Abstract
Fabry disease (FD) is an X-linked lysosomal storage disorder where impaired α-galactosidase A enzyme activity leads to the intracellular accumulation of undegraded glycosphingolipids, including globotriaosylsphingosine (lyso-Gb3) and related analogues. Lyso-Gb3 and related analogues are useful biomarkers for screening and should be routinely monitored for longitudinal patient evaluation. In recent years, a growing interest has emerged in the analysis of FD biomarkers in dried blood spots (DBSs), considering the several advantages compared to venipuncture as a technique for collecting whole-blood specimens. The focus of this study was to devise and validate a UHPLC-MS/MS method for the analysis of lyso-Gb3 and related analogues in DBSs to facilitate sample collection and shipment to reference laboratories. The assay was devised in conventional DBS collection cards and in Capitainer®B blood collection devices using both capillary and venous blood specimens from 12 healthy controls and 20 patients affected with FD. The measured biomarker concentrations were similar in capillary and venous blood specimens. The hematocrit (Hct) did not affect the correlation between plasma and DBS measurements in our cohort (Hct range: 34.3–52.2%). This UHPLC-MS/MS method using DBS would facilitate high-risk screening and the follow-up and monitoring of patients affected with FD.
Keywords: globotriaosylsphingosine, biomarkers, Fabry disease, dried blood spots, mass spectrometry, Capitainer®B, lyso-Gb3 analogues
1. Introduction
Fabry disease (OMIM No. 301500) is an X-linked heterogenous lysosomal storage disease caused by pathogenic mutations in the GLA gene [1]. These mutations lead to impaired α-galactosidase A (EC 3.2.1.22) enzyme activity and to the subsequent intracellular accumulation of undegraded glycosphingolipids, including globotriaosylceramide (Gb3) [2,3,4,5,6], globotriaosylsphingosine (lyso-Gb3) [7,8], galabiosylceramide (Ga2) [9,10], and related isoforms and analogues. The clinical manifestations are progressive and multisystemic. The signs and symptoms in children and adolescents are mainly angiokeratomas, neuropathic pain/acroparesthesia, hypohidrosis/anhidrosis, corneal changes, proteinuria, gastrointestinal problems, and fatigue [11]. Adults are prone to major organ involvement, including an impaired renal function, cardiomyopathy, and stroke [12]. Although Fabry disease is of X-linked inheritance, women are also affected, sometimes as severely as men [13]. Since 2001, enzyme replacement therapy (ERT) is available for the treatment of Fabry disease when the indications for treatment are met, either with agalsidase-alfa (0.2 mg/kg/2 weeks) or agalsidase-beta (1.0 mg/kg/2 weeks) [12]. Migalastat, a pharmacological chaperone therapy, has also been approved (123 mg/2 days) for the treatment of Fabry disease in patients with amenable mutations [14,15]. Future therapies are currently being investigated, including second-generation ERT, substrate reduction therapy, mRNA therapy, and gene therapy [16,17,18].
Our group previously discovered the presence of molecules structurally related to lyso-Gb3 in urine and plasma specimens obtained from Fabry patients as part of metabolomic studies using time-of-flight mass spectrometry. The lyso-Gb3 analogues have modifications on the lyso-Gb3 sphingosine moiety that were confirmed by exact mass measurements: −C2H4 (−28 Da), −C2H4+O (−12 Da), −H2 (−2 Da), −H2+O (+14 Da), +O (+16 Da), +H2O (+18 Da), +H2O2 (+34 Da), and +H2O3 (+50 Da). Lyso-Gb3 and related analogues have been found to be useful biomarkers for screening Fabry patients [19,20] and for evaluating their response to treatment. Our group previously found that levels of lyso-Gb3 and some related analogues had a positive association with the left ventricular mass index and Mainz Severity Score Index in a cohort of patients with the IVS4 + 919G>A late-onset cardiac variant mutation in Taiwan [20]. A recent study highlighted that the serum lyso-Gb3 level and pretreatment exposure to lyso-Gb3 is a significant risk factor associated with adverse clinical outcomes [21]. Another group suggested that plasma lyso-Gb3 might be a useful indicator of the biochemical response to migalastat [22]. A group of experts thus recently suggested that plasma lyso-Gb3 should be routinely monitored in patients with classic Fabry disease at baseline, and then every 6 to 12 months after the treatment initiation or changes in the treatment regimen for a longitudinal evaluation [23].
In recent years, a growing interest has emerged for the analysis of lyso-Gb3 in dried blood spot (DBS) specimens [24,25,26,27,28]. DBS collection cards have several advantages compared to venipuncture as a technique for collecting whole-blood specimens [29,30,31]. The collection of whole blood by venipuncture requires the presence of a certified phlebotomist and is usually collected in a health care facility, while DBS collection cards can be used successfully at home by a minimally trained individual. Blood specimens collected by venipuncture must be processed rapidly by a laboratory technician to avoid sample or analyte degradation, while DBS specimens are simply allowed to dry. The risk of infection transmission is also lowered considering that the specimen is dried, and that a very low volume of whole blood is applied to the sampling card [29,31]. The low volume of whole blood needed to obtain a DBS also facilitates sample collection in pediatric patients [24], and regular mail can be used for the shipment of samples to reference laboratories, without the need for expensive temperature-regulated transport. Biomarker stability is usually good in DBSs, and specimens are easy to store in a cost-effective manner. However, DBS sampling using conventional collection cards can have some drawbacks, including risks of insufficient specimens [32] or uneven blood distribution on the filter card [29]. The hematocrit (Hct) level (or the volume percentage of red blood cells in blood) also influences the spreading of whole blood on the filter card and leads to sample amount variability when a disk with a fixed diameter is punched from the DBS [29,33]. Several next-generation volumetric microsampling devices designed to collect dried blood are now available to overcome these issues [33,34,35,36,37,38]. Delahaye et al. [37] recently published an extensive overview of commercially available devices, including: the Mitra® device with volumetric absorptive microsampling, or VAMS® technology (Neoteryx, Torrance, CA, USA); the hemaPEN® (Trajan Scientific and Medical, Victoria, Australia); the HemaXis™ DB 10 (DBS System SA, Gland, Switzerland); the HemaSpot™ HF (Spot on Sciences, San Francisco, CA, USA); the Tasso-M20 (Tasso Inc., Seattle, WA, USA); and the Capitainer®B (Capitainer Ab, Solna, Sweden) [33,34,35,36,37,38]. The Capitainer®B device consists of a capillary microchannel with a precise volume of 10 µL [33,38]. When a drop of blood comes into contact with the inlet port of the device, 10 µL of blood is aspirated in the capillary microchannel, and then a thin film dissolves and the excess blood is collected onto a waste filter paper. A similar film dissolves at the outlet afterwards, and an exact volume of 10 µL of blood is absorbed in a pre-perforated paper disk by capillary forces. This principle allows the collection of a fixed volume of blood and overcomes the hematocrit bias.
The overall objectives of this research project were thus: (1) to develop and validate a UHPLC-MS/MS method for the analysis of lyso-Gb3 and related analogues in DBSs collected using two different types of collection kits: conventional Whatman-GE 903 collection cards (referred to as W-DBS) and Capitainer®B blood collection devices (referred to as CB-DBS); (2) to establish reference values; (3) to compare the biomarker measurements obtained in plasma and in DBSs; (4) to compare the biomarker measurements in DBSs obtained from venous blood collected by venipuncture (referred to as DBSV) compared to capillary blood obtained by a finger prick (referred to as DBSC); and (5) to evaluate if the difference between the plasma and DBS biomarker measurements is associated with Hct levels.
2. Results
2.1. Method Validation
2.1.1. Accuracy and Precision
The intra-day accuracy and precision (n = 5) was evaluated using spiked quality controls (sQC) at three concentration levels. The results are shown in Table 1. The accuracy was acceptable for W-DBS with mean biases of 6.2% (range: 0.9 to 14.3%), 9.6% (range: 5.1 to 16.5%), and 6.7% (range: 4.6 to 12.6%) at low (L), medium (M), and high (H) lyso-Gb3-13C6 concentrations, respectively. The accuracy was also acceptable for CB-DBS with mean biases of 0.1% (range: −6.5 to 6.5%), 12.3% (range: 0.7 to 35.7%), and 4.8% (range: 1.6 to 9.2%) at low, medium, and high lyso-Gb3-13C6 concentrations, respectively. The precision was acceptable for W-DBS with %RSDs of 4.7%, 3.9%, and 3.2% at low, medium, and high lyso-Gb3-13C6 concentrations, respectively. The precision was also acceptable for CB-DBS with %RSDs of 5.5%, 12.3%, and 2.9% at low, medium, and high lyso-Gb3-13C6 concentrations, respectively.
Table 1.
Intra-day assays (n = 5) performed with spiked quality control (sQC) dried blood spots (DBSs) prepared at three concentration levels by spiking lyso-Gb3-13C6 in a pool of blood from control individuals.
| Whatman-GE 903 | Capitainer®B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sLQC | sMQC | sHQC | sLQC | sMQC | sHQC | |||||||
| nM | Bias% | nM | Bias% | nM | Bias% | nM | Bias% | nM | Bias% | nM | Bias% | |
| Spiked concentration | 0.75 | na | 75.00 | na | 250.00 | na | 0.75 | na | 75.00 | na | 250.00 | na |
| Replicate 1 | 0.79 | 4.7 | 82.93 | 10.6 | 261.79 | 4.7 | 0.79 | 5.2 | 75.56 | 0.7 | 272.94 | 9.2 |
| Replicate 2 | 0.80 | 6.5 | 87.37 | 16.5 | 262.81 | 5.1 | 0.73 | −2.9 | 101.79 | 35.7 | 261.68 | 4.7 |
| Replicate 3 | 0.76 | 0.9 | 80.88 | 7.8 | 261.57 | 4.6 | 0.80 | 6.5 | 79.88 | 6.5 | 255.61 | 2.2 |
| Replicate 4 | 0.78 | 4.4 | 78.81 | 5.1 | 266.48 | 6.6 | 0.70 | −6.5 | 84.77 | 13.0 | 265.91 | 6.4 |
| Replicate 5 | 0.86 | 14.3 | 81.09 | 8.1 | 281.55 | 12.6 | 0.74 | −1.6 | 79.18 | 5.6 | 254.11 | 1.6 |
| Mean | 0.80 | 6.2 | 82.21 | 9.6 | 266.84 | 6.7 | 0.75 | 0.1 | 84.23 | 12.3 | 262.05 | 4.8 |
| SD | 0.04 | na | 3.23 | na | 8.46 | na | 0.04 | na | 10.35 | na | 7.71 | na |
| RSD% | 4.67 | na | 3.93 | na | 3.17 | na | 5.55 | na | 12.28 | na | 2.94 | na |
| Bias% min | na | 0.9 | na | 5.1 | na | 4.6 | na | −6.5 | na | 0.7 | na | 1.6 |
| Bias% max | na | 14.3 | na | 16.5 | na | 12.6 | na | 6.5 | na | 35.7 | na | 9.2 |
na = not applicable; SD = standard deviation; RSD = relative standard deviation; min = minimum; max = maximum; sLQC = spiked low quality control (0.75 nM); sMQC = spiked medium quality control (75 nM); sHQC = spiked high quality control (250 nM).
The intra-day precision (n = 5) was also evaluated for this assay using DBSs obtained from Fabry patients as quality controls (pQCs) at two concentration levels. It was thus possible to evaluate the intra-day precision for lyso-Gb3 and five related analogues. The results are shown in Table 2. The precision was acceptable for W-DBS with %RSDs ranging from 6.9% to 12.8% in pLQC and from 2.8% to 7.8% in pHQC. The precision was also acceptable for CB-DBS with %RSDs ranging from 2.9% to 9.9% in pLQC and from 1.9% to 9.6% in pHQC.
Table 2.
Intra-day assays (n = 5) performed with dried blood spots (DBSs) obtained from Fabry patients as quality controls (pQCs).
| Lyso-Gb3 Analogues | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lyso-Gb3 | −28 Da | −2 Da | +16 Da | +18 Da | +34 Da | ||||||||
| pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | ||
| nM | nM | nM | nM | nM | nM | nM | nM | nM | nM | nM | nM | ||
|
(A) Whatman-
GE 903 |
Replicate 1 | 47.93 | 126.95 | 2.24 | 7.34 | 9.27 | 27.09 | 3.33 | 11.68 | 2.73 | 9.89 | 4.34 | 16.67 |
| Replicate 2 | 45.75 | 120.97 | 2.38 | 7.10 | 8.52 | 25.63 | 3.81 | 11.43 | 2.42 | 10.11 | 3.99 | 15.84 | |
| Replicate 3 | 54.67 | 122.34 | 2.99 | 7.38 | 10.62 | 27.25 | 4.20 | 11.74 | 3.12 | 10.03 | 4.90 | 16.19 | |
| Replicate 4 | 49.73 | 114.15 | 2.33 | 6.83 | 9.16 | 23.91 | 3.65 | 10.41 | 2.64 | 9.49 | 4.49 | 15.04 | |
| Replicate 5 | 51.98 | 114.80 | 2.80 | 6.47 | 9.93 | 23.29 | 3.85 | 9.81 | 2.78 | 9.59 | 4.79 | 16.02 | |
| Mean | 50.01 | 119.84 | 2.55 | 7.02 | 9.50 | 25.43 | 3.77 | 11.01 | 2.74 | 9.82 | 4.50 | 15.95 | |
| SD | 3.47 | 5.38 | 0.33 | 0.38 | 0.80 | 1.80 | 0.32 | 0.86 | 0.25 | 0.27 | 0.36 | 0.60 | |
| RSD% | 6.9 | 4.5 | 12.8 | 5.4 | 8.4 | 7.1 | 8.4 | 7.8 | 9.3 | 2.8 | 8.0 | 3.7 | |
| (B) Capitainer ® B | Replicate 1 | 67.70 | 173.59 | 4.54 | 11.04 | 15.80 | 40.05 | 6.87 | 18.24 | 5.31 | 16.04 | 8.66 | 24.76 |
| Replicate 2 | 70.01 | 189.64 | 4.36 | 11.41 | 14.74 | 40.74 | 6.15 | 18.04 | 5.96 | 16.95 | 8.70 | 25.05 | |
| Replicate 3 | 77.03 | 184.55 | 4.37 | 11.79 | 15.47 | 42.14 | 6.29 | 18.50 | 6.85 | 16.17 | 9.57 | 25.77 | |
| Replicate 4 | 73.57 | 190.14 | 4.13 | 11.40 | 14.95 | 42.13 | 6.17 | 18.81 | 5.85 | 19.35 | 9.34 | 28.32 | |
| Replicate 5 | 75.43 | 185.82 | 3.99 | 11.71 | 14.98 | 42.51 | 6.30 | 18.87 | 6.52 | 19.47 | 9.26 | 29.27 | |
| Mean | 72.75 | 184.75 | 4.28 | 11.47 | 15.19 | 41.51 | 6.36 | 18.49 | 6.10 | 17.59 | 9.10 | 26.63 | |
| SD | 3.85 | 6.68 | 0.22 | 0.30 | 0.43 | 1.06 | 0.29 | 0.36 | 0.60 | 1.69 | 0.41 | 2.03 | |
| RSD% | 5.3 | 3.6 | 5.1 | 2.6 | 2.9 | 2.6 | 4.6 | 1.9 | 9.9 | 9.6 | 4.5 | 7.6 | |
SD = standard deviation; RSD = relative standard deviation; pLQC = low quality control prepared with blood from a Fabry patient; pHQC = high quality control prepared with blood from a Fabry patient.
The inter-day accuracy and precision (n = 5 days) was evaluated for this assay using sQCs at three concentration levels. The results are shown in Table 3. The accuracy was acceptable for W-DBS with mean biases of 3.9% (range: 0.9 to 6.2%), 6.6% (range: 3.2 to 12.0%), and 4.3% (range: −4.0 to 9.0%) at low, medium, and high lyso-Gb3-13C6 concentrations, respectively. The accuracy was also acceptable for CB-DBS with mean biases of 0.0% (range: −9.9 to 6.4%), 5.0% (range: −2.7 to 12.3%), and 5.1% (range: 2.6 to 8.7%) at low, medium, and high lyso-Gb3-13C6 concentrations, respectively. The precision was acceptable for W-DBS with %RSDs of 2.6%, 3.8%, and 5.2% at low, medium, and high lyso-Gb3-13C6 concentrations, respectively. The precision was also acceptable for CB-DBS with %RSDs of 6.8%, 6.9%, and 2.1% at low, medium, and high lyso-Gb3-13C6 concentrations, respectively.
Table 3.
Inter-day (n = 5) assays performed with spiked quality control (sQC) dried blood spots (DBSs) prepared at three concentration levels by spiking lyso-Gb3-13C6 in a pool of blood from control individuals.
| Whatman-GE 903 | Capitainer®B | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| sLQC | sMQC | sHQC | sLQC | sMQC | sHQC | |||||||
| nM | Bias% | nM | Bias% | nM | Bias% | nM | Bias% | nM | Bias% | nM | Bias% | |
| Spiked concentration | 0.75 | na | 75.00 | na | 250.00 | na | 0.75 | na | 75.00 | na | 250.00 | na |
| Day 1 | 0.80 | 6.2 | 82.21 | 9.6 | 266.84 | 6.7 | 0.75 | 0.1 | 84.23 | 12.3 | 262.05 | 4.8 |
| Day 2 | 0.79 | 5.1 | 84.00 | 12.0 | 272.55 | 9.0 | 0.80 | 6.4 | 73.06 | −2.6 | 260.73 | 4.3 |
| Day 3 | 0.76 | 0.9 | 77.69 | 3.6 | 270.24 | 8.1 | 0.80 | 6.3 | 80.39 | 7.2 | 271.74 | 8.7 |
| Day 4 | 0.76 | 1.1 | 77.38 | 3.2 | 239.97 | −4.0 | 0.68 | −9.9 | 72.95 | −2.7 | 262.98 | 5.2 |
| Day 5 | 0.80 | 6.2 | 78.29 | 4.4 | 254.29 | 1.7 | 0.73 | −2.7 | 82.95 | 10.6 | 256.45 | 2.6 |
| Mean | 0.78 | 3.9 | 79.91 | 6.6 | 260.78 | 4.3 | 0.75 | 0.0 | 78.72 | 5.0 | 262.79 | 5.1 |
| SD | 0.02 | na | 3.00 | na | 13.60 | na | 0.05 | na | 5.40 | na | 5.60 | na |
| RSD% | 2.6 | na | 3.8 | na | 5.2 | na | 6.8 | na | 6.9 | na | 2.1 | na |
| Bias% minimum | na | 0.9 | na | 3.2 | na | −4.0 | na | −9.9 | na | −2.7 | na | 2.6 |
| Bias% maximum | na | 6.2 | na | 12.0 | na | 9.0 | na | 6.4 | na | 12.3 | na | 8.7 |
na = not applicable; SD = standard deviation; RSD = relative standard deviation; sLQC = spiked low quality control (0.75 nM); sMQC = spiked medium quality control (75 nM); sHQC = spiked high quality control (250 nM).
The inter-day precision (n = 5 days) was also evaluated for this assay using DBSs obtained from Fabry patients as quality controls at two concentration levels. It was thus possible to evaluate the inter-day precision for lyso-Gb3 and five related analogues. The results are shown in Table 4. The precision was acceptable for W-DBS with %RSDs ranging from 5.4% to 11.5% in pLQC and from 6.7% to 16.4% in pHQC. The precision was also acceptable for CB-DBS with %RSDs ranging from 5.9% to 20.0% in pLQC and from 3.0% to 21.9% in pHQC.
Table 4.
Inter-day (n = 5) assays performed with dried blood spots (DBSs) obtained from Fabry patients as quality controls (pQCs).
| Lyso-Gb3 Analogues | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lyso-Gb3 | −28 Da | −2 Da | +16 Da | +18 Da | +34 Da | ||||||||
| pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | ||
| nM | nM | nM | nM | nM | nM | nM | nM | nM | nM | nM | nM | ||
|
(A) Whatman-
GE 903 |
Day 1 | 50.01 | 119.84 | 2.55 | 7.02 | 9.50 | 25.43 | 3.77 | 11.01 | 2.74 | 9.82 | 4.50 | 15.95 |
| Day 2 | 44.51 | 122.23 | 2.38 | 7.06 | 8.35 | 25.50 | 3.55 | 11.11 | 2.83 | 8.50 | 4.09 | 13.75 | |
| Day 3 | 40.91 | 115.93 | 2.05 | 5.87 | 7.26 | 22.49 | 3.46 | 10.81 | 2.48 | 9.45 | 4.72 | 15.22 | |
| Day 4 | 40.53 | 113.24 | 2.04 | 6.20 | 7.60 | 24.10 | 3.96 | 13.34 | 2.85 | 7.51 | 5.04 | 14.98 | |
| Day 5 | 40.53 | 102.58 | 2.04 | 5.50 | 7.44 | 21.36 | 3.79 | 12.67 | 2.75 | 6.49 | 4.76 | 13.35 | |
| Mean | 43.30 | 114.76 | 2.21 | 6.33 | 8.03 | 23.78 | 3.71 | 11.79 | 2.73 | 8.35 | 4.62 | 14.65 | |
| SD | 4.11 | 7.64 | 0.24 | 0.69 | 0.92 | 1.82 | 0.20 | 1.14 | 0.15 | 1.37 | 0.35 | 1.07 | |
| RSD% | 9.5 | 6.7 | 10.7 | 11.0 | 11.5 | 7.7 | 5.4 | 9.7 | 5.4 | 16.4 | 7.6 | 7.3 | |
| (B) Capitainer ® B | Day 1 | 72.75 | 184.75 | 4.28 | 11.47 | 15.19 | 41.51 | 6.36 | 18.49 | 6.10 | 17.59 | 9.10 | 26.63 |
| Day 2 | 66.06 | 194.13 | 3.66 | 10.37 | 13.50 | 38.14 | 5.59 | 15.69 | 5.89 | 19.45 | 8.39 | 26.80 | |
| Day 3 | 70.66 | 186.87 | 3.70 | 9.66 | 14.02 | 38.99 | 6.70 | 18.68 | 4.22 | 11.66 | 7.22 | 23.71 | |
| Day 4 | 75.98 | 197.24 | 3.80 | 9.62 | 15.09 | 38.51 | 7.71 | 19.81 | 4.57 | 13.21 | 7.69 | 22.67 | |
| Day 5 | 78.69 | 196.27 | 3.43 | 10.29 | 15.54 | 42.21 | 6.94 | 20.63 | 6.87 | 19.08 | 10.07 | 29.24 | |
| Mean | 72.83 | 191.85 | 3.77 | 10.28 | 14.67 | 39.87 | 6.66 | 18.66 | 5.53 | 16.20 | 8.49 | 25.81 | |
| SD | 4.87 | 5.68 | 0.31 | 0.75 | 0.87 | 1.86 | 0.78 | 1.88 | 1.10 | 3.55 | 1.13 | 2.63 | |
| RSD% | 6.7 | 3.0 | 8.3 | 7.3 | 5.9 | 4.7 | 11.7 | 10.1 | 20.0 | 21.9 | 13.3 | 10.2 | |
SD = standard deviation; RSD = relative standard deviation; pLQC = low quality control prepared with blood from a Fabry patient; pHQC = high quality control prepared with blood from a Fabry patient.
2.1.2. Limits of Detection (LODs), Limits of Quantitation (LOQs), Recovery, and Linearity
The LODs and LOQs were evaluated for lyso-Gb3-13C6, lyso-Gb3, and related analogues at −28 Da, −2 Da, +16 Da, +18 Da, and +34 Da. The results are shown in Table 5. The LODs ranged from 0.10 to 0.32 nM for W-DBS, and from 0.15 to 0.40 nM for CB-DBS. The LOQs ranged from 0.32 to 1.08 nM for W-DBS, and from 0.55 to 1.34 nM for CB-DBS. The LODs and LOQs in plasma are shown for reference purposes [19].
Table 5.
Limits of detection (LODs) and limits of quantification (LOQs) for lyso-Gb3 and its analogues in dried blood spots (DBSs).
| LOD | LOQ | |||||
|---|---|---|---|---|---|---|
| Whatman-GE 903 | Capitainer®B | Plasma | Whatman-GE 903 | Capitainer®B | Plasma | |
| nM | nM | nM | nM | nM | nM | |
| Lyso-Gb3-13C6 | 0.21 | 0.40 | na | 0.70 | 1.34 | na |
| Lyso-Gb3 | 0.32 | 0.37 | 0.23 | 1.08 | 1.23 | 0.77 |
| Lyso-Gb3 (Analogue − 28 Da) | 0.11 | 0.18 | 0.06 | 0.36 | 0.59 | 0.21 |
| Lyso-Gb3 (Analogue −2 Da) | 0.22 | 0.15 | 0.29 | 0.73 | 0.55 | 0.97 |
| Lyso-Gb3 (Analogue +16 Da) | 0.10 | 0.39 | 0.22 | 0.32 | 1.29 | 0.72 |
| Lyso-Gb3 (Analogue +18 Da) | 0.13 | 0.23 | 0.14 | 0.42 | 0.77 | 0.47 |
| Lyso-Gb3 (Analogue +34 Da) | 0.13 | 0.19 | 0.24 | 0.43 | 0.64 | 0.79 |
LOD = limit of detection; LOQ = limit of quantification; na = not applicable.
The recoveries from the DBS extraction, and then from the solid phase extraction (SPE) procedures were evaluated at three concentration levels (sLQC: 0.75 nM; sMQC: 75 nM; sHQC: 250 nM) and the results are shown in Table 6. Linearity was evaluated (n = 6 days) and the coefficients of determination (r2) were always ≥ 0.998 in both the calibration curves prepared using W-DBS and CB-DBS.
Table 6.
Dried blood spot (DBS) and solid phase extraction (SPE) recoveries for lyso-Gb3-13C6 in DBSs (n = 5).
| DBS Extraction Recovery (%) | SPE Recovery (%) | |||
|---|---|---|---|---|
| Whatman-GE 903 | Capitainer®B | Whatman-GE 903 | Capitainer®B | |
| sLQC | 69 | 68 | 71 | 72 |
| sMQC | 71 | 66 | 72 | 69 |
| sHQC | 66 | 68 | 74 | 74 |
DBS = dried blood spot; SPE = solid phase extraction; sLQC = spiked low quality control (0.75 nM); sMQC = spiked medium quality control (75 nM); sHQC = spiked high quality control (250 nM).
2.1.3. Stability
The lyso-Gb3 stability was evaluated in DBSs at room temperature for up to 14 days, in a refrigerator (4 °C) for 38 days, in a freezer (−20 °C) for 208 days, and after five freeze/thaw cycles using DBSs prepared with a pool of blood from control individuals fortified with lyso-Gb3-13C6 at low, medium, and high concentrations in duplicate (Table 7). The results showed that the stability was good under these conditions, with biases ranging from −20.5% to 6.4% for W-DBS and from −8.3% to 15.3% for CB-DBS. The stability of lyso-Gb3 was also evaluated in the UHPLC autosampler (4 °C) up to 48 h after the first analysis. The extracts were stable after 48 h, and biases ranged from −9.1% to 13.8%. The stability of the blood pools spiked with lyso-Gb3-13C6 (this solution was used to prepare the sQCs) was evaluated in triplicate just after mixing and after 3 h at room temperature; the biases ranged from −3.3% to 12.9%.
Table 7.
Stability assays (n = 2) performed with spiked quality control (sQC) dried blood spots (DBSs) prepared at three concentration levels by spiking lyso-Gb3-13C6 in a pool of blood from control individuals.
| Whatman-GE 903 | Capitainer®B | |||||
|---|---|---|---|---|---|---|
| sLQC | sMQC | sHQC | sLQC | sMQC | sHQC | |
| Bias% | Bias% | Bias% | Bias% | Bias% | Bias% | |
| Room temperature, 22 °C (7 days) | −9.6 | −1.3 | −4.2 | −8.3 | −1.9 | −4.3 |
| Room temperature, 22 °C (14 days) | −20.5 | −3.2 | −3.0 | −3.2 | −7.8 | 0.7 |
| Refrigerator, 4 °C (38 days) | −8.3 | −6.9 | −2.2 | 15.3 | 3.1 | −3.4 |
| Freezer, −20 °C (208 days) | −6.3 | −0.3 | −1.2 | 3.8 | 1.2 | −1.0 |
| Freeze/thaw cycles (n = 5) | −8.7 | 6.4 | 1.9 | 10.3 | −0.2 | 1.2 |
| UHPLC Autosampler, 4 °C (48 h) | 13.8 | −1.9 | −8.4 | −9.1 | −2.4 | −2.7 |
sLQC = spiked low quality control (0.75 nM); sMQC = spiked medium quality control (75 nM); sHQC = spiked high quality control (250 nM).
The stability of lyso-Gb3 and five related analogues was also evaluated under the same conditions using DBSs prepared using blood from a Fabry patient at low (pLQC) and high (pHQC) concentrations in duplicate (Table 8). The results showed that the stability was good using both types of DBS collection methods for 14 days at room temperature and for 38 days in a refrigerator (4 °C), with biases ranging from −12.6% to 15.6% and from −12.7% to 13.7%, respectively. Regarding the stability in the freezer for 208 days (−20 °C), the stability was good using both types of DBS collection methods, with biases ranging from −19.8% to 7.5%, except for the lyso-Gb3 analogue at −28 Da, with biases ranging from −25.4% to −18.7%. The stability in DBSs was evaluated after five freeze/thaw cycles, and the biases ranged from −23.3% to 15.0% for W-DBS and from −4.8% to 30.1% for CB-DBS. Finally, the stability of the extracts left in the UHPLC autosampler (4 °C) for 48 h after the sample preparation was good for W-DBS (biases ranged from −16.9% to 8.2%). Regarding the stability of the extracts obtained from CB-DBS, the biases ranged from −3.9% to 17.4%, except for the analogues at +18 Da and +34 Da with a lower accuracy (biases ranged from 16.1% to 36.4%).
Table 8.
Stability assays (n = 2) performed with dried blood spots (DBSs) from Fabry patients as quality controls (pQCs).
| Lyso-Gb3 Analogues | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Lyso-Gb3 | −28 Da | −2 Da | +16 Da | +18 Da | +34 Da | ||||||||
| pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | pLQC | pHQC | ||
| Bias% | Bias% | Bias% | Bias% | Bias% | Bias% | Bias% | Bias% | Bias% | Bias% | Bias% | Bias% | ||
|
(A) Whatman-
GE 903 |
RT, 22 °C (7 days) | 1.9 | −4.1 | 2.9 | −4.1 | 4.6 | 3.3 | −1.1 | 3.7 | 10.2 | −2.7 | 0.8 | −0.5 |
| RT, 22 °C (14 days) | −9.2 | 4.2 | −10.3 | 2.6 | −10.4 | 5.7 | −12.6 | 3.1 | 11.4 | 15.6 | −6.6 | 5.5 | |
| Refrigerator, 4 °C (38 days) | −5.0 | 10.8 | 2.2 | 12.0 | −1.1 | 10.9 | 10.5 | 7.5 | −12.7 | 12.0 | −3.8 | 13.7 | |
| Freezer, −20 °C (208 days) | −4.9 | −4.9 | −25.4 | −19.6 | −19.8 | −7.9 | −14.8 | −9.6 | 7.4 | −15.4 | 7.5 | −4.0 | |
| Freeze/thaw cycles (n = 5) | −16.0 | 6.8 | −18.9 | 5.2 | −17.5 | −1.4 | −23.4 | 0.9 | −7.6 | 15.0 | −19.6 | 5.2 | |
| UHPLC Autosampler, 4 °C (24 h) | −2.0 | −2.1 | 2.3 | −4.3 | −1.6 | −3.7 | −4.2 | −2.0 | 27.3 | 16.9 | 18.1 | 12.9 | |
| UHPLC Autosampler, 4 °C (48 h) | −9.4 | −16.9 | −13.8 | −14.4 | −14.8 | −16.7 | −13.1 | −14.4 | 8.2 | −1.7 | 2.6 | −2.6 | |
| (B) Capitainer ® B | RT, 22 °C (7 days) | −4.2 | 0.8 | −0.4 | 3.6 | −0.2 | −0.6 | −1.7 | 6.6 | −7.1 | 4.5 | 2.3 | −0.7 |
| RT, 22 °C (14 days) | −2.7 | −5.2 | 1.6 | −6.6 | 0.3 | −2.3 | −8.2 | −10.0 | 7.0 | −0.9 | 6.6 | −0.5 | |
| Refrigerator, 4 °C (38 days) | −6.0 | −3.0 | −8.5 | −2.8 | −8.4 | −1.2 | 1.0 | −7.0 | −3.1 | 1.6 | −9.1 | −5.1 | |
| Freezer, −20 °C (208 days) | −1.3 | 0.0 | −18.7 | −19.5 | −1.6 | −5.5 | −7.1 | −12.7 | −7.7 | 1.0 | −4.8 | −6.2 | |
| Freeze/thaw cycles (n = 5) | −1.3 | 0.6 | 14.7 | −4.0 | 6.7 | 0.7 | 2.1 | −4.8 | 1.9 | 30.1 | 9.1 | 19.8 | |
| UHPLC Autosampler, 4 °C (24 h) | 0.2 | 1.1 | 4.5 | −0.1 | −2.2 | −1.3 | 0.1 | 0.2 | 11.0 | 15.5 | 23.3 | 16.7 | |
| UHPLC Autosampler, 4 °C (48 h) | −3.9 | −1.8 | 17.4 | 9.8 | 10.8 | 7.8 | 14.0 | 10.0 | 16.1 | 25.9 | 36.4 | 32.4 | |
pLQC = low quality control prepared with blood from a Fabry patient; pHQC = high quality control prepared with blood from a Fabry patient; RT = room temperature.
2.1.4. Matrix Effect
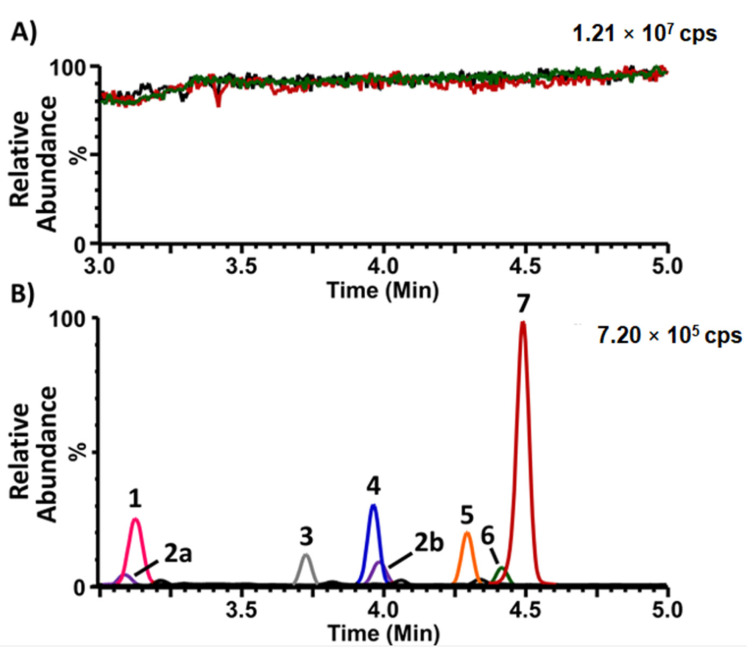
The matrix effect was evaluated using the post-column infusion method. The signal of lyso-Gb3-13C6 infused at a constant flow rate was recorded during the entire chromatographic injection time of the extracts obtained from W-DBS and CB-DBS. The results shown in Figure 1A demonstrate that the signal was mostly stable across the entire chromatogram when the extracts were injected, and comparable to the signal obtained when only the resuspension solvent was injected. The multiple reaction-monitoring (MRM) chromatogram of lyso-Gb3 and five related analogues is shown (Figure 1B) as a reference to compare with the analyte retention times. A minor signal enhancement was observed at the retention time of the analogue at +16 Da in the extracts obtained from the CB-DBS.
Figure 1.
Matrix effect evaluation for the analysis of lyso-Gb3 and its analogues in dried blood spots (DBSs). (A). Signal obtained from the post-column infusion of lyso-Gb3-13C6 (500 nM in: 50% acetonitrile, 49.9% H2O, 0.1% formic acid) at a flow rate of 10 µL/min during the UHPLC injection of: (1) the resuspension solvent (50% acetonitrile, 49.9% H2O, 0.1% formic acid) (green line); (2) a Whatman-GE 903-collected DBS prepared with control blood (post-sample preparation, red line); and (3) a Capitainer®B-collected DBS prepared with control blood (post-sample preparation, black line). (B). Multiple reaction-monitoring (MRM) analysis of lyso-Gb3 and its analogues in a Whatman-GE 903-collected DBS prepared with blood from an untreated Fabry male; 1: analogue (+16 Da); 2a and 2b: (analogue (+34 Da); 3: analogue (−28 Da); 4: analogue (−2 Da); 5: lyso-Gb3-Gly (internal standard); 6: analogue (+18 Da); and 7: lyso-Gb3). cps = counts per second.
2.2. Normal Reference Values
Normal reference values were established for lyso-Gb3 and five related analogues following the analysis of DBSs collected from 12 individuals not affected with Fabry disease. The normal reference values were established at the 99th percentile for W-DBS and CB-DBS for both venous and capillary blood. Table 9 shows a statistical summary of the analyte levels with the following values: mean, minimum, maximum, median, 99th percentile, sensitivity (true positive rate), and specificity (true negative rate). These values are displayed for plasma collected from the same individuals, for comparison purposes.
Table 9.
Normal reference values (99th percentile) established for each analyte under study following the analysis of DBSs from 12 healthy reference controls. Mean, minimum, maximum, and median values are shown, along with the sensitivity and specificity of the biomarkers.
| Biomarker | Blood Type |
Collection Method |
Mean | Min | Max | Median | 99th Percentile |
Sensitivity (%) | Specificity (%) |
|---|---|---|---|---|---|---|---|---|---|
| Lyso-Gb3
(nM) |
Venous | Whatman 903 | 3.96 | 2.25 | 6.25 | 3.86 | 6.14 | 100 | 92 |
| Capitainer®B | 4.60 | 3.16 | 8.43 | 4.18 | 8.19 | 85 | 92 | ||
| Capillary | Whatman 903 | 4.26 | 2.74 | 6.71 | 4.20 | 6.61 | 95 | 92 | |
| Capitainer®B | 4.15 | 2.95 | 5.93 | 4.19 | 5.92 | 100 | 100 | ||
| Plasma | EDTA K2 | 0.74 | 0.33 | 1.09 | 0.78 | 1.08 | 100 | 92 | |
| Lyso-Gb3 −28 Da (nM) |
Venous | Whatman 903 | 0.25 | nd | 0.53 | 0.26 | 0.51 | 55 | 92 |
| Capitainer®B | nd | nd | 0.42 | nd | 0.40 | 85 | 92 | ||
| Capillary | Whatman 903 | 0.28 | nd | 0.58 | 0.27 | 0.56 | 60 | 92 | |
| Capitainer®B | nd | nd | 0.24 | nd | 0.24 | 100 | 92 | ||
| Plasma | EDTA K2 | nd | nd | nd | nd | nd | 100 | 92 | |
| Lyso-Gb3 −2 Da (nM) |
Venous | Whatman 903 | 0.49 | 0.33 | 0.79 | 0.44 | 0.78 | 100 | 92 |
| Capitainer®B | 0.60 | 0.41 | 1.16 | 0.53 | 1.11 | 95 | 92 | ||
| Capillary | Whatman 903 | 0.56 | 0.35 | 1.03 | 0.53 | 1.00 | 100 | 92 | |
| Capitainer®B | 0.55 | 0.36 | 0.82 | 0.52 | 0.80 | 100 | 92 | ||
| Plasma | EDTA K2 | nd | nd | 0.31 | nd | 0.30 | 100 | 92 | |
| Lyso-Gb3 +16 Da (nM) |
Venous | Whatman 903 | nd | nd | 0.21 | nd | 0.20 | 95 | 92 |
| Capitainer®B | nd | nd | nd | nd | nd | 80 | 100 | ||
| Capillary | Whatman 903 | nd | nd | nd | nd | nd | 100 | 100 | |
| Capitainer®B | nd | nd | nd | nd | nd | 84 | 100 | ||
| Plasma | EDTA K2 | nd | nd | nd | nd | nd | 95 | 100 | |
| Lyso-Gb3 +18 Da (nM) |
Venous | Whatman 903 | nd | nd | nd | nd | nd | 100 | 100 |
| Capitainer®B | nd | nd | nd | nd | nd | 100 | 100 | ||
| Capillary | Whatman 903 | nd | nd | nd | nd | nd | 100 | 100 | |
| Capitainer®B | nd | nd | nd | nd | nd | 100 | 100 | ||
| Plasma | EDTA K2 | nd | nd | nd | nd | nd | 100 | 100 | |
| Lyso-Gb3 +34 Da (nM) |
Venous | Whatman 903 | 0.28 | 0.17 | 0.39 | 0.25 | 0.38 | 90 | 92 |
| Capitainer®B | nd | nd | 0.25 | nd | 0.25 | 90 | 92 | ||
| Capillary | Whatman 903 | 0.30 | 0.21 | 0.35 | 0.30 | 0.35 | 85 | 100 | |
| Capitainer®B | nd | nd | 0.20 | nd | 0.20 | 95 | 92 | ||
| Plasma | EDTA K2 | nd | nd | nd | nd | nd | 100 | 92 |
nd = not detected (under the limits of detection).
2.3. Analysis of Collected Specimens
2.3.1. Comparison of Biomarker Levels between Plasma and W-DBSC Matrices
A comparison of lyso-Gb3 and related analogue levels measured in plasma and in W-DBSC specimens was performed. The Deming regression parameters are shown in Table 10. The slope measures the amount of proportional bias, while the Y-intercept represents the systematic bias between the two methods. There was no proportional bias between the plasma and W-DBSC matrices, considering that the 95% confidence interval of the slope included one for lyso-Gb3 and its five related analogues. The examination of the Y-intercept showed a systematic bias for lyso-Gb3, lyso-Gb3 −28 Da, and lyso-Gb3 −2 Da, considering that the 95% confidence interval did not include 0. There was no systematic bias for lyso-Gb3 +16 Da, lyso-Gb3 +18 Da, or lyso-Gb3 +34 Da between the two matrices according to the Deming regression analysis.
Table 10.
Deming regression analysis parameters for lyso-Gb3 and analogue measurements in plasma and in DBSs collected using Whatman-GE 903 filter papers (capillary blood) (n = 32 participants).
| Biomarker | Slope | 95% CI (Slope) | Y-Intercept (nM) | 95% CI (Y-Intercept) | n |
|---|---|---|---|---|---|
| Lyso-Gb3 | 0.993 | 0.956–1.029 | 3.019 | 2.434–3.604 | 32 |
| Lyso-Gb3 −28 Da | 0.973 | 0.800–1.147 | 0.192 | 0.101–0.283 | 32 |
| Lyso-Gb3 −2 Da | 0.910 | 0.775–1.046 | 0.365 | 0.085–0.646 | 32 |
| Lyso-Gb3 +16 Da | 0.825 | 0.547–1.102 | 0.124 | −0.057–0.306 | 32 |
| Lyso-Gb3 +18 Da | 0.962 | 0.421–1.504 | −0.195 | −0.724–0.334 | 32 |
| Lyso-Gb3 +34 Da | 0.988 | 0.454–1.523 | −0.045 | −0.402–0.312 | 32 |
CI: confidence interval.
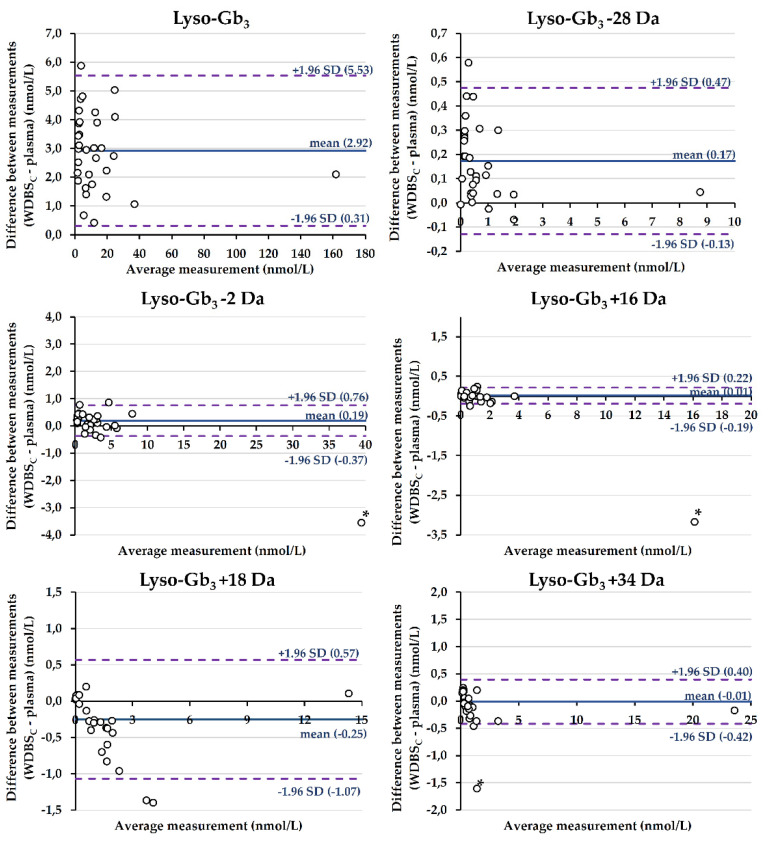
Bland–Altman plots are shown in Figure 2. The difference between the measurements obtained in the two matrices (plasma vs. DBS) was plotted against the average measurement. The mean difference ± standard deviation (95% limits of agreement) was 2.93 ± 1.33 nmol/L (0.31 to 5.53 nmol/L) for lyso-Gb3, 0.17 ± 0.15 nmol/L (−0.13 to 0.47 nmol/L) for lyso-Gb3 −28 Da, and 0.19 ± 0.29 nmol/L (−0.37 to 0.76 nmol/L) for lyso-Gb3 −2 Da. Regarding lyso-Gb3 +16 Da, lyso-Gb3 +18 Da, and lyso-Gb3 +34 Da, the mean differences ± standard deviation (95% limits of agreement) were 0.01 ± 0.10 nmol/L (−0.19 to 0.22 nmol/L), −0.25 ± 0.42 nmol/L (−1.07 to 0.57 nmol/L), and −0.01 ± 0.21 (−0.42 to 0.40 nmol/L), respectively.
Figure 2.
Bland–Altman analyses between lyso-Gb3 and related analogue measurements in plasma and in DBSs collected using Whatman-GE 903 filter papers (capillary blood) (n = 32 participants). Mean differences are shown by solid lines, while the dashed lines represent the limits of agreement. For lyso-Gb3 −2 Da, lyso-Gb3 +16 Da, and lyso-Gb3 +34 Da, an outlier (*) was excluded from the calculations of the mean difference and 95% limits of agreement.
2.3.2. Comparison of Biomarker Levels between Venous and Capillary Blood Matrices
A comparison of lyso-Gb3 and related analogue levels measured in DBSs obtained from venous and capillary blood was performed. The Deming regression parameters are shown in Table 11. There was no proportional bias between the venous and capillary blood matrices, considering that the 95% confidence interval of the slope included one for lyso-Gb3 and its five related analogues, except for slight deviations for lyso-Gb3 −28 Da (95% CI: 1.028 to 1.327) and lyso-Gb3 (+34) (95% CI: 1.058 to 1.097) in the DBSs obtained from Capitainer®B devices. The examination of the Y-intercept showed no systematic bias between the two matrices according to the Deming regression analysis, considering that the 95% confidence interval of the Y-intercept included 0 in all cases.
Table 11.
Deming regression analysis parameters for lyso-Gb3 and analogue measurements in venous and capillary blood.
| Biomarker | Collection Method | Slope | 95% CI (Slope) | Y-Intercept (nM) | 95% CI (Y-Intercept) | n |
|---|---|---|---|---|---|---|
| Lyso-Gb3 | Whatman 903 | 1.243 | 0.650–1.837 | −2.576 | −9.022–3.869 | 32 |
| Capitainer®B | 1.134 | 0.867–1.401 | −1.678 | −4.638–1.282 | 31 | |
| Lyso-Gb3 −28 Da | Whatman 903 | 1.288 | 0.598–1.977 | −0.153 | −0.556–0.250 | 32 |
| Capitainer®B | 1.178 | 1.028–1.327 | −0.066 | −0.156–0.023 | 31 | |
| Lyso-Gb3 −2 Da | Whatman 903 | 1.301 | 0.564–2.037 | −0.643 | −2.203–0.917 | 32 |
| Capitainer®B | 1.119 | 0.933–1.305 | −0.262 | −0.670–0.147 | 31 | |
| Lyso-Gb3 +16 Da | Whatman 903 | 1.233 | 0.617–1.850 | −0.183 | −0.595–0.229 | 32 |
| Capitainer®B | 1.070 | 0.853–1.286 | −0.055 | −0.209–0.099 | 31 | |
| Lyso-Gb3 +18 Da | Whatman 903 | 1.193 | 0.856–1.531 | −0.170 | −0.437–0.098 | 32 |
| Capitainer®B | 0.979 | 0.762–1.197 | 0.007 | −0.184–0.199 | 31 | |
| Lyso-Gb3 +34 Da | Whatman 903 | 1.225 | 0.548–1.903 | −0.170 | −0.595–0.255 | 32 |
| Capitainer®B | 1.077 | 1.058–1.097 | −0.010 | −0.055–0.035 | 31 |
CI: confidence interval.
2.3.3. Evaluation of the Hematocrit Effect
The effect of hematocrit on the correlation between the DBS and plasma biomarker measurements was evaluated using linear regression for both the Whatman-GE 903 and Capitainer®B collection devices. The difference between biomarker measurements in DBSs and plasma was plotted as a function of the hematocrit values. The linear regression parameters are shown in Table 12. The 95% CI of the slopes included 0 in all cases, except for lyso-Gb3 +16 Da in Capitainer®B (95% CI: −2.853 to −0.357 nM) and lyso-Gb3 +18 Da in Whatman-GE 903 (95% CI: 1.552 to 9.022 nM); thus, the differences between the DBS and plasma measurements did not change as a function of hematocrit in general. In all cases, the coefficients of determination (r2) were <0.218, and the p-values for testing the null hypothesis that the overall slope is zero were >0.001. There was thus no association noted between the DBS and plasma biomarker measurement differences and the hematocrit in the present cohort, where the hematocrit values ranged from 34.3 to 52.2%.
Table 12.
Simple linear regression parameters of the difference between DBSs and plasma biomarker measurements as a function of hematocrit for lyso-Gb3 and related analogues.
| Biomarker | Collection Method | Slope | 95% CI (Slope) | Y-Intercept (nM) | 95% CI (Y-Intercept) | r2 | p-Value | n |
|---|---|---|---|---|---|---|---|---|
| Lyso-Gb3 | Whatman 903 | 8.107 | −5.019–21.230 | −0.466 | −5.971–5.040 | 0.050 | 0.217 | 32 |
| Capitainer®B | −7.438 | −23.140–8.261 | 6.358 | −0.214–12.930 | 0.031 | 0.341 | 31 | |
| Lyso-Gb3 −28 Da | Whatman 903 | 1.272 | −0.217–2.762 | −0.358 | −0.983–0.266 | 0.092 | 0.091 | 32 |
| Capitainer®B | −0.439 | −1.538–0.660 | 0.309 | −0.149–0.767 | 0.023 | 0.420 | 30 | |
| Lyso-Gb3 −2 Da | Whatman 903 | −0.070 | −3.106–2.965 | 0.222 | −1.046–1.489 | 0.000 | 0.963 | 31 |
| Capitainer®B | −1.937 | −5.551–1.677 | 0.993 | −0.513–2.499 | 0.041 | 0.282 | 30 | |
| Lyso-Gb3 +16 Da | Whatman 903 | 0.130 | −0.967–1.226 | −0.041 | −0.499–0.417 | 0.002 | 0.811 | 31 |
| Capitainer®B | −1.605 | −2.853–(−0.357) | 0.702 | 0.182–1.222 | 0.199 | 0.014 | 30 | |
| Lyso-Gb3 +18 Da | Whatman 903 | 5.287 | 1.552–9.022 | −2.460 | −4.027–(−0.893) | 0.218 | 0.007 | 32 |
| Capitainer®B | 1.715 | −0.403–3.832 | −0.790 | −1.673–0.092 | 0.089 | 0.108 | 30 | |
| Lyso-Gb3 +34 Da | Whatman 903 | 1.434 | −0.608–3.475 | −0.610 | −1.468–0.247 | 0.066 | 0.162 | 31 |
| Capitainer®B | 0.981 | −0.805–2.767 | −0.465 | −1.210–0.279 | 0.043 | 0.270 | 30 |
CI: confidence interval.
3. Discussion
A method was devised for the analysis of lyso-Gb3 and five related analogues in DBSs. The validation of the assay was performed for both Whatman-GE 903 filter paper cards and in Capitainer®B collection devices, and it showed acceptable intra-day and inter-day accuracy and precision. We have found that the lyso-Gb3 standard could not be used to prepare the calibration curve in whole blood, considering that the lyso-Gb3 concentration measured in healthy controls was significant. To overcome this problem, the lyso-Gb3-13C6 standard was chosen instead of lyso-Gb3 to prepare the calibration curve in whole blood, and lyso-Gb3-Gly was used as the internal standard. Considering that the lyso-Gb3 analogues have different retention times compared to the calibration and internal standards, the matrix effect was evaluated across the entire chromatographic range using the post-column infusion method. No significant ion enhancement or ion suppression regions were observed. During the method development, we found that the SPE procedure was necessary for removing a contaminant present in the Capitainer®B device that was causing noise during the chromatography. The LODs and LOQs were evaluated and were in the same range as those observed previously in plasma. Normal reference values were estimated in capillary and venous blood for both collection devices. There was no difference between capillary and venous blood regarding the biomarker measurements obtained according to Deming’s regression, showing that the two matrices could be used interchangeably. The sensitivity of the assay in our cohort was good, especially when looking at the whole biomarker profile, including the analogues. The sensitivity and specificity of lyso-Gb3 +18 Da was 100% in this cohort. The Deming regression and Bland–Altman analyses showed that there was a systematic positive bias between the measurements obtained in DBSC compared to plasma for lyso-Gb3, lyso-Gb3 −28 Da, and lyso-Gb3 −2 Da. We hypothesized that this might be related to the presence of these molecules in red blood cells, considering that the reference values were much lower in plasma specimens from the same healthy controls. The effect of Hct was not significant in our cohort, potentially because there were no extreme Hct values (range: 34.3 to 52.2%). The main advantage of the Capitainer®B collection devices in this cohort was thus the sample quality, especially in a self-collection setting. There is always a risk that oversampling (multiple drops) or undersampling (insufficient specimen) would occur when using regular filter paper collection cards. In the present study, two DBSs collected with the regular filter cards were slightly insufficient, despite being collected by health care practitioners. The successful sampling indicator was also an appreciated feature on the Capitainer®B device and the full DBS disk was analyzed directly without the need to punch, which is convenient for the Hct effect correction. Lyso-Gb3 and related analogues were stable in DBSs subjected to various conditions, emphasizing the possibility of sending specimens by regular mail. The proposed assay might be useful for the high-risk screening, follow-up, and monitoring of patients affected with Fabry disease.
4. Materials and Methods
4.1. Subject Selection
A total of 20 patients affected with Fabry disease (8 males and 12 females) and 12 healthy controls (6 males and 6 females) were recruited for this study. Fabry disease was confirmed by an enzyme activity assay and/or mutation analysis. All study participants were older than 18 years of age.
4.2. Sample Collection and Storage
For all healthy controls and patients affected with Fabry disease, blood samples were collected by venipuncture into three 4 mL tubes containing potassium EDTA. The first tube was centrifuged at 2200 g for 10 min to retrieve plasma. The second tube was sent to the hematology laboratory to measure Hct, and the third tube was used to prepare the DBS samples. For this purpose, the blood was homogenized by gently inverting the tube 3 times. Thereafter, two blood drops of 50 µL were deposited on a Whatman-GE 903 (GE Healthcare, Chicago, IL, USA) filter paper to obtain two W-DBSV samples. Two CB-DBSV samples were prepared by dropping 30 µL of blood on two different ports of a Capitainer®B collection device (Capitainer Ab, Solna, Sweden). DBS samples were also prepared from capillary blood obtained by a fingertip puncture performed using a BD Microtainer® Contact-Activated Lancet (Becton, Dickinson and Company Limited, Dublin, Ireland). Two drops of blood were deposited on a Whatman-GE 903 filter paper to obtain two W-DBSC samples and two others on two different ports of a Capitainer®B collection device to obtain two CB-DBSC samples. All DBS samples were dried for a minimum of 4 h, then inserted in a hermetic plastic bag containing a desiccant pack of 1 g (GE Healthcare, Chicago, IL, USA). The plasma and DBS samples were stored at −20 °C prior to analysis.
4.3. Reagents
Carbon-13-labelled globotriaosylsphingosine (lyso-Gb3-13C6) was purchased from GelbChem (Seattle, WA, USA). N-glycinated-globotriaosylsphingosine (lyso-Gb3-Gly) was synthesized in-house [39], but is also commercially available at Matreya LLC (State College, PA, USA). HPLC-grade acetonitrile (ACN) was purchased from EMD Chemicals Inc. (Darmstadt, Germany). Formic acid (FA) (99+%) was from Acros Organics (Morris Plains, NJ, USA). A.C.S.-grade o-phosphoric acid (H3PO4) (85%) and ammonium hydroxide (NH4OH) (29%), as well as Optima LC/MS-grade H2O and methanol (MeOH), were from Fisher Scientific (Fair Lawn, NJ, USA).
4.4. Quality Controls and Calibration Curves
Low and high DBS QCs were made with blood from two Fabry patients to evaluate the analytical precision for lyso-Gb3 and its analogues. Since blood always contains a small amount of lyso-Gb3, even in healthy individuals, lyso-Gb3-13C6 was spiked into a pool of freshly collected blood to prepare the QCs used to evaluate the accuracy of the method and for the calibrators. Stock solutions of lyso-Gb3-13C6 (in 50% ACN/50% H2O, 0.1% FA) were prepared to obtain concentrations 50 times higher than those targeted for the QCs and calibrators. Thereafter, the stock solutions were diluted in a ratio of 1:50 with the pool of blood and were agitated for 15 min at 22 °C on a MP980153P7 orbital shaker from VWR (Radnor, PA, USA) at a speed of 200 RPM. Finally, the W-DBS and CB-DBS samples were prepared as described previously. The lyso-Gb3-13C6 concentrations for the low, medium, and high spiked QCs were 0.75, 75, and 250 nM, respectively, whereas the concentrations of the 8 calibrators were 0, 0.5, 1, 2, 10, 50, 200, and 400 nM. All the DBS QCs and calibrators were prepared at the same time with fresh blood and stored at −20 °C until analysis.
4.5. Sample Preparation
For each study participant, a 5 mm disc was punched from a W-DBS, and a whole DBS was removed using forceps from the Capitainer®B collection device. Each filter paper disc was deposited into a 2 mL polypropylene tube and extracted according to a method adapted from Polo et al. [40]. Briefly, 200 µL of extraction solution (80% MeOH/15% ACN/5% H2O) containing 1 nM of lyso-Gb3-Gly used as the internal standard (IS) was added to each tube. The samples were incubated for 60 min at 45 °C in a MP980153P7 orbital shaker from VWR (Radnor, PA, USA) at a speed of 500 RPM. Afterward, 100 µL of water and 325 µL of MeOH were added to each tube. After shaking, the solution was transferred to a glass tube and the paper disc was discarded. A volume of 500 µL of H3PO4 2% in water was added to each sample prior to its SPE purification using a mixed-mode strong cation exchange (MCX) cartridge (Oasis, 1 cc, 30 mg, LP) (Waters Corp., Milford, MA, USA) according to a protocol previously developed for the analysis of lyso-Gb3 and its analogues in plasma [19]. Briefly, the samples were loaded onto cartridges previously conditioned with 1 mL of MeOH and 1 mL of H3PO4 2%. The cartridges were then washed with 1 mL of 2% FA in water followed by 1 mL of 0.2% FA in MeOH, and the samples were eluted with 600 µL of 2% NH4OH in MeOH. Finally, the samples were evaporated under a nitrogen stream and 100 µL of the resuspension solution (50% ACN/50% H2O + 0.1% FA) was added prior to analysis by UHPLC-MS/MS.
4.6. UHPLC-MS/MS Analysis
Lyso-Gb3 and its analogues were analyzed in plasma by UHPLC-MS/MS according to a previously published procedure [19], which was then adapted for the analysis of the same molecules in DBS samples. For the DBS samples, the injection volume was increased from 7.5 to 10 µL and lyso-Gb3-13C6 was used for the calibration curve instead of lyso-Gb3. Unfortunately, it was not possible to analyze the lyso-Gb3 analogue +50 Da in DBS due to a lack of sensitivity and reproducibility. The analyses were performed on a Acquity I-Class ultra-performance liquid chromatography system (Waters Corp., Milford, MA, USA) coupled to a Xevo TQ-S tandem mass spectrometer (Waters Corp., Milford, MA, USA) operated in the multiple reaction monitoring (MRM) mode. The UHPLC-MS/MS results were analyzed with the TargetLynx V4.2 (SCN982) software (Waters Corp., Milford, MA, USA). The calibration curve was quadratic, the origin was excluded, and a 1/x weighing factor was applied.
4.7. Method Validation
Method validation was performed in parallel using the W-DBS and CB-DBS QCs. The intra-day (n = 5) and inter-day precision (n = 5), the stability at −20, 4 and 22 °C (n = 2), the stability of prepared samples at 4 °C in the autosampler (n = 2), and the stability after five freeze/thaw cycles (n = 2) were evaluated using the patient (p) and spiked (s) QCs. A freeze/thaw cycle consisted of leaving the sample for 1 hr in the freezer followed by one hour at 22 °C. It is noteworthy to mention that the pQCs used as part of the method validation were collected using a previous model of the Capitainer®B collection device with a sampling volume of 13.5 µL instead of 10 µL. The intra-day (n = 5) and inter-day (n = 5) accuracy was measured for lyso-Gb3 using the sQCs. For each analyte, the LODs and LOQs were defined as 3 and 10 times the background noise, respectively. The adhesion to plastic and glassware was previously evaluated [19]. To measure the lyso-Gb3 extraction recovery, the three sQCs (n = 5) and control samples (DBS without lyso-Gb3-13C6) (n = 15) were extracted without IS. Just before the SPE, the control samples were spiked with the amount of lyso-Gb3-13C6 contained in the different QCs. The IS was also spiked in all the samples (sQCs and control samples) just before the SPE procedure. Then, the samples were processed as described earlier and the lyso-Gb3-13C6 concentrations measured in the sQCs were compared to the ones obtained for the spiked controls. A similar approach was used to evaluate the recovery from the SPE procedure. This time, 30 control samples were extracted without IS. For each of the three sQC concentrations, 5 control samples were spiked before the SPE and 5 others were spiked after. For all the samples, the IS was added after the SPE procedure. The recovery was obtained by comparing the lyso-Gb3-13C6 concentrations in the samples spiked before the SPE with the ones spiked after. The stability of the blood pools spiked with lyso-Gb3-13C6 and used as sQCs was tested by preparing DBS samples just after their mixture (n = 3) and 3 h later (n = 3). The matrix effect was evaluated by post-column infusion. Lyso-Gb3-13C6 (500 nM in 50% ACN/50% H2O + 0.1% FA) was infused at a flow rate of 10 µL/minute and combined with the effluent of the UHPLC system during the analysis of a blank (reconstitution solution) and control W-DBS and CB-DBS samples.
4.8. Statistical Analyses
Statistical analyses were performed using GraphPad Prism v9.4.1. (Graphpad Software, San Diego, CA, USA). The Deming regression and a Bland–Altman analysis were used to compare biomarker measurements obtained in plasma and W-DBSC. The difference between W-DBSC and plasma measurements was plotted against the mean biomarker measurements. The Deming regression was used to compare the biomarker measurements obtained in capillary and venous blood in both W-DBS and CB-DBS. The effect of Hct on the difference between the biomarker measurements obtained in DBSs and plasma was evaluated using linear regression. The sensitivity of the assay was calculated as follows: TP/(TP+FN), where TP = true positives and FN = false negatives, while the specificity was calculated as follows: TN/(TN+FP), where TN = true negatives and FP = false positives. The normality of the distributions was assessed using the Shapiro–Wilk test and outliers were identified using Grubbs’s test. The statistical significance was set at p < 0.001.
Acknowledgments
The authors would like to thank Waters Corp. for their scientific support and partnership through the Waters Centre of Innovation, directed by Christiane Auray-Blais. We would like to thank Sanofi Genzyme for the supporting grant. The authors are also grateful to Carole Fortier and Caroline Barr for sample collection and Marie-Françoise Arthus for the REB documents. We would also like to thank Capitainer Ab for providing the Capitainer®B collection kits.
Author Contributions
Conceptualization, M.B. (Michel Boutin), P.L. and C.A.-B.; methodology, M.B. (Michel Boutin), P.L. and C.A.-B.; validation, M.B. (Michel Boutin), M.B. (Margot Beaudon) and G.K.N.; formal analysis, M.B. (Michel Boutin), P.L. and M.B. (Margot Beaudon); investigation, M.B. (Michel Boutin), P.L., C.A.-B., D.G.B. and B.M.; writing—original draft preparation, M.B. (Michel Boutin) and P.L.; resources, C.A.-B., D.G.B. and B.M.; writing—review and editing, M.B. (Michel Boutin), P.L., C.A.-B., G.K.N., M.B. (Margot Beaudon), D.G.B. and B.M.; visualization, M.B. (Michel Boutin), P.L., C.A.-B., G.K.N., M.B. (Margot Beaudon), D.G.B. and B.M.; supervision, C.A.-B.; funding acquisition, C.A.-B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of CIUSSS de l’Estrie-CHUS (Project #MP-31-2020-3345, authorization obtained on 17 October 2019).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Not applicable.
Conflicts of Interest
Capitainer®B collection kits were provided free of charge by Capitainer Ab. Capitainer Ab and Sanofi Genzyme had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish these results.
Funding Statement
This research was funded by Sanofi Genzyme and personal research funds from C. Auray-Blais.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Clarke J.T. Narrative review: Fabry disease. Ann. Intern. Med. 2007;146:425–433. doi: 10.7326/0003-4819-146-6-200703200-00007. [DOI] [PubMed] [Google Scholar]
- 2.Najafian B., Tøndel C., Svarstad E., Gubler M.-C., Oliveira J.-P., Mauer M. Accumulation of globotriaosylceramide in podocytes in Fabry nephropathy is associated with progressive podocyte loss. J. Am. Soc. Nephrol. 2020;31:865–875. doi: 10.1681/ASN.2019050497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hsu M.-J., Chang F.-P., Lu Y.-H., Hung S.-C., Wang Y.-C., Yang A.-H., Lee H.-J., Sung S.-H., Wang Y.-F., Yu W.-C., et al. Identification of lysosomal and extralysosomal globotriaosylceramide (Gb3) accumulations before the occurrence of typical pathological changes in the endomyocardial biopsies of Fabry disease patients. Genet. Med. 2019;21:22–232. doi: 10.1038/s41436-018-0010-z. [DOI] [PubMed] [Google Scholar]
- 4.Toupin A., Lavoie P., Arthus M.F., Abaoui M., Boutin M., Fortier C., Ménard C., Bichet D.G., Auray-Blais C. Analysis of globotriaosylceramide (Gb3) isoforms/analogs in unfractionated leukocytes, B lymphocytes and monocytes from Fabry patients using ultra-high performance liquid chromatography/tandem mass spectrometry. Anal. Chim. Acta. 2018;1015:35–49. doi: 10.1016/j.aca.2018.02.022. [DOI] [PubMed] [Google Scholar]
- 5.Manwaring V., Boutin M., Auray-Blais C. A Metabolomic study to identify new globotriaosylceramide-related biomarkers in the plasma of Fabry disease patients. Anal. Chem. 2013;85:9039–9048. doi: 10.1021/ac401542k. [DOI] [PubMed] [Google Scholar]
- 6.Auray-Blais C., Boutin M. Novel gb(3) isoforms detected in urine of fabry disease patients: A metabolomic study. Curr. Med. Chem. 2012;19:3241–3252. doi: 10.2174/092986712800784739. [DOI] [PubMed] [Google Scholar]
- 7.Auray-Blais C., Boutin M., Gagnon R., Dupont F.O., Lavoie P., Clarke J.T. Urinary globotriaosylsphingosine-related biomarkers for Fabry disease targeted by metabolomics. Anal. Chem. 2012;84:2745–2753. doi: 10.1021/ac203433e. [DOI] [PubMed] [Google Scholar]
- 8.Dupont F.O., Gagnon R., Boutin M., Auray-Blais C. A metabolomic study reveals novel plasma lyso-Gb3 analogs as Fabry disease biomarkers. Curr. Med. Chem. 2013;20:280–288. doi: 10.2174/092986713804806685. [DOI] [PubMed] [Google Scholar]
- 9.Boutin M., Auray-Blais C. Metabolomic discovery of novel urinary galabiosylceramide analogs as Fabry disease biomarkers. J. Am. Soc. Mass Spectrom. 2015;26:499–510. doi: 10.1007/s13361-014-1060-3. [DOI] [PubMed] [Google Scholar]
- 10.Touboul D., Roy S., Germain D.P., Baillet A., Brion F., Prognon P., Chaminade P., Laprévote O. Fast fingerprinting by MALDI-TOF mass spectrometry of urinary sediment glycosphingolipids in Fabry disease. Anal. Bioanal. Chem. 2005;382:1209–1216. doi: 10.1007/s00216-005-3239-8. [DOI] [PubMed] [Google Scholar]
- 11.Muntean C., Starcea I.M., Stoica C., Banescu C. Clinical characteristics, renal involvement, and therapeutic options of pediatric patients with Fabry disease. Front. Pediatr. 2022;10:908657. doi: 10.3389/fped.2022.908657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paim-Marques L., de Oliveira R.J., Appenzeller S. Multidisciplinary management of Fabry disease: Current perspectives. J. Multidiscip. Healthc. 2022;15:485–495. doi: 10.2147/JMDH.S290580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang R.Y., Lelis A., Mirocha J., Wilcox W.R. Heterozygous Fabry women are not just carriers, but have a significant burden of disease and impaired quality of life. Genet. Med. 2007;9:34–45. doi: 10.1097/GIM.0b013e31802d8321. [DOI] [PubMed] [Google Scholar]
- 14.Lenders M., Brand E. Fabry disease: The current treatment landscape. Drugs. 2021;81:635–645. doi: 10.1007/s40265-021-01486-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Weidemann F., Jovanovic A., Herrmann K., Vardarli I. Chaperone therapy in Fabry disease. Int. J. Mol. Sci. 2022;23:1887. doi: 10.3390/ijms23031887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Domm J.M., Wootton S.K., Medin J.A., West M.L. Gene therapy for Fabry disease: Progress, challenges, and outlooks on gene-editing. Mol. Genet. Metab. 2021;134:117–131. doi: 10.1016/j.ymgme.2021.07.006. [DOI] [PubMed] [Google Scholar]
- 17.van der Veen S.J., Hollak C.E.M., van Kuilenburg A.B.P., Langeveld M. Developments in the treatment of Fabry disease. J. Inherit. Metab. Dis. 2020;43:908–921. doi: 10.1002/jimd.12228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Azevedo O., Gago M.F., Miltenberger-Miltenyi G., Sousa N., Cunha D. Fabry disease therapy: State-of-the-art and current challenges. Int. J. Mol. Sci. 2020;22:206. doi: 10.3390/ijms22010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boutin M., Auray-Blais C. Multiplex tandem mass spectrometry analysis of novel plasma lyso-Gb3-related analogues in Fabry disease. Anal. Chem. 2014;86:3476–3483. doi: 10.1021/ac404000d. [DOI] [PubMed] [Google Scholar]
- 20.Auray-Blais C., Lavoie P., Boutin M., Ntwari A., Hsu T.R., Huang C.K., Niu D.M. Biomarkers associated with clinical manifestations in Fabry disease with a late-onset cardiac variant mutation. Clin. Chim. Acta. 2017;466:185–193. doi: 10.1016/j.cca.2017.01.018. [DOI] [PubMed] [Google Scholar]
- 21.Nowak A., Beuschlein F., Sivasubramaniam V., Kasper D., Warnock D.G. Lyso-Gb3 associates with adverse long-term outcome in patients with Fabry disease. J. Med. Genet. 2022;59:287–293. doi: 10.1136/jmedgenet-2020-107338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lenders M., Stappers F., Niemietz C., Schmitz B., Boutin M., Ballmaier P.J., Zibert A., Schmidt H., Brand S.-M., Auray-Blais C., et al. Mutation-specific Fabry disease patient-derived cell model to evaluate the amenability to chaperone therapy. J. Med. Genet. 2019;56:548–556. doi: 10.1136/jmedgenet-2019-106005. [DOI] [PubMed] [Google Scholar]
- 23.Germain D.P., Altarescu G., Barriales-Villa R., Mignani R., Pawlaczyk K., Pieruzzi F., Terryn W., Vujkovac B., Ortiz A. An expert consensus on practical clinical recommendations and guidance for patients with classic Fabry disease. Mol. Genet. Metab. 2022;137:49–61. doi: 10.1016/j.ymgme.2022.07.010. [DOI] [PubMed] [Google Scholar]
- 24.Malvagia S., Ferri L., Della Bona M., Borsini W., Cirami C.L., Dervishi E., Feriozzi S., Gasperini S., Motta S., Mignani R., et al. Multicenter evaluation of use of dried blood spot compared to conventional plasma in measurements of globotriaosylsphingosine (LysoGb3) concentration in 104 Fabry patients. Clin. Chem. Lab. Med. 2021;59:1516–1526. doi: 10.1515/cclm-2021-0316. [DOI] [PubMed] [Google Scholar]
- 25.Delarosa-Rodríguez R., Santotoribio J.D., Paula H.A., González-Meneses A., García-Morillo S., Jiménez-Arriscado P., Guerrero J.M., Macher H.C. Accuracy diagnosis improvement of Fabry disease from dried blood spots: Enzyme activity, lyso-Gb3 accumulation and GLA gene sequencing. Clin. Genet. 2021;99:761–771. doi: 10.1111/cge.13936. [DOI] [PubMed] [Google Scholar]
- 26.Olivera S., Iñiguez C., García-Fernández L., Sierra J.L., Camón A.M., Menao S., Torralba M.Á. Usefulness of lyso-globotriaosylsphingosine in dried blood spots in the differential diagnosis between multiple sclerosis and Anderson-Fabry’s disease. Mult. Scler. Relat. Disord. 2020;38:101466. doi: 10.1016/j.msard.2019.101466. [DOI] [PubMed] [Google Scholar]
- 27.Nowak A., Mechtler T., Kasper D.C., Desnick R.J. Correlation of Lyso-Gb3 levels in dried blood spots and sera from patients with classic and later-onset Fabry disease. Mol. Genet. Metab. 2017;121:320–324. doi: 10.1016/j.ymgme.2017.06.006. [DOI] [PubMed] [Google Scholar]
- 28.Gatterer C., Gaggl M., Mundigler G., Rommer P., Graf S., Sunder-Plassmann G. Agreement of dried blood spot lyso-Gb3 concentrations obtained from different laboratories in patients with Fabry disease. Clin. Chem. Lab. Med. 2020;58:e275–e278. doi: 10.1515/cclm-2020-0588. [DOI] [PubMed] [Google Scholar]
- 29.Zakaria R., Allen K.J., Koplin J.J., Roche P., Greaves R.F. Advantages and challenges of dried blood spot analysis by mass spectrometry across the total testing process. EJIFCC. 2016;27:288–317. [PMC free article] [PubMed] [Google Scholar]
- 30.Ostler M.W., Porter J.H., Buxton O.M. Dried blood spot collection of health biomarkers to maximize participation in population studies. J. Vis. Exp. 2014;83:e50973. doi: 10.3791/50973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Malsagova K., Kopylov A., Stepanov A., Butkova T., Izotov A., Kaysheva A. Dried blood spot in the laboratory: Directions and prospects. Diagnostics. 2020;10:248. doi: 10.3390/diagnostics10040248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Su X., Carlson B.F., Wang X., Li X., Zhang Y., Montgomery J.P., Ding Y., Wagner A.L., Gillespie B., Boulton M.L. Dried blood spots: An evaluation of utility in the field. J. Infect. Public Health. 2018;11:373–376. doi: 10.1016/j.jiph.2017.09.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Velghe S., Delahaye L., Stove C.P. Is the hematocrit still an issue in quantitative dried blood spot analysis? J. Pharm. Biomed. Anal. 2019;163:188–196. doi: 10.1016/j.jpba.2018.10.010. [DOI] [PubMed] [Google Scholar]
- 34.Neto R., Gooley A., Breadmore M.C., Hilder E.F., Lapierre F. Precise, accurate and user-independent blood collection system for dried blood spot sample preparation. Anal. Bioanal. Chem. 2018;410:3315–3323. doi: 10.1007/s00216-018-0993-y. [DOI] [PubMed] [Google Scholar]
- 35.Carling R.S., Emmett E.C., Moat S.J. Evaluation of volumetric collection devices for the measurement of phenylalanine and tyrosine to monitor patients with phenylketonuria. Clin. Chim. Acta. 2022;535:157–166. doi: 10.1016/j.cca.2022.08.005. [DOI] [PubMed] [Google Scholar]
- 36.Mazzarino M., Di Costanzo L., Comunità F., Stacchini C., de la Torre X., Botrè F. UHPLC-HRMS method for the simultaneous screening of 235 drugs in capillary blood for doping control purpose: Comparative evaluation of volumetric and non-volumetric dried blood spotting devices. ACS Omega. 2022;7:31845–31868. doi: 10.1021/acsomega.2c01417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Delahaye L., Veenhof H., Koch B.C.P., Alffenaar J.C., Linden R., Stove C. Alternative sampling devices to collect dried blood microsamples: State-of-the-art. Ther. Drug Monit. 2021;43:310–321. doi: 10.1097/FTD.0000000000000864. [DOI] [PubMed] [Google Scholar]
- 38.Velghe S., Stove C.P. Evaluation of the Capitainer-B microfluidic device as a new hematocrit-independent alternative for dried blood spot collection. Anal. Chem. 2018;90:12893–12899. doi: 10.1021/acs.analchem.8b03512. [DOI] [PubMed] [Google Scholar]
- 39.Lavoie P., Boutin M., Auray-Blais C. Multiplex analysis of novel urinary lyso-Gb3-related biomarkers for Fabry disease by tandem mass spectrometry. Anal. Chem. 2013;85:1743–1752. doi: 10.1021/ac303033v. [DOI] [PubMed] [Google Scholar]
- 40.Polo G., Burlina A.P., Ranieri E., Colucci F., Rubert L., Pascarella A., Duro G., Tummolo A., Padoan A., Plebani M., et al. Plasma and dried blood spot lysosphingolipids for the diagnosis of different sphingolipidoses: A comparative study. Clin. Chem. Lab. Med. 2019;57:1863–1874. doi: 10.1515/cclm-2018-1301. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.