Abstract
The filamentous bacterium Streptomyces coelicolor undergoes a complicated process of morphological differentiation that begins with the formation of an aerial mycelium and culminates in sporulation. Genes required for the initiation of aerial mycelium formation have been termed bld (bald), describing the smooth, undifferentiated colonies of mutant strains. By using an insertional mutagenesis protocol that relies on in vitro transposition, we have isolated a bld mutant harboring an insertion in a previously uncharacterized gene, SCE59.12c, renamed here rsuA. The insertion mutant exhibited no measurable growth defect but failed to produce an aerial mycelium and showed a significant delay in the production of the polyketide antibiotic actinorhodin. The rsuA gene encodes an apparent anti-sigma factor and is located immediately downstream of SCE59.13c, renamed here sigU, whose product is inferred to be a member of the extracytoplasmic function subfamily of RNA polymerase sigma factors. The absence of rsuA in a strain that contained sigU caused a block in development, and the overexpression of sigU in an otherwise wild-type strain caused a delay in aerial mycelium formation. However, a strain in which both rsuA and sigU had been deleted was able to undergo morphological differentiation normally. We conclude that the rsuA-encoded anti-sigma factor is responsible for antagonizing the function of the sigma factor encoded by sigU. We also conclude that the sigU-encoded sigma factor is not normally required for development but that its uncontrolled activity obstructs morphological differentiation at an early stage.
Members of the genus Streptomyces are filamentous soil bacteria that undertake a complex developmental process that culminates in the production of unigenomic spores (7, 8, 16). Colonies germinate from spores and grow into the soil by forming a branching network of multinucleoid hyphae termed the substrate mycelium. Morphological differentiation commences with the formation of the aerial mycelium, a fuzzy layer of specialized hyphae that project away from the surface of the colony into the air. These aerial hyphae later undergo septation to produce uninucleoid compartments that mature into pigmented spores, thereby completing the cycle. Morphological differentiation is associated with the production of secondary metabolites, such as pigments and antibiotics, and members of the Streptomyces genus are responsible for the production of a diverse array of such compounds, including the majority of the antibiotics currently in clinical use (7).
Mutant strains of Streptomyces coelicolor have been isolated that fail to initiate differentiation and do not form an aerial mycelium (for examples, see references 6, 22, and 27). These mutants exhibit a bald (bld) phenotype; the surface of mutant colonies remains smooth and does not accumulate the hairlike aerial hyphae. Whereas the corresponding bld genes have been identified from some of these mutant strains (2, 4, 11, 19, 24, 26, 31), it is clear that many genes that influence aerial mycelium formation remain to be uncovered (27). A striking property of bld mutants is their capacity to be rescued by extracellular complementation, a phenomenon that has been interpreted to indicate the existence of signaling molecules that help to regulate aerial mycelium formation under certain conditions (26, 32). But the nature of the signals and the genes involved in the signaling pathways are largely unknown.
As part of an effort to discover additional genes involved in aerial mycelium formation, we have been carrying out insertional mutagenesis with the transposon Tn5apr to generate mutants of S. coelicolor that exhibit a bld phenotype. Here we report the isolation of a mutant that cannot produce an aerial mycelium and shows delayed production of the blue-pigmented antibiotic actinorhodin. The mutant harbors an insertion in a previously uncharacterized gene that appears to encode a membrane-bound anti-sigma factor and is located adjacent to the gene for a putative member of the ECF (extracytoplasmic function) subfamily of RNA polymerase sigma factors. We present evidence that the bld phenotype exhibited by the insertion mutant is an indirect consequence of the unregulated activity of the ECF sigma factor.
MATERIALS AND METHODS
Strains and growth conditions.
S. coelicolor strain M145 (prototrophic, SCP1− SCP2−) was used for mutant construction (18). Strains were grown on solid R2YE, mannitol soya flour, minimal medium (MM) with 1% (wt/vol) glucose or with 0.5% (wt/vol) mannitol, or Difco nutrient agar medium or in liquid yeast extract-malt extract medium (18) at 30°C with, as indicated (from Sigma), 25 μg of apramycin sulfate per ml, 200 μg of spectinomycin dihydrochloride per ml, or 50 μg of thiostrepton per ml.
Extracellular complementation of aerial hyphal growth was assessed by plating pairs of bld mutants in close proximity on solid R2YE medium and then observing them after 1 week (32). The S. coelicolor bld mutant strains used were J774 (bldD53 cysA15 mthB2 pheA1 strA1 SCP1NF SCP2*), J660 (bldC18 cysD18 mthB2 agaA7 SCP1NF SCP2*) (22), J2151 (ΔglkA119 bldM::hyg SCP1− SCP2−) (24), C103 (bldG103 hisA1 uraA1 strA1 Pgl− SCP1− SCP2−), C109 (bldH109 hisA1 uraA1 strA1 Pgl− SCP1− SCP2−) (6), J1700 (bldA39 hisA1 uraA1 strA1 Pgl− SCP1− SCP2−) (20), NS40 (bldK::aadA hisA1 uraA1 strA1 Pgl− SCP1NF SCP2−) (a derivative of NS17 in the J1508 background; 26), and HU261 (bldJ261 hisA1 uraA1 strA1 Pgl− SCP1NF SCP2*) (32).
Insertional mutagenesis of S. coelicolor.
Insertional mutagenesis of M145 was carried out as described previously (13). Briefly, a plasmid library of S. coelicolor DNA in the pSpecoriT vector was subjected to in vitro transposition with Tn5apr. Transposon-disrupted plasmids were isolated and introduced into S. coelicolor M145 by conjugation from E. coli ET12567(pUB307). Exconjugants were selected with apramycin and visually screened for morphological defects leading to the isolation of apramycin-resistant (Aprr), spectinomycin-sensitive (Specs), bald strain NY415. Linkage of the transposon insertion to the bald mutation in NY415 was confirmed by genomic DNA transformation (28), and the chromosomal location of Tn5apr in this strain was determined by sequencing of the transposon-flanking DNA (13).
Construction of a sigU-rsuA deletion strain
A plasmid was constructed as follows for the deletion of sigU and rsuA and their replacement with tsr (conferring resistance to thiostrepton) in S. coelicolor M145. DNAs flanking the rsuA and sigU loci were amplified by PCR from M145 genomic DNA by using the primers 5′-CGAGCTCACCAGGCGTCCAGGAAGCCGGTGAA-3′ and 5′-CTAGCTAGCAGGTCATGACCTTCGACGGGAA-3′ and the primers 5′-CTAGCTAGCTTCATTGGCCACGACGGAAT-3′ and 5′-CGGATATCTCGTCGTCCTCGTGCCGTCA-3′ (restriction enzyme sites are underlined). The PCR products were digested with SacI/NheI and NheI/EcoRV, respectively, and cloned into the vector pSpec (13). The tsr gene, amplified by PCR with the primers 5′-CTAGCTAGCCGGCCACGACACCCCCATCGGCATCGCGT-3′ and 5′-CTAGCTAGCGACCGGCGCCACACCGGCTTGCGCCGGCT-3′ from plasmid pJR35 (laboratory collection), was then inserted into the NheI site to give the knockout construct.
The ΔsigU-rsuA::tsr strain was generated by transformation of M145 with the above-described knockout plasmid. First, the knockout plasmid was passaged through dam dcm mutant Escherichia coli strain SCS110 (Stratagene). M145 protoplasts were then transformed as previously described (18) with the unmethylated plasmids isolated from strain SCS110. Transformants were selected by overlaying with 50 μg of thiostrepton per ml after 16 h of growth. Transformants were streaked onto R2YE containing thiostrepton and then screened for spectinomycin sensitivity by replica plating on R2YE-spectinomycin. One of two identical thiostrepton-resistant (Tsrr) Specs strains, indicating replacement of the sigU and rsuA alleles with tsr, was chosen for further study. The deletion and tsr replacement event was verified by PCR of genomic DNA isolated from this strain.
Construction of plasmids containing sigU or rsuA and complementation experiments.
Complementation plasmids containing the sigU or rsuA gene were constructed in modified versions of the vectors pKC1218 and pSET152 (3, 18). Both of these vectors contain a gene conferring resistance to apramycin that was removed by digestion with SacI and replaced with the aadA gene, which confers spectinomycin-streptomycin resistance. The aadA gene was amplified by PCR from the plasmid pSpec (13) by using primers 5′-CGAGCTCGGCTTGAACGAATTGTTAGAC-3′ and 5′-CGAGCTCGCTTCGGTTTTCATGGCTTG-3′. These modified vectors, which confer spectinomycin-streptomycin resistance, were named pKC1218S and pSET152S.
The sigU gene was amplified by PCR from M145 genomic DNA by using the primers 5′-GGAATTCACAACTGCCCCATCGTGTACGTGGA-3′ and 5′-GGAATTCTGCATGGGGACAGAGTTACCCGTGTC-3′ and cloned into the EcoRI site of pKC1218S and pSET152S to give complementation plasmids pKC1218S-sigU and pSET152S-sigU, respectively. The rsuA gene was amplified from M145 genomic DNA with the primers 5′-GGAATTCTGCTGGGCAGCCGTCAAAAATA-3′ and 5′-GGAATTCTCCTCGTCGAGACGTACTTCA-3′ and cloned into the EcoRI site of pSET152S to give complementation plasmid pSET152S-rsuA. These complementation plasmids were passed through E. coli SCS110 and used to transform protoplasts (18) of S. coelicolor M145 or NY415 or the ΔsigU-rsuA::tsr strain. Transformants were selected by overlaying with spectinomycin dihydrochloride (100 μg/ml) and streptomycin sulfate (30 μg/ml) after 16 h of growth.
Zone of growth inhibition assays.
The sensitivity of S. coelicolor M145 or the ΔsigU-rsuA::tsr strain to various chemicals was tested as follows. Spores of each strain (107 CFU) were mixed in 4 ml of nutrient soft agar (Difco nutrient broth with 0.5% agar) and spread on nutrient agar plates. Glass microfiber filter disks (∼10 mm in diameter) soaked in 20 μl of the compound being tested were then applied to the surface of the soft agar. The zone of growth inhibition was measured after 24 h of growth at 30°C.
RESULTS
A new bld mutant strain generated by insertional mutagenesis.
By using an insertional mutagenesis protocol that relies on in vitro transposition (13), a mutant S. coelicolor strain, NY415, was isolated that did not initiate the morphological differentiation process normally undergone by this organism. NY415 exhibited a classic bald (bld) phenotype (16), failing to produce any aerial mycelium (Fig. 1A). NY415 also showed a severe delay in production of the blue-pigmented polyketide antibiotic actinorhodin. Whereas wild-type strain M145 produces large amounts of this pigment by 2 to 3 days of growth, blue pigmentation was not observed in NY415 until about 1 week of growth.
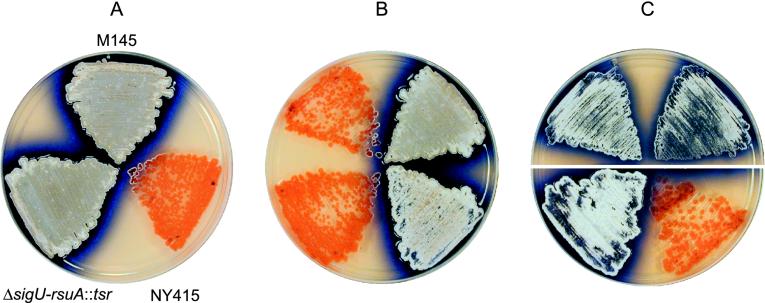
FIG. 1.
(A) Comparison of a wild-type S. coelicolor strain (M145), a bald strain (NY415) generated by transposon disruption of the gene rsuA (SCE59.12c), and a strain in which both rsuA and the upstream gene sigU (SCE59.13c) were deleted and replaced with a gene conferring thiostrepton resistance (ΔsigU-rsuA::tsr). Strains were grown for 4 days at 30°C on R2YE rich medium. (Note the extracellular complementation of NY415 for aerial mycelium formation where this strain approaches the two morphologically normal strains.) (B) Reversal of the bald phenotype of strain NY415 by either deletion of the transposon-disrupted rsuA allele along with sigU (top) or by complementation (bottom). Comparison of NY415 (top left) to a Tsrr Aprs Specs strain (top right) generated by transformation of NY415 protoplasts with a knockout plasmid designed to remove genes rsuA and sigU and replace them with tsr. Comparison of strain NY415 transformed with the integrating vector pSET152S (bottom left) versus strain NY415 transformed with the same vector into which gene rsuA had been inserted (pSET152S-rsuA, bottom right). Growth was for 4 days at 30°C on R2YE medium. (C) Uncoupling of the sigma factor gene sigU from the downstream putative anti-sigma factor gene rsuA results in defects in morphological differentiation. Comparison of the wild-type strain M145 transformed with the low-copy-number vector pKC1218S (top left) and M145 transformed with the same vector containing the gene sigU (pKC1218S-sigU, top right). Note the slight delay in aerial mycelium formation here compared to that of the vector-only strain. Comparison of strain ΔsigU-rsuA::tsr transformed with the integrating vector pSET152S (bottom left) and the same strain transformed with pSET152S containing gene sigU (pSET152S-sigU, bottom right). The presence of the sigma factor gene in the absence of its antagonist anti-sigma factor gene results in a severe bald phenotype. Growth was for 3 days (top) or 4 days (bottom) at 30°C on R2YE medium containing spectinomycin.
For many of the previously characterized bld mutants, it has been observed that aerial mycelium formation can be restored by growth on MM containing mannitol as the carbon source (6, 22). The NY415 strain remained largely bald when plated on MM-mannitol (data not shown). After prolonged incubation (1 to 2 weeks) of the bacteria on this medium, sprouting of small patches of white aerial hyphae on the surfaces of some colonies was observed. Rarely did the colonies become completely covered with aerial hyphae, and many colonies remained bald. As a control, we observed that M145, J1700 (a bldA mutant), and C103 (a bldG mutant), but not J2151 (a bldM mutant), could readily form aerial hyphae after only 2 days of growth on MM-mannitol medium (supplemented with histidine and uracil for J1700 and C103) as previously described (6, 22, 24). When grown on MM-glucose, NY415 colonies remained entirely bald over a 2-week period (data not shown).
Extracellular complementation of the bld mutant strain.
An extracellular signaling cascade has been proposed for the initiation of aerial mycelium formation on rich media based on observations of the ability of bld mutant strains to complement one another for aerial mycelium formation in a unidirectional manner (bldJ < bldK, bldL < bldA, bldH < bldG < bldC < bldD, bldM; 24, 26, 27, 32). The position of NY415 in this extracellular complementation cascade was assessed by plating this strain in close proximity to a number of previously isolated bld strains (data not shown).
NY415 was able to complement the aerial mycelium and pigmentation defects in bldJ (formerly bld261), bldG, and bldH mutants (listed in order of complementation strength). Weak complementation by NY415 of a bldA mutant for pigmentation and aerial mycelium formation was also observed. Conversely, NY415 was complemented for aerial mycelium formation by plating adjacent to the wild-type strain (M145) or a bldD mutant. Given these results, NY415 would seem to fit into the bldC extracellular complementation group. Indeed, NY415 had little effect on the bldC mutant, which develops a light layer of aerial mycelium on its own. However, members of the bldC group should be able to complement mutants in the bldK/bldL group. Whereas NY415 partially restored pigmentation by a bldK mutant, it did not appear to stimulate aerial mycelium formation by the mutant (with the caveat that the bldK mutant is leaky and eventually sporulates on its own). Also, no complementation was observed when NY415 was plated next to the newly described bldM mutant. Thus, whereas NY415 approximately fits in the extracellular complementation cascade proposed to govern aerial mycelium formation, not every expected complementation relationship was observed.
The transposon insertion in the bld mutant disrupts a locus downstream of a putative ECF sigma factor gene.
The site of the insertion in NY415 was determined by sequencing DNA flanking the transposon and searching for the corresponding sequence in the Sanger Centre S. coelicolor genome sequence database (www.sanger.ac.uk/Projects/S_coelicolor). The Tn5apr transposon was found to be inserted in the coding sequence of gene SCE59.12c (Fig. 2). The product of this gene was annotated by the Sanger Centre group as a putative membrane protein with a possible hydrophobic membrane-spanning region.
FIG. 2.
Organization of the S. coelicolor chromosome near the sigU and rsuA loci (SCE59.13c and SCE59.12c, respectively, by the nomenclature of the Sanger Centre genome sequencing project). Numbers on the scale above indicate the base pair positions of these genes in the E59 cosmid DNA sequence, and the Sanger Centre annotation is given below each gene. The chromosomal location of the Tn5apr insertion in strain NY415 is marked with a triangle (base pairs 13788 to 13796 of cosmid E59), and the region deleted and replaced with tsr in strain ΔsigU-rsuA::tsr is indicated below. Finally, DNA regions that were PCR amplified and cloned into vectors pKC1218S and/or pSET152S for complementation experiments are also indicated.
To verify that the Tn5apr insertion in SCE59.12c was the cause of the bald phenotype observed for NY415, this strain was transformed with an integrating plasmid carrying gene SCE59.12c (pSET152S-rsuA; Fig. 2). The resulting transformants exhibited timely pigment production and normal aerial mycelium formation (Fig. 1B) and were unimpaired in sporulation, as judged by light microscopy (data not shown).
Strikingly, SCE59.12c was positioned directly downstream of a gene (SCE59.13c) encoding a probable member of the ECF subfamily of sigma factors. As ECF sigma factors are often regulated by anti-sigma factors encoded by downstream genes (21), the position of SCE59.12c immediately suggested a possible role for its gene product as an anti-sigma factor. A BLAST search of the current protein databases (1) using the SCE59.12c protein sequence as the query indicated that its closest homologs are another S. coelicolor protein (SCM10.32) and Rv0736 (also known as MTV041.10) from Mycobacterium tuberculosis. The genes encoding these proteins are likewise near and downstream of putative ECF sigma factor genes (SCM10.30 and sigL, respectively).
A further indication that SCE59.12c may encode the cognate anti-sigma factor for SCE59.13c was suggested by similarity in the N terminus of this gene product to another S. coelicolor protein, RsrA, whose anti-sigma factor activity has been biochemically demonstrated (15, 29). RsrA is the cognate anti-sigma factor for ςR, an ECF sigma factor that controls expression of the thioredoxin system (29, 30) and more than 29 other genes (M. J. Buttner, personal communication) in response to oxidative stress. RsrA has been shown to bind to and inhibit ςR under reducing conditions (15). The cysteine residues in a conserved N-terminal HisXXXCysXXCys motif have been shown to be required for RsrA anti-sigma factor activity (15, 29). SCE59.12c, as well as at least 10 other S. coelicolor proteins encoded near ECF sigma factor genes, shares this HXXXCXXC motif with RsrA (Fig. 3). In light of this similarity to RsrA and ςR, we propose the nomenclature RsuA and ςU for the products of SCE59.12c and SCE59.13c, respectively, and correspondingly rename these genes rsuA (regulator of ςU) and sigU.
FIG. 3.
Alignment of the N-terminal regions of RsuA and other proteins containing a consensus HXXXCXXC motif. The genes encoding these proteins are all located near, typically downstream and adjacent to, genes encoding members of the ECF family of sigma factors. All sequences are from S. coelicolor, except for Rv0736, which is from M. tuberculosis.
Aerial mycelium formation proceeds normally in strains in which both rsuA and sigU are deleted
As transposon disruption of the putative anti-sigma factor gene rsuA elicits a bald phenotype, it was important to determine the phenotype of a strain in which both rsuA and the upstream ECF sigma factor gene, sigU, were disrupted. In the wild-type strain background, these genes were deleted and replaced with a gene conferring resistance to thiostrepton to give a ΔsigU-rsuA::tsr strain. Unlike NY415, this strain exhibited normal morphological differentiation (Fig. 1A). The only apparent defect in the ΔsigU-rsuA::tsr strain was a short delay in actinorhodin production that was very mild on R2YE medium but more noticeable on nutrient agar medium.
To confirm the phenotype of the ΔsigU-rsuA::tsr strain, the transposon-disrupted rsuA gene and the upstream sigU gene were deleted from bald strain NY415 by transformation with the same plasmid used to generate the ΔsigU-rsuA::tsr strain. As expected, transformants in which the deletion mutation had integrated into the chromosome by double recombination (Tsrr Specs Aprs) lost the bald phenotype of parent strain NY415 and exhibited normal morphological differentiation and the only minor actinorhodin production delay characteristic of the ΔsigU-rsuA::tsr strain (Fig. 1B). Whereas the ECF sigma factor gene sigU appears to be dispensable for the normal growth and differentiation of S. coelicolor, in the absence of the downstream anti-sigma factor gene rsuA, initiation of aerial mycelium formation is blocked.
In addition, analysis of suppressor mutants of NY415 indicated that sigU influences differentiation only if unregulated by rsuA. Suppressor mutants of NY415 that had a wild-type morphological differentiation phenotype were observed to arise spontaneously (data not shown). Eight such suppressor strains were isolated and transformed with an integrating plasmid carrying sigU (pSET152S-sigU). In all cases, the introduction of sigU into these strains reestablished the bald phenotype characteristic of NY415, implying that these suppressors carried a mutation in sigU in addition to the Tn5apr insertion in rsuA.
Expression of sigU uncoupled from rsuA elicits differentiation defects.
To confirm that the presence of the sigU gene in the absence of its apparent antagonist rsuA results in a bald phenotype, the ΔsigU-rsuA::tsr strain was transformed with an integrating plasmid containing only the sigma factor gene sigU (Fig. 2). The resulting transformants showed a phenotype identical to that of NY415—they failed to generate an aerial mycelium and showed severe slowing of blue pigment production (Fig. 1C). Introduction of anti-sigma factor gene rsuA only into the ΔsigU-rsuA::tsr background did not affect morphological differentiation (data not shown).
Extra copies of the sigma factor gene, even in the presence of a wild-type copy of the anti-sigma factor gene, could also elicit a mild delay in morphological differentiation. The wild-type strain was transformed with a low-copy-number vector (pKC1218S) containing sigU. In the resulting transformants, compared to those transformed with the vector alone, the appearance of a full, white aerial mycelium was consistently delayed by several hours (Fig. 1C). This delay in aerial mycelium formation was not observed if sigU was delivered in an integrating vector (pSET152S) that is inserted once per chromosome, presumably because the amount of the RsuA anti-sigma factor in these cells was sufficient to control the activity of the ςU sigma factor.
ςU and the response to stress
ECF sigma factors, as their name suggests, typically regulate a response to environmental stress(es) (23, 33). The ability of the ΔsigU-rsuA::tsr strain to respond to several chemical stresses was therefore examined. The sensitivities of the M145 and ΔsigU-rsuA::tsr strains to various compounds were compared by using a zone of growth inhibition assay. The two strains showed similar responses to oxidants (diamide, hydrogen peroxide, and cumene hydroperoxide), a reductant (dithiothreitol), metal ions (FeCl3, CuCl2, and ZnCl2), EDTA, HCl, NaOH, sodium dodecyl sulfate, and lysozyme. Thus, the particular stress(es) to which ςU presumably responds remains to be uncovered. We note that while the wild-type and ΔsigU-rsuA::tsr strains were able to grow well on nutrient agar supplemented with 0.5 M KCl, the bald mutant NY415 was unable to grow on this medium, suggesting that this strain is osmosensitive.
DISCUSSION
The initiation of aerial mycelium formation in S. coelicolor is a poorly understood process (16). A relatively small number of genes involved in this developmental pathway, the bld genes, have been characterized (2, 4, 11, 19, 24, 26, 31), and only recently have relationships between the activities of certain bld gene products been established (2, 10). Here we describe the identification and initial characterization of a new gene, rsuA, which is indirectly required for the formation of aerial hyphae, as well as for the appropriate timing of pigmented antibiotic production. This gene, located immediately downstream of an ECF sigma factor gene, appears to encode an anti-sigma factor. In the absence of this anti-sigma factor, unchecked activity of the corresponding sigma factor, ςU, blocks the normal developmental process, abrogating aerial mycelium formation and, thus, subsequent sporulation.
The RsuA anti-sigma factor gene product showed similarity in its N terminus to a recently defined anti-sigma factor family termed the ZAS (zinc-binding anti-sigma factor) family (15, 29). Members of this family, although they exhibit limited identity over their lengths, possess an absolutely conserved HXXXCXXC amino acid motif. In S. coelicolor, RsuA and at least 11 additional proteins encoded by genes located near ECF sigma factor genes share this conserved motif (Fig. 3), including the founding member of the ZAS family, RsrA.
RsrA is the cognate anti-sigma factor for ςR, a sigma factor demonstrated to direct the transcription of thioredoxin reductase and thioredoxin genes in response to thiol oxidation (29, 30). Biochemical studies have shown that RsrA binds to ςR under reducing conditions, thereby inhibiting transcription of the thioredoxin system (15). Conversely, under oxidizing conditions, intramolecular disulfide bonds form in RsrA and it loses the ability to bind to and inhibit ςR (15). Three cysteine residues in RsrA, including the two in the conserved HXXX CXXC motif, are required for this anti-sigma factor activity (29). In addition, RsrA has been shown to bind near stoichiometric amounts of zinc. The conserved HXXXCXXC residues have been proposed as possible zinc ligands (ergo, ZAS is the name of this anti-sigma factor family); however, this has not yet been demonstrated experimentally (29). Only one additional member of the proposed ZAS family, Rhodobacter sphaeroides ChrR, has been characterized (25). It was demonstrated that in ChrR, mutation of the second conserved motif cysteine residue to arginine abrogates binding to and inhibition of its cognate sigma factor (ςE).
By analogy to the ςR-RsrA case, we anticipated that the ςU-RsuA pair might play a role in sensing and responding to the redox environment of the cell. However, we were unable to detect a convincing difference in a strain with these loci deleted, compared to wild-type S. coelicolor, in sensitivity to several oxidants and reductants. In mycobacteria, two ECF sigma factor genes located upstream of ZAS family anti-sigma factor genes (15), sigE and sigH, have been implicated in the oxidative stress response (12, 34). A Mycobacterium smegmatis strain in which sigE was disrupted showed a small decrease in survival compared to the wild type when exposed to a variety of stresses, including hydrogen peroxide (34), and an M. smegmatis strain with sigH disrupted, while showing normal susceptibility to hydrogen peroxide, was sensitive to cumene hydroperoxide (12). A sigH sigE double mutant was even more susceptible to cumene hydroperoxide, as well as heat shock (12). Similarly, perhaps the functions of rsuA and sigU overlap those of other members of the S. coelicolor ZAS and ECF sigma factor families (Fig. 3). Alternatively, RsuA and ςU may respond to a signal not directly related to oxidative stress, as previously suggested for other ZAS family members (29). For example, Bacillus subtilis sigW, which is found upstream of a ZAS family gene (15), has no apparent connection to an oxidative stress response (14) but has, instead, been shown to mediate resistance to the antibiotic fosfomycin (5).
The rsuA anti-sigma factor gene is the latest example of a growing number of sigma factor regulatory genes whose presence is required for the normal morphological differentiation of S. coelicolor. A strain in which the ςR regulatory gene rsrA was deleted formed an aerial mycelium but was unable to sporulate, producing straight, white aerial hyphae with little septation, whereas a rsrA sigR double mutant sporulated normally (29). A homolog of the B. subtilis general stress response factor ςB has recently been identified in S. coelicolor (ςH), and the sigH gene is preceded by and cotranscribed with the prsH gene, whose product is homologous to the B. subtilis anti-sigma factors SpoIIAB and RsbW (17). A sigH mutant sporulated normally, but a prsH sigH double mutant exhibited a conditionally bald phenotype (17). This suggests that PrsH may regulate an additional sigma factor(s) whose uncontrolled activity disrupts development analogously to ςR and ςU. Finally, one of the classic bld loci (6), bldG, is now known to encode a protein that is homologous to anti-anti-sigma factors such as B. subtilis SpoIIAA and RsbV, which are antagonists of the anti-sigma factors SpoIIAB and RsbW, respectively (4). A candidate for the corresponding anti-sigma factor gene is present downstream of bldG. Presumably, and opposite to the other cases we have considered, a bldG mutation blocks aerial mycelium formation by preventing the activation of a sigma factor that is required for development. However, a cognate sigma factor for the bldG system, if it exists, is unknown (4).
If the sigU sigma factor gene is not required for development, why does deletion of its anti-sigma factor gene, rsuA, lead to a severe developmental (bald) phenotype? We presume that in the absence of its cognate anti-sigma factor, ςU is constitutively active and free to direct transcription of the genes under its control. Indeed, the trxC target gene of ςR has been shown to be transcribed constitutively and at a very high level in an rsrA mutant (29). One possible explanation for the block in aerial mycelium formation caused by a rsuA mutation is that unregulated and improperly active ςU competes in binding to core RNA polymerase with one or more sigma factors that are needed for development. Currently, only one sigma factor, the ECF sigma factor ςBldN, which directs the transcription of bldM, has been shown to be necessary for aerial mycelium formation (2). Perhaps the sigU-encoded sigma factor competes with ςBldN. However, the introduction of a low-copy-number vector (pKC1218S) containing bldN was insufficient to reverse the bald phenotype of NY415 (A. M. Gehring and R. Losick, unpublished data).
Another possible explanation for the bald phenotype of the rsuA mutant is that ςU directs the transcription of a gene(s) that inhibits aerial mycelium formation. We favor this explanation because it accounts for the fact that an rsuA mutation impairs differentiation at the stage of aerial mycelium formation whereas an rsrA mutation blocks development at the stage of spore formation. It is difficult to imagine how ςU and ςR could differentially compete with different sigma factors, one required for aerial mycelium formation and one required for spore formation. Rather, it seems more likely that ςU directs the transcription of a gene(s) that, when inappropriately expressed or expressed at high levels, causes a bald phenotype whereas ςR directs the transcription of a gene(s) whose product inhibits spore formation.
The developmental defects observed in rsuA and rsrA mutants imply an interconnection between development and the stress response in S. coelicolor. A role for ςR in responding to cytoplasmic thiol-disulfide stress is well established (29, 30), and the homologous ECF sigma factor ςU is presumably active in response to an as yet unidentified stress condition. This is in keeping with earlier evidence pointing to a connection between the regulation of stress and developmental pathways in S. coelicolor, such as the finding that transcription from the p2 promoter of the stress response sigma factor gene sigH is restricted to sporulating aerial hyphae and that BldD, a transcription factor also shown to regulate expression of the developmental genes bldN and whiG (10), represses transcription from the sigHp2 promoter in vegetative hyphae (17). Also, the catalase gene catB, which is required for protection against osmotic stress, has been shown to be necessary for aerial mycelium formation and proper secondary metabolite production (9). Given the increasing evidence that developmental events and the responses to cellular stress are intertwined in S. coelicolor, we suggest that stress-induced activation of ςU and ςR may be a physiologically significant mechanism by which to signal the cell to arrest development under conditions that are incompatible with aerial mycelium formation or sporulation.
ACKNOWLEDGMENTS
We thank M. Buttner and K. Chater for helpful comments on the manuscript.
This work was supported by a grant to R.L. from the National Science Foundation (MCB-9727234). A.M.G. is supported by the Cancer Research Fund of the Damon Runyon-Walter Winchell Foundation Fellowship (DRG-1524).
REFERENCES
- 1.Altschul S F, Madden T L, Schäffer A A, Zhang J, Zhang Z, Miller W, Lipman D J. Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res. 1997;25:3389–3402. doi: 10.1093/nar/25.17.3389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bibb M J, Molle V, Buttner M J. ςBldN, an extracytoplasmic function RNA polymerase sigma factor required for aerial mycelium formation in Streptomyces coelicolor A3(2) J Bacteriol. 2000;182:4606–4616. doi: 10.1128/jb.182.16.4606-4616.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bierman M, Logan R, O'Brien K, Seno E T, Nagaraja Rao R, Schoner B E. Plasmid cloning vectors for the conjugal transfer of DNA from Escherichia coli to Streptomyces spp. Gene. 1992;116:43–49. doi: 10.1016/0378-1119(92)90627-2. [DOI] [PubMed] [Google Scholar]
- 4.Bignell D R D, Warawa J L, Strap J L, Chater K F, Leskiw B K. Study of the bldG locus suggests that an anti-anti-sigma factor and an anti-sigma factor may be involved in Streptomyces coelicolor antibiotic production and sporulation. Microbiology. 2000;146:2161–2173. doi: 10.1099/00221287-146-9-2161. [DOI] [PubMed] [Google Scholar]
- 5.Cao M, Bernat B A, Wang Z, Armstrong R N, Helmann J D. FosB, a cysteine-dependent fosfomycin resistance protein under the control of ςW, an extracytoplasmic-function ς factor in Bacillus subtilis. J Bacteriol. 2001;183:2380–2383. doi: 10.1128/JB.183.7.2380-2383.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Champness W C. New loci required for Streptomyces coelicolor morphological and physiological differentiation. J Bacteriol. 1988;170:1168–1174. doi: 10.1128/jb.170.3.1168-1174.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Champness W. Actinomycete development, antibiotic production, and phylogeny: questions and challenges. In: Brun Y V, Shimkets L J, editors. Prokaryotic development. Washington, D.C.: American Society for Microbiology; 2000. pp. 11–31. [Google Scholar]
- 8.Chater K F. Taking a genetic scalpel to the Streptomyces colony. Microbiology. 1998;144:1465–1478. doi: 10.1099/00221287-144-6-1465. [DOI] [PubMed] [Google Scholar]
- 9.Cho Y-H, Lee E-J, Roe J-H. A developmentally regulated catalase required for proper differentiation and osmoprotection of Streptomyces coelicolor. Mol Microbiol. 2000;35:150–160. doi: 10.1046/j.1365-2958.2000.01685.x. [DOI] [PubMed] [Google Scholar]
- 10.Elliot M A, Bibb M J, Buttner M J, Leskiw B K. BldD is a direct regulator of key developmental genes in Streptomyces coelicolor A3(2) Mol Microbiol. 2001;40:257–269. doi: 10.1046/j.1365-2958.2001.02387.x. [DOI] [PubMed] [Google Scholar]
- 11.Elliot M, Damji F, Passantino R, Chater K, Leskiw B. The bldD gene of Streptomyces coelicolor A3(2): a regulatory gene involved in morphogenesis and antibiotic production. J Bacteriol. 1998;180:1549–1555. doi: 10.1128/jb.180.6.1549-1555.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fernandes N D, Wu Q-L, Kong D, Puyang X, Garg S, Husson R N. A mycobacterial extracytoplasmic sigma factor involved in survival following heat shock and oxidative stress. J Bacteriol. 1999;181:4266–4274. doi: 10.1128/jb.181.14.4266-4274.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gehring A M, Nodwell J R, Beverley S M, Losick R. Genomewide insertional mutagenesis in Streptomyces coelicolor reveals additional genes involved in morphological differentiation. Proc Natl Acad Sci USA. 2000;97:9642–9647. doi: 10.1073/pnas.170059797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Huang X, Gaballa A, Cao M, Helmann J D. Identification of target promoters for the Bacillus subtilis extracytoplasmic function ς factor, ςW. Mol Microbiol. 1999;31:361–371. doi: 10.1046/j.1365-2958.1999.01180.x. [DOI] [PubMed] [Google Scholar]
- 15.Kang J-G, Paget M S B, Seok Y-J, Hahn M-Y, Bae J-B, Hahn J-S, Kleanthous C, Buttner M J, Roe J-H. RsrA, an anti-sigma factor regulated by redox change. EMBO J. 1999;18:4292–4298. doi: 10.1093/emboj/18.15.4292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kelemen G H, Buttner M J. Initiation of aerial mycelium formation in Streptomyces. Curr Opin Microbiol. 1998;1:656–662. doi: 10.1016/s1369-5274(98)80111-2. [DOI] [PubMed] [Google Scholar]
- 17.Kelemen G H, Viollier P H, Tenor J L, Marri L, Buttner M J, Thompson C J. A connection between stress and development in the multicellular prokaryote Streptomyces coelicolor A3(2) Mol Microbiol. 2001;40:804–814. doi: 10.1046/j.1365-2958.2001.02417.x. [DOI] [PubMed] [Google Scholar]
- 18.Kieser T, Bibb M J, Buttner M J, Chater K F, Hopwood D A. Practical Streptomyces genetics. Norwich, England: The John Innes Foundation; 2000. [Google Scholar]
- 19.Lawlor E J, Baylis H A, Chater K F. Pleiotropic morphological and antibiotic deficiencies result from mutations in a gene encoding a tRNA-like product in Streptomyces coelicolor A3(2) Genes Dev. 1987;1:1305–1310. doi: 10.1101/gad.1.10.1305. [DOI] [PubMed] [Google Scholar]
- 20.Leskiw B K, Mah R, Lawlor E J, Chater K F. Accumulation of bldA-specified tRNA is temporally regulated in Streptomyces coelicolor A3(2) J Bacteriol. 1993;175:1995–2005. doi: 10.1128/jb.175.7.1995-2005.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lonetto M A, Brown K L, Rudd K E, Buttner M J. Analysis of the Streptomyces coelicolor sigE gene reveals the existence of a subfamily of eubacterial RNA polymerase ς factors involved in the regulation of extracytoplasmic functions. Proc Natl Acad Sci USA. 1994;91:7573–7577. doi: 10.1073/pnas.91.16.7573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Merrick M J. A morphological and genetic mapping study of bald colony mutants of Streptomyces coelicolor. J Gen Microbiol. 1976;96:299–315. doi: 10.1099/00221287-96-2-299. [DOI] [PubMed] [Google Scholar]
- 23.Missiakas D, Raina S. The extracytoplasmic function sigma factors: role and regulation. Mol Microbiol. 1998;28:1059–1066. doi: 10.1046/j.1365-2958.1998.00865.x. [DOI] [PubMed] [Google Scholar]
- 24.Molle V, Buttner M J. Different alleles of the response regulator gene bldM arrest Streptomyces coelicolor development at distinct stages. Mol Microbiol. 2000;36:1265–1278. doi: 10.1046/j.1365-2958.2000.01977.x. [DOI] [PubMed] [Google Scholar]
- 25.Newman J D, Falkowski M J, Schilke B A, Anthony L C, Donohue T J. The Rhodobacter sphaeroides ECF sigma factor, ςE, and the target promoters cycA P3 and rpoE P1. J Mol Biol. 1999;294:307–320. doi: 10.1006/jmbi.1999.3263. [DOI] [PubMed] [Google Scholar]
- 26.Nodwell J R, McGovern K, Losick R. An oligopeptide permease responsible for the import of an extracellular signal governing aerial mycelium formation in Streptomyces coelicolor. Mol Microbiol. 1996;22:881–893. doi: 10.1046/j.1365-2958.1996.01540.x. [DOI] [PubMed] [Google Scholar]
- 27.Nodwell J R, Yang M, Kuo D, Losick R. Extracellular complementation and the identification of additional genes involved in aerial mycelium formation in Streptomyces coelicolor. Genetics. 1999;151:569–584. doi: 10.1093/genetics/151.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oh S-H, Chater K F. Denaturation of circular or linear DNA facilitates targeted integrative transformation of Streptomyces coelicolor A3(2): possible relevance to other organisms. J Bacteriol. 1997;179:122–127. doi: 10.1128/jb.179.1.122-127.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Paget M S B, Bae J-B, Hahn M-Y, Li W, Kleanthous C, Roe J-H, Buttner M J. Mutational analysis of RsrA, a zinc-binding anti-sigma factor with a thiol-disulphide redox switch. Mol Microbiol. 2001;39:1036–1047. doi: 10.1046/j.1365-2958.2001.02298.x. [DOI] [PubMed] [Google Scholar]
- 30.Paget M S B, Kang J-G, Roe J-H, Buttner M J. ςR, an RNA polymerase sigma factor that modulates expression of the thioredoxin system in response to oxidative stress in Streptomyces coelicolor A3(2) EMBO J. 1998;17:5776–5782. doi: 10.1093/emboj/17.19.5776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pope M K, Green B, Westpheling J. The bldB gene encodes a small protein required for morphogenesis, antibiotic production, and catabolite control in Streptomyces coelicolor. J Bacteriol. 1998;180:1556–1562. doi: 10.1128/jb.180.6.1556-1562.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Willey J, Schwedock J, Losick R. Multiple extracellular signals govern the production of a morphogenetic protein involved in aerial mycelium formation by Streptomyces coelicolor. Genes Dev. 1993;7:895–903. doi: 10.1101/gad.7.5.895. [DOI] [PubMed] [Google Scholar]
- 33.Wösten M M S M. Eubacterial sigma-factors. FEMS Microbiol Rev. 1998;22:127–150. doi: 10.1111/j.1574-6976.1998.tb00364.x. [DOI] [PubMed] [Google Scholar]
- 34.Wu Q-L, Kong D, Lam K, Husson R N. A mycobacterial extracytoplasmic function sigma factor involved in survival following stress. J Bacteriol. 1997;179:2922–2929. doi: 10.1128/jb.179.9.2922-2929.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]