Abstract
An accurate prediction of the hepatotoxicity associated with low-dose methotrexate can provide evidence for a reasonable treatment choice. This study aimed to develop a machine learning-based prediction model to predict hepatotoxicity associated with low-dose methotrexate and explore the associated risk factors. Eligible patients with immune system disorders, who received low-dose methotrexate at West China Hospital between 1 January 2018, and 31 December 2019, were enrolled. A retrospective review of the included patients was conducted. Risk factors were selected from multiple patient characteristics, including demographics, admissions, and treatments. Eight algorithms, including eXtreme Gradient Boosting (XGBoost), AdaBoost, CatBoost, Gradient Boosting Decision Tree (GBDT), Light Gradient Boosting Machine (LightGBM), Tree-based Pipeline Optimization Tool (TPOT), Random Forest (RF), and Artificial Neural Network (ANN), were used to establish the prediction model. A total of 782 patients were included, and hepatotoxicity was detected in 35.68% (279/782) of the patients. The Random Forest model with the best predictive capacity was chosen to establish the prediction model (receiver operating characteristic curve 0.97, accuracy 64.33%, precision 50.00%, recall 32.14%, and F1 39.13%). Among the 15 risk factors, the highest score was a body mass index of 0.237, followed by age (0.198), the number of drugs (0.151), and the number of comorbidities (0.144). These factors demonstrated their importance in predicting hepatotoxicity associated with low-dose methotrexate. Using machine learning, this novel study established a predictive model for low-dose methotrexate-related hepatotoxicity. The model can improve medication safety in patients taking methotrexate in clinical practice.
Keywords: low-dose methotrexate, hepatotoxicity, machine learning, prediction model, risk factor
1. Introduction
Methotrexate (MTX), a folic acid antagonist that inhibits dihydrofolate reductase in the S-phase cell cycle, was first developed as an anticancer treatment in the 1940s [1,2]. Since the 1950s, MTX has been prescribed as an immunosuppressant for treating immune system disorders, including rheumatoid arthritis, psoriasis, psoriatic arthritis, and inflammatory bowel diseases [3]. The overall prevalence of rheumatoid arthritis is 0.24% to 1.1% [4,5,6]. Similarly, a population-based study in the United States found that psoriasis rates increased significantly from 50.8 cases per 100,000 (from 1970 to 1974) to 100.5 cases per 100,000 (from 1995 to 1999) [7]. A worldwide review showed that the prevalence of psoriasis ranged from 0.5 to 11.4% in adults [8]. MTX is now recommended as a first- or second-line treatment for many immune system diseases [9,10,11,12,13,14,15]. Although several biological agents have emerged for immune system diseases in the last two decades, such as adalimumab [16], infliximab [17], canakinumab [18], ustekinumab [19], and secukinumab [19], MTX is still widely used due to its efficacy, low cost, and ease of administration. MTX can be administered orally or subcutaneously as a weekly treatment regimen [11].
Due to lower doses of MTX, life-threatening adverse drug effects (ADE) are rarely observed in MTX treatment for immune system diseases. However, severe ADEs can still occur, especially hepatotoxicity [20]. Abnormal serum levels of alanine aminotransferase (ALT) or aspartate aminotransferase (AST) occurred in 23.47% (315/1342) of patients treated for MTX-treated rheumatoid arthritis [21]. Liver enzyme abnormalities are the leading cause of dose modification or discontinuation of MTX [22]. Furthermore, a systematic review indicated that 33% of patients with psoriasis who received low-dose MTX had liver disease progression, such as liver fibrosis [23]. Therefore, it is crucial to clarify the incidence of liver ADE in MTX-treated patients. Risk factors can affect MTX therapies. These risk factors include alcohol use, history of liver disease, obesity, type 2 diabetes, history of significant exposure to hepatotoxic drugs or chemicals, lack of folate supplementation, and hyperlipidemia [24,25]. However, current research lacks an assessment of the impact of these risk factors. Furthermore, there is a lack of research exploring unknown risk factors and establishing predictive models for hepatotoxicity associated with low-dose MTX.
Machine learning is one of the fastest-growing technical fields [26] and has been widely used in medical fields, such as medical diagnosis and prediction of disease risks [27,28,29]. Machine learning can promote data-driven estimation when selecting multiple variables and processing complex nonlinear relationships among multidimensional variables [30]. Therefore, machine learning can increase the precision of prediction models, especially for analyzing large datasets with many variables [31,32]. The study aimed to compare eight machine learning methods to identify the most optimal model to predict hepatotoxicity and risk factors associated with low-dose MTX.
2. Materials and Methods
2.1. Study Setting and the Study Population
This retrospective study was conducted at the West China Hospital of Sichuan University, a large tertiary teaching hospital in China. This hospital uses an electronic medical record (EMR) and bar code systems to document medication administrations. The study inclusion criteria were (1) patients with immune system diseases and (2) treated with low-dose MTX (≤30 mg per week) [33] during hospital stays at the West China Hospital of Sichuan University. Patients who were treated with other doses of MTX were excluded. The study period was from 1 January 2018 to 31 December 2019.
2.2. Data Extraction
A two-stage review process for medical records was conducted to identify the presence of hepatotoxicity. In the first stage, two trained clinical pharmacists (Hu and Wang) independently reviewed each medical record for the presence of hepatotoxicity. The following sections of the charts were reviewed: basic patient information, diagnostic and progress notes, medication charts, laboratory data, surgical records, nursing flow sheets, and admission and discharge documents. In the second stage, a physician reviewed all medical records identified in the first stage to determine the presence of hepatotoxicity. Disagreements were resolved through a team discussion. Because the clinical interventions usually preceded patients reaching clinical diagnostic criteria of drug-induced liver injury [34], hepatotoxicity was defined as elevated liver enzymes > 1.25 of the upper limits of normal (ULN) and outcomes of liver failure, fibrosis, cirrhosis, or death. The hospital standard cut-off values for ALT are 40 IU/L for women and 50 IU/L for men. AST values are 35 IU/L for women and 40 IU/L for men. Alkaline phosphatase (ALP) values are 135 IU/L for women and 160 IU/L for men. Data collection was carried out from September 2020 to January 2021.
Based on data from included patients’ records, risk factors were screened from multiple patient characteristics to establish a prediction model. Specifically, the following variables were documented: age, gender, height, weight, alcohol use, history of liver diseases (hepatitis B, hepatitis C, and nonalcoholic fatty liver disease), admission, discharge, blood lipid level, antibiotics, other immunosuppressive agents, and Chinese patent medicines. The scores of all risk factors were calculated using the machine learning method and represented by a ranking figure. The factor with a higher score had a more significant impact on the occurrence of hepatotoxicity. Factors with a score of zero were removed because they did not affect the prediction. A Shapley Additive Explanations (SHAP) figure demonstrated the positive or negative correlations between risk factors and hepatotoxicity. Risk factors with a large sample size would affect their impact on the SHAP figure. The SHAP figure was developed by using Python software (version 3.7, Python Software Foundation, Wilmington, DE, USA)
2.3. Model Development
Missing data were imputed using the missForest method, and variables with more than 30% missing data were discarded [35]. Patients included were randomly stratified (8:2) into the training set for modeling development and the testing set to evaluate the performance of the models. Using the selected risk factors as covariates, eight machine-learning models were established and analyzed using algorithms including eXtreme Gradient Boosting (XGBoost), AdaBoost, CatBoost, Gradient Boosting Decision Tree (GBDT), Light Gradient Boosting Machine (LightGBM), Tree-based Pipeline Optimization Tool (TPOT), Random Forest (RF), and Artificial Neural Network (ANN). The area under the curve (AUC) of the receiver operating characteristic (ROC) curve, representing the overall ability to classify and predict, is considered the primary metric for evaluating and comparing models. The accuracy, precision, sensitivity, specificity, recall, F1 scores, and average precision (AP) of precision-recall curve were also calculated. These metrics were used to assess the model performance comprehensively. The best-performing model was selected to establish a hepatotoxicity prediction model associated with low-dose MTX. The missForest method and machine learning models were developed and validated with open-source packages in Python software (version 3.7).
2.4. Statistical Analysis
Categorical variables were summarized using frequency counts and percentages, and continuous variables were presented as means with standard deviations (SD) or medians with ranges. Comparisons between the training set and the testing set were made using the nonparametric Mann–Whitney U test for continuous variables and the χ2 test for categorical variables. By convention, p values of less than 0.05 were considered statistically significant. These analyses were performed using the SPSS 25.0 software (IBM Information Management, Chicago, IL, USA).
3. Results
3.1. Study Population
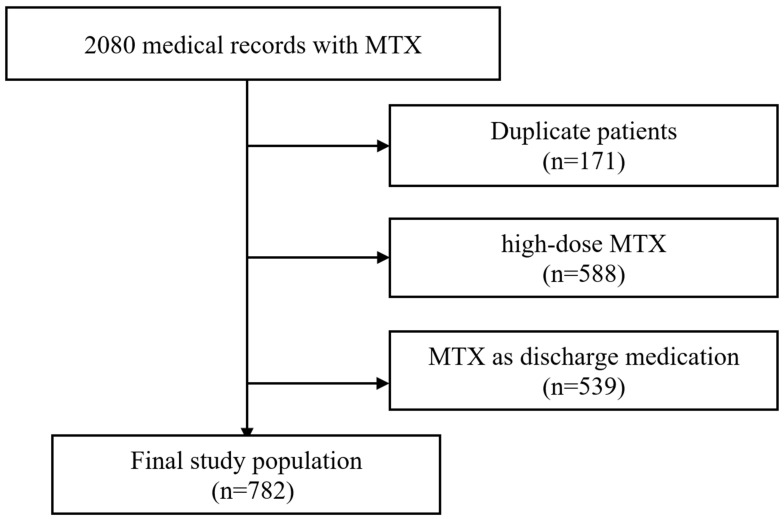
A total of 2080 medical records were registered in the cohort during the study period, and 782 patients were enrolled in this study. The following patients were excluded: 171 had duplicate records, 588 were on high-dose MTX, and 539 had low-dose MTX as discharge medication (Figure 1). Among the patients enrolled, the mean age was 47.85 ± 15.56 years (range from 10 to 87 years), and the females represented 54.99% (430/782). The average body mass index (BMI) was 22.72 ± 3.91 kg/m2 (range from 13.27 to 41.14 kg/m2). A total of 279 (35.68%) patients experienced hepatotoxicity. Among these variables analyzed, BMI had 53 missing data points (6.78%) imputed using the missForest method. There was no significant difference between the processed data and the original data. The enrolled patients were divided into training and testing sets in a ratio of 8:2, with 625 and 157 patients, respectively. There were no significant differences in any variables between the training and testing sets (p > 0.05) (Table 1).
Figure 1.
The flow chart illustrating patient selection.
Table 1.
The characteristics of patients.
| Variable | Total (n = 782) |
Training Set (n = 625) |
Testing Set (n = 157) |
p |
|---|---|---|---|---|
| Hepatotoxicity | ||||
| Yes | 279 | 223 | 56 | 1.00 |
| No | 503 | 402 | 101 | |
| Gender | ||||
| Male | 352 | 281 | 71 | 1.00 |
| Female | 430 | 344 | 86 | |
| Age (years) | 47.85 ± 15.56 (10–87) |
47.67 ± 15.63 (14–78) |
48.58 ± 15.33 (10–87) |
0.40 |
| First time taking MTX | ||||
| Yes | 501 | 400 | 101 | 1.00 |
| No | 272 | 225 | 56 | |
| Body mass index * (kg/m2) | Original data | |||
| 729 | 586 | 143 | ||
| 22.72 ± 3.91 (13.27–41.14) |
22.78 ± 3.94 (13.27–41.14) |
22.45 ± 3.77 (13.74–37.13) |
0.71 | |
| Processed data | ||||
| 22.68 ± 3.81 (13.27–41.14) |
22.77 ± 3.86 (13.27–41.14) |
22.38 ± 3.23 (13.74–37.13) |
0.37 | |
| Alcohol use | ||||
| Yes | 164 | 130 | 34 | 0.83 |
| No | 618 | 495 | 123 | |
| History of kidney disease | ||||
| Yes | 6 | 5 | 1 | 1.00 |
| No | 776 | 620 | 156 | |
| History of liver disease | ||||
| Yes | 32 | 23 | 9 | 0.26 |
| No | 750 | 602 | 148 | |
| Number of comorbidities | 4.98 ± 2.97 (1–17) |
4.90 ± 2.90 (1–17) |
5.28 ± 3.23 (1–16) |
0.69 |
| Type 2 diabetes | ||||
| Yes | 69 | 59 | 10 | 0.27 |
| No | 713 | 566 | 147 | |
| Hyperlipidemia | ||||
| Yes | 41 | 30 | 11 | 0.32 |
| No | 741 | 595 | 146 | |
| Folate supplementation | ||||
| Yes | 723 | 575 | 148 | 0.40 |
| No | 59 | 50 | 9 | |
| Doses of folic acid/week | 9.19 ± 3.33 (0–35) |
9.16 ± 3.39 (0–35) |
9.29 ± 3.07 (0–15) |
0.05 |
| NSAIDs use | ||||
| Yes | 276 | 231 | 45 | 0.06 |
| No | 506 | 394 | 112 | |
| Glucocorticoid use | ||||
| Yes | 441 | 350 | 91 | 0.72 |
| No | 341 | 275 | 66 | |
| Antibiotics use | ||||
| Yes | 153 | 117 | 36 | 0.26 |
| No | 629 | 508 | 121 | |
| Other immunosuppressive agent use | ||||
| Yes | 446 | 351 | 95 | 0.37 |
| No | 336 | 274 | 62 | |
| Number of medications | 5.91 ± 2.93 (0–24) |
5.97 ± 2.93 (0–24) |
5.67 ± 2.92 (0–18) |
0.92 |
| Chinese patent medicines use | ||||
| Yes | 68 | 56 | 12 | 0.75 |
| No | 714 | 569 | 145 | |
*: The difference between processed data and original data was not statistically significant (p values were 0.88, 0.94, and 0.87 in the total, training set, and testing set, respectively). NSAID: non-steroidal anti-inflammatory drugs.
3.2. Model Performance
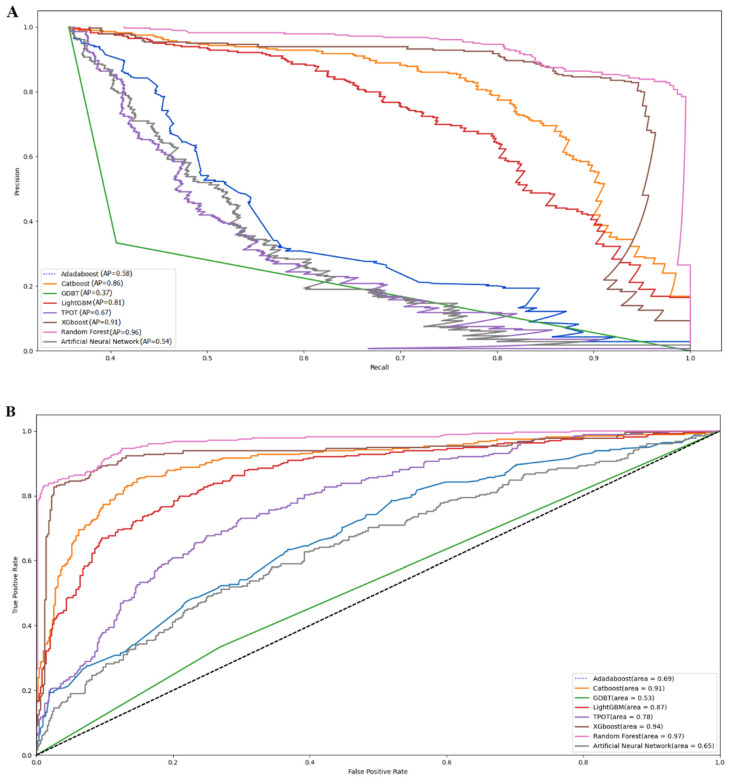
The visual comparisons of the eight models in the total population are shown in Figure 2, including the precision-recall and the ROC curves. Random Forest achieved the highest AUC of 0.97, followed by XGboost (AUC = 0.94), Catboost (AUC = 0.91), LightGBM (AUC = 0.87), and TPOT (AUC = 0.78). The ROC curves of Adaboost, ANN, and GBDT were low, only 0.69, 0.65, and 0.53, respectively. The precision, accuracy, sensitivity, specificity, recall, and F1 values of the eight models are shown in Table 2.
Figure 2.
Visual presentation of eight machine learning models (A) the precision-recall curve, and (B) the receiver operating characteristic (ROC) curve.
Table 2.
Model performance.
| Models | Precision | Accuracy | Sensitivity | Specificity | Recall | F1 |
|---|---|---|---|---|---|---|
| LightGBM | 40.00% | 59.87% | 25.00% | 20.79% | 25.00% | 30.77% |
| GBDT | 50.94% | 59.24% | 41.07% | 30.69% | 41.07% | 41.82% |
| Adaboost | 51.35% | 64.33% | 33.93% | 17.81% | 33.93% | 40.86% |
| Catboost | 42.86% | 60.51% | 32.14% | 23.76% | 32.14% | 36.73% |
| XGboost | 43.18% | 60.51% | 33.93% | 24.75% | 33.93% | 38.00% |
| Random Forest | 50.00% | 64.33% | 32.14% | 17.82% | 32.14% | 39.13% |
| TPOT | 43.90% | 61.15% | 32.14% | 22.77% | 32.14% | 37.11% |
| ANN | 36.36% | 62.42% | 7.14% | 6.93% | 7.14% | 11.94% |
Adaboost and Random Forest had the highest accuracy (64.33%). Adaboost had the highest precision value (51.35%), followed by GBDT (50.94%). GBDT had the highest sensitivity value (41.07%), followed by Adaboost (33.93%) and XGboost (33.93%). GBDT had the highest specificity value (30.69%), followed by XGboost (24.75%). GBDT had the highest recall value (41.07%), followed by Adboost (33.93%) and XGboost (33.93%). GBDT had the highest F1 value (41.82%), followed by Adaboost (40.86%). These results showed that Adaboost had slight advantages in precision and accuracy with good recall, sensitivity, and F1 values. Adaboost had a significantly lower AUC than Random Forest (0.69 versus 0.97). After general consideration of the prediction performance, Random Forest was selected to predict the hepatotoxicity associated with low-dose MTX.
3.3. Hepatotoxicity and Risk Factors
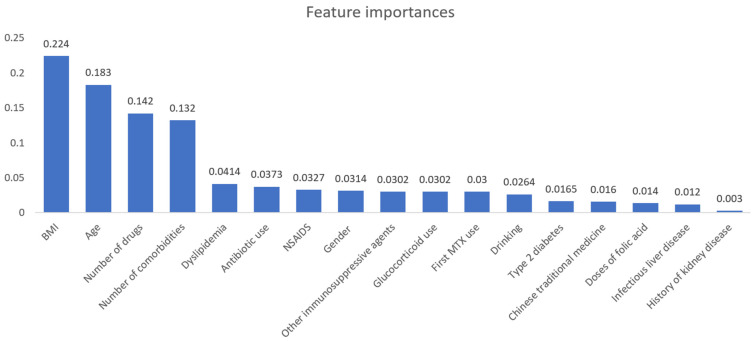
A total of 279 patients experienced hepatotoxicity, with an incidence rate of 35.68%. The importance score ranking in the Random Forest model is shown in Figure 3. Importance scores were above zero for all risk factors, indicating that they had a greater or lesser impact on prediction. Among risk factors, the highest score was BMI (0.237), followed by age (0.198), number of drugs (0.151), and number of comorbidities (0.144), demonstrating their importance in hepatotoxicity associated with low-dose MTX.
Figure 3.
Importance score ranking for risk factors.
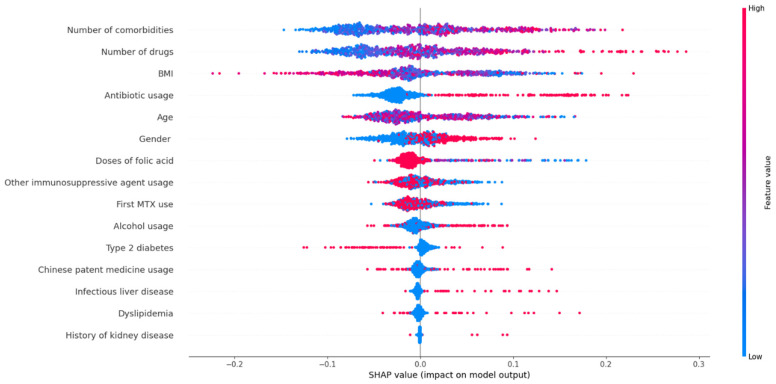
The SHAP values of the risk factors are shown in Figure 4. When analyzed by the following risk factors (the number of comorbidities, the number of drugs, the use of antibiotics, male gender, the use of alcohol, infectious liver disease, dyslipidemia, and the history of kidney disease). The color of the dot became redder as the SHAP value increased. The color was bluer when the SHAP value decreased. The color changes showed degrees of the positive impact of these factors on the risk of hepatotoxicity. In contrast, risk factors, including BMI and doses of folic acid, showed negative effects. Type 2 diabetes, taking MTX for the first time, other immunosuppressive agents, age, and Chinese patent medicines showed unclear influence.
Figure 4.
SHAP values of the important risk factors.
4. Discussion
An effective prediction model is necessary to prevent the hepatotoxicity associated with low-dose MTX. In real-world studies, the variables are not independent but are related nonlinearly. Multivariate analysis methods are challenging for capturing complex relationships. Therefore, we innovatively attempted to apply machine-learning methods that can capture nonlinear relationships between variables. Machine learning can explore risk factors and establish a prediction model for hepatotoxicity associated with low-dose MTX through data learning. Our retrospective study analyzed 15 risk factors for hepatoxicity. The BMI with missing data was imputed using the missForest method, which has been shown to successfully handle missing values, particularly in data sets that include different variables [35]. The results did not show significant differences between the processed and original data.
The eight machine learning methods, including XGBoost, AdaBoost, CatBoost, GBDT, LightGBM, TPOT, RF, and ANN, were applied to establish a prediction model. In these methods, the XGBoost, AdaBoost, CatBoost, GBDT, and LightGBM are boosting algorithms in machine learning. GBDT can combine the predictions from multiple decision trees to generate the final predictions, while it can hardly be adapted to dynamic online data generation, and it tends to be ineffective when facing sparse categorical features [36]. The working procedure of XGBoost is the same as GBDT. XGBoost includes a variety of regularization techniques that can reduce overfitting and improve overall performance, which makes XGBoost slightly better than GBDT. LightGBM is a fast, distributed, high-performance gradient-boosting framework based on a decision tree algorithm. LightGBM uses a histogram-based algorithm, i.e., it buckets continuous feature values into discrete bins that fasten the training procedure [37]. CatBoost is also based on GBDT and has the following two innovations: ordered target statistics and ordered boosting [38]. Therefore, CatBoost works well with the default set of hyperparameters, and the users do not have to spend a lot of time tuning the hyperparameters [38]. Adaboost is relatively robust to overfitting in low-noise datasets. While it is easily defeated by noisy data, the efficiency of the algorithm is highly affected by outliers as the algorithm tries to fit every point perfectly [39]. Random Forest is a bagging algorithm that uses bootstrap aggregation of multiple regression trees to reduce the risk of overfitting and combine the predictions of many trees to produce more accurate predictions [40]. Therefore, Random Forest has a good classification effect for most data. TPOT can automatically optimize feature transformation, feature selection, feature construction, model selection, and parameter optimization via genetic programming using a tree-based structure [41]. The design of ANNs is based on the human brain’s neural network. Neurons in the different layers have their own missions to solve problems, which can be analogous to factory production lines [42]. As a type of parallel distributed system driven by mass data, ANNs are free from the requirements of logical or mathematical associations known beforehand [42].
The performance of different machine learning algorithms should be based on the characteristics of the dataset. Therefore, the choice of models should be based on the calculation results. The results showed that these machine algorithms performed well, especially the Random Forest. The Random Forest showed that its AUC was 0.97. The accuracy and precision were 64.33% and 50.00%, respectively. Both the recall and the F1 scores were satisfactory. Random Forest outperformed other models selected to build the prediction model for hepatotoxicity associated with low-dose MTX.
Analysis of risk factors showed that all 15 variables helped predict low-dose MTX-related hepatotoxicity. The top ten significant risk factors included BMI, age, number of drugs and comorbidities, doses of folic acid, antibiotic use, gender, immunosuppressive agents, taking MTX for the first time, and alcohol use, suggesting physicians should pay more attention to these factors and take the corresponding prevention measures. BMI was considered the most critical risk factor, which had a negative relationship with hepatotoxicity, demonstrating that patients with lower BMI were more likely to experience hepatotoxicity. Therefore, the dose of MTX should be individualized based on height and weight to avoid hepatoxicity. Male gender was also identified as an important risk factor in our study. However, the causal relationship between gender and hepatotoxicity associated with low-dose MTX remains controversial [43,44] and requires further research.
The importance of the number of drugs, the number of comorbidities, and the use of antibiotics was also confirmed. As the primary organ for drug metabolism, the liver is more vulnerable to damage by drugs, active metabolites, or drug interactions [45,46,47]. Multiple drug treatments and comorbid diseases can increase the risks of polypharmacy, drug interactions, and even medication errors [48,49], increasing the risk of hepatotoxicity. Antibiotics are the most common cause of liver damage [50]. However, the potential for liver injury caused by antibacterial drugs was underestimated [51]. Several real-world studies showed that antibiotic-induced liver injury ranged from 13.5% to 65% [52,53,54]. Therefore, to avoid hepatotoxicity during MTX therapy, simplifying treatment regimens should be an important measure for the benefit of patients.
Alcohol consumption is well known to harm the liver, particularly in excess [55]. The American College of Rheumatology and the British Society of Rheumatology recommend limiting alcohol intake for patients on MTX treatment [56,57]. Similarly, we found a positive relationship between alcohol use and hepatotoxicity associated with low-dose MTX. Although the importance score for alcohol consumption was not high in this study due to the relatively small number of patients who drank alcohol, we still recommend limiting or avoiding alcohol intake.
Supplementation with folic or folinic acid during MTX treatment can ameliorate ADEs. Worldwide guidelines currently support the coadministration of folic acid with MTX. The recommended doses range from 0.5 to 2 mg per day [57,58]. However, several studies have suggested that high-dose folinic acid supplementation may reduce the beneficial effects of MTX [59,60,61]. In our study, patients taking high-dose folic acid had a high risk of liver injury. Among patients taking more than 15 mg of folic acid a week, the incidence of liver injury was 41.94%. In contrast, the incidence of liver injury in patients taking 5–10 mg/week was only 34.54%. Furthermore, 59 patients in this study did not take folic acid during MTX treatment, and their liver injury rate was up to 45.76%. Therefore, we recommend daily supplementation with folic acid during MTX treatment.
Metabolic syndrome is a biochemical and clinical condition characterized by visceral obesity, dyslipidemia, hyperglycemia, and hypertension [62,63]. Disorders associated with metabolic syndrome can be significant risk factors for fibrosis and the progression of liver damage. Type 2 diabetes contributed to the biological processes that drove the severity of nonalcoholic fatty liver disease, which was the leading cause of developing chronic liver diseases [64,65]. Several studies showed that nonalcoholic steatohepatitis and hyperlipidemia contributed to MTX hepatotoxicity in patients with psoriasis [43,66]. These were consistent with our results that type 2 diabetes and hyperlipidemia were significant risk factors for hepatotoxicity associated with low-dose MTX.
Hepatitis B and hepatitis C can cause liver damage, increasing the risk of liver toxicity and even liver fibrosis and cirrhosis in patients taking MTX [67]. Infectious liver disease was one of the important risk factors for hepatotoxicity in this study, while its importance score was not high. The reason might be that patients with infectious liver disease were only 4.1% of the study sample. For health and safety reasons in China, many physicians choose other alternative treatments for patients with infectious liver disease instead of MTX. Similarly, only six patients had a history of kidney disease in this study. Therefore, the importance score for the history of kidney disease was low.
Our study has the following limitations (1) knowledge about specific risk factors is still lacking in this study. Although factors such as taking MTX for the first time, other immunosuppressive agents, age, and Chinese patent medicines affected the occurrence of hepatotoxicity, the direction of influence of these factors was unclear. These factors could be influenced by other factors, such as drug regimens (the number of drugs and drug interactions), gender, and BMI; (2) the sample size was small. Future studies should include more patient data from different health care centers; (3) long-term studies are required to verify the association of these risk factors with liver fibrosis or cirrhosis.
5. Conclusions
Machine learning can be applied to establish the prediction model for low-dose hepatotoxicity associated with MTX. The model can help to improve medication safety in patients taking methotrexate in clinical practice. However, due to the above limitations, further studies are required to test our findings.
Acknowledgments
The authors would like to thank Miye Wang, who works in the Information Center of the West China Hospital, for assistance with data extraction. We thank Jinsong Liu’s aid in data analysis.
Author Contributions
All the authors were involved in the study. Study design: Q.H. and T.X. Data extraction: Q.H. and H.W. Analysis and interpretation of data: Q.H. Writing—original draft: Q.H. and H.W. Writing—review and editing: Q.H. and T.X. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
The study was conducted according to the guidelines of the Declaration of Helsinki, and approved by the ethics committee of the West China Hospital of Sichuan University (protocol code 2022-46 and date of approval 13 January 2022).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available in the present manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
The National Key R&D Program of China (grant number: 2020YFC2008302), Science and technology project of Chengdu Health Commission (grant number: 2022020), and the Sichuan Science and Technology Program (grant number: 2023NSFSC1696) supported this study.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.West S.G. Methotrexate hepatotoxicity. Rheum. Dis. Clin. N. Am. 1997;23:883–915. doi: 10.1016/S0889-857X(05)70365-3. [DOI] [PubMed] [Google Scholar]
- 2.Farber S. Chemotherapy in the treatment of leukemia and Wilm’s tumor. JAMA. 1996;198:826–836. doi: 10.1001/jama.1966.03110210076025. [DOI] [PubMed] [Google Scholar]
- 3.Saag K.G., Teng G.G., Patkar N.M., Anuntiyo J., Finney C., Curtis J.R., Paulus H.E., Mudano A., Pisu M., Elkins-Melton M., et al. American college of rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Care Res. 2010;59:762–784. doi: 10.1002/art.23721. [DOI] [PubMed] [Google Scholar]
- 4.Cross M., Smith E., Hoy D., Carmona L., Wolfe F., Vos T., Williams B., Gabriel S., Lassere M., Johns N., et al. The global burden of rheumatoid arthritis: Estimates from the Global Burden of Disease 2010 study. Ann. Rheum. Dis. 2010;73:1316–1322. doi: 10.1136/annrheumdis-2013-204627. [DOI] [PubMed] [Google Scholar]
- 5.Hunter T.M., Boytsov N.N., Zhang X., Schroeder K., Michaud K., Araujo A.B. Prevalence of rheumatoid arthritis in the United States adult population in healthcare claims databases, 2004–2014. Rheumatol. Int. 2017;37:1551–1557. doi: 10.1007/s00296-017-3726-1. [DOI] [PubMed] [Google Scholar]
- 6.Myasoedova E., Crowson C.S., Kremers H.M., Therneau T.M., Gabriel S.E. Is the incidence of rheumatoid arthritis rising?: Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2019;62:1576–1582. doi: 10.1002/art.27425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Icen M., Crowson C.S., McEvoy M.T., Dann F.J., Gabriel S.E., Maradit Kremers H. Trends in incidence of adult-onset psoriasis over three decades: A population-based study. J. Am. Acad. Dermatol. 2008;60:394–401. doi: 10.1016/j.jaad.2008.10.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Michalek I.M., Loring B., John S.M. A systematic review of worldwide epidemiology of psoriasis. J. Eur. Acad. Dermatol. Venereol. 2017;31:205–212. doi: 10.1111/jdv.13854. [DOI] [PubMed] [Google Scholar]
- 9.Lau C.S., Chia F., Dans L., Harrison A., Hsieh T.Y., Jain R., Jung S.M., Kishimoto M., Kumar A., Leong K.P., et al. 2018 update of the APLAR recommendations for treatment of rheumatoid arthritis. Int. J. Rheum. Dis. 2019;22:357–375. doi: 10.1111/1756-185X.13513. [DOI] [PubMed] [Google Scholar]
- 10.Kameda H., Fujii T., Nakajima A., Koike R., Sagawa A., Kanbe K., Tomita T., Harigai M., Suzuki Y. Japan college of rheumatology guideline for the use of methotrexate in patients with rheumatoid arthritis. Mod. Rheumatol. 2019;29:31–40. doi: 10.1080/14397595.2018.1472358. [DOI] [PubMed] [Google Scholar]
- 11.Singh J.A., Guyatt G., Ogdie A., Gladman D.D., Deal C., Deodhar A., Dubreuil M., Dunham J., Husni M.E., Kenny S., et al. Special Article: 2018 American college of rheumatology/national psoriasis foundation guideline for the treatment of psoriatic arthritis. Arthritis Care Res. 2019;71:2–29. doi: 10.1002/acr.23789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuhn A., Aberer E., Bata-Csörgő Z., Caproni M., Dreher A., Frances C., Gläser R., Klötgen H.W., Landmann A., Marinovic B., et al. S2k guideline for treatment of cutaneous lupus erythematosus—Guided by the european dermatology forum (edf) in cooperation with the european academy of dermatology and venereology (eadv) J. Eur. Acad. Dermatol. Venereol. 2017;31:389–404. doi: 10.1111/jdv.14053. [DOI] [PubMed] [Google Scholar]
- 13.Nast A., Spuls P.I., van der Kraaij G., Gisondi P., Paul C., Ormerod A.D., Saiag P., Smith C.H., Dauden E., de Jong E.M., et al. European S3-Guideline on the systemic treatment of psoriasis vulgaris—Update Apremilast and Secukinumab—EDF in cooperation with EADV and IPC. J. Eur. Acad. Dermatol. Venereol. 2017;31:1951–1963. doi: 10.1111/jdv.14454. [DOI] [PubMed] [Google Scholar]
- 14.Warris L.T., van den Heuvel-Eibrink M.M., Aarsen F.K., Pluijm S.M., Bierings M.B., van den Bos C., Zwaan C.M., Thygesen H.H., Tissing W.J., Veening M.A., et al. Hydrocortisone as an intervention for dexamethasone-induced adverse effects in pediatric patients with acute lymphoblastic leukemia: Results of a double-blind, randomized controlled trial. J. Clin. Oncol. 2016;34:2287–2293. doi: 10.1200/JCO.2015.66.0761. [DOI] [PubMed] [Google Scholar]
- 15.Nakase H., Uchino M., Shinzaki S., Matsuura M., Matsuoka K., Kobayashi T., Saruta M., Hirai F., Hata K., Hiraoka S., et al. Evidence-based clinical practice guidelines for inflammatory bowel disease. J. Gastroenterol. 2018;53:305–353. doi: 10.1007/s00535-018-1439-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ramanan A.V., Dick A.D., Jones A.P., McKay A., Williamson P.R., Compeyrot-Lacassagne S., Hardwick B., Hickey H., Hughes D., Woo P., et al. Adalimumab plus Methotrexate for Uveitis in Juvenile Idiopathic Arthritis. N. Engl. J. Med. 2017;376:1637–1646. doi: 10.1056/NEJMoa1614160. [DOI] [PubMed] [Google Scholar]
- 17.Van den Bosch F., Kruithof E., Baeten D., De Keyser F., Mielants H., Veys E.M. Effects of a loading dose regimen of three infusions of chimeric monoclonal antibody to tumour necrosis factor α (infliximab) in spondyloarthropathy: An open pilot study. Ann. Rheum. Dis. 2000;59:428–433. doi: 10.1136/ard.59.6.428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mansouri B., Kivelevitch D., Campa M., Menter A. Palmoplantar pustular psoriasis unresponsive to the interleukin-1β antagonist canakinumab. Clin. Exp. Dermatol. 2016;41:324–326. doi: 10.1111/ced.12759. [DOI] [PubMed] [Google Scholar]
- 19.Bai F., Li G.G., Liu Q., Niu X., Li R., Ma H. Short-term efficacy and safety of IL-17, IL-12/23, and IL-23 inhibitors brodalumab, secukinumab, ixekizumab, ustekinumab, guselkumab, tildrakizumab, and risankizumab for the treatment of moderate to severe plaque psoriasis: A systematic review and network meta-analysis of randomized controlled trials. J. Immunol. Res. 2019;10:2546161. doi: 10.1155/2019/2546161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kremer J.M., Kaye G.I., Kaye N.W., Ishak K.G., Axiotis C.A. Light and electron microscopic analysis of sequential liver biopsy samples from rheumatoid arthritis patients receiving long-term methotrexate therapy. Followup over long treatment intervals and correlation with clinical and laboratory variables. Arthritis Rheum. 1995;8:1194–1203. doi: 10.1002/art.1780380904. [DOI] [PubMed] [Google Scholar]
- 21.Curtis J.R., Beukelman T., Onofrei A., Cassell S., Greenberg J.D., Kavanaugh A., Reed G., Strand V., Kremer J.M. Elevated liver enzyme tests among patients with rheumatoid arthritis or psoriatic arthritis treated with methotrexate and/or leflunomide. Ann. Rheum. Dis. 2010;69:43–47. doi: 10.1136/ard.2008.101378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park S.H., Choe J.Y., Kim S.K. Assessment of liver fibrosis by transient elastography in rheumatoid arthritis patients treated with methotrexate. Joint Bone Spine. 2010;77:588–592. doi: 10.1016/j.jbspin.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 23.Whiting-O’Keefe Q.E., Fye K.H., Sack K.D. Methotrexate and histologic hepatic abnormalities: A meta-analysis. Am. J. Med. 1991;90:711–716. doi: 10.1016/0002-9343(91)90667-M. [DOI] [PubMed] [Google Scholar]
- 24.Kalb R.E., Strober B., Weinstein G., Lebwohl M. Methotrexate and psoriasis: National Psoriasis Foundation Consensus Conference. J. Am. Acad. Dermatol. 2009;60:824–837. doi: 10.1016/j.jaad.2008.11.906. [DOI] [PubMed] [Google Scholar]
- 25.Clary D.D., Reid A.T., Kiani R., Fanciullo J. Methotrexate Hepatotoxicity Monitoring Guidelines in Psoriasis and Rheumatoid Arthritis: Is There a Consensus? South Dak. Med. 2021;74:363–366. [PubMed] [Google Scholar]
- 26.Jordan M.I., Mitchell T.M. Machine learning: Trends, perspectives, and prospect. Science. 2015;349:255–260. doi: 10.1126/science.aaa8415. [DOI] [PubMed] [Google Scholar]
- 27.Esteva A., Kuprel B., Novoa R.A., Ko J., Swetter S.M., Blau H.M., Thrun S. Dermatologist-level classification of skin cancer with deep neural networks. Nature. 2017;542:115–118. doi: 10.1038/nature21056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gunčar G., Kukar M., Notar M., Brvar M., Černelč P., Notar M., Notar M. An application of machine learning to haematological diagnosis. Sci. Rep. 2018;8:411. doi: 10.1038/s41598-017-18564-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Qiu H., Yu H.Y., Wang L.Y., Yao Q., Wu S.N., Yin C., Fu B., Zhu X.J., Zhang Y.L., Xing Y., et al. Electronic health record driven prediction for gestational diabetes mellitus in early pregnancy. Sci. Rep. 2017;7:16417. doi: 10.1038/s41598-017-16665-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Deo R.C. Machine learning in medicine. Circulation. 2015;132:1920–1930. doi: 10.1161/CIRCULATIONAHA.115.001593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Goldstein B.A., Navar A.M., Carter R.E. Moving beyond regression techniques in cardiovascular risk prediction: Applying machine learning to address analytic challenges. Eur. Heart J. 2017;38:1805–1814. doi: 10.1093/eurheartj/ehw302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Meyer A., Zverinski D., Pfahringer B., Kempfert J., Kuehne T., Sündermann S.H., Stamm C., Hofmann T., Falk V., Eickhoff C. Machine learning for real-time prediction of complications in critical care: A retrospective study. Lancet Respir. Med. 2018;6:905–914. doi: 10.1016/S2213-2600(18)30300-X. [DOI] [PubMed] [Google Scholar]
- 33.Mazaud C., Fardet L. Relative risk of and determinants for adverse events of methotrexate prescribed at a low dose: A systematic review and meta-analysis of randomized placebo-controlled trials. Br. J. Dermatol. 2017;177:978–986. doi: 10.1111/bjd.15377. [DOI] [PubMed] [Google Scholar]
- 34.Chalasani N.P., Maddur H., Russo M.W., Wong R.J., Reddy K.R., Practice Parameters Committee of the American College of Gastroenterology ACG Clinical Guideline: Diagnosis and Management of Idiosyncratic Drug-Induced Liver Injury. Am. J. Gastroenterol. 2021;116:878–898. doi: 10.14309/ajg.0000000000001259. [DOI] [PubMed] [Google Scholar]
- 35.Stekhoven D.J., Buhlmann P. MissForest–nonparametric missing value imputation for mixed-type data. Bioinformatics. 2012;28:112–118. doi: 10.1093/bioinformatics/btr597. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Z., Jung C. GBDT-MO: Gradient-Boosted Decision Trees for Multiple Outputs. IEEE Trans. Neural Netw. Learn. Syst. 2021;32:3156–3167. doi: 10.1109/TNNLS.2020.3009776. [DOI] [PubMed] [Google Scholar]
- 37.Zhu J., Su Y., Liu Z., Liu B., Sun Y., Gao W., Fu Y. Real-time biomechanical modelling of the liver using LightGBM model. Int. J. Med. Robot. Comput. Assist. Surg. 2022;18:e2433. doi: 10.1002/rcs.2433. [DOI] [PubMed] [Google Scholar]
- 38.Hancock J.T., Khoshgoftaar T.M. CatBoost for big data: An interdisciplinary review. J. Big Data. 2020;7:94. doi: 10.1186/s40537-020-00369-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wang C., Xu S., Yang J. Adaboost Algorithm in Artificial Intelligence for Optimizing the IRI Prediction Accuracy of Asphalt Concrete Pavement. Sensors. 2021;21:5682. doi: 10.3390/s21175682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Breiman L. Random forests. Mach. Learn. 2001;45:5–32. doi: 10.1023/A:1010933404324. [DOI] [Google Scholar]
- 41.Wang G., Sun Y., Chen Y., Gao Q., Peng D., Lin H., Zhan Z., Liu Z., Zhuo S. Rapid identification of human ovarian cancer in second harmonic generation images using radiomics feature analyses and tree-based pipeline optimization tool. J. Biophotonics. 2020;13:e202000050. doi: 10.1002/jbio.202000050. [DOI] [PubMed] [Google Scholar]
- 42.Cao B., Zhang K.C., Wei B., Chen L. Status quo and future prospects of artificial neural network from the perspective of gastroenterologists. World J. Gastroenterol. 2021;27:2681–2709. doi: 10.3748/wjg.v27.i21.2681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Yeo C.M., Chong V.H., Earnest A., Yang W.L. Prevalence and risk factors for methotrexate hepatoxicity in Asian patients with psoriasis. World J. Hepatol. 2013;5:275–280. doi: 10.4254/wjh.v5.i5.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Amital H., Arnson Y., Chodick G., Shalev V. Hepatotoxicity rates do not differ in patients with rheumatoid arthritis and psoriasis treated with methotrexate. Rheumatology. 2009;48:1107–1110. doi: 10.1093/rheumatology/kep176. [DOI] [PubMed] [Google Scholar]
- 45.Sanoh S. In Vitro and in Vivo Assessments of Drug-induced Hepatotoxicity and Drug Metabolism in Humans. Yakugaku Zasshi. 2015;135:1273–1279. doi: 10.1248/yakushi.15-00200. [DOI] [PubMed] [Google Scholar]
- 46.Ballet F. Hepatotoxicity in drug development: Detection, significance and solutions. J. Hepatol. 1997;2:26–36. doi: 10.1016/S0168-8278(97)80494-1. [DOI] [PubMed] [Google Scholar]
- 47.Demir Y., Duran H.E., Durmaz L., Taslimi P., Beydemir Ş., Gulçin İ. The Influence of Some Nonsteroidal Anti-inflammatory Drugs on Metabolic Enzymes of Aldose Reductase, Sorbitol Dehydrogenase, and α-Glycosidase: A Perspective for Metabolic Disorders. Appl. Biochem. Biotechnol. 2020;190:437–447. doi: 10.1007/s12010-019-03099-7. [DOI] [PubMed] [Google Scholar]
- 48.Marcum Z.A., Arbogast K.L., Behrens M.C., Logsdon M.W., Francis S.D., Jeffery S.M., Aspinall S.L., Hanlon J.T., Handler S.M. The utility of an adverse drug event trigger tool in veterans affairs nursing facilities. Consult. Pharm. 2013;28:99–109. doi: 10.4140/TCP.n.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Hu Q., Qin Z., Zhan M., Chen Z., Wu B., Xu T. Validating the Chinese geriatric trigger tool and analysing adverse drug event associated risk factors in elderly Chinese patients: A retrospective review. PLoS ONE. 2020;15:e0232095. doi: 10.1371/journal.pone.0232095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Einar S.B. Drug-induced liver injury due to antibiotics. Scand. J. Gastroenterol. 2017;52:617–623. doi: 10.1080/00365521.2017.1291719. [DOI] [PubMed] [Google Scholar]
- 51.Leitner J.M., Graninger W., Thalhammer F. Hepatotoxicity of antibacterials: Pathomechanisms and clinical. Infection. 2010;38:3–11. doi: 10.1007/s15010-009-9179-z. [DOI] [PubMed] [Google Scholar]
- 52.Mindikoglu A.L., Magder L.S., Regev A. Outcome of liver transplantation for drug-induced acute liver failure in the United States: Analysis of the United Network for Organ Sharing database. Liver Transplant. 2009;15:719–729. doi: 10.1002/lt.21692. [DOI] [PubMed] [Google Scholar]
- 53.Bjornsson E., Olsson R. Outcome and prognostic markers in severe drug-induced liver disease. Hepatology. 2005;42:481–489. doi: 10.1002/hep.20800. [DOI] [PubMed] [Google Scholar]
- 54.Andrade R.J., Lucena M.I., Fernández M.C., Pelaez G., Pachkoria K., García-Ruiz E., García-Muñoz B., González-Grande R., Pizarro A., Durán J.A., et al. Drug-induced liver injury: An analysis of 461 incidences submitted to the Spanish registry over a 10-year period. Gastroenterology. 2005;129:512–521. doi: 10.1016/j.gastro.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 55.Warner A., Lunt M., Verstappen S. Quantifying the hepatotoxic risk of alcohol consumption in patients with rheumatoid arthritis taking methotrexate. Ann. Rheum. Dis. 2017;76:1509–1514. doi: 10.1136/annrheumdis-2016-210629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kremer J.M., Alarcón G.S., Lightfoot R.W., Jr., Willkens R.F., Furst D.E., Williams H.J., Dent P.B., Weinblatt M.E. Methotrexate for rheumatoid arthritis. Suggested guidelines for monitoring liver toxicity. Arthritis Rheum. 1994;37:316–328. doi: 10.1002/art.1780370304. [DOI] [PubMed] [Google Scholar]
- 57.Chakravarty K., McDonald H., Pullar T., Taggart A., Chalmers R., Oliver S., Mooney J., Somerville M., Bosworth A., Kennedy T., et al. BSR/BHPR guideline for disease-modifying anti-rheumatic drug (DMARD) therapy in consultation with the British Association of Dermatologists. Rheumatology. 2008;47:924–925. doi: 10.1093/rheumatology/kel216a. [DOI] [PubMed] [Google Scholar]
- 58.Griffith S.M., Fisher J., Clarke S., Montgomery B., Jones P.W., Saklatvala J., Dawes P.T., Shadforth M.F., Hothersall T.E., Hassell A.B., et al. Do patients with rheumatoid arthritis established on methotrexate and folic acid 5 mg daily need to continue folic acid supplements long term? Rheumatology. 2000;39:1102–1109. doi: 10.1093/rheumatology/39.10.1102. [DOI] [PubMed] [Google Scholar]
- 59.Singh J.A., Furst D.E., Bharat A., Curtis J.R., Kavanaugh A.F., Kremer J.M., Moreland L.W., O’Dell J., Winthrop K.L., Beukelman T., et al. 2012 Update of the 2008 American College of Rheumatology Recommendations for the Use of Disease-Modifying Antirheumatic Drugs and Biologic Agents in the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2012;64:625–639. doi: 10.1002/acr.21641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Joyce D.A., Will R.K., Hoffman D.M., Laing B., Blackbourn S.J. Exacerbation of rheumatoid arthritis in patients treated with methotrexate after administration of folinic acid. Ann. Rheum. Dis. 1991;50:913–914. doi: 10.1136/ard.50.12.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tishler M., Caspi D., Fishel B., Yaron M. The effects of leucovorin (folinic acid) on methotrexate therapy in rheumatoid arthritis patients. Arthritis Rheum. 1988;31:906–908. doi: 10.1002/art.1780310712. [DOI] [PubMed] [Google Scholar]
- 62.Hurley B.F., Hanson D.E., Sheaff A.K. Strength training as a countermeasure to aging muscle and chronic disease. Sport. Med. 2011;41:289–306. doi: 10.2165/11585920-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 63.Alım Z., Kılıç D., Demir Y. Some indazoles reduced the activity of human serum paraoxonase 1, an antioxidant enzyme: In vitro inhibition and molecular modeling studies. Arch. Physiol. Biochem. 2019;125:387–395. doi: 10.1080/13813455.2018.1470646. [DOI] [PubMed] [Google Scholar]
- 64.Kim H., Lee D.S., An T.H., Park H.J., Kim W.K., Bae K.H., Oh K.J. Metabolic Spectrum of Liver Failure in Type 2 Diabetes and Obesity: From NAFLD to NASH to HCC. Int. J. Mol. Sci. 2021;22:4495. doi: 10.3390/ijms22094495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Sever B., Altıntop M.D., Demir Y., Akalın-Çiftçi G., Beydemir Ş., Özdemir A. Design, synthesis, in vitro and in silico investigation of aldose reductase inhibitory effects of new thiazole-based compounds. Bioorganic Chem. 2020;102:104110. doi: 10.1016/j.bioorg.2020.104110. [DOI] [PubMed] [Google Scholar]
- 66.Langman G., Hall P.M., Todd G. Role of nonalcoholic steatohepatitis in methotrexate-induced liver injury. J. Gastroenterol. Hepatol. 2001;16:1395–1401. doi: 10.1046/j.1440-1746.2001.02644.x. [DOI] [PubMed] [Google Scholar]
- 67.Montaudié H., Sbidian E., Paul C., Maza A., Gallini A., Aractingi S., Aubin F., Bachelez H., Cribier B., Joly P., et al. Methotrexate in psoriasis: A systematic review of treatment modalities, incidence, risk factors and monitoring of liver toxicity. J. Eur. Acad. Dermatol. Venereol. 2011;25((Suppl. S2)):12–18. doi: 10.1111/j.1468-3083.2011.03991.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available in the present manuscript.