Abstract
Analysis of proteins recovered in the S100 precipitate fraction of Streptomyces griseus after ultracentrifugation led to the identification of a 52-kDa protein which is produced during the late growth phase. The gene (eshA) which codes for this protein was cloned from S. griseus, and then its homologue was cloned from Streptomyces coelicolor A3(2). The protein was deduced to be 471 amino acids in length. The protein EshA is characterized by a central region that shows homology to the eukaryotic-type cyclic nucleotide-binding domains. Significant homology was also found to MMPI in Mycobacterium leprae, a major antigenic protein to humans. The eshA gene mapped near the chromosome end and was not essential for viability, as demonstrated by gene disruption experiments, but its disruption resulted in the abolishment of an antibiotic (actinorhodin but not undecylprodigiosin) production. Aerial mycelium was produced as abundantly as by the parent strain. Expression analysis of the EshA protein by Western blotting revealed that EshA is present only in late-growth-phase cells. The eshA gene was transcribed just preceding intracellular accumulation of the EshA protein, as determined by S1 nuclease protection, indicating that EshA expression is regulated at the transcription level. The expression of EshA was unaffected by introduction of the relA mutation, which blocks ppGpp synthesis.
Streptomycetes are gram-positive filamentous soil bacteria which produce a wide variety of secondary metabolites that include about half of the known microbial antibiotics. In addition to antibiotic production (physiological differentiation), the genus Streptomyces is also characterized by the ability to form aerial mycelium from vegetative mycelium when grown in solid culture (morphological differentiation). Streptomyces coelicolor A3(2), the most fully genetically characterized streptomycete, is an appropriate strain for studying the regulation of morphological and physiological differentiation (for a review, see the work by Chater and Hopwood [2]). This strain produces at least four antibiotics, of which the blue-pigmented polyketide antibiotic actinorhodin and the red-pigmented antibiotic undecylprodigiosin are usually produced in stationary-phase cultures (reviewed by Chater and Bibb [3]). Much progress has been made in elucidating not only the organization of antibiotic biosynthesis gene clusters in several Streptomyces species but also a number of pathway-specific regulatory genes that are required for the activation of their cognate biosynthesis genes (reviewed by Hunter and Baumberg [7]). In addition to pathway-specific regulatory genes, S. coelicolor possesses several genes that have pleiotropic effects on antibiotic production. These genes fall into two classes: those that affect only antibiotic production (absA, absB, afsB, afsR, and abaA) and those that affect both antibiotic production and morphological differentiation (bldA, bldB, bldC, bldD, bldF, bldG, bldH, bldI, bldJ, bldK, bldL, bldM, and bldN) (for reviews, see references 2 and 21). However, little is known about the physiological and metabolic signals that trigger antibiotic production and morphological differentiation (briefly reviewed by Okamoto and Ochi [27]). It has been stressed that ppGpp (guanosine 5′-diphosphate, 3′-diphosphate), which is responsible for the so-called stringent response, plays a role in triggering the onset of antibiotic production, including the production of actinorhodin in S. coelicolor. Especially, ppGpp has been shown to function as a positive regulator denoting the onset of antibiotic production. This conclusion came mainly from analyzing relA (coding for ppGpp synthetase) and relC (coding for ribosomal L11 protein) mutants, which are defective in ppGpp synthesis (5, 12, 16, 25).
In the course of studying ribosomes isolated from Streptomyces spp., we recently found a novel protein which was recovered in the sediment after ultracentrifugation. Although this protein has ultimately been shown not to be a component of the ribosome, we attempted to clarify the role of this protein in morphological and/or physiological differentiation in S. coelicolor. The present paper describes the results from the molecular and functional analyses of this particular protein.
MATERIALS AND METHODS
Bacterial strains, plasmids, and culture conditions.
Streptomyces and Escherichia coli strains and plasmids used are listed in Table 1. E. coli was grown at 37°C in Luria-Bertani medium. When necessary, ampicillin and isopropyl-β-d-thiogalactopyranoside (IPTG) were added to the medium at final concentrations of 50 μg/ml and 0.5 mM, respectively. Streptomyces strains were grown at 30°C. YEME, R2YE (6), and SMM media (33) have been described previously. GYM medium (22) and R3 and R4 media (31) have also been described previously. GYC medium is a modified GYM medium supplemented with 0.5% Casamino Acids (Difco). For growing auxotrophic strains, media were supplemented with uracil and histidine (200 μg/ml each). For selection of Streptomyces transformants, media were supplemented with hygromycin (50 μg/ml) or thiostrepton (25 μg/ml).
TABLE 1.
Bacterial strains and plasmids used in this work
| Strain or plasmid | Relevant characteristic(s) | Reference or source |
|---|---|---|
| E. coli | ||
| DH5α | Cloning host | Gibco-BRL |
| DM-1 | dam dcm mutant | Gibco-BRL |
| S. coelicolor A3(2) | ||
| 1147 | Prototrophic wild-type (Pgl+ SCP1+ SCP2+) | Hopwood et al. (6) |
| J1501 | hisA1 strA1 uraA1 (Pgl− SCP1− SCP2−) | Hopwood et al. (6) |
| M600 | Prototroph (Pgl+ SCP1− SCP2−) | Chakraburtty and Bibb (1) |
| M570 | relA null mutant (relA::hyg) of M600 | Chakraburtty and Bibb (1) |
| KO-350 | eshA null mutant (eshA::tsr) of 1147 | This study |
| S. griseus IFO13189 | Prototroph (streptomycin) | Ochi (22) |
| S. lividans 1326 | Prototroph | Hopwood et al. (6) |
| S. antibiotics 3720 | Prototroph (actinomycin) | Ochi (22) |
| S. lavendulae MA406 A-1 | Prototroph (formycin) | Ochi (22) |
| S. griseoflavus FERM 1805 | Prototroph (bicozamycin) | Ochi (22) |
| Plasmids | ||
| Streptomyces replicons | ||
| pV1 | Tsrr Hygr Ampr; cloning vector of low copy number | Kawamoto et al. (11, 12) |
| pV52SC | Tsrr Hygr Ampr; pV1 with a 2.2-kb PstI fragment from pGEM52SC | This study |
| E. coli replicons | ||
| pBluescript SK(+) | Ampr; cloning vector | Stratagene |
| pSGRP52 | Ampr; pBluescript SK(+) with 8.0-kb BamHI fragment containing S. coelicolor eshA gene region | This study |
| pSCP52 | Ampr; pBluescript SK(+) with 7.1-kb PstI fragment containing S. coelicolor eshA gene region | This study |
| pDEL18 | Ampr; cloning vector | Nippon Gene |
| pSKD5 | Ampr; pDEL18 with 5.5-kb PCR-generated fragment containing S. coelicolor eshA::tsr mutant allele | This study |
| pGEM-T | Ampr; direct cloning vector of PCR product | Promega |
| pGEM52SC | Ampr; pGEM-T with 2.2-kb PCR-generated fragment containing S. coelicolor wild-type eshA gene with PstI sites introduced at both ends | This study |
| pGHRB | Ampr; pGEM-T with 175-bp PCR-generated fragment containing 5′ part of S. coelicolor hrdB gene | This study |
| pProEX-1 | Ampr; expression vector | Gibco-BRL |
| pAH0 | Ampr; pProEX-1 derivative producing S. coelicolor EshA protein | This study |
General DNA techniques and transformation.
Restriction and modifying enzymes were used according to the manufacturers' recommendations. General techniques such as plasmid isolation and transformation in Streptomyces and E. coli were employed as described by Hopwood et al. (6) and Sambrook et al. (29). Southern hybridization, PCR, and DNA sequencing were done as described previously (12, 26).
Gene cloning of eshA.
A gene library for S. griseus 13189 was constructed in E. coli DH5α by ligating a BamHI digest (7- to 9-kb fragments) of the genomic DNA into the E. coli vector pBluescript SK+. This library was screened for the genomic eshA gene by colony hybridization. The probe used was an 800-bp PCR-amplified fragment with primers designed from the S. griseus B2682 p3 (=eshA) gene sequence (GenBank accession no. L76204), 5′-GCTGGCTGCTGCGGATGCTTCCCTGGG-3′ and 5′-GGATGCGCTGCTTGAAGTCGGCGTTGTG-3′. We isolated a colony harboring pSGRP52 with an 8.0-kb insert containing the S. griseus eshA gene. A similar S. coelicolor 1147 gene library consisting of a PstI digest (6- to 8-kb fragments) of the genomic DNA was also screened using the same probe. We isolated a colony harboring pSCP52 with a 7.1-kb insert containing the S. coelicolor eshA gene.
Gene disruption of eshA.
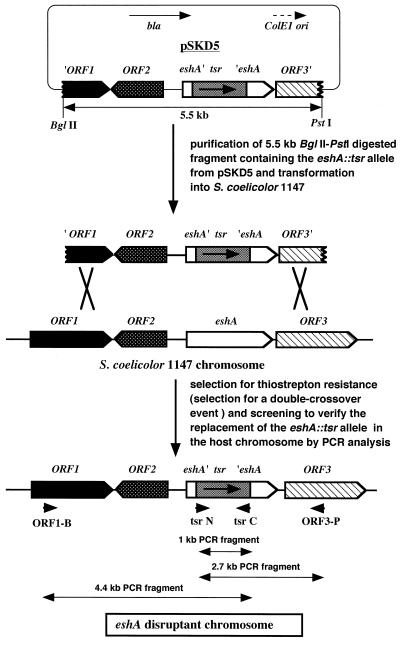
The gene disruption procedure for eshA is outlined in Fig. 3. The three fragments [BglII-SphI (2.7 kb), SphI-EcoRI (1 kb), and EcoRI-PstI (1.7 kb)] were cloned together into the BglII-PstI (3.9 kb) fragment of the vector pDEL18 to yield pSKD5. A 3.3-kb fragment was PCR amplified with pSCP52 as the template to obtain the 2.7-kb restricted fragment. The 1.7-kb fragment was PCR amplified similarly using primers eshA-E (5′-CAACGCCGAATTCAAGCAGCGCATCCAACC-3′) and ORF2-P (5′-GGAGTGCTGCAGCTCGTTGTGGACCTCGAC-3′). The 1-kb fragment was PCR amplified with primers N-tsr (5′-TCAAGGCGCATGCTTCATATGCGGGGATCG-3′) and C-tsr (5′-GACGAATCGAATTCGAGGAACCGAGCGTCC-3′) using pV1 as the template.
FIG. 3.
Gene disruption of the S. coelicolor eshA gene.
Plasmid construction.
Plasmid pV52SC, which contains the S. coelicolor eshA gene, was constructed as follows. A 2.2-kb fragment containing the entire eshA gene was PCR amplified using pSCP52 as the template. The primers used were 5′-GCTCCTCCGGTGTCACCTGCAGTCCTTCG-3′ (ORF2-3P primer) and 5′-GGGGCGGGCACGTCCTGCAGGGTTGTGGGC-3′ (ORF3-5P primer). The PCR fragment was directly cloned into the vector pGEM-T (Promega) to create pGEM52SC. pV52SC was constructed by inserting the 2.2-kb PstI fragment containing the eshA gene from pGEM52SC into the PstI site of the low-copy-number vector pV1, which is an E. coli-Streptomyces shuttle vector (11). For construction of pGHRDB, a 175-bp fragment containing the 5′ part of the S. coelicolor hrdB gene was PCR amplified using the genomic DNA of strain 1147 as the template. The primers used were 5′-CGGCCGCAAGGTACGAGTTG-3′ (HrdB-5 primer) and 5′-GATGCACAGCGCCGTGAACG-3′ (HrdB-3 primer). The PCR fragment was directly cloned into the vector pGEM-T, creating pGHRDB.
Expression and purification of EshA protein.
For preparation of EshA protein, a PstI site was created between the eighth and ninth codons of the S. coelicolor eshA gene by PCR using pSCP52 as the template. The primers were 5′-ATCCCTGCAGCTCAGACCCCACCCGAGACG-3′ and 5′-CTTGCTGCAGCGGAGTGGGCGATGTGTTGC-3′. The PCR fragment was digested with PstI and ligated into pQE30, creating pQ268. The fusion point between the insert and the vector plasmid was verified by DNA sequencing. The recombinant EshA protein possessing an N-terminal extension with a His tag sequence was purified by affinity chromatography using the HisTrap chelating column (Amersham Pharmacia Biotech).
Antiserum and Western blotting.
Polyclonal antiserum against the EshA protein from S. coelicolor was prepared in a rabbit using the purified recombinant protein as described above. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) and Western blotting were performed as described previously (11). The polyclonal antiserum was used as the primary antibody at a dilution rate of 1:10,000 for Western analysis.
Western analysis of EshA protein.
Cells collected by filtration from liquid culture or by scraping off from cellophane sheets were resuspended in buffer A and disrupted by sonication (23). RNase-free DNase I (Takara) was then added at 1 U/ml. After standing for 30 min at 0°C, cell debris was removed by centrifugation at 15,000 × g for 30 min. The supernatant was centrifuged in a Ti70 rotor at 30,000 × g for 1 h. The resulting supernatant (S30 extract) was then subjected to centrifugation at 110,000 × g for 2.5 h. The supernatant was saved as the S100 fraction. The pellet containing the EshA protein (S100 precipitate) was dissolved in a small volume of buffer A and used for the Western blotting experiment.
RNA isolation, S1 mapping, and primer extension.
Mycelium (0.1 to 0.5 g) grown on GYC plates covered with cellophane sheets was harvested quickly with a spatula, immediately frozen in liquid nitrogen, and stored at −80°C. Total RNA was isolated from the frozen mycelium using the Isogene kit (Nippon Gene). Total RNA (50 μg, as estimated spectrophotometrically) was used in each S1 nuclease protection experiment, with hybridizations at 45°C in Na-trichloroacetic acid buffer (17) after denaturation at 65°C for 15 min. S1 nuclease digestion was performed at 37°C for 1 h using 10 U of the enzyme (Takara). The S1 mapping probe for the S. coelicolor eshA transcript was prepared as follows. pGEM52SC was digested with SpeI, which cuts 600 nucleotides (nt) upstream from the initiation codon of the S. coelicolor eshA gene. Generation of the uniquely 5′-end-labeled 650-nt fragment (see Fig. 5) of an antisense single-stranded DNA fragment was done by amplification with the Klenow fragment of DNA polymerase I (Takara) using the linearized plasmid DNA as the template and the primer SCp52R03 (5′-GACGGACATCGGGAGGCCTTCTC-3′) that had been labeled at its 5′ end using [γ-32P]ATP (3,000 Ci/mmol) and T4 polynucleotide kinase. The probe fragment was purified by 6% polyacrylamide gel electrophoresis (29). The S1 mapping probe (240 nt) for the S. coelicolor hrdB transcript was similarly prepared with SpeI-digested pGHRB DNA as the template and the 5′-end-labeled primer SChrdBR21 (5′-GATGCACAGCGCCGTGAACG-3′). The primer extension reaction was carried out by the method of Kelemen et al. (13) with the 5′-end-labeled primer SCp52R03. The sequence ladders were generated by dideoxy chain termination (30) using the same radiolabeled oligonucleotide.
FIG. 5.
Analysis of eshA transcripts by S1 mapping and primer extension analyses. (A) eshA gene locus and DNA sequence of its upstream region. The probe used for S1 mapping is shown in a thick line with a circle. The putative ATG start codon and a potential ribosome-binding site (RBS) are shown in bold and by underline, respectively. A most probable 5′-end nucleotide of the eshA transcript deduced from the S1 mapping and primer extension experiments (see below) is shown in bold with a double underline, and a putative eshA promoter is marked by dots. (B) Identification of the 5′ end of the eshA transcript by S1 mapping analysis. G, A, T, and C show the DNA sequence ladders. The asterisks next to nucleotides A and G at positions −110 and −111 represent the major two 5′ ends identified. The position of a faint band (not visible in the figure) corresponding to the A at position −163 is designated by a circle. (C) Identification of the 5′ end of the eshA transcript by primer extension. The asterisk next to the A at position −111 represents the major 5′ end identified.
RESULTS
Isolation and amino acid sequencing of S. griseus EshA protein.
While analyzing the S100 precipitate from S. griseus 13189 by SDS-PAGE, we found that a protein with an apparent molecular mass of 52 kDa was expressed during the late growth phase (Fig. 1). The 52-kDa protein was present at a high level, and its appearance was coincident in time with the onset of antibiotic production and aerial mycelium formation. We therefore isolated the protein from the gel by blotting and subjected it to protein sequence analysis with a protein sequencer. The N-terminal amino acid sequence was determined to be TVDSTSEARLEVPRQSSLG. A homology search using DNA databases revealed that the N-terminal sequence for the protein matches perfectly with an open reading frame (ORF) in another S. griseus strain, B2682. This ORF had been reported previously as the sporulation-specific p3 gene (GenBank accession no. L76204, as reported from Kendrick's laboratory), and very recently it was renamed eshA (extension of sporogenic hyphae) by Kwak et al. (15) on the basis of morphological characterization of an eshA null mutant. The eshA gene of S. griseus B2682 encodes a protein of 470 amino acids with a calculated size of 51.9 kDa (15), which is in agreement with the apparent size (52 kDa) of our EshA protein from strain 13189.
FIG. 1.
SDS-PAGE analysis of the S100 precipitate from S. griseus 13189. Cells were grown in GYM medium. S100 precipitate was prepared from cells harvested at the indicated times and subjected to SDS–13.5% PAGE. Each lane contained 20 μg of protein. The gel was stained with Coomassie blue R250.
Cloning and sequence analysis of the eshA gene.
Southern analysis (see Materials and Methods) showed that the PstI-digested genomic DNA from S. coelicolor 1147 and the BamHI-digested genomic DNA from S. griseus 13189 give rise to a single hybridization band of ca. 7 and 8.0 kb, respectively. We therefore cloned these fragments from size-fractionated genomic DNA libraries (see Materials and Methods), and the complete nucleotide sequences of the cloned fragments were determined and deposited in the DDJB, EMBL, and GenBank databases under accession no. AB035202 and AB040071, respectively. Codon preference analysis of the sequenced S. coelicolor genomic eshA gene region revealed six ORFs (eshA, ORF1, and ORFs 3 to 5, all five with the same orientation, and one, ORF2, in the opposite direction), while the S. griseus genomic eshA gene region was shown to contain seven ORFs in addition to eshA, all in the same orientation (Fig. 2A). The S. coelicolor eshA gene (located on cosmid 1A4 [28]) encodes a protein of 471 amino acids with a calculated size of 51.7 kDa, and there is high homology (76% amino acid identity) between the EshA proteins of S. coelicolor and S. griseus. In addition, it is notable that in S. coelicolor there is another sequence highly homologous to eshA (64% amino acid identity; located on a cosmid 27G11 [=7G11]), which encodes 460 amino acids. Databases also revealed two other proteins, MMPI of Mycobacterium leprae (36) and SrpI of Synechococcus sp. (19, 20), that have significant homology to the EshA protein. It is notable that the characteristic central region (ca. 140 amino acids) of the EshA protein has considerable homology (30 to 32% amino acid identity) to previously known eukaryotic-type cyclic nucleotide-binding domains (Fig. 2B).
FIG. 2.
(A) Gene organization of the eshA locus for S. griseus 13189 and S. coelicolor 1147. The ORFs are shown by horizontal arrows that designate the direction of transcription. The number of base pairs in the intergenic, noncoding area is indicated below the genes, and the loop shapes show putative rho-independent inverted repeat transcriptional terminators. (B) Schematic representation of homologous region of amino acid sequences between S. coelicolor EshA and M. leprae MMPI and alignment of amino acid sequences of the central segments of S. coelicolor and S. griseus EshA proteins with eukaryotic-type cyclic nucleotide-binding domain sequences. The hatched boxes show homologous regions (40% identity) between S. coelicolor EshA and M. leprae MMPI protein (36). The white box represents a sequence of the EshA protein which shows significant homology (30 to 32%) to the cyclic nucleotide-binding domain sequences. Drosophila, cGMP-dependent protein kinase of Drosophila melanogaster (4, 9); Bovine alpha and beta, cGMP-dependent protein kinase isozymes α and β, respectively (34).
The gene organization of three genes (eshA-orf5-orf6) in S. griseus is the same as in S. coelicolor (eshA-orf3-orf4), and each gene product showed homology as high as 71 to 80% with its counterpart.
Disruption of the S. coelicolor eshA gene.
Although the EshA protein was originally found in the S100 precipitate fraction of the S. griseus cell extract, hereafter we shall deal solely with S. coelicolor because it offers a better genetic system with which to work. To verify the significance of the EshA protein in regulating growth and development, we attempted gene disruption, working with strain 1147, a prototrophic wild-type strain. We employed a double-crossover strategy (Fig. 3) because gene disruption by integration of plasmid DNA with a single crossover event might have a polar effect on gene expression. Of 24 transformants developed as thiostrepton-resistant clones, four transformants were subjected to PCR analysis; they all displayed a PCR product of the expected size. In addition, no EshA protein was detected in the transformants, as examined by Western analysis (data not shown). One of the disruptants (designated KO-350) was used for further experiments. KO-350 grew as well as the parental strain, indicating that the eshA gene is not essential for viability. However, the disruptant KO-350 revealed a severely impaired ability to produce the blue-pigmented antibiotic actinorhodin (decreased by 10-fold), as examined on various solid media such as R2YE, R3, GYM, and GYC, except for R4 medium, on which the disruptant produced actinorhodin normally. The results with R2YE medium are presented in Fig. 4. Abolishment of actinorhodin production was also confirmed in liquid culture (data not shown). In contrast, production of another red-pigmented antibiotic, undecylprodigiosin, was unaffected by eshA gene disruption. The amount of aerial mycelium formation was also not affected (Fig. 4).
FIG. 4.
Effects of eshA disruption on antibiotic production. Spores were inoculated on GYM (for aerial mycelium formation; upper panel) and R2YE (for antibiotic production; lower panel) plates, followed by 3 days of incubation. Plasmid pV52SC contains the wild-type eshA gene. Blue and red in the lower panel represent actinorhodin and undecylprodigiosin, respectively.
Complementation of eshA::tsr disruption allele.
To determine whether the disrupted allele could be overcome, we introduced low-copy-number plasmid pV52SC containing the wild-type eshA gene into KO-350 by transformation. The transformants all showed extensive restoration of actinorhodin production [as shown for transformant KO-350(pV52SC) in Fig. 4] when accompanied by reexpression of the EshA protein as examined by Western analysis (data not shown). Plasmid pV1(vector control) had no effect. When plasmid pV52SC with the wild-type eshA gene was introduced into the wild-type strain 1147, actinorhodin production was accelerated, eventually resulting in twofold more actinorhodin (data not shown). These results, together with the results from gene disruption, indicate unambiguously that the eshA gene plays an essential role in initiating antibiotic production in this organism.
Transcriptional analysis of eshA.
For determining the transcription start point, we performed high-resolution S1 nuclease mapping of the eshA transcripts (Fig. 5A). The major RNA-protected DNA fragments suggest that the transcription start point is located at either A or G (Fig. 5B), which is positioned 111 or 110 bp upstream from the translational initiation codon, respectively (Fig. 5A). Another band with weak intensity was detected 52 bp upstream from the major protected band in the original film (but not in the photograph), as designated by a circle. Full-length protection of the probe was not observed. A was determined to be the first base of the transcript because of results from reverse transcriptase-mediated primer extension experiments using the same RNA as the template (Fig. 5C).
We next conducted eshA transcription analysis during mycelial development, using the S1 nuclease protection method. In these experiments, we used strain J1501 instead of strain 1147 because J1501 produces much more antibiotic than strain 1147. When cells were grown on solid GYC medium, a growth pause was detected (Fig. 6A), as is usually observed with streptomycetes. The cells commenced to produce the red-pigmented antibiotic undecylprodigiosin and aerial mycelium during the first transition phase, while production of the blue-pigmented antibiotic actinorhodin and spore formation started at the second transition phase (Fig. 6A). RNA samples were isolated at different time points from plate-grown cultures. The hrdB transcript was used as a positive internal control because hrdB transcription has been reported to be relatively constant throughout development (13). S1 nuclease protection assays with the eshA-specific probe (see Fig. 5A) revealed that eshA mRNA is transcribed during the late growth phase (30 to 37 h), not the early growth phase (14 to 23 h) (Fig. 6B). Thus, transcription of the eshA gene is thought to be developmentally regulated in S. coelicolor.
FIG. 6.
Expression analysis of EshA protein in S. coelicolor J1501 during growth. Cells were grown on GYC agar plates covered with cellophane sheets. At each time point, cells (ca. 1 g) were collected and used for preparation of S100 precipitate fractions. (A) Growth and development of strain J1501. (B) Profile of eshA transcription. The level of eshA transcripts was determined by S1 mapping analysis, using 50 μg of sample RNA as in Fig. 5. The hrdB transcript was also determined using the same RNA sample as an internal standard. (C) Profile of EshA protein expression. EshA protein expression was examined by Western blot analysis. Samples equivalent to 0.1 g (wet weight) of cells were used.
Expression of the EshA protein.
We examined the expression pattern for the EshA protein by Western analysis. Expression of EshA was slightly if at all detectable during the early growth phase (17 to 23 h), but a burst of EshA expression was detected at the first transition phase (30 h), just following eshA gene expression, and then remained constant (Fig. 6C). The expression level of the EshA protein was virtually the same in the relA (M570) mutant (data not shown).
Distribution of eshA homologues in Streptomyces spp.
The distribution of the sequence homologous to eshA was investigated by Southern hybridization. All of the DNA samples prepared from S. antibioticus, S. lividans, S. griseoflavus, and S. lavendulae (Table 1) showed clear hybridization signals even under highly stringent washing conditions (0.2× SSC [1× SSC is 0.015 M NaCl plus 0.015 M sodium citrate] at 65°C), indicating that genes homologous to eshA are widely distributed among streptomycetes.
DISCUSSION
In this study we have demonstrated the existence and significance of EshA in the S. coelicolor life cycle by disruption of eshA and subsequent complementation of the disrupted allele using a low-copy-number plasmid. Just preceding our report, Kwak et al. (15) reported, based on work with S. griseus B-2682, that EshA of S. griseus is required for the growth of sporogenic hyphae, localization of septation, and spore maturation of this organism; this conclusion came from the fact that an eshA null mutant strain which produces no EshA cannot extend sporogenic hyphae from new branch points but instead accelerates septation and spore maturation at the existing vegetative filaments. Although in our present study we did not focus on morphological changes, it is evident that in S. coelicolor EshA plays an important role(s) as a positive regulator of antibiotic production. However, it should be pointed out that, unlike actinorhodin, the production of another antibiotic, undecylprodigiosin, was unaffected by the disruption of the eshA gene. A recent study by Hesketh et al. (5) indicates that induction of ppGpp synthesis in S. coelicolor grown under conditions of nutritional sufficiency can elicit production of actinorhodin but not undecylprodigiosin. Apparently, these results are indicative of the existence of different mechanisms for initiating the production of actinorhodin and undecylprodigiosin, although production of both of these antibiotics commences in stationary-phase cells (1, 25). It is notable that our eshA null mutants from S. coelicolor show considerable similarity in phenotype to relA null mutant M570, as characterized by (i) severely impaired ability to produce actinorhodin but no impairment of undecylprodigiosin production or aerial mycelium formation and (ii) acquisition of the ability to produce actinorhodin under phosphate-limited conditions, as represented by cultivation on R4 medium (see references 5 and 25 for the phenotype of the relA null mutant). Since the eshA homologue is widely distributed among streptomycetes (see Results), EshA may offer a good tool for uncovering and analyzing the still unknown biological events which take place in stationary-phase cells. In this regard it can be stressed that the characteristic central region within the EshA protein of S. coelicolor and S. griseus has considerable homology (30 to 32%) to the previously known eukaryotic-type cyclic nucleotide-binding domains (9, 34). Although the biochemical role of EshA remains entirely unknown, it is probable that the EshA protein exerts its influence via the nucleotide-binding domain. Although Bacillus subtilis is known not to produce cyclic AMP, recent studies have demonstrated that cyclic AMP accumulates to a high extracellular level during the late growth phase of S. coelicolor (32) and S. griseus (10, 18). It is also notable that eshA is located near the chromosome end (cosmid 1A4 [28]), which is believed to be genetically unstable.
In this study we confirmed the expression of eshA at both the transcription and translation levels (Fig. 6), leading to the conclusion that eshA expression is controlled at least in part at the transcriptional level, without depending on ppGpp, as the EshA protein was expressed normally in the relA mutant (see Results). Both primer extension analysis and S1 nuclease protection experiments measured the end of the RNA isolated from cells. Although multiple transcript 5′ endpoints were detected in either case (Fig. 5B and C), we believe that the A site mapped 111 bp upstream of the translation start codon would be the transcription start point because (i) the transcript at this site was the major one in either case, and other shorter or longer transcripts were detected only in S1 nuclease protection experiments or primer extension analysis, and (ii) the −10 (TAGCTT) and −35 (TTGGTG) regions in the putative promoter (Fig. 5A) show extensive similarity to those for S. griseus eshA (TAGTGT for −10 and TTGGTC for −35) as previously assigned by Kwak et al. (15). Thus, the longer signal detected in S1 nuclease protection analysis (Fig. 5B) could be attributed to an artifact, while the shorter signals that can be seen in Fig. 5B and C could reflect the processing of RNAs at the 5′ end as the message is degraded. The appearance of the EshA protein in S. griseus (Fig. 1) and S. coelicolor cells in late growth phase (Fig. 6C) is in good agreement with the previous finding by Triccas et al. (35) and Kwak et al. (15) that EshA (termed P3 in the former article) in S. griseus is induced by deprivation of nutrients. Likewise, the SrpI protein found in a cyanobacterium, Synechococcus sp., which is analogous to the S. griseus EshA, is also induced under sulfur deprivation (35). Therefore, it is likely that EshA is involved in the response to environmental stress, as represented by nutritional deficiency. Importantly, recent studies indicate that the EshA protein (and also the MMPI protein of M. leprae [35, 36]) are recovered in the membrane fraction and exist in a multimer form in cells (15). Our preliminary experiments (unpublished data) have also demonstrated that EshA exists as a multimer in its native state.
ACKNOWLEDGMENTS
This work was supported by a grant from the Organized Research Combination System (ORCS) of the Science and Technology Agency of Japan.
We are grateful to Kenji Morimoto and Hui Zhang for preliminary work performed in several of the experiments. The relA null mutant (M570) was generously provided by Mervyn Bibb.
REFERENCES
- 1.Chakraburtty R, Bibb M J. The ppGpp synthetase gene (relA) of Streptomyces coelicolor A3(2) plays a conditional role in antibiotic production and morphological differentiation. J Bacteriol. 1997;179:5854–5861. doi: 10.1128/jb.179.18.5854-5861.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chater K F, Hopwood D A. Streptomyces. In: Sonenshein A L, Hoch J A, Losick R, editors. Bacillus subtilis and other gram-positive bacteria: biochemistry, physiology, and molecular genetics. Washington, D.C.: American Society for Microbiology; 1993. pp. 83–89. [Google Scholar]
- 3.Chater K F, Bibb M J. Regulation of bacterial antibiotic production. In: Kleinkauf H, Von Dohren H, editors. Bio/Technology. 7: products of secondary metabolism. Weinheim, Germany: VCH; 1996. pp. 57–105. [Google Scholar]
- 4.Foster J L, Higgins G C, Jackson F R. Cloning, sequence, and expression of the Drosophila cAMP-dependent protein kinase catalytic subunit gene. J Biol Chem. 1988;263:1676–1681. [PubMed] [Google Scholar]
- 5.Hesketh A, Sun J, Bibb M J. Induction of ppGpp synthesis in Streptomyces coelicolor A3(2) grown under conditions of nutritional sufficiency elicits actII-ORF4 transcription and actinorhodin biosynthesis. Mol Microbiol. 2001;39:136–144. doi: 10.1046/j.1365-2958.2001.02221.x. [DOI] [PubMed] [Google Scholar]
- 6.Hopwood D A, Bibb M J, Chater K F, Kieser T, Bruton C J, Kieser H M. Genetic manipulation of Streptomyces:a laboratory manual. Norwich, England: John Innes Foundation; 1985. [Google Scholar]
- 7.Hunter I S, Baumberg S. . S. Baumberg, I. S. Hunter, and P. M. Rhodes (ed.), Microbial products: new approaches. Cambridge, England: Cambridge University Press; 1989. Molecular genetics of antibiotic formation pp. 121–162. [Google Scholar]
- 8.Hunter S W, Rivorie B, Mehra V, Bloom B R, Brennan P J. The major native proteins of the leprosy bacillus. J Biol Chem. 1990;265:14065–14068. [PubMed] [Google Scholar]
- 9.Kalderon D, Rubin G M. cGMP-dependent protein kinase genes in Drosophila. J Biol Chem. 1989;264:10738–10748. [PubMed] [Google Scholar]
- 10.Kang D-K, Li X-M, Ochi K, Horinouchi S. Possible involvement of cAMP in aerial mycelium formation and secondary metabolism in Streptomyces griseus. Microbiology. 1999;145:1161–1172. doi: 10.1099/13500872-145-5-1161. [DOI] [PubMed] [Google Scholar]
- 11.Kawamoto S, Watanabe H, Hesketh A, Ensign J C, Ochi K. Expression analysis of the ssgA gene product, associated with sporulation and cell division in Streptomyces griseus. Microbiology. 1997;143:1077–1086. doi: 10.1099/00221287-143-4-1077. [DOI] [PubMed] [Google Scholar]
- 12.Kawamoto S, Zhang D, Ochi K. Molecular analysis of the ribosomal L11 protein (rplK=relC) of Streptomyces griseus and identification of a deletion allele. Mol Gen Genet. 1997;255:549–560. doi: 10.1007/s004380050528. [DOI] [PubMed] [Google Scholar]
- 13.Kelemen G H, Cundliffe E, Financsek I. Cloning and characterization of gentamicin-resistance genes from Micromonospora purpurea and Micromonospora rosea. Gene. 1991;98:53–60. doi: 10.1016/0378-1119(91)90103-i. [DOI] [PubMed] [Google Scholar]
- 14.Kelemen G H, Brown G L, Kormanec J, Potuckova L, Chater K F, Buttner M J. The positions of the sigma-factor genes, whiG and sigF, in the hierarchy controlling the development of spore chains in the aerial hyphae of Streptomyces coelicolor A3(2) Mol Microbiol. 1996;21:593–603. doi: 10.1111/j.1365-2958.1996.tb02567.x. [DOI] [PubMed] [Google Scholar]
- 15.Kwak J, McCue L A, Trczianka K, Kendrick K E. Identification and characterization of a developmentally regulated protein, EshA, required for sporogenic hyphal branches in Streptomyces griseus. J Bacteriol. 2001;183:3004–3015. doi: 10.1128/JB.183.10.3004-3015.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Martinez-Costa O H, Arias P, Romeo N M, Parro V, Mellado R P, Malpartida F. A relA/spoT homologous gene from Streptomyces coelicolor A3(2) controls antibiotic biosynthetic genes. J Biol Chem. 1996;271:10627–10634. doi: 10.1074/jbc.271.18.10627. [DOI] [PubMed] [Google Scholar]
- 17.Murray M G. Use of sodium trichloroacetate and mung bean nuclease to increase sensitivity and precitsion during transcript mapping. Anal Biochem. 1986;158:165–170. doi: 10.1016/0003-2697(86)90605-6. [DOI] [PubMed] [Google Scholar]
- 18.Neumann T, Piepersberg W, Distler J. Decision phase regulation of streptomycin production in Streptomyces griseus. Microbiology. 1996;142:1953–1963. [Google Scholar]
- 19.Nicholson M L, Laudenbach D E. Genes encoded on a cyanobacterial plasmid that are transcriptionally regulated by sulfur availability and CysR. J Bacteriol. 1995;177:2143–2150. doi: 10.1128/jb.177.8.2143-2150.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nicholson M L, Gaasenbeek M, Laudenbach D E. Two enzymes together capable of cysteine biosynthesis are encoded on a cyanobacterial plasmid. Mol Gen Genet. 1995;247:623–632. doi: 10.1007/BF00290354. [DOI] [PubMed] [Google Scholar]
- 21.Nodwell J R, Yang M, Kuo D, Losick R. Extracellular complementation and the identification of additional genes involved in aerial mycelium formation in Streptomyces coelicolor. Genetics. 1999;151:569–584. doi: 10.1093/genetics/151.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ochi K. Metabolic initiation of differentiation and secondary metabolism by Streptomyces griseus: significance of the stringent response (ppGpp) and GTP content in relation to A-factor. J Bacteriol. 1987;169:608–616. doi: 10.1128/jb.169.8.3608-3616.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ochi K. Heterogeneity of ribosomal proteins among Streptomyces species and its application to identification. J Gen Microbiol. 1989;135:2635–2642. doi: 10.1099/00221287-135-10-2635. [DOI] [PubMed] [Google Scholar]
- 24.Ochi K. Streptomyces relC mutants with an altered ribosomal protein ST-L11 and genetic analysis of a Streptomyces griseus relC mutant. J Bacteriol. 1990;172:4008–4016. doi: 10.1128/jb.172.7.4008-4016.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ochi K, Zhang D, Kawamoto S, Hesketh A. Molecular and functional analysis of the ribosomal L11 and S12 protein genes (rplK and rpsL) of Streptomyces coelicolor A3(2) Mol Gen Genet. 1997;256:488–498. doi: 10.1007/pl00008614. [DOI] [PubMed] [Google Scholar]
- 26.Okamoto S, Itoh M, Ochi K. Molecular cloning and characterization of the obg gene of Streptomyces griseus in relation to the onset of morphological differentiation. J Bacteriol. 1997;179:170–179. doi: 10.1128/jb.179.1.170-179.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Okamoto S, Ochi K. An essential GTP-binding protein functions as a regulator for differentiation in Streptomyces coelicolor. Mol Microbiol. 1998;30:107–119. doi: 10.1046/j.1365-2958.1998.01042.x. [DOI] [PubMed] [Google Scholar]
- 28.Redenbach M, Kieser H M, Denapaite D, Eichner A, Cullum J, Kinashi H, Hopwood D A. A set of ordered cosmids amd a detailed genetic and physical map for the 8Mb Streptomyces coelicolor A3(2) chromosome. Mol Microbiol. 1996;21:77–96. doi: 10.1046/j.1365-2958.1996.6191336.x. [DOI] [PubMed] [Google Scholar]
- 29.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 30.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Shima J, Hesketh A, Okamoto S, Kawamoto S, Ochi K. Induction of actinorhodin production by rpsL (encoding ribosomal protein S12) mutations that confer streptomycin resistance in Streptomyces lividans and Streptomyces coelicolor A3(2) J Bacteriol. 1996;178:7276–7284. doi: 10.1128/jb.178.24.7276-7284.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Susstrunk U, Pidoux J, Taubert S, Ullmann A, Thompson C J. Pleiotropic effects of cAMP on germination, antibiotic biosynthesis and morphological development in Streptomyces coelicolor. Mol Microbiol. 1998;30:33–46. doi: 10.1046/j.1365-2958.1998.01033.x. [DOI] [PubMed] [Google Scholar]
- 33.Takano E, Gramajo H C, Strauch E, Andres N, White J, Bibb M J. Transcriptional regulation of the redD transcriptional activator gene accounts for growth-phase-dependent production of the antibiotic undecylprodigiosin in Streptomyces coelicolor A3(2) Mol Microbiol. 1992;6:2797–2804. doi: 10.1111/j.1365-2958.1992.tb01459.x. [DOI] [PubMed] [Google Scholar]
- 34.Takio K, Wade R D, Smith S B, Krebs E G, Walsh K A, Titani K. Guanosine cyclic 3′, 5′-phosphate dependent protein kinase, a chimeric protein homologous with two separate protein families. Biochemistry. 1984;23:4207–4218. doi: 10.1021/bi00313a030. [DOI] [PubMed] [Google Scholar]
- 35.Triccas J A, Winter N, Roche P W, Gilpin A, Kendrick K E, Britton W J. Molecular and immunological analysis of the Mycobacterium avium homolog of the immunodominant Mycobacterium leprae 35-kilodalton protein. Infect Immun. 1998;66:2684–2690. doi: 10.1128/iai.66.6.2684-2690.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Winter N, Triccas J A, Rivoire B, Pessolani M C V, Eiglmeier K, Hunter S W, Brennan P J, Britton W J. Characterization of the gene encoding the immunodominant 35 kDa protein of Mycobacterium leprae. Mol Microbiol. 1995;16:865–876. doi: 10.1111/j.1365-2958.1995.tb02314.x. [DOI] [PubMed] [Google Scholar]