Abstract
Mutations in any one of three genes, kdpA, -B, or -C, in Escherichia coli abolish the activity of Kdp, a multisubunit K+-ATPase that belongs to the P-type ATPase family of cation transporters. We found in this study that expression in vivo of a 135-amino-acid-long N-terminal fragment (KdpA′), less than one-quarter the length of native KdpA, was able to mediate an improvement in K+-limited growth rates in two different contexts, even in the absence of both KdpC and the ATPase subunit KdpB. The first context was when KdpA′ was overexpressed in cells from a heterologous inducible promoter, and the second was when KdpA′ was provided with a C-terminally altered extension (following a spontaneous genetic rearrangement). Our results suggest that KdpA′ provides an incipient pathway for K+ translocation which can serve to transport K+ into the cells in response to the cytoplasmic membrane potential.
The intracellular concentration of K+ in Escherichia coli under ordinary growth conditions is around 150 mM, and there is evidence that several metabolic activities occur optimally at this concentration of K+. In order to cope with much lower environmental concentrations of K+ ([K+]e), E. coli cells possess several active transport systems for K+ uptake (29). They include (i) TrkA (which has subsequently been shown to comprise two related yet distinct uptake systems, TrkG and TrkH), (ii) TrkD (also called Kup), and (iii) Kdp, which are rendered defective in trkA, trkD, and kdp mutants, respectively.
The Kdp transporter has a Km for K+ of around 2 μM, and its synthesis is induced (at the transcriptional level) only under K+-limiting growth conditions (reviewed in references 11 and 28). Transcriptional control of Kdp is effected by a pair of proteins, KdpD and KdpE, which constitute a dual-component regulatory system. The Kdp transporter belongs to the family of P-type ATPases (for a review, see reference 23), and comprises four subunits (the numbers of amino acid residues are indicated in parentheses): KdpF (29), KdpA (557), KdpB (682), and KdpC (190); the proteins are encoded by the appropriately designated genes organized as a single operon in the order kdpFABC, with the promoter-operator region situated upstream of kdpF. Mutations in any one of the genes kdpA, -B, and -C abolish Kdp transporter activity; on the other hand, mutations in kdpF, which represents a very short open reading frame (ORF), have no discernible phenotype (12). KdpB is the ATPase catalytic subunit, and KdpA has been implicated in binding of the substrate (K+) as well as providing the path for its translocation across the cytoplasmic membrane (6, 7).
Strains simultaneously defective in the Kdp, TrkA, and TrkD transporters can grow only in medium with a sufficiently high [K+]e. We report that a polypeptide (KdpA′) comprising the N-terminal 135 amino acid residues of KdpA is sufficient to permit the triple-transporter-defective cells to grow at lower [K+]es, even in the absence of the KdpB and KdpC polypeptides. Our studies suggest that KdpA′ may be able by itself to mediate K+ translocation and K+ uptake across the membrane in response to the cytoplasmic-membrane potential.
Strains, plasmid vectors, and growth conditions.
The E. coli K-12 strains that were used are listed in Table 1. Cloned plasmid derivatives were constructed from the following three vectors: pUC18 (high copy number; ampicillin resistant) (26), pMU575 (single copy number with lacZ reporter gene; trimethoprim resistant) (2), and pTrc99A (for isopropyl β-d-thiogalactopyranoside [IPTG]-inducible gene expression; ampicillin-resistant) (1).
TABLE 1.
E. coli K-12 strains
| Strain | Genotypea | Reference |
|---|---|---|
| TK2205 | lacZ(Am) ΔkdpABC5 | 25 |
| TL1105A | Δlac kdpA::[Mu lacZ(λ)] | 19 |
| GJ1400 | TL1105A kdp-208 | This study |
| GJ1413 | Δlac kdpA::[Mu lacZ4525::Tn10dKan(λ)] | This study |
| GJ1414 | GJ1413 kdp-208 | This study |
| GJ1417 | TK2205 Δ(argF-lac)U169 [λimm21] | This study |
Genotype designations are as in the work of Berlyn (4). All strains are F−. Allele numbers are given where they are known. In addition to the listed mutations, all strains are thi rha nagA trkA405 trkD1.
Media and growth conditions were essentially as described previously (27). Unless otherwise specified, the growth temperature was 30°C and the medium pH was 7. For growth rate experiments, phosphate-buffered minimal media with 0.2% glucose as a C source and with reciprocally varying concentrations of Na+ and K+ were prepared as described previously (10) by mixing together 115 mM K+-phosphate medium with 115 mM Na+-phosphate medium in the appropriate proportions to achieve the desired [K+]e. Phosphate-buffered minimal medium of pH 6.2 was also prepared similarly, after appropriate adjustments in the ratios of monobasic to dibasic phosphates in the Na+ and K+ stock buffers (26). Trimethoprim was used at a final concentration of 30 μg/ml; other antibiotics were added at the concentrations specified earlier (3).
The procedures for P1 transduction (13), in vitro DNA manipulations and transformation (26), and determination of β-galactosidase activity in cultures (22, 27) were as described previously.
Isolation and genetic characterization of kdp-208 mutant.
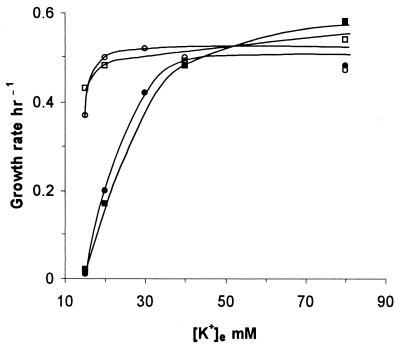
The starting point for this study was the isolation, from a kdp trkA trkD (that is, triple-transporter-defective) strain, TL1105A, of a spontaneous mutant, GJ1400, following selection for growth on medium with 10 mM [K+]e. The growth rates of GJ1400 and TL1105A were comparable in media of high [K+]e (Fig. 1), whereas the former grew significantly faster than the latter at low [K+]e (up to 40 mM) in liquid media (Fig. 1) as well as on agar plates (data not shown and Fig. 2). These results suggested that the growth advantage of GJ1400 is K+ specific, and we refer to it as the K+-sparing phenotype.
FIG. 1.
K+-sparing phenotype in liquid cultures. Growth rates of isogenic pairs of strains are plotted as a function of [K+]e. The pairs were TL1105A (●) and its kdp-208 derivative GJ1400 (○) and transformants of TL1105A with either plasmid vector pTrc99A (■) or its derivative, pHYD724, carrying the cloned parental kdpA′ ORF (□). All cultures were grown in media of pH 7 at 34°C; for the latter pair of strains, the media were additionally supplemented with ampicillin and 0.03 mM IPTG.
FIG. 2.
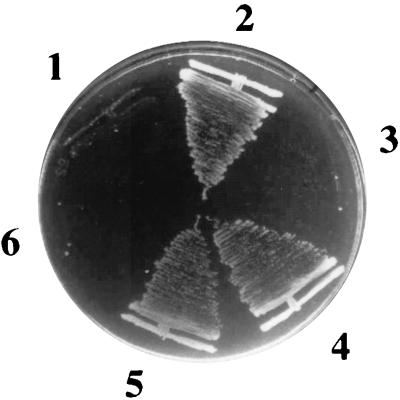
K+-sparing plate phenotype associated with kdp-208. The following isogenic pairs of strains (designated parental and kdp-208 within each pair) were streaked on medium containing 7 mM [K+]e and incubated for 40 h. Sectors 1 and 2, lysogens of GJ1417 with phages λpTL1105 (parental) and λpGJ1400 (kdp-208), respectively; sectors 3 and 4, transformants of TK2205 with pHYD712 (parental) and pHYD711 (kdp-208), respectively; and sectors 5 and 6, pHYD700 transformants of GJ1414 (kdp-208) and GJ1413 (parental), respectively.
Phage P1 transductional mapping experiments indicated that the responsible mutation in GJ1400 is tightly linked to the kdpFABC operon (data not shown), and the mutation was designated kdp-208. The mutation disrupting Kdp transporter function in parental strain TL1105A is a Mu d1lac(λ) insertion in kdpA (19), and we used the genetic technique of F′(Ts) lac-mediated chromosome mobilization described earlier (3, 13) to determine whether kdp-208 is upstream or downstream of the lacZYA genes resident within kdpA. Our results indicated that kdp-208 is upstream of (that is, promoter proximal to) the lac genes (data not shown).
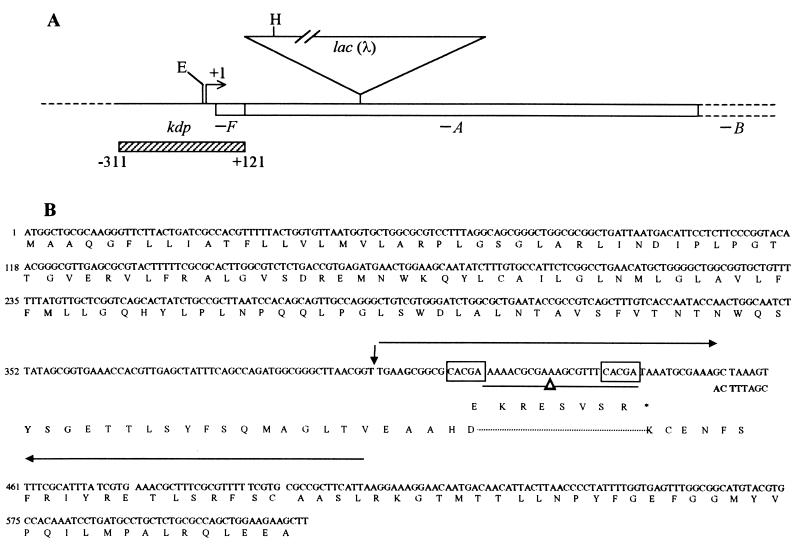
A 432-bp region extending from nucleotide position −311 (relative to the start site of kdp transcription [30], taken as +1) up to the second codon of kdpA, that is, encompassing the kdp promoter-operator region (Fig. 3A), was PCR amplified from each of the strains TL1105A and its kdp-208 derivative, GJ1400. The two DNA sequences were identical. We then performed an activator titration experiment by cloning the PCR product into the multicopy plasmid vector pUC18 and introducing the resulting derivative (pHYD702) into GJ1400. (Multiple copies of the kdp operator are expected to titrate KdpE activator protein in the cells, and consistent with this prediction, introduction of pHYD702 into strain TL1105A was associated with a reduction of β-galactosidase specific activity expressed from the chromosomal kdp-lac fusion in the latter, from a value of 103 to around 9 Miller units.) We observed that whereas a control derivative, GJ1400/pUC18, continued to exhibit a K+-sparing phenotype, GJ1400/pHYD702 no longer did so (data not shown). This result established that the K+-sparing phenotype associated with kdp-208 is dependent on KdpE-activated transcription of the chromosomal kdp locus in GJ1400.
FIG. 3.
Molecular characterization of kdp-208 mutation. (A) Proximal part (to scale) of the kdp operon, including its start site of transcription (+1), extent of the kdpF and kdpA ORFs (open bars), and location in TL1105A of kdpA::Mu d1lac(λ) insertion (shown as inverted triangle; length not to scale). The hatched bar denotes the extent of a 432-bp PCR product used in several experiments described in the text. Also marked are the EcoRI (E) and HindIII (H) sites in the kdp promoter and Mu S end, respectively, that are discussed in the text. (B) Nucleotide sequence (top strand) of the kdp::Mu-lac fusion region in parental strain TL1105A from the start of the kdpA ORF to the HindIII site in Mu S; the nucleotide numbering is indicated to the left of each line. The Mu S region sequence is taken from published data (21, 32), and the junction between the kdpA (17) and Mu S sequences is indicated by a vertical arrow. The perfect inverted repeat within Mu S is shown overlined by the pair of convergent arrows. The 22-bp deletion (-Δ-) and the sequence of the 8-nucleotide substitution that together make up the kdp-208 mutation are depicted beneath the wild-type sequence. Pentameric direct repeats in the vicinity of the deletion are boxed. Beneath the nucleotide sequence is the sequence of the conceptual translation product (in the one-letter amino acid code) for the kdp-208 mutant; also included in the region of the deletion is the short altered C-terminal sequence (ending with ∗) inferred for the polypeptide encoded by the parental strain.
Molecular characterization of kdp-208 mutation.
Taking advantage of the resident λ prophage adjacent to the kdp-lac fusion in each of the strains TL1105A and GJ1400 (Fig. 3A), we prepared specialized λ transducing phages carrying the lac fusion with the kdp promoter-proximal regions of the two strains by the method of Komeda and lino (18). The phages were designated λpTL1105 and λpGJ1400, respectively. Results from physical mapping and subcloning experiments indicated that the two phages carried around 2 and 3 kb of DNA, respectively, from the chromosomal kdp locus upstream of the Mu-lac insertion in kdpA (data not shown). When isogenic lysogens of the two phages were constructed in another triple-transporter-defective strain, GJ1417, and compared on low-[K+]e agar plates, the latter exhibited the K+-sparing phenotype (Fig. 2). This result suggested that (i) the DNA region cloned from GJ1400 on λpGJ1400 phage carries the kdp-208 mutation and (ii) exhibition of the K+-sparing phenotype by the kdp-208 mutant does not require KdpB and KdpC (since GJ1417 is derived from strain TK2205, which bears a chromosomal kdpABC deletion [25]).
We exploited the presence of an EcoRI site in the kdp promoter region to subclone an EcoRI-HindIII fragment from each of the phages λpTL1105 and λpGJ1400 into a single-copy-number trimethoprim resistance plasmid (pHYD700) that carried the PCR-amplified 432-bp kdp promoter-operator region. We thus constructed a pair of plasmids (pHYD712 and pHYD711) that carried the contiguous DNA segments from the kdp loci of the parental and mutant strains, respectively, extending in each case from position -311 up to the HindIII site situated 208 bp within the Mu S end of the kdpA::lac fusion (Fig. 3A). When the plasmids were introduced into strain TK2205, the latter but not the former conferred a K+-sparing phenotype (Fig. 2).
Nucleotide sequence determination of the insert DNAs of pHYD712 and pHYD711 established the following (Fig. 3B). (i) The Mu-lac insertion in parental strain TL1105A occurred immediately after the second base of codon 135 in the kdpA ORF. (ii) The kdp-208 mutation present on plasmid pHYD711 does not alter the kdp sequence at all but instead is a complex rearrangement within the Mu S end region downstream of codon 135 of kdpA. The mutation comprises a 22-bp deletion (between a pair of pentameric direct repeats) in one arm of the 48-bp inverted repeat located at the Mu S end along with a change of the 8-bp loop sequence to its inverse complement (Fig. 3B). We subsequently verified the existence of the same sequence alterations in the original mutant, GJ1400, following PCR amplification of the chromosomal kdp locus in the strain (data not shown).
K+-sparing phenotype is also elicited by overproduction of KdpA′ from TL1105A.
As depicted in Fig. 3B, the Mu-lac insertion mutation in the kdp operon of TL1105A is expected to result in synthesis of a truncated KdpA′ polypeptide 135 amino acids long (with an additional 12 amino-acid-long C-terminal extension provided by the Mu S end sequence). The kdp-208 mutation which confers the K+-sparing phenotype in GJ1400 is predicted to alter and substantially lengthen the C-terminal extension for KdpA′ (Fig. 3B). Two alternative possibilities that can be envisaged are (i) that the C-terminally altered KdpA′ in GJ1400 has acquired a completely novel function that contributes to the K+-sparing phenotype and (ii) that the C-terminal alteration merely serves to enhance an activity that is innate or latent in the KdpA′ polypeptide expressed by the parent TL1105A, perhaps by increasing the protein's stability. In order to distinguish between these possibilities, we undertook an experiment aimed at achieving controlled overproduction of the TL1105A-derived KdpA′ polypeptide in triple-transporter-defective cells.
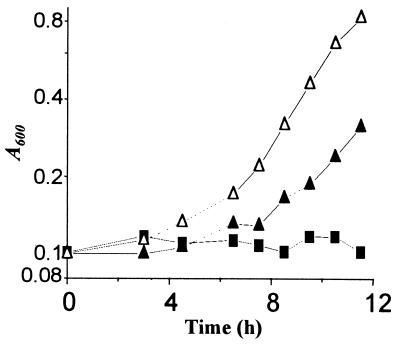
For this purpose, we subcloned the DNA fragment from λpTL1105 that extends from the EcoRI site in the middle of the kdp promoter to the HindIII site in the Mu S end (Fig. 3A) into the appropriate sites of plasmid vector pTrc99A (1). The plasmid (pHYD724) carries both the lacIq gene and the trc promoter (inducible by IPTG) driving expression of the truncated KdpA′ polypeptide encoded by the parental strain, TL1105A. The growth rates of TL1105A/pHYD724 were compared with those of TL1105A/pTrc99A (as a control) in media of different [K+]es, each supplemented with 0.03 mM IPTG as an inducer (Fig. 1). The results indicated that, as with the kdp-208 mutation, IPTG-induced expression of the native KdpA′ polypeptide from pHYD724 was also associated with an increased growth rate of the triple-transporter-defective strain specifically in media of low [K+]e. In an experiment performed in medium of 7 mM [K+]e, a dose dependence in growth rate improvement of TL1105A/pHYD724 with increasing IPTG concentration was demonstrable (Fig. 4; compare growth curves at 0, 10, and 40 μM IPTG). Very similar growth results were also obtained in another triple-transporter-defective strain, TK2205, that had been transformed with plasmid pHYD724, with the difference that a somewhat higher IPTG concentration was needed to sustain a given growth rate at the low [K+]e (data not shown). These findings therefore served to validate the hypothesis that the KdpA′ polypeptide of TL1105A itself possesses the innate ability to confer a K+-sparing phenotype.
FIG. 4.
K+-sparing phenotype associated with IPTG-induced overexpression of parental KdpA′. Growth curves for strain TL1105A/pHYD724 in 7 mM [K+]e medium supplemented with 0 (■), 10 (▴), or 40 (▵) μM IPTG are shown.
K+-sparing phenotype is associated with a repressing signal for kdp-lac expression.
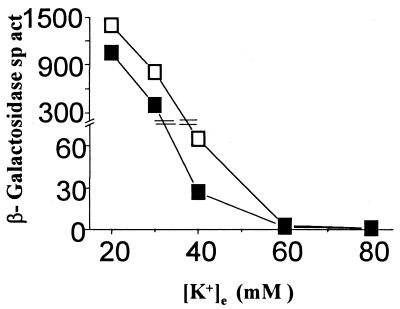
As mentioned above, K+ limitation serves as the signal for induction of kdp operon transcription. We tested whether the kdp-208 mutation, by conferring the K+-sparing phenotype, also affects the strength of the signal controlling kdp expression in media of low [K+]e. Although the kdp-208 derivative GJ1400 also carries a chromosomal kdp-lac fusion, we chose to study reporter gene expression in trans so as to avoid any confounding cis effect caused by the mutation on the lac genes downstream of it. For this purpose, the lacZ gene in cis was inactivated by Tn10dKan insertion in GJ1400 as well as in its parent, TL1105A (as a control), to generate the strains GJ1414 and GJ1413, respectively, and the single-copy-number plasmid pHYD700, carrying the PCR-amplified 432-bp kdp promoter-operator region cloned upstream of lacZ, was introduced into the two strains. As expected, GJ1414/pHYD700 exhibited the K+-sparing phenotype (Fig. 2). The results, shown in Fig. 5, indicated that expression of kdp-lac in this strain was lowered relative to that in the control GJ1413/pHYD700 precisely at the low [K+]es at which kdp-208 confers an improvement in growth rate in GJ1400.
FIG. 5.
K+-sparing phenotype and kdp-lac repression in trans. Plotted are the β-galactosidase specific activities (in Miller units [22]) as a function of [K+]e for isogenic strains carrying the kdp-lac plasmid pHYD700: GJ1413 (parental [□]) and GJ1414 (kdp-208 [■]).
Likewise, we showed that IPTG-induced overexpression of native KdpA′ from plasmid pHYD724 is also associated with concomitant repression of kdp-lac expression in trans. When the pHYD724 derivative of strain TL1105A (which carries the chromosomal wild-type kdp-lac fusion) was grown in 30 mM [K+]e medium supplemented with 0, 10, or 40 μM IPTG, a dose-dependent reduction in kdp-lac expression was observed (with β-galactosidase specific activity values of 273, 90, and 45 Miller units, respectively).
GJ1400 does not exhibit K+-sparing phenotype in low-pH medium.
As discussed below, the results so far had suggested that the N-terminal KdpA′ fragment is able to mediate K+ uptake even in the absence of the ATPase subunit KdpB. In order to test whether the K+-sparing phenotype associated with kdp-208 is dependent on the cytoplasmic membrane potential, Δψ, we examined the growth rates of TL1105A and its kdp-208 derivative, GJ1400, in low-pH medium at different values of [K+]e. It is known that the Δψ component of the homeostatically maintained proton-motive force is reduced under these conditions (16).
It is also known that a higher [K+]e is required to sustain a given growth rate of triple-transporter-defective strains such as TL1105A in low-pH media (3, 20). We found that, in medium of pH 6.2 at the three different values of [K+]e tested with correspondingly varying degrees of severity of K+-limited growth of TL1105A, the kdp-208 derivative, GJ1400, failed to exhibit a K+-sparing phenotype. The [K+]e values and the corresponding growth rates (per hour) of TL1105A and GJ1400 were, respectively, 40 mM, <0.05, and <0.05; 60 mM, 0.13, and 0.16; and 80 mM, 0.26, and 0.23. These results suggest that the improvement in growth rate associated with kdp-208 at low [K+]e occurs only under conditions where Δψ is substantial.
Discussion.
Strains simultaneously defective in the kdp, trkA, and trkD loci exhibit a very low level of residual, nonsaturable K+ uptake activity called TrkF (24), which might represent gratuitous transport of the ion through several unrelated transport systems (cited as unpublished observations of Buurman et al. in reference 7). The results of the present study suggest that in such strains, a truncated KdpA′ polypeptide 135 amino acids in length (in the absence of KdpB and KdpC) can contribute to a K+-sparing phenotype in vivo in two distinct contexts. The first is under conditions when synthesis of the polypeptide within the cell is increased by regulated expression of the ORF from a heterologous (trc) promoter, and the second is that associated with a C-terminal extension to the polypeptide as a consequence of the kdp-208 mutation (in conjunction with KdpDE-mediated activation of kdpA′ transcription). The kdpA′ ORF extension brought about by kdp-208 extends even beyond the HindIII site at the Mu S end (Fig. 3B), but we have been able to demonstrate in subcloning experiments that the placement of tandem stop codons immediately beyond the HindIII site does not abrogate the K+-sparing phenotype associated with kdp-208 (data not shown).
The fact that the K+-sparing phenotype (in the contexts of both KdpA′ overexpression and the kdp-208 mutation) is correlated with generation of an appropriate repressing signal for control of kdp transcription indicates that the K+ concentration within the cells is increased under these conditions. Although formally this could occur by either increased uptake or decreased efflux of the cation, we believe that the former is very much more likely given that KdpA is a known component of an active K+ uptake system in E. coli. A plausible model is that KdpA′ is able to constitute a K+ carrier or uniporter that serves to concentrate K+ intracellularly in response to the Δψ component of the proton-motive force. Similar explanations have been offered for the K+-sparing phenotype in E. coli associated with the expression of various tetracycline efflux proteins (8, 14, 15) as well as of an inwardly rectifying K+ channel of Arabidopsis (31). Previous studies of the topological disposition of native KdpA suggest that its N-terminal 135-amino-acid segment (which constitutes KdpA′) has two transmembrane spans, and there is also genetic evidence for a periplasmic K+-binding site located in this region (6). Dose-dependent increase in KdpA′-mediated K+ uptake may be explained on the assumption that the ability of the carrier to carry out bulk ion movements is rate limiting and therefore that the system at all times is operating far from equilibrium.
Should the KdpA′ carrier hypothesis be valid, one needs to consider whether the truncated polypeptide alone is sufficient to mediate ion transport or whether it is interacting with other polypeptides for the purpose. Notable candidates for the latter are KdpF (whose coordinate expression with KdpA′ is expected to occur from all the constructs employed in the present study) and Kch, a putative K+ channel in E. coli. However, mutations in neither kdpF (12) nor kch (5) confer any demonstrable phenotype. Biochemical and structural studies of KdpA′ in heterologous or reconstituted membrane systems may help address some of these issues. Such studies may also provide clues to the possible mechanism by which ion translocations occur through native Kdp and the other K+ symporters with which it has been postulated to share evolutionary kinship (9), as well as through members of the larger P-type ATPase family.
Acknowledgments
We acknowledge Wolf Epstein, N. C. Mandal, and Jim Pittard for strains, phage, and plasmids, and Mehar Sultana and N. Nagesh for assistance with synthesis of oligonucleotide primers and automated DNA sequencing. We also thank Amit Chattopadhyay for useful discussions.
A.A.S. was the recipient of Junior and Senior Research Fellowships of the Council of Scientific and Industrial Research. J. G. is Honorary Faculty Member of the Jawaharlal Nehru Centre for Advanced Scientific Research.
REFERENCES
- 1.Amann E, Ochs B, Abel K J. Tightly regulated tac promoter vectors useful for the expression of unfused and fused proteins in Escherichia coli. Gene. 1988;69:301–315. doi: 10.1016/0378-1119(88)90440-4. [DOI] [PubMed] [Google Scholar]
- 2.Andrews A E, Lawley B, Pittard A J. Mutational analysis of repression and activation of the tyrP gene in Escherichia coli. J Bacteriol. 1991;173:5068–5078. doi: 10.1128/jb.173.16.5068-5078.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Asha H, Gowrishankar J. Regulation of kdp operon expression in Escherichia coli: evidence against turgor as signal for transcriptional control. J Bacteriol. 1993;175:4528–4537. doi: 10.1128/jb.175.14.4528-4537.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Berlyn M K B. Linkage map of Escherichia coli K-12, edition 10: the traditional map. Microbiol Mol Biol Rev. 1998;62:814–984. doi: 10.1128/mmbr.62.3.814-984.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Booth I R, Jones M A, McLaggan D, Nikolaev Y, Ness L S, Wood C M, Miller S, Tötemeyer S, Ferguson G P. Bacterial ion channels. In: Konings W N, Kaback H R, Lolkema J S, editors. Handbook of biological physics. Vol. 2. Amsterdam, The Netherlands: Elsevier; 1996. pp. 693–729. [Google Scholar]
- 6.Buurman E T, Kim K-T, Epstein W. Genetic evidence for two sequentially occupied K+ binding sites in the Kdp transport ATPase. J Biol Chem. 1995;270:6678–6685. doi: 10.1074/jbc.270.12.6678. [DOI] [PubMed] [Google Scholar]
- 7.Dorus S, Mimura H, Epstein W. Substrate-binding clusters of the K+-transporting Kdp ATPase of Escherichia coli investigated by amber suppression scanning mutagenesis. J Biol Chem. 2001;276:9590–9598. doi: 10.1074/jbc.M009365200. [DOI] [PubMed] [Google Scholar]
- 8.Dosch D C, Salvacion F F, Epstein W. Tetracycline resistance element of pBR322 mediates potassium transport. J Bacteriol. 1984;160:1188–1190. doi: 10.1128/jb.160.3.1188-1190.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Durell S R, Bakker E P, Guy H R. Does the KdpA subunit from the high affinity K+-translocating P-type KDP-ATPase have a structure similar to that of K+ channels? Biophys J. 2000;78:188–199. doi: 10.1016/S0006-3495(00)76584-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Epstein W, Kim B S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971;108:639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Epstein W, Walderhaug M O, Polarek J W, Hesse J E, Dorus E, Daniel J M. The bacterial Kdp K+-ATPase and its relation to other transport ATPases, such as the Na+/K+- and Ca2+-ATPases in higher organisms. Phil Trans R Soc Lond. 1990;326:479–487. doi: 10.1098/rstb.1990.0026. [DOI] [PubMed] [Google Scholar]
- 12.Gassel M, Mollenkamp T, Puppe W, Altendorf K. The KdpF subunit is part of the K(+)-translocating Kdp complex of Escherichia coli and is responsible for stabilization of the complex in vitro. J Biol Chem. 1999;274:37901–37907. doi: 10.1074/jbc.274.53.37901. [DOI] [PubMed] [Google Scholar]
- 13.Gowrishankar J. Identification of osmoresponsive genes in Escherichia coli: evidence for participation of potassium and proline transport systems in osmoregulation. J Bacteriol. 1985;164:434–445. doi: 10.1128/jb.164.1.434-445.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Griffith J K, Kogoma T, Corvo D L, Anderson W L, Kazim A L. An N-terminal domain of the tetracycline resistance protein increases susceptibility to aminoglycosides and complements potassium uptake defects in Escherichia coli. J Bacteriol. 1988;170:598–604. doi: 10.1128/jb.170.2.598-604.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guay G G, Tuckman M, McNicholas P, Rothstein D M. The tet(K) gene from Staphylococcus aureus mediates the transport of potassium in Escherichia coli. J Bacteriol. 1993;175:4927–4929. doi: 10.1128/jb.175.15.4927-4929.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harold F M, Maloney P C. Energy transduction by ion currents. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 283–306. [Google Scholar]
- 17.Hesse J E, Wieczorek L, Altendorf K, Reicin A S, Dorus E, Epstein W. Sequence homology between two membrane transport ATPases, the Kdp-ATPase of Escherichia coli and the Ca2+-ATPase of sarcoplasmic reticulum. Proc Natl Acad Sci USA. 1984;81:4746–4750. doi: 10.1073/pnas.81.15.4746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Komeda Y, Iino T. Regulation of expression of the flagellin gene (hag) in Escherichia coli K-12: analysis of hag-lac gene fusions. J Bacteriol. 1979;139:721–729. doi: 10.1128/jb.139.3.721-729.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Laimins L A, Rhoads D B, Epstein W. Osmotic control of kdp operon expression in Escherichia coli. Proc Natl Acad Sci USA. 1981;78:464–468. doi: 10.1073/pnas.78.1.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malli R, Epstein W. Expression of the Kdp ATPase is consistent with regulation by turgor pressure. J Bacteriol. 1998;180:5102–5108. doi: 10.1128/jb.180.19.5102-5108.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metcalf W W, Steed P M, Wanner B L. Identification of phosphate starvation-inducible genes in Escherichia coli K-12 by DNA sequence analysis of psi::lacZ(MudI) transcriptional fusions. J Bacteriol. 1990;172:3191–3200. doi: 10.1128/jb.172.6.3191-3200.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Miller J H. A short course in bacterial genetics: a laboratory manual and handbook for Escherichia coli and related bacteria. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1992. [Google Scholar]
- 23.Møller J V, Juul B, Maire M. Structural organization, ion transport, and energy transduction of P-type ATPases. Biochim Biophys Acta. 1996;1286:1–51. doi: 10.1016/0304-4157(95)00017-8. [DOI] [PubMed] [Google Scholar]
- 24.Rhoads D B, Epstein W. Energy coupling to net K+ transport in Escherichia coli K-12. J Biol Chem. 1977;252:1394–1401. [PubMed] [Google Scholar]
- 25.Rhoads D B, Laimins L, Epstein W. Functional organization of the kdp genes of Escherichia coli K-12. J Bacteriol. 1978;135:445–452. doi: 10.1128/jb.135.2.445-452.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1989. [Google Scholar]
- 27.Sardesai A A, Gowrishankar J. trans-Acting mutations in loci other than kdpDE that affect kdp operon regulation in Escherichia coli: effects of cytoplasmic thiol oxidation status and nucleoid protein H-NS on kdp expression. J Bacteriol. 2001;183:86–93. doi: 10.1128/JB.183.1.86-93.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siebers A, Altendorf K. K+-translocating Kdp-ATPases and other bacterial P-type ATPases. In: Bakker E P, editor. Alkali cation transport systems in prokaryotes. Boca Raton, Fla: CRC Press; 1993. pp. 225–252. [Google Scholar]
- 29.Silver S. Transport of inorganic cations. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: American Society for Microbiology; 1996. pp. 1091–1102. [Google Scholar]
- 30.Sugiura A, Nakashima K T, Mizuno T. Clarification of the structural and functional features of the osmoregulated kdp operon of Escherichia coli. Mol Microbiol. 1992;6:1769–1776. doi: 10.1111/j.1365-2958.1992.tb01349.x. [DOI] [PubMed] [Google Scholar]
- 31.Uozumi N, Nakamura T, Schroeder J I, Muto S. Determination of transmembrane topology of an inward-rectifying potassium channel from Arabidopsis thaliana based on functional expression in Escherichia coli. Proc Natl Acad Sci USA. 1998;95:9773–9778. doi: 10.1073/pnas.95.17.9773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zieg J, Kolter R. The right end of MudI(Ap,lac) Arch Microbiol. 1989;153:1–6. doi: 10.1007/BF00277532. [DOI] [PubMed] [Google Scholar]