Abstract
Herpes simplex virus (HSV) infection, the most prevalent viral infection that typically lasts for a lifetime, is associated with frequent outbreaks of oral and genital lesions. Oral herpes infection is mainly associated with HSV-1 through oral contact, while genital herpes originates due to HSV-2 and is categorized under sexually transmitted diseases. Immunocompromised patients and children are more prone to HSV infection. Over the years, various attempts have been made to find potential targets for the prevention of HSV infection. Despite the global distress caused by HSV infections, there are no licensed prophylactic and therapeutic vaccines available on the market against HSV. Nevertheless, there are numerous promising candidates in the pre-clinical and clinical stages of study. The present review gives an overview of two herpes viruses, their history, and life cycle, and different treatments adopted presently against HSV infections and their associated limitations. Majorly, the review covers the recent investigations being carried out globally regarding various vaccine strategies against oral and genital herpes virus infections, together with the recent and advanced nanotechnological approaches for vaccine development. Consequently, it gives an insight to researchers as well as people from the health sector about the challenges and upcoming solutions associated with treatment and vaccine development against HSV infections.
Keywords: herpes simplex virus, herpes labialis, vaccine, antiviral, acyclovir, immune
1. Introduction
For a century, the term ‘herpes’ meaning to crawl or to creep has been used in the Greek medicine system. The term cold sore (herpes fibrils) was described by Roman physician Herodotus. Later, in 1736, the term genital herpes was coined by physician John Astruc, and in 1754 its first English translation appeared in his treatise on venereal disease. In the 18th century, herpes simplex virus (HSV) was denoted as a vocational disease of women as it was more prevalent in prostitutes [1]. It was observed in the late 19th century that the orolabial lesion was transferred to other people. According to Nahmias and Dawdle’s report presented in 1960, there are two types of antigenic HSV based on different sites of viral infection. The two serotypes of HSV, HSV-1 and HSV-2, belong to the family Herpesviridae. The three subfamilies of the Herpesviridae family are alpha-herpesviruses, beta-herpesviruses, and gamma-herpesviruses. Although all members of the Herpesviridae family can enter latency (the state of being dormant within the host cell), the cells in which they do so differ. HSV-1 and HSV-2 are members of the alpha-herpesvirus subfamily, have a short replication cycle, and can infect a variety of hosts [2].
The most common diagnostic indication of HSV infection is herpes labialis and herpes genitalis. At the beginning of the infection, the development of vesicular sores/ulcers in the oro-facial and genital regions may appear, slowly drying out into crusts that last up to 10–14 days [3,4]. Despite antiviral therapy, HSV-1 and HSV-2 can cause serious illnesses in immunocompromised children and adults, such as encephalitis with complications [5].
Synthetic medications for the treatment of HSV infection were developed in 1960, particularly drugs that restricted viral replication. First, an antiviral drug that was successfully administered systemically for HSV treatment included vidarabine as it showed better therapeutic efficacy and less toxicity [6]. Currently, acyclovir (ACV), a synthetic acyclic guanosine analogue is frequently used to treat HSV-1 and HSV-2 infections because of its accessibility, tolerability, and effectiveness [7,8,9] Some other Food and Drug Administration (FDA)-approved antiviral medications including penciclovir, famciclovir, ganciclovir, valacyclovir, cidofovir, and foscarnet are currently being used for the treatment of skin lesions caused by HSV-1 and HSV-2. However, the limited efficacy of these antiviral medications is a major concern, as they only shorten the recovery period of infection by usually 1–2 days [10,11,12]. In addition, viral resistance and side effects of these antiviral therapies pose challenges in the treatment of HSV infection [13]. It has also been reported that the effectiveness of these therapies is not noticeable for some people [14]. Apart from synthetic medicines, numerous attempts have been made to develop vaccines at a global level to manage HSV infection. Considering the genetic similarities between HSV-1 and HSV-2, establishing a vaccine that shows success against one kind of the HSV might be beneficial for the other type. However, the development of vaccines to combat the virus has proven extremely difficult because of viral latency and HSV immune evasion [15,16]. Thus, until now, no vaccine has been authorized for the use in humans. As per clinical and pre-clinical data, it has been observed that a vaccination strategy which increases effector memory T cell responses will be required for developing a potent HSV vaccine [17]. In the present review, we highlight the current treatment strategies, their limitations, and ongoing clinical studies for the development of a vaccine for managing HSV infections. The current review provides in-depth understanding about various vaccination strategies to develop an effective, yet safe vaccine for HSV infection.
2. Herpes Simplex Virus (HSV): An Overview
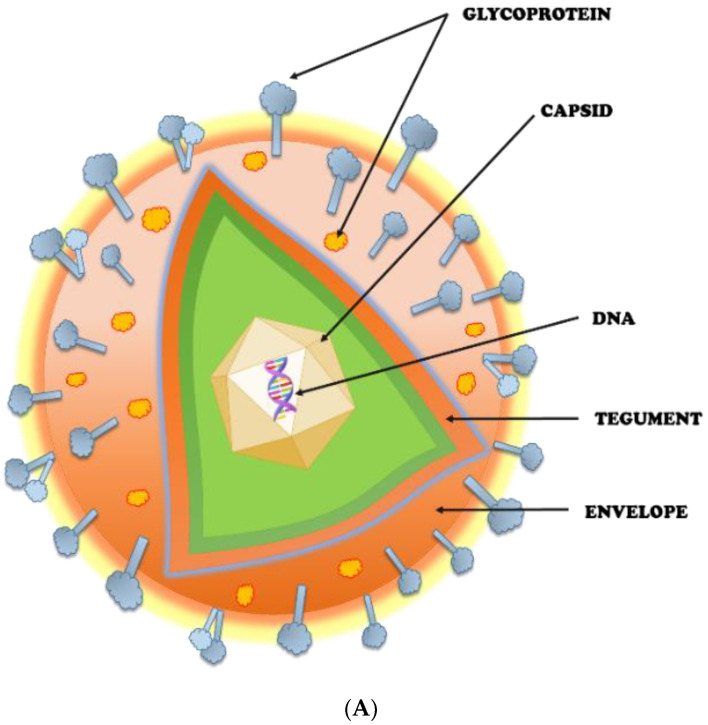
The Herpesviridae family includes HSVs, which are double-stranded DNA viruses having icosahedral symmetry. HSV-1 primarily spreads through oral-to-oral contact and produces an infection in or around the mouth (oral herpes), but it can also result in genital herpes. Genital herpes is mostly caused by the sexually transmitted HSV-2 virus [18]. WHO estimates that 3.7 billion people worldwide under the age of 50 have HSV-1 infection (67%) and 491 million people (13%) between the ages of 15 and 49 have HSV-2 infection [19]. HSV-1 mostly causes oral, labial, and possibly facial lesions. This primary infection is more serious and can cause ulcerative, painful stomatitis in children, and is associated with fever, malnutrition, and severe oral mucosal swelling [20]. Almost 30% of individuals have been affected due to repetitive orolabial infection with serologic evidence of HSV-1 and 40% people encounter frequent infections every year. Viral reactivation is spontaneous but has been linked to stress (either physical or mental), fever, UV exposure, tissue injury, and immunological suppression [21]. Recently, it has been stated that genital herpes is caused by both HSV-1 and HSV-2 mostly in industrialized nations [22,23]. Primary genital herpes develops in the form of papules and macules which are followed by vesicles, pustules, and ulcers. The non-primary genital infection is less severe than the primary infection as it gets healed within two weeks and has minor lesions, less pain, and a lower likelihood of negative consequences [21]. In genital herpes, clinical symptoms include painful genital vesicles and ulcers followed by inguinal adenopathy and systemic flu-like condition [24]. In genital infection, females are more susceptible to the formation of ulcers than males, and the progression of lesions is like that of oral herpes, where males experience a longer vesicular phase [25]. Both HSV-1 and HSV-2 use the same mechanism to bind, fuse, and proliferate from cell to cell [26]. They exhibit tremendous structural analogy, sharing about 82% of the amino acid sequence [27]. Since few studies have been reported for HSV-2, most of the concepts developed for HSV-1 are applied to HSV-2 owing to their high structural similarity [28]. HSV consists of a distinctive four-layered structure: a DNA core, capsid, tegument, and lipid membrane [29]. The electron-dense core of both HSV viruses consists of linear DNA made up of two components, L (long) and S (short), that are covalently linked to each other in addition to unique sequences, Ul (unique long) and Us (unique short) [30,31]. The genome length of HSV-1 and HSV-2 are approximately 152, kbp and 154, kbp, respectively. The genomic sequence of both HSV-1 and HSV-2 are closely related with only a major difference in the Us region, which is 1349 bp longer in HSV-2 [32]. The UL components of HSV-1 and HSV-2 genomes have the highest similarities, while the inverted repeats that bind UL share the least similarities (TRL and IRL). The nucleotide sequence of HSV-1 and HSV-2 reveals that the similarity is greatest across UL, except for UL 42 through UL 44 and UL 49. Most of the peaks correspond to protein-coding areas, and the valleys to intergenic regions. The peak of similarity is in the region encoding ICP0 (RL2), whereas the L component repeats are substantially less conserved. The ICP4 gene (RS1) contains the S component repeats, which is the most conserved region. Overall, US is not as well-conserved as UL. This region contains the US 4 (gG) gene, which is about 1500 bp longer in HSV-2 than in HSV-1 and constitutes the region with the highest divergence between HSV-1 and HSV-2 [33].
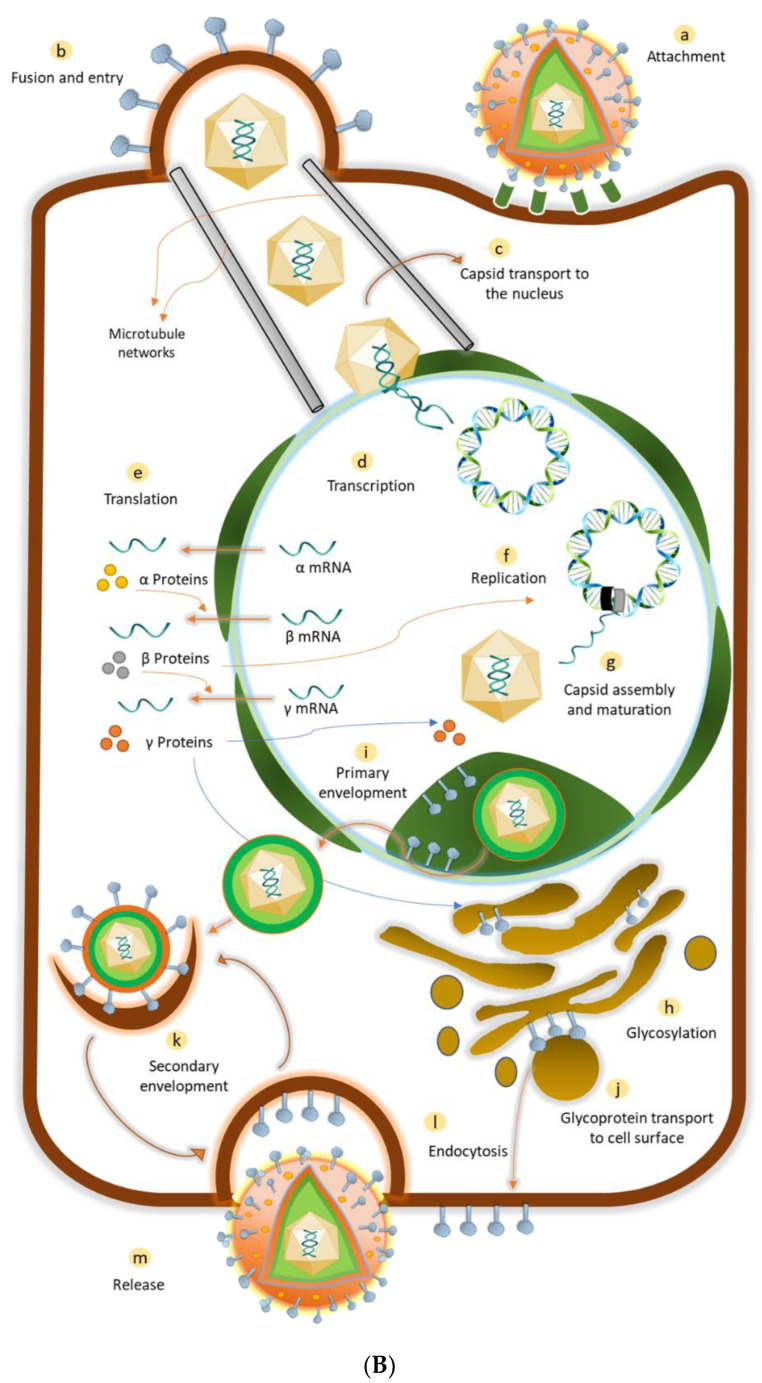
The capsid of HSV is a well-defined icosahedral made of four proteins, the major one being VP5 (149 kDa, 1374 amino acids) [34]. It helps to guard the functionality of viral DNA when outside the host cell and to initiate the infection process [35]. The tegument is an amorphous protein, whose function includes modulation of viral and host protein expression, targeting viral components, and assembly of virus [36]. Approximately twenty-two viral proteins are found in the tegument underneath the envelope [37]. The important HSV-1 teguments include pUS3, pUL13, pUL36, and pUL37 [36], and HSV-2 teguments include VP11/12, VP13/14, and VP22 [38]. This entire structure is enclosed in a glycoprotein-embedded lipid bilayer envelope. HSV has about 85 genes, out of which 12 genes code for glycoproteins [39]. These glycoproteins mediate the attachment and penetration of the virus into the host cell. These 12 different types of glycoproteins (gB, gC, gD, gE, gG, gH, gI, gJ, gK, gL, gM, and gN) are present in the envelope, out of which four glycoproteins have been associated with the process of viral entry, viz., gB, gD, gH, and gL, commonly called core fusion machinery. Figure 1: A and B represent the structure and life cycle of a typical herpesvirus. Generally, HSV-1 mostly spreads by contact with sores, saliva, or surfaces in or near the mouth that have been exposed to the virus. HSV-2 is the virus that typically causes genital HSV infections, while HSV-1 is responsible for an increasing percentage of cases. Compared to HSV-2 infections, genital HSV-1 infections are often less severe and less likely to reoccur. HSV-2 mostly spreads during intercourse by contact with the anal or genital surfaces, skin, lesions, or fluids of an infected person. HSV-2 can even spread if the skin seems normal and frequently does so without causing any symptoms [40,41]. HSV ensures its survival by sustaining a lifelong invasion of its host by establishing latency in peripheral neurons. HSV penetrates through breaches in the skin surface or mucosa by direct contact, rapidly establishing acute infections in the skin while also gaining access to the nerves that innervate the infection site. The virus invades peripheral neuron cell bodies, which are gathered in sensory and autonomic ganglia, by parasitizing axonal transport [42,43]. There are several outcomes for these neurons, ranging from productive infection at one end of the spectrum to the rapid development of latency at the other [44,45]. Furthermore, some acutely infected neurons block this function and live to join the population of latently infected neurons [46,47]. Latently infected neurons serve as a reservoir of virus for further episodes of productive infection if reactivation takes place after active HSV has been eradicated. A well-known cascade of lytic gene expression is linked to the active stage of productive or lytic infection. Contrarily, the latent state is typically thought to include strict viral gene regulation, and HSV has been seen as a model for viral latency in general, and in fact served as the inspiration for the early treatment of other viruses [48].
Figure 1.
(A) Structure of herpes virus; (B) Life cycle of herpes virus.
2.1. Potential Targets for HSV Prevention
There have been major attempts over the last several years to build up a variety of methodologies for the discovery of prospective new antiviral medications (enlisted in Table 1). The discovery and confirmation of viral targets have been the topic of several studies employing several techniques. One of the major targets is DNA polymerase; the viral DNA polymerase from HSV-1-infected cells is a heterodimer of two catalytic subunits encoded by UL30 and UL42. The DNA polymerase enzyme is inhibited by drugs like 4-hydroxy-quinoline-3-carboxamide and 8-hydroxyquinoline [49,50,51]. Since the interaction between UL30 and UL42 is essential for viral replication, its disruption suggests a potential target for developing novel antiviral therapeutics [52,53]. At least five of the HSV-1 viral envelope glycoproteins-gC, gB, gD, gH, and gL, along with host-cell membrane receptors, including herpes virus entry mediator (HVEM), nectin-1, nectin-2, and extracellular glycosaminoglycans, are required for HSV attachment and penetration into the cell [54]. One strategy is direct inhibition of viral attachment factors by using substances such as PRO 2000, polymethylene hydroquinone sulfonate, and cellulose sulphate that stop the virus from invading or replicating by building a stable complex with these proteins [55,56]. Three viral proteins, UL5, UL8, and UL52, which make up the enzyme helicase primase, are involved in the synthesis of DNA by viral DNA polymerase. Therapeutic medications such as 2-amino-thiazole and thiazolylsulfonamide decrease helicase primase production and limit HSV replication [57]. Ribonucleotide reductase (RR), which transforms ribonucleoside diphosphate into deoxyribonucleotides and is required for DNA synthesis, is another target. The interaction of two dimeric subunits, designated as RR1 and RR2, is essential for enzymatic activity. The interaction of the two subunits is selectively inhibited by therapeutic drugs like peptidomimetic inhibitors, which stop replication of HSV [58,59]. Interaction with viral particle, cellular receptor, or both to prevent virus entry is an alternate strategy since virus–host interactions are dynamic and complicated processes. Targeting certain genes to prevent viral infections seems possible with the advent of specialised genome editing tools, such as ZFNs (zinc-finger nucleases), TALENs (transcription activator-like endonucleases), and the CRISPR/Cas9 (CRISPR-associated protein 9) system [60]. A possible therapy for viral genome replication at the RNA level is synthetic siRNA. Targeting the viral DNA polymerase enzyme pUL54, kinase pUL97, and immediate-early genes UL123 and UL122 by siRNAs has been demonstrated in in vitro tests to successfully limit HCMV infection [61]. In HSV-1-infected cells, the polyamine biosynthesis pathway is still active, and S-adenosyl methionine decarboxylase (SAMDC) and ornithine decarboxylase expression is increased [62]. This supports the idea that polyamines play a part in the HSV-1 replication cycle [63,64]. The in vitro replication of HSV-1 and HSV-2 laboratory strains, as well as clinical mutant isolates that are resistant to traditional antiviral medications like ACV and foscarnet, is also inhibited by suppression of SAMDC activity [64]. SAMDC and other important polyamine pathway enzymes thus provide viable targets for anti-HSV therapy. Recent research has demonstrated that certain cyclin-dependent kinases (CDKS) are necessary for HSV replication. Pharmacological CDK inhibitors (PCIs) can prevent HSV-1 infection through several different methods. The PCIs stop viral DNA synthesis and immediate-early and early gene expression activation. The viral proteins ICP4 and ICP0 with ability to phosphorylate are disrupted by roscovitine [65,66]. By interfering with nuclear factor-κB (NF-κB) signalling at an early stage of virus infection, toll-like receptor (TLR) inhibitors greatly reduce the ability of viruses to replicate. Treatment with five adjacent guanosine residues (G-ODN) 2 h before infection suppressed TLR9 signalling and NF- κB activity and markedly decreased the production of lytic virus in herpes-susceptible cells. Additionally, the TLR9 inhibitory action of CpG oligonucleotide has been linked to downregulation of essential immediate early HSV proteins, reduced viral entry and adhesion, virucide activity, and reduced virus multiplication [67].
Table 1.
Potential targets for HSV prevention.
| Mechanism of Action | Potential Target | Drugs | References |
|---|---|---|---|
| Suppresses herpes virus genome replication at the protein level | DNA polymerase | 4-hydroxy-quinoline-3-carboxamide molecule and 8-hydroxyquinoline. | [52,53] |
| Blocks attachments and invasion by targeting viral proteins | Glycoproteins | PRO 2000, polymethylene hydroquinone sulfonate, and cellulose sulphate | [55,56] |
| Inhibits HSV DNA replication | Helicase primase complex | 2-amino-thiazole and thiazolylsulfonamide | [57] |
| Ribonucleotide reductase inhibitors | Ribonucleotide reductase | Peptidomimetic inhibitors | [58,59] |
| Inhibits polyamine biosynthetic pathway to block replication of HSV | Polyamine pathway | SAMDC inhibitors | [63,64] |
| CDK-2 inhibitors | CDK-2 | Roscovitine | [65,66] |
| Blocks attachment and penetration | Entry receptors (HVEM, glycosaminoglycans) | Anti-HVEM antibodies | [54] |
| TLR inhibitors | TLR | G-ODN, CpG oligonucleotide | [67] |
2.2. Interplay of HSV and Immune System
The immune response generated against these viruses is complex and involves an interplay between innate and adaptive immune pathways. The host–pathogen interaction could lead to a diseased state. The pathogen’s virulent factors (encoded proteins) may aid it in overcoming the host’s immune defences. The detection of HSV-1 and the induction of interferon type 1 and inflammatory cytokines have been reported to be mediated by toll-like receptors 2 and 3 [68]. TLR-3 expression and interferon type 1 production have both been shown to be inhibited by HSV-1 tegument kinase US3 [69]. Additionally, the HSV-1-infected cell polypeptide 0 (ICP0) significantly lowers the amounts of myD88, a crucial adaptor protein of the TLR pathway, in the cells [70]. It has been widely reviewed how this ICP0 protein contributes to HSV-1 infection [71]. Furthermore, NF-κB activation of genes related to an inflammatory response has been shown to be inhibited by HSV [72]. However, HSV-1 γ134.5 protein suppresses NF-κB expression in CD8+ dendritic cells [73]. In addition, it has been noted that the tegument protein VHS inhibits viral replication-independent NF-κB expression [74]. The TNF-α dependent activation of NF-κB is prevented by the HSV-1 protein UL42 [72]. It was reported that UL42 binds to the p65 and p50 subunits of NF-κB and prevents their translocation into the nucleus. As a result, the transcription of genes implicated in inflammatory responses is suppressed. The RLR cytosolic signalling pathway can trigger other cytokines such as type I interferon. Like this, the NF-κB is stimulated by the TLR2/TIR/MyD88/Mal signalling pathway, which facilitates the production of pro-inflammatory cytokines such as interleukins 6, 8, and 12 [75]. IRF3 and IRF7 are activated by TLR2/TIR/MyD88 signalling, which stimulates the synthesis of interferon-alpha and beta [76]. In addition, CD8+ TRM, a subtype of memory lymphocyte that dwells in non-lymphoid tissues [77], has been shown to activate the adaptive immune response in HSV-1 infection [78] via IFN-Y and granzyme B effectors. Following initial infection, it has been observed that CD4+ cells prevent HSV-1 infection and eradicate it from vaginal infection sites [79]. The depletion of CD4+ cells enhanced the vulnerability of mice to HSV-1 infection [80], and CD4+ CD25+ cells have been implicated in the HSV-1 response [81]. There has been a thorough study of the function of adaptive immune cells in HSV-1 infection [82]. Studies have reported that HSV inhibits the process of autophagy via the process of phosphorylation, using HSV protein Us3 ser/thr kinase of two factors involved in autophagy, namely, ULK1 and beclin1 [83]. Other suggested mechanisms exerted by HSV for modulating autophagy include downregulation of adaptor optineurin protein, autophagy adaptor protein sequestosome, and mitophagy, which are important mediators for decreasing the viral load [84]. Furthermore, PML-NBs, promyelocytic leukaemia nuclear bodies, form structures termed as viral DNA-containing PML-NBs that restrict HSV genetic expression and its subsequent replication. However, this mechanism to restrict HSV gene expression is counteracted by the ICP0 protein produced by the virus [85]. Inhibition of this apoptosis by HSV is significant for the reactivation of the virus from latency. Us3, which is a serine/threonine kinase, inhibits bad-induced apoptosis, whereas Us5 is a glycosylated J protein that inhibits fas-mediated apoptosis [86]. HSV moves into and out of the neuronal cell body throughout its life cycle within neuronal processes. HSV first affects the mucous membranes of the mouth or eyes before moving on to invade sensory nerve terminals. Retrograde transport allows HSV to go from the neural process back to the cell body, where it either enters latency or starts to multiply. Once replicated, HSV uses an anterograde transport mechanism, i.e., transit from the cell body, towards the mucosal membrane to disseminate the virus within the host as well as to other hosts. The virus has successfully spread through this cycle of retrograde transit, latency, replication, and anterograde transport. Kinesin1 proteins such as KIF5A, 5B, and 5C play a significant role in this anterograde transport mechanism. Targeting this transport mechanism can prove to be a potential therapeutic intervention [87]. The proteins expressed by HSV are known to hamper the translocation of sting protein from the endoplasmic reticulum to the Golgi apparatus, which is an essential mechanism for cellular immunity. This results in decreased levels of interferon responses [88]. Moreover, IFIT3 is an antiviral factor produced by the host that limits the replication of viral nucleic acid. UL41, a tegument protein, is reported to disrupt the IFIT3 protein activity [89]. Downregulating the CD1d expression is yet another mechanism employed by the virus to evade the immune response [90]. Peroxisomes play an important role in signalling pathways against invading pathogens. Another study revealed that VP16, a viral tegument protein, dampens the early antiviral immunity signalling from peroxisomes [91]. Finally, microRNAs (miRNA) are non-coding ribonucleic acids of 20–24 nucleotide short sequences that are responsible for post-transcriptional regulation. The decreased lytic gene expression through latency, increased viral replication, and antagonistic effect on innate and intrinsic immunity are among the hypothesised roles of HSV-1 miRNAs. Understanding the regulation of miRNA expression is crucial since miRNAs have been hypothesised to affect the switch from lytic to latent stage of HSV. Six miRNAs, namely, miR-592, miR-1245b-5p, miR-150, miR-342-5p, miR-1245b-3p, and miR-124, are known to reduce the expression of antiviral protein produced by the host cell as well as reduce viral recognition and clearance by natural killer cells [92].
3. Therapeutic Strategies Available for Herpes Infections
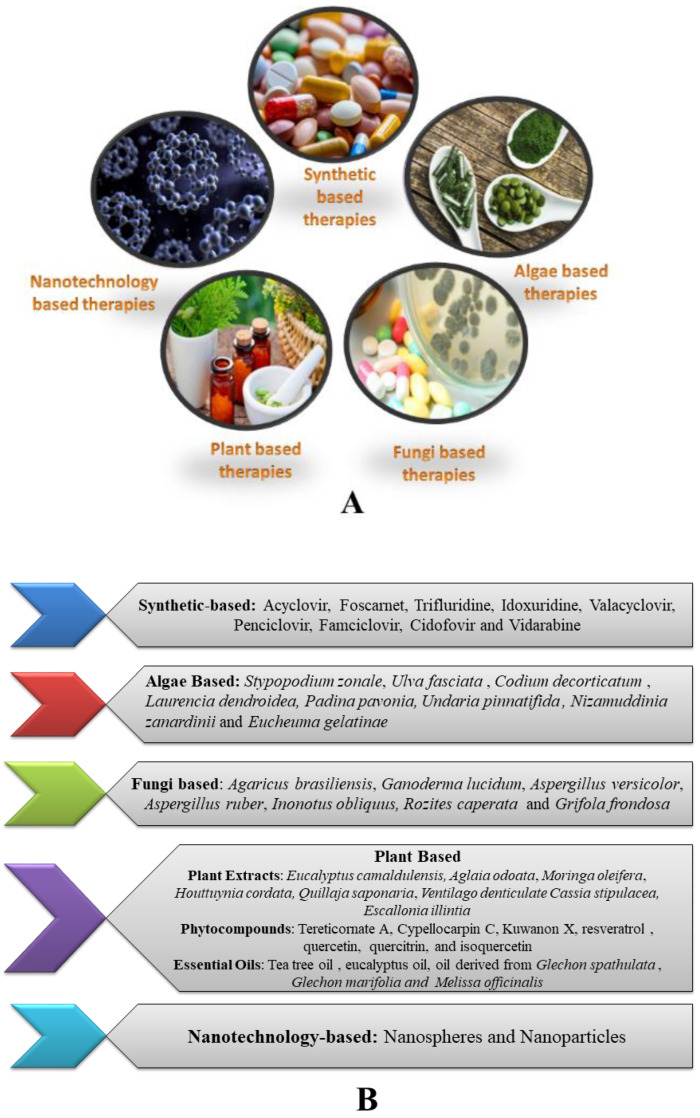
Numerous therapeutic strategies are available to combat infection caused by HSV-1 and HSV-2. These strategies include synthetic drugs, plant extracts, plant-derived compounds, algae-based therapies, fungi-based therapies, and nanotechnology-based therapies. Examples of each category are illustrated in Figure 2. These various therapeutic regimens act through different mechanisms and help to reduce severe damage caused by the herpes virus [93]. The majority of formulations made from natural substances and synthetic medicines are administered to reduce symptoms caused by HSV-1 and HSV-2. [94].
Figure 2.
(A): Various therapeutic strategies for the treatment of HSV infection; (B): Examples under different categories of HSV therapeutic strategies.
3.1. Synthetic Therapeutics
Modern medicines or antivirals are currently available on the market to treat HSV-1 and HSV-2 infections. Acyclic nucleoside and nucleotide analogues are a large class of antivirals that have been licenced for the treatment of HSV-1 and HSV-2 infections [40,41]. These antivirals prevent viral DNA polymerase from extending the viral genome during replication (UL30). ACV is the most common first-line treatment drug used to treat herpes caused by HSV-1 and HSV-2. ACV must be phosphorylated into acyclovir triphosphate in order to exert its antiviral action intracellularly. This mechanism is accomplished by the viral thymidine kinase (TK, UL23 gene), which catalyses the conversion of acyclovir into acyclovir monophosphate, thus decreasing its escape from the cell and thereby its levels within the infected cells [9,93]. ACV undergoes further phosphorylation, and on reaching its triphosphate form, it functions as a substrate for the viral DNA polymerase, thus inhibiting DNA synthesis [95]. ACV is not the only treatment option for herpes simplex skin lesions; additional options include valacyclovir, penciclovir, and famciclovir, which are also utilised frequently and are first-line medications for treating oral and genital herpetic lesions caused by HSV-1 and HSV-2 [96]. These drugs, which share a similar mode of action as ACV, are nucleic acid analogues which work by interfering with the viral DNA polymerase functioning [95]. When applied topically, these drugs alleviate herpetic lesions and accompanying discomfort in around 1–3 days as compared to untreated groups. These drugs differ from one another primarily in their bioavailability, half-life, and dose [97]. Valacyclovir is an ACV prodrug that has improved intestinal absorption [98]. Penciclovir was produced later with the goal of being phosphorylated more quickly than ACV and, as a result, achieving a longer half-life than ACV. Famciclovir is a prodrug that converts into penciclovir and has a higher oral bioavailability [99]. The efficacy of these medications in treating skin lesions brought on by HSV infections has also been sparked by their positive clinical results [14,100]. Additionally, valacyclovir has been licenced for the treatment of HSV-1 and HSV-2 infections and clinical manifestations caused by HSV-1 and HSV-2, including cold sores and recurrent genital herpes [101]. Moreover, famciclovir is authorised for the treatment of genital herpes and orolabial herpes [102].
Nucleoside analogues including idoxuridine and vidarabine are very popular to show effective results against HSV. Idoxuridine is a pyrimidine analogue used for the treatment of HSV infection [103]. It is primarily applied topically as an ointment to treat epithelial keratitis brought on by HSV-1 infection of the corneal epithelium [104]. Its weak hydrosolubility and toxicity to the corneal epithelium of the eye, however, cast doubt on its usefulness, and it is now being replaced by other more efficient, more tolerable, and less toxic drugs [103]. Vidarabine, on the other hand, is a purine analogue that has less adverse effects than idoxuridine but is also sparingly soluble and can only be used in topical formulations, making it less effective than other drugs that are already on the market. Similarly, on the other hand, trifluridine is routinely used topically to treat herpetic keratitis. This medication was given FDA approval in 1980 for the treatment of HSV-related keratitis as a 1% solution, and it is currently one of the most widely used topical antivirals for this condition in the United States, with significant efficacy documented [105]. Brivudine is also a pyrimidine analogue that functions as a prodrug and is phosphorylated by viral thymidine kinase exclusively to target the viral DNA polymerase. It has been demonstrated that this drug is effective in treating HSV-1 infection and is currently used mostly in treating VZV infections in various nations [103]. It has been observed that the nucleic acid analogue ganciclovir, which is used to treat cytomegalovirus (CMV) infection, especially in immunocompromised individuals with systemic and ocular infections, is also effective against HSV-1 and HSV-2 [105,106].
The acyclic nucleotide and pyrophosphate analogues, namely, foscarnet and cidofovir, are alternative options for the treatment of HSV. Foscarnet usually blocks viral DNA polymerase to activate polymerase activity [106,107], whereas cidofovir has been shown to be effective in vitro against HSV-1 or HSV-2 isolates that are resistant to acyclovir [108]. Immunosuppressed people, specifically those with bone marrow transplants, have been found to have resistance to the drug foscarnet. However, few studies have described HSV isolates that are resistant to cidofovir. In one instance, cidofovir was used as a treatment for three bone marrow transplant patients who yet displayed signs of an HSV-related condition. One of the three cases was proven to be resistant to cidofovir, and the isolate displayed alterations that reduced the viral DNA polymerase C-terminal length [109]. Notably, cidofovir resistance has also been recorded in children, namely, in three patients with hematopoietic stem cell transplants who received acyclovir and cidofovir in combination as prophylaxis since ganciclovir had negative side effects. Unfortunately, throughout their cidofovir treatment, these children had HSV-related stomatitis, and the investigators speculated that the medication did not stop the patients’ HSV-1 from reactivation [110]. However, cidofovir is regarded as a useful alternative for dealing with HSV isolates that exhibit decreased levels of enzymes involved in the phosphorylation of nucleoside analogues or foscarnet resistance [111]. In a clinical case of a child with HSV-1 that was resistant to ACV and foscarnet, cidofovir therapy was successful in treating this drug-resistant HSV-1 [112]. Other research found that only cidofovir therapy was effective in preventing recurrent oral stomatitis in a girl with lymphatic leukaemia who had HSV-1 isolates resistant to both ACV and foscarnet [113]. New therapeutic options have arisen in recent years as the medications indicated above have considerably diminished clinical efficacy in the treatment of HSV-induced skin lesions. Acyclovir and hydrocortisone for topical application are two of these substitutes (Xerese®, Medivir), Huddinge, Sweden. In comparison to acyclovir, this formulation lowered the size of the lesion area by 50% and shortened the duration of herpetic lesions by 1.6 days [114].
Moreover, formulations like docosanol, Viroxyn®, and Novitra® attract a lot of attention in the market for showing effectiveness against viral infection caused by HSV [115,116,117].
Docosanol 10% cream, which is easily accessible over the counter (OTC) to treat herpes labialis, is a relatively new medication to treat skin lesions brought on by the virus. It is packaged as a topical cream (Abreva®, Avanir, San Diego, CA, USA) [115]. According to one study, using docosanol 10% cream for herpes labialis shortened recovery time by 18 h [118]. Docosanol’s mechanism of action is carried out through the prevention of viral fusion with cell membranes [119]. Viroxyn® (Quadex Pharmaceuticals, UT, USA) is another medication that is marketed to treat herpes labialis. It contains benzalkonium chloride, a Category III antiseptic that is also used for other uses, primarily as a biocidal preservative. According to a study, benzalkonium chloride has virucidal properties against HSV [116]. However, the FDA declared in 2016 that it would no longer allow the sale of several bactericidal ingredients, putting benzalkonium chloride on “stand-by” while awaiting clinical data demonstrating its safety in humans. As a result, it may be recalled [120]. Finally, Novitra®, a zinc oxide-based cream, is another drug promoted for treating skin lesions brought on by the HSV-1 virus. In a clinical trial, this formulation has been proven to shorten HSV-1 skin lesions by up to 1.5 days as compared to untreated individuals [117].
3.2. Plant-Based Therapeutics
Antiviral medications are used to reduce and lower the intensity of recurring outbreaks of oral and genital herpes caused by HSV-1 and HSV-2 [121]. The major antivirals mentioned above are used for the treatment of oral and genital herpes viral infections [122]. However, conflicting data supporting the use of acyclovir and other nucleoside analogues to treat herpes infection is also present [94,123,124]. Some other antiherpes medicines such as penciclovir and famciclovir work by interfering with viral DNA polymerase but have some inconsistent results [93,94]. Consequently, there is a great necessity to identify new alternative antiviral substances that are derived from plants or natural compounds and demonstrate antiviral efficacy against HSV strains. Interestingly, several plant extracts and phytocompounds derived from plants have been shown to prevent HSV multiplication.
3.2.1. Plant Extracts
In the search for novel molecules against HSV infection, plant extracts have drawn a lot of attention. The in vitro and in vivo studies that have been conducted so far on plant extracts were compiled by Garber A et al. [125]. A cell culture study on ethanolic extract of Eucalyptus camaldulensis has shown the effectiveness of plant leaf extract against HSV-1 and HSV-2 infection [126]. Alternatively, extracts from Aglaia odoata, Moringa oleifera, and Ventilago denticulate have also been demonstrated to exhibit antiviral activity against wild-type and drug-resistant HSV-1 isolates; however, the mechanisms of action seem to be unknown or not documented [127]. A Chinese herbal plant, namely, Houttuynia cordata, prevents HSV-2 infection by preventing NF-κB activation, an important target that has been suggested to be necessary for HSV infection effectiveness [128]. The antiviral efficacy of aqueous and hydroalcoholic extracts obtained from native Chilean plants against HSV-1 and HSV-2 has also been studied. The IC50 value of hydroalcoholic extract of Cassia stipulacea (80 μg/mL) and Escallonia illintia (40 μg/mL) revealed potential antiviral activity against HSV-1. Furthermore, Aristotelia chilensis, Drymis winteri, and Elytropus chilensis also produced satisfactory results against HSV-2 [129]. According to Roner et al. (2007), aqueous extracts of a Chilean soapbark tree, Quillaja saponaria, have antiviral activity against HSV-1 and other viruses [130]. Animal models of plant extract also suggest antiviral efficacy against HSV-1 and HSV-2. A study includes the combination of plant extracts and acyclovir to treat antiherpes activity upon infection of the skin in a BALB/c mice model [127]
3.2.2. Phytocompounds
Eucalyptus species are well-known for their antiviral activity. The main natural compounds present in eucalyptus oil are cineole, alpha-pinene, and others. In a study, 24 compounds were isolated from the leaves of Eucalyptus sideroxylon using UPLC/PDA/ESIqTOF-MS method and evaluated for antiviral activity. Out of all the compounds, four compounds were found effective against HSV-1 and HSV-2 [131]. Similarly, it was shown that 12 compounds isolated from E. globulus also have proven antiviral activity against HSV-1. The compounds, namely, Tereticornate A and Cypellocarpin C, had potent antiviral activity against HSV-1 and HSV-2, respectively, as compared to acyclovir [132]. The leaves of the plant Morus alba have also shown anti-HSV activity, and an active compound present in this plant known as Kuwanon X, a stilbene polyphenol derivative exhibited antiviral activity against different strains of HSV [133]. In addition, some important flavonoids present in Houttuynia cordata such as quercetin, quercitrin, and isoquercetin demonstrate stronger inhibition towards HSV-2 [128]. A plant flavonoid, resveratrol, obtained from Veratum grandiflorum prevents activation of NF-κB [134]. Animal models and cell culture studies have also confirmed antiviral activity of resveratrol against HSV-1 and HSV-2 [135,136]. Two major compounds isolated, namely, Chikusetsusaponin Iva and calenduloside from the plant Alternanthera philoxeroides, were assessed for antiviral activity against HSV-1 and HSV-2. The mode of action of Chikusetsusaponin Iva was found to be virucidal action while evaluating through animal model [137]. Furthermore, it has been revealed that a glycopeptide meliacine (MA) from the plant Melia azedarach, shows antiviral efficacy against HSV-1 [138]. Topical application of MA shows antiviral activity against HSV-2 in mouse model [139]
3.2.3. Essential Oils
It has been demonstrated that essential oils derived from plants of the family Labiatae and Verbenaceae have shown antiviral action against HSV. A study conducted on Vero cells against HSV infection has found that in the first phases of infection, essential oils from the Labiatae have shown inhibitory action with Rf values of 1 × 102 and 1 × 103 [140]. The essential oil derived from Glechon spathulata and Glechon marifolia also showed effectiveness against HSV infection [141]. In another investigation, tea tree oil and eucalyptus oil were examined for antiviral efficacy against HSV-1 and HSV-2 using RC-37 cells. Tea tree oil showed a high virucidal effect as compared to eucalyptus oil in the viral suspension test [142]. Volatile oils from Melissa officinalis, often known as lemon palm, a member of the Laminae family, have demonstrated antiherpes virus action [143]. A similar study showed the effectiveness of these essential oils against HSV-2 [144]
3.3. Algae-Based Therapeutics
Numerous studies have shown that algae contain bioactive compounds with potent antiviral activity against a range of viruses like human papillomavirus (HPV) [145], picornavirus [146], HIV [147,148], influenza [149], and dengue [150]. Additionally, multiple research projects have shown that algae have antiviral action against HSV-1 and HSV-2 [122,151,152]. Four of the more than 36 species of algae collected from Brazil’s beaches were shown to have strong antiviral activity against both HSV-1 and HSV-2. Stypopodium zonale, Ulva fasciata, and Codium decorticatum are some of the examples of green algae that showed antiviral activity against HSV-1 and HSV-2. Laurencia dendroidea, a red alga, also showed potential action against HSV-1 which may be due to the presence of sesquiterpenes [152]. According to research, the alga Padina pavonia, which also prevents HSV-1 reproduction, has a bioactive substance made of sulphated polysaccharides called fucoidan which prevents the virus from adhering to the surface of cells. An in vivo study on the plant fucoidan represented a decrease in herpetic lesions in the HSV-1 corneal-infected animals who received the compound pretreatments for a week. The same compound obtained from the brown alga Undaria pinnatifida also exhibited antiviral properties against HSV-1 and HSV-2 [153]. One more study confirms the antiherpes activity of fucoidan which was isolated from the brown seaweed Nizamuddinia zanardinii. The cell line activity of the compound demonstrated that this compound prevented HSV-2 from adhering to Vero cells, hence preventing the first stage of HSV-2 infection [154].
It has been observed that polysaccharides from the various algae represent anti-HSV activity. For instance, Eucheuma gelatinae, a red alga, has been investigated using Vero cells. The in vitro activity of polysaccharides derived from this alga demonstrates antiviral activity against HSV. It was clear by analysing the expression of the viral protein VP5 as well as the cellular location of this protein that viral protein synthesis was impacted [155]. Another polysaccharide isolated from the green seaweed E. compressa exhibits antiviral action against HSV-1 infection. Plaque reduction assays were performed to evaluate the antiherpes activity of these polysaccharides after viral penetration [156]. In another study, polysaccharides isolated from brown alga Sargassum henslowianum were purified and subjected to plaque reduction assay. The results confirmed the antiviral activity of polysaccharides SHAP-1 and SHAP-2 with IC50 values of 0.89 and 0.82 g/mL, respectively, against HSV-1 [157].
Osmundaria obtusiloba, an alga collected from the Brazilian shore, was shown to exhibit activity against both HSV-1 and HSV-2 by interacting with viral glycoproteins [158]. Hypnea musciformis, a red seaweed, is another example of an alga having antiviral activities against HSV. It has demonstrated high antiviral activity against HSV-1 in various aqueous fractions. Virucidal activity and the prevention of viral binding to cells are the main mechanisms [159]. Additionally, Haematococcus pluvialis extracts have demonstrated antiherpetic efficacy. The major mechanisms were found as reduced virus attachment to the host cell, the viral-cell fusion process, and/or the virus’s entrance into the cell [160]. Cystoseira myrica is another source of alga extract with antiviral action against HSV-1, and it significantly reduces the virus’s capacity to replicate [161].
3.4. Fungi-Based Therapeutics
In order to find new molecules with antiviral activity against HSV-1 and HSV-2, substances produced from fungi have also been investigated. Polysaccharides-derived Agaricus brasiliensis have shown antiherpetic activity against HSV-1 and HSV-2 The cell line study using Vero cells demonstrate a synergistic effect of polysaccharides with acyclovir [162]. It is interesting to note that proteins from fungi also have been found to prevent HSV infection. Two proteins from the fungus Ganoderma lucidum that bind polysaccharides were discovered to have antiviral properties against HSV-1 and HSV-2. The neutral protein bound to polysaccharide (NPBP) and the acidic protein bound to polysaccharide (APBP), respectively, prevented plaque formation by HSV-1 and HSV-2. However, APBP has more antiviral activity than NPBP. It is further noted that APBP inhibits virus’s attachment and penetration into Vero cells [163]. The in vitro activity of three anthraquinones reported from the fungus Aspergillus versicolor exhibits antiviral activities against HSV-1 [164]. Two secondary metabolites from another species, Aspergillus ruber fungus, flavoglaucin, and isodihydroauroglaucin, were also investigated and showed anti-HSV-1 action [165]. Moreover, it was shown that an aqueous extract from the plant Inonotus obliquus (AEIO) prevented HSV-1 infection in Vero cells. Further, the antiviral activity of the plant extract suggests prevention of virus entry and membrane fusion. The RC38 protein from Rozites caperata also inhibited the growth of HSV-1 in Vero cells and reduced the severity of stromal keratitis in animal models [166]. In contrast, proteins from Grifola frondosa (GFAHP) were shown to exhibit antiviral activity against HSV-1 by reducing viral entrance into Vero cells and thus showing virucidal effects. In animal models, GFAHP proteins cause reduction in viral replication, particularly in the cornea, along with vascularization, and thus indicated antiviral efficacy against HSV-1 [167].
3.5. Nanotechnology-Based Therapeutics
Nanotechnology offers innovative techniques to create antiviral therapies. The significance of nanotech-derived delivery systems is rising for demonstrating critical pharmacological qualities of bioavailability, faster circulation times, and site-specificity, as well as a reduction in the overall toxicity profile [168,169,170]. Acyclovir was delivered via chitosan nanospheres (NS), a novel topical formulation. The developed nanospheres showed much greater antiviral efficacy against HSV-1 and HSV-2 as compared to acyclovir alone [171]. To enhance systemic bioavailability of buccal films, acyclovir-loaded nanospheres were placed into the films. A significantly substantial increase in acyclovir absorption relative to oral dosage was seen in in vivo testing of the prepared films. Additionally, extended circulation times of the produced film showed that acyclovir continued to release over time [172].
Metal and inorganic nanoparticles (NPs) prevent viral multiplication at the subcellular level, and thus prevent viral invasion. Silver and gold nanoparticles represent significant antiviral action against viruses. Recent research findings have indicated that metal NPs have an anti-HSV-1 action [173,174].
The silver nanoparticles (AgNPs) of aqueous extract from the leaves of Melaleuca alternifolia exhibit strong antiviral activity against HSV-1, HSV-2 and many other microbes [175]. A hydrogel prepared by tannic acid and silver nanoparticles (TA-AgNP) showed significant capacity to limit HSV-1 infection. The findings suggested that this hydrogel reduced viral adherence, invasion, and post-infection dissemination. It has been also reported that TA-AgNP may bind with proline-rich proteins to prevent viral invasion [176]. The aqueous and hexane extract of Lampranthus coccineus and Malephora lutea were used to synthesize AgNPs. These nanoparticles possess antiviral activities against HSV-1 infection. A further study shows that AgNPs can represent antiviral action which is connected to the virus’s ability to spread more slowly after being blocked by the interaction with viral envelope glycoproteins [177]. Greenly biosynthesized AgNPs from Oscillatoria spp. Demonstrate inhibition of HSV-1 replication in a dose-dependent manner. The algae-biosynthesized gold nanoparticles (AuNPs) from Spirulina platensis suppressed HSV-1 proliferation in a dose-dependent manner, culminating in a 90% decrease in cytopathic effect (CPE) at 31.25 μL/mL. [178]. The AuNPs of Sargassum wightii (Sw-AgNPs) seaweed also could fight HSV1 infection by antiviral action. The CPE reduction approach was used to assess the anti-HSV-1 activity of AuNPs. The cytotoxicity experiment revealed that Sw-AgNPs were safe at concentrations > 25 μL/mL and demonstrated 85.1% cell survival, while the dose-response inhibition assay revealed that Sw-AuNPs decreased HSV-1 CPE at 10 μL/mL [179]. Three different gold nanoparticles, namely, NPAuG1-S2, NPAuG2-S4, and NPAuG3-S8, were evaluated for antiviral activity against HSV-1. The results suggested that these nanoparticles prevented viral entry and thus reduced HSV-1 infection [180]. In one study, nonfunctionalized AuNPs were used for the evaluation of antiviral efficacy. The results showed less cytopathic effect of these nanoparticles in Vero cells against HSV-1 [181].
Recently, gold nanoparticles of lactoferrin (LF-Ag/AuNPs) have been found effective against genital herpes infection caused by HSV-2. LF-Ag/AuNPs significantly outperformed lactoferrin alone at preventing HSV-2 attachment and penetration into human keratinocytes. Moreover, results found lower viral titres, particularly in genital tissues and spinal cord of infected mice. There was a large decrease in B+T, NK, CD8+, and dendritic cells in the vaginal tissues [182]. In female BALB/c mice, intravaginal application of zinc oxide tetrapod nanoparticles (ZOTEN) has been demonstrated to be an efficient HSV-2 genital infection suppressor. The clinical indications of vaginal infection were significantly reduced by ZOTEN’s potent ability to bind HSV-2, and animal mortality was significantly diminished as well [183,184].
Dendrimers, polymeric nanostructures with 100 nm-diameter size that can be used to deliver DNA, siRNA, and antiviral medicines, have been reported to have antiviral activity [185]. The dendrimers interact with viral glycoproteins and prevent HSV-1 infection at early stages. The poly(amide)-based dendrimers exhibited a 35% viral suppression, and at the same concentration as peptidodendrimers, exhibit more than 80% viral inhibition at a dosage of 280 nM/mL. This finding demonstrates the antiviral activity of the dendrimer structure, which could be enhanced by including a specific amino acid sequence into its terminal locations [186].
3.6. Clinical Trial Evidence
In addition to in vitro investigations, a variety of synthetic compounds have been tested in clinical trials to confirm potential new drugs for the treatment of HSV symptoms. Some of these include antiviral drugs such as amenamevir [187], nelfinavir mesylate [188], brincidofovir [189,190], and pritelivir [191]. The clinical investigations of pritelivir [191] (NCT01047540, NCT01658826, and NCT02871492) and brincidofovir (NCT01143181) showed activity in phase III trials and were found effective against HSV infection. However, the findings have not been published. One more clinical study of pritelivir tablets on immunocompromised individuals is currently ongoing for the treatment of acyclovir-resistant HSV-1 or HSV-2 infection. The results of this study are expected in the year 2024 (NCT03073967). It has been further reported that brincidofovir has up to 1000 times the antiviral activity of cidofovir [192]. A clinical trial was conducted to assess the efficacy of topical cidofovir for refractory mucocutaneous HSV-1 and HSV-2 in AIDS; the findings of this clinical trial, however, have not been published (NCT00002116). An oxadiazolephenyl derivative, amenamevir, mainly focused on the viral DNA helicase/primase complex (H/P) [106,193], has undergone evaluation in at least three clinical studies (NCT02209324, NCT01959295, and NCT02852876) [187]. Unfortunately, a study shows that amenamevir had side effects in an early clinical phase [187]. In addition to being an inhibitor of the HIV-1 protease [194,195], the antiviral drug nelfinavir mesylate was also found to have antiviral efficacy against HSV-1 [188]. The clinical study of this antiviral drug is under evaluation for the treatment of Kaposi’s sarcoma and HSV infection (NCT03077451).
Clinical experiments have been also conducted on several phytocompounds based on the research performed in vivo, in order to verify new medications that might be used to cure HSV infection. Various Chinese herbs including Huangbo (Cortex Phellodendri), Zhizi (Scutellaria baicalensis), and Huanglian (Rhizoma coptidis) have been tested for the treatment of herpes labialis (NCT03469232) A Thera Neem Lip therapy containing numerous active ingredients like coconut oil, sesame oil, jojoba oil, neem oil, peppermint oil, and tocopherol is in clinical trials for the treatment of HSV (NCT00985335). Additionally, clinical research evaluated Viblock®, a cream that is allegedly made entirely of botanical ingredients but whose composition has not been disclosed (NCT03080961). The cream’s ability to prevent HSV-2 infection was investigated during formulation. However, the outcomes of the clinical trials mentioned above have not yet been published. In 2019, a randomized controlled clinical trial of medical grade kakua honey was conducted in comparison with acyclovir against herpes labialis. The topical application of natural extract and 5% acyclovir honey was given five times a day, and results found that kakua honey was no more effective than acyclovir 5% [196].
4. Limitations of the Current Treatments and Challenges for Vaccine Development
Treatments that are commercially available to treat HSV-1 and HSV-2 infection have some serious side effects, which is a matter of deep concern. Currently, acyclovir and valacyclovir are widely being used for the treatment of HSV-1 and HSV-2 infections. However, certain limitations are also associated with these antiviral drugs [197]. The developing resistance of some antiviral medications such as valacyclovir, penciclovir, and famciclovir in immunocompromised patients is also a major challenge [111,198,199]. Moreover, these antiviral drugs are not able to control latency or latency reactivation processes. Some of the limitations of current therapies are described below.
4.1. Limitations of Current Treatments
Current therapies such as acyclovir and valacyclovir, two of the most-often used treatments for HSV, work well to shorten the length and intensity of symptoms as well as viral shedding [197]. However, the short treatment window of HSV infections for effective therapy is limited. Furthermore, they are only marginally helpful, with only slight improvements in symptom intensity and duration [200]. It is noted that oral administration of ACV has an absorption efficiency of just 15–30%, and prior studies suggested that elderly patients with renal issues could develop severe neurotoxicity as a result of their inability to excrete this medication correctly [201,202]. The propensity of HSV-1 and HSV-2 to evolve and create forms that are resistant to this drug by attaining mutations in the gene that encodes for the viral thymidine kinase, as well as the gene that encodes for viral DNA polymerase (UL30), is another significant drawback of ACV [203]. However, it is possible for patients to develop resistance to valacyclovir, penciclovir, and famciclovir. As valacyclovir is derived from acyclovir, there are chances of development of cross-resistance between valacyclovir- and acyclovir-resistant HSV isolates [111,198]. Acyclovir-resistant HSV-1 isolates in immunocompromised individuals, on the other hand, may develop cross-resistance to penciclovir and the prodrug famciclovir [199,204,205,206]. If administered as soon as prodrome or erythema symptoms are noticed, oral ACV treatment for skin lesions caused by HSV-1 and HSV-2 only shortens the recovery process by around 2 days (time to disappearance of scab), from 7.9 days (placebo arm) to 5.8 days [207]. The therapeutic value of these antiviral medications for the treatment of herpetic lesions has been somewhat disputed because these changes are mild yet statistically significant [208]. Importantly, a substantial impact for ACV is not shown when the medication is administered at the stage of papule [208]. Additionally, in this case, it was discovered that the treatment group required longer time for cutaneous lesions to heal than the placebo arm did (8 days vs. 7.2 days, respectively). Unfortunately, over 50% of patients are unable to recognise the prodrome and erythema stages before papule development, and as a result, they are not able to begin a successful oral therapy with ACV in time to considerably shorten the duration of the herpetic lesions [208]. ACV therapy is thus not beneficial in these situations [209]. ACV can be used topically as a cream instead of being consumed orally. However, topical administration of ACV to herpetic lesions at the papule stage has therapeutic effects, which are generally modest as they only shorten the recovery period in herpes labialis by one to two days and in genital herpes infections by three days [10,11]. It should be noted that ganciclovir has been reported to cause significant negative side effects in an elevated proportion of individuals, including nephrotoxicity, myelosuppression, neutropenia, confusion, anxiety, altered mental status, ataxia, convulsions, tremors, fever, abnormal liver enzyme levels in serum, nausea, and diarrhoea [96]. Cidofovir and foscarnet are also associated with a variety of negative side effects in patient populations, including nephrotoxicity, azotemia, proteinuria, crystalluria, interstitial nephritis, acute tubular necrosis, rise in creatinine levels of up to 50%, hypo- and hypercalcemia, hypo- and hyperphosphatemia, and the development of urogenital ulcers [210]. Due to these side effects, it is advised that foscarnet patients be closely monitored to prevent the anomalies mentioned above. Foscarnet resistance, unlike that of nucleoside analogues, is exclusively brought on by alterations in the viral DNA polymerase gene. Immunosuppressed people, specifically those who have bone marrow transplants, have been found to have resistance to the drug foscarnet [111].
4.2. Various Approaches Investigated for Meeting out the Limitations and Challenges
The incidence and prevalence of HSV-2 infection at the community level are unlikely to be impacted by current HSV preventive or treatment methods. Therefore, HSV vaccines may be the only practical and approachable means of achieving this objective. Even though currently there is no vaccine for herpes labialis and genital herpes, there are a number of candidates in clinical and pre-clinical stages of research. There are two main areas of vaccine development: preventive and therapeutic, with some having dual purposes [103,200]. The main goal of preventative vaccination is to keep a seronegative individual from developing a primary infection. Therapeutic immunizations are intended to stop HSV reactivation, lessen the frequency of relapses, or lessen the intensity or duration of clinical symptoms [211]. In terms of vaccine development, a successful vaccination would most likely induce both humoral and cell-mediated responses. In each vaccination study, it is critical to pay attention to the adjuvants and assess how they contribute to the development of a robust humoral and cell-mediated response [212].
5. Preventive Methods for HSV through Vaccines
To combat HSV-1 and HSV-2 infections, four main vaccine strategies have been developed, including replication-defective HSV vaccines, inactivated killed HSV vaccines, live-attenuated HSV vaccines, and subunit HSV vaccines. In terms of safety and efficacy, these vaccine strategies have various advantages and disadvantages.
5.1. Inactivated Killed HSV Vaccines
In the 1970s and 1980s, an approach to kill the whole virus led to the development of the first inactivated killed HSV vaccine [213,214]. Since these inactivated HSV vaccines produced only antibodies instead of T cells, they were ineffective at preventing recurring HSV-1 or HSV-2 infections and illnesses [214,215,216]. The ineffectiveness of inactivated viral vaccines has been overcome by live-attenuated viral vaccines by showing their ability to replicate. These two vaccines vary in important ways; the inactivated vaccines have the potential to modify the antigens while the antigens produced by live-attenuated vaccines retain their native structure, thus increasing vaccine effectiveness. In contrast to live-attenuated vaccines, inactivated vaccines stimulate the body to produce antibodies, but the immunological response is significantly delayed [217,218]. Additionally, as is the case with the poliomyelitis vaccines, inactivated and live-attenuated vaccines can be administered through several routes against a specific type of virus. Thus, it is impossible to say with certainty that live-attenuated vaccines are better than inactivated vaccines due to their persisting capacity for replication [217,219,220].
5.2. Live-Attenuated Vaccines
Over the past 24 years, numerous live-attenuated HSV vaccines have been developed and evaluated in animal models mostly as preventative therapy. However, only a handful of these live vaccines have advanced into clinical investigations due to tolerability and safety issues [221]. For the purpose of creating in vitro viral stocks, the mutant HSV-2 gD/+gD-1 virus needs a gD1-complementing cell line. The gD-2 virus can only replicate itself in vivo for one replication cycle. It was established that this vaccine’s protection was brought about through antibody-dependent cell-mediated cytotoxicity (ADCC). Following intravaginal injection, this vaccine provided protection against genital disease and decreased skin lesions and inflammation in mice [222,223]. The vaccine offered defence against several clinical isolates, some of which originated in Africa, as well as laboratory strains of HSV-1 and HSV-2 [222]. Another live-attenuated vaccine, AD472, had been tested in guinea pigs in the year 2005 [224]. The guinea pig genital HSV model was also employed in early tests of R2 to assess its capacity to reproduce at the site of injection, induce illness, and infect brain tissues. The results indicated good efficacy of R2 vaccine when administered through an intradermal route [225]. The effectiveness of herpes vaccination is frequently investigated using female inbred mice in case of genital herpes infection [226]. These mice are widely accessible, simple to keep, and have transgenic or knockout strains to study defence systems [227]. Recent investigations in a murine model have demonstrated that both HSV-1 seronegative and seropositive animals challenged with HSV-2 did not develop latent infection after receiving the gD-2 vaccine [228].
5.3. Replication-Defective Virus Vaccines
Also known as disabled infectious single cycle virus vaccines, these contain one or more dysfunctional genes that are crucial for the replication of the viral genome. Immune responses can be induced by disabled infectious single cycle virus vaccines, but no viral offspring are created. These vaccines are less immunogenic since they have reduced capacity to elicit antigens presenting cells such as dendritic cells and macrophages, which are necessary to produce CD4+ and CD8+ T cell responses. In 1996, McLean evaluated DISC HSV-1 vaccine in guinea pigs [229]. Later, gH-deleted HSV-2 mutant was evaluated in guinea pigs for recurrent genital herpes infection by the same researcher [230].
The HSV-2 dl5-29 (HSV529) vaccine, which has been the subject of the most research, was created by Knipe in 2008 and other researchers in 2010 [215,221,231]. HSV529 has mutations in the vital viral genes UL5 and UL29 that prevent replication. Both a preventive and a therapeutic vaccine were attempted. A trial demonstrated safety of HSV529 by inducing CD4+ T-cell responses and neutralising antibodies in seronegative individuals who received the vaccine [232]. Recent research has shown that the generation of antibodies mediates the activation of NK cells. Moreover, the cervicovaginal fluid contained HSV-2 gD antibodies at a level about one-third that of serum [233]. Table 2 includes vaccines in different phases of trials.
Table 2.
Vaccination strategies for the prevention of HSV-1 and HSV-2 infection.
| Sr. No. | Type of Vaccine | Candidate Name | Modification in Vaccine | Host | Phase | Results | Origin | Refs. |
|---|---|---|---|---|---|---|---|---|
| 1 | Inactivated vaccine | HSV-2 dl5-29 | deletions of UL5 and UL29 | mice and guinea pigs | Pre-clinical | enhance immune responses, protection against reactivates HSV infection | Harvard Medical School, Boston | [234] |
| 2 | Inactivated vaccine | SC16ΔgH | deletion of HSV-1 gH deletion | mice | Clinical | has no impact on viral shedding and inability to demonstrate immunity against reactivated genital herpes infections | Boston, Massachusetts | [16] |
| 3 | Replication-Defective Viral Vaccines | CJ2-gD2 | Dominant negative HSV-2 with CJ2-gD2 | guinea pigs | Pre-Clinical | provide protection against primary infection and reactivates HSV-2 genital infections | - | [235] |
| 4 | Replication-Defective Viral Vaccines | HSV-1 vhs-/ICP8- | deletions of vhs in an ICP8 | mice | Pre-Clinical | significantly enhances protective efficacy | - | [236] |
| Δ41Δ29 | deletions in the gene for virion host shutoff (vhs) protein |
BALB/c mice | Pre-Clinical | decreased the frequency of UV-B-induced recurrent viral shedding in mice with latent infection | Washington University School of Medicine | [237] | ||
| 5 | Replication-Defective Viral Vaccines | HSV529 | Deletion of UL5, UL29 in HSV-2 dl5-29 mice | mice and HSV-1 seropositive guinea pigs | immunogenicity reported | Sanofi Pasteur | [238] | |
| HSV529 | - | human participants | Phase I | induced moderate CD4+ T-cell responses and neutralising antibodies in HSV-seronegative vaccine recipients | Sanofi Pasteur | [232,233] | ||
| 6 | Live-attenuated vaccine | HSV-2 ΔgD2 | Deletion of gD-2 | C57BL/6 or BALB/c mice |
Pre-clinical | protection by non-neutralising Fc-mediated humoral responses | Albert Einstein College of Medicine | [222] |
| C57BL/6 mice | Pre-clinical | infected mice were protected by gD-2 | Albert Einstein College of Medicine | [223] | ||||
| 7 | Live-attenuated vaccine | AD472 | Deletion of γ134.5 gene, UL55-56, UL43.5 and the US10-12 region |
guinea pigs | Pre-clinical | reduce lesion development and infection severity in guinea pigs | Albert Einstein College of Medicine | [224] |
| 8 | Live-attenuated vaccine | NE-HSV-2 | Antigens such as gB2 and gD2 were used to make nanoemulsion | C57BL/6 mice | Pre-Clinical | reduction of reactivated lesions and viral shedding by more than 50% | BlueWillow Biologics | [239] |
| 9 | Live-attenuated vaccine | R2 | Mutated pUL37 gene in the R2 region | guinea pigs | Pre-Clinical | reduction of reactivated virus shedding | Thyreos LLC | [225,240] |
| 10 | Live-attenuated vaccine | HSV-1 VC2 | Mutations in glycoprotein K (gK) and UL20 | rhesus macaques | Pre-Clinical | induction of immune response | Louisiana State University | [241] |
| - | guinea pigs | Pre-Clinical | diminishes HSV-2 replication | Louisiana State University | [242] | |||
| Mutations in glycoprotein K (gK) and UL20 | mice | Pre-Clinical | protect mice against lethal intravaginal infection | Louisiana State University | [243] | |||
| 11 | Live-attenuated vaccine | HSV-2 0ΔNLS | Deletion of ICP0− | mice | Phase II | represent avirulence and immunogenicity | Southern Illinois University | [244] |
| Pre-Clinical | protected against lethal challenge with wild-type HSV-2 | Southern Illinois University | [245] | |||||
| 12 | Subunit Vaccine | gD2 subunit vaccine | gD2 adjuvant with AS04 | HSV-1– and HSV-2–seronegative women | Phase 3 | efficacy against HSV-1 and HSV-1 genital disease was 58% and 35% respectively | GlaxoSmithKline | [246] |
| girls aged 10–17 years | Phase 3 | vaccine was tolerated and immunogenic | GlaxoSmithKline | [247] | ||||
| 13 | Subunit Vaccine | HerpV | 32 synthetic 35mer HSV-2 peptides complexed with Hsc70 protein + QS21 (saponin adjuvant) | HLA-A2 transgenic mice | Pre-clinical | immunogenic, CD4(+) and CD8(+) T cell activators | Agenus | [248] |
| HerpV+ QS-21 | human participant | Phase II (DC) | significant CD4+ T and CD8(+) T cell response | Agenus | [249] | |||
| 14 | Subunit Vaccine | HSV-2 trivalent vaccine | Combination of glycoproteins C, D, and E (gC2, gD2, gE2) | guinea pig | Pre-clinical | trivalent protein vaccine provides protection to prevent herpes infection | University of Pennsylvania | [250,251,252] |
| rhesus macaques and guinea pig | Pre-clinical | viral shedding was reduced | University of Pennsylvania | [253] | ||||
| guinea pigs | Pre-clinical | immune evasion domains on HSV-2 glycoproteins | University of Pennsylvania | [254] | ||||
| 15 | Subunit Vaccine | HSV1 gB lentiviral vector | HSV-1 glycoprotein B (gB1) with feline immunodeficiency virus (FIV) vector | C57BL/6 mice | Pre-Clinical | significant reduction in viral infectivity | University of Pisa, Italy | [255] |
| 16 | Subunit Vaccine | GEN-003 | gD2ΔTMR + M2 | seropositive participants | Completed Phase II (DC) | reduction in shedding and lesion rate was reported | University of Cincinnati, | [256] |
| gD2 (truncated)+ICP4 fragment | healthy participants | Phase 2 | reduction in shedding rate and lesion rate | Genocea | [257,258] | |||
| GEN-003/MM-2 | guinea pigs | Pre-clinical | elicits humoral immune responses, CD4+ and CD8+ T cells | Genocea | [259] | |||
| 17 | Subunit Vaccine | G103 | HSV-2 gD, deletions in UL19 and UL25 | Mice and guinea pigs | Pre-clinical | reductions of no. of lesions and lesion area | Immune Design | [226] |
| 18 | DNA Vaccines | COR-1 | gD2 ubiquitin tag with COR-1 DNA or placebo | HSV-2 seropositive subjects | Phase II | reduction in viral shedding | Admedus | [260] |
| BALB/c mice | Pre-clinical | protects against lethal viral challenge and reduces ganglionic latency | University of Washington | [261] | ||||
| 19 | DNA Vaccines | pRSC-gD-IL-2123 | HSV-1 gD combined with IL-21 | mice | Pre-clinical | inhibition of HSK in HSV-1-infected mice | Southeast University, China | [262] |
| 20 | DNA Vaccines | Vaxfectin®-gD2/UL46/UL47 | HSV-2 glycoprotein D and UL46 and UL47 genes/Vaxfectin | mice | Pre-clinical | reduction of frequency of reactivated disease and viral shedding | Vical | [263,264] |
| Vaxfectin-gD2 pDNA | pDNA + FL gD2 Vaxfectin | guinea pig | Pre-clinical | reduction in HSV-2 DNA copy number | University of Washington | [265] | ||
| 21 | DNA Vaccines | gB1s-NISV | NISV+HSV-1 gB+CpG | mice | Pre-Clinical | provides protection against a heterologous lethal vaginal challenge with HSV-2 | - | [266] |
5.4. Subunit HSV Vaccines
Despite being less dangerous than live-attenuated vaccines, the development of subunit vaccines faces difficulties in producing an immune response that is both efficient and long-lasting. Because of this, adjuvants are frequently used while administering subunit vaccines [267]. Proteins, DNA, and peptide epitope-based vaccines are a few of the several subunit HSV vaccination strategies that have been explored [259,263]. The first therapeutic vaccination experiment was conducted in 1994. In this study, 98 symptomatic genital herpes patients were selected who experienced four to fourteen recurrences annually; they received the gD vaccine with aluminium salt (often known as “Alum”) adjuvant (96). Regrettably, while the vaccine was able to increase neutralising antibodies to HSV-2 fourfold over baseline levels, this vaccine only managed to lower the prevalence of HSV-infection by 24% [268]. The GlaxoSmithKline glycoprotein D2 vaccine is a subunit vaccine made of the HSV-2 glycoprotein D (gD2-AS04) with adjuvants such as aluminium hydroxide and 3-O-deacylated monophosphoryl lipid A (MPL) [237,246,247,252].
A subunit vaccine, HerpV, consisting of 32 HSV-2 peptides complexed with Hsc70 human recombinant protein has shown significant CD4+ and CD8+ T cell responses in HSV-2-positive healthy volunteers. Previous in vivo models demonstrated protection from viral assaults. Mononuclear cell reactivity and CD8+ T-cell proliferation were seen in recent clinical trial results from HSV-2+ patients who received the vaccine [248,249]. Another subunit vaccine, GEN-003, consist of ICP4 and gD2 truncated proteins that are further combined with a novel adjuvant, Matrix M2 (MM-2) [259]. This saponin adjuvant shows immunostimulatory effect for both B and T cells. This trial significantly reduces the viral shedding and recurrent lesions of herpes. This defence seems to be linked to antiviral CD4+ and CD8+ T cell responses produced from blood [256,257,258]. A subunit trivalent vaccine, namely, gC2/gD2/gE2 with a CpG/aluminium adjuvant contains three main glycoproteins, namely, C, D, and E. These glycoproteins are immune-resistant molecules. According to research done on rhesus macaques, the trivalent vaccine can produce neutralising antibodies which prevent the action of gC2 and gE2 immune evasion but induced CD4+ T-cell responses. The guinea pig model was used to assess the vaccine’s effectiveness, and it was discovered to be extremely effective in lowering the severity and frequency of genital lesions [252,253]. Recombinant HSV-2 proteins gD, UL19, and UL25 gene products, namely, G103, together with a TLR4 agonist adjuvant demonstrated full protection against deadly HSV-2 infection in HSV-2-infected mice. An increase in CD4 and CD8 T cells is also reported in the study. A 50% reduction has been observed in the number of lesions in a guinea pig model [226].
5.5. Nucleic Acid Vaccines
Two primary types of nucleic acid vaccines are DNA plasmid vaccines and mRNA vaccines. These vaccines are designed to deliver genetic information to cells so that they may express and synthesize proteins. These proteins act as antigens for the body’s immune system. Recently, mRNA technology has advanced substantially, offering important benefits. It exhibits rapid protein synthesis for a controlled period, does not integrate into the host genome, and can be expressed in both proliferating and non-proliferating cells [269]. A DNA vaccine named COR-1 showed humoral and cellular responses along with decreased viral latency and protection from fatal virus challenge. Two main codons, such as HSV-2 envelope gD2 and the shorter version of gD2, are linked with COR-1 [261]. The clinical study states a reduction in viral shedding; thus, the vaccine was considered to be safe for HSV-infected people [260]. The immune responses for the management and prevention of HSV infection may be different from each other. For controlling recurrent herpes, CD8+ T lymphocytes are very essential. Consequently, recurrent genital herpes therapy antigens may be different from those used for prevention [270,271,272].
6. Conclusions
The wide variety of antiherpetic medicines are known only for shortening the length and severity of symptoms as well as virus shedding, but that too in the later stage. For best results in limiting viral replication, therapy must be initiated during the febrile phase as late treatment is ineffective. It also has been reported that inhibition therapies are only 50% effective in recurrent infections [9]. Increasing resistance and side effects of FDA-approved drugs such as acyclovir and foscarnet also limit the use of synthetic drugs [200]. The cell culture model and animal studies state that various plant, fungus, and algae-derived substances have been shown to have potent antiviral effects against HSV-1 and HSV-2. It has been reported that various plant extracts and bioactive compounds exert antiviral properties [273]. Some important modes of action of these natural compounds include prevention of virus entrance, replication, and viral protein expression [274]. However, certain side effects have also been reported of these natural medicines in comparison to others [275,276,277,278].
The complexity of HSV pathophysiology and immune invasion is continually being studied. Considering the widespread distribution of HSV, several attempts have been made to develop an effective vaccine. Existing vaccine candidates for HSV are not very successful in clinical trials. Thus, combinatorial vaccines appear to be the most suited vaccines. The major aim of developing a vaccine is to prevent viral transmission and lessen severity of disease. However, understanding these mechanisms in more detail is necessary in order to create a therapeutic or preventive vaccine.
It is anticipated that this review will have helped to highlight significant gaps in the available therapeutic options, which are essential to understand for the development of a successful and novel HSV vaccine.
Acknowledgments
We highly acknowledge the Department of Science and Technology for providing financial support to Divya Sharma under the scheme of DST-Inspire fellowship (IF200174), Government of India.
Author Contributions
Conceptualization: M.D. and D.P., Original draft preparation, writing and editing: D.S., S.S., N.A., A.D. and N.Y. Draft revision and editing: M.D. and S.K.P. Final approval: S.K.P., D.P. and M.D. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This study received no external funding.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Mustafa M., Illzam E., Muniandy R., Sharifah A., Nang M., Ramesh B. Herpes simplex virus infections, Pathophysiology and Management. IOSR J. Dent. Med. Sci. 2016;15:85–91. doi: 10.9790/0853-150738591. [DOI] [Google Scholar]
- 2.Madavaraju K., Koganti R., Volety I., Yadavalli T., Shukla D. Herpes Simplex Virus Cell Entry Mechanisms: An Update. Front. Cell. Infect. Microbiol. 2021;10:617578. doi: 10.3389/fcimb.2020.617578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farooq A.V., Shukla D. Herpes Simplex Epithelial and Stromal Keratitis: An Epidemiologic Update. Surv. Ophthalmol. 2012;57:448–462. doi: 10.1016/j.survophthal.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kaye S., Choudhary A. Herpes simplex keratitis. Prog. Retin. Eye Res. 2006;25:355–380. doi: 10.1016/j.preteyeres.2006.05.001. [DOI] [PubMed] [Google Scholar]
- 5.Whitley R., Davis E.A., Suppapanya N. Incidence of Neonatal Herpes Simplex Virus Infections in a Managed-Care Population. Sex. Transm. Dis. 2007;34:704–708. doi: 10.1097/01.olq.0000258432.33412.e2. [DOI] [PubMed] [Google Scholar]
- 6.Mostafa H.H., Thompson T.W., Konen A.J., Haenchen S.D., Hilliard J.G., Macdonald S.J., Morrison L.A., Davido D.J. Herpes Simplex Virus 1 Mutant with Point Mutations in UL39 Is Impaired for Acute Viral Replication in Mice, Establishment of Latency, and Explant-Induced Reactivation. J. Virol. 2018;92:e01654-17. doi: 10.1128/JVI.01654-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gonçalves B.C., Barbosa M.G.L., Olak A.P.S., Terezo N.B., Nishi L., Watanabe M.A., Marinello P., Rechenchoski D.Z., Rocha S.P.D., Faccin-Galhardi L.C. Antiviral therapies: Advances and perspectives. Fundam. Clin. Pharmacol. 2020;35:305–320. doi: 10.1111/fcp.12609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mlynarczyk-Bonikowska B., Majewska A., Malejczyk M., Mlynarczyk G., Majewski S. Antiviral medication in sexually transmitted diseases. Part I: HSV, HPV. Mini-Rev. Med. Chem. 2013;13:1837–1845. doi: 10.2174/13895575113136660088. [DOI] [PubMed] [Google Scholar]
- 9.Birkmann A., Zimmermann H. HSV antivirals—current and future treatment options. Curr. Opin. Virol. 2016;18:9–13. doi: 10.1016/j.coviro.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 10.Evans T.G., Bernstein D.I., Raborn G.W., Harmenberg J., Kowalski J., Spruance S.L. Double-Blind, Randomized, Placebo-Controlled Study of Topical 5% Acyclovir-1% Hydrocortisone Cream (ME-609) for Treatment of UV Radiation-Induced Herpes Labialis. Antimicrob. Agents Chemother. 2002;46:1870–1874. doi: 10.1128/AAC.46.6.1870-1874.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LeFlore S., Anderson P.L., Fletcher C.V., Fletcher C.V., Fletcher C.V. A Risk-Benefit Evaluation of Aciclovir for the Treatment and Prophylaxis of Herpes Simplex Virus Infections. Drug Saf. 2000;23:131–142. doi: 10.2165/00002018-200023020-00004. [DOI] [PubMed] [Google Scholar]
- 12.Arduino P.G., Porter S. Herpes Simplex Virus Type 1 infection: Overview on relevant clinico-pathological features. J. Oral Pathol. Med. 2007;37:107–121. doi: 10.1111/j.1600-0714.2007.00586.x. [DOI] [PubMed] [Google Scholar]
- 13.Javaly K., Wohlfeiler M., Kalayjian R., Klein T., Bryson Y., Grafford K., Martin-Munley S., Hardy W.D. Treatment of Mucocutaneous Herpes Simplex Virus Infections Unresponsive to Acyclovir with Topical Foscarnet Cream in AIDS Patients: A Phase I/II Study. Am. J. Ther. 1999;21:301–306. doi: 10.1097/00126334-199908010-00007. [DOI] [PubMed] [Google Scholar]
- 14.Chen F., Xu H., Liu J., Cui Y., Luo X., Zhou Y., Chen Q., Jiang L. Efficacy and safety of nucleoside antiviral drugs for treatment of recurrent herpes labialis: A systematic review and meta-analysis. J. Oral Pathol. Med. 2017;46:561–568. doi: 10.1111/jop.12534. [DOI] [PubMed] [Google Scholar]
- 15.Johnston C., Gottlieb S.L., Wald A. Status of vaccine research and development of vaccines for herpes simplex virus. Vaccine. 2016;34:2948–2952. doi: 10.1016/j.vaccine.2015.12.076. [DOI] [PubMed] [Google Scholar]
- 16.Akhrameyeva N.V., Zhang P., Sugiyama N., Behar S.M., Yao F. Development of a Glycoprotein D-Expressing Dominant-Negative and Replication-Defective Herpes Simplex Virus 2 (HSV-2) Recombinant Viral Vaccine against HSV-2 Infection in Mice. J. Virol. 2011;85:5036–5047. doi: 10.1128/JVI.02548-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khan A.A., Srivastava R., Lopes P.P., Wang C., Pham T.T., Cochrane J., Thai N.T.U., Gutierrez L., BenMohamed L. Asymptomatic memory CD8+T cells. Hum. Vaccines Immunother. 2014;10:945–963. doi: 10.4161/hv.27762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cliffe A., Chang L., Colgrove R., Knipe D.M. Reference Module in Biomedical Sciences. Elsevier; Amsterdam, The Netherlands: 2014. Herpes Simplex Virus. [Google Scholar]
- 19.Taylor T.J., Brockman M.A., McNamee E.E., Knipe D.M. Herpes simplex virus. Front. Biosci.-Landmark. 2002;7:752–764. doi: 10.2741/taylor. [DOI] [PubMed] [Google Scholar]
- 20.Krishnan R., Stuart P.M. Developments in Vaccination for Herpes Simplex Virus. Front. Microbiol. 2021;12:798927. doi: 10.3389/fmicb.2021.798927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Whitley R.J., Roizman B. Herpes simplex virus infections. Lancet. 2001;357:1513–1518. doi: 10.1016/S0140-6736(00)04638-9. [DOI] [PubMed] [Google Scholar]
- 22.Kortekangas-Savolainen O., Orhanen E., Puodinketo T., Vuorinen T. Epidemiology of Genital Herpes Simplex Virus Type 1 and 2 Infections in Southwestern Finland During a 10-Year Period (2003–2012) Sex. Transm. Dis. 2014;41:268–271. doi: 10.1097/OLQ.0000000000000101. [DOI] [PubMed] [Google Scholar]
- 23.Akkus S.E.a.A. Fundamentals of Sexually Transmitted Infections. IntechOpen; London, UK: 2017. Genital Herpes. [Google Scholar]
- 24.Benedetti J., Corey L., Ashley R. Recurrence Rates in Genital Herpes after Symptomatic First-Episode Infection. Ann. Intern. Med. 1994;121:847–854. doi: 10.7326/0003-4819-121-11-199412010-00004. [DOI] [PubMed] [Google Scholar]
- 25.Roizman B., Zhou G. The 3 facets of regulation of herpes simplex virus gene expression: A critical inquiry. Virology. 2015;479–480:562–567. doi: 10.1016/j.virol.2015.02.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Banerjee A., Kulkarni S., Mukherjee A. Herpes Simplex Virus: The Hostile Guest That Takes Over Your Home. Front. Microbiol. 2020;11:733. doi: 10.3389/fmicb.2020.00733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Luo S., Hu K., He S., Wang P., Zhang M., Huang X., Du T., Zheng C., Liu Y., Hu Q. Contribution of N-linked glycans on HSV-2 gB to cell–cell fusion and viral entry. Virology. 2015;483:72–82. doi: 10.1016/j.virol.2015.04.005. [DOI] [PubMed] [Google Scholar]
- 28.Akhtar J., Shukla D. Viral entry mechanisms: Cellular and viral mediators of herpes simplex virus entry. FEBS J. 2009;276:7228–7236. doi: 10.1111/j.1742-4658.2009.07402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang J., Yuan S., Zhu D., Tang H., Wang N., Chen W., Gao Q., Li Y., Wang J., Liu H., et al. Structure of the herpes simplex virus type 2 C-capsid with capsid-vertex-specific component. Nat. Commun. 2018;9:1–10. doi: 10.1038/s41467-018-06078-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mahiet C., Ergani A., Huot N., Alende N., Azough A., Salvaire F., Bensimon A., Conseiller E., Wain-Hobson S., Labetoulle M., et al. Structural Variability of the Herpes Simplex Virus 1 Genome In Vitro and In Vivo. J. Virol. 2012;86:8592–8601. doi: 10.1128/JVI.00223-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Slobedman B., Zhang X., Simmons A. Herpes Simplex Virus Genome Isomerization: Origins of Adjacent Long Segments in Concatemeric Viral DNA. J. Virol. 1999;73:810–813. doi: 10.1128/JVI.73.1.810-813.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dolan A., Jamieson F.E., Cunningham C., Barnett B.C., McGeoch D.J. The Genome Sequence of Herpes Simplex Virus Type 2. J. Virol. 1998;72:2010–2021. doi: 10.1128/JVI.72.3.2010-2021.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Baines J.D., Pellett P.E. Human Herpesviruses: Biology, Therapy, Immunoprophylaxis. Cambridge University Press; Cambridge, UK: 2007. Genetic comparison of human alphaherpesvirus genomes. [PubMed] [Google Scholar]
- 34.Bowman B.R., Baker M.L., Rixon F.J., Chiu W., Quiocho F.A. Structure of the herpesvirus major capsid protein. EMBO J. 2003;22:757–765. doi: 10.1093/emboj/cdg086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lee J.-H., Shim J., Kim S.J. Stunning symmetries involved in the self-assembly of the HSV-1 capsid. J. Korean Phys. Soc. 2021;78:357–364. doi: 10.1007/s40042-020-00044-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kelly B.J., Fraefel C., Cunningham A.L., Diefenbach R.J. Functional roles of the tegument proteins of herpes simplex virus type 1. Virus Res. 2009;145:173–186. doi: 10.1016/j.virusres.2009.07.007. [DOI] [PubMed] [Google Scholar]
- 37.Diefenbach R., Miranda-Saksena M., Douglas M.W., Cunningham A.L. Transport and egress of herpes simplex virus in neurons. Rev. Med. Virol. 2007;18:35–51. doi: 10.1002/rmv.560. [DOI] [PubMed] [Google Scholar]
- 38.Muller W.J., Dong L., Vilalta A., Byrd B., Wilhelm K.M., McClurkan C.L., Margalith M., Liu C., Kaslow D., Sidney J., et al. Herpes simplex virus type 2 tegument proteins contain subdominant T-cell epitopes detectable in BALB/c mice after DNA immunization and infection. J. Gen. Virol. 2009;90:1153–1163. doi: 10.1099/vir.0.008771-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jaggi U., Wang S., Tormanen K., Matundan H., Ljubimov A.V., Ghiasi H. Role of Herpes Simplex Virus Type 1 (HSV-1) Glycoprotein K (gK) Pathogenic CD8+ T Cells in Exacerbation of Eye Disease. Front. Immunol. 2018;9:2895. doi: 10.3389/fimmu.2018.02895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Saleh D., Yarrarapu S.N.S., Sharma S. Herpes Simplex Type 1. StatPearls Publishing; Treasure Island, FL, USA: 2022. [PubMed] [Google Scholar]
- 41.Mathew J.J., Sapra A. Herpes Simplex Type 2. StatPearls Publishing; Treasure Island, FL, USA: 2022. [PubMed] [Google Scholar]
- 42.Cook M.L., Stevens J.G. Pathogenesis of Herpetic Neuritis and Ganglionitis in Mice: Evidence for Intra-Axonal Transport of Infection. Infect. Immun. 1973;7:272–288. doi: 10.1128/iai.7.2.272-288.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rødahl E., Stevens J.G. Differential accumulation of herpes simplex virus type 1 latency-associated transcripts in sensory and autonomic ganglia. Virology. 1992;189:385–388. doi: 10.1016/0042-6822(92)90721-Z. [DOI] [PubMed] [Google Scholar]
- 44.Speck P.G., Simmons A. Divergent molecular pathways of productive and latent infection with a virulent strain of herpes simplex virus type 1. J. Virol. 1991;65:4001–4005. doi: 10.1128/jvi.65.8.4001-4005.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Speck P.G., Simmons A. Synchronous appearance of antigen-positive and latently infected neurons in spinal ganglia of mice infected with a virulent strain of herpes simplex virus. J. Gen. Virol. 1992;73:1281–1285. doi: 10.1099/0022-1317-73-5-1281. [DOI] [PubMed] [Google Scholar]
- 46.Simmons A., Tscharke D. Anti-CD8 impairs clearance of herpes simplex virus from the nervous system: Implications for the fate of virally infected neurons. J. Exp. Med. 1992;175:1337–1344. doi: 10.1084/jem.175.5.1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Russell T.A., Tscharke D.C. Lytic Promoters Express Protein during Herpes Simplex Virus Latency. PLoS Pathog. 2016;12:e1005729. doi: 10.1371/journal.ppat.1005729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sawtell N.M., Thompson R.L. Herpes simplex virus and the lexicon of latency and reactivation: A call for defining terms and building an integrated collective framework. F1000Research. 2016;5:2038. doi: 10.12688/f1000research.8886.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Rohde W., Mikelens P., Jackson J., Blackman J., Whitcher J., Levinson W. Hydroxyquinolines Inhibit Ribonucleic Acid-Dependent Deoxyribonucleic Acid Polymerase and Inactivate Rous Sarcoma Virus and Herpes Simplex Virus. Antimicrob. Agents Chemother. 1976;10:234–240. doi: 10.1128/AAC.10.2.234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomsen D.R., Oien N.L., Hopkins T.A., Knechtel M.L., Brideau R.J., Wathen M.W., Homa F.L. Amino Acid Changes within Conserved Region III of the Herpes Simplex Virus and Human Cytomegalovirus DNA Polymerases Confer Resistance to 4-Oxo-Dihydroquinolines, a Novel Class of Herpesvirus Antiviral Agents. J. Virol. 2003;77:1868–1876. doi: 10.1128/JVI.77.3.1868-1876.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schnute M.E., Cudahy M.M., Brideau R.J., Homa F.L., Hopkins T.A., Knechtel M.L., Oien N.L., Pitts T.W., Poorman R.A., Wathen M.W., et al. 4-Oxo-4,7-dihydrothieno[2,3-b]pyridines as Non-Nucleoside Inhibitors of Human Cytomegalovirus and Related Herpesvirus Polymerases. J. Med. Chem. 2005;48:5794–5804. doi: 10.1021/jm050162b. [DOI] [PubMed] [Google Scholar]
- 52.Brideau R.J., Knechtel M.L., Huang A., Vaillancourt V.A., Vera E.E., Oien N.L., Hopkins T.A., Wieber J.L., Wilkinson K.F., Rush B.D., et al. Broad-spectrum antiviral activity of PNU-183792, a 4-oxo-dihydroquinoline, against human and animal herpesviruses. Antivir. Res. 2001;54:19–28. doi: 10.1016/S0166-3542(01)00208-X. [DOI] [PubMed] [Google Scholar]
- 53.Oien N.L., Brideau R.J., Hopkins T.A., Wieber J.L., Knechtel M.L., Shelly J.A., Anstadt R.A., Wells P.A., Poorman R.A., Huang A., et al. Broad-Spectrum Antiherpes Activities of 4-Hydroxyquinoline Carboxamides, a Novel Class of Herpesvirus Polymerase Inhibitors. Antimicrob. Agents Chemother. 2002;46:724–730. doi: 10.1128/AAC.46.3.724-730.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Spear P.G., Eisenbergbc R.J., H.Cohenbd G. Three Classes of Cell Surface Receptors for Alphaherpesvirus Entry. Virology. 2000;275:1–8. doi: 10.1006/viro.2000.0529. [DOI] [PubMed] [Google Scholar]
- 55.Gopinath S.C.B., Hayashi K., Kumar P.K.R. Aptamer That Binds to the gD Protein of Herpes Simplex Virus 1 and Efficiently Inhibits Viral Entry. J. Virol. 2012;86:6732–6744. doi: 10.1128/JVI.00377-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yadavalli T., Agelidis A., Jaishankar D., Mangano K., Thakkar N., Penmetcha K., Shukla D. Targeting Herpes Simplex Virus-1 gD by a DNA Aptamer Can Be an Effective New Strategy to Curb Viral Infection. Mol. Ther.-Nucleic Acids. 2017;9:365–378. doi: 10.1016/j.omtn.2017.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Betz U.A.K., Fischer R., Kleymann G., Hendrix M., Rübsamen-Waigmann H. Potent In Vivo Antiviral Activity of the Herpes Simplex Virus Primase-Helicase Inhibitor BAY 57-1293. Antimicrob. Agents Chemother. 2002;46:1766–1772. doi: 10.1128/AAC.46.6.1766-1772.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Dutia B.M., Frame M.C., Subak-Sharpe J.H., Clark W.N., Marsden H.S. Specific inhibition of herpesvirus ribonucleotide reductase by synthetic peptides. Nature. 1986;321:439–441. doi: 10.1038/321439a0. [DOI] [PubMed] [Google Scholar]
- 59.McClements W., Yamanaka G., Garsky V., Perry H., Bacchetti S., Colonno R., Stein R. Oligopeptides inhibit the ribonucleotide reductase of herpes simplex virus by causing subunit separation. Virology. 1988;162:270–273. doi: 10.1016/0042-6822(88)90421-7. [DOI] [PubMed] [Google Scholar]
- 60.Roehm P.C., Shekarabi M., Wollebo H.S., Bellizzi A., He L., Salkind J., Khalili K. Inhibition of HSV-1 Replication by Gene Editing Strategy. Sci. Rep. 2016;6:23146. doi: 10.1038/srep23146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hamilton S.T., Milbradt J., Marschall M., Rawlinson W.D. Human Cytomegalovirus Replication Is Strictly Inhibited by siRNAs Targeting UL54, UL97 or UL122/123 Gene Transcripts. PLoS ONE. 2014;9:e97231. doi: 10.1371/journal.pone.0097231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Greco A., Bausch N., Couté Y., Diaz J.J. Characterization by two-dimensional gel electrophoresis of host proteins whose synthesis is sustained or stimulated during the course of herpes simplex virus type 1 infection. Electrophoresis. 2000;21:2522–2530. doi: 10.1002/1522-2683(20000701)21:12<2522::AID-ELPS2522>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 63.Francke B. Cell-free synthesis of herpes simplex virus DNA: The influence of polyamines. Biochemistry. 1978;17:5494–5499. doi: 10.1021/bi00618a026. [DOI] [PubMed] [Google Scholar]
- 64.Greco A., Callé A., Morfin F., Thouvenot D., Cayre M., Kindbeiter K., Martin L., Levillain O., Diaz J. S-adenosyl methionine decarboxylase activity is required for the outcome of herpes simplex virus type 1 infection and represents a new potential therapeutic target. FASEB J. 2005;19:1128–1130. doi: 10.1096/fj.04-2108fje. [DOI] [PubMed] [Google Scholar]
- 65.Schang L.M., Bantly A., Schaffer P.A. Explant-Induced Reactivation of Herpes Simplex Virus Occurs in Neurons Expressing Nuclear cdk2 and cdk4. J. Virol. 2002;76:7724–7735. doi: 10.1128/JVI.76.15.7724-7735.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mostafa H.H., Thompson T.W., Davido D.J. N-Terminal Phosphorylation Sites of Herpes Simplex Virus 1 ICP0 Differentially Regulate Its Activities and Enhance Viral Replication. J. Virol. 2013;87:2109–2119. doi: 10.1128/JVI.02588-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jahanban-Esfahlan R., Seidi K., Majidinia M., Karimian A., Yousefi B., Nabavi S.M., Astani A., Berindan-Neagoe I., Gulei D., Fallarino F., et al. Toll-like receptors as novel therapeutic targets for herpes simplex virus infection. Rev. Med. Virol. 2019;29:e2048. doi: 10.1002/rmv.2048. [DOI] [PubMed] [Google Scholar]
- 68.Su C., Zhan G., Zheng C. Evasion of host antiviral innate immunity by HSV-1, an update. Virol. J. 2016;13:38. doi: 10.1186/s12985-016-0495-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Peri P., Mattila R.K., Kantola H., Broberg E., Karttunen H.S., Waris M., Vuorinen T., Hukkanen V. Herpes Simplex Virus Type 1 Us3 Gene Deletion Influences Toll-like Receptor Responses in Cultured Monocytic Cells. Virol. J. 2008;5:140. doi: 10.1186/1743-422X-5-140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.van Lint A.L., Murawski M.R., Goodbody R.E., Severa M., Fitzgerald K.A., Finberg R.W., Knipe D.M., Kurt-Jones E.A. Herpes Simplex Virus Immediate-Early ICP0 Protein Inhibits Toll-Like Receptor 2-Dependent Inflammatory Responses and NF-κB Signaling. J. Virol. 2010;84:10802–10811. doi: 10.1128/JVI.00063-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Gu H. What role does cytoplasmic ICP0 play in HSV-1 infection? Futur. Virol. 2018;13:375–378. doi: 10.2217/fvl-2018-0038. [DOI] [Google Scholar]
- 72.Zhang J., Wang S., Wang K., Zheng C. Herpes simplex virus 1 DNA polymerase processivity factor UL42 inhibits TNF-α-induced NF-κB activation by interacting with p65/RelA and p50/NF-κB1. Med. Microbiol. Immunol. 2013;202:313–325. doi: 10.1007/s00430-013-0295-0. [DOI] [PubMed] [Google Scholar]
- 73.Jin H., Ma Y., Yan Z., Prabhakar B.S., He B. Activation of NF-κB in CD8 + Dendritic Cells Ex Vivo by the γ 1 34.5 Null Mutant Correlates with Immunity against Herpes Simplex Virus 1. J. Virol. 2012;86:1059–1068. doi: 10.1128/JVI.06202-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Cotter C.R., Kim W.-K., Nguyen M.L., Yount J.S., López C.B., Blaho J.A., Moran T.M. The Virion Host Shutoff Protein of Herpes Simplex Virus 1 Blocks the Replication-Independent Activation of NF-κB in Dendritic Cells in the Absence of Type I Interferon Signaling. J. Virol. 2011;85:12662–12672. doi: 10.1128/JVI.05557-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Horng T., Barton G.M., Flavell R.A., Medzhitov R. The adaptor molecule TIRAP provides signalling specificity for Toll-like receptors. Nature. 2002;420:329–333. doi: 10.1038/nature01180. [DOI] [PubMed] [Google Scholar]
- 76.Finberg R.W., Knipe D.M., Kurt-Jones E.A. Herpes Simplex Virus and Toll-Like Receptors. Viral Immunol. 2005;18:457–465. doi: 10.1089/vim.2005.18.457. [DOI] [PubMed] [Google Scholar]
- 77.Schenkel J.M., Masopust D. Tissue-Resident Memory T Cells. Immunity. 2014;41:886–897. doi: 10.1016/j.immuni.2014.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Schenkel J.M., Fraser K.A., Beura L.K., Pauken K.E., Vezys V., Masopust D. Resident memory CD8 T cells trigger protective innate and adaptive immune responses. Science. 2014;346:98–101. doi: 10.1126/science.1254536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zheng S.G. Regulatory T cells versus Th17: Differentiation of Th17 versus treg, are they mutually exclusive? Am. J. Clin. Exp. Immunol. 2012;2:91–107. doi: 10.1007/978-3-0348-0522-3_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Manickan E., Rouse R.J., Yu Z., Wire W.S., Rouse B.T. Genetic immunization against herpes simplex virus. Protection is mediated by CD4+ T lymphocytes. J. Immunol. 1995;155:259–265. doi: 10.4049/jimmunol.155.1.259. [DOI] [PubMed] [Google Scholar]
- 81.Suvas S., Kumaraguru U., Pack C.D., Lee S., Rouse B.T. CD4+CD25+ T Cells Regulate Virus-specific Primary and Memory CD8+ T Cell Responses. J. Exp. Med. 2003;198:889–901. doi: 10.1084/jem.20030171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang J., Liu H., Bin Wei B. Immune response of T cells during herpes simplex virus type 1 (HSV-1) infection. J. Zhejiang Univ. B. 2017;18:277–288. doi: 10.1631/jzus.B1600460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Rubio R.M., Mohr I. Inhibition of ULK1 and Beclin1 by an α-herpesvirus Akt-like Ser/Thr kinase limits autophagy to stimulate virus replication. Proc. Natl. Acad. Sci. USA. 2019;116:26941–26950. doi: 10.1073/pnas.1915139116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Waisner H., Kalamvoki M. The ICP0 Protein of Herpes Simplex Virus 1 (HSV-1) Downregulates Major Autophagy Adaptor Proteins Sequestosome 1 and Optineurin during the Early Stages of HSV-1 Infection. J. Virol. 2019;93:e01258-19. doi: 10.1128/JVI.01258-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rodríguez M.C., Dybas J.M., Hughes J., Weitzman M.D., Boutell C. The HSV-1 ubiquitin ligase ICP0: Modifying the cellular proteome to promote infection. Virus Res. 2020;285:198015. doi: 10.1016/j.virusres.2020.198015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Xu X., He Y., Fan S., Feng M., Jiang G., Wang L., Zhang Y., Liao Y., Li Q. Reducing Viral Inhibition of Host Cellular Apoptosis Strengthens the Immunogenicity and Protective Efficacy of an Attenuated HSV-1 Strain. Virol. Sin. 2019;34:673–687. doi: 10.1007/s12250-019-00156-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.DuRaine G., Wisner T.W., Howard P., Johnson D.C. Kinesin-1 Proteins KIF5A, -5B, and -5C Promote Anterograde Transport of Herpes Simplex Virus Enveloped Virions in Axons. J. Virol. 2018;92:1–44. doi: 10.1128/JVI.01269-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Pan S., Liu X., Ma Y., Cao Y., He B. Herpes Simplex Virus 1 γ 1 34.5 Protein Inhibits STING Activation That Restricts Viral Replication. J. Virol. 2018;92:e01015-18. doi: 10.1128/JVI.01015-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Jiang Z., Su C., Zheng C. Herpes Simplex Virus 1 Tegument Protein UL41 Counteracts IFIT3 Antiviral Innate Immunity. J. Virol. 2016;90:11056–11061. doi: 10.1128/JVI.01672-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rao P., Wen X., Lo J.H., Kim S., Li X., Chen S., Feng X., Akbari O., Yuan W. Herpes Simplex Virus 1 Specifically Targets Human CD1d Antigen Presentation to Enhance Its Pathogenicity. J. Virol. 2018;92:e01490-18. doi: 10.1128/JVI.01490-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Zheng C., Su C. Herpes simplex virus 1 infection dampens the immediate early antiviral innate immunity signaling from peroxisomes by tegument protein VP16. Virol. J. 2017;14:35. doi: 10.1186/s12985-017-0709-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Wilson S.S., Fakioglu E., Herold B.C. Novel approaches in fighting herpes simplex virus infections. Expert Rev. Anti-Infect. Ther. 2009;7:559–568. doi: 10.1586/eri.09.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Kukhanova M.K., Korovina A.N., Kochetkov S.N. Human herpes simplex virus: Life cycle and development of inhibitors. Biochem. Pharmacol. 2014;79:1635–1652. doi: 10.1134/S0006297914130124. [DOI] [PubMed] [Google Scholar]
- 94.Hammer K.D., Dietz J., Lo T.S., Johnson E.M. A Systematic Review on the Efficacy of Topical Acyclovir, Penciclovir, and Docosanol for the Treatment of Herpes Simplex Labialis. Dermatology. 2018;6:118–123. doi: 10.33590/emjdermatol/10311121. [DOI] [Google Scholar]
- 95.De Clercq E. Selective Anti-Herpesvirus Agents. Antivir. Chem. Chemother. 2013;23:93–101. doi: 10.3851/IMP2533. [DOI] [PubMed] [Google Scholar]
- 96.Kimberlin D.W., Whitley R.J. In: Antiviral Therapy of HSV-1 and -2, in Human Herpesviruses: Biology, Therapy, and Immunoprophylaxis. Arvin A., Campadelli-Fiume G., Mocarski E., editors. Cambridge University Press; Cambridge, UK: 2007. [PubMed] [Google Scholar]
- 97.Emmert D.H. Treatment of common cutaneous herpes simplex virus infection. Am. Fam. Physician. 2000;61:1697–1704. [PubMed] [Google Scholar]
- 98.Soul-Lawton J., Seaber E., On N., Wootton R., Rolan P., Posner J. Absolute bioavailability and metabolic disposition of valaciclovir, the L-valyl ester of acyclovir, following oral administration to humans. Antimicrob. Agents Chemother. 1995;39:2759–2764. doi: 10.1128/AAC.39.12.2759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Luber A.D., Flaherty J.F. Famciclovir for Treatment of Herpesvirus Infections. Ann. Pharmacother. 1996;30:978–985. doi: 10.1177/106002809603000913. [DOI] [PubMed] [Google Scholar]
- 100.Chi C.-C., Wang S.-H., Delamere F.M., Wojnarowska F., Peters M.C., Kanjirath P.P. Interventions for prevention of herpes simplex labialis (cold sores on the lips) Cochrane Database Syst. Rev. 2015;2016:CD010095. doi: 10.1002/14651858.CD010095.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Ormrod D., Scott L.J., Perry C.M. Valaciclovir: A review of its long term utility in the management of genital herpes simplex virus and cytomegalovirus infections. Drugs. 2000;59:839–863. doi: 10.2165/00003495-200059040-00013. [DOI] [PubMed] [Google Scholar]
- 102.Simpson D., Lyseng-Williamson K.A. Famciclovir: A review of its use in herpes zoster and genital and orolabial herpes. Drugs. 2006;66:2397–2416. doi: 10.2165/00003495-200666180-00016. [DOI] [PubMed] [Google Scholar]
- 103.Wilhelmus K.R. Antiviral treatment and other therapeutic interventions for herpes simplex virus epithelial keratitis. Cochrane Database Syst. Rev. 2015;1:CD002898. doi: 10.1002/14651858.CD002898.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Roozbahani M., Hammersmith K.M. Management of herpes simplex virus epithelial keratitis. Curr. Opin. Ophthalmol. 2018;29:360–364. doi: 10.1097/ICU.0000000000000483. [DOI] [PubMed] [Google Scholar]
- 105.Chou T.Y., Hong B.Y. Ganciclovir ophthalmic gel 0.15% for the treatment of acute herpetic keratitis: Background, effectiveness, tolerability, safety, and future applications. Ther. Clin. Risk Manag. 2014;10:665–681. doi: 10.2147/TCRM.S58242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Poole C.L., James S.H. Antiviral Therapies for Herpesviruses: Current Agents and New Directions. Clin. Ther. 2018;40:1282–1298. doi: 10.1016/j.clinthera.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Crumpacker C.S. Mechanism of action of foscarnet against viral polymerases. Am. J. Med. 1992;92:S3–S7. doi: 10.1016/0002-9343(92)90329-A. [DOI] [PubMed] [Google Scholar]
- 108.Chilukuri S., Rosen T. Management of acyclovir-resistant herpes simplex virus. Dermatol. Clin. 2003;21:311–320. doi: 10.1016/S0733-8635(02)00093-1. [DOI] [PubMed] [Google Scholar]
- 109.Wyles D.L., Patel A., Madinger N., Bessesen M., Krause P.R., Weinberg A. Development of Herpes Simplex Virus Disease in Patients Who Are Receiving Cidofovir. Clin. Infect. Dis. 2005;41:676–680. doi: 10.1086/432477. [DOI] [PubMed] [Google Scholar]
- 110.Dvorak C.C., Weintrub P.S., Cowan M.J., Horn B. Development of Herpes Simplex Virus Stomatitis during Receipt of Cidofovir Therapy. Clin. Infect. Dis. 2009;49:e92–e95. doi: 10.1086/605678. [DOI] [PubMed] [Google Scholar]
- 111.Reusser P. Herpesvirus resistance to antiviral drugs: A review of the mechanisms, clinical importance and therapeutic options. J. Hosp. Infect. 1996;33:235–248. doi: 10.1016/S0195-6701(96)90010-9. [DOI] [PubMed] [Google Scholar]
- 112.Blot N., Schneider P., Young P., Janvresse C., Dehesdin D., Tron P., Vannier J. Treatment of an acyclovir and foscarnet-resistant herpes simplex virus infection with cidofovir in a child after an unrelated bone marrow transplant. Bone Marrow Transplant. 2000;26:903–905. doi: 10.1038/sj.bmt.1702591. [DOI] [PubMed] [Google Scholar]
- 113.Bryant P., Sasadeusz J., Carapetis J., Waters K., Curtis N. Successful treatment of foscarnet-resistant herpes simplex stomatitis with intravenous cidofovir in a child. Pediatr. Infect. Dis. J. 2001;20:1083–1086. doi: 10.1097/00006454-200111000-00016. [DOI] [PubMed] [Google Scholar]
- 114.Strand A., Böttiger D., Gever L.N., Wheeler W. Safety and Tolerability of Combination Acyclovir 5% and Hydrocortisone 1% Cream in Adolescents with Recurrent Herpes Simplex Labialis. Pediatr. Dermatol. 2011;29:105–110. doi: 10.1111/j.1525-1470.2011.01570.x. [DOI] [PubMed] [Google Scholar]
- 115.Woo S.-B., Challacombe S. Management of recurrent oral herpes simplex infections. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontol. 2007;103:S12.e1–S12.e18. doi: 10.1016/j.tripleo.2006.11.004. [DOI] [PubMed] [Google Scholar]
- 116.Bélec L., Tevi-Benissan C., Bianchi A., Cotigny S., Beumont-Mauviel M., Si-Mohamed A., Malkin J.-E. In vitro inactivation of Chlamydia trachomatis and of a panel of DNA (HSV-2, CMV, adenovirus, BK virus) and RNA (RSV, enterovirus) viruses by the spermicide benzalkonium chloride. J. Antimicrob. Chemother. 2000;46:685–693. doi: 10.1093/jac/46.5.685. [DOI] [PubMed] [Google Scholar]
- 117.Godfrey H.R., Godfrey N.J., Godfrey J.C., Riley D. A randomized clinical trial on the treatment of oral herpes with topical zinc oxide/glycine. Altern. Ther. Health Med. 2001;7:49–56. [PubMed] [Google Scholar]
- 118.Sacks S.L., Thisted R.A., Jones T.M., Barbarash R.A., Mikolich D.J., Ruoff G.E., Jorizzo J.L., Gunnilla L.B., Katz D.H., Khalil M., et al. Clinical efficacy of topical docosanol 10% cream for herpes simplex labialis: A multicenter, randomized, placebo-controlled trial. J. Am. Acad. Dermatol. 2001;45:222–230. doi: 10.1067/mjd.2001.116215. [DOI] [PubMed] [Google Scholar]
- 119.E Pope L., Marcelletti J.F., Katz L.R., Lin J.Y., Katz D.H., Parish M.L., Spear P.G. The anti-herpes simplex virus activity of n-docosanol includes inhibition of the viral entry process. Antivir. Res. 1998;40:85–94. doi: 10.1016/S0166-3542(98)00048-5. [DOI] [PubMed] [Google Scholar]
- 120.Safety and Effectiveness of Health Care Antiseptics Topical Antimicrobial Drug Products for Over-the-Counter Human Use. Final rule. Fed. Regist. 2017;82:60474–60503. [PubMed] [Google Scholar]
- 121.Crimi S., Fiorillo L., Bianchi A., D’Amico C., Amoroso G., Gorassini F., Mastroieni R., Marino S., Scoglio C., Catalano F., et al. Herpes Virus, Oral Clinical Signs and QoL: Systematic Review of Recent Data. Viruses. 2019;11:463. doi: 10.3390/v11050463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Hassan S.T.S., Masarčíková R., Berchová-Bímová K. Bioactive natural products with anti-herpes simplex virus properties. J. Pharm. Pharmacol. 2015;67:1325–1336. doi: 10.1111/jphp.12436. [DOI] [PubMed] [Google Scholar]
- 123.Le Cleach L., Trinquart L., Do G., Maruani A., Lebrun-Vignes B., Ravaud P., Chosidow O. Oral antiviral therapy for prevention of genital herpes outbreaks in immunocompetent and nonpregnant patients. Cochrane Database Syst. Rev. 2014;8:Cd009036. doi: 10.1002/14651858.CD009036.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Heslop R., Roberts H., Flower D., Jordan V. Interventions for men and women with their first episode of genital herpes. Cochrane Database Syst. Rev. 2016;2016:CD010684. doi: 10.1002/14651858.CD010684.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Garber A., Barnard L., Pickrell C. Review of Whole Plant Extracts with Activity Against Herpes Simplex Viruses In Vitro and In Vivo. J. Evid.-Based Integr. Med. 2021;26:2515690x20978394. doi: 10.1177/2515690X20978394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Mahmoud H., Abu-Jafar A., Huleihel M. Antiviral activity of Eucalyptus camaldulensis leaves ethanolic extract on herpes viruses infection. Int. J. Clin. Virol. 2017;1:001–009. doi: 10.29328/journal.ijcv.1001001. [DOI] [Google Scholar]
- 127.Lipipun V., Kurokawa M., Suttisri R., Taweechotipatr P., Pramyothin P., Hattori M., Shiraki K. Efficacy of Thai medicinal plant extracts against herpes simplex virus type 1 infection in vitro and in vivo. Antivir. Res. 2003;60:175–180. doi: 10.1016/S0166-3542(03)00152-9. [DOI] [PubMed] [Google Scholar]
- 128.Chen X., Wang Z., Yang Z., Wang J., Xu Y., Tan R.-X., Li E. Houttuynia cordata blocks HSV infection through inhibition of NF-κB activation. Antivir. Res. 2011;92:341–345. doi: 10.1016/j.antiviral.2011.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Pacheco P., Sierra J., Schmeda-Hirschmann G., Potter C.W., Jones B.M., Moshref M. Antiviral activity of chilean medicinal plant extracts. Phytother. Res. 1993;7:415–418. doi: 10.1002/ptr.2650070606. [DOI] [Google Scholar]
- 130.Roner M.R., Sprayberry J., Spinks M., Dhanji S. Antiviral activity obtained from aqueous extracts of the Chilean soapbark tree (Quillaja saponaria Molina) J. Gen. Virol. 2007;88:275–285. doi: 10.1099/vir.0.82321-0. [DOI] [PubMed] [Google Scholar]
- 131.Okba M.M., El Gedaily R.A., Ashour R.M. UPLC–PDA–ESI–qTOF-MS profiling and potent anti-HSV-II activity of Eucalyptus sideroxylon leaves. J. Chromatogr. B. 2017;1068–1069:335–342. doi: 10.1016/j.jchromb.2017.10.065. [DOI] [PubMed] [Google Scholar]
- 132.Brezáni V., Leláková V., Hassan S.T.S., Berchová-Bímová K., Nový P., Klouček P., Maršík P., Dall’Acqua S., Hošek J., Šmejkal K. Anti-Infectivity against Herpes Simplex Virus and Selected Microbes and Anti-Inflammatory Activities of Compounds Isolated from Eucalyptus globulus Labill. Viruses. 2018;10:360. doi: 10.3390/v10070360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Ma F., Shen W., Zhang X., Li M., Wang Y., Zou Y., Li Y., Wang H. Anti-HSV Activity of Kuwanon X from Mulberry Leaves with Genes Expression Inhibitory and HSV-1 Induced NF-κB Deactivated Properties. Biol. Pharm. Bull. 2016;39:1667–1674. doi: 10.1248/bpb.b16-00401. [DOI] [PubMed] [Google Scholar]
- 134.Chen X., Qiao H., Liu T., Yang Z., Xu L., Xu Y., Ge H.M., Tan R.-X., Li E. Inhibition of herpes simplex virus infection by oligomeric stilbenoids through ROS generation. Antivir. Res. 2012;95:30–36. doi: 10.1016/j.antiviral.2012.05.001. [DOI] [PubMed] [Google Scholar]
- 135.Faith S.A., Sweet T.J., Bailey E., Booth T., Docherty J.J. Resveratrol suppresses nuclear factor-κB in herpes simplex virus infected cells. Antivir. Res. 2006;72:242–251. doi: 10.1016/j.antiviral.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 136.Leyton L., Hott M., Acuña F., Caroca J., Nuñez M., Martin C., Zambrano A., Concha M.I., Otth C. Nutraceutical activators of AMPK/Sirt1 axis inhibit viral production and protect neurons from neurodegenerative events triggered during HSV-1 infection. Virus Res. 2015;205:63–72. doi: 10.1016/j.virusres.2015.05.015. [DOI] [PubMed] [Google Scholar]
- 137.Rattanathongkom A., Lee J.-B., Hayashi K., Sripanidkulchai B.-O., Kanchanapoom T., Hayashi T. Evaluation of Chikusetsusaponin IV a Isolated from Alternanthera philoxeroides for Its Potency Against Viral Replication. Planta Med. 2009;75:829–835. doi: 10.1055/s-0029-1185436. [DOI] [PubMed] [Google Scholar]
- 138.Barquero A.A., Alché L.E., Coto C.E. Antiviral activity of meliacine on the replication of a thymidine kinase-deficient mutant of Herpes simplex virus type 1 alone and in combination with acyclovir. Int. J. Antimicrob. Agents. 1997;9:49–55. doi: 10.1016/S0924-8579(97)00023-X. [DOI] [PubMed] [Google Scholar]
- 139.Petrera E., Coto C.E. Therapeutic effect of meliacine, an antiviral derived from Melia azedarach L., in mice genital herpetic infection. Phytother. Res. 2009;23:1771–1777. doi: 10.1002/ptr.2850. [DOI] [PubMed] [Google Scholar]
- 140.Brand Y.M., Roa-Linares V.C., Betancur-Galvis L.A., Durán-García D.C., Stashenko E. Antiviral activity of Colombian Labiatae and Verbenaceae family essential oils and monoterpenes on Human Herpes viruses. J. Essent. Oil Res. 2015;28:130–137. doi: 10.1080/10412905.2015.1093556. [DOI] [Google Scholar]
- 141.Venturi C.R., Danielli L.J., Klein F., Apel M.A., Montanha J.A., Bordignon S.A.L., Roehe P.M., Fuentefria A.M., Henriques A.T. Chemical analysis and in vitro antiviral and antifungal activities of essential oils from Glechon spathulate and Glechon marifolia. Pharm. Biol. 2014;53:682–688. doi: 10.3109/13880209.2014.936944. [DOI] [PubMed] [Google Scholar]
- 142.Schnitzler P., Schön K., Reichling J. Antiviral activity of Australian tea tree oil and eucalyptus oil against herpes simplex virus in cell culture. Die Pharm. 2001;56:682–688. [PubMed] [Google Scholar]
- 143.Mazzanti G., Battinelli L., Pompeo C., Serrilli A., Rossi R., Sauzullo I., Mengoni F., Vullo V. Inhibitory activity of Melissa officinalis L. extract on Herpes simplex virus type 2 replication. Nat. Prod. Res. 2008;22:1433–1440. doi: 10.1080/14786410802075939. [DOI] [PubMed] [Google Scholar]
- 144.Allahverdiyev A., Duran N., Ozguven M., Koltas S. Antiviral activity of the volatile oils of Melissa officinalis L. against Herpes simplex virus type-2. Phytomedicine. 2004;11:657–661. doi: 10.1016/j.phymed.2003.07.014. [DOI] [PubMed] [Google Scholar]
- 145.Buck C., Thompson C.D., Roberts J.N., Müller M., Lowy D.R., Schiller J.T. Carrageenan Is a Potent Inhibitor of Papillomavirus Infection. PLoS Pathog. 2006;2:e69. doi: 10.1371/journal.ppat.0020069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Lee C.-K., Kim H.S., Nam J.R., Lee M.-J., Yim J.-H., Lee H.K., De Clercq E. Anti-Picornavirus Activity and Other Antiviral Activity of Sulfated Exopolysaccharide from the Marine Microalga Gyrodinium impudicum Strain KG03. Antivir. Res. 2009;82:A40. doi: 10.1016/j.antiviral.2009.02.084. [DOI] [Google Scholar]
- 147.Lee J.-B., Hayashi K., Hayashi T., Sankawa U., Maeda M. Antiviral Activities against HSV-1, HCMV, and HIV-1 of Rhamnan Sulfate from Monostroma latissimum. Planta Med. 1999;65:439–441. doi: 10.1055/s-2006-960804. [DOI] [PubMed] [Google Scholar]
- 148.Thuy T.T.T., Ly B.M., Van T.T.T., Van Quang N., Tu H.C., Zheng Y., Seguin-Devaux C., Mi B., Ai U. Anti-HIV activity of fucoidans from three brown seaweed species. Carbohydr. Polym. 2015;115:122–128. doi: 10.1016/j.carbpol.2014.08.068. [DOI] [PubMed] [Google Scholar]
- 149.Gerber P., Dutcher J.D., Adams E.V., Sherman J.H. Protective Effect of Seaweed Extracts for Chicken Embryos Infected with Influenza B or Mumps Virus. Exp. Biol. Med. 1958;99:590–593. doi: 10.3181/00379727-99-24429. [DOI] [PubMed] [Google Scholar]
- 150.Pujol C.A., Ray S., Ray B., Damonte E.B. Antiviral activity against dengue virus of diverse classes of algal sulfated polysaccharides. Int. J. Biol. Macromol. 2012;51:412–416. doi: 10.1016/j.ijbiomac.2012.05.028. [DOI] [PubMed] [Google Scholar]
- 151.Faral-Tello P., Mirazo S., Dutra C., Pérez A., Geis-Asteggiante L., Frabasile S., Koncke E., Davyt D., Cavallaro L., Heinzen H., et al. Cytotoxic, Virucidal, and Antiviral Activity of South American Plant and Algae Extracts. Sci. World J. 2012;2012:174837. doi: 10.1100/2012/174837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Soares A.R., Robaina M.C.S., Mendes G.S., Silva T.S.L., Gestinari L.M.S., Pamplona O.S., Yoneshigue-Valentin Y., Kaiser C.R., Romanos M.T.V. Antiviral activity of extracts from Brazilian seaweeds against herpes simplex virus. Rev. Bras. De Farm. 2012;22:714–723. doi: 10.1590/S0102-695X2012005000061. [DOI] [Google Scholar]
- 153.Hayashi K., Nakano T., Hashimoto M., Kanekiyo K., Hayashi T. Defensive effects of a fucoidan from brown alga Undaria pinnatifida against herpes simplex virus infection. Int. Immunopharmacol. 2008;8:109–116. doi: 10.1016/j.intimp.2007.10.017. [DOI] [PubMed] [Google Scholar]
- 154.Alboofetileh M., Rezaei M., Tabarsa M., Rittà M., Donalisio M., Mariatti F., You S., Lembo D., Cravotto G. Effect of different non-conventional extraction methods on the antibacterial and antiviral activity of fucoidans extracted from Nizamuddinia zanardinii. Int. J. Biol. Macromol. 2018;124:131–137. doi: 10.1016/j.ijbiomac.2018.11.201. [DOI] [PubMed] [Google Scholar]
- 155.Jin F., Zhuo C., He Z., Wang H., Liu W., Zhang R., Wang Y. Anti-herpes simplex virus activity of polysaccharides from Eucheuma gelatinae. World J. Microbiol. Biotechnol. 2015;31:453–460. doi: 10.1007/s11274-015-1798-1. [DOI] [PubMed] [Google Scholar]
- 156.Lopes N., Ray S., Espada S.F., Bomfim W.A., Ray B., Faccin-Galhardi L.C., Linhares R.E.C., Nozawa C. Green seaweed Enteromorpha compressa (Chlorophyta, Ulvaceae) derived sulphated polysaccharides inhibit herpes simplex virus. Int. J. Biol. Macromol. 2017;102:605–612. doi: 10.1016/j.ijbiomac.2017.04.043. [DOI] [PubMed] [Google Scholar]
- 157.Sun Q.-L., Li Y., Ni L.-Q., Li Y.-X., Cui Y.-S., Jiang S.-L., Xie E.-Y., Du J., Deng F., Dong C.-X. Structural characterization and antiviral activity of two fucoidans from the brown algae Sargassum henslowianum. Carbohydr. Polym. 2019;229:115487. doi: 10.1016/j.carbpol.2019.115487. [DOI] [PubMed] [Google Scholar]
- 158.Souza T.M.L., Abrantes J.L., Epifanio R.D.A., Fontes C.F.L., Frugulhetti I.C.P.P. The Alkaloid 4-Methylaaptamine Isolated from the Sponge Aaptos aaptos Impairs Herpes simplex Virus Type 1 Penetration and Immediate-Early Protein Synthesis. Planta Med. 2007;73:200–205. doi: 10.1055/s-2007-967109. [DOI] [PubMed] [Google Scholar]
- 159.Mendes G.D.S., Bravin I.C., Yoneshigue-Valentin Y., Yokoya N.S., Romanos M.T.V. Anti-HSV activity of Hypnea musciformis cultured with different phytohormones. Rev. Bras. De Farm. 2012;22:789–794. doi: 10.1590/S0102-695X2012005000054. [DOI] [Google Scholar]
- 160.Santoyo S., Jaime L., Plaza M., Herrero M., Rodriguez-Meizoso I., Ibañez E., Reglero G. Antiviral compounds obtained from microalgae commonly used as carotenoid sources. J. Appl. Phycol. 2012;24:731–741. doi: 10.1007/s10811-011-9692-1. [DOI] [Google Scholar]
- 161.Keivan Z., Moradali F., Parisa P., Kohzad S. Evaluation of in vitro antiviral activity of a brown alga (Cystoseira myrica) from the Persian Gulf against herpes simplex virus type 1. Afr. J. Biotechnol. 2007;6:2511–2514. doi: 10.5897/AJB2007.000-2399. [DOI] [Google Scholar]
- 162.Cardozo F.T.G.D.S., Camelini C.M., Leal P.C., Kratz J.M., Nunes R.J., de Mendonça M.M., Simões C.M.O. Antiherpetic Mechanism of a Sulfated Derivative of Agaricus brasiliensis Fruiting Bodies Polysaccharide. Intervirology. 2014;57:375–383. doi: 10.1159/000365194. [DOI] [PubMed] [Google Scholar]
- 163.Eo S.-K., Kim Y.-S., Lee C.-K., Han S.-S. Possible mode of antiviral activity of acidic protein bound polysaccharide isolated from Ganoderma lucidum on herpes simplex viruses. J. Ethnopharmacol. 2000;72:475–481. doi: 10.1016/S0378-8741(00)00266-X. [DOI] [PubMed] [Google Scholar]
- 164.Huang Z., Nong X., Ren Z., Wang J., Zhang X., Qi S. Anti-HSV-1, antioxidant and antifouling phenolic compounds from the deep-sea-derived fungus Aspergillus versicolor SCSIO 41502. Bioorganic Med. Chem. Lett. 2017;27:787–791. doi: 10.1016/j.bmcl.2017.01.032. [DOI] [PubMed] [Google Scholar]
- 165.Liang T.-M., Fang Y.-W., Zheng J.-Y., Shao C.-L. Secondary Metabolites Isolated from the Gorgonian-Derived Fungus Aspergillus ruber and Their Antiviral Activity. Chem. Nat. Compd. 2018;54:559–561. doi: 10.1007/s10600-018-2406-z. [DOI] [Google Scholar]
- 166.Pradeep P., Manju V., Ahsan M.F. Antiviral Potency of Mushroom Constituents. In: Agrawal D., Dhanasekaran M., editors. Medicinal Mushrooms: Recent Progress in Research and Development. Springer; Berlin/Heidelberg, Germany: 2019. pp. 275–297. [Google Scholar]
- 167.Gu C.-Q., Li J.-W., Chao F., Jin M., Wang X.-W., Shen Z.-Q. Isolation, identification and function of a novel anti-HSV-1 protein from Grifola frondosa. Antivir. Res. 2007;75:250–257. doi: 10.1016/j.antiviral.2007.03.011. [DOI] [PubMed] [Google Scholar]
- 168.Mamo T., Moseman E.A., Kolishetti N., Salvador-Morales C., Shi J., Kuritzkes D.R., Langer R., von Andrian U., Farokhzad O.C. Emerging nanotechnology approaches for HIV/AIDS treatment and prevention. Nanomedicine. 2010;5:269–285. doi: 10.2217/nnm.10.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Szunerits S., Barras A., Khanal M., Pagneux Q., Boukherroub R. Nanostructures for the Inhibition of Viral Infections. Molecules. 2015;20:14051–14081. doi: 10.3390/molecules200814051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Mahajan S.D., Law W.-C., Aalinkeel R., Reynolds J.L., Nair B.B., E Sykes D., Yong K.-T., Roy I., Prasad P.N., A Schwartz S. Anti-HIV-1 nanotherapeutics: Promises and challenges for the future. Int. J. Nanomed. 2012;7:5301–5314. doi: 10.2147/IJN.S25871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Donalisio M., Leone F., Civra A., Spagnolo R., Ozer O., Lembo D., Cavalli R. Acyclovir-Loaded Chitosan Nanospheres from Nano-Emulsion Templating for the Topical Treatment of Herpesviruses Infections. Pharmaceutics. 2018;10:46. doi: 10.3390/pharmaceutics10020046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Al-Dhubiab B.E., Nair A.B., Kumria R., Attimarad M., Harsha S. Formulation and evaluation of nano based drug delivery system for the buccal delivery of acyclovir. Colloids Surf. B Biointerfaces. 2015;136:878–884. doi: 10.1016/j.colsurfb.2015.10.045. [DOI] [PubMed] [Google Scholar]
- 173.Rai M., Shegokar R. Metal Nanoparticles in Pharma. Springer; Berlin/Heidelberg, Germany: 2017. [Google Scholar]
- 174.Jeyaraj M., Gurunathan S., Qasim M., Kang M.-H., Kim J.-H. A Comprehensive Review on the Synthesis, Characterization, and Biomedical Application of Platinum Nanoparticles. Nanomaterials. 2019;9:1719. doi: 10.3390/nano9121719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Ramadan M.A., Shawkey A.E., Rabeh M.A., Abdellatif A.O. Promising antimicrobial activities of oil and silver nanoparticles obtained from Melaleuca alternifolia leaves against selected skin-infecting pathogens. J. Herb. Med. 2019;20:100289. doi: 10.1016/j.hermed.2019.100289. [DOI] [Google Scholar]
- 176.Szymańska E., Orłowski P., Winnicka K., Tomaszewska E., Bąska P., Celichowski G., Grobelny J., Basa A., Krzyżowska M. Multifunctional Tannic Acid/Silver Nanoparticle-Based Mucoadhesive Hydrogel for Improved Local Treatment of HSV Infection: In Vitro and In Vivo Studies. Int. J. Mol. Sci. 2018;19:387. doi: 10.3390/ijms19020387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Haggag E.G., Elshamy A.M., Rabeh M.A., Gabr N.M., Salem M., Youssif K.A., Samir A., Bin Muhsinah A., Alsayari A., Abdelmohsen U.R. Antiviral potential of green synthesized silver nanoparticles of Lampranthus coccineus and Malephora lutea. Int. J. Nanomed. 2019;14:6217–6229. doi: 10.2147/IJN.S214171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.El-Sheekh M.M., Shabaan M.T., Hassan L., Morsi H.H. Antiviral activity of algae biosynthesized silver and gold nanoparticles against Herps Simplex (HSV-1) virus in vitro using cell-line culture technique. Int. J. Environ. Health Res. 2020;32:616–627. doi: 10.1080/09603123.2020.1789946. [DOI] [PubMed] [Google Scholar]
- 179.Dhanasezhian A., Srivani S., Govindaraju K., Parija P., Sasikala S., Kumar M. Anti-Herpes Simplex Virus (HSV-1 and HSV-2) activity of biogenic gold and silver nanoparticles using seaweed Sargassum wightii. Indian J. Mar. Sci. 2019;48 [Google Scholar]
- 180.I R.-I., Mj S., R G., Fj D.L.M., Mj B., Ma M.-F. Gold Nanoparticles Crossing Blood-Brain Barrier Prevent HSV-1 Infection and Reduce Herpes Associated Amyloid-βsecretion. J. Clin. Med. 2020;9:155. doi: 10.3390/jcm9010155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Paradowska E., Studzińska M., Jabłońska A., Lozovski V., Rusinchuk N., Mukha I., Vitiuk N., Leśnikowski Z.J. Antiviral Effect of Nonfunctionalized Gold Nanoparticles against Herpes Simplex Virus Type-1 (HSV-1) and Possible Contribution of Near-Field Interaction Mechanism. Molecules. 2021;26:5960. doi: 10.3390/molecules26195960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Krzyzowska M., Chodkowski M., Janicka M., Dmowska D., Tomaszewska E., Ranoszek-Soliwoda K., Bednarczyk K., Celichowski G., Grobelny J. Lactoferrin-Functionalized Noble Metal Nanoparticles as New Antivirals for HSV-2 Infection. Microorganisms. 2022;10:110. doi: 10.3390/microorganisms10010110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Antoine T.E., Mishra Y.K., Trigilio J., Tiwari V., Adelung R., Shukla D. Prophylactic, therapeutic and neutralizing effects of zinc oxide tetrapod structures against herpes simplex virus type-2 infection. Antivir. Res. 2012;96:363–375. doi: 10.1016/j.antiviral.2012.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Mishra Y.K., Adelung R., Röhl C., Shukla D., Spors F., Tiwari V. Virostatic potential of micro–nano filopodia-like ZnO structures against herpes simplex virus-1. Antivir. Res. 2011;92:305–312. doi: 10.1016/j.antiviral.2011.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Shen M.-X., Ma N., Li M.-K., Liu Y.-Y., Chen T., Wei F., Liu D.-Y., Hou W., Xiong H.-R., Yang Z.-Q. Antiviral Properties of R. tanguticum Nanoparticles on Herpes Simplex Virus Type I In Vitro and In Vivo. Front. Pharmacol. 2019;10:959. doi: 10.3389/fphar.2019.00959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Tarallo R., Carberry T.P., Falanga A., Vitiello M., Galdiero S., Galdiero M., Weck M. Dendrimers functionalized with membrane-interacting peptides for viral inhibition. Int. J. Nanomed. 2013;8:521–534. doi: 10.2147/ijn.s37739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Chono K., Katsumata K., Kontani T., Kobayashi M., Sudo K., Yokota T., Konno K., Shimizu Y., Suzuki H. ASP2151, a novel helicase-primase inhibitor, possesses antiviral activity against varicella-zoster virus and herpes simplex virus types 1 and 2. J. Antimicrob. Chemother. 2010;65:1733–1741. doi: 10.1093/jac/dkq198. [DOI] [PubMed] [Google Scholar]
- 188.Kalu N.N., Desai P.J., Shirley C.M., Gibson W., Dennis P.A., Ambinder R.F. Nelfinavir Inhibits Maturation and Export of Herpes Simplex Virus 1. J. Virol. 2014;88:5455–5461. doi: 10.1128/JVI.03790-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Quenelle D.C., Lampert B., Collins D.J., Rice T.L., Painter G.R., Kern E.R. Efficacy of CMX001 against Herpes Simplex Virus Infections in Mice and Correlations with Drug Distribution Studies. J. Infect. Dis. 2010;202:1492–1499. doi: 10.1086/656717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Prichard M.N., Kern E.R., Hartline C.B., Lanier E.R., Quenelle D.C. CMX001 Potentiates the Efficacy of Acyclovir in Herpes Simplex Virus Infections. Antimicrob. Agents Chemother. 2011;55:4728–4734. doi: 10.1128/AAC.00545-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Wald A., Corey L., Timmler B., Magaret A., Warren T., Tyring S., Johnston C., Kriesel J., Fife K., Galitz L., et al. Helicase–Primase Inhibitor Pritelivir for HSV-2 Infection. New Engl. J. Med. 2014;370:201–210. doi: 10.1056/NEJMoa1301150. [DOI] [PubMed] [Google Scholar]
- 192.Hostetler K.Y. Alkoxyalkyl prodrugs of acyclic nucleoside phosphonates enhance oral antiviral activity and reduce toxicity: Current state of the art. Antivir. Res. 2009;82:A84–A98. doi: 10.1016/j.antiviral.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Kleymann G., Fischer R., Betz U.A., Hendrix M., Bender W., Schneider U., Handke G., Eckenberg P., Hewlett G., Pevzner V., et al. New helicase-primase inhibitors as drug candidates for the treatment of herpes simplex disease. Nat. Med. 2002;8:392–398. doi: 10.1038/nm0402-392. [DOI] [PubMed] [Google Scholar]
- 194.Patick A.K., Mo H., Markowitz M., Appelt K., Wu B., Musick L., Kalish V., Kaldor S., Reich S., Ho D., et al. Antiviral and resistance studies of AG1343, an orally bioavailable inhibitor of human immunodeficiency virus protease. Antimicrob. Agents Chemother. 1996;40:292–297. doi: 10.1128/AAC.40.2.292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Markowitz M., Conant M., Hurley A., Schluger R., Duran M., Peterkin J., Chapman S., Patick A., Hendricks A., Yuen G.J., et al. A preliminary evaluation of nelfinavir mesylate, an inhibitor of human immunodeficiency virus (HIV)-1 protease, to treat HIV infection. J. Infect. Dis. 1998;177:1533–1540. doi: 10.1086/515312. [DOI] [PubMed] [Google Scholar]
- 196.Semprini A., Singer J., Braithwaite I., Shortt N., Thayabaran D., McConnell M., Weatherall M., Beasley R. Kanuka honey versus aciclovir for the topical treatment of herpes simplex labialis: A randomised controlled trial. BMJ Open. 2019;9:e026201. doi: 10.1136/bmjopen-2018-026201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Gupta R., Wald A., Krantz E., Selke S., Warren T., Vargas-Cortes M., Miller G., Corey L. Valacyclovir and Acyclovir for Suppression of Shedding of Herpes Simplex Virus in the Genital Tract. J. Infect. Dis. 2004;190:1374–1381. doi: 10.1086/424519. [DOI] [PubMed] [Google Scholar]
- 198.Moomaw M.D., Cornea P., Rathbun R.C., A Wendel K. Review of antiviral therapy for herpes labialis, genital herpes and herpes zoster. Expert Rev. Anti-Infect. Ther. 2003;1:283–295. doi: 10.1586/14787210.1.2.283. [DOI] [PubMed] [Google Scholar]
- 199.Boyd M.R., Safrin S., Kern E.R. Penciclovir: A review of its spectrum of activity, selectivity, and cross-resistance pattern. Antivir. Chem. Chemother. 1993;4:3–11. doi: 10.1177/095632029300401S01. [DOI] [Google Scholar]
- 200.Harmenberg J., Öberg B., Spruance S. Prevention of Ulcerative Lesions by Episodic Treatment of Recurrent Herpes Labialis: A Literature Review. Acta Derm. -Venereol. 2010;90:122–130. doi: 10.2340/00015555-0806. [DOI] [PubMed] [Google Scholar]
- 201.Paintsil E., Cheng Y.-C. Antiviral Agents. In: Schaechter M., editor. Encyclopedia of Microbiology. 3rd ed. Academic Press; Oxford, UK: 2009. pp. 223–257. [Google Scholar]
- 202.Sadjadi S.-A., Regmi S., Chau T. Acyclovir Neurotoxicity in a Peritoneal Dialysis Patient: Report of a Case and Review of the Pharmacokinetics of Acyclovir. Am. J. Case Rep. 2018;19:1459–1462. doi: 10.12659/AJCR.911520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Frobert E., Ooka T., Cortay J.C., Lina B., Thouvenot D., Morfin F. Herpes Simplex Virus Thymidine Kinase Mutations Associated with Resistance to Acyclovir: A Site-Directed Mutagenesis Study. Antimicrob. Agents Chemother. 2005;49:1055–1059. doi: 10.1128/AAC.49.3.1055-1059.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Sarisky R.T., Bacon T., Boon R., Locke L., Nguyen T.T., Leary J., Esser K., Saltzman R. Penciclovir Susceptibilities of Herpes Simplex Virus Isolates from Patients Using Penciclovir Cream for Treatment of Recurrent Herpes Labialis. Antimicrob. Agents Chemother. 2002;46:2848–2853. doi: 10.1128/AAC.46.9.2848-2853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Sarisky R.T., Bacon T.H., Boon R.J., Duffy K.E., Esser K.M., Leary J., Locke L.A., Nguyen T.T., Quail M.R., Saltzman R. Profiling penciclovir susceptibility and prevalence of resistance of herpes simplex virus isolates across eleven clinical trials. Arch. Virol. 2003;148:1757–1769. doi: 10.1007/s00705-003-0124-7. [DOI] [PubMed] [Google Scholar]
- 206.Bacon T.H., Levin M.J., Leary J.J., Sarisky R.T., Sutton D. Herpes Simplex Virus Resistance to Acyclovir and Penciclovir after Two Decades of Antiviral Therapy. Clin. Microbiol. Rev. 2003;16:114–128. doi: 10.1128/CMR.16.1.114-128.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Spruance S.L. Prophylactic chemotherapy with acyclovir for recurrent herpes simplex labialis. J. Med. Virol. 1993;41:27–32. doi: 10.1002/jmv.1890410507. [DOI] [PubMed] [Google Scholar]
- 208.Mindel A. Is it meaningful to treat patients with recurrent herpetic infections? Scand. J. Infect. Dis. Suppl. 1991;80:27–32. [PubMed] [Google Scholar]
- 209.Spruance S.L., Stewart J.C.B., Rowe N.H., McKeough M.B., Wenerstrom G., Freeman D.J. Treatment of Recurrent Herpes Simplex Labialis with Oral Acyclovir. J. Infect. Dis. 1990;161:185–190. doi: 10.1093/infdis/161.2.185. [DOI] [PubMed] [Google Scholar]
- 210.Sauerbrei A. Optimal management of genital herpes: Current perspectives. Infect. Drug Resist. 2016;9:129–141. doi: 10.2147/IDR.S96164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Sela M., Hilleman M.R. Therapeutic vaccines: Realities of today and hopes for tomorrow. Proc. Natl. Acad. Sci. USA. 2004;101((Suppl. S2)):14559. doi: 10.1073/pnas.0405924101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Truong N.R., Smith J.B., Sandgren K.J., Cunningham A.L. Mechanisms of Immune Control of Mucosal HSV Infection: A Guide to Rational Vaccine Design. Front. Immunol. 2019;10:373. doi: 10.3389/fimmu.2019.00373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Metcalf J.F. Protection from Experimental Ocular Herpetic Keratitis by a Heat-Killed Virus Vaccine. Arch. Ophthalmol. 1980;98:893–896. doi: 10.1001/archopht.1980.01020030887017. [DOI] [PubMed] [Google Scholar]
- 214.Rajcani J., Kutinová L., Vonka V. Restriction of latent herpes virus infection in rabbits immunized with subviral herpes simplex virus vaccine. Acta. Virologica. 1980;24:183–193. [PubMed] [Google Scholar]
- 215.Hoshino Y., Pesnicak L., Dowdell K.C., Lacayo J., Dudek T., Knipe D.M., Straus S.E., Cohen J.I. Comparison of immunogenicity and protective efficacy of genital herpes vaccine candidates herpes simplex virus 2 dl5-29 and dl5-29-41L in mice and guinea pigs. Vaccine. 2008;26:4034–4040. doi: 10.1016/j.vaccine.2008.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Liu X., Broberg E., Watanabe D., Dudek T., DeLuca N., Knipe D.M. Genetic engineering of a modified herpes simplex virus 1 vaccine vector. Vaccine. 2009;27:2760–2767. doi: 10.1016/j.vaccine.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Peng B., Wang L.R., Gómez-Román V.R., Davis-Warren A., Montefiori D.C., Kalyanaraman V.S., Venzon D., Zhao J., Kan E., Rowell T.J., et al. Replicating Rather than Nonreplicating Adenovirus-Human Immunodeficiency Virus Recombinant Vaccines Are Better at Eliciting Potent Cellular Immunity and Priming High-Titer Antibodies. J. Virol. 2005;79:10200–10209. doi: 10.1128/JVI.79.16.10200-10209.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 218.Halford W.P., Weisend C., Grace J., Soboleski M., Carr D.J.J., Balliet J.W., Imai Y., Margolis T.P., Gebhardt B.M. ICP0 antagonizes Stat 1-dependent repression of herpes simplex virus: Implications for the regulation of viral latency. Virol. J. 2006;3:44. doi: 10.1186/1743-422X-3-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Huang X., Lu B., Yu W., Fang Q., Liu L., Zhuang K., Shen T., Wang H., Tian P., Zhang L., et al. A Novel Replication-Competent Vaccinia Vector MVTT Is Superior to MVA for Inducing High Levels of Neutralizing Antibody via Mucosal Vaccination. PLoS ONE. 2009;4:e4180. doi: 10.1371/journal.pone.0004180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Liu H., Yu W., Tang X., Wang H., Ouyang W., Zhou J., Chen Z. The route of inoculation determines the tissue tropism of modified vaccinia tiantan expressing the spike glycoprotein of SARS-CoV in mice. J. Med. Virol. 2010;82:727–734. doi: 10.1002/jmv.21667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Diaz F., Gregory S., Nakashima H., Viapiano M.S., Knipe D.M. Intramuscular delivery of replication-defective herpes simplex virus gives antigen expression in muscle syncytia and improved protection against pathogenic HSV-2 strains. Virology. 2017;513:129–135. doi: 10.1016/j.virol.2017.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Petro C., A González P., Cheshenko N., Jandl T., Khajoueinejad N., Bénard A., Sengupta M., Herold B.C., Jacobs W.R. Herpes simplex type 2 virus deleted in glycoprotein D protects against vaginal, skin and neural disease. Elife. 2015;4:e06054. doi: 10.7554/eLife.06054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Petro C.D., Weinrick B., Khajoueinejad N., Burn C., Sellers R., Jacobs W.R., Herold B.C. HSV-2 ΔgD elicits FcγR-effector antibodies that protect against clinical isolates. J. Clin. Investig. 2016;1:e88529. doi: 10.1172/jci.insight.88529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Prichard M.N., Kaiwar R., Jackman W.T., Quenelle D.C., Collins D.J., Kern E.R., Kemble G.M., Spaete R.R. Evaluation of AD472, a live attenuated recombinant herpes simplex virus type 2 vaccine in guinea pigs. Vaccine. 2005;23:5424–5431. doi: 10.1016/j.vaccine.2005.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Bernstein D.I., Cardin R.D., Smith G.A., Pickard G.E., Sollars P.J., Dixon D.A., Pasula R., Bravo F.J. The R2 non-neuroinvasive HSV-1 vaccine affords protection from genital HSV-2 infections in a guinea pig model. NPJ Vaccines. 2020;5:104. doi: 10.1038/s41541-020-00254-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Odegard J.M., Flynn P., Campbell D.J., Robbins S.H., Dong L., Wang K., ter Meulen J., Cohen J.I., Koelle D. A novel HSV-2 subunit vaccine induces GLA-dependent CD4 and CD8 T cell responses and protective immunity in mice and guinea pigs. Vaccine. 2015;34:101–109. doi: 10.1016/j.vaccine.2015.10.137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gardner J.K., Swaims-Kohlmeier A., Herbst-Kralovetz M.M. IL-36γ Is a Key Regulator of Neutrophil Infiltration in the Vaginal Microenvironment and Limits Neuroinvasion in Genital HSV-2 Infection. J. Immunol. 2019;203:2655–2664. doi: 10.4049/jimmunol.1900280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Aschner C.B., Knipe D.M., Herold B.C. Model of vaccine efficacy against HSV-2 superinfection of HSV-1 seropositive mice demonstrates protection by antibodies mediating cellular cytotoxicity. Vaccines. 2020;5:35. doi: 10.1038/s41541-020-0184-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.McLean C., Níchallanáin D., Duncan I., Boursnell M., Jennings R., Inglis S. Induction of a protective immune response by mucosal vaccination with a DISC HSV-1 vaccine. Vaccine. 1996;14:987–992. doi: 10.1016/0264-410X(95)00259-4. [DOI] [PubMed] [Google Scholar]
- 230.Boursnell M., Entwisle C., Blakeley D., Roberts C., Duncan I.A., Chisholm S.E., Martin G.M., Jennings R., Ni Challanain D., Sobek I., et al. A Genetically Inactivated Herpes Simplex Virus Type 2 (HSV-2) Vaccine Provides Effective Protection against Primary and Recurrent HSV-2 Disease. J. Infect. Dis. 1997;175:16–25. doi: 10.1093/infdis/175.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Dudek T., Mathews L.C., Knipe D.M. Disruption of the UL41 gene in the herpes simplex virus 2 dl5-29 mutant increases its immunogenicity and protective capacity in a murine model of genital herpes. Virology. 2008;372:165–175. doi: 10.1016/j.virol.2007.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Dropulic L.K., Oestreich M.C., Pietz H.L., Laing K.J., Hunsberger S., Lumbard K., Garabedian D., Turk S.P., Chen A., Hornung R.L., et al. A Randomized, Double-Blinded, Placebo-Controlled, Phase 1 Study of a Replication-Defective Herpes Simplex Virus (HSV) Type 2 Vaccine, HSV529, in Adults with or Without HSV Infection. J. Infect. Dis. 2019;220:990–1000. doi: 10.1093/infdis/jiz225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Wang K., Dropulic L., Bozekowski J., Pietz H.L., Jegaskanda S., Dowdell K., Vogel J.S., Garabedian D., Oestreich M., Nguyen H., et al. Serum and Cervicovaginal Fluid Antibody Profiling in Herpes Simplex Virus–Seronegative Recipients of the HSV529 Vaccine. J. Infect. Dis. 2021;224:1509–1519. doi: 10.1093/infdis/jiab139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Reszka N.J., Dudek T., Knipe D.M. Construction and properties of a herpes simplex virus 2 dl5-29 vaccine candidate strain encoding an HSV-1 virion host shutoff protein. Vaccine. 2010;28:2754–2762. doi: 10.1016/j.vaccine.2010.01.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Zhang P., Xie L., Balliet J.W., Casimiro D.R., Yao F. A Herpes Simplex Virus 2 (HSV-2) Glycoprotein D-expressing Nonreplicating Dominant-Negative HSV-2 Virus Vaccine Is Superior to a gD2 Subunit Vaccine against HSV-2 Genital Infection in Guinea Pigs. PLoS ONE. 2014;9:e101373. doi: 10.1371/journal.pone.0101373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Geiss B.J., Smith T.J., Leib D.A., Morrison L.A. Disruption of Virion Host Shutoff Activity Improves the Immunogenicity and Protective Capacity of a Replication-Incompetent Herpes Simplex Virus Type 1 Vaccine Strain. J. Virol. 2000;74:11137–11144. doi: 10.1128/JVI.74.23.11137-11144.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 237.Keadle T., Morrison L., Morris J., Pepose J., Stuart P. Therapeutic immunization with a virion host shutoff (vhs) defective, replication-incompetent HSV-1 strain limits recurrent herpetic ocular infection. Investig. Ophthalmol. Vis. Sci. 2002;43:3854. doi: 10.1128/JVI.76.8.3615-3625.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Bernard M.-C., Barban V., Pradezynski F., De Montfort A., Ryall R., Caillet C., Londoño-Hayes P. Immunogenicity, Protective Efficacy, and Non-Replicative Status of the HSV-2 Vaccine Candidate HSV529 in Mice and Guinea Pigs. PLoS ONE. 2015;10:e0121518. doi: 10.1371/journal.pone.0121518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Ann Arbor M. BlueWillow Biologics Awarded Patent for Intranasal Genital Herpes Vaccine. BlueWillow News, 23 July 2019.
- 240.Richards A.L., Sollars P.J., Pitts J.D., Stults A.M., Heldwein E.E., Pickard G.E., Smith G.A. The pUL37 tegument protein guides alpha-herpesvirus retrograde axonal transport to promote neuroinvasion. PLoS Pathog. 2017;13:e1006741. doi: 10.1371/journal.ppat.1006741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Stanfield B.A., Pahar B., Chouljenko V.N., Veazey R., Kousoulas K.G. Vaccination of rhesus macaques with the live-attenuated HSV-1 vaccine VC2 stimulates the proliferation of mucosal T cells and germinal center responses resulting in sustained production of highly neutralizing antibodies. Vaccine. 2017;35:536–543. doi: 10.1016/j.vaccine.2016.12.018. [DOI] [PubMed] [Google Scholar]
- 242.Stanfield B.A., Rider P.J., Caskey J., Del Piero F., Kousoulas K.G. Intramuscular vaccination of guinea pigs with the live-attenuated human herpes simplex vaccine VC2 stimulates a transcriptional profile of vaginal Th17 and regulatory Tr1 responses. Vaccine. 2018;36:2842–2849. doi: 10.1016/j.vaccine.2018.03.075. [DOI] [PubMed] [Google Scholar]
- 243.Stanfield B.A., Stahl J., Chouljenko V.N., Subramanian R., Charles A.-S., Saied A.A., Walker J.D., Kousoulas K.G. A Single Intramuscular Vaccination of Mice with the HSV-1 VC2 Virus with Mutations in the Glycoprotein K and the Membrane Protein UL20 Confers Full Protection against Lethal Intravaginal Challenge with Virulent HSV-1 and HSV-2 Strains. PLoS ONE. 2014;9:e109890. doi: 10.1371/journal.pone.0109890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Halford W.P., Püschel R., Gershburg E., Wilber A., Gershburg S., Rakowski B. A Live-Attenuated HSV-2 ICP0− Virus Elicits 10 to 100 Times Greater Protection against Genital Herpes than a Glycoprotein D Subunit Vaccine. PLoS ONE. 2011;6:e17748. doi: 10.1371/journal.pone.0017748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Halford W.P., Püschel R., Rakowski B. Herpes Simplex Virus 2 ICP0− Mutant Viruses Are Avirulent and Immunogenic: Implications for a Genital Herpes Vaccine. PLoS ONE. 2010;5:e12251. doi: 10.1371/journal.pone.0012251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Belshe R.B., Leone P.A., Bernstein D.I., Wald A., Levin M.J., Stapleton J.T., Gorfinkel I., Morrow R.L.A., Ewell M.G., Stokes-Riner A., et al. Efficacy Results of a Trial of a Herpes Simplex Vaccine. N. Engl. J. Med. 2012;366:34–43. doi: 10.1056/NEJMoa1103151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.HSV-040 Study Group Safety and immunogenicity of a glycoprotein D genital herpes vaccine in healthy girls 10–17 years of age: Results from a randomised, controlled, double-blind trial. Vaccine. 2013;31:6136–6143. doi: 10.1016/j.vaccine.2013.06.081. [DOI] [PubMed] [Google Scholar]
- 248.Mo A., Musselli C., Chen H., Pappas J., LeClair K., Liu A., Chicz R.M., Truneh A., Monks S., Levey D.L., et al. A heat shock protein based polyvalent vaccine targeting HSV-2: CD4+ and CD8+ cellular immunity and protective efficacy. Vaccine. 2011;29:8530–8541. doi: 10.1016/j.vaccine.2011.07.011. [DOI] [PubMed] [Google Scholar]
- 249.Wald A., Koelle D.M., Fife K., Warren T., LeClair K., Chicz R.M., Monks S., Levey D.L., Musselli C., Srivastava P.K. Safety and immunogenicity of long HSV-2 peptides complexed with rhHsc70 in HSV-2 seropositive persons. Vaccine. 2011;29:8520–8529. doi: 10.1016/j.vaccine.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 250.Egan K., Hook L.M., Naughton A., Friedman H.M., Awasthi S. Herpes simplex virus type 2 trivalent protein vaccine containing glycoproteins C, D and E protects guinea pigs against HSV-1 genital infection. Hum. Vaccines Immunother. 2020;16:2109–2113. doi: 10.1080/21645515.2020.1749509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 251.Awasthi S., Lubinski J.M., Shaw C.E., Barrett S.M., Cai M., Wang F., Betts M., Kingsley S., DiStefano D.J., Balliet J.W., et al. Immunization with a Vaccine Combining Herpes Simplex Virus 2 (HSV-2) Glycoprotein C (gC) and gD Subunits Improves the Protection of Dorsal Root Ganglia in Mice and Reduces the Frequency of Recurrent Vaginal Shedding of HSV-2 DNA in Guinea Pigs Compared to Immunization with gD Alone. J. Virol. 2011;85:10472–10486. doi: 10.1128/jvi.00849-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Awasthi S., Hook L.M., Pardi N., Wang F., Myles A., Cancro M.P., Cohen G.H., Weissman D., Friedman H.M. Nucleoside-modified mRNA encoding HSV-2 glycoproteins C, D, and E prevents clinical and subclinical genital herpes. Sci. Immunol. 2019;4:eaaw7083. doi: 10.1126/sciimmunol.aaw7083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Awasthi S., Hook L.M., Shaw C.E., Pahar B., Stagray J.A., Liu D., Veazey R.S., Friedman H.M. An HSV-2 Trivalent Vaccine Is Immunogenic in Rhesus Macaques and Highly Efficacious in Guinea Pigs. PLoS Pathog. 2017;13:e1006141. doi: 10.1371/journal.ppat.1006141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Hook L.M., Awasthi S., Dubin J., Flechtner J., Long D., Friedman H.M. A trivalent gC2/gD2/gE2 vaccine for herpes simplex virus generates antibody responses that block immune evasion domains on gC2 better than natural infection. Vaccine. 2018;37:664–669. doi: 10.1016/j.vaccine.2018.11.076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 255.Chiuppesi F., Vannucci L., De Luca A., Lai M., Matteoli B., Freer G., Manservigi R., Ceccherini-Nelli L., Maggi F., Bendinelli M., et al. A Lentiviral Vector-Based, Herpes Simplex Virus 1 (HSV-1) Glycoprotein B Vaccine Affords Cross-Protection against HSV-1 and HSV-2 Genital Infections. J. Virol. 2012;86:6563–6574. doi: 10.1128/JVI.00302-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Bernstein D.I., Flechtner J.B., McNeil L.K., Heineman T., Oliphant T., Tasker S., Wald A., Hetherington S. Therapeutic HSV-2 vaccine decreases recurrent virus shedding and recurrent genital herpes disease. Vaccine. 2019;37:3443–3450. doi: 10.1016/j.vaccine.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 257.Flechtner J.B., Long D., Larson S., Clemens V., Baccari A., Kien L., Chan J., Skoberne M., Brudner M., Hetherington S. Immune responses elicited by the GEN-003 candidate HSV-2 therapeutic vaccine in a randomized controlled dose-ranging phase 1/2a trial. Vaccine. 2016;34:5314–5320. doi: 10.1016/j.vaccine.2016.09.001. [DOI] [PubMed] [Google Scholar]
- 258.Van Wagoner N., Fife K., A Leone P., I Bernstein D., Warren T., Panther L., Novak R.M., Beigi R., Kriesel J., Tyring S., et al. Effects of Different Doses of GEN-003, a Therapeutic Vaccine for Genital Herpes Simplex Virus-2, on Viral Shedding and Lesions: Results of a Randomized Placebo-Controlled Trial. J. Infect. Dis. 2018;218:1890–1899. doi: 10.1093/infdis/jiy415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 259.Skoberne M., Cardin R., Lee A., Kazimirova A., Zielinski V., Garvie D., Lundberg A., Larson S., Bravo F.J., Bernstein D.I., et al. An Adjuvanted Herpes Simplex Virus 2 Subunit Vaccine Elicits a T Cell Response in Mice and Is an Effective Therapeutic Vaccine in Guinea Pigs. J. Virol. 2013;87:3930–3942. doi: 10.1128/JVI.02745-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Chandra J., Woo Y., Dutton J.L., Xu Y., Li B., Kinrade S., Druce J., Finlayson N., Griffin P., Laing K.J., et al. Immune responses to a HSV-2 polynucleotide immunotherapy COR-1 in HSV-2 positive subjects: A randomized double blinded phase I/IIa trial. PLoS ONE. 2019;14:e0226320. doi: 10.1371/journal.pone.0226320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Dutton J.L., Li B., Woo Y., Marshak J.O., Xu Y., Huang M.-L., Dong L., Frazer I.H., Koelle D.M. A Novel DNA Vaccine Technology Conveying Protection against a Lethal Herpes Simplex Viral Challenge in Mice. PLoS ONE. 2013;8:e76407. doi: 10.1371/journal.pone.0076407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 262.Hu K., Dou J., Yu F., He X., Yuan X., Wang Y., Liu C., Gu N. An ocular mucosal administration of nanoparticles containing DNA vaccine pRSC-gD-IL-21 confers protection against mucosal challenge with herpes simplex virus type 1 in mice. Vaccine. 2011;29:1455–1462. doi: 10.1016/j.vaccine.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 263.Veselenak R.L., Shlapobersky M., Pyles R.B., Wei Q., Sullivan S.M., Bourne N. A Vaxfectin®-adjuvanted HSV-2 plasmid DNA vaccine is effective for prophylactic and therapeutic use in the guinea pig model of genital herpes. Vaccine. 2012;30:7046–7051. doi: 10.1016/j.vaccine.2012.09.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 264.Riedmann E.M. Vical initiates vaccine trials against HSV-2 and CMV. Hum. Vaccines Immunother. 2014;10:255. [PubMed] [Google Scholar]
- 265.Shlapobersky M., Marshak J.O., Dong L., Huang M.-L., Wei Q., Chu A., Rolland A., Sullivan S., Koelle D.M. Vaxfectin-adjuvanted plasmid DNA vaccine improves protection and immunogenicity in a murine model of genital herpes infection. J. Gen. Virol. 2012;93:1305–1315. doi: 10.1099/vir.0.040055-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Cortesi R., Ravani L., Rinaldi F., Marconi P., Drechsler M., Manservigi M., Argnani R., Menegatti E., Esposito E., Manservigi R. Intranasal immunization in mice with non-ionic surfactants vesicles containing HSV immunogens: A preliminary study as possible vaccine against genital herpes. Int. J. Pharm. 2013;440:229–237. doi: 10.1016/j.ijpharm.2012.06.042. [DOI] [PubMed] [Google Scholar]
- 267.Karch C.P., Burkhard P. Vaccine technologies: From whole organisms to rationally designed protein assemblies. Biochem. Pharmacol. 2016;120:1–14. doi: 10.1016/j.bcp.2016.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 268.Straus S., Savarese B., Krause P., Kost R., Meier J., Corey L., Barnum G., Burke R., Sekulovich R., Adair S., et al. Placebo-controlled trial of vaccination with recombinant glycoprotein D of herpes simplex virus type 2 for immunotherapy of genital herpes. Lancet. 1994;343:1460–1463. doi: 10.1016/S0140-6736(94)92581-X. [DOI] [PubMed] [Google Scholar]
- 269.Pardi N., Hogan M., Naradikian M.S., Parkhouse K., Cain D.W., Jones L., Moody M.A., Verkerke H.P., Myles A., Willis E., et al. Nucleoside-modified mRNA vaccines induce potent T follicular helper and germinal center B cell responses. J. Exp. Med. 2018;215:1571–1588. doi: 10.1084/jem.20171450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Zhang X., Dervillez X., Chentoufi A.A., Badakhshan T., Bettahi I., BenMohamed L. Targeting the Genital Tract Mucosa with a Lipopeptide/Recombinant Adenovirus Prime/Boost Vaccine Induces Potent and Long-Lasting CD8+ T Cell Immunity against Herpes: Importance of MyD88. J. Immunol. 2012;189:4496–4509. doi: 10.4049/jimmunol.1201121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Khanna K.M., Bonneau R.H., Kinchington P.R., Hendricks R.L. Herpes Simplex Virus-Specific Memory CD8+ T Cells Are Selectively Activated and Retained in Latently Infected Sensory Ganglia. Immunity. 2003;18:593–603. doi: 10.1016/S1074-7613(03)00112-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Posavad C.M., Huang M.L., Barcy S., Koelle D.M., Corey L. Long Term Persistence of Herpes Simplex Virus-Specific CD8+ CTL in Persons with Frequently Recurring Genital Herpes. J. Immunol. 2000;165:1146–1152. doi: 10.4049/jimmunol.165.2.1146. [DOI] [PubMed] [Google Scholar]
- 273.Sitarek P., Merecz-Sadowska A., Kowalczyk T., Wieczfinska J., Zajdel R., Śliwiński T. Potential Synergistic Action of Bioactive Compounds from Plant Extracts against Skin Infecting Microorganisms. Int. J. Mol. Sci. 2020;21:5105. doi: 10.3390/ijms21145105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Ibáñez F.J., Farías M.A., Gonzalez-Troncoso M.P., Corrales N., Duarte L.F., Retamal-Díaz A., Gonzalez P.A. Experimental Dissection of the Lytic Replication Cycles of Herpes Simplex Viruses in vitro. Front. Microbiol. 2018;9:2406. doi: 10.3389/fmicb.2018.02406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Tachjian A., Maria V., Jahangir A. Use of Herbal Products and Potential Interactions in Patients with Cardiovascular Diseases. J. Am. Coll. Cardiol. 2010;55:515–525. doi: 10.1016/j.jacc.2009.07.074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Ekor M. The growing use of herbal medicines: Issues relating to adverse reactions and challenges in monitoring safety. Front. Pharmacol. 2014;4:177. doi: 10.3389/fphar.2013.00177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Lee J.Y., Jun S.A., Hong S.S., Ahn Y.C., Lee D.S., Son C.G. Systematic Review of Adverse Effects from Herbal Drugs Reported in Randomized Controlled Trials. Phytother. Res. 2016;30:1412–1419. doi: 10.1002/ptr.5647. [DOI] [PubMed] [Google Scholar]
- 278.Borse S.P., Singh D.P., Nivsarkar M. Understanding the relevance of herb–drug interaction studies with special focus on interplays: A prerequisite for integrative medicine. Porto Biomed. J. 2019;4:e15. doi: 10.1016/j.pbj.0000000000000015. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.