Abstract
Alzheimer’s disease (AD) is the most common neurodegenerative disease in the world. It is classified as familial and sporadic. The dominant familial or autosomal presentation represents 1–5% of the total number of cases. It is categorized as early onset (EOAD; <65 years of age) and presents genetic mutations in presenilin 1 (PSEN1), presenilin 2 (PSEN2), or the Amyloid precursor protein (APP). Sporadic AD represents 95% of the cases and is categorized as late-onset (LOAD), occurring in patients older than 65 years of age. Several risk factors have been identified in sporadic AD; aging is the main one. Nonetheless, multiple genes have been associated with the different neuropathological events involved in LOAD, such as the pathological processing of Amyloid beta (Aβ) peptide and Tau protein, as well as synaptic and mitochondrial dysfunctions, neurovascular alterations, oxidative stress, and neuroinflammation, among others. Interestingly, using genome-wide association study (GWAS) technology, many polymorphisms associated with LOAD have been identified. This review aims to analyze the new genetic findings that are closely related to the pathophysiology of AD. Likewise, it analyzes the multiple mutations identified to date through GWAS that are associated with a high or low risk of developing this neurodegeneration. Understanding genetic variability will allow for the identification of early biomarkers and opportune therapeutic targets for AD.
Keywords: Alzheimer’s disease, genetics, GWAS, loci, molecular mechanisms, neurodegeneration, neuropathology
1. Introduction
Alzheimer’s disease (AD) is a neurodegenerative disease and represents the most common form of dementia (60–80% of all cases of dementia) [1]. At present, an estimated 50 million people worldwide suffer from some form of dementia; however, as a result of the increase in life expectancy rates, it is expected that by 2050, 139 million people worldwide will suffer from some type of dementia [2,3], which will cause major socioeconomic and health system impacts [4].
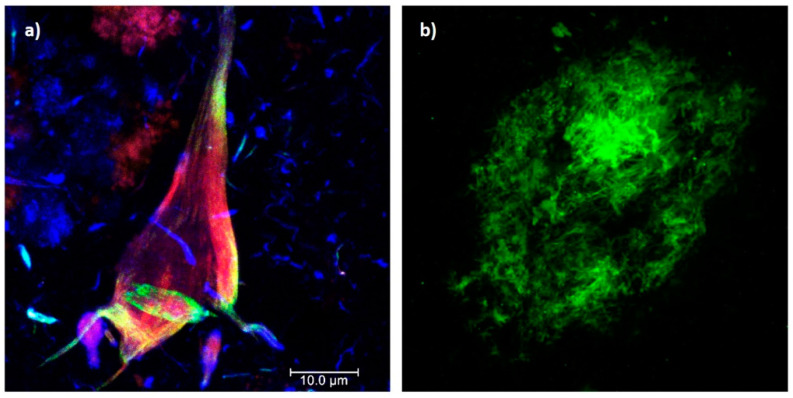
AD is characterized by chronic and acquired memory impairment and cognitive deficits in domains, such as language, spatio-temporal orientation, and executive capacity, as well as behavior alterations, all of which lead to progressive loss of personal autonomy [5]. Histopathologically, AD is characterized by two pathognomonic hallmarks (Figure 1) [6]: (1) the intracellular deposition of abnormally phosphorylated Tau protein that promotes the formation of neurofibrillary tangles (NFTs) in the cerebral cortex and subcortical gray matter (Figure 1a); and (2) extracellular aggregates of Amyloid-beta peptide (Aβ) fibrils in the form of neuritic plaques (NPs; Figure 1b). In this context, it has been postulated that endogenous “damage signals”, such as Aβ oligomers, could cause the activation of microglial cells with the consequent release of proinflammatory cytokines, which would trigger signaling cascades in neurons causing hyperphosphorylation and the aggregation of Tau protein. This protein is released when neurons die, triggering microglial cell activation and, therefore, becomes a cyclic pathological process that culminates in neurodegeneration [7,8]. Therefore, both NPs and NFTs are involved in several neuronal processes and ultimately trigger neuronal death [9,10], synaptic alteration, oxidative stress, mitochondrial disturbance, neuroinflammation, alterations in the permeability of the blood–brain barrier (BBB), and neurovascular unit dysfunction [11].
Figure 1.
Histopathological hallmarks of Alzheimer’s disease. (a) Triple immunofluorescence showing a neurofibrillary tangle (conformational change: green channel; C-terminal tail: red channel; N-terminal of intact tau protein: blue channel). (b) Immunofluorescence showing an amyloid plaque (Aβ 1-40: green channel). Photomicrographs at 100X, calibration bar = 10 µm.
Two forms of AD have been characterized [6]: familial and sporadic. The familial presentation is autosomal dominant, early onset (EOAD) in individuals under 65 years of age (representing 1 to 5% of cases), and characterized by the alteration of specific genes, such as the presenilin 1 gene (PSEN1, 14q24.2), identified in up to 70% of cases with familial AD; the presenilin 2 gene (PSEN2, 1q42.13) and the Amyloid precursor protein gene (APP, 21q21.3). The sporadic presentation is late-onset (LOAD) and occurs in individuals older than 65 years of age. The main risk factor is considered to be age [1,9,12], but sporadic AD is a complex disorder and other risk factors have been identified, such as the female sex, traumatic brain injury, depression, environmental pollution, physical inactivity, social isolation, low academic level, metabolic syndrome [9,13] and genetic susceptibility, mainly mutations in the ε4 allele of apolipoprotein E (APOE, 19q13.32) [1,9], considering a heritability of up to 60–80% [14].
Multiple hypotheses have been formulated to explain the development of AD, including the amyloidogenic cascade, tauopathy, vascular theory, oxidative stress, neuroinflammation, and bacterial infections theory, among others [6,11,15]. However, due to the intricate complexity of the human brain and partial characterization of the polymorphisms associated with AD, the molecular mechanisms involved in each of these hypotheses and their correlation with the genetic load or predisposition of each individual are poorly understood [16]. Therefore, characterizing the genetic risk factors in AD is a priority to understand the several neuropathological events involved.
Recently, genome-wide association studies (GWAS) have allowed the identification of several genes associated with AD; however, the relationship of many loci to the risk of developing AD has not been elucidated [17]. For this reason, this paper aims to analyze the genes that have been associated with a predisposition to developing AD and the possible neuropathological mechanisms involved. This will facilitate the identification of possible early biomarkers and therapeutic targets that can further the development of AD-modifying drugs.
2. The Four Classical Genes Associated with Alzheimer’s Disease
AD is a complex and multigenic disease. Familial AD is associated with the genes that encode PSEN1, PSEN2, and APP, which interfere with the physiological processing of the Aβ peptide [18].
More than 300 mutations (221 pathogenic) of the PSEN1 gene and 80 mutations (19 pathogenic) of the PSEN2 gene have been identified [19,20]. These mutations have been related to the genesis of familial AD. The spectrum of PSEN genes includes missense mutations, small insertions, deletions, and genomic deletions, specifically in PSEN1 [18]. PSEN1 mutations cause the most severe forms of AD with complete penetrance, and the onset of the disease can occur as early as 25 years of age. Missense mutations in the PSEN2 gene may show incomplete penetrance, and carriers show an older age of disease onset than those with PSEN1 mutations [21]. The missense mutations give rise to altered proteins, which interfere with the fusion of the γ-secretase complex and, secondarily, alter the processing of APP with a consequent increase in the Aβ42/Aβ40 ratio [21].
The APP gene encoding this protein consists of a total of 17 exons and encodes several isoforms resulting from the alternative splicing of exons 7 and 8 [22], three of which are relevant to AD (isoforms 695, 751, and 770) and are expressed only in the central nervous system [23]. The last two exons, 16 and 17, encode the portion of APP that, after proteolytic processing, constitutes the Aβ fragment [24]. Interestingly, 73 mutations (32 pathogenic) have been identified [19,20]; the most frequent is the Val717Ile/London mutation. Mutations in the APP gene cause an increase in the Aβ42/Aβ40 ratio, as well as an increase in levels of total Tau and phosphorylated Tau in neurons [9,25]. Most patients with trisomy 21 present early pathological changes similar to those of AD [1,26]; however, not all develop the disease. This suggests that the increase in the genetic load of APP, which has also been observed in cases of familial AD [27,28], does not cause the development of the disease in all cases.
On the other hand, the main gene that is consistently associated with sporadic AD is APOE, which increases the risk for AD three- to eightfold [6,29]:
The physiological isoform, or ApoE-ε3 (Cis112Arg158), is present in 50 to 90% of the healthy population. The main polymorphisms of the APOE gene consist of alleles that translate the isoforms of the protein that differ only by a single amino acid substituted at positions 112 and 158: (i) ApoE-ε2 isoform (Cis112Cis158) is associated with the presentation of hyperlipoproteinemia type III; (ii) ApoE-ε4 isoform (Arg112Arg158) has been implicated in atherosclerosis [30,31], and at least one copy of this allele has been identified in 40–65% of patients with AD. However, not all people with copies of this allele develop the disease, representing only a susceptibility risk factor [1]. APOE competitively binds to Aβ receptors (such as the low-density lipoprotein receptor-related protein 1; LRP1) on the surface of astrocytes, thus blocking Aβ uptake, affecting its clearance and promoting the initial seeding of fibrillar Aβ deposition [6,9]. Growing evidence suggests that APOE influences tau-mediated neurodegeneration and microglial responses to AD-related pathologies [30]. For example, one study revealed that the APOE3ch variant may have a regionally specific role in modifying the effect of Tau and thus in the severity, progression, and clinical presentation of AD [32]. This suggests that APOE polymorphisms would also affect Tau protein processing and strongly predispose one to AD.
3. Unraveling the Genetics of Alzheimer’s Disease: What Is New?
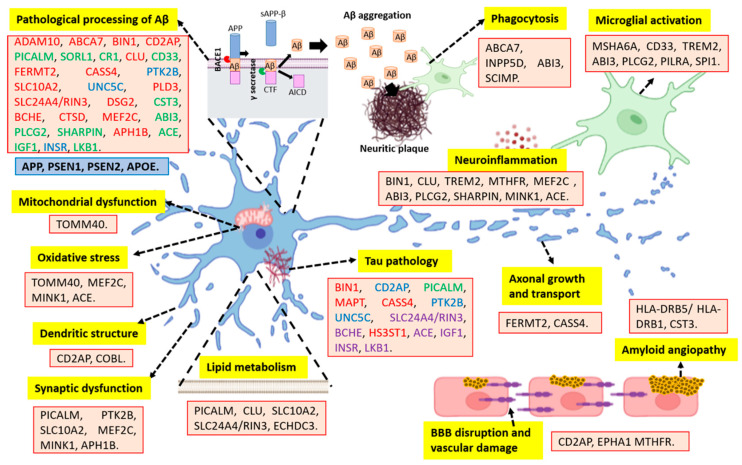
A wide variety of genes in the development of AD have been identified as possible early biomarkers and therapeutic targets. Together, these genes could explain up to 70% of LOAD cases and point to new disease-related phenomena [5], such as the association of these genes with multiple neuropathological events (Figure 2 and Table 1).
Figure 2.
Schematic representation of multiple genes and their association with neuropathological events in AD. The four classical genes associated with familial AD are in the blue box, whereas other novel genes related to sporadic AD are in the red boxes. Word color-coding: red: increased production/aggregation of Aβ or tau; green: alteration in the clearance of Aβ or tau; blue: Aβ- or tau-mediated neuronal damage; purple: Tau phosphorylation. Furthermore, the respective associations with neuropathological mechanisms are indicated in yellow boxes. Abbreviations: AICD: amyloid precursor protein intracellular domain; APP: amyloid precursor protein; BACE1: β secretase; BBB: blood–brain barrier; CTF: C-terminal fragment; sAPP-β: soluble amyloid precursor protein β. This figure was created with biorender.com.
According to the amyloid hypothesis, a wide variety of genes identified are involved in pathological Aβ processing (Figure 2 and Table 1), such as the disintegrin and metalloproteinase domain-containing protein 10 (ADAM10), which is the most important α-secretase in the brain that could cause increases in the Aβ42/Aβ40 ratio [33,34,35]. In the same way, the phosphatidylinositol-binding clathrin assembly protein (PICALM) initiates the polymerization of clathrin, which contributes to NP formation [36,37,38]; clustered mitochondria protein homolog (CLU), a chaperone that prevents the aggregation of foreign proteins, has been found to inhibit the formation of amyloid fibrils by APP and other genes involved [39,40,41].
However, the pathological Aβ processing is not the only mechanism involved, and it does not explain the complexity of AD. More than 29 risk loci have been identified, as well as more than 215 potential causative genes. These genes are strongly expressed in immune-related tissues and cell types, including the spleen, liver, and microglia cells [42]. In this context, the neuroimmunomodulation hypothesis has been postulated, which proposes that the appearance of AD is a consequence of the response of glial cells to signals of damage that trigger a neuroinflammatory response and the subsequent deregulation of protein kinases and phosphatases that promote Tau protein hyperphosphorylation and oligomerization. Tau oligomers and filaments released after neuronal apoptosis are in turn capable of reactivating microglial cells, thus promoting a deleterious molecular signaling cycle responsible for the neurodegeneration observed in AD and other tauopathies [43,44,45,46]. Supporting this hypothesis, several genes involved in the immune response have been identified (Figure 2 and Table 1). For instance, transcription factor PU.1 (SPI1), involved in the expression of immune-related genes in myeloid cells, could contribute to AD by regulating key pathways associated with the immune response and altering the epigenetic landscape [47,48]. Furthermore, the myeloid cell surface antigen CD33, expressed on the surface of microglial cells, is involved in the negative regulation of cytokine production and, therefore, could play a role in AD by modulating microglial activation [49,50]. In the same way, according to the neuroimmunomodulation hypothesis, diverse genes involved in Tau metabolism have also been identified (Figure 2 and Table 1), such as bridging integrator 1 (BIN1), which participates in membrane tubulation through an interaction with microtubule-associated proteins, such as Tau, and other functions, such as endocytosis and intracellular endosome trafficking [29]. Triggering receptor expressed on myeloid cells 2 (TREM2) has been associated with cerebrospinal fluid Tau levels [51]. Likewise, the loss of function of scaffolding CD2-associated protein (CD2AP), which is involved in intracellular trafficking and cytoskeletal reorganization, has also been related to Tau-induced neurotoxicity, abnormal neurite structure modulation, and BBB disruption [52].
Similar to the immunomodulation hypothesis, there is the infectious one, which could also explain the pathological processing of both Tau and Aβ. The infectious hypothesis proposes that a pathogen (virus, bacterium, prion, etc.) triggers an inflammatory response and promotes the aggregation of Tau and Aβ, which in turn can cause further inflammation [53]. In this context, the paired immunoglobulin-like type 2 receptor alpha (PILRA), an inhibitory immunoglobulin receptor involved in the regulation of the immune system and a co-receptor of herpes simplex virus type 1 (HSV-1), is related to dysfunctions in microglial activation and may represent a link to infectious factors that have been connected to AD [24,54,55]. Likewise, APO-ε4 has also been associated with HSV-1 infections [56]. This is extremely important since a close relationship is established between infectious diseases and AD and, therefore, represents an opportunity both for diagnostic and therapeutic strategies.
In addition, the pathological processing of Tau and Aβ, such as other neuropathological events, could be triggered by some genes involved in metabolic disorders (Figure 2 and Table 1); mainly lipid/atherosclerosis pathways and insulin resistance [57]. In this context, the solute carrier family 10 member 2 (SLC10A2) plays an important role in the regulation of cholesterol metabolism, and it has been shown that cholesterol accumulation in neurons leads to neuronal death, memory impairment, and increased Aβ generation [58,59]. Since AD could be considered a metabolic disease mediated in part by insulin resistance [57], it is postulated that the zinc finger CW-type PWWP domain protein 1 (ZCWPW1), a protein involved in the positive regulation of the DNA metabolic process, could decrease the risk of LOAD by suppressing insulin resistance [60]. Furthermore, genes involved in the AMP-activated protein kinase (AMPK) pathway have also been associated with the risk of AD by regulation of energy balance and glucose and lipid metabolism [61], autophagy dysfunctions leading to Aβ and Tau pathology [57], and alteration of the synaptic plasticity of hippocampal neurons [62]. Correspondingly, methylenetetrahydrofolate reductase (MTHFR), a rate-limiting enzyme in the methyl cycle, can be linked, as high levels of homocysteine have been found in AD patients. Homocysteine is associated with vascular damage, increased inflammation, and endothelial cell dysfunction [63]. Therefore, further studies are required to determine the genetic predisposition between chronic-degenerative diseases (such as diabetes mellitus) and AD, and the neuropathological pathways involved.
Table 1 summarizes the main genes, their physiological role, and the possible molecular mechanisms involved in the neuropathology of this neurodegenerative disorder, highlighting that it is possible to differentiate genes associated with neuronal homeostasis, including the physiological metabolism of Tau protein and Aβ peptide, calcium, lipids, synaptic plasticity, neuronal proliferation and differentiation, immunoregulation, cytoskeleton stabilization, and mitochondrial complexes, among others. However, in pathological conditions, they are associated with Tau pathology, increased Aβ accumulation, neuroinflammation, mitochondrial and synaptic dysfunctions, oxidative stress, and disruption of BBB, among others. Understanding the physiological and pathological roles of these genes will facilitate the identification of early biomarkers and opportune therapeutic targets.
Table 1.
New genes linked to Alzheimer’s disease and their physiological and pathological roles.
| Gene/Location | Physiological Role * | Molecular Mechanisms Implicated in AD | Ref. |
|---|---|---|---|
| ADAM10 (15q21.3) | It is the most important α-secretase in the brain and contributes to the non-amyloidogenic pathway of APP metabolism. | Alteration in APP metabolism (through non-amyloidogenic pathway), synaptic plasticity, and hippocampal neurogenesis. | [33,34,35] |
| ABCA7 (19p13.3) |
Modulates lipid metabolism, phagocytosis, apoptosis, and phagocytic of Aβ by microglia. | Alteration in Aβ processing and regulation of APP by β-secretase. | [64,65,66] |
| BIN1 (2q14.3) |
Participates in immune response, calcium homeostasis, apoptosis, endocytosis of synaptic vesicles, and plasma membrane dynamic. | Contributes to Amyloid (through β-secretase activity) and Tau pathology and is related to inflammation, apoptosis, and calcium homeostasis. | [67,68,69] |
| CD2AP (6p12.3) |
Regulates actin cytoskeleton and membrane trafficking through endocytosis and cytokinesis. | Associated with increased Aβ production, Tau neurotoxicity, abnormal modulation of the neurite structure, and altered integrity of the BBB. | [52,70,71] |
| EPHA1 (7q34) |
Plays a role in synaptic development and plasticity, modulating cell migration, angiogenesis, cell proliferation, and apoptosis. | Alterations in immune response and endocytosis, as well as disruption of BBB integrity. | [29,72] |
| PICALM (11q14.2) |
Modulates autophagy, membrane metabolism, internalization of cell receptors, synaptic transmission, removal of apoptotic cells, and endocytic pathways for APP processing. | Dysfunction of Aβ metabolism and APP processing. Possible association with tauopathy, synaptic dysfunction, and altered lipid metabolism. | [36,37,38,73,74] |
| SORL1 (11q24.1) |
Plays a role in endocytosis. | Restricts delivery of precursors to endocytic compartments that promote amyloidogenic clearance. | [75,76] |
| MS4A6A (11q12.2) |
Regulates Ca2+ entry and signal transduction of several proteins. | Its variants affect the processing of the TREM2 microglial receptor increasing the risk of AD. | [71,77] |
| CR1 (1q32.2) |
Facilitates the capture and clearance of complement-opsonized pathogens by erythrocytes, monocytes, macrophages, and microglia. | Decreases complement-mediated clearance of Aβ42. Metabolism and clearance of Aβ and its interaction with APOE-4 are related to cognitive impairment and the appearance of LOAD. | [78,79,80] |
| CLU (8p21.1) |
Participates in lipid metabolism, cell proliferation, apoptosis, immune response, and neuronal differentiation. | Alteration in Aβ aggregation, lipid metabolism, regulation of cell cycle and neuronal apoptosis, and neuroinflammation. | [40,41,81] |
| CD33 (19q13.3) |
Involved in the inhibition of immune cell function and cytokine production. | Modulates microglial activation (neuroinflammation) and Aβ clearance through microglial cells. | [49,50,82] |
| TREM2 (6p21.1) |
This microglia receptor regulates proliferation, survival, phagocytosis, and inflammation. | Related to microglial dysfunctions, stress of the endoplasmic reticulum, Tau and amyloid pathology, and neuroinflammation. | [83,84,85] |
| TOMM40 (19q13.32) |
Plays a role in the stabilization of the mitochondrial membrane respiratory chain (Complex I). | Dysfunction of the mitochondrial membrane and subsequent oxidative stress. | [86,87,88] |
| MAPT (17q21.21) |
Related to the assembly and stability of microtubules and neuronal polarity. | Encoding for the Tau protein that is involved in the microtubule disassembly during AD. | [89,90] |
| FERMT2 (14q22.1) |
Participates in cell differentiation, biogenesis, and connection between extracellular matrix adhesion sites and the actin cytoskeleton. | Associated with increasing levels of mature APP at the cell surface, resulting in increased Aβ-peptide production. | [91,92] |
| CASS4 (20q13.31) |
Associated with cell adhesion, cell spreading, calcium signaling, and microtubule stabilization. | Involved in the formation of neuritic plaques and NFT, neuroinflammation, and dysfunction in synapsis, calcium signaling, and microtubule stabilization. | [93,94] |
| PTK2B (8p21.2) |
Involved in the regulation of calcium flux, LTP, neurite growth, synapsis, and angiogenesis. | Contributes to hypoperfusion, vascular permeability, and Tau toxicity. | [93,94,95] |
| INPP5D (2q37.1) |
Participates in the regulation of microglia gene expression. | Involved in microglia with deficient phagocytic capacity, resulting in increased Aβ deposition. | [96,97] |
| SLC10A2 (13q33.1) |
Has an important role in encoding the sodium/bile acid cotransporter, as well as in cholesterol metabolism. | Associated with LOAD by dysfunctional cholesterol metabolism, neuronal death, memory impairment, and increased Aβ generation. | [58,59] |
| COBL (7p12.1) |
Regulates actin cytoskeleton reorganization and neuron morphogenesis and increases the branching of axons and dendrites. | Reduction in the number of dendritic branch points and neurites. | [59] |
| UNC5C (4q22.3) |
Favors apoptosis and directs axon extension and cell migration during neural development. | Contributes to neuronal death, Tau pathology, and Aβ-associated pathways. | [98,99] |
| PLD3 (19q13.2) |
Hydrolysis of membrane phospholipids, which influences the processing of APP. | Promotes the generation of amyloid plaques and cognitive decline. | [100,101] |
| SLC24A4/RIN3 (14q32.12) | Plays a role in calcium transport and lipid and glucose metabolism. | Associated with Aβ loading and Tau pathology. | [102] |
| HLA-DRB5/DRB1 (6p21.32) |
Encodes proteins for MHC and plays an important role in the immune response, including antigen processing and presentation, and self-recognition by immune cells. | It is associated with the presence of capillary β-amyloid and the development of cerebral Amyloid angiopathy. It also induces microglial activation. | [103,104] |
| DSG2 (18ql2.1) |
Encodes adhesion molecule proteins to promote contact between epithelial cells and other cells. | Mediates APOE-associated Aβ aggregation in neurons. | [105] |
| MTHFR (1p36.3) | The regulatory connection between the folate and methionine cycles. | Associated with high levels of homocysteine and subsequent vascular damage and neuroinflammation. | [63,106] |
| CST3 (20p11.21) | Promotes neurogenesis, reduces Aβ deposition, and inhibits fibril formation. | Involved with amyloid angiopathy and cell death induced by oligomeric and fibrillar Aβ. | [107,108,109] |
| BCHE (3q26.1) | Contributes to acetylcholine inactivation and hydrolysis of neurotoxic organophosphate esters. | Related to NFT and neuritic plaque formation. | [16,110,111] |
| CTSD (11p15.5) | Favors the activation/degradation of polypeptide hormones and growth factors. | Implicated in the processing of APP/Aβ deposition and autophagy dysfunction. | [112,113] |
| ZCWPW1 (7q22.1) | Regulation of the DNA metabolic process. Additionally, it is involved in epigenetic modulation. | Suppresses insulin resistance. It may activate the PI3K signaling pathway in neurons. | [60] |
| MEF2C (5q14.3) | Involved in vascular development, neurogenesis, inflammatory processes, and hippocampal-dependent learning and memory. | Promotes neuroinflammation, cell apoptosis, Aβ aggregates, synaptic plasticity dysfunction, and increases the oxidative stress level. | [114,115,116] |
| ABI3 (17q21.32) | Regulates actin cytoskeleton organization, cytokinesis, migration, endocytosis, and phagocytosis. | Associated with alterations in microglial migration and phagocytosis, Aβ accumulation, and neuroinflammation. | [117,118] |
| PLCG2 (16q24.1) | Regulates divergent microglial functions through TREM2 signaling and is involved in the transition to a microglial state. | Correlation with amyloid plaque density, expression levels of microglial marker genes, and neuroinflammation. | [119,120,121,122] |
| SCIMP (17p13.2) | Regulates MHC-II signaling in B cells and the host’s innate immune responses to danger signals. | Associated with alteration in TLR-mediated microglial phagocytosis via MHC II. | [123] |
| SHARPIN (8q24.3) | Induces NF-kB activation and regulation of inflammation, and cell death. Furthermore, it regulates angiogenesis and NLRP3 activation. | Associated with neuroinflammation, and defective Aβ clearance that leads to pathogenic Aβ accumulation. | [124,125,126,127,128] |
| MINK1 (17p13.2) | Controls glutamate receptor signaling, synaptic density, dendrite complexity, actin cytoskeleton reorganization, cell-matrix adhesion, cell–cell adhesion, and cell migration. | It is related to glutamatergic synapse impairment, dysfunction of axon regeneration, neuronal degeneration, neuroinflammation, and increased ROS levels. | [129,130,131] |
| APH1B (15q22.2) | Serves as a scaffold for the assembly of γ-secretase complex; therefore, it cleaves APP. It also favors excitatory synaptic transmission and plasticity. | Decreases excitatory synapsis and promotes Aβ aggregation and spine formation. | [132,133,134] |
| HS3ST1 (4p15.33) | Modulates stem cell differentiation and neuronal targeting. | Promotes Tau spreading mediated by Tau binding to the cell surface heparan sulfate. | [135,136] |
| ECHDC3 (10p14) | Involved in fatty acid biosynthesis in mitochondria and insulin sensitivity. | Alterations in lipid and cholesterol metabolism, such as cognitive functions. | [137,138] |
| ACE (17q23.3) | Regulates blood pressure, electrolyte homeostasis, synaptic plasticity, and Aβ metabolism. | Associated with cognitive decline, oxidative stress, neuroinflammation, and higher levels of Aβ and Tau load. | [139,140,141] |
| PILRA (7q22.1) | Involved in immune system regulation and plays a key role in the life cycle of HSV-1. | Correlation between AD and HSV-1. In addition, it is related to a decrease in the inhibition of microglial activation. | [54,55] |
| SPI1 (11p11.2) | Regulates the immune response and learning-related neuronal activity in the cerebral cortex. | Alters the microglial phenotype and transcriptome, involving interferon pathways. | [47,48] |
| IGF1 (12q23.2) | Inhibits abnormal Tau phosphorylation and Aβ deposition. Stimulates neurogenesis and prevents apoptosis in the hippocampus. | Associated with Tau and Aβ pathology. | [142,143] |
| INSR (19p13.2) |
The insulin-INSR signaling pathway regulates glucose uptake and release, as well as the synthesis and storage of carbohydrates, lipids, and proteins. | Can activate GSK-3β by initiating the PI3K-AKT signaling pathway and lead to Aβ accumulation and Tau phosphorylation. | [57] |
| LKB1 (19p13.3) | Plays a role in cell metabolism, cell polarity, apoptosis, and the response to DNA damage by regulating AMPK activity. | Autophagy dysfunction, Aβ accumulation, and Tau phosphorylation. | [57] |
Abbreviations: ABCA7: ATP-binding cassette subfamily A member 7; ABI3: ABI gene family member 3; ACE: Angiotensin-converting enzyme; ADAM10: Disintegrin and metalloproteinase domain-containing protein 10; AMPK: AMP-activated protein kinase; APH1B: Gamma-secretase subunit APH-1B; APOE-4: Apolipoprotein E allele 4; APP: Amyloid beta precursor protein; Aβ: Amyloid beta peptide; BBB: Blood–brain barrier; BCHE: Butyrylcholinesterase; BIN1: Bridging integrator 1; Ca2+: Calcium; CASS4: Cas scaffolding protein family member 4; CD2AP: CD2-associated protein; CD33: Myeloid cell surface antigen CD33; CLU: Clustered mitochondria protein homolog; COBL: Protein cordon-bleu; CR1: Complement receptor type 1; CST3: Cystatin-C; CTSD: Cathepsin D; DSG2: Desmoglein-2; ECHDC3: Enoyl-CoA hydratase domain-containing protein 3, mitochondrial; EPHA1: Ephrin type-A receptor 1; FERMT2: Fermitin family homolog 2; GSK-3β: glycogen synthase kinase 3; HLA-DRB5: HLA class II histocompatibility antigen, DR beta 5 chain; HS3ST1: Heparan sulphate glucosamine 3-O-sulfotransferase 1; HSV-1: Herpes simplex virus type 1; IGF1: Insulin-like growth factor 1; INPP5D: Inositol polyphosphate-5-phosphatase D; INSR: Insulin receptor; LKB1: Liver kinase B1 LOAD: Late-onset Alzheimer’s disease; LTP: Long-term potentiation; MAPT: Microtubule-associated protein tau; MEF2C: Myocyte-specific enhancer factor 2C; MHC II: MHC Class II; MHC: Major histocompatibility complex; MINK1: Misshapen-like kinase 1; MS4A6A: Membrane-spanning 4-domains, subfamily A, member 6A; MTHFR: Methylenetetrahydrofolate reductase; NF-kB: Nuclear factor kappa-light-chain enhancer of activated B cells; NFTs: Neurofibrillary tangles; NLRP3: NLR family pyrin domain containing 3; PICALM: Phosphatidylinositol-binding clathrin assembly protein; PI3K: Phosphoinositide 3-kinase; PILRA: Paired immunoglobulin-like type 2 receptor alpha; PLCG2: Phospholipase C gamma 2; PLD3: Phospholipase D family member 3; PTK2B: Protein-tyrosine kinase 2-beta; ROS: Reactive oxygen species; SCIMP: SLP adapter and CSK-interacting membrane protein; SHARPIN: SHANK-associated RH domain interactor; SLC10A2: Solute carrier family 10 member 2; SLC24A4: Solute carrier family 24 (sodium/potassium/calcium exchanger) member 4; SORL1: Sortilin-related receptor 1; SPI1: Transcription factor PU.1; TLR: Toll-like receptor; TOMM40: Translocase of outer mitochondrial membrane 40; TREM2: Triggering receptor expressed on myeloid cells 2; UNC5C: Netrin receptor UNC5C; ZCWPW1: zinc finger CW-type PWWP domain protein 1. * Data source: UniProt.org (accessed on 20 December 2022).
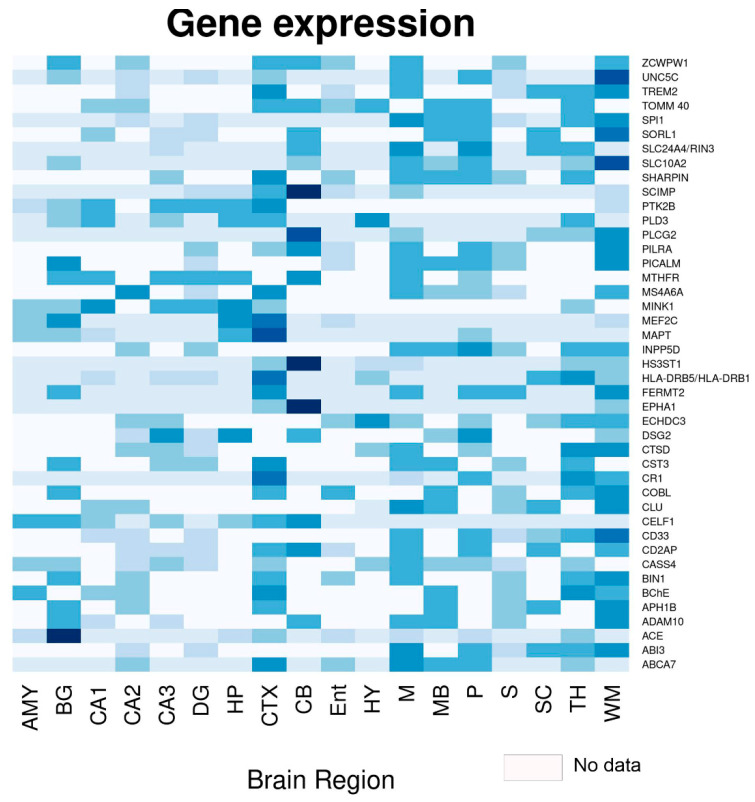
Figure 3 illustrates the expressions of several genes in regions of the brain, highlighting that many of these are highly expressed in the hippocampus, the main neuroanatomical region affected in AD. Remarkably, CD33, DSG2, MEF2C, MINK1, PTK2B, CD2AP, PICALM, BIN1, MTHFR, PLD3, and TOMM40 are highly expressed in the hippocampus. They are related to various pathological processes (Figure 2, Table 1), mainly abnormal Tau and Aβ processing, synaptic dysfunction, and neuroinflammation, highlighting the causal role of the immune system, rather than the immune response as a consequence of the disease. The neuroimmunomodulation hypothesis postulates that AD is a consequence of the response mainly of microglial cells that trigger a neuroinflammatory response and the subsequent neurodegeneration [43,44,45,46]. Furthermore, CD2AP, CD33, BIN1, CR1, PICALM, ABCA7, TREM2, and CLU are strongly expressed in the hippocampus (Figure 3) and represent highly expressed risk genes in microglia; hence, they are associated with the immune response in AD [144]. This is highly relevant since these genes represent a window for the establishment of early biomarkers and therapeutics focused on limiting the neuroinflammatory environment.
Figure 3.
Heat map of the different genes associated with Alzheimer’s disease and their expressions in various regions of the brain and hippocampus. Abbreviations: AMY: Amygdala; BG: basal ganglia; CA1, CA2, CA3: Hippocampal regions; CB: Cerebellum; CTX: Cerebral cortex; DG: Dentate gyrus; Ent: Entorhinal gyrus; HP: Hippocampus; HY: Hypothalamus; M: Medulla oblongata; MB: Midbrain; P: Pons; S: Subiculum; SC: Spinal cord; TH: Thalamus; WM: White matter. Gene expression by brain region was obtained from the human protein atlas (https://www.proteinatlas.org/, accessed on 20 December 2022).
4. Impact of GWAS on Understanding Alzheimer’s Disease
From 2005 to the present, multiple genetic studies have tracked most of the genes that conform to the human genome. These genetic studies are known as Genome-wide Association Studies (GWAS) and their objective is to associate certain genes with multiple pathologies or disorders [145,146]. Until 2007, only mutations in the APOE-ε4 allele were reliably associated with increased susceptibility to LOAD. Nonetheless, to date, multiple analyses have been performed with GWAS technology, demonstrating many possible genes associated with LOAD (Table 2). Targeted genetic approaches and next-generation sequencing studies have also identified several low-frequency genes that are associated with a relatively high risk of developing AD, therefore providing insight into the pathogenesis [5]. GWAS have associated more than 40 risk alleles with AD, identifying variants that trigger neurodegeneration, such as lipid metabolism, inflammation, innate immunity, Aβ production and clearance, and endosomal vesicle recycling (as shown in Table 1 and Figure 2) [147,148]. In particular, GWAS have also allowed us to identify those genes related to the development of both EOAD and LOAD [14].
Since genetic variations have been evidenced between different ethnic groups [149], it is essential that researchers perform GWAS in AD across ethnicities and identify polymorphisms associated with each of them. In Table 2, note that most GWAS analyses have been conducted in the Caucasian population and, therefore, are biased by not including other populations and determining the susceptibility of other possible genetic variations involved. The African American population is twice as likely to develop the disease [14]; hence, it is necessary to increase the studies on these populations. In the same way, only one of the studies [150] focused on identifying possible genes involved in AD taking into account the gender of the patients, although women are at a higher risk of developing AD and have worse clinical and pathological outcomes [151]. In addition, some of these studies could be biased, as the sample between controls and patients with AD was not balanced and neither were the risk factors or modifiers of the study subjects, including comorbidities, gender, age range, environmental exposure, and medication, among others.
On the other hand, GWAS have allowed the identification of genetic components that are not related to the neuropathological processes mentioned above (Figure 2), affecting only cognitive reserve, which refers to individual differences in susceptibility to age-related brain changes or AD-related pathology [152]; thus, some people would tolerate more of these changes and still maintain function. In this context, growing evidence suggests that, among cognitively healthy patients with a genetic risk of developing AD, women exhibit better global cognition than men. This event is maintained during the early stages of the disease, despite showing increased Tau pathology, hippocampal atrophy, and metabolic dysfunction [151]. This indicates that although women have greater resilience to the disease, there are still unidentified factors that cause them to have a greater risk of developing the disease.
Although GWAS have been invaluable in identifying candidate genetic variants associated with the disease, the AD risk loci identified to date explain only a small fraction of the heritability of AD [42]; therefore, part of it remains unexplained. One solution would be to increase the sample size of GWAS, as they have already been used to characterize new genetic risk factors in other diseases [14]. Interestingly, GWAS in AD have transitioned from identifying only a couple of novel genes to identifying a large number of previously unreported associations. This is probably due to an increase in the size of the samples and the diversification of the populations studied.
However, the increase in sample size is due to the use of a Russian doll-like design where larger studies include all smaller studies [153], which could limit results when analyzing the same samples multiple times instead of generating new patient data. In support of this, some GWAS [14,42,59,154] have shown that the use of a proxy patient (self-report of the parental history of AD) is useful to favor an increase in the sample size without affecting the validity of the results.
Therefore, future GWAS must adopt a better definition of cases and controls, as well as designs based on the comparison of ethnic groups, sex, and age, since there may be various environmental, exposure, and genetic factors that modify the risk load conferred by each locus. Likewise, these associations should be replicated and validated in multiple populations and followed by a downstream functional dissection to benefit knowledge of pathophysiology [155].
Table 2.
Genome-wide association studies (GWAS) for Alzheimer’s disease.
| Year | Population (Ethnicity/Race) | New Genes Found * | Ref. |
|---|---|---|---|
| 2007 | British and Americans (n = 3870); LOAD = 1808; Ctrl = 2062 | APOE | [156] |
| 2008 | European ancestry (n = 1376). | CD33 | [157] |
| 2009 | 1. French (n = 7360); LOAD = 2032; Ctrl = 5238 2. Belgians, Finns, Italians, Spanish (n = 7275); LOAD = 3978; Ctrl = 3297 |
CR1, CLU | [158] |
| 2009 | 1. British, Germans, and Americans (n = 11,789); LOAD = 3941; Ctrl = 7848 2. Europeans (n = 4372); LOAD = 2032; Ctrl = 2340. |
PICALM | [41] |
| 2010 | Spanish (n = 2349); LOAD = 1140; Ctrl = 1209 | EXOC3L2, BIN1 | [159] |
| 2010 | Caucasians of German extraction (n = 970); LOAD = 491; Ctrl = 479 | TOMM40 | [160] |
| 2011 | 1. Europeans (n = 9799); LOAD = 4896; Ctrl = 4903 2. Europeans (n = 29,544); LOAD = 8286; Ctrl = 21,258 |
ABCA7, MS4A6, EPHA1, CD2AP | [71] |
| 2011 | European Americans (n = 3839); LOAD = 1848; Ctrl = 1991 | CUGBP2 | [161] |
| 2011 | 1. African Americans (n = 1009); LOAD = 513; Ctrl = 496 2. White (n = 9773); LOAD = 3568; Ctrl = 6205 |
PVRL2 | [162] |
| 2012 | Caribbean Hispanics (n = 1093); LOAD = 549; Ctrl = 544 | DGKB, GWA-10q23.1 (PCDH21, LRIT1, RGR), HPCAL1 | [163] |
| 2012 | Polish (n = 282); Cases = 141 (94 LOAD, and 47 MCI); Ctrl = 141 | GWA-9q21.33 | [164] |
| 2012 | 1. European Americans (n = 2440); LOAD = 1440; Ctrl = 1000 2. European Americans (n = 6063); LOAD = 2727; Ctrl = 3336 |
PPP1R3B | [165] |
| 2013 | European ancestry and African Americans (n = 4689); LOAD = 2493; Ctrl = 2196 | PLD3 | [166] |
| 2013 | 1. Icelanders (n = 4786); LOAD = 3550; Ctrl = 1236. 2. Americans, Norwegians, Germans, Dutch (n = 11,764); LOAD = 2037; Ctrl = 9727 |
TREM2 | [167] |
| 2014 | European Americans (n = 3656); LOAD = 2151; Ctrl = 1505 | APOC1, CAMK1D, FBXL13 | [168] |
| 2014 | Multiethnic (mostly Caucasians; n = 32,346); LOAD = 14,967; Ctrl = 17,379 | PLXNA4 | [169] |
| 2015 | 1. Caribbean Hispanics (n = 4514); LOAD = 2451; Ctrl = 2063 2. Caribbean Hispanics (n = 5300); LOAD = 3001; Ctrl = 2299 |
FBXL7, FRMD4A, CELF1, FERMT2, SLC24A4-RIN3. | [170] |
| 2015 | Japanese (n = 8808); LOAD = 816; Ctrl = 7992 | CNTNAP2, GWA-18p11.32, GWA-12q24.23 | [171] |
| 2016 | European ancestry (n = 3467) LOAD = 2488; Ctrl = 979 | OSBPL6, PTPRG, PDCL3, | [172] |
| 2017 | African Americans (n = 5609); LOAD = 1825; Ctrl = 3784 | COBL, SLC10A2 | [59] |
| 2018 | Multiethnic (n = 54,162); LOAD = 17,008; Ctrl = 37,154 | MLH3, FNBP4, CEACAM19, CLPTM1. | [173] |
| 2018 | British. Maternal AD = 27,696; Ctrl = 260,980 Paternal AD= 14,338; Ctrl = 245,941 |
ADAM10, BCKDK/KAT8 (VKORC1), ACE, TREML2, PLCG2, IL-34. | [154] |
| 2019 | European ancestry (n = 455,258); LOAD = 71,880; Controls = 383,378 | ADAMTS4, INPP5D, HESX1, CLNK, HS3ST1, HLA-DRB1, ZCWPW1 (SPDYE3), CNTNAP2, CLU/PTK2B, ECHDC3, APH1B, SCIMP, AB13, BZRAP1-AS1, SUZ12P1, ALPK2, AC074212.3. | [42] |
| 2019 | Caucasian ancestry (n = 17,480); LOAD = 2741; Ctrl = 14,739 | CEACAM16, BCL3, MIR8085, CBLC, BCAM, PVRL2, APOC1, APOC1P1, APOC4, APOC2, LINC00158, MIR155HG, MIR155, LINC00515, MRPL39, JAM2, C2orf74, ATG10, MS4A6A, ABCB9, ZNF815, TRA2A, MED30, LPXN, IRAK3, N4BP2L2, UQCC, APOBEC3F, SFNa, ESPN, GNAI3, C9orf72, MTMR3 | [150] |
| 2019 | Non-Hispanic Whites (n = 94,437); LOAD = 35,274; Ctrl = 59,163 | NYAP1 (SPDYE3), ECHDC3 (USP6NL), IQCK, WWOX. | [174] |
| 2020 | African Americans (n = 8006); LOAD = 2784; Ctrl = 5222 | TRANK1, FABP2, LARP1B, TSRM, ARAP1, STARD10, SPHK1, SERPINB13, EDEM1, ALCAM, GPC6, VRK3, SIPA1L2, WDR70, API5, ACER3, PIK3C2G, ARRDC4, IGF1R, RBFOX1, MSX2, AKAP9. | [175] |
| 2021 | British (n = 495,000); LOAD = 75,000; Ctrl = 420,000 |
PILRA, NCK2, SPI1, TSPAN14, SPPL2A, ACE, CCDC6, ADAMTS1, SHARPIN, GRN, SPRED2, ADAMTS4, TMEM163, SIGLEC11, PLCG2, IGHG1, IKZF1, TSPOAP1. | [147] |
| 2022 | Japanese (n = 3777); LOAD = 1744; Ctrl = 2033 | FAM126A, ZFHX4, LGR5, ZFC3H1, OR51G1, OR4X2, ARHGEF4, PRUNE2, MLKL, NCOR2, DMD, NEDD4, PLEC | [176] |
| 2022 | European (n = 788,989); LOAD = 111,326; Ctrl = 677,663 | UNC5CL, EPDR1, WNT3, SORT1, ADAM17, PRKD3, MME, IDUA, RHOH, ANKH, COX7C, TNIP1, RASGEF1C, HS3STS, UMAD1, ICA1, TMEM106B, JAZF1, SEC61G, CTSB, ABCA1, ANK3, BLNK, PLEKHA1, TPCN1, SNX1, DOC2A, MAF, FOXF1, PRDM7, WDR91, MYO15A, KLF16, LILRB2, RBCK1, SLC2A4RG, APP. | [14] |
Abbreviations: AD: Alzheimer’s disease; Ctrl: controls; LOAD: Late-onset Alzheimer disease; MCI: Mild cognitive impairment. * Only newly identified genes are shown.
Furthermore, studies that have made associations between genetics and epigenetics through epigenome-wide assays should not be left aside. DNA methylation, an epigenetic modification, plays an important role in regulating gene expression and thus in a wide range of diseases and biological processes [177]. Interestingly, one of these analyses was conducted in a Mexican-American population with mild cognitive impairment (MCI) [62], finding various methylation regions between control and MCI patients, as well as some genes involved in neuronal death, metabolic dysfunction, and inflammatory processes. Highlighting that methylation is transgenerationally heritable and affected by environmental factors [177,178], these processes could explain not only the relationship of genetics with the disease, but also its relationship with environmental and lifestyle factors. For example, exposure to organophosphate pesticides has been shown to promote Tau hyperphosphorylation and microtubule dysfunction [179]. Likewise, it has been shown that the methylation of genes, including ABCA7, BIN1, SORL1, and SLC24A4 (previously described in Figure 2 and Table 1), were significantly associated with the pathological processing of Tau protein and Aβ peptide. In addition, it was demonstrated that histone acetyltransferase and histone deacetylase inhibitors could increase the level of histone acetylation, and thus have different beneficial effects on AD [180]: (i) improve the expression of genes related to memory; (ii) prevent cognitive degeneration; (iii) decrease the deposition of the Aβ peptide; and (iv) avoid hyperphosphorylation of the Tau protein and the NFT formation. Therefore, the identification of polymorphisms associated with multiple environmental factors through GWAS could favor the development of effective diagnostic and therapeutic strategies.
However, GWAS present certain limitations [181]: (1) they eventually involve the entire genome in disease predisposition; (2) the identification of multiple loci without a clear mechanism can be uninformative and cause confusion with respect to AD; (3) the vast majority of GWAS focus on the European population and lack population stratification; and (4) the scarce data can lead to the fact that the heritability of diseases is not fully explained and therefore fail to detect epistasis in humans, which is the main component of the genetic architecture of complex traits [182].
Despite these limitations, as has been analyzed throughout the manuscript, GWAS have multiple benefits given their diverse clinical applications and allow the identification of new biological mechanisms as well as ethnic variations in the health-disease process. Similarly, GWAS should be promoted in understudied populations, with a different methodological and study design, larger sample sizes, with more clinical data, and extrapolating the associations identified to in silico and preclinical models to take full advantage of the potential this technology offers us.
Notwithstanding the significant advances in our understanding of AD pathobiology through GWAS technology, we have yet to identify a disease-modifying therapy that has demonstrated efficacy in humans. Most of the research conducted over the past 30 years has focused on anti-Aβ therapies [183]; however, all clinical trials with anti-Aβ therapies have been unsuccessful [184]. There are several reasons why these therapies may have failed [16,183]: (i) they are administered too late in the course of the disease when neuronal damage has already become irreversible; (ii) the misfolding and accumulation of the Aβ peptide is not the only cause of neurodegeneration and therefore it is not clear whether the reduction of Aβ levels effectively treats the disease; and (iii) since Aβ is produced by multiple pathways, inhibiting its production is complex. Despite the factors listed above, an IgG1 Aβ monoclonal antibody recently proved effective in phase 3 clinical trials to reduce AD biomarkers and reduce cognitive decline [185] and has already been approved for the treatment of AD by the Federal Drug Administration (FDA) [186].
The multiple genes identified by GWAS support the various theories that have been postulated to explain the development of AD, some of them involved in more than one mechanism, such as PICALM, CLU, and CD2AP, among others (Figure 2, Table 1 and Table 2). This supports the hypothesis that the disease and its treatment should be considered from the multiconvergent theory and not through isolated mechanisms. It has been proposed that our genetic program should protect us from diseases, such as AD by working better under the conditions under which it was designed [187]; however, multiple behavioral and environmental factors could play a greater role in the development of the disease than their consideration by single mechanisms. This becomes even more important due to the aforementioned fact that most therapies against a single mechanism continue to show limited success and, therefore, understanding the disease from a unified theory would improve the results of preventive and therapeutic measures. In this context, multiple pathway-directed therapies may be more effective in slowing or preventing the progression of AD. For example, combined therapy with anti-Tau drugs, such as anti-amyloids, has been suggested, as well as the combination of neuroprotective drugs with anti-inflammatory and anti-oxidative stress effects [188].
On the other hand, it should be noted that genetics is only one factor that can influence the treatment of AD, since other factors, such as age, general state of health, and stage of the disease, can also influence the efficacy of the treatment. Similarly, the use of more general approaches to prevent or delay the onset of AD, such as lifestyle interventions or changes to prevent or delay the onset of AD [12], could be useful: maintaining a healthy diet, engaging in regular physical activity, and participating in cognitively stimulating activities [189,190,191].
In conclusion, this review shows the substantial role that genetics plays in the development of not only EOAD, but also LOAD. The GWAS and other genetic analyses will facilitate the identification of possible biomarkers that allow an early diagnosis of the disease or the risk of developing it in susceptible people, as well as its relationship with other pathological processes. Furthermore, post-GWAS functional experiments can elucidate new targets and avenues for therapeutic intervention. Therefore, the selection of genetically supported drug targets could reduce time and costs and improve the success rate of new drug development.
Acknowledgments
J.A.-G., P.J.-A. and I.V.-R. received M.Sc. and Ph.D. fellowships from CONACYT. Additionally, the authors wish to thank Jessica González Norris for proofreading the manuscript.
Author Contributions
J.L.-M. and L.O.S.-R. contributed to the conceptualization, writing, review, and editing of the manuscript. J.A.-G., A.S.-B., P.J.-A., I.V.-R. and A.R.C.-S. contributed to the writing and review of the manuscript. A.S.-B., G.P.-M., C.F.M.-C., C.S.-G., M.-d.-C.C.-A., S.D.-C. and M.P.-H. contributed to the review and editing of the manuscript. G.P.-M., C.F.M.-C., C.S.-G., M.-d.-C.C.-A., S.D.-C., M.P.-H., J.L.-M. and L.O.S.-R. contributed to the acquisition of funding for the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was funded by UNAM-PAPIIT (IA202021, IA205423, IN206322), by the Consejo Nacional de Ciencia y Tecnología (CONACYT), Ciencia Básica y/o Ciencia de Frontera Modalidad: Paradigmas y Controversias de la Ciencia 2022, grant numbers 319433 and 319578, and by Fondo Nacional de Ciencia, Tecnología del Ministerio de Educación Superior Ciencia y Tecnología. República Dominicana 2018-2019-2A3-208 to J.L.-M. and M.P.-H.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.2022 Alzheimer’s disease facts and figures. Alzheimers Dement. 2022;18:700–789. doi: 10.1002/alz.12638. [DOI] [PubMed] [Google Scholar]
- 2.GBD 2016 Neurology Collaborators Global, regional, and national burden of Alzheimer’s disease and other dementias, 1990–2016: A systematic analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019;18:88–106. doi: 10.1016/S1474-4422(18)30403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.World Health Organization (WHO) [(accessed on 3 November 2022)]. Available online: https://www.who.int/news-room/fact-sheets/detail/dementia.
- 4.Matthews K.A., Xu W., Gaglioti A.H., Holt J.B., Croft J.B., Mack D., McGuire L.C. Racial and ethnic estimates of Alzheimer’s disease and related dementias in the United States (2015–2060) in adults aged >/=65 years. Alzheimers Dement. 2019;15:17–24. doi: 10.1016/j.jalz.2018.06.3063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lane C.A., Hardy J., Schott J.M. Alzheimer’s disease. Eur. J. Neurol. 2018;25:59–70. doi: 10.1111/ene.13439. [DOI] [PubMed] [Google Scholar]
- 6.Long J.M., Holtzman D.M. Alzheimer Disease: An Update on Pathobiology and Treatment Strategies. Cell. 2019;179:312–339. doi: 10.1016/j.cell.2019.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maccioni R.B., Tapia J.P., Guzman-Martinez L. Pathway to Tau Modifications and the Origins of Alzheimer’s Disease. Arch. Med. Res. 2018;49:130–131. doi: 10.1016/j.arcmed.2018.05.002. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez A., Singh S.K., Churruca M., Maccioni R.B. Alzheimer’s Disease and Tau Self-Assembly: In the Search of the Missing Link. Int. J. Mol. Sci. 2022;23:4192. doi: 10.3390/ijms23084192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Silva M.V.F., Loures C.M.G., Alves L.C.V., de Souza L.C., Borges K.B.G., Carvalho M.D.G. Alzheimer’s disease: Risk factors and potentially protective measures. J. Biomed. Sci. 2019;26:33. doi: 10.1186/s12929-019-0524-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Reiss A.B., Arain H.A., Stecker M.M., Siegart N.M., Kasselman L.J. Amyloid toxicity in Alzheimer’s disease. Rev. Neurosci. 2018;29:613–627. doi: 10.1515/revneuro-2017-0063. [DOI] [PubMed] [Google Scholar]
- 11.Soto-Rojas L.O., Pacheco-Herrero M., Martinez-Gomez P.A., Campa-Cordoba B.B., Apatiga-Perez R., Villegas-Rojas M.M., Harrington C.R., de la Cruz F., Garces-Ramirez L., Luna-Munoz J. The Neurovascular Unit Dysfunction in Alzheimer’s Disease. Int. J. Mol. Sci. 2021;22:2022. doi: 10.3390/ijms22042022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Breijyeh Z., Karaman R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules. 2020;25:5789. doi: 10.3390/molecules25245789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livingston G., Sommerlad A., Orgeta V., Costafreda S.G., Huntley J., Ames D., Ballard C., Banerjee S., Burns A., Cohen-Mansfield J., et al. Dementia prevention, intervention, and care. Lancet. 2017;390:2673–2734. doi: 10.1016/S0140-6736(17)31363-6. [DOI] [PubMed] [Google Scholar]
- 14.Bellenguez C., Kucukali F., Jansen I.E., Kleineidam L., Moreno-Grau S., Amin N., Naj A.C., Campos-Martin R., Grenier-Boley B., Andrade V., et al. New insights into the genetic etiology of Alzheimer’s disease and related dementias. Nat. Genet. 2022;54:412–436. doi: 10.1038/s41588-022-01024-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Du X., Wang X., Geng M. Alzheimer’s disease hypothesis and related therapies. Transl. Neurodegener. 2018;7:2. doi: 10.1186/s40035-018-0107-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharma P., Srivastava P., Seth A., Tripathi P.N., Banerjee A.G., Shrivastava S.K. Comprehensive review of mechanisms of pathogenesis involved in Alzheimer’s disease and potential therapeutic strategies. Prog. Neurobiol. 2019;174:53–89. doi: 10.1016/j.pneurobio.2018.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Wingo A.P., Liu Y., Gerasimov E.S., Gockley J., Logsdon B.A., Duong D.M., Dammer E.B., Robins C., Beach T.G., Reiman E.M., et al. Integrating human brain proteomes with genome-wide association data implicates new proteins in Alzheimer’s disease pathogenesis. Nat. Genet. 2021;53:143–146. doi: 10.1038/s41588-020-00773-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cacace R., Sleegers K., Van Broeckhoven C. Molecular genetics of early-onset Alzheimer’s disease revisited. Alzheimers Dement. 2016;12:733–748. doi: 10.1016/j.jalz.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 19.Lanoiselee H.M., Nicolas G., Wallon D., Rovelet-Lecrux A., Lacour M., Rousseau S., Richard A.C., Pasquier F., Rollin-Sillaire A., Martinaud O., et al. APP, PSEN1, and PSEN2 mutations in early-onset Alzheimer disease: A genetic screening study of familial and sporadic cases. PLoS Med. 2017;14:e1002270. doi: 10.1371/journal.pmed.1002270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Alzforum. [(accessed on 3 November 2022)]. Available online: https://www.alzforum.org/mutations.
- 21.Van Cauwenberghe C., Van Broeckhoven C., Sleegers K. The genetic landscape of Alzheimer disease: Clinical implications and perspectives. Genet. Med. 2016;18:421–430. doi: 10.1038/gim.2015.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nalivaeva N.N., Turner A.J. The amyloid precursor protein: A biochemical enigma in brain development, function and disease. FEBS Lett. 2013;587:2046–2054. doi: 10.1016/j.febslet.2013.05.010. [DOI] [PubMed] [Google Scholar]
- 23.Belyaev N.D., Kellett K.A., Beckett C., Makova N.Z., Revett T.J., Nalivaeva N.N., Hooper N.M., Turner A.J. The transcriptionally active amyloid precursor protein (APP) intracellular domain is preferentially produced from the 695 isoform of APP in a β-secretase-dependent pathway. J. Biol. Chem. 2010;285:41443–41454. doi: 10.1074/jbc.M110.141390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhang X., Zhang C.M., Prokopenko D., Liang Y., Zhen S.Y., Weigle I.Q., Han W., Aryal M., Tanzi R.E., Sisodia S.S. An APP ectodomain mutation outside of the Abeta domain promotes Abeta production in vitro and deposition in vivo. J. Exp. Med. 2021;218:e20210313. doi: 10.1084/jem.20210313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Muratore C.R., Rice H.C., Srikanth P., Callahan D.G., Shin T., Benjamin L.N., Walsh D.M., Selkoe D.J., Young-Pearse T.L. The familial Alzheimer’s disease APPV717I mutation alters APP processing and Tau expression in iPSC-derived neurons. Hum. Mol. Genet. 2014;23:3523–3536. doi: 10.1093/hmg/ddu064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doran E., Keator D., Head E., Phelan M.J., Kim R., Totoiu M., Barrio J.R., Small G.W., Potkin S.G., Lott I.T. Down Syndrome, Partial Trisomy 21, and Absence of Alzheimer’s Disease: The Role of APP. J. Alzheimers Dis. 2017;56:459–470. doi: 10.3233/JAD-160836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kasuga K., Shimohata T., Nishimura A., Shiga A., Mizuguchi T., Tokunaga J., Ohno T., Miyashita A., Kuwano R., Matsumoto N., et al. Identification of independent APP locus duplication in Japanese patients with early-onset Alzheimer disease. J. Neurol. Neurosurg. Psychiatry. 2009;80:1050–1052. doi: 10.1136/jnnp.2008.161703. [DOI] [PubMed] [Google Scholar]
- 28.Sleegers K., Brouwers N., Gijselinck I., Theuns J., Goossens D., Wauters J., Del-Favero J., Cruts M., van Duijn C.M., Van Broeckhoven C. APP duplication is sufficient to cause early onset Alzheimer’s dementia with cerebral amyloid angiopathy. Pt 11Brain. 2006;129:2977–2983. doi: 10.1093/brain/awl203. [DOI] [PubMed] [Google Scholar]
- 29.Karch C.M., Goate A.M. Alzheimer’s disease risk genes and mechanisms of disease pathogenesis. Biol. Psychiatry. 2015;77:43–51. doi: 10.1016/j.biopsych.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yamazaki Y., Zhao N., Caulfield T.R., Liu C.C., Bu G. Apolipoprotein E and Alzheimer disease: Pathobiology and targeting strategies. Nat. Rev. Neurol. 2019;15:501–518. doi: 10.1038/s41582-019-0228-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Safieh M., Korczyn A.D., Michaelson D.M. ApoE4: An emerging therapeutic target for Alzheimer’s disease. BMC Med. 2019;17:64. doi: 10.1186/s12916-019-1299-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sepulveda-Falla D., Sanchez J.S., Almeida M.C., Boassa D., Acosta-Uribe J., Vila-Castelar C., Ramirez-Gomez L., Baena A., Aguillon D., Villalba-Moreno N.D., et al. Distinct tau neuropathology and cellular profiles of an APOE3 Christchurch homozygote protected against autosomal dominant Alzheimer’s dementia. Acta Neuropathol. 2022;144:589–601. doi: 10.1007/s00401-022-02467-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manzine P.R., Ettcheto M., Cano A., Busquets O., Marcello E., Pelucchi S., Di Luca M., Endres K., Olloquequi J., Camins A., et al. ADAM10 in Alzheimer’s disease: Pharmacological modulation by natural compounds and its role as a peripheral marker. Biomed. Pharmacother. 2019;113:108661. doi: 10.1016/j.biopha.2019.108661. [DOI] [PubMed] [Google Scholar]
- 34.Peron R., Vatanabe I.P., Manzine P.R., Camins A., Cominetti M.R. Alpha-Secretase ADAM10 Regulation: Insights into Alzheimer’s Disease Treatment. Pharmaceuticals. 2018;11:12. doi: 10.3390/ph11010012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yuan X.Z., Sun S., Tan C.C., Yu J.T., Tan L. The Role of ADAM10 in Alzheimer’s Disease. J. Alzheimers Dis. 2017;58:303–322. doi: 10.3233/JAD-170061. [DOI] [PubMed] [Google Scholar]
- 36.Narayan P., Sienski G., Bonner J.M., Lin Y.T., Seo J., Baru V., Haque A., Milo B., Akay L.A., Graziosi A., et al. PICALM Rescues Endocytic Defects Caused by the Alzheimer’s Disease Risk Factor APOE4. Cell Rep. 2020;33:108224. doi: 10.1016/j.celrep.2020.108224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ponomareva N., Andreeva T., Protasova M., Konovalov R., Krotenkova M., Malina D., Mitrofanov A., Fokin V., Illarioshkin S., Rogaev E. Genetic Association Between Alzheimer’s Disease Risk Variant of the PICALM Gene and EEG Functional Connectivity in Non-demented Adults. Front. Neurosci. 2020;14:324. doi: 10.3389/fnins.2020.00324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xu W., Tan L., Yu J.T. The Role of PICALM in Alzheimer’s Disease. Mol. Neurobiol. 2015;52:399–413. doi: 10.1007/s12035-014-8878-3. [DOI] [PubMed] [Google Scholar]
- 39.Reddy A.P., Ravichandran J., Carkaci-Salli N. Neural regeneration therapies for Alzheimer’s and Parkinson’s disease-related disorders. Biochim. Biophys. Acta Mol. Basis Dis. 2020;1866:165506. doi: 10.1016/j.bbadis.2019.06.020. [DOI] [PubMed] [Google Scholar]
- 40.Uddin M.S., Kabir M.T., Begum M.M., Islam M.S., Behl T., Ashraf G.M. Exploring the Role of CLU in the Pathogenesis of Alzheimer’s Disease. Neurotox. Res. 2021;39:2108–2119. doi: 10.1007/s12640-020-00271-4. [DOI] [PubMed] [Google Scholar]
- 41.Harold D., Abraham R., Hollingworth P., Sims R., Gerrish A., Hamshere M.L., Pahwa J.S., Moskvina V., Dowzell K., Williams A., et al. Genome-wide association study identifies variants at CLU and PICALM associated with Alzheimer’s disease. Nat. Genet. 2009;41:1088–1093. doi: 10.1038/ng.440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jansen I.E., Savage J.E., Watanabe K., Bryois J., Williams D.M., Steinberg S., Sealock J., Karlsson I.K., Hagg S., Athanasiu L., et al. Genome-wide meta-analysis identifies new loci and functional pathways influencing Alzheimer’s disease risk. Nat. Genet. 2019;51:404–413. doi: 10.1038/s41588-018-0311-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maccioni R.B., Gonzalez A., Andrade V., Cortes N., Tapia J.P., Guzman-Martinez L. Alzheimer s Disease in the Perspective of Neuroimmunology. Open Neurol. J. 2018;12:50–56. doi: 10.2174/1874205X01812010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cortes N., Andrade V., Guzman-Martinez L., Estrella M., Maccioni R.B. Neuroimmune Tau Mechanisms: Their Role in the Progression of Neuronal Degeneration. Int. J. Mol. Sci. 2018;19:956. doi: 10.3390/ijms19040956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Maccioni R.B., Farias G., Morales I., Navarrete L. The revitalized tau hypothesis on Alzheimer’s disease. Arch. Med. Res. 2010;41:226–231. doi: 10.1016/j.arcmed.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 46.Morales I., Farias G., Maccioni R.B. Neuroimmunomodulation in the pathogenesis of Alzheimer’s disease. Neuroimmunomodulation. 2010;17:202–204. doi: 10.1159/000258724. [DOI] [PubMed] [Google Scholar]
- 47.Cao H., Zhou X., Chen Y., Ip F.C.F., Chen Y., Lai N.C.H., Lo R.M.N., Tong E.P.S., Mok V.C.T., Kwok T.C.Y., et al. Association of SPI1 Haplotypes with Altered SPI1 Gene Expression and Alzheimer’s Disease Risk. J. Alzheimers Dis. 2022;86:1861–1873. doi: 10.3233/JAD-215311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jones R.E., Andrews R., Holmans P., Hill M., Taylor P.R. Modest changes in Spi1 dosage reveal the potential for altered microglial function as seen in Alzheimer’s disease. Sci. Rep. 2021;11:14935. doi: 10.1038/s41598-021-94324-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Wissfeld J., Nozaki I., Mathews M., Raschka T., Ebeling C., Hornung V., Brustle O., Neumann H. Deletion of Alzheimer’s disease-associated CD33 results in an inflammatory human microglia phenotype. Glia. 2021;69:1393–1412. doi: 10.1002/glia.23968. [DOI] [PubMed] [Google Scholar]
- 50.Griciuc A., Federico A.N., Natasan J., Forte A.M., McGinty D., Nguyen H., Volak A., LeRoy S., Gandhi S., Lerner E.P., et al. Gene therapy for Alzheimer’s disease targeting CD33 reduces amyloid beta accumulation and neuroinflammation. Hum. Mol. Genet. 2020;29:2920–2935. doi: 10.1093/hmg/ddaa179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Cruchaga C., Kauwe J.S., Harari O., Jin S.C., Cai Y., Karch C.M., Benitez B.A., Jeng A.T., Skorupa T., Carrell D., et al. GWAS of cerebrospinal fluid tau levels identifies risk variants for Alzheimer’s disease. Neuron. 2013;78:256–268. doi: 10.1016/j.neuron.2013.02.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tao Q.Q., Chen Y.C., Wu Z.Y. The role of CD2AP in the Pathogenesis of Alzheimer’s Disease. Aging Dis. 2019;10:901–907. doi: 10.14336/AD.2018.1025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sochocka M., Zwolinska K., Leszek J. The Infectious Etiology of Alzheimer’s Disease. Curr. Neuropharmacol. 2017;15:996–1009. doi: 10.2174/1570159X15666170313122937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lopatko Lindman K., Jonsson C., Weidung B., Olsson J., Pandey J.P., Prokopenko D., Tanzi R.E., Hallmans G., Eriksson S., Elgh F., et al. PILRA polymorphism modifies the effect of APOE4 and GM17 on Alzheimer’s disease risk. Sci. Rep. 2022;12:13264. doi: 10.1038/s41598-022-17058-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Agostini S., Costa A.S., Mancuso R., Guerini F.R., Nemni R., Clerici M. The PILRA G78R Variant Correlates with Higher HSV-1-Specific IgG Titers in Alzheimer’s Disease. Cell Mol. Neurobiol. 2019;39:1217–1221. doi: 10.1007/s10571-019-00712-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Linard M., Letenneur L., Garrigue I., Doize A., Dartigues J.F., Helmer C. Interaction between APOE4 and herpes simplex virus type 1 in Alzheimer’s disease. Alzheimers Dement. 2020;16:200–208. doi: 10.1002/alz.12008. [DOI] [PubMed] [Google Scholar]
- 57.Yuan X., Wang H., Zhang F., Zhang M., Wang Q., Wang J. The common genes involved in the pathogenesis of Alzheimer’s disease and type 2 diabetes and their implication for drug repositioning. Neuropharmacology. 2023;223:109327. doi: 10.1016/j.neuropharm.2022.109327. [DOI] [PubMed] [Google Scholar]
- 58.Loera-Valencia R., Goikolea J., Parrado-Fernandez C., Merino-Serrais P., Maioli S. Alterations in cholesterol metabolism as a risk factor for developing Alzheimer’s disease: Potential novel targets for treatment. J. Steroid Biochem. Mol. Biol. 2019;190:104–114. doi: 10.1016/j.jsbmb.2019.03.003. [DOI] [PubMed] [Google Scholar]
- 59.Mez J., Chung J., Jun G., Kriegel J., Bourlas A.P., Sherva R., Logue M.W., Barnes L.L., Bennett D.A., Buxbaum J.D., et al. Two novel loci, COBL and SLC10A2, for Alzheimer’s disease in African Americans. Alzheimers Dement. 2017;13:119–129. doi: 10.1016/j.jalz.2016.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gao Y., Tan M.S., Wang H.F., Zhang W., Wang Z.X., Jiang T., Yu J.T., Tan L. ZCWPW1 is associated with late-onset Alzheimer’s disease in Han Chinese: A replication study and meta-analyses. Oncotarget. 2016;7:20305–20311. doi: 10.18632/oncotarget.7945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen M., Huang N., Liu J., Huang J., Shi J., Jin F. AMPK: A bridge between diabetes mellitus and Alzheimer’s disease. Behav. Brain Res. 2021;400:113043. doi: 10.1016/j.bbr.2020.113043. [DOI] [PubMed] [Google Scholar]
- 62.Pathak G.A., Silzer T.K., Sun J., Zhou Z., Daniel A.A., Johnson L., O’Bryant S., Phillips N.R., Barber R.C. Genome-Wide Methylation of Mild Cognitive Impairment in Mexican Americans Highlights Genes Involved in Synaptic Transport, Alzheimer’s Disease-Precursor Phenotypes, and Metabolic Morbidities. J. Alzheimers Dis. 2019;72:733–749. doi: 10.3233/JAD-190634. [DOI] [PubMed] [Google Scholar]
- 63.Reagan A.M., Christensen K.E., Graham L.C., Bedwell A.A., Eldridge K., Speedy R., Figueiredo L.L., Persohn S.C., Bottiglieri T., Nho K., et al. The 677C > T variant in methylenetetrahydrofolate reductase causes morphological and functional cerebrovascular deficits in mice. J. Cereb. Blood Flow Metab. 2022;42:2333–2350. doi: 10.1177/0271678X221122644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.De Roeck A., Van Broeckhoven C., Sleegers K. The role of ABCA7 in Alzheimer’s disease: Evidence from genomics, transcriptomics and methylomics. Acta Neuropathol. 2019;138:201–220. doi: 10.1007/s00401-019-01994-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Aikawa T., Holm M.L., Kanekiyo T. ABCA7 and Pathogenic Pathways of Alzheimer’s Disease. Brain Sci. 2018;8:27. doi: 10.3390/brainsci8020027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Zhao Q.F., Yu J.T., Tan M.S., Tan L. ABCA7 in Alzheimer’s Disease. Mol. Neurobiol. 2015;51:1008–1016. doi: 10.1007/s12035-014-8759-9. [DOI] [PubMed] [Google Scholar]
- 67.Lambert E., Saha O., Soares Landeira B., Melo de Farias A.R., Hermant X., Carrier A., Pelletier A., Gadaut J., Davoine L., Dupont C., et al. The Alzheimer susceptibility gene BIN1 induces isoform-dependent neurotoxicity through early endosome defects. Acta Neuropathol. Commun. 2022;10:4. doi: 10.1186/s40478-021-01285-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Perdigao C., Barata M.A., Burrinha T., Guimas Almeida C. Alzheimer’s disease BIN1 coding variants increase intracellular Abeta levels by interfering with BACE1 recycling. J. Biol. Chem. 2021;297:101056. doi: 10.1016/j.jbc.2021.101056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Gao P., Ye L., Cheng H., Li H. The Mechanistic Role of Bridging Integrator 1 (BIN1) in Alzheimer’s Disease. Cell Mol. Neurobiol. 2021;41:1431–1440. doi: 10.1007/s10571-020-00926-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Naj A.C., Jun G., Beecham G.W., Wang L.S., Vardarajan B.N., Buros J., Gallins P.J., Buxbaum J.D., Jarvik G.P., Crane P.K., et al. Common variants at MS4A4/MS4A6E, CD2AP, CD33 and EPHA1 are associated with late-onset Alzheimer’s disease. Nat. Genet. 2011;43:436–441. doi: 10.1038/ng.801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Hollingworth P., Harold D., Sims R., Gerrish A., Lambert J.C., Carrasquillo M.M., Abraham R., Hamshere M.L., Pahwa J.S., Moskvina V., et al. Common variants at ABCA7, MS4A6A/MS4A4E, EPHA1, CD33 and CD2AP are associated with Alzheimer’s disease. Nat. Genet. 2011;43:429–435. doi: 10.1038/ng.803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Talebi M., Delpak A., Khalaj-Kondori M., Sadigh-Eteghad S., Talebi M., Mehdizadeh E., Majdi A. ABCA7 and EphA1 Genes Polymorphisms in Late-Onset Alzheimer’s Disease. J. Mol. Neurosci. 2020;70:167–173. doi: 10.1007/s12031-019-01420-x. [DOI] [PubMed] [Google Scholar]
- 73.Moreau K., Fleming A., Imarisio S., Lopez Ramirez A., Mercer J.L., Jimenez-Sanchez M., Bento C.F., Puri C., Zavodszky E., Siddiqi F., et al. PICALM modulates autophagy activity and tau accumulation. Nat. Commun. 2014;5:4998. doi: 10.1038/ncomms5998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tian Y., Chang J.C., Fan E.Y., Flajolet M., Greengard P. Adaptor complex AP2/PICALM, through interaction with LC3, targets Alzheimer’s APP-CTF for terminal degradation via autophagy. Proc. Natl. Acad. Sci. USA. 2013;110:17071–17076. doi: 10.1073/pnas.1315110110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Campion D., Charbonnier C., Nicolas G. SORL1 genetic variants and Alzheimer disease risk: A literature review and meta-analysis of sequencing data. Acta Neuropathol. 2019;138:173–186. doi: 10.1007/s00401-019-01991-4. [DOI] [PubMed] [Google Scholar]
- 76.Yin R.H., Yu J.T., Tan L. The Role of SORL1 in Alzheimer’s Disease. Mol. Neurobiol. 2015;51:909–918. doi: 10.1007/s12035-014-8742-5. [DOI] [PubMed] [Google Scholar]
- 77.Kunkle B.W., Jaworski J., Barral S., Vardarajan B., Beecham G.W., Martin E.R., Cantwell L.S., Partch A., Bird T.D., Raskind W.H., et al. Genome-wide linkage analyses of non-Hispanic white families identify novel loci for familial late-onset Alzheimer’s disease. Alzheimers Dement. 2016;12:2–10. doi: 10.1016/j.jalz.2015.05.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Lu L., Yao Q.Y., Ruan S.S., Hu J.W., Long W.J., Dai W.Z., Ma T., Zhu X.C. Explore the role of CR1 genetic variants in late-onset Alzheimer’s disease susceptibility. Psychiatr. Genet. 2021;31:216–229. doi: 10.1097/YPG.0000000000000291. [DOI] [PubMed] [Google Scholar]
- 79.Zhu X.C., Yu J.T., Jiang T., Wang P., Cao L., Tan L. CR1 in Alzheimer’s disease. Mol. Neurobiol. 2015;51:753–765. doi: 10.1007/s12035-014-8723-8. [DOI] [PubMed] [Google Scholar]
- 80.Crehan H., Holton P., Wray S., Pocock J., Guerreiro R., Hardy J. Complement receptor 1 (CR1) and Alzheimer’s disease. Immunobiology. 2012;217:244–250. doi: 10.1016/j.imbio.2011.07.017. [DOI] [PubMed] [Google Scholar]
- 81.Han S., Nho K., Lee Y. Alternative Splicing Regulation of an Alzheimer’s Risk Variant in CLU. Int. J. Mol. Sci. 2020;21:7079. doi: 10.3390/ijms21197079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Griciuc A., Serrano-Pozo A., Parrado A.R., Lesinski A.N., Asselin C.N., Mullin K., Hooli B., Choi S.H., Hyman B.T., Tanzi R.E. Alzheimer’s disease risk gene CD33 inhibits microglial uptake of amyloid beta. Neuron. 2013;78:631–643. doi: 10.1016/j.neuron.2013.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Qin Q., Teng Z., Liu C., Li Q., Yin Y., Tang Y. TREM2, microglia, and Alzheimer’s disease. Mech. Ageing Dev. 2021;195:111438. doi: 10.1016/j.mad.2021.111438. [DOI] [PubMed] [Google Scholar]
- 84.Shi Y., Holtzman D.M. Interplay between innate immunity and Alzheimer disease: APOE and TREM2 in the spotlight. Nat. Rev. Immunol. 2018;18:759–772. doi: 10.1038/s41577-018-0051-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ulland T.K., Song W.M., Huang S.C., Ulrich J.D., Sergushichev A., Beatty W.L., Loboda A.A., Zhou Y., Cairns N.J., Kambal A., et al. TREM2 Maintains Microglial Metabolic Fitness in Alzheimer’s Disease. Cell. 2017;170:649–663.e13. doi: 10.1016/j.cell.2017.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Lee E.G., Chen S., Leong L., Tulloch J., Yu C.E. TOMM40 RNA Transcription in Alzheimer’s Disease Brain and Its Implication in Mitochondrial Dysfunction. Genes. 2021;12:871. doi: 10.3390/genes12060871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Bezuch N., Bradburn S., Robinson A.C., Pendleton N., Payton A., Murgatroyd C. Superior Frontal Gyrus TOMM40-APOE Locus DNA Methylation in Alzheimer’s Disease. J. Alzheimers Dis. Rep. 2021;5:275–282. doi: 10.3233/ADR-201000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Zhu Z., Yang Y., Xiao Z., Zhao Q., Wu W., Liang X., Luo J., Cao Y., Shao M., Guo Q., et al. TOMM40 and APOE variants synergistically increase the risk of Alzheimer’s disease in a Chinese population. Aging Clin. Exp. Res. 2021;33:1667–1675. doi: 10.1007/s40520-020-01661-6. [DOI] [PubMed] [Google Scholar]
- 89.Sengoku R. Aging and Alzheimer’s disease pathology. Neuropathology. 2020;40:22–29. doi: 10.1111/neup.12626. [DOI] [PubMed] [Google Scholar]
- 90.Zhu J.B., Tan C.C., Tan L., Yu J.T. State of Play in Alzheimer’s Disease Genetics. J. Alzheimers Dis. 2017;58:631–659. doi: 10.3233/JAD-170062. [DOI] [PubMed] [Google Scholar]
- 91.Eysert F., Coulon A., Boscher E., Vreulx A.C., Flaig A., Mendes T., Hughes S., Grenier-Boley B., Hanoulle X., Demiautte F., et al. Alzheimer’s genetic risk factor FERMT2 (Kindlin-2) controls axonal growth and synaptic plasticity in an APP-dependent manner. Mol. Psychiatry. 2021;26:5592–5607. doi: 10.1038/s41380-020-00926-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Chapuis J., Flaig A., Grenier-Boley B., Eysert F., Pottiez V., Deloison G., Vandeputte A., Ayral A.M., Mendes T., Desai S., et al. Genome-wide, high-content siRNA screening identifies the Alzheimer’s genetic risk factor FERMT2 as a major modulator of APP metabolism. Acta Neuropathol. 2017;133:955–966. doi: 10.1007/s00401-016-1652-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hassan M., Shahzadi S., Alashwal H., Zaki N., Seo S.Y., Moustafa A.A. Exploring the mechanistic insights of Cas scaffolding protein family member 4 with protein tyrosine kinase 2 in Alzheimer’s disease by evaluating protein interactions through molecular docking and dynamic simulations. Neurol. Sci. 2018;39:1361–1374. doi: 10.1007/s10072-018-3430-2. [DOI] [PubMed] [Google Scholar]
- 94.Beck T.N., Nicolas E., Kopp M.C., Golemis E.A. Adaptors for disorders of the brain? The cancer signaling proteins NEDD9, CASS4, and PTK2B in Alzheimer’s disease. Oncoscience. 2014;1:486–503. doi: 10.18632/oncoscience.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Dourlen P., Fernandez-Gomez F.J., Dupont C., Grenier-Boley B., Bellenguez C., Obriot H., Caillierez R., Sottejeau Y., Chapuis J., Bretteville A., et al. Functional screening of Alzheimer risk loci identifies PTK2B as an in vivo modulator and early marker of Tau pathology. Mol. Psychiatry. 2017;22:874–883. doi: 10.1038/mp.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Tsai A.P., Lin P.B., Dong C., Moutinho M., Casali B.T., Liu Y., Lamb B.T., Landreth G.E., Oblak A.L., Nho K. INPP5D expression is associated with risk for Alzheimer’s disease and induced by plaque-associated microglia. Neurobiol. Dis. 2021;153:105303. doi: 10.1016/j.nbd.2021.105303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Yoshino Y., Yamazaki K., Ozaki Y., Sao T., Yoshida T., Mori T., Mori Y., Ochi S., Iga J.I., Ueno S.I. INPP5D mRNA Expression and Cognitive Decline in Japanese Alzheimer’s Disease Subjects. J. Alzheimers Dis. 2017;58:687–694. doi: 10.3233/JAD-161211. [DOI] [PubMed] [Google Scholar]
- 98.Chen G., Kang S.S., Wang Z., Ahn E.H., Xia Y., Liu X., Sandoval I.M., Manfredsson F.P., Zhang Z., Ye K. Netrin-1 receptor UNC5C cleavage by active delta-secretase enhances neurodegeneration, promoting Alzheimer’s disease pathologies. Sci. Adv. 2021;7:eabe4499. doi: 10.1126/sciadv.abe4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Li Q., Wang B.L., Sun F.R., Li J.Q., Cao X.P., Tan L. The role of UNC5C in Alzheimer’s disease. Ann. Transl. Med. 2018;6:178. doi: 10.21037/atm.2018.04.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Nackenoff A.G., Hohman T.J., Neuner S.M., Akers C.S., Weitzel N.C., Shostak A., Ferguson S.M., Mobley B., Bennett D.A., Schneider J.A., et al. PLD3 is a neuronal lysosomal phospholipase D associated with beta-amyloid plaques and cognitive function in Alzheimer’s disease. PLoS Genet. 2021;17:e1009406. doi: 10.1371/journal.pgen.1009406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Wang J., Yu J.T., Tan L. PLD3 in Alzheimer’s disease. Mol. Neurobiol. 2015;51:480–486. doi: 10.1007/s12035-014-8779-5. [DOI] [PubMed] [Google Scholar]
- 102.Stage E., Duran T., Risacher S.L., Goukasian N., Do T.M., West J.D., Wilhalme H., Nho K., Phillips M., Elashoff D., et al. The effect of the top 20 Alzheimer disease risk genes on gray-matter density and FDG PET brain metabolism. Alzheimers Dement. 2016;5:53–66. doi: 10.1016/j.dadm.2016.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Makela M., Kaivola K., Valori M., Paetau A., Polvikoski T., Singleton A.B., Traynor B.J., Stone D.J., Peuralinna T., Tienari P.J., et al. Alzheimer risk loci and associated neuropathology in a population-based study (Vantaa 85+) Neurol. Genet. 2018;4:e211. doi: 10.1212/NXG.0000000000000211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Zhang Z.G., Li Y., Ng C.T., Song Y.Q. Inflammation in Alzheimer’s Disease and Molecular Genetics: Recent Update. Arch. Immunol. Ther. Exp. 2015;63:333–344. doi: 10.1007/s00005-015-0351-0. [DOI] [PubMed] [Google Scholar]
- 105.Kim H., Yoo J., Shin J., Chang Y., Jung J., Jo D.G., Kim J., Jang W., Lengner C.J., Kim B.S., et al. Modelling APOE varepsilon3/4 allele-associated sporadic Alzheimer’s disease in an induced neuron. Brain. 2017;140:2193–2209. doi: 10.1093/brain/awx144. [DOI] [PubMed] [Google Scholar]
- 106.You M., Zhou X., Yin W., Wan K., Zhang W., Li C., Li M., Zhu W., Zhu X., Sun Z. The Influence of MTHFR Polymorphism on Gray Matter Volume in Patients with Amnestic Mild Cognitive Impairment. Front. Neurosci. 2021;15:778123. doi: 10.3389/fnins.2021.778123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Kaur G., Levy E. Cystatin C in Alzheimer’s disease. Front. Mol. Neurosci. 2012;5:79. doi: 10.3389/fnmol.2012.00079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sun B., Zhou Y., Halabisky B., Lo I., Cho S.H., Mueller-Steiner S., Devidze N., Wang X., Grubb A., Gan L. Cystatin C-cathepsin B axis regulates amyloid beta levels and associated neuronal deficits in an animal model of Alzheimer’s disease. Neuron. 2008;60:247–257. doi: 10.1016/j.neuron.2008.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Mi W., Pawlik M., Sastre M., Jung S.S., Radvinsky D.S., Klein A.M., Sommer J., Schmidt S.D., Nixon R.A., Mathews P.M., et al. Cystatin C inhibits amyloid-beta deposition in Alzheimer’s disease mouse models. Nat. Genet. 2007;39:1440–1442. doi: 10.1038/ng.2007.29. [DOI] [PubMed] [Google Scholar]
- 110.Greig N.H., Utsuki T., Ingram D.K., Wang Y., Pepeu G., Scali C., Yu Q.S., Mamczarz J., Holloway H.W., Giordano T., et al. Selective butyrylcholinesterase inhibition elevates brain acetylcholine, augments learning and lowers Alzheimer beta-amyloid peptide in rodent. Proc. Natl. Acad. Sci. USA. 2005;102:17213–17218. doi: 10.1073/pnas.0508575102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Guillozet A.L., Smiley J.F., Mash D.C., Mesulam M.M. Butyrylcholinesterase in the life cycle of amyloid plaques. Ann. Neurol. 1997;42:909–918. doi: 10.1002/ana.410420613. [DOI] [PubMed] [Google Scholar]
- 112.Letronne F., Laumet G., Ayral A.M., Chapuis J., Demiautte F., Laga M., Vandenberghe M.E., Malmanche N., Leroux F., Eysert F., et al. ADAM30 Downregulates APP-Linked Defects Through Cathepsin D Activation in Alzheimer’s Disease. EBioMedicine. 2016;9:278–292. doi: 10.1016/j.ebiom.2016.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mariani E., Seripa D., Ingegni T., Nocentini G., Mangialasche F., Ercolani S., Cherubini A., Metastasio A., Pilotto A., Senin U., et al. Interaction of CTSD and A2M polymorphisms in the risk for Alzheimer’s disease. J. Neurol. Sci. 2006;247:187–191. doi: 10.1016/j.jns.2006.05.043. [DOI] [PubMed] [Google Scholar]
- 114.Ren J., Zhang S., Wang X., Deng Y., Zhao Y., Xiao Y., Liu J., Chu L., Qi X. MEF2C ameliorates learning, memory, and molecular pathological changes in Alzheimer’s disease in vivo and in vitro. Acta Biochim. Biophys. Sin. 2022;54:77–90. doi: 10.3724/abbs.2021012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Zhang Z., Zhao Y. Progress on the roles of MEF2C in neuropsychiatric diseases. Mol. Brain. 2022;15:8. doi: 10.1186/s13041-021-00892-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Xue F., Tian J., Yu C., Du H., Guo L. Type I interferon response-related microglial Mef2c deregulation at the onset of Alzheimer’s pathology in 5xFAD mice. Neurobiol. Dis. 2021;152:105272. doi: 10.1016/j.nbd.2021.105272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Karahan H., Smith D.C., Kim B., Dabin L.C., Al-Amin M.M., Wijeratne H.R.S., Pennington T., Viana di Prisco G., McCord B., Lin P.B., et al. Deletion of Abi3 gene locus exacerbates neuropathological features of Alzheimer’s disease in a mouse model of Abeta amyloidosis. Sci. Adv. 2021;7:eabe3954. doi: 10.1126/sciadv.abe3954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Satoh J.I., Kino Y., Yanaizu M., Tosaki Y., Sakai K., Ishida T., Saito Y. Microglia express ABI3 in the brains of Alzheimer’s disease and Nasu-Hakola disease. Intractable Rare Dis. Res. 2017;6:262–268. doi: 10.5582/irdr.2017.01073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Li K., Ran B., Wang Y., Liu L., Li W. PLCgamma2 impacts microglia-related effectors revealing variants and pathways important in Alzheimer’s disease. Front. Cell Dev. Biol. 2022;10:999061. doi: 10.3389/fcell.2022.999061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Claes C., England W.E., Danhash E.P., Kiani Shabestari S., Jairaman A., Chadarevian J.P., Hasselmann J., Tsai A.P., Coburn M.A., Sanchez J., et al. The P522R protective variant of PLCG2 promotes the expression of antigen presentation genes by human microglia in an Alzheimer’s disease mouse model. Alzheimers Dement. 2022;18:1765–1778. doi: 10.1002/alz.12577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Kleineidam L., Chouraki V., Prochnicki T., van der Lee S.J., Madrid-Marquez L., Wagner-Thelen H., Karaca I., Weinhold L., Wolfsgruber S., Boland A., et al. PLCG2 protective variant p.P522R modulates tau pathology and disease progression in patients with mild cognitive impairment. Acta Neuropathol. 2020;139:1025–1044. doi: 10.1007/s00401-020-02138-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sims R., van der Lee S.J., Naj A.C., Bellenguez C., Badarinarayan N., Jakobsdottir J., Kunkle B.W., Boland A., Raybould R., Bis J.C., et al. Rare coding variants in PLCG2, ABI3, and TREM2 implicate microglial-mediated innate immunity in Alzheimer’s disease. Nat. Genet. 2017;49:1373–1384. doi: 10.1038/ng.3916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li Y., Laws S.M., Miles L.A., Wiley J.S., Huang X., Masters C.L., Gu B.J. Genomics of Alzheimer’s disease implicates the innate and adaptive immune systems. Cell. Mol. Life Sci. 2021;78:7397–7426. doi: 10.1007/s00018-021-03986-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Yu B., Wang F., Wang Y. Advances in the Structural and Physiological Functions of SHARPIN. Front. Immunol. 2022;13:858505. doi: 10.3389/fimmu.2022.858505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Park J.Y., Lee D., Lee J.J., Gim J., Gunasekaran T.I., Choi K.Y., Kang S., Do A.R., Jo J., Park J., et al. Alzheimer’s Disease Neuroimaging, I. A missense variant in SHARPIN mediates Alzheimer’s disease-specific brain damages. Transl. Psychiatry. 2021;11:590. doi: 10.1038/s41398-021-01680-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Krishnan D., Menon R.N., Gopala S. SHARPIN: Role in Finding NEMO and in Amyloid-Beta Clearance and Degradation (ABCD) Pathway in Alzheimer’s Disease? Cell. Mol. Neurobiol. 2022;42:1267–1281. doi: 10.1007/s10571-020-01023-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Krishnan D., Menon R.N., Mathuranath P.S., Gopala S. A novel role for SHARPIN in amyloid-beta phagocytosis and inflammation by peripheral blood-derived macrophages in Alzheimer’s disease. Neurobiol. Aging. 2020;93:131–141. doi: 10.1016/j.neurobiolaging.2020.02.001. [DOI] [PubMed] [Google Scholar]
- 128.Asanomi Y., Shigemizu D., Miyashita A., Mitsumori R., Mori T., Hara N., Ito K., Niida S., Ikeuchi T., Ozaki K. A rare functional variant of SHARPIN attenuates the inflammatory response and associates with increased risk of late-onset Alzheimer’s disease. Mol. Med. 2019;25:20. doi: 10.1186/s10020-019-0090-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Zhu K., Jin X., Chi Z., Chen S., Wu S., Sloan R.D., Lin X., Neculai D., Wang D., Hu H., et al. Priming of NLRP3 inflammasome activation by Msn kinase MINK1 in macrophages. Cell. Mol. Immunol. 2021;18:2372–2382. doi: 10.1038/s41423-021-00761-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lawingco T., Chaudhury S., Brookes K.J., Guetta-Baranes T., Guerreiro R., Bras J., Hardy J., Francis P., Thomas A., Belbin O., et al. Genetic variants in glutamate-, Abeta-, and tau-related pathways determine polygenic risk for Alzheimer’s disease. Neurobiol. Aging. 2021;101:299.e13–299.e21. doi: 10.1016/j.neurobiolaging.2020.11.009. [DOI] [PubMed] [Google Scholar]
- 131.Larhammar M., Huntwork-Rodriguez S., Rudhard Y., Sengupta-Ghosh A., Lewcock J.W. The Ste20 Family Kinases MAP4K4, MINK1, and TNIK Converge to Regulate Stress-Induced JNK Signaling in Neurons. J. Neurosci. 2017;37:11074–11084. doi: 10.1523/JNEUROSCI.0905-17.2017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Park Y.H., Pyun J.M., Hodges A., Jang J.W., Bice P.J., Kim S., Saykin A.J., Nho K., AddNeuroMed consortium and the Alzheimer’s Disease Neuroimaging Initiative Dysregulated expression levels of APH1B in peripheral blood are associated with brain atrophy and amyloid-beta deposition in Alzheimer’s disease. Alzheimers Res. Ther. 2021;13:183. doi: 10.1186/s13195-021-00919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Barao S., Gartner A., Leyva-Diaz E., Demyanenko G., Munck S., Vanhoutvin T., Zhou L., Schachner M., Lopez-Bendito G., Maness P.F., et al. Antagonistic Effects of BACE1 and APH1B-gamma-Secretase Control Axonal Guidance by Regulating Growth Cone Collapse. Cell Rep. 2015;12:1367–1376. doi: 10.1016/j.celrep.2015.07.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Fazzari P., Snellinx A., Sabanov V., Ahmed T., Serneels L., Gartner A., Shariati S.A., Balschun D., De Strooper B. Cell autonomous regulation of hippocampal circuitry via Aph1b-gamma-secretase/neuregulin 1 signalling. eLife. 2014;3:e02196. doi: 10.7554/eLife.02196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Zhao J., Zhu Y., Song X., Xiao Y., Su G., Liu X., Wang Z., Xu Y., Liu J., Eliezer D., et al. 3-O-Sulfation of Heparan Sulfate Enhances Tau Interaction and Cellular Uptake. Angew. Chem. Int. Ed. Engl. 2020;59:1818–1827. doi: 10.1002/anie.201913029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Holmes B.B., DeVos S.L., Kfoury N., Li M., Jacks R., Yanamandra K., Ouidja M.O., Brodsky F.M., Marasa J., Bagchi D.P., et al. Heparan sulfate proteoglycans mediate internalization and propagation of specific proteopathic seeds. Proc. Natl. Acad. Sci. USA. 2013;110:E3138–E3147. doi: 10.1073/pnas.1301440110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Tesi N., van der Lee S.J., Hulsman M., Jansen I.E., Stringa N., van Schoor N., Meijers-Heijboer H., Huisman M., Scheltens P., Reinders M.J.T., et al. Centenarian controls increase variant effect sizes by an average twofold in an extreme case-extreme control analysis of Alzheimer’s disease. Eur. J. Hum. Genet. 2019;27:244–253. doi: 10.1038/s41431-018-0273-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Jun G.R., Chung J., Mez J., Barber R., Beecham G.W., Bennett D.A., Buxbaum J.D., Byrd G.S., Carrasquillo M.M., Crane P.K., et al. Transethnic genome-wide scan identifies novel Alzheimer’s disease loci. Alzheimers Dement. 2017;13:727–738. doi: 10.1016/j.jalz.2016.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.MacLachlan R., Kehoe P.G., Miners J.S. Dysregulation of ACE-1 in Normal Aging and the Early Stages of Alzheimer’s Disease. J. Gerontol. A Biol. Sci. Med. Sci. 2022;77:1775–1783. doi: 10.1093/gerona/glac083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Braae A., Medway C., Carrasquillo M., Younkin S., Alzheimer’s Research U.K.C., Kehoe P.G., Morgan K. Blood type gene locus has no influence on ACE association with Alzheimer’s disease. Neurobiol. Aging. 2015;36:1767.e1–1767.e2. doi: 10.1016/j.neurobiolaging.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 141.Narain Y., Yip A., Murphy T., Brayne C., Easton D., Evans J.G., Xuereb J., Cairns N., Esiri M.M., Furlong R.A., et al. The ACE gene and Alzheimer’s disease susceptibility. J. Med. Genet. 2000;37:695–697. doi: 10.1136/jmg.37.9.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Westwood A.J., Beiser A., Decarli C., Harris T.B., Chen T.C., He X.M., Roubenoff R., Pikula A., Au R., Braverman L.E., et al. Insulin-like growth factor-1 and risk of Alzheimer dementia and brain atrophy. Neurology. 2014;82:1613–1619. doi: 10.1212/WNL.0000000000000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Zoidis E., Ghirlanda-Keller C., Schmid C. Stimulation of glucose transport in osteoblastic cells by parathyroid hormone and insulin-like growth factor I. Mol. Cell. Biochem. 2011;348:33–42. doi: 10.1007/s11010-010-0634-z. [DOI] [PubMed] [Google Scholar]
- 144.Spangenberg E.E., Lee R.J., Najafi A.R., Rice R.A., Elmore M.R., Blurton-Jones M., West B.L., Green K.N. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-beta pathology. Pt 4Brain. 2016;139:1265–1281. doi: 10.1093/brain/aww016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Bekdash R.A. Early Life Nutrition and Mental Health: The Role of DNA Methylation. Nutrients. 2021;13:3111. doi: 10.3390/nu13093111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Iraola-Guzman S., Estivill X., Rabionet R. DNA methylation in neurodegenerative disorders: A missing link between genome and environment? Clin. Genet. 2011;80:1–14. doi: 10.1111/j.1399-0004.2011.01673.x. [DOI] [PubMed] [Google Scholar]
- 147.Schwartzentruber J., Cooper S., Liu J.Z., Barrio-Hernandez I., Bello E., Kumasaka N., Young A.M.H., Franklin R.J.M., Johnson T., Estrada K., et al. Genome-wide meta-analysis, fine-mapping and integrative prioritization implicate new Alzheimer’s disease risk genes. Nat. Genet. 2021;53:392–402. doi: 10.1038/s41588-020-00776-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Jia L., Li F., Wei C., Zhu M., Qu Q., Qin W., Tang Y., Shen L., Wang Y., Shen L., et al. Prediction of Alzheimer’s disease using multi-variants from a Chinese genome-wide association study. Brain. 2021;144:924–937. doi: 10.1093/brain/awaa364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Huang T., Shu Y., Cai Y.D. Genetic differences among ethnic groups. BMC Genom. 2015;16:1093. doi: 10.1186/s12864-015-2328-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Nazarian A., Yashin A.I., Kulminski A.M. Genome-wide analysis of genetic predisposition to Alzheimer’s disease and related sex disparities. Alzheimers Res. Ther. 2019;11:5. doi: 10.1186/s13195-018-0458-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Vila-Castelar C., Tariot P.N., Sink K.M., Clayton D., Langbaum J.B., Thomas R.G., Chen Y., Su Y., Chen K., Hu N., et al. Sex differences in cognitive resilience in preclinical autosomal-dominant Alzheimer’s disease carriers and non-carriers: Baseline findings from the API ADAD Colombia Trial. Alzheimers Dement. 2022;18:2272–2282. doi: 10.1002/alz.12552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurol. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Escott-Price V., Hardy J. Genome-wide association studies for Alzheimer’s disease: Bigger is not always better. Brain Commun. 2022;4:fcac125. doi: 10.1093/braincomms/fcac125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Marioni R.E., Harris S.E., Zhang Q., McRae A.F., Hagenaars S.P., Hill W.D., Davies G., Ritchie C.W., Gale C.R., Starr J.M., et al. GWAS on family history of Alzheimer’s disease. Transl. Psychiatry. 2018;8:99. doi: 10.1038/s41398-018-0150-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Gallagher M.D., Chen-Plotkin A.S. The Post-GWAS Era: From Association to Function. Am. J. Hum. Genet. 2018;102:717–730. doi: 10.1016/j.ajhg.2018.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Grupe A., Abraham R., Li Y., Rowland C., Hollingworth P., Morgan A., Jehu L., Segurado R., Stone D., Schadt E., et al. Evidence for novel susceptibility genes for late-onset Alzheimer’s disease from a genome-wide association study of putative functional variants. Hum. Mol. Genet. 2007;16:865–873. doi: 10.1093/hmg/ddm031. [DOI] [PubMed] [Google Scholar]
- 157.Bertram L., Lange C., Mullin K., Parkinson M., Hsiao M., Hogan M.F., Schjeide B.M., Hooli B., Divito J., Ionita I., et al. Genome-wide association analysis reveals putative Alzheimer’s disease susceptibility loci in addition to APOE. Am. J. Hum. Genet. 2008;83:623–632. doi: 10.1016/j.ajhg.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Lambert J.C., Heath S., Even G., Campion D., Sleegers K., Hiltunen M., Combarros O., Zelenika D., Bullido M.J., Tavernier B., et al. Genome-wide association study identifies variants at CLU and CR1 associated with Alzheimer’s disease. Nat. Genet. 2009;41:1094–1099. doi: 10.1038/ng.439. [DOI] [PubMed] [Google Scholar]
- 159.Seshadri S., Fitzpatrick A.L., Ikram M.A., DeStefano A.L., Gudnason V., Boada M., Bis J.C., Smith A.V., Carassquillo M.M., Lambert J.C., et al. Genome-wide analysis of genetic loci associated with Alzheimer disease. JAMA. 2010;303:1832–1840. doi: 10.1001/jama.2010.574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Feulner T.M., Laws S.M., Friedrich P., Wagenpfeil S., Wurst S.H., Riehle C., Kuhn K.A., Krawczak M., Schreiber S., Nikolaus S., et al. Examination of the current top candidate genes for AD in a genome-wide association study. Mol. Psychiatry. 2010;15:756–766. doi: 10.1038/mp.2008.141. [DOI] [PubMed] [Google Scholar]
- 161.Wijsman E.M., Pankratz N.D., Choi Y., Rothstein J.H., Faber K.M., Cheng R., Lee J.H., Bird T.D., Bennett D.A., Diaz-Arrastia R., et al. Genome-wide association of familial late-onset Alzheimer’s disease replicates BIN1 and CLU and nominates CUGBP2 in interaction with APOE. PLoS Genet. 2011;7:e1001308. doi: 10.1371/journal.pgen.1001308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Logue M.W., Schu M., Vardarajan B.N., Buros J., Green R.C., Go R.C., Griffith P., Obisesan T.O., Shatz R., Borenstein A., et al. Multi-Institutional Research on Alzheimer Genetic Epidemiology Study, G. A comprehensive genetic association study of Alzheimer disease in African Americans. Arch. Neurol. 2011;68:1569–1579. doi: 10.1001/archneurol.2011.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Lee J.H., Cheng R., Barral S., Reitz C., Medrano M., Lantigua R., Jimenez-Velazquez I.Z., Rogaeva E., St George-Hyslop P.H., Mayeux R. Identification of novel loci for Alzheimer disease and replication of CLU, PICALM, and BIN1 in Caribbean Hispanic individuals. Arch. Neurol. 2011;68:320–328. doi: 10.1001/archneurol.2010.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Gaj P., Paziewska A., Bik W., Dabrowska M., Baranowska-Bik A., Styczynska M., Chodakowska-Zebrowska M., Pfeffer-Baczuk A., Barcikowska M., Baranowska B., et al. Identification of a late onset Alzheimer’s disease candidate risk variant at 9q21.33 in Polish patients. J. Alzheimers Dis. 2012;32:157–168. doi: 10.3233/JAD-2012-120520. [DOI] [PubMed] [Google Scholar]
- 165.Kamboh M.I., Demirci F.Y., Wang X., Minster R.L., Carrasquillo M.M., Pankratz V.S., Younkin S.G., Saykin A.J., Alzheimer’s Disease Neuroimaging I., Jun G., et al. Genome-wide association study of Alzheimer’s disease. Transl. Psychiatry. 2012;2:e117. doi: 10.1038/tp.2012.45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 166.Cruchaga C., Karch C.M., Jin S.C., Benitez B.A., Cai Y., Guerreiro R., Harari O., Norton J., Budde J., Bertelsen S., et al. Rare coding variants in the phospholipase D3 gene confer risk for Alzheimer’s disease. Nature. 2014;505:550–554. doi: 10.1038/nature12825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Jonsson T., Stefansson H., Steinberg S., Jonsdottir I., Jonsson P.V., Snaedal J., Bjornsson S., Huttenlocher J., Levey A.I., Lah J.J., et al. Variant of TREM2 associated with the risk of Alzheimer’s disease. N. Engl. J. Med. 2013;368:107–116. doi: 10.1056/NEJMoa1211103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Floudas C.S., Um N., Kamboh M.I., Barmada M.M., Visweswaran S. Identifying genetic interactions associated with late-onset Alzheimer’s disease. BioData Min. 2014;7:35. doi: 10.1186/s13040-014-0035-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jun G., Asai H., Zeldich E., Drapeau E., Chen C., Chung J., Park J.H., Kim S., Haroutunian V., Foroud T., et al. PLXNA4 is associated with Alzheimer disease and modulates tau phosphorylation. Ann. Neurol. 2014;76:379–392. doi: 10.1002/ana.24219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Tosto G., Fu H., Vardarajan B.N., Lee J.H., Cheng R., Reyes-Dumeyer D., Lantigua R., Medrano M., Jimenez-Velazquez I.Z., Elkind M.S., et al. F-box/LRR-repeat protein 7 is genetically associated with Alzheimer’s disease. Ann. Clin. Transl. Neurol. 2015;2:810–820. doi: 10.1002/acn3.223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Hirano A., Ohara T., Takahashi A., Aoki M., Fuyuno Y., Ashikawa K., Morihara T., Takeda M., Kamino K., Oshima E., et al. A genome-wide association study of late-onset Alzheimer’s disease in a Japanese population. Psychiatr. Genet. 2015;25:139–146. doi: 10.1097/YPG.0000000000000090. [DOI] [PubMed] [Google Scholar]
- 172.Herold C., Hooli B.V., Mullin K., Liu T., Roehr J.T., Mattheisen M., Parrado A.R., Bertram L., Lange C., Tanzi R.E. Family-based association analyses of imputed genotypes reveal genome-wide significant association of Alzheimer’s disease with OSBPL6, PTPRG, and PDCL3. Mol. Psychiatry. 2016;21:1608–1612. doi: 10.1038/mp.2015.218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Hao S., Wang R., Zhang Y., Zhan H. Prediction of Alzheimer’s Disease-Associated Genes by Integration of GWAS Summary Data and Expression Data. Front. Genet. 2018;9:653. doi: 10.3389/fgene.2018.00653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kunkle B.W., Grenier-Boley B., Sims R., Bis J.C., Damotte V., Naj A.C., Boland A., Vronskaya M., van der Lee S.J., Amlie-Wolf A., et al. Genetic meta-analysis of diagnosed Alzheimer’s disease identifies new risk loci and implicates Abeta, tau, immunity and lipid processing. Nat. Genet. 2019;51:414–430. doi: 10.1038/s41588-019-0358-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Kunkle B.W., Schmidt M., Klein H.U., Naj A.C., Hamilton-Nelson K.L., Larson E.B., Evans D.A., De Jager P.L., Crane P.K., Buxbaum J.D., et al. Novel Alzheimer Disease Risk Loci and Pathways in African American Individuals Using the African Genome Resources Panel: A Meta-analysis. JAMA Neurol. 2021;78:102–113. doi: 10.1001/jamaneurol.2020.3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Shigemizu D., Asanomi Y., Akiyama S., Mitsumori R., Niida S., Ozaki K. Whole-genome sequencing reveals novel ethnicity-specific rare variants associated with Alzheimer’s disease. Mol. Psychiatry. 2022;27:2554–2562. doi: 10.1038/s41380-022-01483-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Yong W.S., Hsu F.M., Chen P.Y. Profiling genome-wide DNA methylation. Epigenetics Chromatin. 2016;9:26. doi: 10.1186/s13072-016-0075-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Visscher P.M., Wray N.R., Zhang Q., Sklar P., McCarthy M.I., Brown M.A., Yang J. 10 Years of GWAS Discovery: Biology, Function, and Translation. Am. J. Hum. Genet. 2017;101:5–22. doi: 10.1016/j.ajhg.2017.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Sabarwal A., Kumar K., Singh R.P. Hazardous effects of chemical pesticides on human health-Cancer and other associated disorders. Environ. Toxicol. Pharmacol. 2018;63:103–114. doi: 10.1016/j.etap.2018.08.018. [DOI] [PubMed] [Google Scholar]
- 180.Gao X., Chen Q., Yao H., Tan J., Liu Z., Zhou Y., Zou Z. Epigenetics in Alzheimer’s Disease. Front. Aging Neurosci. 2022;14:911635. doi: 10.3389/fnagi.2022.911635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Tam V., Patel N., Turcotte M., Bosse Y., Pare G., Meyre D. Benefits and limitations of genome-wide association studies. Nat. Rev. Genet. 2019;20:467–484. doi: 10.1038/s41576-019-0127-1. [DOI] [PubMed] [Google Scholar]
- 182.Wang H., Bennett D.A., De Jager P.L., Zhang Q.Y., Zhang H.Y. Genome-wide epistasis analysis for Alzheimer’s disease and implications for genetic risk prediction. Alzheimers Res. Ther. 2021;13:55. doi: 10.1186/s13195-021-00794-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Vaz M., Silvestre S. Alzheimer’s disease: Recent treatment strategies. Eur. J. Pharmacol. 2020;887:173554. doi: 10.1016/j.ejphar.2020.173554. [DOI] [PubMed] [Google Scholar]
- 184.Panza F., Lozupone M., Seripa D., Imbimbo B.P. Amyloid-beta immunotherapy for alzheimer disease: Is it now a long shot? Ann. Neurol. 2019;85:303–315. doi: 10.1002/ana.25410. [DOI] [PubMed] [Google Scholar]
- 185.van Dyck C.H., Swanson C.J., Aisen P., Bateman R.J., Chen C., Gee M., Kanekiyo M., Li D., Reyderman L., Cohen S., et al. Lecanemab in Early Alzheimer’s Disease. N. Engl. J. Med. 2023;388:9–21. doi: 10.1056/NEJMoa2212948. [DOI] [PubMed] [Google Scholar]
- 186.U.S. Food & Drug Administration [(accessed on 10 January 2023)]; Available online: https://www.fda.gov/news-events/press-announcements/fda-grants-accelerated-approval-alzheimers-disease-treatment.
- 187.Nehls M. Unified theory of Alzheimer’s disease (UTAD): Implications for prevention and curative therapy. J. Mol. Psychiatry. 2016;4:3. doi: 10.1186/s40303-016-0018-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Gauthier S., Alam J., Fillit H., Iwatsubo T., Liu-Seifert H., Sabbagh M., Salloway S., Sampaio C., Sims J.R., Sperling B., et al. Combination Therapy for Alzheimer’s Disease: Perspectives of the EU/US CTAD Task Force. J. Prev. Alzheimers Dis. 2019;6:164–168. doi: 10.14283/jpad.2019.12. [DOI] [PubMed] [Google Scholar]
- 189.Rosenberg A., Mangialasche F., Ngandu T., Solomon A., Kivipelto M. Multidomain Interventions to Prevent Cognitive Impairment, Alzheimer’s Disease, and Dementia: From FINGER to World-Wide FINGERS. J. Prev. Alzheimers Dis. 2020;7:29–36. doi: 10.14283/jpad.2019.41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Kivipelto M., Mangialasche F., Ngandu T. Lifestyle interventions to prevent cognitive impairment, dementia and Alzheimer disease. Nat. Rev. Neurol. 2018;14:653–666. doi: 10.1038/s41582-018-0070-3. [DOI] [PubMed] [Google Scholar]
- 191.Ngandu T., Lehtisalo J., Solomon A., Levalahti E., Ahtiluoto S., Antikainen R., Backman L., Hanninen T., Jula A., Laatikainen T., et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): A randomised controlled trial. Lancet. 2015;385:2255–2263. doi: 10.1016/S0140-6736(15)60461-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.