Abstract
Swarming motility plays an important role in surface colonization by several flagellated bacteria. Swarmer cells are specially adapted to rapidly translocate over agar surfaces by virtue of their more numerous flagella, longer cell length, and encasement of slime. The external slime provides the milieu for motility and likely harbors swarming signals. We recently reported the isolation of swarming-defective transposon mutants of Salmonella enterica serovar Typhimurium, a large majority of which were defective in lipopolysaccharide (LPS) synthesis. Here, we have examined the biofilm-forming abilities of the swarming mutants using a microtiter plate assay. A whole spectrum of efficiencies were observed, with LPS mutants being generally more proficient than wild-type organisms in biofilm formation. Since we have postulated that O-antigen may serve a surfactant function during swarming, we tested the effect of the biosurfactant surfactin on biofilm formation. We report that surfactin inhibits biofilm formation of wild-type S. enterica grown either in polyvinyl chloride microtiter wells or in urethral catheters. Other bio- and chemical surfactants tested had similar effects.
Swarming bacteria are prime examples of how prokaryotes interact with and adapt to surface environments (5, 6, 8, 11, 12). Upon propagation on a surface, many flagellated bacteria turn on a new genetic program that leads to formation of swarmer cells, which are typically longer and have more flagella than cells of the same species grown in aqueous conditions (swimmer cells). These morphological changes appear to be necessary to overcome frictional forces presented by a surface, allowing colony expansion. Another important feature of swarming colonies is their encapsulation in slime (a generic term for a mixture of carbohydrates, proteins, peptides, surfactants, etc.), which not only provides an essential milieu for swarming motility, but likely harbors signals for swarmer cell differentiation (18).
We recently reported the isolation of a large number of transposon mutants of Salmonella enterica serovar Typhimurium that affect swarming motility (18). A majority of these mutants were defective in lipopolysaccharide (LPS) synthesis, many were defective in chemotaxis, and some had defects in putative two-component signaling components. While the latter two classes were defective in swarmer cell differentiation, representative LPS mutants were not and could be rescued for swarming by external addition of a biosurfactant. We have suggested that the O-antigen improves surface wettability, required for swarm colony expansion, and that multiple two-component systems cooperate to promote swarmer cell differentiation.
In contrast to the expansionist behavior of swarming colonies, there is a prevalence in nature of microbial colonies that remain attached to a surface in associations called biofilms (2). Biofilms are composed of exopolysaccharides or slime secreted by the adherent bacteria (20). Genetic studies have shown that, like the swarming bacteria, single-species biofilms form in multiple steps (21), require intercellular signaling (4), and show a pattern of gene expression that is distinct from that of planktonic cells (16). Bacteria that have formed adherent biofilms exist not as a tightly packed unit but rather as columns of loosely associated cells, some fixed, others motile. Water channels between pillars of cells in such biofilms allow nutrients to disperse. Biofilms are medically and industrially important because they can accumulate on a wide variety of substrates and are resistant to antimicrobial agents and detergents. What feature of a biofilm allows adherence in one case and expansion in another?
It is clear that swarming and biofilm formation have common features, such as generation of a large bacterial mass within which cells interact closely and generation of slime, which promotes association or colonization of the growth surface. In order to investigate whether our swarming mutants might shed light on the expansionist versus adherent nature of the two types of surface colonies, we tested these mutants for their ability to form biofilms in polyvinyl chloride (PVC) microtiter wells. We find an inverse relationship between the presence of a wettability factor (either LPS or surfactin) and biofilm formation; thus, the presence of the wettability factor favors swarming but inhibits biofilm formation, and vice versa.
MATERIALS AND METHODS
Bacterial strains, media, and chemicals.
S. enterica serovar Typhimurium SJW1103 (a fliC stable derivative of serovar Typhimurium LT2) and its transposon mutants have been described (18). The bacteria were grown in Luria-Bertani (LB) medium. Surfactin, sodium dodecyl sulfate (SDS), and Tween 80 were purchased from Sigma Chemical Co. Rhamnolipid (extracted from Pseudomonas aeruginosa) was a gift from Jeneil Biosurfactant Co. Inc. PVC 96-well microtiter plates were from Costar (2596; nontreated). Clear vinyl urethral catheters (14Fr) were from Kendall Co., Mansfield, Mass.
Quantification of biofilm formation in microtiter wells.
The quantification assay was performed as previously described (14). Typically, 10 μl of an overnight culture was used to inoculate PVC microtiter wells containing 90 μl of LB without NaCl but with 2% glucose. The covered microtiter dish was sealed with Parafilm during incubation at 30°C. Cultures were removed to determine the optical density at 630 nm (OD630), and the wells were rinsed with distilled water. After drying at room temperature for 15 min, 200 μl of crystal violet (1%) was added to the wells for 20 min. The stained biofilms were rinsed several times with distilled water, allowed to dry at room temperature for 15 min, and extracted twice with 200 μl of 95% ethanol. The OD550 was estimated using a Beckman DU-640B spectrophotometer after adjusting the volume to 1 ml with distilled water.
Visualizing biofilm formation in catheters.
An overnight culture of S. enterica (10 μl) was inoculated into 500 μl of medium and injected into clear vinyl urethral catheters. The catheters were capped at both ends and incubated at 30°C overnight. Media and growth conditions were as described above for PVC wells. Cultures were removed to determine the OD630, and the catheters were rinsed with distilled water. After drying at room temperature for 15 min, 700 μl of crystal violet (1%) was added to the catheters for 20 min. The stained biofilms were rinsed several times with distilled water and allowed to dry at room temperature for 15 min before examination.
RESULTS AND DISCUSSION
Analysis of biofilm formation by wild-type S. enterica in microtiter wells.
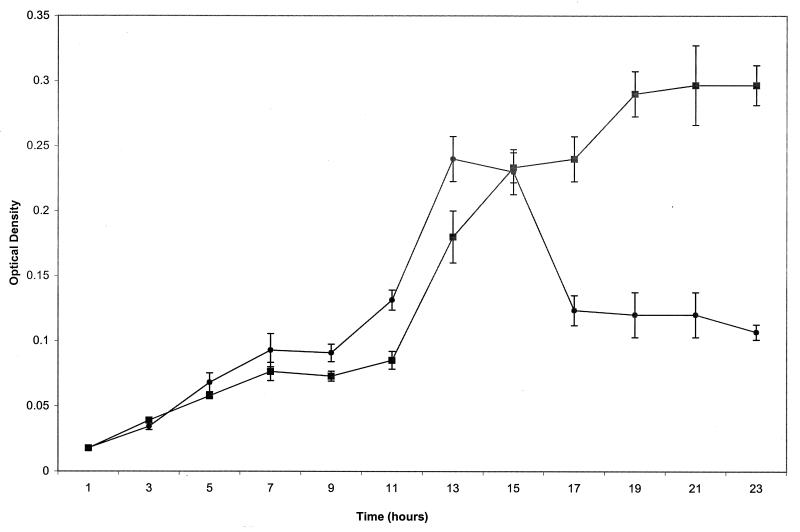
The biofilm assay used in this study monitors the ability of S. enterica to attach to the wells of microtiter dishes (14). The biofilm forms at the interface between the air and the liquid medium and is quantitated by staining with crystal violet as described in Materials and Methods. Initial experiments with different abiotic materials (PVC, polystyrene, and borosilicate glass) showed that the wild-type strain SJW1103 forms the best biofilms on PVC, in LB broth without NaCl but with 0.2% glucose, and at 30°C; these conditions were used throughout. SJW1103 did not form a detectable biofilm in M9 minimal medium, consistent with the results reported for Escherichia coli (15). Figure 1 shows the kinetics of biofilm formation by wild-type S. enterica. The exponential phase of biofilm formation coincided with that of cell growth. Biofilm formation began to slow around 13 h, decreased up to 17 h, and then leveled off, coincident with entry of the culture into stationary phase.
FIG. 1.
Kinetics of biofilm formation by wild-type S. enterica. SJW1103 was cultured at 30°C in PVC 96-well plates containing LB without NaCl plus 0.2% glucose. Samples were harvested at designated time points to determine growth (OD630) and biofilm formation after crystal violet staining (OD550) as described in the text. ▪, OD630; ●, OD550.
Analysis of biofilm formation by swarming mutants.
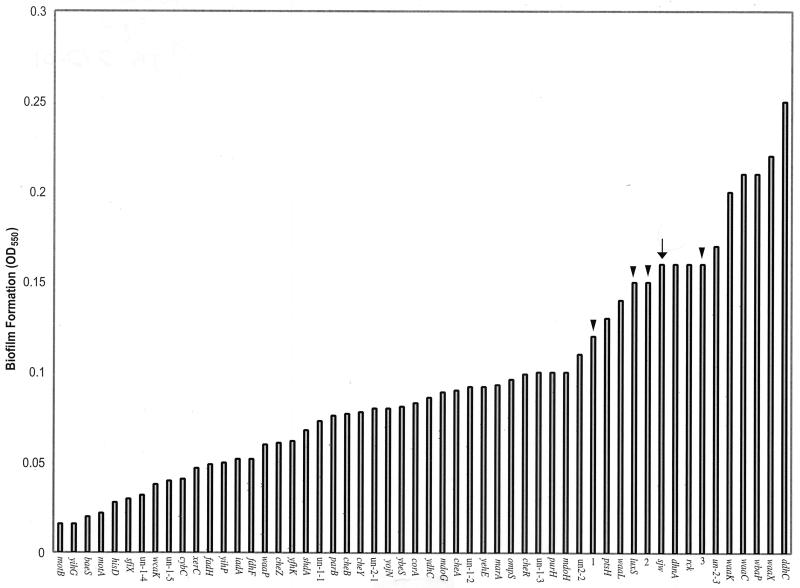
Fifty-nine S. enterica swarming mutants (18), all completely defective in swarming, were tested for their ability to form biofilms. A subset of these is shown in Fig. 2. Also included in this analysis were four mutants with mutations that did not affect swarming (luxS [18]; 1, 2, and 3 are random Tn10dCm insertions in SJW1103). Biofilms were harvested when cultures reached an OD630 of approximately 0.2. The data (arranged in increasing biofilm formation efficiency) are shown in Fig. 2. Overall, a continuous spectrum of biofilm formation was observed in the mutants, from 10 to 156% of the wild-type level. Except for the waaP and waaL mutants, all the LPS mutants clustered at the far end of this spectrum. Mutants not affected in swarming showed biofilm formation similar to that of wild-type cells (Fig. 2, arrowheads and arrow, respectively). Specific mutants are discussed below.
FIG. 2.
Biofilm formation by S. enterica swarming mutants. Growth conditions were as described in the legend to Fig. 1. The OD630 of all samples was approximately 0.2. Arrow points to biofilm levels (OD550) of wild-type SJW1103, and arrowheads point to those of mutants not affected in swarming. The data are means of triplicate samples monitored on the same day. There was a significant variation in the absolute biofilm values of samples processed on different days, but the relative values with respect to the wild-type value were very reproducible.
As shown previously with E. coli (15), both motA and motB mutants were significantly defective in biofilm formation, reaching only about 10% of the wild-type level, while the che mutants were less defective, attaining between 38 and 62% of wild-type levels. Biofilm formation by mutants not defective in swarming (Fig. 2, arrowheads) was within 10 to 20% of the wild-type level. Among the swarming mutants, a whole range of biofilm formation levels was observed. We note that the crystal violet staining technique is only a measure of adherence and that this analysis only measures gross biofilm formation. For example, in the lasI mutant of P. aeruginosa, total biofilm formation was unaffected but the structure was physically different and, in contrast to the wild-type parent, could be dislodged with 0.2% SDS (4). Similarly, a colanic acid mutant of E. coli showed normal crystal violet staining, but when examined under the microscope, the mutant did not display the complex pillars of cells typical of the wild-type strain (3). Thus, it is possible that mutants that appear to be unaffected by this assay could have different structures to their biofilms. If we consider only the very low or very high end of the biofilm formation spectrum, then of the uncharacterized two-component signaling mutations, baeS is at the low end and yfhk and yojN are towards the middle. At the high end there are a cluster of LPS mutations, waaK, waaC, wbaP, wzx, and ddhC. The kinetics of biofilm formation for one of these mutants (ddhC) was determined to be similar to the wild-type kinetics in the initial phase of growth; however, the mutant continued to accumulate more biofilm after the wild type had begun to slow (data not shown). None of the LPS mutants (or the wild type) formed biofilm on a hydrophilic surface such as glass under these growth conditions (data not shown). Attempts to measure cell surface hydrophobicity of the wild type and the mutants by measuring adherence to hydrocarbons (17) were not successful.
Effect of a surfactant on biofilm formation.
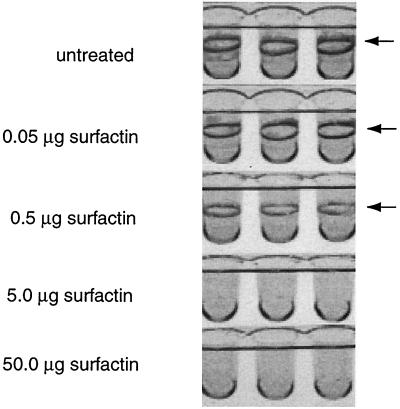
We have recently shown that the swarming defect of the LPS mutants could be rescued by addition of the biosurfactant surfactin isolated from Bacillus subtilis (18). To analyze the effect of surfactin on biofilm formation, either the PVC wells were coated with surfactin or surfactin was included in the growth medium. Figure 3 shows that increasing amounts of surfactin led to a decrease in the amount of biofilm formed by the wild-type strain and that 5 μg of surfactin was more than sufficient to completely abolish biofilm formation. Bacterial growth was unaffected under all surfactin concentrations tested (data not shown). Surfactin had a similar inhibitory effect on biofilm formation by the ddhC mutant (data not shown).
FIG. 3.
Surfactin inhibits biofilm formation by wild-type S. enterica. PVC wells were coated with the indicated amounts of surfactin before inoculation with S. enterica. After overnight incubation at 30°C, the wells were rinsed out and stained with crystal violet. The biofilm is concentrated at the interface between the air and the liquid medium (indicated by arrows).
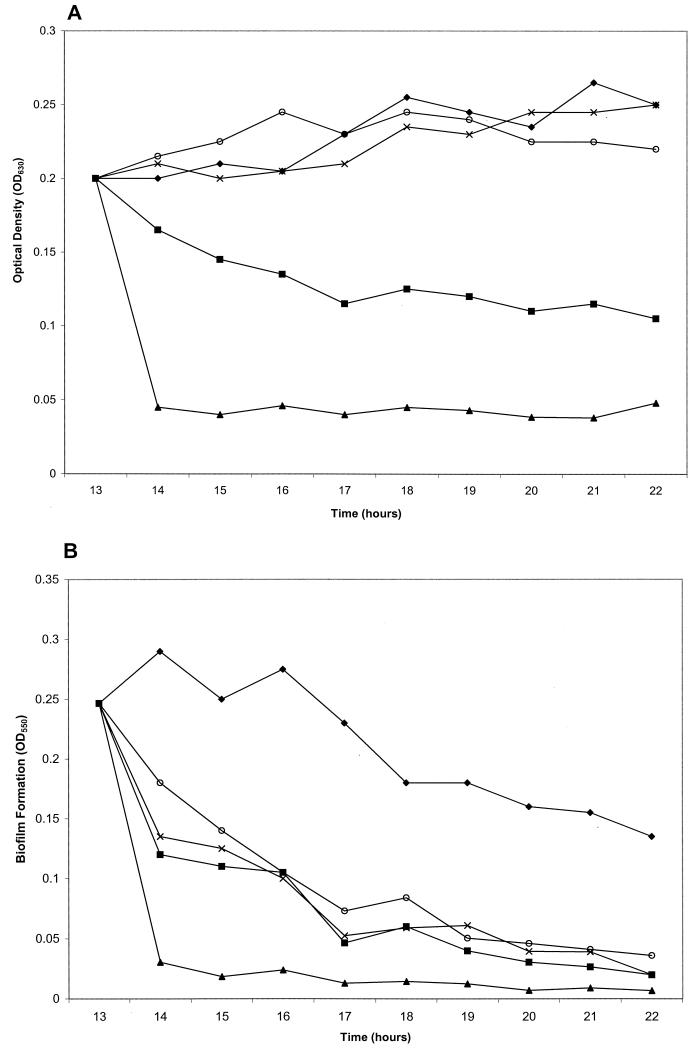
To test if surfactin would dislodge a preformed biofilm, surfactin was added to the PVC wells after the culture had reached an OD630 of ≈0.15 to 0.2 (Fig. 4A). The OD550 of the surfactin-treated sample decreased at a faster rate than that of the untreated sample for the initial sloughing phase of biofilm formation, resulting in an approximately 85% decrease in total biofilm by the end of the experiment at 22 h (Fig. 4B). To determine if other surfactants would have similar effects on preformed biofilms, we tested SDS (ionic surfactant), Tween 80 (anionic surfactant), and rhamnolipid. While all the chemicals tested dispersed the preformed biofilm, SDS and rhamnolipid decreased the OD630 of the culture as well (Fig. 4A). (Surfactin concentrations in this and the rest of the experiments were maintained at 100 μg in order to compare its activity to that of the biosurfactant rhamnolipid, which affected biofilm formation at higher concentrations.)
FIG. 4.
Addition of surfactin and other surfactants to a preformed biofilm accelerates biofilm dispersal. After S. enterica had reached an OD630 of ≈0.15 to 0.2, the indicated surfactants were gently mixed into the cultures in microtiter wells. Samples were harvested, and either growth (A), as determined by OD630, or biofilm levels (B), as measured by OD550 of crystal violet-stained material, were analyzed at the indicated time points. ⧫, no treatment; ○, 100 μg of surfactin; ×, 0.25% Tween 80; ▪, 100 μg of rhamnolipid; ▴, 0.2% SDS. The data are means of triplicate samples monitored on the same day.
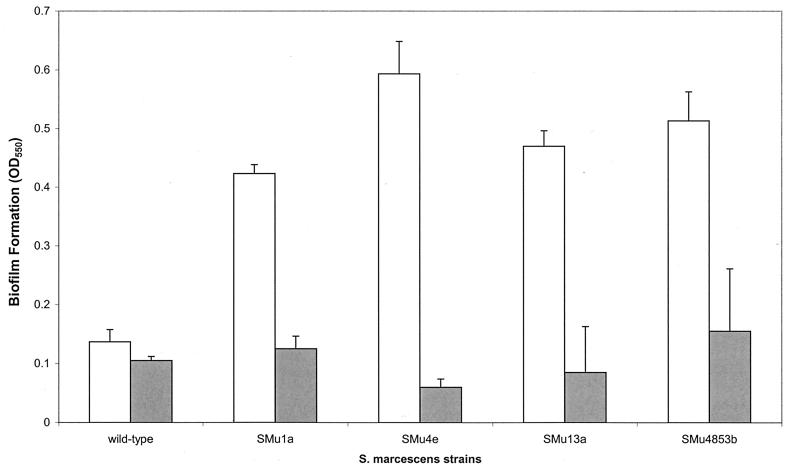
To investigate the biofilm-forming ability of bacteria known to produce surfactants, we tested Serratia marcescens and B. subtilis as well as mutants defective in surfactant production. S. marcescens mutants defective in production of the surfactant serrawettin are unable to swarm (9, 13), as are surfactin mutants of B. subtilis (obtained from P. Zuber, Oregon Graduate Institute of Science and Technology; our unpublished data). Mutants of S. marcescens that were defective in serrawettin synthesis made approximately threefold more biofilm that their wild-type parent (Fig. 5). These results are consistent with the notion that absence of the biosurfactant promotes biofilm formation. To test if surfactin would inhibit biofilm formation by the serrawettin mutants, surfactin was included in parallel wells (Fig. 5). A significant reduction in biofilm formation was observed for all the strains in the presence of surfactin. Purified serrawettin (gift from T. Matsuyama, University of Niigata, Niigata, Japan), had an inhibitory effect on biofilm formation but was not as effective as surfactin (data not shown). We were unable to observe biofilm formation by wild-type B. subtilis or its surfactin mutants under these conditions (data not shown).
FIG. 5.
Biofilm formation by S. marcescens and its mutants. Wild-type S. marcescens 274 and its serrawettin mutants (SMu1a, SMu4e, SMu13a, and SMu4853b [9]) were analyzed for biofilm formation in the absence and presence of surfactin as described in the legend to Fig. 3. The OD630 of all the cultures was ≈0.15 (not shown). Open bars, no surfactin; shaded bars, 100 μg of surfactin.
Surfactin prevents biofilm formation on urethral catheters.
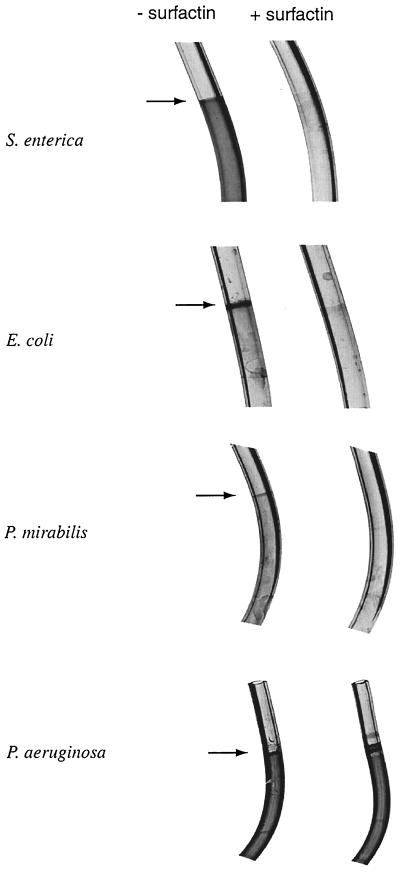
To test the effect of surfactin on medically relevant objects, we grew S. enterica in clear vinyl urethral catheters. The stained catheters are shown in Fig. 6. The biofilm formed by S. enterica was dispersed all along the growth surface. Surfactin eliminated formation of the biofilm. Precoating the catheters by running the surfactin solution through them prior to inoculation with medium was just as effective as including surfactin in the growth medium (data not shown). Among other surfactants tested on S. enterica, Tween 80 (0.25%) was as effective as surfactin, while rhamnolipid seemed to be only half as effective (data not shown). Given the importance of opportunistic infections with Salmonella species, including urinary tract infections of AIDS patients (7), these results have the potential for practical applications.
FIG. 6.
Surfactin inhibits biofilm formation on urethral catheters. The indicated organisms were grown overnight at 30°C in sterile urethral catheters containing medium with and without 100 μg of surfactin as described in the text. Biofilms were analyzed by staining with crystal violet.
To test the effect of surfactin on biofilm formation by other medically relevant organisms, we grew E. coli, Proteus mirabilis, and Pseudomonas aeruginosa in urethral catheters. E. coli and Proteus mirabilis formed a biofilm mainly at the air-liquid interface, while the biofilm formed by P. aeruginosa, like that formed by S. enterica, was dispersed all along the catheter (Fig. 6). Surfactin inhibited biofilm formation (but not growth) by all organisms except P. aeruginosa. Similar inhibition profiles were obtained with Tween 80 (data not shown). We note that there are earlier reports on inhibition of biofilms formed by uropathogens and yeasts on silicone rubber by biosurfactants produced by Streptococcus thermophilus and lactobacilli (1, 19).
Summary.
The main conclusions from this work can be summarized as follows. At least one difference between adherent and moving biofilms may lie in the surfactant composition of the slime, since the absence of surfactants inhibits swarming but promotes biofilm formation and vice versa. O-antigen mutants of S. enterica, which are defective in swarming (18), were generally more proficient in biofilm formation than the wild type (Fig. 2). O-antigen has been postulated to provide a surfactant or wettability function that allows spreading of the swarmer colony (18). Surfactin, a cyclic lipopeptide from B. subtilis which was shown to rescue the swarming defect of the LPS mutants (18), inhibited biofilm formation (Fig. 3). Mutants of S. marcescens defective in serrawettin production (serrawettin is an analog of surfactin [10]) are defective in swarming (9, 13) and make better biofilms (Fig. 5). Surfactin (as well as the chemical surfactant Tween 80) dispersed preformed biofilms without affecting cell growth (Fig. 4) and also prevented biofilm formation in urethral catheters by organisms such as S. enterica, E. coli, and P. mirabilis (Fig. 6). The nonbactericidal nature of these compounds would be advantageous in preventing the selection of resistant organisms if used for inhibiting biofilm formation. Biofilms formed by P. aeruginosa were not affected by the surfactants tested in this study (Fig. 6). Our results suggest the need for additional research into the use of this genre of compounds for biofilm containment. Since P. aeruginosa did not form biofilms on a hydrophilic glass surface under our growth conditions, manipulating the hydrophilicity of the growth surface might provide another avenue to the control of this class of biofilms.
ACKNOWLEDGMENT
This work was supported by a grant from NIH (GM57400).
REFERENCES
- 1.Busscher H J, van Hoogmoed C G, Geertsema-Doornbusch G I, van der Kuijl-Booij M, van der Mei H C. Streptococcus thermophilus and its biosurfactants inhibit adhesion by Candida spp. on silicone rubber. Appl Environ Microbiol. 1997;63:3810–3817. doi: 10.1128/aem.63.10.3810-3817.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Costerton J W, Lewandowski Z, Caldwell D E, Korber D R, Lappin-Scott H M. Microbial biofilms. Annu Rev Microbiol. 1995;49:711–745. doi: 10.1146/annurev.mi.49.100195.003431. [DOI] [PubMed] [Google Scholar]
- 3.Danese P N, Pratt L A, Kolter R. Exopolysaccharide production is required for development of Escherichia coli K-12 biofilm architecture. J Bacteriol. 2000;182:3593–3596. doi: 10.1128/jb.182.12.3593-3596.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Davies D G, Parsek M R, Pearson J P, Iglewski B H, Costerton J W, Greenberg E P. The involvement of cell-to-cell signals in the development of a bacterial biofilm. Science. 1998;280:295–298. doi: 10.1126/science.280.5361.295. [DOI] [PubMed] [Google Scholar]
- 5.Eberl L, Molin S, Givskov M. Surface motility of Serratia liquefaciens MG1. J Bacteriol. 1999;181:1703–1712. doi: 10.1128/jb.181.6.1703-1712.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fraser G M, Hughes C. Swarming motility. Curr Opin Microbiol. 1999;2:630–635. doi: 10.1016/s1369-5274(99)00033-8. [DOI] [PubMed] [Google Scholar]
- 7.Gruenewald R, Blum S, Chan J. Relationship between human immunodeficiency virus infection and salmonellosis in 20- to 59-year-old residents of New York City. Clin Infect Dis. 1994;18:358–363. doi: 10.1093/clinids/18.3.358. [DOI] [PubMed] [Google Scholar]
- 8.Harshey R M. Bees aren't the only ones: swarming in gram-negative bacteria. Mol Microbiol. 1994;13:389–394. doi: 10.1111/j.1365-2958.1994.tb00433.x. [DOI] [PubMed] [Google Scholar]
- 9.Matsuyama T, Bhasin A, Harshey R M. Mutational analysis of flagellum-independent surface spreading of Serratia marcescens 274 on a low-agar medium. J Bacteriol. 1995;177:987–991. doi: 10.1128/jb.177.4.987-991.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Matsuyama T, Kaneda K, Nakagawa Y, Isa K, Hara-Hotta H, Yano I. A novel extracellular cyclic lipopeptide which promotes flagellum-dependent and -independent spreading growth of Serratia marcescens. J Bacteriol. 1992;174:1769–1776. doi: 10.1128/jb.174.6.1769-1776.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McCarter L. The multiple identities of Vibrio parahaemolyticus. J Mol Microbiol Biotechnol. 1999;1:51–57. [PubMed] [Google Scholar]
- 12.Mobley H L, Belas R. Swarming and pathogenicity of Proteus mirabilis in the urinary tract. Trends Microbiol. 1995;3:280–284. doi: 10.1016/s0966-842x(00)88945-3. [DOI] [PubMed] [Google Scholar]
- 13.O'Rear J, Alberti L, Harshey R M. Mutations that impair swarming motility in Serratia marcescens 274 include but are not limited to those affecting chemotaxis or flagellar function. J Bacteriol. 1992;174:6125–6137. doi: 10.1128/jb.174.19.6125-6137.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.O'Toole G A, Kolter R. Flagellar and twitching motility are necessary for Pseudomonas aeruginosa biofilm development. Mol Microbiol. 1998;30:295–304. doi: 10.1046/j.1365-2958.1998.01062.x. [DOI] [PubMed] [Google Scholar]
- 15.Pratt L A, Kolter R. Genetic analysis of Escherichia coli biofilm formation: roles of flagella, motility, chemotaxis and type I pili. Mol Microbiol. 1998;30:285–293. doi: 10.1046/j.1365-2958.1998.01061.x. [DOI] [PubMed] [Google Scholar]
- 16.Prigent-Combaret C, Vidal O, Dorel C, Lejeune P. Abiotic surface sensing and biofilm-dependent regulation of gene expression in Escherichia coli. J Bacteriol. 1999;181:5993–6002. doi: 10.1128/jb.181.19.5993-6002.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rosenberg M, Gutnick D, Rosenberg E. Adherence of bacteria to hydrocarbons: a simple method for measuring cell-surface hydrophobicity. FEMS Microbiol Lett. 1980;9:29–33. [Google Scholar]
- 18.Toguchi A, Siano M, Burkart M, Harshey R M. Genetics of swarming motility in Salmonella enterica serovar Typhimurium: critical role for lipopolysaccharide. J Bacteriol. 2000;182:6308–6321. doi: 10.1128/jb.182.22.6308-6321.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Velraeds M M, van de Belt-Gritter B, Busscher H J, Reid G, van der Mei H C. Inhibition of uropathogenic biofilm growth on silicone rubber in human urine by lactobacilli—a teleologic approach. World J Urol. 2000;18:422–426. doi: 10.1007/pl00007084. [DOI] [PubMed] [Google Scholar]
- 20.Watnick P, Kolter R. Biofilm: city of microbes. J Bacteriol. 2000;182:2675–2679. doi: 10.1128/jb.182.10.2675-2679.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Watnick P I, Kolter R. Steps in the development of a Vibrio cholerae El Tor biofilm. Mol Microbiol. 1999;34:586–595. doi: 10.1046/j.1365-2958.1999.01624.x. [DOI] [PMC free article] [PubMed] [Google Scholar]