Abstract
Cancer is the leading cause of death around the globe, followed by heart disease and stroke, with the highest mortality to this day. We have reached great levels of understanding of how these various types of cancer operate at a cellular level and this has brought us to what we call “precision medicine” where every diagnostic examination and the therapeutic procedure is tailored to the patient. FAPI is among the new tracers that can be used to assess and treat many types of cancer. The aim of this review was to gather all the known literature on FAPI theranostics. A MEDLINE search was conducted on four web libraries, PUBMED, Cochrane, Scopus, and Web of Sciences. All of the available articles that included both diagnoses and therapy with FAPI tracers were collected and put through the CASP (Critical Appraisal Skills Programme) questionnaire for systematic reviewing. A total of 8 records were deemed suitable for CASP review, ranging from 2018 to November 2022. These studies were put through the CASP diagnostic checklist, in order to assess the goal of the study, diagnostic and reference tests, results, descriptions of the patient sample, and future applications. Sample sizes were heterogeneous, both for size as well as for tumor type. Only one author studied a single type of cancer with FAPI tracers. Progression of disease was the most common outcome, and no relevant collateral effects were noted. Although FAPI theranostics is still in its infancy and lacks solid grounds to be brought into clinical practice, it does not show any collateral effects that prohibit administration to patients, thus far, and has good tolerability profiles.
Keywords: FAPI, theranostics, fibroblast activation, FDG, PET
1. Introduction
Cancer is the leading cause of death around the globe, outranking stroke and coronary heart disease mortality rates in many countries [1]. The COVID-19 pandemic did not aid in the overall management of cancer patients, delaying screening, diagnosis, and treatment for these patients. The Ukraine invasion also played its role last year, as many refugees and other citizens will miss or delay their treatment or screening in the aftermath of this war [2]. Cancer diagnosis can be done through different imaging modalities, among which positron emission tomography (PET) plays a central role. It permits in vivo imaging in patients by injection of a radiopharmaceutical, made of a β+-emitting isotope and a carrier, a molecule with a known biodistribution in the organism. The annihilation of a β+ particle and an electron, results in the emission of γ-rays in opposite directions, which are detected by the scanner. PET can tell, within some degree of error, where a metabolic process is taking place. Lately, we have seen great improvement in these tracers, bringing us to theranostics, where we are able to image and conduct diagnosis at the same time [3,4,5,6]. 18Fluoro-2-deoxyglucose (FDG) is the most used tracer in PET for oncologic imaging. FDG becomes internalized by the cell through the glucose transporters (GLUTs). As FDG enters the cell, it is phosphorylated by the hexokinase enzyme and does not undergo any other interaction in the cells, where it is trapped. Malignant, proliferating cells have modified metabolic pathways, which are seen because FDG overexpression in relation to the surrounding tissue, thus the contrast between structures is high [7]. The physiopathology lies in the Warburg effect, described by Otto Warburg 100 years ago, where the malignant cells could meet their energy demand by aerobic glycolysis [8]. PET finds a central role in TNM staging, as far as N and M are concerned, whereas, in T staging, CT plays a more pivotal role [9,10,11]. Although FDG accounts for the majority of PET, we are slowly but surely shifting to a more specific molecular targeted imaging with more specific tracers. FAPI-PET is an example of this shift that we are already living. Many studies have already been conducted evaluating FAPI in many types of tumor, both in diagnosis and theranostics settings. The tumor microenvironment (TME) plays a fundamental role in understanding cancer biology. TME is mainly found in the extracellular matrix (ECM), which is made up of blood vessels, growth factors, cytokines, and fibroblasts. The latter’s role is mainly collagen production and regulation of homeostasis and inflammation of the surrounding cells. Some fibroblasts have contraction properties that make them a special subpopulation, called myofibroblasts, with smooth muscle characteristics. These cells express fibroblast activation protein (FAP), which is the main subject of this review [12]. Cancer-associated fibroblasts (CAFs) are the main component of the TME and are found in most solid tumors. They have either tumor-suppressive or promoting activity. Both normal fibroblasts and CAFs share a spindle shape, although CAFs exhibit slight differences in cytoplasm branches and nuclei under a light microscope. They show high ECM synthesis and remodeling, leading to fibrosis and cancer progression [13]. It has been proven that FAP inhibition leads to a decrease in tumor vascularization [14]. FAP belongs to the dipeptidyl peptidase (DPP) protein family, which includes DPP4, FAP, DDP8, and DDP9. Therefore, FAP functions as an endopeptidase enzyme for substrates with glycine–proline motifs. A gelatinase function was also identified for FAP [15]. The monomeric form, known as FAPα, is an inactive state which is then activated by dimerization into FAPα/FAPα or the heterodimer, FAPα/FAPβ. This protein plays a crucial role in the remodeling of the TME [16]. Stromal fibroblast studies actually date back to the nineties, as imaging was done through labelled monoclonal antibody F19. mAB-F19 was cultivated in hybridoma cells or lung cancer cells in mice [17,18]. It was first studied in 1994 in metastatic colorectal cancer with 131I-mABF19. The rational was that the stromal compartment in TME makes up almost 50% of the normal tissue and imaging 3 to 5 days after injection. All lesions with positive uptake were then found to be sites of metastasis on biopsy. No therapeutic effect was seen whatsoever, as the patients were already in a very late stage of disease.
FDG dominates the oncologic nuclear imaging field, but FAPI might be a good candidate to dethrone the reigning tracer, as many oncologic imaging studies showed that FAPI had a higher potential for detecting tumor lesions. Benefits are also seen in patient compliance as imaging is done 10 min after injection, and so fasting beforehand is required, as uptake is independent of glucose blood levels [19].
The purpose of this review was to collect all known literature on FAPI theranostics, a weapon targeting the tumor microenvironment.
2. Methods
2.1. Search Strategy and Study Selection
This systematic review was set up according to Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Publications gathered included papers from 2018 to November 2022. The research was conducted on Pubmed, Cochrane Library, Web of Sciences, and Scopus. The following keywords were used as research terms: “FAPI” or “FAP” and “theranostic” or theragnostic”. Clinical studies involving the use of radiobound FAP for both diagnosis and therapy that included at least two patients were gathered and assessed for inclusion.
2.2. Data Extraction and Methodological Quality Assessment
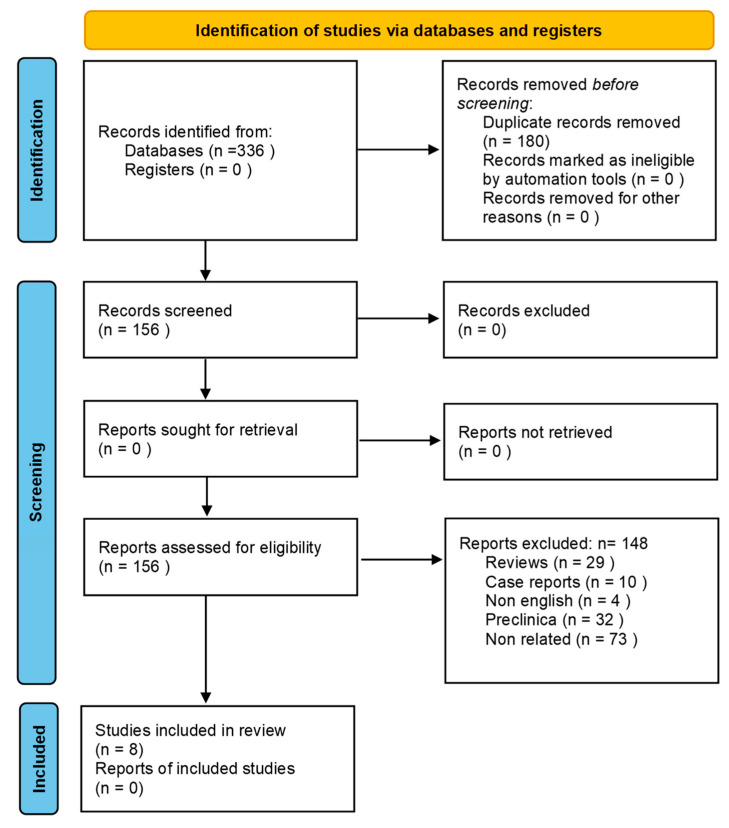
Study design, patient characteristics, year of publication, country and authors’ generalities were retrieved for the included clinical studies. The Clinical Appraisal Skills Program (CASP), a tool frequently used for the systematic review of diagnostic accuracy was used to evaluate clinical studies [20] (See Figure 1).
Figure 1.
PRISMA flow-chart.
3. Results
All eight studies that were deemed suitable for the review went through the CASP signaling question for the CASP diagnostic checklist. All studies had the clear goal of assessing radioligand therapy (RLT) in patients who showed a marked uptake on FAPI-PET and or FAPI scintigraphy, making these patients then eligible for therapy. All patients received therapy and pre-therapy scans with the same ligand, with a few exceptions because of adjustments in the ligand formula for scarce bioretention that would make therapy non-effective. These cases encompassed three studies, and this topic is expanded in the discussion session. All patients were oncologic and selected after the failure of previous lines of therapy and therefore were submitted to compassionate use of the RLT approved by the respective ethic committees. No study showed a relevant collateral effect whatsoever and there was a decent safety profile overall. As most of the patients were terminal, Progressive Disease was the most frequent outcome, followed by Stable Disease according to PERCIST and RECIST 1.1 criteria. Nevertheless, pain medication was greatly reduced with a non-negligible improvement in quality of life for the patients who underwent treatment. Results were matching for all the assessed studies. Most papers were produced in Germany, followed by India and then Iran and Turkey. A total of 74 patients were evaluated, 22 of which had thyroid cancer, 16 breast, 11 pancreas, 6 sarcoma, 5 ovarian, 3 colon, 2 prostate, 1 lung, 1 cervical, 1 rectal, 1 neuroendocrine, 1 paragangliomal, 1 cholangiocarcinoma, 1 chordoma, 1 thymic and 1 round cell tumor (See Table 1, Table 2 and Table 3).
Table 1.
CASP diagnostic checklist.
| Ballal S. et al. 2021 [21] | Ballal S. et al. 2022 [22] | Ferdinandus J. et al. 2022 [23] | Assadi M et al. 2021 [24] | Baum RP et al. 2022 [25] | Kuyumcu S. et al. 2021 [26] | Lindner T. et al. 2018 [27] | Lindner T. et al. 2020 [28] | |
|---|---|---|---|---|---|---|---|---|
| 1. Was there a clear question for the study to address? | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 2. Was there a comparison with an appropriate reference standard? | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 3. Did all patients get the diagnostic test and reference standard? | x | ✔ | ✔ | x | x | ✔ | ✔ | ✔ |
| 4. Could the results of the test have been influenced by the results of the reference standard? | x | x | x | x | x | x | x | x |
| 5. Is the disease status of the tested population clearly described? | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 6. Were the methods for performing the test described in sufficient detail? | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 7. What are the results? | Both tracers were well tolerated. The first one had low tumor retention and was subbed out for the second one which had slower excretion and higher tumor retention and uptake. | No relevant adverse effect. Decrease in thyroglobulin was noted. 4 PR, 3 SD. | All tumors showed FAPI uptake. No major adverse events were noted. 7 PMD, 1 SMD, 1 PMR. | Toxicity profile was acceptable as only one patient experienced adverse effects. 12/18 patients who received the treatment had SD, whereas 6 had PD | These four types of cancer showed high FAP uptake. Decent safety profile with little retention. 9 PD, 2 SD | Bone involvement was noted with highest uptake. Critical organs absorbed dose was lower than other radioligands like 177Lu-PSMA-617 and 177Lu-DOTATATE | Imaging showed rapid uptake in tumor after 10 min from injection and high renal excretion with no retention. | Tumor lesions could be seen on both SPECT and PET. |
| 8. How sure are we about the results, consequences and cost of alternatives performed? | All patients were considered terminal and received compassionate care through radioligand therapy. Stable Disease and Progressive Disease were to be expected. When Partial Response was achieved, palliative care medication was decreased. | |||||||
| 9. Can the results be applied to your patients or the population of interest? | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 10. Can the test be applied to your patients or population of interest? | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 11. Were all outcomes important to the individual or population considered? | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ | ✔ |
| 12. What would be the impact of using this test on your patients/population? | As reported in these studies, palliative care medication was significantly reduced for a period of time with a slight non-negligible improvement in quality of life. | |||||||
SD = Stable Disease; PD = Progression Disease; SMD = Stable Metabolic Disease; PMD = Progressive Metabolic Disease; PMR = Partial Metabolic response.
Table 2.
Characteristics of studies.
| Author | Year of Publication | Country | Tracer | Population | Cancers |
|---|---|---|---|---|---|
| Baum et al. [25] | 2022 | Germany |
177Lu-FAP-2286; 68Ga-FAPI-2286; 68Ga-FAPI-04 |
11 patients | 5 pancreas; 4 breast; 1 rectum; 1 ovary. |
| Ferdinandus et al. [23] | 2022 | Germany | 90Y-FAPI-46; 68Ga-FAPI-46 | 9 patients | 3 pancreatic ductal adenocarcinoma; 4 sarcomas, 1 chordoma, 1 neuroendocrine tumor. |
| Lindner et al. [28] | 2020 | Germany | 99mTc-FAP-34; 68Ga-FAPI-46; 90Y-FAPI-46 | 2 patients | 1 pancreas; 1 ovarian |
| Lindner et al. [27] | 2018 | Germany | 90Y-FAPI-04; 68Ga-FAPI-04 | 2 patients | 2 breast |
| Ballal et al. [21] | 2021 | India |
177Lu-DOTA.SA.FAPI; 177Lu-DOTAGA.(SA.FAPI)2 68Ga-DOTA.SA.FAPI |
10 patients | 5 thyroid; 4 breast; 1 paraganglioma |
| Ballal et al. [22] | 2022 | India | 68Ga-DOTA.SA.FAPI; 177Lu-DOTAGA.(SA.FAPI)2 | 15 patients | 15 thyroid cancers |
| Assadi et al. [24] | 2021 | Iran |
177Lu-FAPI-46; 68Ga-FAPI-46 |
21 patients | 2 ovarian cancer; 2 sarcomas, 3 colon cancer; 5 breast cancer; 2 pancreatic cancer; 2 prostate cancer; 1 cervical cancer; 1 lung cancer; 1 cholangiocarcinoma; 1 thyroid |
| Kuyumcu et al. [26] | 2021 | Turkey |
177Lu-DOTA-FAPI-04; 68Ga-FAPI-04 |
4 patients | 1 breast; 1 thymic carcinoma, 1 thyroid cancer, 1 ovarian cancer |
Table 3.
Direct comparison of detection rate of FAPI vs. FDG in various types of cancer.
| Author | Year of Publication | Country | Tracer | Population | Cancers | Primary Lesion Detection Rate FAPI vs. FDG |
|---|---|---|---|---|---|---|
| Chen et al. [29] | 2020 | China |
68Ga-DOTA-FAPI-04; 18F-FDG |
75 patients | Heterogeneous types of cancer | 98.2% vs. 82.1% |
| Pang et al. [30] | 2020 | China | 68Ga-FAPI; | 35 patients | GI tumor | 100% vs. 53% |
| Jiang et al. [31] | 2021 | China | 68Ga-FAPI-04; 18F-FDG | 38 patients | Gastric cancer | 100% vs. 82% |
| Zhao et al. [32] | 2021 | China | 68Ga-FAPI; 18F-FDG | 45 patients | Nasopharingeal cancer | 100% vs. 97% |
| Qin et al. [33] | 2021 | China | 68Ga-FAPI-04; 18F-FDG | 15 patients | Nasopharingeal cancer | 100% vs. 100% |
| Zhao et al. [34] | 2021 | China | 68Ga-FAPI-04; 18F-FDG | 46 patients | Peritoneal carcinomatosis | 97.67% vs. 72.09% |
| Qin et al. [35] | 2022 | China | 68Ga-DOTA-FAPI-04; 18F-FDG | 21 patients | Gastric cancer | 100% vs. 71.43% |
| Shi et al. [36] | 2020 | China | 68Ga-FAPI; 18F-FDG | 20 patients | Hepatic tumors | 100% vs. 58.8% |
| Pang et al. [37] | 2021 | China | 68Ga-FAPI; 18F-FDG | 36 patients | Pancreatic cancer | 100% vs. 73.1% |
| Kuten et al. [38] | 2021 | Israel | 68Ga-FAPI-04; 18F-FDG | 10 patients | Gastric cancer | 100% vs. 50% |
4. Discussion
4.1. FDG vs. FAPI: A Brief Overview
Although FAPI-PET has been studied in various tumors, it seems to find a particular role in the oncologic evaluation of gastric cancer. Nevertheless, this does not exclude FDG usage in gastric cancer, as coupling of these two tracers greatly increases sensitivity and specificity for distant lesions when compared to each single tracer alone. FAPI was still superior to FDG for primary lesions [39]. FAPI was also found to be better than FDG in detecting lymph node metastasis and peritoneal carcinomatosis, with higher mean and median SUV compared to FDG and with higher tumor to background and tumor to liver ratios. These additional findings are important for patient management as it upstages the lesion overall [40]. FAPI’s superiority to FDG is yet to be confirmed in lung cancer. The use of FAPI-PET in lung cancer is, to this day, very low and studies show discrepancies as to which tracer shows a higher uptake or higher tumor to blood ratio. Some authors report similar results for TBR for both FAPI and FDG with no significant SUV difference [41]. Lung fibrosis and interstitial lung disease are a challenge, as they may be comparable to tumor lesion uptake, although later imaging shows faster washout from the scarred lung than the lung tumor [42]. To this date there is no literature that focuses primarily on FAPI-PET/CT for prostate cancer, aside from case reports of FAPI-positive scans that were negative on PSMA scans [43,44]. Breast cancer might also get good use out of FAPI. The first FAPI application in humans was reported by Baum et al., using 68Ga-FAP-2286 and 177Lu-FAP-2286 alongside other cancer patients, and no adversities were reported. Another case report of metastatic breast cancer imaged with 68Ga-DOTA.SA.FAPI and treated with 177Lu-DOTA.SA.FAPI reported a beneficial outcome for the patients, as her pain medication was reduced overall [25,45]. Differentiated thyroid cancer (DTC) might need both tracers to accurately stage the disease, as FAPI shows higher uptake than FDG in lymphatic lesions and local recurrences of disease, whereas other sites seem to be more FDG avid, making the diagnostic performance for FDG comparable to that of FAPI PET [46]. Another important use of these tracers worth discussing is in bone metastasis evaluation. The most common tools to assess bone metabolism are bone scans with diphosphonates and FDG-PET, the first one assesses the bone reaction of the lesion, whereas the second assesses the glucose metabolism within the lesion [47,48]. In another review evaluating bone metastasis with FAPI and FDG PET/CT, sensitivity was always close to 100% for FAPI, a value reached only in one study by FDG. On the other hand, FDG performed better on specificity [49].
4.2. FAPI Non-Oncologic Uptake
FAPI uptake has been described in non-tumor lesions. Since fibroblast activation can remodel the tissue in fibrosis, inflammation and tissue healing, this may lead to tracer uptake in non-cancerous tissue. It is also worth noting that FAPI tracers have also been studied in non-oncologic diseases, such as IgG4-related disease [50], cardiac amyloidosis [51,52,53], risk of sudden cardiac death [54], tuberculosis [55], Crohn’s disease [56], Erdheim–Chester disease [57], arthritis and fibrosis. A slight uptake is also seen in the thyroid that may increase in cases of chronic thyroiditis and immune related thyroiditis [58,59]. Physiological activity is also seen transiting in the intestines [30]. Bone marrow also exhibits low physiological activity, which increases in bone degenerative diseases and bone fractures [60]. A common pitfall to beware of is related to reactive lymph nodes, as authors have reported 7.7% of lymph nodes to be FAPI positive, most commonly in mediastinum, neck, and axilla and inguinal regions, but still with low uptake compared to metastatic lymph nodes [61].
4.3. CASP Clinical Studies
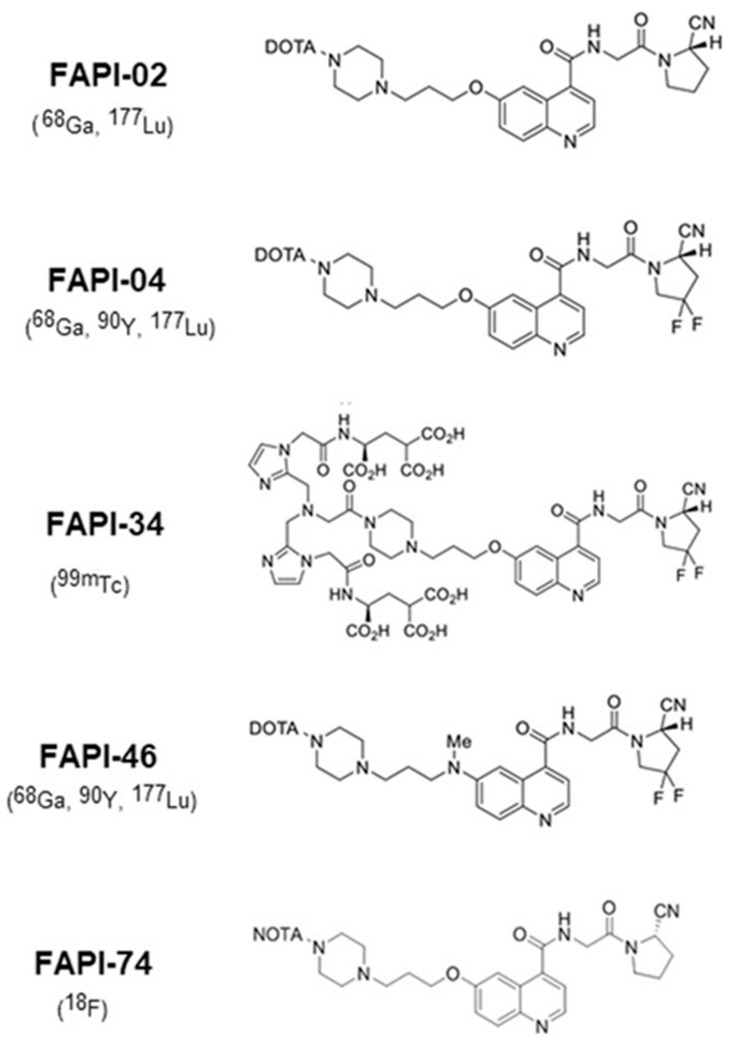
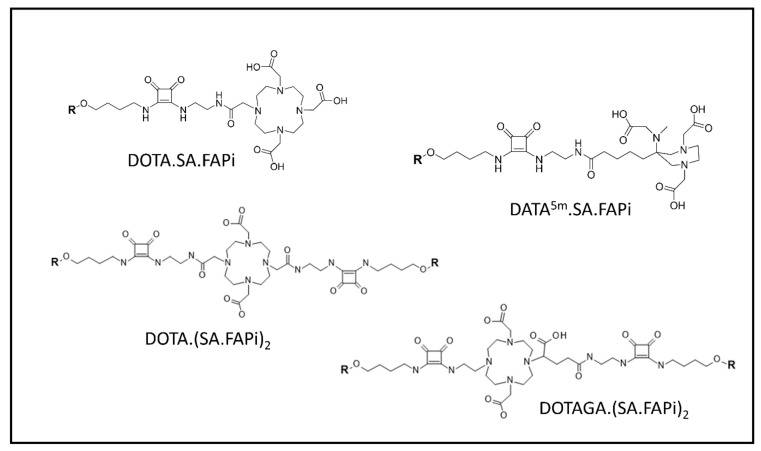
In Figure 2 and Figure 3 we can see the FAPI tracers that were used in both and clinical and pre-clinical settings, with and without chelators, based on bioretention and tumor affinity.
Figure 2.
Chemical structures of FAPI precursors used in clinical application with nuclides in brackets [62]. Reprinted/Adapted from ref. [62].
Figure 3.
FAPI molecules developed by the Mainz research group [63]. Reprinted/Adapted from ref. [62].
Lindner’s group was the first to report in 2018 development of the FAPI tracer and then use it in imaging and therapeutic settings. FAPI-04 turned out to be the most promising because of good stability in human serum, high affinity for FAP, and high tumor uptake. Two patients with metastatic breast cancer were evaluated with 68Ga-FAPI-04 and then treated with 90Y-FAPI-04. No adverse reactions were reported, and the clinical response was seen as a significant reduction in pain medication. The authors had developed numerous FAPI ligands prior to therapy. The best performing were FAPI-04 and FAPI-13. Compared to existing FAPI-02 data, these two tracers had better accumulation in tumor lesions, mainly 24h after injection [27]. In 2020 the same group developed another FAPI tracer (FAPI-34) for imaging purposes. Biodistribution was assessed in animal samples. Human administration of FAPI-34 was done in two metastatic pancreatic and ovarian cancer patients who had already received a 68Ga-FAPI-46 scan and 90Y-FAPI-46 therapy. Follow-up was done with 99mTc-FAP-34, which showed the same lesions both in SPECT and in PET. The first bound ligand, FAPI-19, had very high internalization rate (95%). Interestingly, follow-up cold (unlabeled) FAPI-19 addition in tumor cells showed suppression of 99Tc-FAPI-19, owing to high affinity and specificity of the compound. Out of the compared FAPI ligands (-19 -28, -29, -33, -34, -43), FAPI-34 had the lowest uptake in excretory organs and higher uptake in tumor lesion in mice. [28].
In 2021 Ballal et al. developed two FAPI tracers, 177Lu-DOTA.SA.FAPI and 177Lu-DOTAGA.(SA.FAPI)2, with the aim of using them in patients with high FAP expression confirmed with 68Ga-DOTA.SA.FAPI PET/CT. The tracers were administered to 10 patients (3 and 7, respectively) with a variety of metastatic tumors. 177Lu and 68Ga PET/CT lesions were concordant. The downsides of the study were, in the first place, the small patient sample, as well as the use of two tracers, further subdividing the patient samples; 177Lu-DOTA.SA.FAPI did not prove effective because of rapid clearance (within 48 h p.i.). Thus, the first group received one therapy cycle, whereas the second group received three cycles. In general, patients treated with 177Lu-DOTAGA.(SA.FAPI)2 reported a clinical response, whereas patients treated with 177Lu-DOTA.SA.FAPI relapsed after an initial improvement and two of them died [21]. Again in 2022, the Indian group published their work on radioiodine refractory differentiated thyroid cancer (RR-DTC). Fifteen patients who were positive on a 68Ga-DOTA.SA.FAPI scan received 177Lu-DOTAGA.(SA.FAPI)2. Four patients had partial response whereas three showed Stable Disease. Tireoglobuln was overall lower than baseline. Again, no relevant adverse events were noted [22].
Ferdinandus et al., in 2022, evaluated nine patients with heterogenous tumors using 90Y-FAPI-46. All patients underwent 68Ga-FAPI-46 PET/CT to assess tumor lesions with FAPI uptake, and renal function with 99mTc-MAG3. No significant adverse effects linkable with certainty to the radioligand therapy were reported. Out of nine patients, only one received three cycles of therapy, two patients received two cycles of therapy, whereas the rest received just the first administration. This was reported as being due to patient death, clinical deterioration, or lack of focal 90Y-FAPI-46 uptake in a post-treatment 90Y-scan. Post-treatment evaluation in eight patients showed four Progressive and 4 Stable Diseases according to RECIST 1.1. Metabolic evaluation according to PERCIST 1.0 in seven patients showed five Progressive Metabolic Diseases, one Stable Metabolic Disease and one Partial Metabolic Response [23]. Another German group in 2022 administered 177Lu-FAP-2286 to cancer patients after a positive 68Ga-FAPI scan. Different cancer patients were included in the study in order to achieve a broader spectrum of cancers. All of them showed high tracer uptake and retention as well as no critical adverse effects whatsoever [25]. Still, all patients but two showed Progression Disease on RECIST 1.1 and the rest showed Stable Disease. Assadi et al., in 2021, enrolled 21 patients with various cancers after a positive diagnostic 68Ga-FAPI-46 PET/CT or 177Lu-FAPI-46 scintigraphy. Eighteen patients underwent 177Lu-FAPI-46 cycles. One-third of the patients had stable disease after therapy, with a slight improvement of symptoms, whereas the other had progressive disease. The toxicity profile was overall good, with only one patient experiencing adverse effects [24]. Kuyumcu et al., in 2021, also evaluated 177Lu-DOTA-FAPI-04 in patients with 68Ga-FAPI-04 uptake in different tumors, with the highest involvement being in bone, followed by lymph node and hepatic metastasis [26]. Other authors have tried different isotopes, such as 153Sm bound to FAPI, obtaining similar results to the abovementioned [64] and the use of 188Re as a theranostic couple with 99mTc has been hypothesized [28]. In diagnostic settings, PET FAPI tracers have been shown to be superior to FDG in many types of cancer, including gastrointestinal carcinoma, lung adenocarcinoma, and breast and nasopharyngeal cancer [40,65,66,67,68]. Because of the low number of studies, we still cannot determine which FAPI ligand has the best distribution and retention properties, and validation of a gold standard is still to be made.
The most used FAPI tracers are FAPI-04 and FAPI-46 because of their high tumor uptake and high target-to-background ratio. These properties are due to the lipophilicity and the chemical modification of the linker region. In vitro studies showed fast clearance from cells for FAPI-04 after 4 hours, and very slow clearance for FAPI-21 and FAPI-46. FAPI-36 had a poorer image contrast, probably due to increased albumin binding in the blood [69]. FAPI-113 shares the same characteristic because of binding to albumin or other plasma proteins, although it did have the lowest half maximal effective concentration and highest tumor accumulation [27].
Another limitation lies in the low and very heterogeneous amount of cancer patients, with no clear benefits in specific tumors or specific mutations whatsoever. Although the therapeutic isotopes tested have mainly been the pure β emitters 177Lutetium and 90Yttrium, the clinical utilization of which has been well-established for the management of neuroendocrine tumors and liver cancer [70], α-therapy could find an even more fitting role in small and aggressive lesions because of its high linear energy transfer (LET) [71]. To this day FAPI α-therapy has only been reported in preclinical settings in mice with 225-Actinium (225Ac-FAPI-04) [72]. 225Ac is a pure α emitter that has seen application in prostate and neuroendocrine cancers [73,74,75]. This would require FAPI ligands with a long retention time in tumor lesions to make up for the long half-life of 225Ac (9920 days) as well as 177Lu (66,443 days). Furthermore, prospective studies in non-terminal patients with a relatively satisfying quality of life and low ECOG (Eastern Cooperative Oncology Group) score could bring attention to radioligand therapy in earlier lines of oncologic treatment.
5. Conclusions
FAPI theranostics is definitely at the vanguard of personalized medicine. Studies so far encourage this direction of targeting the tumor microenvironment both in diagnosis, in which it is proving to be superior to FDG, as well as in therapy, where no relevant adverse effect or bad tolerability profiles prohibit administration. Although these studies showed improvement of symptoms at best, it should be noted that the patients received the radioligand therapy for compassionate use, and we cannot exclude a better outcome if the treatment is administered in an earlier stage of the disease.
Author Contributions
Conceptualization, V.F.; methodology, L.F.; validation, M.S.D.F. and M.C.; formal analysis, F.C.; investigation, M.M.A.S.; data curation, J.G.; writing—original draft preparation, M.M.A.S.; supervision, O.S., V.F. and G.D.V. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interests.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Sung H., Ferlay J., Siegel R.L., Laversanne M., Soerjomataram I., Jemal A., Bray F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021;71:209–249. doi: 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- 2.Wells C.R., Galvani A.P. Impact of the COVID-19 pandemic on cancer incidence and mortality. Lancet Public Health. 2022;7:e490–e491. doi: 10.1016/S2468-2667(22)00111-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Berry J.D., Cook G.J. Positron emission tomography in oncology. Br. Med. Bull. 2006;79–80:171–186. doi: 10.1093/bmb/ldl013. [DOI] [PubMed] [Google Scholar]
- 4.Saleem A., Charnley N., Price P. Clinical molecular imaging with positron emission tomography. Eur. J. Cancer. 2006;42:1720–1727. doi: 10.1016/j.ejca.2006.02.021. [DOI] [PubMed] [Google Scholar]
- 5.Cimini A., Ricci M., Chiaravalloti A., Filippi L., Schillaci O. Theragnostic Aspects and Radioimmunotherapy in Pediatric Tumors. Int. J. Mol. Sci. 2020;21:3849. doi: 10.3390/ijms21113849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cistaro A., Albano D., Alongi P., Laudicella R., Pizzuto D.A., Formica G., Romagnolo C., Stracuzzi F., Frantellizzi V., Piccardo A., et al. The Role of PET in Supratentorial and Infratentorial Pediatric Brain Tumors. Curr. Oncol. 2021;28:2481–2495. doi: 10.3390/curroncol28040226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kernstine K.H., Faubert B., Do Q.N., Rogers T.J., Hensley C.T., Cai L., Torrealba J., Oliver D., Wachsmann J.W., Lenkinski R.E., et al. Does Tumor FDG-PET Avidity Represent Enhanced Glycolytic Metabolism in Non-Small Cell Lung Cancer? Ann. Thorac. Surg. 2020;109:1019–1025. doi: 10.1016/j.athoracsur.2019.10.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhong X., He X., Wang Y., Hu Z., Huang H., Zhao S., Wei P., Li D. Warburg effect in colorectal cancer: The emerging roles in tumor microenvironment and therapeutic implications. J. Hematol. Oncol. 2022;15:160. doi: 10.1186/s13045-022-01358-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Farsad M. FDG PET/CT in the Staging of Lung Cancer. Curr. Radiopharm. 2020;13:195–203. doi: 10.2174/1874471013666191223153755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Albano D., Laudicella R., Ferro P., Allocca M., Abenavoli E., Buschiazzo A., Castellino A., Chiaravalloti A., Cuccaro A., Cuppari L., et al. The Role of 18F-FDG PET/CT in Staging and Prognostication of Mantle Cell Lymphoma: An Italian Multicentric Study. Cancers. 2019;11:1831. doi: 10.3390/cancers11121831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bauckneht M., Albano D., Annunziata S., Santo G., Guglielmo P., Frantellizzi V., Branca A., Ferrari C., Vento A., Mirabile A., et al. Somatostatin Receptor PET/CT Imaging for the Detection and Staging of Pancreatic NET: A Systematic Review and Meta-Analysis. Diagnostic. 2020;10:598. doi: 10.3390/diagnostics10080598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuzet S.E., Gaggioli C. Fibroblast activation in cancer: When seed fertilizes soil. Cell Tissue Res. 2016;365:607–619. doi: 10.1007/s00441-016-2467-x. [DOI] [PubMed] [Google Scholar]
- 13.Chen X., Song E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019;18:99–115. doi: 10.1038/s41573-018-0004-1. [DOI] [PubMed] [Google Scholar]
- 14.Santos A.M., Jung J., Aziz N., Kissil J.L., Puré E. Targeting fibroblast activation protein inhibits tumor stromagenesis and growth in mice. J. Clin. Invest. 2009;119:3613–3625. doi: 10.1172/JCI38988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hamson E.J., Keane F.M., Tholen S., Schilling O., Gorrell M.D. Understanding fibroblast activation protein (FAP): Substrates, activities, expression and targeting for cancer therapy. Proteomics. Clin. Appl. 2014;8:454–463. doi: 10.1002/prca.201300095. [DOI] [PubMed] [Google Scholar]
- 16.Cheng J.D., Dunbrack R.L., Jr., Valianou M., Rogatko A., Alpaugh R.K., Weiner L.M. Promotion of tumor growth by murine fibroblast activation protein, a serine protease, in an animal model. Cancer Res. 2002;62:4767–4772. [PubMed] [Google Scholar]
- 17.Welt S., Divgi C.R., Scott A.M., Garin-Chesa P., Finn R.D., Graham M., Carswell E.A., Cohen A., Larson S.M., Old L.J. Antibody targeting in metastatic colon cancer: A phase I study of monoclonal antibody F19 against a cell-surface protein of reactive tumor stromal fibroblasts. J. Clin. Oncol. 1994;12:1193–1203. doi: 10.1200/JCO.1994.12.6.1193. [DOI] [PubMed] [Google Scholar]
- 18.Garin-Chesa P., Old L.J., Rettig W.J. Cell surface glycoprotein of reactive stromal fibroblasts as a potential antibody target in human epithelial cancers. Proc. Natl. Acad. Sci. USA. 1990;87:7235–7239. doi: 10.1073/pnas.87.18.7235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ferdinandus J., Kessler L., Hirmas N., Trajkovic-Arsic M., Hamacher R., Umutlu L., Nader M., Zarrad F., Weber M., Fendler W.P. Equivalent tumor detection for early and late FAPI-46 PET acquisition. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:3221–3227. doi: 10.1007/s00259-021-05266-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh J. Critical appraisal skills programme. J. Pharmacol. Pharmacother. 2013;4:76–77. doi: 10.4103/0976-500X.107697. [DOI] [Google Scholar]
- 21.Ballal S., Yadav M.P., Moon E.S., Kramer V.S., Roesch F., Kumari S., Bal C. First-In-Human Results on the Biodistribution, Pharmacokinetics, and Dosimetry of [(177)Lu]Lu-DOTA.SA.FAPi and [(177)Lu]Lu-DOTAGA.(SA.FAPi)(2) Pharmaceuticals. 2021;14:1212. doi: 10.3390/ph14121212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ballal S., Yadav M.P., Moon E.S., Roesch F., Kumari S., Agarwal S., Tripathi M., Sahoo R.K., Mangu B.S., Tupalli A., et al. Novel Fibroblast Activation Protein Inhibitor-Based Targeted Theranostics for Radioiodine-Refractory Differentiated Thyroid Cancer Patients: A Pilot Study. Thyroid. 2022;32:65–77. doi: 10.1089/thy.2021.0412. [DOI] [PubMed] [Google Scholar]
- 23.Ferdinandus J., Costa P.F., Kessler L., Weber M., Hirmas N., Kostbade K., Bauer S., Schuler M., Ahrens M., Schildhaus H.U., et al. Initial Clinical Experience with (90)Y-FAPI-46 Radioligand Therapy for Advanced-Stage Solid Tumors: A Case Series of 9 Patients. J. Nucl. Med. 2022;63:727–734. doi: 10.2967/jnumed.121.262468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Assadi M., Rekabpour S.J., Jafari E., Divband G., Nikkholgh B., Amini H., Kamali H., Ebrahimi S., Shakibazad N., Jokar N., et al. Feasibility and Therapeutic Potential of 177Lu-Fibroblast Activation Protein Inhibitor-46 for Patients With Relapsed or Refractory Cancers: A Preliminary Study. Clin. Nucl. Med. 2021;46:e523–e530. doi: 10.1097/RLU.0000000000003810. [DOI] [PubMed] [Google Scholar]
- 25.Baum R.P., Schuchardt C., Singh A., Chantadisai M., Robiller F.C., Zhang J., Mueller D., Eismant A., Almaguel F., Zboralski D., et al. Feasibility, Biodistribution, and Preliminary Dosimetry in Peptide-Targeted Radionuclide Therapy of Diverse Adenocarcinomas Using (177)Lu-FAP-2286: First-in-Humans Results. J. Nucl. Med. 2022;63:415–423. doi: 10.2967/jnumed.120.259192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kuyumcu S., Kovan B., Sanli Y., Buyukkaya F., Has Simsek D., Özkan Z.G., Isik E.G., Ekenel M., Turkmen C. Safety of Fibroblast Activation Protein-Targeted Radionuclide Therapy by a Low-Dose Dosimetric Approach Using 177Lu-FAPI04. Clin. Nucl. Med. 2021;46:641–646. doi: 10.1097/RLU.0000000000003667. [DOI] [PubMed] [Google Scholar]
- 27.Lindner T., Loktev A., Altmann A., Giesel F., Kratochwil C., Debus J., Jäger D., Mier W., Haberkorn U. Development of Quinoline-Based Theranostic Ligands for the Targeting of Fibroblast Activation Protein. J. Nucl. Med. 2018;59:1415–1422. doi: 10.2967/jnumed.118.210443. [DOI] [PubMed] [Google Scholar]
- 28.Lindner T., Altmann A., Krämer S., Kleist C., Loktev A., Kratochwil C., Giesel F., Mier W., Marme F., Debus J., et al. Design and Development of (99m)Tc-Labeled FAPI Tracers for SPECT Imaging and (188)Re Therapy. J. Nucl. Med. 2020;61:1507–1513. doi: 10.2967/jnumed.119.239731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H., Pang Y., Wu J., Zhao L., Hao B., Wu J., Wei J., Wu S., Zhao L., Luo Z., et al. Comparison of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F] FDG PET/CT for the diagnosis of primary and metastatic lesions in patients with various types of cancer. Eur. J. Nucl. Med. Mol. Imaging. 2020;47:1820–1832. doi: 10.1007/s00259-020-04769-z. [DOI] [PubMed] [Google Scholar]
- 30.Pang Y., Zhao L., Luo Z., Hao B., Wu H., Lin Q., Sun L., Chen H. Comparison of (68)Ga-FAPI and (18)F-FDG Uptake in Gastric, Duodenal, and Colorectal Cancers. Radiology. 2021;298:393–402. doi: 10.1148/radiol.2020203275. [DOI] [PubMed] [Google Scholar]
- 31.Jiang D., Chen X., You Z., Wang H., Zhang X., Li X., Ren S., Huang Q., Hua F., Guan Y., et al. Comparison of [(68) Ga]Ga-FAPI-04 and [(18)F]-FDG for the detection of primary and metastatic lesions in patients with gastric cancer: A bicentric retrospective study. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:732–742. doi: 10.1007/s00259-021-05441-w. [DOI] [PubMed] [Google Scholar]
- 32.Zhao L., Pang Y., Zheng H., Han C., Gu J., Sun L., Wu H., Wu S., Lin Q., Chen H. Clinical utility of [(68)Ga]Ga-labeled fibroblast activation protein inhibitor (FAPI) positron emission tomography/computed tomography for primary staging and recurrence detection in nasopharyngeal carcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:3606–3617. doi: 10.1007/s00259-021-05336-w. [DOI] [PubMed] [Google Scholar]
- 33.Qin C., Liu F., Huang J., Ruan W., Liu Q., Gai Y., Hu F., Jiang D., Hu Y., Yang K., et al. A head-to-head comparison of (68)Ga-DOTA-FAPI-04 and (18)F-FDG PET/MR in patients with nasopharyngeal carcinoma: A prospective study. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:3228–3237. doi: 10.1007/s00259-021-05255-w. [DOI] [PubMed] [Google Scholar]
- 34.Zhao L., Pang Y., Luo Z., Fu K., Yang T., Zhao L., Sun L., Wu H., Lin Q., Chen H. Role of [(68)Ga]Ga-DOTA-FAPI-04 PET/CT in the evaluation of peritoneal carcinomatosis and comparison with [(18)F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1944–1955. doi: 10.1007/s00259-020-05146-6. [DOI] [PubMed] [Google Scholar]
- 35.Qin C., Shao F., Gai Y., Liu Q., Ruan W., Liu F., Hu F., Lan X. (68)Ga-DOTA-FAPI-04 PET/MR in the Evaluation of Gastric Carcinomas: Comparison with (18)F-FDG PET/CT. J. Nucl. Med. 2022;63:81–88. doi: 10.2967/jnumed.120.258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shi X., Xing H., Yang X., Li F., Yao S., Congwei J., Zhao H., Hacker M., Huo L., Li X. Comparison of PET imaging of activated fibroblasts and (18)F-FDG for diagnosis of primary hepatic tumours: A prospective pilot study. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1593–1603. doi: 10.1007/s00259-020-05070-9. [DOI] [PubMed] [Google Scholar]
- 37.Pang Y., Zhao L., Shang Q., Meng T., Zhao L., Feng L., Wang S., Guo P., Wu X., Lin Q., et al. Positron emission tomography and computed tomography with [(68)Ga]Ga-fibroblast activation protein inhibitors improves tumor detection and staging in patients with pancreatic cancer. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:1322–1337. doi: 10.1007/s00259-021-05576-w. [DOI] [PubMed] [Google Scholar]
- 38.Kuten J., Levine C., Shamni O., Pelles S., Wolf I., Lahat G., Mishani E., Even-Sapir E. Head-to-head comparison of [(68)Ga]Ga-FAPI-04 and [(18)F]-FDG PET/CT in evaluating the extent of disease in gastric adenocarcinoma. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:743–750. doi: 10.1007/s00259-021-05494-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miao Y., Feng R., Guo R., Huang X., Hai W., Li J., Yu T., Qu Q., Zhang M., Shangguan C., et al. Utility of [(68)Ga]FAPI-04 and [(18)F]FDG dual-tracer PET/CT in the initial evaluation of gastric cancer. Eur. Radiol. 2022:1–12. doi: 10.1007/s00330-022-09321-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Li C., Tian Y., Chen J., Jiang Y., Xue Z., Xing D., Wen B., He Y. Usefulness of [(68)Ga]FAPI-04 and [(18)F]FDG PET/CT for the detection of primary tumour and metastatic lesions in gastrointestinal carcinoma: A comparative study. Eur. Radiol. 2022:1–13. doi: 10.1007/s00330-022-09251-y. [DOI] [PubMed] [Google Scholar]
- 41.Giesel F.L., Kratochwil C., Schlittenhardt J., Dendl K., Eiber M., Staudinger F., Kessler L., Fendler W.P., Lindner T., Koerber S.A., et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:4377–4385. doi: 10.1007/s00259-021-05307-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Röhrich M., Leitz D., Glatting F.M., Wefers A.K., Weinheimer O., Flechsig P., Kahn N., Mall M.A., Giesel F.L., Kratochwil C., et al. Fibroblast Activation Protein-Specific PET/CT Imaging in Fibrotic Interstitial Lung Diseases and Lung Cancer: A Translational Exploratory Study. J. Nucl. Med. 2022;63:127–133. doi: 10.2967/jnumed.121.261925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aryana K., Manafi-Farid R., Amini H., Divband G., Moghadam S.Z. 68 Ga-FAPI-46 PET/CT in a Metastatic Castration-Resistant Prostate Cancer Patient With Low PSMA Expression. Clin. Nucl. Med. 2022;47:972–973. doi: 10.1097/RLU.0000000000004315. [DOI] [PubMed] [Google Scholar]
- 44.Pang Y., Meng T., Xu W., Shang Q., Chen H. 68 Ga-FAPI PET/CT Detected Non-PSMA/FDG-Avid Primary Tumor in De Novo Metastatic Prostate Cancer. Clin. Nucl. Med. 2022;47:1108–1111. doi: 10.1097/RLU.0000000000004349. [DOI] [PubMed] [Google Scholar]
- 45.Ballal S., Yadav M.P., Kramer V., Moon E.S., Roesch F., Tripathi M., Mallick S., ArunRaj S.T., Bal C. A theranostic approach of [(68)Ga]Ga-DOTA.SA.FAPi PET/CT-guided [(177)Lu]Lu-DOTA.SA.FAPi radionuclide therapy in an end-stage breast cancer patient: New frontier in targeted radionuclide therapy. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:942–944. doi: 10.1007/s00259-020-04990-w. [DOI] [PubMed] [Google Scholar]
- 46.Mu X., Huang X., Jiang Z., Li M., Jia L., Lv Z., Fu W., Mao J. [(18)F]FAPI-42 PET/CT in differentiated thyroid cancer: Diagnostic performance, uptake values, and comparison with 2-[(18)F]FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2022:1–11. doi: 10.1007/s00259-022-06067-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Peterson J.J., Kransdorf M.J., O’Connor M.I. Diagnosis of occult bone metastases: Positron emission tomography. Clin. Orthop. Relat. Res. 2003;415:S120–S128. doi: 10.1097/01.blo.0000093051.96273.7c. [DOI] [PubMed] [Google Scholar]
- 48.Guo W., Pang Y., Yao L., Zhao L., Fan C., Ke J., Guo P., Hao B., Fu H., Xie C., et al. Imaging fibroblast activation protein in liver cancer: A single-center post hoc retrospective analysis to compare [(68)Ga]Ga-FAPI-04 PET/CT versus MRI and [(18)F]-FDG PET/CT. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1604–1617. doi: 10.1007/s00259-020-05095-0. [DOI] [PubMed] [Google Scholar]
- 49.Li L., Hu X., Ma J., Yang S., Gong W., Zhang C. A systematic review of [(68)Ga]Ga-DOTA-FAPI-04 and [(18)F]FDG PET/CT in the diagnostic value of malignant tumor bone metastasis. Front. Oncol. 2022;12:978506. doi: 10.3389/fonc.2022.978506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Li M., Younis M.H., Zhang Y., Cai W., Lan X. Clinical summary of fibroblast activation protein inhibitor-based radiopharmaceuticals: Cancer and beyond. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:2844–2868. doi: 10.1007/s00259-022-05706-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wang X., Guo Y., Gao Y., Ren C., Huang Z., Liu B., Li X., Chang L., Shen K., Ding H., et al. Feasibility of (68)Ga-Labeled Fibroblast Activation Protein Inhibitor PET/CT in Light-Chain Cardiac Amyloidosis. JACC Cardiovasc. Imaging. 2022;15:1960–1970. doi: 10.1016/j.jcmg.2022.06.004. [DOI] [PubMed] [Google Scholar]
- 52.Treutlein C., Distler J.H.W., Tascilar K., Fakhouri S.C., Györfi A.H., Atzinger A., Matei A.E., Dees C., Büttner-Herold M., Kuwert T., et al. Assessment of myocardial fibrosis in patients with systemic sclerosis using [(68)Ga]Ga-FAPI-04-PET-CT. Eur. J. Nucl. Med. Mol. Imaging. 2022:1–7. doi: 10.1007/s00259-022-06081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo W., Chen H. (68)Ga FAPI PET/MRI in Cardiac Amyloidosis. Radiology. 2022;303:51. doi: 10.1148/radiol.211951. [DOI] [PubMed] [Google Scholar]
- 54.Wang L., Wang Y., Wang J., Xiao M., Xi X.Y., Chen B.X., Su Y., Zhang Y., Xie B., Dong Z., et al. Myocardial Activity at (18)F-FAPI PET/CT and Risk for Sudden Cardiac Death in Hypertrophic Cardiomyopathy. Radiology. 2022;306:221052. doi: 10.1148/radiol.221052. [DOI] [PubMed] [Google Scholar]
- 55.Gu B., Luo Z., He X., Wang J., Song S. 68Ga-FAPI and 18F-FDG PET/CT Images in a Patient With Extrapulmonary Tuberculosis Mimicking Malignant Tumor. Clin. Nucl. Med. 2020;45:865–867. doi: 10.1097/RLU.0000000000003279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Luo Y., Pan Q., Xu H., Zhang R., Li J., Li F. Active uptake of (68)Ga-FAPI in Crohn’s disease but not in ulcerative colitis. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:1682–1683. doi: 10.1007/s00259-020-05129-7. [DOI] [PubMed] [Google Scholar]
- 57.Wu S., Pang Y., Chen Y., Sun H., Chen H. 68Ga-DOTA-FAPI-04 PET/CT in Erdheim-Chester Disease. Clin. Nucl. Med. 2021;46:258–260. doi: 10.1097/RLU.0000000000003491. [DOI] [PubMed] [Google Scholar]
- 58.Can C., Gündoğan C., Güzel Y., Kaplan İ., Kömek H. 68Ga-FAPI Uptake of Thyroiditis in a Patient With Breast Cancer. Clin. Nucl. Med. 2021;46:683–685. doi: 10.1097/RLU.0000000000003637. [DOI] [PubMed] [Google Scholar]
- 59.Hotta M., Sonni I., Benz M.R., Gafita A., Bahri S., Shuch B.M., Yu R., Liu S.T., Czernin J., Calais J. (68)Ga-FAPI-46 and (18)F-FDG PET/CT in a patient with immune-related thyroiditis induced by immune checkpoint inhibitors. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:3736–3737. doi: 10.1007/s00259-021-05373-5. [DOI] [PubMed] [Google Scholar]
- 60.Kessler L., Ferdinandus J., Hirmas N., Zarrad F., Nader M., Kersting D., Weber M., Kazek S., Sraieb M., Hamacher R., et al. Pitfalls and Common Findings in (68)Ga-FAPI PET: A Pictorial Analysis. J. Nucl. Med. 2022;63:890–896. doi: 10.2967/jnumed.121.262808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Zheng S., Lin R., Chen S., Zheng J., Lin Z., Zhang Y., Xue Q., Chen Y., Zhang J., Lin K., et al. Characterization of the benign lesions with increased (68)Ga-FAPI-04 uptake in PET/CT. Ann. Nucl. Med. 2021;35:1312–1320. doi: 10.1007/s12149-021-01673-w. [DOI] [PubMed] [Google Scholar]
- 62.Alfteimi A., Lützen U., Helm A., Jüptner M., Marx M., Zhao Y., Zuhayra M. Automated synthesis of [(68)Ga]Ga-FAPI-46 without pre-purification of the generator eluate on three common synthesis modules and two generator types. EJNMMI Radiopharm. Chem. 2022;7:20. doi: 10.1186/s41181-022-00172-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Imlimthan S., Moon E.S., Rathke H., Afshar-Oromieh A., Rösch F., Rominger A., Gourni E. New Frontiers in Cancer Imaging and Therapy Based on Radiolabeled Fibroblast Activation Protein Inhibitors: A Rational Review and Current Progress. Pharmaceuticals. 2021;14:1023. doi: 10.3390/ph14101023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kratochwil C., Giesel F.L., Rathke H., Fink R., Dendl K., Debus J., Mier W., Jäger D., Lindner T., Haberkorn U. [(153)Sm]Samarium-labeled FAPI-46 radioligand therapy in a patient with lung metastases of a sarcoma. Eur. J. Nucl. Med. Mol. Imaging. 2021;48:3011–3013. doi: 10.1007/s00259-021-05273-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Tang S., Li C., Wang Y., He Y. 68Ga-FAPI is superior to 18F-FDG in detection of micro-sized lung adenocarcinoma. Jpn. J. Clin. Oncol. 2022;53:91–92. doi: 10.1093/jjco/hyac153. [DOI] [PubMed] [Google Scholar]
- 66.Elboga U., Sahin E., Kus T., Cayirli Y.B., Aktas G., Uzun E., Cinkir H.Y., Teker F., Sever O.N., Aytekin A., et al. Superiority of (68)Ga-FAPI PET/CT scan in detecting additional lesions compared to (18)FDG PET/CT scan in breast cancer. Ann. Nucl. Med. 2021;35:1321–1331. doi: 10.1007/s12149-021-01672-x. [DOI] [PubMed] [Google Scholar]
- 67.Zheng J., Liu F., Lin K., Zhang L., Huang N., Zheng W., Zhang J., Yao S., Miao W. [(68)Ga]Ga-FAPI PET/CT Improves the T Staging of Patients with Newly Diagnosed Nasopharyngeal Carcinoma: A Comparison with [(18)F]F-FDG. Mol. Imaging Biol. 2022;24:973–985. doi: 10.1007/s11307-022-01748-8. [DOI] [PubMed] [Google Scholar]
- 68.Calais J., Mona C.E. Will FAPI PET/CT Replace FDG PET/CT in the Next Decade? Point-An Important Diagnostic, Phenotypic, and Biomarker Role. AJR Am. J. Roentgenol. 2021;216:305–306. doi: 10.2214/AJR.20.24302. [DOI] [PubMed] [Google Scholar]
- 69.Loktev A., Lindner T., Burger E.M., Altmann A., Giesel F., Kratochwil C., Debus J., Marmé F., Jäger D., Mier W., et al. Development of Fibroblast Activation Protein-Targeted Radiotracers with Improved Tumor Retention. J. Nucl. Med. 2019;60:1421–1429. doi: 10.2967/jnumed.118.224469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Filippi L., Schillaci O., Cianni R., Bagni O. Yttrium-90 resin microspheres and their use in the treatment of intrahepatic cholangiocarcinoma. Future Oncol. 2018;14:809–818. doi: 10.2217/fon-2017-0443. [DOI] [PubMed] [Google Scholar]
- 71.Liu Y., Watabe T., Kaneda-Nakashima K., Shirakami Y., Naka S., Ooe K., Toyoshima A., Nagata K., Haberkorn U., Kratochwil C., et al. Fibroblast activation protein targeted therapy using [(177)Lu]FAPI-46 compared with [(225)Ac]FAPI-46 in a pancreatic cancer model. Eur. J. Nucl. Med. Mol. Imaging. 2022;49:871–880. doi: 10.1007/s00259-021-05554-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Watabe T., Liu Y., Kaneda-Nakashima K., Shirakami Y., Lindner T., Ooe K., Toyoshima A., Nagata K., Shimosegawa E., Haberkorn U., et al. Theranostics Targeting Fibroblast Activation Protein in the Tumor Stroma: (64)Cu- and (225)Ac-Labeled FAPI-04 in Pancreatic Cancer Xenograft Mouse Models. J. Nucl. Med. 2020;61:563–569. doi: 10.2967/jnumed.119.233122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Busslinger S.D., Tschan V.J., Richard O.K., Talip Z., Schibli R., Müller C. [(225)Ac]Ac-SibuDAB for Targeted Alpha Therapy of Prostate Cancer: Preclinical Evaluation and Comparison with [(225)Ac]Ac-PSMA-617. Cancers. 2022;14:5651. doi: 10.3390/cancers14225651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.King A.P., Gutsche N.T., Raju N., Fayn S., Baidoo K.E., Bell M.M., Olkowski C.S., Swenson R.E., Lin F.I., Sadowski S.M., et al. (225)Ac-Macropatate: A Novel Alpha Particle Peptide Receptor Radionuclide Therapy for Neuroendocrine Tumors. J. Nucl. Med. 2022 doi: 10.2967/jnumed.122.264707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Alam M.R., Singh S.B., Thapaliya S., Shrestha S., Deo S., Khanal K. A Review of 177Lutetium-PSMA and 225Actinium-PSMA as Emerging Theranostic Agents in Prostate Cancer. Cureus. 2022;14:e29369. doi: 10.7759/cureus.29369. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.