Abstract
Borrelia miyamotoi is an emerging tick-borne pathogen in the Northern Hemisphere and is the causative agent of Borrelia miyamotoi disease (BMD). Borrelia miyamotoi is vectored by the same hard-bodied ticks as Lyme disease Borrelia, yet phylogenetically groups with relapsing fever Borrelia, and thus, has been uniquely labeled a hard tick-borne relapsing fever Borrelia. Burgeoning research has uncovered new aspects of B. miyamotoi in human patients, nature, and the lab. Of particular interest are novel findings on disease pathology, prevalence, diagnostic methods, ecological maintenance, transmission, and genetic characteristics. Herein, we review recent literature on B. miyamotoi, discuss how findings adapt to current Borrelia doctrines, and briefly consider what remains unknown about B. miyamotoi.
Keywords: Borrelia miyamotoi disease (BMD), Ixodes, Lyme disease, relapsing fever, reservoir species, tick-borne disease, vector
1. Introduction
The genus Borrelia contains Gram-negative, obligate extracellular parasitic bacteria with a unique spiral morphology. Known as spirochetes, Borrelia owe their shape and spiraling motility to their periplasmic flagella [1]. Borrelia can be divided into two well-defined groups: the Lyme disease (LD) group, vectored by hard ticks, and the relapsing fever (RF) group, vectored by soft ticks and lice. Classically, these groups were highly distinct with separate vector genera, unique transmission dynamics, and differing disease outcomes [2,3]. Yet, the discovery of Borrelia miyamotoi, phylogenetically grouped with RF spirochetes, but vectored by the same hard ticks as LD spirochetes, disturbed this archetype and fueled research [4].
In this review, we discuss novel B. miyamotoi research in recent publications and examine how new data fit into existing knowledge.
2. Background
Ticks are capable of vectoring numerous pathogens, with the most prevalent vector-borne illness in the Northern Hemisphere being Lyme disease. Also known as Lyme borreliosis, LD is the result of infection with species in the Borrelia burgdorferi sensu lato (Bbsl) complex, which are vectored by hard-bodied Ixodes ticks. The primary species responsible for human LD are B. burgdorferi, B. afzelii, and B. garinii [2]. Lyme disease is commonly characterized by the formation of an erythema migrans skin lesion, often at the bite location, in conjunction with flu-like symptoms of headache, fatigue, muscle aches, and fever along with joint pain and swelling. Bbsl spirochetes can disseminate beyond the bite site to additional tissues. This colonization can result in serious clinical manifestations of the joints, heart, and central nervous system (CNS), known as Lyme arthritis, carditis, and neuroborreliosis, respectively [5,6,7,8]. The treatment for LD is an antibiotic regimen; however, symptoms can persist, resulting in post-treatment Lyme disease syndrome [5,7].
Another borreliosis found in the Northern Hemisphere, Africa, and Central America is relapsing fever. Relapsing fever is the result of infection by several species of Borrelia vectored primarily by soft-bodied argasid ticks, the exception being B. recurrentis vectored by lice. Clinical presentations of RF typically consist of a high fever for a few days, followed by an approximately week-long period of well-being, followed by another relapse. Relapses can repeat multiple times without antibiotic treatment. Additional RF symptoms are headache, body aches, and abdominal pain. Historically, outbreaks are caused by louse-borne B. recurrentis and occur during wartime, periods of poor hygienic conditions, and refugee crowding [3].
Borrelia miyamotoi, a new tick-borne Borrelia species, was discovered in Japan in 1995 when spirochetes were isolated from Ixodes persulcatus midguts and Apodemus argenteus blood [4]. Despite being vectored by hard ticks, B. miyamotoi was found to be genetically distinct from the Bbsl complex and was more closely related to RF species. At the time, there were no cases of RF in the region; this led researchers to speculate that this new Borrelia species was a tick endosymbiont maintained by an enzootic transmission cycle [4].
The pathogenicity of B. miyamotoi was realized in Russia in 2011 when 51 patients with suspected tick bites presented with a nonspecific febrile illness. The patients were found to be infected with B. miyamotoi by B. miyamotoi-specific polymerase chain reaction (PCR) of the blood or anti-borreliae immunoglobulins in the serum. The patients’ symptoms were somewhat dissimilar to RF with fever, headache, chills, fatigue, and myalgia. One patient had a relapse, which may have been prevented in other patients due to antibiotic treatment [9]. The RF-like illness caused by B. miyamotoi, now known as B. miyamotoi disease (BMD), has since been widely diagnosed and is considered an emerging public health threat [10].
3. Disease
Human infection with B. miyamotoi can result in BMD. Symptoms vary depending on the constitution of the patient. Immunocompetent, and otherwise healthy, patients present with milder, flu-like symptoms: fever, fatigue, sleepiness, chills, muscle and joint stiffness, aches and pains, and nausea [11,12,13]. While uncommon, relapses of febrile episodes can occur [11]. It is possible that many patients with BMD do not seek medical attention due to short-lived and mild symptoms. Similar to the mechanism of relapses in other RF species, B. miyamotoi exhibits antigenic variation, allowing spirochetes to evade adaptive immune responses [14,15,16,17]. In the blood, B. miyamotoi has numerous mechanisms to evade complement-mediated killing, allowing for the rapid growth and multiplication of spirochetes that result in the symptoms of BMD [11,14,15,16].
The adaptive immune response is important for controlling B. miyamotoi infection [15]. Studies of RF species B. hermsii show that infection clearance is antibody mediated [16]. Borrelia miyamotoi activates dendritic cells which phagocytose the bacteria and produce interleukins IL-8, IL-6, and IL-12, as well as tumor necrosis factor alpha (TNF-α) [18]. These cytokines further stimulate and signal immune activation for inflammation, recruitment of additional immune cells, infection clearance, host-defense, and T-cell differentiation [18]. Immunocompetent mouse models of BMD only develop transient infections, whereas immunodeficient mice mouse models develop persistent infections [19]. This suggests that healthy patients infected with B. miyamotoi may be able to clear the infection without medical intervention.
Borrelia miyamotoi infection in immunocompromised patients can be much more severe [20]. In combination with generalized flu-like symptoms, immunocompromised patients often exhibit reduced cognition, disturbed gait, memory deficits, confusion, and other neurological deficiencies resultant of meningoencephalitis [21]. Additionally, hearing loss, weight loss, uveitis, iritis, neck stiffness, and photophobia have been reported [20]. The designation “immunocompromised” is broad; however, hospitalization reports of severe BDM are frequently seen in patients prescribed B-cell depletion therapies, such as rituximab, other cancer immunotherapeutics, or immunosuppressants for rheumatoid arthritis [20,21,22,23,24,25]. While these medications are often seen in conjunction with meningoencephalitis and other serious symptoms, there is no finite list of medications, treatments, or immunodeficiencies that can give rise to severe BMD.
In patients lacking a complete immune response, the austerity of symptoms is possibly due to unchecked growth of B. miyamotoi in the blood. Similar to B. hermsii, it can be speculated that the humoral immune response is necessary to control B. miyamotoi infection [16]. This suggests that defects in antibody production allow spirochetes to disseminate from the blood and colonize farther tissues, resulting in severe BMD symptoms. It can be inferred that BMD-associated meningoencephalitis is the result of B. miyamotoi colonizing the CNS, as microscopic analysis of cerebrospinal fluid (CSF) collected from BMD patients with meningoencephalitis shows visible spirochetes [21,22,23]. However, the dynamics of host–pathogen interactions leading to inflammation are unknown.
There are no official clinical guidelines for the treatment of diagnosed or suspected BMD. Thereby, treatment falls to the guidelines for LD and RF: an antibiotic regimen, commonly doxycycline and ceftriaxone. Ampicillin, azithromycin, and vancomycin, or a combination thereof, have also been used to treat BMD. Antibiotic treatment engenders a full recovery with rare cases of persistent fatigue [20,26]. A systematic review and meta-analysis by Hoornstra et al. affirmed that doxycycline is the preferred treatment for adults presenting with no neurological complications, and ceftriaxone is the preferred treatment for adults with BMD-associated meningoencephalitis [26]. Research has confirmed in vitro that both clinical and tick isolates of B. miyamotoi are susceptible to doxycycline, ceftriaxone, and azithromycin, but show resistance to amoxicillin [27]. Albeit rarely, Jarisch-Herxheimer reactions have occurred with antibiotic treatment of BMD [22].
4. Diagnosis
Diagnosis of BMD and B. miyamotoi infection is possible using microscopy, PCR, and serodiagnosis, either as single tests or in combination, with the latter two methods being the most common [11]. However, there is no official standardized diagnostic technique [28].
4.1. Microscopy
Spirochetes can be visualized in blood and CSF using dark-field microscopy, or with Giemsa staining or acridine orange staining [21,22,23]. Microscopy can confirm a Borrelia species infection; however, it cannot be used to distinguish between species and has low sensitivity [29]. In order to see spirochetes in a blood smear or CSF sample, the density needs to be greater than 104 cells/mL [11,26]. During the acute phase of BMD, usually within a few days of symptom onset, spirochete density in the blood ranges from 101–105 cells/mL [30]. The presence of B. miyamotoi in the CSF is poorly understood. Thus, there is no guarantee that spirochete density will be high enough for visualization.
Borrelia miyamotoi can be cultured from a patient’s whole blood, serum, or CSF into specialized media, and organisms can then be visualized. However, as spirochetes may take longer than two weeks to grow to a visible density and culturing Borrelia species is notoriously tricky, it is neither timely nor practical to use culturing for diagnosis [31].
The visualization of spirochetes in the blood or CSF confirms Borrelia species infection, but a lack of visible spirochetes does not rule out infection. Microscopy can support a BMD diagnosis but should be used in combination with other methods.
4.2. PCR
Diagnosis of BMD often uses PCR assays performed on whole blood, serum, or CSF and typically targets glpQ and/or flaB [11,22]. It is best to use PCR to diagnose BMD when spirochetes are most likely to be detectable during symptom presentation and prior to antibiotic treatment [20,30]. Human borrelioses all have detectable periods of spirochetemia, albeit brief for Bbsl, and overlapping geographical regions [32,33,34]. Thus, a PCR assay must be sensitive enough to detect B. miyamotoi and specific enough to distinguish between B. miyamotoi, Bbsl, and RF species.
A clinically relevant assay was developed to detect and differentiate between B. miyamotoi, louse-borne RF, and soft tick-borne RF species. The semi-multiplex real-time PCR assay is highly sensitive with a lower detection limit of ten genome copies and uses a nested approach to first broadly amplify and detect RF species and B. miyamotoi. A series of probes then differentiate between groups [35]. If implemented in a clinical setting, this assay would readily test for RF and BMD in acute phase patients.
4.3. Serodiagnosis
Borrelia miyamotoi infection results in the production of immunoglobulins to spirochete proteins, and serodiagnosis utilizes the presence of these reactive antibodies to diagnose a past or present infection. The quantity and variety of antibodies produced fluctuates depending on the route of inoculation, whether via tick bite or needle stick, if antigenic variation occurs, and the duration and dissemination of the infection [14,15,16,36,37]. Immunoglobulins IgM and IgG are produced in response to B. miyamotoi infection, with IgM peaking around one week post infection and IgG peaking around three weeks post infection. While IgG is highly specific for the antigen against which it is produced, IgM is not and is more prone to higher background and cross-reactivity [38,39].
Timing is important when utilizing serology for a diagnosis. At the early onset of BMD symptoms, IgM may be present at low levels, while IgG will not be present [38,39]. Absent or undetectable antibodies could result in a false negative test. Microscopy paired with PCR is more suited for acute diagnosis [20,21,22,23,30]. Patients presenting with chronic BMD symptoms multiple weeks after disease onset, or for retrospective studies, are better suited to diagnosis via serology. The indefatigable nature of anti-B. miyamotoi antibodies is not fully understood; however, the persistence of Borrelia-specific antibodies is correlated to the duration and dissemination of the infection [36]. As antibody production is integral for serodiagnosis, the ability to diagnose immunocompromised patients via serology may be limited due to complications surrounding reduced humoral responses to B. miyamotoi.
Glycerophosphodiester phosphodiesterase (GlpQ) is widely used for serodiagnosis, but its reliability as a serological indicator of BMD has recently been called into question [13,15,40,41]. For PCR, glpQ is reliable as it directly detects the bacteria. However, as serodiagnosis indirectly detects infection by measuring reactive antibodies to a particular antigen or antigens, cross-reactivity can potentially occur between similar proteins from different pathogens [42].
Anti-B. miyamotoi antibodies can be detected using conventional assay systems such as an ELISA or a Western blot [43,44]. Ideally, the best candidate antigens for serodiagnosis should be highly conserved and detectable in both early and late infection with low cross-reactivity to other tick-borne and common bacterial pathogens. Reactivity to GlpQ is often used in combination with flagellin and p66 proteins; however, neither of these are species specific [20]. Despite widespread use for serodiagnosis, GlpQ has been shown to be neither highly sensitive nor specific [41]. Fortunately, researchers have strove to uncover methods to improve GlpQ accuracy and identify additional reliable markers.
Assaying for GlpQ reactivity in conjunction with variable major proteins (Vmps), particularly variable large protein (Vlp)-15/16, improves the sensitivity and specificity of B. miyamotoi detection [37]. This coalition leans heavily on cooperation as Vmps alone are prone to cross-react with orthologous Bbsl protein Vmp-like sequence expressed (VlsE) [45,46]. This dual-marker technique using both GlpQ and Vlp-15/16 was found to be an excellent diagnostic option as IgM against GlpQ and Vmps peaks between 11 and 20 days and IgG peaks between 21 and 50 days. This combination increased specificity to 100% and sensitivity to 79% on days 11–20, and increased specificity to 98.3% and sensitivity to 86.7% on days 21–50 [37]. Thus, testing for seroreactivity to both Vmps and GlpQ can improve BMD diagnostic accuracy.
Whereas GlpQ and Vlp-15/16 test best in combination, Borrelia miyamotoi membrane antigen (BmaA) is a solitary candidate for serodiagnosis. This protein is an externally located cellular putative lipoprotein of B. miyamotoi, and anti-BmaA antibodies can be detected in the serum of confirmed BMD patients between 38 and 100 days after disease onset [47]. Results after 100 days were inconclusive and additional testing is necessary. BmaA has little to no observable reactivity in LD patients or with RF species B. turicate, indicating that BmaA may be species specific [47]. Beyond BmaA, more than 400 immunoreactive peptides, most of which mapped to Vmps, were recently identified as potential targets for future serology studies [48]. Hence, while GlpQ may not be ideal for serodiagnosis, there are multiple other options.
5. Human Cases and Infection Prevalence
The National Institute of Allergy and Infectious Diseases classifies B. miyamotoi as an emerging infectious pathogen and BMD as an emerging infectious disease [10]. Even as B. miyamotoi becomes more prominent, the ambiguity and often self-limiting nature of BMD symptoms suggest that many cases are likely subclinical. Patients who do seek medical treatment may be misdiagnosed with other tick-borne diseases (TBD), particularly LD. This mistaken serodiagnosis of BMD as LD can be due to the C6 peptide of the Bbsl VlsE protein. Reactivity to the C6 peptide is widely used for LD serodiagnosis, and infection with B. miyamotoi leads to cross-reactive antibodies, which may result in a false-positive LD test [49]. As antibiotic treatment for LD is successful in treating BMD, an accurate diagnosis of BMD may never be reached. All of this, combined with low awareness among physicians and the general public, indicates that the known prevalence of BMD may not be wholly accurate. The most updated and thorough conglomeration of reported diagnoses of BMD and retrospective studies of banked human sera reactivity is by Hoornstra et al. in their systematic review and meta-analysis [26].
Including the first cases of BMD diagnosed in Russia, there have been 561 total diagnoses: 367 in Russia, 101 in the United States, 57 in France, 30 elsewhere in Asia, and 6 elsewhere in Europe [26,50,51,52,53,54]. The higher number of cases in Russia may be due to increased awareness of B. miyamotoi prompting more testing, increased transmission abilities of I. persulcatus ticks, or greater virulence of Russian strains, leading to more hospitalizations. As there are no official diagnostic guidelines for BMD, criteria for BMD diagnosis may vary between institutions.
The reactivity of banked sera to B. miyamotoi recombinant proteins, primarily GlpQ and FlaB, can be measured using ELISA and Western blots, either singly or in conjunction as a two-tier test. Across these retrospective studies, 45,608 samples were tested from North America, Europe, and Asia and separated into varying categories depending on risk, confirmed infection, and cross-reactivity. Seroprevalence was 4.6% in the high risk for B. miyamotoi infection group, 4.8% in the suspected LD group, 11.9% in the suspected TBD group, and 1.3% in healthy controls [26]. Prevalence across all groups could indicate that B. miyamotoi infection is far more common than previously thought, detection methods are prone to false positives and cross-reactivity, or coinfections with other tick-borne pathogens (TBP) produce convoluted results [11].
6. Ticks
The geographic distribution of B. miyamotoi in ticks is far greater than that of BMD in humans. This may be an insight on transmission capabilities, or it may be that more surveys have been conducted on ticks. Borrelia miyamotoi is vectored by I. scapularis in the northeastern and northcentral United States and Canada, I. pacificus in the western United States, I. ricinus in Europe, I. persulcatus in Europe and Asia, and I. ovatus, I. nipponensis and I. pavoloskyi in Asia [26,50,55]. Borrelia miyamotoi has been detected in all known vectors of Bbsl [43]. There are reports of B. miyamotoi in other tick species, including Dermacentor reticulatus in Russia and Haemaphysalis species in China and Slovakia [56,57,58,59]. These species are not competent vectors for Bbsl; however, it is unclear if they are capable of successfully vectoring B. miyamotoi [60,61]. Vector competence is the ability of a tick to serve as a disease vector. Vector competence is influenced by environmental factors, such as vector density, longevity, and fitness, and genetic factors, such as host preference and duration of attachment [62]. Table 1 has an updated list of countries with reports of ticks infected with B. miyamotoi. The systematic review and meta-analysis by Hoornstra et al. analyzed B. miyamotoi prevalence in ticks, which is outlined in Table 2. The overall prevalence of B. miyamotoi in questing Ixodes ticks is 1.1%, but varies by region and dominant tick species in that region [26].
A survey of Ixodes ticks in the United States from 2013 to 2019 screened nine Ixodes species, but only detected B. miyamotoi in I. pacificus (14/1497, 0.94%) and I. scapularis (594/34,621, 1.72%). The average infection prevalence in nymphs and adults was similar; there was no mention of larval infection. Borrelia miyamotoi was found in ticks in 19 states, with infection prevalence being 0.5–3.2%. Ticks tested in 20 additional states were all negative [63]. Borrelia miyamotoi has been detected in all Canadian provinces, excluding Newfoundland, in I. scapularis ticks [34]. There are certain states and provinces in which B. miyamotoi infection has not been reported, which may be due to a paucity of competent vector species in that region, spirochetes present but below detection limits, or simply that B. miyamotoi is not in that region.
Natural coinfections in Ixodes ticks occur, as these arthropods vector a number of TBP beyond Borrelia species [33]. A survey of Ixodes ticks in the United States found over half of all B. miyamotoi-infected I. scapularis ticks had concurrent infections (351/594, 59.09%). Half of all B. miyamotoi-infected ticks had a dual infection (293/594, 49.33%) with either B. burgdorferi (220/594, 37.04%), Anaplasma phagocytophilum (43/594, 7.24%), or Babesia microti (30/594, 5.05%). Additionally, 52 ticks (8.75%) had triple infections and six ticks (1.01%) had quadruple infections. No concurrent B. miyamotoi infections were seen with B. mayonii or Ehrlichia muris-like agent [33]. This could be due to coincidence, the result of geographic constraints, and/or competitive habitation in the vector ticks. Regardless, these data indicate that concurrent infections in ticks are common, and physicians should be aware that tick bite patients may be exposed to multiple TBPs.
In the tick, B. miyamotoi disseminates throughout the body into the acini, salivary gland ducts, basal lamina of the midgut, epithelium of the Malpighian tubes, female ovarian tissues, male testes, CNS, and near the mouthparts [64]. During tick colonization, B. burgdorferi species have outer surface membrane proteins that adhere to receptors in the midgut lumen and protect spirochetes from the ingested host blood containing complement and antibodies [62,65]. It is unknown if B. miyamotoi uses similar mechanisms for tick colonization as Bbsl.
Additionally, ticks infected with B. miyamotoi are found to have significantly higher numbers of Borrelia cells than those infected with Bbsl [66,67]. This is a proposed evolutionary trait to compensate for the lower prevalence of B. miyamotoi in tick populations (~1%), compared with Bbsl (~12%) [26,67,68]. Furthermore, B. miyamotoi reach higher levels in the blood than Bbsl species, and thus, ticks ingest more bacteria per blood meal [69]. Other Borrelia species, including Bbsl and B. hermsii, have been shown to replicate within ticks; however, it is not known if B. miyamotoi replicates within ticks [70,71].
When transmitted from tick to host, Bbsl uses outer surface protein C (OspC) to bind tick salivary protein Salp15, which provides protection from the mammalian immune response [72]. Borrelia hermsii variable tick proteins (Vtp) are homologous to OspC and convey a similar function [73]. The variable small proteins (Vsp) in B. miyamotoi are homologous to OspC and Vtp and likely aid in transmission from tick to vertebrate host [14]. There is still much to learn about the intricacies of B. miyamotoi tick colonization and transmission.
Table 1.
Countries with B. miyamotoi-infected ticks.
| Country | Tick |
|---|---|
| Austria | I. ricinus [74] |
| Belarus | I. ricinus [75] |
| Belgium | I. ricinus [76,77] |
| Canada | I. scapularis [78] |
| China | I. persulcatus [79], H. longicornis [57], H. concinna [58] |
| Czechia | I. ricinus [80] |
| Denmark | I. ricinus [81] |
| Estonia | I. ricinus, I. persulcatus [82] |
| Finland | I. ricinus [83,84,85] |
| France | I. ricinus [81,86,87] |
| Germany | I. ricinus [76,88] |
| Ireland | I. ricinus [89] |
| Italy | I. ricinus [76] |
| Japan | I. persulcatus [4], I. ovatus [90] |
| Moldova | I. ricinus [91] |
| Mongolia | I. persulcatus [90,92] |
| Netherlands | I. ricinus [76,81,93,94] |
| Norway | I. ricinus [95] |
| Poland | I. ricinus [96,97] |
| Portugal | I. ricinus [98] |
| Russia | I. persulcatus [99] |
| Serbia | I. ricinus [100] |
| Slovakia | I. ricinus [59,101], H. inermis [59] |
| South Korea | I. nipponensis [55] |
| Spain | I. ricinus [102] |
| Sweden | I. ricinus [67,76] |
| Switzerland | I. ricinus [103] |
| Turkey | I. ricinus [104] |
| Ukraine | I. ricinus [105] |
| United Kingdom | I. ricinus [76] |
| United States | I. pacificus [106], I. scapularis [107] |
Table 2.
Prevalence of B. miyamotoi in ticks a.
| Questing Tick Prevalence | B. miyamotoi Prevalence | |
|---|---|---|
| I. scapularis | 28.0% | 1.1% |
| I. pacificus | 14.8% | 0.7% |
| I. ricinus | 52.2% | 1.0% |
| I. persulcatus | 5.0% | 2.8% |
The combined surveys listed in Hoornstra et al., 2022 covered a total of 165,637 questing ticks. Tick species prevalence is calculated as a proportion of total questing ticks surveyed. B. miyamotoi prevalence was calculated as proportion of the tick species. a adapted from Hoornstra et al., 2022 [26] under creative commons license CC BY 4.0.
7. Animal Infections
The majority of animals infected with B. miyamotoi are small mammals, particularly rodents, with a minority of infections being in larger mammals and birds [108]. An important distinction needs to be made between animal infections, where the animal tissue tested positive for B. miyamotoi (Table 3), and animal-associated tick infections, where the animal tissue was not tested, but the tick(s) attached to the animal tested positive for B. miyamotoi (Table 4). Borrelia miyamotoi has also been detected in both birds and bird-associated ticks; however, there has been minimal follow-up research to interpret the role of birds in B. miyamotoi perpetuation and maintenance (Table 3 and Table 4) [94,109,110].
The multiple reservoir species identified for B. miyamotoi, all of which are rodents, are Peromyscus, Apodemus, and Myodes species [69,108,111,112]. Reservoir rodent species have been identified carrying B. miyamotoi in both rural and urban environments, posing an increased public health risk [83,84,85]. A notable potential new reservoir species in North America is the jumping mouse, Napaeozapus insignis. In a survey of B. miyamotoi in Atlantic-Canadian wildlife, N. insignis had a much higher infection prevalence than other known reservoir species tested in the same survey. Unfortunately, the role of the jumping mouse in B. miyamotoi transmission is unknown, as specimens were collected via public participation and transmission studies cannot be conducted post-mortem [113].
It has been speculated that white-tailed deer may be a reservoir host for B. miyamotoi, as a study found that deer-associated ticks had higher infection prevalence than questing ticks [114]. Unlike B. burgdorferi that is lysed by deer blood complement upon tick ingestion, it appears that B. miyamotoi remains viable in the tick upon ingestion of deer blood. A study found that engorged female I. scapularis ticks collected from white-tailed deer produced infected offspring [114,115,116]. Reservoir competence has not been characterized in white-tailed deer.
Reservoir species must be able to be infected with B. miyamotoi and remain infected long enough to allow naïve vector ticks to acquire infection via feeding. An indirect method to determine a reservoir species tests both wildlife and ticks in a survey plot for B. miyamotoi infection. A direct method live-traps animals and tests both the animal and any ticks on them for B. miyamotoi. Xenodiagnostic ticks are then fed on the animal(s) and tested for B. miyamotoi to determine transmission capabilities.
It is unclear if animals, particularly reservoir species, remain persistently infected with B. miyamotoi. Bbsl species are known to cause persistent infections in rodents [117]. A survey of B. miyamotoi in rodents and their ticks found that there was no correlation between rodent age, month of infection, and infection presence. Infection rates remained stable across the survey. In the same study, B. burgdorferi infection rates increased throughout the survey [118]. Furthermore, while Bbsl species have a strong positive association with rodent population density, maintenance of B. miyamotoi appears to be independent of rodent population density [119]. This suggests that B. miyamotoi and Bbsl use different maintenance strategies [69].
Borrelia miyamotoi has been found in a variety of mammals, birds, and associated ticks. Discerning how B. miyamotoi is maintained in reservoir species, other hosts, and vector ticks is critical for surveillance, risk assessment, and mitigation.
Table 3.
Borrelia miyamotoi animal infections.
| % Positive | Countries | |
|---|---|---|
| Small Mammals | ||
| Striped field mouse (Apodemus agrarius) | 3.13% (1/32); 13.2% (7/53); 7.0% (11/157) | Austria [120], Croatia [121], Poland [112] |
| Small Japanese field mouse (Apodemus argenteus) | 0.7% (1/137) | Japan [118] |
| Yellow-necked mouse (Apodemus flavicollis) * | 0.7% (1/131); 0.9% (1/102) a & 1.5% (1/67) b; 3.6% (3/84); 2.0% (1/49); 0.4% (1/251) c & 2.2% (1/46) d | Croatia [121], Hungary [111], Poland [112], Romania [122], Slovenia [123] |
| Large Japanese field mouse (Apodemus speciosus) | 2.2% (10/446) | Japan [118] |
| Wood mouse (Apodemus sylvaticus) | 14.3% (3/21) | Netherlands [94] |
| European hedgehog (Erinaceus europaeus) | 5.0% (3/60) | Czechia [124] |
| Common vole (Microtus arvalis) | 12.5% (1/8) | Netherlands [94], Slovakia [59] |
| Meadow vole (Microtus pennsylvanicus) |
0.7% (1/146) | Canada [113] |
| Bank vole (Myodes glareolus) * |
5.5% (4/72); 8.8% (3/34); 3.1% (1/32) | France [86], Netherlands [94], Romania [122], Switzerland [103] |
| Grey red-backed vole (Myodes rufocanus) | 1.0% (2/195) | Japan [118] |
| Jumping mouse (Napaeozapus insignis) |
14.3% (3/21) | Canada [113] |
| Dusky-footed woodrat (Neotoma fuscipes) | 16.7% (1/6) | United States [125] |
| Brush mouse (Peromyscus boylii) |
2.8% (2/71) | United States [125] |
| California mouse (Peromyscus californicus) | 16.7% (4/24) | United States [125] |
| White-footed mouse (Peromyscus leucopus) * | 6.4% (36/556) d & 2.3% (2/86) e; 0.5% (3/625) | United States [69], Canada [126] |
| Deer mouse (Peromyscus maniculatus) |
2.9% (1/34) | Canada [113] |
| Eastern grey squirrel (Sciurus carolinensis) | 25.0% (1/4) | Canada [113] |
| Red squirrel (Sciurus vulgaris) |
13.6% (3/22) | Czechia [124], Hungary [127] |
| Common Shrew (Sorex araneus) |
16.7% (1/6) | Croatia [121] |
| Muller’s giant Sunda rat (Sundamys muelleri) | 33.3% (1/3) | Malaysia [128] |
|
Eastern chipmunk (Tamias striatus) |
15.4% (2/13) | Canada [126] |
| Large mammals | ||
| Père David Deer (Elaphurus davidianus) | 2.3% (1/43) | China [57] |
| Birds | ||
| European greenfinch (Carduelis chloris) | 25% (1/4) | Netherlands [94] |
| Wild turkey (Meleagris gallopavo) | 56.0% (35/60) | United States [109] |
| Great tits (Parus major) | 50% (1/2) | Netherlands [94] |
| Ostrich (Struthio camelus) | 16.7% (1/6) | Czechia [110] |
a Positive skin samples, b Positive spleen samples, c Retrospective study, d Prospective study, e Positive blood samples. * Xenodiagnostic ticks were used to confirm species as a reservoir host.
Table 4.
Animal-associated B. miyamotoi tick infections.
| Tick | Countries | |
|---|---|---|
| Mid-Sized Mammals | ||
| Beech marten (Martes foina) | I. ricinus | Belgium, Netherlands [129] |
| European pine marten (Martes martes) | I. ricinus | Belgium, Netherlands [129] |
| European polecat (Mustela putorius) | I. ricinus | Belgium, Netherlands [129] |
| Large Mammals | ||
| Cattle (Bos primigenius tarus) | I. ricinus | Germany [130] |
| Dog (Canis lupus familiaris) | I. ovatus, I. hexagonus, I. ricinus, I. persulcatus, Dermacentor reticulatus | Germany [131,132], Japan [133], Latvia [134], Russia [56] |
| Goat (Capra aegagrus hircus) | I. ricinus | Germany [130] |
| Roe deer (Capreolus capreolus) | I. ricinus | Germany [132], Poland [135], Spain [136] |
| Red deer (Cervus elaphus) | I. ricinus | Poland [137] |
| Raccoon dog (Nyctereutes procyonoides) | I. ricinus | Denmark [138] |
| White-tailed deer (Odocoileus virginianus) |
I. scapularis | United States [114,115,139] |
| Wild boar (Sus scrofa) | I. ricinus | Poland [136] |
| Birds | ||
| Northern cardinal (Cardinalis cardinalis) |
I. dentatus | United States [140] |
| Veery (Catharus fuscescens) | I. scapularis | Canada [126] |
| Hermit Thrush (Catharus guttatus) |
I. scapularis, I. dentatus | Canada [126], United States [140] |
| European robin (Erithacus rubecula) |
I. ricinus | Netherlands [93], Sweden [67] |
| Song Sparrow (Melospiza melodia) |
I. scapularis | Canada [126] |
| Common redstart (Phoenicurus phoeincurus) |
I. ricinus | Sweden [67] |
| Common chiffchaff (Phylloscopus collybita) |
I. ricinus | Netherlands [93] |
| Eurasian wren (Troglodytes troglodytes) |
I. ricinus | Sweden [67] |
| Common blackbird (Turdus merula) |
I. ricinus | Moldova [91], Netherlands [93], Poland [135] |
| American robin (Turdus migratorius) |
I. dentatus | United States [140] |
| Song thrush (Turdus philomelos) | I. ricinus | Netherlands [93] |
8. Transmission
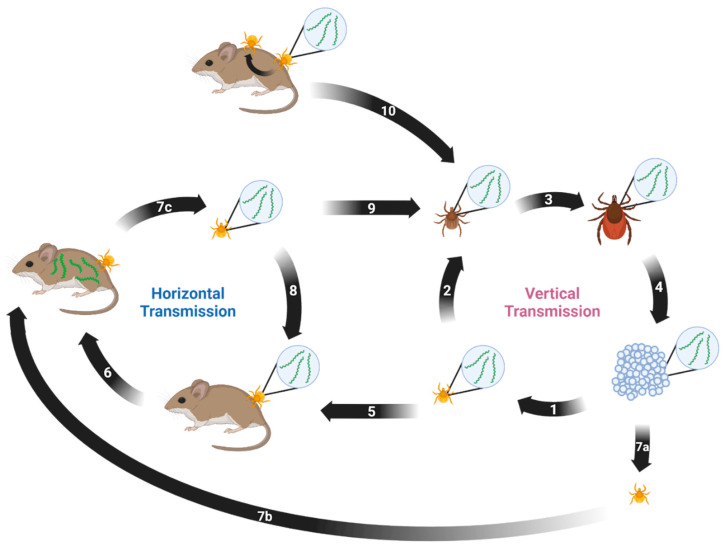
Borrelia miyamotoi can be transmitted both horizontally and vertically [141]. Horizontal transmission occurs between a vector and a host or vice versa. Vertical transmission occurs transovarially between a female tick and her progeny. Both modes are suspected to play key roles in persistence of B. miyamotoi in the environment. A proposed enzootic transmission cycle depicting both transmission methods and their potential interactions is shown in Figure 1.
Figure 1.
Proposed enzootic transmission cycle for Borrelia miyamotoi. (1) Larvae hatch from eggs laid by a B. miyamotoi-infected female Ixodes tick. Transovarial transmission occurs, resulting in partial larval infection. (2) The infected larva takes first blood meal and molts, remaining infected. (3) The infected nymph takes second blood meal and molts, remaining infected. (4) The infected adult female takes third blood meal, mates, and lays eggs. (5) The infected tick feeds on naïve host. (6) The host acquires B. miyamotoi from the infected tick. (7) (a) The naïve larva (b) feeds on the infected host and (c) acquires B. miyamotoi via blood meal. (8) The infected tick feeds on the naïve host. (9) The infected larva takes blood meal and molts into a nymph, remaining infected. (10) The naïve tick acquires B. miyamotoi via co-feeding near an infected tick. Created with BioRender.com (accessed on 9 January 2023).
Horizontal transmission of B. miyamotoi from tick to host appears to have varied success using laboratory models. Mice challenged with B. miyamotoi by horizontally infected ticks resulted in low infection prevalence in both mice and xenodiagnostic larvae [64]. Yet, B. miyamotoi can be transmitted from infected ticks to naïve mice during the first 24 h of feeding. Within this window of time, only 10% of the mice had detectable B. miyamotoi DNA in their blood. If ticks were allowed to feed to repletion, 73% of the mice had detectable B. miyamotoi DNA, though no xenodiagnostic ticks were fed on these mice [142]. This likely indicates that B. miyamotoi in the tick salivary glands enter the host first. Then, as the tick feeds, spirochetes in the midgut migrate to the salivary glands to contribute to the infection [3,64].
Tick acquisition of B. miyamotoi via feeding on an infected immunocompetent host is variable and potentially limited by periods of spirochetemia. Only 1–12% of post-molt ticks were positive for B. miyamotoi when fed to repletion on infected reservoir species P. leucopus and infected CD-1 mice [64]. This may be due to B. miyamotoi colonizing the skin at a low rate combined with brief periods of spirochetemia, or that infection was not sustained through the tick molting process [69]. All naïve ticks fed on infected severe combined immunodeficient (SCID) mice with high, persistent spirochetemia became infected, though infection was frequently lost during the molt [19]. This suggests that spirochetemia may play a role in horizontal transmission from host to tick and that vertical transmission may be more important for B. miyamotoi maintenance as horizontal transmission is often inefficient.
Borrelia miyamotoi can be vertically transmitted from the female tick to her offspring. The rate of transovarial transmission appears to be positively correlated with the maternal bacterial load. The higher the infection density in the female, the more likely her egg clutches will be infected with higher bacterial loads. Seven out of ten I. scapularis females that tested positive for B. miyamotoi produced infected larvae, with the filial infection rate of infected clutches being 3.3–100%, median 71%. The three females that did not produce infected clutches had the lowest bacterial loads [64]. Another group saw 10/11 infected I. scapularis females produce infected clutches, with infection in progeny being 36–100%. This study also saw similar oviposition rates in B. miyamotoi-infected I. scapularis females compared with uninfected females, indicating that B. miyamotoi infection does not appear to impact tick fecundity [115]. These studies support that vertical transmission is relied on more heavily for B. miyamotoi maintenance than horizontal transmission.
Though there is minimal evidence, additional suggested transmission routes of B. miyamotoi are vertical transmission in mammals and co-feeding in ticks. A study found a pregnant jumping mouse to have a fully disseminated infection, resulting in B. miyamotoi detectable in placental and fetal tissues [113]. It is unclear if the pregnancy had reached full term if the offspring would have been viable or remained infected. Furthermore, it is unclear if the pregnancy led to the disseminated infection or if dissemination was due to unknown factors. Additionally, it has been suggested when both infected and naïve ticks feed simultaneously on the same naïve host, B. miyamotoi may be transmitted to the naïve tick, known as co-feeding [64]. Further studies need to be conducted to better understand these potential transmission routes.
As ticks can be infected with B. miyamotoi prior to their first blood meal, it is important to note that larval, nymphal, and adult ticks can transmit B. miyamotoi to humans. A study by Breuner et al. showed that over half (57%) of CD-1 mice exposed to a single transovarially infected larva had evidence of B. miyamotoi infection [143].
9. Phylogenetics
The earliest known B. miyamotoi isolates were collected in Japan in 1995: isolate FR64 from A. argenteus field mice and isolates HT24, HT31, Hk004, and NB103/1 from unfed I. persulcatus ticks [4]. In the decades since, numerous additional B. miyamotoi isolates have been recovered and described from around the world. Table 5 shows reference genomes and chromosomes for 36 different strains. These strains have been utilized in multifarious research objectives including phylogenetics studies.
Table 5.
Borrelia miyamotoi strains with reference genome or chromosome sequences.
| Strain | Origin | Source | Accession |
|---|---|---|---|
| LB-2001 | North America | I. scapularis | CP006647 |
| CT13-2396 | North America | I. scapularis | NZ_CP017126 |
| C14D4 | North America | Human, blood | NZ_CP010308 |
| CA17-2241 | North America | I. pacificus | NZ_CP021872 |
| FR64b | Japan | A. argenteus, blood | NZ_CP004217 |
| HT31 | Japan | I. persulcatus | NZ_AP024371 |
| HT24 | Japan | I. persulcatus | NZ_AP024372 |
| Hk004 | Japan | I. persulcatus | NZ_AP024373 |
| NB103/1 | Japan | A. argenteus, blood | NZ_AP024374 |
| MYK1 | Japan | I. pavlovskyi | NZ_AP024375 |
| MYK2 | Japan | I. persulcatus | NZ_AP024391 |
| MYK3 | Japan | I. persulcatus | NZ_AP024392 |
| MYK4 | Japan | I. persulcatus | NZ_AP024393 |
| MYK5 | Japan | I. persulcatus | NZ_AP024394 |
| Y14T1 | Japan | I. persulcatus | NZ_AP024398 |
| Y15T1 | Japan | I. persulcatus | NZ_AP024399 |
| Y14T18 | Japan | I. ovatus | NZ_AP024400 |
| Yekat-1 | Russia | Human, plasma | NZ_CP024333 |
| Yekat-6 | Russia | Human, plasma | NZ_CP024316 |
| Yekat-17 | Russia | Human, plasma | NZ_CP037215 |
| Yekat-18 | Russia | Human, plasma | CP037471 |
| Yekat-19 | Russia | Human, plasma | NZ_CP037058 |
| Yekat-21 | Russia | Human, plasma | NZ_CP036914 |
| Yekat-31 | Russia | Human, plasma | NZ_CP036726 |
| Yekat-76 | Russia | Human, plasma | NZ_CP036557 |
| Izh-4 | Russia | Human, plasma | NZ_CP024390 |
| Izh-5 | Russia | Human, plasma | NZ_CP024205 |
| Izh-14 | Russia | Human, plasma | CP024371 |
| Izh-16 | Russia | Human, plasma | CP024351 |
| NL-IR-1 | Europe | I. ricinus, eggs | NZ_CP044783 |
| NL-IR-2 | Europe | I. ricinus, eggs | NZ_CP044625 |
| CZ-F1E | Europe | I. ricinus, eggs | NZ_CP046389 |
| CZ-F190E | Europe | I. ricinus, eggs | NZ_CP046388 |
| M12C4 | Mongolia | I. persulcatus | NZ_AP024395 |
| M15A8 | Mongolia | I. persulcatus | NZ_AP024396 |
| M20E6 | Mongolia | I. persulcatus | NZ_AP024397 |
Accession numbers taken from GenBank.
Borrelia miyamotoi isolates collected from Asia, Europe, and North America have high identity; however, isolates are genetically distinct between continents and can be divided into three geography-based lineages [144]. Isolates within these large geographic lineages often are vectored by more than one tick species and cluster based on vector species. Sequence variation also exists between isolates vectored by the same tick species [144]. The genetic diversity of B. miyamotoi isolates can likely be attributed to genetic drift, natural selection, and vector tick speciation [145]. This variability has an unknown impact on population phenotypes [144]. Inferences of isolate infectivity can be made based on B. miyamotoi infection data from the different regions, but this has not been a focus of research yet.
10. Laboratory Studies on B. miyamotoi
10.1. Culturing
Borrelia species are known for their fastidious nature regarding in vitro cultivation, and B. miyamotoi is no exception. The ability to successfully cultivate an organism is at the basis of research and of utmost importance. Indeed, the development of Barbour-Stoener-Kelly (BSK) media was groundbreaking for Bbsl research [146]. Unfortunately, B. miyamotoi does not grow consistently well in BSK media. Several modified versions have been developed and tested including BSKII, BSK-H, and Modified-Kelly-Pettenkofer (MKP) media, all of which have different composing concentrations, but use rabbit serum for the serum source [147,148,149].
Modified-Kelly-Pettenkofer medium was tested with strains HT31 and M1029. Optimal growth for both strains (maximum growth densities (MGD) 2.5 × 107 and 1 × 107 cells/mL, respectively) occurred in media containing 50% pooled human serum [150]. These strains also grew successfully in a modified version of MKP containing 10% fetal calf serum (MKP-F) [151].
Barbour-Stoener-Kelly-IIB medium was tested with nine North American isolates and three Japanese isolates. Barbour-Stoener-Kelly-IIB media supported all Japanese isolates tested, HT31, HT24, and FR64b (MGD 9.0 × 107, 9.0 × 107, and 8.5 × 107 cells/mL, respectively), but only supported the growth of one North American isolate to a lesser degree, CA17-2241 (MGD 4.2 × 106 cells/mL) [31].
Barbour-Stoener-Kelly-R medium, a diluted version of BSK-II that is supplemented with Lebovitz’s L-15, mouse serum, and fetal calf serum, was found to support the growth of all nine North American isolates tested: RI13-2395, CT13-2396, CT15-0838, CT15-0839, CT15-0840, CT15-0841 (MGD 1.2–1.5 × 108 cells/mL), MN16-2304 (MGD 6.8 × 107 cells/mL), CA17-2241 (MGD 6.2 × 107 cells/mL), and LB-2001 (MGD 3.6 × 107 cells/mL), and all three Japanese isolates tested, HT31 (MGD 7.0 × 107 cells/mL), HT24 (MGD 1.5 × 108 cells/mL), and FR64b (MGD 1.3 × 108 cells/mL). Barbour-Stoener-Kelly-R media also supports the growth of B. miyamotoi from blood of infected patients and infected tick homogenate [31]. It has also been shown that B. miyamotoi can be isolated from SCID blood if co-cultured in BSK-R medium with I. scapularis embryonic cells (ISE6) [31].
10.2. Genomics and Pathogenesis
The development of successful B. miyamotoi culturing techniques and complete genome sequencing of B. miyamotoi has allowed for genetic manipulations of B. miyamotoi.
Borrelia species have a genomic structure of an approximate 1-megabase chromosome and numerous linear and circular plasmids [152]. A recent study investigated plasmid differences between North American isolates LB-2001 and CT13-2396. Both isolates have the standard chromosome, with plasmid variation: LB-2001 has 12 plasmids, 8 linear and 4 circular, and CT13-2396 has 14 plasmids, 9 linear and 5 circular. Upon further investigation, the differences in the plasmid contents were found to be the result of alternative rearrangements [153]. This revealed that B. miyamotoi has the capability for genetic rearrangement among isolates of the same geographic lineage [154]. The same group also investigated the genetic stability of B. miyamotoi and found that when passaged over 15 months in vitro, LB-2001 retained plasmids and infectious phenotype [136].
Conversely, a study found that B. miyamotoi loses complement binding inhibitory protein (CbiA), an outer surface protein that conveys serum resistance, with prolonged in vitro passage. As CbiA binds classical and alternative pathway complement factors FH, C3, C3b, C4b, and C5, the loss decreases serum resistance [155]. It is unclear if the loss of CbiA can be attributed to plasmid loss, rearrangement, or alternative gene expression. A caveat of these studies is that plasmid loss was investigated in North American strain LB-2001, while CbiA loss was investigated in Japanese strain HT31, which may exhibit differing stability. Furthermore, other Borrelia species, especially Bbsl, demonstrate reduced genome and plasmid content with extended in vitro passage, often impacting growth and infectivity [156,157,158]. This research suggests that plasmid loss in B. miyamotoi may occur.
As genetic manipulation of B. miyamotoi is still developing, heterologous expression of B. miyamotoi genes in Bbsl species has allowed researchers to characterize in vitro effects [155,159,160]. This method has identified multiple novel B. miyamotoi proteins that convey serum resistance including fibronectin binding proteins FbpA and FbpB, BOM1093, Vlp-15/16, and Vlp18. Fibronectin binding proteins A and B were identified in strains FR64b and LB-2001 with 85% and 95% similarity, respectively. These proteins are orthologs of Bbsl protein BBK32 and inhibit the classical complement pathway by binding factor C1r [161]. Fibronectin binding protein A binds human fibronectin and is capable of restoring serum resistance in a serum-sensitive B. burgdorferi strain, while FbpB does not [161]. Though the role of FbpA in fibronectin binding is unknown, FbpA may be a virulence determinant, as fibronectin binding often mediates Borrelia dissemination and colonization [162]. BOM1093 was identified in strain MYK3 and is a vitronectin-binding protein that confers serum resistance through the inhibition of complement C5b7 complex formation and C9 polymerization. The capability of BOM1093 to bind vitronectin is suspected to convey additional unknown serum resistance mechanisms [163]. Variable major proteins Vlp-15/16 and Vlp18 were identified in strain HT31 to inhibit classical and alternative pathway complement. Additionally, Vlp-15/16 binds plasminogen in an FH-independent manner, which potentially plays a role in tissue dissemination [17]. All of these proteins exhibiting redundancy in complement inhibition and serum resistance likely play an important role in innate immune system evasion.
11. Conclusions
While B. miyamotoi research has rapidly expanded from its discovery in 1995, its pathogenicity in 2011, to the current classification of BMD as an emerging infectious disease, much remains unknown. The host–pathogen interactions that result in BMD, with symptoms ranging from sub-clinical to severe, are mostly speculative. Little is known of tissue colonization, dissemination, immune evasion, and the related mechanisms in ticks, animal hosts, and human patients. While herein, we proposed a B. miyamotoi enzootic transmission cycle, this is based on inferences and has not been explicitly analyzed. Borrelia miyamotoi maintenance in nature appears to rely on both horizontal and vertical transmission; however, the extent to which these processes occur is unclear.
It can be concluded that there is a great need for increased awareness of B. miyamotoi in the public and in healthcare, and a standardized diagnostic method for B. miyamotoi infection needs to be developed. Borrelia miyamotoi research still has a great breadth for expansion.
Acknowledgments
The authors would like to thank Tim Casselli, Yvonne Tourand, Becker Lindner, and Rylee Nelson for their critical reading of the manuscript and continued support.
Author Contributions
Conceptualization, D.W.C., C.C.A. and C.A.B.; writing—original draft preparation, D.W.C. and C.C.A.; writing—review and editing D.W.C., C.C.A. and C.A.B. All authors have read and agreed to the published version of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the National Institutes of Health, grant number R21AI149220, to C.A.B.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Charon N.W., Goldstein S.F. Genetics of Motility and Chemotaxis of a Fascinating Group of Bacteria: The Spirochetes. Annu. Rev. Genet. 2002;36:47–73. doi: 10.1146/annurev.genet.36.041602.134359. [DOI] [PubMed] [Google Scholar]
- 2.Steere A.C., Strle F., Wormser G.P., Hu L.T., Branda J.A., Hovius J.W.R., Li X., Mead P.S. Lyme Borreliosis. Nat. Rev. Dis. Prim. 2016;2:16090. doi: 10.1038/nrdp.2016.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lopez J., Hovius J.W., Bergström S. Pathogenesis of Relapsing Fever. Curr. Issues Mol. Biol. 2021;42:519–550. doi: 10.21775/cimb.042.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fukunaga M., Takahashi Y., Tsuruta Y., Matsushita O., Ralph D., McClelland M., Nakao M. Genetic and Phenotypic Analysis of Borrelia Miyamotoi Sp. Nov., Isolated from the Ixodid Tick Ixodes Persulcatus, the Vector for Lyme Disease in Japan. Int. J. Syst. Bacteriol. 1995;45:804–810. doi: 10.1099/00207713-45-4-804. [DOI] [PubMed] [Google Scholar]
- 5.Bratton R.L., Whiteside J.W., Hovan M.J., Engle R.L., Edwards F.D. Diagnosis and Treatment of Lyme Disease. Mayo Clin. Proc. 2008;83:566–571. doi: 10.1016/S0025-6196(11)60731-3. [DOI] [PubMed] [Google Scholar]
- 6.Halperin J.J. Nervous System Lyme Disease. Curr. Infect. Dis. Rep. 2015;17:445. doi: 10.1007/s11908-014-0445-6. [DOI] [PubMed] [Google Scholar]
- 7.Wormser G.P., Dattwyler R.J., Shapiro E.D., Halperin J.J., Steere A.C., Klempner M.S., Krause P.J., Bakken J.S., Strle F., Stanek G., et al. Erratum: The Clinical Assessment, Treatment, and Prevention of Lyme Disease, Human Granulocytic Anaplasmosis, and Babesiosis: Clinical Practice Guidelines by the Infectious Diseases Society of America (Clinical Infectious Diseases (2006) 43, (1089–1134)) Clin. Infect. Dis. 2007;45:941. doi: 10.1086/522848. [DOI] [PubMed] [Google Scholar]
- 8.Marques A. Lyme Neuroborreliosis. Contin. (Minneap Minn) 2015;21:1729–1744. doi: 10.1212/CON.0000000000000252. [DOI] [PubMed] [Google Scholar]
- 9.Platonov A.E., Karan L.S., Kolyasnikova N.M., Makhneva N.A., Toporkova M.G., Maleev V.V., Fish D., Krause P.J. Humans Infected with Relapsing Fever Spirochete Borrelia Miyamotoi, Russia. Emerg. Infect. Dis. 2011;17:1816–1823. doi: 10.3201/eid1710.101474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.NIAID Emerging Infectious Diseases/Pathogens. [(accessed on 9 January 2023)]; Available online: https://www.niaid.nih.gov/research/emerging-infectious-diseases-pathogens.
- 11.Krause P.J., Fish D., Narasimhan S., Barbour A.G. Borrelia Miyamotoi Infection in Nature and in Humans. Clin. Microbiol. Infect. 2015;21:631–639. doi: 10.1016/j.cmi.2015.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Delaney S.L., Murray L.A., Aasen C.E., Bennett C.E., Brown E., Fallon B.A. Borrelia Miyamotoi Serology in a Clinical Population with Persistent Symptoms and Suspected Tick-Borne Illness. Front. Med. 2020;7:567350. doi: 10.3389/fmed.2020.567350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Molloy P.J., Telford S.R., Chowdri H.R., Lepore T.J., Gugliotta J.L., Weeks K.E., Hewins M.E., Goethert H.K., Berardi V.P. Borrelia Miyamotoi Disease in the Northeastern United States a Case Series. Ann. Intern. Med. 2015;163:91–98. doi: 10.7326/M15-0333. [DOI] [PubMed] [Google Scholar]
- 14.Barbour A.G. Multiple and Diverse vsp and Vlp Sequences in Borrelia Miyamotoi, a Hard Tick-Borne Zoonotic Pathogen. PLoS ONE. 2016;11:e0146283. doi: 10.1371/journal.pone.0146283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wagemakers A., Koetsveld J., Narasimhan S., Wickel M., Deponte K., Bleijlevens B., Jahfari S., Sprong H., Karan L.S., Sarksyan D.S., et al. Variable Major Proteins as Targets for Specific Antibodies against Borrelia Miyamotoi. J. Immunol. 2016;196:4185–4195. doi: 10.4049/jimmunol.1600014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Crowder C.D., Langeroudi A.G., Estabragh A.S., Lewis E.R.G., Marcsisin R.A., Barbour A.G. Pathogen and Host Response Dynamics in a Mouse Model of Borrelia Hermsii Relapsing Fever. Vet. Sci. 2016;3:19. doi: 10.3390/vetsci3030019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barbour A.G., Hayes S.F. Biology of Borrelia Species. Microbiol. Rev. 1986;50:381–400. doi: 10.1128/mr.50.4.381-400.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mason L.M.K., Koetsveld J., Trentelman J.J.A., Kaptein T.M., Hoornstra D., Wagemakers A., Fikrig M.M., Ersoz J.I., Oei A., Geijtenbeek T.B.H., et al. Borrelia Miyamotoi Activates Human Dendritic Cells and Elicits T Cell Responses. J. Immunol. 2020;204:386–393. doi: 10.4049/jimmunol.1801589. [DOI] [PubMed] [Google Scholar]
- 19.Lynn G.E., Breuner N.E., Eisen L., Hojgaard A., Replogle A.J., Eisen R.J. An Immunocompromised Mouse Model to Infect Ixodes Scapularis Ticks with the Relapsing Fever Spirochete, Borrelia Miyamotoi. Ticks Tick. Borne Dis. 2019;10:352–359. doi: 10.1016/j.ttbdis.2018.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gandhi S., Narasimhan S., Workineh A., Mamula M., Yoon J., Krause P.J., Farhadian S.F. Borrelia Miyamotoi Meningoencephalitis in an Immunocompetent Patient. Open Forum Infect. Dis. 2022;9:ofac295. doi: 10.1093/ofid/ofac295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hovius J.W.R., De Wever B., Sohne M., Brouwer M.C., Coumou J., Wagemakers A., Oei A., Knol H., Narasimhan S., Hodiamont C.J., et al. A Case of Meningoencephalitis by the Relapsing Fever Spirochaete Borrelia Miyamotoi in Europe. Lancet. 2013;382:658. doi: 10.1016/S0140-6736(13)61644-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gugliotta J.L., Goethert H.K., Berardi V.P., Telford S.R. Meningoencephalitis from Borrelia Miyamotoi in an Immunocompromised Patient. N. Engl. J. Med. 2013;368:240–245. doi: 10.1056/NEJMoa1209039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boden K., Lobenstein S., Hermann B., Margos G., Fingerle V. Borrelia Miyamotoi-Associated Neuroborreliosis in Immunocompromised Person. Emerg. Infect. Dis. 2016;22:1617–1620. doi: 10.3201/eid2209.152034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Henningsson A.J., Asgeirsson H., Hammas B., Karlsson E., Parke Å., Hoornstra D., Wilhelmsson P., Hovius J.W. Two Cases Of Borrelia miyamotoi Meningitis, Sweden, 2018. Emerg. Infect. Dis. 2019;25:2017–2020. doi: 10.3201/eid2510.190416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mukerji S.S., Ard K.L., Schaefer P.W., Branda J.A. Case 32-2020: A 63-Year-Old Man with Confusion, Fatigue, and Garbled Speech. N. Engl. J. Med. 2020;383:1578–1586. doi: 10.1056/NEJMcpc2004996. [DOI] [PubMed] [Google Scholar]
- 26.Hoornstra D., Azagi T., van Eck J.A., Wagemakers A., Koetsveld J., Spijker R., Platonov A.E., Sprong H., Hovius J.W. Prevalence and Clinical Manifestation of Borrelia Miyamotoi in Ixodes Ticks and Humans in the Northern Hemisphere: A Systematic Review and Meta-Analysis. Lancet Microbe. 2022;3:e772–e786. doi: 10.1016/S2666-5247(22)00157-4. [DOI] [PubMed] [Google Scholar]
- 27.Koetsveld J., Manger A., Hoornstra D., Draga R.O., Oei A., Kolyasnikova N.M., Toporkova M.G., Sarksyan D.S., Wagemakers A., Platonov A.E., et al. In Vitro Antimicrobial Susceptibility of Clinical Isolates of Borrelia Miyamotoi. Antimicrob. Agents Chemother. 2018;62:e00419–e00518. doi: 10.1128/AAC.00419-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rodino K.G., Theel E.S., Pritt B.S. Tick-Borne Diseases in the United States. Clin. Chem. 2020;66:537–548. doi: 10.1093/clinchem/hvaa040. [DOI] [PubMed] [Google Scholar]
- 29.Telford S.R., Goethert H.K., Molloy P.J., Berardi V. Blood Smears Have Poor Sensitivity for Confirming Borrelia Miyamotoi Disease. J. Clin. Microbiol. 2019;57:e01468–e01518. doi: 10.1128/JCM.01468-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karan L., Makenov M., Kolyasnikova N., Stukolova O., Toporkova M., Olenkova O. Dynamics of Spirochetemia and Early PCR Detection of Borrelia Miyamotoi. Emerg. Infect. Dis. 2018;24:860–867. doi: 10.3201/eid2405.170829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Replogle A.J., Sexton C., Young J., Kingry L.C., Schriefer M.E., Dolan M., Johnson T.L., Connally N.P., Padgett K.A., Petersen J.M. Isolation of Borrelia Miyamotoi and Other Borreliae Using a Modified BSK Medium. Sci. Rep. 2021;11:1926. doi: 10.1038/s41598-021-81252-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sage K.M., Johnson T.L., Teglas M.B., Nieto N.C., Schwan T.G. Ecological Niche Modeling and Distribution of Ornithodoros Hermsi Associated with Tick-Borne Relapsing Fever in Western North America. PLoS Negl. Trop. Dis. 2017;11:e0006047. doi: 10.1371/journal.pntd.0006047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu G., Luo C.-Y., Ribbe F., Pearson P., Ledizet M., Rich S.M. Borrelia Miyamotoi in Human-Biting Ticks, United States, 2013–2019. Emerg. Infect. Dis. 2021;27:3193–3195. doi: 10.3201/eid2712.204646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dibernardo A., Cote T., Ogden N.H., Lindsay L.R. The Prevalence of Borrelia Miyamotoi Infection, and Co-Infections with Other Borrelia Spp. in Ixodes Scapularis Ticks Collected in Canada. Parasites Vectors. 2014;7:183. doi: 10.1186/1756-3305-7-183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dietrich E.A., Replogle A.J., Sheldon S.W., Petersen J.M. Simultaneous Detection and Differentiation of Clinically Relevant Relapsing Fever Borrelia with Semimultiplex Real-Time PCR. J. Clin. Microbiol. 2021;59:e0298120. doi: 10.1128/JCM.02981-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Aguero-Rosenfeld M.E., Nowakowski J., Bittker S., Cooper D., Nadelman R.B., Wormser G.P. Evolution of the Serologic Response to Borrelia Burgdorferi in Treated Patients with Culture-Confirmed Erythema Migrans. J. Clin. Microbiol. 1996;34:1–9. doi: 10.1128/jcm.34.1.1-9.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Koetsveld J., Kolyasnikova N.M., Wagemakers A., Stukolova O.A., Hoornstra D., Sarksyan D.S., Toporkova M.G., Henningsson A.J., Hvidsten D., Ang W., et al. Serodiagnosis of Borrelia Miyamotoi Disease by Measuring Antibodies against GlpQ and Variable Major Proteins. Clin. Microbiol. Infect. 2018;24:1338.e1–1338.e7. doi: 10.1016/j.cmi.2018.03.009. [DOI] [PubMed] [Google Scholar]
- 38.Landry M.L. Immunoglobulin M for Acute Infection: True or False? Clin. Vaccine Immunol. 2016;23:540–545. doi: 10.1128/CVI.00211-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schriefer M.E. Lyme Disease Diagnosis: Serology. Clin. Lab. Med. 2015;35:797–814. doi: 10.1016/j.cll.2015.08.001. [DOI] [PubMed] [Google Scholar]
- 40.Molloy P.J., Weeks K.E., Todd B., Wormser G.P. Seroreactivity to the C6 Peptide in Borrelia Miyamotoi Infections Occurring in the Northeastern United States. Clin. Infect. Dis. 2018;66:1407–1410. doi: 10.1093/cid/cix1023. [DOI] [PubMed] [Google Scholar]
- 41.Reiter M., Stelzer T., Schötta A.M., Markowicz M., Leschnik M., Harsch A., Reiß E., Kneusel R.E., Stockinger H., Stanek G. Glycerophosphodiester Phosphodiesterase Identified as Non-Reliable Serological Marker for Borrelia Miyamotoi Disease. Microorganisms. 2020;8:1846. doi: 10.3390/microorganisms8121846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mayer C., Kluj R.M., Mühleck M., Walter A., Unsleber S., Hottmann I., Borisova M. Bacteria’s Different Ways to Recycle Their Own Cell Wall. Int. J. Med. Microbiol. 2019;309:151326. doi: 10.1016/j.ijmm.2019.06.006. [DOI] [PubMed] [Google Scholar]
- 43.Krause P.J., Narasimhan S., Wormser G.P., Rollend L., Fikrig E., Lepore T., Barbour A., Fish D. Human Borrelia Miyamotoi Infection in the United States. N. Engl. J. Med. 2013;368:291–293. doi: 10.1056/NEJMc1215469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Krause P.J., Narasimhan S., Wormser G.P., Barbour A.G., Platonov A.E., Brancato J., Lepore T., Dardick K., Mamula M., Rollend L., et al. Borrelia Miyamotoi Sensu Lato Seroreactivity and Seroprevalence in the Northeastern United States. Emerg. Infect. Dis. 2014;20:1183–1190. doi: 10.3201/eid2007.131587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J.R., Hardham J.M., Barbour A.G., Norris S.J. Antigenic Variation in Lyme Disease Borreliae by Promiscuous Recombination of VMP-like Sequence Cassettes. Cell. 1997;89:275–285. doi: 10.1016/S0092-8674(00)80206-8. [DOI] [PubMed] [Google Scholar]
- 46.Liang F., Alvarez A., Gu Y., Nowling J., Ramamoorthi R., Philipp M. An Immunodominant Conserved Region within the Variable Domain of VlsE, the Variable Surface Antigen of Borrelia Burgdorferi. J. Immunol. 1999;163:5566–5573. doi: 10.4049/jimmunol.163.10.5566. [DOI] [PubMed] [Google Scholar]
- 47.Harris E.K., Brandt K.S., Van Gundy T.J., Goodrich I., Wormser G.P., Armstrong B.A., Gilmore R.D. Characterization of a Borrelia Miyamotoi Membrane Antigen (BmaA) for Serodiagnosis of Borrelia Miyamotoi Disease. Ticks Tick Borne Dis. 2020;11:101476. doi: 10.1016/j.ttbdis.2020.101476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tokarz R., Tagliafierro T., Caciula A., Mishra N., Thakkar R., Chauhan L.V., Sameroff S., Delaney S., Wormser G.P., Marques A., et al. Identification of Immunoreactive Linear Epitopes of Borrelia Miyamotoi. Ticks Tick Borne Dis. 2020;11:101314. doi: 10.1016/j.ttbdis.2019.101314. [DOI] [PubMed] [Google Scholar]
- 49.Koetsveld J., Platonov A.E., Kuleshov K., Wagemakers A., Hoornstra D., Ang W., Szekeres S., van Duijvendijk G.L.A., Fikrig E., Embers M.E., et al. Borrelia Miyamotoi Infection Leads to Cross-Reactive Antibodies to the C6 Peptide in Mice and Men. Clin. Microbiol. Infect. 2020;26:513.e1–513.e6. doi: 10.1016/j.cmi.2019.07.026. [DOI] [PubMed] [Google Scholar]
- 50.Scoles G.A., Papero M., Beati L., Fish D. A Relapsing Fever Group Spirochete Transmitted by Ixodes Scapularis Ticks. Vector Borne Zoonotic Dis. 2001;1:21–34. doi: 10.1089/153036601750137624. [DOI] [PubMed] [Google Scholar]
- 51.Franck M., Ghozzi R., Pajaud J., Lawson-Hogban N.E., Mas M., Lacout A., Perronne C. Borrelia Miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients with Persistent Polymorphic Signs and Symptoms. Front. Med. 2020;7:55. doi: 10.3389/fmed.2020.00055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Franck M., Ghozzi R., Pajaud J., Lawson-Hogban N.E., Mas M., Lacout A., Perronne C. Response: Commentary: Borrelia Miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients with Persistent Polymorphic Signs and Symptoms. Front. Med. 2020;7:586694. doi: 10.3389/fmed.2020.586694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wagemakers A., Sprong H., Platonov A., Hovius J.W. Commentary: Borrelia Miyamotoi: 43 Cases Diagnosed in France by Real-Time PCR in Patients with Persistent Polymorphic Signs and Symptoms. Front. Med. 2020;7:474. doi: 10.3389/fmed.2020.00474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Boyer P.H., Koetsveld J., Zilliox L., Sprong H., Talagrand-Reboul É., Hansmann Y., De Martino S.J., Boulanger N., Hovius J.W., Jaulhac B. Assessment of Borrelia Miyamotoi in Febrile Patients and Ticks in Alsace, an Endemic Area for Lyme Borreliosis in France. Parasites Vectors. 2020;13:199. doi: 10.1186/s13071-020-04071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kim C.M., Seo J.W., Kim D.M., Yun N.R., Park J.W., Chung J.K., Song H.J. Detection of Borrelia Miyamotoi in Ixodes Nipponensis in Korea. PLoS ONE. 2019;14:e0220465. doi: 10.1371/journal.pone.0220465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Livanova N.N., Fomenko N.V., Akimov I.A., Ivanov M.J., Tikunova N.V., Armstrong R., Konyaev S.V. Dog Survey in Russian Veterinary Hospitals: Tick Identification and Molecular Detection of Tick-Borne Pathogens. Parasites Vectors. 2018;11:591. doi: 10.1186/s13071-018-3161-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yang Y., Yang Z., Kelly P., Li J., Ren Y., Wang C. Borrelia Miyamotoi Sensu Lato in Pere David Deer and Haemaphysalis Longicornis Ticks. Emerg. Infect. Dis. 2018;24:928–931. doi: 10.3201/eid2405.171355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Jiang B.G., Jia N., Jiang J.F., Zheng Y.C., Chu Y.L., Jiang R.R., Wang Y.W., Liu H.B., Wei R., Zhang W.H., et al. Borrelia Miyamotoi Infections in Humans and Ticks, Northeastern China. Emerg. Infect. Dis. 2018;24:236–241. doi: 10.3201/eid2402.160378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Heglasová I., Rudenko N., Golovchenko M., Zubriková D., Miklisová D., Stanko M. Ticks, Fleas and Rodent-Hosts Analyzed for the Presence of Borrelia Miyamotoi in Slovakia: The First Record of Borrelia Miyamotoi in a Haemaphysalis Inermis Tick. Ticks Tick Borne Dis. 2020;11:101456. doi: 10.1016/j.ttbdis.2020.101456. [DOI] [PubMed] [Google Scholar]
- 60.Breuner N.E., Ford S.L., Hojgaard A., Osikowicz L.M., Parise C.M., Rosales Rizzo M.F., Bai Y., Levin M.L., Eisen R.J., Eisen L. Failure of the Asian Longhorned Tick, Haemaphysalis Longicornis, to Serve as an Experimental Vector of the Lyme Disease Spirochete, Borrelia Burgdorferi Sensu Stricto. Ticks Tick Borne Dis. 2020;11:101311. doi: 10.1016/j.ttbdis.2019.101311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Rudolf I., Hubálek Z. Effect of the Salivary Gland and Midgut Extracts from Ixodes Ricinus and Dermacentor Reticulatus (Acari: Ixodidae) on the Growth of Borrelia Garinii in Vitro. Folia Parasitol. 2003;50:159–160. doi: 10.14411/fp.2003.029. [DOI] [PubMed] [Google Scholar]
- 62.de la Fuente J., Antunes S., Bonnet S., Cabezas-Cruz A., Domingos A.G., Estrada-Peña A., Johnson N., Kocan K.M., Mansfield K.L., Nijhof A.M., et al. Tick-Pathogen Interactions and Vector Competence: Identification of Molecular Drivers for Tick-Borne Diseases. Front. Cell. Infect. Microbiol. 2017;7:114. doi: 10.3389/fcimb.2017.00114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Lehane A., Maes S.E., Graham C.B., Jones E., Delorey M., Eisen R.J. Prevalence of Single and Coinfections of Human Pathogens in Ixodes Ticks from Five Geographical Regions in the United States, 2013–2019. Ticks Tick Borne Dis. 2021;12:101637. doi: 10.1016/j.ttbdis.2020.101637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lynn G.E., Breuner N.E., Hojgaard A., Oliver J., Eisen L., Eisen R.J. A Comparison of Horizontal and Transovarial Transmission Efficiency of Borrelia Miyamotoi by Ixodes Scapularis. Ticks Tick Borne Dis. 2022;13:102003. doi: 10.1016/j.ttbdis.2022.102003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Kurokawa C., Lynn G.E., Pedra J.H.F., Pal U., Narasimhan S., Fikrig E. Interactions between Borrelia Burgdorferi and Ticks. Nat. Rev. Microbiol. 2020;18:587–600. doi: 10.1038/s41579-020-0400-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Wilhelmsson P., Lindblom P., Fryland L., Ernerudh J., Forsberg P., Lindgren P.E. Prevalence, Diversity, and Load of Borrelia Species in Ticks That Have Fed on Humans in Regions of Sweden and Åland Islands, Finland with Different Lyme Borreliosis Incidences. PLoS ONE. 2013;8:e81433. doi: 10.1371/journal.pone.0081433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Wilhelmsson P., Jaenson T.G.T., Olsen B., Waldenström J., Lindgren P.E. Migratory Birds as Disseminators of Ticks and the Tick-Borne Pathogens Borrelia Bacteria and Tick-Borne Encephalitis (TBE) Virus: A Seasonal Study at Ottenby Bird Observatory in South-Eastern Sweden. Parasites Vectors. 2020;13:607. doi: 10.1186/s13071-020-04493-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Strnad M., Hönig V., Ružek D., Gribhoffer L., Rego R.O.M. Europe-Wide Meta-Analysis of Borrelia Burgdorferi Sensu Lato Prevalence in Questing Ixodes Ricinus Ticks Martin. Appl. Environ. Microbiol. 2017;83:e00609–e00617. doi: 10.1128/AEM.00609-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Barbour A.G., Bunikis J., Travinsky B., Hoen A.G., Diuk-Wasser M.A., Fish D., Tsao J.I. Niche Partitioning of Borrelia Burgdorferi and Borrelia Miyamotoi in the Same Tick Vector and Mammalian Reservoir Species. Am. J. Trop. Med. Hyg. 2009;81:1120–1131. doi: 10.4269/ajtmh.2009.09-0208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.De Silva A.M., Fikrig E. Growth and Migration of Borrelia Burgdorferi in Ixodes Ticks during Blood Feeding. Am. J. Trop. Med. Hyg. 1995;53:397–404. doi: 10.4269/ajtmh.1995.53.397. [DOI] [PubMed] [Google Scholar]
- 71.Schwan T.G., Raffel S.J. Transovarial Transmission of Borrelia Hermsii by Its Tick Vector and Reservoir Host Ornithodoros Hermsi. Microorganisms. 2021;9:1978. doi: 10.3390/microorganisms9091978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Ramamoorthi N., Narasimhan S., Pal U., Bao F., Yang X.F., Fish D., Anguita J., Norgard M.V., Kantor F.S., Anderson J.F., et al. The Lyme Disease Agent Exploits a Tick Protein to Infect the Mammalian Host. Nature. 2005;436:573–577. doi: 10.1038/nature03812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Schwan T.G., Raffel S.J., Battisti J.M. Transgenic Functional Complementation with a Transmission-Associated Protein Restores Spirochete Infectivity by Tick Bite. Ticks Tick Borne Dis. 2020;11:101377. doi: 10.1016/j.ttbdis.2020.101377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Schötta A.M., Stelzer T., Stanek G., Stockinger H., Wijnveld M. Bacteria and Protozoa with Pathogenic Potential in Ixodes Ricinus Ticks in Viennese Recreational Areas. Wien. Klin. Wochenschr. 2022 doi: 10.1007/s00508-022-02046-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kniazeva V., Pogotskaya Y., Higgs S., Krasko A. The Prevalence of Different Human Pathogenic Microorganisms Transmitted by Ixodes Tick Vectors in Belarus. Vector Borne Zoonotic Dis. 2021;21:6–10. doi: 10.1089/vbz.2020.2675. [DOI] [PubMed] [Google Scholar]
- 76.Garcia-Vozmediano A., Tomassone L., Fonville M., Bertolotti L., Heylen D., Fabri N.D., Medlock J.M., Nijhof A.M., Hansford K.M., Sprong H., et al. The Genetic Diversity of Rickettsiella Symbionts in Ixodes Ricinus Throughout Europe. Microb. Ecol. 2022;84:613–626. doi: 10.1007/s00248-021-01869-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Lernout T., De Regge N., Tersago K., Fonville M., Suin V., Sprong H. Prevalence of Pathogens in Ticks Collected from Humans through Citizen Science in Belgium. Parasites Vectors. 2019;12:550. doi: 10.1186/s13071-019-3806-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Burrows H., Talbot B., McKay R., Slatculescu A., Logan J., Thickstun C., Lindsay L.R., Dibernardo A., Koffi J.K., Ogden N.H., et al. A Multi-Year Assessment of Blacklegged Tick (Ixodes Scapularis) Population Establishment and Lyme Disease Risk Areas in Ottawa, Canada, 2017–2019. PLoS ONE. 2021;16:e0246484. doi: 10.1371/journal.pone.0246484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Gaowa, Wulantuya, Sato K., Liu D., Cui Y., Yin X., Zhang L., Li H., Wang T., Liu R., et al. Surveillance of Borrelia Miyamotoi-Carrying Ticks and Genomic Analysis of Isolates in Inner Mongolia, China. Parasites Vectors. 2021;14:368. doi: 10.1186/s13071-021-04809-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Janeček J., Nováková M., Oppelt J., Pospíšilová P., Cunha A., Silva A.C., Dantong L., Šmajs D. Complete Chromosomal Sequences of Two Borrelia Miyamotoi Samples Obtained from Ixodes Ricinus Eggs in Czechia. Microbiol. Resour. Announc. 2020;9:11–13. doi: 10.1128/MRA.01504-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Michelet L., Delannoy S., Devillers E., Umhang G., Aspan A., Juremalm M., Chirico J., van der Wal F.J., Sprong H., Boye Pihl T.P., et al. High-Throughput Screening of Tick-Borne Pathogens in Europe. Front. Cell. Infect. Microbiol. 2014;4:103. doi: 10.3389/fcimb.2014.00103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Geller J., Nazarova L., Katargina O., Järvekülg L., Fomenko N., Golovljova I. Detection and Genetic Characterization of Relapsing Fever Spirochete Borrelia Miyamotoi in Estonian Ticks. PLoS ONE. 2012;7:e51914. doi: 10.1371/journal.pone.0051914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sormunen J.J., Penttinen R., Klemola T., Hänninen J., Vuorinen I., Laaksonen M., Sääksjärvi I.E., Ruohomäki K., Vesterinen E.J. Tick-Borne Bacterial Pathogens in Southwestern Finland. Parasites Vectors. 2016;9:168. doi: 10.1186/s13071-016-1449-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Sormunen J.J., Andersson T., Aspi J., Bäck J., Cederberg T., Haavisto N., Halonen H., Hänninen J., Inkinen J., Kulha N., et al. Monitoring of Ticks and Tick-Borne Pathogens through a Nationwide Research Station Network in Finland. Ticks Tick Borne Dis. 2020;11:101449. doi: 10.1016/j.ttbdis.2020.101449. [DOI] [PubMed] [Google Scholar]
- 85.Zakham F., Jääskeläinen A.J., Castrén J., Sormunen J.J., Uusitalo R., Smura T., Von Troil G., Kuivanen S., Sironen T., Vapalahti O. Molecular Detection and Phylogenetic Analysis of Borrelia Miyamotoi Strains from Ticks Collected in the Capital Region of Finland. Ticks Tick Borne Dis. 2021;12:101608. doi: 10.1016/j.ttbdis.2020.101608. [DOI] [PubMed] [Google Scholar]
- 86.Cosson J.F., Michelet L., Chotte J., Le Naour E., Cote M., Devillers E., Poulle M.L., Huet D., Galan M., Geller J., et al. Genetic Characterization of the Human Relapsing Fever Spirochete Borrelia Miyamotoi in Vectors and Animal Reservoirs of Lyme Disease Spirochetes in France. Parasites Vectors. 2014;7:233. doi: 10.1186/1756-3305-7-233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Lejal E., Marsot M., Chalvet-Monfray K., Cosson J.F., Moutailler S., Vayssier-Taussat M., Pollet T. A Three-Years Assessment of Ixodes Ricinus-Borne Pathogens in a French Peri-Urban Forest. Parasites Vectors. 2019;12:551. doi: 10.1186/s13071-019-3799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rǎileanu C., Tauchmann O., Vasić A., Wöhnke E., Silaghi C. Borrelia Miyamotoi and Borrelia Burgdorferi (Sensu Lato) Identification and Survey of Tick-Borne Encephalitis Virus in Ticks from North-Eastern Germany. Parasites Vectors. 2020;13:106. doi: 10.1186/s13071-020-3969-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Lambert J.S., Cook M.J., Healy J.E., Murtagh R., Avramovic G., Lee S.H. Metagenomic 16S RRNA Gene Sequencing Survey of Borrelia Species in Irish Samples of Ixodes Ricinus Ticks. PLoS ONE. 2019;14:e0209881. doi: 10.1371/journal.pone.0209881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Iwabu-Itoh Y., Bazartseren B., Naranbaatar O., Yondonjamts E., Furuno K., Lee K., Sato K., Kawabata H., Takada N., Andoh M., et al. Tick Surveillance for Borrelia Miyamotoi and Phylogenetic Analysis of Isolates in Mongolia and Japan. Ticks Tick Borne Dis. 2017;8:850–857. doi: 10.1016/j.ttbdis.2017.06.011. [DOI] [PubMed] [Google Scholar]
- 91.Morozov A., Tischenkov A., Silaghi C., Proka A., Toderas I., Movila A., Frickmann H., Poppert S. Prevalence of Bacterial and Protozoan Pathogens in Ticks Collected from Birds in the Republic of Moldova. Microorganisms. 2022;10:1111. doi: 10.3390/microorganisms10061111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lagunova E.K., Liapunova N.A., Tuul D., Otgonsuren G., Nomin D., Erdenebat N., Abmed D., Danchinova G.A., Sato K., Kawabata H., et al. Co-Infections with Multiple Pathogens in Natural Populations of Ixodes Persulcatus Ticks in Mongolia. Parasites Vectors. 2022;15:236. doi: 10.1186/s13071-022-05356-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Heylen D., Fonville M., Docters Van Leeuwen A., Stroo A., Duisterwinkel M., Van Wieren S., Diuk-Wasser M., De Bruin A., Sprong H. Pathogen Communities of Songbird-Derived Ticks in Europe’s Low Countries. Parasites Vectors. 2017;10:497. doi: 10.1186/s13071-017-2423-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Wagemakers A., Jahfari S., de Wever B., Spanjaard L., Starink M.V., de Vries H.J.C., Sprong H., Hovius J.W. Borrelia Miyamotoi in Vectors and Hosts in The Netherlands. Ticks Tick Borne Dis. 2017;8:370–374. doi: 10.1016/j.ttbdis.2016.12.012. [DOI] [PubMed] [Google Scholar]
- 95.Kjelland V., Rollum R., Korslund L., Slettan A., Tveitnes D. Borrelia Miyamotoi Is Widespread in Ixodes Ricinus Ticks in Southern Norway. Ticks Tick Borne Dis. 2015;6:516–521. doi: 10.1016/j.ttbdis.2015.04.004. [DOI] [PubMed] [Google Scholar]
- 96.Kiewra D., Stańczak J., Richter M. Ixodes Ricinus Ticks (Acari, Ixodidae) as a Vector of Borrelia Burgdorferi Sensu Lato and Borrelia Miyamotoi in Lower Silesia, Poland—Preliminary Study. Ticks Tick Borne Dis. 2014;5:892–897. doi: 10.1016/j.ttbdis.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 97.Kubiak K., Szymańska H., Dmitryjuk M., Dzika E. Abundance of Ixodes Ricinus Ticks (Acari: Ixodidae) and the Diversity of Borrelia Species in Northeastern Poland. Int. J. Environ. Res. Public Health. 2022;19:7378. doi: 10.3390/ijerph19127378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Nunes M., Parreira R., Lopes N., Maia C., Carreira T., Sousa C., Faria S., Campino L., Vieira M.L. Molecular Identification of Borrelia Miyamotoi in Ixodes Ricinus from Portugal. Vector-Borne Zoonotic Dis. 2015;15:515–517. doi: 10.1089/vbz.2014.1765. [DOI] [PubMed] [Google Scholar]
- 99.Pukhovskaya N.M., Morozova O.V., Vysochina N.P., Belozerova N.B., Ivanov L.I. Prevalence of Borrelia Burgdorferi Sensu Lato and Borrelia Miyamotoi in Ixodid Ticks in the Far East of Russia. Int. J. Parasitol. Parasites Wildl. 2019;8:192–202. doi: 10.1016/j.ijppaw.2019.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Banović P., Díaz-Sánchez A.A., Galon C., Simin V., Mijatović D., Obregón D., Moutailler S., Cabezas-Cruz A. Humans Infested with Ixodes Ricinus Are Exposed to a Diverse Array of Tick-Borne Pathogens in Serbia. Ticks Tick Borne Dis. 2021;12:101609. doi: 10.1016/j.ttbdis.2020.101609. [DOI] [PubMed] [Google Scholar]
- 101.Hamšíková Z., Coipan C., Mahríková L., Minichová L., Sprong H., Kazimírová M. Borrelia Miyamotoi and Co-Infection with Borrelia Afzelii in Ixodes Ricinus Ticks and Rodents from Slovakia. Microb. Ecol. 2017;73:1000–1008. doi: 10.1007/s00248-016-0918-2. [DOI] [PubMed] [Google Scholar]
- 102.Remesar S., Díaz P., Venzal J.M., Prieto A., Estrada-Peña A., López C.M., Panadero R., Fernández G., Díez-Baños P., Morrondo P. Longitudinal Study of Infection with Borrelia Spp. in Questing Ticks from North-Western Spain. Vector-Borne Zoonotic Dis. 2019;19:785–792. doi: 10.1089/vbz.2019.2442. [DOI] [PubMed] [Google Scholar]
- 103.Hornok S., Daccord J., Takács N., Kontschán J., Tuska-Szalay B., Sándor A.D., Szekeres S., Meli M.L., Hofmann-Lehmann R. Investigation on Haplotypes of Ixodid Ticks and Retrospective Finding of Borrelia Miyamotoi in Bank Vole (Myodes Glareolus) in Switzerland. Ticks Tick Borne Dis. 2022;13:101865. doi: 10.1016/j.ttbdis.2021.101865. [DOI] [PubMed] [Google Scholar]
- 104.Sakakibara K., Sen E., Sato K., Kawabata H., Ohashi N., Masuzawa T. Detection and Characterization of the Emerging Relapsing Fever Pathogen, Borrelia Miyamotoi, from the Ixodes Ricinus Tick in the Rural Trakya (Thrace) Region of Northwestern Turkey. Vector-Borne Zoonotic Dis. 2016;16:797–799. doi: 10.1089/vbz.2016.2012. [DOI] [PubMed] [Google Scholar]
- 105.Rogovskyy A., Batool M., Gillis D.C., Holman P.J., Nebogatkin I.V., Rogovska Y.V., Rogovskyy M.S. Diversity of Borrelia Spirochetes and Other Zoonotic Agents in Ticks from Kyiv, Ukraine. Ticks Tick Borne Dis. 2018;9:404–409. doi: 10.1016/j.ttbdis.2017.12.006. [DOI] [PubMed] [Google Scholar]
- 106.Lynn G.E., Graham C.B., Horiuchi K., Eisen L., Johnson T.L., Lane R.S., Eisen R.J. Prevalence and Geographic Distribution of Borrelia Miyamotoi in Host-Seeking Ixodes Pacificus (Acari: Ixodidae) Nymphs in Mendocino County, California. J. Med. Entomol. 2018;55:711–716. doi: 10.1093/jme/tjx258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Han S., Hickling G.J., Ogden N.H., Ginsberg H.S., Kobbekaduwa V., Rulison E.L., Beati L., Tsao J.I. Seasonality of Acarological Risk of Exposure to Borrelia Miyamotoi from Questing Life Stages of Ixodes Scapularis Collected from Wisconsin and Massachusetts, USA. Ticks Tick Borne Dis. 2021;12:101556. doi: 10.1016/j.ttbdis.2020.101556. [DOI] [PubMed] [Google Scholar]
- 108.Burri C., Schumann O., Schumann C., Gern L. Are Apodemus Spp. Mice and Myodes Glareolus Reservoirs for Borrelia Miyamotoi, Candidatus Neoehrlichia Mikurensis, Rickettsia Helvetica, R. Monacensis and Anaplasma Phagocytophilum? Ticks Tick Borne Dis. 2014;5:245–251. doi: 10.1016/j.ttbdis.2013.11.007. [DOI] [PubMed] [Google Scholar]
- 109.Scott M.C., Rosen M.E., Hamer S.A., Baker E., Edwards H., Crowder C., Tsao J.I., Hickling G.J. High-Prevalence Borrelia Miyamotoi [Scapin Wild Turkeys (Meleagris Gallopavo) in Tennessee. J. Med. Entomol. 2010;47:1238–1242. doi: 10.1603/ME10075. [DOI] [PubMed] [Google Scholar]
- 110.Hrnková J., Golovchenko M., Musa A.S., Needham T., Italiya J., Ceacero F., Kotrba R., Grubhoffer L., Rudenko N., Cerný J. Borrelia Spirochetes in European Exotic Farm Animals. Front. Vet. Sci. 2022;9:996015. doi: 10.3389/fvets.2022.996015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Szekeres S., Coipan E.C., Rigó K., Majoros G., Jahfari S., Sprong H., Földvári G. Eco-Epidemiology of Borrelia Miyamotoi and Lyme Borreliosis Spirochetes in a Popular Hunting and Recreational Forest Area in Hungary. Parasites Vectors. 2015;8:309. doi: 10.1186/s13071-015-0922-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gryczyńska A., Sokół M., Gortat T., Kowalec M. Borrelia Miyamotoi Infection in Apodemus Spp. Mice Populating an Urban Habitat (Warsaw, Poland) Int. J. Parasitol. Parasites Wildl. 2021;14:138–140. doi: 10.1016/j.ijppaw.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Zinck C.B., Lloyd V.K. Borrelia Burgdorferi and Borrelia Miyamotoi in Atlantic Canadian Wildlife. PLoS ONE. 2022;17:e0262229. doi: 10.1371/journal.pone.0262229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Han S., Hickling G.J., Tsao J.I. High Prevalence of Borrelia Miyamotoi among Adult Blacklegged Ticks from White-Tailed Deer. Emerg. Infect. Dis. 2016;22:316–318. doi: 10.3201/eid2202.151218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Han S., Lubelczyk C., Hickling G.J., Belperron A.A., Bockenstedt L.K., Tsao J.I. Vertical Transmission Rates of Borrelia Miyamotoi in Ixodes Scapularis Collected from White-Tailed Deer. Ticks Tick Borne Dis. 2019;10:682–689. doi: 10.1016/j.ttbdis.2019.02.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kurtenbach K., Sewell H.S., Ogden N.H., Randolph S.E., Nuttall P.A. Serum Complement Sensitivity as a Key Factor in Lyme Disease Ecology. Infect. Immun. 1998;66:1248–1251. doi: 10.1128/IAI.66.3.1248-1251.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Barthold S.W., De Souza M.S., Janotka J.L., Smith A.L., Persing D.H. Chronic Lyme Borreliosis in the Laboratory Mouse. Am. J. Pathol. 1993;143:959–972. [PMC free article] [PubMed] [Google Scholar]
- 118.Taylor K.R., Takano A., Konnai S., Shimozuru M., Kawabata H., Tsubota T. Borrelia Miyamotoi Infections among Wild Rodents Show Age and Month Independence and Correlation with Ixodes Persulcatus Larval Attachment in Hokkaido, Japan. Vector-Borne Zoonotic Dis. 2013;13:92–97. doi: 10.1089/vbz.2012.1027. [DOI] [PubMed] [Google Scholar]
- 119.Krawczyk A.I., Van Duijvendijk G.L.A., Swart A., Heylen D., Jaarsma R.I., Jacobs F.H.H., Fonville M., Sprong H., Takken W. Effect of Rodent Density on Tick and Tick-Borne Pathogen Populations: Consequences for Infectious Disease Risk. Parasites Vectors. 2020;13:34. doi: 10.1186/s13071-020-3902-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Jeske K., Herzig-Straschil B., Răileanu C., Kunec D., Tauchmann O., Emirhar D., Schmidt S., Trimpert J., Silaghi C., Heckel G., et al. Zoonotic Pathogen Screening of Striped Field Mice (Apodemus Agrarius) from Austria. Transbound. Emerg. Dis. 2022;69:886–890. doi: 10.1111/tbed.14015. [DOI] [PubMed] [Google Scholar]
- 121.Tadin A., Tokarz R., Markotic A., Margaletic J., Turk N., Habu J., Svoboda P., Vucelja M., Desai A., Jain K., et al. Molecular Survey of Zoonotic Agents in Rodents and Other Small Mammals in Croatia. Am. J. Trop. Med. Hyg. 2016;94:466–473. doi: 10.4269/ajtmh.15-0517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Kalmár Z., Sándor A.D., Matei I.A., Ionicǎ A., D’Amico G., Gherman C.M., Mihalca A.D. Borrelia Spp. in Small Mammals in Romania. Parasites Vectors. 2019;12:461. doi: 10.1186/s13071-019-3713-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Cerar T., Korva M., Avšič-Županc T., Ružić-Sabljić E. Detection, Identification and Genotyping of Borrellia Spp. In Rodents in Slovenia by PCR and Culture. BMC Vet. Res. 2015;11:188. doi: 10.1186/s12917-015-0501-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Majerová K., Hönig V., Houda M., Papežík P., Fonville M., Sprong H., Rudenko N., Golovchenko M., Bolfíková B.Č., Hulva P., et al. Hedgehogs, Squirrels, and Blackbirds as Sentinel Hosts for Active Surveillance of Borrelia Miyamotoi and Borrelia Burgdorferi Complex in Urban and Rural Environments. Microorganisms. 2020;8:1908. doi: 10.3390/microorganisms8121908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Salkeld D.J., Nieto N.C., Bonilla D.L., Yoshimizu M.H., Padgett K.A. Borrelia Miyamotoi Infections in Small Mammals, California, USA. Emerg. Infect. Dis. 2018;24:2356–2359. doi: 10.3201/eid2412.171632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Dumas A., Bouchard C., Dibernardo A., Drapeau P., Robbin Lindsay L., Ogden N.H., Leighton P.A. Transmission Patterns of Tick-Borne Pathogens among Birds and Rodents in a Forested Park in Southeastern Canada. PLoS ONE. 2022;17:e0266527. doi: 10.1371/journal.pone.0266527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Szekeres S., Docters van Leeuwen A., Tóth E., Majoros G., Sprong H., Földvári G. Road-Killed Mammals Provide Insight into Tick-Borne Bacterial Pathogen Communities within Urban Habitats. Transbound. Emerg. Dis. 2019;66:277–286. doi: 10.1111/tbed.13019. [DOI] [PubMed] [Google Scholar]
- 128.Lau A.C.C., Qiu Y., Moustafa M.A.M., Nakao R., Shimozuru M., Onuma M., Mohd-Azlan J., Tsubota T. Detection of Borrelia Burgdorferi Sensu Lato and Relapsing Fever Borrelia in Feeding Ixodes Ticks and Rodents in Sarawak, Malaysia: New Geographical Records of Borrelia Yangtzensis and Borrelia Miyamotoi. Pathogens. 2020;9:846. doi: 10.3390/pathogens9100846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Hofmeester T.R., Krawczyk A.I., Van Leeuwen A.D., Fonville M., Montizaan M.G.E., Van Den Berge K., Gouwy J., Ruyts S.C., Verheyen K., Sprong H. Role of Mustelids in the Life-Cycle of Ixodid Ticks and Transmission Cycles of Four Tick-Borne Pathogens. Parasites Vectors. 2018;11:600. doi: 10.1186/s13071-018-3126-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Richter D., Matuschka F.R. Elimination of Lyme Disease Spirochetes from Ticks Feeding on Domestic Ruminants. Appl. Environ. Microbiol. 2010;76:7650–7652. doi: 10.1128/AEM.01649-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Schreiber C., Krücken J., Beck S., Maaz D., Pachnicke S., Krieger K., Gross M., Kohn B., Von Samson-Himmelstjerna G. Pathogens in Ticks Collected from Dogs in Berlin/Brandenburg, Germany. Parasites Vectors. 2014;7:535. doi: 10.1186/s13071-014-0535-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Regier Y., Komma K., Weigel M., Kraiczy P., Laisi A., Pulliainen A.T., Hain T., Kempf V.A.J. Combination of Microbiome Analysis and Serodiagnostics to Assess the Risk of Pathogen Transmission by Ticks to Humans and Animals in Central Germany 11 Medical and Health Sciences 1108 Medical Microbiology. Parasites Vectors. 2019;12:11. doi: 10.1186/s13071-018-3240-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Seto J., Tanaka S., Kawabata H., Ito Y., Ikeda T., Mizuta K. Detection of Tick-Borne Pathogens in Ticks from Dogs and Cats in the Yamagata Prefecture of Japan in 2018. Jpn. J. Infect. Dis. 2021;74:122–128. doi: 10.7883/yoken.JJID.2020.462. [DOI] [PubMed] [Google Scholar]
- 134.Namina A., Capligina V., Seleznova M., Krumins R., Aleinikova D., Kivrane A., Akopjana S., Lazovska M., Berzina I., Ranka R. Tick-Borne Pathogens in Ticks Collected from Dogs, Latvia, 2011–2016. BMC Vet. Res. 2019;15:398. doi: 10.1186/s12917-019-2149-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Wodecka B., Rymaszewska A., Skotarczak B. Host and Pathogen DNA Identification in Blood Meals of Nymphal Ixodes Ricinus Ticks from Forest Parks and Rural Forests of Poland. Exp. Appl. Acarol. 2014;62:543–555. doi: 10.1007/s10493-013-9763-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Díaz P., Remesar S., Venzal J.M., Vázquez-López M.E., Fernández G., López C., Díez-Baños P., Morrondo P., Panadero R. Occurrence of Borrelia and Borreliella Species in Ixodes Ricinus Collected from Roe Deer in Northwestern Spain. Med. Vet. Entomol. 2019;33:427–430. doi: 10.1111/mve.12364. [DOI] [PubMed] [Google Scholar]
- 137.Wodecka B. Znaczenie Jeleni (Cervus Elaphus) w Ekologii Borrelia Burgdorferi Sensu Lato. Wiadomości Parazytol. 2007;53:231–237. [PubMed] [Google Scholar]
- 138.Kjær L.J., Jensen L.M., Chriél M., Bødker R., Petersen H.H. The Raccoon Dog (Nyctereutes Procyonoides) as a Reservoir of Zoonotic Diseases in Denmark. Int. J. Parasitol. Parasites Wildl. 2021;16:175–182. doi: 10.1016/j.ijppaw.2021.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Rosen M.E., Hamer S.A., Gerhardt R.R., Jones C.J., Muller L.I., Scott M.C., Hickling G.J. Borrelia Burgdorferi Not Detected in Widespread Ixodes Scapularis (Acari: Ixodidae) Collected from White-Tailed Deer in Tennessee. J. Med. Entomol. 2012;49:1473–1480. doi: 10.1603/ME11255. [DOI] [PubMed] [Google Scholar]
- 140.Hamer S.A., Hickling G.J., Keith R., Sidge J.L., Walker E.D., Tsao J.I. Associations of Passerine Birds, Rabbits, and Ticks with Borrelia Miyamotoi and Borrelia Andersonii in Michigan, U.S.A. Parasites Vectors. 2012;5:231. doi: 10.1186/1756-3305-5-231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Rollend L., Fish D., Childs J.E. Transovarial Transmission of Borrelia Spirochetes by Ixodes Scapularis: A Summary of the Literature and Recent Observations. Ticks Tick Borne Dis. 2013;4:46–51. doi: 10.1016/j.ttbdis.2012.06.008. [DOI] [PubMed] [Google Scholar]
- 142.Breuner N.E., Dolan M.C., Replogle A.J., Sexton C., Hojgaard A., Boegler K.A., Clark R.J., Eisen L. Transmission of Borrelia Miyamotoi Sensu Lato Relapsing Fever Group Spirochetes in Relation to Duration of Attachment by Ixodes Scapularis Nymphs. Ticks Tick Borne Dis. 2017;8:677–681. doi: 10.1016/j.ttbdis.2017.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Breuner N.E., Hojgaard A., Replogle A.J., Boegler K.A., Eisen L. Transmission of the Relapsing Fever Spirochete, Borrelia Miyamotoi, by Single Transovarially-Infected Larval Ixodes Scapularis Ticks. Ticks Tick Borne Dis. 2018;9:1464–1467. doi: 10.1016/j.ttbdis.2018.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Hojgaard A., Osikowicz L.M., Maes S., Eisen L., Eisen R.J. Detection of Genetic Variability in Borrelia Miyamotoi (Spirochaetales: Spirochaetaceae) between and within the Eastern and Western United States. J. Med. Entomol. 2021;58:2154–2160. doi: 10.1093/jme/tjab075. [DOI] [PubMed] [Google Scholar]
- 145.Nakao R., Kasama K., Boldbaatar B., Ogura Y., Kawabata H., Toyoda A., Hayashi T., Takano A., Maeda K. The Evolution of Hard Tick-Borne Relapsing Fever Borreliae Is Correlated with Vector Species Rather than Geographical Distance. BMC Ecol. Evol. 2021;21:105. doi: 10.1186/s12862-021-01838-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Kelly R. Cultivation of Borrelia Hernsii. Science. 1971;173:443–444. doi: 10.1126/science.173.3995.443. [DOI] [PubMed] [Google Scholar]
- 147.Barbour A.G. Isolation and Cultivation of Lyme Disease Spirochetes. Yale J. Biol. Med. 1984;57:521–525. [PMC free article] [PubMed] [Google Scholar]
- 148.Preac-Mursic V., Wilske B., Schierz G. European Borrelia Burgdorferi Isolated from Humans and Ticks Culture Conditions and Antibiotic Susceptibility. Zent. Bakteriol. Mikrobiol. Hyg.—Abt. 1 Orig. A. 1986;263:112–118. doi: 10.1016/S0176-6724(86)80110-9. [DOI] [PubMed] [Google Scholar]
- 149.Wang G., Iyer R., Bittker S., Cooper D., Small J., Wormser G.P., Schwartz I. Variations in Barbour-Stoenner-Kelly Culture Medium Modulate Infectivity and Pathogenicity of Borrelia Burgdorferi Clinical Isolates. Infect. Immun. 2004;72:6702–6706. doi: 10.1128/IAI.72.11.6702-6706.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Margos G., Stockmeier S., Hizo-Teufel C., Hepner S., Fish D., Dautel H., Sing A., Dzaferovic E., Rieger M., Jungnick S., et al. Long-Term in Vitro Cultivation of Borrelia Miyamotoi. Ticks Tick Borne Dis. 2015;6:181–184. doi: 10.1016/j.ttbdis.2014.12.001. [DOI] [PubMed] [Google Scholar]
- 151.Wagemakers A., Oei A., Fikrig M.M., Miellet W.R., Hovius J.W. The Relapsing Fever Spirochete Borrelia Miyamotoi Is Cultivable in a Modified Kelly-Pettenkofer Medium, and Is Resistant to Human Complement. Parasites Vectors. 2014;7:4–9. doi: 10.1186/1756-3305-7-418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Casjens S., Palmer N., Van Vugt R., Huang W.M., Stevenson B., Rosa P., Lathigra R., Sutton G., Peterson J., Dodson R.J., et al. A Bacterial Genome in Flux: The Twelve Linear and Nine Circular Extrachromosomal DNAs in an Infectious Isolate of the Lyme Disease Spirochete Borrelia Burgdorferi. Mol. Microbiol. 2000;35:490–516. doi: 10.1046/j.1365-2958.2000.01698.x. [DOI] [PubMed] [Google Scholar]
- 153.Gilmore R.D., Mikula S., Harris E.K., Van Gundy T.J., Goodrich I., Brandt K.S. Borrelia Miyamotoi Strain LB-2001 Retains Plasmids and Infectious Phenotype throughout Continuous Culture Passages as Evaluated by Multiplex PCR. Ticks Tick Borne Dis. 2021;12:101587. doi: 10.1016/j.ttbdis.2020.101587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Gilmore R.D., Kneubehl A.R., Lopez J.E., Armstrong B.A., Brandt K.S., Van Gundy T.J. Modification of the Multiplex Plasmid PCR Assay for Borrelia Miyamotoi Strain LB-2001 Based on the Complete Genome Sequence Reflecting Genomic Rearrangements Differing from Strain CT13–2396. Ticks Tick Borne Dis. 2022;13:101843. doi: 10.1016/j.ttbdis.2021.101843. [DOI] [PubMed] [Google Scholar]
- 155.Röttgerding F., Wagemakers A., Koetsveld J., Fingerle V., Kirschfink M., Hovius J.W., Zipfel P.F., Wallich R., Kraiczy P. Immune Evasion of Borrelia Miyamotoi: CbiA, a Novel Outer Surface Protein Exhibiting Complement Binding and Inactivating Properties. Sci. Rep. 2017;7:303. doi: 10.1038/s41598-017-00412-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Barbour A.G. Plasmid Analysis of Borrelia Burgdorferi, the Lyme Disease Agent. J. Clin. Microbiol. 1988;26:475–478. doi: 10.1128/jcm.26.3.475-478.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Schwan T.G., Burgdorfer W., Garon C.F. Changes in Infectivity and Plasmid Profile of the Lyme Disease Spirochete, Borrelia Burgdorferi, as a Result of in Vitro Cultivation. Infect. Immun. 1988;56:1831–1836. doi: 10.1128/iai.56.8.1831-1836.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Grimm D., Elias A.F., Tilly K., Rosa P.A. Plasmid Stability during in Vitro Propagation of Borrelia Burgdorferi Assessed at a Clonal Level. Infect. Immun. 2003;71:3138–3145. doi: 10.1128/IAI.71.6.3138-3145.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Sato K., Kumagai Y., Sekizuka T., Kuroda M., Hayashi T., Takano A., Gaowa, Taylor K.R., Ohnishi M., Kawabata H. Vitronectin Binding Protein, BOM1093, Confers Serum Resistance on Borrelia Miyamotoi. Sci. Rep. 2021;11:5462. doi: 10.1038/s41598-021-85069-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Schmidt F.L., Sürth V., Berg T.K., Lin Y.P., Hovius J.W., Kraiczy P. Interaction between Borrelia Miyamotoi Variable Major Proteins Vlp15/16 and Vlp18 with Plasminogen and Complement. Sci. Rep. 2021;11:4964. doi: 10.1038/s41598-021-84533-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Booth C.E., Powell-Pierce A.D., Skare J.T., Garcia B.L. Borrelia Miyamotoi FbpA and FbpB Are Immunomodulatory Outer Surface Lipoproteins with Distinct Structures and Functions. Front. Immunol. 2022;13:886733. doi: 10.3389/fimmu.2022.886733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Fischer J.R., LeBlanc K.T., Leong J.M. Fibronectin Binding Protein BBK32 of the Lyme Disease Spirochete Promotes Bacterial Attachment to Glycosaminoglycans. Infect. Immun. 2006;74:435–441. doi: 10.1128/IAI.74.1.435-441.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Singh B., Su Y.C., Riesbeck K. Vitronectin in Bacterial Pathogenesis: A Host Protein Used in Complement Escape and Cellular Invasion. Mol. Microbiol. 2010;78:545–560. doi: 10.1111/j.1365-2958.2010.07373.x. [DOI] [PubMed] [Google Scholar]