Abstract
Domestic chicks walk within 3–4 hr after hatching following 21 days of incubation. However, differences in light exposure can vary incubation duration. Based on pilot studies, we predicted that there would be a positive relationship between incubation duration and locomotor competence at hatching. Embryos were incubated in one of three conditions that varied light duration and intensity, and overground locomotor performance was tested on the day of hatching. Chicks incubated in continuous bright light hatched 1–2 days earlier than chicks incubated in less or no light. Kinematic findings indicated that locomotor skill was similar across incubation conditions and led us to reject our hypothesis. We propose that light may accelerate locomotor development without adversely affecting skill. Our findings raise two important implications for future studies: whether light exposure accelerates locomotor circuit development; and/or it unmasks adaptive motor skill by accelerating development of other physiological systems.
Keywords: motor development, interlimb coordination, bipedal, prenatal condition
INTRODUCTION
Domestic chicksare precocious walkers.Within a few hours after hatching they walk overground and negotiate physical barriers like ramps and miniature stairs. Chicks typically hatch at 21 days of incubation (Hamburger & Hamilton, 1951, reprinted 1992; Noy & Sklan, 1997; Romijn & Roos, 1938) but they can be induced to hatch 1–2 days earlier when exposed to bright light during incubation (Fairchild & Christensen, 2000; Ghatpande, Ghatpande, & Khan, 1995; Lauber, 1975; Siegel, Isakson, Coleman, & Huffman, 1969; Shutze, Lauber, Kato, & Wilson, 1962). Reports also indicate that viability of hatchlings incubated in continuous light is equal to that of chicks incubated in the dark (Lauber & Shutze, 1964). These features make chicks a valuable model for examining the impact of light on locomotor development. Lessons learned may provide useful insights relevant to concerns regarding bright light exposure during intensive care of the neonate (Brandon, Holditch-Davis, & Belyea, 2002; Miller, White, Whitman, O’Callaghan, & Maxwell, 1995).
It is not known if chicks incubated in bright light acquire the same level of locomotor competence at hatching as chicks incubated in less light or no light. Studies examining the effects of light exposure during embryogenesis indicate that bright light accelerates morphological development. For example, light exposure during the first 40 hr of embryogenesis can significantly increase the number of nuclei in the chick blastoderm (Ghatpande et al., 1995). Light exposure during the first days of embryogenesis can increase body weight of embryos by 17% compared to embryos incubated in the dark (Lauber, 1975). Light exposure during incubation can also modify posthatching behavior in chicks. For example, light exposure during the last week of incubation can increase the rate of feather picking between hatchlings, a social behavior that appears to be important for discriminating between familiar and unfamiliar individuals (Riedstra & Groothuis, 2004). Further, it has also been shown that 4 hr of light exposure (500–800 lx) on embryonic day 19 (E19) is sufficient to induce lateralization of some brain functions that regulate attack and copulation behaviors (Zappia & Rogers, 1983).
A number of studies provide evidence that control of stepping is established by the time of hatching when embryos are incubated under standard conditions. Electromyographic (EMG) and kinematic studies of leg movements during embryonic motility indicate that locomotor-related circuits for stepping are established 1–3 days before hatching (Bradley, Ryu, & Lin, 2008; Bradley, Solanki, & Zhao, 2005; Ryu & Bradley, 2009). EMG and kinematic studies also indicate that intralimb and interlimb coordination during locomotion are well established within 1 or more days after hatching (Johnston & Bekoff, 1992, 1996; Jacobson & Hollyday, 1982a). Both intralimb and interlimb EMG patterns for stepping are expressed even in the absence of sensory or descending inputs (Bekoff, Kauer, Fulstone, & Summers, 1989; Bekoff, Nusbaum, Sabichi, & Clifford, 1987; Jacobson & Hollyday, 1982b). However, locomotor skill is not mature at hatching. In a kinetic study of intralimb control, hatchlings needed almost 14 days of practice to acquire weight transfer skills for energy efficient walking (Muir, Gosline, & Steeves, 1996). To date, studies have not examined the acquisition of interlimb coordination during overground walking at hatching. Our study extends previous studies of locomotor development in chicks by providing the first analyses of the spatial and temporal parameters for interlimb coordination during unrestrained overground walking on the day of hatching.
Our primary goal in this study was to determine if interlimb control for locomotion is accelerated when embryogenesis is accelerated by light exposure. Using the kinematic methods typically used for human gait analysis, this study provides analyses of unconstrained overground walking in 1-day-old chicks. Based on pilot studies, we predicted that locomotor skills would be negatively impacted by a light-induced shortening of the incubation period and positively impacted by a dark-induced delay.
METHODS
Subjects
Fertile Leghorn chicken (Gallus gallus) eggs were obtained from a local hatchery and incubated in force draft, humidified incubators at standard temperature (37.5°C) and humidity (62%). Onset of incubation was considered embryonic day E0. All eggs were moved to nonrotating shelves within the same incubator 3 days before anticipated hatching. The eggs were examined at 2 hr intervals thereafter to determine approximate time of pipping, a crack in the shell indicating the onset of hatching, and when hatching was completed. After hatching, chicks were weighed and moved to a brooder (47 cm × 47 cm × 20 cm). At the end of data collection, animals were euthanized and morphologic maturation was estimated. Two measures, the length of third toe and the length of left and right tibia, were obtained as both measures are indicators of physical size on the day of hatching (Hamburger & Hamilton, 1951, reprinted 1992; Romanoff, 1960). All activities were approved by the University Institutional Animal Care and Use Committee.
Incubation Conditions
Prior to the onset of incubation, eggs were weighed and randomly assigned to one of three incubation conditions: continuous light exposure 24 hr daily (24 L) at 4,000–7,000 lx; 12 hr light exposure daily (12L) at 650–3,000 lx; continuous dark exposure 24 hr daily (24D) at ≤1 lx. The duration and exposure intensity for 24L conditions were selected because they have been shown to significantly accelerate embryogenesis (Coleman & McDaniel, 1976; Ghatpande et al., 1995; Lauber, 1975; Siegel et al., 1969). The 12L exposure conditions were selected to approximate normal indoor/outdoor exposure. The 24D conditions were selected as control for the effects of light exposure.
Training and Testing Procedures
On the day of hatching, chicks were weighed and prepared for kinematic analyses of interlimb coordination during overground walking. A circular marker (2.5 mm diameter) was glued to the metatarsal pads bilaterally as shown in Figure 1C. Training began 2–3 hr after hatching once the chicks’ feathers were dry. Chicks were first trained to attend to the experimenter’s voice and finger tapping. Training continued until the hatchlings consistently walked in the direction of the cues. The chicks were then allowed to rest for a few minutes.
FIGURE 1.
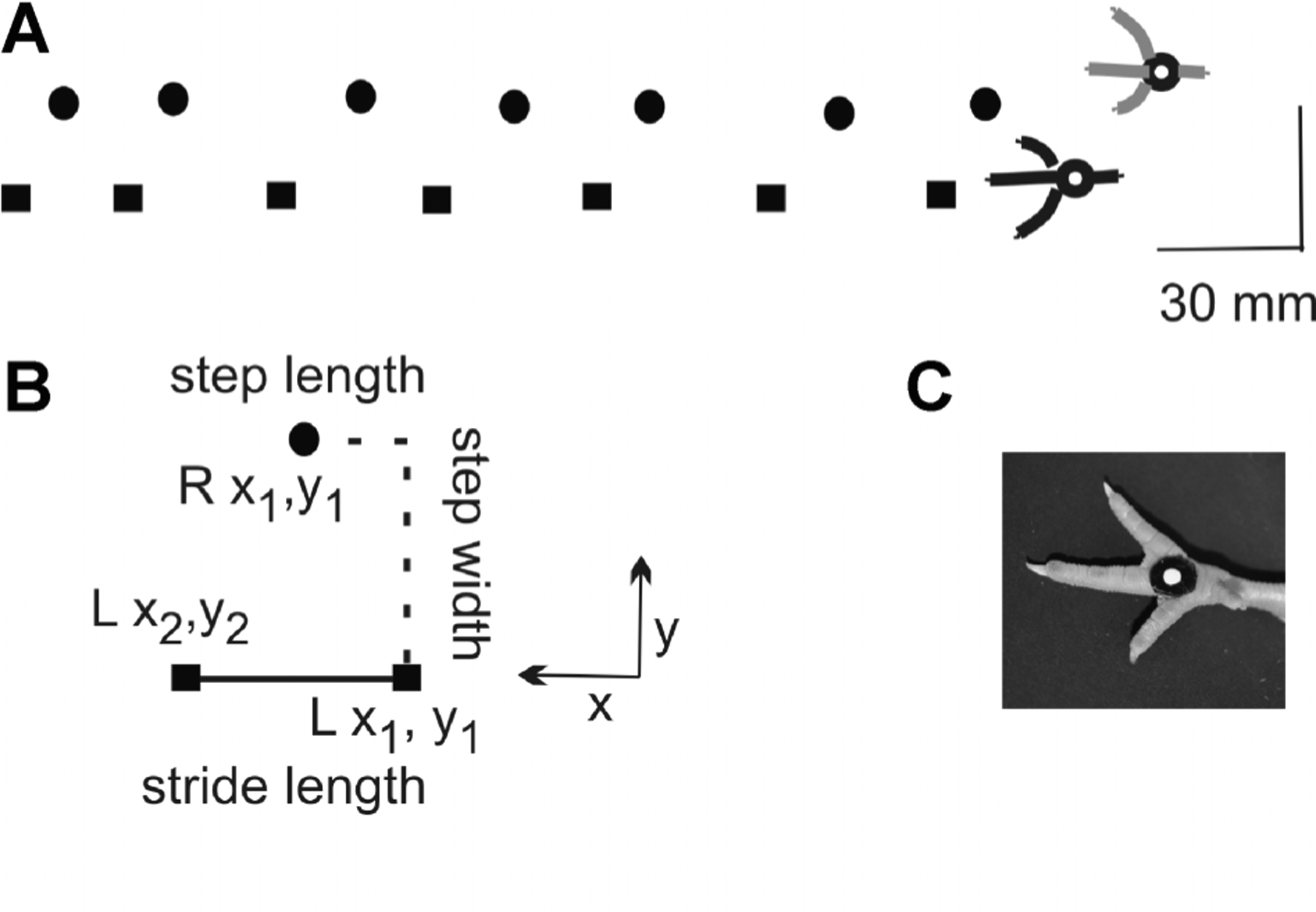
Kinematic methods. (A) Left (square) and right (circle) foot placements within the recording region are shown for one walk trial as the chick progressed from right to left. The scale bar of 30 mm applies to both x and y directions.(B) Methods for measuring spatial parameters are shown. Stride length was defined as the distance between consecutive foot strikes of the same foot (i.e., Lx2 − Lx1). Step length was equal to the distance between consecutive strikes of the left and right foot(i.e., Rx1 − Lx1). Step width was equal to the distance between consecutive left and right foot strikes in the mediolateral direction (i.e., Ry1 − Ly1). (C) Circular markers were applied to the metatarsal pads of both feet. A white dot and black contrasting surround optimized automatic tracking during digitizing of foot placements.
The chicks were then trained to walk on a Plexiglas walkway (90 cm × 9 cm × 12 cm). At the start of each training trial, they were placed for 90 s in a darkened chamber with a trap door attached to one side of the walkway. Pilot training indicated that brief confinement increased the likelihood that chicks would walk the full length of thewalkway with fewer interruptions once the trap door was lifted. The experimenter stood at the opposite end of the walkway providing voice and tapping cues to encourage uninterrupted walking. Only chicks that walked the full length of the walkway within three practice trials were selected for testing to minimize possible training effects. Performance during training was not recorded.
Chicks were tested twice on the day of hatching, the first session beginning immediately after the training and brief rest, the second session 4 hr later. Chicks were placed in the darkened chamber for 90 s at the start of each walk trial and then encouraged to walk the full length of the walkway. The procedure was repeated until chicks completed four walk trials, each having no more than one pause ≤2 s. The total number of walking attempts rarely exceeded six trials. At the beginning of the second test session, chicks were given one or two practice trials before testing resumed.
Kinematic Analysis
Test trials were recorded by a video camera positioned beneath the central region of the walkway to capture foot placements during the most stable portion of a walk trial (Fig. 1A). Initial and final steps of the walk trial occurred outside this recording region. Prior to each recording session a carpenter’s level was used to align the camera lens perpendicular with the walkway, and a circular marker (7.6 cm diameter) was video recorded to calibrate distance in the plane of the walkway (camera focal length .5 m, field of view 40 cm). Walk trials were video recorded at 30 frames/s and directly written to disk (Datapac 2K2, Run Technologies, Laguna Hill, CA).
Video files were de-interlaced during data processing to digitize metatarsal markers at 60 pictures/s and to obtain two-dimensional coordinates (x, y). Three spatial parameters were calculated: stride length, step length, and step width (Fig. 1B). Stride length was defined as the distance between consecutive foot strikes of the same foot (i.e., Lx2 − Lx1). Step length was equal to the distance between consecutive strikes of the left and right foot (i.e., Rx1 − Lx1). Step width was equal to the distance between consecutive left and right foot strikes in the mediolateral direction (i.e., Ry1 − Ly1).
Four temporal parameters were calculated: stride duration, stance duration, double limb support duration, and swing duration (Perry, 1992). Stride duration was defined as the total time between consecutive foot strikes of the same foot. Stance duration was equal to the total time the foot was in contact with the ground. Double limb support duration was equal to the time both feet were in contact with the ground. Swing duration was equal to the interval of time the foot was off the ground. Walking velocity was estimated for each trial using the ratio of average stride length to average stride duration.
The temporal stability between left and right steps during a walk trial was quantified by calculating the phase coordination angle for each stride. This method normalizes step onset time of the contralateral leg relative to the concurrent stride duration and expresses the ratio in degrees. A foot strike of the contralateral leg occurring exactly mid stride produces a phase coordination angle of 180°. The phase coordination angle is a preferred method for examining the timing of presumed coupled oscillators, such as the left and right leg during locomotion (Hamill, Haddad, & McDermott, 2000). In the event asymmetry is detected, phase coordination angles can be used to further quantify asymmetries or variable coupling between oscillators (Hamill et al., 2000; Plotnik, Giladi, & Hausdorff, 2007). Phase coordination angles were calculated for only those trials having at least three consecutive strides, each less than 2 s in duration.
Statistical Analysis
We tested for the effects of light exposure, using a between groups (three light conditions) repeated measure design (sessions I and II). Each gait parameter was averaged across all steps per walk trial and the four trial averages were used to calculate the session average for each chick. Similarly, SD was calculated for each of the four walk trials and averaged to obtain the mean SD for each session. Two-way ANOVAs were performed to test for differences in subject mean and SD across the three light conditions and between the two sessions for each parameter. Statistical significance was set at p < .05. The Student’s t-test with a Bonferroni correction for multiple comparisons was employed in post hoc comparisons.
Owing to the substantial variability characteristic of unconstrained walking, mean and median statistics were compared to determine if it was reasonable to assume a normal distribution for each data set. Regression analyses were used to compare the descriptive statistics. In 90% of the comparisons (N = 63), the regression analyses for mean and median values yielded a correlation coefficient (R2) ≥.7, with the remaining R2 ≥.5, indicating that the assumption of a normal distribution for each data set was reasonable.
RESULTS
Results describe the kinematic findings for overground walking on the day of hatching in a total sample of 30 chicks, 10 per incubation condition. Each data set represents eight successful walk trials per animal, equally divided between experiment sessions I and II. The complete kinematic data set represents 240 walk trials and 1,626 overground strides. We report the hatching results, body weight and toe length data for these animals and tibia length data for 30 hatchlings not included in locomotor testing. Results then focus on the analyses of locomotor performance for each of the three incubation conditions.
Pipping, Hatching, and Morphologic Measures
Pipping and hatching varied significantly across the three incubation conditions but measures of morphologic maturity appeared similar. One-way ANOVAs indicated that differences in age were highly significant for pipping, F(2, 27) = 63.3, p < 10−9, and hatching, F(2,27) = 58.5, p < 10−9 (Tab. 1). Embryos incubated in 24L conditions were first to pip and hatch, preceding those in 12L conditions by 1 day. Embryos incubated in 24D conditions were last to pip and hatch. One-way ANOVA analyses also indicated that neither the egg weight at E0, nor body weight at hatching varied across incubation conditions (Tab. 2). Further, one-way ANOVA analysis indicated that 3rd toe length was similar across conditions, F(2,27) = .3, p > .7. A two-way ANOVA for left and right tibia length indicated that there was a significant main effect for conditions, F(2,54) = 6.5, p < .003, but not between limbs, F(2,54) = .3, p > .5 and no significant interaction, F(2,54) = .5, p > .6. Post hoc comparisons indicated that tibia length was greater in chicks incubated in 24L conditions than chicks in 24D (p < .002) (Tab. 2).
Table 1.
Embryonic Ages at Pipping and Hatching
| Incubation Conditions | Pip age (E)* | Hatch age (E)* |
|---|---|---|
| 12L | 20.8 ± .4 | 21.7 ± .5 |
| 24L | 19.8 ± .3 | 20.4 ± .4 |
| 24D | 21.7 ± .4 | 22.7 ± .4 |
Values represent mean ± SD.
p < 10−9.
Table 2.
Morphological Data for the Incubation Conditions
| Tibia Length (mm)d | |||||
|---|---|---|---|---|---|
| Incubation Conditions | Egg wt at E0 (g)a | Body wt at Hatch (g)b | 3rd Toe Length (mm)c | Left Tibia (mm) | Right Tibia (mm) |
| 12L | 61.4 ± 5.0 | 43.6 ± 3.8 | 25.5 ± .8 | 29.9 ± 1.4 | 29.9 ± 1.1 |
| 24L | 61.6 ± 4.6 | 42.5 ± 3.0 | 25.2 ± 1.2 | 30.4 ± .9 | 30.8 ± .8 |
| 24D | 62.5 ± 3.7 | 44.1 ± 2.7 | 25.4 ± 1.2 | 29.5 ± .6 | 29.5 ± .7 |
Values represent mean ± SD.
p > .8.
p > .5.
p > .7.
p < .003.
Spatial Parameters
At the beginning of each trial, chicks took two to three steps to enter the recording region of the walkway. They typically took seven to eight steps inside the recording region (Fig. 1A) and two to three additional steps to reach the end of the walkway. Thus data analyzed and reported represent the most consistent portion of the self-paced walk trials. The two-way ANOVA comparison of spatial parameters for left and right legs indicated there were no significant differences to suggest there were asymmetries in locomotor performance (see Appendix A). Therefore, we report findings for the left leg as representative of performance.
Mean stride length ranged from 45 to 60 mm and was typically longer during session II (Fig. 2A). A two-way ANOVA indicated that stride length varied significantly across incubation conditions, F(2,54) = 4.84, p < .02 and sessions, F(2,54) = 11.6, p < .002 but that the interaction was not significant, F(2,54) = 2.9, p > .06. Post hoc comparisons indicated that chicks incubated in 24D conditions took longer strides than chicks incubated in 12L (p < .008). Mean step length was approximately half that for stride length (Fig. 2B). Step length did not vary across conditions, F(2,54) = 2.2, p > .1. Nonetheless, step length increased from sessions I to II, F(2,54) = 6.9, p < .02. Mean step width ranges were similar to that for step length (Fig. 2C). ANOVAs indicated that step width varied across conditions, F(2,54) = 6.2, p < .005, but not between sessions, F(2,54) = 1.7, p > .1 and there was no significant interaction, F(2,54) = .02, p > .9. Post hoc comparisons across conditions indicated that chicks incubated in 24D took wider steps than chicks incubated in 24L (p < .005).
FIGURE 2.
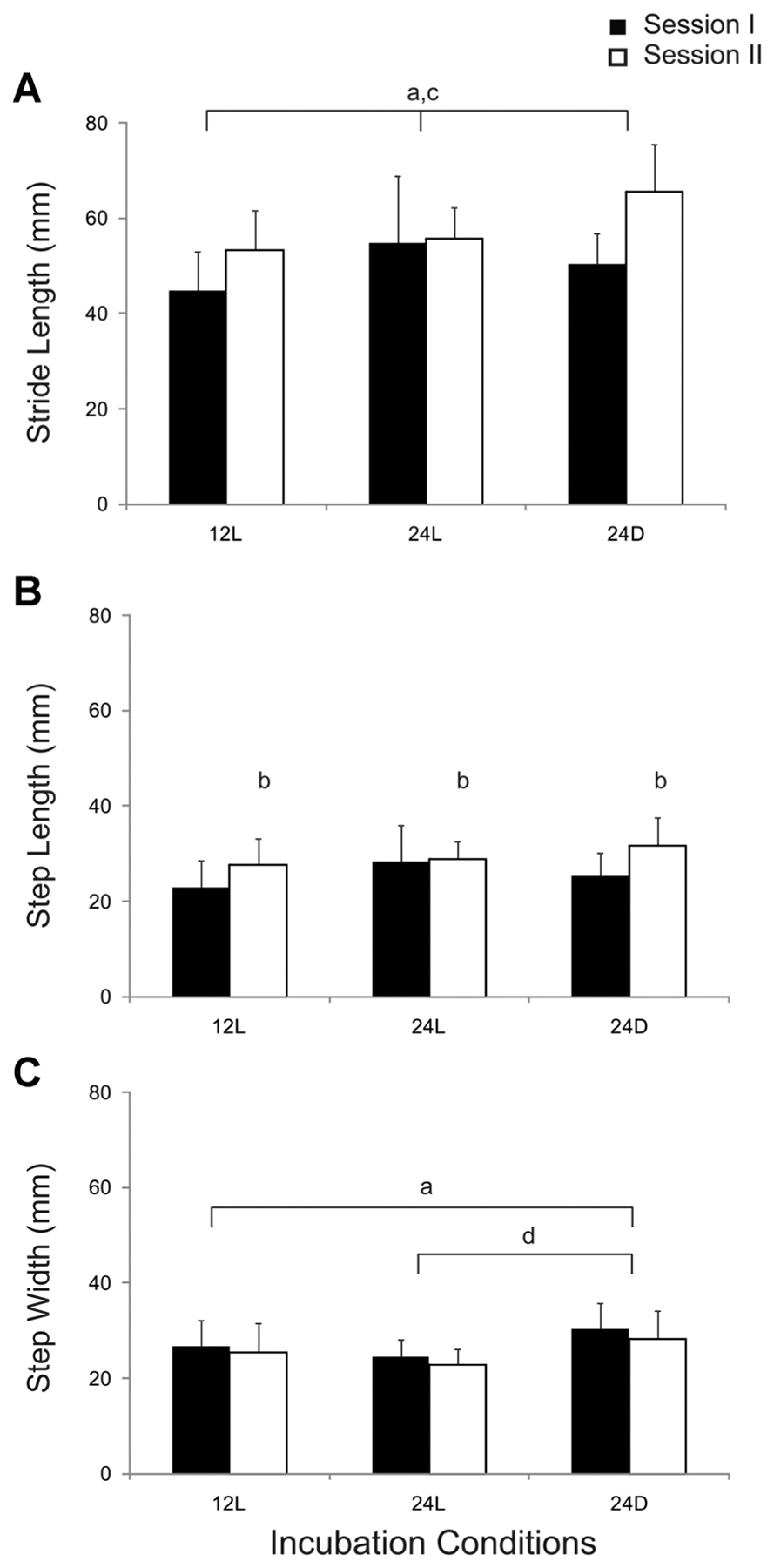
Comparison of spatial parameters for locomotion across incubation conditions during test sessions I and II. Group means (N = 10) and SD are shown. Significant differences are indicated by lower case letters: across incubation conditions (a), between sessions (b), and post hoc comparisons for 12L versus 24D (c), and 24L versus 24D (d).
Considerable within-subject variability was commonly observed during a test session. Within-subject SD values ranged from 6.3 to 13.9 mm for stride length, 3.7 to7.6 mm for step length and 3.3 to 6.2 mm for step width. Thus, we also tested for possible differences in SD for each spatial parameter. SD varied for all three spatial parameters across incubation conditions, but did not vary between sessions. Two-way ANOVAs indicated that SD varied significantly across conditions for stride length, F(2,54) = 4.3, p < .02, step length, F(2,54) = 3.7, p < .04 and step width, F(2,54) = 3.6, p < .04. However, post hoc comparisons indicated that there were no significant differences between incubation conditions.
To further explore the differences in spatial variability across conditions, we examined step-to-step variability for each parameter. Parametric measures for consecutive strides or steps were subtracted from the subject mean for the session and the difference was plotted for each step in sequence within a session. A linear trend line for the scatter was also calculated (Fig. 3). We then examined the distribution of the scatter around the mean (0 on y-axis) across consecutive steps, the slope of the regression line and the regression coefficient (R2) to establish criteria for categorizing the plots. For example, step-to-step variability in stride length for a hatchling during session I is shown in Figure 3A. Most values in Figure 3A fell similarly above or below 0 mm during both early steps (1–20) and later steps (30–50) in the session, suggesting a uniform distribution in variability. Plots with a scatter uniformly distributed around 0 mm across consecutive steps had a slope <.2 and R2 < .2 (Fig. 3A). Plots with a scatter that systematically deviated from 0 mm had a slope >.2 and R2 > .2 (Fig. 3B). We found that the majority of animals in each condition exhibited a pattern of uniformly distributed variability for each of the three spatial parameters (Fig. 3C). More than 90% of animals exhibited uniformly distributed variability in step width for both session I and II. Though there were fewer instances of uniformly distributed variability in stride length and step length during session II, it still represented the majority of animals. Closer inspection of stride length variability indicated that the relative occurrence of uniformly distributed variability was similar across conditions for both sessions I and II (Fig. 3D and E). In addition, one animal in 24L conditions exhibited a linear trend in variability having a negative slope for both sessions I and II.
FIGURE 3.
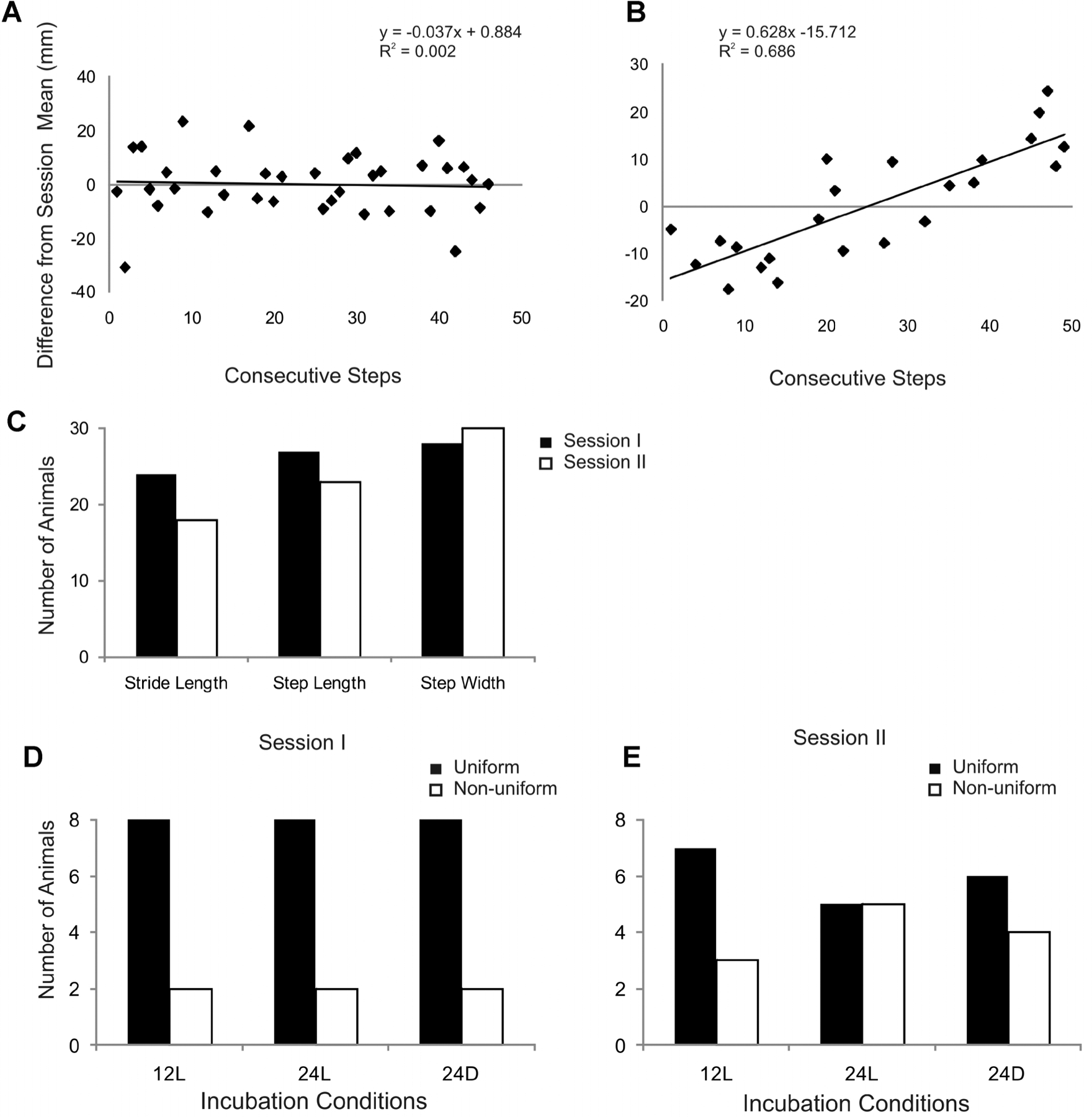
Analyses for step-to-step variability in spatial parameters. (A) Variability in stride length for consecutive steps during walk trials 1–4 in session I are shown for a chick incubated in 12L conditions. Variability was similarly distributed across steps relative to the chick’s session mean (0 mm). (B) Stride length variability in session I for a chick incubated in 24L conditions. Variability shifted across steps relative to this chick’s session mean. (C) Bar graphs indicate the number of animals that exhibited a uniform distribution pattern of step-to-step variability (e.g., A) for each spatial parameter during sessions I and II. (D and E) Bar graphs indicate the number of animals that exhibited a uniform or nonuniform distribution (e.g., B) for stride length in session I(D) and session II (E).
Temporal Parameters
Temporal parameters were also examined for potential left and right leg differences that might suggest asymmetries in locomotor performance. Two-way ANOVAs indicated that there were no differences between limbs for stride, stance, double support, or swing duration (see Appendix B). Therefore, we report findings for the left leg as representative of performance. Stride duration typically exceeded 500 ms, most of which was spent in stance (Fig. 4). The two-way ANOVAs indicated that stride duration did not vary across incubation conditions, F(2,54) = .9, p > .4, but decreased from sessions I to II, F(2,54) = 4.6, p < .05 (Fig. 4A). Stance duration represented approximately 80% of stride duration (Tab. 3) and did not vary across conditions, F(2,54) = 1.1, p > .3, but decreased from sessions I to II, F(2,54) = 6.7, p < .02 (Fig. 4B). Double support duration did not vary across incubation conditions, F(2,54) = .5, p > .6, but decreased from sessions I to II, F(2,54) = 10.4, p < .003 (Fig. 4C). Swing duration represented approximately 20% of stride duration (Tab. 3) and did not vary across conditions, F(2,54) = 2.3, p > .2 or between sessions I and II, F(2,54) = .7, p > .1 (Fig. 4D).
FIGURE 4.
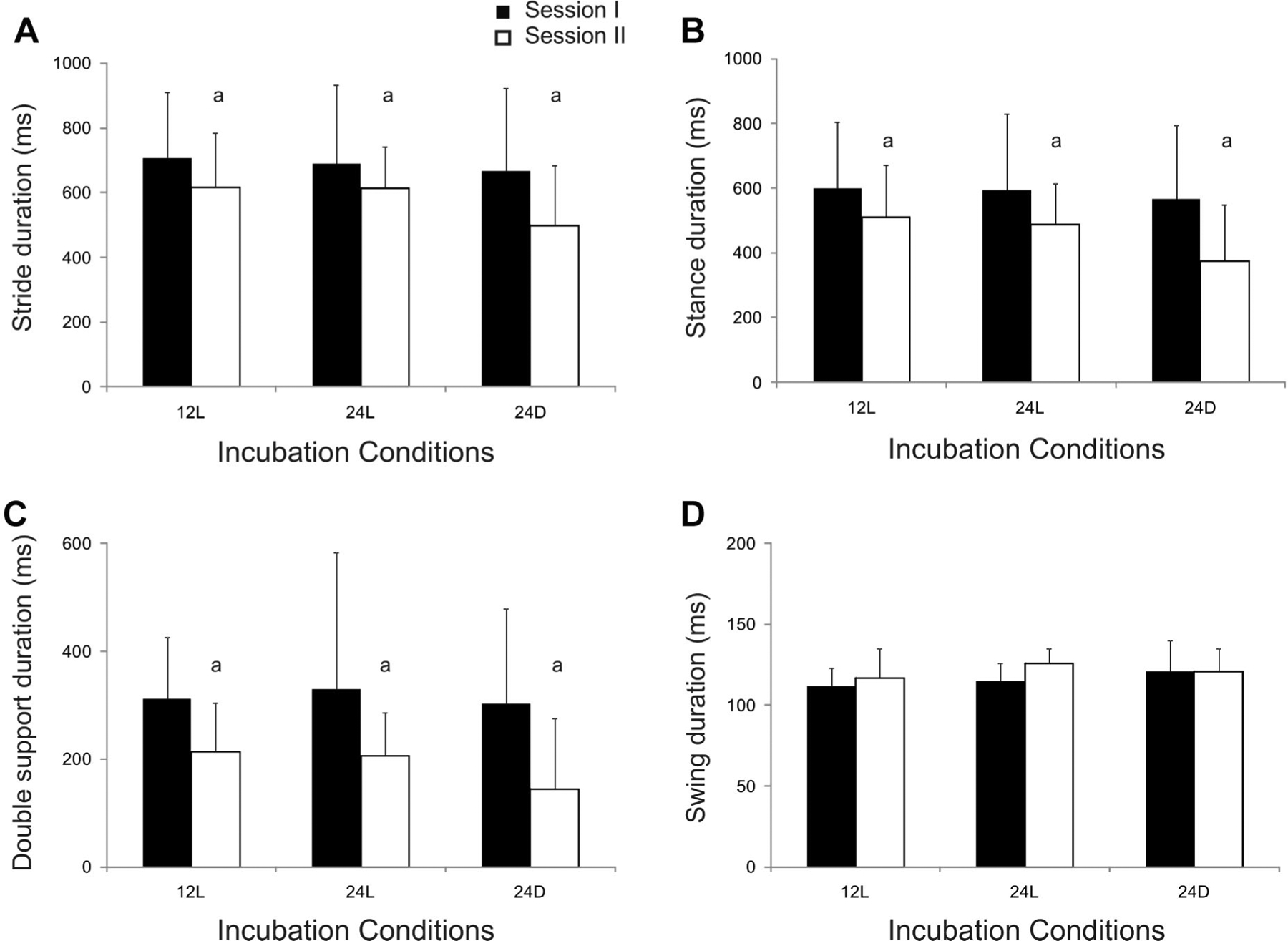
Comparison of temporal parameters for locomotion across incubation conditions during sessions I and II. Group means and SD are shown for stride duration (A), stance duration (B), double support duration (C) and swing duration (D). Significant differences between sessions are indicated (a). Note the change in scale for figures (C and D).
Table 3.
Stance and Swing as a Percent of Stride Duration
| Incubation Condition | Session I | Session II | ||
|---|---|---|---|---|
| Stance (%) | Swing (%) | Stance (%) | Swing (%) | |
| 12L | 84.3 ± 28.7 | 15.7 ± 1.5 | 81.3 ± 25.9 | 18.7 ± 2.8 |
| 24L | 83.7 ± 33.3 | 16.3 ± 1.6 | 79.4 ± 20.3 | 20.6 ± 1.5 |
| 24D | 82.4 ± 33.3 | 17.6 ± 2.8 | 75.5 ± 35.3 | 24.5 ± 2.8 |
Values represent mean ± SD.
Temporal parameters also exhibited considerable within-subject variability. Subject SD values ranged from 129 to 256 ms for stride, 125 to 236 ms for stance, 92 to 253 ms for double support and 9 to 18 ms for swing. Thus, we also tested for possible differences in SD for each temporal parameter. Two-way ANOVAs indicated that SD for stride duration did not vary across conditions, F(2, 54) = .2, p > .7 or between sessions I and II, F(2,54) = 2.3, p > .1. SD for stance duration did not vary across conditions, F(2,54) = .8, p > .4 but decreased between sessions I and II, F(2,54) = 6.9, p < .02. SD for double limb support duration did not vary across conditions, F(2,54) = .4, p > .6 but decreased between sessions I and II, F(2,54) = 10.2, p < .003. SD for swing duration did not vary across conditions, F(2,54) = .5, p > .5 or between sessions I and II, F(2,54) = 1.9, p > .1.
Phase coordination angle analyses indicated that chicks in all three incubation conditions walked with a symmetrically alternating gait. Phase coordination angles typically varied between 170° and 182° (Tab. 4). Two-way ANOVAs indicated there were no significant differences across conditions, F(2,54) = 2.4, p > .09 or between sessions, F(2,54) = .1, p > .7.
Table 4.
Phase Coordination Angles
| Incubation Conditions | Phase Coordination Angles (°)a,b | |
|---|---|---|
| Session I | Session II | |
| 12L | 180 ± 15 | 179 ± 12 |
| 24L | 170 ± 12 | 174 ± 44 |
| 24D | 181 ± 21 | 182 ± 17 |
Values represent mean ± SD.
p > .09 across conditions.
p > .7 between sessions.
Chicks walked at average velocities of 70–160 mm/s (Tab. 5). The two-way ANOVA indicated that walk velocity varied across incubation conditions, F(2,54) = 3.3, p < .05 and increased between sessions I and II, F(2,54) = 5.5, p < .03 but there was no significant interaction, F(2,54) = 1.3, p > .2. Post hoc comparisons indicated there were no significant differences between incubation conditions.
Table 5.
Average Walk Velocity
| Incubation Conditions | Velocity (mm/s)a,b | |
|---|---|---|
| Session I | Session II | |
| 12L | 71.6 ± 34.7 | 5.0 ± 34.8 |
| 24L | 82.9 ± 55.2 | 95.1 ± 28.5 |
| 24D | 92.1 ± 20.8 | 156.6 ± 60.1 |
Values represent mean ± SD.
p < .05 across conditions.
p < .03 between sessions.
DISCUSSION
Our findings indicate that the onset of precocious locomotor performance began a day earlier in chicks that were exposed to continuous bright light throughout incubation (24L) than chicks incubated in less light (12L). The earlier onset of locomotion is at least partly accounted for by the fact that these chicks also hatched 1–2 days earlier than chicks exposed to less light or no light (24D). Chicks incubated in 24L conditions began walking soon after hatching and within a time frame typical of chicks hatching at E21 or E22. Furthermore, they did not require more or different training to cooperate in data acquisition. Kinematic analyses of walking confirmed that they were free of motor deficits that might otherwise compromise their survival after early hatching.
The kinematic results for chicks incubated in 24L lead us to propose that locomotor performance was accelerated by exposure to continuous bright light without any negative effects. Results indicated that all spatial and temporal measures of locomotion for chicks incubated in 24L conditions were similar to those for hatchlings incubated in 12L. Post hoc analyses indicated that the significant differences in spatial parameters were attributed to measures for chicks incubated in 24D: chicks in 24D differed from chicks incubated in 12L (stride length), and both 12L and 24L (step width). Chicks incubated in 24L conditions also exhibited trends in step-to-step variability that were similar to trends for chicks incubated in 12L or 24D conditions. During session I, 80–93% of all hatchlings exhibited variability uniformly distributed about the mean for each spatial parameter, and this trend persisted across parameters in 60–100% of hatchlings during session II (Fig. 3C). Of the three spatial parameters, uniformly distributed variability in stride length appeared to be least frequent in session II, and this was attributed to a modest linear trend across consecutive steps in a portion of chicks for all three incubation conditions. Temporal gait parameters for chicks incubated in 24L were also equivalent to those for hatchlings in both 12L and 24D conditions. There were no differences across conditions for temporal parameters of stride, stance, double support, swing, phase coordination angle, or average walk velocity. A longer average tibia length was the only other measure that distinguished chicks incubated in 24L, but the difference was only significant in comparison to chicks in 24D. Given the absence of significant differences between chicks incubated in 24L and 12L, and only a narrower step width in comparison with chicks in 24D, it does not appear that the difference in tibia length had a significant impact on the locomotor competence of chicks incubated in 24L conditions.
Conversely, the question arises whether or not the absence of light exposure and associated delay in time to hatch significantly impacted locomotor competence. Our findings indicate chicks incubated in 24D conditions took longer strides than chicks incubated in 12L, took wider steps than both chicks in 12L and 24L, and had a shorter tibia length than chicks in 24L. A wider step is often presumed to indicate the need for a broader base of support to control the center of mass (Bril & Brenière, 1992). However, these hatchlings had longer strides than chicks incubated in 12L conditions and their temporal parameters did not differ from either chicks in 12L or 24L. These findings suggest that locomotor competence was not significantly altered by dark-induced delay of hatching and walking.
We cannot rule out the possibility that more subtle differences in locomotor skill might exist across the three conditions. In this study, walking performance was relatively unconstrained and resulted in considerable variability in almost all parameters measured. It is possible that the gait parameters were not sufficiently sensitive to detect subtle differences in locomotor skill across incubation conditions. Finer measurements such as center of foot pressure excursion during stance phase may identify subtle but functionally important differences in mechanical efficiency or the control of balance during locomotion. It is possible, for example, that the longer stride length and greater step width observed in chicks incubated in the 24D conditions indicate these animals exhibited a different strategy for control of posture than chicks incubated in the other light conditions. Thus we interpret our results acknowledging these possible limitations.
The similarity in locomotor parameters between embryos incubated in 24L and embryos hatching and walking 1–2 days later may indicate that light accelerated the development of locomotor circuits. Several studies have shown that incubating chicken embryos in continuous bright light, fluorescent or incandescent, can accelerate growth rate and induce early hatching (Ghatpande et al., 1995; Lauber, 1975; Siegel et al., 1969). Effects of photoaccelaration can be detected in the blastoderms within 10 hr of exposure and somite count is increased within 42 hr (Siegel et al., 1969). In addition, light appears to accelerate onset of respiratory motor control in chicks (Bradley & Jhang, 2003). Light also appears to have positive effects on morphologic development of premature infants (Brandon et al., 2002). Evidence suggests that cycled light exposure (12 hr on/12 hr off) during neonatal intensive care enhances gains in body weight (Brandon et al., 2002). Cycled light exposure may also enhance motor coordination, facilitate an earlier onset of oral feedings, and reduce the duration of ventilator support and phototherapy (Miller et al., 1995). However, the earlier onset of walking in chicks incubated in 24L may be due to an unmasking of latent locomotor competence by early hatching. In other words, locomotor circuits may be ready and adaptive if the context affords adaptive expression of these circuits. It is well established that spinal pattern generating circuits for locomotion emerge early in embryogenesis in chicks and are well established by the time of hatching (Bekoff, 1976; Bekoff et al., 1987; Bradley & Bekoff, 1990; Bradley et al., 2005). Some attributes of precocious walking such as muscle burst frequency range and burst patterns emerge between E15 and E18, and alternating interlimb activity is established by E20 (Bradley et al., 2008; Ryu & Bradley, 2009). Although walking does not emerge until 3–4 weeks of age in kittens, treadmill walking and airstepping can be elicited within the first postnatal week following spinal transaction at birth, suggesting that spinal circuits are sufficiently developed but masked by other brain systems until they can be adaptively integrated with other requirements for walking (Bradley & Smith, 1988a,b,c). Similar observations of unmasking have been made in infants. Infants do not typically initiate repetitive stepping between 2 and 12 months of age, but if the limbs are made buoyant by supporting the infant upright in a tank of water, sustained stepping can be triggered (Thelen, 1983).
At the outset of our study and based on pilot observations, we predicted that light-induced shortening of the incubation period would negatively impact locomotor development. Our assumption was that there is an advantage to the normal delay in onset of hatching until E21, perhaps due to a greater period of practice and/or refinement of limb movements, and that excessive exposure to light under artificial conditions might have undesirable secondary effects. Excessive light exposure during early embryogenesis can elevate the production of reactive oxygen species to toxic levels and alter normal cell division processes (Takenaka, Horiuchi, & Yanagimachi, 2007). Artificially accelerated activity during embryogenesis can induce pathfinding errors in both motor (Hanson, Milner, & Landmesser, 2008) and sensory circuits (S. Butler, personal communication, March 23, 2010). Light-induced acceleration may trigger morphological abnormalities including malformed legs and feet (Tamimie & Fox, 1967). In the neonatal intensive care unit, evidence suggests that continuous light exposure can induce metabolic stress (Brandon et al., 2002; Mann, Haddow, Stokes, Goodley, & Rutter, 1986). Further, continuous light exposure does not appear to have the positive effects on weight gain, motor coordination, or other measures of developmental progress that are found when premature infants are exposed to cycled light (Miller et al., 1995). It is also possible that the continuous bright light of fluorescent lamps used in our study could have had a deleterious impact on motor development, but as 12L conditions also used fluorescent lighting, any effect would still have been associated with the amount of light exposure. We cannot rule out the possibility that performance results might have been different if more naturalistic conditions were used, such as the light exposure conditions experienced by birds in arctic regions during midsummer or during continuous migratory flight (Gwinner & Brandstätter, 2001).
In our study light exposure conditions during incubation were sufficient to induce significant differences in time to pip and hatch. Chicks incubated in continuous bright light acquired locomotor skills within the same relative time frame post hatching as chicks incubated in 12L and 24D conditions. Thus chicks incubated in 24L began walking 1–2 days earlier than all other hatchlings. All measures indicated that hatching early did not compromise locomotor competence. Our findings raise the possibility that light may have also accelerated locomotor development, though our results may not extend to locomotor development of birds in natural settings. We did not find that a dark-induced delay in time to hatch appreciably impacted locomotor performance, though the few effects observed may indicate these hatchlings employed a different locomotor strategy. Further study using kinetic analyses will be required to clarify the significance of these differences. Whether precocious locomotor competence at E20 was due to accelerated development of neural mechanisms underlying adaptive motor skill or the accelerated development of other physiological systems supporting posthatching motor behaviors cannot be answered by the current study. Study of locomotor-related behavior during embryonic development under variable light conditions is required to clarify if circuits for adaptive locomotion are accelerated by light exposure or unmasked by affordances associated with early hatching.
Acknowledgments
Anil Sindhurakar, Program in Systems Biology and Disease, Keck School of Medicine; Nina S. Bradley, Biokinesiology and Physical Therapy, Ostrow School of Dentistry, University of Southern California. This study was supported by NIH National Institute of Child Health and Human Development grant R01 HD-053367. The authors gratefully acknowledge the assistance of John Lin for his assistance in designing and building the walkway, and in developing the training protocol for this study.
APPENDIX A
ANOVA Results for Comparison of Spatial Parameters for Left and Right Leg
| Spatial Parameters | Session I | Session II | ||||
|---|---|---|---|---|---|---|
| df | F | P | df | F | P | |
| Stride length | 1 | .00 | .9 | 1 | .03 | .9 |
| Step length | 1 | .00 | .9 | 1 | .16 | .7 |
| Step width | 1 | .14 | .7 | 1 | .07 | .8 |
APPENDIX B
ANOVA Results for Comparison of Temporal Parameters for Left and Right Leg
| Temporal Parameters | Session I | Session II | ||||
|---|---|---|---|---|---|---|
| df | F | P | df | F | P | |
| Stride duration | 1 | .36 | .6 | 1 | .00 | .9 |
| Stance duration | 1 | .19 | .7 | 1 | .00 | .9 |
| Double support duration | 1 | .25 | .6 | 1 | .00 | .9 |
| Swing duration | 1 | .12 | .7 | 1 | .03 | .9 |
REFERENCES
- Bekoff A (1976). Ontogeny of leg motor output in the chick embryo: A neural analysis. Brain Research, 106, 271–291. [DOI] [PubMed] [Google Scholar]
- Bekoff A, Kauer JA, Fulstone A, & Summers TR (1989). Neural control of limb coordination. II. Hatching and walking motor output patterns in the absence of input from the brain. Experimental Brain Research, 74, 609–617. [DOI] [PubMed] [Google Scholar]
- Bekoff A, Nusbaum MP, Sabichi AL, & Clifford M (1987). Neural control of limb coordination. I. Comparison of hatching and walking motor output patterns in normal and deafferented chicks. The Journal of Neuroscience, 7, 2320–2330. [PMC free article] [PubMed] [Google Scholar]
- Bradley NS, & Bekoff A (1990). Development of coordinated movement in chicks: I. Temporal analysis of hindlimb muscle synergies at embryonic days 9 and 10. Developmental Psychobiology, 23, 763–782. [DOI] [PubMed] [Google Scholar]
- Bradley NS, & Jhang DY (2003). Selective effects of light exposure on distribution of motility in the chick embryo at E18. Journal of Neurophysiology, 90, 1408–1417. [DOI] [PubMed] [Google Scholar]
- Bradley NS, Ryu YU, & Lin J (2008). Fast locomotor burst generation in late stage embryonic motility. Journal of Neurophysiology, 99, 1733–1742. [DOI] [PubMed] [Google Scholar]
- Bradley NS, & Smith J (1988a). Neuromuscular patterns of stereotypic hindlimb behaviors in the first two postnatal months. I. Stepping in normal kittens. Developmental Brain Research, 38, 37–52. [DOI] [PubMed] [Google Scholar]
- Bradley NS, & Smith J (1988b). Neuromuscular patterns of stereotypic hindlimb behaviors in the first two postnatal months. II. Stepping in spinal kittens. Developmental Brain Research, 38, 53–67. [DOI] [PubMed] [Google Scholar]
- Bradley NS, & Smith J (1988c). Neuromuscular patterns of stereotypic hindlimb behaviors in the first two postnatal months. III. Scratching and the paw-shake response in kittens. Developmental Brain Research, 38, 69–82. [DOI] [PubMed] [Google Scholar]
- Bradley NS, Solanki D, & Zhao D (2005). Limb movements during embryonic development in the chick: Evidence for a continuum in limb motor control antecedent to locomotion. Journal of Neurophysiology, 94, 4401–4411. [DOI] [PubMed] [Google Scholar]
- Brandon DH, Holditch-Davis D, & Belyea M (2002). Preterm infants born at less than 31 weeks’ gestation have improved growth in cycled light compared with continuous near darkness. The Journal of Pediatrics, 140, 192–199. [DOI] [PubMed] [Google Scholar]
- Bril B, & Brenière Y (1992). Postural requirements and progression velocity in young walkers. Journal of Motor Behavior, 24, 105–116. [DOI] [PubMed] [Google Scholar]
- Coleman MA, & McDaniel GR (1976). Light alterated changes in the embryonic age versus incubation age of White Leghorn embryos. Poultry Science, 55, 2483–2485. [DOI] [PubMed] [Google Scholar]
- Fairchild BD, & Christensen VL (2000). Photostimulation of turkey eggs accelerates hatching times without affecting hatchability, liver or heart growth, or glycogen content. Poultry Science, 79, 1627–1631. [DOI] [PubMed] [Google Scholar]
- Ghatpande A, Ghatpande S, & Khan MZ (1995). Effect of different intensities of fluorescent light on the early development of chick embryo in ovo. Cellular & Molecular Biology Research, 41, 613–621. [PubMed] [Google Scholar]
- Gwinner E, & Brandstätter R (2001). Complex bird clocks. Philosophical Transaction of the Royal Society London B, 356, 1801–1810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamburger V, & Hamilton HL (1992). A series of normal stages in the development of the chick embryo (reprinted from Journal of Morphology, 88, 49–92, 1951). Developmental Dynamics, 195, 231–272. [DOI] [PubMed] [Google Scholar]
- Hamill J, Haddad JM, & McDermott WJ (2000). Issues in quantifying variability from a dynamical systems perspective. Journal of Applied Biomechanics, 16, 407–418. [Google Scholar]
- Hanson MG, Milner LD, & Landmesser LT (2008). Spontaneous rhythmic activity in early chick spinal cord influences distinct motor axon pathfinding decisions. Brain Research Reviews, 57, 77–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobson RD, & Hollyday M (1982a). A behavioral and electromyographic study of walking in the chick. Journal of Neurophysiology, 48, 238–256. [DOI] [PubMed] [Google Scholar]
- Jacobson RD, & Hollyday M (1982b). Electrically evoked walking and fictive locomotion in the chick. Journal of Neurophysiology, 48, 257–270. [DOI] [PubMed] [Google Scholar]
- Johnston RM, & Bekoff A (1992). Constrained and flexible features of rhythmical hindlimb movements in chicks: Kinematic profiles of walking, swimming and airstepping. Journal of Experimental Biology, 171, 43–66. [DOI] [PubMed] [Google Scholar]
- Johnston RM, & Bekoff A (1996). Patterns of muscle activity during different behaviors in chicks: Implications for neural control. Journal of Comparative Physiology A, 179, 169–184. [DOI] [PubMed] [Google Scholar]
- Lauber JK (1975). Photoacceleration of avian embryogenesis. Comparative Biochemistry & Physiology A, 51, 903–907. [DOI] [PubMed] [Google Scholar]
- Lauber JK, & Shutze JV (1964). Accelerated growth of embryo chicks under the influence of light. Growth, 28, 179–190. [PubMed] [Google Scholar]
- Mann NP, Haddow R, Stokes L, Goodley S, & Rutter N (1986). Effect of night and day on preterm infants in a newborn nursery: Randomized trial. British Medical Journal, 293, 1265–1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CL, White R, Whitman TL, O’Callaghan MF, & Maxwell SE (1995). The effects of cycled versus noncycled lighting on growth and development in preterm infants. Infant Behavior and Development, 18, 87–95. [Google Scholar]
- Muir GD, Gosline JM, & Steeves JD (1996). Ontogeny of bipedal locomotion: Walking and running in the chick. Journal of Physiology, 493, 589–601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noy Y, & Sklan D (1997). Posthatch development in poultry. The Applied Journal of Poultry Research, 6, 344–354. [Google Scholar]
- Perry J (1992). Gait analysis: Normal and pathological function. New Jersey: Slack Incorporated. [Google Scholar]
- Plotnik M, Giladi N, & Hausdorff JM (2007). A new measure for quantifying the bilateral coordination of human gait: Effects of aging and Parkinson’s disease. Experimental Brain Research, 181, 561–570. [DOI] [PubMed] [Google Scholar]
- Riedstra B, & Groothuis TGG (2004). Prenatal light exposure affects early feather-pecking behavior in the domestic chick. Animal Behaviour, 67, 1037–1042. [Google Scholar]
- Romanoff AL (1960). The avian embryo: Structural and functional development. New York: The Macmillan Company. [Google Scholar]
- Romijn C, & Roos J (1938). The air space of the hen’s egg and its changes during the period of incubation. Journal of Physiology, 94, 365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryu YU, & Bradley NS (2009). Precocious locomotor behavior begins in the egg: Development of leg muscle patterns for stepping in the chick. Public Library of Science ONE, 4, e6111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shutze JV, Lauber JK, Kato M, & Wilson WO (1962). Influence of incandescent and coloured light on chicken embryos during incubation. Nature, 196, 594–595. [DOI] [PubMed] [Google Scholar]
- Siegel PB, Isakson ST, Coleman FN, & Huffman BJ (1969). Photoacceleration of development in chick embryos. Comparative Biochemistry & Physiology, 28, 753–758. [Google Scholar]
- Takenaka M, Horiuchi T, & Yanagimachi R (2007). Effects of light on development of mammalian zygotes. Proceedings in National Academy of Sciences, 104, 14289–14293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamimie HS, & Fox MW (1967). Effect of continuous and intermittent light exposure on embryonic development of chicken eggs. Comparative Biochemistry and Physiology, 20, 793–799. [Google Scholar]
- Thelen E (1983). Learning to walk is still an “old” problem: A reply to Zelazo. Journal of Motor Behavior, 15, 139–161. [DOI] [PubMed] [Google Scholar]
- Zappia JV, & Rogers LJ (1983). Light experience during development affects asymmetry of forebrain function in chickens. Developmental Brain Research, 11, 93–106. [DOI] [PubMed] [Google Scholar]


