Abstract
In Staphylococcus aureus RN4220, lipoteichoic acid (LTA) is anchored in the membrane by a diglucosyldiacylglycerol moiety. The gene (ypfP) which encodes diglucosyldiacylglycerol synthase was recently cloned from Bacillus subtilis and expressed in Escherichia coli (P. Jorasch, F. P. Wolter, U. Zahringer, and E. Heinz, Mol. Microbiol. 29:419–430, 1998). To define the role of ypfP in this strain of S. aureus, a fragment of ypfP truncated from both ends was cloned into the thermosensitive replicon pVE6007 and used to inactivate ypfP. Chloramphenicol-resistant (ypfP::cat) clones did not synthesize the glycolipids monoglucosyldiacylglycerol and diglucosyldiacylglycerol. Thus, YpfP would appear to be the only diglucosyldiacylglycerol synthase in S. aureus providing glycolipid for LTA assembly. In LTA from the mutant, the glycolipid anchor is replaced by diacylglycerol. Although the doubling time of the mutant was identical to that of the wild type in Luria-Bertani (LB) medium, growth of the mutant in LB medium containing 1% glycine was not observed. This inhibition was antagonized by either l- or d-alanine. Moreover, viability of the mutant at 37°C in 0.05 M phosphate (pH 7.2)-saline for 12 h was reduced to <0.1%. Addition of 0.1% d-glucose to the phosphate-saline ensured viability under these conditions. The autolysis of the ypfP::cat mutant in the presence of 0.05% Triton X-100 was 1.8-fold faster than that of the parental strain. Electron microscopy of the mutant revealed not only a small increase in cell size but also the presence of pleomorphic cells. Each of these phenotypes may be correlated with either (or both) a deficiency of free glycolipid in the membrane or the replacement of the usual glycolipid anchor of LTA with diacylglycerol.
Lipoteichoic acid (LTA) plays a vital role in the growth and physiology of gram-positive microorganisms. It is postulated that LTA modulates the activities of autolysins (4, 15), the binding of cations required for enzyme function (2, 22, 24, 34), and the electromechanical properties of the cell wall (41).
The proposed pathway for the synthesis of this macroamphiphile may be divided into three phases: (i) the glycolipid anchor, (ii) the poly(glycerophosphate) [poly(Gro-P)] moiety, and (iii) the d-alanyl esters linked to poly(Gro-P) (18, 38). In Staphylococcus aureus, the β-gentiobiosyldiacylglycerol (diglucosyldiacylglycerol [DGlcDAG]) moiety of LTA functions as the membrane anchor (18). The gene encoding UDP-glucose:1,2-diacylglycerol-3-β-d-glucosyl transferase (YpfP), designated DGlcDAG synthase in this paper, was cloned from Bacillus subtilis (27). Inactivation of this gene resulted in the loss of glycolipid and the appearance of a rough colony phenotype (28). In a separate study, ypfP from this bacterium was inactivated, resulting in a phenotype characterized by an effect on cell shape and viability on solid medium (46). The homologous gene in S. aureus (ugt106B1) also encodes a processive DGlcDAG synthase (26). Thus, it would appear that ypfP (ugt106B1) encodes an enzyme involved in the synthesis of the membrane anchor of LTA from S. aureus.
In addition to the role of DGlcDAG as the glycolipid anchor of LTA (6 mol%), the membranes of S. aureus contain 8 mol% of this free glycolipid (29). It was proposed that DGlcDAG hydrogen bonds to the phospholipid neighbors in the membrane bilayer. It has also been speculated that the ratio of monoglucosyldiacylglycerol (MGlcDAG) to DGlcDAG may play an important role in determining bilayer stability (27). Since MGlcDAG is non-bilayer forming and DGlcDAG is bilayer forming, this ratio could play an important role in determining the limit of stability of the membrane.
The goals of this study were threefold: (i) to inactivate ypfP in S. aureus RN4220, (ii) to correlate the deficiency in glycolipid with characteristic phenotypes, and (iii) to determine whether YpfP (DGlcDAG synthase) is a potential target for the rational design of antibacterial agents. To accomplish these goals, the gene was cloned from S. aureus RN4220 and insertionally inactivated in this strain. The ypfP::cat mutant does not synthesize either MGlcDAG or DGlcDAG. These results indicate that YpfP would appear to be the only synthase in S. aureus providing the source of glycolipid anchor for LTA as well as free membrane glycolipid. Characterization of the mutant in a variety of growth experiments has revealed four phenotypes of S. aureus RN4220 ypfP::cat.
MATERIALS AND METHODS
Bacterial strains and plasmids.
All bacterial strains and plasmids used in this study are listed in Table 1.
TABLE 1.
Bacterial strains and plasmids used in this study
| Strain or plasmid | Description | Reference or source |
|---|---|---|
| Strains | ||
| S. aureus RN4220 | NCTC 8325-4-r (restriction mutant) | 31 |
| S. aureus RN4220 ypfP::cat | Cmr, ypfP interrupted by integration of pΔYpfP | This study |
| E. coli XL1-Blue MRF′ | Δ(mcrA)183 Δ(mcrCB-hsdSMR-mrr)173 end1A supE44 thi-1recA1 gyrA96 relA1 lac [F′ proAB lacqZΔM15 Tn10(Tetr)] | Stratagene |
| E. coli BL21(DE3) | F−ompT hsdSB(rB− mB−) gal dcm(DE3) | Novagene |
| L. lactis NZ9800 | NZ9700 derivative, ΔnisA | 32 |
| Plasmids | ||
| pET-3d | Expression vector | Novagene |
| pYpfP | Expression plasmid, ypfP cloned into pET-3d | This study |
| pΔYpfP | Internal 0.56-kb DraI fragment of ypfP cloned into pVE6007 | This study |
| pVE6007 | Cmr thermosensitive shuttle vector | 37 |
Chemicals and reagents.
DGlcDAG from S. aureus was the generous gift of Werner Fischer. UDP-[14C]glucose (308 mCi/mmol), 1,2-di[1-14C]palmitoyl-l-3-phosphatidylcholine (114 mCi/mmol), d-[14C]alanine (55 mCi/mmol), [U-14C]glycerol (44 mCi/mmol), and dithiothreitol were the products of ICN Biomedicals Inc. 1,2-Di[1-14C]palmitoylglycerol was synthesized by the procedure of Taron et al. (53). Media were obtained from Difco Laboratories, Detroit, Mich. Ampicillin, EDTA, Tris, bis-Tris, α-naphthol, phospholipase C, lysostaphin (affinity purified), disodium ATP, and egg yolk tellurite emulsion were purchased from Sigma Chemical Co. Restriction enzymes were obtained from various suppliers and used according to the manufacturers' instructions. DNase I was the product of Boehringer Mannheim. Isopropylthiol-β-d-galactoside (IPTG) was purchased from GIBCO BRL. Phosphate-buffered saline, solutions of phenol, and phenol-chloroform were products of Amresco Inc. Silica Gel 60 thin-layer plates (250 μm [20 by 20 cm]) were obtained from E. Merck AG. Taq DNA polymerase was purchased from MBI Fermentas. The QIAprep spin miniprep, QUAquick nucleotide removal, and QUAquick gel extraction kits were obtained from Qiagen. Oligonucleotides were synthesized by Integrated DNA Technologies, Inc. Monoclonal antibody to LTA (MAB981) was obtained from Chemicon, Inc.
Growth of bacteria.
Escherichia coli and S. aureus RN4220 were cultured in Luria-Bertrani (LB) broth, plated on LB agar, and preserved by freezing in LB broth containing 10% glycerol at −80°C (48). Lactococcus lactis was grown in GM17 medium (23). Ampicillin and chloramphenicol (CHL) (100 and 10 μg/ml, respectively) were used to maintain plasmids while CHL (5 μg/ml) was used to select for integrants. Competent cultures of E. coli were prepared by the method of Hanahan (20). For the preparation of electroporation-competent cells, S. aureus (200 ml) was grown overnight on LB medium, diluted 1:100, and grown to an optical density at 600 nm (OD600) of 0.5. The cells were chilled, harvested, and suspended in 80 ml of 7 mM HEPES (pH 7.4) containing 272 mM sucrose (buffer A). Cells were collected by centrifugation, washed in buffer A, suspended in a final volume of 2.4 ml buffer A, frozen in 160-μl aliquots, and stored at −70°C. The electroporation-competent L. lactis cells were prepared as described by Holo and Nes (23).
DNA techniques.
Restriction digests, agarose gel electrophoresis, small-and large-scale plasmid preparations from E. coli, and E. coli transformations were performed by standard techniques (48). Plasmids from L. lactis were prepared by the miniprep method (40), and S. aureus chromosomal DNA was isolated by a previously published protocol (33).
Sequence analysis.
The protein database searches were performed by the BLAST algorithm (1), whereas the DNA and deduced amino acid sequences were analyzed with the University of Wisconsin Genetics Computer Group sequence analysis software package, version 10 (13). The MacVector sequence analysis program (Kodak Eastman Chemical Co.) was used for the design of PCR and sequencing primers and for the calculation of molecular mass and isoelectric point.
Construction of pYpfP.
To construct the plasmid for the expression of ypfP, this gene was amplified by PCR using S. aureus chromosomal DNA and two mutagenic primers. One primer, 5′-CGGGAGGGTGGCTATccATGGTTACTC-3′ (the mutagenized nucleotides are in lowercase; the restriction site is underlined) positioned a new NcoI site to span the ATG codon of ypfP. The second primer, 5′-ccttggatCCATTAAAAATAAGATAATAAGC-3′, positioned a new BamI site 30 nucleotides (nt) downstream of the termination codon of ypfP. DNA was denatured at 95°C for 1 min, annealed at 56°C for 1 min, and extended at 72°C for 1.5 min (30 cycles). The PCR product (1,220 bp) was digested with NcoI and BamHI and ligated into the pET-3d vector (51). The resulting ligation mixture was used to transform E. coli XL-1. Clones containing plasmids with the ypfP insert, designated pYpfP, were identified by restriction and sequence analysis (Fig. 1A).
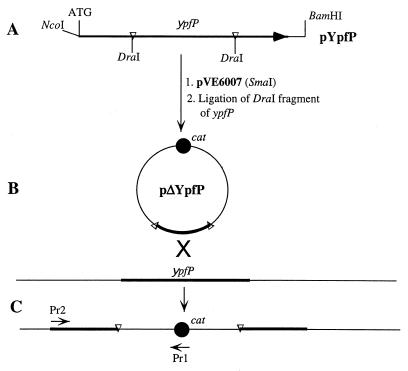
FIG. 1.
Cloning strategy for the expression and insertional inactivation of ypfP. (A) Fragment containing ypfP in pYpfP. The NcoI and BamHI sites were introduced by PCR with the mutagenic primers described in Materials and Methods. (B) Cloning of the DraI fragment of ypfP into the SmaI site of pVE6007 to obtain the plasmid for insertional inactivation (pΔYpfP). (C) Integration of pΔYpfP by a single crossover. Pr1 and Pr2 are primers used in the PCR analysis of integration. Pr1 is complementary to nt 1284 to 1265 of cat (chloramphenicol resistance gene) in pC194 (GenBank accession number J01754), and Pr2 is complementary to nt 2 to 21 of ypfP (GenBank accession number Y14370).
Expression of ypfP in E. coli.
To express ypfP, E. coli BL21(DE3) transformed with pYpfP was grown in 100 ml of LB broth with carbenicillin (100 μg/ml) at 37°C. At an OD600 of 0.7, the cultures were induced with 0.4 mM IPTG, and the cells were harvested 4 h after induction. To prepare soluble and insoluble protein fractions for this analysis, cells were suspended in 0.1 culture volume of buffer B (50 mM Tris-HCl [pH 8.0] containing 20% [vol/vol] glycerol). The cells were subjected to one cycle of freezing and thawing and sonicated (three times for 1 min each) with a B. Braun Labsonic ultrasonic oscillator. Inclusion bodies were obtained by centrifugation at 10,000 × g for 15 min. The supernatant fraction was centrifuged for 90 min at 200,000 × g to obtain membrane and soluble fractions. Membranes were homogenized in a minimal amount of buffer B and washed by three cycles of differential centrifugation at 10,000 × g and 200,000 × g. The washed membranes (15 mg of protein) were suspended in 1 ml of buffer B, frozen in liquid nitrogen, and stored in aliquots at −80°C. Protein was determined with the Bradford reagent (Pierce Chemical Co.).
Insertional inactivation of ypfP.
For the insertional inactivation of ypfP, a fragment of this gene, truncated from both ends, was cloned into pVE6007 (Fig. 1B). The 1,220-bp ypfP PCR product (see above, “Construction of pYpfP”) was digested with DraI, and a 560-bp internal ypfP fragment was isolated and ligated into the SmaI site. Since pVE6007 was unstable in E. coli (data not shown), L. lactis was used for transformation with the ligation mixture (23). The recombinant plasmid, pΔypfP, was isolated and transformed into S. aureus (1 μg of DNA per 160 μl of cells, 2.5 kV, resistance = 186 ohms, 2-mm gap cuvette, BTX electro cell manipulator 600). After the pulse, the cells were diluted with 1 ml of LB medium, incubated 10 min on ice and 1 h at 30°C, and plated on LB agar containing CHL at 30°C. To stop replication of the plasmid and select for integrants, the resulting colonies were grown on LB medium overnight at 37°C, diluted 1:100, and grown 24 h at 37°C. These cells were diluted 1:100 and grown on LB medium containing CHL for 24 h at 37°C. Serial dilutions were plated on LB medium containing CHL at 37°C. The DNA was isolated from the resulting colonies and analyzed by PCR (Fig. 1C).
Preparations of membranes and permeabilized cells.
E. coli BL21(DE3) was grown and membranes were prepared as described above (“Expression of ypfP in E. coli”). To prepared the S. aureus membranes by a modified method of Koo et al. (30), the cells were grown overnight in LB medium. The cells were collected by centrifugation and suspended to an OD600 of 1.0 in phosphate buffer (pH 7.2) containing 0.14 mM NaCl and 3 mM KCl. This suspension (25 ml) was centrifuged at 5,000 × g for 15 min, and the pellet was suspended in 600 μl of digestion buffer (20% sucrose, 0.05 M Tris-HCl [pH 7.6], 0.145 M NaCl). The staphylococcal walls were digested with lysostaphin (34 μg/ml) in the presence of DNase I (10 μg/ml) for 1 h at 37°C. The sucrose-stabilized protoplasts were collected by centrifugation (10,000 × g, 15 min) and resuspended in 1 ml of digestion buffer. The adequacy of wall digestion was confirmed by osmotic shock in ice-cold 0.05 M Tris-HCl (pH 7.6). Protoplast lysis was detected by the rapid decrease in OD420. To isolate the membranes, S. aureus protoplasts were lysed by resuspendion in 0.05 M Tris-HCl (pH 7.6), layered on 5 ml of 55% sucrose, and centrifuged for 2 h at 60,000 × g. The membrane fraction was collected and stored in aliquots at −78°C.
Permeabilized cells were prepared as described by Childs and Neuhaus (10), with several modifications. S. aureus was grown overnight in 100 ml of LB medium. The cells (300 mg) were collected by centrifugation at 2,000 × g and suspended in 1 ml of 20 mM Tris-HCl buffer (pH 7.2) containing 20 mM MgCl2, 1 mM dithiothreitol, and 0.6% toluene. Prior to the permeabilization, the toluene was suspended in this buffer by 1 min of sonication at maximal power on the low setting on a B. Braun Labsonic ultrasonic sonicator. The cells were incubated in this buffer for 15 min at 25°C with occasional vortexing, collected by centrifugation (2,000 × g, 10 min), and suspended in 0.5 ml of the same buffer without toluene. The cells were either used immediately or frozen in aliquots at −70°C.
UDP-[14C]glucose and 1,2-di[1-14C]palmitoylglycerol incorporation assay.
To monitor the incorporation of UDP-[14C]glucose into glycolipids catalyzed by YpfP in intact cells, permeabilized cells, and membranes, the reaction mixture contained, in a total volume of 40 μl, 50 mM Tris-HCl (pH 8.0), 20% (vol/vol) glycerol, and 0.35 nmol UDP-[14C]glucose (286 mCi/mmol). The mixture was incubated at 37°C for the indicated time.
For the filtration assay, the reaction was stopped by adding 0.9 ml of 10% trichloroacetic acid and maintained on ice for 30 min. The mixture was transferred to a 0.45-μm-pore-size Metricel GN-6 filter and washed three times with 1 ml and one time with 10 ml of 10% trichloroacetic acid. The damp filters were dissolved in 1 ml of ethyl acetate (10 min, 25°C) before the addition of 15 ml of Triton-toluene scintillation cocktail, and the samples were assayed for radiolabel.
For the assay of mono- and diglucosyldiacylglycerol formation from radiolabeled diacylglycerol, the 1,2-di[1-14C]palmitoylglycerol was isolated from a chloroform solution, suspended in 35 μl of 50 mM Tris-HCl (pH 8.0) containing 20% (vol/vol) glycerol by 15 min of sonication in an Ultrasonic Dismembrator (Laboratory Supplies Co., Inc.), and added to the reaction mixture containing YpfP (E. coli membranes) and UDP-glucose. The reaction was stopped, and the mixture was extracted as described for the UDP-glucose incorporation assay.
For the analysis of glycolipids, the reaction mixture was extracted by methanol-chloroform (5), with partitioning into the aqueous and organic phases as described by Brautigan et al. (8). The chloroform fraction was evaporated with nitrogen and resuspended in 20 μl of chloroform. These samples were used for separation by thin-layer chromatography (TLC) in the following solvent systems: (i) chloroform-methanol-H2O (65:25:4, vol/vol/vol) and (ii) butanol-acetic acid-H2O (120:30:50, vol/vol/vol). Glycolipids were visualized by lightly spraying with a solution of 3.2% (wt/vol) α-naphthol in methanol-H2SO4-H2O (25:3:2, vol/vol/vol) and heating the plate at 110°C for 25 min.
LTA analyses.
Cells of S. aureus RN4220 were lysed by lysostaphin as described above (“Preparations of membranes and permeabilized cells”). LTA was extracted from this lysate with phenol-water (16) and analyzed by polyacrylamide gel electrophoresis (44). d-Alanine incorporation into LTA into permeabilized cells of S. aureus was determined by the method of Ntamere et al. (39). For the analysis of glycolipid anchor, LTA was hydrolyzed by 48% HF for 24 h at 4°C. The acid was removed by placing the tubes in a polycarbonate desiccator in vacuum containing NaOH pellets (45). Products of hydrolysis were suspended in 40 μl of 10 mM lithium acetate by 15 min of sonication in a model G112SP1T Ultrasonic Dismembrator (Laboratory Supplies). Lipids were extracted by methanol-chloroform (5), and glycolipids were analyzed as described above. The detection of diacylglycerol was performed by TLC in (i) petroleum ether-diethyl ether-acetic acid (40:60:4) and (ii) diethylether-hexane-acetic acid (50:50:1). Diacylglycerol was visualized by spraying the plate with a solution of 10% phosphomolybdic acid in ethanol and heating at 160°C for 20 min.
For quantifying the amount of LTA in cells and medium, the parental strain and the mutant were grown in LB medium (2 ml) with either 5 μCi of [U-14C]glycerol or 5 μCi of d-[14C]alanine from an OD600 of 0.08 to 0.35. The radiolabeled cells were separated from the medium by centrifugation, and the cellular LTA was isolated by the method outlined above. The secreted LTA was isolated from the medium which had been concentrated fourfold and dialyzed using a 10K Nanosep centrifugal filter (Pall-Filtron Corp.). The amount of radiolabeled LTA was determined by precipitation with monoclonal antibody specific for poly(GroP). The resulting radiolabeled antigen antibody complex was isolated by filtration on a 0.2-μm-pore-size filter and washed with 30 mM bis-Tris buffer (pH 6.5); the retained radiolabel was quantified.
Scanning electron microscopy (SEM).
S. aureus RN4220 and its ypfP::cat mutant were grown in 0.5 × amino acid assay medium (Difco) supplemented with methionine (0.05 g/liter) to an OD600 of 0.35. The cells were fixed with 2.5% (vol/vol) glutaraldehyde in 0.1 M cacodylate buffer (pH 7.4) for 6 h and dehydrated with a graded series of ethanol concentrations. A drop of each sample in 100% ethanol on a slide was subjected to critical-point drying, and the cells were coated with gold-palladium. Micrographs were obtained with a JEOL JSM-33 CF scanning electron microscope.
LD50 determination.
LD50 (numbers of CFU required to induce a lethal infection in 50% of the infected animals) were determined in 20-g C3H mice (Charles River, Portage, Mich.). Log dilutions of the S. aureus wild-type and mutant strains were prepared from frozen stocks in BIII broth (Difco) containing 4% (wt/vol) dried brewer's yeast. Each dilution in the series was injected intraperitoneally into 10 mice. The mice were observed for a period of 1 week, after which the observed mortality was used to calculate the LD50 by probit analysis.
RESULTS
Insertional inactivation of ypfP.
To define the role of YpfP in both LTA biosynthesis and free glycolipid formation in S. aureus, a mutant strain was constructed by insertional mutagenesis. A fragment of ypfP truncated from both ends was cloned into a plasmid with a thermosensitive replicon pVE6007 (37), and the resulting plasmid, pΔypfP, was used to obtain a Cmr mutant with ypfP inactivated by plasmid integration (ypfP::cat) (Fig. 1C). Integration of the plasmid by a single crossover was confirmed by (i) PCR using one primer complementary to the cat gene in pΔYpfP (Pr1) and another primer complementary to the chromosomal ypfP sequence outside of the ΔypfP fragment (Pr2) and (ii) DNA sequencing of the region containing the insertion (data not shown).
Growth characteristics of the ypfP::cat mutant.
To characterize the insertion mutant, a variety of growth experiments were undertaken. The doubling times of the mutant strain in LB medium (25 min) and 0.5 × amino acid assay medium (45 min) were identical to those of the parental strain. However, the mutant failed to grow on Baird-Parker agar supplemented with egg yolk and tellurite, a traditional medium for identifying S. aureus (3). Analysis of this inhibition revealed that growth was inhibited by the 1.2% glycine found in this medium. Addition of either 1% glycine or 0.4% d-serine to LB medium also gave no growth of the mutant. The inhibition of mutant growth by glycine is reversed by 0.4% d- or l-alanine. While the parental strain shows the same sensitivity to penicillin as the mutant, it is threefold more sensitive to the action of d-cycloserine, as measured by disk diffusion on LB agar.
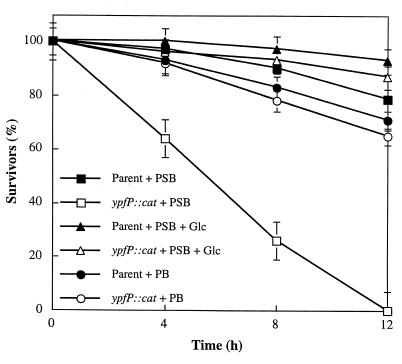
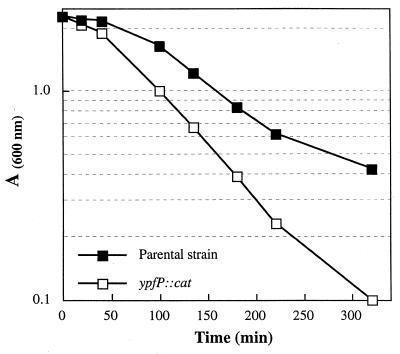
Viability studies revealed that the ypfP::cat mutant does not survive (<0.1%) in phosphate-saline for 12 h at 37°C (Fig. 2). In contrast, the parental strain gave 75% viability under these conditions. When 0.1% d-glucose was added to the phosphate-saline solution, the viability of the mutant was 80% while that of the parental strain was 90%. In phosphate buffer alone, the mutant strain showed the same viability as the parental strain. Thus, the ypfP::cat insertion mutant is not tolerant to 0.85% NaCl in the absence of the nutrient, d-glucose. The autolysis of the ypfP::cat insertion mutant was compared with that of the parental strain. As shown in Fig. 3, the rate of autolysis in the presence of 0.05% Triton X-100, determined by the method of Gustafson et al. (19), is 1.8 times that of the parental strain. Thus, the ypfP::cat mutant differs from the parental strain in (i) its sensitivity to either glycine, d-serine, or d-cycloserine, (ii) its viability in phosphate-saline, and (iii) its rate of autolysis.
FIG. 2.
Viability of the ypfP::cat mutant in the presence of phosphate-saline buffer. The mutant (3 × 106 cells) and wild type (3 × 106 cells) were maintained in 0.05 M phosphate-saline buffer (pH 7.2) (PSB) at 37°C for the indicated time. The numbers of CFU were determined from LB agar plates and are reported as percent survivors (viability). For comparison, the wild type and mutant were also maintained in 0.05 M phosphate buffer (pH 7.2) (PB). In addition, 0.1% d-glucose (Glc) added to PSB containing the parent and mutant are compared.
FIG. 3.
Triton X-100-stimulated cell autolysis of ypfP::cat and parental strains. Autolysis of whole cells was measured at 37°C in 50 mM Tris-HCl buffer (pH 7.2) containing 0.05% (vol/vol) Triton X-100 according to the method of Gustafson et al. (19).
Morphology of the ypfP::cat mutant.
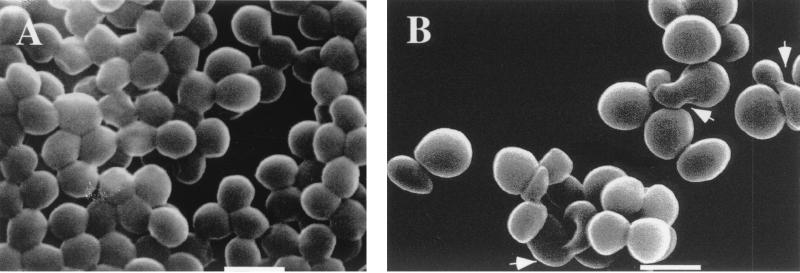
SEM of the mutant strain revealed not only a small increase in size but also a modification in cell shape. Some of the cells were characterized by misshapen coccal forms (pleomorphs) (Fig. 4), while others were characterized by a larger size. The parental strain had an average mean diameter of 0.76 ± 0.06 μm, while the mutant had an average mean diameter of 0.99 ± 0.06 μm. The small increase in diameter and the aberrant cell shapes suggested that cell division may be partly compromised.
FIG. 4.
Scanning electron micrographs of exponential-phase cells of S. aureus RN4220 (A) and the ypfP::cat mutant (B). Bars, 1 μm. SEM was performed as described in Materials and Methods. Arrowheads in panel B show aberrant pleomorphs.
Glycolipid synthesis in S. aureus RN4220 and ypfP::cat.
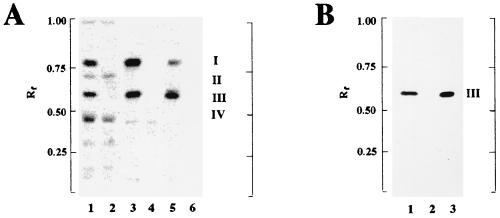
To test for glycolipid biosynthesis in the ypfP::cat mutant, two systems (permeabilized cells and membranes) were used in the UDP-[14C]glucose incorporation assay. MGlcDAG (compound I) and DGlcDAG (compound III) were synthesized by permeabilized cells of the parental strain of S. aureus (Fig. 5A, lane 1) and membranes (Fig. 5A, lane 3). In addition, two unidentified radiolabeled compounds, II and IV, were also synthesized in permeabilized cells (Fig 5A, lane 1). Thus, membranes provided a more defined system for monitoring the synthesis of the glycolipids MGlcDAG and DGlcDAG. α-Naphthol–H2SO4 staining was used to visualize glycolipid in the permeabilized cells of S. aureus. DGlcDAG, identified by comparison with the standard (Fig. 5B, lane 3), was the major glycolipid identified in this strain of S. aureus (Fig. 5B, lane 1). Small amounts of MGlcDAG were observed when larger samples of extract were chromatographed (data not shown).
FIG. 5.
Incorporation of [14C]glucose from UDP-[14C]glucose into the glycolipids (A) and detection of α-naphthol-staining glycolipids (B). The synthesis of [14C]glucose-labeled glycolipids (Materials and Methods) was performed with 6 mg of permeabilized cells of either S. aureus (A, lane 1) or the ypfP::cat mutant (A, lane 2), with 20 μg of membranes from either the parent (A, lane 3) or the ypfP::cat mutant (A, lane 4), and with 20 μg of membranes from either E. coli containing pYpfP (A, lane 5) or E. coli BL21(DE3) (A, lane 6). The reaction conditions were 30 min at 37°C (lanes 1 to 4) and 5 min at 15°C for (lanes 5 and 6). I and III are MGlcDAG and DGlcDAG, respectively; II and IV were not identified. α-Naphthol-staining glycolipids from the parent (B, lane 1) and ypfP::cat mutant (B, lane 2) were compared with the standard β-gentiobiosyl diacylglycerol (DGlcDAG) (B, lane 3).
To establish that ypfP::cat is defective for the synthesis of DGlcDAG, permeabilized cells and membranes of the mutant were also assayed for the incorporation of UDP-[14C]glucose. Permeabilized ypfP::cat (Fig. 5A, lane 2) and its membrane fraction (Fig. 5A, lane 4) were defective for the incorporation of UDP-[14C]glucose into MGlcDAG and DGlcDAG. The syntheses of compounds II and IV detected with permeabilized cells of the parental strain were not affected by the inactivation of ypfP. α-Naphthol–H2SO4 staining was used to compare the glycolipid compositions of the parental strain and the mutant. DGlcDAG was detected in the parental strain (Fig. 5B, lane 1) but was absent in the ypfP::cat mutant (Fig. 5B; lane 2). Thus, YpfP would appear to be a DGlcDAG synthase.
Characterization of recombinant YpfP in transformed E. coli.
To ascertain the function of YpfP from S. aureus RN4220, the gene was cloned into the pET-3d vector pYpfP (Fig. 1A) and expressed in E. coli strain BL21(DE3). A membrane protein with the molecular mass predicted for YpfP (44 kDa) accumulated in the pellet and in the membrane fraction (data not shown). Membranes from this strain of E. coli (Fig. 5A, lane 5) catalyzed the synthesis of two radiolabeled glycolipids, MGlcDAG (I) and DGlcDAG (III), identical to those synthesized by S. aureus permeabilized cells (Fig. 5A, lane 1) and membranes (Fig. 5A, lane 3). Thus, YpfP of S. aureus is a DGlcDAG synthase similar to that demonstrated for B. subtilis (27). While this work was in progress, Jorasch et al. (26) also cloned ypfP from S. aureus and expressed it in E. coli. In contrast to B. subtilis YpfP, which adds up to four glucosyl residues, the S. aureus enzyme adds two residues (26). This observation was confirmed in the present work and is consistent with the glycolipid anchor composition of S. aureus LTA (18).
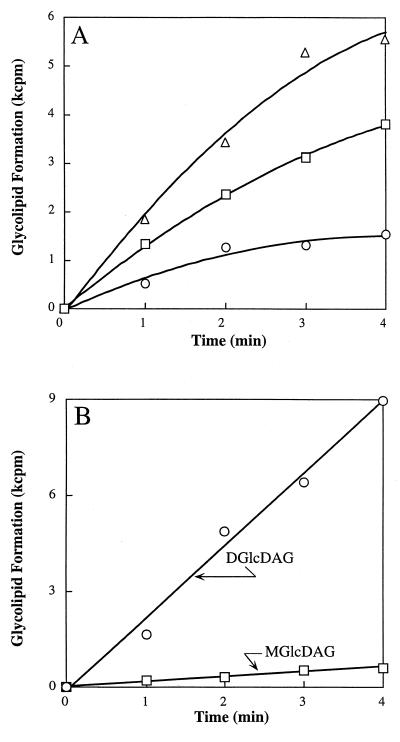
The kinetics of combined MGlcDAG and DGlcDAG formation were monitored at 15°C in incubations containing recombinant YpfP. At this temperature, linear time-dependent and membrane concentration-dependent incorporation of UDP-[14C]glucose was observed in the interval of 3 min (Fig. 6A). At 25°C, the reaction was essentially complete in 1 min. The Km for UDP-glucose measured at 15°C was determined to be 0.7 mM, a value similar to that of the glucosyltransferase involved in teichuronic acid biosynthesis in Micrococcus luteus (0.3 mM) (35). The optimal pH was 7.5. Divalent cations were not required for this reaction. The activity of the recombinant enzyme did not change during 3 months of storage and upon multiple cycles of freezing and thawing. The kinetics of individual MGlcDAG and DGlcDAG formation (Fig. 6B) demonstrated that the ratio (1:20) between MGlcDAG and DGlcDAG is invariant during the 4-min time course, an observation consistent with the processive synthesis of DGlcDAG:
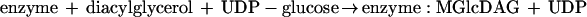 |
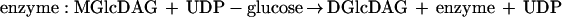 |
Under the conditions of our in vitro assays with recombinant YpfP, only 5% of the MGlcDAG dissociated from the enzyme complex (Fig. 6B). In S. aureus RN4220, only traces of MGlcDAG were detected in isolated membranes by α-naphthol–H2SO4 staining (Fig. 6B). Thus, our studies with recombinant YpfP reflect the observations of glycolipid composition made in intact cells of S. aureus.
FIG. 6.
(A) Incorporation of [14C]glucose from UDP-[14C]glucose into MGlcDAG and DGlcDAG (summation) by recombinant YpfP; (B) incorporation of [14C]glucose from UDP-[14C]glucose into MGlcDAG and DGlcDAG by recombinant YpfP. In panel A, the reaction mixture (40 μl) contained 50 mM Tris-HCl buffer (pH 8.0), membrane-associated recombinant YpfP, and 0.35 nmol of UDP-[14C]glucose (286 mCi/mmol). Membranes concentrations were 2 (○), 4 (□), and 8 (▵) mg of protein/ml. The reaction mixtures were incubated at 15°C for 5 min. The radiolabeled glycolipids were extracted and analyzed by TLC as described in Materials and Methods.
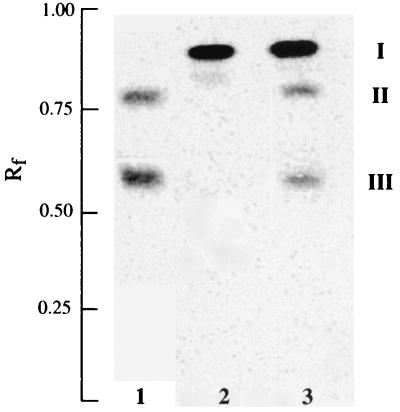
The proposed acceptor of the glucosyl residues, diacylglycerol, is an endogenous component of the membranes. To confirm that diacylglycerol is a sugar acceptor in the reaction catalyzed by DGlcDAG synthase, [14C]diacylglycerol (Fig. 7, compound I) was incubated with recombinant YpfP. Incorporation of the radiolabeled diacylglycerol into MGlcDAG (Fig. 7, compound II) and DGlcDAG (compound III) was demonstrated (Fig. 7, lane 3). These compounds were not synthesized by the E. coli membranes lacking YpfP (Fig. 7, lane 2). Compounds II and III were identical to those synthesized by S. aureus membranes (Fig. 7, lane 1) with UDP-[14C]glucose. Thus, diacylglycerol is the sugar acceptor in the reaction catalyzed by the DGlcDAG synthase, YpfP.
FIG. 7.
Incorporation of 1,2-di[1-14C]palmitoylglycerol into MGlcDAG and DGlcDAG by YpfP. The reaction mixture (40 μl) contained 1 mM UDP-glucose, 20 μg of membrane-associated recombinant YpfP, 3.7 pmol of 1,2 di[14C]palmitoylglycerol (937 cpm), and 44 mM Tris-HCl buffer (pH 8.0) containing 18% (vol/vol) glycerol. The mixtures were incubated at 37°C for 1 h. The incorporation of 1,2-di[1-14C]palmitoylglycerol (I) into II and III by the membranes from E. coli BL21(DE3) (lane 2) and from E. coli BL21(DE3) with YpfP (lane 3) is shown. The procedures for preparing the reaction mixture containing the radiolabeled diacylglycerol and the analyses of the lipids are described in Materials and Methods. For comparison, the incorporation of [14C]glucose from UDP-[14C]glucose into MGlcDAG (II) and DGlcDAG (III) by S. aureus membranes (lane 1) is shown.
Analysis of LTA in the parental S. aureus and ypfP::cat strains.
The deficiency of DGlcDAG in the ypfP::cat mutant suggested that either the mutant was deficient in LTA or an alternative membrane anchor was used. To distinguish between these possibilities, LTA was isolated from the parental strain and the ypfP::cat mutant. Samples were hydrolyzed with 48% HF to release the lipid anchor. The lipid of the parental strain was identified as DGlcDAG, whereas no glycolipid could be detected in the hydrolysate of the LTA from the mutant strain. Therefore, an alternative nonglycolipid anchor appeared to be utilized in the synthesis of the mutant LTA. In contrast to the LTA from the parental strain, the LTA from the ypfP::cat mutant contained diacylglycerol in the HF hydrolysate (data not shown). No trace of this degradation product was found in the hydrolysate of the parental LTA. Thus, a major difference in the lipid anchors between the parental LTA and the mutant LTA was observed. It was concluded that diacylglycerol is the anchor of the LTA in the mutant strain.
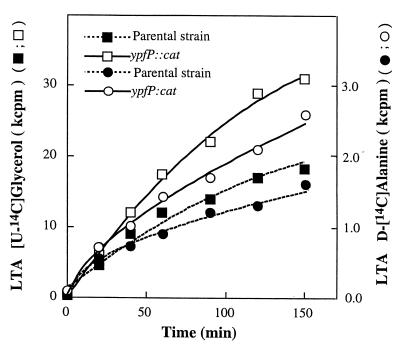
To quantify the amounts of LTA in the parental and ypfP::cat strains, cells were grown with radiolabeled glycerol and d-alanine. The amounts of cellular LTA and secreted LTA were quantitated with antibody directed to the poly(Gro-P) moiety of LTA. As shown in Table 2, the amount of LTA labeled with [14C]glycerol in the mutant strain was 50% greater than that in the parental strain. Moreover, the amount of LTA secreted into the medium was threefold greater for the mutant than for the parental strain (Table 2). When d-[14C]alanine was used as the radiolabel, the amount of labeled d-[14C]alanyl-LTA was 2.4-fold higher in the mutant, while the amount secreted was 3.2-fold higher than in the parental strain. Prelabeling of these strains with either d-[14C]alanine or [14C]glycerol also provided the basis for measuring the turnover of LTA under growth conditions. As shown in Fig. 8, the turnover of LTA from the mutant was twofold greater than that observed with the parental strain. Since the chain length distributions of parental and mutant LTAs were similar (data not shown), the difference in turnover in the two strains does not reflect LTAs of different chain length.
TABLE 2.
Quantitation of cellular LTA and secreted LTA of ypfP::cat and parental strains
| LTA fraction | Amt (103 cpm)a
|
|
|---|---|---|
| Parental strain | ypfP::cat mutant | |
| [U-14C]glycerol radiolabeled | ||
| Cellular LTA | 70.0 | 105.0 |
| Secreted LTA | 12.0 | 36.0 |
| d-[14C]alanine radiolabeled | ||
| Cellular LTA | 4.8 | 11.4 |
| Secreted LTA | 1.6 | 5.1 |
Determined by antibody precipitation. Labeled cells were prepared by growth of the parental or mutant strain in LB medium containing 5 μCi of either [U-14C]glycerol or d-[14C]alanine. After growth, the labeled cells were separated from the medium, washed two times in 50 mM phosphate buffer (pH 7.0), and resuspended in 2 ml of fresh LB medium. After growth for 180 min, the cells were separated from the medium by using a 0.45-μm-pore-size filter. The amount of LTA was quantitated as described in Materials and Methods. The nonprecipitating radiolabel in both series was approximately 0.5%. Although not determined, the specific activity of the d-alanine radiolabel had decreased six- to sevenfold.
FIG. 8.
Release of d-[14C]alanine- and [14C]glycerol-labeled LTA by the ypfP::cat mutant and the parental strain. Samples of d-alanine (8,000 cpm)- and glycerol (36,000 cpm)-labeled cells were suspended in LB medium (2 ml) and grown with aeration at 37°C for the indicated times. Samples of the growth medium were separated from the cells using Nanosep MF centrifugal filters (0.45-μm pore size; Pall-Filtron), and the amounts of released radiolabel were quantified.
The in vitro incorporation of radiolabeled d-alanine into LTA of permeabilized ypfP::cat cells was twofold that of the parental cells (data not shown). These results with d-alanine incorporation system imply that the mutant strain has approximately twofold the number of LTA acceptor sites, an observation consistent with the LTA content described in Table 2.
Pathogenesis of S. aureus strain RN4220 and its ypfP::cat mutant.
With the mutant fully deficient in glycolipid and with its LTA terminated by diacylglycerol, it was of interest to test whether the ypfP::cat mutant retained its pathogenicity in the mouse model. In the mean of two independent experiments, the parental strain caused a lethal infection in 50% of the infected animals with 3.8 × 103 CFU. In the case of the mutant strain, 1.3 × 104 CFU was required to produce the same lethality. The data indicated that the glycolipid deficient mutant strain is not significantly impaired in its ability to produce an acute mouse infection compared with the parental strain. Thus, the mutant strain most likely displays the same amount of endotoxin as does the parental strain.
DISCUSSION
A primary role of d-alanyl-LTA in microbial physiology has been implied by the absence of mutants with defects in the assembly of the poly(Gro-P) moiety (17, 52). Well-characterized mutations in the dlt operon (7, 12, 14, 42, 43, 50) have implicated the importance of the d-alanyl esters in the function of LTA. In this study, we inactivated ypfP of S. aureus RN4220, which encodes DGlcDAG synthase, and correlated this deficiency with the physiology of the mutant.
In 1998, the gene encoding DGlcDAG synthase, ypfP, was cloned from B. subtilis based on its similarity to the monogalactosyldiacylglycerol synthase from cucumber (27, 49). It was demonstrated that ypfP of B. subtilis encodes a UDP-glucosyltransferase which successively transfers up to four glucosyl residues to 1,2-diacylglycerol. Our results confirmed that the ypfP homolog in S. aureus also encodes a protein with essentially the same function, though it transfers only two glucosyl residues to 1,2-diacylglycerol with the synthesis of DGlcDAG. While this research was in progress, the corresponding gene was also cloned from S. aureus (26, 36) and shown to encode the DGlcDAG synthase (26). In addition, the homologous gene was inactivated in B. subtilis (28, 46), resulting in the loss of glycolipid. Two questions remained unanswered in S. aureus: (i) is LTA synthesized in the mutant lacking DGlcDAG? and (ii) if so, is the LTA membrane anchor replaced by either another glycolipid or by another membrane component?
To answer these questions, we inactivated ypfP in S. aureus and compared glycolipid synthesis in the parental strain and the insertion mutant. Our results demonstrated that YpfP appears to be the only DGlcDAG synthase and that the glycolipid of LTA is not replaced by another glycolipid. The lipid which replaces DGlcDAG in this mutant is diacylglycerol. No glycolipid was detected in the LTA of the insertion mutant.
In 1976, Button and Hemmings (9) observed the loss of β-gentiobiosyl-diacylglycerol in a phosphoglucomutase mutant of Bacillus licheniformis. However, the poly(Gro-P) moiety of the LTA was unchanged. These workers proposed that the poly(Gro-P) moiety may be assembled on phosphatidylglycerol and that the completed poly(Gro-P) chains are transferred to the glycolipid in the parental strain. If the glycolipid acceptor is absent, the chains remain attached to phosphatidylglycerol. Alternatively, it was also suggested (9) that the enzymes which function in the biosynthesis of LTA have substrate specificities sufficiently low to tolerate the replacement of β-gentiobiosyl-diacylglycerol by diacylglycerol. While these are not the only mechanisms for assembling LTA (11, 47), they provide a rationale for interpreting the results described for the ypfP::cat mutant. In Bacillus coagulans and Bacillus megaterium, diacylglycerol is normally found as the anchor of LTA under laboratory growth conditions (25). Thus, in those organisms which lack the usual glycolipid, the LTA is anchored into the membrane by diacylglycerol.
In addition to serving as a membrane anchor of LTA, glycolipids have been proposed to play a role in determining membrane stability. It has been hypothesized that the structure of the lipid bilayer is expected to be kept at its limit of stability by the regulated ratio of non-bilayer-forming (MGlcDAG) and bilayer-forming (DGlcDAG) glycolipids, thus allowing a flexible response to various triggering events (27, 54). In studying the velocities of MGlcDAG and DGlcDAG syntheses by YpfP in this work, we observed a fixed ratio of the two glycolipids (1:20). Whether a processive enzyme such as that described for S. aureus and B. subtilis can provide a flexible response with variable amounts of the two glycolipids is not known. The presence of individual enzymes catalyzing the successive addition of glucosyl residues to diacylglycerol such as those found in Acholeplasma laidlawii provides greater opportunity to sense the metabolic status of the cell (54).
Although the doubling time of the ypfP::cat mutant was identical to that of the parental strain in LB medium, growth of the insertion mutant in this medium containing 1% glycine was not observed. The inhibition of growth by glycine was originally detected on Baird-Parker agar plates supplemented with egg yolk and tellurite in studies designed to characterize the mutant. The inhibition was also observed when glycine is replaced by either 0.4% d-serine or 10 μg of d-cycloserine per ml. In each case, the inhibition was reversed by including either l- or d-alanine in the medium. Why a glycolipid-deficient mutant is more sensitive to glycine is not obvious. However, permeabilized cells of the mutant strain incorporated twofold more d-alanine into its LTA than did the parental strain. This observation coupled with a higher turnover rate of LTA in the mutant may reflect the fact that the mutant under certain growth conditions cannot produce the d-alanine needed for the enhanced biosynthesis of LTA as well as for peptidoglycan synthesis. Using mutants of B. subtilis with defective synthesis of d-alanine, Heaton et al. (21) observed that the addition of high concentrations of glycine to the growth medium greatly facilitated the lysis of the mutant. Enhanced d-alanyl-LTA biosynthesis may trigger a higher d-alanine requirement than can be met by the alanine racemase activity of the cell.
Another phenotype became apparent during the course of characterizing the mutant. The viability of the mutant in 0.05 M phosphate buffer (pH 7.2) containing 0.85% NaCl was significantly lower than that of the parental strain. Addition to the phosphate-saline buffer of 0.1% d-glucose reversed the inhibition. Thus, the ypfP::cat mutant differs from the parental strain in (i) its sensitivity to glycine, d-serine, and d-cycloserine, (ii) its viability in phosphate saline, (iii) its autolysis rate, and (iv) the presence of pleomorphic forms. Each of these phenotypes may be the result of one or both (i) deficiency in the free membrane glycolipid and (ii) deficiency in the glycolipid anchor for usual LTA formation.
The efficient expression of recombinant YpfP in E. coli provided enzyme for testing the effect of a number of potential inhibitors. Since Boaretti et al. (6) reported that LTA assembly is a target for daptomycin, this antibiotic was tested as an inhibitor of the reaction catalyzed by DGlcDAG synthase (Ki = 100 μM). Tunicamycin (Ki = 200 μM) and dl-threo-1- phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol (Ki = 300 μM) were also tested as inhibitors of this synthase. All other potential inhibitors tested had Kis of 0.5 mM or higher. None of the inhibitors were sufficiently effective to provide a tool for studying the in vivo inhibition of the DGlcDAG synthase.
S. aureus RN4220 produces an exoprotein that is thought to have an etiologic role in toxic shock syndrome (31). Our results suggest that the deficiency of either free membrane glycolipid or the glycolipid anchor of LTA does not greatly affect the action of this exoprotein in producing the toxic shock response. In addition, one of our goals was to establish whether the synthesis of DGlcDAG would represent a potential target for the rational design of antimicrobial agents against gram-positive bacteria. While a variety of phenotypes are characteristic of this mutant, the glycolipid deficiency did not result in growth inhibition of the mutant in at least two laboratory growth media. However, two of the phenotypes, (i) sensitivity to glycine, d-serine, and d-cycloserine and (ii) and decrease in viability in phosphate-saline, argue for additional studies of this potential target, DGlcDAG synthase.
ACKNOWLEDGMENTS
Michael Y. Kiriukhin and Dmitri V. Debabov made equal contributions to this paper.
The research was supported by Pharmacia postdoctoral fellowship AGRMT 3-25-97 to D.V.D. and by Public Health Service grant RO1 GM51623 to F.C.N.
We are especially grateful to Werner Fischer for DGlcDAG, discussions, and methodology. We also thank Brian Wilkinson and Rebecca Giorno-McConnell for discussions and methodology. We are indebted to Eugene W. Minner for his generous help in the Electron Microscope Facility of the Department of Neurobiology and Physiology, Northwestern University.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers E W, Lipman D J. Basic local alignment search tool. J Mol Biol. 1990;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Archibald A R, Baddiley J, Heptinstall S. The alanine ester content and magnesium binding capacity of walls of Staphylococcus aureus H grown at different pH values. Biochim Biophys Acta. 1973;291:629–634. doi: 10.1016/0005-2736(73)90468-9. [DOI] [PubMed] [Google Scholar]
- 3.Baird-Parker A C. An improved diagnostic and selective medium for isolating coagulase positive staphylococci. J Appl Bacteriol. 1962;25:12–19. [Google Scholar]
- 4.Bierbaum G, Sahl H-G. Induction of autolysis of Staphylococcus simulans 22 by Pep5 and nisin and influence of the cationic peptides on the activity of the autolytic enzymes, p 386–396. In: Jung G, Sahl H-G, editors. Nisin and novel lantibiotics. Leiden, The Netherlands: ESCOM; 1991. [Google Scholar]
- 5.Bligh E G, Dyer W J. A rapid method of total lipid extraction and purification. Can J Biochem Physiol. 1959;37:911–917. doi: 10.1139/o59-099. [DOI] [PubMed] [Google Scholar]
- 6.Boaretti M, Canepari P, Lleò M M, Satta G. The activity of daptomycin on Enterococcus faecium protoplasts: indirect evidence supporting a novel mode of action on lipoteichoic acid synthesis. J Antimicrob Chemother. 1993;31:227–235. doi: 10.1093/jac/31.2.227. [DOI] [PubMed] [Google Scholar]
- 7.Boyd D A, Cvitkovitch D G, Bleiweis A S, Kiriukhin M Y, Debabov D V, Neuhaus F C, Hamilton I R. Defects in d-alanyl-lipoteichoic acid synthesis in Streptococcus mutans results in acid sensitivity. J Bacteriol. 2000;182:6055–6065. doi: 10.1128/jb.182.21.6055-6065.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brautigan V M, Childs III W C, Neuhaus F C. Biosynthesis of d-alanyl-lipoteichoic acid in Lactobacillus casei: d-alanyl-lipophilic compounds as intermediates. J Bacteriol. 1981;146:239–250. doi: 10.1128/jb.146.1.239-250.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Button D, Hemmings N L. Teichoic acids and lipids associated with the membrane of a Bacillus licheniformis mutant and the membrane lipids of the parental strain. J Bacteriol. 1976;128:149–156. doi: 10.1128/jb.128.1.149-156.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Childs W C, III, Neuhaus F C. Biosynthesis of d-alanyl-lipoteichoic acid: characterization of ester-linked d-alanine in the in vitro-synthesized product. J Bacteriol. 1980;143:293–301. doi: 10.1128/jb.143.1.293-301.1980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu T-H, Morimoto H, Baker J J. Biosynthesis and characterization of phosphatidylglycerophosphoglycerol, a possible intermediate in lipoteichoic acid biosynthesis in Streptococcus sanguis. Biochim Biophys Acta. 1993;1166:222–228. doi: 10.1016/0005-2760(93)90101-e. [DOI] [PubMed] [Google Scholar]
- 12.Debabov D V, Kiriukhin M Y, Neuhaus F C. Biosynthesis of lipoteichoic acid in Lactobacillus rhamnosus: role of DltD in d-alanylation. J Bacteriol. 2000;182:2855–2864. doi: 10.1128/jb.182.10.2855-2864.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Duwat P, Cochu A, Ehrlich S D, Gruss A. Characterization of Lactococcus lactis UV-sensitive mutants obtained by ISS-1 transposition. J Bacteriol. 1997;179:4473–4479. doi: 10.1128/jb.179.14.4473-4479.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fischer W, Rosel P, Koch H U. Effect of alanine ester substitution and other structural features of lipoteichoic acids on their inhibitory activity against autolysins of Staphylococcus aureus. J Bacteriol. 1981;146:467–475. doi: 10.1128/jb.146.2.467-475.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer W, Koch H U, Haas R. Improved preparation of lipoteichoic acids. Eur J Biochem. 1983;133:523–530. doi: 10.1111/j.1432-1033.1983.tb07495.x. [DOI] [PubMed] [Google Scholar]
- 17.Fischer W. Physiology of lipoteichoic acids in bacteria. Adv Microb Physiol. 1988;29:233–302. doi: 10.1016/s0065-2911(08)60349-5. [DOI] [PubMed] [Google Scholar]
- 18.Fischer W P. Lipoteichoic acid and lipids in the membrane of Staphylococcus aureus. Med Microbiol Immunol. 1994;183:61–76. doi: 10.1007/BF00277157. [DOI] [PubMed] [Google Scholar]
- 19.Gustafson J E, Berger-Bächi B, Strässle A, Wilkinson B J. Autolysis of methicillin-resistant and -susceptible Staphylococcus aureus. Antimicrob Agents Chemother. 1992;36:566–572. doi: 10.1128/aac.36.3.566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hanahan D. Studies on transformation of Escherichia coli with plasmids J. Mol Biol. 1983;166:557–580. doi: 10.1016/s0022-2836(83)80284-8. [DOI] [PubMed] [Google Scholar]
- 21.Heaton M P, Johnston R B, Thompson T L. Controlled lysis of bacterial cells utilizing mutants with defective synthesis of d-alanine. Can J Microbiol. 1988;34:256–261. doi: 10.1139/m88-047. [DOI] [PubMed] [Google Scholar]
- 22.Heptinstall S, Archibald A R, Baddiley J. Teichoic acids and membrane function in bacteria. Nature. 1970;225:519–521. doi: 10.1038/225519a0. [DOI] [PubMed] [Google Scholar]
- 23.Holo H, Nes I F. High frequency transformation, by electroporation, of Lactococcus lactis subsp. cremoris grown with glycine in osmotically stabilized media. Appl Environ Microbiol. 1989;55:3119–3123. doi: 10.1128/aem.55.12.3119-3123.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hughes A H, Hancock I C, Baddiley J. The function of teichoic acids in cation control in bacterial membranes. Biochem J. 1973;132:83–93. doi: 10.1042/bj1320083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iwasaki H, Shimada A, Ito E. Comparative studies of lipoteichoic acids from several Bacillus strains. J Bacteriol. 1986;167:508–516. doi: 10.1128/jb.167.2.508-516.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jorasch P, Warnecke D C, Lindner B, Zähringer U, Heinz E. Novel processive and nonprocessive glycosyltransferases from Staphylococcus aureus and Arabidopsis thaliana synthesize glycoglycerolipids, glycophospholipids, glycosphingolipids and glycosylsterols. Eur J Biochem. 2000;267:3770–3783. doi: 10.1046/j.1432-1327.2000.01414.x. [DOI] [PubMed] [Google Scholar]
- 27.Jorasch P, Wolter F P, Zähringer U, Heinz E. A UDP-glucosyltransferase from Bacillus subtilis successively transfers up to four glucose residues to 1,2-diacylglycerol: expression of ypfP in Escherichia coli and structural analysis of its reaction products. Mol Microbiol. 1998;29:419–430. doi: 10.1046/j.1365-2958.1998.00930.x. [DOI] [PubMed] [Google Scholar]
- 28.Jorasch P, Wolter F P, Zähringer U, Heinz E. Complete loss of glycolipids in a glucosyltransferase knock-out mutant of Bacillus subtilis. In: Sánchez J, Cerdá-Olmedo E, Martínez-Force E, editors. Advances in plant lipid research—1998. Seville, Spain: Secretariado de Publicaciones de la Universidad de Sevilla; 1998. pp. 243–246. [Google Scholar]
- 29.Koch H U, Haas R, Fischer W. The role of lipoteichoic acid biosynthesis in membrane lipid metabolism of growing Staphylococcus aureus. Eur J Biochem. 1984;138:357–363. doi: 10.1111/j.1432-1033.1984.tb07923.x. [DOI] [PubMed] [Google Scholar]
- 30.Koo S-P, Yeaman M R, Nast C C, Bayer A S. The cytoplasmic membrane is a primary target for the staphylocidal action of thrombin-induced platelet microbicidal protein. Infect Immun. 1997;65:4795–4800. doi: 10.1128/iai.65.11.4795-4800.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kreiswirth B N, Lofdahl S, Betley M J, O'Reilly M, Schlievert P M, Bergdoll M S, Novick R P. The toxic shock syndrome exotoxin structural gene is not detectably transmitted by a prophage. Nature. 1983;305:709–712. doi: 10.1038/305709a0. [DOI] [PubMed] [Google Scholar]
- 32.Kuipers O P, Beerthuyzen M M, Siezen R J, de Vos W M. Characterization of the nisin gene cluster nisABTCIPR of Lactococcus lactis: requirement of expression of the nisA and nisI genes for development of immunity. Eur J Biochem. 1993;216:281–291. doi: 10.1111/j.1432-1033.1993.tb18143.x. [DOI] [PubMed] [Google Scholar]
- 33.Kullik I, Giachino P. The alternative sigma factor sigma B in Staphylococcus aureus: regulation of the sigB operon in response to growth phase and heat shock. Arch Microbiol. 1997;167:151–159. doi: 10.1007/s002030050428. [DOI] [PubMed] [Google Scholar]
- 34.Lambert P A, Hancock I C, Baddiley J. Influence of alanyl ester residues on the binding of magnesium ions to teichoic acid. Biochem J. 1975;151:671–676. doi: 10.1042/bj1510671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lingyi D, Anderson J S. Biosynthesis of teichuronic acid in the bacterial cell wall. Purification and characterization of the glucosyltransferase of Micrococcus luteus. J Biol Chem. 1997;272:479–485. doi: 10.1074/jbc.272.1.479. [DOI] [PubMed] [Google Scholar]
- 36.Ludovice A M, Wu S W, de Lancastre H. Molecular cloning and DNA sequencing of the Staphylococcus aureus UDP-N-acetylmuramyl tripeptide synthetase (murE) gene, essential for the optimal expression of methicillin resistance. Microb Drug Resist. 1998;4:85–90. doi: 10.1089/mdr.1998.4.85. [DOI] [PubMed] [Google Scholar]
- 37.Maguin E, Duwat P, Hege T, Ehrlich D, Gruss A. New thermosensitive plasmid for gram-positive bacteria. J Bacteriol. 1992;174:5633–5638. doi: 10.1128/jb.174.17.5633-5638.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Neuhaus F C, Heaton M P, Debabov D V, Zhang Q. The dlt operon in the biosynthesis of d-alanyl-lipoteichoic acid in Lactobacillus casei. Microb Drug Resist. 1996;2:77–84. doi: 10.1089/mdr.1996.2.77. [DOI] [PubMed] [Google Scholar]
- 39.Ntamere A S, Taron D J, Neuhaus F C. Assembly of d-alanyl-lipoteichoic acid in Lactobacillus casei: mutants deficient in the d-alanyl ester content of this amphiphile. J Bacteriol. 1987;169:1702–1711. doi: 10.1128/jb.169.4.1702-1711.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.O'Sullivan D J, Klaenhammer T R. Rapid mini-prep isolation of high-quality plasmid DNA from Lactococcus and Lactobacillus spp. Appl Environ Microbiol. 1993;59:2730–2733. doi: 10.1128/aem.59.8.2730-2733.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ou L-T, Marquis R E. Electromechanical interactions in cell walls of gram-positive cocci. J Bacteriol. 1970;101:92–101. doi: 10.1128/jb.101.1.92-101.1970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Perego M, Glaser P, Minutello A, Strauch M A, Leopold K, Fischer W. Incorporation of d-alanine into lipoteichoic acid and wall teichoic acid in Bacillus subtilis: identification of genes and regulation. J Biol Chem. 1995;270:15595–15606. doi: 10.1074/jbc.270.26.15598. [DOI] [PubMed] [Google Scholar]
- 43.Peschel A, Otto M, Jack R W, Kalbacher H, Jung G, Götz F. Inactivation of the dlt operon in Staphylococcus aureus confers sensitivity to defensins, protegrins, and other antimicrobial peptides. J Biol Chem. 1999;274:8405–8410. doi: 10.1074/jbc.274.13.8405. [DOI] [PubMed] [Google Scholar]
- 44.Pollack J H, Ntamere A S, Neuhaus F C. d-Alanyl-lipoteichoic acid in Lactobacillus casei: secretion of vesicles in response to benzylpenicillin. J Gen Microbiol. 1992;138:849–859. doi: 10.1099/00221287-138-5-849. [DOI] [PubMed] [Google Scholar]
- 45.Pollack J H, Neuhaus F C. Changes in wall teichoic acid during the rod-sphere transition of Bacillus subtilis 168. J Bacteriol. 1994;176:7252–7259. doi: 10.1128/jb.176.23.7252-7259.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Price K D, Roels S, Losick R. A Bacillus subtilis gene encoding a protein similar to nucleotide sugar transferases influences cell shape and viability. J Bacteriol. 1997;179:4959–4961. doi: 10.1128/jb.179.15.4959-4961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Roethlisberger P, Iida-Tanaka N, Hollemeyer K, Heinzle E, Ishizuka I, Fischer W. Unique poly(glycerophosphate) lipoteichoic acid and the glycolipids of Streptococcus sp. closely related to Streptococcus pneumoniae. Eur J Biochem. 2000;267:5520–5530. doi: 10.1046/j.1432-1327.2000.01613.x. [DOI] [PubMed] [Google Scholar]
- 48.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 49.Shimojima M, Ohta H, Iwamatsu A, Masuda T, Shioi Y, Takamiya K. Cloning of the gene for monogalactosyl diacylglycerol synthase and its evolutionary origin. Proc Natl Acad Sci USA. 1997;94:333–337. doi: 10.1073/pnas.94.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Spatafora G, Sheets A M, June R, Luylmbezi D, Howard K, Holbert R, Barnard D, El Janne M, Hudson M C. Regulated expression of the Streptococcus mutans dlt genes correlate with intracellular polysaccharide accumulation. J Bacteriol. 1999;181:2363–2372. doi: 10.1128/jb.181.8.2363-2372.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Studier F W, Rosenberg A H, Dunn J J, Dubendorff J W. Use of T7 RNA polymerase to direct expression of cloned genes. Methods Enzymol. 1990;185:60–89. doi: 10.1016/0076-6879(90)85008-c. [DOI] [PubMed] [Google Scholar]
- 52.Sutcliffe I C, Shaw N. Atypical lipoteichoic acids of gram-positive bacteria. J Bacteriol. 1991;173:7065–7069. doi: 10.1128/jb.173.22.7065-7069.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Taron D J, Childs III W C, Neuhaus F C. Biosynthesis of d-alanyl-lipoteichoic acid: role of diglyceride kinase in the synthesis of phosphatidylglycerol for chain elongation. J Bacteriol. 1983;154:1110–1116. doi: 10.1128/jb.154.3.1110-1116.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Vikström S, Li L, Wieslander Å. The nonbilayer/bilayer lipid balance in membranes: regulatory enzyme in Acholeplasma laidlawii is stimulated by metabolic phosphates, activator phospholipids, and double-stranded DNA. J Biol Chem. 2000;275:9296–9302. doi: 10.1074/jbc.275.13.9296. [DOI] [PubMed] [Google Scholar]
- 55.Wecke J, Perego M, Fischer W. d-Alanine deprivation of Bacillus subtilis teichoic acids is without effect on cell growth and morphology but affects autolytic activity. Microb Drug Resist. 1997;2:2953–2960. doi: 10.1089/mdr.1996.2.123. [DOI] [PubMed] [Google Scholar]