Abstract
Stasis dermatitis is a chronic inflammatory skin disease of the lower extremities. It typically occurs in older individuals and is the cutaneous manifestation of venous hypertension caused by venous reflux. Such retrograde venous blood flow is the result of incompetent venous valves, valve destruction, or venous obstruction. Stasis dermatitis is eczematous. The associated impairment of venous valves may cause swelling of the legs, leading to serious conditions including venous ulcerations. Diagnosis can be challenging because of its clinical resemblance to other skin conditions and poor clinical recognition by physicians. The cornerstones of stasis dermatitis treatment are compression therapy to ameliorate pain and swelling, topical treatments to alleviate secondary skin changes, and interventional treatment options to correct the underlying causes of venous reflux. Given the central role of inflammation of the lower extremities in driving the cutaneous changes characteristic of stasis dermatitis, new therapeutic approaches that target the inflammation are under clinical evaluation in patients with stasis dermatitis.
Plain Language Summary
Stasis dermatitis is a skin disease that can affect a person for a long time. It affects the legs of older people who have a disease called chronic venous insufficiency. This is when a person’s veins have difficulty sending blood from their limbs back to their heart. Stasis dermatitis is caused by increased pressure inside a person’s veins. Its signs and symptoms are skin discoloration, itch, dryness, and scaling and can be similar to the signs and symptoms of cellulitis and allergic contact dermatitis. Cellulitis is a common skin infection caused by bacteria. Cellulitis causes redness, swelling, and pain. Allergic contact dermatitis is an itchy skin rash caused by contact with something that irritates the skin. Stasis dermatitis is usually diagnosed after a healthcare provider has looked at person’s skin and their medical history. Treatment for stasis dermatitis should treat the chronic venous insufficiency that causes the disease. It should also treat the skin lesions caused by stasis dermatitis. One way to treat stasis dermatitis is to reduce pain and swelling. This is done by applying pressure with compression stockings or bandages. Minor surgery can treat the venous insufficiency that causes stasis dermatitis. No treatments have been approved for the skin symptoms associated with stasis dermatitis. New ways to treat such symptoms need to be developed.
Key Points
| Stasis dermatitis is caused by venous hypertension. |
| Treatment often involves the use of compression therapy and minor surgical procedures. |
| No treatments are approved for the management of the skin changes associated with stasis dermatitis, although topical corticosteroids are frequently used. |
Introduction
Statis dermatitis (SD), also known as venous eczema, stasis eczema, venous SD, or gravitational dermatitis, is a chronic inflammatory skin disease driven by underlying chronic venous insufficiency that typically affects the lower extremities of older individuals [1, 2]. In SD, alterations in the function of the deep venous plexus at the lower extremities cause blood to backflow to the superficial venous system, leading to venous hypertension, cutaneous inflammation, and a range of potential complications, including venous ulcers [3]. This review describes the incidence, clinical presentation, diagnosis, pathogenesis, and treatment of SD. Literature was selected via a high-level PubMed search using keywords related to SD, such as “stasis dermatitis,” “stasis ulcers,” “venous ulcers,” “venous hypertension,” “venous stasis dermatitis,” “venous eczema,” “chronic venous insufficiency,” “chronic venous disease,” and “chronic venous insufficiency.”
Epidemiology
Chronic venous disease, including chronic venous insufficiency, is a common disease that increases with age and is more prevalent in women [4, 5]. Estimates for the prevalence of chronic venous insufficiency vary widely, highlighting a knowledge gap in the extant epidemiological data, with the observed numbers often depending on the clinical characteristics and lifestyles of the populations examined. Geographically, the highest reported prevalence of chronic venous insufficiency is across Western countries, ranging from < 1 to 40% in women and from < 1 to 17% in men [1, 6]. A Saudi Arabian study estimated the overall prevalence of chronic venous insufficiency as about 45.6% [7]. A Scottish study found the age-adjusted prevalence of chronic venous insufficiency to be 9% in men and 7% in women, with the prevalence increasing with age [8]. In a cross-sectional study of chronic venous insufficiency in patients in 24 cities in Italy, only 22.7% of approximately 5200 adult participants demonstrated no visible signs of the disease [9]. It is estimated that between 2 and 6 million individuals in the United States have advanced forms of chronic venous insufficiency, including swelling and skin changes, with about 500,000 individuals displaying painful venous ulcers [10]. Approximately 37–44% of individuals with leg ulcers had been diagnosed with SD [11]. SD is an advanced cutaneous presentation of chronic venous insufficiency that is more common in older patients; age is positively correlated with the number of insufficient venous segments [2]. In one study, SD was identified in 1.4% of 773 individuals aged ≥ 15 years who had varicose veins [12]. In another study, SD was found in 6.2% of 4099 individuals aged ≥ 65 years [13]. Similar studies have found an SD prevalence of 5.9% in 68 individuals with a mean age of 74 years (range 50–91), increasing to 6.9% in 584 individuals with a mean age of 80 years (range 55–106) [2]. With the increasing age of the US population, rising rates of obesity, and more sedentary lifestyles, the prevalence of chronic venous insufficiency (and, consequently, SD) is expected to increase.
Venous ulcerations of the lower extremities are a significant complication in patients with advanced venous insufficiency. Although compression therapy remains the most frequently used treatment for venous ulcerations, with the healing of ulcers reported in approximately 70% of treatment-adherent patients, a significant portion of patients do not fully respond to this treatment [14]. Compression stockings may be too painful for patients with stasis ulcers to don, so surgical treatments may be necessary (see Sect. 7 on treatments). After healing, the 5-year frequency for the recurrence of venous ulcerations ranges between 26 and 51%, with the exact value depending on the initial degree of ulceration [15]. Moreover, healing does not guarantee the full restoration of proper function in patients, nor the resolution of discomfort or pain.
Clinical Presentation
SD, as a cutaneous manifestation of chronic venous insufficiency caused by retrograde venous blood flow, is classified as C4 (changes in skin and subcutaneous tissue secondary to chronic venous disease) on the Clinical-Etiology-Anatomy-Pathophysiology (CEAP) classification of venous disorders [1, 16]. This is an internationally accepted standard for describing patients with chronic venous disorders based on the clinical manifestations, the current understanding of disease etiology, the involved anatomy, and the underlying venous pathology [16]. The typical clinical presentation involves poorly demarcated erythematous and eczematous patches and plaques of the lower extremities, with the medial malleolus region being the most frequently and severely involved [1, 2]. The eczematous nature of SD is represented by scaling, erythema, and xerosis of the skin. These skin changes may extend to the foot as well as to the knee. Characteristic symptoms include pruritus (itch) on the medial ankle (which can extend to the shin), pain, lesional inflammation, scaling, and skin color changes and thickening [1, 3]. Itch is the most troublesome symptom, impacting patient quality of life (QOL) and leading to scratching that can aggravate wounds and increase risk of skin infection. Chronic scratching due to itching can also result in skin thickening and lichenification [1, 17]. Brown speckles (hyperpigmentation), the result of hemosiderin deposition, which is the byproduct of hemoglobin from extravasated erythrocytes, also represents a classic chronic sign (Fig. 1). SD may be associated with other typical presentations of chronic venous disease, including cramps, leg aching/pain, itching, swelling, and lipodermatosclerosis [2]. Due to the disruption of the epidermal barrier, secondary infections may also occur, leading to superficial infections such as impetiginization, cellulitis, and erysipelas. Both cellulitis and erysipelas are skin infections caused by bacteria with the main difference being the depth of infection—erysipelas are more superficial, markedly affecting the face and lower limbs, while cellulitis reaches the deeper dermis and subcutaneous fat layers, markedly affecting the palm of the hands and the lower limbs [1, 18, 19]. Importantly, if the underlying venous alterations remain untreated, the venous disease patient may develop chronic venous ulcerations along with oozing and erythema (Fig. 2) [2]. Patients with SD may also develop acroangiodermatitis (pseudo–Kaposi sarcoma), and a biopsy may be required to differentiate it from Kaposi sarcoma [1, 2, 20].
Fig. 1.
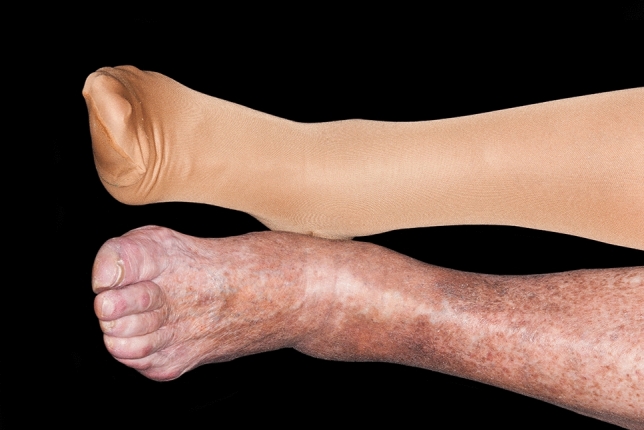
Stasis dermatitis with hyperpigmentation. Figure used with permission from https://www.sciencephoto.com
Fig. 2.
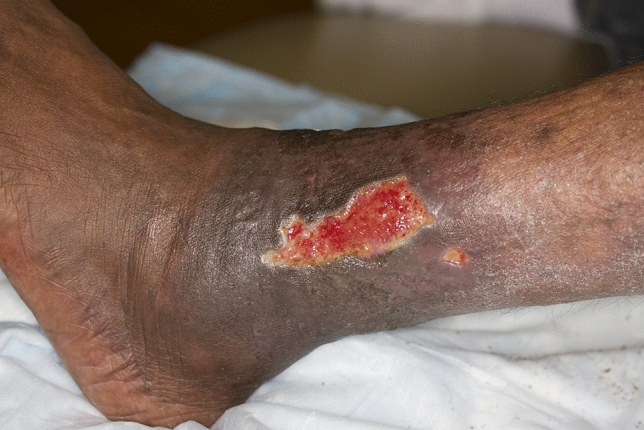
Stasis dermatitis with leg ulcers. Figure used with permission from https://www.sciencephoto.com
It is clinically important to differentiate between acute and chronic SD. Acute SD is often observed with acute or worsening edema and is characterized by substantial erythema, bullae, weeping, and vesicles (referred to as a flare). Both chronic and acute SD are often misdiagnosed as bilateral cellulitis. Chronic SD often presents with poorly demarcated hyperpigmentation and erythema that is linked with chronic edema and underlying inflammation of the lower extremities. Patients with chronic SD can develop acute flares that are clinically shown as worsening of the eczema, with signs of inflammation, such as pain and swelling. The lipodermatosclerosis that is observed in chronic SD is a severe fibrosing panniculitis of the subcutaneous tissue and may lead to an “inverted champagne bottle” appearance of the lipodermatosclerosis of the leg. In acute SD settings, lipodermatosclerosis may present with painful subcutaneous plaques or nodules [1, 3].
Burden of Disease
Chronic venous disease, including SD, is associated with pain and a reduced QOL, including limitations in physical function, sleep, and mobility. Reductions in physical functioning and mobility can limit the patient’s ability to apply topical medications or to put on or take off compression stockings, thereby worsening the situation. Patients with SD also report social isolation and depression [4, 14]. Emotional and social well-being can be impacted by the cosmetic skin changes (including hyperpigmentation and scaling) typically observed [2, 21]. Post-inflammatory hyperpigmentation can persist even after other signs and symptoms have resolved with therapy [2]. A study of 2404 patients using the generic Medical Outcomes Study 36-Item Short-Form General Health Survey questionnaire identified a significant correlation between the severity of venous disease and the decline in the affected patient’s QOL [21]. Similarly, a correlation was found between CEAP class and QOL using a disease-specific questionnaire [22]. A study examining QOL in patients with advanced venous insufficiency found that patients suffered from severe pain and discomfort, with patients’ overall level of independence being lower than that of healthy individuals [14]. As previously noted, pruritus is one of the troublesome symptoms reported in SD. In a survey involving an elderly population, SD was the most commonly reported dermatitis to cause itch [23]. Patients with pruritus of the lower extremities exhibited lower health-related QOL and higher leg pain ratings than those without pruritus [24]. Compounding this issue, the itch in SD can be refractory to conventional therapies [17].
Chronic venous diseases, including SD, directly impact healthcare resources and are associated with a loss of annual workdays and an increase in overall treatment costs. In the United States, treatment costs of venous diseases are between US$2.5 and US$3 billion, with a loss of 2 million workdays per year [25]. Overall, chronic venous diseases account for 1–3% of the total healthcare budgets in nations with developed healthcare systems [4], although there is not a robust body of literature that describes the economic impact of SD. Moreover, chronic venous insufficiency in general is often underdiagnosed and undertreated for prolonged periods of time, and the low recognition of SD within the healthcare system and a lack of dermatology services in many hospitals, emergency rooms, and urgent care settings further compounds the burden on the patient [26, 27]. Several healthcare specialists (including general physicians, dermatologists, geriatricians, vascular surgeons, and wound care specialists) may be involved in the initial interaction with and subsequent care of the patient.
Diagnosis and Histopathology
Several risk factors were established with the development of venous insufficiency leading to SD, including older age, female sex, pregnancy, obesity, prolonged sitting or standing, heart failure, and family history of venous disease (Table 1) [1, 28]. The association with pregnancy and obesity is due to extra stress placed on the patient’s lower extremities [1, 3]. Patients who are unable to move may experience leg edema caused by venous stasis that is due to immobility itself and not to anatomical venous complications [29]. Risk factors such as a sedentary lifestyle and obesity can be addressed, potentially resulting in disease improvement.
Table 1.
Established risk factors for developing stasis dermatitis
| Risk factors | |
|---|---|
|
Older age (usually > 50 years) Female sex Pregnancy Obesity Prolonged periods of sitting or standing High blood pressure Heart failure Kidney failure Chronic edema History of cellulitis Varicose veins History of blood clots in the legs (e.g., deep vein thrombosis) Leg vein surgeries Leg injuries Family history of venous disease |
SD is typically diagnosed by clinical evaluation of the affected skin and medical history [1, 28]. If the initial diagnosis is inconclusive, duplex ultrasounds can be used to detect the direction of blood flow, assess venous reflux, and identify potential venous obstructions. Widespread use of duplex ultrasound is supported by its accuracy, non-invasiveness, and cost-effectiveness [1, 30]. Computed tomography and magnetic resonance imaging can also be used to detect obstructions in proximal veins and surrounding structures [2]. A diagnosis of SD can be confirmed through biopsy and demonstration of classic histological features of SD, including hemosiderin-laden macrophages, dermal fibrosis, extravasated erythrocytes, perivascular lymphocytic infiltration, and the proliferation of dilated blood vessels in the papillary dermis [1]. Biopsies are generally avoided because the impaired blood flow in the affected areas of skin can result in poor healing and development of ulcers [1, 28].
Several cutaneous disorders can present similarly to SD, leading to delayed or inaccurate diagnosis and subsequent disease mismanagement and the associated impact on patient anxiety and healthcare costs. Indeed, over 10% of the diagnoses of cellulitis are incorrect, with SD being the most common misdiagnosed condition. Cellulitis is associated with previous trauma or a disrupted epithelial barrier in the affected region, marked by unilateral erythematous plaque along with tenderness, swelling, and warmth [1]. Cellulitis is often accompanied by a variety of systemic symptoms, including mild leukocytosis, fever, and, less commonly, regional lymphadenopathy and/or ascending lymphangitis [1, 31]. Indications of cellulitis also include a history of acute progression and improvement with antibiotics [2]. However, SD is often bilateral, not associated with significant tenderness, and chronic and frequently occurs with pitting edema [31]. However, unilateral presentations of SD may be observed when the venous insufficiency is limited to one extremity [1]. Due to the many similarities between cellulitis and SD, patients with SD are often treated for recurrent cellulitis. Given that cellulitis is potentially a medical emergency, a misdiagnosis can result in unnecessary hospitalization and exposure to antibiotics. Not surprisingly, early consultation with a dermatologist was shown to improve the accuracy of diagnosis of inflammatory skin diseases in hospitalized patients [1, 32].
Allergic contact dermatitis is also a risk factor in patients with a longer history of SD and ulcerations and is often mistaken for SD [1, 31, 33]. Due to the breakdown of the epidermal barrier in patients with SD and frequent and extended use of topical treatments containing sensitizing ingredients, allergic contact dermatitis may present on a background of SD, further complicating the diagnosis. Patch testing is often needed to confirm allergic contact dermatitis [1, 2]. A patient’s history, including their previous application of products, stockings, and dressings, is essential when determining which allergens to patch test [1]. Autoeczematization, also known as an id reaction, which is the acute onset of a highly erythematous, pruritic, morbilliform, or papulovesicular eruption due to an exposure to a stimulus, commonly occurs with SD [1, 34].
Pigmented purpuric dermatoses (PPDs) and exercise-induced vasculitis may also resemble SD due to their main clinical feature of punctate petechiae secondary to the extravasated erythrocytes [1, 35]. Like SD, PPDs are limited to the lower limbs bilaterally and are often chronic with recurrent exacerbations. Hemosiderin deposition is found in the superficial dermis in PPDs, with the deposition being deeper in SD [1, 2]. Other SD mimickers include atopic dermatitis (AD), diabetic dermopathy, and xerotic eczema [1, 36]. Being familiar with the appearance of such conditions, as well as pursuing the correct diagnostic workup that includes both the history and the clinical presentation, is important to correctly diagnose cutaneous disorders associated with the inflammation of lower extremities [1, 28]. Moreover, general physicians may not recognize venous disorders or appreciate their seriousness and, consequently, may not refer the patient to a specialist [37].
Pathogenesis
Venous reflux that drives venous hypertension can be the result of incompetent venous valves, obstruction to venous flow, or failure of the lower extremity muscle pump and may occur in the superficial or deep venous systems [1, 4]. In a study of patients with chronic venous disease, primary valvular incompetence was present in 70–80% of cases; valvular incompetence was due to trauma or deep vein thrombosis in 18–25% of cases [4]. In chronic venous insufficiency, increased venous hypertension initiates changes in the local subcutaneous tissue and skin, including activation of the endothelial cells, extravasation of red blood cells and macromolecules, diapedesis of leukocytes, and inflammatory changes that are often noted at and above the ankles [38]. There is an accumulation of clinical evidence to support the concept that venous hypertension causes SD. One study that examined 360 lower limbs indicated a positive correlation between increased ambulatory venous pressure and severe skin damage [39]. In another study, venous hypertension was found to be the only plausible cause of SD in patients with superficial venous insufficiency, with negative patch testing and without insufficiency of the deep veins. Interestingly, in this second study, surgical intervention targeting the venous pressure resulted in the complete and rapid resolution of SD for all patients who underwent surgery [40].
In terms of both the pathogenesis of SD and developing effective therapies, it is important to understand that inflammation causes the skin changes characteristic of SD [2, 4]. The accumulation of leukocytes in the microcirculation of extremities with venous hypertension was reported in the 1980s, and such leukocyte trapping is now considered typical of the early stages of chronic venous insufficiency, particularly in SD [40, 41]. Studies demonstrated that the passively dependent blood in the feet of individuals with chronic venous diseases has low levels of leukocytes, suggesting leukocyte accumulation in regions of high venous pressure, leading to the observed inflammatory response [41, 42]. Here, leukocytes attach to the endothelium of vein walls and valve leaflets, causing the necrosis and/or apoptosis of the endothelium, fibroblasts, smooth muscle cells, and parenchymal cells of the venous wall. This causes weakening and destruction of the venous walls and valve leaflets, leading to the observed venous changes in SD [43, 44]. Increased numbers of T lymphocytes, macrophages, and mast cells were also observed in skin biopsies of the lower legs of individuals with chronic venous insufficiency, further supporting the association of inflammatory mediators with cutaneous damage [2, 43]. In SD, venous hypertension promotes the cellular accumulation of inflammatory cells, such as macrophages, as well as the extravasation of erythrocytes at the affected areas. Erythrocyte extravasation is followed by hemoglobin decomposition, which results in the excessive buildup of iron that is stored at the affected tissues as hemosiderin. Further macrophage accumulation is induced by the buildup of hemosiderin and hemoglobin. Such macrophages, alongside other cells, promote inflammation and induce several histological features seen in SD, including epidermal spongiotic changes and capillary proliferation [17].
It was also demonstrated that expression of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 on microvascular endothelial cells, and their ligands lymphocyte function-associated antigen-1 and very late antigen-4 on leukocytes, is upregulated in the early stages of chronic venous insufficiency, including SD. This process remains upregulated as SD progresses. This would facilitate the transmigration of leukocytes into nearby tissues. Epidermal basal keratinocytes were also found to express elevated levels of intercellular adhesion molecule-1 and vascular cell adhesion molecule-1 in SD, potentially promoting skin tissue damage through a perpetuated and upregulated influx of activated leukocytes and eventually leading to skin ulcerations [45].
Studies also demonstrated the role of matrix metalloproteinases (MMPs) in remodeling lesional skin in SD via degradation of extracellular matrix proteins and impairment of healing. In a study that examined skin samples from 19 patients with SD, elevated levels of MMP-1, MMP-2, and MMP-13 were found, with diminished levels of tissue inhibitor of metalloproteinases (TIMP)-1 and TIMP-2, compared to healthy control samples (Figs. 3, 4). Such overexpression and production of MMP-1, MMP-2, and MMP-13, without the inhibitory effects of TIMP-1 and TIMP-2, may be the result of cytokine-mediated induction [46]. Increased expression of MMPs and other proteinases by the vessel wall promotes further degradation of the vascular extracellular matrix, resulting in abnormal vascular permeability as well as edema [1]. Moreover, the release of ferritin and ferric ion from extravasated erythrocytes may result in oxidative stress and additional MMP activation, promoting skin tissue damage, ulcer formation, and delaying healing [2, 4, 47]. MMPs as well as other forms of inflammation may also cause skin hyperpigmentation in SD [2].
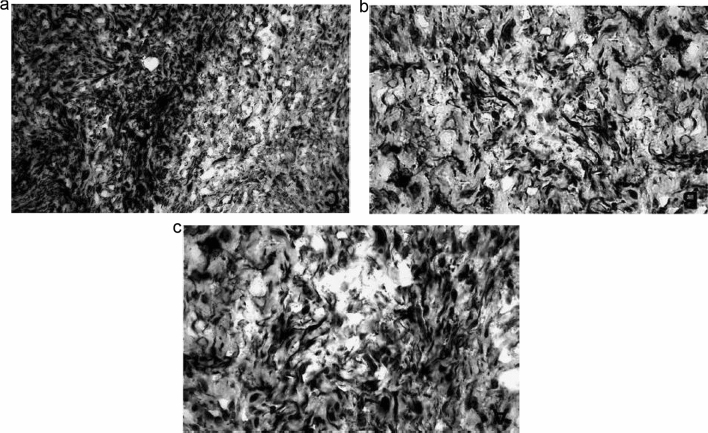
Fig. 3.
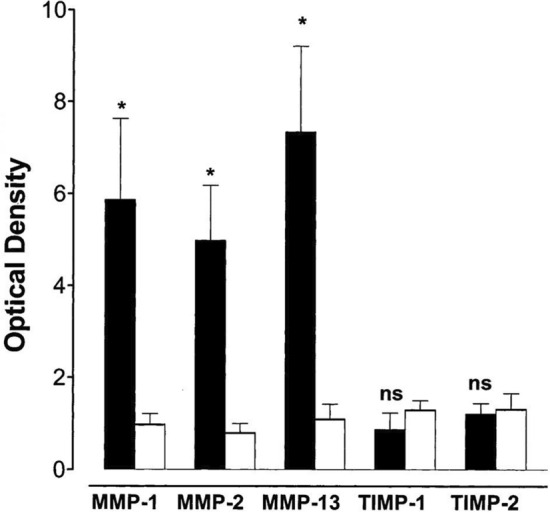
Protein expression of MMPs and their TIMPs in extracts of skin samples from patients with stasis dermatitis. Data were taken from Western blots with monoclonal antibodies against MMP-1, MMP-2, MMP-13, TIMP-1, and TIMP-2 in stasis dermatitis and control skin lesions. Densitometric evaluations of immunoreactive blots for MMP-1, MMP-2, MMP-13, TIMP-1, TIMP-2 from stasis dermatitis (black bars) and healthy skin (white bars) are shown. Data are mean ±SEM; n = 10 for stasis dermatitis and n = 4 for control. The significance of difference was determined by an unpaired Student t test and is indicated in each mapped group. Figure used with permission from Herouy et al. [46] J Dermatol Sciences, 2001. MMP matrix metalloproteinase, ns not significant, SEM standard error of the mean, TIMP tissue inhibitor of metalloproteinase. *p < 0.05
Fig. 4.
Immunohistochemical detection of MMP-1, MMP-2, and MMP-13 in lesions of stasis dermatitis. Increased immunoreactive staining was observed in skin from stasis dermatitis lesions for a MMP-1, b MMP-2, and c MMP-13. Representative photographs from one patient are shown. The experiment was repeated with comparable results (n = 2 for stasis dermatitis; n = 2 for control). Figure used with permission from Herouy et al. [46] J Dermatol Sciences, 2001. MMP matrix metalloproteinase
Very little is known regarding the pathophysiological mechanisms of SD-linked pruritus. Pruritus is either histaminergic or nonhistaminergic. SD-related pruritus is likely nonhistaminergic because antihistamines do not improve SD-associated pruritus. Such nonhistaminergic pruritus involves various mediators, including cytokines/chemokines (e.g., interleukin [IL]-31), amines, neuropeptides and their receptors, proteases and their receptors, ion channels, and immune cells (e.g., T cells, eosinophils, mast cells, and basophils). IL-31 has been implicated in the induction of pruritus in several diseases, including AD, psoriasis, prurigo nodularis, and cutaneous T cell lymphoma. A recent study by Hashimoto et al. has demonstrated the potential involvement of dermal IL-31 from macrophages in inducing SD-associated pruritus. The majority of IL-31–expressing cells in SD are CD68+ macrophages. Such CD68+ macrophages, which coexpress CD163 (a macrophage-specific membrane marker), are classified as M2 macrophages. The number of IL31+CD68+ macrophages was higher in patients with SD with severe pruritus compared to SD without severe pruritus. The number of IL31+CD68+ macrophages was positively correlated with the number of substance P+ cells, dermal C-C chemokine receptor type 4+ T helper type 2 cells, and basophils and the dermal deposition of hemosiderin and periostin [17].
Treatments
The goals of current treatment options in patients with SD include addressing the clinical impact of the underlying venous insufficiency, edema, and inflammation (itch and pain), improving skin lesions, and healing ulcers (Table 2). Initial options for the treatment of SD include exercise, walking, and leg elevation, although such approaches are generally effective only for mild cases of SD [1, 2]. The cornerstone of treatment for SD remains compression therapy through bandages or stockings, using a high pressure of about 60 mmHg or a median pressure of about 30 mmHg [48], respectively, to reduce the ambulatory venous pressure and resultant hypertension. Although compression was demonstrated to alleviate swelling, pain, and stasis skin changes in patients with SD, this approach often fails due to nonadherence or loss of stocking elasticity after repeated use [1, 2, 49–52]. Indeed, only about 50–60% of patients comply with compression therapy, with bandages being more challenging to apply correctly (versus stockings) without professional guidance [2, 50, 53]. In addition, the synthetic rubber in compression stockings can result in allergic contact dermatitis of the lower extremities, further impacting treatment adherence [54]. Topical dressings, such as the Unna boot, which is a bandage that contains moist zinc oxide, can also be utilized. Such dressings serve as non-elastic compressions that contain a topical treatment for SD. The Unna boot can also be combined with other treatments, including topical corticosteroids. Use of the Unna boot alleviates various SD symptoms, including cutaneous inflammation, pruritus, and eczema [1]. One study found the use of the Unna boot over a topical ointment to be more efficacious than Unna boot compression alone [55].
Table 2.
Summary of the treatments used for stasis dermatitis
| Edema | Feeling of heaviness in legs | Hyperpigmentation | Inflammation | Leg cramps | Pain | Prevent ulcer recurrence | Promote healing of ulcers | Pruritus | Xerosis | |
|---|---|---|---|---|---|---|---|---|---|---|
| Nonpharmacological: exercise, walking, leg elevation, stockings, bandages, topical dressings (Unna boot) | X | X | X | X | ||||||
| Non-soap cleansers, barrier preparations, bland emollients | X | |||||||||
| Moisturizers | X | |||||||||
| Topical high- and mid-potency corticosteroids | X | |||||||||
| Topical calcineurin inhibitors (topical tacrolimus [off-label]) | X | |||||||||
| Antihistamines | X | |||||||||
| Pulsed light treatments | X | |||||||||
| Oral pentoxifylline | X | X | X | |||||||
| Topical pentoxifylline and phlebotonics | X | X | ||||||||
| Venoactive medications (escin, micronized purified flavonoid fraction), calcium channel blocker (amlodipine), angiotensin-converting enzyme inhibitors, angiotensin receptor blockers | X | |||||||||
| Hydroxyethylrutosides | X | X | X | |||||||
| Ultrasound-guided foam sclerotherapy, endovenous thermal ablation, ambulatory phlebectomy | X | X | ||||||||
| Topical phosphodiesterase 4 inhibitors (crisaborole [off-label]) | X | X | ||||||||
| Oral analgesics (anti-inflammatory medications, acetaminophen, metamizole) | X | X | ||||||||
| Topical anesthetic creams (prilocaine and lidocaine) | X | |||||||||
| Antibiotics and antiseptics | X |
Although no drugs are currently approved for the treatment of SD symptoms, several topical options are used to relieve the symptoms associated with SD. Skin care treatments for SD include non-soap cleansers, barrier preparations, and bland emollients [2]. Moisturization through the liberal application of emollients is recommended to address the skin dryness that is associated with the concomitant xerosis [56]. High- or mid-potency topical corticosteroids can also be used intermittently to relieve pruritus, although prolonged use may lead to cutaneous atrophy and systemic side effects [1, 57]. Topical calcineurin inhibitors are also an option given their effectiveness in other steroid-responsive dermatoses. Although topical tacrolimus (used off-label) has been shown to be effective for the treatment of SD, topical calcineurin inhibitors are associated with a burning sensation upon application and require patient education due to a box warning for an increased risk of lymphoma [57–59]. Cutaneous hyperpigmentation often persists even if the chronic venous insufficiency is treated; although responses are often poor, noncoherent intense pulsed light or laser treatments do represent an approach to removing hyperpigmentation [60].
Multiple oral treatments have shown promise in improving some of the symptoms experienced by patients with SD. Oral pentoxifylline is often used with or without compression therapy to reduce leg pain and to increase the healing rate of venous ulcers [61]. Pentoxifylline is an inhibitor of platelet aggregation that reduces blood viscosity, thereby improving microcirculation. Pentoxifylline has been shown to be effective for the treatment of venous ulcers when added to compression therapy [62]. Edema may be reduced through the use of venoactive drugs, which reduce capillary permeability, increase venous tone, decrease blood viscosity and improve lymphatic drainage [2]. Escin, a horse chestnut seed extract that stimulates F-series prostaglandins, is used to improve symptoms of chronic venous insufficiency on a short-term basis [63]. Another venoactive drug, micronized purified flavonoid fraction, was shown to be more potent than other venoactive drugs at reducing ankle edema [64].
Hydroxyethylrutosides can also be utilized to relieve symptoms of chronic venous insufficiency, including pain, cramps, and a feeling of heaviness in the legs. Hydroxyethylrutosides is a standardized mixture of semisynthetic flavonoids that is derived through hydroxylation of the natural substance rutin. The benefits of hydroxyethylrutosides for chronic venous insufficiency may be due to their ability to inhibit capillary filtration via reducing microvascular permeability, leading to reduced edema and improved microcirculation. However, a meta-analysis that assessed the effectiveness of hydroxyethylrutosides for chronic venous insufficiency found only modest improvements in several associated symptoms; all analyzed trials were of limited quality [65]. Secondary bacterial infections, if present, can be treated with appropriate oral antibiotics [1]. Antihistamines have been used in an attempt to relieve pruritus, but responses were poor; such results are not unexpected because the pruritus associated with SD is not driven by the histamine pathway, as noted earlier [1, 17].
Calcium channel blocker (CCB)-related edema is common in clinical practice and is due to a decrease in arteriolar resistance, which increases the hydrostatic pressure in the precapillary circulation, causing fluid shifts into the interstitial compartment. Antihypertensive medications, such as amlodipine, are CCBs that increase capillary permeability [66]. When used as monotherapy, such CCBs are associated with a substantial risk of developing peripheral edema, which is the most common reason for their discontinuation [67]. Discontinuing the CCB or switching to an alternative antihypertensive therapy, such as angiotensin-converting enzyme inhibitors or angiotensin-receptor blockers, may reduce the associated peripheral edema [66].
Interventional options that aim to treat the underlying venous reflux are also available. Classic open surgical techniques have been replaced with minimally invasive procedures, such as ultrasound-guided foam sclerotherapy, endovenous thermal ablation, and ambulatory phlebectomy. Compared with conventional techniques, minimally invasive procedures are less painful, are associated with a lower number of complications, are more cost-effective, and have quicker recovery times [2, 38, 68–70].
Given the central role in the pathogenesis of SD, inflammation represents a logical focus for the development of new therapeutic options. Phosphodiesterase 4 is an intracellular nonreceptor enzyme that modulates inflammation, and patients with inflammatory diseases (AD, discoid lupus erythematosus, and psoriasis) show elevated expressions of phosphodiesterase 4 compared to healthy individuals [57, 71]. Moreover, targeting phosphodiesterase 4 was verified as an effective therapeutic strategy for several inflammatory conditions, including psoriasis and AD [71]. Crisaborole, a topical phosphodiesterase 4 inhibitor approved for the treatment of AD in multiple countries, underwent evaluation in the completed phase 2 trial (NCT04091087) in adults with SD [72, 73]. Crisaborole is rapidly metabolized to inactive metabolites that have no effect on cytokine release or phosphodiesterase 4 activity, limiting systemic exposure and reducing the risk of adverse effects. A topical phosphodiesterase inhibitor could lead to the targeted inhibition of inflammation observed in skin diseases while avoiding unwanted side effects [57].
In the case of venous ulcers, treatment options include management, mechanical modalities, wound therapy, medications, and/or surgery. Management options include the use of compression therapy, which is the standard treatment modality for long-term treatment for venous ulcers, or the use of dressings. Compression therapy reduces pain and edema, improves venous reflux, enhances ulcer healing, and prevents ulcer recurrence. Stockings or bandages can also be used. The use of various layers is more effective than single layers, with elastic systems being more efficacious than nonelastic ones [74]. In patients with healed ulcers, compression stockings help reduce the risk of ulcer recurrence by about half [75]. Topical dressings are recommended to promote moist wound healing and cover ulcers, although no dressing has been shown to be superior [62, 74]. The most commonly used drugs are oral analgesics (such as anti-inflammatory drugs, acetaminophen and metamizole) and topical anesthetic creams (such as prilocaine and lidocaine). Topical medications, which include pentoxifylline and phlebotonics, can be applied as monotherapies or in combination with compression therapy [37, 74, 76]. Antibiotics and antiseptics can be used to slow bacterial colonization in venous ulcers, thereby improving wound healing; however, a review of systemic and topical antibiotics and antiseptics for venous ulcer treatment found no correlation between the routine use of oral antibiotics and ulcer healing [62, 77]. Surgical options, which include endovenous ablation and skin grafting, can also be utilized to promote healing and prevent ulcer recurrence [74]. A large review study that evaluated 832 patients with advanced chronic venous insufficiency (CEAP clinical scores of 4–6) found that surgical intervention resulted in an ulcer healing rate of 92%, with recurrence rates as low as 4% [78]. Surgical options have also been studied in comparison to non-surgical compression therapy where surgery reduced ulcer recurrence compared to non-surgical treatment after 4 years (31% vs 56%, respectively) [79]. In patients with recurring ulcers, the addition of ablative superficial surgery to standard treatment may be an option [79]. Besides the addition of surgery for recurring ulcers, standard treatment with compression therapy remains the first-line treatment of choice for patients with venous ulcers [80].
Conclusion
SD represents the cutaneous manifestation of venous hypertension caused by venous reflux and most commonly occurs in the lower extremities of elderly individuals. Cutaneous changes associated with SD are driven by inflammation. The typical clinical presentation involves erythematous and eczematous patches and plaques. Itch is one of the most troublesome symptoms and often leads to scratching that can aggravate wounds and increase the risk of skin infection. SD may also progress to chronic venous ulcerations with oozing and erythema. Accordingly, prompt diagnosis and treatment are essential to preventing serious complications. However, diagnosis is challenging due to the resemblance of SD to other skin conditions. The cornerstone of treatment includes compression therapy to ameliorate pain and swelling, together with topical treatments to alleviate secondary skin changes and associated signs and symptoms. No treatments are currently approved for alleviating the symptoms associated with SD; new therapeutic approaches that target inflammation in SD are thereby needed.
Acknowledgements
Editorial/medical writing support under the guidance of authors was provided by Mark Bloom, Ph.D. (ApotheCom, Yardley, PA, USA), and David Gibson, Ph.D., CMPP (ApotheCom, San Francisco, CA, USA), and was funded by Pfizer Inc., New York, NY, USA, in accordance with Good Publication Practice (GPP 2022) guidelines (Ann Intern Med. 2022; 10.7326/M22-1460).
Author Contributions
All authors prepared and revised the draft manuscript. All authors approved the final version of the manuscript.
Declarations
Conflict of interest
GY has been a consultant and an advisor for Pfizer Inc., Bellus, Eli Lilly and Company, Galderma, Kiniksa, LEO Pharma Novartis, Sanofi-Regeneron, and Trevi Therapeutics and is a principal investigator for Pfizer Inc., Kiniksa, LEO Pharma, Galderma, and Sanofi-Regeneron. JS served as an investigator for Celgene, Eli Lilly and Company, F. Hoffmann-LaRoche, Menlo Therapeutics, Realm Therapeutics, Regeneron, and Sanofi; as a consultant for Pfizer Inc., AbbVie, Anacor, AnaptysBio, Arena Pharmaceuticals, Dermavant, Dermira, Eli Lilly and Company, Galderma, GlaxoSmithKline, Glenmark, Incyte, Kiniksa Pharmaceuticals, LEO Pharma, Menlo Therapeutics, Novartis, Realm Therapeutics, Regeneron, and Sanofi; and as a speaker for Regeneron and Sanofi. JMC and AC are employees and stockholders of Pfizer Inc. AF has been a consultant and advisor for Pfizer Inc., AbbVie, Aveeno, Dermavant, Boehringer Ingelheim, Bristol Myers Squibb, Eli Lilly and Company, Graegis, Galderma, Janssen, L'Oréal, Microcures, Mino Labs, Novartis, Sanofi-Regeneron, and Zylo Therapeutics and is a speaker for AbbVie, Bristol Myers Squibb, Dermavant, Janssen, and Sanofi-Regeneron. SN has received a Pfizer Learning and Change grant and has been an advisor for Pfizer Inc. and is Dermatology editor for the New England Journal of Medicine Healer educational resource.
Availability of data and material
Data sharing is not applicable to this article because no datasets were generated or analyzed during the current study.
Ethics approval
Not applicable.
Consent
Not applicable.
Funding
The preparation of this review was supported by Pfizer Inc.
Code availability
Not applicable.
References
- 1.Rzepecki AK, Blasiak R. Stasis dermatitis: differentiation from other common causes of lower leg inflammation and management strategies. Curr Geriat Rep. 2018;7(4):222–227. doi: 10.1007/s13670-018-0257-x. [DOI] [Google Scholar]
- 2.Sundaresan S, Migden MR, Silapunt S. Stasis dermatitis: pathophysiology, evaluation, and management. Am J Clin Dermatol. 2017;18(3):383–390. doi: 10.1007/s40257-016-0250-0. [DOI] [PubMed] [Google Scholar]
- 3.Theodosat A. Skin diseases of the lower extremities in the elderly. Dermatol Clin. 2004;22(1):13–21. doi: 10.1016/S0733-8635(03)00113-X. [DOI] [PubMed] [Google Scholar]
- 4.Bergan JJ, Schmid-Schönbein GW, Smith PDC, Nicolaides AN, Boisseau MR, Eklof B. Chronic venous disease. N Engl J Med. 2006;355(5):488–498. doi: 10.1056/NEJMra055289. [DOI] [PubMed] [Google Scholar]
- 5.Labropoulos N. Hemodynamic changes according to the CEAP classification. Phlebolymphol. 2003;40(4):130–136. [Google Scholar]
- 6.Beebe-Dimmer JL, Pfeifer JR, Engle JS, Schottenfeld D. The epidemiology of chronic venous insufficiency and varicose veins. Ann Epidemiol. 2005;15(3):175–184. doi: 10.1016/j.annepidem.2004.05.015. [DOI] [PubMed] [Google Scholar]
- 7.Bawakid KO, Al-Raddadi RM, Sabban SS, Alturky KA, Mohamed MS. Prevalence of chronic venous insufficiency in the Saudi adult population. Saudi Med J. 2005;26(2):225–229. [PubMed] [Google Scholar]
- 8.Evans C, Fowkes F, Ruckley C, Lee A. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J Epidemiol Comm Heal. 1999;53(3):149–153. doi: 10.1136/jech.53.3.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiesa R, Marone EM, Limoni C, Volonté M, Schaefer E, Petrini O. Chronic venous insufficiency in Italy: the 24-cities cohort study. Eur J Vascul Endovascul Surg. 2005;30(4):422–429. doi: 10.1016/j.ejvs.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 10.White JV, Ryjewski C. Chronic venous insufficiency. Perspect Vascul Surg Endovascul Therap. 2005;17(4):319–327. doi: 10.1177/153100350501700406. [DOI] [PubMed] [Google Scholar]
- 11.Nazarko L. Diagnosis and treatment of venous eczema. Br J Community Nurs. 2009;14(5):188–194. doi: 10.12968/bjcn.2009.14.5.42076. [DOI] [PubMed] [Google Scholar]
- 12.Maffei FHDA, Magaldi C, Pinho S, Lastoria S, Pinho W, Yoshida W, et al. Varicose veins and chronic venous insufficiency in Brazil: prevalence among 1755 inhabitants of a country town. Int J Epidemiol. 1986;15(2):210–217. doi: 10.1093/ije/15.2.210. [DOI] [PubMed] [Google Scholar]
- 13.Yalçin B, Tamer E, Toy GG, Oztaş P, Hayran M, Alli N. The prevalence of skin diseases in the elderly: analysis of 4099 geriatric patients. Int J Dermatol. 2006;45(6):672–676. doi: 10.1111/j.1365-4632.2005.02607.x. [DOI] [PubMed] [Google Scholar]
- 14.Tracz E, Zamojska E, Modrzejewski A, Zaborski D, Grzesiak W. Quality of life in patients with venous stasis ulcers and others with advanced venous insufficiency. Holis Nurs Pract. 2015;29(2):96–102. doi: 10.1097/HNP.0000000000000072. [DOI] [PubMed] [Google Scholar]
- 15.Nelson EA, Harper DR, Prescott RJ, Gibson B, Brown D, Ruckley CV. Prevention of recurrence of venous ulceration: randomized controlled trial of class 2 and class 3 elastic compression. J Vasc Surg. 2006;44(4):803–808. doi: 10.1016/j.jvs.2006.05.051. [DOI] [PubMed] [Google Scholar]
- 16.Lurie F, Passman M, Meisner M, Dalsing M, Masuda E, Welch H, et al. The 2020 update of the CEAP classification system and reporting standards. J Vascul Surg Ven Lymph Disord. 2020;8(3):342–352. doi: 10.1016/j.jvsv.2019.12.075. [DOI] [PubMed] [Google Scholar]
- 17.Hashimoto T, Kursewicz CD, Fayne RA, Nanda S, Shah SM, Nattkemper L, et al. Mechanisms of itch in stasis dermatitis: significant role of IL-31 from macrophages. J Invest Dermatol. 2020;140(4):850–859. doi: 10.1016/j.jid.2019.09.012. [DOI] [PubMed] [Google Scholar]
- 18.Morris AD. Cellulitis and erysipelas. BMJ Clin Evid. 2008;2008:1708. [PMC free article] [PubMed] [Google Scholar]
- 19.Institute for Quality and Efficiency in Health Care (IQWiG). Erysipelas and cellulitis: Overview. 2015 Jun 03 [cited 2022 Nov 11]; https://www.ncbi.nlm.nih.gov/books/NBK303996/
- 20.Palmer B, Xia Y, Cho S, Lewis FS. Acroangiodermatitis secondary to chronic venous insufficiency. Cutis. 2010;86(5):239–240. [PubMed] [Google Scholar]
- 21.Kaplan RM, Criqui MH, Denenberg JO, Bergan J, Fronek A. Quality of life in patients with chronic venous disease: San Diego population study. J Vasc Surg. 2003;37(5):1047–1053. doi: 10.1067/mva.2003.168. [DOI] [PubMed] [Google Scholar]
- 22.Kahn SR, M'lan CE, Lamping DL, Kurz X, Bérard A, Abenhaim LA, et al. Relationship between clinical classification of chronic venous disease and patient-reported quality of life: results from an international cohort study. J Vasc Surg. 2004;39(4):823–8. [DOI] [PubMed]
- 23.Valdes-Rodriguez R, Mollanazar NK, González-Muro J, Nattkemper L, Torres-Alvarez B, López-Esqueda FJ, et al. Itch prevalence and characteristics in a Hispanic geriatric population: a comprehensive study using a standardized itch questionnaire. Acta Derm Venereol. 2015;95(4). [DOI] [PubMed]
- 24.Paul JC, Pieper B, Templin TN. Itch: association with chronic venous disease, pain, and quality of life. J Wound Ostomy Continence Nurs. 2011;38(1):46–54. doi: 10.1097/WON.0b013e318202c47a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McGuckin M, Waterman R, Brooks J, Cherry G, Porten L, Hurley S, et al. Validation of venous leg ulcer guidelines in the United States and United Kingdom. Am J Surg. 2002;183(2):132–137. doi: 10.1016/S0002-9610(01)00856-X. [DOI] [PubMed] [Google Scholar]
- 26.Spiridon M, Corduneanu D. Chronic venous insufficiency: a frequently underdiagnosed and undertreated pathology. Maedica (Bucur). 2017;12(1):59–61. [PMC free article] [PubMed] [Google Scholar]
- 27.Sung CT, Taguines PR, Jacob SE. Stasis dermatitis. J Dermatol Nur Assoc. 2019;11(3):134–136. [Google Scholar]
- 28.Fiebig A, Krusche P, Wolf A, Krawczak M, Timm B, Nikolaus S, et al. Heritability of chronic venous disease. Hum Gen. 2010;127(6):669–674. doi: 10.1007/s00439-010-0812-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suehiro K, Morikage N, Murakami M, Yamashita O, Ueda K, Samura M, et al. A study of leg edema in immobile patients. Circ J. 2014;78(7):1733–1739. doi: 10.1253/circj.CJ-13-1599. [DOI] [PubMed] [Google Scholar]
- 30.Zygmunt JA. Duplex ultrasound for chronic venous insufficiency. J Invasive Cardiol. 2014;26(11):E149–E155. [PubMed] [Google Scholar]
- 31.Keller EC, Tomecki KJ, Alraies MC. Distinguishing cellulitis from its mimics. Cleve Clin J Med. 2012;79(8):547–552. doi: 10.3949/ccjm.79a.11121. [DOI] [PubMed] [Google Scholar]
- 32.Strazzula L, Cotliar J, Fox LP, Hughey L, Shinkai K, Gee SN, et al. Inpatient dermatology consultation aids diagnosis of cellulitis among hospitalized patients: a multi-institutional analysis. J Amer Acad Dermatol. 2015;73(1):70–75. doi: 10.1016/j.jaad.2014.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Renner R, Wollina U. Contact sensitization in patients with leg ulcers and/or leg eczema: comparison between centers. Int J Low Extrem Wounds. 2002;1(4):251–255. doi: 10.1177/1534734602239654. [DOI] [PubMed] [Google Scholar]
- 34.Id reaction (autoeczematization): background, pathophysiology, epidemiology 2018 Mar 23 [cited 2022 Jan 26]; https://emedicine.medscape.com/article/1049760-overview#a6
- 35.Sardana K, Sarkar R, Sehgal VN. Pigmented purpuric dermatoses: an overview. Int J Dermatol. 2004;43(7):482–488. doi: 10.1111/j.1365-4632.2004.02213.x. [DOI] [PubMed] [Google Scholar]
- 36.Naik PP, Farrukh SN. Clinical significance of diabetic dermatopathy. Diabetes Metab Syndr Obes. 2020;13:4823–4827. doi: 10.2147/DMSO.S286887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davies AH. The seriousness of chronic venous disease: a review of real-world evidence. Adv Ther. 2019;36(Suppl 1):5S–12S. doi: 10.1007/s12325-019-0881-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gloviczki P, Comerota AJ, Dalsing MC, Eklof BG, Gillespie DL, Gloviczki ML, et al. The care of patients with varicose veins and associated chronic venous diseases: clinical practice guidelines of the Society for Vascular Surgery and the American Venous Forum. J Vasc Surg. 2011;53(Suppl 5):2S–48S. doi: 10.1016/j.jvs.2011.01.079. [DOI] [PubMed] [Google Scholar]
- 39.Payne SP, London NJ, Newland CJ, Thrush AJ, Barrie WW, Bell PR. Ambulatory venous pressure: correlation with skin condition and role in identifying surgically correctible disease. Eur J Vasc Endovasc Surg. 1996;11(2):195–200. doi: 10.1016/S1078-5884(96)80051-7. [DOI] [PubMed] [Google Scholar]
- 40.Sippel K, Mayer D, Ballmer B, Dragieva G, Läuchli S, French L, et al. Evidence that venous hypertension causes stasis dermatitis. Phlebology. 2011;26(8):361–365. doi: 10.1258/phleb.2010.010043. [DOI] [PubMed] [Google Scholar]
- 41.Thomas PR, Nash GB, Dormandy JA. White cell accumulation in dependent legs of patients with venous hypertension: a possible mechanism for trophic changes in the skin. Br Med J (Clin Res Ed) 1988;296(6638):1693–1695. doi: 10.1136/bmj.296.6638.1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyses C, Cederholm-Williams SA, Michel CC. Haemoconcentration and accumulation of white cells in the feet during venous stasis. Int J Microcirc Clin Exp. 1987;5(4):311–320. [PubMed] [Google Scholar]
- 43.Wilkinson L, Bunker C, Edwards J, Scurr J, Smith PC. Leukocytes: their role in the etiopathogenesis of skin damage in venous disease. J Vasc Surg. 1993;17(4):669–675. doi: 10.1016/0741-5214(93)90109-Y. [DOI] [PubMed] [Google Scholar]
- 44.Takase S, Pascarella L, Lerond L, Bergan JJ, Schmid-Schönbein GW. Venous hypertension, inflammation and valve remodeling. Eur J Vasc Endovasc Surg. 2004;28(5):484–493. doi: 10.1016/j.ejvs.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 45.Peschen M, Lahaye T, Hennig B, Weyl A, Simon JC, Vanscheidt W. Expression of the adhesion molecules ICAM-1, VCAM-1, LFA-1 and VLA-4 in the skin is modulated in progressing stages of chronic venous insufficiency. Acta Dermat Venereol. 1999;79(1):27–32. doi: 10.1080/000155599750011651. [DOI] [PubMed] [Google Scholar]
- 46.Herouy Y, Mellios P, Bandemir E, Dichmann S, Nockowski P, Schöpf E, et al. Inflammation in stasis dermatitis upregulates MMP-1, MMP-2 and MMP-13 expression. J Dermatol Sci. 2001;25(3):198–205. doi: 10.1016/S0923-1811(00)00128-6. [DOI] [PubMed] [Google Scholar]
- 47.Wenk J, Foitzik A, Achterberg V, Sabiwalsky A, Dissemond J, Meewes C, et al. Selective pick-up of increased iron by deferoxamine-coupled cellulose abrogates the iron-driven induction of matrix-degrading metalloproteinase 1 and lipid peroxidation in human dermal fibroblasts in vitro: a new dressing concept. J Invest Dermatol. 2001;116(6):833–839. doi: 10.1046/j.1523-1747.2001.01345.x. [DOI] [PubMed] [Google Scholar]
- 48.Mosti G, Picerni P, Partsch H. Compression stockings with moderate pressure are able to reduce chronic leg oedema. Phlebology. 2012;27(6):289–296. doi: 10.1258/phleb.2011.011038. [DOI] [PubMed] [Google Scholar]
- 49.Cullum N, Nelson E, Fletcher A, Sheldon T. Compression for venous leg ulcers. Cochr Data Syst Rev. 2001;2:CD0000265. doi: 10.1002/14651858.CD000265. [DOI] [PubMed] [Google Scholar]
- 50.Sippel K, Seifert B, Hafner J. Donning devices (foot slips and frames) enable elderly people with severe chronic venous insufficiency to put on compression stockings. Eur J Vasc Endovasc Surg. 2015;49(2):221–229. doi: 10.1016/j.ejvs.2014.11.005. [DOI] [PubMed] [Google Scholar]
- 51.Stolk R, Wegen Van Der‐Franken C, Neumann H. A method for measuring the dynamic behavior of medical compression hosiery during walking. Dermatol Surg. 2004;30(5):729–36. [DOI] [PubMed]
- 52.Raju S, Hollis K, Neglen P. Use of compression stockings in chronic venous disease: patient compliance and efficacy. Annals Vasc Surg. 2007;21(6):790–795. doi: 10.1016/j.avsg.2007.07.014. [DOI] [PubMed] [Google Scholar]
- 53.Suehiro K, Morikage N, Yamashita O, Harada T, Ueda K, Samura M, et al. Adherence to and efficacy of different compression methods for treating chronic venous insufficiency in the elderly. Phlebolog. 2016;31(10):723–728. doi: 10.1177/0268355515608992. [DOI] [PubMed] [Google Scholar]
- 54.Mizuno J, In-Nami H. Allergic contact dermatitis to synthetic rubber, neoprene in compression stockings. Masui Jpn J Anesthesiol. 2011;60(1):104–106. [PubMed] [Google Scholar]
- 55.Kucharzewski M, Kózka M, Urbanek T. Topical treatment of nonhealing venous leg ulcer with propolis ointment. Evid-Bas Complem Alt Med. 2013;2013. [DOI] [PMC free article] [PubMed]
- 56.Kirkup ME. Xerosis and stasis dermatitis. Preventive Dermatology. London: Springer; 2011. p. 71–79.
- 57.Paller AS, Tom WL, Lebwohl MG, Blumenthal RL, Boguniewicz M, Call RS, et al. Efficacy and safety of crisaborole ointment, a novel, nonsteroidal phosphodiesterase 4 (PDE4) inhibitor for the topical treatment of atopic dermatitis (AD) in children and adults. J Am Acad Dermatol. 2016;75(3):494–503. doi: 10.1016/j.jaad.2016.05.046. [DOI] [PubMed] [Google Scholar]
- 58.Dissemond J, Knab J, Lehnen M, Franckson T, Goos M. Successful treatment of stasis dermatitis with topical tacrolimus. Vasa. 2004;33(4):260–262. doi: 10.1024/0301-1526.33.4.260. [DOI] [PubMed] [Google Scholar]
- 59.Eichenfield LF, Tom WL, Berger TG, Krol A, Paller AS, Schwarzenberger K, et al. Guidelines of care for the management of atopic dermatitis: section 2. Management and treatment of atopic dermatitis with topical therapies. J Am Acad Dermatol. 2014;71(1):116–32. [DOI] [PMC free article] [PubMed]
- 60.Tafazzoli A, Rostan EF, Goldman MP. Q-switched ruby laser treatment for postsclerotherapy hyperpigmentation. Dermatol Surg. 2000;26(7):653–656. doi: 10.1046/j.1524-4725.2000.99268.x. [DOI] [PubMed] [Google Scholar]
- 61.Parsa H, Zangivand AA, Hajimaghsoudi L. The effect of pentoxifylline on chronic venous ulcers. Wounds. 2012;24(7):190–194. [PubMed] [Google Scholar]
- 62.Collins L, Seraj S. Diagnosis and treatment of venous ulcers. Am Fam Physician. 2010;81(8):989–996. [PubMed] [Google Scholar]
- 63.Pittler MH, Ernst E. Horse chestnut seed extract for chronic venous insufficiency. Cochr Data System Rev. 2012;11:CD003230. doi: 10.1002/14651858.CD003230.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Allaert F. Meta-analysis of the impact of the principal venoactive drugs agents on malleolar venous edema. Int Angiol. 2012;31(4):310–315. [PubMed] [Google Scholar]
- 65.Aziz Z, Tang W, Chong N, Tho L. A systematic review of the efficacy and tolerability of hydroxyethylrutosides for improvement of the signs and symptoms of chronic venous insufficiency. J Clin Pharm Therap. 2015;40(2):177–185. doi: 10.1111/jcpt.12247. [DOI] [PubMed] [Google Scholar]
- 66.Sica DA. Calcium channel blocker-related peripheral edema: can it be resolved? J Clin Hyperten. 2003;5(4):291–295. doi: 10.1111/j.1524-6175.2003.02402.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Khadka S, Joshi R, Shrestha DB, Shah D, Bhandari N, Maharjan M, et al. Amlodipine-induced pedal edema and its relation to other variables in patients at a tertiary level hospital of Kathmandu, Nepal. J Pharm Tech. 2019;35(2):51–55. doi: 10.1177/8755122518809005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Hamdan A. Management of varicose veins and venous insufficiency. JAMA. 2012;308(24):2612–2621. doi: 10.1001/jama.2012.111352. [DOI] [PubMed] [Google Scholar]
- 69.Chapman-Smith P, Browne A. Prospective five-year study of ultrasound-guided foam sclerotherapy in the treatment of great saphenous vein reflux. Phlebolog. 2009;24(4):183–188. doi: 10.1258/phleb.2009.008080. [DOI] [PubMed] [Google Scholar]
- 70.Almeida JI, Raines JK. Ambulatory phlebectomy in the office. Perspect Vasc Surg Endovasc Therap. 2008;20(4):348–355. doi: 10.1177/1531003508325054. [DOI] [PubMed] [Google Scholar]
- 71.Schick MA, Schlegel N. Clinical implication of phosphodiesterase-4-inhibition. Int J Mol Sci. 2022;23(3):1209. doi: 10.3390/ijms23031209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Schlessinger J, Shepard JS, Gower R, Su JC, Lynde C, Cha A, et al. Safety, effectiveness, and pharmacokinetics of crisaborole in infants aged 3 to < 24 months with mild-to-moderate atopic dermatitis: a phase IV open-label study (CrisADe CARE 1) Am J Clin Dermatol. 2020;21(2):275–284. doi: 10.1007/s40257-020-00510-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.ClinicalTrials.gov. Identifier NCT04091087 Study Evaluating Efficacy and Safety of Crisaborole in Adults With Stasis Dermatitis. 2019 Sept 16 [cited 2022 Feb 01]; https://clinicaltrials.gov/ct2/show/NCT04091087
- 74.Millan SB, Gan R, Townsend PE. Venous ulcers: diagnosis and treatment. Am Fam Physic. 2019;100(5):298–305. [PubMed] [Google Scholar]
- 75.Health Quality Ontario Compression stockings for the prevention of venous leg ulcer recurrence: a health technology assessment. Ontario Health Technol Assess Ser. 2019;19(2):1–86. [PMC free article] [PubMed] [Google Scholar]
- 76.Imbernon-Moya A, Ortiz-de Frutos F, Sanjuan-Alvarez M, Portero-Sanchez I, Merinero-Palomares R, Alcazar V. Treatment of chronic venous ulcers with topical sevoflurane: a retrospective clinical study. Br J Anaesth. 2017;119(4):846–847. doi: 10.1093/bja/aex269. [DOI] [PubMed] [Google Scholar]
- 77.O'Meara S, Al-Kurdi D, Ologun Y, Ovington LG, Martyn-St James M, Richardson R. Antibiotics and antiseptics for venous leg ulcers. Cochr Data System Rev. 2014;10(1):CD003557. doi: 10.1002/14651858.CD003557.pub5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Tawes RL, Barron ML, Coello AA, Joyce DH, Kolvenbach R. Optimal therapy for advanced chronic venous insufficiency. J Vasc Surg. 2003;37(3):545–551. doi: 10.1067/mva.2003.131. [DOI] [PubMed] [Google Scholar]
- 79.Gohel MS, Barwell JR, Taylor M, Chant T, Foy C, Earnshaw JJ, et al. Long term results of compression therapy alone versus compression plus surgery in chronic venous ulceration (ESCHAR): randomised controlled trial. BMJ. 2007;335:83. doi: 10.1136/bmj.39216.542442.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Rai R. Standard guidelines for management of venous leg ulcer. Ind Dermatol Onlin J. 2014;5(3):408–411. doi: 10.4103/2229-5178.137830. [DOI] [PMC free article] [PubMed] [Google Scholar]