Abstract
Abscisic acid (ABA) is a major phytohormone affecting seed dormancy and germination in plants. ABA is synthesized mainly through the C40 carotenoid pathway. In the ABA biosynthesis pathway, 9-cis-epoxycarotenoid dioxygenase (NCED) is a key rate-limiting enzyme that regulates the accumulation and content of ABA. However, the role of the NCED gene in perennial plants with complex seed dormancy remains largely unknown. Here, we cloned two differentially expressed paralogs of herbaceous peony NCED genes, named PlNCED1 and PlNCED2, and further identified their involvement in seed dormancy from perennial herbaceous peony experiencing complex double seed dormancy. The deduced PlNCED amino acid sequences had high sequence homology with NCED sequences from other plants and contained the typical conserved RPE65 domain of the NCED family. Phylogenetic analysis showed that PlNCED1 and PlNCED2 have a close relationship with PoNCED in Paeonia ostii and VvNCED6 in Vitis vinifera, respectively. A subcellular localization assay demonstrated that the PlNCED1 protein resided within the nucleus, while the PlNCED2 protein was located in the cytoplasm, indicating their different roles in the biosynthesis of ABA. Furthermore, the content of endogenous ABA in transgenic calluses showed that PlNCEDs were positively correlated with ABA content. Both PlNCED transgenic Arabidopsis lines and the functional complementation of Arabidopsis NCED mutants found that PlNCEDs promoted seed dormancy and delayed seed germination. These results reveal that PlNCEDs participate in the seed dormancy of herbaceous peony by regulating the accumulation of endogenous ABA.
Keywords: Paeonia lactiflora pall., abscisic acid, NCEDs, seed dormancy, germination
1. Introduction
Phytohormones are important regulators of seed dormancy, among which ABA plays a major role [1,2,3]. Studies have shown that ABA-deficient mutants of A. thaliana, tomato, and maize undergo early dormancy breaking and enter the germination stage, whereas plants overexpressing the ABA biosynthetic enzyme show prolonged dormancy [4,5,6,7]. Studies also demonstrated that the change in endogenous ABA content significantly positively correlates with the degree of seed dormancy [8,9].
In plants, it is known that several phytohormones are involved in seed dormancy and germination [10,11,12]. Among them, endogenous ABA content is significantly positively correlated with herbaceous peony seed dormancy, while low ABA content promotes seed germination [13]. The final concentration of endogenous ABA in plant seeds depends on the dynamic balance of ABA synthesis and catabolism [1,14]. NCED and CYP707A are two key enzymes in ABA anabolic and catabolic pathways, respectively. Studies show that NCED has a common role in regulating ABA synthesis and seed dormancy in plants. For example, A. thaliana contains five NCED family members, of which AtNCED5 is up-regulated at the late stage of seed maturation and cooperates with AtNCED6 and AtNCED9 to enhance seed dormancy by controlling ABA levels [15,16]. LeNCED1 transgenic tomato plants enhanced ABA biosynthesis and increased seed dormancy [17]. The variation trend of ABA content was consistent with that of AhNCED1 gene transcription in peanut [18]. PtNCED1 directly regulated orchid seed dormancy and was involved in ABA content [19]. In addition, NCED can also affect other physiological functions of plants by changing endogenous ABA content. Overexpressing PvNCED1 enhanced drought tolerance by manipulating ABA levels in tobacco [4]. Silencing AcNCED1 blocked ABA biosynthesis and delayed kiwifruit softening [20]. PpNCED1 and PpNCED5 can cooperatively control ABA biosynthesis and affect fruit ripening and senescence in peach fruit [21]. However, not all NCED family members regulate ABA synthesis. For example, a total of 23 NCED genes were identified in cotton. Among them, only the expression of GhNCED5, GhNCED6, and GhNCED13 was similar to the change in ABA content, which could play a role in ABA biosynthesis [22].
Herbaceous peony (Paeonia lactiflora Pall.) is an herbaceous perennial flower of Paeoniaceae. In the long-term systematic evolutionary process, herbaceous peony seeds evolved a unique double dormancy characteristic of the epicotyl and hypocotyl [23]. In the breeding process, seed dormancy is often not released or incompletely released, which greatly reduces the germination rate and seriously affects cultivation and production [24]. Thus, understanding the mechanisms associated with herbaceous peony seed dormancy is beneficial to greatly promote the breeding of new varieties. At present, research on the seed dormancy release technology for herbaceous peony mainly focuses on mechanical scarification, low temperature, and exogenous hormone treatment [25,26,27]. However, the knowledge of the molecular mechanisms underlying the seed dormancy of herbaceous peony is relatively limited. Using previously published transcriptomes from herbaceous peony seeds pre- and post-double dormancy release [28], here, we were able to identify candidate genes that were associated with double dormancy in herbaceous peony. Specifically, we identified ten family members of PlNCEDs involved in ABA biosynthesis. Among them, PlNCED1 (c53147_g1) and PlNCED2 (c69372_g1) showed significantly differential expression. We subsequently cloned and performed expressional analysis, subcellular localization analysis, and functional characterization of PlNCED1 and PlNCED2 in Paeonia lactiflora. Our results demonstrate that the genes encoding NCED1 and NCED2 regulate ABA synthesis and consequentially affect the herbaceous peony seed dormancy process.
2. Materials and Methods
2.1. Plant Material and Growth Condition
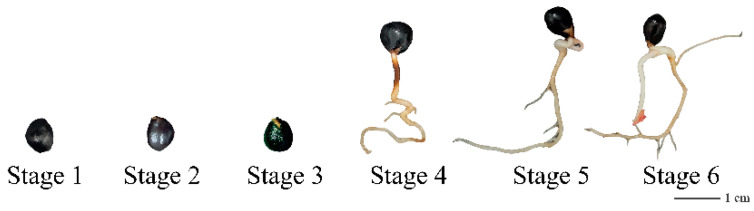
Herbaceous peony hybrid seeds (‘Fen Yu Nu’ × ‘Fen Yu Lou’) were harvested in the Shenyang Agricultural University germplasm resources nursery (Shenyang, Liaoning, China) in August 2019. Filled hybrid seeds were used for variable temperature stratification using a previous method [29]. According to the observation of the seed anatomical structure [29], seeds in six key dormancy release stages were collected: stage 1 (S1: dry seed), stage 2 (S2: imbibition seed), stage 3 (S3: the radicle breaking of seed coat), stage 4 (S4: the length of the seed root is 3–4 cm), stage 5 (S5: the basal part of the seed root turns red), and stage 6 (S6: seed germ breakout) (Figure 1). The cotyledons used as explants were obtained using the conventional embryo induction method [30]. Then, the explants were transferred to an MS callus induction and proliferation medium containing 0.5 mg/L 2,4-dichlorophenoxyacetic acid, 0.5 mg/L α-naphthalene acetic acid, 0.5 mg/L thidiazuron, and 1 g/L polyvinyl pyrrolidone (PVP). The nced5-2 (GK_328D05) and nced9-1 (SALK_033388) genes, which are in the Col-0 background, were obtained from the Arabidopsis Biological Resource Center (ABRC, http://abrc.osu.edu). Homozygous mutants were screened and validated by PCR using the left and right genomic primers (LP and RP) and the T-DNA left border primer (LB) (Supplementary Table S1). Seeds of A. thaliana WT (Col-0) and mutants were grown following previously reported methods [31].
Figure 1.
Seeds of herbaceous peony at six different dormancy release stages.
2.2. RNA Extraction, cDNA Synthesis, and qRT-PCR
Total RNA was extracted using the RNAprep pure Plant Kit (TianGen, Beijing, China). cDNA was synthesized using the PrimeScript™ RT Master Mix kit (Perfect Real Time) (Takara, Dalian, China). Based on the full-length coding sequences (CDSs) of PlNCEDs in transcriptome data, qRT-PCR primers were designed with the Primer Premier 5.0 software. PlACTIN (GenBank accession number JN105299.1) was used as the reference gene [32]. The primers used for qRT-PCR are listed in Supplementary Table S1. qRT-PCR was performed using TB Green® Premix Ex Taq™ II (Tli RNaseH Plus) (Takara, Dalian, China). The reactions were accomplished according to the two-step method—holding stage: 95 °C for 30 s; cycling stage: 40 cycles of 95 °C for 5 s, 60 °C for 30 s; and melt curve stage: 95 °C for 15 s, 60 °C for 1 min, 95 °C for 15 s. Each experiment was performed with three biological and three technical replicates. The relative expression levels of genes were calculated according to the 2−ΔΔCt method, and the error bars represent the standard error from three independent experiments. The results were analyzed by GraphPad Prism 8.0 for ANOVA.
2.3. Cloning and Sequence Analysis
We obtained the CDSs of PlNCEDs from transcriptome data. The amino acid sequences of PlNCEDs were deduced using ORF Finder (https://www.ncbi.nlm.nih.gov/orffinder/) (accessed on 8 December 2022). A phylogenetic tree was constructed using MEGA 7.0 software with the neighbor-joining method, applying bootstrap analysis with 1000 replicates, and iTOL v6 (https://itol.embl.de/) (accessed on 13 January 2023) was used to optimize the trees. The conserved domains were predicted by National Center for Biotechnology Information (NCBI) online software (https://www.ncbi.nlm.nih.gov/Structure/cdd/wrpsb.cgi) (accessed on 13 January 2023). The protein physicochemical properties were analyzed using the Expasy ProtParam tool (http://web.expasy.org/protparam/) (accessed on 8 December 2022).
Using total RNA as the template, 1st-strand cDNA was synthesized using 3’ RACE adaptor primers. According to the CDSs of PlNCEDs, we designed the specific outer and inner primers (Supplementary Table S1) to amplify the 3’ untranslated region (UTR) sequences of PlNCEDs using 3’-Full RACE Core Set with PrimeScript™ RTase Kit (Takara, Dalian, China). The miRNA binding sites of 3’ UTR sequences were predicted using MiRanda software.
Genomic DNA was extracted using the Plant Genome DNA Rapid Extraction Kit (Aidlab, Beijing, China). According to the known verified intronless sequences, three specific primers were designed, namely, SP1, SP2, and SP3 (Supplementary Table S1), to amplify the 5’ end sequences of PlNCEDs containing 5’ UTR and promoter regions using the Genome Walking Kit (Takara, Dalian, China). Cis-acting elements of the promoter were analyzed using PlantCARE.
2.4. Subcellular Localization Analysis
Arabidopsis thaliana leaf protoplasts were extracted using the Arabidopsis Protoplast Preparation and Transformation Kit (Coolaber, Beijing, China) for subcellular localization. The CDSs of PlNCEDs were cloned into the 16318-hGFP vector and fused in-frame with the hGFP sequence under the control of the CaMV 35S promoter. The 16318-hGFP empty vector was used as a blank control. After 16 h of incubation in darkness, the green fluorescence protein (GFP) fluorescence was captured by an ultra-high-resolution laser scan confocal microscope (Leica TCS SP8 STED, Wetzlar, Germany).
2.5. Functional Analysis
To silence PlNCED expression in the herbaceous peony callus, the fragments of PlNCEDs (PlNCED1: 565 bp; PlNCED2: 387 bp) were each amplified and recombined into the linearized pTRV2 empty vector. The CDSs of PlNCEDs were separately inserted downstream of 35S in the pCAMBIA1300-35S-flag vector. The pTRV2-PlNCED and 35S::PlNCED vectors were transformed into EHA105-competent cells. Agrobacterium containing pTRV1 and Agrobacterium containing pTRV2-PlNCEDs were mixed in a 1:1 volume ratio for the preparation of the callus infection solution. The overexpression and silencing experiment in the herbaceous peony callus was performed according to our previous infection method [33]. After the resistance screening of the culture, part of the PlNCED transgenic callus was taken for qRT-PCR identification. The extraction, purification, and determination of the endogenous levels of ABA in the positive transgenic callus of PlNCEDs using an enzyme-linked immunosorbent assay (ELISA) were performed as described by He [34].
We obtained two A. thaliana homozygous T-DNA mutants (Figure S1): nced5-2 and nced9-1. NCED mutants have interrupted 9-cis-epoxycarotenoid dioxygenase genes that result in plants that are deficient in the plant growth regulator abscisic acid. To make the transgenic line and functional complementation line of A. thaliana, the 35S::PlNCED vectors were transformed into Agrobacterium strain GV3101 and then used to infect inflorescences of A. thaliana WT (Col-0) and homozygous mutants (nced5-2 and nced9-1) using the floral-dip method [35], respectively. The transgenic line and functional complementation line were screened on ½ MS medium plates that contained 50 mg/L kanamycin. The seed germination rate was measured in WT, homozygous mutants, functional complementation lines, and transgenic lines (stable T3-generation genetic lines) of A. thaliana, which were grown at the same time under 16 h light and 8 h dark conditions at 22 °C.
3. Results
3.1. Cloning and Sequence Analysis of PlNCEDs
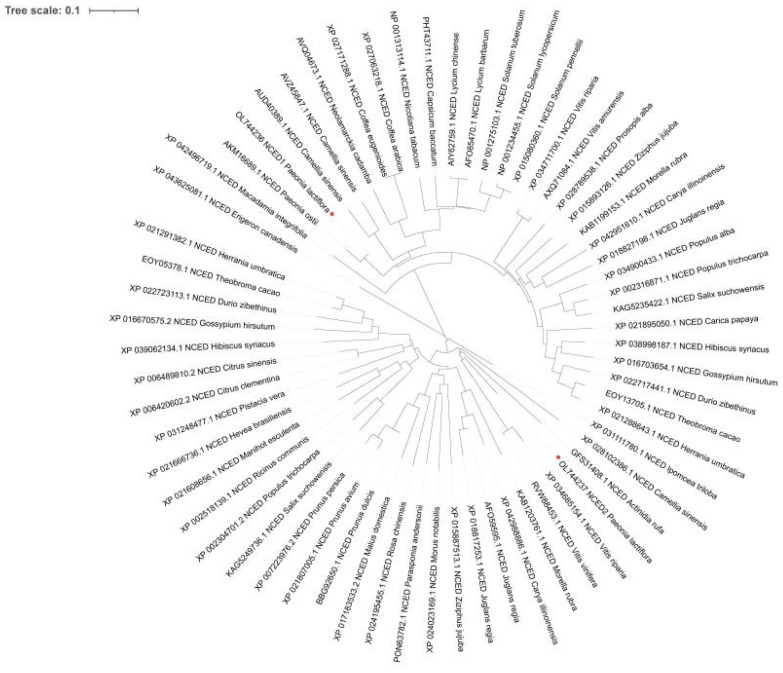
The full-length cDNA sequences of PlNCEDs were isolated and deposited in GenBank (GenBank accession numbers—PlNCED1: OL744236; PlNCED2: OL744237). PlNCED1 and PlNCED2 contained 1518 bp and 1326 bp open reading frames, encoding 505 and 441 amino acids, respectively (Figure S2). NCBI tblastx results displayed their homology to the NCED genes of other plant species. PoNCED from Paeonia ostii (74.70%) and JrNCED from Juglans regia (62.43%) had the highest identity with PlNCED1 and PlNCED2, respectively (Supplementary Tables S2 and S3). This similarity demonstrated that PlNCEDs are relatively conserved among diverse plant species. PlNCEDs had the typical conserved RPE65 domain of the NCED family (Figure S3), which is related to the degradation of carotenoids in plants. Phylogenetic analysis results indicated that PlNCED1 and PlNCED2 are closely related to the NCED proteins in Paeonia ostii, Vitis riparia, and Vitis vinifera, respectively (Figure 2). In addition, based on DNA sequences, we identified putative microRNA (miRNA) and transcription factor binding sites in the 3’UTR and promoter regions of PlNCEDs (Supplementary Tables S4–S7). Among the miRNAs that may target the 3’UTRs of PlNCEDs genes, six miRNAs, namely, miR837-5p, miR5640, miR319c, miR6425a/b/c/d/e-5p, miR168a, and miR5304, are related to plant development (Supplementary Tables S4 and S5). Among the cis-acting elements that may bind to the promoter sequences of PlNCED genes, six cis-acting elements, namely, TCA-element, ABRE, AuxRR-core, TGA-box, TGACG-motif, and CGTCA-motif, are related to phytohormone responses (Supplementary Tables S6 and S7). These results suggest that PlNCEDs may be involved in plant development mediated by ABA and other phytohormones.
Figure 2.
Neighbor-joining phylogenetic tree of PlNCEDs. PlNCED1 and PlNCED2 are indicated with red dots. A neighbor-joining tree was constructed based on amino acid sequences of NCED protein from different plants using MEGA 7.0 software. The bootstrap values of the branches were obtained by testing the tree 1000 times.
3.2. Expression Analysis of PlNCEDs
To explore the correlation between the expression of PlNCED genes and ABA accumulation in herbaceous peony seeds, we performed qRT-PCR using seeds at stages 1-6. Our results indicated that the expression levels and trends of PlNCED1 and PlNCED2 dynamically varied during seed dormancy release (Figure S4). The expression level of PlNCED1 increased sharply from stage 1 to stage 2 and then decreased significantly at stage 3, where seeds experienced hypocotyl dormancy release (Figure S4). After the completion of the hypocotyl dormancy release process, the expression level of PlNCED1 increased incrementally from stage 4 to stage 6 (Figure S4). Conversely, we observed different trajectories for the expression of the PlNCED2 gene. Overall, PlNCED2 maintained a high level of expression at stages 1-2, and it displayed a decreasing trend from stages 2 to 6 (Figure S4). Correspondingly, the ABA content in each of the six seed dormancy release stages largely declined from S1 to S6 (Figure S5) [36]. By comparing the dynamics of PlNCED expression and ABA content during the process of dormancy release, we demonstrated that only PlNCED2 expression was positively associated with ABA accumulation.
3.3. Subcellular Localization of PlNCEDs
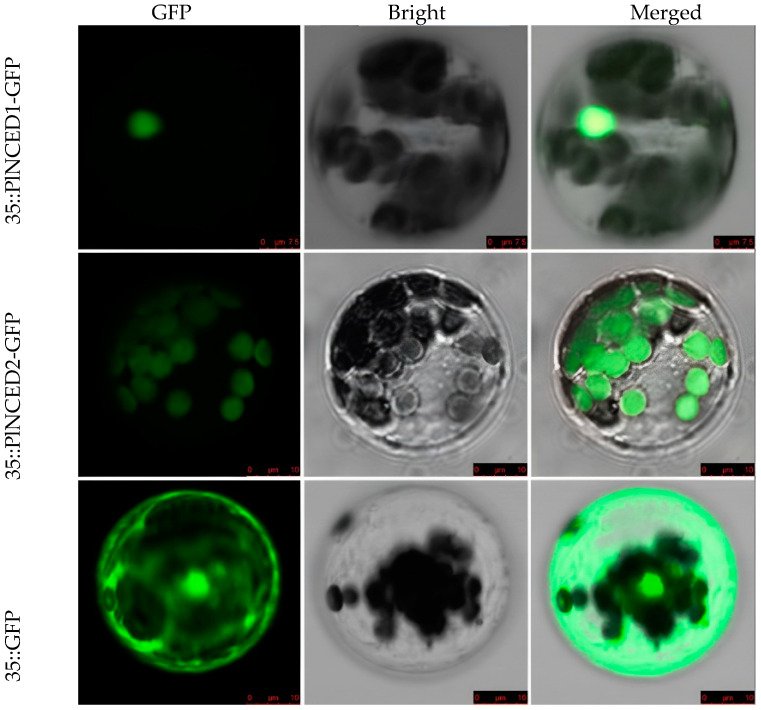
Protein maintains its optimal function in a specific subcellular localization. Therefore, to unravel the cellular functions of PlNCEDs during seed dormancy and its release process, we also carried out fluorophore tagging of the protein using green fluorescent protein to locate the presence of PlNCED proteins within the cell. Fluorescence microscopic analysis showed that the GFP fluorescence signal was distributed in the cell membrane, nucleus, and cytoplasm of A. thaliana protoplast containing the empty 16318-hGFP vector (Figure 3). In contrast, the 16318-hGFP-PlNCED1 and 16318-hGFP-PlNCED2 fusion proteins were only observed in the nucleus and cytoplasm, respectively (Figure 3). This differential cellular localization of two PlNCEDs implies that PlNCED1 may play a genetic role similar to that of transcription factors, whereas PlNCED2 may act as a functional enzyme to synthesize ABA.
Figure 3.
Subcellular localization of the 16318-hGFP-PlNCED fusion protein in protoplast.
3.4. Functional Analysis of PlNCEDs
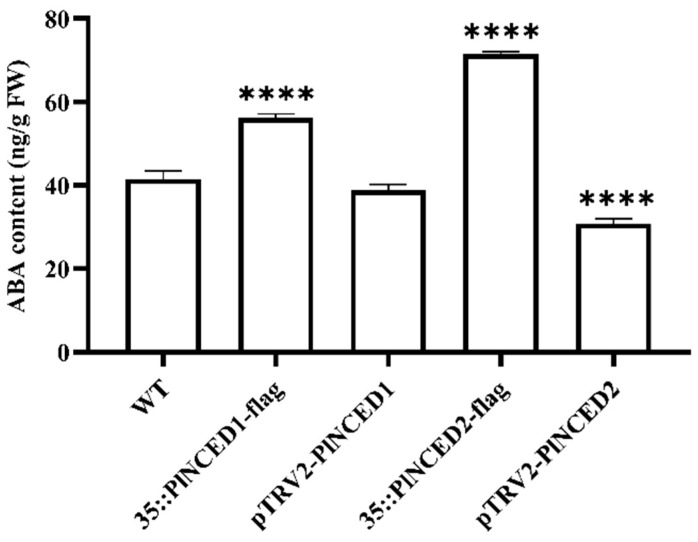
We first used the transgenic herbaceous peony callus to identify the impact of PlNCED1 and PlNCED2 on in vivo ABA content. The results showed that the expression of PlNCED1 and PlNCED2 in transgenic herbaceous peony callus was significantly altered compared with the control groups. The expression levels of PlNCED1 and PlNCED2 in the overexpressed callus were about 14.5-fold higher than that in the control callus, and the expression levels in the silenced callus were about 0.35-fold lower than that in the control callus, indicating that transgenic calluses were successfully obtained (Figure S6). As shown in Figure 4, the ABA content of the overexpressed PlNCED transgenic callus was significantly higher than that of the wild-type callus, while that of the silenced PlNCED transgenic callus was much lower, indicating a positive correlation between PlNCEDs and endogenous ABA content. In particular, the increase in ABA content caused by PlNCED2 overexpression was larger than that generated by PlNCED1 overexpression. We illustrated that PlNCED2 specifically affects endogenous ABA content by regulating its biosynthesis.
Figure 4.
Changes in ABA content in the overexpressed callus of PlNCEDs. PlNCED1 expression in the experimental group (35S::PlNCED1-flag/pTRV2-PlNCED1), PlNCED2 expression in the experimental group (35S::PlNCED2-flag/pTRV2-PlNCED2), and empty control group (WT). Significant differences (**** p ≤ 0.0001) are indicated by asterisks. One-Way ANOVA (F-test) analysis was performed using GraphPad Prism 8.0. WT was used as a control.
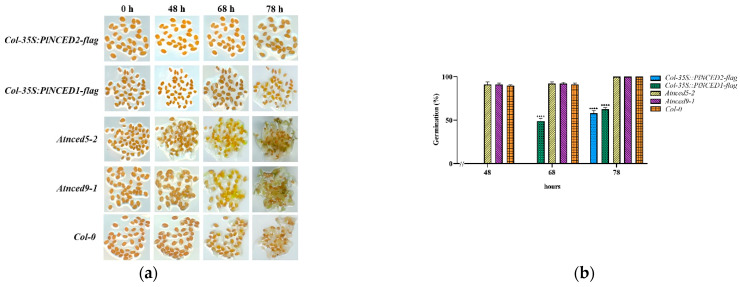
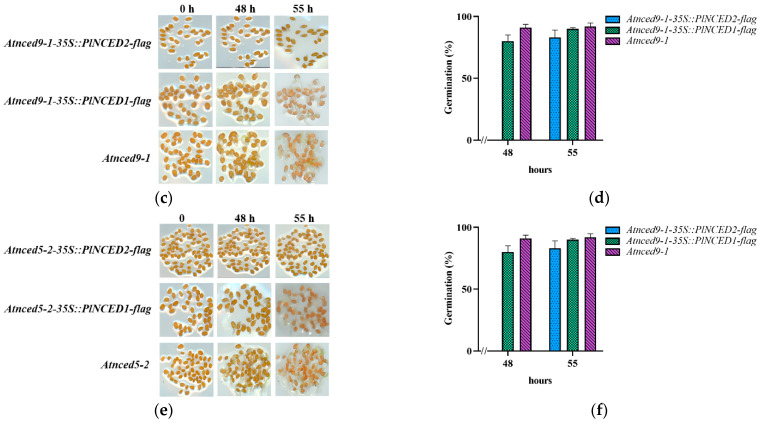
To identify the functional involvement of PlNCEDs in seed dormancy, we recorded the seed germination times of A. thaliana WT, mutant, transgenic lines, and complementation lines. The germination rates of A. thaliana WT and mutant seeds reached about 90% at 48 h, but the seeds of overexpression transgenic lines did not germinate at 48h (Figure 5a,b), indicating that the overexpression of PlNCEDs inhibited seed germination. At 68 h, the PlNCED1 overexpression transgenic line seeds began to germinate, while the PlNCED2 overexpression transgenic line seeds began to germinate at 78 h (Figure 5a,b), indicating that the inhibitory effects of PlNCEDs on seed germination were different, with PlNCED1 having a slightly weaker impact on seed germination. Compared to Atnced9-1 and/or Atnced5-2 mutants, complementation lines induced seed dormancy with a delay and a lower rate of seed germination. In particular, the PlNCED2 complementation line had a stronger effect (Figure 5c–f). Overall, we demonstrated that PlNCEDs inhibited seed dormancy release, and the inhibitory effect of PlNCED2 was stronger.
Figure 5.
Observation of seed germination rate of different types of A. thaliana. (a,b) Seed germination rate of overexpressed PlNCED transgenic lines (under Col-0 background), Col-0, and mutants (nced9-1 and nced5-2); (c,d) seed germination rate of overexpressed PlNCED transgenic lines (under nced9-1 background) and nced9-1; (e,f) seed germination rate of overexpressed PlNCED transgenic lines (under nced5-2 background) and nced5-2. Significant differences (**** p ≤ 0.0001) are indicated by asterisks. One-Way ANOVA (F-test) analysis was performed using GraphPad Prism 8.0. Col-0 and Atnced5-2-35S::PlNCED2-flag in (b) and (f) were used as controls, respectively.
4. Discussion
Paeonia lactiflora is the most familiar herbaceous peony seen in gardens and produces some of the best cut flowers in the floral industry. Though herbaceous peony is one of the most easily grown hardy perennials, its complex double seed dormancy hinders seed germination and consequently imposes adverse effects on breeding and cultivar improvements [24]. Practically, breaking herbaceous peony seed dormancy can be handily achieved through physical (e.g., cold treatments, slitting the seed coat) and biological means (e.g., hormone treatment) [25,26]. The content and level of phytohormones, particularly ABA, are the key factors for natural seed dormancy release. The final concentration of endogenous ABA depends on the dynamic balance between ABA synthesis and catabolism. Therefore, it is critical to know the genes encoding ABA metabolic enzymes and their impacts on herbaceous peony seed dormancy and germination.
To search for genes related to ABA biosynthesis, we identified two NCED genes (PlNCED1 and PlNCED2) with differential transcription pre- and post-germination (Figures S4 and S7) based on previously published transcriptome data. Studies have shown that NCED genes are the key factors that control the responses of endogenous ABA content to environmental stimuli [37]. The Conserved Domain Database (CDD) search for protein sequences of PlNCED1 and PlNCED2 in the NCBI database indicates that PlNCED1 and PlNCED2 proteins belong to the RPE65 family, a characteristic conserved domain of enzymes involved in carotenoid cleavage dioxygenase [38,39]. Furthermore, the phylogenetic analysis of NCED2 clearly revealed its intimate genetic relationship among P. lactiflora, P. ostii, and V. vinifera. This conclusion suggests that PlNCED1 and PlNCED2 have similar functions to PoNCED and VvNCED, which play an important rate-limiting role in ABA biosynthesis [40,41].
Previous reports indicate that most NCED proteins are located in chloroplasts [19,42,43]. However, our data show that PlNCED1 is located in the nucleus, but it has no Nuclear Localization Signal (NLS), a short peptide acting as a signal fragment and mediating the transport of proteins from the cytoplasm into the nucleus. Previous studies have shown that not all nucleus-expressed proteins require an NLS, and multiple additional pathways can also mediate their nuclear import [44,45]. One of these pathways is that these proteins without NLSs enter the nucleus by interacting with proteins with NLSs or with other nuclear localization proteins [46]. Therefore, our experiments imply that PlNCED1 may enter the nucleus by relying on the NLSs of other proteins. Additionally, our results suggest that the expression of PlNCEDs may be regulated by several miRNAs located in the 3’UTR regions of PlNCEDs, as well as cis-acting elements located upstream of transcripts (Supplementary Tables S4 and S5). Given the characteristics of these miRNAs, we further demonstrated that PlNCED1 and PlNCED2 might play a certain role in seed development and biotic and abiotic stresses, which is consistent with the role of ABA in plant growth. These cis-acting elements from the promoter sequences of PlNCED genes were presumed to be involved in salicylic acid, ABA, auxin, and jasmonic acid (Supplementary Tables S6 and S7). By combining PlNCED genes and their associated transcriptional regulatory binding site predictions, our data provide a preliminary path to explore the molecular mechanisms of ABA and other phytohormones involved in seed dormancy and germination.
The ABA content is proportionally related to the process of herbaceous peony seed dormancy and germination, while ABA accumulation in seeds gradually decreases from dormancy to germination (Figure S5) [36]. By comparing the transcription levels of PlNCED genes with ABA contents, we clearly show that the expression dynamics of PlNCED2 are directly associated with ABA biosynthesis and accumulation after seeds imbibe water (Figures S4 and S5) [36], suggesting that PlNCED2 is the crucial causal factor for ABA-mediated seed dormancy release in herbaceous peony. The deviation of PlNCED expression from ABA content in the imbibition stage may be due to the self-regulation of ABA metabolism genes to adapt to the dynamic balance among endogenous phytohormones after seed imbibition (Figures S4 and S5) [36]. Lee et al. (2018) also found that the level of NCED1 in orchids (PtNCED1) was low in the early stage of seed development but gradually increased and then declined slightly when seeds germinated, and the resulting changes in the seed’s endogenous ABA content played a key role in seed germination [19]. In contrast, the expression level of NCED in habanero pepper was high during seed germination, but the content of ABA gradually decreased during the same time span. This indicates that NCED has less of an effect on ABA synthesis and a weaker impact on seed germination in habanero pepper [47]. All of these reports indicate that NCED has different regulatory effects on ABA content in different plant species. Lastly, our transgenic experiment in herbaceous peony has also proved that PlNCED2 plays a major role in ABA biosynthesis and accumulation and subsequently contributes to seed dormancy maintenance.
The ultimate way to dissect the biological function of PlNCED genes involved in seed dormancy is to generate transgenic lines of herbaceous peony. However, it is difficult to establish a stable and efficient genetic transformation system for herbaceous peony [48]. Although the genetic transformation system of herbaceous peony has been used for the functional analysis of some genes in recent years [49,50,51], it takes at least 3 years to obtain transgenic seeds from transgenic seedlings due to the growth characteristics of herbaceous peony. In order to efficiently verify the effect of PlNCED genes on the seed germination rate, we used A. thaliana, a typical short-growth-cycle model plant, for subsequent gene transformation experiments in this study [52]. Arabidopsis thaliana mutants play an important role in revealing the growth and development of different plants. AtNCED5 and AtNCED9 mutants in A. thaliana have been found to affect ABA production in the embryo and endosperm, leading to seed dormancy [15,16]. Therefore, we built PlNCED transgenic lines in wild-type A. thaliana as well as PlNCED complementation lines in A. thaliana NCED mutants to explore the role of PlNCED in seed dormancy and germination. Phenotyping PlNCED transgenic lines indeed showed that weak seed dormancy was induced to a certain extent by the overexpression of PlNCEDs in A. thaliana (Figure 5a,b). More significantly, the seed germination rate of the PlNCED2 complementation line was significantly lower than that of the nced5-2 mutant (Figure 5f), indicating that overexpressed PlNCED2 rescued the partial function of Arabidopsis NCED genes. Collectively, the biological function of PlNCEDs is consistent to that of A. thaliana NCEDs by promoting seed dormancy and delaying germination.
5. Conclusions
In summary, we identified and cloned two genes from the NCED gene family, which are related to ABA synthesis in herbaceous peony. By comparing their protein sequences and phylogenetics with those of homologs in other plant species, we were able to detect their functional conserved domain. The dynamics of PlNCED2 transcription epistatically regulated endogenous ABA biosynthesis and accumulation. Using transgenic and complementation rescue lines in Arabidopsis, we were able to demonstrate the phenotypic traits of PlNCED genes, which induce seed dormancy and hinder seed germination. Our data and analysis provide the first step to understanding the underlying molecular and genetic mechanisms of complex double seed dormancy in herbaceous peony.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/plants12040710/s1. Figure S1: Identification of nced homozygous mutants in A. thaliana.; Figure S2: Nucleotide and amino acid sequences of the PlNCED gene coding regions; Figure S3: Conserved domain prediction for the proteins encoded by PlNCEDs; Figure S4: PlNCED expression at different stages of seed dormancy release in herbaceous peony; Figure S5: Changes in ABA content at different seed dormancy release stages in herbaceous peony; Figure S6: Identification of PlNCED transgenic callus; Figure S7: Other PlNCED family members’ expression at different stages of seed dormancy release in herbaceous peony; Table S1: Primers used in this study; Table S2: The identification of PlNCED1 from different plant species (%); Table S3: The identification of PlNCED1 from different plant species (%); Table S4: Prediction of miRNA targets in 3’UTR of PlNCED1; Table S5: Prediction of miRNA targets in 3’UTR of PlNCED2; Table S6: Prediction of cis-acting elements in promoter sequence of PlNCED1; Table S7: Prediction of cis-acting elements in promoter sequence of PlNCED2.
Author Contributions
Conceptualization, X.S. and S.G.; methodology, X.S.; software, S.D.; validation, R.F., J.G., and T.S.; formal analysis, R.F.; investigation, S.G.; resources, X.S.; data curation, S.G.; writing—original draft preparation, R.F.; writing—review and editing, S.D.; visualization, R.F.; supervision, X.S.; project administration, S.G.; funding acquisition, X.S. All authors have read and agreed to the published version of the manuscript.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The datasets generated and analyzed during the current study are available in the NCBI repository—PlNCED1: OL744236, https://www.ncbi.nlm.nih.gov/nuccore/OL744236, PlNCED2: OL744237, https://www.ncbi.nlm.nih.gov/nuccore/OL744237.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Funding Statement
This research was funded by the National Natural Science Foundation of China, grant number 32071814, and the China Agriculture Research System of MOF and MARA, grant number CARS-23.
Footnotes
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content.
References
- 1.Eggels S., Avramova V., Schon C.C., Poppenberger B., Rozhon W. Assay for abscisic acid 8’-hydroxylase activity of cloned plant cytochrome P450 oxidases in Saccharomyces cerevisiae. Anal. Biochem. 2018;553:24–27. doi: 10.1016/j.ab.2018.05.011. [DOI] [PubMed] [Google Scholar]
- 2.Pan J.J., Wang H.P., Hu Y.R., Yu D.Q. Arabidopsis VQ18 and VQ26 proteins interact with ABI5 transcription factor to negatively modulate ABA response during seed germination. Plant J. 2018;95:529–544. doi: 10.1111/tpj.13969. [DOI] [PubMed] [Google Scholar]
- 3.Shen W., Yao X., Ye T., Ma S., Liu X., Yin X., Wu Y. Arabidopsis aspartic protease ASPG1 affects seed dormancy, seed longevity and seed germination. Plant Cell Physiol. 2018;59:1415–1431. doi: 10.1093/pcp/pcy070. [DOI] [PubMed] [Google Scholar]
- 4.Qin X.Q., Zeevaart J.A.D. Overexpression of a 9-cis-epoxycarotenoid dioxygenase gene in Nicotiana plumbaginifolia increases abscisic acid and phaseic acid levels and enhances drought tolerance. Plant Physiol. 2002;128:544–551. doi: 10.1104/pp.010663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wu X.L., Gong F.P., Yang L., Hu X.L., Tai F.J., Wang W. Proteomic analysis reveals differential accumulation of small heat shock proteins and late embryogenesis abundant proteins between ABA-deficient mutant vp5 seeds and wild-type Vp5 seeds in maize. Front. Plant Sci. 2014;5:801. doi: 10.3389/fpls.2014.00801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martin-Rodriguez J.A., Huertas R., Ho-Plagaro T., Ocampo J.A., Tureckova V., Tarkowska D., Ludwig-Muller J., Garcia-Garrido J.M. Gibberellin-abscisic acid balances during arbuscular mycorrhiza formation in tomato. Front. Plant Sci. 2016;7:1273. doi: 10.3389/fpls.2016.01273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jia K.P., Mi J., Ali S., Ohyanagi H., Moreno J.C., Ablazov A., Balakrishna A., Berqdar L., Fiore A., Diretto G., et al. An alternative, zeaxanthin epoxidase-independent abscisic acid biosynthetic pathway in plants. Mol. Plant. 2021;14:1–6. doi: 10.1016/j.molp.2021.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Wang D.L., Gao Z.Z., Du P.Y., Xiao W., Tan Q.P., Chen X.D., Li L., Gao D.S. Expression of ABA metabolism-related genes suggests similarities and differences between seed dormancy and bud dormancy of Peach (Prunus persica) Front. Plant Sci. 2015;6:1248. doi: 10.3389/fpls.2015.01248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tognacca R.S., Botto J.F. Post-transcriptional regulation of seed dormancy and germination: Current understanding and future directions. Plant Commun. 2021;2:100169. doi: 10.1016/j.xplc.2021.100169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Xia Q., Saux M., Ponnaiah M., Gilard F., Perreau F., Huguet S., Balzergue S., Langlade N., Bailly C., Meimoun P., et al. One way to achieve germination: Common molecular mechanism induced by ethylene and after-ripening in sunflower seeds. Int. J. Mol. Sci. 2018;19:2464. doi: 10.3390/ijms19082464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kim S.Y., Warpeha K.M., Huber S.C. The brassinosteroid receptor kinase, BRI1, plays a role in seed germination and the release of dormancy by cold stratification. J. Plant Physiol. 2019;241:153031. doi: 10.1016/j.jplph.2019.153031. [DOI] [PubMed] [Google Scholar]
- 12.Munguia-Rodriguez A.G., Lopez-Bucio J.S., Ruiz-Herrera L.F., Ortiz-Castro R., Guevara-Garcia A.A., Marsch-Martinez N., Carreon-Abud Y., Lopez-Bucio J., Martinez-Trujillo M. YUCCA4 overexpression modulates auxin biosynthesis and transport and influences plant growth and development via crosstalk with abscisic acid in Arabidopsis thaliana. Genet. Mol. Biol. 2020;43:e20190221. doi: 10.1590/1678-4685-gmb-2019-0221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Li X.T. Ph.D. Thesis. Shenyang Agricultural University; Shenyang, China: 2020. Functional analysis of PlCYP707As related to dormancy release in Paeonia lactiflora seed based on transcriptome analysis. [Google Scholar]
- 14.Yan M., Yao Y.D., Mou K.P., Dan Y.Y., Li W.T., Wang C.L., Liao W.B. The involvement of abscisic acid in hydrogen gas-enhanced drought resistance in tomato seedlings. Sci. Hortic. 2022;292:110631. doi: 10.1016/j.scienta.2021.110631. [DOI] [Google Scholar]
- 15.Frey A., Effroy D., Lefebvre V., Seo M., Perreau F., Berger A., Sechet J., To A., North H.M., Marion-Poll A. Epoxycarotenoid cleavage by NCED5 fine-tunes ABA accumulation and affects seed dormancy and drought tolerance with other NCED family members. Plant J. 2012;70:501–512. doi: 10.1111/j.1365-313X.2011.04887.x. [DOI] [PubMed] [Google Scholar]
- 16.Lefebvre V., North H., Frey A., Sotta B., Seo M., Okamoto M., Nambara E., Marion-Poll A. Functional analysis of Arabidopsis NCED6 and NCED9 genes indicates that ABA synthesized in the endosperm is involved in the induction of seed dormancy. Plant J. 2006;45:309–319. doi: 10.1111/j.1365-313X.2005.02622.x. [DOI] [PubMed] [Google Scholar]
- 17.Thompson A.J., Jackson A.C., Symonds R.C., Mulholland B.J., Dadswell A.R., Blake P.S., Burbidge A., Taylor I.B. Ectopic expression of a tomato 9-cis-epoxycarotenoid dioxygenase gene causes over-production of abscisic acid. Plant J. 2000;23:363–374. doi: 10.1046/j.1365-313x.2000.00789.x. [DOI] [PubMed] [Google Scholar]
- 18.Hu B., Wan X.R., Liu X.H., Guo D.L., Li L. Abscisic acid (ABA)-mediated inhibition of seed germination involves a positive feedback regulation of ABA biosynthesis in Arachis hypogaea L. Afr. J. Biotechnol. 2010;9:1578–1586. doi: 10.5897/ajb10.1819. [DOI] [Google Scholar]
- 19.Lee Y.I., Chen M.C., Lin L., Chung M.C., Leu W.M. Increased expression of 9-Cis-Epoxycarotenoid dioxygenase, PtNCED1, associated with inhibited seed germination in a terrestrial orchid, Phaius tankervilliae. Front. Plant Sci. 2018;9:1043. doi: 10.3389/fpls.2018.01043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gan Z., Shan N., Fei L., Wan C., Chen J. Isolation of the 9-cis-epoxycarotenoid dioxygenase (NCED) gene from kiwifruit and its effects on postharvest softening and ripening. Sci. Hortic. 2020;261:109020. doi: 10.1016/j.scienta.2019.109020. [DOI] [Google Scholar]
- 21.Wang P., Lu S., Zhang X., Hyden B., Qin L., Liu L., Bai Y., Han Y., Wen Z., Xu J., et al. Double NCED isozymes control ABA biosynthesis for ripening and senescent regulation in peach fruits. Plant Sci. 2021;304:110739. doi: 10.1016/j.plantsci.2020.110739. [DOI] [PubMed] [Google Scholar]
- 22.Li Q., Yu X., Chen L., Zhao G., Li S., Zhou H., Dai Y., Sun N., Xie Y., Gao J., et al. Genome-wide identification and expression analysis of the NCED family in cotton (Gossypium hirsutum L.) PLoS ONE. 2021;16:e0246021. doi: 10.1371/journal.pone.0246021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang K., Yao L., Zhang Y., Baskin J.M., Baskin C.C., Xiong Z., Tao J. A review of the seed biology of Paeonia species (Paeoniaceae), with particular reference to dormancy and germination. Planta. 2019;249:291–303. doi: 10.1007/s00425-018-3017-4. [DOI] [PubMed] [Google Scholar]
- 24.Li J.Y. Chinese Tree Peony and Herbaceous Peony. China Forestry Publishing House; Beijing, China: 1999. pp. 153–155. [Google Scholar]
- 25.Ren X.X. Master’s Thesis. Chinese Academy of Agricultural Sciences; Beijing, China: 2016. Mechanism of Low Temperature Regulating Paeonia ostii ‘Fengdan’ Seed Hypocotyl Germination. [Google Scholar]
- 26.Sun X.M., Zhang M.M., Gao H.D., Yang H.G. Study on Characteristic for Seed Coat of Paeonia lactiflora. North. Hort. 2012;36:55–57. doi: 10.11937/bfyy.2012006022. (In Chinese) [DOI] [Google Scholar]
- 27.Jia X., Zhang M.X., Li Y., Xue Y., Zhang Y., Yu J., Wang X.Q. Study on activities of seed germination inhibitors and methods of inhibition elimination in Paeonia lactiflora. J. Chin. Med. Mater. 2021;44:2521–2525. doi: 10.13863/j.issn1001-4454.2021.11.003. (In Chinese) [DOI] [Google Scholar]
- 28.Ma Y.L., Cui J.Q., Lu X.J., Zhang L.J., Chen Z.J., Fei R.W., Sun X.M. Transcriptome Analysis of Two Different Developmental Stages of Paeonia lactiflora Seeds. Int. J. Genom. 2017;2017:8027626. doi: 10.1155/2017/8027626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fei R.W., Sun X.M., Yang P.P., Chen Z.J., Ma Y.L. Anatomical observation of Paeonia lactiflora seeds during stratification process. J. Shenyang Agric.Univ. 2017;48:354–359. doi: 10.3969/j.issn.1000-1700.2017.03.015. (In Chinese) [DOI] [Google Scholar]
- 30.Liu X.T., Sun X.M., Li Y., Li H., Li X.T., Song L.L. Study on initiation culture of Paeonia lactiflora embryo and induction of multiple shoots. J. Shenyang Agric.Univ. 2020;51:312–320. doi: 10.3969/j.issn.1000-1700.2020.03.008. (In Chinese) [DOI] [Google Scholar]
- 31.Fei R.W. Master dissertation. Shenyang Agricultural University; Shenyang, China: 2018. Cloning of the PlSAURs Genes from Paeonia lactiflora Pall. and Transformation of Arabidopsis thaliana. [Google Scholar]
- 32.Zhao D.Q., Tao J., Han C.X., Ge J.T. An actin gene as the internal control for gene expression analysis in herbaceous peony (Paeonia lactiflora Pall.) Afr. J. Agric. Res. 2012;7:2153–2159. doi: 10.5897/ajar11.1613. [DOI] [Google Scholar]
- 33.Duan S., Xin R., Guan S., Li X., Fei R., Cheng W., Pan Q., Sun X. Optimization of callus induction and proliferation of Paeonia lactiflora Pall. and Agrobacterium-mediated genetic transformation. Front. Plant Sci. 2022;13:996690. doi: 10.3389/fpls.2022.996690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.He Z. A Laboratory Guide to Chemical Control Technology on Field Crop. Beijing Agricultural University Press; Beijing, China: 1993. pp. 60–68. [Google Scholar]
- 35.Clough S.J., Bent A.F. Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 1998;16:735–743. doi: 10.1046/j.1365-313x.1998.00343.x. [DOI] [PubMed] [Google Scholar]
- 36.Li X.T., Fei R.W., Chen Z.J., Fan C.Z., Sun X.M. Plant hormonal changes and differential expression profiling reveal seed dormancy removal process in double dormant plant-herbaceous peony. PLoS ONE. 2020;15:e0231117. doi: 10.1371/journal.pone.0231117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nambara E., Marion-Poll A. Abscisic acid biosynthesis and catabolism. Annu. Rev. Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- 38.Kiser P.D. Retinal pigment epithelium 65 kDa protein (RPE65): An update. Prog. Retin. Eye Res. 2021;88:101013. doi: 10.1016/j.preteyeres.2021.101013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Tian X., Ji J., Wang G., Jin C., Guan C., Wu D., Li Z. Cloning and Expression Analysis of 9-cis-Epoxycarotenoid Dioxygenase Gene 1 Involved in Fruit Maturation and Abiotic Stress Response in Lycium chinense. J. Plant Growth Regul. 2015;34:465–474. doi: 10.1007/s00344-015-9481-1. [DOI] [Google Scholar]
- 40.Xue J.Q., Wang S.L., Zhang P., Zhu F.Y., Ren X.X., Liu C.J., Zhang X.X. On the role of physiological substances, abscisic acid and its biosynthetic genes in seed maturation and dormancy of tree peony (Paeonia ostii ‘Feng Dan’) Sci. Hortic. 2015;182:92–101. doi: 10.1016/j.scienta.2014.11.021. [DOI] [Google Scholar]
- 41.Parada F., Noriega X., Dantas D., Bressan-Smith R., Perez F.J. Differences in respiration between dormant and non-dormant buds suggest the involvement of ABA in the development of endodormancy in grapevines. J. Plant Physiol. 2016;201:71–78. doi: 10.1016/j.jplph.2016.07.007. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y., Xiang Z., Liu M., Wang S., Zhang L., Cai D., Huang Y., Mao D., Fu J., Chen L. ABA biosynthesis gene OsNCED3 contributes to preharvest sprouting resistance and grain development in rice. Plant Cell Environ. 2022 doi: 10.1111/pce.14480. [DOI] [PubMed] [Google Scholar]
- 43.Jia Y., Liu J., Bai Z., Ding K., Li H., Liang Z. Cloning and functional characterization of the SmNCED3 in Salvia miltiorrhiza. Acta Physiol. Plant. 2018;40:133. doi: 10.1007/s11738-018-2704-x. [DOI] [Google Scholar]
- 44.Lu J., Wu T., Zhang B., Liu S., Song W., Qiao J., Ruan H. Types of nuclear localization signals and mechanisms of protein import into the nucleus. Cell Commun. Signal. 2021;19:60. doi: 10.1186/s12964-021-00741-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wagstaff K.M., Jans D.A. Importins and beyond: Non-conventional nuclear transport mechanisms. Traffic. 2009;10:1188–1198. doi: 10.1111/j.1600-0854.2009.00937.x. [DOI] [PubMed] [Google Scholar]
- 46.Poon I.K., Jans D.A. Regulation of nuclear transport: Central role in development and transformation? Traffic. 2005;6:173–186. doi: 10.1111/j.1600-0854.2005.00268.x. [DOI] [PubMed] [Google Scholar]
- 47.Santos H.O., Von Pinho E.V.R., Von Pinho I.V., Dutra S.M.F., Andrade T., Guimarães R.M. Physiological quality and gene expression during the development of habanero pepper (Capsicum chinense Jacquin) seeds. Gen. Mol. Res. 2015;14:5085–5098. doi: 10.4238/2015.May.12.11. [DOI] [PubMed] [Google Scholar]
- 48.Shen M.M., Tang Z.J., da Silva J.A.T., Yu X.N. Induction and proliferation of axillary shoots from in vitro culture of Paeonia lactiflora Pall. mature zygotic embryos. N. Z. J. Crop Hortic. Sci. 2015;43:42–52. doi: 10.1080/01140671.2014.944548. [DOI] [Google Scholar]
- 49.Tang Y., Lu L., Sheng Z., Zhao D., Tao J. An R2R3-MYB Network Modulates Stem Strength by Regulating Lignin Biosynthesis and Secondary Cell Wall Thickening in Herbaceous Peony. Plant J. 2023 doi: 10.1111/tpj.16107. [DOI] [PubMed] [Google Scholar]
- 50.Zhao D., Luan Y., Shi W., Tang Y., Huang X., Tao J. Melatonin enhances stem strength by increasing lignin content and secondary cell wall thickness in herbaceous peony. J. Exp. Bot. 2022;73:5974–5991. doi: 10.1093/jxb/erac165. [DOI] [PubMed] [Google Scholar]
- 51.Zhang T., Tang Y., Luan Y., Cheng Z., Wang X., Tao J., Zhao D. Herbaceous peony AP2/ERF transcription factor binds the promoter of the tryptophan decarboxylase gene to enhance high-temperature stress tolerance. Plant Cell Environ. 2022;45:2729–2743. doi: 10.1111/pce.14357. [DOI] [PubMed] [Google Scholar]
- 52.Gepstein S., Horwitz B.A. The impact of Arabidopsis research on plant biotechnology. Biotechnol. Adv. 1995;13:403–414. doi: 10.1016/0734-9750(95)02003-L. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets generated and analyzed during the current study are available in the NCBI repository—PlNCED1: OL744236, https://www.ncbi.nlm.nih.gov/nuccore/OL744236, PlNCED2: OL744237, https://www.ncbi.nlm.nih.gov/nuccore/OL744237.