Abstract
Antioxidant small molecules can prevent or delay the oxidative damage caused by free radicals. Herein, a structure-based hybridization of two natural antioxidants (caffeic acid and melatonin) afforded a novel hybrid series of indole-based amide analogues which was synthesized with potential antioxidant properties. A multiple-step scheme of in vitro radical scavenging assays was carried out to evaluate the antioxidant activity of the synthesized compounds. The results of the DPPH assay demonstrated that the indole-based caffeic acid amides are more active free radical scavenging agents than their benzamide analogues. Compared to Trolox, a water-soluble analogue of vitamin E, compounds 3a, 3f, 3h, 3j, and 3m were found to have excellent DPPH radical scavenging activities with IC50 values of 95.81 ± 1.01, 136.8 ± 1.04, 86.77 ± 1.03, 50.98 ± 1.05, and 67.64 ± 1.02 µM. Three compounds out of five (3f, 3j, and 3m) showed a higher capacity to neutralize the radical cation ABTS•+ more than Trolox with IC50 values of 14.48 ± 0.68, 19.49 ± 0.54, and 14.92 ± 0.30 µM, respectively. Compound 3j presented the highest antioxidant activity with a FRAP value of 4774.37 ± 137.20 μM Trolox eq/mM sample. In a similar way to the FRAP assay, the best antioxidant activity against the peroxyl radicals was demonstrated by compound 3j (10,714.21 ± 817.76 μM Trolox eq/mM sample). Taken together, compound 3j was validated as a lead hybrid molecule that could be optimized to maximize its antioxidant potency for the treatment of oxidative stress-related diseases.
Keywords: bioactive molecules, oxidative stress, antioxidant activity, indole, caffeic acid, DPPH, ABTS, ORAC, FRAP, spectroscopic characterization
1. Introduction
Since the majority of our biological activities only take place in the presence of oxygen, it is necessary for life. However, the oxidation reaction may lead to cell damage, leading to the degradation of various oxygen substrates, proteins, lipids, and DNA. This may cause numerous diseases including inflammation, obesity, diabetes, arthritis, etc. [1,2]. The oxygen paradox contributes to the generation of free radicals which possess a single electron on an oxygen or nitrogen atom, known as reactive oxygen species (ROS) or reactive nitrogen species (RNS) [3,4]. Free radicals are highly unstable. They could be valuable when they are involved in physiological functions or harmful if there is no balance between the defense systems and ROS/RNS, or when the organism is incapable of restricting the destruction triggered by the free radicals, which is known by the oxidative stress [5].
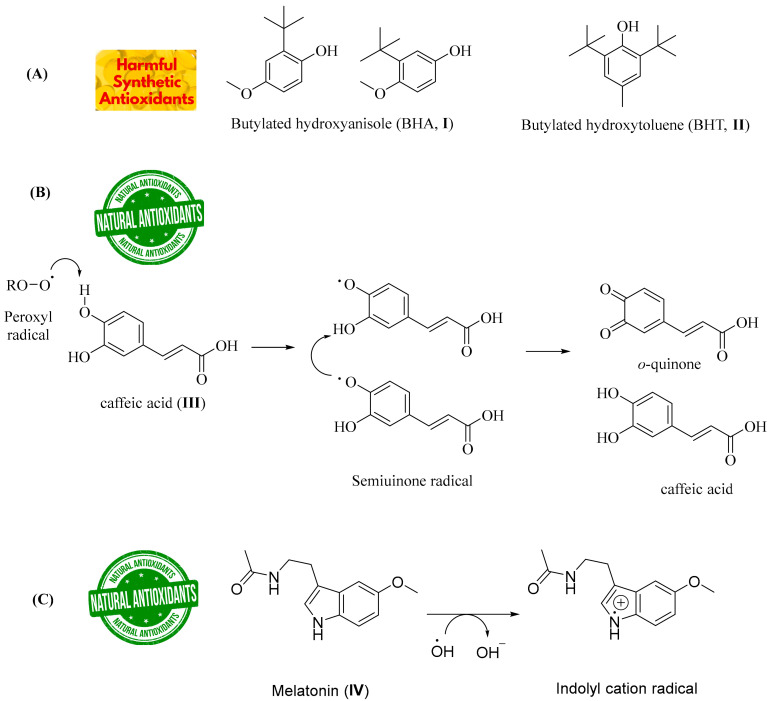
Antioxidants are compounds able to inhibit or postpone the oxidation process via neutralizing free radicals. Some synthetic antioxidants including butylated hydroxyanisole (BHA; I, Figure 1A) and butylated hydroxytoluene (BHT; II, Figure 1A) have recently been reported to be toxic to the environment and the human health [6,7]. Recently, numerous natural antioxidants’ effectiveness has been reported [8]. Antioxidants, whether they are produced naturally or artificially, reduce the severity of oxidative damage via scavenging ROS or stopping ROS-mediated chain reactions. As a result, finding naturally occurring compounds with antioxidant activity followed by hybridizing these natural antioxidant chemical scaffolds becomes a significant scientific issue with several socioeconomic interests. Despite being found in nature with many health advantages [9], the research being done on hybridizing essential antioxidant pharmacophores is still very important today [10,11,12,13,14].
Figure 1.
(A) Chemical structures of some well-known synthetic antioxidants (BHA; I and BHT; II); (B) Chemical structure of the natural antioxidant caffeic acid (III) and a proposed mechanism for caffeic acid antioxidant activity; (C) Chemical structure of the natural antioxidant melatonin (IV) and the proposed mechanism of how the electron-rich aromatic indole ring system of melatonin scavenges the hydroxyl radical (HO•).
Caffeic acid ((E)-3-(3,4-dihydroxyphenyl)prop-2-enoic acid, III, Figure 1B) is one of the main hydroxycinnamic acids possessing active antioxidant activity, as sketched in Figure 1B. It was reported that caffeic acid has potent free radical scavenging activities [15]. Melatonin (IV, Figure 1C), which is also a remarkable antioxidant natural compound [16,17], can scavenge oxygen free radicals, such as super-oxide radicals, hydroxyl radicals, and others. Along with its antioxidant and neuroprotective activities [18,19,20,21,22,23], melatonin was reported to have a therapeutic potential for inflammation [24], cancer [25], pain [26], cardiovascular disorders, etc. [27,28,29,30,31,32]. Thus, numerous derivatives of melatonin were reported with several biological activities [33,34,35,36,37,38,39,40]. The potent activity of melatonin as an ROS-scavenging agent including 1O2, O2•−, H2O2, hydroxyl radical (HO•), and peroxyl radical (ROO•) is due to the electron-rich aromatic indole chemical scaffold (Figure 1C), which enables indoleamine to serve as an electron donor, forming an indolyl cation [23,41,42,43]. In this context, various indole derivatives with promising activities against oxidative stress and monoamine oxidase B enzyme (MAO-B) were recently reported by our research group [44,45,46].
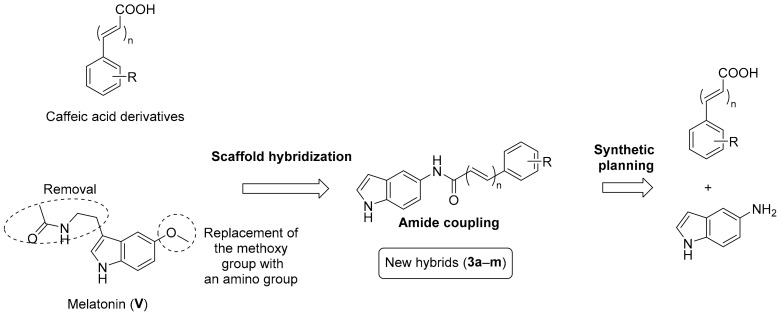
The potent antioxidant activity of both chemical scaffolds (indole and caffeic acid) as shown in Figure 1 encourages our team to design a hybrid scaffold that could have potential antioxidant power. Consequently, development of an efficient indole–caffeic pharmacophore could be enormously important. As illustrated in Figure 2, the methoxy group of melatonin was substituted with an amino group, followed by amide formation by reacting with various caffeic acid analogues to generate the desired amide derivatives (3a–m). The selection process of the functional groups in compounds 3a–m was inspired by their existence in neuroprotectant compounds which showed powerful activities against oxidative stress [47,48,49]. For this objective, a straightforward synthetic approach was used to synthesize the new hybrid indole–caffeic amide analogues 3a–m, and their therapeutic potential against ROS was preliminary assessed (Figure 2).
Figure 2.
Design of structural hybridization of the caffeic acid derivatives with melatonin scaffold, in addition to the synthetic planning, to obtain the novel indole–caffeic amide analogues 3a–m.
2. Materials and Methods
2.1. Chemical Reagents, Purification, and Instrumentation
The general protocols utilized for the chemical synthesis, structure elucidation, and purity of the newly synthesized indole–caffeic acid hybrids are provided in the Supplementary File.
2.2. Synthesis of Indole–caffeic Amide Analogues 3a–m
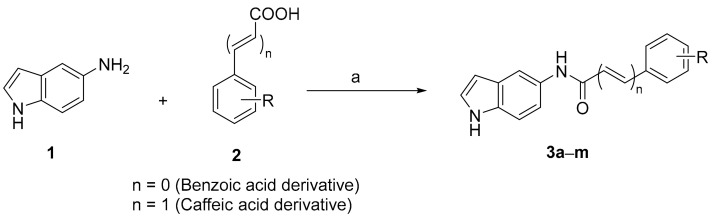
5-Aminoindole (1, 0.1 g, 0.75 mmol), 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide (EDCI, 0.21 g, 1.1 mmol), and hydroxybenzotriazole (HOBt, 0.14 g, 1.1 mmol) were mixed in the presence of N,N-diisopropylethylamine (DIPEA, 0.19 mL, 1.1 mmol) and acetonitrile solvent (5 mL). The appropriate carboxylic acid reagent (2, 0.75 mmol, 1 eq.) was then added. The reaction was carried out at room temperature (25 °C) for 2 h. The excess acetonitrile was evaporated. Work-up was performed using ethyl acetate (EA) and water. The organic solution was evaporated, dried, and purified via flash column chromatography (SiO2, n-hexane:EA = 10:1) to obtain the indole–caffeic amide derivatives in suitable yields (Table 1).
Table 1.
Yields and chemical structures of the synthesized analogues 3a–m.
| Comp. | Chemical Structure | Isolated Yield (%) |
|---|---|---|
| 3a |
|
85 |
| 3b |
|
64 |
| 3c |
|
32 |
| 3d |
|
61 |
| 3e |
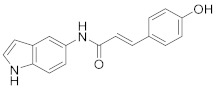
|
47 |
| 3f |
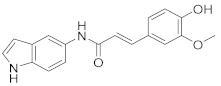
|
79 |
| 3g |
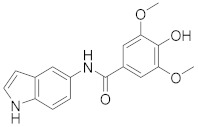
|
82 |
| 3h |
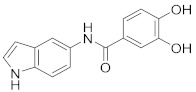
|
93 |
| 3i |
|
85 |
| 3j |
|
91 |
| 3k |
|
76 |
| 3l |
|
57 |
| 3m |
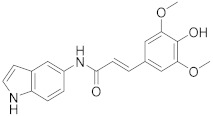
|
46 |
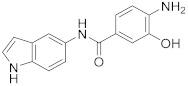
2.2.1. 4-Amino-3-hydroxy-N-(1H-indol-5-yl)benzamide (3a)
Yellowish-white solid. M.p.: 182–183 °C. HPLC purity: 5.614 min, 96.71%. 1H NMR (400 MHz, DMSO-d6) δ 10.96 (s, 1H), 9.58 (s, 1H), 9.24 (s, 1H), 7.93 (s, 1H), 7.36–7.29 (m, 5H), 6.62 (d, J = 8.00 Hz, 1H), 6.37 (s, 1H), 5.09 (br, 2H). 13C NMR (100 MHz, DMSO-d6) δ 165.59, 143.47, 140.75, 133.08, 131.98, 127.80, 126.06, 123.17, 120.17, 116.54, 114.44, 112.88, 112.27, 111.23, 102.00. HRMS (ESI) m/z calculated for C15H14N3O2 [M+H]+: 268.1086, found: 268.1076.
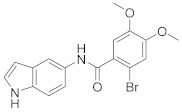
2.2.2. 2-Bromo-N-(1H-indol-5-yl)-4,5-dimethoxybenzamide (3b)
White solid. M.p.: 256–257 °C. HPLC purity: 11.235 min, 99.73%. 1H NMR (400 MHz, DMSO-d6) δ 11.02 (s, 1H), 10.09 (s, 1H), 7.99 (s, 1H), 7.33–7.31 (m, 3H), 7.21 (s, 1H), 7.14 (s, 1H), 6.60 (m, 1H), 3.82 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 165.55, 150.40, 148.38, 133.33, 132.04, 131.51, 127.82, 126.35, 116.90, 115.60, 112.65, 111.59, 111.49, 110.08, 101.57, 55.57. HRMS (ESI) m/z calculated for C17H16BrN2O3 [M+H]+: 375.0344, found: 375.0331.
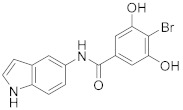
2.2.3. 4-Bromo-3,5-dihydroxy-N-(1H-indol-5-yl)benzamide (3c)
Yellow solid. M.p.: 211–212 °C. HPLC purity: 7.414 min, 99.60%. 1H NMR (400 MHz, DMSO-d6) δ 11.01 (s, 1H), 10.29 (br, 2H), 10.03 (s, 1H), 7.95 (s, 1H), 7.35–7.32 (m, 3H), 6.93 (s, 2H), 6.40 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 165.57, 155.60, 136.38, 133.35, 131.41, 127.80, 126.32, 116.28, 112.36, 111.44, 106.41, 101.57, 101.37. HRMS (ESI) m/z calculated for C15H12BrN2O3 [M+H]+: 347.0031, found: 347.0018.
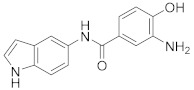
2.2.4. 3-Amino-4-hydroxy-N-(1H-indol-5-yl)benzamide (3d)
Orange solid. M.p.: 182–183 °C. HPLC purity: 12.966 min, 99.87%. 1H NMR (400 MHz, DMSO-d6) δ 11.63 (br, 1H), 11.04 (s, 1H), 10.15 (s, 1H), 8.57 (s, 1H), 8.17 (d, J = 8.00 Hz, 1H), 7.95 (s, 1H), 7.36–7.32 (m, 3H), 7.24 (d, J = 8.00 Hz, 1H), 6.41 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 163.15, 154.68, 136.93, 134.60, 133.48, 131.08, 127.82, 126.45, 126.37, 125.25, 119.31, 116.54, 112.78, 111.47, 101.59. HRMS (ESI) m/z calculated for C15H14N3O2 [M+H]+: 268.1086, found: 268.1094.
2.2.5. (E)-3-(4-Hydroxyphenyl)-N-(1H-indol-5-yl)acrylamide (3e)
White solid. M.p.: 153–154 °C. HPLC purity: 8.983 min, 96.06%. 1H NMR (400 MHz, DMSO-d6) δ 10.98 (s, 1H), 9.85 (br, 2H), 7.99 (s, 1H), 7.47–7.43 (m, 3H), 7.33–7.27 (m, 3H), 6.82 (d, J = 8.00 Hz, 2H), 6.64 (d, J = 16.00 Hz, 1H), 6.38 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 163.98, 159.32, 139.72, 133.10, 131.92, 129.77, 127.98, 126.38, 126.30, 119.73, 116.22, 115.15, 111.65, 110.93, 101.57. HRMS (ESI) m/z calculated for C17H15N2O2 [M+H]+: 279.1134, found: 279.1126.
2.2.6. (E)-3-(4-Hydroxy-3-methoxyphenyl)-N-(1H-indol-5-yl)acrylamide (3f)
Yellowish-white solid. M.p.: 177–178 °C. HPLC purity: 9.259 min, 97.06%. 1H NMR (400 MHz, DMSO-d6) δ 10.98 (s, 1H), 9.86 (s, 1H), 9.44 (s, 1H), 8.00 (s, 1H), 7.45 (d, J = 12.00 Hz, 1H), 7.33–7.30 (m, 3H), 7.18 (s, 1H), 7.05 (d, J = 8.00 Hz, 1H), 6.82 (d, J = 8.00 Hz, 1H), 6.67 (d, J = 12.00 Hz, 1H), 6.38 (s, 1H), 3.83 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 163.92, 148.82, 148.29, 140.02, 133.09, 131.95, 127.94, 126.88, 126.30, 122.11, 120.01, 116.17, 115.09, 111.65, 111.22, 110.93, 101.36, 55.93. HRMS (ESI) m/z calculated for C18H17N2O3 [M+H]+: 309.1239, found: 309.1234.
2.2.7. 4-Hydroxy-N-(1H-indol-5-yl)-3,5-dimethoxybenzamide (3g)
White solid. M.p.: 198–199 °C. HPLC purity: 7.354 min, 97.64%. 1H NMR (400 MHz, DMSO-d6) δ 11.01 (s, 1H), 9.82 (s, 1H), 8.93 (s, 1H), 7.89 (s, 1H), 7.35–7.31 (m, 5H), 6.40 (s, 1H), 3.85 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 165.01, 147.86, 139.08, 133.39, 131.36, 127.84, 126.29, 125.32, 116.94, 113.03, 111.38, 105.83, 101.52, 56.55. HRMS (ESI) m/z calculated for C17H17N2O4 [M+H]+: 313.1188, found: 313.1178.
2.2.8. 3,4-Dihydroxy-N-(1H-indol-5-yl)benzamide (3h)
White solid. M.p.: 145–146 °C. HPLC purity: 5.485 min, 99.53%. 1H NMR (400 MHz, DMSO-d6) δ 10.98 (s, 1H), 9.75 (s, 1H), 9.35 (br, 2H), 7.94 (s, 1H), 7.40–7.30 (m, 5H), 6.81 (d, J = 8.00 Hz, 1H), 5.38 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 165.31, 148.86, 145.29, 133.22, 131.71, 127.80, 126.94, 126.18, 119.78, 116.56, 115.81, 115.28, 112.46, 111.31, 101.50. HRMS (ESI) m/z calculated for C15H13N2O3 [M+H]+: 269.0926, found: 269.0913.
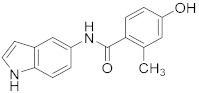
2.2.9. 4-Hydroxy-N-(1H-indol-5-yl)-2-methylbenzamide (3i)
White solid. M.p.: 180–181 °C. HPLC purity: 7.529 min, 98.76%. 1H NMR (400 MHz, DMSO-d6) δ 10.97 (s, 1H), 9.82 (s, 1H), 9.65 (s, 1H), 7.97 (s, 1H), 7.34–7.29 (m, 4H), 6.66–6.64 (m, 2H), 6.37 (s, 1H), 2.34 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 167.78, 158.70, 138.10, 133.15, 131.96, 129.63, 128.97, 127.82, 126.20, 117.59, 115.79, 112.52, 111.56, 111.38, 101.50, 20.29. HRMS (ESI) m/z calculated for C16H15N2O2 [M+H]+: 267.1134, found: 267.1121.
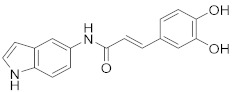
2.2.10. (E)-3-(3,4-Dihydroxyphenyl)-N-(1H-indol-5-yl)acrylamide (3j)
Yellow solid. M.p.: 171–172 °C. HPLC purity: 7.262 min, 99.59%. 1H NMR (400 MHz, DMSO-d6) δ 10.99 (s, 1H), 9.87 (s, 1H), 9.28 (br, 2H), 8.00 (s, 1H), 7.39–7.30 (m, 4H), 7.01 (s, 1H), 6.90 (d, J = 8.00 Hz, 1H), 6.78 (d, J = 8.00 Hz, 1H), 6.58 (d, J = 12.00 Hz, 1H), 6.38 (d, J = 4.00 Hz, 1H). 13C NMR (100 MHz, DMSO-d6) δ 163.95, 147.88, 146.01, 140.11, 133.08, 131.92, 127.93, 126.87, 126.28, 121.08, 119.51, 116.27, 115.13, 114.26, 111.64, 110.91, 101.56. HRMS (ESI) m/z calculated for C17H15N2O3 [M+H]+: 295.1083, found: 295.1070.
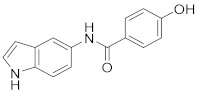
2.2.11. 4-Hydroxy-N-(1H-indol-5-yl)benzamide (3k)
White solid. M.p.: 168–169 °C. HPLC purity: 6.908 min, 98.94%. 1H NMR (400 MHz, DMSO-d6) δ 10.99 (s, 1H), 10.01 (s, 1H), 9.81 (s, 1H), 7.95 (s, 1H), 7.87 (d, J = 8.00 Hz, 2H), 7.36–7.31 (m, 3H), 6.86 (d, J = 8.00 Hz, 2H), 6.39 (s, 1H). 13C NMR (100 MHz, DMSO-d6) δ 165.17, 160.61, 133.28, 131.62, 129.92, 127.83, 126.39, 126.19, 116.64, 115.23, 112.57, 111.35, 101.54. HRMS (ESI) m/z calculated for C15H13N2O2 [M+H]+: 253.0977, found: 253.0966.
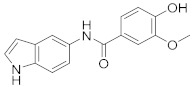
2.2.12. 4-Hydroxy-N-(1H-indol-5-yl)-3-methoxybenzamide (3l)
White solid. M.p.: 190–191 °C. HPLC purity: 7.301 min, 99.75%. 1H NMR (400 MHz, DMSO-d6) δ 11.01 (s, 1H), 9.83 (s, 1H), 9.60 (s, 1H), 7.93 (s, 1H), 7.56 (d, J = 4.00 Hz, 1H), 7.51 (dd, J = 8.0, 4.0 Hz, 1H), 7.35–7.31 (m, 3H), 6.87 (d, J = 8.00 Hz, 1H), 6.40 (s, 1H), 3.86 (s, 3H). 13C NMR (100 MHz, DMSO-d6) δ 165.10, 150.00, 147.58, 133.33, 131.51, 127.84, 126.64, 126.22, 121.56, 116.80, 115.22, 122.78, 112.03, 111.37, 101.53, 56.12. HRMS (ESI) m/z calculated for C16H15N2O3 [M+H]+: 283.1083, found: 283.1073.
2.2.13. (E)-3-(4-Hydroxy-3,5-dimethoxyphenyl)-N-(1H-indol-5-yl)acrylamide (3m)
Yellow solid. M.p.: 199–200 °C. HPLC purity: 8.923 min, 99.88%. 1H NMR (400 MHz, DMSO-d6) δ 11.00 (s, 1H), 9.90 (s, 1H), 8.84 (s, 1H), 8.03 (s, 1H), 7.48 (d, J = 16.00 Hz, 1H), 7.35–7.30 (m, 3H), 6.92 (s, 2H), 6.71 (d, J = 16.00 Hz, 1H), 6.39 (s, 1H), 3.83 (s, 6H). 13C NMR (100 MHz, DMSO-d6) δ 163.84, 148.52, 140.30, 137.82, 133.09, 131.98, 127.94, 126.31, 125.74, 120.36, 115.04, 111.66, 110.82, 105.67, 101.56, 56.37. HRMS (ESI) m/z calculated for C19H19N2O4 [M+H]+: 339.1345, found: 339.1340.
2.3. In Vitro Antioxidant Assays
2.3.1. 2,2-Diphenyl-1-picrylhydrazyl Radical-Scavenging Activity (DPPH Assay)
Final concentrations of 20 mM of the tested compounds and 0.1 mM DMSO were prepared to determine the range of the inhibitory concentration 50 (EC50). A solution of Trolox (a water-soluble analogue of vitamin E, 1000 μM) was prepared in DMSO, from which 5 final concentrations were prepared including 5, 10, 20, 40, and 80 μM. DPPH (2,2-diphenyl-1-picryl-hydrazyl-hydrate) free radical assay was performed as reported [50]. Further details were provided in the Supplementary Materials.
2.3.2. 2,2’-Azino-bis(3-ethylbenzothiazoline-6-sulfonate (ABTS) Assay
The assay was performed as reported [51], with minor modifications. For details, please refer to the Supplementary Materials.
2.3.3. Ferric Reducing Power (FRAP) Assay
A stock solution of Trolox (3 mM in methanol) was made, and the following dilutions were prepared at the concentrations of 1500, 1000, 800, 400, 200, 100, and 50 μM. Samples were initially dissolved in DMSO to obtain a 40 mM concentration (depending on the molecular weight of each compound). Then, they were diluted to reach the concentration of 0.2 mM with methanol. The assay was performed as reported [52], with minor modifications. For details, please refer to the Supplementary Materials.
2.3.4. Oxygen Radical Absorbance Capacity (ORAC Assay)
A stock solution of Trolox (2 mM in MeOH) was prepared, and the following dilutions were prepared: 1200, 900, 600, 500, 400, 300, 200, 100, and 50 μM. Samples were initially dissolved in DMSO at concentrations of 40 mM according to the provided molecular weights. Then, samples were diluted with methanol until reaching the concentration of 0.1 mM. The assay was carried out as reported [53], with modifications. Further details are provided in the Supplementary Materials.
3. Results and Discussion
3.1. Chemical Synthesis
As sketched in Scheme 1, a series of indole-based benzamide and caffeic acid amide analogues 3a–m was synthesized. The amide formation reaction was accomplished via reacting 5-aminoindole (1) in acetonitrile solvent with a variety of commercially available benzoic or caffeic acid derivatives (2). The coupling reagents EDCI and HOBt were used, in addition to DIPEA as an organic base. As shown in Table 1, a variety of indole-based benzamide and caffeic acid amide analogues possessing different chemical substituents were acquired in acceptable yields.
Scheme 1.
(a) Appropriate carboxylic acid derivative (0.75 mmol), EDCI (1.1 mmol), HOBt (1.1 mmol), DIPEA (1.1 mmol), acetonitrile (5 mL), 25 °C, 12 h. (Full description is indicated in the Materials and Methods section).
3.2. Structure Elucidation of the Newly Synthesized Amide Derivatives 3a–m
The chemical structures of the newly synthesized indole-based benzamide and caffeic acid amide analogues (3a–m) were elucidated using different spectroscopic techniques. The purity of compounds 3a–m was found to be more than 96%. The 1H NMR spectra of all the synthesized analogues were characterized by two major singlet peaks with high chemical shifts (>9.00 ppm); the proton of the NH group of the indole scaffold and the proton of the amide group (CONH).
As provided in the Supplementary File, the 1H NMR spectrum of the indole-based benzamide analogue 3a displayed the free para NH2 group at 5.09 ppm as a broad peak (br) representing the two protons of the amino group. In addition, the amide carbon (CO) appeared clearly in the 13C NMR spectrum of compound 3a at 165.59 ppm, which confirmed the formation of the amide group. Indeed, 13C NMR spectra of all the newly synthesized indole-based benzamide and caffeic acid amide analogues (3a–m) showed signals resonating around 163.00–168.00 ppm (CO group of the amide moiety). For compound 3b, the two methoxy groups were found at 3.82 and 55.57 ppm in the 1H and 13 C NMR, respectively. The 1H NMR chart of compound 3c was characterized by a singlet long aromatic peak at 6.93 ppm representing the two para phenyl protons of the 4-bromo-3,5-dihydroxy benzoyl moiety. In addition, the protons of the two hydroxyl moieties were detected as a singlet broad peak at 10.29 ppm. In its 13C NMR chart, compound 3c showed a long peak at 155.60 attributable to the two carbons carrying the two hydroxyl moieties. The 1H NMR spectrum of the benzamide derivative 3d was characterized presence of three singlet peaks with chemical shifts higher than 9.00 ppm (the indole NH, the amide NH, and the free phenolic OH groups).
The first synthesized caffeic acid amide ((E)-3-(4-hydroxyphenyl)-N-(1H-indol-5-yl)acrylamide, 3e) showed these three protons in the range of 9.85–10.98 ppm. In the meantime, one vinylic proton was successfully detected with its characteristic trans J coupling constant of 16.00 Hz at 6.64 ppm. The carbon of the amide moiety appeared at 163.98 in the 13C NMR spectrum of the caffeic acid amide 3e, while the carbon holding the free phenolic OH group was detected at 159.32 ppm. Similarly, the 1H NMR spectrum confirmed the synthesis and the final chemical structure of the second caffeic acid amide derivative in this series (3f) by the presence of three singlet peaks in the range of 9.44–10.98 ppm representing the indole NH, amide NH, and phenolic OH groups, in addition to the three protons of the meta methoxy group (m-OCH3) in the aliphatic region (3.83 ppm). Its 13C NMR chart showed the amide carbon peak at 163.92 ppm, the two carbons bearing the free OH and the methoxy groups at 148.82 and 148.29 ppm, in addition to the aliphatic carbon of the methoxy group at 55.93 ppm.
The 1H NMR spectrum of compound 3g was characterized by a long peak in the aliphatic region at 3.85 representing the six protons of the two methoxy groups. Meanwhile, its 13C NMR chart showed the amide carbon chemical shift at 165.01 ppm, a long peak at 147.86 ppm attributable to the two carbons that hold the two methoxy groups, and the characteristic peak of the carbon bearing the free OH at 139.08 ppm. Similarly, 3,4-dihydroxy-N-(1H-indol-5-yl)benzamide (3h) was characterized by the two common singlet peaks at 10.98 and 9.75 ppm representing the NH protons of the amide linkage and the indole ring. In addition, a broad singlet peak of 2H was found at 9.35 ppm, attributable to the two free OH phenolic moieties. The two carbons bearing these phenolic hydroxyl groups were detected in its 13C NMR spectrum at 148.82 and 145.20 ppm. The methyl group (CH3) of analogue 3i was represented by a singlet peak (3H) in the aliphatic region at 2.34 ppm and a peak at 20.29 ppm (13C NMR).
In addition to the two common singlet peaks of the NH groups of the amide linker and the indole ring at 10.99 and 9.87 ppm, the third caffeic amide analogue 3j also showed a broad peak of 2H representing the protons of the two hydroxyl groups at 9.28 ppm and a doublet peak at 6.58 with a coupling constant of 12.00 Hz attributable to a vinylic proton. The amide CO group was represented at 163.95 ppm (13C NMR), while the two carbons bearing the two hydroxyl groups were represented by two peaks at 147.88 and 146.01 ppm. A vinylic carbon of compound 3j was also successfully detected at 140.11 ppm. In the 13C NMR spectrum of compound 3k, the amide carbon and the carbon atom holding the free OH group were displayed above 160.00 ppm. In compound 3l, the amide carbon appeared at 165.10 ppm, while the two carbons bearing the free OH and the methoxy groups were displayed at 150.00 and 147.58 ppm. The chemical shifts of the methoxy group in 3l were represented by a singlet peak in the aliphatic region at 56.12 ppm (13C NMR) and 3.86 ppm (1H NMR).
Finally, the chemical structure of the final indole-based caffeic acid amide analogue 3m was confirmed by detecting three singlet peaks with chemical shifts of more than 8.00 ppm representing the three protons of the free OH, the amide NH, and the indole NH. Moreover, the two vinylic protons were clearly identified as two doublet peaks with J values of 16.00 Hz at 7.48 and 6.71 ppm. In addition, the six protons of the two methoxy groups were found as a long singlet peak at 3.83 ppm. The amide carbon appeared at 163.84 ppm, the two carbons bearing the two methoxy groups appeared as a long peak at 148.52 ppm, and the two aliphatic carbons of the methoxy groups showed a singlet peak at 56.37 ppm. These data proved and confirmed the formation and purity of the desired amide derivatives 3a–m.
3.3. In Silico Druggability Studies of the Newly Synthesized Amide Derivatives 3a–m
There is no guarantee that a small molecule that possesses a potent interaction with its target protein could be a successful therapeutic candidate. Poor absorption, distribution, metabolism, and excretion (ADME) characteristics may be the reason for this failure. Thus, many promising small molecules fail during the drug discovery process. Moreover, the drug development process is expensive. Accordingly, the pharmacokinetic (PK) characteristics of the indole-based benzamide and caffeic acid amide analogues (3a–m) were evaluated via the SwissADME platform by using distance/pharmacophore models coded as graph-based marks [54]. Using this platform, numerous crucial characteristics can be anticipated including the solubility of the final compounds, their gastrointestinal absorption, and brain entry abilities. During the different steps of the new drug development, these PK factors would constitute the foundation stone of the outcome’s anticipation [55].
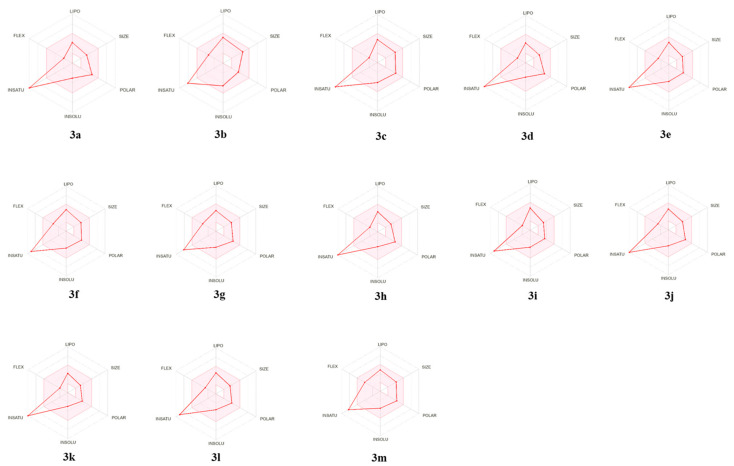
Another major PK property is the topological polar surface area (TPSA) of a compound which refers to the surface sum over the entire polar atoms, mainly nitrogen and oxygen, together with their associated hydrogen atoms. The TPSA is obtained by subtracting from the molecular surface the area of carbon atoms, halogens, and hydrogen atoms bonded to carbon atoms (i.e., nonpolar hydrogen atoms). TPSA is considered a great metric to improve the ability of a drug to penetrate the cells, which could enhance the efficacy of the synthesized drug candidate. Molecules possessing TPSA > 140 Å2 are predicted to not be able to cross cell membranes. On the other hand, a TPSA value < 90 Å2 was found to be essential for a drug candidate to cross the blood–brain barrier (BBB) [56]. Furthermore, compliance with the Lipinski rule of five [57] is another important guide on whether a compound can be taken orally. The outcomes of the in silico PK study are presented in Table 2 and Figure 3.
Table 2.
Predicted lipophilicity, physicochemical, solubility (sol.), and drug-likeness properties of the newly synthesized indole-based benzamide and caffeic acid amide analogues 3a–m.
| Comp. | MW | TPSA | GTI Absorption | Lipinski #Violations | ESOL Log S | ESOL Class |
|---|---|---|---|---|---|---|
| 3a | 267.28 | 91.14 | High | 0 | −3.02 | Sol. |
| 3b | 375.22 | 63.35 | High | 0 | −4.54 | Moderately sol. |
| 3c | 347.16 | 85.35 | High | 0 | −4.12 | Moderately sol. |
| 3d | 267.28 | 91.14 | High | 0 | −3.02 | Sol. |
| 3e | 278.31 | 65.12 | High | 0 | −3.69 | Sol. |
| 3f | 308.33 | 74.35 | High | 0 | −3.75 | Sol. |
| 3g | 312.32 | 83.58 | High | 0 | −3.49 | Sol. |
| 3h | 268.27 | 85.35 | High | 0 | −3.23 | Sol. |
| 3i | 266.29 | 65.12 | High | 0 | −3.67 | Sol. |
| 3j | 294.30 | 85.35 | High | 0 | −3.54 | Sol. |
| 3k | 252.27 | 65.12 | High | 0 | −2.96 | Sol. |
| 3l | 282.29 | 74.35 | High | 0 | −3.43 | Sol. |
| 3m | 338.36 | 83.58 | High | 0 | −3.81 | Sol. |
Figure 3.
PK properties of the newly synthesized amides (3a–m) predicted by the SwissADME platform.
The predicted PK properties of all the newly synthesized indole-based benzamide and caffeic acid amide analogues (3a–m) revealed that all compounds would have high gastrointestinal absorption. In addition, all compounds displayed compliance to the Lipinski rule of five, indicating their great potential to be promising drug candidates with acceptable PK characteristics. Most compounds showed TPSA < 90 Å2, suggesting a potential antioxidant effect also in the brain to battle different neurodegenerative diseases, as hypothesized. These results suggest the PK stability of the indole-based benzamide and caffeic acid amide series.
3.4. In Vitro Antioxidant Assays (Free Radical Scavenging Effects)
3.4.1. DPPH Radical Scavenging Activity
First, all the synthesized analogues (3a–m) were initially screened for their scavenging effects on the DPPH radical. Trolox was used as a reference (IC50 = 33.84 ± 1.01 µM). As illustrated in Table 3, our goal was to discover the antioxidant properties of incorporating the phenolic OH group(s) in addition to some other moieties such as methoxy, bromo, and amino groups in different positions on the phenyl ring, which is attached to position 5 of the antioxidant indole core via two different linkers (depending on the amide type, benzamide or caffeic acid amide). While compounds 3b–e, 3g, 3i, 3k, and 3l showed moderate to weak activity as compared to Trolox, compounds 3a, 3f, 3h, 3j, and 3m were found to have excellent radical scavenging activities with IC50 values of 95.81 ± 1.01, 136.8 ± 1.04, 86.77 ± 1.03, 50.98 ± 1.05, and 67.64 ± 1.02 µM. It was noted that compounds possessing the 3,4-dihydroxyphenyl moiety exhibited promising activities (the benzamide derivative 3h and the caffeic acid amide derivative 3j). On the other hand, the 4-amino-3-hydroxyphenyl moiety was only able to demonstrate its free radical scavenging effect in compound 3a, which has the 4-amino-3-hydroxy phenyl moiety. The 4-hydroxy-3-methoxyphenyl and 4-hydroxy-3,5-dimethoxyphenyl moieties were only able to show their activities in the caffeic acid amide analogues 3f and 3m, respectively. It was also noticed that the majority of the synthesized caffeic acid amide derivatives (three out of four) were able to show higher free radical scavenging activity compared to their benzamide analogues. It could be the double-bond moiety in these caffeic acid amide derivatives that may increase the capacity of the molecule to interact with the free radicals via enhancing the electron conjugation effect in the whole chemical structure so that they do not engage in a destructive biochemical reaction. Based on this primary screening, the position and nature of substitutions on the phenyl moiety and the presence of the double bond in the middle of the structure were found to be essential factors directly affecting the free radical scavenging activity of these new indole-based amides. Consequently, the most potent derivatives (3a, 3f, 3h, 3j, and 3m) were further evaluated.
Table 3.
Results of the in vitro radical scavenging activity assays (DPPH, ABTS, FRAP, and ORAC).
| Comp. | DPPH Radical Scavenging Activity EC50
(Mean ± SD) µM |
ABTS Radical Scavenging Activity IC50
(Mean ± SD) µM |
FRAP Assay (μM Trolox eq/mM Sample) |
ORAC Assay (μM Trolox eq/mM Sample) |
|---|---|---|---|---|
| 3a | 95.81 ± 1.01 | 33.33 ± 1.96 | - | - |
| 3b | 12,527 ± 1.03 | - | - | - |
| 3c | 15,422 ± 1.04 | - | - | - |
| 3d | 14,451 ± 1.06 | - | - | - |
| 3e | 2320 ± 1.01 | - | - | - |
| 3f | 136.8 ± 1.04 | 14.48 ± 0.68 | 1951.45 ± 75.97 | 9253.47 ± 806.00 |
| 3g | 5100 ± 1.03 | - | - | - |
| 3h | 86.77 ± 1.03 | 39.98 ± 0.92 | - | - |
| 3i | >20,000 | - | - | - |
| 3j | 50.98 ± 1.05 | 19.49 ± 0.54 | 4774.37 ± 137.20 | 10,714.21 ± 817.76 |
| 3k | >20,000 | - | - | - |
| 3l | 8912 ± 1.02 | - | - | - |
| 3m | 67.64 ± 1.02 | 14.92 ± 0.30 | 2308.7 ± 73.73 | 7293.46 ± 208.48 |
| Trolox | 33.84 ± 1.01 | 29.62 ± 1.86 | - | - |
3.4.2. ABTS•+ Radical Cation Scavenging Assay
ABTS activity was measured in terms of percentage inhibition (%) of the ABTS•+ radical cation by each of the five most active compounds (3a, 3f, 3h, 3j, and 3m). The ABTS values of the five samples are presented in Table 3. While compound 3a (with the 4-amino-3-hydroxy phenyl moiety) was able to scavenge the radical cation ABTS•+ with an IC50 value of 33.33 ± 1.96 µM, which is almost the similar potency of the standard Trolox (29.62 ± 1.86 µM), compound 3h possessing 3,4-dihydroxy phenyl moiety showed a higher IC50 value of 39.98 ± 0.92 µM. Interestingly, three compounds out of the five (3f, 3j, and 3m) showed higher capacities to neutralize the radical cation ABTS•+ than Trolox with IC50 values of 14.48 ± 0.68, 19.49 ± 0.54, and 14.92 ± 0.30 µM, respectively.
3.4.3. FRAP Assay
The three highly potent analogues (3f, 3j, and 3m) were considered for FRAP and ORAC assays. The FRAP assay assesses the antioxidant properties of the tested compound based on its reducing ability. The values obtained, shown in Table 3, were consistent with the DPPH and ABTS assays. In this study, compound 3j (the caffeic acid derivative possessing 3,4-dihydroxyphenyl moiety) presented the highest antioxidant capacity with a FRAP value of 4774.37 ± 137.20 μM Trolox eq/mM sample, followed by compounds 3m (4-hydroxy-3,5-dimethoxyphenyl moiety containing caffeic acid derivative) and 3f (4-hydroxy-3-methoxyphenyl moiety containing caffeic acid derivative) with values of 2308.7 ± 73.73 and 1951.45 ± 75.97 μM Trolox eq/mM sample, respectively. Based on these findings, it could be concluded that caffeic amide analogue 3j possessing the two phenolic OH groups not only offered the top free radical scavenging capability, but also the strongest reducing power among the tested compounds. Indeed, the antioxidant activity of a small molecule largely depends on both the chemical structure of the compound and the test system. Accordingly, it cannot be fully assessed by one single technique due to the various mechanisms of antioxidant action. As a result, the ORAC test was chosen to be the next further test for these three promising analogues (3f, 3j, and 3m).
3.4.4. ORAC Assay
Through the ORAC test, the antioxidant capacity was investigated of the three highly active compounds (3f, 3j, and 3m) that had demonstrated high antioxidant activity with the previous DPPH, ABTS, and FRAP tests. The ORAC test was intended to validate the results obtained with the previous approaches and extend the activity profile for each tested derivative. All tested compounds exhibited a dynamic ability to reduce the oxidative degradation of the fluorescent molecule, caused by peroxyl radicals. Compounds 3m and 3f showed very high ORAC antioxidant power (9253.47 ± 806.00 and 7293.46 ± 208.48 μM Trolox eq/mM sample, respectively). In a similar way to the previous assay (FRAP), the best antioxidant capacity against the peroxyl radicals was observed for compound 3j (10,714.21 ± 817.76 μM Trolox eq/mM sample).
4. Conclusions
As a step toward the development of novel free-radical scavenging hybrid agents for oxidative stress-related therapy, a new series of indole-based benzamide and caffeic acid amide analogues (3a–m) was successfully designed and synthesized. Among them, compounds 3a (4-amino-3-hydroxy benzamide derivative), 3f (4-hydroxy-3-methoxyphenyl containing caffeic acid derivative), 3h (3,4-dihydroxy benzamide), 3j (3,4-dihydroxyphenyl containing caffeic acid derivative), and 3m (4-hydroxy-3,5-dimethoxyphenyl containing caffeic acid derivative) were able to show promising DPPH radical scavenging activities with IC50 values of 95.81 ± 1.01, 136.8 ± 1.04, 86.77 ± 1.03, 50.98 ± 1.05, and 67.64 ± 1.02 µM, respectively The three caffeic acid derivatives 3f, 3j, and 3m neutralized the free radical cation ABTS•+ more than Trolox with IC50 values of 14.48 ± 0.68, 19.49 ± 0.54, and 14.92 ± 0.30 µM, respectively. Using FRAP and ORAC assays, compound 3j was the most active antioxidant agent with values of 4774.37 ± 137.20 and 10,714.21 ± 817.76 μM Trolox eq/mM sample, respectively. Most small molecules were anticipated to be soluble and to penetrate the brain. No violations of the Lipinski rule of five were noticed, indicating a pharmacokinetically stable profile. Consequently, the hybrid compound 3j is reported as a new antioxidant candidate with highly potent and promising free radical scavenging activities.
Acknowledgments
The authors would like to thank the Deanship of scientific research at Umm Al-Qura University for supporting this work by grant code (23UQU4290565DSR030).
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/metabo13020141/s1, chemical reagents, purification, and instrumentation details, 1HNMR, 13CNMR, purity, and HRMS data of the compounds reported in this study, in addition to the detailed calibration curves of Trolox used in RFAP and ORAC assays.
Author Contributions
Conceptualization, A.E.; methodology, A.E. and N.K.O., validation, N.K.O. and N.A.G.; formal analysis, A.E. and N.K.O.; investigation, M.H.A., S.O.A., A.B. and A.A.A.-K.; resources, M.A.S.A. and Q.A.A.; data curation, A.E. and M.K.; visualization, A.E. and A.A.A.-K.; writing—original draft preparation, A.E.; writing—review and editing, all authors; supervision, K.L.; project administration, A.E.; funding acquisition, K.L. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korean government (MSIT) (No. NRF-2018R1A5A2023127). The authors would like to thank the Deanship of scientific research at Umm Al-Qura University for supporting this work by grant code (23UQU4290565DSR030).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Kumar S., Gupta E., Kaushik S., Kumar Srivastava V., Mehta S.K., Jyoti A. Evaluation of oxidative stress and antioxidant status: Correlation with the severity of sepsis. Scand. J. Immunol. 2018;87:e12653. doi: 10.1111/sji.12653. [DOI] [PubMed] [Google Scholar]
- 2.Darenskaya M.A., Kolesnikova L.I., Kolesnikov S.I. Oxidative Stress: Pathogenetic Role in Diabetes Mellitus and Its Complications and Therapeutic Approaches to Correction. Bull. Exp. Biol. Med. 2021;171:179–189. doi: 10.1007/s10517-021-05191-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pohanka M. Role of oxidative stress in infectious diseases. A review. Folia Microbiol. 2013;58:503–513. doi: 10.1007/s12223-013-0239-5. [DOI] [PubMed] [Google Scholar]
- 4.Sies H. Oxidative stress: A concept in redox biology and medicine. Redox Biol. 2015;4:180–183. doi: 10.1016/j.redox.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Poprac P., Jomova K., Simunkova M., Kollar V., Rhodes C.J., Valko M. Targeting Free Radicals in Oxidative Stress-Related Human Diseases. Trends Pharmacol. Sci. 2017;38:592–607. doi: 10.1016/j.tips.2017.04.005. [DOI] [PubMed] [Google Scholar]
- 6.Liu R., Mabury S.A. Synthetic Phenolic Antioxidants: A Review of Environmental Occurrence, Fate, Human Exposure, and Toxicity. Environ. Sci. Technol. 2020;54:11706–11719. doi: 10.1021/acs.est.0c05077. [DOI] [PubMed] [Google Scholar]
- 7.Wang W., Xiong P., Zhang H., Zhu Q., Liao C., Jiang G. Analysis, occurrence, toxicity and environmental health risks of synthetic phenolic antioxidants: A review. Environ. Res. 2021;201:111531. doi: 10.1016/j.envres.2021.111531. [DOI] [PubMed] [Google Scholar]
- 8.Neha K., Haider M.R., Pathak A., Yar M.S. Medicinal prospects of antioxidants: A review. Eur. J. Med. Chem. 2019;178:687–704. doi: 10.1016/j.ejmech.2019.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Kundu T., Pramanik A. Expeditious and eco-friendly synthesis of new multifunctionalized pyrrole derivatives and evaluation of their antioxidant property. Bioorganic Chem. 2020;98:103734. doi: 10.1016/j.bioorg.2020.103734. [DOI] [PubMed] [Google Scholar]
- 10.Kundu T., Bhattacharjee B., Hazra S., Ghosh A.K., Bandyopadhyay D., Pramanik A. Synthesis and Biological Assessment of Pyrrolobenzoxazine Scaffold as a Potent Antioxidant. J. Med. Chem. 2019;62:6315–6329. doi: 10.1021/acs.jmedchem.9b00717. [DOI] [PubMed] [Google Scholar]
- 11.Bandeira P.T., Dalmolin M.C., de Oliveira M.M., Nunes K.C., Garcia F.P., Nakamura C.V., de Oliveira A.R.M., Piovan L. Synthesis, Antioxidant Activity and Cytotoxicity of N-Functionalized Organotellurides. Bioorganic Med. Chem. 2019;27:410–415. doi: 10.1016/j.bmc.2018.12.017. [DOI] [PubMed] [Google Scholar]
- 12.Tailor N., Sharma M. Antioxidant hybrid compounds: A promising therapeutic intervention in oxidative stress induced diseases. Mini Rev. Med. Chem. 2013;13:280–297. [PubMed] [Google Scholar]
- 13.Vavříková E., Křen V., Jezova-Kalachova L., Biler M., Chantemargue B., Pyszková M., Riva S., Kuzma M., Valentová K., Ulrichová J., et al. Novel flavonolignan hybrid antioxidants: From enzymatic preparation to molecular rationalization. Eur. J. Med. Chem. 2017;127:263–274. doi: 10.1016/j.ejmech.2016.12.051. [DOI] [PubMed] [Google Scholar]
- 14.Carullo G., Mazzotta S., Koch A., Hartmann K.M., Friedrich O., Gilbert D.F., Vega-Holm M., Schneider-Stock R., Aiello F. New Oleoyl Hybrids of Natural Antioxidants: Synthesis and In Vitro Evaluation as Inducers of Apoptosis in Colorectal Cancer Cells. Antioxidants. 2020;9:77. doi: 10.3390/antiox9111077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gülçin I. Antioxidant activity of caffeic acid (3,4-dihydroxycinnamic acid) Toxicology. 2006;217:213–220. doi: 10.1016/j.tox.2005.09.011. [DOI] [PubMed] [Google Scholar]
- 16.Sofic E., Rimpapa Z., Kundurovic Z., Sapcanin A., Tahirovic I., Rustembegovic A., Cao G.J. Antioxidant capacity of the neurohormone melatonin. J. Neural Transm. 2005;112:349–358. doi: 10.1007/s00702-004-0270-4. [DOI] [PubMed] [Google Scholar]
- 17.Cipolla-Neto J., Amaral F.G.D. Melatonin as a Hormone: New Physiological and Clinical Insights. Endocr. Rev. 2018;39:990–1028. doi: 10.1210/er.2018-00084. [DOI] [PubMed] [Google Scholar]
- 18.Reiter R.J., Mayo J.C., Tan D.X., Sainz R.M., Alatorre-Jimenez M., Qin L. Melatonin as an antioxidant: Under promises but over delivers. J. Pineal Res. 2016;61:253–278. doi: 10.1111/jpi.12360. [DOI] [PubMed] [Google Scholar]
- 19.Reiter R.J., Tan D.X., Cabrera J., D’Arpa D. Melatonin and tryptophan derivatives as free radical scavengers and antioxidants. Adv. Exp. Med. Biol. 1999;467:379–387. doi: 10.1007/978-1-4615-4709-9_48. [DOI] [PubMed] [Google Scholar]
- 20.Reiter R.J., Tan D.X., Rosales-Corral S., Galano A., Zhou X.J., Xu B. Mitochondria: Central Organelles for Melatonin’s Antioxidant and Anti-Aging Actions. Molecules. 2018;23:509. doi: 10.3390/molecules23020509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reiter R.J., Rosales-Corral S., Tan D.X., Jou M.J., Galano A., Xu B. Melatonin as a mitochondria-targeted antioxidant: One of evolution’s best ideas. Cell. Mol. Life Sci. CMLS. 2017;74:3863–3881. doi: 10.1007/s00018-017-2609-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Velkov Z.A., Velkov Y.Z., Galunska B.T., Paskalev D.N., Tadjer A.V. Melatonin: Quantum-chemical and biochemical investigation of antioxidant activity. Eur. J. Med. Chem. 2009;44:2834–2839. doi: 10.1016/j.ejmech.2008.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Estevão M.S., Carvalho L.C., Ribeiro D., Couto D., Freitas M., Gomes A., Ferreira L.M., Fernandes E., Marques M.M.B. Antioxidant activity of unexplored indole derivatives: Synthesis and screening. Eur. J. Med. Chem. 2010;45:4869–4878. doi: 10.1016/j.ejmech.2010.07.059. [DOI] [PubMed] [Google Scholar]
- 24.Esposito E., Cuzzocrea S. Antiinflammatory activity of melatonin in central nervous system. Curr. Neuropharmacol. 2010;8:228–242. doi: 10.2174/157015910792246155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Di Bella G., Mascia F., Gualano L., Di Bella L. Melatonin anticancer effects. Int. J. Mol. Sci. 2013;14:2410–2430. doi: 10.3390/ijms14022410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.de Zanette S.A., Vercelino R., Laste G., Rozisky J.R., Schwertner A., Machado C.B., Xavier F., de Souza I.C.C., Deitos A., Torres I.L., et al. Melatonin analgesia is associated with improvement of the descending endogenous pain-modulating system in fibromyalgia: A phase II, randomized, double-dummy, controlled trial. BMC Pharmacol. Toxicol. 2014;15:40. doi: 10.1186/2050-6511-15-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lochner A., Marais E., Huisamen B. Melatonin and cardioprotection against ischaemia/reperfusion injury: What’s new? A review. J. Pineal Res. 2018;65:e12490. doi: 10.1111/jpi.12490. [DOI] [PubMed] [Google Scholar]
- 28.Chen J., Xia H., Zhang L., Zhang H., Wang D., Tao X. Protective effects of melatonin on sepsis-induced liver injury and dysregulation of gluconeogenesis in rats through activating SIRT1/STAT3 pathway. Biomed. Pharmacother. 2019;117:109150. doi: 10.1016/j.biopha.2019.109150. [DOI] [PubMed] [Google Scholar]
- 29.Rahman A., Hasan A.U., Kobori H. Melatonin in chronic kidney disease: A promising chronotherapy targeting the intrarenal renin–angiotensin system. Hypertens. Res. 2019;42:920–923. doi: 10.1038/s41440-019-0223-9. [DOI] [PubMed] [Google Scholar]
- 30.Tordjman S., Chokron S., Delorme R., Charrier A., Bellissant E., Jaafari N., Fougerou C. Melatonin: Pharmacology, Functions and Therapeutic Benefits. Curr. Neuropharmacol. 2017;15:434–443. doi: 10.2174/1570159X14666161228122115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Chitimus D.M., Popescu M.R., Voiculescu S.E., Panaitescu A.M., Pavel B., Zagrean L., Zagrean A.M. Melatonin’s Impact on Antioxidative and Anti-Inflammatory Reprogramming in Homeostasis and Disease. Biomolecules. 2020;10:1211. doi: 10.3390/biom10091211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Salehi B., Sharopov F., Fokou P.V.T., Kobylinska A., Jonge L., Tadio K., Sharifi-Rad J., Posmyk M.M., Martorell M., Martins N., et al. Melatonin in Medicinal and Food Plants: Occurrence, Bioavailability, and Health Potential for Humans. Cells. 2019;8:681. doi: 10.3390/cells8070681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rodríguez-Franco M.I., Fernández-Bachiller M.I., Pérez C., Hernández-Ledesma B., Bartolomé B. Novel tacrine—Melatonin hybrids as dual-acting drugs for Alzheimer disease, with improved acetylcholinesterase inhibitory and antioxidant properties. J. Med. Chem. 2006;49:459–462. doi: 10.1021/jm050746d. [DOI] [PubMed] [Google Scholar]
- 34.Luo X.-T., Wang C.-M., Liu Y., Huang Z.-G. New multifunctional melatonin-derived benzylpyridinium bromides with potent cholinergic, antioxidant, and neuroprotective properties as innovative drugs for Alzheimer’s disease. Eur. J. Med. Chem. 2015;103:302–311. doi: 10.1016/j.ejmech.2015.08.052. [DOI] [PubMed] [Google Scholar]
- 35.Bautista-Aguilera O.M., Esteban G., Bolea I., Nikolic K., Agbaba D., Moraleda I., Iriepa I., Samadi A., Soriano E., Unzeta M., et al. Design, synthesis, pharmacological evaluation, QSAR analysis, molecular modeling and ADMET of novel donepezil-indolyl hybrids as multipotent cholinesterase/monoamine oxidase inhibitors for the potential treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2014;75:82–95. doi: 10.1016/j.ejmech.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 36.Rivara S., Pala D., Bedini A., Spadoni G. Therapeutic uses of melatonin and melatonin derivatives: A patent review (2012–2014) Expert Opin. Ther. Pat. 2015;25:425–441. doi: 10.1517/13543776.2014.1001739. [DOI] [PubMed] [Google Scholar]
- 37.Wang S.Y., Shi X.C., Laborda P. Indole-based melatonin analogues: Synthetic approaches and biological activity. Eur. J. Med. Chem. 2020;185:111847. doi: 10.1016/j.ejmech.2019.111847. [DOI] [PubMed] [Google Scholar]
- 38.Mor M., Rivara S., Silva C., Bordi F., Plazzi P.V., Spadoni G., Diamantini G., Balsamini C., Tarzia G., Fraschini F., et al. Melatonin receptor ligands: Synthesis of new melatonin derivatives and comprehensive comparative molecular field analysis (CoMFA) study. J. Med. Chem. 1998;41:3831–3844. doi: 10.1021/jm9810093. [DOI] [PubMed] [Google Scholar]
- 39.Landagaray E., Ettaoussi M., Leclerc V., Traoré B., Perez V., Nosjean O., Boutin J.A., Caignard D.H., Delagrange P., Berthelot P., et al. New melatonin (MT1/MT2) ligands: Design and synthesis of (8,9-dihydro-7H-furo[3,2-f]chromen-1-yl) derivatives. Bioorganic Med. Chem. 2014;22:986–996. doi: 10.1016/j.bmc.2013.12.054. [DOI] [PubMed] [Google Scholar]
- 40.Angelova V.T., Rangelov M., Todorova N., Dangalov M., Andreeva-Gateva P., Kondeva-Burdina M., Karabeliov V., Shivachev B., Tchekalarova J. Discovery of novel indole-based aroylhydrazones as anticonvulsants: Pharmacophore-based design. Bioorganic Chem. 2019;90:103028. doi: 10.1016/j.bioorg.2019.103028. [DOI] [PubMed] [Google Scholar]
- 41.Tan D.X., Reiter R.J., Manchester L.C., Yan M.T., El-Sawi M., Sainz R.M., Mayo J.C., Kohen R., Allegra M., Hardeland R. Chemical and physical properties and potential mechanisms: Melatonin as a broad spectrum antioxidant and free radical scavenger. Curr. Top. Med. Chem. 2002;2:181–197. doi: 10.2174/1568026023394443. [DOI] [PubMed] [Google Scholar]
- 42.Stasica P., Ulanski P., Rosiak J.M. Melatonin as a hydroxyl radical scavenger. J. Pineal Res. 1998;25:65–66. doi: 10.1111/j.1600-079X.1998.tb00387.x. [DOI] [PubMed] [Google Scholar]
- 43.Reiter R.J. Cytoprotective properties of melatonin: Presumed association with oxidative damage and aging. Nutrition. 1998;14:691–696. doi: 10.1016/S0899-9007(98)00064-1. [DOI] [PubMed] [Google Scholar]
- 44.Elkamhawy A., Kim H.J., Elsherbeny M.H., Paik S., Park J.H., Gotina L., Abdellattif M.H., Gouda N.A., Cho J., Lee K., et al. Discovery of 3,4-dichloro-N-(1H-indol-5-yl)benzamide: A highly potent, selective, and competitive hMAO-B inhibitor with high BBB permeability profile and neuroprotective action. Bioorganic Chem. 2021;116:105352. doi: 10.1016/j.bioorg.2021.105352. [DOI] [PubMed] [Google Scholar]
- 45.Elsherbeny M.H., Kim J., Gouda N.A., Gotina L., Cho J., Pae A.N., Lee K., Park K.D., Elkamhawy A., Roh E.J. Highly Potent, Selective, and Competitive Indole-Based MAO-B Inhibitors Protect PC12 Cells against 6-Hydroxydopamine- and Rotenone-Induced Oxidative Stress. Antioxidants. 2021;10:1641. doi: 10.3390/antiox10101641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Elkamhawy A., Paik S., Kim H.J., Park J.H., Londhe A.M., Lee K., Pae A.N., Park K.D., Roh E.J. Discovery of N-(1-(3-fluorobenzoyl)-1H-indol-5-yl)pyrazine-2-carboxamide: A novel, selective, and competitive indole-based lead inhibitor for human monoamine oxidase B. J. Enzym. Inhib. Med. Chem. 2020;35:1568–1580. doi: 10.1080/14756366.2020.1800666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yeon S.K., Choi J.W., Park J.-H., Lee Y.R., Kim H.J., Shin S.J., Jang B.K., Kim S., Bahn Y.-S., Han G., et al. Synthesis and evaluation of biaryl derivatives for structural characterization of selective monoamine oxidase B inhibitors toward Parkinson’s disease therapy. Bioorganic Med. Chem. 2018;26:232–244. doi: 10.1016/j.bmc.2017.11.036. [DOI] [PubMed] [Google Scholar]
- 48.Cores Á., Abril S., Michalska P., Duarte P., Olives A.I., Martín M.A., Villacampa M., León R., Menéndez J.C. Bisavenathramide Analogues as Nrf2 Inductors and Neuroprotectors in In Vitro Models of Oxidative Stress and Hyperphosphorylation. Antioxidants. 2021;10:941. doi: 10.3390/antiox10060941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Elkamhawy A., Park J.E., Hassan A.H.E., Pae A.N., Lee J., Park B.G., Roh E.J. Synthesis and evaluation of 2-(3-arylureido)pyridines and 2-(3-arylureido)pyrazines as potential modulators of Aβ-induced mitochondrial dysfunction in Alzheimer’s disease. Eur. J. Med. Chem. 2018;144:529–543. doi: 10.1016/j.ejmech.2017.12.045. [DOI] [PubMed] [Google Scholar]
- 50.Boly R., Lamkami T., Lompo M., Dubois J., Guissou I. DPPH free radical scavenging activity of two extracts from Agelanthus dodoneifolius (Loranthaceae) leaves. Int. J. Toxicol. Pharmacol. Res. 2016;8:29–34. [Google Scholar]
- 51.Arnao M.B., Cano A., Acosta M. The hydrophilic and lipophilic contribution to total antioxidant activity. Food Chem. 2001;73:239–244. doi: 10.1016/S0308-8146(00)00324-1. [DOI] [Google Scholar]
- 52.Benzie I.F.F., Strain J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996;239:70–76. doi: 10.1006/abio.1996.0292. [DOI] [PubMed] [Google Scholar]
- 53.Liang Z., Cheng L., Zhong G.Y., Liu R.H. Antioxidant and antiproliferative activities of twenty-four Vitis vinifera grapes. PLoS ONE. 2014;9:e105146. doi: 10.1371/journal.pone.0105146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Daina A., Michielin O., Zoete V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017;7:42717. doi: 10.1038/srep42717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Alavijeh M.S., Chishty M., Qaiser M.Z., Palmer A.M. Drug metabolism and pharmacokinetics, the blood-brain barrier, and central nervous system drug discovery. NeuroRx J. Am. Soc. Exp. NeuroTherapeutics. 2005;2:554–571. doi: 10.1602/neurorx.2.4.554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pajouhesh H., Lenz G.R. Medicinal chemical properties of successful central nervous system drugs. NeuroRx J. Am. Soc. Exp. NeuroTherapeutics. 2005;2:541–553. doi: 10.1602/neurorx.2.4.541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Lipinski C.A. Lead- and drug-like compounds: The rule-of-five revolution. Drug Discov. Today: Technol. 2004;1:337–341. doi: 10.1016/j.ddtec.2004.11.007. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.