Abstract
Retinal degeneration (RD) is a significant cause of incurable blindness worldwide. Photoreceptors and retinal pigmented epithelium are irreversibly damaged in advanced RD. Functional replacement of photoreceptors and/or retinal pigmented epithelium cells is a promising approach to restoring vision. This paper reviews the current status and explores future prospects of the transplantation therapy provided by pluripotent stem cell-derived retinal organoids (ROs). This review summarizes the status of rodent RD disease models and discusses RO culture and analytical tools to evaluate RO quality and function. Finally, we review and discuss the studies in which RO-derived cells or sheets were transplanted. In conclusion, methods to derive ROs from pluripotent stem cells have significantly improved and become more efficient in recent years. Meanwhile, more novel technologies are applied to characterize and validate RO quality. However, opportunity remains to optimize tissue differentiation protocols and achieve better RO reproducibility. In order to screen high-quality ROs for downstream applications, approaches such as noninvasive and label-free imaging and electrophysiological functional testing are promising and worth further investigation. Lastly, transplanted RO-derived tissues have allowed improvements in visual function in several RD models, showing promises for clinical applications in the future.
Keywords: retinal disease, retinal organoids, retinal degenerative model, functional test, transplantation
INTRODUCTION
Vision is critical for humans to perceive the world. The retina originates as an outgrowth of the forebrain during embryonic development. The visual pathways start at the retina where light is transduced into neuronal signals that are ultimately conveyed to the visual cortex for visual perception. The retina is a laminated organ that is broadly composed of retinal ganglion cells (RGCs), amacrine cells (ACs), bipolar cells (BCs), horizontal cells, Muller cells, and photoreceptors (PRs). Upon absorption of photons by visual photopigments in the PRs, a series of biochemical reactions occurs whereby light signals are transduced into neuronal signals. Whereas surgical treatments for diseases that damage light transmission through the cornea and the lens have been well established, permanent vision losses caused by damage to the RGCs as a result of glaucoma, loss of PRs and retinal pigmented epithelium (RPE) from age-related macular degeneration (AMD) and inherited retinal degenerations (IRDs), and damage to all layers of the retina from diabetic retinopathy, are irreversible and no therapies to reverse cell death are available.
Recent decades have witnessed the development of stem cell technology. Under specific culturing conditions, stem cells can be differentiated into self-assembled and layered retinal tissue spheroids that are called retinal organoids (ROs). ROs have been applied to different applications such as disease modeling,1–5 developmental biology,6–9 drug screening,10 gene therapy testing,2,11–14 and transplantation therapies.15–21 In this review, we focus on transplantation studies in recent years. We briefly review common retinal degeneration (RD) diseases, summarize common rodent models with IRD used for RO transplantation studies, and explore current methodologies used for RO culture and analysis. Lastly, we focus on post-transplantation evaluations and their functional effectiveness. Gene therapy in a dish is outside the scope of this review and is not discussed.
RD DISEASES AND RODENT DISEASE MODELS
AMD is marked by the degeneration of the PRs and RPE in the human macula and is the leading cause of irreversible blindness in people over 65 years old in industrialized countries.22 In the early and intermediate stages, AMD is marked by the accumulation of drusen, a yellowish retinoid breakdown product in the macula beneath the retina. Advanced AMD consists of 2 main categories: wet and dry AMD. Wet AMD involves abnormal choroidal blood vessel growth and can be treated by anti-vascular endothelial growth factor.23 However, there is no proven treatment for dry AMD characterized by RPE and subsequent PR death. The only promising approach may be cellular replacement therapy with transplantation.24
Retinitis pigmentosa (RP) is an IRD disease initially affecting peripheral vision progressing to loss of central vision in the end stage. Many gene mutations can yield the RP phenotype, and this heterogeneous genotypic etiology leads to significant difficulties in studying the disease and developing effective treatment.25 In mutations affecting rod-specific proteins, rod PRs will gradually deteriorate over decades, causing losses of night vision in adolescence, peripheral vision in young adulthood, and central vision in later life.26 The functional progression of vision loss is consistent with the characteristic death of rod PRs prior to cone PR death.
Neurons and PRs are highly differentiated cells and lack the ability to repair or regenerate after irreversible damage. Gene therapy has gained popularity in IRD treatment in recent years as summarized in several reviews.27,28 For example, a recent study applied subretinal gene therapy that delivered human melanopsin gene (OPN4) and showed vision restoration in retinal degeneration 1 (rd1) mutation mouse model.29 Several additional studies demonstrated an improvement in PR survival in RP models when animals were administered oral N-acetylcysteine.30–32 While oral and gene therapy approaches demonstrated promise to prevent or halt disease progression, they were not able to restore PRs or RPE that were already lost.33 Cell and tissue replacement therapy offers an additional avenue for hope to patients with advanced RD. Transplantation of human pluripotent stem cell (hPSC)–derived ROs offers 1 pathway to replace segments of dead tissue.
Rodent models used in transplantation studies are summarized in Table 1. Mutations in rodent models primarily yielded RD marked by PR loss. Preclinical studies have also focused on immune rejection of transplantable RO materials. The native retina is known to be immune-privileged similar to the brain.17 A recent study showed that ROs elicited minimal immune response when transplanted,46 thereby allaying some concerns for future clinical application. However, to use allogeneic cells for transplantation research, immune rejection is still an important factor to consider in the long term,47 as cell rejection can occur months after transplantation.48 Human ROs xenografted into animal models raises concern of heterologous tissue rejection. Zhu et al42 reported that immunosuppression before transplantation allowed for better integration of graft cells and improved functionality. Thus, for RO transplantation studies, immunosuppression remains a primary consideration, in which animal models for the studies may receive immunosuppression using pharmacological agents (eg, cyclosporine A, mycophenolate, and tacrolimus), or genetically immunodeficient animals are used.16,19,49,50
TABLE 1.
Summary of Rodent Disease Models
| Rodent Diseases Models | Gene Modification | Affected Cell Type | Degeneration Time Frame | References |
|---|---|---|---|---|
| rd1 mice | Null mutation in the Pde6-β | Rod photoreceptor cells | 97% of rods lost by P17 and cone apoptosis around P30 | 34–38 |
| Loss of a functional ONL by 6 to 10 postnatal weeks | ||||
| rd1/Foxn1 nude mice | Null mutation in Pde6-β | Photoreceptor cells | Immunodeficient | 39 |
| Null mutation in Foxn1 | Immune cells (no T cells) | Complete loss of rods Absence of mouse cone arrestin+ cells from the central retina at 3 months postnatal | ||
| NOG-rd1 −2J mice | Pde6-β allele from rd1 mouse into NOG mice | Photoreceptor cells | Immunodeficient | 40 |
| Immune cells (loss of T, B, and NK cells) | Loss of photoreceptors within 3 to 4 postnatal weeks | |||
| L7-GFP/rd1 mice | Crossing rd1–2J and L7-GFP mice | Photoreceptor cells | Labeled bipolar cells | 21,41 |
| Rod bipolar cells express GFP | End-stage RD marked by the loss of majority of rod cells by P30 | |||
| IL2rγ−/− mice | IL2rγ knockdown | Photoreceptor cells (slow photoreceptor degeneration | Immunodeficient | 42 |
| CRX mutant | Immune cells (10-fold reduction of lymphocytes, absence of NK cells) | Mutation in the CRX gene leads to congenital blindness | ||
| Cpfl1/Rho−/− mice | Rhodopsin knockdown Cpfl1 mutation, cone function loss | Photoreceptor cells (dysfunctional rods and cones) | 2 to 3 rows of photoreceptors at the age of 9 wk | 37 |
| SD-Foxn1 Tg(S334ter) 3LavRrrc nude rats | Crossing SD-Tg(S334ter) 3Lav rat and NTac:NIH-Whn rats | Photoreceptor cells | Immunodeficient | 16,43,44 |
| Immune cells (loss of T cells) | Loss of ONL thickness and photoreceptors as early as P30 | |||
| Loss of most photoreceptor by 10 postnatal weeks | ||||
| RCS nude (Hsd:RH-Foxn1rnu) rats | Deletion in the MerTK receptor | RPE cells | Immunodeficient | 19,45 |
| Null mutation in Foxn1 | Immune cells (loss of T cells) | Failed RPE phagocytosis, causing outer segment debris accumulations and leading to photoreceptor death |
CRX indicates cone-rod homeobox; GFP, green fluorescent protein; MerTK, Mer tyrosine kinase; NOG, NOD/Shi-scid IL2rgamma(null); ONL, outer nuclear layer; RCS, Royal College of Surgeons; RD, retinal degeneration; RPE, retinal pigment epithelium.
RO CULTURE AND ANALYTICAL METHODS
Stage-Specific RO Development
Culture protocols for pluripotent stem cell (PSC)–derived mouse and human ROs were summarized and evaluated in previous reviews.51–53 Although timing is different, in most protocols, the basic procedure consists of 2 steps: (1) initiation of embryonic bodies (EBs) from stem cells by neuro induction media; and (2) long-term differentiation of ROs by adding retinal differentiation media. Stage-specific morphologies are shared by PSC-derived ROs regardless of induction protocols. Capowski et al54 identified 3 distinct morphological stages of RO development by investigating 16 hPSC lines (Figs. 1A–C). ROs in stage 1 are characterized by a neuroblast layer, rich in RGCs and rare ACs. Stage 2 ROs represent a transition period, when different cell types such as PRs, horizontal cells, and ACs start to differentiate and RGCs start to degenerate. Lastly, stage 3 ROs are marked by PR layer and outer segment structures with very few RGCs left in the inner layer. The emergence of Muller glia that form the structural framework of ROs is also one of the stage 3 markers.54 The stage-specific morphological features are accompanied by a shift in metabolic activity, which was confirmed by recent research. Xue et al55 identified these 3 stages of RO differentiation by analyzing the free to bound nicotinamide adenine dinucleotide ratio of the ROs’ surface using fluorescence lifetime imaging microscopy (FLIM). ROs in the early stage were more glycolytic because they mostly consisted of progenitor cells. During the differentiation stage, a metabolic shift from glycolysis to oxidative phosphorylation was observed (Fig. 1). At the maturation stage, the ROs developed glycolytic PR layers.55
FIGURE 1.
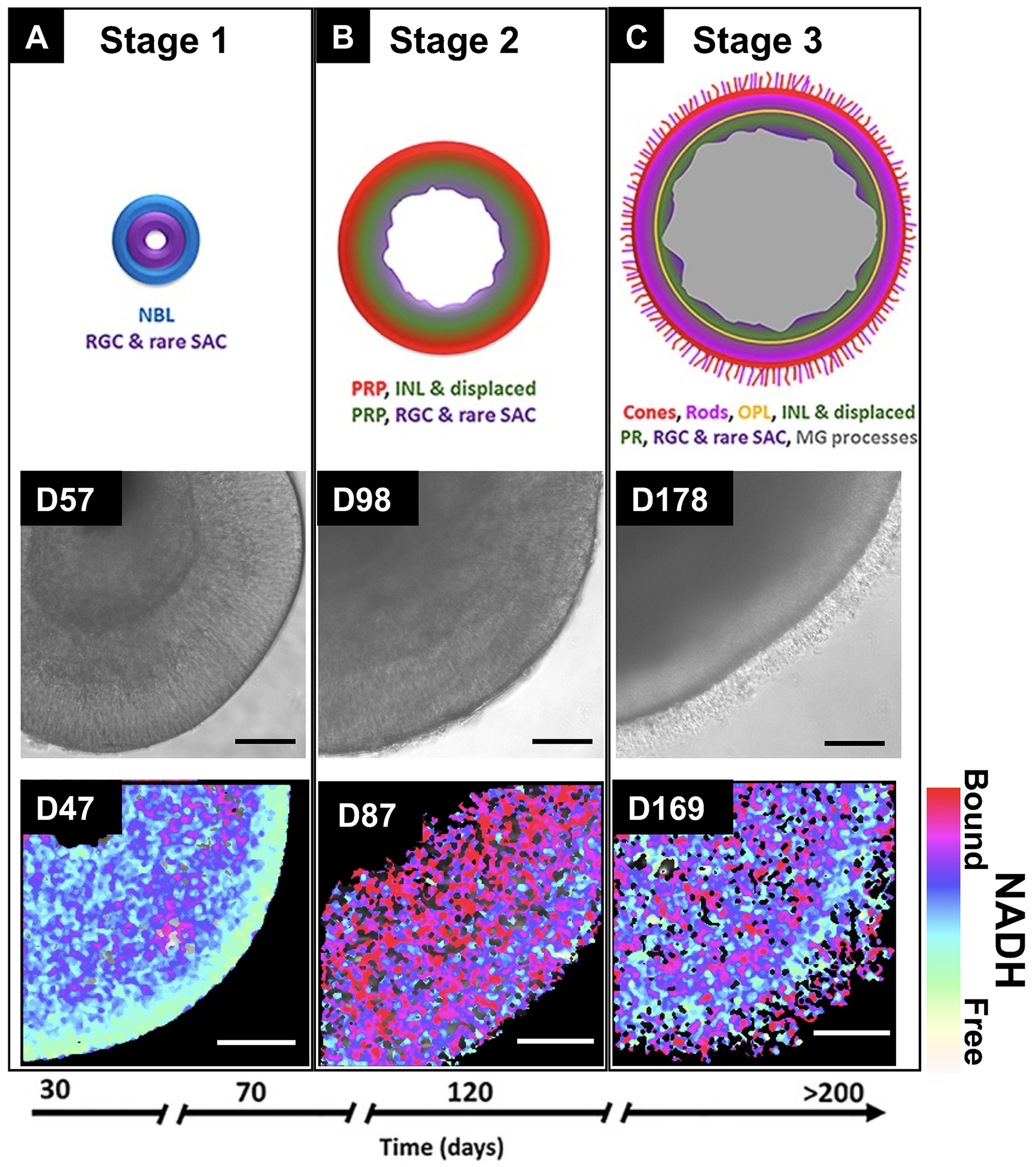
Three developmental stages of retinal organoids as shown by phase-contrast microscopy and fluorescence lifetime imaging (FLIM). The schematic diagram in the first row was taken from Capowski et al.54 (Fig. 10, republished with permission of The Company of Biologists Ltd, from Capowski et al, Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development. 2019;146: dev171686. doi:10.1242/dev.17168; permission conveyed through Copyright Clearance Center, Inc). The FLIM NADH map in the third row was taken from Xue et al.55 (Fig. 1A) (scale bars: second row—200 μm; third row—50 μm). INL indicates inner nuclear layer; MG, Muller glia; NADH, nicotinamide adenine dinucleotide; NBL, neuroblastic layer; OPL, outer plexiform layer; PR, photoreceptor; PRP, photoreceptor precursors; RGC, retinal ganglion cell; SAC, starburst amacrine cells.
RO Differentiation Methods
Methodologies for optimizing RO quality published in recent years can be categorized into 3 types: (1) adjustment of the supplemental reagents in culture media; (2) testing different EB formation approaches; and (3) investigation of alternative 3D suspension culture approaches beyond conventional tissue plate culture.
For the first category, Zerti et al56 found that the addition of specific reagents such as retinoic acid and triiodothyronine (T3) at selected differentiation duration stages could provide high-quality ROs that contained specific PR subtypes. Protocols to accelerate development of rod PRs by supplementing with 9-cis-retinal are reported.57–59 Pan et al employed COCO (a multifunctional antagonist of the Wnt, transforming growth factor-β, and bone morphogenetic protein pathways) to promote RO differentiation. They found increased number of PR precursors in early-stage ROs (main difference observed was cone-rod homeobox transcription factor (CRX+) cells showing on day 45). While the difference was not significant in later stages, they found COCO treatment reduced neural retina leucine zipper (NRL), rhodopsin (RHO), and green opsin (OPN1MW) expression and increased blue opsin expression (OPN1SW), which indicated that an enhanced fate of cones and decreased fate of rods were apparent in late stages.60
The latter 2 categories will be expanded in the following paragraphs according to the chronological order of RO differentiation.
In most differentiation protocols, the first step in RO production is to initiate EBs, which are 3-dimensional (3D) aggregates of PSCs to develop into neurospheres. Different EB formation methods were tested by Mellough et al61 where they studied 3 approaches: (1) mechanical cutting; (2) enzymatic dissociation of stem cell colonies into small pieces; and (3) dissociation into single cells followed by force reaggregation.62,63 Their results showed that mechanically cutting EBs from 2-dimensional (2D) culture under static conditions (vs shaker condition) produced most consistently laminated, mature, and functional ROs.61
Once EBs are formed, they are further differentiated in 2D matrix culture using growth factor–reduced Matrigel or other hydrogels. When the eye field structures are formed, the ROs are excised and transferred to 3D suspension culture.64,65 Afterwards, the 3D culture continues for months while ROs follow typical gestational development and eventually develop mature PR layers on their outermost surface.
To improve 2D differentiation, Dorgau and colleagues placed EBs onto an extracellular matrix that contained decellularized peptides from neural retina and RPE. They observed an improvement in RPE differentiation, ROs synaptogenesis, and light responsiveness.66 Compared to conventional extracellular matrix, decellularization provided necessary biochemical and biophysical components, as well as the biological scaffold for cell engraftment and differentiation.66
However, the 2D differentiation on extracellular matrix is not necessary for all protocols. Hunt and colleagues skipped the 2D differentiation and encapsulated EBs into different hydrogels including RDG-alginate, hyaluronic acid (HA), and HA/gelatin hydrogels. They found that up to day 45 in culture, the 0.5% of RGD-alginate enhanced the derivation of RPE and increased the yield of EBs compared to suspension cultured control group.67 However, to confirm that hydrogel-assisted 3D differentiation is better than suspension culture, longer differentiation duration is needed. In another example, Kim and colleagues mixed human embryonic stem cell (hESC) aggregates in ice-cold Matrigel and dispersed in medium supplemented with N2 and B27 on day 0 for floating culture. They transferred the single-lumen cysts to 24-well plates for attachment culture on day 4 to 5, and enzymatically lift by dispase on day 15 with 3D RO culture immediately initiated. Using this protocol, they successfully developed cone-rich ROs, which are of particular interests in transplantation studies.68
Some studies for RO production focused on improving the long-term 3D differentiation of ROs. Besides conventional 3D suspension culture in tissue culture plates, several research teams designed and fabricated autonomous long-term culture devices to improve ROs long-term culture quality and to reduce variability. Ovando-Roche et al69 applied a stirred-tank bioreactor to culture ROs and improved the laminar stratification and increased the yield of PR cells. Similarly, DiStefano et al70 used a rotating wall vessel for 3D RO culture and as a result accelerated differentiation and improved overall quality. Microfluidic and/or millifluidic bioreactors can minimize shear stress on developing RO while allowing targeted long-term imaging and reduce the total culture medium consumption.71–73 Xue et al73 developed a shear stress-free micromillifluidic bioreactor that produced ROs with comparable quality as those in static culture, while allowing real time functional imaging with the all-transparent design. Studies comparing rotating wall vessel and low-shear systems will address whether shear stresses damage the outer segment structures in mature organoids.
RO Validation and Characterization
The heterogeneity and variability of RO production necessitates validation of RO tissues prior to their use in downstream applications. Common methods for organoid validation include immunohistochemistry (IHC), flow cytometry, single-cell transcriptomics,74 and single-cell RNA sequencing (scRNA seq).75–77 Transmission electron microscopy enables visualization of micro/nanostructures such as outer segments, inner segments with mitochondria, connecting cilia and disc structures. However, the detrimental nature of these commonly used methods is the mortal requirement to either fix the tissue or to dissociate the tissue into single cells. Destructive characterization halts organoid use in downstream applications including transplantation. Therefore, noninvasive and nondestructive characterization methods are gaining popularity in organoid research.
Several noninvasive characterization methods are reviewed in this article, including optical coherence tomography (OCT), confocal imaging of genetically engineered reporters, FLIM, and hyperspectral imaging.
OCT was proposed for assessing 3D-cultured ROs by Browne et al in 2017.78 Further, OCT was implemented to visualize surface topography and internal anatomy by Capowski et al.54 Scholler et al79 developed a dynamic full-field OCT system to achieve label-free visualization of organelle motility with submicrometer spatial resolution and millisecond temporal resolution. OCT performs well in cross-sectional and surface imaging. However, OCT cannot be used to identify cell types within ROs.
To visualize the lamination and cellular composition in ROs at cellular resolution, confocal laser scanning microscopy shows better performance. PSC reporter lines have been widely used for identifying cell lineages, subtypes, and ROs’ developmental stages in live culture. Using clustered regularly interspaced short palindromic repeats/CRISPR-associated protein 9 (CRISPR/Cas-9) genome editing, Philips et al created the first human rod reporter line, which tagged green fluorescent protein (GFP) to the NRL gene of the WA09 hESC line.80 Using zinc finger nuclease technology, Collin et al generated a cone-rod homeobox (CRX)-reporter hESC line,81 which could be applied to isolate PR precursors81 and for use in transplantation.34 Vergara et al82 developed a 3D automated reporter quantification (3D-ARQ) system to effectively monitor the ROs’ developmental process, fluorescence intensity changes, reproducibility evaluation, and realized high throughput screening. Compared to reporter lines that required genetically engineered fluorescence label, 2-photon imaging that integrates FLIM and hyperspectral imaging on ROs can realize label-free imaging by exciting intrinsic fluorophores, offering the advantage of visualizing the metabolic signatures and molecular distribution within ROs.55,78 Further investigation is required to identify metabolic signatures with specific cell types.
Another important aspect is to evaluate the functionalities of ROs in advanced stages for light sensitivity and synapses generation. Common methods for RO electrophysiological functional analysis include patch-clamp,64,83 fluorescent calcium imaging,84–86 2-photon microscopy87 and microelectrode arrays (MEAs),88 reviewed by Afanasyeva et al.89 In more recent studies, Li et al90 systematically characterized the electrophysiology of ROs at different stages (D90, D150, and D200) using patch-clamp recording and found that PR cells in ROs after D200 showed similar characteristic currents as those in human retina. Cowan et al91 compared ROs with human retina in transcriptomes, and they further characterized the functionality of ROs by measuring the light responsiveness, imaging synaptic layers, and functional synapses. Furthermore, Bharathan et al9 applied human ROs as a model system to study the synaptogenesis in human retina, identified stages of human outer plexiform layer development, and successfully recapitulated key aspects of synaptogenesis between PRs and BCs.
ROS FOR TRANSPLANTATION
RO transplantation is becoming a promising therapeutic approach for RD diseases. The current transplantation strategies for treating degenerative diseases can be categorized into 4 types: selected types of cells, transplanting RO sheets, RPE and cograft of RPE and RO pieces. In this section, we summarize recent research of each method and discussed their pros and cons (Table 2, Fig. 2).
TABLE 2.
Advantages and Disadvantages of 3 Tissue Sources for Transplantation
| Tissue Type | Advantages | Disadvantages | References |
|---|---|---|---|
| Single cells | Larger contact area between host and graft tissue likely improved chance of integration | Lacks integrity and mechanical stability | 34–39,42,92–94 |
| Reduced survival rate prevents further development within the host tissue | |||
| Targeted treatment for loss of certain cell types and avoiding inappropriate synapse formation | Difficult to control the orientation of photoreceptor cells in the graft | ||
| Cytoplasmic transfer to host cells if host ONL is present resulting in rescue of host photoreceptors but not replacement | |||
| Easy control of purity and quality of cells to avoid tumorigenesis | |||
| RO sheet | Complete layered structure of retina easier for integration into host retina | Highly trained surgical skills required | 16,21,40,41,43 |
| Intact interneural connectivity improved survival rate | Uniformity and retinal cell purity within the ROs sheet critically needed to avoid tumorigenesis or fibrosis | ||
| Higher mechanical support and better microenvironment for the retinal cells to differentiate and function | Potentially excessive and inappropriate bipolar to bipolar cell synapses between graft and host | ||
| Rosette formation | |||
| RPE-RO Cograft | Physical cell-cell interactions between RPE and photoreceptor layer already formed at time of transplantation | More complex tissue culture and preparation process before transplantation | 45 |
| Reduced apoptosis, gliosis, and increased glutamate synthesis | Extensive manual labor required to transplant the cograft tissue | ||
| Improved developmental environment in the host retina | RO transplants still forming rosettes; optimal embedding matrix yet to be determined | ||
| Rosette formation |
ONL indicates outer nuclear layer; RO, retinal organoid; RPE, retinal pigment epithelium.
FIGURE 2.
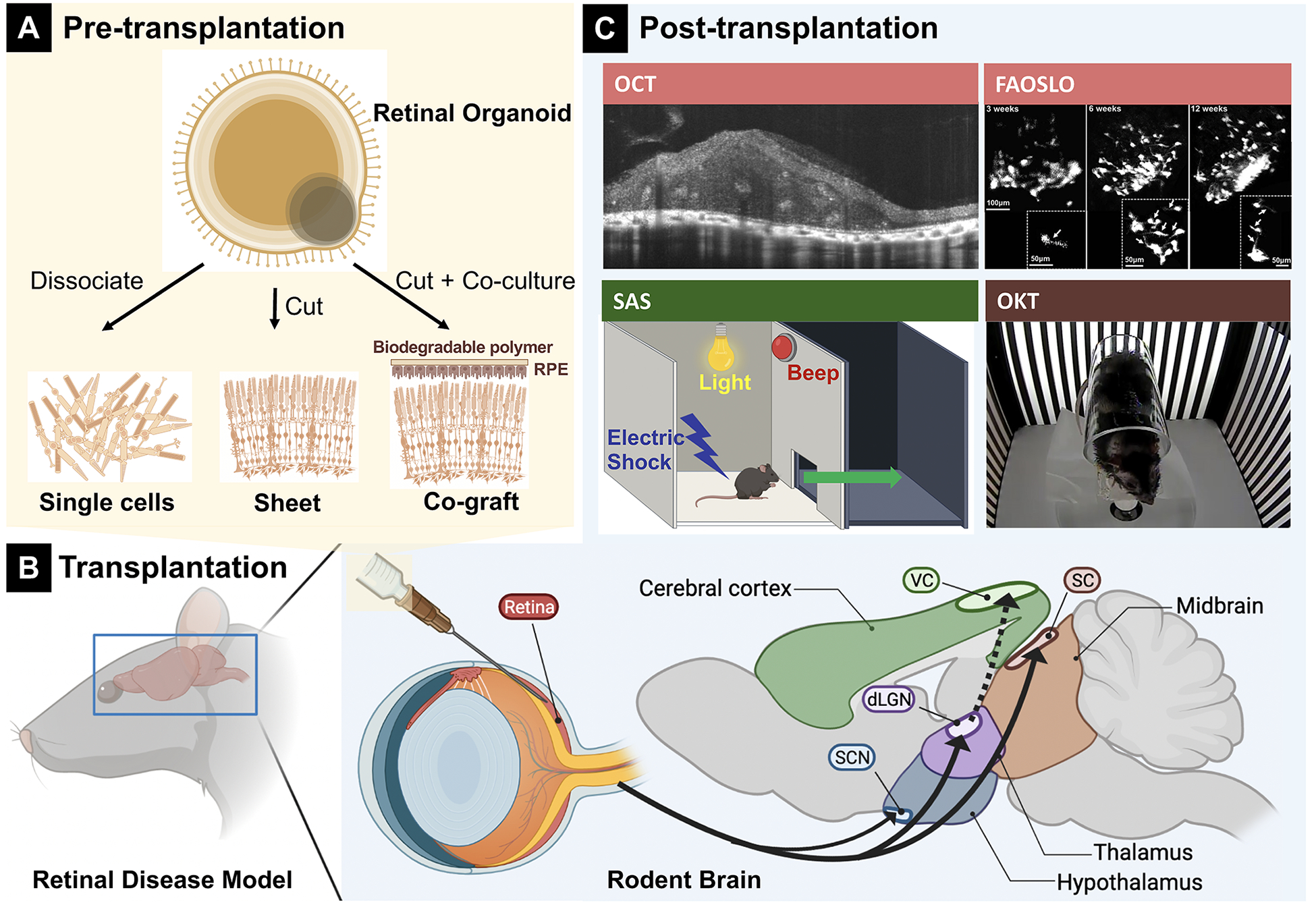
Overview of different transplant types from retinal organoids (ROs) and post-transplantation testing. A, Three different transplants obtained from RO. B, Schematic diagram of transplantation procedure. C, post-transplantation analysis that targets on different regions in the brain. Fluorescence adaptive optics scanning light ophthalmoscopy image was taken from Aboualizadeh et al95 (Fig. 4C); shuttle-avoidance system (SAS) schematic diagram was modified from Mandai et al41 (Fig. 3A). Optical coherence tomography and fluorescence adaptive optics scanning light ophthalmoscopy (FAOSLO) targeted on retina, shuttle-avoidance system (SAS) targeted on visual cortex (VC), and optokinetic test (OKT) targeted on superior colliculus (SC) (color-coded).
Transplant Selected Cells
Single-cell transplantation offers advantages including: (1) targeted treatment for loss of certain cell types; (2) controllable purity and quality of the isolated cells; and (3) a potentially larger contact area between host and graft cells because the cells can spread over a larger area in the subretinal space.
So far, neural and retinal progenitors,41,96 immature PR precursors,35,92,97–100 and fully mature101 PRs have been used for transplantation. Among them, immature but no longer dividing rod and cone precursor cells that can continue differentiation in the host retina are considered as the most feasible donor cell types.36,102 For cell selection and purification, fluorescence-activated cell sorting was used. Lakowski et al36 established a cell surface biomarker combination for PR precursor enrichment from hPSC-differentiated ROs and fetal retinae (CD73+/CD29−/SSEA1−). This combination of markers was also capable of eliminating mitotically active cells to avoid possible tumor development.36 Collin et al34,76 developed a hESC line that produced transplantable cone dominant PR precursors. Recently, Zerti and colleagues transplanted CRX-GFP labeled hESC-derived PR precursors [dissociated from 90 days of differentiation (DD) ROs] into end-stage degeneration Pde6brd1 mouse models. Light sensitivity restoration and up to 1.5% of cell integration into the putative host outer nuclear layer were observed.35 Ribeiro et al39 transplanted purified cone precursors from hPSCs to immunodeficient rd1 mice and demonstrated vision improvements (Fig. 3A).
FIGURE 3.
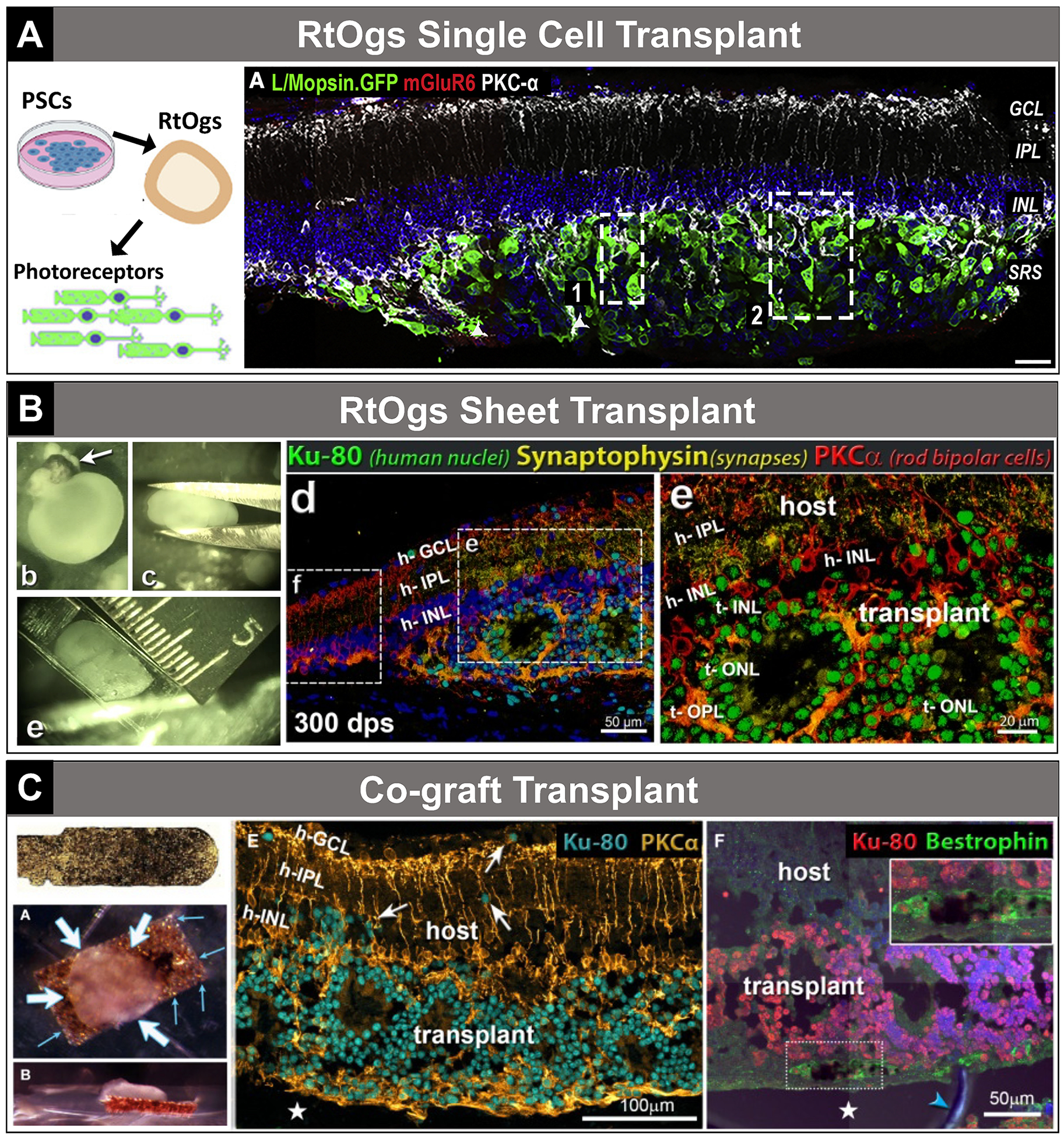
Transplantation examples—single cell, sheet, cograft. A, Single-cell transplantation. Taken from Ribeiro et al39 (graphical abstract; Fig. 3A). B, Sheet transplantation. Taken from McLelland et al16 (Supplemental Fig. 1; Figs. D–E; republished with permission of Investigative Ophthalmology & Visual Sciences, from McLelland et al, Transplanted hESC-derived retina organoid sheets differentiate, integrate, and improve visual function in retinal degenerate rats. Invest Ophthalmol Vis Sci. 2018;59:2586–2603; doi:10.1167/iovs.17-23646; permission conveyed through Copyright Clearance Center, Inc). C, Cograft transplantation. Taken from Thomas et al45 (Fig. 1I; Figs. 3A–B; Figs. 7E–F). h-GCL indicates host ganglion cell layer; h-IPL, host inner plexiform layer; h-INL, host inner nuclear layer; PSCs, pluripotent stem cells; RtOgs, retinal organoids; t-INL, transplant inner nuclear layer; t-OPL, transplant outer plexiform layer; t-ONL, transplant outer nuclear layer.
Retinal progenitor cells are also a common source for transplantation. Chao and colleagues injected 1 million retinal progenitor cells into a nonhuman primate, Saimiri sciureus, and observed extended axonal projections into the host retina and optic nerve without the need for immunosuppression for 3 months. No obvious PR integration was detected.93
However, compared to sheet transplantation, single-cell transplants lack integrity and mechanical stability, which reduced the donor cell survival and further development within the host tissue. Cells injected as a bolus usually aggregated in the subretinal space but only a subpopulation would migrate into the host retina and there were issues with long-term survival.36,103–105 Further, the orientation of PR cells was also hard to control.
Transplant RO Sheets
Compared to single-cell transplantation, the advantages of transplanting ROs sheets are that: (1) the RO sheet preserves the complete layered structure of retina, which is easier for integration into host retina; (2) the survival rate of transplanted tissue is higher due to the intact interneural connectivity; and (3) the tissue piece offers higher mechanical support and provides a better microenvironment for the retinal cells to differentiate and function.
Mandai et al41 transplanted mouse-induced pluripotent stem cell (iPSC)–derived RO pieces (DD11–17) into end-stage rd1 mice model and observed light-responsive behaviors. Iraha and colleagues transplanted hESC/iPSC-derived RO sheets (DD64–66) into immunodeficient IRD mouse models with the graft tissue showing long-term survival and maturation (DD200–220). Host-graft synapse formation was observed and light responses were detected from retinal wholemounts.40 Tu and colleagues transplanted human iPSC-retinas (DD58–78) into rhodopsin mutant SD-Foxn1 Tg (S334ter)3LavRrrc nude rats and performed IHC and electrophysiology recording with a MEA after sacrificing the animal (5–10.5 months). Light responses were detected at the grafted area in 4 of 7 transplanted rat retinas.43 In the same study they also transplanted ROs (DD62 and DD53) into a cynomolgus monkey and a rhesus monkey. The visually guided saccades test revealed a mild recovery of light perception after 1.5 years of transplantation in rhesus monkey.43 In different studies, RO sheets (DD 30–65 and 70) were transplanted into immunodeficient rhodopsin mutant SD-Foxn1 Tg(S334ter)3LavRrrc nude rats16 (Fig. 3B) and immunodeficient Royal College of Surgeons (RCS) rats.19 Improvement of visual responses was demonstrated by optokinetic tests (OKTs) and recording from the superior colliculus (SC) in both IRD models. Interestingly, RO transplants improved visual responses in RCS rats in spite of the absence of functional RPE cells. PR development and synaptic connectivity were identified with IHC.
However, the disadvantage of this method includes the requirement of a highly trained operational skillset and a larger retinal incision compared to transplantation of dissociated cells since the RO sheet needs to be placed flat into the subretinal space in the correct orientation. Also, uniformity and retinal cell purity of the RO sheets are critical to avoid tumorigenesis or fibrosis resulting from contamination with undifferentiated or nonretinal cells. In addition, although the transplants form retinal layers, PRs frequently form spherical structures called rosettes, with PR outer segments in the center (mostly disconnected from RPE) (Fig. 3B).16,19,41,43,46 This may be related to possible rosette formation in organoids before transplantation, and trauma to organoid pieces during transplantation.
Transplant Cograft of RPE and RO Sheet
Besides RO sheets, PSCs-derived RPE is also a promising tissue source for transplantation and vision restoration. RPE plays critical roles in vision by performing vital functions such as: (1) transporting nutrients, ions, and water to the PRs; (2) supplementing 11-cis-retinal in the visual cycle by isomerization of all-trans-retinal; (3) protecting against photooxidation and light absorption; (4) removing shed PR outer segment membranes with phagocytosis; and (5) secreting essential extracellular molecules (eg, laminin, collagen, and HA) to maintain retinal integrity, functionality, and PR viability.106,107 Several studies used hESC/iPSC-derived RPE sheets (or “patches”) for retinal degenerative therapy in animal models108–111 and clinical trials112–115 (reviews116,117). These studies reported maintenance or improvement of visual function and delated RD. However, this approach has not been successful in stopping disease progression.
Considering the limited performance of mere RPE or RO transplantation, some research groups proposed that combination of these 2 tissues might provide enhanced effects. Early studies found that in vitro coculture of rat neural retina and RPE cells promoted PR integration and axonal growth by increasing the synthesis of rhodopsin.118 Further, reduced apoptosis, gliosis, and increased glutamate synthesis were observed compared to retinal culture alone.119 However, since the culturing conditions are different for RPE and RO, the cocultures of these 2 tissues were usually short-term in the range of a few days.118,119 As a result, it was challenging to coculture RPE and RO to the stage ready for transplantation.
A more promising option was to culture RPE and RO separately until ready for transplantation, and then put them together with bioadhesives as cograft and transplant into the host.45 Previous research demonstrated the feasibility of transplanting grafted sheets of fetal retinal progenitor cells with its RPE into animal models44,120 and human121 to address the challenges of the lack of physical cell-cell interactions and undesirable host environment for development.122 However, the use of fetal retina was ethically controversial, and access to the tissue has been very limited. Recently, Thomas et al combined ROs and polarized RPE sheets using bioadhesives (gelatin, growth factor–reduced Matrigel, and medium viscosity alginate). Long-term survival (up to 6.5 months) of the cograft in immunodeficient RCS rats’ subretinal space and improvement in visual function were observed (Fig. 3C).45 This study has proven the feasibility of cograft transplantation for severely degenerated retina.45 Challenges remain due to the complexity of the donor tissue preparation and rosette formation in the RO transplants.
Transplant With Biomaterial Scaffolds
Researchers also turned to engineering approaches to realize outer retinal reconstruction. Specifically, biomaterial scaffolds constructed by synthetic polymers, silk, alginate, HA, and extracellular matrix were used as reviewed by Hunt et al.123 Recently, Lee et al124 designed and fabricated an ultrathin (30 μm) biodegradable scaffold patterned with micrometer-level precision, which was called “poly(glycerol sebacate) ice cube tray.” Compared to their previous “wineglass” design125 that only achieved single-layer PR seeding, the ice cube tray design supported multiple layers of hPSC-PRs with more than 300k cells in a single 5-mm-diameter scaffold similar to the area of a human macula. This design presented slower degradation in vitro (up to 30 days).124 However, more investigations are needed to scale up manufacturing, delivery strategies to animal models, and in vivo functional tests.
POST-TRANSPLANTATION ANALYSIS
Finally, to evaluate the effectiveness of transplantation, different post-transplantation tests have been performed with animal models. The host used in these studies had intact neural pathway from the optical nerve to the visual cortex, despite the loss of PRs (Fig. 2C). Therefore, the transplantation performance was a direct result of the integration, differentiation, and function of the grafted tissue within the host retina. Thus, post-transplantation tests normally focused on examining the following performance: (1) light and contrast sensitivities and visual acuity of subjects with behavioral tests; (2) connectivity of the visual pathway between retina and visual cortex with retinal and brain electrophysiology recordings; and (3) integration, differentiation, and synaptogenesis between graft and host tissue with OCT, histology, and analysis of retinal and synaptic markers in correlation to functional results. Common post-transplantation tests are categorized and summarized in Table 3 and shown schematically in Figure 2.
TABLE 3.
Summary of Post-Transplantation Tests
| Categories | Methods | Examined Features | In Vivo | References |
|---|---|---|---|---|
| Behavior tests | SAS | Light-dark discrimination | Yes | 21,35,41 |
| Light threshold (shock) | ||||
| Light-avoidance test | Light-dark discrimination | Yes | 39 | |
| OKT | Visual acuity | Yes | 16,19,45 | |
| Contrast sensitivity | ||||
| Electrophysiology | VGS | Eye movement: latency, amplitude, and peak velocity | Yes | 43 |
| ERG | Electrical activity of retina in response to light stimulation | Yes | 19 | |
| MEA-based mERG (micro-ERG) | Local electrical potential changes evoked by light | No | 41,40,43,37 | |
| SC recording | Spike counts after light stimulus (different light intensity) | Yes | 16,19,45 | |
| Correlate visual responses in SC to certain retinal areas | ||||
| Graft differentiation, integration and synaptogenesis | SDOCT | Location and overview of transplant | Yes | 16,19,45 |
| Graft thickness | ||||
| FAOSLO | Survival, migration, and neurite outgrowth of fluorescent labeled transplant cells | Yes | 38 | |
| IHC | Labels specific proteins in tissue to reveal certain cell types, synapses and the overall structure of transplant and host | No | Almost all transplantation research projects applied this technique |
ERG indicates electroretinogram; FAOSLO, fluorescence adaptive optics scanning light ophthalmoscopy; IHC, Immunohistochemistry; MEA, microelectrode array; OKT, optokinetic test; SAS, shuttle-avoidance system; SC, superior colliculus; SDOCT, spectral-domain optical coherence tomography; VGS, visually guided saccades.
Behavioral Tests
Behavioral tests are advantageous because they are noninvasive and can be repeated at any time points after transplantation. In particular, OKT is one of the most popular behavioral tests. Rodents show slow horizontal head and body movements when a virtual-reality visual field (black and white stripes of varying density) is rotated around them. The stripe density eliciting a response determines the spatial threshold. For each eye, only a field rotation in the temporal-to-nasal direction evokes the tracking response, making it possible to distinguish between a transplanted and a nonsurgery eye in the same animal. Lesions of the visual cortex had no effect on OKT, suggesting that OKT was driven by subcortical and contralateral pathways.126 Several studies have shown improvements in optokinetic responses after RO sheet transplantation.16,19,45
Multiple behavioral tests for visual functions had been used in different studies. For example, Mandai and colleagues adapted a shuttle-avoidance system to test for light sensitivity and response in animals after transplantation. A warning light was presented to the mouse before an electric shock was administered to train the mouse to move into another chamber through a small opening as soon as it saw the warning light (Fig. 2C).41 Similarly, a light avoidance system used bright light as a cue to test the animal’s light response capability.21,35 Another light avoidance test measured the animal’s preference to evade light without using electric shocks.37,39 Tu and colleagues applied a visually guided saccades test on rhesus monkeys, in which the animal facing a color LCD monitor was trained to gaze at a central fixation spot followed by a random presentation of a target spot somewhere else in the monitor. The resulting saccades landing within a 50 × 50 pixels square containing the visual target were judged as correct responses.43
Electrophysiological Tests
Global or full-field electroretinogram (ERG) represents mass electrical response of the retina to photic stimulation. The basic approach of global ERG is to stimulate the eye with a bright light source such as a flash produced by LEDs or a strobe lamp while monitoring electrical activities in the eye. The flashes of light should elicit a biphasic waveform (the a- and b-waves) recordable from the cornea. Full-field ERGs are in general not sensitive enough to detect visual improvements once RD has progressed too far. For example, Lin et al19 could only detect ERG response improvements at 2 months post-transplantation of RO sheets to immunodeficient RCS rats, but rodent models with more severe RD had never shown recordable ERGs.127
To circumvent this shortcoming, MEA-based microelectroretinography (mERG) technique was used to ascertain the effectiveness of transplantation.37,40,41,43 Compared to full-field ERGs, which only detected changes in mass retinal field potentials, local and multilocal ERGs offer higher signal-to-noise ratio and thus are more suitable for tracking degenerative processes or functional recovery. Fujii et al128 had tested an MEA-based mERG system on rd1 mice with progressive PR degeneration, and were able to record light-evoked mERGs with consistent RGC spike responses. Garita-Hernandez transplanted optogenetically transformed iPSC PR precursors to Rho−/− mice. They were either derived from neonatal mice expressing Natronomonas pharaonis halorhodopsin (NpHR) coupled to a rod promoter; or derived from iPSC-ROs expressing hyperpolarizing chloride pump Jaws, a redshifted cruxhalorhodopsin couple to a cone promoter.37 Function of the transplanted PRs was demonstrated by behavioral tests (light-dark box), MEA recordings, and patch-clamp recording from GFP+ donor PRs (in the absence of functional outer segments) that were specific for the action spectrum of these bacterial opsins (580 nm).37
Another very sensitive technique is electrophysiological recording from the SC16,19,45 in the midbrain, which plays a central role in integrating multiple sensory inputs to motor behaviors such as eye and head movements.129 In this test, a microelectrode is directly placed on the surface of SC; under full-field retinal stimulation at specific light intensities, visual thresholds, and visual responses (spike counts) of specific retinotopic areas of the SC were recorded.
In Vivo Imaging Tools to Determine Transplant Survival and Differentiation
Spectral-domain OCT (SDOCT) is widely used to examine the transplanted regions.16,19 SDOCT offers high axial resolution to show different layers of the retina and visualize the transplanted region thickness. However, SDOCT cannot provide specific morphological information, and the resolution is not high enough to visualize single cells.
Aboualizadeh and colleagues studied the dynamic nature of transplanted cells at cellular resolution utilizing near infrared fluorescence adaptive optics scanning light ophthalmoscopy. They tracked the survival, migration, and neurite outgrowth of individual fluorescent PR precursors in the living monkey eyes in the long term (Fig. 2C).95 Similarly, Liu and colleagues applied confocal scanning laser ophthalmoscopy to evaluate in vivo biomarkers of transplanted PR cells qualitatively and quantitatively. They were able to observe migration of the transplanted tissue as well.38 While these 2 techniques demonstrated high resolution and dynamic imaging, it relied on genetically engineered reporter cell lines (CRX+/tdTomato and Rho+/GFP) to emit fluorescent light, which is not applicable for future clinical use in human subjects.
Analysis of Transplant Differentiation and Connectivity
RO sheets and retinal progenitor cells derived from ROs were usually transplanted while they were in an immature state to facilitate integration and further development in the host. IHC for specific retinal markers was commonly used to identify the differentiation within the transplant over time (examples please find references16,40,41).
A critical indicator of transplanted tissue viability was the formation of synapses between neurons or within the PR ribbon synapse. IHC was considered a robust and high throughput analytical tool to visualize synaptogenesis. This included combining donor label with staining for synaptic markers.16,19,40 Akiba and colleagues has proposed an automatic synapse quantification method that could not only quantify the number of synapses, but also estimate the probability of “synapse-ness” from IHC images. This method was named as “Qualitative and Quantitative Analysis using Bayes Theorem Optimized for Synapse Evaluation (QUANTOS).”18 Because the transplanted RO sheet also contained BCs, which might cause inappropriate bipolar to BC synapses between graft and host, Matsuyama et al21 generated mouse RO retinal sheets with reduced numbers of retinal BCs and demonstrated improved visual recovery and better integration after retinal transplantation. Similar results were achieved with genetically modified human ROs.130 He et al131 transplanted retinal progenitor cells derived from mouse C-Kit-mXCherry and Rosa-lsl-CGaMP5 mESC-derived ROs to the subretinal space of 21-day old RCS rats. Retinal progenitor cells expressing CaMP5 were enriched by cell sorting for C-Kit. Transplanted cells were observed to have migrated into the degenerating retina. The development of functional synapses was shown by IHC for presynaptic and postsynaptic markers and with 2-photon calcium recording of donor cells.131
Cytoplasmic Material Transfer Between Transplant and Host
Several studies in recent years have demonstrated that transplanted dissociated PR precursors exchanged cytoplasmic material (proteins and RNA) with remaining host PRs and thus might result in rescue of host PR function103–105,132,133 (review134,135). This transfer can be bidirectional, from donor to host, and vice versa.103,132,133 In addition, transfer of mitochondria between mesenchymal stem cells and different ocular cell lines has been demonstrated in vitro.136 This may explain the beneficial effect of transplants on host PRs. It was thought that material exchange required PR-to-PR communication, which could not occur in severe RD when the PR layer is completely gone.134,137 Cytoplasmic transfer between PRs also occurs during normal retinal development.138 However, transfer can also be seen from PRs to the Muller cells and ACs in the inner nuclear layer when grafting cells to rats with normal outer nuclear layer.103 Thus, the identity of donor cells in the host retina needs to be clearly demonstrated by nuclear labels (eg, male donor into female host,37,103,105,132 or a human nuclear marker for hPSC-derived transplants in rodent hosts16,19,43).
CONCLUSIONS
In conclusion, methods to derive RO from PSCs have significantly improved and become more efficient in recent years. Meanwhile, more novel technologies are applied to characterize and validate RO quality. However, there is still room for differentiation protocol optimization to achieve better RO reproducibility. In order to screen high quality ROs for downstream applications, approaches such as noninvasive and label-free imaging, and electrophysiological functional testing are promising and worth more investigation. Lastly, transplanted RO-derived tissues have allowed improvements in visual function in several RD models, and this is promising for clinical applications in the future.
Acknowledgments
Supported-by CIRM TRAN1-10995 (M.J.S.); NIH R01 EY031834 (M.J.S.); RPB unrestricted grant to UCI Department of Ophthalmology (M.J.S. and A.W.B.); ICTS KL2 TR001416 (A.W.B.); Koehler Foundation Fund #6630 (M.J.S. and A.B.).
Footnotes
The authors have no conflicts of interest to declare.
REFERENCES
- 1.Kallman A, Capowski EE, Wang J, et al. Investigating cone photoreceptor development using patient-derived NRL null retinal organoids. Commun biol. 2020;3:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chirco KR, Chew S, Moore AT, et al. Allele-specific gene editing to rescue dominant CRX-associated LCA7 phenotypes in a retinal organoid model. Stem Cell Rep. 2021;16:2690–2702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huang KC, Wang ML, Chen SJ, et al. Morphological and molecular defects in human three-dimensional retinal organoid model of X-linked juvenile retinoschisis. Stem Cell Rep. 2019;13:906–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao ML, Lei XL, Han F, et al. Patient-specific retinal organoids recapitulate disease features of late-onset retinitis pigmentosa. Front Cell Dev Biol. 2020;8:128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang X, Wang W, Jin ZB. Retinal organoids as models for development and diseases. Cell Regen. 2021;10:33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gamm DM, Clark E, Capowski EE, et al. The role of FGF9 in the production of neural retina and RPE in a pluripotent stem cell model of early human retinal development. Am J Ophthalmol. 2019;206:113–131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Dorgau B, Felemban M, Sharpe A, et al. Laminin γ3 plays an important role in retinal lamination, photoreceptor organisation and ganglion cell differentiation. Cell Death Dis. 2018;9:615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eldred KC, Hadyniak SE, Hussey KA, et al. Thyroid hormone signaling specifies cone subtypes in human retinal organoids. Science. 2018;362:aau6348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bharathan SP, Ferrario A, Stepanian K, et al. Characterization and staging of outer plexiform layer development in human retina and retinal organoids. Development. 2021;148:dev199551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Aasen DM, Vergara MN. New drug discovery paradigms for retinal diseases: a focus on retinal organoids. J Ocul Pharmacol Ther. 2020;36:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kruczek K, Qu Z, Gentry J, et al. Gene therapy of dominant CRX-leber congenital amaurosis using patient stem cell-derived retinal organoids. Stem Cell Rep. 2021;16:252–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang X, Zhang D, Thompson JA, et al. Gene correction of the CLN3c.175G > A variant in patient-derived induced pluripotent stem cells prevents pathological changes in retinal organoids. Mol Genet Genomic Med. 2021;9:e1601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Garita-Hernandez M, Chaffiol A, Guibbal L, et al. Control of microbial opsin expression in stem cell derived cones for improved outcomes in cell therapy. Front Cell Neurosci. 2021;15:648210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Völkner M, Pavlou M, Büning H, et al. Optimized adeno-associated virus vectors for efficient transduction of human retinal organoids. Hum Gene Ther. 2021;32:694–706. [DOI] [PubMed] [Google Scholar]
- 15.Santos-Ferreira TF, Borsch O, Ader M. Rebuilding the missing part-a review on photoreceptor transplantation. Front Syst Neurosci. 2016;10:105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McLelland BT, Lin B, Mathur A, et al. Transplanted hESC-derived retina organoid sheets differentiate, integrate, and improve visual function in retinal degenerate rats. Invest Ophthalmol Vis Sci. 2018;59:2586–2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gasparini SJ, Llonch S, Borsch O, et al. Transplantation of photoreceptors into the degenerative retina: current state and future perspectives. Prog Retin Eye Res. 2019;69:1–37. [DOI] [PubMed] [Google Scholar]
- 18.Akiba R, Matsuyama T, Tu H-Y, et al. Quantitative and qualitative evaluation of photoreceptor synapses in developing, degenerating and regenerating retinas. Front Cell Neurosci. 2019;13:16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lin B, McLelland BT, Aramant RB, et al. Retina organoid transplants develop photoreceptors and improve visual function in RCS rats with RPE dysfunction. Invest Ophthalmol Vis Sci. 2020;61:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh RK, Binette F, Seiler M, et al. Pluripotent stem cell-based organoid technologies for developing next-generation vision restoration therapies of blindness. J Ocul Pharmacol Ther. 2021;37:147–156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Matsuyama T, Tu HY, Sun J, et al. Genetically engineered stem cell-derived retinal grafts for improved retinal reconstruction after transplantation. iScience. 2021;24:102866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gehlbach P, Li T, Hatef E. Statins for age-related macular degeneration. Cochrane Database Syst Rev. 2016;2016:Cd006927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Lim LS, Mitchell P, Seddon JM, et al. Age-related macular degeneration. Lancet. 2012;379:1728–1738. [DOI] [PubMed] [Google Scholar]
- 24.Chichagova V, Hallam D, Collin J, et al. Cellular regeneration strategies for macular degeneration: past, present and future. Eye (Lond). 2018;32:946–971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125:151–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–1809. [DOI] [PubMed] [Google Scholar]
- 27.Botto C, Rucli M, Tekinsoy MD, et al. Early and late stage gene therapy interventions for inherited retinal degenerations. Prog Retin Eye Res. 2021;86:100975. [DOI] [PubMed] [Google Scholar]
- 28.Wood EH, Korot E, Storey PP, et al. The retina revolution: signaling pathway therapies, genetic therapies, mitochondrial therapies, artificial intelligence. Curr Opin Ophthalmol. 2020;31:207–214. [DOI] [PubMed] [Google Scholar]
- 29.De Silva SR, Barnard AR, Hughes S, et al. Long-term restoration of visual function in end-stage retinal degeneration using subretinal human melanopsin gene therapy. Proc Natl Acad Sci U S A. 2017;114:11211–11216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Campochiaro PA, Iftikhar M, Hafiz G, et al. Oral N-acetylcysteine improves cone function in retinitis pigmentosa patients in phase I trial. J Clin Invest. 2020;130:1527–1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Komeima K, Rogers BS, Lu L, Campochiaro PA. Antioxidants reduce cone cell death in a model of retinitis pigmentosa. Proc Natl Acad Sci U S A. 2006;103:11300–11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Komeima K, Rogers BS, Campochiaro PA. Antioxidants slow photoreceptor cell death in mouse models of retinitis pigmentosa. J Cell Physiol. 2007;213:809–815. [DOI] [PubMed] [Google Scholar]
- 33.Farrar GJ, Millington-Ward S, Chadderton N, et al. Gene therapies for inherited retinal disorders. Vis Neurosci. 2014;31:289–307. [DOI] [PubMed] [Google Scholar]
- 34.Collin J, Zerti D, Queen R, et al. CRX expression in pluripotent stem cell-derived photoreceptors marks a transplantable subpopulation of early cones. Stem Cells. 2019;37:609–622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Zerti D, Hilgen G, Dorgau B, et al. Transplanted pluripotent stem cell-derived photoreceptor precursors elicit conventional and unusual light responses in mice with advanced retinal degeneration. Stem Cells. 2021;39:882–896. [DOI] [PubMed] [Google Scholar]
- 36.Lakowski J, Welby E, Budinger D, et al. Isolation of human photoreceptor precursors via a cell surface marker panel from stem cell-derived retinal organoids and fetal retinae. Stem Cells. 2018;36:709–722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Garita-Hernandez M, Lampič M, Chaffiol A, et al. Restoration of visual function by transplantation of optogenetically engineered photoreceptors. Nat Commun. 2019;10:4524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Liu YV, Sodhi SK, Xue G, et al. Quantifiable in vivo imaging biomarkers of retinal regeneration by photoreceptor cell transplantation. Transl Vis Sci Technol. 2020;9:5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Ribeiro J, Procyk CA, West EL, et al. Restoration of visual function in advanced disease after transplantation of purified human pluripotent stem cell-derived cone photoreceptors. Cell Rep. 2021;35:109022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Iraha S, Tu H-Y, Yamasaki S, et al. Establishment of immunodeficient retinal degeneration model mice and functional maturation of human ESC-derived retinal sheets after transplantation. Stem Cell Rep. 2018;10:1059–1074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mandai M, Fujii M, Hashiguchi T, et al. iPSC-derived retina transplants improve vision in rd1 end-stage retinal-degeneration mice. Stem Cell Rep. 2017;8:69–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Zhu J, Cifuentes H, Reynolds J, et al. Immunosuppression via loss of IL2rγ enhances long-term functional integration of hESC-derived photoreceptors in the mouse retina. Cell Stem Cell. 2017;20:374–384.e375 [DOI] [PubMed] [Google Scholar]
- 43.Tu H-Y, Watanabe T, Shirai H, et al. Medium-to long-term survival and functional examination of human iPSC-derived retinas in rat and primate models of retinal degeneration. EBioMedicine. 2019;39:562–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lin B, McLelland BT, Mathur A, et al. Sheets of human retinal progenitor transplants improve vision in rats with severe retinal degeneration. Exp Eye Res. 2018;174:13–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thomas BB, Lin B, Martinez-Camarillo JC, et al. Co-grafts of human embryonic stem cell derived retina organoids and retinal pigment epithelium for retinal reconstruction in immunodeficient retinal degenerate Royal College of Surgeons rats. Front Neurosci. 2021;15:752958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Yamasaki S, Sugita S, Horiuchi M, et al. Low immunogenicity and immunosuppressive properties of human ESC- and iPSC-derived retinas. Stem Cell Rep. 2021;16:851–867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kramer J, Chirco KR, Lamba DA. Immunological considerations for retinal stem cell therapy. Adv Exp Med Biol. 2019;1186:99–119. [DOI] [PubMed] [Google Scholar]
- 48.West EL, Pearson RA, Barker SE, et al. Long-term survival of photoreceptors transplanted into the adult murine neural retina requires immune modulation. Stem Cells. 2010;28:1997–2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Zhu J, Reynolds J, Garcia T, et al. Generation of transplantable retinal photoreceptors from a current good manufacturing practice-manufactured human induced pluripotent stem cell line. Stem Cells Transl Med. 2018;7:210–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomas BB, Zhu D, Lin TC, et al. A new immunodeficient retinal dystrophic rat model for transplantation studies using human-derived cells. Graefes Arch Clin Exp Ophthalmol. 2018;256:2113–2125. [DOI] [PubMed] [Google Scholar]
- 51.Llonch S, Carido M, Ader M. Organoid technology for retinal repair. Dev Biol. 2018;433:132–143. [DOI] [PubMed] [Google Scholar]
- 52.Völkner M, Kurth T, Schor J, et al. Mouse retinal organoid growth and maintenance in longer-term culture. Front Cell Dev Biol. 2021;9:645704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Bell CM, Zack DJ, Berlinicke CA. Human organoids for the study of retinal development and disease. Annu Rev Vis Sci. 2020;6:91–114. [DOI] [PubMed] [Google Scholar]
- 54.Capowski EE, Samimi K, Mayerl SJ, et al. Reproducibility and staging of 3D human retinal organoids across multiple pluripotent stem cell lines. Development (Cambridge, England). 2019;146:dev171686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xue Y, Browne AW, Tang WC, et al. Retinal organoids long-term functional characterization using two-photon fluorescence lifetime and hyperspectral microscopy. Front Cell Neurosci. 2021;15:796903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zerti D, Dorgau B, Felemban M, et al. Developing a simple method to enhance the generation of cone and rod photoreceptors in pluripotent stem cell-derived retinal organoids. Stem Cells. 2020;38:45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Ueda K, Onishi A, Ito SI, et al. Generation of three-dimensional retinal organoids expressing rhodopsin and S- and M-cone opsins from mouse stem cells. Biochem Biophys Res Commun. 2018;495:2595–2601. [DOI] [PubMed] [Google Scholar]
- 58.Kaya KD, Chen HY, Brooks MJ, et al. Transcriptome-based molecular staging of human stem cell-derived retinal organoids uncovers accelerated photoreceptor differentiation by 9-cis retinal. Mol Vis. 2019;25:663–678. [PMC free article] [PubMed] [Google Scholar]
- 59.Kelley RA, Chen HY, Swaroop A, et al. Accelerated development of rod photoreceptors in retinal organoids derived from human pluripotent stem cells by supplementation with 9-cis retinal. STAR Protoc. 2020;1:100033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Pan D, Xia XX, Zhou H, et al. COCO enhances the efficiency of photoreceptor precursor differentiation in early human embryonic stem cell-derived retinal organoids. Stem Cell Res Ther. 2020;11:366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Mellough CB, Collin J, Queen R, et al. Systematic comparison of retinal organoid differentiation from human pluripotent stem cells reveals stage specific, cell line, and methodological differences. Stem Cells Transl Med. 2019;8:694–706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Nakano T, Ando S, Takata N, et al. Self-formation of optic cups and storable stratified neural retina from human ESCs. Cell Stem Cell. 2012;10:771–785. [DOI] [PubMed] [Google Scholar]
- 63.Wahlin KJ, Maruotti JA, Sripathi SR, et al. Photoreceptor outer segment-like structures in long-term 3d retinas from human pluripotent stem cells. Sci Rep. 2017;7:766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Zhong X, Gutierrez C, Xue T, et al. Generation of three-dimensional retinal tissue with functional photoreceptors from human iPSCs. Nat Commun. 2014;5:4047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Meyer JS, Shearer RL, Capowski EE, et al. Modeling early retinal development with human embryonic and induced pluripotent stem cells. Proc Natl Acad Sci U S A. 2009;106:16698–16703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dorgau B, Felemban M, Hilgen G, et al. Decellularised extracellular matrix-derived peptides from neural retina and retinal pigment epithelium enhance the expression of synaptic markers and light responsiveness of human pluripotent stem cell derived retinal organoids. Biomaterials. 2019;199:63–75. [DOI] [PubMed] [Google Scholar]
- 67.Hunt NC, Hallam D, Karimi A, et al. 3D culture of human pluripotent stem cells in RGD-alginate hydrogel improves retinal tissue development. Acta Biomater. 2017;49:329–343. [DOI] [PubMed] [Google Scholar]
- 68.Kim S, Lowe A, Dharmat R, et al. Generation, transcriptome profiling, and functional validation of cone-rich human retinal organoids. Proc Natl Acad Sci U S A. 2019;116:10824–10833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Ovando-Roche P, West EL, Branch MJ, et al. Use of bioreactors for culturing human retinal organoids improves photoreceptor yields. Stem Cell Res Ther. 2018;9:156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.DiStefano T, Chen HY, Panebianco C, et al. Accelerated and improved differentiation of retinal organoids from pluripotent stem cells in rotating-wall vessel bioreactors. Stem Cell Rep. 2018;10:300–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yu F, Hunziker W, Choudhury D. Engineering microfluidic organoid-on-a-chip platforms. Micromachines. 2019;10:165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Achberger K, Probst C, Haderspeck J, et al. Merging organoid and organ-on-a-chip technology to generate complex multi-layer tissue models in a human retina-on-a-chip platform. Elife. 2019;8:e46188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xue Y, Seiler MJ, Tang WC, et al. Retinal organoids on-a-chip: a micro-millifluidic bioreactor for long-term organoid maintenance. Lab Chip. 2021;21:3361–3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Langer KB, Ohlemacher SK, Phillips MJ, et al. Retinal ganglion cell diversity and subtype specification from human pluripotent stem cells. Stem Cell Rep. 2018;10:1282–1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Zerti D, Collin J, Queen R, et al. Understanding the complexity of retina and pluripotent stem cell derived retinal organoids with single cell RNA sequencing: current progress, remaining challenges and future prospective. Curr Eye Res. 2020;45:385–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Collin J, Queen R, Zerti D, et al. Deconstructing retinal organoids: single cell RNA-Seq reveals the cellular components of human pluripotent stem cell-derived retina. Stem Cells. 2019;37:593–598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Phillips MJ, Jiang P, Howden S, et al. A novel approach to single cell RNA-sequence analysis facilitates in silico gene reporting of human pluripotent stem cell-derived retinal cell types. Stem Cells. 2018;36:313–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Browne AW, Arnesano C, Harutyunyan N, et al. Structural and functional characterization of human stem-cell-derived retinal organoids by live imaging. Invest Ophthalmol Vis Sci. 2017;58:3311–3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Scholler J, Groux K, Goureau O, et al. Dynamic full-field optical coherence tomography: 3D live-imaging of retinal organoids. Light Sci Appl. 2020;9:140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Phillips MJ, Capowski EE, Petersen A, et al. Generation of a rod-specific NRL reporter line in human pluripotent stem cells. Sci Rep. 2018;8:2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Collin J, Mellough CB, Dorgau B, et al. Using zinc finger nuclease technology to generate CRX-reporter human embryonic stem cells as a tool to identify and study the emergence of photoreceptors precursors during pluripotent stem cell differentiation. Stem Cells. 2016;34:311–321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Vergara MN, Flores-Bellver M, Aparicio-Domingo S, et al. Three-dimensional automated reporter quantification (3D-ARQ) technology enables quantitative screening in retinal organoids. Development (Cambridge, England). 2017;144:3698–3705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Meyer JS, Howden SE, Wallace KA, et al. Optic vesicle-like structures derived from human pluripotent stem cells facilitate a customized approach to retinal disease treatment. Stem Cells. 2011;29:1206–1218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Mellough CB, Collin J, Khazim M, et al. IGF-1 signaling plays an important role in the formation of three-dimensional laminated neural retina and other ocular structures from human embryonic stem cells. Stem Cells. 2015;33:2416–2430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Reichman S, Slembrouck A, Gagliardi G, et al. Generation of storable retinal organoids and retinal pigmented epithelium from adherent human ips cells in xeno-free and feeder-free conditions. Stem Cells. 2017;35:1176–1188. [DOI] [PubMed] [Google Scholar]
- 86.Gagliardi G, Ben M’Barek K, Chaffiol A, et al. Characterization and transplantation of CD73-positive photoreceptors isolated from human iPSC-derived retinal organoids. Stem Cell Rep. 2018;11:665–680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Garita-Hernandez M, Guibbal L, Toualbi L, et al. Optogenetic light sensors in human retinal organoids. Front Neurosci. 2018;12:789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Hallam D, Hilgen G, Dorgau B, et al. Human-induced pluripotent stem cells generate light responsive retinal organoids with variable and nutrient-dependent efficiency. Stem Cells. 2018;36:1535–1551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Afanasyeva TAV, Corral-Serrano JC, Garanto A, et al. A look into retinal organoids: methods, analytical techniques, and applications. Cell Mol Life Sci. 2021;78:6505–6532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Li L, Zhao H, Xie H, et al. Electrophysiological characterization of photoreceptor-like cells in human inducible pluripotent stem cell-derived retinal organoids during in vitro maturation. Stem Cells. 2021;39:959–974. [DOI] [PubMed] [Google Scholar]
- 91.Cowan CS, Renner M, De Gennaro M, et al. Cell types of the human retina and its organoids at single-cell resolution. Cell. 2020;182:1623–1640.e1634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zou T, Gao L, Zeng Y, et al. Organoid-derived C-Kit(+)/SSEA4(−) human retinal progenitor cells promote a protective retinal microenvironment during transplantation in rodents. Nat Commun. 2019;10:1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Chao JR, Lamba DA, Klesert TR, et al. Transplantation of human embryonic stem cell-derived retinal cells into the subretinal space of a non-human primate. Transl Vis Sci Technol. 2017;6:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wu Y-R, Hashiguchi T, Sho J, et al. Transplanted mouse embryonic stem cell-derived retinal ganglion cells integrate and form synapses in a retinal ganglion cell-depleted mouse model. Invest Ophthalmol Vis Sci. 2021;62:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Aboualizadeh E, Phillips MJ, McGregor JE, et al. Imaging transplanted photoreceptors in living nonhuman primates with single-cell resolution. Stem Cell Rep. 2020;15:482–497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Klassen HJ, Ng TF, Kurimoto Y, et al. Multipotent retinal progenitors express developmental markers, differentiate into retinal neurons, and preserve light-mediated behavior. Invest Ophthalmol Vis Sci. 2004;45:4167–4173. [DOI] [PubMed] [Google Scholar]
- 97.MacLaren RE, Pearson RA, MacNeil A, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. [DOI] [PubMed] [Google Scholar]
- 98.Lakowski J, Gonzalez-Cordero A, West EL, et al. Transplantation of photoreceptor precursors isolated via a cell surface biomarker panel from embryonic stem cell-derived self-forming retina. Stem Cells. 2015;33:2469–2482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Lakowski J, Han YT, Pearson RA, et al. Effective transplantation of photoreceptor precursor cells selected via cell surface antigen expression. Stem Cells. 2011;29:1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lakowski J, Baron M, Bainbridge J, et al. Cone and rod photoreceptor transplantation in models of the childhood retinopathy Leber congenital amaurosis using flow-sorted Crx-positive donor cells. Hum Mol Genet. 2010;19:4545–4559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Gust J, Reh TA. Adult donor rod photoreceptors integrate into the mature mouse retina. Invest Ophthalmol Vis Sci. 2011;52:5266–5272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Pearson RA, Barber AC, Rizzi M, et al. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Santos-Ferreira T, Llonch S, Borsch O, et al. Retinal transplantation of photoreceptors results in donor–host cytoplasmic exchange. Nat Commun. 2016;7:13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Ortin-Martinez A, Tsai EL, Nickerson PE, et al. A Reinterpretation of cell transplantation: gfp transfer from donor to host photoreceptors. Stem Cells. 2017;35:932–939. [DOI] [PubMed] [Google Scholar]
- 105.Waldron PV, Di Marco F, Kruczek K, et al. Transplanted donor- or stem cell-derived cone photoreceptors can both integrate and undergo material transfer in an environment-dependent manner. Stem Cell Rep. 2018;10:406–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simó R, Villarroel M, Corraliza L, et al. The retinal pigment epithelium: something more than a constituent of the blood-retinal barrier—implications for the pathogenesis of diabetic retinopathy. J Biomed Biotechnol. 2010;2010:190724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Steinberg RH. Interactions between the retinal pigment epithelium and the neural retina. Doc Ophthalmol. 1985;60:327–346. [DOI] [PubMed] [Google Scholar]
- 108.Nishida M, Tanaka Y, Tanaka Y, et al. Human iPS cell derived RPE strips for secure delivery of graft cells at a target place with minimal surgical invasion. Sci Rep. 2021;11:21421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Takagi S, Mandai M, Gocho K, et al. Evaluation of transplanted autologous induced pluripotent stem cell-derived retinal pigment epithelium in exudative age-related macular degeneration. Ophthalmol Retina. 2019;3:850–859. [DOI] [PubMed] [Google Scholar]
- 110.Sugita S, Iwasaki Y, Makabe K, et al. Successful transplantation of retinal pigment epithelial cells from MHC homozygote iPSCs in MHC-matched models. Stem Cell Rep. 2016;7:635–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Kamao H, Mandai M, Ohashi W, et al. Evaluation of the surgical device and procedure for extracellular matrix-scaffold-supported human iPSC-derived retinal pigment epithelium cell sheet transplantation. Invest Ophthalmol Vis Sci. 2017;58:211–220. [DOI] [PubMed] [Google Scholar]
- 112.Mandai M, Watanabe A, Kurimoto Y, et al. Autologous induced stem-cell-derived retinal cells for macular degeneration. N Engl J Med. 2017;376:1038–1046. [DOI] [PubMed] [Google Scholar]
- 113.Kashani AH, Lebkowski JS, Rahhal FM, et al. A bioengineered retinal pigment epithelial monolayer for advanced, dry age-related macular degeneration. Sci Transl Med. 2018;10:eaao4097. [DOI] [PubMed] [Google Scholar]
- 114.da Cruz L, Fynes K, Georgiadis O, et al. Phase 1 clinical study of an embryonic stem cell-derived retinal pigment epithelium patch in age-related macular degeneration. Nat Biotechnol. 2018;36:328–337. [DOI] [PubMed] [Google Scholar]
- 115.Kashani AH, Lebkowski JS, Rahhal FM, et al. One-year follow-up in a phase 1/2a clinical trial of an allogeneic RPE cell bioengineered implant for advanced dry age-related macular degeneration. Transl Vis Sci Technol. 2021;10:13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Vitillo L, Tovell VE, Coffey P. Treatment of age-related macular degeneration with pluripotent stem cell-derived retinal pigment epithelium. Curr Eye Res. 2020;45:361–371. [DOI] [PubMed] [Google Scholar]
- 117.Uyama H, Mandai M, Takahashi M. Stem-cell-based therapies for retinal degenerative diseases: Current challenges in the establishment of new treatment strategies. Dev Growth Differ. 2021;63:59–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.German OL, Buzzi E, Rotstein NP, et al. Retinal pigment epithelial cells promote spatial reorganization and differentiation of retina photoreceptors. J Neurosci Res. 2008;86:3503–3514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kaempf S, Walter P, Salz AK, et al. Novel organotypic culture model of adult mammalian neurosensory retina in co-culture with retinal pigment epithelium. J Neurosci Methods. 2008;173:47–58. [DOI] [PubMed] [Google Scholar]
- 120.Aramant RB, Seiler MJ, Ball SL. Successful cotransplantation of intact sheets of fetal retina with retinal pigment epithelium. Invest Ophthalmol Vis Sci. 1999;40:1557–1564. [PubMed] [Google Scholar]
- 121.Radtke ND, Aramant RB, Petry HM, et al. Vision improvement in retinal degeneration patients by implantation of retina together with retinal pigment epithelium. Am J Ophthalmol. 2008;146:172–182. [DOI] [PubMed] [Google Scholar]
- 122.Ghareeb AE, Lako M, Steel DH. Coculture techniques for modeling retinal development and disease, and enabling regenerative medicine. Stem Cells Transl Med. 2020;9:1531–1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hunt NC, Hallam D, Chichagova V, et al. The application of biomaterials to tissue engineering neural retina and retinal pigment epithelium. Adv Healthc Mater. 2018;7:1800226. [DOI] [PubMed] [Google Scholar]
- 124.Lee I-K, Ludwig AL, Phillips MJ, et al. Ultrathin micromolded 3D scaffolds for high-density photoreceptor layer reconstruction. Sci Adv. 2021;7:eabf0344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Jung YH, Phillips MJ, Lee J, et al. 3D Microstructured Scaffolds to Support Photoreceptor Polarization and Maturation. Adv Mater. 2018;30:1803550. [DOI] [PubMed] [Google Scholar]
- 126.Prusky GT, Alam NM, Beekman S, et al. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. [DOI] [PubMed] [Google Scholar]
- 127.Seiler MJ, Lin RE, McLelland BT, et al. Vision recovery and connectivity by fetal retinal sheet transplantation in an immunodeficient retinal degenerate rat model. Invest Ophthalmol Vis Sci. 2017;58:614–630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Fujii M, Sunagawa GA, Kondo M, et al. Evaluation of micro electroretinograms recorded with multiple electrode array to assess focal retinal function. Sci Rep. 2016;6:30719–30719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Ito S, Feldheim DA. The mouse superior colliculus: an emerging model for studying circuit formation and function. Front Neural Circuits. 2018;12:10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Yamasaki S, Tu HY, Matsuyama T, et al. A genetic modification that reduces ON-bipolar cells in hESC-derived retinas enhances functional integration after transplantation. iScience. 2022;25:103657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.He XY, Zhao CJ, Xu H, et al. Synaptic repair and vision restoration in advanced degenerating eyes by transplantation of retinal progenitor cells. Stem Cell Rep. 2021;16:1805–1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Pearson RA, Gonzalez-Cordero A, West EL, et al. Donor and host photoreceptors engage in material transfer following transplantation of post-mitotic photoreceptor precursors. Nat Commun. 2016;7:13029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Singh MS, Balmer J, Barnard AR, et al. Transplanted photoreceptor precursors transfer proteins to host photoreceptors by a mechanism of cytoplasmic fusion. Nat Commun. 2016;7:13537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Nickerson PEB, Ortin-Martinez A, Wallace VA. Material exchange in photoreceptor transplantation: updating our understanding of donor/host communication and the future of cell engraftment science. Front Neural Circuits. 2018;12:17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Boudreau-Pinsonneault C, Cayouette M. Cell lineage tracing in the retina: could material transfer distort conclusions? Dev Dyn. 2018;247:10–17. [DOI] [PubMed] [Google Scholar]
- 136.Jiang D, Chen FX, Zhou H, et al. Bioenergetic crosstalk between mesenchymal stem cells and various ocular cells through the intercellular trafficking of mitochondria. Theranostics. 2020;10:7260–7272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Santos-Ferreira T, Völkner M, Borsch O, et al. Stem cell-derived photoreceptor transplants differentially integrate into mouse models of cone-rod dystrophy. Invest Ophthalmol Vis Sci. 2016;57:3509–3520. [DOI] [PubMed] [Google Scholar]
- 138.Heisterkamp P, Borsch O, Lezama ND, et al. Evidence for endogenous exchange of cytoplasmic material between a subset of cone and rod photoreceptors within the adult mammalian retina via direct cell-cell connections. Exp Eye Res. 2022:109033. [DOI] [PubMed] [Google Scholar]


