Abstract
Rapid Whole Genome Sequencing (rWGS) represents a valuable exploration in critically ill pediatric patients. Early diagnosis allows care to be adjusted. We evaluated the feasibility, turnaround time (TAT), yield, and utility of rWGS in Belgium. Twenty-one unrelated critically ill patients were recruited from the neonatal intensive care units, the pediatric intensive care unit, and the neuropediatric unit, and offered rWGS as a first tier test. Libraries were prepared in the laboratory of human genetics of the University of Liège using Illumina DNA PCR-free protocol. Sequencing was performed on a NovaSeq 6000 in trio for 19 and in duo for two probands. The TAT was calculated from the sample reception to the validation of results. Clinical utility data were provided by treating physicians. A definite diagnosis was reached in twelve (57.5%) patients in 39.80 h on average (range: 37.05–43.7). An unsuspected diagnosis was identified in seven patients. rWGS guided care adjustments in diagnosed patients, including a gene therapy, an off-label drug trial and two condition-specific treatments. We successfully implemented the fastest rWGS platform in Europe and obtained one of the highest rWGS yields. This study establishes the path for a nationwide semi-centered rWGS network in Belgium.
Keywords: rapid whole genome sequencing, rWGS, critically ill newborn, NICU, NICU-Seq, Dragen, Base Space Sequence Hub
1. Introduction
Genetic diseases are recognized as an important cause of admission and death in neonatal and pediatric intensive care units [1,2], and the proportion of genetic diagnoses is the highest among critically ill patients [3]. Early testing helps in diagnosing those diseases, and providing useful information at a time when it is still possible to modify the path of the disease. The traditional hypothesis-driven diagnostic approach may not be efficient in these patients, since the clinical expression during the neonatal period is often nonspecific, and phenotypes with reduced survival for many genetic diseases are not fully clinically recognizable because of very early deaths [4]. In addition, the standard turnaround time (TAT) for genome-wide sequencing tests (Mendeliome or exomes) does not allow the diagnosis to impact most clinical decisions in a timely fashion. Therefore, severely ill neonatal and infant populations may be considered an uncharacterized niche for early-onset severe and lethal forms of known diseases and for early onset of yet-unknown lethal diseases, for which hypothesis-free genetic testing would be required.
Whole Genome Sequencing (WGS) has been widely proven to be the best exploration method in critically ill pediatric patients, as it allows a hypothesis-free process and the exploration of multiple types of genetic defects, including single nucleotide variants (SNVs), copy number variations (CNVs), complex structural variants (SNVs), repeat expansions and mosaic aneuploidies [3,5,6,7,8,9]. A few teams worldwide are progressively compressing the time from sampling to the return of results (turnaround time, or TAT) of the WGS for critically ill pediatric patients. The current TAT of this fast-track WGS, also referred to as rapid or ultra-rapid whole genome sequencing (rWGS), varies from barely more than 7 h to a few weeks, depending on the workflow and the sequencing technology [3,5,10,11,12,13]. Although these improvements have mostly been reported by USA teams, reports on the implementation of rWGS in understudied populations are also surfacing [5]. The shortest reported TATs in Europe thus far, regardless of the approach (exome sequencing or genome sequencing), ranged between 5 and 21 days depending on the study [5,14,15,16].
According to recent statistics, about 113,000 births were recorded in Belgium in 2020 [17], and about 10% of those newborns were admitted to neonatology units, including about 3.8 to 5.2% in Neonatal Intensive Care Units (NICU) [18]. Based on those statistics, a non-negligible fraction of newborns in Belgium is potentially eligible for fast-track genomic testing for diagnosis and care. We, therefore, initiated the present pilot study aiming to evaluate the feasibility, time to diagnosis, yield, and utility of rWGS in Belgium.
2. Results
2.1. Demographic and Enrollment Criteria
Twenty-one (11 girls and 10 boys) unrelated patients were recruited and offered rWGS. The median age at recruitment was 0.27 years (98.62 days, range, 2 days–18 years), nine patients (42.86%) were admitted to NICU, six (28.57%) to Pediatric Intensive Care Unit (PICU), and the remaining six (28.57%) to neuropediatric ward (NEURO) (Table 1). None of them received a postnatal genetic test before their recruitment. Given NIPT has been offered systematically in Belgium since April 2017, we assumed that the NIPT was performed in the 18 patients born in Belgium and was normal. We did not find a record of invasive of prenatal testing in any of the patients.
Table 1.
Demographic details.
| rWGS_1 | rWGS_2 | rWGS_3 | rWGS_4 | rWGS_5 | rWGS_6 | rWGS_7 | rWGS_8 | rWGS_9 | rWGS_10 | rWGS_11 | rWGS_12 | rWGS_13 | rWGS_14 | rWGS_15 | rWGS_16 | rWGS_17 | rWGS_18 | rWGS_19 | rWGS_20 | rWGS_21 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age in year (days) | 0.24 (86) | 1.25 (457) | 9.33 (3408) | 0.03 (11) | 0.05 (20) | 18.31 (6688) | 0.08 (28) | 0.03 (11) | 1.67 (609) | 0.02 (9) | 0.01 (2) | 1.28 (467) | 0.39 (142) | 0.07 (25) | 0.47 (170) | 1.25 (457) | 0.07 (24) | 0.27 (100) | 0.05 (18) | 1.32 (481) | 5.37 (1963) |
| Gender | F | M | F | M | M | M | F | F | F | F | M | F | M | F | M | F | M | F | F | M | M |
| Recruitment unit | PICU | NEURO | NEURO | NICU | NICU | PICU | NICU | NICU | NEURO | NICU | NICU | NEURO | NEURO | NICU | PICU | NEURO | NICU | PICU | NICU | PICU | PICU |
Abbreviations: NICU, neonatal intensive care unit; PICU, pediatric intensive care unit.
Our inclusion check list (Table S1), slightly adapted from the Blue Shield checklist, comprised nine major clinical categories: (1) developmental delay/mental retardation; (2) specific anomaly highly suggestive of a genetic etiology; (3) an abnormal laboratory test suggests a genetic disease or a complex metabolic phenotype; (4) an abnormal response to standard treatment for a major underlying condition; (5) significant hypotonia; (6) persistent seizures; (7) infant with high-risk stratification on assessment of a Brief Resolved Unexplained Event (BRUE); (8) significantly abnormal electrocardiogram (ECG); and (9) positive family history of the above 8 criteria. Sixteen different congenital malformations were encountered among these 21 patients (Table S2). The single most common indication for rWGS was severe hypotonia (10 patients, 47.72%), whereas abnormal biochemical status and persistent seizures followed, with 38.09 (eight patients) and 23.81% (five patients), respectively.
2.2. Molecular Diagnostic Yield
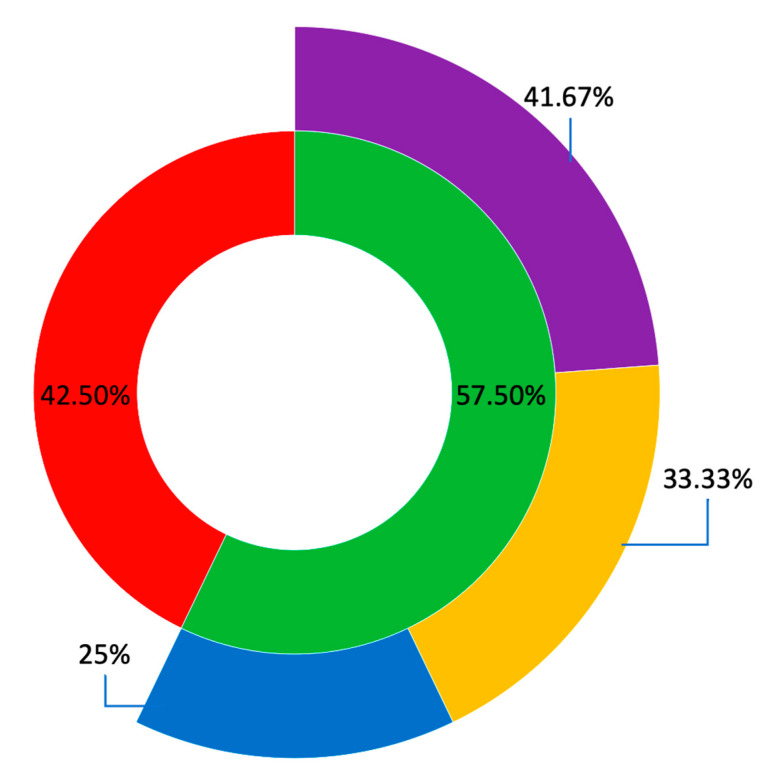
Molecular diagnoses were reached for 12 probands (57.50%), including five probands from NICU (41.67% of solved cases), four from PICU (33.33%), and three from the NEURO (25.00%) (Figure 1). The yield per unit was 5/9 (55.56%) for patients enrolled in NICU, 4/6 (66.67%) in PICU, and 3/6 (50%) in the NEURO.
Figure 1.
Diagnostic yield of rWGS in this cohort. Legend: the inner donut presents the solved cases (green) and the unsolved cases (red). The outer donut spreads the solved cases across the three recruitment units, namely NICU in purple, PICU in orange and NEURO in blue.
In all 12 solved patients, the molecular defect was a SNV. The analyses of CNVs and mitochondrial DNA captured with the nuclear DNA during the library preparation, did not return additional diagnoses (Table 2).
Table 2.
Molecular findings and HPO terms used in the clinical interpretation.
| Patients | Pedigree | Phenotype | OMIM Diseases | Gene | Variant Coordinate (GRCh37) | HGVS c. | Protein Position | Zygocity | Inheritance | ACMG-AMP Classification |
|---|---|---|---|---|---|---|---|---|---|---|
| rWGS_1 | Trio | Muscle hypotonia; Abnormality of brainstem morphology; Frontotemporal cerebral atrophy; Encephalopathy; Progressive encephalopathy; Apneic episodes in infancy; Strabismus; Nystagmus; Abnormal corpus callosum morphology; Enlarged sylvian cistern; Laryngomalacia; Abnormal eye movements | KLF7-related Developmental delay/intellectual disability with neuromuscular and psychiatric symptoms | KLF7 | 2:207,953,249 | NM_003709.4: c.790G > A | p.Asp264As | Heterozygous | De novo | Pathogenic |
| rWGS_2 | Trio | Seizures; Hypotonia, early; Hypotonia, generalized; Large fontanelles; Neurodevelopmental delay; Respiratory infections in early life; Recurrent respiratory infections; Dystonia; Increased serum lactate; Tented upper lips vermilions; Anteverted nares; High palate | Aromatic L-amino acid decarboxylase deficiency (AADCD) | DDC | 7:50,566,899 | NM_001082971.2: c.823G > A | p.Ala275Thr | Heterozygous | Paternaly inherited | Pathogenic |
| 7:5,054,432 | NM_005859.5: c.1037A > G | p.Tyr346Cys | Heterozygous | Maternaly inherited | Likely Pathogenic | |||||
| rWGS_3 | Trio | Mixed demyelinating and axonal polyneuropathy; Progressive polyneuropathy; Progressive degeneration of movement; Difficulty in walking; Progressive muscle weakness; Feeding difficulties; Weight loss; Sensorimotor polyneuropathy affecting arms more than legs; Motor conduction block; Gastroesophageal reflux; Quadriparesis; Toe-walking | Unsolved | |||||||
| rWGS_4 | Trio | Generalized hypotonia; Generalized neonatal hypotonia; Neonatal hypotonia; Severe muscular hypotonia; Respiratory distress | Neurodevelopmental disorder with neonatal respiratory insufficiency, hypotonia, and feeding difficulties | PURA | 5:139,494,456–139,494,458 | NM_005859.4: c.697_699delTTC | p.Phe233del | Heterozygous | De novo | Pathogenic |
| rWGS_5 | Trio | Chylothorax; Facial dysmorphism | Lymphedema, hereditary, III | PIEZO1 | 16:88,851,308 | NM_001142864.4: c.64 + 1G > A | Heterozygous | De novo | Pathogenic | |
| 16:88,792,770 | NM_001142864.4: c.3890T > C | p.Leu1297Pro | Heterozygous | Paternaly inherited | VUS | |||||
| 16:88,783,093 | NM_001142864.4: c.6800C > T | p.Thr2267Met | Heterozygous | Maternaly inherited | VUS | |||||
| rWGS_6 | Duo | Brain calcification; Intellectual impairment; Thrombocytopenia; Leukopenia; Hepatic cirrhosis; Brain imaging abnormality; Neurodevelopmental delay; Retinitis pigmentosa; Speech delay | COACH syndrome 1 | TMEM67 | 8:94,811,875 | NM_153704.6: c.2130G > A | p.Met710Ile | Homozygous | Biparental | VUS |
| Urbach-Wiethe disease | ECM1 | 1:150,480,710 | NM_004425.4: c.25T > A | p.Leu9Met | Homozygous | Biparental | VUS | |||
| rWGS_7 | Trio | Intrauterine growth retardation; Hydrocephaly; Aqueduct of sylvius stenosis; Severe intrauterine growth retardation; Leukopenia; Cholestasis; Thrombocytopenia; Neutropenia; Metabolic acidosis; Atrial septum defect | Unsolved | |||||||
| rWGS_8 | Trio | Severe muscular hypotonia; Lactic acidosis; Hyperammonemia, acute; Hyperammonemia; Neonatal asphyxia | Unsolved | |||||||
| rWGS_9 | Trio | Renal insufficiency; Failure to thrive; Hyperlaxity; Hemiparesis; Unilateral multifocal epileptiform discharges; Hemiclonic seizures; Seizure; Global developmental delay | Mitochondrial DNA depletion syndrome 4A (Alpers type) | POLG | 15:89,864,238 | NM_002693.3: c.2740A > C | p.Thr914Pro | Heterozygous | Maternaly inherited | Pathogenic |
| 15:89,870,432 | NM_002693.2: c.1399G > A | p.Ala467Thr | Heterozygous | Paternaly inherited | Pathogenic | |||||
| rWGS_10 | Trio | Abnormality of the brainstem white matter; Increased urine alpha-ketoglutarate concentration; Leukodystrophy; Multifocal seizures; Generalized clonic seizure; Tonic-clonic convulsions; Generalized convulsive status epilepticus; Bilateral tonic-clonic seizure with generalised onset | Unsolved | |||||||
| rWGS_11 | Trio | Brain imaging abnormality; Increased troponin i level in blood; Abnormal metabolic brain imaging; EEG with burst suppression; Opacification of the corneal epithelium; 3-methylglutaric aciduria; Lactic acidosis; Leukoencephalopathy; Abnormality of the basal ganglia; Corneal opacity | Unsolved | |||||||
| rWGS_12 | Duo | High arched palate; Hypertelorism; Hypotonia; Facial dysmorphism; Abnormal interventricular septum morphology; Poor speech; Failure to thrive | Noonan syndrome 1 | PTPN11 | 12:112,915,524 | NM_002834.5: c.923A > G | p.Asn308Ser | Heterozygous | Absent in mother, father not available for testing | Pathogenic |
| rWGS_13 | Trio | Hypernatremia; metabolic acidosis; generalized seizures, loss of consciousness | Unsolved | |||||||
| rWGS_14 | Trio | Cholestasis; Aortic valve stenosis; Conjugated hyperbilirubinemia; Jaundice; Episodic vomiting; Feeding difficulties in infancy; Meconium ileus; Elevated gamma-glutamyltransferase level; Prolonged neonatal jaundice; Nasogastric tube feeding in infancy | Alagille syndrome 1 | JAG1 | 20:10,621,792 | NM_000214.3: c.3016_3017delCC | p.Pro1006PhefsTer5 | Heterozygous | De novo | Pathogenic |
| rWGS_15 | Trio | Retinal dystrophy; Generalized hypotonia; Tracheomalacia; Sensorineural hearing impairment; Generalized neonatal hypotonia; Ptosis; Unilateral ptosis; Feeding difficulties; Absent or decreased deep tendon reflexes; Respiratory distress | Unsolved | |||||||
| rWGS_16 | Trio | Hypoplasia of the maxilla; Rhinitis; Central apnea; Hypotonia; Growth delay; Hypothermia; Night sweats; Dysautonomia; Failure to thrive; Cerebellar hemorrhage; Delayed gross motor development | Unsolved | |||||||
| rWGS_17 | Trio | Thick vermilion border; Plagiocephaly; Polyhydramnios; Seizure; Hypotonia; Prominent nose; Respiratory distress; Anteverted ears; Preauricular pit; Broad nose; Thick upper lip vermilion; Status epilepticus | Epileptic encephalopathy, early infantile, 14 | KCNT1 | 9:138,656,902 | NM_020822.3: c.1061T > A | p.Met354Lys | Heterozygous | De novo | Likely Pathogenic |
| rWGS_18 | Trio | Dysphagia; Respiratory distress; Stridor; Facial paralysis; Psychomotor retardation; Retrognathia; Camptodactyly; Agenesis of cerebellar vermis HP | TUBB3-related spectrum syndrome | TUBB3 | 16:90,001,644 | NM_006086.4: c.785G > A | p.Arg262His | Heterozygous | De novo | Pathogenic |
| rWGS_19 | Trio | Hypoglycemia; Metabolic acidosis; Lactic acidosis;3-methylglutaconic aciduria;Noncompaction cardiomyopathy; Neonatal hypoglycemia; Hyperammonemia | 3-hydroxy-3-methylglutaryl-Coa lyase deficiency | HMGCL | 1:24,143,241 | NM_000191.3: c.272T > A | p.Val91Asp | Homozygous | Likely Pathogenic | |
| rWGS_20 | Trio | Renal insufficiency; Increased intracranial pressure; Lactic acidosis; Acute rhabdomyolysis; Convulsive status epilepticus; Recurrent hypoglycemia; Cerebral edema |
Unsolved | |||||||
| rWGS_21 | Trio | Conjunctivitis; Failure to thrive; Macrocytic anemia; Steatorrhea; Abnormality of the face; Short stature; Decreased body weight; low vitamine A and D; hypoalbuminemia | Infantile isolated exocrine pancreatic insufficiency, SPINK1-related | SPINK1 | 5:147,211,114 | NM_001379610.1: c.27delC | p.Ser10ValfsTer6 | Homozygous | Biparental | Pathogenic |
Interestingly, one patient (rWGS_6) presented with two different molecular diagnoses (1/21, 4.76%), each explaining part of the phenotype. In another patient (rWGS_5), we identified 3 variants in the PIEZO gene, among which one was inherited from the mother, one from the father and one de novo. Further analyses are planned to determine on which allele the de novo variant occurred.
The molecular diagnosis validated the clinical hypothesis in five patients: hereditary Lymphedema (rWGS_5), mitochondrial depletion syndrome (rWGS_9), Alagille syndrome (rWGS_14), TUBB-related spectrum syndrome (rWGS_18) and 3-hydroxy-3-methylglutaryl-Coa lyase deficiency (rWGS_19).
2.3. Turnaround Time
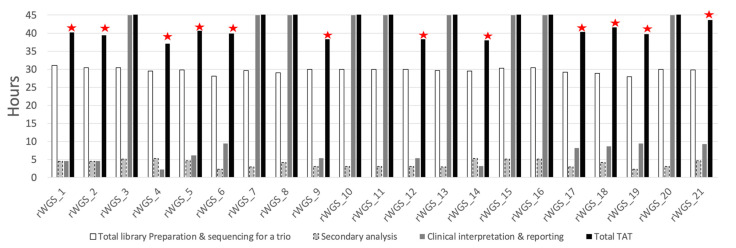
The average time from the sample reception in the laboratory to the signature of the report was 39.80 h (ranges, 37.05–43.7) in solved patients (Figure 2). The shortest TAT in this series was 37.05 h (rWGS_4). In unsolved patients, the clinical interpretation was deliberately kept open for one week in total to include 5 days of additional clinical investigation of extra abnormalities such as repeat expansion and Run Of Homozygosity (ROH). In all patients, the report was returned prior to hospital discharge.
Figure 2.
Time metrics of rWGS in the 21 patients. Legend: for each patient, the white bar indicates library preparation, sequencing, and demultiplexing time, the grey bar with dashed borders represents the secondary analysis (from fastq to vcf), whereas the darker grey bar corresponds to the clinical interpretation time. Though the scale of the graph is limited to 45 h, the analysis was closed after a total of 7 days in unsolved patients. The red stars indicate patients molecularly diagnosed in this study.
The longest component varied depending on the resolution status. For solved patients, the sequencing run time for a S1 PE300 cycles on the NovaSeq 6000 (25 h) was the longest component. In unsolved patients, the further clinical analysis and interpretation time of about 5 days constituted the longest component. Of note, none of the prolonged analysis led to a conclusive diagnosis.
2.4. Clinical Utility
Based on the rWGS results, an adjustment was made to the clinical management for the 12 diagnosed patients, consisting of inclusion in a clinical trial for two, implementation of a disease-specific treatment for two, and adjustment of multidisciplinary care in the remaining eight patients.
Of the two patients included in clinical trials, one patient (rWGS_2) was diagnosed with Aromatic l-amino acid decarboxylase deficiency (AADCD, MIM #608643). Their treatment first consisted of Pramipexole, selegiline, and Artane with a non-satisfactory response. At an age of 30 months, he was included in an in vivo gene therapy trial using an adeno-associated virus 2 delivery of human aromatic L-amino acid decarboxylase (AAV2-hAADC) in the nervous system (Upstaza, 2.8 × 1011 genome vector, from PTC TherapeuticsTM). The evaluation performed six months after treatment indicated significant clinical improvement. The second patient to be included in a clinical trial (rWGS_17) was diagnosed with developmental and epileptic encephalopathy type 14 due to de novo mutation in KCNT1 (MIM #614959). Treating clinicians initiated an off-label clinical trial with quinidine. Dose escalation is still going on at the time of the submission of this manuscript.
The disease-specific treatment consisted of Leucine restricted diet in a patient (rWGS_19) with HMG-CoA lyase deficiency (MIM #246450) and pancrelipase in a homozygous proband and her heterozygous parents for a pathogenic SPINK1 variant (rWGS_21).
Genetic counseling was offered to all diagnosed families, especially the family with exocrine pancreas insufficiency since heterozygous carriers of the mutation are symptomatic as well.
3. Discussion
We present the implementation of the first rapid Whole Genome Sequencing program for severely ill pediatric patients in Belgium. This study was designed as a proof of concept for the feasibility, the efficiency and utility of rWGS in Belgium.
3.1. Feasibility
Twenty-one probands were recruited within 21 months for the project, among which 16 were from the main study site, the CHR de Liège. Our recruitment pace was 0.76 patients per month at the main study site and one patient per month over the four recruitment sites. The Baby Bear project in California (USA) recruited 184 infants over eighteen months, corresponding to two patients per hospital per month [19]. Implementing rWGS with the TAT achieved in this study was challenging and demanded multiple adjustments to avoid delays (use of an easy-to-score checklist and direct pick-up of samples from the pediatric service between 8:00 and 8:30) and to prevent accumulation of work overtime for the personnel (implementation of flexible schedules for people involved in the wet lab and bioinformatics analysis). Although our recruitment pace may increase with the clinical utility being demonstrated, we recommend a semi-centered model for Belgium. In this model, one lab will be in charge for the wet lab. The other genetic laboratories may opt to outsource clinical interpretation as well or performing this last step in-house. A similar approach is applied by the Australian Genomics Health Alliance Acute Care [20].
3.2. Yield
Twelve definite diagnoses were made in the 21 patients (57.5%). This yield is among the highest reported in prospective rWGS studies, despite the small sample size [21]. Our current high yield may be the result of the inclusion of severely ill patients, combined with the administration of WGS as the first-tier test. Causal variants identified in this study were all single nucleotide variants located in the coding or splicing sequences. Although the benefits of WGS for CNV, SV, MT-SNVs and STRs analyses have been well documented [22], these approaches did not yield additional diagnoses in this study. A larger cohort will allow the evaluation of the added value of those approaches in Belgian patients.
In this cohort, one patient (4.76%) was affected by two distinct autosomal recessive disorders, both contributing to the phenotype. Double mendelian diagnoses are being documented in large studies of non-consanguineous patients with developmental disorders [23,24].
3.3. TAT
The TAT has become an important concern in genomic medicine. One of the major outcomes of the rWGS is the reduction of the TAT. However, most of the TATs reported in implementation studies similar to ours are often evaluated in a very controlled experimental design with fixed schedules for sample reception and processing [21,25], not addressing issues of human labor and laboratory work shifts, which may naturally generate delays due to evenings, weekends and holidays. To return a fast diagnosis, we applied on-demand sample processing, including during weekends, holidays, and evenings. We addressed human labor and laboratory work shifts as described in the feasibility subsection, above. Most samples were collected in the evening, because of the availability of parents.
Our TAT did not consider the delay caused by the unavailability of one parent or the transportation to the laboratory in the morning. We rather calculated the TAT based on the steps we could control, meaning from the sample reception to the report. We reduced the delay between sampling and reception in the laboratory by collecting samples the next morning from the three hospitals located in Liège, between 8 and 8:30. However, this approach may not be suited for big sample volumes. A study from Australia showed that for nationwide implementation, the distance between hospitals and the laboratory, and the means of transportation, may delay sample reception in the laboratory [20]. A nationwide implementation phase would need to address these issues upfront. Likewise, a nationwide implementation may also consider testing singletons or duos to avoid delays due to the unavailability of one or both parent(s).
We present the first implementation of rWGS in Belgium. We were able to return the diagnosis in about 40 h despite multiple manual steps. To the best of our current knowledge, this is the shortest delivery time for WGS in Europe. Several improvements are still possible, including the automation of data transfer between workflow steps, the use of FC S1 v1.5 200Cy in order to reduce sequencing run time from 25 h to 19 along with the sequencing cost.
3.4. Clinical Utility
The rWGS was very useful to this series of 21 patients. First, rWGS allowed correction of the diagnosis in multiple patients. The majority of clinical diagnoses were not confirmed by WGS. Unsuspected diagnoses were identified instead. This confirms previous observations that the clinical diagnosis is a poor predictor of the genetic findings in very young patients and supports gene-agnostic testing in NICU/PICU [5].
Second, the rWGS results informed the implementation of care, which included gene therapy, the use of an off-label drug in a trial, and disease-specific treatment. In the remainder, the multidisciplinary care was tailored to the diagnosis. The fact that all results were returned before the hospital discharge the integration of rWGS results in the care and disease surveillance. This represents the biggest advantage of fast-tract analysis as opposed to the standard of care.
Generally, multidisciplinary care may include speech therapy, physical therapy, behavior therapy, specialized education program, dietary care, medications for neurological manifestations and specific surveillance. Although multidisciplinary care may be initiated based on the clinical manifestation, before the diagnostic is known, multidisciplinary care is not generic. It varies depending on the diagnosis. In this study, the majority of patients did not have a multidisciplinary care plan in place yet at the time of diagnosis, probably due to their younger age. The diagnosis allowed a multidisciplinary care plan to be started.
3.5. Challenges/Limitations
The amount of overtime and the cost are the two main limitations to the broad implementation of rWGS in Belgium. In almost all patients, libraries were loaded onto the NovaSeq after office hours, between 8:00 p.m. and 10:00 p.m., and the bioinformatic analyses started 25 h later, meaning the next night, and continued through the morning. For this pilot project, the overtimes were mitigated by using a flexible shift schedules, and the cost was covered by a grant from the government of Wallonia in Belgium. The current reagent price, including TVA, is EUR 6124 for a trio. The semi-centered model will allow the pooling of up to six trios on a single S4 FC PE300 cycle, thus reducing the reagent costs. Ultimately, a specific reimbursement by the health system, as previously implemented for Non-Invasive Prenatal Testing (NIPT) [26], will offer equal access to the Belgian population given the undeniable benefit of rWGS.
4. Materials and Methods
4.1. Patients
Patients were recruited between February 2021 and October 2022. The main study site was the CHR de Liège, an affiliate hospital to the University hospitals of Liège, where 16 probands were recruited. Three other hospitals also contributed patients to the study: the Notre Dame des Bruyères with one patient, the CHC Mont-Légia with three patients, and the University Hospitals of Saint-Luc with one patient.
We used a recruitment checklist adapted from the Blue Shield checklist (checklist in the supplemental material). To be included, a patient had to (1) be admitted to NICU, PICU, or NEURO; (2) meet at least one inclusion criterion and have none of the exclusion criteria. (3) Babies born following sperm or egg donation, or who had been adopted, were excluded. In addition, (4) the patient’s clinical condition must permit drawing at least 1 mL of venous blood.
The neuropediatric unit is a branch of the pediatric service of the University Hospital of Liège (located in the CHR de Liège) where pediatric patients with neurologic pathologies or neurological phenotypes are admitted and treated. Those patients are coming from the pediatric emergency room, the pediatric intensive care unit or external referrals for neurologic assessment. This unit is composed of 16 beds and a certified neuropediatricians is always on duty.
4.2. Study Workflow
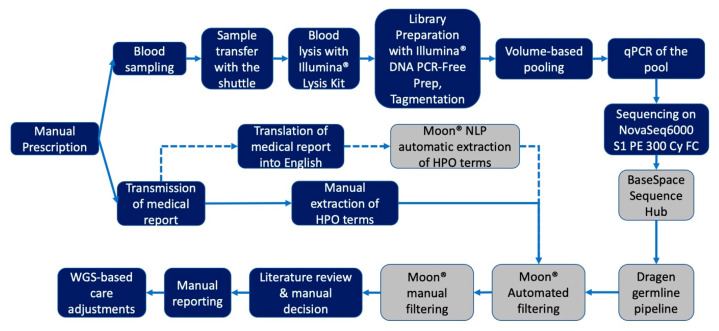
We combined an expedited test prescription approach with multiple cloud-based data analysis platforms into a single data workflow (Figure 3). This study workflow is described in detail in the Appendix A.
Figure 3.
Workflow diagram of the pilot rWGS study. Legend: grey shapes represent steps performed using Cloud-based bioinformatic tools and platforms.
As opposed to the Australian model [20], where the mean sample transportation time was 1.6 days, the most distant points in Belgium are located within a radius of 2 h and half around our sequencing facility.
4.3. Library Preparation and Sequencing
Blood samples were lysed using the Illumina Lysis Kit (catalogue number 20042221) following the manufacturer’s protocol with the exceptions that we started with 40 µL of blood instead of 20 µL, as recommended, and we doubled the lysis solution’s volume. Lysis product was purified with Illumina purification beads (provided with the lysis kit) as recommended by the manufacturer and beads were resuspended in 30 µL of resuspension buffer provided in the lysis kit. DNA content in the lysis product was assayed with the Qubit fluorometer 4.0 and then normalized to the concentration of the least concentrated sample in the trio (see Supplementary Materials). Then, 30 µL of normalized lysis product was used for library preparation with Illumina DNA PCR-Free Prep, Tagmentation library preparation kit (Cat number 20041795) and IDT® for Illumina® DNA/RNA Unique Dual Indexes Set A, Tagmentation (96 Indexes, Cat number 20027213). Library preparation was performed manually in a diagnostic grade pre-PCR room using a VeritiTM Thermal Cycler instrument and standard laboratory equipment. Libraries were pooled per volume, as recommended. From rWGS_3, we also applied the unique dual index correction factor as recommended by Illumina in order to improve the balance of the coverage across samples within the same run [27]. No intermediate Quality Control was performed. The volume to be loaded on the flow cell was determined by either Qubit fluorometer 4.0 (for rWGS_1) or qPCR of the pool (from rWGS_2). Between 1.27 to 1.87 nM of pooled libraries were loaded onto a Flow cell. After rWGS_1, S1 FC version 1.0 (Cat number 20012863) was discontinued by the manufacturer, and we shifted to S1 PE300cy FC v1.5 (Cat number 20028317). For the duo, (rWGS_6) SP v1.5 PE 300 cycles was used instead. Sequencing was performed on a NovaSeq6000 using a standard workflow with PhiX Control v3 (Cat number FC-110-3001). Despite variations in the percentage of reads passing filter, duplicate rates, and yield imbalance across samples, we achieved a mean coverage over the genome between 39 and 75×. The lowest coverage was achieved in rWGS_6 and rWGS_12, which were sequenced on a SP flow cell.
4.4. Analysis of Genomic Data
The standard duration of sequencing for an S1 PE300cycles is 25 h. Data transferred to the local server was used for backup (after demultiplexing) and the review of InterOp files using the Sequencing Analysis Viewer (SAV) Software v2.4.7 (Illumina, San Diego, CA, USA). A Base Space Sequencing Hub (BSSH) connection was established on the NovaSeq 600, allowing data to be directly streamed to the Cloud. BSSH is a bioinformatic environment that contains software and hardware tools such as bcl2fastq and the DRAGEN. Our data were hosted in the European instance of the BSSH, in Frankfurt. Upon the completion of the sequencing run and subsequent data transfer to BSSH, bcl2fastq was automatically launched for the generation of FASTQ files in BSSH. The output Fastq files were stored in BSSH and the research team was automatically notified by email upon the completion of the bcl2fastq. Next, DRAGEN Germline Pipeline 3.2.8 (DRAGEN Host Software Version 05.011.281.3.2.8 and Bio-IT Processor Version 0x04261818) was launched manually in BSSH for SNV and CNV detection. Dragen germline Pipeline 3.2.8 is highly optimized for speed and performs alignment and variant calling with high sensitivity and accuracy [28,29]. We aligned our patients’ data on the GRCh37 reference genome (3.1 Gb). In addition to SNV and CNV detection from nucleic DNA, this pipeline contains multiple additional but optional analysis modules such as structural variation detection, detection of Runs Of Homozygosity (ROH), and detection of repeat expansion analysis (short tandem repeats, STRs) using Expansion Hunter [6]. The output files consisted of vcf files, as well as cram files and related index files.
All molecular findings were validated with Sanger sequencing.
4.5. Phenotype Based Clinical Interpretation
The vcf files produced by DRAGEN were imported in Moon® v3.3.2 (Invitae, San Francisco, CA, USA). This platform uses Artificial Intelligence and multiple knowledge sources to conduct phenotype-based filtering. First, Moon performs an autonomous analysis and provides a shortlist of variants best matching the phenotype (HPO terms) provided by the user along with genomic data. MOON uses a probabilistic model to rank the pathogenicity of variants retained during the autonomous filtering. This framework takes into account 15 in silico prediction tools, ClinVar records, expected inheritance pattern and inheritance pattern–based allele frequencies [12]. In addition, the user has the possibility of performing manual filtering of variants based on the depth, genotype quality, the frequency in gnomAD, the predicted effect, the inheritance, the zygosity and the region or gene of interest. Moon has been optimized for import and analysis of WGS data in short time. The following annotation sources were used in Moon: ClinVar: 21 November 2020, dbNSFP: 4.0, dbSNP: 151, dbscSNV: 1.1, Apollo: 26 November 2020, RefSeq: 37, gnomAD: 2.1.1, HPO: 12 February 2019, Moon KB: 9 March 2021, DGV: 1 March 2016, dbVar: 7 July 2019, Mitomap: 14 January 2019, Mitimpact: 2.9.1, Mastermind: 2 October 2020, InvitaeKB: 2 December 2020.
This pilot study focused on primary findings. Therefore, consent for analysis of incidental findings was not sought in the consent form
4.6. Outcome Measures for the Clinical Utility
The primary outcomes were the diagnostic yield and the TAT. For such a multisite recruitment, the most accurately recordable initial point is the time of sample reception by the laboratory which was thus considered the starting time. The final time point was the electronic signature of the analysis report. Those time points were collected from the local laboratory information management software.
Sanger sequencing was not a requirement before returning the results, since we knew, based on our local experience, that false positive SNVs were very unlikely with our coverage of 39× to 75×. However, given that our genetic service is accredited and that this rWGS is not accredited yet, our results had to be validated by an accredited test (sanger sequencing) to be included in the EHR. We performed Sanger sequencing and added a complementary paragraph to the report with the Sanger results. The time for the sanger sequencing was beyond out TAT.
Other outcomes included the molecular diagnostic yield and the clinical utility. The changes in clinical management were reported by referring physicians using a clinical utility survey form adapted from Scocchia et al. [30]. The level of satisfaction was not assessed during this pilot phase.
5. Conclusions
We have conducted the first implementation study of rapid Whole Genome Sequencing in Belgium. Twenty-one patients aged 2 days to 18 years were recruited. Our average TAT of 39.80 h (ranges, 37.05–43.7) is the fastest rWGS platform in Europe. We achieved a yield of 57.5% (12/21), one of the highest in Europe. rWGS triggered care adjustments in diagnosed patients, including clinical trials, condition-specific treatment, and multidisciplinary care. This study establishes the feasibility and demonstrates the utility of rWGS in Belgium.
Acknowledgments
The authors are grateful to patients and parents for their participation in this study. Special thanks to Marie Christine Seghaye, Emeline Bequet, Benoît Florkin, Angelique Lhomme, Aurélie Janssen, the nurses of participating pediatric services, and all their colleagues for their strong support of this study. Vinciane Dideberg, Jean-Hubert Caberg and the whole team of the molecular diagnostic laboratory at the CHU Liège for their support. We are particularly thankful to the teams of the Dispatching unit and the Secteur Commun for their contribution. Thanks to Illumina for the in kind donation of five sequencing NovaSeq reagents kits used for the technical validation for the rWGS process. This work took place in part in the framework of the European Reference Network for Rare Hereditary Metabolic Disorders (MetabERN).
Supplementary Materials
The supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/ijms24044003/s1.
Appendix A
The patient selection was made by the pediatrician and the test prescription was confirmed by a clinical geneticist. The final inclusion decision was made by the research team based on the presence of at least one inclusion criteria and the absence of any exclusion criteria from our BlueShield-based study checklist (Supplemental Materials). The informed consent from the parents was collected by a clinical geneticist. Once the consent was obtained, blood samples were collected from the trio (affected child and both parents) and transferred to the genetic laboratory for library preparation and sequencing. The Sequencing instrument was set up to transfer sequencing data to our BaseSpace Sequence Hub (BSSH) account, Frankfurt instance (Illumina Inc.). In addition, raw sequencing data were also simultaneously transferred to the hospital server for backup. Demultiplexing was automatically performed in BSSH once the sequencing run and data transfer were finished and data were fully transferred to the Cloud. Fastq biosamples were manually uploaded to DRAGEN Germline Pipeline 3.2.8 (Illumina Inc.) within BSSH. Resulting vcf files for SNVs and CNVs were manually exported from Dragen® to Moon® (Invitae Inc.) and HPO terms were fed to Moon for phenotype-based clinical interpretation. For the last 3 patients, we tested the new clinical natural language processing (CNLP) feature in Moon, which allows the automatic extraction of HPO terms from the medical file. VCF files for SNVs and CNVs were manually moved from BSSH to Moon. We complemented the interpretation with manual filtering based on inheritance hypothesis and population frequencies. Plausible candidate variants were retained for manual literature and database review. The final plausible variants were manually classified according to the ACMG-AMP. A manual report was written and returned to the clinician for reverse phenotyping and action when possible.
Author Contributions
Conceptualization, A.L. and V.B.; methodology, A.L. and C.F.; software, A.L. and C.F.; validation, A.L., C.F., A.B., L.P. and B.C.; formal analysis, A.L., C.F., L.P., B.C. and F.B.; investigation, F.-G.D., S.A., A.J., J.H., A.M., S.B., S.V., R.V., V.R. and V.B.; resources, L.P. and B.C.; writing—original draft preparation, A.L.; writing—review and editing, A.L., C.F., A.B., L.P., B.C., F.-G.D., S.A., A.J., J.H., A.M., S.B., S.V., R.V., V.R. and V.B.; supervision, V.B.; project administration, A.L.; funding acquisition, V.B. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki, and approved by the ethical committee of the Faculty of Medicine and the University hospitals of the University of Liège (Ref 2020/14) and by the ethical committee of the CHR Liège (Ref 2020/143).
Informed Consent Statement
Informed consent was obtained from parents for themselves as well as on behalf of their children involved in the study.
Data Availability Statement
Genomic variants supporting the diagnoses in this publication are submitted to ClinVar and accession numbers will be provided after the review. Reasonable requests for additional anonymized data may be sent to the corresponding author and will be considered in case per case.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the region of Wallonia (Belgium), grant number RWAL1710180 (WALGEMED).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Wojcik M.H., Schwartz T.S., Thiele K.E., Paterson H., Stadelmaier R., Mullen T.E., VanNoy G.E., Genetti C.A., Madden J.A., Gubbels C.S., et al. Infant mortality: The contribution of genetic disorders. J. Perinatol. 2019;39:1611–1619. doi: 10.1038/s41372-019-0451-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy S.L., Kochanek K.D., Xu J. Deaths: Final Data for 2015. Natl. Vital. Stat. Rep. 2017;66:1–75. [PubMed] [Google Scholar]
- 3.Meng L., Pammi M., Saronwala A., Magoulas P., Ghazi A.R., Vetrini F., Zhang J., He W., Dharmadhikari A.V., Qu C., et al. Use of exome sequencing for infants in intensive care units. JAMA Pediatr. 2017;171:e173438. doi: 10.1001/jamapediatrics.2017.3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Halabi N., Ramaswamy S., El Naofal M., Taylor A., Yaslam S., Jain R., Alfalasi R., Shenbagam S., Bitzan M., Yavuz L., et al. Rapid whole genome sequencing of critically ill pediatric patients from genetically underrepresented populations. Genome Med. 2022;14:56. doi: 10.1186/s13073-022-01061-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.French C.E., Delon I., Dolling H., Sanchis-Juan A., Shamardina O., Mégy K., Abbs S., Austin T., Bowdin S., Branco R.G., et al. Whole genome sequencing reveals that genetic conditions are frequent in intensively ill children. Intensive Care Med. 2019;45:627–636. doi: 10.1007/s00134-019-05552-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mc Laughlin R., Hardiman O. Detection of long repeat expansions from PCR-free whole-genome sequence data. Genome Res. 2017;27:1895–1903. doi: 10.1101/gr.225672.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nicholas T.J., Al-Sweel N., Farrell A., Mao R., Bayrak-Toydemir P., Miller C.E., Bentley D., Palmquist R., Moore B., Hernandez E.J., et al. Comprehensive variant calling from whole-genome sequencing identifies a complex inversion that disrupts ZFPM2 in familial congenital diaphragmatic hernia. Mol. Genet. Genom. Med. 2022;10:e1888. doi: 10.1002/mgg3.1888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jiao Q., Sun H., Zhang H., Wang R., Li S., Sun D., Yang X.A., Jin Y. The combination of whole-exome sequencing and copy number variation sequencing enables the diagnosis of rare neurological disorders. Clin. Genet. 2019;96:140–150. doi: 10.1111/cge.13548. [DOI] [PubMed] [Google Scholar]
- 9.Choudhry N., Golding J., Manry M.W., Rao R.C. Whole genome sequencing increases molecular diagnostic yield compared with current diagnostic testing for inherited retinal disease. Ophthalmology. 2016;123:1143–1150. doi: 10.1016/j.ophtha.2016.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gorzynski J.E., Goenka S.D., Shafin K., Jensen T.D., Fisk D.G., Grove M.E., Spiteri E., Pesout T., Monlong J., Baid G., et al. Ultrarapid nanopore genome sequencing in a critical care setting. N. Engl. J. Med. 2022;386:700–702. doi: 10.1056/NEJMc2112090. [DOI] [PubMed] [Google Scholar]
- 11.Farnaes L., Hildreth A., Sweeney N.M., Clark M.M., Chowdhury S., Nahas S., Cakici J.A., Benson W., Kaplan R.H., Kronick R., et al. Rapid whole-genome sequencing decreases infant morbidity and cost of hospitalization. NPJ Genom. Med. 2018;3:10. doi: 10.1038/s41525-018-0049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Farnaes L., Hildreth A., Sweeney N.M., Clark M.M., Chowdhury S., Nahas S., Cakici J.A., Benson W., Kaplan R.H., Kronick R., et al. Does genomic sequencing early in the diagnostic trajectory make a difference? A follow-up study of clinical outcomes and cost-effectiveness. Genet. Med. 2019;21:173–180. doi: 10.1038/s41436-018-0006-8. [DOI] [PubMed] [Google Scholar]
- 13.Fishman V., Battulin N., Nuriddinov M., Maslova A., Zlotina A., Strunov A., Chervyakova D., Korablev A., Serov O., Krasikova A. Diagnosis of genetic diseases in seriously ill children by rapid whole-genome sequencing and automated phenotyping and interpretation. Sci. Transl. Med. 2019;11:eaat6177. doi: 10.1126/scitranslmed.aat6177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Van Diemen C.C., Kerstjens-Frederikse W.S., Bergman K.A., De Koning T.J., Sikkema-Raddatz B., Van Der Velde J.K., Abbott K.M., Herkert J.C., Löhner K., Rump P., et al. Rapid targeted genomics in critically ill newborns. Pediatrics. 2017;140:e20162854. doi: 10.1542/peds.2016-2854. [DOI] [PubMed] [Google Scholar]
- 15.Mestek-Boukhibar L., Clement E., Jones W.D., Drury S., Ocaka L., Gagunashvili A., Stabej P.L.Q., Bacchelli C., Jani N., Rahman S., et al. Rapid paediatric sequencing (RaPS): Comprehensive real-life workflow for rapid diagnosis of critically ill children. J. Med. Genet. 2018;55:721–728. doi: 10.1136/jmedgenet-2018-105396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Śmigiel R., Biela M., Szmyd K., Błoch M., Szmida E., Skiba P., Walczak A., Gasperowicz P., Kosińska J., Rydzanicz M., et al. Rapid whole-exome sequencing as a diagnostic tool in a neonatal/pediatric intensive care unit. J. Clin. Med. 2020;9:2220. doi: 10.3390/jcm9072220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Statbel Births and Fertility. Belgium in Figure. [(accessed on 11 February 2022)]; Available online: https://statbel.fgov.be/en.
- 18.Leroy C., Van Leeuw V., Rigo V. Grossesses Gémellaires (Santé Périnatale en Wallonie—Année 2018) Centre D’Épidémiologie Périnatale (CePIP); Bruxelles, Belgium: 2020. [Google Scholar]
- 19.Franck L.S., Kriz R.M., Rego S., Garman K., Hobbs C., Dimmock D. Implementing rapid whole-genome sequencing in critical care: A qualitative study of facilitators and barriers to new technology adoption. J. Pediatr. 2021;237:237–243. doi: 10.1016/j.jpeds.2021.05.045. [DOI] [PubMed] [Google Scholar]
- 20.Ference B.A., Kastelein J.J., Ray K.K., Ginsberg H.N., Chapman M.J., Packard C.J., Laufs U., Oliver-Williams C., Wood A.M., Butterworth A.S., et al. Feasibility of ultra-rapid exome sequencing in critically ill infants and children with suspected monogenic conditions in the Australian public health care system. JAMA. 2020;323:2503. doi: 10.1001/jama.2020.7671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stark Z., Ellard S. Rapid genomic testing for critically ill children: Time to become standard of care? Eur. J. Hum. Genet. 2022;30:142–149. doi: 10.1038/s41431-021-00990-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Minoche A.E., Lundie B., Peters G.B., Ohnesorg T., Pinese M., Thomas D.M., Zankl A., Roscioli T., Schonrock N., Kummerfeld S., et al. ClinSV: Clinical grade structural and copy number variant detection from whole genome sequencing data. Genome Med. 2021;13:32. doi: 10.1186/s13073-021-00841-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dong X., Liu B., Yang L., Wang H., Wu B., Liu R., Chen H., Chen X., Yu S., Chen B., et al. Clinical exome sequencing as the first-tier test for diagnosing developmental disorders covering both CNV and SNV: A Chinese cohort. J. Med. Genet. 2020;57:558–566. doi: 10.1136/jmedgenet-2019-106377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wright C.F., Fitzgerald T.W., Jones W.D., Clayton S., McRae J.F., Van Kogelenberg M., King D.A., Ambridge K., Barrett D.M., Bayzetinova T., et al. Genetic diagnosis of developmental disorders in the DDD study: A scalable analysis of genome-wide research data. Lancet. 2015;385:1305–1314. doi: 10.1016/S0140-6736(14)61705-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dimmock D., Caylor S., Waldman B., Benson W., Ashburner C., Carmichael J.L., Carroll J., Cham E., Chowdhury S., Cleary J., et al. Project baby bear: Rapid precision care incorporating rWGS in 5 California children’s hospitals demonstrates improved clinical outcomes and reduced costs of care. Am. J. Hum. Genet. 2021;108:1231–1238. doi: 10.1016/j.ajhg.2021.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Van Den Bogaert K., Lannoo L., Brison N., Gatinois V., Baetens M., Blaumeiser B., Boemer F., Bourlard L., Bours V., De Leener A., et al. Outcome of publicly funded nationwide first-tier noninvasive prenatal screening. Genet. Med. 2021;23:1137–1142. doi: 10.1038/s41436-021-01101-4. [DOI] [PubMed] [Google Scholar]
- 27.Illumina Balancing Sample Coverage for Whole-Genome Sequencing. 2021. [(accessed on 28 December 2022)]. Available online: https://www.illumina.com/content/dam/illumina/gcs/assembled-assets/marketing-literature/illumina-dna-prep-pcr-free-index-correction-tech-note-m-gl-00005/illumina-dna-pcr-free-index-correction-tech-note-m-gl-00005.pdf.
- 28.Miller N.A., Farrow E.G., Gibson M., Willig L.K., Twist G., Yoo B., Marrs T., Corder S., Krivohlavek L., Walter A., et al. A 26-hour system of highly sensitive whole genome sequencing for emergency management of genetic diseases. Genome Med. 2015;7:100. doi: 10.1186/s13073-015-0221-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Illumina DRAGEN v3.2.8 Software Release Notes. 2019. [(accessed on 28 December 2022)]. Available online: https://support.illumina.com/content/dam/illumina-support/documents/downloads/software/dragen/dragen-v3-2-8-release-notes-1000000076767-00.pdf.
- 30.Scocchia A., Wigby K.M., Masser-Frye D., Del Campo M., Galarreta C.I., Thorpe E., McEachern J., Robinson K., Gross A., Ajay S.S. Clinical whole genome sequencing as a first-tier test at a resource-limited dysmorphology clinic in Mexico. NPJ Genom. Med. 2019;4:5. doi: 10.1038/s41525-018-0076-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Genomic variants supporting the diagnoses in this publication are submitted to ClinVar and accession numbers will be provided after the review. Reasonable requests for additional anonymized data may be sent to the corresponding author and will be considered in case per case.