inouye M. A sequence downstream of the initiation codon is essential for cold shock induction of cspB in Escherichia coli. J Bacteriol. 1999;181:5852–5854. doi: 10.1128/jb.181.18.5852-5854.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Faxen M, Plumbridge J, Isakson L A. Codon choice and potential complementarity between mRNA downstream of the initiation codon and bases 1471–1480 in 16S ribosomal RNA affects expression of glnS. Nucleic Acids Res. 1991;19:5247–5251. doi: 10.1093/nar/19.19.5247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firpo M A, Dahlberg A E. The importance of base pairing in the penultimate stem of Escherichia coli 16S rRNA for ribosomal subunit association. Nucleic Acids Res. 1998;26:2156–2160. doi: 10.1093/nar/26.9.2156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gabashvili I S, Agrawal K A, Spahn C M T, Grassucci R, Svergun D I, Frank J, Penczek P. Solution structure of the E. coli 70S ribosome at 11.5A resolution. Cell. 1999;100:537–549. doi: 10.1016/s0092-8674(00)80690-x. [DOI] [PubMed] [Google Scholar]
- 12.Grill S, Gualerzi C O, Londei P, Bläsi U. Selective stimulation of translation of leaderless mRNA by initiation factor 2: evolutionary implications for translation. EMBO J. 2000;19:4101–4110. doi: 10.1093/emboj/19.15.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gründling A. Diploma thesis. Vienna, Austria: University of Vienna; 1997. [Google Scholar]
- 14.Hall L, Emery D C. A rapid and efficient method for site-directed mutagenesis by PCR, using biotinylated universal primers and streptavidin-coated magnetic beads. Protein Eng. 1991;4:601. doi: 10.1093/protein/4.5.601. [DOI] [PubMed] [Google Scholar]
- 15.Hüttenhofer A, Noller H F. Footprinting mRNA-ribosome complexes with chemical probes. EMBO J. 1994;13:3892–3901. doi: 10.1002/j.1460-2075.1994.tb06700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ito K, Kawakami K, Nakamura Y. Multiple control of Escherichia coli lysyl-tRNA synthetase expression involves a transcriptional repressor and a translational enhancer element. Proc Natl Acad Sci USA. 1993;90:302–306. doi: 10.1073/pnas.90.1.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lanzer M, Bujard H. Promoters determine largely the efficiency of repressor action. Proc Natl Acad Sci USA. 1988;85:8973–8977. doi: 10.1073/pnas.85.23.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.La Teana A, Brandi A, O'Connor M, Freddi S, Pon C L. Translation during cold adaptation does not involve mRNA-rRNA base pairing through the downstream box. RNA. 2000;6:1393–1402. doi: 10.1017/s1355838200000595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Messing J, Crea R, Seeburg P H. A system for shot gun DNA sequencing. Nucleic Acids Res. 1981;9:309–321. doi: 10.1093/nar/9.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 21.Mitta M, Fang L, Inouye M. Deletion analysis of cspA of Escherichia coli: requirement of the AT-rich UP element for cspA transcription and the downstream box in the coding region for its cold shock induction. Mol Microbiol. 1997;26:321–335. doi: 10.1046/j.1365-2958.1997.5771943.x. [DOI] [PubMed] [Google Scholar]
- 22.Moll I, Resch A, Bläsi U. Discrimination of 5′-terminal start codons by translation initiation factor 3 is mediated by ribosomal protein S1. FEBS Lett. 1998;436:213–217. doi: 10.1016/s0014-5793(98)01131-4. [DOI] [PubMed] [Google Scholar]
- 23.Morita M, Kanemori M, Yanagi H, Yura T. Heat-induced synthesis of sigma32 in Escherichia coli: structural and functional dissection of rpoH secondary structure. J Bacteriol. 1999;181:401–410. doi: 10.1128/jb.181.2.401-410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mueller F, Brimacombe R. A new model for the three-dimensional folding of E. coli 16S rRNA. I. Fitting the RNA to a 3D electron microscopic map at 20A. J Mol Biol. 1997;271:524–544. doi: 10.1006/jmbi.1997.1210. [DOI] [PubMed] [Google Scholar]
- 25.Mueller F, Brimacombe R. A new model for the three-dimensional folding of E. coli 16S rRNA. II. The RNA-protein interaction data. J Mol Biol. 1997;271:545–565. doi: 10.1006/jmbi.1997.1211. [DOI] [PubMed] [Google Scholar]
- 26.Nagai H, Yuzawa H, Yura T. Interplay of two cis-acting mRNA regions in translational control of sigma32 synthesis during the heat shock response of E. coli. Proc Natl Acad Sci USA. 1991;88:10515–10519. doi: 10.1073/pnas.88.23.10515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nauta A, van Sinderen D, Karsens H, Smit E, Venema G, Kok J. Inducible gene expression mediated by a repressor-operator system isolated from Lactococcus lactis bacteriophage r1t. Mol Microbiol. 1996;19:1331–1341. doi: 10.1111/j.1365-2958.1996.tb02477.x. [DOI] [PubMed] [Google Scholar]
- 28.O'Connor M, Asai T, Squires C L, Dahlberg A E. Enhancement of translation by the downstream box does not involve mRNA-rRNA base pairing of mRNA with the penultimate stem sequence of 16S rRNA. Proc Natl Acad Sci USA. 1999;96:8973–8978. doi: 10.1073/pnas.96.16.8973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Olsen G J, Pace N R, Nuell M, Daine B P, Gupta R, Woese C R. Sequence of the 16S rRNA gene from the thermoacidophilic archaebacterium Sulfolobus solfataricus and its evolutionary implications. J Mol Evol. 1985;22:301–307. doi: 10.1007/BF02115685. [DOI] [PubMed] [Google Scholar]
- 30.Resch A, Tedin K, Graschopf A, Haggård-Ljungquist E, Bläsi U. Ternary complex formation on leaderless phage mRNA. FEMS Microbiol Rev. 1995;17:151–157. doi: 10.1111/j.1574-6976.1995.tb00197.x. [DOI] [PubMed] [Google Scholar]
- 31.Resch A, Tedin K, Gründling A, Mündlein A, Bläsi U. Downstream/anti-downstream-box interactions are dispensable for translation initiation of leaderless mRNAs. EMBO J. 1996;15:4740–4748. [PMC free article] [PubMed] [Google Scholar]
- 32.Rocha E P C, Danchin A, Viari A. Translation in B. subtilis: roles and trends of initiation and termination, insights from a genome analysis. Nucleic Acids Res. 1999;27:3567–3576. doi: 10.1093/nar/27.17.3567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanchez R, Roovers M, Glansdorff N. Organization and expression of a Thermus thermophilus arginine cluster: presence of unidentified open reading frames and absence of a Shine-Dalgarno sequence. J Bacteriol. 2000;182:5911–5915. doi: 10.1128/jb.182.20.5911-5915.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schluenzen F, Tocilj A, Zarivach R, Harms J, Gluehman M, Janell D, Bashan A, Bartels H, Agmon I, Franceschi F, Yonath A. Structure of functionally activated small ribosomal subunit at 3.3 A resolution. Cell. 2000;102:615–623. doi: 10.1016/s0092-8674(00)00084-2. [DOI] [PubMed] [Google Scholar]
- 35.Sergiev P V, Lavrik I N, Wlasoff V A, Dokudovskaya S S, Dontsova O A, Bogdanov A A, Brimacombe R. The path of mRNA through the bacterial ribosome: a site-directed cross-linking study using new photoreactive derivatives of guanosine and uridine. RNA. 1997;3:464–475. [PMC free article] [PubMed] [Google Scholar]
- 36.Shean C S, Gottesman M. Translation of the prophage λ cI transcript. Cell. 1992;70:513–522. doi: 10.1016/0092-8674(92)90175-c. [DOI] [PubMed] [Google Scholar]
- 37.Sprengart M L, Fatscher H P, Fuchs E. The initiation of translation in E. coli: apparent base pairing between the 16S rRNA and downstream sequences of the mRNA. Nucleic Acids Res. 1990;18:1719–1723. doi: 10.1093/nar/18.7.1719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sprengart M L, Fuchs E, Porter A G. The downstream box: an efficient and independent translation initiation signal in Escherichia coli. EMBO J. 1996;15:665–674. [PMC free article] [PubMed] [Google Scholar]
- 39.Sprengart M L, Porter A G. Functional importance of RNA interactions in selection of translation initiation codons. Mol Microbiol. 1997;24:19–28. doi: 10.1046/j.1365-2958.1997.3161684.x. [DOI] [PubMed] [Google Scholar]
- 40.Steiner M, Lubitz W, Bläsi U. The missing link in phage lysis of Gram positive bacteria: gene 14 of B. subtilis phage φ29 encodes the functional homolog of λ S protein. J Bacteriol. 1993;175:1038–1042. doi: 10.1128/jb.175.4.1038-1042.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tedin K, Resch A, Bläsi U. Requirements for ribosomal protein S1 for translation initiation of mRNAs with and without a 5′-leader sequence. Mol Microbiol. 1997;25:189–199. doi: 10.1046/j.1365-2958.1997.4421810.x. [DOI] [PubMed] [Google Scholar]
- 42.Wimberley B T, Brodersen D E, Clemons W M, Morgan-Warren R J, Carter A P, Vornhelm C, Hartsch T, Ramakrishnan V. Structure of the 30S ribosomal subunit. Nature. 2000;407:327–339. doi: 10.1038/35030006. [DOI] [PubMed] [Google Scholar]
- 43.Winzeler E, Shapiro L. Translation of the leaderless Caulobacter dnaX mRNA. J Bacteriol. 1997;179:3981–3988. doi: 10.1128/jb.179.12.3981-3988.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wu C J, Janssen G R. Translation of vph mRNA in Streptomyces lividans and Escherichia coli after removal of the 5′-untranslated leader. Mol Microbiol. 1996;22:339–355. doi: 10.1046/j.1365-2958.1996.00119.x. [DOI] [PubMed] [Google Scholar]