Abstract
Legionella pneumophila is an intracellular pathogen that can cause severe pneumonia after the inhalation of contaminated aerosols and replication in alveolar macrophages. Several pattern recognition receptors (PRRs) have been identified that contribute to the recognition of L. pneumophila by the innate immune system. However, the function of the C-type lectin receptors (CLRs), which are mainly expressed by macrophages and other myeloid cells, remains largely unexplored. Here, we used a library of CLR-Fc fusion proteins to search for CLRs that can bind the bacterium and identified the specific binding of CLEC12A to L. pneumophila. Subsequent infection experiments in human and murine macrophages, however, did not provide evidence for a substantial role of CLEC12A in controlling innate immune responses to the bacterium. Consistently, antibacterial and inflammatory responses to Legionella lung infection were not significantly influenced by CLEC12A deficiency. Collectively, CLEC12A is able to bind to L. pneumophila-derived ligands but does not appear to play a major role in the innate defense against L. pneumophila.
Keywords: Legionella pneumophila, C-type lectin receptors, CLEC12A, macrophages
1. Introduction
L. pneumophila is an intracellular bacterial pathogen that persists in the environment as a parasite of freshwater protozoans such as Acanthamoeba castellanii [1]. After the inhalation of contaminated aerosols from, e.g., cooling towers, hot and cold water systems and whirlpools, humans can develop an infection [2]. The severity of Legionella infections range from mild flu-like Pontiac fever to Legionnaires’ disease, an atypical, often severe form of pneumonia that is associated with 10% mortality [3]. Within the lung, L. pneumophila is engulfed by alveolar macrophages, where it replicates inside an endoplasmic reticulum (ER)-like organelle called the Legionella-containing vacuole (LCV) [4,5]. The establishment of the LCV and other manipulations of the host cell require the bacterial dot/icm-encoded type IV secretion system (T4SS), which injects around 300 effector molecules into the host’s cytosol [6,7].
The innate immune system uses various PRRs to detect L. pneumophila infection. These receptors include the transmembrane Toll-like receptors (TLRs) and the cytosolic NOD-like receptors (NLRs), as well as cyclic GMP-AMP synthase (cGAS), which senses, e.g., bacterial cell wall components, flagellin and nucleic acids. The sensing of Legionella by these PRRs initiates, for example, the production of proinflammatory cytokines and IFNs, as well as the activation of so-called inflammasomes [8,9,10,11]. Antibacterial immune defense in the lung against L. pneumophila partly relies on TNFα- and type I and II IFN-dependent macrophage-intrinsic resistance mechanisms, as well as on an inflammasome-mediated type of cell death named pyroptosis [12,13,14,15,16,17].
CLRs are PRRs that often bind to carbohydrate ligands, although proteins and lipids have also been identified as CLR ligands [18,19,20]. C-type lectin domain family 12 member A (CLEC12A/MICL) is a type II transmembrane protein that is primarily expressed on myeloid cells, including granulocytes, monocytes, macrophages and dendritic cells [21]. CLEC12A is an evolutionarily conserved inhibitory CLR containing an immunoreceptor tyrosine-based inhibitory motif (ITIM), thus recruiting inhibitory phosphatases upon activation [22]. While human and murine CLEC12A are structurally and functionally similar, human CLEC12A is a heavily glycosylated monomer, whereas murine CLEC12A is expressed as a less glycosylated dimer [23]. In previous studies, CLEC12A was described as a receptor involved in the regulation of homeostasis and in the control of inflammation, including rheumatoid arthritis and experimental autoimmune encephalomyelitis (EAE) [24,25]. CLEC12A was identified as a receptor for dead cells sensing uric acid crystals (monosodium urate, MSU) as a key danger signal for cell-death-induced immunity [26]. Lately, we identified CLEC12A as a receptor for another crystalline ligand, hemozoin, and showed the crucial role of CLEC12A in the development of cerebral malaria [27]. To date, there are only few studies on the role of CLEC12A in the context of bacterial infections. In a murine Salmonella infection model, it was shown that CLEC12A contributed to antibacterial autophagy, as CLEC12A was recruited to bacteria–autophagosome complexes and interacted with an E3-ubiquitin ligase complex functionally involved in autophagy [28]. Recently, mycolic acids from various mycobacterial species were described as binding to mouse CLEC12A and, more potently, to human CLEC12A. Innate immune responses were augmented in CLEC12A-deficient mice after M. tuberculosis infection, suggesting that mycobacteria dampened the host’s immune response by hijacking CLEC12A through their mycolic acids [29]. Currently, the role of CLEC12A in the recognition of L. pneumophila and in the induction of innate immune responses during Legionella infection in vivo is unknown.
Since the role of CLRs in Legionella infection was unknown, here, we screened for CLRs capable of binding the bacterium, and functionally characterized the role of CLEC12A in L. pneumophila infection. We used a comprehensive CLR-Fc fusion protein library to screen for CLRs sensing L. pneumophila and identified CLEC12A as a candidate receptor binding to different L. pneumophila strains. A distinct Legionella-derived ligand for CLEC12A remains to be identified; nevertheless, we could already exclude lipopolysaccharide (LPS) from L. pneumophila and putative (glyco-)proteins as CLEC12A ligands. In a murine L. pneumophila infection model, bacterial loads and effector functions such as cytokine secretion were assessed but showed no significant difference between WT and Clec12a−/− mice. We therefore conclude that CLEC12A recognizes L. pneumophila but has a limited role in antibacterial defense.
2. Results
2.1. The CLR CLEC12A Recognizes L. pneumophila
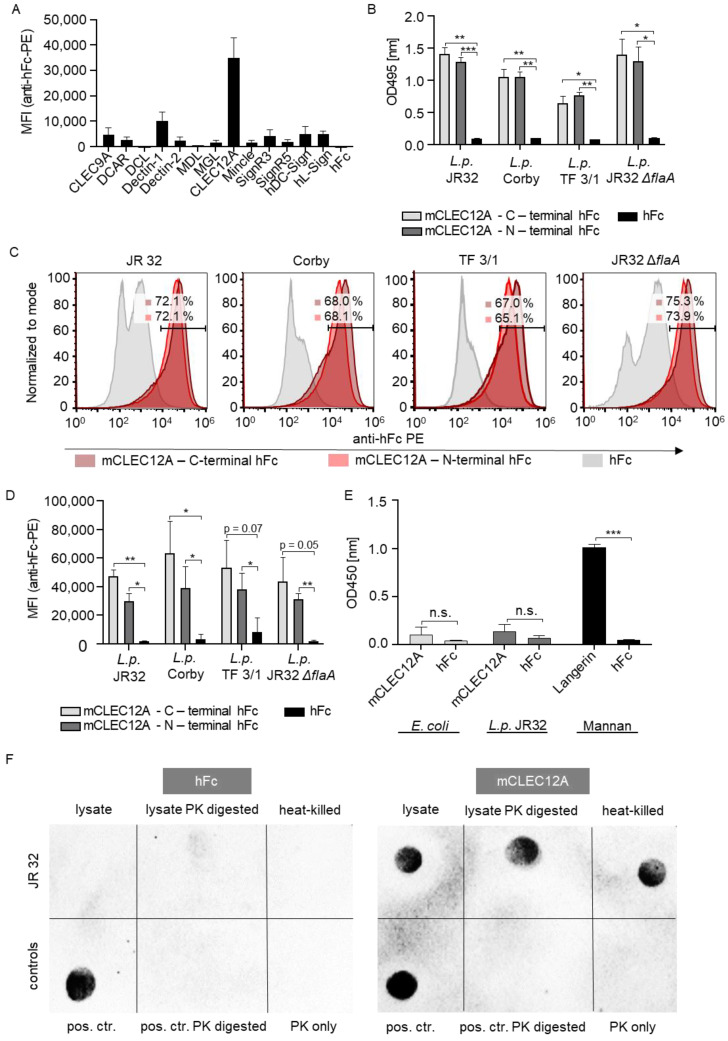
To investigate whether CLRs bind to L. pneumophila, we initially performed a flow cytometry-based binding assay using a comprehensive CLR-Fc library [30,31]. To this end, L. pneumophila JR32 was incubated with the respective CLR-Fc fusion proteins. Indeed, several CLR-Fc fusion proteins (CLEC12A, Dectin-1, DC-SIGN and L-SIGN) exhibited moderate to substantial binding to L. pneumophila, with CLEC12A displaying the highest MFI signal compared with the hFc control and the other CLRs included in the library (Figure 1A). Since L. pneumophila serogroup 1 causes most of the worldwide outbreaks [32], we decided to test the wild-type strains JR32 and Corby, both categorized as serotype 1, for binding to CLEC12A [12,33]. To gain further insights into the role of major L. pneumophila pathogenicity factors in CLEC12A binding, a LPS mutant [33] and a flagellin mutant (ΔflaA) [34] were included in the binding assay. The LPS mutant TF 3/1 carries a point mutation in the lag-1 gene, resulting in a loss of the 8-O-acetyl groups in legionaminic acid [33], while the flagellin mutant is characterized by an in-frame deletion of the gene lpg1340, leading to a cessation of flagellin expression [34]. To exclude the potential impact of the CLR-Fc’s structure on the CLR–L. pneumophila interaction, we used C- and N-terminal hFc-fused murine CLEC12A in the binding study. An ELISA-based binding study revealed the binding of both CLEC12A fusion protein constructs to all tested heat-killed L. pneumophila strains (Figure 1B). Although the TF 3/1 mutant exhibited slightly reduced binding to CLEC12A, this finding suggests that CLEC12A recognition is independent of the Legionella LPS. A flow cytometry-based binding study demonstrated the recognition of intact L. pneumophila by murine CLEC12A (Figure 1C,D), thereby confirming and extending the results obtained by the ELISA-based assay (Figure 1B). In conclusion, CLEC12A recognizes L. pneumophila as well as the LPS- and flagellin-deficient mutants.
Figure 1.
The murine CLEC12A-hFc fusion protein recognizes L. pneumophila (A) Flow cytometry-based binding study of a comprehensive CLR-Fc fusion protein library to L. pneumophila wild-type strain JR32. A PE-conjugated anti-hFc antibody was used for CLR detection. The results are presented as the mean fluorescence intensities (MFI). (B) ELISA-based binding study using the L. pneumophila strains JR32, Corby, TF 3/1 and JR32 ΔflaA. C-terminal hFc (N-CTLD-hFc-C) and N-terminal hFc (N-hFc-CTLD-C) murine CLEC12A fusion proteins were incubated with the respective strains, and binding was detected using an anti-hFc HRP antibody. (C,D) Flow cytometry-based binding study using the L. pneumophila strains JR32, Corby, TF 3/1 and JR32 ΔflaA. The binding study was performed with C-terminal hFc (N-CTLD-hFc-C) and N-terminal hFc (N-hFc-CTLD-C) murine CLEC12A fusion proteins. The detection was performed using a PE-conjugated anti-hFc antibody. (C) Representative histograms of flow cytometry-based binding studies are shown. Values within the histograms show the percentage of L. pneumophila strains that were positive for a PE-conjugated anti-hFc antibody. The first values represent the percentages of the C-terminal hFc murine CLEC12A fusion proteins binding to the respective L. pneumophila strain; the second values represent the percentages of the N-terminal hFc murine CLEC12A fusion protein. (D) The results of the flow cytometry-based binding studies are presented as the mean fluorescence intensities (MFI). (E) ELISA-based binding study using LPS isolated from E. coli and L. pneumophila JR32. The binding of the CLR Langerin to its ligand (mannan) was included as a positive control. (F) Representative dot blot (of 3 independent experiments) using L. pneumophila JR32. The L. pneumophila JR32 samples were pipetted on the upper row as bacterial lysate (“lysate”), proteinase K-digested lysate (“lysate PK digested”) and heat-killed untreated L. pneumophila (“heat-killed”) (from left to right). On the bottom, the following controls were pipetted: hFc as a positive control (“pos. ctr.”), proteinase K-digested hFc (“pos. ctr. PK digested”) and proteinase K only (“PK only”) (from left to right). The left membrane was incubated with hFc (“hFc”) as a control; the right membrane was incubated with murine CLEC12A—C-terminal hFc (“mCLEC12A”). The detection was performed using an anti-hFc HRP antibody. (A–E) All data are shown as the mean ± SD (n = 3). Data were analyzed using a paired Student’s t-test. Asterisks indicate significant differences (n.s. = not significant, * p < 0.05, ** p < 0.01, *** p < 0.001).
To unravel the nature of the putative CLEC12A ligand in L. pneumophila, we extracted LPS from the wild-type strain JR32, as described by Lück et al. [35]. This extraction method allowed the separation of the L. pneumophila LPS into three fractions: cell-wall-bound compounds, outer membrane vesicles and soluble non-vesicular LPS. For the ELISA-based binding study, we used E. coli LPS as a control and compared the binding of murine CLEC12A and hFc to the different LPS preparations. As no significant binding of murine CLEC12A to LPS was observed (Figure 1E), LPS does not seem to play a major role in the interaction of L. pneumophila with murine CLEC12A. To test the proteinaceous nature of the putative murine CLEC12A ligand, L. pneumophila JR32 was lysed by sonication, followed by proteinase K digestion. The lysates were pipetted on a nitrocellulose membrane and incubated with hFc or murine CLEC12A-hFc followed by detection using an HRP-conjugated anti-hFc antibody (Figure 1F). Interestingly, CLEC12A still bound to the proteinase K-digested L. pneumophila lysate, thus rendering a L. pneumophila-derived protein ligand for CLEC12A unlikely (Figure 1F). While the exact nature of the CLEC12A ligand in L. pneumophila still needs to be identified, here, we identified murine CLEC12A as a CLR recognizing L. pneumophila.
2.2. CLEC12A Does Not Affect the Replication of L. pneumophila in Murine Macrophages and Infection-Induced Cytokine Responses
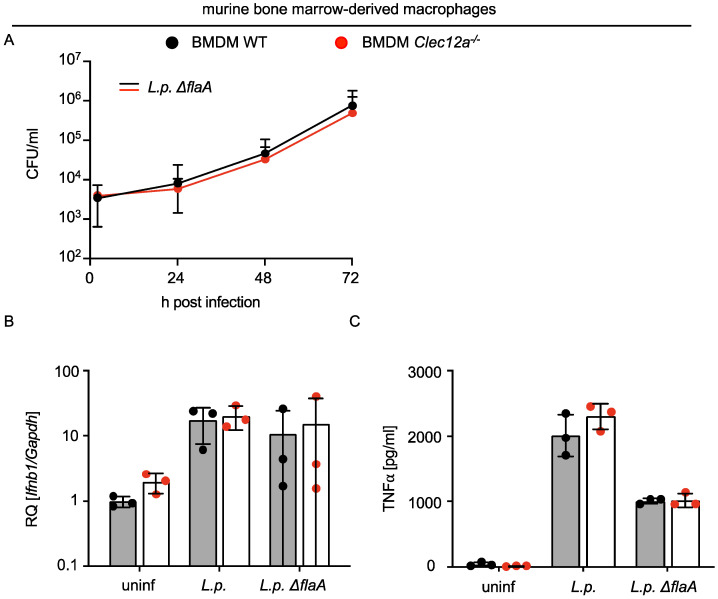
Given that murine CLEC12A binds L. pneumophila, we tested whether bacterial replication in murine macrophages or the production of cytokines was affected. Since the growth of L. pneumophila is restricted in C57Bl/6 macrophages by the NAIP5/NLRC4 inflammasome [15,16], L. pneumophila ΔflaA was used for the replication assay. The infection of bone marrow-derived macrophages (BMDMs) from WT and Clec12a−/− mice with L. pneumophila ΔflaA for over 72 h did not reveal any effect of CLEC12A on bacterial replication (Figure 2A). Moreover, the L. pneumophila-induced expression of Ifnb1 and the production of TNFα was not significantly influenced by a CLEC12A deficiency (Figure 2B,C). We therefore concluded that CLEC12A does not play a major role in controlling bacterial replication or in regulating cytokine production during L. pneumophila infection of murine macrophages.
Figure 2.
Replication of Legionella in murine BMDMs as well as type I IFN responses and TNFα production are not influenced by CLEC12A. (A) WT and Clec12a−/− BMDMs were infected with L. pneumophila ΔflaA at MOI 0.1, and replication was assessed by evaluating CFUs in the cells and supernatants after 2, 24, 48 and 72 h. (B,C) WT and Clec12a−/− BMDMs were infected with L. pneumophila JR32 (“L.p.”) or ΔflaA at MOI 10, and the expression of Ifnb1 was evaluated at 8 h after infection by qRT-PCR. TNFα levels were quantified from supernatants after 18 h. All data represent the mean ± SD of 3 independent experiments carried out in triplicate. Differences were assessed using multiple paired t-tests. Comparisons with p < 0.05 were considered significant.
2.3. The Limited Role of Human CLEC12A in L. pneumophila Infection
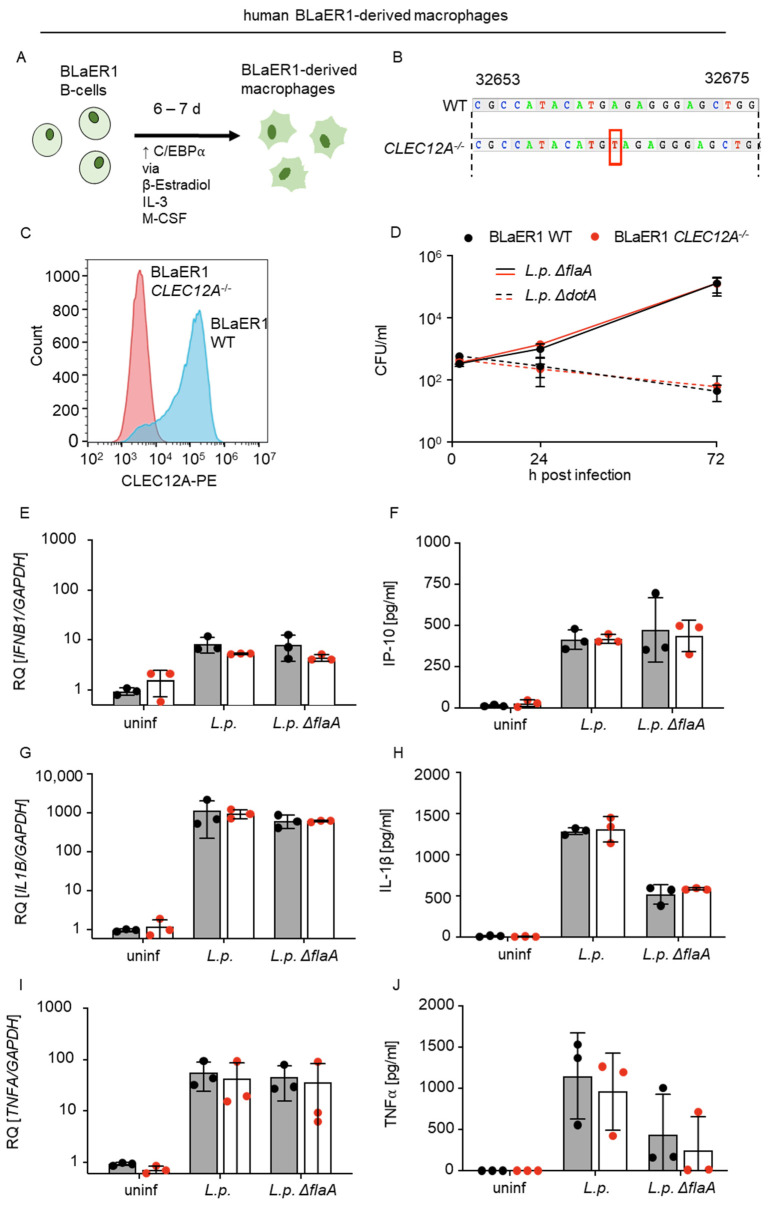
To investigate the role of CLEC12A during L. pneumophila infection in human cells, we used BLaER1-derived macrophage-like cells (Figure 3A). BlaER1-derived macrophages were generated from the immortalized B cell line BlaER1 by heterologous inducible expression of the myeloid transcription factor C/EBPα [36]. After transdifferentiation, BlaER1 macrophages are adherent, non-proliferative, highly phagocytic, have a macrophage-like transcriptional profile and morphology, and are well suited for studies of the innate immune response, as they behave more like primary human monocytes/macrophages than many of commonly used cell lines such as THP1 and U937 [37,38]. BLaER1-derived macrophages also support the replication of L. pneumophila to a similar extent to human alveolar macrophages and express CLEC12A (Figure S1A,B). We constructed a BLaER1 CLEC12A−/− line by using CRISPR/Cas9 (Figure 3B), then confirmed the deficiency in CLEC12A by flow cytometry (Figure 3C) and used the infected WT and CLEC12A−/− cells to assess bacterial replication and cytokine production. We observed no differences in bacterial load in the WT and CLEC12A−/− cells infected with L.p. ΔflaA or ΔdotA over the course of 72 h (Figure 3D). The L. pneumophila-induced expression of IFNB1, IL1B and TNFA (Figure 3E,G,I), and the production of the IFN-inducible cytokine IP-10 as well as IL-1β and TNFα (Figure 3F,H,J) were not significantly affected by the CLEC12A deficiency. Thus, our results do not support an important role of human CLEC12A in L. pneumophila infection.
Figure 3.
CLEC12A does not affect the replication of L. pneumophila or the production of proinflammatory cytokines and type I IFNs by BLaER1-derived human macrophages. (A) BLaER1 cells were transdifferentiated into BLaER1-derived macrophages by stimulation of the transcription factor C/EBPα with β-estradiol, IL-3 and M-CSF for 5 to 7 days. (B) BLaER1 CLEC12A−/− cells were generated by introducing a frameshift of one base into CLEC12A (see red box) by CRISPR/Cas9. (C) The loss of CLEC12A in BLaER1 cells was confirmed by flow cytometry, using an anti-CLEC12A-PE labeled antibody. (D) The replication of L. pneumophila (L.p.) ΔflaA and ΔdotA in BLaER1 WT and CLEC12A−/− cells was assessed by infecting cells at MOI 0.1 and evaluating CFUs at 2, 24 and 72 h after infection. BLaER1 WT and CLEC12A−/− cells were infected with L.p. WT and ΔflaA at MOI 10. The expression levels of IFNB1 (E), IL1B (G) and TNFA (I) were measured after 8 h by qRT-PCR and compared with uninfected controls. Data are shown as the relative quantification (RQ) of the target mRNAs relative to GAPDH. (F,H,J) Production of IP-10 (CXCL10) (F), IL-1β (H) and TNFα (J) was measured in supernatants of the infected BLaER1 WT and CLEC12A−/− cells after 18 h. (D–J) All data represent the mean ± SD of 3 independent experiments carried out in triplicate. Differences were assessed using multiple paired t-tests. Comparisons with p < 0.05 were considered significant.
2.4. Role of CLEC12A in Pulmonary L. pneumophila Infection In Vivo
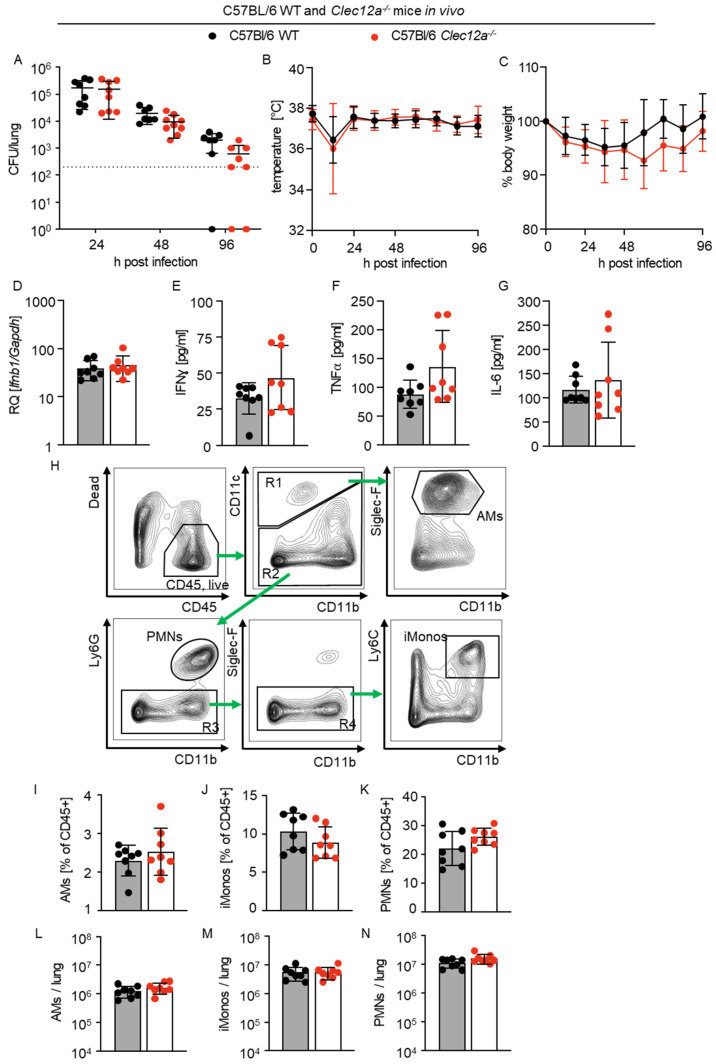
Finally, we investigated the role of CLEC12A during L. pneumophila infection in vivo [9,12,17]. C57BL/6 WT and Clec12a−/− mice were intranasally infected, and the bacterial loads in the lung were evaluated at different time points after infection. We observed a trend towards lower bacterial loads in the lungs of Clec12a−/− mice at 48 and 96 h after infection, which, however, was not significant (Figure 4A). Monitoring of temperature and body weight did also not reveal differences between WT and Clec12a−/− animals (Figure 4B,C). Next, we measured the gene expression and proinflammatory cytokine levels in the lungs of WT and Clec12a−/− mice at 24 h after infection. We found that neither the expression of pulmonary Ifnb1 nor the production of IFNy, TNFα or IL-6 were significantly influenced by a deficiency in CLEC12A (Figure 4D–G). Moreover, the ratio and number of alveolar macrophages (AMs), inflammatory monocytes (iMonos) and polymorphonuclear neutrophils (PMNs) in the lungs of WT and Clec12a−/− mice were similar (Figure 4H–N). Taken together, our data indicate that CLEC12A does not play an important role in pulmonary L. pneumophila infection in mice.
Figure 4.
CLEC12A plays a limited role in lung infection by L. pneumophila in mice. (A) WT and Clec12a−/− C57BL/6 mice were infected with L. pneumophila at a dose of 106 CFU/mouse, and the bacterial loads from their lungs were assessed at 24, 48 and 96 h after infection by plating serial dilutions of homogenized lungs on BYCE agar. (B,C) Infected WT and Clec12a−/− C57BL/6 mice were monitored for temperature (B) and body weight (C) over the course of the infection experiment. The expression levels of Ifnb1 in homogenized murine lungs 24 h after infection were assessed by qRT-PCR and normalized to the lungs of PBS-treated WT mice (D). (E–G) The levels of IFNγ, TNFα and IL-6 in mouse lungs were measured 24 h after infection. (H) Representative flow cytometric analyses of alveolar macrophages (AMs; CD45+, CD11c+, CD11b−, Siglec-F+), inflammatory monocytes (iMonos; CD45+, CD11c−, CD11b+, Ly6C+) and polymorphonuclear neutrophils (PMNs; CD45+, CD11c−, CD11b+, Ly6G+). Green arrows indicate the gating strategy. (I–N) Percentages and numbers of AMs, iMonos and PMNs in the lungs of WT and Clec12a−/− mice 24 h after infection. All data represent the means ± SD of 2 independent experiments, with 4 to 5 mice per experiment. Differences were assessed using a two-way ANOVA and the Mann–Whitney U-test. Comparisons with p < 0.05 were considered significant.
3. Discussion
The role of CLRs in the innate immune responses to bacterial pathogens in general and to L. pneumophila in particular is poorly understood. We therefore screened for CLRs capable of binding to L. pneumophila by using a library of CLR fusion proteins and identified CLEC12A as the CLR with the strongest bacterial binding capacity. Subsequent functional experiments, however, did not reveal any influence of CLEC12A on the infection of human and murine macrophages by L. pneumophila or antibacterial innate immunity. Moreover, CLEC12A did not influence L. pneumophila-induced pneumonia in mice. We thus conclude that CLEC12A is able to bind L. pneumophila but does not play a role in the host’s antibacterial responses to the infection.
CLEC12A has been described as an innate immune receptor for uric acid crystals, plasmodial hemozoin and mycobacterial mycolic acids with either anti-inflammatory or adaptive immunity-promoting functions [26,27,29]. Moreover, CLEC12A was found to potentiate the type I IFN responses induced by cytosolic RNA sensors, and to stimulate antibacterial autophagy during Salmonella infection by interacting with the endogenous host proteins [28,39]. Considering that all of these downstream effects are also of potential importance for L. pneumophila infection, that CLEC12A is highly expressed in the macrophages and that it strongly binds to the bacterium, we assumed that a deficiency in CLEC12A might influence L. pneumophila infection and the antibacterial defense. Unexpectedly, however, we observed no effect of CLEC12A on the replication of L. pneumophila in both human and murine macrophages, or on bacteria-induced cytokine production. Consistent with these findings, CLEC12A deficiency did not significantly influence L. pneumophila lung infections in vivo, although a non-significant trend towards lower bacterial loads in Clec12a−/− mice compared with the WT control mice was observed. Thus, while the possibility of a partly redundant immune regulatory function of CLEC12A during L. pneumophila lung infections cannot be fully excluded, our results do not support the important role of CLEC12A in L. pneumophila infection. Since L. pneumophila possesses RavZ-dependent and -independent mechanisms to prevent autophagic degradation of intravacuolar bacteria [40,41], it is possible that effects of CLEC12A on autophagy are masked by these bacterial mechanisms in the context of Legionella infection. Moreover, the role of individual CLRs in bacterial infections might also depend on the mouse model and the bacterial strain used, as recently shown, for example, for Mincle in pneumococcal infections [42,43]. Thus, we cannot exclude the possibility that we would have seen the effects of CLEC12A if we had used a different L. pneumophila strain.
To identify the interactions of L. pneumophila with the host CLRs, we used a comprehensive CLR-Fc fusion protein library that was established to identify novel CLR–ligand interactions [22,42]. Screening with this library revealed the substantial binding of CLEC12A to both intact L. pneumophila in a flow-cytometry based binding assay and heat-killed L. pneumophila in an ELISA-based study. While CLEC12A has been regarded so far as a CLR recognizing dead cells [26], pathogen-derived molecules were also reported as ligands for CLEC12A more recently [27,29]. In particular, the latter study highlighted that the recognition of CLEC12A is not restricted to ligands of crystalline nature such as uric acid or plasmodial hemozoin crystals, but it may also bind to lipidic ligands, thereby affecting the innate immune response and serving as a target for bacterial immune evasion mechanisms [29]. We initially considered L. pneumophila LPS as a candidate CLEC12A ligand, as it is the main antigen recognized by the antibodies contained in the serum of patients and plays a critical role in the early stages of infection by anchoring the bacteria to the host cell membrane [44]. L. pneumophila LPS differs from LPS from other Gram-negative bacteria by its high hydrophobicity caused by the presence of deoxy groups and N- and O-acyl substituents in legionaminic acid [44]. However, the binding of CLEC12A to the L. pneumophila LPS mutant TF 3/1 exhibiting a loss of the 8-O-acetyl groups in the legionaminic acid was only marginally affected compared with the wild-type strains Corby and JR32. Furthermore, we purified the L. pneumophila LPS according to established protocols and did not observe significant binding to CLEC12A in an ELISA-based assay, thus rendering a CLEC12A–LPS interaction unlikely. Subsequent binding assays evaluated by flow cytometry using a flagellin mutant (ΔflaA) ruled out flagellin as a CLEC12A ligand and, importantly, a dot-blot-based lectin assay using proteinase K-treated L. pneumophila lysates even excluded (glyco-)proteins as putative CLEC12A ligands. In light of a recent publication showing the binding of CLEC12A to mycolic acids from various mycobacterial species [29], it would be interesting to isolate L. pneumophila-derived lipid fractions and test them for binding to CLEC12A and for agonistic activity. However, a distinct CLEC12A ligand identification would go beyond the scope of the present study.
In order to examine CLEC12A’s functional role in human macrophages, we employed CRISPR/Cas9 technology to generate human CLEC12A-deficient BLaER1-derrived macrophages. BlaER1 macrophages have emerged as a suitable model for the study of innate immune recognition, since they behave more similarly to primary human monocytes/macrophages than many of commonly used cell lines such as THP1 and U937, and because genetic loss-of-function studies are possible [37,38]. They are adherent, non-proliferative and highly phagocytic, and develop a macrophage-like transcriptional profile [38]. Moreover, we demonstrated that BLaER1-derived macrophages express CLEC12A and enable the replication of L. pneumophila, similar to human alveolar macrophages. Thus, BLaER1-derived macrophages represent a suitable model for studying L. pneumophila infection in human macrophages.
In summary, our study identified that CLEC12A binds to L. pneumophila. While the exact Legionella ligands of CLEC12A remain unidentified, both LPS and (glyco-)proteins are unlikely to be involved in the binding of CLEC12A. Our functional experiments in murine and human macrophages, as well in vivo experiments in mice, did not provide any evidence for an important functional role of CLEC12A in L. pneumophila infection.
4. Materials and Methods
4.1. Ethical Statement
All animal experiments were carried out with strict adherence to German law (Tierschutzgesetz, TierSchG), following the approval of the corresponding institutional (Charité-Universitätsmedizin Berlin) and governmental animal welfare authorities (LAGeSo Berlin, approval ID G0334/17). Experiments with human alveolar macrophages were performed with the ethical approval of Charité EA2/079/13.
4.2. Bacteria and Culturing
The L. pneumophila strain JR32 of serogroup type I was used, as well as the isogenic mutants ΔflaA and ΔdotA. Further, the Corby strain and the isogenic mutant TF 3/1 were used [33]. The isogenic mutant TF3/1 was kindly provided by Dr. Christian Lück (Technische Universität Dresden). The L. pneumophila strains were cultured on buffered charcoal yeast extract (BCYE) agar plates for two days at 37 °C. For the binding studies, bacteria were grown in a medium of N-(2-acetamido)-2-aminoethanesulfonic acid (ACES)-buffered yeast extract (AYE) and were washed twice with PBS. Both the BCYE agar plates and the AYE medium were supplemented with L-cysteine and ferric nitrate. Heat-killing was performed at 75 °C for 1 h and verified by plating the bacteria on BCYE agar.
4.3. Mice
The generation of the Clec12a−/− mice was described previously [26] and the mice were backcrossed to a C57BL6/J background. The genotype of the Clec12a−/− mice was confirmed by PCR and flow cytometry analysis of spleen and bone marrow cells using PE-conjugated anti-mouse CLEC12A antibody (5D3/CLEC12A, Bio-Legend, San Diego, CA, USA, 1:200) [27]. C57BL6/J WT and Clec12a−/− mice were bred at the Institute for Biochemistry at Stiftung Tierärztliche Hochschule Hannover. Infection experiments were performed at the Charité-Universitätsmedizin Berlin, Department of Infectious Diseases, Respiratory Medicine and Critical Care.
4.4. Murine Model of Legionnaires’ Disease
Anesthetized 8- to 16-week-old female WT and Clec12a−/− mice were intranasally infected with 106 CFU L. pneumophila JR32 suspended in 40 µL PBS. Control mice were treated with 40 µL PBS. Mice were sacrificed at 24 and 48 h after infection, and their bacterial loads were evaluated by lysing cells of a homogenized lung suspension in 0.2% Triton X-100 and then plating a defined volume on BYCE agar. Additionally, cytokine and mRNA levels as well as the numbers of different cell populations were evaluated after 24 h and compared with the PBS-treated control group.
4.5. CLR-Fc Fusion Proteins
The production of CLR-Fc fusion protein was performed as described [30,31]. Briefly, cDNA encoding the C-type lectin-like domain (CTLD) of the respective CLR was ligated into the pFuse-hIgG1-Fc (hFc) expression vector (Invivogen, Toulouse, France). For the murine CLEC12A (mCLEC12A)–C-terminal hFc fusion protein (N-CTLD-hFc-C), the amplified cDNA was fused to the N-terminus of hFc. Vice versa, for N-terminal hFc fusion proteins (N-hFc-CTLD-C), the cDNA was fused to the C-terminus of hFc. For protein expression, CHO cells were transfected with the respective plasmid. Proteins were purified using HiTrap Protein G HP columns (GE Healthcare, Chicago, IL, USA) [45]. The purity and identity of the fusion proteins were tested by SDS-PAGE followed by Coomassie staining and Western blotting using a HRP-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch Labs, Cambridgeshire, UK, RRID: AB_2337586) [30]. Subsequently, the functionality of the fusion proteins was tested. For CLEC12A-hFc, binding studies with monosodium urate (MSU) were performed [26].
4.6. ELISA-Based Binding Studies
The ELISA-based binding studies were conducted as described in Mayer et al. [31]. Briefly, heat-killed bacteria or bacterial LPS from L. pneumophila and E. coli (Sigma-Aldrich, St. Louis, MO, USA) were coated on half-area microplates (Greiner Bio-One GmbH, Frickenhausen, Germany) and incubated with 250 ng of the respective fusion proteins in a lectin-binding buffer (50 mM HEPES, 5 mM magnesium chloride and 5 mM calcium chloride; pH 7.4). Binding of the fusion proteins was detected using a 1:5.000-diluted HRP-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch Labs, Cambridgeshire, UK, RRID: AB_2337586). An OPD-Solution (o-phenylenediamine dihydrochloride substrate tablet (Thermo Fisher, Waltham, MA, USA), 24 mM oxalosuccinic acid, 0.04% H2O2 and 50 mM disodium hydrogen phosphate in H2O) was used as the HRP substrate, and the reaction was stopped with 2.5 M sulfuric acid. Finally, the optical density was measured at 495 nm using a Multiskan Go microplate spectrophotometer (Thermo Fisher Scientific, Waltham, MA, USA). For the LPS binding studies, substrate oxidization was measured in a kinetic loop at 450 nm.
4.7. Flow Cytometry-Based Binding Studies
Samples were prepared as described in Mayer et al. [31]. Live bacteria were incubated with 250 ng of fusion proteins in a lectin binding buffer. The binding of the fusion proteins was detected using a 1:200-diluted polyclonal goat anti-human IgG (Fc)-PE (Jackson ImmunoResearch Labs, Cambridgeshire, UK, RRID: AB_2337675). Samples were analyzed using an Attune NxT Flow Cytometer (Thermo Fisher Scientific, MA, USA). For data analysis, FlowJo Software (FlowJo, Ashland, OR, USA) was used.
4.8. LPS Extraction
Extraction of the LPS from L. pneumophila WT JR32 was performed according to Lück et al. [35]. Briefly, the bacterial culture was centrifuged (with the pellet considered to be cell-wall-bound components), and the supernatant was sequentially filtered through a 0.2 µm strainer and filters with a 300 and 10 kDA molecular weight cut-off (MWCO) (Corning Spin-X UF concentrators, Corning Inc., Corning, NY, USA). Fractions that were retained by the 300 kDa MWCO filter were considered to be outer membrane vesicles. Flow-through that had passed the 300 kDa MWCO filter but was retained by the 10 kDa MWCO filter was considered to be soluble, non-vesicular LPS. Both the cell-wall-bound components and the soluble non-vesicular LPS were heat-inactivated and treated with Proteinase K (Thermo Fisher, MA, USA) to exclude protein-based interactions in the subsequent binding studies.
4.9. Immunoblotting
L. pneumophila JR32 was lysed by sonication (Bandelin, Sonopuls, Berlin, Germany) in a lysis buffer (150 mM sodium chloride, 1% Triton X-100, 50 mM Tris; pH 8.0), followed by Proteinase K treatment (0.05 mg/mL). Afterwards, the samples were pipetted onto a nitrocellulose membrane (Macherey-Nagel, Düren, Germany), and an immunoblot test was performed as described by Raulf et al. [46]. Briefly, the membrane was blocked with a 5% milk solution and then incubated with the respective CLR-Fc fusion proteins (1 µg/mL). For detection, an HRP-conjugated goat anti-human IgG antibody (Jackson ImmunoResearch Labs, RRID: AB_2337586) was diluted 1:10.000 in TBS + 0.05% Tween-20. The signals were visualized using Amersham ECL Prime Western Blotting Detection Reagent (Thermo Fisher, MA, USA). Chemiluminescence was measured using a chemiluminescence imager (Bio-Rad Laboratories, Hercules, CA, USA).
4.10. Isolation and Differentiation of Murine BMMs
Bone marrow cells from WT and Clec12a−/− mice were isolated from the tibia and femur of 8- to 16-week-old mice of both sexes. The bones were washed in 70% ethanol and flushed with IMDM + 10% FCS + 2mM L-glutamine + 100 U/mL pen/strep. The collected cell suspension was passed through a 40 µm cell strainer and centrifuged at 300× g for 5 min. RBC lysis (90% 160 mM NH4CL + 10% 100mM Tris-HCL (pH 7.5)) was performed, and bone marrow cells were washed and stored at -150 °C in 10% DMSO. For differentiation into bone-marrow-derived macrophages (BMMs), cells were cultivated in a BMM growth medium (RPMI 1640 + 20% FCS + 30% L929 fibroblast supernatant + 4.5 mM L-glutamine + 100 µg/mL pen/strep) for 10 days, and fresh medium was added on Day 4 of cultivation. Differentiated cells were replated in a BMM replating medium (RPMI 1640 + 10% FCS + 15% L929 fibroblast supernatant + 4.5 mM L-glutamine) at a density of 4 × 105 cells/mL in 48-well plates one day before the experiment.
4.11. Isolation of Human Alveolar Macrophages
Primary human alveolar macrophages (AM) were isolated by repeated perfusion of the human lung tissue (with ethical approval from Charité EA2/079/13) with HBSS as described previously [47].
4.12. Human BLaER1 Cell Transdifferentiation and Generation of a Human BLaER1
CLEC12A−/− Line
Human BLaER1 B-cells were kindly provided by Prof. Thomas Graf (Center for Genomic Regulation, Barcelona, Spain). The cells were grown in a BLaER1 cultivation medium (RPMI 1640 + 10% FCS + 100 µg/mL pen/strep) and passaged every 4 days. For transdifferentiation into BLaER1-derived macrophages, the cells were seeded into a 48-well plate in a BLaER1 differentiation medium (RPMI 1640 + 10% FCS + 100 µg/mL pen/strep + 10 ng/mL IL-3 and 10 ng/mL M-CSF + 100 nM β-estradiol). The cells were incubated for 6 days, and the stage of differentiation was confirmed via flow cytometry by evaluating the expression of CD11b and CD19. The generation of a BLaER1 CLEC12A−/− cell line was achieved using the CRISPR/Cas9 system. The CAS9 enzyme and specific guide RNA (GCTGGACGCCATACATGAGA) (IDT, IA, USA) were assembled in vitro, and the ribonucleoprotein was mixed with the cell suspension, followed by electroporation in a 4-D nucleofector (Lonza, Basel, Switzerland) with the program EH-140.
Electroporated cells were collected in a prewarmed medium and incubated at 37 °C and 5% CO2 for 72 h. Single cells were sorted by flow cytometry and expanded in a 96-well plate and incubated at 37 °C and 5% CO2. Screening for indel mutations was achieved by extracting the DNA from 105 cells of each clone and performing PCR, followed by Sanger sequencing. The primers used for PCR and sequencing were as follows: CLEC12A forward primer: tgacatgccacaattgtctactca; reverse primer: ttgccaagactcccaatccaa. Loss-of-function mutations in the BLaER1 clones were confirmed by flow cytometry.
4.13. Short-Term Infection of Murine BMMs and Human BLaER1 Cells
Mouse BMMs and human BLaER1 cells were seeded in 48-well plates and infected with L. pneumophila JR32 WT and ΔflaA at MOI 10. Infected cells were centrifuged at 200× g for 5 min and then incubated for 8 h (gene expression) or 18 h (cytokine production) at 37 °C and 5% CO2, respectively.
4.14. Quantitative RT-PCR
Total RNA was isolated from the cultured cells or lung homogenates using the RNeasy Plus Mini purification system (QIAGEN, Düsseldorf, Germany) or Trizol (Life Technologies, Darmstadt, Germany), respectively. Total RNA was reverse-transcribed using the high-capacity reverse transcription kit (Applied Biosystems, Darmstadt, Germany), and quantitative PCR was performed using TaqMan assays for murine Ifnb1 and human IFNB1 as well as human IL1B and TNFA (Thermo Fisher, MA, USA), on a qTOWER3 G instrument (Analytik Jena AG, Jena, Germany). The input was normalized to the average expression of murine and human GAPDH, and the relative expression (relative quantity, RQ) of the respective gene in untreated cells or PBS-treated mice was set to 1.
4.15. ELISA
The cytokine levels of TNFα, IFNβ and IL-6 from homogenized lung lysates of mice were measured with the LEGENDplex Mouse Anti-Virus Response Panel Multi-Analyte Flow Assay Kit (BioLegend, CA, USA). The concentrations of human IP-10 as well as human IL-1β and human TNFα (R&D System, MN, USA) and murine TNFα (Thermo Fischer, MA, USA) in the supernatants from cells infected in vitro were quantified by commercially available sandwich ELISA kits. The protein concentrations were determined in a FilterMax F5 Multi-Mode Microplate Reader (Molecular Devices, Sunnyvale, CA, USA) at 450 nm.
4.16. Flow Cytometry Assay
For determination of the number of specific cell populations in murine lungs, the cell suspensions were labeled with anti-CD45.2 (BioLegend, CA, USA), anti-CD11b (BioLegend, CA, USA), anti-Ly6G (BioLegend, CA, USA), anti-Ly6C (BioLegend, CA, USA) and anti-CD11c (BioLegend CA, USA). Additionally, cells were labeled with a live–dead fixable viability cell marker (Thermo Fischer, MA, USA). The differentiation stages of the BLaER1 cells were assessed by labeling the cells with anti-CD19 (BioLegend, CA, USA) and anti-CD11b (BioLegend, CA, USA). Confirmation of functional CLEC12A knock-out was evaluated by labeling with anti-CLEC12A (Milteny Biotec, Bergisch Gladbach, Germany). The analyses were conducted on a Becton Dickinson LSRFortessa flow cytometer using FACS DIVA software (BD Bioscience). Data analysis was carried out using FlowJo software (Tree Star).
4.17. Intracellular Bacterial Replication Assay
Murine BMMs, human BLaER1 cells and human alveolar macrophages were infected with a MOI of 0.1. Plates with the bacterial suspension added to the cells were centrifuged at 200× g for 5 min and subsequentially incubated at 37 °C and 5% CO2. After 30 min, the cells were washed with PBS and cell media supplemented with 50 µg/mL gentamycin. After 1 h, cells were washed twice with PBS, and fresh medium was added. The cells were incubated at 37 °C and 5% CO2, and lysis was performed with a 1% saponin solution at 2, 24, 48 and 72 h after infection. The combined CFUs of the cell lysate and the supernatants were evaluated by plating a defined volume of different serial dilutions on BCYE agar.
4.18. Statistical Analyses
All data are presented as the mean ± SD. The data were analyzed using GraphPad Prism Version 8 (GraphPad Software, La Jolla, CA, USA), and paired Student’s t-tests or multiple paired t-tests were performed for the in vitro assays, while the in vivo assays were analyzed using a two-way ANOVA and the Mann–Whitney U-test. Asterisks indicate significant differences (n.s. = not significant, * p < 0.05, ** p < 0.01, *** p < 0.001).
Acknowledgments
We thank Silke Schöneberg for expert technical assistance.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/ijms24043891/s1.
Author Contributions
Conceptualization, B.L. and B.O.; methodology, A.-B.K. and C.D., J.L., K.H., S.C. and F.F.V.; formal analysis, A.-B.K. and C.D.; investigation, A.-B.K., C.D., J.L., F.S., S.M.-L. and S.C.; resources, S.F., J.R., K.H., A.C.H. and M.W.; data curation, A.-B.K. and C.D.; writing—original draft preparation, A.-B.K. and C.D.; writing—review and editing, A.-B.K., C.D., B.L. and B.O.; visualization, A.-B.K. and C.D.; supervision, B.L. and B.O.; funding acquisition, B.L. and B.O. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
All animal experiments were carried out with strict adherence to German law (Tierschutzgesetz, TierSchG), following the approval of the corresponding institutional (Charité Universitätsmedizin Berlin) and governmental animal welfare authorities (LAGeSo Berlin, approval ID G0334/17). Experiments with human alveolar macrophages were performed following ethical approval by Charité EA2/079/13.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
All supporting information is provided in the submitted Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
Parts of this work are going to be included in the PhD theses of A.B.K. and C.D. The work was supported by the IMPRS-IDI to A.B.K., by the Deutsche Forschungsgemeinschaft (LE 2498/11-1 to B.L., OP 86/12-1 to B.O. and SFB/TR84 to B.O., M.W. and A.H.) and the German Federal Ministry of Education and Research (MAPVAP FKZ 16GW0247 to B.O.). J.R. acknowledges funding from the Deutsche Forschungsgemeinschaft (Project-ID 360372040—SFB 1335), and the European Research Council (ERC) under the European Union’s Horizon 2020 research and innovation program (grant agreement No. 834154).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Fields B.S. The Molecular Ecology of Legionellae. Trends Microbiol. 1996;4:286–290. doi: 10.1016/0966-842X(96)10041-X. [DOI] [PubMed] [Google Scholar]
- 2.Cunha B.A., Burillo A., Bouza E. Legionnaires’ Disease. Lancet. 2016;387:376–385. doi: 10.1016/S0140-6736(15)60078-2. [DOI] [PubMed] [Google Scholar]
- 3.Phin N., Parry-Ford F., Harrison T., Stagg H.R., Zhang N., Kumar K., Lortholary O., Zumla A., Abubakar I. Epidemiology and Clinical Management of Legionnaires’ Disease. Lancet Infect. Dis. 2014;14:1011–1021. doi: 10.1016/S1473-3099(14)70713-3. [DOI] [PubMed] [Google Scholar]
- 4.Isberg R.R., O’Connor T., Heidtman M. The Legionella pneumophila Replication Vacuole: Making a Cozy Niche inside Host Cells. Nat. Rev. Microbiol. 2009;7:13–24. doi: 10.1038/nrmicro1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Horwitz M.A. Formation of a Novel Phagosome by the Legionnaires’ Disease Bacterium (Legionella pneumophila) in Human Monocytes. J. Exp. Med. 1983;158:1319–1331. doi: 10.1084/jem.158.4.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hubber A., Roy C.R. Modulation of Host Cell Function by Legionella pneumophila Type IV Effectors. Annu. Rev. Cell Dev. Biol. 2010;26:261–283. doi: 10.1146/annurev-cellbio-100109-104034. [DOI] [PubMed] [Google Scholar]
- 7.Asrat S., de Jesús D.A., Hempstead A.D., Ramabhadran V., Isberg R.R. Bacterial Pathogen Manipulation of Host Membrane Trafficking. Annu. Rev. Cell Dev. Biol. 2014;30:79–109. doi: 10.1146/annurev-cellbio-100913-013439. [DOI] [PubMed] [Google Scholar]
- 8.Naujoks J., Lippmann J., Suttorp N., Opitz B. Innate Sensing and Cell-Autonomous Resistance Pathways in Legionella pneumophila Infection. Int. J. Med. Microbiol. 2018;308:161–167. doi: 10.1016/j.ijmm.2017.10.004. [DOI] [PubMed] [Google Scholar]
- 9.Ruiz-Moreno J.S., Hamann L., Shah J.A., Verbon A., Mockenhaupt F.P., Puzianowska-Kuznicka M., Naujoks J., Sander L.E., Witzenrath M., Cambier J.C., et al. The Common HAQ STING Variant Impairs CGAS-Dependent Antibacterial Responses and Is Associated with Susceptibility to Legionnaires’ Disease in Humans. PLoS Pathog. 2018;14:e1006829. doi: 10.1371/journal.ppat.1006829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Massis L.M., Zamboni D.S. Innate Immunity to Legionella pneumophila. Front. Microbiol. 2011;2:109. doi: 10.3389/fmicb.2011.00109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Liu X., Shin S. Viewing Legionella pneumophila Pathogenesis through an Immunological Lens. J. Mol. Biol. 2019;431:4321–4344. doi: 10.1016/j.jmb.2019.07.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Naujoks J., Tabeling C., Dill B.D., Hoffmann C., Brown A.S., Kunze M., Kempa S., Peter A., Mollenkopf H.-J., Dorhoi A., et al. IFNs Modify the Proteome of Legionella-Containing Vacuoles and Restrict Infection Via IRG1-Derived Itaconic Acid. PLoS Pathog. 2016;12:e1005408. doi: 10.1371/journal.ppat.1005408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ziltener P., Reinheckel T., Oxenius A. Neutrophil and Alveolar Macrophage-Mediated Innate Immune Control of Legionella pneumophila Lung Infection via TNF and ROS. PLoS Pathog. 2016;12:e1005591. doi: 10.1371/journal.ppat.1005591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Skerrett S.J., Bagby G.J., Schmidt R.A., Nelson S. Antibody-Mediated Depletion of Tumor Necrosis Factor-a Impairs Pulmonary Host Defenses to Legionella pneumophila. J. Infect. 1997;176:1019–1028. doi: 10.1086/516530. [DOI] [PubMed] [Google Scholar]
- 15.Zamboni D.S., Kobayashi K.S., Kohlsdorf T., Ogura Y., Long E.M., Vance R.E., Kuida K., Mariathasan S., Dixit V.M., Flavell R.A., et al. The Birc1e Cytosolic Pattern-Recognition Receptor Contributes to the Detection and Control of Legionella pneumophila Infection. Nat. Immunol. 2006;7:318–325. doi: 10.1038/ni1305. [DOI] [PubMed] [Google Scholar]
- 16.Molofsky A.B., Byrne B.G., Whitfield N.N., Madigan C.A., Fuse E.T., Tateda K., Swanson M.S. Cytosolic Recognition of Flagellin by Mouse Macrophages Restricts Legionella pneumophila Infection. J. Exp. Med. 2006;203:1093–1104. doi: 10.1084/jem.20051659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lippmann J., Müller H.C., Naujoks J., Tabeling C., Shin S., Witzenrath M., Hellwig K., Kirschning C.J., Taylor G.A., Barchet W., et al. Dissection of a Type I Interferon Pathway in Controlling Bacterial Intracellular Infection in Mice: Role of Type I IFNs in L. pneumophila Infection. Cell. Microbiol. 2011;13:1668–1682. doi: 10.1111/j.1462-5822.2011.01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Prado Acosta M., Lepenies B. Bacterial Glycans and Their Interactions with Lectins in the Innate Immune System. Biochem. Soc. Trans. 2019;47:1569–1579. doi: 10.1042/BST20170410. [DOI] [PubMed] [Google Scholar]
- 19.Sancho D., Reis e Sousa C. Signaling by Myeloid C-Type Lectin Receptors in Immunity and Homeostasis. Annu. Rev. Immunol. 2012;30:491–529. doi: 10.1146/annurev-immunol-031210-101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brown G.D., Willment J.A., Whitehead L. C-Type Lectins in Immunity and Homeostasis. Nat. Rev. Immunol. 2018;18:374–389. doi: 10.1038/s41577-018-0004-8. [DOI] [PubMed] [Google Scholar]
- 21.Marshall A.S.J., Willment J.A., Pyż E., Dennehy K.M., Reid D.M., Dri P., Gordon S., Wong S.Y.C., Brown G.D. Human MICL (CLEC12A) Is Differentially Glycosylated and Is down-Regulated Following Cellular Activation. Eur. J. Immunol. 2006;36:2159–2169. doi: 10.1002/eji.200535628. [DOI] [PubMed] [Google Scholar]
- 22.Fischer S., Stegmann F., Gnanapragassam V.S., Lepenies B. From Structure to Function—Ligand Recognition by Myeloid C-Type Lectin Receptors. Comput. Struct. Biotechnol. J. 2022;20:5790–5812. doi: 10.1016/j.csbj.2022.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pyż E., Huysamen C., Marshall A.S.J., Gordon S., Taylor P.R., Brown G.D. Characterisation of Murine MICL (CLEC12A) and Evidence for an Endogenous Ligand. Eur. J. Immunol. 2008;38:1157–1163. doi: 10.1002/eji.200738057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Redelinghuys P., Whitehead L., Augello A., Drummond R.A., Levesque J.-M., Vautier S., Reid D.M., Kerscher B., Taylor J.A., Nigrovic P.A., et al. MICL Controls Inflammation in Rheumatoid Arthritis. Ann. Rheum. Dis. 2016;75:1386–1391. doi: 10.1136/annrheumdis-2014-206644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sagar D., Singh N.P., Ginwala R., Huang X., Philip R., Nagarkatti M., Nagarkatti P., Neumann K., Ruland J., Andrews A.M., et al. Antibody Blockade of CLEC12A Delays EAE Onset and Attenuates Disease Severity by Impairing Myeloid Cell CNS Infiltration and Restoring Positive Immunity. Sci. Rep. 2017;7:2707. doi: 10.1038/s41598-017-03027-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Neumann K., Castiñeiras-Vilariño M., Höckendorf U., Hannesschläger N., Lemeer S., Kupka D., Meyermann S., Lech M., Anders H.-J., Kuster B., et al. Clec12a Is an Inhibitory Receptor for Uric Acid Crystals That Regulates Inflammation in Response to Cell Death. Immunity. 2014;40:389–399. doi: 10.1016/j.immuni.2013.12.015. [DOI] [PubMed] [Google Scholar]
- 27.Raulf M.-K., Johannssen T., Matthiesen S., Neumann K., Hachenberg S., Mayer-Lambertz S., Steinbeis F., Hegermann J., Seeberger P.H., Baumgärtner W., et al. The C-Type Lectin Receptor CLEC12A Recognizes Plasmodial Hemozoin and Contributes to Cerebral Malaria Development. Cell Rep. 2019;28:30–38.e5. doi: 10.1016/j.celrep.2019.06.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Begun J., Lassen K.G., Jijon H.B., Baxt L.A., Goel G., Heath R.J., Ng A., Tam J.M., Kuo S.-Y., Villablanca E.J., et al. Integrated Genomics of Crohn’s Disease Risk Variant Identifies a Role for CLEC12A in Antibacterial Autophagy. Cell Rep. 2015;11:1905–1918. doi: 10.1016/j.celrep.2015.05.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nishimura N., Tomiyasu N., Torigoe S., Mizuno S., Fukano H., Ishikawa E., Katano H., Hoshino Y., Matsuo K., Takahashi M., et al. Mycobacterial Mycolic Acids Trigger Inhibitory Receptor Clec12A to Suppress Host Immune Responses. Tuberculosis. 2023;138:102294. doi: 10.1016/j.tube.2022.102294. [DOI] [PubMed] [Google Scholar]
- 30.Maglinao M., Eriksson M., Schlegel M.K., Zimmermann S., Johannssen T., Götze S., Seeberger P.H., Lepenies B. A Platform to Screen for C-Type Lectin Receptor-Binding Carbohydrates and Their Potential for Cell-Specific Targeting and Immune Modulation. J. Control. Release. 2014;175:36–42. doi: 10.1016/j.jconrel.2013.12.011. [DOI] [PubMed] [Google Scholar]
- 31.Mayer S., Moeller R., Monteiro J.T., Ellrott K., Josenhans C., Lepenies B. C-Type Lectin Receptor (CLR)–Fc Fusion Proteins As Tools to Screen for Novel CLR/Bacteria Interactions: An Exemplary Study on Preselected Campylobacter Jejuni Isolates. Front. Immunol. 2018;9:213. doi: 10.3389/fimmu.2018.00213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ricci M.L., Fillo S., Ciammaruconi A., Lista F., Ginevra C., Jarraud S., Girolamo A., Barbanti F., Rota M.C., Lindsay D., et al. Genome Analysis of Legionella pneumophila ST23 from Various Countries Reveals Highly Similar Strains. Life Sci. Alliance. 2022;5:e202101117. doi: 10.26508/lsa.202101117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lück P.C., Freier T., Steudel C., Knirel Y.A., Lüneberg E., Zähringer U., Helbig J.H. A Point Mutation in the Active Site of Legionella pneumophila O-Acetyltransferase Results in Modified Lipopolysaccharide but Does Not Influence Virulence. Int. J. Med. Microbiol. 2001;291:345–352. doi: 10.1078/1438-4221-00140. [DOI] [PubMed] [Google Scholar]
- 34.Tao L., Zhu W., Hu B.-J., Qu J.-M., Luo Z.-Q. Induction of Rapid Cell Death by an Environmental Isolate of Legionella pneumophila in Mouse Macrophages. Infect. Immun. 2013;81:3077–3088. doi: 10.1128/IAI.00252-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lück C., Helbig J.H. Characterization of Legionella Lipopolysaccharide. In: Buchrieser C., Hilbi H., editors. Legionella. Volume 954. Humana Press; Totowa, NJ, USA: 2013. pp. 381–390. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 36.Rapino F., Robles E.F., Richter-Larrea J.A., Kallin E.M., Martinez-Climent J.A., Graf T. C/EBPα Induces Highly Efficient Macrophage Transdifferentiation of B Lymphoma and Leukemia Cell Lines and Impairs Their Tumorigenicity. Cell Rep. 2013;3:1153–1163. doi: 10.1016/j.celrep.2013.03.003. [DOI] [PubMed] [Google Scholar]
- 37.Gaidt M.M., Ebert T.S., Chauhan D., Schmidt T., Schmid-Burgk J.L., Rapino F., Robertson A.A.B., Cooper M.A., Graf T., Hornung V. Human Monocytes Engage an Alternative Inflammasome Pathway. Immunity. 2016;44:833–846. doi: 10.1016/j.immuni.2016.01.012. [DOI] [PubMed] [Google Scholar]
- 38.Gaidt M.M., Rapino F., Graf T., Hornung V. Modeling Primary Human Monocytes with the Trans–Differentiation Cell Line BLaER1. In: De Nardo D., De Nardo C.M., editors. Innate Immune Activation. Volume 1714. Springer New York; New York, NY, USA: 2018. pp. 57–66. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 39.Li K., Neumann K., Duhan V., Namineni S., Hansen A.L., Wartewig T., Kurgyis Z., Holm C.K., Heikenwalder M., Lang K.S., et al. The Uric Acid Crystal Receptor Clec12A Potentiates Type I Interferon Responses. Proc. Natl. Acad. Sci. USA. 2019;116:18544–18549. doi: 10.1073/pnas.1821351116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Omotade T.O., Roy C.R. Legionella pneumophila Excludes Autophagy Adaptors from the Ubiquitin-Labeled Vacuole in Which It Resides. Infect. Immun. 2020;88:e00793-19. doi: 10.1128/IAI.00793-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Choy A., Dancourt J., Mugo B., O’Connor T.J., Isberg R.R., Melia T.J., Roy C.R. The Legionella Effector RavZ Inhibits Host Autophagy Through Irreversible Atg8 Deconjugation. Science. 2012;338:1072–1076. doi: 10.1126/science.1227026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rabes A., Zimmermann S., Reppe K., Lang R., Seeberger P.H., Suttorp N., Witzenrath M., Lepenies B., Opitz B. The C-Type Lectin Receptor Mincle Binds to Streptococcus pneumoniae but Plays a Limited Role in the Anti-Pneumococcal Innate Immune Response. PLoS ONE. 2015;10:e0117022. doi: 10.1371/journal.pone.0117022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Behler-Janbeck F., Takano T., Maus R., Stolper J., Jonigk D., Tort Tarrés M., Fuehner T., Prasse A., Welte T., Timmer M.S.M., et al. C-Type Lectin Mincle Recognizes Glucosyl-Diacylglycerol of Streptococcus pneumoniae and Plays a Protective Role in Pneumococcal Pneumonia. PLoS Pathog. 2016;12:e1006038. doi: 10.1371/journal.ppat.1006038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kowalczyk B., Chmiel E., Palusinska-Szysz M. The Role of Lipids in Legionella-Host Interaction. Int. J. Mol. Sci. 2021;22:1487. doi: 10.3390/ijms22031487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Johannssen T., Lepenies B. Identification and Characterization of Carbohydrate-Based Adjuvants. In: Lepenies B., editor. Carbohydrate-Based Vaccines. Volume 1331. Springer New York; New York, NY, USA: 2015. pp. 173–187. Methods in Molecular Biology. [DOI] [PubMed] [Google Scholar]
- 46.Raulf M.-K., Lepenies B., Strube C. Toxocara Canis and Toxocara Cati Somatic and Excretory-Secretory Antigens Are Recognised by C-Type Lectin Receptors. Pathogens. 2021;10:321. doi: 10.3390/pathogens10030321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Berg J., Zscheppang K., Fatykhova D., Tönnies M., Bauer T.T., Schneider P., Neudecker J., Rückert J.C., Eggeling S., Schimek M., et al. Tyk2 as a Target for Immune Regulation in Human Viral/Bacterial Pneumonia. Eur. Respir. J. 2017;50:1601953. doi: 10.1183/13993003.01953-2016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
All supporting information is provided in the submitted Supplementary Materials.