Abstract
The most recent monkeypox (Mpox) outbreak is mostly affecting men who have sex with men (MSM) who participate in high-risk sexual behaviors, which is typically the case among human immunodeficiency virus (HIV) carriers, according to clinical and epidemiological statistics. The objective of this research is to determine the epidemiological situation of HIV and smallpox co-infection. Until 1 October 2022, a thorough evaluation of the literature was conducted utilizing the databases PubMed, Embase, Scopus, and Web of Science. Studies were evaluated based on the criteria for selection. Fifty-three studies met the selection criteria. A total of 6345 confirmed cases of monkeypox were recorded, and 40.32% (n = 2558) of these cases also had HIV co-infection. In addition, 51.36% (n = 3259) of the men (91.44%; n = 5802), whose ages ranged from 18 to 71 years, exhibited MSM-specific sexual behaviors. Co-infection with these two viruses can be especially dangerous because it can exacerbate the symptoms of both diseases and make them more difficult to treat. People with HIV are more vulnerable to certain infections, including monkeypox, because their immune systems are weakened. Therefore, it is important that they take measures to prevent infection, such as avoiding contact with infected animals, risky behaviors, and maintaining good hygiene.
Keywords: monkeypox, HIV, MSM, co-infection, STIs
1. Introduction
The zoonotic disease known as monkeypox (Mpox) is caused by a double-stranded DNA virus belonging to the genus Orthopoxvirus (monkeypox virus) [1,2]. Humans can contract the Mpox virus through direct contact (sexual or skin-to-skin), respiratory droplets, and fomites that have been exposed to the virus [3].
The World Health Organization (WHO) designated the current outbreak of Mpox disease as a Public Health Problem of International Concern on 23 July 2022 [4]. In addition, 83,487 cases were found in 110 countries by 23 December 2022 [5].
The current global outbreak of Mpox continues to affect mainly homosexuals, bisexuals, and men who have sex with men (MSM), with evidence of an increase in the prevalence of human immunodeficiency virus (HIV) and other sexually transmitted infections (STIs) [6]. According to WHO, current epidemiological data show that 51% (13,769/26,992) of confirmed cases of Mpox have HIV [7]; this is because HIV, STIs, and Mpox can be transmitted through sexual contact [7,8].
HIV infection and being immunocompromised may or may not affect the presentation of monkeypox [9]. Although it is reasonable to assume that, because of underlying immunosuppression, the course of monkeypox should be more severe in persons living with HIV, the effects of Mpox in this patient population have yet to be determined [10].
Given the prevalence of HIV in Mpox cases during the current outbreak, the present study aimed to assess the epidemiology of HIV and Mpox co-infection.
2. Materials and Methods
2.1. Protocol and Registration
This protocol adheres to the standards specified by the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) statement and has been registered in the Prospective International Registry of Systematic Reviews (PROSPERO) database (CRD42022363632) [11].
2.2. Eligibility Criteria
To explore the epidemiological situation of HIV and Mpox co-infection, we included primary research articles that had information on patients older than 18 years with serological diagnosis, polymerase chain reaction (PCR), electron microscopy or immunohistochemical findings positive for Mpox and with a current or previous diagnosis of HIV. We included articles published up to 1 October 2022, with study designs of case reports, case series, and observational studies (cross-sectional, cohort, and case-control). Systematic reviews, scoping reviews, narrative reviews, randomized clinical trials, editorials, conference proceedings, letters to the editor that did not present original results, and other studies that did not answer our research question were excluded. No language restriction was established.
2.3. Information Sources and Search Strategy
A comprehensive search strategy composed of phrases related to “HIV” and “Monkeypox” was used to search Pubmed, Embase, Scopus, and Web of Science (Table 1). Searches were completed on 1 October 2022, and results were evaluated separately by four different investigators.
Table 1.
Search approach for the literature. * NS: Not Specified.
| Base | Search Strategy |
|---|---|
| PUBMED | #1 “Monkeypox” [MH] OR “Monkeypox” [All Fields] OR “Monkeypox virus” [MH] OR “Monkeypox virus” [All Fields] OR “Monkeypoxvirus*” [TIAB] #2 “HIV” [MH] OR “HIV” [All Fields] OR “Human Immunodeficiency Virus*” [All Fields] OR “Acquired Immunodeficiency Syndrome” [MH] OR “Acquired Immunodeficiency Syndrome” [All Fields] OR “AIDS*” [All Fields] OR “HIV Infection*” [MH] OR “HIV Infection*” [All Fields] OR “HIV Coinfection*” [All Fields] OR “HIV Long-Term Survivors” [MH] OR “HIV Long-Term Survivor*” [All Fields] OR “Sexually Transmitted Diseases” [MH] OR “Sexually Transmitted Disease*” [All Fields] OR “Venereal Disease*” [All Fields] OR “STD” [TIAB] OR “Sexually Transmitted Infection*” [TIAB] OR “STI” [TIAB] OR “Sexual Behavior” [MH] OR “Sexual Behavior” [All fields] OR “MSM” [All fields] OR “Men Who Have Sex With Men” [All fields] #3 = #1 AND #2 |
| SCOPUS | #1 TITLE-ABS-KEY (“Monkeypox” OR “Monkeypox virus” OR “Monkey Pox” OR “Monkeypoxvirus*”) #2 TITLE-ABS-KEY (“HIV*” OR “Human Immunodeficiency Virus*” OR “Acquired Immunodeficiency Syndrome” OR “AIDS*” OR “HIV Infection*” OR “HIV Coinfection*” OR “HIV Long-Term Survivor*” OR “Sexually Transmitted Disease*” OR “Venereal Disease*” OR “STD*” OR “Sexually Transmitted Infection*” OR “STI*” OR “Sexual Behavior” OR “MSM” OR “Men Who Have Sex With Men”) #3 = #1 AND #2 |
| WEB OF SCIENCE | #1 ALL = (“Monkeypox” OR “Monkeypox virus” OR “Monkey Pox” OR “Monkeypoxvirus*”) #2 ALL = (“HIV*” OR “Human Immunodeficiency Virus*” OR “Acquired Immunodeficiency Syndrome” OR “AIDS*” OR “HIV Infection*” OR “HIV Coinfection*” OR “HIV Long-Term Survivor*” OR “Sexually Transmitted Disease*” OR “Venereal Disease*” OR “STD*” OR “Sexually Transmitted Infection*” OR “STI*” OR “Sexual Behavior” OR “MSM” OR “Men Who Have Sex With Men”) #3 = #1 AND #2 |
| EMBASE | #1 ‘monkeypox’/exp OR ‘monkeypox’ #2 ‘human immunodeficiency virus’ #3 = #1 AND #2 |
2.4. Study Selection
Using Rayyan QCRI (https://rayyan.qcri.org/, accessed on 7 October 2022), two writers (B.O.S. and E.S.M.M.) independently reviewed the titles and abstracts in accordance with the inclusion and exclusion criteria. The full texts of chosen pertinent studies were searched for an in-depth examination. Conflicts were settled by consensus and, if necessary, input from a third author (D.A.L.F.). Selected articles were stored using the Endnote 20 program.
2.5. Outcomes
Reporting the epidemiological condition of HIV and Mpox co-infection in adult patients was the main result.
2.6. Data Collection Process and Data Items
Three researchers independently retrieved data into an Excel spreadsheet from the chosen objects. The following details were taken out: First author, date of publication, study design, country, N° of patients, sex, age, diagnostic test for Mpox, HIV and Mpox co-infection, other sexually transmitted infections (STIs), Acute HIV, antiretroviral treatment (ART), viral load, CD4+ T-cell count, clinical features, location of skin lesions, treatment and outcomes. To guarantee that there were no duplicate articles or material, a fourth investigator reviewed the final list of included articles.
3. Results
3.1. Study Selection
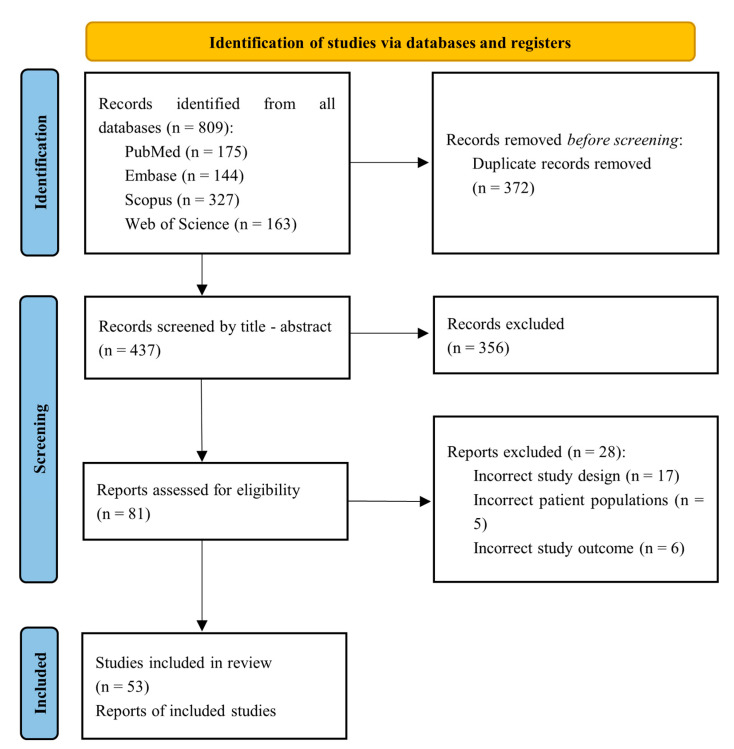
The search method initially yielded a total of 809 articles. The PRISMA flow chart shows the selection procedure (Figure 1). A total of 437 articles were reviewed after eliminating duplicates (n = 372). Fifty-three articles qualified for inclusion in this systematic review after being chosen from 81 after being screened for titles and abstracts [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64].
Figure 1.
PRISMA flowchart summarizing the process of choosing the studies.
3.2. Study Characteristics
The studies (n = 53) described cases of concurrent Mpox and HIV infection, including the number of cases, HIV infection, history of sexually transmitted diseases, method of Mpox diagnosis, clinical manifestations, location, and progression of skin lesions, CD4+ T-cell count, HIV viral load, treatment, and outcome (Table 2 and Table 3). Table 2 presents a summary of the general features of the publications included in this review [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. A total of 6345 confirmed cases of simian pox were reported, distributed across countries: United States (n = 3169) [19,22,28,37,55], Spain (n = 937) [18,25,33,42,49,60], Germany (n = 869) [15,27,30,31,41,43,54], United Kingdom (n = 300) [23,24,29,51,62], France (n = 264) [36], Italy (n = 90) [13,17,34,35,39,40,56,57,58,59], Nigeria (n = 77) [44,45,46], Portugal (n = 71) [12,21,52,53], Israel (n = 26) [63], Belgium (n = 4) [20], Brazil (n = 3) [16,38], Romania (n = 2) [47,48], Czech Republic (n = 2) [14,64], Taiwan (n = 1) [32], Greece (n = 1) [50], and Australia (n = 1) [26] (Table 1). Of the total cases, 40.32% (n = 2558) had co-infection between HIV and Mpox [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64].
Table 2.
Characteristics of included studies.
| Author | Year | Design | Country | N° of Participants |
Age | Sex (M/F) | Diagnostic Method for Mpox | HIV and Mpox Coinfection | Previous STIs | Sexual Behavior |
|---|---|---|---|---|---|---|---|---|---|---|
| Alpalhão M, et al. [12] | 2022 | Case series | Portugal | 42 | HIV: 37.7 ± 9.2 a | M (n = 42) | NR | 22 | NR | MSM (n = 37) |
| Without-HIV: 32.5 ± 8.1 a | ||||||||||
| Antironi A, et al. [13] | 2022 | Case reports | Italy | 4 | Median: 30 | M (n = 4) | RT-PCR | 2 | Syphilis (n = 3), HBV (n = 1), HCV (n = 1) |
MSM (n = 4) |
| Boesecke C, et al. [15] | 2022 | Case report | Germany | 1 | 40 | M (n = 1) | RT-PCR | 1 | Syphilis | NR |
| Bížová B, et al. [14] | 2022 | Case report | Czech Republic | 1 | 34 | M (n = 1) | RT-PCR | 1 | Syphilis | MSM (n = 1) |
| Brites C, et al. [16] | 2022 | Case reports | Brazil | 2 | 37 31 |
M (n = 2) | RT-PCR | 1 | NR | MSM (n = 2) |
| Brundu M, et al. [17] | 2022 | Case report | Italy | 1 | 35 | M (n = 1) | RT-PCR | 1 | NR | MSM (n = 1) |
| Catála A, et al. [18] | 2022 | Cohort study | Spain | 185 | 38.7 ± 8.2 a | M (n = 185) | RT-PCR | 78 | Yes (n = 140) * | MSM (n = 184) |
| Curran KG, et al. [19] | 2022 | Cohort study | USA | 1969 | 35 (30–42) b | M (n = 1466) F (n = 10) |
RT-PCR | 755 | Gonorrhea (n = 546), Chlamydia (n = 489), and Syphilis (n = 165) | NR |
| de Baetselier I, et al. [20] | 2022 | Cases series | Belgium | 4 | Range: 30–50 | M (n = 4) | RT-PCR | 3 | Yes (n = 3) * | MSM (n = 3) |
| de Sousa D, et al. [21] | 2022 | Case report | Portugal | 1 | 24 | M (n = 1) | RT-PCR | 1 | No | MSM (n = 1) |
| Perez-Duque M, et al. [53] | 2022 | Cases series | Portugal | 27 | 33 (22–51) c | M (n = 27) | RT-PCR | 14 | NR | MSM (n = 18) |
| Gandrakota N, et al. [22] | 2022 | Case report | USA | 1 | 34 | Male-to-Female transgender (n = 1) | RT-PCR | 1 | Neurosyphilis | MSM (n = 1) |
| Gedela K, et al. [23] | 2022 | Cases series | UK | 2 | Range: 30–40 | M (n = 2) | RT-PCR | 1 | HSV (n = 2), and Chlamydia (n = 1) | MSM (n = 2) |
| Girometti N, et al. [24] | 2022 | Cohort study | UK | 54 | 41 (34–45) b | M (n = 54) | RT-PCR | 13 | Syphilis (n = 14), HSV (n = 24) and Gonorrhea (n = 13) | MSM (n = 54) |
| Gomez-Garberi M, et al. [25] | 2022 | Cases series | Spain | 14 | 42 (20–56) c | M (n = 14) | RT-PCR | 8 | Chlamydia (n = 2) Syphilis (n = 1), Gonorrhea (n = 1), Mycoplasma, HSV-2 (n = 1) | MSM (n = 10) |
| Hammerschlag Y, et al. [26] | 2022 | Case report | Australia | 1 | 30 | M (n = 1) | RT-PCR | 1 | Syphilis (n = 1) | MSM (n = 1) |
| Heskin J, et al. [29] | 2022 | Case reports | UK | 2 | NR | M (n = 2) | RT-PCR | 1 | Negative | MSM (n = 2) |
| Hermanussen L, et al. [27] | 2022 | Case series | Germany | 3 | 44 (31–54) c | M (n = 3) | RT-PCR | 1 | Syphilis (n = 1) | MSM (n = 2) |
| Hernandez LE, et al. [28] | 2022 | Case report | USA | 1 | 37 | M (n = 1) | RT-PCR | 1 | Syphilis (n = 1) | NR |
| Hoffman C, et al. [31] | 2022 | Cohort study | Germany | 546 | 39 (20–69) c | M (n = 546) | RT-PCR | 256 | Yes (n = 286) * | MSM (n = 546) |
| Hoffmann C, et al. [30] | 2022 | Cohort study | Germany | 301 | 39 (20–64) c | M (n = 301) | RT-PCR | 141 | Yes (n = 177) * | MSM (n = 301) |
| Huang S, et al. [32] | 2022 | Case report | Taiwan | 1 | 24 | M (n = 1) | RT-PCR | 1 | NR | MSM (n = 1) |
| Iñigo-Martínez J, et al. [33] | 2022 | Case series | Spain | 508 | 35 (18–67) c | M (n = 503) F (n = 5) |
RT-PCR | 225 | NR | MSM (n = 397) |
| Lapa D, et al. [34] | 2022 | Case report | Italy | 1 | 39 | M (n = 1) | RT-PCR | 1 | NR | MSM (n = 1) |
| Loconsole D, et al. [35] | 2022 | Case series | Italy | 10 | 36 (25–71) c | M (n = 8) F (n = 2) |
RT-PCR | 4 | NR | MSM (n = 6), heterosexual intercourse (n = 3) |
| Mailhe M, et al. [36] | 2022 | Cohort study | France | 264 | 35 (30–41) b | M (n = 262) F (n = 1) Trans (n = 1) |
RT-PCR | 73 | Yes (n = 209) * | MSM (n = 245) |
| Matias WR, et al. [37] | 2022 | Cases series | USA | 3 | 20 (20–40) c | M (n = 3) | RT-PCR | 1 | Gonococcal urethritis (n = 1) | MSM (n = 3) |
| Rodrigues Menezes Y, et al. [38] | 2022 | Case report | Brazil | 1 | 41 | M (n = 1) | RT-PCR | 1 | NR | MSM (n = 1) |
| Mileto D, et al. [39] | 2022 | Case report | Italy | 1 | 33 | M (n = 1) | RT-PCR | 1 | No | NR |
| Moschese D, et al. [40] | 2022 | Case series | Italy | 32 | 38 (34–42) b | M (n = 32) | RT-PCR | 17 | NR | MSM (n = 32) |
| Noe S, et al. [41] | 2022 | Case report | Germany | 2 | 26 32 |
M (n = 2) | RT-PCR | 1 | NR | MSM (n = 2) |
| Nolasco S, et al. [42] | 2022 | Case report | Spain | 1 | 36 | M (n = 1) | RT-PCR | 1 | Syphilis | MSM (n = 1) |
| Norz D, et al. [43] | 2022 | Cohort study | Germany | 10 | Range: 20–50 | M (n = 16) | RT-PCR | 2 | NR | MSM (n = 16) |
| Ogoina D, et al. [44] | 2020 | Cohort study | Nigeria | 40 | 32 (28–54) c | M (n = 31) F (n = 9) |
RT-PCR and IgM serology |
9 | NR | NR |
| Ogoina D, et al. [45] | 2018 | Case series | Nigeria | 21 | 29 (6–45) c | M (n = 17) F (n = 4) |
RT-PCR and IgM serology |
2 | Syphilis (n = 2/8) | NR |
| Ogoina D, et al. [46] | 2022 | Case series | Nigeria | 16 | 28 (22–43) c | M (n = 12) F (n = 6) |
RT-PCR and IgM serology | 3 | Yes (n = 4) * | Heterosexual intercourse (n = 16) |
| Oprea C, et al. [47] | 2022 | Case report | Romania | 1 | 26 | M (n = 1) | RT-PCR | 1 | No | MSM (n = 1) |
| Oprea C, et al. [48] | 2022 | Case report | Romania | 1 | 30 | M (n = 1) | RT-PCR | 1 | Syphilis (n = 1) | MSM (n = 1) |
| Orviz E, et al. [49] | 2022 | Descriptive | Spain | 48 | 35 (29–44) b | M (n = 48) | RT-PCR | 19 | Gonorrhea (n = 6), Syphilis (n = 4)), and Mycoplasma genitalium (n = 1) | MSM (n = 42) |
| Paparizos V, et al. [50] | 2022 | Case report | Greece | 1 | 59 | M (n = 1) | RT-PCR | 1 | No | MSM (n = 1) |
| Patel A, et al. [51] | 2022 | Descriptive | UK | 197 | 38 (32–42) b | M (n = 197) |
RT-PCR | 70 | Gonorrhea (n = 43/161), Chlamydia (n = 13/161), Syphilis (n = 6/163), and HSV (n = 11/157) |
MSM (n = 197) |
| Patrocinio-Jesus R, et al. [52] | 2022 | Case report | Portugal | 1 | 31 | M (n = 1) | RT-PCR | 1 | No | MSM(n = 1) |
| Pfäfflin F, et al. [54] | 2022 | Cases series | Germany | 6 | Range: 21–50 | M (n = 6) | RT-PCR | 2 | Gonorrhea (n = 3) and Syphilis (n = 1) | MSM (n = 6) |
| Philpott F, et al. [55] | 2022 | Descriptive | USA | 1195 | 35 (30–41) b | M (n = 1178) F (n = 5) Transgender man (n = 3) Transgender woman (n = 5) |
RT-PCR | 490 | NR | MSM (n = 337) |
| Pisano L, et al. [57] | 2022 | Case report | Italy | 1 | 45 | M (n = 1) | RT-PCR | 1 | NR | MSM (n = 1) |
| Pipitò L, et al. [56] | 2022 | Case reports | Italy | 2 | 45 69 |
M (n = 2) | RT-PCR | 2 | Syphilis (n = 2), Gonorrhea (n = 1), and HCV (n = 1) | MSM (n = 2) |
| Quattri E, et al. [58] | 2022 | Case reports | Italy | 2 | 35 29 |
M (n = 2) | RT-PCR | 1 | Syphilis (n = 2) and Gonorrhea (n = 1) | MSM (n = 2) |
| Raccagni AR, et al. [59] | 2022 | Cases series | Italy | 36 | 41.5 (31.25–35.5) b | M (n = 36) | RT-PCR | 15 | Yes (n = 4) * | MSM (n = 36) |
| Tarin-Vicente EJ, et al. [60] | 2022 | Cohort study | Spain | 181 | 37 (31–42) b | M (n = 175) F (n = 6) |
RT-PCR | 72 | Syphilis (n = 13) and Chlamydia (n = 10) | MSM (n = 166) and MSW (n = 15) |
| Thornhill JP, et al. [61] | 2022 | Cases series | 16 countries | 528 | 38 (18–68) c | M (n = 527) Trans (n = 1) |
RT-PCR | 218 | Gonorrhea (n = 32/377), Chlamydia (n = 20/377), Syphilis (n = 33/377), HSV (n = 3/377), Lympho-granuloma venereum (n = 2/377), Chlamydia and Gonorrhea (n = 5/377) |
Heterosexual (n = 9) Homosexual (n = 509) Bisexual (n = 10) |
| Vusirikala A, et al. [62] | 2022 | Descriptive | UK | 45 | 40 (32–43) b | M (n = 45) | RT-PCR | 11 | Yes (n = 27) * | MSM (n = 45) |
| Yakubovsky M, et al. [63] | 2022 | Descriptive | Israel | 26 | 34 (24–53) c | M (n = 26) | RT-PCR | 7 | Gonorrhea (n = 3) and C. trachomatis (n = 3) | MSM (n = 26) |
| Zlámal M, et al. [64] | 2022 | Case report | Czech Republic | 1 | 38 | M (n = 1) | RT-PCR | 1 | HSV, Syphilis, Chlamydia, Gonorrhea, and HCV | MSM (n = 1) |
Mpox: Monkeypox; UK: United Kingdom; USA: United States of America; MSM: men who have sex with men; STI: sexually transmitted infection; HIV: human immunodeficiency virus; HBV: hepatitis B virus, HCV: hepatitis C virus, HSV: herpes simplex virus, RT-PCR: Polymerase chain reaction with reverse transcriptase; M/F: Male/Female; NR: No report. a Media ± SD. b Median (IQR). c Median (Range). * NS: Not Specified.
Table 3.
Characteristics of eligible studies. HIV status, clinical manifestations, localization, antiretroviral therapy, viral load, CD4+ T-cell count, treatment and outcomes.
| Author | N° of Patients |
HIV and Mpox Coinfection |
Clinical Manifestations | Localization of Skin Lesions | Antiretroviral Therapy | Mpox and Acute HIV | HIV Viral Load | CD4+ T-Cell Count (cells/μL) | Treatment | Outcome |
|---|---|---|---|---|---|---|---|---|---|---|
| Alpalhão M, et al. [12] | 42 | 22 | Fever (n = 22), myalgias/arthralgias (n = 23), headache (n = 21), lymphadenopathy (n = 28) | Genital (n = 28), perianal (n = 22), and perioral (n = 12) | Yes (n = 22) | No (n = 1) | NR | NR | NR | Recovered (n = 42) |
| Antinori A, et al. [13] | 1 | Yes | Skin lesions and lymphadenopathy | Genital, thorax and calf area | Yes | No (n = 1) | NR | NR | Ciprofloxacin, acyclovir, and benzylpenicillin | Recovered |
| 2 | No | Skin lesions, fever, asthenia, and lymphadenopathy | Anal, back, legs and foot sole | Anti-inflammatory and antihistaminic drugs | Recovered | |||||
| 3 | Yes | Skin lesions and fever | Anal, head, thorax, legs, arms, hand, and genital area | Yes | No (n = 1) | NR | NR | NR | Recovered | |
| 4 | No | Skin lesions, myalgia | Genital and pubic area | NR | Recovered | |||||
| Boesecke C, et al. [15] | 1 | Yes | Skin lesions | Nose, penis, and oral mucosa | Bictegravir/emtricitabine/tenofovir alafenamide | Yes (n = 1) | NR | 127 | Oral tecovirimat 600 mg bid for 7 days and ceftriaxone 2 g intravenous for 10 days | Recovered |
| Bížová B, et al. [14] | 1 | Yes | High fever, chills, lymphadenopathy, rash, painless perianal erosions, painless ulceration on his left tonsil, and perianal umbilicated papules | Perianal and left side of the body | NR | No (n = 1) | NR | NR | Cephalosporins | Recovered |
| Brites C, et al. [16] | 1 | Yes | Skin lesions, fever, chills, myalgia, lymphadenopathy, and urethral burning sensation during urination | Forehead, nose, thorax, left leg, glans, and scrotal sac | Lamivudine/tenofovir/efavirenz | No (n = 1) | Undetectable | 604 | Doxycycline and ceftriaxone | Recovered |
| 2 | NR | Skin lesions, fever, headache, back pain, and lymphadenopathy | Legs, trunk, hands, and perianal area | - | - | - | - | - | Recovered | |
| Brundu M, et al. [17] | 1 | Yes | Skin lesions, fever, lymphadenopathy, chills, myalgia, headache, malaise, sore throat, and episodes of rectal bleeding | Perianal, abdomen, chest, and back | Darunavir/cobicistat/emtricitabine /tenofovir alafenamide | Yes (n = 1) | NR | NR | Analgesics | Recovered |
| Catála A, et al. [18] | 185 | 78 | Skin lesions (n = 185), lymphadenopathy (n = 104), fever (n = 100), asthenia (n = 81), myalgia (n = 81), headache (n = 59), proctalgia (n = 40), throat ache (n = 34), arthralgia (n = 21), lumbar pain (n = 12), and oral ulcer (n = 10) | Genital (n = 98), face (n = 72), arms (n = 70), perianal (n = 62), legs (52), thorax (n = 47), pubis (n = 30), abdomen (n = 29), back (n = 28), mouth (n = 26), plantar (22), palmar (n = 12), and eyelids (n = 2) | NR | NR | Detectable viral load (n = 63) | CD4 count: 698 (549–930) a CD4 nadir: 396 (249–575) a |
NR | Recovered (n = 185) |
| Curran KG, et al. [19] | 1969 | 755 | NS | NR | Yes (n = 713) | Yes (n = 19) | <200 copies/mL (n = 618) | 639 (452–831) a <350 (n = 91) |
NR | Recovered (n = 1969) |
| de Baetselier I, et al. [20] | 4 | 3 | Asymptomatic (n = 3), Painful vesicular perianal rash (n = 1) | Perianal (n = 1) | Yes (n = 3) | NR | Viral load <20 µL (n = 3) | >350 (n = 3) | NR | Recovered (n = 4) |
| de Sousa D, et al. [21] | 1 | Yes | Skin lesions, fatigue, anal pain, lymphadenopathy, and fever | Perianal, genital, mouth, face, and trunk | No (n = 1) | Yes (n = 1) | >10,000,000 copies/mL | 208 | Paracetamol, tramadol, and fusidic acid cream | Recovered |
| Perez-Duque M, et al. [53] | 27 | 14 | Exanthema (n = 14), inguinal lymphadenopathy (n = 14), fever (n = 13), genital ulcers (n = 6), genital vesicles (n = 6), asthenia (n = 7), headache (n = 7), and myalgia (n = 5) |
Genital (n = 6), anal (n = 4) | NR | NR | NR | NR | NR | Recovered (n = 27) |
| Gandrakota N, et al. [22] | 1 | Yes | Skin lesions, anal pain, headache, fever, photophobia, neck stiffness, and bilateral lower extremity weakness | Perianal | Irregular | No (n = 1) | NR | 200 | Tecovirimat, penicillin, vancomycin, ceftriaxone, ampicillin, doxycycline, and dexamethasone | Recovered |
| Gedela K, et al. [23] | 2 | 1 | Myalgia (n = 2), fever (n = 2), rectal pain (n = 2), lymphadenopathy (n = 2), skin lesions (n = 1), and throat pain (n = 1) | Perianal (n = 1) | Yes (n = 1) | NR | NR | NR | Aciclovir (n = 2), paracetamol (n = 2), topical lidocaine (n = 2) ibuprofen (n = 1), codein (n = 1), and morphine (n = 1) | Recovered (n = 2) |
| Girometti N, et al. [24] | 54 | 13 | Skin lesions (n = 54), Fatigue (n = 36), fever (n = 31), lymphadenopathy (n = 30), myalgia (n = 16), and sore throat (n = 11) | Genital (n = 33), perianal (n = 24), upper and lower extremities (n = 27), facial (n = 11), oropharyngeal (n = 4), and torso (n = 14) | Yes (n = 13) | Yes (n = 2) | <50 copies/mL (n = 11), 200–500 copies/mL (n = 2) | >500 (n = 13) | Ceftriaxone (n = 3), doxycyclin (n = 2), metronidazole (n = 1), and tecovirimat (n = 1) | Recovered (n = 54) |
| Gomez-Garberi M, et al. [25] | 14 | 8 | Skin lesions (n = 14), lymphadenopathy (n = 8), penile oedema (n = 6), fever (n = 5), malaise (n = 4), proctalgia (n = 1), and rectal itching (n = 1) | Genital (n = 12), inguinal (n = 1), and perianal (n = 1) | Yes (n = 8) | Yes (n = 1) | NR | Median: 663 | Antihistamines, analgesics, and nonsteroidal anti-inflammatory drugs (n = 14) and surgical (n = 2) | Recovered (n = 14) |
| Hammerschlag Y, et al. [26] | 1 | Yes | Skin lesions, fever, lymphadenopathy and general malaise | Penis, trunk, face, extremities, hand, calf, and nasal throat |
Abacavir/lamivudine/dolutegravir | No (n = 1) | < 100 copies/mL | 700 | Ceftriaxone, doxycycline, cephalexin, and oral analgesia |
Recovered |
| Heskin J, et al. [29] | 2 | 1 | Skin lesions (n = 2) | Genital (n = 1), pubic (n = 1), oral and buccal mucous membranes (n = 1), perioral (n = 1), and perianal (n = 1) |
Yes (n = 1) | No (n = 1) | NR | NR | Oral antiviral, antibacterial medication (ceftriaxone) (n = 2) | Recovered (n = 2) |
| Hermanussen L, et al. [27] | 1 | No | Skin lesions, fever, and malaise | Face, hairy scalp, trunk, extremities, oral, palmar, and plantar regions | - | - | - | NR | Tecovirimat, amoxicillin and clavulanic acid | Recovered |
| 2 | No | Skin lesions, lymphadenopathy, and myalgia | Trunk and extremities | - | - | - | NR | Tecovirimat and penicillin | Recovered | |
| 3 | Yes | Skin lesions, fever, malaise, and weakness | All over the body, but sparing the genital area | Irregular | No (n = 1) | 1.29 × 106 copies/mL | 50 | Tecovirimat | Recovered | |
| Hernandez LE, et al. [28] | 1 | Yes | Skin lesions | Trunk, upper and lower extremities, groin, and perianal region | Emtricitabine/tenofovir /doravarine/darunavir/cobicistat | No (n = 1) | <20 copies/mL | 262 | Tecovirimat, doxycycline, ceftriaxone, and valacyclovir | Recovered |
| Hoffman C, et al. [31] | 256 | 256 | Fever (n = 126), headache and pain in the limbs (n = 98), night sweats (n = 40), and lymph node swelling (n = 95) | Genital (n = 110), anal (n = 127), oral, perioral, head and neck (n = 64), and extremities and/or trunk (n = 92) | NR | Yes (n = 1) | >50 copies/mL (n = 10), >200 copies/mL (n = 4) | 691 (185–1603) b <500 (n = 42) and <350 (n = 7) |
NR | Recovered (n = 256) |
| 232 (PrEP User) | 0 | Fever (n = 118), headache and pain in the limbs (n = 91), night sweats (n = 30), and lymph node swelling (n = 95) | Genital (n = 114), anal (n = 116), oral, perioral, head and neck (n = 47), and extremities and/or trunk (n = 90) | - | - | - | - | NR | Recovered (n = 232) | |
| 58 (Without HIV or PrEP) | 0 | Fever (n = 28), headache and pain in the limbs (n = 19), night sweats (n = 3), and lymph node swelling (n = 23) | Genital (n = 43), anal (n = 14), oral, perioral, head and neck (n = 12), and extremities and/or trunk (n = 14) | - | - | - | - | NR | Recovered (n = 58) | |
| Hoffmann C, et al. [30] | 301 | 177 | Fever (n = 168/274), headache and pain in the limbs (n = 126/270), night sweats (n = 53/266), and lymph node swelling (n = 116/264) | Genital (n = 146/298), anal (n = 152/299), oral, perioral, and head (n = 72/296), and extremities and/or trunk (n = 122/292) | NR | NR | <50 copies/mL (n = 123/130), 50–200 copies/mL (n = 4/130), ≥200 copies/mL (n = 3/130) | <350 (n = 4/127), 350–500 (n = 21/127), and ≥500 (n = 102/127) | NR | Recovered (n = 301) |
| Huang S, et al. [32] | 1 | Yes | Skin lesions, lymphadenopathy, fever, sore throat, and myalgia | Face, limbs, trunk, genital, and perianal | Yes (n = 1) | No (n = 1) | NR | 517 | NR | Recovered |
| Iñigo-Martínez J, et al. [33] | 508 | 225 | Skin lesions (n = 498), fever (n = 324), lymphadenopathy (n = 311), asthenia (n = 238), myalgia (n = 185), headache (n = 162), odynophagia (n = 143), and proctitis (n = 81) |
Anogenital and/or perineal area (n = 359), legs and/or arms (n = 222), face (n = 177), chest and/or abdomen (n = 159), back (n = 132), palms and/or plants (n = 124) |
NR | NR | NR | NR | NR | Recovered (n = 508) |
| Lapa D, et al. [34] | 1 | Positive | Skin lesions, fever | Head, thorax, legs, arms, hand, and penis | Dolutegravir/lamivudine | No (n = 1) | NR | NR | NR | Recovered |
| Loconsole D, et al. [35] | 10 | 6 | Skin lesions (n = 10), fever (n = 10), shivering and sweating (n = 10), and lymphadenopathy (n = 10) | Genital (n = 7), face (n = 6), palms (n = 3), arms (n = 4), trunk (n = 4), back (n = 3), and oral (n = 2) | NR | NR | NR | NR | NR | Recovered (n = 10) |
| Mailhe M, et al. [36] | 264 | 73 | Skin lesions (n = 264), lymphadenopathy (n = 174), fever (n = 171), pharyngitis (n = 51), angina (n = 41), respiratory signs (n = 31), and headaches (n = 89) | Genital area (n = 135), limbs (n = 121), trunk (n = 105), perianal (n = 100), face (n = 88), and palmoplantar area (n = 36) | NR | NR | NR | >500 (n = 4) | Cidofovir (n = 1), valaciclovir (n = 1), tobramycin (n = 1), ocular dexamethasone (n = 1), ganciclovir (n = 1), opioids (n = 6), acetaminophen (n = 9), surgical (n = 4) | Recovered (n = 264) |
| Matias WR, et al. [37] | 1 | No | Skin lesions, lymphadenopathy, fever, chills, and general malaise | Penis, pubis, and arm | - | - | - | - | Tecovirimat | Recovered |
| 2 | Yes | Skin lesions, lymphadenopathy, fever, chills, myalgias, left tonsillar pain, and odynophagia | Forearms and hands | Yes (n = 1) | No (n = 1) | Suppressed viral load | >500 | Tecovirimat | Recovered | |
| 3 | No | Skin lesions, lymphadenopathy, malaise, and subjective fevers | Penis, chest, and arm | - | - | - | - | Tecovirimat | Recovered | |
| Rodrigues Menezes Y, et al. [38] | 1 | Yes | Skin lesions, lymphadenopathy, dyspnea, penis and glans edema, throat pain, diarrhea, weakness, and malaise | Chest, abdomen, back, upper and lower limbs, palms of the hands, soles of the feet, genitalia, perineum, anorectal region, tongue, and oropharynx | Yes | No (n = 1) | Undetectable | 53 | Meropenem and vancomycin | Death |
| Mileto D, et al. [39] | 1 | Yes | Skin lesions, fever, lymphadenopathy, asthenia, malaise, faryngodynia, sneezing, and anorexia | Face, both elbows, the trunk, the buttock and the right foot | Dolutegravir/rilpivirine | No (n = 1) | <20 copies/mL | 771 | NR | Recovered |
| Moschese D, et al. [40] | 32 | 0 | Skin lesions (n = 7), fever (n = 3), lymphadenopathy (n = 2), fatigue, asthenia, and malaise (n = 9), back pain (n = 2), myalgia (n = 1), abdominal symptoms (n = 2), sore throat (n = 2), and headache (n = 5) | Genital (n = 2), face (n = 6), oral (n = 2), anal/perianal (n = 8), palms (n = 3), and soles (n = 1) | - | - | - | NR | NR | Recovered (n = 15) |
| 15 | Skin lesions (n = 9), fever (n = 6), lymphadenopathy (n = 1), fatigue, asthenia, and malaise (n = 11), back pain (n = 3), myalgia (n = 1), abdominal symptoms (n = 2), sore throat (n = 4), and headache (n = 2) | Genital (n = 9), face (n = 10), oral (n = 1), anal/perianal (n = 10), and palms (n = 1) | Yes (n = 17) | NR | <50 copies/mL (n = 16) | 678 (526–933) a | NR | Recovered (n = 17) | ||
| Noe S, et al. [41] | 1 | Yes | Skin lesions, malaise, fever, arthralgia, myalgia, back pain, headache, dysphagia, and presence of white spots on his tonsils. |
Trunk, extremities, and head | Bictegravir/emtricitabine/tenofovir alafenamide | NR | NR | NR | NR | Recovered |
| 2 | No | Skin lesions, fever, fatigue, cough, inguinal lymphadenopathy, and anal pain |
Trunk | - | - | - | - | Topical zinc oxide suspension | Recovered | |
| Nolasco S, et al. [42] | 1 | Yes | Skin lesions, fever, sore throat, fatigue, headache and lymphadenopathy | Perianal, torso, lower limbs, palms, face and glutes | Dolutegravir/abacavir/lamivudine | Yes (n = 1) |
234,000 copies/mL | 812 | Sotrovimab | Recovered |
| Norz D, et al. [43] | 10 | 2 | Skin lesions (n = 10), lymphadenopathy (n = 5), fever (n = 3), malaise (n = 3), muscle and joint pains (n = 2), penile swelling and pain (n = 1), and anal pain (n = 1) | Genital (n = 8), perianal (n = 3), oral (n = 2), legs (n = 2), anal (n = 2), arms (n = 2), back (n = 1), and face (n = 1) | Bictegravir/Emtricitabin/Tenofovir alafenamide (n = 1) Dolutegravir/lamivudin (n = 1) |
NR | 22 copies/mL (n = 1) and not detectable (n = 1) | 360 (n = 1), and 279 (n = 1) | Local therapy (n = 10), antibiotic therapy (n = 2), analgesic (n = 2) | Recovered (n = 10) |
| Ogoina D, et al. [44] | 40 | 9 | Skin lesions (n = 40), fever (n = 36), lymphadenopathy (n = 35), body aches (n = 25), headache (n = 19), sore throat (n = 18), pruritus (n = 15), and conjunctivitis and photophobia (n = 9). | Face (97.5%), trunk (92.5%), arms (87.5%), legs (85%), genitalia (67.5%), scalp (62.5%), palms (55%), soles (50%), mouth (37.5%), and eyes (25%) | Yes (n = 5) | Yes (n = 4) | 4798 copies/mL (n = 1) | 20 (n = 1), 55 (n = 1), 300 (n = 1), 101 (n = 1), 354 (n = 1), and 357 (n = 1) | Symptomatic and supportive care according to the Nigerian interim guidelines for management of HMPOX | HIV: death (n = 2) Without-HIV: death (n = 3) |
| Ogoina D, et al. [45] | 21 | 2 | Skin lesions (n = 21), fever (n = 18), itching (n = 14), malaise (n = 13), headache (n = 12), lymphadenopathy (n = 13), sore throat (n = 9), myalgia (n = 5), conjuctivitis (n = 4), and diarrhoea (n = 1) | NR | NR | Yes (n = 2/8) | NR | 354 (n = 1), and 280 (n = 1) | NR | Recovered (n = 20) Death (n = 1, suicide) |
| Ogoina D, et al. [46] | 16 | 3 | Skin lesions (n = 7), and fever (n = 9) | NR | NR | NR | NR | NR | NR | NR |
| Oprea C, et al. [47] | 1 | Yes | Skin lesions, fever, lymphadenopathy, chills, rectal pain, and dysphagia | Genital, perineal, anal, neck, trunk, and upper and lower limbs | 3TC/ABC/DTG | No (n = 1) | 40 copies/mL | 988 | Symptomatic treatment, fluids and topic treatment | Recovered |
| Oprea C, et al. [48] | 1 | Yes | Skin lesions, fever, lymphadenopathy, malaise, nausea, loss of appetite, and jaundice | Genital, anal, trunk, lumb., face, ear flap, limbs, palms, soles, and oral mucosa | Tenofovir/lamivudine/dolutegravir (adherence problems) | No (n = 1) | 2820 copies/mL | 936 | Glucose, arginine, benzathine benzylpenicillin, dexamethasone 8 mg/day, vitamin K and fresh frozen plasma | Recovered |
| Orviz E, et al. [49] | 48 | 19 | Skin lesions (n = 45), fever (n = 25), asthenia (n = 32), myalgia (n = 25), lymphadenopathy (n = 39), headache (n = 25), proctitis (n = 13), urethritis (n = 7), rash (n = 4), nasal congestion (n = 4), and cough (n = 8) |
Genital (n = 26), upper extremities (n = 20), perianal (n = 17), trunk (n = 16), facial (n = 12), periorally (n = 9), lower extremities (n = 10), and palms and soles (n = 2) |
Yes (n = 18) | Yes (n = 1) | NR | NR | NR | Recovered (n = 48) |
| Paparizos V, et al. [50] | 1 | Yes | Skin lesions, fever, lymphadenopathy, myalgia and fatigue | Genital | Yes (n = 1) | No (n = 1) | < 20 copies/mL | 648 | Topical octenidine and antibiotic ointment | Recovered (n = 1) |
| Patel A, et al. [51] | 197 | 70 | Mucocutaneous manifestations (n = 197), fever (n = 122), lymphadenopathy (n = 114), headache (n = 49), fatigue/lethargy (n = 46), myalgia (n = 62), arthralgia (n = 21), back pain (n = 21), and rectal pain or pain on defecation (n = 71) | Face (n = 71), trunk (n = 70), arms/legs (n = 74), hands/feet (n = 56), genitals (n = 111), anus or perianal area (n = 82), and oropharyngeal (n = 27) | Yes (n = 64/70) | Yes (n = 1) | <200 copies/mL (n = 55/70) | 664 (522–894) b | Paracetamol, ibuprofen, opioids, and lidocaine gel | Recovered (n = 197) |
| Patrocinio-Jesus R, et al. [52] | 1 | Yes | Skin lesions, lymphadenopathy, fever and sore throat | Genital, face and hands | NR | NR | NR | NR | NR | Recovered (n = 1) |
| Pfäfflin F, et al. [54] | 1 | Yes | Skin lesions, fever, perianal pain, anal abscess, and lymphadenopathy |
Limbs | Yes | NR | NR | 870 | Ibuprofen | Recovered |
| 2 | No | Skin lesions, fever, malaise, anal pain, and anal fissure | Left arm | - | - | - | - | Metamizole, tramadol, lidocaine topical | Recovered | |
| 3 | No | Skin lesions, anal pain, rectal ulcer, and proctitis | Limbs | - | - | - | - | Ibuprofen, metamizole, lidocaine topical | Recovered | |
| 4 | No | Skin lesions, fatigue, anal pain, and anal ulcer | Arms, trunk, genital | - | - | - | - | Metamizole, lidocaine topical | Recovered | |
| 5 | No | Skin lesions, fever, malaise, myalgia, sweats, anal pain, inflammation of rectum and anal canal |
Head, neck, trunk, limbs | - | - | - | - | Metamizole, lidocaine topical | Recovered | |
| 6 | Yes | Skin lesions, anal ulcer, myalgia, fever, malaise, anal pain, and proctitis | Legs | Yes | NR | NR | > 500 | Metamizole, lidocaine topical | Recovered | |
| Philpott F, et al. [55] | 1195 | 490 | Skin lesions (n = 1004), fever (n = 596), chills (n = 550), lymphadenopathy (n = 545), malaise (n = 531), myalgia (n = 507), headache (n = 469), rectal pain (n = 201), pus or blood in stools (n = 184), abdominal pain (n = 96), rectal bleeding (n = 90), tenesmus (n = 90), and vomiting or nausea (n = 83) |
Genital (n = 333), arms (n = 284), face (n = 276), legs (n = 265), perianal (n = 225), mouth, lips, or oral mucosa (n = 179), palms of hands (n = 157), trunk (n = 156), neck (n = 130), head (n = 97), and soles of feet (n = 77) |
NR | NR | NR | NR | NR | NR |
| Pisano L, et al. [57] | 1 | Yes | Skin lesions, lymphadenopathy, asthenia, headache, mild myalgia and cold. | Face, neck, genital, limbs and trunk | Elvitegravir/tenofovir/emtricitabine/cobicistat | No | Undetectable | NR | NR | Recovered (n = 1) |
| Pipitò L, et al. [56] | 1 | Yes | Skin lesions, fever, malaise, sore throat and painful cervical lymphadenopathy | Oral mucosa and trunk | Yes | NR | Undetectable | NR | NR | Recovered |
| 2 | Yes | Skin lesions, sore throat and painful cervical lymphadenopathy | Oral mucosa and nipple | Yes | NR | Undetectable | NR | NR | Recovered | |
| Quattri E, et al. [58] | 1 | Yes | Skin lesion | Genital | NR | NR | NR | NR | NR | Recovered |
| 2 | No | Skin lesion | Genital | - | - | - | - | NR | Recovered | |
| Raccagni AR, et al. [59] | 36 | 15 | Skin lesions (n = 36) | Genital (n = 13), rectal (n = 18), and cutaneous (n = 20) | NR | NR | NR | NR | NR | Recovered (n = 36) |
| Tarin-Vicente EJ, et al. [60] | 181 | 72 | Skin lesions (n = 181), lymphadenopathy (n = 153), influenza-like illness (n = 147), fever (n = 131), headache (n = 96), and sore throat (n = 66) | Genital (n =100), perianal area (n = 66), oral ulcer (n = 45), perioral (n = 51), hands and feet (n = 108), trunk and extremities (n = 104) | Yes (n = 71) | NR | NR | <500 (n = 8) | Cidofovir (n = 6) | Recovered (n = 181) |
| Thornhill JP, et al. [61] | 528 | 218 | Rash or skin lesions (n = 500), fever (n = 330), lymphadenopathy (n = 295), lethargy or exhaustion (n = 216), myalgia (n = 165), headache (n = 145), pharyngitis (n = 113), low mood (n = 54), and proctitis or anorectal pain (n = 75). | Anogenital area (n = 383), trunk or limbs (n = 292), face (n = 134), palms or soles (n = 51), and mucosal lesions (n = 217). | Tenofovir-based three-drug regimen (n = 102/210), abacavir-based three-drug regimen (n = 20/210), zidovudine-based three-drug regimen (n = 2/210), two-drug regimen (n = 48/210) | NR | < 50 copies/mL (n = 180/190), < 200 copies/mL (n = 185/190) | 680 (513–861) a | Cidofovir (n = 12), tecovirimat (n = 8), and vaccinia immune globulin (n = 1) | Recovered (n = 528) |
| Vusirikala A, et al. [62] | 45 | 11 | NR | NR | Yes (n = 11) | NR | Undetectable (n = 10) | NR | NR | Recovered (n = 45) |
| Yakubovsky M, et al. [63] | 26 | 7 | Skin lesions (n = 26), proctitis (n = 26), fever (n = 19), and inguinal lymphadenopathy (n = 17) | Anorrectal (n = 19), genital (n = 11), and other (n = 18) | NR | NR | NR | NR | NR | Recovered (n = 26) |
| Zlámal M, et al. [64] | 1 | Yes | Skin lesions, fever, rash, groin lymphadenopathy, and rectal pain | Anal, perianal, and trunk | Yes (n = 1) | No | Undetectable (n = 1) | >200 | Valaciclovir, ceftriaxone, azithromycin, and metronidazole | Recovered (n = 1) |
Mpox: Monkeypox; NR: No report. a Median (IQR). b Median (Range).
3.3. Demographical Characteristics and Diagnostic Method for Monkeypox
Males accounted for 91.44% (n = 5802) of the total cases registered with Mpox [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. The patients ranged in age from 18 to 71 years. In addition, 51.36% (n = 3259) presented sexual behaviors of being MSM [12,13,14,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,40,41,42,43,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64]. The most frequent previous or current sexually transmitted infections were 40.32% HIV (n = 2558) [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64], 10.26% Gonorrhea (n = 651) [19,24,25,37,49,51,54,56,58,61,63,64], 3.81% Syphilis (n = 242) [13,14,15,19,22,24,25,26,27,28,42,45,48,49,51,54,56,58,60,61,64], and less than 1% HSV (n = 42) [23,24,25,51,61,64]. Overall, almost all confirmed Mpox cases were diagnosed by PCR [12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,62,63,64] and only three studies conducted in Nigeria used positive IgM serology [44,45,46] (Table 2).
3.4. Clinical Symptoms, Skin Lesion Localization, CD4+ T-Cells, Treatment and Outcomes
The most frequent clinical manifestations in patients with Mpox were: 50% skin lesions (n = 3173) [13,14,15,16,17,18,20,21,22,23,24,25,26,27,28,29,32,33,34,35,36,37,38,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,57,58,59,60,61,63,64], 38.53% fever (n = 2445) [12,13,14,16,17,18,21,22,23,24,25,26,27,30,31,32,33,34,35,36,37,39,40,41,42,43,44,45,46,47,48,49,50,51,52,53,54,55,56,60,61,63,64], 35.56% lymphadenopathy (n = 2256) [12,13,14,16,17,18,21,23,24,25,26,27,30,31,32,33,35,36,37,38,39,40,41,42,43,44,45,47,48,49,50,51,52,53,54,55,56,57,60,61,63,64], and 23.61% headache (n = 1498) [12,16,17,18,22,30,31,33,36,40,41,42,44,45,49,51,53,55,57,60,61](Table 3). The most frequent locations of lesions were: 33.16% genitalia (n = 2104) [12,13,15,16,18,21,24,25,26,27,29,30,31,32,33,34,35,36,37,38,40,43,44,47,48,49,50,51,52,53,54,55,57,58,59,60,61,63], 28.51% anus or perianal area (n = 1809) [12,13,14,16,17,18,20,21,22,23,24,25,28,29,30,31,32,33,36,38,39,40,42,43,47,48,49,51,53,55,59,60,61,63,64], and 11.49% mouth, lips, or oral mucosa (n = 729) [12,15,18,21,24,27,29,30,31,35,38,40,43,44,45,46,48,49,51,55,56,60] (Table 3). The majority of patients received empirical treatment targeting sexually transmitted diseases and symptomatic, and only Mpox-targeted treatment received: cidofovir (n = 19) [36,60,61] and tecovirimat (n = 18) [15,22,24,27,28,37,61]. In HIV-positive patients, 428 patients were reported to be receiving ART [12,15,16,17,19,20,23,24,25,26,28,29,32,34,37,38,39,40,41,42,43,44,47,48,49,50,51,54,56,57,60,61,62,64], 114 had a CD4+ T-cell count <350/μL [15,19,21,22,27,28,30,31,38,43,44,45], and 15 had a viral load >200 copies/mL [21,24,27,30,31,42,44,48,51,61]. Finally, six deaths were reported, one of these being of an HIV-positive patient with an undetectable viral load and a CD4+ T-cell count of 74/μL who was receiving chemotherapy for diffuse large B-cell lymphoma with metastases to the spine, skull, and liver [38] and two others in HIV-1 positive patients, one who developed sepsis and another with a CD4+ T-cell count < 20/μL and who died after multiple episodes of seizures [44].
4. Discussion
Monkeypox emerged this last year as an important epidemiological topic to approach due to the rapid spread of confirmed cases [5]. Likewise, diverse investigations identified the association between Mpox and people living with HIV [65,66]. There have been reports of higher HIV and other STI prevalence in the current worldwide Mpox outbreak, which has largely afflicted gay, bisexual, and MSM people [19]. A theoretical idea that HIV may enhance Monkeypox virus transmission and vice versa was also identified [44]. However, there is still limited scientific information about Mpox co-infection with HIV. For that reason, we summarize the cases of this co-infection in order to have a better epidemiological view. In this study, the epidemiological situation of HIV and Mpox co-infection was determined.
We assessed 53 studies. Our principal findings reveal that most of the population (91.44%) was male. Moreover, the main diagnostic test for Mpox was PCR, and that finding shows the relevance of this test in the diagnosis of Mpox. Another relevant result is that 51.36% of the cases were MSM, which demonstrate the importance to explore this risk factor. The HIV and Mpox co-infection were 40.3%, and the most frequent clinical signs were skin lesions, fever, lymphadenopathy, and headache. Identifying all these characteristics or possible risk factors generates a better prescription of the early system of vaccination for Mpox. The Centers for Disease Control and Prevention (CDC) support the recommendation of the vaccine to people who have already been exposed to Mpox or someone who might be in risk of exposure [67].
The largest number of cases registered with co-infection of Mpox and HIV are male (91.44%), which is consistent with other reviews in which it is reported that the population most affected by Mpox are men [6,68,69,70]. The prevalence of Mpox and HIV co-infection was 40% of the cases. This could be due to the fact that most of the cases occurred in MSM (51%) and that MSM has a greater HIV prevalence than the overall population [19,71]. In addition, HIV-positive patients are more likely to attend a health care facility and have a diagnostic test for Mpox compared to HIV-negative patients [72]. However, the 40% prevalence reported in this systematic review exceeds the prevalence of HIV in MSM in the USA (23%) [19] and Europe (7.7%) [73]. This disparity would suggest that transmissibility could be higher in people with HIV [74]. Mpox can be transmitted by respiratory secretions, skin lesions, contaminated fomites and through seminal fluid [69,75]. Reda et al. [69] found that seminal fluid from Mpox-infected patients had a high Monkeypox virus DNA positivity rate (72.4%), behind the positivity rate of anogenital/rectal lesion samples (74.3%). Therefore, such sexual behaviors in HIV patients could predispose to Mpox infection.
Of the three reported deaths of patients with Mpox and HIV co-infection, two cases had a CD4+ T-cells count < 200/μL. Agrati et al. [76] found rapid activation and expansion of CD4+ and CD8+ T cells with effector memory phenotype and a good Th1 cell response that persisted even after clinical recovery in Mpox patients. However, it was also found that paucisymptomatic patients had a less active T-cell response [76]. This suggests a link between the immune response and clinical severity from Mpox. In addition, it was previously reported that Mpox has the ability to trigger a state of T-cell unresponsiveness via a unique major histocompatibility complex (MHC), the independent mechanism that prevents the activation of CD4+ and TCD8+ T-cell antiviral responses and cytokine production [77,78]. This is probably related to viral dissemination and clinical severity in the infected host. Therefore, a state of immunosuppression, characterized by a low CD4+ T-cell count and response in HIV patients, could be associated with clinical severity, dissemination, and mortality from Mpox infection.
Early and consistent ART delivery according to modern combination regimens reduces viraemia in HIV-infected individuals in a few weeks [79]. The level of viral suppression can be so high that viral evolution is halted and the immune system is restored [79]. In the study by Agrati et al. [76] it was found that the T-cell response in patients with Mpox did not differ according to HIV status. This was due to the fact that patients with Mpox and HIV had a good viroimmunological status. In addition, although there is currently no strong evidence to support the use of antiviral drugs directed against Mpox [80,81], such as tecovirimat or cidofovir, the CDC’s “Interim Guidance for Prevention and Treatment of Monkeypox in Persons with HIV Infection” [82] recommends the use of tecovirimat according to the viroimmunological status of the patient and thus avoid possible complications [83]. Therefore, in patients with HIV and Mpox co-infection it is necessary to start or continue the administration of ART and, if indicated, a therapy directed against Mpox such as tecovirimat. Potential drug interactions between ART and tecovirimat are not grounds for discontinuation of either [82].
Limitations and Strengths
Among the limitations of this systematic review is that most of the studies correspond to case reports and case series, while longitudinal observational studies are scarce. Therefore, in order to draw reliable conclusions about the severity and mortality of Mpox in HIV patients, it is necessary to have observational studies with an established control group and to control for different confounding factors, such as previous vaccination status or other comorbidities. Likewise, the information reported regarding HIV stage, antiviral therapy regimen, adherence to treatment, CD4+ T-cells counts and viral load is scarce. It would be important to perform subgroup analyses for these variables to determine their influence during disease development in patients with Mpox and HIV. The available research does not allow us to draw conclusions about the severity and mortality of Mpox in HIV patients. In terms of strengths, the current study had a rigorous methodology because it was carried out in accordance with the PRISMA criteria. Similarly, all steps for selecting research were carried out independently by two or more authors.
5. Conclusions
HIV and Mpox are spread through sexual contact and are more frequent in those who engage in male-male sexual behavior. Co-infection between HIV and Mpox occurred in 40.32%. Co-infection with these two viruses can be especially dangerous, as it can exacerbate the symptoms of both diseases and make them more difficult to treat.
Author Contributions
Conceptualization, B.O.-S., E.S.M.-M. and C.C.-R.; methodology, D.A.L.-F. and R.S.; software, J.J.B.; validation, A.R.-M. and N.A.; formal analysis, B.O.-S. and R.S.; investigation, D.A.L.-F.; resources, J.J.B.; data curation, C.C.-R.; writing—original draft preparation, B.O.-S., E.S.M.-M. and N.A.; writing—review and editing, D.A.L.-F., B.O.-S. and J.J.B.; visualization, A.R.-M.; supervision, N.A.; project administration, A.M., B.K.P. and R.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data included within the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Saied A.A., Dhawan M., Metwally A.A., Fahrni M.L., Choudhary P., Choudhary O.P. Disease History, Pathogenesis, Diagnostics, and Therapeutics for Human Monkeypox Disease: A Comprehensive Review. Vaccines. 2022;10:2091. doi: 10.3390/vaccines10122091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.León-Figueroa D.A., Bonilla-Aldana D.K., Pachar M., Romaní L., Saldaña-Cumpa H.M., Anchay-Zuloeta C., Diaz-Torres M., Franco-Paredes C., Suárez J.A., Ramirez J.D., et al. The Never-Ending Global Emergence of Viral Zoonoses after COVID-19? The Rising Concern of Monkeypox in Europe, North America and Beyond. Travel Med. Infect. Dis. 2022;49:102362. doi: 10.1016/j.tmaid.2022.102362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Melgosa Ramos F.J., Parra Civera M., Pons Fuster J.J. Skin Lesions Due to Monkeypox Virus in a Well-Controlled HIV Patient. Med. Clin. 2022;159:e87–e88. doi: 10.1016/j.medcli.2022.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Satapathy P., Mohanty P., Manna S., Shamim M.A., Rao P.P., Aggarwal A.K., Khubchandani J., Mohanty A., Nowrouzi-Kia B., Chattu V.K., et al. Potentially Asymptomatic Infection of Monkeypox Virus: A Systematic Review and Meta-Analysis. Vaccines. 2022;10:2083. doi: 10.3390/vaccines10122083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.WHO . 2022 Mpox Outbreak Global Map. Centers for Disease Control and Prevention; Geneva, Switzerland: 2022. [Google Scholar]
- 6.León-Figueroa D.A., Barboza J.J., Garcia-Vasquez E.A., Bonilla-Aldana D.K., Diaz-Torres M., Saldaña-Cumpa H.M., Diaz-Murillo M.T., Cruz O.C.-S., Rodriguez-Morales A.J. Epidemiological Situation of Monkeypox Transmission by Possible Sexual Contact: A Systematic Review. Trop. Med. 2022;7:267. doi: 10.3390/tropicalmed7100267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Multi-Country Outbreak of Mpox (Monkeypox)—External Situation Report 12, Published 14 December 2022—World|Relief Web. [(accessed on 28 December 2022)]. Available online: https://reliefweb.int/report/world/multi-country-outbreak-mpox-monkeypox-external-situation-report-12-published-14-december-2022.
- 8.León-Figueroa D.A., Barboza J.J., Saldaña-Cumpa H.M., Moreno-Ramos E., Bonilla-Aldana D.K., Valladares-Garrido M.J., Sah R., Rodriguez-Morales A.J. Detection of Monkeypox Virus According to The Collection Site of Samples from Confirmed Cases: A Systematic Review. Trop. Med. Infect. Dis. 2023;8:4. doi: 10.3390/tropicalmed8010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wong K., Chaudhary M., Magadia R. A Case of Monkeypox Infection in an Unvaccinated HIV-Positive Male in Rural Alabama. Cureus. 2022;14:e31383. doi: 10.7759/cureus.31383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Farahat R.A., Sah R., El-Sakka A.A., Benmelouka A.Y., Kundu M., Labieb F., Shaheen R.S., Abdelaal A., Abdelazeem B., Bonilla-Aldana D.K., et al. Human Monkeypox Disease (MPX) Infez. Med. 2022;30:372–391. doi: 10.53854/liim-3003-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Page M.J., McKenzie J.E., Bossuyt P.M., Boutron I., Hoffmann T.C., Mulrow C.D., Shamseer L., Tetzlaff J.M., Akl E.A., Brennan S.E., et al. The PRISMA 2020 Statement: An Updated Guideline for Reporting Systematic Reviews. BMJ. 2021;2021:n71. doi: 10.1136/bmj.n71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Alpalhão M., Sousa D., Frade J.V., Patrocínio J., Garrido P.M., Correia C., Brazão C., Mancha D., Núncio M.S., Carvalho I.L., et al. Human Immunodeficiency Virus Infection May Be a Contributing Factor to Monkeypox Infection: Analysis of a 42-Case Series. J. Am. Acad. Dermatol. 2022;2022:S0190962222027724. doi: 10.1016/j.jaad.2022.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Antinori A., Mazzotta V., Vita S., Carletti F., Tacconi D., Lapini L.E., D’Abramo A., Cicalini S., Lapa D., Pittalis S., et al. Epidemiological, Clinical and Virological Characteristics of Four Cases of Monkeypox Support Transmission through Sexual Contact, Italy, May 2022. Eurosurveillance. 2022;27:421. doi: 10.2807/1560-7917.ES.2022.27.22.2200421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bížová B., Veselý D., Trojánek M., Rob F. Coinfection of Syphilis and Monkeypox in HIV Positive Man in Prague, Czech Republic. Travel Med. Infect. Dis. 2022;49:102368. doi: 10.1016/j.tmaid.2022.102368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boesecke C., Monin M.B., van Bremen K., Schlabe S., Hoffmann C. Severe Monkeypox-Virus Infection in Undiagnosed Advanced HIV Infection. Infection. 2022;50:1633–1634. doi: 10.1007/s15010-022-01901-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Brites C., Deminco F., Sá M.S., Brito J.T., Luz E., Stocker A. The First Two Cases of Monkeypox Infection in MSM in Bahia, Brazil, and Viral Sequencing. Viruses. 2022;14:1841. doi: 10.3390/v14091841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Brundu M., Marinello S., Scaglione V., Ferrari A., Franchin E., Mazzitelli M., Cattelan A.M. The First Case of Monkeypox Virus and Acute HIV Infection: Should We Consider Monkeypox a New Possible Sexually Transmitted Infection? J. Dermatol. 2022;2022:1346–8138. doi: 10.1111/1346-8138.16556. [DOI] [PubMed] [Google Scholar]
- 18.Català A., Clavo-Escribano P., Riera-Monroig J., Martín-Ezquerra G., Fernandez-Gonzalez P., Revelles-Peñas L., Simon-Gozalbo A., Rodríguez-Cuadrado F.J., Castells V.G., de la Torre Gomar F.J., et al. Monkeypox Outbreak in Spain: Clinical and Epidemiological Findings in a Prospective Cross-sectional Study of 185 Cases*. Br. J. Dermatol. 2022;187:765–772. doi: 10.1111/bjd.21790. [DOI] [PubMed] [Google Scholar]
- 19.Curran K.G., Eberly K., Russell O.O., Snyder R.E., Phillips E.K., Tang E.C., Peters P.J., Sanchez M.A., Hsu L., Cohen S.E., et al. HIV and Sexually Transmitted Infections Among Persons with Monkeypox—Eight U.S. Jurisdictions, May 17–July 22, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1141–1147. doi: 10.15585/mmwr.mm7136a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.De Baetselier I., Van Dijck C., Kenyon C., Coppens J., Michiels J., de Block T., Smet H., Coppens S., Vanroye F., Bugert J.J., et al. Retrospective Detection of Asymptomatic Monkeypox Virus Infections among Male Sexual Health Clinic Attendees in Belgium. Nat. Med. 2022;28:2288–2292. doi: 10.1038/s41591-022-02004-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.de Sousa D., Patrocínio J., Frade J., Correia C., Borges-Costa J., Filipe P. Human Monkeypox Coinfection with Acute HIV: An Exuberant Presentation. Int. J. STD AIDS. 2022;33:936–938. doi: 10.1177/09564624221114998. [DOI] [PubMed] [Google Scholar]
- 22.Gandrakota N., Lee H., Nwosu O., Kulshreshtha A. Monkeypox Coinfection with Neurosyphilis in a Transgender with HIV in Atlanta, USA. Travel Med. Infect. Dis. 2022;50:102454. doi: 10.1016/j.tmaid.2022.102454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gedela K., Da Silva Fontoura D., Salam A., Gorman G., Golden J., O’Hara G., Elowaidy A., Tittle V., Girometti N., Whitlock G., et al. Infectious Proctitis Due to Human Monkeypox. Clin. Infect. Dis. 2022;2022:ciac713. doi: 10.1093/cid/ciac713. [DOI] [PubMed] [Google Scholar]
- 24.Girometti N., Byrne R., Bracchi M., Heskin J., McOwan A., Tittle V., Gedela K., Scott C., Patel S., Gohil J., et al. Demographic and Clinical Characteristics of Confirmed Human Monkeypox Virus Cases in Individuals Attending a Sexual Health Centre in London, UK: An Observational Analysis. Lancet Infect. Dis. 2022;22:1321–1328. doi: 10.1016/S1473-3099(22)00411-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gomez-Garberi M., Sarrio-Sanz P., Martinez-Cayuelas L., Delgado-Sanchez E., Bernabeu-Cabezas S., Peris-Garcia J., Sanchez-Caballero L., Nakdali-Kassab B., Egea-Sancho C., Olarte-Barragan E.H., et al. Genitourinary Lesions Due to Monkeypox. Eur. Urol. 2022;82:625–630. doi: 10.1016/j.eururo.2022.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hammerschlag Y., MacLeod G., Papadakis G., Adan Sanchez A., Druce J., Taiaroa G., Savic I., Mumford J., Roberts J., Caly L., et al. Monkeypox Infection Presenting as Genital Rash, Australia, May 2022. Eurosurveillance. 2022;27:411. doi: 10.2807/1560-7917.ES.2022.27.22.2200411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hermanussen L., Grewe I., Tang H.T., Nörz D., Bal L.C., Pfefferle S., Unger S., Hoffmann C., Berzow D., Kohsar M., et al. Tecovirimat Therapy for Severe Monkeypox Infection: Longitudinal Assessment of Viral Titers and Clinical Response Pattern—A First Case-series Experience. J. Med. Virol. 2022;2022:jmv. 28181. doi: 10.1002/jmv.28181. [DOI] [PubMed] [Google Scholar]
- 28.Hernandez L.E., Jadoo A., Kirsner R.S. Human Monkeypox Virus Infection in an Immunocompromised Man: Trial with Tecovirimat. Lancet. 2022;400:e8. doi: 10.1016/S0140-6736(22)01528-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Heskin J., Belfield A., Milne C., Brown N., Walters Y., Scott C., Bracchi M., Moore L.S., Mughal N., Rampling T., et al. Transmission of Monkeypox Virus through Sexual Contact—A Novel Route of Infection. J. Infect. 2022;85:334–363. doi: 10.1016/j.jinf.2022.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hoffmann C., Jessen H., Wyen C., Noe S., Kreckel P., Köppe S., Krauss A.-S., Schuler C., Bickel M., Lenz J., et al. Monkeypox in Germany. Dtsch. Ärzteblatt Int. 2022;199:551. doi: 10.3238/arztebl.m2022.0287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoffmann C., Jessen H., Wyen C., Grunwald S., Noe S., Teichmann J., Krauss A., Kolarikal H., Scholten S., Schuler C., et al. Clinical Characteristics of Monkeypox Virus Infections among Men with and without HIV: A Large Outbreak Cohort in Germany. HIV Med. 2022:hiv.13378. doi: 10.1111/hiv.13378. [DOI] [PubMed] [Google Scholar]
- 32.Huang S.-T., Wu Y.-H., Lin H.-H., Yang J.Y., Hsieh P.-Y., Chiang S.-J., Wang S.-P., Chan Y.-H., Lin L.-F., Chen Y.-J., et al. The First Imported Case of Monkeypox in Taiwan. J. Formos. Med. Assoc. 2022;1:73–77. doi: 10.1016/j.jfma.2021.01.012. S0929664622003242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Iñigo Martínez J., Gil Montalbán E., Jiménez Bueno S., Martín Martínez F., Nieto Juliá A., Sánchez Díaz J., García Marín N., Córdoba Deorador E., Nunziata Forte A., Alonso García M., et al. Monkeypox Outbreak Predominantly Affecting Men Who Have Sex with Men, Madrid, Spain, 26 April to 16 June 2022. Eurosurveillance. 2022;27:471. doi: 10.2807/1560-7917.ES.2022.27.27.2200471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lapa D., Carletti F., Mazzotta V., Matusali G., Pinnetti C., Meschi S., Gagliardini R., Colavita F., Mondi A., Minosse C., et al. Monkeypox Virus Isolation from a Semen Sample Collected in the Early Phase of Infection in a Patient with Prolonged Seminal Viral Shedding. Lancet Infect. Dis. 2022;22:1267–1269. doi: 10.1016/S1473-3099(22)00513-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Loconsole D., Sallustio A., Centrone F., Casulli D., Accogli M., Saracino A., Foti C., Grandolfo M., Buccoliero G.B., Vitale V., et al. Monkeypox Virus Infections in Southern Italy: Is There a Risk for Community Spread? IJERPH. 2022;19:11719. doi: 10.3390/ijerph191811719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mailhe M., Beaumont A.-L., Thy M., Le Pluart D., Perrineau S., Houhou-Fidouh N., Deconinck L., Bertin C., Ferré V.M., Cortier M., et al. Clinical Characteristics of Ambulatory and Hospitalized Patients with Monkeypox Virus Infection: An Observational Cohort Study. Clin. Microbiol. Infect. 2022:S1198743X22004281. doi: 10.1016/j.cmi.2022.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Matias W.R., Koshy J.M., Nagami E.H., Kovac V., Moeng L.R., Shenoy E.S., Hooper D.C., Madoff L.C., Barshak M.B., Johnson J.A., et al. Tecovirimat for the Treatment of Human Monkeypox: An Initial Series from Massachusetts, United States. Open Forum Infect. Dis. 2022;9:ofac377. doi: 10.1093/ofid/ofac377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Menezes Y.R., Miranda A.B. de Severe Disseminated Clinical Presentation of Monkeypox Virus Infection in an Immunosuppressed Patient: First Death Report in Brazil. Rev. Soc. Bras. Med. Trop. 2022;55:e0392-2022. doi: 10.1590/0037-8682-0392-2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mileto D., Riva A., Cutrera M., Moschese D., Mancon A., Meroni L., Giacomelli A., Bestetti G., Rizzardini G., Gismondo M.R., et al. New Challenges in Human Monkeypox Outside Africa: A Review and Case Report from Italy. Travel Med. Infect. Dis. 2022;49:102386. doi: 10.1016/j.tmaid.2022.102386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Moschese D., Pozza G., Giacomelli A., Mileto D., Cossu M.V., Beltrami M., Rizzo A., Gismondo M.R., Rizzardini G., Antinori S. Natural History of Human Monkeypox in Individuals Attending a Sexual Health Clinic in Milan, Italy. J. Infect. 2022;1:S0163445322005035. doi: 10.1016/j.jinf.2022.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Noe S., Zange S., Seilmaier M., Antwerpen M.H., Fenzl T., Schneider J., Spinner C.D., Bugert J.J., Wendtner C.-M., Wölfel R. Clinical and Virological Features of First Human Monkeypox Cases in Germany. Infection. 2022 doi: 10.1007/s15010-022-01874-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Nolasco S., Vitale F., Geremia A., Tramuto F., Maida C.M., Sciuto A., Coco C., Manuele R., Frasca E., Frasca M., et al. First Case of Monkeypox Virus, SARS-CoV-2 and HIV Co-Infection. J. Infect. 2022;1:S0163445322004790. doi: 10.1016/j.jinf.2022.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nörz D., Brehm T.T., Tang H.T., Grewe I., Hermanussen L., Matthews H., Pestel J., Degen O., Günther T., Grundhoff A., et al. Clinical Characteristics and Comparison of Longitudinal QPCR Results from Different Specimen Types in a Cohort of Ambulatory and Hospitalized Patients Infected with Monkeypox Virus. J. Clin. Virol. 2022;155:105254. doi: 10.1016/j.jcv.2022.105254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ogoina D., Iroezindu M., James H.I., Oladokun R., Yinka-Ogunleye A., Wakama P., Otike-odibi B., Usman L.M., Obazee E., Aruna O., et al. Clinical Course and Outcome of Human Monkeypox in Nigeria. Clin. Infect. Dis. 2020;71:e210–e214. doi: 10.1093/cid/ciaa143. [DOI] [PubMed] [Google Scholar]
- 45.Ogoina D., Izibewule J.H., Ogunleye A., Ederiane E., Anebonam U., Neni A., Oyeyemi A., Etebu E.N., Ihekweazu C. The 2017 Human Monkeypox Outbreak in Nigeria—Report of Outbreak Experience and Response in the Niger Delta University Teaching Hospital, Bayelsa State, Nigeria. PLoS ONE. 2019;14:e0214229. doi: 10.1371/journal.pone.0214229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ogoina D., Yinka-Ogunleye A. Sexual History of Human Monkeypox Patients Seen at a Tertiary Hospital in Bayelsa, Nigeria. Int J. STD AIDS. 2022;33:928–932. doi: 10.1177/09564624221119335. [DOI] [PubMed] [Google Scholar]
- 47.Oprea C., Ianache I., Piscu S., Tardei G., Nica M., Ceausu E., Popescu C.P., Florescu S.A. First Report of Monkeypox in a Patient Living with HIV from Romania. Travel Med. Infect. Dis. 2022;49:102395. doi: 10.1016/j.tmaid.2022.102395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Oprea C., Popa I., Ianache I., Păun A., Vasile S., Grațiela Țârdei, Nica M.M., Popescu C.P., Ceausu E., Florescu S.A. Monkeypox, Severe Hepatitis A, and Syphilis in an HIV Returning Traveler from Spain to Romania. Travel Med. Infect. Dis. 2022;50:102455. doi: 10.1016/j.tmaid.2022.102455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Orviz E., Negredo A., Ayerdi O., Vázquez A., Muñoz-Gomez A., Monzón S., Clavo P., Zaballos A., Vera M., Sánchez P., et al. Monkeypox Outbreak in Madrid (Spain): Clinical and Virological Aspects. J. Infect. 2022;85:412–417. doi: 10.1016/j.jinf.2022.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Paparizos V., Nicolaidou E., Tryfinopoulou K., Papa A., Rigopoulos D., Tsiodras S., Stratigos A. Monkeypox Virus Infection: First Reported Case in Greece in a Patient with a Genital Rash. Acad. Dermatol. Venereol. 2022:jdv.18521. doi: 10.1111/jdv.18521. [DOI] [PubMed] [Google Scholar]
- 51.Patel A., Bilinska J., Tam J.C.H., Da Silva Fontoura D., Mason C.Y., Daunt A., Snell L.B., Murphy J., Potter J., Tuudah C., et al. Clinical Features and Novel Presentations of Human Monkeypox in a Central London Centre during the 2022 Outbreak: Descriptive Case Series. BMJ. 2022;378:e072410. doi: 10.1136/bmj-2022-072410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patrocinio-Jesus R., Peruzzu F. Monkeypox Genital Lesions. N. Engl. J. Med. 2022;387:66. doi: 10.1056/NEJMicm2206893. [DOI] [PubMed] [Google Scholar]
- 53.Perez Duque M., Ribeiro S., Martins J.V., Casaca P., Leite P.P., Tavares M., Mansinho K., Duque L.M., Fernandes C., Cordeiro R., et al. Ongoing Monkeypox Virus Outbreak, Portugal, 29 April to 23 May 2022. Eurosurveillance. 2022;27:424. doi: 10.2807/1560-7917.ES.2022.27.22.2200424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Pfäfflin F., Wendisch D., Scherer R., Jürgens L., Godzick-Njomgang G., Tranter E., Tober-Lau P., Stegemann M.S., Corman V.M., Kurth F., et al. Monkeypox In-Patients with Severe Anal Pain. Infection. 2022;32 doi: 10.1007/s15010-022-01896-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Philpott D., Hughes C.M., Alroy K.A., Kerins J.L., Pavlick J., Asbel L., Crawley A., Newman A.P., Spencer H., Feldpausch A., et al. Epidemiologic and Clinical Characteristics of Monkeypox Cases—United States, May 17–July 22, 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1018–1022. doi: 10.15585/mmwr.mm7132e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pipitò L., Cascio A. Monkeypox Virus Infection and Creatine Phosphokinase Increase: A Case from Italy. Travel Med. Infect. Dis. 2022;50:102412. doi: 10.1016/j.tmaid.2022.102412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Pisano L., Turco M., Mancuso F.R., Lastrucci I., Pimpinelli N. Atypical Oral Presentation of Monkeypox Virus: A Report of Two Cases from Florence, Italy. Travel Med. Infect. Dis. 2022;50:102457. doi: 10.1016/j.tmaid.2022.102457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Quattri E., Avallone G., Maronese C.A., Cusini M., Carrera C.G., Marzano A.V., Ramoni S. Unilesional Monkeypox: A Report of Two Cases from Italy. Travel Med. Infect. Dis. 2022;49:102424. doi: 10.1016/j.tmaid.2022.102424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Raccagni A.R., Candela C., Mileto D., Canetti D., Bruzzesi E., Rizzo A., Castagna A., Nozza S. Monkeypox Infection among Men Who Have Sex with Men: PCR Testing on Seminal Fluids. J. Infect. 2022;85:573–607. doi: 10.1016/j.jinf.2022.07.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarín-Vicente E.J., Alemany A., Agud-Dios M., Ubals M., Suñer C., Antón A., Arando M., Arroyo-Andrés J., Calderón-Lozano L., Casañ C., et al. Clinical Presentation and Virological Assessment of Confirmed Human Monkeypox Virus Cases in Spain: A Prospective Observational Cohort Study. Lancet. 2022;400:661–669. doi: 10.1016/S0140-6736(22)01436-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Thornhill J.P., Barkati S., Walmsley S., Rockstroh J., Antinori A., Harrison L.B., Palich R., Nori A., Reeves I., Habibi M.S., et al. Monkeypox Virus Infection in Humans across 16 Countries—April–June 2022. N. Engl. J. Med. 2022;387:679–691. doi: 10.1056/NEJMoa2207323. [DOI] [PubMed] [Google Scholar]
- 62.Vusirikala A., Charles H., Balasegaram S., Macdonald N., Kumar D., Barker-Burnside C., Cumiskey K., Dickinson M., Watson M., Olufon O., et al. Epidemiology of Early Monkeypox Virus Transmission in Sexual Networks of Gay and Bisexual Men, England, 2022. Emerg. Infect. Dis. 2022;28:2082–2086. doi: 10.3201/eid2810.220960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Yakubovsky M., Shasha D., Reich S., Tau L., Friedel N., Halutz O., Dekel M., Meijer S., Ben-Ami R., Paran Y. Monkeypox Presenting as Proctitis in Men Who Have Sex with Men. Clin. Infect. Dis. 2022:ciac737. doi: 10.1093/cid/ciac737. [DOI] [PubMed] [Google Scholar]
- 64.Zlámal M., Bartovská Z., Burantová A., Zákoucká H., Jiřincová H., Chmel M., Holub M. Monkeypox and Herpes Simplex Virus Type 2 Coinfection: Case Report of Perianal Lesions in HIV-Positive Patient. Sex. Trans. Dis. 2022;49:769–770. doi: 10.1097/OLQ.0000000000001694. [DOI] [PubMed] [Google Scholar]
- 65.Ortiz-Martínez Y., Zambrano-Sanchez G., Rodríguez-Morales A.J. Monkeypox and HIV/AIDS: When the Outbreak Faces the Epidemic. Int. J. STD AIDS. 2022;33:949–950. doi: 10.1177/09564624221114191. [DOI] [PubMed] [Google Scholar]
- 66.UK Health Security Agency Investigation into Monkeypox Outbreak in England: Technical Briefing 6. GOV.UK 2022. [(accessed on 23 September 2022)]; Available online: https://www.gov.uk/government/publications/monkeypox-outbreak-technical-briefings/investigation-into-monkeypox-outbreak-in-england-technical-briefing-1.
- 67.CDC Mpox in the U.S. [(accessed on 8 January 2023)]; Available online: https://www.cdc.gov/poxvirus/monkeypox/response/2022/world-map.html.
- 68.Benites-Zapata V.A., Ulloque-Badaracco J.R., Alarcon-Braga E.A., Hernandez-Bustamante E.A., Mosquera-Rojas M.D., Bonilla-Aldana D.K., Rodriguez-Morales A.J. Clinical Features, Hospitalisation and Deaths Associated with Monkeypox: A Systematic Review and Meta-Analysis. Ann. Clin. Microbiol. Antimicrob. 2022;21:36. doi: 10.1186/s12941-022-00527-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Reda A., Abdelaal A., Brakat A.M., Lashin B.I., Abouelkheir M., Abdelazeem B., Rodriguez-Morales A.J., Sah R. Monkeypox Viral Detection in Semen Specimens of Confirmed Cases: A Systematic Review and Meta-analysis. J. Med. Virol. 2023;95 doi: 10.1002/jmv.28250. [DOI] [PubMed] [Google Scholar]
- 70.DeWitt M.E., Polk C., Williamson J., Shetty A.K., Passaretti C.L., McNeil C.J., Fairman R.T., Sampson M.M., Dalton C., Sanders J.W. Global Monkeypox Case Hospitalisation Rates: A Rapid Systematic Review and Meta-Analysis. eClin. Med. 2022;54:101710. doi: 10.1016/j.eclinm.2022.101710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hessou P.H.S., Glele-Ahanhanzo Y., Adekpedjou R., Ahouada C., Johnson R.C., Boko M., Zomahoun H.T.V., Alary M. Comparison of the Prevalence Rates of HIV Infection between Men Who Have Sex with Men (MSM) and Men in the General Population in Sub-Saharan Africa: A Systematic Review and Meta-Analysis. BMC Public Health. 2019;19:1634. doi: 10.1186/s12889-019-8000-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ghaffar R.A., Shahnoor S., Farooq M. Increased Prevalence of HIV among Monkeypox Patients—An Alarming Update. New Microbes New Infect. 2022;49–50:101039. doi: 10.1016/j.nmni.2022.101039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mirandola M., Gios L., Sherriff N., Marcus U., Toskin I., Rosinska M., Schink S., Kühlmann-Berenzon S., Suligoi B., Folch C., et al. Quantifying Unmet Prevention Needs among MSM in Europe through a Multi-Site Bio-Behavioural Survey. Eurosurveillance. 2018;23:97. doi: 10.2807/1560-7917.ES.2018.23.49.1800097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Alakunle E., Moens U., Nchinda G., Okeke M.I. Monkeypox Virus in Nigeria: Infection Biology, Epidemiology, and Evolution. Viruses. 2020;12:1257. doi: 10.3390/v12111257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.CDC . How It Spreads |Monkeypox| Poxvirus. CDC Centers for Disease Control and Prevention; New York, NY, USA: 2022. [Google Scholar]
- 76.Agrati C., Cossarizza A., Mazzotta V., Grassi G., Casetti R., De Biasi S., Pinnetti C., Gili S., Mondi A., Cristofanelli F., et al. Immunological Signature in Human Cases of Monkeypox Infection in 2022 Outbreak: An Observational Study. Lancet Infect. Dis. 2022:S1473309922006624. doi: 10.1016/S1473-3099(22)00662-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Hammarlund E., Dasgupta A., Pinilla C., Norori P., Früh K., Slifka M.K. Monkeypox Virus Evades Antiviral CD4+ and CD8+ T Cell Responses by Suppressing Cognate T Cell Activation. Proc. Natl. Acad. Sci. USA. 2008;105:14567–14572. doi: 10.1073/pnas.0800589105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rodriguez-Morales A.J., Barbosa-Quintero Z.M., Villamil-Gomez W.E. Is It Possible That Monkeypox Can Behave as an Opportunistic Infection in People Living with HIV? Rev. Chil. Infectol. 2022;39:233–237. doi: 10.4067/s0716-10182022000200233. [DOI] [PubMed] [Google Scholar]
- 79.Deeks S.G., Overbaugh J., Phillips A., Buchbinder S. HIV Infection. Nat. Rev. Dis. Primers. 2015;1:15035. doi: 10.1038/nrdp.2015.35. [DOI] [PubMed] [Google Scholar]
- 80.Ortiz-Saavedra B., León-Figueroa D.A., Montes-Madariaga E.S., Ricardo-Martínez A., Alva N., Cabanillas-Ramirez C., Barboza J.J., Siddiq A., Coaguila Cusicanqui L.A., Bonilla-Aldana D.K., et al. Antiviral Treatment against Monkeypox: A Scoping Review. Trop. Med. 2022;7:369. doi: 10.3390/tropicalmed7110369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kuroda N., Shimizu T., Hirano D., Ishikane M., Kataoka Y. Lack of Clinical Evidence of Antiviral Therapy for Human Monkeypox: A Scoping Review. J. Infect. Chemother. 2022;20:S1341321X22002926. doi: 10.1016/j.jiac.2022.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.O’Shea J., Filardo T.D., Morris S.B., Weiser J., Petersen B., Brooks J.T. Interim Guidance for Prevention and Treatment of Monkeypox in Persons with HIV Infection—United States, August 2022. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1023–1028. doi: 10.15585/mmwr.mm7132e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuehn B.M. Interim Guidance for Monkeypox among Patients with HIV. JAMA. 2022;328:1173. doi: 10.1001/jama.2022.14727. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data included within the manuscript.