Abstract
Chagas disease, a neglected disease caused by the protozoan Trypanosoma cruzi, is endemic in 21 Latin American countries, affecting 6–8 million people. Increasing numbers of Chagas disease cases have also been reported in non-endemic countries due to migration, contamination via blood transfusions or organ transplantation, characterizing Chagas as an emerging disease in such regions. While most individuals in the chronic phase of Chagas disease remain in an asymptomatic clinical form named indeterminate, approximately 30% of the patients develop a cardiomyopathy that is amongst the deadliest cardiopathies known. The clinical distinctions between the indeterminate and the cardiac clinical forms are associated with different immune responses mediated by innate and adaptive cells. In this review, we present a collection of studies focusing on the human disease, discussing several aspects that demonstrate the association between chemokines, cytokines, and cytotoxic molecules with the distinct clinical outcomes of human infection with Trypanosoma cruzi. In addition, we discuss the role of gene polymorphisms in the transcriptional control of these immunoregulatory molecules. Finally, we discuss the potential application of cytokine expression and gene polymorphisms as markers of susceptibility to developing the severe form of Chagas disease, and as targets for disease control.
Keywords: cytokines, immunoregulation, gene polymorphism, chagas disease, Trypanosoma cruzi
1. Introduction
According to the World Health Organization (WHO), Chagas disease, a parasitic disease caused by infection with Trypanosoma cruzi, leads to approximately 14,000 deaths annually and is one of the main causes of sudden death, which often occurs in the most productive phase of the patient’s life. The disease is still considered a serious social and public health problem, despite the advances made in its control and prevention [1,2,3,4].
The main form of T. cruzi infection in endemic setting is via the contact with contaminated excreta from the invertebrate host, a triatomine of the Reduviidae family, that occurs during the vector’s blood meal. However, other forms of parasite transmission via blood transfusion, organ transplantation, congenital transmission, ingestion of contaminated food and, less frequently, laboratory accidents, may occur [5,6,7,8].
After infection, an acute phase of short duration is followed by the chronic phase of the disease, which can last for years or decades. The acute phase is characterized by local tissue degeneration and inflammatory changes due to high parasitemia [9,10]. In approximately 90% of cases, the clinical manifestations last from a few weeks to a few months, and spontaneously regress with a decrease in parasitemia. In most cases, the acute phase can be asymptomatic, or patients may display unspecific symptoms [11]. Lack of specific symptoms is one of the challenges in the early diagnosis of CD, which has implications for therapeutic interventions. Thus, finding new diagnostics, especially a point-of-care that can be applied in the field is a pressing matter. Regarding this effort, new approaches have recently been developed and have been extensively reviewed in a recent paper focused on Chagas disease diagnosis [12].
Around 70% of patients who progress to the chronic phase of CD remain asymptomatic, characterizing the indeterminate clinical form (IND). In these patients, the disease is only detected by positive results from at least two specific serological tests since the patients do not display any clinical signs or symptoms of the disease. Approximately 30% of chronic patients display digestive and/or cardiac alterations during the chronic phase, which may lead to death. Amongst the symptomatic forms, the cardiac clinical form has the highest morbidity and mortality rate [13,14]. The distinct clinical evolution in the chronic phase results from multifactorial mechanisms. For instance, parasite aspects, such as strain variability and tropism [15,16], and host aspects, such as age, sex, nutritional, socioeconomic factors, and immunological characteristics [17,18,19,20] are known to influence disease progression.
2. The Role of Cytokines in Immune Regulation during the Chronic Phase of Chagas Disease
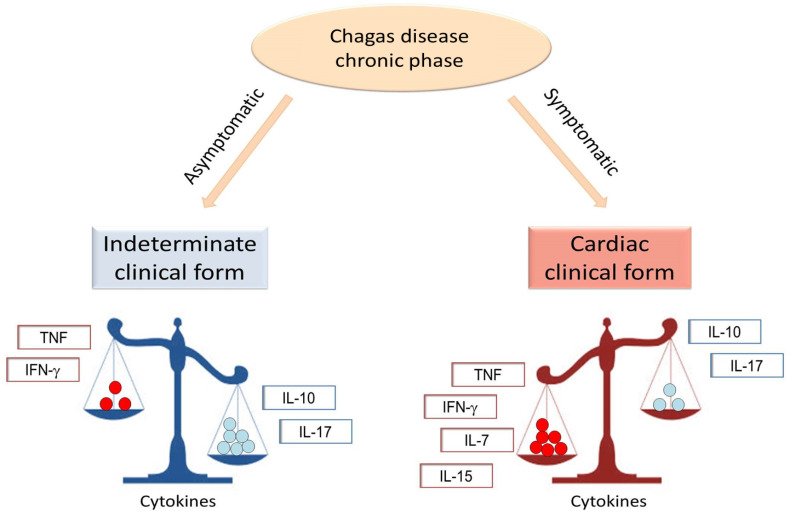
Cytokines are molecules actively involved in immune regulation during the chronic phase of Chagas disease (Figure 1) and, although their role in cell activation is essential for infection control, it can contribute to myocardial dysfunction [21,22]. While a high expression of mRNA that code for pro- and anti-inflammatory cytokines has been observed in mononuclear cells from patients chronically infected with T. cruzi [23], studies performed by several groups showed that the production of such cytokines was distinct in patients with different clinical forms of the disease (Table 1). While the role of these cytokines provides insight as to the mechanism by which they act, more studies are needed to address this issue. The release of pro-inflammatory cytokines in the plasma, as well as their expression by peripheral blood mononuclear cells (PBMC) and by the myocardium, characterize the intense inflammation and Th-1-like response that is observed in patients with chronic Chagas cardiomyopathy (CCC) [24,25,26,27,28,29,30,31,32,33]. This exuberant inflammatory immune activation observed in CCC patients is high, even if compared to inflammatory cardiomyopathies of other etiologies, such as rheumatic heart disease and idiopathic cardiomyopathy [34,35]. On the other hand, anti-inflammatory cytokines are predominant in the immune milieu of IND patients, despite the concurrent production of anti- and pro-inflammatory cytokines observed in these patients [36].
Figure 1.
Schematic representation of cytokine expression in the indeterminate and cardiac clinical forms of Chagas disease. In the indeterminate clinical form, an increased expression of anti-inflammatory cytokines, such as IL-10 and IL-17 is observed. However, in the cardiac clinical form, the increased expression of pro-inflammatory cytokines, such as IFN-gamma and TNF, favor the establishment of the inflammatory environment. Cytokines, such as IL-7 and IL-15, have been associated with the cardiac clinical form.
Table 1.
Cytokines, chemokines, and cytotoxic molecules associated with distinct clinical outcomes of human Chagas disease.
| Cytokines and Their Receptors | Role | Source | References |
| Association with the Development of CCC | |||
| IFN-gamma | Inflammation, T-cell activation | PBMC, heart | [22,24,29,30,37,38,39] |
| TNF | Inflammation, T-cell activation, worse cardiac function | Plasma, blood, PBMC, heart | [18,28,31,32,38,40,41,42] |
| sTNFR1 and sTNFR2 | Inflammation, worse cardiac function |
Plasma | [43] |
| IL-6 | Inflammation, worse cardiac function |
Plasma | [30,44,45] |
| IL-1β | Inflammation | Plasma | [30,46] |
| TGF-β | Tissue fibrosis | Serum, heart | [47] |
| MIF | Inflammation, progression | Serum | [48] |
| Association with protection to CCC | |||
| IL-2 | Low in CCC | PBMC | [49] |
| IL-10 | Protective immune response, better cardiac function | Plasma, PBMC, whole blood | [18,30,38,39,40,41,50] |
| IL-17 | Protective immune response, better cardiac function | Plasma, PBMC | [38,51,52,53] |
| Fibrotic and Cytotoxic Molecules | Description | Source | References |
| Association with the Development of CCC | |||
| MMP-2 | Tissue fibrosis | Plasma | [54,55] |
| MMP-9 | Tissue fibrosis | Plasma, PBMC | [54,56] |
| MMP-2/MMP-9 ratio | Pathological cardiac remodeling | Plasma, heart | [57,58] |
| Fibronectin | Recruitment of inflammatory T cells to the heart | Heart | [42] |
| Granzyme A | Myocyte dysfunction | Heart | [28] |
| Chemokines and Their Receptors | Description | Source | References |
| Association with the Development of CCC | |||
| CXCL9 | Recruitment of inflammatory cells |
Plasma, heart | [46,59,60] |
| CXCL10 | Recruitment of inflammatory cells |
Plasma | [46,61,62] |
| CCL5 | Recruitment of inflammatory cells |
Serum | [63] |
| CCL2 | Recruitment of inflammatory cells |
Serum and plasma | [32,63] |
| CCR5 | Recruitment of inflammatory cells, Worse prognosis | PBMC | [62,64] |
| CXCR3 | Recruitment of inflammatory cells | PBMC | [64] |
Cytokines can be produced by a plethora of distinct cell populations, and identifying the source of the cytokines, as well as the antigens that induce their expression may guide immunotherapeutic interventions. The functional analysis of human monocytes after in vitro infection with T. cruzi trypomastigotes showed that, in CCC patients, these cells display high expression of TNF. Conversely, monocytes from IND patients are compromised with the production of IL-10 [18,51]. In addition, classical (CD14++CD16-) and inflammatory (CD14++CD16+) monocytes are positively correlated with IL-6 production, described as a biomarker of cardiac failure and severity in CCC patients [44,45,65]. Although monocytes are an essential source of cytokine in chronic Chagas disease, they are also crucial for antigenic presentation to T lymphocytes, which are highly activated in the chronic phase of infection [66].
The presence of circulating CD4+ T cells that produce high levels of IFN-gamma and low IL-10/IFN-gamma ratio reported in CCC patients confirms the Th1 profile associated with intense inflammation. Menezes et al. (2004) demonstrated a positive correlation between the frequency of CD4+ TNF+ cells and CD4+CD28- cells in CCC patients. Alternatively, CD4+ CD28- cells were positively correlated with the frequency of CD4+IL-10+ cells in IND patients, suggesting the involvement of distinct mechanisms of immunoregulation between the symptomatic and asymptomatic clinical forms of the disease [67].
This immunological polarization is also observed among other cytokine-producing cell subpopulations. T cells that do not express the co-receptors CD4 and CD8, named double-negative (DN) T cells, have been described as potent cytokine producers in many diseases [68]. After in vitro stimulation with T. cruzi trypomastigotes, DN T cells expressing the T-cell receptor (TCR) alpha-beta from CCC patients show high expression of inflammatory cytokines (IFN-gamma, TNF). On the other hand, the TCR gamma-delta+ DN T cells are involved with high IL-10 expression and better cardiac function in IND patients [50]. Interestingly, stimulation of TCR gamma-delta+ DN T cells with a glycolipid-rich fraction of T. cruzi increases the IFN-gamma-mediated inflammatory profile in CCC patients [69]. Conversely, blocking the in vitro activation of DN T cells from CCC patients reduces the frequency of IFN-gamma in TCR gamma-delta+ DN T cells and increases the frequency of IL-10 in effector memory TCR gamma-delta+ DN T cells, favoring the establishment of a less inflammatory environment [70,71]. Furthermore, in IND patients, central memory DN T cells show a balanced immune response characterized by the co-expression of circulating IL-10+ IFN+ cells [70].
In addition to the DN T cells, another minority circulating cell population that displays a dichotomic cytokine profile in Chagas disease associated with distinct clinical forms are the B1 B cells. These cells represent a subset of B cells involved with the production of natural and auto-reactive antibodies [72,73], as well as significant IL-10 expression [74]. Previous studies have shown that the frequency of B1 B cells is increased in chronic Chagas disease patients [66], and that the frequency of these cells is restored to normal levels in Chagas patients submitted to successful chemotherapy [75]. It has been shown that B-1 B cells from Chagas patients respond to parasite antigens [75], and are preferentially activated by a component of this antigen that is enriched in proteins, but not lipids and carbohydrates [41]. In addition, these activated B1 B cells produce different cytokine profiles depending on whether they come from IND or CCC patients: while activated TNF-producing B1 B cells are observed in cultures of PBMC from CCC-stimulated protein-rich parasite-derived antigens, a concomitant production of TNF and IL-10 was observed in cultures of PBMC from IND under the same condition [41]. Importantly, these potentially regulatory activated B1 B cells are correlated with better cardiac function in Chagas disease [41]. Moreover, the presence of regulatory B2 B-cells, the great majority of antibody-producing B-cells, have been correlated with the indeterminate form of Chagas disease [56]. These data support the hypothesis that a balanced anti-inflammatory/pro-inflammatory profile in IND patients, established with the involvement of different cell subpopulations, prevents tissue damage and progression to symptomatic forms of the disease.
The systemic and cellular inflammatory profile observed in CCC patients is mirrored by the expression of cytokines in the cardiac tissue [28,31,37]. This scenario contributes to cardiomyocyte apoptosis and, consequently, to heart tissue damage and remodeling, which involves fibrosis [39,64,76,77]. In addition, these immune mediators may contribute to cellular recruitment and survival of inflammatory T cells in the myocardium [42,78].
Therefore, understanding the cytokine networks involved with the pathogenic immune response in CCC and with the protection in IND patients may elucidate important immunological targets to prevent progression to symptomatic forms of the disease, as well as control the intense inflammatory response associated with the cardiac pathology.
3. Cell Cytotoxicity in the Pathogenesis of Chronic Chagas Cardiomyopathy (CCC)
CCC is characterized by the development of myocarditis, tissue fibrosis, and cardiac hypertrophy [79,80]. The inflammatory infiltrates present in the myocardium of CCC patients are composed mainly of CD8+ T cells, which reinforces the role of cytotoxic activity in cardiac tissue damage [81,82,83,84].
CD8+ T cells release cytolytic granules, composed of perforin and granzymes, toward the target cells. Perforin forms pores in cell membranes and allows the entry of granzymes that activate caspase pathways. This can result in apoptosis induction of target cells infected by intracellular microorganisms, such as T. cruzi [83,84,85].
The presence of lysosomal-associated membrane protein-1 (LAMP-1 or CD107a) on the surface of cytolytic cells characterizes the process of cell degranulation and the effector immune response in infectious diseases [86]. In 2015, Lasso et al. showed that CD8+ T cells from patients with CCC displayed an increase in the expression of CD107a/b, perforins, and granzyme B when stimulated with T. cruzi antigens and the KMP-11 recombinant protein, indicating that these cells increase their cytotoxic potential during chronic infection [87]. Reis et al. (1993) had previously reported the expression of CD8+ granzyme A+ cells in myocardial lesions of CCC patients, supporting the idea that cell cytotoxic mechanisms mediate cardiomyocyte destruction [28].
CD4+ cells have also been described as potentially cytotoxic in CD. A positive correlation was demonstrated between CD4+ cells expressing the variable region beta 5 chain (Vβ5) of the T-cell receptor (TCR), and the expression of granzyme A in CCC patients [88]. Furthermore, in 2012, Keesen et al. (2012) demonstrated an increased frequency of granzyme B and CD107 on circulating CD4+ T cells from IND and CCC patients, when compared to healthy donors. Interestingly, the authors showed an association between the expression of granzyme B and CD107a with the memory marker CD45RO in CD4+ cells from patients in the IND group after stimulation with T. cruzi antigen. This indicates that the expression of cytotoxic markers by this cell subpopulation may be necessary to control the immune response in asymptomatic patients [89]. However, it is worth speculating which factors are associated with the immunological imbalance that leads to cellular cytotoxicity in CCC patients. It is also important to highlight that the frequency of CD4+ granzyme A+ T cells is higher in patients with CCC compared to idiopathic dilated cardiomyopathy (IDC) [35]. Furthermore, the potentially cytotoxic CD4+CD28 cells are increased in patients with chronic Chagas disease, when compared to uninfected individuals [90,91].
The mechanisms of cell recruitment from the blood to cardiac tissue are not fully understood. However, it is known that the expression of adhesion molecules and chemokines may contribute to the migration of cell subpopulations with cytotoxic and inflammatory potential. In 1996, Laucella and collaborators demonstrated an association between serum s-p-selectin and the severity of chronic Chagas disease [92]. Furthermore, the upregulation of ICAM-1, VCAM-1, and LFA-1 in the myocardium of CCC patients points to the role of T-cell recruitment and inflammation in cardiac pathogenesis [92].
The engagement of adhesion molecules and chemokines are essential orchestrators of leukocyte migration. In 2004, Talvani et al. demonstrated that the release of CCL2 in plasma and the supernatant of PBMC from patients infected with T. cruzi is higher in those with severe cardiac impairment than in the group with mild heart disease [32]. Furthermore, samples from CCC patients showed elevated plasma levels of CCL3 and CCL4 when compared to patients with digestive disorders (32). The serum levels of chemokines CXCL10 and CXCL9 were increased in Chagas patients when compared to uninfected individuals [46]. Moreover, the plasma of CCC patients, when compared to healthy donors, showed increased levels of different systemic chemokines involved with the cellular recruitment of leukocytes and granulocytes [34]. This reinforces the role of strong immune activation and cellular recruitment in the pathogenesis of CD.
In 2005, Gomes et al. reported that CCC displayed an increased frequency of CXCR3 and/or CCR5 expression by CD4+ and CD8+ T cells. Interestingly, these cells produce the inflammatory cytokines IFN-gamma and TNF [64]. Additionally, when studying the progressive evolution of CCC, Roffe et al., in 2019, demonstrated a gradual increase in the expression of effector cells expressing CCR5 [62]. Consistent with these data, other studies showed that circulating and infiltrating mononuclear cells in the myocardial tissue of CCC patients present the expression of CXCR3, CCR5, CXCL9, and CCL5 [60,93].
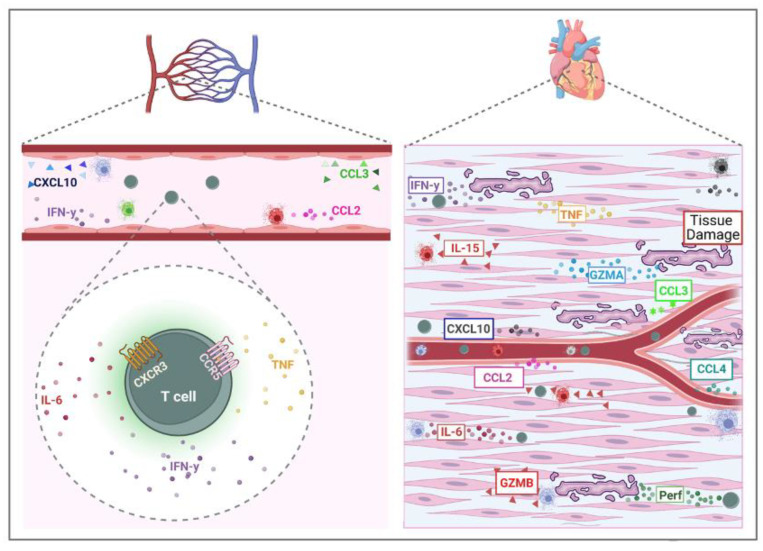
Importantly, circulating CD8+ T cells from CCC patients show higher co-expression of CCR5 and cMet, chemotactic receptors involved in T-cell cardiac tropism, compared to IDC patients. Furthermore, the association between CD8+ CCR5+ cMet+ cells with IFN-gamma and the Eomes transcription factor highlights the potential of these cells for the recruitment of inflammatory and cytotoxic molecules capable of mediating cardiac tissue damage in CCC patients [35]. These findings demonstrate the participation of chemokines and their receptors in mediating the recruitment of immune cells with inflammatory and cytotoxic activity in the myocardium of CCC patients, which can induce tissue destruction and cardiac pathology (Figure 2). However, studies in this area are still scarce and further analysis evaluating the association of these molecules with cytotoxic activity may elucidate the mechanisms involved in cardiac tissue destruction in CD.
Figure 2.
Cytotoxic and inflammatory immune response in chronic Chagas cardiomyopathy. T cells mediate cytotoxicity in chronic Chagas cardiomyopathy. These cells are recruited to the heart by adhesion molecules and chemokines, and can release inflammatory cytokines and cytotoxic molecules, such as granzymes and perforins, that contribute to cardiac tissue damage, fibrosis, and disease severity (Designed with Biorender).
4. Control of Cytokine Expression
Factors that lead IND patients to develop CCC are still unknown. However, studies indicate that mechanisms of gene regulation and cytokine-mediated signaling may be involved in the susceptibility to developing cardiomyopathy [94]. Amongst these mechanisms, the presence of single nucleotide polymorphisms (SNPs) and alteration of transcription factors emerges as important.
SNPs are changes in the DNA that occur in at least 1% of a given population, where a nucleotide is exchanged for another. SNPs can occur in every region of the genome, such as introns, exons, promoters, enhancers, or in between genes [95,96]. Some gene polymorphisms can lead to changes in the levels of expression of the final protein, as well as alterations in its functions, and are, thus, classified as functional polymorphisms.
Several studies have demonstrated that genotypic alterations can influence the production of biological molecules in CD [97,98,99], suggesting that SNPs may be related to susceptibility to CD and/or to the establishment of severe CCC. SNPs are often used as biological markers to locate genes that are associated with the disease. For over 30 years, numerous studies related to polymorphism in genes that encode cytokines, chemokines, multiple receptors, and important molecules in the antigens presentation (MHC) have been published. The selected studies are presented in Table 2 displaying the molecules by their effector characteristics. While this data show the implication of polymorphisms in disease outcomes, it is important to emphasize that some characteristics may vary between populations causing different implications and results according to the sample studied [100].
Table 2.
Polymorphisms in genes associated with the immune response and their association with distinct clinical outcomes of human Chagas disease.
| Gene Polymorphism | Description | References |
| Association with Development of CCC | ||
| IL4RA | Interleukin 4 receptor | [101] |
| IKBL/NFKBIL1 | NF-kappa-B inhibitor-like protein 1 | [102] |
| IL12ß; IL12 | Pro-inflammatory cytokine | [99,103] |
| IL17A; IL17F | Protective cytokine | [104,105,106] |
| NLRP1 | Protein involved in inflammasome | [107] |
| CASP1 | Protein involved in inflammatory cascade | [108] |
| Lymphotoxin | Member of the TNF superfamily of cytokines; are responsible for regulating the growth and function of lymphocytes | [109,110] |
| PI3 kgamma | Molecules involved in signaling pathway of the efficient immune response against T. cruzi |
[111] |
| Association with Protection to CCC | ||
| CXCL10, CCL5, CXCL9 | Chemokine Ligand | [60,63] |
| CTLA-4 | Cytotoxic T-lymphocyte-associated antigen 4 | [112] |
| VPAC1 | Vasoactive intestinal peptide (VIP) receptors 1 | [113] |
| No association with CCC | ||
| IL4 | Anti-inflammatory cytokine | [101,103,114] |
| MIF | Macrophage migration inhibitory factor | [115] |
| IL1A, IL6 | Pro-inflammatory cytokine | [103,116] |
| TGF-β1 | Multifunctional cytokine | [99,117,118] |
| TLR1, TLR2 TLR4, TLR6 | Toll-like receptor (TLR) | [119,120] |
| TNFR1; TNFR2; | Tumor necrosis factor receptor | [99] |
| Galectin-3 | Member of the lectin family/ cell–cell adhesion | [121] |
| CARD11 | Protein involved in the function of immune system cells | [107] |
| FOXP3 | Protein involved in immune system responses | [122] |
| Variable According to the Population Studied | ||
| MHC genes | Major histocompatibility complex/presentation of internal or external antigens to the T cells | [123,124,125,126] |
| CCR5; CCR2 | Chemokine receptor type | [60,109,127,128,129,130] |
| TNFA; TNFB; IL1B; IFN-g | Pro-inflammatory cytokine | [99,103,131,132,133,134,135,136,137,138] |
| IL10 | Anti-inflammatory cytokine | [97,99,114] |
| IL1RN | Interleukin-1 receptor antagonist | [131,132] |
| MAL/TIRAP | Encodes an adaptor protein for TLR | [120,139,140] |
| BAT-1 | Anti-inflammatory activity associated with reduced expression of HLA-B-1 | [99,141] |
| CCL2/MCP-1 | Chemokine ligand 2 | [63,142] |
Despite the number of studies performed related to polymorphism association with disease susceptibility and severity, there are still important candidates that have not been evaluated. For example, studies related to CD and polymorphism in genes encoding cytotoxic molecules, critical for pathology, are lacking. Until now, few genome-wide association studies (GWAS) have been conducted, and there are no GWAS studies linking SNPs and changes in immune response in Chagas disease. Thus, studies on different molecules associated with CD and the assessment of their potential use as genetic biomarkers are necessary. In addition to gene polymorphisms, epigenetic alterations and activation of transcription factors are also important in controlling the expression and function of cytokines and other immune proteins.
Epigenetics is characterized by reversible changes in the expression and activity of one or more genes, without modifying the DNA sequence. Epigenetic changes occur by chemical changes in DNA bases which can result in changes in chromosome structure and packaging, maintaining the same nucleotide sequence [143]. The main epigenetic mechanisms are DNA methylation, histone modification, and non-coding RNA expression. DNA methylation consists of the addition of a methyl group (-Ch3) at the 5’ position of the DNA cytosine (C) and it occurs most frequently in cytosines that are immediately followed by a guanine (G). Regions rich in this sequence are called CpG islands and are commonly located in the promoter region of genes. Thus, methylation is associated with gene silencing, blocking gene transcription [144,145,146].
Another epigenetic mechanism is post-translational modifications in histone proteins. Histones are proteins that DNA binds around that are important for the condensation of the genetic material in the nucleus, regulating gene expression [147]. These modifications include acetylation (addition of an acetyl group), methylation (addition of a methyl group), phosphorylation (inclusion of a negative phosphate group to the histone tail), and ubiquitylation, which is the annexation of a large molecule of ubiquitin to lysine residues. Each of these changes interferes with DNA-histone interactions and can activate or block gene transcription [147,148,149,150].
Another class of epigenetic mechanisms is non-coding RNAs (ncRNAs). The ncRNAs are transcribed but not translated into proteins. Among ncRNAs, we can highlight the microRNAs (miRNAs) that are small, endogenous, and participate in the post-transcriptional regulation of cell signaling pathways [151,152]. miRNAs bind to complementary mRNAs, regulating expression through their destruction or the avoidance of protein translation (89). Several studies demonstrate the influence of miRNAs in various biological processes such as cell death, cell proliferation/differentiation, and immune regulation [153,154].
There are a few studies in the literature on epigenetic mechanisms and CD. Among these mechanisms, the most studied are microRNAs. A lower expression of miR-1, miR-133a, miR-133b, miR-208a, and miR-208b microRNAs were identified in heart tissue samples of CCC patients when compared to the healthy control group [155]. In addition, it was observed that during the chronic phase of the disease, IND patients have overexpression of microRNA-208a in plasma, when compared to cardiac patients, which may be a possible biomarker of disease progression [156]. In 2019, Nonaka et al., showed that the microRNAs MiR-19a-3p, miR-21-5p, miR-29b-3p, miR-30a, and miR-199b were differentially expressed in patients with CCC when compared to the indeterminate form. Additionally, their expression presented a positive correlation with cardiac dysfunction and fibrosis, and a negative correlation with ejection fraction and left ventricular tension, suggesting a relationship between the expression of these microRNAs and disease progression [157]. The difficulty of obtaining human samples and the limitation in simulating in vitro a biological environment are factors that may explain why there are so few epigenetic studies in Chagas disease patients. Thus, experiments in animal models and in silico analysis are strategies used to better understand the role of miRNA in this disease.
In 2015, Navarro et al., infected C57BL/6 mice with Colombian T. cruzi strain, evaluated the expression of different miRNAs at 15, 30, and 45 days after infection, and identified which miRNAs were differentially expressed. They reported that an association was observed between changes in the QT interval and the expression of miRNAs. miR-20, miR-20b, miR-21, miR-142, miR-146a, miR-146b, miR-155, miR-182, miR203, miR-222 had an increased expression, while in miR-139, miR -145, miR-149, miR-322, miR-503, a reduction of expression was observed [158]. The same group, in 2017, evaluated the role of miRNAs in the regulation of transcriptional changes during the acute phase of T. cruzi infection in mice. Using the computational evaluation of the integrated genome-wide analysis of genes and miRNA expression changes, the authors predicted that several miRNAs are related to disease progression of arrhythmia, fibrosis, myocarditis, and hypertrophy of the heart and that miR-238-3p, miR-149-5p, miR-143-3p, miR-145-5p, and miR-486-5p are present in these four pathological changes [159]. It was observed that miR-21, miR-146a, and miR-155 were overexpressed in cardiac tissue and plasma of mice infected with T. cruzi, both in the chronic and acute phases. Of these microRNAs, only miR-146a was found to be expressed in both stages of the disease, thus emerging as a potential biomarker of infection [160]. The upregulation of miR-21 and collagen expression was observed in serum samples from CCC patients, cardiac tissue from infected mice, and tests in cardiac fibroblast culture. Thus, this specific microRNA would be a mediator involved in the pathogenesis of cardiac fibrosis, being a potential therapeutic target for CCC [161]. Furthermore, it was observed that the lack of miR-155 caused strong parasitic infection and decreased the survival of infected mice, and these showed a reduction in the production of IFN-gamma and TNF, which are pro-inflammatory cytokines [162].
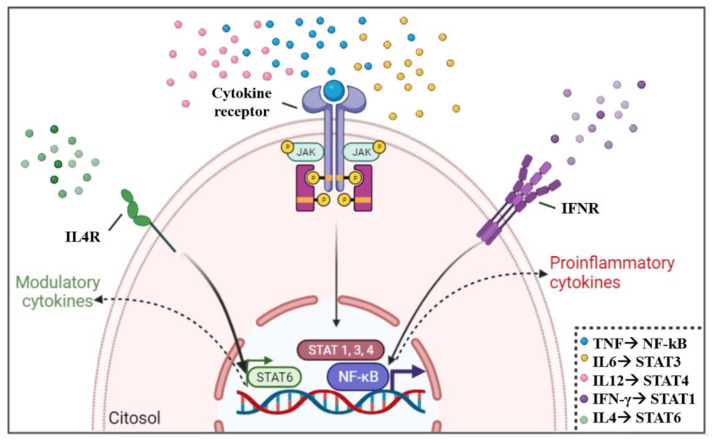
Cytokines bind to their receptors on the cell surface, leading to activation or inactivation of STATs and NF-kB, which are transcription factors capable of interfering with gene transcription. Target genes regulated by STATs are related to cell survival, growth, apoptosis, host defense, cell stress, and differentiation functions depending on the signaling pathway and target tissue. The NF-kB pathway plays a critical role in regulating the survival, activation, and differentiation of innate immune cells and inflammatory T cells [163,164]. For example, Th1 lymphocyte subpopulations respond to the binding of proinflammatory cytokines to their receptors, such as IFN-γ, IL-6, IL-12, and TNF, which will activate STATs 1, 3, 4, and NF-kB, respectively [39,164]. However, Th2 cells develop a regulatory response, mainly mediated by IL-4 binding, promoting STAT6 pathway activation [165]. Although not directly associated with Th1 and Th2 activation, STAT2 is critical for macrophage activation and type 1 interferon responses [166] and STAT5 is associated with cell proliferation and apoptosis [167]. Activation pathways of transcription factors by the engagement of cytokines and their receptors are shown in Figure 3.
Figure 3.
Cytokine activation of STAT and association with Th1/Th2 development. The engagement of inflammatory cytokines, such as IFN-gamma, IL6, IL12, and TNF, with their receptors favors the activation of transcription factors STAT1, STAT3, STAT4, and NF-kB, which contributes to the production of Th1 cytokines. While in modulatory environments, the presence of IL4 cytokine activates STAT6, which contributes to the production of Th2 cytokines. The association of STAT with cytokines (right corner of figure) emphasizes the main STAT associated with the cytokine, although other STAT may also be activated by the same cytokine (Designed with Biorender).
Despite the importance of these mechanisms in influencing protein expression and function, to the best of our knowledge, fewer studies regarding cytokine signaling have been performed in Chagas disease. It has been shown that STAT4 is associated with heart failure in patients with dilated cardiomyopathy [31]. Furthermore, it has been described that T. cruzi can release extracellular vesicles that act on macrophages activating the NF-kB signaling pathway, which in turn, produces inflammatory cytokines contributing to cardiac damage and exacerbated inflammatory pathology observed in CCC [168]. In addition, the blockade of this pathway is important for the reduction of cardiac damage [169] and may emerge as a potential target for intervention. Therefore, analysis of the expression of cytokine receptors and activated STATs will also provide important information regarding cytokine-mediated immune control and unveil additional targets.
5. Concluding Remarks
The significance of this review is that it provides a comprehensive assessment of the role of cytokine and immunoregulation in the differential clinical evolution of human Chagas disease, presenting critical aspects related to their role in controlling or exacerbating the inflammatory response, as well as pre- and post-transcriptional control of these molecules. Below, we highlight some of the potential future directions that can arise from the current knowledge in the area. There is convincing evidence that cytokine networks can influence the clinical outcome of CD. In fact, the studies reviewed in this article reinforce the correlation of inflammatory cytokines, such as IFN-gamma and TNF, with the worst clinical outcome and progression of CCC. Cytokines with a modulatory profile are correlated with the maintenance of IND, without impairment of cardiac and digestive functions. However, the mechanisms by which these molecules act, as well as the complex signaling pathways activated by cytokines, are not completely understood. Additionally, there are a few studies of SNPs in cytokine coding genes in CD patients. Despite that, signaling pathways and SNPs certainly have an impact on the clinical course of the disease. It is important to emphasize that several other aspects of the host, as well as the parasite’s intrinsic characteristics, are key determinants in cytokine responses. In addition, although many studies assess the profile of cytokines in individuals with established chronic disease, longitudinal study cohorts that follow the profile of this immune network from the beginning of the infection should be encouraged to understand the importance and impact of these molecules in CD evolution. Given the solid and extensive knowledge gained over the years related to cytokine influence in Chagas disease severity, future studies focused on their modulation, or on their receptors and signaling pathways, to control disease pathology may provide important immunotherapeutic alternatives to treat Chagas disease alone or in combination with anti-parasite drugs.
Acknowledgments
We would like to thank Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), Fundação de Amparo ’a Pesquisa do Estado de Minas Gerais (FAPEMIG) and the National Institutes of Health (NIH) for supporting our work over the years.
Author Contributions
C.C.K., E.G.A.N., T.G.d.S.-S., A.C.C. and C.H.R.P. wrote the manuscript; C.C.K., E.G.A.N. and T.G.d.S.-S. prepared the figures; A.G. and K.J.G. revised and edited the manuscript; W.O.D. conceptualized, wrote, and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Our research has been funded by the National Institutes of Health (grant NIH-RO1), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq, grant Universal), Fundação de Amparo ’a Pesquisa do Estado de Minas Gerais (FAPEMIG, grant Universal/FAP), and Instituto Nacional de Ciência e Tecnologia em Doenças Tropicais (grant INCT-DT).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Olivera M.J., Porras-Villamil J.F., Villar J.C., Herrera E.V., Buitrago G. Chagas Disease-Related Mortality in Colombia from 1979 to 2018: Temporal and Spatial Trends. Rev. Soc. Bras. Med. Trop. 2021;54:e07682020. doi: 10.1590/0037-8682-0768-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Stanaway J.D., Roth G. The Burden of Chagas Disease Estimates and Challenges. Glob. Heart. 2015;10:139–144. doi: 10.1016/j.gheart.2015.06.001. [DOI] [PubMed] [Google Scholar]
- 3.Valdez Tah A.R. Making Sense of Chagas Disease among Mexican Immigrants in California. Med. Anthropol. 2021;40:511–524. doi: 10.1080/01459740.2021.1894560. [DOI] [PubMed] [Google Scholar]
- 4.WHO Chagas Disease in Latin America: An Epidemiological Update Based on 2010 Estimates. Wkly. Epidemiol. Rec. 2015;90:33–43. [PubMed] [Google Scholar]
- 5.Schmunis G.A., Yadon Z.E. Chagas Disease: A Latin American Health Problem Becoming a World Health Problem. Acta. Trop. 2010;115:14–21. doi: 10.1016/j.actatropica.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 6.Jackson Y., Gétaz L., Wolff H., Holst M., Mauris A., Tardin A., Sztajzel J., Besse V., Loutan L., Gaspoz J.M., et al. Prevalence, Clinical Staging and Risk for Blood-Borne Transmission of Chagas Disease among Latin American Migrants in Geneva, Switzerland. PLoS Negl. Trop. Dis. 2010;4:e592. doi: 10.1371/journal.pntd.0000592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Shikanai-Yasuda M.A., Carvalho N.B. Oral Transmission of Chagas Disease. Clin. Infect. Dis. 2012;54:845–852. doi: 10.1093/cid/cir956. [DOI] [PubMed] [Google Scholar]
- 8.Bern C., Messenger L.A., Whitman J.D., Maguire J.H. Chagas Disease in the United States: A Public Health Approach. Clin. Microbiol. Rev. 2019;33:e00023–e00119. doi: 10.1128/CMR.00023-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Dias E., Laranja F.S., Miranda A., Nobrega G. Chagas’ Disease: A Clinical, Epidemiologic, and Pathologic Study. Circulation. 1956;14:1035–1060. doi: 10.1161/01.CIR.14.6.1035. [DOI] [PubMed] [Google Scholar]
- 10.Echavarría N.G., Echeverría L.E., Stewart M., Gallego C., Saldarriaga C. Chagas Disease: Chronic Chagas Cardiomyopathy. Curr. Probl. Cardiol. 2021;46:100507. doi: 10.1016/j.cpcardiol.2019.100507. [DOI] [PubMed] [Google Scholar]
- 11.Dutra W.O., Rocha M.O.C., Teixeira M.M. The Clinical Immunology of Human Chagas Disease. Trends Parasitol. 2005;21:581–587. doi: 10.1016/j.pt.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 12.Candia-Puma M.A., Machaca-Luque L.Y., Roque-Pumahuanca B.M., Galdino A.S., Giunchetti R.C., Coelho E.A.F., Chávez-Fumagalli M.A. Accuracy of Diagnostic Tests for the Detection of Chagas Disease: A Systematic Review and Meta-Analysis. Diagnostics. 2022;12:2752. doi: 10.3390/diagnostics12112752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nunes M.C.P., Beaton A., Acquatella H., Bern C., Bolger A.F., Echeverría L.E., Dutra W.O., Gascon J., Morillo C.A., Oliveira-Filho J., et al. Chagas Cardiomyopathy: An Update of Current Clinical Knowledge and Management: A Scientific Statement From the American Heart Association. Circulation. 2018;138:e169–e209. doi: 10.1161/CIR.0000000000000599. [DOI] [PubMed] [Google Scholar]
- 14.Benziger C.P., do Carmo G.A.L., Ribeiro A.L.P. Chagas Cardiomyopathy: Clinical Presentation and Management in the Americas. Cardiol. Clin. 2017;35:31–47. doi: 10.1016/j.ccl.2016.08.013. [DOI] [PubMed] [Google Scholar]
- 15.Franco D.J., Vago A.R., Chiari E., Meira F.C.A., Galvão L.M.C., Machado C.R.S. Trypanosoma cruzi: Mixture of two populations can modify virulence and tissue tropism in tat. Exp. Parasitol. 2003;104:54–61. doi: 10.1016/S0014-4894(03)00119-X. [DOI] [PubMed] [Google Scholar]
- 16.Vago A.R., Andrade L.O., Leite A.A., d’Ávila Reis D., Macedo A.M., Adad S.J., Tostes S., da Consolação M.V.M., Filho G.B., Pena S.D.J. Genetic Characterization of Trypanosoma cruzi Directly from Tissues of Patients with Chronic Chagas Disease: Differential Distribution of Genetic Types into Diverse Organs. Am. J. Pathol. 2000;156:1805–1809. doi: 10.1016/S0002-9440(10)65052-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rodrigues C.M., Valadares H.M.S., Francisco A.F., Arantes J.M., Campos C.F., Teixeira-Carvalho A., Martins-Filho O.A., Araujo M.S.S., Arantes R.M.E., Chiari E., et al. Coinfection with Different Trypanosoma cruzi Strains Interferes with the Host Immune Response to Infection. PLoS Negl. Trop. Dis. 2010;4:e846. doi: 10.1371/journal.pntd.0000846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Souza P.E.A., Rocha M.O.C., Rocha-Vieira E., Menezes C.A.S., Chaves A.C.L., Gollob K.J., Dutra W.O. Monocytes from Patients with Indeterminate and Cardiac Forms of Chagas’ Disease Display Distinct Phenotypic and Functional Characteristics Associated with Morbidity. Infect. Immun. 2004;72:5283–5291. doi: 10.1128/IAI.72.9.5283-5291.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Magalhães L.M.D., Gollob K.J., Zingales B., Dutra W.O. Pathogen Diversity, Immunity, and the Fate of Infections: Lessons Learned from Trypanosoma cruzi Human-Host Interactions. Lancet Microbe. 2022;3:e711–e722. doi: 10.1016/S2666-5247(21)00265-2. [DOI] [PubMed] [Google Scholar]
- 20.Casares-Marfil D., Strauss M., Bosch-Nicolau P., lo Presti M.S., Molina I., Chevillard C., Cunha-Neto E., Sabino E., Ribeiro A.L.P., González C.I., et al. A Genome-Wide Association Study Identifies Novel Susceptibility Loci in Chronic Chagas Cardiomyopathy. Clin. Infect. Dis. 2021;73:672–679. doi: 10.1093/cid/ciab090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bartekova M., Radosinska J., Jelemensky M., Dhalla N.S. Role of Cytokines and Inflammation in Heart Function during Health and Disease. Heart Fail Rev. 2018;23:733–758. doi: 10.1007/s10741-018-9716-x. [DOI] [PubMed] [Google Scholar]
- 22.Llaguno M., da Silva M.V., Batista L.R., da Silva D.A.A., de Sousa R.C., de Resende L.A.P.R., da Silva V.J.D., Lages-Silva E., Oliveira C.J.F., Machado J.R., et al. T-Cell Immunophenotyping and Cytokine Production Analysis in Patients with Chagas Disease 4 Years after Benznidazole Treatment. Infect. Immun. 2019;87:e00103–e00119. doi: 10.1128/IAI.00103-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dutra W.O., Gollob K.J., Pinto-Dias J.C., Gazzinelli G., Correa-Oliveira R., Coffman R.L., Carvalho-Parra J.F. Cytokine MRNA Profile of Peripheral Blood Mononuclear Cells Isolated from Individuals with Trypanosoma cruzi Chronic Infection. Scand. J. Immunol. 1997;45:74–80. doi: 10.1046/j.1365-3083.1997.d01-362.x. [DOI] [PubMed] [Google Scholar]
- 24.Abel L.C.J., Rizzo L.v., Ianni B., Albuquerque F., Bacal F., Carrara D., Bocchi E.A., Teixeira H.C., Mady C., Kalil J., et al. Chronic Chagas’ Disease Cardiomyopathy Patients Display an Increased IFN-gammaResponse to Trypanosoma cruzi Infection. J. Autoimmun. 2001;17:99–107. doi: 10.1006/jaut.2001.0523. [DOI] [PubMed] [Google Scholar]
- 25.Benvenuti L.A., Roggério A., Nishiya A.S., Campos S.V., Fiorelli A.I., Levi J.E. Trypanosoma Cruzi Persistence in the Native Heart Is Associated with High-Grade Myocarditis, but Not with Chagas’ Disease Reactivation after Heart Transplantation. J. Heart Lung Transplant. 2014;33:698–703. doi: 10.1016/j.healun.2014.01.920. [DOI] [PubMed] [Google Scholar]
- 26.Bestetti R.B., Dellalibera-Joviliano R., Lopes G.S., Faria-Jr M., Furlan-Daniel R., Lopes K.C., Batista D.R. Determination of the Th1, Th2, Th17, and Treg Cytokine Profile in Patients with Chronic Chagas Heart Disease and Systemic Arterial Hypertension. Heart Vessels. 2019;34:123–133. doi: 10.1007/s00380-018-1228-z. [DOI] [PubMed] [Google Scholar]
- 27.Ferreira R.C., Ianni B.M., Abel L.C.J., Buck P., Mady C., Kalil J., Cunha-Neto E. Increased Plasma Levels of Tumor Necrosis Factor-Alpha in Asymptomatic/“indeterminate” and Chagas Disease Cardiomyopathy Patients. Mem. Inst. Oswaldo. Cruz. 2003;98:407–411. doi: 10.1590/S0074-02762003000300021. [DOI] [PubMed] [Google Scholar]
- 28.Reis D.D., Jones E.M., Tostes S., Lopes E.R., Gazzinelli G., Colley D.G., McCurley T.L. Characterization of Inflammatory Infiltrates in Chronic Chagasic Myocardial Lesions: Presence of Tumor Necrosis Factor-Alpha+ Cells and Dominance of Granzyme A+, CD8+ Lymphocytes. Am. J. Trop. Med. Hyg. 1993;48:637–644. doi: 10.4269/ajtmh.1993.48.637. [DOI] [PubMed] [Google Scholar]
- 29.Reis M.M., Higuchi M.D.L., Benvenuti L.A., Aiello V.D., Gutierrez P.S., Bellotti G., Pileggi F. An in Situ Quantitative Immunohistochemical Study of Cytokines and IL-2R+ in Chronic Human Chagasic Myocarditis: Correlation with the Presence of Myocardial Trypanosoma Cruzi Antigens. Clin. Immunol. Immunopathol. 1997;83:165–172. doi: 10.1006/clin.1997.4335. [DOI] [PubMed] [Google Scholar]
- 30.Sousa G.R., Gomes J.A.S., Fares R.C.G., Damásio M.P.D.S., Chaves A.T., Ferreira K.S., Nunes M.C.P., Medeiros N.I., Valente V.A.A., Corrêa-Oliveira R., et al. Plasma Cytokine Expression Is Associated with Cardiac Morbidity in Chagas Disease. PLoS ONE. 2014;9:e87082. doi: 10.1371/journal.pone.0087082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rodrigues D.B.R., dos Reis M.A., Romano A., Pereira S.A.D.L., Teixeira V.D.P.A., Tostes Junior S., Rodrigues V. In Situ Expression of Regulatory Cytokines by Heart Inflammatory Cells in Chagas’ Disease Patients with Heart Failure. Clin. Dev. Immunol. 2012;2012:361730. doi: 10.1155/2012/361730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Talvani A., Rocha M.O.C., Barcelos L.S., Gomes Y.M., Ribeiro A.L., Teixeira M.M. Elevated Concentrations of CCL2 and Tumor Necrosis Factor-α in Chagasic Cardiomyopathy. Clin. Infect. Dis. 2004;38:943–950. doi: 10.1086/381892. [DOI] [PubMed] [Google Scholar]
- 33.Cunha-Neto E., Coelho V., Guilherme L., Fiorelli A., Stolf N., Kalil J. Autoimmunity in Chagas’ Disease. Identification of Cardiac Myosin-B13 Trypanosoma cruzi Protein Crossreactive T Cell Clones in Heart Lesions of a Chronic Chagas’ Cardiomyopathy Patient. J. Clin. Investig. 1996;98:1709–1712. doi: 10.1172/JCI118969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Neves E.G.A., Koh C.C., Padilha da Silva J.L., Passos L.S.A., Villani F.N.A., dos Santos J.S.C., Menezes C.A.S., Silva V.R., Tormin J.P.A.S., Evangelista G.F.B., et al. Systemic Cytokines, Chemokines and Growth Factors Reveal Specific and Shared Immunological Characteristics in Infectious Cardiomyopathies. Cytokine. 2021;148:155711. doi: 10.1016/j.cyto.2021.155711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Neves E.G.A., Koh C.C., Souza-Silva T.G., Passos L.S.A., Silva A.C.C., Velikkakam T., Villani F., Coelho J.S., Brodskyn C.I., Teixeira A., et al. T-Cell Subpopulations Exhibit Distinct Recruitment Potential, Immunoregulatory Profile and Functional Characteristics in Chagas versus Idiopathic Dilated Cardiomyopathies. Front. Cardiovasc. Med. 2022;9:787423. doi: 10.3389/fcvm.2022.787423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Dutra W.O., Menezes C.A.S., Magalhães L.M.D., Gollob K.J. Immunoregulatory Networks in Human Chagas Disease. Parasite Immunol. 2014;36:377–387. doi: 10.1111/pim.12107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nogueira L.G., Santos R.H.B., Fiorelli A.I., Mairena E.C., Benvenuti L.A., Bocchi E.A., Stolf N.A., Kalil J., Cunha-Neto E. Myocardial Gene Expression of T-Bet, GATA-3, Ror-Γt, FoxP3, and Hallmark Cytokines in Chronic Chagas Disease Cardiomyopathy: An Essentially Unopposed TH1-Type Response. Mediat. Inflamm. 2014;2014:914326. doi: 10.1155/2014/914326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guedes P.M.M., Gutierrez F.R.S., Silva G.K., Dellalibera-Joviliano R., Rodrigues G.J., Bendhack L.M., Rassi Jr A., Rassi A., Schmidt A., Maciel B.C., et al. Deficient Regulatory T Cell Activity and Low Frequency of IL-17-Producing T Cells Correlate with the Extent of Cardiomyopathy in Human Chagas’ Disease. PLoS Negl. Trop. Dis. 2012;6:e1630. doi: 10.1371/journal.pntd.0001630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gomes J.A.S., Rocha M.O.C., Gazzinelli G., Gomes J.A.S., Rocha M.O.C. Evidence That Development of Severe Cardiomyopathy in Human Chagas’ Disease Is Due to a Th1-Specific Immune Response Evidence That Development of Severe Cardiomyopathy in Human Chagas’ Disease Is Due to a Th1-Specific Immune Response. Infect. Immun. 2003;71:1185–1193. doi: 10.1128/IAI.71.3.1185-1193.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vitelli-Avelar D.M., Sathler-Avelar R., Teixeira-Carvalho A., Pinto Dias J.C., Gontijo E.D., Faria A.M., Elói-Santos S.M., Martins-Filho O.A. Strategy to Assess the Overall Cytokine Profile of Circulating Leukocytes and Its Association with Distinct Clinical Forms of Human Chagas Disease. Scand. J. Immunol. 2008;68:516–525. doi: 10.1111/j.1365-3083.2008.02167.x. [DOI] [PubMed] [Google Scholar]
- 41.Passos L.S.A., Magalhães L.M.D., Soares R.P., Marques A.F., Alves M.L.R., Giunchetti R.C., Nunes M.d.C.P., Gollob K.J., Dutra W.O. Activation of Human CD11b+ B1 B-Cells by Trypanosoma cruzi-Derived Proteins Is Associated with Protective Immune Response in Human Chagas Disease. Front. Immunol. 2019;9:3015. doi: 10.3389/fimmu.2018.03015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Berbert L.R., González F.B., Villar S.R., Vigliano C., Lioi S., Beloscar J., Bottasso O.A., Silva-Barbosa S.D., Savino W., Pérez A.R. Enhanced Migratory Capacity of T Lymphocytes in Severe Chagasic Patients Is Correlated With VLA-4 and TNF-α Expression. Front. Cell. Infect. Microbiol. 2021;11:782. doi: 10.3389/fcimb.2021.713150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Silva W.T., Costa H.S., de Lima V.P., Xavier D.M., Mendonça V.A., Lacerda A.C.R., Lage V.K.S., Lima M.M.O., Rocha M.O.C., Figueiredo P.H.S. Plasma Levels of Soluble TNF Receptors Are Associated with Cardiac Function in Patients with Chagas Heart Disease. Int. J. Cardiol. 2020;316:101–103. doi: 10.1016/j.ijcard.2020.04.053. [DOI] [PubMed] [Google Scholar]
- 44.de Alba-Alvarado M., Salazar-Schettino P.M., Jiménez-Álvarez L., Cabrera-Bravo M., García-Sancho C., Zenteno E., Vazquez-Antona C., Cruz-Lagunas A., Zúñiga J., Bucio-Torres M.I. Th-17 Cytokines Are Associated with Severity of Trypanosoma cruzi Chronic Infection in Pediatric Patients from Endemic Areas of Mexico. Acta Trop. 2018;178:134–141. doi: 10.1016/j.actatropica.2017.11.009. [DOI] [PubMed] [Google Scholar]
- 45.López L., Arai K., Giménez E., Jiménez M., Pascuzo C., Rodríguez-Bonfante C., Bonfante-Cabarcas R. Las Concentraciones Séricas de Interleucina-6 y Proteína C Reactiva Se Incrementan a Medida Que La Enfermedad de Chagas Evoluciona Hacia El Deterioro de La Función Cardíaca. Rev. Esp. Cardiol. 2006;59:50–56. doi: 10.1157/13083649. [DOI] [PubMed] [Google Scholar]
- 46.de Araújo F.F., Lima Torres K.C., Viana Peixoto S., Pinho Ribeiro A.L., Vaz Melo Mambrini J., Bortolo Rezende V., Lima Silva M.L., Loyola Filho A.I., Teixeira-Carvalho A., Lima-Costa M.F., et al. CXCL9 and CXCL10 Display an Age-Dependent Profile in Chagas Patients: A Cohort Study of Aging in Bambui, Brazil. Infect. Dis. Poverty. 2020;9:51. doi: 10.1186/s40249-020-00663-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferreira R.R., Waghabi M.C., Bailly S., Feige J.J., Hasslocher-Moreno A.M., Saraiva R.M., Araujo-Jorge T.C. The Search for Biomarkers and Treatments in Chagas Disease: Insights From TGF-Beta Studies and Immunogenetics. Front. Cell. Infect. Microbiol. 2022;11:1310. doi: 10.3389/fcimb.2021.767576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cutrullis R.A., Petray P.B., Schapachnik E., Sánchez R., Postan M., González M.N., Martín V., Corral R.S. Elevated Serum Levels of Macrophage Migration Inhibitory Factor Are Associated with Progressive Chronic Cardiomyopathy in Patients with Chagas Disease. PLoS ONE. 2013;8:e57181. doi: 10.1371/journal.pone.0057181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cruz-Robles D., Vargas-Alarcón G., Ortíz-Muñiz R., Reyes P.A., Monteon V.M. Serum Cytokines and Activation Ex Vivo of CD4+ and CD8+ T Cells in Chagasic Chronic Mexican Patients. Ann. Parasitol. 2017;63:299–308. doi: 10.17420/AP6304.116. [DOI] [PubMed] [Google Scholar]
- 50.Villani F.N.A., Rocha M.O.D.C., Nunes M.D.C.P., Antonelli L.R.D.V., Magalhães L.M.D., dos Santos J.S.C., Gollob K.J., Dutra W.O. Trypanosoma cruzi-Induced Activation of Functionally Distinct ab and gd CD4- CD8- T Cells in Individuals with Polar Forms of Chagas’ Disease. Infect. Immun. 2010;78:4421–4430. doi: 10.1128/IAI.00179-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Souza P.E.A., Rocha M.O.C., Menezes C.A.S., Coelho J.S., Chaves A.C.L., Gollob K.J., Dutra W.O. Trypanosoma cruzi Infection Induces Differential Modulation of Costimulatory Molecules and Cytokines by Monocytes and T Cells from Patients with Indeterminate and Cardiac Chagas’ Disease. Infect. Immun. 2007;75:1886–1894. doi: 10.1128/IAI.01931-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Magalhães L.M.D., Villani F.N.A., Nunes M.D.C.P., Gollob K.J., Rocha M.O.C., Dutra W.O. High Interleukin 17 Expression Is Correlated with Better Cardiac Function in Human Chagas Disease. J. Infect. Dis. 2013;207:661–665. doi: 10.1093/infdis/jis724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Campi-Azevedo A.C., Gomes J.A.S., Teixeira-Carvalho A., Silveira-Lemos D., Vitelli-Avelar D.M., Sathler-Avelar R., Peruhype-Magalhães V., Béla S.R., Silvestre K.F., Batista M.A., et al. Etiological Treatment of Chagas Disease Patients with Benznidazole Lead to a Sustained Pro-Inflammatory Profile Counterbalanced by Modulatory Events. Immunobiology. 2015;220:564–574. doi: 10.1016/j.imbio.2014.12.006. [DOI] [PubMed] [Google Scholar]
- 54.Medeiros N.I., Gomes J.A.S., Fiuza J.A., Sousa G.R., Almeida E.F., Novaes R.O., Rocha V.L.S., Chaves A.T., Dutra W.O., Rocha M.O.C., et al. MMP-2 and MMP-9 Plasma Levels Are Potential Biomarkers for Indeterminate and Cardiac Clinical Forms Progression in Chronic Chagas Disease. Sci. Rep. 2019;9:14170. doi: 10.1038/s41598-019-50791-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Bautista-López N.L., Morillo C.A., López-Jaramillo P., Quiroz R., Luengas C., Silva S.Y., Galipeau J., Lalu M.M., Schulz R. Matrix Metalloproteinases 2 and 9 as Diagnostic Markers in the Progression to Chagas Cardiomyopathy. Am. Heart J. 2013;165:558–566. doi: 10.1016/j.ahj.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 56.Fares R.C.G., Correa-Oliveira R., de Araújo F.F., Keesen T.S.L., Chaves A.T., Fiuza J.A., Ferreira K.S., Rocha M.O.C., Gomes J.A.S. Identification of Phenotypic Markers of B Cells from Patients with Chagas Disease. Parasite Immunol. 2013;35:214–223. doi: 10.1111/pim.12038. [DOI] [PubMed] [Google Scholar]
- 57.Okamoto E.E., Sherbuk J.E., Clark E.H., Marks M.A., Gandarilla O., Galdos-Cardenas G., Vasquez-Villar A., Choi J., Crawford T.C., Q R., et al. Biomarkers in Trypanosoma cruzi-Infected and Uninfected Individuals with Varying Severity of Cardiomyopathy in Santa Cruz, Bolivia. PLoS Negl. Trop. Dis. 2014;8:e3227. doi: 10.1371/journal.pntd.0003227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Baron M.A., Ferreira L.R.P., Teixeira P.C., Moretti A.I.S., Santos R.H.B., Frade A.F., Kuramoto A., Debbas V., Benvenuti L.A., Gaiotto F.A., et al. Matrix Metalloproteinase 2 and 9 Enzymatic Activities Are Selectively Increased in the Myocardium of Chronic Chagas Disease Cardiomyopathy Patients: Role of TIMPs. Front. Cell. Infect. Microbiol. 2022;12:249. doi: 10.3389/fcimb.2022.836242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.De Oliveira A.P., Ayo C.M., Bestetti R.B., de Mattos C.C.B., Cavasini C.E., de Mattos L.C. The Role of CCR5 in Chagas Disease—A Systematic Review. Infect. Genet. Evol. 2016;45:132–137. doi: 10.1016/j.meegid.2016.08.012. [DOI] [PubMed] [Google Scholar]
- 60.Nogueira L.G., Santos R.H.B., Ianni B.M., Fiorelli A.I., Mairena E.C., Benvenuti L.A., Frade A., Donadi E., Dias F., Saba B., et al. Myocardial Chemokine Expression and Intensity of Myocarditis in Chagas Cardiomyopathy Are Controlled by Polymorphisms in CXCL9 and CXCL10. PLoS Negl. Trop. Dis. 2012;6:e1867. doi: 10.1371/journal.pntd.0001867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Luz P.R., Velavan T.P., Kremsner P.G., Messias-Reason I.J.T. Association of IP-10 and PDGF-BB Levels with Clinical Forms of Chronic Chagas Disease. Int. J. Cardiol. 2013;169:e53–e55. doi: 10.1016/j.ijcard.2013.08.110. [DOI] [PubMed] [Google Scholar]
- 62.Roffe E., dos Santos L.I., Santos M.O., Henriques P.M., Teixeira-Carvalho A., Martins-Filho O.A., Rocha M.O.C., Eloi-Santos S.M., Correa-Oliveira R., Antonelli L.R.V. Increased Frequencies of Circulating CCR5+ Memory T Cells Are Correlated to Chronic Chagasic Cardiomyopathy Progression. J. Leukoc. Biol. 2019;106:641–652. doi: 10.1002/JLB.MA1118-472R. [DOI] [PubMed] [Google Scholar]
- 63.Batista A.M., Alvarado-Arnez L.E., Alves S.M., Melo G., Pereira I.R., de Souza Ruivo L.A., da Silva A.A., Gibaldi D., da Silva T.d.E.S., de Lorena V.M.B., et al. Genetic Polymorphism at CCL5 Is Associated with Protection in Chagas’ Heart Disease: Antagonistic Participation of CCR1+ and CCR5+ Cells in Chronic Chagasic Cardiomyopathy. Front. Immunol. 2018;9:615. doi: 10.3389/fimmu.2018.00615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gomes J.A.S., Bahia-Oliveira L.M.G., Rocha M.O.C., Busek S.C.U., Tekeira M.M., Silva J.S., Correa-Oliveira R. Type 1 Chemokine Receptor Expression in Chagas’ Disease Correlates with Morbidity in Cardiac Patients. Infect. Immun. 2005;73:7960–7966. doi: 10.1128/IAI.73.12.7960-7966.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Gómez-Olarte S., Bolaños N.I., Echeverry M., Rodríguez A.N., Cuéllar A., Puerta C.J., Mariño A., González J.M. Intermediate Monocytes and Cytokine Production Associated with Severe Forms of Chagas Disease. Front. Immunol. 2019;10:1671. doi: 10.3389/fimmu.2019.01671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dutra W.O., Martins-Filho O.A., Caņado J.R., Pinto-dias J.C., Brener Z., Freeman G.L., Colley D.G., Gazzinelll G., Parra J.C. Activated T and B Lymphocytes in Peripheral Blood of Patients with Chagas’ Disease. Int. Immunol. 1994;6:499–506. doi: 10.1093/intimm/6.4.499. [DOI] [PubMed] [Google Scholar]
- 67.Menezes C.A.S., Rocha M.O.C., Souza P.E.A., Chaves A.C.L., Gollob K.J., Dutra W.O. Phenotypic and Functional Characteristics of CD28+ and CD28- Cells from Chagasic Patients: Distinct Repertoire and Cytokine Expression. Clin. Exp. Immunol. 2004;137:129–138. doi: 10.1111/j.1365-2249.2004.02479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Velikkakam T., Gollob K.J., Dutra W.O. Double-Negative T Cells: Setting the Stage for Disease Control or Progression. Immunology. 2022;165:371–385. doi: 10.1111/imm.13441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Passos L.S.A., Magalhães L.M.D., Soares R.P., Marques A.F., Nunes M.C.P., Gollob K.J., Dutra W.O. Specific Activation of CD4- CD8- Double-Negative T Cells by Trypanosoma cruzi-Derived Glycolipids Induces a Proinflammatory Profile Associated with Cardiomyopathy in Chagas Patients. Clin. Exp. Immunol. 2017;190:122–132. doi: 10.1111/cei.12992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Passos L.S.A., Koh C.C., Magalhães L.M.D., Nunes M.C.P., Gollob K.J., Dutra W.O. Distinct CD4-CD8- (Double-Negative) Memory T-Cell Subpopulations Are Associated With Indeterminate and Cardiac Clinical Forms of Chagas Disease. Front. Immunol. 2021;12:4667. doi: 10.3389/fimmu.2021.761795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Passos L.S.A., Villani F.N.A., Magalhães L.M.D., Gollob K.J., Antonelli L.R.D.V., Nunes M.C.P., Dutra W.O. Blocking of CD1d Decreases Trypanosoma cruzi-Induced Activation of CD4-CD8-T Cells and Modulates the Inflammatory Response in Patients with Chagas Heart Disease. J. Infect. Dis. 2016;214:935–944. doi: 10.1093/infdis/jiw266. [DOI] [PubMed] [Google Scholar]
- 72.Choi Y.S., Dieter J.A., Rothaeusler K., Luo Z., Baumgarth N. B-1 Cells in the Bone Marrow Are a Significant Source of Natural IgM. Eur. J. Immunol. 2012;42:120–129. doi: 10.1002/eji.201141890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ding C., Ma Y., Chen X., Liu M., Cai Y., Hu X., Xiang D., Nath S., Zhang H., Ye H., et al. Integrin CD11b Negatively Regulates BCR Signalling to Maintain Autoreactive B Cell Tolerance. Nat. Commun. 2013;4:2813. doi: 10.1038/ncomms3813. [DOI] [PubMed] [Google Scholar]
- 74.Zhang X. Regulatory Functions of Innate-like B Cells. Cell. Mol. Immunol. 2013;10:113–121. doi: 10.1038/cmi.2012.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dutra W.O., Profeta da Luz Z.M., Cançado J.R., Pereira M.E., Brígido-Nunes R.M., Galvão L.M.C., Colley D.G., Brener Z., Gazzinelli G., Carvalho-Parra J.F. Influence of Parasite Presence on the Immunologic Profile of Peripheral Blood Mononuclear Cells from Chagasic Patients after Specific Drug Therapy. Parasite Immunol. 1996;18:579–585. doi: 10.1046/j.1365-3024.1996.d01-29.x. [DOI] [PubMed] [Google Scholar]
- 76.Cunha-Neto E., Dzau V.J., Allen P.D., Stamatiou D., Benvenutti L., Higuchi M.L., Koyama N.S., Silva J.S., Kalil J., Liew C.C. Cardiac Gene Expression Profiling Provides Evidence for Cytokinopathy as a Molecular Mechanism in Chagas’ Disease Cardiomyopathy. Am. J. Pathol. 2005;167:305–313. doi: 10.1016/S0002-9440(10)62976-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Teixeira P.C., Ducret A., Langen H., Nogoceke E., Santos R.H.B., Silva Nunes J.P., Benvenuti L., Levy D., Bydlowski S.P., Bocchi E.A., et al. Impairment of Multiple Mitochondrial Energy Metabolism Pathways in the Heart of Chagas Disease Cardiomyopathy Patients. Front. Immunol. 2021;12:4779. doi: 10.3389/fimmu.2021.755782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Fonseca S.G., Reis M.M., Coelho V., Nogueira L.G., Monteiro S.M., Mairena E.C., Bacal F., Bocchi E., Guilherme L., Zheng X.X., et al. Locally Produced Survival Cytokines IL-15 and IL-7 May Be Associated to the Predominance of CD8+ T Cells at Heart Lesions of Human Chronic Chagas Disease Cardiomyopathy. Scand. J. Immunol. 2007;66:362–371. doi: 10.1111/j.1365-3083.2007.01987.x. [DOI] [PubMed] [Google Scholar]
- 79.Barretto A.C.P., Mady C., Arteage-Fernandez E., Stolf N., Lopes E.A., de Lourdes Higuchi M., Bellotti G., Pileggi F. Right Ventricular Endomyocardial Biopsy in Chronic Chagas’ Disease. Am. Heart J. 1986;111:307–312. doi: 10.1016/0002-8703(86)90144-4. [DOI] [PubMed] [Google Scholar]
- 80.Reis M.M., Higuchi M.L., Aiello V.D., Benvenuti L.A. Growth Factors in the Myocardium of Patients with Chronic Chagasic Cardiomyopathy. Rev. Soc. Bras. Med. Trop. 2000;33:509–518. doi: 10.1590/S0037-86822000000600001. [DOI] [PubMed] [Google Scholar]
- 81.Argüello R.J., Vigliano C., Cabeza-Meckert P., Viotti R., Garelli F., Favaloro L.E., Favaloro R.R., Laguens R., Laucella S.A. Presence of Antigen-Experienced T Cells with Low Grade of Differentiation and Proliferative Potential in Chronic Chagas Disease Myocarditis. PLoS Negl. Trop. Dis. 2014;8:e2989. doi: 10.1371/journal.pntd.0002989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Higuchi M.d.L., Gutierrez P.S., Aiello V.D., Palomino S., Bocchi E., Kalil J., Bellotti G., Pileggi F. Immunohistochemical Characterization of Infiltrating Cells in Human Chronic Chagasic Myocarditis: Comparison with Myocardial Rejection Process. Virchows Archiv. A. 1993;423:157–160. doi: 10.1007/BF01614765. [DOI] [PubMed] [Google Scholar]
- 83.Barry M., Bleackley R.C. Cytotoxic T Lymphocytes: All Roads Lead to Death. Nat. Rev. Immunol. 2002;2:401–409. doi: 10.1038/nri819. [DOI] [PubMed] [Google Scholar]
- 84.Dotiwala F., Mulik S., Polidoro R.B., Ansara J.A., Burleigh B.A., Walch M., Gazzinelli R.T., Lieberman J. Killer Lymphocytes Use Granulysin, Perforin and Granzymes to Kill Intracellular Parasites. Nat. Med. 2016;22:210–216. doi: 10.1038/nm.4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Trapani J.A., Smyth M.J. Functional Significance of the Perforin/Granzyme Cell Death Pathway. Nat. Rev. Immunol. 2002;2:735–747. doi: 10.1038/nri911. [DOI] [PubMed] [Google Scholar]
- 86.Ferraz R., Cunha C.F., Pimentel M.I.F., Lyra M.R., Pereira-Da-Silva T., Schubach A.O., Da-Cruz A.M., Bertho A.L. CD3+CD4negCD8neg (Double Negative) T Lymphocytes and NKT Cells as the Main Cytotoxic-Related-CD107a+ Cells in Lesions of Cutaneous Leishmaniasis Caused by Leishmania (Viannia) Braziliensis. Parasites Vectors. 2017;10:219. doi: 10.1186/s13071-017-2152-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lasso P., Mateus J., Pavía P., Rosas F., Roa N., Thomas M.C., López M.C., González J.M., Puerta C.J., Cuéllar A. Inhibitory Receptor Expression on CD8 + T Cells Is Linked to Functional Responses against Trypanosoma cruzi Antigens in Chronic Chagasic Patients. J. Immunol. 2015;195:3748–3758. doi: 10.4049/jimmunol.1500459. [DOI] [PubMed] [Google Scholar]
- 88.Menezes C.A.S., Sullivan A.K., Falta M.T., Mack D.G., Freed B.M., Rocha M.O.C., Gollob K.J., Fontenot A.P., Dutra W.O. Highly Conserved CDR3 Region in Circulating CD4 +Vβ5 + T Cells May Be Associated with Cytotoxic Activity in Chagas Disease. Clin. Exp. Immunol. 2012;169:109–118. doi: 10.1111/j.1365-2249.2012.04608.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Keesen T.S.L., Gomes J.A.S., Fares R.C.G., de Araújo F.F., Ferreira K.S., Chaves A.T., Rocha M.O.C., Correa-Oliveira R. Characterization of CD4 + Cytotoxic Lymphocytes and Apoptosis Markers Induced by Trypanossoma Cruzi Infection. Scand. J. Immunol. 2012;76:311–319. doi: 10.1111/j.1365-3083.2012.02730.x. [DOI] [PubMed] [Google Scholar]
- 90.Dutra W.O., Martins-Filho O.A., Cançado J.R., Pinto-Dias J.C., Brener Z., Gazzinelli G., Carvalho J.F., Colley D.G. Chagasic Patients Lack CD28 Expression on Many of Their Circulating T Lymphocytes. Scand. J. Immunol. 1996;43:88–93. doi: 10.1046/j.1365-3083.1996.d01-9.x. [DOI] [PubMed] [Google Scholar]
- 91.Giraldo N.A., Bolaños N.I., Cuellar A., Roa N., Cucunubá Z., Rosas F., Velasco V., Puerta C.J., González J.M. T Lymphocytes from Chagasic Patients Are Activated but Lack Proliferative Capacity and Down-Regulate CD28 and CD3ζ. PLoS Negl. Trop. Dis. 2013;7:e2038. doi: 10.1371/journal.pntd.0002038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Laucella S., de Titto E.H., Segura E.L., Orn A., Rottenberg M.E. Soluble Cell Adhesion Molecules in Human Chagas’ Disease: Association with Disease Severity and Stage of Infection. Am. J. Trop. Med. Hyg. 1996;55:629–634. doi: 10.4269/ajtmh.1996.55.629. [DOI] [PubMed] [Google Scholar]
- 93.Talvani A., Rocha M.O., Ribeiro A.L., Correa-Oliveira R., Teixeira M.M. Chemokine Receptor Expression on the Surface of Peripheral Blood Mononuclear Cells in Chagas Disease. J. Infec. Dis. 2004;189:214–220. doi: 10.1086/380803. [DOI] [PubMed] [Google Scholar]
- 94.Cunha-Neto E., Chevillard C. Chagas Disease Cardiomyopathy: Immunopathology and Genetics. Mediat. Inflamm. 2014;2014:683230. doi: 10.1155/2014/683230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Flórez O., Martín J., González C.I. Genetic Variants in the Chemokines and Chemokine Receptors in Chagas Disease. Hum. Immunol. 2012;73:852–858. doi: 10.1016/j.humimm.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 96.Knight J. Polymorphisms in Tumor Necrosis Factor and Other Cytokines as Risks for Infectious Diseases and the Septic Syndrome. Curr. Infect. Dis. Rep. 2001;3:427–439. doi: 10.1007/BF03160478. [DOI] [PubMed] [Google Scholar]
- 97.Costa G.C., Rocha M.O.D.C., Moreira P.R., Menezes C.A.S., Silva M.R., Gollob K.J., Dutra W.O. Functional IL-10 Gene Polymorphism Is Associated with Chagas Disease Cardiomyopathy. J. Infect. Dis. 2009;199:451–454. doi: 10.1086/596061. [DOI] [PubMed] [Google Scholar]
- 98.Deghaide N.H.S., Dantas R.O., Donadi E.A. HLA Class I and II Profiles of Patients Presenting with Chagas’ Disease. Dig. Dis. Sci. 1998;43:246–252. doi: 10.1023/A:1018829600200. [DOI] [PubMed] [Google Scholar]
- 99.Alvarado-Arnez L.E., Batista A.M., Alves S.M., Melo G., Maria V., de Lorena B., Cardoso C.C., Pereira I.R., Carrazzone C., Pacheco A.G., et al. Single Nucleotide Polymorphisms of Cytokine-Related Genes and Association with Clinical Outcome in a Chagas Disease Case-Control Study from Brazil. Mem. Inst. Oswaldo Cruz. 2018;113:e170489. doi: 10.1590/0074-02760170489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Tajima F. The Effect of Change in Population Size on DNA Polymorphism. Genetics. 1989;123:597–601. doi: 10.1093/genetics/123.3.597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Arnez L.E.A., Venegas E.N., Ober C., Thompson E.E. Sequence Variation in the IL4 Gene and Resistance to Trypanosoma cruzi Infection in Bolivians. J. Allergy Clin. Immunol. 2011;127:279–282. doi: 10.1016/j.jaci.2010.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Ramasawmy R., Faé K.C., Cunha-Neto E., Borba S.C.P., Ianni B., Mady C., Goldberg A.C., Kalil J. Variants in the Promoter Region of IKBL/NFKBIL1 Gene May Mark Susceptibility to the Development of Chronic Chagas’ Cardiomyopathy among Trypanosoma cruzi-Infected Individuals. Mol. Immunol. 2008;45:283–288. doi: 10.1016/j.molimm.2007.04.015. [DOI] [PubMed] [Google Scholar]
- 103.Frade-Barros A.F., Ianni B.M., Cabantous S., Pissetti C.W., Saba B., Lin-Wang H.T., Buck P., Marin-Neto J.A., Schmidt A., Dias F., et al. Polymorphisms in Genes Affecting Interferon-γ Production and Th1 T Cell Differentiation Are Associated with Progression to Chagas Disease Cardiomyopathy. Front. Immunol. 2020;11:1386. doi: 10.3389/fimmu.2020.01386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Reis P.G., Ayo C.M., de Mattos L.C., de Mattos C.D.C.B., Sakita K.M., de Moraes A.G., Muller L.P., Aquino J.S., Macedo L.C., Mazini P.S., et al. Genetic Polymorphisms of IL17 and Chagas Disease in the South and Southeast of Brazil. J. Immunol. Res. 2017;2017:1017621. doi: 10.1155/2017/1017621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Rodriguez D.A.L., Echeverría L.E., González C.I., Martin J. Investigation of the Role of IL17A Gene Variants in Chagas Disease. Genes Immun. 2015;16:536–540. doi: 10.1038/gene.2015.42. [DOI] [PubMed] [Google Scholar]
- 106.Strauss M., Palma-Vega M., Casares-Marfil D., Bosch-Nicolau P., lo Presti M.S., Molina I., González C.I., Paglini P.A., Schijman A.G., Robello C., et al. Genetic Polymorphisms of IL17A Associated with Chagas Disease: Results from a Meta-Analysis in Latin American Populations. Sci. Rep. 2020;10:5015. doi: 10.1038/s41598-020-61965-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Clipman S.J., Henderson-Frost J., Fu K.Y., Bern C., Flores J., Gilman R.H. Genetic Association Study of NLRP1, CARD, and CASP1 Inflammasome Genes with Chronic Chagas Cardiomyopathy among Trypanosoma cruzi Seropositive Patients in Bolivia. PLoS ONE. 2018;13:e0192378. doi: 10.1371/journal.pone.0192378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Fu K.Y.J., Zamudio R., Frost J.H., Almuedo A., Steinberg H., Clipman S.J., Duran G., Marcus R., Crawford T., Alyesh D., et al. Association of Caspase-1 Polymorphisms with Chagas Cardiomyopathy among Individuals in Santa Cruz, Bolivia. Rev. Soc. Bras. Med. Trop. 2017;50:516–523. doi: 10.1590/0037-8682-0015-2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Pissetti C.W., de Oliveira R.F., Correia D., Nascentes G.A.N., Llaguno M.M., Rodrigues V. Association between the Lymphotoxin-Alpha Gene Polymorphism and Chagasic Cardiopathy. J. Interferon Cytokine Res. 2013;33:130–135. doi: 10.1089/jir.2012.0024. [DOI] [PubMed] [Google Scholar]
- 110.Ramasawmy R., Fae K.C., Cunha-Neto E., Müller N.G., Cavalcanti V.L., Ferreira R.C., Drigo S.A., Ianni B., Mady C., Goldberg A.C., et al. Polymorphisms in the Gene for Lymphotoxin-Alpha Predispose to Chronic Chagas Cardiomyopathy. J. Infect. Dis. 2007;196:1836–1843. doi: 10.1086/523653. [DOI] [PubMed] [Google Scholar]
- 111.Silva M.C., da Silva Medina T., Fuzo C.A., Dias F.C., Freitas-Castro F., Fukutani K.F., Donadi E.A., Cunha-Neto E., Cunha T.M., Silva J.S. Polymorphism in the Catalytic Subunit of the PI3Kγ Gene Is Associated with Trypanosoma cruzi-Induced Chronic Chagasic Cardiomyopathy. Infect. Genet. Evol. 2021;88:104671. doi: 10.1016/j.meegid.2020.104671. [DOI] [PubMed] [Google Scholar]
- 112.Dias F.C., Medina T.d.S., Mendes-Junior C.T., Dantas R.O., Pissetti C.W., Rodrigues Junior V., Dellalibera-Joviliano R., Marin-Neto J.A., Gutierrez F.R.S., Moreau P., et al. Polymorphic Sites at the Immunoregulatory CTLA-4 Gene Are Associated with Chronic Chagas Disease and Its Clinical Manifestations. PLoS ONE. 2013;8:e78367. doi: 10.1371/journal.pone.0078367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Corrêa M.V., da Costa Rocha M.O., de Sousa G.R., Nunes M.C.P., Gollob K.J., Dutra W.O., da Silva Menezes C.A. Low Levels of Vasoactive Intestinal Peptide Are Associated with Chagas Disease Cardiomyopathy. Hum. Immunol. 2013;74:1375–1381. doi: 10.1016/j.humimm.2013.06.028. [DOI] [PubMed] [Google Scholar]
- 114.Flórez O., Martín J., González Rugeles C.I. Interleukin 4, Interleukin 4 Receptor-α and Interleukin 10 Gene Polymorphisms in Chagas Disease. Parasite Immunol. 2011;33:506–511. doi: 10.1111/j.1365-3024.2011.01314.x. [DOI] [PubMed] [Google Scholar]
- 115.Torres O.A., Calzada J.E., Beraún Y., Morillo C.A., González C.I., González A., Martín J. Association of the Macrophage Migration Inhibitory Factor −173G/C Polymorphism with Chagas Disease. Hum. Immunol. 2009;70:543–546. doi: 10.1016/j.humimm.2009.04.022. [DOI] [PubMed] [Google Scholar]
- 116.Torres O.A., Calzada J.E., Beraún Y., Morillo C.A., González A., González C.I., Martín J. Lack of Association between IL-6 -174G/C Gene Polymorphism and Chagas Disease. Tissue Antigens. 2010;76:131–134. doi: 10.1111/j.1399-0039.2010.01478.x. [DOI] [PubMed] [Google Scholar]
- 117.Calzada J.E., Beraún Y., González C.I., Martín J. Transforming Growth Factor Beta 1 (TGFβ1) Gene Polymorphisms and Chagas Disease Susceptibility in Peruvian and Colombian Patients. Cytokine. 2009;45:149–153. doi: 10.1016/j.cyto.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 118.Ferreira R.R., da Silva Madeira F., Alves G.F., da Costa Chambela M., de Oliveira Vaz Curvo E., Moreira A.D.S., de Sá R.A., Mendonça-Lima L., Cabello P.H., Bailly S., et al. TGF-β Polymorphisms Are a Risk Factor for Chagas Disease. Dis. Markers. 2018;2018:4579198. doi: 10.1155/2018/4579198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zafra G., Flórez O., Morillo C.A., Echeverría L.E., Martín J., González C.I. Polymorphisms of Toll-like Receptor 2 and 4 Genes in Chagas Disease. Mem. Inst. Oswaldo Cruz. 2008;103:27–30. doi: 10.1590/S0074-02762008000100004. [DOI] [PubMed] [Google Scholar]
- 120.Weitzel T., Zulantay I., Danquah I., Hamann L., Schumann R.R., Apt W., Mockenhaupt F.P. Mannose-Binding Lectin and Toll-like Receptor Polymorphisms and Chagas Disease in Chile. Am. J. Trop. Med. Hyg. 2012;86:229–232. doi: 10.4269/ajtmh.2012.11-0539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Da Silva Cruz G., Angelo A.L.D., Larocca T.F., Macedo C.T., Noya-Rabelo M., Correia L.C.L., Torreão J.A., de Freitas Souza B.S., dos Santos R.R., Soares M.B.P. Assessment of Galectin-3 Polymorphism in Subjects with Chronic Chagas Disease. Arq. Bras. Cardiol. 2015;105:472–478. doi: 10.5935/abc.20150105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Rodriguez D.A.L., González C.I., Martin J. Analysis of Association of FOXO3 Gene with Trypanosoma cruzi Infection and Chronic Chagasic Cardiomyopathy. HLA. 2016;87:449–452. doi: 10.1111/tan.12808. [DOI] [PubMed] [Google Scholar]
- 123.Faé K.C., Drigo S.A., Cunha-Neto E., Ianni B., Mady C., Kalil J., Goldberg A.C. HLA and β-Myosin Heavy Chain Do Not Influence Susceptibility to Chagas’ Disease Cardiomyopathy. Microbes Infect. 2000;2:745–751. doi: 10.1016/S1286-4579(00)00501-3. [DOI] [PubMed] [Google Scholar]
- 124.Layrisse Z., Fernandez M.T., Montagnani S., Matos M., Balbas O., Herrera F., Colorado I.A., Catalioti F., Acquatella H. HLA-C*03 Is a Risk Factor for Cardiomyopathy in Chagas Disease. Hum. Immunol. 2000;61:925–929. doi: 10.1016/S0198-8859(00)00161-0. [DOI] [PubMed] [Google Scholar]
- 125.Nieto A., Beraún Y., Callado M.D., Caballero A., Alonso A., González A., Martín J. HLA Haplotypes Are Associated with Differential Susceptibility to Trypanosoma Cruzi Infection. Tissue Antigens. 2000;55:195–198. doi: 10.1034/j.1399-0039.2000.550301.x. [DOI] [PubMed] [Google Scholar]
- 126.Del Puerto F., Nishizawa J.E., Kikuchi M., Roca Y., Avilas C., Gianella A., Lora J., Velarde F.U.G., Miura S., Komiya N., et al. Protective Human Leucocyte Antigen Haplotype, HLA-DRB1*01-B*14, against Chronic Chagas Disease in Bolivia. PLoS Negl. Trop. Dis. 2012;6:e1587. doi: 10.1371/journal.pntd.0001587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.De Oliveira A.P., Bernardo C.R., da Silveira Camargo A.V., Ronchi L.S., Borim A.A., de Mattos C.C.B., de Campos E., Castiglioni L., Netinho J.G., Cavasini C.E., et al. Genetic Susceptibility to Cardiac and Digestive Clinical Forms of Chronic Chagas Disease: Involvement of the CCR5 59029 A/G Polymorphism. PLoS ONE. 2015;10:e0141847. doi: 10.1371/journal.pone.0141847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Machuca M.A., Suárez E.U., Echeverría L.E., Martín J., González C.I. SNP/Haplotype Associations of CCR2 and CCR5 Genes with Severity of Chagasic Cardiomyopathy. Hum. Immunol. 2014;75:1210–1215. doi: 10.1016/j.humimm.2014.09.023. [DOI] [PubMed] [Google Scholar]
- 129.Fernández-Mestre M.T., Montagnani S., Layrisse Z. Is the CCR5-59029-G/G Genotype a Protective Factor for Cardiomyopathy in Chagas Disease? Hum. Immunol. 2004;65:725–728. doi: 10.1016/j.humimm.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 130.Calzada J.E., Nieto A., Beraún Y., Martín J. Chemokine Receptor CCR5 Polymorphisms and Chagas’ Disease Cardiomyopathy. Tissue Antigens. 2001;58:154–158. doi: 10.1034/j.1399-0039.2001.580302.x. [DOI] [PubMed] [Google Scholar]
- 131.Cruz-Robles D., Chvez-Gonzlez J.P., Cavazos-Quero M.M., Prez-Mndez O., Reyes P.A., Vargas-Alarcn G. Association between IL-1B and IL-1RN Gene Polymorphisms and Chagas’ Disease Development Susceptibility. Immunol. Investig. 2009;38:231–239. doi: 10.1080/08820130902729637. [DOI] [PubMed] [Google Scholar]
- 132.Flórez O., Zafra G., Morillo C., Martín J., González C.I. Interleukin-1 Gene Cluster Polymorphism in Chagas Disease in a Colombian Case-Control Study. Hum. Immunol. 2006;67:741–748. doi: 10.1016/j.humimm.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 133.Torres O.A., Calzada J.E., Beraún Y., Morillo C.A., González A., González C.I., Martín J. Role of the IFNG +874T/A Polymorphism in Chagas Disease in a Colombian Population. Infect. Genet. Evol. 2010;10:682–685. doi: 10.1016/j.meegid.2010.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Beraún Y., Nieto A., Collado M.D., González A., Martín J. Polymorphisms at Tumor Necrosis Factor (TNF) Loci Are Not Associated with Chagas’ Disease. Tissue Antigens. 1998;52:81–83. doi: 10.1111/j.1399-0039.1998.tb03028.x. [DOI] [PubMed] [Google Scholar]
- 135.Drigo S.A., Cunha-Neto E., Ianni B., Mady C., Faé K.C., Buck P., Kalil J., Goldberg A.C. Lack of Association of Tumor Necrosis Factor-Alpha Polymorphisms with Chagas Disease in Brazilian Patients. Immunol. Lett. 2007;108:109–111. doi: 10.1016/j.imlet.2006.10.008. [DOI] [PubMed] [Google Scholar]
- 136.Pissetti C.W., Correia D., de Oliveira R.F., Llaguno M.M., Balarin M.A.S., Silva-Grecco R.L., Rodrigues V. Genetic and Functional Role of TNF-Alpha in the Development Trypanosoma Cruzi Infection. PLoS Negl. Trop. Dis. 2011;5:e976. doi: 10.1371/journal.pntd.0000976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Criado L., Flórez O., Martín J., González C.I. Genetic Polymorphisms in TNFA/TNFR2 Genes and Chagas Disease in a Colombian Endemic Population. Cytokine. 2012;57:398–401. doi: 10.1016/j.cyto.2011.12.007. [DOI] [PubMed] [Google Scholar]
- 138.Rodríguez-Pérez J.M., Cruz-Robles D., Hernández-Pacheco G., Pérez-Hernández N., Murguía L.E., Granados J., Reyes P.A., Vargas-Alarcón G. Tumor Necrosis Factor-Alpha Promoter Polymorphism in Mexican Patients with Chagas’ Disease. Immunol. Lett. 2005;98:97–102. doi: 10.1016/j.imlet.2004.10.017. [DOI] [PubMed] [Google Scholar]
- 139.Ramasawmy R., Cunha-Neto E., Fae K.C., Borba S.C.P., Teixeira P.C., Ferreira S.C.P., Goldberg A.C., Lanni B., Mady C., Kalil J. Heterozygosity for the S180L Variant of MAL/TIRAP, a Gene Expressing an Adaptor Protein in the Toll-like Receptor Pathway, Is Associated with Lower Risk of Developing Chronic Chagas Cardiomyopathy. J. Infect. Dis. 2009;199:1838–1845. doi: 10.1086/599212. [DOI] [PubMed] [Google Scholar]
- 140.Frade A.F., Pissetti C.W., Ianni B.M., Saba B., Lin-Wang H.T., Nogueira L.G., de Melo Borges A., Buck P., Dias F., Baron M., et al. Genetic Susceptibility to Chagas Disease Cardiomyopathy: Involvement of Several Genes of the Innate Immunity and Chemokine-Dependent Migration Pathways. BMC Infect. Dis. 2013;13:587. doi: 10.1186/1471-2334-13-587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Ramasawmy R., Cunha-Neto E., Faé K.C., Müller N.G., Cavalcanti V.L., a Drigo S., Ianni B., Mady C., Kalil J., Goldberg A.C. BAT1, a Putative Anti-Inflammatory Gene, Is Associated with Chronic Chagas Cardiomyopathy. J. Infect. Dis. 2006;193:1394–1399. doi: 10.1086/503368. [DOI] [PubMed] [Google Scholar]
- 142.Ramasawmy R., Cunha-Neto E., Faé K.C., Martello F.G., Müller N.G., Cavalcanti V.L., Iaani B., Mady C., Kalil J., Goldberg A.C. The Monocyte Chemoattractant Protein-1 Gene Polymorphism Is Associated with Cardiomyopathy in Human Chagas Disease. Clin. Infect. Dis. 2006;43:305–311. doi: 10.1086/505395. [DOI] [PubMed] [Google Scholar]
- 143.Peschansky V.J., Wahlestedt C. Non-Coding RNAs as Direct and Indirect Modulators of Epigenetic Regulation. Epigenetics. 2014;9:3–12. doi: 10.4161/epi.27473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Feinberg A.P. The Key Role of Epigenetics in Human Disease Prevention and Mitigation. N. Engl. J. Med. 2018;378:1323–1334. doi: 10.1056/NEJMra1402513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Saxonov S., Berg P., Brutlag D.L. A Genome-Wide Analysis of CpG Dinucleotides in the Human Genome Distinguishes Two Distinct Classes of Promoters. Proc. Natl. Acad. Sci. USA. 2006;103:1412–1417. doi: 10.1073/pnas.0510310103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.McMahon K.W., Karunasena E., Ahuja N. The Roles of DNA Methylation in the Stages of Cancer. Cancer J. 2017;23:257–261. doi: 10.1097/PPO.0000000000000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Alaskhar Alhamwe B., Khalaila R., Wolf J., Bülow V., Harb H., Alhamdan F., Hii C.S., Prescott S.L., Ferrante A., Renz H., et al. Histone Modifications and Their Role in Epigenetics of Atopy and Allergic Diseases. Allergy Asthma Clin. Immunol. 2018;14:39. doi: 10.1186/s13223-018-0259-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Bannister A.J., Kouzarides T. Regulation of Chromatin by Histone Modifications. Cell. Res. 2011;21:381–395. doi: 10.1038/cr.2011.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Esteller M. Epigenetics in Cancer. N. Engl. J. Med. 2008;358:1148–1159. doi: 10.1056/NEJMra072067. [DOI] [PubMed] [Google Scholar]
- 150.Rossetto D., Avvakumov N., Côté J. Histone Phosphorylation: A Chromatin Modification Involved in Diverse Nuclear Events. Epigenetics. 2012;7:1098–1108. doi: 10.4161/epi.21975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Frías-Lasserre D., Villagra C.A. The Importance of NcRNAs as Epigenetic Mechanisms in Phenotypic Variation and Organic Evolution. Front. Microbiol. 2017;8:2483. doi: 10.3389/fmicb.2017.02483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Paul S.C., Sharma A., Mehta R., Paul S. Genome Wide Computational Identification of Tuna (Thunnus Orientalis) MicroRNAs and Their Targets. Ocean. Sci. J. 2018;53:727–734. doi: 10.1007/s12601-018-0041-z. [DOI] [Google Scholar]
- 153.Miska E.A. How MicroRNAs Control Cell Division, Differentiation and Death. Curr. Opin. Genet. Dev. 2005;15:563–568. doi: 10.1016/j.gde.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 154.Alemohammad H., Najafzadeh B., Asadzadeh Z., Baghbanzadeh A., Ghorbaninezhad F., Najafzadeh A., Safarpour H., Bernardini R., Brunetti O., Sonnessa M., et al. The Importance of Immune Checkpoints in Immune Monitoring: A Future Paradigm Shift in the Treatment of Cancer. Biomed. Pharmacother. 2022;146:112516. doi: 10.1016/j.biopha.2021.112516. [DOI] [PubMed] [Google Scholar]
- 155.Ferreira L.R.P., Frade A.F., Santos R.H.B., Teixeira P.C., Baron M.A., Navarro I.C., Benvenuti L.A., Fiorelli A.I., Bocchi E.A., Stolf N.A., et al. MicroRNAs MiR-1, MiR-133a, MiR-133b, MiR-208a and MiR-208b Are Dysregulated in Chronic Chagas Disease Cardiomyopathy. Int. J. Cardiol. 2014;175:409–417. doi: 10.1016/j.ijcard.2014.05.019. [DOI] [PubMed] [Google Scholar]
- 156.Linhares-Lacerda L., Granato A., Gomes-Neto J.F., Conde L., Freire-de-Lima L., de Freitas E.O., Freire-de-Lima C.G., Barroso S.P.C., Guerra R.J.d.A., Pedrosa R.C., et al. Circulating Plasma MicroRNA-208a as Potential Biomarker of Chronic Indeterminate Phase of Chagas Disease. Front. Microbiol. 2018;9:269. doi: 10.3389/fmicb.2018.00269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Nonaka C.K.V., Macêdo C.T., Cavalcante B.R.R., de Alcântara A.C., Silva D.N., da Rocha Bezerra M., Caria A.C.I., Tavora F.R.F., de Souza Neto J.D., Noya-Rabelo M.M., et al. Circulating MiRNAs as Potential Biomarkers Associated with Cardiac Remodeling and Fibrosis in Chagas Disease Cardiomyopathy. Int. J. Mol. Sci. 2019;20:4064. doi: 10.3390/ijms20164064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Navarro I.C., Ferreira F.M., Nakaya H.I., Baron M.A., Vilar-Pereira G., Pereira I.R., Silva A.M.G., Real J.M., de Brito T., Chevillard C., et al. MicroRNA Transcriptome Profiling in Heart of Trypanosoma cruzi-Infected Mice: Parasitological and Cardiological Outcomes. PLoS Negl. Trop. Dis. 2015;9:3828. doi: 10.1371/journal.pntd.0003828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Ferreira L.R.P., Ferreira F.M., Laugier L., Cabantous S., Navarro I.C., Cândido D.D.S., Rigaud V.C., Real J.M., Pereira G.V., Pereira I.R., et al. Integration of MiRNA and Gene Expression Profiles Suggest a Role for MiRNAs in the Pathobiological Processes of Acute Trypanosoma Cruzi Infection. Sci. Rep. 2017;7:17990. doi: 10.1038/s41598-017-18080-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Ballinas-Verdugo M.A., Jiménez-Ortega R.F., Martínez-Martínez E., Rivas N., Contreras-López E.A., Carbó R., Sánchez F., Bojalil R., Márquez-Velasco R., Sánchez-Muñoz F., et al. Circulating MiR-146a as a Possible Candidate Biomarker in the Indeterminate Phase of Chagas Disease. Biol. Res. 2021;54:21. doi: 10.1186/s40659-021-00345-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Nonaka C.K.V., Sampaio G.L., Silva K.N., Khouri R., Macedo C.T., Consortium C.T.R., Rogatto S.R., dos Santos R.R., de Freitas Souza B.S., Soares M.B.P. Therapeutic Mir-21 Silencing Reduces Cardiac Fibrosis and Modulates Inflammatory Response in Chronic Chagas Disease. Int. J. Mol. Sci. 2021;22:3307. doi: 10.3390/ijms22073307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Jha B.K., Varikuti S., Seidler G.R., Volpedo G., Satoskar A.R., McGwire B.S. MicroRNA-155 Deficiency Exacerbates Trypanosoma cruzi Infection. Infect. Immun. 2020;88:e00948–e01019. doi: 10.1128/IAI.00948-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Liu T., Zhang L., Joo D., Sun S.C. NF-ΚB Signaling in Inflammation. Signal Transduct. Target. Ther. 2017;2:17023. doi: 10.1038/sigtrans.2017.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Stark G.R., Cheon H., Wang Y. Responses to Cytokines and Interferons That Depend upon JAKs and STATs. Cold Spring Harb. Perspect. Biol. 2018;10:a028555. doi: 10.1101/cshperspect.a028555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Takeda H., Tanaka T., Shi W., Matsumoto M., Minami M., Kashiwamura S.I., Nakanishi K., Yoshida N., Kishimoto T., Akira S. Essential Role of Stat6 in IL-4 Signalling. Nature. 1996;380:627–630. doi: 10.1038/380627a0. [DOI] [PubMed] [Google Scholar]
- 166.Choudhuri S., Garg N.J. PARP1-CGAS-NF-ΚB Pathway of Proinflammatory Macrophage Activation by Extracellular Vesicles Released during Trypanosoma cruzi Infection and Chagas Disease. PLoS Pathog. 2020;16:e1008474. doi: 10.1371/journal.ppat.1008474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Campos-Estrada C., Liempi A., González-Herrera F., Lapier M., Kemmerling U., Pesce B., Ferreira J., López-Muñoz R., Maya J.D. Simvastatin and Benznidazole-Mediated Prevention of Trypanosoma cruzi-Induced Endothelial Activation: Role of 15-Epi-Lipoxin A4 in the Action of Simvastatin. PLoS Negl. Trop. Dis. 2015;9:e0003770. doi: 10.1371/journal.pntd.0003770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Cevey Á.C., Mirkin G.A., Penas F.N., Goren N.B. Low-Dose Benznidazole Treatment Results in Parasite Clearance and Attenuates Heart Inflammatory Reaction in an Experimental Model of Infection with a Highly Virulent Trypanosoma cruzi Strain. Int. J. Parasitol. Drugs Drug Resist. 2015;6:12–22. doi: 10.1016/j.ijpddr.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Wan X., Wen J.J., Koo S.J., Liang L.Y., Garg N.J. SIRT1-PGC1α-NFκB Pathway of Oxidative and Inflammatory Stress during Trypanosoma Cruzi Infection: Benefits of SIRT1-Targeted Therapy in Improving Heart Function in Chagas Disease. PLoS Pathog. 2016;12:e1005954. doi: 10.1371/journal.ppat.1005954. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.