Abstract
Simple Summary
Antibiotic growth promoters have long been used in pig diets to maintain gut health and improve growth performance. However, the overuse of antibiotics has led to the emergence of antibiotic-resistance microbes, an imbalance of the healthy intestinal microflora, and antibiotic residues. As a result, probiotic bacteria have been used as an alternative to antibiotics in pig production. Nevertheless, in-feed probiotics may be inconsistent due to differences in preparation methods, feed storage, and ability to survive through the gastrointestinal tract. Heat-killed Lactobacillus plantarum L-137 (HK L-137), also known as a paraprobiotic, has been shown to have beneficial effects on the immune response and has no limitations. Our study indicates that supplementing HK L-137 in pig diets can improve production performance and promote immune function.
Abstract
In the present study, the effects of dietary heat-killed Lactobacillus plantarum L-137 (HK L-137) on the productive performance, intestinal morphology, and cytokine gene expression of suckling-to-fattening pigs were investigated. A total of 100 suckling pigs [(Large White × Landrace) × Duroc; 4.5 ± 0.54 kg initial body weight (BW)] were used and assigned to each of the four dietary treatments as follows: (1) a control diet with antibiotics as a growth promoter (AGP) from the suckling phase to the grower phase and no supplement in the finisher phases; (2) a control diet without antibiotics as a growth promoter (NAGP); (3) a control diet with HK L-137 at 20 mg/kg from the suckling phase to the starter phase and no supplement from the grower phase to the finisher phases (HKL1); and (4) a control diet with HK L-137 at 20 mg/kg from the suckling phase to the weaner phase, at 4 mg/kg from the starter phase to the finisher 1 phase, and no supplement in the finisher 2 phase (HKL2). During the weaner–starter period, the pigs fed on the AGP and HKL2 diets showed significantly higher weight gain and average daily gain (ADG) than those in the NAGP group (p < 0.05). The pigs in the AGP, HKL1, and HKL2 groups showed greater ADG than those in the NAGP groups (p < 0.05) throughout the grower–finisher period. The suckling pigs in the HKL1 and HKL2 groups showed a higher platelet count (484,500 and 575,750) than in the others (p < 0.05); however, there were no significant differences in the other hematological parameters among the treatment groups. The relative mRNA expression level of IFN- ß of the suckling and starter pigs were significantly higher in the HKL1 and HKL2 groups than in the others (p < 0.05), while the IFN-γ showed the highest level in the HKL2 suckling pigs (p < 0.05). These results demonstrate that a HK L-137 supplementation could stimulate the immune response in suckling and starter pigs and promote the growth performance in finishing pigs.
Keywords: cytokine gene expression, growth performance, HK L-137, immune response, swine
1. Introduction
The weaning of piglets is acutely stressful during the initial stage of life, causing a reduction in feed intake, increased incidence of diarrhea, growth retardation, susceptibility to pathogens, and increased mortality [1,2]. These influences may have an effect on pig performance throughout the finishing stage; thus, antibiotic growth promoters (AGPs) have been widely used in weaning pig diets to maintain gut health and increase growth performance [2,3]. However, the continuing use of antibiotics in animal feed has resulted in problems; for example, antibiotic resistance in bacteria has emerged, along with an imbalance of the healthy intestinal microflora and antibiotic residues in animal products [3]. As a result, several countries have banned or restricted the use of AGPs [4,5]. Therefore, there is an ongoing search for non-antibacterial growth promoters that act in vivo, are fast acting, possess a broad spectrum of activity, and can subsequently promote the growth performance of pigs [6]. Due to the aforementioned problems, livestock producers are looking for alternative natural supplements with aspects that can enhance physiological functions and activate animal immune responses. Famous natural and widely used growth promoters are probiotic bacteria [7,8].
Probiotics are live microorganisms that will benefit the host when administered appropriately [9,10]. Health-promoting probiotics contribute to healthy gut microbiota and activate the host immune response, thus positively enhancing growth performances. Lactic acid bacteria (LAB), which are non-pathogenic Gram-positive inhabitants of conventional human and animal intestines, are identified as being associated with health-promoting results by facilitating non-specific immune system enhancement and safety against intestinal infection [11]. Previous research on the use of dietary supplementation with probiotics has improved piglets’ immune response [12,13,14,15,16]. Nevertheless, the effects of probiotic supplementation may be inconsistent due to the differences in preparation methods, feed storage, ability to survive passage through the gastrointestinal tract, and antibiotic-resistance gene transfer to other microorganisms [8,17,18,19,20].
Recently, metabolic by-products, dead microorganisms, or other microbial-based non-viable products (for example, yeast cell walls) are being extensively used as a non-antibiotic nutritional approach to improving the productive performance, gut function, and immune system. Previous studies have reported that non-viable microbes, referred to as immunobiotics or paraprobiotics, also exhibit beneficial effects on the immune response that are equivalent to or greater than probiotics. Moreover, they still have no limitations on the processing, storage, and ability to survive in the gastrointestinal tract [21,22,23,24].
Heat-killed Lactobacillus plantarum strain L-137 (HK L-137), a strain isolated from fermented foods, is a non-viable, heat-treated Lactobacillus plantarum that is resistant to high temperatures in feed processing. It plays a role as an immunostimulant and a growth promoter. HK L-137 has been studied in many land animals, such as mice [21,22,25], broiler chickens [8,17], pigs [26], and aquatic animals, such as kuruma shrimp [27], red sea beam [18,20], and amberjack [19]. These studies indicate that HK L-137 may activate intestinal function, regulate immunological response, and cause an increase in productive performance during the supplementation time. However, its effects on suckling-to-fattening pigs have not been completely investigated [26]. Our study aimed to evaluate the effects of dietary supplementation of HK L-137 on growth performance, hematological parameters, morphology of the small intestine, and expression of cytokine-encoding genes in pigs from the suckling to the finishing stages.
2. Materials and Methods
The current study was carried out in the research farm of the Faculty of Agriculture, Natural Resources and Environment, Naresuan University, Thailand. The experimental procedure was approved by the Naresuan University Animal Care and Use Committee (NUACUC; reference no. NU-AG600613).
2.1. Preparation of Heat-Killed Lactobacillus Plantarum L-137
Heat-killed Lactobacillus plantarum L-137 (HK L-137) was prepared based on the method previously described [21]. In this study, LP ProTM (House Wellness Foods Corp, Itami, Japan) that contained 10% of HK L-137 and 90% of whey protein, dextrin, and sunflower lecithin was used. It contained 1 × 1011 cfu/g of Lactobacillus plantarum in the dry product stored at room temperature (information from product instruction).
2.2. Experimental Design and Pig Management
Crossbred [(Large White × Landrace) × Duroc; 4.5 ± 0.54 kg initial body weight (BW)] suckling pigs were used and assigned to each of the 4 dietary treatments, with piglets from 10 lactating sows per treatment (Landrace × Large White, 250 ± 50 kg of BW) at a parity of 2–6; the sows gave birth at full term and had the same litter size (more than ten live piglets per litter). The experimental feeds (Creep feed) were offered from 14 days (suckling pigs) to 24 days during the weaning age. The number of piglets per treatment in this period was 118, 133, 117, and 125, respectively.
After that, a total of 100 (castrated males) weaning pigs were selected and arranged from the experimental suckling pigs in each treatment using a completely randomized design, with 25 pigs per treatment (5 pigs per replicate and 5 replicates per treatment). They were separated into the weaning pig phase (24–52 days) and starting pig phase (52–66 days). The pigs were weighed on arrival and every week during the weaner-to-starter phase. The ambient temperature was maintained at 30 °C for the first week after weaning, dropped by 1 °C each week after that, and maintained at 26–28 °C. All pigs were given ad libitum feed and water. The pigs from the starting pig phase were transferred to the growing–finishing pig house and weighed every two weeks during the growing–finishing pig phase. The ambient temperature was maintained at 26–28 °C, and the humidity was controlled between 60 and 70%. All pigs were given ad libitum feed and water.
2.3. Dietary Treatments
All nutrients of the dietary treatment met or exceeded the NRC requirements [28]. The formula and chemical composition of the experimental diet are presented in Table 1 as follows: (1) a control diet with antibiotics as a growth promoter (AGP) (amoxicillin and colistin) at 300 mg/kg from the suckling phase to the grower phase and no supplement during the finisher phases; (2) a control diet without antibiotics as a growth promoter (NAGP); (3) a control diet with HK L-137 at 20 mg/kg from the suckling phase to the starter phase and no supplement from the grower to the finisher phases (HKL1); and (4) a control diet with HK L-137 at 20 mg/kg from the suckling phase to the weaner phase, at 4 mg/kg from the starter phase to the finisher 1 phase, and no supplement during the finisher 2 phase (HKL2).
Table 1.
Composition and calculated nutrient content of the experimental diets from the suckling to the finisher 2 phases (g/100 g as the feed basis).
| Item 1 | Suckling | Weaner | Starter | Grower | Finisher 1 | Finisher 2 |
|---|---|---|---|---|---|---|
| (14 Day–Weaned) | (Weaned–10 kg) | (10–25 kg) | (25–50 kg) | (50–75 kg) | (75–100 kg) | |
| Ingredients | ||||||
| Broken rice | 50.40 | 45.85 | 46.00 | 35.00 | 38.00 | 54.00 |
| Full fat soybean | 18.00 | 30.00 | 30.00 | 20.00 | - | - |
| Soybeans meal (44%) | 3.00 | 10.00 | 14.00 | 18.00 | 26.00 | 16.00 |
| Skim milk | 13.00 | 5.00 | 1.00 | - | - | - |
| Cornmeal | - | - | - | 14.00 | 10.00 | - |
| Defatted rice bran | - | 5.00 | 5.00 | - | - | - |
| Rice bran | - | - | - | 10.00 | 20.00 | 25.00 |
| Palm oil | - | - | - | - | 3.00 | 2.00 |
| Fish meals 60% | 4.00 | - | - | - | - | - |
| Fermented soybean meal | 8.00 | - | - | - | - | - |
| Mono-dicalcium phosphate | 0.10 | - | - | - | - | - |
| Dicalcium phosphate | - | 2.00 | 2.00 | 1.80 | 1.30 | 1.30 |
| Bone meals | 2.00 | - | - | - | - | - |
| Calcium carbonate | 0.10 | 0.60 | 0.50 | 0.20 | 0.70 | 0.60 |
| Salt | 0.40 | 0.50 | 0.50 | 0.50 | 0.50 | 0.50 |
| L-lysine | 0.30 | 0.40 | 0.30 | - | - | 0.10 |
| DL-methionine | 0.30 | 0.20 | 0.10 | - | - | - |
| Threonine | 0.15 | 0.20 | 0.10 | - | - | - |
| Premix | 0.25 | 0.25 | 0.50 | 0.50 | 0.50 | 0.50 |
| Chemical composition | ||||||
| Crude Protein, % | 22.32 | 20.09 | 21.24 | 20.15 | 18.00 | 14.76 |
| ME, kcal/kg | 3346 | 3341 | 3396 | 3315 | 3316 | 3290 |
| Crude Fiber, % | 1.43 | 2.79 | 3.06 | 3.68 | 3.97 | 3.59 |
| Ether Extract, % | 4.48 | 6.93 | 6.91 | 5.37 | 1.44 | 1.19 |
| Lysine, % | 1.71 | 1.62 | 1.53 | 1.14 | 1.00 | 0.86 |
| Methionine, % | 0.75 | 0.66 | 0.45 | 0.33 | 0.31 | 0.27 |
| Threonine, % | 1.08 | 1.02 | 0.93 | 0.79 | 0.69 | 0.56 |
| Tryptophan, % | 0.29 | 0.29 | 0.29 | 0.27 | 0.24 | 0.20 |
| Calcium, % | 1.05 | 0.90 | 0.83 | 0.64 | 0.68 | 0.62 |
| Available Phosphorus, % | 0.61 | 0.44 | 0.40 | 0.37 | 0.30 | 0.29 |
1 The dietary treatments were divided into four treatments as follows: (1) AGP = basal feeds supplemented with antibiotics as a growth promoter (amoxicillin and colistin) at 300 mg/kg from the suckling to grower phases; (2) NAGP = basal feeds without AGP or any feed additive from the suckling to the finisher phases; (3) HKL1 = basal feeds supplemented with HK L-137 at 20, 20, 20, 0, 0, and 0 mg/kg; and (4) HKL2 = basal feeds supplemented with HK L-137 at 20, 20, 4, 4, 4, and 0 mg/kg during the suckling phase, weaner phase, starter phase, grower phase, finisher 1 phase, and finisher 2 phase, respectively.
2.4. Sample Collection
For sample collection, ten pigs from each group (two pigs per replication) at 24 days of age and at 66 days of age (starter pigs), with live weights close to the group average, were randomly selected. EDTA-blood samples were collected [29], and all blood parameters were analyzed using an Abbott Cell-Dyn 3700 hematological analyzer (GMI, Ramsey, MN, USA). Finally, all pigs were euthanized to collect the entire small intestine for gut morphology analysis and spleen for gene expression analysis.
2.5. Microscopic Gut Morphology
Each intestinal specimen of about 2–3 cm length was transversely cut from the midpoint of the duodenum, jejunum, and ileum, and gently flushed with 0.1 M phosphate buffered saline (PBS). The intestine was divided into segments based on the length: duodenum (first 10%), jejunum (middle 75%), and ileum (final 15%) [30]. Then, the washed specimens were immersed in buffered formalin and kept in a refrigerator at 4 °C. The samples were dehydrated consecutively with 70, 80, 90, and 100% (absolute) ethanol. An intestinal specimen was embedded in paraffin wax and cut into thin slices of 5 µm thickness. Finally, all sample slides were studied using a compound microscope equipped with a digital camera and an OPTIKA Vision lite software (OPTIKA B-380 series, OPTIKA Microscopes, Bergamo, Italy). The variables of intestinal morphology included villus height, villus area, crypt depth, and villus height per crypt depth. These measurements were analyzed separately for each intestinal segment, according to Incharoen et al. [8].
2.6. Quantitative RT-PCR Analysis
After sample collection, total RNA was extracted from the splenic tissue using the RiboZolTM RNA solution (VWR Life Science, PA, USA) and a tissue lysis buffer, according to the manufacturer’s instructions. Each sample was diluted and used in a reverse transcriptase-polymerase chain reaction (qRT-PCR). The resulting cDNA samples were divided into aliquots and subjected to a real-time polymerase chain reaction (real-time PCR). The primers used in the semi-quantification of cytokine expression, i.e., IL-12, IFN-β, IFN-γ, and reference gene (ß-actin), were designed based on the gene sequences deposited at https://www.ncbi.nlm.nih.gov (accessed on 8 March 2021) (Table 2). The real-time PCR was performed using the fluorescent dye LightCycler® 480 SYBR Green I Master Mix in the LightCycler machine (Real-Time PCR System; Roche Life Science, PA, USA). The ΔCt values were calculated by subtracting the experimental Ct values from the Ct values for the housekeeping gene targets amplified within each sample and were normalized with the ß-actin gene. A relative mRNA expression analysis was carried out using the 2−∆∆Ct method to account for the exponential amplification of the PCR products, as described in our previous study [8].
Table 2.
Primer sequences for the semi-quantitative real-time PCR assay.
| Gene * | Primer Sequences (5′–3′) | PCR Size (bp) | Accession No. |
|---|---|---|---|
| IL-12 | F: AAG CTG TTC ACA AGC TCA AGT ATG A | 81 | NM_214013.1 |
| R: TCT TGG GAG GGT CTG GTT TG | |||
| IFN-ß | F: TGC AAC CAC CAC AAT TCC | 79 | NM_001003923.1 |
| R: CTG AGA ATG CCG AAG ATC TG | |||
| IFN-γ | F: TGG TAG CTC TGG GAA ACT GAA TG | 79 | NM_213948.1 |
| R: GGC TTT GCG CTG GAT CTG | |||
| ß-actin | F: CTC CTT CCT GGG CAT GGA | 65 | U07786.1 |
| R: CGC ACT TCA TGA TCG AGT TGA |
* IL-12 = Interleukin-12, IFN-ß = Interferon-β, IFN-γ = Interferon-γ, and ß-actin = Beta actin (control gene).
2.7. Statistical Analysis
The various parameters tested for the dietary treatments were compared using a one-way analysis of variance (ANOVA), followed by a multiple comparison test. Each pen represented the experimental unit for growth performance traits, while each pig was examined for hematology, intestinal morphology, and gene expression. The data were presented as means ± SEM. Significant differences between the treatment means were determined using the Duncan’s multiple-range test. The significance of the difference was defined at p < 0.05. All statistical analyses were conducted using the statistical software SPSS, version 17.0 (SPSS Inc., Chicago, USA) [31].
3. Results
3.1. Growth Performances
In the randomly selected litters of all treatment groups, no significant differences (p > 0.05) were observed in the litter size of both the total born and live-born between the groups (Table 3). There were no significant differences (p > 0.05) in piglet BW, WG, ADG, ADFI, G:F, and incidence of diarrhea between the groups of pigs during the pre-challenge (birth to 14 days old), post-challenge (14 to 24 days old), and overall suckling period (birth to 24 days old).
Table 3.
Effects of the dietary treatments on the growth performances of piglets fed on the experimental diets from 14 days of age until weaned (24 days old).
| Parameters | Dietary Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| AGP | NAGP | HKL1 | HKL2 | |||
| Number of suckling pigs | 118 | 133 | 117 | 125 | ||
| Litter size | ||||||
| Total born | 12.22 | 14.3 | 12.3 | 13.90 | 0.96 | 0.385 |
| Born alive | 11.89 | 13.3 | 11.7 | 12.50 | 0.89 | 0.642 |
| Piglet body weight | ||||||
| At birth, kg | 1.53 | 1.48 | 1.60 | 1.58 | 0.06 | 0.570 |
| At 14 days old, kg | 4.64 | 4.26 | 4.44 | 4.67 | 0.17 | 0.192 |
| At 24 days old, kg | 5.70 | 5.48 | 5.67 | 5.81 | 0.20 | 0.712 |
| Weight gain, kg | ||||||
| At birth–d14 | 3.11 | 2.78 | 2.84 | 3.09 | 0.18 | 0.241 |
| At 14–24 days old | 1.08 | 1.22 | 1.23 | 1.14 | 0.19 | 0.716 |
| At birth–24 days old | 4.19 | 3.99 | 4.07 | 4.23 | 0.21 | 0.853 |
| Average daily gain, g/d | ||||||
| At birth–14 days old | 222 | 198 | 203 | 221 | 12.70 | 0.241 |
| At 14–24 days old | 108 | 122 | 123 | 114 | 19.28 | 0.716 |
| At birth–24 days old | 182 | 174 | 183 | 184 | 9.20 | 0.853 |
| Average daily feed intake, g/d | 205 | 189 | 209 | 204 | 19.99 | 0.912 |
| Feed efficiency (Gain: Feed) | 0.53 | 0.64 | 0.59 | 0.56 | 0.09 | 0.881 |
| Diarrhea incidence, % | ||||||
| Average at birth–14 days old | 11.83 | 10.97 | 12 | 11.49 | 1.12 | 0.924 |
| Average at 14–24 days old | 6.00 | 6.96 | 7.68 | 7.68 | 0.90 | 0.528 |
AGP = basal feeds supplemented with antibiotics as a growth promoter (amoxicillin and colistin) at 300 ppm from the suckling phase to the grower phase; NAGP = basal feeds without AGP or any feed additive from the suckling to finisher phases; HKL1 = basal feeds supplemented with HK L-137 at 20, 20, 20, 0, 0 and 0 ppm; and HKL2 = basal feeds supplemented with HK L-137 at 20, 20, 4, 4, 4, and 0 ppm during the suckling phase, weaner phase, starter phase, grower phase, finisher 1 phase 1, and finisher 2 phase, respectively. SEM is the standard error mean.
The effects of the dietary supplementation of HK L-137 on the growth performances of the pigs from the weaner to the starter phases are presented in Table 4. Our study showed that BW, WG, ADG, ADFI, and G:F were similar in all treatment groups at 24–38 days, at 52–66 days, and during the entire 66-day experimental period. However, from days 38 to 52 post-weaning, WG and ADG were greater (p < 0.05) in the AGP and HKL2 groups compared to the NAGP group. Table 5 shows the effects of the supplementation of HKL1 and HKL2 on the growth performance of the pigs from the grower to finisher phases, compared to the AGP and NAGP groups. During the grower phase (25–50 kg BW), we observed no significant differences in ADG, ADFI, and G:F between the treatment groups. However, the experimental period in the AGP group was the shortest (p < 0.05), with no significant difference from the HKL1 group.
Table 4.
Effects of the dietary treatments on the growth performances of the pigs from the weaner phase to the starter phase (24 to 66 days old).
| Parameters | Dietary Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| AGP | NAGP | HKL1 | HKL2 | |||
| Initial weight (24 days old) | 6.10 | 5.94 | 5.93 | 6.13 | 0.07 | 0.699 |
| Body Weight, kg | ||||||
| 38 days | 8.11 | 7.60 | 7.64 | 7.71 | 0.10 | 0.232 |
| 52 days | 14.45 | 13.07 | 13.46 | 13.80 | 0.20 | 0.079 |
| 66 days | 21.42 | 19.90 | 20.14 | 20.42 | 0.26 | 0.164 |
| Weight gain, kg | ||||||
| 24–38 days | 2.02 | 1.66 | 1.72 | 1.58 | 0.07 | 0.157 |
| 38–52 days | 6.34 a | 5.47 b | 5.82 ab | 6.09 a | 0.12 | 0.034 |
| 52–66 days | 6.97 | 6.83 | 6.67 | 6.63 | 0.10 | 0.652 |
| Overall | 15.32 | 13.97 | 14.21 | 14.30 | 0.23 | 0.153 |
| Average daily gain, g/d | ||||||
| 24–38 days | 144 | 119 | 123 | 113 | 5.17 | 0.157 |
| 38–52 days | 453 a | 391 b | 416 ab | 435 a | 8.22 | 0.034 |
| 52–66 days | 498 | 488 | 477 | 473 | 7.27 | 0.652 |
| Overall | 365 | 333 | 338 | 340 | 5.42 | 0.153 |
| Average daily feed intake, g/d | ||||||
| 24–38 days | 239 | 203 | 207 | 199 | 6.18 | 0.081 |
| 38–52 days | 745 | 662 | 645 | 709 | 18.57 | 0.214 |
| 52v66 days | 908 | 915 | 885 | 891 | 13.27 | 0.862 |
| Overall | 631 | 593 | 579 | 600 | 9.00 | 0.225 |
| Feed efficiency (Gain: Feed) | ||||||
| 24–38 days | 0.60 | 0.58 | 0.54 | 0.56 | 0.02 | 0.839 |
| 38–52 days | 0.62 | 0.59 | 0.64 | 0.62 | 0.01 | 0.619 |
| 52v66 days | 0.55 | 0.53 | 0.54 | 0.53 | 0.01 | 0.755 |
| Overall | 0.58 | 0.56 | 0.58 | 0.57 | 0.01 | 0.532 |
AGP = basal feeds supplemented with antibiotics as a growth promoter (amoxicillin and colistin) at 300 ppm from the suckling phase to the grower phase; NAGP = basal feeds without AGP or any feed additive from the suckling to the finisher phases; HKL1 = basal feeds supplemented with HK L-137 at 20, 20, 20, 0, 0, and 0 ppm; and HKL2 = basal feeds supplemented with HK L-137 at 20, 20, 4, 4, 4, and 0 ppm during the suckling phase, weaner phase, starter phase, grower phase, finisher 1 phase, and finisher 2 phase, respectively. ab Means with different superscripts differ significantly. SEM is the standard error mean.
Table 5.
Effects of the dietary treatments on the growth performances of the pigs from the starter to the finisher phases to reach 100 kg in body weight.
| Parameters | Dietary Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| AGP | NAGP | HKL1 | HKL2 | |||
| Grower phase, 25–50 kg | ||||||
| ADG, kg/d | 0.67 | 0.54 | 0.60 | 0.58 | 0.05 | 0.450 |
| ADFI, kg/d | 1.66 | 1.43 | 1.49 | 1.42 | 0.12 | 0.645 |
| Feed efficiency (Gain: Feed) | 0.41 | 0.38 | 0.41 | 0.41 | 0.01 | 0.179 |
| Experimental periods | 37 a | 46 c | 42 ab | 43 bc | 1.56 | 0.002 |
| Finisher phase 1, 50–75 kg | ||||||
| ADG, kg/d | 0.90 | 0.79 | 0.92 | 0.92 | 0.06 | 0.418 |
| ADFI, kg/d | 2.46 | 2.20 | 2.52 | 2.50 | 0.17 | 0.530 |
| Feed efficiency (Gain: Feed) | 0.36 | 0.36 | 0.36 | 0.37 | 0.10 | 0.596 |
| Experimental periods | 28 | 32 | 27 | 27 | 1.53 | 0.063 |
| Finisher phase 2, 75–100 kg | ||||||
| ADG, kg/d | 0.98 | 1.00 | 0.95 | 1.10 | 0.03 | 0.065 |
| ADFI, kg/d | 2.82 | 2.99 | 2.79 | 3.05 | 0.09 | 0.273 |
| Feed efficiency (Gain: Feed) | 0.35 ab | 0.34 b | 0.35 ab | 0.36 a | 0.02 | 0.017 |
| Experimental periods | 26 | 25 | 26 | 23 | 1.10 | 0.194 |
| Grower–Finisher phase 2, 25–100 kg | ||||||
| ADG, kg/d | 0.82 a | 0.73 b | 0.79 a | 0.81 a | 0.06 | 0.015 |
| ADFI, kg/d | 2.31 | 2.21 | 2.27 | 2.32 | 0.06 | 0.512 |
| Feed efficiency (Gain: Feed) | 0.36 a | 0.33 b | 0.35 a | 0.35 a | 0.01 | 0.018 |
| Experimental periods | 91 a | 103 b | 95 a | 93 a | 1.91 | <0.001 |
AGP = basal feeds supplemented with antibiotics as a growth promoter (amoxicillin and colistin) at 300 ppm from the suckling phase to the grower phase; NAGP = basal feeds without AGP or any feed additive from the suckling to finisher phases; HKL1 = basal feeds supplemented with HK L-137 at 20, 20, 20, 0, 0, and 0 ppm; and HKL2 = basal feeds supplemented with HK L-137 at 20, 20, 4, 4, 4, and 0 ppm during the suckling phase, weaner phase, starter phase, grower phase, finisher 1 phase, and finisher 2 phase, respectively. abc Means with different superscripts differ significantly (p < 0.05). SEM is the standard error mean.
The experimental period of the NAGP group was the longest (p < 0.05) compared to the other groups, with no significant differences from the HKL2 group. There were no significant differences in any growth performance parameters among the treatment groups during the finisher 1 phase (50–75 kg BW) and the finisher 2 phase (75–100 kg BW). An exception was that the best G:F (p < 0.05) was observed in the HKL2 group, with no significant differences compared to the AGP group in the pigs during the finisher 2 phase. The growth performance throughout the grower–finisher phase (25–100 kg BW) of the pigs did not significantly differ in terms of ADFI and G:F between the treatment groups. However, the pigs in the AGP, HKL1, and HKL2 groups showed higher ADG than the pigs in the NAGP groups (p < 0.05). It was also observed that the group of pigs supplemented with HK L-137 (HKL1 and HKL2) and AGP took less feeding period to attain a BW of 100 kg from 25 kg, compared to the pigs in the NAGP group (p < 0.05).
3.2. Hematological Parameters and Intestinal Morphology
There was no effect of the dietary HK L-137 supplementation on the suckling pigs’ hematological parameters until weaning (p > 0.05), except that the platelet counts in the HKL1 and HKL2 groups were significantly higher (p < 0.003) than the AGP and NAGP groups. However, no differences in the hematological parameters of the suckling pigs were observed between the treatment groups (Table 6).
Table 6.
Effects of the dietary treatments on the hematological parameters of the pigs at the end of the suckling phase (24 days old) and starter phase (73 days old).
| Parameters | Dietary Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| AGP | NAGP | HKL1 | HKL2 | |||
| Suckling phase (24 days old) | ||||||
| RBC count, 106/μL | 7.16 | 7.39 | 7.61 | 7.70 | 0.33 | 0.371 |
| Hb, g/dl | 13.3 | 14.15 | 14.13 | 14.88 | 0.44 | 0.169 |
| Hct, % | 42.5 | 44.35 | 44.75 | 47.75 | 1.39 | 0.130 |
| MCV, fl | 58.50 | 60.75 | 58.50 | 60.75 | 1.76 | 0.737 |
| MCH, fg | 18.88 | 19.40 | 18.58 | 19.28 | 0.59 | 0.825 |
| MCHC, g/dl | 32.08 | 31.90 | 31.75 | 31.83 | 0.16 | 0.648 |
| RDW, % | 17.55 | 16.53 | 15.28 | 16.15 | 0.45 | 0.064 |
| WBC count, cell/cu.mm. | 21,700 | 17,825 | 19,475 | 13,800 | 2304 | 0.190 |
| Neutrophil, % | 42.75 | 45.75 | 44.75 | 39.75 | 3.23 | 0.602 |
| Lymphocyte, % | 56.50 | 53.75 | 54.75 | 60.25 | 3.19 | 0.535 |
| Monocyte, % | 0.00 | 1.00 | 1.00 | 0.00 | 0.14 | 0.248 |
| Platelet count, cell/cu.mm. | 264,000 b | 218,250 b | 484,500 a | 575,750 a | 58,279 | 0.003 |
| Starter phase (73 days old) | ||||||
| RBC, 106/µL | 7.33 | 7.11 | 7.18 | 7.50 | 0.15 | 0.314 |
| Hb, g/dl | 13.18 | 12.94 | 13.30 | 12.94 | 0.28 | 0.787 |
| Hct, % | 41.60 | 40.80 | 41.60 | 41.00 | 0.87 | 0.905 |
| MCV, fl | 56.80 | 56.80 | 57.80 | 54.40 | 1.09 | 0.204 |
| MCH, fg | 18.02 | 18.18 | 18.5 | 17.28 | 0.35 | 0.139 |
| MCHC, g/dl | 31.70 | 31.86 | 31.98 | 31.68 | 0.16 | 0.641 |
| RDW, % | 14.30 | 14.22 | 14.06 | 14.40 | 0.29 | 0.877 |
| WBC, cell/cu.mm. | 16,640 | 19,000 | 16,420 | 20,040 | 1958 | 0.536 |
| Neutrophil, % | 26.80 | 31.80 | 27.40 | 25.40 | 5.12 | 0.864 |
| Lymphocyte, % | 70.80 | 67.00 | 71.40 | 71.60 | 5.21 | 0.933 |
| Monocyte, % | 1.00 | 1.25 | 1.00 | 2.67 | 0.41 | 0.413 |
| Eosinophil, % | 4.50 | 1.00 | 1.00 | 1.60 | 0.50 | 0.386 |
| Platelet count, cell/cu.mm. | 259,200 | 233,400 | 281,400 | 249,000 | 27,492 | 0.706 |
AGP = basal feeds supplemented with antibiotics as a growth promoter (amoxicillin and colistin) at 300 ppm from the suckling phase to the grower phase; NAGP = basal feeds without AGP or any feed additive from the suckling to the finisher phases; HKL1 = basal feeds supplemented with HK L-137 at 20, 20, 20, 0, 0, and 0 ppm; and HKL2 = basal feeds supplemented with HK L-137 at 20, 20, 4, 4, 4, and 0 ppm during the suckling phase, weaner phase, starter phase, grower phase, finisher 1 phase, and finisher 2 phase, respectively. ab Means with different superscripts differ significantly (p < 0.05). SEM is the standard error mean.
The morphological measurements of the duodenal, jejunal, and ileal mucosae in the suckling pigs and the weaning pigs fed with HK l-137 are shown in Table 7. There are no significant differences in the villus height, crypt depth, villus height/crypt depth ratio, and villus area among the treatment groups.
Table 7.
Effects of the dietary treatments on the small intestine (duodenum, jejunum, and ileum) histology of suckling pigs (24 days old).
| Items | Dietary Treatments | SEM | p-Value | |||
|---|---|---|---|---|---|---|
| AGP | NAGP | HKL1 | HKL2 | |||
| Suckling phase (24 days old) | ||||||
| Duodenum | ||||||
| Villus height, µm | 300.13 | 278.27 | 275.57 | 347.00 | 28.96 | 0.363 |
| Crypt depth, µm | 118.50 | 112.14 | 101.37 | 137.37 | 10.69 | 0.197 |
| Villus height/crypt depth ratio | 2.67 | 2.48 | 2.94 | 2.54 | 0.40 | 0.885 |
| Villus area, mm2 | 20.43 | 21.38 | 19.91 | 28.64 | 2.65 | 0.178 |
| Jejunum | ||||||
| Villus height, µm | 293.82 | 270.91 | 262.52 | 227.71 | 32.62 | 0.610 |
| Crypt depth, µm | 121.88 | 108.92 | 106.99 | 109.91 | 12.82 | 0.876 |
| Villus height/crypt depth ratio | 2.45 | 2.56 | 2.45 | 2.07 | 0.26 | 0.602 |
| Villus area, mm2 | 21.93 | 18.45 | 20.28 | 14.88 | 3.56 | 0.629 |
| Ileum | ||||||
| Villus height, µm | 210.61 | 159.16 | 257.26 | 198.46 | 25.79 | 0.120 |
| Crypt depth, µm | 111.29 | 88.57 | 120.83 | 96.55 | 11.51 | 0.263 |
| Villus height/crypt depth ratio | 2.00 | 1.78 | 2.16 | 2.22 | 0.33 | 0.827 |
| Villus area, mm2 | 13.07 | 10.72 | 21.35 | 11.24 | 3.09 | 0.222 |
| Starter phase (73 days old) | ||||||
| Duodenum | ||||||
| Villus height, µm | 265.52 | 286.05 | 272.43 | 250.12 | 18.79 | 0.620 |
| Crypt depth, µm | 136.25 | 130.52 | 125.55 | 127.55 | 9.77 | 0.882 |
| Villus height/crypt depth ratio | 1.96 | 2.20 | 2.20 | 2.00 | 0.14 | 0.510 |
| Villus area, mm2 | 21.97 | 22.94 | 27.30 | 22.80 | 3.21 | 0.740 |
| Jejunum | ||||||
| Villus height, µm | 322.31 | 317.52 | 318.96 | 289.37 | 19.18 | 0.622 |
| Crypt depth, µm | 125.76 | 127.56 | 125.55 | 133.87 | 11.89 | 0.958 |
| Villus height/crypt depth ratio | 2.62 | 2.61 | 2.69 | 2.19 | 0.28 | 0.598 |
| Villus area, mm2 | 27.13 | 24.24 | 27.10 | 22.95 | 1.54 | 0.228 |
| Ileum | ||||||
| Villus height, µm | 223.02 | 249.66 | 245.43 | 228.44 | 20.53 | 0.765 |
| Crypt depth, µm | 108.62 | 118.15 | 113.67 | 112.98 | 10.70 | 0.940 |
| Villus height/crypt depth ratio | 2.11 | 2.12 | 2.20 | 2.06 | 0.17 | 0.959 |
| Villus area, mm2 | 16.40 | 19.00 | 16.93 | 16.28 | 2.1 | 0.784 |
AGP = basal feeds supplemented with antibiotics as a growth promoter (amoxicillin and colistin) at 300 ppm from the suckling phase to the grower phase; NAGP = basal feeds without AGP or any feed additive from the suckling to the finisher phases; HKL1 = basal feeds supplemented with HK L-137 at 20, 20, 20, 0, 0, and 0 ppm; and HKL2 = basal feeds supplemented with HK L-137 at 20, 20, 4, 4, 4, and 0 ppm during the suckling phase, weaner phase, starter phase, grower phase, finisher 1 phase, and finisher 2 phase, respectively.
3.3. Expression of Cytokine-Encoding Genes in Pig Spleen
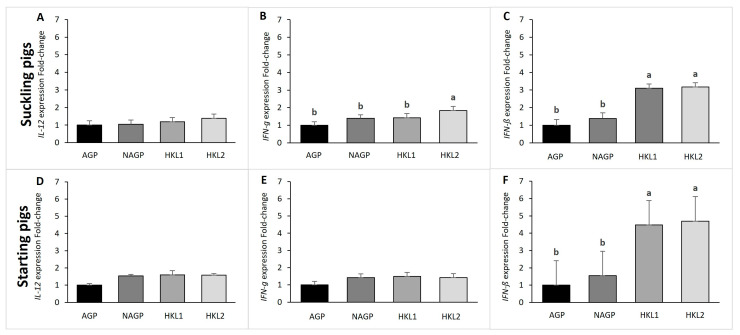
The spleen acts as a filter for blood as part of the immune system. To determine the effects of HK L-137 on cytokine production, we evaluated the expression of interferon (IFN-γ and IFN-β) and interleukin (IL-12β) genes in the spleen of the pigs at the end of the suckling phase (24 days old) and at the end of the starter phase (73 days old). The mean values of relative mRNA expression are presented in Figure 1. At 24 days of age, the HKL2 group showed the highest relative RNA expression level of IFN-γ compared to the other groups. On the other hand, in both the 24- and 73-day-old pigs, the HKL1 and HKL2 groups showed a higher relative expression of IFN-β than the AGP and NAGP groups (p < 0.05). However, there was no significant difference in the expression of IL-12 at 24 and 73 days of age (p > 0.05).
Figure 1.
Effects of the dietary treatments on relative gene expression in the pigs at the end of the suckling phase (24 days old; (A) = IL-12, (B) = IFN-γ, (C) = IFN-ß) and the starter phase (73 days old; (D) = IL-12, (E) = IFN-γ, (F) = IFN-ß). AGP = basal feeds supplemented with antibiotics as a growth promoter (amoxicillin and colistin) at 300 ppm from the suckling to the grower phase; NAGP = basal feeds without AGP or any feed additive from the suckling to the finisher phases; HKL1 = basal feeds supplemented with HK L-137 at 20, 20, 20, 0, 0, and 0 ppm; and HKL2 = basal feeds supplemented with HK L-137 at 20, 20, 4, 4, 4, and 0 ppm during the suckling phase, weaner phase, starter phase, grower phase, finisher 1 phase, and finisher 2 phase, respectively. ab Means with different superscripts differ significantly (p < 0.05). SEM is the standard error mean. IL-12 = Interleukin-12, IFN-ß = Interferon-β, IFN-γ = Interferon-γ, and ß-actin = Beta actin (control gene).
4. Discussion
Weaning pigs usually have poor growth performance, and their gastrointestinal tract is susceptible to infections that can readily cause post-weaning diarrhea. This effect may have an impact on pig performance throughout the finishing stage. Therefore, antibiotic growth promoters (AGPs) are used in pig diets to support gut function and enhance growth performance [2,3]. Probiotic bacteria have been employed as an alternative to antibiotics in pig production. Nonetheless, in-feed probiotics may be uneven due to inconsistencies in preparation methods, feed storage, and ability to survive the gastrointestinal tract [18,19,20]. Previous studies have shown that heat-killed probiotics, which can be obtained by the heat-treated method, can complement probiotics’ risk and stability and exhibit beneficial effects that are equal to or better than living ones [8,21,22]. Moreover, heat-killed probiotics may release exopolysaccharides (EPS), lipoteichoic acids (LTA), and other components with immune-regulatory actions against pathogens [21,22,24]. However, the effect of heat-killed probiotics in suckling-to-fattening pigs has remained unclear [26].
In this study, the effects of dietary supplementation of heat-killed Lactobacillus plantarum strain L-137 (HK L-137) were investigated concerning the growth performance, hematological parameters, intestinal morphology, and expression of cytokine-encoding genes in pigs from the suckling to the finisher phases. Such variations in pig development and growth are probably the result of adaptations to various supplements, farm hygiene, dietary composition, and feed forms [6,32,33,34]. Our results indicated increased weight gain and ADG of weaning pigs that were fed dietary HK L-137 at both tested levels, compared to the NAGP group, for 38–52-day-old pigs. These results for pigs at the 38–52-day stage were in accordance with other studies that reported an enhancement in the growth performance of pigs that were fed diets supplemented with Lactobacillus spp. [32,33,34]. As growing–finishing pigs have a mature gastrointestinal tract with higher digestive enzyme activity, immune capacity, and disease resistance, the influence of HK L-137 in the grower–finisher pigs in this study might be relatively limited. However, our results indicated that the dietary HK L-137 in the treatment HKL groups significantly improved feed efficiency during the finisher phase. Furthermore, both the HKL1 and HKL2 groups showed improved ADG throughout the grower–finisher phase (25–100 kg BW), which was similar to the AGP group.
One possible explanation for this finding is that small changes in FE and ADG during the weaner–starter and grower–finisher stages accumulated up to a point of showing a significant difference across the trial period. These findings agree with the results of Kim et al. [6,35]. They observed that bacteriophages and probiotics could improve different performance parameters, and bacteriophages had a more pronounced effect than probiotics. Moreover, Balasubramanian et al. [36] indicated that supplementation with Bacillus spp. probiotics had a linear correlation with ADG and G:F at week 16 and had a significant linear effect on ADG and G:F in the overall experiment, with no differences observed on ADFI between the dietary treatments during the entire period. Comparably, Meng et al. [37] reported a rise in ADG following a dietary supplementation of Bacillus subtilis and Clostridium butyricum endospores during the experiment. However, G:F was not enhanced throughout the finisher phase. Furthermore, Shon et al. [38] did not find a difference in the growth performance of grower–finisher pigs fed with a diet supplemented with 0.2% probiotics containing L. reuteri, L. salivarius, L. plantarum, and a yeast complex. Although previous studies have reported that live or heat-killed Lactobacillus strains provide a functional component that supports nutrient utilization and immune function, leading to improved growth performance in weaning pigs [26,33,34,39], our findings suggest that supplementing HK L-137 during the suckling to fattening stages can promote the growth performance of the finisher pigs.
Blood hematological parameters can reveal information about body metabolism and health. In this study, the suckling and starter pigs did not show statistically significant differences among the treatment groups, with the exception that the suckling pigs in the groups supplemented with HK L-137 (HKL1 and HKL2) having a significantly higher platelet count than the AGP and NAGP groups. Tao et al. [40] reported the blood platelet counts of suckling pigs at the age of one, four, and seven days as approximately 586.25 ± 115.42, 304.25 ± 123.00, and 406.75 ± 119.59 × 109 cells per liter, respectively. Other factors that alter platelet count include animal health, age, stress, and internal bleeding [40]. The elevated platelet count in the HKL1 and HKL2 groups in this study suggests that HK L-137 may have some potential properties that modulate platelet creations.
It is well known that intestinal morphology is an essential index for gut health analysis. Increasing length and size of the intestinal villi induce absorptive ability by providing a greater surface area. Sayan et al. [41] reported that supplementation with probiotics improves the villus height, although there were no effects on suckling pigs’ crypt depth and villus height/crypt depth ratio. Supplementation with probiotics improved the villus height of the small intestine, thus enhancing the absorption capacity of the small intestine. Lactobacillus spp. can produce short-chain fatty acids to stimulate epithelial cells, enterocytes, mucus secretion, and the villus height and to promote the growth of the intestinal microflora [42,43,44]. However, there were no significant differences in the villus height, crypt depth, villus height/crypt depth ratio, and villus area among the treatment groups in the suckling and weaning pigs. This might be explained by the fact that HK l-137 is a paraprobiotic consisting of dead microbial cells, which cannot produce short-chain fatty acids to stimulate the morphological development of the intestinal epithelium in swine.
Weaning pigs can confront social, physical, and physiological stresses, increasing their intestinal absorption and depressing their immune function [39]. Previous studies demonstrated that HK L-137 could activate intestinal function and immune response [8,17,21,22,25,26]. The immunomodulating activity of paraprobiotics or non-viable microbes might result in some microbial components, for instance, exopolysaccharides (EPS), lipoteichoic acids (LTA), and other components, with anti-pathogen abilities [21,22,24,39]. Lipoteichoic acids (LTA) are cell membrane-bound polyglycerophosphate polymers anchored by a glycolipid and are highly substituted by D-alanyl esters [44]. Several studies reported that LTA is an effective activator or suppressor of the innate immune response via several mechanisms [35,45,46,47]. Lactobacillus strains can also produce EPS, which shields the surface polymer to prevent pathogens from attaching to intestinal barrier and regulate the release of pro-inflammatory cytokines [39,48].
Cytokines are a group of small proteins that specifically affect cell communications and are crucial in the regulation of the immune and inflammatory responses [45]. HK L-137 has been shown to trigger a significant increase in the production of several cytokines in human and animal models [21,22,26,27]. It has been shown to enhance beta (IFN-β) and gamma interferon (IFN-γ) production, promote NK-cell-killing activity, and activate macrophages, which protect against viral infection. To our best knowledge, the present study is the first to investigate the influence of HK L-137 on the expression of cytokine-encoding genes in suckling and starter pigs. A higher mRNA expression level of IFN-β was observed in the spleens of the suckling pigs (24-days-old) in the HK L-137 group, compared to the AGP and NAGP groups. These results are in agreement with the findings of Arimori et al. [26]. They reported that the daily intake of HK L-137 in pigs resulted in significantly higher mRNA expression levels of IFN-β in the whole blood cells than in the control group, which could enhance the host defense against influenza virus infection. Wang et al. [48] showed that a supplementation of L. reuteri I5007 could enhance T-cell differentiation and induce the mRNA expression of the ileal cytokine gene, indicating that L. reuteri I5007 strain can improve immune function in weaned piglets. The results are in line with Yu et al. [38], who demonstrated that probiotic supplementation increased serum-specific anti-OVA IgG levels. Moreover, L. reuteri has been observed to decrease proinflammatory cytokine mRNA expression, such as IL-1β, in the pig ileum [49].
A study by Hatano et al. [50] showed that HK L-137 was a more significant inducer of interleukin-12p40 (IL-12p40), a proinflammatory cytokine in the spleen cells of mice, which, if produced appropriately, will promote the immune response against the influenza virus. It was also demonstrated that this expression, which leads to the induction of IL-12 in mouse spleen and splenic dendritic cells, may be attributed to the putative genes related to LTA synthesis in an internal plasmid of L. plantarum L-137. However, no significant difference in IL-12 gene expression was found in this study. The higher levels of cytokine mRNA expression in the starter pigs in the HKL2 group compared to the AGP group might be attributed to antibiotic influence, which eliminated or disrupted the gut microbiota in the pigs. The cytokine system and its reaction are a complex trait resulting from an animal’s genotype and environment. To better understand the consequences of paraprobiotics on the interaction among the gut microbiome, the immune systems, and nutrient metabolism in swine and other livestock, a shotgun metagenomic analysis using high-throughput sequencing (HTS) should be performed.
5. Conclusions
A dietary supplementation at 20 mg/kg during the suckling phase to the weaning phase and at 4 mg/kg during the starter to the finisher phases increased growth performance and upregulated the expression of IFN-β and IFN-γ in pigs. It did not affect the villus height to crypt depth ratio. This study shows that the use of HK L-137 supplementation in suckling-to-fattening pigs might stimulate the immune response and promote growth performance in the finisher phase. Our study implies that HK L-137 supplementation may be a viable alternative to antibiotic growth promoters (AGPs) in the swine production industry.
Author Contributions
Conceptualization: W.T., S.O. and R.C.; data curation: W.T., T.P. and R.C.; formal analysis: S.N., T.P. and R.C.; methodology: W.T., R.C. and S.O.; software: W.T. and T.P.; validation: W.T., S.N. and R.C.; Investigation: W.T., R.C., S.O. and B.B.; writing—original draft: W.T., R.C., T.I. and T.P.; writing—review and editing: W.T., R.C., S.O. and B.B.; supervision, S.O., G.S. and B.B.; project administration, W.T. and R.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The animal study protocol was approved by the Naresuan University Animal Care and Use Committee (NUACUC; reference no. NU-AG600613).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by the House Wellness Foods Corporation (HWF), Japan, and the Division of Research and Innovation (DRI), Naresuan University, Thailand (grant no. R2560A121).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Frydendahl K. Prevalence of serogroups and virulence genes in Escherichia coli associated with postweaning diarrhoea and edema disease in pigs and a comparison of diagnostic approaches. Vet. Microbiol. 2002;85:169–182. doi: 10.1016/S0378-1135(01)00504-1. [DOI] [PubMed] [Google Scholar]
- 2.Rhouma M., Fairbrother J.M., Beaudry F., Letellier A. Post weaning diarrhea in pigs: Risk factors and non-colistin-based control strategies. Acta Vet. Scand. 2017;59:31. doi: 10.1186/s13028-017-0299-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarz S., Kehrenberg C., Walsh T.R. Use of antimicrobial agents in veterinary medicine and food animal production. Int. J. Antimicrob. Ag. 2001;17:431–437. doi: 10.1016/S0924-8579(01)00297-7. [DOI] [PubMed] [Google Scholar]
- 4.Pettigrew J.E. Reduced use of antibiotic growth promoters in diets fed to weanling pigs: Dietary tools, part 1. Anim. Biotechnol. 2006;17:207–215. doi: 10.1080/10495390600956946. [DOI] [PubMed] [Google Scholar]
- 5.GAIN . Korea Phases out Antibiotic Usage in Compound Feed. USDA Foreign Agricultural Service; Seoul, Republic of Korea: 2011. p. 2. [Google Scholar]
- 6.Kim K.H., Ingale S.L., Kim J.S., Lee S.H., Lee J.H., Kwon I.K., Chae B.J. Bacteriophage and probiotics both enhance the performance of growing pigs but bacteriophage are more effective. Anim. Feed Sci. Technol. 2014;196:88–95. doi: 10.1016/j.anifeedsci.2014.06.012. [DOI] [Google Scholar]
- 7.Allen H.K., Levine U.Y., Looft T., Bandrick M., Casey T.A. Treatment, promotion, commotion: Antibiotic alternatives in food-producing animals. Trends Microbiol. 2013;21:114–119. doi: 10.1016/j.tim.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 8.Incharoen T., Charoensook R., Onoda S., Tartrakoon W., Numthuam S., Pechrkong T. The effects of heat-killed Lactobacillus plantarum L-137 supplementation on growth performance, intestinal morphology, and immune-related gene expression in broiler chickens. Anim. Feed. Sci. Technol. 2019;257:114272. doi: 10.1016/j.anifeedsci.2019.114272. [DOI] [Google Scholar]
- 9.Hill C., Guarner F., Reid G., Gibson G.R., Merenstein D.J., Plot B., Morelli L., Cannani R.B., Flint H.J., Salminen S., et al. The International Scientific Association for Probiotics and Prebiotics consensus statement on the scope and appropriate use of the term probiotic. Nat. Rev. Gastroenterol. Hepatol. 2014;11:506–514. doi: 10.1038/nrgastro.2014.66. [DOI] [PubMed] [Google Scholar]
- 10.FAO . FAO Animal Production and Health Paper. Food and Agriculture Organization of the United Nations; Rome, Italy: 2016. [(accessed on 8 March 2021)]. Probiotics in Animal Nutrition—Production, Impact, and Regulation. no. 179 [Internet] Available online: http://www.fao.org/3/a-i5933e.pdf. [Google Scholar]
- 11.Nomoto K. Prevention of postoperative microbial infection by symbiotic. Indian. J. Exp. Biol. 2008;46:557–561. [PubMed] [Google Scholar]
- 12.European Food Safety Authority Scientific opinion on the safety and efficacy of Calsporin (Bacillus subtilis) as feed additive for piglets. EFSA J. 2010;8:1426–1437. doi: 10.2903/j.efsa.2010.1426. [DOI] [Google Scholar]
- 13.Lee S.H., Ingale S.L., Kim J.S., Kim K.H., Lokhande A., Kim E.K., Kwon I.K., Kim Y.H., Chae B.J. Effects of dietary supplementation with Bacillus subtilis LS 1–2 fermentation biomass on growth performance, nutrient digestibility, cecal microflora and intestinal morphology of weanling pig. Anim. Feed. Sci. Technol. 2014;188:102–110. doi: 10.1016/j.anifeedsci.2013.12.001. [DOI] [Google Scholar]
- 14.Ayichew T., Belete A., Alebachew T., Tsehaye H., Berhanu H., Minyuyelet A. Bacterial Probiotics their Importances and Limitations: A Review. J. Nutr. Health. Sci. 2017;4:202. [Google Scholar]
- 15.Galdeano C.M., Cazorla S.I., Dumit J.M.L., Vélez E., Perdigón G. Beneficial Effects of Probiotic Consumption on the Immune System. Ann. Nutr. Metab. 2019;74:115–124. doi: 10.1159/000496426. [DOI] [PubMed] [Google Scholar]
- 16.Barba-Vidal E., Martín-Orúe S.M., Castillejos L. Practical aspects of the use of probiotics in pig production: A review. Livest. Sci. 2019;223:84–96. doi: 10.1016/j.livsci.2019.02.017. [DOI] [Google Scholar]
- 17.Khonyoung D., Yamauchi K. Effects of heat-killed Lactobacillus plantarum L-137 on morphology of intestinal villi and epithelial cells in broiler chickens. J. Appl. Ani. Res. 2012;40:140–147. doi: 10.1080/09712119.2011.640208. [DOI] [Google Scholar]
- 18.Dawood M.A., Koshio S., Ishikawa M., Yokoyama S. Interaction effects of dietary supplementation of heat-killed Lactobacillus plantarum and beta-glucan on growth performance, digestibility and immune response of juvenile red sea bream, Pagrus major. Fish Shellfish. Immunol. 2015;45:33–42. doi: 10.1016/j.fsi.2015.01.033. [DOI] [PubMed] [Google Scholar]
- 19.Dawood M.A., Koshio S., Ishikawa M., Yokoyama S. Effects of partial substitution of fish meal by soybean meal with or without heat-killed Lactobacillus plantarum (LP20) on growth performance, digestibility, and immune response of amberjack, Seriola dumerili juveniles. BioMed Res. Int. 2015;2015:514196. doi: 10.1155/2015/514196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dawood M.A., Koshio S., Ishikawa M., Yokoyama S. Effects of heat-killed Lactobacillus plantarum (LP20) supplemental diets on growth performance, stress resistance and immune response of red sea bream, Pagrus major. Aquaculture. 2015;442:29–36. doi: 10.1016/j.aquaculture.2015.02.005. [DOI] [Google Scholar]
- 21.Murosaki S., Yamamoto Y., Ito K., Inokushi T., Kusaka H., Ikeda H., Yoshikai Y. Heat-killed Lactobacillus plantarum L-137 suppresses naturally fed antigen-specific IgE production by stimulation of IL-12 production in mice. J. Allergy Clin. Immunol. 1998;102:57–64. doi: 10.1016/S0091-6749(98)70055-7. [DOI] [PubMed] [Google Scholar]
- 22.Murosaki S., Muroyama K., Yamamoto Y., Yoshikai Y. Antitumor effect of heat-killed Lactobacillus plantarum L-137 through restoration of impaired interleukin-12 productiog mice. Cancer Immunol. Immunother. 2000;49:157–164. doi: 10.1007/s002620050615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bosi P., Trevisi P. New topics and limits related to the use of beneficial microbes in pig feeding. Benef. Microbes. 2010;1:447–454. doi: 10.3920/BM2010.0036. [DOI] [PubMed] [Google Scholar]
- 24.de Almada C.N., Almada C.N., Martinez R.C.R., Sant’Ana A.S. Paraprobiotics: Evidences on their ability to modify biological responses, inactivation methods and perspectives on their application in foods. Trends Food Sci. Technol. 2016;58:96–114. doi: 10.1016/j.tifs.2016.09.011. [DOI] [Google Scholar]
- 25.Maeda N., Nakamura R., Hirose Y., Murosaki S., Yamamoto Y., Kase T., Yoshikai Y. Oral administration of heat-killed Lactobacillus plantarum L-137 enhances protection against influenza virus infection by stimulation of type I interferon production in mice. Int. Immunopharmacol. 2009;9:1122–1125. doi: 10.1016/j.intimp.2009.04.015. [DOI] [PubMed] [Google Scholar]
- 26.Arimori Y., Nakamura R., Hirose Y., Murosaki S., Yamamoto Y., Shidara O., Ichikawa H., Yoshikai Y. Daily intake of heat-killed Lactobacillus plantarum L-137 enhances type I interferon production in healthy humans and pigs. Immunophar. Immunotoxicol. 2012;34:937–943. doi: 10.3109/08923973.2012.672425. [DOI] [PubMed] [Google Scholar]
- 27.Tung H.T., Koshio S., Traifalgar R.F. Effects of dietary heat-killed Lactobacillus plantarum on larval and post-larval kuruma shrimp, Marsupenaeus japonicus Bate. J. World Aquacul. Soc. 2010;41:16–27. doi: 10.1111/j.1749-7345.2009.00329.x. [DOI] [Google Scholar]
- 28.NRC . Nutrient Requirements of Swine. 11th ed. National Academy Press; Washington, DC, USA: 2012. [Google Scholar]
- 29.FASS . Guide for the Care and Use of Agricultural Animals in Research and Teaching. 3rd ed. 2441 Village Green Place; Champaign, IL, USA: 2010. p. 169. [Google Scholar]
- 30.Hartke J.L., Monaco M.H., Wheeler M.B., Donovan S.M. Effect of a short-term fast on intestinal disaccharidase activity and villus morphology of piglets suckling insulin-like growth factor-I transgenic sows. J. Anim. Sci. 2005;83:2404–2413. doi: 10.2527/2005.83102404x. [DOI] [PubMed] [Google Scholar]
- 31.SPSS . Statistical Package for Social Sciences. SPSS Inc.; Chicago, IL, USA: 2010. [Google Scholar]
- 32.Giang H.H., Viet T.Q., Ogle B., Lindberg J.E. Growth performance, digestibility, gut environment and health status in weaned piglets fed a diet supplemented with potentially probiotic complexes of lactic acid bacteria. Livest. Sci. 2010;129:95–103. doi: 10.1016/j.livsci.2010.01.010. [DOI] [Google Scholar]
- 33.Liu H., Ji H.F., Zhang D.Y., Zhang S.X., Wang J., Shan D.C., Wang Y.M. Effects of Lactobacillus brevis preparation on growth performance, fecal microflora and serum profile in weaned pigs. Livest. Sci. 2015;178:251–254. doi: 10.1016/j.livsci.2015.06.002. [DOI] [Google Scholar]
- 34.Lähteinen T., Rinttilä T., Koort J.M.K., Kant R., Levonen K., Jakava-Viljanen M., Bjorkroth J., Palva A. Effect of a multispecies lactobacillus formulation as a feeding supplement on the performance and immune function of piglets. Livest. Sci. 2015;80:164–171. doi: 10.1016/j.livsci.2015.07.016. [DOI] [Google Scholar]
- 35.Kim J.S., Hosseindoust A., Lee S.H., Choi Y.S., Kim M.J., Lee J.H., Kwon I.K., Chae B. Bacteriophage cocktail and multi-strain probiotics in the feed for weanling pigs: Effects on intestine morphology and targeted intestinal Coliforms and Clostridium. Animal. 2017;11:45–53. doi: 10.1017/S1751731116001166. [DOI] [PubMed] [Google Scholar]
- 36.Balasubramanian B., Li T., Kim I.H. Effects of supplementing growing-finishing pig diets with Bacillus spp. probiotic on growth performance and meat-carcass grade quality traits. Rev. Bras. Zootec. 2016;45:93–100. doi: 10.1590/S1806-92902016000300002. [DOI] [Google Scholar]
- 37.Meng Q.W., Yan L., Ao X., Zhou T.X., Wang J.P., Lee J.H., Kim I.H. Influence of probiotic in different energy and nutrient density diets on growth performance, nutrient digestibility, meat quality, and blood characteristics in growing finishing pig. J. Anim. Sci. 2010;88:3320–3326. doi: 10.2527/jas.2009-2308. [DOI] [PubMed] [Google Scholar]
- 38.Shon K.S., Hong J.W., Kwon O.S., Min B.J., Kim I.H., Park Y.H., Lee I.S. Effects of Lactobacillus reuteri based direct-fed microbial supplementation for growing-finishing pigs. Asian-Australas. J. Anim. Sci. 2005;18:370–374. doi: 10.5713/ajas.2005.370. [DOI] [Google Scholar]
- 39.Kang J., Lee J.J., Cho J.H., Choe J., Kyoung H., Kim S.H., Kim H.B., Song M. Effects of dietary inactivated probiotics on growth performance and immune responses of weaned pigs. J. Anim. Sci. Technol. 2021;63:520–530. doi: 10.5187/jast.2021.e44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tao X., Xu Z., Men X. Transient effects of weaning on the health of newly weaning piglets. Czech J. Anim. Sci. 2016;61:82–90. doi: 10.17221/8731-CJAS. [DOI] [Google Scholar]
- 41.Sayan H., Assavacheep P., Angkanaporn K., Assavacheep A. Effect of Lactobacillus salivarius on growth performance, diarrhea incidence, fecal bacterial population and intestinal morphology of suckling pigs challenged with F4+ enterotoxigenic Escherichia coli. Asian-Australas. J. Anim. Sci. 2018;8:1308–1314. doi: 10.5713/ajas.17.0746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Estienne M., Hartsock T., Harper A. Effects of antibiotics and probiotics on suckling pig and weaned pig performance. Intern. J. Appl. Res. Vet. Med. 2005;3:303–308. [Google Scholar]
- 43.Yu H.F., Wang A.N., Li X.J., Qiao S.Y. Effect of viable Lactobacillus fermentum on the growth performance, nutrient digestibility and immunity of weaned pigs. J. Anim. Feed Sci. 2008;17:61–69. doi: 10.22358/jafs/66470/2008. [DOI] [Google Scholar]
- 44.Suo C., Yin Y., Wang X., Lou X., Song D., Wang X., Gu Q. eEffects of Lactobacillus plantarum ZJ316 on pig growth and pork quality. BMC Vet. Res. 2012;8:89. doi: 10.1186/1746-6148-8-89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J.M., An J. Cytokines, inflammation and pain. Int. Anesthesiol. Clin. 2007;45:27–37. doi: 10.1097/AIA.0b013e318034194e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Von Aulock S., Hartung T., Hermann C. Comment on Not lipoteichoic acid but lipoproteins appear to be the dominant immunobiologically active compounds in Staphylococcus aureus. J. Immunol. 2007;178:2610–2611. doi: 10.4049/jimmunol.178.5.2610. [DOI] [PubMed] [Google Scholar]
- 47.Hirose Y., Murosaki S., Fujiki T., Yamamoto Y., Yoshikai Y., Yamashita M. Lipoteichoic acids on Lactobacillus plantarum cell surfaces correlate with induction of interleukin-12p40 production. Microbiol. Immunol. 2010;54:143–151. doi: 10.1111/j.1348-0421.2009.00189.x. [DOI] [PubMed] [Google Scholar]
- 48.Wang A., Yu H., Gao X., Li X., Qiao S. Influence of Lactobacillus fermentum I5007 on the intestinal and systemic immune responses of healthy and E. coli challenged piglets. Antonie. Van Leeuwenhoek. 2009;96:89–98. doi: 10.1007/s10482-009-9339-2. [DOI] [PubMed] [Google Scholar]
- 49.Azevedo M.S.P., Zhang W., Wen K., Gonzalez A.M., Saif L.J., Yousef A.E., Yuan L. Lactobacillus acidophilus and Lactobacillus reuteri modulate cytokine responses in gnotobiotic pigs infected with human rotavirus. Benef. Microbes. 2012;3:33–42. doi: 10.3920/BM2011.0041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hatano S., Hirose Y., Yamamoto Y., Murosaki S., Yoshikai Y. Scavenger receptor for lipoteichoic acid is involved in the potent ability of Lactobacillus plantarum stain L-137 to stimulate production of interleukin-12p40. Int. Immunopharmacol. 2015;25:321–331. doi: 10.1016/j.intimp.2015.02.011. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.