Abstract
JAK inhibitors (JAKi) are new targeted-synthetic drugs, approved for various immune-mediated inflammatory diseases (IMIDs), including inflammatory arthritides (rheumatoid arthritis—RA, psoriatic arthritis—PsA, ankylosing spondylitis—AS) and ulcerative colitis (UC). JAKi have been associated with increased risk for herpes zoster (HZ), but the relative risk among different JAKi in these IMIDs remains unclear. We aimed to systematically review the incidence of HZ among RA, PsA, AS and UC patients treated with the approved doses of tofacitinib (TOFA), baricitinib (BARI) or upadacitinib (UPA). PubMed, Embase, Scopus, Cochrane and Web-of-Science were searched up to 30 March 2022. Clinical trials and real-world studies (RWS) were included. Outcomes assessed were the incidence rate (/100 patient-years) or/and cumulative incidence of HZ. From 1710 records, 53 clinical trials and 25 RWS were included (RA: 54, PsA: 8, AS: 4, and UC: 12). In clinical trials, the HZ-incidence was higher in TOFA-treated patients with RA (2.2–7.1/100 patient-years) or UC (1.3–7.6/100 patient-years) compared to PsA (1.7/100 patient-years), and with higher doses of TOFA in UC (10 mg/twice daily: 3.2–7.6/100 patient-years vs. 5 mg/twice daily: 1.3–2.3/100 patient-years). Evidence for HZ-risk in JAKi-treated patients with AS and in UPA-treated patients was limited. The HZ-incidence between TOFA and BARI groups in 2 RA RWS did not differ significantly. Concomitant glucocorticoid, but not methotrexate, use in RA increased the HZ-risk. This systematic review showed higher HZ-risk in RA or UC than PsA patients treated with TOFA, in those treated with higher TOFA doses or with concomitant glucocorticoids. Preventive measures and monitoring of JAKi-treated patients with IMIDs are essential in daily practice.
Supplementary Information
The online version contains supplementary material available at 10.1007/s00296-022-05270-6.
Keywords: JAK inhibitors, Herpes zoster, Rheumatoid arthritis, Spondyloarthritis, Ulcerative colitis, Systematic review
Introduction
Herpes zoster (HZ), caused by varicella zoster virus (VZV) reactivation, is a common cause of acute and occasionally long-term morbidity associated with increasing age, ethnicity, female sex, certain diseases such as diabetes mellitus and immunosuppressive therapies [1, 2]. In the general population, the incidence rate (IR) of HZ is 3–5/1000 person-years (PY), rising to more than 11/1000 PY in individuals > 80 years [1, 2].
Patients with immune-mediated inflammatory diseases (IMIDs) are more susceptible to develop HZ compared to the general population [3, 4]; this increased risk may in part be related to certain immune-suppressive/modulatory therapies, such as glucocorticoids (GCs) and cyclophosphamide [5–8]. Janus kinase inhibitors (JAKi), a new class of targeted-synthetic agents approved for the treatment of various IMIDs, have been also associated with increased HZ-risk [9].
JAKs are part of a 4-member cytoplasmic tyrosine kinase family (JAK1, JAK2, JAK3, and TYK2) which engage the intracellular domains of distinct cytokine and growth factor receptors and act by phosphorylating either themselves or adjacent molecules like activators of transcription (STATs) [10]. Thus, JAKi block the downstream signaling of a variety of pro-inflammatory cytokines and so modulate pivotal pathogenetic pathways operating in several IMIDs, including rheumatoid arthritis (RA), psoriatic arthritis (PsA), ankylosing spondylitis (AS) and ulcerative colitis (UC).
Tofacitinib (TOFA) is a JAK1/3 inhibitor, baricitinib (BARI) a JAK1/2 inhibitor, while upadacitinib (UPA) is predominantly a JAK1 inhibitor [10]. TOFA and UPA have been licensed for RA, PsA, AS and UC, while BARI only for RA (Supplementary Table-1). JAK/STATs apart from their role in downstream pro-inflammatory cytokine and growth factor signaling, participate also in interferon (IFN) signaling and thus in antiviral defense [11]. Signaling via JAK1 and JAK3 is also crucial for the differentiation, maturation and survival of CD4+ Th cells, CD8+ T cells and B cells, responsible for VZV control [12–14]. Moreover, the JAK/STAT pathway is involved in the development and activation of natural killer cells, whose deficiency predisposes to VZV infection [15].
At the same time, VZV life cycle is dependent on several JAK-family functions. VZV blocks the IFN-induced JAK/STAT signaling through inhibition of STAT1 and STAT2 and downregulation of JAK2 and IFN regulatory factor-9. In addition, VZV induces the STAT3 promoting the viral replication and spread into host tissues [16–18], as well modulates the antigen presentation on VZV-infected cells protecting them from CD4+ T-cell immune surveillance [19]. Therefore, JAKi may mimic the inhibitory action of VZV on IFN-induced JAK/STAT pathway, increasing the risk for VZV reactivation.
Studies underpin higher HZ-rates in IMID patients treated with JAKi than those receiving placebo or immunomodulating drugs [20–22]. Although HZ has been associated with all JAKi (“class effect”), it is unclear whether the risk differs across IMIDs. Furthermore, the role of concomitant therapies such as GCs or methotrexate (MTX) on HZ-risk has not been clarified.
We conducted a systematic literature review (SLR) of clinical trials and real-world studies (RWS) to: (i) evaluate the HZ-incidence among patients with RA, PsA, AS and UC after the initiation of treatment with the approved doses of 3 different JAKi (TOFA, BARI or UPA) and (ii) synthesize the available data for patient and treatment characteristics that could support the clinical management of HZ-risk.
Methods
This SLR was undertaken according to the Cochrane Handbook [23] and PRISMA 2020 statement [24]. The review protocol was submitted in the International Prospective Register of Systematic Reviews (https://www.crd.york.ac.uk/prospero/display_record.php?ID=CRD42022323423) [25].
Literature search strategy
PubMed, Embase, Cochrane-Library, Scopus and Web-of-Science were searched from inception up to 30 March 2022. JAKi of interest were tofacitinib-TOFA, baricitinib-BARI and upadacitinib-UPA. Our search strategy was guided from the PICO format: Population: RA, PsA, AS or UC patients; Intervention: treatment with TOFA, BARI or UPA; Comparator: placebo or active comparator [GCs, immuno-suppressants/-modulators] or none; Outcome: incident HZ events.
The search query was formed, in collaboration with an experienced librarian (ED), as follows: (“rheumatoid arthritis” OR “psoriatic arthritis” OR “ankylosing spondylitis” OR “spondyloarthritis” OR “ulcerative colitis”) AND (“Janus kinases inhibitors” OR “JAK inhibitors” OR “tofacitinib” OR “upadacitinib” OR “baricitinib” OR “DMARDs”) AND (“herpes zoster” OR “varicella zoster virus” OR “varicella zoster virus reactivation” OR “shingles”).
Inclusion and exclusion criteria
Clinical trials (randomized and non-randomized) and RWS were included. We excluded: (a) case reports and case series with < 20 participants, editorials, letters, reviews, comments, surveys, recommendations, guidelines, expert opinions, and study protocols; (b) non-English studies; and (c) articles not referring to population/intervention/outcome of interest. SLRs, meta-analyses and pooled analyses were excluded from the final synthesis, but their references were screened to identify additional eligible publications. Articles and supplementary material non-available online were requested from the study investigators and were finally excluded if they were not provided up until the study submission.
Study selection
All eligible studies had to fulfill the pre-specified PICO format. Two reviewers (CGG and SM) independently screened all records, in two steps: first, the titles and the abstracts were screened to determine relevance and excluded conference abstracts and then the full-texts to detect the adherence to inclusion criteria.
Data extraction
Data extraction was performed independently by CGG and SM. First, CGG extracted data from all the eligible studies and hand-searched their reference lists to detect further suitable studies. Subsequently, SM independently and randomly extracted data from 25% of these studies (n = 20) and verified the rest 75%. Across all stages, any discrepancies were resolved by consensus or by a third reviewer (DV).
Review Manager 5.4 software was used to record the following data: (a) study characteristics: first author’s last name, publication year, study acronym, study type, country, number of participants in treatment arm(s), and observational period; (b) patients’ characteristics: disease, age, JAKi dose, comparators (when available), concomitant MTX and/or GC therapy, and baseline history of HZ; (c) reports on incident HZ events: cumulative incidence (%), incidence/event rate (IR/ER) per 100 patient-years (PY) with 95% confidence interval (CI) and/or hazard ratio (HR) (95% CI); and (d) recurrence of HZ during JAKi therapy.
Data synthesis
Only data pertaining to groups treated with the approved JAKi doses per disease were analyzed (Supplementary Table-1). For the final synthesis, studies were clustered as per disease, class of JAKi and study type. The range of HZ-incidence in terms of cumulative incidence or IR/ER was estimated separately in clinical trials and RWS, including all the respective studies, regardless of the RoB.
Risk of bias assessment
Risk of bias (RoB) was assessed independently by CGG and SM using the Cochrane RoB-2 tool [26] and the Newcastle–Ottawa-Scale [27, 28] for randomized and non-randomized studies, respectively. Discordances were openly discussed and if necessary DV made the final decision.
Results
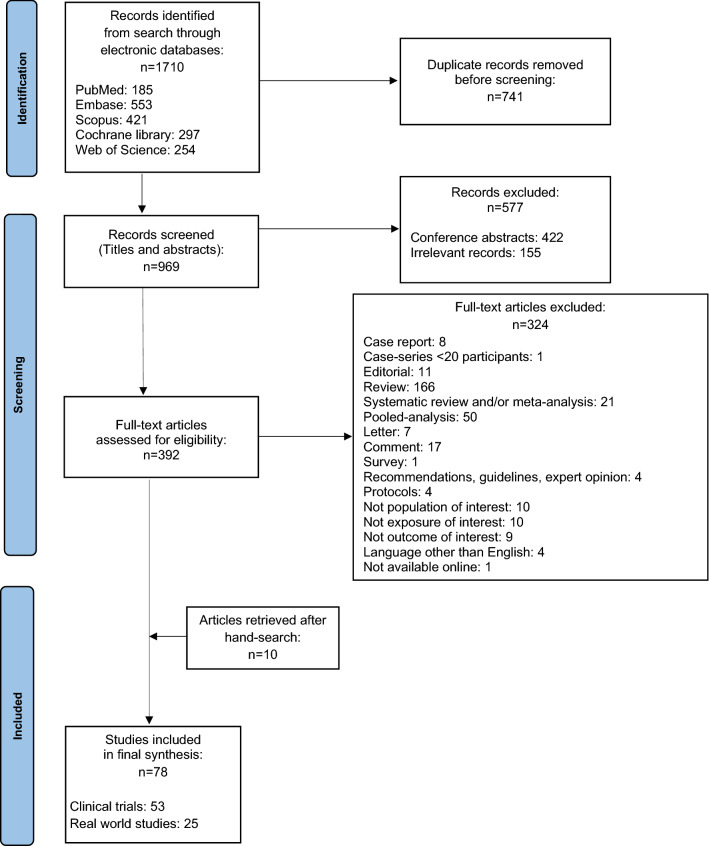
Our query initially retrieved 1710 references (Fig. 1). From them, after deduplication and titles/abstract screening, 392 were relevant to our search question and proceeded to full-text review. Finally, 68 articles met the inclusion criteria, in addition to 10 references identified after hand-search.
Fig. 1.
Flow-chart of the screening process performed for identification and selection of studies included in the systematic literature review
Agreement between reviewers in articles’ screening, validation of extracted data and RoB assessment was 91%, 97% and 85%, respectively.
Characteristics of the included studies
Overall, 44 original articles of randomized controlled trials (RCT) were included (RA: 30, PsA: 6, AS: 4, and UC: 4), 9 of long-term extension studies (LTEs) (RA: 5, PsA: 1, and UC: 1) and 25 of RWS (RA: 19 and UC: 6). No RWS was identified for AS or UC patients.
Characteristics of the included studies are summarized in Supplementary Table-2 (RCTs and LTEs) and Supplementary Table-3 (RWS).
Most of the randomized studies included in this SLR (30/46) [44 RCTs and 2 LTEs with randomized arms at baseline [29–31]] had unclear RoB, attributed mainly to insufficient reporting on random sequence generation and allocation concealment. 8 RCTs showed high RoB; 6 included in their analysis an open-label period for participants/investigators and/or outcomes’ assessor [29, 32–36], 2 had selection bias in terms of the randomization process [33, 35] and 3 reported incompletely the outcome data [35, 37, 38] (see details in the Supplementary figure-1).
Among the 32 non-randomized studies (25 RWS and 7 LTEs), 5 had low, 20 intermediate and 7 high RoB (Supplementary table 4). RoB derived foremost from the absence of adjustments for confounders. In addition, in RWS high rates of study discontinuation (with ≥ 15% lost to follow-up deemed more likely to introduce bias) and/or inadequate follow-up assessment (defined for our outcome of interest as less than 1 year) were the most frequent bias-related issues.
HZ-incidence after JAKi exposure
Rheumatoid arthritis
Tofacitinib
In total, 23 RA studies for TOFA at the approved dose of 5 mg twice daily (BID) were eligible: 9 RCTs (10 original articles: 2 low, 7 unclear and 1 high RoB), 2 LTEs (intermediate RoB) (Supplementary Table-2) and 12 RWS (4 low, 5 intermediate, 3 high RoB) (Supplementary Table-3).
TOFA monotherapy was studied in 2 clinical trials [39, 40] and 1 RWS [41]. TOFA + MTX was administered in 1 RCT [42, 43] and 1 RWS [40, 41], while MTX was permitted in 2 clinical trials [44, 45] and 9 RWS [45–52]. Concomitant GCs were used in 8 RCTs [40, 53, 54] and 9 RWS [45–52].
The HZ-incidence in RCTs (within 12–96 weeks) ranged between 0 and 7.5% [38, 39, 42–44, 53–56]. In 2 longer duration studies (1 RCT and 1 LTE with 192- and 456-week duration, respectively), the HZ-incidence was higher at 12.4% and 10.7% [36, 40]. Of note, the IR was higher in a Japanese open-label LTEs (7.1/100 PY) compared to a global one (2.3/100 PY) [40, 45]. In RWS (mean observation period: 24–81.6 weeks), the incidence ranged between 1.3 and 16.7% and the IR between 1.4 and 6.5/100 PY [41, 46–52, 57–59]. Finally, in a post-marketing surveillance study for TOFA in RA, 7 HZ events out of 9291 adverse events were reported [60].
Baricitinib
9 RCTs (1 low, 3 unclear and 5 high RoB), 1 LTE (intermediate RoB) (Supplementary Table-2) and 9 RWS (1 low, 7 intermediate, 1 high RoB) (Supplementary Table-3) were retrieved for BARI 2 mg and/or 4 mg once daily (QD).
BARI combined with MTX was administered in 5 clinical trials [33, 34, 37, 61–64], MTX was permitted in 2 RCTs [65, 66] and 5 RWS [58, 59, 67–69], while GCs were used in 8 clinical trials [61–63, 65, 66] and 5 RWS [58, 59, 67–69].
The HZ-incidence in RCTs ranged between 0 and 1.7% for the 2 mg dose and between 1.3 and 7.0% for the 4 mg dose [33–35, 37, 61, 62, 64–66]. In 1 LTE (4 mg QD), the incidence was 5.6% the first 76 weeks and decreased to 1.3% between 76 and 128 weeks [63]. In RWS (follow-up: 24–48 weeks), the HZ-incidence in groups receiving 4 mg QD ranged between 1.3 and 4.9% [58, 67, 68]. In another 2 cohorts, the pooled analysis of both BARI dose-groups revealed an incidence of 3.6% and 6.2%, respectively [59, 69]. Finally, a post-marketing surveillance study in RA found 49 HZ cases out of 1598 reported adverse events [70].
Upadacitinib
For UPA, 12 primary reports of RCTs (1 low, 9 unclear and 2 high RoB) and 1 LTE (unclear RoB) were included (Supplementary Table-2). UPA combined with MTX was studied in 1 RCT [71], while MTX was permitted in 5 clinical trials [30, 72–76].
The range of HZ-incidence in RCTs (12–48 weeks) was 0.5–2.2% [29, 32, 71–78], whereas in a subgroup analysis of Japanese patients from a 24-week RCT, the incidence was higher at 7.4% [79]. The overall HZ-incidence and ER in a long-term trial (within 108 weeks) were 6.1% and 3.1/100 PY, respectively [29], while in another Japanese LTEs (84-week duration), the incidence and IR were likewise higher (21.9% and 12.3/100 PY, respectively) [30, 74].
Psoriatic arthritis
Tofacitinib
3 RCTs (low RoB) and 1 LTE (intermediate RoB), but no RWS, were eligible for TOFA in PsA (Supplementary Table-2). In 3 of them, MTX and/or GCs were co-administered [80–82]. In RCTs (12–48-week duration), the HZ-incidence ranged between 0 and 3.3% [80, 81, 83] with a similar incidence (2.5%) in a LTE (144 weeks) [82].
Upadacitinib
Evidence for HZ under UPA therapy were provided by 2 RCTs (unclear RoB). The HZ-incidence ranged between 0.9 and 1.4% (up to week 24) [84, 85] while the respective ER in 1 study was 3.8/100 PY (up to week 56) [86]. RWS relevant to our PICO format were not found.
Ankylosing spondylitis
Tofacitinib
Overall, 2 RCTs (unclear RoB) were included for TOFA in AS patients. In the first, no HZ event was documented up to 12 weeks on treatment followed by 4 weeks of follow-up [87], while in the second trial, an incidence of 0%, 1.5% and 2.3% was reported at weeks 16, 32 and 48 on treatment, respectively [88]. No RWS was identified.
Upadacitinib
Data for HZ during UPA therapy in AS patients were retrieved only from 1 RCT (2 original articles: 1 low and 1 unclear RoB). The interim analysis (up to week 64) indicated an incidence of 2.5% [89]. RWS were not found.
Ulcerative colitis
Tofacitinib
Overall, 5 RCTs (2 low and 3 unclear RoB), 1 LTE (intermediate RoB) (Supplementary Table-2) and 6 RWS (3 low and 3 high RoB) (Supplementary Table-3) were eligible. GCs were permitted in 5 clinical trials [90–92] and 4 RWS [93–96]. TOFA monotherapy was studied in 1 RCT [97] and 1 RWS [98].
In RCTs (8–52 weeks), the HZ-incidence ranged between 0.5 and 5.1% for the 10 mg BID dose [59, 61, 62] and 1.4–1.5% for the 5 mg BID dose [91, 97]. In the LTEs (up to week 240), the respective incidence for these doses was 7.8% and 7.4% [92]. Similarly, in 1 RCT and 1 LTE, the IRs for the 10 mg BID dose were higher, at 3.2 and 3.6/100 PY, compared to 1.3 and 2.1/100 PY for the 5 mg BID dose [92, 97]. In addition, Vermeire et al. randomized patients at remission, who had received TOFA 10 mg BID for ≥ 2 consecutive years, either to remain at the 10 mg BID dose or to de-escalate to 5 mg BID. Analyses up to week 24 showed higher HZ-rates in the 10 mg BID group compared to the 5 mg BID group (4.3% vs. 1.4%) with IRs of 3.2/100 PY and 1.3/100 PY, respectively [97].
In RWS (mean follow-up: 24–60 weeks), the pooled HZ-incidence ranged from 0 to 7.9% [93–96, 98, 99]. Interestingly, across all of them, 9 of the 13 (69%) HZ cases occurred in patients receiving the high TOFA dose (10 mg BID).
Upadacitinib
Limited evidence is available for UPA in AS, since only 1 RCT (unclear RoB), and none RWS, was eligible. Data from the first 8 weeks on treatment showed an HZ-incidence of 0% for the 15 mg and 30 mg groups and 0.8% for the 45 mg group [100].
Comparative studies of HZ-incidence among different JAKi
We identified only 2 RWS in Japanese RA patients at which the HZ-rate was compared between TOFA and BARI groups (Table 1). Iwamoto et al. demonstrated a slightly higher HZ-incidence in TOFA than BARI group (5.6% vs. 4.9%) [58]. Inversely, Miyazaki et al. found higher incidence for BARI (3.6% vs. 1.3%) [59]. However, in both studies, the differences were non-significant. Head-to-head studies comparing the HZ-incidence among different JAKi were not found.
Table 1.
Studies included in this SLR, which reported the difference on the HZ-incidence among patients receiving different JAKi
| Author (ref), year, region | Study type | Observational period | Age, yearsa | Concomitant GCs b % (dosec) | Cumulative HZ-incidence, % (n/N) | p-value | RoB | |
|---|---|---|---|---|---|---|---|---|
| TOFA | BARI | |||||||
|
Miyazaki [59] 2021, Japan |
Cohort | 24 weeks | 59.1 (13.4) |
TOFA*: 16% (5.0) BARI*: 17% (7.5) |
1.3¥ (2/156) | 3.6¥¥ (5/138) | 0.18 | Intermediate |
|
Iwamoto [58] 2021, Japan |
Cohort | 24 weeks | 66.5 (12.2) |
TOFA*: 53% (4.8) BARI*: 47% (4.8) |
5.6§ (9/161) |
4.9§§ (4/81) |
NR¶ | Intermediate |
aMean/median (SD/IQR)
bReported at baseline
cMean daily dose (mg)
*Non-significant difference of concomitant GC use between TOFA and BARI groups
¥ 89.7% received 10 mg and 10.3% received 5 mg BID
¥¥ 88.4% received 4 mg and 11.6% 2 mg QD
§All received 5 mg BID
§§All received 4 mg QD
¶ Not reported. The authors only mentioned that the difference was non-significant
GCs glucocorticoids, HZ herpes zoster, RoB risk of bias, TOFA tofacitinib, BARI baricitinib
The effect of concomitant MTX and/or GCs on HZ-incidence
In 1 LTE with 1123 RA patients, the IR of HZ was similar between TOFA monotherapy and TOFA combined with MTX (2.4 vs. 2.2/100 PY) [40]. In line, Curtis et al. analyzed 8030 RA patients from a US insurance database and found only a small difference in HZ-rates between those treated with TOFA vs. TOFA and MTX (3.7 vs. 3.4/100 PY) [41]. Similar results were reported in an Italian study [67]. On the other hand, 1 RCT with PsA patients reported a higher incidence (up to week 48) in the combination than the monotherapy group (2.2% vs. 1.1%) [83].
In contrast, data from RA studies have shown that concomitant GC use increases the HZ-risk (Table 2). Curtis et al. in the same study showed that the addition of GCs increased the HZ-risk in both TOFA monotherapy and combination groups (TOFA: 3.7/100 PY and TOFA + MTX: 3.4/100 PY vs. TOFA + GCs: 6.0/100 PY and TOFA + MTX + GCs: 6.5/100 PY) [41]. In addition, in a cohort of 446 RA patients, HZ was the most frequent infection reported among BARI-treated patients under concomitant GCs compared to other infections [67].
Table 2.
Studies included in this SLR that examined the role of concomitant glucocorticoids on the risk of HZ development in JAKi-treated patients
| Author, year (ref), country | Study type follow-up | Disease JAKi | Age, yearsa | Key findings | RoB | ||
|---|---|---|---|---|---|---|---|
| Curtis 2019 [41] US | Cohort NR | RA TOFA | 60.3 (12.6) | IR (95% CI)* | HR (95% CI) | Low | |
| TOFA monotherapy | 3.7 (2.9–4.6) | 1.0 (ref) | |||||
| TOFA + MTX | 3.4 (2.3–5.0) | 0.99 (0.64–1.54) | |||||
| TOFA + GCs | 6.0 (4.9–7.5) | 1.75 (1.28–2.41) | |||||
| TOFA + MTX + GCs | 6.5 (4.8–8.8) | 1.96 (1.33–2.88) | |||||
| Guidelli 2021 [67] Italy | Prospective cohort ≤ 48 weeks | RA BARI | 59.0 (11.9) | In BARI-treated patients on GCs, the rate of VZV reactivation was significantly higher compared to other infections (83% vs. 25%; p = 0.034) | Intermediate | ||
| Redeker 2022 [101] Germany | Prospective cohort ≤ 480 weeks | RA TOFA, BARI or UPA | 57.9 (12.5) |
Among JAKi-treated patients, the ER of HZ was comparable between those with and those without concomitant GC use Dose-dependent relationship; HR (95% CI): GCs 5–10 vs. 0 mg/day: 1.24 (0.98 to 1.57) GCs > 10 vs. 0 mg/day: 3.45 (2.14 to 5.55) |
Intermediate | ||
aMean/median (SD/IQR)
*Per 100 patient-years
ref reference, NR not reported, ER event rate, IR incidence rate, HR hazard ratio, CI confidence interval, JAKi Janus kinases inhibitor, TOFA tofacitinib, BARI baricitinib, UPA upadacitinib, RoB risk of bias, VZV varicella zoster virus, GCs glucocorticoids
This enhanced HZ-risk in GC-treated patients could be partially dose-dependent. Indeed, in a large prospective cohort of JAKi-treated patients with RA, although the ER of HZ was comparable between patients with and those without concomitant GC use, the adjusted HZ-risk was significantly increased in patients on a “high” GC dose (> 10 mg/day), but not in those on a “low” GC dose (5–10 mg/day), compared to patients who did not receive GCs [101].
Different risk of HZ between JAKi and non-biologic/biologic immunomodulators
In 5 RA trials, the HZ-incidence in patients treated with TOFA, BARI or UPA was higher compared to MTX [39, 42, 61, 78]. Similarly, 1 RA RWS showed a 3.6-fold increased risk of HZ with JAKi compared to conventional-synthetic disease-modifying anti-rheumatic drugs (csDMARDs) [101].
Regarding biologics, a higher HZ-rate was found for JAKi compared to TNFi in 3 RA and 2 PsA head-to-head RCTs [29, 36, 37, 81, 85, 101]. In addition, 5 RA RWS studies showed a higher risk for TOFA compared to various biologics [47, 48, 50, 51, 101]. However, in a Korean study with RA patients treated with JAKi or biologics, after adjustment for disease duration, number of previous targeted therapies, concomitant MTX and/or GC use, the difference was attenuated (7.6% vs. 4.8%) [102]. Conversely, in 3 RA RCTs, the incidence was similar between TOFA/UPA and ADA/ABA groups [53, 54, 75]. More details are given in Supplementary Tables 2 and 3.
Association of previous history of HZ with a new HZ episode after JAKi exposure
Among the included studies, only 2 clinical trials and 4 RWS provided information about patients’ previous HZ history. In 5 of them, less than 15% of patients who developed HZ during JAKi treatment had history of an HZ event [30, 34, 47, 57, 67, 94, 103]. In contrast, in a cohort with 125 RA patients, 100% (7/7) of patients who developed HZ during TOFA treatment had HZ history compared to 14.4% (17/118) of those who did not develop HZ during TOFA therapy (p < 0.001)[57].
Recurrence of HZ during JAKi therapy
Limited data are available for the rate of HZ recurrence in patients who develop the first HZ episode during JAKi therapy. In the ORAL Sequel trial, an open-label LTE with 4481 RA patients, HZ recurrence was 2.6/100 PY within 456 weeks [40]. Moreover, in a small cohort with 29 RA patients who had previously experienced an HZ event during TOFA or BARI therapy, only 1 patient developed a second episode within a median follow-up of 12 months. Interestingly, in this dataset, the continuation or resumption (after completion of HZ therapy) of the same JAKi did not seem to affect the risk of HZ recurrence [103].
Discussion
In this SLR, we reviewed the HZ-incidence among patients with different IMIDs (RA, PsA, AS and UC) who were treated with different JAKi (TOFA, BARI or UPA). These results provide a better understanding of the relative HZ-risk across the expanding spectrum of JAKi indications and inform physicians and patients alike for the necessary steps for its prevention.
In RA, the overall HZ-rates in RCTs ranged between 0 and 12.4% for TOFA [36, 39, 40, 42–44, 53–56], 0–7% for BARI [33–35, 37, 61–66] and 0.5–7.4% for UPA [29, 30, 32, 71–79, 104]. Similar rates were reported in RWS: TOFA: 1.3–16.7% [41, 46–50, 52, 57] and BARI: 1.3–6.2% [58, 59, 67–69]] (Table 3).
Table 3.
Incidence of herpes zoster reported in the included RCTs, LTEs and RWS
| Disease | JAKi | Dose | RCTs | LTEs | RWS | |||
|---|---|---|---|---|---|---|---|---|
| Cumulative incidence % [weeks¥] | IR/ER* (/100 PY) | Cumulative incidence % [weeks¥] | IR/ER (/100 PY) | Cumulative incidence % [weeks¥] | IR/ER (/100 PY) | |||
| RA | TOFA | 5 mg BID | 0–7.5% [12–96] 12.4% [192§] | – | 10.7% [456] | 2.2–7.1 | 1.3–16.7% [24–81.6§] | 1.3–6.5 |
| UPA | 0.5–7.4% [12–48] | 2.3*–3.1* | 6.1–21.9% [84–108] | 3.1–12.3 | – | – | ||
| BARI | 2 mg QD | 0–1.7% [12–24] | – | – | – | 1.3–6.2% [24–48] (2 or 4 mg QD) | 8.4 (2 or 4 mg QD) | |
| 4 mg QD | 1.3–2.3% [12–28] 7% [50] | – | 1.3–5.6% [52] | 1.3–5.8 | ||||
| PsA | TOFA | 5 mg BID | 0–3.3% [12–48] | – | 2.5% [144] | 1.7 | – | – |
| UPA | 15 mg QD | 0.9–1.4% [24] | 3.8* | – | – | – | – | |
| AS | TOFA | 5 mg BID | 0–2.3% [16–48] | – | – | – | – | – |
| UPA | 15 mg QD | 2.5% [64] | 2.1* | – | – | – | – | |
| UC | TOFA | 5 mg BID | 1.4–1.5% [24–52] | 1.3 | 4.5–7.4% [48–180] | 2.1–2.3 | 0–7.9% [24–60§] (5 or 10 mg BID) | – |
| 10 mg BID | 0.5–5.1% [8–52] | 3.2 | 7.8–12.3% [48–180] | 3.6–7.6 | – | |||
| UPA | 15 mg QD | 0% [8] | – | – | – | – | – | |
| 30 mg QD | 0% [8] | – | – | – | – | – | ||
| 45 mg QD | 0.8% [8] | – | – | – | – | – | ||
¥Observational period
§Mean/median
RCT randomized controlled trial, LTEs long-term extension studies, RWS real-world study, RA rheumatoid arthritis, PsA psoriatic arthritis, AS ankylosing spondylitis, UC ulcerative colitis, TOFA tofacitinib, BARI baricitinib, UPA upadacitinib, BID twice daily, QD once daily, IR incidence rate, ER event rate, PY patient-years
*ER/100 PY
For PsA and AS, in general, the rates were lower for the 2 approved JAKi (TOFA, UPA); however, data were limited and mainly derived from RCTs. More specifically, in PsA patients, the HZ-rate for TOFA was 0–3.3% [80–83] and for UPA 0.9–1.4% [84, 85]. The respective rates in AS patients were 0–2.3% for TOFA [87, 88] and 2.5% for UPA [89, 105] (Table 1). Long-term trials and RWS are required to investigate the real HZ-incidence under JAKi therapy in these patient populations.
Finally for UC, more studies are available for TOFA, showing a higher HZ-rate for the higher dose [10 mg BID; RCTs: 0.5–5.1% [90, 91, 97], LTEs: 7.8–12.3% [92, 106]] compared to the lower [5 mg BID; RCTs: 1.4–1.5% [91, 97], LTEs: 4.5–7.4% [92, 106]].
From indirect comparisons of TOFA clinical trials, we appraised that HZ-incidence was similar between RA (2.2–7.1/100 PY) and UC patients (1.3–7.6/100 PY), but lower in PsA (1.7/100 PY) patients. Higher age of RA patients, more frequent GC use and/or higher TOFA doses in UC patients, could explain this finding. In addition, differences in underlying disease mechanisms may play a role. In accordance, Burmester et al. in a pooled analysis of RA, PsA and UC RCTs and LTEs found higher IRs in RA and UC than PsA cohorts. This difference was attributed to the highest proportion of Asian patients enrolled in RA and UC studies and/or to concomitant GC use [107].
In the absence of head-to-head studies comparing the HZ-risk among different JAKi, we looked for evidence from non-randomized studies for an indirect comparison. In 2 Japanese RA observational studies, there were non-significant differences in the HZ-incidence between TOFA and BARI groups. Similar results were reported in recent meta-analyses of RA clinical trials that compared the BARI- and UPA-related HZ-risk [20, 107, 108]. Conversely, another meta-analysis of clinical trials and RWS on patients with different IMIDs showed a higher pooled IR of HZ for UPA (3.92/100 PY), followed by BARI (2.16/100 PY) and TOFA (1.62/100 PY) therapy [109]. Notably, in this study, results were not stratified per type of IMID and so comparisons were not feasible.
Another important question is whether there is a dose-dependent increase of HZ-risk in JAKi-treated patients. In the included clinical trials, we found a higher IR of HZ with the high dose of TOFA in UC (10 mg BID: 3.2–7.6/100 PY vs. 5 mg BID: 1.3–2.3/100 PY). To compare the BARI doses in RA, only the cumulative HZ-incidence was available from the short-term RCTs (up to 28-week duration); the HZ-rate was slightly higher with the high dose (4 mg QD: 1.3–2.3% vs. 2 mg QD: 0–1.7%) (Table 1). Accordingly, in a meta-analysis of 4 BARI RCTs, which have been included in this SLR, the HZ-rate was estimated to be similar between the two doses up to week 12, but higher with 4 mg vs. 2 mg QD at 24 weeks’ follow-up. However, this difference was non-significant [110]. In addition, there are conflicting data for TOFA dosing in RA, with 1 meta-analysis showing similar HZ-rates between TOFA 5 mg and 10 mg BID [111], whereas in a pooled analysis of RA RCTs by Winthrop et al., the TOFA dose was an independent risk factor for HZ [112].
Ethnicity appears to be also an important risk factor for HZ. In general, we found higher HZ-rates in Japanese patient-groups treated with JAKi. So far, there is not a clear explanation for this finding. A recent meta-analysis of genome-wide association studies revealed multiple loci associated with faster onset of HZ in European and East Asian patients with RA or PsO treated with TOFA [113].
As for the role of concomitant therapies, we identified GCs as an additional risk factor for HZ in several RA studies, with some of them displaying a dose-dependent relationship. In contrast, MTX did not appear to confer additional risk in RA. In agreement, Winthrop et al. in a pooled analysis of 6192 RA demonstrated that concomitant GCs, at any dose, independently increased the HZ-risk, whereas the addition of csDMARDs (mainly MTX) had no effect when compared to TOFA monotherapy [112]. Similar results for the concomitant GC use have been reported for UC patients within the TOFA clinical program [114], as well for PsA patients in a previous SLR [115].
In general, JAKi when used as monotherapy displayed higher HZ-rates compared to non-biologics (mainly MTX) in RA and biologics in RA and PsA. In accordance, the IR of HZ in a previous SLR was estimated between 3–4/100 PY (in Western Europe, USA, Australia) and 9/100 PY (in Japan, Korea) with JAKi vs. 2–3/100 PY with TNFi [116]. However, more well-designed studies are needed to examine whether these differences are influenced by confounders.
The timing of HZ development after JAKi initiation could be clinically relevant. We observed that in RA trials for TOFA, the HZ-incidence was higher in longer duration studies (> 96 weeks); however, the timepoint of HZ occurrence was not reported. In addition, IRs were higher in LTEs than RCTs for TOFA in UC patients. Studies for PsA were limited, but the HZ-incidence in one LTE was within the range reported in RCTs. In addition, in SELECT-COMPARE trial, the IR did not differ between 48- and 108-week durations of UPA therapy [29, 71]. In line, pooled analyses of RA and UC clinical trials have indicated that longer duration of TOFA exposure did not increase the HZ-risk [114, 117]. More details were provided by RWS. In 3 cohorts, most events occurred within the first 16 weeks of JAKi initiation [57, 67, 98], while in another 4 cohorts, the median/mean time to VZV reactivation since JAKi commencement ranged between 7 and 44 weeks [49, 69, 94, 103].
Thus far, studies examining whether patients with HZ history have a higher risk for HZ recurrence after JAKi initiation are lacking. Of note, most clinical trials excluded patients with a history of > 1 HZ episode or a single episode of disseminated HZ. Thus, the association of HZ history with a new HZ episode after exposure to JAKi was examined only in 1 RA cohort at which was found to be significant [57]. In accordance, an integrated analysis of the SELECT program for UPA in RA showed that the history of HZ was a significant risk factor for incident HZ (HR: 24.19; 95% CI, 15.94–36.72) [118].
The risk of HZ recurrence after the first episode during JAKi treatment is also unclear. Data from the included studies are scarce, but it seems that the HZ recurrence is rare even in IMID patients. In a large RA trial for TOFA, the recurrence rate was 2.6/100 PY [40]. In addition, Cohen et al. in a post hoc analysis of clinical trials found that about 5% (36/783) of PsA and 11% (783/7061) of RA patients had at least 1 HZ event, but only 3% (1/36) and 8% (63/783), respectively, had more than 2 events during TOFA therapy [119].
The availability of vaccines against HZ is a crucial development for the prevention of therapy-related HZ in patients with IMIDs. Data regarding the efficacy and safety of the older, live attenuated and of the most recently available, non-live recombinant zoster vaccine are scarce [120]. In this SLR, the rate of vaccination against HZ at baseline was reported in a few clinical trials [30, 40, 54, 73, 77, 78] and RWS [41, 47, 51, 93, 94, 102, 103], and it was generally low, less than 10%. Notably, in a retrospective analysis of US insurance databases for TOFA-treated RA patients, prior vaccination with a live-attenuated zoster vaccine was associated with a trend towards lower HZ-risk (HR: 0.60; 95% CI 0.34–1.05)[41]. Thus, meaningful conclusions about the protective role of zoster vaccination in JAKi-treated patients cannot be made.
In the 2019 EULAR recommendations, HZ vaccination was recommended for high-risk patients with IMIDs [120], while the 2022 Guidelines from the Advisory Committee on Immunization Practices of the United States Centers for Disease Control and Prevention recommend the use of the newer recombinant zoster vaccine for patients ≥ 19 years who are or will be immune-deficient/suppressed because of disease or therapy [121]. The new recombinant vaccine, which has been licensed in Europe since March 2018, has shown moderate to high efficacy and an acceptable safety profile in immunocompromised persons and is thus appropriate for patients on high-risk for HZ such as those treated with JAKi for IMIDs.
Most of the HZ events that occurred during JAKi therapy were classified as non-serious and involved 1 or 2 adjacent dermatomes both in clinical trials and RWS. Furthermore, we observed discrepancies in the management of JAKi treatment after HZ development, mainly in RWS, given that some RCTs required discontinuation of treatment in case of HZ occurrence [34, 122]. Even so, for most of the cases, JAKi therapy was continued after HZ resolution. Similarly, in a recent post hoc analysis of 21 RA and 3 PsA clinical studies, the majority of first or recurrent HZ events were non-serious, mild, or moderate in severity and clinically manageable with antiviral therapy and/or with temporary drug discontinuation [119]. Recently, the EULAR expert committee proposed temporarily interruption of JAKi therapy until the HZ episode is resolved, while antiviral prophylaxis could be considered in individuals with recurrent zoster [116].
This study has some limitations. First, an accurate estimate of the relative HZ-risk among different IMIDs and different JAKi cannot be provided, given that this requires a meta-analysis which was out of our scope. Second, in this SLR, many of the included studies had unclear/intermediate RoB. However, in RCTs, this derived mainly from insufficient reporting of randomization procedure that we consider not to significantly influence our outcomes. Third, to assess the RoB in non-randomized studies, we implemented the Newcastle–Ottawa-Scale, which although is not the most powerful tool, is suggested from Cochrane handbook for non-Cochrane Reviews [123]. Besides, we still found at least intermediate RoB in the majority of the included non-randomized studies, and so the quality of them was not overestimated. Fourth, the true impact of concomitant MTX and/or GC use on HZ-risk could not be estimated since neither doses nor duration of exposure were reported in detail. Finally, some studies (mainly RWS) provided pooled data from groups receiving different JAKi and/or doses, thus the HZ-incidence with specific JAKi/dose could not be ascertained.
This SLR has also important strengths. We searched 5 electronic databases covering the largest part of the literature. Importantly, aside from RCTs and LTEs, we also included RWS most of which have recently been published and so have not been reviewed previously. In addition, we analyzed data referring only to the approved JAKi doses in order to provide a useful overview applicable to daily clinical practice. In addition, the search strategy was supported by an experienced librarian, while the screening process and RoB assessment were fully and independently conducted by two reviewers. Finally, the quality of studies was assessed using reliable tools recommended for SLRs.
Conclusion
This SLR provides useful updated information regarding the incidence and management of HZ during JAKi treatment in patients with IMIDs. Overall, this evidence supports that HZ-risk is a “class” effect of JAKi (higher risk compared to other non-biologic and biologic drugs) and is partially disease- (higher risk for TOFA-treated patients with RA or UC compared to PsA) and dose- (higher risk with the higher dose of TOFA) dependent, while concomitant GC use remains an additional risk factor. It is reassuring that most episodes were non-serious and JAKi therapy could be continued without consequences. These findings are instructive for the optimal use of JAKi and the management of HZ-risk in clinical practice, highlighting the need for more real-world data on the efficacy and safety of the newly available HZ vaccines, as well for the comparative HZ-risk among different JAKi.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1: Table 1 Approved doses of tofacitinib, baricitinib and upadacitinib for the treatment of RA, PsA, AS and UC. Table 2 RCTs and LTEs included in this SLR. Study and patient characteristics, incident herpes zoster events and risk of bias. Table 3 Real-world studies included in this SLR. Study and patient characteristics, incident herpes zoster events and risk of bias. Table 4 Risk of bias in non-randomized studies according to the Newcastle-Ottawa scale. Figure 1 Risk of bias in randomized studies according to the Cochrane RoB 2 tool.
Acknowledgements
We would like to thank Athanasios Vassilopoulos, MD for his constructive comments and the librarian Eirini Delikoura for her assistance in the search of electronic databases. This work was partially supported by the Special Account for Research Grants (S.A.R.G.), National and Kapodistrian University of Athens, Athens, Greece (DV, Grants #12085, 12086) and the Greek Rheumatology Society and Professional Association of Rheumatologists (ERE-EPERE).
Author contributions
Conception of the study: DV. Study design: DV, CGG, and SM. Screening and selection of the included studies: CGG and SM. Risk of bias appraisal: CGG and SM. Data analysis and interpretation: all the authors. Draft manuscript preparation: CGG and SM. Critical review and final approval of the manuscript: DV, EH, and KT.
Funding
Open access funding provided by HEAL-Link Greece. No specific funding has been received.
Data availability
Data for this study are presented in the manuscript, tables and supplementary material. Additional data are available upon request to the corresponding author.
Declarations
Conflict of interest
The authors declare no conflict of interest.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Kawai K, Yawn BP. Risk factors for herpes zoster: a systematic review and meta-analysis. Mayo Clin Proc. 2017;92(12):1806–1821. doi: 10.1016/j.mayocp.2017.10.009. [DOI] [PubMed] [Google Scholar]
- 2.Johnson RW, Alvarez-Pasquin MJ, Bijl M, Franco E, Gaillat J, Clara JG, et al. Herpes zoster epidemiology, management, and disease and economic burden in Europe: a multidisciplinary perspective. Therapeutic Adva Vaccines. 2015;3(4):109–120. doi: 10.1177/2051013615599151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Smitten AL, Choi HK, Hochberg MC, Suissa S, Simon TA, Testa MA, et al. The risk of herpes zoster in patients with rheumatoid arthritis in the United States and the United Kingdom. Arthritis Rheum. 2007;57(8):1431–1438. doi: 10.1002/art.23112. [DOI] [PubMed] [Google Scholar]
- 4.Yun H, Yang S, Chen L, Xie F, Winthrop K, Baddley JW, et al. Risk of herpes zoster in autoimmune and inflammatory diseases: implications for vaccination. Arthritis Rheumatol (Hoboken, NJ) 2016;68(9):2328–2337. doi: 10.1002/art.39670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Strangfeld A, Listing J, Herzer P, Liebhaber A, Rockwitz K, Richter C, et al. Risk of herpes zoster in patients with rheumatoid arthritis treated with anti-TNF-alpha agents. JAMA. 2009;301(7):737–744. doi: 10.1001/jama.2009.146. [DOI] [PubMed] [Google Scholar]
- 6.Galloway J, Raine T, Rivett L, Roberts J, Dews SA, Choy EH. Herpes zoster and Janus kinase inhibition in rheumatology and gastroenterology patients: managing risk and vaccination. Clin Exp Rheumatol. 2022;40(7):1432–1441. doi: 10.55563/clinexprheumatol/0jdyse. [DOI] [PubMed] [Google Scholar]
- 7.Mok CC, Tse SM, Chan KL, Ho LY. Prevalence and risk factors of herpes zoster infection in patients with biopsy proven lupus nephritis undergoing immunosuppressive therapies. Lupus. 2020;29(8):836–844. doi: 10.1177/0961203320923739. [DOI] [PubMed] [Google Scholar]
- 8.Schub D, Assmann G, Sester U, Sester M, Schmidt T. VZV-specific T-cell levels in patients with rheumatic diseases are reduced and differentially influenced by antirheumatic drugs. Arthritis Res Ther. 2018;20(1):252. doi: 10.1186/s13075-018-1742-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thomas K, Vassilopoulos D. Infections in patients with rheumatoid arthritis in the era of targeted synthetic therapies. Mediterr J Rheumatol. 2020;31(Suppl 1):129–136. doi: 10.31138/mjr.31.1.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fragoulis GE, McInnes IB, Siebert S. JAK-inhibitors new players in the field of immune-mediated diseases, beyond rheumatoid arthritis. Rheumatology (Oxford) 2019;58(Suppl 1):43–54. doi: 10.1093/rheumatology/key276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raftery N, Stevenson NJ. Advances in anti-viral immune defence: revealing the importance of the IFN JAK/STAT pathway. Cell Mol Life Sci. 2017;74(14):2525–2535. doi: 10.1007/s00018-017-2520-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ghoreschi K, Laurence A, O'Shea JJ. Janus kinases in immune cell signaling. Immunol Rev. 2009;228(1):273–287. doi: 10.1111/j.1600-065X.2008.00754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hambleton S, Gershon AA. Preventing varicella-zoster disease. Clin Microbiol Rev. 2005;18(1):70–80. doi: 10.1128/CMR.18.1.70-80.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sharfe N, Dadi HK, O'Shea JJ, Roifman CM. Jak3 activation in human lymphocyte precursor cells. Clin Exp Immunol. 1997;108(3):552–556. doi: 10.1046/j.1365-2249.1997.4001304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Winthrop KL. The emerging safety profile of JAK inhibitors in rheumatic disease. Nat Rev Rheumatol. 2017;13(4):234–243. doi: 10.1038/nrrheum.2017.23. [DOI] [PubMed] [Google Scholar]
- 16.Verweij MC, Wellish M, Whitmer T, Malouli D, Lapel M, Jonjic S, et al. Varicella viruses inhibit interferon-stimulated JAK-STAT signaling through multiple mechanisms. PLoS Pathog. 2015;11(5):e1004901. doi: 10.1371/journal.ppat.1004901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nagel MA, James SF, Traktinskiy I, Wyborny A, Choe A, Rempel A, et al. Inhibition of phosphorylated-STAT1 nuclear translocation and antiviral protein expression in human brain vascular adventitial fibroblasts infected with varicella-zoster virus. J Virol. 2014;88(19):11634–11637. doi: 10.1128/JVI.01945-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sen N, Che X, Rajamani J, Zerboni L, Sung P, Ptacek J, et al. Signal transducer and activator of transcription 3 (STAT3) and survivin induction by varicella-zoster virus promote replication and skin pathogenesis. Proc Natl Acad Sci USA. 2012;109(2):600–605. doi: 10.1073/pnas.1114232109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abendroth A, Slobedman B. Modulation of MHC and MHC-like molecules by varicella zoster virus. Curr Top Microbiol Immunol. 2022 doi: 10.1007/82_2022_254. [DOI] [PubMed] [Google Scholar]
- 20.Bechman K, Subesinghe S, Norton S, Atzeni F, Galli M, Cope AP, et al. A systematic review and meta-analysis of infection risk with small molecule JAK inhibitors in rheumatoid arthritis. Rheumatology (Oxford) 2019;58(10):1755–1766. doi: 10.1093/rheumatology/kez087. [DOI] [PubMed] [Google Scholar]
- 21.Adas MA, Alveyn E, Cook E, Dey M, Galloway JB, Bechman K. The infection risks of JAK inhibition. Expert Rev Clin Immunol. 2022;18(3):253–261. doi: 10.1080/1744666X.2022.2014323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kerschbaumer A, Smolen JS, Nash P, Doerner T, Dougados M, Fleischmann R, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a systematic literature research. RMD Open. 2020 doi: 10.1136/rmdopen-2020-001374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Cochrane Handbook for systematic reviews of interventions https://training.cochrane.org/handbook Accessed 9 July 2022.
- 24.Liberati A, Altman DG, Tetzlaff J, Mulrow C, Gotzsche PC, Ioannidis JP, et al. The PRISMA statement for reporting systematic reviews and meta-analyses of studies that evaluate healthcare interventions: explanation and elaboration. BMJ. 2009;339:b2700. doi: 10.1136/bmj.b2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.PROSPERO-International prospective register of systematic reviews https://www.crd.york.ac.uk/prospero/ Accessed 7 July 2022.
- 26.Higgins JPT SJ, Page MJ, Elbers RG, Sterne JAC. Chapter 8: assessing risk of bias in a randomized trial. cochrane handbook for systematic reviews of interventions version 6.3. Cochrane, 2022. www.training.cochrane.org/handbook Accessed Feb 2022.
- 27.Wells GA SB, O’Connell D, et al. The Newcastle-Ottawa scale (NOS) for assessing the quality if nonrandomized studies in meta-analyses. https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp.
- 28.Zeng X, Zhang Y, Kwong JS, Zhang C, Li S, Sun F, et al. The methodological quality assessment tools for preclinical and clinical studies, systematic review and meta-analysis, and clinical practice guideline: a systematic review. J Evid Based Med. 2015;8(1):2–10. doi: 10.1111/jebm.12141. [DOI] [PubMed] [Google Scholar]
- 29.Fleischmann R, Mysler E, Bessette L, Peterfy CG, Durez P, Tanaka Y, et al. Long-term safety and efficacy of upadacitinib or adalimumab in patients with rheumatoid arthritis: results through 3 years from the SELECT-COMPARE study. RMD Open. 2022 doi: 10.1136/rmdopen-2021-002012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kameda H, Takeuchi T, Yamaoka K, Oribe M, Kawano M, Yokoyama M, et al. Efficacy and safety of upadacitinib over 84 weeks in Japanese patients with rheumatoid arthritis (SELECT-SUNRISE) Arthritis Res Ther. 2021;23(1):9. doi: 10.1186/s13075-020-02387-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Julian PT Higgins JS, Matthew J Page, Jonathan AC Sterne. Revised Cochrane risk of bias tool for randomized trials (RoB 2.0) 2016 https://www.unisa.edu.au/contentassets/72bf75606a2b4abcaf7f17404af374ad/rob2-0_indiv_main_guidance.pdf Accessed 16 11 2022.
- 32.Fleischmann R, Pangan AL, Song IH, Mysler E, Bessette L, Peterfy C, et al. Upadacitinib versus placebo or adalimumab in patients with rheumatoid arthritis and an inadequate response to methotrexate: results of a phase III, double-blind, randomized controlled trial. Arthritis Rheumatol (Hoboken, NJ) 2019;71(11):1788–1800. doi: 10.1002/art.41032. [DOI] [PubMed] [Google Scholar]
- 33.Li Z, Hu J, Bao C, Li X, Li X, Xu J, et al. Baricitinib in patients with rheumatoid arthritis with inadequate response to methotrexate: results from a phase 3 study. Clin Exp Rheumatol. 2020;38(4):732–741. [PubMed] [Google Scholar]
- 34.Tanaka Y, Ishii T, Cai Z, Schlichting D, Rooney T, Macias W. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis: A 52-week, randomized, single-blind, extension study. Mod Rheumatol. 2018;28(1):20–29. doi: 10.1080/14397595.2017.1307899. [DOI] [PubMed] [Google Scholar]
- 35.Yang Y, Li XF, Zhang X, Bao CD, Hu JK, Xu JH, et al. Efficacy and safety of baricitinib in Chinese rheumatoid arthritis patients and the subgroup analyses: results from study RA-BALANCE. Rheumatol Ther. 2020;7(4):851–866. doi: 10.1007/s40744-020-00231-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ytterberg SR, Bhatt DL, Mikuls TR, Koch GG, Fleischmann R, Rivas JL, et al. Cardiovascular and cancer risk with tofacitinib in rheumatoid arthritis. N Engl J Med. 2022;386(4):316–326. doi: 10.1056/NEJMoa2109927. [DOI] [PubMed] [Google Scholar]
- 37.Taylor PC, Keystone EC, Van Der Heijde D, Weinblatt ME, Del Carmen ML, Gonzaga JR, et al. Baricitinib versus placebo or adalimumab in rheumatoid arthritis. N Engl J Med. 2017;376(7):652–662. doi: 10.1056/NEJMoa1608345. [DOI] [PubMed] [Google Scholar]
- 38.Fleischmann R, Kremer J, Cush J, Schulze-Koops H, Connell CA, Bradley JD, et al. Placebo-controlled trial of tofacitinib monotherapy in rheumatoid arthritis. N Engl J Med. 2012;367(6):495–507. doi: 10.1056/NEJMoa1109071. [DOI] [PubMed] [Google Scholar]
- 39.Lee EB, Fleischmann R, Hall S, Wilkinson B, Bradley JD, Gruben D, et al. Tofacitinib versus methotrexate in rheumatoid arthritis. N Engl J Med. 2014;370(25):2377–2386. doi: 10.1056/NEJMoa1310476. [DOI] [PubMed] [Google Scholar]
- 40.Wollenhaupt J, Lee EB, Curtis JR, Silverfield J, Terry K, Soma K, et al. Safety and efficacy of tofacitinib for up to 9.5 years in the treatment of rheumatoid arthritis: final results of a global, open-label, long-term extension study. Arthritis Res Ther. 2019;21(1):89. doi: 10.1186/s13075-019-1866-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Curtis JR, Xie F, Yang S, Bernatsky S, Chen L, Yun H, et al. Risk for herpes zoster in tofacitinib-treated rheumatoid arthritis patients with and without concomitant methotrexate and glucocorticoids. Arthritis Care Res. 2019;71(9):1249–1254. doi: 10.1002/acr.23769. [DOI] [PubMed] [Google Scholar]
- 42.van der Heijde D, Tanaka Y, Fleischmann R, Keystone E, Kremer J, Zerbini C, et al. Tofacitinib (CP-690,550) in patients with rheumatoid arthritis receiving methotrexate: twelve-month data from a twenty-four-month phase III randomized radiographic study. Arthritis Rheum. 2013;65(3):559–570. doi: 10.1002/art.37816. [DOI] [PubMed] [Google Scholar]
- 43.van der Heijde D, Strand V, Tanaka Y, Keystone E, Kremer J, Zerbini CAF, et al. Tofacitinib in combination with methotrexate in patients with rheumatoid arthritis: clinical efficacy, radiographic, and safety outcomes from a twenty-four–month phase III study. Arthritis Rheumatol. 2019;71(6):878–891. doi: 10.1002/art.40803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kremer J, Li ZG, Hall S, Fleischmann R, Genovese M, Martin-Mola E, et al. Tofacitinib in combination with nonbiologic disease-modifying antirheumatic drugs in patients with active rheumatoid arthritis: a randomized trial. Ann Intern Med. 2013;159(4):253–261. doi: 10.7326/0003-4819-159-4-201308200-00006. [DOI] [PubMed] [Google Scholar]
- 45.Yamanaka H, Tanaka Y, Takeuchi T, Sugiyama N, Yuasa H, Toyoizumi S, et al. Tofacitinib, an oral Janus kinase inhibitor, as monotherapy or with background methotrexate, in Japanese patients with rheumatoid arthritis: an open-label, long-term extension study. Arthritis Res Ther. 2016;18:34. doi: 10.1186/s13075-016-0932-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bilgin E, Ceylan F, Duran E, Farisoğullari B, Bölek E, Yardimci GK, et al. Efficacy, retention, and safety of tofacitinib in real-life: Hur-bio monocentric experience. Turkish J Med Sci. 2021;51(1):297–308. doi: 10.3906/sag-2007-123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Curtis JR, Xie F, Yun H, Bernatsky S, Winthrop KL. Real-world comparative risks of herpes virus infections in tofacitinib and biologic-treated patients with rheumatoid arthritis. Ann Rheum Dis. 2016;75(10):1843–1847. doi: 10.1136/annrheumdis-2016-209131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chen SK, Lee H, Jin Y, Liu J, Kim SC. Use of biologic or targeted-synthetic disease-modifying anti-rheumatic drugs and risk of diabetes treatment intensification in patients with rheumatoid arthritis and diabetes mellitus. Rheumatol Adva Prac. 2020;4(2):1–11. doi: 10.1093/rap/rkaa027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Iwamoto N, Tsuji S, Takatani A, Shimizu T, Fukui S, Umeda M, et al. Efficacy and safety at 24 weeks of daily clinical use of tofacitinib in patients with rheumatoid arthritis. PLoS ONE. 2017;12(5):e0177057. doi: 10.1371/journal.pone.0177057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kremer JM, Bingham CO, Cappelli LC, Greenberg JD, Madsen AM, Geier J, et al. Postapproval comparative safety study of tofacitinib and biological disease-modifying antirheumatic drugs: 5-year results from a United States-Based rheumatoid arthritis registry. ACR Open Rheumatol. 2021;3(3):173–184. doi: 10.1002/acr2.11232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Pawar A, Desai RJ, Gautam N, Kim SC. Risk of admission to hospital for serious infection after initiating tofacitinib versus biologic DMARDs in patients with rheumatoid arthritis: a multidatabase cohort study. Lancet Rheumatol. 2020;2(2):e84–e98. doi: 10.1016/S2665-9913(19)30137-7. [DOI] [PubMed] [Google Scholar]
- 52.Mori S, Ueki Y. Outcomes of dose reduction, withdrawal, and restart of tofacitinib in patients with rheumatoid arthritis: a prospective observational study. Clin Rheumatol. 2019;38(12):3391–3400. doi: 10.1007/s10067-019-04721-z. [DOI] [PubMed] [Google Scholar]
- 53.Van Vollenhoven RF, Fleischmann R, Cohen S, Lee EB, Meijide JAG, Wagner S, et al. Tofacitinib or adalimumab versus placebo in rheumatoid arthritis. N Engl J Med. 2012;367(6):508–519. doi: 10.1056/NEJMoa1112072. [DOI] [PubMed] [Google Scholar]
- 54.Fleischmann R, Mysler E, Hall S, Kivitz AJ, Moots RJ, Luo Z, et al. Efficacy and safety of tofacitinib monotherapy, tofacitinib with methotrexate, and adalimumab with methotrexate in patients with rheumatoid arthritis (ORAL Strategy): a phase 3b/4, double-blind, head-to-head, randomised controlled trial. The Lancet. 2017;390(10093):457–468. doi: 10.1016/S0140-6736(17)31618-5. [DOI] [PubMed] [Google Scholar]
- 55.Kremer JM, Bloom BJ, Breedveld FC, Coombs JH, Fletcher MP, Gruben D, et al. The safety and efficacy of a JAK inhibitor in patients with active rheumatoid arthritis: Results of a double-blind, placebo-controlled phase IIa trial of three dosage levels of CP-690,550 versus placebo. Arthritis Rheum. 2009;60(7):1895–1905. doi: 10.1002/art.24567. [DOI] [PubMed] [Google Scholar]
- 56.Tanaka Y, Takeuchi T, Yamanaka H, Nakamura H, Toyoizumi S, Zwillich S. Efficacy and safety of tofacitinib as monotherapy in Japanese patients with active rheumatoid arthritis: A 12-week, randomized, phase 2 study. Mod Rheumatol. 2015;25(4):514–521. doi: 10.3109/14397595.2014.995875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chen YJ, Chen YM, Huang WN, Chen HH, Liao TL, Chen JP, et al. Herpes Zoster in rheumatoid arthritis patients receiving tofacitinib, a single center experience from Taiwan. Medicine. 2020;99(41):e22504. doi: 10.1097/MD.0000000000022504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Iwamoto N, Sato S, Kurushima S, Michitsuji T, Nishihata S, Okamoto M, et al. Real-world comparative effectiveness and safety of tofacitinib and baricitinib in patients with rheumatoid arthritis. Arthritis Res Ther. 2021;23(1):197. doi: 10.1186/s13075-021-02582-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Miyazaki Y, Nakano K, Nakayamada S, Kubo S, Inoue Y, Fujino Y, et al. Efficacy and safety of tofacitinib versus baricitinib in patients with rheumatoid arthritis in real clinical practice: analyses with propensity score-based inverse probability of treatment weighting. Ann Rheum Dis. 2021;80(9):1130–1136. doi: 10.1136/annrheumdis-2020-219699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Cohen S, Curtis JR, DeMasi R, Chen Y, Fan H, Soonasra A, et al. Worldwide, 3-year, post-marketing surveillance experience with tofacitinib in rheumatoid arthritis. Rheumatol Therapy. 2018;5(1):283–291. doi: 10.1007/s40744-018-0097-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Fleischmann R, Schiff M, van der Heijde D, Ramos-Remus C, Spindler A, Stanislav M, et al. Baricitinib, methotrexate, or combination in patients with rheumatoid arthritis and no or limited prior disease-modifying antirheumatic drug treatment. Arthritis Rheumatol. 2017;69(3):506–517. doi: 10.1002/art.39953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Keystone EC, Taylor PC, Drescher E, Schlichting DE, Beattie SD, Berclaz PY, et al. Safety and efficacy of baricitinib at 24 weeks in patients with rheumatoid arthritis who have had an inadequate response to methotrexate. Ann Rheum Dis. 2015;74(2):333–340. doi: 10.1136/annrheumdis-2014-206478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keystone EC, Genovese MC, Schlichting DE, De La Torre I, Beattie SD, Rooney TP, et al. Safety and efficacy of baricitinib through 128 weeks in an open-label, longterm extension study in patients with rheumatoid arthritis. J Rheumatol. 2018;45(1):14–21. doi: 10.3899/jrheum.161161. [DOI] [PubMed] [Google Scholar]
- 64.Tanaka Y, Emoto K, Cai Z, Aoki T, Schlichting D, Rooney T, et al. Efficacy and safety of baricitinib in Japanese patients with active rheumatoid arthritis receiving background methotrexate therapy: a 12-week, double-blind, randomized placebo-controlled study. J Rheumatol. 2016;43(3):504–511. doi: 10.3899/jrheum.150613. [DOI] [PubMed] [Google Scholar]
- 65.Dougados M, Van Der Heijde D, Chen YC, Greenwald M, Drescher E, Liu J, et al. Baricitinib in patients with inadequate response or intolerance to conventional synthetic DMARDs: results from the RA-BUILD study. Ann Rheum Dis. 2017;76(1):88–95. doi: 10.1136/annrheumdis-2016-210094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Genovese MC, Kremer J, Zamani O, Ludivico C, Krogulec M, Xie L, et al. Baricitinib in patients with refractory rheumatoid arthritis. N Engl J Med. 2016;374(13):1243–1252. doi: 10.1056/NEJMoa1507247. [DOI] [PubMed] [Google Scholar]
- 67.Guidelli GM, Viapiana O, Luciano N, De Santis M, Boffini N, Quartuccio L, et al. Efficacy and safety of baricitinib in 446 patients with rheumatoid arthritis: a real-life multicentre study. Clin Exp Rheumatol. 2021;39(4):868–873. doi: 10.55563/clinexprheumatol/pudtpo. [DOI] [PubMed] [Google Scholar]
- 68.Tesei G, Cometi L, Nacci F, Terenzi R, Tofani L, Capassoni M, et al. Baricitinib in the treatment of rheumatoid arthritis: clinical and ultrasound evaluation of a real-life single-centre experience. Therapeutic Adva Musculoskeletal Dis. 2021 doi: 10.1177/1759720X211014019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Takahashi N, Asai S, Kobayakawa T, Kaneko A, Watanabe T, Kato T, et al. Predictors for clinical effectiveness of baricitinib in rheumatoid arthritis patients in routine clinical practice: data from a Japanese multicenter registry. Sci Rep. 2020 doi: 10.1038/s41598-020-78925-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Peng L, Xiao K, Ottaviani S, Stebbing J, Wang YJ. A real-world disproportionality analysis of FDA adverse event reporting system (FAERS) events for baricitinib. Expert Opin Drug Saf. 2020;19(11):1505–1511. doi: 10.1080/14740338.2020.1799975. [DOI] [PubMed] [Google Scholar]
- 71.Fleischmann RM, Genovese MC, Enejosa JV, Mysler E, Bessette L, Peterfy C, et al. Safety and effectiveness of upadacitinib or adalimumab plus methotrexate in patients with rheumatoid arthritis over 48 weeks with switch to alternate therapy in patients with insufficient response. Ann Rheum Dis. 2019;78(11):1454–1462. doi: 10.1136/annrheumdis-2019-215764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Burmester GR, Kremer JM, Van den Bosch F, Kivitz A, Bessette L, Li Y, et al. Safety and efficacy of upadacitinib in patients with rheumatoid arthritis and inadequate response to conventional synthetic disease-modifying anti-rheumatic drugs (SELECT-NEXT): a randomised, double-blind, placebo-controlled phase 3 trial. Lancet (London, England) 2018;391(10139):2503–2512. doi: 10.1016/S0140-6736(18)31115-2. [DOI] [PubMed] [Google Scholar]
- 73.Genovese MC, Fleischmann R, Combe B, Hall S, Rubbert-Roth A, Zhang Y, et al. Safety and efficacy of upadacitinib in patients with active rheumatoid arthritis refractory to biologic disease-modifying anti-rheumatic drugs (SELECT-BEYOND): a double-blind, randomised controlled phase 3 trial. Lancet (London, England) 2018;391(10139):2513–2524. doi: 10.1016/S0140-6736(18)31116-4. [DOI] [PubMed] [Google Scholar]
- 74.Kameda H, Takeuchi T, Yamaoka K, Oribe M, Kawano M, Zhou Y, et al. Efficacy and safety of upadacitinib in Japanese patients with rheumatoid arthritis (SELECT-SUNRISE): a placebo-controlled phase IIb/III study. Rheumatology (Oxford) 2020;59(11):3303–3313. doi: 10.1093/rheumatology/keaa084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Rubbert-Roth A, Enejosa J, Pangan AL, Haraoui B, Rischmueller M, Khan N, et al. Trial of upadacitinib or abatacept in rheumatoid arthritis. N Engl J Med. 2020;383(16):1511–1521. doi: 10.1056/NEJMoa2008250. [DOI] [PubMed] [Google Scholar]
- 76.Zeng X, Zhao D, Radominski SC, Keiserman M, Lee CK, Meerwein S, et al. Upadacitinib in patients from China, Brazil, and South Korea with rheumatoid arthritis and an inadequate response to conventional therapy. Int J Rheum Dis. 2021;24(12):1530–1539. doi: 10.1111/1756-185X.14235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.van Vollenhoven R, Takeuchi T, Pangan AL, Friedman A, Mohamed MF, Chen S, et al. Efficacy and safety of upadacitinib monotherapy in methotrexate-naive patients with moderately-to-severely active rheumatoid arthritis (SELECT-EARLY): a multicenter, multi-country, randomized, double-blind, active comparator-controlled trial. Arthritis Rheumatology (Hoboken, NJ) 2020;72(10):1607–1620. doi: 10.1002/art.41384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Smolen JS, Pangan AL, Emery P, Rigby W, Tanaka Y, Vargas JI, et al. Upadacitinib as monotherapy in patients with active rheumatoid arthritis and inadequate response to methotrexate (SELECT-MONOTHERAPY): a randomised, placebo-controlled, double-blind phase 3 study. Lancet (London, England) 2019;393(10188):2303–2311. doi: 10.1016/S0140-6736(19)30419-2. [DOI] [PubMed] [Google Scholar]
- 79.Takeuchi T, Rischmueller M, Blanco R, Xavier RM, Ueki Y, Atsumi T, et al. Upadacitinib monotherapy versus methotrexate monotherapy in methotrexate-naïve Japanese patients with rheumatoid arthritis: a sub-analysis of the Phase 3 SELECT-EARLY study. Mod Rheumatol. 2021;31(3):534–542. doi: 10.1080/14397595.2020.1847776. [DOI] [PubMed] [Google Scholar]
- 80.Gladman D, Rigby W, Azevedo VF, Behrens F, Blanco R, Kaszuba A, et al. Tofacitinib for psoriatic arthritis in patients with an inadequate response to TNF Inhibitors. N Engl J Med. 2017;377(16):1525–1536. doi: 10.1056/NEJMoa1615977. [DOI] [PubMed] [Google Scholar]
- 81.Mease P, Hall S, FitzGerald O, van der Heijde D, Merola JF, Avila-Zapata F, et al. Tofacitinib or adalimumab versus placebo for psoriatic arthritis. N Engl J Med. 2017;377(16):1537–1550. doi: 10.1056/NEJMoa1615975. [DOI] [PubMed] [Google Scholar]
- 82.Nash P, Coates LC, Kivitz AJ, Mease PJ, Gladman DD, Covarrubias-Cobos JA, et al. Safety and efficacy of tofacitinib in patients with active psoriatic arthritis: interim analysis of opal balance, an open-label long-term extension study. Rheumatol Therapy. 2020;7(3):553–580. doi: 10.1007/s40744-020-00209-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Nash P, Mease PJ, Fleishaker D, Wu J, Coates LC, Behrens F, et al. Tofacitinib as monotherapy following methotrexate withdrawal in patients with psoriatic arthritis previously treated with open-label tofacitinib plus methotrexate: a randomised, placebo-controlled substudy of OPAL balance. Lancet Rheumatol. 2021;3(1):e28–e39. doi: 10.1016/S2665-9913(20)30339-8. [DOI] [PubMed] [Google Scholar]
- 84.Mease PJ, Lertratanakul A, Anderson JK, Papp K, Van Den Bosch F, Tsuji S, et al. Upadacitinib for psoriatic arthritis refractory to biologics: SELECT-PsA 2. Ann Rheum Dis. 2021;80(3):312–320. doi: 10.1136/annrheumdis-2020-218870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McInnes IB, Anderson JK, Magrey M, Merola JF, Liu Y, Kishimoto M, et al. Trial of upadacitinib and adalimumab for psoriatic arthritis. N Engl J Med. 2021;384(13):1227–1239. doi: 10.1056/NEJMoa2022516. [DOI] [PubMed] [Google Scholar]
- 86.Mease PJ, Lertratanakul A, Papp KA, van den Bosch FE, Tsuji S, Dokoupilova E, et al. Upadacitinib in patients with psoriatic arthritis and inadequate response to biologics: 56-week data from the randomized controlled phase 3 SELECT-PsA 2 study. Rheumatol Therapy. 2021;8(2):903–919. doi: 10.1007/s40744-021-00305-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Van Der Heijde D, Deodhar A, Wei JC, Drescher E, Fleishaker D, Hendrikx T, et al. Tofacitinib in patients with ankylosing spondylitis: A phase II, 16-week, randomised, placebo-controlled, dose-ranging study. Ann Rheum Dis. 2017;76(8):1340–1347. doi: 10.1136/annrheumdis-2016-210322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Deodhar A, Sliwinska-Stanczyk P, Xu H, Baraliakos X, Gensler LS, Fleishaker D, et al. Tofacitinib for the treatment of ankylosing spondylitis: a phase III, randomised, double-blind, placebo-controlled study. Ann Rheum Dis. 2021;80(8):1004–1013. doi: 10.1136/annrheumdis-2020-219601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Deodhar A, van der Heijde D, Sieper J, Van den Bosch F, Maksymowych WP, Kim TH, et al. Safety and efficacy of upadacitinib in patients with active ankylosing spondylitis and an inadequate response to nonsteroidal antiinflammatory drug therapy: one-year results of a double-blind, placebo-controlled study and open-label extension. Arthritis Rheumatol. 2022;74(1):70–80. doi: 10.1002/art.41911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Sandborn WJ, Ghosh S, Panes J, Vranic I, Su C, Rousell S, et al. Tofacitinib, an oral Janus kinase inhibitor, in active ulcerative colitis. N Engl J Med. 2012;367(7):616–624. doi: 10.1056/NEJMoa1112168. [DOI] [PubMed] [Google Scholar]
- 91.Sandborn WJ, Su C, Sands BE, D'Haens GR, Vermeire S, Schreiber S, et al. Tofacitinib as induction and maintenance therapy for ulcerative colitis. N Engl J Med. 2017;376(18):1723–1736. doi: 10.1056/NEJMoa1606910. [DOI] [PubMed] [Google Scholar]
- 92.Sandborn WJ, Lawendy N, Danese S, Su C, Loftus EV, Jr, Hart A, et al. Safety and efficacy of tofacitinib for treatment of ulcerative colitis: final analysis of OCTAVE open, an open-label, long-term extension study with up to 7.0 years of treatment. Aliment Pharmacol Ther. 2022;55(4):464–478. doi: 10.1111/apt.16712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Avni-Biron I, Bar-Gil Shitrit A, Koslowsky B, Levartovsky A, Kopylov U, Weisshof R, et al. Short-term effectiveness and safety of tofacitinib in ulcerative colitis - real world data from tertiary medical centers in Israel. Digestive Liver Dis. 2022;54(2):192–197. doi: 10.1016/j.dld.2021.11.009. [DOI] [PubMed] [Google Scholar]
- 94.Deepak P, Alayo QA, Khatiwada A, Lin B, Fenster M, Dimopoulos C, et al. Safety of tofacitinib in a real-world cohort of patients with ulcerative colitis. Clin Gastroenterol Hepatol. 2021;19(8):1592–601.e3. doi: 10.1016/j.cgh.2020.06.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Jameshorani M, Vahedi H, Sadeghi A, Sima AR, Anushiravani A, Nateghi Beige H, et al. Efficacy and safety of tofacitinib for treatment of moderate to severe active ulcerative colitis: first report from Iran. Arch Iran Med. 2021;24(5):354–363. doi: 10.34172/aim.2021.52. [DOI] [PubMed] [Google Scholar]
- 96.Straatmijer T, van Gennep S, Duijvestein M, Ponsioen CIJ, Gecse KB, D'Haens GR, et al. Real-world clinical and endoscopic outcomes after one year tofacitinib treatment in ulcerative colitis. Eur J Gastroenterol Hepatol. 2021;33(10):1288–1297. doi: 10.1097/MEG.0000000000002028. [DOI] [PubMed] [Google Scholar]
- 97.Vermeire S, Su C, Lawendy N, Kobayashi T, Sandborn WJ, Rubin DT, et al. Outcomes of tofacitinib dose reduction in patients with ulcerative colitis in stable remission from the randomised RIVETING trial. J Crohns Colitis. 2021;15(7):1130–1141. doi: 10.1093/ecco-jcc/jjaa249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lair-Mehiri L, Stefanescu C, Vaysse T, Laharie D, Roblin X, Rosa I, et al. Real-world evidence of tofacitinib effectiveness and safety in patients with refractory ulcerative colitis. Digestive Liver Dis. 2020;52(3):268–273. doi: 10.1016/j.dld.2019.10.003. [DOI] [PubMed] [Google Scholar]
- 99.Chaparro M, Garre A, Mesonero F, Rodríguez C, Barreiro-De Acosta M, Martínez-Cadilla J, et al. Tofacitinib in ulcerative colitis: real-world evidence from the ENEIDA registry. J Crohns Colitis. 2021;15(1):35–42. doi: 10.1093/ecco-jcc/jjaa145. [DOI] [PubMed] [Google Scholar]
- 100.Sandborn WJ, Ghosh S, Panes J, Schreiber S, D'Haens G, Tanida S, et al. Efficacy of upadacitinib in a randomized trial of patients with active ulcerative colitis. Gastroenterology. 2020;158(8):2139–49.e14. doi: 10.1053/j.gastro.2020.02.030. [DOI] [PubMed] [Google Scholar]
- 101.Redeker I, Albrecht K, Kekow J, Burmester GR, Braun J, Schäfer M, et al. Risk of herpes zoster (shingles) in patients with rheumatoid arthritis under biologic, targeted synthetic and conventional synthetic DMARD treatment: data from the German RABBIT register. Ann Rheum Dis. 2022;81(1):41–47. doi: 10.1136/annrheumdis-2021-220651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Song YJ, Cho SK, Kim H, Kim HW, Nam E, Choi CB, et al. Risk factors for herpes zoster in Korean patients with rheumatoid arthritis treated with JAK inhibitor: a nested case-control study. RMD Open. 2022 doi: 10.1136/rmdopen-2021-001892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Choi W, Ahn SM, Kim YG, Lee CK, Yoo B, Hong S. Safety of JAK inhibitor use in patients with rheumatoid arthritis who developed herpes zoster after receiving JAK inhibitors. Clin Rheumatol. 2022 doi: 10.1007/s10067-022-06096-0. [DOI] [PubMed] [Google Scholar]
- 104.Pavelka K, Szekanecz Z, Damjanov N, Anić B, Tomšič M, Mazurov V, et al. Upadacitinib versus placebo or adalimumab with background methotrexate in patients with rheumatoid arthritis and an inadequate response to methotrexate: a subgroup analysis of a phase III randomized controlled trial in Central and Eastern European patients. Drugs Context. 2020 doi: 10.7573/dic.2020-7-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.van der Heijde D, Song IH, Pangan AL, Deodhar A, van den Bosch F, Maksymowych WP, et al. Efficacy and safety of upadacitinib in patients with active ankylosing spondylitis (SELECT-AXIS 1): a multicentre, randomised, double-blind, placebo-controlled, phase 2/3 trial. Lancet (London, England) 2019;394(10214):2108–2117. doi: 10.1016/S0140-6736(19)32534-6. [DOI] [PubMed] [Google Scholar]
- 106.Sands BE, Armuzzi A, Marshall JK, Lindsay JO, Sandborn WJ, Danese S, et al. Efficacy and safety of tofacitinib dose de-escalation and dose escalation for patients with ulcerative colitis: results from OCTAVE Open. Aliment Pharmacol Ther. 2020;51(2):271–280. doi: 10.1111/apt.15555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Burmester GR, Nash P, Sands BE, Papp K, Stockert L, Jones TV, et al. Adverse events of special interest in clinical trials of rheumatoid arthritis, psoriatic arthritis, ulcerative colitis and psoriasis with 37 066 patient-years of tofacitinib exposure. RMD Open. 2021 doi: 10.1136/rmdopen-2021-001595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Alves C, Penedones A, Mendes D, Marques FB. The risk of infections associated with JAK inhibitors in rheumatoid arthritis: a systematic review and network meta-analysis. J Clin Rheumatol. 2022;28(2):e407–e414. doi: 10.1097/RHU.0000000000001749. [DOI] [PubMed] [Google Scholar]
- 109.Olivera PA, Lasa JS, Bonovas S, Danese S, Peyrin-Biroulet L. Safety of Janus kinase inhibitors in patients with inflammatory bowel diseases or other immune-mediated diseases: a systematic review and meta-analysis. Gastroenterology. 2020;158(6):1554–73.e12. doi: 10.1053/j.gastro.2020.01.001. [DOI] [PubMed] [Google Scholar]
- 110.Huang F, Luo ZC. Risk of adverse drug events observed with baricitinib 2 mg Versus Baricitinib 4 mg once daily for the treatment of rheumatoid arthritis: a systematic review and meta-analysis of randomized controlled trials. BioDrugs. 2018;32(5):415–423. doi: 10.1007/s40259-018-0304-3. [DOI] [PubMed] [Google Scholar]
- 111.Huang F, Luo ZC. Adverse drug events associated with 5mg versus 10mg Tofacitinib (Janus kinase inhibitor) twice daily for the treatment of autoimmune diseases: a systematic review and meta-analysis of randomized controlled trials. Clin Rheumatol. 2019;38(2):523–534. doi: 10.1007/s10067-018-4299-4. [DOI] [PubMed] [Google Scholar]
- 112.Winthrop KL, Curtis JR, Lindsey S, Tanaka Y, Yamaoka K, Valdez H, et al. Herpes zoster and tofacitinib: clinical outcomes and the risk of concomitant therapy. Arthritis Rheumatol (Hoboken, NJ) 2017;69(10):1960–1968. doi: 10.1002/art.40189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bing N, Zhou H, Chen X, Hirose T, Kochi Y, Tsuchida Y, et al. Contribution of a European-prevalent variant near CD83 and an East Asian-Prevalent Variant near IL17RB to Herpes Zoster Risk in Tofacitinib Treatment: results of genome-wide association study meta-analyses. Arthritis Rheumatol (Hoboken, NJ) 2021;73(7):1155–1166. doi: 10.1002/art.41655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Winthrop KL, Melmed GY, Vermeire S, Long MD, Chan G, Pedersen RD, et al. Herpes zoster infection in patients with ulcerative colitis receiving tofacitinib. Inflamm Bowel Dis. 2018;24(10):2258–2265. doi: 10.1093/ibd/izy131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Baumrin E, Van Voorhees A, Garg A, Feldman SR, Merola JF. A systematic review of herpes zoster incidence and consensus recommendations on vaccination in adult patients on systemic therapy for psoriasis or psoriatic arthritis: from the medical board of the national psoriasis foundation. J Am Acad Dermatol. 2019;81(1):102–110. doi: 10.1016/j.jaad.2019.03.017. [DOI] [PubMed] [Google Scholar]
- 116.Nash P, Kerschbaumer A, Dorner T, Dougados M, Fleischmann RM, Geissler K, et al. Points to consider for the treatment of immune-mediated inflammatory diseases with Janus kinase inhibitors: a consensus statement. Ann Rheum Dis. 2021;80(1):71–87. doi: 10.1136/annrheumdis-2020-218398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cohen SB, Tanaka Y, Mariette X, Curtis JR, Lee EB, Nash P, et al. Long-term safety of tofacitinib up to 9.5 years: a comprehensive integrated analysis of the rheumatoid arthritis clinical development programme. RMD Open. 2020 doi: 10.1136/rmdopen-2020-001395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Cohen SB, van Vollenhoven RF, Winthrop KL, Zerbini CAF, Tanaka Y, Bessette L, et al. Safety profile of upadacitinib in rheumatoid arthritis: integrated analysis from the SELECT phase III clinical programme. Ann Rheum Dis. 2020;80(3):304–311. doi: 10.1136/annrheumdis-2020-218510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Winthrop KL, Curtis JR, Yamaoka K, Lee EB, Hirose T, Rivas JL, et al. Clinical management of herpes zoster in patients with rheumatoid arthritis or psoriatic arthritis receiving tofacitinib treatment. Rheumatol Therapy. 2022;9(1):243–263. doi: 10.1007/s40744-021-00390-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Furer V, Rondaan C, Heijstek MW, Agmon-Levin N, van Assen S, Bijl M, et al. 2019 update of EULAR recommendations for vaccination in adult patients with autoimmune inflammatory rheumatic diseases. Ann Rheum Dis. 2020;79(1):39–52. doi: 10.1136/annrheumdis-2019-215882. [DOI] [PubMed] [Google Scholar]
- 121.Anderson TC, Masters NB, Guo A, Shepersky L, Leidner AJ, Lee GM, et al. Use of recombinant zoster vaccine in immunocompromised adults aged >/=19 years: recommendations of the advisory committee on immunization practices—United States, 2022. MMWR Morb Mortal Wkly Rep. 2022;71(3):80–84. doi: 10.15585/mmwr.mm7103a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Smolen JS, Genovese MC, Takeuchi T, Hyslop DL, Macias WL, Rooney T, et al. Safety profile of baricitinib in patients with active rheumatoid arthritis with over 2 years median time in treatment. J Rheumatol. 2019;46(1):7–18. doi: 10.3899/jrheum.171361. [DOI] [PubMed] [Google Scholar]
- 123.Cochrane methods: ROBINS-I tool for non-randomized studies https://methods.cochrane.org/methods-cochrane/robins-i-tool Accessed 15 Nov 2022.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary file 1: Table 1 Approved doses of tofacitinib, baricitinib and upadacitinib for the treatment of RA, PsA, AS and UC. Table 2 RCTs and LTEs included in this SLR. Study and patient characteristics, incident herpes zoster events and risk of bias. Table 3 Real-world studies included in this SLR. Study and patient characteristics, incident herpes zoster events and risk of bias. Table 4 Risk of bias in non-randomized studies according to the Newcastle-Ottawa scale. Figure 1 Risk of bias in randomized studies according to the Cochrane RoB 2 tool.
Data Availability Statement
Data for this study are presented in the manuscript, tables and supplementary material. Additional data are available upon request to the corresponding author.