Organisms utilize a wide range of regulatory mechanisms to control gene expression. While regulation of transcription initiation is a common regulatory strategy, it is now apparent that this is only the starting point. Bacteria have developed several sophisticated regulatory mechanisms that allow the organism to modulate gene expression after transcription has initiated. In addition, several subtle mechanisms allow organisms to fine-tune the final level of any particular gene product. Several of these mechanisms, which operate after transcription initiation, are crucial for regulating tryptophan metabolism in Bacillus subtilis.
The B. subtilis trpEDCFBA operon contains six of the seven genes that are required for the biosynthesis of tryptophan from chorismic acid, the common aromatic amino acid precursor (Fig. 1). The trp operon is present within a histidine and aromatic amino acid supraoperon. In addition to the trp operon promoter driving expression of this operon, the promoter from the upstream aroFBH operon contributes to trp operon expression. Since the first terminator for the aro operon is the terminator in the trp leader, transcriptional readthrough results in transcription of the trp operon structural genes (23). trpG, the remaining tryptophan biosynthetic gene, is present in an operon primarily concerned with folic acid biosynthesis (Fig. 1) (38). Since there is no evidence for regulation of initiation from the trpEDCFBA promoter, it appears that the greater than 1,000-fold regulation observed for TrpE synthesis occurs after transcription has initiated. The trp RNA-binding attenuation protein (TRAP) plays a central role in controlling tryptophan metabolism by sensing the concentration of tryptophan in the cell (for previous reviews, see references 3, 20, 23). Another recently identified protein called anti-TRAP (AT) antagonizes TRAP activity (41). Since expression of the gene encoding AT responds to the accumulation of uncharged tRNATrp, it is now apparent that B. subtilis regulates tryptophan biosynthesis by sensing the levels of both tryptophan and uncharged tRNATrp in the cell (35, 41).
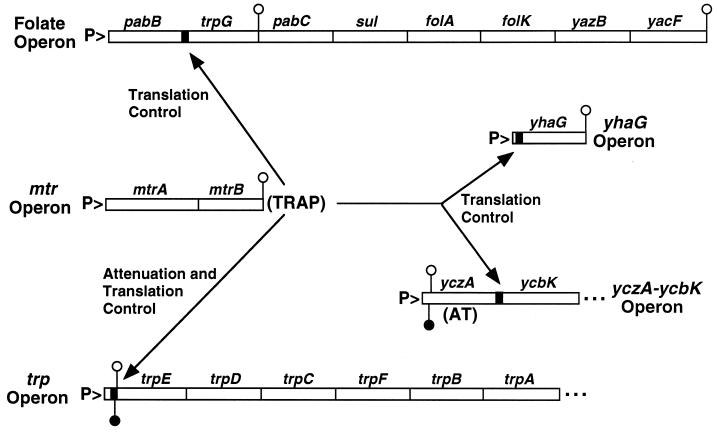
FIG. 1.
Folate, mtr, trp, yhaG, and yczA-ycbK operons. trpG is located within the folate operon, while the rest of the trp genes are clustered in the trpEDCFBA operon. yhaG encodes a putative tryptophan transport protein. mtrB encodes TRAP, while yczA encodes the AT protein (41). TRAP is responsible for regulating expression of the trp operon by transcription attenuation and a translational control mechanism. TRAP regulates translation of trpG, yhaG, and probably ycbK. P> marks the position of the promoters for each operon, while the black boxes show the positions of the TRAP binding sites. The positions of terminator hairpins (open circles) and antiterminator hairpins (filled circles) are shown.
TRAP regulates tryptophan biosynthesis by participating in transcription attenuation and translational control mechanisms. In the transcription attenuation mechanism, TRAP is responsible for the decision to terminate transcription in the trp operon leader region or to allow transcription to proceed into the trp structural genes by sensing the level of tryptophan in the cell (e.g., 4, 9, 27, 33). TRAP is also responsible for regulating translation of trpE and trpG. In the case of trpE, TRAP binding promotes formation of an RNA structure that sequesters the trpE Shine-Dalgarno (SD) sequence (14, 27, 31). Interestingly, the trpE SD sequence is more than 100 nucleotides downstream from the TRAP binding site. In contrast, TRAP regulates TrpG synthesis by binding to a segment of the trpG message that contains the SD sequence (7, 16, 38, 44). In addition to the tryptophan biosynthetic genes, TRAP regulates expression of a putative tryptophan transport gene (yhaG) (Fig. 1). As with trpG, it appears that TRAP regulates YhaG synthesis by binding to the cognate SD sequence (34). Thus, TRAP coordinately regulates tryptophan synthesis and transport by three distinct mechanisms; transcription attenuation of the trpEDCFBA operon, promoting formation of the trpE SD blocking hairpin, and blocking ribosome access to the trpG and yhaG ribosome binding sites. In these situations TRAP functions by binding to RNA targets in a tryptophan-dependent manner.
TRANSCRIPTION ATTENUATION OF THE TRP OPERON
Transcription of the trpEDCFBA operon initiates 203 nucleotides upstream of the trpE start codon (24, 36). The B. subtilis trp leader transcript contains several inverted repeats that are capable of forming three RNA secondary structures involved in the transcription attenuation mechanism (Fig. 2). All three of these structures are conserved in Bacillus pumilus, Bacillus stearothermophilus, and Bacillus caldotenax.
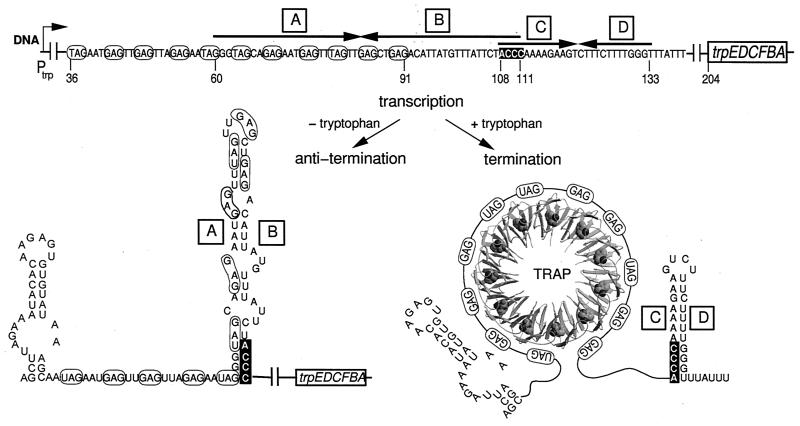
FIG. 2.
Transcription attenuation model of the trp operon. When tryptophan is limiting (−tryptophan) TRAP is not activated. During transcription, antiterminator formation (A and B) prevents formation of the terminator (C and D), which results in transcription of the trp operon structural genes. When tryptophan is in excess (+tryptophan) TRAP is activated. Tryptophan-activated TRAP can bind to the (G/U)AG repeats and promote termination by preventing antiterminator formation. The overlap between the antiterminator and terminator structures is shown. Numbering is from the start of transcription.
In B. subtilis, an antiterminator structure can form just upstream of an intrinsic terminator. Since these two structures overlap by four nucleotides, their formation is mutually exclusive (4, 27, 37). In cells growing in the presence of excess tryptophan, TRAP is activated to bind to 11 (G/U)AG repeats (7 GAG and 4 UAG) that overlap the 5′ portion of the antiterminator (7). Inhibition of antiterminator formation by bound TRAP favors formation of the terminator hairpin, and transcription halts in the trp leader region. In limiting-tryptophan growth conditions, TRAP is not activated and does not bind to the nascent trp leader transcript. Under these conditions the antiterminator forms and the operon is expressed. Thus, the ability of TRAP to modulate which of these two alternative structures forms in response to changes in the intracellular tryptophan concentration serves as the basis for the transcription attenuation mechanism of this operon (Fig. 2).
In addition to the antiterminator and terminator structures, an RNA hairpin forms at the 5′ end of the trp leader transcript (5′ stem-loop) and participates in the transcription attenuation mechanism (Fig. 2). Disruption of this structure increases trp operon expression in vivo and transcriptional readthrough in vitro (40). TRAP–5′ stem-loop interaction increases the affinity of TRAP for trp leader RNA and reduces the number of (G/U)AG repeats that are necessary for tight TRAP binding (15). Thus, it is possible that TRAP–5′ stem-loop interaction increases the rate of TRAP binding to the nascent trp leader transcript. This would increase the probability that TRAP binding to the triplet repeats takes place before the antiterminator forms, thereby increasing the likelihood that transcription termination will occur before RNA polymerase can reach the trp operon structural genes. Several TRAP-RNA binding studies and in vitro transcription experiments using B. subtilis RNA polymerase have firmly established that TRAP binds to trp leader RNA, that binding is tryptophan dependent, and that TRAP enhances termination in the leader region of the trp operon (e.g., see references 4 and 33). Similar in vitro transcription results have also been obtained using T7, SP6, or Escherichia coli RNA polymerase (33), consistent with the model in which TRAP functions by interacting with the RNA rather than with RNA polymerase.
TRAP is encoded by the second gene of the mtrAB operon (Fig. 1) (21). While it is not known how expression of this operon is regulated, mtrAB is not regulated in response to tryptophan nor is TRAP involved in controlling its own expression (20, 29). Since mtrA encodes GTP cyclohydrolase I, an enzyme involved in folic acid biosynthesis (9), it is possible that folic acid or an intermediate in its biosynthesis is involved in regulating expression of the mtr operon by activating an unidentified repressor protein. A potential candidate would be chorismic acid since this compound is an intermediate in the biosynthesis of both folic acid and tryptophan (3). The 75-amino-acid TRAP polypeptide does not show significant similarity to the sequences of other characterized RNA binding motifs. The only known protein that shows significant sequence homology to TRAP is SplA, a regulator of the spore photoproduct lyase gene (splB) in B. subtilis (19).
TRANSLATIONAL CONTROL OF TRPE
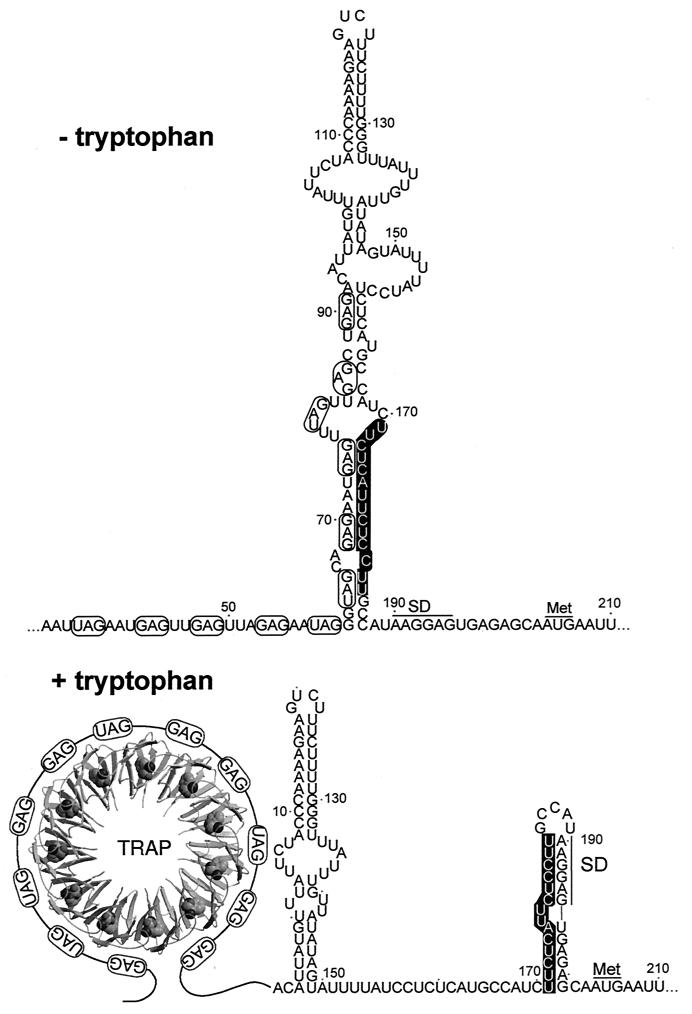
In addition to regulating transcription of the trpEDCFBA operon, TRAP regulates translation of trpE. In vivo studies indicate that TRAP regulates transcription termination in the trp leader approximately 100-fold and that TRAP inhibits translation of trpE an additional 13-fold (14, 27, 31). When TRAP binds to a trp operon readthrough transcript, the trp leader RNA adopts a structure in which the trpE SD sequence is sequestered in a stable RNA hairpin (trpE SD blocking hairpin) (Fig. 3) (14, 27, 31). Translational control of trpE expression requires a higher intracellular concentration of tryptophan than that required for attenuation (43a). This recent finding adds to the significance of previous results where it was shown that the affinity of TRAP for trp leader RNA increases with increasing tryptophan concentrations (33, 43) and that the dissociation rate of TRAP-trp leader RNA complexes decreases as the concentration of tryptophan increases (10). In the transcription attenuation mechanism, only transient TRAP interaction with the (G/U)AG repeats is necessary to block formation of the antiterminator. In contrast, for translational control TRAP must remain associated with the triplet repeats to maintain formation of the trpE SD blocking hairpin (14). In the absence of bound TRAP the trp leader transcript adopts a structure in which the trpE SD sequence is single stranded (14). Studies carried out in vitro support the TRAP-dependent trpE translational control model. TRAP binding promotes formation of the trpE SD blocking hairpin, and formation of this structure inhibits ribosome binding and, as a consequence, TrpE synthesis (14). Thus, TRAP regulates TrpE synthesis by promoting the formation of a trp leader RNA secondary structure that is more than 100 nucleotides downstream from the 3′ end of the TRAP binding site. The ability to form the trpE SD blocking hairpin is conserved in B. pumilus, B. stearothermophilus, and B. caldotenax.
FIG. 3.
trpE translational control model. Under tryptophan-limiting conditions, TRAP is not activated and is unable to bind to the trp leader transcript. In this case the trp leader RNA adopts a structure such that the trpE SD sequence is single stranded and available for translation. Under excess tryptophan conditions, TRAP is activated and binds to the (G/U)AG repeats. As a consequence, the trpE SD blocking hairpin forms, which prevents ribosome binding and translation. The overlap between the two alternative structures is shown. Numbering is from the start of transcription.
TRANSLATIONAL COUPLING AND TRANSCRIPTIONAL POLARITY IN THE TRP OPERON
With the exception of trpC and trpF, all of the coding sequences in the trp operon overlap by several nucleotides. This sequence arrangement suggested that translational coupling, a process in which translation of an upstream gene in a polycistronic transcript influences translation of the gene immediately downstream, plays a role in trp operon expression (24, 30, 32). For example, the coding sequences of trpE and trpD overlap by 29 nucleotides. Recent results establish that translation of trpD is coupled to translation of trpE and that formation of the trpE SD blocking hairpin regulates expression of trpD via translational coupling (43a). Thus, it is possible that TRAP-dependent formation of the trpE SD blocking hairpin coordinately regulates translation of the entire operon via translational coupling. The overlapping trp genes, and presumably translational coupling, are conserved in B. stearothermophilus as well (12).
Another interesting finding is that Rho, a transcription termination factor, influences expression of the trp operon when cells are grown under conditions that promote translational control of trpE expression. When cells are grown under tryptophan excess conditions, expression of the trp operon increases severalfold in Rho-deficient strains compared to isogenic wild-type strains. It is likely that the increase in translational inhibition that occurs in the presence of excess tryptophan leads to the appearance of a relatively ribosome-free transcript segment downstream from the trpE SD blocking hairpin. Thus, it appears that Rho gains access to the nascent trp transcript downstream from this structure, thereby causing transcriptional polarity of the trp operon (43a).
TRANSLATIONAL CONTROL OF TRPG
trpG is the second gene in an operon primarily concerned with folic acid biosynthesis (38) (Fig. 1). The TrpG polypeptide functions as a glutamine amidotransferase in the biosynthesis of tryptophan (TrpG-TrpE) and folic acid (TrpG-PabB) (26). Translation of trpG is regulated by TRAP in response to tryptophan, whereas translation of pabB is not (44). TRAP binds to nine triplet repeats that surround and overlap the trpG SD sequence and inhibits TrpG synthesis (7 GAG, 1 UAG, and 1 AAG) (7, 16). Deletion of the five triplet repeats located just upstream from the trpG SD sequence abolishes TRAP-dependent regulation (44). These findings indicate that binding of tryptophan-activated TRAP to the nine triplet repeats present in the trpG message regulates translation of trpG by blocking ribosome access to the trpG ribosome binding site. In addition, TRAP binding to the folate transcript reduces the steady-state level of the message that encodes all eight of the polypeptides, but not the mRNA segment that corresponds only to the first two genes of the operon (13). Since the steady-state level of folate transcripts is increased when cells are transferred to fresh medium, it also appears that the folate operon is influenced by the growth phase of the cells (13). Neither the mechanism by which TRAP regulates folate operon transcript levels nor the mechanism of growth phase control is understood.
TRANSLATIONAL CONTROL OF A PUTATIVE TRYPTOPHAN TRANSPORT GENE
A computer search of the B. subtilis genome identified a gene of previously unknown function (yhaG) that appears to contain a TRAP binding site that overlaps the yhaG SD sequence and translation initiation region (34). This presumed TRAP target contains as many as nine triplet repeats (5 GAG, 3 UAG, and 1 AAG). In vivo expression studies demonstrated that translation of yhaG is regulated by TRAP. The findings that YhaG is predicted to be a transmembrane protein and that YhaG-deficient strains are more resistant to the tryptophan analog 5-fluorotryptophan than are wild-type strains suggest that YhaG functions in tryptophan transport (34).
REGULATION OF TRP GENE EXPRESSION BY TRNATRP AND THE ANTI-TRAP PROTEIN
In E. coli and many other bacterial species, the well-characterized transcription attenuation mechanism for the trp operon senses the availability of charged tRNATrp (28). It is now known that the availability of charged tRNATrp also influences expression of the B. subtilis trp genes (29). In vivo studies examining the effect of a mutant tryptophanyl-tRNA synthetase gene (trpS) suggested that B. subtilis has a mechanism for recognizing an increase in the level of uncharged tRNATrp and of responding to this accumulation by increasing expression of the trp genes (29, 39). A search of the B. subtilis genome sequence identified an additional operon (yczA-ycbK) with a presumed TRAP binding site (35). This site contains as many as 11 triplet repeats (7 GAG, 3 AAG, and 1 CAG) that overlap the ycbK SD sequence and translation initiation region. Furthermore, the features of this operon suggested that it could be regulated by uncharged tRNATrp (35). yczA is preceded by a leader regulatory region with the characteristics of operons regulated by uncharged tRNA via the T-box antitermination mechanism (reviewed in reference 22). Indeed, transcription of this operon was shown to be regulated by antitermination in response to changes in the level of uncharged tRNATrp (35). Moreover, overexpression of yczA abolished TRAP-mediated regulation of trp operon expression, whereas replacing the yczA start codon with a stop codon eliminated this effect (41). In addition, it was shown that purified YczA inhibits TRAP binding to trp leader transcripts via protein-protein interaction. Since these results indicate that YczA functions as an inhibitor of TRAP activity, this protein is now referred to as AT, for anti-TRAP protein (41). While the function of yczA has been determined, the function of ycbK is unknown.
TRAP-RNA RECOGNITION
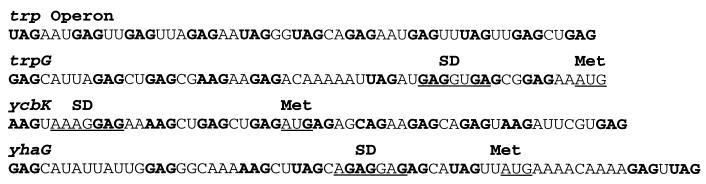
Numerous in vitro (4, 6–8, 11) and in vivo (2) studies have established that TRAP recognizes RNAs containing multiple GAG and UAG (and rarely AAG) trinucleotide repeats. These triplet repeats are separated from each other by nonconserved spacer nucleotides. Two nucleotide spacers are optimal (8), with pyrimidines generally favored over purines (11, 42). The TRAP binding site in the B. subtilis trp leader RNA consists of 11 repeats (7 GAG and 4 UAG), each separated by two or three nucleotide spacers (Fig. 4). The B. pumilus trp leader also contains 11 (G/U)AG repeats (25), whereas this region from B. stearothermophilus and B. caldotenax contains 12 triplet repeats, including 1 CAG in B. stearothermophilus (12). The TRAP binding site in trpG of B. subtilis contains nine triplet repeats (7 GAG, 1 UAG, and 1 AAG) (14, 38), while the presumed TRAP binding sites in yhaG (34) and yczA-ycbK (35) of B. subtilis contain as many as 9 or 11 triplet repeats, respectively (Fig. 4). In these last three binding sites there are a few exceptionally long spacers separating some of the repeats. Thus, it is apparent that these binding sites are suboptimal. A plausible reason for the suboptimal trpG TRAP target is that it would allow a basal level of TrpG synthesis which would maintain folate synthesis in the presence of tryptophan-activated TRAP (16). The finding that TrpG synthesis is regulated only sevenfold in response to tryptophan is consistent with this hypothesis (44). As mentioned above, the 5′ stem-loop also participates in the attenuation mechanism (40). This structure is located two nucleotides upstream from the first triplet repeat (Fig. 2). Exactly how this structure acts is not firmly established, but it appears to interact with TRAP to increase the affinity of TRAP for trp leader RNA (15).
FIG. 4.
Comparison of the TRAP binding sites. The (G/U)AG repeats are shown in bold type. The positions of the SD sequences and the start codons are shown for trpG, ycbK, and yhaG.
TRAP STRUCTURE
The structures of TRAP complexed with tryptophan from B. subtilis (2) and B. stearothermophilus (12) have been determined by X-ray crystallography. The amino acid sequences of the two proteins are 77% identical, and their structures are also very similar, consisting of 11 identical subunits arranged in a ring around a central hole (Fig. 5). The TRAP oligomer is composed of 11 seven-stranded antiparallel β-sheets, each containing four β-strands from one subunit and three strands from the adjacent subunit. This structural arrangement generates extensive interfaces between adjacent subunits that greatly stabilize the oligomer structure (2, 11, 12). TRAP is activated to bind RNA by the cooperative binding of tryptophan (2, 5). The crystal structure reveals that each tryptophan molecule binds between adjacent subunits with its indole ring buried in a hydrophobic pocket. Nine hydrogen bonds are formed between the amino group, the carboxyl group, and the indole nitrogen of each bound tryptophan with amino acids residing in two loops on adjacent subunits. The hydrogen bond formed between Thr30 and the amino group of tryptophan, as well as the hydrogen bond between Thr49 and the carboxyl group of tryptophan, appears to be essential for TRAP function. Conversely, the hydrogen bond between Ser53 and the carboxyl group of tryptophan is dispensable for TRAP activity (43). The mechanism by which tryptophan binding activates TRAP to bind RNA is not known. While the structure of TRAP in the absence of bound tryptophan has not yet been determined, several biochemical studies have established that TRAP remains as an 11-subunit complex in the absence of bound tryptophan.
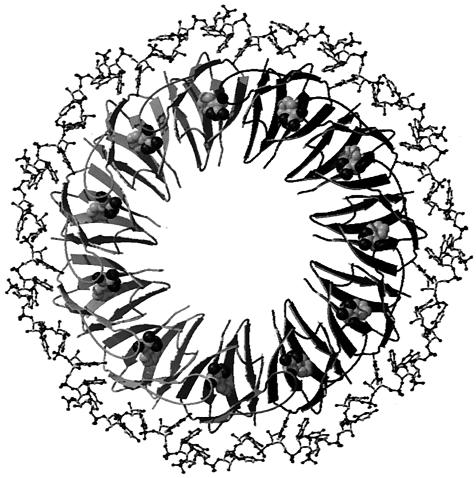
FIG. 5.
Ribbon diagram of TRAP complexed with an RNA containing 11 GAG repeats separated by AU spacers. TRAP binds to the linear transcript and wraps the RNA around the periphery of the TRAP complex. The TRAP subunits are shown in various shades of gray, and the RNA is shown in ball-and-stick models. The bound l-tryptophan molecules are shown as van der Waals spheres.
The discoveries that TRAP contains 11 subunits and that its RNA binding sites contain multiple trinucleotide repeats, including 11 in the B. subtilis and B. pumilus trp operon leaders, suggested a model in which the bound RNA wraps around the TRAP protein (Fig. 5) (2). Further support for this model came from the identification of three amino acid residues from each subunit (Lys37, Lys56, and Arg58), whose replacement by alanine specifically interferes with RNA binding (45). These three residues are aligned on the perimeter of the TRAP ring, suggesting that the (G/U)AG repeats interact with the 11 KKR patches on the protein. Nucleoside analog studies identified several important functional groups for interaction with TRAP on the second A and third G of each triplet repeat. These studies also suggested that the bases in the first position (G or U), as well as the spacer bases, are not crucial for TRAP recognition and binding (17).
Several unusual properties of the TRAP-RNA complex may be relevant to the mechanism by which TRAP binds RNA to regulate gene expression in vivo. Several studies of TRAP binding to RNAs containing various numbers of NAG repeats (where N is any nucleotide) showed that the stability of the TRAP-RNA complex is not directly proportional to the number of triplet repeats in the RNA (8, 18). Measurable affinity for TRAP was seen with RNA containing as few as three GAG repeats. The affinity then increased as the number of repeats was raised from three to six GAGs. However, raising the number of repeats from 6 to 11 repeats resulted in only a slight increase in the stability of the complex with TRAP. These studies suggested a cooperative mechanism of RNA binding to TRAP. Additional studies using nucleoside analogs (18) as well as studies of heteromeric TRAP proteins containing mixtures of wild-type and mutant subunits (P. Li and P. Gollnick, submitted for publication) further support these findings. Together these results suggest that the mechanism of TRAP binding to RNA involves initial interaction with a small subset of the repeats followed by a cooperative wrapping of the remainder of the RNA binding site around the protein (see below).
The crystal structure of B. stearothermophilus TRAP complexed with a 53-base RNA consisting of 11 GAG repeats separated by AU spacers was recently solved (1). As predicted, the RNA wraps around the outside of the protein ring (Fig. 5). The phosphodiester backbone is on the outside of the RNA ring, and the bases point in toward the protein. Nearly all of the contacts with RNA are to groups on the bases; there are no contacts to phosphates. The only direct hydrogen bond to the phosphodiester backbone is a hydrogen bond to the 2′ OH of the ribose of the third G in each repeat. This contact, which was predicted by deoxyribonucleoside substitution studies (17), explains how TRAP distinguishes RNA from DNA. As predicted, Lys37, Lys56, and Arg58 all form hydrogen bonds with the RNA. Lys37 hydrogen bonds to the second A of each repeat, while both Lys56 and Arg58 hydrogen bond with the third G of each repeat. In addition, Glu36 forms hydrogen bonds with the third G of each repeat, and Phe32 stacks on this base. The AU spacer bases do not directly contact the protein. This is consistent with the lack of sequence conservation in spacers of natural TRAP binding sites (12) and the observations that their composition is generally not critical for TRAP binding (8, 10, 42). The preference for G and U in the first position of the triplet repeats is not clarified by the crystal structure. This simple mechanism of wrapping the RNA around the circular TRAP complex explains both the specificity of the protein for its RNA targets as well as how TRAP binding alters RNA structure to function in regulating both transcription and translation.
FUTURE CONSIDERATIONS
While several mechanistic features concerning the regulation of tryptophan biosynthesis in B. subtilis and its relatives are understood, many fundamental questions have not been answered. Since the structure of TRAP in the absence of bound tryptophan has not been determined, the mechanism of TRAP activation by tryptophan is not known. Furthermore, the reason that G or U is preferred over A or C in the first position of triplet repeats in TRAP binding sites is unresolved. In addition, structural information will be required to understand how AT inhibits TRAP activity. It will also be important to elucidate the mechanism of 5′ stem-loop function and to determine if translational coupling influences expression of the entire trp operon. Finally, it will be necessary to investigate the mechanisms responsible for regulating expression of yhaG, yczA, and ycbK and to determine the functions of YhaG and YcbK.
ACKNOWLEDGMENTS
We thank Angela Valbuzzi and Charles Yanofsky for sharing data prior to publication. We also thank Fred Antson of York University for making the TRAP-RNA structure figure.
This work was supported by grant GM52840 from the National Institutes of Health to P.B. and grant GM62750 from the National Institutes of Health and grant MCB 9982652 from the National Science Foundation to P.G.
REFERENCES
- 1.Antson A A, Dodson E J, Dodson G, Greaves R B, Chen X, Gollnick P. Structure of the trp RNA-binding attenuation protein, TRAP, bound to RNA. Nature. 1999;401:235–242. doi: 10.1038/45730. [DOI] [PubMed] [Google Scholar]
- 2.Antson A A, Otridge J, Brzozowski A M, Dodson E J, Dodson G G, Wilson K S, Smith T M, Yang M, Kurecki T, Gollnick P. The structure of the trp RNA attenuation protein. Nature. 1995;374:693–700. doi: 10.1038/374693a0. [DOI] [PubMed] [Google Scholar]
- 3.Babitzke P. Regulation of tryptophan biosynthesis: Trp-ing the TRAP or how Bacillus subtilis reinvented the wheel. Mol Microbiol. 1997;26:1–9. doi: 10.1046/j.1365-2958.1997.5541915.x. [DOI] [PubMed] [Google Scholar]
- 4.Babitzke P, Yanofsky C. Reconstitution of Bacillus subtilis trp attenuation in vitro with TRAP, the trp RNA-binding attenuation protein. Proc Natl Acad Sci USA. 1993;90:133–137. doi: 10.1073/pnas.90.1.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Babitzke P, Yanofsky C. Structural features of L-tryptophan required for activation of TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis. J Biol Chem. 1995;270:12452–12456. doi: 10.1074/jbc.270.21.12452. [DOI] [PubMed] [Google Scholar]
- 6.Babitzke P, Bear D G, Yanofsky C. TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis, is a toroid-shaped molecule that binds transcripts containing GAG or UAG repeats separated by two nucleotides. Proc Natl Acad Sci USA. 1995;92:7916–7920. doi: 10.1073/pnas.92.17.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Babitzke P, Stults J T, Shire S J, Yanofsky C. TRAP, the trp RNA-binding attenuation protein of Bacillus subtilis, is a multisubunit complex that appears to recognize G/UAG repeats in the trpEDCFBA and trpG transcripts. J Biol Chem. 1994;269:16597–16604. [PubMed] [Google Scholar]
- 8.Babitzke P, Yealy J, Campanelli D. Interaction of the trp RNA-binding attenuation protein (TRAP) of Bacillus subtilis with RNA: effects of the number of GAG repeats, the nucleotides separating adjacent repeats, and RNA secondary structure. J Bacteriol. 1996;178:5159–5163. doi: 10.1128/jb.178.17.5159-5163.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Babitzke P, Gollnick P, Yanofsky C. The mtrAB operon of Bacillus subtilis encodes GTP cyclohydrolase I (MtrA), an enzyme involved in folic acid biosynthesis, and MtrB, a regulator of l-tryptophan biosynthesis. J Bacteriol. 1992;174:2059–2064. doi: 10.1128/jb.174.7.2059-2064.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Baumann C, Otridge J, Gollnick P. Kinetic and thermodynamic analysis of the interaction between TRAP (trp RNA-binding attenuation protein) of Bacillus subtilis and trp leader RNA. J Biol Chem. 1996;271:12269–12274. doi: 10.1074/jbc.271.21.12269. [DOI] [PubMed] [Google Scholar]
- 11.Baumann C, Xirasagar S, Gollnick P. The trp RNA-binding attenuation protein (TRAP) from Bacillus subtilis binds to unstacked trp leader RNA. J Biol Chem. 1997;272:19863–19869. doi: 10.1074/jbc.272.32.19863. [DOI] [PubMed] [Google Scholar]
- 12.Chen X-P, Antson A A, Yang M, Li P, Baumann C, Dodson E J, Dodson G G, Gollnick P. Regulatory Features of the trp operon and the crystal structure of the trp RNA-binding attenuation protein from Bacillus stearothermophilus. J Mol Biol. 1999;289:1003–1016. doi: 10.1006/jmbi.1999.2834. [DOI] [PubMed] [Google Scholar]
- 13.de Saizieu A, Vankan P, Vockler C, van Loon A P G M. The trp RNA-binding attenuation protein (TRAP) regulates the steady-state levels of transcripts of the Bacillus subtilis folate operon. Microbiol. 1997;143:979–989. doi: 10.1099/00221287-143-3-979. [DOI] [PubMed] [Google Scholar]
- 14.Du H, Babitzke P. trp-RNA binding attenuation protein-mediated long-distance RNA refolding regulates translation of trpE in Bacillus subtilis. J Biol Chem. 1998;273:20494–20503. doi: 10.1074/jbc.273.32.20494. [DOI] [PubMed] [Google Scholar]
- 15.Du H, Yakhnin A V, Dharmaraj S, Babitzke P. trp RNA-binding attenuation protein-5′ stem-loop RNA interaction is required for proper transcription attenuation control of the Bacillus subtilis trpEDCFBA operon. J Bacteriol. 2000;182:1819–1827. doi: 10.1128/jb.182.7.1819-1827.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Du H, Tarpey R, Babitzke P. The trp-RNA binding attenuation protein regulates TrpG synthesis by binding to the trpG ribosome binding site of Bacillus subtilis. J Bacteriol. 1997;179:2582–2586. doi: 10.1128/jb.179.8.2582-2586.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Elliott M B, Gottlieb P A, Gollnick P. Probing the TRAP-RNA interaction with nucleoside analogs. RNA. 1999;5:1277–1289. doi: 10.1017/s1355838299991057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Elliott M B, Gottlieb P A, Gollnick P. The mechanism of RNA binding to TRAP: initiation and cooperative interactions. RNA. 2001;7:85–93. doi: 10.1017/s135583820100173x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fajardo-Cavazos P, Nicholson W L. The TRAP-like SplA protein is a trans-acting negative regulator of spore photoproduct lyase synthesis during Bacillus subtilis sporulation. J Bacteriol. 2000;182:555–560. doi: 10.1128/jb.182.2.555-560.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gollnick P. Regulation of the Bacillus subtilis trp operon by an RNA-binding protein. Mol Microbiol. 1994;11:991–997. doi: 10.1111/j.1365-2958.1994.tb00377.x. [DOI] [PubMed] [Google Scholar]
- 21.Gollnick P, Ishino S, Kuroda M I, Henner D J, Yanofsky C. The mtr locus is a two gene operon required for transcription attenuation in the trp operon of Bacillus subtilis. Proc Natl Acad Sci USA. 1990;87:8726–8730. doi: 10.1073/pnas.87.22.8726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henkin T M. Control of transcription termination in prokaryotes. Annu Rev Genet. 1996;30:35–57. doi: 10.1146/annurev.genet.30.1.35. [DOI] [PubMed] [Google Scholar]
- 23.Henner D, Yanofsky C. Biosynthesis of aromatic amino acids. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 269–280. [Google Scholar]
- 24.Henner D, Band L, Shimotsu H. Nucleotide sequence of the Bacillus subtilis tryptophan operon. Gene. 1984;34:169–177. doi: 10.1016/0378-1119(85)90125-8. [DOI] [PubMed] [Google Scholar]
- 25.Hoffman R J, Gollnick P. The mtrB gene of Bacillus pumilus encodes a protein with sequence and functional homology to the trp RNA-binding attenuation protein (TRAP) of Bacillus subtilis. J Bacteriol. 1995;177:839–842. doi: 10.1128/jb.177.3.839-842.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kane J F. Regulation of a common amidotransferase subunit. J Bacteriol. 1977;132:419–425. doi: 10.1128/jb.132.2.419-425.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuroda M I, Henner D, Yanofsky C. cis-acting sites in the transcript of the Bacillus subtilis trp operon regulate expression of the operon. J Bacteriol. 1988;170:3080–3088. doi: 10.1128/jb.170.7.3080-3088.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Landick R, Turnbough C L, Jr, Yanofsky C. Transcription attenuation. In: Neidhardt F C, editor. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 1263–1286. [Google Scholar]
- 29.Lee A I, Sarsero J P, Yanofsky C. A temperature-sensitive trpS mutation interferes with TRAP regulation of trp gene expression in Bacillus subtilis. J Bacteriol. 1996;178:6518–6524. doi: 10.1128/jb.178.22.6518-6524.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McCarthy J E G, Gualerzi C. Translational control of prokaryotic gene expression. Trends Genet. 1990;6:78–85. doi: 10.1016/0168-9525(90)90098-q. [DOI] [PubMed] [Google Scholar]
- 31.Merino E, Babitzke P, Yanofsky C. trp RNA-binding attenuation protein (TRAP)-trp leader RNA interactions mediate translational as well as transcriptional regulation of the Bacillus subtilis trp operon. J Bacteriol. 1995;177:6362–6370. doi: 10.1128/jb.177.22.6362-6370.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Oppenheim D S, Yanofsky C. Translational coupling during expression of the tryptophan operon of Escherichia coli. Genetics. 1980;95:785–795. doi: 10.1093/genetics/95.4.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Otridge J, Gollnick P. MtrB from Bacillus subtilis binds specifically to trp leader RNA in a tryptophan dependent manner. Proc Natl Acad Sci USA. 1993;90:128–132. doi: 10.1073/pnas.90.1.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sarsero J P, Merino E, Yanofsky C. A Bacillus subtilis gene of previously unknown function, yhaG, is translationally regulated by tryptophan-activated TRAP and appears to be involved in tryptophan transport. J Bacteriol. 2000;182:2329–2331. doi: 10.1128/jb.182.8.2329-2331.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sarsero J P, Merino E, Yanofsky C. A Bacillus subtilis operon containing genes of unknown function senses tRNATrp charging and regulates expression of the genes of tryptophan biosynthesis. Proc Natl Acad Sci USA. 2000;97:2656–2661. doi: 10.1073/pnas.050578997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shimotsu H, Henner D J. Characterization of the Bacillus subtilis tryptophan promoter region. Proc Natl Acad Sci USA. 1984;43:85–94. doi: 10.1073/pnas.81.20.6315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shimotsu H, Kuroda M I, Yanofsky C, Henner D J. Novel form of transcription attenuation regulates expression of the Bacillus subtilis tryptophan operon. J Bacteriol. 1986;166:461–471. doi: 10.1128/jb.166.2.461-471.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Slock J, Stahly D P, Han C-Y, Six E W, Crawford I P. An apparent Bacillus subtilis folic acid biosynthetic operon containing pab, an amphibolic trpG gene, a third gene required for synthesis of para-aminobenzoic acid, and the dihydropteroate synthase gene. J Bacteriol. 1990;172:7211–7226. doi: 10.1128/jb.172.12.7211-7226.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Steinberg W. Temperature-induced derepression of tryptophan biosynthesis in a tryptophanyl-transfer ribonucleic acid synthetase mutant of Bacillus subtilis. J Bacteriol. 1974;117:1023–1034. doi: 10.1128/jb.117.3.1023-1034.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sudershana S, Du H, Mahalanabis M, Babitzke P. A 5′ RNA stem-loop participates in the transcription attenuation mechanism that controls expression of the Bacillus subtilis trpEDCFBA operon. J Bacteriol. 1999;181:5742–5749. doi: 10.1128/jb.181.18.5742-5749.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Valbuzzi A, Yanofsky C. Inibition of TRAP, the B. subtilis trp RNA-binding attenuation protein, by the anti-TRAP protein. 2001. AT. Science, in press. [Google Scholar]
- 42.Xirasager S, Elliott M B, Bartolini W, Gollnick P, Gottlieb P. RNA structure inhibits the TRAP (trp RNA-binding attenuation protein)-RNA interaction. J Biol Chem. 1998;273:27146–27153. doi: 10.1074/jbc.273.42.27146. [DOI] [PubMed] [Google Scholar]
- 43.Yakhnin A V, Trimble J J, Chiaro C R, Babitzke P. Effects of mutations in the L-tryptophan binding pocket of the trp RNA-binding attenuation protein of Bacillus subtilis. J Biol Chem. 2000;275:4519–4524. doi: 10.1074/jbc.275.6.4519. [DOI] [PubMed] [Google Scholar]
- 43a.Yakhnin H, Babiarz J E, Yakhnin A V, Babitzke P. Expression of the Bacillus subtilis trpEDCFBA operon is influenced by translational coupling and Rho termination factor. J Bacteriol. 2001;183:5918–5926. doi: 10.1128/JB.183.20.5918-5926.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yang M, de Saizieu A, van Loon A P G M, Gollnick P. Translation of trpG in Bacillus subtilis is regulated by the trp RNA-binding attenuation protein (TRAP) J Bacteriol. 1995;177:4272–4278. doi: 10.1128/jb.177.15.4272-4278.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Yang M, Chen X-P, Gollnick P. Alanine-scanning mutagenesis of Bacillus subtilis trp RNA-binding attenuation protein (TRAP) reveals residues involved in tryptophan binding and RNA binding. J Mol Biol. 1997;270:696–710. doi: 10.1006/jmbi.1997.1149. [DOI] [PubMed] [Google Scholar]