Abstract
For many decades, the proper functioning of the human body has become a leading scientific topic. In the course of numerous experiments, a striking impact of probiotics on the human body has been documented, including maintaining the physiological balance of endogenous microorganisms, regulating the functioning of the immune system, enhancing the digestive properties of the host, and preventing or alleviating the course of many diseases. Recent research, especially from the last decade, shows that this health-benefiting activity of probiotics is largely conditioned by the production of extracellular vesicles. Although the importance of extracellular vesicles in the virulence of many live-threatening pathogens is widely described in the literature, much less is known with respect to the health-promoting effect of extracellular vesicles secreted by non-pathogenic microorganisms, including probiotics. Based on this, in the current review article, we decided to collect the latest literature data on the health-inducing properties of extracellular vesicles secreted by probiotics. The characteristics of probiotics’ extracellular vesicles will be extended by the description of their physicochemical properties and the proteome in connection with the biological activities exhibited by these structures.
Keywords: extracellular vesicles, membrane vesicles, probiotics, probiotic bacteria, postbiotics
1. Introduction
For time immemorial, the proper functioning of the human body has become a leading topic undertaken by scientists [1]. It quickly became clear that there is a very strong relationship between human health and its resident microbiota [2,3]. As a resultant, the idea of using probiotics was created, i.e., live microorganisms that, when administered in the proper dose, have a beneficial effect on the host [1,4]. One of the first well-documented example of the usefulness of probiotics dates back to 1907, from the observations made by Elie Metchnikoff, who showed the existence of a positive correlation between the consumption of fermented food containing probiotics and the lifespan of the Bulgarian population [1]. This was the starting point for further observations of health-benefiting properties of this microbial group and the development of a powerful trend of research on probiotics in the following years [1,5]. After several decades of experiments, a striking impact of probiotics on the human body has been documented, including maintaining the physiological balance of microbiota, regulating the functioning of the immune system, enhancing the digestive properties of the host, and preventing or alleviating the course of many diseases [1,5,6]. The growing awareness of the benefits of probiotics has contributed to the exponential growth of commercial products containing these microorganisms [4]. The use of probiotics has undoubtedly become very popular, however, their viability in such preparations is often questioned, which is related to their exposure to various unfavorable parameters, such as processing (e.g., dehydration), storage conditions, and physiology of the product’s target site (e.g., passage through the physicochemically diverse, harsh environment of the digestive system) [7].
Although probiotics are currently in the center of interest of pharmaceutical concerns, more and more research focuses on searching for alternatives to these classically used products [8,9]. Difficulties in maintaining the viability of probiotics in commercial products and the fact that the viability of these microorganisms is not always necessary to obtain therapeutic effects have led to the concept of postbiotics [5,7,10,11]. Postbiotics are a mixture of metabolic products or non-viable fragments of probiotics that have a beneficial effect on the functioning of the human body [8,12]. Despite the fact that research on postbiotics is still in its infancy, a number of health-promoting properties of these new products have already been demonstrated (maintenance of the proper structure of the resident microbiota, strengthening the host epithelial barrier, modulation of the local and systemic immune response, or increase of the host metabolic activity) [12]. As non-viable elements of probiotics, postbiotics present their strictly defined technological properties and thus represent a promising tool for obtaining therapeutic effects [10,11,13]. These parameters include a favorable level of absorption and distribution of postbiotics [13] and the lack of risk to spread resistance mechanisms, as documented between probiotics and microbiota or pathogens [12]. In 2021, the International Scientific Association of Probiotics and Prebiotics established a new definition of postbiotic as a “preparation of inanimate microorganisms and/or their components that confers a health benefit on the host” [14]. The use of the word ‘components’ was made because the whole microbial cells are not always required to present health-promoting activity and such an impact may be related to the presence of cellular structures produced by probiotics. The above modification of the meaning of the term ‘postbiotic’ has opened a new avenue for many new categories of these preparations, including probiotic extracellular vesicles [12].
Extracellular vesicles (EVs) are nanoscale lipid particles secreted by virtually every type of living cell [15]. For this reason, the use of various nomenclature in the description of these structures is frequent. This richness in the nomenclature of the vesicles is related to their physical properties (mostly size), the way they are formed, or the cells that secrete them [15,16,17]. In order to standardize the nomenclature as much as possible, in accordance with the recommendations of the International Society for Extracellular Vesicles [18], in this review, we will use the term “extracellular vesicles”. This is a collective term classically referring to various types of small compartments released from cells, which are surrounded by a lipid bilayer and incapable of spontaneous replication [18]. The main function of EVs is the transport of various classes of macromolecules, including lipids, polysaccharides, proteins, and nucleic acids [9,16,19]. Through this cargo, these structures perform a number of key functions in microorganisms, including long-distance transport of nutrients, protection against environmental stressors, or communication during microorganism–microorganism or microorganism–host interactions [16,17,20]. The importance of EVs in the virulence of many live-threatening pathogens is well known and widely described in the literature [21,22]. Much less is reported with respect to the health-promoting effect of EVs secreted by non-pathogenic microorganisms, including probiotics. For the last few years, however, awareness on probiotic EVs as a very promising therapeutic platform is growing rapidly [15,17,19]. This seems to be strictly related with both the ability of EVs to carry many different bioactive macromolecules and with nanometric dimensions of these structures.
Based on the above facts, in this review article, we decided to collect the latest literature data on the health-promoting properties of extracellular vesicles secreted by probiotics. These characteristics will be extended by the description of physicochemical properties and the proteome of EVs produced by probiotic microorganisms.
2. Review Strategy and Literature Included
To obtain articles comprising the central core of the current review paper, we used the Scopus and PUBMED databases. In that respect, only English-language original articles from the last decade (1 January 2012–30 June 2022) were included. The search terms were “membrane vesicles” or “extracellular vesicles” together with “probiotics”. In order to obtain as many records as possible, an additional search phase was performed involving the use of “membrane vesicles” or “extracellular vesicles” together with different genera of the most important probiotic microorganisms, e.g., “Lactobacillus” (and all new genera from the Lactobacillaceae family), “Bifidobacterium”, “Lactococcus”, “Pediococcus”, “Propionibacterium”, etc. As a result of this, we were finally able to obtain 73 original articles, which were subjected to our further analysis and description in subsequent parts of the current review.
3. Discussion
3.1. Physicochemical Properties of EVs Produced by Probiotics
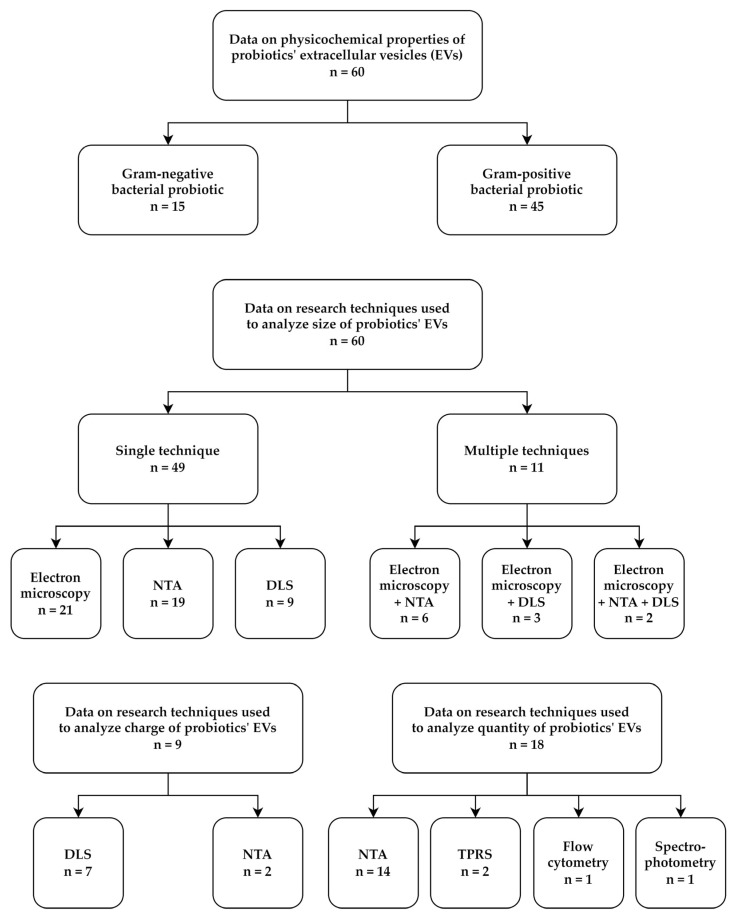
Our review of the literature on the ability of probiotic bacteria to produce EVs began with the collection of data describing EVs’ physicochemical properties (Table S1). We noticed that this information was available for 60 out of the 73 publications being the central core of this review. Interestingly, in 75% of the cases (45/60), the results concerned Gram-positive bacteria, in particular from the Lactobacillaceae family (Figure 1 and Table S1). The group of Gram-negative bacteria consisted of only two representatives—Escherichia coli Nissle 1917 and Akkermansia muciniphila ATCC BAA-835 (Figure 1 and Table S1). In our opinion, this situation is the result of frequent interchangeable use of two words with different definitions—‘probiotics’ and ‘lactic acid bacteria (LAB)’ [23,24]. The term ‘probiotics’ is broader and refers to many different groups of microorganisms with beneficial properties for the host, while the term ‘LAB’ refers only to Gram-positive, lactic acid-producing bacteria for which, many decades ago, numerous health-promoting properties were proved, and, hence, these bacteria were quickly classified as the most important representatives of probiotics [23,24,25,26]. In recent years, a strong separation between these two terms is made, contributing to the gradual expansion of research on probiotic properties of Gram-negative bacteria and their EVs. In this context, a mucin-degrading, Gram-negative bacterium A. muciniphila deserves special attention, as it was isolated for the first time as lately as in 2004 [27], while, in recent years, there has been an undoubted bloom in interest in this bacterium and its EVs [28]. In our opinion, the above situation has a chance to encourage other researchers focusing on the subject of probiotic microorganisms to expand their search with new, valuable species of probiotics from the group of Gram-negative bacteria.
Figure 1.
A graph presenting the categorization of articles constituting the core of this review, in which the physicochemical properties of extracellular vesicles (EVs) of probiotics were described. Among the 60 of them, 15 and 45 concerned Gram-negative and Gram-positive bacteria, respectively. All articles assessed the size of the produced EVs, of which 49 determined it using only one technique (21 with electron microscopy [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66,67,68,69,70,71], 19 with NTA [72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90], and 9 with DLS [42,45,49,50,91,92,93,94,95]), while 11 used more than one technique (6 with electron microscopy + NTA [29,30,31,32,33,34], 3 with electron microscopy + DLS [35,36,37], and 3 with electron microscopy + NTA + DLS [38,39]). Only 9 papers analyzed the electrical charge of EVs, of which 7 used DLS [31,36,37,38,39,42,45] and 2 used NTA [84,85]. The quantity of EVs produced was examined in 18 articles, of which 14 used NTA [31,33,38,39,72,73,74,76,77,79,83,87,88,89], 2 used TRSP [49,50], 1 used flow cytometry [45], and 1 used spectrophotometry [51]. Detailed information on the numerical data of the above-presented results can be found in Table S1. Abbreviations: DLS, dynamic light scattering; NTA, nanoparticle tracking analysis; TRSP, tunable resistive pulse sensing.
A careful analysis of the data collected by us showed that, in all 60 articles, the dimensions of probiotics’ EVs were determined, while, for this purpose, various research techniques were used (Figure 1 and Table S1). The most frequently applied methods were electron microscopy (21/60; 35%) or nanoparticle tracking analysis (NTA) (19/60; 31.7%). Dynamic light scattering (DLS) (9/60; 15%) was used less commonly for this purpose (Figure 1 and Table S1). In 11 articles, more than one technique was applied to determine the size of EVs, including 6 with microscopy + NTA [29,30,31,32,33,34], 3 with microscopy + DLS [35,36,37], and 2 with microscopy + NTA + DLS [38,39] (Figure 1 and Table S1). Among other parameters of EVs measured by researchers, the surface charge/zeta potential and the quantity of EVs can be distinguished. In both cases, the subject was, however, undertaken relatively rarely, i.e., 9/60 (15%) and 18/60 (30%), respectively (Figure 1 and Table S1). The surface charge of EVs was detected most often with the use of DLS (7 of 9 articles) and the quantity of EVs with the use of NTA (14 of 18 articles).
Looking closer at the size analysis of probiotics’ EVs, we noticed a large spectrum of results, which depended on both the tested strains/species/genera of bacteria and the analytical techniques used. For Gram-negative bacteria, most of the dimensions were in the range of 20–200 nm (Table S1). On the other hand, for Gram-positive bacteria, the range of the obtained results was greater, although often equal to 50–300 nm (on average, approx. 150 nm) (Table S1). In a comparative context, the articles in which the dimensions of EVs were measured using two or three techniques seem particularly valuable. For example, Hu et al. [29], Liu et al. [30], and Müller et al. [31] showed the convergence of EVs’ size values between electron microscopy and NTA, in which the former showed a wider range of detected sizes, while the latter often narrowed this range down to specific values (Table S1). In line with this, electron microscopy might be better than NTA in the analysis of EVs with very low dimensions, as the former suffers from sensitivity and resolution limitations [40,41]. On the other hand, in contrast to NTA, using electron microscopy, it is difficult to precisely determine the mean size of EVs (often being presented as a range of sizes) and it is impossible to determine the concentration of EVs in the sample [40,41]. Nevertheless, the usefulness of both techniques is reflected in the frequency of their use in the articles we analyzed (in total, 51 out of the 60 cases applied one or both of them) (Figure 1 and Table S1). In the context of DLS, according to Shao et al. [40], this method seems also to be quite useful in measuring the dimensions of EVs, but it should be remembered that the critical step during the analysis is to use number distribution, as original size distribution is intensity-weighted and large EVs may over-dominate the obtained results.
As mentioned previously, the parameters of EVs other than dimensions—surface charge/zeta potential and quantity—are of little interest to scientists. In the studies in which this topic was addressed, the zeta potential of EVs had negative values between −0.5 mV [37] and −45 mV [42], with an average ranging from −10 mV to −20 mV (Table S1). The phenomenon of negative charge of EVs is most often related to the presence of extracellular DNA on the surface of these structures, which translates into their important function in supporting adhesion, aggregation, and biofilm formation of microbes [43,44]. Due to this, the electric charge of the EVs of probiotic bacteria can directly affect their colonization capacity of the host [31,39,45]. It seems to us that the low interest in this parameter among the scientific community can be explained by the willingness to administer to patients purified EVs of probiotics, without applying microorganisms secreting them (e.g., in a form of postbiotics) [46]. Although the influence of EVs on the colonization capacity of the probiotics producing them is not widely investigated, it should still be kept in mind that EVs applied in this way could affect the diversity and properties of the host microbiota [47,48].
Taking into account the amount of EVs produced by probiotics, these values were in the range of 108–1012 per mL when focusing on the most commonly used technique—NTA (14/18; 77.8%) (Table S1). For the other two techniques, tunable resistive pulse sensing (TRPS) [49,50] and flow cytometry [45], these values were equal to approximately 1010 and 107 per mL, respectively (Table S1). For spectrophotometry [51], the unit used (relative fluorescence units/colony forming units of probiotics) makes it impossible to compare the results with the others (Table S1). Although the frequency in measuring quantity of probiotic EVs was quite low, the high homogeneity of applied research techniques allows for a relatively objective comparison of EVs’ production by different probiotics. In our opinion, the lack of universality in measuring the efficiency of production of probiotic EVs is, as stated before, associated with the frequent perception of these EVs as a therapeutic agent with designation to be administered in a purified form to patients (as postbiotics) and not necessarily as structures that would be secreted by the probiotic into the local environment, e.g., the intestines [46].
Other aspects of the biological and physicochemical properties of EVs secreted by probiotics, including the spatial orientation of EVs’ membranes or their biological origin (including the participation or lack of cell lysis), were examined extremely rarely—only in the case of single original articles. Therefore, the above properties were not included in the main part of our discussion. The description of the aforementioned problem will be additionally deliberated in Section 3.5. “Challenges and Limitations of Articles Focusing on EVs Produced by Probiotics”.
3.2. Proteomic Profile of EVs Produced by Probiotics
The second aspect analyzed by us was the evaluation of the proteome of EVs produced by probiotic bacteria. Out of 73 articles constituting the core of this review, 17 took up this topic (Table 1). Since the methodology of isolation and purification of EVs or analysis of their proteins may influence the obtained results, we decided to collect this information in Table 1. We noticed that, in both cases, there is a relatively high homogeneity of the research techniques used. Most probiotics’ EVs were isolated by ultracentrifugation (14/17; 82.4%), while, in the remaining cases, chemical precipitation (1/17) or size exclusion chromatography (SEC) (2/17) was applied (Table 1). In the analysis of the EVs’ proteome, the most frequently used technique was electrophoresis combined with liquid chromatography with tandem mass spectrometry (LC-MS/MS) or matrix-assisted laser desorption/ionization-time of flight (MALDI-TOF) (12/17; 70.6%). Less frequently, chemical (4/17) or magnetic (1/17) precipitation combined with LC-MS/MS was used for this purpose (Table 1).
Table 1.
Proteomic data on extracellular vesicles produced by probiotics.
| Bacterial Producer | Methodology of Isolation/Determination | Proteomic Data | Reference | ||
|---|---|---|---|---|---|
| Total Number of Identified Proteins * | Cellular Localization of Proteins | The Most Abundant Representatives/ Proteins Highlighted by Authors |
|||
|
Escherichia coli Nissle 1917 |
Ultracentrifugation (vesicles) Electrophoresis + LC-MS/MS (proteome) |
192 | Outer membrane ~ 40% Cytoplasm 40% Periplasm 15% Inner membrane < 5 % |
|
[52] |
| Ultracentrifugation + DGC (vesicles) Electrophoresis + LC-MS/MS (proteome) |
189 | Outer membrane 28% Cytoplasm 36.5% Periplasm 20% Inner membrane 9.5% Secretory 6% |
|
[29] | |
| Ultracentrifugation (vesicles) Electrophoresis + LC-MS/MS (proteome) |
295 | ND |
|
[51] | |
| Ultracentrifugation + DGC or SEC (vesicles) Chemical precipitation + LC-MS/MS (proteome) |
189 | Membrane ~ 60% Cytoplasm ~ 40% |
|
[72] | |
| Lactiplantibacillus plantarum BGAN8 | Ultracentrifugation (vesicles) Electrophoresis + MALDI-TOF (proteome) |
1149 | Membrane ~ 45% Cytoplasm + ribosomes ~ 52% Secretory < 1% |
|
[54] |
| Lactiplantibacillus plantarum WCFS1 | Chemical precipitation (vesicles) Electrophoresis + LC-MS/MS (proteome) |
31 | Membrane 42% Cytoplasm 13% Secretory 16% |
|
[32] |
|
Lacticaseibacillus casei ATCC 393 |
Ultracentrifugation (vesicles) Chemical precipitation + LC-MS/MS (proteome) |
43 | Membrane ~ 20% Cytoplasm 65% Secretory 14% |
|
[38] |
|
Lacticaseibacillus casei BL23 |
Ultracentrifugation (vesicles) Electrophoresis + LC-MS/MS (proteome) |
103 | Membrane + secretory 43% Cytoplasm 57% |
|
[36] |
| Limosilactobacillus reuteri ATCC 23272 | Ultracentrifugation (vesicles) Chemical precipitation + LC-MS/MS (proteome) |
17 | Membrane 18% Cytoplasm 82% |
|
[38] |
|
Limosilactobacillus reuteri BBC3 |
Ultracentrifugation + DGC (vesicles) Chemical precipitation + LC-MS/MS (proteome) |
92 | Membrane 27% Cytoplasm 56.5% Secretory 16% |
|
[34] |
|
Ligilactobacillus animalis ATCC 35046 |
Ultracentrifugation + DGC (vesicles) Chemical precipitation + nLC-MS/MS (proteome) |
340 | From the top 74 proteins: Membrane 25.7% Cytoplasm 25.7% Secretory 1.3% Unknown 47.3% |
|
[76] |
|
Lactobacillus acidophilus ATCC 53544 |
Ultracentrifugation (vesicles) Chemical precipitation + LC-MS/MS (proteome) |
26 | Membrane ~ 30% Cytoplasm 62% Secretory 12% |
|
[38] |
|
Lactobacillus gasseri BC12 |
Ultracentrifugation (vesicles) Electrophoresis + LC-ESI-MS/MS (proteome) |
15 | Membrane ~ 44% Cytoplasm ~ 44% Secretory ~ 11% |
|
[74] |
|
Lactobacillus crispatus BC5 |
Ultracentrifugation (vesicles) Electrophoresis + LC-ESI-MS/MS (proteome) |
11 | Membrane ~ 45% Cytoplasm ~ 45% Secretory ~ 9% |
|
[74] |
|
Lactobacillus johnsonii N6.2 |
Ultracentrifugation (vesicles) Electrophoresis + LC-MS/MS (proteome) |
366 | Cytoplasm + ribosomes 86% Secretory 14% |
|
[33] |
|
Bifidobacterium longum NCC 2705 |
Ultracentrifugation (vesicles) Electrophoresis + LC-MS/MS (proteome) |
24 | Membrane 21% Cytoplasm 75% |
|
[64] |
| Propionibacterium freudenreichii CIRM-BIA 129 | SEC (vesicles) Electrophoresis + LC-ESI-MS/MS (proteome) |
319 | Membrane 16% Cytoplasm 75% Secretory 9% |
|
[78] |
| SEC (vesicles) Electrophoresis + LC-MS/MS (proteome) |
391 (medium-dependent; 358 common for all) |
Membrane 16.5% Cytoplasm 74% Secretory 9.5% |
|
[79] | |
|
Lactococcus lactis FM-YL11 |
Ultracentrifugation (vesicles) Magnetic precipitation + LC-MS/MS (proteome) |
1283 | From the top 320 proteins: Membrane 16.5% Cytoplasm 74% Secretory 9.5% |
|
[62] |
| Pediococcus pentosaceus ** | Ultracentrifugation (vesicles) Electrophoresis + LC-MS/MS (proteome) |
103 | Membrane 9% Cytoplasm + ribosomes ~ 83% Secretory 5.5% |
|
[42] |
Abbreviations: ND, no data; DGC, density gradient centrifugation; LC-MS/MS, liquid chromatography with tandem mass spectrometry; nLC-MS/MS, nano-scale liquid chromatographic tandem mass spectrometry; LC-ESI-MS/MS, liquid chromatography electrospray ionization with tandem mass spectrometric; MALDI-TOF, matrix-assisted laser desorption/ionization-time of flight; SEC, size exclusion chromatography. * Total number of whole-length gene products, ** bacterial strain was not reported.
Despite the relatively high methodological homogeneity, a large discrepancy within the data was noticed. The total number of identified proteins (understood as whole-length gene products) ranged from a dozen [74] to over a thousand (1149 [54] and 1283 [62]), while, in most articles, these values were within the range of several hundred (10/17; 58.8%) (Table 1). In this context, it is also worth highlighting the strong correlation between cellular localization and the number of isolated EVs’ proteins. If the protein localization was classified as ‘membrane’, then the proteome was narrower (11–192 proteins) than when the most abundant protein representation was derived from the cytoplasm (11–1286 proteins; in all cases where the proteome was >300 proteins, ‘cytoplasm’ dominated) (Table 1). This phenomenon may be caused by two sources—the sensitivity of the research techniques and the level of contamination of the EVs’ proteome with proteins derived from bacterial cells producing these structures [40,96,97]. Insufficient level of sensitivity may contribute to the loss of proteins with low representation in the EVs’ proteome, the function of which, however, may be of key importance for microorganisms secreting them [98,99]. On the other hand, too high representation of proteins in the EVs’ proteome may suggest its contamination and the need to include/improve the purification step of the obtained EVs [96,100]. According to the review by Nagakubo et al. [96], some researchers consider numerous representations of ribosomal proteins (30S and 50S), which are typically of cytoplasmic origin, as an independent indicator of the EVs’ proteome contamination. However, there are articles showing that extracellular secretion of ribosomal proteins may have important, extra-ribosomal functions for the physiology of microorganisms, including biofilm formation [101] or resistance against translation-targeting antibiotics [102,103]. Therefore, in our opinion, the detection of ribosomal proteins in the EVs’ proteome should not be automatically interpreted as contamination, however, serious consideration for improving the techniques of isolation and analysis of EVs should be made if the proteome of these structures is both too numerous and over-represented by cytoplasmic proteins, including, in particular, the ribosome subunits.
In addition to information about the methodology of isolation of EVs and analysis of their proteome, in Table 1 we also included some details about the most abundant/most important proteins constituting the EVs’ proteome of probiotics. To simplify this issue, once again, we have decided to divide the discussion into a part covering Gram-negative and Gram-positive bacteria.
Gram-negative bacteria described in Table 1 were represented only by E. coli Nissle 1917, while as many as 4 out of the 17 articles focused on this aspect [29,51,52,72]. It is worth noting that within the most numerous proteins secreted by this bacterium in EVs many adhesive proteins were highlighted, including fimbrial (FocA, Fim1C, FocF, FocG, and FocH) and flagellar (FliC, FliD, FlgA, FlgE, FlgK, and FlgL) subunits, and outer membrane proteins (OmpA, OmpC, OmpF, and NmpC) (Table 1). According to many, the presence of adhesins anchored on the surface of EVs of Gram-negative bacteria is an important element facilitating the colonization of the intestines [104,105,106]. The second important group of proteins produced by this bacterium within EVs was related to peptidoglycan and cell membrane rearrangement, i.e., murein hydrolases (MltA, MltB, and MltC), murein-interacting protein MipA, and peptidoglycan-associated lipoproteins (Pal, TolB, Sat, LpoA, YbaY, and SlyB) (Table 1). It is indicated that the rearrangement of murein and cell membranes are important steps in the biogenesis of EVs, hence, the presence of proteins related to the above processes within EVs should not come as a surprise [107]. On the other hand, researchers pay attention to the participation of murein hydrolases encased within EVs in the competitive fight against other bacteria [108]. In summary, the main components of the EVs’ proteome of E. coli Nissle 1917 were adhesins and proteins associated with peptidoglycan rearrangement, which are involved in the effective colonization of the host and its protection against pathogenic microorganisms.
Gram-positive bacteria described in Table 1 were mainly representatives of the Lactobacillaceae family (Lactiplantibacillus, Lacticaseibacillus, Limosilactobacillus, Ligilactobacillus, and Lactobacillus), and, additionally, by Bifidobacterium, Propionibacterium, Lactococcus, and Pediococcus. Among the dominant group of proteins located within EVs of these bacteria were metabolic proteins, while, in single cases, peptidoglycan rearrangement proteins (including putative murein hydrolases or lysozyme-like proteins [32,34,36,38]) and proteins related to adhesion or aggregation (including surface proteins, mucus-binding proteins, or aggregation-promoting factors [33,38,78,79]) were also noticed (Table 1). Whereas the function of adhesins and peptidoglycan rearrangement proteins in EVs has been described above and is similar in Gram-positive bacteria, proteins responsible for metabolism deserve special attention. Based on the data collected in Table 1, it can be noticed that this group includes proteins related to the biosynthesis or breakdown of various classes of nutrients, and they are produced by many representatives of probiotics, such as Lactiplantibacillus [32,54], Lacticaseibacillus [36], Limosilactobacillus [34,38], Ligilactobacillus [76], Lactobacillus [33,74], Bifidobacterium [64], Propionibacterium [78,79], Lactococcus [62], and Pediococcus [42]. In addition to this, numerous proteins determining an uptake of glycerol [54], vitamins [54], amino acids and peptides [54,76], phosphates [74,76], inorganic acid ions [76], and iron [64] were detected. All the above-mentioned proteins participate in the transport of nutrients from the local environment and their delivery in an assimilable form. Many scientists point out that this system can provide nutrients not only to the EVs-producing microorganisms, but also to the host, especially in areas with high availability of nutrients, e.g., intestines [109,110,111]. On this basis, it can be concluded that EVs of Gram-positive probiotic bacteria determine not only their colonization abilities and competition with pathogens, but also may improve digestive processes of the host.
As it can be easily observed, EVs produced by probiotics surely have a different role in the host than those released by pathogens. With regards to probiotics, we speculate that the role of EVs secreted by different probiotics is strain-dependent and, in fact, influenced by the bacterial phenotype, culture conditions (i.e., culture media used or the age of microbial culture), and biogenesis mechanisms by which such EVs are released—including differences between Gram-positive and Gram-negative species [112,113]. For this reason, currently, we cannot state that a “universal molecular mechanism” conditioning benefit of probiotic EVs for the host exists. Unquestionably, further studies based on a deeper proteomic and metabolomic analysis of probiotic EVs, which compares different microbial strains and culture conditions, should be performed to discover microbial components capable of conferring beneficial properties on the host.
3.3. Biological Activities and Properties of EVs Produced by Probiotics
The last aspect considered in our review was the analysis of biological activities and properties of probiotics’ EVs. As reported in Table 2, a total amount of 54 articles were considered. In 23 of them, experiments were performed only in in vitro models, and 6 used only in vivo models, while the other 25 analyzed both (amongst these, 5 used ex vivo models). In our opinion, inclusion of both in vitro and in vivo models provides, undoubtedly, a better understanding of EVs’ activity. For example, as reported by Chen et al. [76], the use of murine models allows us to assess whether EVs could be transported to the femoral heads of glucocorticoid-treated mice after intragastric administration, thus providing information about EVs’ tissue distribution. We can assume that studying EVs’ properties addresses the same challenges of characterizing new probiotics. As described by Papadimitriou et al. [114], one of the most important advantage of in vitro assays is the ability to perform different screenings simultaneously, helping to evaluate potential interactions between probiotics and their products with the host. At the same time, these models are affected by some biases since the laboratory conditions only partially reproduce the in vivo situation. As well as probiotics needing to reach the desired body niches alive, EVs’ stability needs to be established since it depends on multiple factors. Thus, in vivo assays may be more appropriate because they can reproduce the complexity of the existing interactions. On the other hand, the main weakness of in vivo models is that they cannot be used for high throughput screening due to the increased cost and for ethical issues. For the above-mentioned reasons, a combination of in vitro and in vivo tests could represent an appropriate approach for EVs’ study [115].
Table 2.
Biological activity of extracellular vesicles produced by probiotics reported in in vitro and in vivo models.
| Bacterial Producer | In Vitro Model EVs Treatment (Duration) |
In Vivo Model EVs Treatment (Duration) |
Observations on EVs Activity | EVs Properties | Reference |
|---|---|---|---|---|---|
|
Escherichia coli Nissle 1917 |
Caco-2 and T-84 cells (EPEC-infected) 0.1 mg/mL (24 h) |
ND |
|
|
[131] |
| RAW264.7 murine macrophages1 µg/mL (16 h) |
ND |
|
|
[29] | |
| OVA-Escherichia coli Nissle 1917, Escherichia coli BL21 ΔompA |
ND | Tumor in C57BL/6 and BALB/c female 4–8-week-old mice (administration: oral gavage) 10 µg (3–5 times) |
|
|
[122] |
| Escherichia coli serotype O6:K5:H1 | Caco-2 and HT-29 cells 10 mg/mL (8 h) |
ND |
|
|
[132] |
| Caco-2 and PMBCs cells 50 µg/mL (5–24 h) |
Colon organ culture (ex vivo model) 50 µg/mL (5 h) |
|
|
[63] | |
| Human Monocyte-Derived DCs 10 µg/mL (24 h) |
ND |
|
|
[133] | |
|
Akkermansia muciniphila ATCC BAA-835 |
Caco-2 cells 0.1, 0.5, and 5 μg (24 h) |
ND |
|
|
[68] |
| Caco-2 cells 10 μg (24 h) |
HFD induced and ND thirty male C57BL/6 mice (administration: oral gavage) 10 μg (5 weeks) |
|
|
[67] | |
|
Akkermansia muciniphila ATCC BAA-835 |
ND | NFD induced and NF 8-week-old male C57BL/6 mice (administration: oral gavage) 10 μg protein/200 μL (5 weeks) |
|
|
[69] |
| Caco-2 cells (inflammation model) 0.1, 1, and 10 μg (4–8 h) |
HFD in 6–8-week-old male C57BL/6 mice (administration: oral gavage) 10 μg (14 days) |
|
|
[91] | |
| THP-1 and RAW264.7 10 μg/mL (24 h) |
Prostate cancer RM-1 mice model (administration: injection) 40 μg per mouse (13 days) |
|
|
[92] | |
| LX-2 cells (inflammation model) 1, 10, 50 µg/mL (24 h) |
Chronic liver injury in 7–8-week-old male C57BL/6 mice (administration: intraperitoneal injection) 50 µg protein/200 µL (4 weeks) |
|
|
[70] | |
| Caco-2 and Hep-G2 cells 50, 100 µg/mL (24 h) |
ND |
|
|
[53] | |
|
Akkermansia muciniphila ATCC BAA-835 |
LX-2 cells (inflammation model) 1, 10, 50 µg/mL (24 h) |
Livery injury in 8-week-old male C57BL/6 mice (administration: oral gavage) 50 µg protein/200 µL (4 weeks) |
|
|
[134] |
|
A. muciniphila ATCC BAA-835, Faecalibacterium prausnitzii A2-165c |
Caco-2 cells 1 and 50 μg/mL (24 h) |
ND |
|
|
[15] |
|
A. muciniphila ATCC BAA-835, L. plantarum KCTC 11401BP, Bacillus subtilis * |
HT22 cells (stress model) 20 μg (24 h) |
Chronic stress in 7-week-old male C57BL6 mice (administration: intraperitoneal injection) 6 μg/100 μL mouse per day (14 days) |
|
|
[130] |
| Lactiplantibacillus plantarum KCTC 11401BP | HT22 cells (stress model) 20 μg/mL (24 h) |
Depression in 7-week-old male C57BL/6J mice (administration: intraperitoneal injection) 0.1, 0.18, and 0.27 μg/kg (1–35 days) |
|
|
[129] |
| HaCaT cells and keratinocytes 0.1, 1, and 10 μg/mL (12 h) |
S. aureus atopic dermatitis-induced mouse model (administration: oral gavage) |
|
|
[55] | |
| Lactiplantibacillus plantarum APsulloc 331261 | THP1 cells 10 μg/mL (48 h) |
Human skin organ culture (ex vivo) 50 μg/mL (2–4 days) |
|
|
[50] |
| Lactiplantibacillus plantarum WCFS1 | Caco-2 cells 500 µL (24 h) |
C. elegans Bristol N2 EVs isolated from 109 CFU (1–15 days) |
|
|
[32] |
| Lactiplantibacillus plantarum Q7 | ND | Colitis in 4–5-week-old SPF male C57BL/6J mice(administration: gavage) 10/20 mg Q7-EVs group (0.5/1 mg/kg body weight) (1–18 days) |
|
|
[93] |
| Lactiplantibacillus plantarum * | CCD-986Sk cells 0.625%, 1.25%, 5%, and 10% EVs (24 h) |
Korean women in their 50s (administration: topically on the skin) (twice a day, 4 weeks) |
|
|
[87] |
| Lactiplantibacillus plantarum YW11 | Primary cortical neurons from C57BL/6 mice (OGD model) Co-culturing with EVs (24 h) |
tMCAO (ischemic stroke model) in 10–12-week-old male C57BL/6 mice (administration: injection through the tail vein) 100 μg/day (3 days) |
|
|
[86] |
|
Lacticaseibacillus casei BL23 |
T84 and HT-29 cells 20 ng/mL to 10 μg/mL (24 h) |
ND |
|
|
[35] |
|
Lacticaseibacillus casei ATCC 393 |
Caco-2 cells 100 and 150 μg/mL (24 h) |
ND |
|
|
[37] |
|
L. casei DSMZ 20011, L. plantarum NCIMB 8826 |
Caco-2 and THP-1 cells (inflammation model) 5 × 1011–5 × 1012 EVs/mL (24 h) |
ND |
|
|
[83] |
| THP-1 cells (inflammation model) 1:2 EVs per well (48 h) |
ND |
|
|
[31] | |
| Lacticaseibacillus paracasei * | RAW 264.7 cells (inflammation model) 0.1, 1, 10, 50 μg/mL (12 h) HT 29 cells (inflammation model) 500 ng/mL (12 h) |
Acute colitis-induced 7-week-old male C57BL/6 mice (administration: oral gavage) 5 mg/day (12 days) |
|
|
[58] |
|
Lacticaseibacillus paracasei PC-H1 |
Colorectal cancer cell line, HCT116, SW1116, and SW620 cells 200 μg/mL (24 h) |
4-week-old female BALB/c nude mice (administration with HCT116 and EVs through subcutaneous injection) 200 μg/mL (30 days) |
|
|
[90] |
|
Lactobacillus crispatus BC3, BC5; Lactobacillus gasseri BC12, BC13 |
Human T-lymphocyte MT-4 and Jurkat-tat cell lines 50 μL (1–72 h) |
Human tissue cultures (ex vivo model) 108 EVs/mL (12 days) |
|
|
[74] |
|
Limosilactobacillus reuteri BBC3 |
HD11 cells 10 µg/mL (6 h or 12 h) Splenic lymphocytes 10 μg/mL (12 h) |
Broiler chicks (inflammation model) (administration: oral gavage) 200 μg/bird (21 days) Jejunum explant culture (ex vivo model) (inflammation model) 10 µg/mL (6 h) |
|
|
[34] |
|
Lacticaseibacillus rhamnosus GG |
HepG2 cells 50, 100, 150, and 200 μg (24 h) |
ND |
|
|
[56] |
| SW480 and HT 29 cells (human colon cancer cell lines) 5–200 µg/mL (24 h) |
ND |
|
|
[57] | |
| ND | Colitis-induced 4–5-week-old C57BL/6J male mice (administration: oral gavage) 1.2 mg/kg of body weight (14 days) |
|
|
[94] | |
|
Lacticaseibacillus rhamnosus JB-1 |
HT-29 and MODE-K cells 3 × 1010 EVs (2 h) |
8- to 10-week-old SPF BALB/c male mice (administration: oral gavage) 3 × 1010 EVs (2 h) |
|
|
[88] |
|
L. rhamnosus GG, L. reuteri DSM 17938 |
PBMCs cells 500:1, 100:1, and 20:1 (48 h) |
ND |
|
|
[135] |
| Lentilactobacillus kefirgranum PRCC-1301 | Caco-2 and HCT116 cells 0, 10, and 100 µg/mL (6–48 h) |
Colitis-induced 6-week-old male C57BL/6 mice (administration: oral gavage) 3 mg/kg (3–14 days) |
|
|
[118] |
|
Lentilactobacillus kefir KCTC 3611, Lentilactobacillus kefiranofaciens KCTC 5075, Lentilactobacillus kefirgranum KCTC 5086 |
Caco-2 cells (inflammation model) 1 × 109 EVs/mL (24 h) |
IBD-induced 8-week-old male BALB mice (administration: oral gavage) (3 × 108 or 3 × 1010 EVs/head) |
|
|
[60] |
|
Latilactobacillus sakei NBRC 15893 |
PP and BMDCs from BALB/c mice (female, 7–14 weeks old) 30 μg protein/mL (4 days) |
ND |
|
|
[125] |
| PP cells 37 μg/mL EVs (1–4 days) |
ND |
|
|
[61] | |
|
Ligilactobacillus animalis ATCC 35046 |
HMECs, MLO-Y4, MC3T3-E1, and BMSCs(MPS-treated) 10 μg/mL (6–24 h) |
GC-induced ONFH male C57BL/6J mice (administration: oral gavage) 30 μg/200 μL (once a week) |
|
|
[76] |
|
Lactobacillus johnsonii N6.2 |
Pancreatic cell line βlox5, Caco-2, Jurkat, and THP-1 cells 108 or 1010 EVs/mL (2–8 h) |
Pancreatic islets isolated from human donors(ex vivo model) 6 × 109 EVs/mL (5 h) |
|
|
[136] |
| Lactococcus lactis * | Dendritic cells isolated from asthmatic patients 10 μg/mL (24 h) |
Allergic asthma-induced 6-week-old female BALB/c mice (administration: intranasally) 10 μg/20 μL PBS (5 days) |
|
|
[95] |
|
Bifidobacterium longum KACC 91563 |
PP, T cells, B cells, eosinophils, and BMCCs from mice 2 µg/mL (2 h) |
Food allergy-induced 6- to 8-week-old BALB/c mice (administration: oral gavage) EVs from 109 CFU/mouse (2 weeks) |
|
|
[137] |
|
Bifidobacterium bifidum LMG 13195 |
Monocyte-derived DCs and naïve T cells 0.1 μg/mL (48 h) |
ND |
|
|
[138] |
|
B. longum *, L. plantarum WCFS1 |
DC2.4 and RAW264.7 cells 0.01 or 0.1 μg/well (6–24 h) |
ND |
|
|
[85] |
| DC2.4 and RAW264.7 cells 0.5 μg/well (6 h) |
ND |
|
|
[84] | |
| Propionibacterium freudenreichii CIRM-BIA 129 | HT-29 cells (inflammation model) 109 EVs/mL (1 h) |
ND |
|
|
[78] |
| HT-29 cells (inflammation model) 109 EVs/mL (24 h) |
ND |
|
|
[79] | |
|
Bacillus subtilis 168 |
Caco-2 cells 1.3 × 109 EVs (0–4 h) |
ND |
|
|
[80] |
|
Clostridium butyricum MIYAIRI 588 |
ND | Ulcerative colitis in 40–60-day-old male C57BL/6 mice (administration: intragastrically) 15 μg/200 μL (once a day, 5 days) |
|
|
[82] |
| ND | Ulcerative colitis-induced male C57BL6J mice (administration: oral gavage) 50 μg/day (11 days) |
|
|
[81] | |
|
Leuconostoc holzapfelii GFC1203H, L. plantarum *, B. longum *, B. animalis *, L. acidophilus * |
Human HFDP cells 1, 2.5, 5, and 10 μg/mL (6 h, 12 h, 24 h) |
ND |
|
|
[77] |
|
Pediococcus pentosaceus *, Ligilactobacillus salivarius * |
E.G7–EL4 and HEK-BLUE hTLR2, BMDCsMouse splenocytes (inflammation model) 0.2, 1, and 5 mg/mL (24 h) |
Liver-fibrosis in 6- to 8-week-old male C57BL/6 mice (administration: injection) 10 μg/mouse (14 days) |
|
|
[42] |
Abbreviations: ND, no data; MVs, membrane vesicles; EPEC, enteropathogenic Escherichia coli; TJ, tight junctions; TNF, tumor necrosis factor; IL, interleukin; IκBα, nuclear factor of kappa light polypeptide gene enhancer in B-cells inhibitor; NOD, nucleotide-binding oligomerization domain; TGF, transforming growth factor; TLR. toll-like receptor; HFD, high-fat diet; ND, normal diet; PP, Peyer’s Patch; IgA, immunoglobulin A; ZO, zonula occludens; OCLDN, occludin; CLDN, claudin; TLR, toll-like receptor; PPAR, peroxisome proliferator-activated receptors; Angptl4, angiopoietin-like 4; HT-29, human colonic epithelial; MODE-K, mouse duodenal epithelial; GC, glucocorticoid; TST, tail suspension test; BDNF, brain-derived neurotrophic factor; Sirt1, sirtuin 1; GM-CFS, granulocyte-macrophage colony-stimulating factor; CTSB, cysteine proteinase; REG3G, C-type Lectin; SIT, allergen-specific immunotherapy; LTA, lipoteichoic acid; NFKβ, nuclear factor kappa-light-chain-enhancer of activated B cells; LPS, lipopolysaccharides; CG, glucocorticoid; Sirt1, Sirtuin 1; PMBCs, peripheral blood mononuclear cells; ER, endoplasmatic reticulum; BMCCs, bone marrow-derived mast cells; MPO, myeloperoxidase; HMECs, human microvascular endothelial cells; MLO-Y4, mouse long bone osteocyte-Y4; MC3T3-E1, mouse preosteoblast cells; BMSCs, mouse bone marrow mesenchymal stem cells; ONFH, osteonecrosis of the femoral head; MPS, methylprednisolone; CB1R and CB2R, cannabinoid receptors; FAAH, fatty acid amide hydrolase; PPARs, peroxisome proliferator-activated receptors; ECS, endocannabinoid system; CCD-986Sk, human dermal fibroblasts; MMP-1, matrix metalloproteinase-1; ECM, extracellular matrix; HAS2, hyaluronidase 2; CEA, carcinoembryonic antigen; OVA, ovalbumin; BALF, bronchoalveolar lavage fluid; UC, ulcerative colitis; MUC2, mucin 2; ZO-1, zonula occludens protein 1; M2, M2 macrophages; M1, M1 macrophages; OAS, 2′,5′-oligoadenylate synthetase; AHR, aryl hydrocarbon receptor; CYP1A1 and CYP1B1, cytochrome P450 superfamily enzymes; GLUT6, glucose transporter 6; SREBF1, sterol regulatory element binding transcription factor 1; GLP1R, glucagon-like peptide 1 receptor; PRKACA, protein kinase catalytic subunit α; MTA2, metastasis-associated 1 family member 2; STC2, stanniocalcin-2; SOD1, superoxide dismutase 1; tMCAO, transient middle cerebral artery occlusion; OGD, oxygen-glucose deprivation; HFDPCs, human hair follicle dermal papilla cells. * Bacterial strain was not reported.
Of the 54 articles considered, a large number of studies focused on the evaluation of EVs’ activity on the gastrointestinal system. From them, the most commonly applied models were Caco-2 cells, a model of the intestinal epithelial barrier, and C57BL/6 mice, one of the most adaptable animal models (Table 2). It is widely known that probiotics have a beneficial effect on the intestinal homeostasis, and this is obtained via multifactorial health-promoting activity [1,5,6]. In fact, several authors reported ability of probiotic EVs to enhance the intestinal barrier integrity by increasing the expression of tight junction (TJ) proteins, such as ocldn, zo1, zo2, and zo3 [68], and by reducing cldn-2 [67,69] (Table 2). Alterations of TJ barrier function and paracellular permeability are closely associated with the onset of metabolic diseases. All the previously-mentioned proteins aggregate into complexes located at the apical site of the lateral membranes of intestinal epithelial cells and regulate the selective passage of ions, solutes, and water. Occludins, the first identified integral membrane TJ proteins, create a barrier against macromolecules through the hemophilic interactions of their extracellular loops and so they have a crucial role in TJ structure and function [116]. ZO proteins are multi-domain proteins that provide an intracellular scaffold in TJs, creating a direct connection with the actin cytoskeleton and cytoskeleton-associated proteins; it is also recognized that ZO proteins have an important role in the regulation of TJ assembly [116]. Claudins, on the contrary to the two previously-described proteins, confer pore-like properties on TJs and regulate the selective passage of molecules in the paracellular pathways [116]. Of note is that increased claudin-2 expression by intestinal epithelial cells is correlated with colitis and inflammatory bowel disease [117]. As reported in Table 2, EVs produced by A. muciniphila can decrease the expression of cldn-2, thus regulating the integrity of the intestinal barrier and reducing inflammation [67]. Moreover, in the context of inflammatory bowel diseases (IBD), Hao et al. [93], Tong et al. [94], and Kang et al. [118] detected colitis amelioration in mice treated with EVs. In their studies, these authors used similar models, C57BL/6 and C57BL/6J mice with specific pathogen-free conditions, and colitis was induced by dextran sulfate sodium (DSS). DSS-treated mouse is the most widely used to obtain a good experimental model of ulcerative colitis (UC) since it leads to pathological alternations that are similar to what occurs in human UC [119]. In all three articles [93,94,118], EVs were administered by oral gavage in similar dosage and, despite the difference in the producer strain (L. plantarum Q7 [93], L. rhamnosus GG (ATCC 53103) [94], and L. kefirgranum PRCC-1301 [118]), the results obtained were comparable. They all reported a reversion of colon shortening and a downregulation of pro-inflammatory cytokines, including IL-1β, IL-2, IL-6, and TNF-α. It is worth noting that another common finding was the restoration of the gut microbiota homeostasis [82,93,94,118] (Table 2). Dysbiosis of intestinal microbiota, with reduction of probiotics and rise in pathogenic bacteria, represents a significant feature in UC patients. Consequently, the therapeutic potential of probiotic strains in UC has been examined by several researchers, who have identified different related mechanisms of action [120]. Thus, regarding the articles considered in this review, we can assume that EVs could be considered one of the bacterial products involved. In this context, Ma et al. [81] also highlighted the correlation between EVs treatment and mucus barrier integrity enhancement. This association was widely studied by Petersson et al. [121], who observed that the colonic mucus layer in germ-free mice was very thin compared with that observed in conventionally housed mice. Moreover, the administration of bacterial products (lipopolysaccharide and peptidoglycan) restored the normal mucus levels.
Five studies focused on the correlation between EVs and tumor development (Table 2). Using animal models, Tomasi et al. [122], Luo et al. [92], and Shi et al. [90] tested EVs of E. coli Nissle 1917, A. muciniphila ATCC BAA-835, and L. paracasei PC-H1, respectively. Melanoma, prostate cancer, and colorectal cancer were investigated, and comparable results were obtained despite different routes of administration and dosage; each author reported a reduction in tumor growth. Luo et al. [92] observed an upregulation of M1 macrophages and CD8+ lymphocytes expressing IFN-γ and GZMB, concluding that A. muciniphila-EVs stimulate anti-tumor immunity against prostate cancer. Shi et al. [90], instead, found out that the treatment with L. paracasei-EVs increased the expression level of Bax and decreased Bcl-2. These results were confirmed in both in vivo and in vitro models, and the authors confirmed that EVs can be taken up by colon cancer cells and inhibit their growth through apoptosis induction. As is well documented, many tumor types induce extensive systemic perturbations in the activity of the immune system, although the microbiome can modulate the systemic immunity and thus influence the outcome of tumor control strategies [123]. Fessler et al. [124] summarized the potential biological mechanisms of microbiome-mediated immune modulation: (1) bacterial translocation to different body districts may stimulate the immune response by providing microbial-derived, conserved antigens; (2) cross-reactive T cells primed against bacterial antigens might exert anti-tumor effects; (3) gut bacteria can release soluble immunomodulatory factors (IL-12, IFN-γ, and TNF-α) that then disseminate systematically and can activate dendritic cells. In this context, given the results obtained by the authors previously mentioned, it can be assumed that EVs could represent one of the effectors of these processes. For the research of Tomasi et al. [122] it is necessary to highlight that they tested E. coli-EVs engineered with a cancer-specific epitope and showed that the administration of these EVs, but not of wild type EVs, induced a reduction in tumor growth (Table 2). Given these results, we can assume that, unsurprisingly, not all probiotic strains may have the same health-promoting potential, although engineered EVs could represent a promising tool in cancer therapies. The remaining two articles [56,57] noted the same EVs’ properties on HepG2, SW480, and HT29 cells, highlighting an anti-proliferative effect on cancer cell lines (Table 2). According to Behzadi et al. [56], L. rhamnosus-EVs can increase the apoptotic index (bax/bcl2 expression ratio) in liver cancer cells in a dose-dependent manner. Keyhani et al. [57], analyzing EVs of the same probiotic strain, reported an inhibitory effect on colon cancer cells too. Otherwise, in the latter article, a specific mechanism of action was not considered.
Other authors frequently reported that EVs have an immunomodulatory effect, which is related to their role in the regulation of different types of cytokines, chemokines, and antibodies [35,37,63,125] (Table 2). The influence of probiotics on the human immune system is not strictly related to pathological conditions, as previously discussed; a large amount of research, in fact, proved that the gut microbiota play a crucial role in the development and regulation of the host immune system and this complex interplay starts already during the birthing process. Under the stimulus of probiotic-derived products, intestinal epithelial cells may release thymic stromal lymphopoietin (TSLP), transforming growth factor-β (TGF-β), IL-25, and B cell activating factor (BAFF) [126]. Furthermore, TH17, TReg, and IgA-producing cells development is also regulated by gut microbiota [127]. Different strains of probiotics can increase the number of dendritic cells and macrophages, and activate the latter through proinflammatory mediators, such as cytokines, reactive oxygen species or nuclear factor kB, and Toll-like receptor 2 pathways [128]. Fabrega et al. [63] determined that the presence of LPS in E. coli-EVs may explain the activation of IL-6, IL-8, and TNF-α, while the upregulation of IL-10 seems to be attributed to the presence of other vesicle factors (Table 2). At the same time, Morishita et al. [84,85] elucidated that EVs-mediated cytokine production is strictly related to their internalization. The release of TNF-α and IL-6 from cells treated with EVs was reduced in the presence of endocytosis and TLR2 inhibitors, with only one exception for RAW264.7 cells, for which no reduction in TNF-α was observed even after blocking clathrin-mediated endocytosis and micropinocytosis (Table 2). This suggests that several pathways could be involved in the EVs–cell interaction, and characterizing the main effectors is the key for the understanding of the immunomodulation in the host.
Interestingly, two articles considered the correlation between gut microbiota and the nervous system when focusing on EVs’ activity (Table 2). Choi et al. [129,130] found that EVs treatment reversed the expression of brain-derived neurotrophic factors (BDNFs) in HT22 cells and afforded antidepressant-like effects in C57BL/6 mice with stress-induced depression. In the first study, they examined whether L. plantarum-EVs treatment could block stress-induced, depressive-like behaviours in mice during the stress induction phase and during the post-stress phase. In both cases L. plantarum-EVs treatment restored the expression levels of BDNFs in the hippocampus and reduced depressive-like behaviors. This correlation was confirmed by their results obtained in vitro using HT22 cells. In the second study, they also considered EVs of B. subtilis and A. muciniphila and obtained comparable, although not identical, results. These data were also confirmed by several experiments that have proved the existence of the so-called ‘gut–brain axis’. Activation of proinflammatory cytokines, such as IL-1 and IL-6, has a certain association with the development of depression, so the impact of probiotics on immune homeostasis could help in the prevention or treatment of depression [128]. However, the detailed mechanisms of the action of EVs and their tissue distribution remain to be explored further.
Considering the influence of microbiota on the host health, two articles evaluated the effect of probiotics on another extraintestinal tissue—the skin [55,87] (Table 2). In this regard, Kim et al. [55] evaluated the therapeutic properties of L. plantarum-EVs on S. aureus-induced mouse atopic dermatitis model and on keratinocytes. The results showed that L. plantarum-EVs decrease skin inflammation by reducing the level of proinflammatory cytokines (IL-4 and IL-6). Since current treatment of atopic dermatitis involves the use of anti-inflammatory drugs and emollients, in order to compensate poor immune tolerance and barrier dysfunction, probiotics and their byproducts could represent an alternative option in the prevention or treatment of this disorder. Another article by Jo et al. [87] evaluated the effect of probiotic EVs on skin aging by using human dermal fibroblasts (CCD986sk) and a clinical trial among Korean women. As reported in Table 2, authors discovered that L. plantarum-EVs exert an anti-aging and anti-pigmentation effect and were able to positively regulate multiple pathways in fibroblasts.
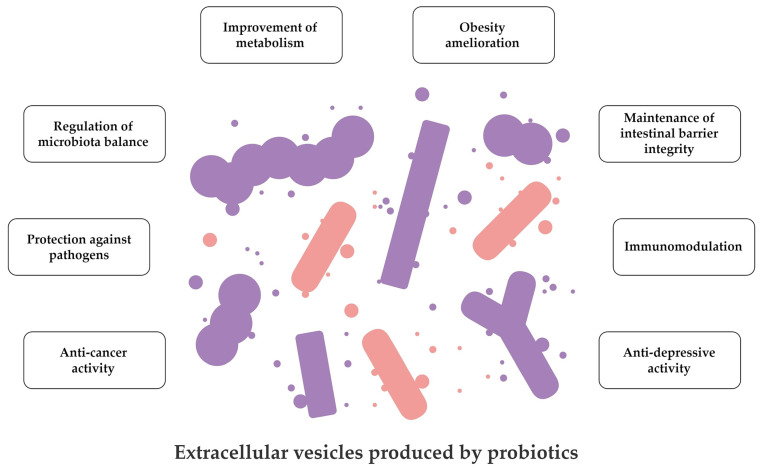
Taken together, all the data summarised in Figure 2 and Table 2 suggest a huge variety of applications of probiotic EVs. Most of the studies confirmed their potential in protecting the intestinal barrier integrity and modulating host immune response in both physiological and disease-induced conditions. At the same time, there are many fields that still require further investigations on mechanisms by which probiotic EVs exert their activity. This knowledge could be then used to design innovative approaches in prevention and therapy of difficult-to-treat diseases.
Figure 2.
Graphical summary of data on the biological activities of extracellular vesicles secreted by probiotics.
3.4. Future Application and Perspectives on EVs Produced by Probiotics
Unquestionably, we consider it very optimistic to find an exponential increase over the last decade in the number of publications on the production of probiotic EVs. For example, in 2012, only a single research article was published, while, in the first half of 2022 alone, as many as 15 original papers were released. Because of such a high dynamic of new articles appearing, during our analysis of the already collected literature and preparation of the central core of the manuscript’s discussion, we were unable to include newly-published articles from the second half of 2022. In this regard, however, we would like to point out that seven more original articles appeared during this time. These studies showed the protective effect of probiotic EVs on atopic dermatitis [139] and various intestinal pathologies (chemoresistant colorectal cancer [140] or intestinal infections made by enterotoxigenic E. coli [141]). It was also noticed that probiotic EVs have a strong immunomodulatory effect on the human body (including the intestines) [142], and that they can be used in the design of innovative vaccines against infectious diseases [143] and cancers [122]. In the last publication, attention was drawn to the participation of prophages in the secretion of EVs by the tested probiotic bacteria [144].
The latest research, together with the papers already analyzed in this review, lead us to highlight multiple applications of probiotic EVs. As previously mentioned, the close interconnection between probiotics and the gastrointestinal system is of great interest. In this context, EVs could represent a new strategy for the treatment of metabolic diseases, such as diabetes and obesity. There is evidence supporting the role of diet in shaping the host microbiota and the release of gut microbiota EVs, which, in turn, can exert their beneficial effect on human gut homeostasis [145]. In addition, another study showed that probiotic-derived EVs have a protective effect on Caco-2/HT29-MTX co-cultures exposed to enterotoxigenic E. coli, confirming the role of this bacterial structure in the maintenance of intestinal barrier integrity [141]. Considering their application in perspective, some authors also suggest that the administration of probiotic EVs alone could be a safer alternative than delivering live probiotics, especially in immunocompromised patients [146,147,148]. Moreover, in relation to some probiotic strains, the use of EVs alone could also represent an advantage in terms of efficacy. For example, Pang et al. [141] noted that EVs of L. reuteri DSM 17938 are more effective in the treatment of infantile colic compared to the bacterial cells of this strain.
Taking into account their biological properties, probiotic EVs could also be used for the treatment of certain neurological diseases. In fact, having the potential to cross the blood–brain barrier, they could represent efficient transporters for the delivery of drugs into the central nervous system [11]. Yuan et al. [149] and Sun et al. [150] extensively reported in their reviews the potential application of EVs-based strategies in the treatment of neurological disorders. Although in those papers only eukaryotic cells-based vesicles were considered, we cannot ignore the fact that they show comparable structure and properties to bacterial ones, which lead us to believe that a similar application of probiotics-derived EVs is reasonable. In a recent work, González-Lozano et al. [148] reported several examples of EVs’ applications in neurological disorders, focusing on their ability to transport active bacterial-produced compounds through different body districts. In addition, it is worth highlighting that the possibility to engineer EVs to improve their properties also exists. In this context, the surface modification can improve the EVs’ targeting capability and, as a consequence, their therapeutic potential [150]. To sum up, EVs biocompatibility, size, and drug delivering capabilities make them promising tools for future biomedical applications [147].
3.5. Challenges and Limitations of Articles Focusing on EVs Produced by Probiotics
As a summary, below, we would like to draw attention to a few challenges and limitations, the consideration of which may help the scientific community in an even more robust and reliable study of EVs produced by probiotics in the future.
We discovered that most research on the above topic focuses on the properties of EVs produced by Gram-positive bacteria; however, research on E. coli or A. muciniphila shows that the health-promoting properties of EVs of Gram-negative bacteria can be equally valuable (Table S1 and Table 2). It is also worth mentioning that other probiotic microorganisms, including yeasts of the genus Saccharomyces, have many health-benefiting properties [151] and EVs produced by them could possibly constitute very valuable therapeutics. For this reason, we would like to encourage scientists to increase the pool of tested microorganisms not only with new strains of the bacterial species tested so far, but also with novel genera or families of microorganisms [152,153].
The second aspect we would like to discuss is insufficiently low attention paid to characterizing biological and physicochemical properties of EVs produced by probiotics. As we proved in Figure 1 and Table S1, when undertaking such an analysis, it is mostly limited only to estimating the size of EVs. Other parameters were determined either rarely (electric charge and quantity) or extremely rarely (spatial orientation of EVs’ membranes or biological origin). Since we have already discussed the subject of electric charge and quantity of probiotic EVs, here, we would like to pay special attention to the second group of parameters. In none of the articles we analyzed, the spatial orientation of EVs (inside-out or right-side-out) was determined. The issue of the biological origin of EVs, and, thus, the precise nomenclature of isolated structures, has also been addressed sporadically. In most of the original articles, the term ‘extracellular vesicles’ (43) or ‘membrane vesicles’ (16) was used, while the presence of EVs’ subpopulations was included in only one article [51]. It is worth mentioning that cell lysis can be a significant source of EVs, and, hence, determining its intensity is also of importance [96,112]. Again, however, this phenomenon was rarely established (only in two publications [62,66]). In connection to the above description, we would like to sensitize scientists to increase their attention toward characterizing the properties of probiotic EVs and include “minimal information for studies of extracellular vesicles”, as recommended by the International Society for Extracellular Vesicles [18].
Another aspect we would like to highlight is the proteomic analysis of probiotic EVs. We noticed that, despite the relatively high methodological homogeneity, consisting of ultracentrifugation followed by electrophoresis combined with LC-MS/MS or MALDI-TOF, a large discrepancy within the data was noticed (Table 1). The total number of identified proteins (understood as whole-length gene products) ranged from as low as a dozen [74] to as high as over a thousand [54,62]. Still, however, in most articles, these values were within the range of several hundred. The phenomenon of under- or over-representation of identified EVs’ proteins may be caused by two sources—the sensitivity of the research techniques and the level of contamination of the EVs’ proteome with proteins derived from bacterial cells secreting these structures [40,96,97]. In line with this, we propose to make serious considerations for changing the techniques of isolation and analysis of EVs if the proteome of these structures is either too sparse or too numerous (especially when over-represented by cytoplasmic proteins).
Finally, the last issue worth recalling is the scope of the conducted research. In Table 2, we extensively presented the data of original articles describing biological activities of EVs produced by probiotics. As it can be relatively easily observed, a large amount of research on EVs produced by probiotics focuses on the evaluation of the biological effect of these structures only on the gastrointestinal system. For obvious reasons, including the ingestible administration of probiotic EVs, the action of these structures on the digestive system is highly intuitive and justified [154,155]. However, it should still be remembered that EVs, due to their nanometric dimensions, can reach various tissues of the host, and, therefore, their effect on different types of human cells should be discovered [13,156]. As described in this review, a good example of a different approach to evaluating the biological activity of probiotic EVs is the original article aimed at the skin [55,87] or the nervous system [129,130]. Undoubtedly, the health-promoting impact of probiotic EVs on the host should be extended to many other organs untested yet, such as the oral cavity, cardiovascular system, respiratory system, or genitourinary system.
4. Conclusions
Many decades of numerous studies on probiotics have confirmed their health-promoting effect on humans. Despite this, knowledge about the activity of EVs produced by probiotic microorganisms is still in its infancy. As our detailed review of the literature shows, in the last decade, the awareness on the usefulness of these structures is, however, dynamically growing. A broad variety of benefits of using EVs secreted by probiotics have already been shown, including regulation of intestinal homeostasis on both microbiota and host metabolism levels, anti-depressive activity, and immunostimulation, leading to a better control of microbial and carcinogenic disorders. We hope that the coming years will bring even more groundbreaking discoveries on these topics.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pharmaceutics15020522/s1, Table S1: Detailed data on physicochemical properties of extracellular vesicles produced by probiotics.
Author Contributions
Conceptualization, P.K. and R.G.; methodology, P.K.; formal analysis, P.K., B.M., I.V.; writing—original draft preparation, P.K., B.M. and I.V.; writing—review and editing, P.K., B.M., I.V. and R.G.; visualization, P.K.; supervision, P.K. and R.G.; funding acquisition, P.K. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The study was supported by the Wroclaw Medical University grant No: SUBZ.A130.23.070. The funders had no role in the study design, data collection, and analysis, decision to publish, or preparation of the manuscript.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.You S., Ma Y., Yan B., Pei W., Wu Q., Ding C., Huang C. The Promotion Mechanism of Prebiotics for Probiotics: A Review. Front. Nutr. 2022;9:1000517. doi: 10.3389/fnut.2022.1000517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ogunrinola G.A., Oyewale J.O., Oshamika O.O., Olasehinde G.I. The Human Microbiome and Its Impacts on Health. Int. J. Microbiol. 2020;2020:8045646. doi: 10.1155/2020/8045646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hou K., Wu Z.X., Chen X.Y., Wang J.Q., Zhang D., Xiao C., Zhu D., Koya J.B., Wei L., Li J., et al. Microbiota in Health and Diseases. Signal Transduct. Target. Ther. 2022;7:135. doi: 10.1038/s41392-022-00974-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Depoorter L., Vandenplas Y. Probiotics in Pediatrics. A Review and Practical Guide. Nutrients. 2021;13:2176. doi: 10.3390/nu13072176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Varela-Trinidad G.U., Domínguez-Díaz C., Solórzano-Castanedo K., Íñiguez-Gutiérrez L., Hernández-Flores T.D.J., Fafutis-Morris M. Probiotics: Protecting Our Health from the Gut. Microorganisms. 2022;10:1428. doi: 10.3390/microorganisms10071428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ranjha M.M.A.N., Shafique B., Batool M., Kowalczewski P.Ł., Shehzad Q., Usman M., Manzoor M.F., Zahra S.M., Yaqub S., Aadil R.M. Nutritional and Health Potential of Probiotics: A Review. Appl. Sci. 2021;11:11204. doi: 10.3390/app112311204. [DOI] [Google Scholar]
- 7.Wang G., Chen Y., Xia Y., Song X., Ai L. Characteristics of Probiotic Preparations and Their Applications. Foods. 2022;11:2472. doi: 10.3390/foods11162472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Thorakkattu P., Khanashyam A.C., Shah K., Babu K.S., Mundanat A.S., Deliephan A., Deokar G.S., Santivarangkna C., Nirmal N.P. Postbiotics: Current Trends in Food and Pharmaceutical Industry. Foods. 2022;11:3094. doi: 10.3390/foods11193094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maccelli A., Carradori S., Puca V., Sisto F., Lanuti P., Crestoni M.E., Lasalvia A., Muraro R., Bysell H., Sotto A.D., et al. Correlation between the Antimicrobial Activity and Metabolic Profiles of Cell Free Supernatants and Membrane Vesicles Produced by Lactobacillus reuteri DSM 17938. Microorganisms. 2020;8:1653. doi: 10.3390/microorganisms8111653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sabahi S., Homayouni Rad A., Aghebati-Maleki L., Sangtarash N., Ozma M.A., Karimi A., Hosseini H., Abbasi A. Postbiotics as the New Frontier in Food and Pharmaceutical Research. Crit. Rev. Food Sci. Nutr. 2022:1–28. doi: 10.1080/10408398.2022.2056727. [DOI] [PubMed] [Google Scholar]
- 11.Srivastava P., Kim K. Membrane Vesicles Derived from Gut Microbiota and Probiotics: Cutting-Edge Therapeutic Approaches for Multidrug-Resistant Superbugs Linked to Neurological Anomalies. Pharmaceutics. 2022;14:2370. doi: 10.3390/pharmaceutics14112370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aggarwal S., Sabharwal V., Kaushik P., Joshi A., Aayushi A., Suri M. Postbiotics: From Emerging Concept to Application. Front. Sustain. Food Syst. 2022;6:887642. doi: 10.3389/fsufs.2022.887642. [DOI] [Google Scholar]
- 13.Viswanathan K., Muthusamy S. Review on the Current Trends and Future Perspectives of Postbiotics for Developing Healtheir Foods. eFood. 2022;3:e47. doi: 10.1002/efd2.47. [DOI] [Google Scholar]
- 14.Salminen S., Collado M.C., Endo A., Hill C., Lebeer S., Quigley E.M.M., Sanders M.E., Shamir R., Swann J.R., Szajewska H., et al. The International Scientific Association of Probiotics and Prebiotics (ISAPP) Consensus Statement on the Definition and Scope of Postbiotics. Nat. Rev. Gastroenterol. Hepatol. 2021;18:649–667. doi: 10.1038/s41575-021-00440-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou C., Zhang Y., Liu H., Wu Y., Zhou X. Extracellular Vesicles: Recent Insights Into the Interaction Between Host and Pathogenic Bacteria. Front. Immunol. 2022;13:840550. doi: 10.3389/fimmu.2022.840550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Combo S., Mendes S., Nielsen K.M., da Silva G.J., Domingues S. The Discovery of the Role of Outer Membrane Vesicles against Bacteria. Biomedicines. 2022;10:2399. doi: 10.3390/biomedicines10102399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Srivatsav A.T., Kapoor S. The Emerging World of Membrane Vesicles: Functional Relevance, Theranostic Avenues and Tools for Investigating Membrane Function. Front. Mol. Biosci. 2021;8:59. doi: 10.3389/fmolb.2021.640355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Théry C., Witwer K.W., Aikawa E., Alcaraz M.J., Anderson J.D., Andriantsitohaina R., Antoniou A., Arab T., Archer F., Atkin-Smith G.K., et al. Minimal Information for Studies of Extracellular Vesicles 2018 (MISEV2018): A Position Statement of the International Society for Extracellular Vesicles and Update of the MISEV2014 Guidelines. J. Extracell. Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Liu H., Zhang Q., Wang S., Weng W., Jing Y., Su J. Bacterial Extracellular Vesicles as Bioactive Nanocarriers for Drug Delivery: Advances and Perspectives. Bioact. Mater. 2022;14:169–181. doi: 10.1016/j.bioactmat.2021.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Huang Y., Nieh M.P., Chen W., Lei Y. Outer Membrane Vesicles (OMVs) Enabled Bio-applications: A Critical Review. Biotechnol. Bioeng. 2022;119:34–47. doi: 10.1002/bit.27965. [DOI] [PubMed] [Google Scholar]
- 21.Macion A., Wyszyńska A., Godlewska R. Delivery of Toxins and Effectors by Bacterial Membrane Vesicles. Toxins. 2021;13:845. doi: 10.3390/toxins13120845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang W., Chanda W., Zhong M. The Relationship between Biofilm and Outer Membrane Vesicles: A Novel Therapy Overview. FEMS Microbiol. Lett. 2015;362:fnv117. doi: 10.1093/femsle/fnv117. [DOI] [PubMed] [Google Scholar]
- 23.Reid G., Gadir A.A., Dhir R. Probiotics: Reiterating What They Are and What They Are Not. Front. Microbiol. 2019;10:424. doi: 10.3389/fmicb.2019.00424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Quinto E.J., Jiménez P., Caro I., Tejero J., Mateo J., Girbés T., Quinto E.J., Jiménez P., Caro I., Tejero J., et al. Probiotic Lactic Acid Bacteria: A Review. Food Nutr. Sci. 2014;5:1765–1775. doi: 10.4236/fns.2014.518190. [DOI] [Google Scholar]
- 25.Wang Y., Wu J., Lv M., Shao Z., Hungwe M., Wang J., Bai X., Xie J., Wang Y., Geng W. Metabolism Characteristics of Lactic Acid Bacteria and the Expanding Applications in Food Industry. Front. Bioeng. Biotechnol. 2021;9:612285. doi: 10.3389/fbioe.2021.612285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nataraj B.H., Shivanna S.K., Rao P., Nagpal R., Behare P.V. Evolutionary Concepts in the Functional Biotics Arena: A Mini-review. Food Sci. Biotechnol. 2021;30:487–496. doi: 10.1007/s10068-020-00818-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Derrien M., Vaughan E.E., Plugge C.M., de Vos W.M. Akkermansia muciniphila gen. nov., sp. nov., a Human Intestinal Mucin-Degrading Bacterium. Int. J. Syst. Evol. Microbiol. 2004;54:1469–1476. doi: 10.1099/ijs.0.02873-0. [DOI] [PubMed] [Google Scholar]
- 28.Si J., Kang H., You H.J., Ko G.P. Revisiting the Role of Akkermansia muciniphila as a Therapeutic Bacterium. Gut Microbes. 2022;14:2078619. doi: 10.1080/19490976.2022.2078619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hu R., Lin H., Li J., Zhao Y., Wang M., Sun X., Min Y., Gao Y., Yang M. Probiotic Escherichia coli Nissle 1917-Derived Outer Membrane Vesicles Enhance Immunomodulation and Antimicrobial Activity in RAW264.7 Macrophages. BMC Microbiol. 2020;20:268. doi: 10.1186/s12866-020-01953-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu H., Zhao F., Zhang K., Zhao J., Wang Y. Investigating the Growth Performance, Meat Quality, Immune Function and Proteomic Profiles of Plasmal Exosomes in Lactobacillus plantarum-Treated Broilers with Immunological Stress. Food Funct. 2021;12:11790–11807. doi: 10.1039/D1FO01936H. [DOI] [PubMed] [Google Scholar]
- 31.Müller L., Kuhn T., Koch M., Fuhrmann G. Stimulation of Probiotic Bacteria Induces Release of Membrane Vesicles with Augmented Anti-inflammatory Activity. ACS Appl. Bio Mater. 2021;4:3739–3748. doi: 10.1021/acsabm.0c01136. [DOI] [PubMed] [Google Scholar]
- 32.Li M., Lee K., Hsu M., Nau G., Mylonakis E., Ramratnam B. Lactobacillus-Derived Extracellular Vesicles Enhance Host Immune Responses against Vancomycin-Resistant Enterococci. BMC Microbiol. 2017;17:66. doi: 10.1186/s12866-017-0977-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Harrison N.A., Gardner C.L., da Silva D.R., Gonzalez C.F., Lorca G.L. Identification of Biomarkers for Systemic Distribution of Nanovesicles from Lactobacillus johnsonii N6.2. Front. Immunol. 2021;12:3491. doi: 10.3389/fimmu.2021.723433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hu R., Lin H., Wang M., Zhao Y., Liu H., Min Y., Yang X., Gao Y., Yang M. Lactobacillus reuteri-Derived Extracellular Vesicles Maintain Intestinal Immune Homeostasis against Lipopolysaccharide-Induced Inflammatory Responses in Broilers. J. Anim. Sci. Biotechnol. 2021;12:25. doi: 10.1186/s40104-020-00532-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bäuerl C., Coll-Marqués J.M., Tarazona-González C., Pérez-Martínez G. Lactobacillus casei Extracellular Vesicles Stimulate EGFR Pathway Likely Due to the Presence of Proteins P40 and P75 Bound to Their Surface. Sci. Rep. 2020;10:19237. doi: 10.1038/s41598-020-75930-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rubio A.P.D., Martínez J.H., Casillas D.C.M., Leskow F.C., Piuri M., Pérez O.E. Lactobacillus casei BL23 Produces Microvesicles Carrying Proteins That Have Been Associated with Its Probiotic Effect. Front. Microbiol. 2017;8:1783. doi: 10.3389/fmicb.2017.01783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Vargoorani M.E., Modarressi M.H., Vaziri F., Motevaseli E., Siadat S.D. Stimulatory Effects of Lactobacillus casei Derived Extracellular Vesicles on Toll-Like Receptor 9 Gene Expression and Cytokine Profile in Human Intestinal Epithelial Cells. J. Diabetes Metab. Disord. 2020;19:223–231. doi: 10.1007/s40200-020-00495-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Dean S.N., Leary D.H., Sullivan C.J., Oh E., Walper S.A. Isolation and Characterization of Lactobacillus-Derived Membrane Vesicles. Sci. Rep. 2019;9:877. doi: 10.1038/s41598-018-37120-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grande R., Celia C., Mincione G., Stringaro A., Di Marzio L., Colone M., Di Marcantonio M.C., Savino L., Puca V., Santoliquido R., et al. Detection and Physicochemical Characterization of Membrane Vesicles (MVs) of Lactobacillus reuteri DSM 17938. Front. Microbiol. 2017;8:1040. doi: 10.3389/fmicb.2017.01040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shao H., Im H., Castro C.M., Breakefield X., Weissleder R., Lee H. New Technologies for Analysis of Extracellular Vesicles. Chem. Rev. 2018;118:1917–1950. doi: 10.1021/acs.chemrev.7b00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hilton S.H., White I.M. Advances in the Analysis of Single Extracellular Vesicles: A Critical Review. Sens. Actuators Rep. 2021;3:100052. doi: 10.1016/j.snr.2021.100052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Alpdundar Bulut E., Bayyurt Kocabas B., Yazar V., Aykut G., Guler U., Salih B., Surucu Yilmaz N., Ayanoglu I.C., Polat M.M., Akcali K.C., et al. Human Gut Commensal Membrane Vesicles Modulate Inflammation by Generating M2-like Macrophages and Myeloid-Derived Suppressor Cells. J. Immunol. 2020;205:2707–2718. doi: 10.4049/jimmunol.2000731. [DOI] [PubMed] [Google Scholar]
- 43.Campoccia D., Montanaro L., Arciola C.R. Extracellular DNA (eDNA). A Major Ubiquitous Element of the Bacterial Biofilm Architecture. Int. J. Mol. Sci. 2021;22:9100. doi: 10.3390/ijms22169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Okshevsky M., Meyer R.L. The Role of Extracellular DNA in the Establishment, Maintenance and Perpetuation of Bacterial Biofilms. Crit. Rev. Microbiol. 2015;41:341–352. doi: 10.3109/1040841X.2013.841639. [DOI] [PubMed] [Google Scholar]
- 45.Puca V., Ercolino E., Celia C., Bologna G., Di Marzio L., Mincione G., Marchisio M., Miscia S., Muraro R., Lanuti P., et al. Detection and Quantification of eDNA-Associated Bacterial Membrane Vesicles by Flow Cytometry. Int. J. Mol. Sci. 2019;20:5307. doi: 10.3390/ijms20215307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zingl F.G., Leitner D.R., Thapa H.B., Schild S. Outer Membrane Vesicles as Versatile Tools for Therapeutic Approaches. microLife. 2021;2:uqab006. doi: 10.1093/femsml/uqab006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Caruana J.C., Walper S.A. Bacterial Membrane Vesicles as Mediators of Microbe—Microbe and Microbe—Host Community Interactions. Front. Microbiol. 2020;11:432. doi: 10.3389/fmicb.2020.00432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Díaz-Garrido N., Badia J., Baldomà L. Microbiota-Derived Extracellular Vesicles in Interkingdom Communication in the Gut. J. Extracell. Vesicles. 2021;10:e12161. doi: 10.1002/jev2.12161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kim H., Kim M., Myoung K., Kim W., Ko J., Kim K.P., Cho E.G. Comparative Lipidomic Analysis of Extracellular Vesicles Derived from Lactobacillus plantarum APsulloc 331261 Living in Green Tea Leaves Using Liquid Chromatography-Mass Spectrometry. Int. J. Mol. Sci. 2020;21:8076. doi: 10.3390/ijms21218076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kim W., Lee E.J., Bae I.H., Myoung K., Kim S.T., Park P.J., Lee K.H., Pham A.V.Q., Ko J., Oh S.H., et al. Lactobacillus plantarum-Derived Extracellular Vesicles Induce Anti-Inflammatory M2 Macrophage Polarization In Vitro. J. Extracell. Vesicles. 2020;9:1793514. doi: 10.1080/20013078.2020.1793514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pérez-Cruz C., Cañas M.A., Giménez R., Badia J., Mercade E., Baldomà L., Aguilera L. Membrane Vesicles Released by a Hypervesiculating Escherichia coli Nissle 1917 tolR Mutant Are Highly Heterogeneous and Show Reduced Capacity for Epithelial Cell Interaction and Entry. PLoS ONE. 2016;11:e0169186. doi: 10.1371/journal.pone.0169186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Aguilera L., Toloza L., Giménez R., Odena A., Oliveira E., Aguilar J., Badia J., Baldomà L. Proteomic Analysis of Outer Membrane Vesicles from the Probiotic Strain Escherichia coli Nissle 1917. Proteomics. 2014;14:222–229. doi: 10.1002/pmic.201300328. [DOI] [PubMed] [Google Scholar]
- 53.Ghaderi F., Sotoodehnejadnematalahi F., Hajebrahimi Z., Fateh A., Siadat S.D. Effects of Active, Inactive, and Derivatives of Akkermansia muciniphila on The Expression of The Endocannabinoid System and PPARs Genes. Sci. Rep. 2022;12:10031. doi: 10.1038/s41598-022-13840-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Bajic S.S., Cañas M.A., Tolinacki M., Badia J., Sánchez B., Golic N., Margolles A., Baldomá L., Ruas-Madiedo P. Proteomic Profile of Extracellular Vesicles Released by Lactiplantibacillus plantarum BGAN8 and Their Internalization by Non-Polarized HT29 Cell Line. Sci. Rep. 2020;10:21829. doi: 10.1038/s41598-020-78920-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim M.H., Choi S.J., Choi H., II, Choi J.P., Park H.K., Kim E.K., Kim M.J., Moon B.S., Min T.K., Rho M., et al. Lactobacillus plantarum-Derived Extracellular Vesicles Protect Atopic Dermatitis Induced by Staphylococcus aureus-Derived Extracellular Vesicles. Allergy. Asthma Immunol. Res. 2018;10:516–532. doi: 10.4168/aair.2018.10.5.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Behzadi E., Mahmoodzadeh Hosseini H., Imani Fooladi A.A. The Inhibitory Impacts of Lactobacillus rhamnosus GG-Derived Extracellular Vesicles on the Growth of Hepatic Cancer Cells. Microb. Pathog. 2017;110:1–6. doi: 10.1016/j.micpath.2017.06.016. [DOI] [PubMed] [Google Scholar]
- 57.Keyhani G., Hosseini H.M., Salimi A. Effect of Extracellular Vesicles of Lactobacillus rhamnosus GG on The Expression of CEA Gene and Protein Released by Colorectal Cancer Cells. Iran. J. Microbiol. 2022;14:90–96. doi: 10.18502/ijm.v14i1.8809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Choi J.H., Moon C.M., Shin T.S., Kim E.K., McDowell A., Jo M.K., Joo Y.H., Kim S.E., Jung H.K., Shim K.N., et al. Lactobacillus paracasei-Derived Extracellular Vesicles Attenuate the Intestinal Inflammatory Response by Augmenting the Endoplasmic Reticulum Stress Pathway. Exp. Mol. Med. 2020;52:423–437. doi: 10.1038/s12276-019-0359-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Shiraishi T., Yokota S., Sato Y., Ito T., Fukiya S., Yamamoto S., Sato T., Yokota A. Lipoteichoic Acids are Embedded in Cell Walls during Logarithmic Phase, but Exposed on Membrane Vesicles in Lactobacillus gasseri JCM 1131 T. Benef. Microbes. 2018;9:653–662. doi: 10.3920/BM2017.0124. [DOI] [PubMed] [Google Scholar]
- 60.Seo M.K., Park E.J., Ko S.Y., Choi E.W., Kim S. Therapeutic Effects of Kefir Grain Lactobacillus-Derived Extracellular Vesicles in Mice with 2,4,6-Trinitrobenzene Sulfonic Acid-Induced Inflammatory Bowel Disease. J. Dairy Sci. 2018;101:8662–8671. doi: 10.3168/jds.2018-15014. [DOI] [PubMed] [Google Scholar]
- 61.Yamasaki-Yashiki S., Miyoshi Y., Nakayama T., Kunisawa J., Katakura Y. IgA-Enhancing Effects of Membrane Vesicles Derived from Lactobacillus sakei subsp. sakei NBRC15893. Biosci. Microbiota, Food Health. 2019;38:29. doi: 10.12938/bmfh.18-015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Liu Y., Tempelaars M.H., Boeren S., Alexeeva S., Smid E.J., Abee T. Extracellular Vesicle Formation in Lactococcus lactis is Stimulated by Prophage-Encoded Holin–Lysin System. Microb. Biotechnol. 2022;15:1281–1295. doi: 10.1111/1751-7915.13972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Fábrega M.J., Aguilera L., Giménez R., Varela E., Cañas M.A., Antolín M., Badía J., Baldomà L. Activation of Immune and Defense Responses in the Intestinal Mucosa by Outer Membrane Vesicles of Commensal and Probiotic Escherichia coli Strains. Front. Microbiol. 2016;7:705. doi: 10.3389/fmicb.2016.00705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Nishiyama K., Takaki T., Sugiyama M., Fukuda I., Aiso M., Mukai T., Odamaki T., Xiao J.Z., Osawa R., Okada N. Extracellular Vesicles Produced by Bifidobacterium longum Export Mucin-Binding Proteins. Appl. Environ. Microbiol. 2020;86:e01464-20. doi: 10.1128/AEM.01464-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fábrega M.J., Rodríguez-Nogales A., Garrido-Mesa J., Algieri F., Badía J., Giménez R., Gálvez J., Baldomà L. Intestinal Anti-inflammatory Effects of Outer Membrane Vesicles from Escherichia coli Nissle 1917 in DSS-Experimental Colitis in Mice. Front. Microbiol. 2017;8:1274. doi: 10.3389/fmicb.2017.01274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hirayama S., Nakao R. Glycine Significantly Enhances Bacterial Membrane Vesicle Production: A Powerful Approach for Isolation of LPS-Reduced Membrane Vesicles of Probiotic Escherichia coli. Microb. Biotechnol. 2020;13:1162–1178. doi: 10.1111/1751-7915.13572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Ashrafian F., Shahriary A., Behrouzi A., Moradi H.R., Keshavarz Azizi Raftar S., Lari A., Hadifar S., Yaghoubfar R., Ahmadi Badi S., Khatami S., et al. Akkermansia muciniphila-Derived Extracellular Vesicles as a Mucosal Delivery Vector for Amelioration of Obesity in Mice. Front. Microbiol. 2019;10:2155. doi: 10.3389/fmicb.2019.02155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Ashrafian F., Behrouzi A., Shahriary A., Badi S.A., Davari M., Khatami S., Jamnani F.R., Fateh A., Vaziri F., Siadat S.D. Comparative Study of Effect of Akkermansia muciniphila and Its Extracellular Vesicles on Toll-Like Receptors and Tight Junction. Gastroenterol. Hepatol. Bed Bench. 2019;12:168. [PMC free article] [PubMed] [Google Scholar]
- 69.Ashrafian F., Keshavarz Azizi Raftar S., Lari A., Shahryari A., Abdollahiyan S., Moradi H.R., Masoumi M., Davari M., khatami S., Omrani M.D., et al. Extracellular Vesicles and Pasteurized Cells Derived from Akkermansia muciniphila Protect against High-Fat Induced Obesity in Mice. Microb. Cell Fact. 2021;20:219. doi: 10.1186/s12934-021-01709-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Keshavarz Azizi Raftar S., Ashrafian F., Yadegar A., Lari A., Moradi H.R., Shahriary A., Azimirad M., Alavifard H., Mohsenifar Z., Davari M., et al. The Protective Effects of Live and Pasteurized Akkermansia muciniphila and Its Extracellular Vesicles against HFD/CCl4-Induced Liver Injury. Microbiol. Spectr. 2021;9:e0048421. doi: 10.1128/Spectrum.00484-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Yaghoubfar R., Behrouzi A., Zare Banadkoki E., Ashrafian F., Lari A., Vaziri F., Nojoumi S.A., Fateh A., Khatami S., Siadat S.D. Effect of Akkermansia muciniphila, Faecalibacterium prausnitzii, and Their Extracellular Vesicles on the Serotonin System in Intestinal Epithelial Cells. Probiotics Antimicrob. Proteins. 2021;13:1546–1556. doi: 10.1007/s12602-021-09786-4. [DOI] [PubMed] [Google Scholar]
- 72.Hong J., Dauros-Singorenko P., Whitcombe A., Payne L., Blenkiron C., Phillips A., Swift S. Analysis of the Escherichia coli Extracellular Vesicle Proteome Identifies Markers of Purity and Culture Conditions. J. Extracell. Vesicles. 2019;8:1632099. doi: 10.1080/20013078.2019.1632099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Lee B.H., Wu S.C., Shen T.L., Hsu Y.Y., Chen C.H., Hsu W.H. The Applications of Lactobacillus plantarum-Derived Extracellular Vesicles as a Novel Natural Antibacterial Agent for Improving Quality and Safety in Tuna Fish. Food Chem. 2021;340:128104. doi: 10.1016/j.foodchem.2020.128104. [DOI] [PubMed] [Google Scholar]
- 74.Ñahui Palomino R.A., Vanpouille C., Laghi L., Parolin C., Melikov K., Backlund P., Vitali B., Margolis L. Extracellular Vesicles from Symbiotic Vaginal Lactobacilli Inhibit HIV-1 Infection of Human Tissues. Nat. Commun. 2019;10:5656. doi: 10.1038/s41467-019-13468-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.West C.L., Stanisz A.M., Mao Y.K., Champagne-Jorgensen K., Bienenstock J., Kunze W.A. Microvesicles from Lactobacillus reuteri (DSM-17938) Completely Reproduce Modulation of Gut Motility by Bacteria in Mice. PLoS ONE. 2020;15:e0225481. doi: 10.1371/journal.pone.0225481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Chen C.Y., Rao S.S., Yue T., Tan Y.J., Yin H., Chen L.J., Luo M.J., Wang Z., Wang Y.Y., Hong C.G., et al. Glucocorticoid-Induced Loss of Beneficial Gut Bacterial Extracellular Vesicles Is Associated with The Pathogenesis of Osteonecrosis. Sci. Adv. 2022;8:eabg8335. doi: 10.1126/sciadv.abg8335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Yoon Y.C., Ahn B.H., Min J.W., Lee K.R., Park S.H., Kang H.C. Stimulatory Effects of Extracellular Vesicles Derived from Leuconostoc holzapfelii That Exists in Human Scalp on Hair Growth in Human Follicle Dermal Papilla Cells. Curr. Issues Mol. Biol. 2022;44:845–866. doi: 10.3390/cimb44020058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.de Rodovalho V.R., da Luz B.S.R., Rabah H., do Carmo F.L.R., Folador E.L., Nicolas A., Jardin J., Briard-Bion V., Blottière H., Lapaque N., et al. Extracellular Vesicles Produced by the Probiotic Propionibacterium freudenreichii CIRM-BIA 129 Mitigate Inflammation by Modulating the NF-κB Pathway. Front. Microbiol. 2020;11:1544. doi: 10.3389/fmicb.2020.01544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.de Rezende Rodovalho V., da Luz B.S.R., Nicolas A., do Carmo F.L.R., Jardin J., Briard-Bion V., Jan G., Loir Y.L., de Carvalho Azevedo V.A., Guédon E. Environmental Conditions Modulate the Protein Content and Immunomodulatory Activity of Extracellular Vesicles Produced by the Probiotic Propionibacterium freudenreichii. Appl. Environ. Microbiol. 2020;87:e02263-20. doi: 10.1128/AEM.02263-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rubio A.P.D., Martínez J., Palavecino M., Fuentes F., López C.M.S., Marcilla A., Pérez O.E., Piuri M. Transcytosis of Bacillus subtilis Extracellular Vesicles through an In Vitro Intestinal Epithelial Cell Model. Sci. Rep. 2020;10:3120. doi: 10.1038/s41598-020-60077-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ma L., Shen Q., Lyu W., Lv L., Wang W., Yu M., Yang H., Tao S., Xiao Y. Clostridium butyricum and Its Derived Extracellular Vesicles Modulate Gut Homeostasis and Ameliorate Acute Experimental Colitis. Microbiol. Spectr. 2022;10:e0136822. doi: 10.1128/spectrum.01368-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Liang L., Yang C., Liu L., Mai G., Li H., Wu L., Jin M., Chen Y. Commensal Bacteria-Derived Extracellular Vesicles Suppress Ulcerative Colitis through Regulating The Macrophages Polarization and Remodeling The Gut Microbiota. Microb. Cell Fact. 2022;21:88. doi: 10.1186/s12934-022-01812-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kuhn T., Koch M., Fuhrmann G., Kuhn T., Fuhrmann G., Koch M. Probiomimetics—Novel Lactobacillus-Mimicking Microparticles Show Anti-Inflammatory and Barrier-Protecting Effects in Gastrointestinal Models. Small. 2020;16:2003158. doi: 10.1002/smll.202003158. [DOI] [PubMed] [Google Scholar]
- 84.Morishita M., Sagayama R., Yamawaki Y., Yamaguchi M., Katsumi H., Yamamoto A. Activation of Host Immune Cells by Probiotic-Derived Extracellular Vesicles via TLR2-Mediated Signaling Pathways. Biol. Pharm. Bull. 2022;45:354–359. doi: 10.1248/bpb.b21-00924. [DOI] [PubMed] [Google Scholar]
- 85.Morishita M., Horita M., Higuchi A., Marui M., Katsumi H., Yamamoto A. Characterizing Different Probiotic-Derived Extracellular Vesicles as a Novel Adjuvant for Immunotherapy. Mol. Pharm. 2021;18:1080–1092. doi: 10.1021/acs.molpharmaceut.0c01011. [DOI] [PubMed] [Google Scholar]
- 86.Yang Z., Gao Z., Yang Z., Zhang Y., Chen H., Yang X., Fang X., Zhu Y., Zhang J., Ouyang F., et al. Lactobacillus plantarum-Derived Extracellular Vesicles Protect against Ischemic Brain Injury via The MicroRNA-101a-3p/c-Fos/TGF-β Axis. Pharmacol. Res. 2022;182:106332. doi: 10.1016/j.phrs.2022.106332. [DOI] [PubMed] [Google Scholar]
- 87.Jo C.S., Myung C.H., Yoon Y.C., Ahn B.H., Min J.W., Seo W.S., Lee D.H., Kang H.C., Heo Y.H., Choi H., et al. The Effect of Lactobacillus plantarum Extracellular Vesicles from Korean Women in Their 20s on Skin Aging. Curr. Issues Mol. Biol. 2022;44:526–540. doi: 10.3390/cimb44020036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Champagne-Jorgensen K., Mian M.F., McVey Neufeld K.A., Stanisz A.M., Bienenstock J. Membrane Vesicles of Lacticaseibacillus rhamnosus JB-1 Contain Immunomodulatory Lipoteichoic Acid and Are Endocytosed by Intestinal Epithelial Cells. Sci. Rep. 2021;11:13756. doi: 10.1038/s41598-021-93311-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Champagne-Jorgensen K., Jose T.A., Stanisz A.M., Mian M.F., Hynes A.P., Bienenstock J. Bacterial Membrane Vesicles and Phages in Blood After Consumption of Lacticaseibacillus rhamnosus JB-1. Gut Microbes. 2021;13:e1993583. doi: 10.1080/19490976.2021.1993583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Shi Y., Meng L., Zhang C., Zhang F., Fang Y. Extracellular Vesicles of Lacticaseibacillus paracasei PC-H1 Induce Colorectal Cancer Cells Apoptosis via PDK1/AKT/Bcl-2 Signaling Pathway. Microbiol. Res. 2021;255:126921. doi: 10.1016/j.micres.2021.126921. [DOI] [PubMed] [Google Scholar]
- 91.Chelakkot C., Choi Y., Kim D.K., Park H.T., Ghim J., Kwon Y., Jeon J., Kim M.S., Jee Y.K., Gho Y.S., et al. Akkermansia muciniphila-Derived Extracellular Vesicles Influence Gut Permeability through the Regulation of Tight Junctions. Exp. Mol. Med. 2018;50:e450. doi: 10.1038/emm.2017.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Luo Z.W., Xia K., Liu Y.W., Liu J.H., Rao S.S., Hu X.K., Chen C.Y., Xu R., Wang Z.X., Xie H. Extracellular Vesicles from Akkermansia muciniphila Elicit Antitumor Immunity Against Prostate Cancer via Modulation of CD8 + T Cells and Macrophages. Int. J. Nanomed. 2021;16:2949–2963. doi: 10.2147/IJN.S304515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hao H., Zhang X., Tong L., Liu Q., Liang X., Bu Y., Gong P., Liu T., Zhang L., Xia Y., et al. Effect of Extracellular Vesicles Derived From Lactobacillus plantarum Q7 on Gut Microbiota and Ulcerative Colitis in Mice. Front. Immunol. 2021;12:5167. doi: 10.3389/fimmu.2021.777147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Tong L., Zhang X., Hao H., Liu Q., Zhou Z., Liang X., Liu T., Gong P., Zhang L., Zhai Z., et al. Lactobacillus rhamnosus GG Derived Extracellular Vesicles Modulate Gut Microbiota and Attenuate Inflammatory in DSS-Induced Colitis Mice. Nutrients. 2021;13:3319. doi: 10.3390/nu13103319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Lee D.H., Park H.K., Lee H.R., Sohn H., Sim S., Park H.J., Shin Y.S., Kim Y.K., Choi Y., Park H.S. Immunoregulatory Effects of Lactococcus lactis-Derived Extracellular Vesicles in Allergic Asthma. Clin. Transl. Allergy. 2022;12:e12138. doi: 10.1002/clt2.12138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Nagakubo T., Nomura N., Toyofuku M. Cracking Open Bacterial Membrane Vesicles. Front. Microbiol. 2020;10:3026. doi: 10.3389/fmicb.2019.03026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lee J., Kim O.Y., Gho Y.S. Proteomic Profiling of Gram-negative Bacterial Outer Membrane Vesicles: Current Perspectives. PROTEOMICS—Clin. Appl. 2016;10:897–909. doi: 10.1002/prca.201600032. [DOI] [PubMed] [Google Scholar]
- 98.Pedersen S.K., Harry J.L., Sebastian L., Baker J., Traini M.D., McCarthy J.T., Manoharan A., Wilkins M.R., Gooley A.A., Righetti P.G., et al. Unseen Proteome: Mining Below the Tip of the Iceberg To Find Low Abundance and Membrane Proteins. J. Proteome Res. 2003;2:303–311. doi: 10.1021/pr025588i. [DOI] [PubMed] [Google Scholar]
- 99.Ahmed N., Rice G.E. Strategies for Revealing Lower Abundance Proteins in Two-Dimensional Protein Maps. J. Chromatogr. B. Analyt. Technol. Biomed. Life Sci. 2005;815:39–50. doi: 10.1016/j.jchromb.2004.10.070. [DOI] [PubMed] [Google Scholar]
- 100.Klimentová J., Stulík J. Methods of Isolation and Purification of Outer Membrane Vesicles from Gram-Negative Bacteria. Microbiol. Res. 2015;170:1–9. doi: 10.1016/j.micres.2014.09.006. [DOI] [PubMed] [Google Scholar]
- 101.Graf A.C., Leonard A., Schäuble M., Rieckmann L.M., Hoyer J., Maass S., Lalk M., Becher D., Pané-Farré J., Riedel K. Virulence Factors Produced by Staphylococcus aureus Biofilms Have a Moonlighting Function Contributing to Biofilm Integrity. Mol. Cell. Proteomics. 2019;18:1053. doi: 10.1074/mcp.RA118.001120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Kesavan D., Vasudevan A., Wu L., Chen J., Su Z., Wang S., Xu H. Integrative Analysis of Outer Membrane Vesicles Proteomics and Whole-Cell Transcriptome Analysis of Eravacycline Induced Acinetobacter baumannii Strains. BMC Microbiol. 2020;20:31. doi: 10.1186/s12866-020-1722-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Sung K., Chon J., Kweon O., Nho S., Kim S., Park M., Paredes A., Lim J.H., Khan S.A., Phillips K.S., et al. Dynamic Adaptive Response of Pseudomonas aeruginosa to Clindamycin/Rifampicin-Impregnated Catheters. Antibiotics. 2021;10:752. doi: 10.3390/antibiotics10070752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Rolhion N., Barnich N., Bringer M.A., Glasser A.L., Ranc J., Hébuterne X., Hofman P., Darfeuille-Michaud A. Abnormally Expressed ER Stress Response Chaperone Gp96 in CD Favours Adherent-Invasive Escherichia coli Invasion. Gut. 2010;59:1355–1362. doi: 10.1136/gut.2010.207456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Taheri N., Mahmud A.K.M.F., Sandblad L., Fällman M., Wai S.N., Fahlgren A. Campylobacter jejuni Bile Exposure Influences Outer Membrane Vesicles Protein Content and Bacterial Interaction with Epithelial Cells. Sci. Rep. 2018;8:16996. doi: 10.1038/s41598-018-35409-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kim S.I., Kim S., Kim E., Hwang S.Y., Yoon H. Secretion of Salmonella Pathogenicity Island 1-Encoded Type III Secretion System Effectors by Outer Membrane Vesicles in Salmonella enterica Serovar Typhimurium. Front. Microbiol. 2018;9:2810. doi: 10.3389/fmicb.2018.02810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Avila-Calderón E.D., del Ruiz-Palma M.S., Aguilera-Arreola M.G., Velázquez-Guadarrama N., Ruiz E.A., Gomez-Lunar Z., Witonsky S., Contreras-Rodríguez A. Outer Membrane Vesicles of Gram-Negative Bacteria: An Outlook on Biogenesis. Front. Microbiol. 2021;12:345. doi: 10.3389/fmicb.2021.557902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Clarke A.J. The “Hole” Story of Predatory Outer-Membrane Vesicles. Can. J. Microbiol. 2018;64:589–599. doi: 10.1139/cjm-2017-0466. [DOI] [PubMed] [Google Scholar]
- 109.Peng X.-P., Nie C., Guan W.-Y., Qiao L.-D., Lu L., Cao S.-J. Regulation of Probiotics on Metabolism of Dietary Protein in Intestine. Curr. Protein Pept. Sci. 2019;21:766–771. doi: 10.2174/1389203720666191111112941. [DOI] [PubMed] [Google Scholar]
- 110.Díez-Sainz E., Lorente-Cebrián S., Aranaz P., Riezu-Boj J.I., Martínez J.A., Milagro F.I. Potential Mechanisms Linking Food-Derived MicroRNAs, Gut Microbiota and Intestinal Barrier Functions in the Context of Nutrition and Human Health. Front. Nutr. 2021;8:85. doi: 10.3389/fnut.2021.586564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Villard A., Boursier J., Andriantsitohaina R. Microbiota-Derived Extracellular Vesicles and Metabolic Syndrome. Acta Physiol. 2021;231:e13600. doi: 10.1111/apha.13600. [DOI] [PubMed] [Google Scholar]
- 112.McMillan H.M., Kuehn M.J. The Extracellular Vesicle Generation Paradox: A Bacterial Point of View. EMBO J. 2021;40:e108174. doi: 10.15252/embj.2021108174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bitto N.J., Zavan L., Johnston E.L., Stinear T.P., Hill A.F., Kaparakis-Liaskos M. Considerations for the Analysis of Bacterial Membrane Vesicles: Methods of Vesicle Production and Quantification Can Influence Biological and Experimental Outcomes. Microbiol. Spectr. 2021;9:e01273-21. doi: 10.1128/Spectrum.01273-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Papadimitriou K., Zoumpopoulou G., Foligné B., Alexandraki V., Kazou M., Pot B., Tsakalidou E. Discovering Probiotic Microorganisms: In Vitro, In Vivo, Genetic And Omics Approaches. Front. Microbiol. 2015;6:58. doi: 10.3389/fmicb.2015.00058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sagheddu V., Guidesi E., Galletti S., Elli M. Selection and Characterization Criteria of Probiotics Intended for Human Use from the Past to the Future. Food Sci. Nutr. Stud. 2019;3:p73. doi: 10.22158/fsns.v3n2p73. [DOI] [Google Scholar]
- 116.Suzuki T. Regulation of Intestinal Epithelial Permeability By Tight Junctions. Cell. Mol. Life Sci. 2013;70:631–659. doi: 10.1007/s00018-012-1070-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Turner J.R. Intestinal Mucosal Barrier Function in Health and Disease. Nat. Rev. Immunol. 2009;9:799–809. doi: 10.1038/nri2653. [DOI] [PubMed] [Google Scholar]
- 118.Kang E.A., Choi H.I., Hong S.W., Kang S., Jegal H.Y., Choi E.W., Park B.S., Kim J.S. Extracellular Vesicles Derived from Kefir Grain Lactobacillus Ameliorate Intestinal Inflammation via Regulation of Proinflammatory Pathway and Tight Junction Integrity. Biomedicines. 2020;8:522. doi: 10.3390/biomedicines8110522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Xu X., Lin S., Yang Y., Gong X., Tong J., Li K., Li Y. Histological and Ultrastructural Changes of the Colon in Dextran Sodium Sulfate-Induced Mouse Colitis. Exp. Ther. Med. 2020;20:1987. doi: 10.3892/etm.2020.8946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Hu Y., Ye Z., Wu M., She Y., Li L., Xu Y., Qin K., Hu Z., Yang M., Lu F., et al. The Communication Between Intestinal Microbiota and Ulcerative Colitis: An Exploration of Pathogenesis, Animal Models, and Potential Therapeutic Strategies. Front. Med. 2021;8:766126. doi: 10.3389/fmed.2021.766126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Petersson J., Schreiber O., Hansson G.C., Gendler S.J., Velcich A., Lundberg J.O., Roos S., Holm L., Phillipson M. Importance and Regulation of the Colonic Mucus Barrier In A Mouse Model of Colitis. Am. J. Physiol. 2011;300:G327. doi: 10.1152/ajpgi.00422.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tomasi M., Caproni E., Benedet M., Zanella I., Giorgetta S., Dalsass M., König E., Gagliardi A., Fantappiè L., Berti A., et al. Outer Membrane Vesicles From The Gut Microbiome Contribute to Tumor Immunity by Eliciting Cross-Reactive T Cells. Front. Oncol. 2022;12:912639. doi: 10.3389/fonc.2022.912639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Hiam-Galvez K.J., Allen B.M., Spitzer M.H. Systemic Immunity in Cancer. Nat. Rev. Cancer. 2021;21:345–359. doi: 10.1038/s41568-021-00347-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Fessler J., Matson V., Gajewski T.F. Exploring the Emerging Role of the Microbiome in Cancer Immunotherapy. J. Immunother. Cancer. 2019;7:108. doi: 10.1186/s40425-019-0574-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Miyoshi Y., Saika A., Nagatake T., Matsunaga A., Kunisawa J., Katakura Y., Yamasaki-Yashiki S. Mechanisms Underlying Enhanced IgA Production in Peyer’s Patch Cells by Membrane Vesicles Derived from Lactobacillus sakei. Biosci. Biotechnol. Biochem. 2021;85:1536–1545. doi: 10.1093/bbb/zbab065. [DOI] [PubMed] [Google Scholar]
- 126.Geuking M.B., Köller Y., Rupp S., McCoy K.D. The Interplay Between the Gut Microbiota and the Immune System. Gut Microbes. 2014;5:411. doi: 10.4161/gmic.29330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kamada N., Seo S.U., Chen G.Y., Núñez G. Role of The Gut Microbiota in Immunity and Inflammatory Disease. Nat. Rev. Immunol. 2013;13:321–335. doi: 10.1038/nri3430. [DOI] [PubMed] [Google Scholar]
- 128.Wang X., Zhang P., Zhang X. Probiotics Regulate Gut Microbiota: An Effective Method to Improve Immunity. Molecules. 2021;26:6076. doi: 10.3390/molecules26196076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Choi J., Kim Y.K., Han P.L. Extracellular Vesicles Derived from Lactobacillus plantarum Increase BDNF Expression in Cultured Hippocampal Neurons and Produce Antidepressant-Like Effects in Mice. Exp. Neurobiol. 2019;28:158–171. doi: 10.5607/en.2019.28.2.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Choi J., Kwon H., Kim Y.K., Han P.L. Extracellular Vesicles from Gram-positive and Gram-negative Probiotics Remediate Stress-Induced Depressive Behavior in Mice. Mol. Neurobiol. 2022;59:2715–2728. doi: 10.1007/s12035-021-02655-9. [DOI] [PubMed] [Google Scholar]
- 131.Alvarez C.S., Giménez R., Cañas M.A., Vera R., Díaz-Garrido N., Badia J., Baldomà L. Extracellular Vesicles and Soluble Factors Secreted by Escherichia coli Nissle 1917 and ECOR63 Protect against Enteropathogenic E. coli-Induced Intestinal Epithelial Barrier Dysfunction. BMC Microbiol. 2019;19:166. doi: 10.1186/s12866-019-1534-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Cañas M.A., Fábrega M.J., Giménez R., Badia J., Baldomà L. Outer Membrane Vesicles From Probiotic and Commensal Escherichia coli Activate NOD1-Mediated Immune Responses in Intestinal Epithelial Cells. Front. Microbiol. 2018;9:498. doi: 10.3389/fmicb.2018.00498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Díaz-Garrido N., Bonnin S., Riera M., Gíménez R., Badia J., Baldomà L. Transcriptomic microRNA Profiling of Dendritic Cells in Response to Gut Microbiota-Secreted Vesicles. Cells. 2020;9:1534. doi: 10.3390/cells9061534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Raftar S.K.A., Ashrafian F., Abdollahiyan S., Yadegar A., Moradi H.R., Masoumi M., Vaziri F., Moshiri A., Siadat S.D., Zali M.R. The Anti-Inflammatory Effects of Akkermansia muciniphila and Its Derivates in HFD/CCL4-Induced Murine Model of Liver Injury. Sci. Rep. 2022;12:2453. doi: 10.1038/s41598-022-06414-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mata Forsberg M., Björkander S., Pang Y., Lundqvist L., Ndi M., Ott M., Escribá I.B., Jaeger M.C., Roos S., Sverremark-Ekström E. Extracellular Membrane Vesicles from Lactobacilli Dampen IFN-γ Responses in a Monocyte-Dependent Manner. Sci. Rep. 2019;9:17109. doi: 10.1038/s41598-019-53576-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Teixeira L.D., Harrison N.A., da Silva D.R., Mathews C.E., Gonzalez C.F., Lorca G.L. Nanovesicles From Lactobacillus johnsonii N6.2 Reduce Apoptosis in Human Beta Cells by Promoting AHR Translocation and IL10 Secretion. Front. Immunol. 2022;13:899413. doi: 10.3389/fimmu.2022.899413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Kim J.H., Jeun E.J., Hong C.P., Kim S.H., Jang M.S., Lee E.J., Moon S.J., Yun C.H., Im S.H., Jeong S.G., et al. Extracellular Vesicle-Derived Protein from Bifidobacterium longum Alleviates Food Allergy through Mast Cell Suppression. J. Allergy Clin. Immunol. 2016;137:507–516.e8. doi: 10.1016/j.jaci.2015.08.016. [DOI] [PubMed] [Google Scholar]
- 138.López P., González-Rodríguez I., Sánchez B., Gueimonde M., Margolles A., Suárez A. Treg-Inducing Membrane Vesicles from Bifidobacterium bifidum LMG13195 as Potential Adjuvants in Immunotherapy. Vaccine. 2012;30:825–829. doi: 10.1016/j.vaccine.2011.11.115. [DOI] [PubMed] [Google Scholar]
- 139.Kim S., Han S.-Y., Lee J., Kim N.-R., Lee B.R., Kim H., Kwon M., Ahn K., Noh Y., Kim S.J., et al. Bifidobacterium longum and Galactooligosaccharide Improve Skin Barrier Dysfunction and Atopic Dermatitis-Like Skin. Allergy. Asthma Immunol. Res. 2022;14:564. doi: 10.4168/aair.2022.14.5.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.An J.J., Ha E.M. Extracellular Vesicles Derived from Lactobacillus plantarum Restore Chemosensitivity through the PDK2-Mediated Glucose Metabolic Pathway in 5-FU-Resistant Colorectal Cancer Cells. J. Microbiol. 2022;60:735–745. doi: 10.1007/s12275-022-2201-1. [DOI] [PubMed] [Google Scholar]
- 141.Pang Y., Ermann Lundberg L., Mata Forsberg M., Ahl D., Bysell H., Pallin A., Sverremark-Ekström E., Karlsson R., Jonsson H., Roos S. Extracellular Membrane Vesicles from Limosilactobacillus reuteri Strengthen the Intestinal Epithelial Integrity, Modulate Cytokine Responses and Antagonize Activation of TRPV1. Front. Microbiol. 2022;13:1032202. doi: 10.3389/fmicb.2022.1032202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kurata A., Kiyohara S., Imai T., Yamasaki-Yashiki S., Zaima N., Moriyama T., Kishimoto N., Uegaki K. Characterization of Extracellular Vesicles from Lactiplantibacillus plantarum. Sci. Rep. 2022;12:13330. doi: 10.1038/s41598-022-17629-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nakao R., Kobayashi H., Iwabuchi Y., Kawahara K., Hirayama S., Ramstedt M., Sasaki Y., Kataoka M., Akeda Y., Ohnishi M. A Highly Immunogenic Vaccine Platform against Encapsulated Pathogens Using Chimeric Probiotic Escherichia coli Membrane Vesicles. NPJ Vaccines. 2022;7:153. doi: 10.1038/s41541-022-00572-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.da Silva Barreira D., Lapaquette P., Novion Ducassou J., Couté Y., Guzzo J., Rieu A. Spontaneous Prophage Induction Contributes to the Production of Membrane Vesicles by the Gram-positive Bacterium Lacticaseibacillus casei BL23. MBio. 2022;13:e0237522. doi: 10.1128/mbio.02375-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Díez-Sainz E., Milagro F.I., Riezu-Boj J.I., Lorente-Cebrián S. Effects of Gut Microbiota-Derived Extracellular Vesicles on Obesity and Diabetes and Their Potential Modulation through Diet. J. Physiol. Biochem. 2022;78:485–499. doi: 10.1007/s13105-021-00837-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Jahromi L.P., Fuhrmann G. Bacterial Extracellular Vesicles: Understanding Biology Promotes Applications as Nanopharmaceuticals. Adv. Drug Deliv. Rev. 2021;173:125–140. doi: 10.1016/j.addr.2021.03.012. [DOI] [PubMed] [Google Scholar]
- 147.Domínguez Rubio A.P., D’Antoni C.L., Piuri M., Pérez O.E. Probiotics, Their Extracellular Vesicles and Infectious Diseases. Front. Microbiol. 2022;13:864720. doi: 10.3389/fmicb.2022.864720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.González-Lozano E., García-García J., Gálvez J., Hidalgo-García L., Rodríguez-Nogales A., Rodríguez-Cabezas M.E., Sánchez M. Novel Horizons in Postbiotics: Lactobacillaceae Extracellular Vesicles and Their Applications in Health and Disease. Nutrients. 2022;14:5296. doi: 10.3390/nu14245296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yuan Y., Sun J., You T., Shen W., Xu W., Dong Q., Cui M. Extracellular Vesicle-Based Therapeutics in Neurological Disorders. Pharmaceutics. 2022;14:2652. doi: 10.3390/pharmaceutics14122652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Sun K., Zheng X., Jin H., Yu F., Zhao W. Exosomes as CNS Drug Delivery Tools and Their Applications. Pharmaceutics. 2022;14:2252. doi: 10.3390/pharmaceutics14102252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Abid R., Waseem H., Ali J., Ghazanfar S., Ali G.M., Elasbali A.M., Alharethi S.H. Probiotic Yeast Saccharomyces: Back to Nature to Improve Human Health. J. Fungi. 2022;8:444. doi: 10.3390/jof8050444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Saarela M.H. Safety Aspects of Next Generation Probiotics. Curr. Opin. Food Sci. 2019;30:8–13. doi: 10.1016/j.cofs.2018.09.001. [DOI] [Google Scholar]
- 153.Cunningham M., Azcarate-Peril M.A., Barnard A., Benoit V., Grimaldi R., Guyonnet D., Holscher H.D., Hunter K., Manurung S., Obis D., et al. Shaping the Future of Probiotics and Prebiotics. Trends Microbiol. 2021;29:667–685. doi: 10.1016/j.tim.2021.01.003. [DOI] [PubMed] [Google Scholar]
- 154.Cieślik M., Nazimek K., Bryniarski K. Extracellular Vesicles-Oral Therapeutics of the Future. Int. J. Mol. Sci. 2022;23:7554. doi: 10.3390/ijms23147554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Chang X., Wang S.L., Zhao S.B., Shi Y.H., Pan P., Gu L., Yao J., Li Z.S., Bai Y. Extracellular Vesicles with Possible Roles in Gut Intestinal Tract Homeostasis and IBD. Mediators Inflamm. 2020;2020:1945832. doi: 10.1155/2020/1945832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Hendrix A., De Wever O. Systemically Circulating Bacterial Extracellular Vesicles: Origin, Fate, and Function. Trends Microbiol. 2022;30:213–216. doi: 10.1016/j.tim.2021.12.012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.