Abstract
GerN, a Bacillus cereus spore germination protein, exhibits homology to a widely distributed group of putative cation transporters or channel proteins. GerN complemented the Na+-sensitive phenotype of an Escherichia coli mutant that is deficient in Na+/H+ antiport activity (strain KNabc). GerN also reduced the concentration of K+ required to support growth of an E. coli mutant deficient in K+ uptake (strain TK2420). In a fluorescence-based assay of everted E. coli KNabc membrane vesicles, GerN exhibited robust Na+/H+ antiport activity, with a Km for Na+ estimated at 1.5 mM at pH 8.0 and 25 mM at pH 7.0. Li+, but not K+, served as a substrate. GerN-mediated Na+/H+ antiport was further demonstrated in everted vesicles as energy-dependent accumulation of 22Na+. GerN also used K+ as a coupling ion without completely replacing H+, as indicated by partial inhibition by K+ of H+ uptake into right-side-out vesicles loaded with Na+. K+ translocation as part of the antiport was supported by the stimulatory effect of intravesicular K+ on 22Na+ uptake by everted vesicles and the dependence of GerN-mediated 86Rb+ efflux on the presence of Na+ in trans. The inhibitory patterns of protonophore and thiocyanate were most consistent with an electrogenic Na+/H+-K+ antiport. GerN-mediated Na+/H+-K+ antiport was much more rapid than GerN-mediated Na+/H+ antiport.
Endospore germination completes the developmental program of Bacillus species, which supports the formation of a dormant, stress-resistant spore under conditions of nutrient deprivation and then allows the emergence of a vegetative cell upon appropriate signaling, e.g., by specific nutrients (10, 25, 26). Features of many of the ger genes that are required for optimal germination of Bacillus spores suggest that they are receptors for particular nutrient germinants and/or transport proteins (18, 19). Transporters are likely to be centrally involved in endospore germination, since there are significant outward fluxes of Na+, K+, and H+, as well as the subsequent reuptake of K+ in early stages of germination (27). Recently, a new spore germination gene that is required for inosine-dependent germination of Bacillus cereus spores has been identified and designated gerN (30). The deduced product of gerN, like a previously reported spore germination gene, grmA, from Bacillus megaterium (29), is a member of the CPA-2 monovalent cation:proton antiporter family of membrane transport proteins (23). This large family of cation transporters contains a putative iron transport protein, MagA, from a Magnetospirillum sp. (20), Kef(C) and Kef(B) proteins, which are K+ efflux systems activated by glutathione adducts with electrophilic compounds (3, 7), and NapA, an Na+/H+ antiporter that has a role in Na+ resistance in Enteroccocus hirae (33). Among these three types of proteins within the CPA-2 family, both GerN and GrmA most closely resemble NapA, to which they are, respectively, 43 and 48% identical and 67 and 71% similar in deduced amino acid sequence by BLAST (1) analysis. The possibility that NapA-like antiporters are involved in Bacillus spore germination and may be associated with receptors for specific nutrient germinants has been suggested (30). However, the actual catalytic activities of GerN and GrmA have not been documented. In the present study, two different mutants of Escherichia coli were first transformed with a plasmid that expressed B. cereus gerN and then used in complementation and membrane transport assays to clarify the activity of this putative transport protein.
MATERIALS AND METHODS
Bacterial strains, plasmids, and growth conditions.
The E. coli strains used in this study were DH5αMCR (Gibco-BRL), Na+/H+ antiporter-deficient KNabc (Δcha ΔnhaA ΔnhaB) (21), and potassium uptake-deficient TK2420 (Kdp− Kup− Trk−) (8). E. coli KNabc has a normal complement of K+ uptake systems, but it has reduced levels of K+/H+ antiport (mediating K+ efflux) relative to the wild type, presumably because of the cation substrate spectrum of one or more of the disrupted antiporter genes. E. coli TK2420 contains the normal complement of Na+(K+)/H+ antiporters. E. coli strains were routinely grown at 37°C in LBK medium (11). The effects of increasing concentrations of Na+ on the growth of E. coli KNabc were measured as described previously using LBK medium supplemented with various concentrations of NaCl (12).The effects of increasing concentrations of KCl on the growth of E. coli TK2420 were determined as described previously either in a defined medium (9) containing Na+ or in a medium in which the Na+-based buffer was replaced by morpholinepropanesulfonic acid (MOPS) (12).
The plasmids used were pGEM3zf(+) (Promega) and pGerN, the same pGEM plasmid into which the open reading frame of the gerN gene from B. cereus (30) was cloned behind the T7 promoter and a ribosome binding site. For construction of this recombinant plasmid, a promoterless copy of gerN was amplified from the B. cereus chromosome by PCR. The first primer, 5′-CCAGAGCTCGATGAGGAGGGGATCAGATG-3′, contains 9 bases at the 5′ end that provide a SacI restriction site. Bases 10 to 29 represent positions 458 to 477 in the GenBank AF246294 sequence except that A at 467 in the ribosome binding site was altered to G. The second primer, 5′-CCAGTCGACGATGATTATGGTATTAAGGTA-3′, contains an AccI restriction site at the 5′ end. Bases 10 to 30 represent the complement of the sequence of those bases that are 49 to 69 bases beyond the end of theGenBank gerN sequence. After digestion with SacI and AccI and purification of the product, the product was ligated with appropriately digested pGEM3zf(+) and used to transform E. coli. Recombinant transformants were selected by conventional techniques, and the presence of the insert was confirmed. The recombinant plasmid pGerN was sequenced and found to contain only one base change, a silent change of C to T at position 1161.
Preparation of membrane vesicles and transport assays.
Everted membrane vesicles were prepared by a method described by Rosen and colleagues (2, 22). Right-side-out membrane vesicles were prepared by the method of Kaback (14).
A fluorescence assay of Na+/H+ antiport activity using acridine orange (AO) in everted membrane vesicles was performed as described by Goldberg et al. (11). The assay buffer contained 10 mM Tris-HEPES, 140 mM choline Cl, and 5 mM MgCl2. The pH of the assay buffer was varied as indicated for the individual experiments. For measurements of the fluorescence of ACMA (9-amino-6-chloro-methoxyacridine), right-side-out membrane vesicles were assayed in the same buffer as that for AO, at pH 8.0. ACMA was added to 500 nM, and a Perkin-Elmer LS50B luminescence spectrometer with an excitation wavelength of 410 nm and an emission wavelength of 490 nm was used. Right-side-out membrane vesicles were either loaded with 10 mM NaCl or not preloaded. A baseline of ACMA fluorescence was established, at which time 60 μg of vesicle protein was added, and the quenching of the fluorescence was monitored. All fluorescence assays were conducted at room temperature. The high concentration of chloride ion in both assays ensures that the transmembrane electrical component, Δψ, of the respiration-generated electrochemical proton gradient is dissipated by chloride ion movements, maximizing the pH gradient, ΔpH (more protons inside than out, in these assays), which is directly monitored by the probes. In experiments in which antiport is driven by substrate gradients and may, by its own electrogenic activity, generate a Δψ that would then constrain further antiport, the chloride ion fluxes would at least partially offset this constraint too, depending on the relative rates of antiport versus chloride ion equilibration.
Transport of radioactive 22Na+ was measured as described elsewhere (13). Everted membrane vesicles in 10 mM Tris-HEPES, pH 8.0, were either loaded with 10 mM potassium phosphate or not loaded. The assay, performed at 15°C, was initiated by diluting the vesicles 1:10 in Tris-HEPES buffer containing 2 mM 22Na phosphate. Samples were taken at various times by filtration onto 0.22-μm-pore-size GSWP0025 (Millipore) membrane filters, followed by immediate washing with 2 ml of cold Tris-HEPES buffer. Radioactivity was determined by liquid scintillation spectrometry. For assays of 86Rb+ efflux, everted membrane vesicles were prepared from E. coli TK2420 or KNabc transformants. Vesicles were passively loaded with 2 mM potassium phosphate-86Rb+. The efflux assay, conducted at 15°C, was initiated by diluting the vesicles 1:10 in 10 mM Tris-HEPES, pH 8.0, with or without the addition of 5 mM sodium phosphate. Samples were filtered, washed, and processed as for the 22Na+ uptake assays. Protein was measured by the method of Lowry et al. (16) using lysozyme as the standard.
RESULTS
Complementation studies in E. coli mutant strains.
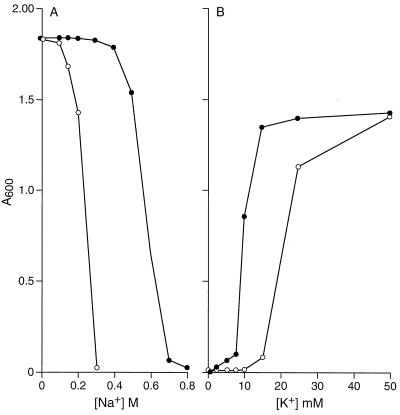
As shown in Fig. 1, the recombinant plasmid that expresses gerN, pGerN, strongly complemented both the Na+-sensitive phenotype of E. coli KNabc and the phenotype of the K+ uptake-deficient E. coli strain TK2420. This pattern was consistent with the possibility that GerN is an antiporter that catalyzes both Na+ extrusion and K+ uptake, either with or without additional coupling ions. The possibility that the Na+ efflux was coupled to GerN-mediated K+ uptake was further suggested by the dependence of complementation of E. coli TK2420 by GerN on the presence of Na+; when the growth experiment with that strain was conducted in a Na+-free, MOPS-based buffer, there was no complementation (data not shown). Direct assays were necessary to test these inferences and explore other properties of the catalytic capacities of GerN.
FIG. 1.
Effects of different concentrations of NaCl or KCl on the growth of E. coli strain KNabc or TK2420 transformed with control plasmid or pGerN. (A) Growth of E. coli KNabc transformants in the presence of increasing NaCl concentrations was measured after 15 h at 37°C. (B) Growth of E. coli TK2420 transformants in the presence of increasing KCl concentrations was measured after 15 h at 37°C. Open circles, control vector; solid circles, pGerN.
Fluorescence-based assays of monovalent cation/H+ antiport in membrane vesicles.
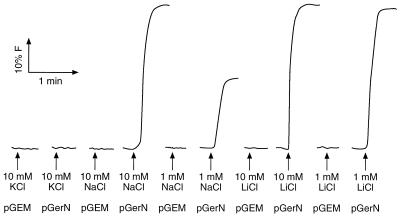
Everted membrane vesicles prepared from an E. coli KNabc transformant with a control plasmid exhibited no Na+, K+, or Li+/H+ antiport in a fluorescence-based assay using AO. In this assay, addition of the electron donor d-lactate to the vesicles under the conditions described in Materials and Methods establishes a pH gradient (ΔpH), acid in, that results from respiration-dependent proton pumping into the vesicles. The fluorescence of the AO probe is quenched in response to that ΔpH. Antiport can then be assessed by the dequenching of AO fluorescence upon addition of a cation that is taken up into the vesicles in antiport with the intravesicular protons. The changes in probe quenching directly monitor the decrease in the ΔpH arising from the efflux of protons coupled to cation uptake. The transformant of E. coli KNabc with pGerN exhibited strong Na+ and Li+/H+ antiport, i.e., significant dequenching (Fig. 2), but no K+/H+ antiport. Consistently, addition of K+ before the electron donor did not alter the Na+/H+ antiport, whereas Na+ and Li+ were cross-competitive (data not shown). The strong signal in the fluorescence assay made it possible to assess an apparent Km for Na+ for GerN-dependent Na+/H+ antiport. As shown in a reciprocal plot (Fig. 3), the Km for Na+ was highly pH dependent, decreasing with increasing pH over the pH range of 7.0 to 8.0.
FIG. 2.
Na+ or Li+/H+ antiport activity in everted membrane vesicles prepared from E. coli KNabc transformed with pGEM3Zf(+) or pGerN. Formation of a ΔpH was monitored via AO quenching at pH 8.0 after the addition of 2 mM Tris-d-lactate to a mixture containing 70 μg of vesicle protein. The figure shows the effects of adding (at the arrows) various amounts of KCl, LiCl, or NaCl after the steady-state level of ΔpH (100% quenching) had been established.
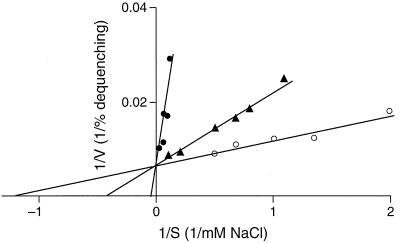
FIG. 3.
Double-reciprocal plot of NaCl concentration versus percent dequenching in the AO fluorescence assay. The assay was performed as for Fig. 2 at pH 7.0 (●), 7.5(▴), or 8.0 (○) by measuring dequenching after addition of NaCl at various concentrations.
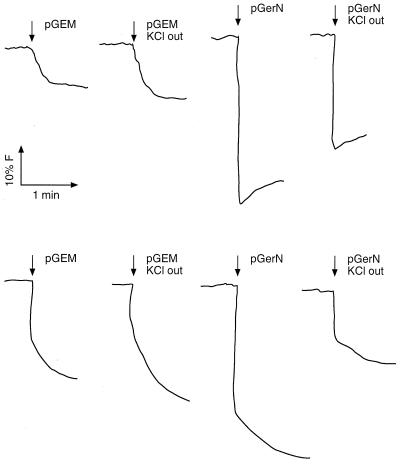
A fluorescence assay was next performed to address the question of whether GerN could use K+ as the coupling ion for Na+ antiport either instead of H+ or together with H+. Right-side-out vesicles were prepared from E. coli TK2420 and KNabc transformants and preloaded with 10 mM NaCl. At the start of the experiment, control vesicles (empty vector transformant) and GerN vesicles (pGerN transformant) were diluted into buffer either containing no added monovalent cation or containing 10 mM KCl, with no electron donor added. It was anticipated that there would be GerN-dependent uptake of H+ in exchange for the intravesicular Na+ in this right-side-out system, i.e., Na+/H+ antiport driven by the outwardly directed Na+ gradient. If K+ could substitute for all or some of the H+ as the coupling ion, then fewer H+ ions would move inward. The development of a ΔpH, acid in, was monitored as described in Materials and Methods via ACMA fluorescence. As shown in Fig. 4, GerN-dependent H+ accumulation did indeed occur in both transformants. In addition, whereas the small H+ accumulation in the control vesicles was increased by addition of K+ to the extravesicular buffer, GerN-dependent H+ uptake was significantly reduced in the presence of K+. The patterns were somewhat different in the two mutant strains, as expected from the absence of a different set of background transporters. In the E. coli KNabc transformants, in particular (Fig. 4, top), it was evident that there was still rapid initial movement of H+ dependent upon GerN, albeit reduced, when K+ was present. That is, H+ ions still move in antiport with Na+ when K+ is added, but fewer H+ ions move; some are likely to have been replaced by K+.
FIG. 4.
Effect of KCl on ACMA fluorescence in right-side-out membrane vesicles prepared from E. coli KNabc or TK2420 transformed with pGEM3Zf(+) or pGerN. Right-side-out membrane vesicles were prepared with 10 mM NaCl inside and assayed for changes in ACMA fluorescence as described in Materials and Methods. Where indicated, 10 mM KCl was included in the assay buffer. (Top) Fluorescence in vesicles prepared from E. coli KNabc transformants. (Bottom) Fluorescence in vesicles prepared from E. coli TK2420 transformants.
Assays of 22Na+ accumulation and 86Rb+ efflux by everted vesicles.
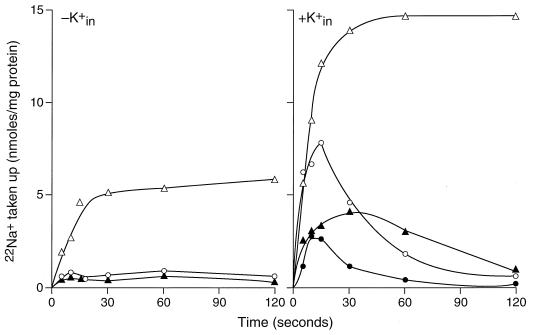
In order to monitor the GerN-dependent fluxes of monovalent cations directly, assays of 22Na+ and 86Rb+ fluxes were next undertaken in everted membrane vesicle preparations. First, the accumulation of 22Na+ was assayed, with or without addition of an electron donor, and as a function of whether the vesicles had been preloaded with 10 mM K+. The buffers used in these assays did not contain high chloride concentrations, so both the ΔpH and Δψ components of the total electrochemical proton gradient, Δp, were part of the available chemiosmotic driving force for the antiport as opposed to the completely ΔpH-driven protocol used in the initial fluorescence assays (Fig. 2). The effect of the protonophore carbonyl cyanide-m-chlorophenylhydrazone (CCCP) was also examined. These experiments were conducted with control and pGerN transformants of E. coli KNabc. As shown in Fig. 5, GerN-dependent uptake of 22Na+ was significantly enhanced by the presence of intravesicular K+. In the absence of intravesicular K+ (Fig. 5, left panel), lactate-dependent uptake of 22Na+ was observed. Essentially no uptake was observed if lactate was omitted or CCCP was added. In the vesicles that contained K+ inside, more uptake of Na+ was observed even in the absence of lactate than in the vesicles lacking K+ inside; this uptake was transient (Fig. 5, right panel). The further addition of lactate to K+-loaded vesicles resulted in rapid and sustained 22Na+ uptake without the subsequent loss of accumulated 22Na+ that was observed in the absence of the electron donor. CCCP inhibited both the lactate-dependent uptake of 22Na+ and the uptake that occurred in the absence of lactate, driven by the combined inward gradient of Na+ and outward gradient of K+. Plasmid controls conducted under the same sets of conditions exhibited no Na+ uptake (data not shown).
FIG. 5.
Uptake of 22Na+ by everted membrane vesicles from E. coli KNabc transformed with pGerN upon energization and/or loading with K+. Membrane vesicles were either loaded (right) or not loaded (left) with K+ as indicated in Materials and Methods. Uptake of 22Na+ was measured either with no further additions (○) or in the presence of either 10 μM CCCP (●), 2.5 mM Tris-d-lactate (▵), or lactate plus CCCP (▴).
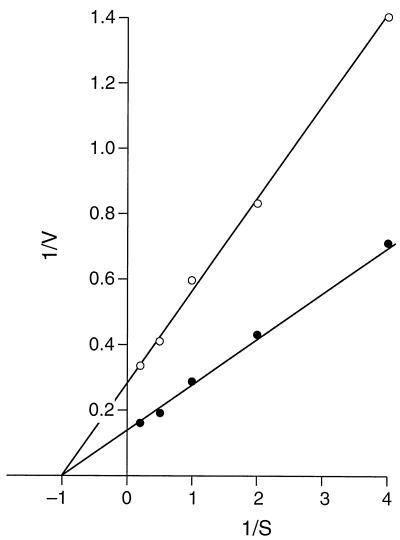
It was of interest to ascertain whether the Km determined by the 22Na+ uptake assay for Na+ for the Na+/H+ antiport, i.e., in vesicles with no K+ inside, was in the same range as that calculated from the fluorescence assay and whether intravesicular K+ affected Km and/or Vmax. As shown in Fig. 6, in assays conducted at pH 8.0, a Km of about 0.8 mM was calculated for both vesicles with and vesicles without intravesicular K+. Thus, the Km was indeed in the same general range estimated by the fluorescence assay, and it was not changed by the addition of K+ as a coupling ion. On the other hand, the Vmax was increased approximately twofold by the inclusion of intravesicular K+. The Km and Vmax patterns at pH 7.0 were consistent both with the earlier finding of a higher Km at the lower pH than at pH 8.0 and with an increased Vmax in the presence of intravesicular K+ (data not shown).
FIG. 6.
Effect of loading with K+ on a double-reciprocal plot of 22Na+ uptake by everted membrane vesicles from E. coli KNabc transformed with pGerN. Initial uptake (10 s) of 22Na+ was measured in Tris-d-lactate-energized membrane vesicles loaded (●) or not loaded (○) with K+.
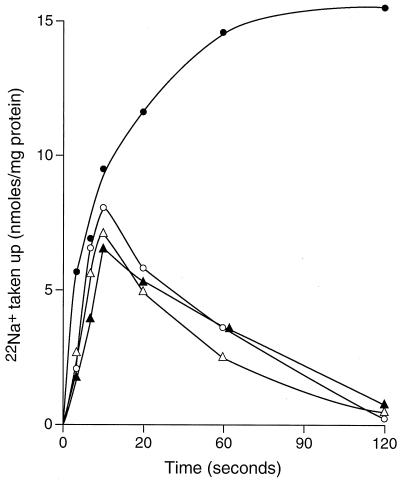
The partial, rather than complete, inhibition by K+ of GerN- and Na+-dependent H+ uptake into right-side-out vesicles (Fig. 4) was most consistent with an Na+/H+-K+ antiport, wherein a proton is a required counterion even when K+ replaces at least 1 H+ ion as part of the coupling ion complement and increases the antiport velocity. An Na+/H+-K+ exchange could be electroneutral, e.g., if each turnover involved 2 Na+ ions effluxing in exchange for 1 H+ and 1 K+ ion, or electrogenic, e.g., if a turnover involved efflux of 1 Na+ ion in exchange for 1 H+ and 1 K+ ion. In order to assess this property, we examined the effect of thiocyanate on 22Na+ uptake by K+-loaded everted vesicles prepared from E. coli KNabc cells expressing gerN. The protocol for thiocyanate treatment, described in Materials and Methods, included the permeant anion on both sides of the membrane at the start of the experiment. Transmembrane movements of the thiocyanate in response to a Δψ developed during lactate-dependent respiratory activity would be expected to reduce that Δψ. As shown in Fig. 7, thiocyanate treatment completely abolished the stimulation of 22Na+ uptake by lactate but did not affect lactate-independent, solute gradient-driven antiport.
FIG. 7.
Effect of thiocyanate on 22Na+ uptake by K+-loaded everted membrane vesicles prepared from E. coli KNabc transformed with pGerN. Vesicles were loaded with 2 mM KPi (circles) or 2 mM KSCN (triangles) and diluted 1:10 into buffer containing 2 mM 22NaPi (circles) or 2 mM 22NaSCN (triangles) in the absence (open symbols) or presence (closed symbols) of 2.5 mM Tris-d-lactate.
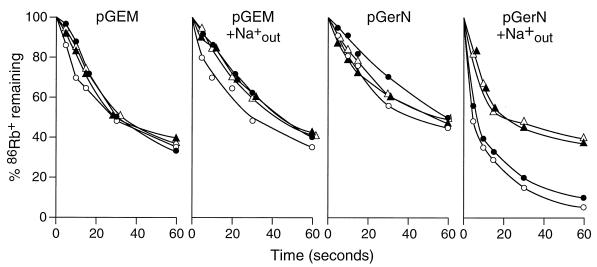
Finally, GerN-dependent efflux of 86Rb+ from everted membrane vesicles of E. coli TK2420 was assessed both in the presence and in the absence of each of the following: Na+ in the extravesicular buffer, added electron donor, and CCCP (Fig. 8). Significant 86Rb+ efflux from control everted vesicles was observed even in this triple mutant, consistent with the likelihood that additional K+ uptake pathways exist in E. coli. However, this background efflux exhibited no Na+ dependence; in fact, extravesicular Na+ modestly reduced the rate of 86Rb+ efflux whether lactate or CCCP was added to the control vesicles. GerN-dependent 86Rb+ efflux was much more rapid than in the control vesicles only when extravesicular Na+ was present. Addition of CCCP significantly reduced the rate of GerN-dependent 86Rb+ efflux in the presence of extravesicular Na+ both in the absence and in the presence of lactate. There was no stimulation by lactate. Stimulation would be expected, given lactate stimulation of GerN-dependent Na+ transport in E. coli KNabc (Fig. 5). However, the experiments for which results are shown in Fig. 8 were conducted with E. coli TK2420, in which GerN-dependent Rb+ uptake was optimally observed against the reduced K+ uptake background of this strain. This mutant strain, however, contains the full normal complement of Na+(K+)/H+ antiporters. We hypothesized that the apparent absence of lactate stimulation of Rb+ efflux from the everted vesicles might reflect the offsetting of such stimulation, which is really there, by simultaneous energization of an antiport that returns Rb+ to the intravesicular space. This was confirmed by conducting the same experiments with E. coli KNabc. The Rb+ efflux was less pronounced relative to the background but was clearly discernible, and stimulation by lactate was now observed in this antiporter-deficient background (data not shown).
FIG. 8.
Effects of combinations of energization, Na+ in trans, and CCCP on GerN-dependent efflux of 86Rb+ from everted membrane vesicles prepared from E. coli TK2420 transformants. Vesicles were loaded with K+-86Rb+ as indicated in Materials and Methods. Efflux was initiated by 10-fold dilution into buffer containing no Na+ or 10 mM Na+ as indicated. Efflux was performed either with no further additions (○) or in the presence of either 2.5 mM Tris-d-lactate (●), 10 μM CCCP (▵), or both lactate and CCCP (▴).
DISCUSSION
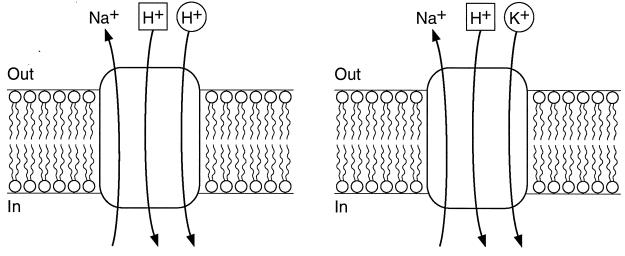
The major result of this study is the demonstration that GerN catalyzes Na+/H+ antiport, as was anticipated by its sequence similarity to NapA, and also catalyzes Na+/H+-K+ antiport, which is significantly more rapid than Na+/H+ antiport. Li+, but not K+, can also serve as the cytoplasmic substrate. The Na+/H+ antiport observed was not just a reflection of contaminating K+. Rather, GerN must have a capacity for Na+/H+ antiport as one of its catalytic modes, since, in the fluorescence assays of antiport, Na+- and Li+-dependent movements of protons were observed. Evidence for coupling between Na+ efflux and K+ uptake included the dependence of the rate of Na+ uptake by everted vesicles on the presence of intravesicular K+ and the dependence of the rate of K+ (Rb+) efflux on extravesicular Na+. K+ apparently replaced some of the H+ ions that move in exchange for Na+ during Na+/H+ antiport but not all. There remained a significant Na+-dependent rapid H+ flux into right-side-out vesicles when abundant K+ was present on the outside (Fig. 4), consistent with an Na+/H+-K+ antiport. The two proposed GerN antiport modes are depicted in Fig. 9. The 2:1 stoichiometry of coupling ions entering to Na+ effluxing was chosen arbitrarily as the simplest stoichiometry to use for diagrammatic purposes in representing the electrogenic antiports. The two H+ sites are distinguished because only part of the H+ ion complement can be replaced by K+ as the coupling ion. It will be worthwhile to confirm and extend deductions from these membrane vesicle assays in proteoliposomes in which purified GerN is the only protein. That system will be particularly useful, inasmuch as proteoliposomes are less leaky than natural membranes, for determinations of the actual stoichiometry and maximal turnover number for the antiport.
FIG. 9.
Proposed antiport activities of GerN. Two modes of GerN-mediated antiport are shown. (Left) GerN-mediated Na+/H+ antiport. The assignment of a stoichiometry of 2H+ entering in exchange for 1 Na+ effluxing is completely hypothetical and represents the apparent electrogenicity of the antiport, i.e., the number of total coupling ions translocated per turnover is greater than the number of effluxing Na+ ions, so that a net positive charge moves inward. The geometric figures surrounding the two coupling ions suggest that these ions have distinct binding sites. The pH profile of Na+/H+ antiport suggests that protons compete with Na+ on the cytoplasmic side of the membrane. (Right) Na+/H+-K+ antiport by GerN is supported by the the finding of GerN-mediated and Na+-dependent Rb+ (K+) translocation with K+ replacing some, but not all, of the H+ ions that are transported in antiport with Na+. In the diagram, K+ is shown, hypothetically, as able to compete with H+ at only one of the H+ binding sites, and the antiport is shown as still requiring the full complement of coupling ions in this mode. GerN-mediated Na+/H+-K+ antiport is much more rapid than Na+/H+ antiport; K+ increases the velocity of the antiport without affecting the Km for Na+.
GerN-mediated antiport is probably electrogenic as depicted in Fig. 9. That is, the ratio of H+ plus K+ moving into a right-side-out preparation to the Na+ moving out is greater than unity, so that a net positive charge moves inward during an antiporter turnover. This proposal is best supported in the present studies by the profound inhibition by thiocyanate of energy-dependent Na+ uptake by everted vesicles containing K+ (Fig. 7). Thiocyanate would be expected to abolish the Δψ component of the electrochemical proton gradient (Δp) established during respiration. Thus, its inhibition of the entire lactate-dependent component of the antiport suggests that the Δψ is a dominant energizing force for the antiport. This, in turn, suggests that the antiport is electrogenic. Energization of GerN-mediated Na+/H+ antiport can, however, be mediated by the ΔpH alone. This was clearly detected in the sensitive fluorescence assay under conditions in which the high chloride ion concentration in the assay buffer would have abolished the Δψ (12) and maximized the initial ΔpH produced upon addition of the electron donor (Fig. 2).
One of the notable properties of GerN-mediated antiport was the extremely high speed observed, especially when K+ was serving as one of the coupling ions. These high rates of antiport necessitated use of a low temperature for the radioactivity-based assays in order to observe a time course. They almost certainly reflect high turnover numbers given the expression conditions. Turnover numbers are best assessed in proteoliposomes and require determinations of the actual number of transporter molecules, neither of which has yet been accomplished for GerN. However, in the present experiments, gerN expression was under the control of the T7 promoter in E. coli strains that do not express a T7 polymerase. This is a device that we have found useful for producing very low levels of expression of membrane transport proteins that are toxic to particular E. coli strains when expressed at higher levels (13). Attempts to express gerN from stronger promoters in multicopy plasmids were unsuccessful with the strains used here (data not shown). No transformants that retained the correct recombinant plasmid were found. High levels of GerN may be toxic. It is not yet known whether gerN is expressed in vegetative cells of B. cereus or whether its expression is sporulation specific. High rates of GerN activity could be transiently important for some of the extensive early cation fluxes involved in germination (27). The rates are also of special note because some of the members of the CPA-2 family of transporters have been hypothesized to be channels (4); it may be that this structural group of transporters generally catalyzes rapid fluxes.
We hypothesize that it is the high speed of antiport catalyzed by GerN that accounts for certain features of the pattern of inhibition by thiocyanate. It was anticipated that electrogenic Na+/H+-K+ antiport would be stimulated by thiocyanate when driven entirely by inwardly directed Na+ and outwardly directed K+ gradients in everted vesicles. Under these conditions the antiport would be generating a Δψ that would constrain the rate of antiport unless this back force was dissipated. The absence of a stimulatory effect by thiocyanate suggests that GerN-mediated antiport outpaces the rate at which thiocyanate can equilibrate across the membrane and dissipate the Δψ that the antiport produces. Thiocyanate equilibration, like the high chloride ion concentration used in the fluorescence assays of antiport, is rapid enough to dissipate the Δψ generated by respiration. It may also keep pace with Δψ generation by GerN-mediated Na+/H+ antiport, but not with the much more rapid GerN-mediated Na+/H+-K+ antiport. There is a precedent for the notion that the rate of a secondary antiport, but not respiration-dependent proton extrusion, might outpace the rate at which a permeant anion such as thiocyanate could equilibrate and dissipate the Δψ. The turnover number reported for E. coli NhaA is 89,000 min−1 (at pH 8.5) (28), whereas turnover rates measured for cytochrome oxidase are cited in a range around 125 O2 s−1 (34).
CCCP also had an unanticipated effect on gradient-driven Na+/H+-K+ antiport, as assayed by 22Na+ uptake in everted vesicles containing K+. CCCP had an inhibitory effect (Fig. 5, right graph). CCCP abolishes both components of the Δp set up by respiration-dependent H+ pumping into the everted vesicles. It was expected to inhibit respiration-driven antiport. By contrast, under conditions in which antiport was driven solely by opposing chemical gradients of two of the antiporter substrates, it might have stimulated or had no effect, as explained above for thiocyanate. The inhibition observed could have its basis in a greater intrinsic velocity of the Na+/H+-K+ antiport than the Na+/H+ antiport. When the gradient-driven Na+/H+-K+ antiport generated a Δψ in the presence of CCCP, protons would have accumulated inside the vesicles in response to that potential. Perhaps the intravesicular H+ concentration was sufficient to compete effectively with K+. Any positive effect of dissipating the Δψ may have been more than offset because the velocity of the ensuing Na+/H+ antiport was much slower than the Na+/H+-K+ antiport that occurred in the absence of CCCP. The reduction of Na+-dependent efflux of 86Rb+ by CCCP (Fig. 8) is consistent with this explanation.
Another notable property of GerN-mediated antiport is the decrease in the Km for Na+ at a neutral versus an alkaline pH. Other Na+/H+ antiporters, especially NhaA of E. coli, are relatively inactive at neutral or low pHs. NhaA is tremendously activated at high pHs via increased specific activity and by regulation of expression (6, 28). The pattern of the pH effect on GerN activity in vesicles suggests that it is mediated by competition of H+ with Na+. Perhaps, during germination, this competition contributes to a temporal order of cation fluxes. Since the internal spore compartment is usually acidic (17, 24, 27), GerN-mediated activities might be suppressed until after the efflux of H+ that occurs during germination has sufficiently alkalinized the internal spore compartment. Such speculations must be advanced with caution, since it is possible that additional features of GerN, relevant to its role in germination, have yet to be discovered. Moreover, the number of other ion transporters involved in germination, their identities, properties, and any ordering of their activities relative to GerN still need to be clarified. Also to be elucidated is the mechanistic basis for the specificity of GerN function with respect to the nature of the germination stimulus. That is, inosine-initiated germination appears to be much more dependent on GerN function than l-alanine-initiated germination (30). One of several possibilities is that the different germinant receptors sequester particular transporters in an inactive state and that when the receptor is stimulated by its germinant, its associated antiporter or group of transporters is activated either by their release or by some other mechanism.
The present findings on GerN raise the question of whether NapA, GrmA, and other homologues might also have the capacity for Na+/H+ + K+ as well as Na+/H+ antiport. One or more of these proteins might then have a physiological role in K+ acquisition and pH homeostasis in vegetative cells in addition to the role in Na+ exclusion already proposed for NapA (33). Secondary Na+/K+ or Na+/H+-K+ antiport activities may be more widespread than has been appreciated. Verkhovskaya et al. (31) have suggested that E. coli has such an activity, but it has yet to be identified with a specific gene. In Bacillus species and other gram-positive organisms, Tet(L) and Tet(K) proteins can mediate electrogenic exchange of a tetracycline–divalent-metal complex or Na+ or K+ for external H+ or K+ (12, 15). The possibility that an H+ ion is involved in the Tet-mediated antiport even when K+ is also a coupling ion, as suggested here for GerN, has not yet been tested. It has been shown, however, that in Bacillus subtilis, the chromosomally encoded Tet(L) protein plays a physiological role in pH homeostasis, Na+ exclusion, and K+ acquisition as well as in antibiotic resistance (5, 32). Thus, there is precedent for an antiporter that is involved in a particular stress response, i.e., antibiotic stress, also having roles in meeting other physiological challenges.
ACKNOWLEDGMENTS
This work was supported by a BBSRC project grant to A.M., research grants GM28454 and GM52837 from the National Institute of General Medical Sciences to T.A.K., and a BBSRC studentship to T.W.S.
REFERENCES
- 1.Altschul S F, Madden T L, Schaffer A A, Zhang J H, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ambudkar S V, Zlotnick G U, Rosen B P. Calcium efflux from Escherichia coli: evidence for two systems. J Biol Chem. 1984;259:6142–6146. [PubMed] [Google Scholar]
- 3.Bakker E P, Booth I R, Dinnbier U, Epstein W, Gajewska A. Evidence for multiple potassium export systems in Escherichia coli. J Bacteriol. 1987;169:3743–3749. doi: 10.1128/jb.169.8.3743-3749.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Booth I R, Jones M A, McLaggan D, Nikolaev Y, Ness L S, Wood C M, Miller S, Totemeyer S, Ferguson G P. Bacterial ion channels. In: Konings W N, Kaback H R, Lolkema J S, editors. Handbook of biological physics. Vol. 2. Amsterdam, The Netherlands: Elsevier Science; 1996. pp. 693–729. [Google Scholar]
- 5.Cheng J, Guffanti A A, Wang W, Krulwich T A, Bechhofer D H. Chromosomal tetA(L) gene of Bacillus subtilis: regulation of expression and physiology of a tetA(L) deletion strain. J Bacteriol. 1996;178:2853–2860. doi: 10.1128/jb.178.10.2853-2860.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dover N, Higgins C, Carmel O, Rimon A, Pinner E, Padan E. Na+-induced transcription of nhaA, which encodes an Na+/H+ antiporter in Escherichia coli, is positively regulated by nhaR and affected by hns. J Bacteriol. 1996;178:6508–6517. doi: 10.1128/jb.178.22.6508-6517.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Elmore M J, Lamb A J, Ritchie G Y, Douglas R M, Munro A, Gajewska A, Booth I R. Activation of potassium efflux from Escherichia coli by glutathione metabolites. Mol Microbiol. 1990;4:405–412. doi: 10.1111/j.1365-2958.1990.tb00607.x. [DOI] [PubMed] [Google Scholar]
- 8.Epstein W, Buurman E, McLaggan D, Naprstek J. Multiple mechanisms, roles and controls of K+ transport in Escherichia coli. Biochem Soc Trans. 1993;21:1006–1010. doi: 10.1042/bst0211006. [DOI] [PubMed] [Google Scholar]
- 9.Epstein W, Kim B S. Potassium transport loci in Escherichia coli K-12. J Bacteriol. 1971;108:639–644. doi: 10.1128/jb.108.2.639-644.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Errington J. Bacillus subtilis sporulation: regulation of gene expression and control of morphogenesis. Microbiol Rev. 1993;57:1–33. doi: 10.1128/mr.57.1.1-33.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Goldberg E B, Arbel T, Chen J, Karpel R, Mackie G A, Schuldiner S, Padan E. Characterization of a Na+/H+ antiporter gene of Escherichia coli. Proc Natl Acad Sci USA. 1987;84:2615–2619. doi: 10.1073/pnas.84.9.2615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guffanti A A, Cheng J, Krulwich T A. Electrogenic antiport activities of the Gram-positive Tet proteins include a Na+(K+)/K+ mode that mediates net K+ uptake. J Biol Chem. 1998;273:26447–26454. doi: 10.1074/jbc.273.41.26447. [DOI] [PubMed] [Google Scholar]
- 13.Guffanti A A, Cheng J, Krulwich T A. Tetracycline/H+ antiport and Na+/H+ antiport catalyzed by Bacillus subtilis TetA(L) transporter expressed in Escherichia coli. J Bacteriol. 1995;177:4557–4561. doi: 10.1128/jb.177.15.4557-4561.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kaback H R. Bacterial membranes. Methods Enzymol. 1971;22:99–120. [Google Scholar]
- 15.Krulwich T A, Jin J, Guffanti A A, Bechhofer D H. Functions of tetracycline efflux proteins that do not involve tetracycline. J Mol Microbiol Biotechnol. 2001;3:237–246. [PubMed] [Google Scholar]
- 16.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 17.Magill N G, Cowan A E, Koppel D E, Setlow P. The internal pH of the forespore compartment of Bacillus megaterium decreases by about 1 pH unit during sporulation. J Bacteriol. 1994;176:2252–2258. doi: 10.1128/jb.176.8.2252-2258.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moir A, Kemp E H, Robinson C, Corfe B M. The genetic analysis of bacterial spore germination. J Appl Bacteriol. 1994;76:9S–16S. [PubMed] [Google Scholar]
- 19.Moir A, Smith D A. The genetics of bacterial spore germination. Annu Rev Microbiol. 1990;44:531–553. doi: 10.1146/annurev.mi.44.100190.002531. [DOI] [PubMed] [Google Scholar]
- 20.Nakamura C, Burgess J G, Sode K, Matsunaga T. An iron-regulated gene, magA, encoding an iron transport protein of Magnetospirillum sp. strain AMB-1. J Biol Chem. 1995;270:28392–28396. doi: 10.1074/jbc.270.47.28392. [DOI] [PubMed] [Google Scholar]
- 21.Nozaki K, Inaba K, Kuroda T, Tsuda M, Tsuchiya T. Cloning and sequencing of the gene for Na+/H+ antiporter of Vibrio parahaemolyticus. Biochem Biophys Res Commun. 1996;24:774–779. doi: 10.1006/bbrc.1996.0820. [DOI] [PubMed] [Google Scholar]
- 22.Rosen B P. Ion extrusion systems in Escherichia coli. Methods Enzymol. 1986;125:328–336. doi: 10.1016/s0076-6879(86)25028-4. [DOI] [PubMed] [Google Scholar]
- 23.Saier M H, Eng B H, Fard S, Garg J, Haggerty D A, Hutchinson W J, Jack D L, Lai E C, Liu H J, Nusinew D P, Omar A M, Pao S A, Paulsen I T, Quan J A, Siwinski M, Tseng T-T, Wachi S, Young G B. Phylogenetic characterisation of novel transport protein families revealed by genome analyses. Biochim Biophys Acta. 1999;1422:1–56. doi: 10.1016/s0304-4157(98)00023-9. [DOI] [PubMed] [Google Scholar]
- 24.Setlow B, Setlow P. Measurement of the pH within dormant and germinated bacterial spores. Proc Natl Acad Sci USA. 1980;77:2474–2476. doi: 10.1073/pnas.77.5.2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sonenshein A L. Bacterial sporulation: a response to environmental signals. In: Storz G, Hengge-Aronis R, editors. Bacterial stress responses. Washington, D.C.: ASM Press; 2000. pp. 199–215. [Google Scholar]
- 26.Stragier P, Losick R. Molecular genetics of sporulation in Bacillus subtilis. Annu Rev Genet. 1996;30:297–341. doi: 10.1146/annurev.genet.30.1.297. [DOI] [PubMed] [Google Scholar]
- 27.Swerdlow B M, Setlow B, Setlow P. Levels of H+ and other monovalent cations in dormant and germinating spores of Bacillus megaterium. J Bacteriol. 1981;148:20–29. doi: 10.1128/jb.148.1.20-29.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taglicht D, Padan E, Schuldiner S. Overproduction and purification of a functional Na+/H+ antiporter coded by nhaA (ant) from Escherichia coli. J Biol Chem. 1991;266:11289–11294. [PubMed] [Google Scholar]
- 29.Tani K, Watanabe T, Matsuda H, Nasu M, Kondo M. Cloning and sequencing of the spore germination gene of Bacillus megaterium ATCC 12872: similarities to the NaH-antiporter gene of Enterococcus hirae. Microbiol Immunol. 1996;40:99–105. doi: 10.1111/j.1348-0421.1996.tb03323.x. [DOI] [PubMed] [Google Scholar]
- 30.Thackray P D, Behravan J, Southworth T W, Moir A. GerN, an antiporter homologue important in germination of Bacillus cereus endospores. J Bacteriol. 2001;183:476–482. doi: 10.1128/JB.183.2.476-482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Verkhovskaya M L, Verkhovsky M I, Wikstrom M. K+-dependent Na+ transport driven by respiration in Escherichia coli cells and membrane vesicles. Biochim Biophys Acta. 1996;1273:207–216. doi: 10.1016/0005-2728(95)00142-5. [DOI] [PubMed] [Google Scholar]
- 32.Wang W, Guffanti A A, Wei Y, Ito M, Krulwich T A. Two types of Bacillus subtilis tetA(L) deletion strains reveal the physiological importance of TetA(L) in K+ acquisition as well as in Na+, alkali, and tetracycline resistance. J Bacteriol. 2000;182:2088–2095. doi: 10.1128/jb.182.8.2088-2095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Waser M, Hess-Beinz D, Davies K, Solioz M. Cloning and disruption of a putative NaH-antiporter gene of Enterococcus hirae. J Biol Chem. 1992;267:5396–5400. [PubMed] [Google Scholar]
- 34.Zaslavsky D, Gennis R B. Proton pumping by cytochrome oxidase: progress, problems and postulates. Biochim Biophys Acta. 2000;1458:164–179. doi: 10.1016/s0005-2728(00)00066-9. [DOI] [PubMed] [Google Scholar]