Abstract
Aeromonas salmonicida containing the cloned gene for proaerolysin secretes the protein via the type II secretory pathway. Here we show that altering a region near the beginning of aerA led to a dramatic increase in the amount of proaerolysin that was produced and that a large amount of the protein was cell associated. All of the cell-associated protein had crossed the cytoplasmic membrane, because the signal sequence had been removed, and all of it was accessible to processing by trypsin during osmotic shock. Enlargement of the periplasm was observed by electron microscopy in overproducing cells, likely caused by the osmotic effect of the very large concentrations of accumulated proaerolysin. Immunogold electron microscopy localized nearly all of the proaerolysin in the enlarged periplasm; however, only half of the protoxin was released from the cells by osmotic shocking. Cross-linking studies showed that this fraction contained normal dimeric proaerolysin but that proaerolysin in the fraction that was not shockable had not dimerized, although it appeared to be correctly folded. Both periplasmic fractions were secreted by the cells; however, the nonshockable fraction was secreted much more slowly than the shockable fraction. We estimated a rate for maximal secretion of proaerolysin from the bacteria that was much lower than the rates that have been estimated for inner membrane transit, which suggests that transit across the outer membrane is rate limiting and may account for the periplasmic accumulation of the protein. Finally, we show that overproduction of proaerolysin inhibited the release of the protease that is secreted by A. salmonicida.
Many gram-negative bacteria use type II secretion, first identified in Klebsiella oxytoca (25), to release proteins into the environment. These proteins cross the inner membrane while still unfolded by means of the well-characterized Sec system (for a recent review, see reference 9), directed by N-terminal signal sequences that are removed either co- or posttranslationally (17). Surprisingly, folding occurs in the periplasm before the next step in the process, which is transit across the outer membrane by means of a poorly understood process that requires a large number of components.
Aeromonas spp. use type II secretion to release the lipolytic enzyme GCAT (3) and the inactive precursor of the channel-forming toxin aerolysin (13, 15). It is likely that other proteins, including at least one protease, are also secreted in this way (34). Proaerolysin secretion has been studied using Aeromonas hydrophila expressing the chromosomally encoded aerA gene or using Aeromonas salmonicida expressing cloned, plasmid-borne A. hydrophila aerA. Proaerolysin was the first protein secreted by the type II pathway that was shown to pass through the periplasm on its way out of the cell (13), and it has been shown more recently that it is there that the protein folds and even dimerizes before it is translocated across the outer membrane (12). Periplasmic concentrations of proaerolysin are much higher in A. salmonicida expressing the cloned gene than in A. hydrophila, but they do not appear to reduce the secretion of chromosomally encoded GCAT or protease by A. salmonicida, indicating that if the proteins are secreted by the same pathway, either there is no competition for the secretory machinery or there is not enough proaerolysin in the periplasm for competition to occur (34).
It has been observed that the beginning of the aerA gene contains a sequence that could form a secondary structure that could reduce the rate of transcription or translation of the gene (7). In this communication we show that altering this sequence to prevent the structure from forming leads to a striking increase in the amount of proaerolysin produced by the A. salmonicida strain containing the cloned gene. This increase results in the accumulation of a very large amount of cell-associated proaerolysin, which in turn has several important consequences for the cell.
MATERIALS AND METHODS
Culture conditions.
CB3/pNB5 (31), CB3/pγ123 (7), and Rif-1/pγ123 (this study) were grown in Luria-Bertani medium supplemented with Davis salts (19) and 0.2% (wt/vol) glucose and containing 40 μg of rifampin/ml and 40 μg of kanamycin/ml. Ampicillin (100 μg/ml) was added for selection. All clones were grown at 27°C with mild agitation (250 rpm). Overnight cultures were subcultured at a 1/100 dilution into fresh media and induced with isopropyl-β-d-thiogalactopyranoside (IPTG) once the cells had entered the log phase.
Cell fractionation.
Osmotic shock was carried out according to the procedure of Willis et al. (32). Cells were centrifuged and resuspended in sucrose shock solution (20% [wt/vol] sucrose, 33 mM Tris-HCl, 1 mM EDTA [pH 7.5]). After 5 min of incubation at room temperature (RT), the cells were pelleted and subjected to osmotic shock by rapidly suspending them in ice-cold distilled H2O containing 1 mM phenanthroline. The samples were then incubated on ice for 2 min. Finally, the cells were centrifuged to separate the shock fluid from the shocked cells.
Treatment with lysozyme-EDTA involved resuspending harvested cells in a mixture of 20% (wt/vol) sucrose, 33 mM Tris-HCl, 1 mM EDTA, and 70 μg of lysozyme/ml (pH 7.5). The samples were then incubated at RT for 15 min before being centrifuged to remove the lysozyme solution. The cells were shocked in ice-cold H2O containing 1 mM phenanthroline and finally centrifuged to separate the supernatant and cells.
Chloroform extraction, cell fractionation using polymyxin B, and Triton X-100 extraction were all performed according to the methods of Thorstenson et al. (30). During chloroform extraction, cells were harvested and resuspended in TE buffer (10 mM Tris-HCl, 1 mM EDTA [pH 8.0]). An equal volume of chloroform was added, and the cells were vortexed briefly. The samples were then diluted 10-fold with TE buffer and incubated on ice for 30 min. Finally, the cells and supernatant were separated by centrifugation.
Fractionation of cells with polymyxin B was performed by resuspending cells in a solution containing 0.5 M sucrose, 0.2 M Tris-HCl (pH 8.0), 0.5 mM EDTA, and 2 μg of polymyxin B/ml. The samples were then incubated on ice for 30 min and centrifuged to recover the supernatant fraction.
Triton X-100 extraction involved first harvesting induced cells and then resuspending them in TEX buffer (50 mM Tris-HCl, 3 mM EDTA, and 0.025% Triton X-100 [pH 8.0]). The samples were incubated on ice for 30 min, pelleted, and then washed with 1 volume of TEX buffer. The supernatants were then pooled.
French pressing.
Cells were harvested by centrifugation at an optical density at 600 nm of approximately 2 4 h after induction with 0.1 mM IPTG. They were resuspended to their original volume in 20 mM HEPES–0.15 M NaCl–10 μg of DNase/ml–10 μg of RNase/ml (pH 7.4) and passed through a prechilled French pressure cell three times at a pressure of 1,100 kg/cm3 (24). The resulting lysate was centrifuged at 39,000 rpm (260,000 × g) for 2 h. The pellet containing the cell envelope was suspended in 20 mM HEPES–0.15 M NaCl (pH 7.4). A rabbit polyclonal antibody prepared against the A. hydrophila equivalent of OmpF that cross-reacts with the corresponding A. salmonicida porin was used as an outer membrane marker.
Electron microscopy and immunogold labeling.
CB3/pγ123 cells that had been induced with 0.1 mM IPTG were harvested and fixed in primary glutaraldehyde fixative containing 0.1% glutaraldehyde, 4% paraformaldehyde, 150 mM NaCl, and 200 mM NaH2PO4 (pH 7.4). Fixation continued for 1 h on ice. After this time, cells were postfixed in 1% OsO4–150 mM NaCl–200 mM NaH2PO4 (pH 7.4). Postfixation was also carried out for 1 h on ice. Cells were then dehydrated in a graded series of ethanol and then propylene oxide before being embedded in Epon-Araldite resin (Marivac). Thin sections (thicknesses, 60 to 90 nm) were cut on an ultramicrotome using glass knives and placed on Formvar-coated nickel grids.
Sections were etched for 30 min using 1% sodium metaperiodate. Blocking was then carried out for 15 min in fetal calf serum diluted 1/20 in a solution containing 0.5% bovine serum albumin, 0.05% Tween 20, and phosphate-buffered saline (PBS). Sections were labeled for 1 h at RT with an antiaerolysin polyclonal antiserum diluted 1/1,000 in the bovine serum albumin-Tween 20-PBS, followed by labeling with the secondary antibody, a 10-nm-diameter gold particle-conjugated goat anti-rabbit immunoglobulin G (British BioCell) diluted 1/50. After 1 h, the sections were postfixed in primary glutaraldehyde fixative for 15 min. The sections were stained with 2% aqueous uranyl acetate and counterstained with 0.1% lead citrate. Samples were examined with a Hitachi 7000 transmission electron microscope using an accelerating voltage of 75 kV.
Proaerolysin secretion.
Two hours following induction with 0.1 mM IPTG, cells were harvested and then resuspended in fresh medium containing 120 μg of chloramphenicol/ml. Incubation continued at 27°C. At the indicated time intervals, samples were taken and the cells were osmotically shocked. The presence of proaerolysin in the shock fluid and shocked cells was then assayed by both immunoblotting and hemolytic titer assay.
Hemolytic titers.
Proaerolysin concentrations were determined according to our published procedure (13). Briefly, the toxin was activated by incubation with 20 μg of trypsin/ml for 10 min at RT. After this time, serial twofold dilutions were made in 96-well plates. Washed human erythrocytes (0.8%) were then added to each well, and the plates were incubated at 37°C. The degree of cell lysis was then measured and compared to that of known quantities of proaerolysin.
Colorimetric assays.
The β-lactamase activity in cell extracts was assayed spectrophotometrically using the synthetic substrate CENTA (Calbiochem) according to the method of Jones et al. (16). Isocitrate dehydrogenase activity was determined as described by Smith et al. (29). Protease activity in the supernatant of Rif-1/pγ123 cultures was assayed according to the method of Young and Broadbent (36). Total protein was determined using the dye binding assay of Bradford (2).
Electrophoresis and Western blotting.
Proteins were separated on 12% acrylamide slabs by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) according to the method of Neville (23). The proteins were either stained with Coomassie brilliant blue or electroblotted onto nitrocellulose membranes. Membranes were blocked overnight at 4°C in PBS containing 0.5% Tween 20 and 0.5% skim milk. In order to detect proaerolysin, the membranes were incubated with a monoclonal or polyclonal antibody against the toxin (diluted 1:4,000 in PBS containing 0.5% Tween 20) for 1 h at RT, followed by incubation with anti-mouse or anti-rabbit horseradish peroxidase conjugate (Amersham). The blots were then developed by enhanced chemiluminescence according to the instructions of the manufacturer (NEN Life Science).
RESULTS
Removal of potential secondary structure increases proaerolysin production.
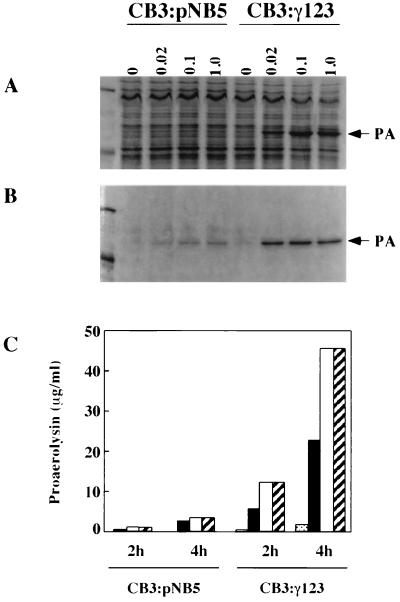
Sequence analysis of the 5′ end of the aerolysin gene (aerA) has revealed the presence of two inverted repeats upstream of the start site (14). The second of these repeats is part of a stretch of 27 bp (including the ribosome binding site and start codon) that, when transcribed, could form an extensive secondary structure (Fig. 1) and affect expression of the aerolysin gene at the level of either transcription or translation (1, 6). In an effort to determine if this region does indeed affect proaerolysin production, PCR was used to remove half of the loop sequence and replace it with a new sequence (7). We then compared the levels of proaerolysin produced by A. salmonicida CB3/pNB5 cells expressing intact aerA (aerA+loop) with those produced by the CB3/pγ123 cells expressing the modified gene (aerA−loop). The results presented in Fig. 2 indicate that far more proaerolysin was detected in both the cells and the culture supernatant when the loop was not present.
FIG. 1.
Potential secondary structure located at the beginning of the aerolysin gene. (A) Schematic presentation of the 5′ end of the aerolysin mRNA. The ribosome binding site and start codon (indicated in bold) are confined within the stem structure. (B) New DNA sequence used in engineering the pγ123 construct. This sequence replaces the original sequence that encoded the stem structure.
FIG. 2.
Comparison of proaerolysin production in CB3/pNB5 and CB3/pγ123 cells. Cells (A) and culture supernatants (B) were collected after 4 h of induction with the indicated concentrations of IPTG (in millimolar units). Proteins were separated using SDS-PAGE and stained with Coomassie brilliant blue as described in Materials and Methods. The arrows indicate the position of proaerolysin (PA). Corresponding amounts of cells and supernatants were applied to each lane. Molecular mass markers in the leftmost lane are 97.4 and 45 kDa. (C) Comparison of the amounts of proaerolysin found within culture supernatants after 2 and 4 h of induction, expressed as micrograms of proaerolysin per milliliter of culture. At each time point, the bars correspond to IPTG concentrations of 0, 0.02, 0.1, and 1.0 mM (left to right). Proaerolysin concentrations were determined by hemolytic titration as described in the text. Results are representative of several assays.
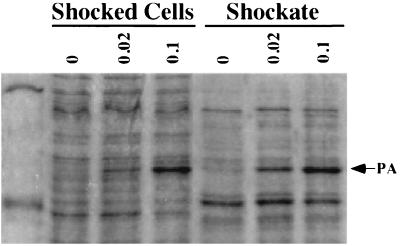
We have previously shown that there is a sizable amount of intracellular proaerolysin in CB3/pNB5 cells (cells expressing the wild-type construct aerA+loop), and we have found that nearly all of it (approximately 1.2 × 10−15 g per cell [1.4 × 104 molecules per cell] is located within the periplasm, as it can be released from the cells by osmotic shock (18, 34). Assuming that the periplasm makes up approximately 10% of the volume of the cell, this corresponds to a periplasmic concentration of 15 mg of proaerolysin/ml. Far more proaerolysin was found in the shockable fraction of CB3/pγ123 cells (expressing the new construct aerA−loop) when they were grown under similar conditions. For example, by measuring the amount of proaerolysin released by shocking, we calculated that at least 14 × 10−15 g (1.6 × 105 molecules) of proaerolysin could be shocked from each cell 2 h following induction with 0.1 mM IPTG, corresponding to periplasmic concentrations on the order of 165 mg/ml. Remarkably, this accounted for only approximately half of the total intracellular proaerolysin associated with CB3/pγ123 cells. The remainder of the cell-associated protoxin was not released by osmotic shock (Fig. 3).
FIG. 3.
Accumulation and distribution of proaerolysin within CB3/pγ123 cells. Cells were collected after 4 h of induction with the indicated concentrations of IPTG (millimolar) and subjected to osmotic shock. Shocked cells and shockates (periplasmic fractions) were analyzed as described in the legend to Fig. 2. The arrow indicates the position of proaerolysin (PA). Molecular mass markers in the leftmost lane are 97.4 and 45 kDa.
Secretion of cell-associated proaerolysin.
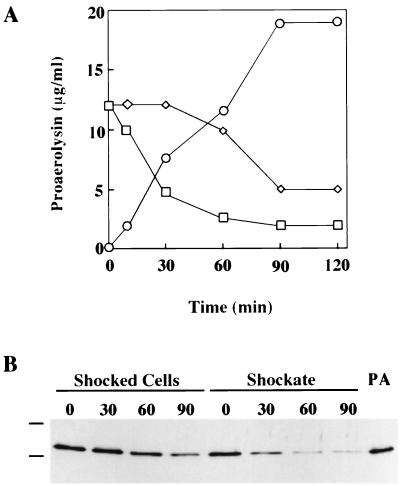
Letellier et al. (18) have previously reported that the periplasmic pool of proaerolysin found within CB3/pNB5 cells is rapidly secreted into the extracellular environment. Indeed, such cells treated with chloramphenicol to arrest further synthesis of the protein are able to secrete 90% of this proaerolysin pool within the first 20 min following addition of the poison. We were interested in measuring the ability of CB3/pγ123 cells to secrete the much larger amount of proaerolysin that they had accumulated. To accomplish this, CB3/pγ123 cells were first induced for 2 h with 0.1 mM IPTG in order to accumulate the intracellular pool. The cells were then moved into fresh medium, and chloramphenicol was added. Changes in the levels of proaerolysin were then monitored in the shockable and nonshockable cell-associated pools as well as in the culture supernatant. In agreement with the results of Letellier et al., a rapid decrease in the shockable pool of proaerolysin from the CB3/pγ123 cells and a corresponding increase in the amount of protein appearing in the culture supernatant was observed. More than 60% of the shockable pool had left the cells after 30 min of incubation (Fig. 4). The results in Fig. 4 also indicate that the cell-associated pool of proaerolysin that was not shockable behaved very differently. It did not decrease at all during the first 30 min after chloramphenicol addition. Most of it had left the cell after 90 min, but some was still cell associated at our longest time point. It should be noted that the decreases in the amounts of proaerolysin recovered in the two cell-associated fractions were due not to breakdown of the protoxin but rather to secretion, as they corresponded to the increase in the amount of protoxin recovered in the culture supernatant. The near absence of bands migrating faster than that of proaerolysin in Western blots obtained with either a polyclonal or a monoclonal antiaerolysin antibody is another indication that neither cell-associated fraction was undergoing proteolysis.
FIG. 4.
Time-dependent changes in proaerolysin levels in cell-associated and culture supernatant fractions. CB3/pγ123 cells were induced for 2 h with 0.1 mM IPTG and then treated with chloramphenicol according to the procedure described in the text. (A) Proaerolysin levels in shocked cells (◊), the shockate (□), and the culture supernatant (○). Proaerolysin concentrations were determined by hemolytic titer assay as described in the text. (B) Immunoblot of the shocked cells and shockate samples taken at the indicated time intervals (in minutes) following the addition of chloramphenicol. Purified proaerolysin (PA) is visible in the rightmost lane, and horizontal lines mark the locations of molecular mass markers (77 and 50 kDa). Proteins found in the shocked cells and shockates were separated by SDS-PAGE and immunoblotted as described in Materials and Methods.
Folding and dimerization of cell-associated proaerolysin.
One possible explanation for the delay in secretion of the pool of proaerolysin associated with the shocked cells is that the protein in this pool had not correctly folded. To examine this possibility, induced CB3/pγ123 cells were subjected to osmotic shock in the presence of 40 μg of trypsin/ml. Correctly folded proaerolysin is converted to aerolysin by trypsin, and aerolysin resists further breakdown by the protease (10). Improperly folded protein should not be correctly processed by trypsin; rather, it should be reduced to smaller peptides. The results in Fig. 5 show that all of the proaerolysin associated with the cells was converted to aerolysin by shocking in the presence of trypsin, and there was little or no evidence of further breakdown. Isocitrate dehydrogenase activity remained nearly entirely associated with the shocked cells during this procedure (data not shown), indicating that the cells had not lysed. Thus, we can conclude not only that the cell-associated pool of proaerolysin is correctly folded but also that it is not on the cytoplasmic side of the plasma membrane, where it would have been inaccessible to the protease. Other evidence that this proaerolysin had crossed the inner membrane was the observation that it migrated with purified proaerolysin by SDS-PAGE (Fig. 5). We would have expected to see a band of higher molecular weight, corresponding to preproaerolysin (containing the 23-amino-acid signal sequence), if the protein had not crossed the inner membrane.
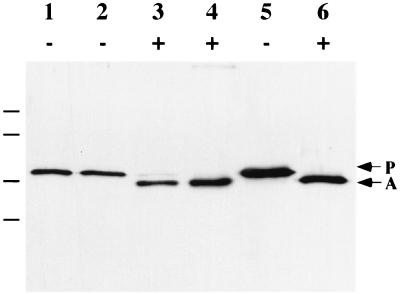
FIG. 5.
Activation of the cell-associated pool of proaerolysin. CB3/pγ123 cells were induced for 2 h with 0.1 mM IPTG and then osmotically shocked in the presence (+) or absence (−) of 40 μg of trypsin/ml as indicated. Lanes 1 and 3, shockate; lanes 2 and 4, shocked cells; lanes 5 and 6, purified proaerolysin. Samples were separated by SDS-PAGE and immunoblotted. Arrows indicate the positions of proaerolysin (P) and aerolysin (A), and horizontal lines indicate the locations of the molecular mass markers (from top: 103, 77, 50, and 34.3 kDa).
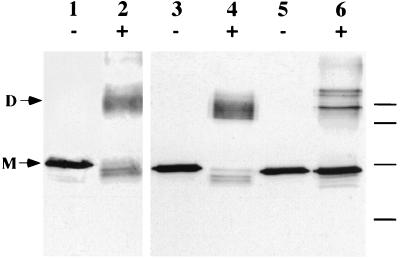
Proaerolysin is a dimer in solution (31), and previous results from our laboratory have indicated that dimerization occurs in the periplasm and is required for secretion to occur (12). When CB3/pγ123 cells were shocked in the presence of the cross-linking reagent dithiobis(succinimidylpropionate) (DSP), the majority of the proaerolysin recovered in the shockable fraction migrated as a covalent dimer in an SDS-PAGE gel (Fig. 6), indicating that the protoxin in this pool was dimeric. Surprisingly, however, only a small fraction of the proaerolysin that remained cell associated after shocking migrated as a dimer after cross-linking. Most of this protein migrated as a monomer, although we observed two additional bands that were not detected in the shockable fraction. These results suggest that the cell-associated pool was not dimeric and that at least part of it might be associated with other cell components.
FIG. 6.
Chemical cross-linking of CB3/pγ123 cells. CB3/pγ123 cells were induced for 2 h with 0.1 mM IPTG and then osmotically shocked in the presence (+) or absence (−) of 0.25 mM DSP as indicated. Lanes 1 and 2, purified proaerolysin; lanes 3 and 4, shock fluid; lanes 5 and 6, shocked cells. Samples were separated by SDS-PAGE and immunoblotted. Horizontal lines mark the locations of molecular mass markers (from top; 116.0, 80.0, 52.5, and 34.9 kDa). D, dimer; M, monomer.
Further characterization of the cell-associated fraction.
The fact that the cell-associated pool of proaerolysin was correctly folded and on the periplasmic side of the cytoplasmic membrane appeared to be inconsistent with the fact that it was not released by osmotic shock. This was not because the shocking procedure was inefficient; we found that virtually all of the CB3/pγ123 β-lactamase (a periplasmic marker) was recovered in the shockate (not shown). Other procedures which have previously been shown to selectively release periplasmic proteins were also not successful in releasing all of the cell-associated pool of proaerolysin (data not shown). These included polymyxin B treatment (30), chloroform treatment (30) and treatment with lysozyme-EDTA (35).
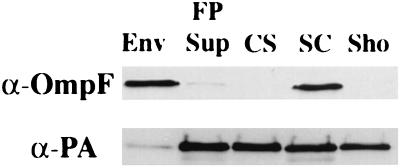
We next determined whether association with the inner or outer membranes could account for the resistance of the cell-associated fraction to osmotic shocking. The results presented in Fig. 7 show that, although a large fraction of the proaerolysin was cell associated after shocking, only a small amount was recovered with the cell envelope after French pressing. Instead, nearly all of the protein was found in the soluble fraction. Triton X-100 extraction (30), which also causes cell disruption, resulted in the solubilization of the cell-associated proaerolysin fraction (data not shown).
FIG. 7.
Comparison of the distributions of proaerolysin after shocking or French pressing induced CB3/pγ123 cells. Cells were induced with 0.1 mM IPTG for 4 h and then either shocked or French pressed as described in the text. Equivalent amounts of all fractions were electrophoresed and blotted with anti-OmpF rabbit polyclonal serum (α-OmpF) (A) or antiaerolysin mouse monoclonal serum (α-PA) (B). Samples were applied in the following order: envelope (Env), French press supernatant (FP Sup), culture supernatant (CS), shocked cells (SC), and shockate (Sho).
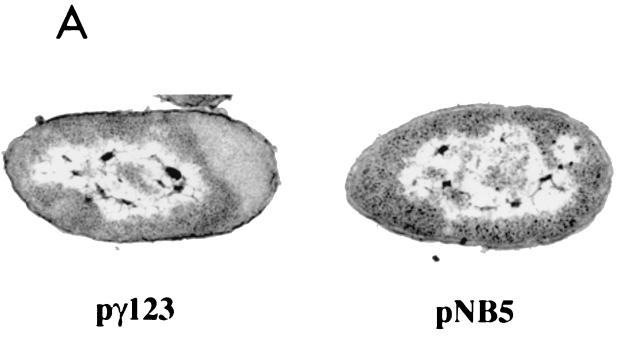
Proaerolysin accumulates within an enlarged periplasm.
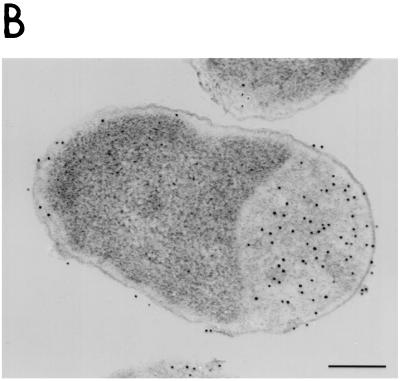
We used electron microscopy to examine CB3/pγ123 cells induced with 0.1 mM IPTG. We found no evidence for the presence of inclusion bodies (11, 27) or periplasmic vesicles; however, our results did reveal that the periplasm of the cells was enlarged at one pole (Fig. 8). No enlargement was observed in CB3/pNB5 cells induced in the same manner (Fig. 8A), suggesting that the periplasmic swelling in the CB3/pγ123 cells was a consequence of the increased amounts of proaerolysin that they accumulated. Immunogold labeling of thin sections from CB3/pγ123 cells (again induced with 0.1 mM IPTG) with polyclonal antisera raised against proaerolysin revealed that most of the proaerolysin was indeed localized within the enlarged periplasmic compartment (Fig. 8B).
FIG. 8.
Periplasmic accumulation of proaerolysin in CB3/pγ123 cells. (A) Electron micrographs comparing strains CB3/pγ123 and CB3/pγNB5 after induction with IPTG as described in Materials Methods. (B) Immunogold labelling of thin sections of induced CB3/pγ123 cells. Bar, 0.25 μm.
Overexpression of proaerolysin reduces protease release.
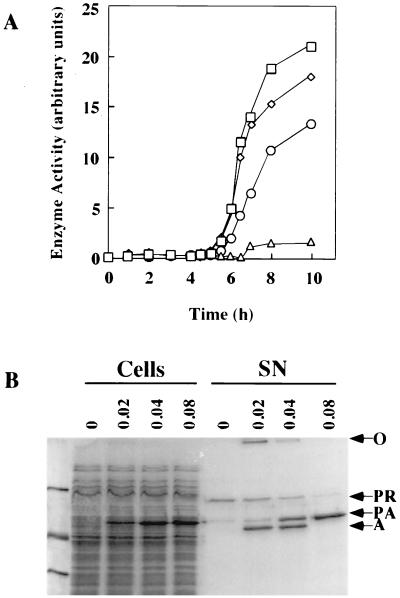
We have previously shown that secretion of plasmid-encoded proaerolysin by A. salmonicida Rif-1/pNB5 cells has little or no effect on the apparent rate of secretion of the bacterium's chromosomally encoded extracellular protease (34). In order to determine if the much larger amounts of proaerolysin produced by CB3/pγ123 cells could affect protease secretion, we introduced the plasmid containing the aerA−loop gene into the A. salmonicida strain Rif-1. Unlike strain CB3, this strain secretes a chromosomally encoded protease (4). Induction of the resulting strain, Rif-1/pγ123, clearly demonstrated that as the production of proaerolysin increased there was a dramatic decrease in the secretion of the protease (Fig. 9). The protease is clearly visible as a band migrating at 90 kDa after SDS-PAGE of the supernatant of uninduced cells (Fig. 9B), and its activity was readily detected (Fig. 9A). As the amount of proaerolysin produced by the cells increased, the amount of supernatant protease declined, and cells induced with 0.08 mM IPTG secreted less than 10% of the protease separated by uninduced cells. The effect of protease production on aerolysin activation is worth noting (Fig. 9B). At the lowest IPTG concentration, where substantial amounts of the protease and proaerolysin were secreted, most of the protoxin was converted to aerolysin by the protease. Oligomeric aerolysin was also observed, migrating at the top of the gel. Considerable processing and some oligomerization were also observed in supernatant samples induced with 0.04 mM IPTG, but at 0.08 mM IPTG, there was not enough protease secreted to process the proaerolysin and there was no evidence of aerolysin or the oligomer.
FIG. 9.
Effect of proaerolysin production on the secretion of a chromosomally encoded protease. (A) Proteolytic activity in the supernatants of Rif-1/pγ123 cultures induced with increasing IPTG concentrations of 0 mM (□), 0.02 mM (◊), 0.04 mM (○), and 0.08 mM (▵). Proteolytic activity was determined using the Hide powder Asuze assay; 1 arbitrary unit corresponds to an optical density at 595 nm of 1.0. (B) Distribution of the protease and proaerolysin in Rif-1/pγ123 cells and culture supernatants. Samples were collected after 8 h of induction with the indicated (millimolar) concentrations of IPTG and analyzed as described in the legend to Fig. 2. Arrows indicate the positions of the aerolysin oligomer (○), chromosomal protease (PR) proaerolysin (P), and aerolysin (A). Molecular mass markers in the leftmost lane are (from the top) 97.4, 45, and 31 kDa.
DISCUSSION
Replacing part of the aerA DNA sequence so that stem-loop formation was prevented resulted in a dramatic increase in the amount of proaerolysin produced by A. salmonicida containing the new construct. This result suggests that the loop may normally provide the cell with a means to vary aerolysin production in response to environmental changes. An analogous example of such a system may be the Escherichia coli heat shock factor ς32, encoded by the rpoH gene. Because of the secondary structure at the 5′ end of the rpoH mRNA, production of ς32 ordinarily proceeds at low rates. Melting of the loop as a result of an increase in temperature results in an increase in the rate of translation of the mRNA, leading to increased levels of ς32 (20, 21).
Overproduction of proaerolysin after removal of part of the loop sequence led to accumulation of the protein and a number of important consequences to the cell. The fact that the protein accumulated in the periplasm after crossing the inner membrane suggests that transit across the outer membrane, rather than transit across the inner membrane via the Sec system, was the rate-limiting step in the secretory process. This conclusion was supported by our estimate of the rate at which proaerolysin appears in the culture supernatant of overproducing cells. We determined the rate in two ways: by measuring the increase with time in the amount of proaerolysin in the culture supernatant during the logarithmic growth of induced cells and by measuring the rate of decline of the periplasmic pool (and the corresponding appearance of proaerolysin in the supernatant) after cells had been induced to accumulate proaerolysin and had then been transferred to fresh medium containing chloramphenicol to block further protein synthesis. In both cases the rate of secretion was determined to be approximately 103 molecules cell−1 min−1. This is much lower than the rate at which polypeptides are thought to cross the E. coli inner membrane via the Sec system. In that system, it has been estimated that approximately 4 × 104 polypeptide molecules are transferred per min, based on 500 Sec translocons per cell and 80 polypeptides/translocon/min (26). If the rate of inner membrane transfer is similar for A. salmonicida, it is higher than the maximal rate of proaerolysin appearance outside the cell and would account for the periplasmic accumulation of the protein that we observed.
The accumulation of proaerolysin in the shockable fraction 2 h after induction with 0.1 mM IPTG was estimated to be 1.6 × 105 molecules cell−1, and it increased to 2.5 × 105 molecules cell−1 after 4 h. The total amount of protein in the shockable fraction nearly doubled in the first 2 h after induction due to the accumulation of proaerolysin, and it had more than doubled after 4 h of induction, when proaerolysin accounted for more than half of the total protein released by shocking. The increase in osmotic pressure accompanying the increased periplasmic protein levels presumably accounted for the increase in the size of the periplasm that we observed. Periplasmic expansion due to proaerolysin overproduction was consistently restricted to the poles of the cells; it was not observed along their lengths. This may be evidence of periplasmic compartmentalization (reviewed in reference 28) or of localization of secretion to the poles; however, it is worth noting that mild osmotic upshock has been shown to cause the formation of plasmolysis bays at the poles of E. coli (22, 33).
Overexpression of proaerolysin quickly resulted in a decline in the amount of protease that was secreted by the bacteria. We did not determine whether this was because the Sec system was being saturated by outgoing proaerolysin or whether it was because a chaperone or some component required for outer membrane transit was being titrated by the protoxin. However, our observation that proaerolysin accumulated in large quantities in the periplasm suggests that the latter explanation is more likely. It seems unlikely that the effect on protease was due to a nonspecific inhibitory effect of proaerolysin on the secretory system, since protease release was decreased while the cells were still actively releasing proaerolysin.
All of our results suggest that there were two distinct pools of proaerolysin in the overexpressing A. salmonicida cells. One of these pools appears to be the same shockable pool of proaerolysin that we have described previously for A. hydrophila and A. salmonicida (13, 34). This pool contains correctly folded dimeric proaerolysin, which can leave the cell very rapidly and which is likely to be part of the normal secretory pathway. The other pool of proaerolysin behaves quite differently from the shockable pool, and it has not been described previously nor has a comparable pool been described for other systems. The presence of this pool appears to be a consequence of the very high expression levels of the protoxin upon removal of the secondary structure from the gene. We can conclude that this pool is also on the periplasmic side of the inner membrane, because it contains virtually no preproaerolysin, evidence that the signal sequence has been removed, and it is entirely accessible to proteolytic processing by trypsin under conditions where cytoplasmic contents are not released. The protein in this pool also appears to be correctly folded because it is correctly processed by trypsin to aerolysin, which is not degraded further. It is also not degraded by periplasmic proteases, in contrast to a number of proaerolysin variants that we have studied that are unable to fold (unpublished observations). Preliminary evidence obtained from pulse-chase experiments, not shown here, indicates that proaerolysin moves from this pool into the shockable pool before it leaves the cell.
Using a chemical cross-linking agent, we were able to confirm our earlier findings that proaerolysin in the shockable fraction of A. salmonicida is dimeric, as is the secreted form of the protein, but we were very surprised to discover that the nonshockable fraction did not appear to have dimerized. Most of this fraction still migrated as a monomer on SDS-PAGE after cross-linking, although some higher-molecular-weight bands were also observed, suggesting that the protein might be associated with other periplasmic components. These bands were not observed after the cross-linking of the shockable fraction. The fact that this pool of proaerolysin had not dimerized might account for its very slow release from the cell, as we have shown earlier that dimerization is required for the secretion of proaerolysin by A. salmonicida (12). Why the protein in this pool was still monomeric is not at all clear, especially considering that it was correctly folded. It is conceivable that disulfide bond isomerases or other chaperones that could be required for dimer formation are limiting under these circumstances. It is also possible that this protein pool was associated with the inner or outer membrane in a way that prevented dimerization. This explanation might also account for the fact that it was not released by osmotic shock; however, the association would have to be loose enough to account for the fact that nearly all of the cell-associated proaerolysin was soluble after French pressing. It is hard to imagine that the cell-associated pool is associated with a periplasmic protein that prevents its dimerization, or its release from the cell by shocking, if only because it seems very unlikely that another protein could be expressed in large-enough amounts to allow a stoichiometric interaction with proaerolysin.
The barrier properties of peptidoglycan, which have recently been reviewed (8), could conceivably be responsible for the generation of two periplasmic pools of proaerolysin. The rate of production of periplasmic proaerolysin may be so high that diffusion of the protein through the peptidoglycan barrier, however it occurs, becomes rate limiting: the two pools may represent proaerolysin on both sides of the sacculus. Evidence obtained from studies of the peptidoglycan of E. coli suggests that only molecules that are smaller than 25 kDa can move freely through the meshwork of the sacculus (5). Thus, some of the proaerolysin, which is 50 kDa as a monomer, may not be released by osmotic shock because it is on the wrong side of the sacculus, but it may be processed by trypsin added during shocking because the protease is small enough (23.5 kDa) to rapidly penetrate the meshwork. French pressing would release all of the cell-associated proaerolysin, as we observed, by destroying the barrier.
ACKNOWLEDGMENTS
This work was supported by a grant from the Natural Sciences and Engineering Research Council of Canada.
The technical assistance of Vanessa Thompson is gratefully acknowledged.
REFERENCES
- 1.Abe H, Aiba H. Differential contributions of two elements of rho-independent terminator to transcription termination and mRNA stabilization. Biochimie. 1996;78:1035–1042. doi: 10.1016/s0300-9084(97)86727-2. [DOI] [PubMed] [Google Scholar]
- 2.Bradford M M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 3.Brumlik M J, van der Goot F G, Wong K R, Buckley J T. The disulfide bond in the Aeromonas hydrophila lipase/acyltransferase stabilizes the structure but is not required for secretion or activity. J Bacteriol. 1997;179:3116–3121. doi: 10.1128/jb.179.10.3116-3121.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Buckley J T. Purification of cloned proaerolysin released by a low protease mutant of Aeromonas salmonicida. Biochem Cell Biol. 1990;68:221–224. doi: 10.1139/o90-029. [DOI] [PubMed] [Google Scholar]
- 5.Demchick P, Koch A L. The permeability of the wall fabric of Escherichia coli and Bacillus subtilis. J Bacteriol. 1996;178:768–773. doi: 10.1128/jb.178.3.768-773.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeSmit M H, van Duin J. Secondary structure of the ribosome binding site determines translational efficiency: a quantitative analysis. Proc Natl Acad Sci USA. 1990;87:7668–7672. doi: 10.1073/pnas.87.19.7668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Diep D B, Lawrence T S, Ausio J, Howard S P, Buckley J T. Secretion and properties of the large and small lobes of the channel-forming toxin aerolysin. Mol Microbiol. 1998;30:341–352. doi: 10.1046/j.1365-2958.1998.01068.x. [DOI] [PubMed] [Google Scholar]
- 8.Dijkstra A J, Keck W. Peptidoglycan as a barrier to transenvelope transport. J Bacteriol. 1996;178:5555–5562. doi: 10.1128/jb.178.19.5555-5562.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Economou A. Following the leader: bacterial protein export through the Sec pathway. Trends Microbiol. 1999;7:315–320. doi: 10.1016/s0966-842x(99)01555-3. [DOI] [PubMed] [Google Scholar]
- 10.Garland W J, Buckley J T. The cytolytic toxin aerolysin must aggregate to disrupt erythrocytes, and aggregation is stimulated by human glycophorin. Infect Immun. 1988;56:1249–1253. doi: 10.1128/iai.56.5.1249-1253.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Georgiou G, Telford J N, Shuler M L, Wilson D B. Localization of inclusion bodies in Escherichia coli overproducing β-lactamase or alkaline phosphatase. Appl Environ Microbiol. 1986;52:1157–1161. doi: 10.1128/aem.52.5.1157-1161.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hardie K R, Schulze A, Parker M W, Buckley J T. Vibrio spp. secrete proaerolysin as a folded dimer without the need for disulphide bond formation. Mol Microbiol. 1995;17:1035–1044. doi: 10.1111/j.1365-2958.1995.mmi_17061035.x. [DOI] [PubMed] [Google Scholar]
- 13.Howard S P, Buckley J T. Protein export by a gram-negative bacterium: production of aerolysin by Aeromonas hydrophila. J Bacteriol. 1985;161:1118–1124. doi: 10.1128/jb.161.3.1118-1124.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Howard S P, Buckley J T. Molecular cloning and expression in Escherichia coli of the structural gene for the hemolytic toxin aerolysin from Aeromonas hydrophila. Mol Gen Genet. 1986;204:289–295. doi: 10.1007/BF00425512. [DOI] [PubMed] [Google Scholar]
- 15.Jiang B, Howard S P. The Aeromonas hydrophila ExeE gene, required both for protein secretion and normal outer membrane biogenesis, is a member of a general secretion pathway. Mol Microbiol. 1992;6:1351–1361. doi: 10.1111/j.1365-2958.1992.tb00856.x. [DOI] [PubMed] [Google Scholar]
- 16.Jones R N, Wilson H W, Novick W J, Jr, Barry A L, Thornsberry C. In vitro evaluation of CENTA, a new beta-lactamase-susceptible chromogenic cephalosporin reagent. J Clin Microbiol. 1982;15:954–958. doi: 10.1128/jcm.15.5.954-958.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Josefsson L G, Randall L L. Different exported proteins in E. coli show differences in the temporal mode of processing in vivo. Cell. 1981;25:151–157. doi: 10.1016/0092-8674(81)90239-7. [DOI] [PubMed] [Google Scholar]
- 18.Letellier L, Howard S P, Buckley J T. Studies in the energetics of proaerolysin secretion across the outer membrane of Aeromonas species. J Biol Chem. 1997;272:11109–11113. doi: 10.1074/jbc.272.17.11109. [DOI] [PubMed] [Google Scholar]
- 19.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1972. [Google Scholar]
- 20.Morita M, Kanemori M, Yanagi H, Yura T. Heat-induced synthesis of ς32 in Escherichia coli: structural and functional dissection of rpoH mRNA secondary structure. J Bacteriol. 1999;181:401–410. doi: 10.1128/jb.181.2.401-410.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Morita M T, Tanaka Y, Kodama T S, Kyogoku Y, Yanagi H, Yura T. Translational induction of heat shock transcription factor ς32: evidence for a built-in RNA thermosensor. Genes Dev. 1999;13:655–665. doi: 10.1101/gad.13.6.655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mulder E, Woldringh C L. Plasmolysis bays in Escherichia coli: are they related to development and positioning of division sites? J Bacteriol. 1993;175:2241–2247. doi: 10.1128/jb.175.8.2241-2247.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Neville D M. Molecular weight determination of protein dodecyl sulphate complexes by gel electrophoresis in a discontinuous buffer system. J Biol Chem. 1971;246:6328–6334. [PubMed] [Google Scholar]
- 24.Osborn M J, Munson R. Separation of the inner (cytoplasmic) and outer membranes of gram-negative bacteria. Methods Enzymol. 1974;31:642–653. doi: 10.1016/0076-6879(74)31070-1. [DOI] [PubMed] [Google Scholar]
- 25.Pugsley A P, Kornacker M G, Pouquet I. The general protein-export pathway is directly required for extracellular pullulanase secretion in Escherichia coli K12. Mol Microbiol. 1991;5:343–352. doi: 10.1111/j.1365-2958.1991.tb02115.x. [DOI] [PubMed] [Google Scholar]
- 26.Pugsley A P. The complete general secretory pathway in gram-negative bacteria. Microbiol Rev. 1993;57:50–108. doi: 10.1128/mr.57.1.50-108.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Reverbel-Leroy C, Belaich A, Bernadac A, Gaudin C, Belaich J P, Tardif C. Molecular study and overexpression of the Clostridium cellulolyticum celF cellulase gene in Escherichia coli. Microbiology. 1996;142:1013–1023. doi: 10.1099/00221287-142-4-1013. [DOI] [PubMed] [Google Scholar]
- 28.Shapiro L, Losick R. Protein localization and cell fate in bacteria. Science. 1997;276:712–718. doi: 10.1126/science.276.5313.712. [DOI] [PubMed] [Google Scholar]
- 29.Smith H, Bron S, Van Ee J, Venema G. Construction and use of signal sequence selection vectors in Escherichia coli and Bacillus subtilis. J Bacteriol. 1987;169:3321–3328. doi: 10.1128/jb.169.7.3321-3328.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thorstenson Y R, Zhang Y, Olson P S, Mascarenhas D. Leaderless polypeptides efficiently extracted from whole cells by osmotic shock. J Bacteriol. 1997;179:5333–5339. doi: 10.1128/jb.179.17.5333-5339.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.van der Goot F G, Ausio J, Wong K R, Pattus F, Buckley J T. Dimerization stabilizes the pore-forming toxin aerolysin in solution. J Biol Chem. 1993;268:18272–18279. [PubMed] [Google Scholar]
- 32.Willis R C, Morris R G, Cirakoglu C, Schellenberg G D, Gerber N H, Furlong C E. Preparations of periplasmic proteins from Salmonella typhimurium and Escherichia coli. Arch Biochem Biophys. 1974;161:64–75. [Google Scholar]
- 33.Woldringh C L. Significance of plasmolysis spaces as markers for periseptal annuli and adhesion sites. Mol Microbiol. 1994;14:597–607. doi: 10.1111/j.1365-2958.1994.tb01299.x. [DOI] [PubMed] [Google Scholar]
- 34.Wong K R, Green M J, Buckley J T. Extracellular secretion of cloned aerolysin and phospholipase by Aeromonas salmonicida. J Bacteriol. 1989;171:2523–2527. doi: 10.1128/jb.171.5.2523-2527.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Yamato I, Anraku Y, Hirosawa K. Cytoplasmic membrane vesicles of Escherichia coli. A simple method for preparing the cytoplasmic and outer membranes. J Biochem (Tokyo) 1975;77:705–718. doi: 10.1093/oxfordjournals.jbchem.a130774. [DOI] [PubMed] [Google Scholar]
- 36.Young D B, Broadbent D A. Biochemical characterization of extracellular proteases from Vibro cholerae. Infect Immun. 1982;37:875–883. doi: 10.1128/iai.37.3.875-883.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]