Abstract
Purpose
Evidence suggests low energy availability (LEA) is prevalent in elite male Gaelic football (GF) players. Previous research in male and female team sports found LEA may negatively impact endocrine function. The aim of this study was to examine the seasonal variations in energy availability (EA) and its associations with salivary measures in elite male GF players across the competitive season.
Methods
Energy availability was assessed using field-based methods in conjunction with salivary testosterone (s-T), cortisol (s-C) and immunoglobin A (s-IgA) concentrations at pre-season (PRE), in-season (IN) and post-season (POST).
Results
38% reported LEA at PRE, 33% at IN, and 28.5% at POST. s-C, s-T and s-IgA levels were within normal ranges at PRE, IN and POST. Salivary cortisol declined significantly from PRE to IN, remaining reduced at POST. Salivary testosterone decreased significantly from PRE to IN but was significantly elevated at POST compared to IN. Salivary IgA was significantly greater at POST than IN. No significant associations were found between s-C or s-T and EA at any time point. Pre-season s-IgA exhibited a significant inverse association with PRE EA. Decreased s-IgA flow rate and s-IgA secretion rate were significantly associated with decreased EA at PRE. Reduced carbohydrate (CHO) intake was also associated with decreased s-IgA secretion rate at PRE.
Conclusion
This study suggests that LEA is prevalent in elite male GF players, but is not associated with s-C or s-T. However, EA is associated with s-IgA which may impact the immune system. Therefore, education and interventions surrounding the prevalence and associations of EA should be implemented in this population.
Keywords: Nutrition, Gaelic football, Energy availability, Hormones
Introduction
Low energy availability (LEA) is prevalent in elite male Gaelic football (GF) players at pre-season (PRE) and in-season (IN) [1]. Previous research has reported seasonal variations in anthropometric and performance measures, distance covered and high-speed running distance across the competition season in GF players [2, 3]. However, little is known about the associations of LEA and hormonal factors in this cohort, and if variations occur across a competitive season.
Periods of excessive training load [4] and LEA [5] may decrease testosterone and/or elevate cortisol concentrations in male athletes. Testosterone is a steroid hormone, mainly produced in the testes, that promotes protein synthesis and inhibits protein degradation [6] and is considered an indicator of anabolism [7]. In conditions of reduced EA, a disruption in the release of gonadotropin-releasing hormone can occur, resulting in irregular pulsatility rates of luteinising hormone, which may result in reduced plasma testosterone levels and a reduction in spermatogenesis [8]. Cortisol, the main form of glucocorticoid in humans, is secreted in response to prolonged exercise, LEA and/or stress [9]. It exerts catabolic effects which inhibit protein synthesis, increases protein degradation [10] and inhibit elements of immune function [11]. The testosterone-cortisol ratio (T:C) represents the overall anabolic/catabolic balance and is a useful marker in assessing an athlete’s readiness to train and level of recovery [12]. An imbalance between training load and recovery is suggested when T:C is reduced by > 30%, reflective of an increase in cortisol, a decrease in testosterone, or a combination of both [13]. Previous research investigating the seasonal hormonal variations in soccer [14, 15], rugby [16] and basketball [17] players reported alterations in testosterone and cortisol throughout the season, however, EA was not measured in these studies. Elite GF players play at a level posing similar physiological demands to elite-level soccer such that they have similar fitness characteristics and distance covered during match-play [18, 19]. Therefore, the hormonal responses throughout a GF season may be similar. To the best of the author’s knowledge, only one previous study [20] has investigated the associations between LEA and hormonal markers in a small sample of 10 male soccer collegiate athletes during PRE. LEA was not significantly correlated with reduced testosterone or elevated cortisol levels at PRE, however, further research is warranted throughout the season for example at IN and POST to validate these findings. It was hypothesised that GF players will present with LEA at PRE and IN, which will be associated with reduced testosterone levels and elevated cortisol levels.
Salivary immunoglobulin A (s-IgA) is an indirect biomarker of immune function [21]. Prolonged periods of high-volume training in individual and team sports, in particular soccer and rugby, may decrease s-IgA concentrations, thus leaving athletes at increased risk of illness [22, 23]. Furthermore, a significant relationship was reported between elevated s-C and reduced s-IgA levels in basketball players [24]. Similarly, decreased levels of testosterone have been found to significantly correlate with suppressed IgA levels in soccer players [25]. Gaelic football poses similar physiological demands as soccer on athletes [18], therefore elevated cortisol levels and reduced testosterone may also occur in GF players, particularly at IN, suppression of s-IgA may also be evident. Although no research to date has directly investigated the association between LEA and IgA, studies in energy-deprived male soldiers [26], and judo players undergoing a weight loss strategy [27] suggested energy deficiency may be associated with reduced levels of IgA. Therefore, this study aimed to investigate EA status and its associations with seasonal variations of s-C, s-T and s-IgA in elite male GF players at PRE, IN and POST.
Methods
Participants
Thirty elite division 3 inter-county male GF players (age = 24.1 ± 3.8 y; stature = 1.76 ± 0.35 m; body mass (BM) = 88.6 ± 5.6 kg; lean body mass (LBM) 75.8 ± 3.1 kg; training age = 5.1 years; nutritional support provision = 2 years), who ranked in the second quartile of the GF team ranking system [28] participated in this study, during the 2021 (COVID-19) season. Inter-county GF players are defined as elite, as they are deemed the best players in their county [3]. During a pre-testing session, written and verbal details regarding study design and participation were explained to the management and players, and written informed consent was obtained. Participants had to be current members of a male inter-county GF team and aged 18–40 years. The study was approved by the Waterford Institute of Technology Ethics Committee (17/ HSES/03).
Study design
During 1 week at PRE (April 2021), IN (June 2021), and POST (October 2021) repeated measures of energy intake (EI) and exercise energy expenditure (EEE) were obtained for two training days (one gym day and one pitch day) and a rest day and used in the calculation of EA. Body composition and estimation of LBM were assessed by a level 1 ISAK-trained practitioner and morning salivary hormone collections were completed once at PRE, IN and POST.
Energy availability
Energy availability was defined as EI minus EEE relative to kilograms of LBM per day [29]. EA levels were classified as optimal (> / = 40 kcal.kg LBM−1.d−1); subclinical (30- 40 kcal.kg LBM−1.d−1) and clinical (< 30 kcal.kg LBM−1.d−1) [30]. Energy intake and EEE measurements were performed for the same three consecutive days (gym, pitch and rest day) at PRE, IN and POST.
Energy intake was assessed from a 3-day weighed and photographed food diary log. Three days have been suggested to provide a reasonably accurate estimation of dietary intake without resulting in reduced compliance, that has been reported when using 7-day food records [31]. Players were instructed to follow their typical diet throughout the testing period. Each participant was provided with instructions on how to accurately record all foods and beverages, including time of day, location, supplements, and leftovers. To minimise the issues associated with estimating dietary intake, the players provided photographs for each eating occasion and the researcher clarified any inconclusive information by interview following the submission of the food diary. Energy intake and macronutrient composition were calculated using dietary analysis software (Nutritics Ltd, Ireland). The average EI from the 3-day food diary records provided the EI to calculate energy availability. The possibility of misreporting was assessed by calculating the mean reported EI to predicted RMR ratio (rEI:RMR) [32] on an individual level:
SDmin = − 2; SDmax was + 2 (95% confidence limits); CVwEI = within-subject variation in energy intake (23%); CV2wB = within -subject RMR accuracy (8.5%); CV2tP = within-subject variation in physical activity level (PAL) (15%); d = days of dietary intake (3). A PAL value of 1.6 was selected based on previous research on GF players [33]. At the individual level, calculated cut – off points were < 1.03 for under-reporters and > 2.46 for over-reporters.
Training logs recording training type, duration of activity and rest periods for both gym and pitch-based sessions were completed and assigned a Metabolic Equivalent (MET) value from the compendium of physical activities [34] to estimate EEE. To accurately assess EEE, calories expended for RMR for the duration of exercise were subtracted from the estimated EEE [35]. Resting metabolic rate was estimated using the Cunningham equation [36].
Body mass was measured using a digital scale (OMRON BF511, Shanghai, China) to the nearest 0.01 kg during both testing occasions. Height was measured to the nearest 0.1 cm without shoes using a SECA stadiometer during the pre-season. Participants wore gym shorts and no shoes. BMI was calculated as a ratio of body mass to the squared height (kg/m2). Measures of LBM were obtained via skinfold assessment during the week leading up to the testing periods. Skinfold measurements were conducted at 7 sites (biceps, triceps, subscapular, iliac crest, supraspinale, abdominal, front thigh and medial calf) using International Society for the Advancement of Kinanthropometry (ISAK) techniques previously described by Norton, [37]. Measurements were taken by a Level I ISAK accredited anthropometrist with a technical error of measurement of ≤ 3.0% using Harpenden callipers (Baty International, Burgess Hill, England). The estimation of LBM was calculated using the recently validated predictive equations, specific to male elite GF players [38].
Salivary hormones
Salivary testosterone (s-T), cortisol (s-C) and IgA (s-IgA) were collected within 1 h of the players’ awakening to limit the effect of diurnal variation on sample collection [39]. To ensure standardised testing, players were instructed to consume the same breakfast and refrain from brushing their teeth, eating chewing gum or consuming caffeine before each saliva collection [23]. To evaluate training load, each player recorded a session rating of perceived exertion (sRPE) 30 min after each training session in the 7 days leading up to the saliva collection, which was then multiplied by the number of minutes spent training [40]. Any stressful situations in the previous 24 h and sleep quality were also recorded using the Modified Sleep Questionnaire and Stressful Situations Form [40, 41]. During saliva collection, players placed the oral fluid collector swab (Soma Bioscience, Wallingford, United Kingdom) on top of their tongue and closed their mouth until the swab collected 0.5 ml of fluid and turned blue. The swab was then placed in the OFC buffer bottle of assays, in line with the manufacturer’s guidelines, sealed and sent to a private laboratory, where it was assessed using enzyme immunoassay test kits (EIA; SOMA Bioscience, Wallingford, UK), and an automated analyser (Tecan Nanoquant, Tecan, Männedorf, Switzerland) as per manufacturers guidelines. The intra- and inter- assay CV for s-T, s-C and s-IgA was 7.94% and 9.4%, 4.26% and 6.88%, 4.71% and 11.4%, respectively.
Following analysis, s-T was converted to its molar value to calculate T:C. All analyses were completed by the same laboratory technician. For s-IgA analysis, saliva flow rate and secretion rate were also calculated. Saliva flow rate (ml/min) was calculated by dividing the total volume of saliva by the number of minutes it took for the swab to turn blue. Secretion rate (ug/min) is the total volume of IgA present in saliva per minute and was calculated by multiplying the IgA concentration by the flow rate [23].
Statistical analysis
Statistical analysis was completed using SPSS version 28.0 (SPSS, Inc., Chicago, IL, USA). Cohen’s effect size (d) used to describe the differences in variables across PRE, IN and POST was categorized with: d < 0.20 (trivial), 0.20–0.59 (small), 0.60–1.19 (moderate), 1.20–1.99 (large), and > 2.00 (very large) [42]. Normality of data was assessed using the Shapiro–Wilks test. Repeated measures ANOVA were used to determine whether there was a significant difference between PRE, IN and POST in data normally distributed. Friedman’s test was used for data not normally distributed. When a significant effect was found, a post hoc Bonferroni test was performed to identify the difference between the means. The level of significance was set at P < 0.05. To assess any relationship between EA and associated variables Pearson bivariate correlations and Spearman’s correlation coefficients were conducted for normally and not normally distributed data.
Results
Due to injury (n = 2), unsuccessful selection to the squad (n = 3) and under-reporting (n = 4), twenty-one participants completed this study. Demographics of the final subset (n = 21) are described in Table 1.
Table 1.
Anthropometric characteristics of elite male Gaelic football players (n = 21)
| Characteristics | PRE | IN | POST | Effect size (d) | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| Height (m) | 1.77 ± 0.12 | N/A | 1.77 ± 0.12 | N/A | 1.77 ± 0.12 | N/A | N/A |
| Body mass (kg) | 86.29 ± 70.2 | 83.07–89.51 | 85.59 ± 7.21 | 82.30–88.77 | 85.54 ± 7.73 | 82.03–89.05 | 0.110 |
| Fat mass (kg) | 15.04 ± 2.95 | 13.69–16.38c | 14.49 ± 2.58 | 13.31–15.66c | 16.24 ± 3.04 | 14.86–17.63ab | 0.075 |
| Lean body mass (kg) | 71.24 ± 5.65 | 68.67–73.82c | 71.09 ± 60.3 | 68.36–73.83c | 69.29 ± 6.31 | 66.42–72.17ab | 0.711 |
| Sum of 7 skinfolds (mm) | 66.96 ± 13.91 | 60.63–73.29 | 64.1 ± 12.43 | 58.44–69.75 | 74.7 ± 15.04 | 67.93–81.62 | 0.265 |
Values expressed as means ± SD standard deviation, 95% CI 95% confidence intervals, d Effect Sizes
aSignificantly different than PRE (P < 0.05)
bSignificantly different than IN (P < 0.05)
CSignificantly different than POST (P < 0.05)
Anthropometrics
Table 1 provides a summary of the anthropometric data for PRE, IN and POST. Fat Mass (FM) was significantly increased at POST compared to PRE and IN. Lean Body Mass (LBM) was significantly lower at POST compared to PRE and IN. No significant differences were reported between PRE, IN or POST for the remaining anthropometric characteristics.
Components of EA, macronutrient intakes and salivary measures
Data for components of EA, macronutrient intakes and salivary measures are presented in Table 2. Pre-season EA was reported as clinical in 38% (n = 8), subclinical in 43% (n = 9) and optimal in 19% (n = 4) of athletes. In-season EA analysis showed 33% (n = 7) were categorised as clinical, 62% (n = 13) as subclinical, 5% (n = 1) as high. Post-season EA was reported as clinical in 28.5% (n = 6), subclinical in 62% (n = 13) and high in 9.5% (n = 2) of athletes. There were no significant differences in EA between PRE, IN, or POST.
Table 2.
EA, EI, macronutrient intake, RMR, exercise energy expenditure adjusted for RMR (EEEadj) and training load in elite male Gaelic football players (n = 21)
| Variable | PRE | IN | POST | Effect size (d) | |||
|---|---|---|---|---|---|---|---|
| Mean ± SD | 95% CI | Mean ± SD | 95% CI | Mean ± SD | 95% CI | ||
| EA (kcal/kg LBM/d) | 33.9 ± 6.1 | 31.1–36.6 | 31.7 ± 4.2 | 29.7–33.5 | 33.1 ± 4.1 | 31.2–34.9 | 0.124 |
| EI (kcal) | 2548 ± 922 | 2128–2968 | 2154 ± 931 | 1730–2578 | 2552 ± 365 | 2385–2718 | 0.359 |
| CHO (g/kg/day) | 4.0 ± 0.6 | 3.7–4.2 | 3.7 ± 0.7 | 3.3–4.1 | 3.6 ± 0.7 | 3.5–3.8a | 0.273 |
| Protein (g/kg/day) | 1.8 ± 0.5 | 1.5–2.1 | 1.6 ± 0.3 | 1.5–1.7 | 1.8 ± 0.3 | 1.7–1.9 | 0.173 |
| Fat (g/kg/day) | 1.1 ± 0.3 | 0.9–1.2 | 1.0 ± 0.2 | 0.9–1.1 | 1.9 ± 0.2 | 0.9–1.1 | 0.069 |
| RMR (kcal/day) | 2067 ± 124 | 2010–2124c | 2064 ± 132 | 2004–2124c | 2024 ± 138 | 1961–2087ab | 0.711 |
| EEEadj (kcal/day) | 431.6 ± 37 | 414–449bc | 341.7 ± 30 | 327–356ac | 257.8 ± 25 | 246–269ab | 0.991 |
| Training load (sRPE x min) | 634.9 ± 25.7 | 611–654bc | 535.7 ± 44.27 | 497–574ac | 388.4 ± 14.7 | 378–399ab | 0.985 |
Values expressed as means ± SD standard deviation, EA Energy Availability, EI Energy Intake, RMR Resting Metabolic Rate, CHO Carbohydrates, EEE Exercise Energy Expenditure, T:C Testosterone:Cortisol ratio, IgA Immunoglobin A, 95% CI 95% confidence intervals, d Effect Sizes
aSignificantly different than PRE ( P < 0.05)
bSignificantly different than IN ( P < 0.05)
CSignificantly different than POST (P < 0.05)
Mean carbohydrate (CHO) intake was below (5-7 g/kg/day), protein intake was above (1.4-17 g/kg/day), and fat intake was above (30% of total daily calories) the recommended intake for team sport athletes [43–45] at PRE, IN and POST. Carbohydrate intake was significantly higher at PRE compared to POST (d = 0.273). There was no significant difference between protein or fat intakes at PRE, IN or POST. Exercise energy expenditure, RMR and training load were significantly higher at PRE compared to IN and POST (d = 0.991; d = 0.711; d = 0.985).
Salivary testosterone, s-C and s-IgA concentrations were within normal range at all time points (Figs. 1, 2 and 4) [43, 46]. Salivary testosterone was significantly higher at PRE (395.6 ± 289.6) and POST (442.3 ± 163.2) than IN (352.1 ± 182.1) (d = 0.566). Salivary cortisol was significantly higher at PRE (12.69 ± 4.08) than IN (8.49 ± 3.16) or POST (8.35 ± 3.35) (d = 0.354). Testosterone:Cortisol was significantly reduced at IN (34.4 ± 22.6) when compared to PRE (50.1 ± 37.3) and significantly greater at POST (64.5 ± 40.3) when compared to PRE and IN (d = 0.412) (Fig. 3). Salivary IgA was significantly higher at POST (541.7 ± 256.3) than IN (373.7 ± 190.3) (d = 0.343) (Fig. 4).
Fig. 1.
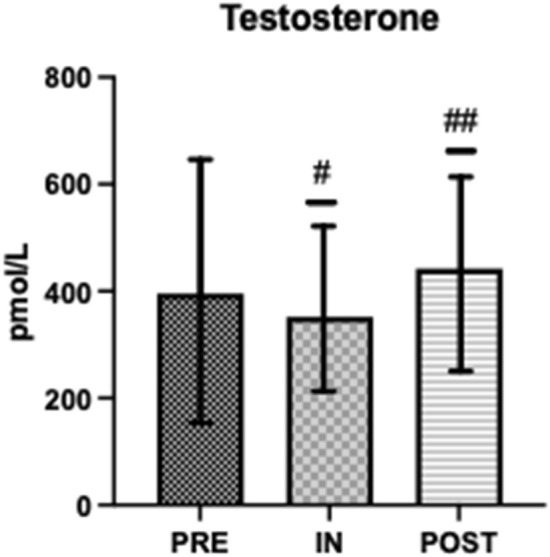
Pre, In and Post mean salivary testosterone. #Significantly different to PRE (P < 0.05); ##Significantly different to IN (P < 0.05)
Fig. 2.
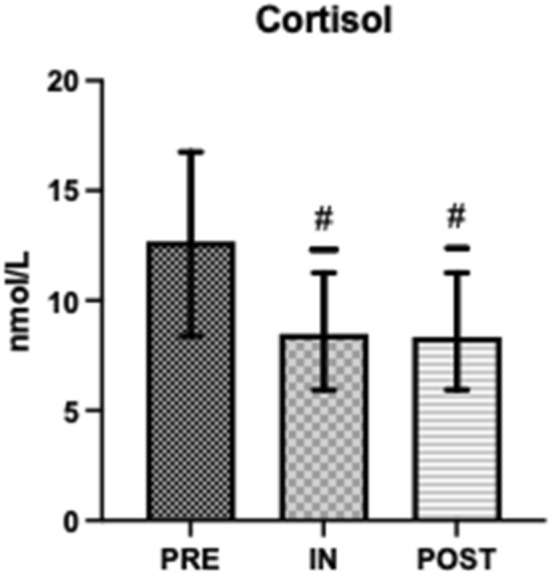
Pre, In and Post mean salivary cortisol. #Significantly different to PRE (P < 0.05)
Fig. 4.
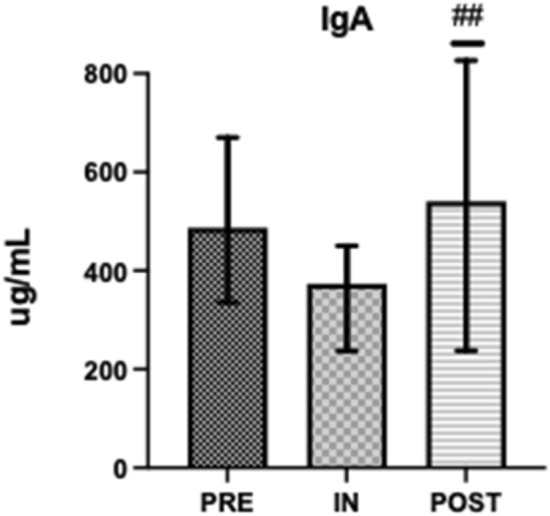
Pre, In and Post mean salivary IgA. ##Significantly different to IN (P < 0.05)
Fig. 3.
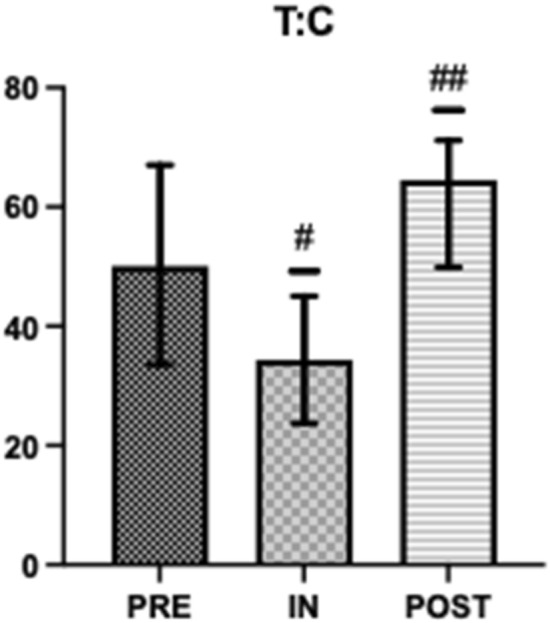
Pre, In and Post mean testosterone:cortisol. #Significantly different to PRE (P < 0.05) ##Significantly different to PRE and IN (P < 0.05)
Table 3 presents the associations between EA and EI, macronutrient intake, body composition, EEE, RMR, s-C, s-T, and s-IgA. Energy availability was positively associated with EI, CHO, fat, protein, and s-IgA flow rate and negatively associated with BM, LBM, RMR and s-IgA concentration at PRE. Energy availability was positively associated with EI and all macronutrients at IN, and EI and fat intake at POST.
Table 3.
Associations between EA and EI, macronutrient intake, body composition, EEE, RMR, salivary hormones and IgA in elite male Gaelic football players (n = 21)
| Variable | PRE r value | IN r value | POST r value |
|---|---|---|---|
| EI (kcal) | 0.844*** | 0.734*** | 0.762*** |
| CHO (g/kg/day) | 0.447*** | 0.687*** | 0.399 |
| Protein (g/kg/day) | 0.765*** | 0.503* | 0.391 |
| Fat (g/kg/day) | 0.790*** | 0.531* | 0.834*** |
| Body Mass (kg) | – 0.442* | – 0.277 | – 0.125 |
| Fat Mass (kg) | – 0.051 | 0.175 | – 0.277 |
| Skinfolds (mm) | 0.030 | 0.328 | – 0.273 |
| Lean Body Mass (kg) | – 0.636* | – 0.423 | 0.010 |
| RMR (kcal/day) | – 0.505* | – 0.365 | 0.004 |
| EEEadj (kcal/day) | – 0.427 | – 0.258 | – 0.152 |
| s-T (pmol/L) | – 0.427 | 0.295 | – 0.012 |
| s-C (nmol/L) | – 0.001 | 0.279 | 0.058 |
| T:C | – 0.257 | 0.273 | – 0.070 |
| s-IgA (ug/mL) | – 0.443* | 0.230 | – 0.51 |
| s-IgA Flow Rate | 0.567* | 0.197 | 0.213 |
EI Energy Intake, CHO Carbohydrates, RMR Resting Metabolic Rate, EEE Exercise Energy Expenditure, s-T salivary testosterone, s-C salivary cortisol, T:C Testosterone:Cortisol ratio, s-Ig Immunoglobin A, IgA Immunoglobin A
*P < 0.05
**P < 0.01
*** P < 0.001
Table 4 presents the associations between macronutrient intake and salivary measures. Significant inverse correlations were reported between s-IgA concentrations and s-IgA flow rate at PRE and IN. Significant inverse associations were also found between s-IgA concentrations and secretion rate at PRE, IN, and POST.
Table 4.
Associations between macronutrient intake, training load and salivary measures in elite male Gaelic football players (n = 21)
| s-T | s-C | s-IgA | |||||||
|---|---|---|---|---|---|---|---|---|---|
| PRE | IN | POST | PRE | IN | POST | PRE | IN | POST | |
| CHO (g/kg/day) | 0.048 | 0.284 | 0.212 | – 0.018 | 0.025 | 0.197 | – 0.001 | 0.124 | 0.006 |
| Protein (g/kg/day) | – 0.362 | 0.010 | 0.109 | – 0.244 | 0.087 | – 0.033 | – 0.397 | 0.078 | – 0.173 |
| Fats (g/kg/day) | – 0.047 | – 0.029 | – 0.151 | – 0.042 | 0.350 | 0.345 | – 0.425 | – 0.006 | 0.120 |
| Training load (min x RPE) | – 0.041 | – 0.038 | – 0.166 | 0.107 | 0.096 | 0.121 | – 0.234 | – 0.089 | – 0.176 |
| IgA Flow (ml/min) | – 0.477* | – 0.630** | 0.399 | ||||||
| IgA Secretion (ug/min) | – 0.821** | – 0.830** | – 0.937** | ||||||
CHO Carbohydrates, RPE Rate of Perceived Exertion, s-T salivary testosterone, s-C salivary cortisol, s-Ig Immunoglobin A
*P < 0.05
**P < 0.01
*** P < 0.001
Discussion
There is a dearth of studies investigating the associations between EA and salivary hormone levels in male athletes. To the author’s knowledge, no previous research has been conducted to investigate the associations between EA and salivary hormone levels in elite male team sport athletes. This study is the first to examine seasonal variations in s-C, s-T and s-IgA in elite male GF athletes. The main findings were 38%, 33% and 28.5% of elite male GF players presented with clinical EA during PRE, IN, and POST. However, EA did not correlate with s-T, s-C or s-IgA levels, which were all within the normative range, at all-time points, with the exception of a small inverse association between EA and IgA at PRE.
Prevalence of LEA in the present study is less than previously reported in elite male GF players (65–70%) [1]. However, the current study calculated LBM using the newly validated Dunne method of assessing FM in elite male GF players [38]. It is worth noting that the mean sum of 7 skinfolds in both the current study and McGuire et al. [1] are the same for PRE and IN (65 mm). It is now a global trend to communicate the sum of skinfolds as opposed to just body fat % [47, 48], with many practitioners choosing to omit body fat % or FM results. However, for the estimation of EA, LBM and, therefore, FM and body fat % measurements are needed. Therefore, caution should be taken when choosing what predictive equations to use. Dunne et al. [38] evaluated the accuracy and variability of various predictive equations in estimating BF% and FM of male elite GF players in comparison to DXA [38]. Findings indicated that previous skinfold prediction equations commonly used to estimate BF% and FM in GF players all underestimated BF% and absolute FM [38], meaning previous estimations of EA in this population may have been overestimated, due to the overestimation of LBM. In addition to this, the athletes in the current study had more experience working with a Sports Nutritionist and therefore may have had a better understanding of how to fuel more adequately. Mean EA was 31.7–33.9 kcal/kg LBM/day across the season in the current study, which is similar to EA previously reported in male soccer players [20, 49], cyclists [50] and endurance athletes [51]. This indicates that suboptimal EA status exists within various male sporting populations and highlights the need to identify and manage the potential associations that may occur.
No significant differences in EI across the season observed in the current study are similar to results previously reported [1]. Similar EI at PRE (2655.7 ± 448.7 kcal) in male professional soccer players (n = 26) has previously been reported [40]. EI in the current study at IN was substantially less than that seen in elite soccer players at the same time point (3186 ± 367 kcals) [52], despite GF players being exposed to similar physiological demands [18]. POST EI in the present study is similar to that reported in elite male soccer players [53]. Macronutrient intakes at PRE and IN in the present study correspond with previous findings [1] and research investigating EI of elite male GF [33, 54]. The possible reasons and consequences of these intakes have already been discussed in detail in a previous study investigating the EA status and nutritional intake in elite male GF players, conducted by our research group [1].
No significant differences in BM were seen between PRE, IN and POST. These results are similar to those previously reported [1]. In contrast, FM was significantly higher at POST than PRE and IN. Contrasting research found FM returned to PRE values, with no significant difference in FM between PRE and POST [46], but POST assessment was conducted one week after the last competitive game, whereas the current study performed POST measurements a minimum of 6 weeks after the last competitive game. The longer off-season time frame may have resulted in higher FM gains in elite male GF players in the current study. LBM was significantly higher at PRE and IN, in comparison to POST. Reduced EEE during POST, coupled with inadequate dietary intake, may have resulted in reduced LBM in the current study. Suboptimal calorie intake has been shown to blunt responses to anti-catabolic stimuli [55] and potentially reduce insulin levels which can stimulate proteolysis and possibly increase LBM loss [55]. Although these athletes consumed adequate protein for team sport athletes at POST (1.80 ± 0.28 g/kg/day) [44], a range of 2.3–3.1 g/kg of FFM has been shown to optimally protect against LBM loss when calorie intake is insufficient [56].
Despite Gaelic football’s amateur status, elite male GF players have been shown to train at a level similar to professional athletes and in some instances, with more intensified training periods over the course of the season [57, 58]. In the current study, elite male GF players mean s-T was within a normal range (175−870 pmol/L) [46] and not significantly associated with EA at any time point. However, closer examination of the data revealed that 8, 4, and 2 players at PRE, IN and POST, respectively, had testosterone levels within the lowest quartile of the reference range. Furthermore, seasonal variations did occur whereby s-T was significantly higher at PRE and POST compared to IN. Although no significant association was reported between training loads and any salivary measures, when coupled with higher training loads elevated testosterone may represent stimulation of the hypothalamic–pituitary–adrenal axis, one of the main mechanisms of adaptation to PRE training [59]. The current study shows a significant decrease in s-T at IN with similar findings reported in professional soccer players [4, 15]. Conversely, increases in serum testosterone levels were reported in professional basketball players [17] during IN. Protein intake, in particular, appears to be inversely related to resting testosterone levels [60, 61]. Although there were no significant associations found between s-T and protein intake at PRE, the mean calorie contribution from protein at IN for the current study was 22%, with 38% of GF players consuming more than the recommended amounts for team sport athletes [44]. A recent meta-analysis showed that highly trained soccer players also tend to exceed the recommended intake for protein [62]. Conversely, unlike elite GF players, professional basketball players tend to under-consume protein throughout the season [63], which may account for the disparity in testosterone levels at IN. s-T was elevated at POST, which was also seen in male professional [4] and collegiate soccer players [64] and may reflect a reduction in stress following the cessation of competition. However, other research reported no change or reduction in serum testosterone levels at the end of the season [11, 59]. Differences between those studies and the current study may be explained by different experimental designs. For example, it is unclear exactly how long after the last competitive game Michailidis [65] conducted the POST assessment. The POST assessment of the current study took place at least 6 weeks after the last competitive game as previous research has suggested that a minimum of 35 days is required to allow hormonal levels to return to baseline following periods of restricted calorie intake and intensive physical activity [66]. Furthermore, both Michailidis [65] and Silva et al. [67], assessed total testosterone concentration in blood serum which may have led to inaccurate results due to the globin-binding proteins in total testosterone.
Mean s-C was within the normative range (2.8–27.5 nmol/L) [46]. No significant associations were reported between s-C and EA at any time point, which is in line with previous findings in male endurance runners [68] and collegiate soccer players [20]. Regardless of the lack of association with EA, the current study is the first of its kind to determine s-C levels at various time points throughout the season in elite male GF players. s-C decreased significantly from PRE to IN and POST, with no significant associations with training load. Similar variations in cortisol levels were seen in professional soccer players at PRE, IN, POST and after the end of the recovery period [67]. However, cortisol concentrations were assessed in plasma, and it is unclear whether the protein-bound form or free form was measured. Only free-form cortisol is found in saliva [69], therefore it is difficult to accurately compare these findings. A recent study investigating s-C in elite male Irish rugby union players reported elevated s-C throughout the training week during PRE, indicating inadequate recovery and the onset of fatigue because of training [40]. Like the current study, Tiernan et al. [40] found no significant relationship between salivary cortisol levels and training load [40]. The authors suggested that a potential reason for a lack of association between training load and s-C could be due to large individual variability in s-C concentrations, therefore future studies should assess results on an individual basis. Conversely, higher levels of cortisol during IN in professional soccer players have been reported [2, 11]. However, these studies [2, 11] reported higher training loads at IN, whereas the current study described the highest training loads at PRE. Furthermore, difficult pitch conditions were suggested to result in a higher stress response at IN [11], while in the current study IN occurred during the summer months with more favourable pitch conditions. Dietary CHO intake could affect cortisol levels during periods of higher training loads. GF players did not consume adequate amounts of CHO at PRE in the current study. Without adequate dietary CHO to replenish glycogen stores and maintain optimal blood glucose levels, cortisol will be secreted to maintain blood glucose through muscle breakdown, and amino acid oxidation [14, 70]. No significant correlations were established between CHO intake and s-C in this study, however, future research should consider investigating this further.
To the author’s best knowledge, this is the first study to investigate T:C in elite GF players at PRE, IN and POST. There is a paucity of evidence outlining the effect EA may have on T:C, with no association established in a previous study on cyclists [71]. Similarly, no significant correlations were found in these elite male GF players at any time point during the season. However, seasonal fluctuations in T:C were observed. T:C increased significantly from PRE to IN and IN to POST. Similar increases from PRE to IN were reported in elite male soccer players [11] whereby the T:C significantly increased from PRE to IN by 12.1%. However, in the current study, the T:C significantly decreased by 24.3% from IN to POST. As previously mentioned, serum measures of cortisol and total testosterone were taken so readings may be affected due to reduced hormonal bioavailability. Further research investigating T:C via salivary sampling should be considered including measurement of resting metabolic rate, as evidence has shown males with suppressed RMR exhibited an association with lower T:C [30].
s-IgA was within the normative range (79.26–679.50 ug/mL) [72] at all time points in the present study. Novel findings of this study were the significant association between decreased s-IgA concentration and reduced EA at PRE, reduced CHO intake and lower IgA secretion rate at PRE, and a significant association between elevated s-IgA concentrations and reduced EA at PRE, indicating players’ immune function may be compromised at this phase of the season. It has been recommended that IgA secretion rate, s-IgA flow rate and s-IgA concentration are provided when discussing results, as measurements can vary highly between individuals [73]. Flow rate has been shown to be reduced by both exercise [74] and fluid restriction [75] and in turn may account for an increase in s-IgA concentration [75], similar to findings in this study. The current study also suggests that reduced EA is associated with a lower s-IgA flow rate, thus providing an elevated s-IgA concentration reading which may not be an accurate reflection of what is truly at play. Tiernan et al. [23] investigated the use of s-IgA as a predictor of upper respiratory tract infections (URTI) in nine male elite rugby union players over a 10-week period. Results showed that the onset of a URTI significantly increased when s-IgA significantly decreased. When s-IgA decreased by > 65%, a player was at a greater risk of contracting an URTI within the subsequent 2-week period. In the current study, s-IgA concentrations decreased by 20% from PRE to IN, and increased from IN to POST by 45%, indicating improved immunity once competition had ceased. In contrast to Filaire et al. [4], who reported unchanged s-IgA levels across four-time points throughout a professional soccer season, the current study found a significant increase in s-IgA concentrations from IN to POST. In the study by Filaire et al., [4] fasted saliva samples were obtained, in contrast to the current study where a CHO-rich breakfast was consumed prior to saliva collection. Previous research investigating the effect of dietary CHO on IgA levels in Ironman athletes found that IgA concentrations were significantly increased following high CHO ingestion [76]. Therefore, it would be naïve to assume that results from fasted saliva measurements would be appropriate to compare against.
Although this study provides novel findings, it is not without limitations. Firstly, this study only provides a snapshot of one week of EA assessment and one saliva collection during PRE, IN and POST, further research should consider taking weekly or bi-weekly saliva samples and EA assessment across PRE, IN and POST. The pitfalls of assessing EA were discussed in detail in our previous research [1]. Whilst efforts were made to ensure valid methods were used to measure EA, such as the inclusion of the Dunne method of body composition assessment specific to male GF players [38] and conducting interviews after the EI assessments, limitations still exist. In the absence of objective measures of EEE, Physical Activity Level (PAL) was used. A PAL value of 1.6 was selected based on previous research in elite GF players [33], however, the PAL range for sedentary and light activity lifestyles is between 1.40 and 1.69, for moderately active or active lifestyles between 1.70 and 1.99 [77]. Considering the professional approach GF players take to training, it could be assumed that a PAL range for at least moderately active lifestyles would be more applicable. Furthermore, salivary measures of testosterone, cortisol and IgA appear to exhibit a high degree of individual variability. This is supported by the findings of Francis et al., [78] who reported that s-IgA concentrations in elite swimmers could vary by up to 100 mg/L when collected on consecutive days. Similarly, large inter-individual variability was found between s-C and s-T in rugby Union players [40] and elite track and field athletes [79]. Therefore, future studies should consider bi-weekly or daily saliva collections to account for possible variations.
The current study offers novel insights into EA and salivary hormone status of elite male GF players. A substantial percentage (28–38%) of elite male GF players were in a state of LEA at PRE, IN and POST, with a strong correlation between CHO intake and EA. The EA status, inadequate carbohydrate intake and overconsumption of dietary protein in this cohort is of concern. Athletes often increase protein intake and reduce carbohydrate intake to reduce adiposity, indicating a need for nutritional education specifically related to EA and EI to support the development of optimal health and performance of these athletes. Significant fluctuations in salivary hormones and IgA concentrations were apparent across the season, albeit within the normative range. However, GF players appear to manage their training load well, as reflected in the increase in T:C as the season progressed. To minimise the risk of non-functional overreaching, possible overtraining and compromised immune function, training load should be monitored effectively and managed accordingly, and these biomarkers should be frequently monitored. Similarly, these athletes’ s-IgA levels did not decrease by > 65%, which suggests there is minimal risk of contracting URTIs. However significant associations were found between s-IgA flow rate and EA at PRE, which warrants further investigation and indicates the need to educate GF players on immune health. Furthermore, interesting trends were observed between macronutrients and salivary measures, in particular protein and s-T, and CHO and s-IgA in this study. Therefore, future research should explore the associated effects of macronutrients on s-C, s-T and s-IgA in various sporting populations across all genders.
Data availability
The data that support the findings of this study are available from the corresponding author, [AMcG], upon reasonable request.
Declarations
Conflict of interest
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Ethical approval
This study has been approved by the SETU ethics committee and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
References
- 1.McGuire A, Warrington G, Doyle L. Energy availability and macronutrient intake in elite male Gaelic football players. Sci Med Football. 2022 doi: 10.1080/24733938.2022.2029551. [DOI] [PubMed] [Google Scholar]
- 2.Kelly RA, Collins K. The seasonal variations in anthropometric and performance characteristics of elite intercounty Gaelic football players. J Strength Cond Res. 2018;32(12):3466–3473. doi: 10.1519/JSC.0000000000001861. [DOI] [PubMed] [Google Scholar]
- 3.Mangan S, Ryan M, Shovlin A, McGahan J, Malone S, O'Neill C, Burns C, Collins K. Seasonal changes in Gaelic football match-play running performance. J Strength Cond Res. 2019;33(6):1685–1691. doi: 10.1519/JSC.0000000000002269. [DOI] [PubMed] [Google Scholar]
- 4.Filaire E, Lac G, Pequignot JM. Biological, hormonal, and psychological parameters in professional soccer players throughout a competitive season. Perceptual Motor Skills. 2003;97(3_suppl):1061–1072. doi: 10.2466/pms.2003.97.3f.1061. [DOI] [PubMed] [Google Scholar]
- 5.Heikura IA, Uusitalo ALT, Stellingwerff T, Bergland D, Mero AA, Burke LM. Low energy availability is difficult to assess but outcomes have large impact on bone injury rates in elite distance athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):403–411. doi: 10.1123/ijsnem.2017-0313. [DOI] [PubMed] [Google Scholar]
- 6.Vingren JL, Kraemer WJ, Ratamess NA, Anderson JM, Volek JS, Maresh CM. Testosterone physiology in resistance exercise and training: the up-stream regulatory elements. Sports Med. 2010;40(12):1037–1053. doi: 10.2165/11536910-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 7.West DWD, Phillips SM. Anabolic processes in human skeletal muscle restoring the identities of growth hormone and testosterone. Phys Sportsmed. 2010;38(3):97–104. doi: 10.3810/psm.2010.10.1814. [DOI] [PubMed] [Google Scholar]
- 8.Martin B, Golden E, Carlson OD, Egan JM, Mattson MP, Maudsley S. Caloric restriction: impact upon pituitary function and reproduction. Ageing Res Rev. 2008;7(3):209–224. doi: 10.1016/j.arr.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mastorakos G, Pavlatou M, Diamanti-Kandarakis E, Chrousos GP. Exercise and the stress system. Hormones (Athens) 2005;4(2):73–89. [PubMed] [Google Scholar]
- 10.Viru A, Viru M. Cortisol - essential adaptation hormone in exercise. Int J Sports Med. 2004;25(06):461–464. doi: 10.1055/s-2004-821068. [DOI] [PubMed] [Google Scholar]
- 11.Cain DW, Cidlowski JA. Immune regulation by glucocorticoids. Nat Rev Immunol. 2017;17(4):233–247. doi: 10.1038/nri.2017.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.de Luccia TPB. Use of the testosterone/cortisol ratio variable in sports. Open Sports Sci J. 2016;9(1):104–113. doi: 10.2174/1875399x01609010104. [DOI] [Google Scholar]
- 13.Adlercreutz H, Harkonen M, Kuoppasalmi K, Näveri H, Huhtaniemi I, Tikkanen H, Remes K, Dessypris A, Karvonen J. Effect of training on plasma anabolic and catabolic steroid hormones and their response during physical exercise. Int J Sports Med. 1986 doi: 10.1055/s-2008-1025798. [DOI] [PubMed] [Google Scholar]
- 14.Michailidis Y. Stress hormonal analysis in elite soccer players during a season. J Sport Health Sci. 2014;3(4):279–283. doi: 10.1016/j.jshs.2014.03.016. [DOI] [Google Scholar]
- 15.Handziski Z, Maleska V, Petrovska S, Nikolik S, Mickoska E, Dalip M, Kostova E. The changes of ACTH, cortisol, testosterone and testosterone/cortisol ratio in professional soccer players during a competition half-season. Bratisl Lek Listy. 2006;107(6/7):259. [PubMed] [Google Scholar]
- 16.Argus CK, Gill ND, Keogh JWL, Hopkins WG, Beaven CM. Changes in strength, power, and steroid hormones during a professional rugby union competition. J Strength Cond Res. 2009;23(5):1583–1592. doi: 10.1519/JSC.0b013e3181a392d9. [DOI] [PubMed] [Google Scholar]
- 17.Martínez AC, Calvo JS, Marí JAT, Inchaurregui LCA, Orella EE, Biescas AP. Testosterone and cortisol changes in professional basketball players through a season competition. J Strength Cond Res. 2010;24(4):1102–1108. doi: 10.1519/JSC.0b013e3181ce2423. [DOI] [PubMed] [Google Scholar]
- 18.Malone S, Solan B, Collins K. The running performance profile of elite Gaelic football match-play. J Strength Cond Res. 2017;31(1):30–36. doi: 10.1519/JSC.0000000000001477. [DOI] [PubMed] [Google Scholar]
- 19.Reilly T, Collins K. Science and the Gaelic sports: Gaelic football and hurling. Eur J Sport Sci. 2008;8(5):231–240. doi: 10.1080/17461390802251851. [DOI] [Google Scholar]
- 20.Lee S, Kuniko M, Han S, Oh T, Taguchi M. Association of low energy availability and suppressed metabolic status in korean male collegiate soccer players: a pilot study. Am J Men’s Health. 2020 doi: 10.1177/1557988320982186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bishop NC, Gleeson M. Acute and chronic effects of exercise on markers of mucosal immunity. Front Biosci. 2009;14(2):4444–4456. doi: 10.2741/3540. [DOI] [PubMed] [Google Scholar]
- 22.Papacosta E, Nassis GP. Saliva as a tool for monitoring steroid, peptide and immune markers in sport and exercise science. J Sci Med Sport. 2011;14(5):424–434. doi: 10.1016/j.jsams.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 23.Tiernan C, Lyons M, Comyns T, Nevill AM, Warrington G. Salivary IgA as a predictor of upper respiratory tract infections and relationship to training load in elite rugby union players. J Strength Cond Res. 2020;34(3):782–790. doi: 10.1519/JSC.0000000000003019. [DOI] [PubMed] [Google Scholar]
- 24.He CS, Tsai ML, Ko MH, Chang CK, Fang SH. Relationships among salivary immunoglobulin A, lactoferrin and cortisol in basketball players during a basketball season. Eur J Appl Physiol. 2010;110(5):989–995. doi: 10.1007/s00421-010-1574-8. [DOI] [PubMed] [Google Scholar]
- 25.Peñailillo L, Maya L, Niño G, Torres H, Zbinden-Foncea H. Salivary hormones and IgA in relation to physical performance in football. J Sports Sci. 2015;33(20):2080–2087. doi: 10.1080/02640414.2015.1064151. [DOI] [PubMed] [Google Scholar]
- 26.Gomez-Merino D, Chennaoui M, Burnat P, Drogou C, Guezennec CY. Immune and hormonal changes following intense military training. Mil Med. 2003;168(12):1034–1038. doi: 10.1093/milmed/168.12.1034. [DOI] [PubMed] [Google Scholar]
- 27.Hiraoka H, Hanaoka Y, Jesmin S, Kimura F, Matsuish Y, Shimizu K, Watanabe K. Variation of salivary IgA during weight loss period before a competition among university judo players. J Clin Med Res. 2019;11(12):798. doi: 10.14740/jocmr3998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mangan S, Collins K. A rating system for Gaelic football teams: factors that influence success. Int J Comput Sci Sport. 2016;15(2):78–90. doi: 10.1515/ijcss-2016-0006. [DOI] [Google Scholar]
- 29.Loucks AB, Thuma JR. Luteinizing hormone pulsatility is disrupted at a threshold of energy availability in regularly menstruating women. J Clin Endocrinol Metab. 2003;88(1):297–311. doi: 10.1210/jc.2002-020369. [DOI] [PubMed] [Google Scholar]
- 30.Melin AK, Heikura IA, Tenforde A, Mountjoy M. Energy availability in athletics: health, performance and physique. Int J Sport Nutr Exerc Metab. 2019;29(2):152–164. doi: 10.1123/ijsnem.2018-0201. [DOI] [PubMed] [Google Scholar]
- 31.Magkos F, Yannakoulia M. Methodology of dietary assessment in athletes: concepts and pitfalls. Curr Opin Clin Nutr Metab Care. 2003;6(5):539–549. doi: 10.1097/00075197-200309000-00007. [DOI] [PubMed] [Google Scholar]
- 32.Goldberg GR, Black AE, Jebb SA, Cole TJ, Murgatroyd PR, Coward WA, Prentice AM. Critical evaluation of energy intake data using fundamental principles of energy physiology: 1. Derivation of cut-off limits to identify under-recording. Eur J Clin Nutr. 1991;45(12):569–581. [PubMed] [Google Scholar]
- 33.McCrink CM, McSorley EM, Grant K, McNeilly AM, Magee PJ. An investigation of dietary intake, nutrition knowledge and hydration status of Gaelic football players. Eur J Nutr. 2021;60:1465–1473. doi: 10.1007/s00394-020-02341-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Ainsworth BE, Haskell WL, Herrmann SD, Meckes N, Bassett DR, Tudor-Locke C, Greer JL, Vezina J, Whitt-Glover MC, Leon AS. 2011 compendium of physical activities: a second update of codes and MET values. Med Sci Sports Exerc. 2011;43(8):1575–1581. doi: 10.1249/MSS.0b013e31821ece12. [DOI] [PubMed] [Google Scholar]
- 35.Torstveit MK, Fahrenholtz I, Stenqvist TB, Sylta Ø, Melin A. Within-day energy deficiency and metabolic perturbation in male endurance athletes. Int J Sport Nutr Exerc Metab. 2018;28(4):419–427. doi: 10.1123/ijsnem.2017-0337. [DOI] [PubMed] [Google Scholar]
- 36.Cunningham JJ. A reanalysis of the factors influencing basal metabolic rate in normal adults. Am J Clin Nutr. 1980;33(11):2372–2374. doi: 10.1093/ajcn/33.11.2372. [DOI] [PubMed] [Google Scholar]
- 37.Norton KI. Kinanthropometry and exercise physiology. Routledge; 2018. Standards for anthropometry assessment; pp. 68–137. [Google Scholar]
- 38.Dunne A, O’Neill C, Warrington G, Rackard G, Cullen S. Variability and accuracy of body fat estimates using skinfolds and DXA in elite Gaelic football players: a validation study and development of novel prediction equations. Sport Sci Health. 2022;6:1–100. doi: 10.1007/s11332-022-00896-2. [DOI] [Google Scholar]
- 39.Pritchard BT, Stanton W, Lord R, Petocz P, Pepping GJ. Factors affecting measurement of salivary cortisol and secretory immunoglobulin a in field studies of athletes. Front Endocrinol. 2017;8:168. doi: 10.3389/fendo.2017.00168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Tiernan C, Lyons M, Comyns T, Nevill AM, Warrington G. Investigation of the relationship between salivary cortisol, training load, and subjective markers of recovery in elite rugby union players. Int J Sports Physiol Perform. 2020;15(1):113–118. doi: 10.1123/ijspp.2018-0945. [DOI] [PubMed] [Google Scholar]
- 41.Engeland CG, Hugo FN, Hilgert JB, Nascimento GG, Junges R, Lim HJ, Marucha PT, Bosch JA. Psychological distress and salivary secretory immunity. Brain Behav Immun. 2016;52:11–17. doi: 10.1016/j.bbi.2015.08.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hopkins W. Magnitude matters: effect size in research and clinical practice. Med Sci Sports Exerc. 2006;38(5):56. doi: 10.1249/00005768-200605001-00463. [DOI] [Google Scholar]
- 43.Mujika I, Burke LM. Nutrition in team sports. Ann Nutr Metab. 2011;57(Suppl. 2):26–35. doi: 10.1159/000322700. [DOI] [PubMed] [Google Scholar]
- 44.Tarnopolsky M. Protein and amino acid needs for training and bulking up. Clin Sports Nutrition. 2010;8(2):90–123. [Google Scholar]
- 45.Jenner SL, Buckley GL, Belski R, Devlin BL, Forsyth AK. Dietary intakes of professional and semi-professional team sport athletes do not meet sport nutrition recommendations—a systematic literature review. Nutrients. 2019;11(5):1160. doi: 10.3390/nu11051160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Walker RF, Wilson DW, Read GF, Riad-Fahmy D. Assessment of testicular function by the radioimmunoassay of testosterone in saliva. Int J Androl. 1980;3(1–6):105–120. doi: 10.1111/j.1365-2605.1980.tb00100.x. [DOI] [PubMed] [Google Scholar]
- 47.Reilly T, Maughan RJ, Hardy L. Body fat consensus statement of the steering groups of the British olympic association. Sports Exercise Injury. 1996;2:46–49. [Google Scholar]
- 48.Shovlin A, Roe M, Malone S, Collins K. Positional anthropometric and performance profile of elite Gaelic football players. J Strength Cond Res. 2018;32(8):2356–2362. doi: 10.1519/JSC.0000000000002071. [DOI] [PubMed] [Google Scholar]
- 49.Ksiażek A, Zagrodna A, Słowińska-Lisowska M. Assessment of the dietary intake of high-rank professional male football players during a preseason training week. Int J Environ Res Public Health. 2020;17(22):8567. doi: 10.3390/ijerph17228567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Schofield KL, Thorpe H, Sims ST. Case study: energy availability and endocrine markers in elite male track cyclists. Int J Sports Physiol Perform. 2021;1(aop):1–4. doi: 10.1123/ijspp.2020-0521. [DOI] [PubMed] [Google Scholar]
- 51.Jurov I, Keay N, Hadžić V, Spudić D, Rauter S. Relationship between energy availability, energy conservation and cognitive restraint with performance measures in male endurance athletes. J Int Soc Sports Nutr. 2021;18(1):1. doi: 10.1186/s12970-021-00419-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Anderson L, Orme P, Naughton RJ, et al. Energy intake and expenditure of professional soccer players of the English premier league: evidence of carbohydrate periodization. Int J Sport Nutr Exerc Metab. 2017;27(3):228–238. doi: 10.1123/ijsnem.2016-0259. [DOI] [PubMed] [Google Scholar]
- 53.Devlin BL, Kingsley M, Leveritt MD, Belski R. Seasonal changes in soccer players’ body composition and dietary intake practices. J Strength Cond Res. 2017;31(12):3319–3326. doi: 10.1519/JSC.0000000000001751. [DOI] [PubMed] [Google Scholar]
- 54.O’Brien L, Collins K, Doran D, Khaiyat O, Amirabdollahian F. Dietary intake and energy expenditure assessed during a pre-season period in elite Gaelic football players. Sports. 2019;7(3):62. doi: 10.3390/sports7030062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Tipton KD, Hamilton DL, Gallagher IJ. Assessing the role of muscle protein breakdown in response to nutrition and exercise in humans. Sports Med. 2018;48(1):53–64. doi: 10.1007/s40279-017-0845-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Helms ER, Zinn C, Rowlands DS, Brown SR. A systematic review of dietary protein during caloric restriction in resistance trained lean athletes: a case for higher intakes. Int J Sport Nutr Exerc Metab. 2014;24:127–138. doi: 10.1123/ijsnem.2013-0054. [DOI] [PubMed] [Google Scholar]
- 57.Malone S, Solan B, Collins K. The Influence of pitch size on running performance during Gaelic football small sided games. Int J Perform Anal Sport. 2016;16(1):111–121. doi: 10.1080/24748668.2016.11868874. [DOI] [Google Scholar]
- 58.Malone S, Hughes B, Roe M, Collins K, Buchheit M. Monitoring player fitness, fatigue status and running performance during an in-season training camp in elite Gaelic football. Sci Med Football. 2017;1(3):229–236. doi: 10.1080/24733938.2017.1361040. [DOI] [Google Scholar]
- 59.Ziemba A, Adamczyk JG, Barczak A, Boguszewski D, Kozacz A, Dąbrowski J, Steczkowska M, Pepłońska B, Żekanowski C. Changes in the hormonal profile of athletes following a combat sports performance. Biomed Res Int. 2020;1:1–7. doi: 10.1155/2020/9684792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Volek JS, Kraemer WJ, Bush JA, Incledon T, Boetes M. Testosterone and cortisol in relationship to dietary nutrients and resistance exercise. J Appl Physiol. 1997;82(1):49–56. doi: 10.1152/jappl.1997.82.1. [DOI] [PubMed] [Google Scholar]
- 61.Anderson KE, Rosner W, Khan MS, New MI, Pang S, Wissel PS, Kappas A. Diet-hormone interactions: protein/carbohydrate ratio alters reciprocally the plasma levels of testosterone and cortisol and their respective binding globulins in man. Life Sci. 1987;40(18):1761–1768. doi: 10.1016/0024-3205(87)90086-5. [DOI] [PubMed] [Google Scholar]
- 62.Steffl M, Kinkorova I, Kokstejn J, Petr M. Macronutrient intake in soccer players–a meta-analysis. Nutrients. 2019;11(6):1305. doi: 10.3390/nu11061305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Zamora AJ, María Belmonte L. Evaluation of anthropometric and nutritional assessment of basketball players. Arch Med Deporte. 2020;37:244–252. [Google Scholar]
- 64.Kraemer WJ, French DN, Paxton NJ, et al. Changes in exercise performance and hormonal concentrations over a big ten soccer season in starters and nonstarters. J Strength Cond Res. 2004;18(1):121–128. doi: 10.1519/1533-4287(2004)018<0121:ciepah>2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 65.Michailidis Y. Stress hormonal analysis in elite soccer players during a season. J Sport Health Sci. 2014;3:279–283. doi: 10.1016/j.jshs.2014.03.016. [DOI] [Google Scholar]
- 66.Nindl BC, Friedl KE, Frykman PN, Marchitelli LJ, Shippee RL, Patton JF. Physical performance and metabolic recovery among lean, healthy men following a prolonged energy deficit. Int J Sports Med. 1997;18(05):317–324. doi: 10.1055/s-2007-972640. [DOI] [PubMed] [Google Scholar]
- 67.Silva JR, Rebelo A, Marques F, Pereira L, Seabra A, Ascensão A, Magalhães J. Biochemical impact of soccer: an analysis of hormonal, muscle damage, and redox markers during the season. Appl Physiol Nutr Metab. 2014;39(4):432–438. doi: 10.1139/apnm-2013-0180. [DOI] [PubMed] [Google Scholar]
- 68.Hooper DR, Kraemer WJ, Saenz C, Schill KE, Focht BC, Volek JS, Maresh CM. The presence of symptoms of testosterone deficiency in the exercise-hypogonadal male condition and the role of nutrition. Eur J Appl Physiol. 2017;18(05):317–324. doi: 10.1007/s00421-017-3623-z. [DOI] [PubMed] [Google Scholar]
- 69.Sakihara S, Kageyama K, Oki Y, et al. Evaluation of plasma, salivary, and urinary cortisol levels for diagnosis of cushing’s syndrome. Endocr J. 2010;57(4):331–337. doi: 10.1507/endocrj.K09E-340. [DOI] [PubMed] [Google Scholar]
- 70.Lane AR, Duke JW, Hackney AC. Influence of dietary carbohydrate intake on the free testosterone: cortisol ratio responses to short-term intensive exercise training. Eur J Appl Physiol. 2010;108(6):1125–1131. doi: 10.1007/s00421-009-1220-5. [DOI] [PubMed] [Google Scholar]
- 71.Stenqvist TB, Torstveit MK, Faber J, Melin AK. Impact of a 4-week intensified endurance training intervention on markers of relative energy deficiency in sport (RED-S) and performance among well-trained male cyclists. Front Endocrinol. 2020 doi: 10.3389/fendo.2020.512365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pawlow LA, Jones GE. The impact of abbreviated progressive muscle relaxation on salivary cortisol and salivary immunoglobulin A (sIgA) Appl Psychophysiol Biofeedback. 2005;30(4):375–387. doi: 10.1007/s10484-005-8423-2. [DOI] [PubMed] [Google Scholar]
- 73.Brandtzaeg P. Do salivary antibodies reliably reflect both mucosal and systemic immunity? Ann N Y Acad Sci. 2007;1098(1):288–311. doi: 10.1196/annals.1384.012. [DOI] [PubMed] [Google Scholar]
- 74.Ligtenberg AJM, Brand HS, van den Keijbus PAM, Veerman ECI. The effect of physical exercise on salivary secretion of MUC5B, amylase and lysozyme. Arch Oral Biol. 2015;60(11):1639–1644. doi: 10.1016/j.archoralbio.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 75.Oliver SJ, Laing SJ, Wilson S, Bilzon JLJ, Walters R, Walsh NP. Salivary immunoglobulin a response at rest and after exercise following a 48 h period of fluid and/or energy restriction. Br J Nutr. 2007;97(6):1109–1116. doi: 10.1017/S0007114507682919. [DOI] [PubMed] [Google Scholar]
- 76.Costa RJS, Jones GE, Lamb KL, Coleman R, Williams JHH. The effects of a high carbohydrate diet on cortisol and salivary immunoglobulin A (s-IgA) during a period of increase exercise workload amongst olympic and ironman triathletes. Int J Sports Med. 2005;26(10):880–885. doi: 10.1055/s-2005-837467. [DOI] [PubMed] [Google Scholar]
- 77.Westerterp KR. Physical activity and physical activity induced energy expenditure in humans: measurement, determinants, and effects. Front Physiol. 2013;4:1–12. doi: 10.3389/fphys.2013.00090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Francis JL, Gleeson M, Pyne DB, Callister R, Clancy RL. Variation of salivary immunoglobulins in exercising and sedentary populations. Med Sci Sports Exerc. 2005;37(4):571–578. doi: 10.1249/01.MSS.0000158191.08331.04. [DOI] [PubMed] [Google Scholar]
- 79.Guilhem G, Hanon C, Gendreau N, Bonneau D, Guével A, Chennaoui M. Salivary hormones response to preparation and pre-competitive training of world-class level athletes. Front Physiol. 2015;6:333. doi: 10.3389/fphys.2015.00333. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding author, [AMcG], upon reasonable request.


