Abstract
During hemorrhagic shock, blood loss causes a fall in blood pressure, decreases cardiac output, and, consequently, O2 transport. The current guidelines recommend the administration of vasopressors in addition to fluids to maintain arterial pressure when life-threatening hypotension occurs in order to prevent the risk of organ failure, especially acute kidney injury. However, different vasopressors exert variable effects on the kidney, depending on the nature and dose of the substance chosen as follows: Norepinephrine increases mean arterial pressure both via its α-1-mediated vasoconstriction leading to increased systemic vascular resistance and its β1-related increase in cardiac output. Vasopressin, through activation of V1-a receptors, induces vasoconstriction, thus increasing mean arterial pressure. In addition, these vasopressors have the following different effects on renal hemodynamics: Norepinephrine constricts both the afferent and efferent arterioles, whereas vasopressin exerts its vasoconstrictor properties mainly on the efferent arteriole. Therefore, this narrative review discusses the current knowledge of the renal hemodynamic effects of norepinephrine and vasopressin during hemorrhagic shock.
Keywords: hemodynamics, hemorrhagic shock, kidney, norepinephrine, renal hemodynamics, renal perfusion, vasopressin, vasopressor, ischemia/reperfusion
1. Introduction
Hemorrhagic shock may cause arterial hypotension and, consecutively, acute circulatory failure. Together with the control of the source of bleeding, fluid resuscitation and transfusion of blood products are recommended by the current guidelines [1]. Norepinephrine is referred to as the drug of first choice if vasopressors are additionally required to maintain adequate perfusion pressure [1]. However, the use of vasopressors per se is still a matter of debate, especially due to the risk of excessive vasoconstriction. In addition, the respective effect on the kidney of the vasopressor of the molecule chosen remains an open question. Therefore, this review discusses the pathophysiological rationale for the administration of the two most frequently used vasopressors during hemorrhagic shock, i.e., norepinephrine and vasopressin, with a special focus on the kidney.
2. Pathophysiology of Hemorrhagic Shock: Why Could We Need Vasopressor?
During hemorrhagic shock, blood loss causes the fall of blood pressure, decreases cardiac output, and, consequently, O2 transport [2], in turn leading to activation of the sympathetic system, which comprises arterial and venous vasoconstriction. Via this “compensatory phase” during the early period of hemorrhagic shock, the body aims at restoring macro-circulatory perfusion [3]. However, beyond a certain amount of blood loss, a sympatho-inhibitory response with hypo-responsiveness to vasopressor occurs, resulting in a vasoplegic state with arterial hypotension and, ultimately, potentially cardiac arrest [4]. Under these conditions, the administration of vasopressors appears to be a sensible approach.
In the second phase of hemorrhagic shock, once control of bleeding has been obtained, the patient may develop a sepsis-like response induced by the ischemia/reperfusion (I/R) sequence comprising oxidative and nitrosative stresses [5] as well as the systemic release of cytokines [6]. In addition, the use of analgesic and sedative drugs, which is mandatory for the management of patients with hemorrhagic shock, may further impair the vasoconstrictor response [7]. Finally, vasopressors, in particular norepinephrine, may help to stabilize hemodynamics as a result of splanchnic veno-constriction and the consecutive shift of blood volume into the central circulation caused by the increased pressure in capacitance vessels [1]. Therefore, the administration of vasopressors seems to be useful as an adjunct measure to restore vasomotor tone in both the early resuscitation as well as the post-reperfusion phase of the management of hemorrhagic shock [1,2,4].
3. Hemorrhagic Shock and Acute Kidney Injury
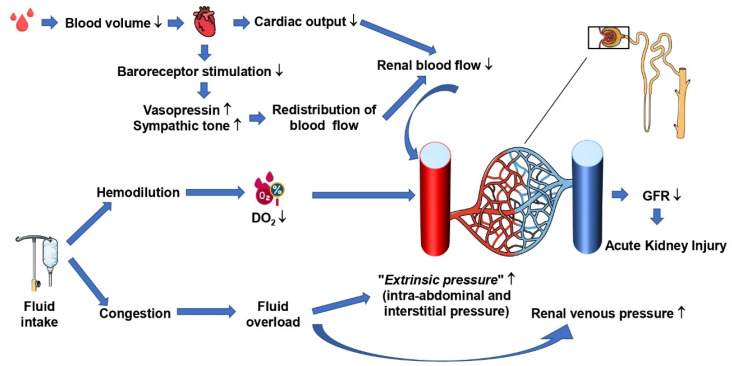
During hemorrhagic shock, several mechanisms may induce acute kidney injury (AKI): (1) The fall of MAP and the consecutive decrease in CO are associated with reduced renal blood flow (RBF), O2 delivery, and microcirculatory perfusion [8,9,10] (Figure 1).
Figure 1.
Physiopathology of acute kidney injury in patients with hemorrhagic shock. DO2: oxygen delivery; GFR: glomerular filtration rate.
(2) Through the hormonal activity, i.e., the above-mentioned activation of the sympathetic system, redistribution of blood to the “vital” organs, heart, and brain further reduce renal blood flow.
(3) Within the renal parenchyma, RBF is redistributed at the expense of the renal cortex and outer medulla [11,12]. Since the microvascular O2 partial pressure (PμvO2) declines much earlier in the kidney than in other organs [13], AKI with glomerular and tubular injuries may occur [11]. In the absence of severe hypotension, the loss of hemoglobin associated with fluid resuscitation-induced hemodilution may decrease renal O2 supply with redistribution of PμvO2 away from the cortex and outer medullar [14], ultimately leading to impaired renal function [15,16] (Figure 1). During normovolemic hemodilution, despite preserved or even increased total RBF, cortex, and medulla PμvO2 drops immediately, and renal O2 consumption (VO2R) becomes dependent on renal O2 delivery (DO2R) [14]. Both Legrand et al. and Ergin et al. reported in rats that fluid resuscitation during hemorrhagic shock alone without additional vasopressor administration did not allow for restoring renal PμvO2 [8,9]. In addition, the re-transfusion of shed blood after canine hemorrhagic shock failed to restore VO2R and lactate uptake despite the increased renal PμvO2 [10].
While hemorrhagic shock situation per se may be responsible for the development of AKI, resuscitation therapies may also contribute to kidney injury as follows: restoration of blood flow due to the resuscitation procedure may induce renal I/R-injury as a result of oxidative and nitrosative stress [17]. Moreover, excessive fluid administration may cause congestive renal edema that decreases the glomerular filtration rate (GFR). Finally, as mentioned above, fluid administration may lead to hemodilution and thereby decrease O2 supply.
4. Renal Hemodynamics and Autoregulation
Within the normal MAP range, RBF and GFR are auto-regulated as follows: in fact, in conscious mammals, RBF and GFR remained unchanged above the MAP thresholds of 65 and 80 mmHg, respectively [18,19,20]. RBF autoregulation is based on the following two mechanisms: the myogenic response and the tubuloglomerular feedback.
The myogenic response is related to vascular smooth muscle cells’ contraction in response to stretching force [21]. In the kidney, an increase in arterial pressure leads to vasoconstriction of the renal afferent arteries. This mechanism appears to be protective against a rise in the glomerular capillary pressure, allowing it to maintain the glomerular flow unchanged [22].
The tubuloglomerular feedback leads to vasoconstriction of the renal afferent arteries in response to an increase in sodium chloride concentration in the macula densa in the early distal tubule [23]. An increase in sodium, chloride, and osmolarity concentration in the intra-distal tubular fluid leads to the activation of chemoreceptors in the macula densa [23]. This mechanism induces vasoconstriction of renal afferent arterioles in order to decrease the glomerular perfusion flow. As sodium reabsorption from the ascending part of the loop of Henle was an active and limited process, conversely to the passive diffusion of water out the descending loop of Henle, the concentration of sodium chloride reaching the macula densa was dependent on the rate of tubular flow as follows: the higher the renal tubular flow rate, the higher the distal tubular NaCl concentration. Therefore, the vasoconstriction of the renal arterial afferent led to a decrease in the RBF and a decrease in sodium chloride concentration in the distal tubular fluid.
Therefore, a decrease in MAP will decrease tubular flow through a decreased glomerular filtration [23]. This will induce a decreased sodium chloride concentration at the macula densa, responsible for afferent arteriolar vasodilatation, providing restoration of RBF.
In this way, the nature and amount of chloride among resuscitation fluids became the object of many controversies in the setting of critically ill patients. No trial found improved mortality nor renal outcomes with the use of balanced fluids when compared to sodium chloride 0.9% [24].
However, below these critical thresholds of mean renal artery pressure, both RBF and GFR decreased and became MAP dependent.
5. Rationale for the Use of Norepinephrine in Patients with Hemorrhagic Shock
Norepinephrine increases systemic vascular resistance through α-1 receptor activation. Furthermore, through β-1 activation, norepinephrine increases CO [25], and both effects together mediate an increase in MAP. Equivocal data are available on the renal hemodynamic effects of norepinephrine in healthy animals, in as much as increased [26], unchanged [27,28,29], or decreased [30,31] RBF has been reported. These different RBF responses have been referred to different effects on CO and/or to variable basal sympathetic tone.
In a model of a hemorrhagic pig, RBF was preserved between the range of 60 and 100 mmHg of MAP [32]. During hemorrhagic shock, the autoregulation mechanism may be impaired, and hence, RBF may become dependent on MAP [33,34]. To illustrate autoregulation failure, Vatner et al. induced moderate hypotensive controlled hemorrhage in dogs with a moderate MAP decreased, RBF remained unchanged, and renal arteries resistance decreased. Then, a further bleeding was induced, leading to a severe hemorrhage with a marked MAP decreased, RBF decreased, and renal resistance increased. These results suggested a loss of RBF autoregulation mechanism in the most severe hemorrhage [35]. In addition to the loss of autoregulation during severe hemorrhagic shock, Adams et al. reported that compared to control, kidney challenge with ischemia-reperfusion stress completely lost the autoregulation mechanism [36].
In healthy humans, norepinephrine infusion has been reported to reduce RBF [37,38,39,40,41] as a result of increased afferent and efferent glomerular arteriolar resistances [37]. Interestingly, vasoconstriction was more pronounced in efferent glomerular arteries, fostering the maintenance of the GFR [41,42]. In a situation with well-maintained autoregulation, norepinephrine increases renal vascular resistance through an α-receptor-mediated, direct vasoconstriction of both afferent and efferent renal arteries. The increase in glomerular capillary pressure leads to vasoconstriction of the afferent arteriole through the autoregulation phenomenon [43,44].
To summarize, the severity and ischemia-reperfusion stress during hemorrhagic shock both contribute to the loss of kidney autoregulation. Therefore, these results suggest a rationale for the use of norepinephrine to target MAP within physiological ranges in hemorrhagic shock states in order to maintain glomerular perfusion pressure.
6. Renal Hemodynamic Effects of Norepinephrine during Hemorrhagic Shock
As mentioned above, during hemorrhagic shock, norepinephrine induces veno-constriction, which may help to mobilize the unstressed blood venous compartment in order to increase the circulating blood volume [1,39]. Various experimental studies are available on the effects of norepinephrine on the kidney during hemorrhagic shock (for details, see Table 1).
Table 1.
Norepinephrine and kidney during hemorrhagic shock. MAP: mean arterial pressure, SAP: systolic arterial pressure.
| References | Species | Models of Hemorrhagic Shock | Arterial Pressure Target during Resuscitation | Intervention | Group Compare |
Main Results | Limitations |
|---|---|---|---|---|---|---|---|
| Dunberry-Poissant et al. [45] |
Anesthetized wistar rats | Blood exsanguination to target MAP 30 mmHg during 60 min, resuscitation after control of bleeding | 55–60 mmHg of MAP target | Resuscitation with 40% of the shed blood withdrawn then used of norepinephrine |
|
|
|
| Libert et al. [46] |
Anesthetized pigs |
Blood exsanguination to MAP target 30 mmhg–35 mmHg during 90 min, resuscitation after stop of exsanguination | 80–85 mmHg of SAP target | Resuscitation with norepinephrine and fluid |
|
|
|
| Murakawa et al. [47] | Anesthetized dogs | Blood exsanguination to MAP target 50 mmHg during 60 min, | >100 mmHg of MAP target for 90 min | Resuscitation with norepinephrine |
|
|
|
| Prunet et al. [48] | Anesthetized pigs | Chest trauma and blood exsanguination to reach MAP target of 50 mmHg during 90 min | MAP to 70 mmHg | Resuscitation with limited fluid and norepinephrine |
|
|
Lower cardiac output in group with use of norepinephrine |
In a dog model of hemorrhagic shock, norepinephrine-related titration of MAP values above 100 mmHg was associated and did not allow for restoring renal PμvO2 to pre-shock levels [47]. However, the MAP achieved was 110 mmHg, thus possibly causing excessive vasoconstriction. Moreover, in this experiment, shed blood was not re-transfused, suggesting a further decrease in renal DO2.
In rats undergoing hemorrhagic shock with MAP ~30 mmHg over 60 min, resuscitation with fluid resuscitation alone (i.e., re-transfusion of shed blood and Ringer’s lactate) was compared with a pre-established limited fluid volume (i.e., a bolus totalizing 40% of the blood volume initially withdrawn) plus norepinephrine both aiming at a MAP target of 50–55 mmHg. At day 1 or 3 post-shock, neither renal function nor markers of renal tissue injury showed any inter-group difference [45]. Moreover, in particular, this study confirmed the fluid-sparing effect of vasopressor administration during hemorrhagic shock demonstrated by others [49].
In a pig model of combined hemorrhagic shock (25–30 mL/kg of blood loss over 30 min) and blunt chest trauma, Prunet et al. reported lower urine output in the group resuscitated to a MAP of 70 mmHg with combined norepinephrine and fluids when compared to fluids alone [48]. However, it should be noted that CO and pulmonary artery occlusion pressure were significantly lower and stroke volume variation higher in the group treated with norepinephrine and fluid, as compared to the group treated with fluid only, suggesting more severe hypovolemia in that group.
In another model of porcine hemorrhagic shock, resuscitation to a systolic arterial pressure target of 80–90 mmHg with norepinephrine in combination with fluid administration restored kidney microcirculation and oxygenation, as well as renal function in a manner comparable to fluid resuscitation alone. However, at 48 h post-shock, additional norepinephrine administration led to a fluid volume-sparing effect with less hemodilution and, subsequently, an attenuated drop of hemoglobin concentration, as compared with fluid-alone resuscitation [46].
In summary, norepinephrine, in combination with fluids, probably does not alter the renal microcirculation and function during resuscitation hemorrhagic shock and allows the sparing fluids [49]. However, the association between excessive fluid overload and acute kidney injury [50] is well established as follows: accumulation of fluid and the consecutively increased renal venous and interstitial pressure will result in a reduced transrenal pressure gradient for RBF (Figure 1). Nevertheless, albeit beneficial effects of norepinephrine were reported in experimental models of hemorrhagic shock achieved by controlled bleeding. This situation is not comparable to the situation of major trauma with its inherent inflammatory responses, rhabdomyolysis, and potentially abdominal compartment that may further threaten kidney function. In addition, for obvious ethical reasons, in these experimental models, animals were under general anesthesia, and any benefit of infusing norepinephrine may have been the result of counteracting the anesthesia-related decrease in sympathetic tone.
7. Rationale for the Use of Vasopressin in Patients with Hemorrhagic Shock
Vasopressin is synthesized in the hypothalamus and stored in the post-pituitary gland. Vasopressin secretion is regulated by plasma osmolarity as well as by blood volume and pressure. Vasopressin has well-known specific renal effects through the activation of V2 receptors located on the basolateral surface of renal tubular cells in collecting ducts. There, vasopressin induces aquaporine-2 recruitment, leading to increased permeability of the epithelial membrane to water and, consecutively, allowing water reabsorption [51]. In addition to V2 receptors, vasopressin also binds to V1a receptors, the stimulation of which induces vascular smooth cell contraction and, consequently, vasoconstriction [51]. Interestingly, V1a receptors distribution is heterogeneous in renal circulation. As a result, the vasoconstrictive properties of vasopressin infused at low doses has a predominant effect on the renal efferent arterioles, while their negligible effects are on the renal afferent arterioles. Apart from V1a receptors distribution, this variable effect on renal arterioles vasomotor tone appears to be related to a local phenomenon of nitrogen monoxide release [52,53]. Through the increase in efferent vasoconstriction, theoretically, glomerular renal perfusion pressure rises, and, consequently, also GFR. In fact, this rationale is consistent with clinical data that, outside the context of hemorrhagic shock, reports higher diuresis, higher creatinine clearance [54,55,56], and reduced need for renal replacement therapy [57] in patients receiving vasopressin administration. Moreover, when blood loss is severe, the initial activation of the sympathetic system to maintain MAP is no longer sufficient, with abnormal vascular bed reactions mediated by nitric oxide-dependent mechanisms that reduce the response to endogenous and exogenous norepinephrine [58]. Furthermore, a rapid fall in the levels of the circulating arginine-vasopressin peptide during hemorrhagic shock was reported [59]. Both phenomena theoretically support vasopressin administration during the management of the hemorrhagic shock.
8. Renal Hemodynamic Effects of Vasopressin during Hemorrhagic Shock
Various experimental studies are available on the renal effects of vasopressin on the kidney during hemorrhagic shock (for details, see Table 2). In anesthetized and hypovolemic animals, vasopressin was shown to not only increase MAP but also CO and, consecutively, RBF. In fact, such macro-hemodynamic effects were reported by Voelcker et al. in swine undergoing uncontrolled, near-fatal hemorrhagic shock (MAP < 20 mmHg, shock-related > 30% fall of heart rate): not only did vasopressin administration improve survival when compared to crystalloid fluid resuscitation alone [60,61], but also compared favorably with epinephrine after liver laceration-induced hemorrhage [62] and even after hemorrhage-induced cardiac arrest [62]. So far, no studies are available evaluating the effect of vasopressin on renal microcirculation and oxygenation during and after hemorrhagic shock; nevertheless, in rats undergoing decompensated hemorrhagic shock with MAP ~40 mmHg and resuscitated with lactated Ringer’s over 60 min, combining fluids with vasopressin renal tissue mitochondrial respiratory activity, attenuated formation of reactive oxygen species and thereby lipid peroxidation-mirrored oxidative damage and histological injury at 18 h post-shock [63].
Table 2.
Vasopressin and kidney during hemorrhagic shock. MAP: mean arterial pressure.
| References | Species | Models of Hemorrhagic Shock | Arterial Pressure Target during Resuscitation |
Intervention | Group Compare | Main Results | Limitations |
|---|---|---|---|---|---|---|---|
| Voelckel et al. [62] | Anesthetized pigs | Models of very severe haemorrhagic shock: Dissection of the right liver lobe allowing blood loss, to reach MAP target < 30 mmHg (near fatal hypotension) |
Increase MAP without a specific target. |
Resuscitation with vasopressin during uncontrolled shock |
|
In vasopressin group, renal artery blood flow was restored and remains higher than epinephrine or placebo groups |
No information on the effect on renal function |
| Voelckel et al. [64] | Anesthetized pigs | Models of very severe haemorrhagic shock: Blood exsanguination and ventricular fibrillation was induced with single administration of alternating current. | Return of spontaneous circulation with a MAP ≥ baseline value before exsanguination. | Injection of vasopressin after 4 min of untreated ventricular fibrillation and 4 min of cardiopulmonary resuscitation |
|
In vasopressin group, renal artery blood flow was restored and remains higher than epinephrine or placebo groups |
No information on the effect on renal function |
| Sims et al. [65] | Humans | Trauma patients who received at least 6 units of blood products | MAP target ≥ 65 mmHg for 48 h | Randomized study: use of Vasopressin (bolus of 4 U then 0.04 U/min) ± norepinephrine to target ≥ 65 mmHg of MAP |
|
|
|
These experimental findings prompted the authors’ group to perform the so far single clinical study that investigated the impact of vasopressin on renal function in patients with hemorrhagic shock. In a randomized, double-blind, placebo-controlled trial in a total of 100 patients with trauma, who had received at least 6 units of blood products, the authors tested the hypothesis of whether vasopressin (bolus of 4 U, thereafter ≤0.04 U/min) vs. other vasopressors and titrated to target a MAP ~65 mmHg would reduce the total volume of blood product transfused. Patients who received AVP indeed required significantly fewer blood products, while none of the other secondary end points (i.e., total fluid balance (p = 0.10), vasopressor requirements, secondary complications, and mortality at day 30) showed any significant intergroup difference. Of note, albeit not significant either (p = 0.19), AKI was less frequent in the AVP-treated patients (n = 8, 16%) when compared to the control group (n = 14, 27%) [65]. This result is interesting as blood product administration may independently be associated with the following adverse events: venous thromboembolism, multiple-organ failure, and death [66,67].
9. Potential Adverse Effect of Vasopressor during Hemorrhagic Shock
During hemorrhagic shock, arterial vascular resistances are increased, and cardiac output is low as well. The use of norepinephrine alone may theoretically worsen the hemodynamics, may induce excessive vasoconstriction, and may further induce ischemic injuries. Experimental studies, due to their short-term assessment, do not allow to answer this concern, and in a clinical setting, there is no data on patients with hemorrhagic shock treated with vasopressor alone.
Furthermore, there is a theoretical risk of lowered cardiac output through higher cardiac afterload induced by arterial vasoconstriction with norepinephrine use. This was not reported in the literature. This may be explained by the beta1 effect of norepinephrine.
10. Conclusions
The European guidelines recommend the administration of vasopressors in addition to fluids to maintain the target arterial pressure in the presence of life-threatening hypotension [1]. These recommendations are based on studies where patients with hemorrhagic shock resuscitated with restricted volume and permissive hypotension had either improved survival [68,69,70] or at least unchanged mortality [71,72] when compared to patients resuscitated with a non-restrictive fluid strategy. In addition, aggressive volume administration has been shown to aggravate the incidence of secondary abdominal compartment syndrome [73], coagulopathy [74], and multiple organ failure [75] and, thereby, decrease the likelihood of survival [75,76,77,78].
To date, no randomized studies compared the outcome of patients with hemorrhagic shock resuscitated with fluids alone vs. fluids with vasopressor. Moreover, no randomized study compared the choice of vasopressor used in this situation. In this mini-review, we highlighted the importance of this unanswered question and the utmost importance of renal hemodynamic effects of vasopressors in the setting of hemorrhagic shock. Nevertheless, according to the current knowledge, we might suggest that vasopressor treatment could be used during hemorrhagic shock in association with fluid therapy. Norepinephrine administration appears to be a safe approach, as it does not threaten kidney function and allows a fluid-sparing effect. To date, there is not enough data to evaluate and conclude the impact of vasopressin on kidney hemodynamics and/or function.
Abbreviations
| AKI | Acute kidney injury |
| CO | Cardiac output |
| DO2 | Oxygen delivery |
| DO2R | Renal oxygen delivery |
| GFR | Glomerular filtration rate |
| I/R | Ischemia/Reperfusion |
| MAP | Mean arterial pressure |
| PμvO2 | Microvascular O2 partial pressure |
| RBF | Renal blood flow |
| SAP | Systolic arterial pressure |
| VO2R | Renal oxygen consumption |
Author Contributions
N.F., P.A., P.R. and J.D. wrote the manuscript. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analysed in this article.
Conflicts of Interest
The authors declare no conflict of interest.
Funding Statement
This research was supported by the Deutsche Forschungsgemeinschaft (DFG, German Research Foundation, project 251293561—collaborative research center CRC 1149).
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Spahn D.R., Bouillon B., Cerny V., Duranteau J., Filipescu D., Hunt B.J., Komadina R., Maegele M., Nardi G., Riddez L., et al. The European guideline on management of major bleeding and coagulopathy following trauma: Fifth edition. Crit. Care. 2019;23:98. doi: 10.1186/s13054-019-2347-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Asfar P., May C. A Rationale for the Use of Norepinephrine after the Control of Bleeding in Hemorrhagic Shock? Am. J. Respir. Crit. Care Med. 2022;206:1–2. doi: 10.1164/rccm.202203-0521ED. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schadt J.C., Ludbrook J. Hemodynamic and neurohumoral responses to acute hypovolemia in conscious mammals. Pt 2Am. J. Physiol.-Heart Circ. Physiol. 1991;260:H305–H318. doi: 10.1152/ajpheart.1991.260.2.H305. [DOI] [PubMed] [Google Scholar]
- 4.Levy B., Fritz C., Tahon E., Jacquot A., Auchet T., Kimmoun A. Vasoplegia treatments: The past, the present, and the future. Crit. Care. 2018;22:52. doi: 10.1186/s13054-018-1967-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Douzinas E.E., Livaditi O., Tasoulis M.-K., Prigouris P., Bakos D., Goutas N., Vlachodimitropoulos D., Andrianakis I., Betrosian A., Tsoukalas G.D. Nitrosative and Oxidative Stresses Contribute to Post-Ischemic Liver Injury Following Severe Hemorrhagic Shock: The Role of Hypoxemic Resuscitation. PLoS ONE. 2012;7:e32968. doi: 10.1371/journal.pone.0032968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Baker T.A., Romero J., Bach H.H., Strom J.A., Gamelli R.L., Majetschak M. Systemic release of cytokines and heat shock proteins in porcine models of polytrauma and hemorrhage. Crit. Care Med. 2012;40:876–885. doi: 10.1097/CCM.0b013e318232e314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vatner S.F., Braunwald E. Cardiovascular Control Mechanisms in the Conscious State. N. Engl. J. Med. 1975;293:970–976. doi: 10.1056/NEJM197511062931906. [DOI] [PubMed] [Google Scholar]
- 8.Legrand M., Mik E.G., Balestra G.M., Lutter R., Pirracchio R., Payen D., Ince C. Fluid resuscitation does not improve renal oxygenation during hemorrhagic shock in rats. Anesthesiology. 2010;112:119–127. doi: 10.1097/ALN.0b013e3181c4a5e2. [DOI] [PubMed] [Google Scholar]
- 9.Ergin B., Kapucu A., Guerci P., Ince C. The role of bicarbonate precursors in balanced fluids during haemorrhagic shock with and without compromised liver function. Br. J. Anaesth. 2016;117:521–528. doi: 10.1093/bja/aew277. [DOI] [PubMed] [Google Scholar]
- 10.Nelimarkka O., Halkola L., Niinikoski J. Renal hypoxia and lactate metabolism in hemorrhagic shock in dogs. Crit. Care Med. 1984;12:656–660. doi: 10.1097/00003246-198408000-00011. [DOI] [PubMed] [Google Scholar]
- 11.Mayeur N., Minville V., Jaafar A., Allard J., al Saati T., Guilbeau-Frugier C., Fourcade O., Girolami J.P., Schaak S., Tack I. Morphologic and functional renal impact of acute kidney injury after prolonged hemorrhagic shock in mice. Crit. Care Med. 2011;39:2131–2138. doi: 10.1097/CCM.0b013e31821f04f0. [DOI] [PubMed] [Google Scholar]
- 12.Rector J.B., Stein J.H., Bay W.H., Osgood R.W., Ferris T.F. Effect of hemorrhage and vasopressor agents on distribution of renal blood flow. Am. J. Physiol.-Leg. Content. 1972;222:1125–1131. doi: 10.1152/ajplegacy.1972.222.5.1125. [DOI] [PubMed] [Google Scholar]
- 13.Van Bommel J., Siegemund M., Henny C.P., Ince C. Heart, kidney, and intestine have different tolerances for anemia. Transl. Res. 2008;151:110–117. doi: 10.1016/j.trsl.2007.11.001. [DOI] [PubMed] [Google Scholar]
- 14.Johannes T., Mik E.G., Nohé B., Unertl K.E., Ince C. Acute decrease in renal microvascular Po2 during acute normovolemic hemodilution. Am. J. Physiol.-Ren. Physiol. 2007;292:F796–F803. doi: 10.1152/ajprenal.00206.2006. [DOI] [PubMed] [Google Scholar]
- 15.Konrad F.M., Mik E.G., Bodmer S.I.A., Ates N.B., Willems H.F.E.M., Klingel K., de Geus H.R.H., Stolker R.J., Johannes T. Acute Normovolemic Hemodilution in the Pig Is Associated with Renal Tissue Edema, Impaired Renal Microvascular Oxygenation, and Functional Loss. Anesthesiology. 2013;119:256–269. doi: 10.1097/ALN.0b013e31829bd9bc. [DOI] [PubMed] [Google Scholar]
- 16.Wang L., Pei F., Wu J., Ouyang B., Guan X. Kidney Injury in a Hemodilution Model of Hemorrhagic Shock and Fluid Resuscitation. Am. J. Med. Sci. 2021;362:506–511. doi: 10.1016/j.amjms.2021.06.002. [DOI] [PubMed] [Google Scholar]
- 17.Harrois A., Libert N., Duranteau J. Acute kidney injury in trauma patients. Curr. Opin. Crit. Care. 2017;23:447–456. doi: 10.1097/MCC.0000000000000463. [DOI] [PubMed] [Google Scholar]
- 18.Kirchheim H.R., Ehmke H., Hackenthal E., Löwe W., Persson P. Autoregulation of renal blood flow, glomerular filtration rate and renin release in conscious dogs. Pflügers Arch. 1987;410:441–449. doi: 10.1007/BF00586523. [DOI] [PubMed] [Google Scholar]
- 19.Finke R., Gross R., Hackenthal E., Huber J., Kirchheim H.R. Threshold pressure for the pressure-dependent renin release in the autoregulating kidney of conscious dogs. Pflügers Arch. 1983;399:102–110. doi: 10.1007/BF00663904. [DOI] [PubMed] [Google Scholar]
- 20.Kremser P.C., Gewertz B.L. Effect of pentobarbital and hemorrhage on renal autoregulation. Am. J. Physiol.-Ren. Physiol. 1985;249:F356–F360. doi: 10.1152/ajprenal.1985.249.3.F356. [DOI] [PubMed] [Google Scholar]
- 21.Johnson P.C. The Myogenic Response. In: Bevan J.A., Halpern W., Mulvany M.J., editors. The Resistance Vasculature: A Publication of the University of Vermont Center for Vascular Research [Internet] Humana Press; Totowa, NJ, USA: 1991. pp. 159–168. Vascular Biomedicine. [DOI] [Google Scholar]
- 22.Harder D.R., Gilbert R., Lombard J.H. Vascular muscle cell depolarization and activation in renal arteries on elevation of transmural pressure. Am. J. Physiol.-Ren. Physiol. 1987;253:F778–F781. doi: 10.1152/ajprenal.1987.253.4.F778. [DOI] [PubMed] [Google Scholar]
- 23.Wilcox C.S. Regulation of Renal Blood Flow by Plasma Chloride. J. Clin. Investig. 1983;71:726–735. doi: 10.1172/JCI110820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Semler M.W., Self W.H., Wanderer J.P., Ehrenfeld J.M., Wang L., Byrne D.W., Stollings J.L., Kumar A.B., Hughes C.G., Hernandez A., et al. Balanced Crystalloids versus Saline in Critically Ill Adults. N. Engl. J. Med. 2018;378:829–839. doi: 10.1056/NEJMoa1711584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schetz M. Vasopressors and the kidney. Blood Purif. 2002;20:243–251. doi: 10.1159/000047016. [DOI] [PubMed] [Google Scholar]
- 26.Anderson W.P., Korner P.I., Selig S.E. Mechanisms involved in the renal responses to intravenous and renal artery infusions of noradrenaline in conscious dogs. J. Physiol. 1981;321:21–30. doi: 10.1113/jphysiol.1981.sp013969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Bellomo R., Kellum J.A., Wisniewski S.R., Pinsky M.R., Ondulik B. Effects of Norepinephrine on the Renal Vasculature in Normal and Endotoxemic Dogs. Am. J. Respir. Crit. Care Med. 1999;159:1186–1192. doi: 10.1164/ajrccm.159.4.9802055. [DOI] [PubMed] [Google Scholar]
- 28.Schaer G.L., Fink M.P., Parrillo J.E. Norepinephrine alone versus norepinephrine plus low-dose dopamine: Enhanced renal blood flow with combination pressor therapy. Crit. Care Med. 1985;13:492–496. doi: 10.1097/00003246-198506000-00011. [DOI] [PubMed] [Google Scholar]
- 29.Booke M., Hinder F., McGuire R., Traber L.D., Traber D.L. Noradrenaline and nomega-monomethyl-L-arginine (L-NMMA): Effects on haemodynamics and regional blood flow in healthy and septic sheep. Clin. Sci. 2000;98:193–200. doi: 10.1042/CS19990143. [DOI] [PubMed] [Google Scholar]
- 30.Mills L.C., Moyer J.H., Handley C.A. Effects of various sympathicomimetic drugs on renal hemodynamics in normotensive and hypotensive dogs. Am. J. Physiol.-Leg. Content. 1960;198:1279–1283. doi: 10.1152/ajplegacy.1960.198.6.1279. [DOI] [PubMed] [Google Scholar]
- 31.Peng Z.-Y., Critchley L.A.H., Fok B.S.P. The effects of increasing doses of noradrenaline on systemic and renal circulations in acute bacteraemic dogs. Intensive Care Med. 2005;31:1558–1563. doi: 10.1007/s00134-005-2741-y. [DOI] [PubMed] [Google Scholar]
- 32.Maier M., Starlinger M., Wagner M., Meyer D., Binder B.R. The effect of hemorrhagic hypotension on urinary kallikrein excretion, renin activity, and renal cortical blood flow in the pig. Circ. Res. 1981;48:386–392. doi: 10.1161/01.RES.48.3.386. [DOI] [PubMed] [Google Scholar]
- 33.Bersten A.D., Holt A.W. Vasoactive drugs and the importance of renal perfusion pressure. New Horiz. 1995;3:650–661. [PubMed] [Google Scholar]
- 34.Redfors B., Bragadottir G., Sellgren J., Swärd K., Ricksten S.-E. Effects of norepinephrine on renal perfusion, filtration and oxygenation in vasodilatory shock and acute kidney injury. Intensive Care Med. 2011;37:60–67. doi: 10.1007/s00134-010-2057-4. [DOI] [PubMed] [Google Scholar]
- 35.Vatner S.F. Effects of hemorrhage on regional blood flow distribution in dogs and primates. J. Clin. Investig. 1974;54:225–235. doi: 10.1172/JCI107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Adams P.L., Adams F.F., Bell P.D., Navar L.G. Impaired renal blood flow autoregulation in ischemic acute renal failure. Kidney Int. 1980;18:68–76. doi: 10.1038/ki.1980.111. [DOI] [PubMed] [Google Scholar]
- 37.Pullman T.N., Mcclure W.W. The Response of the Renal Circulation in Man to Constant-Speed Infusions of l -Norepinephrine. Circulation. 1954;9:600–605. doi: 10.1161/01.CIR.9.4.600. [DOI] [PubMed] [Google Scholar]
- 38.Gombos E.A., Hulet W.H., Bopp P., Goldring W., Baldwin D.S., Chasis H. Reactivity of renal and systemic circulations to vasoconstrictor agents in normotensive and hypertensive subjects. J. Clin. Investig. 1962;41:203–217. doi: 10.1172/JCI104472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lang C.C., Rahman A.R., Balfour D.J.K., Struthers A.D. Effect of Noradrenaline on Renal Sodium and Water Handling in Euhydrated and Overhydrated Man. Clin. Sci. 1993;85:487–494. doi: 10.1042/cs0850487. [DOI] [PubMed] [Google Scholar]
- 40.Richer M., Robert S., Lebel M. Renal hemodynamics during norepinephrine and low-dose dopamine infusions in man. Crit. Care Med. 1996;24:1150–1156. doi: 10.1097/00003246-199607000-00014. [DOI] [PubMed] [Google Scholar]
- 41.Hoogenberg K., Smit A.J., Girbes A.R.J. Effects of low-dose dopamine on renal and systemic hemodynamics during incremental norepinephrine infusion in healthy volunteers. Crit. Care Med. 1998;26:260–265. doi: 10.1097/00003246-199802000-00022. [DOI] [PubMed] [Google Scholar]
- 42.Myers B.D., Deen W.M., Brenner B.M. Effects of norepinephrine and angiotensin II on the determinants of glomerular ultrafiltration and proximal tubule fluid reabsorption in the rat. Circ. Res. 1975;37:101–110. doi: 10.1161/01.RES.37.1.101. [DOI] [PubMed] [Google Scholar]
- 43.Hellebrekers L.J., Liard J.F., Laborde A.L., Greene A.S., Cowley A.W. Regional autoregulatory responses during infusion of vasoconstrictor agents in conscious dogs. Am. J. Physiol.-Heart Circ. Physiol. 1990;259:H1270–H1277. doi: 10.1152/ajpheart.1990.259.4.H1270. [DOI] [PubMed] [Google Scholar]
- 44.Metting P.J., Stein P.M., Stoos B.A., Kostrzewski K.A., Britton S.L. Systemic vascular autoregulation amplifies pressor responses to vasoconstrictor agents. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 1989;256:R98–R105. doi: 10.1152/ajpregu.1989.256.1.R98. [DOI] [PubMed] [Google Scholar]
- 45.Dunberry-Poissant S., Gilbert K., Bouchard C., Baril F., Cardinal A.-M., L’Ecuyer S., Hylands M., Lamontagne F., Rousseau G., Charbonney E. Fluid sparing and norepinephrine use in a rat model of resuscitated haemorrhagic shock: End-organ impact. Intensive Care Med. Exp. 2018;6:47. doi: 10.1186/s40635-018-0212-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Libert N., Laemmel E., Harrois A., Laitselart P., Bergis B., Isnard P., Terzi F., Decante B., Mercier O., Vicaut E., et al. Renal Microcirculation and Function in a Pig Model of Hemorrhagic Shock Resuscitation with Norepinephrine. Am. J. Respir. Crit. Care Med. 2022;206:34–43. doi: 10.1164/rccm.202109-2120OC. [DOI] [PubMed] [Google Scholar]
- 47.Murakawa K., Kobayashi A. Effects of Vasopressors on Renal Tissue Gas Tensions During Hemorrhagic Shock in Dogs. Crit. Care Med. 1988;16:789–792. doi: 10.1097/00003246-198808000-00012. [DOI] [PubMed] [Google Scholar]
- 48.Prunet B., Prat N., Couret D., Cordier P.-Y., De Bourmont S., Lambert D., Asencio Y., Meaudre E., Michelet P. Midterm Effects of Fluid Resuscitation Strategies in an Experimental Model of Lung Contusion and Hemorrhagic Shock. Shock. 2014;41:159–165. doi: 10.1097/SHK.0000000000000069. [DOI] [PubMed] [Google Scholar]
- 49.Harrois A., Baudry N., Huet O., Kato H., Dupic L., Lohez M., Ziol M., Vicaut E., Duranteau J. Norepinephrine Decreases Fluid Requirements and Blood Loss While Preserving Intestinal Villi Microcirculation during Fluid Resuscitation of Uncontrolled Hemorrhagic Shock in Mice. Anesthesiology. 2015;122:1093–1102. doi: 10.1097/ALN.0000000000000639. [DOI] [PubMed] [Google Scholar]
- 50.Prowle J.R., Kirwan C.J., Bellomo R. Fluid management for the prevention and attenuation of acute kidney injury. Nat. Rev. Nephrol. 2014;10:37–47. doi: 10.1038/nrneph.2013.232. [DOI] [PubMed] [Google Scholar]
- 51.Demiselle J., Fage N., Radermacher P., Asfar P. Vasopressin and its analogues in shock states: A review. Ann. Intensive Care. 2020;10:9. doi: 10.1186/s13613-020-0628-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Edwards R.M., Trizna W., Kinter L.B. Renal microvascular effects of vasopressin and vasopressin antagonists. Am. J. Physiol.-Ren. Physiol. 1989;256:F274–F278. doi: 10.1152/ajprenal.1989.256.2.F274. [DOI] [PubMed] [Google Scholar]
- 53.Rudichenko V.M., Beierwaltes W.H. Arginine Vasopressin-lnduced Renal Vasodilation Mediated by Nitric Oxide. J. Vasc. Res. 1995;32:100–105. doi: 10.1159/000159082. [DOI] [PubMed] [Google Scholar]
- 54.Morelli A., Rocco M., Conti G., Orecchioni A., De Gaetano A., Cortese G., Coluzzi F., Vernaglione E., Pelaia P., Pietropaoli P. Effects of terlipressin on systemic and regional haemodynamics in catecholamine-treated hyperkinetic septic shock. Intensive Care Med. 2004;30:597–604. doi: 10.1007/s00134-003-2094-3. [DOI] [PubMed] [Google Scholar]
- 55.Patel B.M., Chittock D.R., Russell J.A., Walley K.R. Beneficial Effects of Short-term Vasopressin Infusion during Severe Septic Shock. Anesthesiology. 2002;96:576–582. doi: 10.1097/00000542-200203000-00011. [DOI] [PubMed] [Google Scholar]
- 56.Gordon A.C., Mason A.J., Thirunavukkarasu N., Perkins G.D., Cecconi M., Cepkova M., Pogson D.G., Aya H.D., Anjum A., Frazier G.J., et al. Effect of Early Vasopressin vs Norepinephrine on Kidney Failure in Patients With Septic Shock: The VANISH Randomized Clinical Trial. JAMA. 2016;316:509–518. doi: 10.1001/jama.2016.10485. [DOI] [PubMed] [Google Scholar]
- 57.Nagendran M., Russell J.A., Walley K.R., Brett S.J., Perkins G.D., Hajjar L., Mason A.J., Ashby D., Gordon A.C. Vasopressin in septic shock: An individual patient data meta-analysis of randomised controlled trials. Intensive Care Med. 2019;45:844–855. doi: 10.1007/s00134-019-05620-2. [DOI] [PubMed] [Google Scholar]
- 58.Thiemermann C., Szabó C., Mitchell J.A., Vane J.R. Vascular hyporeactivity to vasoconstrictor agents and hemodynamic decompensation in hemorrhagic shock is mediated by nitric oxide. Proc. Natl. Acad. Sci. USA. 1993;90:267–271. doi: 10.1073/pnas.90.1.267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Sims C.A., Guan Y., Bergey M., Jaffe R., Holmes-Maguire L., Martin N., Reilly P. Arginine vasopressin, copeptin, and the development of relative AVP deficiency in hemorrhagic shock. Am. J. Surg. 2017;214:589–595. doi: 10.1016/j.amjsurg.2017.06.015. [DOI] [PubMed] [Google Scholar]
- 60.Raedler C., Voelckel W.G., Wenzel V., Krismer A.C., Schmittinger C.A., Herff H., Mayr V.D., Stadlbauer K.H., Lindner K.H., Königsrainer A. Treatment of uncontrolled hemorrhagic shock after liver trauma: Fatal effects of fluid resuscitation versus improved outcome after vasopressin. Anesth. Analg. 2004;98:1759–1766. doi: 10.1213/01.ANE.0000117150.29361.5A. [DOI] [PubMed] [Google Scholar]
- 61.Stadlbauer K.H., Wagner-Berger H.G., Krismer A.C., Voelckel W.G., Konigsrainer A., Lindner K.H., Wenzel V. Vasopressin improves survival in a porcine model of abdominal vascular injury. Crit. Care. 2007;11:R81. doi: 10.1186/cc5977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Voelckel W.G., Raedler C., Wenzel V., Lindner K.H., Krismer A.C., Schmittinger C.A., Herff H., Rheinberger K., Königsrainer A. Arginine vasopressin, but not epinephrine, improves survival in uncontrolled hemorrhagic shock after liver trauma in pigs. Crit. Care Med. 2003;31:1160–1165. doi: 10.1097/01.CCM.0000060014.75282.69. [DOI] [PubMed] [Google Scholar]
- 63.Sims C.A., Yuxia G., Singh K., Werlin E.C., Reilly P.M., Baur J.A. Supplemental arginine vasopressin during the resuscitation of severe hemorrhagic shock preserves renal mitochondrial function. PLoS ONE. 2017;12:e0186339. doi: 10.1371/journal.pone.0186339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Voelckel W.G., Lurie K.G., Lindner K.H., Zielinski T., McKnite S., Krismer A.C., Wenzel V. Vasopressin Improves Survival After Cardiac Arrest in Hypovolemic Shock. Anesth. Analg. 2000;91:627–634. doi: 10.1213/00000539-200009000-00024. [DOI] [PubMed] [Google Scholar]
- 65.Sims C.A., Holena D., Kim P., Pascual J., Smith B., Martin N., Seamon M., Shiroff A., Raza S., Kaplan L., et al. Effect of Low-Dose Supplementation of Arginine Vasopressin on Need for Blood Product Transfusions in Patients with Trauma and Hemorrhagic Shock: A Randomized Clinical Trial. JAMA Surg. 2019;154:994–1003. doi: 10.1001/jamasurg.2019.2884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Spinella P.C., Perkins J.G., Grathwohl K.W., Beekley A.C., Niles S.E., McLaughlin D.F., Wade C.E., Holcomb J.B. Effect of plasma and red blood cell transfusions on survival in patients with combat related traumatic injuries. J. Trauma. 2008;64:S69–S77. doi: 10.1097/TA.0b013e318160ba2f. [DOI] [PubMed] [Google Scholar]
- 67.Blet A., McNeil J.B., Josse J., Cholley B., Cinotti R., Cotter G., Dauvergne A., Davison B., Duarte K., Duranteau J., et al. Association between in-ICU red blood cells transfusion and 1-year mortality in ICU survivors. Crit. Care. 2022;26:307. doi: 10.1186/s13054-022-04171-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bickell W.H., Wall M.J., Pepe P.E., Martin R.R., Ginger V.F., Allen M.K., Mattox K.L. Immediate versus Delayed Fluid Resuscitation for Hypotensive Patients with Penetrating Torso Injuries. N. Engl. J. Med. 1994;331:1105–1109. doi: 10.1056/NEJM199410273311701. [DOI] [PubMed] [Google Scholar]
- 69.Sampalis J.S., Tamim H., Denis R., Boukas S., Ruest S.-A., Nikolis A., Lavoie A., Fleiszer D., Brown R., Mulder D., et al. Ineffectiveness of on-site intravenous lines: Is prehospital time the culprit? J. Trauma. 1997;43:608–615. doi: 10.1097/00005373-199710000-00008. [DOI] [PubMed] [Google Scholar]
- 70.Schreiber M.A., Meier E.N., Tisherman S.A., Kerby J.D., Newgard C.D., Brasel K., Egan D., Witham W., Williams C., Daya M., et al. A Controlled Resuscitation Strategy is Feasible and Safe in Hypotensive Trauma Patients: Results of a Prospective Randomized Pilot Trial. J. Trauma Acute Care Surg. 2015;78:687–697. doi: 10.1097/TA.0000000000000600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Dutton R.P., Mackenzie C.F., Scalea T.M. Hypotensive Resuscitation during Active Hemorrhage: Impact on In-Hospital Mortality. J. Trauma Inj. Infect. Crit. Care. 2002;52:1141–1146. doi: 10.1097/00005373-200206000-00020. [DOI] [PubMed] [Google Scholar]
- 72.Turner J., Nicholl J., Webber L., Cox H., Dixon S., Yates D. A randomised controlled trial of prehospital intravenous fluid replacement therapy in serious trauma. Health Technol. Assess. Winch. Engl. 2000;4:1–57. doi: 10.3310/hta4310. [DOI] [PubMed] [Google Scholar]
- 73.Madigan M.C., Kemp C.D., Johnson J.C., Cotton B.A. Secondary Abdominal Compartment Syndrome After Severe Extremity Injury: Are Early, Aggressive Fluid Resuscitation Strategies to Blame? J. Trauma Inj. Infect. Crit. Care. 2008;64:280–285. doi: 10.1097/TA.0b013e3181622bb6. [DOI] [PubMed] [Google Scholar]
- 74.Maegele M., Lefering R., Yucel N., Tjardes T., Rixen D., Paffrath T., Simanski C., Neugebauer E., Bouillon B., The AG Polytrauma of the German Trauma Society (DGU) Early coagulopathy in multiple injury: An analysis from the German Trauma Registry on 8724 patients. Injury. 2007;38:298–304. doi: 10.1016/j.injury.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 75.Kasotakis G., Sideris A., Yang Y., de Moya M., Alam H., King D.R., Tompkins R., Velmahos G. Aggressive Early Crystalloid Resuscitation adversely affects Outcomes in Adult Blunt Trauma Patients: An Analysis of the Glue Grant Database. J. Trauma Acute Care Surg. 2013;74:1215–1222. doi: 10.1097/TA.0b013e3182826e13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Harada M.Y., Ko A., Barmparas G., Smith E.J.T., Patel B.K., Dhillon N.K., Thomsen G.M., Ley E.J. 10-Year trend in crystalloid resuscitation: Reduced volume and lower mortality. Int. J. Surg. 2017;38:78–82. doi: 10.1016/j.ijsu.2016.12.073. [DOI] [PubMed] [Google Scholar]
- 77.Hussmann B., Lefering R., Waydhas C., Touma A., Kauther M.D., Ruchholtz S., Lendemans S., Trauma Registry of the German Society for Trauma Surgery Does increased prehospital replacement volume lead to a poor clinical course and an increased mortality? A matched-pair analysis of 1896 patients of the Trauma Registry of the German Society for Trauma Surgery who were managed by an emergency doctor at the accident site. Injury. 2013;44:611–617. doi: 10.1016/j.injury.2012.02.004. [DOI] [PubMed] [Google Scholar]
- 78.Haut E.R., Kalish B.T., Cotton B.A., Efron D.T., Haider A.H., Stevens K.A., Kieninger A.N., Cornwell E.E., Chang D.C. Prehospital intravenous fluid administration is associated with higher mortality in trauma patients: A National Trauma Data Bank analysis. Ann. Surg. 2011;253:371–377. doi: 10.1097/SLA.0b013e318207c24f. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No new data were created or analysed in this article.