Abstract
A key step in the regulation of heat shock genes in Escherichia coli is the stress-dependent degradation of the heat shock promoter-specific ς32 subunit of RNA polymerase by the AAA protease, FtsH. Previous studies implicated the C termini of protein substrates, including ς32, as degradation signals for AAA proteases. We investigated the role of the C terminus of ς32 in FtsH-dependent degradation by analysis of C-terminally truncated ς32 mutant proteins. Deletion of the 5, 11, 15, and 21 C-terminal residues of ς32 did not affect degradation in vivo or in vitro. Furthermore, a peptide comprising the C-terminal 21 residues of ς32 was not degraded by FtsH in vitro and thus did not serve as a recognition sequence for the protease, while an unrelated peptide of similar length was efficiently degraded. The truncated ς32 mutant proteins remained capable of associating with DnaK and DnaJ in vitro but showed intermediate (5-amino-acid deletion) and strong (11-, 15-, and 21-amino-acid deletions) defects in association with RNA polymerase in vitro and biological activity in vivo. These results indicate an important role for the C terminus of ς32 in RNA polymerase binding but no essential role for FtsH-dependent degradation and association of chaperones.
The expression of heat shock genes in Escherichia coli is positively controlled at the transcriptional level by the product of the rpoH gene, the ς32 subunit of RNA polymerase (RNAP) (5, 8, 30). Stress treatment of the cells, such as a sudden temperature upshift, induces transient heat shock gene expression until the cells have adapted to the applied stress. This heat shock response is mediated by increases in the translation of rpoH, stabilization of ς32, and activation of ς32 by sequestration of the DnaK chaperone and its DnaJ cochaperone from a complex with ς32.
Under steady-state growth conditions, ς32 is an extremely unstable protein with half-lives at 30 and 42°C of less than 1 and 4 min, respectively (17, 23, 26). Heat shock treatment of the cells by transfer from 30 to 42°C increases the half-life of ς32 transiently to approximately 10 min (23). Degradation is mediated mainly by FtsH (HflB), an ATP-dependent metalloprotease associated with the inner membrane (10, 25, 27, 28). The interaction of ς32 with the RNAP core enzyme prevents degradation, indicating that FtsH and RNAP compete for binding to ς32 (28). The DnaK chaperone system plays an active but mechanistically unclear role in ς32 degradation, since ς32 is stabilized in dnaK and dnaJ mutant backgrounds (22, 24, 25, 28).
A conceptually interesting question with respect to ς32 degradation is how this biologically active, and hence seemingly folded, protein can be subject to such efficient proteolysis. Either ς32 carries specific degradation signals within its polypeptide, or it is thermodynamically unstable despite its biological activity. Earlier work showed that the in vivo half-life of fusions between N-terminal fragments of ς32 and β-galactosidase increased when a stretch of 23 residues (R122 to Q144), located between conserved regions 2 and 3 of ς32 and termed region C, is deleted or replaced by another reading frame (17). However, subsequent analysis of ς32 mutants altered in region C showed that this region is not essential for degradation but instead plays a role in ς32 binding to RNAP (1). Another study identified a role for the C terminus of ς32 in degradation by FtsH (3). In this previous study, rpoH genes with truncated 3′ ends were cloned into an expression vector such that fusion proteins between C-terminally truncated ς32 proteins (by 20 residues) and six amino acids from the vector sequence were generated. These fusion proteins were more stable than wild-type ς32 in E. coli cells and in vitro. However, this approach did not exclude the possibility that the vector-encoded extra residues at the C terminus artificially stabilize the fusion proteins.
In this study we investigated further the role of the C terminus of ς32 in the degradation process. Our analysis of C-terminally truncated ς32 proteins shows that the authentic C-terminal residues are not essential for degradation.
MATERIALS AND METHODS
Strains, plasmids, and media.
Cells of strains C600 (thr-1 leuB6 thi-1 lacY supE44 rfbD1 fhuA21) and BB2019 [GW1000 recA441 sulA11 Δ(argF-lac)U169 supC(Ts) rpoH165(Am) pDMI,1] (6) were grown aerobically at 30 or 42°C in Luria broth (LB) or in M9 minimal medium supplemented with glucose (0.2%; M9-Glu), thiamine (20 μg/ml), and appropriate amino acids (50 μg/ml). Growth media were further supplemented with isopropyl-β-d-thiogalactopyranoside (IPTG; 1 mM), kanamycin (20 μg/ml), and ampicillin (50 μg/ml) when required. The wild-type rpoH gene cloned into plasmid pUHE21-2fdΔ12 (7) was used as template for construction of 3′-truncated rpoH alleles. These alleles (rpoH-Δ5aa, rpoH-Δ11aa, rpoH-Δ15aa, and rpoH-Δ21aa) were generated by PCR using appropriate oligonucleotides with HindIII and PstI restriction sites for cloning of the coding sequences into pUHE21-2fdΔ12. For production of hexahistidine-tagged ς32 variants, wild-type and mutant alleles of rpoH were subcloned by inserting the HindIII-PstI fragments into pUHE212-1 (to encode N-His6-ς32). For immunoblotting and pulse-chase experiments, the rpoH alleles encoding wild-type ς32, N-His6-ς32, C-His6-ς32 (7), and C-terminally truncated ς32 proteins were subcloned by inserting the respective EcoRI-HindIII fragments into pFN476 (24). Plasmid pDMI,1 carrying lacIq and encoding kanamycin resistance (14) was used to provide cells with a Lac repressor.
Overproduction of ς32 proteins and ex vivo degradation assay.
C600 cells carrying rpoH expression plasmids and pDMI,1 were grown at 30°C in 20 ml of LB containing ampicillin and kanamycin. IPTG (1 mM final concentration) was added to exponential-phase cultures (optical density at 600 nm [OD600], 0.5 to 0.7) for 1 h, followed by harvesting and washing of the cells in buffer A (50 mM Tris-acetate [pH 8.0], 5 mM magnesium acetate, 2 mM β-mercaptoethanol, 50 mM KCl, 5% glycerol). The cell pellet was dissolved (OD600, 50) in buffer A containing 1 mM phenylmethylsulfonyl fluoride and sonicated. The lysate was transferred to Eppendorf tubes and centrifuged at 2,500 × g for 2 min, and the supernatant was subjected to ultracentrifugation at 100,000 × g for 2 h. The protein content of the soluble cytoplasmic fraction was quantified by Bradford assay using bovine serum albumin as a standard. A 5-μg portion of the cytoplasmic fraction was mixed with FtsH reaction buffer (50 mM Tris-acetate [pH 8.0], 5 mM magnesium acetate, 2 mM β-mercaptoethanol, 50 mM KCl) to reach a final volume of 15 μl. Degradation assays were started by adding 1 μl of 100 mM ATP and 4 μl of 10 μM FtsH activated by the addition of Zn2+ (27) or buffer without FtsH as a control and incubated at 42°C for 1 h.
In vitro degradation of ς32.
Wild-type and C-terminally truncated N-His6-ς32 proteins were purified and radiolabeled with N-succinimidyl[2,3-3H]propionate (Amersham) as previously described (7, 28). These proteins (1 μM each) were incubated with purified FtsH (2 μM) and tested for degradation as described previously (1, 28). After precipitation with trichloroacetic acid (TCA; 10%) followed by centrifugation (15,000 rpm, 3 min), the radioactive peptides generated by proteolysis were quantified in the supernatant by liquid scintillation counting. For assaying degradation of peptides, the final volume of the reaction was 60 μl. The ς32-derived peptides Q132-Q151-C (QRKLFFNLRKTKQRLGWFNQC) and A264-A284-C (AERVRQLEKNAMKKLRAAIEAC) (50 μM each) were mixed and incubated with FtsH (1). At various time points, aliquots of 18 μl were mixed with 92 μl of 0.5% trifluoroacetic acid to stop the reaction. Products were analyzed by reverse-phase chromatography using a 5-to-80% acetonitrile gradient in 0.1% trifluoroacetic acid.
Analysis of protein interactions.
DnaK and RNAP core enzyme were purified as described previously (4, 15). Association of N-His6-ς32 or C-terminally truncated N-His6-ς32 with DnaK, DnaJ, and RNAP core was determined by gel filtration using a Superdex 200 column essentially as described previously (1, 7). To determine association of ς32 with RNAP core, N-His6-ς32 (1 μM) was incubated with RNAP core (1.5 μM) for 10 min at 30°C in transcription buffer (20 μl, final volume). To determine association of ς32 with DnaK, DnaK (5 μM) was incubated for 2 h at 30°C in transcription buffer to disfavor oligomerization, mixed with N-His6-ς32 (1 μM) in a final volume of 20 μl, and further incubated for 30 min at 30°C. These mixtures were placed on ice, adjusted to 100 μl by addition of transcription buffer, and loaded on a Superdex 200 column at 4°C. Labeled N-His6-ς32 was detected in the elution fractions by liquid scintillation counting.
In vivo stability of ς32.
For determination of ς32 stability in vivo, C600 cells carrying rpoH expression plasmids were grown at 30°C in M9-Glu until mid-log phase. One milliliter of culture was labeled for 1 min with 70 μCi of [35S]methionine followed by chase with unlabeled methionine (200 μg/ml, final concentration). After 30 s (time for synthesis of ς32), aliquots of 200 μl were collected at various times, mixed with TCA (10%, vol/vol), and incubated for 15 min on ice. After centrifugation for 15 min at 14,000 rpm, the pellets were washed with acetone and resuspended in 50 mM Tris-HCl (pH 8.0), 1% sodium dodecyl sulfate (SDS), and 1 mM EDTA. Samples were subjected to immunoprecipitation using ς32-specific rabbit antiserum as described previously (25, 29).
SDS-PAGE, immunoblotting, and quantifications.
Polyacrylamide gel electrophoresis (PAGE) was carried out as described by Laemmli (13) using SDS–12% polyacrylamide gels and staining with Coomassie brilliant blue. Immunoblotting was carried out according to standard procedures, using rabbit antisera specific for the relevant proteins as primary antibodies, and blots were developed with a Vistra ECF fluorescence Western blotting kit (Amersham) or 5-bromo-4-chloro-3-indolylphosphate–nitroblue tetrazolium color detection using alkaline phosphatase-conjugated anti-rabbit immunoglobulin G as the secondary antibody (Vector Laboratories, Inc.). Stained gels and developed immunoblots were scanned using a fluoroimager (FLA-2000) and quantified using MacBAS software (Fuji Film Co.).
RESULTS
Construction of truncated ς32 mutant proteins.
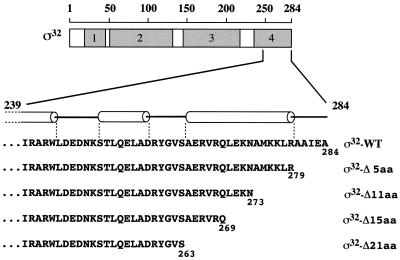
To investigate whether C-terminal residues of ς32 constitute a destabilizing element, we generated ς32 mutant proteins with truncations of their C termini by 5, 11, 15, and 21 residues (ς32-Δ5aa, ς32-Δ11aa, ς32-Δ15aa, and ς32-Δ21aa) (Fig. 1). The truncations were chosen such that the new termini are at two positions within (ς32-Δ11aa and ς32-Δ15aa), N-terminal to (ς32-Δ21aa), or C-terminal to (ς32-Δ5aa) an α-helix predicted by a secondary-structure-predicting algorithm (Fig. 1). PCR fragments of the appropriately truncated rpoH genes were cloned into the expression vector pUHE21-2fdΔ12 (7) such that a stop codon immediately follows the last codon, thus ensuring that no vector-encoded amino acids are fused to the ς32 mutant proteins. The C termini of the truncated ς32 mutant proteins do not resemble the consensus sequence of destabilizing C termini, as described by Sauer and coworkers (21), are not particularly hydrophobic, and do not constitute predicted DnaK binding sites (19).
FIG. 1.
Mutational alteration of ς32. The locations of conserved regions 1 to 4 and details of the C-terminal region and its predicted secondary structure (using the PhD program) are shown. α-helices are shown as cylinders. The end points of the C-terminal truncations of the ς32 mutant proteins are indicated.
In vivo activity of truncated ς32 mutant proteins.
To determine the in vivo activity of the truncated ς32 mutant proteins, we first tested the ability of pUHE21-2fdΔ12-borne rpoH mutant alleles to complement the temperature-sensitive growth of rpoH165(Am) mutant cells on LB agar plates. The rpoH165(Am) mutant cells carry a temperature-sensitive amber suppressor mutation which is active and supports growth at 30°C. Only the rpoH-Δ5aa mutant allele allowed complementation of growth at 42°C to an extent similar to that allowed by wild-type rpoH (data not shown). Even in the absence of IPTG, a condition in which read-through expression of the rpoH mutant alleles produced ς32 levels only approximately fivefold above wild-type levels, ς32-Δ5aa complemented the growth defects of rpoH(Am) mutants at 42°C (data not shown).
We then determined the cellular levels of the ς32 mutant proteins after IPTG-induced overproduction in rpoH165(Am) mutant cells. We observed that wild-type ς32 was poorly overproduced, while ς32-Δ5aa was overproduced to intermediate levels and ς32-Δ11aa, ς32-Δ15aa, and ς32-Δ21aa were overproduced to high levels (Fig. 2).
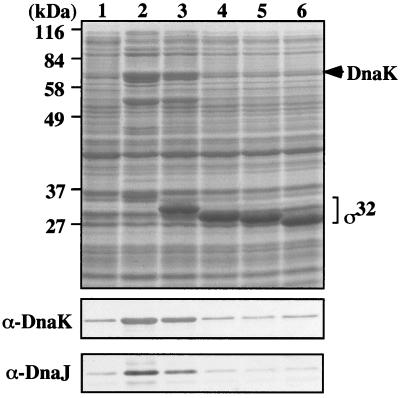
FIG. 2.
In vivo activity of C-terminally truncated ς32 proteins. Cells of strain BB2019 [rpoH165(Am)] were transformed either with pUHE212-1 expressing wild-type or 3′-truncated rpoH alleles (producing N-His6-ς32 proteins) or with pUHE212-1 control vector. Cultures of these cells were induced with IPTG, followed by SDS-PAGE of equal amounts of total protein and staining of the gels with Coomassie brilliant blue (top) or immunoblotting using DnaK- and DnaJ-specific sera (bottom). Lanes: 1, vector control; 2, N-His6-ς32; 3, N-His6-ς32-Δ5aa; 4, N-His6-ς32-Δ11aa; 5, N-His6-ς32-Δ15aa; 6, N-His6-ς32-Δ21aa.
Overproduction of wild-type ς32 led to strong increases in DnaK and DnaJ levels, overproduction of ς32-Δ5aa led to intermediate increases, and overproduction of the other ς32 mutant proteins did not lead to increases in DnaK or DnaJ levels. The changes in DnaK and DnaJ levels were the result of the biological activity of ς32 in transcribing heat shock genes, including dnaK and dnaJ, and of the loss of this activity in the case of the ς32 mutant proteins with C-terminal truncations. Thus, through its ability to promote production of heat shock proteins, including FtsH and DnaK, the active ς32 triggers its own degradation and hence prevents stronger overproduction. Together, these results indicate that deletion of the ultimate C-terminal five residues (280 to 284) do not lead to complete loss of activity, whereas the segment between residues 264 and 279 is essential for the activity of ς32.
In vivo stability of truncated ς32 mutant proteins.
We determined the in vivo half-lives of two of the C-terminally truncated ς32 mutant proteins (ς32-Δ11aa and ς32-Δ15aa) by pulse-chase experiments followed by immunoprecipitation of ς32. Such experiments were complicated by our finding that most of the C-terminally truncated ς32 mutant proteins lack activity in vivo. Consequently, the synthesis of heat shock proteins is lower in cells producing the inactive mutant proteins from plasmids than in cells producing wild-type ς32 from plasmids (Fig. 2). These heat shock proteins include DnaK and DnaJ, which are limiting for degradation of ς32 (24, 25, 28). In the cells expressing the inactive ς32 mutant proteins, ς32 should be less subject to chaperone-mediated degradation. When the ς32 proteins are overexpressed, the efficiency by which ς32 mutant proteins are degraded in vivo will therefore be influenced by their biological activity. This complication led us to perform the ς32 half-life determinations in cells of strain C600 expressing low levels of plasmid-encoded ς32 proteins. Cells were transformed with plasmids expressing wild-type or mutant rpoH alleles under transcriptional control of a promoter specific for T7 polymerase. Since C600 cells lack T7 polymerase, only very little read-through from the T7 promoter occurs, just enough to produce approximately wild-type levels of ς32 (Fig. 3). This fact allowed us to perform the half-life determinations of the ς32 proteins under almost physiological conditions.
FIG. 3.
Cellular levels of plasmid-encoded ς32 mutant proteins. Cells of strain C600 transformed with plasmid pFN476 expressing the appropriate rpoH allele were grown at 30°C in LB medium. Aliquots of exponential-phase cultures (OD600, 0.5 to 0.7) were collected, and equal amounts of total protein were analyzed by SDS-PAGE. Levels of ς32 were determined by immunoblotting.
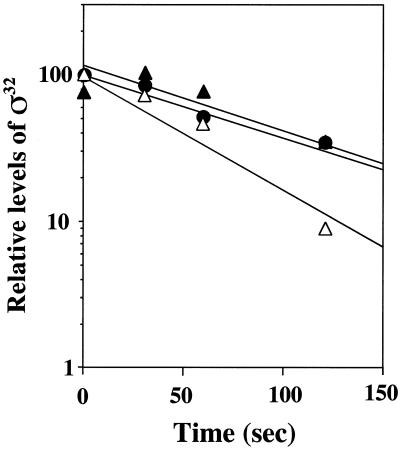
Only minor differences existed between the half-life of wild-type ς32 (approximately 60 to 100 s) and those of ς32-Δ11aa and ς32-Δ15aa (Fig. 4). This result indicates that the C-terminal truncations do not affect significantly the half-life of ς32 in vivo provided that the levels of DnaK and DnaJ are kept constant.
FIG. 4.
In vivo stability of ς32 mutant proteins at 30°C. Cells of strain C600 which harbor pFN476 expressing rpoH-Δ11aa, rpoH-Δ15aa, or wild-type rpoH were grown at 30°C, pulse-labeled with [35S]methionine, and chased with unlabeled methionine. Aliquots were taken at the indicated times, followed by immunoprecipitation of ς32 and quantification of the precipitated proteins relative to the largest value. Closed circles, wild-type ς32; closed triangles, ς32-Δ11aa; open triangles, ς32-Δ15aa.
In vitro degradation of truncated ς32 mutant proteins by FtsH.
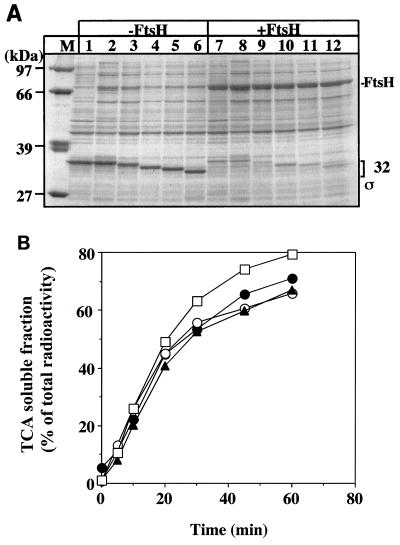
We investigated the susceptibility of N-His6-tagged wild-type and C-terminally truncated ς32 mutant proteins to degradation by FtsH in vitro. These degradation assays were performed only at 42°C, since FtsH is known to be poorly active at 30°C when tested in vitro in the presence of detergent (12). We first determined whether exogenously added FtsH is capable of degrading the ς32 proteins present in extracts of C600 cells after IPTG-induced overproduction. The rationale for this experiment was that for these ex vivo degradation assays we could use authentic ς32 mutant proteins without histidine tags, ruling out any contribution by the tag. All truncated and wild-type ς32 proteins were degraded by FtsH in presence of ATP to similar extents (Fig. 5A).
FIG. 5.
(A) FtsH-dependent degradation of overproduced ς32 in cytoplasmic fractions. Cells of strain C600 which harbor plasmid pUHE21-2fdΔ12 expressing wild-type or truncated rpoH alleles mutant ς32 or the control plasmid were grown at 30°C with or without (lanes 1 and 7) IPTG. The extracts of cells grown without IPTG served as a control to ensure that the endogenous chaperones, whose cellular concentrations increase upon IPTG-induced production of plasmid-encoded wild-type ς32, do not interfere with the degradation of ς32 in the extracts. Cytosolic extracts were prepared and assayed for degradation by exogenously added FtsH. Lane M, molecular weight marker. All other lanes show extracts of cells producing plasmid-encoded wild-type ς32 supplemented with 2 μM purified wild-type ς32 (since wild-type ς32 could not be overproduced to large amounts) (lanes 1, 2, 7, and 8) or plasmid-encoded mutants ς32-Δ5aa (lanes 3 and 9), ς32-Δ11aa (lanes 4 and 10), ς32-Δ15aa (lane 5 and 11), and ς32-Δ21aa (lanes 6 and 12). The extracts were incubated with (+FtsH) or without (−FtsH) added protease. (B) In vitro degradation of ς32 mutant proteins by FtsH. 3H-labeled N-His6-ς32 proteins were incubated with FtsH in the presence of 5 mM ATP, followed by TCA precipitation at the indicated times. The curves represent the percentage of radioactivity in the supernatants which contain the proteolytic fragments. Closed circles, N-His-ς32; open circles, N-His6-ς32-Δ5aa; closed triangles, N-His6-ς32-Δ11aa; open squares, N-His6-ς32-Δ21aa.
We then determined with purified components the efficiency by which FtsH degrades the N-His6-tagged ς32 mutant proteins. All ς32 proteins were soluble after overproduction in E. coli cells and had wild type-like elution profiles during nickel-nitrilotriacetic acid and ion-exchange chromatography. Furthermore, they were indistinguishable from wild-type protein with respect to the proteolysis pattern obtained by partial proteinase K and trypsin digestion (data not shown). Therefore, there is no indication of changes in their overall tertiary structures. All C-terminally truncated ς32 mutant proteins were degraded by FtsH in the presence of ATP, with kinetics similar to that of wild-type ς32 (Fig. 5B). Thus, the C-terminal truncations did not affect the efficiency of ς32 degradation by FtsH in vitro.
C-terminal residues of ς32 do not constitute a substrate for FtsH.
As an independent experimental approach to investigate the possibility that the C-terminal residues of ς32 constitute a destabilizing element for FtsH-dependent degradation, we tested whether the C-terminal sequences themselves provide a substrate motif for FtsH. We designed a peptide comprising the C-terminal 21 residues of ς32 and an additional cysteine at the C-terminal end (AERVRQLEKNAMKKLRAAIEAC) and tested whether it is a substrate for FtsH. The length of this 22-mer peptide was well above the minimal length of approximately 15 residues required for FtsH to degrade peptides (T. Tomoyasu and B. Bukau, unpublished results). As a positive control, we included a peptide of the same length and carrying a cysteine at the C terminus, derived from region C of ς32 (QRKLFFNLRKTKQRLGWFNQC). This peptide has been shown previously to be a good substrate peptide for FtsH (1).
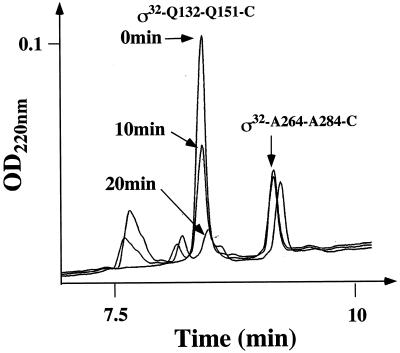
In one experiment, both peptides were mixed at a 1:1 molar ratio and incubated with FtsH and ATP. Aliquots taken at 0, 10, and 20 min were analyzed for peptide degradation by high-pressure liquid chromatography. While the region C peptide was efficiently degraded with a half time of approximately 10 min, the C-terminal peptide was stable within the duration of the experiment (Fig. 6). This result was verified in experiments in which each peptide was tested individually for degradation by FtsH (data not shown). These results show that at the peptide level, the 22 residues of the C terminus of ς32 do not provide a recognition site for degradation by FtsH.
FIG. 6.
FtsH-mediated degradation of peptides derived from ς32. Peptides from region C (ς32-Q132-Q151-C) and the C terminus (ς32-A264-A284-C) of ς32 were mixed and incubated with FtsH. Degradation of the peptides at various times after mixing is shown as high-pressure liquid chromatography–reverse-phase chromatography data.
DnaK and DnaJ bind to truncated ς32 mutant proteins in vitro.
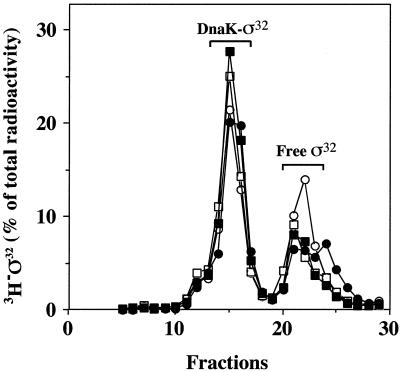
We tested whether DnaK binding to ς32 is impaired by the C-terminal truncations. 3H-labeled wild-type ς32 and mutant N-His6-ς32 were incubated with DnaK followed by gel filtration to separate DnaK-ς32 complexes from free ς32 (Fig. 7). Under the conditions used, approximately 75% of wild-type [3H]ς32 was recovered in complex with DnaK (eluting in fractions 12 to 17). The 3H-labeled C-terminally truncated N-His6-ς32 mutant proteins (ς32-Δ5aa, ς32-Δ11aa, and ς32-Δ21aa) showed similar efficiencies of complex formation. Earlier work established that the interaction of DnaK with ς32 does not occur through the histidine tag (6). Furthermore, no defects in chaperone binding were observed when the N-His6-ς32 mutant proteins were incubated with DnaK together with DnaJ in the presence of ATP (data not shown). These data indicate that the authentic C terminus of ς32 is not essential for interaction with DnaK and DnaJ.
FIG. 7.
Binding of ς32 mutant proteins to DnaK. 3H-labeled N-His6-ς32, N-His6-ς32-Δ5aa, N-His6-ς32-Δ11aa, and N-His6-ς32-Δ21aa were incubated with DnaK, followed by gel filtration of the reaction product. Labeled protein was quantified in the elution fractions. Open circles, wild-type ς32; closed circles, ς32-Δ5aa; open squares, ς32-Δ11aa; closed squares, ς32-Δ21aa.
RNAP binding to truncated ς32 mutant proteins in vitro.
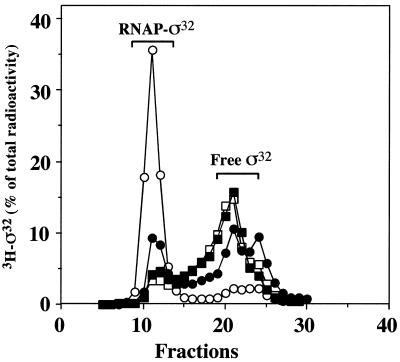
We considered the possibility that the truncated C-terminal segments of ς32 are involved in the interaction with the RNAP core enzyme. We determined the efficiency of association of 3H-labeled N-His6-ς32 with RNAP by gel filtration. The relative amounts of all truncated ς32 proteins (ς32-Δ5aa, ς32-Δ11aa, ς32-Δ15aa, and ς32-Δ21aa) recovered in association with RNAP (eluting in fractions 8 to 15) were lower than that of wild-type ς32, with ς32-Δ5aa showing the highest affinity for RNAP among all truncated proteins (Fig. 8). These results indicate a role for the C terminus of ς32 in the binding to RNAP.
FIG. 8.
Binding of ς32 mutant proteins to RNAP. 3H-labeled N-His6-ς32 proteins were incubated with RNAP, followed by gel filtration. The amount of labeled protein was quantified. Open circles, wild-type ς32; closed circles, ς32-Δ5aa; open squares, ς32-Δ11aa; closed squares, ς32-Δ21aa.
Involvement of N-terminal segments of ς32 in degradation by FtsH in vitro.
Given our finding that the C terminus of ς32 is not an essential degradation signal, we considered that perhaps N-terminal sequences play such a role. However, N-terminal sequences of ς32 are not amenable to mutational analysis, since the efficiency of translation of rpoH mRNA drops dramatically upon mutational alteration of the downstream box located at the 5′ end of the coding sequence (18).
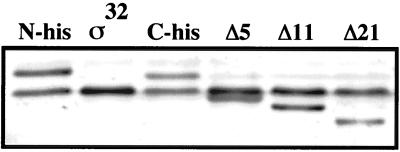
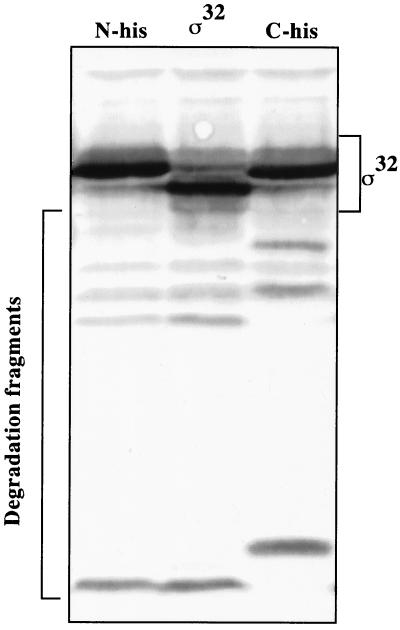
We therefore restricted our efforts to an analysis of fragments of authentic ς32, N-His6-ς32, and C-His6-ς32 which we observed to accumulate during FtsH-dependent proteolysis in vitro. Although these fragments may be dead-end products, as they did not chase into smaller peptides, they may provide a tool for dissecting the degradation process. The immunologically detectable fragments, ranging from approximately 10 to 20 kDa, were identical for authentic ς32 and N-His6-ς32, which indicates that the N-terminal His6 extension of the N-His6-ς32 protein is missing in these fragments (Fig. 9). In contrast, the C-His6-ς32 fragments showed a similar pattern but had higher molecular weights than the ς32 and N-His6-ς32 fragments, which indicates that the C-terminal extension of the C-His6-ς32 protein was still attached. Together these findings suggest that the degradation of ς32 proceeds through cuts in the N-terminal segment of ς32, although they do not indicate whether the N terminus of ς32 is involved in the recognition by FtsH.
FIG. 9.
Determination of in vivo degradation intermediates of ς32. Cells of strain C600 which harbor plasmids expressing IPTG-regulated rpoH alleles encoding N-His-ς32, C-His-ς32, or authentic ς32 were grown at 30°C in LB medium. Aliquots of exponential-phase cultures (OD600, 0.5 to 0.7) were collected, and equal amounts of total protein were analyzed by SDS-PAGE. The degradation intermediates were determined by immunoblotting using ς32-specific antisera.
DISCUSSION
The aim of this study was to investigate the role of the C terminus of ς32 in the degradation by FtsH. C termini frequently constitute the degradation determinants of naturally unstable proteins, which target them to proteolysis by AAA proteases (9, 21). Such a role has been also proposed for the C terminus of ς32 on the basis of an analysis of C-terminal variants of ς32 (3).
We show here that a series of C-terminally truncated ς32 proteins are as unstable as wild-type ς32 in vivo, in cell extracts supplemented with FtsH and ATP, and in a purified ATP-dependent degradation system with FtsH and ς32 as the sole protein components. Furthermore, we show that the C-terminal truncations do not compromise the ability of ς32 to associate with DnaK in vitro. The C-terminal truncations were chosen such that the local predicted secondary structures are considered and that the novel C termini do not constitute predicted DnaK binding sites (16, 19) and do not resemble the consensus sequence of protease targeting sites (9). Taken together, our results do not provide any evidence for an essential role of C-terminal sequences of ς32 in the FtsH-dependent degradation process or in chaperone binding. Our findings agree well with the results of a recent study of truncated ς32 proteins and hybrids of stable Bradyrhizobium japonicum and unstable E. coli proteins (2). That study mapped a region of 85 residues, located between residues 36 to 122 of ς32, as being responsible for degradation of ς32.
We do not know the reason for the discrepancy with a previous study claiming an essential role for the C terminus of ς32 in degradation (3). It is possible that the additional six residues encoded by the vector which were fused to the C-terminally truncated ς32 proteins in the former study generated fusion proteins which became stabilized for unknown reasons. Our results are, however, in agreement with the findings that (i) class I fusions between N-terminal segments of ς32 and β-galactosidase exhibit normal shutoff of the heat shock response and ς32 instability (17) and (ii) blocking the N terminus, but not the C terminus, of ς32 by fusion with green fluorescent protein resulted in stabilization of the fusion protein, indicating the importance of sequences at or near the N terminus of ς32 in its degradation by FtsH (T. Tatsuta and T. Ogura, unpublished results). At this point we cannot exclude an involvement of internal sequences of ς32 in degradation. Furthermore, our conclusion is supported by our finding that fragments of ς32 proteins which were generated by FtsH-dependent degradation in vitro lack N-terminal segments but not C-terminal segments of ς32. Although this finding should still be interpreted with caution, it suggests important roles for N-terminal segments of ς32 in the degradation process. Further genetic and biochemical dissection is required to identify such a role.
Our analysis provided evidence for a role of the C-terminal sequences of ς32 in association with RNAP. Only the mutant protein lacking five C-terminal residues remained partially proficient in binding to RNAP and in in vivo activity, whereas all mutant proteins with longer truncations showed no significant RNAP binding or in vivo activity. The molecular basis for this role of the C terminus of ς32 in the association with RNAP is unclear. However, on the basis of several criteria (solubility and wild type-like partial proteolysis pattern), the C-terminally truncated ς32 proteins are not perturbed in their overall structure. Thus, either local conformational changes induced by the truncations or the lack of C-terminal residues directly involved in the association process may account for the observed loss in affinity. In this respect it is interesting that many regions within the polypeptide chain of ς32, including the regulatory region C and the conserved regions 2.1, 2.2, 3, and 4, have been implicated in the binding of RNAP (1, 3, 11, 20, 31). Structural analysis seems essential to solve this obvious complexity of the interaction between ς32 and RNAP.
ACKNOWLEDGMENTS
We thank A. Schulze-Specking for excellent technical assistance, T. Tatsuta for construction of pFN476 derivatives, and D. Dougan and M. Mayer for review of the manuscript.
This work was support by grants from the Deutsche Forschungsgemeinschaft (Leibniz-Programm; SFB388) and the Fonds der Chemischen Industrie to B.B., a Marie Curie training grant from the EEC to F.A., and a grant from the Japan Society for the promotion of Science to T.O.
The first two authors contributed equally to this study.
REFERENCES
- 1.Arsène F, Tomoyasu T, Mogk A, Schirra C, Schulze-Specking A, Bukau B. Role of region C in regulation of the heat shock gene-specific sigma factor of Escherichia coli, ς32. J Bacteriol. 1999;181:3552–3561. doi: 10.1128/jb.181.11.3552-3561.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bertani D, Oppenheim A B, Narberhaus F. An internal region of the RpoH heat shock transcription factor is critical for rapid degradation by the FtsH protease. FEBS Lett. 2001;493:17–20. doi: 10.1016/s0014-5793(01)02266-9. [DOI] [PubMed] [Google Scholar]
- 3.Blaszczak A, Georgopoulos C, Liberek K. On the mechanism of FtsH-dependent degradation of the ς32 transcriptional regulator of Escherichia coli and the role of the DnaK chaperone machine. Mol Microbiol. 1999;31:157–166. doi: 10.1046/j.1365-2958.1999.01155.x. [DOI] [PubMed] [Google Scholar]
- 4.Buchberger A, Valencia A, McMacken R, Sander C, Bukau B. The chaperone function of DnaK requires the coupling of ATPase activity with substrate binding through residue E171. EMBO J. 1994;13:1687–1695. doi: 10.1002/j.1460-2075.1994.tb06433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bukau B. Regulation of the E. coli heat shock response. Mol Microbiol. 1993;9:671–680. doi: 10.1111/j.1365-2958.1993.tb01727.x. [DOI] [PubMed] [Google Scholar]
- 6.Gamer J, Bujard H, Bukau B. Physical interaction between heat shock proteins DnaK, DnaJ, GrpE and the bacterial heat shock transcription factor ς32. Cell. 1992;69:833–842. doi: 10.1016/0092-8674(92)90294-m. [DOI] [PubMed] [Google Scholar]
- 7.Gamer J, Multhaup G, Tomoyasu T, McCarty J S, Rüdiger S, Schönfeld H-J, Schirra C, Bujard H, Bukau B. A cycle of binding and release of the DnaK, DnaJ and GrpE chaperones regulates activity of the E. coli heat shock transcription factor ς32. EMBO J. 1996;15:607–617. [PMC free article] [PubMed] [Google Scholar]
- 8.Gross C A. Function and regulation of the heat shock proteins. In: Neidhardt F C, et al., editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Vol. 1. Washington, D.C.: ASM Press; 1996. pp. 1382–1399. [Google Scholar]
- 9.Herman C, Thévenet D, Boulox P, Walker G C, D'Ari R. Degradation of carboxy-terminal-tagged cytoplasmic proteins by the Escherichia coli protease HflB (FtsH) Genes Dev. 1998;12:1348–1355. doi: 10.1101/gad.12.9.1348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Herman C, Thévenet D, D'Ari R, Bouloc P. Degradation of sigma 32, the heat shock regulator in Escherichia coli, is governed by HflB. Proc Natl Acad Sci USA. 1995;92:3516–3520. doi: 10.1073/pnas.92.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Joo D M, Nolte A, Calendar R, Zhou Y N, Jin D J. Multiple regions on the Escherichia coli heat shock transcription factor ς32 determine core RNA polymerase binding specificity. J Bacteriol. 1998;180:1095–1102. doi: 10.1128/jb.180.5.1095-1102.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kanemori M, Yanagi H, Yura T. Marked instability of the ς32 heat shock transcription factor at high temperature. J Biol Chem. 1999;274:22002–22007. doi: 10.1074/jbc.274.31.22002. [DOI] [PubMed] [Google Scholar]
- 13.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 14.Lanzer M. Ph.D. thesis. Heidelberg, Germany: University of Heidelberg; 1988. [Google Scholar]
- 15.Lowe P A, Hager D A, Burgess R R. Purification and properties of the ς subunit of Escherichia coli DNA-dependent RNA polymerase. Biochemistry. 1978;18:1344–1352. doi: 10.1021/bi00574a034. [DOI] [PubMed] [Google Scholar]
- 16.McCarty J S, Rüdiger S, Schönfeld H-J, Schneider-Mergener J, Nakahigashi K, Yura T, Bukau B. Regulatory region C of the E. coli heat shock transcription factor, ς32, constitutes a DnaK binding site and is conserved among eubacteria. J Mol Biol. 1996;256:829–837. doi: 10.1006/jmbi.1996.0129. [DOI] [PubMed] [Google Scholar]
- 17.Nagai H, Yuzawa H, Kanemori M, Yura T. A distinct segment of the ς32 polypeptide is involved in DnaK-mediated negative control of the heat shock response in Escherichia coli. Proc Natl Acad Sci USA. 1994;91:10280–10284. doi: 10.1073/pnas.91.22.10280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagai H, Yuzawa H, Yura T. Regulation of the heat shock response in E coli: involvement of positive and negative cis-acting elements in translation control of ς32 synthesis. Biochimie. 1991;73:1473–1479. doi: 10.1016/0300-9084(91)90180-9. [DOI] [PubMed] [Google Scholar]
- 19.Rüdiger S, Germeroth L, Schneider-Mergener J, Bukau B. Substrate specificity of the DnaK chaperone determined by screening cellulose-bound peptide libraries. EMBO J. 1997;16:1501–1507. doi: 10.1093/emboj/16.7.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sharp M M, Chan C L, Lu C Z, Marr M T, Nechaev S, Merritt E W, Severinov K, Roberts J W, Gross C A. The interface of ς with core RNA polymerase is extensive, conserved, and functionally specialized. Gene Dev. 1999;13:3015–3026. doi: 10.1101/gad.13.22.3015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Smith C K, Baker T A, Sauer R T. Lon and Clp family proteases and chaperones share homologous substrate-recognition domains. Proc Natl Acad Sci USA. 1999;96:6678–6682. doi: 10.1073/pnas.96.12.6678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Straus D, Walter W, Gross C A. DnaK, DnaJ, and GrpE heat shock proteins negatively regulate heat shock gene expression by controlling the synthesis and stability of ς32. Genes Dev. 1990;4:2202–2209. doi: 10.1101/gad.4.12a.2202. [DOI] [PubMed] [Google Scholar]
- 23.Straus D B, Walter W A, Gross C A. The heat shock response of E. coli is regulated by changes in the concentration of ς32. Nature. 1987;329:348–350. doi: 10.1038/329348a0. [DOI] [PubMed] [Google Scholar]
- 24.Tatsuta T, Joo D M, Calendar R, Akiyama Y, Ogura T. Evidence for an active role of the DnaK chaperone system in the degradation of ς32. FEBS Lett. 2000;478:271–275. doi: 10.1016/s0014-5793(00)01869-x. [DOI] [PubMed] [Google Scholar]
- 25.Tatsuta T, Tomoyasu T, Bukau B, Kitagawa M, Mori H, Karata K, Ogura T. Heat shock regulation in the ftsH null mutant of Escherichia coli: dissection of stability and activity control mechanisms of sigma32 in vivo. Mol Microbiol. 1998;30:583–593. doi: 10.1046/j.1365-2958.1998.01091.x. [DOI] [PubMed] [Google Scholar]
- 26.Tilly K, Spence J, Georgopoulos C. Modulation of stability of the Escherichia coli heat shock regulatory factor ς32. J Bacteriol. 1989;171:1585–1589. doi: 10.1128/jb.171.3.1585-1589.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tomoyasu T, Gamer J, Bukau B, Kanemori M, Mori H, Rutman A J, Oppenheim A B, Yura T, Yamanaka K, Niki H, Hiraga S, Ogura T. Escherichia coli FtsH is a membrane-bound, ATP-dependent protease which degrades the heat shock transcription factor ς32. EMBO J. 1995;14:2551–2560. doi: 10.1002/j.1460-2075.1995.tb07253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tomoyasu T, Ogura T, Tatsuta T, Bukau B. Levels of DnaK and DnaJ provide tight control of heat shock gene expression and protein repair in E. coli. Mol Microbiol. 1998;30:567–581. doi: 10.1046/j.1365-2958.1998.01090.x. [DOI] [PubMed] [Google Scholar]
- 29.Yano R, Nagai H, Shiba K, Yura T. A mutation that enhances synthesis of sigma 32 and suppresses temperature-sensitive growth of the rpoH15 mutant of Escherichia coli. J Bacteriol. 1990;172:2124–2130. doi: 10.1128/jb.172.4.2124-2130.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Yura T, Nakahigashi K. Regulation of the heat-shock response. Curr Opin Microbiol. 1999;2:153–158. doi: 10.1016/S1369-5274(99)80027-7. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Y N, Walter W A, Gross C A. A mutant sigma 32 with a small deletion in conserved region 3 of sigma has reduced affinity for core RNA polymerase. J Bacteriol. 1992;174:5005–5012. doi: 10.1128/jb.174.15.5005-5012.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]