Abstract
Viral hepatitis is an infection of human hepatocytes resulting in liver damage. Dual infection of two hepatotropic viruses affects disease outcomes. The hepatitis A virus (HAV) and hepatitis E virus (HEV) are two enterically transmitted viruses; they are single-stranded RNA viruses and have common modes of transmission. They are transmitted mainly by the fecal-oral route and ingestion of contaminated food, though the HAV has no animal reservoirs. The HAV and HEV cause acute self-limiting disease; however, the HEV, but not HAV, can progress to chronic and extrahepatic infections. The HAV/HEV dual infection was reported among acute hepatitis patients present in developing countries. The impact of the HAV/HEV on the prognosis for acute hepatitis is not completely understood. Studies showed that the HAV/HEV dual infection increased abnormalities in the liver leading to fulminant hepatic failure (FHF) with a higher mortality rate compared to infection with a single virus. On the other hand, other reports showed that the clinical symptoms of the HAV/HEV dual infection were comparable to symptoms associated with the HAV or HEV monoinfection. This review highlights the modes of transmission, the prevalence of the HAV/HEV dual infection in various countries and among several study subjects, the possible outcomes of this dual infection, potential model systems for studying this dual infection, and methods of prevention of this dual infection and its associated complications.
Keywords: viral hepatitis, HAV, HEV, dual infection, outcomes, treatment, prevention
1. Introduction
Hepatitis A virus (HAV) and hepatitis E virus (HEV) are enterically transmitted viruses that affect significant populations worldwide. HAV infection causes acute viral hepatitis that is associated with a mortality rate of 0.5% according to the WHO, 2016 [1]. The WHO reported that there are about twenty million HEV infections globally per year, 3.3 million clinically manifested patients, and there were 44,000 deaths in 2015 [2]. The WHO reported that 3.3% of mortality caused by viral hepatitis was attributed to HEV infection [2].
Both HAV and HEV have a genome of approximately 7.2–7.5 kb [3,4,5]. HAV is a positive sense single-stranded RNA virus; it belongs to the genus Hepatovirus and family Picornaviridae [6]. The HAV genome includes one open reading frame (ORF), untranslated region (UTR) at the 5′ terminus, and UTR and poly-A tail at the 3′ terminus [3]. The HAV genome encodes one large polyprotein that is processed into structural proteins and non-structural proteins [4]. HEV is a positive sense single-stranded RNA virus; it belongs to the family Hepeviridae which has two subfamilies Orthohepevirinae and Parahepevirinae [7]. The HEV genome encodes three ORFs and a cap at the 5′ terminus [8]. HEV ORF1 encodes non-structural proteins, and HEV ORF2 and HEV ORF3 encode structural protein and ion channel protein, respectively [5,8,9,10,11].
HAV has one serotype, and it has five genotypes (I-V). Genotypes I-III infect humans, and genotypes IV and V infect primates [12]. Human HAV isolates are subgenotyped into A and B (IA, IB, IIA, IIB, IIIA, and IIIB) [12]. HAV genotypes I and III are the most known genotype globally [13]. The HAV infects humans only, and there are no animal reservoirs for HAV. In contrast, humans and animals are the main reservoirs for HEV. The subfamily Orthohepevirinae includes four genera, two of them (Paslahepevirus, Rocahepevirus) including HEV isolates that infect humans [7]. The genus Rocahepevirus includes rat HEV isolates that cross species to humans [14]. The genus Paslahepevirus includes mammalian HEV isolates that are subdivided into eight genotypes [7]. Humans are susceptible to infections by HEV-1, HEV-2, HEV-3, HEV-4, and HEV-7 isolates, and there are no animal reservoirs for HEV-1 and HEV-2 isolates [15]. HEV-1 is subdivided into at least seven subtypes (1a–1g) and HEV-2 is subdivided into two subtypes (2a–2b) [16]. In contrast, there are animal reservoirs for HEV which infect humans, such as HEV-3, HEV-4, and HEV-7, and some genotypes have only been detected in animals, such as HEV-5, HEV-6, and HEV-8 [16]. Swine and wild boars are well-known sources of infection for HEV-3 and HEV-4 [17,18]. HEV-3 is subdivided into at least 13 subtypes (3a–3m) and HEV-4 is categorized into at least nine subtypes (4a–4i) [16]. Rabbits are also reservoirs for rabbit-derived HEV isolates which are close to HEV-3 [16,19]. Wild boars are sources of HEV-5 and HEV-6 [16]. Camels are reservoirs for HEV-7 and HEV-8 isolates; HEV-7 was found in dromedary camels, and HEV-8 was detected in Bactrian camels [20,21,22,23]. Humans are the common host for HAV and HEV [7,12].
HAV and HEV replicate in the liver and there are two forms of viruses produced during viral pathogenesis: naked or non-enveloped virions, which are present mainly in human stool, and quasi-enveloped viruses, which are present in human plasma [24,25,26]. A high viral titer was found in the stool, mediating virus transmission, and spreading the infection [24,25,26].
HAV and HEV share many similarities in genome structure, virus forms, pathogenesis, and mode of transmission (which will be discussed later in the review). In this review, we give an update on the literature reporting the dual infection of HAV/HEV, possible outcomes, and preventive measures to reduce the risk of this dual infection. Since in most literature, it is not clear if infection by both viruses occurred simultaneously or if one virus preceded the other, the term dual infection is used in the review to describe both situations.
2. Modes of Infection and Possible Source(s) of Dual Infection
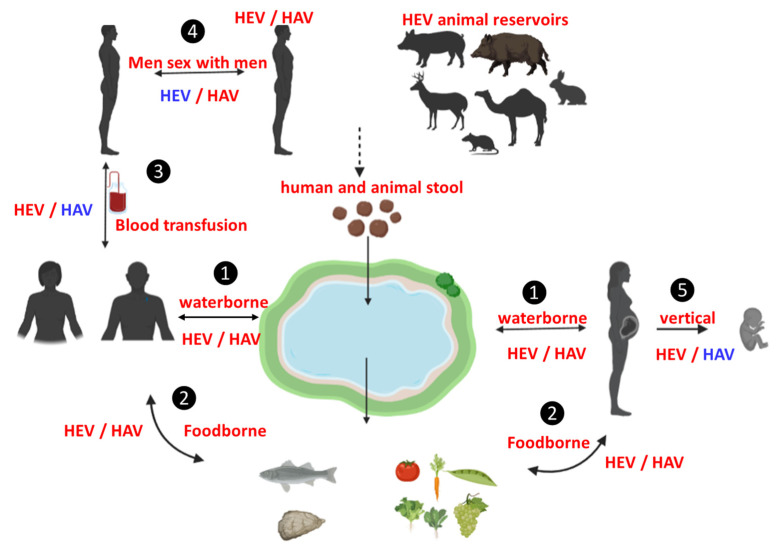
To predict the common modes of HAV/HEV dual infection, it is important to discuss the modes of transmission of each virus. Some modes of transmission are common to both viruses, while others are not (summarized in Figure 1). HAV and HEV infections are transmitted via contaminated water, which represents the fecal-oral route [15,27,28]. Foodborne infections are also caused by both viruses, but there are some differences. Foodborne HAV infections are common. Several products were associated with HAV-foodborne infections such as salads, sandwiches, multiple foods, fruits, and vegetables [29,30]. Recently HAV was detected in milk products and cheese [31,32]. Most foodborne-HAV infection is mediated through food handlers and restaurants and not animal reservoirs [29]. Foodborne HEV infections, caused by zoonotic genotypes, are mainly transmitted through ingestion of contaminated edible products from pigs, wild boars, deer, rabbits, and camels [19,33,34,35]. Also, ingestion of contaminated vegetables and fruits was associated with HEV infection. The water supply has been contaminated with either human or animal stool and has become a source of HEV transmission to these products during irrigation [30,36]. Importantly, HAV and HEV infections have been associated with the ingestion of seafood [37,38,39,40].
Figure 1.
Possible routes of HAV/HEV dual infection. Humans are reservoirs for HAV and HEV, while pigs, wild boar, deer, and other animals are reservoirs for HEV only. (1) Waterborne dual infection has been confirmed for HAV/HEV via the fecal oral route. (2) Foodborne infection—ingestion of vegetables/fruits irrigated by contaminated water or seafood—has been confirmed for both viruses. (3) Blood transfusions, i.e., transmission via transfusion, have been confirmed for HEV (red) but are not a frequent source of HAV (blue). (4) Person-to person-contact such as MSM has been confirmed for HAV (red) but is not a frequent source of HEV (blue). (5) Vertical transmission, i.e., from mother to child, has been confirmed for HEV (red) but is not a frequent source for HAV (blue). For HEV/ HAV: Red means a confirmed source of infection; blue means not a frequent source, which is possible but not confirmed yet.
Person-to-person transmission through sexual contact or sharing drugs with others can greatly spread HAV infection [41,42]. The HAV outbreak has been associated with men who had sex with men (MSM) or who had oral-anal sexual contact [43]. However, HEV infection through person-to-person contact is not common; there are few such cases [44]. Teshale et al. reported that person-to-person transmission of the HEV-1 outbreak in Uganda was probably due to poor sanitation and hygiene [45]. Several studies reported that HEV is not transmitted among MSM or it has a low prevalence of transmission even during HAV outbreaks affecting MSM [46,47,48]. In these studies, there was no significant difference between anti-HEV IgG prevalence among MSM and control groups [46,47,48]. HEV RNA was not recorded in MSM, while two cases were anti-HEV IgM positive [46]. However, there is one study that reported the prevalence of HEV and HAV antibodies was significantly higher in MSM (14.6%) compared to the control group (1%), suggesting that MSM are at higher risk of being infected with the 2 pathogens and that HEV should be considered or investigated during HAV outbreak in the MSM population [49]. Anti-HEV IgM was recorded in 6.1% of MSA, but HEV RNA was not detected in any patients [49].
Few cases of HAV infection have been linked to blood transfusions, and this mode of transmission is rare [50,51]. On the other hand, HEV-3 and HEV-4 infections through blood transfusions were confirmed [52,53,54]. The HEV RNA was detected in blood products and associated with transfusion-transmitted HEV infections [52,53,55]. Dual infection by HAV/HEV was recorded in Israeli hemophiliacs. Higher anti-HAV seroprevalence was recorded in HEV-seropositive patients compared to HEV seronegative patients [56]. In contrast, there has been no difference between HEV seropositive and HEV seronegative patients in the level of bloodborne viruses markers including HBV, HCV, and HIV [56], suggesting that the fecal-oral route could be the mode of HAV/ HEV coinfection.
HAV infection can lead to complications in pregnant women such as premature labor, gestational complication, and acute liver failure [57,58]. However, the transmission of HAV infection from mother to fetus during pregnancy is controversial. Few cases of intrauterine HAV transmission from a pregnant woman have been documented with the fetus developing complications such as ascites, polyhydramnios, and meconium peritonitis [57,59]. In contrast, other studies showed that HAV infection during pregnancy did not impact the fetus, and the fetus tested negative for HAV markers [57,60]. Transmission of HEV from mother to child is confirmed, and the outcomes of HEV infection depend on virus genotype [61,62]. HEV-1, HEV-2, and HEV-4 have adverse feto-maternal outcomes and maternal fatality [62,63,64,65]. HEV-3 causes a mild course of infection with either low complications or none for both mother and child [66].
Collectively, it seems that the fecal-oral route or waterborne infection is the most common method of HAV/HEV coinfection. Although there is no report on dual infection through foodborne infection, both viruses have been detected solely in some common products such as fruits, vegetables, and seafood. Person-to-person contact, especially through MSM and blood transfusions are other potential ways of transmitting HAV/HEV coinfection, but further studies are needed to confirm these modes.
3. Incidence of Dual Infection HAV/HEV
The dual infection by HAV and HEV has been confirmed in many developing countries, and to a small extent in developed countries (Table 1). In developing countries, HAV/ HEV coinfections were reported in India, Mexico, Kenya, Bangladesh, and Egypt (Table 1). HAV/HEV dual infections were associated with several outbreaks in Cuba; the number of HAV/HEV outbreaks was higher than the number of outbreaks caused by HEV alone [67]. In addition, a water-borne outbreak was reported in Hyderabad, where HEV infection caused 78.5% of infections, and HAV/HEV mixed infection was detected in 5.3% of the infected population [68]. In India, several studies assessed the rate of HAV/HEV coinfection, and the results were variable depending on the studied groups, geographic distribution, age, etc. Surveillance was conducted by large laboratories on 24,000 patients during 2014–2017 to assess the prevalence of HAV and HEV. The infection rate was 16.1%, 12.6%, and 1.3% for HEV, HAV, and dual infection (HAV/HEV), respectively [69]. Another study was retrospectively conducted on acute hepatitis patients, pregnant women, and non-pregnant women. HAV/HEV dual infection was detected in 2.07% of acute hepatitis patients, 2.6% among males and 1.7% among females [70]. Additionally, dual infection was detected in 2.5% of non-pregnant women and 1.2% among pregnant women [70]. Liver function values were more abnormal in dual infection cases compared to monoinfection with either HEV or HAV [70]. In some regions in India, HEV/HBV dual infection was the most common haptotropic dual infection among all viral hepatitis cases [71], while in other regions HAV/HEV dual infection was the most prevalent dual infection [72]. Moreover, some studies reported that HAV/HEV dual infection was predominant in children [71], while others showed that adults were more affected by HAV/HEV dual infection [73]. The incidence of HEV/HAV dual infection reported in several studies in India is summarized in Table 1. HEV infection is the most common viral hepatitis detected in India, and the infection is mainly associated with waterborne transmission [74]. Therefore, the proposed mode of HAV/HEV dual infection there is mediated via the fecal-oral route. In Bangladesh, diagnosis of acute hepatitis patients (n = 1925) during the period 2014–2017 revealed that HEV was the most common cause. The mortality rate among acute HEV infections was 5% and increased to 12% among pregnant women. The highest risk of mortality among HEV-infected patients was through dual infection with HBV or HAV [75]. In Egypt, most of the dual infected HAV/HEV cases were children [76,77,78]. HAV/HEV dual infection was confirmed in 70 (26%) out of 268 children who developed acute viral hepatitis [76]. Several studies revealed that HAV circulating in drinking water treatment facilities and sewage in Egypt, where the isolated viruses belonged to the HAV genotype IB, was the same strain isolated from clinical patients [79,80]. HEV RNA was also detected in water treatment plants in Egypt [81]. Therefore, it seems that water is the main source of the HEV/HAV dual infection in Egypt either directly or through using it for treatment and irrigation of plants, vegetables, or fruits; the dual infection may be transmitted via ingestion of these contaminated products. In Venezuela, HAV/HEV dual infection (31%) was slightly higher than HEV monoinfection (29%) among acute hepatitis patients of various ages. Phylogenetic analysis performed on some HEV isolates revealed that HEV genotype 3 was the isolated strain from one of the coinfected cases, while HAV isolates did not characterize the study [82]. The coinfected patient had abnormal liver values. In Mexico, HAV is endemic and HAV/HEV dual infection was reported. The circulating HEV strains in the dual infection were found; they belonged to the HEV genotype 1 [83]. In China, among 46 acute hepatitis patients, five patients (10.87%) were HAV/HEV dual infected. The coinfected patients had higher liver transaminases, bilirubin, and jaundice compared to HEV or HAV-monoinfected patients [84]. The isolated HAV was genotype 1A, but HEV had not been characterized in this dual infection [84]. In addition, HAV, HEV, and HAV/HEV dual infections were assessed in animals present there. HEV RNA was detected only in rabbits and pigs, and HAV was detected only in ferrets, while dual infection HEV/HAV was not detected [84]. In Italy, HEV/HAV dual infection (14%) was recorded in MSM [49]. In Israel, high anti-HAV antibodies, but not HBV or HCV, were recorded in HEV seropositive hemophiliac patients, suggesting a possible HAV/HEV dual infection among those patients [56].
Table 1.
The rate of HAV/HEV dual infection among different groups in different countries.
| Country | Study Groups and Number | Method of Analysis | Results and Dual Infection Rate | Ref | |
|---|---|---|---|---|---|
| India | New Delhi | Acute liver failure, children, (n = 44) |
|
|
[85] |
| Dibrugarh, Assam | Acute hepatitis, adults (14–55 age), (n = 591) |
|
|
[86] | |
| Chandigarh | Acute hepatitis, children (<14 years of age), (n = 172) |
|
|
[87] | |
| south India | Acute hepatitis, children (n = 127) |
|
|
[88] | |
| South India, Vellore |
Acute hepatitis, children, and adults (n = 404) |
|
|
[89] | |
| North India (Lucknow, Uttar Pradesh) |
Acute viral hepatitis, children, and adults (n= 267) |
|
|
[72] | |
| West Bengal | Acute hepatitis, children, and adults (n = 285) |
|
|
[90] | |
| Jabalpur, Madhya Pradesh | Acute hepatitis, children, adults (n = 555) |
|
|
[71] | |
| Hyderabad | Acute hepatitis, children, adults (n= 546) during water outbreak |
|
|
[68] | |
| Mangalore | Acute hepatitis, children, adults (n =958) |
|
|
[91] | |
| Northwest Districts of Punjab | Acute hepatitis, children, adults (n = 95). It is composed of 9 outbreaks |
|
|
[74] | |
| Uttarakhand | Acute hepatitis, children, adults (n = 617). |
|
|
[92] | |
| Northern India. | Acute hepatitis, children, adults, and pregnant (n = 5319) |
|
|
[73]. | |
| Western India, Pune, Maharashtra | Acute hepatitis, children, adults. (n = 1807) |
|
|
[93] | |
| Bangladesh | Hospitalized acute jaundice patients, adults and pregnant (age > 14 years), (n = 1925) |
|
|
[75] | |
| Fulminant hepatic failure, adults (n = 67) |
|
|
[94] | ||
| Acute jaundice syndrome (AJS) among the Rohingya refugees in Cox’s Bazar, an outbreak affecting all ages (n = 275) |
|
|
[95] | ||
| China |
|
|
|
[84] | |
| Korea | Acute hepatitis, adults, (n = 55) |
|
|
[96] | |
| Mexico | Acute hepatitis, children |
|
|
[97]. | |
| Venezuela | Acute hepatitis, children, and adults (1–55 years), (n = 39) |
|
|
[82] | |
| Italy | Men who have sex with Men (MSM), adults (20–85 years), (n = 636) |
|
|
[49] | |
| Mitrovica | Acute hepatitis, children, epidemics, aftermath of the war in Kosovo (1999) (n = 104) |
|
|
[98] | |
| Egypt | Acute hepatitis, children, (n = 268) |
|
|
[76] | |
| Acute hepatitis, children, (n = 180) |
|
|
[77] | ||
| Acute hepatitis, children, adults, aged (1–73 years), (n = 202) |
|
|
[99] | ||
| Acute hepatitis, children, (n = 73) |
|
|
[78] | ||
| Acute hepatitis patients, aged (14–64 years), (n= 18) |
|
|
[100] | ||
| Kenya | Acute hepatitis patients (n = 100) |
|
|
[101] | |
4. Outcomes of Dual HAV/HEV Infection
Both HAV and HEV are hepatotropic and they replicate mainly in human hepatocytes. Therefore, dual infection or superinfection could impact the viral pathogenesis in the liver and disease outcomes.
4.1. Clinical Symptoms of HEV or HAV Monoinfection
HEV or HAV infections are acute self-limiting diseases with comparable symptoms, including gastrointestinal, hepatitis, and/or fever. However, there are some differences in the clinical outcomes between HEV and HAV monoinfection. HAV infection does not develop chronicity, while HEV infection, especially genotype 3 and genotype 4, develops into a chronic infection, especially in immunodeficient patients such as AIDS patients, organ recipients, and patients with hematological diseases [102,103]. Besides, HEV-infected patients could develop extrahepatic disorders such as renal, neurological, and blood disorders [104,105,106]. Extrahepatic disorders are rare in HAV-infected patients, which include renal, arthritis, cutaneous vasculitis, and neurological diseases [107,108,109,110]. HEV infection could result in fulminant hepatic failure (FHF) [100,111], while FHF is rare in HAV monoinfection [112,113].
4.2. Impact of Dual Infection (HAV/HEV)
The impact of dual HAV/HEV infection on disease outcome compared to monoinfection is controversial. Some studies showed that dual infection does not affect the disease pathogenesis, while others reported that dual infection results in severe disease outcomes. Kaur and colleagues reported that dual infection of HEV with other hepatotropic viruses such as HAV, HBV, and HCV was common in India, and there were no differences in symptoms, clinical profile, and disease prognosis between patients infected with a single virus or dual infected patients [114]. Similarly, Kumar et al. assessed hepatotropic viruses (HAV, HBV, HCV, and HEV) and evaluated the impact of dual infection among children diagnosed with acute viral hepatitis (n = 122) and patients who developed FHF (n = 27). There were no differences between HAV/HEV dual infected children (n = 24) and children infected with a single virus in all clinical parameters analyzed, such as jaundice incidence and duration, disease recurrence, ascites, mortality rate, etc. [115]. Similarly, there was no observable difference between monoinfection and HAV/HEV dual infection in the FHF group [115]. The authors hypothesized that dual infection with two or more hepatotropic viruses did not lead to a severe outcome, and HEV infection/seropositivity was linked to 88% of dual infection cases in acute viral hepatitis patients [115]. Similarly, Sarguna and colleagues evaluated the causative agents and outcomes associated with the waterborne outbreak that occurred in Hyderabad [68]. Out of 546 patients, 429 patients were infected with HEV, 53 patients were infected with HAV, and 29 patients were dual infected with HEV/HAV. There was no difference in clinical symptoms and liver function tests between coinfected patients and monoinfected patients. Most patients recovered without complications [68].
However, other studies reported that HEV/HAV dual infection can lead to severe outcomes. Arora et al. assessed the causative agent of acute liver failure in children, and importantly, the authors reported that dual infection by the HAV/HEV (20.45%) was the main cause of FHF in children, of which three children died. The rate of acute liver failure (ALF) caused by dual infection HAV/HEV was higher than ALF caused by a single agent (9% for HAV and 13.6% for HEV) [85]. Several cases of HAV/HEV dual infection developed acalculous cholecystitis, hepatic encephalopathy, and the worst outcomes [116,117,118]. Other studies supported the finding that the HEV/HAV dual infection can lead to a severe course of the disease as shown by abnormalities in liver function tests or liver abnormalities [70,84,119]. Likewise, Paul and colleagues reported that the fatality rate associated with HEV infection was increased in pregnant women, as was the presence of dual infected hepatotropic viruses, especially HBV or HAV [75].
The reasons for the discrepancies among the previous reports about the impact of HAV/HEV on the disease outcomes are not clear, but they could be attributed to different study subjects, geographical locations, risk factors, or viral genotypes, etc.
4.3. HAV/HEV Dual Infection with Another Hepatotropic Virus
HAV/HEV dual infected patients could also be infected with another hepatotropic pathogen such as HCV and HBV. These cases are rare, and few cases were described in the literature. Butt and colleagues described a 12-year boy’s case of acute viral hepatitis, where the boy was coinfected with three hepatotropic viruses, HAV, HBV, and HEV [120]. The case had elevated liver transaminases, but there were no severe complications associated with the case, suggesting that the three hepatotropic viruses did not affect each other [120]. Poddar and colleagues reported another acute hepatitis case in which the patient was coinfected with HAV, HEV, and HCV [87]. The authors did not mention the outcomes of this case. Since these cases are few, we could not draw conclusions on the impact of the pathogenesis of three hepatotropic viruses on the liver. Importantly, the HEV superinfection on chronically HBV-infected patients could lead to a higher mortality rate; the coinfected patients did not respond to anti-HBV therapies and progressed to liver failure [121]. Future studies should assess the outcomes of infection by three hepatotropic viruses.
5. Preventive Measures for HAV and HEV Dual Infection
There is no specific therapy or treatment for acute HAV or HEV infections; only symptomatic and supportive therapies are required [5,122]. The HAV post-exposure prophylaxis includes immunoglobulin and/or a vaccine [123]. Both measures can be given to immunocompromised patients and patients with chronic liver disease [123]. The HAV vaccine is recommended for patients aged 1–40 years, while immunoglobulin is recommended in children less than 12 months or in cases of allergy to the vaccine [123]. Non-specific therapies such as ribavirin and interferon are recommended for chronic HEV infections [124]. However, to our knowledge, most of the reported HAV/ HEV dual infections were acute, not chronic infections (Table 1). Therefore, there is no specific therapy for dual HAV/HEV infections. Preventive measures seem to be the best strategy to reduce the risk of dual HAV/HEV infections and their complications. Since most of the reported dual HAV/HEV infections were linked to the fecal-oral route or waterborne infections, improving hygiene and sanitation practices could reduce the risk of dual infections. Also, frequent washing of hands, vegetables, and fruits could reduce the spread of viruses. The HAV has a vaccine that is given to children, which induces long-lasting immunity. Since most HAV/HEV dual infection was reported in children, the HAV vaccine could reduce the infection rate and virus transmission. However, a recent study showed that some HAV antigenic variants can escape vaccine-mediated immune responses [125]. Hecolin is an approved HEV vaccine in China. This vaccine showed full protection against HEV-4 and partial protection against HEV-C1 infection [126,127]. By conducting a clinical study on four volunteers, Wen and colleagues showed that Hecolin can induce antibodies which could react with several HEV genotypes [128]. Preventive measures during MSM or oral-anal intercourse should be considered to reduce or prevent the infection.
6. Challenges in HAV/HEV Dual Infection Research and Future Perspectives
As mentioned in the previous section, the outcomes of HAV/HEV dual infection are questionable. Limited sequences of HAV and HEV isolates were characterized the dual infections. It is not known if different HAV and HEV genotypes and subtypes can affect the outcome of liver diseases. Huh7-A-I cells can be used for the growth of the wild-type HAV virus [129]. The HAV can adapt to the cell culture after several sub-passages. Highly adapted HAV isolates can replicate efficiently in the cell culture, causing a cytopathic effect and apoptosis in the infected cells [130,131]. HEV genotypes 1 and 2 are the main genotypes associated with waterborne infections [15]. However, the growth of HEV genotype 1 in the hepatoma cell line is limited [132,133]. Therefore, it seems that there is no general culture system for studying HAV/HEV dual infections. A recent study showed that dual infection of HCV with HEV leads to viral interference [134]. Using Huh7.5 cells and the HCV/HEV co-transfection model, HEV replication was reduced, while HCV replication was not altered [134]. HCV infection inhibits HEV replication via HCV protease NS3/4A, which probably cleaves HEV ORF1 to a less active form [134]. On the other hand, the HAV/HCV dual infection with Huh7.5 showed that there were no direct interactions between the two viruses and limited competition [135]. Future studies should study the impact of HAV/HEV dual infection in vitro.
Studies showed that immunodeficient humanized mice (uPA-SCID or FRG) are suitable models for studying HEV infections, especially HEV genotypes 1 and 3 [136,137,138,139,140,141]. HEV replicates in the human hepatocytes occupied in the murine liver and the produced viral particles are excreted in the stool and blood of the infected mice [136,137,138,139,140,141,142]. The replication of HEV in these models is non-cytopathic, i.e., no damage occurs to the human hepatocytes [137]. HEV particles released in the mouse stool were nonenveloped, while HEV particles released in the blood were enveloped [137]. Moreover, uPA-SCID humanized mice were used to study the innate liver transcriptome against the HEV genotype 1 infections [137]. Interestingly, Hirai-Yuki and colleagues showed that uPA-SCID humanized mice can be used for studying the propagation and pathogenesis of HAV [143]. Similar to HEV infection in these mice, HAV replicates specifically in human hepatocytes and the propagated viruses were released in stool as naked viruses and in the blood as quasi-enveloped particles [143]. HAV infection is not cytopathic in these mice and infection induces host interferon responses [143]. Researchers can utilize the humanized mouse model for studying the impact of dual HAV/HEV infections. Humanized mice can be challenged with HAV (monoinfection), HEV (monoinfection), or both viruses (HAV and HEV) via intraperitoneal or intravenous injection. The viral load can be assessed in the mouse plasma and stool, and the viral proteins can be detected in the humanized mouse liver. The impact of dual infection HEV/HAV on the transcriptome changes of hepatocytes and its effect on liver pathogenesis can be compared with monoinfection in this model.
7. Conclusions
The HAV/HEV dual infection is common in developing countries; it is mainly transmitted via the fecal-oral route and may be associated with outbreaks. The impact of this dual infection is questionable, and more research should be conducted in the future to assess the impact of dual infection. Improving practices in hygiene could reduce this dual infection and possible complications.
Acknowledgments
Figure 1 was designed using a free software, www.biorender.com/.
Institutional Review Board Statement
Not applicable for studies not involving humans or animals.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.WHO. [(accessed on 15 October 2022)]; Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-a.
- 2.WHO. [(accessed on 15 October 2022)]; Available online: https://www.who.int/news-room/fact-sheets/detail/hepatitis-e.
- 3.McKnight K.L., Lemon S.M. Hepatitis A Virus Genome Organization and Replication Strategy. Cold Spring Harb. Perspect. Med. 2018;8:a033480. doi: 10.1101/cshperspect.a033480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Martin A., Lemon S.M. Hepatitis Viruses. Springer; Berlin/Heidelberg, Germany: 2002. The Molecular Biology of Hepatitis A Virus; pp. 23–50. [Google Scholar]
- 5.Sayed I.M., Vercouter A.S., Abdelwahab S.F., Vercauteren K., Meuleman P. Is hepatitis E virus an emerging problem in industrialized countries? Hepatology. 2015;62:1883–1892. doi: 10.1002/hep.27990. [DOI] [PubMed] [Google Scholar]
- 6.Wang X., Ren J., Gao Q., Hu Z., Sun Y., Li X., Rowlands D.J., Yin W., Wang J., Stuart D.I., et al. Hepatitis A virus and the origins of picornaviruses. Nature. 2015;517:85–88. doi: 10.1038/nature13806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Purdy M.A., Drexler J.F., Meng X.J., Norder H., Okamoto H., Van der Poel W.H.M., Reuter G., de Souza W.M., Ulrich R.G., Smith D.B. ICTV Virus Taxonomy Profile: Hepeviridae 2022. J. Gen. Virol. 2022;103:001778. doi: 10.1099/jgv.0.001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Koonin E.V., Gorbalenya A.E., Purdy M.A., Rozanov M.N., Reyes G.R., Bradley D.W. Computer-assisted assignment of functional domains in the nonstructural polyprotein of hepatitis E virus: Delineation of an additional group of positive-strand RNA plant and animal viruses. Proc. Natl. Acad. Sci. USA. 1992;89:8259–8263. doi: 10.1073/pnas.89.17.8259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sayed I.M., Vercauteren K., Abdelwahab S.F., Meuleman P. The emergence of hepatitis E virus in Europe. Future Virol. 2015;10:763–778. doi: 10.2217/fvl.15.29. [DOI] [Google Scholar]
- 10.Ding Q., Heller B., Capuccino J.M., Song B., Nimgaonkar I., Hrebikova G., Contreras J.E., PLoSs A. Hepatitis E virus ORF3 is a functional ion channel required for release of infectious particles. Proc. Natl. Acad. Sci. USA. 2017;114:1147–1152. doi: 10.1073/pnas.1614955114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamada K., Takahashi M., Hoshino Y., Takahashi H., Ichiyama K., Nagashima S., Tanaka T., Okamoto H. ORF3 protein of hepatitis E virus is essential for virion release from infected cells. Pt 8J. Gen. Virol. 2009;90:1880–1891. doi: 10.1099/vir.0.010561-0. [DOI] [PubMed] [Google Scholar]
- 12.Hepatovirus F.P.G. [(accessed on 15 October 2022)]. Available online: https://ictv.global/report/chapter/picornaviridae/picornaviridae/hepatovirus.
- 13.Sjogren M.H., Cheatham J.G. Chapter 77-Hepatitis A. In: Feldman M., Friedman L.S., Brandt L.J., editors. Sleisenger and Fordtran’s Gastrointestinal and Liver Disease. 9th ed. W.B. Saunders; Philadelphia, PA, USA: 2010. pp. 1279–1285.e2. [DOI] [Google Scholar]
- 14.Sridhar S., Yip C.C., Wu S., Chew N.F., Leung K.H., Chan J.F., Zhao P.S., Chan W.M., Poon R.W., Tsoi H.W., et al. Transmission of Rat Hepatitis E Virus Infection to Humans in Hong Kong: A Clinical and Epidemiological Analysis. Hepatology. 2021;73:10–22. doi: 10.1002/hep.31138. [DOI] [PubMed] [Google Scholar]
- 15.Rein D.B., Stevens G.A., Theaker J., Wittenborn J.S., Wiersma S.T. The global burden of hepatitis E virus genotypes 1 and 2 in 2005. Hepatology. 2012;55:988–997. doi: 10.1002/hep.25505. [DOI] [PubMed] [Google Scholar]
- 16.Smith D.B., Izopet J., Nicot F., Simmonds P., Jameel S., Meng X.J., Norder H., Okamoto H., van der Poel W.H.M., Reuter G., et al. Update: Proposed reference sequences for subtypes of hepatitis E virus (species Orthohepevirus A) J. Gen. Virol. 2020;101:692–698. doi: 10.1099/jgv.0.001435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meng X.J., Purcell R.H., Halbur P.G., Lehman J.R., Webb D.M., Tsareva T.S., Haynes J.S., Thacker B.J., Emerson S.U. A novel virus in swine is closely related to the human hepatitis E virus. Proc. Natl. Acad. Sci. USA. 1997;94:9860–9865. doi: 10.1073/pnas.94.18.9860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pavio N., Meng X.J., Doceul V. Zoonotic origin of hepatitis E. Curr. Opin. Virol. 2015;10:34–41. doi: 10.1016/j.coviro.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 19.Abravanel F., Lhomme S., El Costa H., Schvartz B., Peron J.M., Kamar N., Izopet J. Rabbit Hepatitis E Virus Infections in Humans, France. Emerg. Infect. Dis. 2017;23:1191–1193. doi: 10.3201/eid2307.170318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Woo P.C., Lau S.K., Teng J.L., Tsang A.K., Joseph M., Wong E.Y., Tang Y., Sivakumar S., Xie J., Bai R., et al. New hepatitis E virus genotype in camels, the Middle East. Emerg. Infect. Dis. 2014;20:1044–1048. doi: 10.3201/eid2006.140140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shirazi R., Pozzi P., Gozlan Y., Wax M., Lustig Y., Linial M., Mendelson E., Bardenstein S., Mor O. Identification of Hepatitis E Virus Genotypes 3 and 7 in Israel: A Public Health Concern? Viruses. 2021;13:2326. doi: 10.3390/v13112326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El-Kafrawy S.A., Hassan A.M., El-Daly M.M., Qadri I., Tolah A.M., Al-Subhi T.L., Alzahrani A.A., Alsaaidi G.A., Al-Abdullah N., Kaki R.M., et al. Seroprevalence of Dromedary Camel HEV in Domestic and Imported Camels from Saudi Arabia. Viruses. 2020;12:553. doi: 10.3390/v12050553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Woo P.C., Lau S.K., Teng J.L., Cao K.Y., Wernery U., Schountz T., Chiu T.H., Tsang A.K., Wong P.C., Wong E.Y., et al. New Hepatitis E Virus Genotype in Bactrian Camels, Xinjiang, China, 2013. Emerg. Infect. Dis. 2016;22:2219–2221. doi: 10.3201/eid2212.160979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Feng Z., Hensley L., McKnight K.L., Hu F., Madden V., Ping L., Jeong S.H., Walker C., Lanford R.E., Lemon S.M. A pathogenic picornavirus acquires an envelope by hijacking cellular membranes. Nature. 2013;496:367–371. doi: 10.1038/nature12029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Das A., Hirai-Yuki A., González-López O., Rhein B., Moller-Tank S., Brouillette R., Hensley L., Misumi I., Lovell W., Cullen J.M., et al. TIM1 (HAVCR1) Is Not Essential for Cellular Entry of Either Quasi-enveloped or Naked Hepatitis A Virions. mBio. 2017;8:e00969-17. doi: 10.1128/mBio.00969-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Montpellier C., Wychowski C., Sayed I.M., Meunier J.C., Saliou J.M., Ankavay M., Bull A., Pillez A., Abravanel F., Helle F., et al. Hepatitis E Virus Lifecycle and Identification of 3 Forms of the ORF2 Capsid Protein. Gastroenterology. 2018;154:211–223.e8. doi: 10.1053/j.gastro.2017.09.020. [DOI] [PubMed] [Google Scholar]
- 27.De Serres G., Cromeans T.L., Levesque B., Brassard N., Barthe C., Dionne M., Prud’homme H., Paradis D., Shapiro C.N., Nainan O.V., et al. Molecular confirmation of hepatitis A virus from well water: Epidemiology and public health implications. J. Infect. Dis. 1999;179:37–43. doi: 10.1086/314565. [DOI] [PubMed] [Google Scholar]
- 28.Bergeisen G.H., Hinds M.W., Skaggs J.W. A waterborne outbreak of hepatitis A in Meade County, Kentucky. Am. J. Public Health. 1985;75:161–164. doi: 10.2105/AJPH.75.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fiore A.E. Hepatitis A transmitted by food. Clin. Infect. Dis. 2004;38:705–715. doi: 10.1086/381671. [DOI] [PubMed] [Google Scholar]
- 30.Terio V., Bottaro M., Pavoni E., Losio M.N., Serraino A., Giacometti F., Martella V., Mottola A., Di Pinto A., Tantillo G. Occurrence of hepatitis A and E and norovirus GI and GII in ready-to-eat vegetables in Italy. Int. J. Food Microbiol. 2017;249:61–65. doi: 10.1016/j.ijfoodmicro.2017.03.008. [DOI] [PubMed] [Google Scholar]
- 31.de Castro Carvalho S.V., Rogovski P., Cadamuro R.D., Viancelli A., Michelon W., Dos Reis D.A., Santana das Chagas I.A., Assenço R., da Silva Lanna M.C., Treichel H., et al. Co-contamination of food products from family farms in an environmental disaster area in Southeast Brazil with pathogenic bacteria and enteric viruses. Arch. Virol. 2020;165:715–718. doi: 10.1007/s00705-019-04501-9. [DOI] [PubMed] [Google Scholar]
- 32.Battistini R., Rossini I., Listorti V., Ercolini C., Maurella C., Serracca L. HAV detection from milk-based products containing soft fruits: Comparison between four different extraction methods. Int. J. Food Microbiol. 2020;328:108661. doi: 10.1016/j.ijfoodmicro.2020.108661. [DOI] [PubMed] [Google Scholar]
- 33.Meng X.J. From barnyard to food table: The omnipresence of hepatitis E virus and risk for zoonotic infection and food safety. Virus Res. 2011;161:23–30. doi: 10.1016/j.virusres.2011.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Feagins A.R., Opriessnig T., Guenette D.K., Halbur P.G., Meng X.J. Inactivation of infectious hepatitis E virus present in commercial pig livers sold in local grocery stores in the United States. Int. J. Food Microbiol. 2008;123:32–37. doi: 10.1016/j.ijfoodmicro.2007.11.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee G.H., Tan B.H., Teo E.C., Lim S.G., Dan Y.Y., Wee A., Aw P.P., Zhu Y., Hibberd M.L., Tan C.K., et al. Chronic Infection With Camelid Hepatitis E Virus in a Liver Transplant Recipient Who Regularly Consumes Camel Meat and Milk. Gastroenterology. 2016;150:355–357.e3. doi: 10.1053/j.gastro.2015.10.048. [DOI] [PubMed] [Google Scholar]
- 36.Maunula L., Kaupke A., Vasickova P., Söderberg K., Kozyra I., Lazic S., van der Poel W.H., Bouwknegt M., Rutjes S., Willems K.A., et al. Tracing enteric viruses in the European berry fruit supply chain. Int. J. Food Microbiol. 2013;167:177–185. doi: 10.1016/j.ijfoodmicro.2013.09.003. [DOI] [PubMed] [Google Scholar]
- 37.Halliday M.L., Kang L.Y., Zhou T.K., Hu M.D., Pan Q.C., Fu T.Y., Huang Y.S., Hu S.L. An epidemic of hepatitis A attributable to the ingestion of raw clams in Shanghai, China. J. Infect. Dis. 1991;164:852–859. doi: 10.1093/infdis/164.5.852. [DOI] [PubMed] [Google Scholar]
- 38.Pintó R.M., Costafreda M.I., Bosch A. Risk assessment in shellfish-borne outbreaks of hepatitis A. Appl. Environ. Microbiol. 2009;75:7350–7355. doi: 10.1128/AEM.01177-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Diez-Valcarce M., Kokkinos P., Söderberg K., Bouwknegt M., Willems K., de Roda-Husman A.M., von Bonsdorff C.H., Bellou M., Hernández M., Maunula L., et al. Occurrence of human enteric viruses in commercial mussels at retail level in three European countries. Food Environ. Virol. 2012;4:73–80. doi: 10.1007/s12560-012-9078-9. [DOI] [PubMed] [Google Scholar]
- 40.Said B., Ijaz S., Kafatos G., Booth L., Thomas H.L., Walsh A., Ramsay M., Morgan D. Hepatitis E outbreak on cruise ship. Emerg. Infect. Dis. 2009;15:1738–1744. doi: 10.3201/eid1511.091094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foster M.A., Hofmeister M.G., Albertson J.P., Brown K.B., Burakoff A.W., Gandhi A.P., Glenn-Finer R.E., Gounder P., Ho P.Y., Kavanaugh T., et al. Hepatitis A Virus Infections Among Men Who Have Sex with Men-Eight U.S. States, 2017–2018. MMWR Morb. Mortal. Wkly. Rep. 2021;70:875–878. doi: 10.15585/mmwr.mm7024a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Foster M.A., Hofmeister M.G., Yin S., Montgomery M.P., Weng M.K., Eckert M., Nelson N.P., Mermin J., Wester C., Teshale E.H., et al. Widespread Hepatitis A Outbreaks Associated with Person-to-Person Transmission-United States, 2016–2020. MMWR Morb. Mortal. Wkly. Rep. 2022;71:1229–1234. doi: 10.15585/mmwr.mm7139a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Raczyńska A., Wickramasuriya N.N., Kalinowska-Nowak A., Garlicki A., Bociąga-Jasik M. Acute Hepatitis A Outbreak Among Men Who Have Sex With Men in Krakow, Poland; February 2017-February 2018. Am J Mens Health. 2019;13:1557988319895141. doi: 10.1177/1557988319895141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dalton H.R., Izopet J. Transmission and Epidemiology of Hepatitis E Virus Genotype 3 and 4 Infections. Cold Spring Harb. Perspect. Med. 2018;8:a032144. doi: 10.1101/cshperspect.a032144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Teshale E.H., Grytdal S.P., Howard C., Barry V., Kamili S., Drobeniuc J., Hill V.R., Okware S., Hu D.J., Holmberg S.D. Evidence of person-to-person transmission of hepatitis E virus during a large outbreak in Northern Uganda. Clin. Infect. Dis. 2010;50:1006–1010. doi: 10.1086/651077. [DOI] [PubMed] [Google Scholar]
- 46.Rodríguez-Tajes S., Perpiñán E., Leonel T., Lens S., Mariño Z., Pérez-Del-Pulgar S., García-López M., Pocurull A., Koutsoudakis G., Forns X. Low seroprevalence and zero incidence rate of hepatitis E in men who have sex with men during a hepatitis A outbreak. J. Med. Virol. 2020;92:1359–1362. doi: 10.1002/jmv.25630. [DOI] [PubMed] [Google Scholar]
- 47.Spada E., Costantino A., Pezzotti P., Bruni R., Pisani G., Madonna E., Chionne P., Simeoni M., Villano U., Marcantonio C., et al. Hepatitis E virus infection prevalence among men who have sex with men involved in a hepatitis A virus outbreak in Italy. Blood Transfus. 2019;17:428. doi: 10.2450/2019.020919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Migueres M., Ducours M., Dimeglio C., Trimoulet P., Abravanel F., Delobel P., Cazanave C., Izopet J. No evidence of sexual transmission of HEV among individuals using HIV pre-exposure prophylaxis. J. Viral Hepat. 2020;27:1495–1501. doi: 10.1111/jvh.13367. [DOI] [PubMed] [Google Scholar]
- 49.Greco L., Uceda Renteria S.C., Guarneri D., Orlandi A., Zoccoli A., Benardon S., Cusini M., Lunghi G. HEV and HAV seroprevalence in men that have sex with men (MSM): An update from Milan, Italy. J. Viral Hepat. 2018;90:1323–1327. doi: 10.1002/jmv.25052. [DOI] [PubMed] [Google Scholar]
- 50.Dahl V., Majeed A., Wikman A., Norda R., Edgren G. Transmission of viral hepatitis through blood transfusion in Sweden, 1968 to 2012. Euro Surveill. 2020;25:1900537. doi: 10.2807/1560-7917.ES.2020.25.29.1900537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherertz R.J., Russell B.A., Reuman P.D. Transmission of hepatitis A by transfusion of blood products. Arch. Intern. Med. 1984;144:1579–1580. doi: 10.1001/archinte.1984.00350200071011. [DOI] [PubMed] [Google Scholar]
- 52.Huzly D., Umhau M., Bettinger D., Cathomen T., Emmerich F., Hasselblatt P., Hengel H., Herzog R., Kappert O., Maassen S., et al. Transfusion-transmitted hepatitis E in Germany, 2013. Euro Surveill. 2014;19:20812. doi: 10.2807/1560-7917.ES2014.19.21.20812. [DOI] [PubMed] [Google Scholar]
- 53.Hewitt P.E., Ijaz S., Brailsford S.R., Brett R., Dicks S., Haywood B., Kennedy I.T., Kitchen A., Patel P., Poh J., et al. Hepatitis E virus in blood components: A prevalence and transmission study in southeast England. Lancet. 2014;384:1766–1773. doi: 10.1016/S0140-6736(14)61034-5. [DOI] [PubMed] [Google Scholar]
- 54.Ren F., Zhao C., Wang L., Wang Z., Gong X., Song M., Zhuang H., Huang Y., Shan H., Wang J., et al. Hepatitis E virus seroprevalence and molecular study among blood donors in China. Pt 2Transfusion. 2014;54:910–917. doi: 10.1111/trf.12530. [DOI] [PubMed] [Google Scholar]
- 55.Satake M., Matsubayashi K., Hoshi Y., Taira R., Furui Y., Kokudo N., Akamatsu N., Yoshizumi T., Ohkohchi N., Okamoto H., et al. Unique clinical courses of transfusion-transmitted hepatitis E in patients with immunosuppression. Transfusion. 2017;57:280–288. doi: 10.1111/trf.13994. [DOI] [PubMed] [Google Scholar]
- 56.Barzilai A., Schulman S., Karetnyi Y.V., Favorov M.O., Levin E., Mendelson E., Weiss P., Fields H.A., Varon D., Martinowitz U. Hepatitis E virus infection in hemophiliacs. J. Med. Virol. 1995;46:153–156. doi: 10.1002/jmv.1890460213. [DOI] [PubMed] [Google Scholar]
- 57.Elinav E., Ben-Dov I.Z., Shapira Y., Daudi N., Adler R., Shouval D., Ackerman Z. Acute hepatitis A infection in pregnancy is associated with high rates of gestational complications and preterm labor. Gastroenterology. 2006;130:1129–1134. doi: 10.1053/j.gastro.2006.01.007. [DOI] [PubMed] [Google Scholar]
- 58.Simsek Y., Isik B., Karaer A., Celik O., Kutlu R., Aydin N.E., Yilmaz S. Fulminant hepatitis A infection in second trimester of pregnancy requiring living-donor liver transplantation. J. Obstet. Gynaecol. Res. 2012;38:745–748. doi: 10.1111/j.1447-0756.2011.01757.x. [DOI] [PubMed] [Google Scholar]
- 59.Leikin E., Lysikiewicz A., Garry D., Tejani N. Intrauterine transmission of hepatitis A virus. Pt 2Obstet. Gynecol. 1996;88:690–691. doi: 10.1016/0029-7844(96)00259-1. [DOI] [PubMed] [Google Scholar]
- 60.Motte A., Blanc J., Minodier P., Colson P. Acute hepatitis A in a pregnant woman at delivery. Int. J. Infect. Dis. 2009;13:e49–e51. doi: 10.1016/j.ijid.2008.06.009. [DOI] [PubMed] [Google Scholar]
- 61.Patra S., Kumar A., Trivedi S.S., Puri M., Sarin S.K. Maternal and fetal outcomes in pregnant women with acute hepatitis E virus infection. Ann. Intern. Med. 2007;147:28–33. doi: 10.7326/0003-4819-147-1-200707030-00005. [DOI] [PubMed] [Google Scholar]
- 62.Sharma S., Kumar A., Kar P., Agarwal S., Ramji S., Husain S.A., Prasad S., Sharma S. Risk factors for vertical transmission of hepatitis E virus infection. J. Viral Hepat. 2017;24:1067–1075. doi: 10.1111/jvh.12730. [DOI] [PubMed] [Google Scholar]
- 63.Li M., Bu Q., Gong W., Li H., Wang L., Li S., Sridhar S., Cy Woo P., Wang L. Hepatitis E virus infection and its associated adverse feto-maternal outcomes among pregnant women in Qinhuangdao, China. J. Matern. Fetal Neonatal Med. 2020;33:3647–3651. doi: 10.1080/14767058.2019.1582630. [DOI] [PubMed] [Google Scholar]
- 64.Ma X.X., Ji Y., Jin L., Baloch Z., Zhang D.R., Wang Y., Pan Q., Ma Z. Prevalence and clinical features of hepatitis E virus infection in pregnant women: A large cohort study in Inner Mongolia, China. Clin. Res. Hepatol. Gastroenterol. 2021;45:101536. doi: 10.1016/j.clinre.2020.08.012. [DOI] [PubMed] [Google Scholar]
- 65.Bustamante N.D., Matyenyika S.R., Miller L.A., Goers M., Katjiuanjo P., Ndiitodino K., Ndevaetela E.E., Kaura U., Nyarko K.M., Kahuika-Crentsil L., et al. Notes from the Field: Nationwide Hepatitis E Outbreak Concentrated in Informal Settlements-Namibia, 2017–2020. MMWR Morb. Mortal. Wkly. Rep. 2020;69:355–357. doi: 10.15585/mmwr.mm6912a6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Charre C., Ramière C., Dumortier J., Abravanel F., Lhomme S., Gincul R., Scholtès C. Chronic Genotype 3 Hepatitis E in Pregnant Woman Receiving Infliximab and Azathioprine. Emerg. Infect. Dis. 2018;24:941–943. doi: 10.3201/eid2405.171845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rodríguez Lay Lde L., Quintana A., Villalba M.C., Lemos G., Corredor M.B., Moreno A.G., Prieto P.A., Guzmán M.G., Anderson D. Dual infection with hepatitis A and E viruses in outbreaks and in sporadic clinical cases: Cuba 1998–2003. J. Med. Virol. 2008;80:798–802. doi: 10.1002/jmv.21147. [DOI] [PubMed] [Google Scholar]
- 68.Sarguna P., Rao A., Sudha Ramana K.N. Outbreak of acute viral hepatitis due to hepatitis E virus in Hyderabad. Indian J. Med. Microbiol. 2007;25:378–382. doi: 10.1016/S0255-0857(21)02055-7. [DOI] [PubMed] [Google Scholar]
- 69.Murhekar M.V., Ashok M., Kanagasabai K., Joshua V., Ravi M., Sabarinathan R., Kirubakaran B.K., Ramachandran V., Shete V., Gupta N., et al. Epidemiology of Hepatitis A and Hepatitis E Based on Laboratory Surveillance Data-India, 2014–2017. Am. J. Trop. Med. Hyg. 2018;99:1058–1061. doi: 10.4269/ajtmh.18-0232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Samaddar A., Taklikar S., Kale P., Kumar C.A., Baveja S. Infectious hepatitis: A 3-year retrospective study at a tertiary care hospital in India. Indian J. Med. Microbiol. 2019;37:230–234. doi: 10.4103/ijmm.IJMM_19_197. [DOI] [PubMed] [Google Scholar]
- 71.Barde P.V., Chouksey V.K., Shivlata L., Sahare L.K., Thakur A.K. Viral hepatitis among acute hepatitis patients attending tertiary care hospital in central India. Virusdisease. 2019;30:367–372. doi: 10.1007/s13337-019-00541-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Jain P., Prakash S., Gupta S., Singh K.P., Shrivastava S., Singh D.D., Singh J., Jain A. Prevalence of hepatitis A virus, hepatitis B virus, hepatitis C virus, hepatitis D virus and hepatitis E virus as causes of acute viral hepatitis in North India: A hospital based study. Indian J. Med. Microbiol. 2013;31:261–265. doi: 10.4103/0255-0857.115631. [DOI] [PubMed] [Google Scholar]
- 73.Bansal Y., Singla N., Garg K., Sharma G., Gill M., Chander J. Seroprevalence of hepatitis A and hepatitis E in patients at a teaching hospital of northern India over a period of 8 years. J. Fam. Med. Prim. Care. 2022;11:567–572. doi: 10.4103/jfmpc.jfmpc_1212_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kaur M., Sidhu S.K., Singh K., Devi P., Kaur M., Singh N.J. Hepatitis E virus: A leading cause of waterborne viral hepatitis in Northwest Districts of Punjab, India. J. Lab. Physicians. 2017;9:121–124. doi: 10.4103/0974-2727.199636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Paul R.C., Nazneen A., Banik K.C., Sumon S.A., Paul K.K., Akram A., Uzzaman M.S., Iqbal T., Tejada-Strop A., Kamili S., et al. Hepatitis E as a cause of adult hospitalization in Bangladesh: Results from an acute jaundice surveillance study in six tertiary hospitals, 2014–2017. PLoS Negl. Trop. Dis. 2020;14:e0007586. doi: 10.1371/journal.pntd.0007586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Fouad H.M., Reyad E.M., El-Din A.G. Acute hepatitis A is the chief etiology of acute hepatitis in Egyptian children: A single-center study. Eur. J. Clin. Microbiol. Infect. Dis. 2018;37:1941–1947. doi: 10.1007/s10096-018-3329-0. [DOI] [PubMed] [Google Scholar]
- 77.Zaki M.E.S., Alsayed M.A.L., Abbas H.R.R., Ahmed D.M., Ashry A.Y.E. Prevalence of hepatitis E virus in children with acute hepatitis: One Egyptian center study. Germs. 2020;10:88–94. doi: 10.18683/germs.2020.1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hyams K.C., McCarthy M.C., Kaur M., Purdy M.A., Bradley D.W., Mansour M.M., Gray S., Watts D.M., Carl M. Acute sporadic hepatitis E in children living in Cairo, Egypt. J. Med. Virol. 1992;37:274–277. doi: 10.1002/jmv.1890370407. [DOI] [PubMed] [Google Scholar]
- 79.Ali M.A., Al-Herrawy A.Z., El-Hawaary S.E. Detection of enteric viruses, Giardia and Cryptosporidium in two different types of drinking water treatment facilities. Water Res. 2004;38:3931–3939. doi: 10.1016/j.watres.2004.06.014. [DOI] [PubMed] [Google Scholar]
- 80.Kamel A.H., Ali M.A., El-Nady H.G., Deraz A., Aho S., Pothier P., Belliot G. Presence of enteric hepatitis viruses in the sewage and population of Greater Cairo. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2011;17:1182–1185. doi: 10.1111/j.1469-0691.2011.03461.x. [DOI] [PubMed] [Google Scholar]
- 81.El-Esnawy N.A., Gamil M.A., El-Wakkad A.S. Detection of hepatitis E virus in greater Cairo. Two wastewater treatment plants and its prevalence among workers of these plants. J. Egypt. Public Health Assoc. 1998;73:597–619. [PubMed] [Google Scholar]
- 82.García C.G., Sánchez D., Villalba M.C., Pujol F.H., de Los Ángeles Rodríguez Lay L., Pinto B., Chacón E.P., Guzmán M.G. Molecular characterization of hepatitis E virus in patients with acute hepatitis in Venezuela. J. Med. Virol. 2012;84:1025–1029. doi: 10.1002/jmv.23277. [DOI] [PubMed] [Google Scholar]
- 83.Realpe-Quintero M., Mirazo S., Viera-Segura O., Copado-Villagrana E.D., Panduro A., Roman S., Arbiza J., Fierro N.A. Hepatitis E Virus Genotype 1 and Hepatitis A Virus Dual Infection in Pediatric Patients with a Low Socioeconomic Status from Mexico. Intervirology. 2018;61:105–110. doi: 10.1159/000492425. [DOI] [PubMed] [Google Scholar]
- 84.Li M., Zhang H., Wang L., Li Z., Wang J., Xu B., Hao R., Liu C., Fu H., Rao H., et al. The investigation of hepatitis A virus and hepatitis E virus co-infection in humans and animals in China. Acta Virol. 2020;64:20–27. doi: 10.4149/av_2020_103. [DOI] [PubMed] [Google Scholar]
- 85.Arora N.K., Nanda S.K., Gulati S., Ansari I.H., Chawla M.K., Gupta S.D., Panda S.K. Acute viral hepatitis types E, A, and B singly and in combination in acute liver failure in children in north India. J. Med. Virol. 1996;48:215–221. doi: 10.1002/(SICI)1096-9071(199603)48:3<215::AID-JMV1>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 86.Das A.K., Ahmed S., Medhi S., Kar P. Changing Patterns of Aetiology of Acute Sporadic Viral Hepatitis in India-Newer Insights from North-East India. Int. J. Curr. Res. Rev. 2014;6:14–20. [Google Scholar]
- 87.Poddar U., Thapa B.R., Prasad A., Singh K. Changing spectrum of sporadic acute viral hepatitis in Indian children. J. Trop. Pediatr. 2002;48:210–213. doi: 10.1093/tropej/48.4.210. [DOI] [PubMed] [Google Scholar]
- 88.Malathi S., Mohanavalli B., Menon T., Srilatha P., Sankaranarayanan V.S., Raju B.B., Ramathilagam B., Thyagarajan S.P. Clinical and viral marker pattern of acute sporadic hepatitis in children in Madras, South India. J. Trop. Pediatr. 1998;44:275–278. doi: 10.1093/tropej/44.5.275. [DOI] [PubMed] [Google Scholar]
- 89.Radhakrishnan S., Raghuraman S., Abraham P., Kurian G., Chandy G., Sridharan G. Prevalence of enterically transmitted hepatitis viruses in patients attending a tertiary--care hospital in south India. Indian J. Pathol. Microbiol. 2000;43:433–436. [PubMed] [Google Scholar]
- 90.Chandra N.S., Ojha D., Chatterjee S., Chattopadhyay D. Prevalence of hepatitis E virus infection in West Bengal, India: A hospital-based study. Pt 7J. Med. Microbiol. 2014;63:975–980. doi: 10.1099/jmm.0.072249-0. [DOI] [PubMed] [Google Scholar]
- 91.Joon A., Rao P., Shenoy S.M., Baliga S. Prevalence of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) in the patients presenting with acute viral hepatitis. Indian J. Med. Microbiol. 2015;33((Suppl. 1)):102–105. doi: 10.4103/0255-0857.150908. [DOI] [PubMed] [Google Scholar]
- 92.Kalita D., Paul M., Deka S., Badoni G., Gupta P. Simultaneous infection of Hepatitis A and Hepatitis E viruses amongst acute viral hepatitis patients: A hospital-based study from Uttarakhand. J. Fam. Med. Prim. Care. 2020;9:6130–6134. doi: 10.4103/jfmpc.jfmpc_1373_20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Palewar M.S., Joshi S., Choudhary G., Das R., Sadafale A., Karyakarte R. Prevalence of Hepatitis A virus (HAV) and Hepatitis E virus (HEV) in patients presenting with acute viral hepatitis: A 3-year retrospective study at a tertiary care Hospital in Western India. J. Family. Med. Prim. Care. 2022;11:2437–2441. doi: 10.4103/jfmpc.jfmpc_1746_21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Alam S., Azam G., Mustafa G., Azad A.K., Haque I., Gani S., Ahmad N., Alam K., Khan M. Natural course of fulminant hepatic failure: The scenario in Bangladesh and the differences from the west. Saudi J. Gastroenterol. 2009;15:229–233. doi: 10.4103/1319-3767.56094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mazhar M.K.A., Finger F., Evers E.S., Kuehne A., Ivey M., Yesurajan F., Shirin T., Ajim N., Kabir A., Musto J., et al. An outbreak of acute jaundice syndrome (AJS) among the Rohingya refugees in Cox’s Bazar, Bangladesh: Findings from enhanced epidemiological surveillance. PLoS ONE. 2021;16:e0250505. doi: 10.1371/journal.pone.0250505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Kang H.M., Jeong S.H., Kim J.W., Lee D., Choi C.K., Park Y.S., Hwang J.H., Kim N., Lee D.H. [Recent etiology and clinical features of acute viral hepatitis in a single center of Korea] Korean J. Hepatol. 2007;13:495–502. doi: 10.3350/kjhep.2007.13.4.495. [DOI] [PubMed] [Google Scholar]
- 97.Copado-Villagrana E.D., Anaya-Covarrubias J.Y., Viera-Segura O., Trujillo-Ochoa J.L., Panduro A., José-Abrego A., Roman S., Fierro N.A. Spatial and Temporal Distribution of Hepatitis A Virus and Hepatitis E Virus Among Children with Acute Hepatitis in Mexico. Viral Immunol. 2021;34:653–657. doi: 10.1089/vim.2021.0045. [DOI] [PubMed] [Google Scholar]
- 98.Rey J.L., Ramadani Q., Soarès J.L., Nicand E., Ibrahime D., Preteni E., Buisson Y., Teyssou R. Sero-epidemiological study of the hepatitis epidemic in Mitrovica in the aftermath of the war in Kosovo (1999) Bull. Soc. Pathol. Exot. 2002;95:3–7. [PubMed] [Google Scholar]
- 99.Divizia M., Gabrieli R., Stefanoni M.L., Renganathan E., El Ghazzawi E., Kader O.A., Gamil F., El Sawaf G., El Sherbini E., Saleh E., et al. HAV and HEV infection in hospitalised hepatitis patients in Alexandria, Egypt. Eur. J. Epidemiol. 1999;15:603–609. doi: 10.1023/A:1007514030062. [DOI] [PubMed] [Google Scholar]
- 100.Ramadan H.K., Sayed I.M., Elkhawaga A.A., Meghezel E.M., Askar A.A., Moussa A.M., Osman A., Elfadl A.A., Khalifa W.A., Ashmawy A.M., et al. Characteristics and outcomes of acute hepatitis of unknown etiology in Egypt: First report of adult adenovirus-associated hepatitis. Infection. 2022:1–9. doi: 10.1007/s15010-022-01945-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Muchiri I., Okoth F.A., Ngaira J., Tuei S. Seroprevalence of Hav, Hbv, Hcv, and Hev Among Acute Hepatitis Patients at Kenyatta National Hospital in Nairobi, Kenya. East Afr. Med. J. 2012;89:199–205. [PubMed] [Google Scholar]
- 102.Kamar N., Selves J., Mansuy J.M., Ouezzani L., Péron J.M., Guitard J., Cointault O., Esposito L., Abravanel F., Danjoux M., et al. Hepatitis E virus and chronic hepatitis in organ-transplant recipients. New Engl. J. Med. 2008;358:811–817. doi: 10.1056/NEJMoa0706992. [DOI] [PubMed] [Google Scholar]
- 103.Wang Y., Chen G., Pan Q., Zhao J. Chronic Hepatitis E in a Renal Transplant Recipient: The First Report of Genotype 4 Hepatitis E Virus Caused Chronic Infection in Organ Recipient. Gastroenterology. 2018;154:1199–1201. doi: 10.1053/j.gastro.2017.12.028. [DOI] [PubMed] [Google Scholar]
- 104.Pischke S., Behrendt P., Manns M.P., Wedemeyer H. HEV-associated cryoglobulinaemia and extrahepatic manifestations of hepatitis E. Lancet Infect. Dis. 2014;14:678–679. doi: 10.1016/S1473-3099(14)70823-0. [DOI] [PubMed] [Google Scholar]
- 105.El-Mokhtar M.A., Sayed I.M. Model systems for studying extrahepatic pathogenesis of hepatitis E virus. Current knowledge and future directions. Rev. Med. Virol. 2021;31:e2218. doi: 10.1002/rmv.2218. [DOI] [PubMed] [Google Scholar]
- 106.Kamani P., Baijal R., Amarapurkar D., Gupte P., Patel N., Kumar P., Agal S. Guillain-Barre syndrome associated with acute hepatitis E. Indian J. Gastroenterol. 2005;24:216. [PubMed] [Google Scholar]
- 107.Jung Y.J., Kim W., Jeong J.B., Kim B.G., Lee K.L., Oh K.H., Yoon J.H., Lee H.S., Kim Y.J. Clinical features of acute renal failure associated with hepatitis A virus infection. J. Viral Hepat. 2010;17:611–617. doi: 10.1111/j.1365-2893.2009.01216.x. [DOI] [PubMed] [Google Scholar]
- 108.Schiff E.R. Atypical clinical manifestations of hepatitis A. Vaccine. 1992;10((Suppl. 1)):S18–S20. doi: 10.1016/0264-410X(92)90534-Q. [DOI] [PubMed] [Google Scholar]
- 109.Lee J.J., Kang K., Park J.M., Kwon O., Kim B.K. Encephalitis associated with acute hepatitis a. J. Epilepsy Res. 2011;1:27–28. doi: 10.14581/jer.11005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Menon D., Jagtap S.A., Nair M.D. Guillain-Barré syndrome following acute viral hepatitis A. J. Neurosci. Rural. Pract. 2014;5:204–205. doi: 10.4103/0976-3147.131695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Harvala H., Reynolds C., Brailsford S., Davison K. Fulminant Transfusion-Associated Hepatitis E Virus Infection Despite Screening, England, 2016–2020. Emerg. Infect. Dis. 2022;28:1805–1813. doi: 10.3201/eid2809.220487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Lemon S.M. Type A viral hepatitis. New developments in an old disease. N. Engl. J. Med. 1985;313:1059–1067. doi: 10.1056/NEJM198510243131706. [DOI] [PubMed] [Google Scholar]
- 113.Xie C., Fenkel J.M., Halegoua-DeMarzio D.L., Civan J.M., Tholey D.M., Herrine S.K., Thapar M., Ambelil M., Arastu S., Frank A.M., et al. Acute Liver Failure Requiring Liver Transplantation due to Acute Hepatitis A Virus Infection. Case Rep. Transplant. 2021;2021:5159934. doi: 10.1155/2021/5159934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Kaur R., Gur R., Berry N., Kar P. Etiology of endemic viral hepatitis in urban North India. Southeast Asian J. Trop. Med. Public Health. 2002;33:845–848. [PubMed] [Google Scholar]
- 115.Kumar A., Yachha S.K., Poddar U., Singh U., Aggarwal R. Does co-infection with multiple viruses adversely influence the course and outcome of sporadic acute viral hepatitis in children? J. Gastroenterol. Hepatol. 2006;21:1533–1537. doi: 10.1111/j.1440-1746.2006.04509.x. [DOI] [PubMed] [Google Scholar]
- 116.Jin Hong P., Byung Seok K., Chang Hyeong L., Sun Young K., Jung Hyun S., Chang Jae H. A Case of Coinfection of Hepatitis A and E Virus with Hepatic Encephalopathy. Korean J. Med. 2011;80:101–105. [Google Scholar]
- 117.Piza Palacios L., Espinoza-Ríos J. Hepatitis A and hepatitis E virus co-infection with right pleural effusion, ascites and acute acalculous cholecystitis. A case report. Rev. Gastroenterol. Peru. 2020;40:77–79. doi: 10.47892/rgp.2020.401.1035. [DOI] [PubMed] [Google Scholar]
- 118.Saeed A., Cheema H.A., Assiri A. Hepatitis Aand E Co-Infection with Worst Outcome. J. Coll. Physicians Surg. Pak. 2016;26:S31–S32. [PubMed] [Google Scholar]
- 119.Mittal A., Bithu R., Vyas N., Maheshwari R. Prevalence of hepatitis a virus and hepatitis e virus in the patients presenting with acute viral hepatitis at a tertiary care hospital Jaipur Rajasthan. New Niger. J. Clin. Res. 2016;5:47–50. doi: 10.4103/2250-9658.197436. [DOI] [Google Scholar]
- 120.Butt N., Khan M.A., Haleem F. Acute Viral Hepatitis: Simultaneous Infection from Hepatitis A, B and E Viruses. J. Coll. Physicians Surg. Pak. 2019;29:S103–S105. doi: 10.29271/jcpsp.2019.12.S103. [DOI] [PubMed] [Google Scholar]
- 121.Cheng S.H., Mai L., Zhu F.Q., Pan X.F., Sun H.X., Cao H., Shu X., Ke W.M., Li G., Xu Q.H. Influence of chronic HBV infection on superimposed acute hepatitis E. World J. Gastroenterol. 2013;19:5904–5909. doi: 10.3748/wjg.v19.i35.5904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Migueres M., Lhomme S., Izopet J. Hepatitis A: Epidemiology, High-Risk Groups, Prevention and Research on Antiviral Treatment. Viruses. 2021;13:1900. doi: 10.3390/v13101900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Nelson N.P., Link-Gelles R., Hofmeister M.G., Romero J.R., Moore K.L., Ward J.W., Schillie S.F. Update: Recommendations of the Advisory Committee on Immunization Practices for Use of Hepatitis A Vaccine for Postexposure Prophylaxis and for Preexposure Prophylaxis for International Travel. MMWR Morb. Mortal. Wkly. Rep. 2018;67:1216–1220. doi: 10.15585/mmwr.mm6743a5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Kamar N., Izopet J., Tripon S., Bismuth M., Hillaire S., Dumortier J., Radenne S., Coilly A., Garrigue V., D’Alteroche L., et al. Ribavirin for chronic hepatitis E virus infection in transplant recipients. N. Engl. J. Med. 2014;370:1111–1120. doi: 10.1056/NEJMoa1215246. [DOI] [PubMed] [Google Scholar]
- 125.Sabrià A., Gregori J., Garcia-Cehic D., Guix S., Pumarola T., Manzanares-Laya S., Caylà J.A., Bosch A., Quer J., Pintó R.M. Evidence for positive selection of hepatitis A virus antigenic variants in vaccinated men-having-sex-with men patients: Implications for immunization policies. EBioMedicine. 2019;39:348–357. doi: 10.1016/j.ebiom.2018.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Su Y.Y., Huang S.J., Guo M., Zhao J., Yu H., He W.G., Jiang H.M., Wang Y.J., Zhang X.F., Cai J.P., et al. Persistence of antibodies acquired by natural hepatitis E virus infection and effects of vaccination. Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2017;23:e331–e336. doi: 10.1016/j.cmi.2016.10.029. [DOI] [PubMed] [Google Scholar]
- 127.Sridhar S., Situ J., Cai J.P., Yip C.C., Wu S., Zhang A.J., Wen L., Chew N.F., Chan W.M., Poon R.W., et al. Multimodal investigation of rat hepatitis E virus antigenicity: Implications for infection, diagnostics, and vaccine efficacy. J. Hepatol. 2021;74:1315–1324. doi: 10.1016/j.jhep.2020.12.028. [DOI] [PubMed] [Google Scholar]
- 128.Wen G.P., He L., Tang Z.M., Wang S.L., Zhang X., Chen Y.Z., Lin X., Liu C., Chen J.X., Ying D., et al. Quantitative evaluation of protective antibody response induced by hepatitis E vaccine in humans. Nat. Commun. 2020;11:3971. doi: 10.1038/s41467-020-17737-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Konduru K., Kaplan G.G. Stable growth of wild-type hepatitis A virus in cell culture. J. Virol. 2006;80:1352–1360. doi: 10.1128/JVI.80.3.1352-1360.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lemon S.M., Murphy P.C., Shields P.A., Ping L.H., Feinstone S.M., Cromeans T., Jansen R.W. Antigenic and genetic variation in cytopathic hepatitis A virus variants arising during persistent infection: Evidence for genetic recombination. J. Virol. 1991;65:2056–2065. doi: 10.1128/jvi.65.4.2056-2065.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Brack K., Frings W., Dotzauer A., Vallbracht A. A cytopathogenic, apoptosis-inducing variant of hepatitis A virus. J. Virol. 1998;72:3370–3376. doi: 10.1128/JVI.72.4.3370-3376.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Shukla P., Nguyen H.T., Torian U., Engle R.E., Faulk K., Dalton H.R., Bendall R.P., Keane F.E., Purcell R.H., Emerson S.U. Cross-species infections of cultured cells by hepatitis E virus and discovery of an infectious virus-host recombinant. Proc. Natl. Acad. Sci. USA. 2011;108:2438–2443. doi: 10.1073/pnas.1018878108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Nguyen H.T., Shukla P., Torian U., Faulk K., Emerson S.U. Hepatitis E virus genotype 1 infection of swine kidney cells in vitro is inhibited at multiple levels. J. Virol. 2014;88:868–877. doi: 10.1128/JVI.02205-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Burkard T., Proske N., Resner K., Collignon L., Knegendorf L., Friesland M., Verhoye L., Sayed I.M., Brüggemann Y., Nocke M.K., et al. Viral Interference of Hepatitis C and E Virus Replication in Novel Experimental Co-Infection Systems. Cells. 2022;11:927. doi: 10.3390/cells11060927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Jiang W., Ma P., Long G. A visualizable hepatitis A virus and hepatitis C virus coinfection model <em>in vitro</em>: Coexistence of two hepatic viruses under limited competition in viral RNA synthesis. bioRxiv. 2019 doi: 10.1101/709071. [DOI] [Google Scholar]
- 136.Sayed I.M., Verhoye L., Montpellier C., Abravanel F., Izopet J., Cocquerel L., Meuleman P. Hepatitis E Virus (HEV) Open Reading Frame 2 Antigen Kinetics in Human-Liver Chimeric Mice and Its Impact on HEV Diagnosis. J. Infect. Dis. 2019;220:811–819. doi: 10.1093/infdis/jiz171. [DOI] [PubMed] [Google Scholar]
- 137.Sayed I.M., Verhoye L., Cocquerel L., Abravanel F., Foquet L., Montpellier C., Debing Y., Farhoudi A., Wychowski C., Dubuisson J., et al. Study of hepatitis E virus infection of genotype 1 and 3 in mice with humanised liver. Gut. 2017;66:920–929. doi: 10.1136/gutjnl-2015-311109. [DOI] [PubMed] [Google Scholar]
- 138.Sayed I.M., Foquet L., Verhoye L., Abravanel F., Farhoudi A., Leroux-Roels G., Izopet J., Meuleman P. Transmission of hepatitis E virus infection to human-liver chimeric FRG mice using patient plasma. Antivir. Res. 2017;141:150–154. doi: 10.1016/j.antiviral.2017.02.011. [DOI] [PubMed] [Google Scholar]
- 139.van de Garde M.D., Pas S.D., van der Net G., de Man R.A., Osterhaus A.D., Haagmans B.L., Boonstra A., Vanwolleghem T. Hepatitis E Virus (HEV) Genotype 3 Infection of Human Liver Chimeric Mice as a Model for Chronic HEV Infection. J. Virol. 2016;90:4394–4401. doi: 10.1128/JVI.00114-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Allweiss L., Gass S., Giersch K., Groth A., Kah J., Volz T., Rapp G., Schöbel A., Lohse A.W., Polywka S., et al. Human liver chimeric mice as a new model of chronic hepatitis E virus infection and preclinical drug evaluation. J. Hepatol. 2016;64:1033–1040. doi: 10.1016/j.jhep.2016.01.011. [DOI] [PubMed] [Google Scholar]
- 141.Sayed I.M., Meuleman P. Updates in Hepatitis E virus (HEV) field; lessons learned from human liver chimeric mice. Rev. Med. Virol. 2020;30:e2086. doi: 10.1002/rmv.2086. [DOI] [PubMed] [Google Scholar]
- 142.Sayed I.M., Meuleman P. Murine Tissues of Human Liver Chimeric Mice Are Not Susceptible to Hepatitis E Virus Genotypes 1 and 3. J. Infect. Dis. 2017;216:919–920. doi: 10.1093/infdis/jix422. [DOI] [PubMed] [Google Scholar]
- 143.Hirai-Yuki A., Whitmire J.K., Joyce M., Tyrrell D.L., Lemon S.M. Murine Models of Hepatitis A Virus Infection. Cold Spring Harb. Perspect. Med. 2019;9:a031674. doi: 10.1101/cshperspect.a031674. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.