Abstract
Hand, foot, and mouth disease (HFMD) is a mild exanthematous, febrile disease, but it also remains a threat to global public health. HFMD is characterized by a brief febrile illness in children and with a typical skin rash of the hand and foot, with or without mouth ulcers. However, the morphology and distribution of vesicles, as well as accompanying symptoms, are varied among atypical HFMD. An upsurge in atypical presentations of HFMD caused by Coxsackievirus A6 (CVA6), including Gianotti–Crosti-like eruptions, eczema coxsackium, petechial/purpuric eruption, and vesiculobullous exanthema, can be difficult to diagnose clinically as it may mimic other severe skin diseases, such as eczema herpeticum, varicella, disseminated zoster, and erythema multiforme major. The recognition of the distinguishing features of atypical HFMD is vital for an accurate and timely diagnosis, as is initiating appropriate laboratory evaluation and supportive care. Clinicians must identify the wide range of cutaneous and mucosal alterations caused by atypical HFMD. A systemic, high-quality overview of atypical HFMD is needed for advances in better strategies for clinical diagnosis and treatment. Hence, this review is aimed at summarizing the available data on clinical investigations and differential diagnostics to provide a scientific guide for the timely diagnosis of HFMD for preventing serious complications.
Keywords: HFMD, atypical manifestations, CVA6, differential diagnosis, genetic evolution
1. Introduction
Hand, foot, and mouth disease (HFMD) is a highly contagious, globally epidemic disease that primarily affects children under 5 years old [1]. Apart from general symptoms such as fever, the most typical symptom is a rash on specific areas such as the hands, feet or/and mouth [2]. Currently, HFMD has received great attention due to increasing evidence that the clinical, epidemiological and etiological features of the disease are significantly different from those initially thought [3]. Although HFMD is usually a mild disease, neurological complications may occur during large-scale epidemics involving HFMD. A few numbers of patients may develop serious and even life-threatening complications within a short time [4,5]. Moreover, an increasing number of studies have reported that atypical HFMD is becoming a new concern for children’s health [6]. At present, there is no official definition for atypical HFMD, but some studies have defined that it occurs at anatomic sites not listed in the World Health Organization HFMD definition [7]. Many pathogens that can cause atypical HFMD have been reported, such as Coxsackievirus A (CVA) 6, CVA16, CVA4, CVB4, CVB5, and CVB15 [8,9], but particularly CVA6. Currently, CVA6, one of the most prevalent strains in the world; it is also a new variant that has undergone genetic recombination and evolution. Recombination may not only cause variation in the clinical symptoms of HFMD; it may also increase the pathogenicity due to the accumulation of nucleotide mutations [10]. Compared with typical virus strains, the new mutant strains affect a broader population and cause more severe disease with a longer duration [11].
The skin lesions of atypical HFMD in the early stage are very similar to the clinical manifestations of other diseases, such as eczema. However, the early diagnosis of HFMD is mainly based on physical examination, which poses a challenge for clinicians aiming to identify and diagnose HFMD in a timely manner. It can lead to misdiagnosis as well as serious complications, ultimately leading to regional or large-scale epidemics [8]. Therefore, improving the ability of clinicians to identify atypical HFMD is a critical component of clinical practice. The current literature is mostly limited to reports of clinical data but lacks systematic reviews and summaries, especially for differential diagnosis. Therefore, this review focuses on the atypical presentations of HFMD and the differential diagnosis with other similar diseases, providing a scientific basis for the timely diagnosis of HFMD and the prevention of serious complications.
2. Literature Search Strategy
Sources consulted for this article included PubMed from the National Library of Medicine, the Centers for Disease Control and Prevention, and the World Health Organization. Search terms included (atypical hand, foot, and mouth disease) OR (atypical HFMD); (atypical presentations/manifestations/characters/clinical features and hand, foot, and mouth disease) OR (atypical presentations/manifestations/characters/clinical features and HFMD). Diseases involved in the differential diagnosis were summarized based on the literature presenting atypical presentation of HFMD. The articles selected for this study were published before December 2022. We also searched for relevant reviews to determine that there were no similar published articles. Then, we performed a manual search of the reference lists from the eligible articles and identified articles to complete our search.
3. Inclusion and Exclusion Criteria
The inclusion criteria were: (1) only peer-reviewed, published studies were included, and the subjects were clearly confirmed HFMD cases; (2) the study included atypical clinical features/presentations/manifestations/characteristics of HFMD.
The exclusion criteria of the studies were as follows: (1) abstract-only articles, editorials, duplicated publications, and so on; (2) data and information in the article were incomplete, suspicious, or inconsistent; (3) the full text could not be accessed; and (4) animal experiments.
4. Gianotti Crosti-like eruptions
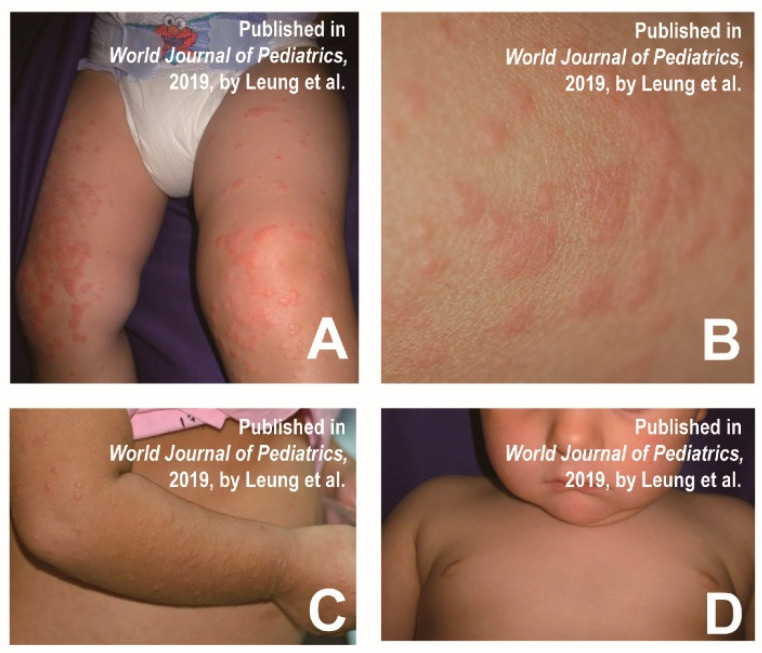
Mathes et al. found that a few cases with HFMD had a similar distribution of skin lesions to Gianotti–Crosti syndrome, involving the cheeks, extremities, and buttocks, but the trunk remained unaffected [9]. Typical Gianotti–Crosti syndrome is characterized by monomorphic lichenoid papules and/or papular vesicles, whereas the 2011–2012 North American outbreak was more common in papular sacs with significant erosions. Epstein–Barr virus and hepatitis B virus are the most common causative agents of Crostyanoti’s disease [9], but other viruses (e.g., CVA16, CVB4, and CVB15) are less commonly involved [12]. Diagnostic criteria for Gianotti–Crosti syndrome include the following symptoms: lesions lasting at least 10 days; papules or papular injuries of 1 mm to 10 mm involving three of the following four areas: extensor side of the forearm, extensor side of the leg, cheek or buttocks; and symmetrical distribution of lesions. The clinical presentation is characterized by small, symmetrical isomorphic papules or pimples on the adductor surfaces of the cheeks, ears, arms, and legs, and buttocks (Figure 1A–D) [13]. HFMD patients representing Gianotti–Crosti-like eruptions are more febrile and generally suffer from oral mucosal stomatitis. Papules are more symmetrically distributed on the face, arms and legs, but relatively sparse on the trunk. The rash and blisters are often accompanied with pain and itching.
Figure 1.
(A) Multiple individual, flat-topped, erythematous papules on the external surface of the lower limbs. (B) Well-defined and non-squamous papules on the right thigh. (C) Multiple monomorphic, flat-topped, erythematous papules on the external surface of the upper limb. (D) Monomorphic erythematous papules on the face, with sparing of the trunk. Reprinted/adapted with permission from Ref. [13]. 2019, Leung et al.
In addition, Gianotti–Crosti-like eruptions of HFMD may be mistaken for other diseases, such as papulosquamous urticaria and erythema infectiosum [13,14]. Papulosquamous urticaria presents with no fever, groups of or disseminated urticarial pimples, vesicles or wheals, exfoliated pruritus and large blisters developing from small blisters [14]. Erythema infectiosum initially presents as an erythematous rash on the cheeks and can progress to diffuse macular erythema. It is accompanied by paroxysmal pruritus, which is mostly worse at night [14]. In addition, the syndrome is associated with bacterial or viral infections, such as β hemolytic streptococcus, mycoplasma pneumoniae, bartonella aureus, hepatitis A virus, cytomegalovirus, herpesvirus type 6, coxsackievirus, rotavirus, parvovirus B19, and human respiratory syncytial virus.
Once a patient presents similar symptoms to Gianotti–Crosti syndrome, it is required to distinguish the associated causative agents described above. Because the pathogens are different, etiological detection is necessary and effective in order to confirm the diagnosis. The pictures below show the typical signs of Gianotti–Crosti syndrome, which is based on the case reports of 2 children with Gianotti–Crosti syndrome (Figure 1). Table 1 shows the main differential diagnoses of Gianotti–Crosti-like eruptions and some similar diseases.
Table 1.
Differential diagnosis of Gianotti–Crosti-like eruptions.
| Differential Points | Gianotti–Crosti-Like Eruptions | Gianotti–Crosti Eruptions | Papulosquamous Urticaria | Erythema Infectiosum |
|---|---|---|---|---|
| Fever |
|
|
|
|
| Skin rash |
|
|
|
|
| Itching |
|
|
|
|
| Blisters |
|
|
|
|
| Recurrence |
|
|
|
|
| Duration |
|
|
|
|
| Pathogens |
|
|
|
|
| Prevalence age |
|
|
|
|
5. Eczema Coxsackium
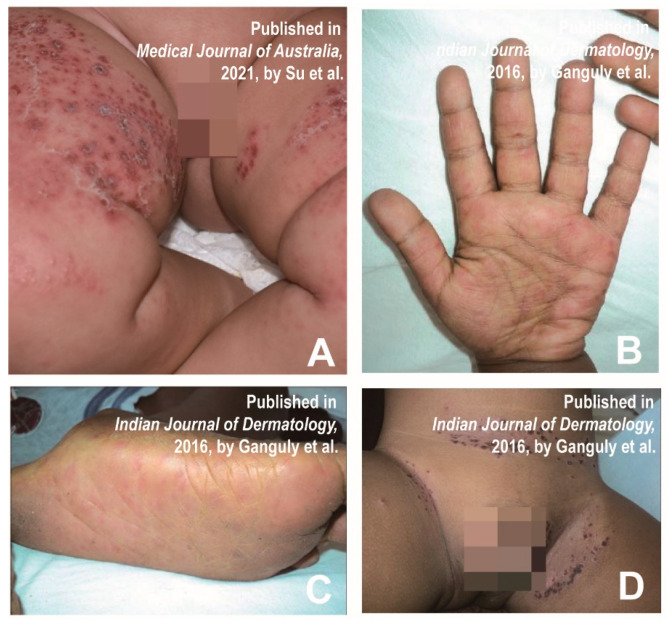
In 1968, a child patient with CVA16 infection presented with a severe widespread papular rash named eczema coxsackium [20]. Eczema coxsackium is clinically characterized by blisters in the area of eczematous dermatitis, which is similar to herpetic eczema caused by the herpes simplex virus (HSV) [21]. In addition, the lesions are more severe at the bends, such as the wrinkled skin of the arms and legs [22]. Most patients have a rash that shows signs of secondary bacterial infection [22]. The skin pattern consists of relatively monomorphic vesicles and/or blisters concentrated in areas that are affected by atopic dermatitis [12]. A girl who was diagnosed with coxsackie eczema presented multiple erythematous papules and blisters around the mouth, trunk and extremities (Figure 2A) [23]. A 3-year-old male patient with multiple elongated blisters on the palms of hands and fingers surrounded by erythema (Figure 2B), as well as on the soles of his feet (Figure 2C) and on his knees, was reported in a previous study. A little erythematous erosion could be seen on the hard palate. Additionally, numerous partially fused crusted papules with a few blisters surrounded by erythema and maceration were observed in the groin, abdominal crease, and scrotum (Figure 2D) [21].
Figure 2.
(A) Multiple erythematous papules and blisters with crusts on the extremities. Reprinted/adapted with permission from Ref. [23].2021, Su et al. (B) Elongated papulovesicles over palm and palmar aspect of fingers. (C) Erythematous papulovesicles over soles. (D) Crusted papules, vesicles and surrounding erythema and maceration in the groins, abdominal folds, and scrotum. Subfigures (B–D) were reprinted/adapted with permission from Ref. [21]. 2016, Ganguly et al.
As eczema coxsackium resembles herpetic eczema, overlapping varicella caused by HSV, the differential diagnosis generally includes varicella-zoster virus infection, herpetic eczema and herpetic impetigo [24]. If necessary, causative agents in the blister fluid could be detected by nucleic acid testing [23]. Table 2 shows the main differential diagnoses of eczema coxsackium and some similar diseases.
Table 2.
Differential diagnosis of eczema coxsackium.
| Differential Points | Eczema Coxsackium | Herpes Zoster | Herpetic Eczema |
|---|---|---|---|
| Fever |
|
|
|
| Skin rash |
|
|
|
| Pain or itching |
|
|
|
| Complications |
|
|
|
| Recurrence |
|
|
|
| Duration |
|
|
|
| Pathogens |
|
|
|
| Prevalence age |
|
|
|
6. Petechial/Purpuric Eruptions
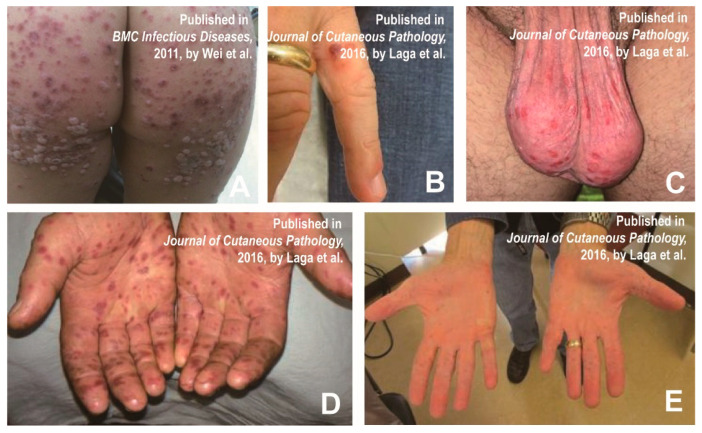
Petechial/purpuric eruptions consisting of relatively single blisters or concentrated blisters in some areas affected by atopic dermatitis or hemorrhagic or purpuric lesions are common atypical presentations of HFMD in children [12]. Some laboratory-confirmed CVA6-infected cases had a generalized purpuric rash around their mouth, trunk and/or neck, and feet, in addition to the typical rash on the hands. Profuse blistering and purpuric rash on a boy’s buttocks are shown in Figure 3A [28]. Laga et al. also reported atypical HFMD associated with CVA6 in adults, presenting erythematous purpura on the hands and scrotum (Figure 3B–E) [29]. In addition, leukocytoclastic vasculitis, as well as glove-and-stocking purpura (parvovirus infection), also have similar symptoms. HFMD is diagnosed clinically mainly by symptoms and pathogenic testing and does not require biopsy [29]. The histopathology of HFMD showed: spongiosis, neutrophilic exocytosis, massive keratinocyte necrosis, shadow cells in the upper epidermis, the vacuolization of basal cells, necrotic cells in follicles and sweat glands, dense superficial dermal infiltrates of CD3 lymphocytes, and strong granulysin expression [30]. Although pathological examination is not routinely used in clinical practice, leukocytoclastic vasculitis and glove and stocking purpura can also be distinguished by histopathology, as they are caused by other viral infections, subepidermal immunodeficiency or nutritional deficiencies [31,32]. Table 3 shows the main differential diagnoses of petechial/purpuric eruptions and some similar diseases.
Figure 3.
(A) A boy experienced prominent skin eruptions and vesicles on his buttocks. Reprinted/adapted with permission from Ref. [28]. 2011, Wei et al. (B–E) Erythematous purpura of adult patients and scattered blisters on the palms and scrotum. Reprinted/adapted with permission from Ref. [29]. 2016, Laga et al.
Table 3.
Differential diagnosis of petechial/purpuric eruptions.
| Differential Points | Petechial/Purpuric Eruptions | Leukocytoclastic Vasculitis | Glove and Stocking Purpura |
|---|---|---|---|
| Fever |
|
|
|
| Skin rash |
|
|
|
| Itching |
|
|
|
| Recurrence |
|
|
|
| Duration |
|
|
|
| Histopathological features |
|
|
|
| Pathogens |
|
|
|
| Prevalence age |
|
|
|
7. Vesiculobullous Exanthema
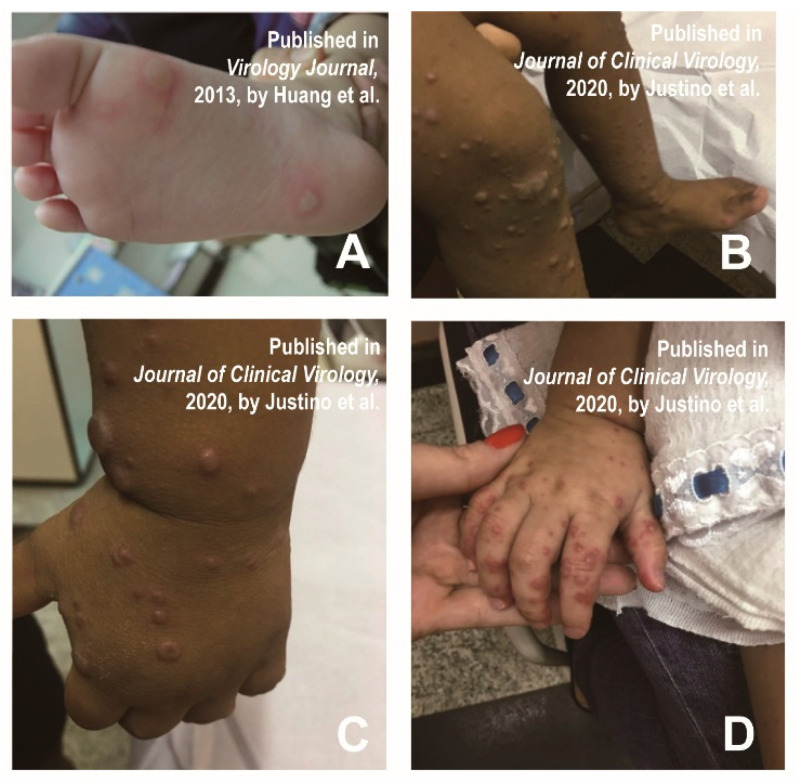
Since the spring of 2010, infantile HFMD infections from China and Taiwan have experienced an atypical skin injury, including large blisters on limbs, trunk or buttocks, and facial papules accompanied by fever, stomatitis and sore throat [35]. Successively, patients with bullous lesions were reported [12,35,36]. Huang et al. found that a 5-year-old male child had bullae on the bottom of his feet, causing slight itching and tingling (Figure 4A) [35]. Atypical lesions were most common on the face (41%), buttocks (31%), and trunk (29%). Bubble-like eruptions were also observed on the thigh, although it was rare in typical HFMD [35]. The large vesicle group was defined as vascular bullous lesions ≥ 1cm in diameter [35]. Mathes et al. found that 2 of the 80 patients presented vesiculobullous exanthema [12]. In a study of the clinical manifestation of 40 HFMD patients, 70% of them had bullous lesions on their faces, extremities (knees and/or elbows), buttocks, and necks lasting approximately two weeks (Figure 4B–D) [36].
Figure 4.
(A) Bullae were formed on a sole, accompanied by mild pruritus and prickling sensations. Reprinted/adapted with permission from Ref. [35]. 2013, Huang et al. (B–D) Bullous lesions on the extremities (knees and/or elbows) in patients with atypical HFMD. Reprinted/adapted with permission from Ref. [36]. 2020, Justino et al.
In addition, the clinical presentation of vesiculobullous exanthema is similar to bullous impetigo, varicella and primary immune bullous, making it susceptible to misdiagnosis [16]. The clinicians can make a differential diagnosis by distinguishing the clinical manifestations, as well as symptom sites. Table 4 shows the main differential diagnoses of vesiculobullous exanthema and some similar diseases.
Table 4.
Differential diagnosis of vesiculobullous exanthema.
| Differential Points | Vesiculobullous Exanthema | Bullous Impetigo | Varicella | Primary Immune Bullous (Take Bullous Pemphigoid, for Example) |
|---|---|---|---|---|
| Fever |
|
|
|
|
| Clinical Presentation |
|
|
|
|
| Preferred site |
|
|
|
|
| Itching |
|
|
|
|
| Recurrence |
|
|
|
|
| Duration |
|
|
|
|
| Pathogens |
|
|
|
|
| Prevalence age |
|
|
|
|
8. Tomato Flu
Tomato flu, possibly caused by a variant HFMD-related pathogen, has been reported recently in India [43]. Recently, it was speculated that the etiological agent that caused ‘tomato flu’ was not a novel virus but an evolutionary CVA16 [43]. With the emergence of new pathogens or the recombination and evolution of classical viruses, the clinical characteristics of HFMD may also change a lot, which poses a challenge for correct and timely diagnosis.
Unlike typical HFMD (mainly diagnosed according to physical examination and medical history), atypical cases may require laboratory examination [44]. Diagnosis can be confirmed by PCR (serum; oropharyngeal and skin swabs). If necessary, viral and bacterial cultures, direct antibody tests for viruses and skin biopsies can also be used to exclude other possibilities.
9. Geographical Distribution of Atypical HFMD
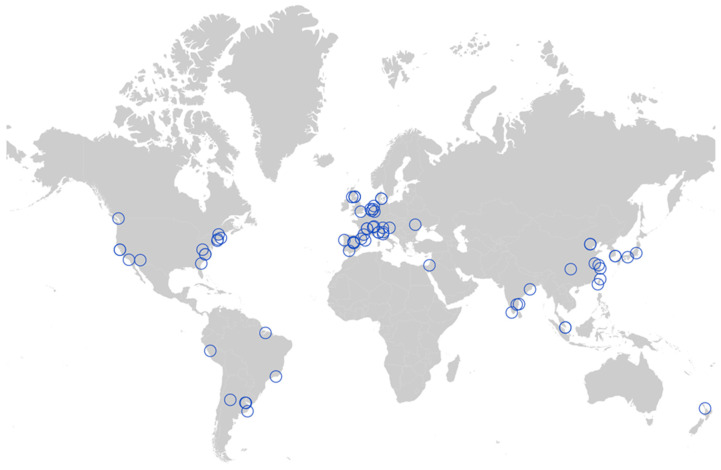
Atypical HFMD has been reported worldwide, mostly in Asia, the Americas and Europe. (Figure 5) [45]. In China, most atypical HFMD cases were reported in coastal areas [46]. The subtropical and tropical monsoon climates characterized by high humidity and temperatures make them an environmental factor in HFMD epidemics [47]. Atypical HFMD also occurs in North America (the United States), South America (Brazil, Peru, and Argentina), Europe (Switzerland, the United Kingdom, the Netherlands, Denmark, Portugal, and Italy). Nevertheless, atypical HFMD is relatively rare in Africa, which may result from the fact that the viral agents causing atypical HFMD, such as CVA6, have not yet caused an outbreak in Africa. Of course, this is also related to the lack of real-time and effective public health surveillance in Africa.
Figure 5.
Geographical distribution of atypical HFMD. Data are visualized with the help of online website services (https://www.chiplot.online/map_plot.html, accessed on 3 January 2023).
10. Evolutionary Characteristics of Virus Strains Causing Atypical HFMD
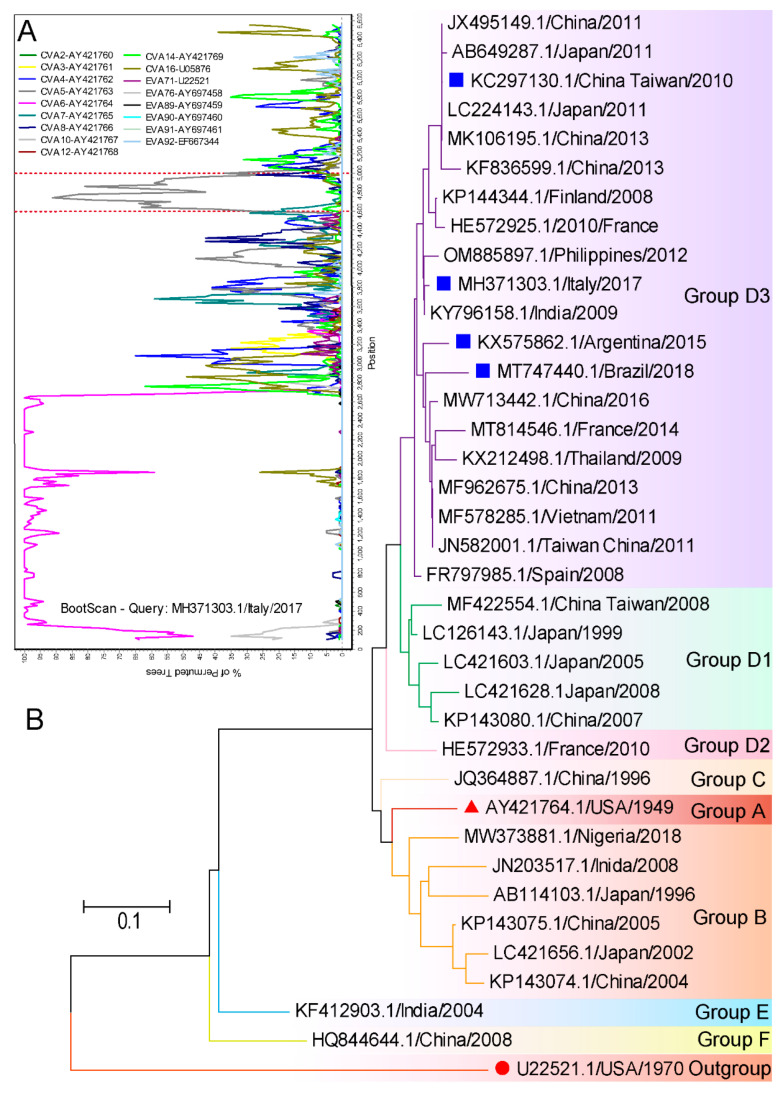
A variety of pathogens could cause atypical HFMD, but CVA6 has been commonly reported. Details such as atypical presentations caused by CVA6 are listed in Table 5. We applied Simplot and MEGA 7 for the evolutionary characterization of these strains from GenBank. The strains (MH371303.1) containing the entire gene coding sequence (CDS) were collected for recombination analysis. There was no recombination in the structural region, but possible recombination with CVA5 in non-structural regions marked by red dotted lines was detected (Figure 6A). CVA6 strains circulating worldwide can be divided into 6 genotypes designated as A to F, and the D genotype can be further subdivided into D1–D3 sub-genotypes. An analysis of these representative strains confirmed that all were sub-grouped into the genetic group D3. The D3 sub-genotype is the predominant genotype that circulates all over the world, particularly in Southeast Asia and Europe in recent years (Figure 6B). Thus, the virus strains causing atypical HFMD are very common. In addition to geographical location, conditions such as host immunity, socioeconomic status and ethnicity are suspected to contribute to the occurrence of atypical symptoms.
Table 5.
Detailed information on virus strains associated with atypical HFMD.
| Serotype | Atypical Presentation | Accession Number | Time | Country | References |
|---|---|---|---|---|---|
| CVA6 |
|
KX575862 - KX575865 |
2015 | Argentina | [48] |
| CVA6 |
|
KC297130 - KC297135 |
2010 | Taiwan, China | [35] |
| CVA6 |
|
TW910141 - TW910142 |
2015 | Taiwan, China | [49] |
| CVA6 |
|
MT747440 | 2018 | Brazil | [50] |
| CVA6 |
|
MH371303 | 2017 | Italy | [44] |
Figure 6.
Evolutionary characteristics of CVA6 causing atypical HFMD available from GenBank were conducted applying MEGA 7 and Simplot 3.5.1 software. (A) The Bootscan analysis of MH371303.1/Italy/2017 based on the CDS region. The reference strains are represented in the legend. (B) Phylogenetic analyses of CVA6 based on a part of VP1 sequence (276 bp). The bootstrap test was performed with 1000 replications. All the strains are labelled using the following format: accession number/country of origin/year of isolation. The prototype strain of EVA71 marked with red circles indicates the outgroups. The prototype strain of CVA6 is marked as red triangles. The blue squares mark the representative strains causing atypical HFMD.
11. Summary and Prospective
Familiarity with the symptoms of the disease, including its atypical manifestations, is essential to make the correct diagnosis and treatment options. In view of the contagious nature of HFMD, a timely diagnosis can help avoid contact with the affected individual and decrease the risk of an outbreak. As an infectious disease primarily affecting children, there can be a delay in establishing the diagnosis in adult cases, which increases the risk of disease transmission. Therefore, we should give adequate attention to atypical HFMD in adults. Atypical HFMD is self-limiting with complete resolution usually within 7–14 days after disease onset. The treatment of atypical HFMD is usually symptomatic. Prompt recognition can reduce unnecessary treatment and hospital admission. The emergence of novel viruses or the recombination of common serotypes has brought considerable challenges to the timely differential diagnosis of HFMD. Clinicians must identify the wide range of cutaneous and mucosal alterations caused by the different EV types and prescribe appropriate diagnostic tests. Hence, establishing an adequate surveillance system for enteroviral diseases is a more appropriate public health intervention to combat HFMD.
In this review, we summarized the current research on atypical manifestations of HFMD. Numerous studies have shown that the clinical presentations of HFMD caused by CVA6 infection are different from that of typical HFMD and may be more severe and widespread. The main clinical manifestations of atypical HFMD include Gianotti–Crosti-like eruptions, eczema coxsackium, petechial/purpuric eruptions and vesiculobullous exanthema. We also described the differential diagnosis of atypical HFMD in terms of rash characteristics, duration, common sites, and pathological features. However, some limitations of this study are worth noting. Firstly, we have only detailed the differential diagnosis of common diseases similar to atypical HFMD, as space is limited. Secondly, we likely underestimated the actual depth of the crisis caused by atypical HFMD due to the lack of real-time and effective public health surveillance in some regions, as well as the underdiagnosis of atypical HFMD. Despite these limitations, the present study provides a scientific guide for the timely diagnosis of HFMD and the prevention of disease progression.
Author Contributions
Conceptualization, Y.C., W.J. and Y.J.; methodology, data curation, writing—original draft preparation, Y.C.; software, supervision, writing—review and editing, W.J. and Y.J.; resources, project administration, Y.J.; funding acquisition, Y.J., S.C. and G.D.; validation, B.D. and S.H.; formal analysis, G.D.; investigation, H.Y.; visualization, S.C. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.
Conflicts of Interest
The authors have declared that no competing interests exist.
Funding Statement
This work was supported by the National Natural Science Foundation of China (NO. 82273695, NO. 82002147 and NO. 82073618); China Postdoctoral Science Foundation (NO. 2019M662543); Key Scientific Research Project of Henan Institution of Higher Education (NO. 21A310026). The funders had no role in the study design, data collection and analysis, decision to publish, or the preparation of the manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Solomon T., Lewthwaite P., Perera D., Cardosa M.J., McMinn P., Ooi M.H. Virology, epidemiology, pathogenesis, and control of enterovirus 71. Lancet Infect. Dis. 2010;10:778–790. doi: 10.1016/S1473-3099(10)70194-8. [DOI] [PubMed] [Google Scholar]
- 2.Ooi M.H., Wong S.C., Lewthwaite P., Cardosa M.J., Solomon T. Clinical features, diagnosis, and management of enterovirus 71. Lancet Neurol. 2010;9:1097–1105. doi: 10.1016/S1474-4422(10)70209-X. [DOI] [PubMed] [Google Scholar]
- 3.Merzel Sabovic E.K., Tockova O., Ursic T., Zgavec B., Dolenc-Voljc M. Atypical hand, foot, and mouth disease in an adult patient: A case report and literature review. Acta Derm. Alp. Pannonica Adriat. 2019;28:85–88. doi: 10.15570/actaapa.2019.21. [DOI] [PubMed] [Google Scholar]
- 4.Bruning A.H., van der Sanden S.M., ten Hoedt A.E., Wolthers K.C., van Kaam A.H., Pajkrt D. An atypical course of coxsackievirus A6 associated hand, foot and mouth disease in extremely low birth weight preterm twins. J. Clin. Virol. 2015;65:20–22. doi: 10.1016/j.jcv.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 5.Zhang Y.C., Jiang S.W., Gu W.Z., Hu A.R., Lu C.T., Liang X.Y., Hu Y.R., Zhu D.D., Xie L. Clinicopathologic features and molecular analysis of enterovirus 71 infection: Report of an autopsy case from the epidemic of hand, foot and mouth disease in China. Pathol Int. 2012;62:565–570. doi: 10.1111/j.1440-1827.2012.02837.x. [DOI] [PubMed] [Google Scholar]
- 6.Lizasoain A., Piegas S., Victoria M., Da Silva E.E., Colina R. Hand-foot-and-mouth disease in uruguay: Coxsackievirus A6 identified as causative of an outbreak in a rural childcare center. J. Med. Virol. 2020;92:167–173. doi: 10.1002/jmv.25590. [DOI] [PubMed] [Google Scholar]
- 7.Mirand A., le Sage F.V., Pereira B., Cohen R., Levy C., Archimbaud C., Peigue-Lafeuille H., Bailly J.L., Henquell C. Ambulatory Pediatric Surveillance of Hand, Foot and Mouth Disease as Signal of an Outbreak of Coxsackievirus A6 Infections, France, 2014-2015. Emerg. Infect. Dis. 2016;22:1884–1893. doi: 10.3201/eid2211.160590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Drago F., Ciccarese G., Gariazzo L., Cioni M., Parodi A. Acute localized exanthem due to Coxsackievirus A4. Infez. Med. 2017;25:274–276. [PubMed] [Google Scholar]
- 9.Brandt O., Abeck D., Gianotti R., Burgdorf W. Gianotti-Crosti syndrome. J. Am. Acad. Derm. 2006;54:136–145. doi: 10.1016/j.jaad.2005.09.033. [DOI] [PubMed] [Google Scholar]
- 10.Kimmis B.D., Downing C., Tyring S. Hand-foot-and-mouth disease caused by coxsackievirus A6 on the rise. Cutis. 2018;102:353–356. [PubMed] [Google Scholar]
- 11.Zhao T.S., Du J., Sun D.P., Zhu Q.R., Chen L.Y., Ye C., Wang S., Liu Y.Q., Cui F., Lu Q.B. A review and meta-analysis of the epidemiology and clinical presentation of coxsackievirus A6 causing hand-foot-mouth disease in China and global implications. Rev. Med. Virol. 2020;30:e2087. doi: 10.1002/rmv.2087. [DOI] [PubMed] [Google Scholar]
- 12.Mathes E.F., Oza V., Frieden I.J., Cordoro K.M., Yagi S., Howard R., Kristal L., Ginocchio C.C., Schaffer J., Maguiness S., et al. “Eczema coxsackium” and unusual cutaneous findings in an enterovirus outbreak. Pediatrics. 2013;132:e149–e157. doi: 10.1542/peds.2012-3175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leung A.K.C., Sergi C.M., Lam J.M., Leong K.F. Gianotti-Crosti syndrome (papular acrodermatitis of childhood) in the era of a viral recrudescence and vaccine opposition. World J. Pediatr. 2019;15:521–527. doi: 10.1007/s12519-019-00269-9. [DOI] [PubMed] [Google Scholar]
- 14.Marcassi A.P., Piazza C.A.D., Seize M., Cestari S. Atypical Gianotti-Crosti syndrome. Bras Derm. 2018;93:265–267. doi: 10.1590/abd1806-4841.20186726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Losey N.A., Stevenson B.S., Busse H.J., Damste J.S.S., Rijpstra W.I.C., Rudd S., Lawson P.A. Thermoanaerobaculum aquaticum gen. nov., sp. nov., the first cultivated member of Acidobacteria subdivision 23, isolated from a hot spring. Int. J. Syst. Evol. Microbiol. 2013;63:4149–4157. doi: 10.1099/ijs.0.051425-0. [DOI] [PubMed] [Google Scholar]
- 16.Allmon A., Deane K., Martin K.L. Common Skin Rashes in Children. Am. Fam. Physician. 2015;92:211–216. [PubMed] [Google Scholar]
- 17.Naimer S.A., Cohen A.D., Mumcuoglu K.Y., Vardy D.A. Household papular urticaria. Isr. Med. Assoc. J. 2002;4:911–913. [PubMed] [Google Scholar]
- 18.Millikan L.E. Papular urticaria. Semin Derm. 1993;12:53–56. [PubMed] [Google Scholar]
- 19.Vafaie J., Schwartz R.A. Erythema infectiosum. J. Cutan Med. Surg. 2005;9:159–161. doi: 10.1007/s10227-005-0101-8. [DOI] [PubMed] [Google Scholar]
- 20.Nahmias A.J., Froeschle J.E., Feorino P.M., McCord G. Generalized eruption in a child with eczema due to coxsackievirus A16. Arch. Derm. 1968;97:147–148. doi: 10.1001/archderm.1968.01610080051008. [DOI] [PubMed] [Google Scholar]
- 21.Ganguly S., Kuruvila S. Eczema Coxsackium. Indian J. Derm. 2016;61:682–683. doi: 10.4103/0019-5154.193691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gin A., King E., Scardamaglia L., Orchard D. Eczema exacerbation caused by Coxsackie virus A6. Australas. J. Derm. 2018;59:64–65. doi: 10.1111/ajd.12677. [DOI] [PubMed] [Google Scholar]
- 23.Su H.J., Chen C.B. Eczema coxsackium. Med. J. Aust. 2021;215:403. doi: 10.5694/mja2.51297. [DOI] [PubMed] [Google Scholar]
- 24.Feder H.M., Jr., Bennett N., Modlin J.F. Atypical hand, foot, and mouth disease: A vesiculobullous eruption caused by Coxsackie virus A6. Lancet Infect Dis. 2014;14:83–86. doi: 10.1016/S1473-3099(13)70264-0. [DOI] [PubMed] [Google Scholar]
- 25.Schmader K. Herpes Zoster. Ann. Intern. Med. 2018;169:ITC19–ITC31. doi: 10.7326/L18-0558. [DOI] [PubMed] [Google Scholar]
- 26.Damour A., Garcia M., Seneschal J., Leveque N., Bodet C. Eczema Herpeticum: Clinical and Pathophysiological Aspects. Clin. Rev. Allergy Immunol. 2020;59:1–18. doi: 10.1007/s12016-019-08768-3. [DOI] [PubMed] [Google Scholar]
- 27.Ventarola D., Bordone L., Silverberg N. Update on hand-foot-and-mouth disease. Clin. Derm. 2015;33:340–346. doi: 10.1016/j.clindermatol.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 28.Wei S.H., Huang Y.P., Liu M.C., Tsou T.P., Lin H.C., Lin T.L., Tsai C.Y., Chao Y.N., Chang L.Y., Hsu C.M. An outbreak of coxsackievirus A6 hand, foot, and mouth disease associated with onychomadesis in Taiwan, 2010. BMC Infect. Dis. 2011;11:346. doi: 10.1186/1471-2334-11-346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Laga A.C., Shroba S.M., Hanna J. Atypical hand, foot and mouth disease in adults associated with coxsackievirus A6: A clinico-pathologic study. J. Cutan Pathol. 2016;43:940–945. doi: 10.1111/cup.12775. [DOI] [PubMed] [Google Scholar]
- 30.Second J., Velter C., Cales S., Truchetet F., Lipsker D., Cribier B. Clinicopathologic analysis of atypical hand, foot, and mouth disease in adult patients. J. Am. Acad. Derm. 2017;76:722–729. doi: 10.1016/j.jaad.2016.10.022. [DOI] [PubMed] [Google Scholar]
- 31.Fraticelli P., Benfaremo D., Gabrielli A. Diagnosis and management of leukocytoclastic vasculitis. Intern. Emerg. Med. 2021;16:831–841. doi: 10.1007/s11739-021-02688-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Zelman B., Muhlbauer A., Kim W., Speiser J. A rare case of papular-purpuric “gloves and socks” syndrome associated with influenza. J. Cutan Pathol. 2022;49:632–637. doi: 10.1111/cup.14213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Halasz C.L., Cormier D., Den M. Petechial glove and sock syndrome caused by parvovirus B19. J. Am. Acad. Derm. 1992;27:835–838. doi: 10.1016/0190-9622(92)70260-m. [DOI] [PubMed] [Google Scholar]
- 34.Segura Saint-Gerons R., Ceballos Salobrena A., Gutierrez Torres P., Gonzalez Ruiz A., Gavilan Fernandez I., Martinez-Sahuquillo Marquez A. Papular purpuric gloves and socks syndrome. Presentation of a clinical case. Med. Oral. Patol. Oral. Cir. Bucal. 2007;12:E4–E6. [PubMed] [Google Scholar]
- 35.Huang W.C., Huang L.M., Lu C.Y., Cheng A.L., Chang L.Y. Atypical hand-foot-mouth disease in children: A hospital-based prospective cohort study. Virol. J. 2013;10:209. doi: 10.1186/1743-422X-10-209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Justino M.C.A., da S.M.D., Souza M.F., Farias F.P., Dos S.A.J.C., Ferreira J.L., Lopes D.P., Tavares F.N. Atypical hand-foot-mouth disease in Belem, Amazon region, northern Brazil, with detection of coxsackievirus A6. J. Clin. Virol. 2020;126:104307. doi: 10.1016/j.jcv.2020.104307. [DOI] [PubMed] [Google Scholar]
- 37.Hartman-Adams H., Banvard C., Juckett G. Impetigo: Diagnosis and treatment. Am. Fam. Physician. 2014;90:229–235. [PubMed] [Google Scholar]
- 38.Arvin A.M. Varicella-zoster virus. Clin. Microbiol. Rev. 1996;9:361–381. doi: 10.1128/CMR.9.3.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bagci I.S., Horvath O.N., Ruzicka T., Sardy M. Bullous pemphigoid. Autoimmun. Rev. 2017;16:445–455. doi: 10.1016/j.autrev.2017.03.010. [DOI] [PubMed] [Google Scholar]
- 40.Johnson M.K. Impetigo. Adv. Emerg. Nurs. J. 2020;42:262–269. doi: 10.1097/TME.0000000000000320. [DOI] [PubMed] [Google Scholar]
- 41.Cole C., Borradori L., Amber K.T. Deciphering the Contribution of BP230 Autoantibodies in Bullous Pemphigoid. Antibodies. 2022;11:44. doi: 10.3390/antib11030044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brazel M., Desai A., Are A., Motaparthi K. Staphylococcal Scalded Skin Syndrome and Bullous Impetigo. Medicina. 2021;57:1157. doi: 10.3390/medicina57111157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chavda V.P., Patel K., Apostolopoulos V. Tomato flu outbreak in India. Lancet Respir. Med. 2023;11:e1–e2. doi: 10.1016/S2213-2600(22)00300-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Broccolo F., Drago F., Ciccarese G., Genoni A., Puggioni A., Rosa G.M., Parodi A., Manukyan H., Laassri M., Chumakov K., et al. Severe atypical hand-foot-and-mouth disease in adults due to coxsackievirus A6: Clinical presentation and phylogenesis of CV-A6 strains. J. Clin. Virol. 2019;110:1–6. doi: 10.1016/j.jcv.2018.11.003. [DOI] [PubMed] [Google Scholar]
- 45.Aswathyraj S., Arunkumar G., Alidjinou E.K., Hober D. Hand, foot and mouth disease (HFMD): Emerging epidemiology and the need for a vaccine strategy. Med. Microbiol. Immunol. 2016;205:397–407. doi: 10.1007/s00430-016-0465-y. [DOI] [PubMed] [Google Scholar]
- 46.Zhang P., Zhang J. Surveillance on other infectious diarrheal diseases in China from 2014 to 2015. Zhonghua Liu Xing Bing Xue Za Zhi. 2017;38:424–430. doi: 10.3760/cma.j.issn.0254-6450.2017.04.003. [DOI] [PubMed] [Google Scholar]
- 47.Mao Y., Zhang N., Zhu B., Liu J., He R. A descriptive analysis of the Spatio-temporal distribution of intestinal infectious diseases in China. BMC Infect. Dis. 2019;19:766. doi: 10.1186/s12879-019-4400-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cisterna D.M., Lema C.L., Martinez L.M., Veron E., Contarino L.P., Acosta D., Freire M.C. Atypical hand, foot, and mouth disease caused by Coxsackievirus A6 in Argentina in 2015. Rev. Argent Microbiol. 2019;51:140–143. doi: 10.1016/j.ram.2018.05.003. [DOI] [PubMed] [Google Scholar]
- 49.Chiu H.H., Liu M.T., Chung W.H., Ko Y.S., Lu C.F., Lan C.E., Lu C.W., Wei K.C. The Mechanism of Onychomadesis (Nail Shedding) and Beau’s Lines Following Hand-Foot-Mouth Disease. Viruses. 2019;11:522. doi: 10.3390/v11060522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.De Sousa I.P., Jr., Giamberardino H.I., Raboni S.M., Debur M.C., de Lourdes Aguiar Oliveira M., Burlandy F.M., da Silva E.E. Simultaneous enterovirus EV-D68 and CVA6 infections causing acute respiratory distress syndrome and hand, foot and mouth disease. Virol. J. 2021;18:88. doi: 10.1186/s12985-021-01560-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the manuscript and its Supporting Information files.